Note: Descriptions are shown in the official language in which they were submitted.
CA 02533678 1997-01-15
WO 97127840 PCT/US97/00722
~ SUSTAINED DELIVERY OF AN ACTIVE AGENT USING -AN
2 IMPLANTABLE SYSTEM
3 4 Technical Field
a This invention is related to the sustained delivery of a biologically
7 active agent. More particularly, the invention is directed to an implantable
a delivery system for the prolonged delivery of an active agent to a fluid
9 environment in a natural or artificial body cavity.
Background of the Invention
12
13 Treatment of disease by prolonged delivery of an active agent at a
14 controiled rate has been a goal in the drug delivery field. Various
approaches
have been taken toward delivering the active agents.
16 One approach involves the use of implantable diffusional systems.. For
17 example, subdermal'implants for contraception are described by Philip D.
18 Darney in Current Opinion in Obstetrics and Gynecology 1991, 3:470-4.76.
19 Norplant requires the placement of 6 levonorgestrel-filled silastic
capsules
under the skin. Protection from conception for up to 5 years is achieved. The
21 implants operate by simple diffusion, that is, the active agent diffuses
through
22 the polymeric material at a rate that is controlled by the characteristics
of the
23 active agent formulation and the polymeric material. Damey further
describes
24 biodegradable implants, namely CapranorTM' and norethindrone pellets.
25. These. systems are designed to deliver contraceptives for about one year
and
26 then dissolve. The CapranorTM systems consist of poly(E-caprolactone)
27 capsules that are filled with -levonorgestrel and the pellets are 10% pure
28 cholesterol with 90% norethindrone.
29 Implantable infusion pumps have also been described for delivering
3o drugs by intravenous, intra-arterial, intrathecal, intraperitoneal,
intraspinal and
31 epidural pathways. The pumps are usually surgically inserted into a
CA 02533678 1997-01-15
67696-260
2
I subcutaneous pocket of tissue in the lower abdomen. Systems for pain
2 management, chemotherapy and insulin delivery are described in the BBI
3 Newsletter,'Vol. 17, No. 12, pages 209-211, December 1994. These systems
4 provide for more accurately controlled delivery than simple diffusional
systems. ;
6 One particularly promising approach invoives osmotically driven7 devices
such as those described in U.S. Patent Nos. 3,987,790, 4,865,845,
a 5,057,318, 5,059,423, 5,112,614, 5,137,727, 5,234,692 and 5,234,693.
9 These devices can be implanted
into an animal to release the active agent in a controlled manner.for a
11 predetermined administration period. In general, these devices operate by
12 imbibing fluid from the outside environment and n:leasing corresponding
13 amounts of the active agent.
14 The above-described devices have been useful for delivering active
is agents to a fluid environment of use. Atthough these devices have found
16 application for human and veterinary purposes, there remains a need for
17 devices that are capable of delivering active agents, particulariy potent
,8 unstable agents, reliably to a human being at a controlled rate over a
19 prolonged period of time.
21 Summary of the Invention
22
23 Implantable osmotic systems for delivery of an active agent to an
24 animal are well known. Adaptation of these systems for human use raises a
zs number of difficutt issues. The size of the device may need to be decreased
26 for human implantation. The strength of the device must be suffiaent to
27 ensure a robust system. Accurate and reproducible deliveryrates and
sa durations must'be ensured and the period from implantation to start-up of
29 delivery must be minimized. The active agent must return its purity and
so activity for extended periods of time at the elevated temperatures
31 encountered in the body cavity.
CA 02533678 1997-01-15
67696-260
2a
According to one aspect of the present invention,
there is provided a fluid-imbibing device for delivering an
active agent to a fluid environment of use, said fluid-
imbibing device comprising a water-swellable semipermeable
plug that conforms to an interior surface of an end of an
impermeable reservoir and creates a liquid-tight seal
between the water-swellable semipermeable plug and the
interior surface and an active agent to be displaced from
the fluid-imbibing device when the water-swellable
semipermeable plug swells.
According to another aspect of the present
invention, there is provided the device as described above,
further comprising a piston received within the interior
surface of the impermeable reservoir, wherein the piston
divides the reservoir into an active agent containing
chamber and a water-swellable agent containing chamber.
According to still another aspect of the present
invention, there is provided the device as described above,
further comprising: (a) a piston that divides the reservoir
into a first and a second chamber, the first and second
chambers each having an open end; (b) a water-swellable
semipermeable plug in the first chamber; and (c) the active
agent in the second chamber, wherein the water-swellable
semipermeable plug is located in the open end of the first
chamber.
According to yet another aspect of the present
invention, there is provided the device as described above
wherein the water-swellable semipermeable plug contains at
least about 64 mg NaCl.
According to a further aspect of the present
invention, there is provided the device as described above
wherein the water-swellable semipermeable plug contains
CA 02533678 2007-09-27
67696-260D
2b
NaCl, a gelling osmopolymer and tabletting agents and
viscosity modifying agents.
According to another aspect of the present
invention, there is provided an implantable device for
delivering an active agent to a fluid environment of use,
said device comprising a reservoir and a back-diffusion
regulating outlet in mating relationship, wherein a flow
path for the active agent comprises a pathway formed between
the mating surfaces of the reservoir and the back-diffusion
regulating outlet.
According to another aspect of the present
invention, there is provided an implantable device for
storing an active agent in a fluid environment of use during
a predetermined administration period, the device comprising
a reservoir containing an active agent, said reservoir being
formed at least in part from a metallic material, the
portion of said reservoir contacting said active agent being
non-reactive with the active agent, said metallic material
in contact with active agent being formed of a material
selected from the group consisting of titanium and its
alloys.
According to still another aspect of the present
invention, there is provided a fluid-imbibing implantable
active agent delivery system for delivering an active agent
to a fluid environment of use for a predetermined
administration period, wherein the time to start-up is less
than 10% of the predetermined administration period.
According to yet another aspect of the present
invention, there is provided a back-diffusion regulating
outlet useful in an implantable active agent delivery system
for delivering active agent to a fluid environment of use,
said outlet defining a flow path wherein the length,
CA 02533678 2007-09-27
67696-260D
2c
interior cross-sectional shape and area provide for an
average linear velocity of the active agent that is higher
than the linear inward flux of the fluid environment of use.
According to a further aspect of the present
invention, there is provided a semipermeable plug useful in
an implantable active agent delivery system for delivering
an active agent to a fluid environment of use, said plug
being water-swellable and expanding linearly in said
delivery system to commence pumping of active agent upon
insertion of the delivery system in the fluid environment of
use.
