Note: Descriptions are shown in the official language in which they were submitted.
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
A PROCESS FOR THE DESULFURIZATION OF HYDROCARBONACEOUS OIL
BACKGROUND OF THE INVENTION
[0001] There is an increasing demand to reduce the sulfur content of
hydrocarbonaceous oil
to produce products which have very low concentrations of sulfur and are
thereby marketable
in the ever more demanding marketplace. With the increased environmental
emphasis on the
requirement for more environmentally friendly transportation fuels, those
skilled in the art have
sought to find feasible and economical techniques to reduce the sulfur content
of
hydrocarbonaceous oil to low concentrations.
[0002] Traditionally, hydrocarbons containing sulfur have been subjected to a
catalytic
hydrogenation zone to remove sulfur and produce hydrocarbons having lower
concentrations of
sulfur. Hydrogenation to remove sulfur is very successful for the removal of
the sulfur from
hydrocarbons that have sulfur components that are easily accessible to contact
with the
hydrogenation catalyst. However, the removal of sulfur components that are
sterically hindered
becomes exceedingly difficult and therefore the removal of sulfur components
to a sulfur level
below 100 ppm is very costly by known current hydrotreating techniques. It is
also known that
a hydrocarbonaceous oil containing sulfur may be subjected to oxygenation to
convert the
hydrocarbonaceous sulfur compounds to compounds containing sulfur and oxygen,
such as
sulfoxide or sulfone for example, which have different chemical and physical
characteristics
which make it possible to isolate or separate the sulfur bearing compounds
from the balance of
2o the original hydrocarbonaceous oil. The disadvantage to this approach is
that the isolated
sulfur bearing hydrocarbon compounds are still not useful as a sulfur-free
material and
therefore the yield of a sulfur-free material from the original
hydrocarbonaceous oil is less than
desirable and therefore uneconomic.
INFORMATION DISCLOSURE
[0003] US 2,769,760 discloses a hydrodesulfurization process which reduces the
organic
sulfur concentration in a hydrocarbon feedstock. The resulting hydrocarbon
product from the
first stage hydrodesulfurization zone contains sulfur and is subsequently
introduced into a
second stage partial desulfurization and/or chemical reaction wherein the
second stage
treatment is conducted at a temperature of approximately 232°C
(450°F) and at atmospheric
-1-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
pressure in the absence of hydrogen. The contact material for the reaction in
the second stage
is of the same type as used for the hydrodesulfurization reaction. Preferred
contact materials
contain cobalt and molybdenum.
[0004] Published European Patent Application No. 565324 discloses a method of
recovering an organic sulfur compound from a liquid oil wherein the method
comprises
treating the liquid oil containing an organic sulfur compound with an oxygen
agent and
separating the oxidized organic sulfur compound by separation means such as
distillation,
solvent extraction and/or adsorption means.
[0005] US 3,551,328 discloses a process for reducing the sulfur content of
heavy
hydrocarbon petroleum fractions by oxidizing the sulfur compounds present in
such heavy
hydrocarbon fractions and contacting the heavy hydrocarbon fractions
containing such oxidized
sulfur compounds with a lower paraffinic hydrocarbon solvent in a
concentration sufficient to
separate the oxidized sulfur compounds from the heavy hydrocarbon fractions
and recovering a
heavy hydrocarbon fraction of reduced sulfur content.
[0006] US 6,277,271 Bl discloses a process for the desulfurization of
hydrocarbonaceous
oil wherein the hydrocarbonaceous oil and a recycle stream containing sulfur-
oxidated
compounds is contacted with a hydrodesulfurization catalyst in a
hydrodesulfurization reaction
zone to reduce the sulfur level to a relatively low level and then contacting
the resulting
hydrocarbonaceous stream from the hydrodesulfurization zone with an oxidizing
agent to
convert the residual, low level of sulfur compounds into sulfur-oxidated
compounds. The
residual oxidizing agent is decomposed and the resulting hydrocarbonaceous oil
stream
containing the sulfur-oxidated compounds is separated to produce a stream
containing the
sulfur-oxidated compounds and a hydrocarbonaceous oil stream having a reduced
concentration of sulfur-oxidated compounds. At least a portion of the sulfur-
oxidated
compounds is recycled to the hydrodesulfurization reaction zone.
