Note: Descriptions are shown in the official language in which they were submitted.
CA 02536036 2006-02-10
a
AUTOMATED REAGENT DISPENSING SYSTEM
AND METHOD OF OPERATION
Field Of The Invention
The present invention is directed generally to tissue sample processing
systems and in
particular to systems and methods of dispensing reagents.
20 Background Of The Invention
Tissue processors can be operated with varying levels of automation to process
human
or animal tissue specimens for histology or pathology uses. Various types of
chemical reagents
can be used at various stages of tissue processing and various systems have
been developed for
delivering reagents to microscope slides containing specimens. Examples of
known reagent
25 delivery systems include small quantity release dispensers, technicians
manually pouring
reagents into reagent vats, and bulk containers connected with a specimen
processor via tubing.
There are various disadvantages of known systems. For example, a technician
manually
pouring reagents into, or draining, reagent vats suffers the disadvantages of
being time
consuming and requiring pouring accuracy which decreases the overall
efficiency of the tissue
30 processing system. Another disadvantage is that manually pouring and
draining reagents can be
1
CA 02536036 2006-02-10
sloppy, requiring clean-up of spills and conseauential instrument down-time. A
further
disadvantage is that selecting the correct reagent requires operator attention
and accuracy and
there is an increased possibility of reagent application errors, which
decreases test accuracy and
operational efficiency.
In the previously known automated systems, there are also disadvantages. In
those
systems, reagents are selected and administered to slides during processing,
frequently via
gravity promoted dispensing from above. Such delivery systems require
specialized equipment
for reagent delivery such as specialized reagent dispensers, drivers or
automated pipetting
systems. Such systems suffer various drawbacks such as the amount of effort
required to set up
and dispense the reagents, the possibilities of evaporation during processing
or contamination
and difficulties in handling minute quantities of large numbers of reagents.
Summary Of The Invention
The present invention alleviates to a great extent the disadvantages of the
known
automated slide staining systems. The invention reduces errors and increases
efficiency in
1 S tissue processing by providing a central controller that may
simultaneously operate multiple
stainers in a scheduled manner. The central controller initiates tissue
processing either
automatically, for example, on a scheduled basis, or manually upon receiving a
start event
condition. The stainers undergo an inventory procedure that determines the
number and types
of tissue samples provided on slides that have been placed on trays in the
stainers. This
procedure may include scanning a bar code or otherwise obtaining patient,
tissue, reagent,
and/or other types of information from the slides andfor trays provided in the
stainer.
The stainer transmits all or a portion of this information to the central
controller.
Preferably, the central controller at least receives primary reagent
information regarding the
tissue samples. Based on the primary reagent information, the central
controller determines a
staining protocol to be applied to the tissue samples. The staining protocols
are communicated
to and stored by the stainer. The stainer may then operate independently of
the central
controller.
The central controller enables a user to obtain a status regarding one or more
tissue
processes, generate reports, modify reagent cartridge information, select
programs to run, and
2
CA 02536036 2006-02-10
initiate other functions. The central controller may also enable the user to
manually initiate
tissue processing for one or more of the stainers.
These and other features and advantages of the present invention will be
appreciated
from review of the following detailed description of the invention, along with
the
accompanying figures in which like reference numerals refer to like parts
throughout.
Brief Descriution Of The Drawings
FIG. l is a schematic of an automated reagent dispensing system in accordance
with the
present invention;
FIG. 2 is a top view of a tissue processing system suitable for use with one
or more slide
retaining trays in accordance with the present invention;
FIG. 3 is a side partial cross-sectional view of the tissue processing system
of FIG. 1;
FIG. 4 is a flowchart depicting a method of manufacturing a retaining tray in
accordance
with the present invention;
FIG. 5 is a flowchart depicting a tray loading procedure in accordance with
the present
invention;
FIG. 6 is a flowchart depicting an overall system procedure in accordance with
the
present invention;
FIG. 7 is a display associated with creating a user account in accordance with
the
present invention;
FIG. 8 is a display associated with creating a user account in accordance with
the
present invention;
FIG. 9 is a display associated with a user login in accordance with the
present invention;
FIG. 10 is a display associated with changing a user password in accordance
with the
present invention;
FIG. 11 is a display associated with system setup in accordance with the
present
invention;
FIG. 12 is a flowchart depicting an initialization procedure in accordance
with the
present invention;
3
CA 02536036 2006-02-10
FIG. 13 is a flowchart depicting a scanning procedure in accordance with the
present
invention;
FIG. 14 is a flowchart depicting an inventory procedure in accordance with the
present
invention;
FIG. 15 is a display associated with slide information table setup in
accordance with the
present invention;
FIG.16 is a display associated with barcode format setup in accordance with
the present
invention;
FIG. 17 is a display of a main control window in accordance with the present
invention;
I 0 FIG. 18 is a display of a main control window in accordance with the
present invention;
FIG. 19 is a display associated with editing slide information in accordance
with the
present invention;
FIG. 20 is a display associated with editing slide information tables
accordance with the
present invention;
1 S FIG. 21 is a flowchart depicting an interrupt event procedure in
accordance with the
present invention;
FIG. 22 is a flowchart depicting an overall procedure in accordance with the
present
mvenrion;
FIG. 23 is a display associated with manual operations iri accordance with the
present
20 invention;
FIG. 24 is a flowchart depicting a method of using a slide retaining tray in
accordance
with the present invention;
FIG. 25 is a flowchart depicting run protocols sub-steps in accordance with
the present
invention;
25 FIG. 26 is a display associated with viewing cartridge information in
accordance with
the present invention;
FIG. 27 is a flowchart depicting an audit logging procedure in accordance with
the
present invention;
4
CA 02536036 2006-02-10
FIG. 28 is a display associated with selecting a program in accordance with
the present
invention;
FIG. 29 is a display associated with creating/editing a program in accordance
with the
present invention;
FIG. 30 is a display associated with selecting a macro in accordance with the
present
tnvenrion;
FIG. 31 is a display associated with creating/editing a macro in accordance
with the
presentinvention;
FIG. 32 is a display associated with module information setup in accordance
with the
present invention;
FIG. 33 is a display associated with sending commands in accordance with the
present
invention;
FIG. 34 is a display associated with selecting a tray reagent in accordance
with the
presentinvention;
FIG. 35 is a display associated with adding a new tray reagent in accordance
with the
present invention;
FIG. 36 is a display associated with editing a tray reagent in accordance with
the present
invention;
FIG. 37 is a display associated with selecting a cartridge reagent in
accordance with the
present invention;
FIG. 38 is a display associated with adding a new cartridge reagent in
accordance with
the present invention;
FIG. 39 is a display associated with editing a cartridge reagent in accordance
with the
present invention;
FIG. 40 is a display associated with selecting a bulk solution in accordance
with the
present invention;
FIG. 41 is a display associated with adding a new bulk solution in accordance
with the
present invention;
5
CA 02536036 2006-02-10
FIG. 42 is a display associated with editing a bulk solution in accordance
with the
present invention;
FIG. 43 is a display associated with bulk solution bottles setup in accordance
with the
present invention;
S FIG. 44 is a display associated with waste bottles setup in accordance with
the present
invention;
FIG. 45 is a display associated with creating a user account in accordance
with the
present invention;
FIG. 46 is a display associated with a delayed start in accordance with the
present
invention;
FIG. 47 is a display associated with setting up an auto print schedule in
accordance with
the present invention;
FIG. 48 is a display associated with scheduled maintenance setup in accordance
with the
present invention;
FIG. 49 is a display associated with setting up days off in accordance with
the present
invention;
FIG. 50 is a display associated with debugging the system in accordance with
the
present invention;
FIG. 51 is a display associated with viewing bulk solution bottle information
in
accordance with the present invention;
FIG. 52 is a display associated with viewing waste bottle information in
accordance
with the present invention;
FIG. 53 is a display associated with creating a program report in accordance
with the
present invention;
FIG. 54 is a display associated with setting up a program report in accordance
with the
present invention;
FIG. 55 is a display associated with creating a macro report in accordance
with the
present invention;
6
CA 02536036 2006-02-10
FIG. 56 is a display associated with setting up a macro report in accordance
with the
present invention;
FIG. 57 is a display associated with creating a primary antibody/probe report
in
accordance with the present invention;
FIG. 58 is a display associated with setting up a primary antibody/probe
report in
accordance with the present invention;
FIG. 59 is a display associated with creating a secondary reagent report in
accordance
with the present invention;
FIG. 60 is a display associated with setting up a secondary reagent report in
accordance
with the present invention;
FIG. 61 is a display associated with creating a bulk solution report in
accordance with
the present invention;
FIG. 62 is a display associated with setting up a bulk solution report in
accordance with
the present invention;
FIG. 63 is a display associated with creating a waste bottle report in
accordance with the
present invention; and
FIG. 64 is a display associated with viewing reports in accordance with the
present
invention.
Detailed Description
In the following paragraphs, the present invention will be described in detail
by way of
example with reference to the figures. Throughout this description, the
preferred embodiment
and examples shown should be considered as exemplars, rather than as
limitations on the
present invention. As used herein, the "present invention" refers to any one
of the
embodiments of the invention described herein, and any equivalents.