According to yet a further aspect of the present
invention, there is provided an implantable device for
delivering leuprolide acetate to a fluid environment of use,
said device comprising a reservoir and a back-diffusion
regulating outlet in mating relationship, wherein a flow
path for the active agent comprises a pathway formed between
the mating surfaces of the reservoir and the back-diffusion
regulating outlet.
According to still a further aspect of the present
invention, there is provided a use of the implantable device
as described herein for the treatment of prostate cancer.
CA 02533678 1997-01-15
~ =
WO 97/27840 PCT/US97/00722
3
1 Accordingly, in one aspect, the inventipn is a fluid-imbibing device for
2 delivering an active agent formulation to a fluid environment of use. The
3 device comprises a water-swellable, semipermeable material that is received
4 in sealing relationship with the interior surface at one end of an
impermeable
reservoir. The device further contains an active agent to be displaced from
6 the device when the water-swellable material swells.
7 In another aspect, the invention is directed to an implantable device for
e delivering an active agent to a fluid environment of use. The device
9 comprises a reservoir and a back diffusion regulating outlet in a mating
relationship. The flow path of the active agent comprises a pathway formed
11 between the mating surfaces of the back diffusion regulating outlet and the
12 reservoir.
13 In yet another aspect, the present invention is directed to a device for
14 storing an active agent in a fluid environment of use during a
predetermined
administration period, the device comprising a reservoir containing an active
16 agent. The reservoir is impermeable and formed at least in part from a
17 metallic material. The portion of the reservoir contacting the active agent
is
18 non-reactive with the active agent, and is formed of a material selected
from
19 the group consisting of titanium and its alloys.
In a further aspect, the invention is an implantable fluid-imbibing active
21 agent delivery system that comprises an impermeable reservoir. The
iz reservoir contains a piston that divides the reservoir into an active agent
23 containing chamber and a water-swellable agent containing chamber. The
24 active agent containing chamber is provided with a back-diffusion
regulating
outlet. The water-swellable agent containing chamber is provided with a
26 semipermeable plug. Either the plug or the outlet is releasable from the
27 reservoir at an internal pressure that is lower than the maximum osmotic
28 pressure generated by the water-swellable agent.
29 The invention is further directed to a fluid-imbibing implantable active
3o agent delivery system where the time to start-up of delivery is less than
10%
31 of the predetermined administration period.
CA 02533678 1997-01-15
. = =
WO 97/27840 PCTIUS97/00722
4
1 In another aspect, the invention is directed to a method for preparing a
2 fluid-imbibing implantable active agent delivery system. The method
3 comprises injection molding a semipermeable plug into the end of an
4 impermeable reservoir such that the plug is protected by the reservoir.
In still another aspect, the invention is directed to an impermeable
6 active agent delivery system for delivering an active agent that is
susceptible
7 to degradation. The reservoir contains a piston that divides the reservoir
into
8 a water-swellable agent chamber and an active agent chamber. The open
9 end of the water-swellable agent chamber contains a semipermeable
membrane and the open end of the active agent chamber contains a back-
11 diffusion regulating outlet. The system effectively seals the active agent
12 chamber and isolates it from the environment of use.
13 In a further aspect, the invention is directed to a back-diffusion
14 regulating outlet useful in an active agent delivery system. The outlet
defines
1s a flow path wherein the length, interior cross-sectional shape and area
16 provide for an average linear velocity of active agent that is higher than
the
17 linear inward flow of fluid in the environment of use.
18 The invention is also directed to a semipermeable plug useful in an
19 active agent delivery system. The plug is water-swellable and must expand
linearly in the delivery system to commence pumping upon insertion of the
21 system into the fluid environment of use.
22 The invention is further directed to implantable delivery systems useful
23 for delivering leuprolide.
24
Descrintion of the Drawings
26
27 The figures are not drawn to scale, but are set forth to illustrate various
28 embodiments of the invention. Like numbers refer to like structures.
29 Figs. 1 and 2 are partial cross-sectional views of two embodiments of
the delivery device of the invention.
CA 02533678 1997-01-15
= =
WO 97/27840 PCT/US97/00722
1 Fig. 3 is an eniarged cross-sectional view of the back-diffusion
2 regulating outlet of Fig. 1.
3 Fig. 4 is a graph that shows the effect of orifice diameter and length on
4 drug diffusion.
5 Figs. 5, 6, 7 and 8 are enlarged cross-sectional views of further
6 embodiments of the semipermeable plug end of the reservoir according to the
7 invention.
8 Figs. 9, 10 and 11 are graphs of release rates for systems with
9 leuprolide (Fig. 9) and with blue dye and with different membranes (Figs. 10
and 11).
11
12 Detailed Description of the Invention
13
,a The present invention provides a device for the delivery of an active
agent to a fluid environment of use in which the active agent must be
16 protected from the fluid environment until it is delivered. Prolonged and
17 controlled delivery is achieved.
18
19 Definitions
21 The term "active agent" intends the active agent(s) optionally in
22 combination with pharmaceutically acceptable carriers and, optionally
23 additional ingredients such as antioxidants, stabilizing agents, permeation
24 enhancers, etc.
By a "predetermined administration period" is intended a period of
26 greater than 7 days, often between about 30 days and 2 years, preferably
27 greater than about I month and usually between about 1 month and 12
28 months.
29 By the time to "start-up" of delivery is intended the time from insertion
into the fluid environment of use until the active agent is actually delivered
at
31 a rate not less than approximately 70% of the intended steady-state rate.
CA 02533678 1997-01-15
= ~. .
WO 97/27840 PCT/US97/00722
6
, The term "impermeable" intends that the material is sufficiently
2 impermeable to environmental fluids as well as ingredients contained within
3 the dispensing device such that the migration of such materials into or out
of
4 the device through the impermeable device is so low as to have substantially
no adverse impact on the function of the device during the delivery period.
6 The term "semipermeable" intends that the material is permeable to
7 external fluids but substantially impermeable to other ingredients contained
8 within the dispensing device and the environment of use.
9 As used herein, the terms "therapeutically effective amount" or
"therapeutically effective rate" refer to the amount or rate of the active
agent
õ needed to effect the desired biologic or pharmacologic effect.
12 The active agent delivery devices of the invention find use where the
13 prolonged and controlled delivery of an active agent is desired. In many
14 cases the active agent is susceptible to degradation if exposed to the
environment of use prior to delivery and the delivery devices protect the
agent
16 from such exposure.
