Note: Descriptions are shown in the official language in which they were submitted.
CA 02530460 2006-07-06
METHOD OF TREATING ACUTE CORONARY SYNDROMES
1. FIELD OF INVENTION
[0002] The present invention relates to methods and compositions designed for
the
treatment or management of acute coronary syndromes, particularly, unstable
angina and
acute myocardial infarction. The methods of the invention comprise the
administration of an
effective amount of a formulation containing one or more therapeutic agents
which
specifically decreases or inhibits the activity of and/or eliminates or
diminishes the amount of
phagocytic cells including, but not limited to, macrophages and monocytes.
2. BACKGROUND OF THE INVENTION
[0003] Coronary artery disease is a leading cause of death in industrialized
countries.
In the United States, 50-60% of heart attacks occur in people without
documented coronary
artery disease. A chief contributor to the pathology of the disease is the
formation of
atherosclerotic plaques. Atherosclerotic plaques are thickened areas in vessel
walls which
result from an accumulation of cholesterol, proliferating smooth muscle cells,
and
inflammatory cells.
Atherosclerotic Plaques
[00041 In general, an atherosclerotic plaque consists of a raised focal point
within the
intima having a central core of extra-cellular lipids covered by a fibrous
cap. The core within
the plaque contains crystalline cholesterol, cholesterol esters,
phospholipids, cellular
degradation products and collagen remnants. The fibrous cap separates the core
of the plaque
from the lumen of the blood vessel or artery and is comprised mainly of
connective tissues
that are a dense, fibrous extracellular matrix made up of collagens, elastins,
proteoglycans
and other extracellular matrix materials. The fibrous cap varies in thickness,
number of
smooth muscle cells and macrophages, and collagen content. (Vallabhajosula et
al., 1997, J.
Nucl. Med. 38(11): 1788-1796).
[00051 Atherosclerotic plaques can be characterized as active and prone to
rupture
("vulnerable or high-risk plaques") or inactive and relatively stable ("stable
plaques"). A
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
vulnerable, high-risk or rupture-prone plaque is characterized by an abundance
of
inflammatory cells (such as macrophages), a thin fibrous cap, and a large
lipid core. The size
of the lipid pool within the atherosclerotic plaque and the thickness of the
overlying fibrous
cap are important characteristics predicting the stability of the plaque. The
edge of the
fibrous cap (the shoulder region) is a location of high stress and predisposed
to rupture, in
part, due to the accumulation of inflammatory cells (such as macrophages) in
the area and
their secretion of enzymes that cause degradation of the material that makes
up the fibrous
cap (Moreno et al, Circulation., 1994, 90:775-8; van der Wal et al., 1994,
Circulation 89:36-
44.; Jander et al, 1998, Stroke, 29:1625-1630) which can lead to rupture of
the plaque.
[00061 Rupture of the lipid-laden plaque exposes the highly thrombogenic core
and
the sub-endothelial vascular smooth muscle cell component of the arterial wall
to circulating
blood. Platelet activation, adhesion and aggregation follow this almost
immediately. Platelet
adhesion and activation results in the release of coagulation factors and the
initiation of the
coagulation cascade. The released growth factors, specifically platelet-
derived growth factor
(PDGF) stimulate, the proliferation and migration of vascular smooth muscle
cells.
Proliferation and migration of vascular smooth muscle cells can lead to plaque
remodeling
and increased vascular stenosis, or interact with the platelets leading to
enhanced
thrombogenesis (Pasterkamp et al., 2000, J. Clin. Basic Cardiol. 3:81-86). The
resulting
thrombosis caused by the vulnerable plaque can cause unstable angina, acute
myocardial
infarction, stroke, acute deterioration in peripheral artery disease, or
sudden coronary death.
Unstable Angina
[0007] The heart requires oxygen-rich blood to function. The right and left
coronary
arteries branch from the aorta and carry oxygenated blood to the tissues of
the heart. When
the coronary arteries fail to deliver an adequate amount of oxygen-rich blood
(a condition
called hypoxia) to the heart, chest pain, pressure, or discomfort, commonly
known as angina,
result. If this situation is prolonged, oxygen depravation can damage the
heart muscle itself
(a situation known as ischemia) either reversibly or irreversibly.
[00081 Angina is classified broadly as stable or unstable, depending on its
severity
and pattern of occurrence. Stable angina occurs when increased physical
activity (e.g.,
hurrying across a street or climbing a long flight of stairs) raises the
demand for oxygen-rich
2
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
blood. Due to a possible multitude of factors (the most common of which is one
or more
occluded coronary arteries), the supply created by the coronary blood flow
cannot meet this
increased demand and hypoxia results. Unstable angina is understood as anginal
pain that
occurs with lesser degrees of exertion, increasing frequency, or at rest
(i.e., without exertion).
Unstable angina that occurs at rest represents the condition in its most
serious form. It
usually is caused by the formation of a blood clot in a coronary artery at the
site of a ruptured
plaque and, if left untreated, it may result in a heart attack and
irreversible damage to the
heart.
[00091 Unstable angina is likely due to the partial rupture of a vulnerable
plaque that
has become unstable. The plaque's partial rupture causes a thrombus to
develop, but does not
completely occlude the artery. Endogenous clot-fighting mechanisms serve to
break up the
clot but, over time, the plaque continues to rupture and the clotting episodes
repeat. Although
this patient may not have yet suffered a myocardial infarction, he or she is
at high risk of
doing so (e.g., if the unstable plaque completely ruptures or if the
endogenous clot fighting
mechanisms cannot eliminate the clot before total occlusion of the artery).
Disrupted fibrous
caps taken post mortem from patients with unstable angina are often more
heavily infiltrated
with macrophages at the plaque rupture site than plaque from cases of stable
angina.
Acute Myocardial Infarction
[0010] Acute myocardial infarction ("AMI") refers to a common clinical
condition
that leads to necrosis of myocardial tissue. This condition is well known in
the art and is
characterized by the occurrence of pain (in most cases precordial),
characteristic
electrocardiographic changes, and an increase in plasma levels of
intracellular enzymes (such
as creatinine phosphokinase and c -hydroxybutyrate dehydrogenase) or cardiac
proteins (such
as components of the troponin complex, and myoglobin) released by the necrotic
cardiac
tissue. AM may be accompanied by hypotension, circulatory failure, pulmonary
edema and
arrhythmia. In most cases, but not exclusively, AMI results from vascular
injury and
thrombosis in the coronary vessels, which causes these vessels to become
occluded with
subsequent impaired blood flow to the jeopardized myocardium (Fuster et al.,
1992, New
Engl.J Med., 326:242-310). In most cases, the time of the occlusion of the
coronary vessel
3
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
can be estimated from the medical history, the course of plasma levels of
intracellular heart
muscle enzymes and electrocardiographic changes.