Furthermore, reference to
various features) of the "present invention" throughout this document does not
mean that all
claimed embodiments or methods must include the referenced feature(s).
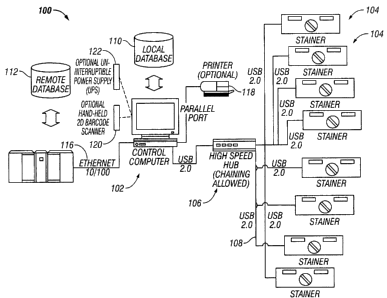
FIG. 1 is an illustration of an automated reagent dispensing system 100
according to an
embodiment of the present invention. A control computer 102 is in
communication with a
plurality of stainers 104 and may provide a centralized user interface for
controlling the
7
CA 02536036 2006-02-10
plurality of stainers 104. Stainers 104 may be used to process biological
specimens as
described below. Control computer 102 may communicate with stainers 104 in any
manner
known in the art, for example control computer 102 may communicate with
stainers 104 via a
high-speed hub 106. High-speed hub 106 enables dispensing system 100 to
quickly convey
information between the plurality of stainers 104 and the other components
such as control
computer 102. For example, stainers 104 may download staining protocols to be
applied to
slides placed in the stainers over a network formed by data lines 108 and high-
speed hub 106.
It shall be appreciated that control computer 102 and stainers 104 may be
configured to
communicate through hardwires or wirelessly, for example, the system may
utilize data lines
108, as described above, which may be conventional conductors or fiber optics.
Additionally,
the components may communicate wirelessly such as using radio frequency
communication,
such as BLUETOOTH (a registered trademark of Bluetooth SIG, Inc., of Bellevue,
Washington), or any other wireless technology.
Control computer 102 may also communicate with one or more local databases 110
so
that data may be transferred to or from local databases 110. For example,
local database 110
may store a plurality of staining protocols that are designed to be performed
by stainers 104.
The staining protocols implemented by stainers 104 may be chosen based on
information
obtained from identifiers (e.g., barcodes, radio frequency identification
devices (RFID), etc.)
associated with slides and/or trays used in the stainers, as further described
below. Control
computer 102 may process identification data received from stainers 104 and
retrieve staining
protocols from local database 110 and transmit the staining protocols to
stainers 104.
Furthermore, control computer 102 may use local databases 110 for storage of
information
received from stainers 104, such as reports and/or status information.
Control computer 102 may also communicate with one or more remote databases
112
and/or a server 114. Control computer 102 may communicate with remote database
112
directly or through server 114, which may be a laboratory information system
(LIS). Control
computer 102 may communicate with server 114 via a network 116. As noted
above, server
114 may communicate with remote database 112. Server 114 and remote database
112 may be
8
CA 02536036 2006-02-10
used to provide staining protocols to be used by stainers 104 in a similar
fashion as local
database 110 or to supplement the protocols provided by local database 110.
Automated reagent dispensing system 100 may optionally include one or more
printers
118. Printer 118 may communicate directly with control computer 102, as shown,
or directly
with stainers 104. Furthermore, stainers 104 may each have a dedicated printer
118 that may be
integrated into the stainers or free-standing, or multiple stainers 104 may
share one or more
printers.
Automated reagent dispensing system 100 may also include a hand-held or
desktop
scanner 120 for reading identifiers that may be included throughout the system
components
(e.g., on microscope slides, trays, reagent containers, etc.). Any type of
scanner 120 may be
utilized that is capable of interpreting the identifiers. For example, scanner
120 may be an
RF>D scanner, a 2D or 3D barcode scanner, or any other type of scanner known
in the art.
Scanner 120 may communicate directly with control computer 102 or stainers 104
and each
component may have a dedicated scanner.
The system may also be powered by an uninterruptible power supply 122.
Uninterruptible power supply 122 may be used to limit the susceptibility of
the system to
general power failures that may invalidate tests that are interrupted. Such an
interruption in
power could also result in the tissue samples becoming unusable which could
require gathering
additional specimens. Power supply 122 may be used to power any or all of the
components of
automated reagent dispensing system 100.
Although control computer 102 is shown networked with multiple stainers 104 in
FIG.
l, it shall be appreciated that a stainer may combined in single unit with an
onboard control
computer, in addition to any other component described above in the automated
reagent
dispensing system. Such a combination may provide a compact, stand-alone unit
that may be
used to process lower volumes of biological specimens.
Referring to FIGS. 2 and 3, a stainer 104 suitable for use in automated
reagent
dispensing system 100 is shown. Stainer 104 generally includes a housing 224,
a fluid
dispensing apparatus 226 and a plurality of tray support stations 228. Stainer
104 is configured
so that multiple biological specimens each of which is supported by a
retaining tray 230, may
9
CA 02536036 2006-02-10
be efficiently processed. Housing 224 supports fluid dispensing apparatus 226
and tray support
surface 228 and provides an enclosure in which the processing of the
biological specimens may
be contained.
Fluid dispensing apparatus 226 includes a plurality of stations 232 at which a
plurality
of fluid dispensing cartridges 234 may be mounted. Stations 232 include
mounting apertures
236 selectively positioning a plurality of fluid dispensing cartridges 234
adjacent to an actuator
assembly 238, which is used to trigger the ejection of a desired amount of a
fluid, such as a
secondary reagent or a de-waxing fluid, from a fluid dispenser 340 that may be
integrated into
fluid dispensing cartridge 234. An example of a fluid dispensing apparatus
including a
multiplicity of fluid dispensing cartridges is described in U.S. Patent
Application Serial No.
10/639,021, the content of which is hereby incorporated by reference in its
entirety.
Alternatively, a fluid dispensing system using tubing or pipetting may be
used, such as the
system described for example in U.S. Patent No. 5,338,358.
Retaining trays 230 are positioned on tray support stations 228 and may be
configured
to hold microscope slides, as shown, and/or specimen containers. As shown in
FIG. 3,
retaining trays 230 are located in rows 246, 247, 248 generally beneath fluid
dispensing
apparatus 226. As a result, the system may take advantage of gravity to
deliver fluids from a
cartridge 234 onto a drip surface 233 of a desired retaining tray 230. Trays
230 may include a
specimen support plateau 231 that is outlined by a raised sidewall and may
include fluid inlet
and outlet ports 237 and 239, respectively. When a microscope slide is placed
onto tray 230 it
is supported by the raised sidewall thereby forming a gap between plateau 231
and the slide.
As described below, the specimen may be exposed to a fluid by flowing the
fluid through the
gap. Trays 230 may also include a reagent recess 235 for holding a reagent-
containing gel or
solid.
Preferably, fluid dispensing apparatus 226 and retaining trays 230 are movable
with
respect to one another so that cartridges 234 may be positioned to dispense
fluids on any
desired tray 230. Any combination of movability of fluid dispensing apparatus
226 and
retaining trays 230 may be utilized. For example, both fluid dispensing
apparatus 226 and
retaining trays 230 may be movable or only one may be movable and the other
stationary.
CA 02536036 2006-02-10
Actuator assembly 238 optionally includes a plurality of actuators 242, 243,
?44, w~~ch
may be used to selectively dispense fluid onto respective rows 246, 247, 248,
of retaining trays
230. In the embodiment shown, dispensing actuator 242 is configured to
dispense fluids onto
retaining trays 230 disposed in row 246, dispensing actuator 243 is configured
to dispense
fluids onto retaining trays 230 disposed in row 247 and dispensing actuator
244 is configured to
dispense fluids onto retaining trays 230 disposed in row 248. Of course, as
will be understood
by those of skill in the art, any number of actuators and/or slide retaining
trays can be employed
without departing from the scope of the present invention.
In an example of operation of stainer 104, fluid dispensing apparatus 226 is
rotated so
that individual cartridges 234 are selectively positioned adjacent actuators
242, 243, 244 of
actuator assembly 238. Alternatively, an actuator may be positioned adjacent
to each cartridge
234 such that rotation of the fluid dispensing apparatus 226 with respect to
actuator assembly
238 is not required. Actuator assembly 238 may be any activation device that
triggers cartridge
234 to emit a controlled amount of fluid. Preferably, fluid dispensing
apparatus 226 may be
both translated and rotated with respect to retaining trays 230 so that an
individual cartridge
234 can be selectively positioned above any retaining tray 230. After
cartridge 234 is
positioned above a selected retaining tray 230, actuator assembly 238 triggers
cartridge 234 to
emit a controlled amount of fluid onto retaining tray 230.
In a preferred embodiment, the fluid dispensing apparatus 226 may be coupled
to a
support member 350 such that cartridges 234 maybe rotated with respect to
actuator assembly
238. Actuator assembly 238 may be fixedly attached to support member 350,
optionally
beneath fluid dispensing cartridges 234. Preferably, support member 350 may be
translated
horizontally such that cartridges 234 can be both rotated and translated with
respect to the trays
230. In this manner, any cartridge 234 can be selectively positioned above any
retaining tray
230.
