Note: Descriptions are shown in the official language in which they were submitted.
CA 02534031 2006-02-03
METHOD AND APPARATUS FOR OPERATdNG A METHANE-
FUELLED ENGINE AND TREATING EXHAUST GAS WITH A
METHANE OXIDATION CATALYST
Field of the Invention
[0001] The present invention relates to a method and apparatus for
operating a methane-fuelled engine and treating exhaust gas with a
methane oxidation catalyst. The invention can be applied to vehicle
engines or other engines that are operated with variable load cycles to
reduce emissions of carbon monoxide, methane and other unburned
hydrocarbons.
Background of the Invention
[0002] Natural gas is comprised mostly of methane. Natural gas is burned
as a fuel in internal combustion engines because in many markets around
the world, natural gas is less expensive on an energy basis compared to
diesel or gasoline. In addition, natural gas is cleaner burning compared to
diesel or gasoline, which can help to improve air quality, providing
another incentive to replace vehicle engines that burn diesel or gasoline
with engines that burn natural gas. However, in the exhaust gases expelled
from the combustion chambers of an internal combustion engine, there can
remain unburned fuel, and the same is true for engines fuelled with natural
gas. Because methane is a greenhouse gas, it is desirable to oxidize
unburned methane before the exhaust gases leave a vehicle's exhaust pipe.
[0003] There are a number of approaches for burning natural gas in an
engine. So-called stoichiometric natural gas engines use an actual air to
fuel ratio of about 14.6:1, which corresponds to a lambda of 1, since
lambda is calculated by dividing the actual air-fuel ratio by 14.6 (which is
the theoretical stoichiometric ideal air-fuel ratio). As defined herein an
engine operating in a stoichiometric mode need not necessarily operate
with a lambda of exactly 1.0, but with a lambda that is at or near 1.0
CA 02534031 2006-02-03
-2-
whereby there is substantially no excess oxygen in the charge of air and
fuel that is combusted in the engine's combustion chamber. U.S. Patent
No. 5,131,224 discloses a method of reducing methane exhaust emissions
from a natural gas fuelled engine. The '224 patent teaches operating a
natural gas engine in a stoichiometric operating mode with an air-fuel
mixture that is on average slightly fuel-rich (that is, with lambda being, on
average, less than 1.0), and using a platinum or platinum-palladium (non-
rhodium) catalytic converter for exhaust gas treatment. As is known in the
prior art, emissions of NOx and methane can be reduced by using exhaust
gas recirculation and a three-way catalyst, but these techniques add
significantly to the overall cost of the system.
[0004] So-called lean burn spark ignition ("LBSI") natural gas engines
bum a lean mixture of natural gas with ignition triggered by a spark plug.
A lean mixture means that there is a surplus of oxygen in the combustion
chamber, so lambda is greater than 1. LBSI natural gas engines can be less
complicated and less costly, while producing lower emissions of nitrogen
oxide (NOx), compared to stoichiometric natural gas engines operating
without a three-way catalyst and exhaust gas recirculation, because higher
air-fuel ratios result in cooler combustion temperatures. LBSI natural gas
engines can also produce lower emissions of carbon dioxide compared to
stoichiometric natural gas engines. Accordingly, LBSI engines offer cost
and performance advantages over stoichiometric natural gas engines that
don't employ exhaust gas recirculation or three-way catalysts. However, a
problem with LBSI natural gas engines is that methane oxidation catalysts
can be inhibited by exposure to sulfur oxides (SOx), even at very low
concentrations, resulting in deteriorating methane oxidation conversion
efficiencies. It is believed that the SOx are chemisorbed onto the catalyst
wash coat, effectively blocking the conversion site for methane. For
CA 02534031 2006-02-03
-3-
example, experimental results have shown that a SOx concentration level
of only 1 ppm (w/w) can result in a 25% reduction in methane conversion
efficiency in less than 50 hours of operation, and such a concentration can
be introduced into the engine exhaust gas from sulfur that is present in the
natural gas and engine lubricating oil. For example, a contributor to SOx
in the exhaust gas can be the odorant that is normally added to natural gas
for olfactory detection.
[0005] A report prepared by Engelhard Corporation for the Gas Research
Institute, entitled "Catalyst Development for Methane Emissions
Abatement From Lean Burn Natural Gas Vehicles" dated November 1997
reports on work done between January 1994 through May 1997, with the
objective of developing a lean burn natural gas vehicle catalytic converter
that will continue to oxidize methane over the lifetime of the vehicle. A
catalyst containing palladium (Pd) such as Pd/alumina (A1203) was found
to be the most active catalyst for methane emissions abatement from
natural gas engines. In its final report, Engelhard Corporation reported
that it failed to achieve its objective because the presence of SOx in the
engine's exhaust gas inhibited catalyst activity. Engelhard Corporation's
research focused on developing a sulfur-resistant catalyst and investigating
the de-activation mechanism of palladium oxidation catalysts under
laboratory conditions that closely resemble actual natural gas vehicle
exhaust.
[0006] Published SAE technical paper 961971, entitled, "Methane
Emissions Abatement from Lean Burn Natural Gas Vehicle Exhaust:
Sulfur's Impact on Catalyst Performance" is authored by employees of
Engelhard Corporation. On page 18 of this SAE technical paper the
authors disclose a strategy for periodic thermal-reduction regeneration so
that the catalyst maintains acceptable activity in the presence of sulfur.
