Note: Descriptions are shown in the official language in which they were submitted.
CA 02536069 1994-03-14
-1-
USE OF GREEN PORPHYRINS
IN OCULAR DIAGNOSIS AND THERAPY
This application is a division of Canadian Application Serial Number
2,185,644, which is the national phase applicat=ion of PCT International
Application PCT/US94/02639, filed 14 March 1994.
Technical Field
The invention is in the field of photodynamic
therapy, specifically related to ocular conditions. More
particularly, the invention concerns the use of green
porphyrins in photodynamic therapeutic treatment of
pigmented tumors and conditions characterized by unwanted
neovasculature in the eye. Green porphyrins are also
useful as dyes in ocular angiography.
Bac round Art
Choroidal neovascularization leads to
hemorrhage and fibrosis, with resultant visual loss in a
number of eye diseases, including macular degeneration,
ocular histoplasmosis syndrome, myopia, and inflammatory
diseases. Age-related macular degeneration is the
leading cause of new blindness in the elderly, and
choroidal neovascularization is responsible for 60t of
the severe visual loss in patients with this diseases.
Although the natural history of the disease is eventual
quiescence and regression of the neovascularization
process, this usually occurs at the cost of sub-retinal
fibrosis and vision loss.
Current treatment relies on occlusion of the
blood vessels using laser photocoagulation. However,
such treatment requires thermal destruction of the
neovascular tissue, and is accompanied by full-thickness
retinal damage, as well as damage to medium and large
choroidal vessels. Further, the subject is lef t with an
atrophic scar and visual scotoma. Moreover, recurrences
are common, and visual prognosis is poor.
CA 02536069 1994-03-14
-2-
Developing strategies have sought more
selective closure of the blood vessels to preserve the
overlying neurosensory retina. One such strategy is
photodynamic therapy, which relies on low intensity light
exposure of photosensitized tissues to produce
photochemical effects. Photosensitizing dyes are
preferentially retained in tumors and neovascular tissue,
which allows for selective treatment of the pathologic
tissue. As a result of the i.nvention, PDT may be used to
cause vascular occlusion in tumors by damaging
endothelial cells, as well as a direct cytotoxic effect
on tumor cells.
Photodynamic therapy of conditions in the eye
characterized by neovascularization has been attempted
over the past several decades:using the conventional
porphyrin derivatives such as hematoporphyrin derivative
and Photofrin porfimer sodium. Problems have been
encountered in this context due to interference from eye
pigments. In addition, phthalocyanine has been used in
photodynamic treatment.
A newer photosensitizer, a member of the group
designated "green porphyrins", is in the class of
compounds called benzoporphyrin derivatives (BPD). This
photosensitizer has also been tested to some extent in
connection with ocular conditions. For example, Schmidt,
U. et al. described experiments using BPD for the -
treatment of Greene melanoma (a nonpigmented tumor)
implanted into rabbit eyes and achieved ne.crosis in this
context (JQVS (1992) 3a:1253 Abstract 2802). Lin, C.P.
et al. describe the measurement of kinetics and
distribution in retinal and choroidal vessels by
fluorescence imaging using a 458 nm line from an argon-
ion laser to excite BPD (IOVS (1993) 3g:1168 Abstract
2293). In addition, Lin, S.C. et al. described
CA 02536069 2007-02-26
-3-
photodynamic closure. of choroidal vessels using BPD in IOVS (1993)
34:1303 Abstract 2953.
The present applicants have described treating choroidal
neovascularization using BPD in several abstracts published 15 March
1993. These abstracts include Schmidt-Erfurth, U. et al.
"Photothrombosis of Ocular Neovascularization Using BPD"; Haimovici,
R. et al. "Localization of Benzoporphyrin Derivative Monoacid in the
Rabbit Eye"; and Walsh, A.W. et al. "Photodynamic Therapy of
Experimental Choroidal Neovascularization Using BPD-MA." All of the
foregoing are published in IOVS (1993) 34:1303 as abstracts 256,
255 and 254, and Moulton, R.S. et al. "Response of Retinal and
Choroidal Vessels to Photodynamic Therapy Using Benzoporphyrin
Derivative Monoacid", IOVS (1993) 34:1169 Abstract 58.
The green porphyrins offer advantages in their selectivity
for neovasculature and in their ability to effect photodynamically
mediated destruction of nonpigmented tumors of the eye.
Gonzales et al. reported photodynamic therapy of pigmented
melanomas in the eye using phthalocyanine as a photoactive compound
(Gonzales, Y.H. et al. (IOVS (1993) 34:891 Abstract 949). The use
of BPD-MA in photodynamic treatment of the nonpigmented Greene
hamster melanoma in rabbit eyes was also described by Schmidt-Erfurth,
U. et al. in Ophthalmology (1994) 101:89-99. This report was
presented in part at the ARVO annual meeting in Sarasota, Florida,
May 1992.
Disclosure of the Invention
The invention is directed to diagnosis and treatment of
certain conditions of the eye using photodynamic methods and
employing green porphyrins as the photoactive compounds. The green
porphyrins of the invention are described in U.S. Patent Nos.
