Note: Descriptions are shown in the official language in which they were submitted.
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
TITLE: NOVEL COMPOUNDS AND COMPOSITIONS COMPRISING STEROLS
ANDIOR STANOLS AND CHOLESTEROL BIOSYNTHESIS INHIBITORS AND USE
THEREOF IN TREATING OR PREVENTING A VARIETY OF DISEASES AN0
CONDITIONS.
INVENTORS: JAMES P. KUTNEY
P. HADYN PRITCHARD
TATJANA LUKIC
ASSIGNEE: FORBES MEDI-TECH INC.
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
FIELD OF THE INVENTION
This present invention relates to the field of sterols and stanols and novel
derivatives
thereof and their use in treating and preventing cardiovascular disease and
other-
disorders.
BACKGROUND OF THE INVENTION
While recent advances in science and technology are helping to improve quality
and add
years to human life, the prevention of atherosclerosis, the underlying cause
of
cardiovascular disease ("CVD") has not been sufficiently addressed. In fact,
cardiovascular diseases account for more deaths annually than any other
disease,
including all forms of cancer combined'. In the USA alone, more than one
million heart
attacks 'occur each year and more than half a million people die as a result.
This
enormous toll has necessitated continued research to determine the causes of
CVD and
means by which it can be prevented and treated.
The primary cause of CVD is atherosclerosis, a disease characterized by the
deposition
of lipids, including cholesterol, in the arterial vessel wall resulting in a
narrowing of the
vessel passages and ultimately a hardening of the vascular system.
Atherosclerosis is a
degenerative process resulting from aninterplay of inherited (genetic) factors
and
environmental factors such as diet and lifestyle. Research to date suggest
that
cholesterol may play a role in atherosclerosis by forming atherosclerotic
plaques in blood
vessels, ultimately cutting off blood supply to the heart muscle or
alternatively to the brain
2
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
or limbs, depending on the location of the plaque in the arterial tree'~2. A
total cholesterol
in excess of 225-250 mg/dl is associated with significantly elevated risk of
CVD, including
vascular disease. Overviews have indicated that a 1 % reduction in a person's
total serurtZ
cholesterol yields a 2% reduction in risk of a coronary artery event4.
Statistically, a 10°/v
decrease in average serum cholesterol (e.g. from 6.0 mmol/L to 5.3 mmol/L) may
result in
the preventiori of 100,000 deaths in the United States annually5.
Cholesteryl esters are a major component of atherosclerotic lesions and the
major
storage form of cholesterol in arterial wall cells. Formation of cholesteryl
esters is also a
step in the intestinal absorption of dietary cholesterol through homeostatic
control
mechanisms. These control mechanisms involve the inter-related regulation of
dietary
cholesterol, cholesterol biosynthesis and catabolism of cholesterol-containing
plasma
lipoproteins. Cholesterol biosynthesis and catabolism occur primarily in the
liver and
hence, it is a prime determinant of plasma cholesterol levels.
Lipoproteins are complexes of lipids and proteins held together by non-
covalent bonds.
Each type of lipoprotein class has a characteristic mass, chemical
composition, density
and physiological role. Irrespective of density or particle size, circulating
lipids consist of
a core of cholesteryl esters and triglycerides, and an envelope of
phospholipids, free
cholesterol and apolipoproteins. The apolipoproteins are involved in the
assembly and
secretion of the lipoprotein, provide structural integrity, activate
lipoprotein-modifying
enzymes, and are the ligand for a large assortment of receptors and membrane
proteins. Lipoprotein classes found in plasma include HDL, LDL, intermediate
density
lipoproteins (IDL) and very low density lipoproteins (VLDL).
Each type of lipoprotein has a characteristic apolipoprotein composition or
ratio. The
most prominent apolipoprotein in HDL is apolipoprotein-AI (apo-AI), which
accounts for
approximately 70% of the protein mass, with apo-All accounting for another
20%. The
ratio of apoA_I to apoA-II may determine HDL functional and anti-atherogenic
3
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
properties. Circulating HDL particles consist of a heterogeneous mixture of
discoidal
and spherical particles with a mass of 200 to 400 kilo-daltons and a diameter
of 7 to 1 O
nm.
HDL is one of the major classes of lipoproteins that function in the transport
of lipids inr
plasma, and has multiple functions within the body, including reverse
cholesterol
transport, providing the cholesterol molecule substrate for bile acid
synthesis, transport
of clusterin, transport of paraoxanase, prevention of lipoprotein oxidation
and selective
uptake of cholesterol by adrenal cells. The major lipids associated with HDL
include
cholesterol, cholesteryl ester, triglycerides, phospholipids and fatty acids.
To better understand how HDL is anti-atherogenic, a brief explanation of the
atherosclerotic process is necessary. The atherosclerotic process begins when
LDL
becomes trapped within the vascular wall. Oxidation of this LDL results in the
binding of
monocytes to the endothelial cells lining the vessel wall. These monocytes are
activated
and migrate into the endothelial space where they are transformed into
macrophages,
leading to further oxidation of the LDL. The oxidized LDL is taken up through
the
scavenger rece~ntor on the macrophage, leading to the formation of foam cells.
A,
fibrous cap is generated through the proliferation and migration of arterial
smooth
muscle cells, thus creating an atherosclerotic plaque.
HDL is essential for the transport of cholesterol from extra-hepatic tissues
to the liver,
where it is excreted into bile as free cholesterol or as bile acids that are
formed from
cholesterol. The rop cess requires several steps. The first is the formation
of nascent or
pre-beta HDL particles in the liver and intestine. Excess cholesterol moves
across cell
membranes into the nascent HDL through the action of the ABC A1 transporter_
Lecithin cholesterol acyl transferase (LCAT) converts the cholesterol to
cholesteryl
ester and the subsequent conversion of nascent HDL to mature HDL. Esterified
cholesterol is then transferred by cholesteryl ester transfer protein (CETP)
from HDL to
4
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
apolipoprotein-B containing lipoproteins, which are taken up by numerous
receptors in,,
the liver.' Nascent HDL is regenerated via. hepatic triglyceride lipase and
phospholipid
transfer protein and the c ycle c ontinues. In a ddition t o t he c holesterol
r emoved f rorn.
peripheral cells, HDL accepts cholesterol from LDL and erythrocyte membranes.
Another m echanism o f r everse c holesterol t ransport m ay involve passive
diffusion of=
cholesterol between cholesterol-poor membranes and HDL or other acceptor
molecules.
HDL protects against the development of atherosclerosis both through its role
in
reverse cholesterol transport and possibly by impeding LDL oxidation. Several
HDL-
associated enzymes are involved in the process. Paroxonase (PON1 ), LCAT, and
platelet activating factor acetylhydrolase (PAFAH) all participate by
hydrolyzing
phospholipid hydroperoxides generated during LDL oxidation and act in tandem
to
prevent the accumulation of oxidized lipid in LDL. These enzymes are
responsible for
the anti-oxidative and anti-inflammatory properties of HDL. Studies have shown
that a
low plasma concentration of HDL cholesterol is a significant risk factor for
the
development of atherosclerosiss and that high levels are protective.
The liver is the major organ responsible for synthesis and secretion of VLDLs,
which, as
noted above, are metabolized to LDL in circulation. LDLs are the predominant
cholesterol carrying lipoproteins in plasma and hence an increase in their
concentration
is directly correlated with atherosclerosis. Simply put, when intestinal
cholesterol
absorption is reduced, by any means, less cholesterol is d elivered t o t he I
fiver. A s a
result, VLDL production is reduced and there is a concomitant increase in
hepatic
clearance of plasma cholesterol, mostly in the form of LDL.
Accordingly, cholesterol acts on three different levels to regulate its own
synthesis. Firstly,
it suppresses endogenous cholesterol synthesis by inhibiting the enzyme HMG
CoA
reductase. Secondly, it activates LCAT. Thirdly, it regulates the synthesis of
the LDL-
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
receptor ensuring that a cell already having a sufficient amount of
cholesterol will not take
up additional cholesterol.
Sterols are naturally occurring compounds that perform many critical cellular
functions_
Sterols such as campesterol, stigmasterol and beta-sitosterol in plants,
ergosterol in fungi
and cholesterol in animals are each primary components of cellular and sub-
cellular
membranes in their respective cell types. The dietary source of phytosterols
in humans
comes from plant materials i.e. vegetables and plant oils. The estimated daily
phytosterol
content in the conventional western-type diet is approximately 60-80
milligrams in contrast
to a vegetarian diet which would provide about 500 milligrams per day.
Phytosterols have received a great deal of attention due to their ability to
decrease serum
cholesterol levels when fed to a number of mammalian species, including
humans. While:
the precise mechanism of action remains largely unknown, the relationship
between
cholesterol and phytosterols is apparently due in part to the similarities
between the
respective chemical structures (the differences occurring in the side chains
of the
molecules). It is assumed that phytosterols displace cholesterol from the
micellar phase
and thereby reduce its absorption or possibly compete with receptor and/or
carrier sites irt
the cholesterol absorption process.