CA 02534031 2006-02-03
-4-
Specifically, the disclosed strategy involves operating a natural gas engine
to cycle the catalyst between a lean, high space velocity mode lasting 14.5
minutes and a rich, low space velocity mode for 30 seconds. The catalyst
temperature increases from 550 degrees Celsius during the lean mode to
approximately 650 degrees Celsius during the rich mode. The authors
concluded from their experimental results that this strategy only delays the
eventual decay in catalyst methane activity.
[0007] Published Japanese patent application number JP2000200005 8777
(publication number JP2003254117A2), entitled "Exhaust Emission
Controlling Method" (the '58777 Application), like SAE technical paper
961971, discloses a method of alternately burning the fuel in a lean
atmosphere of excessive air and a rich atmosphere of excessive fuel. The
exhaust gas from burning the fuel in a rich atmosphere subjects the
inhibited catalyst sites to a reducing atmosphere that regenerates the
inhibited catalyst sites thereby recovering methane oxidizing catalyst
activity and restoring methane oxidization conversion rates. According to
the '58777 Application, the timing for regenerating the inhibited catalyst
sites can be determined by calculating when the catalyst's methane
conversion efficiency has deteriorated by a predetermined amount, with
this calculation being based upon a number of parameters including sulfur
concentration in the exhaust gas, air-fuel ratio, and exhaust gas
temperature. A problem with this approach is that it adds complexity to
the control of the engine, since this approach involves calculating the
timing for desulphation of the methane oxidation catalyst, and two control
strategies for operating the engine in a lean bum or fuel rich mode, since
the timing for operating in a fuel rich mode is dependent on a calculated
timing which can occur at any point on the engine map.
CA 02534031 2006-02-03
-5-
[0008] Accordingly, there is a need for an improved control strategy for
operating a methane-fuelled engine to simply and effectively achieve some
of lower emissions benefits associated with LBSI methane-fuelled engines,
while reducing emissions of unburned methane by managing the
performance and desulphation of a methane oxidation catalyst.
Summary of the Invention
[0009] A method is provided for operating a methane-fuelled engine and
of treating exhaust gas coming from the engine to reduce emissions of
methane and nitrogen oxides. In preferred embodiments of the method is
engine is fuelled with natural gas. The disclosed method comprises
fuelling the engine with a lean fuel mixture when operating the engine at
one of a predetermined first set of points on an engine map; fuelling the
engine with a rich fuel mixture when operating the engine at one of a
predetermined second set of points on the engine map; and flowing an
exhaust gas from the engine through a methane oxidation catalyst.
[0010] For example, the predetermined second set of points on the engine
map can be associated with when the engine is operating within a
predetermined high engine speed range and within a predetermined low
engine load range. In a preferred method, the predetermined low engine
load range is defined by when the engine is operating at less than 20
percent of maximum engine load and the predetermined high engine speed
range is defined by when the engine is operating with a speed that is at
least 80 percent of maximum engine speed. Preferably the predetermined
second set of points on the engine map are associated with when the
engine is operating within a predetermined engine speed range and within
a predetermined engine load range in which the engine can be, and the
engine is controlled to be, operated in a stoichiometric operating mode
CA 02534031 2006-02-03
-6- .
with an exhaust gas temperature between the engine and the methane
oxidation catalyst of at least 600 degrees Celsius and more preferably a
temperature between 650 and 800 degrees Celsius. In one embodiment the
predetermined engine load range for operating with a rich fuel mixture
corresponds to when inlet manifold pressure is less than about 85 kPa
absolute (about 12 psia).
[0011] When the engine is operating in a stoichiometric operating mode,
the rich fuel mixture can be produced by reducing the air mass flow rate
through the intake air manifold and into the engine's combustion chamber.
In some embodiments the air mass flow rate can be reduced by throttling
air flow through the intake air passage. Methane-fuelled engines equipped
with a turbocharger can reduce air mass flow rate by opening a wastegate
valve so that some of the exhaust gas is by-passed around the
turbocharger's turbine. If the turbocharger is a variable geometry
turbocharger or a variable nozzle turbocharger, the respective geometry or
nozzle can be controlled to reduce air mass flow rate.
[0012] In a preferred method, when the engine is operated with a lean fuel
mixture, the average lambda of the charge formed in the combustion
chamber is at least 1.3, and preferably between 1.3 and 1.7. The method
can further comprise fuelling the methane-fuelled engine with a fuel
mixture comprising methane and hydrogen, and controlling the lean fuel
mixture to have an average lambda between 1.3 and 2Ø When the engine
is operated with a rich fuel mixture, the average lambda of the charge
formed in the combustion chamber is less than or equal to 1.0, and
preferably between 0.95 and 1Ø
[0013] The preferred method further comprises activating a spark plug to
promote ignition of the lean and rich fuel mixtures and advancing timing
CA 02534031 2006-02-03
-7-
for activating the spark plug to an earlier time when fuelling the engine
with the lean fuel mixture.
[0014] The method preferably comprises oxidizing said methane with in
the exhaust gas in the presence of palladium provided by the methane
oxidation catalyst. In one embodiment the palladium can be impregnated
into a washcoat comprising alumina, and the washcoat can be deposited on
a ceramic support comprising silicon carbide or magnesium aluminum
silicate (known as "Cordierite"). In other embodiments the method can
comprise depositing the washcoat on a metallic support.