Retaining trays 230 preferably are mounted to tray support stations 228 on
spring loaded
heating/cooling pads 352, thereby providing selective and/or independent
heating and/or
cooling of retaining trays 230 and their associated slides and/or specimen
containers.
Additionally, heating/cooling pads 352 are capable of independently heating
the plateau or
11
CA 02536036 2006-02-10
platen region and the recess region. In an embodiment, each tra« 230 has a
corresponding
heating and/or cooling element 352, maintaining retaining tray 230 at a
particular desired
temperature. In an alternative embodiment, there may be two or more heating
andlor cooling
elements for each retaining tray 230.
Stainer 104 optionally includes bulk fluid supply containers 354, waste fluid
containers
356 and one or more fluid delivery manifolds 358. Supply containers 354 may be
used to hold
liquids such as water for rinsing or flushing the gap between a microscope
slide, or specimen
container, and platen, or plateau, 231 of a respective retaining tray 230.
Fluid delivery
manifold 358 preferably includes valves and switches (not shown) for directing
the flow of
fluids from supply containers 354, through an inlet port and conduit of
manifold 358, to
retaining trays 230. In addition, fluid delivery manifold 358 may include
valves and switches
(not shown) for directing the flow of excess fluids and waste material from
fluid evacuation
ports and conduits of manifold 358 into waste fluid containers 356.
Stainer 104 may also include a scanning device (not shown) for scanning
identifiers
included on retaining trays 230 or specimen slides or containers. The scanning
device may be
coupled to fluid dispensing apparatus 226, for example, so that information
may be read from
identifiers on retaining trays 230 as fluid dispensing apparatus 226 is
translated over retaining
trays 230. The scanning device may also be configured so that stations 232 for
reagent
cartridges 234 may be moved relative to the scanning device so that
identifiers included on
cartridges 234 may also be read by the scanning device.
A method of manufacturing a slide retaining tray 230 according to the
principles of the
present invention will now be described with respect to FIG. 4. As illustrated
diagrammatically
as box 400, the initial step involves fabricating retaining tray 230.
According to a preferred
embodiment, retaining tray 230 is fabricated from a polymeric material that is
injection molded
to form the desired structural shape. However, as would be understood to those
of ordinary
skill in the art, any fabrication process can be used or material selected
that can achieve the
desired structural features, without departing from the scope of the present
invention. For
example, retaining tray 230 may be constructed using any known technique such
as injection
molding, machining, vacuum/pressure forming, die casting, etc. Furthermore,
any material
12
CA 02536036 2006-02-10
known in the art may be used including polymeric and metallic materials. For
example, tray
materials may include urethane, polyurethane, acetal, stainless steel,
aluminum, etc.
The next step involves dispensing a desired quantity of reagent into reagent
recess 235
(shown in FIG. 2) as indicated by box 410. For example, a predetermined amount
of a gel
matrix containing a reagent may inserted through apertures in the bottom
surface of the recess,
or alternatively such a material can be inserted from above. Examples of the
gel matrix include
distilled water, distilled water with surfactant, buffering solution, etc.
After filling the recess, it may be sealed. As illustrated diagrammatically as
box 420,
the next step involves sealing the recess. The recess may be sealed in any way
known in the
art, for example the recess may be sealed by applying tape, or another sealing
material such as a
meltable material that can allow the recess to become open upon melting of the
reagent
containing matrix. Any form of seal may be selected that can retain the
reagent in place and
reduce vaporization and/or fluidic flow loss. For example, a mechanical seal
can be applied as
discussed above.
1 S After a reagent is loaded into the reagent recess and the recess is
sealed, an identifier
may be affixed to the retaining tray as shown by box 430. The identifier may
be used to track
the location and processing of a particular biological specimen. It may also
be used to correlate
a biological specimen with the reagent contained in the recess of the
retaining tray so that
reagent specific processing may be performed. The identifier may also contain
information
about the specimen, requested processing and/or requesting physician. Any type
of identifier
may be used such as, for example, two or three dimensional barcodes, RF>D
devices, scanable
microchips, etc. as will be appreciated by a person having ordinary skill in
the art.
Prior to initiating a procedure, specimens on retaining trays are loaded into
a stainer
that is included in the system. As shown in FIG. 5, a process for loading
retaining trays into
the system may be performed as shown. In the first step, a cover on the
housing of a stainer is
opened as indicated by box S 10. Next, a slide containing a biological
specimen or a specimen
container holding a specimen is selected, as shown diagrammatically as box
520. After a slide
or specimen container is selected a reagent protocol may be determined, as
indicated by box
530, based on the tissue type, a diagnostician's or pathologist's directions,
a predetermined
13
CA 02536036 2006-02-10
procedure or in any other manner. A tray may then be selected based on the
selected reagent
protocol, as indicated by box 540. As described above, the retaining tray may
be preloaded
with a reagent containing matrix so the tray may be specific to certain
reagent protocols.
Therefore, preferably a tray is selected to match the selected reagent
protocol. The slide, or
specimen container, may then be loaded onto the selected tray, as shown by box
550. The slide
may be placed on the retaining tray face down to facilitate the exposure of
the specimen to
fluids flowing through the retaining tray. After the slide or container is
loaded onto the
retaining tray, the retaining tray may be loaded into a stainer, or other
processing apparatus,
included in the automated system, as shown by box 560.
The steps of selecting a slide, determining a reagent protocol, selecting a
tray,
positioning the slide on a tray and positioning the tray on the stainer may be
repeated until the
tray capacity of the stainer is met as indicated by box 570. For example, in
an embodiment, a
stainer of the present system may have a twenty-four (24) tray capacity as
shown in FIG. 2.
Finally, after all of the desired specimens are loaded into the stainer, the
operator may close the
cover as indicated by box 580.
FIG. 6 illustrates diagrammatically an overall system procedure according to
one
embodiment of the invention. An initial step, illustrated diagrammatically as
box 600, a user
account is created. Examples of displays associated with creating a user
account are shown in
FIGS. 7-8. A user account may be created by establishing a user identification
700, name 710
and password 720 through a user setup display shown in FIG. 7. Each user
account may be
assigned an authorization level 730. Such authorization levels 730 may
include, for example,
developer, service, administrator, operator, etc. Each authorization level 730
may have
different privileges enabled for use with the system.
Additionally, an administrator setting up a user may manually limit that
user's ability to
perform various operations. For example, the administrator may prohibit the
user from editing
programs or performing manual operations. An administrator may limit the
user's access to
various operations through a function selection display, as shown in FIG. 8.
The administrator
may select the functions to which a particular user may have access, such as
by selecting a
checkbox 800 associated with the particular function. As shown, an
administrator may control
14
CA 02536036 2006-02-10
the ability of a user to edit programs, tray reagents, cartridge reagents,
bulk solutions
information tables worklists and inventory. The administrator may also control
the user's
ability to enter manual operations, import or export data, archive
information, link to the
Internet, run reports, schedule reports, or to set up system defaults, barcode
format, solution
S bottles, waste bottles, module names, printers or maintenance schedules.
This may be
performed by selecting a checkbox, radio button, toggle switch or other
selectable feature
adjacent a desired function. Any combination of functions may be selected.
After creating the user account, the user may login as illustrated
diagrammatically as
box 610 of FIG. 6. The user may be presented with a login display as shown in
FIG. 9. 'The
user may enter the username 710 and password 720 that were selected during
step 600 of FIG.
6, during which a user account was created, to login to the automated system.
Next, the user may elect or be required to change his/her password as
indicated by box
620 in FIG. 6. In that step, a determination is made by the system to
determine whether a
password change request has been received. If a password change request has
been received, a
change password display, shown in FIG. 10, may be presented to the user as
illustrated
diagrammatically as box 630.
After a user has created an account, they may edit system preferences through
a system
setup display. FIG. 11 illustrates a display 1100 that may be presented to a
user to during an
initial system setup according to an embodiment of the present invention. The
display 1100
may include a name section 1110 that enables the user to assign a name to a
particular system
setup. One or more preference sections 1120 may be provided to enable the user
to assign
system preferences. For example, the preference sections 1120 may enable the
user to assign a
run mode (automatic/manual start), external barcode reader status
(enabled/disabled), language
(English, German, Japanese, Italian, Spanish, French, Portuguese or other
language), short date
format (M/d/yyyy, MMldd/yy, yy/MMlDD, dd-MMM-yy, M/d/yy, MM/dd/yyyy, and yyyy-
MM-dd), long date format (dddd MMMM dd yyyy, ddd dd MMMM yyyy), auto-abort
selection
status (enabled/disabled), overdue worklist time limits (first warning (hrs.),
time limits repeat
interval (hrs)), and product expiry warning.
CA 02536036 2006-02-10
A contact information section 1130 may also be provided to enable the user to
input
contact information such as name, phone number, electronic mail address, web
site or other
information for a sales, customer support or other person associated with the
system.
Selectable function keys 1140 may also be presented to enable the user to save
the settings and
exit display 1100.