Over forty years ago, Eli Lilly marketed a sterol preparation from tall oil
and later from
soybean oil called CyteIlinTM which was found to lower serum cholesterol by
about 9%
according to one report'. Various subsequent researchers have explored the
effects of
sitosterol preparations on plasma lipid and lipoprotein concentrations8 and
the effects of
sitosterol and campesterol from soybean and tall oil sources on serum
cholesterols9. A
composition of phytosterols which has been found to be highly effective in
lowering serum
cholesterol is disclosed in US Patent Serial No. 5,770,7~48:to Kutney et ~al.
and comprises
no more than 70% b y w eight b eta-sitosterol, a t I east 1 0% b y w eight c
ampesterol a nd
stigmastanol (beta-sitostanol). It is noted in this patent that there is some
form of synergy
6
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
between the constituent phytosterols, affording even b etter c holesterol-
lowering r esults
than had been previously achieved.
Many other compounds and compositions have been developed over the last
decade=
with a view either to lowering serum LDL cholesterol, increasing serum HDL
cholesterol
and preventing other significant risk factors for CVD.
It is an object of the present invention to provide novel compounds which may
obviate or
mitigate the disadvantages of prior known compounds used to treat CVD and
underlying
disorders including lipid disorders.
It is an object of the present invention to provide novel compositions which
may obviate or
mitigate the disadvantages of prior known compositions used to treat CVD and
underlying
disorders including lipid disorders.
SUMMARY OF THE INVENTION
The present invention provides, in one aspect, novel compounds having one or
more of
the following formulae:
a)
0
~I
R2-(CH2)n -C-O-R
b)
R2-R
C)
O O
II II
R2 -C -C -O -R
d)
7
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
O
I I
R3 -P -O -R
I
OX
e)
0
R2 -R4 -P-O-R
I
OX
f)
O O
II II
R3 -P -R4 -P -O -R
OX OX
wherein R is a sterol or stanol moiety, R2 is a cholesterol biosynthesis
inhibitor with at
least one free and reactive carboxyl group; R3 is a cholesterol biosynthesis
inhibitor with
at least one free and reactive hydroxyl group; R4 is derived from ascorbic
acid, X is either
hydrogen or is selected from the group consisting of metals, alkali earth
metals and alkali
metals and n=1-5, including all biologically acceptable salts or solvates or
prodrugs of at
least one such compound or of the salts or of the solvates thereof.
The present invention provides, in another aspect, a composition comprising:
a) at least one cholesterol absorption inhibitor selected from compounds
having the
general formulae:
0
I I
-P -O -R
I
OH
O O
II II
-C -C -O -R
8
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
iii)
R4-R
iv)
O
I I
R4 -(CFi2)n -C-O-R
wherein R is a sterol or stanol moiety, R4 is derived from ascorbic acid and
n=1-5,
including all biologically acceptable salts or solvates or prodrugs of at
least one such
compound or of the salts or of the solvates thereof; and
b1 at least one cholesterol biosynthesis inhibitor.
The present invention provides, in another aspect, a method of achieving one
or more of
the following therapeutic goals:
a) preventing, treating or alleviating one or more conditions associated with
CVD
generally and including arteriosclerosis, atherosclerosis, arteriolosclerosis,
angina pectoris, and thrombosis;
b) reducing and/or eliminating one or more of the risk factors associated with
CVD;
c) preventing, treating or alleviating atherosclerosis;
d) preventing, treating or alleviating hypercholesterolemia;
e) preventing, treating or alleviating a hyperlipidic condition;
f) preventing, treating or alleviating dislipidemia;
g) preventing, treating or alleviating hypertension;
h) preventing, treating or alleviating coronary artery disease;
i) preventing, treating or alleviating coronary plaque development;
j) preventing, treating or alleviating coronary plaque inflammation;
k) lowering serum LDL cholesterol;
9
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
I) increasing serum HDL cholesterol;
m) decreasing serum triglycerides levels;
n) decreasing cholesterol biosynthesis;
o) preventing, reducing, eliminating or ameliorating a dislipidemic condition
or
disorder;
p) preventing, reducing, eliminating or ameliorating hypercholesterolemia or
hypoalphalipoproteinemia;
q) preventing, reducing, eliminating, stabilizing or ameliorating the
development of
atherosclerotic lesions or plaque;
r) preventing, reducing, eliminating, or ameliorating the development of
inflammation
associated with the development of. cardiovascular disease and coronary artery
disease;
s) preventing, reducing, eliminating or ameliorating any condition, disease or
disorder which has as its basis or which is exacerbated by a deficiency in
plasma HDL, or by an excess of either LDL, VLDL, Lp(a), beta-VLDL, IDL or
remnant lipoproteins;
t) decreasing the risk of a stroke;
u) inhibiting isoprenoid synthesis;
v) preventing, treating or alleviating Alzheimer's disease;
w) preventing, treating or alleviating dementia;
x) preventing, treating or alleviating osteoporosis;
y) preventing, reducing, eliminating or ameliorating injuries due to oxidative
stress;
z) enhancing and/or preserving the stability of HDL from oxidation;
aa)enhancing and/or preserving the stability of LDL, VLDL or IDL from
oxidation;
bb)enhancing and/or preserving the stability of triglyceride (TG) from
oxidation;
cc) exhibiting anti-coagulatant properties;
dd)exhibiting anti-proliferative properties;
ee)exhibiting immunomodulatory properties;
ff) exhibiting angiogenic properties;
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
gg)preventing, treating or alleviating tumour growth;
hh)increasing bone mass and/or bone turnover; and
ii) enhancing any of the non-lipid related, pleiotropic effects achieved by
the
administration of statins, in particular at the cellular and molecular level,
which comprises administering to an animal, a non-toxic and therapeutically
effective
amount of a compound or composition as described and claimed herein.
The present invention provides, in yet another aspect, a method for treating
or preventing
cardiovascular disease and its underlying conditions including, without
limitation,
atherosclerosis, hypercholesterolemia, hyperlipidemia, dislipidemia,
hypertension,
thrombosis, coronary artery disease, and for treating and reducing
inflammation including
coronary plaque inflammation, which comprises administering to an animal, a
non-toxic
and therapeutically effective amount of one or more of the compounds, as shown
above.
In a further aspect of the present invention, there is provided a method for
treating or
preventing cardiovascular disease and its underlying conditions including,
without
limitation, atherosclerosis, hypercholesterolemia, hyperlipidemia,
dislipidemia,
hypertension, thrombosis, coronary artery disease, and for treating and
reducing
inflammation including coronary plaque inflammation, which comprises
administering to
an animal, a non-toxic and therapeutically effective amount of the
composition, as
described in summary above.
In yet another aspect, the present invention relates to a pharmaceutical
composition
comprising an effective or therapeutic amount of one or more of the novel
compounds
described herein and a pharmaceutically acceptable carrier. In a further
aspect, the
present invention relates to a pharmaceutical composition comprising an
effective or
therapeutic amount of at least one cholesterol absorption inhibitor having one
of formulae
i)-iv) together with an effective or therapeutic amount at least one
cholesterol biosynthesis
inhibitor and a pharmaceutically acceptable carrier.
11
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
In a final aspect, the present invention provides a kit comprising, in one
container, are
effective amount at least one cholesterol absorption inhibitor having one of
formulae i)-ivy
and a pharmaceutically acceptable carrier and in another separate container,
an effective
amount at least one cholesterol biosynthesis inhibitor and a pharmaceutically
acceptable
carrier.
The crux of the present invention is the provision and co-administration of
sterols and/or
stanols with cholesterol biosynthesis inhibitors, for example and preferably,
statins. This
can be accomplished in two ways:
1 ) via the formation of novel compounds wherein sterols and/or stanols are
chemically joined to the selected cholesterol biosynthesis inhibitor in a
unified
structure; and
2) via the formation of novel compositions, wherein selected cholesterol
absorption
inhibitors (in the form of sterol andlor.stanol esters or derivatives) are
admixed with
the selected cholesterol biosynthesis inhibitor.
It is believed that when the cholesterol biosynthesis inhibitors are either
derivatized with
the sterol/stanol component as described herein, or merely co-adminstered with
sterols/stanols i n c omposition, a I ower d osage o f the selected
cholesterol biosynthesis
inhibitor m ay b a required t o a chieve t he d esired a ffects. T his i s
important due to the
documented adverse side-effects of some cholesterol biosynthesis inhibitors,
including
some statins. The reduction of potential side-effects is also considered
important from the
perspective of patient compliance. ,
Some of the compounds of the present invention (those depicted in formulae (e)
and (f)
and in i) through iv) above) comprise an ascorbyl moiety. These particular
compounds
have numerous advantages. In particular, solubility in aqueous solutions such
as water is
improved by the ascorbyl moiety thereby allowing oral administration per se.
Likewise,
12
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
other modes of administration are facilitated. Accordingly, these selected
compounds of
the present invention can be prepared and used as such or they can be easily
incorporated into pharmaceutical preparations regardless of whether such
preparations
are water-based. This enhanced solubility generally translates into lower
administration
dosages of the compounds in order to achieve the desired therapeutic effect.
