Note: Descriptions are shown in the official language in which they were submitted.
CA 02531615 2005-12-23
HIGH STRENGTH THIN STEEL SHEET HAVING HIGH HYDROGEN
EMBRITTLEMENT RESISTING PROPERTY
BACKGROUND OF THE INVENTION
Field of the Invention
[0001]
The present invention relates to a high strength thin
steel sheet that has high hydrogen embrittlement resisting
property (particularly the hydrogen embrittlement resisting
property after being subjected to forming process) and high
workability, especially to a high strength thin steel sheet
that has high resistance against fractures due to hydrogen
embrittlement such as season crack and delayed fracture that
pose serious problems for steel sheets having tensile
strength of 1180 MPa or higher, and has high workability.
Description of the Related Art
[0002]
There are increasing demands for the steel sheet, that
is pressed or bent into a form of a high-strength component
of automobile or industrial machine, to have both high
strength and high ductility at the same time. In recent
years, there are increasing needs for high strength steel
sheets having strength of 1180 MPa or higher, as the
automobiles are being designed with less weight. A type of
- 1 -
CA 02531615 2005-12-23
steel sheet that is regarded as promising to satisfy these
needs is TRIP (transformation induced plasticity) steel sheet.
[0003]
The TRIP steel sheet includes residual austenite
structure and, when processed to deform, undergoes
considerable elongation due to induced transformation of the
residual austenite (residual y) into martensite by the action
of stress. Known examples of the TRIP steel include TRIP
type composite-structure steel (TPF steel) that consists of
polygonal ferrite as the matrix phase and residual austenite;
TRIP type tempered martensite steel (TAM steel) that consists
of tempered martensite as the matrix phase and residual
austenite; and TRIP type bainitic steel (TBF steel) that
consists of bainitic ferrite as the matrix phase and residual
austenite. Among these, the TBF steel has long been known
(described, for example, in NISSIN STEEL TECHNICAL REPORT, No.
43, Dec. 1980, ppl-10), and has such advantages as the
capability to readily provide high strength due to the hard
bainitic ferrite structure, and the capability to show
outstanding elongation because fine residual austenite grains
can be easily formed in the boundary of lath-shaped bainitic
ferrite in the bainitic ferrite structure. The TBF steel
also has such an advantage related to manufacturing, that it
can be easily manufactured by a single heat treatment process
(continuous annealing process or plating process).
- 2 -
CA 02531615 2005-12-23
[0004]
In the realm of high strength of 1180 MPa upward,
however, the TRIP steel sheet is known to suffer a newly
emerging problem of delayed fracture caused by hydrogen
embrittlement, similarly to the conventional high strength
steel. Delayed fracture refers to the failure of high-
strength steel under stress, that occurs as hydrogen
originating in corrosive environment or the atmosphere
infiltrates and diffuses in microstructural defects such as
dislocation, void and grain boundary, and makes the steel
brittle. This results in decreases in ductility and
toughness of the metallic material.
[0005]
It has been well known that the high strength steel
that is widely used in the manufacture of PC steel wire and
line pipe experiences hydrogen embrittlement (pickling
embrittlement, plating embrittlement, delayed fracture, etc.)
caused by the infiltration of hydrogen into the steel when
tensile strength of the steel becomes 980 MPa or higher.
Accordingly, most of technologies of improving hydrogen
embrittlement resisting property have been developed aiming
at steel members such as bolt. "New Development in
Elucidation of Delayed Fracture" (published by The Iron and
Steel Institute of Japan in January, 1997), for example,
describes that it is effective in improving the resistance
- 3 -
CA 02531615 2005-12-23
against delayed fracture to add element such as Cr, Mo or V
that demonstrates resistance against temper softening to the
metal structure that is based on tempered martensite as the
major phase. This technology is intended to cause the
delayed fracture to take place within grains instead of in
the grain boundaries, thereby to constrain the fracture from
occurring, by precipitating alloy carbide and making use
thereof as the site for trapping hydrogen.
[0006]
Thin steel sheets having strength higher than 780 MPa
have rarely been used for the reason of workability and
weldability. Also hydrogen embrittlement has rarely been
regarded as a problem for thin steel sheets where hydrogen
that has infiltrated therein is immediately released due to
the small thickness. For these reasons, much efforts have
not been dedicated to counter the hydrogen embrittlement. In
recent years, however, higher strength is required of the
reinforcement members such as bumper, impact beam and seat
rail, etc., in order to meet the requirement of weight
reduction of the automobile and to improve the collision
safety. As a result, there have been increasing demands for
high strength steel sheet having strength of 980 MPa or
higher for the manufacture of these parts. This makes it
necessary to improve hydrogen embrittlement resisting
property of the high strength steel sheet.
- 4 -
CA 02531615 2005-12-23
[0007]
Use of the technology addressed to the bolt steel
described above may be considered for improving the hydrogen
embrittlement resisting property of the high strength steel
sheet. However, in the case of ~~New Development in
Elucidation of Delayed Fracture" (published by The Iron and
Steel Institute of Japan in January, 1997), for example, 0.4%
or higher C content and much alloy elements are contained,
and therefore application of this technology to a thin steel
sheet compromises the workability required of the thin steel
sheet. The technology also has a drawback related to the
manufacturing process, since it takes several hours or longer
period of heat treatment to cause the alloy carbide to
precipitate. Therefore, improving the hydrogen embrittlement
resisting property of a thin steel sheet requires it to
develop a novel technology.
[0008]
Japanese Unexamined Patent Publication (Kokai) No. 11-
293383 describes a technology to improve the hydrogen
embrittlement resisting property of steel sheet, where
hydrogen-induced defects can be suppressed by having oxides
that include Ti and Mg exist as the main components in the
structure. However, this technology is intended for thick
steel sheets and, although consideration is given to delayed
fracture after welding with a large input heat, no
- 5 -
CA 02531615 2005-12-23
consideration is given to the environment (for example,
corrosive environment, etc.) in which automobile parts
manufactured by using thin steel sheets are used. Japanese
Unexamined Patent Publication (Kokai) No. 2003-166035
describes that it is made possible to improve the ductility
and delayed fracture resistance after being subjected to
forming process, by controlling the mutual relationships
between 1)the form (standard deviation and mean grain size)
in which oxide, sulfide, composite crystallization product or
composite precipitate of Mg is dispersed, 2)volumetric
proportion of residual austenite and 3)strength of the steel
sheet. However, it is difficult to improve the hydrogen
embrittlement resisting property in such an environment as
hydrogen is generated through corrosion of the steel sheet
simply through the trapping effect achieved by controlling
the form of precipitate.
[0009]
Tomohiko HOJO et. al "Hydrogen Embrittlement of High
Strength Low Alloy TRIP Steel (Part I: Hydrogen Absorbing
Characteristic and Ductility", The Society of Materials
Science, Japan, proceedings of 51St academic lecture meeting,
2002, vol. 8, ppl7-18 and Tomohiko HOJO et. al "Influence of
Austempering Temperature on Hydrogen Embrittlement of High,
for example, describe investigations into the hydrogen
embrittlement resisting property of the TRIP steel. It is
- 6 -
CA 02531615 2005-12-23
pointed out that, among the TRIP steels, TBF steel has
particularly high hydrogen absorbing capacity, and
observation of a fracture surface of the TBF steel shows the
restriction of quasi cleavage fracture due to storage of
hydrogen. However, the TBF steels reported in the documents
described above show delayed fracture characteristic of about
1000 seconds at the most in terms of the time before crack
occurrence measured in cathode charging test, indicating that
these steels are not meant to endure the harsh operating
environment such as that of automobile parts over a long
period of time. Moreover, since the heat treatment
conditions reported in the documents described above involve
heating temperature being set higher, there are such problems
as low efficiency of practical manufacturing process. Thus
it is strongly required to develop a new species of TBF steel
that provides high production efficiency as well.
SUMMARY OF THE INVENTION
[0010]
The present invention has been made with the background
described above, and the object of the present invention is
to make available a high strength thin steel sheet that shows
high hydrogen embrittlement resisting property with
workability improved under the tensile strength of 1180 MPa
or higher.
[0011]
CA 02531615 2005-12-23
Hydrogen-induced delayed fracture is believed to occur
in such a steel that contains tempered martensite or
martensite +ferrite, that has been commonly used as a high-
strength steel ever before, because hydrogen is concentrated
in grain boundaries of prior austenite thereby to form voids
or other defects that become the starting points of the
fracture. Common practice that has been employed to decrease
the sensitivity to delayed fracture is to diffuse fine grains
of carbide or the like uniformly as the site for trapping
hydrogen, thereby to decrease the concentration of diffusive
hydrogen. However, even when a large number of carbide
grains or the like are diffused as the trap site for hydrogen,
there is a limitation to the hydrogen trapping capability and
delayed fracture attributable to hydrogen cannot be fully
suppressed.
[0012]
Thus, as a result of a hard study of the present
inventors, they found that (a) decreasing the points of
destroying grains and (b) neutralizing hydrogen by improving
the ability of trapping hydrogen are satisfied in order to
make it possible to show a satisfactory hydrogen
embrittlement resisting property (delayed fracture resisting
property) with its use environment sufficiently considered .
[0013]
In order to achieve condition (a), it is desirable to
_ g _
CA 02531615 2005-12-23
form the matrix phase of the steel structure after processing
from a binary phase structure of bainitic ferrite and
martensite with the bainitic ferrite acting as the main phase,
instead of the single phase structure of martensite that is
generally used for high strength steels. Because in the case
of the single phase structure of martensite, a carbide (for
example, film-like cementite) is likely to precipitate in the
grain boundaries, thus making intergranular fracture likely
to occur. On the other hand, in the case of the binary
structure of bainitic ferrite and martensite acting as the
main phase, the bainitic ferrite is easy to increase the
strength of the entire structure as in the case of the single
phase of martensite, because a dislocation density of the
bainitic ferrite is high as it is in the form of plates,
differing from polygonal ferrite used commonly. The hydrogen
embrittlement resisting property can also be improved as much
hydrogen is trapped in the dislocations. It also has such an
advantage that coexistence of the bainitic ferrite and the
residual austenite which will be described later prevents the
generation of carbide that acts as the intergranular fracture
initiating points.
[0014]
The bainitic ferrite is a hard phase and therefore it
is easy to increase the strength of the entire structure. It
can also absorb much hydrogen compared to the other TRIP
_ g _
CA 02531615 2005-12-23
steel as much hydrogen is trapped in the dislocations. It
also has such an advantage that in the boundaries of the
lath-shaped bainitic ferrite, it becomes easier to produce
the lath-shaped residual austenite, thus providing an
excellent elongation with it. Accordingly, it is found that
it is required in the present invention that the binary
structure of bainitic ferrite and martensite occupy 800 or
more in order to effectively achieve the above action.
[0015]
In order to achieve condition (b), it is desirable to
form the lath-shaped residual austenite. In the past it has
been thought that the residual austenite exerts a negative
impact on its hydrogen embrittlement resisting property and
fatigue. The present inventor has studied that although the
residual austenite which is in the form of cluster in the
submicron order exerts a negative impact on its hydrogen
embrittlement resisting property and fatigue, if the residual
austenite above described is controlled to be produced the
lath-shaped residual austenite in the order of submicron, it
can absorb and trap much hydrogen, thus improving the
hydrogen embrittlement resisting property to a large degree
due to the ability of absorbing hydrogen which the residual
austenite naturally has. In particular, hydrogen
embrittlement risk index sharply decreases when the mean axis
ratio (major axis/minor axis) of the residual austenite
- 10 -
CA 02531615 2005-12-23
grains increases beyond 5. This is supposedly because, when
the mean axis ratio of the residual austenite grains becomes
or higher, intrinsic capability of the residual austenite
to absorb hydrogen is put into full play, so that the
5 residual austenite attains far higher capacity of trapping
hydrogen than carbide and substantially neutralizes the
hydrogen that infiltrates from the outside through
atmospheric corrosion thereby to achieve remarkable
achievement in hydrogen embrittlement resisting property.
