Note: Descriptions are shown in the official language in which they were submitted.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-1-
Substituted Triazole Derivatives as Oxytocin Antagonists
The present invention relates to a class of substituted 1,2,4-triazoles with
activity
as oxytocin antagonists, uses thereof, processes for the preparation thereof
and
compositions containing said inhibitors. These inhibitors have utility in a
variety
of therapeutic areas including sexual dysfunction, particularly premature
ejaculation (P.E.).
Eur. J. Med. Chem. 1985, 20(3), pp257-266, refers to derivatives of
1,2,4-triazoles having analgesic and antiinflammatory properties.
WO 03/053437 refers to 1,2,4-triazoles having activity as oxytocin
antagonists.
EP 1,293,503 refers to derivatives of 1,2,4-triazoles having glycine
transporter
inhibiting properties.
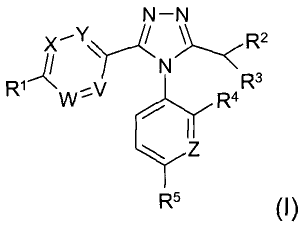
The first aspect of the present invention provides for compounds of formula
(I)
N-N
x,Y / \~ 'R2
/ ~N/~
R1w,V R4R3
Z
R5 (I)
wherein
V, W, X and Y, which may be the same or different, represent C-R6 or N;
ZisC-HorN;
R' is selected from:
(i) a phenyl ring substituted with two or more substituents, which may be
the same or different, each independently selected from halo,
(C1-C6)alkyl, (C1-C6)alkoxy, cyano, C(O)NR'R8, NR'R8, NR'C(O)R10 and
N[C(O)R10]2; and
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-2-
(ii) a five to seven membered aromatic heterocyclic ring containing 1-3
hetero atoms selected from N, 0 and S and N-oxides thereof; said ring
being optionally substituted with two or more substituents, which may be
the same or different, selected from halo, (C1-C6)alkyl, (C1-C6)alkoxy,
cyano, C(O)NR'Ra, NR'R8, NR'C(O)R1 and N[C(O)R10]2;
R2 is selected from:
(i) H, OH, OR9, NR7R8, NR'C(O)R10 and N[C(O)R'0]2;
(ii) a 5-7 membered N-linked heterocycle containing 1-3 heteroatoms
selected from N,O and S; said ring being optionally substituted with one or
more groups selected from (C1-C6)alkyl, (C1-C6)alkoxy and C(O)NR'R8;
and
(iii) (Ci-C6)alkyl optionally substituted with an N-linked 5-7 membered
heterocycle containing 1-3 heteroatoms selected from N, 0 and S;
R3 is selected from H and (Ci-C6)alkyl;
R4 is selected from H, (C1-C6)alkyl and OR9;
R5 is selected from halo, (C1-C6)alkyl, (C1-C6)alkoxy, NR'R8, NR'C(O)R10 and
N[C(O)R10]2i
R6 is selected from H, halo, (C1-C6)alkyl, (Cl-C6)alkoxy, cyano, NR~RB,
NR'C(O)R10, N[C(O)R10]2 and C(O)NR'R8;
R7 and R8, which may be the same or different, are selected from H and
(C1-Cg)alkyl;
CA 02539297 2006-03-16
Priitted 31-03-20061 FAx
i DESCPAIVID , EPOnTaxr.nrm!PCT/IB04%029 77
003 06.06:2005-_17e2-10'-c49.'
PC26064A PCT REPLACEMENT PAGE
-3-
Ra is (C,-Ca)aikyl, which is optionally substituted with with one or more
groups
each independentiy selected from (C,-Cs)aikoxy and an N-linked 5-7 membered
heterocycle containing 1-3 heteroatoms selected from N, 0 and S; and
R10 Is selected from (Cj-Cg)alkyl and (CI-C6)alkoxy,
a tautomer thereof or a pharmaceutically acaeptable salt, solvate or polymorph
of
said compound or tautomer;
with the proviso that the compound of formula (i) is not
3-ethyi-5-(4-imidazoi-l-ylphenyl)-4-(4-methoxyphenyl)-4H-[1,2,4]triazole.
Unless otherwise indicated, alkyl and alkoxy groups may be straight or
branched
and contain 1 to 6 carbon atoms and preferably 1 to 4 carbon atoms. Examples
- 15 of alkyl include methyl, ethyl, n-propyi, isopropyl, n-butyl, isobutyl,
sec-butyl,
pentyl and hexyl. Examples of alkoxy Include methoxy, ethoxy, isopropoxy and
n-butoxy.
Halo means fluoro, chloro, bromo or ioqo and Is preferably fluoro,
A heterocycle may be saturated, parqal(y saturated or aromatic_ Examples of
heterocyclic groups are thiolanyl, pyrroiidinyi, pyrrolinyl, imidazolidinyl,
imidazolinyl, suifolanyl, dioxolanyl, dihydropyranyi, tetrahydropyranyl,
piperidinyl,
pyrazolinyl, pyrazoiidinyl, dioxanyl, morpholinyl, dithianyl, thiomorpholinyl,
piperazinyl, azepinyl, oxazepinyl, thiazepinyl, thiazolinyl and diazapanyl.
Examples of aromatio heterocyclic groups are furyl, thienyl, pyrrolyl,
oxazolyl,
thiazolyl, imidazolyl, pyrazolyi, isoxazolyi, isothiazolyl, oxadiazolyl,
triazolyl,
thiadiazolyi, pyridyl, pyrimidinyi, pyrazinyl, pyridazinyl, triazinyi.
;~ ~ AMENDED SHEET ,06-0E`r~(lEl5i
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-4-
" ,
Uniess` otherwise indicated, the term substituted means substituted by one or
more defined groups. In the case where groups may be selected from a number
of alternative groups, the selected groups may be the same or different.
Preferred aspects of the invention are defined below.
In a preferred aspect, the present invention comprises compounds of formula
(I)
wherein
1 or 2 of the groups V, W, X and Y represent N when the remainder represent
C-R6;
Z is C-H or N;
R' is selected from:
(i) a phenyl ring substituted with two or more substituents, which may be
the - same or different, each independently selected from halo,
(C1-C6)alkyl, (C1-Cs)alkoxy, cyano, C(O)NR'R8, NR'R8, NR'C(O)R10 and
N[C(O)R10]2; and
(ii) a five to seven membered aromatic heterocyclic ring containing 1-3
hetero atoms selected from N, 0 and S and N-oxides thereof; said ring
being optionally substituted with two or more substituents, which may be
the same or different, selected from halo, (C1-C6)alkyl, (C1-C6)alkoxy,
cyano, C(O)NR7R8, NR'R8, NR'C(O)R10 and N[C(O)R'0]2;
Preferably, R2 is selected from:
(i) H, (Ci-C6)alkoxy, (C1-C6)aikoxy-(C1-C6)alkoxy, NR'R8, NR'C(O)R10 and
N[C(O)R10]2; and
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-5-
(ii) a 5-7 membered N-linked heterocycle containing 1-3 heteroatoms
selected from N,O and S; said ring optionally substituted with one or more
groups selected from (C1-C6)alkyl, (C1-C6)alkoxy and C(O)NR'R8;
R3 is selected from H and (C1-C6)alkyl;
R4 is selected from H, (C1-C6)alkyl and OR9;
R5 is (Cl-C3)alkyl, (C1-C3)alkoxy or NR'R8;
R6 is H, halo, (C1-C6)alkyl, (C1-C6)alkoxy, cyano or NR'R8;
R' and R8, which may be the same or different, are selected from H and
(C1-C6)alkyl;
R9 IS (C1-Cs)alkyl optionally substituted with (C1-C6)alkoxy; and
R10 is selected from (Ci-C6)aikyl and (C1-C6)alkoxy;
a tautomer thereof or a pharmaceutically acceptable salt, solvate or polymorph
of said compound or tautomer.
In another preferred aspect, the present invention comprises compounds of
formula (I) wherein
1 or 2 of the groups V, W and Y represent N when the remainder represent C-R6
and X is CH;
Z is C-H or N;
R' is selected from:
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-6-
(i) a phenyl ring substituted with two substituents, which may be the same
or different, each independently selected from halo, (C1-C3)alkyl,
(C1-C3)alkoxy, and cyano; and
(ii) a pyridyl ring or N-oxide thereof each substituted with two substituents,
which may be the same or different, each independently selected from
halo, (C1-C3)alkyl, (C1-C3)alkoxy, and cyano;
R2 is selected from:
(i) H, (Ci-C3)alkoxy, (C1-C3)alkoxy-(C1-C3)alkoxy and N((C1-C3)alkyl)2; and
(ii) a 5 membered N-linked heterocycle containing 1-3 nitrogen atoms,
said ring optionally substituted with C(O)NR7R8;
R3 is selected from H and (C1-C6)alkyl;
R4 is selected from H, (Cl-C6)alkyl and OR9;
R5 is (C1-C3)alkyl, (C1-C3)alkoxy or NR'Ra;
R6 is H, (C1-C6)alkyl, (Ci-C6)alkoxy or NR'R8;
R' and R8, which may be the same or different, are selected from H and
(C1-C6)alkyl; and
R9 is (C1-C6)alkyl optionally substituted with methoxy;
a tautomer thereof or a pharmaceutically acceptable salt, solvate or polymorph
of said compound or tautomer.
In another preferred aspect, the present invention comprises compounds of
formula (I) wherein
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-7-
W and Y are each independently CH or N and X and V are each CH;
Z is N;
R' is a phenyl ring substituted with two substituents, which may be the same
or
different, each independently selected from fluoro, chloro, methyl, methoxy,
and
cyano;
R2 is selected from H, methoxy, ethoxy, 2-methoxyethoxy, dimethylamino,
1,2,3-triazol-2-yl and pyrollidinyl, the latter being optionally substituted
by
CONH2;
R3 is selected from H and (C1-C6)al{cyl;
R4 is H; and
R5 is methoxy;
a tautomer thereof or a pharmaceutically acceptable salt, solvate or polymorph
of said compound or tautomer.
Preferred embodiments of the compounds of formula (I) according to the above
aspects are those that incorporate two or more of the following preferences.
Preferably, 1 or 2 of the groups V, W, X and Y represent N when the remainder
represent C-R6.
In a preferred emodiment, X is CH.
In a preferred emodiment, 1 or 2 of the groups V, W and Y represent N when the
remainder represent C-R6 and X is CH;
Preferably, Y is N or CR6.
More preferably, V, W and Y are each independently CH, C-OCH3 or N.
Most preferably, W and Y are each independently CH or N.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-8-
In the most preferred embodiment, W and Y are each independently CH or N
and X and V are each CH.
In a preferred emodiment, Z is N.
In another preferred emodiment, Z is CH.
Preferably, R' is selected from:
(i) a phenyl ring substituted with two or more substituents, which may be
the same or different, each independently selected from halo,
(C1-C6)alkyl, (C1-C6)alkoxy, cyano, C(O)NR'R8, NR'R8, NR'C(O)R10 and
N[C(O)R10]2a and
(ii) a five to seven membered aromatic heterocyclic ring containing 1-3
hetero atoms selected from N, 0 and S and N-oxides thereof; said ring
being optionally substituted with two or more substituents, which may be
the same or different, selected from halo, (C1-C6)alkyl, (C1-C6)alkoxy,
cyano, C(O)NR'R8, NR'R8, NR'C(O)R10 and N[C(O)R'0]2;
More preferably, R' is selected from:
(i) a phenyl ring substituted with two substituents, which may be the same
or different, each independently selected from halo, (C1-C6)aikyl,
(Ci-C6)alkoxy, cyano, C(O)NR'R8, NR'R8, NR'C(O)R10 and N[C(O)R10]2;
. 25 and
(ii) a pyridyl ring or N-oxide thereof each substituted with two substituents,
which may be the same or different, each independently selected from
halo, (C1-C6)alkyl, (Ci-C6)alkoxy, cyano, C(O)NR'R8, NR'R8, NR'C(O)R10
and N[C(O)R10]2.
Yet more preferably, Ri is selected from:
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-9-
(i) a phenyl ring substituted with two substituents, which may be the same
or different, each independently selected from halo, (C1-C3)alkyl,
(C1-Cs)alkoxy and cyano; and
(ii) a pyridyl ring or N-oxide thereof each substituted with two substituents,
which may be the same or different, each independently selected from
halo, (Ci-C3)alkyl, (C1-C3)alkoxy and cyano.
In a preferred embodiment, R' is a phenyl ring substituted with two
substituents,
which may be the same or different, each independently selected from fluoro,
chloro, methyl, methoxy and cyano.
In another preferred embodiment, R' is pyridine-N-oxide substituted with two
methyl groups.
Preferably, R2 is selected from:
(i) H, (C1-C6)alkoxy, (C1-C6)alkoxy-(C1-C6)alkoxy, NR7 R8, NR'C(O)R10 and
N[C(O)R10]2; and
(ii) a 5-7 membered N-linked heterocycle containing 1-3 heteroatoms
selected from N,O and S; said ring optionally substituted with one or more
groups selected from (C1-C6)alkyl, (C1-C6)alkoxy and C(O)NR7R8.
More preferably, R2 is selected from:
(i) H, (C1-C3)alkoxy, (C1-C3)alkoxy-(C1-C3)alkoxy and N((C1-C3)alkyl)2i and
(ii) a 5 membered N-linked heterocycle containing 1-3 nitrogen atoms,
8
said ring optionally substituted with C(O)NR'R.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-10-
Yet more preferably, R2 is selected from, H, methoxy, ethoxy, 2-methoxyethoxy,
dimethylamino, 1,2,3-triazol-2-yl and pyrollidinyl, the latter being
optionally
substituted by CONH2.
Most preferably, R2 is selected from H and methoxy.
Preferably, R3 is H or (C1-C3)alkyl.
Most preferably, R3 is H.
Preferably, R4 is H, (C1-C3)alkyl or OR9.
More preferably, R4 is H, (Ci-C3)alkyl or (C1-C3)alkoxy.
Most preferably, R4 is H, methyl or methoxy.
In a preferred embodiment, R4 is H.
Preferably, R5 is (C1-C3)alkyl, (C1-C3)aikoxy or NR'R8.
More preferably, R5 is (Ci-C3)alkoxy or NR7R8.
Most preferably, R5 is methoxy or NHCH3.
In a preferred embodiment, R5 is methoxy.
Preferably,- R6 is H, halo, (C1-C6)alkyl, (C1-C6)alkoxy, cyano or NR'R8.
More preferably, R6 is H, (C1-C6)alkyl, (C1-C6)alkoxy or NR7R8.
Yet more preferably, R6 is H, (C1-C3)alkyl or (CI-C3)alkoxy.
Most preferably, R6 is H, methyl or methoxy.
In a preferred embodiment, R6 is H or methyl.
In a most preferred embodiment, R6 is H.
Preferably, R' is H or (C1-C3)alkyl.
Most preferably, R' is H or methyl.
Preferably, R 8 is H or (C1-C3)alkyl.
Most preferably, R 8 is H or methyl.
Preferably, R9 is (C1-C6)alkyl optionally substituted with (C1-C6)alkoxy.
More preferabiy, R9 is (C1-Cg)alkyl optionally substituted with methoxy.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-11- Most preferably, R9 is methyl.
Preferred compounds of formula (I) are:
2-(4-fluoro-2-methylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
2-(2,3-dimethylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
5-(4-fluoro-2-methylphenyl)-2-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
5-(2,3-dimethylphenyl)-2-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
1-[5-[5-(2,3-dimethylphenyl)-pyridin-2-yl]-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-ylmethyl]-pyrrolidine-(2S)-2-carboxylic acid amide;
5-(2,3-dimethylphenyl)-2-(5-pyrrolidin-1-ylmethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
2-(4-fluoro-2-methylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-Pyrazine;
2-(2,3-dimethylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyrazine; "
2-(4-fluoro-2-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(2,3-dimethylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yI]-pyrazine;
2-(4-cyano-2-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(5-fluoro-2-methoxyphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(4-cyano-2-methylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyrazine;
- 5-(4-cyano-2-methylphenyl)-2-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yi]-pyridine;
2-(5-fluoro-2-methoxyphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyridine;
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-12-
2-(2-fluoro-5-methoxyphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(2-fluoro-5-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(2,5-difluorophenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yI]-pyrazine;
2-(3,5-dimethylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yI]-pyrazine;
2-(2,5-dimethylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yi]-pyrazine;
2-(2,5-dichlorophenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yI]-pyrazine;
2-(2-fluoro-5-methoxyphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4
H-[1,2,4]triazol-3-yl]-pyrazine;
2-(3,5-difluoro-phenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-
yi]-pyrazine;
2-(2,3-dimethylphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-methoxypyridin-3-
yl)-
4H-1,2,4-triazol-3-yl]pyridine;
2-(5-chloro-2-methoxyphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-
methoxypyridin-3-yl)-4H-1,2,4-triazol-3-yl]pyridine;
2-(4-fluoro-2-methoxyphenyl)-5-[5-[(2-methoxyethoxy) methyl]-4-(6-
methoxypyridin-3-yl)-4H-1,2,4-triazol-3-yl]pyridine;
2-(5-fluoro-2-methoxyphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-
methoxypyridin-3-yl)-4H-1,2,4-triazol-3-yl]pyridine;
2-(5-fluoro-2-methylphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-methoxypyridin-
3-yI)-4H-1,2,4-triazol-3-yl]pyridine;
2-methoxy-5-{3-[(2-methoxyethoxy) methyl]-5-[6-(2-methoxy-5-
methylphenyl)pyridin-3-yi]-4H-1,2,4-triazol-4-yl}pyridine;
2-(2-fluoro-3-methoxyphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-
methoxypyridin-3-yl)-4H-1,2,4-triazol-3-yl]pyridine;
2-(3,5-difluorophenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-methoxypyridin-3-
yl)-
4H-1,2,4-triazol-3-yl]pyridine;
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-13-
2-(2,5-dimethoxyphenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-methoxypyridin-3-
yl)-4H-1,2,4-triazol-3-yl]pyridine;
2-(3-chloro-4-fIuorophenyl)-5-[5-[(2-methoxyethoxy)methyl]-4-(6-methoxypyridin-
3-yl)-4H-1,2,4-triazol-3-yl]pyridine; and
2-(3-fluoro-2-methoxyphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-
yI)-4H-[1,2,4]triazol-3-yl]-pyrazine;
2-(3-fluoro-2-methoxy-phenyl)-5-[4-(6-methoxy-pyridi n-3-yl)-5-methyl-4-H-
[1,2,4]triazol-3-yl]-pyrazine;
and tautomers thereof and pharmaceutically acceptable salts, solvates and
polymorphs of said compound or tautomer.
Most preferred compounds of formula (I) are:
2-(4-fluoro-2-methylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
2-(2,3-dimethylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
5-(4-fluoro-2-methylphenyl)-2-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
5-(2,3-dimethylphenyl)-2-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yi)-pyridine;
1-[5-[5-(2,3-dimethylphenyl)-pyridin-2-yl]-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-ylmethyl]-pyrrolidine-(2S)-2-carboxylic acid amide;
5-(2,3-dimethylphenyl)-2-(5-pyrrolidin-1-ylmethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl)-pyridine;
2-(4-fluoro-2-methylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(2,3-dimethylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(4-fluoro-2-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(2,3-dimethytphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-[1,2,4]triazol-
3-
yl]-pyrazine;
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-14-
2-(4-cyano-2-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(5-fluoro-2-methoxyphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,4]triazol-3-yl]-pyrazine;
2-(4-cyano-2-methylphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyrazine;
5-(4-cyano-2-methylphenyl)-2-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyridine;
2-(5-fluoro-2-methoxyphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4]triazol-3-yl]-pyridine; and
2-(3-fl uoro-2-methoxyphenyl)-5-[5-methoxymethyl-4-(6-methoxypyri d i n-3-
yl)-4H-[1,2,4]triazol-3-yl]-pyrazi ne;
2-(3-fl uoro-2-methoxy-phenyl)-5-[4-(6-methoxy-pyridin-3-yl)-5-methyl-4-H-
[1,2,4]triazoi-3-yI]-pyrazine;
and tautomers thereof and pharmaceutically acceptable salts, solvates and
polymorphs of said compound or tautomer.
Pharmaceutically acceptable salts of the compounds of formula (I) comprise the
acid addition salts thereof.
Suitable acid addition salts are formed from acids which form non-toxic salts.
Examples include the acetate, aspartate, benzoate, besylate,
bicarbonate/carbonate, bisulphate/sulphate, borate, camsylate, citrate,
edisylate,
esylate, formate, fumarate, gluceptate, gluconate, glucuronate,
hexafluorophosphate, hibenzate, hydrochloride/chloride, hydrobromide/bromide,
hydroiodide/iodide, isethionate, lactate, malate, maleate, malonate, mesylate,
methylsulphate, naphthylate, 2-napsylate, nicotinate, nitrate, orotate,
oxalate,
palmitate, pamoate, phosphate/hydrogen phosphate/dihydrogen phosphate,
saccharate, stearate, succinate, tartrate, tosylate and trifluoroacetate
salts.
Hemisalts of acids may also be formed, for example, hemisulphate salts.
For a review on suitable salts, see Handbook of Pharmaceutical Salts:
Properties, Selection, and Use by Stahl and Wermuth (Wiley-VCH, Weinheim,
Germany, 2002).
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-15-
Pharmaceutically acceptable salts of compounds of formula (I) may be prepared
by one or more of three methods:
(i) by reacting the compound of formula (I) with the desired acid;
(ii) by removing an acid-labile protecting group from a suitable precursor of
the compound of formula (I) using the desired acid; or
(iii) by converting one salt of the compound of formula (I) to another by
reaction with an appropriate acid or by means of a suitable ion exchange
column.
All three reactions are typically carried out in solution. The resulting salt
may
precipitate out and be collected by filtration or may be recovered by
evaporation
of the solvent. The degree of ionisation in the resulting salt may vary from
completely ionised to almost non-ionised.
The compounds of the invention may exist in both unsolvated and solvated
forms. The term `solvate' is used herein to describe a molecular complex
comprising- the compound of the invention and a stoichiometric or non-
stoichiometric amount of one or more pharmaceutically acceptable solvent
molecules, for example, ethanol. The term `hydrate' is employed when said
solvent is water.
Included within the scope of the invention are complexes such as clathrates,
drug-host inclusion complexes wherein the drug and host are present in
stoichiometric or non-stoichiometric amounts. Also included are complexes of
the drug containing two or more organic and/or inorganic components which may
be in stoichiometric or non-stoichiometric amounts. The resulting complexes
may be ionised, partially ionised, or non-ionised. For a review of such
complexes, see J Pharm Sci, 64 (8), 1269-1288, by Haleblian (August 1975).
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-16- '
Hereinafter all references to compounds of formula (I) include references to
salts, solvates and complexes thereof and to solvates and complexes of salts
thereof.
The compounds of the invention include compounds of formula (I) as
hereinbefore defined, including all polymorphs and crystal habits thereof,
prodrugs and isomers thereof (including optical, geometric and tautomeric
isomers) as hereinafter defined and isotopically-labeled compounds of formula
(I)
As indicated, so-called `pro-drugs' of the compounds of formula (I) are also
within the scope of the invention. Thus certain derivatives of compounds of
formula (I) which may have little or no pharmacological activity themselves
can,
when administered into or onto the body, be converted into compounds of
formula (I) having the desired activity, for example, by hydrolytic cleavage.
Such
derivatives are referred to as `prodrugs'. Further information on the use of
prodrugs may be found in Pro-drugs as Novel Delivery Systems, Vol. 14, ACS
Symposium Series (T. Higuchi and W. Stella) and Bioreversible Carriers in Drug
Design, Pergamon Press, 1987 (ed. E. B. Roche, American Pharmaceutical
Association).
Prodrugs in accordance with the invention can, for example, be produced by
replacing appropriate functionalities present in the compounds of formula (I)
with
certain moieties known to those skilled in the art as `pro-moieties' as
described,
for example, in Design of Prodrugs by H. Bundgaard (Elsevier, 1985).
Some examples of prodrugs in accordance with the invention include
(i) where the compound of formula (I) contains an alcohol functionality (-OH),
an ether thereof, for example, a compound wherein the hydrogen of the
alcohol functionality of the compound of formula (I) is replaced by
(C1-C6)alkanoyloxymethyl; and
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-17-
(ii) where the compound of formula (I) contains a primary or secondary
amino functionality (-NH2 or -NHR where R# H), an amide thereof, for
example, a compound wherein, as the case may be, one or both
hydrogens of the amino functionality of the compound of formula (I) is/are
replaced by (Ci-C1o)alkanoyl.
Further examples of replacement groups in accordance with the foregoing
examples and examples of other prodrug types may be found in the
aforementioned references.
Moreover, certain compounds of formula (I) may themselves act as prodrugs of
other compounds of formula (I).
Also included within the scope of the invention are metabolites of compounds
of
formula (I), that is, compounds formed in vivo upon administration of the
drug.
Some examples of metabolites in accordance with the invention include
(i) where the compound of formula (I) contains a methyl group, an
hydroxymethyl derivative thereof (-CH3 -> -CH2OH): -
(ii) where the compound of formula (I) contains an alkoxy group, an hydroxy
derivative thereof (-OR -> -OH);
(iii) where the compound of formula (I) contains a tertiary amino group, a
secondary amino derivative thereof (-NR1R2 -> -NHR1 or -NHR2);
(iv) where the compound of formula (I) contains a secondary amino group, a
primary derivative thereof (-NHR1-> -NH2);
(v) where the compound of formula (I) contains a phenyl moiety, a phenol
derivative thereof (-Ph -> -PhOH); and
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-18-
(vi) where the compound of formula (I) contains an amide group, a carboxylic
acid derivative thereof (-CONH2 -> COOH).
