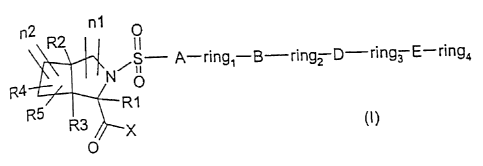Note: Descriptions are shown in the official language in which they were submitted.
CA 02539731 2006-03-21
WO 2005/030728 1 PCT/EP2004/010248
Bicyclic imino acid derivatives used as inhibitors of matrix-
metalloproteinases
The invention relates to novel derivatives of saturated bicyclic imino acids,
such as, in particular, decahydroisoquinoline-1-carboxylic acid, to methods
for preparing them, and to the use thereof as pharmaceuticals.
In diseases such as osteoarthritis and rheumatism, destruction of the joint
takes place, with this destruction being caused, in particular, by the
proteolytic breakdown of collagen due to collagenases. Collagenases
belong to the metalloproteinase (MP) or matrix metalloproteinase (MMP)
supen'amily. The MMPs form a group of Zn-dependent enzymes which are
involved in the biological breakdown of the extracellular matrix (D. Yip et
al.
in Investigational New Drugs 17 (1999), 387-399 and Michaelides et al. in
Current Pharmaceutical Design 5 (1999) 787 - 819). These MMPs are, in
particular, able to break down fibrillar and nonfibrillar collagen and also
proteoglycans, both of which are important matrix constituents. MMPs are
involved in processes of wound healing, tumor invasion and metastasis
migration, and also in angiogenesis, multiple sclerosis and heart failure
(Michaelides, page 788; see above). In particular, they play an important
role in the breakdown of the joint matrix in arthrosis and arthritis, whether
this be osteoarthrosis, osteoarthritis or rheumatoid arthritis.
The activity of the MMPs is furthermore essential for many of the processes
which play a role in atherosclerotic plaque formation, such as the
infiltration
of inflammatory cells and smooth muscle cell migration, as well as
proliferation and angiogenesis (S.J. George, Exp. Opin. Invest. Drugs
(2000), 9 (5), 993-1007). Furthermore, the degradation of matrix by MMPs
can cause anything from plaque instabilities through to ruptures, with these
conditions being able to give rise to the clinical symptoms of
atherosclerosis, unstable angina pectoris, myocardial infarction or stroke
(E.J.M. Creemers et al., Circulation Res. 89, 201-210 (2001 )). All in all,
the
MMP family as a whole is able to break down all the components of the
extracellular matrix of the blood vessels; in normal blood vessels, therefore,
their activity is to a very great degree subject to regulatory mechanisms.
The increase in MMP activity during plaque formation and plaque instability
is caused by an increase in cytokine-stimulated and growth factor-
stimulated gene transcription, an increase in zymogen activation and an
CA 02539731 2006-03-21
2
imbalance in the MMPITIMP (tissue inhibitors of metalloproteases) ratio. It
therefore seems plausible that an MMP inhibition or the reattainment of the
MMP-TIMP balance, would be helpful in treating atherosclerotic diseases. It
is likewise becoming ever more clear that an increase in MMP activity is at
least partially responsible for other cardiovascular diseases in addition to
atherosclerosis, such as restenosis, dilated cardiomyopathy and the
previously mentioned myocardial infarction. It has been shown that
administering synthetic inhibitors to experimental animal models of these
diseases is able to achieve marked improvements, for example in regard to
the formation of atherosclerotic lesions, neointima formation, left
ventricular
remodeling, pump performance malfunction or infarction healing. In various
preclinical studies using MMP inhibitors, detailed tissue analyses indicated
a reduction in collagen damage, an improvement in extracellular matrix
remodeling and an improvement in the structure and function of cardiac
muscle and blood vessels. Among these processes, matrix remodeling
processes and MMP-regulated fibroses, in particular, are regarded as
being important components in the progression of heart diseases
(infarction) (Drugs 61, 1239-1252 (2001 )).
Under physiological conditions, MMPs cleave matrix proteins such as
collagen, laminin, proteoglycans, elastin or gelatine, and also process (i.e.
activate or inactivate), by means of cleavage, a large number of other
proteins and enzymes, which means that they play an important role in the
entire body, with this role being particularly significant in the connective
tissue and bones.
A large number of different MMP inhibitors are known (EP 0 606 046; WO
94/28889; WO 96/27583; cf. reviews such as Current Medicinal Chemistry
8, 425-74 (2001 ) as welt). Following the first clinical studies in humans, it
has now been found that MMPs give rise to side-effects. The side-effects to
be mentioned are musculoskeletal pain or anthralgias. The prior art makes
it clear that it is expected that more selective inhibitors would be able to
reduce these side-effects (Yip, page 387, see above). Specificity towards
MMP-1 is particularly to be emphasized in this connection since these
unwanted side-effects evidently appear to an increased extent when MMP-
1 is inhibited.
A disadvantage of the known MMP inhibitors is therefore that they
frequently lack specificity. Most MMP inhibitors inhibit many MMPs
CA 02539731 2006-03-21
3
simultaneously because the catalytic domains of the MMPs possess similar
structures. As a consequence, the inhibitors also undesirably affect the
enzymes which have a vital function (Massova I, et al., The FASEB Journal
(1998) 12, 1075-1095).
In the endeavor to find effective compounds for treating connective tissue
diseases, it has now been found that the derivatives which are employed in
accordance with the invention are powerful inhibitors of the matrix
metalloproteinases MMP-2, MMP-3, MMP-8, MMP-9 and MMP-13 while
only having a weak inhibitory effect on MMP-1.
The invention therefore relates to a compound of the formula I
n1 0
n2 R2
N,S A-ring-B ' ring2 D-ring3 E-ring4
R4
R5 ~ R1
R3 X (I)
0
andlor to all the stereoisomeric forms of the compound of the formula I
and/or to mixtures of these forms in any ratio, and/or to a physiologically
tolerated salt of the compound of the formula I, wherein
A is -(Co-C4)-alkylene,
B, D and E are identical or different and are, independently of each other,
-(Co-C4)-alkylene or the radical -B1-B2-B3- in which
B1 is -(CH2)~-, in which n is the integer zero, 1 or 2,
B3 is -(CH2)m , in which m is the integer zero, 1 or 2,
with the proviso that the sum of n and m amounts to zero, 1 or 2, and
B2 is
1 ) -C(O)-
2) -(C2-C4)-alkenylene,
3) -S(O)o , where o is the integers zero, 1 or 2,
4) -N(R6)-, in which R6 is hydrogen atom, methyl or ethyl,
5) -N(R6)-C(Y)-, in which Y is oxygen atom or sulfur atom and
R6 is defined as above,
CA 02539731 2006-03-21
4
6) -C(Y)-N(R6)-, in which Y is oxygen atom or sulfur
atom and
R6 is defined as above,
7) -N(R6)-S02-, in which R6 is defined as above,
8) -SOZ-N(R6)-, in which R6 is defined as above,
9) -N(R6)-S02-N(R6)-, in which R6 is defined as
above,
10) -N(R6)-C(Y)-N(R6)-, in which Y is oxygen atom
or sulfur atom
and R6 is defined as above,
11 ) -O-C(O)-N(R6)-,
12) -NH-C(O)-O-,
