Note: Descriptions are shown in the official language in which they were submitted.
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
The claimed invention relates to novel 4-piperidinecarboxamide and the use
thereof~f_or th.e
preparation of medicaments against 5-HT2A receptor-related disorders.
RELATED APPPLICATIONS
This application claims priority to Swedish application number 0302369-4,
filed on
September 3, 2003, and U.S. provisional application 60/505,295, filed on
September 23,
2003, the contents of which are incorporated herein by reference.
TECHNICAL FIELD
The present invention relates to novel compounds, to pharmaceutical
compositions
comprising the compomds, to processes for their preparation, as well as to the
use of the
compounds for the preparation of a medicament against 5-HT2A receptor-related
disorders.
BACKGROUND OF THE INVENTION
Many disorders and conditions of the central nervous system are influenced by
the
adrenergic, the dopaminergic, and the serotonergic neurotransmitter systems.
For example,
2o serotonin (5-HT; 5-hydroxytryptamine) has been implicated in a number of
disorders and
conditions which originate in the central nervous system.
The HT2A receptor has been implicated as a therapeutic target for the
treatment or
prevention of abnormalities of the serotonergic system, including psychotic
disorders such
as schizophrenia (A. Carlsson, N. Waters and M. L. Carlsson, Biol. Psychiatry,
46, 1388
(1999); G.J. Marek and G. K. Aghajanian, Biol. Psychiatry, 44, 1118 (1998); E.
Sibelle, Z.
Sarnyai, D. Benjamin, J. Gal, H. Baker and M. Toth, Mol. Placzrmezcol., 52,
1056 (1997)).
Abnormality of this system has also been implicated in a number of human
diseases such
as mental depression (Arias B, Gutierrez B, Pintor L, Gasto C, Fananas L, Mol.
Psychiatry
(2001) 6, 239-242), migraine, epilepsy and obsessive-compulsive disorder
(Luisa de
Angelis, Cu~reht ~pinioh iyi Ifavestigcctionczl Drugs (2002) 3 (1) 106-112). 5-
HT2A
antagonists may also be useful in the treatment of sleep disorders such as
insomnia and
obstntctive sleep apnea, anorexia nervosa (Zlegler A, Gorg T, Lancet (1999)
353, 929),
cardiovascular conditions such as hypertension, vasospasm, angina, Raynaud's
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
phenomenon and thrombotic illness including stroke, glaucoma (T. Mano et al.
and H.
Takanelca et al., Investigative Ophthalmology and Visual Science, 1995, vol.
36, pages 719
and 734, respectively) and in the inhibition of platelet aggregation. Evidence
also implies
that selective 5-HT2A receptor antagonists rnay also be useful in the
treatment of alcohol
and cocaine dependence (Maurel S, De Vry J, De Beun R, Schreiber, Pha~macol.
Biocl2em
Behav (1999) 89-96; McMahan LR, Cunningham KA, Plzarmacol Exp They (2001) 297,
357-363).
No publications disclose the use of the compounds according to the present
invention against 5-HT2A receptor-related disorders.
to
DISCLOSURE OF THE INVENTION
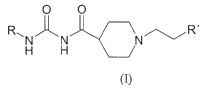
One object of the present invention is a compound of the Formula (I)
O O
R~N~N NCR'
H H
is
Formula (I)
wherein R is either
20 - aryl optionally independently substituted with one or more of C1_6-
allcyl, C1_6-
allcoxy, halogen, and halo-C1_6-alkyl; or
- aryl-C1_6-allcyl optionally independently substituted with one or more of
C1_6-
alkoxy; or
- C3_$-cycloalliyl;
25 R' is either
- aryl optionally independently substituted with one or more of halogen, Cl_6-
alkoxy,
halo-C1_6-alkyl, and cyano; or
- aryloxy optionally independently substituted with one or more of halogen and
CI_6-
allcoxy; or
2
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
- heteroaryl optionally independently substituted with one aryl andlor one or
more of
halogen, C1-6-alkyl, and C1_~-alkoxy, which aryl is optionally independently
substituted with one or more of halogen, Cl_6-allcyl, and C1_6-alkoxy;
and pharmaceutically acceptable salts, hydrates, solvates, geometrical
isomers, tautomers,
optical isomers, and prodrug forms thereof.
It is preferred that R is selected from
- phenyl independently substiW ted with one or more of methyl, methoxy,
ethoxy,
l0 fluoro, and trifluoromethyl;
- benzyl independently substitLited with one or more of methoxy; and
- cyclohexyl.
It is especially preferred that R is selected from 2-ethoxyphenyl, 2,4-
difluorophenyl, 3-
(trifluoromethyl)phenyl, 3,4,5-trimethoxybenzyl, and cyclohexyl.
