Note: Descriptions are shown in the official language in which they were submitted.
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
USES OF SPATIAL CONFIGURATION TO MODULATE PROTEIN FUNCTION
The application disclosed herein claims priority of U.S.
Serial No. 60/498,449, filed August 28, 2003; U.S. Serial
No. 60/498,785, filed August 28, 2003; and U.S. Serial No.
60/498,923, filed August 28, 2003. This application claims
priority of Indian Application No. 279/MUM/2004, filed
March 5, 2004, and Indian Application No. 280/MUM/2004,
filed March 5, 2004. The contents of the preceding
1 0 applications are hereby incorporated in their entireties by
reference into this application.
Throughout this application, various publications are
referenced. Disclosures of these publications in their
1 5 entireties are hereby incorporated by reference into this
application in order to more fully describe the state of
the art to which this invention pertains.
BACKGROUND OF THE INVENTION
2 0 The completion of the human genome project verified the
therapeutic effects of many genes, and some of them have
been developed into therapeutic proteins, but most of them
cannot be controlled by gene or protein techniques in the
art. They cannot be correctly translated into proteins
2 5 which maintain the whole therapeutic effects possessed by
their genes. The biggest obstacle on the road to successful
protein translation is the correct protein-folding. The
field of research on how to obtain a protein with efficient
spatial configuration is filled with competition.
Changing the spatial configuration of proteins without
disturbing amino acid sequence may change functions of
certain proteins. For example, some proteins with abnormal
3-dimensional structure can cause diseases in humans and
3 5 animals, such as: bovine spongiform encephalopathy (BSE),
Alzheimer's Disease, cystic fibrosis, familial
hypercholestrolacemia, familial amyloid disease, certain
carcinoma or cataract. These diseases also have been
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
cabled fo~:~.i~~-diseases". The °Prion" protein causes BSE
and can infect normal proteins and transmit among them.
During the research of protein structure, most researchers
consider that the most important part in retrieving the
correct spatial structure of proteins are the techniques of
denaturation and refolding. Masses of literature reported
improvement in refolding associated with various chaperons
or reverse micelles, etc. Many secretion expression vectors
1 0 have been developed to allow those proteins expressed in
more natural environments, but all these efforts only
result in an increase in the yields of proteins, not in
qualitative changes.
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
DETA2T~EI7 DE'S~lfIPT'I'f~~1' OF THE FIGURES
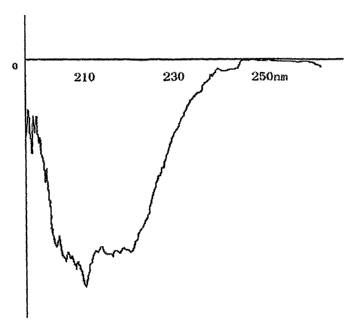
Figure 1. Circular Dichroism spectrum of Infergeri
Spectrum range: 250nm - 190nm
Sensitivity: 2 m°/cm
Light path: 0.20 cm
Equipment: Circular Dichroism J-500C
Samples :contain 30~1g/ml IFN-conl, 5.9 mg/ml of NaCl and
3.8 mg/ml of Na2P04, pH7Ø
Figure 2. Circular Dichroism spectrum of rSIFN-co
Spectrum range: 250nm - 190nm
Sensitivity: 2 m°/cm
Light path: 0.20 cm
Equipment: Circular Dichroism J-500C
Samples :contain 30~1g/ml rSIFN-co, 5.9 mg/ml of NaCl and
3.8 mg/ml of Na2P04, pH7Ø
Figure 3. Comparison of Inhibition Effects of Different
2 0 Interferons on HBV Gene Expression
Figure 4A-1. Curves of Changes of Body Temperature in Group
A (5 patients)
This figure is the record of body temperature changes of 5
patients in Group A.
Figure 4A-2. Curves of Changes of Body Temperature in Group
A (6 patients)
This figure is the record of body temperature changes of
the other 6 patients in Group A.
Figure 4B-1. Curves of Changes of Body Temperature in Group
B (5 patients)
This figure is the record of body temperature changes of 5
patients in Group B.
4 0 Figure 4B-2. Curves of Changes of Body Temperature
- 3 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
irt G~o~p~ ~ ~"~ p~a'ti'~rlts)
This figure is the record of body temperature changes of
the other 5 patients in Group B.
Figure 5. rsIFN-co Crystal I
Figure 6. rsIFN-co Crystal II
Figure 7. The X-ray Diffraction of rsIFN-co Crystal
- 4 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
DEV''A~~'ED' 'DE'S'CI~'~'i~'~~~TN OF THE INVENTION
This invention provides a set of methods for modulating
protein spatial configuration. First, select the amino-acid
codon for encoding the target protein according to host
codon usage. Second, choose combinations which can modulate
the spatial configuration and construct into different
vectors which can transfect a series of hosts. Therefore,
an appropriate vector with appropriate host may be chosen.
Third, choose the vector promoter by monitoring a
1 0 combination of base pairs after combining the code sequence
of the promoter and the target protein. Finally, choose the
appropriate expression host to express the target protein,
refold and purify, measure the activity and spatial
configuration.
This invention discovered that during the protein-
constructing process, the variation of codon that encodes
the amino acid of target protein, the difference of
choosing vectors, the modulation of the promoter and the
2 0 selection of host expression vector, even conditions of
denaturation and renaturation, agents etc. are all
adjustable factors for modulating the spatial configuration
of target proteins. Accordingly, modulation of the spatial
configuration of proteins to obtain new functions and to
2 5 improve activity is the result of systematic analysis.
This invention provides a method for modulating the
function of proteins without changing the primary amino
acid sequence of said protein comprising steps of: a)
3 0 altering the codon usage of said protein; b) expressing the
protein using the altered codon to obtain purified protein;
and c) comparing the expressed protein with altered codon
usage to one without, wherein an increase in function or
identification of new function indicates that the function
3 5 of the protein has been modulated.
In an embodiment, the altered codon usage results in high
expression of said protein.
- 5 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
This invention also provides a method for preparing protein
with enhanced or new functions without changing the primary
amino acid sequence of said protein comprising steps of: a)
altering the codon usage of said protein; b) expressing the
protein using the altered codon to obtain purified protein;
and c) comparing the expressed protein with altered codon
usage to one without, wherein an increase in function or
identification of new function indicates that a protein
1 0 with enhanced and new function has been prepared.
In an embodiment, the altered codon usage results in high
expression of said protein. This invention also provides
the protein prepared by the above method. In an embodiment,
the protein has unique secondary or tertiary structure.
This invention further provides a synthetic gene with
altered codon, which, when expressed, produces enhanced or
new functions. In an embodiment, the invention provides a
2 0 vector comprising the gene. In a further embodiment, this
invention provides an expression system comprising the
gene. In yet a further embodiment, this invention provides
a host cell comprising the gene.