These effects and other significant advantages will become apparent herein
below.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention is illustrated by way the following non-limiting
drawings in which:
Figure 1 is a schematic showing a process of preparing ascorbyl sitostanyl or
campestanyl atorvastatin phosphate ester and its sodium salt;
Figure 2 is a schematic showing a process of preparing ascorbyl sitostanyl or
campestanyl simavastatin phosphate ester and its sodium salt;
Figure 3 is a schematic showing a process of preparing sitostanyl or
campestanyl
simavastatin phosphate ester; and
Figure 4 is a schematic showing a process of preparing sitostanyl or
campestanyl
atorvastatin carboxylic ester.
PREFERRED EMBODIMENTS OF THE INVENTION
The following detailed description is provided to aid those skilled in the art
in practising
the present invention. However, this detailed description should not be
construed so as
to unduly limit the scope of the present invention. Modifications and
variations to the
13
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
embodiments discussed herein may be made by those with ordinary skill in the
art without
departing from the spirit or scope of the present invention.
As used herein, "animal" means any member of the animal kingdom, including
alt:
mammals and most preferably humans.
As used herein, the term "prodrug" refers to compounds that are drug
precursors, which,
following administration to a patient, release the drug in vivo via some
chemical or
physiological process (for example, a prodrug, on being brought to
physiological pH or
through enzyme action is converted to the desired drug form).
As used herein, the term "solvate" refers to a molecular or ionic complex of
molecules or
ions of solvent with those of solute (for example the compounds of formulae a)
to f) or
prodrugs of compounds a) to f)). Non-limiting examples of useful solvents
include polar,
protic solvents such as water and/or alcohols (for example methanol).
As used herein, the term "compound" is interchangeable with the terms
"derivative",
"structure" and "analogue".
As used herein, the terms "effective" or "therapeutically effective", are
intended to qualify
the amount of the compounds) or composition administered to an animal, in
particular a
human, in order to elicit a biological or medical response of a tissue,
system, animal or
mammal that is being sought by the person administering the compounds) or
composition and which amount achieves one or more of the following goals:
a) preventing, treating or alleviating one or more conditions associated with
CVD
generally and including arteriosclerosis, atherosclerosis, arteriolosclerosis,
angina
pectoris, and thrombosis;
b) reducing and/or eliminating one or more of the risk factors associated with
CVD
c) preventing, treating or alleviating atherosclerosis;
14
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
d) preventing, treating or alleviating hypercholesterolemia;
e) preventing, treating or alleviating a hyperlipidic condition;
f) preventing, treating or alleviating dislipidemia;
g) preventing, treating or alleviating hypertension;
h) preventing, treating or alleviating coronary artery disease;
i) preventing, treating or alleviating coronary plaque development;
j) preventing, treating or alleviating coronary plaque inflammation;
k) lowering serum LDL cholesterol;
I) increasing serum HDL cholesterol;
m) decreasing serum triglycerides levels;
n) decreasing cholesterol biosynthesis;
o) preventing. reducing, eliminating or ameliorating a dislipidemic condition
or disorder;
p) preventing, reducing, eliminating or ameliorating hypercholesterolemia or
hypoalphalipoproteinemia,
q) preventing, reducing, eliminating, stabilizing or ameliorating the
development of
atherosclerotic lesions or plaque;
r) preventing, reducing, eliminating, or ameliorating the development of
inflammation
associated with the development of cardiovascular disease and coronary artery
disease;
s) preventing, r educing, eliminating or ameliorating any condition, disease
or disorder
which has as its basis or which is exacerbated by a deficiency in plasma HDL,
or by
an excess of either LDL, VLDL, Lp(a), beta-VLDL, IDL or remnant lipoproteins;
t) decreasing the risk of a stroke;
u) inhibition of isoprenoid synthesis;
v) preventing, treating or alleviating Alzheimer's disease;
w) preventing, treating or alleviating dementia;
x) preventing, treating or alleviating osteoporosis;
y) preventing, reducing, elimiriating or ameliorating injuries due to
oxidative stress;
z) enhancing and/or preserving the stability of HDL from oxidation;
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
aa)enhancing and/or preserving the stability of LDL, VLDL or IDL from
oxidation
bb)enhancing and/or preserving the stability of triglyceride (TG) from
oxidation;
cc) exhibiting anti-coagulatant properties;
dd) exhibiting anti-proliferative properties;
ee)exhibiting immunomodulatory properties;
ff) exhibiting angiogenic properties;
gg)preventing, treating or alleviating tumour growth;
hh)increasing bone mass and/or bone turnover; and
ii) enhancing any of the non-lipid related, pleiotropic effects . achieved by
the
administration of statins, in particular at the cellular and molecular level.
As used herein, the term "statin" includes any naturally occurring or
synthetic compound
that inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase by competing
with 3-
hydroxy-3-methylglutaric acid for the substrate binding site on HMG CoA
reductase.
As used herein, the term "sterol" includes all sterols without limitation, for
example: (from
any source and in any form: a, ~i and Y) sitosterol, campesterol,
stigmasterol,
brassicasterol (including dihydrobrassicasterol), desmosterol, chalinosterol,
poriferasterol,
clionasterol, ergosterol, coprosterol, codisterol, isofucosterol, fucosterol,
clerosterol,
nervisterol, lathosterol, stellasterol, spinasterol, chondrillasterol,
peposterol, avenasterol,
isoavenasterol, fecosterol, pollinastasterol, cholesterol and all natural or
synthesized
forms and derivatives thereof, including isomers.
The term "stanol" refers to, for example: (from any source and in any form: a,
~i and y)
saturated or hydrogenated sterols including all natural or synthesized forms
and
derivatives thereof, and isomers, including sitostanol, campestanol,
stigmastanol,
brassicastanol (including dihydrobrassicastanol), desmostanol, chalinostanol,
poriferastanol, clionastanol, ergostanol, coprostanol, codistanol,
isofucostanol, fucostanol,
clerostanol, nervistanol, lathostanol, stellastanol, spinastanol,
chondrillastanol,
16
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
pepostanol, avenastanol, isoavenastanol, fecostanol, and pollinastastanol.
It is to be understood that modifications to the sterols and stanols i.e. to
include side
chains also falls within the purview of this invention. It is also to be
understood that,
when in doubt throughout the specification, and unless otherwise specified,
the term
"sterol" encompasses both sterol and stanol. The terms "phytosterol" and
"phytostanol"
may also be used and refer to all plant-derived sterols or stanols
respectively.
The sterols and stanols for use in forming derivatives in accordance with this
invention
may be procured from a variety of natural sources or they may be artificially
synthesized.
For example, they may be obtained from the processing of plant oils (including
aquatic
plants) such as corn oil and other vegetable oils, wheat germ oil, soy
extract, rice extract,
rice bran, rapeseed oil, sunflower oil, sesame oil and fish (and other marine-
source) oils.
They may also be derived from yeasts and fungi, for example ergosterol.
Accordingly,
the present invention is not to be limited to any one source of sterols. US
Patent Serial
No. 4,420,427 teaches the preparation of sterols from vegetable oil sludge
using solvents
such as methanol. Alternatively, phytosterols and phytostanols may be obtained
from tall
oil pitch or soap, by-products of forestry practises as described in US Patent
Serial
No.5,770,749, incorporated herein by reference. A further method of extracting
sterols
and stanols from tall oil pitch is described in Canadian Patent Application
Serial No.
2,230,373 which was filed on February 20, 1998 (corresponding to
PCT/CA99/00150
which was filed on February 19, 1999) and US Patent Application Serial No
10/060,022
which was filed on January 28, 2002 the contents of all of which are
incorporated herein
by reference.
Accordingly, it is to be understood that the widest possible definition is to
be accorded to
the terms "sterol" and "stanol" as used herein, including, but not limited to:
free sterols
and stanols, esterified sterols and stanols with aliphatic or aromatic acids
(thereby
forming aliphatic or aromatic esters, respectively), phenolic acid esters,
cinnamate esters,
17
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
ferulate esters, phytosterol and phytostanol glycosides and acylated
glycosides or
acylglycosides. Thus, the terms "sterols" and "stanols" encompasses all
analogues, which
may further h ave a d ouble b and a t t he 5 -position i n t he c yclic a nit
as in most natural
sterols, or one or more double bonds at other positions in the rings (for
example, 6, 7,
8(9), 8(14), 14 5/7) or no double bonds in the cyclic unit as in stanols.
Further, there may
be additional methyl groups as, for example, in a~-sitosterol.
Cholesterol Biosynthesis Inhibitors
Within the scope of the present invention, and as used herein, the term
"cholesterol
biosynthesis inhibitor" refers to any compound having a negative effect on
systemic
cholesterol production by whatever mechanism. Non-limiting examples of such
compounds include: competitive inhibitors of 1 ) 3-hydroxy-3-methylglutaryl
coenzyme A
reductase " HMG CoA reductase", 2) 3-hydroxy-3-methylglutaryl coenzyme A
synthase
"HMG CoA synthase", 3) squalene synthase, and 4) squalene epoxidase..
The HMG CoA reductase inhibitors are more commonly known as "statins". These
agents have been used for primary and secondary prevention of coronary artery
disease. HMG CoA reductase is a key enzyme in the cholesterol biosynthetic
pathway.