[00111 The initiating event of many myocardial infarctions (heart attacks) is
rupture
of an atherosclerotic plaque. Such rupture may result in formation of a
thrombus or blood
clot in the coronary artery which supplies the infarct zone. The infarct zone
or area, as it is
commonly referred to, is an area of necrosis which results from an obstruction
of blood
circulation. The thrombus formed is composed of a combination of fibrin and
blood cells.
The location, degree and duration of the occlusion caused by the clot
determine the mass of
the infarct zone and the extent of damage. Ultimately, the extent of
myocardial damage
caused by the coronary occlusion depends upon the "territory" supplied by the
affected
vessel, the degree of occlusion of the vessel, the amount of blood supplied by
collateral
vessels to the affected tissue, and the demand for oxygen of the myocardium
whose blood
supply has suddenly been limited (Pasternak and Braunwald, 1994, Acute
Myocardial
Infarction, Harrison's Principles ofInternal Medicine, 13th Ed., pgs. 1066-
77).
Macrophages and the Inflammatory Response
[00121 Macrophages are involved in the cause and/or pathology of some coronary
syndromes. Macrophage secretion of proteolytic proteins that degrade the
fibrous caps of
plaques decrease cap thickness as well as increase additional macrophage
infiltration thus
contributing to plaque instability. Therefore macrophages are considered to
have a central
role in plaque rupture and their presence in large concentrations is
considered predictive to
such rupture. Indeed, erosion and/or disruption of the fibrous cap of
atherosclerotic plaques
is known to modulate arterial thrombus formation, leading to the onset of
acute ischemic
events. It is clear that rupture at the site of a vulnerable atherosclerotic
plaque is the most
frequent cause of acute coronary syndromes, such as unstable angina,
myocardial infarction
or sudden death.
[00131 Inflammation has been related both to the pathogenesis of acute
myocardial
infarctions and to the healing and repair following AMI. Myocardial ischemia
prompts an
inflammatory response. In addition, reperfusion, the mainstay of current acute
therapy of
AMI, also enhances inflammation. Reperfusion involves the rapid dissolution of
the
occluding thrombus and the restoration of blood flow to the area of the heart
which has had
4
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
its blood supply cut off. The presence of inflammatory cells in the ischemic
myocardial
tissues has traditionally been believed to represent the pathophysiological
response to injury.
However, experimental studies have shown that while crucial to healing, the
influx of
inflammatory cells into tissues, specifically macrophages which are phagocytic
cells, results
in tissue injury beyond that caused by ischemia alone.
[00141 Macrophages and other leukocytes infiltrate the myocardium soon after
ischemia ensues. Macrophages secrete several cytokines, which stimulate
fibroblast
proliferation. However, the activated macrophages also secrete cytokines and
other
mediators that promote myocardial damage. Accordingly, the influx of
macrophages into the
myocardium increases myocardial necrosis and expands the zone of infarct.
Thus, although
the acute phase of inflammation is a necessary response for the healing
process, persistent
,activation is in fact harmful to the infarct area as well as the area
surrounding it, the so-called
`peri-infarct zone'.
[00151 The inflammatory response that follows myocardial ischemia is critical
in
determining the severity of the resultant damage caused by the activated
macrophages.
Plasma levels of inflammatory chemotactic factors (macrophage chemoattractant
protein-1
(MCP-1), macrophage inflammatory protein-1 alpha (MIP-1 alpha), have been
shown to
correlate with subsequent heart failure and left ventricular dysfunction (see,
for example,
Parissis, et al., 2002, J. Interferon Cytokine Res., 22(2):223-9). Peripheral
monocytosis (an
elevated number of monocytes) at two and three days after AMI is associated
with left
ventricular dysfunction and left ventricular aneurysm, suggesting a possible
role of
monocytes in the development of left ventricular remodeling after reperfused
AMI
(Maekawa, Y. et al., 2002, J. Am. Coll. Cardiol., 39(2):241-6). Left
ventricular remodeling
after acute myocardial infarction is the process of infarct expansions
followed by progressive
left ventricular dilation and is associated with an adverse clinical outcome.
Furthermore,
plasma levels of macrophage chemoattractant protein-1 (MCP-1) are elevated in
patients with
acute myocardial infarction. MCP-1 is induced by myocardial
ischemia/reperfusion injury
and neutralization of this chemokine significantly reduced infarct size.
[00161 Suppression of the inflammatory response by nonspecific anti-
inflammatory
composites after coronary occlusion, in several coronary occlusion/reperfusion
models, was
shown to reduce the infarct area (See, for example, Squadrito, et al., 1997,
Eur. J.
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
Pharmacol.; 335:185-92; Libby, et al., 1973, J. Clin. Invest., 3:599-607;
Spath, et al., 1974,
Circ. Res., 35: 44-51). However, these nonspecific regimens are associated
with adverse
effects, such as interference with scar formation and healing, and, in some
patients, the
development of aneurysm and rupture of the ventricular wall. As such, these
regimens are
precluded from clinical use. However, animal models that have a decreased
ability to
suppress macrophage function due to a deficiency in the anti-inflammatory
cytokine
interleukin-10 were shown to suffer from increased infarct size and myocardial
necrosis in a
coronary occlusion model (Yang, Z. et al., 2000, Circulation, 101:1019-1026.)
[00171 One object of the present invention is the identification of
therapeutic agents
capable of blocking the accumulation of and/or the biological function
including secretion of
factors from phagocytic cells (particularly macrophages and monocytes) in the
patient
suffering from an acute coronary syndrome (particularly unstable angina or and
acute
myocardial infarction).
[00181 Another object of the invention is the development of methods for
treating an
acute coronary syndrome (particularly unstable angina or and acute myocardial
infarction) as
well as stabilizing the plaques associated with these syndromes.
3. SUMMARY OF THE INVENTION
[00191 The present invention relates to methods and compositions designed for
the
treatment or management of acute coronary syndromes, particularly, unstable
angina and
acute myocardial infarction. The methods of the invention comprise the
administration of an
effective amount of a formulation containing one or more therapeutic agents
which
specifically inhibits the activity of and/or diminishes the amount of
phagocytic cells
including, but not limited to, macrophages and monocytes. Administration of a
formulation
containing one or more therapeutic agents according to the invention acts as
an acute,
treatment aimed at stabilizing the patient's coronary syndrome condition. In
one
embodiment, a formulation containing one or more therapeutic agents is
administered to a
patient suffering from unstable angina to stabilize a vulnerable or unstable
plaque. In another
embodiment, a formulation is administered to a patient currently suffering or
recently having
suffered an acute myocardial infarction to minimize infarct size and
myocardial necrosis.
