Note: Descriptions are shown in the official language in which they were submitted.
CA 02536047 2006-02-16
Boehringer Ingelheim International GmbH Case 1/1547
55216 Ingelheim foreign filing text
84772fft
Spray-dried amorphous BIBN 4096, process for preparing and the use thereof
as inhalative
s The invention relates to the CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-
dihydro-
2(11~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine (A) and the physiologically acceptable salts thereof which are
stable in the
amorphous state under normal conditions (T < 50°C, relative humidity <
75%) and
are in the form of microparticles, processes for preparing such microparticles
from
~o these substances and the use of these particles for preparing a
pharmaceutical
composition of the inhalable powder type for pulmonary and nasal inhalation,
particularly for preparing a pharmaceutical composition for the treatment of
headaches, migraine and cluster headache.
15 Background to the invention
The CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-~-oxoquinazolin-
3-
yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A)
is known
from International Patent Application PCT/EP97/04862 (published as WO
98/11128)
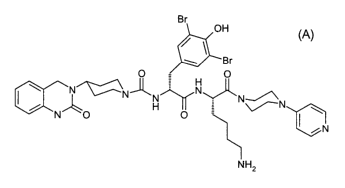
and has the following structure:
Br
OH
w ~Br
O
\ N'~N ~ N
N
N" O H O
(A)
Prior art
The active substance base (A) is a highly effective CGRP antagonist for the
acute
2s and prophylactic treatment of headaches, particularly migraine and cluster
headache,
which cannot be administered orally using conventional formulations, as the
substance has very limited oral bioavailability.
CA 02536047 2006-02-16
- 2 - Case 1/1547
foreign filing text
For treating attacks of migraine it is essential that an active substance is
systemically
available as quickly as possible. The treatment should be uncomplicated for
the
patient to administer and no other conditions which could affect
bioavailability (e.g.
the food effect) should restrict the use of the medicament for the patient.
Active substances which are intended to be systemically available are usually
administered by oral route. If this route is unsuitable or undesirable on
account of
particular properties of the active substance or particular demands made of
the
1o application, other possible ways of administering substances systemically
are known
in the art. For example, inhalation, by means of which active substances may
be
administered systemically as well as topically, has been under discussion for
some
time. For substances which prove critical on account of their decomposition in
solution or which have poor solubility per se, powder inhalation is an option.
The
absolute amount of the active substance which has to be administered per
application makes particular demands of the formulation. On the other hand,
the
physical stability (e.g. aerodynamic particle size, dispersibility,
physicochemical
properties) of the active substance has proved to be a critical requirement
for the
development and production of an inhalable powder.
With formulations of the powder inhalant type, inhalable powders, which are
packaged for example in suitable capsules (inhalettes), are delivered to the
lungs by
means of powder inhalers. Similarly, other systems in which the quantity of
powder
to be administered is pre-dosed (e.g. blisters),are also known as multidose
powder
systems. Alternatively, the medicament may also be inhaled by the use of
suitable
powdered inhalable aerosols which are suspended for example in HFA134a,
HFA227 or mixtures thereof as propellant gas.
In powder inhalation, the microparticles of a pure active substance are
administered
so through the airways onto the surface of the lungs, e.g. in the alveoli, by
the inhalation
process. These particles settle on the surtace and can only be absorbed into
the
body after the dissolving process by active and passive transporting
processes.
CA 02536047 2006-02-16
- 3 - Case 1/1547
foreign filing text
Inhalation systems are known in the literature in which the active substance
is
present in the form of solid particles either as a micronised suspension in a
suitable
solvent system as carrier or in the form of a dry powder.
Usually, powder inhalants, e.g. in the form of capsules for inhalation, are
prepared on
s the basis of the general teaching as described in DE-A-179 22 07.
A critical factor in multi-substance systems of this kind is the uniform
distribution of
the pharmaceutical composition in the powder mixture.
The pharmaceutical active substance used to prepare the above-mentioned
pharmaceutical composition should be as pure as possible and its stability on
long-
~o term storage must be guaranteed under different environmental conditions.
This is
absolutely essential in order to prevent the use of pharmaceutical
compositions in
which breakdown products, for example, are present together with the active
substance itself.
