Note: Descriptions are shown in the official language in which they were submitted.
CA 02537669 2013-05-17
1
INHIBITORS OF NUCLEOSIDE PHOSPHORYLASES AND
NUCLEOSIDASES FOR TREATING CANCER
TECHNICAL FIELD
The present invention relates to methods of treating cancer by administering
to a
patient in need thereof one or more inhibitors of 5'-methylthioadenosine
phosphorylase (MTAP). In particular, the invention relates to methods of
treating
prostate cancer or head and neck cancer.
BACKGROUND
Certain nucleoside analogues have been identified as potent inhibitors of 5'-
methylthioadenosine phosphorylase (MTAP) and 5'-methylthioadenosine
nucleosidase (MTAN). These are the subject of WO 03/080620.
Compounds where the location of the nitrogen atom in the sugar ring is varied
or
where two nitrogen atoms form part of the sugar ring, have also been
identified as
inhibitors of MTAP and MTAN. These compounds are described in WO
2004/018496.
MTAP and MTAN function in the polyamine biosynthesis pathway, in purine
salvage
in mammals, and in the quorum sensing pathways in bacteria. MTAP catalyses the
reversible phosphorolysis of methylthioadenosine (MTA) to adenine and 5-
methylthio-
a-D-ribose-1-phosphate (MTR-1P). MTAN catalyses the reversible hydrolysis of
MTA
to adenine and 5-methylthio-a-D-ribose and of S-adenosyl-L-homocysteine (SAH)
to
adenine and S-ribosyl-homocysteine (SRH). The adenine formed is subsequently
recycled and converted into nucleotides. Essentially, the only source of free
adenine
in the human cell is a result of the action of these enzymes. The MTR-1P is
subsequently converted into methionine by successive enzymatic actions.
CA 02537669 2006-02-24
2
MTA is a by-product of the reaction involving the transfer of an aminopropyl
group
from decarboxylated S-adenosylmethionine to putrescine during the formation of
spermidine. The reaction is catalyzed by spermidine synthase. Likewise,
spermine
synthase catalyses the conversion of spermidine to spermine, with concomitant
production of MTA as a by-product. The spermidine synthase is very sensitive
to
product inhibition by accumulation of MTA. Therefore, inhibition of MTAP or
MTAN
severely limits the polyamine biosynthesis and the salvage pathway for adenine
in the
cells.
Although MTAP is abundantly expressed in normal cells and tissues, MTAP
deficiency due to a genetic deletion has been reported with many malignancies.
The
loss of MTAP enzyme function in these cells is known to be due to homozygous
deletions on chromosome 9 of the closely linked MTAP and p16/MTS1 tumour
suppressor gene. As absence of p16/MTS1 is probably responsible for the
tumour,
the lack of MTAP activity is a consequence of the genetic deletion and is not
causative for the cancer. However, the absence of MTAP alters the purine
metabolism in these cells so that they are mainly dependent on the de novo
pathway
for their supply of purines.
MTA has been shown to induce apoptosis in dividing cancer cells, but to have
the
opposite, anti-apoptotic effect on dividing normal cells such as hepatocytes
(E.
Ansorena et at., Hepatology, 2002, 35: 274-280).
MTAP inhibitors may therefore be used in the treatment of cancer. Such
treatments
are described in WO 03/080620 and WO 2004/018496.
The need for new cancer therapies remains ongoing. For some prevalent cancers
the
treatment options are still limited. Prostate cancer, for example, is the most
commonly
diagnosed non-skin cancer in the United States. Current treatment options
include
radical prostatectomy, radiation therapy, hormonal therapy, and watchful
waiting.
Although the therapies may offer successful treatment of an individual's
condition, the
pitfalls are quite unfavorable and lead to a decrease in a man's overall
quality of life.
Surgery may inevitably result in impotence, sterility, and urinary
incontinence. Side
CA 02537669 2006-02-24
3
effects associated with radiation therapy include damage to the bladder and
rectum
as well as slow-onset impotence. Hormonal therapy will not cure the cancer and
eventually most cancers develop a resistant to this type of therapy. The major
risk
associated with watchful waiting is that it may result in tumour growth,
cancer
progression and metastasis. It is therefore desirable that a better treatment
option is
made available to patients diagnosed with prostate cancer.
It is an object of the invention to provide a method of treating cancer,
particularly
prostate or head and neck cancer, or at least to provide a useful choice.
STATEMENTS OF INVENTION
Ina first aspect, the invention provides a method of treating cancer
comprising
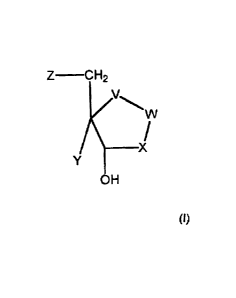
administering to a patient in need thereof a compound of the formula (I):
Z-C H2
V
__________________________________ X
OH
(I)
wherein:
V is selected from CH2 and NH, and W is selected from NR1 and NR2; or V
is selected from NR1 and NR2, and W is selected from CH2 and NH;
X is selected from CH2 and CHOH in the R or S-configuration;
Y is selected from hydrogen, halogen and hydroxy, except where V is
selected from NH, NR1 and NR2 then Y is hydrogen;
Z is selected from hydrogen, halogen, hydroxy, SQ, OQ and Q, where Q
is alkyl, aralkyl or aryl, each of which is optionally substituted with one or
CA 02537669 2006-02-24
4
more substituents selected from hydroxy, halogen, methoxy, amino, or
carboxy;
R1 is a radical of the formula (II)
/ N
A
)1
G
(II)
R2 is a radical of the formula (Ill)
Al
'-(LN
G
(Ill)
A is selected from N, CH and CR3, where R3 is alkyl, aralkyl or aryl, each
of which is optionally substituted with one or more substituents selected
from hydroxy and halogen; or R3 is hydroxyl, halogen, NH2, NHR4, NR4R5;
or SR6, where R4, R5 and R6 are alkyl, aralkyl or aryl groups, each of
which is optionally substituted with one or more substituents selected from
hydroxy and halogen;
B is selected from NH2 and NHR7, where R7 is alkyl, aralkyl or aryl, each
of which is optionally substituted with one or more substituents selected
from hydroxy and halogen;
CA 02537669 2006-02-24
, .
