Note: Descriptions are shown in the official language in which they were submitted.
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
PROCESSING AIDS FOR ENHANCED HYDROCARBON RECOVERY
FROM OIL SANDS, OIL SHALE AND OTHER PETROLEUM RESIDUES
This application claims priority from U.S. provisional applications 60/505,083
filed on September 22, 2003 and 60/604,212 filed on August 25, 2004.
BACKGROUND OF THE INVENTION
Field of the Invention
[0001 ] The present invention relates to the recovery of hydrocarbons from tar
sands, oil
shale and other petroleum residues. The invention also relates to the settling
of solids
from the tailings slurry of a hydrocarbon recovery process. More specifically,
the
invention relates to compositions and methods for improving the recovery of
hydrocarbons and, in some cases, also the settling of solids in the tailings.
Description of the Related Art
[0002] The Athasbasca Oil Sands Deposit is, by itself, the largest petroleum
resource in
the world. These oil sands or tar sands are located in northwestern Alberta,
Canada and
represent the equivalent of roughly 1.6 to 2.7 trillion barrels of oil. Oil
sand is visible on
the banks of the Athabasca River, north and south of Fort McMurray, but most
of the oil
sand in the area lies buried 50 meters or deeper under muskeg and overburden.
[0003] To utilize this resource, as one means, truck and shovel operations
first mine the
oil sand and deliver it to a crusher. The crushed oil sand is then conveyed to
a mixing
operation that combines the oil sand with hot water to create an aqueous
slurry that is
pumped via pipeline to a bitumen extraction plant.
[0004] Bichard (U.S. 3,330,757) discloses a bitumen extraction process that
mixes
water at a temperature between 140° F and 200° F with the tar
sand to remove most of
the sand and other solids. Under these conditions, a froth or emulsion
comprising water,
nonseparated solids and oil is produced. This froth is introduced into a
secondary
processing zone where the froth is contacted with a combination of water, a
transfer agent
1
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
and a chelating agent in order to obtain a froth that is substantially solids-
free. Bichard
says that this process results in substantially 100% of the sand being removed
from the
froth. The froth can then be further processed to recover hydrocarbons.
[0005] Tipman et al. (U.S. Patent 5,876,592) discloses that bitumen separation
and
recovery from sand may be accomplished by following what is known as the Clark
hot
water extraction process. In the front end of this process, the oil sand is
mixed with hot
water and caustic in a rotating tumbler to produce an aqueous slurry. The
slurry is
screened to remove large rocks and the like. The screened slurry is diluted
with
additional hot water and the product is them temporarily retained in a primary
separation
vessel (PSV). In this vessel, bitumen globules contact and coat air bubbles
that are
entrained in the slurry in the tumbler. The buoyant bitumen-coated air bubbles
rise
through the slurry and form bitumen froth. The sand in the slurry settles and
is
discharged from the base of the vessel, together with some water and bitumen.
This
stream or portion is referred to as the "PSV underflow" or tailings. A
"middlings"
portion may also be obtained, the middlings comprising water with non-buoyant
bitumen
and fines, collected from the middle of the PSV. The froth overflows the lip
of the vessel
and is recovered as the primary froth, typically comprising 65 wt. % bitumen,
28 wt.
water and 7 wt. % particulate solids.
[0006] The PSV underflow is introduced into a deep cone vessel, referred to as
the
tailings oil recovery vessel ("TORV"). Here the PSV underflow is contacted and
mixed
with a stream of aerated middlings from the PSV. Again, bitumen and air
bubbles contact
and unite to form buoyant globules that rise and form froth. This "secondary"
froth
overflows the lip of the TORY and is recovered. The secondary froth typically
comprises
45 wt. % bitumen, 45 wt. % water and 10 wt. % solids. The middlings from the
TORV
are withdrawn and processed in a series of sub-aerated, impeller-agitated
flotation cells.
Secondary froth, typically comprising 40 wt. % bitumen, 50 wt. % water and 10
wt.
solids, is produced from these cells.
[0007] The primary and secondary froth streams are combined to yield a product
froth
stream, typically comprising 60 wt. % bitumen, 32 wt. % water and 8 wt. %
solids. This
2
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
stream will typically have a temperature of about 80 °C. The water and
solids in the froth
are contaminants which need to be reduced in concentration before the froth
can be
treated in a downstream refinery-type upgrading facility. This cleaning
operation is
carried out using what is referred to as a "dilution centrifuging circuit".
[0008] More particularly, the combined froth product is first deaerated and
then diluted
with sufficient solvent, specifically naphtha, to provide a solvent to froth
("S/F") ratio of
about 0.45 (w/w). This is done to increase the density differential between
the bitumen on
the one hand and the water and solids on the other. The diluted froth is then
processed in
a scroll-type centrifuge, to remove coarse solids. The bitumen product from
the scroll
machine is subsequently processed in a disc-type centrifuge, to remove water
and fine
clay solids.
[0009] However, the process of separating bitumen from sand, clay, and water
is
complicated by variations in the composition of the oil sand deposits. While
the amount
of sand, clay, water and bitumen in the oil sands are quite determinable,
variations in the
processability that are experienced between different oils sands are not self
evident. In
fact, various oil sands have been classified as "problem ores" or "poor
processing ores"
while others are referred to as "good processing ores." Problem ores, such as
transition
ores, oxidized ores, "type-X" ores and those having high fines or clays, bring
about
reduced bitumen yield or increased bitumen losses to waste streams. These
problem ores
typically yield only 40 to 60 % recovery of their total bitumen content. By
contrast, good
processing ores typically yield around 98% recovery of their total bitumen
content.
Because of the relative amounts of problem ores and good processing ores, the
problem
ores reduce the industry average recovery to about 92% of total bitumen
content.
Heretofore, there has been no explanation for why these problem ores are
difficult to
process and there has been no solution that allows these ores to be processed
efficiently.
As a result, problem ores are avoided or pushed aside in the mining operation
in order to
take advantage of the good processing ores. Eventually, the problem ores must
be
processed.
3
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
[0010] Therefore, there remains a need for a bitumen recovery process that
improves
the separation of bitumen from other constituents of the oil sands. It would
be desirable
if the process allowed problem ores to be processed in due order as
encountered in the
mining operation. It would be further desirable to identify a processing aid
that would
allow problem ores to be processed in the same extraction plant as good
processing ores
without any major redesigns, additional equipment, or process interruptions.
Additional
benefits would be enjoyed if there were a method for controlling the addition
of the
processing aid as needed to effectively recovery bitumen from oil sands of
different
quality.