This invention also provides a process for production of a
protein of enhanced function or new function comprising
introducing an artificial gene with selected codon
preference into an appropriate host, culturing said
introduced host under appropriate conditions for the
3 0 expression of said protein, and harvesting the expressed
protein. ,
This invention provides the above process, wherein the
artificial gene is operatively linked to a vector. In an
3 5 embodiment, the process comprises extraction of the protein
from fermentation broth, or collection of the inclusion
body, and denaturation and renaturation of the harvested
- 6 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
pr'r~~t'~ ~'~. .
This invention also provides the protein produced by any of
the above processes.
This invention provides a composition comprising any of the
above proteins and a suitable carrier. This invention
further provides a pharmaceutical composition comprising
any of the above produced proteins and a pharmaceutically
acceptable carrier.
One significance of this invention is that it modulates the
spatial configuration of protein during the process of
translating genes with therapeutic effects into proteins
1 5 which possess functions originating from the genes, or
functions not seen in proteins produced using traditional
techniques, or even with improved activity compared with
those existing proteins.
2 0 Taking the interferon as an example, construct the gene of
human IFN-a into reverse transcriptive expression vector to
produce PDOR-INF-a expression vector, then transfect 2.2.15
cell. HBsAg and HBeAg in the culturing supernatant of cell
is measured. The results indicate that the suppression rate
2 5 of rSIFN-co to HBsAg was 62o and 67.7% to HBeAg, but the
recombinant interferon protein produced by gene
recombination techniques do not have the effect in vitro.
In addition, the experiment of constructing the human INF-
a2 expression vector using the reverse trans~criptive viral
3 0 vector and transfecting it into HIV cell strain-A3.01
proved that IFN-a2 can completely restrain the replication
and transcript of HIV-DNA. However, the effect of
interferon is limited in the treatment of HIV disease.
3 5 This invention will be better understood from the examples
which follow. However, one skilled in the art will readily
appreciate that the specific methods and results discussed
are merely illustrative of the invention as described more
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
fu'il~y in the claims which follow thereafter.
Example 1:
CONFORMATION RECONSTRUCTION OF IFN-CONL
rSIFN-co is a new interferon molecule constructed according
to conservative amino acids in human IFN-a subtype with
genetic engineering methods. The interferon has been
described in United States Patent Nos. 4,695,263 and
4,897,471, and has been proven in literature and patents to
have broad-spectrum interferon activity with strong
antiviral, anti-tumor and natural cell-killing effects.
The DNA coding sequence was redesigned according to E.
Coli. codon usage by first constructing an insert into pHY-
4 vector, mediating down-stream expression with PBAD
promoter, then choosing E. Coli. as host. The high-purity
products are gained by denaturation with 6 mol/L guanidine
hydrochloride --> renatured with 4 mol/L arginine .-> purified
2 0 with Cu2+-chelating affinity chromatography after POROS HS/M
canon exchange chromatography.
The comparison test of duplicates of hepatitis B virus DNA
and secretion of HBsAg and HBeAg inhibition between rSIFN-
2 5 co and IFN-cons proved that rSIFN-co has the effect of
inhibiting the secretion of HBsAg and HBeAg which is not
possessed by IFN-conl. In another test, the HBV core/
pregenomic(C/P) promoter and associate cis-acting element
were placed upstream of luciferase-encoding plasmid. This
3 0 reporter construct was transfected into HpeG2 cells. The
cells were treated with different interferons and
luciferase reporter gene expression was measured. Results
show that rSIFN-co can suppress 680 of luciferase reporter
gene expression; whereas IFN-conl and IFN-a2b only suppress
3 5 35% and 270 of it. Therefore, the suppression effect of
rSIFN-co on HBcAg has been obviously improved.
_ g _
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
Meanwhile, circular dichroism spectrum also proved there
are differences in the secondary structure of rSIFN-co by
comparison with IFN-conl.
The following are those comparison experiments in detail:
1) Comparison of circular dichroism spectrum
Address: The Center of Analysis and Test in Sichuan
University
Apparatus: J-500C Circular Dichroism equipment (spectrum
range: 250-190nm / sensibility . 2 m°/cm / light path:
0.2cm. (See Figure 1 and Figure 2.)
2) rSIFN-co inhibits HBV-DNA duplication and secretion of
HBsAg and HBeAg.
Materials
Solvent and Dispensing Method: Add 1m1 saline into each
vial, dissolve, and mix with MEM culture medium at
2 0 different concentrations. Mix on the spot.
Control drugs: IFN-a2b (Intron A) as lyophilized powder,
purchased from Schering Plough. 3x106U each, mix to
3x106IU/ml with culture medium; INFERGEN (liquid solution),
2 5 purchased from Amgen, 9~g, 0.3m1 each, equal to 9X106IU, and
mix with 9X106IU/ml culture medium preserve at 4°C; 2.2.15
cell: 2.2.15 cell line of hepatoma (Hep G2) cloned and
transfected by HBV DNA, constructed by Mount Sinai Medical
Center.
Reagent: MEM powder, Gibco American Ltd. cattle fetal blood
serum, HycloneLab American Ltd. G-418(Geneticin); MEM
dispensing, Gibco American Ltd.; L-Glutamyl, imported and
packaged by DING KE Chemical Ltd.; HBsAg and HBeAg solid-
3 5 phase radioimmunoassay box, Northward Reagent Institute of
Chinese Isotope Ltd.; Biograncetina, Northern China
Medicine; and Lipofectin, Gibco American Ltd.
- 9 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
Experimental goods and equipment: culture bottle, Denmark
TunclonTM; 24-well and 96-well culture board, Corning
American Ltd.; Carbon Dioxide hatching box, Shel-Lab
American Ltd.; MEM culture medium 100m1: loo cattle fetal
blood serum, 0.030 Glutamine, 6418 380~Zg/ml, biograncetina
50U/ml.
Method:
2.2.15 cell culture: Add 0.25% pancreatic enzyme into
culture box with full of 2.2.15 cell. Digest at 37°C for 3
minutes and add culture medium to stop digestion and
disperse the cells. Reproduce with a ratio of 1:3. They
will reach full growth in 10 days.
Toxicity test: Set groups of different concentrations and a
control group in which cells are not acted on with
medicine. Digest cells, and dispense to a 100,000 cell/ml
solution. Inoculate to 96-well culture board, 2001 per
well. Culture at 37°C for 24h with 5o CO2. Test when simple
2 0 cell layer grows.
Dispense rSIFN-co to 1.8x10'IU/ml solution then prepare a
series of solutions diluted at two-fold gradients. Add into
96-well culture board, 3 wells per concentration. Change
2 5 the solution every 4 days. Test cytopathic effect by
microscope after 8 days. Fully destroy as 4, 75o as 3, 50%
as 2, 25~ as 1, zero as 0. Calculate average cell lesions
and inhibition rates at different concentrations. Calculate
TC50 and TCO according to the Reed Muench method.