Compounds of formula (I) containing one or more asymmetric carbon atoms can
exist as two or more stereoisomers. Where a compound of formula (I) contains
an alkenyl or alkenylene group, geometric cis/trans (or Z/E) isomers are
possible. Where structural isomers are interconvertible via a low energy
barrier,
tautomeric isomerism ('tautomerism') can occur. This can take the form of
proton
tautomerism in compounds of formula (I) containing, for example, an imino,
keto,
or oxime group, or so-called valence tautomerism in compounds which contain
an aromatic moiety. It follows that a single compound may exhibit more than
one
type of isomerism.
Included within the scope of the present invention are all stereoisomers,
geometric isomers and tautomeric forms of the compounds of formula (I),
including compounds exhibiting more than one type of isomerism, and mixtures
of one or more thereof. Also included are acid addition salts wherein the
counterion is optically active, for example, d-lactate, or racemic, for
example, di-
tartrate.
Cisltrans isomers may be separated by conventional techniques well known to
those skilled in the art, for example, chromatography and fractional
crystallisation.
25. Conventional techniques for the preparation/isolation of individual
enantiomers
include chiral synthesis from a suitable optically pure precursor or
resolution of
the racemate (or the racemate of a salt or derivative) using, for example,
chiral
high pressure liquid chromatography (HPLC).
Alternatively, the racemate (or a racemic precursor) may be reacted with a
suitable optically active compound, for example, an alcohol, or, in the case
where the compound of formula (I) contains an acidic or basic moiety, a base
or
acid such as 1-phenylethylamine or tartaric acid. The resulting diastereomeric
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-19-
mixture may be separated by chromatography and/or fractional crystallization
and orie or both of the diastereoisomers converted to the corresponding pure
enantiomer(s) by means well known to a skilled person.
Chiral compounds of the invention (and chiral precursors thereof) may be
obtained in enantiomerically-enriched form using chromatography, typically
HPLC, on an asymmetric resin with a mobile phase consisting of a hydrocarbon,
typically heptane or hexane, containing from 0 to 50% by volume of
isopropanol,
typically from 2% to 20%, and from 0 to 5% by volume of an alkylamine,
typically
0.1% diethylamine. Concentration of the eluate affords the enriched mixture.
Stereoisomeric conglomerates may be separated by conventional techniques
known to those skilled in the art - see, for example, Stereochemistry of
Organic
Compounds by E. L. Eliel and S. H. Wilen (Wiley, New York, 1994).
The present invention includes all pharmaceutically acceptable isotopically-
labelled compounds of formula (I) wherein one or more atoms are replaced by
atoms having the same atomic number, but an atomic mass or mass number
different from the atomic mass or mass number which predominates in nature.
Examples of isotopes suitable for inclusion in the compounds of the invention
include isotopes of hydrogen, such as 2H and 3H, carbon, such as 11C, 13C and
14C, chlorine, such as 36CI, fluorine, such as 18F, iodine, such as 1231 and
1251,
nitrogen, such as 13N and 15N, oxygen, such as 150, 17 0 and 180, phosphorus,
such as 32P, and sulphur, such as 35S.
Certain isotopically-labelled compounds of formula (I), for example, those
incorporating a radioactive isotope, are useful in drug and/or substrate
tissue
distribution studies. The radioactive isotopes tritium, i.e. 3H, and carbon-
14, i.e.
14C, are particularly useful for this purpose in view of their ease of
incorporation
and ready means of detection.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-20-=
Substitution with heavier isotopes such as deuterium, i.e. 2H, may afford
certain
therapeutic advantages resulting from greater metabolic stability, for
example,
increased in vivo half-life or reduced dosage requirements, and hence may be
preferred in some circumstances.
Substitution with positron emitting isotopes, such as "C,'$F,150 and13N, can
be
useful in Positron Emission Topography (PET) studies for examining substrate
receptor occupancy.
Isotopically-labeled compounds of formula (I) can generally be prepared by
conventional techniques known to those skilled in the art or by processes
analogous to those described in the accompanying Examples and Preparations
using an appropriate isotopically-labeled reagent in place of the non-labeled
reagent previously employed.
Pharmaceutically acceptable solvates in accordance with the invention include
those wherein the solvent of crystallization may be isotopically substituted,
e.g.
D20, d6-acetone, d6-DMSO.
Also within the scope of the invention are intermediate compounds as
hereinafter defined, all salts, solvates and complexes thereof and all
solvates
and complexes of salts thereof as defined hereinbefore for compounds of
formula (I). The invention includes all polymorphs of the aforementioned
species
and crystal habits thereof.
When preparing compounds of formula (I) in accordance with the invention, it
is
open to a person skilled in the art to routinely select the form of
intermediate
which provides the best combination of features for this purpose. Such
features
include the melting point, solubility, processability and yield of the
intermediate
form and the resulting ease with which the product may be purified on
isolation.
Drug Product
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-21-
Compounds of the invention intended for pharmaceutical use may be
administered as crystalline or amorphous products. They may be obtained, for
example, as solid plugs, powders, or films by methods such as precipitation,
crystallization, freeze drying, spray drying, or evaporative drying. Microwave
or
radio frequency drying may be used for this purpose.
They may be administered alone or in combination with one or more other
compounds of the invention or in combination with one or more other drugs (or
as any combination thereof). Generally, they will be administered as a
formulation in association with one or more pharmaceutically acceptable
excipients. The term 'excipient' is used herein to describe any ingredient
other
than the compound(s) of the invention. The choice of excipient will to a large
extent depend on factors such as the particular mode of administration, the
effect of the excipient on solubility and stability, and the nature of the
dosage
form.
Pharmaceutical compositions suitable for the delivery of compounds of the
present invention and methods for their preparation will be readily apparent
to
those skilled in the art. Such compositions and methods for their preparation
may be found, for example, in Remington's Pharmaceutical Sciences, 19th
Edition (Mack Publishing Company, 1995).
Oral Administration
The compounds of the invention may be administered orally. Oral administration
may involve swallowing, so that the compound enters the gastrointestinal
tract,
or buccal or sublingual administration may be employed by which the compound
enters the blood stream directiy from the mouth.
Formulations suitable for oral administration include solid formulations such
as
tablets, capsules containing particulates, liquids, or powders, lozenges
(including
liquid-filled), chews, multi- and nano-particulates, gels, solid solution,
liposome,
films, ovules, sprays and liquid formulations.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-22-
Liquid formulations include suspensions, solutions, syrups and elixirs. Such
formulations may be employed as fillers in soft or hard capsules and typically
comprise a carrier, for example, water, ethanol, polyethylene glycol,
propylene
glycol, methylcellulose, or a suitable oil, and one or more emulsifying agents
and/or suspending agents. Liquid formulations may also be prepared by the
reconstitution of a solid, for example, from a sachet.
The compounds of the invention may also be used in fast-dissolving, fast-
disintegrating dosage forms such as those described in Expert Opinion in
Therapeutic Patents, 11 (6), 981-986, by Liang and Chen (2001).
For tablet dosage forms, depending on dose, the drug may make up from 1"
weight % to 80 weight % of the dosage form, more typically from 5 weight % to
60 weight % of the dosage form. In addition to the drug, tablets generally
contain
a disintegrant. Examples of disintegrants include sodium starch glycolate,
sodium carboxymethyl cellulose, calcium carboxymethyl cellulose,
croscarmellose sodium, crospovidone, polyvinylpyrrolidone, methyl cellulose,
microcrystalline cellulose, lower alkyl-substituted hydroxypropyl celluiose,
starch,
pregelatinised starch and sodium alginate. Generally, the disintegrant will
comprise from 1 weight % to 25 weight %, preferably from 5 weight % to 20
weight % of the dosage form.
Binders are generally used to impart cohesive qualities to a tablet
formulation.
Suitable binders include microcrystalline cellulose, gelatin, sugars,
polyethylene
glycol, natural and synthetic gums, polyvinylpyrrolidone, pregelatinised
starch,
hydroxypropyl cellulose and hydroxypropyl methylcellulose. Tablets may also
contain diluents, such as lactose (monohydrate, spray-dried monohydrate,
anhydrous and the like), mannitol, xylitol, dextrose, sucrose, sorbitol,
microcrystalline cellulose, starch and dibasic calcium phosphate dihydrate.
Tablets may also optionally comprise surface active agents, such as sodium
lauryl sulfate and polysorbate 80, and glidants such as silicon dioxide and
talc.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-23-
When present, surface active agents may comprise from 0.2 weight % to 5
weight % of the tablet, and glidants may comprise from 0.2 weight % to 1
weight
% of the tablet.
Tablets also generally contain lubricants such as magnesium stearate, calcium
stearate, zinc stearate, sodium stearyl fumarate, and mixtures of magnesium
stearate with sodium lauryl sulphate. Lubricants generally comprise from 0.25
weight % to 10 weight %, preferably from 0.5 weight % to 3 weight % of the
tablet.
Other possible ingredients include anti-oxidants, colourants, flavouring
agents,
preservatives and taste-masking agents.
Exemplary tablets contain up to about 80% drug, from about 10 weight % to
about 90 weight % binder, from about 0 weight % to about 85 weight % diluent,
from about 2 weight % to about 10 weight % disintegrant, and from about 0.25
weight % to about 10 weight % lubricant.
Tablet blends may be compressed directiy or by roller to form tablets. Tablet
blends or portions of blends may alternatively be wet-, dry-, or melt-
granulated,
melt congealed, or extruded before tabletting. The final formulation may
comprise one or more layers and may be coated or uncoated; it may even be
encapsulated.
The formulation of tablets is discussed in Pharmaceutical Dosage Forms:
Tablets, Vol. 1, by H. Lieberman and L. Lachman (Marcel Dekker, New York,
1980).
Consumable oral films for human or veterinary use are typically pliable water-
soluble or water-swellable thin film dosage forms which may be rapidly
dissolving
or mucoadhesive and typically comprise a compound of formula (I), a film-
forming polymer, a binder, a solvent, a humectant, a plasticiser, a stabiliser
or
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-24-
emulsifier, a viscosity-modifying agent and a solvent. Some components of the
t I I .
formulation may perform more than one function.
The compound of formula (I) may be water-soluble or insoluble. A water-soluble
compound typically comprises from 1 weight % to 80 weight %, more typically
from 20 weight % to 50 weight %, of the solutes. Less soluble compounds may
comprise a greater proportion of the composition, typically up to 88 weight %
of
the solutes. Alternatively, the compound of formula (I) may be in the form of
multiparticulate beads.
The film-forming polymer may be selected from natural polysaccharides,
proteins, or synthetic hydrocolloids and is typically present in the range
0.01 to
99 weight %, more typically in the range 30 to 80 weight %.
Other possible ingredients include anti-oxidants, colorants, flavourings and
flavour enhancers, preservatives, salivary stimulating agents, cooling agents,
co-"'
solvents (including oils), emollients, bulking agents, anti-foaming agents,
surfactants and taste-masking agents.
Films in accordance with the invention are typically prepared by evaporative
drying of thin aqueous films coated onto a peelable backing support or paper.
This may be done in a drying oven or tunnel, typically a combined coater
dryer,
or by freeze-drying or vacuuming.
Solid formulations for oral administration may be formulated to be immediate
and/or modified release. Modified release formulations include delayed-,
sustained-, pulsed-, controlled-, targeted and programmed release.
Suitable modified release formulations for the purposes of the invention are
described in US Patent No. 6,106,864. Details of other suitable release
technologies such as high energy dispersions and osmotic and coated particles
are to be found in Pharmaceutical Technology On-line, 25(2), 1-14, by Verma et
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-25-.
al (2001). The use of chewing gum to achieve controlled release is described
in
WO 00/35298.
Parenteral Administration
The compounds of the invention may also be administered directly into the
blood
stream, into muscle, or into an internal organ. Suitable means for parenteral
administration include intravenous, intraarterial, intraperitoneal,
intrathecal,
intraventricular, intraurethral, intrasternal, intracranial, intramuscular and
subcutaneous. Suitable devices for parenteral administration include needle
(including microneedle) injectors, needle-free injectors and infusion
techniques.
Parenteral formulations are typically aqueous solutions which may contain
excipients such as salts, carbohydrates and buffering agents (preferably to a
pH
of from 3 to 9), but, for some applications, they may be more suitably
formulated
as a sterile non-aqueous solution or as a dried form to be used in conjunction
with a suitable vehicle such as sterile, pyrogen-free water.
The preparation of parenteral formulations under sterile conditions, for
example,
by lyophilisation, may readily be accomplished using standard pharmaceutical
techniques well known to those skilled in the art.
The solubility of compounds of formula (I) used in the preparation of
parenteral
solutions may be increased by the use of appropriate formulation techniques,
such as the incorporation of solubility-enhancing agents.
Formulations for parenteral administration may be formulated to be immediate
and/or modified release. Modified release formulations include delayed-,
sustained-, pulsed-, controlled-, targeted and programmed release. Thus
compounds of the invention may be formulated as a solid, semi-solid, or
thixotropic liquid for administration as an implanted depot providing modified
release of the active compound. Examples of such formulations include drug-
coated stents and poly(dl-lactic-coglycolic) acid (PGLA) microspheres.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-26-
Topical Administration
The compounds of the invention may also be administered topically to the skin
or mucosa, that is, dermally or transdermally. Typical formulations for this
purpose include gels, hydrogels, lotions, solutions, creams, ointments,
dusting
powders, dressings, foams, films, skin patches, wafers, implants, sponges,
fibres, bandages and microemulsions. Liposomes may also be used. Typical
carriers include alcohol, water, mineral oil, liquid petrolatum, white
petrolatum,
glycerin, polyethylene glycol and propylene glycol. Penetration enhancers may
be incorporated - see, for example, J Pharm Sci, 88 (10), 955-958, by Finnin
and
Morgan (October 1999).
Other means of topical administration include delivery by electroporation,
iontophoresis, phonophoresis, sonophoresis and microneedle or needle-free
(e.g. PowderjectT"', SiojectT"', etc.) injection.
Formulations for topical administration may be formulated to be immediate
and/or modified release. Modified release formulations _include delayed-,
sustained-, pulsed-, controlled-, targeted and programmed release.
Inhaled/Intranasal Administration
The compounds of the invention can also be administered intranasally or by
inhalation, typically in the form of a dry powder (either alone, as a mixture,
for
example, in a dry blend with lactose, or as a mixed component particle, for
example, mixed with phospholipids, such as phosphatidylcholine) from a dry
powder inhaler or as an aerosol spray from a pressurised container, pump,
spray, atomiser (preferably an atomiser using electrohydrodynamics to produce
a fine mist), or nebuliser, with or without the use of a suitable propellant,
such as
1,1,1,2-tetrafluoroethane or 1,1,1,2,3,3,3-heptafluoropropane. For intranasal
use, the powder may comprise a bioadhesive agent, for example, chitosan or
cyclodextrin.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-27-
The pressurised container, pump, spray, atomizer, or nebuliser contains a
solution or suspension of the compound(s) of the invention comprising, for
example, ethanol, aqueous ethanol, or a suitable alternative agent for
dispersing, solubilising, or extending release of the active, a propellant(s)
as
solvent and an optional surfactant, such as sorbitan trioleate, oleic acid, or
an
oligolactic acid.
Prior to use in a dry powder or suspension formulation, the drug product is
micronised to a size suitable for delivery by inhalation (typically less than
5
microns). This may be achieved by any appropriate comminuting method, such
as spiral jet milling, fluid bed jet milling, supercritical fluid processing
to form
nanoparticles, high pressure homogenisation, or spray drying. "
Capsules (made, for example, from gelatin or hydroxypropylmethylcellulose),
blisters and cartridges for use in an inhaler or insufflator may be formulated
to
contain a powder mix of the compound of the invention, a suitable powder base
such as lactose or starch and a performance modifier such as I-leucine,
mannitol, or magnesium stearate. The lactose may be anhydrous or in the form
of the monohydrate, preferably the latter. Other suitable excipients include
dextran, glucose, maltose, sorbitol, xylitol, fructose, sucrose and trehalose.
A suitable solution formulation for use in an atomiser using
electrohydrodynamics to produce a fine mist may contain from 1 pg to 20mg of
25, the compound of the invention per actuation and the actuation volume may
vary
from 1 pl to 100 fal. A typical formulation may comprise a compound of formula
(I),
propylene glycol, sterile water, ethanol and sodium chloride. Alternative
solvents
which may be used instead of propylene glycol include glycerol and
polyethylene
glycol.
Suitable flavours, such as menthol and levomenthol, or sweeteners, such as
saccharin or saccharin sodium, may be added to those formulations of the
invention intended for inhaled/intranasal administration.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-28-
Formulations for inhaled/intranasal administration may be formulated to be
immediate and/or modified release using, for example, PGLA. Modified release
formulations include delayed-, sustained-, pulsed-, controlled-, targeted and
programmed release.
In the case of dry powder inhalers and aerosols, the dosage unit is determined
by means of a valve which delivers a metered amount. Units in accordance with
the invention are typically arranged to administer a metered dose or "puff"
containing from 2 to 30mg of the compound of formula (I). The overall daily
dose will typically be in the range 50 to 100mg which may be administered in a
single dose or, more usually, as divided doses throughout the day.
Rectal/Intravaginal Administration
The compounds of the invention may be administered rectally or vaginally, for'
example, in the form of a suppository, pessary, or enema. Cocoa butter is a
traditional suppository base, but various alternatives may be used as
appropriate.
Formulations for rectal/vaginal administration may be formulated to be
immediate and/or modified release. Modified release formulations include
delayed-, sustained-, pulsed-, controlled-, targeted and programmed release.
Ocular/Aural Administration
The compounds of the invention may also be administered directly to the eye or
ear, typically in the form of drops of a micronised suspension or solution in
isotonic, pH-adjusted, sterile saline. Other formulations suitable for ocular
and
aural administration include ointments, biodegradable (e.g. absorbable gel
sponges, collagen) and non-biodegradable (e.g. silicone) implants, wafers,
lenses and particulate or vesicular systems, such as niosomes orliposomes. A
polymer such as crossed-linked polyacrylic acid, polyvinylalcohol, hyaluronic
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-29-.
acid, a cellulosic polymer, for example, hydroxypropyimethylcellulose,
hydroxyethyicellulose, or methyl cellulose, or a heteropoiysaccharide polymer,
for
example, gelan gum, may be incorporated together with a preservative, such as
benzalkonium chloride. Such formulations may also be delivered by
iontophoresis.
Formulations for ocular/aural administration may be formulated to be immediate
and/or modified release. Modified release formulations include delayed-,
sustained-, pulsed-, controlled-, targeted, or programmed release.
Other Technologies
The compounds of the invention may be combined with soluble macromolecular
entities, such as cyclodextrin and suitable derivatives thereof or
polyethylene
glycol-containing polymers, in order to improve their solubility, dissolution
rate,
taste-masking, bioavailability and/or stability for use in any of the
aforementioned
modes of administration.
Drug-cyclodextrin complexes, for example, are found to be generally useful for
most dosage forms and administration routes. Both inclusion and non-inclusion
complexes may be used. As an alternative to direct complexation with the drug,
the cyclodextrin may be used as an auxiliary additive, i.e. as a carrier,
diluent, or
solubiliser. Most commonly used for these purposes are alpha-, beta- and
gamma-cyclodextrins, examples of which may be found in International Patent
Applications Nos. WO 91/11172, WO 94/02518 and WO 98/55148.
Kit-of-Parts
Inasmuch as it may desirable to administer a combination of active compounds,
for example, for the purpose of treating a particular disease or condition, it
is
within the scope of the present invention that two or more pharmaceutical
compositions, at least one of which contains a compound in accordance with the
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-30-
invention, may conveniently be combined in the form of a kit suitable for
coadministration of the compositions.
Thus the kit of the invention comprises two or more separate pharmaceutical
compositions, at least one of which contains a compound of formula (I) in
accordance with the invention, and means for separately retaining said
compositions, such as a container, divided bottle, or divided foil packet. An
example of such a kit is the familiar blister pack used for the packaging of
tablets, capsules and the like.
The kit of the invention is particularly suitable for administering different
dosage
forms, for example, oral and parenteral, for administering the separate
compositions at different dosage intervals, or for titrating the separate
compositions against one another. To assist compliance, the kit typically
comprises directions for administration and may be provided with a so-called
memory aid.
Dosage
For administration to human patients, the total daily dose of the compounds of
the invention is typically in the range 50mg to 100mg depending, of course, on
the mode of administration and efficacy. For example, oral administration may
require a total daily dose of from 50mg to 100mg. The total daily dose may be
administered in single or divided doses and may, at the physician's
discretion,
fall outside of the typical range given herein.
These dosages are based on an average human subject having a weight of
about 60kg to 70kg. The physician will readily be able to determine doses for
subjects whose weight falls outside this range, such as infants and the
elderly.
For the avoidance of doubt, references herein to. "treatment" include
references
to curative, palliative and prophylactic treatment.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-31-
Processes
Compounds of general formula (I) where R2 is H, R3 is H and where R1, R4, R5,
R6, X, V, W, Y and Z are as described herein may be prepared according to
reaction scheme 1.
O 0 N-N
i Y ii '\ Y /\
jY\OCH3 \ H,H2 hahaloW~V halo.~W,V lo--~W,V
(II) (III) (IV)
halo = Ci or Br
III
N-N
N-N X-Y, .[/ I
jj /YN~CH3 iv / ~~'" N CH3
RW,V R4 haloW,V R4
I
z Z
R$ (I) R (V)
Scheme 1
Compounds of formula (II) are either commercially available or can be prepared
by analogy with the methods described in J. Org. Chem., 66(2), 605-608; 2001
and UK Pat. Appi., 2219793, 20 Dec 1989.
Alternatively, when V, W, X or Y= CR6, compounds of formula (II) can be
prepared from commercial compounds using standard chemical reactions and
transformations of R6.
When R6 is alkoxy and preferably methoxy, R6 is incorporated by substitution
of
a functional group, preferably chloro, as exemplified in preparations 84-86.
Compounds of formula (III) may be prepared from compounds of formula (II) by
process step (i), whcih comprises reaction with hydrazine monohydrate in a
suitable solvent such as methanol or ethanol between -10 C and reflux. Typical
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-32-
conditions comprise heating 1 equivalent of aryl ester (II) and 1.2-3
equivalents
of hydrazine monohyrate in methanol at reflux for 18-48 hours.
Compounds of formula (IV) may be prepared from compounds of formula (III) by
process step (ii), which comprises reaction with N,N-dimethylacetamide
dimethyl
acetal (available from Aldrich) in a suitable solvent such as
N,N-dimethylformamide, N-methyl pyrrolidine or toluene followed by the
addition
of a suitable acid catalyst such as trifluoroacetic acid, para-toluenesulfonic
acid,
camphor sulfonic acid, or hydrochloric acid. Typical conditions comprise
heating
1 equivalent of aryl hydrazide (III) and 1.3 equivalents of N,N-
dimethylacetamide
dimethyl acetal in N,N-dimethylformamide to 60 C for 2 hours, followed by
concentration in vacuo, addition of toluene and 0.025 equivalents of para-
toluenesulfonic acid, which is then heated to reflux for 2 hours.
Compounds of formula (V) may be prepared from compounds of formula (IV) by
process step (iii), which comprises reaction with a suitable aniline or
3-aminopyridine in the presence of a suitable acid, such as trifluoroacetic
acid,
para-toluenesulfonic acid, camphor sulfonic acid, or hydrochloric acid in a
suitable solvent, such as xylene, heated at 150 C. Typical conditions comprise
heating 1 equivalent of 1,2,4-oxadiazole (IV), 2-3 equivalents of aniline or
aminopyridine and 0.04-0.1 equivalents of para-toluenesulfonic acid in xylene
at
150 C for 18-23 hours.
Compounds of formula (I) may be prepared from compounds of formula (V) by
process step (iv), which comprises a Suzuki coupling reaction with a suitable
boronic acid such as 2,3-dimethylphenyl boronic acid (commercially available),
in
a suitable solvent, in the presence of a base and a palladium catalyst such as
[2-[(Dimethylamino-xN)methyl]phenyl-xC](tricyclohexylphosphine)
(trifluoroacetato-xO-(SP-4-3)-palladium, prepared as described in
Organometallics, 2003, 22 (5), 987-999.
The Suzuki coupling reaction can be carried out as described in the
literature:
Suzuki, A. Pure & Appl. Chem. 1985, 57, 1749 and reference contained within;
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-33-
Angew. Chem. Int. Ed. 2002, 41, 4176-4211 and references contained within.
,
Typica'l conditions comprise heating 1 equivalent of aryl bromide (V), 2.5
equivalents of boronic acid, 3 equivaients cesium carbonate. 0.06 equivalents
of
palladium catalyst from preparation 3 in 1,4-dioxane at 120 C for 4 hours.
Compounds of general formula (I) where R3 is H and and where Ri, R2, R4, R5,
R6, X, V, W, Y and Z are as described herein, except R2 0 H, may be prepared
according to reaction scheme 2.
0 0
~' ~~N,NH2 v X'Y~N N~Rz
halo W.V H halo'.,Q W =V H 0
halo = Cl or Br (III) (VI)
vi
X,Y N R2
I
halo~W ~V N R4 Y N-N R2
~ I .E--_-- x' ~
Z halo W -V
(VIII) R5 iv (VII)
,Y N z
\\ R
X
1 \NJ~
R W,V R4
I
z
R5 (I)
Scheme 2
Compounds of formula (VI) can be prepared from aryl hydrazides of formula
(III)
by process step (v), which comprises reaction with an acid chloride, such as
methoxyacetyl chloride (for R2 = OCH3), in the presence of base such as
triethylamine, N-methyl morpholine, sodium carbonate or potassium hydroxide.