It is preferred that R' is selected from
- phenyl independently substituted with one or more of fluoro;
- phenoxy independently substituted with one or more of methoxy; and
- indolyl independently substituted with one phenyl andlor one or more of
fluoro,
chloro, methyl, and methoxy, which phenyl is optionally independently
substituted
with one or more of fluoro, chloro, methyl, and methoxy.
It is especially preferred that R' is selected from 4-fluorophenyl, 2,6-
dimethoxyphen~3xy,
and 2-phenyl-3-indolyl.
Preferred compounds are given in Examples 1-5.
Another object of the present invention is a process for the preparation of a
3o compound as mentioned above, which process comprises the step of
a) reacting an amine RNHa
wherein R is either
- aryl optionally independently substituted with one or more of C1_6-allcyl,
C1_6-
allcoxy, halogen, and halo-C1_6-alkyl; or
3
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
- aryl-C1_6-alkyl optionally independently substituted with one or more of C1-
s-
allcoxy; or
- C3_8-cycloalkyl;
with a cyanate, to give a compound of formula R-NH-CO-NH2,
wherein R is as defined above,
b) allcylation of a compound of Formula (II)
O
R"O ~N H
to
Formula (II)
wherein R'° is CI_6-alkyl,
via displacement of a leaving group by reaction of the compound of Formula
(Il' with an
allcylating agent of the Formula R'-CHz-CH2-LG,
wherein R° is either
- aryl optionally independently substituted with one or more of halogen, CI_6-
alkoxy,
halo-C1_6-alkyl, and cyano; or
- aryloxy optionallyindependently substituted with one or more of halogen and
C1_s-
2o alkoxy; or
- heteroaryl optionally independently substituted with one aryl and/or one or
more of
halogen, Cl_6-alkyl, and Cl_6-allcoxy, which aryl is optionally independently
substituted with one or more of halogen, C1_6-alkyl, and C1_6-alkoxy; and
- LG is a leaving group,
to give a compound of Formula (III)
O
\ ~R'
R.'O ~N
Formula (III)
4
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
wherein R' and R" are as defined above,
c) reacting the products from steps a) and b) in the presence of a base, such
as sodium
methoxide or potassium tert-butoxide, to gi~sre a compound of Formula (I)
O O
R~N~~N NCR'
H H
Formula (I)
wherein R and R' are as defined above.
to Another object of the present invention is a compound as mentioned above
for use
in therapy, especially for use in the prophylaxis or treatment of a 5-HT~A
receptor-related
disorder.
Another obj ect of the present invention is a pharmaceutical formulation
comprising
a compound as mentioned above as active ingredient, in combination with a
15 pharmaceutically acceptable diluent or carrier, especially for use in the
prophylaxis or
treatment of a 5-HT~A receptor-related disorder.
Another obj ect of the present invention is a method for treating a human or
animal
subject suffering from a 5-HT2A receptor-related disorder. The method can
include
administering to a subject (e.g., a human or an animal, dog, cat, horse, cow)
in need thereof
2o an effective amount of one or more compounds of any of the formulae herein,
their salts, or
compositions containing the compounds or salts.
The methods delineated herein can also include the step of identifying that
the
subject is in need of treatment of the 5-HT2A receptor-related disorder.
Identifying a
subject in need of such treatment can be in the judgment of a subject or a
health care
25 professional and can be subjective (e.g., opinion) or objective (e.g.,
measurable by a test or
diagnostic method).
Another object of the present invention is a method for the prophylaxis of a 5-
HT2A receptor-related disorder, which comprises administering to a subject in
need of
such treatment an effective amount of a compound as mentioned above.
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
Another object of the present invention is a method for modulating (i a
prompting
or inhibiting) 5-HT2A receptor activity, which comprises administering to a
subject in
need of such treatment an effective amount of a compound as mentioned above.
Another object of the present invention is the use of a compound as mentioned
above for the manufacture of a medicament for use in the prophylaxis or
treatment of a 5-
HT2A receptor-related disorder.
The compounds as mentioned above may be agonists, partial agonists or
antagonists for the 5-HT2A receptor.
Examples of 5-HT2A receptor-related disorders are schizophrenia, mental
to depression, migraine, epilepsy, obsessive-compulsive disorder, sleep
disorders such as
insomnia and obstructive sleep apnea, anorexia nervosa, cardiovascular
conditions such as
hypertension, vasospasm, angina, Raynaud's phenomenon and thrombotic illness
including
stroke, glaucoma, alcohol and cocaine dependence.
The compounds and compositions are useful for treating diseases, including
15 schizophrenia, mental depression, migraine, epilepsy, obsessive-compulsive
disorder, sleep
disorders such as insomnia and obstructive sleep apnea, anorexia nervosa,
cardiovascular
conditions such as hypertension, vasospasm, angina, Raynaud's phenomenon and
thrombotic illness including stroke, glaucoma, alcohol and cocaine dependence.