D is selected from hydroxy, NH2, NHR8, hydrogen, halogen and SCH3,
where R8 is alkyl, aralkyl or aryl, each of which is optionally substituted
with one or more substituents selected from hydroxy and halogen;
E is selected from N and CH;
G is selected from CH2 and NH, or G is absent, provided that where W is
NR1 or NR2 and G is NH then V is CH2, and provided that where V is NR1
or NR2 and G is NH then W is CH2;
or a tautomer thereof, or a pharmaceutically acceptable salt thereof, or an
ester
thereof, or a prodrug thereof.
Preferably the cancer is prostate cancer or head and neck cancer.
In another aspect, the invention provides a method of treating cancer
comprising
administering to a patient in need thereof a compound of the formula (IV):
Z-9H2
V
K,
X
Y ______________________________________________
OH
(IV)
wherein:
V is selected from CH2 and NH, and W is selected from NR1 and NR2; or V
is selected from NR1 and NR2, and W is selected from CH2 and NH;
X is selected from CH2 and CHOH in the R or S-configuration;
Y is selected from hydrogen, halogen and hydroxy, except where V is
selected from NH, NR1 and NR2 then Y is hydrogen;
CA 02537669 2006-02-24
6
Z is selected from hydrogen, halogen, hydroxy, SQ, OQ and Q, where Q
is alkyl, aralkyl or aryl, each of which is optionally substituted with one or
more substituents selected from hydroxy, halogen, methoxy, amino, or
carboxy;
R1 is a radical of the formula (V)
A/
)E=
G
(V)
R2 is a radical of the formula (VI)
Ai
(VI)
A is selected from N, CH and CR3, where R3 is alkyl, aralkyl or aryl, each
of which is optionally substituted with one or more substituents selected
from hydroxy and halogen; or R3 is hydroxyl, halogen, NH2, NHR4, NR4R5;
or SR6, where R4, R5 and R6 are alkyl, aralkyl or aryl groups, each of
which is optionally substituted with one or more substituents selected from
hydroxy and halogen;
B is selected from NH2 and NHR7, where R7 is alkyl, aralkyl or aryl, each
of which is optionally substituted with one or more substituents selected
from hydroxy and halogen;
CA 02537669 2006-02-24
7
D is selected from hydroxy, NH2, NHR8, hydrogen, halogen and SCH3,
where R8 is alkyl, aralkyl or aryl, each of which is optionally substituted
with one or more substituents selected from hydroxy and halogen;
E is selected from N and CH;
G is selected from CH2 and NH, or G is absent, provided that where W is
NR1 or NR2 and G is NH then V is CH2, and provided that where V is NR1
or NR2 and G is NH then W is CH2;
or a tautomer thereof, or a pharmaceutically acceptable salt thereof, or an
ester
thereof, or a prodrug thereof;
provided that the compound (3R,4S)-1-[(9-deazaadenin-9-yl)methy1]-3-hydroxy-
4-(methylthiomethyl)pyrrolidine is excluded.
Preferably Z is SQ.
Preferably Q is an alkyl group, optionally substituted with one or more
substituents
selected from hydroxy, halogen, methoxy, amino, or carboxy. It is further
preferred
that Q is a C1-C6 alkyl group, optionally substituted with one or more
substituents
selected from hydroxy, halogen, methoxy, amino, or carboxy. Most preferably Q
is a
methyl group
Alternatively it is preferred that Z is not methylthio.
It is also preferred that Q is an aryl group, optionally substituted with one
or more
substituents selected from hydroxy, halogen, methoxy, amino, or carboxy. More
preferably Q is a phenyl or benzyl group, optionally substituted with one or
more
substituents selected from hydroxy, halogen, methoxy, amino, or carbon.
Preferably G is CH2. It is also preferred that V is CH2 and W is NR1. It is
further
preferred that B is NH2. It is also preferred that D is H, and it is preferred
that A is CH.
Preferably any halogen is chlorine or fluorine.
CA 02537669 2006-02-24
8
In another aspect, the invention provides a method of treating cancer
comprising
administering to a patient in need thereof a compound of the formula (VII):
NH2
\S N
HO
(VII)
where J is aryl, aralkyl or alkyl, each of which is optionally substituted
with one
or more substituents selected from hydroxy, halogen, methoxy, amino, or
carboxy.
Preferably the cancer is prostate cancer or head and neck cancer.
Preferably J is C1-C7 alkyl. More preferably J is methyl, ethyl, n-propyl, i-
propyl, n-
butyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexylmethyl or cycloheptyl.
It is also preferred that J is phenyl, optionally substituted with one or more
halogen
substituents. More preferably J is phenyl, p-chlorophenyl, p-fluorophenyl or m-
chlorophenyl.
It is also preferred that J is heteroaryl. More preferably J is 4-pyridyl.
It is also preferred that J is aralkyl. More preferably J is benzylthio.
Preferably J is ¨CH2CH2(NH2)COOH.
In another aspect, the invention provides a method of treating cancer
comprising
administering to a patient in need thereof a compound of the formula (VIII):
CA 02537669 2006-02-24
9
NH2
T\s
\ 'I
HO OH
(VIII)
where T is aryl, aralkyl or alkyl, each of which is optionally substituted
with one
or more substituents selected from hydroxy, halogen, methoxy, amino,
carboxy or straight- or branched-chain C1-C6 alkyl.
Preferably the cancer is prostate cancer or head and neck cancer.
Preferably T is C1-C6 alkyl, optionally substituted with one or more
substituents
selected from halogen and hydroxy. More preferably T is methyl, ethyl, 2-
fluoroethyl,
or 2-hydroxyethyl. Most preferably T is methyl.