15 Apart from the requirements concerning chemical stability of the active
substance s
outlined above it must be generally borne in mind that any change to the solid
state
of a pharmaceutical composition or to the active substance used which improves
its
physical and chemical stability gives a considerable advantage over less
stable forms
of the same pharmaceutical composition. Different physical / physicochemical
2o properties may, however, bring about improved pharmacological /
pharmacokinetic
properties of the pharmaceutical composition in some cases. In particular,
depending
on the formulation, special morphological properties of solid particles may be
beneficial to the preparation of a pharmaceutical composition.
25 It is known from the literature that particles in the submicron range can
be produced
by spray-drying. Usually, industrially suitable formulations which exhibit
sufficient
dispersibility in medical use (inhalation) may be prepared from spray-dried
particles
of this kind in accordance with the method cited above (DE-A-179 22 07) [Y.-F.
Maa,
P.-A. Ngyuyen, J.D. Andya, N. Dasovich, T.D. Sweeny, S.J. Shire, C.C. Hsu,
so Pharmaceutical Research, 15, No. 5 (1998), 768-775; M.T. Vidgren, P.A.
Vidgr~n,
T.P. Paronen, Int. J. Pharmaceutics, 35 (1987), 139-144; R.W. Niven, F.D.
Lott, A.Y.
Ip, J.M. Cribbs, Pharmaceutical Research, 11, No. 8 (1994), 1101-1109].
CA 02536047 2006-02-16
- 4 - Case 1/1547
foreign filing text
It is also known from the literature that using special methods it is possible
to
produce so-called "large porous particles" which have proved particularly
suitable for
use in powder inhalants (D.A. Edwards, J. Hanes, G. Caponetti, J. Hrkach, A.
Ben-
Jebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lotan, R. Langer, Science, 276
(1997)
1868-1871 ). By these are meant particles with a mean geometric size of more
than 5
Nm (e.g. 8.5 pm to 20 pm), which behave aerodynamically in the same way as
particles less than 5 pm in size, these powders also being characterised by an
extremely low density (< 0.4 g/cm3).
1o Statement of the problem
The complex problem of the present invention was thus primarily to provide
novel
stable microparticles of the active substance base 1-[N2-[3,5-dibromo-N-[[4-
(3,4-
dihydro-2(11-~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-
4-(4-
pyridinyl)-piperazine (A) and the physiologically acceptable salts thereof,
which meet
the stringent requirements mentioned above that are imposed on a
pharmaceutical
active substance for a powder inhalant for pulmonary and nasal inhalation and
compared with conventional micronised starting material (obtained e.g. by air-
jet
grinding) have proved suitable for use as powder inhalants in terms of their
pharmacological / pharmacokinetic properties. According to the invention the
2o morphology of the microparticles was to be optimised so that the
formulation
consisting thereof preferably contains no excipient and hence consists
exclusively of
active substance.
The formulation according to the invention should also exhibit a rapid onset
of activity
2s for the treatment of the acute pain which occurs very suddenly in the case
of
migraine. This means that rapid absorption of the active substance and a rapid
increase in the plasma level must be guaranteed.
Detailed description of the invention
so A rapid onset of activity for the treatment of acute pain as well as a high
plasma level
of the CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1l-~-
oxoquinazolin-3-
yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A)
and the
physiologically acceptable salts thereof within a very short time can best be
achieved
through the lungs as the site of absorption.
CA 02536047 2006-02-16
- 5 - Case 1/1547
foreign filing text
It has been found that when the active substance (A) is administered by
inhalation in
the form of a powder inhalant a bioavailability of about 60% can be achieved
based
on the fine content of the formulation (corresponding to FPD "fine particle
dose"',
determined according to USP 24 Suppl. 2000).
The present invention therefore consists in the preparation of novel, stable
microparticles of the CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-
2(11~-
oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine (A) and the physiologically acceptable salts thereof, which are
surprisingly
1o especially suitable for preparing powder inhalants for pulmonary and nasal
inhalation.
