Note: Descriptions are shown in the official language in which they were submitted.
CA 02534302 2008-07-07
RECIPROCATING MOVEMENT PLATFORM FOR THE EXTERNAL ADDITION OF
PULSES TO THE FLUID CHANNELS OF A SUBJECT
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0002] The present invention relates generally to a reciprocating motion
platform for
oscillating a subject in a back and forth, headward to footward manner in
order to externally add
pulses to the fluid channels of the subject. The external addition of pulses
caused by the periodic
acceleration of the subject results in many therapeutic benefits.
2. Description of the Related Art
[0003] This application builds on the work previously done in this field by
Non-Invasive
Monitoring Systems, Inc., located at 1666 Kennedy Causeway, Suite 400 in North
Bay Village,
Florida, as exemplified in U. S. Patent No. 6,155, 976 to Sackner et al.
entitled "Reciprocating
Movement Platform For Shifting Subject To and Fro in Headwards-Footwards
Direction"
(hereinafter referred to as the '976 patent), U. S. Patent Application
Publication Serial No.
2002/0103454 filed by Dr. Marvin Sackner and D. Michael Inman, entitled
"External Addition of
Pulses To Fluid Channels Of Body To Release Or Suppress Endothelial Mediators
And To
Determine Effectiveness Of Such Intervention" (hereinafter referred to as the
'454 publication), and
U. S. Patent Application Publication Serial No. 2003/0236476, filed by Dr.
Marvin Sackner and D.
Michael Inman, entitled "Reciprocating Movement Platform For The External
Addition of Pulses
of The Fluid Channels of a Subject", (hereinafter referred to as the '476
publication).
[0004] Both the '976 patent and the '476 publication describe reciprocating
movement
platforms which can be used in medical treatments based on the external
addition of pulses, as well
as various medical treatments based on the external addition of pulses. The
'454 publication is
directly mostly to medical treatments. Although the present application builds
on these three
works, it is not limited by them.
[0005] A description of one embodiment of a reciprocating movement platform in
the '476
publication is presented below to provide a background by which to understand
the present
invention. The placement of this description in the background section does
not mean to suggest by
any means that the applicant considers or admits that the '476 publication is
necessarily prior art to
the present application, Its placement here is merely to demarcate the
material disclosed in the '476
publication from the new material described herein.
1
CA 02534302 2007-07-17
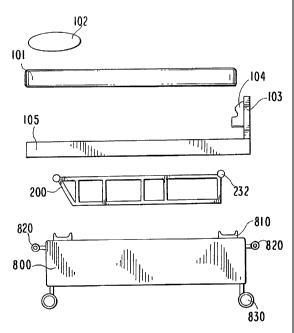
[0006] The '476 publication described one embodiment of a reciprocating
movement
platform as shown in FIGS. 1,4, 5, and 6. FIGS. 1,4, 5, and 6 show a
completely constructed
reciprocating movement platform comprised of a mattress 101 for the subject to
lie upon, a pillow
102 for the subject's head, a footboard frame 103 with cast shoes 104 attached
in order to secure
the subject, a mattress support 105 to hold the mattress 101 and to which the
footboard frame 103
is attached, a box frame 800 which holds the drive machinery (or "drive") 200
onto which the
mattress support 105 is attached, bumpers 820 attached to the top and bottom
of the box frame 800,
and casters 830 at the four corners of the bottom of the box frame 800 for
moving the reciprocating
movement platform.
[0007] The entire reciprocating movement platform system (without patient,
i.e., mattress
101 and mattress support 105, footboard support 105, box frame 800, and drive
macHnery 200)
weighs between 400 and 500 lbs. The entire reciprocating movement platform
system is 30" wide,
which is the standard width of a hospital gurney, so that it may be easily
moved through doorways,
semi-crowded offices, etc. The length of the entire system from bumper to
bumper is 88", which is
as long as a standard twin or king size bed. The mattress 101 is 30" above the
floor, and the top of
the footboard support 103 is 42" above the floor.
[0008] The mattress support 105 secures the mattress 101 by means of Velcro
strips. The
mattress support 105 and footboard support 105 together weigh roughly 120 lbs.
total, When
assembled, the combined mattress support 105 and footboard support 105 are 30"
wide and 82"
long. The mattress 101 is 6" thick, 30" wide, 80" long, and weighs
approximately 30 lbs. The top
3" of the mattress foam is the "visco-elastic" type foam for form-fitting
comfort while the subject is
on the platform. The mattress 101 can be designed to fold in half for easier
transport and storage.
[0009] FIG. 7 shows the cast shoes 104 and the footboard frame 103 to which
they are
attached. The cast shoes 104 of the footboard frame 103 are the only means by
which the subject is
secured to the mattress support 105, and thus, is the means by which the
subject is "pulsed" by the
reciprocating platform. The two cast shoes 104 are rigidly attached by nuts
and bolts to the
footboard frame 103. Once the subject is lying on the mattress 101, he or she
will put his or her feet
(with shoes on) into the cast shoes 104 and then the cast shoes 104 will be
secured around the
shoes by a system of Velcro and straps and cloth. Experiments have shown that
"one size fits
many", with the cast shoes 104 servicing most adults quite adequately due to
the flexibility of the
Velcro closure system. The feet may be fastened in the cast shoes 104 by other
means, such as a ski
boot-like apparatus, or another fastening means, such as a snap, a buckle, a
lock, etc. connection.
2
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
[0010] The casters 830 on the bottom portion of the reciprocating movement
platform are 6" hospital
bed casters with central locking features; these provide easy rolling and
maneuvering, good ground
clearance, easy locking (as shown by the brake petal), and an attractive
appearance. The ground
clearance is approximately 8", which accommodates the use of equipment (such
as hoists) to lift the
reciprocating movement platform. The bumpers 820 make sure the reciprocating
platform is not set too
close to a wall by extending further out than the mattress support 105. The
mattress support 105 is 82"
long and, when the platform is engaged in a reciprocating movement, has a
range of movement of +/- 2".
The bumpers 820 are built to extend 1" beyond the furthest limit the mattress
support 105 can travel so that
the reciprocating movement platform will not be accidentally set too close to
a wall where it might bump the
wall during operation,
[0011] The drive machinery (or "drive") 200 is enclosed within the box frame
800 and, as such,
cannot be seen from the outside of the fully assembled movement platform.
Supported by the box frame
800 and attached to the mattress support 105, the drive 200 provides the
reciprocating movement of the
device. The reciprocating (headwards-footwards) movement preferably has a rate
of about 120-180 rpm
with a force in the range of about +/-0.2 to about +/-0.3g. The relationship
between the parts can be seen
in the exploded view of the reciprocating movement platform shown in FIG. 1.
Starting from the top, the
mattress 101 attaches to the mattress support 105 with Velcro strips, while
the footboard frame 103 (with
attached cast shoes 104) is bolted onto the mattress support 105. The mattress
support 105 is securely
attached to the drive 200. The drive 200 has four track wheels 232 located in
the four top corners of the
drive 200. These wheels 232 sit in four similarly placed tracks in the box
frame 800. Hence, the drive 200,
mattress support 105, and mattress 101 form one part of the assembled movement
platform, and the only
physical connection between this top part and the bottom box frame 800 is the
four wheels 232 of the drive
200 sitting in the four tracks of the box frame 800.
[0012] When the drive 200, by means which will be discussed further below,
moves within the box
frame 800, the wheels 232 move within the tracks, which serve to both support
the drive 200 and limit the
reciprocating motion of the drive 200. The track 810 on top of the box frame
800 has rounded ends so that
the wheel 232 of the drive 200 may only move a certain distance in either
direction. The track is beveled so
that the track wheel 232 of the drive 200 will rest naturally in the center of
the track. The track is also
located near the metal support struts of the box frame 800 which thus transfer
the weight of the drive 200
(and the attached mattress support 105, mattress 101, and subject) directly
down to the caster 830 in the
corner below.
[0013] The box frame 800 weighs about 120 lbs. and serves at least the
following five purposes: 1)
supporting the rest of the platform (the drive 200, mattress support 105,
mattress 101, and subject); 2)
providing a foundation that can be moved or anchored by means of the casters
830; 3) maintaining an
adequate distance from surrounding walls by means of its bumpers 820; (4)
carrying the system
-3-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
electronics; and (5) encasing the drive 200 for safety and noise reduction. In
addition, the box frame 800
provides ground clearance for the hoist legs.
[0014] The drive 200 weighs 200 lbs and is 24" wide. The displacement modules
in the drive 200
take the form of two pairs of rotating counterweights, connecting belts,
pulleys, springs, and motors. FIGS.
2A and 2B are drawings of a side view and a top view, respectively, of the
drive 200 and its various
mechanisms. In FIGS. 2A and 2B, the two pairs of drive weights 215A & 215B and
225A & 225B are
shown attached to their respective horizontal shafts 210 and 220. These shafts
are attached by means of
struts to the frame of the drive 200. The four track wheels 232 can be seen in
FIGS. 2A-2B. There are two
motors, the drive rotation motor 1700 which drives the drive weights and a
linear displacement motor 260
which sets the phase difference between the two pairs of drive weights. The
drive rotation motor 1700 is a
180VDC 1/2hp 0-1750RPM motor, although only 1/10hp is actually used. The
linear displacement motor
260 is a 9" per minute 400 lb. 110VAC linear displacer with 12" of travel.
[0015] The movement of counterweights 215A and 215B as seen from above is
shown in FIGS. 3A-
E. In FIG. 3A, the centers of gravity of both drive weights 215A and 215B are
on the same line 401 from
center drive shaft 210. As center drive shaft 210 continues to rotate in FIG.
3B, drive weights 215A and
215B continue their rotations in opposite directions: drive weight 215A in a
clockwise direction, drive weight
215B in a counter-clockwise direction. In FIG. 3C, the drive weights have
moved into positions opposite
each other. This is beneficial because the force of the two drive weights are
also in opposite directions and
thus, negate each other's effect. The` rotation continues in FIG. 3D and then
the drive weights end up
adding the force of their weights in the same direction in FIG. 3E. FIGS. 3A-E
show how the motion of the
drive weights moves the drive 200 up and down the box frame tracks (i.e.,
headwards and footwards for a
subject on the mattress 101), but not sideways within the box frame 800. If
FIG. 3A is the position which
causes the headward movement, FIG. 3C is the position which negates any
movement, and FIG. 3E
causes the footward movement.
[0016] As can be seen in FIGS. 2A-2B and 3A-3E, the drive weights are of
unequal size. This is
because the weights are located at different distances from the center of
drive shaft 210. If the drive
weights were the same mass, their effects would not be balanced and the drive
200 would rock sideways in
the box frame 800. However, if drive weight 215B is a predetermined amount of
mass less than drive
weight 215A, the effect of the drive weights when rotating in opposite
directions will cancel each other out.
Because of this arrangement, the drive weights are in the same horizontal
plane as shown in FIG. 2, which
greatly reduces any shimmy effect that was produced in previous platform
versions which had their drive
weights in different horizontal planes. The outer edge of drive weight 215A is
12" from drive shaft 210 and
this outer edge travels past the very outside edge of the drive itself when
rotating.
[0017] FIG. 2B shows the pulley system with drive belt 370 and the phase
control belt 380. The
drive belt 370 runs from drive rotation motor 1700 to drive shaft 210 and
provides the power to rotate drive
-4-
CA 02534302 2007-08-31
weights 215A and 215B around drive shaft 210 and 'undiireetly provides the
power to rotate drive
weights 225A and 225B around shaft 220, Drive belt 370 is a 3/4" L pitch tin;-
ing belt, although a
timing belt is not required in this position. Beeatise of the size of the
wheel aroimd drive shaft 210
which is driven by drive belt 370 in comparison to the size of rotation shaft,
there is a 5:1 speed
reduetion from the drive rotation motor 1700 to the actual rotational speed of
the drive weights.
[0018] Phase control belt 380 runs around four pulley wheels of equal size : a
release pulley
wheel, a drive shaft pulley wheel, secondary shaft pulley wheel, and a linear
displacement pulley wheel.
Because it is also attached to drive shaft 210, the drive pulley wheel drives
the phase control belt.
Secondary shaft pulley wheel receives the power to rotate the drive weights
around shaft 220 froin the
drive shaft pulley wheel tbrough phase oantrol belt 380. The release pulley
wheel provides required
tension for phase control belt 380, and can also be used to release the
ten,sion on phase control belt 380
in order that phase control belt 3 80 can be taken off for repair or
transport. Linear displacement pulley
wheel can be moved in position up and down linear sha$ under the control of
linear displacement motor
260. It is by this means that the relative phases of the two pairs of drive
weights are controlled.
[0019] The drive weights a.round each shaft make the same movements as shown
in FIGS. 3A-
3E. However, one pair of drive rveights can be moved in and out of phase with
the other pair of drive
weights. The two pairs of drive weigbts are in phase when they are in the same
rotational positions at
the same time. Both pairs would look ltlce FIG. 3A at the same time, like FIG.
3B at tbe same time, etc.
The two pairs are out of phase when they are not in the same rotational
positions at the same time. For
instance, drive weights 215A & 215B might be in the position shown in k'IG_
3A, while drive weights
225A & 225B might be in the positions shown in FIG. 313_ In that case, they
would be 45 out of phase
with each otber. Although the sideways forces ofthese out-of-phase pairs of
drive weights would still
cancel themselves out (and thus not produce a rocking effect in the movement
platform), the force
produced in the headwards-footwards directions would lessen in comparison to
when the pairs of drive
weights are in phase.
[0020] The relative phases of the pairs of drive weights are controlled by the
linear
displacement motor 360, whicli coxrtrols the pulley system. The speed of
rotation of the pairs of drive
weights are controlled by increasing or decreasing the speed ot'the drive
rotation motor 1700. Thus, one
can control both the speed of the headwards-footwards movement (by increasing
or decreasing the
speed of the drive rotation motor 1700) and the force applied by the headwards-
footwards movement
(by moving the pairs of drive weights in and out of phase with each other
through linear displacement
pulley wheel uiider the control of linear displacement motor 360). In its
simplest form, the control
electronics of the present invention merely control these two variables in
order to get the desired effect
on the subject (as described, for example, in the '962 patent, the '454
publication, and the '416
publication). A handheld controller with a communication link to the control
electronics of the drive
200 may be used by the health care provider or the subject him-or herself,
Readings of the speed and
peak acceleration could also be available. The
5
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
control electronics also incorporate a "patient stop switch" which may be
given to the subject to hold. The
motors would stop whenever the switch was activated.
[0021] Although this reciprocating movement platform is well designed for
providing a wide range of
controlled motions to a subject on it, it is fairly heavy, and, as such, may
not be appropriate for usage in
the more. Thus, there is a need for a reciprocating movement platform with
reduced weight.