[00151 In preferred methods the methane oxidation catalyst promotes the
oxidation of methane when the engine is fuelled with a lean fuel mixture
and promotes the reduction of nitrogen oxides to nitrogen when the engine
is fuelled with a rich fuel mixture.
[0016] The method can further comprise commanding the engine to
operate at a point that is one of the predetermined second set of points on
the engine map for a predetermined time as a step in the start-up sequence
for the engine. If the engine is a prime mover for a vehicle or machine, the
controller can be programmed to recognize predefmed conditions when the
vehicle or machine is stationary, and to command the engine to operate at
a point that is one of the predetermined second set of points on the engine
map for the lesser of a predetermined time or until the vehicle is no longer
stationary.
[0017] A methane-fuelled engine is disclosed that comprises an intake air
manifold defining a passage through which air can flow into a combustion
chamber of the engine; a fuel metering valve operable to regulate the mass
flow rate of a fuel comprising methane that is introduced into the
combustion chamber through a fuel supply pipe; a throttle disposed inside
the intake air manifold for regulating the mass flow rate of air that is
CA 02534031 2006-02-03
-8-
introduced into the combustion chamber; an exhaust manifold defining a
passage that is in communication with the combustion chamber for
receiving combustion products from the combustion chamber and
directing the combustion products to an exhaust pipe; a methane oxidation
catalyst disposed in the exhaust pipe; at least one sensor associated with
the methane-fuelled engine for calculating or measuring lambda in the
exhaust manifold or in the intake air manifold; and an electronic controller
programmed to operate the engine in one of a lean bum mode and a fuel-
rich mode at respective predetermined points on an engine map.
[0018] In a preferred embodiment the at least one sensor is a lambda
sensor with a sensing probe disposed in the exhaust manifold or the
exhaust pipe upstream from the methane oxidation catalyst, and the
lambda sensor is operable to send signals representative of the measured
lambda value to the electronic controller.
[0019] In another preferred embodiment the at least one sensor comprises
a first mass flow sensor associated with the intake air manifold and a
second mass flow sensor associated with-the fuel supply pipe, and the first
and second mass flow sensors are operable to send signals representative
of the respective air and fuel mass flow rates to the electronic controller,
and the electronic controller is programmable to calculate lambda of a
charge formed in the combustion chamber.
[0020] In yet another preferred embodiment, the at least one sensor
comprises a first temperature sensor associated with the intake air
manifold; a first pressure sensor associated with the intake air manifold; a
second temperature sensor associated with the fuel supply pipe; a second
pressure sensor associated with the fuel supply pipe; and the electronic
controller is programmable to process data collected from the first and
CA 02534031 2006-02-03
-9-
temperature sensor and the first and second pressure sensor to
second
calculate lambda of a charge formed in the combustion chamber.
[0021] In the preferred apparatus, the methane oxidation catalyst
comprises palladium. The palladium can be impregnated in a washcoat
comprising alumina and the washcoat can be deposited on a ceramic
support comprising silicon carbide or magnesium aluminum silicate. In
another embodiment the washcoat can be deposited on a metallic support.
[0022] The methane oxidation catalyst consists of a number of
components. Generally, it is preferred that the methane oxidation catalyst
comprises at least one catalytically active component, namely a noble
metal selected from the group consisting of palladium, platinum and
rhodium. The methane oxidation catalyst preferably further comprises at
least one oxygen storage component selected from the group consisting of
cerium oxide (known as "Ceria"), and a combination of cerium and
zirconium. In addition, the methane oxidation catalyst preferably further
comprises a scavenger for hydrogen sulfide. The methane oxidation
catalyst can be a three-way catalyst of the type that has been developed for
automotive applications, though such catalyst formulations have not been
developed for use with lean burn methane-fuelled engines.
[0023] In a preferred embodiment, the methane oxidation catalyst
comprises palladium for oxidizing methane when the engine is operating
in a lean burn mode, and also rhodium for reducing nitrogen oxides to
nitrogen when the engine is operating in a stoichiometric mode. The
methane oxidation catalyst can further comprise cerium oxide to serve as
an oxygen storage component, and a scavenger for hydrogen sulfide.
[0024] The fuel can be introduced into the intake air manifold of the
engine in a number of ways. For example, the engine can comprise a port
fuel injection valve for introducing the fuel into an intake port between the
CA 02534031 2006-02-03
-10- =
intake air manifold and the combustion chamber. In another embodiment,
a sparger can be employed for introducing the fuel from the fuel supply
pipe into the intake air manifold upstream from a throttle. Instead of a
sparger, the engine can comprise a plurality of ports provided in a wall of
the intake air manifold upstream from the throttle, wherein the fuel from
the fuel supply pipe is flowable from a plenum through the plurality of
ports into the intake air manifold. An advantage of this arrangement over
a sparger is that it does not block any of the flow area through the intake
air manifold.
[0025] In yet another embodiment, the engine can comprise a fuel
injection valve with a nozzle disposed in the combustion chamber for
introducing the fuel directly into the combustion chamber. The fuel can be
introduced into the combustion chamber during the intake stroke or early
in the compression stroke while the combustion chamber pressure is still
relatively low.
[0026] The disclosed invention has advantages over the prior art.