13) -O-,
14) -C(O)-O-,
15) -O-C(O)-,
16) -O-C(O)-O-,
17) -O-CH2-C(O)-,
18) -O-CH2-C(O)-O-,
19) -O-CH2-C(O)-N(R6)-, in which R6 is defined as
above,
20) -C(O)-CHZ-O-,
21 ) -O-C(O)-CH2-O-,
22) -N(R6)-C(O)-CH2-O-, in which R6 is defined as
above,
23) -O-(CH2)"-O-, in which n is the integer 2 or
3, or
24) -O-(CHZ)m-N(R6)-, in which m is the integer 2
or 3 and R6 is
defined as above,
25) -N(R6)-(CH2)m-O-, in which m is the integer 2
or 3 and R6 is
defined as above,
26) -N(R6)-N(R6)-, in which R6 is defined as above,
27) -N=N-,
28) -N(R6)-CH=N-, in which R6 is defined as above,
29) -N=CH-N(R6)-, in which R6 is defined as above,
30) -N(R6)-C(R7)=N-, in which R6 is defined as above
and R7 is
-N H-R6,
31 ) -N=C(R7)-N(R6)-, in which R6 is defined as above
and R7 is
-NH-R6, or
32) -(CZ-C6)-alkynylene,
ring1, and ring3 are identical or different and are,
ring2 independently of
each other,
1 ) covalent bond,
2) -(C6-C~4)-aryl, in which aryl is unsubstituted
or substituted,
independently of each other, once, twice or three
times, by G,
or
CA 02539731 2006-03-21
3) 4- to 15-membered Het ring, in which Het ring is
unsubstituted or substituted, independently of each other,
once, twice or three times, by G,
ring4 is
5 1 ) -(C6-C~4)-aryl, in which aryl is unsubstituted or substituted,
independently of each other, once, twice or three times, by G,
2) 4- to 15-membered Het ring, in which the Het ring is
unsubstituted or substituted, independently of each other,
once, twice or three times, by G, or
3) is one of the following radicals
O N O O N O
H H
and these radicals are unsubstituted or substituted once by
G,
G is 1 ) hydrogen atom,
2) halogen,
3) =O,
4) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or substituted,
once, twice or three times, by halogen, -(C3-C6)-cycloalkyl,
-
(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring,
5) -(Cs-C,a)-aryl,
6) Het ring,
7) -C(O)-O-R10, in which R10 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(CZ-C6)-alkynyl, -(Cs-C~4)-aryl or Het ring,
or
b) -(C6-C~4)-aryl or Het ring,
8) -C(S)-O-R10, in which R10 is defined as above,
9) -C(O)-NH-R11, in which R11 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(C6-C,4)-aryl or Het ring, or
b) -(C6-C~4)-aryl or Het ring,
10) -C(S)-NH-R11, in which R11 is defined as above,
11 ) -O-R12, in which R12 is
CA 02539731 2006-03-21
6
a) hydrogen atom,
b) -(C~-C6)-alkyl, in which alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen, -(C3-C6)-cycloalkyl, -(C2-C6)-alkynyl,
(Cs-Cia)-aryl or Het ring,
c) -(Cs-C,a)-aryl,
d) Het ring,
e) -C(O)-O-R13, in which R13 is
e)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by -(C3-C6)-cycloalkyl, -(CZ-Cs)
alkynyl, -(C6-C~4)-aryl, or Het ring, or
e)2) -(C6-C~4)-aryl or Het ring,
f) -C(S)-O-R13, in which R13 is defined as above,
g) -C(O)-NH-R14, in which R14 is
g)1 ) -(C~-Cs)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by -(C3-C6)-cycloalkyl, -(CZ-C6)-
alkynyl, -(C6-C~4)-aryl or Het ring, or
g)2) -(C6-C~4)-aryl or Het ring, or
h) -C(S)-NH-R14, in which R14 is defined as above,
12) -C(O)-R10, in which R10 is defined as above,
13) -S(O)p R12, in which R12 is defined as above and p is the
integers zero, 1 or 2,
14) -N02,
15) -CN, or
16) -N(R15)-R12, in which R15 is
16)1 ) hydrogen atom,
16)2) -(C~-C6)-alkyl, or
16)3) -S02-(C~-C6)-alkyl, in which alkyl is unsubstituted or
substituted, once or twice, by -(C3-C6)-cycloalkyl, -
(CZ-C6)-alkynyl, -(C6-C1a)-aryl or Het ring, and R12 is
defined as above, or
17) -S02-N(R12)-R1, in which R12 is defined as above and R1 is
defined as below,
X is -OH or -NH-OH,
n1 is the integer zero, 1, 2 or 3,
n2 is the integer zero, 1, 2, 3 or 4, with the proviso that the sum of n1 and
n2 amounts to 1, 2, 3, 4, 5, 6 or 7,
CA 02539731 2006-03-21
7
R1, R2, R3, R4 and R5 are identical or different and are, independently of
each other,
1 ) hydrogen atom,
2) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or substituted,
once or twice, by -(C3-C6)-cycloalkyl, -(C2-Cs)-alkynyl,
-(C6-
C~a)-aryl or Het ring,
3. -C(O)-O-R8, in which R8 is
3)1 ) hydrogen atom,
3)2) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring,
or substituted
once to five times, by fluorine, or
3)3) -(C6-C~4)-aryl or Het ring,
4) -O-R8, in which R8 has the abovementioned meaning,
or
5) -(C3-C6)-cycloalkyl.
The invention also relates to the compound of the formula I where
A is -(Co-C4)-alkylene,
B, D and E are identical or different and are, independently of each other,
-(Co-C4)-alkylene or the radical -B1-B2-B3- in which
B1 is -(CHZ)~-, in which n is the integer zero, 1 or 2,
B3 is -(CH2)m-, in which m is the integer zero, 1 or 2,
with the proviso that the sum of n and m amounts to zero, 1 or 2, and
B2 is
1 ) -C(O)-
2) -(CZ-C4)-alkenylene,
3) -S(O)o-, where o is the integers zero, 1 or
2,
4) -N(R6)-, in which R6 is hydrogen atom, methyl
or ethyl,
5) -N(R6)-C(Y)-, in which Y is oxygen atom or sulfur
atom and
R6 is defined as above,
6) -C(Y)-N(R6)-, in which Y is oxygen atom or sulfur
atom and
R6 is defined as above,
7) -N(R6)-S02-, in which R6 is defined as above,
- 8) -SOz-N(R6)-, in which R6 is defined as above,
9) -N(R6)-S02-N(R6)-, in which R6 is defined as
above,
10) -N(R6)-C(Y)-N(R6)-, in which Y is oxygen atom
or sulfur atom
and R6 is defined as above,
11 ) -O-C(O)-N(R6)-,
12) -NH-C(O)-O-,
CA 02539731 2006-03-21
8
13) -O-,
14) -C(O)-O-,
15) -O-C(O)-,
16) -O-C(O)-O-,
17) -O-CH2-C(O)-,
18) -O-CH2-C(O)-O-,
19) -O-CH2-C(O)-N(R6)-, in which R6 is defined as
above,
20) -C(O)-CHZ-O-,
21 ) -O-C(O)-CH2-O-,
22) -N(R6)-C(O)-CH2-O-, in which R6 is defined as
above,
23) -O-(CH2)~-O-, in which n is the integer 2 or
3, or
24) -O-(CH2)m-N(R6)-, in which m is the integer 2
or 3 and R6 is
defined as above,
25) -N(R6)-(CH2)m-O-, in which m is the integer 2
or 3 and R6 is
defined as above,
26) -N(R6)-N(R6)-, in which R6 is defined as above,
27) -N=N-,
28) -N(R6)-CH=N-, in which R6 is defined as above,
29) -N=CH-N(R6)-, in which R6 is defined as above,
30) -N(R6)-C(R7)=N-, in which R6 is defined as above and R7 is
-N H-R6,
31 ) -N=C(R7)-N(R6)-, in which R6 is defined as above and R7 is
-NH-R6, or
32) -(C2-C6)-alkynylene,
ring1, ring2 and ring3 are identical or different and are, independently of
each other,
1 ) covalent bond,
2) phenyl or naphthyl and are unsubstituted or substituted,
independently of each other, once, twice or three times, by G,
or
3) 4- to 15-membered Het ring, in which the Het ring is a radical
from the series acridinyl, azepinyl, azetidinyl, aziridinyl,
benzimidazalinyl, benzimidazolyl, benzofuranyl,
benzothiofuranyl, benzothiophenyl, benzoxazolyl,
benzothiazolyl, benzotriazolyl, benzotetrazolyl,
benzisoxazolyl, benzisothiazolyl, carbazolyl, 4aH-carbazolyl,
carbolinyl, quinazolinyl, quinolinyl, 4H-quinolizinyl,
quinoxalinyl, quinuclidinyl, chromanyl, chromenyl, cinnolinyl,
deca-hydroquinolinyl, dibenzofuranyl, dibenzothiophenyl,
CA 02539731 2006-03-21
9
dihydrofuran[2,3-b]tetrahydrofuranyl, dihydrofuranyl,
dioxolyl,
dioxanyl, 2H,6H-1,5,2-dithiazinyl, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, 1 H-indazolyl,