Statins decrease liver cholesterol biosynthesis (approximately 50% of
circulating
cholesterol is endogenously synthesized, principally as LDL cholesterol),
which in turn
increases the production of LDL receptors thereby decreasing plasma total and
LDL
cholesterol'°. Depending on the agent and dose used, statins may also
decrease serum
triglycerides levels and increase serum HDL. Statins have become the standard
therapy
for LDL cholesterol lowering.
Dyspepsia, abdominal pain and flatulence are among the most common side
effects of
statin administration. The most severe adverse effects of statins are
elevations of the
serum transaminase levels and development of myositis.
18
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Myotoxicity is a common effect of all statins at high doses" The mechanism
appears to
be oxidative damage to mitochondria. Statins cause a drop in the
lactate/pyruvate
level'2. The lactate/pyruvate ratio is a sensitive measure of mitochondria)
dysfunction
and oxidative status'3 . It has been shown in clinical studies that statins
deplete an
essential cofactor required for energy production, coenzyme Q'4. The depletion
of
ctienzyme Q is dose dependent's. Coenzyme Q is an essential part of the
mitochondria) electron transport process which provides energy derived from
oxidative
processes. Statins work by blocking cholesterol synthesis at the HMG CoA
reductase
catalyzed step.
HMG CoA reductase (enzyme)
HMGCoA ------------------------------~ mevalonate
Mevalonate, through a series of enzymatic steps, is used to synthesize
cholesterol.
Mevalonate is also a precursor to coenzyme Q. The inhibition of cholesterol
synthesis
thereby inhibits the synthesis of coenzyme Q. While muscle cells, which have
high
energy requirements are the most susceptible to damage by statins, liver cells
are also
subject to injury. The latter is probably the result of the relatively hypoxic
condition of
the centrilobular liver cells in which the primary blood supply is from the
hepatic portal
system. The most serious form of muscle damage occurs when the muscle cell
contents are released into the systemic circulation (rhabdomyolysis). Major
complications include acute renal failure and cardiac abnormalities. The
cardiac toxicity
may be a direct effect of the statins on the heart muscle coenzyme Q levels.
These adverse effects are more common when statins are used in combination
with
other medications that inhibit the cytochrome P450 system, such as azole
antifungal
agents, cimetadine and methotrexate. The risk for statin-related myositis
increases in
patients taking gemfibrozil, nicotinic acid or macrolides.
19
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
References describing reported statin indications are widespread.'s,"''8
Even without the possibility of drug interactions, the primary side effect of
statins on
muscle is a considerable disincentive to patients to remain on such
medication. Since
muscle toxicity is dose dependent, any safe adjunct therapy that will allow
use of a
lower statin dose and still achieve the target LDL cholesterol level is highly
desirable.
Accordingly, there is a niche for compounds and compositions as provided
within the
scope of the present invention that can work additively with a statin to
provide an
additional lowering of LDL cholesterol without, at the same time, increasing
the risk of
an adverse reaction.
The follovring list comprises prefer r ed statins in actor dance ~~,~ith the
present inwnt~on
and their patent references, the latter of which are incorporated herein by
reference as
fully as if set forth herein:
Statin Patent Reference
Lovastatin (Mevacor) US Patent 4,231,938
Pravastatin (Pravachol) US patent 4,346,227
Simvastatin (Zocor) US Patent 4,739,073
Fluvastatin (Lesol) [syntheticallyUS Patent 4,739,073
derived]
Atorvastatin US Patent 5,273,995
Cerivastatin (Baycol) US Patent 5,177,080
Mevastatin US Patent 3,983,140
Cerivastatin US Patent 5,502,199
Velostatin US Patent 4,448,784
Compactin US Patent 4,804,770
Dalvastatin EP 738510 A2
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Fluindostatin EP 363934 A1
Dihydrocompactin US Patent 4,450,171
Pitavastatin --
Itavastatin --
The following list describes the chemical formulae of some preferred statins:
lovastatin: [1 S[1 a(R) 3 alpha, 7 beta, 8 beta (2S, 4S), 8a beta]]-
1,2,3,7,8,8a-
hexahydro-3,7-d imethyl-8-[2-(tetrahydro-4-hyd roxy-6-oxo-2H-pyran-2-yl)ethyl]-
1-
naphthalenyl-2-methylbutanoate.
pravastatin sodium: 1-naphthalene-heptanoic acid, 1,2,6,7,8a-hexahydro-beta,
delta,
6-trihydroxy-2-methyl-8-(2-ethyl-1-oxybutoxy)-1-monosodium salt [1S-[1
alpha(beta s,
delta, S), 2 alpha, 6 alpha, 8 beta (R), 8a alpha
simvastatin: butanoic acid, 2,2 dimethyl- 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-
8-[2-
(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester [1 S-[1
alpha, 3
alpha, 7 beta, 8 beta, (2S, 4S),-8a beta
sodium fluvastatin: [R, S(E)]-(+/-)-7-[3(4-fluurophenyl)-1-(1 methylethyl)-1 H-
indole-2-
yl]-3,5-dihydroxy-6-heptenoic acid, monosodium salt.
The present invention should not be considered to be limited to the foregoing
statins.
Naturally occurring statins are derivatives of fungi metabolites (ML-
236B/compactin/monocalin K) isolated from Pythium ultimum, Monacus ruber,
Penicillium citrinum, Pencillium brevicompactum and Aspergillus terreus,
though as
shown above, they can be prepared synthetically as well. Statin derivatives
are well
known in the literature and can be prepared by methods as disclosed in US
Patent
4,397,786. Other methods are well known in the art today.
21
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Structures of the most preferred statins for use in accordance with the
present invention
are as follows:
/ OH3C CH3 OH OH HO O
COOH
NH ~ ~N O
H
\/ ~ H3C O
CH3 H '~~H CH3
F
H3C, . ~ / /
Htorvastatin Lovastatin
,,OH HO O
Na+ '00C
HO
O ., O .
'H
H3C ~ ~O
CH3 n ~ ~ ~ ~HiCH3
Pravastatin sodium Simvastatin
Squalene synthase inhibitors decrease the activity of squalene synthase, thus
inhibiting
the conversion of farnesyl pyrophosphate into squalene. Squalene synthase
inhibitors
can act on squalene synthase directly or indirectly by:
1 ) decreasing the activity of one or more enzymes or cofactors involved in
the
22
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
activation or squalene synthase;
2) increasing the activity of one or more enzymes or cofactors involved in the
down
regulation of squalene synthase
Suitable squalene synthase inhibitors include, but are not limited to a-
phosphono-
sulfonates disclosed in US Patent No 5,712,396 including isoprenoid
(phosphinyl-
methyl)phosphonates as well as other known squalene synthetase inhibitors, for
example
as disclosed in US Patent No. 4,871,721, terpenoid pyrophosphates, farnesyl
diphosphate analog A and presqualene pyrophosphate analogs.
Preferably, for use accordance with the present invention, the cholesterol
biosynthesis
inhibitor is selected is from the group consisting of: a HMG Co A reductase
inhibitor
selected from the group consisting of lovastatin (for example MEVACOR~ which
is
available from Merck & Co.), pravastatin (for example PRAVACHOL~ which is
available
from Bristol Meyers Squibb), fluvastatin, simvastatin (for example ZOCOR~
which is
available from Merck & Co.), atorvastatin, cerivastatin, CI-981 and
pitavastatin (such as
NK-104 of Negma Kowa of Japan); HMG CoA synthetase inhibitors, for example L-
659,699 ((E,E)-11-[3'R-(hydroxyl-methyl)-4'-oxo-2'R-oxetanyl]-3,5,78-trimethyl-
2,4-
uridecadienoic acid); squalene synthesis inhibitors, for example squalestatin
1; and
squalene epoxidase inhibitors, for example, NB-598 ((E)-N-ethyl-N-(6,6-
dimethyl-2-
hepten-4-ynyl)-3-[(3,3'-bithiophen-5-yl)methoxy]benzene-methanamine
hydrochloride).
23
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Compounds
The compounds of the present invention comprise a sterol or stanol moiety and
a
cholesterol biosynthesis inhibitor moiety represented by one or more of the
following°
formulae:
a)
0
I~
Rz -(CHz)n -C -O
-R
b)
Rz - R
C)
O O
II II
Rz -C -C -O -R
d)
O
I I
R3 -P -O -R
I
OX
e)
0
I I
R2 R4 -P -O-R
-
I
OX
f)
O O
II II
R3 P -R4 -P -O
- -R
I I
OX OX
24
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
wherein R is the sterol or stanol moiety, R2 is a cholesterol biosynthesis
inhibitor with at
least one free and reactive carboxyl group; R3 is a cholesterol biosynthesis
inhibitor with
at least one free and reactive hydroxyl group; R4 is derived from ascorbic
acid, X is either
hydrogen or is selected from the group consisting of and n=1-5. The compounds
within
the scope of the present invention include all biologically acceptable salts
or solvates or
prodrugs of at least one such compound or of the salts or of the solvates
thereof.