Typical conditions comprise heating 1.0 equivalents of aryl hydrazide (III),
1.0-
1.3 equivalents of acid chloride, 1.2-2.0 equivalents of N-methyl morpholine
in
dichloromethane at 0-25 C for 3-18 hours.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-34-.
Compounds of formula (VII) can be prepared from compound (VI) by process
step (vi), which comprises reaction with a suitable dehydrating agent such as
phosphorous oxychloride, trifluoromethanesulfonic anhydride, or phosphorous
pentachloride between a temperature of 25 C and 110 C. Typical conditions
comprise heating 1.0 equivalents of (VI) in phosphorous oxychloride at 110 C
for
4 hours.
Compounds of formula (VIII) may be prepared from compounds of formula (VII)
by process step (iii), which comprises reaction with a suitable aniline or
3-aminopyridine in the presence of a suitable acid, such as trifluoroacetic
acid,
para-toluenesulfonic acid, camphor sulfonic acid, or hydrochloric acid, in a
suitable solvent such as xylene, which is heated at 150 C. Typical conditions
comprise heating 1 equivalent of 1,2,4-oxadiazole (VII), 3 equivalents of
aniline/aminopyridine and 0.04-0.1 equivalents of para-toluenesulfonic acid in
xylene at 150 C for 18-22 hours.
Compounds of formula (I) may be prepared from compounds of formula (VIII) by
process step (iv), which comprises a Suzuki coupling reaction as described in
scheme 1.
Compounds of general formula (I) where R2 is NR'R$ or a 5-7 membered
N-linked heterocycle as described herein, R3 is H and where R1, R4, R5, R6,
R7,
R8, X, V, W, Y and Z are as described herein may be prepared according to
reaction scheme 3.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-35-
X-Y NH Y 0 H N-N
~ ~ N ~ X~ ~N,NCCI ~ X-Y / \
Halo WV H Halo~ :V H 0 ~ ~ ~~
W Halo W,V a
halo = CI or Br (III) (IX) (X)
N-N
X-Y~~R~ N-N
N
~,V R4 E iii X,Y / ~R2
Halo W ~ 0
I Halo W,V
z (XI)
(XII) R5 iv
X,~1Y'~ N ~~ Rz
,'l N.~.~
R W :V Ra
z
R5 (I)
Scheme 3
Compounds of formula (IX) can be prepared from aryl hydrazides of formula
(III)
by process step (v), which comprises reaction with a suitable acid chloride,
such
as chloroacetyl chloride, in the presence of a base, such as triethylamine,
N-methyl morpholine, sodium carbonate or potassium hydroxide. Typical
conditions comprise reacting 1.0 equivalents of aryl hydrazide (III), 1.0-1.3
equivalents of chloroacetyl chloride, 1.2-2.0 equivalents of N-methyl
morpholine
in dichloromethane at 25 C.
Compounds of formula (X) can be prepared from compounds of formula (IX) by
process step (vi), which comprises reaction with a suitable dehydrating agent
such as phosphorous oxychloride, trifluoromethanesulfonic anhydride, or
phosphorous pentachloride between a temperature of 25 C and 110 C. Typical
conditions comprise heating 1.0 equivalent of compound (IX) in phosphorous
oxychloride at 110 C for 4 hours.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-36-
Compounds of formula (XI) can be prepared from alkyl chlorides of formula (X)
by process step (vii), which comprises reaction with a suitable primary or
secondary amine (HNR'R$) or a 5-7 membered N-linked heterocycle, optionally
in the presence of a base such as potassium carbonate, sodium carbonate or
cesium carbonate, in a suitable solvent such as acetonitrile or
N,N-dimethylformamide, by heating at 25-50 C for 2-18 hours. Typical
conditions
comprise reacting 1 equivalent of alkyl chloride (X), 1.5 equivalent of amine
(HNR'R$) or 5-7 membered N-linked heterocycle and 2 equivalents of potassium
carbonate in acetonitrile for 18 hours at 25 C.
Compounds of formula (XII) may be prepared from compounds of formula (XI)
by process step (iii), which comprises reaction with a suitable aniline or
3-aminopyridine, in the presence of a suitable acid, such as trifluoroacetic
acid,
para-toluenesulfonic acid, camphor sulfonic acid, or hydrochloric acid, in a
suitable solvent such as xylene, heated at 150 C. Typical conditions comprise
heating 1 equivalent of 1,2,4-oxadiazole (XI), 3 equivalents of
aniline/aminopyridine and 0.04-0.1 equivalents of para-toluenesulfonic acid in
xylene at 150 C for 18-24 hours.
Compounds of formula (I) may be prepared from compounds of formula (XII) by
process step (iv), which comprisies reaction with a suitable boronic acid such
as
2,3-dimethylphenyl boronic acid (commercially available), in a suitable
solvent, in
the presence of a suitable base and palladium catalyst as described in
scheme 1.
25.
Compounds of general formula (I) where Ri, R2, R3, R4, R5, R6, X, V, W, Y and
Z
are as described herein may alternatively be prepared according to reaction
scheme 4.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-37-
0 0 0
~-~~o.CH3 iv X' ~`O'CH3 i ~~ \N'NH2
halo W;V R wsV ~ R' W;V H
(II) (XIII) (XIV).
N-N viii
X-~ õ ~R2
R
~ ~N R3 iii X,Y N-N 2
R
W ,V I R 4
' " ~C~3
Z R ~W
R5 (I) (XV)
Scheme 4
Compounds of formula (II) are prepared as described in scheme 1.
Compounds of general formula (XIII) can be prepared from compounds of
general formula (II) by process step (iv) as described in scheme 1.
Compounds of general formula (XIV) can be prepared from compounds of
general formula (XIII) by process step (i) as described in scheme 1.
When R2=H, compounds of general formuia (XV) can be prepared from
compounds of general formula (XIV) by process step (viii), using a method
analogous to process step (ii), as described in scheme 1.
When R2OH, compounds of general formula (XV) can be prepared from
compounds of general formula (XIV) by process step (viii), using methods
analogous to steps (v) and (vi), as described in scheme 2 or steps (v), (vi)
and
(vii) as described in scheme 3.
Compounds of general formula (I) can be prepared from compounds of general
formula (XV) by process step (iii), as described in scheme 1.
Compounds of general formula (I) where X is C-R6, R3 is H and where R1, R2,
R4, R5, R6, V, W, Y and Z are as described herein may alternatively be
prepared
according to reaction scheme 5.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
_3g_ .
z
X-Y~N.NHz viii X-Y~O~R
halo~W;V H haloW,V R3
(III) (IV)
iv
N-N Rz
X- Y
3 _
Ri~ V N 4 R iii X-Y N\L Rz
3
W~ I R I ~O,R
Z R~ -'\W
R5
(I) (XV)
Scheme 5
Compounds of formula (III) are prepared as described in scheme 1.
When R2=H, compounds of general formula (IV) can be prepared from
compounds of general formula (III) by process step (viii), using a method
analogous to process step (ii), as described in scheme 1.
When R2OH, compounds of general formula (IV) can be prepared from
compounds of general formula (Ilt) by process step (viii), using methods
analogous to steps (v) and (vi), as described in scheme 2 and steps (v), (vi)
and
(vii) as described in Scheme 3.
Compounds of general formula (XV) may be prepared from compounds of
general formula (IV) by process step (iv) as described in scheme 1.
Compounds of general formula (I) may be prepared from compounds of general
formula (XV) by process step (iii) as described in scheme 1.
Compounds of general formula (I) and (VIII) where R1, R2, R4, R5, V, W, X, and
Y are described herein and R3 = H may be prepared according to reaction
scheme 6.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-39-
O Y N-NR2
X- ~ NH ix ~N R3
/ N- 2 R W,V R4
R W-,V H I
(XIV) z
(I) Rs
0 X- Y N ~R2
X-Y~N,NH2 ix 30 ~ ~N R3
haloW-;V H halo W;V R4
I
z
(Ili)
(VIII) R5
Scheme 6
Compounds of formula (I) and (VIII) may be prepared from compounds of
formula (XIV) and (III) respectively by process step (ix), which comprises
sequential reaction with a dimethylacetamide dimethylacetal in a suitable
solvent
such as tetrahydofuran or acetic acid heated at 55-60 C followed by reaction
with a suitable aniline or aminopyridine in the presence of a suitable acid
such
as acetic acid heated at 90-100 C. Typical conditions comprise heating 1.0
equivalent of acyl hydrazide, 1.5 equivalents of dimethylacetamide
dimethylacetal (Aldrich) in THF at 55 C for 2 hours followed by the addition
of
1.5 equivalents of 2-methoxy-5-aminopyridine (Aldrich) and heating in acetic
acid
at 90 C for 5 hours.
All of the above reactions and the preparations of novel starting materials
disclosed in the preceding methods are conventional and appropriate reagents
and reaction conditions for their performance or preparation as well as
procedures for isolating the desired products will be well-known to those
skilled
in the art with reference to literature precedents and the Examples and
Preparations hereto.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-40-
Utili
The compounds of the invention are useful because they have pharmacological
activity in mammals, including humans. More particularly, they are useful in
the
treatment or prevention of a disorder in which modulation of the levels of
oxytocin could provide a beneficial effect. Disease states that may be
mentioned include sexual dysfunction, particularly premature ejaculation,
preterm labour, complications in labour, appetite and feeding disorders,
benign
prostatic hyperplasia, premature birth, dysmenorrhoea, congestive heart
failure,
arterial hypertension, liver cirrhosis, nephrotic hypertension, occular
hypertension, obsessive compulsive disorder and neuropsychiatric disorders.
Sexual dysfunction (SD) is a significant clinical problem which can affect
both males and females. The causes of SD may be both organic as well as
psychological. Organic aspects of SD are typicaily caused by underlying
vascular diseases, such as those associated with hypertension or diabetes
mellitus, by prescription medication and/or by psychiatric disease such as
depression. Physiological factors include fear, performance anxiety and
interpersorral conflict. SD impairs sexual performance, diminishes self-esteem
and disrupts personal relationships thereby inducing personal distress. In the
clinic, SD disorders have been divided into female sexual dysfunction (FSD)
disorders and male sexual dysfunction (MSD) disorders (Melman et al, J.
Urology, 1999, 161, 5-11).
FSD can be defined'as the difficulty or inability of a woman to find
satisfaction in
sexual expression. FSD is a collective term for several diverse female sexual
disorders (Leiblum, S.R. (1998). Definition and classification of female
sexual
disorders. Int. J. Impotence Res., 10, S104-S106; Berman, J.R., Berman, L. &
Goldstein, I. (1999). Female sexual dysfunction: Incidence, pathophysiology,
evaluations and treatment options. Urology, 54, 385-391). The woman may
have lack of desire, difficulty with arousal or orgasm, pain with intercourse
or a
combination of these problems. Several types of disease, medications, injuries
or psychological problems can cause FSD. Treatments in development are
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-41-
targeted to treat specific subtypes of FSD, predominantly desire and arousal
disorders.
The categories of FSD are best defined by contrasting them to the phases of
normal female sexual response: desire, arousal and orgasm (Leiblum, S.R.
(1998). Definition and classification of female sexual disorders, Int. J.
Impotence
Res., 10, S104-S106). Desire or libido is the drive for sexual expression. Its
manifestations often include sexual thoughts either when in the company of an
interested partner or when exposed to other erotic stimuli. Arousal is the
vascular response to sexual stimulation, an important component of which is
genital engorgement and includes increased vaginal lubrication, elongation of
the vagina and increased genital sensation/sensitivity. Orgasm is the release
of
sexual tension that has culminated during arousal.
Hence, FSD occurs when a woman has an inadequate or unsatisfactory
response in any of these phases, usually desire, arousal or orgasm. FSD
categories include hypoactive sexual desire disorder, sexual arousal disorder,
orgasmic disorders and sexual pain disorders. Although the compounds of the
invention will improve the genital response to sexual stimulation (as in
female
sexual arousal disorder), in doing so it may also improve the associated pain,
distress and discomfort associated with intercourse and so treat other female
sexual disorders.
Thus, in accordance with a further aspect of the invention, there is provided
the
use of a compound of the invention in the preparation of a medicament for the
treatment or prophylaxis of hypoactive sexual desire disorder, sexual arousal
disorder, orgasmic disorder and sexual pain disorder, more preferably for the
treatment or prophylaxis of sexual arousal disorder, orgasmic disorder, and
sexual pain disorder, and most preferably in the treatment or prophylaxis of
sexual arousal disorder.
Hypoactive sexual desire disorder is present if a woman has no or little
desire to
be sexual, and has no or few sexual thoughts or fantasies. This type of FSD
can
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-42-
be caused by low testosterone levels, due either to natural menopause or to
surgical menopause. Other causes include illness, medications, fatigue,
depression and anxiety.
Female sexual arousal disorder (FSAD) is characterised by inadequate genital
response to sexual stimulation. The genitalia do not undergo the engorgement
that characterises normal sexual arousal. The vaginal walls are poorly
lubricated, so that intercourse is painful. Orgasms may be impeded. Arousal
disorder can be caused by reduced oestrogen at menopause or after childbirth
and during lactation, as well as by illnesses, with vascular components such
as
diabetes and atherosclerosis. Other causes result from treatment with
diuretics,
antihistamines, antidepressants eg SSRIs or antihypertensive agents.
Sexual pain disorders (includes dyspareunia and vaginismus) is characterised
by
pain resulting from penetration and may be caused by medications which reduce
lubrication, endometriosis, pelvic inflammatory disease, inflammatory bowel
disease or urinary tract problems.
The preval'ence of FSD is difficult to gauge because the term covers several
types of problem, some of which are difficult to measure, and because the
interest in treating FSD is relatively recent. Many women's sexual problems
are
associated either directly with the female ageing process or with chronic
illnesses such as diabetes and hypertension.
Because FSD consists of several subtypes that express symptoms in separate
phases of the sexual response cycle, there is not a single therapy. Current
treatment of FSD focuses principally on psychological or relationship issues.
Treatment of FSD is gradually evolving as more clinical and basic science
studies are dedicated to the investigation of this medical problem. Female
sexual complaints are not all psychological in pathophysiology, especially for
those individuais who may have a component of vasculogenic dysfunction (eg
FSAD) contributing to the overall female sexual complaint. There are at
present
no drugs licensed for the treatment of FSD. Empirical drug therapy includes
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-43- oestrogen administration (topically or as hormone replacement therapy),
androgens or mood-altering drugs such as buspirone or trazodone. These
treatment options are often unsatisfactory due to low efficacy or unacceptable
side effects.
The Diagnostic and Statistical Manual (DSM) IV of the American Psychiatric
Association defines Female Sexual Arousal Disorder (FSAD) as being:
"a persistent or recurrent inability to attain or to maintain until
completion of the sexual activity adequate lubrication-swelling
response of sexual excitement. The disturbance must cause
marked distress or interpersonal difficulty."
The arousal response consists of vasocongestion in the pelvis, vaginal
lubrication and expansion and swelling of the external genitalia. The
disturbance
causes marked distress and/or interpersonal difficulty.
FSAD is a highly prevalent sexual disorder affecting pre-, peri- and post
menopausal ( HRT) women. It is associated with concomitant disorders such as
depression, cardiovascular diseases,- diabetes and UG disorders.
The primary consequences of FSAD are lack of engorgement/swelling, lack of
lubrication and lack of pleasurable genital sensation. The secondary
consequences of FSAD are reduced sexual desire, pain during intercourse and
difficulty in achieving an orgasm.
Male sexual dysfunction (MSD) is generally associated with either erectile
dysfunction, also known as male erectile dysfunction (MED) and/or ejaculatory
disorders such as premature ejaculation, anorgasmia (unable to achieve
orgasm) or desire disorders such as hypoactive sexual desire disorder (lack of
interest in sex).
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-44-
PE is a relativeiy common sexual dysfunction in men. It has been defined in
several different ways but the most widely accepted is the Diagnostic and
Statistical Manual of Mental Disorders IV one which states:
"PE is a lifelong persistent or recurrent ejaculation with minimal
sexual stimulation before, upon or shortly after penetration and
before the patient wishes it. The clinician must take into account
factors that affect duration of the excitement phase, such as age,
noveity of the sexual partner or stimulation, and frequency of
sexual activity. The disturbance causes marked distress of
interpersonal difficulty."
The International Classification of Diseases 10 definition states:
"There is an inability to delay ejaculation sufficiently to enjoy
lovemaking, manifest as either of the following: (1) occurrence of
ejaculation before or very soon after the beginning of intercourse (if
a time limit is required: before or within 15 seconds of the
beginning of intercourse); (2) ejaculation occurs in the absence of
sufficient erection to make intercourse possible. The problem is not
the result of prolonged abstinence from sexual activity"
Other definitions which have been used include classification on the following
criteria:
= Related to partner's orgasm = Duration between penetration and ejaculation
= Number of thrust and capacity for voluntary control
Psychological factors may be involved in PE, with relationship problems,
anxiety,
depression, prior sexual failure all playing a role.
Ejaculation is dependent on the sympathetic and parasympathetic nervous
systems. Efferent impulses via the sympathetic nervous system to the vas
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-45-
deferens and the epididymis produce smooth muscle contraction, moving sperm
into the posterior urethra. Similar contractions of the seminal vesicles,
prostatic
glands and the bulbouretheral glands increase the volume and fluid content of
semen. Expulsion of semen is mediated by efferent impulses originating from a
population of lumber spinothalamic cells in the lumbosacral spinal cord
(Coolen
& Truitt, Science, 2002, 297, 1566) which pass via the parasympathetic nervous
system and cause rhythmic contractions of the bulbocavernous, ischiocavernous
and pelvic floor muscles. Cortical control of ejaculation is still under
debate in
humans. In the rat the medial pre-optic area and the paraventricular nucleus
of
the hypothalamus seem to be involved in ejaculation.
Ejaculation comprises two separate components - emission and ejaculation.
Emission is the deposition of seminal fluid and sperm from the distal
epididymis,
vas deferens, seminal vesicles and prostrate into the prostatic urethra.
Subsequent to this deposition is the forcible expulsion of the seminal
contents
from the urethral meatus. Ejaculation is distinct from orgasm, which is purely
a
cerebral event. Often the two processes are coincidental.
A pulse of oxytocin in peripheral serum accompanies ejaculation in mammals. In
man oxytocin but not vasopressin plasma concentrations are significantly
raised
at or around ejaculation. Oxytocin does not induce ejaculation itself; this
process is 100% under nervous control via a1-adrenoceptor/sympathetic nerves
originating from the lumbar region of the spinal cord. The systemic pulse of
oxytocin may have a role in the peripheral ejaculatory response. It could
serve
' to modulate the contraction of ducts and glandular lobules throughout the
male
genital tract, thus influencing the fluid volume of different ejaculate
components
for example. Oxytocin released centrally into the brain could influence sexual
behaviour, subjective appreciation of arousal (orgasm) and latency to
subsequent ejaculation.
Accordingly, one aspect of the invention provides for the use of a compound of
formula (I), without the proviso, in the preparation of a medicament for the
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-46-
prevention or treatment of sexual dysfunction, preferably male sexual
dysfunction, most preferably premature ejaculation.
It has been demonstrated in the scientific literature that the number of
oxytocin
receptors in the uterus increases during pregnancy, most markedly before the
onset of labour (Gimpl & Fahrenholz, 2001, Physiological Reviews, .81 (2), 629-
683.). Without being bound by any theory it is known that the inhibition of
oxytocin can assist in preventing preterm labour and in resolving
complications
in labour.
Accordingly, another aspect of the invention provides for the use of a
compound
of formula (I), without the proviso, in the preparation of a medicament for
the
prevention or treatment of preterm labour and complications in labour.
Oxytocin has a role in feeding; it reduces the desire to eat (Arietti et al.,
Peptides, 1989, 10, 89). By inhibiting oxytocin it is possible to increase the
desire
to eat. Accordingly oxytocin inhibitors are useful in treating appetite and
feeding
disorders.
Accordingly, a further aspect of the invention provides for the use of a
compound
of formula (I), without the proviso, in the preparation of a medicament for
the
prevention or treatment of appetite and feeding disorders.
Oxytocin is implicated as one of the causes of benign prostatic hyperplasia
(BPH). Analysis of prostate tissue have shown that patients with BPH have
increased levels of oxytocin (Nicholson & Jenkin, Adv. Exp. Med. & BioL, 1995,
395, 529). Oxytocin antagonists can help treat this condition.
Accordingly, another aspect of the invention provides for the use of a
compound
of formuia (I), wihout the proviso, in the preparation of a medicament for the
prevention or treatment of benign prostatic hyperplasia.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-47- '
Oxytocin has a role in the causes of dysmenorrhoea due to its activity as a
uterine vasoconstrictor (Akerlund, Ann. NY Acad. Sci., 1994, 734, 47).
Oxytocin
antagonists can have a therapeutic effect on this condition.
Accordingly, a further aspect of the invention provides for the use of a
compound
of formula (I), without the proviso, in the preparation of a medicament for
the
prevention of treatment of dysmenorrhoea.
It is to be appreciated that all references herein to treatment include
curative,
palliative and prophylactic treatment.
The compounds of the present invention may be coadministered with one or
more agents selected from:
1) One or more selective serotonin reuptake inhibitors (SSRIs) such as
dapoxetine, paroxetine, 3-[(dimethylamino)methyl]-4-[4-
(methylsulfanyl)phenoxy]benzenesulfonamide (Example 28, WO
0172687), 3-[(dimethylamino)methyl]-4-[3-methyl-4-
(methylsulfanyl)phenoxy]benzenesulfonamide (Example 12, WO
0218333), N-methyl-N-({3-[3-methyl-4-(methylsulfanyl)phenoxy]-4-
pyridinyl}methyl)amine (Example 38, PCT Application no
PCT/IB02/01032).
2) One or more local anaesthetics;
3) one or more a-adrenergic receptor antagonists (also known as
a-adrenoceptor blockers, a-receptor blockers or a-blockers); suitable a1-
adrenergic receptor antagonists include: phentolamine, prazosin,
phentolamine mesylate, trazodone, alfuzosin, indoramin, naftopidil,
tamsulosin, phenoxybenzamine, rauwolfa alkaloids, Recordati 15/2739,
SNAP 1069, SNAP 5089, RS17053, SL 89.0591, doxazosin, Example 19
of W09830560, terazosin and abanoquil; suitable a2- adrenergic receptor
antagonists include dibenarnine, tolazoline, trimazosin, efaroxan,
yohimbine, idazoxan clonidine and dibenarnine; suitable non-selective a-
adrenergic receptor antagonists include dapiprazole; further a- adrenergic
CA 02539297 2008-08-11
-69387-571
-48-
receptor antagonists are described in PCT application W099/30697
published on 14th June 1998 and US patents: 4,188,390; 4,026,894;
3,511,836; 4,315,007; 3,527,761; 3,997,666; 2,503,059; 4,703,063;
3,381,009; 4,252,721 and 2,599,000.
4) one or more cholesterol lowering agents such as statins (e.g.
atorvastatin/Lipitor- trade mark) and fibrates;
5) one or more of a serotonin receptor agonist, antagonist or modulator,
more particularly agonists, antagonists or modulators for example 5HT1A,
5HT2A, 5HT2C, 5HT3, 5HT6 and/or 5HT7 receptors, including those
described in WO-09902159, WO-00002550 and/or WO-00028993;
6) one or more NEP inhibitors, preferably wherein said NEP is EC 3.4.24.11
and more preferably wherein sald NEP inhibitor is a selective inliibitor fo[
EC 3.4.24.11, more preferably a selective NEP inhibitor is a selective
inhibitor for EC 3.4.24.11, which has an IC50 of less than 100nM (e.g.
ompatrilat, sampatrilat) suitable NEP inhibitor compounds are described
in EP-A-1097719; IC50 values against NEP and ACE may be determined
using methods described in published patent application EP1097719-A1,
paragraphs [0368] to [0376];
7) one or more of an antagonist or modulator for vasopressin receptors,
such as relcovaptan (SR 49059), conivaptan, atosiban, VPA-985, CL-
385004, Vasotocin.