[0016]
The metal structure may include other structure such as
ferrite (the term ferrite used herein refers to polygonal
ferrite, that is a ferrite structure that includes no or very
few dislocations) or pearlite to such an extent that the
effect of the present invention is not compromised. The less
the concentration of additional components is, more
preferable it is. It is found that in particular, its
concentration is preferably within 90.
[0017]
The present inventors conducted a research on a steel
sheet to find that if it is controlled so that the below
conditions are satisfied at a time, high hydrogen
embrittlement resisting property is achieved even if too much
alloys are not added and they brought to a completion of the
present invention.
- 11 -
CA 02531615 2005-12-23
Obainitic ferrite and martensite: 80% or more in
total;
the mean axis ratio (major axis/minor axis) of said
residual austenite grains is 5 or higher; and
Oferrite and pearlite: 9% or less (may be 0%) in total.
[0018]
Thus, a first high strength thin steel sheet having
high hydrogen embrittlement resisting property according to
the present invention is constituted from 0.10 to 0.25% of C
(contents of components given in terms of percentage in this
patent application all refer to percentage by weight), 1.0 to
3.0% of Si, 1.0 to 3.5% of Mn, 0.15% or less P, 0.02% or less
S and 1.5% or less (higher than 0%) of A1, with balance of
iron and inevitable impurities, wherein the metallurgical
structure comprises:
1% or more residual austenite;
80% or more in total of bainitic ferrite and martensite; and
9% or less (may be 0%) in total of ferrite and pearlite in
the proportion of area to the entire structure, and wherein
the mean axis ratio (major axis/minor axis) of the residual
austenite grains is 5 or higher, and the steel has tensile
strength of 1180 MPa or higher.
[0019]
The present inventors also found that if its average
length of minor axis of the lath-shaped grains in the
- 12 -
CA 02531615 2005-12-23
residual austenite is 1 um or less (submicrometer order), the
surface area (interface) of the grains in the residual
austenite becomes larger, thus improving the ability of
trapping hydrogen and effectively improving high hydrogen
embrittlement resisting property. And it has been found that
hydrogen embrittlement resisting property can be improved
further by controlling the minimum distance between adjacent
residual austenite grains so that it is 1 um or less. This
is supposedly because propagation of cracks is suppressed so
that the structure demonstrates higher resistance against
fracture, when a large number of fine lath-shape grains of
residual austenite are dispersed in proximity to each other.
[0020]
Thus, a second high strength thin steel sheet having
high hydrogen embrittlement resisting property according to
the present invention is constituted from 0.10 to 0.25% of C,
1.0 to 3.0% of Si, 1.0 to 3.5% of Mn, 0.15% or less P, 0.02%
or less S, 0.5% or less (higher than 0%) Al, with balance of
iron and inevitable impurities, and wherein the metal
structure comprises:
1% or more residual austenite;
the mean axis ratio (major axis/minor axis) of the residual
austenite grains is 5 or higher;
mean length of minor axes of the residual austenite grains is
1 um or less;
- 13 -
CA 02531615 2005-12-23
minimum distance between the residual austenite grains is 1
~m or less; and
tensile strength is 1180 MPa or higher.
[0021]
Preferably, the high strength thin steel sheet
according to the present invention may contain 0.5% or less
(higher than 0%) by weight of A1. If the A1 content
increases over 0.5%, inclusions such as alumina increase and
thus workability becomes poorer. With the content of Al to
be restricted within 0.50 or less, it can prevent a steel
sheet from having a poorer workability.
[0022]
Preferably, the high strength thin steel sheets
according to the present invention may further contain 0.003
to 0.5% of Cu and/or 0.003 to 1.0% of Ni in terms of
percentage by weight. The effect of improving hydrogen
embrittlement resisting property through control of the
structure can be achieved further by containing 0.003 to 0.50
of Cu and/or 0.003 to l.Oo of Ni.
Further, it is preferred that the high strength thin
steels sheet according to the present invention may further
contain 0.003 to 1.0% of Ti and/or V. Ti and/or V has/have
the effect of assisting in the generation of protective rust,
the effect of rendering steel high corrosion resistance, and
the effect of cleaning the steel. And V has the effect of
- 14 -
CA 02531615 2005-12-23
increasing the strength of the steel sheet and decreasing the
size of crystal grains, in addition to having the effect of
improving hydrogen embrittlement resistance.
Preferably, the high strength thin steels sheet
according to the present invention may further contain;
l.Oo or less (higher than 0%) of Mo and O.lo or less
(higher than Oo) of Nb,
0.0002 to O.Ols of B, or
at least one kind selected from the group consisting
of:
0.0005 to 0.0050 of Ca;
0.0005 to O.Olo of Mgt and
0.0005 to 0.01s of REM
in terms of percentage by weight.
[0023]
According to the present invention, it is made possible
to manufacture, with a high level of productivity, a high
strength thin steel sheet having tensile strength of 1180 MPa
or higher that neutralizes hydrogen that infiltrates from the
outside after the steel sheet has been formed into a part
thereby to maintain satisfactory hydrogen embrittlement
resisting property, and demonstrates high workability during
the forming process. Use of the high strength thin steel
sheet makes it possible to manufacture high strength parts
that hardly experience delayed fracture, such as bumper,
- 15 -
CA 02531615 2005-12-23
impact beam and other reinforcement members and other
automobile parts such as seat rail, pillar, etc.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024]
Fig. 1 is a graph showing the relationship between the
mean axis ratio of the residual austenite grains and hydrogen
embrittlement risk index.
Fig. 2 is a diagram schematically showing the minimum
distance between residual austenite grains.
Fig. 3 is a schematic perspective view of a part used
in pressure collapse test in Example 1.
Fig. 4 is a side view schematically showing the setup
of pressure collapse test in Example 1.
Fig. 5 is a schematic perspective view of a part used
in impact resistance test in Example 1.
Fig. 6 is a sectional view along A-A in Fig. 5.
Fig. 7 is a side view schematically showing the setup
of impact resistance test in Example 1.
Fig. 8 is a photograph of TEM observation
(magnification factor 15000) of No.101 (inventive steel) of
Example 1.
Fig. 9 is a photograph of TEM observation
(magnification factor 15000) of No.120 (comparative steel) of
Example 1.
- 16 -
CA 02531615 2005-12-23
Fig. 10 is a photograph of TEM observation
(magnification factor 15000) of No.201 (inventive steel) of
Example 2.
Fig. 11 is a photograph of TEM observation
(magnification factor 15000) of No.220 (comparative steel) of
Example 2.
Fig. 12 is a photograph of TEM observation
(magnification factor 15000) of No.301 (inventive steel) of
Example 3.
Fig. 13 is a photograph of TEM observation
(magnification factor 60000) of No.301 (inventive steel) of
Example 3.
Fig. 14 is a photograph of TEM observation
(magnification factor 15000) of No.313 (comparative steel) of
Example 3.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0025]
The high strength thin steel sheet of non-limiting
embodiment according to the present invention is explained in
more detail below with reference to the drawing. The
inventions can be made variable in the scope of the present
invention.
[0026]
(First Embodiment)
- 17 -
CA 02531615 2005-12-23
The first high strength thin steel sheet according to
the present invention comprises higher than 0.10 and up to
0.250 of C (contents of components given in terms of
percentage in this patent application all refer to percentage
by weight), 1.0 to 3.Oo of Si, 1.0 to 3.50 of Mn, 0.150 or
less P, 0.020 or less S, 1.50 or less (higher than Oo) of Al,
l.Oo or less (higher than Oo) of Mo and 0.1% or less (higher
than Oo) of Nb, with balance of iron and inevitable
impurities, and
the metal structure comprises:
to or more residual austenite;
800 or more in total of bainitic ferrite and
martensite; and
90 or less (may be Oo) in total of ferrite and pearlite
in terms of the proportion of area to the entire structure,
and
the mean axis ratio (major axis/minor axis) of the
residual austenite grains is 5 or higher.
[0027]
(lo or more residual austenite in the area proportion to the
entire structure)
It is necessary that the metal contains to or more
residual austenite in the area proportion to the entire
structure, because residual austenite contributes not only to
the improvement of hydrogen embrittlement resisting property,
- 18 -
CA 02531615 2005-12-23
but also to the improvement of total elongation. Content of
the residual austenite is preferably 3% or higher, and more
preferably 5% or higher.
[0028]
Since the desired level of high strength cannot be
obtained when an excessive amount of residual austenite is
contained, it is recommended to set an upper limit of 15%
(more preferably l00) to the residual austenite content.
[0029]
It is recommended to increase the C content in the
steel sheet as described above, thereby to maintain the
concentration of C in the residual austenite (CyR) of 0.8% or
higher. Controlling the value of CyR to 0.8% or higher
enables it to effectively improve the elongation property,
which is preferably l.Oo or higher and more preferably 1.20
or higher. While it is preferable that CyR is as high as
possible, it is considered that in practice there is an upper
limit of around 1.6%.
[0030]
The residual austenite refers to a region that is
observed as FCC (face centered cubic lattice) by the FE-
SEM/EBSP method which will be described later. Measurement
by the EBSP may be done, for example, by measuring a
measurement area (about 50 by 50 ~Zm) at an arbitrarily chosen
position in a surface parallel to the rolled surface at a
- 19 -
CA 02531615 2005-12-23
position of one quarter of the thickness at measuring
intervals of 0.1 um. The measuring surface is prepared by
electrolytic polishing in order to prevent the residual
austenite from transforming. Then the test piece is set in
the lens barrel of an FE-SEM equipped with the EBSP detector
(of which details will be described later) and is irradiated
with electron beam. An EBSP image projected onto a screen is
captured by a high sensitivity camera (VE-1000-SIT
manufactured by Dage-MTI Inc.) and is sent to a computer.
The computer carries out image analysis and generates color
mapping of the FCC phase through comparison with a structural
pattern simulated with a known crystal system (FCC (face
centered cubic lattice) phase in the case of residual
austenite). Area proportion of the region that is mapped as
described above is taken as the area proportion of the
residual austenite. This analysis was carried out by means
of hardware and software of OIM (Orientation Imaging
MicroscopyTM) system of TexSEM Laboratories Inc.
[0031]
(Mean axis ratio (major axis/minor axis) of the residual
austenite grains: 5 or higher)
In the past it has been thought that the residual
austenite exerts a negative impact on its hydrogen
embrittlement resisting property and fatigue. The present
inventor's studies show that although the residual austenite
- 20 -
CA 02531615 2005-12-23
which is in the form of cluster in the order of submicron
exerts a negative impact on its hydrogen embrittlement
resisting property and fatigue, Lath-shaped grains of
residual austenite have far higher capacity of trapping
hydrogen than carbide, if the residual austenite above
described is controlled to be produced the lath-shaped
residual austenite.
[0032]
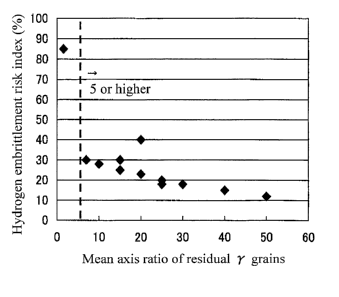
Fig. 1 is a graph showing the relationship between the
mean axis ratio of the residual austenite grains measured by
a method to be described later and hydrogen embrittlement
risk index (measured by a method to be described later in an
example, lower value of this index means better hydrogen
embrittlement resisting property). From Fig. 1, it can be
seen that hydrogen embrittlement risk index sharply decreases
when the mean axis ratio (major axis/minor axis) of the
residual austenite grains increases beyond 5. This is
supposedly because, when the mean axis ratio of the residual
austenite grains becomes 5 or higher, intrinsic capability of
the residual austenite to absorb hydrogen is put into full
play, so that the residual austenite attains far higher
capacity of trapping hydrogen than carbide and substantially
neutralizes the hydrogen that infiltrates from the outside
through atmospheric corrosion thereby to achieve remarkable
achievement in hydrogen embrittlement resisting property.