After changing the password, or if a password change request has not been
received, the
user may be presented with a main control display as illustrated
diagrammatically as box 640 in
FIG. 6. An initialization procedure may be run after the specimens are loaded
into the stainer
so that specimen and reagent information may be gathered by the system and
presented in the
main control display. An example of an initialization procedure in combination
with
processing protocols are shown in FIG. 12. The initialization procedure occurs
after a start
condition is detected as indicated by box 1210 and may include taking an
inventory of the
retaining trays and reagent cartridges and containers. A start condition may
be, for example,
closing the cover on the housing of a stainer included in the automated
reagent dispensing
I 5 system, receiving a start signal from a control computer, or any other
condition. If a start
condition is not detected, the automated reagent dispensing system may
continually check
whether a start condition is detected until a start condition is detected.
After detecting a start condition, an inventory procedure may be run as
diagrammatically illustrated as box 1220. The inventory procedure may include
the scanning
procedure illustrated in FIG. 13. The scanning procedure scans all trays and
cartridges
provided in the automated reagent dispensing system to determine a status for
the system. As
illustrated diagrammatically as box 1310, a reagent carousel for the automated
reagent
dispensing system is positioned in a home position. The home position is
preferably a zero (0),
or start, position from which the carousel begins each scanning procedure. The
start position
may correspond to a location of the carousel with respect to the slides in
addition to a rotational
start position of the cartridges. For example, the start position may
correspond to the position
of the carousel shown in FIG. 2. The carousel moves by each tray as shown
diagrammatically
as box 1320. As described above, the carousel may include a scanner that scans
an identifier
associated with each tray as shown diagrammatically as box 1330. This enables
the system to
16
CA 02536036 2006-02-10
determine which trays include specimens. After scanning the tray identifiers,
the carousel
returns to the home position (box 1340).
Identifiers associated with reagent cartridges are then scanned as illustrated
diagrammatically as box 1350. By scanning the reagent cartridges, the system
may indicate the
type and quantity of a reagent present in each reagent cartridge. A
determination is made
regarding a cartridge condition for each reagent cartridge (box 1360). The
determination may
indicate a particular type of reagent contained in the cartridge and that the
quantity of reagent in
the cartridge such as in percentage fill of the cartridge (e.g., cartridge is
seventy-five (75)
percent fitll).
Maintaining a history of the quantity of a reagent that has been dispensed may
further
assist in making the determination regarding the quantity of reagent that is
present in the
cartridge. For example, in an embodiment, a cartridge may have a one-hundred (
100) milliliter
capacity for reagent and in an embodiment each time reagent is dispensed from
the cartridge
using the automated reagent dispensing system, one ( 1 ) milliliter of reagent
is dispensed. If the
history indicates that reagent has been dispensed twenty five (25) times from
the cartridge, then
seventy-five (75) milliliters (or seventy-five (75) percent) of the reagent
remains in the
cartridge. After determining the cartridge condition, a cartridge condition
signal may be output
to provide the user with an indication regarding how much and what types of
reagents are
stored in the cartridges (box 1370). As an example of this, a number of
dispenses is designated
as a maximum for each cartridge. With each dispense a mechanical or software
counter is
incremented, and once the maximum number is reached or exceeded, a replace
signal or other
indicator is provided. In a further example, a cartridge depleted signal is
provided which the
system understands as requiring no further usage of the cartridge, and
requiring replacement.
Alternatively, the cartridge can continue being used, but a warning is
provided, notifying an
operator that a fresh cartridge should be installed. As a further example, the
maximum number
of dispenses for each cartridge is pre-programmed in a computer memory, or
alternatively is
noted on a readable (machine or human) indicator on a carnidge label.
Different number of
maximum dispenses can be set for different cartridges or different volume of
dispensing
chambers on particular cartridges.
17
CA 02536036 2006-02-10
If a determination is made that a cartridge i~ empty or that the cartridge
contains an
insufficient amount of reagent to perform a predetermined staining process,
the automated
reagent dispensing system may await an override or replacement signal as
diagrammatically
illustrated as box 1380. The override signal indicates that the user desires
to continue with a
staining process regardless of the cartridge conditions. The replacement
signal indicates that
one or more of the cartridges having an insufficient amount of reagent have
been filled or
replaced. In addition, the system may scan the bulk solution and waste
containers to determine
whether they require refilling, emptying or replacement.
In another embodiment of an initialization process, shown in FIG. 14, an
initial step of
an initialization procedure, illustrated diagrammatically as box 1410, is to
determine whether a
start event (described above) has been detected. If a start event has not been
detected, the
automated reagent dispensing system may continually check whether a start
event has been
detected. After a start event has been detected, the automated reagent
dispensing system may
scan the trays (box 1415) to determine a status of the automated reagent
dispensing system
(described in further detail above). Based on information obtained during
scanning of the trays,
a list sequence may be generated as shown diagrammatically as box 1420.
A scanner of the automated reagent dispensing system is returned to a home
position,
provided the scanner is not already located at the home position, shown
diagrammatically as
box 1425. The scanner then scans the cartridges (box 1430). The cartridges are
scanned to
determine a number of cartridges present and what reagents are present in the
cartridges. After
scanning the cartridges, the scanner may be returned to the home position,
illustrated
diagrammatically as box 1435. The containers provided in the automated reagent
dispensing
system are then scanned to determine a number of containers and which reagents
are present
(box 1440).
Based on the scan of the containers, a determination is made regarding whether
each of
the containers has an acceptable fluid level, illustrated diagrammatically as
box 1445. If a
determination is made that the fluid levels in each of the containers is
acceptable, a ready signal
may be output to, for example, a controller of the automated reagent
dispensing system (box
18
CA 02536036 2006-02-10
1450). The ready signal indicates that the automated reagent dispensing system
is ready to
operate and the staining protocols are run (box 1455).
If a determination is made that the fluid level in any of the containers is
not acceptable,
a container condition signal may be output as illustrated diagrammatically as
box 1460. The
automated reagent dispensing system may then await an override or replacement
signal as
described in further detail above with reference to FIG. 6.
The information that is gathered during the inventory procedures may be stored
in one
or more databases either locally or externally. The databases may be formatted
through
displays that allow the user to input and organize data. FIG. 1 S illustrates
a display 1500 that
may be presented to a user to setup slide information tables. The display 1500
may include a
name field 1510 that enables the user to assign a name to a slide information
table and a
description field 1520 that enables the user to input a description of the
slide information table.
For example, a table associating patient information with a particular slide
may be created. In
addition, a table associating physician information with a particular slide
may be created. It
should be appreciated that any information that would be useful to track may
be recorded in a
table, such as tissue type, dates of collection of a specimen and/or dates of
performing steps in
the processing protocols, etc. Selectable function keys 1530 may also be
presented to enable
the user to save slide information tables and exit the display 1500.
FIG.16 illustrates a display 1600 that may be presented to a user to setup
slide barcode
format according to one embodiment of the present invention. The display 1600
may include a
number field 1610 that indicates a number assigned to a slide barcode, a
length field 1620 that
enables the user to assign a length to the barcode, and a data format field
1630 that enables the
user to assign a data format to the barcode. Selectable function keys 1640 may
also be
presented to enable the user to save slide barcode formats and exit the
display 1600.
As mentioned above, after the system has run the inventory procedures it may
display
the gathered information through a main control display. Examples of main
control displays
1700, 1800 are shown in FIGS. 17 and 18, respectively. The main control window
preferably
displays a substantial graphical replica of the automated reagent dispensing
system. For
example, display 1700 includes a circular graphical representation of
cartridges and an array of
19
CA 02536036 2006-02-10
retaining trays. Display 1800 also provides an illustration of the capacities
of bulk solution
waste bottles. Alternatively, main control display 1800 may only display
retaining trays
included in the automated reagent dispensing system, as shown in FIG. 18. It
shall be
appreciated that both displays may be available and a user may select the
preferred display
using a button 1710, toggle switch or other selector included on the displays.
The main control display preferably enables the user to view which trays and
cartridges
are in use, an amount of reagent available, an amount of waste collected,
pause operation of the
automated reagent dispensing system, add/remove trays/cartridges from
operation, and other
desired functions. The main control display preferably also provides a status
of a staining
protocol being run or the last staining protocol run. Additional information
such as tray and/or
slide information obtained by scanning associated identifiers may also be
displayed.
Upon completion of the inventory procedure, the automated reagent dispensing
system
may receive instruction sequences from a controller as diagrammatically
illustrated as box
1130. The instruction sequences define one or more staining processes to be
applied to the
specimens contained on the slides, or in the containers, provided on the
retaining trays. The
staining processes, as described above, identify the type and quantity of each
reagent that will
be applied to each specimen over a specified period.
During the process of using the system shown in FIG. 6, a determination is
made
regarding whether a request to run a program has been received, as illustrated
diagrammatically
as box 650. A user may make such a request through a main control display such
as those
illustrated in FIGS. 17 and 18. Using the main control display, the user may
request that one or
more pre-programmed routines and/or manual operations be run.
After the slide information is scanned and displayed through the main control
display.