In one
aspect, the invention relates to a method for treating or preventing an
aforementioned
2o disease comprising administrating to a subject in need of such treatment an
effective
amount of a compou~ld or composition delineated herein.
Deftnitiohs
25 The following definitions shall apply throughout the specification and the
appended
claims.
Unless otherwise stated or indicated, the term "C1_6-alkyl" denotes a straight
or
branched alkyl group having from 1 to 6 carbon atoms. Examples of said lower
alkyl
include methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, t-
butyl and
3o straight- and branched-chain pentyl and hexyl. For parts of the range "Cl_6-
alleyl" all
subgroups thereof are contemplated such as C1_5-alkyl, C1_4-alkyl, CI_3-alkyl,
Cl_Z-alkyl, CZ_
6-alkyl, CZ_5-alkyl, CZ_4-allcyl, CZ_3-alkyl, C3_6-alkyl, C4_5-alkyl, etc.
"Halo-C1_6-alkyl"
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
means a Cl_~-alkyl group substituted with one or more halogen atoms. Likewise,
"aryl-C~_~-
alkyl" means a C1_~-allcyl group substituted with one or more aryl groups.
Unless otherwise stated or indicated, the term "C3_8-cycloalkyl" denotes a
cyclic
allcyl group having a ring size from 3 to ~ carbon atoms. Examples of said
cycloalkyl
include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, methylcyclohexyl,
cycloheptyl,
and cyclooctyl. For parts of the range "C3_8-cycloallcyl" all subgroups
thereof are
contemplated such as C3_~-cycloallcyl, C3_6-cycloallcyl, C3_5-cycloalkyl, C3_4-
cycloalkyl, C4_
8-cycloalkyl, C4_7-cycloalkyl, C4_G-cycloalkyl, C4_5-cycloalkyl, CS_~-
cycloalkyl, C6_7-
cycloalkyl, etc.
to Unless otherwise stated or indicated, the term "C1_6 alkoxy" denotes a
straight or
branched allcoxy group having from 1 to 6 carbon atoms. Examples of said lower
alkoxy
include methoxy, ethoxy, n-propoxy, iso-propoxy, n-butoxy, iso-butoxy, sec-
butoxy, t-
butoxy and straight- and branched-chain pentoxy and hexoxy. For parts of the
range "Ci_6-
alkoxy" all subgroups thereof are contemplated such as C1_5-alkoxy, C1_4-
alkoxy, C1_~-
alkoxy, C1_2-alkoxy, Cz_6-alkoxy, CZ_5-alkoxy, C2_4-alkoxy, C2_3-alkoxy, C3_6-
allcoxy, C4_s-
alkoxy, etc.
Unless otherwise stated or indicated, the term "halogen" shall mean fluorine,
chlorine, bromine or iodine.
Unless otherwise stated or indicated, the term "aryl" refers to a hydrocarbon
ring
2o system having at least one aromatic ring. Examples of aryls are phenyl,
pentalenyl,
indenyl, indanyl, isoindolinyl, chromanyl, naphthyl, fluorenyl, anthryl,
phenanthryl and
pyrenyl. The aryl rings may optionally be substituted with C1_6-alkyl.
Examples of
substituted' aryl groups are 2-methylphenyl aazd 3-methylphenyl. Likewise,
"aryloxy" refers
to an aryl group bonded to an oxygen atom.
The term "heteroaryl" refers to a hydrocarbon ring system having at least one
aromatic ring having one or more ring atoms are a heteroatorn such as O, N, or
S, and the
remaining ring atoms are carbon. Examples of heteroaryl groups include furyl,
pyrrolyl,
thienyl, oxazolyl, isoxazolyl, imidazolyl, thiazolyl, isothiazolyl, pyridinyl,
pyrimidinyl,
quin.azolinyl, indolyl, pyrazolyl, pyridazinyl, quinolinyl, benzofuranyl,
3o dihydrobenzofuranyl, benzodioxolyl, benzodioxinyl, benzothiazolyl,
benzothiadiazoiyl,
and benzotriazolyl groups.
The term "leaving group" refers to a group to be displaced from a molecule
during
a nucleophilic displacement reaction. Examples of leaving groups are iodide,
bromide,
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
chloride, methanesulfonate, hydroxy, methoxy, thiomethoxy, tosyl, or suitable
protonated
forms thereof (e.g., H20, MeOH), especially bromide and methanesulfonate.