- 21 -
CA 02531615 2005-12-23
The mean axis ratio of the residual austenite grains is
preferably 10 or higher, and more preferably 15 or higher.
[0033]
While no upper limit of the mean axis ratio is
specified for the consideration of improvement in hydrogen
embrittlement resisting property, the residual austenite
grains are required to have certain level of thickness in
order to achieve the TRIP effect during processing. Thus it
is preferable to set an upper limit to 30, more preferably to
20 or less.
The mean axis ratio was determined by observing with
TEM (magnification factor of 15000) in each of three
arbitrarily chosen fields of view and averaging the distances
measured in the three fields of view.
[0034]
(800 or more in total of bainitic ferrite and martensite)
In the case of the binary structure of bainitic ferrite
and martensite (with the bainitic ferrite acting as the main
phase in the binary structure), the bainitic ferrite is a
hard phase and therefore it is easy to increase the strength
of the entire structure. The hydrogen embrittlement
resisting property can also be improved as much hydrogen is
trapped in the dislocations. It also has such an advantage
that coexistence of the bainitic ferrite and the residual
austenite which will be described later prevents the
- 22 -
CA 02531615 2005-12-23
generation of carbide that acts as the intergranular fracture
initiating points, and it becomes easier to create the lath-
shaped residual austenite in the boundaries of lath-shaped
bainitic ferrite.
Accordingly, it is required in the present invention
that the binary structure of bainitic ferrite and martensite
occupy 800 or more, preferably 85% or more and more
preferably 900 or more. Upper limit of the proportion may be
determined by the balance with other structure (residual
austenite), and is set to 99o when the other structures
(ferrite, etc.) than the residual austenite is not contained.
[0035]
The bainitic ferrite referred to in the present
invention is plate-shaped ferrite having a lower structure of
high density of dislocations. It is clearly distinguished
from polygonal ferrite that has lower structure including no
or very low density of dislocations, by SEM observation as
follows.
[0036]
Area proportion of bainitic ferrite structure is
determined as follows. A test piece is etched with Nital
etchant. A measurement area (about 50 by 50 um) at an
arbitrarily chosen position in a surface parallel to the
rolled surface at a position of one quarter of the thickness
is observed with SEM (scanning electron microscope)
- 23 -
CA 02531615 2005-12-23
(magnification factor of 1500) thereby to determine the area
proportion.
[0037]
Bainitic ferrite is shown with dark gray color in SEM
photograph (bainitic ferrite, residual austenite and
martensite may not be distinguishable in the case of SEM
observation), while polygonal ferrite is shown black in SEM
photograph and has polygonal shape that does not include
residual austenite and martensite inside thereof.
[0038]
The SEM used in the present invention is a high-
resolution FE-SEM (Field Emission type Scanning Electron
Microscope XL30S-FEG manufactured by Philips Inc.) equipped
with an EBSP (Electron Back Scattering Pattern) detector,
that has a merit of being capable of analyzing the area
observed by the SEM at the same time by means of the EBSP
detector. EBSP detection is carried out as follows. When the
sample surface is irradiated with electron beam, the EBSP
detector analyzes the Kikuchi pattern obtained from the
reflected electrons, thereby to determine the crystal
orientation at the point where the electron beam has hit upon.
Distribution of orientations over the sample surface can be
measured by scanning the electron beam two-dimensionally over
the sample surface while measuring the crystal orientation at
predetermined intervals. The EBSP detection method has such
- 24 -
CA 02531615 2005-12-23
an advantage that different structures that are regarded as
the same structure in the ordinary microscopic observation
but have different crystal orientations can be distinguished
by the difference in color tone.
[0039]
(90 or less (may be Oo) in total of ferrite and pearlite)
The steel sheet after the processing may be constituted
either from only the structures described above (namely, a
mixed structure of bainitic ferrite + martensite and residual
austenite), or may include other structure such as ferrite
(the term ferrite used herein refers to polygonal ferrite,
that is a ferrite structure that includes no or very few
dislocations) or pearlite to such an extent that the effect
of the present invention is not compromised. Such additional
components are structures that can inevitably remain in the
manufacturing process of the present invention, of which
concentration is preferably as low as possible, within 90,
preferably less than 5o and more preferably less than 30
according to the present invention.
[0040]
As described above, the present high strength steel is
characterized in that the amount and form of residual
austenite are controlled and the easy control of the amount
and form of residual austenite can be achieved to provide the
desired high strength steel according to the following
- 25 -
CA 02531615 2005-12-23
composition.
[0041]
<C: 0.10 to 0.25%>
C is an element required to achieve a high strength of
1180 MPa or higher. And C is an important element that can
remain the desired austenite at room temperature by providing
a sufficient amount of C into the phase of austenite. In the
present invention, O.lOo or higher of C is contained, and
0.12% or more, preferably 0.15% or more C is contained.
However, in order to ensure corrosion resistance and
weldability, concentration of C is limited within 0.25%,
preferably 0.230 or lower in the present invention.
[0042]
<Si: 1.0 to 3.Oo>
Si is an important element that effectively suppresses
the residual austenite from decomposing and carbide from
being generated, and is also effective in enhancing
substitution solid solution for hardening the material. In
order to make full use of these effects, it is necessary to
include Si in a concentration of 1.0% or higher, preferably
1.2% or higher and more preferably 1.50 or higher. However,
excessively high content of Si leads to conspicuous formation
of scales due to hot rolling and makes it necessary to remove
flaws, thus adding up to the manufacturing cost and resulting
in economical disadvantage. Therefore Si content is
- 26 -
CA 02531615 2005-12-23
controlled within 3.0%, preferably within 2.5o and more
preferably within 2.Oo.
[0043]
<Mn: 1.0 to 3.50>
Mn is an element required to stabilize austenite and
obtain desired residual austenite. In order to make full use
of this effect, it is necessary to add Mn in concentration of
l.Oo or higher, preferably 1.20 or higher, and more
preferably 1.50 or higher. However, adding an excessive
amount Mn leads to conspicuous segregation and poor
workability. Therefore upper limit to the concentration of
Mn is set to 3.5o and more preferably to 3.Oo or less.
[0044]
<P: 0.15°s or lower (higher than 0%)>
P intensifies intergranular fracture due to
intergranular segregation, and the content thereof is
therefore preferably as low as possible. Upper limit to the
concentration of P is set to 0.150, preferably 0.1°s or less
and more preferably to 0.05% or less.
[0045]
<S: 0.020 or lower (higher than Oo)>
S intensifies the absorption of hydrogen into the steel
sheet in corrosive environment, and the content thereof is
therefore preferably as low as possible. Upper limit to the
concentration of S is set to 0.020.
- 27 -
CA 02531615 2005-12-23
[0046]
<Al: 1.50 or less (higher than Oo)> (In the case of inventive
steel 1)
<A1: 0.50 or less (higher than Oo)> (In the case of inventive
steel 2)
O.Olo or higher content of A1 may be included for the
purpose of deoxidation. In addition to deoxidation, Al also
has the effects of improving the corrosion resistance and
improving hydrogen embrittlement resisting property.
[0047]
The mechanism of improving the corrosion resistance is
supposedly based on the improvement of corrosion resistance
of the matrix phase per se and the effect of formation rust
generated by atmospheric corrosion, while the effect of the
formation rust presumably has greater contribution. This is
supposedly because the formation rust is denser and better in
protective capability than ordinary iron rust, and therefore
checks the progress of atmospheric corrosion so as to
decrease the amount of hydrogen generated by the atmospheric
corrosion, thereby to effectively suppress the occurrence of
hydrogen embrittlement, and hence the delayed fracture.
[0048]
While details of the mechanism of improvement of the
hydrogen embrittlement resistance by Al is not known, it is
supposed that condensing of A1 on the surface of the steel
- 28 -
CA 02531615 2005-12-23
makes it difficult for hydrogen to infiltrate into the steel,
and the decreasing diffusion rate of hydrogen in the steel
makes it difficult for hydrogen to migrate so that hydrogen
embrittlement becomes less likely to occur. In addition,
stability of lath-shaped residual austenite improved by the
addition of Al is believed to contribute to the improvement
of hydrogen embrittlement resisting property.
[0049]
In order to effectively achieve the effects of Al in
improving the corrosion resistance and improving the hydrogen
embrittlement resisting property, Al content is controlled to
0.2% or higher, preferably 0.50 or higher.
[0050]
However, A1 content must be controlled within 1.5o in
order to keep inclusions such as alumina from increasing in
number and size so as to ensure satisfactory workability,
ensure the generation of fine residual austenite grains,
suppress corrosion from proceeding from the inclusion
containing Al as the starting point, and prevent the
manufacturing cost from increasing. In view of the
manufacturing process, it is preferable to control so that A3
point is not higher than 1000°C.
[0051]
As the Al content increases, inclusions such as alumina
increase and workability becomes poorer. In order to
- 29 -
CA 02531615 2005-12-23
suppress the generation of the inclusions such as alumina and
make a steel sheet having higher workability, A1 content is
restricted within 0.50, preferably within 0.3% and more
preferably within 0.1%.
[0052]
While constituent elements (C, Si, Mn, P, S, A1, Mo,
Nb) of the steel of this embodiment is as described above
with the rest substantially being Fe, it may include
inevitable impurities introduced into the steel depending on
the stock material, production material, manufacturing
facility and other circumstances, containing O.OOlo or less N
(nitrogen). In addition, other elements as described below
may be intentionally added to such an extent that does not
adversely affect the effects of the present invention.
[0053]
The case in which 1.5% or less (higher than 0%) of A1
is contained is referred to as an inventive steel 1. And the
case in which 0.50 or less (higher than Oo) of Al is
contained is referred to as an inventive steel 2
[0054]
(Cu: 0.003 to 0.5% and/or Ni: 0.003 to l.Oo)
It was found that by including 0.003 to 0.5o of Cu
and/or 0.003 to l.Oo of Ni, the generation of hydrogen
leading to the hydrogen embrittlement and the infiltration of
hydrogen that has been generated can be sufficiently
- 30 -
CA 02531615 2005-12-23
suppressed.
[0055]
Specifically, presence of Cu and Ni improves the
corrosion resistance of the steel, and effectively suppresses
the generation of hydrogen due to corrosion of the steel
sheet. These elements also have the effect of promoting the
generation of iron oxide, a-Fe00H, that is believed to be
particularly stable thermodynamically and have protective
property among various forms of rust generated in the
atmosphere. By assisting the generation of this rust, it is
made possible to suppress hydrogen that has been generated
from infiltrating into the spring steel thereby to
sufficiently improve the hydrogen embrittlement resisting
property to endure in harsh corrosive environment. This
effect can be achieved particularly satisfactorily when Cu
and Ni are contained at the same time.
[0056]
In order to achieve the effects described above,
concentration of Cu, if added, should be 0.0030 or higher,
preferably 0.050 or higher and more preferably O.lo or higher.
Concentration of Ni, if added, should be 0.0030 or higher,
preferably 0.05°s or higher and more preferably O.lo or higher.
[0057]
Since excessively high concentration of either Cu or Ni
is detrimental to workability, it is preferable to limit the
- 31 -
CA 02531615 2005-12-23
Cu content to 0.5% or lower and limit the Ni content to 1.0~
or lower.