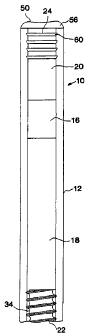
17 Fig. 1 shows one embodiment of the device according to the invention.
18 In Fig. 1 a fluid-imbibing system 10 is shown that comprises an impermeable
19 reservoir 12. The reservoir 12 is divided into two chambers by a piston 16.
The first chamber 18 is adapted to contain an active agent and the second
21 chamber 20 is adapted to contain a fluid-imbibing agent. A back-diffusion
22 regulating outlet 22 is inserted into the open end of the first compartment
18
23 and a water-swellable semipermeable plug 24 is inserted into the open end
of
24 the second chamber 20. In Fig. 1, the back-diffusion regulating outlet 22
is
shown as a male threaded member in a mating relationship with the smooth
26 interior surface of the reservoir 12 thereby forming therebetween helical
flow
27 path 34. The pitch (x), the amplitude (y), and the cross-sectional area and
28 shape of the helical path 34 formed between the mating surfaces of the back-
29 diffusion regulating outlet 22 and the reservoir 12 as shown in Fig. 3 are
factors that affect both the efficiency of path 34 preventing back-diffusion
of
31 external fluid into the formulation in chamber 18 and the back pressure in
the
CA 02533678 1997-01-15
WO 97/27840 PCTIUS97/00722
7
, device. The geometry of outlet 22 prevents water diffusion into the
reservoir.
2 In general, it is desired that these characteristics be selected so that the
3 length of the helical flow path 34 and the velocity of flow of active agent
4 therethrough is sufficient to prevent back-diffusion of external fluid
through the
flow path 34 without significantly increasing the back pressure, so that,
6 following start-up, the release rate of the active agent is govemed by the
7 osmotic pumping rate.
8 Fig. 2 is a second embodiment of the device of the invention with a
9 reservoir 12, piston 16 and plug 26. In this embodiment, the flow path 36 is
formed between a threaded back-diffusion regulating outlet 40 and threads 38
õ formed on the interior surface of the reservoir 12. The amplitudes of the
12 threaded portions of the back-diffusion regulating outlet 40 and reservoir
12
13 are different so that a flow path 36 is formed between the reservoir 12 and
the
14 back-diffusion regulating outlet 40.
The water-swellable semipermeable plugs 24 and 26 shown in Figs. 1
16 and 2 respectively are inserted into the reservoir such that the reservoir
wall
17 concentrically surrounds and protects the plug. In Fig. 1, the top portion
50 of
18 the plug 24 is exposed to the environment of use and may form a flanged end
19 cap portion 56 overlaying the end of reservoir 12. The semipermeable plug
zo 24 is resiliently engaged with the interior surface of the reservoir 12 and
in
21 Fig. I is shown to have ridges 60 that serve to frictionally engage the
22 semipermeable plug 24 with the interior of reservoir 12. In addition, the
23 ridges 60 serve to produce redundant circumferential seals that function
24 before the semipermeable plug 24 expands due to hydration. The clearance
between ridges 60 and the interior surface of the reservoir 12 prevents
26 hydration swelling from exerting stresses on the reservoir 12 that can
result in
27 tensile failure of the reservoir 12 or compression or shear failure of the
plug
28 24. Fig. 2 shows a second embodiment of the semipermeable plug 26 where
29 the plug is injection molded into the top portion of the reservoir and
where the
top of the semipermeable plug 26 is flush with the top 62 of the reservoir 12.
31 In this embodiment, the diameter of the plug is substantially less than the
CA 02533678 1997-01-15
WO 97/27840 PCTIUS97/00722
8
~ diameter of the reservoir 12. In both embodiments the plugs 24 and 26 will
2 swell upon exposure to the fluid in body cavity forming an even tighter seal
3 with the reservoir 12.
4 The novel configurations of the components of the above-described
embodiments provide for implantable devices that are uniquely suited for
6 implantation into humans and can provide delivery devices which are capable
7 of storing unstable formulations at body temperatures for extended periods
of
a time, which devices have start-up times of less than 10% of,the
administration
s period and can be designed to be highly reliable and with predictable fail
safe
modes.
>> Reservoir 12 must be sufficiently strong to ensure that it will not leak,
12 crack, break or distort so as to expel its active agent contents under
stresses
13 it would be subjected to during use while being impermeable. In particular,
it
14 should be designed to withstand the maximum osmotic pressure that could be
generated by the water-swellable material in chamber 20. Reservoir 12 must
16 also be chemically inert and biocompatible, that is, it must be non-
reactive
17 with the active agent formulation as well as the body. Suitable materials
,e generally comprise a non-reactive polymer or a biocompatible metal or
alloy.
19 The polymers include acrylonitrile polymers such as acrylonitrile-butadiene-
styrene terpolymer, and the like; halogenated polymers such as
21 polytetrafluoroethylene, polychlorotrifluoroethylene, copolymer
22 tetrafluoroethylene and hexafluoropropylene; polyimide; polysulfone;
23 polycarbonate; polyethylene; polypropylene; polyvinylchloride-acrylic
24 copolymer; polycarbonate-acrylonitrile-butadiene-styrene; polystyrene; and
the like. The water vapor transmission rate through compositions useful for
2s forming the reservoir are reported in J. Pharm. Sci., Vol. 29, pp. 1634-37
27 (1970), Ind. Eng. Chem., Vol. 45, pp. 2296-2306 (1953); Materials
28 Engineering, Vol. 5, pp. 38-45 (1972); Ann. Book of ASTM Stds., Vol. 8.02,
29 pp. 208-211 and pp. 584-587 (1984); and Ind. and Eng. Chem., Vol. 49, pp.
1933-1936 (1957). The polymers are known in the Handbook of Common
31 Polymers by Scott and Roff, CRC Press, Cleveland Rubber Co., Clevefand,
CA 02533678 1997-01-15
. . ~ =
WO 97/27840 PCT/US97/00722
9
I OH. Metallic materials useful in the invention include. stainless steel,
titanium,
2 platinum, tantalum, gold and their alloys as well as gold-plated ferrous
alloys,
3 platinum-plated ferrous alloys, cobalt-chromium ailoys and titanium nitride
4 coated stainless steel. A reservoir made from titanium or a titanium alloy
having greater than 60%, often greater than 85% titanium is particularly
6 preferred for the most size-critical applications, for high payload
capability and
7 for long duration applications and for those applications where the
formulation
8 is sensitive to body chemistry at the implantation site or where the body is
9 sensitive to the formulation. Preferred systems maintain at least 70% active
agent after 14 months at 37 C and have a shelf stability of at least about 9
>> months, or more preferably at least about two years, at 2-8 C. Most
12 preferably, systems may be stored at room temperature. In certain
13 , embodiments, and for applications other than the fluid-imbibing devices
14 specifically described, where unstable formulations are in chamber 18,
particularly protein and/or peptide formulations, the metallic components to
16 which the formulation is exposed must be formed of titanium or its alloys
as
17 described above.