The term "alkylating agent" refers to a compound containing one or more alkyl
groups which can be added to another compound. Examples of alkylating agents
include,
but are not limited to, iodomethane, iodoethane, 1-iodopropane, 2-iodopropane,
straight-
and branched-iodobutane, iodopentane, iodohexane, bromomethane, bomoethane, 1-
bromopropane, 2-bromopropane, straight- and branched- bromobutane,
bromopentane,
bromohexane, allyl bromide, ethyl methanesulfonate, methyl methanesulfonate,
and propyl
methanesulfonate.
to "Pharmaceutically acceptable" means being useful in preparing a
pharmaceutical
composition that is generally safe, non-toxic and neither biologically nor
otherwise
undesirable and includes being useful for veterinary use as well as human
pharmaceutical
use.
"Treatment" as used herein includes prophylaxis of the named disorder or
condition, or amelioration or elimination of the disorder once it has been
established.
"An effective amount" refers to an amount of a compound that confers a
therapeutic effect on the treated subject. The therapeutic effect may be
objective (i.e.,
measurable by some test or marlcer) or subjective (i.e., subject gives an
indication of or
feels an effect).
2o The term "prodrug forms" means a pharmacologically acceptable derivative,
such
as an ester or an amide, which derivative is biotransformed in the body to
form the active
dmg. Reference is made to Goodman and Gilman's, The Pharmacological basis of
Therapeutics, 8th ed., Mc-Graw-Hill, Int. Ed. 1992, "Biotransformation of
Drugs", p. 13-
15.
The following abbreviations have been used:
ACN means acetonitrile,
CHO means Chinese hamster ovary,
DEA means diethylamine,
DEPT means distortion enhancement polarisation transfer,
3o DMSO means dimethyl sulfoxide,
ELS means electron light scattering,
HPLC means high performance liquid chromatography,
Rt means retention time,
TFA means trifluoroacetic acid,
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
THF means tetrahydrofuran,
TLC means thin layer chromatography.
All isomeric forms possible (pure enantiomers, diastereomers, tautomers,
racemic
mixtures and unequal mixtures of two enantiomers) for the compounds delineated
are
within the scope of the invention. Such compounds can also occur as cis- or
trans-, E- or 2-
double bond isomer forms. All isomeric forms are contemplated.
The compounds of the formula (I) may be used as such or, where appropriate, as
pharmacologically acceptable salts (acid or base addition salts) thereof. The
to pharmacologically acceptable addition salts mentioned above are meant to
comprise the
therapeutically active non-toxic acid and base addition salt forms that the
compounds are
able to form. Compounds that have basic properties can be converted to their
pharmaceutically acceptable acid addition salts by treating the base form with
an
appropriate acid. Exemplary acids include inorganic acids, such as hydrogen
chloride,
15 hydrogen bromide, hydrogen iodide, sulfuric acid, phosphoric acid; and
organic acids such
as formic acid, acetic acid, propanoic acid, hydroxyacetic acid, lactic acid,
pynivic acid,
glycolic acid, malefic acid, malonic acid, oxalic acid, benzenesulfonic acid,
toluenesulfonic
acid, methanesulfonic acid, trifluoroacetic acid, fumaric acid, succinic acid,
malic acid,
tartaric acid, citric acid, salicylic acid, p-aminosalicylic acid, pamoic
acid, benzoic acid,
2o ascorbic acid and the like. Exemplary base addition salt forms are the
sodium, potassium,
calcium salts, and salts with pharmaceutically acceptable amines such as, for
example,
ammonia, alkylamines, benzathine, and amino acids, such as, e.g. arginine and
lysine. The
term addition salt as used herein also comprises solvates which the compounds
and salts
thereof are able to form, such as, far example, hydrates, alcoholates and the
like.
25 For clinical use, the compounds of the invention are formulated into
pharmaceutical
formulations for oral, rectal, parenteral or other mode of administration.
Pharmaceutical
formulations are usually prepared by mixing the active substance, or a
pharmaceutically
acceptable salt thereof, with conventional pharmaceutical excipients. Examples
of
excipients are water, gelatin, gum arabicum, lactose, microcrystalline
cellulose, starch,
3o sodium starch glycolate, calcium hydrogen phosphate, magnesium stearate,
talcum,
colloidal silicon dioxide, and the like. Such formulations may also contain
other
pharmacologically active agents, and conventional additives, such as
stabilizers, wetting
agents, emulsifiers, flavouring agents, buffers, and the like.
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
The formulations can be further prepared by known methods such as
granulation, compression, microencapsulation, spray coating, etc. The
formulations may be prepared by conventional methods in the dosage form of
tablets, capsules, granules, powders, synips, suspensions, suppositories or
injections. Liquid formulations may be prepared by dissolving or suspending
the
active substance in water or other suitable vehicles. Tablets and granules may
be
coated in a conventional manner.
In a further aspect the invention relates to methods of making compounds of
any of
the formulae herein comprising reacting any one or more of the compounds of
the
to formulae delineated herein, including any processes delineated herein. The
compounds of
the formula (I) above may be prepared by, or in analogy with, conventional
methods.