In a preferred form of the compound, the cholesterol biosynthesis inhibitors,
R2 and R3,
are selected from the group consisting of competitive inhibitors of HMG CoA
reductase,
HMG CoA synthase, squalene synthase, and squalene epoxidase. In a further
preferred
embodiment of the present invention, R2 is either atorvastatin or pravastatin
sodium. In a
further preferred embodiment, R3 is either simvastatin or lovastatin.
It is important to note that, the formation of compounds a), b), c) and e) can
only be
achieved by selecting a cholesterol biosynthesis inhibitor with at least one
free and
reactive carboxyl group. Likewise, the formation of compounds d) and f) can
only be
achieved by selecting a cholesterol biosynthesis inhibitor with at least one
free and
reactive hydroxyl group. While, appropriate cholesterol biosynthesis
inhibitors must be
selected on this basis, it is entirely within the purview of even a student of
chemistry to do
so.
In a most preferred aspect of th.e present invention, the compound formed
between the
sterol and/or stanol moiety and selected cholesterol biosynthesis inhibitor is
selected from
the group consisting of:
Compound 1: sodium ascorbyl phytostanyl atorvastatin phosphate ester
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Compound 2: disodium ascorbyl phytostanyl pravastatin phosphate ester
R = H, CH3
O
~ ~ ~''H
H3C~0 H ,.H
H3 ~~ /~3
Compound 3: sodium ascorbyl phytostanyl simavastatin phosphate ester
O H
HO O 0
H Fi
HO ~p~ ~~O
O' Na' H R = H, CH3
~~O
Na' ~O/ ~~ O
O
H
H3C~0
H3C CH3 ' H '~H CH
3
H C,~~ / /
26
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Compound 4: disodium ascorbyl phytostanyl lovastatin phosphate ester
R
0 H /
HO O O
II H R
0~~~0 =
n O~ Na' I-I R = H, G'i3
Na'
O
~ ~ _ H
H3C' Y '0
I H ,.H
~3 ~3
HC,,~ / /
Compound 5 Sitostanol-simvastatin phosphate ester
0 O H
O H R = H, CH3
H3C~0
H3C CH3 - H ~'H CH
3
H3C, , ~ / /
Compound 6 Sodium salt of sitostanol (or campestanol) lovastatin phosphate
ester
27
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
R
H /
O H H v
Na' -O~ ~~O
H R = H, CH3
O O
O
H
H3~~ H ..H
CH3 CH3
~C,. / / .
Compound 7 Sitostanol atorvastatin ester
Compound 8 Sitostanol pravastatin ester
H3C~
28
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Optionally, the compounds of the present invention are formed of naturally-
derived or
artificially synthesized beta-sitosterol, campestanol, sitostanol, and
campesterol and each
of these compounds so formed is then admixed in a pharmaceutical composition
prior to
delivery in various ratios. In the most preferred form, the compound of the
present
invention comprises a chemical linkage between one or more disodium ascorbyl
phytostanyl phosphates (referred to herein as "FM-VP4") which comprises two
major
components: disodium ascorbyl campestanyl phosphate ("DACP") and disodium
ascorbyl
sitostanyl phosphate ("DASP").
Salts
As a sed h ere!n, the ter,~; "biologically acceptable salts" refers any salts
that retain the
desired biological and/or physiological activity of the compounds as described
herein and
exhibit minimal undesired toxicological effects. Accordingly, reference to
compounds of
formulae a) through f) thereby includes reference to acidic and/or base salts
thereof,
formed with inorganic and/or organic acids and bases.
Exemplary acid addition salts include acetates (such as those formed with
acetic acid or
trihaloacetic acid, for example trifluroacetic acid), adipates, alginates,
ascorbates,
aspartates, benzoates, benzenesulfonates, bisulfates, borates, butyrates,
citrates,
camphorates, camphorsulfonates, cyclpentanepropionates, digluconates,
dodecylsulfates, heptanoates, hexanoates, hydrochlorideshyrobromides,
hydroiodides, 2-
hydroethanesulfonates, lactates, maleates, methanesulfonates, 2-
naphthalenesulfonates,
nicotinates, nitrates, oxalates, pectinates, persulfonates, 3-
phenylpropionates,
phosphates, picrates, pivalates, propionates, salicylates, succinates,
sulfates, sulfonates,
tartrates, thiocyantes, toluenesulfonates, undecanoates and the like.
Those compounds which contain an acid moiety may form salts with a variety or
organic
and inorganic bases.
29
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
Accordingly, the present invention encompasses not only the parent compounds
comprising the selected sterol and/or stanol and cholesterol biosynthesis
inhibitor but:
also, where possible (i.e. where the parent contains a free hydroxyl group),
the present
invention encompasses the biologically acceptable metal, alkali earth metal,
or alkali
metal salts of the disclosed compounds.
The salts, as described herein, are even more water soluble than the
corresponding
parent compounds and therefore their efficacy and evaluation both in vitro and
in vivo
may be enhanced.
Salt formation of the compounds of the present invention can be readily
performed; for
example, by treatment of any parent compound containing a free OH group with a
series
of bases (for example, sodium methoxide or other metal alkoxides) to produce
the
corresponding alkali metal salts. Other metal salts of calcium, magnesium,
manganese,
copper, .zinc, and the like can be generated by reacting the parent with
suitable metal
alkoxides.
Compound Formation
There. are many processes by which novel compounds comprising sterols and/or
stanols and the selected cholesterol biosynthesis inhibitors can be formed. In
one
process, wherein the cholesterol biosynthesis inhibitor has at least one free
and
reactive carboxyl group, the selected sterol or stanol (or halophosphate,
halocarbonate
or halo-oxalate derivatives thereof) and the cholesterol biosynthesis
inhibitor are mixed
together under reaction conditions to permit condensation of the "acid" moiety
with the
"alcohol" (phytosterol). These conditions are the same as those used in other
common
esterification reactions in which the acid chloride formed from the acid
component and
the alcohol component are allowed to react directly or in the presence of a
suitable acid
catalyst such as mineral acid, sulfuric acid, phosphoric acid, p-
toluenesulfonic acid. The
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
organic solvents generally employed in such esterification reactions are
ethers such as
diethyl ether, tetrahydrofuran, or benzene, toluene or similar aromatic
solvents and th~
temperatures can vary from room to elevated temperatures depending on the
reactivit~r
of the reactants undergoing the reaction.
In another preferred embodiment, the process to form the ester derivative
comprises
"protecting" the hydroxyl groups of the cholesterol biosynthesis inhibitor as
esters (for
example, as acetate esters) or ethers (for example, methyl ethers) and then
condensing
the protected cholesterol biosynthesis inhibitor with the reactive
sterol/stanol (or its
halophosphate, halocarbonate or halo-oxalate) under suitable reaction
conditions. In
general, such condensation reactions are conducted in an organic solvent such
as diethyl
ether, tetrahydrofuran, or benzene, toluene or similar aromatic solvents.
Depending on
the nature and reactivity of the reactants, the reaction temperatures may vary
from low (-
15°C) to elevated temperatures.
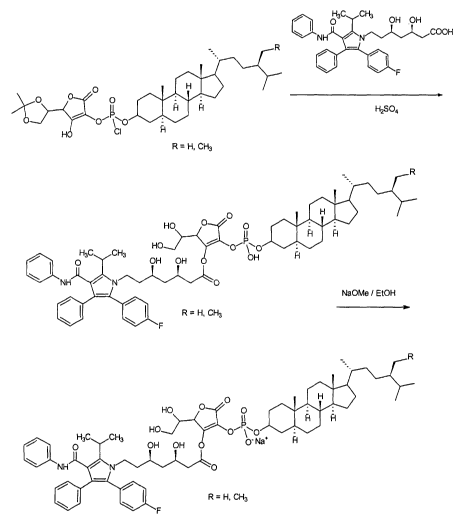
By way of non-limiting example, Figure 1 is a schematic showing the formation
of
ascorbyl sitostanyl or campestanyl atorvastatin phosphate esters and their
sodium salts.
The starting material, prepared by a previously developed method , is
condensed with
atorvastatin in the presence of sulfuric acid as catalyst, in a classical
esterification
process, to form the coupled product. The latter is then treated with sodium
methoxide to
obtain the sodium salt as the final product.
In another non-limiting alternative, exemplified in the schematic of Figure 2,
the process
for the preparation of ascorbyl sitostanyl or campestanyl simvastatin
phosphate ester and
their sodium salts is described. The starting material is obtained by treating
the starting
material already shown in Figure 1, with phosphorus oxychloride to afford the
dichlorophosphate shown. The latter is then treated with simvastatin whereupon
the free
hydroxyl group of the statin, displaces the halogen atom of the more reactive
chlorophosphate group, to afford the coupled product. Acidification with
mineral acid,
31
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
followed by subsequent treatment with sodium methoxide generates the final
product as
the disodium salt.
In another non-limiting alternative, exemplified in the schematic of Figure 3,
the process.
for the preparation of sitostanyl or campestanyl simvastatin phosphate ester
is described.