indolinyl,
indolizinyl, indolyl, 3H-indolyl, isobenzofuranyl,
isochromanyl,
isoindazolyl, isoindolinyl, isoindolyl, isoquinolinyl
(benzimidazolyl), isothiazolidinyl, 2-isothiazolinyl,
isothiazolyl,
isoxazolyl, isoxazolidinyl, 2-isoxazolinyl, morpholinyl,
naphthyridinyl, octahydroisoquinolinyl, oxadiazolyl,
1,2,3-
oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-oxadiazolyl,
1,3,4-
oxadiazolyl, oxazolidinyl, oxazolyl, oxothiolanyl,
pyrimidinyl,
phenanthridinyl, phenanthrolinyl, phenazinyl, phenothiazinyl,
phenoxathiinyl, phenoxazinyl, phthalazinyl, piperazinyl,
piperidinyl, pteridinyl, purinyl, pyranyl, pyrazinyl,
pyroazolidinyl, pyrazolinyl, pyrazolyl, pyridazinyl,
pyridooxazolyl, pyridoimidazolyl, pyridothiazolyl,
pyridothiophenyl, pyridinyl, pyridyl, pyrimidinyl,
pyrrolidinyl,
pyrrolinyl, 2H-pyrrolyl, pyrrolyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl, tetrahydroquinolinyl, tetrahydro-
pyridinyl, 6H-1,2,5-thiadazinyl, 1,2,3-thiadiazolyl,
1,2,4-thia-
diazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl,
thianthrenyl,
thiazolyl, thienyl, thienothiazolyl, thienooxazolyl,
thienoimidazolyl, thiomorpholinyl, thiophenyl, triazinyl,
1,2,3-
triazolyl, 1,2,4-triazolyl, 1,2,5-triazolyl, 1,3,4-triazolyl
and
xanthenyl, and these radicals are unsubstituted or
substituted, independently of each other, once, twice
or three
times, by G,
ring4 is
1 ) -(C6-C~4)-aryl, in which aryl is a radical from the series phenyl,
naphthyl, 1-naphthyl, 2-naphthyl, anthryl and fluorenyl, and
these radicals are unsubstituted or substituted, independently
of each other, once, twice or three times, by G,
2) 4- to 15-membered Het ring, in which the Het ring is a radical
from the series acridinyl, azepinyl, azetidinyl, aziridinyl,
benzimidazalinyl, benzimidazolyl, benzofuranyl,
benzothiofuranyl, benzothiophenyl, benzoxazolyl,
benzothiazolyl, benzotriazolyl, benzotetrazolyl,
benzisoxazolyl, benzisothiazolyl, carbazolyl, 4aH-carbazolyl,
carbolinyl, quinazolinyl, quinolinyl, 4H-quinolizinyl,
quinoxalinyl, quinuclidinyl, chromanyl, chromenyl, cinnolinyl,
CA 02539731 2006-03-21
deca-hydroquinolinyl, dibenzofuranyl, dibenzothiophenyl,
dihydrofuran[2,3-b]tetrahydrofuranyl, dihydrofuranyl, dioxolyl,
dioxanyl, 2H,6H-1,5,2-dithiazinyl, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, 1 H-indazolyl, indolinyl,
5 indolizinyl, indolyl, 3H-indolyl, isobenzofuranyl, isochromanyl,
isoindazolyl, isoindolinyl, isoindolyl, isoquinolinyl
(benzimidazolyl), isothiazolidinyl, 2-isothiazolinyl, isothiazolyl,
isoxazolyl, isoxazolidinyl, 2-isoxazolinyl, 2'-methylbiphenyl-
2-0l, morpholinyl, naphthyridinyl, octahydroisoquinolinyl,
10 oxadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-
oxadiazolyl, 1,3,4-oxadiazolyl, oxazolidinyl, oxazolyl,
oxothiolanyl, pyrimidinyl, phenanthridinyl, phenanthrolinyl,
phenazinyl, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, piperazinyl, piperidinyl, pteridinyl, purinyl,
pyranyl, pyrazinyl, pyroazolidinyl, pyrazolinyl, pyrazolyl,
pyridazinyl, pyridooxazolyl, pyridoimidazolyl, pyridothiazolyl,
pyridothiophenyl, pyridinyl, pyridyl, pyrimidinyl, pyrrolidinyl,
pyrrolinyl, 2H-pyrrolyl, pyrrolyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl, tetrahydroquinolinyl, tetrahydro-
pyridinyl, 6H-1,2,5-thiadazinyl, 1,2,3-thiadiazolyl, 1,2,4-thia-
diazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl, thianthrenyl,
thiazolyl, thienyl, thienothiazolyl, thienooxazolyl,
thienoimidazolyl, thiomorpholinyl, thiophenyl, triazinyl, 1,2,3-
triazolyl, 1,2,4-triazolyl, 1,2,5-triazolyl, 1,3,4-triazolyl and
xanthenyl, . and are unsubstituted or substituted,
independently of each other, once, twice or three times, by G,
or
3) is one of the following radicals
O N O O N O
H H
and these radicals are unsubstituted or substituted once by
G,
G is 1 ) hydrogen atom,
2) halogen,
3) =O,
CA 02539731 2006-03-21
11
4) -(C~-C6)-alkyl, in which alkyl is unsubstituted or substituted,
once, twice or three times, by halogen, -(C3-C6)-cycloalkyl,
-
(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring,
where aryl and Het
ring are defined as above,
5) -(C6-C~4)-aryl, where aryl is defined as above,
6) Het ring, where Het ring is defined as above,
7) -C(O)-O-R10, in which R10 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring,
where
aryl and Het ring are defined as above, or
b) -(C6-C~4)-aryl or Het ring, where aryl and
Het ring
are defined as above,
8) -C(S)-O-R10, where R10 is defined as above,
9) -C(O)-NH-R11, in which R11 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(C2-C6)-alkynyl, -(C6-C14)-aryl or Het ring,
where
aryl and Het ring are defined as above, or
b) -(C6-C~4)-aryl or Het ring, where aryl and
Het ring
are defined as above,
10) -C(S)-NH-R11, in which R11 is defined as above,
11 ) -O-R12, in which R12 is
a) hydrogen atom,
b) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once, twice or three times, by
halogen, -(C3-C6)-cycloalkyl, -(C2-Cs)-alkynyl,
-
(C6-C~4)-aryl or Het ring, where aryl and Het
ring
are defined as above,
c) -(C6-C~4)-aryl, where aryl is defined as
above,
d) Het ring, where Het ring is defined as above,
e) -C(O)-O-R13, in which R13 is
e)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by -(C3-C6)-cycloalkyl, -(C2-C6)-
alkynyl, -(C6-C~a)-aryl, or Het ring, where
aryl and Het ring are defined as above, or
e)2) -(C6-C~4)-aryl or Het ring, where aryl
and
Het ring are defined as above,
CA 02539731 2006-03-21
12
f) -C(S)-O-R13, in which R13 is defined as above,
g) -C(O)-NH-R14, in which R14 is
g)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by -(C3-C6)-cycloalkyl, -(C2-C6)-
alkynyl, -(C6-C~4)-aryl or Het ring, where
aryl and Het ring are defined as above, or
g)2) -(C6-C~4)-aryl or Het ring, where aryl
and
Het ring are defined as above, or
h) -C(S)-NH-R14, in which R14 is defined as
above,
12) -C(O)-R10, in which R10 is defined as above,
13) -S(O)p-R12, in which R12 is defined as above
and p is the
integers zero, 1 or 2,
14) -N02,
15) -CN, or
16) -N(R15)-R12, in which R15 is
16)1 ) hydrogen atom, or
16.2) -(C~-C6)-alkyl and R12 is defined as above,
16.3) -S02-(C~-C6)-alkyl, in which alkyl is
unsubstituted or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-
(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring,
17) -SOZ-N(R12)-R1, in which R12 is defined as above
and R1 is
defined as below,
X is -OH or -NH-OH,
n1 is the integer 1 or 2,
n2 is the integer 2 or 3,
R1, R2, R3, R4 and R5 are identical or different and are, independently of
each other,
1 ) hydrogen atom,
2) -(C~-C6)-alkyl, in which alkyl is unsubstituted or substituted,
once or twice, by -(C3-C6)-cycloalkyl, -(CZ-C6)-alkynyl, -(C6-
C,4)-aryl or Het ring,
3) -C(O)-O-R8, in which R8 is
3)1 ) hydrogen atom,
3)2) -(C~-Cs)-alkyl, in which alkyl is unsubstituted or
substituted, once or twice, by -(C3-C6)-cycloalkyl,
-(C2-C6)-alkynyl, -(C6-C~4)-aryl or Het ring, or is
substituted once to five times by fluorine, or
3)3) -(C6-C~a)-aryl or Het ring,
CA 02539731 2006-03-21
13
4) -O-R8, in which R8 has the abovementioned meaning, or
5) -(C3-C6)-cycloalkyl.