The processes described above may be carried out to give a compound of the
invention in the form of a free base or as an acid addition salt. A
pharmaceutically
acceptable acid addition salt may be obtained by dissolving the free base in a
suitable
15 organic solvent and treating the solution with an acid, in accordance with
conventional
procedures for preparing acid addition salts from base compounds. Examples of
addition
salt forming acids are mentioned above.
The compounds of formula (I) may possess one or more chiral carbon atoms, and
they may therefore be obtained in the form of optical isomers, e.g. as a pure
enantiomer, or
2o as a mixture of enantiomers (racemate) or as a mixture containing
diastereomers. The
separation of mixtures of optical isomers to obtain pure enantiomers is well
known in the
art and may, for example, be achieved by fractional crystallization of salts
with optically
active (chiral) acids or by chromatographic separation on chiral columns.
The chemicals used in the synthetic routes delineated herein may include, for
25 example, solvents, reagents, catalysts, and protecting group and
deprotecting group
reagents. The methods described above may also additionally include steps,
either before
or after the steps described specifically herein, to add or remove suitable
protecting groups
in order to ultimately allow synthesis of the compounds. In addition, various
synthetic
steps may be performed in an alternate sequence or order to give the desired
compounds.
30 Synthetic chemistry transformations and protecting group methodologies
(proteetiowand
deprotection) useful in synthesizing applicable compounds are known in the art
and
include, for example, those described in R. Larock, Compr~eherasive Organic
TransfoYmc~tions, VCH Publishers (1989); T.W. Greene and P.G.M. Wuts,
Protective
GYOUps in O>~garaic Sy>zt7Zesis, 3rd Ed., John Wiley and Sons (1999); L.
Fieser and M.
to
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
Fieser, Fiesef~ grad Fiese~'s Reagefzts for Organic Synthesis, John Wiley and
Sons (1994);
and L. Paquette, ed., Encyclopedia of Reczgerats for O~gahie Synthesis, John
Wiley and
Sons (1995) and subsequent editions thereof.
The necessary starting materials for preparing the compounds of formula (I)
;.re
either known or may be prepared in analogy with the preparation of known
compounds.
The dose level and frequency of dosage of the specific compound will vary
depending on a
variety of factors including the potency of the specific compound employed,
the metabolic
stability and length of action of that compound, the patient's age, body
weight, general
health, sex, diet, mode and time of administration, rate of excretion, drug
combination, the
to severity of the condition to be treated, and the patient undergoing
therapy. The daily
dosage may, for example, range from about 0.001 mg to about 100 mg per kilo of
body
weight, administered singly or multiply in doses, e.g. from about 0.01 mg to
about 2:i mg
each. Normally, such a dosage is given orally but parenteral administration
may also be
chos en.
The invention will now be further illustrated by the following non-limiting
Examples.
EXAMPLES
Experin2e~ctal methods
All reagents were commercial grade and were used as received without further
purification, unless otherwvise specified. Cormnercially available anhydrous
solvents were
used for reactions conducted under inert atmosphere. Reagent grade solvents
were used in
all other cases, unless otherwise specified. Column chromatography was
performed on
Matrex~ silica gel 60 (35-70 micron). TLC was carried out using pre-coated
silica gel F-
254 plates (thickness 0.25 mm). 1H NMR spectra were recorded on a Bruker
Avance250 at
250 MHz. Chemical shifts for iH NMR spectra are given in part per million and
eith ~r
tetramethylsilane (0.00 ppm) or residual solvent peaks were used as internal
reference.
Splitting patterns are designated as follows: s, singlet; d, doublet; t,
triplet; q, quartet; p,
3o pentet; m, multiplet; br, broad. Coupling constants are given in Hertz
(Hz). Only selected
data are reported. The 13C NMR spectra were recorded at 62.5 MHz. DEPT
experiments
were used to help assign 13C NMR resonances where necessary. Chemical shifts
for 13C
NMR spectra are expressed in parts per million and residual solvent peaks were
used as
11
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
internal reference. HPLC analyses were performed using a Waters Xterra MS C18
column
(100 x 4.6 mm, 5~.) eluting with a gradient of 5% ACN in 95% water to 95% ACN
in 5%
water (0.2% TFA buffer) over 3.5 mins, then 95% ACN in 5% water (0.2% TFA
buffer)
for a further 2.5 mins at a flow rate of 3 ml/min on a Waters 600E or Gilson
system with
monitoring at 254 nm. Reverse phase preparative HPLC was carried out using a
Xterra MS
C18 column (100 x 19 mm, 5~.m) eluting with a gradient of 5% ACN in 95% water
to 95%
ACN in 5% water (0.05% DEA) over 12.0 mins, then 95% ACN in 5% water (0.05%
DEA) for a further 5.0 mins at a flow rate of 25 ml/min with monitoring at 254
nm. The
fractions that contained the desired product were concentrated under reduced
pressure and
l0 the resultant residue was lyophilised from a mixW re of dioxane and water.
