Note: Descriptions are shown in the official language in which they were submitted.
CA 02534596 2006-01-30
-1-
TITLE: MEDICATION VERIFICATION SYSTEMS AND METHODS
FIELD OF THE INVENTION
[0001] The invention relates to medical informatics, and in particular to
systems, methods and apparatus for safely administering medications.
BACKGROUND OF THE INVENTION
5[0002] Pharmaceutical companies often package medications in liquid
form in containers such as vials or ampoules. For the sake of brevity, the
containers that pharmaceutical companies use to package medications in are
referred to as primary coiitainers hereinafter. A label on a primary container
often includes a machine-readable barcode and/or RFID (Radio Frequency
IDentification) tag to aid in the identification of the contents of the
primary
container in addition to human-readable text and symbols. The barcodes
andlor RFID tags store information such as Drug Identification Numbers
(DIN), UPC codes and National Drug Codes (that are used specifically in the
United States).
[0003] Within a health-care facility a medication in liquid form is often
transferred from a primary container to a secondary container. Secondary
containers include, for example and without limitation, syringes, cups,
solution
bowls and basins. Secondary containers are used to temporarily store andlor
administer medications.
[0004] For example, in an operating theatre, medications are brought
into a sterile field around a patient using syringes andlor another type of
secondary container. Each syringe may contain a different medication for use
during the operation. Most medications are clear and colorless, so it is
almost
impossible to simply identify the contents of a secondary container (e.g. a
syringe) without some type of visual aid.
[0005] Accordingly, medical professionals employ a number of ad hoc
methods for identifying medications in secondary containers. For example,
specific medications are sometimes paired with a specific size andlor type of
CA 02534596 2006-01-30
-2-
secondary container. However, such practices are not standardized and
different medical professionals often pair medications with secondary
containers differently from their colleagues. In another example, a secondary
container is provided with a label and/or color-code indicia. However, user
applied labels are often quite small and the text on the labels can be smudged
or covered by blood (or other fluids) that may cause the user applied labels
to
be misread. As a result of the ad hoc methods employed, numerous patients
are incorrectly administered medications causing a number of side effects
ranging from the relatively harmless to loss of vital organ function and
sometimes even death.
[0006] Human error is the primary source of the errors made. However,
human error in a health-care environment is difficult to address, since
medical
staff act according to strict operating procedures that are hard to adjust
without introducing added liability. Subsequently, medical staff are often
averse to procedural changes because such changes are thought to
inherently include increased liability.
[0007] Additionally, within an inpatient health-care facility, such as a
nursing home or a hospital, nurses routinely administer medication(s) to
patients as prescribed by a doctor and/or on an as needed basis. In many
jurisdictions nurses are required to record the details relating to the
distribution of the medications in order to comply with regional health care
regulations. The details may include a listing of inedications provided, time,
reason, outcome (1=e= observations) and dosage of medication(s) provided to
each patient.
[0008] The workflow described above is widely susceptible to human
error, as there are few points at which the activities of individuals can be
checked to ensure that individuals (e.g. nurses) working within an inpatient
health-care facility are complying with regional health care regulations.
[0009] For example, while nurses are supposed to record the time and
dosage of medications at the same time the medications are administered to
a patient, some nurses first distribute medications to a number of patients
and
CA 02534596 2006-01-30
-3-
then update and initial the patient-specific charts, thereby separating the
tasks
of distribution and recordation. This practice can lead to accidentally
providing
the wrong medication to one or more patients, providing the correct
medication at the wrong times, failing to provide the medication to a patient
and/or misjudging the effects of particular medications on respective
patients.
In more extreme cases, a nurse may actually distribute medication before or
after the respective prescribed time, but nevertheless update a patient-
specific chart as though the medication was provided at the appropriate time.
Additionally, nurses may forget to document the time, reason and outcome for
providing medication that is to be administered to a particular patient only
as
needed, since scheduled times for providing such medication are not listed on
a patient-specific chart. Unfortunately, there is no practical way for anyone
else to detect that an individual in charge of distributing medications at
particular times is not following health-care regulations set out by the
inpatient
health-care facility and/or a governing body (e.g. a state andtor federal
agency).