-6-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
SUMMARY OF THE INVENTION
[0022] It is an object of the present invention to provide an apparatus and
method of causing the
external addition of pulses to the fluid channels of a subject based on the
periodic acceleration of the subject's
body,
[0023] It is another object of the present invention to provide a simplified
apparatus which is more
suitable and economical for home treatments than past devices.
[0024] It is yet another object of the present invention to provide medical
treatments based on the
periodic acceleration of the subject's body, where said periodic acceleration
causes the external addition of
pulses to the fluid channels of the subject.
[0025] The presently preferred embodiment of an apparatus of the present
invention comprises a box
frame, a drive module, and a support connected to the drive module. The
support has a planar surface for
supporting the subject, and a footboard to hold the subject's feet. The drive
module provides periodic
acceleration to the subject by moving in a line parallel to the planar surface
of the support.
[0026] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention include the treatment and
prevention of cancer as well as
diminishing the unwanted side effects of chemotherapy and radiotherapy, and
the chronic preconditioning,
immediate preconditioning, and/or postconditioning of subjects, such as
athletes, to prevent and/or treat
prevent/treat any of the insalubrious conditions which may be caused by
athletic activity, whether such
activity is continuous, periodic, or intermittent as well as to improve sports
performance,
[0027] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention also include chronic
treatments to minimize organ damage
caused by an unforeseen future stroke, coronary artery thrombosis, pulmonary
embolism, etc. The
presently preferred medical treatments possible with externally applied
periodic acceleration according to
the present invention include attenuation of left ventricular remodeling and
promotion of reverse left
ventricular remodeling, The presently preferred medical treatments possible
with externally applied
periodic acceleration according to the present invention include attenuation
of the inflammatory and
cognitive deficit complications of coronary artery bypass surgery, diminution
of cardiac allograph
vasculopathy as well as aiding angiogenesis in ischemic tissue,
[0028] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention include the cognitive and
learning deficits as well as
behavioral abnormalities in early cognitive impairment, Alzheimer's disease,
vascular dementias,
Parkinson's disease, amyotrophic lateral sclerosis, Huntington's chorea,
Wilson's disease, suprabulbar
palsy and possibly Tourette syndrome. The presently preferred medical
treatments possible with externally
applied periodic acceleration according to the present invention includes
hereditary hemorrhagic
-7-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
telangiectasia. The presently preferred medical treatments possible with
externally applied periodic
acceleration according to the present invention include migraine and prion
diseases.
[0029] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention includes the ageing process
and management of Sjogren's
syndrome, Lyme disease, and the Gulf War syndrome. The presently preferred
medical treatments
possible with externally applied periodic acceleration according to the
present invention includes treatment
of cystic fibrosis, chronic bronchitis, asthma, chronic sinusitis and adult
and infant respiratory distress
syndrome, SARS and chronic otitis media as well as the adverse effects of
mechanical ventilation that
cause damage to the lung. The presently preferred medical treatments possible
with externally applied
periodic acceleration according to the present invention includes
corticosteroid resistant asthma, Crohn's
disease and ulcerative colitis.
[0030] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention include improving nail growth
and nail brittleness.
[0031] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention includes preventing and
treating the serious side effects of
cell free hemoglobin transfusions.
[0032] The presently preferred medical treatments possible with externally
applied periodic
acceleration according to the present invention includes treatment of the
consequences of injuries from a
nuclear explosion, "dirty bomb" or nuclear power plant attack.
[0033] The various features of novelty which characterize the invention are
pointed out with
particularity in the claims annexed to and forming a part of the disclosure.
For a better understanding of the
invention, its operating advantages, and specific objects attained by its use,
reference should be had to the
drawing and descriptive matter in which there are illustrated and described
preferred embodiments of the
invention. It is to be understood, however, that the drawings are designed
solely for purposes of illustration
and not as a definition of the limits of the invention, for which reference
should be made to the appended
claims. It should be further understood that the drawings are not necessarily
drawn to scale and that,
unless otherwise indicated, they are merely intended to conceptually
illustrate the structures and
procedures described herein.
-8-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
BRIEF DESCRIPTION OF THE DRAWINGS
[0034] In the drawings:
FIG. 1 is an exploded view of the components in a reciprocating movement
platform according
to embodiments of the present invention;
FIG. 2A is a schematic drawing of a side view of a drive according to a
previously described
preferred embodiment of the present invention;
FIG. 2B is a schematic drawing of a top view of a drive according to a
previously described
preferred embodiment of the present invention;
FIGS. 3A-3E are diagrams showing the movement of a single pair of drive
weights according
to a previously described embodiment of the present invention;
FIGS. 4, 5, and 6 are different views of a completely assembled reciprocating
movement
platform according to a previously described preferred embodiment of the
present invention; and
FIG. 7 shows cast shoes and a footboard support according to a previously
described.
preferred embodiment of the present invention.
-9-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
DETAILED DESCRIPTION OF THE PRESENTLY PREFERRED EMBODIMENTS
[0035] The present invention relates to both an apparatus and methods of
treatment using periodic
acceleration. This portion of the application is broken into two sections:
section I will describe some
preferred embodiments of the apparatus, and section II will describe methods
of treatment.
1. The Reciprocating Movement Platform
[0036] In the current commercial model of the reciprocating movement platform,
controls are
available to adjust cycling speed and amplitude of platform displacement. This
combination can be
monitored by an accelerometer to estimate of the magnitude of applied periodic
gravitational (i.e.,
acceleration-based) forces. This current model is capable of fine-tuning, but
is also fairly heavy, and is
thus appropriate for use in an institution, such as a clinic or hospital, or a
doctor's office. ,
[0037] Using a dose response curve of nitric oxide released from periodic
acceleration (obtained from
analysis of the descent of the dicrotic notch of the finger pulse using a
photoelectric-plethysmographic
sensor), it has been determined that, with the motion platform cycling rate
fixed at 140 cycles per minute,
periodic acceleration values between 0.20 and 0.30 g produced more effective
release of nitric oxide
than 0.15 or 0.10 g. (Sackner MA, Gummels EM, Adams JA. Dose responsiveness
of dicrotic notch
position in periodic acceleration, Am.J.Respir.Crit.Care Med. 169[7], A178.
2004.). For adults with normal
body weight, settings of approximately 0.20 g and rate of 140 cycles per
minute and for obese adults,
settings of approximately 0.17 and rate of 140 cycles per minute provide a
proper balance between nitric
oxide release and subject comfort during periodic acceleration treatments.
However, to be more certain of
such settings, it may be necessary to analyze the position of the dicrotic
notch and the amplitude of cycling
and the time of the cycle as a measure of nitric oxide release with the
current periodic acceleration motion
platform to more precisely set the appropriate parameters for the home model.
[0038] Thus, since periodic acceleration gravitational forces within a narrow
range produce an
effective release of nitric oxide, it was realized that a simplified
reciprocating platform is possible, where the
amount of machinery in the embodiments described in the '957 application can
be sharply reduced,
resulting in a reduction of the weight of the platform by hundreds of pounds,
and thereby making a more
suitable and economical home model reciprocating platform.
[0039] In one embodiment of the presently preferred invention, the amount of
displacement of drive
module 200 is fixed, rather than variable, as it is in the '957 application.
With such a change, the complex
machinery in the embodiments of the '957 application are no longer necessary.
Specifically, there is no
need for the two large, heavy electrical motors, or the complex system of fly
wheel belt assemblies which
provide both the speed and displacement adjustments in the '957 application.
-10-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
[0040] The new "home unit" motion platform will provide variable speed, but
fixed displacement. It
will use the existing welded box frame 800, or a bolted, wrought aluminum
modular box frame (with greatly
reduced weight), such as the modular elements made by Item, Solingen, Germany.
A simplified motor
driving system will be attached to the box frame 800 rather than being
contained within drive module 200,
as it is in the '957 application. This simplified motor driving system will
'push' and 'pull' drive 200 through a
sinusoidal horizontal, head to foot motion of approximately 2.5 cm or less.
The stroke distance is fixed
during manufacture for the desired periodic acceleration gravitational
setting. The cycling speed is
adjusted to a given range of speeds. The presently preferred drive for the
home model device is a rotary
eccentric mounted to box frame 800, but coupled to drive 200. It is powered by
an adjustable AC drive
motor, powered via a 120 VAC, single-phase input (50 or 60 Hz). The adjustable
AC drive motor provides
an adjustable frequency and voltage output of 0-60 Hz, 0-230 VAC, 3-phase
power. An example of such a
variable speed AC drive motor rated at % HP is manufactured by AC Tech (Model
SM005S, AC
Technology, Usbridge, MA 01569). It has an adjustable
acceleration/deceleration control; the variable
speed can be controlled thereby via a front panel,
[0041] The adjustable AC drive motor powers a three-phase induction, brushless
motor from 0 to
1800 rpm with a totally enclosed, non-ventilated, flange (AC induction motor,
56C flange, TENV
construction, 230VAC, 3 ph, 60 Hz, Baldor Electric Company, Fort Smith AR
72901) mounted to an
industrial rated right angle worm reducer of 10:1 reduction that converts the
rotary motion of the shaft of the
AC motor into linear displacement. The worm reducer is provided with an output
shaft, whereupon the
rotary eccentric drive is fixed and keyed. The worm reducer with 56C face
input is scaled for lift lubrication
(Model NMRV-040-10:1-56 C, Motovario, Alpharetta, Ga 30005). On the end of the
worm reducer output
shaft is a plate that can be securely bolted to another plate attached to the
flat surface of one end of the
frame of drive module 200, thereby imparting a head to foot linear sinusoidal
motion. It should be
recognized that other models of the components of the drive and frame and
manufacturers could be
substituted for these "off the shelf' parts in the fabrication of the home
periodic acceleration, motion
platform device. Furthermore, different constructions and architectures may be
used, as would be known
to one skilled in the art, for having a motor attached to box frame 800 which
imparts the head to foot motion
of drive module 200.
[0042] Treatments with periodic acceleration are self-limited for acute soft
tissue and bone injuries.
They serve as a jump-start for patients in whom aerobic exercise is one of the
recommended first line
treatments such as coronary artery disease, peripheral vascular disease,
fibromyalgia and chronic fatigue
syndrome. However, treatment is lifetime for most chronic inflammatory
diseases that encompass 1)
neurological diseases such as Alzheimer's disease, Parkinson's disease,
multiple sclerosis, amyotrophic
lateral sclerosis, neuropathy, etc., 2) rheumatological diseases such as
osteoarthritis, rheumatoid arthritis,
etc., 3) gastrointestinal diseases such as Crohn's disease, ulcerative
colitis, etc., 4) liver diseases such as
-11-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
hepatitis C and portal cirrhosis, 5) kidney diseases such as
glomerulonephritis, etc. 6) urinary tract
diseases such as interstitial cystitis, etc. Therefore, a cheaper, simplified,
periodic acceleration device of
less weight than the current design is needed for home applications. Further,
the external appearance may
take several forms depending upon use and consumer preferences, viz., a stand
alone medical device, to
also serve as a single or queen size bed for sleeping, or to be incorporated
into a sofa design and also
serve for sitting. The dimensions of the frame can be increased in width to
accommodate two individuals or
side-to-side periodic acceleration.
[0043] The use of the digital pulse wave and R wave trigger recording from the
electrocardiograph
during varied settings of periodic acceleration amplitude and rate in cycles
per minute allows analysis of the
descent of the dicrotic,notch as described in the '957 application. The
magnitude of descent of the dicrotic
notch and/or the cycle length and magnitude of the rise and fall of the
dicrotic notch during periodic
acceleration provides an estimate of the effectiveness of nitric oxide
released from activation of endothelial
nitric oxide synthase by pulsatile shear stress. This enables transfer of
optimal settings from the current
device to the home device that allows control of rate with a fixed setting for
amplitude adjusted in the
factory where the home device is manufactured.
II. Methods of Treatment
[0044] This section will describe preferred embodiments of medical treatments
using a reciprocating
movement platform. Although use of the preferred embodiment of the
reciprocating movement platform is
preferred and the descriptions below are based on its use, another type of
device which could apply pulses
in the manner appropriate for the particular treatment (as discussed below)
may be used.
[0045] In addition to the treatments previously disclosed in the '976 patent,
the '454 publication, and
the '957 application, periodic acceleration according to the present invention
may be used to
A) treat and/or prevent cancer, as well as provide relieve to the unwanted
side effects of
cancer treatment,
B) serve as a means of preconditioning or conditioning,
C) manage obesity and weight control generally;
D) promote ventricular remodeling;
E) treat and/or prevent atrial fibrillation;
F) managing complications of coronary artery bypass surgery;
G) treat and/or prevent cognitive and learning deficits, behavioral
abnormalities, and/or
diseases which affect the cognitive function;
H) treat and/or prevent atherosclerosis;
I) promote angiogenesis in ischemic tissues;
J) treat and/or prevent talangiectasia;
-12
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
K) treat and/or prevent migraines;
L) treat and/or prevent prion diseases;
M) manage the aging process;
N) manage Sjogren's Syndrome;
0) manage Lyme Disease;
P) manage Gulf War Syndrome;
Q) manage miscellaneous pulmonary effects;
R) treat corticosteroid resistance;
S) treat chronic otitis media;
T) promote nail growth and strength;
U) manage the side effects of cell free hemoglobin transfusions; and
V) treat radiation injuries.
A. Treatment of Cancer
[0046] Tumors in which nuclear factor kappa beta is present in the nucleus of
cells (constitutive
activation) include the following among others (Garg A, Agawam B. Nuclear
transcription factor-kappa B as
a target for cancer drug development. Leukemia 2002; 16:1053-68); Bus-Ramos
CE, Roche FC, Shishodia
S, Medeiros LJ, Kantarjian HM, Vadhan-Raj S, Estrov Z, Smith TL, Nguyen MH
Aggarwal BB. Expression
of constitutively active nuclear-kappa B ReIA transcription factor in blasts
of acute myeloid leukemia. Hum
Patho12004; 35: 246-253) :
B cell lymphoma
Hodgkin's disease
T cell lymphoma
adult T cell lymphoma
acute lymphoblastic leukemia
mantle cell lymphoma
myeloid leukemias
gastric cancer
breast cancer
liver cancer
thyroid cancer
cervical cancer
pancreatic cancer
prostate cancer
mesothelioma
-13-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
melanoma
head and neck squamous cell carcinoma
colorectal cancer
multiple myeloma
ovarian cancer
bladder cancer
lung cancer
vulvar cancer
brain tumors
fibrosarcoma
osteosarcoma
neuroblastoma
[0047] Tumorigenesis is characterized by self-sufficiency in growth signals,
insensitivity to growth
inhibition, evasion of apoptosis, immortalization, sustained angiogenesis,
tissue invasion and metastasis.