SUMMARY OF THE INVENTION
[0011 ] One embodiment of the present invention provides a method, comprising
contacting a hydrocarbon-containing source with a processing aid, and
separating the
hydrocarbon-containing source into a hydrocarbon-containing froth portion and
a tailings
portion. Preferably, the method further comprises determining an amount of
hydrocarbons in the tailings portion, and varying the amount of the processing
aid added
to the hydrocarbon-containing source to control the amount of hydrocarbons in
the
tailings portion. Most preferably, the hydrocarbon-containing source is
separated in the
presence of water and a transfer agent, such as a caustic, phosphate,
silicate, carbonate, or
combinations thereof. An exemplary hydrocarbon-containing source is selected
from tar
sand, oil shale, petroleum residues, and combinations thereof. Without being
limited to a
single theory, it is believed that the processing aids are effective by
associating with or
sequestering one or more cations selected from calcium, magnesium, iron,
alumina,
silica, titanium, zirconium, and combinations thereof.
[0012] The invention provides a method comprising the step of providing a
processing
aid to sequester canons in an aqueous mixture of hydrocarbons and inorganic
solids and
liberating the hydrocarbons from the inorganic material. It is preferred to
then separate
the aqueous mixture into a hydrocarbon-containing froth portion and a tailings
portion,
4
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
for example by mechanical separation including a centrifuge. Optionally, the
method
may further include determining an amount of hydrocarbons in the tailings
portion, such
as by fluorescence, and varying the amount the processing aid added to the
aqueous
mixture to control the amount of hydrocarbons in the tailings portion. The
hydrocarbons
may be liberated in the presence of water and a transfer agent. Suitable
transfer agents
are selected from a caustic, a phosphate, a silicate, a carbonate and
combinations thereof.
Furthermore, the aqueous mixture may include a hydrocarbon-containing source
selected
from tar sand, oil shale, petroleum residues, and combinations thereof. The
hydrocarbon-
containing source may be characterized by having a combined concentration of
calcium
ions, magnesium ions and iron ions that is greater than 40 parts per million.
[0013] The processing aid may be a carboxylic acid or a salt or partial salt
thereof. The
processing aid may be a carboxylic acid derivative of a nitrogen containing
organic
compound, or a salt or partial salt thereof. The processing aid may be a
carboxylic acid, a
salt of the carboxylic acid, a partial salt of the carboxylic acid, or a
combination thereof.
The processing aid may be selected from glycine, nitrilotriacetic acid,
ethylenediaminetetraacetic acid, diethylenetriaminepentaacetic acid,
dicarboxymethyl
glutamic acid, diaminocyclohexane-N,N,N',N'-tetraacetic acid,
ethylenebis(oxyethylenenitrilo)tetraacetic acid, hydroxyethyliminodiacetic
acid,
hydroxyethylethylenediaminetriacetic acid, salts of these acids, partial salts
of these
acids, and combinations thereof. The processing aid may be a carbohydrate. The
processing aid may be an organic alcohol. The processing aid may be an organic
amine, a
derivative of an organic amine, a salt of an organic amine, or a combination
thereof, for
example wherein the organic amine is selected from a hydroxy amine, an
aromatic
amines, derivatives or salts of the amines, and combinations thereof, or
wherein the
organic amine is selected from ethylene diamine, benzotriazole,
triethanolamine, 2,2'-
dipyridyl, or combinations thereof. The processing aid may be an organic acid
selected
from acetic acid, malonic acid, citric acid, isocitric acid, humic acid,
vulvic acid, salts of
these acids, partial salts of these acids, and combinations thereof. The
processing aid
may be a hydroxy organic acid selected from glycolic acid, malic acid,
gluconic acid,
glucoheptonic acid, tartaric acid, malefic acid, ferulic acid, salicylic acid,
salts of these
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
acids, partial salts of these acids, and combinations thereof. The processing
aid may be a
polymeric acid selected from polyacrylic acid, polymethacrylic acid, salts of
these acids,
partial salts of these acids, and combinations thereof. The processing aid may
be a
polymer based on monomers of malefic anhydride. The processing aid may be a
salt of a
carboxylic acid having cations selected from sodium, potassium, and
combinations
thereof. The processing aid may be a phosphoric acid derivative selected from
sodium
triphosphate, potassium triphosphate, sodium polyphosphate, disodium
dihydrogenpyrophosphate, and combinations thereof. The processing aid may be
an
organophosphonate selected from methylphosphonic acid, hydroxyethylidine
diphosphoric acid, any of the salts or partial salts of these acids, and
combinations
thereof. The processing aid may be an organophosphonate based on a nitrogen
containing organic compound selected from aminomethanephosphonic acid,
nitrilotris(methylene)triphosphonic acid,
ethylenediaminetetra(methylenephosphonic
acid), diethylene triamine penta(methylene phosphonic acid), hexamethylene-
diaminetetra(methylenephosphonic acid), hydroxy containing derivatives
thereof, and
combinations thereof. The processing aid may be an organic compound containing
both
carboxylic and phosphonic functional groups, the organic compound selected
from 2-
phosphonobutane 1,2,4-tricarboxylic acid, any of the salts or partial salts of
these acids,
and combinations thereof. The processing aid may be a polymer selected from a
homo
polymer, a co-polymer, a salt or partial salt of these polymers, and
combinations thereof,
wherein the polymer includes a functional group selected from hydroxyl,
carboxylic,
amine, or combinations thereof. The processing aid may be a sulfur-containing
organic
acid selected from 2,3-dimercapto-1-propanol, sulfosalicylic acid,
lignosulfonate, a salt
or partial salt thereof, and combinations thereof. The processing aid may be
an
oxyalkylate derivative of an amine. The processing aid may be used to control
the
amount of hydrocarbons in the tailings portion. The processing aid may be
suitable for
sequestering canons selected from calcium, magnesium, iron, alumina, silica,
titanium,
zirconium, and combinations thereof.
[0014] Another embodiment of the invention provides a method that includes
providing
a polymeric processing aid to sequester canons in an aqueous mixture of
hydrocarbons
6
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
and selectively flocculate inorganic solids, and liberating the hydrocarbons
from the
inorganic material. Preferably, the polymeric processing aid is provided in an
amount
that is effective to increase the liberation of the hydrocarbons. The
hydrocarbons and
inorganic solids may optionally be obtained from oil sand, oil shale,
petroleum residue, or
a combination thereof. The process is especially beneficial for use wherein
the
hydrocarbons and inorganic solids are obtained from a poor processing ore.
[0015] The method may also include recovering liberated hydrocarbons from the
mixture, such as by forming a froth having the hydrocarbons attached to air
bubbles.