[0058]
<Ti and/or V: 0.003 to l.Oo in total>
Ti has the effect of assisting in the generation of
protective rust, similarly to Cu and Ni. The protective rust
has a very valuable effect of suppressing the generation of
(3-Fe00H that appears in chloride environment and has adverse
effect on the corrosion resistance (and hence on the hydrogen
embrittlement resisting property). Formation of such a
protective rust is promoted particularly by adding Ti and V
(or Zr). Ti renders the steel high corrosion resistance, and
also has the effect of cleaning the steel.
V is effective in increasing the strength of the steel
sheet and decreasing the size of crystal grains, in addition
to having the effect of improving hydrogen embrittlement
resistance through cooperation with Ti, as described
previously.
[0059]
In order to fully achieve the effect of Ti and/or V
described above, it is preferable to add Ti and/or V in total
concentration of 0.0030 or higher (more preferably O.Olo or
higher). For the purpose of improving hydrogen embrittlement
resisting property, in particular, it is preferable to add
more than 0.03% of Ti, more preferably 0.050 or more Ti.
- 32 -
CA 02531615 2005-12-23
However, the effects described above reach saturation when an
excessive amount of Ti is added, resulting in economical
disadvantage. Excessive V content also increases the
precipitation of much carbonitride and leads to poor
workability and lower hydrogen embrittlement resisting
property. Therefore, it is preferable to control the total
concentration of Ti and/or V to within l.Oo, more preferably
within 0.50.
[0060]
<Zr: 0.003 to l.Oo>
Zr is effective in increasing the strength of the steel
sheet and decreasing the crystal grain size, and also has the
effect of improving hydrogen embrittlement resisting property
through cooperation with Ti. In order to sufficiently
achieve these effects, it is preferable that 0.0030 or more
Zr is contained. However, excessive Zr content increases the
precipitation of carbonitride and leads to poor workability
and lower hydrogen embrittlement resisting property.
Therefore, it is preferable to control the concentration of
Zr to within l.Oo.
[0061]
<Mo: 1.0% or less (higher than 0%)>
Mo has the effects of stabilizing austenite so as to
retain the residual austenite, and suppress the infiltration
of hydrogen thereby to improve hydrogen embrittlement
- 33 -
CA 02531615 2005-12-23
resisting property. Mo also has the effect of improving the
hardenability of the steel sheet. In addition, Mo
strengthens the grain boundary so as to suppress hydrogen
embrittlement from occurring. It is recommended to add
0.0050 or more Mo in order to achieve these effects. More
preferably 0.1% or more Mo is added. However, since the
effects described above reach saturation when the Mo content
exceeds 1.0%, resulting in economical disadvantage, Mo
content is limited to 0.80 or less and more preferably to
0.50 or less.
[0062]
<Nb: 0.1~ or less (higher than Oo)>
Nb is very effective in increasing the strength of the
steel sheet and decreasing the grain size of the structure.
Nb achieves these effects particularly effectively in
cooperation with Mo. In order to achieve these effects, it
is recommended to include 0.005 or more Nb. More preferably
0.01% or more Nb is added. However, since the effects
described above reach saturation when an excessive Nb content
is included, resulting in economical disadvantage, Nb content
is limited to 0.1% or less and more preferably to 0.08% or
less.
[0063]
<B: 0.0002 to O.Olo>
B is effective in increasing the strength of the steel
- 34 -
CA 02531615 2005-12-23
sheet, and it is preferable that 0.0002% or more (more
preferably 0.0005% or more) B is contained in order to
achieve these effects. However, an excessive content of B
leads to poor hot processing property. Therefore, it is
preferable to control the concentration of B to within 0.01%
(more preferably within 0.005%).
[0064]
<At least one kind selected from among a group consisting of
Ca: 0.0005% to 0.005%, Mg: 0.0005% to 0.01% and REM: 0.0005%
to 0.01%)
Ca, Mg and REM (rare earth element) are effective in
suppressing an increase in hydrogen ion concentration, that
is, a decrease in pH in the atmosphere of the interface due
to corrosion of the steel sheet surface, thereby to improve
the corrosion resistance of the steel sheet. It is also
effective in controlling the form of sulfide in the steel and
improving the workability of the steel. In order to achieve
the effects described above, it is recommended to add each of
Ca, Mg and REM in concentration of 0.0005% or higher.
However, since excessive contents of these elements leads to
poor workability, it is preferable to keep the concentrations
of Ca within 0.005%, Mg and REM each within 0.01%.
[0065]
While the present invention does not specify the
manufacturing conditions, it is recommended to apply heat
- 35 -
CA 02531615 2005-12-23
treatment in the following procedure after hot rolling or
cold rolling conducted thereafter, in order to form the
structure described above that can be easily worked and has
high strength and high hydrogen embrittlement resistance
after the processing, by using the steel material of the
composition described above.
[0066]
The recommended procedure is to keep the steel the
composition described above at a temperature (T1) in a range
from A3 point to (A3 point + 50°C) for a period of 10 to 1800
seconds (tl), cool down the steel at a mean cooling rate of
3°C/s or higher to a temperature (T2) in a range from Ms
point to Bs point and keep the material at this temperature
for a period of 60 to 3600 seconds (t2).
[0067]
It is not desirable that the temperature T1 becomes
higher than (A3 point + 50°C) or the period tl is longer than
1800 seconds, in which case austenite grains grow resulting
in poor workability (elongation flanging property). When the
temperature T1 is lower than A3 point, on the other hand,
desirable bainitic ferrite structure cannot be obtained.
When the period tl is shorter than 10 seconds,
austenitization does not proceed sufficiently and therefore
cementite and other alloy carbides remain. The period t1 is
preferably in a range from 30 to 600 seconds, more preferably
- 36 -
CA 02531615 2005-12-23
from 60 to 400 seconds.
[0068]
Then the steel sheet is cooled down. The steel is
cooled at a mean cooling rate of 3°C/s or higher, for the
purpose of preventing pearlite structure from being generated
while avoiding the pearlite transformation region. The mean
cooling rate should be as high as possible, and is preferably
5°C/s or higher, and more preferably 10°C/s or higher.
[0069]
After quenching to the temperature between Ms point and
Bs point at the rate described above, the steel is subjected
to isothermal transformation so as to transform the matrix
phase into binary phase structure of bainitic ferrite and
martensite. When the heat retaining temperature T2 is higher
than Bs, much pearlite that is not desirable for the present
invention is formed, thus hampering the formation of the
predetermined bainitic ferrite structure. When T2 is below
Ms, on the other hand, the amount of residual austenite
decreases.
[0070]
When the temperature holding period t2 is longer than
1800 seconds, density of dislocations in bainitic ferrite
becomes low, the amount of trapped hydrogen decreases and the
desired residual austenite cannot be obtained. When t2 is
less than 60 second, on the other hand, desired bainitic
- 37 -
CA 02531615 2005-12-23
ferrite structure cannot be obtained. The length of t2 is
preferably from 90 to 1200 seconds, and more preferably from
120 to 600 seconds. There is no restriction on the method of
cooling after maintaining the heating temperature, and air
cooling, quenching or air-assisted water cooling may be
employed.
[0071]
In the practical manufacturing process, the annealing
process described above can be carried out easily by
employing a continuous annealing facility or a batch
annealing facility. In case a cold rolled sheet is plated
with zinc by hot dipping, the heat treatment process may be
replaced by the plating process by setting the plating
conditions so as to satisfy the heat treatment conditions.
The plating may also be alloyed.
[0072]
There is no restriction on the hot rolling process (or
cold rolling process as required) that precedes the
continuous annealing process described above, and commonly
employed process conditions may be used. Specifically, the
hot rolling process may be carried out in such a procedure as,
after hot rolling at a temperature above Ar3 point, the steel
sheet is cooled at a mean cooling rate of about 30°C/s and is
wound up at a temperature from about 500 to 600°C. In case
the hot rolled steel sheet has unsatisfactory appearance,
- 38 -
CA 02531615 2005-12-23
cold rolling may be applied in order to rectify the
appearance. It is recommended to set the cold rolling ratio
in a range from 1 to 70°s. Cold rolling beyond 700 leads to
excessive rolling load that makes it difficult to carry out
the cold rolling.
[0073]
While the present invention is addressed to thin steel
sheet, there is no limitation to the form of product, and may
be applied, in addition to steel sheet made by hot rolling or
steel sheet made by cold rolling, to those subjected to
annealing after hot rolling or cold rolling, followed by
chemical conversion treatment, hot-dip coating,
electroplating, vapor deposition, painting, priming for
painting, organic coating treatment or the like.
[0074]
The plating process may be either galvanizing or
aluminum plating. The method of plating may be either hot-
dip coating or electroplating, and the plating process may
also be followed by alloying heat treatment or multi-layer
plating. A steel sheet, that is plated or not plated, may
also be laminated with a film.
[0075]
When the coating operation described above is carried
out, chemical conversion treatment such as phosphating or
electrodepositing coating may be applied in accordance to the
- 39 -
CA 02531615 2005-12-23
application. The coating material may be a known resin that
can be used in combination with a known hardening agent such
as epoxy resin, fluorocarbon resin, silicone acrylic resin,
polyurethane resin, acrylic resin, polyester resin, phenol
resin, alkyd resin, or melamine resin. Among these, epoxy
resin, fluorocarbon resin or silicone acrylic resin is
preferably used in consideration of corrosion resistance.
Known additives that are added to coating materials such as
coloring agent, coupling agent, leveling agent, sensitization
agent, antioxidant agent, anti-UV protection agent, flame
retarding agent or the like may be used.
[0076]
There is also no restriction on the coating and
solvent-based coating, powder coating, water-based coating,
water-dispersed coating, electrodeposition coating or like
may be employed. Desired coating layer of the coating
material described above can be formed on the steel by a
known technique such as dipping, roll coater, spraying, or
curtain flow coater. The coating layer may have any proper
thickness.
[0077]
The high strength thin steel sheet of the present
invention may be applied to high-strength automotive
components such as bumper, door impact beam, pillar and other
reinforcement members and interior parts such as seat rail,
- 40 -
CA 02531615 2005-12-23
etc. Automobile components that are manufactured by forming
process also have sufficient properties (strength) and high
hydrogen embrittlement resisting property.
[0078]
(Second Embodiment)
The second high strength thin steel sheet according to
the present invention comprises:
C: higher than 0.10 up to 0.250; Si: 1.0 to 3.0%; Mn: 1.0 to
3.5%; P: 0.15% or less; S: 0.020 or less; and A1: 1.5o or
less (higher than Oo) in terms of percentage by weight,
with balance of iron and inevitable impurities, wherein
the metal structure comprises:
residual austenite; 1$ by area or more in proportion to the
entire structure;
while the mean axis ratio (major axis/minor axis) of said
residual austenite grains is 5 or higher;
mean length of minor axes of said residual austenite grains
is 1 um or less; and
a minimum distance between said residual austenite grains is
1 ~m or less; and the steel has tensile strength of 1180 MPa
or higher. The structure may further contain 800 or more
bainitic ferrite and martensite in total and/or 9% or less
(may be 0%) ferrite and pearlite in total, and the structure
may not contain them.
The reason why the above requirements are defined and a
- 41 -
CA 02531615 2005-12-23
measuring method are explained in detail below. Requirements
explained in the first embodiment are omitted below.
[0079]
<Mean length of minor axes of the residual austenite grains
is 1 um or less>
According to the present invention, it has been found
that hydrogen embrittlement resisting property can be
effectively improved by dispersing fine grains of residual
austenite of lath shape. Specifically, hydrogen
embrittlement resisting property can be surely improved by
dispersing the lath-shape grains of residual austenite having
sizes of 1 um or less (submicrometer order). This is
supposedly because surface area of the residual austenite
grains (interface) increases resulting in larger hydrogen
trapping capability, when larger number of fine lath-shape
grains of residual austenite having smaller mean length of
minor axis are dispersed. Mean length of minor axes of the
residual austenite grains is preferably 0.5 um or less, more
preferably 0.25 um or less.