The invention furthermore relates to the compound of the formula I wherein
A is -(Co-C4)-alkylene,
B, D and E are identical or different and are, independently of each other,
-(Co-C4)-alkylene or the radical -B1-B2-B3- in which
B1 is -(CH2)~ , in which n is the integer zero, 1 or 2,
B3 is -(CH2)m-, in which m is the integer zero, 1 or 2,
with the proviso that the sum of n and m amounts to zero, 1 or 2, and
B2 is
1 ) -(Co-C2)-alkylene,
2) ethenylene,
3) ethynylene,
4) -C(O)-
5) -N(R6)-C(O)-, in which R6 is hydrogen atom,
methyl or ethyl,
6) -C(O)-N(R6)-, in which R6 is defined as above,
7) -O-, or
8) _S_,
ring1, and ring3 are identical or different and are,
ring2 independently of
each other,
1 ) covalent bond,
2) phenyl or naphthyl and are unsubstituted or
substituted,
independently of each other, once or twice,
by G, or
3) Het ring, in which the Het ring is a radical
from the series
dihydrofuranyl, furanyl, pyridinyl, pyrimidinyl,
pyrrolyl,
thiadiazolyl, thiazolyl or thiophenyl, and the
radicals are
unsubstituted or substituted, independently
of each other,
once or twice, by G,
ring4 is
1 ) phenyl or naphthyl and is unsubstituted or substituted,
independently of each other, once or twice, by G,
2) Het ring, in which the Het ring is a radical from the series
benzofuranyl, dihydrofuranyl, dibenzofuranyl,
dibenzothiophenyl, furanyl, 2'-methylbiphenyl-2-ol,
morpholinyl, piperazinyl, piperidinyl, pyridinyl, pyrimidinyl,
pyridothiophenyl, pyrrolyl, pyrrolidinyl, thiazolyl or thiophenyl
and is unsubstituted or substituted, independently of each
other, once or twice, by G, or
CA 02539731 2006-03-21
14
3) the following radical
O N O
and this radical is unsubstituted or substituted
once by G,
G is 1 ) hydrogen atom,
2) Br, CI or F,
3) -(C~-C4)-alkyl, in which alkyl is unsubstituted
or substituted
once or twice by F, phenyl, -C3-cycloalkyl or
Het ring, where
Het ring is defined as above,
4) phenyl,
5) Het ring, where Het ring is defined as above,
6) -C(O)-O-R10, in which R10 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by cyclopropyl, phenyl
or Het ring, where Het ring is defined as above,
b) phenyl, or
c) Het ring, where Het ring is defined as above,
7) -C(O)-NH-R11, in which R11 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by cyclopropyl, phenyl
or Het ring, where Het ring is defined as above,
b) phenyl, or
c) Het ring, where Het ring is defined as above,
8) -O-R12, in which R12 is
a) hydrogen atom,
b) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once, twice or three times, by
halogen, cyclopropyl, phenyl or Het ring, where
Het ring is defined as above,
c) phenyl,
d) Het ring, where Het ring is defined as above,
e) -C(O)-O-R13, in which R13 is
e)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by cyclopropyl, phenyl or Het ring,
where Het ring is defined as above, or
CA 02539731 2006-03-21
e)2) phenyl or Het ring, where Het ring is
defined as above,
f) -C(S)-O-R13, in which R13 is defined as above,
or
5 g) -C(O)-NH-R14, in which R14 is
g)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by phenyl or Het ring, where Het
ring is defined as above, or
10 g)2) phenyl or Het ring, where Het ring is
defined as above,
9) -C(O)-R10, in which R10 is defined as above,
10) -S(O)p-R12, in which R12 is defined as above and p is the
integers 1 or 2,
15 11 ) -N 02,
12) -CN, or
13) -N(R15)-R12, in which R15 is
13)1 ) hydrogen atom, or
13)2) -(C1-Cs)-alkyl and R12 is defined as above,
X is -OH or -NH-OH,
n1 is the integer 1 or 2,
n2 is the integer 2 or 3,
R1, R2 and R3 are in each case hydrogen atom,
R4 and R5 are identical or different and are, independently of each other,
1 ) hydrogen atom,
2) methyl,
3) ethyl, or
4) -OH.
The invention furthermore relates to the compound of the formula I wherein
A is -(Co-C4)-alkylene,
B, D and E are identical or different and are, independently of each other,
-(Co-C4)-alkylene or the radical -B1-B2-B3- in which
B1 is -(CH2)~-, in which n is the integer zero, 1 or 2,
B3 is -(CHZ)m-, in which m is the integer zero, 1 or 2,
with the proviso that the sum of n and m amounts to zero, 1 or 2, and
B2 is
1 ) -(Co-C2)-alkylene,
2) ethenylene, or
CA 02539731 2006-03-21
16
3) ethynylene,
ring1, ring2 and ring3 are identical or different and are, independently of
each other,
1 ) covalent bond,
2) phenyl, and are unsubstituted or substituted, independently of
each other, once or twice, by G, or
3) Het ring, in which the Het ring is a radical from the series
dihydrofuranyl, furanyl, morpholinyl, piperazinyl, piperidinyl,
pyridinyl, pyrimidinyl, pyrrolyl, thiazolyl or thiophenyl, and are
unsubstituted or substituted, independently of each other,
once or twice, by G,
ring4 is
1 ) phenyl, and is unsubstituted or substituted, independently of
each other, once or twice, by G,
2) Het ring, in which the Het ring is a radical from the series
benzofuranyl, dihydrofuranyl, dibenzofuranyl,
dibenzothiophenyl, furanyl, 2'-methylbiphenyl-2-ol,
morpholinyl, piperazinyl, piperidinyl, pyridinyl, pyrimidinyl,
pyridothiophenyl, pyrrolyl, pyrrolidinyl, thiazolyl or thiophenyl
and is unsubstituted or substituted, independently of each
other, once or twice, by G, or
3) the following radical
O N O
and this radical is unsubstituted or substituted once by G,
G is 1 ) hydrogen atom,
2) Br, CI or F,
3) -(C~-C4)-alkyl, in which alkyl is unsubstituted or substituted
once, twice or three times, by Br, CI, F, -C3-cycloalkyl, phenyl
or Het ring, where Het ring is defined as above,
4) phenyl,
5) Het ring, where Het ring is defined as above,
6) -C(O)-O-R10, in which R10 is
a) -(C,-C6)-alkyl, in which alkyl is unsubstituted or
substituted, once or twice, by cyclopropyl, phenyl
or Het ring, where Het ring is defined as above,
CA 02539731 2006-03-21
17
b) phenyl, or
c) Het ring, where Het ring is defined
as above,
7) -C(O)-NH -R11, in which R11 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by cyclopropyl,
phenyl
or Het ring, where Het ring is defined
as above,
b) phenyl or naphthyl, or
c) Het ring, where Het ring is defined
as above,
8) -O-R12, in which
R12 is
a) hydrogen atom,
b) -(C1-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once, twice or three times,
by
halogen, cyclopropyl, phenyl or Het
ring, where
Het ring is defined as above,
c) phenyl,
d) Het ring, where Het ring is defined
as above,
e) -C(O)-O-R13, in which R13 is
e)1 ) -(C~-C6)-alkyl, in which alkyl
is
unsubstituted or substituted, once or
twice, by cyclopropyl, phenyl, naphthyl,
or Het ring, where Het ring is defined
as
above, or
e)2) phenyl or Het ring, where Het ring is
defined as above,
f) -C(S)-O-R13, in which R13 is defined as above,
or
g) -C(O)-NH-R14, in which R14 is
g)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
twice, by phenyl or Het ring, where Het
ring is defined as above, or
g)2) phenyl or Het ring, where Het ring is
defined as above,
9) -C(O)-R10, in which R10 is defined as above,
10) -S(O)P-R12, in which R12 is defined as above and p is the
integers 1 or 2,
11 ) -N02,
12) -CN, or
13) -N(R15)-R12, in which R15 is
CA 02539731 2006-03-21
18
13)1 ) hydrogen atom, or
13)2) -(C~-C6)-alkyl and R12 is defined as above,
X is -OH or -NH-OH,
n1 is the integer 2,
n2 is the integer 3,
R1, R2, R3, R4 and R5 are in each case hydrogen atom.
The invention furthermore relates to the compound of the formula I wherein
A is a covalent bond or -CH2-CH2-,
B, D and E are identical or different and are, independently of each other,
-(Co-C4)-alkylene or the radical -B1-B2-B3- in which
B1 is -(CH2)"-, in which n is the integer zero, 1 or 2,
B3 is -(CHZ)m-, in which m is the integer zero, 1 or 2,
with the proviso that the sum of n and m amounts to zero, 1 or 2, and
B2 is
1 ) -C(O)-
2) -(C2-C4)-alkynylene,
3) -S(O)o-, where o is the integers zero or 1,
4) -N(R6)-C(Y)-, in which Y is oxygen atom and R6 is hydrogen
atom,
5) -C(Y)-N(R6)-, in which Y is oxygen atom and R6 is hydrogen
atom, or
6 ) -O-,
ring1, ring2 and ring3 are identical or different and are, independently of
each other,
1 ) covalent bond,
2) phenyl and are unsubstituted or substituted, independently of
each other, once or twice, by G, or
3) Het ring, in which the Het ring is a radical from the series
furanyl, pyridinyl, pyrimidinyl or thiophenyl, and are
unsubstituted or substituted, independently of each other,
once or twice, by G,
ring4 is
1 ) phenyl and is unsubstituted or substituted, independently of
each other, once or twice, by G,
2) Het ring, in which the Het ring is a radical from the series
benzofuranyl, dibenzofuranyl, furanyl, 2'-methylbiphenyl-2-ol,
morpholinyl, piperazinyl, piperidinyl, pyridinyl, pyrimidinyl,
pyridothiophenyl, pyrrolyl, pyrrolidinyl, thiazolyl or thiophenyl
CA 02539731 2006-03-21
19
and is unsubstituted or substituted, independently of each
other, once or twice, by G, or
3) the following radical
o~o
s
and this radical is unsubstituted or substituted once by G,
G is 1 ) hydrogen atom,
2) Br, CI or F,
3) -(C~-C4)-alkyl, in which alkyl is unsubstituted
or once, twice or
three times, by Br, CI, F, -C3-cycloalkyl, phenyl
or Het ring,
where Het ring is defined as above,
4) phenyl,
5) Het ring, where Het ring is defined as above,
6) -C(O)-O-R10, in which R10 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by cyclopropyl, phenyl
or Het ring, where Het ring is defined as above,
b) phenyl, or
c) Het ring, where Het ring is defined as above,
7) -C(O)-NH-R11, in which R11 is
a) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once or twice, by cyclopropyl, phenyl
or Het ring, where Het ring is defined as above,
b) phenyl, or
c) Het ring, where Het ring is defined as above,
8) -O-R12, in which R12 is
a) hydrogen atom,
b) -(C~-C6)-alkyl, in which alkyl is unsubstituted
or
substituted, once, twice or three times, by
halogen, cyclopropyl, phenyl or Het ring, where
Het ring is defined as above,
c) phenyl,
d) Het ring, where Het ring is defined as above,
e) -C(O)-O-R13, in which R13 is
e)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
CA 02539731 2006-03-21
twice, by cyclopropyl, phenyl or Het ring,
where Het ring is defined as above, or
e)2) phenyl or Het ring, where Het ring is
defined as above,
5 f) -C(S)-O-R13, in which R13 is defined as above,
or
g) -C(O)-NH-R14, in which R14 is
g)1 ) -(C~-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once or
10 twice, by phenyl or Het ring, where Het
ring is defined as above, or
g)2) phenyl or Het ring, where Het ring is
defined as above,
9) -C(O)-R10, in which R10 is defined as above,
15 10) -S(O)p-R12, in which R12 is defined as above and p is the
integers zero, 1 or 2,
11 ) -N02,
12) -CN, or
13) -N(R15)-R12, in which R15 is
20 13)1 ) hydrogen atom, or
13)2) -(C~-C6)-alkyl and R12 is defined as above,
X is -NH-OH,
n1 is the integer 2,
n2 is the integer 3, and
R1, R2, R3, R4 and R5 are in each case hydrogen atom.