Sitostanol or campestanol, upon treatment with phosphorus oxychloride, affords
the
corresponding chlorophosphate ester. This intermediate is then reacted with
simvastatin
in a m fixture o f p yridine/THF i n o rder t o a ffect t he d isplacement of
the chloride by the
hydroxyl group of the statin. The resulting coupled product is treated with
water to
complete the synthesis of the final phosphate ester.
In another nonlimiting alternative; exemplified in the schematic of Figure 4,
the process
for the preparation of sitostanyl or campestanyl atorvastatin carboxylic ester
is described.
Sitostanol or campestanol is treated with atorvastatin in the presence of
sulfuric acid as
catalyst to afford the ester via the classical esterification process.
With respect to the formation of these derivatives, it is to be appreciated
that, while
selected synthesis processes are described, there are a number of other means
by which
the variety of derivatives disclosed and claimed can be made. It is well
within the purview
of a skilled person in this chemical field, once a particular derivative is
chosen, to
undertake the synthesis using commonly available techniques in the art. For
this reason,
the complete synthesis of each and every claimed derivative is not described.
To the extent that the compounds as described herein and salts thereof, may
exist in their
tautomeric form; all such tautomeric forms are contemplated herein as part of
the present
invention.
All stereoisomers of the compounds of the present invention, such as those
which may
exist due to asymmetric carbons on various constituents, including
enantiomeric forms
32
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
(which may exist even in the absence of asymmetric carbons) and diastereomeric
forms,.
are contemplated within the scope of the present invention. Individual
stereoisomers of=
the compounds of the present invention may, for example, be admixed as
racemates or
with all other, or other selected sterioisomers. The chiral centres of the
compounds can
have the S or R configuration as defined by the IUPAC 1974 Recommendations.
When
diastereomeric or enantomeric products are prepared, they can be separated by-
conventional methods, for example, chromatographic or fractional
crystallization.
Compositions
According to another aspect of the present invention, there are provided novel
compositions comprising at least one sterol/stanol based cholesterol
absorption
inhibitor, as described herein, admixed with at least one cholesterol
biosynthesis
inhibitor, which compositions are suitable for use in treating or preventing
CVD and its
underlying conditions including, without limitation, atherosclerosis,
hypercholesterolemia,
hyperlipidemia, dislipidemia, hypertension, thrombosis, coronary artery
disease, and .
inflammation including coronary plaque inflammation.
More specifically, the composition comprises:
a) at least one cholesterol absorption inhibitor selected from compounds
having the
general formulae:
i)
i1
R4 -P -O -R
I
OH
ii)
O O
fl II
R4 -C -C -O -R
iii)
33
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
R4-R
iv)
O
R4 -(CH2)n -C -0 -R
wherein R is a sterol or stanol moiety, R4 is derived from ascorbic acid and
n=1-5,
including all biologically acceptable salts or solvates or prodrugs of at
least one such
compound or of the salts or of the solvates thereof; and
b) at least one cholesterol biosynthesis inhibitor.
The compounds of formulae i) to iv) can be prepared by known methods, for
example
those described below and in PCT/CA00/00730, which was filed on June 20, 2000
and
claims priority back to US Patent Application 09/339,903 filed on June 23,
1999, the
entire contents of which are incorporated herein by reference..
In general, compounds of formulae i) to iv) can be prepared as follows: the
selected
sterol o r s tanol ( or h alophosphate, h alocarbonate or halo-oxalate
derivatives thereof)
and ascorbic acid are mixed together under reaction conditions ~fo permit
condensation
of the "acid" moiety with the "alcohol" (sterol). These conditions are the
same as those
used in other common esterification reactions such as the Fisher
esterification process
in which the acid component and the alcohol component are allowed to react
directly or
in the presence of a suitable acid catalyst such as mineral acid, sulfuric
acid,
phosphoric acid, p-toluenesulfonic acid. The organic solvents generally
employed in
such esterification reactions are ethers such as diethyl ether,
tetrahydrofuran, or
benzene, toluene or similar aromatic solvents and the temperatures can vary
from room
to elevated temperatures depending on the reactivity of the reactants
undergoing the
reaction.
34
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
In a preferred embodiment, the process to form the ester comprises
"protecting" the
hydroxyl groups of the ascorbic acid or derivatives thereof as esters (for
example, as.
acetate esters) or ethers (for example, methyl ethers) and then condensing the
protected
ascorbic acid with the sterol/stanol halophospahte, halocarbonate or halo-
oxalate under
suitable reaction conditions. In general, such condensation reactions are
conducted in an
organic solvent such as diethyl ether, tetrahydrofuran, or benzene, toluene or
similar
aromatic solvents. Depending on the nature and reactivity of the reactants,
the reaction
temperatures may vary from low (-15°C) to elevated temperatures.
In more detail, the following is one preferred mode of preparing the compounds
of
formulae i) to iv) and in particular formula i): ascorbic acid is initially
protected from
decomposition by the formation of 5,6-isopropylidene-ascorbic acid. This can
be
achieved by mixing acetone with ascorbic acid and an acidic catalyst such as
sulfuric acid
or hydrochloric acid under suitable reaction conditions. Phytostanol
chlorophosphate is
prepared by forming a solution of phytostanol in toluene and pyridine
(although other
nitrogen b ases s uch a s a liphatic a nd a romatic a mines m ay a
Iternatively be used) and
treating this solution with a phosphorus derivative such as phosphorus
oxychloride. The
residue so formed after filtration and concentration of t he m other I iquor i
s p hytostanol
chlorophosphate. The latter is then mixed with 5,6-isopropylidene-ascorbic
acid and,
after the addition of a suitable alcohol such as ethanol and HCI,
concentrated.
Alternatively, pyridine/THF may be added and the product concentrated. After
final
washing and drying, the resultant novel product a stanol-phosphate-ascorbate.
In another preferred form of the process to prepare compounds of formulae i)
to iv),
ascorbic a cid is protected at the hydroxyl sites not as 5,6-isopropylidene-
ascorbic acid
but as esters (for example as acetates, phosphates and the like..). The latter
may then
be condensed with sterols or stanols, derivatized as described above, using
known
esterification methods ultimately to produce the compounds. The formation of
mono and
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
diphosphates of ascorbic acid is described thoroughly in the literature. For
example, US
Patent Serial No. 4,939,128 to Kato et al., the contents of which are
incorporated herein
by reference, teaches the formation of phosphoric acid esters of ascorbic
acid. Similarly,
US Patent Serial No. 4,999,437 to Dobler et al., the contents of which are
also fully
incorporated herein by reference, describes the preparation of ascorbic acid 2-
phosphate.
In Dobler et al., the core reaction of phosphorylating ascorbic acid or
ascorbic acid
derivatives with POCI3 in the presence of tertiary amines (described in German
Laid
Open Application DOS 2,719,303) is improved by adding to the reaction solution
a
magnesium compound, preferably an aqueous solution of a magnesium compound.
Any
of these known ascorbic acid derivatives can be used.
In more detail, the following is another preferred mode of preparing the
compounds of
formulae i) to iv) and in particular formula ii): prepare the "protected"
ascorbic acid and
follow the same process outlined in detail above; however, the phosphorus
oxylchloride is
replaced by oxalyl chloride thereby yielding a stanol-oxalate-ascorbate.
In a preferred form, the composition of the present invention comprises one or
more
disodium ascorbyl phytostanyl phosphates (referred to as "FM-VP4") which
comprises
two major components: disodium ascorbyl campestanyl phosphate ("DACP") and
disodium ascorbyl sitostanyl phosphate ("DASP") together with at least one
statin.
In another preferred embodiment, the sterol and/or stanol moiety may be
incorporated
into a micelle prior to or after combining with the selected cholesterol
biosynthesis
inhibitor. T his m icelle c an b a p roduced a sing I ecithin o r a ny other
suitable emulsifying
agent and using techniques known and applied widely in the art.
The compositions of the present invention allow for a "combination therapy"
wherein the
cholesterol absorption inhibitor and the cholesterol biosynthesis inhibitor
are either co-
administered in a substantially simultaneous manner, for example, in a single
tablet or
36
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
capsule having a fixed ratio of active ingredients or in multiple, separate
administrations
for each therapeutic agent. This separate administration includes sequential
dosage
forms.