(Hanahan D, Weinberg R A. The hallmarks of cancer, Cell. 2000;100:57-70).
Nuclear factor kappa beta
that is constitutively activated in tumor cells promotes tumorigenesis since
this gene produces negative
feedback of nuclear factor kappa beta, causes cancer cell proliferation,
prevents apoptosis (programmed
cell death), increases angiogenesis, and increases metastatic potential. (Garg
A, Aggarwal B B. Nuclear
transcription factor-kappa B as a target for cancer drug development. Leukemia
2002:16:1053-68; Bharti A
C, Aggarwal B B. Nuclear factor-kappa B and cancer.- its role in prevention
and therapy.
Biochem.Pharmacol 2002; 64:883-88). Because of these factors, Karin suggested
that nuclear factor kappa
beta should receive as much attention from cancer researchers as it has
already from immunologists (Karin
M, Cao Y, Greten F R, Li Z W. NF-kappaB in cancer: from innocent bystander to
major culprit,
Nat. Rev.Cance. 2002; 2:301-10).
[0048] Indeed, pharmacological agents that block nuclear factor kappa beta
activity have been
employed to treat cancerous cell lines with success (Fujioka S, Sclabas GM,
Schmidt C, Niu J, Frederick
WA, Dong QG et al. Inhibition of constitutive NF-kappa B activity by I kappa B
alpha M suppresses
tumorigenesis, Oncogene 2003; 22:1365-70; Liptay S, Weber CK, Ludwig L, Wagner
M, Adler G, Schmid R
M. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in
pancreatic cancer.
Int.J.Cancer 2003; 735-46; Umezawa K, Ariga A, Matsumoto N. Naturally
occurring and synthetic inhibitors
of NF-kappaB functions, Anticancer Drug Des 2003; 15:239-44).
[0049] Although activation of nuclear factor kappa beta in cancer cells plays
a major role in
tumorigenesis, other factors as well contribute including overexpression of 1)
vascular endothelial growth
factor (VEGF), 2) interleukin 8(IL-8), 3) large quantities of nitric oxide
from inducible nitric oxide synthase
(iNOS) activity, 4) mutated p53, 5) epidermal growth factor receptor (EGFR),
6) tumor necrosis factor
-14-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
superfamily, and 7) COX2. Vascular endothelial growth factor (VEGF) and
interleukin- 8 (IL-8) expressed
by tumors promote angiogenesis thereby providing a blood supply to fuel tumor
growth. VEGF induces
proliferation of endothelial cells, increases vascular permeability, and
induces activation of plasminogen
activators by such cells. VEGF and IL-8 are directly expressed by tumor cells
and also stimulated by
nuclear factor kappa beta activation. (Xiong HQ, Abbruzzese JL, Lin E et al.
NF-kappaB activity blockade
impairs the angiogenic potential of human pancreatic cancer cells. Int J
Cancer 2004; 108(2):181-188;
Huang S, Robinson JB, Deguzman A et ai. Blockade of nuclear factor-kappaB
signaling inhibits
angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing
expression of vascular
endothelial growth factor and interieukin 8. Cancer Res 2000; 60(19):5334-
5339).
[0050] Large quantities of nitric oxide expressed through activation of iNOS
in cancer cells facilitate
tumor progression. Nitric oxide produced from iNOS in at a low level in
ulcerative colitis and sporadic
colorectal cancer activates p53, which is anti-tumorigenic, but at a high
level of production, NO may cause
mutations in p53 thereby acting in a pro-tumorigenic role. Under normal
circumstances, the tumor
suppressor p53 is a sensor of diverse cellular stresses including DNA damage,
oxidative stress, and
hypoxia, and aids in directing cell cycle arrest and apoptosis (physiological
programmed death of cells)
through transcriptional activation of target genes like p21. p53 is the most
commonly mutated gene in a
broad spectrum of cancers and is often associated with tumor progression,
resistance to therapy, and poor
prognosis. In melanoma, p53 rarely mutates but increased expression associated
with tumor progression
correlates well with iNOS activity. Increased nitric oxide from activation of
iNOS in melanoma cells also
correlates with resistance to chemotherapeutic agents. (Goodman JE, Hofseth
LJ, Hussain SP et al. Nitric
oxide and p53 in cancer-prone chronic inflammation and oxyradical overload
disease, Environ Mol
Mutagen 2004; 44(1):3-9; Tang CH, Grimm EA. Depletion of endogenous nitric
oxide enhances cisplatin-
induced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol
Chem 2004; 279(1):288-298.)
[0051] Epidermal growth factor receptor (EGFR) is overexpressed in tumors such
as lung, colon,
kidney, prostate, breast, and head and neck carcinomas, which are mostly
resistant to current
chemotherapies, EGF, prevents apoptosis or programmed death of cancer cells
like nuclear factor kappa
beta, thereby rendering them immortal. Activation of the EGFR-TK enzyme also
results in
autophosphorylation, which drives signal transduction pathways leading to
tumor growth and malignant
progression. When EGFR activation is blocked with anti EGFR drugs, VGFR and IL-
8 production falls.
However, use of anti-EGFR agents may be associated with the side effects of
skin rash and diarrhea and
less frequently interstitial pneumonitis. The EGFR-TK inhibitor gefitinib
(Iressa) shows clinical benefits in
patients with advanced non-small cell lung cancer whose disease had previously
progressed on platinum-
and docetaxel-based chemotherapy regimens. (Lage A, Crombet T, Gonzalez G.
Targeting epidermal
growth factor receptor signaling: early results and future trends in oncology.
Ann Med 2003; 35(5):327-336;
Vlahovic G, Crawford J. Activation of tyrosine kinases in cancer. Oncologist
2003; 8(6):531-538.)
-15-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
[0052] The tumor necrosis factor (TNF) superfamily of inflammatory cytokines
mediates either
proliferation, survival, or apoptosis of cells. Although distinct receptors,
all members share a common cell
signaling pathway that mediates the activation of nuclear factor-kappaB (NF-
kappaB) and mitogen-
activated protein kinases (e.g. c-jun N-terminal kinase). Under specific
conditions TNF alpha is a tumor
promoter and helps to produce the toxic effects associated with conventional
cancer therapy, such as the
cytokine release syndrome and cisplatin-induced nephrotoxicity. (Gaur U,
Aggarwal BB. Regulation of
proliferation, survival and apoptosis by members of the TNF superfamily.
Biochem Pharmacol 2003;
66(8):1403-1408; Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a
potential target for the
therapy of solid tumours. Lancet Oncol 2003; 4(9):565-573.)
[0053] Cyclooxygenase 2 (COX2) overexpression that is found most commonly in
lung and colorectal
cancers contributes to the tumorigenesis by at least five different mechanisms
including transformation of
procarcinogens on carcinogens, pro-inflammatory and immunomodulatory effect,
resistance to apoptosis,
angiogenesis and invasion progression. Therefore, treatment with a COX2
inhibitor in such tumors retards
tumor progression. (Gasparini G, Longo R, Sarmiento R et al. Inhibitors of
cyclo-oxygenase 2: a new class
of anticancer agents? Lancet Oncol 2003; 4(10):605-615).
[0054] Adhesion of circulating tumor cells to microvascular endothelium plays
an important role in
tumor metastasis. Tumor cells are more likely to adhere to postcapillary
venules than to corresponding
precapillary arterioles thereby playing an important role in tumor metastasis
to distant organs. (Kong L,
Dunn GD, Keefer LK et al. Nitric oxide reduces tumor cell adhesion to isolated
rat postcapillary venules,
Clin Exp Metastasis 1996; 14(4):335-3).
[0055] A major problem in human cancers is to distribute the pharmacological
agent to the tumor
without producing toxicity to normal cells. Nitric oxide released from eNOS
with periodic acceleration offers
a non-toxic means to suppress activated nuclear factor kappa beta (Stefano GB,
Prevot V, Cadet P, Dardik
1. Vascular pulsations stimulating nitric oxide release during cyclic exercise
may benefit health: a molecular
approach (review), Int.J.MoI.Med. 2001; 7:119-29). Further, since tumors are
characterized by a well-
developed blood supply, distribution of the nitric oxide suppressant activity
on activated nuclear factor
kappa beta does not pose a problem. Both aerobic exercise and periodic
acceleration increase shear
stress to the endothelium but there are differences between the two with
respect to distribution of blood
flow and NO from eNOS, particularly with regard to the viscera. Aerobic
exercise causes diminution of
blood flow to the internal organs whereas periodic acceleration increases
blood flow to these organs, e.g.,
liver, gastrointestinal tract, and kidneys. (Adams JA, Mangino MJ, Bassuk J et
al. Regional blood flow
during periodic acceleration. Crit Care Med 2001; 29(10):1983-1988.). Miyauchi
et al. showed that both
eNOS activity and NOx are diminished in the kidneys along with decreased blood
flow with exercise while
the opposite takes place with pulmonary blood flow. (Miyauchi T, Maeda S,
Lemitsu M et al. Exercise
-16-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
causes a tissue-specific change of NO production in the kidney and lung. J
Appl Physiol 2003; 94(1):60-
68.)
[0056] Periodic acceleration through nitric oxide release from activation of
eNOS suppresses activity
of nuclear factor kappa beta. This was recently demonstrated in a sheep model
of asthma, which is an
example of an nuclear factor kappa beta disease. Periodic acceleration (pGz)
stimulates NO release from
endothelial nitric oxide synthase (eNOS) through pulsatile shear stress (Adams
et al. Effects of periodic
body acceleration on the in vivo vasoactive response to N-w-nitro-L-arginine
and the in vitro nitric oxide
production. Ann.Biomed.Engineer. 2003;31:1337). It was found that: a)
pretreatment with pGz significantly
blunts the early (EAR) probably through NO preventing mast cell degranulation
and blocks the late (LAR)
allergen-induced airway responses in allergic sheep; b) pGz-induced protection
is lost if the eNOS inhibitor,
L-NAME, is given 30 min before pGz treatment and c) initiating pGz 2h after
antigen challenge still blocks
the LAR (Abraham et al. Periodic acceleration via nitric oxide modifies
antigen-induced airway responses in
sheep. Am.J.Respir.Crit Care Med. 2004;169:A321). NF-kB is a transcription
factor for inflammatory
cytokines involved in the LAR. There are reports that eNOS generated NO
suppresses NF-kB activity
(Blues'., Rivet's. Inhibitory action of nitric oxide on circulating tumor
necrosis factor-induced NF-kappaB
activity and COX-2 transcription in the endothelium of the brain capillaries.
Neuropathol.
Exp.Neurol.2001;60:893). To determine if pGz suppresses NF-kB activity thereby
affecting antigen-induced
airway responses, we performed bronchoalveolar lavage 6h after antigen
challenge and measured free p65
levels in lavage cell nuclear extracts (an indicator of NF-kB activation) by
ELISA. Peak LAR (% increase
over baseline) in control, pGz-treated and L-NAME+pGz treated sheep (all n=6)
were 118 2%, 21 4% and
130 4%, respectively. Levels of p65/106 cells were 1.9- and 1.8-fold higher in
the control and L-
NAME+pGz groups (both p<0.05) when compared to pGz treated animals. Therefore,
pGz stimulates
eNOS and increases NO throughout the body, which can block NF-kB -mediated
inflammation.
(Sackner,M.A., Laredo,I.T., Serebriakov,l., Adams,J.A., Bassuk,J.,
Abraham,W.M. Periodic acceleration
modifies antigen-induced ainvay responses in sheep by nitric oxide (NO)-
mediated down regulation of
nuclear factor kappa beta (NF-k8). Eur.Resp.J. 2004; 24: in press).
[0057] Nitric oxide released from eNOS with periodic acceleration acts on
other tumorigenic
mediators directly or indirectly through nuclear factor kappa beta. Nuclear
factor kappa beta regulates the
expression of vascular endothelial growth factor (VEGF) and IL-8, The
decreased expression of VEGF and
interleukin 8 directly correlate with decreased tumorigenicity, decreased
vascularization of lesions,
decreased formation of malignant ascites, and prolonged survival in several
cancers, e.g., pancreatic,
ovarian, etc. (Xiong HQ, Abbruzzese JL, Lin E et al. NF-kappaB activity
blockade impairs the angiogenic
potential of human pancreatic cancer cells. Int J Cancer 2004; 108(2):181-
188.; Gilmore T, Gapuzan ME,
Kalaitzidis D et al. Rel/NF-kappa 8/I kappa B signal transduction in the
generation and treatment of human
cancer. Cancer Lett 2002; 181(1):1-9.) Thus, suppression of nuclear factor
kappa beta activity with NO
-17-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
released from eNOS with pGz indirectly suppresses tumorigenesis by
downregulating VEGF and IL-8.
Although its action on nuclear factor kappa beta activity is favorable as anti-
tumorigenic, NO released from
eNOS by periodic acceleration might be considered potentially harmful because
under certain
circumstances it stimulates VEGF that might help tumor spread by angiogenesis.
However, there are
conflicting data of NO effects on VEGF that relate to the amount of released
NO which can be a positive or
negative modulator of the VEGF gene under the same conditions, The VEGF-
mediated angiogenesis
requires NO production from activated endothelial NO synthase (eNOS).
Activation of eNOS by VEGF
involves several pathways including Akt/PKB, Ca(2+)/calmodulin, and protein
kinase C. The NO-mediated
VEGF expression can be regulated by HIF-1 and heme oxygenase 1(HO-1) activity,
and the VEGF-
mediated NO production by eNOS can be also modulated by HIF-1 and HO-1
activity, depending upon the
amount of produced NO. These reciprocal relations between NO and VEGF may
contribute to regulated
angiogenesis in normal tissues. (Kimura H, Esumi H. Reciprocal regulation
between nitric oxide and
vascular endothelial growth factor in angiogenesis. Acta Biochim Pol 2003;
50(1):49-59.) In 10 normal
subjects who received 35 daily periodic acceleration treatments, plasma VEGF
doubled from baseline
measurements at the end of seven weeks but was still within the upper range of
normal values and similar
to the VEGF elevation experienced by endurance athletes after acute exercise.
