Note: Descriptions are shown in the official language in which they were submitted.
CA 02531459 2010-05-12
1
Device and method for simultaneously carrying out blood group determination,
serum
cross-check and antibody detection test
The invention relates to a device for lateral-diagonal flow multi-parameter
tests, in
particular in the fields of immune haematology and of infection serology, for
the
simultaneous, qualitative or quantitative determination of a plurality of
analytes in a
liquid sample, including at least one membrane with an application zone for
the
application of the liquid sample, at least two indicator zones which are able
to interact
with the analyte(s) and at least one absorption region which absorbs the
liquid after
having passed the indicator zones, the indicator zones being positioned
between the
application zone and the absorption region, characterized in that the
directions of flow
from the application zone through the respective indicator zones to the
absorption region
(flow tracks) are essentially parallel and there being present at least two
different flow
tracks.
The invention further relates to a process for analyzing a plurality of
analytes in a liquid
sample, comprising applying the sample onto the application zone of a membrane
of the
apparatus according to the invention, wherein this sample is present in
adequate quantity
in order to induce the sample liquid to flow in the direction of the
adsorption region
through the indicator zones and in order to induce the analytes or their
derivatives in the
sample liquid to form a complex in the indicator zones, in particular for the
simultaneous
determination of cellular and plasmatic parameters, preferably for the
simultaneous
conduction of blood group determination and serum cross-check (serum reverse
regrouping) and/or antibody detection tests as well as for simultaneously
conducting
blood group determinations and the determination of transfusion relevant
infection
serological markers as well as for the simultaneous conduction of blood group
CA 02531459 2006-01-05
WO 2005/005986 2 PCT/EP2004/007525
Ln\hhh\bardeh le\30\ 12\2005
determination and the determination of antibodies which are directed against
blood cells
other than erythrocytes, in particular anti-thrombocytes and anti-lymphocytes
antibodies.
In order to prevent risks of complications, e.g. during a transfusion, in
particular blood
group incompatibilities, viral and/or bacterial contaminations it is known to
perform a
variety of laboratory tests on the donor and patient blood in order to make
available
components which are compatible in a blood group serological sense with the
recipient
and are free of known transmitable pathogens. The respective serological tests
as a rule
include the determination of the blood group of the donor and the recipient,
in particular
of blood groups of the blood group systems ABO, Rh and Kell, serum cross-
checks on
the donor and recipient, antibody detection tests in respect of irregular
antibodies of the
donor and recipient as well as antibody identifications in the case of the
recipient where
irregular antibodies are present. The detection of antibodies against
thrombocytes and or
lymphocytes is performed likewise in the context of transfusions and
transplantations.
Infection serological tests on the donor include in a known manner the routine
determination of antibodies, in particular against HIV-I, HIV-II, against HCV
against
treponema pallidum (syphilis), as well as the determination of the hepatitis B
surface
antigen (=HbsAg: hepatitis surface antigen).
In blood group serological diagnostics, parameters are generally tested which
are of
particular relevance in the context of transfusions or of morbus haemolyticus
neonatorum
(Mhn). This includes inter alia the detection of antigens on the surface of
erythrocytes
which are characteristic for the blood groups (blood group determination).
Further
important antigen systems are present also on thrombocytes, granulocytes,
lymphocytes
which likewise play a role in the context of transfusions and
transplantations. In the
event of thrombocytes and granulocytes antigen non-identity between mother and
fetus
pathological symptoms may occur with neonatants comparable with Mhn. In
addition the
detection of regular blood group antibodies (isoagglutinine) and of irregular
blood group
antibodies in serum or plasma is applicable here.
CA 02531459 2006-01-05
WO 2005/005986 3 PCT/EP2004/007525
Ln\hhh\bardeh 1e\3 0\ 12\2005
The isoagglutinines or regular antibodies are acquired particularly by humans
soon after
their birth and correspond to the respective blood group of the ABO system.
They are
directed against those blood group antigens A or B which the individual itself
lacks, i.e.
persons which have the blood group A have anti-B, persons of blood group B
have anti-A
persons of blood group 0 have anti-A and anti-B; persons of blood group AB
have no
isoagglutinine. The regular antibodies are also known as "complete" because
they are
able to directly agglutinate erythrocytes in NaCl medium.
The irregular or allo-antibodies, in contrast to the isoagglutinines are
acquired by
immunizations in later life, in particular by transfusion or pregnancy. For
that reason
most humans have no irregular blood group antibodies. The transfusion relevant
irregular
antibodies are as a rule heat reactive and belong mostly to the IgG class. In
contrast to
the regular antibodies they are not able to directly agglutinize erythrocytes
in an NaCl
medium.
It is known that for determining blood groups the erythrocytes of the persons
to be tested,
(donors or recipients) are brought together with reagents which contain blood
group
specific antibodies. Generally these tests are performed in the liquid state,
in which by
mixing of an erythrocyte containing sample with a sample containing antibodies
directed
against a specific blood group characteristic a testing batch is produced. The
testing
batch is then incubated over a defined period and under defined conditions and
after
conclusion of the incubation, either directly or after a centrifugation step,
is tested
visually or by optical methods for a possible agglutination or adsorption of
the
erythrocytes. The predominant end point measurement in blood group serology is
still
the haemaglutination test. For each blood group to be determined a separate
batch must
be pipetted, i.e. e.g. for the determination of the nine most important blood
groups A, B,
D, C, c, E, e, Cw and K, nine separate batches are needed, without counting
any control.
For the serum cross-check cell reagents with known ABO blood groups (Al, A2,
B, 0)
are used in a known manner which are incubated with the serum or plasma of the
person
to be tested. After a centrifugations step a possible agglutination of the
erythrocytes is
CA 02531459 2006-01-05
WO 2005/005986 4 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
tested for visually or by optical methods. For a serum cross-check with the
aforesaid test
cells it is conventionally necessary to pipette four batches.
In order to search for irregular antibodies panels are generally used
comprising two or
three blood group 0 cells the combined antigen profile of which contains the
most
important antigens, in particular the blood group systems Rh, Kell, Duffy,
Kidd, MNS, P,
Lewis, Lutheran. The cell reagent is brought together with the serum or plasma
of the
person to be tested, is incubated and after a step of centrifugation is tested
visually or by
optical methods for any agglutination of the erythrocytes. Two to three
batches must be
pipetted for testing one patient sample.
In order to identify the irregular antibodies which as a rule takes place
after a positive
antibody detection test, panels comprising up to 16 blood group 0 cells are
used the
antigen profile of which covers the most important antigens, in particular the
blood group
systems Rh, Kell, Duffy, Kidd, MNS, P, Lewis, Lutheran in an exactly
predetermined
manner. The cell reagent is brought together with the serum or blood plasma of
the
person to be tested and by visual or optical methods is tested for any
agglutination of the
erythrocytes. For testing a patient sample up to 16 batches must be pipetted.
Since most transfusion relevant irregular antibodies are of the IgG type and
are therefore
incomplete the reactions for antibody detection and identification as
described must as a
rule be reinforced in order to be able to detect the end point of the
haemaglutination. The
most common reagent for this purpose is a polyclonal anti-humanglobulin
reagent to
which frequently anti-complement antibodies have been added (typically anti-
C3d and/or
anti-C3b).
A commonly used method for detecting thrombocyte antibodies is the so-called
MAIPA
test (monoclonal antibody immobilization of platelet antigens). In this case
test
thrombocytes are incubated with the serum to be tested. After a rinsing step,
incubation
is performed with a monoclonal, e.g. mouse antibody which is specific for a
particular
thrombocyte glycoprotein. The thrombocytes are thereafter subjected to lysing
and the
CA 02531459 2006-01-05
WO 2005/005986 5 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\12\2005
diluted lysate is introduced into a reaction vessel coated e.g. with goat anti-
mouse
antibodies of a microtitration panel. The goat anti-mouse antibody binds the
mouse
antibody and the thrombocyte glycoprotein human antibody complex attached
thereto.
The human antibody is tested for by the addition of an enzyme-conjugated goat
anti-
human IgG.
With conventional diagnostic tests it is possible only to either determine
cellular or
plasmatic parameters. In order to determine blood components it is invariably
necessary
to first separate cells from plasma.
Lateral flow tests nowadays are frequently applied as quick tests e.g. as
pregnancy tests,
for determining infection markers or for drug screening. A lateral flow test
device in a
known manner includes a rigid support on which an application zone for the
sample to be
tested is provided, a separating membrane, on which bonding elements, e.g.
catcher
antibodies or antigens are bound and on which the bonding reactions can be
detected, and
a suction generating absorption region which causes the sample to be tested to
flow in a
linear manner through the separating membrane.
Test membranes of conventional lateral flow tests are generally described
involving a
chromatography-like separation. The analyte in the sample binds specifically
to the
bonding elements fixed in a membrane which as a rule are present in
consecutive or
superimposed bands serving as indicator zones. The binding complex is rendered
visible
by indicator particles which as a rule are already present in the device in
dehydrated form
in a conjugate liberation pad. The conjugate liberation pad is provided
between the
application zone and the membrane. The pre-coated coloured indicator particles
are
coated for example with an antibody directed against the analyte to be tested
for.
The conventional lateral flow test format corresponds to a so-call "sandwich
assay", in
which both the indicator zone as well as the indicator particles are coated
with a ligand
aimed at the analyte tested for, normally an antibody. In that context the
ligand (bonding
element) is immobilized on the membrane. The detector reagent, normally an
antibody
CA 02531459 2006-01-05
WO 2005/005986 6 PCT/EP2004/007525
Ln\hhh\bardeh le\30\12\2005
bonded to a coloured polystyrene particle or to colloidal metals, is deposited
in the
conjugate liberation pad in a leachable manner. This bonding complex serves as
indicator particle. Once the sample to be tested has been applied it very
rapidly wets the
conjugate liberation pad, whereby the indicator particles are mobilized. The
indicator
particles migrate with the liquid front along the porous membrane. An analyte
present in
the sample becomes bonded by the antibody coupled to the indicator particle.
As the
sample passes the indicator zone, the analyte/indicator particle complex in
the indicator
zone is immobilized by reaction of the analyte with the antibody bonded in the
indicator
zone, resulting in a visible signal.
A further known test format for small analytes comprising but a single
antigenic
determinant, incapable of simultaneously bonding two antibodies, is the so-
called
"competition assay". The detector reagent bonded to the indicator particle is
normally a
molecule identical to or analogous with the analyte. The indicator particles
are deposited
in the conjugate liberation pad. The indicator particles migrate with the
liquid front along
the porous membrane. If the sample contains the analytes, and if the indicator
particles
(which effectively likewise contain analyte) pass the indicator zone, part of
the analyte
molecules in the sample bond to part of the indicator particles. The more
analyte is
present in the sample the more effective will it compete with the bonding of
the indicator
particle and the weaker will the signal become.
According to the prior art these indicator particles are predominantly
composed of
colloidal gold or of polystyrene, manufactured and coated according to methods
known to
the skilled person. In the typical lateral flow tests formats the analytes are
determined
indirectly. In this context a direct determination of an analyte denotes that
the analyte is
already bonded naturally to the indicator particle (e.g. erythrocyte). In the
more common
situation of indirect determination of the analytes the sample to be tested as
a rule
contains a non-cellularly bonded, e.g. plasmatic component as the analyte and,
besides
the sample to be tested, two reagent components are required, i.e. indicator
particles and a
bonding element. In the indirect determination the analyte initially bonds to
the indicator
CA 02531459 2006-01-05
WO 2005/005986 7 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\12\2005
particle dissolved out of the conjugate liberation pad, before this complex
becomes
immobilized in the indicator zones with the bonding element by way of a second
reaction.
When using conventional lateral flow tests with erythrocytes as indicator
particles which
have been bonded to the analytes to be determined, for example blood group
specific
antigens, it is at present usual for antibodies to be provided in the
indicator zones against
corresponding blood group antigens serving as bonding elements in successive
or
superimposed bands in but a single flow track such as for example anti-A, anti-
B against
the Rh blood group system. In this context conventional lateral flow tests
suffer from the
disadvantage that the erythrocytes bonded to the antibodies form a flow
barrier against
the analytes still to be tested for, for example further cell associated
antigens, in a sample.