Furthermore, the method may include separating the inorganic solids into a
tailings
portion. Optionally, an additional amount of a polymeric processing aid may be
added to
the tailings portion to flocculate solids. The polymeric processing aid
provided to the
tailings may or may not be the same polymeric processing aid provided to the
aqueous
mixture. Preferably, the polymeric processing aid separates along with the
tailing portion
in order to also flocculate solids.
[0016] Preferably, the aqueous mixture is provided with between 1 and 150 ppm
of the
polymeric processing aid, more preferably between 10 and 100 ppm, and most
preferably
between 15 and 60 ppm. The addition of the polymeric processing aid has been
shown to
increase the bitumen recovery by at least 5 percent, or even at least 10
percent.
[0017] The canons in the aqueous mixture may originate from the source of the
hydrocarbons and inorganic material and the cations may include multivalent
ions
selected from calcium, magnesium, iron and combinations thereof. Depending
upon the
specific hydrocarbon source and/or water source, the aqueous mixture may have
greater
than 10 ppm, or even greater than 30 ppm of these cations. Still further, the
aqueous
mixture can have a combined concentration of calcium ions, magnesium ions and
iron
ions that is greater than 40 parts per million.
[0018] Accordingly, the processing aids of this invention should be suitable
for
sequestering cations selected from calcium, magnesium, iron, alumina, silica,
titanium,
7
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
zirconium, and combinations thereof. Preferably, the method is characterized
by a
reduction in the cation concentration and allows greater bubble attachment of
the
hydrocarbons. Optionally, the method includes reducing the concentration of
unsequestered multivalent ions in the aqueous mixture to less than 30 ppm.
Still, the
method may further comprise monitoring the efficiency of the hydrocarbon
recovery; and
varying the amount of processing aid added to the aqueous mixture to control
the
efficiency.
[0019] Suitably, the polymeric processing aid may comprise a polymer,
copolymer or
terpolymer based on monomers selected from acrylic acid, acrylamide,
acrylonitrile, or
substituted derivatives of these monomers. Optionally, these polymeric
processing aids
may be a derivative selected from sulfonates, phosphates, phosphonates,
betaines,
sulfobetaines, and combinations thereof. Furthermore, the polymeric processing
aid may
be a salt selected from amine salts, ammonium salts, phosphonium salts,
thiouronium salts,
and combinations thereof. The polymeric processing aids may be oil soluble or
water
soluble. In one embodiment, the polymeric processing aid is supplied or
provided as an
aqueous solution.
[0020] In another embodiment, the polymeric processing aid comprises an
acrylic acid-
based polymer or a polymer based on a substituted polyacrylic acid. Preferred
examples
include polyacrylic acid or polymethacrylic acid. Furthermore, the polymeric
processing
aid may be a polymeric acid selected from polyacrylic acid, polymethacrylic
acid, salts of
these acids, partial salts of these acids, and combinations thereof.
[0021 ] In a further embodiment, the polymeric processing aid comprises an
acrylamid-
based polymer or a polymer based on a substituted acrylamide, such as
polyacrylamide or
polymethacrylamid. Optionally, the polymeric processing aids of these
embodiments may
have a hypophosphite in the polymer backbone. An example of this is where the
hypophosphite backbone is formed by reacting the polyacrylic acid or the
polymethacrylic acid with hypophosphorous acid.
8
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
[0022] In a still further embodiment, the polymeric processing aid is a
hydrolysis
product or salt capable of sequestering calcium. Optionally, such a polymeric
processing
aid hydrolyzes under conditions of the aqueous mixture.
[0023] In a yet further embodiment, the polymeric processing aid comprises a
methylene
sulfonate modified polyacrylamide, a methylene sulfonate modified
polyrnethacrylamide,
a hydroxamic acid modified polyacrylamide, or a hydroxamic acid modified
polymethacrylamide.
[0024] The polyacrylamides useful to the present invention should not be
limited by
molecular weight, anionic character, or cationic character. However, a
suitable
polyacrylamide processing aid may be prepared by the reaction of
polyacrylonitrile with
water. The polyacrylamide processing aid is preferably characterized by
forming a
polycarboxylic acid at alkaline pH. Specifically, the polymeric processing aid
may be a
hydrolysed polyacrylamide polymer characterized by an ability to sequester
cations and
an ability to flocculate solids. The polyacrylamide may have a molecular
weight between
that of an acrylamide monomer and 1 billion, preferably between 1 and 50
million, and
more preferably between 1 and 30 million. It should be noted that while this
discussion
refers primarily to polymers, acrylamide monomers, dimers, trimers and other
acrylamide
polymers may be used in accordance with the invention. The polyacrylamide may
have
any percentage anionic or cationic character, depending upon the intended
functions) of
the polyacrylamide, but preferably has between 10 and 90 percent anionic
charge. In a
polyacrylamide prepared primarily for water softening (canon removal), the
percentage
anionic charge will be higher, such as SO to 100 percent, and the molecular
weight will be
lower, such as a between a monomer and 8 million. One preferred polyacrylamide
for
water softening has a molecular weight of about 5 million and an anionic
charge of about
80% and is preferably added at the front end of the process to improve bitumen
liberation. In a polyacrylamide prepared primarily for flocculation, the
percentage anionic
charge will be lower, such as 10 and 30 percent, and the molecular weight will
be higher,
such as between 10 million and 1 billion. One preferred polyacrylamide for
flocculating
has a molecular weight of 20 million and an anionic charge of about 30% and is
9
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
preferably added into the primary separation vessel. Alternatively, a cationic
polyacrylamide is useful for the removal of fine silica particles that carry a
positive
charge. Still further, the polyacrylamide may be further selected to optimize
pH and/or
viscosity effects, such as increasing the pH or reducing the viscosity. Any of
the
acrylamides may be used alone or in combinations. It should be recognized that
the exact
composition of the acrylamide may be tailored the given conditions.
[0025] The polymeric processing aid may be a polymer with a nitrogen
functionality,
such as a polyetheramine that has been reacted with methylchloride or salted
with a
polyacid or a triethanolamine-based polymer having a molecular weight up to
150,000.
[0026] The polymeric processing aid may be a polymer selected from a homo
polymer,
a co-polymer, a salt or partial salt of these polymers, and combinations
thereof, wherein
the polymer includes a functional group selected from hydroxyl, carboxylic,
amine, or
combinations thereof. The polymeric processing aid may also be a polymer based
on
monomers of malefic anhydride.
[0027] In further embodiments, the liberation is performed at a pH between
about 7 and
about 10, between about 8 and about 9, between about 8.2 and about 8.7, or
between
about 8.2 and about 8.5. Similarly, the method may include adjusting the pH of
the
aqueous mixture to between about 8 and about 9.