Methods of Use
The present invention provides a method of achieving one or more of the
following
therapeutic goals:
a) preventing, treating or alleviating one or more conditions associated with
CVD
generally and including arteriosclerosis, atherosclerosis, arteriolosclerosis,
angina pectoris, and thrombosis;
b) reducing a nd/or a liminating o ne o r more of the risk factors associated
with
CVD;
c) preventing, treating or alleviating atherosclerosis;
d) preventing, treating or alleviating hypercholesterolemia;
e) preventing, treating or alleviating a hyperlipidic condition;
f) preventing, treating or alleviating dislipidemia;
g) preventing, treating or alleviating hypertension;
h) preventing, treating or alleviating coronary artery disease;
i) preventing, treating or alleviating coronary plaque development;
j) preventing, treating or alleviating coronary plaque inflammation;
k) lowering serum LDL cholesterol;
I) increasing serum HDL cholesterol;
m) decreasing serum triglycerides levels;
n) decreasing cholesterol biosynthesis;
o) preventing, reducing, eliminating or ameliorating a dislipidemic condition
or
disorder;
p) preventing, reducing, eliminating or ameliorating hypercholesterolemia or
37
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
hypoalphalipoproteinemia;
q) preventing, reducing, eliminating, stabilizing or ameliorating the
development of=
atherosclerotic lesions or plaque;
r) preventing, reducing, eliminating, or ameliorating the development of=
inflammation associated with the development of cardiovascular disease and
coronary artery disease;
s) preventing, reducing, eliminating or ameliorating any condition, disease or
disorder which has as its basis or which is exacerbated by a deficiency in
plasma HDL, or by an excess of either LDL, VLDL, Lp(a), beta-VLDL, IDL or
remnant lipoproteins;
t) decreasing the risk of a stroke;
u) inhibiting isoprenoid synthesis;
v) preventing, treating or alleviating Alzheimer's disease;
w) preventing, treating or alleviating dementia;
x) preventing, treating or alleviating osteoporosis;
y) preventing, reducing, eliminating or ameliorating injuries due to oxidative
stress;
z) enhancing and/or preserving the stability of HDL from oxidation;
aa)enhancing and/or preserving the stability of LDL, VLDL or IDL from
oxidation;
bb)enhancing and/or preserving the stability of triglyceride (TG) from
oxidation;
cc) exhibiting anti-coagulatant properties;
dd)exhibiting anti-proliferative properties;
ee)exhibiting immunomodulatory properties;
ff) exhibiting angiogenic properties;
gg) preventing, treating or alleviating tumour growth;
hh)increasing bone mass and/or bone turnover; and
ii) enhancing any of the non-lipid related, pleiotropic effects achieved by
the
administration of statins, in particular at the cellular and molecular level,
which comprises administering to an animal, a non-toxic and therapeutically
effective
amount of a compound or composition as described and claimed herein. This
invention
38
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
further comprises the use of any of the disclosed compounds and compositions
for any
indications described herein, more specifically, for use in achieving one or
more of the
therapeutic goals as defined above.
In particular, the compounds and compositions of the present invention have
been found
to be especially useful in addressing at least two significant factors
contributing to the
multi-factorial presentation of cardiovascular disease: intestinal cholesterol
absorption
and systemic cholesterol biosynthesis. Serum cholesterol levels are controlled
primarily
by two organs: the liver, which produces cholesterol and bile acids (which are
used in
digestion), and the intestine, which absorbs cholesterol both from food and
from the bile
(produced by the fiver). Sterols lower LDL serum cholesterol levels through a
unique
mechanism of action by inhibiting cholesterol absorption in the intestine.
This
mechanism of action makes sterols complementary to cholesterol biosynthesis
inhibitors, such as statins, which work in the liver. Therefore, patients who
take sterols
with statins can achieve additional reductions in LDL and total cholesterol.
Accordingly, it is highly advantageous to administer one moiety which
simultaneously
lowers cholesterol absorption (for example: sterols/stanols), and another
moiety which
decreases cholesterol biosynthesis (for example: statins). This can be
achieved either
through the administration of at least one of the compounds of formulae a)
through f) or
through the administration of a composition comprising a cholesterol
absorption inhibitor
(having one of the formulae i) through iv)) and a cholesterol biosynthesis
inhibitor.
Ultimately, the most important benefits derived from use of the compounds and
compositions described herein are a decrease in serum LDL cholesterol and a
decrease
in total serum cholesterol, with additional beneficial effects being achieved
via an increase
in serum HDL cholesterol and a decrease in serum triglycerides. These benefits
are
achieved without the adverse effects associated with statin administration.
39
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
In addition, with respect to the compounds of the present invention, there is
a significant
biological consequence (and benefit) of changing the overall hydophobicity of
the
cholesterol biosynthesis inhibitors (such as the statins) by covalently
modifying them
with the sterol-based cholesterol absorption inhibitors as described herein.
Specifically, the compounds of the present invention have a higher overall
hydrophobicity than the native statins. This is important on at least two
fronts. Firstly,
there may be an enhancement of the pleotropic effects of the statins.
Secondly, if the
compounds are absorbed in tact, it is likely that the ADME properties of the
statins
might be different, thereby decreasing their potential toxicities, among other
benefits.
While not intending to be bound by any one theory as to mechanism of action,
it is
possible that since the structural changes in the compound as compared to free
statins
will result in a higher proportional distribution into the plasma lipoprotein
pool, the active
components will be delivered in greater proportion to peripheral tissues,
especially if the
drug is carried by HDL. This may significantly enhance the potential effect of
the
compounds of the present invention on many reported "statin" indications,
including
Alzheimer's disease and osteoporosis.
Furthermore, some of the compounds of the present invention (those depicted in
formulae (e) and (f) and in i) through iv)) comprise an ascorbyl moiety. These
particular
compounds have numerous added advantages. First and foremost, solubility of
the
compounds is greatly enhanced, both in aqueous solutions and non-aqueous media
such
as oils and fats. With this greater solubility, effective dietary and
therapeutic dosages and
concomitantly costs, can be reduced. Secondly, it is very likely that there is
even a
synergistic or additive effect between the phytosterol moiety and the ascorbic
acid, when
united in one structure, in treating or preventing not only cardiovascular
disease and its
underlying conditions including atherosclerosis and hyperlipidemia. Thirdly,
the formation
of these compounds allows the full potential of ascorbic acid to be realized
while
eliminating decomposition. Fourthly, these derivatives are heat stable (stable
to oxidation
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
and hydrolysis) which is essential for some processing mechanisms.
The desired effects d escribed h erein m ay b a a chieved i n a n umber o f d
ifferent w ays.
The compounds and compositions of the present invention may be administered by
any
conventional means available for use in conjunction with pharmaceuticals.
Accordingly,
the present invention relates, on aspect, to a pharmaceutical composition
comprising one
or more of the compounds of formulae a) to f) and a pharmaceutically
acceptable carrier.
The present invention relates, in another- respect, to a pharmaceutical
composition
comprising an effective or therapeutic amount of at least one cholesterol
absorption
inhibitor having one of formulae i)-iv) together with an effective or
therapeutic amount at
least one cholesterol biosynthesis inhibitor and a pharmaceutically acceptable
carrier.
These compounds and/or compositions can be administered in any conventional
dosage
form, preferably an oral dosage form such as a tablet, capsule, powder,
cachet,
suspension or solution. The pharmaceutical compositions can comprise from
about 1 % to
99% of the "active" components (cholesterol absorption inhibitors and
cholesterol
biosynthesis inhibitors) and preferably from about 5% to 95% of the active
components.
The formulations and pharmaceutical compositions can be prepared using
conventional,
pharmaceutically available excipients, and additives and by conventional
techniques.
Such pharmaceutically acceptable excipients and additives include non-toxic
compatible
fillers, binders, disintegrants, buffers, preservatives, anti-oxidants,
lubricants, flavourings,
thickeners, colouring agents, emulsifiers and the like.
The exact amount or dose of the compound or composition which is required to
achieve
the desired effects will, of course, depend on a number of factors such as the
particular
compound or composition chosen, the potency of the compound or composition
administered, the mode of administration and the age, weight, condition and
response of
the patient. All of these factors, among others, will be considered by the
attending
clinician with respect to each individual or patient.
41
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
For the pharmaceutical compositions comprising at least one cholesterol
absorption
inhibitor having one of formulae i)-iv) together with at least one cholesterol
biosynthesis
inhibitor and .a pharmaceutically acceptable carrier, the typical daily dose
of the
cholesterol biosynthesis inhibitor can range from about 0.1 mg to 160mg/kg and
preferably
from 2mg to 80mg of mammalian body weight per day administered in single or
divided
doses, a sually o nce o r twice a d ay. F or example, for HMG CoA reductase
inhibitors,
about 0.25mg to 40mg per dose is given one to two times per day, giving a
total daily
dose of from about 0.5mg to 80mg. With respect to other cholesterol
biosynthesis
inhibitors, about 1 mg to about 1000mg per dose is given one or two times per
day, giving
a total daily dose of 1 mg to about 2000mg per day.
Generally, a total daily dose the cholesterol absorption inhibitor having one
of formulae
i)-iv) and comprising sterols and/or stanols may be administered in a daily
dosage range
of from 10mg to about 20 g, more preferably 10mg to 1.5g, per day in single or
multiple
divided doses.
When the components of this composition are administered separately, the
number of
doses and the amount of such dosage of each component given per day may not
necessarily be the same. For example, it is possible that the cholesterol
absorption
inhibitor may require either a greater number of administrations per day than
the
cholesterol biosynthesis inhibitor and/or may require a larger dosage.
The daily dose of these compositions and compounds can be administered to an
individual in a single dose or in multiple doses, as required. Sustained
release dosages
can be used.