[0007] US 6,171,47 B 1 discloses a process for the desulfurization of
hydrocarbonaceous
oil wherein the hydrocarbonaceous oil is contacted with a hydrodesulfurization
catalyst in a
hydrodesulfurization reaction zone to reduce the sulfur level to a relatively
low level and then
contacting the resulting hydrocarbonaceous stream from the
hydrodesulfurization zone with an
oxidizing agent to convert the residual, low level of sulfur compounds into
sulfur-oxidated
compounds. The resulting hydrocarbonaceous oil stream containing the sulfur-
oxidated
-2-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
compounds is separated by solvent extraction after decomposing any residual
oxidizing agent
to produce a stream containing the sulfur-oxidated compounds and a
hydrocarbonaceous oil
stream having a reduced concentration of sulfur-oxidated compounds.
SUMMARY OF THE INVENTION
[0008] The present invention provides a process for the desulfurization of
hydrocarbonaceous oil wherein the hydrocarbonaceous oil is contacted with a
hydrodesulfurization catalyst in a hydrodesulfurization reaction zone to
reduce the sulfur level
to a relatively low level and then contacting the resulting hydrocarbonaceous
stream from the
hydrodesulfurization zone with an oxidizing agent to convert the residual, low
level of sulfur
compounds into sulfur-oxidated compounds. The residual oxidizing agent is
decomposed and
the resulting hydrocarbonaceous oil stream containing the sulfur-oxidated
compounds is
separated to produce a stream comprising the sulfur-oxidated compounds and a
hydrocarbonaceous oil stream having a reduced concentration of sulfur-oxidated
compounds.
(0009] In a preferred embodiment of the present invention, the
hydrocarbonaceous effluent
stream from the hydrodesulfurization zone is contacted with an aqueous
oxidizing solution to
convert the residual, low level of sulfur compounds into sulfur-oxidated
compounds. The
resulting hydrocarbonaceous oil stream containing the sulfur-oxidated
compounds is treated to
decompose any residual oxidizing agent and is contacted with a selective
adsorbent having a
greater selectivity for the sulfur-oxidated compounds than for the sulfur-free
hydrocarbonaceous oil to produce an adsorbent containing at least a portion of
the sulfur-
oxidated compounds and a hydrocarbonaceous oil stream having a reduced
concentration of
sulfur-oxidated compounds.
(001Oj The present invention discloses a novel integrated process that is
capable of easily
and economically reducing the sulfur content of hydrocarbonaceous oil while
achieving high
recovery of the original feedstock. Important elements of the present
invention are the
minimization of the cost of hydrotreating in the integrated two-stage
desulfurization process
and the ability to economically desulfurize hydrocarbonaceous oil to a very
low level while
maximizing the yield of the desulfurized hydrocarbonaceous oil.
-3-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
BRIEF DESCRIPTION OF THE DRAWING
[0011] The drawing is a simplified process flow diagram of a preferred
embodiment of the
present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0012] The present invention provides an improved integrated process for the
deep
desulfurization of hydrocarbonaceous oil in a two-stage desulfurization
process. In accordance
with the present invention, a preferred hydrocarbonaceous oil feedstock
contains distillable
hydrocarbons boiling in the range from 93°C (200°F) to
565°C (1050°F) and more preferably
from 149°C (300°F) to 538°C (1000°F). The
hydrocarbonaceous oil feedstock is contemplated
to contain from 0.1 to 5 weight percent sulfur and the process is most
advantageously utilized
when the feedstock contains high levels of sulfur and the desired desulfurized
product contains
a very low concentration of sulfur. Preferred product sulfur levels are less
than 100 wppm,
more preferably less than 50 wppm, and even more preferably less than 30 wppm.