[0028] Optionally, the polymeric processing aid is added into a pipeline
transporting the
aqueous mixture andlor into a separation vessel. The method may further
include
supplying a transfer agent to the aqueous mixture. An exemplary transfer agent
may be
selected from a caustic, a phosphate, a silicate, a carbonate, and
combinations thereof.
Examples of suitable caustics include sodium hydroxide, potassium hydroxide,
and
combinations thereof.
[0029] The foregoing and other objects, features and advantages of the
invention will be
apparent from the following more particular description of a preferred
embodiment of the
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
invention, as illustrated in the accompanying drawing wherein like reference
numbers
represent like parts of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0030] FIG. 1 is a schematic process flow diagram of a simplified embodiment
of a
bitumen recovery process.
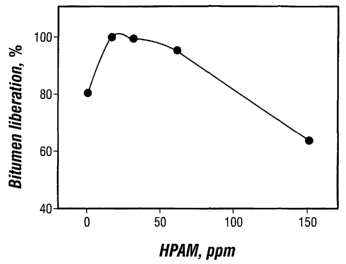
[0031 ] FIG. 2 is a graph of bitumen liberation at various HPAM dosages.
[0032] FIG. 3 is a graph of cumulative bitumen recovery after 50 minutes
flotation at
various HPAM dosages.
[0033] FIG. 4 is a graph of initial settling rate of the tailings flurry at
various HPAM
dosages.
[0034] FIG. 5 is a graph of the concentrations of calcium ions (Ca++) and
magnesium
ions (Mg++) in Aurora process water showing the concentrations without
treatment and
after treatment with various concentrations of HPAM.
[0035] FIG. 6 is a graph of bitumen recovery percentage as a function of the
flotation
time using 50 ppm citric acid.
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
[0036] The present invention is a method for recovering hydrocarbons. The
method
comprises contacting a hydrocarbon-containing source with a processing aid,
and
separating the hydrocarbon-containing source into a hydrocarbon-containing
froth portion
and a tailings portion. Preferably, the hydrocarbon-containing source is
separated in the
presence of water and a transfer agent. The hydrocarbon-containing source is
contacted
with the processing aid before, during and/or after the primary separation,
but the contact
most preferably occurs before the primary separation in order to increase
bitumen
11
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
recovery. The contact may even occur before or during an optional
hydrotransport step
used to move hydrocarbon-containing slurries (such as oil sand slurries) to an
extraction
plant. Providing the processing aid early in the process stream allows for
better mixing
and longer contact time with the hydrocarbons, resulting in greater bitumen
recovery
effectiveness of the processing aid.
[0037] The processing aids of the present invention include polymeric
compounds or
agents, non-polymeric compounds or agents, and combinations thereof. The
advantages
or disadvantages of the various polymeric and non-polymeric processing aids
may dictate
their individual use under certain processing conditions or hydrocarbons
sources, or
benefit from their combined use in any beneficial ratios or amounts. At the
present time,
it has been confirmed that the polymeric processing aids not only improve
bitumen
recovery, but also improve the settling of fines in the tailings. Other
advantages or
benefits of polymeric or non-polymeric processing aids may be discovered over
time that
may provide a basis for using one or the other, using mixtures of one or the
other, using
mixtures of both, or optimizing the ratios of components in a mixture.
[0038] The non-polymeric processing aids of this invention include, for
example, any
one of the following chemical families:
I . Carbohydrates, including polysaccharides, or organic alcohols such as
sorbitol,
mannitol, etc., or organic amines such as ethylene diamine or benzotriazole,
etc.
2. Organic amines such as ethylene diamine, etc., or hydroxy amines such as
triethanolamine, etc., or aromatic amines such as 2,2'-dipyridyl, etc., or
derivatives or salts of the amines.
3. Organic acids such as acetic acid, malonic acid, citric acid, isocitric
acid, etc.,
or hydroxy organic acids, such as glycolic acid, malic acid, gluconic acid,
glucoheptonic acid, tartaric acid, etc., or unsaturated hydroxy organic acids,
such
as malefic acid, ferulic acid, etc., or aromatic hydroxy acids such as
salicylic acid,
or complex mixtures of organic acids such as humic acid, vulvic acid, etc., or
polymeric acids such as polyacrylic acid, polymethacrylic acid or polymers
based
12
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
on monomers such as malefic anhydride, etc, or any of the salts, or partial
salts of
these acids, with cations such as sodium, potassium, etc.
4. Carboxylic acid derivatives of nitrogen containing organic compounds, such
as
glycine, or such as nitrilotriacetic acid, NTA, or such as
ethylenediaminetetraacetic acid, EDTA, or such as
diethylenetriaminepentaacetic
acid, DTPA, or such as dicarboxymethyl glutamic acid, etc., or cyclic
derivatives
such as diaminocyclohexane-N,N,N',N'-tetraacetic acid, CDTA, etc., or ether
derivatives such as ethylenebis(oxyethylenenitrilo)tetraacetic acid, EGTA,
etc., or
hydroxy containing derivatives such as hydroxyethyliminodiacetic acid,
hydroxyethylethylenediaminetriacetic acid, HEDTA, etc., or any of the salts,
or
partial salts of these acids, with cations such as sodium, potassium, etc.
5. Phosphoric acid derivatives or salts, such as sodium triphosphate,
potassium
triphosphate, sodium polyphosphate, disodium dihydrogenpyrophosphate, etc.
6. Organophosphonates such as methylphosphonic acid, etc., or hydroxy
containing derivatives such as hydroxyethylidine diphosphoric acid, etc. or
any of
the salts, or partial salts of these acids, with cations such as sodium,
potassium,
etc.
7. Organophosphonates based on nitrogen containing organic compounds such as
amino tri(lower alkylene phosphonic acids), such as aminomethanephosphonic
acid, AMPH, nitrilotris(methylene)triphosphonic acid, ATMP,
ethylenediaminetetra(methylenephosphonic acid), EDTMP, diethylene triamine
penta(methylene phosphonic acid), DTPMP, hexamethylene-
diaminetetra(methylenephosphonic acid),etc., or hydroxy containing
derivatives,
such as
8. Organic compounds containing both carboxylic and phosphonic functional
groups, such as 2-phosphonobutane 1,2,4-tricarboxylic acid, PBTC, etc., or any
of
the salts, or partial salts of these acids, with cations such as sodium,
potassium,
etc.
9. Homo polymers containing hydroxyl, or carboxylic or amine functional groups
or co-polymers containing any combination of these functional groups, or
13
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
mixtures of these polymers or any of the salts, or partial salts of these
polymers,
with cations such as sodium, potassium, etc.