18 The devices of this invention provide a sealed chamber 18 which
19 effectively isolates the formulation from the fluid environment. The
reservoir
12 is made of a rigid, impermeable and strong material. The water-swellable
21 semipermeable plug 24 is of a lower durometer material and will conform to
22 the shape of the reservoir to produce a liquid-tight seal with the interior
of
23 reservoir 12 upon wetting. The flow path 34 isolates chamber 18 from back-
24 diffusion of environmental fluid. Piston 16 isolates chamber 18 from the
environmental fluids that are permitted to enter chamber 20 through
26 semipermeable plugs 24 and 26 such that, in use at steady-state flow,
active
27 agent is expelled through outlet 22 at a rate corresponding to the rate at
28 which water from the environment flows into the water-swellable material in
29 chamber 20 through semipermeable plugs 24 and 26. As a result, the plug
and the active agent formulation will be protected from damage and their
31 functionality will not be compromised even if the reservoir is deformed. In
CA 02533678 1997-01-15
~ =
WO 97/27840 PCTIUS97/00722
1 addition, the use of sealants and adhesives will be avoided and the
attendant
2 issues of biocompatibility and ease of manufacture resolved.
3 Materials from which the semipermeable plug are made are those that
4 are semipermeable and that can conform to the shape of the reservoir upon
5 wetting and adhere to the rigid surface of the reservoir. The semipermeable
6 plug expands as it hydrates when placed in a fluid environment so that a
seal
7 is generated between the mating surfaces of the plug and the reservoir. The
8 strength of the seals between the reservoir 12 and the outlet 22 and the
9 reservoir 12 and the plugs 24 and 26 can be designed to withstand the
10 maximum osmotic pressure generated by the device. In a preferred
11 alternative, the plugs 24 and 26 may be designed to withstand at least 10X
12 the osmotic agent compartment 20 operating pressure. In a further
13 altemative the plugs 24 and 26 may be releasable from the reservoir at an
14 intemal pressure that is lower than the pressure needed to release the back
diffusion regulating outlet. In this fail safe embodiment, the water-swellable
16 agent chamber will be opened and depressurized, thus avoiding dispelling
17 the diffusion regulating outlet and attendant release of a large quantity
of the
18 active agent. In other cases, where a fail-safe system requires the release
of
19 the active agent formulation rather than the water=swellable agent
formulation, the semipermeable plug must be releasable at a pressure that, is
21 higher than the outlet.
22 In either case, the semipermeable plug must be long enough to
23 sealably engage the reservoir wall under the operating conditions, that is,
it
24 should have an aspect ratio of between 1:10 and 10:1 length to diameter,
preferably at least about 1:2 length to diameter, and often between 7:10 and
26 2:1. The plug must be able to imbibe between about 0.1% and 200% by
27 weight of water. The diameter of the plug is such that it will sealingly
fit inside
28 the reservoir prior to hydration as a result of sealing contact at one or
more
29 circumferential zones and will expand in place upon wetting to form an even
tighter seal with the reservoir. The polymeric materials from which the
31 semipermeable plug may be made vary based on the pumping rates and
CA 02533678 1997-01-15
= =
WO 97/27840 PCT/US97/00722
11
1 device configuration requirements and include but are not limited to
2 plasticized cellulosic materials, enhanced polymethylmethacrylate such as
3 hydroxyethylmethacrylate (HEMA) and elastomeric materials such as
4 polyurethanes and polyamides, polyether-polyamide copolymers,
thermoplastic copolyesters and the like.
6 The piston 16 isolates the water-swellable agent in chamber 20 from
7 the active agent in chamber 18 and must be capable of sealably moving
8 under pressure within reservoir 12. The piston 16 is preferably made of a
9 material that is of lower durometer than the reservoir 12 and that will
deform
to fit the lumen of the reservoir to provide a fluid-tight compression seal
with
11 the reservoir 12. The materials from which the piston are made are
12 preferably elastomeric materials that are impermeable and include but are
not
13 limited to polypropylene, rubbers such as EPDM, silicone rubber, butyl
14 rubber, and the like, and thermoplastic elastomers such as plasticized
polyvinylchloride, polyurethanes, Santoprene , C-Flex TPE (Consolidated
16 Polymer Technologies Inc.), and the like. The piston may be of a self-
loading
17 or compression-loaded design.
18 The back-diffusion regulating outlet 22 forms the delivery pathway
19 through which the active agent flows from the chamber.18 to the
implantation
site where absorption of the active agent takes place. The seal between the
21 outlet 22 and the reservoir 12 can be designed to withstand the maximum
22 osmotic pressure generated within the device or to fail-safe in the modes
23 described above. In a preferred embodiment, the pressure required to
24 release back-diffusion regulating outlet 22 is at least 10X the pressure
required to move piston 16 and/or at least 1 OX the pressure in chamber 18.
26 The exit flow path of the active agent is the pathway 34 formed
27 between the mating surfaces of the back-diffusion regulating outlet 22 and
the
28 reservoir 12. The pathway length, interior cross-sectional shape and area
of
29 the outlet path 34 or 36 are chosen such that the average linear velocity
of
the exiting active agent is higher than that of the linear inward flux of
materials
31 in the environment of use due to diffusion or osmosis, thereby attenuating
or
CA 02533678 1997-01-15
= =
WO 97/27840 PCT/US97/00722
12
1 moderating back-diffusion and its deleterious effects of contaminating the
2 interior of the pump, destabilizing, diluting, or otherwise altering the
3 formulation. 'The release rate of active agent can be modffied by modifying
4 the outlet pathway geometry, which relationship is shown below.