The user may be presented with displays that allow them to manually edit the
gathered
information. FIG. 19 is an illustration of a display 1900 that may be
presented to enable the
user to edit slide information according to one embodiment of the present
invention. The
display 1900 may include a slide identifier 1910. The slide identifier 1910
may be used to
display a name or other identification for a particular slide. The display
1900 may also include
one or more input fields 1920. The input fields 1920 enable the user to
editJinput information
CA 02536036 2006-02-10
regarding a particular slide. The input fields I 920 may include, for example,
patient, physician,
and table information. The display 1900 may also include a tray identifier
1930 and tray
reagent identifier 1940 to identify a tray associated with the particular
slide. Function keys
1950 may also be provided to enable the user to, for example, save information
entered or exit
the display 1900.
FIG. 20 illustrates a display 2000 that may be presented to a user to edit
slide
information tables according to an embodiment of the present invention. The
display 2000 may
include an edit section 2010 that includes one or more edit fields 2020. The
edit fields 2020
may enable the user to edit slide information such as, for example, entry
number, abbreviated
name, and description. An information section 2030 may also be presented that
identifies an
entry number, abbreviated name, and description for one or more slides. One or
more
selectable function keys 2040 may be used to perform various functions. For
example, the user
may add, edit, delete or print a slide information table using the function
keys 2040 or exit the
display 2000.
I 5 After receiving a run program request, the automated reagent dispensing
system runs the
program request as illustrated diagrammatically as box 660 and as further
detailed in FIG. 12.
As shown in FIG. I 2, upon receiving the instruction sequences (step 1230),
staining protocols
are run by the automated reagent dispensing system as diagrammatically
illustrated as box
1240.
While the staining protocols are run, the automated reagent dispensing system
determines whether an interrupt signal has been received, as indicated by box
1250. An
interrupt signal may be caused by, for example, opening of the cover of the
automated reagent
dispensing system, a command received from the controller or other event. If
an interrupt
signal has been received, the automated reagent dispensing system stops
processing as
diagrammatically illustrated as box 1260. A determination is then made
regarding whether a
resume processing signal has been received (box 1270). If a resume processing
signal has not
been received, the automated reagent dispensing system continues to stall
processing (box
1260). If a resume processing signal has been received, however, the automated
reagent
21
CA 02536036 2006-02-10
dispensing system continues to run the staining protocols as dsagrammatscally
shown by box
1240.
If an interrupt signal has not been received, a determination is made whether
processing
has been completed, as indicated by box 1280. Processing may include
completing all staining
protocols for each of the tissue samples provided in the automated reagent
dispensing system.
If the processing has not been completed, the automated reagent dispensing
system continues to
run the staining protocols as diagrammatically shown as box 1240. If a
determination is made
that processing is complete, a processing complete signal may be output to a
controller (box
1290) and the automated reagent dispensing system stops processing as
diagrammatically
illustrated as box 1260.
FIG. 21 illustrates in further detail an interrupt event procedure according
to an
embodiment of the present invention. A determination is made regarding whether
an interrupt
signal has been received (shown diagrammatically as box 2110). If an interrupt
signal has been
received, a determination is made regarding whether a hazardous condition
exists as illustrated
diagrammatically as box 2120. A hazardous condition may be, for example, that
a reagent
having a poisonous gas associated therewith has just been dispensed. Should a
user come in
contact with the poisonous gas, the user may experience illness. If a
determination is made that
a hazardous condition exists, the hazardous process may be completed as
illustrated
diagrammatically as box 2130. A carousel or other reagent cartridge holder is
then moved to a
home position as shown diagrammatically as box 2140. The user is enabled
access to an
interior portion of the automated reagent dispensing system (box 2150). The
user may be
enabled access by, for example, unlocking a lock or other mechanism that
prevents the cover of
the automated reagent dispensing system from being opened.
The automated reagent dispensing system then determines whether a resume
signal has
been received as shown diagrammatically as box 2160. A resume signal may be
caused by
closing the cover or a command output by a controller as described above. If a
resume signal
has not been received, the automated reagent dispensing system continues to
position the
cartridge carousel or holder at a home position (box 2140). If, however, a
resume signal has
22
CA 02536036 2006-02-10
been received, the automated reagent dispensing system resumPa the staining
protocols) as
illustrated diagrammatically as box 2170.
In another embodiment, the process may be fully automated. FIG. 11 illustrates
diagrammatically a fully automated procedure that may be performed by an
automated reagent
dispensing system according to an embodiment of the invention. As an initial
step, a script or
processing program that defines the steps required to perform an automated
staining procedure
is downloaded from a controller. This step is illustrated diagrammatically as
box 2210. The
downloaded script may be based on information obtained by scanning an
identifier associated
with the slide as described above. A primary reagent to be applied to the
slide is determined as
shown diagrammatically as box 2220. The primary reagent information may also
be obtained
from the identifier associated with the slide. One or more staining protocols
to be applied to a
particular slide are determined based on the primary reagent identified as
illustrated
diagrammatically as box 2230. The staining protocols) determined are then
transmitted to a
manifold controller as shown diagrammatically as box 2240. The manifold
controller controls
the carousel on which the cartridges are mounted and the dispensing of
reagents from the
cartridges. The automated reagent dispensing system then runs the protocols,
as
diagrammatically illustrated as box 2250.
One or more status reports may be transmitted from the automated reagent
dispensing
system to, for example, a central controller such as a personal computer or
other controller, as
illustrated by box 2260. Status reports may be transmitted automatically, for
example, on a
periodic basis, or manually upon request by a user using the central
controller. Upon receiving
the status reports, the central controller may display the reports as
diagrammatically illustrated
by box 2270.
Referring back to FIG. 6, a user may elect to create or modify program
requests that are
received, as indicated by box 670. If the user requests that one or more
manual operations be
run, the system enables creating or modifying operations, indicated by box 680
and the user
may be presented with a manual operations display as shown in FIG. 23. Manual
operations
that the user may initiate may include, for example, flush all lines 2310,
flush selected lines
2320, pump selected solution 2330, exercise barcode reader 2340, test printer
2350, etc. The
23
CA 02536036 2006-02-10
display may also present function keys 2360 that allow the user to send a
manual operation
request or to exit the screen. After all operation requests are received by
the system, the user
may elect that the system display the requests that were received as indicated
by boxes 690 and
695 of FIG. 6.
Methods of processing tissue samples in accordance with the present invention
may
include various steps. In an embodiment, a method of processing a tissue
sample using a slide
retaining tray in accordance with the present invention is shown in to FIG.
24. As illustrated
diagrammatically as box 2400, the initial step involves selecting a slide
retaining tray based
upon the type of gel or reagents) contained therein. Of course, the type of
gel (i.e. reagent)
contained within an individual tray is dependent upon the type of test to be
performed on a
tissue sample. In other words, the initial step of selecting a slide retaining
tray may include the
step of determining the type of test to be performed on the tissue sample.
As illustrated diagrammatically as box 2410, the next step involves optionally
swiping a
bar code on a slide or tray. It should be noted that such a step is not
necessary, and
alternatively, no slide data may be read or input, or slide data may be input
manually. As
illustrated diagrammatically as box 2420, the next step involves pulling the
seal from the tray,
thereby exposing the recess and reagent therein. Referring to box 2430, the
next step involves
positioning the slide on the tray. Preferably, the slide is positioned such
that the tissue sample
is disposed between the slide and a platen. As illustrated diagrammatically as
box 2440, the
next step involves optionally positioning the slide retaining tray on a spring
loaded
heating/cooling pad.
As illustrated diagrammatically as box 2450, the next step involves liquefying
a reagent
matrix (i.e., the gel). This step may include the step of heating the matrix
to form a melt.
Alternatively, the matrix may be soluble in a solvent, which is added to the
recess to dissolve it.
Thus, the step of liquefying the matrix alternatively may include the step of
dissolving the gel
using a solvent. Refernng to box 2460, the next step involves flowing the
liquefied reagent
matrix over a drip surface into a gap, or reaction chamber, between the platen
and the slide.
This step may be accomplished with the assistance of gravity.
24
CA 02536036 2006-02-10
Referring to box 2470, the next step optionally involves flushing the gap with
wash
fluids to prepare the tissue sample for subsequent tissue processing steps. As
illustrated
diagrammatically as box 2480, the next step involves optionally dispensing non-
primary
reagents from the fluid dispensing apparatus onto the drip surface of the
retaining tray. Finally,
S referring to box 2490, the next step involves drawing waste and excess fluid
through a fluid
return conduit into a waste reservoir.
With further reference to FIG. 24, the steps illustrated by boxes 2410, 2420,
and 2430
may be performed in any order without departing from the scope of the present
invention.
Additionally, the step of swiping the bar code on the tray (box 2410) can
optionally be
performed after the step of positioning the slide on the tray (box 2430), and
either of these steps
can be eliminated. Further, the step of pulling the seal from the tray (box
2420) can be
performed at any time after the initial step of selecting a tray based upon
the type of gel
contained therein (box 2400).