Due to agglutination or adsorption of cells in a band of bonding elements
arranged
proximally to the application zone, additional analytes, in particular cells
or cell
fragments in the sample to be tested, can no longer be separated unimpededly
and visibly
and can therefore not be tested for unambiguously or completely. For example
in a
person who is blood group AB Rh D positive this may result in a weakening or
elimination of the B and the D bands, which may result in a faulty
interpretation of being
blood group A Rh negative. For that reason it was hitherto not possible,
specifically in
blood group serological diagnostics to employ a lateral flow test with more
than one
indicator zone. In order to determine a plurality of, in particular cellular
and plasmatic
blood group parameters, it is to date necessary to conduct single parameter
tests
separately.
It is an object of the invention to overcome the disadvantages referred to of
the state-of-
the-art, in particular of the successive or superposed indicator or testing
zones of
conventional lateral flow tests for a simultaneous determination of different
sample
parameters, in particular of cellular and plasmatic parameters.
The object is attained according to the invention on the one hand by a device
for the
simultaneous qualitative or quantitative determination of one or more analytes
in a liquid
sample or a plurality of liquid samples comprising at least one membrane with
an
CA 02531459 2006-01-05
WO 2005/005986 8 PCT/EP2004/007525
Ln\hhh\bardeh le\30\ 12\2005
application zone for applying the liquid sample, at least one group of at
least two
indicator zones which can enter into interaction with the analyte(s) or with
which analytes
can interact and an absorption region which takes up the liquid after having
passed the
indicator zones, wherein the indicator zones are positioned between the
applications zone
and the absorption region characterized in that the flow directions from the
applications
zone through the respective indicator zones of a group towards an absorption
region,
representing flow tracts are substantially parallel, there being present at
least two
different flow tracks.
The indicator zones of the apparatus according to the invention are present on
the
membrane and comprise bonding elements which capture or bond the analytes to
be
determined in the sample. The bonding reactions between the analyte and the
bonding
element are detected in the indicator zones. Antibodies or antibody fragments,
lectines,
antigens or antigen epitopes and/or cells or cell fragments are fixed to the
porous
membrane as particularly preferred bonding elements. The indicator zones
preferably
each comprise one bonding element against one analyte to be tested for.
In an embodiment of the invention the indicator zones are so arranged that the
sample
liquid for each flow track will flow through not more than one indicator zone.
For
example, the indicator zones are provided on the membrane in staggered
relationship.
This arrangement of the indicator zones is, in this context, preferably
configured in a row
extending diagonally from proximal to distal or vice versa. Particular
embodiments are
V-shaped, W-, M- or N-shaped or reversed V-shaped W-, M- or N-shaped. In a
further
embodiment the indicator zones are staggered parallel side by side in a linear
row.
The provision of staggered indicator zones is a precondition for a multi-
parameter testing
with erythrocytes as indicator particles in a lateral arrangement. The
particularly
preferred embodiment of a diagonal arrangement offers the advantage that the
denotation
of the results can be applied to the device according to the invention in a
particularly
practical and easily readable manner; because each parameter to be tested for
occupies a
defined X and Y position the arrangement of the device according to the
invention can be
CA 02531459 2006-01-05
WO 2005/005986 9 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
considered as a coordinate system having an ordinate (plane of the direction
of flow) and
an abscissar (plane of the application zone).
In a further embodiment of the invention more than one such rows of indicator
zones,
extending preferably each from proximal to distal or vice versa in a diagonal
direction or
for example even V-shaped, W-, M- or N-shaped or reversed V-shaped W-, M- or N-
shaped, extending in the direction of flow sequentially and/or laterally
staggered, and the
indicator zones of the various rows are either arranged in spaced apart
relationship so that
the sample liquid for each flow track flows through not more than a single
indicator zone
or extend in contact with one another such that the sample liquid for each
flow track
flows through more than one indicator zone.
More than one such row of indicator zones, for example two rows of indicator
zones
having different distances from the application zone are particularly
advantageous in the
event that from a complete blood sample cellular and plasmatic parameters are
to be
determined. In one embodiment, for example in a test batch containing complete
blood
as sample, the bonding elements are so selected that the analytes contained in
the plasma,
for example any kind of antibodies, which flow through the apparatus from the
application zone to the adsorption region through the indicator zones, become
bonded to
the bonding elements in the row of indicator zones arranged proximally to the
application
zone. The bonding elements for detecting the cellularly bonded analytes, for
examples
erythrocyte antigens, are, on the other hand, so selected that these become
bonded to the
bonding elements in that row of indicator zones which is arranged distally to
the
application zone. In this preferred embodiment one preferably operates, in
addition to the
erythrocytes, with a further type of indicator particle, preferably of
colloidal gold or
polystyrene or with immobilized erythrocytes. These indicator particles are
employed in
particular in order to render visible in the bonding complex in an indicator
zone analytes
which are not bonded to erythrocytes, for example antibodies occurring freely
in plasma.
If for example two types of indicator particles are employed one of which is
not
erythrocytes, the indicator zones of the two rows can be arranged side by side
in contact
with one another or sequentially in a single flow tract. In this context the
arrangement is
CA 02531459 2006-01-05
WO 2005/005986 10 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
advantageous in which the analytes to be tested for in plasma are detected in
the proximal
indicator zones and where the erythrocytes-bonded analytes are detected in the
distal
indicator zones. An embodiment of the invention comprising more than one row
of
indicator zones as aforesaid in which the bonding elements of each row react
or bond to
erythrocytes serving as indicator particles, the rows of indicator zones are
in spaced apart
interrelationship or in a single flow tract and not in succession.
In a further and particularly preferred embodiment of the invention more than
one such
row of indicator zones are provided preferably in one row extending diagonally
from
proximal to distal or vice versa or for example in a row extending in a V-
shaped, W-, M-
or N-shaped manner or reversed V-shaped, W-, M- or N-shaped row or even for
example
in a row extending parallel side by side in staggered relationship, bi-
directionally (e.g. at
an angle of 180 to one another) in relation to a central application zone.
Such an
arrangement is advantageous in particular in the event that from a complete
blood sample
cellular and plasmatic parameters are to be detected.
In an embodiment for example in a test batch comprising complete blood as the
sample,
the bonding elements are so selected that the analytes contained in the
plasma, for
example any type of antibodies which flow from the application zone to the
absorption
region through the indicator zones, become bonded to the bonding elements in
the row of
indicator zones provided on the one side of the application zone. The bonding
elements
for detecting the cellularly bonded analytes, for example erythrocyte
antigens, are on the
other hand so selected that these become bonded to the bonding elements of the
end row
of indicator zones arranged on the opposite side of the application zone. In
the case of
this preferred embodiment one preferably operates, in addition to the
erythrocytes, with a
further kind of indicator particles, preferably of colloidal gold or
polystyrene. These
indicator particles are employed in particular in order to render visible in
the bonding
complex in an indicator zone, analytes which are not bonded to erythrocytes,
for example
antibodies occurring freely in the plasma.
CA 02531459 2006-01-05
WO 2005/005986 1 1 PCT/EP2004/007525
L n\hhh\b and eh 1 e\3 0\ 12\200 5
In a preferred embodiment the one application zone furthermore comprises two
different
membranes of differing porosaties. In this preferred embodiment one preferably
operates, in addition to the erythrocytes, with a further kind of indicator
particles,
preferably of colloidal gold or polystyrene. These indicator particles are
particularly
employed in order to render visible in the bonding complex in an indicator
zone analytes
which are not bonded to erythrocytes, for example antibodies occurring freely
in plasma.
In a particularly preferred embodiment the one application zone includes a
membrane or
two different membranes of differing porosities. Furthermore, one of the
membranes
includes a conjugate pad arranged between a sealing element and indicator
zones. In this
preferred embodiment one preferably operates in addition to the erythrocytes
with a
further kind of indicator particles, particularly of colloidal gold or
polystyrene. The
indicator particles are employed in particular in order to render visible in
the bonding
complex in an indicator zone analytes which are not bonded to erythrocytes,
for example
antibodies occurring freely in the plasma. This further kind of indicator
particles,
employed in addition to the erythrocytes, is preferably present in dried form
in the
conjugate pad. Furthermore the conjugate pad takes care that the flow of
erythrocytes is
slowed down. This has the affect that in the bi-directional arrangement
including a single
common application zone optimized conditions are maintained in the one
direction for
detecting cellular properties and in the opposite direction optimized
conditions are
maintained for the detection of plasmatic properties.
By means of the device according to the invention a lateral flow test, in
particular for
blood serological diagnostics is made available by means of which erythrocytes
can be
used as indicator particles and wherein in a single test batch simultaneously
several
cellular parameters, in particular erythrocyte antigens or antigen epitopes,
plasmatic
parameters and/or blood cell properties, in particular of complete blood
components can
be determined in each sample to be tested. Moreover, in this manner a test
system is
made available which can be manufactured particularly easily and can be
employed in
particular with few test series and without sample preparation, as well as
being cost-
CA 02531459 2006-01-05
WO 2005/005986 12 PCT/EP2004/007525
Ln\hhh\bardeh le\30\ 12\2005
effective, but by means of which simultaneously a variety of cellular
parameters and/or
plasmatic parameters of a sample or a plurality of samples to be tested can be
determined.
The device according to the invention affords these advantages in all medical
diagnostic
fields in which simultaneously different cellular parameters and plasmatic
parameters are
to be determined, in particular also in the field of blood group and
infections serology, in
particular in the context of any diagnostics relevant to transfusion medicine,
e.g. for the
simultaneous conductance of blood group determinations wherein in particular
erythrocytes bonded antigens or antigen epitopes are determined and for serum
cross-
checking, wherein in particular regular antibodies (isoagglutinines) are
determined and/or
antibody detection tests, wherein in particular irregular antibodies are
determined, for
example for the simultaneous conduction of blood group determinations and the
detection
of transfusion relevant infection serological markers, for example antibodies
against HIV-
I, HIV-2, HCV, treponema pallidum, as well as the surface antigen of hepatitis
B virus
(HbsAg) as well as for the simultaneous conduction of blood group
determinations and
the detection of antibodies against other blood cells such as erythrocytes in
particular
anti-thrombocyto and anti-lymphocyto antibodies.
In this context anti-coagulation treated or native complete blood can be used,
where it is
not necessary, prior to the testing to extensively separate from one another
erythrocytes
and serum or plasma fractions. The determination may be performed in a manual
format,
i.e. completely without instruments (including electric current).
In a preferred embodiment of the device according to the invention the
indicator zones
include, preferably in a row of indicator zones arranged distally to the
application zone,
antibodies or antibody fragments or lectines which capture or bind the blood
group
antigens to be determined of all conceivable blood group systems and
accordingly the
cells serving as their carriers, present in the sample. Antibodies or antibody
fragments or
lectines against antigens or antigen epitopes, in particular of the blood
group systems
ABO, Rh and Kell, for example anti-A, anti-B, anti-D and anti-K are.applied as
preferred
CA 02531459 2006-01-05
WO 2005/005986 13 PCT/EP2004/007525
Ln\h hh\bardeh ] e\30\ 12\2005
bonding elements in the indicator zones on the porous membrane, preferably in
a row
distal to other indicator rows.
Preferably a control bonding element (control = ctl) is applied in an
indicator zone of this
row of indicator zones, preferably in an indicator zone distal to all
remaining indicator
zones of this row, which gives a positive indication of the flow of the
samples through the
indicator zones. The control bonding element is preferably a polyclonal anti-
erythrocyte
antibody.
This preferred embodiment of the device according to the invention comprises
in a
further row of indicator zones, preferably arranged proximally to the
application zone,
antigens or antigen epitopes which capture or bond the regular antibodies in
the sample.
For this purpose, serving as preferred bonding elements, Al, A2, B, 0 blood
group
antigens or antigen epitopes, for example erythrocyte membranes of
erythrocytes of
defined blood groups (Al, A2, B, 0) or synthetically produced blood group
substances
are applied to the porous membrane. Preferably, in an indicator zone of this
row of
indicator zones, preferably in an indicator zone distal to all remaining
indicator zones of
this row, a control bonding element (control = ctl) is applied which
positively indicates
the flow of the sample through the indicator zones. The control bonding
element is
preferably an anti-IgG antibody.
