Note: Descriptions are shown in the official language in which they were submitted.
CA 02532749 2006-O1-13
-1-
TITLE OF THE INVENTION
PROCESS FOR PARTICLE SIZE REDUCTION OF
GLASS-LIKE POLYSACCHARIDES
FIELD OF THE INVENTION
The present invention relates to a process for reducing
the particle size of glass-like polysaccharides.
BACKGROUND OF THE INVENTION
Glass-like polysaccharides are a special class of
physically modified polysaccharides. Unlike their native, crystalline
counterpart, glass-like polysaccharides are amorphous and have glass-like
characteristics. Glass-like polysaccharides do not possess an organized
crystalline pattern, making them more suitable as absorbent materials.
Glass-like polysaccharides have found use in a variety of applications.
Glass-like polysaccharides have been described as being
useful in a variety of food related applications. More specifically, they have
been used to encapsulate organoleptic additives (Carrell, P. K. US P
3,706,598; Spratt et al. CA 1,319,045; Sair et al. US P 4,232,047; Galluzzi
et al. US P 3,922,354; Saleeb et al. US P 5,972,395, US P 4,820,534, US
P 4,532,145; Levine et al. US P 5,009,900; Fulger et al. US P 5,958,502;
Franke et al. US P 5,846,580; Willibald-Ettle et al. US P 6,582,753, US P
6,248,386).
Glass-like polysaccharides may sometimes comprise
occluded moisture. This occluded moisture has an effect on the hardness
and the brittleness of the glass-like polysaccharides, rendering them less
abrasive and making them very suitable as gentle abrasive grits (Lane et
al. US P 5,066,335, US P 5,360,903, US P US 5,367,068; Koutlakis et al.
US P 6,159,257, US App. 2004/157532; Drake et al. US P 6,726,536, US
App. 2004/121,707).
CA 02532749 2006-O1-13
-2-
Glass-like polysaccharides have also been described as
water absorbent materials (Huppe et al. CA 2,308,537; Thibodeau et al. CA
2,462,053; Berrada et al. CA 2,483,049; Drake et al. US App.
2004/244,652).
In the above-cited applications, the glass-like
polysaccharides are ground to obtain a particulate form. Several useful
techniques for particle size reduction have been described by Richard et al.
(ferry's Chemical Engineers' Handbook, 7t" ed., ferry et al., 1997,
McGraw-Hill, s. 20, p. 1-56). Moreover, Richard et al. classified crushing
and grinding equipment in 11 classes: Jaw crushers, Gyratory crushers,
Heavy-duty impact mills, Roll mills, Dry pans and chaser mills, Shredders,
Rotary cutters and dicers, Media mills, Medium peripheral-speed mills,
High peripheral-speed mills and Fluid-energy superfine mills.
The use of heavy-duty impact mills and among them
hammer mills, for grinding glass-like polysaccharides for abrasive grits or
absorbent materials, has been previously described. However, the use of
such heavy-duty impact mills generally results in a very broad particle size
distribution having a high content in fine and large particles. Large
particles generally exceed the average particle size of the glass-like
polysaccharide by more than about 350 Nm. Fine particles generally have
a particle size about 350 Nm less than the average particle size of the
glass-like polysaccharide. For applications in the absorbent industry, the
desired average particle size is about 500 Nm. Hence, within the context of
the absorbent industry, large particles are defined as being in excess of
about 850 Nm and fine particles are defined as being less than about 150
Nm.
Fine particles are generally unwanted, causing dusting
and particle size migration problems. Moreover, a narrow particle size
distribution is often preferred for some applications, such as water
absorption. Fine particles are known to create gel blocking problems, as
CA 02532749 2006-O1-13
-3-
reported by Berg et al. (US P 5,300,565). Moreover, fine particles tend to
adhere to movable parts of various industrial process equipments,
especially greased parts. Such adhesion will create a crust which may
eventually lead to equipment damage. Additionally, fine particles are also
more prone to generate airborne dusts which may become a serious
occupational health concern. Finally, airborne dusts, especially in the case
of polysaccharides or grains, could cause explosions and fires.
Larger particles are generally not suitable in applications
comprising thin hygiene products such as sanitary napkins or airlaids.
Moreover, larger particles will tend to only slowly absorb fluids such as
water and will cause pinholes in hygiene products.