The convective flow of active agent out of outlet 22 is set by the
s pumping rate of the system and the concentration of active agent in chamber
7 20 and can be represented as follows:
8
9 Qca = (Q) (Ca) (1)
where
11 Qc. is the convective transport of agent A in mg/day
12 Q is the overall convective transport of the agent and its
13 diluents in cm3/day
14 C. is the concentration of agent A in the formulation within
,s chamber 20 in mg/cm3
16
17 The diffusive flow of agent A through the material in the outlet 22 is a
18 function of agent concentration, cross-sectional configuration of flow path
34
19 or 36, agent diffusivity and length of flow path 34 or 36, and can be
represented as follows:
21
22 Qda=DnrzA Ca/L (2)
23 where
24 Qda is the diffusive transport of agent A in mg/day
D is the diffusivity through the material in path 34 or 36 in
26 cm2/day
27 r is the effective inner radius of the flow path in cm
28 OCa is the difference between the concentration of agent A in
29 the reservoir and in the body outside of the outlet 22 in
mg/cm3
31 L is the length of the flow path in cm
CA 02533678 1997-01-15
WO 97/27840 PCT/US97/00722
13
2 In general, the concentration of agent in the reservoir is much greater
3 than the concentration of agent in the body outside of the orifice such that
the
4 difference, ACa can be approximated by the concentration of agent within the
reservoir, Ca.
6
7 Qda=Dnr2Ca/L (3)
8
s It is generally desirable to keep the diffusive flux of agent at less than
10% of the convective flow. This is represented as follows:
11
12 Qda/Q,a = D n~ Ca/QCaL = Dn rz/QL _ 0.1 (4)
13
14 Equation 4 indicates that the relative diffusive flux decreases with
increasing volumetric flow rate and path length and increases with increasing
16 diffusivity and channel radius and is independent of drug concentration.
17 Equation 4 is plotted in Figure 4 as a function of length (L) and diameter
(d)
18 for D = 2 x 10'6 cm2/sec and Q = 0.36 Uday.
19 The diffusive flux of water where the orifice opens into chamber 18
can be approximated as:
21
22 Qm,d (res) = C,Qe (-QUDwA) (5)
23 where
24 C. is the concentration profile of water in mg/cm3
Q is the mass flow rate in mg/day
26 L is the length of the flow path in cm
27 D,, is the diffusivity of water through the material in the flow path in
28 cm2/day
29 A is the cross-sectional area of the flow path in cm2
CA 02533678 1997-01-15
~
=
WO 97/27840 PCT/US97/00722
14 -
1 The hydrodynamic pressure drop across the orifice can be calculated
2 as follows:
3
4 OP = $Ou (6)
7tr4
6
7 Simultaneously solving equations (4), (5) and (6) gives the values
8 shown in Table 1 where:
s
Q =0.38 l/day
11 Ca = 0.4 mg/ l
12 L =5cm
13 Da = 2.00 E-06 cm2/sec
14 =5.00E+02cp
C,,,,o = 0 mg/ l
is DW =6.00E+06cm2/sec
17
18
19 Table I
21
Drug Diffusion & Pumping Water Intrusion Pressure Drop
Effective Pump rate Diffusion Diff/Conv
Orifice dia Cross Sec QC, QD, QD QC QD, Qdw deRa P
(mil) area (mm2) mgiday mg/day m da mglyear psi
1 0.00051 0.152 0.0001 0.0005 0 0 1.55800
2 0.00203 0.152 0.0003 0.0018 1.14E-79 4.16E-77 0.09738
3 0.00456 0.152 0.0006 0.0041 4.79E-36 1.75E-33 0.01923
4 0.00811 0.152 0.0011 0.0074 8.89E-21 3.25E-18 0.00609
5 0.01267 0.152 0.0018 0.0115 1.04E-13 3.79E-11 0.00249
6 0.01824 0.152 0.0025 0.0166 7.16E-10 2.61E-07 0.00120
7 0.02483 0.152 0.0034 0.0226 1.48E-07 5.4E-05 0.00065
8 0.03243 0.152 0.0045 0.0295 4.7E-06 0.001715 0.00038
9 0.04105 0.152 0.0057 0.0373 5.04E-05 0.018381 0.00024
10 0.05068 0.152 0.0070 0.0461 0.000275 0.100263 0.00016
11 0.06132 0.152 0.0085 0.0558 0.000964 0.351771 0.00011
12 0.07298 0.152 0.0101 0.0664 0.002504 0.913839 0.00008
13 0.08564 0.152 0.0118 0.0779 0.005263 1.921027 0.00005
14 0.09933 0.152 0.0137 0.0903 0.00949 3.463836 0.00004
15 0.11402 0.152 0.0158 0.1037 0.015269 5.573195 0.00003
16 0.12973 0.152 0.0179 0.1180 0.022535 8.225224 0.00002
17 0.14646 0.152 0.0202 0.1332 0.031114 11.35656 0.00002
18 0,16419 0.152 0.0227 0.1493 0.040772 14.88166 0.00001
19 0.18295 0.152 0.0253 0.1664 0.051253 18.70728 0.00001
20 0.20271 0.152 0.0280 0.1844 0.062309 22.7427 0.00001
22
CA 02533678 1997-01-15
16696-260
~. ~ The calculations indicate that an orifice diameter of between about.3
zand 10 mil and a length of 2 to 7 cm is optimal for a device with the
operating
3 conditions described. In a preferred embodiment, the pressure drop across-
4 the orifice is less than 10% of the pressure required to release the back-
s diffusion regulating outlet 22.
6 The back-diffusion regulating outlet 22 preferably fomns a belical
7 pathway 34 or 36 incorporating a long flow path with a means of
mechanically.
s attaching the outlet into the reservoir without using adhesives or other
s sealants. The back-diffusion regulating outlet is made of an inert and
10 biocompatible material selected from but not limited to metals including
but
11 not limited to titanium, stainless steel, platinum and their alloys and
coba6-
12 chromium alloys and the like, and polymers including but not limited to
13 polyethylene, polypropylene, polycarbonate and poiymethylmethacrylate and
14 the like. The flow path is usually between about 0.5 and 20 cm long,
~s preferably between about I and'10 cm long and between about 0.001 and
16 0.020 inches in diameter, preferably between about 0.003 and 0.015 inches
to allow for a flow of between about 0.02 and 50 Vday, usually0.2 to 10
ie Uday and often 0.2 to 2.0 Uday. Additionally, a catheter ot other system
is may be attached to the end of the back-diffusion tegulating outlet to
provide.
zo for delivery of the active agent formulation at a site removed from the
implant.
21 Such systems are known in the art and are described, for example. in U.S.
ss Patent Nos. 3,732,865 and 4,340,054.
23 Further, the flow path design may be useful in systems other than
24 the fluid-imbibing devices specifically described herein.
The inventive device configurations described above also allow for a
26 minimal period of delay from start-up to steady-state flow rate. This is
27 accomplished in part as a resutt of the configuration of the semipermeable
28 -plug 24 or 26. As water is imbibed by the semipermeable plug, it swells.