The invention also relates to the compound of the formula I from the series
2-(4'-nitrobiphenyl-4-sulfonyl)decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-(4'-chlorobiphenyl-4-sulfonyl)decahydroisoquinoline-1-carboxylic acid,
2-(4'-chlorobiphenyl-4-sulfonyl)decahydroisoquinoline-1-(N-hydroxy)-
carboxamide,
2-(6-phenoxypyridine-3-sulfonyl)decahydroisoquinoline-1-carboxylic acid;
trifluoroacetate,
2-(6-phenoxypyridine-3-sulfonyl)decahydroisoquinoline-1-(N-hydroxy)-
carboxamide; trifluoroacetate,
2-[2-(4'-chlorobiphenyl-4-yl)ethanesulfonyl]decahydroisoquinoline-1-
carboxylic acid,
CA 02539731 2006-03-21
21
2-[2-(4'-chlorobiphenyl-4-yl)ethanesulfonyl]decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-[4-(pyridin-4-yloxy)benzenesulfonyl]decahydroisoquinoline-1-carboxylic
acid; trifluoroacetate,
2-[4-(pyridin-4-yloxy)benzenesulfonyl]decahydroisoquinoline-1-(N-hydroxy)-
carboxamide; trifluoroacetate,
2-[4-(4-methoxyphenoxy)benzenesulfonyl]decahydroisoquinoline-1-
carboxylic acid,
2-[4-(4-methoxyphenoxy)benzenesulfonyl]decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-{4-[4-(2,2,2-trifluoroethoxy)phenoxy]benzenesulfonyl}decahydro-
isoquinoline-1-carboxylic acid,
2-{4-[4-(2,2,2-trifluoroethoxy)phenoxy]benzenesulfonyl}decahydro-
isoquinoline-1-(N-hydroxy)carboxamide,
2-[4'-(2,2,2-trifluoroethoxy)biphenyl-4-sulfonyl]decahydroisoquinoline-1-
carboxylic acid,
2-(4'-isopropoxycarbonylaminobiphenyl-4-sulfonyl)decahydroisoquinoline-
1- carboxylic acid,
[4'-(1-hydroxycarbamoyloctahydroisoquinoline-2-sulfonyl)biphenyl-4-
yl]carboxamide isopropyl ester,
2-[4'-(2,2,2-trifluoroethoxy)biphenyl-4-sulfonyl]decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-(4'-trifluoromethoxybiphenyl-4-sulfonyl)decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-[4-(4-fluorophenoxy)benzenesulfonyl]decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-[4-(4-trifluoromethoxyphenoxy)benzenesulfonyl]decahydroisoquinoline-1-
(N-hydroxy)carboxamide,
2-[4-(4-trifluoromethoxyphenoxy)benzenesulfonyl]decahydroisoquinoline-1-
carboxylic acid,
2-(biphenyl-4-sulfonyl)decahydroisoquinoline-1-(N-hydroxy)carboxamide,
2-(biphenyl-4-sulfonyl)decahydroisoquinoline-1-carboxylic acid,
2-[4-(4-cyanophenoxy)benzenesulfonyl]decahydroisoquinoline-1-(N-
hydroxy)carboxamide,
2-(dibenzofuran-2-sulfonyl)decahydroisoquinoline-1-carboxylic acid,
2-(dibenzofuran-2-sulfonyl)decahydroisoquinoline-1-(N-
hydroxy)carboxamide, or
CA 02539731 2006-03-21
22
2-[4-(4-fluorophenoxy)benzenesulfonyl]-6-methoxydecahydroisoquinoline-
1-(N-hydroxy)carboxamide, and also all the isomeric forms of the
abovementioned compounds.
The term "(C~-C6)alkyl" is understood as meaning hydrocarbon radicals
whose carbon chain is straight-chain or branched and contains from 1 to 6
carbon atoms, for example methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
tertiary butyl, pentyl, isopentyl, neopentyl, hexyl, 2,3-dimethylbutane or
neohexyl.
The term "-(Co-C4)alkylene" is understood as meaning hydrocarbon
radicals whose carbon chain is straight-chain or branched and contains
from 1 to 4 carbon atoms, for example methylene, ethylene, propylene,
isopropylene, isobutylene, butylene or tertiary butylene. "-Co-Alkylene" is a
covalent bond.
The term "-(CH2)~-, in which n is the integer zero, 1 or 2" is understood as
meaning a covalent bond, where n is zero, the radical methylene, where n
is 1 and the radical ethylene where n is 2.
The term "-(C2-C4)-alkenylene" is understood as meaning hydrocarbon
radicals whose carbon chain is straight-chain or branched and contains
from 2 to 4 carbon atoms and which, depending on chain length, possess
one or two double bonds, for example ethenylene, propenylene,
isopropenylene, isobutenylene or butenylene; insofar as the possibility
exists in principle, the substituents at the double bond can be arranged in
the E position or Z position.
The term "-(C2-C6)-alkynylene" is understood as meaning hydrocarbon
radicals whose carbon chain is straight-chain or branched and contains
from 2 to 6 carbon atoms and which, depending on chain length, possess
one or two triple bonds, for example ethynylene, propenylene,
isopropynylene, isobutynyl, butynylene, pentynylene or isomers of
pentynylene, or hexynyl or isomers of hexynylene.
The term "(C3-C6)-cycloalkyl" is understood as meaning radicals such as
compounds which are derived from 3- to 6-membered monocycles such as
cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
The radicals
CA 02539731 2006-03-21
23
n2
n1
or
are in each case understood as meaning -CH2- radicals in the ring of the
formula I, where the variables n1 or n2 in each case specify the number of
the -CH2- radicals in the ring of the formula I. When n1 has the value zero,
a covalent bond ensues and the resulting part ring has a total of 4 ring
atoms. When n2 has the value zero, a covalent bond ensues and the
resulting part ring has a total of 3 ring atoms. When n1 has the value 1, a
-CH2- radical ensues and the resulting part ring has a total of 5 ring atoms.
When n1 has the value 2, a -CH2-CH2- radical ensues and the resulting
part ring has a total of 6 ring atoms. When n1 has the value 3, a -CH2-CHZ-
CH2- radical ensues and the resulting part ring has a total of 7 ring atoms.
Corresponding part rings ensue in the case of n2.
The term "-(C6-C~4)-aryl" is understood as meaning aromatic hydrocarbon
radicals having from 6 to 14 carbon atoms in the ring. -(C6-C~4)-Aryl
radicals are, for example, phenyl, naphthyl, for example 1-naphthyl or 2
naphthyl, anthryl or fluorenyl. Naphthyl radicals and, in particular, phenyl
radicals are preferred aryl radicals.