This preferred embodiment of the device according to the invention can in a
further row
of indicator zones, preferably proximal to the application zone, comprise
antigens or
antigen epitopes which capture or bond the irregular antibodies or fragments
thereof in
the sample. As preferred bonding elements for this purpose the cell membranes
of
different blood group 0 erythrocyte preparations, the combined antigen profile
of which
covers those antigens which are directed against the important transfusion
relevant
irregular antibodies, are fixed onto the porous membrane. Preferably a control
bonding
element (control = ctl) which positively indicates the flow of the sample
through the
indicator zones is applied in an indicator zone of this row of indicator
zones, preferably in
CA 02531459 2006-01-05
WO 2005/005986 14 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\ 12\2005
an indicator zone distal to all remaining indicator zones of this row. The
control bonding
element is preferably an anti-IgG antibody.
In a further preferred embodiment of the device according to the invention the
indicator
zones, preferably in a row of indicator zones arranged distally to the
application zone,
comprise antibodies or antibody fragments or lectines which capture or bond
the blood
group antigens to be determined in the blood group determination, and thereby
the cells
in the sample which carry those antigens. Antibodies or antibody fragments or
lectines
against antigens or antigen epitopes of the ABO blood group system, for
example anti-A,
anti-B, anti-A and anti-B are fixed to the porous membrane in the indicator
zone serving
as preferred bonding elements, preferably in a row arranged distally to the
application
zone and to other indicator zone rows.
Preferably a control bonding element (control = ctl) which positively
indicates the flow
of the sample through the indicator zones is provided in an indicator zone of
this row of
indicator zones, preferably in an indicator zone which is distal to all
remaining indicator
zones of this row. The control bonding element is preferably a polyclonal anti-
erythrocyte antibody.
This preferred embodiment of the device according to the invention comprises
in a
further row of indicator zones which is preferably proximal to the application
zone,
thrombocytes and/or lymphocytes membranes or membrane components serving as
bonding elements for the detection of anti-thrombocyte/lymphocyte antibodies.
In a further preferred embodiment of the device according to the invention,
the indicator
zones, preferably in a row of indicator zones distal to the application zone,
comprise
antibodies or antibody fragments or lectines which capture or bond the blood
group
antigens to be determined for the blood group determinations and thereby also
the cells in
the sample which carry the aforegoing. Antibodies or antibody fragments or
lectines
against antigens or antigen epitopes of the ABO blood group system, for
example anti-A,
anti-B, anti-A and anti-B are affixed as preferred bonding elements to the
porous
CA 02531459 2006-01-05
WO 2005/005986 15 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
membrane in the indicator zones, preferably in a row which is distal to the
application
zone and to other indicator zone rows.
Preferably in an indicator zone of this row of indicator zones, preferably in
an indicator
zone which is distal to all remaining indicator zones of this row, a control
bonding
element (control = ctl) is applied which positively indicates the flow of the
sample
through the indicator zone. The control bonding element is preferably a
polyclonal anti-
erythrocyte antibody.
This preferred embodiment of the device according to the invention comprises
in a
further row of indicator zones, preferably arranged proximally to the
application zone,
bonding elements for the detection of infective agents, in particular
synthetically
produced peptides or the recombinant antigens expressed with recombinant DNA
methods, and which include diagnostically significant sequences of surface
proteins of
the respective markers (antibody detection) or antibodies which are directed
against
(surface) proteins of infective agents (antigen detection).
As a result of the device according to the invention it is no longer necessary
for the
simultaneous determination of cellular and plasmatic parameters of a sample to
perform
separate pipetting for each individual determination, but rather all desired
parameters can
be determined simultaneously in a sample particularly in the case of
simultaneous
performance of blood group determinations and serum cross-checking and/or
antibody
detection tests as well as in the simultaneous conduction of blood group
determinations
and determinations of transfusion relevant infection serological markers,
wherein it is
possible to combine the blood group determination with the detection of any
infection
serological marker, as well as in the simultaneous performance of blood group
determinations and determination of antibodies against blood cells other than
erythrocytes, in particular anti-thrombocyte and anti-lymphocyte antibodies.
This represents an exceptional rationalization of the procedures. Besides the
advantage
of simultaneous determination of many serological parameters, the virtually
complete
CA 02531459 2006-01-05
WO 2005/005986 16 PCT/EP2004/007525
Ln\hhh\bardeh 1 e\3 0\ 12\2005
redundancy of sample preparations as compared with conventional tests should
be
mentioned. The reading of the results represented in a diagonal pattern is
likewise more
advantageous. Thus, in the device according to the invention it is possible,
for example,
to determine and read in a device, side by side, for example blood groups in
particular
ABO properties and serum cross-checks and antibody detection tests or blood
group in
particular ABO properties and other immune haematological parameters in
particular
anti-thrombocyte and/or anti-lymphocyte antibodies or fragments thereof or
blood group
in particular ABO properties and infection serological markers, in particular
antibodies
against bacterial and/or viral agents or fragments thereof or viral or
bacterial antigens or
epitopes.
The two-dimensional planar display as well as the stable end point of the
reaction,
facilitate not only reading with the naked eye but also the automatic reading
of the results
with conventional display analytical methods, such as e.g. CCD cameras. The
work
expenditure is reduced even where manual evaluation is performed. The device
according to the invention moreover results in a reduction of environmental
impact and in
favourable cost affects. Even in emergency situations under time pressure it
is, for
example, possible to perform in a short period in a single test device, a
complete ABO
blood group determination with serum cross-checks and antibody detection test
for
irregular antibodies, for example a complete ABO blood group determination
with
infection serological marker determination or anti-thrombocyte/lymphocyte
antibody
determination. From a production technical point of view the lateral-diagonal
now
pattern offers considerable advantages compared with the state-of-the-art,
involving a
considerably reduced consumption of reagents employed and in view of the
provision of
a multiplicity of test parameters in a single device which previously had to
be tested for
separately.
Due to the device according to the invention a lateral flow test, in
particular for immune
haematological and infection serological diagnostics is provided by means of
which in a
single testing batch simultaneously several cellular, in particular
erythrocytal antigens or
antigen epitopes, plasmatic parameters and/or blood cell properties, in
particular of
CA 02531459 2010-05-12
17
complete blood components can be determined per sample being tested, there
being
employed at least two kinds of indicator particle of which at least one kind
is represented
by erythrocytes. In addition, there is thereby made available a cost-effective
test system,
easily produceable which can be handled in the simplest possible manner with
few test
runs and without sample preparation by means of which simultaneously different
cellular
parameters and/or plasma parameters of a sample in particular blood group
characteristics, detection of regular and irregular antibodies against
thrombocytes and/or
lymphocytes and/or infection serological markers in particular those with
transfusion
medicinal relevancy can be determined.
The membrane of the device according to the invention is a porous membrane.
Preferred membrane materials are, for example, nitrocellulose (e.g. uniS-
artTM, HiFlowTM
of Millipore, Whatman, AE99 or FF85/100 of Schleicher & Schuell), polyethylene
(Lateral F1oTM of Porex Corporation) or nylon (NovalonTM of CUNO). Preferably
the
membrane has the largest possible pore size because a high porocity of the
membrane
facilitates the influx in particular of cellular components of the sample to
be tested e.g.
of erythrocytes into the porous structure. The use of absorbent membranes is
particularly advantageous. However, the device according to the invention is
not limited
by such properties. Preferred are all membranes having a high capillary flow
rate
(capillary speed) wherein the capillary flow rate represents that time which
is required
by a dye solution in order to travel forty millimeters on a given membrane.
Particularly
preferred are membranes having a capillary flow rate less than 100.
In the bi-directional embodiment using two different membranes, the one
membrane
preferably has a higher capillary flow rate, preferably, less than 100, and
the other
membrane preferably has a lower capillary flow rate, preferably more than 100.
In a preferred embodiment of the invention a sealing element is provided on
the porous
membrane downstream of the application zone of the device in accordance with
the
invention. Two- or three-dimensional sealing elements, which are placed onto
the porous
membrane and by means of which a sample application zone separate from the
remaining
CA 02531459 2006-01-05
WO 2005/005986 18 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\ 12\2005
surface of the porous membrane, are employed. According to the invention the
sealing
element primarily has the effect of a liquid barrier and permits the
directional distribution
of sample liquid and test reagents in the porous membrane. Moreover the
sealing element
according to the invention seals off the sample application zone in order to
prevent an
inadvertent entry of liquid into the remaining parts of the lateral flow
device. Preferred
embodiments of the sealing element are web shapes or trough shapes or funnel
shapes.
The shaping of the sealing element takes place by cutting processes from the
material
used for the production of the sealing element. In the case of the funnel or
trough shape
the sealing element is provided with an inner aperture, the preferred
modifications of
which are round, square or rectangular and tapering towards the underside
(membrane
contact side) of the sealing element in the case of the funnel shape.
Preferred materials for the sealing element are materials which are
hydrophobic. In a
special embodiment, the materials are coated on one side with an adhesive
film, for
example, a pressure sensitive or self-adhesive acrylate adhesive. Accordingly,
the sealing
element can be adhesively bonded directly onto the surface of the porous
membrane.
Alternatively, the sealing element can be bonded to the lateral flow casing,
for example
adhesively bonded such that in this embodiment the lateral flow casing presses
the
sealing element against the surface of the porous membrane such that the
functions of the
sealing element are attained.
Preferred materials for the formation of two-dimensional sealing elements are
any form
of adhesive tape or adhesive foils (e.g. Tesa 4124 of Beiersdorf AG, ARcare
7815 of
Adhesives Research).
Preferred materials for the formation of three-dimensional sealing elements
are flexible,
closed pore elastomer materials or flexible silicon materials of variable
material
thicknesses, preferably 3-5 mm (e.g. cellular caoutchouk EPDM140 of Pitzner,
silicone
rubber or solid caoutchouk, hardness 40 or less of Castan).
CA 02531459 2006-01-05
WO 2005/005986 19 PCT/EP2004/007525
Ln\h hh\bardeh 1e\3 0\ 12\2005
Due to the structure according to the invention the device according to the
invention is
capable of accommodating liquid samples which contain cells, for example
complete
blood without filtering of the cells. Moreover, the sealing element permits
the application
of large volumes of sample onto the porous membrane (application zone) without
flooding thereof. Accordingly the sealing element supports the utilization of
the
absorbent properties of the porous membrane. Furthermore, the sealing element
ensures
a directional flow of sample. Nevertheless, the device according to the
invention can
function well with or without any sealing element.
For the absorption region (absorption pad) of the device according to the
invention,
mechanically stable materials are preferred, preferably having a water
absorption capacity
of 20-30 g/100 cm2 (e.g. Wicking Papier, type 300, Schleicher and Scholl). The
contact
between the absorption pad and the lateral flow membrane of the device
according to the
invention is produced by contact pressure and overlapping with the porous
membrane.
The exact positioning of the absorption pad on the membrane is attained by
adhesive
bonding of the absorption pad to the carrier layer (backing sheet) carrying
the lateral flow
membrane.
The conjugate pad preferably consists of glass fibre or cellulose and
preferably has the
property of retarding the flow of native erythrocytes.
In a further embodiment the components of the device according to the
invention are
applied for purposes of mechanical strengthening onto a support or carrier
layer. The
device according to the invention can however also function without a carrier
layer.
Preferably mechanically stable and non-water absorbent materials, preferably
having
thicknesses of 100 pm or more coated on one or both sides with an adhesive
film e.g. a
pressure sensitive or self-adhesive acrylate adhesive (e.g. 0,005 inch
polyester W/ GL-
187, G & L). On the carrier layer the porous membrane and the absorption pad
are fixed.
In the case of a carrier layer rendered adhesive on both sides, the adhesive
second side is
employed for fixing the stack onto further surfaces, e.g. inside the lateral
flow casing.
CA 02531459 2006-01-05
WO 2005/005986 20 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\12\2005
In a further embodiment the device according to the invention, either with or
without a
carrier layer, onto which the components of the device according to the
invention have
been applied, is integrated in a casing, by which the membrane components are
pressed
onto one another and the casing supports the sealing element function.