Compared to a stoichiometric natural gas fuelled engine or an engine that
operates with an even richer fuel mixture as taught by the '244 patent, the
presently disclosed engine and method can operate most of the time in a
lean bum mode, to achieve lower emissions of NOx and carbon dioxide,
while operating only with a rich fuel mixture when the engine is operating
in a predetermined region of the engine map. The authors of SAE
technical paper 961971 described a method of operating in a lean burn
mode for 14.5 minutes and a fuel rich mode for 30 seconds, but their
conclusion was that this approach only delayed the eventual decay in
catalyst performance. Unlike the approach taught in SAE technical paper
981971, with the presently disclosed method and apparatus experimental
results show that methane oxidation catalyst performance can be restored
CA 02534031 2006-02-03
-11- ~
to methane conversion rates of 85% to 90% at maximum fuelling. The
presently disclosed method is also simpler to implement than a timed
periodic catalyst desulphation cycle because two parallel engine control
strategies are not required for lean burn and fuel rich modes. With a timed
desulphation cycle, the engine could be operating under conditions
anywhere on the engine map when it is time for a desulphation cycle, so
parallel control strategies are needed for every operating point on the
engine map. With the presently disclosed method and apparatus, the
engine only operates in a fuel rich mode when the engine is operating in a
predetermined region of the engine map. Japanese patent application
number JP20002000058777 also disclosed a method of periodically
switching between lean burn and fuel rich operating modes, but this
method has the same disadvantages as the method taught by SAE technical
paper 981971.
[0027] Accordingly, the presently disclosed method and apparatus
provides an approach for operating a methane-fuelled engine that can
reduce emissions of NOx and carbon dioxide compared to a stoichiometric
methane-fuelled engine. Experimental results have shown that the
disclosed method and apparatus can allow a methane-fuelled engine to
operate most of the time in a lean burn mode while maintaining the
conversion efficiency of a methane oxidation catalyst by operating in a
fuel rich mode when operating in a predetermined region of the engine
map.
Brief Description of the Drawings
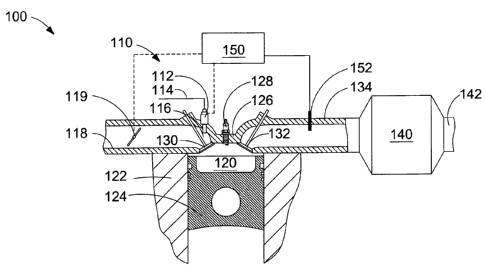
[0028] Figure 1 is a schematic diagram of an apparatus for practicing the
disclosed method. The apparatus comprises a fuel injection system, an
engine combustion chamber, a catalytic converter, and an electronic
CA 02534031 2006-02-03
-12-
controller. In this embodiment a port injector is employed to introduce
fuel into the intake port and a lambda sensor disposed in the exhaust pipe
upstream from the methane oxidation catalyst is employed to control the
air/fuel ratio of the charge that is delivered to the combustion chamber.
[0029] Figure 2 is a schematic diagram of a second embodiment of an
apparatus for practicing the disclosed method. The apparatus of Figure 2
is like the apparatus of Figure 1 except that a sparger is employed to
introduce fuel into the intake air manifold and mass flow sensors for the
intake air and the fuel are employed instead of a lambda sensor to control
the air/fuel ratio of the charge that is delivered to the combustion chamber.
[0030] Figure 3 is a schematic diagram of a third embodiment of an
apparatus for practicing the disclosed method. The apparatus of Figure 3
is like the apparatuses of Figures 1 and 2 except that fuel is introducible
into the intake air manifold through a plurality of ports and temperature
and pressure sensors are employed measure the pressure and temperature
of the intake air and the fuel, so that the electronic controller can
calculate
the mass flow of air and fuel and thereby control the air/fuel ratio of the
charge that is delivered to the combustion chamber. Figure 3 also shows
an embodiment with a turbocharging system that comprises a turbocharger
and a wastegate. Engines that have a turbocharging system can use it to
control the air/fuel ratio of the charge that is delivered to the combustion
chamber.
[0031] Figure 4 is an engine map showing a region on the map where the
engine can be operated with a lean fuel mixture and a region where the
engine can be operated with a rich fuel mixture.
[0032] Figure 5 is a plot of lambda against engine load for different engine
speeds. A plurality of lines are plotted with each line corresponding to a
different engine speed. This plot shows by example an application of the
CA 02534031 2006-02-03
- 13 -
method showing how an engine can be run under lean burn conditions at
most engine speeds and engine loads and that a target lambda can be
determined as a function of engine speed and load.
[0033] Figure 6 is a plot of methane concentration against time, showing
the regenerative effect on the methane oxidation catalyst by application of
the disclosed method. The plotted data is from experimental results that
show the effectiveness of the disclosed method in regenerating a methane
oxidation catalyst.
Detailed Description of Preferred Embodiment(s)
[0034] With reference to the figures, like-named components with like
reference numbers separated by multiples of one hundred refer to like
components in different embodiments.
[0035] Figure 1 is a schematic view of apparatus 100 for practicing the
disclosed method. The apparatus comprises fuel injection system 110,
engine combustion chamber 120, catalytic converter 140, and electronic
controller 150. In this embodiment fuel injection system 110 comprises
port injector 112, which introduces fuel from fuel supply pipe 114 into
intake port 116. Air flows into combustion chamber 120 through intake
air manifold 118, and throttle 119 regulates the flow of air through intake
air manifold 118. Combustion chamber 120 is defined by cylinder block
122, piston 124, and cylinder head 126. The engine can comprise spark
plug 128 for triggering ignition of the charge inside combustion chamber
120. As is well-known for internal combustion engines, intake valve 130
is operable to control the flow of the air and fuel mixture into combustion
chamber 120 from intake air manifold 118, and exhaust valve 132 is
operable to control the flow of combustion products from combustion
chamber 120 to exhaust manifold 134. Exhaust gas flows from exhaust
CA 02534031 2006-02-03
-14-
manifold 134 to a methane oxidation catalyst provided inside catalytic
converter 140. From catalytic converter 140, the exhaust gas flows to
engine exhaust pipe 142.