29 Radial expansion is limited by the rigid reservoir 12, thus the expansion
must
occur linearly, thereby pushing against the water-swellable agent in chamber
31 18, which in tum pushes against the piston 16. This allows pumping to
CA 02533678 1997-01-15
67696-260
16
i_ commence prior to the time that water reaches the water-swellable agent
2 which othenwise would be required before pumping could commence. jo, :
3 facilitate reliable start-up, the flow path 34 can be precharged with the
active
4 agent in chamber 18. Further, the geometry of the outlet 22 allows for
initial
s delivery that is influenced by the concentration gradient of drug along the
6 length of the outlet. The start-up period is less than about 25% of the ~
7 predetermined delivery period and is often less than about 10% and usually
a less than about 5% of the predetermined delivery period. In a preferred
:s embodiment for a one year system, at least 70% of the steady-state flow
rate
-,o is achieved by day 14.
11 The water-swellable agent formulation in chamber 20 is preferably a.
12 tissue tolerable formulation whose high osmotic pressure and high
solubility
13 propels the active agent over a long period of time while remaining in
14 saturated solution in the water admitted by the semipermeable membrane.
15 The water-swellable agent is preferably selected for tolerability by
16 subcutaneous tissue, at least at pumping rates and hypothetically resulting
17 concentrations to allow inadvertent dispensing from implanted devices left
in
,e the patient for a longer than labeled period. In preferred embodiments, the
19 water-swellable agent should not diffuse or permeate through the
zo semipermeable plug 24 or 26 to any appreciable amount (e.g., less than 8%)
21 under normal operating conditions. Osmotic agents, such as NaCI with
.22 appropriate tabletting agents (lubricants and binders) and viscosity
modifying
23 agents, such as sodium carboxymethylcellulose or sodium polyacrylate are
24 preferred water-swellable agents. Other osmotic agents useful as the water-
25. swellable agent inclUde osmopolymers and osmagents and are described, for
26 example, in U.S. Patent No. 5,413,572.
27 The water-swellable agent formulation can be a slurry, a tablet, a
za molded or extruded material or other forrin known in the art. A liquid or
gel
29 additive or filler may be added to chambet 20 to exclude air from spaces
ao around the osmotic engine. Exclusion of air from the devices should mean
CA 02533678 1997-01-15
-~ ~
WO 97/27840 PCT/US97/00722
17
, that delivery rates will be less affected by nominal external pressure
changes
2 (e.g., 7 p.s.i. ( 5 a.t.m.)).
3 The devices of the invention are useful to deliver a wide variety of
4 active agents. These agents include but are not limited to pharmacologically
active peptides and proteins, genes and gene products, other gene therapy
6 agents, and other small molecules. The polypeptides may include but are not
7 limited to growth hormone, somatotropin analogues, somatomedin-C,
8 Gonadotropic releasing hormone, follicle stimulating hormone, luteinizing
s hormone, LHRH, LHRH analogues such as leuprolide, nafarelin and
goserelin, LHRH agonists and antagonists, growth hormone releasing factor,
11 calcitonin, coichicine, gonadotropins such as chorionic gonadotropin,
12 oxytocin, octreotide, somatotropin plus an amino acid, vasopressin,
13 adrenocorticotrophic hormone, epidermal growth factor, prolactin,
14 somatostatin, somatotropin plus a protein, cosyntropin, lypressin,
polypeptides such as thyrotropin releasing hormone, thyroid stimulation
16 hormone, secretin, pancreozymin, enkephalin, glucagon, endocrine agents
17 secreted internally and distributed by way of the bloodstream, and the
like.
18 Further agents that may be delivered include alantitrypsin, factor VIII,
factor
19 IX and other coagulation factors, insulin and other peptide hormones,
adrenal
cortical stimulating hormone, thyroid stimulating hormone and other pituitary
21 hormones, interferon a, R, and S, erythropoietin, growth factors such as
22 GCSF, GMCSF, insulin-like growth factor 1, tissue plasminogen activator,
23 CD4, dDAVP, interieukin-1 receptor antagonist, tumor necrosis factor,
24 pancreatic enzymes, lactase, cytokines, interleukin-1 receptor antagonist,
interieukin-2, tumor necrosis factor receptor, tumor suppresser proteins,
26 cytotoxic proteins, and recombinant antibodies and antibody fragments, and
27 the like.
28 The above agents are useful for the treatment of a variety of conditions
29 including but not limited to hemophilia and other blood disorders, growth
disorders, diabetes, leukemia, hepatitis, renal failure, HIV infection,
hereditary
31 diseases such as cerbrosidase deficiency and adenosine deaminase
CA 02533678 1997-01-15
i =
WO 97/27840 pCT/US97/00722
18
1 deficiency, hypertension, septic shock, autoimmune diseases such as
2 multiple sclerosis, Graves disease, systemic lupus erythematosus and
3 rheumatoid arthritis, shock and wasting disorders, cystic fibrosis, lactose
4 intolerance, Crohn's diseases, inflammatory bowel disease, gastrointestinal
and other cancers.
6 The active agents may be anhydrous or aqueous solutions,
7 suspensions or complexes with pharmaceutically acceptable vehicles or
e carriers such that a flowable formulation is produced that may be stored for
9 long periods on the shetf or under refrigeration, as well as stored in an
implanted delivery system. The formulations may include pharmaceutically
11 acceptable carriers and additional inert ingredients. The active agents may
12 be in various forms, such as uncharged molecules, components of molecular
13 complexes or pharmacologically acceptable salts. Also, simple derivatives
of
14 the agents. (such as prodrugs, ethers, esters, amides, etc.) which are
easily
hydrolyzed by body pH, enzymes, etc., can be employed.
16 It is to be understood that more than one active agent may be
17 incorporated into the active agent formulation in a device of this
invention and
18 that the use of the term "agent" in no way excludes the use of two or more
19 such agents. The dispensing devices of the invention find use, for example,
in humans or other animals. The environment of use is a fluid environment
21 and can comprise any subcutaneous position or body cavity, such as the
22 peritoneum or uterus, and may or may not be equivalent to the point of
23 ultimate delivery of the active agent formulation. A single dispensing
device
24 or several dispensing devices can be administered to a subject during a
therapeutic program. The devices are designed to remain implanted during a
26 predetermined administration period. If the devices are not removed
following
27 the administration, they may be designed to withstand the maximum osmotic
28 pressure of the water-swellable agent or they may be designed with a bypass
29 to release the pressure generated within the device.