6
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
[00201 In preferred embodiments, the formulation specifically targets
phagocytic
cells. Because phagocytic cells possess the unique ability of phagocytosis, in
these
embodiments, the formulations are prepared such that they comprise particles
of such
properties as to enter into a cell primarily or exclusively via phagocytosis.
The formulation
may comprise an encapsulated therapeutic agent, an embedded therapeutic agent,
or a
particulate therapeutic agent. Once phagocytosed, the therapeutic agent is
released from the
formulation into the targeted phagocytic cells, e.g., macrophages and
monocytes, and inhibits
the function of and/or destroys the phagocytic cell.
[00211 In one embodiment, the present invention relates to a method of
treating an
acute coronary syndrome by administering to an individual in need thereof an
effective
amount of a formulation comprising an encapsulated therapeutic agent. The
therapeutic
agent is encapsulated in a suitable carrier of a specific dimension. The
formulation
specifically targets phagocytic cells by virtue of its properties, such as,
for example, size or
charge, which allow the formulation to be taken-up primarily or exclusively by
phagocytosis.
Once the formulation is taken-up by the phagocytic cell, the encapsulated
therapeutic agent is
released and the agent is able to inhibit the activity of and/or destroy the
phagocytic cell.
[00221 In another embodiment, the present invention relates to a method of
treating
an acute coronary syndrome by administering to an individual in need thereof
an effective
amount of a formulation comprising an embedded therapeutic agent. The
therapeutic agent is
embedded in a suitable carrier of a specific dimension. The formulation
specifically targets
phagocytic cells by virtue of its properties, such as, for example, size
and/or charge, which
allow the formulation to be taken-up primarily or exclusively by phagocytosis.
Once inside
the phagocytic cells the embedded therapeutic agent is released and the agent
is able to
inhibit the activity of and/or destroy the phagocytic cell.
[00231 In another embodiment, the present invention relates to a method of
treating
an acute coronary syndrome by administering to an individual in need thereof
an effective
amount of a formulation comprising a particulate therapeutic agent. The
therapeutic agent is
made into particulates of a specific dimension. The formulation specifically
targets
phagocytic cells by virtue of the particulate's properties, such as, for
example, size and/or
charge, which allow the formulation to be taken-up primarily or exclusively by
phagocytosis.
7
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
Once inside the phagocytic cells the particulate therapeutic agent is able to
inhibit the activity
of and/or destroy the phagocytic cell.
[00241 The present invention also relates to a method of stabilizing plaques
associated
with an acute coronary syndrome by administering to an individual in need
thereof an
effective amount of a formulation comprising an encapsulated, embedded, or
particulate
therapeutic agent.
[0025] In a further embodiment, the present invention includes a
pharmaceutical
composition for administration to subjects currently suffering from or having
recently
suffered an acute coronary syndrome such as unstable angina and acute
myocardial infarction
comprising a formulation selected from the group consisting of an encapsulated
therapeutic
agent, an embedded therapeutic agent, and a particulate therapeutic agent
together with a
pharmaceutically acceptable vehicle, carrier, stabilizer or diluent for the
treatment of an acute
coronary syndrome.
[0026] The formulation of present invention is preferably in the size range of
0.03-1.0
microns. However, depending on the type of agent and/or the carrier used, the
more
preferred ranges include, but are not limited to, 0.07-0.5 microns, 0.1-0.3
microns and 0.1 to
0.18.
4. BRIEF DESCRIPTION OF THE FIGURES
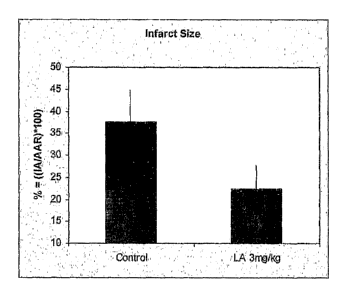
[0027] FIG. 1 illustrates the effect of liposomal alendronate treatment on the
size of
infarct area after transient coronary artery occlusion in rabbits. The size of
the infarct zone
was calculated as the area of the infarcted zone as a % of the left
ventricular area supplied by
the occluded artery and thus at risk for subsequent infarction. Data are
expressed as mean
SD, with n=4/group and a p value of p <0.05.
[0028] FIGS. 2A-2B illustrate the effect of liposomal alendronate treatment on
myocardial morphology after reversible coronary occlusion in rabbits. Control
rabbits (A)
have distorted myocardial morphology while rabbits treated with liposomal
alendronate (B)
have a more normal myocardial morphology.
FIGS. 3A-3B illustrate the reduction in macrophage infiltration following
treatment with liposomal alendronate after reversible coronary occlusion in
rabbits. Control
8
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
rabbits (A) show increased RAMI 1+ macrophage accumulation in the zone of
infarct as
compared to rabbits treated with liposomal alendronate (B).
5. DETAILED DESCRIPTION OF THE INVENTION
[00291 Phagocytic cells, particularly macrophages and monocytes, are involved
in the
cause and/or pathology of some coronary syndromes. Macrophages/monocytes
degrade the
fibrous caps of plaques through the secretion of various substances that not
only decrease cap
thickness, but also serve to recruit additional macrophages/monocytes to the
area.
Degradation of the fibrous cap leads to exposure to blood of the lipid core of
the plaque as
well as initiation of the clotting cascade which culminates in a thrombus. The
thrombus may
partially occlude the lumen leading to unstable angina or it may completely
occlude the
lumen thus causing an acute mycardial infarction. Once an acute myocardial
infarction
occurs, macrophages/monocytes are recruited to the damaged myocardial tissue
and secrete
cytokines and other mediators that promote myocardial damage thus resulting in
tissue injury
beyond that caused by ischemia alone and increases myocardial necrosis which
expands the
zone of infarct. Although a complete and chronic incapacitation and/or
ablation of
phagocytic cells is not desirable, such a decrease in phagocytic cell activity
and/or presence is
desirable in the short term during or after an acute coronary syndrome to
stabilize the patient
and/or reduce the damage of the coronary syndrome.