[0013] The hydrocarbonaceous oil containing sulfur compounds is introduced
into a
catalytic hydrodesulfurization zone containing hydrodesulfurization catalyst
and maintained at
hydrodesulfurization conditions. The catalytic hydrodesulfurization zone may
contain a fixed,
ebullated or fluidized catalyst bed. This reaction zone is preferably
maintained under an
imposed pressure from 101 kPa (14.7 psig) to 13.9 MPa (2000 psig) and more
preferably
under a pressure from 800 kPa (100 psig) to 12.5 MPa (1800 psig). Suitably,
the
hydrodesulfurization reaction is conducted with a maximum catalyst bed
temperature in the
range from 204°C (400°F) to 400°C (750°F) selected
to perform the desired
hydrodesulfurization conversion to reduce the concentration of the sulfur
compounds to the
desired level. In accordance with the present invention, it is contemplated
that the desired
hydrodesulfurization conversion includes, for example, desulfurization,
denitrification and
olefin saturation. Further preferred operating conditions include liquid
hourly space velocities
in the range from 0.05 hr.'1 to 20 hr.'i and hydrogen to feed ratios from 200
standard cubic feet
per barrel (SCFB) (33.7 nm3/m3) to 50,000 SCFB (8425 nm3/m3), preferably from
200 SCFB
(33.7 nm3/m3) to 10,000 SCFB (1685 nm3/m3). The hydrodesulfurization zone
operating
-4-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
conditions are preferably selected to produce a desulfurized
hydrocarborlaceous oil containing
from 100 to 1000 wppm sulfur.
[0014] The preferred catalytic composite disposed within the hereinabove-
described
hydrodesulfurization zone can be characterized as containing a metallic
component having
hydrodesulfurization activity, which component is combined with a suitable
refractory
inorganic oxide carrier material of either synthetic or natural origin. The
precise composition
and method of manufacturing the carrier material is not considered essential
to the present
invention. Preferred carrier materials are alumina, silica, and mixtures
thereof. Suitable
metallic components having hydrodesulfurization activity are those selected
from the group
comprising the metals of Groups V1B and VIII of the Periodic Table, as set
forth in the
Periodic Table of the Elements, E.H. Sargent and Company, 1964. Thus, the
catalytic
composites may comprise one or more metallic components from the group of
molybdenum,
tungsten, chromium, iron, cobalt, nickel, platinum, palladium, iridium,
osmium, rhodium,
ruthenium, and mixtures thereof. The concentration of the catalytically active
metallic
component, or components, is primarily dependent upon a particular metal as
well as the
physical and/or chemical characteristics of the particular hydrocarbon
feedstock. For example,.
the metallic components of Group VIB are generally present in an amount within
the range of
from 1 to 20 weight percent, the iron-group metals in an amount within the
range of 0.2 to 10
weight percent, whereas the noble metals of Group VIII are preferably present
in an amount
within the range of from 0.1 to 5 weight percent, all of which are calculated
as if these
components existed within the catalytic composite in the elemental state. In
addition, any
catalyst employed commercially for hydrodesulfurizing middle distillate
hydrocarbonaceous
compounds to remove nitrogen and sulfur may function effectively in the
hydrodesulfurization
zone of the present invention. It is further contemplated that
hydrodesulfurization catalytic
composites may comprise one or more of the following components: cesium,
francium,
lithium, potassium, rubidium, sodium, copper, gold, silver, cadmium, mercury
and zinc.