However, in this
context the device according to the invention can function as well with as
without a
casing.
A further subject of the invention is the use of the device according to the
invention for
the analysis of blood, in particular for simultaneously performing the blood
group
determination and serum cross-checking and/or antibody detection test and/or
for the
simultaneous performance of the blood determination and the detection of
antibodies
against infectious in particular bacterial and/or viral agents or fragments
thereof or of
antigens of infective agents and/or for the simultaneous performance of blood
group
determinations and the detection of antibodies against erythrocytes other than
blood cells,
in particular anti-thrombocyte and/or anti-lymphocyte antibodies or fragments
thereof.
The object is attained according to the invention also by a process for
determining a
plurality of analytes or their derivatives in a liquid sample comprising the
application of
the sample onto the application zone of a membrane of the device according to
the
invention, wherein the sample is present in adequate amount in order to induce
the
sample liquid to flow in the direction of the absorption region through the
indicator zones
and in order to induce the analytes or their derivatives in the sample liquid
to become
bonded to the respective indicator zones or to form a complex in the indicator
zones.
A specific embodiment of the process according to the invention performs
simultaneously
a blood group determination and a serum cross-checking and/or antibody
detection tests.
For example complete blood or a diluted form thereof is applied onto the
application zone
of the device. All components penetrate, guided by the sealing element into
the porous
membrane and pass during migration in the direction of the absorption pad
initially
through the indicator zone region for serum retesting, taking up fragments of
the cells A 1,
CA 02531459 2006-01-05
WO 2005/005986 21 PCT/EP2004/007525
Ln\hhh\bardehle\30\1 2\2005
A2, B, 0 as well as through the control indicator shown including anti-
IgG/IgM. The
isoagglutinines present in the serum become bonded to the corresponding
cellularly
bound antigens. Serum IgG bonds to the control bonding element (serum cross-
check:
sensibilisation).
The cellular bound antigens migrate onwards into the indicator zone region
distal to the
indicator zone region for serum cross-checking, for blood group determination
wherein in
each indicator zone an antibody is immobilized against a different blood group
characteristic, (e.g. anti-A, anti-B, anti-AB). The indicator zone of this
region most distal
to the application zone for example comprises polyclonal anti-erythrite
antibodies as a
bonding element. In this indicator zone region the erythrocytes become bonded
to the
bonding elements corresponding to the respective blood group characteristics.
Erythrocytes of each and every blood group (blood group determination) become
bonded
to the control bonding element.
In a subsequent rinsing step non-bonded material is washed from the membrane.
In the
subsequent detection step immobilized isoagglutinines and control antibodies
immobilized on the bonding elements of the proximal series of indicator zones
are
rendered visible (serum cross-check: detection) by means of synthetic
particles coated
with anti-IgG/IgM.
In a further embodiment of the process according to the invention for the
simultaneous
blood group determination and serum cross-check and/or antibody detection test
the
blood group characteristics are determined directly as described above whereas
the serum
cross-check and/or antibody detection test is performed as a competition test.
By way of example complete blood or a diluted form thereof is applied onto the
application zone of the device. Guided by the sealing element all components
penetrate
into the porous membrane and pass during their migration in the direction of
the
absorption pad initially through the indicator zone region for serum cross-
checking which
comprises fragments of cells of blood group A and B as well as the control
indicator zone
CA 02531459 2006-01-05
WO 2005/005986 22 PCT/EP2004/007525
Ln\hhh\bardehle\3 0\12\2005
which comprises fragments of cells of blood group 0. The isoagglutinines
present in the
serum become bonded to the corresponding cellularly bound antigens (serum
cross-
check: sensibilisation).
The cellularly bound antigens migrate onwards into the indicator zone region
for blood
group determination located distal to the indicator zone region for serum
cross-checking
and wherein in each indicator zone an antibody against a different blood group
characteristic is immobilized (e.g. anti-A, anti-B, anti-D). The indicator
zone of this
region which is most distal to the application zone may, for example, have
polyclonal
anti-erythite anti-bodies serving as bonding element. In this indicator zone
region the
erythrocytes become bonded to those bonding elements which correspond to the
respective blood group characteristics. Erythrocytes of whatever blood group
bond to
the control bonding element (blood group determination).
In the subsequent rinsing step, non-bonded material is rinsed out of the
membrane. In the
next following step a suspension of different synthetic particles, coated
respectively with
anti-A, anti-B or anti-H is applied onto the application zone. The particles
are only able
to bond each to that bonding element in the indicator zone region for serum
cross-
checking which during the sensibilisation step had not been brought into
contact with the
serum isoagglutinines i.e. displaying a coloured band which in this case
indicates the
absence of the corresponding isoagglutinines. For example in the case of a
blood group
A (persons having isoagglutinines anti-B) the cell fragments of the B cells
are blocked by
the isoagglutinines which results in the cell fragments of the A cells
becoming stained by
the subsequently applied mixture of synthetic particles due to the anti-A
particles
contained therein. The blood group 0 fragments are stained in all conceivable
constellations because they are not blocked by isoagglutinines and are
accordingly always
free to react with the stained anti-H particles, as a result of which a
control element is
also made available in the modification of a competition assay (serum cross-
check:
detection).
CA 02531459 2006-01-05
WO 2005/005986 23 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
In a further embodiment of the process according to the invention for
simultaneous blood
group determination and serum cross-checking and/or antibody detection test
including
direct determination of the blood group characteristics and determination of
the serum
cross-check and/or antibody detection test performed as a competition test,
the following
procedure is followed.
Complete blood or a diluted form thereof is applied onto the asymmetrical
application
zone of the device having two different porous membranes. One of the porous
membranes has a lower capillary flow rate than the other membrane. In the case
of the
latter, a conjugate pad containing dried anti-A/anti-B/anti-H particles has
been applied
onto the membrane between the sealing element and the indicator zones. Guided
by the
sealing element, all components penetrate into both porous membranes,
resulting in the
following flow characteristics:
"Membrane only side": All components during their migration pass in the
direction of
the associated absorption pad through the indicator zone region for blood
group
determination in which in each indicator zone there is provided an antibody
against a
different blood group characteristic in immobilized form (e.g. anti-A, anti-B,
anti-D).
The indicator zone of this region most distal to the application zone
comprises for
example polyclonal anti-erythrocyte antibodies as a bonding element. In this
indicator
zone region the erythrocytes become bonded to those bonding elements which
correspond
to the respective blood group characteristics. Erythrocytes of whatever blood
group
become bonded to the control bonding element.
"Membrane/conjugate pad side": All components having penetrated into the
membrane
and passed the sealing element enter into contact with the conjugate pad
causing the
cellular components to be retained or retarded whereas the liquid components
(plasma)
can flow onward without restraint. The latter cause the anti-A/anti-B/anti-H
particles to
be released from the conjugate pad. The indicator zone region of the serum
cross-check
includes fragments of cells of the blood groups A and B as well as control
element
fragments of cells of the blood group O. The isoagglutinines present in serum
become
CA 02531459 2006-01-05
WO 2005/005986 24 PCT/EP20041007525
Ln\h hh\bardeh le\30\ 12\2005
bonded to the corresponding cellularly bound antigens and compete thereby for
bonding
of the stained anti-A, anti-B particles. This means that the particles can
bond only if the
respective isoagglutinine is absent. For example, a blood group A person has
anti-B
isoagglutinines which results in the blood group B fragments being blocked in
the
indicator zone region so that only the blood group A fragments can be stained
by the
coloured anti-A particles. The control element against which no
isoagglutinines exist
accordingly always remains unblocked and becomes stained as a visible band by
the anti-
H particles contained in the particle mixture of the conjugate pad.
A further particular embodiment of the process according to the invention
results
simultaneously in a blood group determination and a determination of anti-
thrombocyte
and/or anti-lymphocyte antibodies or fragments thereof.
By way of example for the determination of the anti-thrombocyte antibodies the
indicator
zones in the region proximal to the application zone includes thrombocyte
fragments as
bonding elements to which - if present in the sample - anti-thrombocytal
antibodies
contained in the serum will become bonded. The remainder of the process is as
described
in the preceding embodiment of the process according to the invention, in
particular the
blood group determination proceeds as there described.
A further particular embodiment of the process according to the invention
performs
simultaneously a blood group determination and a determination of transfusion
relevant
infection serological markers.
By way of example, for the determination of infection markers, the indicator
zones of the
first proximal indicator zone region include as bonding elements synthetic
peptides
and/or recombinant proteins corresponding to the sequences of proteins of
viral or
bacterial agents, (determination of antibodies against infection markers, e.g.
anti-HIV-1),
as well as antibodies which are directed against infection markers
(determination of
antigens e.g. HbsAg). Antibodies or antigens as the case may be contained in
the serum
bond in the first step to the corresponding antigens or antibodies. The
remainder of the
CA 02531459 2006-01-05
WO 2005/005986 25 PCT/EP2004/007525
Ln\hhh\bardeh 1e\3 0\12\2005
process is as described in the preceding embodiment of the process according
to the
invention, in particular the blood group determination proceeds as there
described.
In the process according to the invention the analytes to be determined more
particular
represent blood group antigens or antigen epitopes, preferably those of the
blood group
systems ABO, Rh, Kell in order to interact with blood group antigens or with
antigen
epitopes against antibodies or fragments thereof preferably regular
antibodies, irregular
antibodies in order to detect antibodies against infective agents (surface)
antigens of
infective agents and/or anti-thrombocytol or anti-lymphocytol antibodies or
fragments
thereof.
The sample to be inspected for example native or anti-coagulated complete
blood or
erythrocyte concentrates or diluted erythrocyte suspensions, blood components
or test
liquids such as control serum or control cells is applied onto the application
zone of the
device according to the invention. The erythrocytes contained in the sample
and which
carry the analyte(s) at the same time serve as indicator particles.
In particular two groups of indicator particles are used. One thereof is
represented
exclusively by erythrocytes for the direct detection of erythrocyte-bound
analytes. The
other group is composed of particles of any conceivable type and combination
by means
of which bonding reactions can be demonstrated, preferably particles of
colloidal gold or
of polystyrene or immobilized erythrocytes. In a preferred embodiment
different
indicator particles are used in each test run, of which at least one type
represents native
erythrocytes.
In the following the invention will be further illucidated by figures and
examples without
being limited thereby. There is shown in:
Fig. I a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determination and serum cross-
checking;
CA 02531459 2006-01-05
WO 2005/005986 26 PCT/EP2004/007525
Ln\hhh\bardeh le\30\12\2005
Fig. 2 an explosive view of the device for lateral flow tests according to the
invention
illustrated in Fig. 1;
Fig. 3 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations and serum cross-
checking
carried out with a three-dimensional sealing element in the form of a web;
Fig. 4 an explosive view of the device according to the invention for lateral
flow tests
illustrated in Fig. 3;
Fig. 5 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations and serum cross-
checking
performed with a three-dimensional sealing element in the form of a trough;
Fig. 6 an explosive view of the device for lateral flow tests according to the
invention
illustrated in Fig. 5;
Fig. 7 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations, serum cross-check
and
antibody detection tests for recipients;
Fig. 8 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations serum cross-checks
and
antibody detection tests for donors;
Fig. 9 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations and the detection
of
infection markers;
CA 02531459 2006-01-05
WO 2005/005986 27 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
Fig. 10 a perspective view of a device according to the invention for lateral
flow tests for
the simultaneous performance of blood group determinations and the detection
of
antibodies against thrombocytal antigens;
Fig. 11 shows a perspective view of a device according to the invention for
lateral flow
tests with bi-directional flow for the simultaneous determination of blood
group testing
and serum cross-checking;
Fig. 12 shows an explosive view of the device according to the invention for
lateral flow
tests according to Fig. 11.