[00301 The present invention relates to methods and compositions designed to
decrease or inhibit the activity of and/or eliminate or diminish the amount of
phagocytic cells
(including, but not limited to, macrophages and monocytes) for an acute, short
term period
during or following an acute coronary syndrome for the treatment or management
of the
acute coronary syndrome (including, but not limited to, unstable angina and
acute myocardial
infarction). The methods of the invention comprise the administration of an
effective amount
of a formulation containing one or more therapeutic agents which specifically
decreases or
inhibits the activity of and/or eliminates or diminishes the amount of
phagocytic cells
(including, but not limited to, macrophages and monocytes) in a patient.
Administration of a
formulation containing one or more therapeutic agents is contemplated as an
acute, short term
treatment aimed at stabilization of the patient and/or minimization of the
immediate and long
term damage from the acute coronary syndrome. In one embodiment, a formulation
containing one or more therapeutic agents are administered to a patient
suffering from
9
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
unstable angina to stabilize a vulnerable or unstable plaque and decrease the
immediate threat
of an acute myocardial infarction. In another embodiment, one or more
therapeutic agents
are administered to a patient currently suffering or recently having suffered
an acute
myocardial infarction to minimize the infarct size and myocardial necrosis.
[0031] The formulations used in the methods of the invention specifically
decrease or
inhibit the activity of phagocytic cells and/or eliminate or diminish the
amount of phagocytic
cells in a patient. Specificity of the formulation is due to the ability of
the composition to
affect only particular cell types (e.g., macrophages and/or monocytes). In
preferred
embodiments, specificity of the fonnulation for phagocytic cells is due to the
physiochemical
properties ,e.g. size or charge, of the formulation such that it can only or
primarily be
internalized by phagocytosis. Once phagocytosed and intracellular, the
therapeutic agent
inhibits or decreases the activity of the phagocytic cell and/or destroys the
phagocytic cell.
Although not intending to be bound by any particular mechanism of action, the
therapeutic
agents of the formulation are released upon becoming intracellular before
disabling an/or
destroying the phagocytic cell.
[00321 The formulation of the present invention, e. g., the encapsulated
therapeutic
agent, embedded therapeutic agent or the particulate therapeutic agent,
suppresses the
inflammatory response by transiently depleting and/or inactivating cells that
are important
triggers in the inflammatory response, namely macrophages and/or monocytes.
The
encapsulated agent, embedded agent and/or particulate agent are taken-up, by
way of
phagocytosis, by the macrophages and monocytes. In contrast, non-phagocytic
cells are
incapable of taking up the formulation due to the large dimension and/or other
physiochemical properties of the formulation.
[00331 The term "phagocytosis" as used herein refers to a preferred means of
entry
into a phagocytic cell and is well understood in the art. However, the term
should be
understood to also encompass other forms of endocytosis which may also
accomplish the
same effect. In particular, it is understood that pinocytosis, receptor-
mediated endocytosis
and other cellular means for absorbing/internalizing material from outside the
cell are also
encompassed by the methods and compositions of the present invention.
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
[0034] The invention also provides pharmaceutical compositions comprising one
or
more therapeutic agents of the invention for administration to subjects
currently suffering
from or recently having suffered an acute coronary syndrome such as unstable
angina and
acute myocardial infarction.
5.1 THERAPEUTIC AGENTS
[00351 The therapeutic agents used in the formulations and in the methods of
the
invention specifically decrease or inhibit the activity of phagocytic cells
and/or eliminate or
diminish the amount of phagocytic cells in a patient, by virtue of the
physiochemical
properties, such as size or charge, of the formulation. The therapeutic agent
may be an intra-
cellular inhibitor, deactivator, toxin, arresting substance and/or
cytostatic/cytotoxic substance
that, once inside a phagocytic cell such as a macrophage or monocyte,
inhibits, destroys,
arrests, modifies and/or alters the phagocytic cell such that it can no longer
function normally
and/or survive.
[00361 As used herein, the term "therapeutic agents" refers to molecules which
either
make up the formulation or form a part of the formulation and provide the
inactivating/toxic
potency to the formulation., e.g., inhibits or decreases phagocytic cell
activity and/or
eliminates or decreases the amount of phagocytic cells. Compounds that can be
therapeutic
agents include, but are not limited to, inorganic or organic compounds; or a
small molecule
(less than 500 daltons) or a large molecule, including, but not limited to,
inorganic or organic
compounds; proteinaceous molecules, including, but not limited to, peptide,
polypeptide,
protein, post-translationally modified protein, antibodies etc.; or a nucleic
acid molecule,
including, but not limited to, double-stranded DNA, single-stranded DNA,
double-stranded
RNA, single-stranded RNA, or triple helix nucleic acid molecules. Compounds
can be
natural products derived from any known organism (including, but not limited
to, animals,
plants, bacteria, fungi, protista, or viruses) or from a library of synthetic
molecules.
Therapeutic agents can be monomeric as well as polymeric compounds.
[00371 In preferred embodiments where the preferred therapeutic agent may be a
bisphosphonate or analog thereof. The term "bisphosphonate" as used herein,
denotes both
geminal and non-geminal bisphosphonates. In a specific embodiment, the
bisphosphonate
has the following formula (1):
11
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
OH 11 OH
O= P C P= O (I)
I I I
OH R 2 OH
wherein R1 is H, OH or a halogen atom; and R2 is halogen; linear or branched
C1-Clo alkyl or
C2-C10 alkenyl optionally substituted by heteroaryl or heterocyclyl C1-CIO
alkylamino or C3-
C8 cycloalkylamino where the amino may be a primary, secondary or tertiary; -
NHY where
Y is hydrogen, C3-C8 cycloalkyl, aryl or heteroaryl; or R2 is -SZ where Z is
chlorosubstituted
phenyl or pyridinyl.
[00381 In a more specific embodiment, the bisphosphoate is alendronate or an
analog
thereof. In such an embodiment, the alendronate has the following formula
(II):
OH OH OH
O= P - C- P= O (II)
OH (CH2)3 OH
NH2
[00391 In other specific embodiments, additional bisphosphonates can be used
in the
methods of the invention. Examples of other bisphosphonates include, but are
not limited to,
clodronate, tiludronate, 3-(N,N-dimethylamino)-1-hydroxypropane-l,1-
diphosphonic acid,
e.g. dimethyl-APD; 1-hydroxy-ethylidene-1,1-bisphosphonic acid, e.g.
etidronate; 1-hydroxy-
3(methylpentylamino)-propylidene-bisphosphonic acid, (ibandronic acid), e.g.