[0015] The hydrocarbonaceous effluent from the hydrodesulfurization reaction
zone is
separated to produce a gaseous stream containing hydrogen, hydrogen sulfide
and normally
gaseous hydrocarbons, and a liquid hydrocarbonaceous stream having a reduced
concentration
of sulfur compounds. This resulting liquid hydrocarbonaceous stream in one
prefewed
embodiment of the present invention is contacted with an aqueous oxidizing
solution in an
-5-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
oxidation zone to convert sulfur-containing compounds into sulfur-oxidated
compounds. Any
suitable known aqueous oxidizing solution may be used to perform the sulfur
oxidation. In a
preferred embodiment, the aqueous oxidizing solution contains acetic acid and
hydrogen
peroxide. Preferably the molar feed ratio of hydrogen peroxide to sulfur
ranges from 1 to 10 or
more and the molar ratio of acetic acid to hydrogen peroxide ranges from 0.1
to 10 or more.
The oxidation conditions including contact time are selected to give the
desired results as
described herein and the pressure is preferably great enough to maintain the
aqueous solution in
a liquid phase during the contacting of the hydrocarbonaceous oil. Preferred
oxidation
conditions include a pressure from atmospheric to 800 kPa (100 psig), and a
temperature from
38°C (100°F) to 149°C (300°F). Since the aqueous
oxidizing solution and the
hydrocarbonaceous oil are immiscible, the oxidation zone must have the ability
to intimately
mix and contact the two phases to ensure the completion of the chemical
oxidation. Any
suitable means may be used for the contacting and preferred methods include
the use of a
packed mixing column with countercurrent flows of the two phases or in-line
mixing
apparatus.
(0016] In the event that there is residual hydrogen peroxide after the
completion of the
oxidation, it is preferred that the stream containing the residual hydrogen
peroxide is contacted
with a suitable catalyst to decompose the hydrogen peroxide. A preferred
hydrogen peroxide
decomposition catalyst is a supported transition metal, a transition metal
complex or a
transition metal oxide. The decomposition of the hydrogen peroxide is
conducted to simplify
the recovery and separation of the reaction products including sulfur-oxidated
compounds
recovered from the oxidation zone. Preferred decomposition operating
conditions include a
pressure from atmospheric to 800 kPa (100 psig) and a temperature from
38°C (100°F) to
149°C (300°F).
[0017] The resulting effluent from the oxidation zone after decomposition of
the oxidizing
agent contains desulfurized hydrocarbonaceous oil, sulfur-oxidated compounds
such as
sulfoxides and sulfones, for example, water and acetic acid. This resulting
effluent from the
oxidation zone is contacted with a selective adsorbent having a greater
selectivity for the
sulfur-oxidated compounds than for the sulfur-free hydrocarbonaceous oil to
produce a
selective adsorbent containing at least a portion of the sulfur-oxidated
compounds and a
hydrocarbonaceous oil having a reduced concentration of sulfur. Any suitable
known selective
-6-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
adsorbent may be used to selectively extract the sulfur-oxidated compounds. In
a preferred
embodiment of the present invention, the selective adsorbent is selected from
the group
consisting of aluminosilicates such as X and Y type zeolites, zeolite beta,
and L-zeolite, highly
silaceous zeolites such as ZSM-5 and silicalite, and silica aluminophosphates
such as
SAPO-34. The preferred selective adsorbents are contacted with the effluent
from the
oxidation zone in an adsorption zone. In a preferred mode, the sulfur-oxidated
compounds are
adsorbed with thermally stable aluminosilicates such as Y-type zeolite,
dealuminated Y type
zeolite, zeolite beta, and zeolite L. The raffinate hydrocarbonaceous oil
recovered from the
adsorption zone preferably contains less than 100 weight ppm, more preferably
less than 50
weight ppm sulfur and even more preferably less than 30 wppm.
[0018] The resulting adsorbent is regenerated to recover the adsorbed sulfur-
oxidated
compounds and the regenerated adsorbent is preferably recycled to the
adsorption zone. The
regeneration of the adsorbent may be accomplished by desorbing the adsorbed
sulfur-oxidated
compounds by heating and/or by contacting with a liquid or vapor desorbent.