(Kraus RM, Stallings HW,
III, Yeager RC et al. Circulating plasma VEGF response to exercise in
sedentary and endurance-trained
men. J Appl Physiol 2004; 96(4):1445-1450.) In metastatic cancers, plasma VEGF
greatly exceeds normal
values of VEGF, for example with metastatic lung cancers , it may be 10 times
the normal value. (Kishiro I,
Kato S, Fuse D et al. Clinical significance of vascular endothelial growth
factor in patients with primary lung
cancer. Respirology 2002; 7(2):93-98) Thus, the periodic acceleration VEGF
suppressant effect through
inhibition of nuclear factor kappa beta activity is of greater importance in
anti-tumorigenesis than the pro-
tumorigenesis effect of VEGF in angiogenesis,
[0058] Periodic acceleration by releasing small quantities of NO from eNOS
suppresses iNOS
activity, which is usually pro-tumorigenic. It also scavenges reactive oxygen
species (ROS) and reactive
nitrogen species (RNS) that are pro-tumorigenic (Stefano GB, Goumon Y,
Bilfinger TV et al. Basal nitric
oxide limits immune, nervous and cardiovascular excitation: human endothelia
express a mu opiate
receptor. Prog Neurobiol 2000; 60(6):513-530 ). This action of periodic
acceleration therefore may help to
prevent and treat colorectal and other cancers. (Hussain SP, Amstad P, Raja K
et al. Increased p53
mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-
prone chronic inflammatory
disease. Cancer Res 2000; 60(13):3333-3337. The tumor suppressor p53 is a
sensor of diverse cellular
stresses including DNA damage, oxidative stress, and hypoxia, and helps to
direct cell cycle arrest and
apoptosis through transcriptional activation of target genes like p21, p53 is
the most commonly mutated
gene in a broad spectrum of cancers and is frequently associated with tumor
progression, resistance to
therapy, and poor prognosis. In melanoma, p53 rarely mutates but increased -
expression associated with
-18-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
tumor progression and correlates well with iNOS activity. The reason for
paradoxical activity of p53 thought
to be some sort of dysregulation problem. Therefore, suppression of iNOS
activity by eNOS activated with
periodic acceleration promotes the normal tumor suppressor function of p53.
(Tang CH, Grimm EA.
Depletion of endogenous nitric oxide enhances cisplatin-induced apoptosis in a
p53-dependent manner in
melanoma cell lines. J Biol Chem 2004; 279(1):288-298; Stefano GB, Goumon Y,
Bilfinger TV et al. Basal
nitric oxide limits immune, nervous and cardiovascular excitation: human
endothelia express a mu opiate
receptor. Prog Neurobiol 2000; 60(6):513-530.)
[0059] Nitric oxide from NO donor drugs transiently and reversibly inhibits
epidermal growth factor
receptors (EGFR) in neuroblastoma cells, (Murillo-Carretero M, Ruano MJ,
Matarredona ER et al.
Antiproliferative effect of nitric oxide on epidermal growth factor-responsive
human neuroblastoma cells. J
Neurochem 2002; 83(1):119-131.). There is overexpression of epidermal growth
factor receptor (EGFR) in
tumors such as lung, colon, kidney breast, prostate, and, head and neck
carcinomas, which are mostly
resistant to current chemotherapy. Activation of the EGFR-TK enzyme results in
autophosphorylation,
which drives signal transduction pathways leading to tumor growth and
malignant progression. EGF
prevents apoptosis of cancer cells prevents apoptosis thereby immortalizing
tumor cells. When EGFR
activation is blocked with drugs, VGFR and IL-8 production also contribute to
tumor regression. (Lage A,
Crombet T, Gonzalez G. Targeting epidermal growth factor receptor signaling:
early results and future
trends in oncology. Ann Med 2003; 35(5):327-336; Vlahovic G, Crawford J.
Activation of tyrosine kinases in
cancer. Oncologist 2003; 8(6):531-538.) Repeated periodic acceleration
treatments by releasing NO from
nitric oxide would decrease EGFR thereby causing regression of tumor growth.
[0060] Nitric oxide from eNOS activation with periodic acceleration suppresses
tumor necrosis factor
superfamily indirectly through inhibition of nuclear factor kappa beta. (Zhou
Z, Wang L, Song Z et al.
Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition
of lipopolysaccharide-induced
tumor necrosis factor-alpha production and liver injury. Am J Pathol 2004;
164(5):1547-1556.). Periodic
acceleration through shear stress suppresses tumor necrosis factor alpha
activity independently of
activation of eNOS. (Chiu JJ, Lee PL, Lee Cl et al. Shear stress attenuates
tumor necrosis factor-alpha-
induced monocyte chemotactic protein-I expressions in endothelial cells. Chin
J Physiol 2002; 45(4):169-
176). Nitric oxide from eNOS activation with periodic acceleration scavenges
COX1 and COX2 and also
inhibits lipoxygenase. (Stefano GB, Magazine HI. Nitric Oxide Autoregulation
and Its Significance. In:
Stefano GB, editor. Biomedical Significance of Nitric Oxide. Warsaw-New York:
Medical Science
International Co., Ltd., 2003: 57-68.). Finally, NO from eNOS reduces tumor
cell adhesion to blood vessels
and also suppresses adhesion molecules thereby suppressing tumor metastases.
(Kong L, Dunn GD,
Keefer LK et al. Nitric oxide reduces tumor cell adhesion to isolated rat
postcapillary venules. Clin Exp
Metastasis 1996; 14(4):335-343; Stefano GB, Goumon Y, Bilfinger TV et al.
Basal nitric oxide limits
-19-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
immune, nervous and cardiovascular excitation: human endothelia express a mu
opiate receptor. Prog
Neurobiol 2000; 60(6):513-530.)
[0061] The availability of nitric oxide released by activation of eNOS with
periodic acceleration may
be improved by vitamin and dietary supplements thereby enhancing its effects
in cancer treatment as well
as treatment of any condition that requires increased levels of nitric oxide
from eNOS. This may be of
importance where endothelial function is compromised such as in
arterioscierosis. Thus, L-ascorbic acid
(vitamin C), increases nitric oxide synthase (NOS) enzyme activity via
chemical stabilization of
tetrahydrobiopterin (BH4). Vitamin C also increases tetrahydrobiopterin and
NOS activity in blood vessels.
The beneficial effect of vitamin C on vascular endothelial function appears to
be mediated in part by
protection of tetrahydrobiopterin and restoration of eNOS enzymatic activity.
Prolonged high activity of
iNOS may be detrimental to vascular function due to "uncoupling" of,eNOS and
subsequent formation of
reactive oxygen species (ROS). (d'Uscio LV, Milstien S, Richardson D et al.
Long-term vitamin C treatment
increases vascular tetrahydrobiopterin levels and nitric oxide synthase
activity. Circ Res 2003; 92(1):88-
95.) An oral glucose challenge causes transient impairment of endothelial
function, probably because of
increased oxidative stress. During oxidative stress, endothelial nitric oxide
(NO) synthase (eNOS) becomes
uncoupled because of decreased bioavailability of tetrahydrobiopterin (BH4),
an essential cofactor of
eNOS. Administration of BH4, which is available commercially in some countries
but not the United States,
reverses downregulation of eNOS. (Ihlemann N, Rask-Madsen C, Perner A et al.
Tetrahydrobiopterin
restores endothelial dysfunction induced by an oral glucose challenge in
healthy subjects. Am J Physiol
Heart Circ Physiol 2003; 285(2):H875-H882.) Also, folic acid administration
exerts direct anti-oxidative
effects and contributes to restoration of impaired NO metabolism. Folate also
reduces plasma
homocysteine levels, enhances eNOS, and has anti-inflammatory actions. It
stimulates endogenous BH4
regeneration, a cofactor necessary for NO synthesis from eNOS, inhibits
intracellular superoxide
generation, and thus enhances the half-life of NO. BH4 in turn enhances NO
generation and augments
arginine transport into the cells. Folic acid increases the concentration of
omega-3 PUFAs, which also
upregulates eNOS synthesis. Vitamin C augments NO synthesis from eNOS by
increasing intracellular
BH4 and stabilization of BH4. (Stanger 0, Weger M. Interactions of
homocysteine, nitric oxide, folate and
radicals in the progressively damaged endothelium. Clin Chem Lab Med 2003;
41(11):1444-1454; Das UN.
Folic acid says NO to vascular diseases. Nutrition 2003; 19(7-8):686-692.). In
addition, there appears to be
a benefit of higher folic acid consumption in reducing risks of colon and
breast cancers, (Willett WC. Diet
and cancer. Oncologist 2000; 5(5):393-404) Niacin in much higher doses than
recommended for daily
requirements elevates high density lipoprotein (HDL) and improves endothelial
function by upregulating
eNOS. The dose of niacin (Niaspan, KOS) is initiated at 375 mg at night and
titrated to a maximal tolerated
dose of 1500 mg. Patients take aspirin 30 minutes prior to niacin to minimize
side effects. (Kuvin JT, Ramet
ME, Patel AR et al. A novel mechanism for the beneficial vascular effects of
high-density lipoprotein
-20-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
cholesterol: enhanced vasprelaxation and increased endothelial nitric oxide
synthase expression. Am Heart
J 2002; 144(1):165-172.)
[0062] Das (Das UN, Folic acid says NO to vascular diseases. Nutrition 2003;
19(7-8):686-692)
recommends the following for supplementation as an aid to achieve good
endothelial function: folic acid 1
to 5 mg /day, vitamin B12 1000ug/day, vitamin B6 5 to 10 mg/day, vitamin C 100
mg/day, L-arginine 500
mg twice a day, BH4 1 to 2 mg/Kg body weight (available in some countries but
not the United States),
polyunsaturated fatty acids (PUFAs) (especially eicosapentaenoic acid 120
mg/day & docosahexaenoic
acid 180 mg/day. Such a supplement plan may be used in conjunction with
periodic acceleration to
enhance its effects on eNOS. However, Das' recommendation for the dose of L-
Arginine is probably an
underestimate. Oral supplementation with large amounts (6-21 g/day) of L-
arginine, the precursor of
endothelial-derived nitric oxide, improves endothelium-mediated vasodilation
in hypercholesterolemia. Flow
mediated vasodilation is improved with 6.6 g of L-Arginine administered for in
the form of a nutrient bar.
(Maxwell AJ, Anderson B, Zapien MP et al. Endothelial dysfunction in
hypercholesterolemia is reversed by
a nutritional product designed to enhance nitric oxide activity. Cardiovasc
Drugs Ther 2000; 14(3):309-
316.) Lower doses are ineffective. High dosage niacin may be administered in
the presence of endothelial
dysfunction to produce further upregulation of eNOS,
[0063] Several chemopreventive phytochemicals have been shown to inhibit COX-2
and iNOS
expression by blocking NF-kappa B activation. Curcumin, a yellow pigment of
turmeric (Curcuma longa L.,
Zingiberaceae), the green tea polyphenol epigallocatechin gallate (EGCG), and
resveratrol from grapes
(Vitis vinifera, Vitaceae) strongly.suppress tumor promotion because they
suppress nuclear factor kappa
beta, (Surh YJ, Chun KS, Cha HH et al. Molecular mechanisms underlying
chemopreventive activities of
anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through
suppression of NF-kappa
B activation, Mutat Res 2001; 480-481:243-268,) These phytochemicals could
potentiate the effect of
periodic acceleration.
[0064] Both the- immediate and late complications of radiation and/or
chemotherapy may be
ameliorated by periodic acceleration as pre-treatment, during treatment and
post-treatment. Because
radiation-induced vascular injury precedes the tissue damage, vascular injury
is regarded as crucial in the
pathogenesis of tissue damage. Radiation injury is marked by activation of
adhesion molecules that
promote leukocyte infiltration of normal tissue. Radiation activates nuclear
factor kappa beta, which in turn
activates adhesion molecules. (Quarmby,S.; Kumar,P.; Kumar,S. Radiation-
induced normal tissue injury:
role of adhesion molecules in leukocyte-endothelial cell interactions.
Int.J.Cancer 1999;82_385-395.)
Radiotherapy and chemotherapy produce numerous adverse early and late
complications. Oral and
gastrointestinal (GI) mucositis, a frequent complication of anticancer
treatment, threatens the effectiveness
of therapy because it leads to dose reductions, increases healthcare costs,
and impairs patients' quality of
life. (Sonis ST, Elting LS, Keefe D et al. Perspectives on cancer therapy-
induced mucosal injury:
-21 -
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
pathogenesis, measurement, epidemiology, and consequences for patients. Cancer
2004; 100(9
Suppl):1995-2025,)
[0065] Thus, periodic acceleration alone or in conjunction with
chemotherapeutic or x-ray or other
cancer suppressing agents or technology offers a way to treat cancers. It may
permit lesser doses of
radiation and/or chemotherapy thereby minimizing deleterious side effects.
Furthermore, application of
periodic acceleration either alone or with other preventative agents can be
used to prevent cancers.
Treatment with periodic acceleration avoids the late effects of radiotherapy
and chemotherapy on normal
tissues. The late onset of necrosis and fibrosis in normal tissues can be a
serious consequence of
radiotherapy and chemotherapy in cancer patients. Because radiation-induced
vascular injury precedes the
tissue damage, vascular injury is regarded as crucial in the pathogenesis of
tissue damage. Radiation
injury is marked by activation of adhesion molecules that promote leukocyte
infiltration of normal tissue.
The stress of radiation or chemotherapy activated nuclear factor kappa beta in
turn activates adhesion
molecules. (Sonis ST, Elting LS, Keefe D et al. Perspectives on cancer therapy-
induced mucosal injury:
pathogenesis, measurement, epidemiology, and consequences for patients. Cancer
2004; 100(9
Suppl):1995-2025) Radiotherapy for abdominal and pelvic malignancies results
in an increased risk of
radiation enteritis. (Bismar MM, Sinicrope FA. Radiation enteritis, Curr
Gastroenterol Rep 2002; 4(5):361-
365.) Late effects of radiotherapy depend upon site that of radiation and may
cause the following: 1) cranial
radiotherapy - neurocognitive deficits, obesity, seizures and strokes,
cataracts, etc. 2) chest or mantle
radiotherapy - breast cancer, thyroid cancer, hypothyroidism, pulmonary
fibrosis, lung cancer, cardiac
fibrosis, pericarditis, etc, 3) abdominal/pelvic radiotherapy - chronic
enteritis, gastrointestinal malignancy,
renal failure, hemorrhagic cystitis, bladder cancer, ovarian failure,
testicular failure, etc., 4) any radiation -
skin cancer, melanoma, sarcoma, etc. Late effects of chemotherapy depend upon
the drug and may cause
the following: 1) alkylating agents (e.g., cyclophosphamide, chlorambucil,
bisulfan, procarbazine, etc.) -
hypogonadism, early menopause, acute myeloid leukemia, pulmonary fibrosis,
bladder fibrosis, renal
failure, etc., 2) cisplatin/carboplatin - hearing loss, vertigo, tinnitis,
renal failure, etc., 3) methotrexate -
neurocognitive deficits, 4) anthracyclines (e.g., doxorubacin, daunorubicin,
etc.) - cardiomyopathy,
arrhythmia, and 4) bleomycin - interstitial pneumonitis, pulmonary fibrosis,
5) corticosteroids - osteopenia,
osteoporosis, avascular necrosis, 6) epepodophylloxins - acute myeloid
leukemia. (Oeffinger KC, Hudson
MM. Long-term complications following childhood and adolescent cancer:
foundations for providing risk-
based health care for survivors, CA Cancer J Clin 2004; 54(4):208-236.)