To solve the problems inherent to fine or large particles,
the ground glass-like polysaccharides could be sieved. However, the
sieving operation will lead to lost product fractions that will need to be
discarded. Moreover, the sieving of glass-like polysaccharide fine particles
is a difficult operation at best, in view of their very irregular geometry.
This
irregular geometry often results in clogging of the sieves, especially by the
fine particles.
The use of roller mills for reducing the particle size of
glass-like polysaccharides is also known in the art. Roller mills compress
the glass-like polysaccharide particles, leading to a "stress" build-up, which
will results in the bursting of the particles between rollers. This type of
size
reduction is very aggressive. In order to reduce the aggressiveness of the
process and thus the fine particle content, the use of multiple pairs of
successive rollers has been described for the grinding of cereal based
products or foods (Taylor, T. US P 453,364; Brunner, H. FR 415230,
Johnston, G. US P 1,396,712; Noll et al. US P 2,986,348; Huessy, E. G.
US P 3,895,121; Sartori, R. FR 1,296,235; Livrieri, F. EP 0949003;
Standing, C. N. US P 3,933,086; Boczewski, M. P. US P 4,220,287; Rusch
et al. US P 4,225,093; Bielskis et al. US P 4,859,484; Gemsjager, H. US P
CA 02532749 2006-O1-13
-4-
5,031,845; Baltensperger et al. WO 89/03245 A1; Wellman, W. US P
5,089,282, US P 5,104,671, US P 5,141,764, US P 5,186,968, US P
5,194,287, US P 5,211,982; Salem et al. US P 6,098,905; Curran, S. P.
US P 5,192,028; t_eusner et al. US P 6,887,509; Giguere, R. J. US P
5,250,313; Hellweg et al. US App. 2005/153,044; Morgan, K. R. WO
05/002343; and Weaver, W. R. US App. 2005/160,996).
The use of roll mills for grinding glass-like
polysaccharides has been described by Sair et al. (US P 4,232,047).
However, Sair et al. describe the use of only one pair of rollers, which, as
mentioned before, is a very aggressive treatment. Moreover, Sair et al. are
silent with respect to the moisture content of the glass-like
polysaccharides, which plays a critical role for a highly effective size
reduction treatment. The moisture content is also a critical feature of
absorbent glass-like polysaccharides, as moisture will have a direct impact
on the absorbent properties. It is well known in the relevant art that
products having high moisture contents will show reduced absorbency.
There thus remains a need for a process for the efficient
particle size reduction of glass-like polysaccharides.
The present invention seeks to meet these and other
needs.
The present invention refers to a number of documents,
the content of which is herein incorporated by reference in their entirety.
CA 02532749 2006-O1-13
-5-
SUMMARY OF THE INVENTION
The present invention relates to a novel process for
reducing the particle size of glass-like polysaccharides. The present
invention also relates to particulate materials obtained by such a process,
as well as to compositions comprising such particulate materials. More
specifically, the present invention relates to a process for reducing the
particle size of glass-like polysaccharides, producing less fine and/or large
particles (i.e. narrower particle size distribution). The process of the
present invention can be conveniently employed for reducing the particle
size of glass-like polysaccharides for use in the absorbent industry. The
process of the present invention offers the additional advantages of being
both cost and energy efficient.
In an embodiment, the present invention relates to a
process for reducing the particle size of glass-like polysaccharides selected
from the group consisting of glass-like polysaccharides having a moisture
content ranging from 0% to about 13% and glass-like polysaccharides
being in a glassy state. The process comprises successively submitting
the glass-like polysaccharide to the particle size reducing action of at least
three pairs of successive rollers.
In an embodiment, the present invention relates to a
process for reducing the particle size of glass-like polysaccharides selected
from the group consisting of glass-like polysaccharides having a moisture
content ranging from 0% to about 13% and glass-like polysaccharides
being in a glassy state, wherein the glass-like polysaccharides comprise
starch. The process comprises successively submitting the glass-like
polysaccharide to the particle size reducing action of at least three pairs of
successive rollers.
In an embodiment, the present invention relates to
absorbent compositions comprising particles of reduced size of glass-like
polysaccharides selected from the group consisting of glass-like
CA 02532749 2006-O1-13
-6-
polysaccharides having a moisture content ranging from 0% to about 13%
and glass-like polysaccharides being in a glassy state.