4,883,790;
CA 02536069 1994-03-14
-4-
4,920,143; 5,095,030; and 5,171,749. These materials offer
advantages of selectivity and effectiveness when employed in
protocols directed to the destruction of unwanted neovasculature and
pigmented tumors. They are also particularly effective as
visualizing agents in angiography of ocular blood vessels. The
visualization of the compounds is enhanced by their ability to
fluoresce.
Accordingly, in one aspect, the invention is directed to a
method to treat conditions of the eye characterized by unwanted
neovasculature, which method comprises administering to a subject in
need of such treatment an amount of a green porphyrin that will
localize in said neovasculature; and irradiating the neovasculature
with light absorbed by the green porphyrin.
In another aspect, the invention is directed to a method to
treat pigmented tumors in the eye, which method comprises
administering to a subject in need of such treatment an amount of a
green porphyrin that will localize in said tumor; and irradiating
the tumor with light absorbed by the green porphyrin.
In still another aspect, the invention is directed to a
method to observe the condition of blood vessels in the eye, which
method comprises administering to a subject comprising at least one
eye for which said observation is desired an amount of green
porphyrin which will provide an observable amount of green porphyrin
in the blood vessels of said eye, permitting sufficient time to
elapse so that an observable amount of said green porphyrin resides
in the eyes; and observing the blood vessels in which the green
porphyrin resides.
In another aspect, the present invention provides a
porphyrin dye for use with light having an irradiance of about 600
mW/cm2 to treat unwanted choroidal neovasculature, the light being
absorbed by the porphyrin dye.
In another aspect, the present invention provides a green
prophyrin dye for use with light having an irradiance of about 600
CA 02536069 1994-03-14
-4a-
mW/cmz to treat age-related macular degeneration, the light being
absorbed by the porphyrin dye.
In another aspect, the present invention provides use of a
porphyrin dye and light having an irradiance of about 600 mW/cm2 for
treatment of unwanted choroidal neovasculature, the light being
absorbed by the porphyrin dye.
In another aspect, the present invention provides use of a
green porphyrin dye and light having an irradiance of about 600 mW/cm2
for treatment of age-related macular degeneration, the light being
absorbed by the porphyrin dye.
In another aspect, the present invention provides a method
of treating unwanted choroidal neovasculature in a shortened
treatment time, the method comprising the steps of: administering to
a primate subject in need of such treatment an amount of a porphyrin
dye sufficient to permit an effective amount to localize in the
neovasculature; and irradiating the neovasculature with light at an
irradiance of about 600 mW/cmz, the light being absorbed by the
porphyrin dye so as to occlude the neovasculature.
In another aspect, the present invention provides a method
of treating age-related macular degeneration in a primate subject
having unwanted choroidal neovasculature in a shortened treatment
time, the method comprising the steps of: administering to the
primate subject an amount of a green porphyrin dye sufficient to
permit an effective amount to localize in the neovasculature; and
irradiating the neovasculature with light at an irradiance of about
600 mW/cm2, the light being absorbed by the porphyrin dye so as to
occlude the neovasculature.
CA 02536069 1994-03-14
-5-
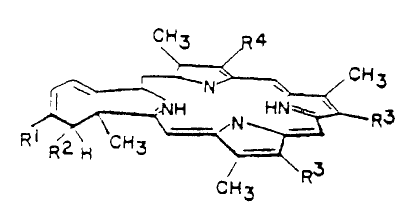
Brief Description of the Drawings
. Figure 1 shows preferred forms of the green
porphyrins useful in the methods of the invention.
Modes of Carrying Out the Invention
The methods of the claimed invention
administering to a subject a green porphyrin,-whic.h is in
the class of compounds called benzoporphyrin derivatives
(BPD). A BPD is a synthetic chlorin-like porphyrin with
a number of structural analogues, as shown in Figure 1.
Preferably, the green porphyrin is
benzoporphyrin derivative mono-acid ring A (BPD-MA),
which absorbs light at-about 692 nm wavelength with
improved tissue penetration properties. BPD-MA is
lipophilic, a potent photosensitizer, and appears to be
phototoxic to neovascular tissues and tumors.
In a preferred embodiment, the green porphyrin
is prepared as a liposomal preparation or is coupled to a
ligand that binds to a specific surface component of the
neovasculature to improve even further its effectiveness
as a photosensitizer. Preferably, the ligand comprises
an antibody or an immunologically reactive fragment
thereof. The capacity for selective localization of a
green porphyrin can also be improved by coupling to a
carrier molecule that potentially delivers higher
concentrations of the green porphyrin to the target
tissue.
A carrier that is appropriate for clinical use
is human low-density lipoprotein (LDL). Human LDL is a
physiologic serum protein rnetabolized by cells via uptake
by high affinity receptors. LDL exhibits desirable
characteristics as a selective carrier because LDL
metabolism is increased in tumor cells. Neoplastic
tissues and neovascularization have been shown to have
increased numbers of LDL receptors. Further, by
CA 02536069 1994-03-14
-6-
increasing the partitioning of the green porphyrin into
the lipoprotein phase of the blood, it appears to be
delivered more efficiently to the target tissue.