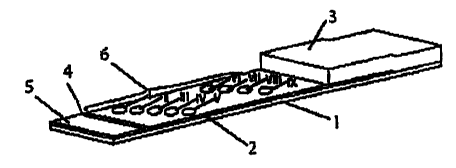
Fig. I shows by way of example a perspective illustration of a device
according to the
invention for lateral flow tests for the simultaneous performance of blood
group
determinations and serum cross-checking. In the present example the device
comprises a
support layer 1, the porous membrane 2 the absorption pad 3 and the two-
dimensional
sealing element 4 in the form of a strip. The porous membrane 2 is fixed onto
the support
layer l by means of a pressure sensitive acrylic adhesive. Likewise, the
absorption pad 3
is fixed onto the support layer 1, part of the absorption pad 3 overlapping
the porous
membrane 2. The sealing element 4 fixed on the upper side of the porous
membrane 2
separates the application zone 5 from the remaining membrane surface and
permits the
directed distribution of sample liquid and test reagents into the porous
membrane 2.
Between the application zone 5 and the region of the porous membrane 2 which
is in
contact with the absorption pad 3 the indicator zone region 6 is provided. The
latter is
formed by diagonally staggered point-shaped indicator zones I-IX arranged in
defined X
and Y positions wherein the indicator zones I-V denote the indicator zone
region "serum
cross-check" and the indicator zones VI-IX denote the indicator zone region
"blood group
determination" and are composed of the following bonding elements:
CA 02531459 2006-01-05
WO 2005/005986 28 PCT/EP2004/007525
Ln\hhh\bardeh le\30\l 2\2005
Indicator Zone Bonding Element Specification
Indicator zone region: serum cross-check
I Erythrocyte ghosts Blood group Al
11 Erythrocyte ghosts Blood group A2
III Erythrocyte ghosts Blood group B
IV Erythrocyte ghosts Blood group 0
V Antibodies Anti-human IgG/IgM
Indicator zone region : Blood group determination
VI Antibodies Anti-A (monoclonal)
VII Antibodies Anti-AB (monoclonal)
IX Antibodies Anti-erythrocytes (polyclonal)
Indicator zone V is the control (ctl) for the serum cross-check and contains
anti-human
IgG/IgM antibodies. Indicator zone X is the control (ctl) for the blood group
determination and contains polyclonal anti-erythrite antibodies. They are
positioned
distally to all remaining indicator zones.
In Fig. 2 an explosive view of the device according to the invention for
lateral flow tests
illustrated in Fig. 1 is shown comprising the components support layer 1,
porous
membrane 2, absorption pad 3 and sealing element 4 which separate the
application zone
5 from the remainder of the membrane which in turn comprises the indicator
zone regions
"serum cross-check" and "blood group determination" including the diagonally
staggered
indicator zones I-IX.
CA 02531459 2006-01-05
WO 2005/005986 29 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
In Fig. 3 a perspective view of a device according to the invention for
lateral flow tests
for the simultaneous performance of blood group determinations and serum cross-
checks
is shown. In the present example the components of the device correspond to
the
components of the device illustrated in Fig. I except for the sealing element
fixed to the
upper side of the porous membrane 2 in the form of a three-dimensional batten
4.
In Fig. 4 an explosive view is shown of the device according to the invention
illustrated
in Fig. 3 for lateral flow tests including the components support layer 1,
porous
membrane 2, absorption pad 3 and a sealing element 4 in the form of a three-
dimensional
batten which separates the application zone from the remaining membrane which
in turn
contains the indicator zone region 6 comprising the indicator zone regions
"serum cross-
check" and "blood group determination" including the diagonally staggered
indicator
zones I-IX.
In Fig. 5 is shown by way of example a perspective illustration of a device
according to
the invention for lateral flow tests for the simultaneous performance of blood
group
determinations and serum cross-checking. In the present example the components
of the
device correspond to the components of the device as illustrated in Fig. I
except for the
sealing element 4 fixed to the porous membrane 2 in the form of a three-
dimensional
trough.
In Fig. 6 an exploded view of the device according to the invention for
lateral flow tests
illustrated in Fig. 5 is shown including the components support layer 1,
porous membrane
2, absorption pad 3 and sealing element 4 in three-dimensional trough form
which
separates the application zone 5 from the remainder of the membrane which in
turn
consists of the indicator zone region 6, of the indicator zone regions "serum
cross-check"
and "blood group determination" including the diagonally staggered indicator
zones I-IX.
Fig. 7 illustrates by way of example a perspective view of a device according
to the
invention for lateral flow tests for the simultaneous performance of blood
group
determinations, serum cross-checking and antibody detection tests for
recipients. In the
CA 02531459 2006-01-05
WO 2005/005986 30 PCT/EP2004/007525
Ln\hhh\bardeh le\30\12\2005
present case the device is composed of a support layer 1, the porous membrane
2, the
absorption pad 3 and the two-dimensional sealing element 4 in the form of a
strip. The
membrane 2 is affixed on the support layer I provided with a pressure
sensitive acrylic
adhesive. Likewise the absorption pad 3 is affixed to the support layer 1 with
part of the
absorption pad 3 overlapping the porous membrane 2. The sealing element 4
fixed to the
upper side of the porous membrane 2 separates the application zone 5 from the
remaining
membrane surface and permits the directional distribution of sample liquid and
test
reagents into the porous membrane 2. Between the application zone 5 and the
region of
the porous membrane 2 which is in contact with the absorption pad 3, the
indicator zone
region 6 is provided. The latter is formed by diagonally staggered point-
shaped indicator
zones l-XII provided in defined X and Y positions, the indicator zones I-VIII
representing the indicator zone region "serum cross-check/antibodies detection
test" and
the indicator zones IX-X1I representing the indicator zone region "blood group
determination" and consists of the following binding elements:
CA 02531459 2006-01-05
WO 2005/005986 31 PCT/EP2004/007525
Ln\h h h\b and eh l e\3 0\ 12\200 5
Indicator Zone Bonding Element Specification
Indicator zone region: serum cross-check/antibody detection
I Erythrocyte ghosts Blood group Al
11 Erythrocyte ghosts Blood group A2
III Erythrocyte ghosts Blood group B
IV Erythrocyte ghosts Blood group 0
V Erythrocyte ghosts Detector cell 1, blood group 0, Rh-formula R1, R1'
VI Erythrocyte ghosts Detector cell 2, blood group 0, Rh-formula R2, R2
VII Erythrocyte ghosts Detector cell 3, blood group 0, Rh-formula R1, R1
VIII Antibodies Anti-human IgG/lgM
Indicator zone region : Blood group determination
IX Antibodies Anti-A (monoclonal)
X Antibodies Anti-B (monoclonal)
XI Antibodies Anti-AB (monoclonal)
XII Antibodies Anti-erythrocytes (polyclonal)
Indicator zone VIII is the control (ctl) for the serum cross-check and
antibody detection
test and contains anti-human IgG/IgM antibodies. Indicator zone XII is the
control (ctl)
for the blood group determination and contains polyclonal anti-erythrocyte
antibodies. It
is provided distal to all remaining indicator zones.
In Fig. 8 by way of example, a perspective view of a device according to the
invention for
lateral flow tests for the simultaneous performance of blood group
determinations, serum
CA 02531459 2006-01-05
WO 2005/005986 32 PCT/EP2004/007525
Ln\hhh\bardeh 1e\3 0\ 12\2005
cross-checking and antibody detection tests for blood donors is shown. In the
present
example the device comprises a support layer 1, the porous membrane 2, the
absorption
pad 3 and the two-dimensional sealing element 4 in the form of a strip. The
porous
membrane 2 is fixed to the support layer provided with a pressure sensitive
acrylate
adhesive. Likewise the absorption pad 3 is fixed to the support layer 1, part
of the
absorption pad 3 overlapping with the porous membrane 2. The sealing element 4
fixed
on the upper side of the porous membrane 2 separates the application zone 5
from the
remaining membrane surface and permits the directed distribution of sample
liquid and
test reagents into the porous membrane 2. Between the application zone 5 and
the region
of the porous membrane 2 which is in contact with the absorption pad 3, the
indicator
region 6 is provided. This is formed by point-shaped indicator zones I-X
provided
diagonally staggered in defined positions X and Y, the indicator zones I-VI
representing
the indicator region serum cross-check/antibody detection tests and the
indicator zones
VII-X comprising the indicator zone region "blood group determination" and
being
composed of the following bonding elements:
CA 02531459 2006-01-05
WO 2005/005986 33 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\12\2005
Indicator Zone Bonding Element Specification
Indicator zone region: serum cross-check/antibody detection
I Erythrocyte ghosts Blood group Al
11 Erythrocyte ghosts Blood group A2
III Erythrocyte ghosts Blood group B
IV Erythrocyte ghosts Blood group 0
V Erythrocyte ghosts Blood group 0, pool of detection cells 1, 2, 3 (see
description Fig. 8)
VI Antibodies Anti-human IgG/IgM
Indicator zone region : Blood group determination
VII Antibodies Anti-A (monoclonal)
VIII Antibodies Anti-B (monoclonal)
IX Antibodies Anti-AB (monoclonal)
X Antibodies Anti-erythrocytes (polyclonal)
Indicator zone VI represents the control (ctl) for the serum cross-check and
antibody
detection tests and contains anti-human IgG/IgM antibodies. Indicator zone X
represents
the control (ctl) for the blood group determination and contains polyclonal
anti-
erythrocyte antibodies. It is situated distally to all remaining indicator
cells.
In Fig. 9 there is shown by way of example a perspective view of a device
according to
the invention for lateral flow tests for the simultaneous performance of the
blood group
determinations and the detection of infection markers. In the present example
the device
CA 02531459 2006-01-05
WO 2005/005986 34 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\ 12\2005
includes a support layer 1, the porous membrane 2, the absorption pad 3 and
the two-
dimensional sealing element 4 in the form of a strip. The porous membrane 2 is
fixed
onto the support layer 1 provided with a pressure sensitive acrylate adhesive.
Likewise
the absorption pad 3 is fixed onto the support layer 1, part of the absorption
pad 3
overlapping with the porous membrane 2. The sealing element 4 fixed to the
upper side
of the porous membrane 2 separates the application zone 5 from the remaining
membrane
surface and permits the directed distribution of sample liquid and test
reagents into the
porous membrane 2. Between the application zone 5 and the region of the porous
membrane 2 which is in contact with the absorption pad 3 the indicator region
6 is
provided. The latter is formed by diagonally staggered point-shaped indicator
zones I-
XII arranged in defined X and Y positions, the indicator zones 1-VI
representing the
indicator zone "detection of infection markers" and the indicator zones VII-
XII the
indicator zone region "blood group determination" and are composed of the
following
bonding elements:
CA 02531459 2006-01-05
WO 2005/005986 35 PCT/EP2004/007525
Ln\hhh\b ardeh 1e\3 0\ 12\2005
Indicator Zone Bonding Element Specification
Indicator region: Detection of infection markers
I Synthetic peptides HIV-1 (gp-14, gp-41)
II Synthetic peptides HIV-2 (gp-36)
III Antibodies Anti-HBsAg (monoclonal)
IV Recombinant antigen HCV (C-100, C-200, C33c, C22)
V Recombinant antigen Syphilis (TpN 15, TpN 17, TpN 47)
VI Antibodies Anti-human IgG/IgM
Indicator zone region : Blood group determination
VII Antibodies Anti-A (monoclonal)
VIII Antibodies Anti-B (monoclonal)
IX Antibodies Anti-AB (monoclonal)
X Antibodies Anti-D (monoclonal)
XI Antibodies Anti-CDE (monoclonal)
XI1 Antibodies Anti-erythrocytes (polyclonal)
Indicator zone VI is the control (ctl) for the determination of antibodies
against infective
agents and contains anti-human IgG/IgM antibodies. Indicator zone XII is the
control
(ctl) for the blood group determinations and contains polyclonal anti-
erythrocyte
antibodies. It is distally arranged in relation to all other indicator zones.
In Fig. 10 a perspective view of a device according to the invention for
lateral flow tests
is shown by way of example for the simultaneous performance of blood group
CA 02531459 2006-01-05
WO 2005/005986 36 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\12\2005
determinations and the detection of antibodies against thrombocytal antigens.