8) Apomorphine - teachings on the use of apomorphine as a pharmaceutical
may be found in US-A-5945117;
9) Dopamine agonists (in particular selective D2, selective D3, selective D4
and selective D2-like agents) such as Pramipexole (Pharmacia Upjohn
compound number PNU95666), ropinirole, apomorphine, surmanirole,
quinelorane, PNU-142774, bromocriptine, carbergoline, Lisuride;
10) Melanocortin receptor agonists (e.g. Melanotan f{ and PT141) and
selective MC3 and MC4 agonists (e.g.THIQ);
11) Mono amine transport inhibitors, particularly Noradrenaline Re-uptake
Inhibitors (NRIs) (e.g. Reboxetine), other Serotonin Re-uptake Inhibitors
CA 02539297 2008-08-11
.69387-571
-49-
(SRIs) (e.g. paroxetine, dapoxetine) or Dopamine Re-uptake lnhibitors
(DRIs);
12) 5-HTlA antagonists (e.g. robalzotan); and
13) PDE inhibitors such as PDE2 (e.g. erythro-9-(2-hydroxyl-3-nonvi)-
adenine) and Example 100 of EP 0771799
and in particular a PDE5 inhibitor such as the pyrazolo [4,3-
d]pyrimidin-7-ones disclosed in EP-A-0463756; the pyrazoio [4,3-
d]pyrimidin-7-ones disclosed in EP-A-0526004; the pyrazolo [4,3-
d]pyrimidin-7-ones disclosed in published international patent application
WO 93/06104; the isomeric pyrazolo [3,4-d]pyrimidin-4-ones disclosed in
published international patent application WO 93/07149; the quinazolin-4-
ones disclosed in published international patent application WO
93/12095; the pyrido [3,2-d]pyrimidin-4-ones disclosed in pubiished
international patent application WO 94/05661; the purin-6-ones. disclosed
in published international patent application WO 94/00453; the pyrazolo
[4,3-d]pyrimidin-7-ones disclosed in published international patent
application WO 98/49166; the pyrazolo [4,3-d]pyrimidin-7-ones disclosed
in published international patent application WO 99/54333; the pyrazolo
[4,3-d]pyrimidin-4-ones disclosed in EP-A-0995751; the pyrazolo [4,3-
d]pyrimidin-7-ones disclosed in published intemational patent application
WO 00/24745; the pyrazolo [4,3-d]pyrimidin-4-ones disclosed in EP-A-
0995750; the compounds disclosed in published international application
W095/19978; the compounds disclosed in published international
application WO 99/24433 and the compounds disclosed in published
intemational application WO 93/07124; the pyrazolo [4,3-d]pyrimidin-7-
ones disclosed in published intemational application WO 01/27112; the
pyrazolo [4,3-d]pyrimidin-7-ones disclosed in published international
application WO 01/27113; the compounds disclosed in EP-A-1092718
and the compounds disclosed in EP-A-1092719.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-50-
Preferred PDE5 inhibitors for use with the invention:
5-[2-ethoxy-5-(4-methyl-1-piperazinylsulphonyl)phenyl]-1-methyl-3-n-
propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (sildenafil) also
known as 1-[[3-(6,7-dihydro-1 -methyl-7-oxo-3-p ropyl- 1 H-pyrazolo[4,3-
d]pyrimidin-5-yl)-4-ethoxyphenyl]sulphonyl]-4-methylpiperazine (see EP-
A-0463756);
5-(2-ethoxy-5-morpholinoacetylphenyl)-1-methyl-3-n-propyl-1,6-dihydro-
7H-pyrazolo[4,3-d]pyrimidin-7-one (see EP-A-0526004);
3-ethyl-5-[5-(4-ethylpiperazin-1-ylsulphonyl)-2-n-propoxyphenyl]-2-
(pyridin-2-yl)methyl-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (see
W098/49166);
3-ethyl-5-[5-(4-ethylpiperazin-1-ylsulphonyl)-2-(2-methoxyethoxy)pyridin-
3-yI]-2-(pyridin-2-yl)methyl-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one
(see W099/54333);
(+)-3-ethyl-5-[5-(4-ethylpiperazin-1 -ylsulphonyl)-2-(2-methoxy-1 (R)-
methylethoxy)pyridin-3-yl]-2-methyl-2,6-dihydro-7H-pyrazolo[4,3-
d]pyrimidin-7-one, also known as 3-ethyl-5-{5-[4-ethylpiperazin-l-
ylsulphonyl]-2-([(1 R)-2-methoxy-1-methylethyl]oxy)pyridin-3-yl}-2-methyl-
2,6-dihydro-7H-pyrazolo[4,3-d] pyrimidin-7-one (see W099/54333);
5-[2-ethoxy-5-(4-ethylpiperazin-1-ylsulphonyl)pyridin-3-yl]-3-ethyl-2-[2-
methoxyethyl]-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, also known
as 1-{6-ethoxy-5-[3-ethyl-6,7-dihydro-2-(2-methoxyethyl)-7-oxo-2H-
pyrazolo[4,3-d]pyrimidin-5-yl]-3-pyridylsulphonyl}-4-ethylpiperazine (see
WO 01/27113, Example 8);
5-[2-iso-Butoxy-5-(4-ethylpiperazin-1-ylsulphonyl)pyridin-3-yl]-3-ethyl-2-
(1-methylpiperidin-4-yl)-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one
(see WO 01/27113, Example 15);
5-[2-Ethoxy-5-(4-ethylpiperazin-1-ylsulphonyl)pyridin-3-yl]-3-ethyl-2-
phenyl-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (see WO 01/27113,
Example 66);
5-(5-Acetyl-2-propoxy-3-pyridinyl)-3-ethyl-2-(1-isopropyl-3-azetidinyl)-2,6-
dihydro-7M pyrazolo[4,3-d]pyrimidin-7-one (see WO 01/27112, Example
124);
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-51-
5-(5-Acetyl-2-butoxy-3-pyridinyl)-3; ethyl-2-(1-ethyl-3-azetidinyl)-2,6-
dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one (see WO 01/27112, Example
132);
(6R, 1 2aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-
methylenedioxyphenyl) -pyrazino[2',1':6,1 ]pyrido[3,4-b]indole-1,4-dione
(IC-351), i.e. the compound of examples 78 and 95 of published
international application W095/19978, as well as the compound of
examples 1, 3, 7 and 8;
2-[2-ethoxy-5-(4-ethyl-piperazin-1-yl-1-sulphonyl)-phenyl]-5-methyl-7-
propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one (vardenafil) also known as 1-
[[3-(3,4-dihydro-5-methyl-4-oxo-7-propylimidazo[5,1-f]-as-triazin-2-yl)-4-
ethoxyphenyl]sulphonyl]-4-ethylpiperazine, i.e. the compound of examples
20, 19, 337 and 336 of published international application W099/24433;
and
the compound of example 11 of published international application
W093/07124 (EISAI); and
compounds 3 and 14 from Rotella D P, J. Med. Chem., 2000, 43, 1257.
Still further PDE5 inhibitors for use with the invention include:
4-bromo-5-(pyridylmethylamino)-6-[3-(4-chlorophenyl)-propoxy]-
3(2H)pyridazinone; 1-[4-[(1,3-benzodioxol-5- ylmethyl)amiono]-6-chloro-2-
quinozolinyl]-4-piperidine-carboxylic acid, monosodium salt; (+)-cis-
5,6a,7,9,9,9a-hexahydro-2-[4-(trifluoromethyl)-phenylmethyl-5-methyl-
cyclopent-4,5]imidazo[2,1-b]purin-4(3H)one; furazlocillin; cis-2-hexyl-5-
methyl-3,4,5,6a,7,8,9,9a- octahydrocyclopent[4,5]-imidazo[2,1-b]purin-4-
one; 3-acetyl-l-(2-chlorobenzyl)-2-propylindole-6- carboxylate; 3-acetyl-l-
(2-chlorobenzyl)-2-propylindole-6-carboxylate; 4-bromo-5-(3-
pyridylmethylamino)-6-(3-(4-chlorophenyl) propoxy)-3- (2H)pyridazinone;
I-methyl-5(5-morpholinoacetyl-2-n-propoxyphenyl)-3-n-propyl-1,6-dihydro-
7H-pyrazolo(4,3-d)pyrimidin-7-one; 1-[4-[(1,3-benzodioxol-5-
ylmethyl)arnino]-6-chloro-2- quinazolinyl]-4-piperidinecarboxylic acid,
monosodium salt; Pharmaprojects No. 4516 (Glaxo Wellcome);
Pharmaprojects No. 5051 (Bayer); Pharmaprojects No. 5064 (Kyowa
CA 02539297 2008-08-11
69387-571
-52-
Hakko; see WO 96/26940); Pharmaprojects No. 5069 (Schering Plough);
GF-196960 (Glaxo Wellcome); E-8010 and E-4010 (Eisai); Bay-38-3045
& 38-9456 (Bayer) and Sch-51866.
More preferred PDE5 inhibitors for use with the invention are selected from
the
group:
5-[2-ethoxy-5-(4-methyi-l-piperazinylsulphonyl)phenyl]-1-methyl-3-n-
propyl-1,6-dlhydro-71-I-pyrazolo[4,3-d]pyrirrridiri-7-one (silderiafil);
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-
methylenedioxyphenyl) -pyrazino[2',1':6,1 ]pyrido[3,4-b]indofe-1,4-dione
(IC-351);
2-[2-ethoxy-5-(4-ethyl-piperazin-1-yl-1-sulphonyl)-phenyl]-5-methyl-7-
propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one (vardenafil); and
5-[2-ethoxy-5-(4-ethylpiperazin-1-ylsulphonyl)pyridin-3-yl]-3-ethyl-2-[2-
methoxyethyl]-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one = or 5-(5-
Acetyl-2-butoxy-3-py(dinyl)-3-ethyl-2-(1-ethyl-3-azetidinyl)-2,6-dihydro-
7H-pyrazolo[4,3-d]pyrimidin-7-one and pharmaceutically acceptable salts
thereof.
A particularly preferred PDE5 inhibitor is 5-[2-ethoxy-5-(4-methyl-1-
piperazinylsulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-
d]pyrimidin-7-one (sildenafil) (also known as 1-[[3-(6,7-dihydro-l-methyl-7-
oxo-3-
propyl-1 H-pyrazolo[4,3-d]py(midin-5-y1)-4-ethoxyphenyl]sulphonyl]-4-
methylpiperazine) and pharmaceutically acceptable salts thereof. Sildenafil
citrate is a preferred salt.
Preferred agents for coadministration with the compounds of the present
invention are PDE5 inhibitors, selective serotonin reuptake inhibitors
(SSRIs),
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-53-
vasopressin V1A antagonists, a-adrenergic receptor antagonists, NEP
inhibitors,
dopamine agonists and melanocortin receptor agonists as described above.
Particularly preferred agents for coadministration are PDE5 inhibitors, SSRIs,
and V1A antagonists as described herein.
Assay
A suitable assay for determining the oxytocin antagonist activity of a
compound
is detailed herein below.
Oxytocin Receptor Beta-lactamase Assay
Materials:
Cell culture/Reagents
A: cell culture
Nutrient Mixture
F12 Ham's
Foetal Bovine Serum (FBS)
Geneticin
Zeocin
Trypsin/EDTA
PBS (phosphate buffered saline)
HEPES
B: reagents
Oxytocin
OT receptor-specific antagonist
Molecular grade Dimethyl Suiphoxide (DMSO)
Trypan Blue Solution 0.4%
CCF4-AM (Solution A)
Plurbnic F127s (Solution B)
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-54-
24%PEG, 18%TR40 (Solution C)
Probenecid (Dissolved at 200mM in 200mM NaOH, Solution D)
Methods
Cell Culture
Cells used are CHO-OTR/NFAT-0-Lactamase. The NFAT-0-lactamase
expression construct was transfected into the CHO-OTR cell line and clonal
populations were isolated via fluorescence activated cell sorting (FACS). An
appropriate clone was selected to develop the assay.
Growth Medium
90% F12 Nutrient Mix, 15mM HEPES
10% FBS
400 g/ml Geneticin
200 g/ml Zeocin
2mM L-Glutamine
Assay media
99.5% F12 Nutrient Mix, 15mM HEPES
0.5% FBS
Recovery of cells- A vial of frozen cells is thawed rapidly in 37 C water bath
and
the cell suspension tr.ansferred into a T225 flask with 50m1 of fresh growth
medium and then incubated at 37 C, 5% CO2 in an incubator until the cells
adhered to the flask Replace media with 50ml of fresh growth media the
following day.
Culturing cells- CHO-OTR-NFAT-PLactamase cells were grown in growth
medium. Cells were harvested when they reached 80-90% confluence removing
the medium and washing with pre-warmed PBS. PBS was then removed and
Trypsin/EDTA added (3mls for T225cm2 flask) before incubating for 5 min in
37 C/5%CO2 incubator. When cells were detached, pre-warmed growth media
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-55-
was added (7mls for T225cm2 flask) and the cells re-suspended and mixed
gently by pipetting to achieve single cell suspension. The cells were split
into
T225 fiask at 1:10 (for 3days growth) and 1:30 (for 5 days growth) ratio in
35m1
growth medium.
P-Lactamase assay Method
DAY 1
Cell plate preparation
Cells grown at 80-90% confluence were harvested and counted. Suspensions of
cells at 2x105 cells/mi in growth medium were prepared and 30 1 of cells
suspension added in 384-well, black clear-bottom plates. A blank plate
containing diluents from each reagent was used for background subtraction.
Plates were incubated at 37 C, 5% CO2 overnight.
DAY 2
Cells stimulation
= 10 l antagonist/compound (diluted in assay media containing 1.25% DMSO
= antagonist diluent) was added to appropriate wells and incubated for 15
minutes at 37 C, 5% C 2,
= 10 i oxytocin, made up in assay media, was added to all wells and incubated
for 4 hours at 37 C, 5% C02.
= A separate 384-well cell plate was used to generate an oxytocin dose
response curve. (10 l antagonist diluent was added to every we11.10 1 of
oxytocin was , then added. The cells are then treated as per
antagonist/compound cell plates).
CA 02539297 2008-08-11
69387-571
-56-
Prepar,ation of 1 ml of 6x Loading Buffer with Enhanced Loading Protocol (this
requires scale-up according to number of plates to be screened)
= 12 1 of solution A(1 mM CCF4-AM in Dry DMSO) was added to 60 i of
solution B(100mg/m1 Pluronicw-F127 in DMSO + 0.1% Acetic Acid) and
vortexed.
= The resulting solution was added to 925pi of solution C (24% w/w PEG400,
18% TR40 v/v in water).
= 75 1 of solution D was added (200mM probenecid in 200mM NaOH).
= 10 1 of 6x Loading Buffer was added to all wells and incubated for 1.5hrs -
2hrs at room temperature in the dark.
= The plates were read using an LJL Analyst, Excitation 405nm, Emission
450nm and 530nm, gain optimal, lagtime 0.40 s integration, 4 flashes,
bottom reading.
Using the assay described above, the compounds of the present invention all
exhibit oxytocin antagonist activity, expressed as a Ki value, of less than
500nM.
Preferred examples have Ki values of less than 200nM and particularly
preferred
examples have Ki values of less than 50nM.
The compound of example 8 has a Ki value of 3nM.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-57- '
The invention is illustrated by the following non-limiting examples in which
the
following abbreviations and definitions are used:
Arbocel Filtration agent, from J. Rettenmaier & Sohne, Germany
APCI+ Atmospheric Pressure Chemical lonisation (positive scan)
CDCI3 Chloroform-d1
d Doublet
dd Doublet of doublets
DMSO Dimethylsulfoxide
ES+ Electrospray ionisation positive scan.
eq Equivalent
'H NMR Proton Nuclear Magnetic Resonance Spectroscopy
MS (Low Resolution) Mass Spectroscopy
m Multiplet
m/z Mass spectrum peak
q Quartet
s Singlet
t Triplet
S Chemical shift "
Preparation 1
bisf2-f(Dimethylamino-xN)methyllphenyl-xClbisfv-(trifluoroacetato-xO: xO')1-
palladium
C NMe2 C
CI -Pdt'
Me2N 10
To a suspension of palladium chloride (3.43g, 19.4mmol) in methanol (200mL)
under nitrogen at room temperature was added N,N-dimethylbenzylamine
(5.82mL, 38.7mmol) via syringe. The resulting red/brown suspension was stirred
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-58-
at room temperature for 24 hours. The now green/brown suspension was
concentrated in vacuo to remove methanol, re-dissolved in dichloromethane
(150mL) and passed through a pad of silica gel washing through with
dichloromethane. The resulting bright yellow filtrate was concentrated in
vacuo
and recrystallised from dichloromethane:ether to give the desired product,
4.66g.
1HNMR(CDCI3, 300MHz) S: 2.86(s, 6H), 2.89(s, 6H), 3.95(s, 4H), 6.84-7.24(m,
8H)
Preparation 2
bis(2-f(Dimethylamino-xN)methyilphenyi-xClbisf -(trifluoroacetato-xO: xO')1-
palladium
QI3Me2 O O
F3C~ >-0F3
O O
Pd
Me2/(
To a solution of silver trifluoroacetate (4.48g, 20.3mmol) in acetone (30mL)
under nitrogen at room temperature was added a solution of the complex of
preparation 1 (5.60g, .10.15mmol) in dichloromethane (100mL). A thick white
precipitate appeared during addition. The suspension was stirred for 15
minutes,
and was then filtered through a pad of silica gel, washing with
dichloromethane.
Concentration in vacuo gave a bright yellow powder that was recrystallised
from
dichloromethane:ether to give the desired product, 7.06g.
iHNMR(CDCI3, 300MHz) S: 2.05(s, 6H), 2.88(s, 6H), 3.18(d, 2H), 3.63(d, 2H),
6.89-6.97(m, 6H), 7.00-7.10(m, 2H)
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-59-
Preparation 3
12-f (Dimethylamino-xN)methyllphenyl-
xC1(tricyclohexylphosphine)(trifIuoroacetato-xO-(SP-4-3)-palladium
Me
2
CcPd i
F3CCO ~ \ PCy3
To a solution of the product of preparation 2 (6.43g, 9.10mmol) in
dichloromethane (50mL) under nitrogen at room temperature was added a
solution of tricyclohexylphosphine (6.89g, 24.5mmol) in dichloromethane
(20mL).
After stirring for 1 hour, the solution was passed through a plug of silica
gel (7cm
x 2cm) washing with dichloromethane (400mL), and the pale yellow filtrate
concentrated in vacuo. Recrystallisation from dichloromethane:ether gave the
desired complex, 10.53g.
1HNMR(CDCI3, 300MHz) 8: 1.05-2.30(m, 33H), 2.57(s, 3H), 2.58(s, 3H), 3.93(s,
2H), 6.86-6.98(m, 3H), 7.10-7.12(m, 1 H)
Preparation 4
2-(4-Bromo-phenyl)-5-methyl-f 1,3,41oxadiazole
--
Br / \ / I
- O~CH3
4-Bromo-benzoic acid hydrazide (12.90g, 60mmol) and N,N-dimethylacetamide
dimethyl acetal (12mL, 82.Ommol) were dissolved in N,N-dimethylformamide
(100mL) and the * solution heated to 60 C for 2 hours. The solution was
concentrated in vacuo and the residue taken up in toluene (80mL) and treated
with para-toluenesulfonic acid monohydrate (200mg, 1.50mmol). The mixture
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-60-
was heated to reflux for 2 hours, allowed to cool, and the product
crystallised
from the solution and was collected by filtration. The crude product was
washed
with ether and dried in vacuo to yield a white solid. The filtrate was
concentrated
in vacuo and the residue combined with the white solid, dissolved in toluene
(50mL) and treated with para-toluenesulfonic acid monohydrate (100mg,
0.75mmol). The mixture was heated to reflux for 3 hours, allowed, to cool and
concentrated in vacuo. The residue was purified by column chromatography on
silica gel eluting with pentane:ethyl acetate 80:20 to 40:60 to yield the
title
product, 12.00g.
'HNMR(CDCI3, 400MHz) S: 2.61(s, 3H), 7.62(d, 2H), 7.88(d, 2H). MS ES+ m/z
239 [MH]+
Preparation 5
3-(4-Bromo-phen rl -4-(4-methoxy-phenyl)-5-methyl-4H-(1,2,41triazole
N-N
~ >-CH3
N
BrI \
/ ~ 1
~
O- CH3
The product of preparation 4(5.OOg, 20.9mmol) was added to a solution of
para-toluenesulfonic . acid monohydrate (1 00mg, 0.75mmol) and
4-methoxyphenylamine (7.70g, 62.5mmol) in xylene (150mL) and the reaction
mixture heated to 150 C for 22 hours. The reaction mixture was concentrated in
vacuo and the residue taken up in dichloromethane and purified by column
chromatography on silica gel eluting with dichloromethane:methano1:0.88
ammonia 100:0:0 to 97:3:0.3 to yield the title product, 7.05g.
1HNMR(DMSO-D6, 400MHz) 8: 2.20(s, 3H), 3.81(s, 3H), 7.07(m, 2H), 7.28(m,
2H), 7.34(m, 2H), 7.56(m, 2H). MS APCI+ mlz 344 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-61-
Preparation 6
5-Bromo-pyridine-2-carboxylic acid hydrazide
/ N O
Br
- H-NH2
5-Bromo-pyridine-2-carboxylic acid methyl ester (J. Org. Chem., 2001, 66(2),
605-608, compound 4) (18.10g, 83mmol) and hydrazine monohydrate (12.5mL,
250mmol) were dissolved in methanol (400mL) and the reaction mixture heated
to reflux for 48 hours. The reaction mixture was then filtered and the
precipitate
collected dried in vacuo to yield the title product, 15.40g.
'HNMR(DMSO-D6, 400MHz) S: 4.57(d, 2H), 7.91(m, 1 H), 8.22(m, 1 H), 8.72(m,
1 H), 9.98(m, 1 H). MS ES+ m/z 217 [MH]+
Preparation 7
5-Chloro-pyrazine-2-carboxylic acid hydrazide
-N O
CI-C/
N H-NH2
The title compound was prepared by the method of preparation 6 using 5-chloro-
pyrazine-2-carboxylic acid methyl ester. 5.01 g, 50% yield of the desired
product
was produced.
'HNMR(CDC13, 400MHz) 8: 4.09(d, 2H), 8.52(s, 1H), 8.66(bs, 1H), 9.14(s, 1H).
Microanalysis: C5H5CIN4O requires: C 34.80; H 2.92; N 32.47; found C 34.89; H
2.91, N 32.32. MS APCI+ m/z 173 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-62-
Preparation 8
5-Bromo-pyridine-2-carboxylic acid N'-(2-methoxy-acetyl)-hydrazide
0
N NN_ OICH3
~ H O
/
Br
The product of preparation 6(2.0g, 9.3mmol) and N-methylmorpholine (1.3mL,
12.Ommol) were dissolved in dichloromethane (60mL) and the solution treated
with methoxyacetyl chloride (868 L, 9.50mmol). The reaction mixture was
stirred
at room temperature for 5 hours and then washed with water and concentrated
in vacuo to afford 2.41 g, 90% yield of the title product.
' HNMR(CDCI3, 400MHz) 8: 3.46(s, 3H), 4.07(s, 2H), 7.98(dd, 1 H), 8.02(dd, 1
H),
8.61 (d, 1 H), 8.89(d, 1 H), 9.95(d, 1 H). MS ES+ m/z 289 [MH]+
Preparation 9
6-Chloro-nicotinic acid N'-(2-methoxy-acetyl)-hydrazide
0
H
\ NNOCH3
H O
cl N
The title product was prepared by the method of preparation 8 using
6-chloronicotinic acid hydrazide. 19.0g, 90% yield of the desired product was
produced.
1 HNMR(CDCI3, 400MHz) 8: 3.36(s, 3H), 3.97(s, 2H), 7.68(d, 1 H), 8.26(dd, 1
H),
8.84(s, 1 H), 9.99(s, 1 H), 10.61(s, 1 H). MS ES+ m/z 246 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-63-
Preparation 10
5-Chloro-pyrazine-2-carboxylic acid N'-(2-methoxy-acetyl)-hydrazide
0
NDZ,' NN ~OCH3
H O
CI N
The title product was prepared by the method of preparation 8 using the
hydrazide of preparation 7. 3.90g 70% yield of the desired product was
produced.
MS APCI+ m/z 245 [MH]+
Preparation 11
5-Chloro-pyrazine-2-carboxylic acid N'-acetyl-hydrazide
0 H
N\ N~NYCH3
~ ~ H O
CI N
The title product was prepared by the method of preparation 8 using the
hydrazide of preparation 7 and acetyl chloride. 4.0g, 64% of the desired
product
was produced.
MS APCI+ m/z 215 [MH]+
Preparation 12
5-Bromo-pyridine-2-carboxylic acid N'-(2-chloro-acetyl)-hydrazide
oo
Br
CI
N H_H
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-64-
The title product was prepared by the method of preparation 8 using the
hydrazide of preparation 6 and chloroacetyl chloride. 4.30g, 59% yield of the
desired product was produced.
'HNMR(CDCI3, 400MHz) 8: 4.20(s, 2H), 8.00(d, 1H), 8.30(d, 1H), 8.80(s, 1H),
10.40(s, 1 H), 10.70(s, 1 H). MS APCI+ m/z 293 [MH]+
Preparation 13
5-Bromo-2-(5-methoxymethyl-(1,3,41oxadiazol-2-yl)-pyridine
N\N
Br ~
CH3
The product of preparation 8 (2.41g, 8.4mmol) and phosphorous oxychloride
(7mL) were combined and heated to 110 C for 4 hours. The reaction mixture
was concentrated in vacuo and the residue taken up in ethyl acetate and water.
The mixture was neutralised by the addition of 10% sodium carbonate solution
and the phases separated. The aqueous phase was extracted with ethyl acetate
and the combined organics dried over magnesium sulfate and concentrated in
vacuo. The residue was purified by column chromatography on silica gel eluting
with ethyl acetate to yield the title product, 1.01 g, 45% yield.
' HNMR(CDCI3a 400MHz) S: 3.48(s, 3H), 4.73(s, 2H), 8.01(dd, 1 H), 8.12(dd, 1
H),
8.81 (dd, 1 H). MS APCI+ m/z 272 [MH]+
Preparation 14
2-Chloro-5-(5-methoxymethyl-f 1,3,41oxadiazol-2-yl)-pyridine
N
CI
- O~O
N ~CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-65-
The title compound was prepared by the method of preparation 13 using the
product of preparation 9 provide 7.93g, 40% yield of title compound as a rust
brown solid .
1HNMR(CDCI3, 400MHz) 8: 3.52(s, 3H), 4.74(s, 2H), 7.50(d, 1H), 8.32(dd, 1H),
9.06(d, 1 H). Microanalysis: C9H$CIN302 requires: C 47.91; H 3.57; N 18.62;
found C 47.75; H 3.50, N 18.46. MS APCI+ m/z 226 [MH]+.