It is also preferred that T is aryl, optionally substituted with one or more
substituents
selected from halogen or straight-chain Cl-Ce alkyl. More preferably T is
phenyl,
naphthyl, p-tolyl, m-tolyl, p-chlorophenyl, m-chlorophenyl or p-fluorophenyl.
It is also preferred that T is aralkyl. More preferably T is benzyl.
Preferably the compound of formula (I) is:
(3R,4R)-1-[(8-aza-9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(hydroxymethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-(2-
phenylethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(benzylthiomethyl)pyrrolidine;
(3R,4S)-1-[(8-aza-9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(benzylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methylj-3-hydroxy-4-(4-
chlorophenylthiomethyl)pyrrolidine;
(3R,4R)-1-[(9-deazaadenin-9-yOmethyl]-3-acetoxy-4-
CA 02537669 2006-02-24
(acetoxymethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-(n-
butylthiomethyppyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yOmethyl]-3-hydroxy-4-(4-
fluorophenylthiomethyppyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-(n-
propylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methy11-3-hydroxy-4-
(cyclohexylthiomethyhpyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-(3-
chlorophenylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yOmethyl]-3-hydroxy-4-
(ethylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methy1]-3-hydroxy-4-
(phenylthiomethyppyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methy1]-3-hydroxy-4-(4-
pyridylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-n-propylpyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(homocysteinylmethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yhmethyI]-3-hydroxy-4-
(benzyloxymethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-Amethyl]-3-hydroxy-4-(i-
propylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yOmethyl]-3-hydroxy-4-
(methoxymethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(cyclohexylmethylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(cycloheptylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methy11-3-hydroxy-4-
(cyclopentylthiomethyl)pyrrolidine;
(3R,4S)-1-[(9-deazaadenin-9-yl)methy11-3-hydroxy-4-
(cyclobutyfthiomethyl)pyrrolidine.
CA 02537669 2006-02-24
11
The compounds defined above may be administered orally, parenterally, by
inhalation, topically, rectally, nasally, buccally or via an implanted
reservoir. Preferably
the compounds are administered orally.
BRIEF DESCRIPTION OF THE FIGURES
Figure la shows the survival of mouse prostate cancer cells (RM1) against
increasing
concentrations of compound (2) R3R,4S)-1-[(9-deazaadenin-9-y1)methyl]-3-
hydroxy-4-
(methylthiomethyl)pyrrolidine], either in the presence or absence of MTA.
Figure lb
shows the survival of human prostate cancer cells (PC3) against increasing
concentrations of compound (2) [(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-
hydroxy-4-
(methylthiomethyl)pyrrolidine], either in the presence or absence of MTA.
Figure 2 is a time dependent proliferation curve, showing the effect of
compound (2)
[(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-
(methylthiomethyl)pyrrolidine]
and MTA on human prostate cancer cells (PC3).
Figure 3 is a time dependent proliferation curve, showing the effect of
compound (2)
[(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-4-(methylth
iomethyppyrrolidine]
and MTA on SCC25 cells.
Figure 4 is a time dependent proliferation curve, showing the effect of
compound (2)
[(3R,4S)-1-[(9-deazaad en in-9-yl)methyI]-3-hydroxy-4-(m ethylth
iomethyl)pyrrolidin el
and MTA on FaDu cells.
Figure 5 shows phase contrast photographs of FaDu cells after 5 days of
treatment
with compound (2) [(3R,4S)-1-
[(9-deazaadenin-9-yl)methyI]-3-hydroxy-4-
(methylthiomethyl)pyrrolidine] and MTA.
Figure 6 shows a cell cycle and apoptosis analysis of FaDu cells after 6 days
of
treatment with compound (2) [(3R,4S)-1-[(9-deazaadenin-9-yl)methyl]-3-hydroxy-
4-
(methylthiomethyl)pyrrolidine] and MTA; (1) untreated results: G1 83.66%, S
8.08%,
G2 8.26%, Apoptosis 6.06%; (2) treated with MTA results: G1 79.67%, S 10.42%,
G2
9.91%, Apoptosis 6.66%; (3) treated with compound (3) results G1 72.06%, S
17.98%, G29.96%, Apoptosis 7.89%; (4) treated with MTA + compound (3) results
G1
8.26%, S 31.25%, G2 60.49%, Apoptosis 29.41%.
CA 02537669 2006-02-24
12
Figures 7 to 19 show oral and IP availability of selected compounds that may
be used
in the methods of the invention.
Figure 20 shows the effects of compound 1 R3R,4S)-14(9-deazaadenin-9-
yl)methyl]-
3-hydroxy-4-(benzylthiomethyl)pyrrolidine] on FaDu xenografts in NOD-SCID
mice.
Figure 21 shows representative tumours from each of the treatment cohorts for
the
above NOD-SCID mouse study.
Figure 22 shows MRI images of TRAMP mice (Panels A and B: Control TRAMP
(transgenic adenocarcinoma of mouse prostate) mice, Panels E and F: TRAMP mice
treated with 1 mM Compound 1 [(3R,4S)-14(9-deazaadenin-9-yl)methy11-3-hydroxy-
4-
(benzylthiomethyppyrrolidine])
Figure 23 shows that compound (2) and MTA alter polyamine levels and induce
cytostasis in PC3 cells (PUT=putrescine, SPD=spermidine, SPN=spermine). PC3
cells were cultured and treated in triplicate as follows: untreated control,
20 RM
substrate (MTA) alone, 1 p.M compound (2) alone, or a combination of both
substrate
and inhibitor. Both cells and spent media were harvested at 1, 6, and 12 days
for
polyamine analysis by HPLC fluorescence.
Figure 24 shows that compound (2) reduces tumour growth and metastasis in
TRAMP mice, but does not alter polyamine levels in vivo. C56B1I6 mice were
treated
with 100 RM compound (2) via their drinking water and sacrificed at 24, 48
hours, and
7 days. Livers were immediately removed for polyamine analysis. TRAMP mice
were treated approximately 6-8 months with 100 RM compound (2) via their
drinking
water and control sacrificed. Livers were removed for polyamine analysis.