Other objects, features and advantages of the present
invention will become apparent from the following detailed description. It
should be understood, however, that the detailed description and the
specific examples, while indicating illustrative embodiments of the
invention, are given by way of illustration only, since various changes and
modifications within the spirit and scope of the invention will become
apparent to those skilled in the art from this detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus generally described the invention, reference
will now be made to the accompanying drawings, showing by way of
illustration a preferred embodiment thereof, and in which:
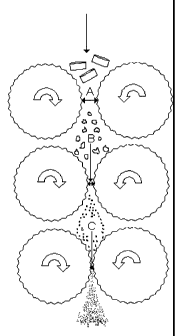
FIG. 1: FIG. 1 illustrates a side elevational view of three
successive pairs of rollers, according to an embodiment of the present
invention.
FIG. 2: FIG. 2 shows a scanning electron micrograph of a
glass-like polysaccharide, according to an embodiment of the present
invention.
FIG. 3: FIG. 3 illustrates a side a side elevational view of
a roller tooth having a "wave" shape, according to an embodiment of the
present invention.
FIG. 4: FIG. 4 illustrates a perspective view of a roller
according to an embodiment of the present invention.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
In order to provide a clear and consistent understanding
of the terms used in the present specification, a number of definitions are
CA 02532749 2006-O1-13
_7-
provided below. Moreover, the present description refers to a number of
routinely used chemical and technical terms; definitions of selected terms
are provided for clarity and consistency.
The use of the word "a" or "an" when used in conjunction
with the term "comprising" in the claims and/or the specification may mean
"one", but it is also consistent with the meaning of "one or more", "at least
one", and "one or more than one". Similarly, the word "another" may mean
at least a second or more.
As used in this specification and claim(s), the words
"comprising" (and any form of comprising, such as "comprise" and
"comprises"), "having" (and any form of having, such as "have" and "has"),
"including" (and any form of including, such as "include" and "includes") or
"containing" (and any form of containing, such as "contain" and "contains"),
are inclusive or open-ended and do not exclude additional, unrecited
elements or method steps.
The term "about" is used to indicate that a value includes
an inherent variation of error for the device or the method being employed
to determine the value.
As used herein, the term "glass-like polysaccharide"
refers to a polysaccharide which is substantially a uniform amorphous
solid. Glass-like polysaccharides are commonly prepared by the rapid
cooling of molten polysaccharides. Rapid cooling reduces the
polysaccharide's mobility, preventing the polysaccharide chains from
packing into a more thermodynamically favorable crystalline state. Glass-
like polysaccharides are often designated as "self entangled"
polysaccharides, such as reported by Thibodeau et al. (CA 2,462,053) or
Berrada et al. (CA 2,483,049). Moreover, glass-like polysaccharides are
characterized as having a broken glass-like shape. A non-limiting example
of such a glass-like shape is illustrated in Figure 2. It should be
understood that the definition of glass-like polysaccharides comprises
CA 02532749 2006-O1-13
-$-
agglomerates, (i.e. inorganic-polysaccharide agglomerates) and
polysaccharide nanocomposites (Berrada et al. CA 2,483,049), all of which
comprise a glass-like polysaccharide component of at least 50%.
As used herein, the term "glassy state" refers to a sub-
state of matter of glassy materials, particularly polymeric materials. In a
glassy state, the polysaccharide chains are relatively strongly associated
with each other, without however having a crystalline pattern. When in a
glassy state, the polysaccharides are more brittle and harder.
As used herein, the term "rubbery state" refers to a sub-
state of matter of glassy materials, particularly polymeric materials. In a
rubbery state, the electrostatic interaction (non-limiting examples of which
include H-bonding and ionic bonding) between the polysaccharide chains
are weaker, allowing for more mobility of the polymeric chains. This
additional freedom of motion makes "rubbery" glass-like polysaccharides
more resilient to stress or more ductile under an applied pressure.
As used herein, the term "molten polysaccharides" refers
to polysaccharides for which a sufficient amount of heat and water has
been provided to rupture their crystalline pattern. A synonymous term, i.e.
"gelatinised", is often used when referring to starch.