10. Sulfur containing organic acids such as 2,3-dimercapto-1-propanol, etc.,
or
sulfur containing aromatic hydroxy acids such as sulfosalicylic acid, etc., or
sulfonates, such as lignosulfonate or any of the salts, or partial salts of
these
polymers, with cations such as sodium, potassium, etc.
11. Oxyalkylate derivatives of amines such as tetraethoxyethylenediamine, etc.
[0039] However, a presently preferred processing aid includes ethylene diamine
tetraacetic acid or a salt thereof, citric acid or a salt thereof, or
combinations thereof.
Furthermore, it is believed that the chelating agents disclosed in U.S. Patent
3,330,757,
which patent is incorporated by reference herein, may be effective as
processing aids
when used in accordance with the present invention.
[0040] The polymeric processing aids include, without limitation, the
following:
1. Acrylic acid-based polymers, such as polyacrylic acid, and polymers based
on
substituted polyacrylic acids, such as polymethacrylic acid.
2. Acrylamid-based polymers, such as polyacrylamides, and polymers based on
substituted acrylamides, such as polymethacrylamid.
3. Copolymers based on acrylic acid, acrylamide and/or acrylonitrile, or
substituted
derivatives of these monomers, or other polymers that hydrolyze to this family
under
conditions of application, such as polyacrylamides and polyacrylonitriles.
4. Derivatives of polymers and copolymers based on acrylic acids, acrylamides
and
acrylonitriles, such as sulfonates, phosphates, phosphonates, betaines, and
sulfobetaines.
5. Salts of derivatives of polymers and copolymers based on acrylic acids,
acrylamides and acrylonitriles, such as amine salts, quaternary salts,
phosphonium salts,
and thiouronium salts.
6. Polyacrylic acids and polymethacrylic acids manufactured using
hypophosphorous acid as a chain transfer agent. This leads to hypophosphite in
the
polymer backbone.
14
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
7. Modified polyacrylamides and polymethacrylamides, such as those modified
with formaldehyde/bisulfite or hydroxylamine. If modified with
formaldehyde/bisulfite, it
will form a "methylene sulfonate" modified polyacrylamide. If modified with
hydroxylamine, it will form a hydroxamic acid modified polyacrylamide. Some
acrylate
is formed in the modification process.
[0041 ] One preferred polymeric processing aid is a polyacrylamide. A
polyacrylamide
can be prepared by the reaction of polyacrylonitrile with water. One suitable
polyacrylamide has an average molecular weight ranging from 1,000,000 to
50,000,000,
preferably between 5,000,000 to 20,000,000, with about 20 to 30 percent ionic
character.
PERCOL 727 from Ciba Specialty Chemicals (New York) is an example.
Polyacrylamides form polycarboxylic acids (polyacrylicacid) at alkaline pH and
can
serve as a chelating agent. Furthermore, this hydrolysed polyacrylamide
polymer
(HPAM), namely PERCOL 727, is well suited as an aid for bitumen recovery as
well as
for solids settling from the produced tailings slurry.
[0042] Other polymeric processing aids include polymers with nitrogen
functionalities,
such as polyetheramines that have been reacted with methylchloride or salted
with a
polyacid. Suitably, a triethanolamine-based polymer having a molecular weight
up to
150,000 can serve as a carrier for citric acid.
[0043] Further still, the processing aids may be or include precursors to
cation-
sequestering polymers, where the processing conditions are suitable to
transform the
precursor into the desired cation-sequestering polymer. For example, the
alkaline
conditions typically present within the hydrotransport line can cause changes
in the
composition or polymerization. Accordingly, the processing aids of the
invention are not
limited to those aids having a composition that is directly capable of
sequestering cations,
but specifically further includes those aids that will be transformed into
cation-
sequestering agents under conditions prevailing in the hydrotransport line or
other
hydrocarbon processing operations. For example, when polyacrylamides are
supplied to
the hydrotransport line, the polyacrylamides form polycarboxylic acids
(polyacrylicacid)
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
at alkaline pH, which typically exists because of the use of an alkaline
transfer agent such
as sodium hydroxide. Accordingly, the polycarboxylic acids serve as a
chelating agent
that improves hydrocarbon recovery.
[0044] In addition to improving hydrocarbon recovery, polymeric processing
aids
provide the benefit of flocculating fine inorganic particles in the tailings
stream. This
flocculation results in faster settling of solids and improved recovery of
water. In order to
accomplish this flocculation, the processing aids may be provided directly to
the tailings
stream. However, it is preferable to supply polymeric processing aids into the
process as
disclosed above with respect to all processing aids, such as before the
primary separator.
By supplying polymeric processing aids early in the process, the polymeric
processing
aids can chelate troublesome cations to improve the primary separation of
bitumen or
other hydrocarbons from inorganic solids. Any unreacted portion of the
polymeric
processing aids remains in the process stream and can subsequently serve as a
flocculating agent in the tailings stream. In this manner, the polymeric
processing aids
may serve dual purposes to a greater extent than other processing aids
described herein.
The polymeric processing aids, as well as their chelated calcium or magnesium
cations
and flocculated mineral solids, will separate out in the tailings stream of
the separator.
[0045] The processing aids may be formulated with other agents, additives or
solvents.
Optionally, the processing aid may be provided as an aqueous solution, such as
a 50
weight percent solution of citric acid in water. Preferred additives for use
in cold weather
or climates include antifreeze, such as methanol. Accordingly, an aqueous
solution
containing the processing aid may further include between 10 and 15 %
antifreeze.
[0046] The processing aids are particularly beneficial when they make contact
with the
hydrocarbon slurry before or during the primary separation, although the
agents are also
beneficial in any secondary separations and any further separations (tertiary,
etc.) of the
hydrocarbons from sand, clay and other inorganic materials when the
hydrocarbons do
not efficiently separate from the inorganic solids. However, it should be
emphasized that
the method may be used to avoid the loss of hydrocarbons in a tailings or
waste stream
16
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
and that using the processing aid at appropriate injection points will improve
the overall
hydrocarbon recovery efficiency, as well as processability. The processing
aids may also
be used to treat middlings, which may include both hydrocarbons and sand,
although the
volume of primary middlings may be reduced if the processing aid is used
before or
during the primary separation. It should be recognized that the processing
aids described
herein may be introduced to the oil sands in various manners. For example, the
processing aids may be injected directly into an aqueous oil sand stream, or
mixed with
other processing aids, such as transfer agents. In one embodiment, it is
preferably to
inject the processing aid into the process at a point located before the air
is introduced
into the transport pipeline. Specifically, it is anticipated that the
processing aids can be
beneficially used in contactors, hydrotransport lines, tumblers, primary
separators and
secondary separators. Other processing vessel types and processing stages may
also
benefit from the use of these processing aids.