Because of its lipophilicity and negative
charge, green porphyrins strongly interact with
lipoproteins. Most preferably, the green.porphyrin is
complexed with low density lipoprotein (LDL).
When injected intravenously, BPD-MA is cleared
from the bloodstream with a half-life of about 10-30
minutes, with the highest tissue levels being reached in
about three hours after administration by injection and
declining rapidly in the first 24 hours. BPD-MA is
cleared primarily via bile and feces (60k), with only 4%
being cleared via the kidneys and urine. Thus, skin
photosensitivity occurs with BPD-MA only transiently,
with minimal reactivity after 24 hours in in vivo models.
The green porphyrin can be administered in any
of a wide variety of ways, for example, orally,
parenterally, or rectally. Parenteral administration,
such as intravenous, intramuscular, or subcutaneous, is
preferred. Intravenous injection is especially
preferred.
The dose of green porphyrin can vary widely
depending on the tissue to be treated; the physical
delivery system in which it is carried, such as in the
form of liposomes, or whether it is coupled to a target-
specific ligand, such as an antibody or an
immunologically active fragment.
It should be noted that the various parameters
used for effective, selective photodynamic therapy in the
invention are interrelated. Therefore, the dose should
also be adjusted with respect to other parameters, for
example, fluence, irradiance, duration of the light used
in photodynamic therapy, and the time interval between
administration of the dose and the therapeutic
CA 02536069 1994-03-14
-7-
irradiation. A11 of these parameters should be adjusted
to produce significant damage to neovascular or tumor
tissue without significant damage to the surrounding
tissue or, on the other hand, to enable the observation
of blood vessels in the eye without significant damage to
the surrounding tissue. Typically, the dose of green
porphyrin used is within the range of from about 0.1 to
about 20 mg/kg, preferably from about 0.15-2.0 mg/kg, and
even more preferably from about 0.25 to about 0.75 mg/kg.
Specifically, as the green porphyrin dose is
reduced from about 2 to about 1 mg/kg, the fluence
required to close choroidal neovascular tissue tends to
increase, for example, from about 50 to about 100
Joules/cmZ.
After the photosensitizing green porphyrin has
been administered, the choroidal neovascular tissue or
tumor being treated or observed in the eye is irradiated
at the wavelength of maximum absorbance of the green
porphyrin, usually between about 550 and 695 nm. A
wavelength in this range is especially preferred for
enhanced penetration into bodily tissues.
As a result of being irradiated, the green
porphyrin in its triplet state is thought to interact
with oxygen and other compounds to form reactive
intermediates, such as singlet oxygen, which can cause
disruption of-cellular structures. Possible cellular
targets include the cell membrane, mitochondria,
lysosomal membranes, and the nucleus. Evidence from
tumor and neovascular models indicates that occlusion of
the vasculature is a major mechanism of photodynamic
therapy, which.occurs by damage to endothelial cells,
with subsequent platelet adhesion, degranulation, and
thrombus formation.
The fluence during the irradiating treatment
can vary widely, depending on type of tissue, depth of
CA 02536069 1994-03-14
-8-
target tissue, and the amount of overlying fluid or
blood, but preferably varies from about 50-200 Joules/cm2.
The irradiance typically varies from about 150-
900 mW/cm2, with the range between about 150-600 mW/cm 2
being preferred. However, the use of higher irradiances
may be selected as effective and having the advantage of
shortening treatment times.
The optimum time following green porphyrin
administration until light treatment can vary widely
depending on the mode of administration; the form of
administration, such as in the form of_liposomes or as a
complex with LDL; and the type of target tissue. As a
specific example, a time interval of 1-20 minutes is
often appropriate for.retinalneovascular tissue, about
120 minutes is allowed for choroidal neovascular tissue,
and up to about three hours may be allowed for tumors.
Thus, effective vascular closure generally occurs at
times in the range of about one minute to about three
hours following administration of the green porphyrin.
The time of light irradiation after
administration of the green porphyrin may be important as
one way of maximizing the selectivity of the treatment,
thus minimizing damage to structures other than the
target tissues. For a primate, it is believed that the
green porphyrin begins to reach the retinal vasculature
by about 7-15 seconds following administration.
Typically, the green porphyrinpersists for a period of
about 5-15 minutes, depending on the dose given.
Treatment within the first five minutes following
administration of the green porphyrin should generally be
avoided to prevent undue damage to retinal vessels still
containing relatively high concentrations of the green
porphyrin.