[0066] In summary, periodic acceleration has a place in management of cancer
either as a stand-
alone modality or complimentary to radiotherapy and chemotherapy regimens.
Periodic acceleration
evokes widespread release of nitric oxide throughout body- wherever there is a
blood vessel, pulsatile
shear stress promotes expression of nitric oxide that suppresses directly or
indirectly pro-tumorigenic
mediators. These include nuclear factor kappa beta, vascular endothelial
growth factor, inteleukin-8,
-22-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
epidermal growth factor receptor, tumor necrosis superfamily, inducible nitric
oxide synthase, mutated p53,
COX2, and adhesion molecules. Periodic acceleration does not harm healthy
cells nor produce deleterious
side effects in contrast to radiotherapy and chemotherapy thereby avoiding the
late fibrotic effects of
radiation and chemotherapy on normal tissues. Periodic acceleration cannot
cause overdose of
endothelial-derived mediators. Periodic acceleration is complementary to
conventional cancer therapies
without adverse "drug" interactions." Periodic acceleration is synergistic or
additive in suppression of
tumorigenesis to radiotherapy and chemotherapy. Periodic acceleration
mitigates the early and late
complications of radiotherapy and chemotherapy. Periodic acceleration may be
utilized chronically as a
cancer prevention measure. Vitamin supplements, antioxidants, and
phytochemicals in conjunction with
periodic acceleration may increase the effectiveness of NO release from eNOS,
with potential for greater
suppression of nuclear factor kappa beta and other inflammatory mediators in
tumors,
B. Preconditioning and/or Conditioning
[0067] Stretch-induced muscle injuries or strains, muscle contusions and
delayed-onset muscle
soreness (DOMS) are common muscle problems in athletes. Anti-inflammatory
treatment is often used for
the pain and disability associated with these injuries. The most recent
studies on non-steroidal anti-
inflammatory drugs (NSAIDs) in sprains and contusions suggest that their use
can result in a modest
inhibition of the initial inflammatory response and its symptoms. This may be
associated with slight
negative effects later in the healing phase. Corticosteroids have generally
been shown to adversely affect
the healing of these acute injuries. The beneficial effect of NSAIDs on
improvement of delayed-onset of
muscle soreness appears to be minimal. (Almekinders L C. Anti-inflammatory
treatment of muscular
injuries in sport. An update of recent studies, Sports Med 1999; 28:383-88).
Prolonged and strenuous
exercise induces significant increases in plasma IL-1beta, IL-6 and tumor
necrosis factor alpha (Brenner I
K, Natale V M, Vasiliou P, Moldoveanu A I, Shek P N, Shephard R J. Impact of
three different types of
exercise on components of the inflammatory response, Eur. J. Appl. Physiol.
Occup. Physiol. 1999; 80:452-
60; Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen T L, MacLean D A,
Pedersen B K. Exercise-
induced increase in serum interleukin-6 in humans is related to muscle damage,
J. Physiol 1997; 499 (Pt
3):833-41; Pedersen B K, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine
response to strenuous
exercise. Can. J. Physiol. Pharmacol. 1998; 76:505-11).
[0068] There is a positive correlation between elevated serum IL-6 levels and
skeletal muscle
damage in terms of creatine kinase elevations (Bruunsgaard H, Galbo H,
Halkjaer-Kristensen J, Johansen
T L, MacLean D A, Pedersen B K. Exercise-induced increase in serum interleukin-
6 in humans is related to
muscle damage, J. Physiol. 1997; 499 (Pt 3):833-41). In football players who
require intravenous hydration
for muscle cramps after training sessions, all have extremely high levels of
serum nitrite, presumably
released from iNOS present in macrophages and leucocytes as a result of the
stress of strenuous exercise
(Maddali S, Rodeo S A, Barnes R, Warren R F, Murrell G A. Postexercise
increase in nitric oxide in football
-23-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
players with muscle cramps. Am. J. Sports Med. 1998; 26:820-24). Athletes seem
to be more prone to
upper respiratory viral infections probably because strenuous exercise
promotes increase of IL-6, tumor
necrosis factor alpha, and large quantities of nitric oxide that compromise
the immune defense system.
These infections usually appear after exercise discontinuation (within 3 days)
particularly in those athletes
practicing sports that require a long term effort and resistance (Gani F,
Passalacqua G, Senna G, Mosca F
M. Sport, immune system and respiratory infections, Allerg. Immunol.(Paris)
2003; 35:41-46).
[0069] Small quantities of nitric oxide released from eNOS suppress strenuous
exercise induced
activation of nuclear factor kappa beta thereby diminishing IL-1 beta, IL-6,
tumor necrosis factor and other
inflammatory cytokines and adhesion molecules. In addition, small quantities
of nitric oxide from eNOS
suppress activity of iNOS (Stefano GB, Prevot V, Cadet P, Dardik I. Vascular
pulsations stimulating nitric
oxide release during cyclic exercise may benefit health: a molecular approach
(review). Int. J. Mol. Med.
2001; 7:119-29). This is important because large amounts of nitric oxide are
released after strenuous
exercise in professional football players and other athletes that are
associated with severe muscle cramps.
[0070] Therefore, periodic acceleration can mitigate skeletal muscular cramps
during an athletic
event, and help to prevent muscle strains during an event as well as delayed
onset muscular soreness
(DOMS) and involuntary muscle cramps and spasms immediately following the
athletic event and delayed
until the sleeping hours. It has been found that an additional periodic
acceleration treatment administered
four to eight hours after the athletic event provides even better relief than
a single pretreatment in relieving
nocturnal muscle cramps.
[0071] In addition to skeletal muscle damage and propensity to viral
infections associated with
strenuous exercise, damage to heart muscle may occur even in normal subjects.
Cardiac troponin T (cTnT)
and troponin I (cTnl) are highly sensitive and 'specific for detecting
myocardial damage even in the
presence of skeletal muscle injury. Ultraendurance exercise may cause
myocardial damage as indicated by
elevations of these biochemical cardiac-specific markers and also by
echocardiography (Rifai N, Douglas P
S, 0'Toole M, Rimm E, Ginsburg G S. Cardiac troponin T and I,
echocardiographic [correction of
electrocardiographic] wall motion analyses, and ejection fractions in athletes
participating in the Hawaii
Ironman Triathlon, Am. J. Cardiol. 1999; 83:1085-89; Shave RE, Dawson E, Whyte
G, George K, Ball D,
Gaze D C et al. Evidence of exercise-induced cardiac dysfunction and elevated
cTnT in separate cohorts
competing in an ultra-endurance mountain marathon race Int. J. Sports Med.
2002: 23:489-94; Ohba H,
Takada H, Musha H, Nagashima J, Mori N, Awaya T et al. Effects of prolonged
strenuous exercise on
plasma levels of atrial natriuretic peptide and brain natriuretic peptide in
healthy men. Am. Heart J. 2001;
141:751-58). There are no studies reported in the literature on normal
subjects with regard to less
strenuous exercise but it stands to reason that in some individuals, 'minor
damage might occur. Minimal
myocardial damage could compromise athletic performance.
-24-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
[0072] Activation of eNOS to release small quantities of nitric oxide
preconditions the heart against
the adverse effects of compromise of the blood supply to the heart that
produces myocardial damage.
Periodic acceleration activates eNOS through increased pulsatile shear stress
and, as such, is a means to
precondition the heart. Endogenous nitric oxide 1) reduces myocardial oxygen
consumption and thus
improves regional myocardial function for any given level of myocardial blood
flow, oxygen consumption
and energetics, 2) preserves contractile calcium sensitivity during myocardial
ischemia, and 3) contributes
to hibernation, i.e., adaptation to myocardial ischemia, by preserving
regional contractile function without
any effect on myocardial energetics (Heusch G, Post H, Michel MC, Kelm M,
Schulz R. Endogenous nitric
oxide and myocardial adaptation to ischemia. Circ. Res. 2000; 87:146-52).
Since the limitation to athletic
activities often is the amount of blood pumped by the heart through the body,
preconditioning with periodic
acceleration serves to optimize athletic performance.
[0073] Based upon animal experiments, upregulation of endothelial nitric oxide
synthase activity
should increase the number of mitochondria present in skeletal muscle cells.
In turn, heat production is
increased within these cells thereby improving sports performances. (Nisoli E,
Clementi E, Paolucci C et al.
Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide.
Science 2003; 299(5608):896-
899; Brown GC. NO says YES to mitochondria. Science 2003; 299:838-839.)
[0074] Exercise-induced bronchospasm (EIB), i.e., an asthmatic episode,
affects up to 35% of
athletes and up to 90% of asthmatics (Kukafka DS, Lang DM, Porter S, Rogers J,
Ciccolella D, Polansky M
et al. Exercise-induced bronchospasm in high school athletes via a free
running test: incidence and
epidemiology. Chest 1998; 114:1613-22). This factor limits athletic
capabilities.
[0075] Since many athletic venues do not permit effective drugs for the
treatment of asthma because
of they also improve performance unrelated to alleviation of asthma,
pretreatment of such athletes can be
accomplished with periodic acceleration to prevent exercise induced asthma.
Here, the beneficial agent,
nitric oxide is generated from the athlete's own body.
[0076] Physical activity protects against ischemic stroke via mechanisms
related to the upregulation
of endothelial nitric oxide synthase (eNOS) in the vasculature. In wild-type
mice that performed voluntary
training on running wheels or exercise on a treadmill apparatus for 3 weeks,
respectively, ligation of the
middle cerebral artery was associated with reduced cerebral infarct size and
functional deficits, improved
endothelium-dependent vasorelaxation, and augmented cerebral blood flow. The
neuroprotective effects of
physical training were completely absent in eNOS-deficient mice, indicating
that the enhanced eNOS
activity by physical training was the predominant mechanism by which this
modality protects against
cerebral injury. (Endres M, Gertz K, Lindauer U et al, Mechanisms of stroke
protection by physical activity.
Ann Neurol 2003; 54(5):582-590.)
[0077] In summary, periodic acceleration treatments administered prior to an
athletic event minimize
delayed onset of muscle soreness (DOMS) and nocturnal muscle spasms. An
additional periodic
-25-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
acceleration treatment administered four to eight hours following cessation of
the athletic event provides
even further relief. Periodic acceleration administered prior to strenuous
athletic events minimizes
microscopic myocardial damage. Chronic treatment with periodic acceleration
improves sports
performance by promoting mitochondrial biogenesis. Periodic acceleration
administered prior to an athletic
event protects against exercise induced asthma. Since many athletic venues do
not permit effective drugs
for the treatment of asthma because of they also improve performance unrelated
to alleviation of asthma,
pretreatment of competitive athletes can be accomplished with periodic
acceleration to prevent exercise
induced asthma. Chronic periodic acceleration treatments should minimize
damage that might occur with
ischemic events such as stroke, coronary thrombosis, pulmonary embolism, etc.
C. Weight Control
[0078] An epidemic of obesity exists in the United States and other countries
of the Western World.
Currently, 65% of American adults are overweight and 31% are obese. Further,
this prevalence parallels
the 29% prevalence of hypertension with blood pressures >140/90 or taking anti-
hypertensive drugs.
Weight loss is critical in the effective management of obesity hypertension
and the accompanying target
organ damage, although recidivism rates are high. Prevention of weight gain
should be the major priority
for combating hypertension and its consequences in the future. (Davy KP, Hall
JE. Obesity and
hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol
2004; 286(5):R803-R813.)
Depression and obesity are linked to elevated CRP suggesting a possible
synergistic effect of obesity and
depressive mood on chronic low-level inflammation, which may play a crucial
role in the pathogenesis of
atherosclerosis. (Ladwig KH, Marten-Mittag B, Lowel H et al. Influence of
depressive mood on the
association of CRP and obesity in 3205 middle aged healthy men. Brain Behav
Immun 2003; 17(4):268-
275.) Exercise and proper diet are key to weight control but numerous studies
have stressed the difficulty in
getting obese patients to comply.
[0079] In a one year study, it was found that nitric oxide deficient mice
(eNOS knockout) were
significantly heavier than normal wild-type mice over the entire observation
period even though dietary
intake and activity were controlled and the same. The NO deficient had lesser
oxygen consumptions and
fewer mitochondria in brown adipocytes than the wild type mice. The
investigators concluded that eNOS
regulates mitochondrial biogenesis, energy expenditure and heat production.
They noted that NOS
deficiency reduced energy expenditure and produced weight gain, insulin
resistance, and hypertension,
which are typical features of the metabolic syndrome. (Nisoli E, Clementi E,
Paolucci C et al. Mitochondrial
biogenesis in mammals: the role of endogenous nitric oxide. Science 2003;
299(5608):896-899.) Based
upon these findings, Brown speculated that if eNOS could be upregulated, then
an increase mitochondrial
number in skeletal muscle would increase sport performances, reduce obesity,
treat the metabolic
syndrome and even reverse aging. (Brown GC. NO says YES to mitochondria.
Science 2003; 299:838-
839.) I have made anecdotal observations in three subjects indicating that
two, 45 minute treatments daily
-26-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
with periodic acceleration cause significant weight reduction over weeks to
months period. A metabolic
chamber study in a single subject using periodic acceleration produced an
excess caloric expenditure of
approximately 100 calories in 24 hours compared to a control day. Long term
periodic acceleration should
produce an accumulative effect as a result of increased mitochondrial
biogenesis. Thus, periodic
acceleration ought to play a role in the management of obesity.
[0080] Cachexia, which is marked by severe weight loss, is the opposite of
obesity yet it too may
respond to upregulation of eNOS but by a totally different mechanism. For
example, weight loss, mostly
due to skeletal muscle atrophy, is a frequent and clinically relevant problem
in patients with chronic
obstructive pulmonary disease (COPD) as well as in patients with neoplasms. In
such patients, activation of
nuclear factor kappa beta and iNOS induction takes place in the skeletal
muscle and contributes to the
molecular pathogenesis of cachexia along with other inflammatory cytokines.
(Agusti A, Morla M, Sauleda J
et al. NF-kappaB activation and iNOS upregulation in skeletal muscle of
patients with COPD and low body
weight. Thorax 2004; 59(6):483-487) Periodic acceleration by increasing
release of small amounts of nitric
oxide from eNOS suppresses nuclear factor kappa beta and iNOS activities
thereby ameliorating the
skeletal muscle pathology. In addition, deficiency of eNOS causes less muscle
oxidative capacity as well
as the activities of energy metabolism enzymes in oxidative (soleus) muscle.