[0047] The addition of the processing aid into the process may be achieved
manually or
automatically, using batch, intermittent or continuous processes, and other
techniques
known in the art. It is preferred to provide the processing aid automatically
using a
process control scheme. For example, the process control may include
determining an
amount of hydrocarbon in the tailings portion, and varying the amount the
processing aid
added to the hydrocarbon-containing source to control the amount of
hydrocarbons in the
tailings portion. This type of process control can be accomplished with an
analog or
digital microcontroller or computer-based process control system having an
input signal
for the hydrocarbons in the tailings and an output signal to a flow control
valve providing
the processing aid into the process. The input signal may be from a detector
that
measures the fluorescence of the tailings as an indicator of hydrocarbon
content. Such a
detector may provide continuous detection by placing the detector in
fluorescent
communication with the process stream, or provide periodic detection by taking
samples
out of the process stream.
[0048] The term "control" as used herein means that one factor is used to
exert some
effect over another factor, and is not limited to a definite and known
relationship between
the factors. Accordingly, it is recognized that the use of a processing aid to
"control"
17
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
hydrocarbon-content in a tailings portion does not mean that a given amount of
the
processing aid will necessarily produce a direct, measurable and known change
in the
hydrocarbon-content, since other factors may change in a manner that offsets
the use of
the processing aid. For example, a good processing ore having a low calcium or
magnesium ion concentration may easily separate to produce a tailings portion
having
little or no hydrocarbons even without use of a processing aid. By contrast, a
problem
ore having a higher calcium or magnesium ion concentration may produce a
tailings
portion having significant hydrocarbon content. While the use of a processing
aid will
reduce the hydrocarbons in the tailings portion of the problem ore, the
hydrocarbon-
content may still be higher than in the tailings of the good processing ore
without using a
processing aid. Still, the hydrocarbon-content in the tailings portion when
processing a
problem ore will be reduced with use of a processing aid relative to the
hydrocarbon-
content in the tailings portion when processing the same problem ore without a
processing aid.
[0049] The concentration of processing aids used may vary accordingly to the
type and
condition of the hydrocarbon source being processed and the specific
processing aids)
being used. However, a processing aid is preferably added in an amount
effective to
improve hydrocarbon recovery. Preferably, the processing aid is provided into
an
aqueous slurry containing tar sands at a concentration between 1 and 150 parts
per
million (ppm) based on the slurry. The processing aid concentration in the
slurry is more
preferably between 10 and 100 ppm and most preferably less than 100 ppm, such
as
between 15 and 60 ppm. Concentrations exceeding 100 ppm have not presently
been
found to provide any additional recovery of bitumen, but may actually recover
less
bitumen than with 100 ppm or less of processing aid. It should be recognized
that the
processing aids) may be used in sufficient concentrations to provide improved
hydrocarbon recovery as well as improved consolidation of solids in the
tailings.
[0050] Without being limited to a particular theory, it is believed that the
processing
aids of the present invention are effective to improve hydrocarbon recovery
because of
the ability of the aids to associate with canons in the hydrocarbon-containing
source, such
as calcium, magnesium, iron, alumina, silica, titanium, zirconium, and
combinations
18
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
thereof. It is considered that these cations (positively charged) may be
responsible for
attracting both hydrocarbons and sand or clay particles (having a slightly
negative surface
charge) due to a charge association. By introducing a processing aid that
associates with
these cations, the charge association can be broken so that the hydrocarbons
can more
easily separate from the sand or clay. The exact mechanism of the attraction
between the
processing aid and the cations has not been determined, and is therefore
referred to as an
"association." This association is intended to encompass partial associations
and perhaps
chelation.
[0051 J The terms "oil," "bitumen," and "hydrocarbons" are used
interchangeably herein
to identify the hydrocarbon content of the tar sand, oil shale, crude oil or
other petroleum
source or residue. These terms encompass hydrocarbons based on carbon chains
or rings
and also containing hydrogen with or without oxygen, nitrogen, sulfur or other
elements,
regardless of the color, viscosity, or condition of the hydrocarbons. These
carbon chains
or rings specifically encompass functional groups selected from alkyl, aryl,
alkenyl and
combinations thereof.
[0052] The hydrocarbon-containing source may be various types of materials.
For
example, the source may be oil sand (also referred to as "tar sand"), oil
shale, petroleum
residues, and combinations thereof. The hydrocarbon-containing sources are
typically
mixtures of hydrocarbons and inorganic materials, such as sand, clay or rock.
The
process of the invention may also be beneficial in enhancing the recovery of
other
hydrocarbons that require separation from a mixture with inorganic substrates
or
particulates. Furthermore, the terms "oil," "bitumen," and "hydrocarbons" are
used
interchangeably herein to broadly identify the hydrocarbon content of the tar
sand, oil
shale, or other petroleum source or residue. These terms encompass
hydrocarbons based
on carbon chains or rings and also containing hydrogen with or without oxygen,
nitrogen,
sulfur or other elements, regardless of the color, viscosity, or condition of
the
hydrocarbons. These carbon chains or rings specifically encompass functional
groups
selected from alkyl, aryl, alkenyl and combinations thereof.
19
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
[0053] The processing aids may be suitable for use in various hydrocarbon
recovery
processes. Even in the extraction of hydrocarbons from oil sands, there are
various
processes, equipment and conditions that can be used, each process having its
own
advantages and disadvantages. For example, there are hot water extraction
processes and
solvent extraction processes. The processing aids disclosed may also prove
beneficial in
hydrocarbon recovery processes not mentioned herein. It is anticipated that
the
processing aids may find use in a variety of applications, especially where
hydrocarbons
must be separated from sand or other inorganic substrates or particulate
components.
Furthermore, it is believed that the processing aids would improve interface
control in
treaters, specifically for heavy crude oils containing asphaltenes and
naphthenic acids.
[0054] However, many of the separation processes include a hot water
separation that is
at least similar to that described by Tipman in U.S. Patent 5,876,592 as the
Clark Hot
Water Extraction Process (discussed above). Accordingly, one particularly
preferred
embodiment of the invention provides for the use of the processing aids in the
hot water
extraction process. It is an important discovery that processing aids will
enhance the
separation of hydrocarbons from sand to provide improved hydrocarbon yields
and
reduce environmental hazards associated with handling the tailings.
[0055] The most preferred transfer agents are caustics, such as sodium
hydroxide,
potassium hydroxide or combinations thereof. However, a suitable transfer
agent may
include a phosphate, such as sodium tripolyphosphate, tetrasodium
pyrophosphate,
trisodium phosphate, sodium hexametaphosphate and condensed phosphates. A
further
suitable transfer agent is sodium silicate or silicated having SiOZ:Na20
moduli of less
than or equal to one. Still other chemicals that may be used as transfer
agents includes
sodium bicarbonate, sodium carbonate, sodium sulphide, sodium hydrosulphide,
sodium
cyanide, sodium hydroxide with carboxymethylcellulose, ammonium hydroxide (and
similar salts of other alkali metal canons, e.g. potassium).