TC50 = Antilog (B + ~_~ x C)
A=log >50% medicine concentration; B=log<50o medicine
concentration; C=log dilution power
Inhibition test for HBeAg and HBsAg: Separate into
positive and negative HBeAg and HBsAg contrast groups, cell
contrast groups and medicine concentration groups.
- 10 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
~nwc'cil~te 7"~'l~', d00 cells/ml of 2 . 2 . 15 cell into 6-well
culture board, 3 ml per well, culture at 37°C for 24h with
5% COz, then prepare 5 gradiently diluted solutions with 3-
fold as the grade (Prepare 5 solutions, each with a
different protein concentration. The concentration of
Solution 2 is 3 times lower than that of Solution 1, the
concentration of Solution 3 is 3 times lower than that of
Solution 2, etc.) 4.5x106IU/ml, 1.5x106IU/ml, 0.5x106IU/ml,
0.17x1061U/ml, and 0.056x1061U/ml, 1 well per concentration,
1 0 culture at 37°C for 24h with 5% COz. Change solutions every
4 days using the same solution. Collect all culture medium
on the 8th day. Preserve at -200 Repeat test 3 times to
estimate HBsAg and HBeAg with solid-phase radioimmunoassay
box (Northward Reagent Institute of Chinese Isotope Ltd.).
1 5 Estimate cpm value of each well with a Y- accounting
machine.
Effects calculation: Calculate cpm mean value of contrast
groups and different-concentration groups and their
2 0 standard deviation, P/N value such as inhibition rate, IC50
and SI.
1) Antigen inhibition rate ( o) - A~B x 100
A
A = cpm of control group; B = cpm of test group;
2 5 2) Counting the half-efficiency concentration of the
medicine
Antigen inhibition IC50 = Antilog (B + ~ _ B x C)
A=log>50% medicine concentration; B=log<50~medicine
concentration; C=log dilution power
3) SI of interspace-conformation changed rSIFN-co effect on
HBsAg and HBeAg in 2.2.15 cell culture:
S I = TC50
IC50
- 11 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
4) ~'stimate the t~'i'f'ferences in cpm of each dilution degree
from the control group using student t test
Southern blot: (1) HBV-DNA extract in 2.2.15 cell: Culture
cell 8 days. Exsuction culture medium (Separate cells from
culture medium by means of draining the culture medium.).
Add lysis buffer to break cells, then extract 2 times with
a mixture of phenol, chloroform and isoamyl alcohol
(1:1:1), 10,0008 centrifuge. Collect the supernatant adding
1 0 anhydrous alcohol to deposit nucleic acid. Vacuum draw, re-
dissolve into 20~1TE buffer. (2) Electrophoresis: Add 6XDNA
loading buffer, electrophoresis on 1.5% agarose gel, IV/cm,
at fixed pressure for 14-18h. (3) Denaturation and
hybridization: respectively dip gel into HC1, denaturaion
1 5 buffer and neutralization buffer. (4) Transmembrane: Make
an orderly transfer of DNA to Hybond-N membrane. Bake,
hybridize and expose with dot blot hybridization. Scan and
analyze relative density with gel-pro software. Calculate
inhibition rate and IC50.
Results
Results from Tables 1, 2 and 3 show: After maximum
innocuous concentration exponent culturing for 8 days with
2.2.15 cell, the maxima is 9.0 ~ Ox106IU/ml average
2 5 inhibition rate of maximum innocuous concentration rSIFN-co
to HBeAg is 46.0~5.25% (P<OD001), IC50 is
4.54~1.32X106IU/ml, SI is 3.96; rate to HBsAg is 44.8~ 6.6%,
IC50 is 6.49~0.42x106IU/ml, SI is 2.77. This shows that
rSIFN-co can significantly inhibit the activity of HBeAg
3 0 and HBsAg, but that the IFN of the contrast group and
INFERGEN cannot. It has also been proven in clinic that
rSIFN-co can decrease HBeAg and HBsAg or return them to
normal levels.
- 12 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
W n .-t M ,~ ~Li m n m m rn
N V~N COif) O N N N rlO rl d'
./", L'.
.t-1 u1M c0d W O .u w N O V~~H r
O O
Itf M N riM d~\ Itf lD01r r 1f1lp
rl ri
u-i 01a'1r M ~ r1 O crr ~D00 M
.U A3
~ n M ~o~ w ~ o M ovr ,-tr
-w -,~
E w o w .-i r E umo M w m r
,Lt .o
-rl ,-1O COO ~ 01COr1tt>N
N ri
N
U W M O O N U LS1N vdO O ri
.~-. .~,'
.5..1 .lt
~
Q,' O O O O O lp Q,' O O O O O l0
--ri -r
f-1 i
W-1
fIf v-td' ODu-Iri to 01'W N '
ri N N ODO101 ri M N l0M V~
01O~ O O O ~ r L~111M r p
N dt 01V~~H ~ r O r O r
G o w InIntno ~ N r r o r V
G
U ttlN O O Q U'1 U ~DM N N O H
O O
t . . . . . i~ t
U U
.,.t rt
rl O ri N M V'H r-) O r-iN M W
f~ R'
J-1 .1.1
.,.t .,.t
.1.1 01 Q1 J.~ N Q1
fIS 0101 M ~p Id ODr M r CO
ri O N M 01 rl lt7Cl1COv-iCD
O1CD CO01 U N O10100tf1
Lr7GD O ch ~ N O1N 01N
d~M 4 O ~ N ~ 01O1N
U ? l!1N O M U ; tnN rt.-I
O p
A,' O O O O O A', O O d O O M
O O
1~ ~'
O O
.-~ a\r tor ~ -~t .-1m n ao G
v r m M ov O v to.-ttnM ao O
.l..t .u
(J7 O 01 CO01 ri t31 N O r-IN Ll1rl
rl .-I
((f ~"r InV~ 1J (6 N r M r N 1!