In the
present example the device comprises a support layer 1, a porous membrane 2,
the
absorption pad 3 and the two-dimensional sealing element 4 in the form of a
strip. The
porous membrane 2 is fixed to the support layer I provided with a pressure
sensitive
acrylate adhesive. Likewise the absorption pad 3 is fixed to the support layer
1, part of
the absorption pad 3 overlapping with the porous membrane 2. The sealing
element 4
fixed to the upper side of the porous membrane 2 separates the application
zone 5 from
the remaining membrane surface and permits the directed distribution of sample
liquid
and test reagents into the porous membrane 2. Between the application zone 5
and the
region of the porous membrane 2 which is in contact with the absorption pad 3
the
indicator region 6 is provided. This is formed of diagonally staggered point-
shaped
indicator zones I-IX in defined X and Y positions, wherein the indicator zones
I-III
include the indicator zone region "detection of antibodies against
thrombocytal antigens"
and the indicator zones IV-IX the indicator zone region "blood group
determination" and
consist of the following bonding elements:
CA 02531459 2006-01-05
WO 2005/005986 37 PCT/EP2004/007525
Ln\hhh\bardehle\30\1 2\2005
Indicator Zone Bonding Element Specification
Indicator region: Detection of antibodies against thrombocydol antigens
I Membrane proteins Thrombocytes, HPA lbb3aa5bb
II Membrane proteins Thrombocytes, HPA l aa3bb5aa
III Antibodies Anti-human IgG/IgM
Indicator zone region : Blood group determination
IV Antibodies Anti-A (monoclonal)
V Antibodies Anti-B (monoclonal)
VI Antibodies Anti-AB (monoclonal)
VII Antibodies Anti-D (monoclonal)
VIII Antibodies Anti-CDE (monoclonal)
IX Antibodies Anti-erythrocytes (polyclonal)
XII Antibodies Anti-erythrocytes (polyclonal)
Indicator zone III is the control (ctl) for the detection of antibodies
against thrombocytal
antigens and contains anti-human IgG/IgM antibodies. Indicator zone IX is the
control
(ctl) for the blood group determination and contains polyclonal anti-
erythrocyte
antibodies. It is positioned distally in relation to all remaining indicator
zones.
In Fig. I 1 is shown by way of example a perspective view of a device
according to the
invention for lateral flow tests with bi-directional flow for the simultaneous
determination
of blood groups and serum cross-checks. In the present example the device
comprises a
CA 02531459 2006-01-05
WO 2005/005986 38 PCT/EP2004/007525
Ln\hhh\bardeh le\30\ 12\2005
support layer 1, a porous membrane 2a for the blood group determination a
porous
membrane for the serum cross-check which differs from the membrane 2a, the
absorption
pad 3a and 3b, a three-dimensional sealing element 4 in trough form and a
conjugate pad
6. The porous membranes 2a and 2b are fixed onto the support layer 1 provided
with a
pressure sensitive acrylate adhesive. Likewise the absorption pads 3a and 3b
are fixed
onto the support layer 1, one part each of the absorption pads 3a and 3b
overlapping with
the porous membranes 2a and 2b. The sealing element 4 fixed to the upper side
of the
porous membranes 2a and 2b separates the application zones 5a and 5b
respectively from
the remaining membrane surfaces and permits the directed distribution of
sample liquid
and test reagents into the porous membranes 2a and 2b. Between the application
zone 5a
and the region of the porous membrane 2a with which the absorption pad 3a is
in contact,
the indicator zone region 7a blood group (blood group determination) is
provided. The
latter is formed by diagonally staggered point-shaped indicator zones I-VI in
defined X
and Y positions, the indicator zones of the indicator zone regions 7a
consisting of the
following bonding elements:
CA 02531459 2006-01-05
WO 2005/005986 39 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
Indicator Zone Bonding Element Specification
I Antibodies Anti-A (monoclonal)
II Antibodies Anti-B (monoclonal)
III Antibodies Anti-AB (monoclonal)
IV Antibodies Anti-D (monoclonal)
V Antibodies Anti-CDE (monoclonal)
VI Antibodies Anti-erythrocytes (polyclonal)
The indicator zone VI represents the control (ctl) for the blood group
determination and
contains polyclonal anti-erythrocyte antibodies. It is provided distally in
respect of the
indicator zones I-V.
Between the application zone 5b and the region of the porous membrane 2b which
is in
contact with the absorption pad 3b the indicator zone region 7b (serum cross-
check) is
provided. This is formed by point-shaped indicator zones VII-IX, diagonally
off-set in
defined X and Y positions, the indicator zones of the indicator zone region 7b
being
composed of the following bonding elements:
Indicator Zone Bonding Element Specification
VII Erythrocyte ghosts Blood group A
VIII Erythrocyte ghosts Blood group B
IX Erythrocyte ghosts Blood group 0 (control)
CA 02531459 2006-01-05
WO 2005/005986 40 PCT/EP2004/007525
Ln\hhh\bardeh le\30\12\2005
In Fig. 12 an exploded view is shown of the device according to the invention
illustrated
in Fig. I for lateral flow tests with bi-directional flow composed of the
components
support layer 1, a porous membrane 2a for the blood group determination, a
porous
membrane 2b for the serum cross-check which differs from the membrane 2a, the
absorption pads 3a and 3b, a three-dimensional sealing element 4 of trough-
shaped design
and a conjugate pad 6. The sample application zone extends over both porous
membranes including the probe application zone 5a of membrane 2a and the probe
application zone 5b of membrane 2b and is separated by the sealing element 4
in trough
form from the remaining surfaces of the membranes 2a and 2b. The membrane 2a
contains the indicator region 7a including the indicator zones I-VI in
diagonally staggered
arrangement from proximal to distal whereas the membrane 2b contains the
indicator
region 7b including the indicator zones VII-IX extending from proximal to
distal in a
diagonally staggered arrangement.
Examples
Example 1: Simultaneous blood group determination (direct assay) and serum
cross-
check (direct binding assay)
Preparation of test strips:
The test strips comprises an application zone an indicator zone region and an
absorption
region. Membranes of the type Millipore HiFlow Plus 065 are cut to size in
strips
measuring 15 mm x 35 mm (width/length; x/y) and are adhesively bonded onto a
support
layer (backing sheet, e.g. of G&L). Diagonally staggered 0,2 l points of the
various
bonding elements are applied in the indicator zone region divided into the
indicator zone
region "serum cross-check" (proximal to the application zone) and blood group
determination (distal to the application zone):
Indicator zone region "serum cross-check" - suspensions of erythrocyte ghosts
of the
specification blood group Al, blood group A2, blood group B and blood group 0
CA 02531459 2006-01-05
WO 2005/005986 41 PCT/EP2004/007525
Ln\hh h\b and eh l e\3 0\ 12\20 05
(produced from erythrocyte concentrates) as well as anti-human IgG/IgM
antibodies
(goat anti-human IgG, goat anti-human IgM, sigma, 1-3382,1-0759) as control,
indicator
zone region "blood group determination" - anti-A, antibodies - clone Birma-1
(serologicals, TLJ0105); anti-B antibodies - clone ES-4 (serologicals,
NCA0201); anti-
AB antibodies - clones AB6, AB26, AB92 (Medion Diagnostics, 010062); anti-
erythrocyte antibodies (rabbit IgG fraction of anti-human RBC, Rockland, 209-
4139).
The positioning of the bonding elements of the indictor zone region "serum
cross-check"
commences with erythrocyte ghosts of the specification blood group Al in
position x=2,5
mm/ y=10 mm. All other bonding elements are dispensed iteratively at distances
of
x=2,5 mm / y=1,5 mm in relation to the position of the erythrocyte ghosts of
the
specification blood group Al. The erythrocyte ghosts are dispensed as 0,1 -
0,5 % (v/v)
suspensions in 15 mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol,
the anti-
human IgG/IgM antibodies as a 1:1 mixture in a concentration of 50 gg/ml in 15
mM
potassium phosphate buffer pH 7,5, 10% (v/v) methanol.
The positioning of the bonding elements of the indicator zone region "blood
group
determination" starts with the anti-A antibody in position x=3 mm/y=20 mm. All
other
bonding elements are dispensed iterating at distances of x=3 mm/y=1,5 mm to
the
position of the anti-A antibody. The dilutions of the antibodies are performed
in 15 mM
potassium phosphate buffer pH 7,5, 10 % (v/v) methanol as follows: anti-A
antibody 1:3,
anti-B antibody 1:2, anti-AB antibody 1:4, anti-RBC antibody 1:3.
After dispensing the bonding elements the membranes are dried for 20 minutes
at 40 C
and subsequently stored at constant air humidity until the test is performed.
At the end
which is distal to the application zone an absorption pad sized 15 x 15 mm
(Schleicher
and Scholl 300) is adhesively applied onto the membrane overlapping by 3 mm.
The
application zone is separated over the entire membrane width from the
remainder of the
membrane by the adhesive application of a 1-2 mm wide adhesive strip (Tesa
4124) in
position y=5 mm.
CA 02531459 2006-01-05
WO 2005/005986 42 PCT/EP2004/007525
Ln\hhh\bard eh 1e\30\ 12\200 5
Testing batch:
For blood samples anti-coagulated complete blood is used. For the test proper
100 l
undiluted or diluted blood 1:3 or 1:6 in diluting buffer (Enlisstll, Medion
Diagnostics or
Diluent 1, DiaMed) are applied into the application zone. Once the blood has
left the
application zone rinsing is performed twice with 100 l EnlisstII in order to
remove
unbonded erythrocytes from the membrane. Thereafter 50 1 anti-IgG/A/M
conjugated
gold particles (20 to 40 nm, Arista Biologicals, CGIGA-0800, CGIGG-0800, CGIGM-
0800), diluted 1:10 (v/v) in TBS, 0,08 % gelatine, 0,5 % albumin are applied
onto the
application zone. Instead of the gold particles it is also possible to employ
coloured
polystyrene particles of 100 to 400 nm, e.g. of Merck Eurolab France/Estapor.
Once the
gold particles have left the application zone the membrane is once again
rinsed once or
twice with 100 l EnlisstII.
Result:
(a) Controls: The test is valid if the anti-RBC control (indicator zone IX,
indicator
zone region "blood group determination") displays a clearly positive signal
(red dot) and
if the anti-IgG/IgM control (indicator zone V, indicator zone region "serum
cross-check")
is characteristically stained purple (gold particles) or in the colour of the
polystyrene
particles used.
(b) Test Results: Depending on the presence or absence of the respective blood
group
antigens red dots (positive) or the almost white background colouration of the
membrane
(negative) is displayed at the corresponding positions in the indicator zone
region "blood
group determination". The corresponding isoagglutinines can be recognized in
the
indicator zone region "serum cross-check" by the characteristic purple of the
gold
particles in the form of purple-shaped dots or in the colour of the
polystyrene particles
used. In the absence of an isoagglutinine no signals differing from background
are to be
perceived in these positions.
CA 02531459 2006-01-05
WO 2005/005986 43 PCT/EP2004/007525
L n\hh h\b and eh le\3 0\ 12\20 0 5
Example 2: Simultaneous determination of blood groups (direct assay) and serum
cross-check (competition assay)
Production of Test Strip:
The test strip consists of an application zone, two indicator zone regions on
both sides of
the application zone and two absorption regions. Membranes of the type
Millipore
HiFlow Plus 065 and HiFlow Plus 140 are cut to size into strips sized 15 mm by
20 mm
(width/length; x/y) and bonded adhesively side by side onto a support layer
(backing
sheet e.g. of G&L). Onto the HiFlow Plus 140 membrane in addition a conjugate
pad
into which the anti-A/anti-B/anti/H conjugated gold particles have been
introduced with
drying, is so applied that it becomes positioned between the sealing element
(adhesive
strip) and the indicator zones. Instead of gold particles it is also possible
to employ
coloured polystyrene particles 100 to 400 nm e.g. of Merck Eurolab
France/Estapor.
Diagonally staggered in the indicator region "blood group determination"
positions on the
HiFlow Plus 065 membrane dots of 0,2 l each of the following bonding elements
are
applied using a dispenser, e.g. AD3200 (Biodot): anti-A antibodies - clone
Birma-1
(serologicals, TLJO105); anti-B antibodies - clone ES-4 (serologicals,
NCA0201); anti-
AB antibodies - clones AB6, AB26, Ab92 (Medion Diagnostics, 010062); anti-
erythrocytes antibodies (rabbit IgG fraction of anti-human RBC, Rockland 209-
4139).