CA 02536069 1994-03-14
-9-
Clinical examination and fundus photography
typically reveal no color change immediately following
photodynamic therapy, although a mild retinal whitening
occurs in some cases after about 24 hours. Closure of
choroidal neovascularization, however, is preferably
confirmed histologically by the observation of damage to
endothelial cells. Vacuolated cytoplasm and abnormal
nuclei can become apparent as early as 1-2 hours
following photodynamic therapy, with disruption of
neovascular tissue typically becoming more apparent by
about 24 hours after light treatment. Associated damage
to the retinal pigment epithelium (RPE), pyknotic nuclei
in the outer nuclear layer, and loss of photoreceptors
may also be observed. However, the inner retina usually
appears relatively undamaged, as shown by control studies
using photodynamic therapy with BPD-MA on a normal retina
and choroid showing no damage to large choroidal and
retinal vessels.
Closure can usually be observed
angiographically by about 40 seconds to a minute in the
early frames by hypofluorescence in the treated areas.
During the later angiographic frames, a corona of
hyperfluorescence begins to appear and then fills the
treated area, possibly representing leakage from the
.25 adjacent choriocapillaris through damaged retinal pigment
epithelium in the treated area. Large retinal vessels in
the treated area perfuse following photodynamic therapy,
but tend to demonstrate late staining.
Minimal retinal damage is generally found on
histopathologic correlation and is dependent on the
fluence and the time interval after irradiation that the
green porphyrin is administered. Histopathologic
examination usually reveals vessel remnants in the area
of choroidal neovascular tissue, but the retinal vessels
typically appear nbrmal. Further, there is no indication
CA 02536069 1994-03-14
-zo-
of systemic toxicity, and cutaneous photosensitization
does not appear to develop.
As a result of.the invention, photodynamic
therapy can be used more selectively, relying on the low
intensity light exposure of green porphyrins that have
become localized within vascular tissue. Complications,
suck as serous detachment and_hemorrhage, are not noted
with the invention. Thus, photodynamic therapy with_a
green porphyrin appears to have broad application to
clinical ophthalmology in treating such diseases as age-
related macular degeneration, neovascular glaucoma, and
persistent disc neovascularization in diabetic
retinopathy.
Photodynamic therapy using a green porphyrin
can also be used advantageously to treat not only
nonpigmented tumors, but also pigmented intraocular
tumors such as pigmented choroidal melanomas, and other
pigmented tumors of-the choroid, retina, iris or cornea.
The admi.nistered green porphyrin accumulates within the
neoplastic lesion and, upon localized light exposure at
an appropriate wavelength, the tumor tissue is thought to
be irreversibly damaged by the- interaction with active
molecular species like singlet oxygen and other radicals
induced by photoactivated dye molecules. Maximal
efficiency in tumor destruction, combined with minimal
irritation to adjacent physiological.structures is a
major benefit in the treatment of intraocular .
malignancies, in particular, choroidal tumors, which are
in close contact with sensitive neuronal and vascular
structures.
Light microscopic evaluation of tumors that
have not been treated with photodynamic therapy show a
mass of polygonal cells. Cell-.nuclei are typically
vesicular with prominent nucleoli, and mitotic figures
are acattered throughout the microscopic field. Tumors
CA 02536069 1994-03-14
-11-
typically exhibit many thin-walled blood vessels, which
are cylindrical in shape, are lined with intact
endothelial cells, and contain few erythrocytes.
After photodynamic therapy with a green
porphyrin, however, tumor blood vessels become dilated
and densely packed with erythrocytes. The tumor
demonstrates clotted erythrocytes within the vascular
lumina. Several days later, homogeneous necrosis is
typically observable throughout the_lesion. The tumor
cells begin toexhibit hyperchromaticnuclei and loss of
cytoplasmic features. Further, vascular structures show
disintegration and endothelial cell loss.
In treating tumors, the green porphyrin dosage
administered may vary widely depending on other
parameters, as described above but, preferably, is within
the range of 0.5 to 3 mg/kg. The radiant exposure can
also range widely, depending on the pigmentation and size
of.the tumor, but typically is in the range from about 60
to about 2600 J/cm2. The level of light exposure is of
particular interest with respect to the time duration of
the treatment.
In addition, green porphyrin can be used to
observe the condition of blood vessels in the either,
either alone or used in concert with other dyes such as
fluorescein or indocyanine green, as described above to
follow the progress of destruction choroidal neovascular
or tumor tissue. In such angiographic systems, a
sufficient amount of green porphyrin is administered to
produce an observable fluorescent emission when excited
by light, preferably light having a wavelength in the
range of about 550-700 nm. Images are recorded by
illuminating the eye with light in the excitation
wavelength range and detecting the amount of fluorescent
light emitted at the emission wavelength. A preferred
camera, which both emits and receives light in the 550-
CA 02536069 1994-03-14
-12-
700 nm range, is the TopCon 50VT camera in the Ophthalmic
Imaging System (Ophthalmic Imaging System Inc_, 221
Lathrop Way, Suite 1, Sacramento CA). An alternative
camera is the TopCon* camera TRC 50IA connected to the
TopConImagenet System (TopCon America Corporation, 65
West Century Road, Taramus NJ).