(0019] In one embodiment, operation of the invention is achieved as a fixed
bed adsorber
by introducing reacted mixture from the sulfur oxidation zone to an adsorption
zone until a
selected portion of the adsorptive capacity of the selective adsorbent is
exhausted. By suitable
valuing means the flow of reacted mixture from the sulfur oxidation zone is
then halted and the
exhausted adsorption zone is regenerated in a desorption zone by desorbing the
sulfur-oxidated
compounds from the adsorbent with desorbent material. In this manner, a single
fixed bed may
be so employed in the desulfurization process of the instant invention.
(0020] In another embodiment, operation of the invention is achieved as a
fixed bed
adsorber by introducing reacted mixture from the sulfur oxidation zone to a
first adsorption
zone until a selected portion of the adsorptive capacity of the selective
adsorbent is exhausted.
By suitable valuing means the flow of reacted mixture from the sulfur
oxidation zone is then
redirected to a second adsorption zone containing selective adsorbent which
has been
previously regenerated and the exhausted adsorption zone is regenerated in a
desorption zone
by desorbed the sulfur-oxidated compounds from the adsorbent with desorbent
material. In
this manner, any desired number of fixed beds may be so arranged in the
desulfitrization of the
instant invention.
_7_
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
[0021] In another embodiment, operation of the invention is achieved as a
fluidized or
ebullated bed adsorber by introducing reacted mixture from the sulfur
oxidation zone to an
fluidized adsorption zone until a selected portion of the adsorptive capacity
of the selective
adsorbent is exhausted. By suitable means at least a portion of the exhausted
adsorbent from
the adsorption zone is then redirected to a desorption zone and the exhausted
adsorbent is
regenerated desorbing the sulfur-oxidated compounds from the adsorbent with
desorbent
material.
[0022] In yet another embodiment, operation of the invention is achieved as
simulated
countercurrent adsorber by introducing reacted mixture from the sulfur
oxidation zone to a
moving bed adsorption zone until a selected portion of the adsorptive capacity
of the selective
adsorbent is exhausted. By suitable means at least a portion of the exhausted
adsorbent from
the adsorption zone is then redirected to a desorption zone and the exhausted
adsorbent is
regenerated desorbing the sulfur-oxidated compounds from the adsorbent with
desorbent
material.
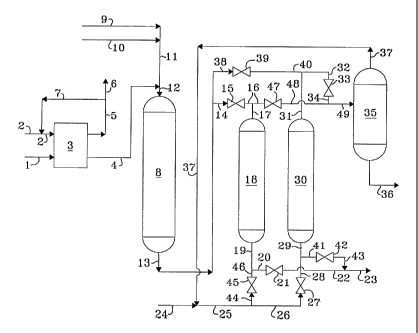
[0023] With reference now to the drawing that illustrates a two-bed fixed
adsorber
operation, with one bed in the adsorption mode and one bed in the desorption
mode, a
hydrocarbonaceous oil containing sulfur is introduced into the process via
line 1 and enters
hydrodesulfurization zone 3. A fresh hydrogen stream is introduced via line 2
and is admixed
with a hydrogen-rich gaseous recycle stream provided via line 7 and the
resulting admixture is
introduced into hydrodesulfurization zone 3 via line 2. A gaseous stream
containing hydrogen,
hydrogen sulfide and normally gaseous hydrocarbons is removed from
hydrodesulfurization
zone 3 via line 5 and at least a portion is recycled via line 7 as described
hereinabove and at
least another portion is removed from the process via line 6. A
hydrocarbonaceous stream
having a reduced concentration of sulfur is removed from hydrodesulfurization
zone 3 via line
4 and introduced into sulfur oxidation zone 8 via line 12 along with a
carboxylic acid stream
provided via lines 9, 11 and 12, and an aqueous hydrogen peroxide stream which
is introduced
into the process via lines 10, 11 and 12. The aqueous stream and the
hydrocarbonaceous
stream are intimately admixed in sulfur oxidation zone 8 in order to oxidize
the sulfur
compounds. A resulting reacted mixture is removed from sulfur oxidation zone 8
via line 13
after decomposing any residual hydrogen peroxide and is transported via line
14, valve 15,
lines 16 and 17 and is introduced into adsorption zone 18 and is contacted