The term "4- to 15-membered Het ring" or "Het ring" is understood as
meaning ring systems which have from 4 to 15 carbon atoms, which are
present in one, two or three ring systems which are linked to each other,
and which contain one, two, three or four identical or different heteroatoms
from the series oxygen, nitrogen or sulfur. Examples of these ring systems
are the radicals acridinyl, azepinyl, azetidinyl, aziridinyl,
benzimidazalinyl,
benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl,
benzoxazolyl, benzothiazolyl, benzotriazolyl, benzotetrazolyl,
benzisoxazolyl, benzisothiazolyl, carbazolyl, 4aH-carbazolyl, carbolinyl,
quinazolinyl, quinolinyl, 4H-quinolizinyl, quinoxalinyl, quinuclidinyl,
chromanyl, chromenyl, cinnolinyl, decahydroquinolinyl, dibenzofuranyl,
dibenzothiophenyl, dihydrofuran[2,3-b]tetrahydrofuranyl, dihydrofuranyl,
dioxolyl, dioxanyl, 2H, 6H-1,5,2-dithiazinyl, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, 1 H-indazolyl, indolinyl,
indolizinyl,
indolyl, 3H-indolyl, isobenzofuranyl, isochromanyl, isoindazolyl,
isoindolinyl,
isoindolyl, isoquinolinyl (benzimidazolyl), isothiazolidinyl, 2-
isothiazolinyl,
isothiazolyl, isoxazolyl, isoxazolidinyl, 2-isoxazolinyl, 2'-methylbiphenyl-2-
CA 02539731 2006-03-21
24
o1, morpholinyl, naphthyridinyl, octahydroisoquinolinyl, oxadiazolyl, 1,2,3-
oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl,
oxazolidinyl, oxazolyl, oxothiolanyl, pyrimidinyl, phenanthridinyl,
phenanthrolinyl, phenazinyl, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, piperazinyl, piperidinyl, pteridinyl, purinyl, pyranyl,
pyrazinyl,
pyroazolidinyl, pyrazolinyl, pyrazolyl, pyridazinyl, pyridooxazolyl,
pyridoimidazolyl, pyridothiazolyl, pyridothiophenyl, pyridinyl, pyridyl,
pyrimidinyl, pyrrolidinyl, pyrrolinyl, 2H-pyrrolyl, pyrrolyl,
tetrahydrofuranyl,
tetrahydroisoquinolinyl, tetrahydroquinolinyl, tetrahydropyridinyl, 6H-1,2,5-
thiadiazinyl, 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl, 1,2,5-thiadiazolyl,
1,3,4-
thiadiazolyl, thianthrenyl, thiazolyl, thienyl, thienothiazolyl,
thienooxazolyl,
thienoimidazolyl, thiomorpholinyl, thiophenyl, triazinyl, 1,2,3-triazolyl,
1,2,4-triazolyl, 1,2,5-triazolyl, 1,3,4-triazolyl and xanthenyl.
Preferred Het rings are the radicals benzofuranyl, benzimidazolyl,
benzoxazolyl, benzothiazolyl, benzothiophenyl, 1,3-benzodioxolyl,
quinazolinyl, quinolinyl, quinoxalinyl, chromanyl, cinnolinyl, furanyl such as
2-furanyl and 3-furanyl; imidazolyl, indolyl, indazolyl, isoquinolinyl,
isochromanyl, isoindolyi, isothiazolyi, isoxazolyl, 2'-methyibiphenyl-2-ol,
oxazolyl, phthalazinyl, pteridinyl, pyrazinyl, pyrazolyl, pyridazinyl,
pyridoimidazolyl, pyridopyridinyl, pyridopyrimidinyl, pyridyl; such as 2-
pyridyl, 3-pyridyl or 4-pyridyl; pyrimidinyl, pyrrolyl; such as 2-pyrrolyl and
3-
pyrrolyl; purinyl, thiazolyl, tetrazolyl or thienyl; such as 2-thienyl and 3-
thienyl.
The term "halogen" is understood as meaning fluorine, chlorine, bromine or
iodine.
The invention furthermore relates to a process for preparing the compound
of the formula I andlor a stereoisomeric form of the compound of formula I
and/or a physiologically tolerated salt of the compound of the formula I,
which comprises
a) reacting a compound of the formula IV,
O
n2 R2 ,Re
~O
R4
NH (IV)
R5 R3
CA 02539731 2006-03-21
in which Re is a hydrogen atom or an ester-protecting group,
with a compound of the formula V,
0
Rz-S- A -rings- B ring2 D ring3 E-ring4 (V)
5 O
in which A, B, D, E and ring1, ring2, ring3 and ring4 are defined as in
formula I, and in which Rz is chlorine atom, imidazoyl or OH,
10 in the presence of a base or following silylation with a suitable
silylating agent, or using a suitable dehydrating agent when Rz = OH,
to give a compound of the formula VI,
n2 O
R2 ,Re
~O
R4 1
R5 R3 N\S O A-ring-B-ringZ D-ring3 E-ring4 (vp
O
in which A, B, D, E, Re and ringl , ring2, ring3 and ring4 are defined
as above, and
b) when Re = ester reacting a compound of the formula VI prepared as
described in a) with a solution of alkali such as NaOH or LiOH, and
then treating the product with acid, to give the carboxylic acid
according to the invention of the formula I, in which X = OH
(corresponding to VII), with modifications in one of the side chains of
the rings ring1-ring4 also having previously been made, where
appropriate; or converting said ester, by treating it with a mineral acid,
such as hydrochloric acid, into the free carboxylic acid VII
CA 02539731 2006-03-21
26
n2 O
R2
'OH
R4 1
N~ %O (VII)
R5 R3 S-A-ring-B-ringZ D-ring3 E-ringa
O
and then converting this into the hydroxamic acid according to the
invention, in which X = NH-OH, of the formula I,
c) using salt formation with enantiomerically pure acids or bases,
chromatography on chiral stationary phases, or derivatization with
chiral, enantiomerically pure compounds such as amino acids,
separation of the resulting diastereomers and elimination of the chiral
auxiliary groups, to separate a compound of formula I prepared as
described in procedure a), or a suitable precursor of the formula I,
which arises in enantiomeric forms due to its chemical structure, into
the pure enantiomers, or
d) either isolating the compound of the formula I prepared as described
in procedures b) or c) in free form, or, when acid or basic groups are
present, converting it into physiologically tolerated salts.
Compounds of the formula IV to VII type are compounds which are only
presented by way of example; in accordance with formula I, it is also
possible to adduce four-membered rings, six-membered rings and seven-
membered rings instead of the five-membered ring.
Compounds of the formula IV type can be prepared using known protocols.
For example, compounds in which n~ = 1 and n2 = 0 (methanoprolines) can
be prepared using a number of known methods. The description of a recent
synthesis can be found, for example, in Tetrahedron 53, 14773-92 (1997).
For example, the bicyclic skeletons of the formula IV in which n~ = 2 and n2
- 3 in accordance with formula I can be prepared by hydrogenation of
isoquinoline-1-carboxylic acid or suitable derivatives of isoquinoline-1
carboxylic acid, such as methyl ester or ethyl ester. This hydrogenation is
described, for example, in US 5430023, US 5726159 and EP 643073.
CA 02539731 2006-03-21
27
In the same way, it is possible to use 1,2,3,4-tetrahydroisoquinoline-1-
carboxylic acid and its derivatives to prepare these compounds by
hydrogenation. This procedure has the advantage that it is possible to use
a broad range of methods for synthesizing the 1,2,3,4-tetrahydro-
isoquinoline-1-carboxylic acids. For example, Pictet-Spengler-type
cyclizations, such as described in US 4902695, are particularly well known
and broadly applicable. Depending on the nature of the starting compounds
employed, such methods can be used, for example, to obtain substituted
compounds, i.e. compounds in which the substituents R1, R4 and R5 are
not H atoms. A novel example of ring-substituted compounds can be found
in WO 2003041641. Innumerable examples of R1 and/or R4 and R5 not
being H exist and are readily available to the skilled person.
Other possible methods for preparing the cyclic skeletons use free radical
cyclization reactions, for example, and are described in Tetrahedron 48,
4659-76 (1992).
Other methods can be used for synthesizing compounds of the IV type
when n~ = 1 and n3 = 3. For example, described syntheses are to be found
in Tetrahedron 55, 8025 (1999) and Tetrahedron Lett. 24, 5339 (1983) or in
the laid-open specifications DE 3322530 and DE 3211676.
Methods for synthesizing compounds of the IV type in which n~ = 1 and n2
- 2 are likewise to be found in Tetrahedron 55, 8025 (1999) and
DE 3322530 or DE 3211676.
Methods for synthesizing the skeletons of related compounds, for example
in which n~ = 1 and n2 = 4, are also described. For example, J. Org. Chem.
61, 7125 (1996) describes syntheses of (3-lactams which contain said
skeleton. The substituted basic structures in analogy with formula IV can
also be prepared from these compounds by opening the (i-lactam.
Methods for synthesizing the skeletons in which n~ = 2 and n2 = 4 are
likewise known and well described, for example in EP 0672665 and the
abovementioned references.
The groups used as protecting groups for esters in "Protective Groups in
Organic Synthesis", T.H. Greene, P.G.M. Wuts, Wiley-Interscience, 1999,
CA 02539731 2006-03-21
28
can be used as the ester-protecting group Re. Examples of preferred ester-
protecting groups are methyl, ethyl, isopropyl, tert-butyl and benzyl.
Under certain conditions, it can be useful to employ compounds of the IV
type in the N-protected state. For example, it is easier to purify compounds
which are protected in this way than it is to purify the free imino acids; in
the same way, these protected compounds can sometimes also be more
readily used for preparing the enantiomerically pure or diastereomerically
pure compounds. The groups described in "Protective Groups in Organic
Synthesis", T.H. Greene, P.G.M. Wuts, Wiley-Interscience, 1999, can be
employed as protecting groups for the amino group. Examples of preferred
amino-protecting or imino-protecting groups are Z, Boc, Fmoc, Aloc, acetyl,
trifluoroacetyl, benzoyl, benzyl and the like.
The starting compounds and reagents employed can either be prepared
using known methods or obtained commercially.
The reactions are carried out as described, for example, in WO 97/18194.