In a preferred observation method of the
invention, the green porphyrin is administered,
preferably by intravenous injection of a bolus followed
by a saline flush. The green.porphyrin typically reaches
the retinal vasculature in about 7-15 seconds, and the
early angiographic frames are recorded after the first 20
seconds, one every 3-5 seconds. Additional frames are
taken periodically for about two hours. A typical
protocol might call for the image to be recorded at about
40 seconds, then at SO seconds, 60 seconds, two minutes,
5 minutes, 10 minutes, 20 minutes, 40 minutes, 60
m.nutes, and two hours.
A camera is usually used to collect the emitted
fluorescent light, digitize the data, and store.it for
later depiction on a video screen, as a hard paper copy,
or in connection with some other imaging system. while a
film recording device may be used when additional dyes
euch as fluorescein are being used in combination with
the green porphyrin, a CCD camera (video recording
device) is preferable as being able to capture emissions
at higher wavelengths, thus producing greater tissue
penetration. As a result, one can obtain more
sophisticated information regarding the pattern and
extent of vascular structures in different ocular tissue
layers, giving the ability to detect the "leakiness" that
is characteristic of new or inflamed blood vessels.
Further, it is preferable to use a camera that is capable
of providing the excitation light, appropriately filtered
to deliver only light of the desired excitation
* Trade-mark
CA 02536069 1994-03-14
-13-
wavelength range, and then to capture the emitted,
fluorescent light with a receiving device, appropriately
filtered to receive only light in the desired emission
wavelength range.
The following examples are to illustrate but
not to limit the invention.
Example 1.
Control Qf xperimental Choroidal NeovascularizatiQn
Using PDT with BPD-MA/TDL at Low Irradiance
Cynomolgus monkeys weighing 3-4 kg were
anesthetized with an intramuscular injection of ketamine
hydrochloride (20 mg/kg), diazepam (1 mg/kg), and
atropine (0.125 mg/kg), with a supplement of 5-6 mg/kg of
ketamine hydrochloride as needed. For topical
anesthesia, proparacaine (0.5!k) was used. The pupils
were dilated with 2.5t phenylephrine and 0.8t
tropicamide.
Choroidal neovascularization was produced in
the eyes of the monkeys using a modification of the Ryan
model, in which burns are placed in the macula, causing
breaks'in Bruch's membrane, with a Coherent Argon Dye
Laser #920, Coherent Medical Laser, Palo Alto, CA
(Ohkuma, H. et al. Arch. Ophthalm4l. (1983) 1,U:1102-
1110; Ryan, S.J. Arch Ophthalmol (1982) 100:1804-1809).
Initially, a power of 300-700 mW for 0.1 seconds was used
to form spots of about 100 m, but improved rates of
neovascularization were obtained with 50 spots formed
using a power of about 300-450 mW for 0.1 second.
The resulting choroidal neovascularizations
were observed by (1) fundus photography (using a Canon
Fundus' CF-60Z camera, Lake Success, Long Island, N.Y.)
(2) by fluorescein angiography (for example, by using
about 0.1 ml/kg body weight of 10% sodium fluorescein via
CA 02536069 1994-03-14
-14-
saphenous vein injection); and (3) histologic examination -
by light and electron microscopy.
Immediately before use, BPD-MA was dissolved in
dimethyl sulfoxide (Aldrich Chemical Co., Inc.,
Milwaukee, WI) at a concentration of about 4 mg/ml.
Dulbeccos phosphate buffered salt solution (Meditech,
Washington, D.C.) was then added to the stock to achieve
a final BPD concentration of 0.8 mg/ml. Human low-
density-lipoprotein (LDL) prepared from fresh frozen
plasma was added at a ratio-of 1:2.5 mg BPD-MA:LDL. The
green porphyrin dye and dye solutions were protected from
light at all times. After mixing, the dye preparation
was incubated at 37 for 30 minutes prior to intravenous
injection. The monkeys were then injected intravenously
via a leg vein with 1-2 mg/kg of the BPD-MA complexed
with LDL over a five-minute period, followed by a flush
of 3-5 cc of normal saline.
Following this intravenous injection, the eyes
of the monkeys were irradiated with 692 nm of light from
.20 an argon/dye laser (Coherent 920 Coherent Medical Laser,
Palo Alto, CA), using a Coherent LDS-20 slit lamp. The
standard fiber was coupled to larger 400 m silica
optical fiber (Coherent Medical Laser, Pal Alto, CA) to
allow larger treatment spots as desired. Seventeen (17)
areas of. choroidal neovascularization were treated using
a 1250 gm spot. Treatment spot sizes were confirmed at
the treatment plane using a Dial caliper micrometer.
Some areas of choroidal neovascularization were treated
with several adjacent treatment spots to treat the whole
area of choroidal neovascularization. One large
choroidal neovascular membrane was treated with
photodynamic the'rapy to the nasal half only.
The photodynamic irradiation treatments were
carried out with a plano fundus contact lens (OGFA,
Ocular Instruments, Inc., Bellvue, MA). Power was
CA 02536069 1994-03-14
-15-
verified at the cornea by a power meter (Coherent
Fieldmaster, Coherent, Auborn, CA). The fluence at each
treatment spot was 50, 75, 100 or 150 Joules/cm2.