[0036] In the embodiment illustrated by Figure 1, lambda sensor 152 is
disposed in exhaust manifold 134 upstream from the methane oxidation
catalyst. Lambda measurements from sensor 152 are employed to control
the air/fuel ratio of the charge that is delivered to the combustion chamber.
According to the disclosed invention, with data received from lambda
sensor 152, electronic controller 150 is programmable to operate the
engine in a lean bum mode under most operating conditions with the
charge delivered to combustion chamber 120 having an average lambda
greater than 1.1. Under certain predetermined operating conditions that
are predefined on an engine map, such as a predefined range of engine
speed and a predefined range of engine torque, the engine is controlled to
operate with a richer air-fuel mixture, with the charge delivered to
combustion chamber 120 having a lambda less than or equal to about 1.0
and preferably between 0.95 and 1Ø In the embodiment of Figure 1,
electronic controller 150 processes the measurement of lambda from
lambda sensor 152 and determines from an engine map what the target
lambda is given the current values for engine speed and engine torque. In
this embodiment, the lambda sensor is located in exhaust manifold 134
because it is difficult to measure lambda inside the combustion chamber,
and with a fuel injection system that uses port injectors it is difficult to
measure lambda upstream from the combustion chamber. However, it is
possible for electronic controller 150 to determine lambda inside
combustion chamber 120 from measurements of lambda taken in exhaust
manifold 134 or at any point in an exhaust pipe upstream from catalytic
converter 140. Accordingly, using the measurement of lambda taken by
CA 02534031 2006-02-03
- 15 -
sensor 152, electronic controller 150 can use throttle 119 and port injector
112 to control lambda according to an engine map so that the engine is
operated in a lean bum mode most of the time (with an average lambda
between 1.3 and 1.7), and in a stoichiometric mode (with a lambda less
than or equal to about 1.0 and preferably between 0.95 and 1.0) in
predetermined areas of the engine map, and by this method, the methane
oxidation catalyst is periodically regenerated to drive sulfur from the
catalyst's active sites and to restore methane conversion efficiency.
[0037] Figure 2 is a schematic diagram of a second embodiment of an
apparatus that can be used to practice the disclosed method. Apparatus
200 is like apparatus 100 except that sparger 212 is employed to introduce
fuel into the intake air manifold and mass flow sensors 254 and 256 for
respectively measuring the mass flow of intake air and fuel are employed
instead of a lambda sensor to control the air/fuel ratio of the charge that is
delivered to the combustion chamber.
[0038] Apparatus 200 comprises fuel injection system 210, engine
combustion chamber 220, catalytic converter 240, and electronic controller
250. Sparger 212 introduces fuel from fuel supply pipe 214 into intake air
manifold 218 upstream from throttle 219. The fuel and air are mixed
inside intake manifold 218 prior to being introduced into combustion
chamber 220. Throttle 219 regulates the flow of the air-fuel mixture into
combustion chamber 220. Combustion chamber 220 is defined by
cylinder block 222, piston 224, and cylinder head 226. The engine can
comprise spark plug 228 for triggering ignition of the charge inside
combustion chamber 220. Apparatus 200 fiirther comprises intake valve
230, which is operable to admit the air and fuel mixture into combustion
chamber 120, and exhaust valve 232, which is operable to allow
combustion products to flow from combustion chamber 220 to exhaust
CA 02534031 2006-02-03
-16-
manifold 234. Exhaust gas flows from exhaust manifold 234 to a methane
oxidation catalyst provided inside catalytic converter 240. From catalytic
converter 240, the exhaust gas flows to engine exhaust pipe 242.
[0039] Apparatus 200 can be employed to practice the subject method in
the same manner as apparatus 100 except that the manner of determining
lambda in the combustion chamber is different. Instead of using lambda
measurements, controller 250 uses mass flow measurements from sensors
254 and 256 to determine lambda. Then electronic controller can operate
fuel metering valve 215 and throttle 219 to make lambda greater than 1.1
to operate in a lean combustion mode under most conditions, and to make
lambda less than or equal to about 1.0 and preferably between 0.95 and 1.0
in a predetermined area on the engine map.
[0040] Figure 3 is a schematic diagram of a third embodiment of an
apparatus that can be used for practicing the disclosed method. Apparatus
300 uses a ring of ports 312 that open onto intake air manifold 318 from
fuel supply pipe 314, and temperature and pressure sensors are employed
to measure the pressure and temperature of the intake air and the fuel, so
that the electronic controller can calculate the mass flow of air and fuel
and thereby control the air/fuel ratio of the charge that is delivered to the
combustion chamber.
[0041] Apparatus 300 comprises fuel injection system 310, engine
combustion chamber 320, catalytic converter 340, and electronic controller
350. An annular plenum supplies fuel to ports 212 and fuel flows into
intake air manifold 318 upstream from throttle 319. Like apparatus 200,
some mixing of the fuel and air can occur in intake air manifold 318 prior
to the air-fuel mixture being introduced into combustion chamber 320 to
form a charge therein. Throttle 319 can be used to regulate the flow of the
air-fuel mixture into combustion chamber 320.