SUMMARY OF THE INVENTION
[0010] According to an aspect of an embodiment of the invention there
is provided a system for verifying the contents of a secondary oontainer, the
secondary container suitable for storing and administering medications in a
liquid form, the system comprising: a User Applied Medication Label (UAML),
for a secondary container, including machine-readable information
corresponding to machine-readable included on a primary container for a
medication in liquid form; a machine-scanner for scanning machine-readable
information and providing a scanned output; and a workstation computer
connectable to the machine-scanner for receiving the scanned output from the
machine-scanner,
[0011] In some embodiments, the UAML includes a barcode containing
the machine-readable information. Additionally and/or alternatively, the UAML
includes a RFID tag containing the machine-readable information.
CA 02534596 2006-01-30
-4-
[0012] In some embodiments the machine-readable information
includes at least one of a Drug Identification Number, a UPC code and a
National Drug Code.
[0013] In some embodiments the workstation computer includes a
computer usable program code including program instructions for: receiving
scanned machine-readable information from a primary container and a
secondary container; comparing the scanned machine-readable information
from the primary container and the secondary container; providing affirmative
feedback if the primary and secondary containers have the same scanned
machine-readable information; and providing dissenting feedback if the
primary and secondary containers do not have the same scanned machine-
readable information.
[0014] Additionally and/or alternatively, in some embodiments the
computer usable program code further includes program instructions for
creating a record of the comparison. Additionally and/or alternatively, in
some
embodiments the computer usable program code including program
instructions for updating a database of records, wherein each record contains
the results and a particular comparison.
[0015] Additionally and/or alternatively, in some embodiments the
UAML are sterilized. Additionally and/or alternatively, in some embodiments
the sterilized UAML are packaged in a sterilized package suitable for use in
an operating theatre.
(0016] According to an aspect of an embodiment of the invention there
is provided a method for verifying the contents of a secondary container, the
secondary container suitable for storing and administering medications in a
liquid form, the method comprising: scanning machine-readable information
from a primary container containing a medication in liquid form; scanning
machine-readable information for a secondary container for use in temporarily
storing and then administering medication; comparing the scanned machine-
readable information from the primary container and the secondary container;
providing affirmative feedback if the primary and secondary containers have
CA 02534596 2006-01-30
-5-
the same scanned machine-readable information; and providing dissenting
feedback if the primary and secondary containers do not have the same
scanned machine-readable information,
[0017] Additionally and/or alternatively, in some embodiments the
method further comprising: preparing the medication in the primary container
according to a set of safe standard operating practices; and transferring the
medication from the primary to secondary container.
[0018] In some more specific embodiments, preparing the medication
includes verifying and recording at least one of the type of medication,
dosage, patient, time and correct route.
[0019] Other aspects and features of the present invention will become
apparent, to those ordinarily skilled in the art, upon review of the following
description of the specific embodiments of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0020] For a better understanding of the present invention, and to show
more clearly how it may be carried into effect, reference will now be made, by
way of example, to the accompanying drawings, which illustrate aspects of
embodiments of the present invention and in which:
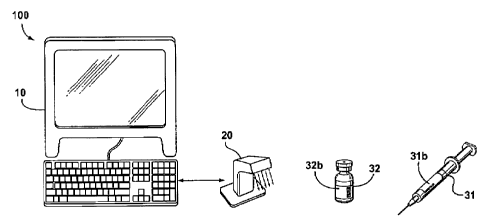
[0021] Figure 'f is a schematic drawing of a system for verifying the
contents of secondary containers in a clinical environment; and
[0022] Figure 2 is a flow chart depicting the general steps of an
electronically-aided method for verifying the contents of secondary containers
in a clinical environment.
DETAILED DESCRIPTION OF THE INVENTION
[0020] Changing the operating procedures within a health-care facility
may inadvertently introduce new sources of liability. Subsequently, health-
care professionals are naturally very cautious and risk averse when
contemplating the adoption of new operating procedures and electronic
CA 02534596 2006-01-30
~G-
systems that involve significant changes to their accepted procedures.