As used herein, the term "polysaccharide" refers to
polymers having a backbone comprising monosaccharide repeating units
and/or derivatized monosaccharide repeating units, wherein such repeating
units make-up at least 90% of the polymers. Non-limiting examples include
starches, modified starches, amylopectin, modified amylopectin, amylose,
modified amyiose, chitosan, chitin, guar gum, modified guar gum, locust
bean gum, tara gum, konjac gum, konjac flour, fenugreek gum, mesquite
gum, aloe mannans, cellulose, modified cellulose (representative examples
include carboxyalkylated cellulose and carboxymethyl cellulose), oxidized
polysaccharides, sulfated polysaccharides, cationic polysaccharides
(representative examples include chitosan, quaternary ammonium
CA 02532749 2006-O1-13
_g_
derivatives of polysaccharides and guanidinated polysaccharides such as
described by Berrada, M. CA 2,519,417), pectin, arabic gum, karaya gum,
xanthan, kappa, iota or lambda carrageenans, agar-agar, alginates and
mixtures thereof.
As used herein, the term "monosaccharide unit" refers to
cyclic C5-Cs aldoses or ketoses. Non limiting examples of C5-C6 aldoses
include allose, altrose, glucose, mannose, gulose, idose, galactose, talose,
ribose, arabinose, xylose, and lyxose. Non limiting examples of C5-C6
ketoses include ribulose, xylulose, fructose, sorbose and tagatose.
As used herein, the term "monosaccharide derivatives"
refers to any chemically or enzymatically modified monosaccharide unit.
As used herein, the term "moisture content" refers to the
amount of water (% w/w) contained in a solid.
As used herein, the term "ambient temperature" refers to
temperatures ranging from about 15 to about 40°C.
As used herein, the term "roller" refers to cylinders
rotating along their longitudinal axis.
As used herein, the term "pair of rollers" refers to 2
counter rotating, substantially parallel rollers, positioned in close
proximity
to each other.
As used herein, the term "gap nip" refers to the spacing
between a pair of rollers. In order to be effective, the gap nip should be
smaller than the particles to be reduced in size.
As used herein, the term "gap nip aggressiveness" refers
to the gap nip ratio between two pairs of successive rollers. The "gap nip
aggressiveness" can be defined by the following equation:
Gapnip, - aggressiveness ratio
Gap nipZ
CA 02532749 2006-O1-13
-10-
As illustrated by the drawing in Figure 1, a first "gap nip aggressiveness
ratio" is obtained by dividing "gap nip A" by "gap nip B". A second "gap nip
aggressiveness ratio" is obtained by dividing "gap nip B" by "gap nip C".
Higher aggressiveness ratios (i.e. higher numerical values) are indicative of
more particles of glass-like polysaccharides being stressed. However, if
the exerted stress is excessive (i.e. an aggressiveness ratio of about 2 or
more), an excessive bursting of the particles will occur, generating a larger
content of fine particles.
As used herein, the term "successive pairs of rollers"
refers to a series of pairs of rollers, each pair having a reducing impact on
the size of the glass-like polysaccharide particles. A series of three
successive pairs of rollers is illustrated in Figure 1.
As used herein, the term "Fine" or "Fine particle" refers to
small particles. The size of fine particles can be calculated by subtracting
350 Nm from the desired average particle size. For applications in the
absorbent industry, a desired average particle size is about 500 Nm; the
fine would thus have a particle size of about 150 pm or less.
As used herein, the term "Large" or "Large particle" refers
to big particles. The size of large particles can be calculated by adding 350
pm to the desired average particle. For applications in the absorbent
industry, a desired average particle size is about 500 pm; the large
particles would thus have a particle size of about 850 pm or more.
As used herein, the term "Free Swell Capacity" (FSC),
also called "Total Absorption", refers to the amount (g) of fluid absorbed
per gram of the composition. Typical fluids are saline solutions (0.9%
WeightNVeight NaCI solution, hereinafter called 0.9% NaCI solution or
saline).
As used herein, the term "Centrifuge Retention Capacity"
(CRC) also called "Retention", refers to the amount (g) of fluid retained per
CA 02532749 2006-O1-13
-11-
gram of the composition, following exposure of the composition to a
centrifugation force of 2506. Typical fluids are saline solutions (0.9%
WeightNVeight NaCI solution, hereinafter called 0.9% NaCI solution or
saline).
In a broad sense, the present invention relates to a novel
process for reducing the particle size of glass-like polysaccharides. In an
embodiment of the present invention, the particle size of the glass-like
polysaccharides is reduced by the action of at least three pairs of
successive rollers. In a further embodiment of the present invention, the
glass-like polysaccharides have a moisture content ranging from 0% to
about 13%. In a further embodiment of the present invention, the glass-like
polysaccharides are in a glassy state.