with previously
_g_
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
regenerated selective adsorbent. A resulting hydrocarbonaceous stream
containing a reduced
concentration of sulfur is removed from adsorption zone 18 via line 19, line
20, valve 21, line
22 and line 23 and recovered. Desorbent stream 25, optionally comprising fresh
desorbent
stream 24 and/or recycled desorbent carried via line 37 is introduced via line
25, line 26, valve
27, line 28, and line 29 into a previously exhausted adsorption zone
comprising desorption
zone 30. A desorption stream rich in oxidized sulfur compounds is removed from
desorption
zone 30 via line 31, line 32, valve 33, line 34, and line 49 and is introduced
into distillation
zone 35. A desorbent stream having a reduced concentration of sulfur-oxidated
compounds is
removed from distillation zone 35 via line 37 and is optionally recycled to
desorption zone 30
as hereinabove described. Oxidized sulfur compounds are removed from
distillation zone 35
via line 36 and recovered. When adsorption zone 18 becomes spent, the stream
carried via line
13 is transported via line 38, valve 39, line 40, and line 31 and is
introduced into previously
regenerated desorption zone 30. The now adsorption zone 30 produces an
effluent stream
having a reduced concentration of sulfur compounds which stream is transported
via line 29,
line 41, valve 42, line 43, and line 23 and recovered. The spent adsorption
zone 18 is
regenerated by the introduction of a desorption stream carried via line 25,
line 44, valve 45,
line 46, and line 19 into spent adsorption zone 18. A resulting effluent is
recovered via line 17,
line 16, valve 47, line 48, and line 49 and introduced into distillation zone
35.
(0024] Selective adsorbents useful in the present invention are identified by
the following
illustrative calculations. These calculations are, however, not presented to
unduly limit the
selective adsorbents useful in this invention, but to illustrate the molecular
properties of the
sulfur-containing and sulfur-oxidated compounds that play a role in the
adsorption and removal
from hydrocarbonaceous oil in the hereinabove described embodiment. The
following results
were not obtained by the actual measurement of the properties of the compounds
but are
considered prospective and reasonably illustrative of the expected performance
of the invention
based upon sound quantum chemistry calculations.
ILLUSTRATIVE CALCULATIONS
[0025] The chemical structures and selected molecular properties of methyl
thiophene,
methyl thiophene sulfone, methyl thiophene sulfoxide, benzothiophene,
benzothiophene
sulfone, and benzothiophene sulfoxide were calculated by the use of semi-
empirical molecular
-g_
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
orbital theory using the PM3 Hamiltonian as described by J.J.P. Stewart,
"Optimization of
Parameters for Semi-Empirical Methods 1 -Method", Journal of Computational
Chemistry,
Volume 10, 1989, pages 209-220, and are presented in Table 1.
Table 1
Sulfur Atom Limiting
Compound Dipole Moment,Partial ChargeDimension,
(debye) (electrostatic(angstroms)
units)
2-methyl thiophene 0.950 0.303 4.588
2-methyl thiophene 4.718 1.063 4.636
sulfone
2-methyl thiophene 5.669 2.285 4.654
sulfoxide
Benzothiophene 1.089 0.261 5.010
Benzothiophene sulfone4.546 1.063 5.006
Benzothiophene sulfoxide5.573 2.285 5.002
(0026] A first property affecting selective adsorption of the sulfur-oxidated
molecules
compared to the molecules comprising is dipole moment. Dipole moment increases
upon
oxidation of the sulfur-containing molecules and reflects an increase in
polarity of the sulfur-
oxidated molecules compared to hydrocarbonaceous oil molecules and un-oxidized
sulfur-
containing molecules. Such an increase in dipole moment increases the
adsorption selectivity
for the sulfur-oxidated molecules compared to un-oxidized sulfur-containing
molecules and
molecules comprising hydrocarbonaceous oil, when using such adsorbents as
silica, alumina,
silicates, aluminosilicates and aluminophosphates in the instant invention.