ibandronate;
6-amino- 1-hydroxyhexane-1,1-diphosphonic acid, e.g. amino-hexyl-BP; 3-(N-
methyl-N-
pentylamino)- 1-hydroxypropane-1,l-diphosphonic acid, e.g. methyl-pentyl-APD;
1-hydroxy-
2-(imidazol-l-yl)ethane-1,1-diphosphonic acid, e.g. zoledronic acid; 1-hydroxy-
2-(3-
pyridyl)ethane-l,l-diphosphonic acid (risedronic acid), e.g. risedronate; 3-[N-
(2-
12
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
phenylthioethyl)-N-methylamino]-1-hydroxypropane-1, l-bishosphonic acid; 1-
hydroxy-3-
(pyrrolidin-1-yl)propane-1,1-bisphosphonic acid, 1-(N-
phenylaminothiocarbonyl)methane-
1,1-diphosphonic acid, e.g. FR 78844 (Fujisawa); 5-benzoyl-3,4-dihydro-2H-
pyrazole-3,3-
diphosphonic acid tetraethyl ester, e.g. U81581 (Upjohn); and 1-hydroxy-2-
(imidazo[1,2-
a]pyridin-3-yl)ethane-1,1-diphosphonic acid, e.g. YM 529, or analogs thereof.
[00401 Other formulations containing therapeutic agents include, but are not
limited
to, gallium, gold, selenium, gadolinium, silica, mithramycin, paclitaxel,
sirolimus,
everolimus, and other similar analogs thereof. Generally, chemotherapeutic
agents, such as,
for example, 5-fluorouracil, cisplatinum, alkylating agents and other anti-
proliferation or anti-
inflammatory compounds, such as, for example, steroids, aspirin and non-
steroidal anti-
inflammatory drugs may also be used in a formulation.
[00411 The present invention is meant to encompass the administration of one
or
more formulations to manage or treat an acute coronary syndrome. More than one
formulation can be administered in combination to the patient. The term "in
combination" is
not limited to the administration of the formulation at exactly the same time,
but rather it is
meant that the formulations are administered to a patient in a sequence and
within a time
interval such that they can act together to provide an increased benefit than
if they were
administered otherwise. For example, each formulation may be administered at
the same
time or sequentially in any order at different points in time; however, if not
administered at
the same time, they should be administered sufficiently close in time so as to
provide the
desired therapeutic effect. Each formulation can be administered separately,
in any
appropriate form and by any suitable route which effectively transports the
therapeutic agent
to the appropriate or desirable site of action. Preferred modes of
administration include
intravenous (IV) and intra-arterial (IA). Other suitable modes of
administration include
intramuscular (IM), subcutaneous (SC), and intraperitonal (IP) and oral (PO).
Such
administration may be bolus injections or infusions. Another mode of
administration may be
by perivascular delivery. The formulation may be administered directly or
after dilution.
Combinations of any of the above routes of administration may also be used in
accordance
with the invention.
[0042] In various embodiments, the formulations are administered less than 1
hour
apart, at about 1 hour apart, at about 1 hour to about 2 hours apart, at about
2 hours to about 3
13
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
hours apart, at about 3 hours to about 4 hours apart, at about 4 hours to
about 5 hours apart, at
about 5 hours to about 6 hours apart, at about 6 hours to about 7 hours apart,
at about 7 hours
to about 8 hours apart, at about 8 hours to about 9 hours apart, at about 9
hours to about 10
hours apart, at about 10 hours to about 11 hours apart, at about 11 hours to
about 12 hours
apart, no more than 24 hours apart or no more than 48 hours apart. In one
embodiment two
or more formulations are administered concurrently or within the same patient
visit.
5.1.1 IDENTIFICATION OF THERAPEUTIC AGENTS
[0043] The invention provides methods of screening for compounds that can be
used
as a therapeutic agent. Although not intending, to be bound by a particular
mechanism of
action, a compound that is a therapeutic agent for use in the methods of the
invention can,
once targeted to the phagocytic cell by the physiochemical properties of the
formulation
itself, i) inhibit phagocytic cell activity, ii) decrease phagocytic cell
activity, iii) eliminate
phagocytic cells from circulation and/or from the area affected by the acute
coronary
syndrome, and/or iv) decrease the number of phagocytic cells in circulation
and/or in the
area affected by the acute coronary syndrome.
[0044] The methods of screening for therapeutic agents generally involve
incubating
a candidate compound with phagocytic cells either in vitro or in vivo and then
assaying for an
alteration (e.g., decrease) in phagocytic cell activity or longevity thereby
identifying a
compound that is a therapeutic agent for use in the present invention. Any
method known in
the art can be used to assay phagocytic cell activity or longevity. In one
embodiment,
phagocytic cell activity is assayed by the level of cell activation in
response to an activating
stimulus. For example, macrophage/monocyte activation can be assayed by
quantifying the
levels of chemotactic factors such as macrophage chemoattractant protein-1
(MCP-1) and
macrophage inflammatory protein-1 alpha (MIP-1 alpha) as well as other
substances
produced by macrophages such as interleukin 1 beta (IL-1 (3) and tissue
necrosis factor alpha
(TNF-a). In another embodiment, phagocytic cell longevity is assayed. For
example, cell
proliferation can be assayed by measuring 3H-thymidine incorporation, by
direct cell count,
by detecting changes in transcriptional activity of known genes such as proto-
oncogenes
(e.g., fos, myc) or cell cycle markers; or by trypan blue staining. Any method
known in the
14
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
art can be used to assay for levels of mRNA transcripts (e.g., by northern
blots, RT-PCR, Q-
PCR, etc.) or protein levels (e.g., ELISA, western blots, etc.).
[0045] In one embodiment, a compound that decreases the activity of a
phagocytic
cell is identified by:
a) contacting a phagocytic cell with a first compound and a second
compound, said first compound being a compound which activates said phagocytic
cell and
said second compound being a candidate compound; and
b) determining the level of activation in said contacted phagocytic cell,
wherein a decrease in activation in said contacted cell as compared to the
level of activation
in a phagocytic cell contacted with said first compound in the absence of said
second (i.e., a
control cell) indicates that said second compound decreases the activity of a
phagocytic cell.
[0046] In another embodiment, a compound that decreases the amount of
phagocytic
cells is identified by:
a) contacting a phagocytic cell with a compound; and
b) determining the viability of said contacted phagocytic cell,
wherein a decrease in viability in said contacted cell as compared to the
viability of a
phagocytic cell not contacted with said compound (i.e., a control cell)
indicates that said
compound decreases the amount of phagocytic cells.
[0047] In other embodiments, candidate compounds are assayed for their ability
to
alter phagocytic cell activity or longevity in a manner that is substantially
similar to or better
than compounds known to alter phagocytic cell activity or longevity in a
therapeutically
desirable way.. As used herein "substantially similar to" refers to an agent
having similar
action on a phagocytic cell as an exemplified agent, i.e., an agent that
inhibits the activity,
function, motility, and/or depletion of phagocytic cells.