Electrospray
MS spectra were obtained on a Micromass platform LCMS spectrometer. Compounds
were named using AutoNom 2000.
EXAMPLE 1
1-[2-(2-phenyl-1H-indol-3-yl)etliyl]-N-~ [(3,4,5-
trimethoxybenzyl)amino]carbonyl~piperidine-4-carboxamide
Step 1; (3,4,5-Trimethoxy-benzyl)-urea
To a solution of 3,4,5-trimethoxybenzylamine (1.0496g, 5.32 mmol) in water
(4mL) were
added conc. HCl (1mL) and potassium cyanate (3.458, 42.5mmo1) and the solution
was
stirred at 90°C for 2h. The mixture was then cooled to room temperature
and the solid was
~5 filtered and washed with water to yield a white solid (lg, 78.2%).
'H-NMR(250MHz, DMSO-d6) 8= 3.60 (s, 3H, -OMe), 3.73 (s, 6H, -OMe), 4.08 (d,
2H,
J=6.OHz, -CH2-Ar), 5.54 (s, 2H, -NHZ), 6.42 (t, 1H, J=6.OHz, -NH), 6.55 (s,
2H, Harom).
HPLC 98%, Rt=1.30 min. MS (ES) m/z 241.24 (M+H).
3o Step 2; Methanesulfonic acid 2-(Z-phenyl-1H-indol-3-yl)-ethyl ester
Methane sulfonyl chloride (0.247mL, 3.18mmo1) was added dropwise at 0°C
to a so~ution
of 2-(2-phenyl-1H-indol-3-yl)-ethanol (606mg, 2.55mmol) and triethylamine
(0.56mL,
12
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
4mmol) in dry dichloromethane (SmL). After 40min the solution was poured into
1N HCl,
the organic layer was separated, washed with water, brine, dried over
magnesium sulfate
and concentrated under vacuum to afford a red oil (0.8g, 100%).
IH-NMR(250MHz, CDCl3) 8= 2.79 (s, 3H, -Me), 3.37 (t, 2H, J=7.4Hz, -CHZ-Ar),
4.48 (t,
2H, J=7.3Hz,-CH2-O-), 7.17-7.34 (m, 4H, Harom), 7.39-7.67 (m, SH, Harom), 8.20
(s, 1H,
-NH). HPLC 94%, Rt=2.95 min. MS (AP) m/z no molecular ion found.
Step 3; 1-[2-(2-Phenyl-1H-indol-3yl)-ethyl]-piperidine-4-carboxylic acid
methyl ester
A solution of methanesulfonic acid 2-(2-phenyl-1H-indol-3-yl)-ethyl ester
(0.8g,
2.Smmol), methyl isonipecotate (0.473mL, 3.Smmol) and sodium hydrogen
carbonate
(0.9g, l lmmol) in dry acetonitrile (5mL) was stirred at 80°C for 20h.
The solution was
cooled, filtered and concentrated under vacuum to afford a yellow oil (O.Sg)
that could not
be purified by column chromatography due to decomposition on silica or on
alumina.. The
intermediate was engaged in the next step (coupling with urea) without further
purification.
Step 4; 1-[2-(2-phenyl-1H-indol-3-yl)etliyl]-N-{[(3,4,5-
trimethoxybenzyl)amino]-
carbonyl}piperidine-4-carboxamide
To a solution of 3,4,5-trimethoxybenzylurea (97.2mg, 0.4nunol), 1-[2-(2-phenyl-
1H-indol-
3y1)-ethyl]-piperidine-4-carboxylic acid methyl ester (220mg, 0.6mmol) in
dimethylacetamide (3mL) was added sodium methoxide (0.45mL, 25% wt sol. in
MeOH,
2.Ommol). The reaction was carried out on a rotary evaporator for lh to remove
any brace
of methanol. Water was then added and the compound extracted with ethyl
acetate. The
compound was purified by preparative-hplc under basic conditions (DEA) to
afford a white
solid (29.3mg, 13%).
1H-NMR (250 MHz, CDC13) 8=1.77-1.90 (m, 4H, piperidine), 2.04 (dd, 2H,
J=2.75/11.4Hz, -CHZ-), 2.12-2.25 (m, 1H, -CH-CO), 2.66-2.72 (m, 2H, -CHZ-),
3.06-3.13
(m, 4H, 2-CHZ), 3.82 (s, 3H, -OMe), 3.85 (s, 6H, -OMe), 4.40 (d, 2H, J=5.8Hz, -
CHZ-Ar),
6.53 (s, 2H, Harom), 7.11-7.25 (m, 2H, Harom), 7.37-7.67 (m, 7H, Harom), 8.05
(d, 2H,
J=7.8Hz, -NH), 8.72 (t, 1H, J=5.56Hz, -NH). HPLC 100%, Rt=3.59 min. MS (ES)
m/z
571.02 (M+H).