They are characterised by special physical and physicochemical properties,
which
lead to an improved pharmacological as well as pharmacokinetic activity when
the
substance is inhaled. One surprising feature is that by varying/optimising the
particle
shape of these particles with a large specific surface area, the aerodynamic
15 properties and also the increase in dispersibility and inhalability can be
improved.
The invention also includes the chief method of preparing microparticles of
this kind
and the use thereof for preparing pharmaceutical compositions in the form of a
powder inhalant.
2o According to the invention in addition to the active substance base the
corresponding
physiologically acceptable acid addition salts are used which are selected for
example from among 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-IJ-oxoquinazolin-
3-
yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine
hydrochloride,
sulphate, phosphate, hydrobromide, carbonate, methanesulphonate, p-
25 toluenesulphonate, nitrate, citrate, malate, tartrate, lactate, succinate,
gluconate,
acetate, formate, propionate, capronate, oxalate, maleate, fumarate, mandelate
and
hydroxysuccinate, while the 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11~-
oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine hydrochloride, the sulphate and the hydrobromide are preferred and
the
30 1-[NZ-[3, 5-d i bromo-N-[[4-(3,4-d i hyd ro-2( 11-x-oxoq a i nazoli n-3-yl)-
1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine
hydrochloride as
well as the free active substance base are particularly preferred.
CA 02536047 2006-02-16
- 6 - Case 1/1547
foreign filing text
Particle geometries of the microparticles which have proved advantageous may
be
described as collapsed hemispheres and have a crinkled structure. In terms of
geometry, particles prepared by the processes described below have particle
shapes
which may be described, depending on the test conditions, between the extremes
of
"spherical shell fragment", "thin-walled, totally collapsed sphere ",
"crinkled, filigree-
flaked platelet structure", as well as "rosette-like crinkled structure".
These particles are characterised in that
(a) they have a specific surface area between 3 mz/g and 35 m2/g, preferably
between 5 m2/g and 30 m2/g and particularly preferably between 10 m2/g and
30 m2/g,
(b) the characteristic Q~S.a~ is between 50% and 100% and
(c) the parameter X5o is in the range from 0.5 Nm to 10 Nm, preferably from
0.5
Nm to 6 Nm.
The crinkled microparticles according to the invention are suitable for
preparing
2o powder inhalants for pulmonary and nasal inhalation, in which no other
excipients or
additives (carrier materials) are needed in order to obtain an industrially
workable
powder which can be further processed directly and which has excellent
properties in
terms of dispersibility and is sufficiently easy to process with regard to its
cohesive
properties.
In a first aspect the present invention thus relates to a powder inhalant for
pulmonary
and nasal inhalation, comprising the CGRP antagonist 1-[NZ-[3,5-dibromo-N-[[4-
(3,4-
dihydro-2(1 f-~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-
lysyl]-4-(4-
pyridinyl)-piperazine (A) or one of the physiologically acceptable salts
thereof in the
so form of crinkled microparticles, characterised in that
(a) they have a specific surface area between 3 m2/g and 35 m2/g, preferably
between 5 m2/g and 30 mZ/g and particularly preferably between 10 m2/g and
m2/g,
CA 02536047 2006-02-16
7 - Case 1 /1547
foreign filing text
(b) the characteristic Q~S.s> is between 50% and 100% and
(c) the parameter X5o is in the range from 0.5 Nm to 10 Nm, preferably from
0.5
s pm to 6 Nm.
The crinkled microparticles according to the invention are however also
suitable for
preparing powder inhalants wherein the active substance is administered
together
with an excipient.
Normal carrier materials or flow adjuvants may be used as physiologically
acceptable
homogeneous excipients according to the invention. The normal carrier
materials
may be selected from among the monosaccharides (e.g. glucose or arabinose),
disaccharides (e.g. lactose, saccharose, maltose, trehalose), oligo- and
polysaccharides (e.g. dextrans, starch, cellulose derivatives), polyalcohols
(e.g.
mannitol, sorbitol, xylitol), salts (e.g. sodium chloride, calcium carbonate),
polylactides, polyglycolides and mixtures of these excipients. The flow
adjuvants may
for example be selected from a group consisting of magnesium stearate, calcium
stearate, stearic acid, stearyl alcohols, calcium behenate, calcium
arachinate,
2o hydrogenated vegetable oils such as for example hydrogenated castor oil or
hydrogenated cottonseed oil, fatty acid esters, sodium stearyl fumarate,
sodium
dodecyl sulphate, magnesium dodecyl sulphate and mixtures of these flow
adjuvants.