FIG. 25 illustrates another embodiment of sub-steps associated with a
processing
procedure. According to the embodiment shown in FIG. 25, the processing
procedure includes
dispensing reagents, as shown diagrammatically as box 2510. This may include,
for example,
dispensing reagents from a bulk container to a reagent cartridge. The bulk
containers may be
used to supply the cartridges with additional reagent. The bulk containers may
be operated
manually such that user intervention is required to transfer the reagent from
the bulk container
to the cartridge. This may be done, for example, by operating a switch or
other mechanism that
causes the reagent to travel from the bulk container, through a supply line or
other conduit, to
the cartridge. Alternatively, the automated reagent dispensing system may
automatically fill the
cartridge. This may be performed after the scanning procedure described above
with respect to
the initialization procedures. For example, the scanning procedure may
identify one or more
cartridges that require reagent through a screen such as that shown in FIG.
26. The automated
reagent dispensing system may initiate filling of the cartridges) by causing
reagent from an
appropriate bulk container to travel to the cartridge(s). This may be
performed using a pump or
other known mechanism.
CA 02536036 2006-02-10
The processing procedure also includes dispensing reagents from the cartridges
as
illustrated diagrammatically as box 2520. The reagent may be dispensed from
the cartridge
using, for example, a pump. The cartridges may be provided with a pump that is
actuated by a
solenoid. If a particular reagent is required to be dispensed, the automated
reagent dispensing
system actuates the solenoid associated with that cartridge by transmitting a
signal to the
solenoid. The solenoid pushes the pump and causes a predetermined amount of
reagent to be
dispensed from the cartridge. Preferably, the reagent is dispensed at desired
times and
according to a staining protocol.
The automated reagent dispensing system may also dispense reagents from tray
reagent
containers, or recesses, as shown diagrammatically as box 2530.
Upon completion of a processing procedure, evacuation ports associated with
the trays
may be activated as illustrated diagrammatically as box 2540. The evacuation
ports may be, for
example, holes provided in the trays. A vacuum may be applied to the tray that
causes reagent
located on the tray to be sucked into a waste conduit. According to an
embodiment of the
present invention, the waste may be divided into hazardous and non-hazardous
waste with each
goW g into a respecrive waste container.
The system may also allow for an audit logging procedure to be performed, as
shown in
FIG. 12. In an embodiment, the audit logging procedure may begin by scanning
or otherwise
obtaining information from an identifier provided on a slide as
diagrammatically illustrated as
box 2710. The identifier may be a bar code or other identifier as described
above. Based on
information obtained from scanning the identifier, the slide having the
identifier may be
associated with a particular tray, as indicated by box 2720. Various pieces of
information may
be stored and related to that tray in an audit log, as indicated by box 2730.
For example, a
record of what processes were run at what time and for which patient may be
maintained based
on the slide, tray, and processing protocol information. The audit log may be
stored in a
database as shown diagrammatically as box 2740.
Referring to FIGS. 28-64, various optional screens that may be presented to a
user
throughout the use of the system are described below. FIG. 28 is an
illustration of a display
2800 that may be presented to enable the user to select a program according to
an embodiment
26
CA 02536036 2006-02-10
of the present invention. The display 2800 may include one or more selectable
fields 2810.
The selectable fields 2810 may include information relating to, for example,
tray reagent
identifiers, tray reagent abbreviated names, program identifiers, and program
abbreviated
names. The selectable fields 2810 may enable the user to select a particular
program using the
program identifier or the program abbreviated name or a program associated
with a particular
tray using the tray reagent identifier or the tray reagent abbreviated name.
Upon selecting a
program, information relating to the program may be presented in an
information section 2820.
The information section 2820 may include, for example, a program description
and name of
the author of the program. Other information may also be presented. The
display 2800 may
also include one or more selectable function keys 2830. The function keys 2830
may be used
to, for example, create a new program, set a particular program as a default
program, edit/delete
a particular program, and exit the display 2800.
FIG. 29 illustrates a display 2900 that may be presented to enable a user to
create/edit a
program according to one embodiment of the present invention. The display 2900
may include
a program information section 2910 that provides information regarding the
program. The
information may include, for example, a tray reagent identifier, tray reagent
name, program
identifier, program abbreviated name, program full name, author, editor,
program description,
creation date, and last modified date. The display 2900 may also include a
macro section 2920.
The macro section 2920 may include a macro identifier and a macro name
associated with the
program being created or edited. The macro section 2920 may also include a
selectable edit
macro function that enables the associated macro to be edited.
The display 2900 may also include a number of cycles section 2930, variability
section
2940, and a hold time section 2950. The number of cycles section 2930 enables
the user to
indicate a minimum, maximum, and/or default number of cycles for the program
to perform.
The variability section 2940 enables the user to indicate a minimum, maximum,
and/or default
number for variability within the program. The hold time section 2950 enables
the user to
indicate a minimum, maximum, and/or default holding time for the program. The
holding time
may be indicated in seconds, minutes, hours or any other increment. The macro,
number of
cycles, variability, and hold time sections 2920, 2930, 2940, and 2950,
respectively, may each
27
CA 02536036 2006-02-10
have function keys 2960 associated therewith. The fiznction keys 2960 may
enable the user to
add/edit/clear alUdelete/undo information input into one or more of the macro,
number of
cycles, variability, and hold time sections 2920, 2930, 2940, and 2950,
respectively.
The display 2900 may also include an information section 2970 and program type
S section 2980. The information section 2970 may include information relating
to the program.
The information may include, for example, step, function, reagent, hold time,
platen
temperature, and variability. The program type section 2980 may enable the
user to select a
particular program to run. The user may elect to use a particular program as
the default
program or allow the user to select a program during worklist development.
FIG. 30 illustrates a display 3000 that may be presented to a user to select a
macro
according to one embodiment of the present invention. The display 3000 may
include an
identifier field 3010 and a name field 3020 that indicates an identifier and a
name for a
particular macro. One or more selectable function keys 3030 may be used to
perform various
functions. For example, the user may create, edit or delete a macro using the
function keys
3030 or exit the display 3000.
FIG. 31 illustrates a display 3100 that may be presented to a user to
create/edit a macro
according to one embodiment of the invention. The display 3100 may include a
plurality of
data fields 3110 that enables the user to input or edit information regarding
a particular macro.
The data fields 3110 may include, for example, macro identifier, macro name,
revision, reagent
A, reagent B, waste bottle identifier, minimum cycles, maximum cycles, default
cycles,
variability, emergency substitution solution name, emergency substitution
solution temperature,
extended incubation, minimum hold time, maximum hold time, default hold times,
minimum
hold temperature, maximum hold temperature, and default hold temperature.
Data fields 3110 may also be provided for each reagent associated with the
macro. Data
fields 3110 regarding reagent information may provide information pertaining
to cartridge
reagent identifier, cartridge reagent abbreviated name, bulk solution
identifier and bulk solution
abbreviated name. Other information may also be provided in data fields 3110
such as, for
example, step sequence number, action, total units, platen temperature, pellet
recess
temperature, cycle step, criticality factor, and time.
28
CA 02536036 2006-02-10
An information section 3120 may be provided that provides additional
information
regarding one or more of the data fields 3110. Selectable function keys 3130
may also be
presented to enable the user to create/edit/delete/save a particular macro,
undo/clear
information input, and exit the display 3100.
FIG. 32 illustrates a display 3200 that may be presented to a user to setup
module names
according to one embodiment of the present invention. The display 3200 may
include a module
identification field 3210 that indicates a module identifier assigned to a
module. A name field
3220 may be provided that enables the user to assign a name to the module. A
serial number
field 3230 may be provided that enables the user to assign a serial number to
the module.
Selectable function keys 3240 may also be presented to enable the user to save
module
information and exit the display 3200.
The user may use a send commands display as shown in FIG. 33 to input specific
commands. The user may input a particular command and receive a response
status from the
automated reagent dispensing system regarding the particular command.
FIG. 34 illustrates a display 3400 that may be presented to a user to select a
tray reagent
according to one embodiment of the present invention. The display 3400 may
include an
identifier field 3410 and a name field 3420 that indicates an identifier and a
name for a
particular tray reagent. One or more selectable function keys 3430 may be used
to perform
various functions. For example, the user may create, edit or delete a nay
reagent using the
function keys 3430 or exit the display 3400.
FIGS. 35-36 illustrates displays 3500, 3600 that maybe presented to a user to
create and
edit a tray reagent, respectively, according to one embodiment of the
invention. The displays
3500, 3600 may include a plurality of data fields 3510, 3610 that enable the
user to input or edit
information regarding a particular tray reagent. The data fields 3510, 3610
may include, for
example, tray reagent identifier, tray reagent full name, abbreviated name,
antibody type,
antibody source, clone, dilution, primary pre-treatment, incubation time,
detection system,
hazard level, waste type, stability, price, inventory, minimum stock quantity,
and last received
date.
29
CA 02536036 2006-02-10
Information sections 3520, 3620 may be provided that provides additional
information
regarding one or more of the data fields 3510, 3610. Selectable function keys
3530, 3630 may
also be presented to enable the user to save a particular tray reagent and
exit the displays 3500,
3600. Furthermore, tray reagent category selectors 3540, 3640 may also be
provided. The tray
S reagent category selectors 3540, 3640 enable the user to indicate whether
the tray reagent is a
basic tray reagent or a user-defined tray reagent.