DETAILED DESCRIPTION
Definitions
The term "alkyl" is intended to include straight- and branched-chain alkyl
groups, as
well as cycloalkyl groups. The same terminology applies to the non-aromatic
moiety
of an aralkyl radical. Examples of alkyl groups include: methyl group, ethyl
group, n-
.
CA 02537669 2006-02-24
13
propyl group, iso-propyl group, n-butyl group, iso-butyl group, sec-butyl
group, t-butyl
group, n-pentyl group, 1,1-dimethylpropyl group, 1,2-dimethylpropyl group, 2,2-
dimethylpropyl group, 1-ethylpropyl group, 2-ethylpropyl group, n-hexyl group
and 1-
methy1-2-ethylpropyl group.
The term "aryl" means an aromatic radical having 6 to 18 carbon atoms and
includes
heteroaromatic radicals. Examples include monocyclic groups, as well as fused
groups such as bicyclic groups and tricyclic groups. Some examples include
phenyl
group, indenyl group, 1-naphthyl group, 2-naphthyl group, azulenyl group,
heptalenyl
group, biphenyl group, indacenyl group, acenaphthyl group, fluorenyl group,
phenalenyl group, phenanthrenyl group, anthracenyl group,
cyclopentacyclooctenyl
group, and benzocyclooctenyl group, pyridyl group, pyrrolyl group, pyridazinyl
group,
pyrimidinyl group, pyrazinyl group, triazolyl group, tetrazolyl group,
benzotriazolyl
group, pyrazoly1 group, imidazoly1 group, benzimidazolyl group, indolyl group,
isoindolyl group, indolizinyl group, purinyl group, indazolyl group, furyl
group, pyranyl
group, benzofuryl group, isobenzofuryl group, thienyl group, thiazolyl group,
isothiazolyl group, benzothiazolyl group, oxazolyl group, and isoxazolyl
group.
The term "halogen" includes fluorine, chlorine, bromine and iodine.
The compounds are useful for the treatment of certain diseases and disorders
in
humans and other animals. Thus, the term "patient" as used herein includes
both
human and other animal patients.
The term "prodrug" as used herein means a pharmacologically acceptable
derivative
of the compound of formula (1), (IV), (VII) or (VIII), such that an in vivo
biotransformation of the derivative gives the compound as defined in formula
(I), (IV),
(VII) or (VIII). Prodrugs of compounds of formulae (I), (IV), (VII) or (VIII)
may be
prepared by modifying functional groups present in the compounds in such a way
that
the modifications are cleaved in vivo to give the parent compound.
Prodrugs include compounds of formulae (I), (IV), (VII) and (VIII), tautomers
thereof
and/or pharmaceutically acceptable salts thereof, which include an ester
functionality,
or an ether functionality. It will be clear to the skilled person that the
compounds of
formulae (I), (IV), (VII) and (VIII) may be converted to corresponding ester
or ether
prodrugs using known chemical transformations.
CA 02537669 2006-02-24
14
Suitable prodrugs include those where the hydroxyl groups of the compounds of
formula (I), (IV), (VII) or (VIII) are esterified to give, for example, a
primary hydroxyl
group ester of propanoic or butyric acid. Other suitable prodrugs are
alkycarbonyoxymethyl ether derivatives on the hydroxyl groups of the compounds
of
formula (I), (IV), (VII) or (VIII) to give, for example, a primary hydroxyl
group ether with
a pivaloyloxymethyl or a propanoyloxymethyl group.
The term "pharmaceutically acceptable salts" is intended to apply to non-toxic
salts
derived from inorganic or organic acids, including, for example, the following
acid
salts: acetate, adipate, alginate, aspartate, benzoate, benzenesulfonate,
bisulfate,
butyrate, citrate, camphorate, camphorsulfonate, cyclopentanepropionate,
digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate,
glucoheptanoate,
glycerophosphate, glycolate, hemisulfate, heptanoate, hexanoate,
hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate, maleate,
malonate,
methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, oxalate,
palmoate,
pectinate, persulfate, 3-phenylpropionate, phosphate, picrate, pivalate,
propionate, p-
toluenesulfonate, salicylate, succinate, sulfate, tartrate, thiocyanate, and
undecanoate.
It will be appreciated that the representation of a compound of formula (I) or
(IV)
where B and/or D is a hydroxy group, is of the enol-type tautomeric form of a
corresponding amide, and this will largely exist in the amide form. The use of
the
enol-type tautomeric representation is simply to allow fewer structural
formulae to
represent the compounds of the invention.
Similarly, it will be appreciated that the representation of a compound of
formula (I) or
(IV), where B and/or D is a thiol group, is of the thioenol-type tautomeric
form of a
corresponding thioamide, and this will largely exist in the thioamide form.
The use of
the thioenol-type tautomeric representation is simply to allow fewer
structural
formulae to represent the compounds of the invention.
Detailed Description of the Invention
The present invention relates to methods of treating cancer by administering
to a
patient in need thereof one or more inhibitors of 5'-methylthioadenosine
CA 02537669 2006-02-24
phosphorylase (MTAP). In particular, the invention relates to methods of
treating
certain cancers, such as prostate cancer or head and neck cancer.
Suitable MTAP inhibitors which may be employed in the method of the present
invention and the methods for preparing these inhibitors are described in WO
03/080620 and VVO 2004/018496.
Certain MTAP inhibitor compounds are surprisingly effective for treating
prostate and
head and neck cancers. These are compounds of general formula (VII).
NH2
Jµs fel" 1
HO
(VII)
This sub-class of MTAP inhibitors incorporates an adenine-like base moiety and
a
pyrrolidine moiety having an alkyl- aryl- or aralkylthiomethyl group at the 4-
position.
Other MTAP inhibitor compounds are also surprisingly effective for treating
prostate
and head and neck cancers. These are compounds of general formula (VIII).