Initially, the irradiance was set at 150 mW/cmZ to avoid
any thermal effect but, as the experiment proceeded, the
irradiance was increased to 300 mW/cm2 or 600 mW/cm2 to
reduce the treatment duration time. The time interval
between injection of the green porphyrin dye and the
treatment irradiating step ranged from about 1 to about
81 minutes.
A number of different combinations of parameter
values were studied and are summarized below in Table 1:
TASLE 1- IRRADIANCE AT 150 mW/cm'
Dura- Time
tion of after Closure
Number Dye Treat- Injec- by
of CNV dose Fluence ment tion Angio-
Treated (mg/kcr) J c 2 m'n (mins) graphy
2 2 50 5.6 18, 38 2/2
1 2 75 8.3 81 1/1
1 2 100 11.2 22 1/1
2 1 50 5.6 5, 30 0/2
3 1 100 11.2 1, 2 3/3
and 5
4 1 150 16.6 14-43 3/4
"Dye only" controls, which were exposed to dye
but not to laser light, were examined in the areas of
normal retina/choroid. Areas of choroidal
neovascularization were examined angiographically and
histologically. "Light only" controls were not
performed, since the irradiances used for photodynamic
therapy were well below the levels used for clinical
CA 02536069 1994-03-14
-16-
laser photocoagulation. (In a related experiment, a
minimally detectable lesion using "light-only" required
an irradiance of 37 W/cm2, about 100 times the light
levels used for photodynamic therapy.)
Following photodynamic therapy, the monkeys
were returned to an-animal care facility. No attempt was
made to occlude the animals' eyes, but the room in which
they were housed-was darkened overnight.
The condition of the choroidal neovasculature
was followed by fundus photography, fluorescein
angiography, and histologic examination. In particular,
the eyes of the monkeys were examined by fluorescein-
angiography acutely and at 24 hours after the -._
photodynamic therapy was given. In some cases, follow-up
by fluorescein angiography was performed at 48 hours and
at one week, until the eyes were harvested and the
animals killed at the following time points: acutely, at
24 hours, 48 hours, and 8 days following photodynamic
therapy. Animals were sacrificed with an intravenous
injection of 25 mg/mg Nembutal.
To perform the histologic examination, all eyes
were enucleated under deep anesthesia and fixed overnight
in modified Karnovsky's fixative, and then transferred to
0.1M phosphate buffer, pH 7.2 at 4 C. Both light
microscopy and electron microscopy were used for these
studies. For light microscopy, tissue samples were
dehydrated, embedded in epon and serially sectioned at
one micron. The sections were stained with tolnizin blue
and examined with an Olympus photomicroscope. For -
electron microscopy, tissue samples were post-fixed in 296
osmium tetroxide and dehydrated in ethanol. Sections
were stained with uranyl acetate in methanol, stained
with Sato's lead stain, and examined with a Philips #CM
10 transmission electron microscope.
CA 02536069 1994-03-14
-17-
Using the low irradiance level of 150 mW/crnZ to
minimize any thermal component of the treatment, green
porphyrin doses of 1-2 mg/kg of BPD-MA/LDL, and fluences
of 50-150 Joules/cm~, choroidal neovascularization was
effectively closed. Using the higher 2 mg/kg dose
effectively closed choroidal neovascularizations at even
the lowest 50 Joules/cmZ fluence. When the green
porphyrin dose was decreased to 1 mg/kg of BPD-MA/LDL to
minimize damage to surrounding tissues, the fluence
required to effectively close choroidal neovascular
tissue increased to 100 Joules/cmz. At 100 and 150
Joules/cm2, the treated choroidal neovascular tissue was
angiographically closed, as shown by hypofluorescence in
the area of treatment.
Prior to photodynamic therapy, the areas of
choroidal neovascularization exhibited a gray sub-retinal
elevation that leaked profusely on fluorescein
angiography. There was no apparent color change in the
treated areas either during or immediately after
photodynamic treatment. However, 24 hours after the
irradiating step, there was mild retinal whitening in the
treated areas.
Further fluorescein angiography showed
hypofluorescence in the treated areas, with no apparent
filling of the associated neovascular tissues. Retinal
vessels within the treated areas were perfused, but
stained later. A hyperfluorescent rim at the border of
the treated area was apparent in the later frames of the
angiograph, and the rim then progressed to fill the
treated area. Although mild staining of retinal vessels
was noted angiographically, no complications, such as
serous retinal detachment or hemorrhage, were noted.
On histopathologic examination of the 2 mg/kg
dose samples, there was marked disruption of the treated
choroidal neovascular tissue with disrupted endothelial
CA 02536069 1994-03-14
-18-
cells. The choriocapillaris was also occluded. Although
large choroidal vessels were unaffected, extravasated red
blood cells were noted in the choroid. Retinal pigment
epithelium (RPE) damage was noted as well with vacuolated
cells, with the outer nuclear.layer demonstrating
pyknotic nuclei-and disrupted architecture. No
histologic abnormality of the retinal vessels was seen.