The method of preparing the microparticles according to the invention is
2s characterised in that the active substance is suitably dissolved, sprayed
and dried in
a spraying tower. The particle morphology including the particle size of these
microparticles can be deliberately controlled by the choice of process
parameters
and production parameters.
ao In a second aspect the present invention thus relates to a process for
producing the
microparticles of the active substance base (A) according to the invention,
comprising the following steps:
CA 02536047 2006-02-16
- $ - Case 1/1547
foreign filing text
(a) dissolving the active substance (A) in an organic solvent or an organic-
aqueous solvent mixture to prepare a sprayable solution with a concentration
of active substance of between 0.2 and 4 wt.%, preferably between 0.2 wt.%
and 3 wt.%, particularly preferably between 0.3 wt.% and 2 wt.%,
(b) spraying the active substance solution thus obtained in the usual way, so
as to
obtain a spray mist with a droplet size having the characteristic X5o from 1
to
50 Nm, preferably from 1 Nm to 30 pm, particularly preferably from 1 Nm to 20
Nm,
(c) drying the spray mist thus obtained using a drying gas while applying the
following parameters:
(i) an entry temperature of the drying gas from 100°C to 350°C,
preferably
from 120°C to 250°C and particularly preferably from
130°C to 200°C
and
(ii) an exit temperature of the drying gas from 40°C to 120°C
and
(d) separating the dried solid fraction from the current of drying gas in the
usual
way.
Preferably the microparticles of the active substance base (A) according to
the
invention are prepared by a method comprising the following steps:
(a) dissolving the active substance (A) in an organic solvent or an organic
aqueous solvent mixture in order to prepare a sprayable solution with a
concentration of active substance of between 0.2 and 4 wt.%, preferably
between 0.2 wt.% and 3 wt.%, particularly preferably between 0.3 wt.% and 2
wt.%,
(b) spraying the active substance solution thus obtained in the usual way with
a
flow volume of spray gas of from 1 Nm3/h to 15 Nm3/h, so as to obtain a spray
CA 02536047 2006-02-16
- g - Case 1 /1547
foreign filing text
mist with a droplet size having the characteristic X5o from 1 to 50 pm,
preferably from 1 Nm to 30 Nm, particularly preferably from 1 pm to 20 Nm,
(c) drying the spray mist thus obtained using a drying gas while applying the
s following parameters:
(i) an entry temperature of the drying gas from 100°C to 350°C,
preferably
from 120°C to 250°C and particularly preferably from
130°C to 200°C,
(ii) an exit temperature of the drying gas from 40°C to 120°C
and
(iii) a flow volume of the drying gas from 15 Nm3/h to 150 Nm3/h and
(d) separating the dried solid fraction from the current of drying gas in the
usual
1 s way.
Organic solvents, organic-aqueous solvent mixtures and water have proved
suitable
as solvents for preparing a sprayable solution of the active substance base.
Preferably an alcoholic or alcoholic-aqueous solvent system is used,
particularly
2o preferably a solvent mixture consisting of ethanol/methanol/water or
ethanol/propanol/water and most particularly preferably the solvent mixture of
ethanol/water or the solvent absolute ethanol, methanol or water.
In a third aspect the present invention relates to a process for preparing the
25 microparticles of the salts of the active substance base (A) according to
the
invention, comprising the following steps:
(a) dissolving the active substance base (A) in water or an aqueous buffer
system
and adding the corresponding acid in order to prepare a sprayable salt
ao solution of the active substance with a concentration of active substance
of
between 0.2 and 4 wt.%, preferably between 0.2 wt.% and 3 wt.%, particularly
preferably between 0.3 wt.% and 2 wt.%,
CA 02536047 2006-02-16
- 10 - Case 1 /1547
foreign filing text
(b) spraying the active substance solution thus obtained in the usual way, so
as to
obtain a spray mist with a droplet size having the characteristic X5o from 1
to
50 Nm, preferably from 1 Nm to 30 Nm, particularly preferably from 1 Nm to 20
Nm,
(c) drying the spray mist thus obtained using a drying gas while applying the
following parameters:
(i) an entry temperature of the drying gas from 100°C to 350°C,
preferably
1o from 120°C to 250°C and particularly preferably from
130°C to 200°C
and
(ii) an exit temperature of the drying gas from 40°C to 120°C
and
(d) separating the dried solid fraction from the current of drying gas in the
usual
way.