The devices of the present invention are preferably rendered sterile
31 prior to use, especially when such use is implantation. This may. be
CA 02533678 1997-01-15
. =
WO 97/27840 PCT/US97/00722
19
, accomplished by separately sterilizing each component, e.g., by gamma
2 radiation, steam sterilization or sterile filtration, then aseptically
assembling
3 the final system. Alternatively, the devices may be assembled, then
4 terminally sterilized using any appropriate method.
6 Preparation of the Devices of the Invention
7
8 Reservoir 12 is prepared preferably by machining a metal rod or by
9 extrusion or injection molding a polymer. The top portion of the reservoir
may
be open as shown in Fig. I or may contain a cavity as shown in Fig. 2.
11 Where the reservoir 12 is open as shown in Fig.. 1, a water-swellable
12 semipermeable plug 24 is inserted mechanically from the outside of the
13 reservoir without using an adhesive before or after insertion of the piston
and
14 water-swellable agent formulation. Reservoir 12 may be provided with
grooves or threads which engage ribs or threads on plug 24.
16 Where the reservoir 12 contains a cavity as shown in Fig. 2, the cavity
17 may be cylindrical in shape, as shown in Fig. 5, it may be stepped, as
shown
18 in Fig. 6, it may be helical, as shown in Fig. 7 or it may be in a spaced
19 configuration, as shown in Fig. 8. The semipermeable plug 26 is then
injected, inserted, or otherwise assembled into the cavity so that it forms a
21 seal with the reservoir wall.
22 Following insertion of the plug 26 either mechanically, by welding or by
23 injection, the water-swellable agent is assembled into the reservoir
foliowed
24 by insertion of the piston, with appropriate steps taken to vent entrapped
air.
The active agent is filled into the device using a syringe or a precision
26 dispensing pump. The diffusion moderator is inserted into the device,
usually
27 by a rotating or helical action, or by axial pressing.
28 The following examples are illustrative of the present invention. They
29 are not to be construed as limiting the scope of the invention. Variations
and
equivalents of these examples will be apparent to those of skill in the art in
31 light of the present disclosure, the drawings and claims herein.
CA 02533678 1997-01-15
= = .
WO 97/27840 PCTIUS97/00722
2 Examples
3
4 Example 1- Preparation of a Device with an HDPE Reservoir
5
6 A system containing leuprolide acetate for the treatment of prostate
7 cancer was assembled from the following components:
8 Reservoir (HDPE) (5 mm outside diameter, 3 mm inside diameter)
9 Piston (Santoprene )
10 Lubricant (silicone medical fluid)
11 Compressed osmotic engine (60% NaCI, 40%. sodium carboxymethyl
12 cellulose)
13 Membrane plug (Hytrel polyether-ester block copolymer, injection
14 molded to desired shape)
15 Back diffusion Regulating Outlet (polycarbonate)
16 Active agent (0.78g of 60% propylene glycol and 40% leuprolide
rn acetate)
18 Assembly
19 The piston and inner diameter of the reservoir were lightly lubricated
20 with silicon medical fluid. The piston 16 was inserted into the open end of
21 chamber 20. Two osmotic engine tablets (40 mg each) were then inserted on
22 top of piston 16. After insertion, the osmotic engine was flush with the
end of
23 the reservoir. The membrane plug 24 was inserted by lining up the plug with
24 the reservoir and pushing gently until the plug was fully engaged in the
reservoir. Active agent was loaded into a syringe which was then used to fill
26 chamber 18 from its open end by injecting the material into the open tube
until
27 the formulation was -3 mm from the end. The filled reservoir was
centrifuged
28 (outlet end "up") to remove any air bubbles that have been trapped in the
29 formulation during filling. The outlet 22 was screwed into the open end of
the
reservoir until completely engaged. As the outlet was screwed in, excess
31 formulation exited out of the orifice ensuring a uniform fill.
CA 02533678 1997-01-15
0 0
WO 97/27840 PCT/US97/00722
21
2 Example 2 - Insertion of the Device of Example 1
3
4 Insertion of the device of Example 1 is done under aseptic conditions
using a trocar similar to that used in the implantation of Norplant
6 contraceptive implants to position the device under the skin. The insertion
7 area is typically in the inside of the upper arm, 8 to 10 cm above the
elbow.
8 The area is anesthetized and an incision is made through the skin.
9 The incision is approximately 4 mm long. The trocar is inserted into the
incision until the tip of the trocar is at a distance of 4 to 6 cm from the
incision.
11 The obturator is then removed from the trocar and the device of Example 1
12 inserted into the trocar. The device is then advanced to the open end of
the
13 trocar using the obturator. The obturator is then held in position, thus
14 immobilizing the device of Example 1 while the trocar is withdrawn over
both
the device and the obturator. The obturator is then removed, leaving the
16 implant behind in a well-controlled position. The edges of the incision are
17 then secured with a skin closure. The area is covered and kept dry for 2 to
3
18 days.
19
Example 3 - Removal of the Device of Example 1
21
22 The device of Example 1 is removed as follows: The device is located
23 by fingertip palpation of the upper arm area. The area at one end of the
24 implant is then anesthetized and an approximately 4 mm, perpendicular
incision is made through the skin and any fibrous capsule tissue surrounding
26 the implant area. The end of the device opposite the incision is pushed so
27 that the device end proximal to the incision is urged out of the incision.
Any
28 further fibrotic tissue is cut with a scalpel. Following removal, the
procedure
29 of Example 2 can be followed to insert a new device.
CA 02533678 1997-01-15
WO 97/27840 PCTlUS97/00722
22
Example 4- Delivery Rate of the Device of Example 1
2
3 Glass test tubes were filled with 35 mi distilled water and then placed
4 in a 37 C water bath. A single device as described in Example 1 was placed
in each test tube and the test tubes were changed periodically. The delivery
6 rate profile from the system is shown in Fig. 9. The system does not have
7 any start-up time because the system exhibits a period of initial high
release
8 followed by a lower steady state release for a period of 200 days.