[0080]
According to the present invention, hydrogen trapping
capability of the fine lath-shape grains of residual
austenite can be made far greater than that in the case of
dispersing carbide, and thereby to substantially neutralize
hydrogen that infiltrates from the outside through
- 42 -
CA 02531615 2005-12-23
atmospheric corrosion, even when the same proportion by
volume of residual austenite is contained, by controlling the
mean axis ratio and mean length of minor axes of the residual
austenite grains as described above.
[0081]
<Minimum distance between residual austenite grains is 1 um
or less>
According to the present invention, it has been found
that hydrogen embrittlement resisting property can be
improved further by controlling the minimum distance between
adjacent residual austenite grains, in addition to the above.
Specifically, hydrogen embrittlement resistance can be surely
improved when the minimum distance between residual austenite
grains is 1 um or less. This is supposedly because
propagation of cracks is suppressed so that the structure
demonstrates higher resistance against fracture, when a large
number of fine lath-shape grains of residual austenite are
dispersed in proximity to each other. Minimum distance
between adjacent residual austenite grains is preferably 0.8
um or less, and more preferably 0.5 um or less.
[0082]
The present invention will now be described below by
way of examples, but the present invention is not limited to
the examples. Various modifications may be conceived without
departing from the technical scope of the present invention.
- 43 -
CA 02531615 2005-12-23
[Example 1]
[0083]
Sample steels A-1 through Y-1 having the compositions
described in Table 1 were melt-refined in vacuum to make test
slabs. The slabs were processed in the following procedure
(hot rolling -~ cold rolling -continuous annealing) thereby to
obtain hot-rolled steel plates measuring 3.2 mm in thickness.
The steel plates were pickled to remove scales from the
surface and then cold rolled so as to reduce the thickness to
1.2 mm.
[0084]
<Hot rolling>
Starting temperature (SRT): Held at a temperature between
1150 and 1250°C for 30 minutes.
Finishing temperature (FDT): 850°C
Cooling rate: 40°C/s
Winding-up temperature: 550°C
<Cold rolling>
Rolling ratio: 500
<Continuous annealing>
Each steel specimen was kept at a temperature of A3
point + 30°C for 120 seconds, then cooled in air at a mean
cooling rate of 20°C/s to temperature TO shown in Table 2,
and was kept at TO for 240 seconds, followed by air-assisted
water cooling to the room temperature.
- 44 -
CA 02531615 2005-12-23
[0085]
No. 116 shown in Table 2 was made by heating a cold-
rolled steel sheet to 830°C, keeping at this temperature for
minutes followed by quenching in water and tempering at
5 300°C for 10 minutes, thereby to form a martensite steel as a
comparative example of the high-strength steel of the prior
art. No. 120 was made by heating a cold-rolled steel sheet
to 800°C, keeping at this temperature for 120 seconds,
cooling down at a mean cooling rate of 20°C/s to 350°C and
keeping at this temperature for 240 seconds.
[0086]
The metal structures of steel sheets obtained as
described above were observed, and their tensile strength
(TS) and elongation (total elongation El) and hydrogen
embrittlement resisting property were measured by the
following procedures.
[0087]
Observation of metal structure
Metal structures of the test pieces were observed
before and after the processing as follows. A measurement
area (about 50 by 50 um) at an arbitrarily chosen position in
a surface parallel to the rolled surface at a position of one
quarter of the thickness was photographed at measuring
intervals of 0.1 um, and area proportions of bainitic ferrite
(BF), martensite (M) and residual austenite (residual y) were
- 45 -
CA 02531615 2005-12-23
measured by the method described previously. Then similar
measurements were made in two fields of view that were
arbitrarily selected, and the measured values were averaged.
Area proportions of other structures (ferrite, pearlite,
etc.) were subtracted from the entire structure. Mean axis
ratio of the residual austenite grains of the steel sheet
before and after the processing were measured by the method
described previously. Test pieces having mean axis ratio of
5 or higher were regarded to satisfy the requirements of the
present invention (o), and those having mean axis ratio of
lower than 5 were regarded to fail to satisfy the
requirements of the present invention (x).
[0088]
Measurement of tensile strength (TS) and elongation (El)
Tensile test was conducted on the JIS No. 5 test piece
before processing, so as to measure the tensile strength (TS)
and elongation (El). Stretching speed of the tensile test
was set to I mm/sec. Among the steel sheets having tensile
strength of 1180 MPa as measured by the method described
previously, those which showed elongation of l00 or more were
evaluated as high in elongation property.
[0089]
Evaluation of hydrogen embrittlement resisting property
In order to evaluate hydrogen embrittlement resisting
property, the JIS No. 5 test piece was stretched. Then after
- 46 -
CA 02531615 2005-12-23
bending with a radius of curvature of 15 mm, load of 1000 MPa
was applied and the test piece was immersed in 5o solution of
hydrochloric acid, and the time before crack occurred was
measured.
[0090]
Hydrogen-charged 4-point bending test was also
conducted for some steel species. Specifically, a
rectangular test piece measuring 65 mm by 10 mm made of each
steel sheet elongated by 3o was immersed in a solution of 0.5
mol of HzS09 and 0.01 mol of KSCN and was subjected to
cathode hydrogen charging. Maximum stress endured without
breaking for 3 hours was determined as the critical fracture
stress (DFL).
[0091]
Results of these tests are shown in Table 2.
[0092]
Evaluation of weldability
Test of weldability was conducted on No.101 and No.114
which are representative steel species.
The test on weldability was conducted on the test
pieces made according to the procedures of JTS Z 3136 and JIS
Z 3137. And then spot welding was conducted on these pieces
under the following conditions. Then tensile shear test (in
which ultimate load was measured in the tensile velocity of
20mm/min) and cross tension test (in which ultimate load was
- 47 -
CA 02531615 2005-12-23
measured in the tensile velocity of 20mm/min) was conducted
on these pieces, so as to measure the tensile shear strength
(TSS) and cross tension strength (CTS). And if the
ductibility ratio(CTS/TSS) of cross tension strength (CTS) to
the tensile shear strength (TSS) is 0.2 or higher, it was
evaluated that the test piece has a better weldability. As a
result, it was found that No.101(present invention) is better
than No.114(prior art) in reference to weldability because
the ratio of ductibility in sample No.114 is 0.19 while that
in sample No.101 is 0.22.
[0093]
[Conditions of spot welding]
Olnitial pressurization time:60 cycles/60Hz,
Pressurized force 450 kgf (4.4kN)
Power distribution time: 1 cycle/60Hz
D Power current for welding: 8.5kA.
- 48 -
CA 02531615 2005-12-23
[0094]
Table 1
I~ I~~ N ~O v1 O V1N cnv1~D vDI~00 ~n~ l~ O ~ d;~ I
~
~ ~ M r 01O O~00 O 00O V1Q100 00~O\p ~ V1M 01M M N
,~ O~ 01_ I~O ~Ol~~ l~l~00 ~OI~~ O ~ ~O O ~ G~ 00O~O~O~ 00
_
~ M M ~ M ~ M M M M M M M M M d'M ~ etM M M M M M M
W n t mn~D d:O O t~N M wt~ O~N O ( mnt!10ov0v0O o0
~ ~
oG cVOiM cV ~ ~ ~ O~~ ~D v0d'cV ~ ~ Ov N riN Oi oo O
N l~ ~ ~ M N l~M 00 N M M N M ~f1~DQOr~ ~'~ ~ M ~ ~ M M
~ V1 ~ ~ U1~D '~V1~ ~!1~ V'1V1~ V W ~ ~ ~O~ V1 n!1V1V~V1 V1
O
l~ N O~~D~ O~O~O ~nO M W n l~ 00M .-rN I~00 N .-..
~ N ~
M vp Vi~OcV~C O O ~ W Os01 ~ 00vC O N I~O fV o000v N o
D O 1 0
V [~ ~ [~Op00 W l~ ~ V1M M M V1 N O N 00<t~1 V1
O
d o0 00000000 000000 000000 00000o a,ooa o00,oo a~a,
0
0
0
0
Gci
0 0
0 0
0 0
0
'~ ~ 0 0 0
o
0 0 0 0 0 0 0
0 0 0 0 0
o ~ o o
Q ~7 _ o ~7C1 o r:7C7 ~qU ~ ~ U CI O r_7Cl o f~D O U U ~ ~.
N N N N N N N o0 N N N N N N N N N N ~nN N N N N N
G O O O O O O G O O G O O G O O O O O O O O O O
~ ~!1~OV1~ ~!100~!1M vo~ ~DV~~O V1~!1~O ~ V7 V1~!1v1V1
.DO O O O O O O O O O O O O O O O O N O O O O O O O
z o 0 0 0 0 0 0 o 0 0 o c o o c o o c o o o
v
M N ~ O ~ O ~ M ~ M M N M .~ --~M M M ~ ~ O V1O O
'
M M M M M M M M M M M M M M M ~ M M M V N ~1M ~1
1 ~ ~ "~
O O O O O O O O O O O O O O O O O O O O M ~ Y1l O
d O O O O O O O O O O O O O C O O O O O O O O O O
N N N N N N N N N N N N N ~n inN N N N N N N N N N U
O O O O O O O O O O O O O O O O O O O O O
~ O O O O O ~ O O ~ O O ~ O O O O O
* O O O O O O O ro
v~0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o
ow i'
23 N .-r.-.~ ..,.-...r.-,.-..~N .-..-.et ~ ~ N N N .-nN .~.-rN .-.
O O O O O O O O O O O O O O O o O O O O O O O O O "i
LhO O O O O O O O O O O O O O O O O O O O O O O O O
G G
o ro
'~ G
O d'-~M O O 00 O M 00 O O O O OO_ O O O O ~-~~ ~ O O
"~~ O V1v1V'1~ N v1~ v1V1~ ~!1v1O 01O\O ~ON V) V1V7V1~!1~DYI
V V V V V cVcV O cJ N N cVN N cV"~
O
N c N N M ( c N ( c N
O
O
U O N N O N 00 N 00O~ O O O O ~D N O O O~O O O~ O N
'
O ~1O v1O O O 01 O 01~ ~n~nO ~n~.O O O V1 ~ ~nV~~ ~n~
_ N cVcVcV N N N N cVO cV cVN
C/~
ro
U G
O l~M O M O~O N N O O M O O viO v7 O I~ 00I~t~l~ 00U
N ~ ~ N N ~ N N N N N N N M N N O N N ~ --~~ ~ ~ ~
U o c o 0 0 0 0 0 0 0 0 0 0 0 0 o c o o c o o c o 0
G
ro
ro
J7
o n o y r ; ~;~ o ~ o ~ o
. .
~ ~ o ~ o 3 F
~ m U r~w w o x ~ .~x a ~ z ~, x ~ ~ ~ > x ~
- 49 -
CA 02531615 2005-12-23
[0095]
Table 2
I
u., 0.. o ~no 0 0 0 0
Ca ~ m v ~ o a ~ ~ o w ~ o m c r o ~ ~ ~ ~ ~ o ~ ~ 0 0
~ ~ ~ ~~
_ .
a~
0
a~
a~
+~
tr~ ~ ~ ~ ~ v ~ ~ ~r~ ~ ~ ~ ~ ~ ~ ~ v rr ~ ~t
+~
O
~~
N N N N N N N N N N N N N N N N N N N N
4 i L
~ w v Y~V iwW Y~4rV Y~YH L F. 4~ 4vi..n4~ Y W
~ N N N N N N N N N ~ W N N N N N N ~ ~
y
>, > > > > > > > > > > > > > N > > > > > > >
N
x ~ O O O O O O O O O O O O O 00M O o o O O O O O O
N
~n N N O N O 01 M N N v1
N
~D~ M ~OV7I~-~ O N l~d'~ M 01.~ O l~.--iM V1M O
_ _ _
I~O~O~ h W 00M ~ N 00v1~n O~ehI~~ t~~ O O ..-~O~ O
..M.~ ~ .-~N'.~w~ ~ v~ V'V1N V1 O\M M \O V1v1et ~f1V1N
, a0 Ov
N
4,
N
J'".