(0027 A second property affecting selective adsorption of the sulfur-oxidated
molecules
compared to the molecules comprising is the atomic partial charge on the
sulfur atom. Positive
partial charge on sulfur increases upon oxidation of the sulfur-containing
molecules and
reflects an increase in electrostatic attraction for the sulfur-oxidated
molecules to adsorbent
atoms bearing negative partial charge compared to hydrocarbonaceous oil
molecules and un-
oxidized sulfur-containing molecules. Such an increase in electrostatic
attraction increases the
-10
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
adsorption selectivity for the sulfur-oxidated molecules compared to un-
oxidized sulfur-
containing molecules and molecules comprising hydrocarbonaceous oil, when
using such
adsorbents such as silica, alumina, silicates, aluminosilicates and
aluminophosphates in the
instant invention.
[0028] A third property that affects selective adsorption of the sulfur-
oxidated molecules in
preference of the un-oxidized sulfur-containing molecules is molecular size.
The crystalline
structure of certain selective adsorbents such as crystalline silicates,
aluminosilicates, and
aluminophosphates may manifest channels and pores that are of molecular
dimensions. In the
present invention, if the sulfur-oxidated molecules are small enough to gain
access to such
channels and pores, they may be selectively adsorbed compared to larger
molecules in the
hydrocarbonaceous oil. The calculated limiting dimensions for the sulfur-
oxidated molecules
indicate that such molecules can gain access to the pore structures and be
selectively adsorbed
by such adsorbents as X and Y-type zeolites, dealuminated Y zeolite, L-
zeolite, beta zeolite,
SAPO-34, and the like. In addition, because many such microporous adsorbents
display a
selectivity for more polar molecules compared to less polar molecules,
enhancement of dipole
moment and sulfur positive partial charge via sulfur oxidation while not
increasing the limiting
molecular dimension makes these adsorbents particularly advantageous for use
in the instant
invention.
ILLUSTRATIVE EMBODIMENT
[0029] A stream of straight run vacuum gas oil boiling in the range of
315°C (600°F) to
482°C (900°F) and containing 2 weight percent sulfur is
introduced into a hydrodesulfurization
zone containing a hydrodesulfurization catalyst which contains alumina,
nickel, molybdenum
and phosphorus. The hydrodesulfurization zone is operated at a pressure of
11.8 MPa (1700
psig), a hydrogen to feed ratio of 843 nm3/m3 (5000 SCFB) and a maximum
catalyst
temperature of 393°C (740°F) to reduce the residual sulfur in
the resulting desulfurized
vacuum gas oil to 500 weight ppm (0.05 weight percent). The desulfurized
vacuum gas oil is
then introduced into an oxidation reaction zone and contacted with acetic acid
and hydrogen
peroxide in water. The molar feed ratio of hydrogen peroxide to sulfur is 5
and the molar ratio
of acetic acid to hydrogen peroxide is 5, and the contacting is conducted at a
temperature of
65°C (150°F) and a pressure of 207 kPa (30 psig). The effluent
from the oxidation reaction
-11-
CA 02535349 2006-02-09
WO 2005/019386 PCT/US2004/027045
zone is passed over a catalyst containing a mixed oxide of iron and molybdenum
to decompose
the unreacted hydrogen peroxide and then introduced into an adsorber wherein
the sulfur-oxide
compounds are extracted with Y zeolite as a selective adsorbent to produce a
finished product
containing less than 30 weight ppm sulfur. A spent selective adsorbent is
regenerated to
remove sulfur-oxide compounds and the subsequently regenerated adsorbent is
returned to
adsorbent service.
(0030 The foregoing description, drawing and illustrative embodiment clearly
illustrate
the advantages encompassed by the process of the present invention and the
benefits to be
afforded with the use thereof.
-12-