Histolopathologic examination of the 1 mg/kg
dose samples showed damage to endothelial cells in the
choroidal neovascular tissue, with abnormal nuclei and
disrupted cytoplasm in the endothelial cells. The lumens
of the vessels in the choroidal neovascular tissue were
occluded by fibrin acutely and were closed by 24 hours
after treatment. Closure of the choroicapillaris was
also noted. At 24 hours, the retinal pigment epithelium
(RPE) appeared abnormal with vacuolated cytoplasm.
Pyknotic nuclei in the inner and outer layer indicated
damage secondary to the laser injury used to induce the
neovascularization in this model. Retinal vessels
appeared to be undamaged.
Choroidal neovascular tissue that was treated
and followed for eight days showed persistent closure, as
shown by hypofluorescence in the early frames of the
angiogram. Histologically, the treated areas
demonstrated degraded vessel lumens empty of debris. The
choriocapillaris was sparse but patent in the treated
area. In contrast, areas of choroidal neovascularization
not treated by photodynamic therapy demonstrated
branching capillaries between Bruch's membrane and the
outer retina.
No adverse effects of photodynamic therapy with
the green porphyrin were noted. There was no associated
serous retinal detachment, retinal or sub-retinal
hemorrhage, or post-treatment inflammation. Further, no
adverse systemic effects of the dye administration were
CA 02536069 1994-03-14
-19-
noted. However, the low irradiance forced treatment
times to be long--about 16.6 minutes to yield 150
Joules/cm2.
ENammple 2
Control of E-xnerimental Choroidal Neovascularization
Using PDT with BPD-MA/LDL at Hi.aher Irradiances
To make clinical treatments shorter, additional
experiments were performed using higher irradiance
values. Experience with higher irradiance indicated that
no thermal damage would take place-with irradiances as
high as 1800 mW/cm2. Moulton et al., "Response of Retinal
and Choroidal Vessels to Photodynamic Therapy Using
Benzoporphyrin Derivative Monoacid", IOVS 34, 1169
(1993), Abstract 2294-58. Therefore, irradiances of 300
mW/cm2 and 600 mW/cm' were also used to treat choroidal
neovascular tissue in accordance with the procedures
described in Example 1. The results showed that
shortened treatment times effectively closed the
choroidal neovascular tissue, as indicated below in Table
2.
TABLE 2 - IRRADIANCE OVER 150 mW/cm2
Dura-
tion Time
Number Dye Flu- Irra- of after Clos-
of CNV dose ence diance Treat- Injec- ure by
Trea- (mg/ (J/ (mW/ ment tion Angio-
ted kg) cm2) cm2) (mins) mins crraphy
2 1 150 300 8.3 5, 53 2/2
2 1 150 600 4.7 22, 69 2/2
Occlusion of the choroidal neovascular tissue
and subjacent choriocapillaris was observed, as well as
damage to the retinal pigment epithelium and outer
ret',i.na.
CA 02536069 1994-03-14
-20-
Example 3
c'ortrol of xperimental ChQroidal Neovaecularzzation
UrLq PDT with BPD-MA LipoeQmee
The following experiment of photodynamic
therapy using a liposomal preparation of BPD-MA was
conducted to determine the optimal time interval after
intravenous injection as a bolus of the BPD-MA over about
20 seconds, followed by a 3-5 cc saline flush, to begin
the irradiating step. Choroidal neovascularization in
cynomolgus monkeys was treated to demonstrate efficacy of
the photodynamic therapy. Normal chorcid tissue was
treated to assess relative damage to adjacent tissues.
The monkeys were initially injected with a
green porphyrin dose of 1 mg/kg. At predeterinined time
intervals following this injection, the eyes of the
monkeys were irradiated with an irradiance of 600 mW/cmZ,
and a fluence of 150J/cm2. The irradiating light was
from an argon/dye laser (Coherent 920 Coherent Medical
Laser, Palo Alto, CA) equipped with a.200 micron fiber
adapted through a LaserLink*(Coherent * Medical Laser) and
a split lamp delivery system (Coherent). Other than
these differences, the eye membranes were treated in the
same manner as described in Example I. A11 areas of
zreated choroidal neovasculature for all time points
after the liposomal BPD-MA injection showed whitening of
the retina and early hypofluorescense on fluorescein
angiography when measured one week after treatment. On
histology, there was evidence of partial closure of
choroidal neovasculature at the early time points, no
effect at mid-time points, and more effective closure at
late irradiation time points, e.g., at 80 and 100
minutes.
The normal choroid treated with the same
parameters showed whitening of the retina, early
hypofluorescence at all time points, and histologic
Trade-mark
CA 02536069 1994-03-14
-21-
evidence of choroicapillaris (c-c) accompanied by damage
to the choroid and retina, particularly at early time
points.