Preparation 15
2-Chloro-5-(5-methoxymethyl-[1 3 4loxadiazol-2-yl)-pyrazine
~- ~ N, N
CI-(\/ ~
N- C
' CH3
The title compound was prepared by the method of preparation 13 using the,
product of preparation 10. 1.38g, 38% yield of the desired product was
produced
as a light brown solid.
1 HNMR(CDCI3, 400MHz) b: 3.52(s, 3H), 4.78(s, 2H), 8.75(s, 1 H), 9.25(s, 1 H)
MS APCI+ m/z 227 [MH]+
Preparation 16
2-Chloro-5-(5-methyl-f 1 3,41oxadiazol-2-yl)-pyrazine
N N-
CI-(\/
N CH3
The title compound was prepared by the method of preparation 13 using the
product of preparation 11. 30g, 35% of the desired product was produced as a
brown solid.
'HNMR(CDCI3, 400MHz) S: 2.68(s, 3H), 8.71(s, 1 H), 9.22(s, 1 H)
MS APCI+ m/z 197 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-66-
Preparation 17
5-Bromo-2-(5-chloromethyl-[1,3,41oxadiazol-2-yl)-pyridine
N-N
Br O~CI
N
The title compound was prepared by the method of preparation 13 using the
product of preparation 12. 2.3g 57% yield of desired product, was obtained as
an
off white solid.
1HNMR(DMSO-D6a 400MHz) S: 4.80(s, 2H), 8.05(d, 1H), 8.15(d, 1H), 8.85(s,
1 H). MS APCI+ m/z 276 [MH]+
Preparation 18
5-Bromo-2-[5-(methoxymethLrl)-4-(6-meth.oxypyridin-3-yl)-4H-1,2,4-triazol-3-
I ridine
N-N ~O-CH
N 3
IN
Br ~ ~ \
N
0- CH3
The product of preparation 13 (1.01 g, 3.74mmol), 5-amino-2-methoxypyridine
(1.40g, 11.3mmol) and para-toluenesulfonic acid monohydrate (50mg,
0.37mmol) were dissolved in xylene (25mL) and the reaction mixture heated to
150 C for 23 hours. The reaction. mixture was concentrated in vacuo and the
residue purified by column chromatography on silica gel eluting with
dichloromethane:methanol 100:0 to 90:10 to yield the title product, 1.0g, 72%
yield as a purple gum.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-67-
'HNMR(CDCI3, 400MHz) b: 3.32(s, 3H), 3.99(s, 3H), 4.46(s, 2H), 6.82(d, 1H),
7.54(dd, 1 H), 7.90(dd, 1 H), 8.05(d, 1 H), 8.13(d, 1 H), 8.37(d, 1 H).
MS ES+ m/z 398 [MH]+
Preparation 19
2-(4-Fluoro-2-methyl-phenyl)-5-(5-methoxymethyl-f 1,3,41oxadiazol-2-yl)-
pyridine
CH3
N- i
F
- N- p O
CH3
The chloro compound of preparation 14 (500mg, 2.22mmol), 4-fluoro-2-
methylphenyl boronic acid (361mg, 2.65mmoi), the palladium complex of
preparation 3 (10mg, cat.) and caesium carbonate (2.16g, 6.66mmol) were
dissolved in 1,4-dioxan (10mL) and the reaction mixture heated to reflux for 2
hours. Additional palladium complex (10mg) was added and the reaction mixture
refluxed for a further 1 hour. The reaction mixture was concentrated in vacuo
and the residue taken up in ethyl acetate and water. The phases were separated
and the ethyl acetate phase washed with brine, dried over magnesium sulfate
and concentrated in vacuo to yield the title product, 690mg in quantitative
yield.
MS APCI+ m/z 300 [MH]+
Preparation 20
2-(2,3-Dimethyl-phenyl)-5-(5-methoxymethyl-r1,3,41oxadiazol-2-yl)-pyridine
H3C CH3
N,
I
N OO.
CH3
The title product was prepared by the method of preparation 19 using
2,3-dimethylphenyl boronic acid (399mg, 1.2eq) and the product of preparation
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-68-
14 (500mg, 2.22mmol). 712mg, quantitative yield, of the desired product was
produced.
MS APCI+ m/z 296 [MH]+
Preparation 21
2-(2,3-Dimethyl-phen rl -5-(5-methoxymethyl-f 1,3,41oxadiazol-2-yl)-pyrazine
H3C CH3
\ N'N
NO O
.
CH3
The title product was prepared by the method of preparation 19 using
2,3-dimethylphenyl boronic acid and the chloro compound of preparation 15.
466mg, quantitative yield, of the desired product was produced.
'HNMR(CDCI3a 400MHz) b: 2.29(s, 3H), 2.39(s, 3H), 3.52(s, 3H), 4.78(s, 2H),
7.23-7.37(m, 3H), 8.84(s, 1 H), 9.54(s, 1 H)
MS APCI+ m/z 297[MH]+
Preparation 22
2-(4-Fluoro-2-methyl-phenyl)-5-(5-methoxymethyl-f 1,3,41oxadiazol-2-yl)-
pyrazine
CH3
F C \ / \ N-N
~
N OO'
CH3
The title product was prepared by the method of preparation 19 using 4-fluoro-
2-
methyl-phenyl boronic acid and the chloro compound of preparation 15. 450mg,
97% of the desired product was produced.
MS APCI+ m/z 301'[MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-69-
Preparation 23
2-(2,3-Dimethyl-phen L)I -5-(5-methyl-[1,3,41oxadiazol-2-yl)-pyrazine
H3C CH3
N-
N
N CH3
The title product was prepared by the method of preparation 19 using
2,3-dimethylphenyl boronic acid and the chloro compound of preparation 16.
404mg, quantitative yield, of the desired product was produced.
MS APCI+ m/z 267 [MH]+
Preparation 24
2-(4-Fluoro-2-methyl-phenyl)-5-(5-methyl-(1 3,41oxadiazol-2-yl)-pyrazine
CH3
F ~ \ ~ \ N-N
_
N CH3
The title product was prepared by the method of preparation 19 using 4-fiuoro-
2-
methyl-phenyl boronic acid and the chloro compound of preparation 16. 377mg,
quantitative yield, of the desired product was produced.
MS APCI+ m/z 271 [MH]+
Preparation 25
1-[5-(5-Bromo-pyridin-2-yl)-[1 3 4loxadiazol-2-ylmethyll-pyrrolidine-(2S)-2-
carboxylic acid amide
N-N
' Br / \
N
N 0~
H2N 0
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-70-
The chloro compound of preparation 17 (500mg, 1.82mmol) and (S)-prolinamide
(312mg, 2.73mmol) were dissolved in acetonitrile (10mL) and the mixture
treated
with potassium carbonate (503mg, 3.64mmol). The reaction mixture was stirred
at room temperature for 18 hours and then at 50 C for 2 hours. The reaction
mixture was concentrated in vacuo and the residue partitioned between ethyl
acetate and water. The precipitate formed was filtered off and the organic
layer
of the filtrate washed with water, 1 M sodium hydroxide solution and brine.
The
organic layer was then concentrated in vacuo to yield the title product,
540mg,
84% yield.
'HNMR(DMSO-D6, 400MHz) b: 1.70(m, 3H), 2.00(m, 1H), 2.60(m, 1H), 3.10(m,
1 H), 3.20(m, 1 H), 4.00(d, 1 H), 4.20(d, 1 H), 7.00-7.20(d, 2H), 8.10(d, 1
H), 8.30(d,
1 H), 8.90(s, 1 H).
Preparation 26
5-Bromo-2-(5-pyrrolidin-1-ylmethyl-f1 3 4loxadiazol-2-yl)-pyridine
~ ~ ~ `I
Br _ ON
N
Pyrrolidine (324mg, 0.38mL, 4.56mmol) was added to a stirred solution of the
chloro compound of preparation 17 (500mg, 1.82mmol) in aceonitrile (15mL) at
room temperature. After stirring for 18 hours the reaction mixture was
concentrated in vacuo and, the residue taken up in ethyl acetate (50mL) and
washed with 2M aqueous sodium hydroxide, followed by water followed by brine.
The organic phase was dried over sodium sulfate, filtered and concentrated in
vacuo to afford 407 mg, 72% yield of the title compound.
1 HNMR(CDC13, 400MHz) 5: 1.90(m, 4H), 2.70(m, 4H), 4.00(s, 2H), 8.00(d, 1 H),
8.20(d, 1 H), 8.80(s, 1 H).
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-71-
Preparation 27
1-[5-(5-Bromo-pyridin-2-Lrl)-4-(6-methoxy-pyridin-3-yl)-4H-[1,2,41triazol-3-
õ 11 methyll-pyrrolidine-(2S)-2-carboxylic acid amide
N-N
~ \ N
Br -~ N O
N
H2N
N
O`CH3
The product of preparation 25 (500mg, 1.20mmol) and 5-amino-2-
methoxypyridine (224mg, 1.81 mmol) were dissolved in xylene (15mL) and the
solution treated with catalytic para-toluenesulfonic acid monohydrate and
heated
to reflux for 18 hours. The ,reaction mixture was concentrated in vacuo and
the
residue taken up in ethyl acetate and washed with water, 2M citric acid
solution,
2M sodium hydroxide solution, dried over magnesium sulfate and concentrated
in vacuo. The residue was purified by column chromatography on silica gel
eluting with dichloromethane:methanol 100:0 to 95:5 to yield the title
product,
252mg, 46% yield.
'HNMR(C C13, 400MHz) S: 1.80(m, 2H), 2.20(m, 1 H), 2.60(m, 1 H), 3.10(m, 1 H),
3.20(m, 1 H), 3.90(m, 2H), 4.00(s, 3H), 5.00(s, 1 H), 6.70(s, 1 H), 6.90(d, 1
H),
7.90(d, 1 H), 8.05(d, 1 H), 8.20(d, 1 H), 8.40(s, 1 H). MS ES+ m/z 458 [MH]+
Preparation 28
5-Bromo-2-(((5-pyrrolidin-1-Ylmethyl)-4-(6-methoxypyridin-3-yl))-4H-
[1,2,41triazol-
3-yi)-pyridine
N-N
Br \ /N
N
ON\
O,
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-72-
412 mg, 77% yield of the title compound was prepared by the method of
preparation 27 using the product of preparation 26.
MS ES+ m/z 417 [MH]+
Preparation 29
3-Methyl-4-(4,4 5 5-tetramethyl-f 1,3,21dioxaborolan-2-yl)-benzonitrile
H3C CH3
91-'~CH3
I ~ B.C CH3
NC ~ CH3
Palladium (II) acetate (224mg, 5 mol%), potassium acetate (3.68g, 61.2 mmot)
10 and bis(pinacolato)diboron (5.4g, 21.4 mmol) were added to a solution of
1-bromo-4-cyano-2-methylbenzene (4.0g, 20.4 mmol) in N,N-dimethylformamide
(40 mL) and heated at 80 C for 18 hours. After such time the mixture was
cooled
and filtered through a pad of Celite , washing with ethyl acetate and water.
The
organic phase was separated, dried over sodium sulfate and concentrated in
vacuo to afford a brown solid. The solid was purified by trituration in
pentane,
filtration and drying to afford the title compound as a beige solid (2.68g,
54%
yield).
1HNMR(CDCI3, 400MHz) 5: 1.35(s, 12H), 2.55(s, 3H), 7.41-7.45(m, 2H), 7.82(d,
1 H). MS APCI+ m/z 261 [MNH4]+
Preparation 30
2-Chloro-5-f4-(6-methoxy-pyridin-3-yl)-5-methyl-4H-f 1,2,41triazol-3-yll-
rpy azine
CI- N-
CH3
~ N
N
H3C-0
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-73-
The title product was prepared by the method of preparation 18 using the
oxadiazole compound of preparation 16 and 5-amino-2-methoxypyridine. 4.3g,
44% yield of the desired product was produced as a beige solid.
1 HNMR(CDCI3, 400MHz) 8: 2.36(s, 3H), 3.99(s, 3H), 6.86(d, 1 H), 7.45(dd, 1
H),
8.02(d, 1 H), 8.27(d, 1 H), 9.23(d, 1 H).
Preparation 31
2-Chloro-5-f 5-methoxymethyl-4-(6-methoxy-pyridin-3-yl)-4H-f 1,2,4]triazol-3-
yll-
rpy azine
CI~ N\N
I
N N01 CH
3
XON
H3C-0
The title product was prepared by the method of preparation 18 using the
oxadiazole compound of preparation 15 and 5-amino-2-methoxypyridine. 10.5g,
59% yield of the desired product was produced as a beige solid.
'HNMR(CDCI3, 400MHz) 8: 3.33(s, 3H), 3.99(s, 3H), 4.47(s, 2H), 6.83(d, 1H),
7.53(dd, 1 H), 8.06(d, 1 H), 8.30(d, 1 H), 9.25(d, 1 H).
MS APCI+ m/z 333 [MH]+
Preparation 32
2-(5-Fluoro-2-methoxy-phenyl)-5-(5-methoxymethyl-f 1,3,41oxadiazol-2-YI)-
rpy idine
H3C
O
N~I
N 0~ p,
F CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-74- -
The title product was prepared by the method of preparation 19 using 5-fluoro-
2-
methoxy-phenyl boronic acid (565mg, 3.33mo1) and the chloro compound from
preparation 14 (500mg, 2.22mmol). 669mg, 96% yield of the desired product
was produced.
i HNMR(CDCI3, 400MHz) 8: 3.51(s, 3H), 3.88(s, 3H), 4.75(s, 2H), 6.97(d, 1 H),
7.11(m, 1 H), 7.70(dd, 1 H), 8.11(d, 1 H), 8.37(dd, 1 H), 9.34 (d, 1 H).
MS APCI+ m/z 316 [MH]+
Preparation 33
6-Chloro-nicotinic acid N'-(2-chloro-acetyl)-hydrazide
D o~
CI _\ N_N CI
H H
Chloroacetyl chloride (2.8mL, 34.9mmol) was added dropwise to an ice-cooled
solution of 6-chloronicotinic acid hydrazide (5g, 29.1 mmol) and
4-methylmorpholine (4.8mL, 43.7mmol) in dichloromethane (100mL) and the
reaction was stirred at room temperature for 3 hours. The resulting
precipitate
was then filtered off, slurried with dichloromethane, re-filtered, washed with
dichloromethane (x3) and dried to afford the title compound as a beige solid
in
57% yield, 4.1 g
iHNMR(DMSO-D6, 400MHz) 8: 4.20(s, 2H), 7.68(d, 1H), 8.23(d, 1H), 8.84(m,
1 H), 10.50(s, 1 H), 10.83(s, 1 H). MS APCI+ m/z 248/250 [MH]+
Preparation 34
5-Chloro-pyrazine-2-carboxylic acid N'-(2-chloro-acetyl)-hydrazide
N p O~
CIN--N CI
N H H
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-75-
The title product was prepared from the product of preparation 7 and
chloroacetylchloride, using the method of preparation 33, as a solid in 37%
yield.
MS APCI+ m/z 249/251 [MH]+
Preparation 35
6-Chloro-nicotinic acid N'-acetyl-hydrazide
O O
CI ~-CH3
N-N
H H
The title compound was prepared from 6-chloronicotinic acid hydrazide and
acetyl chloride, using the method of preparation 33, as a white solid in 64%
yield.
1 HNMR(DMSO-D6, 400MHz) 8: 1.91(s, 3H), 7.68(d, 1H), 8.24(dd, 1H), 8.82(d,
1 H), 10.00(s, 1 H), 10.58(s, 1 H). MS APCI+ m/z 214 [MH]+
Preparation 36
6-Chloro-nicotinic acid N'-f2-(2-methoxy-ethoxy)-acetyll-hydrazide
FU~~ o oCI N_N ~Hs
N H H O
(2-Methoxy-ethoxy)-acetyl chloride (2.13g, 13.99mmol) was added to an ice-cold
solution of 6-chloronicotinic acid hydrazide (2g, 11.66mmol) and
N-methylmorpholine (1.92mL, 17.49mmol) in dichloromethane (60mL) and the
mixture was stirred at room temperature for 18 hours. The mixture was then
treated with sodium hydrogen carbonate solution and concentrated in vacuo.
The aqueous residue was extracted with dichloromethane (x2) and the
combined organic solutions were washed with brine and dried over sodium
sulfate and concentrated in vacuo to give a pale yellow residue. The residue
was
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-76-
then stirred in diethyl ether for 2 hours, filtered and dried to afford the
title
compound as a pale yellow solid in 51 % yield, 1.7g.
'HNMR(CDCI3, 400MHz) 8:3.46(s, 3H), 3.62(m, 2H), 3.78(m, 2H), 4.21(s, 2H),
7.42(d, 1H), 8.07(dd, 1H), 8.82(d, 1H) 9.28(brs, 1H), 9.83(brs, 1H). MS APCI+
m/z 288 [MH]+
Preparation 37
6-Chloro-nicotinic acid N'-(2-ethoxy-acetyl)-hydrazide
o oI
Cl- \
_N-N
CH3
H H ~
(2-Ethoxy)-acetyl chloride [(1.72g, 13.99mmol), Tett. Lett., 35, (39), 7269;
1994)]
was added to an ice-cold solution of 6-chloronicotinic acid hydrazide (2g,
11.66mmol) and N-methylmorpholine (1.92mL, 17.49mmol) in dichloromethane
(60mL) and the mixture was stirred at room temperature for 18 hours. The
mixture was then washed with citric acid, sodium hydrogen carbonate solution
and brine and the solvent was evaporated under reduced pressure to yield some
title product as a white solid, 880mg. The combined aqueous washings were
extracted with ethyl acetate (x2) and the combined organic solutions were
dried
over sodium sulfate and concentrated in vacuo to afford a further crop of
title
compound as a pale yellow solid, 1.6g (total yield of 83%).
iHNMR(CDCI3a 400MHz) 5:1.21(t, 3H), 3.55(q, 2H), 4.06(s, 2H), 7.38(d, 1H),
8.00(dd, 1 H), 8.77(d, 1 H) 8.99(brs, 1 H), 9.15(brs, 1 H). MS APCI+ mlz
258/260
[MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-77-
Preparations 38 to 42
, `.
The following compounds, of the general formula shown below, were prepared
by the method of preparation 13 using the appropriate hydrazide (preparations
33-37) and phosphorus oxychloride.
Y N-N
CI~4 0 ~R2
N
No. R 2 Y Data Yield
38 CI CH 1HNMR(CDCI3, 400MHz) b: 4.80(s, 55%
2H), 7.52(d, 1H), 8.35(dd, 1H),
9.08(d, 1 H). MS APCI+ m/z 230/232
[MH]+
39 CI N 1HNMR(CDCI3, 400MHz) S: 4.83(s, 38%
2H), 8.76(s, 1H), 9.36(s, 1 H). MS
APCI+ m/z 231/233 [MH]+
40 H CH HNMR(CDCI3i 400MHz) 8: 2.66(s, 77%
3H), 7.51(d, 1 H), 8.31(d, 1 H), 9.02(s,
1 H).
Microanalysis: C8H6CIN3O 0.25 H20
requires: C 48.02; H 3.27; N 21.00;
found C 47.89; H 3.23, N 20.95.
41 HCCH HNMR(CDCI3, 400MHz) 8: 3.39(s, 68%
3 3H), 3.59-3.61(m, 2H), 3.78-3.80(m,
2H), 4.86(s, 2H), 7.52(d, 1H),
8.33(dd, 1 H), 9.07(d, 1 H). MS APCI+
m/z 270/272 [MH]+
42 H Co CH HNMR(CDCI3, 400MHz) S: 1.28(t, 75%
3 3H), 3.69(q, 2H), 4.77(s, 2H), 7.49(d,
1 H), 8.34(dd, 1 H), 9.07(d, 1 H).
MS ES+ m/z 262 [MNa]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-78-.
Preparation 42 was purified by column chromatography on silica gel, eluting
with
pentane:ethyl acetate, 100:0 to 90:10.
Preparation 43
2-Chloro-5-(5-f 1,2,31triazol-2-ylmeth rLl-f 1,3,41oxadiazol-2-yl)-pyridine
;~ N"N N-
CI \
N.- O N
1 H-1,2,3-Triazole (264mg, 3.85mmol) was added to a suspension of the chloro
compound of preparation 38 (800mg, 3.5mmol), and potassium carbonate (1.4g,
7mmol) in N,N-dimethylformamide (15mL) and the mixture stirred at room
temperature for 18 hours. The reaction mixture was then partitioned between
ethyl acetate and water and the organic layer was separated, washed with
brine,
dried over sodium sulfate and concentrated in vacuo to afford the title
compound
as a yellow solid in 71 % yield, 650mg.
' HNMR(CDCI3, 400MHz) S: 6.08(s, 2H), 7.66(d, 1H), 7.80(s, 2H), 8.38(dd, 1H),
8.99(d, 1 H). MS APCI+ m/z 263 [MH]+
Preparations 44 to 45
The following compounds, of the general formuia shown below, were prepared
from the product of preparations 38 and 39 using the method of preparation 43.
Y N-N
~ 0 ~R2
CI---C
N
No. R Y Data Yield
44 "N N MS APCI+ m/z 264 [MH]+ 40%
N~~'
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-79-
45 H3C CH 1HNMR(CDCI3, 400MHz) S: 2.34(s, 82%
N-;
H3~ 6H), 3.78(s, 2H), 7.43(d, 1H),
8.28(dd, 1 H), 9.01(d, 1 H).
MS APCI+ m/z 239/241 [MH]+
Preparations 46 to 52
The following compounds, of the general formula shown below, were prepared
by the method of preparation 18 using the appropriate oxadiazole (preparations
14 and 40-45) and 5-amino-2-methoxypyridine.
Y N'-N
R2
N NA
N
H3C~0
No. R Y Data Yield
46 C-7N CH 1HNMR(CDCI3, 400MHz) S: 3.97(s, 25%
N3H), 5.75(s, 2H), 6.78(d, 1H),
7.24(d, 1H), 7.34(d, 1H), 7.56(s,
2H), 7.90(dd, 1H), 7.94(m, 1H),
8.30(d, 1 H)
MS APCI+ m/z 369 [MH]+
47 ~N N MS APCI+ m/z 370 [MH]+ 29%
~
N
48 H3C CH 1HNMR(CDCI3i 400MHz) 8: 2.25(s, 36%
H CN~ 6H), 3.46(s, 2H), 3.99(s, 3H),
3
6.85(d, 1 H), 7.34(d, 1 H), 7.60(dd,
1 H), 7.88(dd, 1 H), 8.10(d, 1 H),
8.34(d, 1 H)
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-80-
MS APCI+ m/z 345/347[MH]+
49 OCH3 CH 1HNMR(CDCI3, 400MHz) S: 3.35(s, 50%
3H), 3.99(s, 3H), 4.48(s, 2H),
6.88(d, 1 H), 7.34(d, 1 H), 7.50(m,
1 H), 7.88(m, 1 H), 8.10(d, 1 H),
8.35(m, 1 H)
MS APCI+ m/z 332 [MH]+
50 H CH 1HNMR(CDCI3, 400MHz) b: 2.38(s, 33%
3H), 4.00(s, 3H), 6.90(d, 1 H),
7.35(d, 1 H), 7.40(dd, 1 H), 7.88(dd,
1 H), 8.06(d, 1 H), 8.31(d, 1 H)
MS APCI+ m/z 302/304 [MH]+
51 0/ CH HNMR(CDCI3, 400MHz) S: 3.33(s, 62%
3H), 3.48(m, 2H), 3.64(m, 2H),
3.99(s, 3H), 4.60(s, 2H), 6.86(d,
1H), 7.35(d, 1H), 7.60(dd, 1H),
7.88(dd, 1H), 8.09(d, 1H), 8.35(d,
1 H). MS APCI+ m/z 376/378 [MH]+
52 H Co CH HNMR(CDCI3, 400MHz) S: 1.14(t,
3c
3H), 3.50(q, 2H), 4.00(s, 3H),
4.52(s, 2H), 6.86(d, 1H), 7.34(d,
1H), 7.51(dd, 1H), 7.90(dd, 1H),
8.11(d, 1H), 8.36(d, 1H). MS
APCI+ m/z 346 [MH]+
Preparation 46 was purified by column chromatography on silica gel, eluting
with
ethyl acetate:pentane, 25:75 to 50:50 to 75:25.
Preparation 48 was purified by column chromatography on silica gel, eluting
with
dichloromethane:methanol:0.88 ammonia, 99:1:0.1 to 97:3:0.1, followed by
trituration with diethyl ether.
Preparation 49 was purified by column chromatography on silica gel, eluting
with
ethyl acetate:pentane, 10:90 to 100:0, followed by trituration with diethyl
ether.
Preparation 50 was purified by re-crystallisation from ethyl acetate.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-81-
Preparation 51 was purified by column chromatography on silica gel, eluting
with
dichloromethane:methanol:0.88 ammonia, 100:0:0 to 99:1:0.1.
Preparation 53
4-Bromo-2,3-dimethyl-pyridine 1-oxide
Br
h+" CH3 NCH3
1 _
O
A mixture of 2,3-dimethyl-4-nitropyridine N-oxide (5g, 29.7mmol) and hydrogen
bromide (30% wt in acetic acid, 100mL) was heated for 48 hours at 100 C. The
mixture was then filtered, washing through with 2M sodium hydroxide and the
filtrate was extracted with dichloromethane (x3). The combined organic
solutions
were washed with brine, dried over sodium sulfate and concentrated in vacuo.
The residue was purified by column chromatography on silica gel, eluting with
ethyl acetate:pentane, 50:50, to afford the title product as a pale yellow
solid.