.Q .Q
H o M avo O H r trso~r N
,~ ,~
v v
Ci)N ePt'W-IO r! N M N O O rl rI
.C, .i;
.4.1 a.1
N ~ ~
N
o 0 0 0 0 .n. ~ o 0 0 0 o A
x
~ w m
O m t ~ ~ r
r~
~ 0 ~
x ''C1If1O1 ~ N W r r
~I sH1D O 1~iO\01ri01r
r1 rl
V ri M M N rf i1,-1.-iO O
rl r-1
~
3
~t ~ ~ E 0 0 0 0 0 ~ ~ H o 0 0 0 0
-r g
va
~ ~
r :0~~:~,-sc,
od
p M m x ~ o g N
p p ~ 2t tn O b a c
o v
,~O ~ a~ut o p G .-wnc m-t.i
O
-.aO M v~ o H O aoM aom o
,-t ~ ,-t
~ N ~ N O ~ M M O O N
-,iN 0 0 --riN o 0 0
co 0 0 0 oU co 0 0 0
3 g
~
N
'
t H 1: '~ ~ (,", tp
,
H litri r M
H
N r O O~ U tf1OD01O tit
.4!l001 10d\ xH' 1i N M t0N CO ,S,"
I1JM 01 OD,-i 1'".,O W d~dtr COW (""
H r1 rl
f-IW M f~1O It1'H ~I c~1N O O O f0
,-1 ri
W3 ~ ~ ~
O
0 0 0 0 o p~ G430 0 0 0 0 W
q
'Lf tom o w w ~ T1 0 0 0
S.i r 01 O N M , H d~t0M r n-1
rl ~".,ri
-r1 !Ho aDm o~ H ~-i ,-ir M u)oD
H f-I r-I
.C., G O N utW .4" r1d O O O
di N
H ,-i,-~,-i.-i.~ H r ov,-a.-i
3 3
O
''t 't~ 'C1
'N ('.. N N l0O O .("., O cr W
O lD00 O O r v-i O O CON .itV rl
r1 rf
U r O O M N O U dtr r ritJ7r
ri rl
N Q1N l!7U1N t0 N N r O rlM ri
N N
O u~ ao.-r,-t.1N ~ rn r r .-t~ rn .-1
3 3
rl
U
I
4-7 W ~
"'~
..n ri ~DD N r r r-l u1 ~D~O,-1V~r r-1
i-1 N ,-1N r .-1r-1 n-i O lf1W r 1O ri
n-1 !-1
rl
.~ o w ootno~v .~ r m o 0 o v
v v
Gu ofov o w-i.-aU fir r cv,-IriH U
3 3
O
,..
~
N ~
E
~ H M n ~ M ri
~ ~
a-I .t -1,-i 1
. J s
R 1.
~ N M n-1O
Q M r-161 "
.U
' ~ M r11J U M ei .
~ J
.1 ~'r O O p x
~ x
Si
ld O O O O M ,-iO O O O O M rl O
,i
H ~ 01M r-1M r1L3 C.7 01M r1M rl U
w ~"' '~
- 13 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
l fl' ri h h ~ m m
N r W 1 m M ~ N d~d~W M N D
f., O d rr t
~ J 1 N M M r W yl m D O m r il
O O ~ l l
0
( fS i M m M O r ~ IlO V~N r 01
rt rl
r t
ri N W -i'~m l r4 M -Sn M M -I
11 n t V i1 f t u
'.~' ~ t7O O\V~M "..l m N m t0N 01
-ri I ri
C~ r h N M ri01 ~ V~N fl'~riO
i M l ' O O
N ! !1~
rl M W 01D M ~ r N o 0 0 .-l
~ h w l o o v
~ ~
~
~
~ o 0 0 0 0
~
~ 0 0 0 0 o M ~ ~
~ .~
sa
(~ N lDM ~ td ~'r N N M
ri 0101N h a rl M V7rle-IN
rnw ~rr m ~ m m m o u~
r O 10l0m ~ M r w-1v-1lD
~' m 19'-IV'N O ~ ~-1O,~-1v-IO O
!~,
U 'd~O m r l0In U ti'1N H f-1r1U
O O
. . . ~.U '
rl O r!riN M H ri O riN M W H
Ff', A;
.13 .!~
riN l!1 1.l m ri
rlM N W ri fa h M d'01.01
-~ m r lflO lI1 w-i r rlM m UU
rlr!lD1Do1 ~ d~V~~ O O
-.la~.-tr r ~ M r w n in
h !nM m rl ~ 01O O O O
U M m ~r~ ri U w w rlo o
~ G
~
~
O O M O O O O O M
m h N M r1G' O M ~ ~ La"
~q N Q avh a tnO N tom a~ m O
.1-~ f ' .u
.~.Ol O cHO l001r1 t31 O 01M O ri
r1 .1 7
tIS N h d~01r .y.1 1~ l0litl0 t17.1.
.la ,Sa
O ~-i riN d~iDH ~ Sa m O 01 O
ri r~
N N
~ tt1W N O ri~ ~ tr1O O
D ~
~ y3
,~ rr; a o 0 0 'oS~ ,~ 0 0 0 0 o p
~ .~
~ S~
U
d~d'N tf1 riN r
Inm r ril0 r V1M 10
O m O O1m lf7W M N
T1 r h d~N vi 'Lth m d~ U1
~, ~1 l0r d~r N fi 01hm-I [-i
r-1 ri
,-Id~d'N r v-1 n-1st'N rl O
ri wl
'~ H ' 0 0
3
''~ ( o a o 0 0 ~ o o 0
-v
g
+~ .N ~
~
'Q S-1 N 01 N ~-1 M tf1N
'"i 01O r1rlc0 fY) 1t1ri'-t
~" .('.,'O t!1N O V~.01 ',.i,Fi'ClM h W
O ~ w w rnr N O s~ N rnh
m O N O r1O toulN
r-I
(ITM n-io N 1~ U V~N O
~
N
.i1CI~O O O O O O U ,QU1 O O O O O O
~ $
o
h 4
-c
W tDW 01l0 W Lf1riW
r rnM uio U toh o~
m ~ r
.u ~ o rnw rnx ~ .a.~ x
~ w ,-iovN o s~o m a~h w
.-r .-r
inw N ,-ao c0~ ~ w M ~
~
~
w~ o o o o o W .~ tr.3o o o o o W
.i1
.'.,
N O '~~ ~
ri O O 1 m ( ri N CO h ttl
0 ,~,
.-1 l(7lDN ~-1W H ri Q1~-iN W M
e-1 rl
.~ M 'MM lDtfi .~.' h M O M rl
N N
N Ol01r-~rlrl H InGo-i~-Iri
3 3
'O
m m W N N f~ N m d~
O 1PN N t-iM ~D O m W ri~DM
rl ri
U rilDN W 1D01 U 01M N M
ri n-1
~ N ll)O W r M 1D N rilt1v~iN ~-1
v Q1
O cn m .-lrl~ ri.-w n w m .a
3 3
i
yrn ~ Wm
~ o o r-i ~ w o o -t~-i
t o
H f,.i riM N M M ri l1 m lf1M
r1 ri
U1 .I ODO N if1h N rl h rim M N fU
Ul N
t~ h ,i~ '-lr-U w u~h m ~ .-lU
3 3
O
v o
.. ~i
~
:y M r-1 i 7 M r l
r~- I- .1.1 ri
..