(Momken I, Fortin D, Serrurier
B et al. Endothelial nitric oxide synthase (NOS) deficiency affects energy
metabolism pattern in murine
oxidative skeletal muscle. Biochem J 2002; 368(Pt 1):341-347.) This, too, is
ameliorated with upregulation
of eNOS by periodic acceleration.
[0081] In summary, periodic acceleration treatments control weight, ameliorate
the metabolic
syndrome, improve sports performance, and improve skeletal muscle pathology
associated with the
cachexia of COPD and cancers.
D. Ventricular Remodeling
[0082] While current therapeutic strategies are designed to restore blood flow
to the ischemic
myocardium and limit infarct size, adverse left ventricular remodeling
progressing to dysfunction remains a
significant complication following myocardial infarction. Ventricular,
remodeling also takes place in
conditions associated with volume overload. Ventricular remodeling consists of
change from an elliptical to
a spherical left ventricular volume with concomitant increase of end-systolic
and end-diastolic diameters.
Reverse remodeling means a reversal of ventricular shape toward a normal
shape. In myocardial
infarctions, the extracellular matrix (ECM) is a key component in the
remodeling process through increases
of collagen in the infarct area that replace necrotic myocytes to form a scar.
In addition, the matrix
metal loprotei n ases (MMP) coordinate ECM turnover through degradation of ECM
components. Several
laboratories have demonstrated that MMP participates in remodeling events that
lead to left ventricular
dilation, and inhibition or targeted deletion of specific MMPs has beneficial
effects post-myocardial
infarction. (Lindsey ML, Mann DL, Entman ML et al. Extracellular matrix
remodeling following myocardial
-27-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
injury. Ann Med 2003; 35(5):316-326) Left ventricular remodeling that occurs
in mitral regurgitation and
other ventricular volume overload conditions are produced by overstretch of
the myocardium. (Oral H,
Sivasubramanian N, Dyke DB et al. Myocardial proinflammatory cytokine
expression and left ventricular
remodeling in patients with chronic mitral regurgitation. Circulation 2003;
107(6):831-837; Wei CC,
Lucchesi PA, Tallaj J et al. Cardiac interstitial bradykinin and mast cells
modulate pattern of LV remodeling
in volume overload in rats. Am J Physiol Heart Circ Physiol 2003; 285(2):H784-
H792; Stewart JA, Jr., Wei
CC, Brower GL et al. Cardiac mast cell- and chymase-mediated matrix
metalloproteinase activity and left
ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardioi
2003; 35(3):311-319.)
[0083] Several mediators regulate ventricular remodeling. Over stretch of the
myocardium due to
volume overload causes expression of tumor necrosis factor alpha. (Oral H,
Sivasubramanian N, Dyke DB
et al. Myocardial proinflammatory cytokine expression and left ventricular
remodeling in patients with
chronic mitral regurgitation. Circulation 2003; 107(6):831-837) As mentioned
above, MMP's activity
increases during ventricular remodeling. In experimental aortocaval fistula in
rats, the number of myocardial
mast cells significantly increases, and there is a close association between
mast cell density and MMP
activity. Cromolyn, a drug that inhibits degranulation of the mast cell
prevents the increase in mast cell
number and MMP activity, Therefore cardiac mast cells play a major role in
regulating MMP activity in
ventricular remodeling. (Brower GL, Chancey AL, Thanigaraj S et al. Cause and
effect relationship between
myocardial mast cell number and matrix metalloproteinase activity. Am J
Physiol Heart Circ Physiol 2002;
283(2):H518-H525.) Further, there is a significant interaction of mast cells
and bradykinin in the cardiac
interstitium that modulates the pattern of LV remodeling in the acute phase of
volume overload. (Wei CC,
Lucchesi PA, Tallaj J et al. Cardiac interstitial bradykinin and mast cells
modulate pattern of LV remodeling
in volume overload in rats. Am J Physiol Heart Circ Physiol 2003; 285(2):H784-
H792.) Cardiac mast cells
and chymase are important modulators of MMP activity and extracellular matrix
degradation that contribute
to adverse left ventricular remodeling in chronic volume overload secondary to
mitral regurgitation. (Stewarf
JA, Jr., Wei CC, Brower GL et al. Cardiac mast cell- and chymase-mediated
matrix metalloproteinase
activity and left ventricular remodeling in mitral regurgitation in the dog. J
Mol Cell Cardiol 2003; 35(3):311-
319.)
[0084] Another mediator that promotes ventricular remodeling is caspase-3 that
is activated by
myocardial stunning following myocardial ischemia. (Ruetten H, Badorff C,
lhling C et al. Inhibition of
caspase-3 improves contractile recovery of stunned myocardium, independent of
apoptosis-inhibitory
effects. J Am Coll Cardiol 2001; 38(7):2063-2070.) Caspase-3 promotes cleavage
of troponin-I, an
important component of the cardiac contractile apparatus, and death or
apoptosis of cardiomyocytes.
Inhibition of caspase-3 by pharmacological agents causes less cleavage of
tropomin-1 and fewer apoptotic
cardiomyocytes. This intervention in turn preserves myocardial contractile
proteins, reduces systolic
dysfunction, and attenuates ventricular remodeling. (Chandrashekhar Y, Sen S,
Anway R et al. Long-term
-28-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I
cleavage, protects left ventricular
function, and attenuates remodeling in rats with myocardial infarction. J Am
Coll Cardiol 2004; 43(2):295-
301.) Nitric oxide from eNOS tonically inhibits myocardial caspase activity
and prevents caspase activation
by upstream caspases. The ability of NO to inhibit downstream caspase 3 has
potential to rescue a cell
from apoptosis (programmed cell death) even after the caspase cascade has been
activated. The reduced
NO in chronic heart failure increases myocardial caspase 3 activity. Agents
that promote NO release from
eNOS such as ACE inhibitors, prevent caspase activation in heart failure and
attenuate ventricular
remodeling. (Mital S, Barbone A, Addonizio LJ et al. Endogenous endothelium-
derived nitric oxide inhibits
myocardial caspase activity: implications for treatment of end-stage heart
failure. J Heart Lung Transplant
2002; 21(5):576-585.)
[0085] Left ventricular reverse remodeling takes place in animals after
administration of drugs that
increase activity of eNOS. (Kobayashi N, Mori Y, Nakano S et al. Celiprolol
stimulates endothelial nitric
oxide synthase expression and improves myocardial remodeling in
deoxycorticosterone acetate-salt
hypertensive rats. J Hypertens 2001; 19(4):795-801; Kobayashi N, Hara K,
Watanabe S et al. Effect of
imidapril on myocardial remodeling in L-NAME-induced hypertensive rats is
associated with gene
expression of NOS and ACE mRNA. Am J Hypertens 2000; 13(2):199-207.) In
humans, cardiac
resynchronization therapy produces reverse left ventricular (LV) remodeling in
patients with congestive
heart failure that might be due to upregulation of eNOS. (Penicka M, Bartunek
J, De Bruyne B et al.
Improvement of left ventricular function after cardiac resynchronization
therapy is predicted by tissue
Doppler imaging echocardiography. Circulation 2004; 109(8):978-983; Hiratsuji
T, Adachi H, Isobe N et al,
[Does cardiac resynchronization therapy improve nitric oxide concentration in
exhaled gas?]. J Cardiol
2004; 43(1):11-15.)
[0086] Periodic acceleration through release of NO from eNOS promotes left
ventricular reverse
remodeling because it suppresses the activity of several mediators that play
in role in promoting left
ventricular remodeling including tumor necrosis factor alpha and caspase-3.
Periodic acceleration also
prevents cardiac mast cell degranulation as a result of the increased nitric
oxide from eNOS. In this
respect, heparin also prevents mast cell degranulation and activated eNOS to
release NO. (Kouretas PC,
Hannan RL, Kapur NK et al. Non-anticoagulant heparin increases endothelial
nitric oxide synthase activity:
role of inhibitory guanine nucleotide proteins. J Mol Cell Cardiol 1998;
30(12):2669-2682. Finally, periodic
acceleration causes a significant increase of myocardial blood flow that
suppresses adverse mediator
expression. (Adams JA, Mangino MJ, Bassuk J et al. Regional blood flow during
periodic acceleration. Crit
Care Med 2001; 29(10):1983-1988.)
[0087] In summary, periodic acceleration administered early in situations that
promote left ventricular
remodeling such as acute myocardial infarction and ventricular volume overload
from mitral regurgitation,
aortic regurgitation and arteriovenous fistula attenuates ventricular
remodeling. Administration of periodic
-29-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
acceleration after ventricular remodeling has developed promotes beneficial
reverse ventricular
remodeling, The combination of periodic acceleration with drugs that stabilize
cardiac mast cells such as
non-coagulant and coagulant heparin and cardiac drugs that activate eNOS such
as ACE inhibitors as well
as heparin produce additive or synergistic effects,
E. Atrial Fibrillation
[0088] Atrial fibrillation may occur in susceptible patients as a result of
electrical remodeling of the
atria due to oxidative stress or inflammation. (Korantzopoulos P, Kolettis T,
Siogas K et al. Atrial fibrillation
and electrical remodeling: the potential role of inflammation and oxidative
stress. Med Sci Monit 2003;
9(9):RA225-RA229,) In this respect, statins have been shown to prevent atrial
fibrillation in patients with
coronary artery disease independent of their cholesterol lowering properties.
(Young-Xu Y, Jabbour S,
Goldberg R et al. Usefulness of statin drugs in protecting against atrial
fibrillation in patients with coronary
artery disease. Am J Cardiol 2003; 92(12):1379-1383.) Further, stains promote
release of nitric oxide from
eNOS, and NO has potent anti-inflammatory and anti-oxidative stress effects.
(Laufs U. Beyond lipid-
lowering: effects of statins on endothelial nitric oxide. Eur J Clin Pharmacol
2003; 58(11):719-731.) Thus,
chronic periodic acceleration treatments that upregulation of eNOS activity
prevents or minimizes
occurrence of atrial fibrillation.
[0089] Atrial fibrillation is associated with decreased expression of eNOS in
left atrial tissue because
of turbulent flow in the atrium. Decreased NO is associated with lack of
inhibition of prothrombotic protein
plasminogen activator inhibitor-1 (PAI-1) and therefore predisposes to atrial
thrombus formation, a serious
complication that can lead to stroke. (Cai H, Li Z, Goette A et al.
Downregulation of endocardial nitric oxide
synthase expression and nitric oxide production in atrial fibrillation:
potential mechanisms for atrial
thrombosis and stroke. Circulation 2002; 106(22):2854-2858.)
[0090] In summary, chronic periodic acceleration treatments is patients
susceptible to atrial fibrillation
because of enlarged left atrium, e.g., mitral stenosis, mitral regurgitation,
chronic heart failure, can be
administered prophylactically. In established atrial fibrillation, chronic
periodic acceleration can prevent the
formations of left atrial thrombosis.
F. Coronary Artery Bypass Surgery
[0091] Myocardial tumor necrosis factor alpha production and nuclear factor
kappa B activation has
been demonstrated in chronic heart failure and experimental models of acute
ischemia-reperfusion injury.
Further, a cause and effect relationship has been established between these
events and cardiomyocyte
apoptosis (cell death) following such conditions. Recently, it as found that
coronary artery bypass grafting
results in activation of NF-kappaB and an increase of tumor necrosis factor
alpha in the heart. (Meldrum
DR, Partrick DA, Cleveland JC, Jr. et al. On-pump coronary artery bypass
surgery activates human
myocardial NF-kappaB and increases TNF-alpha in the heart. J Surg Res 2003;
112(2);175-179,) These
mediators promote the deleterious effect of inflammation from cardiopulmonary
bypass (CPB) is known to
-30-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
cause part of the systemic inflammatory reaction after cardiac surgery that
lead to organ failure. (Fillinger
MP, Rassias AJ, Guyre PM et al. Glucocorticoid effects on the inflammatory and
clinical responses to
cardiac surgery. J Cardiothorac Vasc Anesth 2002; 16(2):163-169.)
[0092] After coronary artery bypass surgery, almost a quarter of patients have
a cognitive deficit
when tested two months after the operation. This improves but cognitive
deficit can be detected in some
patients even five years later. (van Dijk D, Kaiser AM, Daphnis JC et al.
Neurocognitive dysfunction after
coronary artery bypass surgery: a systematic review. J Thorac Cardiovasc Surg
2000; 120(4):632-639;
Stygail J, Newman SP, Fitzgerald G et al. Cognitive change 5 years after
coronary artery bypass surgery.
Health Psychol 2003; 22(6):579-586.) This cognitive deficit has been
attributed to cerebral microembolism.
Nitric oxide released from eNOS improves red cell deformability permitting
easier capillary passage. (Bor-
Kucukatay M, Wenby RB, Meiselman HJ et al. Effects of nitric oxide on red
blood cell deformability. Am J
Physiol Heart Circ Physiol 2003; 284(5):H1577-H1584.
[0093] Long-term potentiation (LTP) is a persistent increase in synaptic
strength of nerves implicated
in certain forms of learning and memory, eNOS from endothelial cells, rather
than nNOS, generates NO
within the postsynaptic cell in the central nervous system in LTP. (Blackshaw
S, Eliasson MJ, Sawa A et al.
Species, strain and developmental variations in hippocampal neuronal and
endothelial nitric oxide synthase
clarify discrepancies in nitric oxide-dependent synaptic plasticity.
Neuroscience 2003; 119(4):979-990;
O'Dell TJ, Huang PL, Dawson TM et al. Endothelial NOS and the blockade of LTP
by NOS inhibitors in
mice lacking neuronal NOS. Science 1994; 265(5171):542-546.) Increased eNOS
activity within the brain
promotes long-term potentiation at cortico-striatal connections thereby
favoring memory and learning.
Blockade of eNOS activity in chicks impairs memory. (Doreulee N, Sergeeva OA,
Yanovsky Y et al.
Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase
deficient mice. Brain Res 2003;
964(1):159-163; Rickard NS, Gibbs ME, Ng KT. Inhibition of the endothelial
isoform of nitric oxide synthase
impairs long-term memory formation in the chick. Learn Mem 1999; 6(5):458-
466.) Recently, it has been
found that LTP may take place in connections to the hypoglossal nerve, which
controls tongue movements.
Long-term depression of such activity may contribute to the pathogenesis of
the obstructive sleep apnea
syndrome. (Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent
plasticity in endogenously
active mammalian motoneurons. Proc Natl Acad Sci U S A 2004; 101(12):4292-
4295.)
[0094] In summary, periodic acceleration by releasing NO from eNOS plays a
major role in
management of complications of coronary artery bypass surgery. In addition to
preconditioning the heart to
the adverse effects of ischemia described in another section above, periodic
acceleration treatments
administered prior to and after coronary artery bypass surgery attenuate the
inflammatory effects of
cardiopulmonary bypass that can lead to the systemic inflammatory response and
organ failure. Periodic
acceleration treatments administered prior to and after coronary artery bypass
surgery can mitigate the
cognitive and learning deficits that are common after cardiopulmonary bypass
surgery in part by improving
-31 -
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
red cell deformability with easier capillary passage. Periodic acceleration
treatments can attenuate the
obstructive sleep apnea syndrome commonly observed in patients with coronary
artery disease.