In the same manner suspensions of erythrocytes ghosts of the blood group A, B
and blood
group 0 (produced from erythrocyte concentrates) are applied in the indicator
zone
region "serum cross-check" positioned on the HiFlow Plus 140 membrane.
The positioning of the bonding elements of the indicator zone region "serum
cross-check"
commence with erythrocyte ghosts of the specification blood group A in
position x=3
mm/y=10 mm. All other bonding elements are dispensed iterating at distances of
x=3
mm/y=1,5 mm from the position of the erythrocyte ghosts of the specification
blood
group A. The erythrocyte ghosts are dispensed as 0,1 - 0,5 % (v/v) suspensions
in 15
mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol.
CA 02531459 2006-01-05
WO 2005/005986 44 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\ 12\2005
The positioning of the bonding elements of the indicator zone region "blood
group
determination" commences with the anti-A antibody in positions x=2,5 mm/y=20
mm.
All other bonding elements are dispensed iterating at distances of x=2
mm/y=1,5 mm
from the position of the anti-A antibody. The dilutions of the antibody are
performed in
mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol as follows: anti-A
antibody 1:3, anti-B antibody 1:2, anti-AB antibody 1:4, anti-RBC antibody
1:3.
After dispensing the bonding elements the membranes are dried at 40 C for 20
minutes
10 and subsequently stored at constant air humidity until performance of the
test. To the two
ends which are distal to the application zone an absorption pad (Schleicher &
SchUll,
300) sized 15 x 15 mm is adhesively bonded to the membrane with a 3 mm
overlap. The
application zone is separated over the entire membrane width from the
remainder of the
membrane by the adhesive application of a 1-2 mm wide adhesive strip (Tesa
4124) in
15 position y=5 mm.
Test Batch:
Anti-coagulated complete blood is used as blood samples. For the actual test
100 1
undiluted or blood diluted 1:3 or 1:6 in dilution buffer (Enlisstll, Medion
Diagnostics or
Diluent 1 DiaMed) is applied to the application zone. Once the blood has left
the
application zone rinsing is performed twice with 100 l Enlisstll in order to
remove
unbound erythrocytes from the membrane.
Result:
(a) Controls: The test is valid if the anti-RBC control (indicator zone region
"blood
group determination") displays a clearly positive signal (red dot) and if the
erythrocyte
blood groups 0 control (indicator zone region "serum cross-check") is stained
characteristically purple (gold particles) or in the colour of the polystyrene
particles used.
CA 02531459 2006-01-05
WO 2005/005986 45 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\12\2005
(b) Test Results: Depending on the presence or absence of the respective blood
group
antigens, red dots (positive) or the almost white background colouration of
the membrane
(negative) are displayed at the respective positions in the indicator zone
region "blood
group determination". The corresponding isoagglutinines are characterized in
the
indicator zone region "serum-cross-check" by an absence of the corresponding
band. If
an isoagglutinine is absent the corresponding band is characteristically
stained.
Example 3: Simultaneous blood group determination serum cross-check and
antibody
detection test on recipients
Production of the testing strip:
The testing strip consists of an application zone, an indicator zone region
and an
absorption region. Membranes of the type Millipore HiFlow Plus 065 are cut to
size in
strips sized 20 mm x 35 mm (width/length; x/y) and adhesively applied onto a
support
layer (backing sheet e.g. of G&L). Dots of 0,2 l each of the various bonding
elements
are applied using a dispenser, e.g. AD3200 (Biodot) digitally staggered in the
indicator
zone region sub-divided into the indicator zone regions "serum cross-
check/antibody
detection" (proximal to the application zone) and "blood group determination"
(distal to
the application zone).
Indicator zone region "serum cross-check/antibody detection" - Suspensions of
erythrocyte ghosts of the specification blood group Al, blood group A2, blood
group B,
blood group 0, blood group 0 Rh-formula R1R1"' (detection cell 1), blood group
0 Rh-
formula R2R2 (detection cell 2), blood group 0 RH-formula R1R1 (detection cell
3), as
well as anti-human IgG/lgM (goat anti-human IgG, goat anti-human IgM, Signma,
I-
3382, 1-0759) as control; indicator zone region "blood group determination" -
anti-A
antibody- clone Birma-I (serologicals, TLJ0105); anti-B antibody - clone ES-4
(serologicals, NCA0201); anti-AB antibodies - (clones AB6, Ab26, AB92 (Medion
Diagnostics, 010062); anti-erythrocyte antibodies (rabbit IgG fraction of anti-
human
RBC, Rockland, 209-4139).
CA 02531459 2006-01-05
WO 2005/005986 46 PCT/EP2004/007525
Ln\hhh\bardehle\30\12\2005
The positioning of the bonding elements of the indicator zone region "serum
cross-check"
starts with erythrocyte ghosts of the specification blood group Al in position
x=3
mm/y=10 mm, the positioning of the erythrocyte ghosts of the specification
blood group
A2, B, 0 follows by iteration at distances of x=2 mm/y=1,5 mm. The positioning
of the
erythrocyte ghosts of the detector cell I proceeds in position x=1I mm/y=10 mm
the
positioning of the erythrocyte ghosts of the detector cells 2 and 3 as well as
the human
IgG/IgM iterates at distances of x=2 mm/y=1,5 mm. The erythrocyte ghosts are
dispensed as 0,1-0,5 % (v/v) suspensions in 15 mM potassium phosphate buffer
pH 7,5,
10 % (v/v) methanol, the anti-human IgG/IgM antibodies as 1:1 mixture in
concentrations
of 50 pg/ml in 15 mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol.
The positioning of the binding elements of the indicator zone region "blood
group
determination" commences with the anti-A antibody in position x=4 mm/y=20 mm.
All
other bonding elements are dispensed by iteration at distances of x=4 mm/y-1,5
mm to
the position of the anti-A antibody. The dilutions of the antibodies take
place in 15 mM
potassium phosphate buffer pH 7,5, 10 % (v/v) methanol as follows: anti-A
antibody 1:3,
anti-B antibody 1:2, anti-AB antibody 1:4, anti-RBC antibody 1:3.
The membranes, after dispensing the bonding elements, are dried for 20 minutes
at 40 C
and subsequently stored at constant air humidity until the test is to be
performed. At the
end distal to the application zone an absorption pad (Schleicher & SchUll,
300) sized
20x15 mm, is adhesively bonded to the membrane with a 3 mm overlap. The
application
zone is separated from the remaining membrane over the entire width of the
membrane
by the adhesive application of an adhesive strip (Tesa 4124) 1-2 mm wide in
position y=5
mm.
Test batch:
Anti-coagulated complete blood is used for the blood samples. For the test
proper, 120 l
undiluted blood or blood diluted 1:3 or 1:6 in dilution buffer (Enlisstll,
Medion
CA 02531459 2006-01-05
WO 2005/005986 47 PCT/EP2004/007525
Ln\hhh\bardeh 1e\3 0\12\2005
Diagnostics or diluent 1, DiaMed) is applied in the application zone. Once the
blood has
left the application zone rinsing is performed twice with 120 l EnlisstlI in
order to
remove unbonded erythrocytes from the membrane. Thereafter 75 l anti-IgG/A/M
conjugated gold particles (20 to 40 nm, Arista Biologicals, CGIGA-0800, CGIGG-
0800,
CGIGM-0800), 1:10 (v/v) diluted in TBS, 0,08 % gelatine, 0,5 % albumin, are
applied
onto the application zone. Instead of the gold particles it is possible also
to use coloured
polystyrene particles 100 to 400 nm e.g. from Merck Eurolab France/Estapor.
Once the
gold particles have left the application zone, the membrane is once again
rinsed once or
twice with 120 p1 Enlisstll.
Result:
(a) Controls: The test is valid if the anti-RBC control (indicator zone XII,
indicator
zone region "blood group determination") displays a clearly positive signal
(red dot) and
if the anti-IgG/IgM control (indicator zone VIII, indicator zone region "serum
reverse
grouping/antibody test") is stained characteristically purple (gold particles)
or in the
colour of the polystyrene particles used. The anti-IgG/IgM control must in
each case be
stained so that in the case of a person of blood group AB having no irregular
antibodies,
the staining of this indicator zone in complete absence of other signals in
the indicator
zone region "serum reverse grouping/antibody test" confirms a correct test
performance.
The indicator zone IV (erythrocyte ghosts blood group 0) is a negative control
for
isoagglutinines. A staining of this indicator zone means that besides
isoagglutinines also
irregular antibodies must be present, i.e. that at least one of the three
indicator zones V,
VI, VII must likewise be stained. If this is not the case, the test is
invalid.
(b) Test results: Depending on the presence or absence of the respective blood
group
antigens, red dots (positive) or almost white background colour (negative) of
the
membrane are displayed in the respective positions in the indicator zone
region "blood
group determination". The corresponding isoagglutinines are detectable in the
indicator
zone region "serum reverse grouping" by the characteristical purple of the
gold particles
in the form of purple coloured dots or in the colour of the polystyrene
particles used. In
CA 02531459 2006-01-05
WO 2005/005986 48 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\ 12\2005
the absence of isoagglutinine no signals differeing from background are
detectable in
those positions. If an irregular antibody is present, one, two or all three of
the indicator
zones which include the erythrocyte ghosts of the detector cells 1, 2 or 3 are
stained by
the characteristic purple of the gold particles or in the colour of the
polystyrene particles
used.
Example 4: Simultaneous blood group determination, serum cross-check (reverse
grouping) and antibody detection test for donor
Production of the test strip:
In principle the structure of the test strip corresponds to the structure of
the test strip in
Example 2. The format of the Millipore HiFlow Plus 065 membrane is 15 mm x 35
mm
(width/length; x/y). The indicator zones of the indicator zone region "blood
group
determination" correspond to Example 2. In the indicator region "serum cross-
check/antibody test" dots of 0,2 l of the following bonding elements are
dispensed using
a dispenser, e.g. AD3200 (Biodot):
Suspensions of erythrocytes ghosts of the specification blood group Al, blood
group A2,
blood group B, blood group 0, erythrocyte ghosts of a mixture of the cells to
be detected
1-3 (see Example 2) as well as anti-human IgG/IgM (goat anti-human IgG, goat
anti-
human IgM, Sigma, 1-3382,1-0759) serve as controls.
The positioning of the bonding elements of the indicator zone region "serum
cross-
check/antibody test" commence with erythrocyte ghosts of the specification
blood group
Al in position x=2,5 mm/y=10 mm, the positioning of the erythrocyte ghosts of
the
specification blood group A2, B, 0 is iterated at distances of x=2 mm/y=1,5
mm. The
positioning of the erythrocyte ghosts of the mixture of the cells for
detection 1-3 takes
place in positions x=10,5 mm/y=13 mm, that of the human IgG/IgM in position
x=12,5
mm/y=14,5 mm. The erythrocyte ghosts are dispensed as 0,1-0,5 % (v/v)
suspensions in
15 mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol, the anti-human
CA 02531459 2006-01-05
WO 2005/005986 49 PCT/EP2004/007525
Ln\hhh\bardehle\30\1 2\2005
IgG/IgM antibodies as 1:1 mixtures in a concentration of 50 g/ml in 15 mM
potassium
phosphate buffer pH 7,5, 10 % (v/v) methanol.
The positioning of the bonding elements of the indicator zone region "blood
group
determination" starts with the anti-A antibody in position x=3 mm/y=20 mm. All
other
bonding elements of the indicator zone are dispensed, iterated at distances of
x=3
mm/y=1,5 mm to the position of the anti-A antibody. The dilutions of the
antibodies
proceed in 50 mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol as
follows:
anti-A antibody 1:3, anti-B antibody 1:2, anti-AB antibody 1:4, anti-RBC
antibody 1:3.
The membranes, after dispensing the bonding elements, are dried for 20 minutes
at 40 C
and thereafter stored at constant air humidity until testing takes place. At
the end distal to
the application zone an absorption pad (Schleicher & SchUll, 300) sized 15 x
15 mm is
adhesively applied overlapping the membrane by 3 mm. The application zone is
separated from the remaining membrane by the adhesive application of a 1-2 mm
wide
test strip (Tesa 4124) in position y=6 mm over the entire membrane width.
Test batch:
The test batch corresponds to the batch in Example 1.