1HNMR(CDC13, 400MHz) S: 2.44(s, 3H), 2.58(s, 3H), 7.30(d, 1 H), 8.01(d, 1 H)
MS APCI+ m/z 202/204 [MH]*
Preparation 54
4-(2,3-Dimethyl-1-oxy-pyridin-4-yl)-benzoic acid methyl ester
O
OCH3
/ \ I
_-N+
O
CH3
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-82-
A mixture of the product of preparation 53 (765mg, 3.78mmol),
4-meth'oxycarbonylphenylboronic acid (750mg, 4.16mmol), caesium carbonate
(3.7g, 11.34mmol) and the product of preparation 3 (50mg, cat.) in 1, 4-dioxan
(20mL) was heated at 110 C for 3 hours. The mixture was then partitioned
between ethyl acetate and water and the aqueous layer was separated and
extracted by dichloromethane (x3). The combined organic solutions were
washed with brine, dried over sodium suifate and concentrated in vacuo to give
a dark yellow solid. This solid was triturated with diethyl ether to afford
the title
compound as a pale brown solid in 87% yield.
1HNMR(CDCI3, 400MHz) b: 2.24(s, 3H), 2.60(s, 3H), 3.95(s, 3H), 7.02(d, 1H),
7.35(m, 2H), 8.12(m, 2H), 8.22(d, 1 H). MS APCI+ m/z 258 [MH]+
Preparation 55
4-(2,3-Dimethyl-l-oxy-pyridin-4_yl)-benzoic acid hydrazide
O
N--NH2
H
_-N+
O
CH3
CH3
A mixture of the product of preparation 54 (850mg, 3.3mmol) and hydrazine
monohydrate (482pL, 9.9mmol) in methanol (15mL) was heated under reflux for
3 hours. A further amount of hydrazine monohydrate (482pL, 9.9mmol) was then
added to the reaction mixture and heating continued for 18 hours. The mixture
was then filtered through Celite , washing through with methanol and the
filtrate
was concentrated in vacuo to give a white solid. The solid was slurried in
ethyl
acetate, filtered off, washed with diethyl ether (x2) and vacuum dried to
afford
the title compound as a white solid in 94% yield, 800mg.
' HNMR(CDCI3i 400MHz) 8: 2.18(s, 3H), 2.41(s, 3H), 4.58(m, 2H), 7.14(d, 1 H),
7.42(d, 2H), 7.96(d, 2H), 8.18(d, 1 H), 9.85(s, 1 H). MS APCI+ m/z 258 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-83- -
Preparation 56
1, 1, 1,2-Tetramethoxy-ethane
H3C
O
H3C-OO,
CH3
CH3
Methoxyacetonitrile (50.0g, 704mmol) was dissolved in a mixture of methanol
(34mL) and diethyl ether- (210mL) and the mixture cooled to 0 C. Hydrogen
chloride gas was bubbled through the solution for 20 minutes and the reaction
mixture was stirred at room temperature for 2 hours. Hydrogen chloride gas was
then bubbled through the mixture for a second time and it was allowed to stand
at room temperature for 18 hours. The mixture was filtered and the resulting
white solid was washed with diethyl ether, dissolved in methanol (340mL) and
stirred for 90 minutes. The solution was then diluted with ether (370mL),
heated
under reflux for 6 hours and left to stand at room temperature for 18 hours.
Additional ether (200mL) was added and the mixture was filtered off. The
filtrate
was washed with 10% sodium carbonate solution, dried over magnesium sulfate
and concentrated in vacuo to yield the title product, 34.5g.
iHNMR(CDCI3, 400MHz) 6: 3.29(s, 9H), 3.39(s, 3H), 3.50(s, 2H)
Preparation 57
4-f4-(5-Methoxymethyl-f 1,3,41oxadiazol-2- rl -phenyll-2,3-dimethyl-pyridine 1-
oxide
N-N
~.O-CH3
O
_,N+
O
CH3
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-84-
para-Toluenesulfonic acid (20mg, cat.) was added to a mixture of the products
of
preparations 55 (400mg, 1.56mmol) and 56 (470mg, 3.12mmol) in methanol
(8mL) and the mixture was heated under reflux for 10 hours. The mixture was
then treated with sodium hydrogen carbonate solution and the aqueous mixture
was extracted with ethyl acetate (x3). The combined organic solutions were
washed with brine, dried over sodium sulfate and concentrated in vacuo to
afford
the title product as a yellow oil in 25% yield, 122mg.
' HNMR(CDCI3a 400MHz) S: 2.25(s, 3H), 2.62(s, 3H), 3.52(s, 3H), 4.70(s, 2H),
7.04(d, 1 H), 7.41(d, 2H), 8.16(d, 2H), 8.25(d, 1 H). MS APCI+ m/z 312 [MH]+
Preparation 58
6-Chloro-pyridazine-3-carboxylic acid methyl ester
0
N~ \ O~CH3
CI
~ /
Oxalyl chloride (1.14mL, 13.09mmol), was added dropwise to an ice-cold
suspension of 6-chloro-pyridazine-3-carboxylic acid [(1.9g, 11.9mmol), J. Het.
Chem. 29(6), 1583-92; 1992] in a mixture of dichloromethane (50mL) and
N,N-dimethylformamide (1 drop) and the mixture was stirred for 1 hour at room
temperature. The reaction mixture was then evaporated under reduced pressure
and the residue was diluted with dichloromethane (30mL) and cooled to 0 C.
Methanol (485pL, 11.9mmol) was added and the mixture was stirred at 0 C for 1
hour. Sodium hydrogen carbonate solution was then added to the reaction
mixture and the aqueous layer was separated and extracted with
dichloromethane (x2). The combined organic solutions were washed with brine,
dried over sodium sulfate and concentrated in vacuo to afford the title
compound
as a white solid in 65% yield, 1.33g.
1 HNMR(CDCI3a 400MHz) S: 4.09 (s, 3H), 7.67(d, 1H), 8.16(d, 1H). MS APCI+
m/z 173 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-85-
Preparation 59
6-(4-Fluoro-2-methyl-phenyl)-pyridazine-3-carboxylic acid methyl ester
0
N~ _' OI-ICH3
F CH3
The title compound was prepared from the product of preparation 58 and
4-fluoro-2-methylphenyl boronic acid, using the method of preparation 54.
Purification of the crude product by column chromatography on silica gel,
eluting
with pentane:ethyl acetate:methanol, 75:25:1 to 50:50:1 afforded the desired
product as a beige solid in 16% yield.
' HNMR(CDCI3a 400MHz) S: 2.44(s, 3H), 4.11(s, 3H), 7.06(m, 2H), 7.47(dd, 1 H),
7.71 (d, 1 H), 8.26(d, 1 H). MS APCI+ m/z 247 [MH]+
Preparation 60
6-(4-Fluoro-2-methyl-phenyl)-pyridazine-3-carboxylic acid hydrazide
0
N _" N~NH2
H
F CH3
Hydrazine monohydrate (69pL, 1.42mmol) was added to a suspension of the
product of preparation 59 (290mg, 1.18mmol) in methanol (5mL) and the mixture
was stirred for 18' hours at room temperature. The resulting precipitate was
filtered off and dried to afford the title compound as a peach solid in 83%
yield,
240mg.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-86-
1HNMR(CDCI3, 400MHz) S: 2.42(s, 3H), 4.18(brs, 2H), 7.06(m, 2H), 7.46(dd,
1 H), 7.75(d, 1 H), 8.33(d, 1 H), 9.18(brs, 1 H). MS APCI+ m/z 247 [MH]+
Preparation 61
6-(4-Fluoro-2-methyl-phenLl)-pyridazine-3-carboxylic acid N'-(2-methox
y-acet Ir)-hydrazide
0
H
\
N N~N0~CH3
H
O
I /
F CH3
The title compound was prepared from the product of preparation 60 and
methoxyacetyl chloride, using the method of preparation 8, as a beige foam in
95% yield.
1 HNMR(CDCI3, 400MHz) S: 2.43(s, 3H), 3.52(s, 3H), 4.14(s, 2H), 7.06(m, 2H),
7.46(m, 1 H), 7.76(d, 1 H), 8.33(d, 1 H), 8.80(brs, 1 H), 10.06(brs, 1 H). MS
APCI+
m/z 319 [MH]+
Preparation 62
3-(4-Fluoro-2-methyl-phenyl)-6-(5-methoxymethyl-f 1,3,41oxadiazol-2-Ll)-
pyridazine
N'N
N
~O-CH3
\ I o 20 F CH3
The title compound was prepared from the product of preparation 61 and
phosphorous oxychloride, using the method of preparation 13. The crude
product was purified by column chromatography on silica gel, eluting with
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-87-=
dichloromethane:methanol, 99:1 to 98:2, to afford the desired compound as a
beige solid in 15% yield.
1HNMR(CDCI3, 400MHz) S: 2.43(s, 3H), 3.53(s, 3H), 4.80(s, 2H), 7.06(m, 2H),
7.46(m, 1 H), 7.74(d, 1 H) 8.43(d, 1 H). MS APCI+ m/z 301 [MH]+
Preparation 63
5-Bromo-pyrimidine-2-carbonitrile
N
N
Br
A solution of 5-bromo-2-chloropyrimidine (10g, 51.8mmol) in dimethylsulfoxide
(26mL) was added to a mixture of sodium cyanide (2.59g, 51.8mmol) and
triethylenediamine (1.2g, 10.4mmol) in dimethylsulfoxide (14mL) and water
(28mL). The resulting mixture was stirred for 18 hours at room temperature.
The
mixture was then diluted with water (130mL) and extracted with diethyl ether
(3xl5OmL). The combined organic solutions were dried over sodium sulfate and
concentrated in vacuo to give a pale yellow solid. Re-crystallisation of the
solid
from hot dichloromethane afforded the title product in 99% yield, 9.4g.
iHNMR(CDCI3, 400MHz) 8: 8.84 (s, 2H).
Preparation 64
5-Bromo-pyrimidine-2-carboxylic acid
/ N"0
Br
N O-H
A mixture of sodium hydroxide (4.88g, 120mmol) and the product of preparation
63 (7.5g, 40.8mmol) in water (122mL) was heated at 60 C for 1 hour. The
mixture was then acidified with 1 M hydrochloric acid, extracted with ethyl
acetate
and dichloromethane and concentrated in vacuo to afford some title compound
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-88-
as a white solid, 300mg. The aqueous solution was also evaporated under
reduced pressure and the residue was extracted into dichloromethane:methanol,
90:10. The precipitate was filtered off and the filtrate was concentrated in
vacuo
to afford further title compound as a white solid, 5.5g.
' HNMR(DMSO-D6, 400MHz) S: 9.10(s, 2H), 13.8(brs, 1H). MS APCI+ m/z 203
[MH]+
Preparation 65
5-Bromo-pyrimidine-2-carboxylic acid methyl ester
/ NO
Br
O CH3
Fuming hydrochloric acid was passed through an ice-cooled solution of the
product of preparation 64 (5.5g, 27mmol) in methanol (5OmL) until saturated.
The reaction mixture was warmed to room temperature and was stirred for 18
hours. The solvent was then evaporated under reduced pressure and the
residue was dissolved in dichloromethane, washed with water and sodium
hydrogen carbonate solution, dried over magnesium sulfate and concentrated in
vacuo to afford the title compound as yellow solid in 57% yield, 3.5g.
'HNMR(CDC13, 400MHz) 5: 4.05(s, 3H), 9.00(s, 2H). MS APCI+ m/z 218 [MH]+
Preparation 66
5-Bromo-pyrimidine-2-carboxylic acid hydrazide
~N O
Br-(' ~
~N H_NH2
The title compound was prepared from the product of preparation 65 and
hydrazine monohydrate, using the method of preparation 6, as a yellow solid in
quantitative yield.
1HNMR(CDCI3, 400MHz) 5: 4.20(s, 2H), 8.90(m, 3H). MS APCI+ m/z 217 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-89-
Preparation 67
5-Bromo-pyrimidine-2-carboxylic acid N'-(2-methoxy-acetyl)-hydrazide
~ N~O ~---~ -
Br
N H-H O CH3
The title compound was prepared from the product of preparation 66 and
methoxyacetyl chloride, using the method of preparation 8, as a white solid in
45% yield.
'HNMR(DMSO-D6i 400MHz) S: 3.32(s, 3H), 3.98(s, 2H), 9.20(s, 2H), 10.02(s,
1 H), 10.66 (s, 1 H). MS APCI+ m/z 290 [MH]+
Preparation 68
5-Bromo-2-(5-methoxymethyl-f 1 3,4]oxadiazol-2-yl)-pyrimidine
N-N
N
I O
Br ~ N O--CH
3
The title compound was prepared from the product of preparation 67, using the
method of preparation 13. The crude product was purified by column
chromatography on silica gel, eluting with dichloromethane:methanol, 99.5:0.5
to
99:1, to afford the desired compound as a white solid in 45% yield.
'HNMR(CDCI3i 400MHz) S: 3.52(s, 3H), 4.78(s, 2H), 9.02(s, 2H). MS APCI+ m/z
271 [MH]+
Preparation 69
5-(4-Fluoro-2-methyl-phenyi)-2-(5-methoxymethyl-[1,3 4loxadiazol-2-yl)-
pyrimidine
N-N
N
= ~ I O
N O--CH
F 3
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-90-
solution of sodium carbonate (295mg, 2.78mmol) in water (3mL) was added to
A
a solution of the product of preparation 68 (376mg, 1.39mmol), 4-fluoro-2-
methylphenyl boronic acid (320mg, 2.08mmol) and palladium triphenylphosphine
(48mg, cat) in 1,2-dimethoxyethane (3mL) and the mixture was heated under
reflux for 2 hours. The reaction mixture was then evaporated under reduced
pressure and the residue was partitioned between water and ethyl acetate. The
resulting precipitate was filtered off, washing through with water, ethyl
acetate
and diethyl ether, and dried to afford the title compound as a beige solid in
59%
yield, 332mg.
iHNMR(CDCI3i 400MHz) S: 2.33(s, 3H), 3.53(s, 3H), 4.81(s, 2H), 7.09(m, 2H)
7.25(m, 1 H), 8.90(s, 2H). MS APCI+ m/z 301 [MH]+
Preparation 70
5-Bromo-2-(5-methyl-r1 3 4loxadiazol-2-yl)-pyridine
`
Br
N CH3
The title compound was prepared from the product of preparation 6 and
N,N-dimethylacetamide dimethyl acetal, using the method of preparation 4, as a
white solid in 47% yield.
' HNMR(CDCI3i 400MHz) S: 2.65(s, 3H), 8.01(m, 1 H), 8.12(m, 1 H), 8.80(m, 1
H).
MS APCI+ m/z 240/242 [MH]+
Preparation 71
3-f3-(5-Bromo-pyridin-2-yl)-5-methyl-[1,2 4ltriazol-4-yll-2,6-dimethoxy-
pyridine
N-N
Br / D ~
NN CH3
CH3
N
H3L.,0
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-91-
3-Amino-2,6-dimethoxypyridine monohydrochloride (2g, 13mmol) was partitioned
between sodium carbonate solution and ethyl acetate. The organic layer was
separated, washed with brine, dried over sodium sulfate and concentrated in
vacuo to afford the free base. The base was then dissolved in xylene (30mL)
and the product of preparation 70 (1.8g, 7.5mmol) and para-toluenesulfonic
acid
(50mg, cat) were added. The resulting mixture was heated under reflux for 18
hours. The reaction mixture was concentrated in vacuo and the residue purified
by column chromatography on silica gel eluting with dichloromethane:methanol
100:0 to 98:2, to afford the title product as a purple solid in 23% yield,
628mg.
1 HNMR(CDCI3, 400MHz) S: 2.49(s, 3H), 3.80(s, 3H), 3.98(s, 3H), 6.41(d, 1H),
7.45(d, 1 H), 7.92(dd, 1 H), 8.11 (d, 1 H), 8.37(d, 1 H). MS APCI+ m/z 377
[MH]+
Preparation 72
5-(4-Fluoro-2-methyl-phenyl)-2-(5-methy[-f 1,3,4]oxadiazol-2-yl)-pyridine
N-N
o ~
~CH
N
F i
CH3
A mixture of the product of preparation 40 (545mg, 2.79mmol), 4-fluoro-2-
methylphenyl boronic acid (643mg, 4.18mmol), caesium carbonate (2.7g,
8.29mmol) and the product- of preparation 3(10mg, cat.) in 1,4-dioxan (25mL)
was heated under reflux for 4 hours. The reaction mixture was then cboled to
room temperature, filtered through Celite and concentrated in vacuo to afford
the title compound as a pale yellow solid in quantitative yield.
'HNMR(CDCI3, 400MHz) 6: 2.40(s, 3H), 2.66(s, 3H), 7.00(m, 2H), 7.43(dd, 1H),
7.53(d, 1 H), 8.37(d, 1 H) 9.29(d, 1 H). MS APCI+ m/z 270 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-92-
Preparations 73 to 81
The following compounds of the general formula shown below were prepared in
quantitative yield, by the method of preparation 72, using the appropriate
oxadiazole (preparations 14-16) and boronic acid.
The progress of the reactions was monitored by tic analysis and the mixtures
were heated under reflux until all of the starting materials had been
consumed.
Y N-N
R/ R2
N 0~ ~~
No. R R Y Data
73 H N HNMR(CDCI3i 400MHz) S: 2.29(s,
( CCH3H), 2.37(s, 3H), 2.70(s, 3H), 7.21-
CH3 7.34(m, 3H), 8.81(d, 1 H), 9.51(d, 1 H).
MS ES+ m/z 289 [MNa]+
74 H N HNMR(CDCI3i 400MHz) 8: 2.46(s,
F CH 3H), 2.70(s, 3H), 7.06(m, 2H), 7.50(m,
3
1 H), 8.82(d, 1 H), 9.49(d, 1 H). MS ES+
m/z 293 [MNa]+
75 OCH3 CH HNMR(CDCI3, 400MHz) 8: 2.41(s,
F CH 3H), 3.52(s, 3H), 4.75(s, 2H), 6.94-
3
7.04(m, 2H), 7.44(dd, 1 H), 7.55(dd,
1 H), 8.42(d, 1 H), 9.35(dd, 1 H). MS
APCI+ m/z 300 [MH]+
76 I~ CH OCH3 N HNMR(CDCI3, 400MHz) S: 2.46(s,
F 3H), 3.53(s, 3H), 4.79(s, 2H), 7.06(m,
3
2H), 7.51(m, 1 H), 8.84(d, 1 H), 9.51(d,
1 H). MS APCI+ m/z 301 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-93-
77 OCH3 N HNMR(CDCI3, 400MHz) S:
2.40(d, 3H), 3.52(s, 3H), 4.51(s, 2H),
F 7.23-7.3Q(m, 3H), 9.06(s, 1 H), 9.40(s,
CH3 1 H)
MS ES+ m/z 433 [MCs]+
78 ~ H3 F OCH3 N 1HNMR(CDCI3, 400MHz) S:
u 3.78(s, 3H), 4.09(s, 3H), 4.80(s, 2H),
7.01-7.18(m, 2H), 7.54-7.72(m, 1 H),
9.26(s, 1 H), 9.57(s, 1 H)
MS ES+ m/z 449 [MCs]+
79 0 I.ICH3 OCH3 N HNMR(CDCI3, 400MHz) S: 2.37(s,
3H), 3.52(s, 3H), 3.89(s, 3H), 4.78(s,
2H), 6.95(d, 1 H), 7.25(m, 1 H), 7.77(m,
1 H), 9.35(d, 1 H), 9.49(d, 1 H). MS ES+
CH3 m/z 445 [MCs]+
80 0 .11CH3 OCH3 N HNMR(CDCI3, 400MHz) S: 3.52(s,
3H), 3.91(s, 3H), 4.78(s, 2H), 6.99(m,
1 H), 7.17(m, 1 H), 7.77(m, 1 H), 9.41(d,
1 H), 9.50(d, 1 H). MS ES+ m/z 461
F [MCs]+
81 CH3 OCH3 N 1HNMR(CDCI3, 400MHz) S: 2.39(s,
~ 3H), 2.40(s, 3H), 3.53(s, 3H), 4.79(s,
2H), 7.03-7.41(m, 2H), 8.86(s, 1 H),
CH3 9.52(d, 1 H). MS ES+ m/z 429 [MCs]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-94-
Preparation 82
Methyl-(5-nitro-pyridin-2-yl)-amine
O-Z~-N+.O
N
~NH
H3C
Methylamine gas was bubbled through a stirred solution of 2-chloro-5-
nitropyridine (4g, 25.2mmol) in dichloromethane (60mL), at room temperature,
until saturation had occurred. The resulting yellow precipitate was then
filtered
off, washed with dichloromethane and dried under vacuum to afford the title
compound as a yellow solid in 80% yield, 3.07g.
'HNMR(CDCI3, 400MHz) S: 3.05(d, 3H), 5.41(bs, 1H), 6.37(d, 1H), 8.21(d, 1H),.
9.03(d, 1 H).
MS APCI+ m/z 154 [MH]+
Preparation 83
N*2*-Methyl-pyridine-2,5-diamine
NH2
N
~NH
H3C
The product of preparation 82 (3.0g, 19.5mmol) and 10% Pd/C (300mg, cat.)
were stirred in ethanol (150mL) under 60psi of hydrogen gas for 18 hours. The
reaction mixture was then filtered through Celite and the filtrate was
concentrated in vacuo to afford the title product in 17% yield, 400mg.
1HNMR(CDCI3, 400MHz) S: 2.85(s, 3H), 3.19(s, 2H), 4.11(s, 1 H), 6.31(d, 1 H),
6.97(dd, 1 H), 7.69(d, 1 H). MS APCI+ m/z 269 [MNa]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-95- Preparation 84
4,6-Dichloro-nicotinic acid methyl ester
CI O
llz~ O~CH3
CI N
3-Pyridinecarboxylic acid ~(27g, 160mmol), J.Het. Chem, 20, 1363; 1983] was
added portionwise to phosphorus oxychloride (180mL) and the mixture was
heated under reflux for 7 hours and stirred at room temperature for 18 hours.
The mixture was then concentrated in vacuo to a low volume and the residue
was quenched with water. The aqueous mixture was neutralised with sodium
hydrogen carbonate soiution and extracted with chloroform (3x150mL). The
combined organic solutions were washed with brine, dried over sodium sulfate
and concentrated in vacuo to afford the titie compound as a red oil in 83%
yield,
27.2g.
1HNMR(CDCI3, 400MHz) S: 3.96(s, 3H), 7.47(s, 1 H), 8.85(s, 1 H). MS APCI+ m/z
206 [M H]+
Preparations 85 and 86
Sodium methoxide (0.5M in methanol, 48.6mL, 24.3mmol) was added dropwise
to an ice-cold solution of the product of preparation 84 (5.0g, 24.3mmol) in
methanol (20mL). The mixture was allowed to warm to room temperature and
was stirred for 2 hours. The soivent was then evaporated under reduced
pressure and the residue was partitioned between water (5OmL) and chloroform
(50mL). The layers were separated and the aqueous layer was extracted with
chloroform (2 x 75mL). The combined organic solutions were then dried over
sodium sulphate and concentrated in vacuo to give an orange oil. The oil was
purified by column 'chromatography on silica gel, eluting with dichloromethane
(100%) to afford the product of preparation 85 as a white solid in 7.6% yield,
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-96-
370mg. Further elution with dichloromethane then isolated the product of
preparation 86 as a white solid in 27% yield, 1.32g.
Preparation 85
4-Chloro-6-methoxy-nicotinic acid methyl ester
CI 0
OCH3
H3C"1
O N
' HNMR(CDCI3, 400MHz) S: 3.90(s, 3H), 3.96(s, 3H), 6.81(s, 1H), 8.71(s, 1H).
MS APCI+ m/z 202 [MH]+
Preparation 86
6-Chloro-4-methoxy-nicotinic acid methyl ester
H3C~0 O
OI-ICH3
CI N
'HNMR(CDCI3, 400MHz) S: 3.87(s, 3H), 3.94(s, 3H), 6.90(s, 1H), 8.68(s, 1H).
MS APCI+ m/z 202 [MH]+
Preparation 87
6-Chloro-4-methoxy-nicotinic acid hydrazide
H3C~0 0
N
H
CI N
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-97-
Hydrazi,ne monohydrate (690pL, 14.2mmol) was added to a suspension of the
product of preparation 86 (2.7g, 13.4mmol) in methanol (35mL), cooled to -5 C,
and the mixture was stirred for 3 hours. The mixture was then warmed to room
temperature and was stirred for 18 hours. The resulting precipitate was
filtered
off and dried to afford some title compound as a white solid, 990mg. TIc
analysis
of the filtrate showed that not all of the starting material had been
corisumed and
so further hydrazine monohydrate (267pL, 5.51 mmol) was added and the
reaction mixture was stirred for 24 hours. The resulting precipitate was
collected
by filtration and dried to afford a further crop of title compound as a yellow
solid,
832mg.
'HNMR(CDC13, 400MHz) b: 3.92(s, 3H), 4.55(bd, 2H), 7.29(s, 1 H), 8.37(s, 1 H),
9.38(m, 1 H). MS APCI+ m/z 247 [MH]+
Preparation 88
6-Chloro-4-methoxy-nicotinic acid N'-(2-methoxy-acet rl -hydrazide
H30I_I 0 O
H
O~CH3
H
O
CI N
Methoxyacetyl chloride (733 L, 8.02mmol) was added to an ice-cold suspension
of the product of preparation 87 (1.16g, 5.73mmol) in dichloromethane (20mL)
and triethylamine (1.2mL, 8.61 mmol) and the mixture was stirred for 18 hours
at
room temperature. The reaction mixture was then washed with water and brine,
dried over sodium sulphate and concentrated in vacuo to give a pale yellow
gum.