The reaction as described in procedural step a) takes place in the presence
of a base, such as KOH, NaOH, LiOH, N-methylmorpholine (NMM), N-
ethylmorpholine (NEM), triethylamine (TEA), diisopropylethylamine
(DIPEA), pyridine, collidin, imidazole or sodium carbonate, in solvents such
as tetrahydrofuran (THF), dimethylformamide (DMF), dimethylacetamide,
dioxane, acetonitrile, toluene, chloroform or methylene chloride, or else in
the presence of water. If the reaction is being carried out using silylating
agents, N,O-bis(trimethylsilyl)acetamide (BSA) or N,O-bis(trimethylsilyl)-
trifluoroacetamide (BSTFA) is used, for example, for silylating the imino
acid in order to subsequently carry out the sulfonamide formation.
Modifications in the side chain F mean that, for example, a nitro group is
hydrogenated using the metal catalyst Pd/C, or reacted with SnCl2 or Zn
under standard conditions, and the resulting amino group can then be
subjected to further modification, for example by reacting it with carbonyl
chlorides, sulfonyl chlorides, chloroformic esters, isocyanates,
isothiocyanates or other reactive or activatable reagents in order to arrive
at the precursors of the compounds of formula I according to the invention.
In this case, it is frequently advantageous for Re in compound VI to be an
ester since side reactions can be expected when the carboxylic acid is
unprotected.
CA 02539731 2006-03-21
29
In procedural step c), the compound of the formula I is separated, insofar
as it arises as a mixture of diastereomers or enantiomers, or accrues in the
chosen synthesis as their mixtures, into the pure stereoisomers, either by
means of chromatography on a support material, which is chiral where
appropriate, or, provided the racemic compound of formula I is capable of
salt formation, by means of fractional crystallization of the diastereomeric
salts which are formed with an optically active base or acid used as
auxiliary substance. Examples of chiral stationary phases which are
suitable for separating enantiomers by means of thin layer chromatography
or column chromatography are modified silica gel supports (what are
termed Pirkle phases) and also high molecular weight carbohydrates such
as triacetylcellulose. For analytical purposes, it is also possible, following
appropriate derivatization known to the skilled person, to use gas-
chromatographic methods on chiral stationary phases. In order to resolve
the racemic carboxylic acids into their enantiomers, an optically active
base, which is as a rule commercially available, such as (-)-nicotine, (+)-
and (-)-phenylethylamine, quinine bases, L-lysine or L- and D-arginine, are
used to form the differently soluble diastereomeric salts, the more difficulty
soluble component is isolated as a solid, the more readily soluble
diastereomer is removed from the mother liquor, and the pure enantiomers
are obtained from the diastereomeric salts which have thus been isolated.
In what is in principle the same way, the racemic compounds of the formula
I which contain a basic group such as an amino group can be converted
into the pure enantiomers using optically active acids, such as
(+)-camphor-10-sulfonic acid, D- and L-tartaric acid, D- and L-lactic acid
and also (+)- and (-)-mandelic acid. It is also possible to convert chiral
compounds which contain alcohol or amine functions into the
corresponding esters or amides using appropriately activated and, where
appropriate, N-protected enantiomerically pure amino acids or, vice versa,
to convert chiral carboxylic acids into the amides using carboxyl-protected
enantiomerically pure amino acids or to convert them into the
corresponding chiral esters using enantiomerically pure hydroxycarboxylic
acids such as lactic acid. The chirality of the amino acid radical or alcohol
radical which has been introduced in enantiomerically pure form can then
be used for separating the isomers by carrying out a separation of the
diastereomers, which are now present, by means of crystallization or by
means of chromatography on suitable stationary phases and, after that,
CA 02539731 2006-03-21
using suitable methods to once again eliminate the entrained chiral
molecule moiety.
In addition, the possibility arises, in the case of some of the compounds
5 according to the invention, of using diastereomerically pure or
enantiomerically pure starting compounds for preparing the skeletal
structures. This then makes it possible, where appropriate, to also use
other methods, or simplified methods, for purifying the end products. These
starting compounds were prepared beforehand in enantiomerically pure
10 form or diastereomerically pure form using methods known from the
literature. For example, it is possible, as mentioned and cited above, to use
isoquinoline-1-carboxylic acid either directly in the method for preparing
decahydroisoquinoline-1-carboxylic acid. As a result of 3 heterocenters
being present, it is possible, in this case, to form a maximum of 8
15 stereoisomers (4 enantiomeric diastereomer pairs). However, the nature of
the preparation, for example hydrogenation, strongly favors certain
stereoisomers. Thus, it should be possible, as described in the literature, to
achieve strong preference for hydrogen attachment at the positions of the
ring linkage, for example, by selecting the hydrogenation conditions
20 (catalyst, pressure, solvent and temperature) appropriately. Thus, it is
possible, under the specified conditions, to achieve formation of the cis-
linked rings. It would then consequently only remain a matter of
determining the position of the carboxylic acid since the number of possible
stereoisomers would already be restricted to 4. As a result of the nature of
25 the hydrogenation mechanism, it is particularly easy to attach the
hydrogens on the same side as that of the bridgehead hydrogens, i.e. a
further restriction in the possibility of isomer formation is thereby to be
expected. It would consequently be possible, in the most favorable case, to
assume that only one enantiomer pair would be formed. It ought then to be
30 possible to use the abovementioned methods to resolve this pair into the
enantiomers. However, in connection with these considerations, it has also
to be assumed that complete stereoselection will never take place and that,
on the contrary, varying proportions of the other isomers will virtually
always also be formed or will be detectable, even in very small quantities,
when suitable methods are used.
When enantiomerically pure 1,2,3,4-tetrahydroisoquinoline-1-carboxylic
acid derivatives are used, it would be expected that, if the reaction
conditions were identical or similar to those used during the hydrogenation
of isoquinoline-1-carboxylic acid, analogous considerations would apply
CA 02539731 2006-03-21
31
and it would once again to a large extent only be preferred stereoisomers
which were formed; in this said case, there should be strong preference for
only one single enantiomer since, when carrying out the hydrogenation
process under conditions which were analogous to those which lead to the
cis ring linkage when hydrogenating isoquinoline-1-carboxylic acid, the H
atoms can once again only be attached from the one side and, as a
consequence, analogous products will be formed. The identity of the
structures can be established by means of suitable 2D NMR experiments,
X ray methods such as cocrystallization and others, as well as reference
analysis or chemical derivatization and suitable analysis or chemical
derivatization which leads to known and described isomers.
Another possibility for synthesizing enantiomerically pure or
diastereomerically pure compounds is that of using suitably chirally
substituted starting compounds in order, by means of the chiral substituent,
to achieve induction of chirality at other chiral centers. For example, chiral
glyoxylic esters could be used in Pictet-Spengler cyclizations in order to
obtain chiral Tic derivatives and then to hydrogenate these derivatives, as
already mentioned above.
Acid or basic products of the compound of formula I can be present in the
form of their salts or in free form. Preference is given to pharmacologically
tolerated salts, for example alkali metal or alkaline earth metal salts, or
hydrochlorides, hydrobromides, sulfates, hemisulfates and all possible
phosphates, as well as salts of the amino acids, natural bases or carboxylic
acids. Physiologically tolerated salts are prepared in a manner known per
se, in accordance with procedural step d), from compounds of formula I,
including their stereoisomeric forms, which are capable of salt formation.
The compounds of formula I form stable alkali metal salts, alkaline earth
metal salts, or optionally substituted ammonium salts, with basic reagents
such as hydroxides, carbonates, hydrogen carbonates or alkoxides, as well
as ammonia or organic bases, for example trimethylamine, triethylamine,
ethanolamine, diethanolamine, triethanolamine or trometamol, or else basic
amino acids, for example lysine, ornithine or arginine. Provided the
compounds of formula I possess basic groups, it is also possible to prepare
stable acid addition salts using strong acids. Both inorganic and organic
acids, such as hydrochloric acid, hydrobromic acid, sulfuric acid,
hemisulfuric acid, phosphoric acid, methanesulfonic acid, benzenesulfonic
acid, p-toluenesulfonic acid, 4-bromobenzenesulfonic acid,
CA 02539731 2006-03-21
32
cyclohexylamidosulfonic acid, trifluoromethylsulfonic acid,
2-hydroxyethanesulfonic acid, acetic acid, oxalic acid, tartaric acid,
succinic
acid, phosphoglyceric acid, lactic acid, malic acid, adipic acid, citric acid,
fumaric acid, malefic acid, gluconic acid, glucuronic acid, palmitic acid or
trifluoroacetic acid are suitable for this purpose.
The invention also relates to pharmaceuticals which are characterized by
an effective content of at least one compound of the formula I and/or a
physiologically tolerated salt of the compound of formula I and/or an
optionally stereoisomeric form of the compound of formula I, together with a
pharmaceutically suitable and physiologically tolerated carrier substance,
additive and/or other active compounds and auxiliary substances.