CA 02534031 2006-02-03
-17-
[0042] Combustion chamber 320 is defined by cylinder block 322, piston
324, and cylinder head 326. The engine can comprise spark plug 328 for
triggering ignition of the charge inside combustion chamber 320.
Apparatus 300 further comprises intake valve 330, which is operable to
admit the air and fuel mixture into combustion chamber 320, and exhaust
valve 332, which is operable to allow combustion products to flow from
combustion chamber 320 to exhaust manifold 334.
[0043] In this embodiment, exhaust gas flowing from exhaust manifold
334 can be directed to a turbine of turbocharger 338 or can be directed to
by-pass the turbine by operation of wastegate valve 336. Air intake 316
directs the intake air to turbocharger 338 and then to intake air manifold
318 through air passage 317. As is known to persons skilled in engine
technology, turbocharger 338 is driven by exhaust gas and can be
employed to boost the pressure of the intake air. After the exhaust gas
exits the turbine, exhaust pipe 339 directs the exhaust gas to catalytic
converter 340. When wastegate valve 336 is open, exhaust gas flows
through exhaust pipe 337 to catalytic converter 340. The turbine of
turbocharger 338 can be a variable geometry turbine or a variable nozzle
turbine. By controlling the wastegate to control how much exhaust gas
flows through the turbine and/or by controlling the variable geometry or
nozzle of the turbine the mass airflow through intake air manifold 118 is
controlled, whereby lambda can be controlled in cooperation with fuel
metering valve 315. That is, one can control lambda by controlling fuel
metering valve 315 and throttle 119, in combination with one of,
wastegate 336, or the variable geometry or the variable nozzle turbine of
turbocharger 338, or wastegate 336 and the variable geometry or the
variable nozzle turbine of turbocharger 338.
CA 02534031 2006-02-03
-18-
[0044] Like the other embodiments, apparatus 300 can be employed to
practice the subject method. Electronic controller 350 can calculate air
mass flow rate from measurements taken by air temperature sensor 358
and air pressure sensor 360. Similarly, electronic controller 350 can
calculate fuel mass flow rate from measurements taken by fuel temperature
sensor 362 and fuel pressure sensor 364. Then electronic controller 350
can make reference to an engine map to determined the desired operating
mode (lean burn or stoichiometric) based upon current engine operating
conditions. When the engine is operating in a predetermined area of the
engine map, electronic controller 350 controls fuel metering valve 315 and
throttle 319 to deliver a richer fuel mixture to the combustion chamber,
preferably with a lambda between 0.95 and 1Ø When the engine is
operating at a point outside the predetermined area for operating with a
richer fuel mixture, electronic controller 350 controls fuel metering valve
315 and throttle 319 to deliver a lean fuel mixture to the combustion
charnber, preferably with an average lambda between 1.1 and 1.7.
[0045] Figures 1 through 3 are illustrative of different embodiments of an
apparatus for practicing the disclosed method. Persons skilled in the
technology will understand that variations can be made to the illustrated
embodiments without departing from the spirit and scope of the disclosed
apparatus. For example, the ring of ports 312 shown in Figure 3 for
introducing the fuel into the intake manifold can be substituted for sparger
212 in Figure 2 with the same effect. In other variations, the lambda
sensor of Figure 1 can be substituted for the sensors of the illustrated
embodiments of Figures 2 and 3, or the fuel mass flow sensor 256 of
Figure 2 can be substituted for pressure and temperature sensors 362 and
364 in the embodiment of Figure 3. An important feature of the disclosed
apparatus is at least one sensor associated with the methane-fuelled engine
CA 02534031 2006-02-03
-19-
that measures a parameter that can be used by the electronic engine
controller to calculate or measure lambda directly in the exhaust manifold
or in the intake air manifold, and the combination of such one or more
sensors with an electronic engine controller that can command and control
the engine to run in a lean burn mode under most operating conditions, and
with a richer fuel mixture, closer to stoichiometric, with lambda less than
or equal to about 1.0, under predetermined conditions defined by an engine
map.
[0046] Figure 4 is an illustration of an engine map with engine torque on
the y-axis and engine speed on the x-axis. Line 400 defines the upper
torque limit for the engine so that the space between line 400 and the x-
axis is the operating range of the engine. The axes in Figure 4 do not show
units because this figure illustrates the approach taught by the presently
disclosed method, which can be applied to the engine map of any engine.
According to the method, the engine is controlled to operate in a lean burn
mode under most operating conditions, and in a stoichiometric mode under
certain predetermined operating conditions. In figure 4, area 401 is the
space on the illustrated engine map where the engine is controlled to
operate in a lean burn mode. That is, when the engine torque and the
engine speed defines a point on the engine map in area 401, an engine
controller controls the air to fuel ratio in the intake air manifold to
deliver
a charge to the engine's combustion chambers with an average lambda
greater than 1.1. Line 402 defines the boundary of area 403, which is a
predetermined region on the engine map where the engine is controlled to
operate with a richer fuel mixture that is closer to stoichiometric. That is,
when the engine torque and the engine speed defines a point on the engine
map in area 403, an engine controller controls the air to fuel ratio to
CA 02534031 2006-02-03
-20-
deliver a charge to the engine's combustion chambers with an average
lambda between about 0.95 and 1Ø
[0047] In Figure 4, the predetermined region for burning a richer fuel
mixture that is defined by line 402 is somewhat arbitrary. This
predetermined region could be anywhere on the engine map, but it is
preferably in a region that is utilized frequently while representing a minor
proportion of the anticipated normal operating cycle, so that the engine
operates mostly in a lean burn mode. In addition, as is described in more
detail below, with respect to Figure 6, for reducing the time for oxidizing
the catalyst at the end of the desulphation cycle, it is preferred to choose
an
area on the engine map for stoichiometric operation where the exhaust gas
temperature is normally higher than 600 degrees Celsius, and more
preferably above 650 degrees Celsius when the engine operates in the
stoichiometric operating mode.