Preferably the microparticles of the salts of the active substance base (A)
according
2o to the invention are prepared by a method comprising the following steps:
(a) dissolving the active substance base (A) in water or an aqueous buffer
system
and adding the corresponding acid in order to prepare a sprayable salt
solution of the active substance with a concentration of active substance of
between 0.2 and 4 wt.%, preferably between 0.2 wt.% and 3 wt.%, particularly
preferably between 0.3 wt.% and 2 wt.%,
(b) spraying the active substance solution thus obtained in the usual way with
a
flow volume of spray gas of from 1 Nm3/h to 15 Nm3/h, so as to obtain a spray
so mist with a droplet size having the characteristic X5o from 1 to 50 Nm,
preferably from 1 Nm to 30 Nm, particularly preferably from 1 Nm to 20 Nm,
while applying the following parameters:
CA 02536047 2006-02-16
- 11 - Case 1/1547
foreign filing text
(c) drying the spray mist thus obtained using a drying gas while applying the
following parameters:
(i) an entry temperature of the drying gas from 100°C to 350°C,
preferably
from 120°C to 250°C and particularly preferably from
130°C to 200°C,
(ii) an exit temperature of the drying gas from 40°C to 120°C
and
(iii) a flow volume of the drying gas from 15 Nm3/h to 150 Nm3/h and
(d) separating the dried solid fraction from the current of drying gas in the
usual
way.
Water or an aqueous buffer system with a pH between 6 and 8 have proved
suitable
1s as solvents for preparing a sprayable solution of the salt forms of the
active
substance base (A). The active substance according to the invention which is
present
in the form of the free base is dissolved in an aqueous solution, which is
combined
with 0.9 to 1.1 equivalents of acid, according to the quantity of active
substance to be
dissolved, in the form of the corresponding salt. The acids according to the
invention
2o are preferably inorganic acids (for example hydrochloric acid, hydrobromic
acid,
phosphoric acid, nitric acid, sulphuric acid, carbonic acid), fruit acids (for
example
citric acid, malic acid, tartaric acid, lactic acid, succinic acid, gluconic
acid), carboxylic
acids (for example formic acid, acetic acid, propionic acid, hexanoic acid) as
well as
other organic acids such as oxalic acid, methanesulphonic acid, p-
toluenesulphonic
25 acid, fumaric acid, mandelic acid or malefic acid; it is particularly
preferable to use
hydrochloric acid, hydrobromic acid or sulphuric acid and particularly
hydrochloric
acid.
The surface qualities of the particles can be optimised by adjusting the ratio
between
so the droplet size and solids concentration. Normally a concentration of
between 0.2
and 4 wt.%, preferably between 0.2 and 3 wt.%, most preferably between 0.3 and
2
wt.% is selected.
CA 02536047 2006-02-16
- 12 - Case 1 /1547
foreign filing text
The droplet size is a crucial parameter in the production of inhalable
particles.
Depending on the nozzle used the throughput of spray gas combined with the
throughput of solution should be selected to achieve the desired droplet size.
As
there are a number of combinations of the parameters nozzle / throughput of
spray
gas / throughput of solution leading to a suitable droplet size, the process
can
usefully be defined by the droplet size which is obtained with the same nozzle
parameters with water at ambient temperature. These may be described by the
characteristic X5o (median value of droplet size below which 50°/a by
volume of the
droplet fraction falls), which should be in the range from 1 Nm to 50 pm.
The critical characteristics which impinge on the drying step are the entry
and exit
temperature of the drying gas, as well as the flow volume of the drying gas
passing
through.