[0056] In another embodiment, the processing aid may be used with lower
concentrations of a transfer agent than is typically used, possibly even in
the absence of a
transfer agent. Reductions in the use of a transfer agent may be possible if
the beneficial
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
effects of the transfer agent can be achieve with the processing aids at a
lower cost of
operation.
[0057] FIG. 1 is a schematic process flow diagram of a simplified embodiment
of a
bitumen recovery process 10. Oils sand ores are removed from natural deposits
and
crushed. The crushed oil sand ore 12 is contacted with water and an optional
transfer
agent to form a slurry that will typically be passed through a hydrotransport
line to a
hydrocarbon recovery facility. A processing aid is also added to the slurry,
preferably at
the upstream end of the hydrotransport line. At the hydrocarbon recovery
facility, air is
injected into the slurry in association with a primary separation 14 of the
hydrocarbon
from the sand or other inorganic material, such as clay. During the primary
separation
14, the components of the oil sand ore are separated into froth, middlings and
tailings.
The froth comprises hydrocarbons attached to the surfaces of air bubbles,
typically
accompanied by small but undesirable amounts of water and solids. The tailings
comprise inorganic material, such as sand and clay, along with water. However,
the
tailings may also include a significant amount of hydrocarbons. The middlings
typically
comprises less hydrocarbon, more water and more solids than the froth, yet
more
hydrocarbon, less water and less solids than the tailings. While the froth,
middlings and
tailings commonly undergo further separation processes 16,18,20, respectively,
in order
to increase the overall hydrocarbon recovery efficiency and to increase the
quality or
purity of each stream. For example, it is desirable to further process the
hydrocarbons
without water or solids, reuse the water without high solids content and
dispose of the
inorganic materials without excessive amounts of water. These objectives are
achieved
while avoiding large capital investments, avoiding further processing steps,
and
maintaining or increasing processing capacity through the use of the present
processing
aids. Specifically, the processing aids serve to increase bitumen separation
from organic
material leading to greater bitumen content and less solids in the froth. The
processing
aids separate out into the tailings where the aids improve consolidation of
the solids from
the water. The improved quality of the froth and the tailings may result in
higher process
capacities or throughput for a given processing facility.
21
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
[0058] Example 1 - Bitumen yields using a pol~rmeric hydrolysed polyacrylamide
rocessin~ aid.
[0059] Bitumen extraction experiments were conducted in a laboratory
hydrotransport
extraction system capable of simulating commercial production conditions. Two
CCD
(charge coupled device) cameras were used for on-line monitoring of bitumen
liberation
and bubble surface loading, respectively, and a computer automatically
recorded the
signals.
[0060] Samples of oil sands, called transition ore that is one of poor
processing ores, and
Aurora recycle process water were obtained from an Aurora commercial plant
operated
by Syncrude Canada Ltd. The bulk ore sample was homogenized, packed in 600 g
plastic
bags and stored in a freezer at -29°C to prevent oxidation. This ore
sample consisted of
9.2 wt% bitumen, 7.3 wt% water and 83.5 wt% solids. The solids contain 33%
clay fines
(less than 44 um in size). The water used for the bitumen extraction
experiments is called
Aurora process water. Atomic absorption spectrometer (AAS) analysis showed
that this
water contained 47 ppm calcium and 15 ppm magnesium. The water had a pH of
8.2.
[0061 ] A polymeric hydrolysed polyacrylamide, PERCOL 727, was purchased from
Ciba
Specialty Chemicals. Each polymer solution was prepared at 0.04 wt% with
deionized
water just before the extraction experiment. All polymer dosages were recorded
as mg of
polymer per liter of slurry. Unless otherwise stated, all bitumen extraction
experiments
were carned out with Aurora industrial process water at a slurry temperature
of 35° C.
[0062] For each bitumen extraction experiment, lkg oil sand was prepared and
fed into
the laboratory hydrotransport pipeline containing 3L Aurora process water pre-
heated to
around 35°C. Bitumen extraction usually involves two steps, bitumen
liberation and
bitumen flotation. Bitumen liberation takes place in the slurry conditioning
stage (oil
sands plus Aurora process water with chemicals added), which runs for 5 min
before air
is introduced into the pipeline. When the air is introduced into the slurry
through a
stainless steel needle with a blunt end and adjusted to 195 mL/min, bitumen
flotation and
flotation timing begin. Six froth samples were collected at times of 3, 10,
20, 30, 40 and
22
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
60 minutes. Pulp slurry pH values were measured by a pH meter and slurry
temperature
was measured by a digital thermometer.
[0063] To evaluate the effect of chemical addition on bitumen liberation from
the sand
grains, an interactive image analysis technique was used. A high-speed black
and white
CCD camera was mounted in front of a specially designed square section of the
glass
pipeline. Images of oil sand slurry were captured and analyzed by image
analysis
software (obtained from Sigman Scan Pro), which used grey scale intensity to
differentiate bitumen from the grayish background. The degree of "darkness" of
the
slurry was taken as an inverse measure of bitumen liberation degree from the
sand grains.
[0064] After each experiment, the weight percent of bitumen, solids and water
was
determined for both the froth and the feed samples. The froth bitumen recovery
i.e.
aerated bitumen, was calculated based on the ratio of bitumen content in the
froth to that
in the feed. This procedure gives bitumen recovery as a function of flotation
time. The
tailings slurry was used for tailings settling tests. Tailings water was taken
from tailings
slurry after standing and was used for AFM measurement and was analyzed by AAS
(Spectr AA 220FS) for calcium and magnesium concentrations.
[0065] Bitumen liberation plays an important role in bitumen extraction.
Generally,
bitumen liberation is controlled by the interaction between bitumen and sand
grain
surfaces. FIG. 2 shows a plot of bitumen liberation during the bitumen
extraction
experiments at various HPAM dosages. Compared to the case of no chemical
addition,
the use of HPAM significantly improved bitumen liberation degree from 80% to
almost
100% at a flotation time of 60 minutes using a low dosage, e.g., 15 or 30 ppm.
However,
bitumen liberation deteriorated beginning at 60 ppm and declined to 60% when
150 ppm
HPAM was added. So, a preferred HPAM dosage for bitumen extraction would be
limited to about 30 ppm or less from the standpoint of bitumen liberation. A
direct benefit
obtained from improved bitumen liberation is increased bitumen recovery.