Result:
(a) Controls: The test is valid if the anti-RBC control (indicator zone X,
indicator
zone region "blood group determination") displays a clearly positive signal
(red dot) or if
the anti-IgG/IgM control (indicator zone VI, indicator zone region "serum
cross-
check/antibody detection") is characteristically purple (gold particles) or is
stained in the
colour of the polystyrene particles used. The anti-IgG/IgM control must in any
event be
stained so that in the case of a person of blood group AB, who has no
irregular
antibodies, the staining of this indicator zone in the complete absence of
other signals in
the indicator zone region "serum cross-check/antibody test" denotes a correct
test
CA 02531459 2006-01-05
WO 2005/005986 50 PCT/EP2004/007525
Ln\hhh\bard eh 1e\30\12\200 5
performance. The indicator zone IV (erythrocyte ghosts blood group 0) is a
negative
control. A staining of this indicator zone denotes, that besides
isoagglutinines also
irregular antibodies must be present, i.e. that at least one of the three
indicator zones V,
VI, VII must likewise be stained. If this is not the case the test is invalid.
(b) Test results: Depending on the presence or absence of the respective blood
group
antigens red dots (positive) or the almost white background colouration of the
membrane
(negative) are displayed in the respective positions in the indicator zone
region. The
corresponding isoagglutinines are detectable in the indicator zone region
"serum reverse
grouping" by the characteristic purple of the gold particles in the form of
purple stained
dots or in the colour of the polystyrene particles used. In the absence of an
isoagglutinine
no signals differing from background are detectable in those positions. In the
presence of
an irregular antibody one, two or all three of the indicator zones including
the erythrocyte
ghosts of the detection cells 1, 2 or 3 are stained by the characteristic
purple of the gold
particles or in the colour of the polystyrene particles used.
Example 5: Simultaneous blood group determination and detection of infection
markers
Production of the tests strips:
The test strips consist of an application zone, an indicator zone region and a
absorption
region. Membranes of the type Millipore HiFlow Plus 065 are cut to size to 15
mm x 35
mm dimensions (width/length; x/y) and are adhesively bonded onto a support
layer
(backing sheet e.g. of G&L). In the indicator region sub-divided into the
indicator zone
region "detection of infection markers" (proximal to the application zone) and
"blood
group determination" (distal to the application zone) dots, each 0,2 l, of
the various
bonding elements are applied diagonally staggered using a dispenser, e.g.
AD3200
(Biodot):
CA 02531459 2006-01-05
WO 2005/005986 51 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\ 12\2005
Indicator zone region "detection of infection markers" - solutions of the
recombinant
antigens (Syphilis; TpN 15, TpN 17, TpN 47), synthetic peptides of sequences
of the
glycoproteins gp-14, gp-41 (HIV-1; HIV-0) and gp-36 (HIV-2) recombinant HCV
antigens (C-100, C-200, C33c, C22), monoclonal antibodies (HBsAg) as well as
anti-
human IgG/IgM (goat anti-human IgG, goat anti-human IgM, Sigma, 1-3382, 1-
0759) as
controls; indicator zone region "blood group determination" - anti-A
antibodies - clone
Birma-1 (serologicals, TLJOI05); anti-B antibodies - clone ES-4 (serologicals,
NCA0201); anti-AB antibodies - clones AB6, AB26, AB92 (Medion Diagnostics,
010062); anti-D antibodies - clones LDM3/ESD1 (SNBTS), anti-CDB antibodies -
clones MS-24/MS-201/MS 80/MS-258 (serologicals), anti-erythrocyte antibodies
(rabbit
IgG fraction of anti-human RBC, Rockland, 209-4139).
The positioning of the bonding elements of the indicator zone region
"detection of
infection markers" commences with synthetic peptides of the specificity HIV-1
(gp-14,
gp-41) in position x=2,5mm/y=10 mm. All other bonding elements are dispensed,
iterating at distances of x=2 mm/y=1,5 mm to the position of the indicator
zone I. The
bonding elements of the indicator zones IN are dispensed in suitable
concentrations in 15
mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol, the anti-human
IgG/IgM
antibodies in a concentration of 50 pg/ml in 15 mM potassium phosphate buffer
pH 7,5,
10 % (v/v) methanol.
The positioning of the bonding elements of the indicator zone region "blood
group
determination" commence with the anti-A antibody in position x=2,5 mm/y=20 mm.
All
other bonding elements of the indicator zone region are iterating at distances
of x=2
mm/y=1,5 mm to the position of the anti-A antibody. The dilutions of the
antibodies
proceed in 15 mM potassium phosphate buffer pH 7,5, 10 % (v/v) methanol as
follows:
anti-A antibody 1:3; anti-B antibody 1:2; anti-AB antibody 1:4; anti-RBC
antibody 1:3.
The membranes, after the dispensing of the bonding elements, are dried for 20
minutes at
40 C and subsequently stored at constant air humidity until testing is
performed. At the
end distal to the application zone an absorption pad (Schleicher & Schi ll,
300), sized
CA 02531459 2006-01-05
WO 2005/005986 52 PCT/EP2004/007525
Ln\hhh\bard eh 1e\30\ 12\2005
15x15 mm, is adhesively applied to the membrane with a 3 mm overlap. The
application
zone is separated from the remaining membrane over the entire membrane width
by the
adhesive application of a 1-2 mm wide adhesive strip (Tesa 4124) in position
y=6 mm.
Test batch:
As blood samples anti-coagulative complete blood lots are used. For the test
proper
blood is applied into the application zone either undiluted or diluted 1:3 or
1:6 in dilution
buffer (Enlisstll, Medion Diagnostics or diluent 1, DiaMed). Once the blood
has left the
application zone rinsing proceeds twice with 100 l Enlisstll in order to
rinse unbound
erythrocytes from the membrane.
Therafter, 50 l of a mixture of anti-IgG/A/M conjugated gold particles (20 to
40 nm,
Arista Biologicals, CGIGA-0800, CGIGG-0800, CGIGM-0800), diluted 1:10 (v/v) in
TBS, 0,08 % gelatine, 0,5 % albumin as well anti-HbsAg conjugated gold
particles
(Arista Biologicals, ABHBS-0500) are applied in suitable dilution onto the
application
zone. Instead of the gold particles it is also possible to use coloured, 100
to 400 nm
polystyrene particles, e.g. from Merck Eurolab France/Estapor. Once these have
left the
application zone the membranes are once again rinsed once or twice with 100 l
EnlisstII.
Result:
The test is valid if the anti-RBC control (indicator zone XII, indicator zone
region "blood
group determination") displays a clearly positive signal (red dot) and if the
anti-IgG/lgM
control (indicator zone VI, indicator zone region "detection of infection
markers") is
characteristically purple (gold particles) or is stained in the colour of the
polystyrene
particles used. Depending on the presence or absence of the respective blood
group
antigens, red dots (positive) or the nearly white background colouration of
the membrane
(negative) appear in the respective positions. In the presence of antibodies
against HIV-
1, HIV-2, Syphilis or of Hepatitis B surface antigen (HBsAg) the respective
position is
CA 02531459 2006-01-05
WO 2005/005986 53 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\12\2005
detectable as purple coloured dots due to the characteristically purple
staining by the gold
particles. If polystyrene particles have been used as indicator particles the
respective
position is stained in the colour of the polystyrene particles used. In the by
far most
frequent situation, namely a negative reaction for all infection markers, only
the anti-
lgG/IgM control (indicator zone VI, indicator zone region "detection of
infection
markers") is stained.
Example 6: Simultaneous blood group determination and detection of antibodies
against
thrombocytal antigens
Production of the test strip:
In principle the structure and the format of the test strip corresponds to the
test strip
structure in Example 4. The indicator zone region is sub-divided into the
indicator zone
regions "blood group determination" and "detection of antibodies against
thrombocyte
antigens". 0,2 l dots of the following bonding elements are dispensed using a
dispenser,
e.g. AD3200 (Biodot):
Indicator zone region "detection of antibodies against thromocytal antigens" -
membrane
proteins of thrombocytes of blood group 0 having distinctive HPA antigen
profiles such
as HPA lbb3aa5bb and HPA laa3bb5aa as well as anti-human 1gG/IgM (goat anti-
human IgG, goat anti-human IgM, Sigma, 1-3382, 1-0759) as controls; indicator
zone
region "blood group determination' - anti-A antibodies - clone Birma-1
(serologicals,
TLJ0105); anti-B antibodies - clone ES-4 (serologicals, NCA0201); anti-AB -
clones
AB6, AB26, AB92 (Medion Diagnostics, 010062); anti-D antibodies - clones
LDM3/ESDI (SNBTS), anti-CDE antibodies - clones MS-24/MS-20I/MS 80/MS-258
(serologicals), anti-erythrocyte antibodies (rabbit IgG fraction of anti-human
RBC,
Rockland, 209-4139). As alternatives to the membrane proteins of the
thrombocyte it is
also possible to apply recombinant antigens having the corresponding
characteristic
features (HPA-antigen profile).
CA 02531459 2006-01-05
WO 2005/005986 54 PCT/EP2004/007525
Ln\hhh\bardeh le\3 0\1 2\2005
The positioning of the bonding elements of the indicator zone region
"detection of
antibodies against thrombocytal antigens" commences with membrane proteins
antigens
profile HPA lbb3aa5bb in position x=4 mm/y=10 mm. All other bonding elements
are
dispensed iterating at distances of x=3,5 mm/y=2 mm to the position of the
indicator
zone 1. The bonding elements of the indicator zones 1 and II are dispensed in
suitable
concentrations in 15 mM potassium phosphate buffer pH 7,5, 10 % (v/v)
methanol, and
the anti-human IgG/IgM antibodies in a concentration of 15 g/ml in 15 mM
potassium
phosphate buffer pH 7,5, 10 % (v/v) methanol.
The positioning of the bonding elements of the indicator zone region "blood
group
determination" corresponds to Example 4.
The membranes, after dispensing the bonding elements, are dried for 20 minutes
at 40 C
and subsequently stored at constant air humidity until testing takes place. At
the end
distal to the application zone an absorption pad (Schleicher & Scholl, 300),
sized 15x15
mm is adhesively applied to the membrane with a 3 mm overlap. The application
zone is
separated from the remainder of the membrane over the entire membrane's width
by the
adhesive application of a 1-2 mm wide adhesive strip (Tesa 4124) in position
y=6 mm.
Test batch:
Anti-coagulated complete blood lots are used for the blood samples. For the
test proper
100 pl of blood, undiluted or diluted 1:3 or 1:6 in dilution buffer
(Enlisstll, Medion
Diagnostics or diluent 1, DiaMed) are applied to the application zone. Once
the blood
has left the application zone rinsing is performed twice with 100 p1 EnlisstIl
in order to
rinse unbound erythrocytes from the membrane.
Thereafter 50 l of a mixture of anti-IgG/A/M conjugated gold particles (20 to
40 nm,
Arista Biologicals, CGIGA-0800, CGIGG-0800, CGIGM-0800), diluted 1:10 (v/v) in
TBS, 0,08 % gelatine, 0,5 % albumin are applied to the application zone.
Instead of gold
particles it is also possible to use coloured, 100 to 400 nm polystyrene
particles, e.g.
CA 02531459 2006-01-05
WO 2005/005986 55 PCT/EP2004/007525
Ln\hhh\bardeh 1e\30\ 12\2005
from Merck Eurolab France/Estapor. Once the gold particles have left the
application
zone the membrane is rinsed again once or twice with 100 l Enlisstll.
Result:
The test is valid if the anti-RBC control (indicator zone IX, indicator zone
region "blood
group determination") displays a clear positive signal (red dot) and if the
anti-IgG/IgM
control (indicator zone III, indicator zone region "detection of antibodies
against
thrombocytal antigens") is stained characteristically purple (gold particles)
or in the
colour of the polystyrene particles used. Depending on the presence or absence
of the
respective blood group antigens, red dots (positive) or the almost white
background
colouration of the membrane (negative) are displayed in the respective
positions. In the
presence of antibodies against thrombocytal antigens the respective position
is
recognizable by the purple staining characteristic for gold particles and as a
purple
coloured spot. If polystyrene particles are used as indicator particles the
respective
position is stained in the colour of the polystyrene particles used.