[0048] Figure 5 shows a plot of lambda against engine load for different
engine speeds that could be used to implement the engine map illustrated
by Figure 4. The legend on the right hand side of Figure 5 sets out the
unique symbols that are employed to plot lines corresponding to different
engine operating speeds in rpm. In accordance with the disclosed method,
for most points on the engine operating map, the engine is operated in a
lean burn mode with an average lambda greater than 1.1. The desired
lambda programmed into the engine map changes with engine speed,
increasing gradually as engine load increases, with the desired lambda
being generally higher for higher engine speeds. The exception to this
pattern is when the engine is operating in the predefined engine speed and
load range when the engine operates in a "stoichiometric" operating mode
which in this example is when lambda is less than or equal to 1.0, or when
the engine is transitioning from the stoichiometric operating mode to the
CA 02534031 2006-10-03
-21-
normal lean burn operating mode. In the example of Figure 5, for the
stoichinmetric operating mode the predefined engine speed range is
between 2500 and 2800 rpm, and the predefined engine load range is from
zero to about 20 percent of the maximum load. When the engine speed is
3000 rpm and engine load is less than 50% of maximum engine load,
lambda is commanded to values that help with the transition from the
stoichiometric operating mode to the lean burn operating mode, and vice
versa.
[0049] Following a set of predetermined values for lambda, determined as
a function of engine load and engine speed, the disclosed method can be
employed to operate an engine under lean burn conditions most of the time
to reduce engine emissions, with periodic desulphation of the methane
oxidation catalyst occurring automatically when under predefined
conditions the engine runs in a stoichiometric operating mode.
[0050] Figure 6 is a plot of experimental data, which shows the
effectiveness of the disclosed method in regenerating a methane oxidation
catalyst that has been inhibited by the adsorption of SOx. The data was
collected from a CumminsTM 5.91iter engine fuelled with natural gas. The
methane oxidation catalyst comprised a catalytic washcoat of alumina
impregnated with palladium deposited on a support comprising
magnesium aluminum silicate. In other embodiments the support could be
metallic, or silicon carbide. Figure 6 plots methane concentration in the
exhaust gas downstream from the methane oxidation catalyst with the
concentration measured in units of parts per million (ppm). The data
plotted in Figure 6 illustrates the effect on methane concentration of
switching from a lean burn operating mode to a stoichiometric operating
mode. On the left hand side, the engine is running in a lean burn operating
mode and methane concentration is about 675 ppm. In the illustrated data,
CA 02534031 2006-02-03
-22-
the conversion efficiency of the methane oxidation catalyst has been
allowed to decline significantly to better demonstrate the regenerative
capabilities of the disclosed method. That is, on the left hand side of the
graph, the methane conversion efficiency of the catalyst is approximately
65% and catalytic activity is severely inhibited by adsorption of SOx to the
active sites of the methane oxidation catalyst. After desulphation of the
methane oxidation catalyst by the disclosed method, the methane
conversion efficiency was restored to 85-90%. By practicing the disclosed
method, desulphation occurs whenever the engine is operated in the part of
the engine map where the engine controller is programmed to operate the
engine in a stoichiometric operating mode and depending upon the size of
the stoichiometric region on the engine map and the normal operating
cycle of the engine, desulphation can be made to occur with enough
frequency that the methane oxidation catalyst is prevented from being
inhibited to the degree shown in Figure 6, and less time is required to
restore methane conversion efficiency to 85-90%.
[0051] In Figure 6, as indicated by the legend, three sets of data are plotted
against the same time scale. Methane concentration measured in parts per
million (ppm) and engine torque measured in Nm share the same scale on
the left-hand y-axis with units from zero to one thousand. Engine speed
measured in revolutions per minute (rpm) use the scale on the right-hand
y-axis with units from zero to three thousand. At about 1220 seconds on
the depicted time scale the engine operating mode was switched from a
lean burn mode with a lambda between 1.4 and 1.5, to a stoichiometric
mode with an average lambda of around 1. In the tests performed to
collect this data, the engine speed was kept constant until the end of the
desulphation cycle but the engine load was reduced, as shown by the
plotted engine speed and engine torque. This simulates switching to a
CA 02534031 2006-02-03
-23-
stoichiometric operating mode at a predetermined region of the engine
map where engine speed is kept at about 2800 rpm, and engine load is well
less than 20 percent of maximum load. There are some transient effects
associated with the change in engine load and the warming of the methane
oxidation catalyst to a higher temperature that helps with desulphation. In
the plotted data for methane concentration because of the location of the
methane sensor downstream from the methane oxidation catalyst there can
be a lag between the measured methane concentration and the methane
concentration in the exhaust stream that is leaving the catalytic converter
that houses the methane oxidation catalyst.