Following the reduction process, reduced glass-like
polysaccharide particles are obtained. The reduction process minimizes
the formation of fine and/or large particles. In an embodiment of the
present invention, the process for reducing the particle size of glass-like
polysaccharides generates particles exhibiting a narrow particle size
distribution. In a further embodiment, the reduction process of the present
invention generates reduced particles having a fine particle content of less
than about 5% and a large particle content of less than about 5%. In yet a
further embodiment, the reduction process of the present invention
generates reduced particles having a fine particle content of less than
about 2% and a large particle content of less than about 2%.
Glass-like polysaccharides, due to their physical
characteristics, constitute a special class of polysaccharides. Glass-like
polysaccharides can be in a glassy state or in a rubbery state. It was
unexpectedly discovered that when at ambient temperature and depending
on the moisture content, glass-like polysaccharides will fracture according
to different mechanisms.
Rubbery glass-like polysaccharides are glass-like
CA 02532749 2006-O1-13
-12-
polysaccharides having high moisture contents and tend to have plastic-
like and rubber-like characteristics. Such glass-like polysaccharides will be
more prone to deform under an applied pressure and will rather fracture
than burst. Moreover, the high moisture content inherent to these rubbery
glass-like polysaccharides imparts elasticity and ductility to the particles,
making their reduction using roller mills substantially ineffective.
Elasticity
and ductility allow for the polysaccharide to absorb energy when submitted
to the action of rollers. More energy will thus be required to properly
fracture the particles. The energy absorption will rapidly increase the
temperature of the polysaccharide which will tend to stick to industrial
equipment. Moreover, high temperature polysaccharide dusts are
flammable.
Glass-like polysaccharides in a glassy state are
characterized by lower moisture contents and tend to be more brittle and
harder. Such glass-like polysaccharides will burst rather than fracture
under an applied stress such as shear and pressure, making them very
suitable for size reduction using roller mills. In an embodiment of the
present invention, such glass-like polysaccharides have a moisture content
ranging from 0% to about 13%. In a further embodiment of the present
invention, such glass-like polysaccharides have a moisture content ranging
from about 7% to about 9%.
It is within the capacity of a skilled technician that
lowering the temperature of glass-like materials will stow the mobility of the
individual polymeric chains making up the glass-like materials. This will
make the material glassier, rendering it more prone to the action of roller
mills. Higher temperatures, however, will soften the glass-like materials
rendering them less suitable for use in roller mills.
In a roller mill, a pair of counter rotating rollers is spaced-
apart defining a space called "gap nip". This spacing is preferably
adjustable in order to control the stress applied on the particles to be
CA 02532749 2006-O1-13
-13-
reduced. As can be observed from Figure 1, the particles to be reduced
are successively conveyed into the gap nip spacing A, B and C, in which
they are stressed by the pressure exerted by the rollers. To be effective,
the gap nip must be smaller than the size of the particles to be reduced.
Depending on the roller mill configuration, several pairs of successive
rollers may be present.
In an embodiment of the present invention, the pair of
rollers is counter rotating at different speeds. This will result in the
particles
being exposed to shear stresses, resulting in an efficient size reduction. In
an embodiment, the rollers are equipped with corrugated teeth, located
along the outer surface of the rollers. The teeth will secure the particles
entering the gap nip and will impact the particles therein. In an
embodiment of the present invention, and as illustrated in Figure 3, the
teeth have a wave-like shape. The concave side of the wave should point
at the glass-like polysaccharide particles to be reduced, providing for the
particles to be guided into the gap nip. In a further embodiment of the
present invention, the corrugated teeth are linear, spanning the length of
the roller, either substantially parallel to the axis of the roller or offset
there
from.
An adequate adjustment of the gap nips) is important if
an efficient particle size reduction is to be achieved. Indeed, the
successive gap nips should be progressively reduced. An excessive gap
nip difference between two successive pairs of rollers results in an
excessively high aggressiveness ratio. Such a high ratio will result in
excessive bursting of the glass-like polysaccharide particles, generating
unwanted amounts of fine particles. In an embodiment of the present
invention, the gap nip aggressiveness ratio ranges from about 1.0 to about
2Ø In a further embodiment, the gap nip aggressiveness ratio ranges
from about 1.2 to about 1.8.