13
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
EXAMPLE 2
1-[2-(2,6-dimethoxyphenoxy)ethyl]-N-(~ [3-(trifluoromethyl)phenyl] amino]
carbonyl)-
piperidine-4-carboxamide
To a solution of (3-trifluoromethyl-phenyl)-urea* (0.1699g, 0.83mmol) and 1-[2-
(2,6-
dimethoxy-phenoxy)-ethyl]-piperidine-4-carboxylic acid methyl ester**
(0.2635g,
0.81mmol) in dimethylacetamide (2.OmL) was added sodimn methoxide (0.9rnL, 25%
in
MeOH, 4.Ommo1). The reaction was stirred for lh under vacumn on a rotary
evaporator at
room temperature. Water (1 OmL) was added, a white precipitate formed. The
solid was
to filtered and wash with water. The solid was dissolved in ethyl acetate and
the solution
washed with water. The organic layer was dried (MgS04) and the solvent
concentrated to
about O.SmL. White crystals formed. The crystals were filtered and washed with
a srlall
amount of ethyl acetate. The product was obtained as white crystals (0.1157g,
29%).
1H-NMR (250 MHz, CDCl3) ~= 1.86-1.95 (m, 4H, CHZ), 2.05-2.19 (m, 2H, -CHZ-),
2.33-
2.43 (m, 1H, -CH-CO), 2.80 (t, 2H, J= 5.9Hz, -CHZ-), 3.14-3.19 (m, 2H, -CHZN),
3.83 (s,
6H, 2x-OMe), 4.10 (t, 2H, J=5.9Hz, -CHZO), 6.57 (d, 2H, J=8.4Hz, Harom), 6.99
(t, 1H,
J=8.4Hz, Harom), 7.34-7.47 (m, 2H, Harom), 7.60 (brd, 1H, J=B.OHz, Harorn),
7.98 (s, 1H,
Harom), 9.78 (s, 1H, -NH) and 10.92 (s, 1H, -NH). 13C-NMR (62.SMHz, CDCl3) 8=
26.3,
41.9, 51.1, 54.1, 56.1, 68.5, 103.2, 115.0, 121.2, 121.7, 127.6, 135.2, 135.8,
150.4, 151.7
2o and 175.9. HPLC 99%, Rt=2.25 min. MS (ES) ~z/z 496 (M+H).
* Was synthesized using a similar procedure to Example 1, Step 1.
*~' Was synthesized using a similar procedure to Example l, Step 3.
EXAMPLE 3
N-[(cyclohexylamino)carbonyl]-1-[2-(2-phenyl-1H-indol-3-yl)ethyl]piperidine-4-
carboxamide
To a solution of cyclohexylurea* (75.1mg, O.Smmol) and 1-[2-(2-phenyl-1H-indol-
3yl)-
ethyl]-piperidine-4-carboxylic acid methyl ester** (253.3mg, 0.75rmnol) in
dimethylacetamide (SmL) was added sodium methoxide (0.7mL, 25% wt in MeOH,
3.14mmol). The reaction was earned out on a rotary evaporator for lh to remove
any trace
of methanol. Water was then added and attempts to extract the compound with
ethyl
acetate or chloroform failed. The aqueous layer was therefore evaporated to
dryness,
acetonitrile was added, the solution dried over magnesium sulfate and
concentrated to give
14
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
a yellow solid which was purified by preparative-hplc under basic condition
(DEA) and
afford a white solid (5.8mg, 3%). HPLC 100%, Rt=3.97 min. MS (ES) rralz 473.00
(M+H).
* Was synthesized using a similar procedure to Example l, Step 1.
:k=k Was synthesized using a similar procedure to Example 1, Step 3.
General procedure A for example 4 and 5: (library compounds):
H H
I I
O N~N
R
O
N~
I
R'
To a stirred solution of urea* (0.20 mmol) in DMSO (0.5 ml) was added
potassium te~t-
l0 butoxide (0.40 mmol) as a DMSO solution amd the reactions shaken at room
temperature.
After 15 minutes a solution of the ester** (0.2 mrnol) in DMSO was added and
the
contents shaken for a further 18 hours. The reactions were subsequently
filtered over
amberliteTM-IR-120(H) resin and purified by preparative chromatography using
the
following conditions:
* Was synthesized using a similar procedure to Example 1, Step 1.
** Was synthesized using a similar procedure to Example 1, Step 3.
Mobile phase.
0.2% TFA/water, ACN
2o Flow rate 25 ml/min.
Gradient: 85/15 H~0 + 0.2% TFA / ACN for 1.5 min.