In a fourth aspect the present invention relates to the use of the crinkled
microparticles produced by the processes described hereinbefore for the
production
of a powder inhalant.
In a fifth aspect the present invention relates to the microparticles
according to the
2o invention, which can be obtained according to the processes described
above.
CA 02536047 2006-02-16
- 13 - Case 1/1547
foreign filing text
Experimental section
1) Methods of measurement
s a) Determining the particle size by laser diffraction (Frauenhofer
diffraction):
Measuring method: In order to determine the particle size the powder is fed
into a laser diffraction spectrometer using a dispersing unit.
The median value X5o refers to the particle size below which
to 50% of the quantity of particles fall. The Q~s.a~ value
describes the percentage of particles which are less than
5.8 Nm in size.
Measuring device: Laser diffraction spectrometer (HELOS), Messrs. Sympatec
Software: WINDOX 4
15 Dispersing unit: RODOS / dispersing pressure: 3 bar
Focal length: 100 mm [measuring range: 0.9.....175 pm]
Evaluation method: HRLD (V 4)
b) Determining the Specific Surface Area:
Measuring method: The specific surface is determined by exposing the powder
sample to a nitrogen atmosphere at different pressures. Cooling
the sample causes the nitrogen molecules to be condensed on
the surface of the particles. The quantity of condensed nitrogen
2s is determined by means of the drop in pressure in the system
and the surface of the sample is calculated by means of the
surface nitrogen requirement and the weight of the sample.
so Measuring device: Tri Star Multi Point BET, Messrs. Micromeritics
Heating station: VacPrep 061, Messrs. Micromeritics
Heating: approx. 12 h / 40 °C
CA 02536047 2006-02-16
- 14 - Case 1 /1547
foreign filing text
Analysis parameters
sample tube: 'h inch; with filler rod
analysis method: 16 point BET surface measurement
0.05 to 0.20 p/p0
absolute pressure tolerance: 5.0 mm Hg
relative pressure tolerance: 5.0%
evacuation rate: 50.0 mm Hg/second
evacuation threshold: 10.0 mm Hg
evacuation time: 0.1 h
1 o free space: lower Dewar, t: 0.5 h
retention time: 20 seconds
minimum equilibration delay: 600 seconds
adsorptive: nitrogen
C) Determining the droplet size by laser diffraction (according to Mie):
Measuring device: Laser diffraction spectrometer (HELOS), Messrs. Sympatec
Software: WINDOX 4
Focal length: 100 mm [measuring range: 0.9.....175 Nm]
2o Measuring method: The droplet size is determined by removing the nozzle
from the spray dryer and placing the spray in the upper
third of the spray cone in the centre of the laser beam.
Measuring is done at ambient temperature with water as
reference medium under otherwise identical conditions.
CA 02536047 2006-02-16
- 15 - Case 1 /1547
foreign filing text
2) Examples of spray parameters
Exa J~le: Spray parameters suitable for an alcoholic solution of (A) (NIRO
Spray
dryer SD Micro):
Concentration solution 0.5 g (A) in 100 mL ethanol
Droplet size X5o 11 Nm
(Reference solution: Hz0 at
ambient
tem erature
Flow volume "spray rate" 4.5 mL / min
Spray pressure 0.7 bar overpressure (N2)
(nozzle type) (Niro spray nozzle 0.5
mm,
Art.-Nr. 248709/A)
Flow volume "Atomising pressure"2.2 kg / h
(nozzle type) (Niro spray nozzle 0.5
mm,
Art.-Nr. 248709/A)
entry temperature 149C
exit temperature 96C
Flow volume of "drying gas" 20.1 kg / h
cross section of drying tower200 mm
3) Characterisation of the solid particles obtained in the above Example:
~ o Example:
Particle size X5o ~ 1.2 ~m
Q~s.s> ~ 99.5%
Specific surface area Sm 21.5 mz/g
CA 02536047 2006-02-16
- 16 - Case 1 /1547
foreign filing text
Brief description of the Figures
Figures 1 and 2 show photographs of microparticles of the active substance
base
(A), prepared from an alcoholic spray solution by the process according to the
invention.