CA 02532749 2006-O1-13
-14-
In an embodiment of the present invention, the glass-like
polysaccharide particles are subjected to the reducing action of at least
three pairs of successive rollers. The use of an increased number of
successive rollers, allows for the aggressiveness ratio between two
successive pairs of rollers to be more gradually reduced. However, it is
within the capacity of a skilled technician to determine the number of
successive pairs of rollers to be used, as well as determining adequate gap
nip settings, in order obtain a desired particle size distribution.
The reduced glass-like polysaccharide particles of the
present invention exhibit a narrow particle size distribution. However,
sieves may still be used to further narrow the particle size distribution.
Moreover, a dust cleaner may be used in proximity to the rollers in order to
remove any airborne particles. The use of such dust cleaners further
narrows the particle size distribution.
The glass-like polysaccharides to be used in the process
of the present invention may be obtained from a variety of sources. Non-
limiting examples include starches, modified starches, amylopectin,
modified amylopectin, amylose, modified amylose, chitosan, chitin, guar
gum, modified guar gum, locust bean gum, tars gum, konjac gum, konjac
flour, fenugreek gum, mesquite gum, aloe mannans, cellulose, modified
cellulose (representative examples include carboxyalkylated cellulose and
carboxymethyl cellulose), oxidized polysaccharides, sulfated
polysaccharides, cationic polysaccharides, pectin, arabic gum, karaya
gum, xanthan, kappa, iota or lambda carrageenans, agar-agar and
alginates. Non-limiting examples of mannose-based polysaccharides
include guar gum, tars gum, locust bean gum, konjac gum, mesquite gum,
and fenugreek extracts. Further non-limiting examples include cross-linked
polysaccharides such as those described by Couture et al. (CA 2,362,006);
mixtures of polysaccharides such as those described by Bergeron, D. (CA
2,426,478); chemically derivatized polysaccharides such as those
CA 02532749 2006-O1-13
-15-
described by Berrada, M. (CA 2,519,417); and polysaccharides-inorganic
agglomerates, and polysaccharides nanocomposites such as those
described by Berrada et al. CA 2,483,049.
In an embodiment of the present invention, the glass-like
polysaccharide is starch. Non-limiting example of starches include corn,
waxy corn, wheat, waxy wheat, rice, waxy rice, potato, cassava, waxy
maize, sorghum, waxy sorghum, sago, buckwheat, beans, peas, rye,
barley, and amaranth.
Glass-like polysaccharides are usually obtained through
extrusion processes. The glass-like extrudates are usually cut into pellets,
such as those described in US P 5,066,335 (Lane et al.). In an
embodiment of the present invention, the diameter of the pellets ranges
from about 2 mm to about 8 mm.
The reduced glass-like polysaccharide particles of the
present invention can be employed in a variety of applications such as in
disposable sanitary products (i.e. diapers, incontinence articles, feminine
hygiene products, and absorbent dressings), airlaids, household articles,
sealing materials, humectants (i.e. agricultural products for soil
conditioning), mining and oil drilling, anti-condensation coatings, water-
storing materials (agriculture/horticulture/forestry), absorbent paper
products, surgical absorbents, pet litter, bandages, wound dressings,
chemical absorbents, polymeric gels for cosmetics and pharmaceuticals,
artificial snow, in fire-fighting techniques, and in applications related to
the
transportation of fresh food or seafood, as well as in food packaging
applications. Moreover, the reduced glass-like polysaccharide particles of
the present invention can be employed to absorb a variety of liquids, non-
limiting examples of which include physiological fluids, saline solutions,
water and aqueous solutions.
The reduced glass-like polysaccharide particles of the
present invention can be mixed with other co-absorbent materials. In an
CA 02532749 2006-O1-13
-16-
embodiment of the present invention, the compositions comprise from
about 1 to about 99% (w/w) of reduced size glass-like polysaccharides,
and from about 99 to about 1 % (w/w) of co-absorbent material. Non-
limiting examples of co-absorbent materials include synthetic
superabsorbent polymers, mannose-based polysaccharides, ionic
polysaccharides, fibers and mixtures thereof.
In an embodiment of the present invention, absorbent
compositions are prepared by mixing the reduced size glass-like
polysaccharide particles with ionic polysaccharides, either cationic or
anionic polysaccharides, or mixtures thereof. In a further embodiment,
absorbent compositions are prepared by mixing the reduced size glass-like
polysaccharide particles with one or more anionic polysaccharides.