NH2
\
\
HO 011
(VIII)
This sub-class of MTAP inhibitors also incorporates the adenine-like base
moiety but
has an iminoribitol moiety with an alkyl- aryl- or aralkylthiomethyl group at
the 5'-
position.
Examples of the first sub-class of inhibitors include compounds (1) and (2).
,
CA 02537669 2006-02-24
16
C(1 H NH/
\ H NH2
¨pH
sJt
HO HO
Compound (1) Compound (2)
BT-DADMe-ImrnA MT-DADMe-ImmA
The present studies show that compounds (1) and (2) are effective both in
vitro and in
vivo against a variety of cell lines (PC3, RM1, SCC25 and FaDu). These
compounds
are therefore particularly useful in the treatment of prostate and head and
neck
cancers.
The MTAP inhibitor compounds inhibit cell growth in vitro of the prostate
cancer cell
lines PC3 and RM1 and the head and neck cancer cell lines SCC25 and FaDu. An
enhanced cell-killing effect is seen in vitro with combined administration of
the MTAP
inhibitor compound plus MTA. Examples of this effect are shown in Figures 1 to
6.
Furthermore, the inhibitor compounds, when co-administered with MTA, exhibit a
cytostatic effect on PC3 cells in vitro.
In order to determine whether the inhibition is selective for malignant cells,
normal
human fibroblast cells (GM02037) were also treated with compound (2) and MTA
for
3 weeks. No cytotoxicity was observed. Compound (2) is therefore cytotoxic for
human HNSCC (human head and neck squamous cell carcinoma) cells at doses that
exhibit minimal toxicity for normal cells. This selectivity is a further
indication that the
MTAP inhibitors described above are useful agents for the treatment of head
and
neck cancer.
The present in vivo studies further demonstrate the surprising efficacy of the
compounds. In a NOD-SCID mouse model, compound (2) significantly delays the
growth of established FaDu xenografts. The effect is seen either with or
without co-
administration of the inhibitor compound with MTA.
In addition, prostate cancer progression in the TRAMP mouse model is inhibited
in
CA 02537669 2006-02-24
17
mice treated with compound (1), either alone or in combination with MTA.
An example of the second sub-class of inhibitors is compound (3).
NH2
\ \N
N-off
HO Compound (3)
MT-ImmA
This compound also inhibits prostate cancer progression in the TRAMP mouse
model, when administered either alone or in combination with MTA.
For the above in vivo models, the inhibitor compounds exhibit activity when
administered with exogenous MTA and when administered alone. There is not a
significant enhancement observed when the inhibitors are administered together
with
MTA. However, the in vitro results clearly demonstrate a surprising
enhancement in
activity when the inhibitors are administered in conjunction with MTA. Thus,
the
combined administration method provides a potential alternative treatment
method for
patients suffering from cancer, where the administration of an MTAP inhibitor
is
indicated.
The MTAP inhibitor compounds of formulae (I), (IV), (VII) and (VIII) (in
particular the
compounds of formulae (VII) and (VIII)) provide an effective alternative
treatment
option for cancer sufferers, especially for patients diagnosed with prostate
and head
and neck cancers.
General Aspects
The MTAP inhibitor compounds are useful in both free base form and in the form
of
salts.
Figures 7, 9, 10, 12, 13, 15 and 16-19 show that the MTAP inhibitor compounds
used
in the methods of the present invention are orally available, and may
therefore be
formulated for oral administration. The compounds may also be administered by
other
CA 02537669 2006-02-24
18
routes. For example, the MTAP inhibitors may be administered to a patient
orally,
parenterally, by inhalation spray, topically, rectally, nasally, buccally or
via an
implanted reservoir. The amount of compound to be administered will vary
widely
according to the nature of the patient and the nature and extent of the
disorder to be
treated. Typically the dosage for an adult human will be in the range less
than 1 to
1000 milligrams, preferably 0.1 to 100 milligrams. The specific dosage
required for
any particular patient will depend upon a variety of factors, including the
patient's age,
body weight, general health, sex, etc.
For oral administration the active compounds can be formulated into solid or
liquid
preparations, for example tablets, capsules, powders, solutions, suspensions
and
dispersions. Such preparations are well known in the art as are other oral
dosage
regimes not listed here. In the tablet form the compounds may be tableted with
conventional tablet bases such as lactose, sucrose and corn starch, together
with a
binder, a disintegration agent and a lubricant. The binder may be, for
example, corn
starch or gelatin, the disintegrating agent may be potato starch or alginic
acid, and the
lubricant may be magnesium stearate. For oral administration in the form of
capsules, diluents such as lactose and dried cornstarch may be employed. Other
components such as colourings, sweeteners or flavourings may be added.
When aqueous suspensions are required for oral use, the active ingredient may
be
combined with carriers such as water and ethanol, and emulsifying agents,
suspending agents and/or surfactants may be used. Colourings, sweeteners or
flavourings may also be added.
The compounds may also be administered by injection in a physiologically
acceptable
diluent such as water or saline. The diluent may comprise one or more other
ingredients such as ethanol, propylene glycol, an oil or a pharmaceutically
acceptable
surfactant.
The compounds may also be administered topically. Carriers for
topical
administration of the compounds include mineral oil, liquid petrolatum, white
petrolatum, propylene glycol, polyoxyethylene, polyoxypropylene compound,
emulsifying wax and water. The compounds may be present as ingredients in
lotions
or creams, for topical administration to skin or mucous membranes. Such creams
may contain the active compounds suspended or dissolved in one or more
CA 02537669 2013-05-17
19
pharmaceutically acceptable carriers. Suitable carriers include mineral oil,
sorbitan
monostearate, polysorbate 60, cetyl ester wax, cetearyl alcohol, 2-
octyldodecanol,
benzyl alcohol and water.
The compounds may further be administered by means of sustained release
systems.
For example, they may be incorporated into a slowly dissolving tablet or
capsule.