G. Cognitive and Learning Impairment in Movement Disorders
[0095] Treatments with periodic acceleration also improve cognitive
impairments and dementia
because nitric oxide released from eNOS improves cerebral blood flow and long-
term potentiation (LTP), a
persistent increase in synaptic strength of nerves implicated in certain forms
of learning and memory as
described above. This is of particular importance in management of mild
cognitive impairment, defined as
memory complaints with objective memory impairment, without dementia,
impairment of general cognitive
functioning, or disability in activities of daily living. In a large
population study, this was a good predictor of
Alzheimer's disease with an annual conversion rate of 8.3% and good
specificity, but very unstable over
time: Within 2 to 3 years, only 6% of the subjects continued to have MCI,
whereas >40% reverted to
normal. (Larrieu S, Letenneur L, Orgogozo JM et al. Incidence and outcome of
mild cognitive impairment in
a population-based prospective cohort. Neurology 2002; 59(10):1594-1599;
Voisin T, Touchon J, Vellas B.
Mild cognitive impairment: a nosological entity? Curr Opin Neurol 2003; 16
Suppl 2:S43-S45.) Mild
cognitive impairment refers to the transitional zone between normal ageing and
dementia and may be the
optimum stage to intervene with preventive therapies such as periodic
acceleration.
[0096] Cognitive dysfunction is a major component of several neurological
diseases such as
Alzheimer's disease and vascular dementia. (De La Torre JC. Alzheimer's
disease is a vasocognopathy: a
new term to describe its nature. Neurol Res 2004; 26(5):517-524.) In its
earliest clinical phase, Alzheimer's
disease characteristically produces a remarkably pure impairment of inemory.
Mounting evidence suggests
that this syndrome begins with subtle alterations of hippocampal synaptic
efficacy prior to frank neuronal
degeneration, and that diffusible oligomeric assemblies of the amyloid beta
protein cause the synaptic
dysfunction. (Selkoe DJ. Alzheimer's disease is a synaptic failure. Science
2002; 298(5594):789-791.)
Patients with Parkinson's disease can also exhibit cognitive and behavioral
impairments. These
impairments may be attributed to dysfunction of multiple systems associated
with the disease process in
Parkinson's disease that are not necessarily related to motor symptoms. In
recent years, considerable
attention has addressed to disruption of the circuits in patients with
Parkinson's disease connecting the
frontal cortical regions and the basal ganglia (i.e., frontostriatal circuits)
and how they mediate cognition
and behavior in humans. (Zgaljardic DJ, Borod JC, Foldi NS et al. A review of
the cognitive and behavioral
sequelae of Parkinson's disease: relationship to frontostriatal circuitry.
Cogn Behav Neurol 2003;
16(4):193-210.) Recently, it has been recognized that cognitive decline may be
present in a population of
patients with amyotrophic lateral sclerosis. (Strong M, Rosenfeld J.
Amyotrophic lateral sclerosis: a review
of current concepts. Amyotroph Lateral Scler Other Motor Neuron Disord 2003;
4(3):136-143.)
Frontalstriatal synaptic circuits are disrupted in an animal model of ALS.
(Geracitano R, Paolucci E, Prisco
S et al. Altered long-term corticostriatal synaptic plasticity in transgenic
mice overexpressing human CU/ZN
-32-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
superoxide dismutase (GLY(93)-->ALA) mutation. Neuroscience 2003; 118(2):399-
408.) Frontalstrial
synapses are also disrupted in other movement disorders such as Huntington's
chorea and Wilson's
disease in animal models, probably suprabulbar palsy and possibly Tourette
syndrome. (Murphy KP, Carter
RJ, Lione LA et al. Abnormal synaptic plasticity and impaired spatial
cognition in mice transgenic for exon 1
of the human Huntington's disease mutation. J Neurosci 2000; 20(13):5115-5123;
Doreulee N, Yanovsky Y,
Haas HL. Suppression of long-term potentiation in hippocampal slices by
copper. Hippocampus 1997;
7(6):666-669; Clark M, Carr L, Reilly S et al. Worster-Drought syndrome, a
mild tetraplegic perisylvian
cerebral palsy. Review of 47 cases. Brain 2000; 123 ( Pt 10):2160-2170; Albin
RL, Koeppe RA, Bohnen NI
et al. Increased ventral striatal monoaminergic innervation in Tourette
syndrome. Neurology 2003;
61(3):310-315.)
[0097] Thus, frontalstriatal circuits appear to be disrupted or impaired in
Alzheimer's disease,
vascular dementia Parkinson's disease and amyotrophic lateral sclerosis,
Huntington's chorea, Wilson's
disease, suprabulbar palsy thereby causing memory and learning deficits
association with long term
depression of the synaptic pathways. Long-term potentiation (LTP), a
persistent increase in synaptic
strength is favorable for certain forms of learning and memory. It has been
found that eNOS from
endothelial cells, rather than nNOS, generates NO within the postsynaptic cell
as a means of producing
LTP. (O'Dell TJ, Huang PL, Dawson TM et al. Endothelial NOS and the blockade
of LTP by NOS inhibitors
in mice lacking neuronal NOS. Science 1994; 265(5171):542-546.) Therefore,
activation of eNOS with
release of nitric oxide attendant with periodic acceleration treatments
improves memory, learning and
behavior in patients with Alzheimer's disease, vascular dementias, Parkinson's
disease, amyotrophic lateral
sclerosis, Huntington's chorea, Wilson's disease, suprabubar palsy and
possibly Tourette syndrome.
[0098] Impairment of NO-synthesis in eNOS deficient mice shifts striatal
plasticity from long term
potentiation to long term depression. Since computation of perivascular NO
gradients from the vessel wall
of capillaries indicates that targets 200 um distant can still be reached by
NO, this is consistent with the
possibility that NO released from eNOS participates in the modulation of
cortico-striatal plasticity. (Doreulee
N, Sergeeva OA, Yanovsky Y et al. Cortico-striatal synaptic plasticity in
endothelial nitric oxide synthase
deficient mice. Brain Res 2003; 964(1):159-163.) Further, this assertion is
consistent with the rapid,
dramatic improvements observed after only one 45 minute periodic acceleration
treatment in patients with
these movement disorders. The added pulses produced with periodic acceleration
is superior to
nonpulsatile blood flow, Thus, Baba et al used a pump to bypass the heart that
produced pulsatile and
nonpulsatile flow in goats and observed bulbar conjunctiva capillaries with a
digital high definition
microscope. When the flow pattern was changed from pulsatile to nonpulsatile,
the velocity of erythrocytes
in many capillaries dropped and remained at a low level, and the number of
perfused capillaries decreased.
After the flow pattern was returned to pulsatile, the velocity of erythrocytes
recovered to the initial level. In
many cases, the flow of nonperfused capillaries recovered to the initial level
as well. Also, pulsatile flow
-33-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
enhanced the basal and flow-stimulated endothelium-derived nitric oxide
release in the microvessels.
(Baba A, Dobsak P, Mochizuki S et al. Evaluation of pulsatile and nonpulsatile
flow in microvessels of the
bulbar conjunctiva in the goat with an undulation pump artificial heart. Artif
Organs 2003; 27(10):875-881.
[0099] In summary, periodic acceleration is indicated as prophylactic and
therapeutic treatment for
cognitive and learning deficits as well as behavioral abnormalities in early
cognitive impairment,
Alzheimer's disease, vascular dementias, Parkinson's disease, amyotrophic
lateral sclerosis, Huntington's
chorea, Wilson's disease, suprabulbar palsy and possibly Tourette syndrome.
H. Cardiac Allograph Vasculopathy
[00100] Accelerated graft atherosclerosis is a key feature of most chronic
rejection syndromes.
Atherosclerosis diffusely involves the coronary circulation. Cardiac allograft
vasculopathy is the most
aggressive form of atherosclerosis in humans and is the leading cause of death
after the first year of heart
transplantation. Endothelial dysfunction is a major contributing factor to the
acceleration of coronary
vascular disease in these individuals. Alteration in endothelial function
contributes to vascular inflammation
and progression of the disease. (Weis M, Cooke JP. Cardiac allograft
vasculopathy and dysregulation of
the NO synthase pathway. Arterioscler Thromb Vasc Biol 2003; 23(4):567-575,).
Periodic acceleration
ameliorates the endothelial dysfunction responsible for this syndrome.
1. Endothelial Progenitor Cells
[00101] Endothelial nitric oxide synthase (eNOS) is essential for
neovascularization. Impaired
neovascularization in mice lacking eNOS is related to a defect in progenitor
cell mobilization owing to
reduced vascular endothelial growth factor (VEGF)-induced mobilization of
endothelial progenitor cells
(EPCs). eNOS expressed by bone marrow stromal cells in response to shear
stress influences recruitment
of stem and progenitor cells. This may contribute to impaired regeneration
processes in ischemic heart
disease patients, who are characterized by a reduced systemic NO bioactivity.
Aerobic exercise by release
of NO from eNOS increases vascular endothelial growth factor (VEGF) with
consequent increase of
endothelial progenitor cells. (Aicher A, Heeschen C, Mildner-Rihm C et al.
Essential role of endothelial nitric
oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;
9(11);1370-1376; Laufs U,
Werner N, Link A et al. Physical training increases endothelial progenitor
cells, inhibits neointima formation,
and enhances angiogenesis. Circulation 2004; 109(2):220-226.) C-Reactive
Protein (CRP) at
concentrations > or =15 microg/mL significantly reduces the number of EPC's.
Human recombinant CRP,
at concentrations known to predict adverse vascular outcomes, directly
inhibits EPC differentiation,
survival, and function, key components of angiogenesis and response to chronic
ischemia. This occurs in
part because CRP reduces EPC eNOS expression, (Verma S, Kuliszewski MA, Li SH
et al. C-reactive
protein attenuates endothelial progenitor cell survival, differentiation, and
function: further evidence of a
mechanistic link between C-reactive protein and cardiovascular disease.
Circulation 2004; 109(17):2058-
2067.)
-34-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
[00102] Periodic acceleration like aerobic exercise promotes mobilization of
endothelial progenitor
cells into the circulation as well as suppression of CRP if elevated. Periodic
acceleration aids in promoting
angiogenesis in ischemic tissues.
J. Hereditary Hemorrhagic Telangiectasis
[00103] Hereditary hemorrhagic telangiectasis (Osler-Weber-Rendu disease) is
caused by a genetic
deficiency of endoglin. Endoglin also regulates transforming growth factor
beta 1; In turn this mediator
causes under-expression of eNOS that leads to development of abnormal blood
flow passages and the
manifestations of the disease. These patients experience frequent bleedings
with increasing age, in
particular from the nasal, gastrointestinal, and cerebral vascular beds.
Vascular arteriovenous
malformations develop that vary in size from 1 mm to several centimeters.
Pulmonary vascular
arteriovenous malformations are particularly life threatening because of
bleeding, or paradoxical embolism
causing brain infarction or brain abscess. The cause of hereditary hemorrhagic
telangiectasis is mutated
genes identified as endoglin and ALK-1. They mediate binding on signaling of
transforming growth factor
beta. The disease develops as a result of deficient TGF beta signaling in
vascular endothelial cells that
produces abnormal blood vessel development. (Jerkic M, Rivas-Elena JV, Prieto
M et al. Endoglin
regulates nitric oxide-dependent vasodilatation. FASEB J 2004; 18(3):609-611;
van den DS, Mummery CL,
Westermann CJ. Hereditary hemorrhagic telangiectasia: an update on
transforming growth factor beta
signaling in vasculogenesis and angiogenesis. Cardiovasc Res 2003; 58(1):20-
31.)
[00104] Periodic acceleration helps in the management of hereditary
hemorrhagic telangiectasia
because it addresses the underlying cause of this disease, i.e., under-
expression of eNOS,
K. Migraine
[00105] Nitric oxide generated from inducible nitric oxide synthase (iNOS)
participates in immune and
inflammatory responses in many tissues. The NO donor glyceryl trinitrate (GTN)
provokes delayed migraine
attacks when infused into migraineurs and also causes iNOS expression and
delayed inflammation within
rodent dura mater, Sodium nitroprusside, an NO donor as well, also increases
iNOS expression.
Intravenous GTN increases NO production within macrophages. iNOS expression is
preceded by
significant nuclear factor kappa beta activity after GTN infusion. Nuclear
factor kappa beta activation and
iNOS expression are attenuated by parthenolide (3mg/kg), the active
constituent of feverfew, an anti-
inflammatory drug used for migraine treatment. Thus, GTN promotes NF-kappaB
activity and inflammation
with a time course consistent with migraine attacks in susceptible
individuals. Therefore, blockade of NF-
kappaB activity provides a target for the anti-migraine treatment. (Reuter U,
Chiarugi A, Bolay H et al.
Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol
2002; 51(4):507-516.) Since
periodic acceleration causes release of NO from eNOS and NO suppresses
activity of nuclear factor kappa
beta and iNOS, this action provides effective anti-migraine treatment.
-35-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
L. Prion Diseases
[00106] Prion diseases, Mad Cow and Creutzfeldt-Jakob diseases, are
devastating lethal neurological
diseases which currently are untreatable. However, examination of brain tissue
from these patients reveals
inflammation marked by accumulation of COX-1-expressing macrophages/microglial
cells and COX-2-
expressing neurons, and increased nuclear factor kappa beta and iNOS activity,
(Bacot SM, Lenz P,
Frazier-Jessen MR et al. Activation by prion peptide PrP106-126 induces a NF-
kappaB-driven
proinflammatory response in human monocyte-derived dendritic cells. J Leukoc
Biol 2003; 74(1):118-125;
Brown DR, Nicholas RS, Canevari L. Lack of prion protein expression results in
a neuronal phenotype
sensitive to stress, J Neurosci Res 2002; 67(2):211-224; Cui T, Holme A,
Sassoon J et al. Analysis of
doppel protein toxicity. Mol Cell Neurosci 2003; 23(1):144-155; Deininger MH,
Bekure-Nemariam K,
Trautmann K et al. Cyclooxygenase-1 and -2 in brains of patients who died with
sporadic Creutzfeldt-Jakob
disease. J Mol Neurosci 2003; 20(1):25-30.) Since all these inflammatory
mediators are suppressed by
nitric oxide released from eNOS during periodic acceleration, this therapy has
a place in treating the
inflammation attendant with prion diseases.