'ClG M ~ O ~ M .-i
H ~ O
Ci N M r l 1 O M r'1
v I O S-1
O U M r l J U M .-1
O 1 H .4J
U ("., o O o ~ ,'F.' O o O
,-I X
N O O O O M r-1 O O O O M a O
X O
t4 U 0 1 - i .-I U o \ r-i H U
~- M M U '-' M M
- 14 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
'O M t'm-1 CO M tn 'Cf M ri 01
N W N N U1 M M N 01 M sN
G,' G,'
1-~ ~ ~"~~ ~ ~ a1 4~ N O r-i
O O
tp 01 O1 ri 01 t11~D td CO O h
-ri -ri
r-1 d1 u7 ~ s-!h ~ r-4 M O1 N
i.1 .1.~
ri to cD l0 tt1c0 V~ O CO 10 O
rl
,L1 01 O N N 'W O
~
'.3' O V~ h c0 v-i - t31 CO
r1 N
N
r d~ ri O O ~
,
U W U 01
(..." ~-,
It1 ttf
Ft,' O O O O O M ~C O O O O O 1D
ri ri
f-1 SI
flf 01 M N ri h (~ 01 In l17 Ifs
rl tD O M M ri ri W h h h
rS O'WD M l,pO "'~ M fD CD CO
~i M t0 N M OD O ~ rl M M M O
(''.,
U m o o~ h vo ut U ~.n N N N m
O O
H U
ri o v-fs-iN M c-t O n1 N V~H
ty' Q
1J ',
.1.J
J.~ U1 M 11 OD
~
M N to im n rtt w M
r-1 CO t17OD rl ,-1 rl N h
~ ~
.t", M W N O f.'. h N
-~ 0 0 0 o m ~ 0 0 0 0 o M
~
~ .-i~ ~ ,-tr-t~ -.~ ~ w
tJ M 1D h O ~O O N II1 h O
~'N 11
p
(31 O M N h Ln -ri l71 ~D V~ rl
-ri r!
.U 1J
O IIS rl M lD O tI1.4.1 tl1 ~D l0
.Cl .O
li1 f6
,~~-I W t17ll101 O ~ St 0~ h
rl ri
!-i Sd
Ul d~ 'd~rl rl O ri N W N r-I
.~'. .C."
O 0 o q ~
~
p
o 0 0 m - o 0 0 0 o Q
N
4-I
h l0 r1 O t!f In h
O
OD O U1 l0 h O N
'
W
-'IN C~ h ~-~It'~ f-iN 10
O ri
rl tCld~ rt N O ri In M
.,i ~-~ ri
E-~O O O O O H O O O O O
$ N lp h N r-1 3 'W N
.i~
sN It1h M ttl tlt
~'
O l0 h d~ O~ M N
H
v O M O tf!s~ a1 N O w M
r! .-I
U cr W N N O 13U cH N
rl '-i
~
~
~ 0 0 0 0 0 0 ~ c 0 0 0 0 0 o h
3 n
3
w
O ~
0
M O o
0
-ri \O N '-1V' ri W tt1 ~"~
If'1
.U ri h N tn 1J lD N '
01
-ri O V~ M 01 ri OD h N
N
a co .cro0 o x .R~ ~ ~r x M
n-1fllN h M a-t f.,"' ri~ Ov M ~."'"~
ri r1 N
N ~
H H ~a~N r-
I
W$ 0 0 0 4 p al H Ga,$O O 0 0 0
A
N
A
t1 O O M CD N ~1 1D lD 01'-1tN
ri r1
ri ed h O~ N h rl 'W 01 M riM
ri ri
H m o ~ M ~ ~ M
3
a . . H ~ r -t.
o -~ -1 3 , -ii
M
Lr" N OD ch 1D 'W Gi O N lDODN
pip
O d~ M CO ~-11p W O CO O l000M N
rl rl
U r1 O lD OD M tn U N 01 M LC!M 111O
rl rl
N lp O N N L!1h t71N N tf1v-tc-iN i-1IJI
N N p~
cn m ~ ,a ,-~~ ~ ~ m vo ao ~-rrrt~ w
3 3 b,
W
x
N W
W N d -1 W N riO r1 ~D N N InW rlD
r! W
71 O ~-IM O 01 H J.1ti v-i V~ cNlDrlrl~1
rl ~-t O
rl h O1 l0 N ~-IU1 i-1 ~D u? t-iN M N
N N
I~ av m ~-trt N U V W tn ao rfr1~-IU H
3 $ U
i
i N N
H
~
N
.. u1.-. of
p a
H
r t
l d
u
O '
~
r ,
f t-1 M ~-1 rid M rl
~ ~.I
~
' p H M r1r1
' i!
H
~ M ,-1 w-iF', ..
U M ~ ~ V M H ~
O O
r+ -~: r.~ 1-1ty'
.J ~
.
' x ~ ~''G'' O O O
X
O O O O M ,-iU H U 0 M ~
v
U O2 M r1 M a-1 '~"' 0 M C s U
1 S -f x,
x
- 15 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
-d ~r ~ w r
(., N N M r~
.C'.
.i-~ r .V r m
O O
it ~ ~a t~o
s~ M
~ r ri u~.-i
.4-m .u
a ~ ~ ,c~a cn
.~
.i
~ ~ ~
~
f O O O O O FL' O O O O O
l r
l
~,
fd M M M M M (d ~f7In!l7t(1
r-1 111!15L1tnlf~ ri O O O O
N N N N N ~ N C~ r r L~
ri~-irirtrl ~ ~ O O O O
F,' M M M M M O ',7 N rl rlrW-1O
'(..,'
U m a~rno1o1~n U m to m m c~v7
O O
r-1 O tiN M d~H wi O ri N M V~H
A', F~
L .u
(6 r fa u7V'
r-! sY r) 41~
~o ~ m rl
A
U o U ,-io
O O
' ~
.1.1 O O O O O M X1',.1.7 O Or O O O M
fy
O
5':
N V~ O N 01 O
.7-~ .1-1
A! N
b1 r ~ tn m ~
,-~ ~
a .u
l0 m .L~ td O m .tJ
.Il .s7
tt1 (d
~ ' ~
' W
41 O rl fU ~'1O r-
.~ .~ I
r
~ q ~ p
O ~
O O
x~ - a o 0 o a a o 0 0 0 o
-, C
~
4.a
GN ' v~N
N l1?r
u1 O N
~ ~
r.C--ri
H r r-1 N
fn+~ -r1 s-1 -ri Lc1('1
r-1 r-1
H ~ ~ $
x~ ~ O O O O O ~ O O O O O
~ ~
.!J~" a-1 dl 1D\D
~ ~
N 13O ri N ~ J O r-i d~!Il
.t
riU n-1 O rlU ri N O
N N
U1 O O o O O o
$
Cl~ O O O O O O 11~
$
H
H
U H 01
, N m
4a d~
~ N
O ~ rl F.'Q7 o t r'.