However, human errors during the distribution of medications to patients
results in a number of accidents that can be attributed to not having a
systematic method for verifying the contents of secondary containers used for
storing and administering medications.
[0024] Some embodiments of the invention provide electronically-aided
systems and methods for validating the contents of a secondary container
after a medication has been transferred from a primary container to the
secondary container and before the medication is administered to a patient. In
accordance with some aspects of the invention, labels for secondary
containers are provided. These labels are referred to as User Applied
Medication Labels (UAML) and these labels are different from conventional
medication primary container labels because the UAML's are applied by
health care professionals onto secondary containers. In some embodiments
the UAML include machine readable information corresponding to the
machine-readable information on the labels on primary containers supplied by
the pharmaceutical companies.
[00251 In accordance with some embodiments, the machine-readable
information may be stored in barcode form andfor within an RFID tag. The
barcodes and/or RFID tags store information such as Drug Identification
Numbers (DIN), UPC codes and National Drug Codes (that are used
specifically in the United States). In some embodiments the same information
is also typically printed on the labels for visual identification.
[0026] In accordance with some aspects of the invention a system is
provided for verifying the contents of a secondary container. With reference
to
Figure 1, shown is a schematic diagram of an example system 100 in
accordance with aspects of the invention. The system 100 includes
workstation computer 10 (e.g. a personal computer, network computer, etc.),
and a machine-scanner 20 coupled to the workstation computer 10. The
workstation computer 10 includes a processor and a memory that is
accessible by the processor. Those skilled in the art will appreciate that the
CA 02534596 2006-01-30
-7-
workstation computer 10 also includes an additional suitable combination of
hardware, software and firmware, and that the functional elements illustrated
in Figure 1 have only been provided to describe aspects of a very specific
embodiment of the invention.
5(0027] In some embodiments the machine-scanner 20 is suitable to
scan information from barcodes. Additionally and/or alternatively, the
machine-scanner 20 is suitable to scan information from RFID tags.
[0028] Also shown in Figure 1, for the sake of example only, is a vial of
medication 32 (the primary container) and a syringe 31 (the secondary
container). The primary container 32 has a label 32b with machine-readable
information, as described above. Similarly, the secondary container 31 also
has a label 31b with machine-readable information.
[0029] In operation, the system 100 is used to scan the labels 32b and
31 b to verify whether or not the information on each is the same. The
machine-scanner 20 is used to scan each label 32b and 31b individually and
provide the information to the workstation computer 10. The workstation
computer 10, having received the scanned information on both labels
compares the scanned information and provides feedback to a user. If the
labeis are the same, the feedback is affirmative. On the other hand if the
information is not the same the feedback is dissenting and the user is
instructed to not administer the medication in the secondary container to a
patient.
[0030] Moreover, by scanning the labels 31b and 32b and storing the
result of the comparison a record is created that can be stored in the memory
of the workstation computer 10. In a hospital or another health-care facility,
records of all medications provided to respective patients can be created in
this way. Each such record can be reviewed at a later time to verify that the
individual(s) responsible for preparing and/or administering the medications
was (were) being diligent while preparing andlor administering the
3p medications. That is, in accordance with aspects of the invention, the
record
CA 02534596 2006-01-30
-8-
created by scanning labels on primary and secondary containers can be used
as a metric for quality control and culpability.
(00311 A number of records created by the system can be stored in a
database. To that end, aspects of the invention may be embodied in a
number of forms. For example, various aspects of the invention can be
embodied in a suitable combination of hardware, software and firmware. In
particular, some embodiments include, without limitation, entirely hardware,
entirely software, entirely firmware or some suitable combination of hardware,
software and firmware. In a preferred embodiment, the invention is
implemented in software, which includes but is not limited to firmware,
resident software, microcode, etc.
[0032] Additionally and/or alternatively, aspects of the invention can be
embodied in the form of a computer program product accessible from a
computer-usable or computer-reedable medium providing program code for
use by or in conneotion with a computer or any instruction execution system.