_ _
O ~lO O N O O M O O O O O V V O O O ~O O O O O
O M
N I~N M N N ~ ~- M M N M 00p~O1 00t~~pO t~~pet ~ N O
Ca I.J
p1OvO~ O~C~O~Ov O~O\O~O\O~ O\00O~01 O~fTO~N O~O~O~ 0101vD
"i
O
rl
x
ro
ro
o
~
~
-~
b
ro
~
.~
o
ro
~
a
~
0 0 0 0 0 0 0 0 0 0 0 0 0 o x X o 0 o x o 0 0 0
0
ro
N
N _ _
fx 0 00M ~O I~00I~~O 0000l~l~00 I~.N-..V V
?-
N M ~ .~ M ~ \D ~D00O~
O O O O O O O O O O O O O O O O O O O O O O O O O
N O O N V~O O O O N N N N N N l~V1V1v1 O O N N N N
F" ~ M
M M M M M M M M M M M M M M ~ M M M M M M M M M M
N
rl
~
-~i
O
Q r~v A w w ~ x x a ~ z o d a c~v Q H ~ > 3 C
. . l ~
N M ~ C ~ ~ 0 0 O N M ~ V1~O I~00O~O ~ N M ~ ~'1~D
O O O O O O N N N N N N N
- 50 -
CA 02531615 2005-12-23
[0096]
The results shown in Tables 1 and 2 can be interpreted
as follows (numbers in the following description are test Nos.
in Table 2).
[0097]
Test pieces Nos. 101 through 113 (inventive steel
sheets 2) and test pieces Nos. 121 through 125 (inventive
steel sheets 1) that satisfy the requirements of the present
invention have high strength of 1180 MPa or higher, and high
hydrogen embrittlement resisting property in harsh
environment after the forming process. They also have high
elongation property required of the TRIP steel sheet, thus
providing steel sheets best suited for reinforcement parts of
automobiles that are exposed to corrosive atmosphere. Test
pieces Nos. 121 through 125, in particular, show even better
hydrogen embrittlement resisting property.
[0098]
Test pieces Nos. 114 through 120 and 126 that do not
satisfy the requirements of the present invention, in
contrast, have the following drawbacks.
[0099]
No. 114 made of steel species N-1 that includes
excessive amounts of C content does not have good weldability.
[0100]
No. 115 made of steel species 0-1 that includes
- 51 -
CA 02531615 2005-12-23
insufficient Mn content does not retain sufficient residual
austenite and is inferior in hydrogen embrittlement resisting
property after the processing.
[0101]
No. 116, martensite steel that is a conventional high
strength steel made of steel species P-1 that includes
insufficient Si content, hardly contains residual austenite
and is inferior in hydrogen embrittlement resisting property.
It also does not show the elongation property required of a
thin steel sheet.
[0102]
No. 117 made of steel species Q-1 that includes
excessive C content has precipitation of carbide and is
inferior in both forming workability and hydrogen
embrittlement resisting property after processing.
[0103]
No. 118 made of steel species R-1 that includes
excessive Mo content and No. 119 made of steel species S-1
that includes excessive Nb content are inferior in forming
workability. Nos. 118 and 119 could not undergo the
processing, making it impossible to investigate the property
after the processing.
[0104]
No. 120, that was made of a steel that has the
composition specified in the present invention but was not
- 52 -
CA 02531615 2005-12-23
manufactured under the recommended conditions, resulted in
the conventional TRIP steel. As a result, the residual
austenite does not have the mean axis ratio specified in the
present invention, while the matrix phase is not formed in
binary phase structure of bainitic ferrite and martensite,
and therefore sufficient level of hydrogen embrittlement
resisting property is not achieved.
[0105]
No. 126 includes A1 content higher than that specified
for the inventive steel sheet 1. As a result, although the
predetermined amount of residual austenite is retained, the
residual austenite does not have the mean axis ratio
specified in the present invention, the desired matrix phase
is not obtained and inclusions such as AIN are generated thus
resulting in poor hydrogen embrittlement resisting property.
[0106]
Then parts were made by using steel species A-1, J-1
shown in Table 1 and comparative steel sheet (590 MPa class
high strength steel sheet of the prior art). Performance
(pressure collapse resistance and impact resistance) of the
formed test piece were studied by conducting pressure
collapse test and impact resistance test as follows.
[0107]
Pressure collapse test
The part 1 (hat channel as test piece) shown in Fig. 1
- 53 -
CA 02531615 2005-12-23
was made by using steel species A-1, J-1 shown in Table 1 and
the comparative steel sheet, and was subjected to pressure
collapse test. The part was spot welded at the positions 2
of the part shown in Fig. 1 at 35 mm intervals as shown in
Fig. 1 by supplying electric current of a magnitude less than
the expulsion generating current by 0.5 kA from an electrode
measuring 6 mm in diameter at the distal end. Then a die 3
was pressed against the part 1 from above the mid portion
thereof in the longitudinal direction as shown in Fig. 2, and
the maximum tolerable load was determined. Absorbed energy
was determined from the area under the load-deformation curve.
The results are shown in Table 3.
[0108]
Table 3
Steel sheet used Evaluation
of test
piece
Residual Maximum Energy
Steel species TS EL load absorbed
mb 1
...............................................................................
...............................................................................
...............................................................................
...............................................................................
Sy o
(Mpa) (%) (Area %) (kN) (kJ)
A-1 1470 11 8 13.8 0.66
H-1 1540 10 8 14.3 0.7
Comparative steel
613 22 0 5.7 0.33
sheet
[0109]
From Table 3, it can be seen that the part (test piece)
made from the steel sheet of the present invention has higher
load bearing capability and absorbs greater energy than a
part made of the conventional steel sheet having lower
- 54 -
CA 02531615 2005-12-23
strength, thus showing high pressure collapse resistance.
[0110]
Impact resistance test
The parts 4 (hat channel as test piece) shown in Fig. 3
were made by using steel species A-1, J-1 shown in Table 1
and the comparative steel sheet, and were subjected to impact
resistance test. Fig. 4 is a sectional view along A-A of the
part 4 shown in Fig. 3. In the impact resistance test, after
the part was spot welded at the positions 5 of the part 4
similarly to the pressure collapse test, the part 4 was
placed on a base 7 as schematically shown in Fig. 5. A
weight 6 (weighing lOkg) was dropped onto the part 4 from a
height of 11 meters, and the energy absorbed before the part
4 underwent deformation of 40 mm in the direction of height.
The results are shown in Table 4.
[0111]
Table 4
Steel sheet Evaluation of test piece
used
Steel species TS EL Residual Energy absorbed
........................__~..__...................-y
....__............-_........................~..._...............-
._..._._.........__._.._.~_._...........
Symbol __. (~)
_..............................................._(kJ)
(MPa) o _
(Area o)
A-1 1476 11 8 6.58
J-1 1540 10 8 6.87
Comparative 613 22 0 3.56
steel sheet
[0112]
From Table 4, it can be seen that the part (test piece)
- 55 -
CA 02531615 2005-12-23
made from the steel sheet of the present invention absorbs
greater energy than a part made of the conventional steel
sheet that has lower strength, thus showing higher impact
resistance.
[0113]
TEM photograph of the test piece made in this example
is shown as reference. Fig. 6 is a photograph of TEM
observation of No. 101 of the present invention. From Fig. 6,
it can be seen that the high strength thin steel sheet of the
present invention contains lath-shaped residual austenite
(black portion of bar shape in Fig. 6) specified in the
present invention dispersed therein. Fig. 7 is a photograph
of TEM observation of No. 120 of a comparative example. From
Fig. 7, it can be seen that the high strength thin steel
sheet of No. 120 contains residual austenite (black portion
of somewhat round shape in Fig. 7), although the residual
austenite has a block shape that does not satisfy the
requirements of the present invention.
[Example 2]
[0114]
By using sample steels A-2 through Y-2 having the
compositions described in Table 5, test slabs were produced
under the same requirements as that in Example 1(hot rolling,
cold rolling and continuous annealing). In addition, No.217
in Table 6 was prepared by the procedure of No.116 in Table 2
- 56 -
CA 02531615 2005-12-23
according to Example 1 to produce a known high strength
martensite steel as a comparative example. No. 220 was
prepared by the procedure of No.120 in Table 2 according to
Example 1.
[0115]
The metal structures of steel sheets obtained as
described above, their tensile strength (TS) and elongation
(total elongation El) and hydrogen embrittlement resisting
property were measured by the procedures in Example 1 and the
following procedures.
[0116]
Evaluation of hydrogen embrittlement resisting property
In order to evaluate hydrogen embrittlement resisting
property, test pieces were produced by the same procedures as
in Example 1. Then the test pieces were immersed in the same
solution of hydrochloric acid as that in Example l, and the
time before crack occurred was measured.
The bent test pieces prepared as described in Example 1
were subjected to accelerated exposure test in which 30
solution of NaCl was sprayed once every day for 30 days
simulating the actual operating environment, and the number
of days before crack occurred was determined. In addition,
hydrogen-charged 4-point bending test was also conducted for
some steel species as is the case with Example 1. Maximum
stress endured without breaking for 3 hours was determined as
- 57 -
CA 02531615 2005-12-23
the critical fracture stress (DFL). Then the ratio (DFL
ratio) of this value to the value of DFL of test No. 203
(steel species C-2) shown in Table 6 was determined.
Results of these tests are shown in Table 6.
[0117]
Evaluation of weldability
Test of weldability was conducted on No.201 and No.215
which are representative steel species. As a result, it was
found that No.201(present invention) is better than
No.215(prior art) in reference to weldability because the
ratio of ductibility in sample No.215 is 0.19 while that in
sample No.201 is 0.22.