Non-limiting examples of anionic polysaccharides include
carboxyalkyl polysaccharides, carboxymethyl cellulose, carboxymethyl
starch, oxidized polysaccharides, xanthan, carrageenans, pectin and
mixtures thereof.
Non-limiting examples of fibers include cellulose, viscose,
rayon, cellulose acetate, NylonT"", polyalkylenes, polyethylene,
polypropylene, bi-component fibers, polyesters, polylactides,
polypropanediols, LyoceIIT"", sphagnum and mixtures thereof.
Non-limiting examples of mannose based
polysaccharides include guar, tara, locust bean, konjac, fenugreek
extracts, mesquite extracts, aloe mannans and mixtures thereof.
The co-absorbent synthetic superabsorbent polymers
can generally be obtained via the polymerization of monomers, non-limiting
examples of which include acrylic acid, acrylate salts, acrylic ester, acrylic
anhydride, methacrylic acid, methacrylate salts, methacrylic esters,
methacrylic anhydride, malefic anhydride, malefic salts, maleate esters,
CA 02532749 2006-O1-13
-17-
acrylamide, acrylonitrile, vinyl alcohol, vinyl pyrrolidone, vinyl acetate,
vinyl
guanidine, aspartic acid, aspartic salts and mixtures thereof.
EXPERIMENTAL
Materials
Glass-like grade A wheat starch pellets having a diameter
ranging from about 2 mm to about 8 mm and having a moisture content of 10
%, were obtained from Archer Daniels Midland, (Decatur, USA, Division
Candiac, Canada). Glass-like corn starch pellets having a diameter ranging
from about 2 mm to about 8 mm and having a moisture content of 8 %, were
obtained from Groupe Lysac Inc. ~ (Boucherville, Canada).
Triale aair roller mill
The glass-like polysaccharide particles were reduced using
a Gran-U-LizerT"" 1052 TP grinder from Modern Processing Equipment
(Chicago, USA). The grinder was equipped with three (3) pairs of rollers.
The rollers, made from centrifugally cast dual metal chilled iron, had a
diameter of 10 inches and were 52 inches in length. Moreover, the rollers
were corrugated with wave-like teeth. The rollers were pneumatically
controlled, permitting a variable gap nip adjustment. All pairs of rollers
were
asynchronous, i.e. rotating at a different pace. The first pair of rollers
rotated
at speeds of 478 rpm and 1160 rpm respectively. The second pair of rollers
rotated at speeds of 512 rpm and 1160 rpm respectively. The third pair of
rollers rotated at speeds of 614 rpm and 1160 rpm respectively. A 25 HP
motor powered each pair of rollers. The grinder was fed using a 1052 PF
roller feeder (1 HP), with variable motor speed drive, from Modern
Processing Equipment (Chicago, USA).
CA 02532749 2006-O1-13
-18-
Siever
Samples were sieved for a period of about 10 minutes
using a Tyler Ro-TapT"" test sieve shaker rotating at 1725 rpm. Samples
were sieved with sieves having openings of 20, 30, 60 and 100 Tyler mesh.
Test methods
As discussed in Modern Superabsorbent Polymer
Technology (Buchholz, F.L. and Graham, A.T. Eds., Wiley-VCH, New York,
1998, section 4.6.1. Swelling Capacity: Theory and Practice, p. 147),
several measurement methods are used in order to characterize the
swelling capacity of a polymer. In the field of superabsorbents, the
Gravimetric Swelling Capacity [also called the Free Swell Capacity (FSC)]
and the Centrifuge Capacity (also called the Centrifuge Retention Capacity
(CRC)] are recommended methods. The FSC and the CRC were used to
compare the swelling capacities of the obtained absorbent products.
Tea bass for FSC and CRC measurements
Tea bags (10 X 10 cm) were made from heat sealable
AhlstromT"" filter paper (16.5 ~0.5 g/m2).
FSC measurements
The Free Swell Capacity (FSC) in a 0.9% NaCI solution
was determined according to the recommended test method 440.2-02 from
EDANA.
CRC measurements
The Centrifuge Retention Capacity (CRC) in a 0.9% NaCI
solution was determined according to the recommended test method
441.2-02 from EDANA.