Examples of suitable pharmaceutical carriers are described in Remington's
Pharmaceutical Sciences (Mack Publishing Company).
EXAMPLES
Inhibitor Compounds Inhibitors of MTAP were synthesized as described earlier
(Singh, V., Shi, W., Evans, G. B., Tyler, P. C., Furneaux, R H, Almo, S C, and
Schramm, V L (2004) Biochemistry 43, 9-18; Evans GB, Furneaux R H, Lenz D H,
et
al., J Med Chem 2005:48, 4679-89). Solutions were standardized by the UV
absorbance of the 9-deazaadenine ring. Sterile solutions of inhibitors were
prepared
by filtration.
Example 1: Clonogenic Assays (Figure 1)
PC3 cells were grown in equal (1:1) portions of Dulbecco's modified Eagle's
medium
and F12 containing 10% fetal bovine serum, 10 U/mL penicillin-G and 10 lig/mL
streptomycin in monolayers to near confluency at 37 C. Cells were lysed in 50
mM
sodium phosphate pH 7.5, 10 mM KCI and 0.5% Triton TM X-100.
Example 2: Effect of Compound 2 and MTA on PC3 cells (Figure 2)
PC3 cells were maintained in MEM Eagle's media supplemented with 10% fetal
bovine serum, 100 units/ml penicillin, 100 g/mL streptomycin, 0.1 mM non
essential
amino acids and 1 mM sodium pyruvate.
Cell survival was evaluated using the WST-1 assay (Kicska G A, long Li, Honig
H, et
al. Proc Nat! Aced Sci USA 2001;98:4593-98). Cells were seeded onto 96 well
plates
at a density of 104 cells per well, with either no additions, 1 M compound 2,
20 1/M
CA 02537669 2006-02-24
MTA or 1 p,M compound 2 + 20 RM MTA. IC50 was determined following the
manufacturer's protocol (Roche Applied Science, IN). Cells were grown and
measured in triplicate or quadruplicate and the error bars show the mean SD
of the
multiple samples.
Example 3: Effect of Compound 2 and MTA on SCC25 cells (Figure 3)
SCC25 cells were maintained in MEM Eagle's media supplemented with 10% fetal
bovine serum, 100 units/rill penicillin, 100 Rg/mL streptomycin, 0.1 mM non
essential
amino acids and 1 mM sodium pyruvate.
Cell survival was evaluated using the WST-1 assay (Kicska G A, long Li, Honig
H, et
al. Proc Nat! Acad Sc! USA 2001;98:4593-98). Cells were seeded onto 96 well
plates
at a density of 104 cells per well, with either no additions, 1 p,M MT-
compound 2, 20
p.M MTA or 1 ELM compound 2 + 20 p,M MTA. IC50 was determined following the
manufacturer's protocol (Roche Applied Science, IN). Cells were grown and
measured in triplicate or quadruplicate and the error bars show the mean SD
of the
multiple samples.
Example 4: Effect of MT-DADMe-ImmA (Compound 1) and MTA on FaDu cells
(Figure 4)
FaDu cells were maintained in MEM Eagle's media supplemented with 10% fetal
bovine serum, 100 units/ml penicillin, 100 pg/mL streptomycin, 0.1 mM non
essential
amino acids and 1 mM sodium pyruvate.
Cell survival was evaluated using the WST-1 assay (Kicska G A, long Li, Honig
H, et
al. Proc Nall Acad Sci USA 2001;98:4593-98). Cells were seeded onto 96 well
plates
at a density of 104 cells per well, with either no additions, 1 p,M compound
2, 20 p,M
MTA or 1 OA compound 2 + 20 p,N1 MTA. IC50 was determined following the
manufacturer's protocol (Roche Applied Science, IN). Cells were grown and
measured in triplicate or quadruplicate and the error bars show the mean SD
of the
multiple samples.
CA 02537669 2006-02-24
21
Example 5: Phase Contrast Microscopy of FaDu Cells (Figure 5)
FaDu cells were subjected to six days in culture using the same conditions
described
as for Example 4.
Example 7: Cell Cycle and Apoptosis Analysis of FaDu cells (Figure 6)
FaDu cells were subjected to six days in culture using the same conditions
described
as for Example 4, before staining with propidium bromide and FAGS cell sorting
analysis.
Example 8: Oral Availability (Method for Compound (2))
Two groups of 3 C57BL6 mice received a single oral dose of compound (2)
dissolved
in sterile, deionized water, pippeted onto a crumb of food. Treated food was
fed to
each mouse individually under close observation at time zero. Two different
single
doses of inhibitor were administered: 50 itg and 100 IQ. Mice were
individually fed
and closely observed for consumption of food. At specific time points, 4 tit
blood
samples were collected from the tail vein. The blood was mixed with 44 0.6%
Triton
X-100 in PBS and stored at -80 C until time of analysis.
The amount of adenine produced was measured by the following MTAP activity
assay:
Cells were harvested, washed three times with PBS and lysed with RIPA buffer.
The
reaction mixture for MTAP activity assays contained the following: - 75 pg
protein
from cell lysates, 50 mM HEPES pH 7.4, 50 01 MTA, and 20,000 dpm [2,8-311]MTA.
Labeled MTA was synthesized from [2,8-3NS-adenosylmethionine by a known
method. Products of the MTAP reaction were resolved using TLC silica plates
with 1
M ammonium acetate, pH 7.55, and 5% isopropanol. Adenine spots were excised
and counted for label incorporation.
Example 9: FaDu Xenograft Studies (Figures 20 and 21)
FaDu cells were injected into the dorsum of the foot of NOD-SCID mice. Groups
were
fed with 250 and 500 tiM of compound 2 p.o. or given i.p. injections of 100 RI
of
CA 02537669 2013-05-17
22
compound 2, daily. Differences between treatment cohorts were determined using
Student's t test. Compound 2 significantly delayed the growth of established
FaDu
xenografts.