[00481 Additionally, candidate compounds can be used in animal models of acute
coronary syndromes to assess their ability to be used in the methods of the
invention. In one
embodiment, a rabbit AMI model can be used (see e.g., Section 6.1).
5.2 FORMULATION OF THERAPEUTIC AGENTS
[0049] Formulations containing one or more therapeutic agents can be prepared
so
that the size of the formulation is large enough to only or primarily be
internalized by
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
phagocytosis, thus imparting specificity to phagocytic cells. Although non-
phagocytic cells
may be affected by such a formulation should it become intracellular, there is
no mechanism
for a non-phagocytic cell to internalize a formulation prepared in this
manner. Formulations
imparting extrinsic specificity to one or more therapeutic agents are
preferably in the size
range of 0.03-1.0 microns, more preferably 0.07-0.5 microns, more preferably
0.1-0.3
microns, and more preferably 0.1 to 0.18 microns.
[00501 Any method known in the art can be used to incorporate a therapeutic
agent
into a formulation such that it can only or primarily be internalized via
phagocytosis.
Formulations of therapeutic agents may sequester the therapeutic agents for a
sufficient time
to enhance delivery of the agent to the target site. Furthermore, formulations
of therapeutic
agents may discharge the therapeutic agent from the particles when they are
within the target
cell (e.g., the phagocytic cell) at the target site.
[00511 In one embodiment, the therapeutic agent is encapsulated in a carrier
(i.e.,
encapsulating agent) of desired properties. In a specific embodiment, the
encapsulating agent
is a liposome. The liposomes may be prepared by any of the methods known in
the art (see,
e.g., Monkkonen, J. et al., 1994, J. Drug Target, 2:299-308; Monkkonen, J. et
al., 1993,
Calcif. Tissue Int., 53:139-145; Lasic DD., Liposomes Technology Inc.,
Elsevier, 1993, 63-
105.( chapter 3); Winterhalter M, Lasic DD, Chem Phys Lipids, 1993 Sep;64(1-
3):35-43).
The liposomes may be positively charged, neutral or, more preferably,
negatively charged.
The liposomes may be a single lipid layer or may be multilamellar. Suitable
liposomes in
accordance with the invention are preferably non-toxic liposomes such as, for
example, those
prepared from phosphatidyl-choline phosphoglycerol, and cholesterol. The
diameter of the
liposomes used preferably ranges from 0.03-1.0 m. However, other size ranges
suitable for
phagocytosis by phagocytic cells may also be used.
[0052] In another embodiment, the therapeutic agent is embedded in a carrier
(i.e.,
embedding agent) of desired properties. A therapeutic agent which is embedded
includes
those therapeutic agents that are embedded, enclosed, and/or adsorbed within a
carrier,
dispersed in the carrier matrix, adsorbed or linked on the carrier surface, or
a combination of
any of these forms. In specific embodiments, the embedding agent (or carrier)
is a
microparticle, nanoparticle, nanosphere, microsphere, microcapsule, or
nanocapsule (see e.g.,
M. Donbrow in: Microencapsulation and Nanoparticles in Medicine and Pharmacy.
CRC
16
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
Press, Boca Raton, FL, 347, 1991). The term carrier includes both polymeric
and non-
polymeric preparations. In a specific embodiment, the embedding agent is a
nanoparticle.
Preferably, nanoparticles are 0.03-1.0 microns in diameter and can be
spherical, non-
spherical, or polymeric particles. The therapeutic agent may be embedded in
the
nanoparticle, dispersed uniformly or non-uniformly in the polymer matrix,
adsorbed on the
surface, or in combination of any of these forms. In a preferred embodiment,
the polymer
used for fabricating nanoparticles is biocompatible and biodegradable, such as
poly(DL-
lactide-co-glycolide) polymer (PLGA). However, additional polymers which may
be used for
fabricating the nanoparticles include, but are not limited to, PLA (polylactic
acid), and their
copolymers, polyanhydrides, polyalkyl-cyanoacrylates (such as
polyisobutylcyanoacrylate),
polyethyleneglycols, polyethyleneoxides and their derivatives, chitosan,
albumin, gelatin and
the like.
10053] In another embodiment, the therapeutic agent is in particulate form,
the
particles each being of desired properties. A particulate therapeutic agent
form includes any
insoluble suspended or dispersed particulate form of the therapeutic agent
which is not
encapsulated, entrapped or absorbed within a carrier. A therapeutic agent
which is in
particulate form includes those therapeutic agents that are suspended or
dispersed colloids,
aggregates, flocculates, insoluble salts, insoluble complexes, and polymeric
chains of an
agent. Such particulates are insoluble in the fluid in which they are
stored/administered (e.g.,
saline or water) as well as the fluid in which they provide their therapeutic
effect (e.g., blood
or serum). Typically, "insoluble" refers to a solubility of one (1) part of a
particulate
therapeutic agent in more than ten-thousand (10,000) parts of a solvent. Any
method known
in the art to make particulates or aggregates can be used. Preferably,
particulates are 0.03-1.0
microns in diameter and can be any particular shape.
5.2.1 DETERMINATION OF PARTICLE SIZE
100541 Formulations containing therapeutic agents are preferably prepared such
that
the size of the formulation is large enough to only or primarily be
internalized by
phagocytosis, that is, preferably larger than 0.03 microns. In preferred
embodiments, such
formulations are 0.03-1.0 microns, more preferably 0.07-0.5 microns, more
preferably 0.1-0.3
microns, and most preferably 0.1 to 0.18 microns. Any method known in the art
can be used
17
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
to determine the size of the formulation before administration to a patient in
need thereof.
For example, a Nicomp Submicron Particle Sizer (model 370, Nicomp, Santa
Barbara, CA)
utilizing laser light scattering can be used.
5.3 ADMINISTRATION OF THE FORMULATION
[00551 Effective amounts of the formulations are contemplated as short term,
acute
therapy and are not meant for chronic administration. Time period of treatment
is preferably
such that it produces inhibition/depletion of phagocytic cells for a period
that is less than a
month, preferably less than two weeks, most preferably up to one week.
Empirically, one can
determine this by administering the compound to an individual in need thereof
(or an animal
model of such an individual) and monitoring the level of inhibition/depletion
at different time
points. One may also correlate the time of inhibition with the appropriate
desired clinical
effect, e.g. reduction in the acute risk of plaque rupture.