5/95 in 9.5 min. for 1.5 min.
85/15 in 0.5 min.
Detector: ELS. (approx. 1.5m1/min flow split to Sedex 55 ELSD)
Gas (Nitrogen) 2.0 bar
Nebulizer 40°C
Column: Waters SyrnmetryPrep TM l9mm x 150mm x 7~,m C18
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
EXAMPLE 4
N-] [(2,4-difluorophenyl)amino] carbonyl}-1-[2-(4-
fluorophenyl)ethyl]piperidine-4-
carboxamide
Example 4 was synthesized according to General procedure A.
HPLC 100%, Rt = 3.88 min. MS (AP) m/z 406 (M+H).
EXAMPLE 5
N-f [(2-ethoxyphenyl)amino]carbonyl}-1-[2-(4-fluorophenyl)ethyl]piperidine-4-
carboxamide
Example 5 was synthesized according to General procedure A.
HPLC 97%, Rt = 3.96 min. MS (AP) m/z 414 (M+H).
PREPARATION OF A PHARMACEUTICAL COMPOSITION
E~~AMPLE
6:
Preparation
of
tablets
Ingredients m ltg
ablet
1. Active compound of formula10.0
(I)
2. Cellulose, microcrystalline57.0
3. Calcium hydrogen phosphate15.0
4. Sodium starch glycolate 5.0
5. Silicon dioxide, colloidal0.25
6. Magnesium stearate 0.75
The active ingredient 1 is mixed with ingredients 2, 3, 4 and 5 for about 10
minutes. The magnesium stearate is then added, and the resultant mixture is
mixed for
about 5 minutes and compressed into tablet form with or without film-coating.
3o BIOLOGICAL METHODS
Experimental methods
16
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
Primary screening and TCSO determination
CHO cells expressing 5-HT2A receptors seeded in 384 well plates are pre-loaded
with Fluo-4AM fluorescent dye and then incubated with compound (10 ~,M for
primary
screen) for 15 min. Fluorescent intensity is recorded using a Fluorometric
imaging plate
reader (FLIPR384, Molecular Devices) and inhibition of the peak response
evoked by 5-
HT (EC~o concentration) is calculated.
ICSO determinations are performed utilizing the same functional assay as
described
for primary screening (15 min antagonist compowd pre-incubation), applying the
compounds in the dose range of 3 nM to 10 ~.M.
Ih vitf~o receptor pharmacology -selectivity determinations
The affinity constants of compounds were determined using recombinant human
serotonin receptors stably expressed in fibroblast cell lines (CHO or HEK293),
measuring
the ability of the compounds to displace radio-labelled tracers using
scintillation proximity
assays or filter binding assays. For 5-HT1B, 5-HT~B and 5-HT~C receptor
binding studies
3H-LSD was used as radio ligand, for 5-HT2A and 5-HT6 3H-5-HT was used as
tracer,
while the binding constant to 5-HT1A was determined using 3H-8-OH-DPAT. The
non-
selective serotonin receptor antagonist mianserine was used as reference
substance.
The activity at 5-HT2C receptors was studied in a FLIPR based assay, measuring
2o the effect of compounds on 10 nM 5-HT induced Ca2+-currents.
Biological summary
The calculation of the K; values for the inhibitors was performed by use of
Activity
Base. The K; value is calculated from IC50 using the Cheng Prushoff equation
(with
reversible inhibition that follows the Michaelis-Menten equation): K; = IC50
(1+[S]/Km)
[Cheng, Y.C.; Prushoff, W.H. Biochem. Pharmacol. 1973, 22, 3099-3108]. The
compounds of Formula (I) exhibit ICSp values for the 5-HT2A receptor in the
range from
1 nM to 10 ECM.
5-HT2A antagonist lead compounds were identified in FLIPR-based functional
screening of the 5-HT2A receptor. One of these compounds were tested in
equilibrium
displacement binding measurements. The results show that Example 2 is a high
affinity
17
CA 02537349 2006-02-28
WO 2005/021505 PCT/SE2004/001236
ligand for the 5-HT2A receptor subtype, with a K; value in the nanomolar
range. The
compound is highly selective over five other serotonin receptors assayed (5-
HT2C, 5-
HT2B, 5-HTlA, 5-HT6 and 5-HT1B). Example 2 is shown also to be selective at 5-
HT2A
versus the 5-HT2C receptor in terms of efficacy.
Functional
Binding
Exam K; (~) K;
le (nM)
p 5-HT~A 5-HT1A 5-HTlg 5-HT2A 5-HT~B 5-HT2~ 5-HT6
Example
32.3 > 1000 > 1000 23 > 1000 > 1000 > 1000
2
The table shows the selectivity of Example 2 for the 5-HT2A over other
serotonin-
binding receptors.
to
18