Example 10: MRI Studies (Figure 22)
MRI experiments were performed using a 9.4T 21 cm bore horizontal bore magnet
(Magnex Scientific) Varian NOVATM MRI system (Fremont, CA) equipped with a 28
mm
inner diameter quadrature birdcage coil. Mice were anesthetized with
isoflurane
inhalation anesthesia (1-1.5% in 100% 02 administered via a nose cone) and
positioned in the MRI coil. Body temperature was maintained (37-38 C) using a
homeothermic warming system. After acquiring scout images, multi-slice spin-
echo
imaging with an echo time of 18 ms and a repetition time of 400ms ms was
performed. A 40 mm field of view with a 256 x 256 matrix size was used. Nine
to 15
slices along the transverse, sagittal, and coronal planes were acquired in
each multi-
slice experiment with a slice thickness of 1 mm and the gap between slices of
0.5
mm. MRI data were processed off-line with MATLAB-based MRI analysis software.
Example 11: Quantitation of Polyamines in Cells, Spent Media and Tissue
Samples (Figure 23)
Spent media and perchloric acid extracts of both PC3 cells and tissue samples
were
subjected to purification via cation exchange chromatography and dansyl-
derivatized
with minor changes. Disposable 10 ml BIO-RAD columns were centrifuged at 4,000
rpm for 3 minutes. Sodium carbonate used for derivatization was adjusted to pH
9.3
and the concentration of dansyl-chloride was adjusted to 100 mM. Dansyl-
polyamines were quantitated by a Waters HPLC/ Fluorescence system. A
Phenomenex Luna 5 t C18 column was used with a mobile phase of 30%
acetonitrile
in a 50 mM ammonium acetate buffer at pH 6.8 (eluent A) and 100% acetonitrile
(eluent B). Fluorescence detection was monitored by excitation at 338 nm and
emission at 500 nm.
Example 12: Treatment of TRAMP Mice (Table 1, Figure 22)
Short-Term: Mice were treated with sterile solutions of 100 ILLM compound (2)
(pH -6.4). Water bottles were autoclaved prior to filling with sterile
inhibitor solutions.
CA 02537669 2006-02-24
23
Mice were sacrificed at 1, 2, and 7 days, with three mice in each group, with
the
control group sacrificed after 7 days. Livers were immediately removed upon
sacrifice for polyamine analysis, conducted as described above.
Long-Term: Sterile solutions of 100 tiM compound (2) (pH ¨6.4). Water bottles
were
autoclaved prior to filling with sterile inhibitor solutions. Water
consumption was
monitored every other day, with fresh inhibitor solution being administered to
prevent
bacterial growth. Mice were control-sacrificed and tissues (genitourinary
system,
liver, lungs) were collected for histology and polyamine analysis. Mass and
dimensions of excised genitourinary system tumours were recorded. Sections of
small intestine were also removed for toxicity analysis via H&E staining.
Discussion of the Examples
Figure la shows the effect of the addition of compound (2) to cultured mouse
prostate
cancer cells (RM1). Figure lb shows the effect of the addition of compound (2)
to
cultured human prostate cancer cells (PC3). Compound (2) was added either
alone or
in the presence of 20 RM MTA. Figures 2, 3 and 4 show the effects of MTA
alone,
compound (2) alone and MTA with compound (2) in time dependent cell
proliferation
experiments (PC3 cells, SCC25 cells and FaDu cells). The combination of
compound
(2) and MTA reduces cell proliferation. These data demonstrate that the
compounds
which are used in the methods of the present invention inhibit cell growth in
vitro,
when administered in combination with MTA.
Figure 5 further demonstrates, showing phase contrast photographs of FaDu
cells
after 5 days of treatment with compound (2)/compound (2) + MTA, that the
inhibitor
compound + MTA is effective in inhibiting cell growth.
Thus, administration of MTA in circumstances where its degradation by MTAP is
inhibited by an MTAP inhibitor leads to greater circulatory and tissue levels
of MTA
and consequently an enhanced effect in the treatment of cancer.
Figure 6 shows that compound (2) in combination with MTA is also effective for
stopping cell cycling (for FaDu cells) such that the cells become apoptotic.
CA 02537669 2006-02-24
24
Figures 20 and 21 show the results of in vivo studies. FaDu cells were
injected into
the dorsum of the foot of NOD-SCID mice. Groups were fed with 250 and 500 [LM
of
compound (2) p.o. or given i.p. injections of 100 iii of 4 mM compound (2),
daily.
Figure 21 shows representative tumours from each of the treatment cohorts.
Differences between treatment cohorts were determined using Student's t test.
Figure
20 is a summary of the data for all treatment cohorts. The results show that
compound (2) significantly delays the growth of established FaDu xenografts.
Longitudinal MRI provides a noninvasive means of monitoring prostate tumour
growth
in mice (Gupta S, Hastak K, Ahmad N, Lewin J S, Mukhtar H Proc Nat! Aced Sci
USA
2001 Aug 28;98(18):10350-5; Eng M H, Charles L G, Ross B D, Chrisp C E, Pienta
K
J, Greenberg N M, Hsu C X, Sande M G Urology 1999 Dec:54(6):1112-9; Song S K,
Qu Z, Garabedian E M, Gordon J I, Milbrandt J, Ackerman J J Cancer Res. 2002
Mar
1:62(5):1555-8.).
MRI is used in the present case to evaluate prostate tumour growth and
progression
longitudinally in TRAMP mice (either untreated or treated with a compound that
may
be used according the methods of the invention). Mice were imaged
approximately
monthly from 12-33 weeks of age. Representative MRI images comparing untreated
control TRAMP and treated TRAMP mice at approximately 30 weeks of age are
shown in Figure 22.
Panels A and B show results from control mice. Panel A shows a coronal section
through of a 30 week old TRAMP mouse with a large tumour (bright tissue) that
weighed 8.76 g upon dissection at 34 weeks of age. The inset shows a more
posterior coronal section. The bright tumour is smaller in this section but
metastasis
to the liver is observed (white arrow). Panel B shows a coronal section
through the
prostate region of a 30 week old TRAMP mouse. The seminal vesicles (SV) are
enlarged. A large tumour (weighing 4.89 g upon dissection at 36 weeks of age)
that
spanned from the kidney to bladder (BL) is visible in the transverse section
shown in
the inset (white arrow).