The gum was purified by column chromatography on silica gel, eluting with
dichloromethane:methanol, 100:0:0 to 95:5, to afford the title compound as a
clear glass in 31 % yield, 480mg.
iHNMR(CDCI3, 400MHz) b: 3.48(s, 3H), 4.08(s, 3H), 4.10(s, 2H), 6.96(s, 1H),
9.03(s, 1 H), 9.25(d, 1 H), 9.99(d, 1 H). MS APCI+ m/z 274/276 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-98-
Preparation 89
2-Chloro-4-methoxy-5-(5-methoxymethyl-[1,3,41oxadiazol-2-yl)_pyridine
H3C,~, 0 N--N
-\o
I O _CH3
CI N
The title compound was prepared form the product of preparation 88 and
phosphorus oxychloride, using the method of preparation 13, as a yellow oil in
quantitative yield.
' HNMR(CDCI3, 400MHz) 8: 3.49(s, 3H), 4.04(s, 3H), 4.73(s, 2H), 7.01(s, 1H),
8.83(s, 1 H). MS APCI+ m/z 256 [MH]+
Preparation 90
2-Chloro-4-methoxy-5-[5-methoxymethyl-4-(6-methoxy-pyridin-3-yl)-4H-[1
,2,41triazol-3-yll-pyridine
H3C~0 N-'"N
N` C CH3
CI N
N
/0
H3C
The title compound was prepared from the product of preparation 89 and
5-amino-2-methoxypyridine, using the method of preparation 18, as a pale
yellow foam in 29% yield.
iHNMR(CDCI,, 400MHz) S: 3.37(s, 3H), 3.61(s, 3H), 3.93(s, 3H), 4.49(s, 2H),
6.76(m, 2H), 7.43(dd, 1 H), 7.98(d, 1 H), 8.42(s, 1 H). MS APCI+ m/z 362 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-99-
Preparation 91
5-(4-Fluoro-2-methyl-phenyl)-pyrazine-2-carboxylic acid methyl ester
O
N
C~,CH3
N
F ~
CH3
4-Fluoro-2-methylphenyl boronic acid (17.36g, 112.7mmol), caesium carbonate
(70.8g, 216.8mmol) and the product of preparation 3 (1.5mg, 8.67mmol.) were
added to a solution of 5-chloropyrazine-2-methylcarboxylate (15g, 86.7mmol) in
1,4-dioxan (2L) and the mixture was heated under reflux for 2 hours. The
reaction mixture was then filtered and concentrated in vacuo to afford the
title
compound in 99% yield, 21.33g.
1 HNMR(CDCI3i 400MHz) b: 2.40(s, 3H), 4.05(s, 3H), 7.05(m, 2H), 7.45(m, 1 H),
8.80(1, 1 H), 9.35(s, 1 H). MS APCI+ m/z 247 [MH]+
Preparation 92
5-(4-Fluoro-2-methyl-phenyl)-pyrazine-2-carboxylic acid hydrazide
0
N
N--NH2
H
N
F ~
CH3
A mixture of the product of preparation 91 (42.5g, 172.8mmol) and hydrazine
monohydrate (9.46mL, 207.3mmol) in methanol (600mL) was heated under
reflux for 30 hours. The reaction mixture was then cooled to room temperature
and the precipitate was filtered off and dried in vacuo to afford the title
compound in 75% yield, 7.10g.
1 HNMR(DMSO, 400MHz) b: 2.35(s, 3H), 4.60(s, 2H), 7.20(m, 2H), 7.60(m, 1H),
8.80(s, 1 H), 9.15(s, 1 H), 10.2(s, 1 H).
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-100-
Examples 1 - 4
N-N
~ >-CH3
N
R1
O- L.Ha
The bromo compound of preparation 5 (100mg, 0.29mmol), the palladium
complex of preparation 3 (10mg, cat.), caesium carbonate (440mg, 1.35mmol)
and the appropriate boronic acid (0.73mmol) were suspended in 1,4-dioxan
(5mL) and the reaction mixture heated to 120 C for 90 minutes. Additional
1,4-dioxan (4mL) was added and the reaction mixture heated to 100 C for a
further 4 hours. The reaction mixture was filtered under vacuum, washing
through with dichloromethane. The filtrate was concentrated in vacuo and the
residue purified by column chromatography on silica gel eluting with
dichloromethane:methanol:0.88 ammonia 95:5:0.5 to yield the desired product.
No. R Data Yield
1 F MS ES+ m/z 390 [MH]+ 9%
( 1 \
/CCH3
3
2 CH3 1HNMR(CDCI3, 400MHz) 8: 2.01(s, 3H), 10%
H3C 2.22(s, 3H), 2.26(s, 3H), 3.75(s, 3H),
/ 6.87(d, 1 H), 7.02(m, 4H), 7.20(m, 2H),
7.24(m, 2H), 7.39(m, 2H)
MS APCI+ m/z 370 [MH]+
3 CH3 MS APCI+ m/z 370 [MH]+ 36%
CH3
4 F 1HNMR CDCI3,
I~ ( 400MHz) b: 2.31(s, 3H), 52%
H3c,0/ 3.79(s, 3H), 3.83(s, 3H), 7.02(m, 3H),
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-101-
7.27(m, 2H),'.7.34(d, 2H), 7.41(d, 2H),
7.57(d, 2H).
MS APCI+ m/z 390 [MH]+
Example 5
2-(4-Fluoro-2-methylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl) -4H-
j1,2,41triazol-3-yl)-pyridine
N-N o-cH
1 3
CH3 I \ N
\ N
N
F
C.Hs
The title product was prepared by the method of preparation 18 using the
product of preparation 19 and 5-amino-2-methoxypyridine. 140mg, 15% yield of
the desired product was produced.
1 HNMR(CDCI3, 400MHz) 8: 2.36(s, 3H), 3.38(s, 3H), 4.01(s, 3H), 4.51(s, 2H),
6.88(d, 1 H), 6.93-7.00(m, 2H), 7.36(dd, 1 H), 7.40(d, 1 H), 7.56(dd, 1 H),
8.00(dd,
1 H), 8.15(d, 1 H), 8.64(d, 1 H). Microanalysis: C22H20FN502 requires; C
65.18, H
4.97, N 17.27; found C 65.01, H 4.96, N 17.27. MS APCI+ m/z 406 [MH]+
Example 6
2-(2,3-Dimethylph6nyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
L1,2,41triazol-3-yl)-pyridine
' ~~ ,O-~CH3
~
CH3 N~ -
H3C
&N~
N
~~CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-102-
The title product was prepared by the method of preparation 18 using the
product of preparation 20 and 5-amino-2-methoxypyridine. 325mg, 36% of the
desired product was produced.
1HNMR(CDCI3, 400MHz) S: 2.21(s, 3H), 2.34(s, 3H), 3.38(s, 3H), 4.00(s, 3H),
4.52(s, 2H), 6.88(d, 1H), 7.13-7.22(m, 3H), 7.40(d, 1H), 7.55(d, 1H), 7.99(dd,
1H), 8.16(d, 1H), 8.64(d, 1 H). Microanalysis: C23H23N502Ø1 H20 requires; C
68.50, H 5.80, N 17.37; found C 68.24, H 5.90, N 17.05. MS APCI+ mlz 402
[MH]+
Example 7
5-(4-Fluoro-2-methylphenyl)-2-(((5-methoxymethyl-4-(6-methoxypyridin-3-yl))-
4H-f 1,2,41triazol-3-yl)-pyridine
i-N O-CH3
CH3 I \ N ---
~ iN
I / N
F
0- CH3
The bromo compound of preparation 18 (250mg, 0.66mmol), 2-methyl-4-fluoro-
phenylboronic acid (235mg, 1.53mmol), the palladium complex of preparation 3
(10mg, cat.) and caesium carbonate (1.OOg, 3.07mmol) were added to
1,4-dioxan (1 5mL) and the reaction mixture heated to 110 C for 4 hours. The
reaction mixture was filtered through Arbocel , washed through with
dichloromethane and the filtrate concentrated in vacuo. The residue was
purified
by column chromatography on silica gel eluting with dichloromethane:methanol
100:0 to 95:5 to yield the title product, 128mg, 48% yield as a pale pink
solid.
1 HNMR(DMSO-D6, 4001VIHz) b: 2.21(s, 3H), 3.18(s, 3H), 3.89(s, 3H), 4.44(s,
2H),
6.94(m, 1 H), 7.09(m, 1 H), 7.19(m, 1 H), 7.28(m, 1 H), 7.81(m, 1 H), 7.94(m,
1 H),
8.15(m, 1 H), 8.23(m, 1 H), 8.32(m, 1 H).
MS APCI+ m/z 406 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-103-
Example 8
5-(2 3-Dimethylphen rl -2-f5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-
1,2,4-
triazol-3-yllpyridine
I- ~O--CH3
CH3 N
H3C \ ~ N
( N
/
O`CH3
The title product was prepared by the method of example 7 using
2,3-dimethylphenylboronic acid. 132mg, 49% yield of the desired product was
prepared as a pale pink solid.
' HNMR(DMSO-D6i 400MHz) 8: 2.07(s, 3H), 2.38(s, 3H), 3.17(s, 3H), 3.89(s, 3H),
4.44(s, 2H), 6.94(m, 1 H), 7.04(m, 1 H), 7.15(m, 1 H), 7.22(m, 1 H), 7.82(m, 1
H),
7.90(m, 1 H), 8.15(m, 1 H), 8.24(m, 1 H), 8.28(m, 1 H). MS APCI+ m/z 402 [MH]+
Example 9
1-f5-f5-(2 3-Dimethylphenyi)-pyridin-2-yl1-4-(6-methoxypyridin-3-yi)-4H-
L1 2 4ltriazol-3-ylmethyll-pyrrolidine-(2S)-2-carboxylic acid amide
H3C C H N" N
~~N
-N N
HZN
O
ON\
0
CH3
The bromo compound of preparation 27 (125mg, 0.27mmol), 2,3-dimethylphenyl
boronic acid (61 mg, 0.41 mmol) and the palladium complex of preparation 3
(10mg) were dissolved in 1,2-dimethoxyethane (4mL) and the solution was
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-104-
treated with sodium carbonate (58mg, 0.55mmol). The reaction mixture was
heated to reflux for 1 hour and then concentrated in vacuo. The residue was
taken up in ethyl acetate (25mL) and washed with water (25mL), 2M sodium
hydroxide solution (25mL) and brine (25mL). The solution was dried over
magnesium sulfate and concentrated in vacuo. The residue was purified by
column chromatography on silica gel eluting with
dichloromethane:methanol:0.88 ammonia 100:0:0 to 97:3:0.3 to yield the title
product, 95mg, 72% yield.
1HNMR(CDCI3, 400MHz) S: 1.80(m, 2H), 2.00(m, 1 H), 2.10(s, 3H), 2.20(m, 1 H),
2.40(s, 3H), 2.60(m, 1 H), 3.20(m, 2H), 3.80(m, 2H), 4.00(s, 3H), 5.00(s, 1
H),
6.80(s, 1 H), 6.90(d, 1 H), 7.00(d, 1 H), 7.10(d, 1 H), 7.20(d, 1 H), 7.60(d,
1 H),
7.70(d, 1 H), 8.80(s, 1 H), 8.20(m, 2H). MS ES+ m/z 484 [MH]+
Example 10
5-(2, 3-Dimethylphe ny)-2-(5-pyrrolidi n-1-vlmethyl-4-(6-methoxypyridin-3-yl)-
4H-
f 1,2,41triazol-3-Lrl)-pyridine
H3C CH3
`
N Y N
O-N
0
CH3
80mg, 38% yield of the title product was prepared by the method of example 9
using the bromo compound of preparation 28.
1HNMR(CDCI3i 400MHz) b: 1.80(s, 4H), 2.10(s, 3H), 2.40(s, 3H), 2.50(s, 4H),
3.70(s, 2H), 4.00(s, 3H), 6.80(d, 1H), 7.00(d, 1H), 7.20(m, 2H), 7.70(t, 2H),
8.10(d, 1 H), 8.20(d, 1 H), 8.30(s, 1 H). MS ES+ m/z 441 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-105-
Example 11
2-(4-Fluoro-2-methylphenyl)-5-f 5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
f 1,2,4ltriazol-3-yll-pyrazine
CH3
F N-N
~
CH3
H3C-O
The product of preparation 22 (450mg, 1.50mmol), para-toluenesulfonic acid
monohydrate (30mg) and 5-amino-2-methoxypyridine (205mg, 1.65mmol) were
added to xylene (8mL) and the reaction mixture heated to 145 C for 18 hours.
The reaction mixture was concentrated in vacuo and the residue purified by
column chromatography on silica gel eluting with
dichloromethane:methanol:0.88 ammonia 100:0:0 to 99.5:0.5:0.05 to 99:1:0.1 to
yield the title product, 300mg, 49% yield as a green solid.
'HNMR(CDCI3, 400MHz) 8: 2.38(s, 3H), 3.34(s, 3H), 3.99(s, 3H), 4.50(s, 2H),
6.85(d, 1 H), 6.95-7.03(m, 2H), 7.36-7.42(m, 1 H), 7.60(dd, 1 H), 8.12(d, 1
H),
8.40(s, 1 H), 9.48(s, 1 H). MS APCI+ m/z 407 [MH]}
Example 12
2-(2 3-Dimethylphenyl)-5-f5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
j1,2,41triazol-3-yll-pyrazine
H3C CH3 N N-
N
NO,
CH3
ONX/
H3C-O
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-106-
42mg, 7% yield of the title product was prepared by the method of example 11,
using the product of preparation 21.
'HNMR(CDCI3i 400MHz) S: 2.23(s, 3H), 2.34(s, 3H), 3.36(s, 3H), 4.00(s, 3H),
4.50(m, 2H), 6.86(d, 1 H), 7.19(d, 1 H), 7.20(s, 1 H), 7.24(m, 1 H), 7.62(dd,
1 H),
8.14(d, 1 H), 8.41(d, 1 H), 9.49(d, 1 H). MS APCI+ m/z 403 [MH]+
Example 13
2-(2,3-Dimethylphenyl)-5-r4-(6-methoxypyridin-3-yl)-5-methyl-4H-(1,2,41triazol-
3-
yll-pyrazine
H3C CH3
N-
N
~
N N~CH3
ONX/
H3C-O
The title product was prepared by the method of example 11 using the product
of preparation 23. 14mg, 3% of the desired product was produced as a white
solid.
1HNMR(CDCI3i 400MHz) S: 2.22(s, 3H), 2.34(s, 3H), 2.39(s, 3H), 4.01(s, 3H),
6.88(d, 1 H), 7.18-7.25(m, 3H), 7.52(dd, 1 H), 8.09(d, 1 H), 8.37(s, 1 H),
9.49(s,
1 H). MS APCI+ m/z 373 [MH]+
Example 14
2-(4-Fluoro-2-methylphenyl)-5-[4-(6-methoxypyridin-3-yl)-5-methyl-4H-
[1,2,41triazol-3-yll-pyrazine
CH3
F ~ N-N
- 0 CH3
~
~ N
H3C-O
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-107-
The chloro compound of preparation 30 (800mg, 2.60mmol), 4-fluoro-2-
methylphenyl boronic acid (470mg, 3.12mmol), the palladium complex of
preparation 3 (5mg) and caesium carbonate (2.50g, 7.90mmol) were added to
1,4-dioxan (80mL) and the reaction mixture heated to reflux for 2 hours. The
mixture was filtered through a filter tube and then filtered through a pad of
silica
eluting with dichloromethane:methanol 96:4. The filtrate was concentrated in
vacuo and purified by column chromatography on silica gel, eluting with
dichloromethane:methanol 100:0 to 95:5 to -afford the title product as a white
solid in 66% yield, 646mg.
' HNMR(CDCI3, 400MHz) 8: 2.39(s, 3H), 2.41(s, 3H), 4.01(s, 3H), 6.89(d, 1 H),
6.95-7.03(m, 2H), 7.38(dd, 1 H), 7.54(dd, 1 H), 8.09(d, 1 H), 8.38(d, 1 H),
9.49(d,
1 H). MS APCI+ m/z 377 [MH]+
Alternative method
Dimethylacetamide dimethylacetal (28mL, 192.1 mmol) was added to a
suspension of the product of preparation 92 (31.5g, 127.9mmol) in glacial
acetic
acid (315mL) and the mixture was heated at 60 C for 5 hours. 5-Amino-2-
methoxy pyridine (23.9g, 192mmol) was added and the mixture was heated at
100 C for a further 6 hours. The mixture was then cooled to room temperature
and evaporated under reduced pressure. The residue was taken up in
dichloromethane (750mL) and washed with saturated sodium hydrogen
carbonate solution (1 L). The organic solution was dried over magnesium
sulfate
and concentrated in vacuo. Re-crystallisation of the residue from hot acetone
then afforded the title compound as a white solid in 31 % yield, 14.81 g.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-108-
Example 15
2-(4-Cyano-2-methylphenLrl)-5-f4-(6-methoxypyridin-3- rl -5-methyl-4H-
f 1,2,41triazol-3-yil-pyrazine
CH3
N
NC N~I
N-~ NCH3
ONX/
H3C-O
The title product was prepared by the method of example 14 using the chloro
compound of preparation 30 (200mg, 0.66mmol) and the product of preparation
29 (240mg, 0.99mmol). 68mg, 27% yield of the title product was prepared as a
white solid.
1 HNMR(CDCI3i 400MHz) S: 2.40(s, 3H), 2.43(s, 3H), 4.01(s, 3H), 6.90(d, 1H),
7.50-7.55(m, 2H), 7.57(s, 1 H), 7.60(s, 1 H), 8.08(d, 1 H), 8.41(d, 1 H),
9.55(d, 1 H).
Microanalysis: C21 H17N700.1 H20 requires; C 65.79, H 4.47, N 25.57; found C
65.23, H 4.48, N 25.09. MS APCI+ m/z 384 [MH]+
Example 16
2-(5-Fluoro-2-methoxyphenyl)-5-f4-(6-methoxypyridin-3-yl)-5-methyl-4H-
f 1,2,41triazol-3-yll-pyrazine
F
N-
N
N_ ACH3
O
_..--
.
H3C
~
~ N
H3C-O
The title product was prepared by the method of example 14 using the chloro
compound of preparation 30 (200mg, 0.66mmol) and 5-fluoro-2-methoxyphenyl
boronic acid (168mg, 0.99mmol). 150mg, 58% yield of the title product was
prepared as a cream solid.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-109-
' HNMR(CDCI3, 400MHz) S: 2.37(s, 3H), 3.85(s, 3H), 4.00(s, 3H), 6.88(d, 1 H),
6.92(dd, 1 H), 7.09(m, 1 H), 7.50(dd, 1 H), 7.68(dd, 1 H), 8.07(d, 1 H),
8.93(d, 1 H),
9.46(d, 1 H).
Example 17
2-(4-Cyano-2-methylphenyl)-5-(5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
j1,2,41triazol-3-yll-pyrazine
CH3
N
NC 6- L ~ ___/N`N
N \
CH3
H3C-o
The title product was prepared by the method of example 14 using the chloro
compound of preparation 31 (1.0g, 3.0mmol) and preparation 29 (1.02g,
4.2mmol). 814mg, 66% yield of the. desired product was prepared as a pale
yellow solid.
'HNMR(CDCI3, 400MHz) 8: 2.35(s, 3H), 3.15(s, 3H), 3.80(s, 3H), 4.45(s, 2H),
6.95(d, 1 H), 7.65-7.90(m, 4H). MS APCI+ m/z 414[MH]+
Example 18
5-(4-Cyano-2-meth I~r phenyl)-2-[5-methoxymethyl-4-(6-methoxypyridin-3-yl)-4H-
[1,2,4ltriazol-3-yll-pyridine
CH3
NC N-N
CH3
O\N/
H3C-0
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-110-
The titt,e product was prepared by the method of example 7 using the product
of
preparation 29 (100mg, 0.41 mmol) and the bromo compound of preparation 18
(1 55mg, 0.41 mmol). 67mg 39% yield of the desired product was prepared as a
white solid.
1HNMR(CDCI3, 400MHz) 8: 2.28(s, 3H), 3.35(s, 3H), 3.99(s, 3H), 4.50(s, 2H),
6.85(d, 1 H), 7.28(d, 1 H), 7.52-7.58(m, 2H), 7.62(dd, 1 H), 7.74(dd, '1 H),
8.11(d,
1H), 8.28(dd, 1H), 8.33(1 H, d). Microanalysis: C23H20N60200.5H20 requires; C
66.55, H 5.02, N 19.94; found C 66.02, H 4.90, N 19.83. MS APCI+ m/z 413
[MH]+
Example 19
2-(5-Fluoro-2-methoxyphenyl)-5-[5-methoxymethyl-4-(6-methoxypyridin-3- IY)-4H-
f 1,2,41triazol-3=yll-pyridine
H3C,
O
N-N
N- Njk,~O,
F CH3
ON\
H3C-O
The title compound was prepared using the method of example 11, using the
oxadiazole compound of preparation 32 and 5-amino-2-methoxy pyridine, as a
pale green solid (325mg, 30%).
iHNMR(CDCI3, 400MHz) 8:'3.36(s, 3H), 3.84(s, 3H), 3.98(s, 3H), 4.49(s, 2H),
6.87(d, 1 H), 6.92(dd, 1 H), 7.06(m, 1 H), 7.54(dd, 1 H), 7.58(dd, 1 H), 7.96-
8.00(m,
2H), 8.14(d, 1 H), 8.60(d, 1 H). MS APCI+ m/z 422 [MH]+
Examples 20-22
The following compounds, of the general formula shown below, were prepared
by the method of examples 1-4 using the products of preparations 46 or 47 and
the appropriate boronic acid.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-111-
The crude compounds were purified firstly by column chromatography on silica
gel, eluting with ethyl acetate:methanol:0.88 ammonia, 100:0:0 to 99:1:0.1 to
95:5:0.5, followed by purification by HPLC using a Phenomenex Luna C18
system, eluting with water/acetonitrile/trifluoroacetic acid
(5:95:0.1):acetonitrile,
95:5 to 5:95.
R1~N~ N
~ '~ N, N ~
\
ON
H3C-0
No. R Y Data Yield
20 ~ CH HNMR(CDCI3i 400MHz) S: 2.37(s, 21%
3H), 3.97(s, 3H), 5.77(s, 2H),
NC CH 6.80(d, 1 H), 7.31(dd, 1 H), 7.45(m,
2H), 7.56(m, 4H), 7.99(d, 1 H),
8:04(dd, 1 H), 8.66(dd, 1 H).
MS APCI+ m/z 450 [MH]+
21 N HNMR(CDCI3, 400MHz) S: 2.40(s, 15%
3H), 3.97(s, 3H), 5.77(s, 2H),
NC CH 6.75(d, 1 H), 7.35(dd, 1 H), 7.50(d,
1 H), 7.54-7.67(m, 4H), 7.95(d, 1 H),
8.41(s, 1 H), 9.58(s, 1 H).
MS APCI+ m/z 450 [MH]+
22 CH
HNMR(CDCI3, 400MHz) 8: 2.34(s, 43%
3H), 3.97(s, 3H), 5.77(s, 2H),
J:X
F CH3 6.78(d, 1 H), 6.95(m, 2H), 7.30(dd,
1 H), 7.35(dd, 1 H), 7.42(dd, 1 H),
7.57(s, 2H), 8.00(m, 2H), 8.60(d,
1 H)
MS APCI+ m/z 443 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-112-
Example 23
4-{5-r5-(Methoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-1,2,4-triazol-3-
yllpyridin-2-
yl}-3-methylbenzonitrile
N-N
~
NC /- N- O\CH3
CH3
H3C~0
The chloro compound of preparation 49 (170mg, 0.87mmol), the palladium
complex of preparation 3 (5mg, cat.), caesium carbonate (847mg, 2.61 mmol)
and the product of preparation 29 (317mg, 1.31 mmol) were suspended in
1,4-dioxan (5mL) and the reaction mixture heated to 110 C for 2 hours. The
reaction mixture was diluted with ethyl acetate and water, and filtered
through
Celite . The layers of the filtrate were separated and the aqueous solution
was
re-extracted with ethyl acetate (x2). The combined organic solutions were then
washed with brine, dried over sodium sulfate and concentrated in vacuo. The
residue was purified by column chromatography on silica gel eluting with
dichloromethane:methanol:0.88 ammonia 100:0:0 to 97.5:2.5:0.25 to afford the,
title compound as a white foam in 45% yield, 160mg.
'HNMR(CDCI3, 400MHz) b: 2.38(s, 3H), 3.38(s, 3H), 4.00(s, 3H), 4.51(s, 2H),
6.90(d, 1 H), 7.45(d, 1 H), 7.48(d, 1 H), 7.55-7.60(m, 3H), 8.03(dd, 1 H),
8.15(d,
1 H), 8.70(dd, 1 H). MS APCI+ m/z 413 [MH]+
Examples 24 to 28
The following compounds, of the general formula shown below, were prepared
by the method of example 23, using the appropriate triazole compounds
(preparations 31 and 50-51) and boronic acids.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-113-
Y N-N
R'~V ~ R2
N N
OA"
3C,0
No. R Y R Yield
24 CH H 37%
NC CH3
Data 'HNMR(CDCI3a 400MHz) S: 2.38(s, 3H), 2.41(s,
3H), 4.00(s, 3H), 6.92(d, 1H), 7.45(m, 3H), 7.55(s,
1 H), 7.58(s, 1 H), 8.02(dd, 1 H), 8.12(d, 1 H), 8.66(d,
1H). Microanalysis: C22Hj$N60 requires; C 69.10,
H 4.74, N 21.98 found C 68.74, H 4.75, N 21.83.