FIG. 37 illustrates a display 3700 that may be presented to a user to select a
cartridge
reagent according to one embodiment of the present invention. The display 3700
may include
an identifier field 3710 and a name field 3720 that indicates an identifier
and a name for a
particular cartridge reagent. One or more selectable function keys 3730 may be
used to perform
various functions. For example, the user may create, edit or delete a
cartridge reagent using the
function keys 3730 or exit the display 3700.
FIGS. 38-39 illustrate displays 3800, 3900 that may be presented to a user to
create and
edit a cartridge reagent, respectively, according to one embodiment of the
invention. The
1 S displays 3800, 3900 may include a plurality of data fields 3810, 3910 that
enable the user to
input or edit information regarding a particular cartridge reagent. The data
fields 3810, 3910
may include, for example, cartridge identifier, full name, abbreviated name,
reagent type,
reagent source, hazard level, waste type, product code, package, stability, on-
board stability,
price, inventory, minimum stock quantity, and last received date.
Information sections 3820, 3920 may be provided that provides additional
information
regarding one or more of the data fields 3810, 3910. Selectable function keys
3830, 3930 may
also be presented to enable the user to save a particular cartridge reagent
and exit the displays
3800, 3900. Furthermore, cartridge reagent category selectors 3840, 3940 may
also be
provided. The cartridge reagent category selectors 3840, 3940 enable the user
to indicate
whether the cartridge reagent is a basic cartridge reagent or a user-defined
cartridge reagent.
FIG. 40 illustrates a display 4000 that may be presented to a user to select a
bulk
solution according to one embodiment of the present invention. The display
4000 may include
an identifier field 4010 and a name field 4020 that indicates an identifier
and a name for a
particular bulk solution. One or more selectable function keys 4030 may be
used to perform
CA 02536036 2006-02-10
various functions. For example, the user may create, edit or delete a bulk
solution using the
function keys 4030 or exit the display 4000.
FIGS. 41-42 illustrate displays 4100, 4200 that may be presented to a user to
create and
edit a bulk solution, respectively, according to one embodiment of the
invention. The displays
4100, 4200 may include a plurality of data fields 4110, 4210 that enable the
user to input or edit
information regarding a particular bulk solution. The data fields 4110, 4210
may include, for
example, solution identifier, full name, abbreviated name, solution type,
hazard level, waste
type, product code, package size, stability, price, inventory, minimum stock
quantity, and last
received date.
Information sections 4120, 4220 may be provided that provides additional
information
regarding one or more of the data fields 4110, 4210. Selectable function keys
4130, 4230 may
also be presented to enable the user to save a particular bulk solution and
exit the displays
4100, 4200. Furthermore, bulk solution category selectors 4140, 4240 may also
be provided.
The bulk solution category selectors 4140, 4240 enable the user to indicate
whether the bulk
solution is a basic bulk solution or a user-defined bulk solution.
FIG. 43 illustrates a display 4300 that may be presented to a user to setup
bulk solution
bottles according to one embodiment of the present invention. The display 4300
may include a
bottle identification field 4310 that indicates an identifier assigned to a
bulk solution bottle. A
bulk solution identification field 4320 may be provided to enable the user to
indicate an
identifier of a bulk solution stored in the bulk solution bottle. A bulk
solution abbreviated
name field 4330 may be provided to enable the user to indicate an abbreviated
name for the
bulk solution. A source field 4340 may be provided to enable the user to
indicate a source for
the bulk solution. A capacity field 4350 may be provided to enable the user to
indicate a
capacity, for example, liters, of the bulk solution bottle. Selectable
function keys 4360 may
also be presented to enable the user to save bulk solution bottle information
and exit the display
4300.
FIG. 44 illustrates a display 4400 that may be presented to a user to setup
waste bottles
according to one embodiment of the present invention. The display 4400 may
include a bottle
identification field 4410 that indicates an identifier assigned to a waste
bottle. A waste type
31
CA 02536036 2006-02-10
identification field 4420 may be provided to enable the user to indicate an
identifier for ~ type
of waste stored in the waste bottle. A waste type field 4430 may be provided
to enable the user
to indicate a type of waste stored in the waste bottle. A location field 4440
may be provided to
enable the user to indicate a location of the waste bottle. A capacity field
4450 may be
S provided to enable the user to indicate a capacity, for example, liters, of
the waste bottle.
Selectable function keys 4460 may also be presented to enable the user to save
bulk solution
bottle information and exit the display 4400.
FIG. 45 is an illustration of a display 4500 that may be presented to enable
the user to
create a worklist according to one embodiment of the present invention. The
display 4500 may
include a worklist identifier 4510 such as a name that identifies the worklist
created. A table
4520 may include sections, rows, columns, etc. providing slide information
4530 and tray
information 4540. The slide information 4530 may include, for example, slide
identification.
The tray information 4540 may include, for example, tray reagent
identification, tray reagent
abbreviated name, program identification, program abbreviated name, and
whether all or a
portion of the tray information 4540 has changed. Other types of slide
information 4530 and
tray information 4540 may also be provided.
The display 4500 may also include selectable function keys 4550 that perform a
desired
function when selected. The keys 4550 may enable users to display slide
details, adjust
program variables, delete entry, print worklist, and close worklist. The keys
4550 may be used
in conjunction with information provided in the table 4520. For example, the
user may select
information related to a particular slide. This selection may be indicated by
having a row in
which the information lies be highlighted. The user may then select one of the
keys 4550 to
perform a particular function related to the information selected. For
example, the user may
select information regarding a particular slide and choose to delete
information regarding that
slide. The user then selects delete entry key 4550 to delete the information
regarding that slide.
Optionally, the user may be presented with a confirmation message requesting
confirmation
from the user that the information selected is to be deleted.
The user may also elect to begin a particular program at a later time. The
user may use
a delayed start function to achieve this. If the user desires to begin a
particular program at a
32
CA 02536036 2006-02-10
later time, the user may be presented with a delayed start display as shown in
FIG. 46. The user
may elect to run a program later the same day, the following day, in two (2)
days, in three (3)
days, etc.
FIG. 47 illustrates a display 4700 that may be presented to a user to setup an
auto print
schedule according to one embodiment of the present invention. The display
4700 may include
a time frame for auto prints section 4710. The time frame for auto prints
section 4710 may
enable the user to select a day of the week, start times, end times, and days
off. The user may
select one or more days of the week to automatically print a schedule by, for
example, selecting
a check box adjacent a named day of the week. The user may also select start
and ends times
by, for example, selecting a time listed in a pull-down menu.
The display 4700 may also include reports fields 4720, schedule fields 4730,
days fields
4740, interval fields 4750, and time fields 4760. The report fields 4720 may
provide a list of
reports for which to automatically schedule to print a particular report. For
example, the user
may select to automatically print reports for overdue worklists, workload
statistics, product
I S expiry warning, overdue maintenance, run history, and other reports. The
schedule fields
section 4730 may provide pull-down menus, radio buttons, check boxes or other
selectable
option to disable or enable scheduled auto prints for a particular report. The
day fields 4740,
interval fields 4750, and time fields 4760 may also enable the user to select
a day, interval, and
time on which to automatically print a particular report. Selectable function
keys 4770 may
also be presented to enable the user to save an auto print schedule and exit
the display 4700.
FIG. 48 illustrates a display 4800 that may be presented to a user to setup
maintenance
schedules according to one embodiment of the present invention. The display
4800 may
include task fields 4810 that enable the user to select one or more tasks for
which to setup a
maintenance schedule. The tasks may include, for example, flush all lines,
flush selected lines,
pump in selected solution, etc. A maintenance schedule field 4820 may be
provided to enable
or disable a maintenance schedule for a particular task. A day field 4830 may
be provided to
enable the user to select a day of the week on which to perform the task. An
interval field 4840
may be provided to enable the user to indicate an interval at which to perform
the task. A time
field 4850 may be provided to indicate a time at which to perform the task.
Selectable function
33
CA 02536036 2006-02-10
keys 4860 may also be presented to enable the user to save the maintenance
schedule and exit
the display 4800.
FIG. 49 illustrates a display 4900 that may be presented to a user to enable
selecting
auto print schedule days off according to one embodiment of the present
invention. The display
4900 may include a calendar view 4910 that enables the user to select a
particular day on the
calendar to designate as a day off. The days off selected by the user may be
presented in a days
off list 4920. Instructions 4930 regarding how to add and delete a day off may
also be
presented in the display 4900. Selectable function keys 4940 may also be
presented to enable
the user to save a days off list and exit the display 4900.
The user may also request that debug commands be run and that response data be
saved
to a particular location. The user may request the debug commands be run using
a debug
system display as shown in FIG. 50. Debug commands may include, for example,
system
status report, error status report, master process memory dump, slave process
memory dump,
etc.
Referring to FIGS. 51-52, screens showing status of bulk solution bottles and
waste
bottles may be displayed. The screens generally indicate information about the
bottles such as
bottle identification. They may also indicate the type. of solution or waste,
as appropriate.
Additionally, they may provide information regarding the capacity, for example
in graphical
form.
FIG. 53 illustrates a display 5300 that may be presented to a user to create a
program
report according to one embodiment of the present invention. The display 5300
may include a
program identifier section 5310 and a program abbreviated name section 5320.