0'e-I l7
~1 ((j S~i ri c~
a-~ r-f O ri .
m 0
u 3
3 u
, 0 0 0 0 o , 0 0 0 0 0
H
w '''
0
a o ~ M ~ ~ m M o m
a o a r-i coi
O c
U ~ u1~ w rio ~ -,-~ n o0 0~o m
~ y
~ ~ ~ N M N o
N ~ . ~ . H N ~ ~ , , .
3 -t-t -11 3 -i-~-t
G
O z3 'd
F.' '4~O lflN M ~ ~"'" O O (V~Dl0
O N ~Ts-tW ancn O ~oV~ 00d'm o0
r1 .-I
~ U r m r o o W U o1M a~M oow
,-s ,-I
U) '~il0v-tll1N r (U r-1O N N O 4
N U1
.~ rra .-r.-~N ~ .-i,-~ u~ ca.-t,-~.-i~ .-i
3 3
.
.u aom o w M .u w o ~r~.o
O w r-W wna~.-i m o w u1M cor~
,-~ 01o r m O ri w N O1 N 10CO~-1
~1 OD 1-~
rl rt
-r-I crep10O N N rt N O N N O N
N 4f
ft. r-1r1.-1N H U Cu m ~-1H .-~~-IU
~ ~
L
E
~ D y M .iW
"
~'
N .U M rl ,.-1 " M rlO r1
,
N
O N M riM !I p M .1M !t
p
r-i U r~rio ~ U M ~ o t~
,1 ~..i
,Lt ~ a o r ~ ~ o o t~G
X X
O O O M s-1 O O O O M H O
E-~ U m a r1r~M U U M r1 M rin'1U
~" "
- 76 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
zi w avaoN r ~ .-irmn ~ ao
Q1 L~ c-Il~O~l~ UJ eii"tit,-lM
("., .("'a
.I-i N ~ t0U7M .1~ rlO 01d'c0
O O
(CS O d'l0lW-I fU t N tD<HCr
-ri ri
n--1 ri N riO O rl V~N O O LD
.1..1 .i~
ri l~ O 01M t0 '3' l0d~LOf~~"'~
rl
.~., d~ N 01L'titW ~ d~O M N N
,!.'~ ll)O N O O t!) .O N e-1O O O
O ri
r1 N
N
U N rlO O O ~ U O O o o O W
..t; .i."
.>, 1~
~
~ o 0 0 0 o fu ~ 0 0 0 0 0
-~ ~
a
4$ tI1V'N N M (f1 tDipl0t~
ri N LOM M cN wl t!1d~~W'WM
OD l~N N O ~ C'LO1DLOd0O
E In h rlr1M E tf1tt1ifTtt1d'
01 h N N 41O ~ 01GDOpODt'~U
~.,"
m h r h to~ U 01atovrnatH
~
~
rl O riN M V~H rl O r1N M ti'
A,' Q',
J.~ .i-1
J~ h h .l~ lfl wi
(IS t~ N .-i01 fO M N 00COCO
ri !faCON 00O1 m1 1DriO O O
r- 0101M M ~ '-!01t0ODW
1D rlM L'~l~ E InO O O O
.~3 O O W N N ~~ N N s-1~-1'-i
U M N O o O U O O O O O
~ ~',
U U
O O
r.~ o 0 0 0 o M ~ o 0 0 o ci~M
-a ~t
rl N N ri La ri ' 'W OD'h'
N h tn~M m O N InO o O
.u .u
N N
X31 ri O tit M ri al N ri 00rf
ri 1-1
.N .U
rti w coto r .u rt w o o s.i
.s7 .U
of ~
~1 O r-1l11 N ~ ~1 O v-! ri,7
rl .-1
Si I1
'
"
N ri r~O O r-I N o O O rl
,1," J
-.
0 q ~ 0 p
~
O
U
0 0 0 o - o 0 0 o
N
4a
".., tn o~ r
w w O
N ~-i N
C3 O N 2f of
f-t OL aD SI N
r-t rl
'
Q) rl
rl O O -rl O
'-1 r1
~ H 0 o H
3 g
x . N 0 0 0 U o 0 0 0 0
~ta' .u
C~
rt G ~ M
H ~ o
d
FC ''iO N txlQ f~'N
r-1N r x
i
U N N ~ U O O
r-a i-i
x ~ C1 O O O O O O ~ ~ U1 O O O O
1 $
$
. O O
O y .~
r U O
H In tpO1 O H (~
it)l~W W M
O ' r ~ ~ w
.u . x .u x
-ss
~ 0 ~ o ~ m o G
~ rl
~ ~ ~'i o m
-1 ~
C~.3o o o o o p ~ Ci,go o o o o
.~
H ,t)
ri
O 'b LO N V'O O .~rd erO lpO W
f-1 O U101crN ,C,t-I M lt!ODN O
r1 rl
O ri c~1N ~-1M M f"..,-,t N r7O O t11
.-1 r1
.::; L~ l0W LDd~ H i~" N N U7M c1
~ N
H ~ w I ~ .-i H ~ .-i~ .-m-t
S 3
G
O ~ b
't.,' LO c0W W N N L.'' N d~tpGOchM
O tt1W M M 117L? O OLCOO~N W V~
e1 r-i
U r-iN aoo t mo U tod~w a~rnao
rm r1
N N N 00l0t~tf1 N r W V'N wlrl
N N -1
m ~ ~ ,a. w-Ir1 m r1~ ~
S 3
rl
N
W ..r N o w N ~ .~ o o .~N w
~ L' 01t0N O rt W ODV~01M 01r1
rl ri
O ~ ,~ m cnc tn,-i ~ o coaso 1~~-i
N r-i ,..i
r1 V~ M W tt1twN rl N N N titriN
N N
W G4 .-~r-i.-~.ir-~U W r-Ir-ir~,-~.-iU
r" ,'3 3
,u
H
r-1
..
N O '
.-. O
(V w1 'i
,ta
H
~ ~
~ 1
M .l.) M rlrl 1J M e-tr(
~1 H H
r~ M ~ O i~ M .-iO
O U M H j~ M ~
O
t , I i . U ri.t
111 .~ n r ~ i
r-
.4 y"., O O O ~,' ~', o O O . O
t-1 ~ x
td O O O O M n-IO O O O o M mlO
r
H U Q1 M rlM riU U a1M riM .-IU
W " '-"
- 17 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
m
y tf1O 00 .F~ I~l0l0M
O O
t0 O r~~ t(f t~00Q1l0
-ri ri
r-i 01~O01 ri rlniV~01
1J J~
~ N rlI~ ~ tt1O d~L~
-rl ri
N V7W O U1 ~
-~
17
l N st'N ri Cn
U N o O ~ U 1 O O O
.L," ,C'.,
.13 .4W
U ~
~
~
.rC 0 0 o O o Gx, -t O O O o 4
, ~
- ~
f .,
-1
M lf1ll1lf1 (lS lDO1V'00OD
M In1010 y -I O1O M M M
~ 4~l~W ~DW
01v-I4l1lflU1
t,' o ~ N N N O
O ~ N ~
O ODt~t~h I~Il'1 U o O a1m o In
1 1 0
~
! O v-1N M erH w-( O r-SN M V~H
rl ~
A', L1
A.
a m .u .-m n
ro u~r m c0 N w ..-itn
ri M lDN ri lDLflr 01
t
O .NO O ~ ~ O O 0
O
A', O O O O O M A', O O p O O M
ri ri
,
r) h M In ("., r-1 d~t~'WlD
N ~DCOCD O ~ O W r 01 O
.U J-1
N ~
dl M M CO ri ~t71 O N OD01 vi
rl rl
.i-1 3J
a.(d c7DOlI1 l.A LO riInO1M .1-1
.4 .~
fIi 10
O a 0110rl O ~1 W M O 1I1
-rl ri
~1 1!
r1O O rl O O O O
~., 0 , ,''
x ~ .',.