5.4 CHARACTERIZATION OF THERAPEUTIC UTILITY
100561 The term "effective amount" denotes an amount of a particular
formulation
which is effective in achieving the desired therapeutic result, namely
inhibited or decreased
phagocytic cell activity and/or elimination or reduction in the amount of
phagocytic cells. In
one embodiment, the desired therapeutic result of inhibiting or decreasing
phagocytic cell
activity and/or eliminating or reducing in the amount of phagocytic cells
stabilizes a
vulnerable or unstable plaque in a patient suffering from unstable angina. In
another
embodiment, the desired therapeutic result of inhibiting or decreasing
phagocytic cell activity
and/or eliminating or reducing in the amount of phagocytic cells minimizes the
infarct size
and/or the amount of myocardial necrosis in a patient having suffered an acute
myocardial
infarction.
100571 Toxicity and efficacy of the therapeutic methods of the instant
invention can
be determined by standard pharmaceutical procedures in cell cultures or
experimental
animals, e.g., for determining the LD50 (the dose lethal to 50% of the
population), the No
Observable Adverse Effect Level (NOAEL) and the ED50 (the dose therapeutically
effective
in 50% of the population). The dose ratio between toxic and therapeutic
effects is the
18
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
therapeutic index and it can be expressed as the ratio LDsotEDso or
NOAEL/ED50.
Formulations that exhibit large therapeutic indices are preferred. While
formulations that
exhibit toxic side effects may be used, care should be taken to design a
delivery system that
targets the agents of such formulations to the site of affected tissue in
order to minimize
potential damage to unaffected cells and, thereby, reduce side effects.
[00581 The data obtained from the cell culture assays and animal studies can
be used
in determining a range of dosage of the formulation for use in humans. The
dosage of such
formulations lies preferably within a range of circulating concentrations that
include the ED50
with little or no toxicity. The dosage may vary within this range depending
upon the dosage
form employed and the route of administration utilized. For any formulation
used in the
method of the invention, the effective dose can be estimated initially from
cell culture assays.
A dose may be formulated in animal models to achieve a circulating plasma
concentration
range that includes the IC50 (i.e., the concentration of the test compound
that achieves a half-
maximal inhibition of symptoms) as determined in cell culture. Such
information can be
used to more accurately determine useful doses in humans. Levels in plasma may
be
measured, for example, by high performance liquid chromatography.
[0059] The protocols and compositions of the invention are preferably tested
in vitro,
and then in vivo, for the desired therapeutic activity, prior to use in
humans. One example, of
such an in vitro assay is an in vitro cell culture assay in phagocytic cells
which are grown in
culture, and exposed to or otherwise administered to cells, and observed for
an effect of this
assay upon the cells, e.g., inhibited or decreased activity and/or complete or
partial cell death.
The phagocytic cells may be obtained from an established cell line or recently
isolated from
an individual as a primary cell line. Many assays standard in the art can be
used to measure
the activity of the formulation on the phagocytic cells; for example,
macrophage/monocyte
activation can be assayed by quantitating the levels of chemotactic factors
such as
macrophage chemoattractant protein-1 (MCP-1), interleukin 1 beta (IL-1(3),
tissue necrosis
factor alpha (TNF-a) and macrophage inflammatory protein-1 alpha (MIP- 1
alpha). Many
assays standard in the art can be used to assess survival and/or growth of the
phagocytic cells;
for example, cell proliferation can be assayed by measuring 3H-thymidine
incorporation, by
direct cell count, by detecting changes in transcriptional activity of known
genes such as
19
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
proto-oncogenes (e.g., fos, myc) or cell "cycle markers; cell viability can be
assessed by
trypan blue staining.
[0060] Selection of the preferred effective dose can be determined (e.g., via
clinical
trials) by a skilled artisan based upon the consideration of several factors
known to one of
ordinary skill in the art. Such factors include the acute coronary syndrome to
be managed or
treated, the symptoms involved, the patient's body mass, the patient's immune
status and
other factors known to the skilled artisan to reflect the accuracy of
administered
pharmaceutical compositions.
5.5 PHARMACEUTICAL COMPOSITIONS AND ROUTES OF
ADMINISTRATION
[00611 Formulations comprising one or more therapeutic agents for use in the
methods of the invention may be in numerous forms, depending on the various
factors
specific for each patient (e.g., the severity and type of disorder, age, body
weight, response,
and the past medical history of the patient), the number and type of
therapeutic agents in the
formulation, the type of formulation (e.g., encapsulated, embedded,
particulate, etc.), the
form of the composition (e.g., in liquid, semi-liquid or solid form), and/or
the route of
administration (e.g., oral, intravenous, intramuscular, intra-arterial,
intramedullary,
intrathecal, intraventricular, transdermal, subcutaneous, intraperitoneal,
intranasal, enteral,
topical, sublingual, vaginal, or rectal means). Pharmaceutical carriers,
vehicles, excipients, or
diluents may be included in the compositions of the invention including, but
not limited to,
water, saline solutions, buffered saline solutions, oils (e.g., petroleum,
animal, vegetable or
synthetic oils), starch, glucose, lactose, sucrose, gelatin, malt, rice,
flour, chalk, silica gel,
sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim
milk, glycerol,
propylene, glycol, ethanol, dextrose and the like. The composition, if
desired, can also
contain minor amounts of wetting or emulsifying agents, or pH buffering
agents. These
compositions can take the form of solutions, suspensions, emulsion, tablets,
pills, capsules,
powders, sustained-release formulations and the like.
[00621 Pharmaceutical formulations suitable for parenteral administration may
be
formulated in aqueous solutions, preferably in physiologically compatible
buffers such as
Hanks' solution, Ringer's solution, or physiologically buffered saline.
Aqueous injection
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
suspensions may contain substances, which increase the viscosity of the
suspension, such as
sodium carboxymethyl cellulose, sorbitol, or dextran. In addition, suspensions
of the active
compounds may be prepared as appropriate oily injection suspensions. Suitable
lipophilic
solvents or vehicles include fatty oils such as sesame oil, or synthetic fatty
acid esters, such as
ethyloleate or triglycerides, or liposomes. Optionally, the suspension may
also contain
suitable stabilizers or agents which increase the solubility of the compounds
to allow for the
preparation of highly concentrated solutions.
[0063] The pharmaceutical composition may be provided as a salt and can be
formed
with many acids, including but not limited to, hydrochloric, sulfuric, acetic,
lactic, tartaric,
malic, succinic, and the like. Salts tend to be more soluble in aqueous
solvents, or other
protonic solvents, than are the corresponding free base forms.