The program
identifier section 5310 and program abbreviated name section 5320 may provide
lists of
program identifiers and abbreviated names, respectively, for which the user
may create or print
a program report. The lists of program identifiers and abbreviated names may
be sorted by
identifier and name, respectively, using sort options 5330. Selectable
function keys 5340 may
also be presented to enable the user to create or print one or more program
reports and exit the
display 5300.
34
CA 02536036 2006-02-10
FIG. 54 illustrates a display 5400 that may be presented to a user to setup a
program
report according to one embodiment of the present invention. The display 5400
may be used to
customize information provided in program reports created by the user. The
display 5400 may
include a program data fields section 5410 that includes one or more data
fields that the user
may select to customize a program report. For example, the program data fields
section 5410
may include selectable options that enable the user to select particular
information to be
included in the report. The information may include, for example, program
identifier, program
full name, program abbreviated name, program description, creation date, last
modify date,
creation author, and modifying author.
The display 5400 may also include a step data fields section 5420 that
includes one or
more fields regarding step data that may be included in the report. The step
data may include,
for example, step sequence number, reagent/primary antibody/probe, time,
platen temperature,
pellet recess temperature, and criticality factor. Selectable function keys
5430 may also be
presented to enable the user to restore or save a program report setup and
exit the display 5400.
FIG. 55 illustrates a display 5500 that may be presented to a user to create a
macro
report according to one embodiment of the present invention. The display 5500
may include a
macro identifier section 5510 and a macro name section 5520. The macro
identifier section
5510 and macro name section 5520 may provide a list of macro identifiers and
names,
respectively, for which the user may create or print a macro report. The lists
of macro
identifiers and names may be sorted by identifier and name, respectively,
using sort options
5530. Selectable function keys 5540 may also be presented to enable the user
to create or print
one or more macro reports and exit the display 5500.
FIG. 56 illustrates a display 5600 that may be presented to a user to setup a
macro report
according to one embodiment of the present invention. The display 5600 may be
used to
customize information provided in macro reports created by the user. The
display 5600 may
include a macro data fields section 5610 that includes one or more data fields
that the user may
select to customize a macro report. For example, the macro data fields section
5610 may
include selectable options that enable the user to select particular
information to be included in
the report. The information may include, for example, macro identifier, macro
name, revision
CA 02536036 2006-02-10
number, product code, reagent location, reagent, waste bottle identifier,
minimum cycle times,
maximum cycle times, default cycle times, minimum hold times, maximum hold
times, default
hold times, minimum hold temperature, maximum hold temperature, default hold
temperature,
extended incubation, emergency substitution solution, and emergency
substitution solution
temperature.
The display 5600 may also include a step data fields section 5620 that
includes one or
more fields regarding step data that may be included in the report. The step
data may include,
for example, step sequence number, time, platen temperature, pellet recess
temperature,
criticality factor, and cycle step (on/off). Selectable function keys 5630 may
also be presented
to enable the user to restore or save a macro report setup and exit the
display 5600.
FIG. 57 illustrates a display 5700 that may be presented to a user to create a
primary
antibody and probe report according to one embodiment of the present
invention. The display
5700 may include an antibody/probe identifier section 5710, an antibody/probe
abbreviated
name section 5720, and an antibody/probe full name section 5730. The
antibody/probe
identifier section 5710, antibody/probe abbreviated name section 5720, and
antibody/probe full
name section 5730 may provide lists of antibody/probe identifiers, abbreviated
names, and full
names, respectively, for which the user may create or print a primary
antibody/probe report.
The lists of antibody/probe identifiers, abbreviated names, and full names may
be sorted by
identifier, abbreviated name, and full name, respectively, using sort options
5740. Selectable
function keys 5750 may also be presented to enable the user to create or print
one or more
primary antibody/probe reports and exit the display 5700.
FIG. 58 illustrates a display 5800 that may be presented to a user to setup a
primary
antibodylprobe report according to one embodiment of the present invention.
The display S 800
may be used to customize information provided in primary antibody/probe
reports created by
the user. The display 5800 may include an antibody and probe data fields
section 5810 that
includes one or more data fields that the user may select to customize a
primary antibody/probe
report. For example, the antibody/probe data fields section 5810 may include
selectable
options that enable the user to select particular information to be included
in the report. The
information may include, for example, antibody identifier, full name,
abbreviated name,
36
CA 02536036 2006-02-10
antibody type, antibody source, clone, dilution, primary pre-treatment,
incubation time,
detective system, MSDS, hazard level, waste type, product code, stability,
price, inventory, last
receive date, and minimum stock quantity. Selectable function keys 5820 may
also be
presented to enable the user to restore or save a primary antibody and probe
report setup and
exit the display 5800.
FIG. 59 illustrates a display 5900 that may be presented to a user to create a
secondary
reagent report according to one embodiment of the present invention. The
display 5900 may
include a secondary reagent identifier section 5910, a secondary reagent
abbreviated name
section 5920, and a secondary reagent full name section 5930. The secondary
reagent identifier
section 5910, secondary reagent abbreviated name section 5920, and secondary
reagent full
name section 5930 may provide lists of secondary reagent identifiers,
abbreviated names, and
full names, respectively, for which the user may create or print a secondary
reagent report. The
lists of secondary reagent identifiers, abbreviated names, and full names may
be sorted by
identifier, abbreviated name, and full name, respectively, using sort options
5940. Selectable
function keys 5950 may also be presented to enable the user to create or print
one or more
secondary reagent reports and exit the display 5900.
FIG. 60 illustrates a display 6000 that may be presented to a user to setup a
secondary
reagent report according to one embodiment of the present invention. The
display 6000 may be
used to customize information provided in secondary reagent reports created by
the user. The
display 6000 may include a secondary reagent data fields section 6010 that
includes one or
more data fields that the user may select to customize a secondary reagent
report. For example,
the secondary reagent data fields section 6010 may include selectable options
that enable the
user to select particular information to be included in the report. The
information may include,
for example, reagent identifier, reagent full name, reagent abbreviated name,
reagent type,
reagent source, MSDS, hazard level, waste type, product code, package size,
stability, on-board
stability, price, inventory, last receive date, and minimum stock quantity.
Selectable function
keys 6020 may also be presented to enable the user to restore or save a
secondary reagent report
setup and exit the display 6000.
37
CA 02536036 2006-02-10
FIG. 61 illustrates a display 6100 that may be presented to a user to create a
bulk
solution report according to one embodiment of the present invention. The
display 6100 may
include a bulk solution identifier section 6110, a bulk solution abbreviated
name section 6120,
and a bulk solution full name section 6130. The bulk solution identifier
section 6110, bulk
solution abbreviated name section 6120, and bulk solution full name section
6130 may provide
lists of bulk solution identifiers, abbreviated names, and full names,
respectively, for which the
user may create or print a bulk solution report. The lists of bulk solution
identifiers,
abbreviated names, and full names may be sorted by identifier, abbreviated
name, and full
name, respectively, using sort options 6140. Selectable function keys 6150 may
also be
presented to enable the user to create or print one or more bulk solution
reports and exit the
display 6100.
FIG. 62 illustrates a display 6200 that may be presented to a user to setup a
bulk
solution report according to one embodiment of the present invention. The
display 6200 may
be used to customize information provided in bulk solution reports created by
the user. The
display 6200 may include a bulk solution data fields section 6210 that
includes one or more
data fields that the user may select to customize a bulk solution report. For
example, the bulk
solution data fields section 6210 may include selectable options that enable
the user to select
particular information to be included in the report. The information may
include, for example,
bulk solution identifier, bulk solution full name, bulk solution abbreviated
name, bulk solution
type, MSDS, hazard level, waste type, product code, package size, stability,
price, inventory,
last receive date, and minimum stock quantity. Selectable function keys 6220
may also be
presented to enable the user to restore or save a bulk solution report setup
and exit the display
6200.
FIG. 63 illustrates a display 6300 that may be presented to a user to create a
waste bottle
report according to one embodiment of the present invention. The display 6300
may include a
module identifier section 6310. The module identifier section 6310 may provide
a list of
module identifiers for which the user may create or print a module report. The
list of module
identifiers may be sorted by identifier using sort option 6320. Selectable
function keys 6330
38
CA 02536036 2006-02-10
may also be presented to enable the user to create or print one or more module
reports and exit
the display 6300.
Finally, the user may be presented with a screen that allows them to manually
print any
of the reports described above, as indicated in FIG. 64. The screen provides
the user with a list
of reports 6410 that the user may select to create. A list of the reports
selected by the user is
also presented (6420). Finally, function keys 6430 may be provided that allow
the user to open
a particular report, print a report, delete a report or exit the screen.
Thus, it is seen that an automated reagent dispensing system and method is
provided.
One skilled in the art will appreciate that the present invention can be
practiced by other than
the various embodiments and preferred embodiments, which are presented in this
description
for purposes of illustration and not of limitation, and the present invention
is limited only by
the claims that follow. It is noted that equivalents for the particular
embodiments discussed in
this description may practice the invention as well.
39