~'..
r.C 0 0 0 o A r.~ 0 0 0 0 0 Ca
~ ~
0 0
0
r ~n
.l.! ODr OD M
H 01
~
4-1 S-t00 ~I d~ N
rl ~-I
..~ ri rlrl r-I4 O
rl .1
H
3
~ 0 0 0 0 0 H 0 0 0 0 0
3
~
~
.-N ~ y un m
t
' gy
~ Cj lfl 10 ( p 'b lDM If?
p
~ '~'U O o '~''NU o o H
~
_ -1 .- r r
'i i 'ii 1
N O tU
H ~ 4! 0 0 N 0 0 0
; cn 0 0 0 o a..,~ cn 0 0 0
3 3
s~
h
M
t-il~ ~ rlt~M l0
u1h W t~O N N
O OD W ral!1~OGO
~
~ ~ M 01N rt
~-i
~-1N O !"', S-1O O O O
ri r1
u o ~ ~
3
, o o o o W ~ W3 o o o o o p
q
b '~ro ~r~ N w ao
H toeho N OD ,Of.1 t~.-1aDw N
r-1 ~-I
N 'WM rl1nt0 H ~ N M M N N
N
H vc~.oaoavr H ri.-i~ .-iri
3 3
ro
O 1PN d~rNODCO O 10O lD'~N N
ri r-I
U r rn~ ~nd~w U N r d~M aoao
.-t .-i
T ~ N N M ~-1N N
U
UI 10C1 ODl~I~ 3 H H v-1H riri
3
i
W J-1 d~O a01UCO
91 CDN O O ODr! U7 l001d W N ~-1
ri ri -t
~I I"01~DM ~ rl fi M L11V~I,pCOrl
rl r1
ra N ~O01U1a0N rl N '-IN N N N
03 N
fra vor ao0or U f=. .-t~ rt,~-i.-tU
3 3
O
_,i
~ M c-1
1J M r-W .1 f1H ri
H -t H
y
N M v-if1 N M riti
p p
x
O O O O M w1O O O O O M
v
U ovM .-IM 'rU U 01M v-1M rlU
"
_.
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
u~w h ~ ,n
o ~ ~ o ~
o
-rl v-1t0M v-i ri l0
rt3 ~ h iow b
.u s.i
_ O1
d
~ -1N LOri ~ ~ t11 W
.IJ 'N
.W i7
fIi O er.-1O ro
ri ~
N U
U~~~ N O O O Ur- O
J
i.t
'as
~ O
R' O O O O O fix, -~ O O O O
~ y,~
ri ,Q,'
N ~
-,.
M ~ ~ ~ M M M M ,M
r-f d~10COO O ri W a0c0t0m
O 4 O O O
m ~Dh N N
i; lDN OJW COO
CDm h h h u7 U o10141O1011f1
O
U
' U
, O rlN (~"1d~1-i ri O riN m d~H
11 .yf'
v-i ,
Fl J3
t.I O~ O
(~ 01h m rl to h
m W M O
h O sHIfl E N
U o O
.E"., N O U p
O O
FC O O O O o M F( O O O O O
'
r!
, M
O
O
5C O
n N h
Q lU InQ1 O O N ' p
1J 0 J.) -ii
1-~b1 O1O N O n-I ~1 Q1
vi ri
-LI
~I m m M O
N ~ U
~;O O O ~ N O
J.1
e~ ~
Li
(l1
r.C 0 0 0 0 o q ~ 0 0 0 0 o p
-,~ ~
y~
p m o~av
o ~ ~ ~
M N N
,N 'i7 s1'c0d' 'U
~ Q O S-1
~ rl
r N
l
r
-1
'
N ,t . . . N ,~
, , v
41
, H 0 0 0 0 0 .uH 0 0 '00 0
3 3
~ b
H ~ .rC
,
b
0 ~ x o
.1 - ~
O ~ U
rt
'u
R W 0 0 0 0 0 0 .c7ai 0 0 0 0 0 0
~ 3
U
p~ tf!
01 tHcr
If1 InN ~H
.!J W M lfl .~Gv 1J h r,,~
,
~I O O O 1(fO H O
rt rl
.'t . . ,1O
N
fx,3o o o o o pp~ u,~ 0 0 0 0 0
0
~ 'Cf N ODt0O N O
d ~
rl lf1 tnh , .r1 o~h W m to
r-I
3 ~ H N
lDlCh h N ~ ~ ~ . ~
3 -tt
A
b
O N t01Oi0O O i tlQ l O
r1 O
N ~ ~ r 7 1 p !!~O
U aDp -i t
ri
N 1 C ~ ~ ~ ~ ~ w D l0b
p ~ ~ N M ~
cf1 ~ aoavaoh h ~ r . m-~ . ~ o
G 3 3 .i- r -i
.~1-~ N .4.1 c0V~1pO N b
!~-i
W O h 10N O rl W W U1V~aoO r-iN
N ri ri p
a erO r-IN w r-1 N o w COW O riU
ri rl
N
.-i N ~-iO tDh v -r1 ,-~M N N '-iN H
N N
U
W h .-1h h h U f~ .--i,-i.-I,-i-iU
3 3
.
N
O H
,.. O
U yt ~ N
rl
,-~
N
H M rir1
~
M r-1O ~"
U O M rit-IUl
O O ~Sl
X 0 M v-iL U m a ~
'
0 0 C ~' O O O ~"N
., ., m
X
H '~'
U a~M ~ m ,-iU U rnm p M ~ U
v
- 19 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
E;xarnpl,e 2:
COMPARISON OF INHIBITORY EFFECTS OF DIFFERENT INTERFERONS
ON HBV GENE EXPRESSION
Hepatitis B virus (HBV) DNA contains consensus elements for
transactivating proteins whose binding activity is
regulated by interferons. Treatment of HBV-infected
hepatocytes with interferons leads to inhibition of HBV
gene expression. The aim of the present study was to
characterize the effects of different interferons on HBV
regulated transcription. Using transient transfection of
human hepatoma cells with reporter plasmids containing the
firefly luciferase gene under the control of HBV-Enhancer
(EnH) I, Enh II and core promoter, Applicant studied the
1 5 biological activities of three different interferons on
transcription.