CA 02532749 2006-O1-13
-19-
~~rennp~ Gc
Example 1: Wheat starch
The gap nip for the first pair of rollers was adjusted to
0.018 inches (457 Nm); the gap nip for the second pair of rollers was
adjusted to 0.010 inches (254 pm); and the gap nip for the third pair of
rollers was adjusted to 0.006 inches (152 Nm). The aggressiveness ratio
as calculated for the first pair of rollers and the second pair of rollers was
1.80; the aggressiveness ratio as calculated for the second pair of rollers
and the third pair of rollers was 1.67.
Glass-like wheat starch pellets were fed into the mill.
Once ground, the reduced glass-like wheat starch
particles were sieved for a period of 10 minutes. The obtained particle size
distribution is illustrated hereinbelow in Table 1.
Table 1: Particle size distribution of Example 1
Mesh size Micron size Percentage (w/w)
> 20 > 833 Nm 4.1
30 589 Nm 26.7
60 246 pm 65.5
100 147 Nm 2.7
<100 <147Nm 0.8%
CA 02532749 2006-O1-13
-20-
Examples 2-5: Effect of gap nip on particle size distribution
The gap nip was modified as illustrated hereinbelow in
Table 2. Glass-like wheat starch pellets were fed into the mill.
Once ground, the reduced glass-like wheat starch
particles were sieved for a period of 10 minutes. The obtained particle size
distribution is illustrated hereinbelow in Table 3.
Table 2: Gap nip settings
Example n Gap nip 1 Gap nip 2 Gap nip 3
2 0.024 in.* 0.014 in. 0.010 in.
3 0.022 in. 0.012 in. 0.008 in.
4 0.014 in. 0.008 in. 0.005 in.
5 0.014 in. 0.008 in. 0.004 in.
*1 in. = 25,400 Nm
Table 3: Particle size distribution of Examples 2-5
Mesh/Micron sizeExample Example Example Example
2 3 4 5
>20Mesh/>833Nm 11.6% 7.2% 1.7% 2.1
30 Mesh / 589 47.1 % 38.1 14.2 % 19.7
Nm %
60 Mesh / 246 39.3 % 52.4 76.9 % 73.1
Nm %
100Mesh/147Nm 1.3% 1.8% 6.0% 4.2%
<100Mesh/<147Nm 0.3% 0.5% 1.1 % 0.7%
As it can be observed from Table 3, the size of the
reduced glass-like polysaccharide particles is directly related to the gap nip
settings. Larger gap nip settings will generate a more significant fraction of
large particles. The aggressiveness ratio as calculated for the first pair of
CA 02532749 2006-O1-13
-21-
rollers and the second pair of rollers for example 4 was 1.75; the
aggressiveness ratio as calculated for the second pair of rollers and the
third pair of rollers for example 4 was 1.6. The aggressiveness ratio as
calculated for the first pair of rollers and the second pair of rollers for
example 5 was 1.75; the aggressiveness ratio as calculated for the second
pair of rollers and the third pair of rollers was 2Ø The third pair of
rollers in
Example 5 is more aggressive that the third pair of rollers in Example 4.
Example 6: Corn starch glass-like polysaccharides and absorbent
properties
The gap nip for the first pair of rollers was adjusted to
0.0235 inches (596 Nm); the gap nip for the second pair of rollers was
adjusted to 0.0115 inches (292 pm); and the gap nip for the third pair of
rollers was adjusted to 0.0085 inches (216 pm). The aggressiveness ratio
as calculated for the first pair of rollers and the second pair of rollers was
2.04; the aggressiveness ratio as calculated for the second pair of rollers
and the third pair of rollers was 1.35.
Glass-like corn starch pellets were fed in the mill. Once
ground, the reduced glass-like corn starch particles were sieved for a
period of 10 minutes. The obtained particle size distribution is illustrated
hereinbelow in Table 4. The absorbent characteristics of the reduced
glass-like corn starch particles were as follows: Free Swell Capacity: 7 g/g;
Centrifuge Retention Capacity: 4.8 g/g. As can be observed from Table 4,
the higher aggressiveness ratio for the first pair of rollers and the second
pair of rollers generated a higher content of fine particles.
CA 02532749 2006-O1-13
-22-
Table 4: Particle size distribution of Example 6
Mesh size Micron size Percentage (w/w)
> 20 > 833 Nm 2.59
30 589 ~m 21.5
60 246 Nm 68.3
100 147 ~m 5.68
< 100 < 147 Nm 2.09