Panels E and F show results for mice treated with 1 mM compound (1). Panel E
shows a coronal section through the prostate region of a 30 week old treated
TRAMP
mouse. The tumour, weighing 0.41 g upon dissection at 34 weeks of age, was not
observed during the imaging session. Panel F shows a similar section through a
30
= =
CA 02537669 2006-02-24
week old treated TRAMP mouse that exhibited a 0.64 g tumour upon dissection at
39
weeks of age. The tumour is indicated by the white arrow in the MRI image
shown in
this panel.
Untreated TRAMP mice therefore demonstrate primary prostate tumour growth.
However, prostate cancer progression in the TRAMP mouse is inhibited in mice
treated with compound (1), either alone or in combination with MTA.
Figure 23 shows that compound (2) and MTA, administered together, alter
polyamine
levels and induce cytostasis in PC3 cells. Combination treatment of PC3 cells
with
compound (2) and MTA for 1 day resulted in a significant 6-fold increase in
intracellular PUT levels (3.03 x 104 t 2.86 x 104, combination treated cells
vs. 5.04 X
104 1.08 x 104, control, p= 0.001, pmoles PUT/mg protein), a 2-fold increase
in
spent media PUT levels [1.19 x 104 t 2.04 x 101, combination treated media vs.
5.85
x 104 t 5.09 x 104', control media, p= 0.0001, pmoles PUT/mL spent media, as
well
as roughly a 2.5-fold increase in intracellular SPD levels (7.19 x 104 t 4.38
x 104,
combination treated cells vs. 3.05 x 103 6.3 x 104, control, p=0.001 pmoles
SPD/mg protein). SPN levels in combination treated spent media also slightly
decreased (p=0.02). After 6 days of treatment, cellular SPN levels were
decreased
roughly 0.5-fold (4.0 x iO3 7.38 x 104, combination treated cells vs. 6.87 x
104
9.68 x 104, control, p= 0.005, pmoles SPN/mg protein), with both PUT and SPD
elevated (p= 0.02 and p= 0.01, respectively in comparison to controls). Most
significantly, levels of PUT in spent media were almost double that of the
control (2.41
x 104 t 7.35 x 10'1, combination treated spent media vs. 1.31 X 10-3 0.0,
control,
p=0.0007, pmoles PUT/mL spent media). By day 12, a significant increase in
cellular
SPD levels were observed (9.05 x 10 1.09 x 10'3, combination treated cells
vs.
3.93 x 104 t 8.4 x 10'1, control, p=0.007, pmoles SPD/mg protein), with a
corresponding decrease in levels of spent media PUT levels (1.65 x 10-3 t 227
x 10-
2, combination treated spent media vs. 2.12 x 104 9.34 x 10.1, control
media,
pmoles PUT/mL spent media, p=0.013). Intracellular PUT levels in combination
treated cells were still significantly higher than controls (p=0.005).
Treatment of PC3 cells with compound (2) therefore results in numerous
significant
alterations in both intracellular and spent media polyamine levels. After 24
hours of
treatment, the increase observed in cellular SPD levels as well as putrescine
(PUT)
cellular and spent media polyamine levels correlates with the effects expected
with
CA 02537669 2006-02-24
26
MTAP inhibition. MTA is accumulating in the cells, beginning to feedback
inhibiting
SPN synthase, resulting in accumulations of SPD and PUT, with PUT being
significantly excreted into the media, and a slight decrease of SPN in the
media. By
day 6, cellular SPN levels are significantly reduced in combination treated
cells, while
maintaining the characteristic elevations in levels of PUT and SPD. Treatment
of
cells for 12 days shows a significant increase in cellular SPD levels and a
slight
decrease in spent media PUT levels, pointing to the fact that a compensatory
pathway has been activated to make up for the block in MTAP. PUT may be being
taken up from the media for SPD synthesis. After combination treatment for
approximately 2 weeks, PC3 cells display a cytostatic effect, as determined by
the
clonogenic assay. Initially, it was believed that MTAP inhibition would lead
to MTA
accumulation, causing feedback inhibition of polyamine biosynthesis, resulting
in
decreases in cellular proliferation. A halt in cellular proliferation is
observed, and it is
now believed that this is not due only to polyamine depletion.
Figure 24 shows that compound (2) reduces tumour growth and metastasis in
TRAMP
mice, but does not alter polyamine levels in vivo. Polyamine levels of mice
livers are
not significantly altered during short-term treatment (Figure 24A). After
extended
treatment with compound (2) inhibitor solutions, no significant alterations in
either
TRAMP liver or GUS polyamine levels were detected (Figures 24B and 24C).
Mass (Table 1) and dimensions of excised genitourinary system tumors were
recorded for all members of the treatment groups. Sections of small intestine
were
also removed for toxicity analysis via H&E staining. Histology of TRAMP mice
revealed all animals showed extensive prostate intraepithelial neoplasia
involving
most prostate acini. However, the size and incidence of preinvasive tumors, as
well
as the incidence of invasive cancer and metastasis were all decreased in
treated
TRAMP mice (Table 1). No alterations, inflammations, or irregularities were
observed
in the intestinal crypts, neither were any hair loss or general GI problems
noted,
indicating a lack of drug toxicity.
CA 02537669 2013-06-04
27
Table 1: Summary of results for TRAMP mice treated with compound (2)
Weeks Metastatic
Tumor
Experimental Animals Size
treated Cancer
Condition (n) (g)
Control 16 4.0 2.8 32 5 44%
100 tiM compound (2) 12 1.7 0.8 29 7 8%
Although the invention has been described by way of example, it should be
appreciated the variations or modifications may be made without departing from
the
scope of the invention.