Use of pharmaceutically acceptable carriers to formulate the compounds and
compositions herein disclosed for the practice of the invention into dosages
suitable for
42
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
systemic administration is within the scope of the invention. With proper
choice of
carrier and suitable manufacturing practice, the compounds and compositions of
the
present invention, in particular, those formulated as solutions, may be
administered
parenterally, such as by intravenous injection. The compounds and compositions
can
be formulated readily using pharmaceutically acceptable carriers well known in
the art
into dosages suitable for oral administration. Such carriers enable the
compounds and
compositions of the invention to be formulated as tablets, pills, capsules,
liquids, gels,
syrups, slurries, suspensions and the like, for oral ingestion by a patient to
be treated.
Pharmaceutical compositions, comprising one or more of the compounds of the
present
invention, include compositions wherein the active ingredients are contained
in an
effective amount to achieve their intended purpose. Determination of the
effective
amounts is well within the capability of those skilled in the art, especially
in light of the
detailed disclosure provided herein.
In addition to the active ingredients these pharmaceutical compositions may
contain
suitable pharmaceutically acceptable carriers comprising excipients and
auxiliaries
which facilitate processing of the active compounds into preparations which
can be
used pharmaceutically. The preparations formulated for oral administration may
be in
the form of tablets, dragees, capsules, or solutions.
The pharmaceutical compositions of the present invention may be manufactured
in a
manner that is itself known, e.g., by means of conventional mixing,
dissolving,
granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping
or
lyophilizing processes.
Pharmaceutical formulations for parenteral administration include aqueous
solutions of
the active compounds in water-soluble form. Additionally, suspensions of the
active
compounds may be prepared as appropriate oily injection suspensions. Suitable
43
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
lipophilic solvents or vehicles include fatty oils such as sesame oil, or
synthetic fatty
acid esters, such as ethyl oleate or triglycerides, or liposomes. Aqueous
injection
suspensions may contain substances which increase the viscosity of the
suspension,
such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the
suspension may also contain suitable stabilizers or agents which increase the
solubility
of the compounds to allow for the preparation of highly concentrated
solutions.
Pharmaceutical preparations for oral use can be obtained by combining the
active
compounds with solid excipient, optionally grinding a resulting mixture, and
processing
the mixture of granules, after adding suitable auxiliaries, if desired, to
obtain tablets or
dragee cores. Suitable excipients include lactose, sucrose, mannitol,
sorbitol, maize
starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth,
methyl
cellulose, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, and
polyvinylpyrrolidone (PVP). If desired, disintegrating agents may be added,
such as the
cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof
such as sodium
alginate.
Dragee cores are provided with suitable coatings. For this purpose,
concentrated sugar
solutions may be used, which may optionally contain gum arabic, talc,
polyvinyl
pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium dioxide,
lacquer solutions,
and suitable organic solvents or solvent mixtures. Dyestuffs or pigments may
be added
to the tablets or dragee coatings for identification or to characterize
different
combinations of active compound doses.
Pharmaceutical preparations which can be used orally include push-fit capsules
made
of gelatin, as well as soft, sealed capsules made of gelatin and a
plasticizer, such as
glycerol or sorbitol. The push-fit capsules can contain the active ingredients
in
admixture with filler such as lactose, binders such as starches, and/or
lubricants such
as talc or magnesium stearate and, optionally, stabilizers. In soft capsules,
the active
44
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
compounds may be dissolved or suspended in suitable liquids, such as fatty
oils, liquid
paraffin, or liquid polyethylene glycols. In addition, stabilizers may be
added.
Oral liquid preparations may be in the form of, for example, emulsions,
syrups, or elixirs,
or may be presented as a dry product for reconstitution with v~iater or other
suitable
vehicle before use. Such liquid preparations may contain conventional
additives such as
suspending agents, for example sorbitol, syrup, methyl cellulose, gelatin,
hydroxyethylcellulose, carboxymethylcellulose, aluminium stearate gel,
hydrogenated
edible fats; emulsifying agents, for example lecithin, sorbitan monooleate, or
acacia; non-
aqueous vehicles (which may include edible oils), for example almond oil,
fractionated
coconut oil, oily esters such as esters of glycerine, propylene glycol, or
ethyl alcohol;
preservatives, for example methyl or propy! p-hydroxybenzoate or sorbic acid;
and !f
desired conventional flavouring or colouring agents.
Since the present invention relates to compositions with a combination of
active
ingredients which may be administered together or separately, there are also
provided
herein kits for such purpose. A kit is contemplated wherein two separate units
are
combined: a pharmaceutical composition comprising at last one cholesterol
biosynthesis
inhibitor, as described herein, and a separate pharmaceutical composition
comprising at
least one cholesterol absorption inhibitor, as described herein. The kit will
preferably
include directions for the administration of the separate components. This
type of kit
arrangement is particularly useful when separate components must be
administered in
different dosage forms (for example, oral and parenteral) or are administered
at different
dosage intervals.
Without further elaboration, the foregoing so fully illustrates the present
invention that
others may, by applying current or future knowledge, adapt the same for use
under the
various conditions described and claimed herein.
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
REFERENCES
1. Levi RI Declining Mortality in Coronary Heart Diseases Atherosclerosis
1981; 1:312-
325
2. Law M.R., Wald N.J., Wu., Hacksaw ZA., Bailey A.; Systemic underestimation
of
association between serum cholesterol concentration and ischemic heart disease
in
observational studies: Data from BUPA Study; 8r. Med. J. 1994; 308:363-366
3. Law M.R., Wald N.J., Thompson S.G.; By how much and how quickly does
reduction in
serum cholesterol concentration lower risk of ischemic heart disease? Br. Med.
J. 1994;
308:367-373
4. La Rosa J.C., Hunninghake D.. Bush D. et al.; The cholesterol facts: A
summary of the
evidence relating to dietary fats, serum cholesterol and coronary heart
disease: A joint
statement by the American Heart Association and the National Heart, Lung and
Blood
Institute. Circulation 1990; 81:1721-1733
5. Have) R.J., Rapaport E.. Drug Therapy: Management of Primary
Hyperlipidemia. Nevi
England Journal of Medicine, 1995; 332:1491-1498
6. Barker and Rye Atherosclerosis 1996; 121:1-12
7. Kuccodkar et al.; Effects of plant sterols on cholesterol metabolism.
Atherosclerosis,
1976; 23:239-248
8. Lees R.S., Lees A.M. Effects of sitosterol therapy on plasma lipid and
lipoprotein
concentrations. In: Greten H (Ed) Lipoprotein Metabolism. Springer-Verlag,
Berlin,
Heidelberg, New York, 1976:119-124
9. Lees A.M., Mok H.Y.I., Lees R.S., McCluskey M.A., Grundy S.M. Plant sterols
as
cholesterol-lowering agents: clinical trials in patients with
hypercholesterolemia and
studies of sterol balance. Atherosclerosis 1977; 28: 325-338
10.
11. von Keutz E, Schluter G. ~Preclinical safety evaluation of cerivastatin, a
novel HMG-
CoA reductase inhibitor. Am J Cardiol 1998 Aug 27;82(4B):11 J-17J
12. De Pinieux G, Chariot P, Ammi-Said M, Louarn F, Lejonc JL, Astier A,
Jacotot B,
Gherardi R. Lipid-lowering drugs and mitochondria) function: effects of HMG-
CoA
reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br
J Clin
Pharmacol 1996 Sep;42(3):333-7.
46
CA 02531836 2006-O1-09
WO 2005/005453 PCT/CA2004/000999
13. Chariot P, Monnet I, Mouchet M, Rohr M, Lefaucheur JP, Dubreuil-Lemaire
ML,
Chousterman M, Gherardi R. Determination of the blood lactate:pyruvate ratio
as a
noninvasive test for the diagnosis of zidovudine myopathy. Arthritis Rheum
1994
Apr;37(4):583-6
~a. Miyake Y, Shouzu A, Nishikawa M, Yonemoto T, Shimizu H, Omoto S, Hayakawa
T,
Inada M. Effect of treatment with 3-hydroxy-3-methylglutaryl coenzyme A
reductase
inhibitors on serum coenzyme Q10 in diabetic patients. Arzneimittelforschung
1999
Apr;49(4):324-9
15. iJloi ei sen SA, Leth A, Agner E, Rohde iii. Dose-related decrease of
serum
coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects
Med
1997;18 SuppI:S137-44
16. Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond
lipid
lowering. Cardiology Division, Department of Medicine, Weill Medical College
of Cornell
University, New York, New York 10021, USA. c'v2t 001(a~med.cornell.edu Am J
Cardiol.
2003 Feb 20;91 (4A):23B-29B.
17. Brinton EA. Lipid abnormalities in the metabolic syndrome. Carl T. Hayden
VA
Medical Center, Section of Metabolism, Endocrinology, & Nutrition, Department
of
Internal Medicine, 111 E, 650 East Indian School Road, Phoenix, AZ 85012, USA.
Curr
Diab Rep. 2003 Feb;3(1 ):65-72.
18. Siegel-Axel DI. Cerivastatin: a cellular and molecular drug for the
future?
Department of Medicine III (Cardiology), University of Tubingen, Otfried-
Muller St. 10,
D-72076 Tubingen, Germany. daaxel a~med.uni-tuebingen.de Cell Mol Life Sci.
2003
Jan;60(1 ):144-64
47