For the purposes of this description, a computer-usable or computer readable
medium can be any apparatus that can contain, store, communicate,
propagate, or transport the program for use by or in connection with the
instruction execution system, apparatus, or device.
[0033] A computer-readable medium can be an electronic, magnetic,
optical, electromagnetic, infrared, or semiconductor system (or apparatus or
device) or a propagation medium. Examples of a computer-readable medium
include a semiconductor andlor solid-state memory, magnetic tape, a
removable computer diskette, a random access memory (RAM), a read-only
memory (ROM), a rigid magnetic disk and an optical disk. Current examples
of optical disks include, without limitation, compact disk - read only memory
(CD-ROM), compact disk - read/write (CD-RNV) and DVD.
[0034] In accordance with aspects of the invention, a data processing
system suitable for storing and/or executing program code will include at
least
one processor coupled directly or indirectly to memory elements through a
system bus. The memory elements can include local memory employed
CA 02534596 2006-01-30
-9-
during actual execution of the program code, bulk storage, and cache
memories which provide temporary storage of at least some program code in
order to reduce the number of times code must be retrieved from bulk storage
during execution.
5[0035] Input/output (i.e. 1/0 devices) - including but not limited to
keyboards, displays, pointing devices, etc. - can be coupled to the system
either directly or through intervening I/Q controllers.
[0036] Network adapters may also be coupled to the system to enable
communication between multiple data processing systems, remote printers, or
storage devices through intervening private or public networks. Modems,
cable modems and Ethernet cards are just a few of the currently available
types of network adapters.
[0037] In some embodiments the "User Applied Medication Labels" are
sterilized using gamma radiation. The sterilized labels are then packaged in a
sterile environment to ensure that the labels remain sterile so that they may
be safely introduced into a sterile field in an operating theatre.
Additionally, in
some embodiments secondary containers include syringes, solution bowls,
basins, medicine cups or similar vessels used to temporarily store andlor
administer a medication.
[0038] In accordance with some aspects of the invention, the "User
Applied Medication Labels" allow for the replacement or rotation of health
care
staff during the administration of inedication. For example, during a long
surgical procedure, a nurse can be relieved for breaks or lunch. Systems and
methods provided by aspects of the invention provide at least some
assurance to the relieving nurse that the medications on the sterile field are
correct because the relieving nurse can validate the contents of secondary
containers by scanning the "User Applied Medication Labels".
[0039] Referring to Figure 2, shown is a flow chart depicting the general
steps of an electronically-aided method for verifying the contents of
secondary
containers in a clinical environment. Starting at step 2-1, a medical
CA 02534596 2006-01-30
-10-
professional (i.e. a user) prepares medications as per hospital standard and
regional health-care regulations. In some cases, this may include verifying:
that the medications to be administered are cdrrect (with reference to
instructions from a doctor): dosage: patient; time; and, correct route as per
normal standards of practice.
[0040] At step 2-2, the label on a primary container (e.g. a vial) for a
medication is scanned and the scanned information is stored. Then at step 2-
3, the medication is transferred from the primary container to a secondary
container labeled for the medication. At step 2-4, the secondary container is
scanned and the corresponding scanned information is stored.
[00411 At step 2-5, the scanned information from the primary and
secondary containers is compared to ensure that the information is the same.
If the scanned information from the primary and secondary containers is the
same, the user is provided with affirmative feedback at step 2-6. On the other
hand, if the scanned information from the primary and secondary containers is
not the same, the user is provided with dissenting feedback at step 2-7.
[0042] While the above description provides example embodiments, it
will be appreciated that the present invention is susceptible to modification
and change without departing from the fair meaning and scope of the
accompanying claims. Accordingly, what has been described is merely
illustrative of the application of aspects of embodiments of the invention.
Numerous modifications and variations of the present invention are possible
in light of the above teachings. It is therefore to be understood that within
the
scope of the appended claims, the invention may be practiced otherwise than
as specifically described herein.