Example 4
lZsincr PDT with BPD-MA Liposomes
at Lower Green Por-phvrin Doses
Using the general procedure of Example 1,
additional experiments were performed using the
intravenous injection of liposomal BPD-MA at doses of
0.25, 0.5 and 1 mg/kg. Photodynamic therapy was
performed with an irradiance of_600 mW/cm2 , a fluence of
150 J/ctn2, and a treatment duration of four minutes, nine
seconds.
The effects of treatment were assessed by
fundus photography and fluorescein angiography, and then
confirmed by light and electron microscopy. Photodynamic
therapy of normal choroid tissue demonstrated the effect
on adjacent structures, such as the retina, while the
treatment of choroid neovascular tissue demonstrated
efficacy.
Table 3 below describes the lesions produced on
normal choroids by administration of 0.5 mg/kg BPD-MA at
time points ranging from 5 to 60 minutes:
TABLE 3 - 0.5 mg/kct, NORMAL CHOROID
Time after
injection Fluorescein
(min) Anaioaraphy Histologv
5 Hypofluorescence c-c and large
choroidal vessel
closure; outer and
inner retina
damage.
20 Hypofluorescence; cc closure; damage
retinal vessels - to outer retina
normal
CA 02536069 1994-03-14
-22-
40 Mild early cc open (not center
hypofluorescence of lesion); outer
retina damage
60 Early cc closed; outer
hypofluorescence; retina damage;
-less than the 20- inner.retina fairly
minute lesion good.
described above
When 0.5 mg/kg BPD-MA was also used to treat choroidal
neovasculature under the same conditions, marked
hypofluorescence corresponding to closure of choroid
neovasculature was exhibited in areas irradiated at times
of 5, 20 and 40 minutes after injection. When 50 minutes
after injection were allowed to elapee before
photodynamic irradiation was begun, therewas less
hypofluorescence and presumabl.y less effective closure.
The study was then repeated with the green
porphyrin dose decreased to 0.25 mg/kg. Table.4 below
describes.the lesions produced on normal choroids by
treatments with 0.25 mg/kg, 600 mW/cm7, and 150 J/cm2 at
time points ranging from 5 to 60 minutes:
TABLE 4 - 0,25 ma/ka, NORMAL CHOROID
Time after
injection Fluorescein
(min) Angiography Histoloav
10 Early c-c closure;
hypofluorescence choroidal vessel -
normal; RPE
damaged; retinal
vessels - normal;
mild damage to
outer retina
20 Early Same as 10-minute
hypofluorescence lesion above
40 Faint early Patchy cc closure;
hypofluorescence; less damage to RPE
late staining and outer retina
CA 02536069 1994-03-14
-23-
60 Not demonstrated No effect on cc;
mild vacuolization
of RPE
When the above study was repeated using the
same green porphyrin dose of 0.25 mg/kg and irradiance of
600 mW/cm2, but with a reduced fluence of 100 J/cmZ, the
same angiographic and histologic pattern was exhibited as
described above. However, cc was open in the 40-minute
lesion. - -
In the last portion of these experimente, a
green porphyrin dose of 0.25 mg/kg was used to treat
experimental choroidal neovascularization with an
irradiance of 600 mW/cmZ and a fluence of 150 J/cm2 at
elapsed time points ranging from 5 to 100 minutes. This
combination of conditions caused effective cc closure
with only minimal damage to the outer retina. The
results are shown in Table 5 below:
TABLE 5 - 0,25 ma/kgr PDT over CNV
Time after
injection Fluorescein
(min ) Angioaratahv Histoloay
5 Early Partially closed
hypofluorescence CNV; c-c closed;
damage to inner
retina
20 Early CNV - open vessel,
hypofluorescence; fibrin and clots;
less than the 5- inner retina looks
minute lesion fine
30 Some Minimal effect on
hypofluorescense cNV
next to CNV
Hypofluorescence; Minimal effect on
questionable change CNV
35 compared to
previous reaction
CA 02536069 1994-03-14
-24-
60 Hypofluorescence Minimal effect on
CNV
80 Hypofluorescence Partial closure of
CNV; retina over-
CNV looks intact
100 Hypofluorescence CNV partially
closed
Thus, fluorescein angiography and
histopathology in the above series of experiments
demonstrated early hypofluorescence at early time points.
Further, the histopathology study showed partial CNV
closure at all time points after injection using 80 and
100 minutes as the post-injection interval before the
irradiating treatment.
In summary, acceptable destruction of choroidal
neovascular tissue at all tested doses of BPD-:MA was
shown by fluorescein angiography and_histology. However,
the lower doses appeared to increase selectivity, as
assessed by treatment of a normal choroid. Effective
choriocapillaris closure in normal choroids with minimal
retinal damage was produced by irradiating at a time
about 10 minutes, 20 seconds after injection of the green
porphyrin at a dose of 0.25 mg/kg. By adjusting the
dose, the time of irradiation after green porphyrin
injection, and fluence, one can improve even further_the
selectivity of the green porphyrin. However, the
liposomal preparation of BPD-MA was clearly demonstrated
to be a potent photosensitizer.