Materials and Methods
1. Interferons: IFN-conl (Infergen~), IFN-Hui-Yang (ySIFN-
2 0 co) and IFN-beta 1b
2. Reporter plasmid: The DNA fragments containing HBV-
Enhancer (EnH) I, Enh II and core promoter were prepared
using PCR and blunt-end cloned into the Smal I site of the
promoter- and enhancer-less firefly luciferase reporter
2 5 plasmid pGL3-Basic (Promega, WI, USA). The resulting
reporter plasmid was named as pGL3-HBV-Luc.
3. Cell Culture and DNA transfection: HepG2 cells were
cultured in DMEM medium supplemented with 10% FBS and 100
U/ml penicillin and 100 ug/ml streptomycin. The cells were
30 kept in 30°C, 5% C02 incubator. The cells were transfected
with pGL3-HBV-Luc reporter plasmid using Boehringer's
Lipofectin transfection kit. After 18 hours, the medium
containing transfection reagents was removed and fresh
medium was added with or without interferons. The cells
3 5 were kept~in culture for another 48 hours.
4. Luciferase Assay: Forty-eight hours after the addition
of interferon, the cells were harvested and cell lysis were
- 20 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
prepared. The protein concentration of cell lysates were
measured using Bio-Rad Protein Assay kit. The luciferase
activity was measured using Promega's Luciferase Reporter
Assay Systems according to the instructions of
manufacturer.
RESULTS
Expression of Luciferase Activity in Different Interferon -
Treated Cell Lysates
No treatment IFN-conl IFN-Hui-Yang IFN-beta lb
100 48+8 29+6 64+10
This result shows that ySIFN-co inhibits most effectively
on the expression of HBV gene expression.
Example 3:
SIDE EFFECTS AND CHANGES IN BODY TEMPERATURE WHEN USING
YSIFN-co
2 5 There are usually more side effects to using interferon.
The side effects include: nausea, muscle soreness, loss of
appetite, hair loss, hypoleucocytosis (hypoleukmia;
hypoleukocytosis; hypoleukia), and decrease in blood
platelets, etc.
METHOD
Sample patients are divided into two groups. 11 patients in
Group A were injected with 9~.g Infergen~. 10 patients in
Group B were injected with 9~g ySIFN-co. Both groups were
3 5 monitored for 48 hours after injections. First monitoring
was recorded 1 hour after injection, after that, records
were taken every 2 hours.
- 21 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
Table 4 is the comparison of side effects between patients
being injected with 9~,g of Infergeri and 9ug of ySIFN-co.
Table 4. Side Effects
ySIFN-co Infergen
9~,g 9,ug
Person: n=10 Person: n=11
Body Systems Reactions Headcount Headcount
In General Feebleness 3 3
Sole heat 1
Frigolability 3 4
Decrease in
leg strength
Mild lumbago 2 1
Body soreness 4 5
Central Nervous Headache 3 6
System/ Dizziness 2 11
Peripheral Drowsiness
Nervous System
GastroenterostomyApoclesis 1
Celiodynia 1
Diarrhea 1
Musculoskeletal Myalgia 1 2
system
Arthralgia 2
Respiratory Stuffy nose 1
system
Paropsia (Swollen eyes ~ ~1
RESULTS
For those patients who were injected with ySIFN-co, the
side effects were minor. They had some common symptoms
1 0 similar to flu, such as: headache, feebleness,
frigolability, muscle soreness, hidrosis, and arthralgia
(arthrodynia; arthronalgia). The side effects of those
patients whom were injected with Infergeno were worse than
those were injected with ySIFN-co.
From Figures 4A-1, 4A-2, 4B-1, and 4B-2, it was obvious
that the body temperatures of sample patients in Group A
were higher than the patients in Group B. It also reflected
that the endurance of ySIFN-co was much better than
2 0 Inf ergen~ .
- 22 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
Evca2npvc~ ~ :
CRYSTAL GROWTH of ySIFN-co AND TEST OF CRYSTALLOGRAPHY
PARAMETER
Crystal of ySIFN-co. Two types of crystal were found after
systematic trial and experiment. (See Figures 5-7)
1. Crystal Growth
Dissolve YSIFN-co protein with pure water (H20) to 3mg/ml
1 0 in density. Search crystallization by using Hampton
Research Crystal Screen I and II which was made by Hampton
Company. By using Drop Suspension Diffusion Method, liquid
500.1, drop 1 ~.1 protein + 1 ~,l liquid, in 293K
temperature. First 2 different types of small crystals were
1 5 found as listed in Table 5.
Table 5. Screen of ySIFN-co Crystallin
Condition I II
Diluent 0.1M Tris-HC1 0.1M HEPES
PH=8.75 PH=7.13
Precipitant 17.5%(w/v) PEG550 l0o(w/v)PEG6K
MME
Additives 0.1M NaCl 3o(v/v)MPD
Temperature 293K 293K
Crystal Size (mm) 0.2x0.2x0.1 0.6x0.02x0.02
Crystallogram Figure 5 Figure 6
2. Data Collection and Processing
Crystal I was used to collect X-Ray diffraction data and
preliminary analysis of crystallography. Testing of
parameters was also completed. The diffraction data was
2 5 collected under room temperature. Crystal I (Condition I)
was inserted into a thin siliconized wall tube. By using
BrukerAXS Smart CCD detector, light source CuKa (A=1.5418A)
generated by Nonius FR591 X-ray generator. Light power 2000
KW (40 kv x 50mA), wave length 1.OOA, under explosion 60
3 0 second, Dep=2°, the distance between crystal and detector
was 50mm. Data was processed using Proteum Procedure
Package by Bruker Company. For crystal diffraction pattern
(partially), see Figure 7. See Table 6 for process results.
- 23 -
CA 02535902 2006-02-15
WO 2005/021777 PCT/US2004/028068
Table 6. Results of Crystallography Parameters
Parameters
a (A) 82.67
b (A) 108.04
c (A) 135
. 01
a ( 90 .
00
)
(3 () 90.00
y ( 98 .35
)
Space Group P2 or P21
Sharpness of separation 5 A
Asymmetric molecule # 10
Dissolution 57.6%
In addition, there was no crystal growth of YSIFN-co based
on previous publications. The closest result to the ySIFN-
co was huIFN-a2b but the screen was very complicated. After
2 0 seeding 3 times, crystal grew to 0.5x0.5x0.3mm, sharpness
of separation was 2.9 A, space group was P21. The crystals
were also big, asymmetric molecule number was 6, and
dissolution was about 60%.
- 24 -