M. Aging
[00107] Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are
widely implicated in
the inflammatory process. During the aging process, both ROS and RNS are
increased along with an
inflammatory response that takes place in the body involving upregulation of
nuclear factor kappa beta, IL-
beta, IL-6, tumor necrosis factor alpha, cyclooxygenase-2, and inducible NO
synthase. Caloric restriction
downregulates these inflammatory mediators. (Chung HY, Kim HJ, Kim JW et al.
The inflammation
hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad
Sci 2001; 928:327-335.)
Activated nuclear factor kappa beta produces oxidative stress via the
induction of MnSOD and contributes
the ageing process. (Bernard D, Gosselin K, Monte D et al. Involvement of
Rel/nuclear factor-kappaB
transcription factors in keratinocyte senescence, Cancer Res 2004; 64(2):472-
481. Chronic inflammation
accounts for effects of susceptibility to infection in aged animals. For
example, the increased expression of
proinflammatory cytokines and inflammatory responsive genes in the lung piays
a role in the increased
susceptibility in aging animals to endotoxic stress. (Chang CK, LoCicero J,
III. Overexpressed nuclear
factor kappaB correlates with enhanced expression of interleukin-1beta and
inducible nitric oxide synthase
in aged murine lungs to endotoxic stress. Ann Thorac Surg 2004; 77(4):1222-
1227). Since NO from eNOS
increases mitochondrial number in skeletal muscle, this might reverse aging.
(Brown GC, NO says YES to
mitochondria. Science 2003; 299:838-839.) Therefore, periodic acceleration
alone and with caloric
restriction as an additive or synergistic effect might favorably modify the
ageing process.
N. Sjogren's Syndrome
[00108] Sjogren's syndrome is marked by xeropthalmia (dry eyes) and xerostomia
(dry mouth) due to
lymphocytic infiltrates of lacrimal and salivary glands. It may occur alone or
in association with several
-36-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
other autoimmune diseases. The clinical features involve a wide variety of
organs, including skin, eyes, oral
cavity and salivary glands, and systems, including nervous, musculoskeletal,
genitourinary and vascular.
The dryness symptoms can be found in a number of other disorders including
rheumatoid arthritis,
systemic lupus erythematosus, scleroderma, primary biliary cirrhosis, and
other rheumatic disorders.
(Rehman HU. Sjogren's syndrome. Yonsei Med J 2003; 44(6):947-954.) Suppression
of tumor necrosis
factor alpha with cepharanthine, an anti-inflammatory, pro-apoptotic anti-
tumorigenesis drug halts induction
of matrix metalloproteinase 9 thereby preventing destruction of acinar tissue
in the salivary glands of
patients with Sjogren's syndrome. (Azuma M, Aota K, Tamatani T et al.
Suppression of tumor necrosis
factor alpha-induced matrix metalloproteinase 9 production in human salivary
gland acinar cells by
cepharanthine occurs via down-regulation of nuclear factor kappaB: a possible
therapeutic agent for
preventing the destruction of the acinar structure in the salivary glands of
Sjogren's syndrome patients.
Arthritis Rheum 2002; 46(6):1585-1594) Periodic acceleration through
activation of eNOS with subsequent
NO release has an anti-inflammatory, pro-apoptotic action through suppression
of nuclear factor kappa
beta which in turn inhibits tumor necrosis factor alpha. Therefore, it has a
place in management of
Sjogren's syndrome.
0. Lyme Disease
[00109] The Lyme disease agent, Borrelia burgdorferi, a spirochete, causes
infection by migration
through tissues, adhesion to host cells, and evasion of immune clearance. The
infection is introduced by a
tick bite and cause a skin rash and persistent flu-like symptoms and fever in
the summer. If inadequately
treated, arthritis, cardiac arrhythmia marked by heart block, facial nerve
palsy, meningitis, polyneuropathy
and encepalopathy may occur. (Steere AC, Coburn J, Glickstein L. The emergence
of Lyme disease. J Clin
Invest 2004; 113(8):1093-1101.) The systemic symptoms of Lyme disease are due
in part to activation of
nuclear factor kappa beta with intense inflammatory cytokine expression with
inflammation of microglia.
(Ebnet K, Brown KD, Siebenlist UK et al. Borrelia burgdorferi activates
nuclear factor-kappa B and is a
potent inducer of chemokine and adhesion molecule gene expression in
endothelial cells and fibroblasts. J
Immunol 1997; 158(7):3285-3292; Rasley A, Anguita J, Marriott I. Borrelia
burgdorferi induces inflammatory
mediator production by murine microglia. J Neuroimmunol 2002; 130(1-2):22-31.)
In the acute phase of
Lyme disease, periodic acceleration is contraindicated because nitric oxide
from eNOS could suppress the
host's immuno-defense mechanisms. But in the chronic phase of Lyme disease,
periodic acceleration
diminishes constitutional and local symptoms in the central nervous system,
heart and joints in conjunction
with antibiotics,
P. Gulf War Syndrome
[00110] The Gulf War syndrome consists of multisymptom illnesses characterized
by persistent pain,
fatigue, and cognitive symptoms that have been reported by many Gulf War
veterans. Vaccinations against
biological warfare using pertussis were utilized as an adjuvant in such
patients. This could trigger
-37-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
neurodegeneration through induction of interleukin-lbeta secretion in the
brain. Particular susceptibility for
IL-1 beta secretion and potential distant neuronal damage could provide an
explanation for the diversity of
the symptoms. The symptoms in many of these patients are similar to the
chronic fatigue syndrome and
those with severe fatiguing illness have shown plasma immunological
abnormalities but not as a universal
finding. No measurements have been made of inflammatory cytokines in the
cerebrospinal fluid where
detection of pathology would be more likely to occur. (Donta ST, Clauw DJ,
Engel CC, Jr. et al. Cognitive
behavioral therapy and aerobic exercise for Gulf War veterans' illnesses: a
randomized controlled trial.
JAMA 2003; 289(11):1396-1404; Tournier JN, Jouan A, Mathieu J et al. Gulf war
syndrome: could it be
triggered by biological warfare-vaccines using pertussis as an adjuvant? Med
Hypotheses 2002; 58(4):291 -
292; Zhang Q, Zhou XD, Denny T et al. Changes in immune parameters seen in
Gulf War veterans but not
in civilians with chronic fatigue syndrome. Clin Diagn Lab Immunol 1999;
6(1):6-13; Everson MP, Shi K,
Aldridge P et al. Immunological responses are not abnormal in symptomatic Gulf
War veterans. Ann N Y
Acad Sci 2002; 966:327-342.)
[00111] Nitric oxide released from eNOS with periodic acceleration has a
potent anti-inflammatory
action through suppression of nuclear factor kappa beta. Perodic acceleration
has been utilized in the
treatment of fibromyalgia and chronic fatigue syndrome, entities with symptoms
similar to those in the Gulf
War syndrome. The dramatic improvement in symptoms of fibromyalgia and chronic
fatigue syndrome was
attributed to suppression of an inflammatory process in the brain. (Sackner
MA, Gummels EM, Adams JA.
Say NO to fibromyalgia and chronic fatigue syndrome: an alternative and
complementary therapy to
aerobic exercise. Med Hypotheses 2004; 63(1):118-123.) However, the cognitive
improvement might also
have been due to nitric oxide from eNOS enhancing long-term potentiation of
frontalstriatal synapses that
deal with memory and learning. (O'Dell TJ, Huang PL, Dawson TM et al,
Endothelial NOS and the
blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science 1994;
265(5171):542-546.)
[00112] In summary, periodic acceleration is indicated in treatment of the
Gulf War syndrome.
Q. Miscellaneous Pulmonary Effects
[00113] Through the action of nitric oxide expressed from activation of eNOS,
periodic acceleration
improves mucociliary clearance, increases pulmonary surfactant productions and
minimizes the volutrauma
and barotrauma of positive pressure mechanical ventilation. Nitric oxide
improves nasal mucociliary
clearance by increasing ciliary beat frequency. (Runer T, Lindberg S.
Ciliostimulatory effects mediated by
nitric oxide. Acta Otolaryngol 1999; 119(7):821-825.) Unpublished experiments
in our laboratory indicate
that periodic acceleration through NO release from eNOS increases tracheal
mucous velocity over baseline
in conscious sheep and after administration of elastin, which is a potent
suppressant of mucociliary
clearance. Therefore, treatment with periodic acceleration is indicated in
medical conditions associated with
production of excessive bronchopulmonary and nasal secretions such as cystic
fibrosis, bronchial asthma,
chronic bronchitis and chronic sinusitis. Periodic acceleration should be
helpful in shortening duration of
-38-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
the mucous surface, Further, constitutional symptoms are alleviated by NO
suppression of nuclear factor
kappa beta activity that directs inflammatory cytokine production.
Physiological concentration as those
released from eNOS stimulate pulmonary surfactant production and therefore
periodical acceleration is
indicated in the management of the adult and infant respiratory distress
syndrome as well as SARS. (Sun
P, Wang J, Mehta P et al. Effect of nitric oxide on lung surfactant secretion,
Exp Lung Res 2003; 29(5):303-
314.) Since pulmonary injury from mechanical ventilation is due to
inflammation owing to activation of
nuclear factor kappa beta, periodic acceleration through release of NO that
blocks nuclear factor kappa
beta activity can serve in prophylactic and therapeutic roles. (Uhlig U,
Fehrenbach H, Lachmann RA et al.
Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell
activation. Am J Respir Crit
Care Med 2004; 169(2):201-208.)
R. Corticosteroid Resistance
[00114] Asthma patients who respond poorly or are resistance to the action of
corticosteroids
constitute slight less than 5% of 20 million patients for a total of about 1
million patients, (Adcock IM, Lane
SJ, Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol
2003; 178(3):347-355.)
Corticosteroid therapy resistance is a common indication for surgery in
inflammatory bowel disease, with as
many as 50% of patients with Crohn's disease and approximately 20% of patients
with ulcerative colitis
requiring surgery in their lifetime. One of the major causes of resistance is
constitutive epithelial activation
of proinflammatory mediators, including nuclear factor kappa B, resulting in
inhibition of glucocorticoid
receptor transcriptional activity, (Farrell RJ, Kelleher D. Glucocorticoid
resistance in inflammatory bowel
disease. J Endocrinol 2003; 178(3):339-346) Periodic acceleration by releasing
NO from eNOS
suppresses nuclear factor kappa beta activity and can used as a stand-alone
therapy in patients with
corticosteroid resistance asthma and inflammatory bowel disease,
S. Chronic Otitis Media
[00115] The chronic inflammation seen in some chronic otitis media patients
appears to be due to
lipopolysaccharide activating adhesion molecule receptors and nuclear factor
kappa beta followed by
release of IL-8. Since periodic acceleration releases NO from eNOS with
subsequent suppression of
nuclear factor kappa beta activity and IL-8, it can be utilized to treat
patients with chronic otitis media,
(Barrett TQ, Kristiansen LH, Ovesen T. NF-kappaB in cultivated middle ear
epithelium. Int J Pediatr
Otorhinolaryngol 2003; 67(8):895-903.)
T. Nail Growth and Nail Brittleness
[00116] In several patients that were chronically treated with periodic
acceleration, nail growth was
more rapid and nail brittleness improved. Presumably, this was related to
increased blood supply to the nail
bed. Therefore, periodic acceleration has a role in nail regeneration.
-39-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
U. Cell Free Hemoglobin Transfusions
[00117] Hemoglobin-based oxygen carriers are being developed for use in blood
replacement
therapies, either for perioperative hemodilution or for resuscitation from
hemorrhagic blood loss. There is a
high demand for these products because of risks associated with blood
transfusions and pending
worldwide blood shortages. The primary adverse effect for the majority of
cross-linked or polymerized cell
free hemoglobin products is increased vascular resistance to blood flow.
(Vandegriff KD. Haemoglobin-
based oxygen carriers. Expert Opin Investig Drugs 2000; 9(9):1967-1984.) This
side effect of cell free
hemoglobin is a serious one and is due to hemoglobin scavenging nitric oxide
from eNOS that renders the
transfusion recipient nitric oxide deficient. Periodic acceleration through
release of increased nitric oxide
from eNOS can be used to prevent or treat the NO deficit.
V. Nuclear Weapons and "Dirty Bombs"
[00118] A major threat facing the world today is the possibility of a nuclear
terrorist attack through a
conventional nuclear weapon or a "dirty bomb" (combination of coventional
explosive and nuclear material)
or through an attack on a nuclear power plant site. Some deaths from an
explosion would result from direct
contact with the explosive and debris but the majority of early deaths would
result from infections related to
bone marrow suppression of neutrophils. Radiation to the abdomen can produce
acute enteritis
characterized by diarrhea and chronic enteropathy (hemorrhage and ulceration)
which leads to
progressively reduced mobility. Fistulas, strictures and malabsorption are
potentially life threatening. Tumor
necrosis factor alpha and IL-6 contribute significantly to leukemias and
radiation pneumonitis. Because
radiation-induced vascular injury precedes the tissue damage, vascular injury
is regarded as crucial in the
pathogenesis of tissue damage. Radiation injury is marked by activation of
adhesion molecules that
promote leukocyte infiltration of normal tissue. The stress of radiation
activated nuclear factor kappa beta in
turn promotes activation of adhesion molecules. The inflammatory mediators
activated in radiation injury
are regulated by nuclear factor kappa beta, the key gene directing the
inflammatory response. (Linard C,
Marquette C, Mathieu J et al. Acute induction of inflammatory cytokine
expression after gamma-irradiation
in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys
2004; 58(2):427-434; Quarmby S,
Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion
molecules in leukocyte-
endothelial cell interactions. Int J Cancer 1999; 82(3):385-395.) Periodic
acceleration through NO released
from eNOS suppresses nuclear factor kappa beta activity and the inflammatory
cytokines. Therefore, it
serves ro treat radiation injuries.
[00119] The invention is not limited by the embodiments described above which
are presented as
examples only but can be modified in various ways within the scope of
protection defined by the appended
patent claims. Thus, while there have shown and described and pointed out
fundamental novel features of
the invention as applied to a preferred embodiment thereof, it will be
understood that various omissions and
substitutions and changes in the form and details of the devices illustrated,
and in their operation, may be
-40-
CA 02534302 2006-01-31
WO 2005/016216 PCT/US2004/025017
made by those skilled in the art without departing from the spirit of the
invention, For example, it is
expressly 'intended that all combinations of those elements and/or method
steps which perform
substantially the same function in substantially the same way to achieve the
same results are within the
scope of the invention, Moreover, it should be recognized that structures
and/or elements and/or method
steps shown and/or described in connection with any disclosed form or
embodiment of the invention may
be incorporated in any other disclosed or described or suggested form or
embodiment as a general matter
of design choice. It is the intention, therefore, to be limited only as
indicated by the scope of the claims
appended hereto.
-41-