[0066] When air is introduced and dispersed into the slurry in the form of air
bubbles
after conditioning (bitumen liberation), bitumen droplets and air bubbles
attach to each
other and bitumen flotation takes place. FIG. 3 shows the final bitumen
extraction results
23
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
after 50 minutes flotation at dosages of 0, 15, 30, 60 and 1 SO ppm. The
recovery is
represented by the cumulative bitumen recovery (total bitumen amount recovered
in
froth).
[0067] On the recovery curve, the cumulative bitumen recovery was found to
increase
with the addition of HPAM at low dosages, and a peak value of 84% recovery
appears at
about 30 to 40 ppm. This indicates an increase of about 20% in bitumen
recovery as
compared to the 64% recovery achieved without chemical addition. As the
polymer
dosage was increased further, the bitumen recovery decreased and dropped below
50%
when a much higher dosage of 150 ppm HPAM was added.
[0068] Example 2 - Tailings settling using a polymeric hydrolysed
polyacrylamide
rocessin~ aid.
[0069] Tailings samples taken directly from the bitumen extraction experiments
of
Example 1, with or without chemical addition, were used to conduct settling
tests in
closed cylinders. The descent of the solid/solution interface (mud line) was
recorded as a
function of time. A plot of the thickness of the supernatant layer versus time
was used to
determine the initial settling rate from the slope of the initial linear
portion of the plot.
[0070] A Nanoscope E atomic force microscope (AFM) with a fluid cell was used
for the
surface force measurements. Force measurements were performed in the fluid
cell where
a colloidal probe (a silica sphere or a clay fine particle) interacted with a
flat silica plate
or a bitumen surface in various tailings waters taken from bitumen extraction
experiments
as soon as a clean layer of water was available. All force measurements were
conducted
after an incubation time of 30 minutes at a room temperature 22~1 °C.
[0071 ] FIG. 4 shows the variation of the initial settling rate with HPAM
polymer dosage.
With no polymer addition as well as with a polymer dosage of 1 SO ppm, the
initial
settling rates were fairy low. A maximum in the settling rate occurred at
about 30 ppm
polymer dosage. FIG. 4 shows a large improvement in the slurry settling rate
due to an
optimal polymer dosage addition. Note that the HPAM dosage is refernng to the
amount
24
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
of HPAM that was added for bitumen liberation with no additional HPAM added
during
bitumen recovery. A distinct layer of clear supernatant was observed at HPAM
dosages
of 15 and 30 ppm, but no such clear supernatant was obtained at 150 ppm at a
settling
time of 150 minutes.
Example 3 - Effect of a polymeric hydrolysed polyacrylamide processing aid on
the
concentration of divalent cations in process water.
[0072] A sample of Aurora process water at a pH of 8.5 was obtained and
divided into
four equal aliquots. Three of the aliquots received a treatment with HPAM at
dosages of
15, 45 and 150 mg/L, respectively. The fourth aliquot was not treated. The
concentrations of calcium ions (Ca++) and magnesium ions (Mg++) in each of the
aliquots were measured using an Atomic Absorption Spectrometer and the results
are
plotted in the graph of FIG. 5. The untreated aliquot showed that the process
water had
initial calcium and magnesium ion concentrations of 39 ppm and 13 ppm,
respectively.
The treated aliquots showed a linear reduction of both calcium and magnesium
ions with
increasing HPAM concentration over the range investigated. The reduction of
calcium
and magnesium ion concentrations indicates ion intake by HPAM. It is believed
that this
ion absorption not only improves bitumen liberation, but leads to cation
bridging between
HPAM and fines in the tailings to improve settling.
Example 4 - Effect of citric acid on bitumen recover
[0073] A bitumen extraction experiment was conducted in accordance with
Example 1,
except that the processing aid was 50 ppm citric acid. FIG. 6 shows the
bitumen recovery
percentage as a function of the flotation time for up to 60 minutes. The
recovery is
represented by the cumulative bitumen recovery (total bitumen amount recovered
in
froth). On the recovery curve, the cumulative bitumen recovery was found to
increase
over time with the addition of 50 ppm citric acid. The peak recovery of about
90% was
obtained at 60 minutes of flotation. When the same experiment was run without
a
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
processing aid, the bitumen recovery was approximately 64%. This indicates an
increase
of about 26% in bitumen recovery attributable to the use of 50 ppm citric
acid. The
amount of solids and water in the recovered bitumen was also measured and the
bitumen-
to-solids ratio and the bitumen-to-water ratio were calculated and plotted on
the same
graph using the right-hand axis. These bitumen ratios increased over time with
the
addition of 50 ppm citric acid by about 50% and 25%, respectively.
Accordingly, citric
acid was found to increase the froth quality as well as the bitumen recovery
efficiency.
[0074] The significance of the bitumen recovery results (Example 1 ) and
tailings settling
results (Example 2) is that they reveal the surprising and beneficial effect
of HPAM as a
bitumen (hydrocarbon) processing aid as well as a solids flocculant that
accelerates the
tailings slurry settling rate.
[0075] Aside from the successful results of Examples 1 and 2, other polymeric
processing aids disclosed herein may enhance both bitumen recovery and solids
settling
derived from tailings streams. It is also possible that certain polymeric
processing aids
would also enhance froth treatment through demulsification of the water
droplets in the
diluted bitumen.
[0076] The terms "comprising," "including," and "having," as used in the
claims and
specification herein, shall be considered as indicating an open group that may
include
other elements not specified. The term "consisting essentially of," as used in
the claims
and specification herein, shall be considered as indicating a partially open
group that may
include other elements not specified, so long as those other elements do not
materially
alter the basic and novel characteristics of the claimed invention. The terms
"a," "an,"
and the singular forms of words shall be taken to include the plural form of
the same
words, such that the terms mean that one or more of something is provided. For
example,
the phrase "a solution comprising a hydrocarbon-containing compound" should be
read
to describe a solution having one or more hydrocarbon-containing compound. The
term
"one" or "single" shall be used to indicate that one and only one of something
is
intended. Similarly, other specific integer values, such as "two," are used
when a specific
26
CA 02535702 2006-02-13
WO 2005/028592 PCT/CA2004/001727
number of things is intended. The terms "preferably," "preferred," "prefer,"
"optionally,"
"may," and similar terms are used to indicate that an item, condition or step
being
referred to is an optional (not required) feature of the invention.
[0077] It should be understood from the foregoing description that various
modifications and changes may be made in the preferred embodiments of the
present
invention without departing from its true spirit. It is intended that this
foregoing
description is for purposes of illustration only and should not be construed
in a limiting
sense. Only the language of the following claims should limit the scope of
this invention.
27