[0064] Pharmaceutical compositions can be administered systemically or
locally, e.g.,
near the site of pathology of an acute coronary syndrome. Additionally,
systemic
administration is meant to encompass administration that can target to a
particular area or
tissue type of interest.
[00651 Pharmaceutical compositions are preferably administered immediately at
the
onset of the first symptoms of actual plaque rupture; such as, for example,
chest pain, pain
that radiates to the shoulder, arm, teeth, jaw, abdomen or back or shortness
of breath or
cough, lightheadedness, fainting, nausea, vomiting, sweating or anxiety
associated with a
plaque rupture. Other symptoms will be apparent to the skilled artisan and
medical doctor,
and may be signals to administer the instant pharmaceutical composition.
Alternatively
and/or additionally, the pharmaceutical compositions may be administered just
after onset of
symptoms, for example, within minutes of symptom onset. Alternatively and/or
additionally,
the compositions may be administered within 1 hour, or about 2 hours, or about
3 hours or
about 4 hours, or about 5 hours or about 6 hours,, up to within 1-3 days after
onset of
symptoms.
[00661 In another regime, pharmaceutical compositions are administered to a
patient
with an increased risk of plaque rupture. For example, the compositions of the
invention may
be administered to a patient prior to a procedure which increases the risk of
plaque rupture,
such as, for example, an angioplasty procedure. It may be preferred to
administer the
21
CA 02530460 2011-08-08
composition up to 3 days before such a procedure. Also preferred,
administration may be 1-6
hours before the procedure or within 1 hour of the procedure or less than 1
hour before or
even within minutes of the procedure. The skilled person can readily determine
the
appropriate timing of administration depending on various physiological
factors, specific to
the individual patient, such as, for example, weight, medical history and
genetic
predisposition, as well as various factors which influence the anticipated
risk of plaque
rupture such as complexity of the procedure to be performed.
6. EXAMPLES
100691 The follownng examples as set forth herein are meant to illustrate and
exemplify the various aspects of carrying out the present invention and are
not intended to
limit the invention in any way.
6.1 Effect of Liposomal Alendronate on the Size of the Zone of Infarct
The effects of treatment with encapsulated bisphosphonates on the zone of
infarct were
studied in a rabbit AMI model. Liposomal Alendronate, approx. 0.1504m in
diameter was
made using the following outline:
a. Dissolve lipids, DSPC, DSPG and cholesterol in 1/1 ethanol/tert-butanol.
b. Dilute solvent into buffer containing Alendronate to generate large
multilamellar
vesicles (MLVs).
c. Extrude MLVs through 200 run polycarbonate filters to generate large
unilamellar 150:1:
20 urn vesicles (LUVs),
d. Ultra-filtrate LUVs to remove un-encapsulated alendronate.
e. Sterile filter
22
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
[0070] Eight New Zealand White male rabbits, 2.5-3.5 kg B.W., were fed normal
chow and water ad libitum. The rabbits were randomly administered saline
(control) or
liposomal alendronate (3 mg/kg, i.v.) as a single infusion simultaneous with
coronary artery
occlusion. The rabbits were anesthetized by KetaminelXylazine (35mg/kg; 5
mg/kg) and
Isoflurane. The experiment was performed with respiratory support given by
intubation and
mechanical ventilation with isoflurane in balance oxygen, and continuous
echocardiogram
(ECG) and arterial blood pressure (catheter in ear artery) monitoring.
Thoracotomy was
performed through the left 4th intercostal space, followed by pericardiotomy
and creation of a
pericardial cradle. The left main coronary artery was identified and a large
branch was
encircled by a 5-0 silk suture and a snare. Thereafter, the snare was
tightened for 30 minutes.
Ischemia was verified by ECG changes (ST-T segment elevation), changes of
segment
coloration and hypokinesia. After thirty minutes, the snare was released and
resumption of
blood flow was confirmed. The suture was left in place, released, and the
chest cavity was
closed in layers. Buprenex was administered to the rabbits for analgesia for 2-
3 additional
days. Following euthanasia with Penthotal, the rabbits were sacrificed after 7
days and the
hearts were harvested. The coronary arteries were perfused through the
ascending aorta with
saline, followed by tightening of the suture on the previously occluded
coronary artery and
perfusion of the coronary arteries with 0.5% Evans blue solution (Sigma) to
stain areas of re-
endothelialization (presence of blood). The left ventricular area unstained by
Evans blue was
defined as the area at risk. The hearts were then frozen at -20 C for 24 hours
and cut into
transverse sections 2 mm apart. Slices of the hearts were incubated for 30
minutes in the vital
stain tritetrazolium chloride (TTC, 1%, Sigma), fixed in 10% natural buffered
formalin to
stain cells that had been alive previous to tissue processing. The left
ventricular area not
stained by TTC (white) was defined as the area of infarct. The stained
sections were then
photographed and processed by digital planimetry (Photoshop).
[00711 Rabbits treated with liposomal alendronate had a zone of infarct that
was
29.5 6% of the area at risk. This was contrasted with the control rabbits
(untreated with
liposomal alendronate) which showed an infarct zone that was 42 5.5% of the
area at risk
(FIG. 1). Accordingly, liposomal alendronate was effective in reducing the
zone of infarct.
No adverse effects were observed in the treatment group.
23
CA 02530460 2005-12-22
WO 2005/002545 PCT/US2004/020487
6.2 Effect of Liposomal Alendronate On Myocardial Morphology
[0072] Rabbits as treated in Section 6.1 showed variation in myocardial
morphology
as exhibited by Hemotoxylin and Eosin staining. The control rabbits have a
distorted
myocardial morphology (FIG. 2A) while the rabbits treated with liposomal
alendronate
exhibit a more normal morphology (FIG. 2B).
6.3 Effect of Liposomal Alendronate on Macrophage Infiltration
[0073] Rabbits as treated in Section 6.1 showed a reduction in macrophage
infiltration
in rabbits treated with liposomal alendronate. Representative sections of the
rabbits' hearts
were subjected to immunostaining for RAM1 1+ macrophages. Sections from
rabbits treated
with liposomal alendronate (FIG. 3B) showed less staining and therefore had
less RAM1 1+
macrophages accumulation than sections from control rabbits (FIG. 3A).
[0074] Liposomal alendronate was also shown to reduce the number of
circulating
monocytes systemically. Rabbits were administered saline (control) or
liposomal alendronate
(3 mg/kg, i.v.) Monocyte levels in circulating blood were determined using
FACS analysis
for CD-14. At 48 hours after injection with liposomal alendronate, the blood
monocyte
population was reduced by 75-95% as compared to the control group.
24