MS APCI+ m/z 383 [MH]+
25 F CH H 83%
0
CH3
Data
iHNMR(CDCI3i 400MHz) S: 2.39(s, 3H), 3.83(s,
3H), 3.99(s, 3H), 6.88-6.94(m, 2H), 7.05(m, 1 H),
7.45(dd, 1 H), 7.58(s, 1 H), 7.97(m, 2H), 8.11(d,
1 H), 8.57(d, 1 H). MS APCI+ m/z 392 [MH]+
26 N OCH3 35%
F) O
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-114-
Data ' HNMR(CDCI3, 40oMHz) S: 3.35(s, 3H), 3.86(s,
3H), 4.00(s, 3H), 4.49(s, 2H), 6.71 (dd, 1H),
6.80(m, 1H), 6.85(d, 1H), 7.59(dd, 1H), 7.91(dd,
1H), 8.12(d, 1H), 8.88(d, 1H), 9.44(d, 1H). MS
APCI+ m/z 423 [MH]+
27 c CH 31 %
FbC' o
NC CH3
Data 1HNMR(CDCI3, 400MHz) b: 2.38(s, 3H), 3.34(s,
3H), 3.50(m, 2H), 3.66(m, 2H), 3.99(s, 3H), 4.62(s,
2H), 6.88(d, 1H), 7.44(d, 1H), 7.48(d, 1H), 7.56(d,
2H), 7.66(dd, 1H), 8.02(dd, 1H), 8.15(d, 1H),
8.70(d, 1 H). Microanalysis: C25H24N603 0.5H20
requires; C 64.50, H 5.41, N 18.05; found C 64.71,
H 5.33, N 17.94. MS APCI+ m/z 457 [MH]+
28 CH 90%
F~C~ O
F CH3
Data 1 HNMR(CDCI3, 400MHz) S: 2.35(s, 3H), 3.34(s,
3H), 3.50(m, 2H), 3.66(m, 2H), 3.98(s, 3H), 4.62(s,
2H), 6.87(d, 1H), 6.93-7.00(m, 2H), 7.36(dd, 1H),
7.41(d, 1H), 7.65(dd, 1H), 7.97(dd, 1H), 8.15(d,
1 H), 8.64(d, 1 H). MS APCI+ m/z 450 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-115-
Example 29
f 5-f 6-(4-Fluoro-2-methLrl-phenyl)-pyridin-3-yll-4-(6-methoxy-pyridin-3-
yi)-4H-f 1,2,41triazol-3-ylmethyll-dimethyl-amine
N-N NH3
~
N CH3
CH3 ON'\/
H3C~
The title compound was prepared from the product of preparation 48 and
4-fluoro-2-methylphenylboronic acid, using the method of examples 1-4, as a
white foam in 70% yield.
'HNMR(CDCI3, 400MHz) S: 2.25(s, 6H), 2.34(s, 3H), 3.47(s, 2H), 3.99(s, 3H),
6.86(d, 1 H), 6.93-7.00(m, 2H), 7.38(m, 2H), 7.64(dd, 1 H), 8.00(dd, 1 H),
8.16(d,
1H), 8.63(d, 1H). Microanalysis: C23H23FN60 0.25 H20 requires; C 65.31, H
5.60, N 19.87 found C 65.19, H 5.63, N 19.58. MS APCI+ m/z 419 [MH]+
Example 30
5-{3-(Ethoxymethyl)-5-f 6-(4-fluoro-2-methylphenyl)pyridin-3-yIl-4H-1,2,4-
triazol-4-
yl}-2-methoxypyridine
N-N
F / \ N' vOCH3
- N
CH3 ON/"--
H3C,-0
The chloro compound of preparation 52 (230mg, 0.67mmol), the palladium
complex of preparation 3 (10mg, cat.), caesium carbonate (648mg, 2.01 mmol)
and 4-fluoro-2-methylphenylboronic acid (143mg, 0.94mmol) were suspended in
1,4-dioxan (4mL) and the reaction mixture heated to 110 C for 2 hours. A
further
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-116-
amount of the product of preparation 3 (5mg) was added and heating continued
for 3.5 hours. The mixture was then partitioned between ethyl acetate and
water,
and the organic layer was separated, dried over sodium sulfate and
concentrated in vacuo. The residue was purified HPLC using a Phenomenex
Luna C18 system, eluting with water/acetonitrile/trifluoroacetic acid
(5:95:0.1):acetonitrile, 95:5 to 5:95 to afford the title compound as a white
powder in 16% yield, 44mg.
iHNMR(CDCI3i 400MHz) 8: 1.16(t, 3H), 2.35(s, 3H), 3.54(q, 2H), 3.99(s, 3H),
4.55(s, 2H), 6.88(d, 1 H), 6.93-7.00(m, 2H), 7.34-7.42(m, 2H), 7.56(dd, 1 H),
8.00(dd, 1 H), 8.16(d, 1 H), 8.65(d, 1 H). Microanalysis: C23H99FN5O2 0.5 H20
requires; C 64.48, H 5.41, N 16.35 found C 64.46, H 5.27, N 16.40. MS APCI+
m/z 420 [MH]+
Example 31
4-{5-f5-(Ethoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-1,2,4-triazol-3-yllpyridin-
2-
Lrl}-3-methylbenzonitrile
N-N
NC Q /N' ,O,CH3
N
CH3
H3C~0
The title compound was prepared from the product of preparation 52 and the
product of preparation 29, using the method of example 30, as a beige solid in
15% yield.
1HNMR(CDCI3i 400MHz) S: 1.16(t, 3H), 2.38(s, 3H), 3.52(q, 2H), 4.00(s, 3H),
4.55(s, 2H), 6.89(d, 1 H), 7.43(d, 1 H), 7.49(d, 1 H), 7.58(m, 3H), 8.02(dd, 1
H),
8.17(d, 1 H), 8.70(d, 1 H). Microanalysis: C24H22N602 0.5 H20 requires; C
66.19,
H 5.32, N 19.30 found C 66.57, H 5.17, N 19.53. MS ES+ mlz 427 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-117-
Example 32
2-(3,4-Dimethyl-phenLl)-5-f 5-methoxymethyl-4-(6-methoxy-pyridin-3-yl)-
4H-f 1,2,41triazol-3-ylLpyrazine
N N-N
H3C N N CH3
H3C O---N/
H3G.-0
The title compound was prepared from the product of preparation 31 and
3,4-dimethylbenzene boronic acid, using the method of example 30. The crude
compound was purified by column chromatography on silica gel, eluting with
dichloromethane:methanol:0.88 ammonia, 96:4:0.4 followed by 100% ethyl
acetate to afford the desired compound as a beige solid in 68 lo yield.
'HNMR(CDCI3, 400MHz) 8: 2.31(s, 3H), 2.33(s, 3H), 3.35(s, 3H), 4.00(s, 3H),
4.50(s, 2H), 6.85(d, 1H), 7.23(s, 1H), 7.58(dd, 1H), 7.71(dd, 1H), 7.80(s,
1H),
8.11 (d, 1 H), 8.71 (d, 1 H), 9.42(d, 1 H). MS APCI+ m/z 403 [MH]+
Example 33
4-{4-f 5-Methoxymethyi-4-(6-methox rL-pyridin-3-yl)-4H-f 1,2,41triazol-3-y
Ii-phenyl}-2,3-dimethyl-pyridine 1-oxide
N-N
~C CH3
/ j N
N IN
CH3
CiH3
~
H3C
A mixture of the product of preparation 57 (230mg, 0.74mmol), 5-amino-2-
methoxy pyridine (100mg, 0.81 mmol) and para-toluenesulfonic acid (30mg, cat.)
in xylene (4mL) was heated under reflux for 18 hours. The mixture was then
acidified with 1 M hydrochloric acid and washed with ethyl acetate and the
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-118-
organic layer was discarded. The aqueous solution was basified with 1 M sodium
hydroxide solution and extracted with ethyl acetate (x2). The combined organic
extracts were washed with brine, dried over sodium sulfate and concentrated in
vacuo to give a brown oil. Purification of the oil by column chromatography on
silica gel, eluting with dichloromethane:methanol:0.88 ammonia, 100:0:0 to
98:2:0.1, afforded the title compound as a beige foam in 24% yield, 74mg.
1HNMR(CDCI3, 400MHz) S: 2.16(s, 3H), 2.53(s, 3H), 3.31(s, 3H), 3.94(s, 3H),
4.43(s, 2H), 6.81(d, 1H), 6.94(d, 1H), 7.19(m, 2H), 7.49(m, 3H), 8.08(d, 1H),
8.16(d, 1 H). MS APCI+ m/z 418 [MH]+
Example 34
2-(4-Fluoro-2-methylphenyl)-5-f4-(6-methoxypyridin-3-yl)-5-methyl-4H-1,2,4-
triazol-3-yilpyridine
N-N
N CH3
/ \ I
N
F ~11-1 I IN
CH3 \
H3C
The title compound was prepared from the product of preparation 72 and
5-amino-2-methoxy pyridine, using the method of example 33, as a brown solid
in 54% yield.
iHNMR(CDCI3i 400MHz) S: 2.34(s, 3H), 2.39(s, 3H), 3.99(s, 3H), 6.87-7.01(m,
3H), 7.35(dd, 1 H), 7.39(dd, 1 H), 7.45(dd, 1 H), 7.97(dd, 1 H), 8.11(d, 1 H),
8.59(d,
1 H). MS APCI+ m/z 376 [MH]+
Examples 35 to 45
The following compounds, of the general formula shown below, were prepared
by the method of example 34 using the appropriate oxadiazole (preparations 73-
81) and aminopyridine.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-119-
The progress of the reactions was monitored by tic analysis and the mixtures
were heated under reflux until all of the starting materials had been
consumed.
Y N-N
Rl~ \ R2
N N~
0__F,4
H3C~0
N-N
\ 2
R - N ~/
N CH3
H3C.0
No. R R2 Y Data Yield
35 OCH3 CH HNMR(CDC13, 400MHz) 8: 25%
F I CH
2.07(s, 3H), 2.34(s, 3H),
3
3.32(s, 3H), 3.97(s, 3H),
4.38(d, 1 H), 4.49(d, 1 H),
6.74(d, 1 H), 6.95(m, 2H),
7.35(dd, 1 H), 7.39(d, 1 H),
7.50(d, 1 H), 8.02(d, 1 H),
8.65(d, 1 H). MS APCI+ m/z
420 [MH]+
36 OCH3 N HNMR(CDCI3, 400MHz) 8: 33%
F CH 3 2.12(s, 3H), 2.38(s, 3H),
3.32(s, 3H), 3.99(s, 3H),
4.39(d, 1 H), 4.49(d, 1 H),
6.66(d, 1 H), 6.99(m, 2H),
7.37(d, 1 H), 7.39(d, 1 H),
8.39(d, 1 H), 9.52(d, 1 H). MS
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-120-
APCI+ m/z 421 [MH]+
37 I~ CH 3 H N HNMR(CDCI3, 400MHz) S: 50%
~ 2.17(s, 3H), 2.23(s, 3H),
F
2.44(s, 3H), 4.00(s, 3H),
6.64(d, 1 H), 7.01(m, 2H),
7.37(m, 2H), 8.37(s, 1 H),
9.43(s, 1 H). MS APCI+ m/z
390 [MH]+
38 H N HNMR(CDCI3, 400MHz) S: 36%
CH3 2.15(s, 3H), 2.21(s, 3H),
CH3 2.31(s, 3H), 2.33(s, 3H),
3.99(s, 3H), 6.68(d, 1 H),
7.18(m, 2H), 7.24(m, 1 H),
7.33(d, 1 H), 8.35(d, 1 H),
9.50(d, 1 H). MS APCI+ m/z
387 [MH]+
N-N
-\ /Nõ Rz
RN ~f
O,
CH3
H3c ,o
No. R' R2 Y Data Yield
39 H N HNMR(CDCI3, 400MHz) S: 69%
F CH 2.33(s, 3H), 2.38(s, 3H),
3.82(s, 3H), 3.98(s, 3H),
6.41(d, 1 H), 6.99(m, 2H),
7.38(dd, 1H), 7.43(d, 1H),
8.38(d, 1H), 9.45(d, 1H). MS
APCI+ m/z 407 [MH]+
40 H N HNMR(CDCI3i 400MHz) S: 49%
(?~CH3 2.21(s, 3H), 2.33(m, 6H),
CH3 3.82(s, 3H), 3.98(s, 3H),
6.41(s, 1 H), 7.21(m, 3H),
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-121-
7.44(d, 1 H), 8.37(d, 1 H),
9.44(d, 1 H). MS APCI+ m/z
403 [M H]+
Y N-N Rz
~
Ri~~ N
N
H3C .0
No. R' R Y Data Yield
41 OCH3 N HNMR(CDCI3, 400MHz) S: 33%
2.34(d, 3H), 3.35(s, 3H),
F 4.00(s, 3H), 4.50(s, 2H),
cH3 6.85(d, 1 H), 7.10(dd, 1 H),
7.58(dd, 1 H), 7.79(m, 1 H),
7.87(dd, 1 H), 8.11(d, 1 H),
8.69(d, 1 H), 9.44(d, 1 H)
MS APCI+ m/z 407 [MH]+
42 ~ H3 F OCH3 N 1HNMR(CDCI3, 400MHz) 8: 40%
u 3.36(s, 3H), 3.93(s, 3H),
4.01(s, 3H), 4.51(s, 2H),
6.86(d, 1 H), 7.06(dd, 1 H),
7.21(dd, 1 H), 7.57(m, 2H),
8.11(d, 1 H), 8.79(d, 1 H),
9.51(d, 1 H)
MS APCI+ m/z 423 [MH]+
43 0 '~CH3 OCH3 N HNMR(CDCI3a 400MHz) b: 31%
2.34(s, 3H), 3.35(s, 3H),
3.83(s, 3H), 4.00(s, 3H),
4.50(s, 2H), 6.85(d, 1 H),
CH3 6.89(d, 1 H), 7.19-7.26(m, 1 H),
7.59(dd, 1 H), 7.68(s, 1 H),
8.12(d, 1 H), 8.91(s, 1 H),
9.44(d, 1 H). MS APCI+ m/z
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-122-
419 [MH]+
44 0 ~CH3 OCH3 N HNMR(CDCI3, 400MHz) S: 39%
3.35(s, 3H), 3.85(s, 3H),
4.00(s, 3H), 4.50(s, 2H),
6.85(d, 1 H), 6.93(d, 1 H),
F 7.10(m, 1 H), 7.59(dd, 1 H),
7.69(s, 1 H), 8.12(d, 1 H),
8.96(s, 1 H), 9.47(d, 1 H). MS
APCI+ m/z 423 [MH]+
45 CH3 OCH3 N 1HNMR(CDCI3, 400MHz) 8: 41%
2.33(s, 3H), 2.34(s, 3H),
3.35(s, 3H), 4.00(s, 3H),
CH3 4.50(s, 2H), 6.85(d, 1 H),
7.17(dd, 2H), 7.24(m, 1 H),
7.60(dd, 1 H), 8.13(d, 1 H),
8.43(d, 1 H), 9.48(d, 1 H). MS
APCI+ m/z 403 [MH]+
Example 42: crude product was re-purified by column chromatography on silica
gel, eluting with ethyl acetate:methanol, 98:2.
Examples 35, 37, 38, 41, 43, 44 and 45: crude products were purified by
trituration with diethyl ether.
Example 46
5-{3-[5-(4-Fluoro-2-methyl-phenyl)-pyrazin-2-yll-5-methyl-f 1,2,41tria
zol-4-L}I -pyridin-2-yl)-methyl-amine
F ~/ \/ \ N-N
N CH3
CH3
= N/
\
H3C H
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-123-
A mixture of the product of preparations 74 (437mg, 1.62mmol) and 83 (434mg,
3.52mmol) and para-toluenesulfonic acid in xylene (10mL) was heated under
reflux for 100 hours. The mixture was then filtered through Celite , washing
through with dichloromethane and the filtrate was concentrated in vacuo.
Purification by column chromatography on silica gel, eluting with
dichloromethane:methano1:0.88 ammonia, 98:2:0.2, followed by ethyl
acetate:methanol, 98:2, afforded the title compound as a beige solid in 9%
yield,
57mg.
' HNMR(CDCI3, 400MHz) 8: 2.38(m, 6H), 2.98(d, 3H), 4.89(m, 1H), 6.47(d, 1H),
6.94-7.02(m, 2H), 7.36(m, 2H), 7.97(d, 1 H) 8.43(s, 1 H), 9.41(d, 1 H). MS
APCI+
m/z 376 [MH]+
Example 47
3-(4-Fluoro-2-methyl-phenyl)-6-[5-methoxymethyl-4-(6-methoxy-pyridin-3
-yl)-4H-f 1,2,41triazol-3-yll-pyridazine
N--N
N
NI \ N 0...._CH3
N
F CH3
0
H3C
The product of preparation 62 (40mg, 0.13mmol), 5-amino-2-methoxypyridine
(16mg, 0.13mmol) and para-toluenesulfonic acid monohydrate (5mg, cat) were
dissolved in xylene (2mL) and the reaction mixture was heated under reflux for
3
hours. The reaction mixture was then partitioned between dichloromethane and
sodium hydrogen carbonate solution and the organic solution was washed with
brine, dried over sodium sulfate and concentrated in vacuo. The residue was
purified by HPLC using a Phenomenex Luna C18 system, eluting with
water/acetonitrile/trifluoroacetic acid (5:95:0.1):acetonitrile, 95:5 to 5:95
to afford
the title compound as a yellow oil in 8% yield, 4mg.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-124-
iHNMR(CDCI3i 400MHz) 8: 2.33(s, 3H), 3.36(s, 3H), 3.94(s, 3H), 4.52(s, 2H),
6.83(d, 1 H), 7.02(m, 2H), 7.39(d, 1 H), 7.70(m, 2H) 8.11(s, 1 H), 8.46(d, 1
H).
Example 48
5-(4-Fluoro-2-methyl-phenyl)-2-f5-methoxymethyl-4-(6-methoxy-pyridin-3
-yl)-4H-f 1,2,41triazol-3-yll-pyrimidine
N-N
N
N 0---CH
F 3
CH3
N
H3C
The product of preparation 69 (80mg, 0.27mmol), 5-amino-2-methoxypyridine
(50mg, 0.39mmol) and para-toluenesulfonic acid monohydrate -(10mg, cat) were
dissolved in xylene (3mL) and the reaction mixture was heated under reflux for
18 hours. `Additional para-toluenesulfonic acid monohydrate (10mg, cat) was
added and heating continued for a further 18 hours. The reaction mixture was
then evaporated under reduced pressure and the residue was dissolved in ethyl
acetate, washed with 1 M hydrochloric acid, sodium hydrogen carbonate solution
and brine, dried over magnesium sulfate and concentrated in vacuo.
Purification
of the residue by column chromatography on silica gel, eluting with
dichloromethane:methanol, 97:3, afforded the title compound in 46% yield,
49.3mg.
'HNMR(CDCI3, 400MHz) 8: 2.23(s, 3H), 3.32(s, 3H), 3.99(s, 3H), 4.51(s, 2H),
6.82(d, 1 H), 6.98(m, 2H), 7.17(d, 1 H), 7.60(d, 1 H), 8.11(s, 1 H), 8.67(s,
2H). MS
APCI+ m/z 407 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-125-
Examples 49 to 52
The following compounds, of the general formula shown below, were prepared
by the method of example 48, using the product of preparation 71 and the
appropriate boronic acid.
N-N
43 R N CH3
NO
`CH3
N
H3C,0
No. R' Data Yield
49 HNMR(CDCI3, 400MHz) S: 2.20(s, 3H), 50%
2.30(s, 3H), 3.79(s, 3H), 3.95(s, 3H),
F CH3 6.38(d, 1H), 6.88-6.98(m, 2H), 7.10(dd,
1H), 7.42(d, 1H), 7.65(dd, 1H), 8.19(d,
1H), .8.21(d, 1 H). MS APCI+ m/z 406
[MH]+
50 HNMR(CDCI3, 400MHz) 5: 2.09(s, 3H), 50%
2.30(s, 3H), 2.39(s, 3H), 3.80(s, 3H),
CH3 3.96(s, 3H), 6.39(d, 1H), 6.98(d, 1H),
CH3 7.09-7.02(m, 2H), 7.46(d, 1H), 7.68(d,
1H), 8.19(d, 1H), 8.24(d, 1 H). MS
APCI+ m/z 402 [MH]+
51 F MS APCI+ m/z 422 [MH]+ 69%
O
CH3
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-126-
52 MS APCI+ m/z 406 [MH]+ 46%
CH3
Example 53
3-{5-f4-(6-Methoxy-pyridin-3-yl)-5-methyl-4H-f 1,2,41triazol-3-yll-pyra
zin-2-yl}-4-methyl-benzonitrile
NC
N-
N
CH3
OA"'
CH3 H3C-0
A mixture of 3-chloro-4-methylbenzonitrile (1g, 6.6mmol),
bis(pinacolato)diboron
(1.8g, 7.Ommol), caesium carbonate (6.4g, 19.8mmol) and the product of
preparation 3 (5mg, cat) in 1,4-dioxan (50mL) was heated under reflux for 4
hours. The reaction mixture was then cooled to room temperature, filtered
through Celite and concentrated in vacuo.
A portion of the residue (145mg, 0.6mmol), the product of preparation 30
(90mg,
0.3mmol), caesium carbonate (293mg, 0.9mmol) and the product of preparation
3 (2mg, cat) were then dissolved in 1,4-dioxan and the mixture was heated
under reflux for 18 hours. The mixture was then cooled to room temperature,
filtered through Celite and concentrated in vacuo. Purification of the
residue by
column chromatography on silica gel, eluting with dichloromethane:methanol,
100:0 to 97:3, afforded the title compound as a white solid in 3% yield, 3mg.
' HNMR(CDCI3, 400MHz) S: 2.41(s, 3H), 2.46(s, 3H), 4.02(s, 3H), 6.90(d, 1H),
7.43(d, 1H), 7.53(dd, 1H), 7.63(dd, 1H), 7.70(d, 1H), 8.09(d, 1H), 8.41(d,
1H),
9.55(d, 1 H). MS APCI+ m/z 383 [MH]+
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-127-
Example 54
2-(4-Fluoro-2-methyl-phenyl)-4-methoxy-5-[5-methoxymethyl-4-(6-methoxy-
pyridin-3-yl)-4H-[1,2,4]triazol-3-yll-pyridine
H3C
O
N~N
CH3
CH3 ON/\
H3C-O
A mixture of the product of preparations 90 (60mg, 0.17mmol) and 3(10mg,
cat), 4-fluoro-2-methylbenzene boronic acid (33mg, 0.21 mmol) and caesium
carbonate (110mg, 0.34mmol) in dioxan (4mL) was heated under reflux for 16
hours. Catalytic amounts of 4-fluoro-2-methylbenzene boronic acid and the
product of preparation 3 were then added to the reaction mixture and heating
continued for a further 3 hours. The mixture was diluted with dichloromethane
and was purified directly by column chromatography on silica gel, eluting with
dichloromethane:methanol, 100:0 to 96:4, to obtain the title compound as a
white foam in 54% yield, 39mg.
'HNMR(CDCI3, 400MHz) 8: 2.34(s, 3H), 3.39(s, 3H), 3.62(s, 3H), 3.94(s, 3H),
4.51(s, 2H), 6.77(m, 2H), 6.96(m, 2H), 7.33(dd, 1 H), 7.50(dd, 1 H), 8.05(d, 1
H),
8.70(d, 1 H). MS APCI+ m/z 436 [MH]+
25
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-128-
Example 55
2-(5-Fluoro-2-methoxy-phenyl)-4-methoxy-5-['5-methoxymethyl-4-(6-methoxV-
pyridin-3-yl)-4H-[1,2,41triazol-3-yil-pyridine
F H3C
O
N-N
N N-k,,O.
CH3
H3C
O ON\
H3C-O
The title compound was prepared from the product of preparation 90 and
5-fluoro-2-methoxybenzene benzoic acid, using the method of example 54, as a
yellow foam in 53% yield.
'HNMR(CDCI3, 400MHz) b: 3.39(s, 3H), 3.63(s, 3H), 3.83(s, 3H), 3.93(s, 3H),
4.51(s, 2H), 6.76(d, 1 H), 6.93(dd, 1 H), 7.07(m, 1 H), 7.41(s, 1 H), 7.49(dd,
1 H),
7.63(dd, 1 H), 8.04(d, 1 H), 8.70(s, 1 H). MS APCI+ m/z 452 [MH]+
Example 56
2-(3-Fluoro-2-methoxy-phenyl)-5-f4-(6-methoxy-pyridin-3-yl)-5-methyl-4-H-
[1,2,41triazol-3-yil-pyrazi ne
N N-
N
NN
F O CH3
H3C ON\
H3C-O
The title product was prepared by the method of example 14 using the
chloro compound of preparation 30 (108mg, 0.32mmol) and 2-methoxy-3-
fluoro-benzene boronic acid (72mg, 0.48mmol). 107mg, 79% yield of the
title product was prepared as a white solid.
CA 02539297 2006-03-15
WO 2005/028452 PCT/IB2004/002977
-129-
'HNMR(CDC13a 400MHz) S: 3.35(s, 3H), 3.85(s, 3H), 4.00(s, 3H), 4.5(s, 2H),
6.85(d, 1H), 7.20(m, 2H), 7.60(m, 2H), 8.15(s, 1H), 8.85(s, 1H), 9.50(s, 1H).
Microanalysis: C21H19FN603O.2H20 requires; C 59.21, H 4.59, N 19.73; found
C 59.27, H 4.69, N 19.33. MS APCI+ m/z 423 [MH]+