9
Example 5- Delivery Rate Profiles
11
12 Glass test tubes were filled with 35 mi distilled water which were then
13 placed in a 37 C water bath. After the test tubes had come up to
14 temperature, a single device as described in Example 1, but with membrane
materials described below and containing 1% FD&C blue dye in water as the
16 drug formulation, was placed in each tube. Water from the test tube
17 permeated through the membrane causing the system to pump formulation
,a (blue dye) into the surrounding water in the test tube. At regular
intervals,
19 systems were switched to fresh test tubes. The amount of dye released was
2o determined by measuring the concentration of blue dye in each test tube
21 using a spectrophotometer. The pumping rate was calculated from the total
22 dye released, the volume of water in the tube, the initial concentration of
dye
23 and the interval over which the system was in the test tube. Results for
two
24 different tests are shown in Figures 10 and 11. Figure 10 shows 3 different
systems with different plug materials (Hytrel 2, 3 and 12 month systems) and
26 Figure 11 shows 4 systems with different plug materials. These materials
are:
27 Membrane Material
28 1 month Pebax 25 (Polyamide)
29 2 month Pebax 22 (Polyamide)
3 month Polyurethane (HP60D)
31 12 month Pebax 24 (Polyamide)
CA 02533678 1997-01-15
= ~
WO 97/27840 PCT/US97/00722
23 -
I The systems were capable of delivering for a period of from 2 to 12
2 months, depending on the membrane used.
3
4 Example 6 - Preparation of a Delivery Device with a Titanium Reservoir
6 A system containing leuprolide acetate for the treatment of prostate
7 cancer was assembled from the following components:
e Reservoir (Titanium, Ti6A14V alloy )(4 mm outside diameter, 3 mm
9 inside diameter)
Piston (C-Flex )
11 Lubricant (silicone medical fluid)
12 Compressed osmotic engine (76.4% NaCI, 15.5% sodium
13 carboxymethyl cellulose, 6% povidone, 0.5% Mg Stearate, 1.6%
14 water)
PEG 400 (8 mg added to osmotic engine to fill air spaces)
16 Membrane plug (polyurethane polymer, injection molded to desired
17 shape)
,s Back diffusion Regulating Outlet (polyethylene)
19 Drug formulation (0.150g of 60% water and 40% leuprolide acetate)
Assemblv
21 The piston and inner diameter of the reservoir were lightly lubricated.
n The piston was inserted -0.5 cm into the reservoir at the membrane end.
23 PEG 400 was added into the reservoir. Two osmotic engine tablets (40 mg
24 each) were then inserted into the reservoir from the membrane end. After
insertion, the osmotic engine was flush with the end of the reservoir. The
26 membrane plug was inserted by lining up the plug with the reservoir and
27 pushing gently until the retaining features of the plug were fully engaged
in
28 the reservoir. Formulation was loaded into a syringe which was then used to
29 fill the reservoir from the outlet end by injecting formulation into the
open tube
until the formulation was --3 mm from the end. The filled reservoir was
31 centrifuged (outlet end "up") to remove any air bubbles that have been
CA 02533678 1997-01-15
WO 97/27840 PCT/US97100722
24
"1 trapped in the formulation during filling. The outlet was screwed into the
open
2 end of the reservoir until completely engaged. As the outlet was screwed in,
3 excess formuiation exited out of the orifice ensuring a uniform fill.
4
Example 7 - Preparation of a Leuprolide Acetate Delivery Device with a
6 Titanium Reservoir
7
a A system containing leuprolide acetate for the treatment of prostate
9 cancer was assembled from the following components:
Reservoir (Titanium Ti6AI4V alloy) (4 mm outside diameter, 3 mm
11 inside diameter, 4.5 cm length)
12 Piston (C-Flex TPE elastomer, available from Consolidated Polymer
13 Technologies, Inc.)
14 Lubricant (silicone medical fluid 360)
Compressed osmotic engine tablet (76.4% NaCI, 15.5% sodium
16 carboxymethyl cellulose, 6% povidone, 0.5% Mg Stearate, 1.5%
17 water, 50 mg total)
18 PEG 400 (8 mg added to osmotic engine to fill air spaces)
19 Membrane plug (polyurethane polymer 20% water uptake, injection
molded to desired shape 3 mm diameter X 4 mm length)
21 Back-diffusion Regulating Outlet (polyethylene, with 6 mil X 5 cm
22 channel)
23 Drug formulation (leuprolide acetate dissolved in DMSO to a measured
24 content of 65 mg leuprolide)
Assembly
26 Systems were assembled as in Example 6, using aseptic procedures
27 to assemble y-irradiated subassemblies and filled aseptically with sterile
28 filtered leuprolide DMSO formulation.
29 Release Rate
These systems delivered about 0.35 Uday leuprolide formulation
31 containing on average 150 g leuprolide in the amount delivered per day.
CA 02533678 1997-01-15
0
WO 97/27840 PCT/US9'7/00722
I They provide delivery of leuprolide at this rate.for at least one year. The
2 systems achieved approximately 70% steady-state delivery by day 14.
3 Implantationand Removal
4 Systems will be implanted under local anesthetic and by means of an
5 incision and trocar as in Example 2 to patient suffering from advanced
6 prostatic cancer.
7 After one year, systems will be removed under locai anesthetic as
a described in Example 3. New systems may be inserted at that time.
9
10 Example 8 - Treatment of Prostatic Cancer
11
12 Leuprolide acetate, an LHRH agonist, acts as a potent inhibitor of
13 gonadotropin secretion when given continuously and in therapeutic doses.
14 Animal and human studies indicate that following an initial stimulation,
chronic
15 administration of leuprolide acetate results in suppression of testicular
16 steroidogenesis. This effect is reversible upon discontinuation of drug
17 therapy. Administration of leuprolide acetate has resulted in inhibition of
the
18 growth of certain hormone-dependent tumors (prostatic tumors in Noble and
19 Dunning male rats and DMBA-induced mammary tumors in female rats) as
20 well as atrophy of the reproductive organs. In humans, administration of
21 leuprolide acetate results in an initial increase in circulating levels of
22 luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to
a
23 transient increase in levels of the gonadal steroids (testosterone and
24 dihydrotestosterone in males). However, continuous administration of
25 leuprolide acetate results in decreased level of LH and FSH. In males,
26 testosterone is reduced to castrate levels. These decreases occur within
two
27 to six weeks after initiation of treatment, and castrate levels of
testosterone in
28 prostatic cancer patients have been demonstrated for multiyear periods.
29 Leuprolide acetate is not active when given orally.
CA 02533678 1997-01-15
~ = =
WO 97/27840 PCT/US97/00722
26
~ Systems will be prepared as in Example 7, then inserted as described.
2 The continuous administration of leuprolide for one year using these systems
3 will reduce testosterone to castrate levels.
4 The above description has been given for ease of understanding only.
No unnecessary limitations should be understood therefrom, as modifications
6 will be obvious to those skilled in the art.