[0052] Figure 6 shows that with the disclosed method, regeneration of the
methane oxidation catalyst by desulphation can occur in a very short
period of time. Desulphation occurs when the excess methane in the
exhaust gas is converted to carbon monoxide and hydrogen, and the
hydrogen strips the SOx from the methane oxidation catalyst by reacting
with the sulfur to produce H2S and H2SO4. In the conducted experiments
the time period for operating in the stoichiometric operating mode is about
60 seconds but the methane oxidation catalyst desulphation is completed
after about 40 seconds. In the experiments, the engine is operated in the
stoichiometric operating mode longer than was needed for the
desulphation to occur because the switching was done manually, and by
operating the engine beyond the time needed for desulphation, it was
confirmed that desulphation was completed to the fullest extent possible.
That is, the increase in methane concentration at around the 1260 second
mark indicates that desulphation is complete. When the engine was
switched back to the lean bum operating mode and the transient effects
had subsided, the measured methane concentration confirmed that the
methane conversion efficiency was restored to 85-90%.
CA 02534031 2006-02-03
-24-
[0053] Though not shown on Figure 6, the temperature of the exhaust gas
directed to the methane oxidation catalyst increases when the engine is
switched to a stoichiometric operating mode. In the testing that produced
the data shown in Figure 6, in the lean burn operating mode preceding the
1220 second mark, even with a higher engine torque relative to the
desulphation time period, the temperature of the exhaust gas entering the
catalytic converter is about 645 degrees Celsius. When the engine
switches to a stoichiometric operating mode, the engine torque is much
lower but the temperature of the exhaust gas entering the catalytic
converter increases to about 700 degrees Celsius. Exhaust gas temperature
is preferably kept below 800 degrees Celsius because temperatures higher
than that can damage the methane oxidation catalyst. When the engine
switches back to a lean burn operating mode after the 1275 second mark,
the temperature of the exhaust gas entering the catalytic converter declines
back to about 645 degrees Celsius. It is believed that desulphation of the
methane oxidation catalyst is worse when the temperature of the methane
oxidation catalyst is below 600 degrees Celsius and with the pre-
determined region of the engine map for operating in a stoichiometric
operating mode that was selected for the experimental data plotted in
Figure 6, when operating in a stoichiometric operating mode with a high
engine speed and low engine torque, the exhaust gas is at a desirable
temperature for desulphation of the methane oxidation catalyst.
[0054] It is believed that the methane oxidation catalyst is more effective
in oxidizing methane when the catalyst is in an oxidized state, and that
after the sulfur is driven from the active sites of the catalyst by the
disclosed desulphation cycle, the methane oxidation catalyst is not
oxidized. Accordingly, after running an engine is a stoichiometric
operating mode and completing a desulphation cycle, when the engine
CA 02534031 2006-02-03
-25-
switches back to a lean bum operating mode the methane conversion
efficiency of the catalyst is not restored until the methane catalyst is
oxidized, which explains some of the transient effects observed in the
measured methane concentration after switching from a desulphation
mode to a normal lean burn operating mode.
[0055] Under normal operating conditions, in practicing the disclosed
method, an engine may run under stoichiometric conditions at random
intervals. For example when a vehicle is downshifting or coasting down a
hill the engine may operate briefly in the region on the engine map that is
designated for stoichiometric operation. The size of the predetermined
engine speed and load ranges on the engine map can be selected based on
the engine's anticipated operating cycle, to provide adequate time periods
for automatically regenerating the methane oxidation catalyst. Whenever
the engine operates in the predefined conditions associated with
stoichiometric operating mode, the methane oxidation catalyst can be fully
regenerated or partially regenerated, depending upon the length of time
that the engine is operating in the stoichiometric operating mode.
[0056] Because the methane oxidation catalyst can be fully regenerated in
a short period of time (about 40 seconds in the experimental example
when the catalyst is severely inhibited), the start up sequence for the
engine can optionally include an engine warm up sequence that includes
desulphation of the methane oxidation catalyst so that the engine can begin
each operating cycle by restoring the methane conversion efficiency to a
higher level by regenerating the methane oxidation catalyst. That is, as
part of the start-up sequence, the engine controller can be programmed to
command the engine to operate for a preset time within the area of the
engine map where the engine is operated in the stoichiometric operating
mode. In another embodiment, the engine controller can be programmed
CA 02534031 2006-02-03
-26-
to run in the stoichiometric operating mode at other predetermined times
when a vehicle is stationary. For example, if the disclosed methane-
fuelled engine is the prime mover for a garbage truck, when the truck is
stationary and the engine controller recognizes that the operator has
stopped the vehicle to pick up or dump out a load, the engine controller
can command the engine to operate in the stoichiometric operating mode
for a preset time or until the vehicle is commanded to move from its
stationary position. In these examples (start-up and predetermined times
when a vehicle is stationary), the same method is employed for
desulphation of the methane oxidation catalyst. That is, the engine is
operated in a stoichiometric operating mode within a predetermined area
of the engine map. The only difference is that the engine controller can be
programmed to operate the engine in that part of the map when the engine
controller recognizes predetermined operating conditions, instead of when
the engine operates in that part of the engine map during the normal course
of operating the engine.
[0057] While particular elements, embodiments and applications of the
present invention have been shown and described, it will be understood, that
the invention is not limited thereto since modifications can be made by those
skilled in the art without departing from the scope of the present disclosure,
particularly in light of the foregoing teachings.