- 58 -
CA 02531615 2005-12-23
[0118]
Table 5
01 ~ v1O ~1 O~O vo 00O 00 ~ M l~I~ N C~O~ O~~Ol~ h l~ ~O
v, O N M N V'1-.V1-~ O ~ O ~ O ~O l~l~ ~ V'1~ O N O OOI~ M
I~ 00 _ l~ V'10000l~ l~0000 l~O~l~ ttO ~~~DN 0000l~ l~~ l~
M ~ M ~ M M M M M M M M M M M ~ tt~ ~ M M M M M M
M ~DM V'101 V'1M O N ~ l~ M O O~~ V'1M M V1~O 00 N
~ O ~G ~G v0vGO 00viOv ~ vil~ I~O I W mi ~ O G~ ~tfJ
t
00 ~OM v~I~ ~ ~ ~ M ~ N N M N V7I~ M N ~D 0000\O ~D\O ~O
m v m n vov~ ~n m mnh ~n ~n v~ vov'~ ~n
~O~ O
O 00~ \O00 O Q~O M ~OI~ O ~DN Q~O~ N N O1 O~M O
V l~ l~O ~ ~ 'Ol~~D \OI~M O I~N ~ O ~O N M N \O ~Ol~ N
v1 v0l~00N ~ ~O~ ~OM l~ t~I~f~ ~ _ O O O v~O~ N m
00 c0000000 000000 0000 000000 00O~ 00 00 00O~01
'n
O
V O O O O
O O
U V~
O o 0 0 o n o 0 0 o N D o td~ n o o D a o o n o o
N N N N N N N
'
0 o n o o a o n a o 0 0 o n o 0 0 0 ;~o o o
0 0 0 0 0 0 0
0 o c~o r~ o o n o 0 0 0 o n o ~ o !~~~0 0 0
m ~n
0 0
V1 V1M V'1~ h V1 V'1~f1V'1 M
_ C G O O O C O C O G O
F~ n O n O O
M M M M O N N N N N N N N M M M M N M N N N
z o 0 0 0 0 0 0 0 0 0 0
0
M M M M M M M M M M M M M M M M
'
U ~ ~ 0 0 0 0 0 ~ o o o
M O O M M N N M N M M M M N ~O~1
M M M M M M M M M M M M M M M M d'M M V1l~V7 ~ N ~O
O O O O O O O O O O O O O O O O O O O N M I~O ~O
d O O O O O O O O O O O O O O O O O O O O O O O
N
N N N N N N N N N N N N N N ~n~n N N N N N N N N N
O O O O O O O O O O S O S O O O O S O O O O O O O
O O O O O O O O O O O O O O O O O O
O O O O a
~n o 0 0 0 0 o
* v
oN N_,~ ~_,'~~ "."~ ~' ~ N ~ ,~..-i.r ~_~_ ~_N_N ,rN_~ ~ ~ ~.-~,-I
U7 , O , O O O O O O O O O O O O O O O O O O O O O O
O O ro
~
N P-~ O O O O O O O O O O O .~
l
'J
N
C
00.~O O M O DO O o0O O O O O O N O O N T N O
F '
'
O - O ~ V N N ~n~n~ V1~ in V1inV1 O O~ ~ O N O O O O~O O
1
"~~ cV cVcJ M N fVN N N N N cV(VfV fV N
ro
C
O O
a
O ~ ~ N o0N oo N Q~O 00 O O v0 N ~Y Ov N O o0
U S
_ O O V1O Q~O Ov O ~ O O O~O O v1 O O O O O~ O O 01O
-ICn N N N N N N N N N N N
U m
m
N et M N O O 01N N O O~ I~O O h O ~ ~ M W M M
O
N _ N N N N N N N N N M N N O N N N N N N N U
U U O O O O O C O O O O O O O O O
N N
N U
~I
U ro
N ~-I
ro
.t1
~ a~
o
fV N N N N N N N N N N N N N N N N N N N N N F
o ~ N : V x N N a ~ O O' v ~ ~ 3 < ~
=
v~ d c U L W u .=~. aG . 0.. cG~ E- > J x
m .
- 59 -
CA 02531615 2005-12-23
[0119]
Tahl A F,
O
a 0
+~
0
w '
ro
a o o r~o m o 0 0 0 0 ~ ~ 0 0 0 0 o a c .... o
a
a
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
M M M M M M M M M M M M M M M M M M M M M
O L L L L L L L L L L L L L L L L L L I-~L L
~ ~ N ~ N ~ N N N N N N N N N N N N N N N N
Q
l~
, 7 7 7 7 7 7 7 7 7 ' 7 ~ 7 ' ~ ' ~ ' 7 7 7 7
o ,~ o,~, O O O
U
.'
O
H
~
U ~ ~ ~ ~ ~ ~ ~r
U
~
o N N N N N N N N N N N N N N N N N N N N N N
ro
L L L L L L L L i-.L L L L L L L L L L L i-.L
'~ N ~ N N N N N N N N N N N N N N ~ N N N ~
.
x O O O O O O O O O O O O O O - ooM O O O O O O O O r
N d' ~ N N N N I~O ~ N N ct
~ 00M
cCN O etM l~.-.N N l~ O~v~ O M N M 00 00 ~nO~ N M ~ O
p"01.-rM M ~O--~~ M ~D~O~ 01 01O ~O~ 00 N ~ 01O -~~ O M
V)v1V1N
~ N N V1~ V1d' ~ V1~ ~ ~ ~t~1V1M ~ ~ ~ ~ ~ ~
h ~
,~ 00 O~
o a o o --0 0 0 0 0 0 0 0 0 0 0 o v v W o 0 0 0 0 o N
i
+
_
w N l~ N M N M N N N M M M ~ M N O~01 O~~ O M N N N
GO O~O~ O~O~O~O~01 O~01O~O~O~ O~O~O~O~Cv 01O~N O~O~ O~01O~~O
~
W
r-i
_
o
ro
x
ro
o
~
+~
m
ro
ro
~
a
a 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o x x x o x o 0 0 0 o x
ro
0
_ _ _
N O
G: ,_00M l~r 00l~00 0000l~I~I~ ~DI~00~/V ~/~ I~00 0000T
?-
O O O O O O O O O O O O O O O O O O O O O O O O O
O O O O
E--I U M M M M M M M M M M M M M M M M M M M M M M M M M
N
r~
r1
~r~
N
U N N N N N N N N N N N N N N N N N N N N ~;N N
N
~
~
~ d ~ v c N ~ x N N ~ ~ z o d ' ~ v~Q ~ ~ ~ 3
' w ~
~ r ~ w t , a ,
N M ~ v1~Ot~ 0001O .~N M ~ n ~ l ~ ~ ~ y
~ O ~
_ _ _ _ _ _ _ _ ~ N N N N N
N O O O O O O O O O
O
H N N N N N N N N N N N N N N N N N N N N N N N N N N
Z
- 60 -
CA 02531615 2005-12-23
[0120]
The results shown in Tables 5 and 6 can be interpreted
as follows (numbers in the following description are test Nos.
in Table 6).
Test pieces Nos. 201 through 214 (inventive steel
sheets 2) and test pieces Nos. 221 through 225 (inventive
steel sheets 1) that satisfy the requirements of the present
invention have high strength of 1180 MPa or higher, and high
hydrogen embrittlement resisting property in harsh
environment after the forming process. They also have high
elongation property required of the TRIP steel sheet, thus
providing steel sheets best suited for reinforcement parts of
automobiles that are exposed to corrosive atmosphere. Test
pieces Nos. 221 through 225, in particular, show even better
hydrogen embrittlement resisting property.
[0121]
Test pieces Nos. 215 through 220 and 226 that do not
satisfy the requirements of the present invention, in
contrast, have the following drawbacks.
[0122]
No. 215 made of steel species 0-2 that includes
insufficient C content has the amount of residual austenite
significantly decreased after the processing, and fails to
show the required level of hydrogen embrittlement resisting
property of the present invention.
- 61 -
CA 02531615 2005-12-23
[0123]
No. 216 made of steel species P-2 that includes
insufficient Mn content does not retain sufficient residual
austenite and is inferior in hydrogen embrittlement resisting
property after the processing.
[0124]
No. 217, martensite steel that is a conventional high
strength steel made of steel species Q-2 that includes
insufficient Si content, hardly contains residual austenite
and is inferior in hydrogen embrittlement resisting property.
It also does not show the elongation property required of a
thin steel sheet.
[0125]
No. 218 made of steel species R-2 that includes
excessive C content has precipitation of carbide and is
inferior in both the forming workability and the hydrogen
embrittlement resisting property after processing. No. 219
made of steel species S-2 that does not include Cu and/or Ni
shows insufficient corrosion resistance and fails to show the
required level of hydrogen embrittlement resisting property
of the present invention.
[0126]
No. 220, that was made of a steel that has the
composition specified in the present invention but was not
manufactured under the recommended conditions, resulted in
- 62 -
CA 02531615 2005-12-23
the conventional TRIP steel. As a result, the residual
austenite does not have the mean axis ratio specified in the
present invention, while the matrix phase is not formed in
binary phase structure of bainitic ferrite and martensite,
and therefore sufficient level of hydrogen embrittlement
resisting property is not achieved.
[0127]
No. 226 includes Al content higher than that specified
for the inventive steel sheet 1. As a result, although the
predetermined amount of residual austenite is retained, the
residual austenite does not have the mean axis ratio
specified in the present invention, the desired matrix phase
is not obtained and inclusions such as AlN are generated thus
resulting in poor hydrogen embrittlement resisting property.
[0128]
Then parts were made by using steel species A-2, I-2
shown in Table 5 and comparative steel sheet (590 MPa class
high strength steel sheet of the prior art). Performance
(pressure collapse resistance and impact resistance) of the
formed test piece were studied by conducting pressure
collapse test and impact resistance test as follows.
[0129]
Pressure collapse test
Maximum tolerable Load was determined similarly to
Example 1 by using steel species A-2, K-2 shown in Table 5
- 63 -
CA 02531615 2005-12-23
and the comparative steel sheet. Absorbed energy was
determined from the area lying under the load-deformation
curve. The results are shown in Table 7.
[0130]
Table 7
Steel sheet used Evaluation
of test
piece
Residual Maximum Energy
Steel species TS EL Y load ab
sorb
ed
Symbol ----------___ _ ___ _---- ----
_____ __.--_ _
_
____
(MPa) ($) (Area $) (kN) (kJ)
A-2 1492 12 8 13.9 0.68
I-2 1567 11 8 14.6 0.71
Comparative steel
613 22 0 5.7 0.33
sheet
[0131]
From Table 7, it can be seen that the part (test piece)
made from the steel sheet of the present invention has higher
load bearing capability and absorbs greater energy than a
part made of the conventional steel sheet that has lower
strength, thus showing higher pressure collapse resistance.
[0132]
Impact resistance test
The impact resistance test was conducted similarly to
Example 1 on the steel sheets made of steel species A-2, K-2
shown in Table 5 and the comparative steel sheet. The
results are shown in Table 8.
[0133]
- 64 -
CA 02531615 2005-12-23
Table 8
Steel sheet used Evaluation
of test
piece
Steel species _ TS EL Residual Energy absorbed
Synbol (MPa~ _~ y ________-~kJ~__________
_ o~___-Area
g~___
A-2 1492 12 8 6.65
I-2 1567 11 8 6.99
Comparative steel sheet613 22 0 3.56
[0134]
From Table 8, it can be seen that the part (test piece)
made from the steel sheet of the present invention absorbs
greater energy than a part made of the conventional steel
sheet having lower strength, thus showing higher impact
resistance.
[0135]
TEM photograph of the test piece made in this example
is shown as reference. Fig. 8 is a photograph of TEM
observation of No. 201 of the present invention. From Fig. 8,
it can be seen that the high strength thin steel sheet of the
present invention contains lath-shaped residual austenite
(black portion of bar shape in Fig. 8) specified in the
present invention dispersed therein. Fig. 9 is a photograph
of TEM observation of No. 220 of a comparative example. From
Fig. 9, it can be seen that the high strength thin steel
sheet of No. 220 contains residual austenite (black portion
of somewhat round shape in Fig. 9), although the residual
- 65 -
CA 02531615 2005-12-23
austenite has a block shape that does not satisfy the
requirements of the present invention.
[Example 3]
[0136]
By using sample steels A-3 through R-3 having the
compositions described in Table 9, test slabs were produced
under the same conditions as that in Example 1(hot rolling,
cold rolling and continuous annealing). In addition, No.312
in Table 10 was prepared by the procedure of No.116 in Table
2 according to Example 1 to produce a known high strength
martensite steel as a comparative example. No. 313 was
prepared by the procedure of No.120 in Table 2 according to
Example 1.
[0137]
The metal structures of steel sheets obtained as
described above, their tensile strength (TS) and elongation
(total elongation E1) and hydrogen embrittlement resisting
property were measured by the procedures in Example 1 and the
following procedures.
[0138]
Evaluation of hydrogen embrittlement resisting property
In order to evaluate the hydrogen embrittlement
resisting property, flat test piece 1.2 mm in thickness was
subjected to slow stretching rate test (SSRT) with a
stretching speed of 1x10-4/sec, to determine hydrogen
- 66 -
CA 02531615 2005-12-23
embrittlement risk index (o) defined by the equation shown
below.
Hydrogen embrittlement risk index (%) - 100 X (1-E1/EO)
[0139]
EO represents the elongation before rupture of a steel
test piece that does not substantially contain hydrogen, El
represents the elongation before rupture of a steel test
piece that has been charged with hydrogen electrochemically
in sulfuric acid. Hydrogen charging was carried out by
immersing the steel test piece in a mixed solution of HZS04
(0.5 mol/L) and KSCN (0.01 mol/L) and supplying constant
current (100A/m2) at room temperature.
[0140]
A steel sheet having hydrogen embrittlement risk index
higher than 50o is likely to undergo hydrogen embrittlement
during use. In the present invention, steel sheets having
hydrogen embrittlement risk index not higher than 50o were
evaluated to have high hydrogen embrittlement resisting
property. Results of the test are shown in Table 10.
[0141]
Evaluation of weldability
Test of weldability was conducted on No.201 and No.215
which are representative steel species. As a result, it was
found that No. 301 (present invention) is better than No.311
(prior art) in reference to weldability because the ratio of
- 67 -
CA 02531615 2005-12-23
ductibility in sample No.311 is 0.19 while that in sample
No.301 is 0.22.
- 68 -
CA 02531615 2005-12-23
[0142]
Table 9
O N v1 ~O h l~O I~ 01 ~ M 'O M V1 V1 O (~
01 O ~ ~ 00 O N Q~ 01 ~ ~ 01 01 O O M 00 V1
l~ l~~ 00 I~ l~ 0000 ~O I~ O ~ 00 l~ I~ ~O
M M M M M M M M M M M et M M M M M M
M ~ p~ ~p ~ 00 M 00 ~O ~ O N ~~ ~O M M ~ M
00 vDvD M t~ O 00O~ O t~ N oo O M l~ ~O ~D O~
M l~ ~ ~ M ~ M N ~ d'~ ~ ~ M M M
~ '
(1] V1 ~flV1 ~ V'1V1 V'1Vl ~1 Vl V1~O V'1~ ~1 ~ V V1
~ 1
~ ~OO N O~ M ~ ~ V1 00 N 00 r~ M d'
M N V)00 vD V1 N O\ 00 01 M O O ~ (~ W O O
V'>rh~O l~ I~ ~n ~WO ~D ~ M _ ~O M 01 N V1
O
M O v1
~
O O
O O
O O O O
Q O
I. p p tp ~
b0
~
p o m o ~~ o ~ o ~ U o ~ o ~ o a U
~ ~
O N N N N
0 o n o m o ~ o o ~ n c o ~ 0 0
0
n o r-io 0 0 0 0 0 ~ 0 0 0 0 ~ 0 0
0 0 0
0 0 0 0 0 0
0 0 0 0 0
0 0
0 0 0 0 0
0 0 0 ~ ~ o ~ ~ 0 0 0
N N N M N N M M
O O O O O O
M M M M M M M
U ~ o o . o ~ 0 0 0 0
m
ro
M M M O ~ O N M M ~ M ~ ~ ~ O _W
M M M M M M M M M M M ~ ~1 V'1I~ ~1 M 00
O O O O O O O O O O O O O N M l~ O ~O
O O O O O O O O O O
N N N N N N N N N N V1N N N N N N N
O O O O O O O O O O O O O O O O O O
O O O O O O O O O O O O O O O O O O
V~ O O O O O O O O O O O O O O O O O O
N
N V ~_ _ N_ N_ N_ N_ _
_ _ _ _ _ _ _ _ _ _ O O O O O O O O
O O O O O O O O O O
p. O O O O O O O O O O O O O O O O O
o C
ro
C
O N M ~ 00 M O O 00 O N o0 O ~ ~ V~ O
C v~ v~O v1 v~ C W vo V~ ~ O ~t ~ v1 ~n v W ~O .,
1
O ~ N N N N N N N N N N N N N N N N N
_ W
O
O
CL _ _
N 00<t 00 N 00 O 00 00 O O O N O\ N O W
~
O O~O O~ O O~ O Q~ O O~ O _ O O O~ O O O
U V7 N N N N N N O N N N N N
O
U U
N cr O N O o0 N O O O N
~
N N N _ N N N N N ~ N N N N N N N U
U U o 0 0 0 0 0 0 0 0 0 0 0 0 0
ro
o v
M M M M M M M M M M M M M M M M H
d a v o w w ~ x M M ~ ~ ~ z o ~ a ~
~
o .~ . .
- 69 -
CA 02531615 2005-12-23
[0143]
Table 10
x
~
;
~
a
'
'
+~
~
o
,
~'
r
b
x
~
.
~
~
ro
..~
x
'~
O ~ ~ N 00 V~ N O O v1 00N 01 01 d' h
N M N N ~ O~ 00 N N I
N .~ O O O O v~ ~ M N
O O O v1 O O O O O O O O O O C1 M V~
O ~ ~' o ~ ~O h o0 l~ l~ N O O 01_ 00 O r. O~
N M d ~ d' ~h et ~ ~ ~ ~ et ~O N ~ ~ ~n v~ N
O~ rr
V
N
0 o O o V ~ o
O N
N --~N N N N M M M M ~f1O~ O N M M M M O~
0
01O~ O~ 01 O~ 01 01 00 N O~O1 ~ V1
O
J~
r1
ro
ro
ro
t.~
W
N
O
'd
-r1
U7
O ~1 O V~ V1 ~n O O O O ~ O M N N
ro
~
l~ N N N M ~ V1 , M
N
~
U
ro
b
3
T
+.~
-~
~J
rl
N
m
~
~
G
a
ro
a~
~
~~
3
0 0 0 0 0 0 0 0 0 0 0 o 0 0 0 0 0 o
m m N N ~ N ~ N ~ ~ ~ N ~ ~ ~ ~ 't N
, N N
w
o
>-
x
a
r:
ro
~
o
o
c
~,
ro
a~
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o
M N ~ N ~ 0 N M O~~ M N O
a ~
0
ro
N
O ~ N
04 n o00~ o0 00 00 00 ~ ~ ~o op
>-
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
~ ~
n n N N N N N N O O V W ~n ~nN N N N ~n
n
M M M M M M M M M M M M M M M
.~
W
O
N
U
N M M M M M ~.,~M M M M M M M M M M M
N ' '
c~ v r~ w w x M ~ s~ a d ~ z a, a
N M ~ v o t~ oo ~ o ~. N M ~ ~w o r oo o
0 ~
0 0 0 o 0 0 0 0 -. .- _ _ _ _ _ _ _ _
M
M M M M M M M M M M M M M M M M M M
7
CA 02531615 2005-12-23
[0144]
The results shown in Tables 9 and 10 can be interpreted
as follows (numbers in the following description are test Nos.
in Table 10).
[0145]
Test pieces Nos. 301 through 310 (inventive steel
sheets 2) and test pieces Nos. 314 through 318 (inventive
steel sheets 1) that satisfy the requirements of the present
invention have high strength of 1180 MPa or higher, and show
high hydrogen embrittlement resisting property in harsh
environment after the forming process. They also have high
elongation property required of the TRIP steel sheet, thus
providing steel sheets best suited for reinforcement parts of
automobiles that are exposed to corrosive atmosphere.
[0146]
Test pieces Nos. 3I1 through 313 and 319 that do not
satisfy the requirements of the present invention, in
contrast, have the following drawbacks.
[0147]
No. 311 made of steel species K-3 that includes
excessive C content has carbide precipitated and residual
austenite of longer mean length of minor axis, thus resulting
poor performance in both workability and hydrogen
embrittlement resisting property after processing.
[0148]
- 71 -
CA 02531615 2005-12-23
No. 312, martensite steel that is a conventional high
strength steel made of steel species L-3 that includes
insufficient Si content, hardly contains residual austenite
and is inferior in hydrogen embrittlement resisting property.
It also does not show the elongation property required of a
thin steel sheet.
[0149]
No. 313, that was made of a steel that has the
composition specified in the present invention but was not
manufactured under the recommended conditions, resulted in
the conventional TRIP steel. As a result, the residual
austenite does not have the mean axis ratio and the mean
length of minor axis specified in the present invention,
while the matrix phase is not formed in binary phase
structure of bainitic ferrite and martensite, thus resulting
in low strength and poor hydrogen embrittlement resisting
property.
[0150]
No. 319 includes Al content higher than that specified
for the inventive steel sheet 1. As a result, although the
predetermined amount of residual austenite is retained, the
residual austenite does not have the mean axis ratio
specified in the present invention, the desired matrix phase
is not obtained and inclusions such as A1N are generated thus
resulting in poor hydrogen embrittlement resisting property.
- 72 -
CA 02531615 2005-12-23
[0151]
Then parts were made by using steel species A-3, D-3
shown in Table 9 and comparative steel sheet (590 MPa class
high strength steel sheet of the prior art). Performance
(pressure collapse resistance and impact resistance) of the
formed test piece were studied by conducting pressure
collapse test and impact resistance test as follows.
[0152]
Pressure collapse test
Maximum tolerable load was determined similarly to
Example 1 by using steel species A-3, D-3 shown in Table 9
and the comparative steel sheet. Absorbed energy was
determined from the area under the load-deformation curve.
The results are shown in Table 11.
[0153]
Table 11
Steel sheet used Evaluation
of test
piece
Residual Maximum Energy
Steel species TS EL Y load absorbed
____________________
S ymb o 1
_________________________________________________
(MPa) (%) (Area (kN) (kJ)
~)
A-3 1280 14 8 12 0.6
D-3 1495 11 8 13.9 0.67
Comparative steel
613 22 0 5.7 0.33
sheet
[0154]
From Table 11, it can be seen that the part (test
- 73 -
CA 02531615 2005-12-23
piece) made from the steel sheet of the present invention has
higher load bearing capability and absorbs greater energy
than a part made of the conventional steel sheet having lower
strength, thus showing high pressure collapse resistance.
[0155]
Impact resistance test
The impact resistance test was conducted similarly to
Example 1 on the steel sheets made of steel species A-3, D-3
shown in Table 9 and the comparative steel sheet. The
results are shown in Table 12.
[0156]
Table 12
Evaluation of test
Steel sheet used
piece
TS EL Residual Energy absorbed
y
Steel species __ ___ _- __________
___ -_ ___
Symbol ($~ ~ _________
__(
(MPa) Area ~) kJ)
A-3 1280 14 8 5.95
D-3 1495 11 8 6.77
Comparative steel
613 22 0 3.56
sheet
[0157]
From Table 12, it can be seen that the part (test
piece) made from the steel sheet of the present invention
absorbs greater energy than a part made of the conventional
steel sheet that has lower strength, thus showing high impact
resistance.
- 74 -
CA 02531615 2005-12-23
[0158]
TEM photographs of the test pieces made in this example
are shown as reference. Fig. 12 is a photograph of TEM
observation (magnification factor 15000) of No. 301 of the
present invention. Fig. 13 is a photograph of TEM
observation (magnification factor 60,000) of a portion shown
in the photograph of Fig. 12. From Figs. 12, 13, it can be
seen that the high strength thin steel sheet of the present
invention contains fine residual austenite grains (black
portion of bar shape in Figs. 12, 13) specified in the
present invention dispersed therein, and that the residual
austenite has the lath shape specified in the present
invention. Fig. 14 is a photograph of TEM observation of No.
313 of a comparative example. From Fig. 14, it can be seen
that the high strength thin steel sheet of No. 313 contains
residual austenite (black portion of somewhat round shape in
Fig. 14), although the residual austenite has a block shape
that does not satisfy the requirements of the present
invention.
- 75 -