On account of their pharmacological properties, the compounds according
to the invention are suitable for the selective prophylaxis and therapy of all
those diseases whose course involves an increase in the activity of the
metalloproteinases. These diseases include degenerative joint diseases
such as osteoarthroses, spondyloses and chondrolysis following joint
trauma or a relatively long period of joint immobilization following meniscus
injuries or patella injuries or ligament ruptures. They furthermore also
include diseases of the connective tissue such as collagenoses,
periodontal diseases, wound healing disturbances and chronic diseases of
the locomotory apparatus such as inflammatory, immunologically
determined or metabolism-determined acute and chronic arthritides,
arthropathies, myalgias and disturbances of bone metabolism. The
compounds of the formula I are furthermore suitable for the treatment of
ulceration, atherosclerosis and stenoses. In addition, the compounds of the
formula I are suitable for the treatment of inflammations, cancer diseases,
tumor metastases formation, cachexia, anorexia, heart failure and septic
shock. The compounds are also suitable for the prophylaxis of myocardial
and cerebral infarctions.
The pharmaceuticals according to the invention can be administered by
means of oral, inhalative, rectal or transdermal administration or by means
of subcutaneous, intraarticular, intraperitoneal or intravenous injection.
Oral
administration is preferred.
The invention also relates to a process for producing a pharmaceutical,
which comprises bringing at least one compound of the formula I, together
CA 02539731 2006-03-21
33
with a pharmaceutically suitable and physiologically tolerated excipient and,
where appropriate, other suitable active compounds, additives or auxiliary
substances, in a suitable form of administration.
Examples of suitable solid or galenic preparation forms are granules,
powders, sugar-coated tablets, tablets, (micro)capsules, suppositories,
syrups, juices, suspensions, emulsions, drops or injectable solutions, as
well as preparations giving a protracted release of active compound, the
production of which makes use of customary adjuvants such as carrier
substances, disintegrants, binders, coating agents, swelling agents,
glidants, lubricants, flavorings, sweeteners and solubilizers. Frequently
employed auxiliary substances which may be mentioned are magnesium
carbonate, titanium dioxide, lactose, mannitol and other sugars, talc, milk
protein, gelatin, starch, cellulose and its derivatives, animal and vegetable
oils, such as cod liver oil, sunflower oil, groundnut oil or sesame oil,
polyethylene glycol and solvents such as sterile water and monohydric or
polyhydric alcohols, such as glycerol.
The pharmaceutical preparations are preferably produced and
administered in dosage units, with each unit containing, as the active
constituent, a defined dose of the compound of the formula I according to
the invention. This dose can be up to about 1000 mg, preferably, however,
from about 50 to 300 mg, in the case of solid dosage units, such as tablets,
capsules, sugar-coated tablets or suppositories, and be up to about 300
mg, preferably, however, from about 10 to 100 mg, in the case of injection
solutions in ampoule form.
Daily doses of from about 2 mg to 1000 mg of active compound, preferably
of from 50 mg to 500 mg, are indicated, in dependence on the activity of
the compound of the formula I, for treating an adult patient weighing about
70 kg. However, higher or lower daily doses may sometimes also be
appropriate. The daily dose can be administered either by means of a
once-only administration in the form of a single dosage unit or of several
smaller dosage units or else by means of the multiple administration of
subdivided doses at defined intervals.
End products are as a rule determined by means of mass-spectroscopic
methods (FAB-MS and ESI-MS) and'H-NMR (400 MHz, in DMSO-D6); the
main peak or the two main peaks are given in each case. Temperatures
CA 02539731 2006-03-21
34
are given in degrees centigrade, RT denotes room temperature (21 °C to
24°C). The abbreviations employed are either explained or in conformity
with the customary conventions. The invention is clarified below with the
aid of examples.
General protocol 1: Sulfonamide from sulfonyl chloride and carboxylic acid
The carboxylic acid (6.45 mmol) was dissolved in 20 ml of dimethylform-
amide (DMF), and 3 equivalents of a 3N solution of NaOH (6.45 ml) were
added at 0°C. After 10 min, a solution of the arylsulfonyl chloride
(1.1
equivalents, 7.1 mmol) in from 10 to 15 ml of DMF was slowly added
dropwise; after room temperature (RT) has been reached, the mixture
continues to be stirred for a maximum of 12 hours (h) at temperatures
between 20°C and 80°C. The precise time depends on when
conversion is
complete, with this being established by mass spectroscopy. After that, the
solvent was removed under reduced pressure. Aqueous working-up
(extracting by shaking with 1 N HCI and a saturated solution of NaCI, drying
of the organic phase such as ethyl acetate, methylene chloride or
chloroform with magnesium sulfate or sodium sulfate, and after that
concentrating) subsequently took place. The crude product was either
subjected directly to further reaction or purified by chromatography.
General protocol 2: Sulfonamide from sulfonyl chloride and carboxylic acid
The carboxylic acid was dissolved in 0.5-2 molar NaOH, where appropriate
in the added presence of 10-50% tetrahydrofuran (THF) or DMF. Acid
chloride (1-1.2 equivalents, preferably 1.1 ) was dissolved in THF
(concentration, from 0.05 to 1 M) and this solution was slowly added
dropwise. 2 N NaOH was automatically added at RT, using an autotitrator,
for the purpose of maintaining a constant pH. The set pH was: from 8 to 12,
preferably from 9 to 11. After the termination of the reaction, recognizable
by no further NaOH being consumed, the organic cosolvent was removed
on a rotary evaporator and the aqueous solution or suspension was treated
with ethyl acetate and acidified with 1 N HCI. After the organic phase had
been separated off, and the aqueous phase had been extracted once again
with ethyl acetate, the organic phases were combined and dried over
sodium sulfate; the solvent was subsequently removed under reduced
pressure. The crude product was either subjected directly to further
reaction or purified by chromatography.
CA 02539731 2006-03-21
General protocol 3: Sulfonamide from sulfonyl chloride and carboxylic acid.
This protocol is particularly suitable for the reaction of
biphenylethylsulfonyl
chloride with iminocarboxylic acids (see Example 6 and Example 7) or
5 similar, more hydrolysis-labile sulfonyl chlorides.
8 mmol of the imino acid were dissolved or suspended in 30 ml of
acetonitrile. 2.3 g (9 mmol) of BSTFA (bis(trimethylsilyl)trifluoroacetamide)
were added at RT and under an inert gas (N2) and the mixture was heated
10 for 2 h under reflux. 2.84 g (9 mmol) of 4-chlorobiphenylethanesulfonyl
chloride, dissolved in 30 ml of acetonitrile, were added to this solution and
the whole was once again heated for 3 h under reflux conditions. After the
reaction mixture had cooled down, aqueous 1 N HCI was added and the
mixture was stirred for 1 h; the solvent was then removed under reduced
15 pressure on a rotary evaporator, after which ethyl acetate or chloroform
was added; the organic phase was then separated off, extracted with a
saturated solution of NaCI, dried over sodium sulfate and concentrated
under reduced pressure. Depending on its purity, it was either possible to
subject the reaction product directly to further reaction or necessary to
20 previously chromatograph it through silica gel.
General protocol 4: Preparing the hydroxamic acid from carboxylic acid by
way of chloroformate activation
25 The sulfonated carboxylic acid was dissolved in 10 ml of DMF after which
1.1 equivalents of ethyl chloroformate, 2.2 equivalents of N-ethylmorpholine
and, after a preactivation time of from 30 min to 1 h, 3 equivalents of
trimethylsilylhydroxylamine were added at 0°C. After the mixture had
been
heated at 80°C for at least 4 h, the solvent was removed under reduced
30 pressure and the crude product purified using chromatographic methods.
General protocol 5: Preparing the hydroxamic acid by way of the
corresponding carbonyl chloride
35 The sulfonated carboxylic acid was initially introduced in dry chloroform
(ethanol-free) (about 5 ml for 0.5 mmol) and 3 equivalents of oxalyl chloride
were added at RT. The mixture was then heated at 45°C for about 30 min.
In order to monitor the chloride formation, a small sample was removed
from the reaction flask and treated with a little benzylamine in THF. It was
CA 02539731 2006-03-21
36
possible to ascertain when the reaction was complete by the quantitative
formation of benzylamide; it was no longer possible to detect the carboxylic
acid (monitoring by HPLC-MS). It may be necessary to heat for a longer
period or to heat under reflux conditions. The solvent was then distilled off
under reduced pressure, after which the residue was taken up repeatedly in
dry toluene and once again subjected to rotary evaporation. The acid
chloride was now once again taken up in chloroform (10 ml per 0.5 mmol)
and this mixture was treated, at RT, with 3 equivalents of
O-trimethylsilylhydroxylamine. After a reaction period of at least 30 min
(reaction monitored by HPLC-MS), the reaction mixture was evaporated
under reduced pressure and the residue was purified directly by
chromatography.
Special protocols
4-Chlorobiphenyiethanesulfonyl chloride (intermediate for Example 7)
Step 1: 1-(2-Bromoethenone)-4-(4-chlorophenyl)benzene
4-Chlorobiphenyl (23.6 g, 0.125 mol) was introduced, in portions and at
0°C, into a stirred suspension of AICl3 (34.7 g, 0.26 mol) and
bromoacetyl
bromide (25.2 g, 0.125 mol) in 400 ml of CS2 and the reaction mixture was
then heated under reflux for 3 h. After that, it was slowly poured onto ice
and subsequently extracted with ethyl acetate; the organic phase was then
washed with an aqueous solution of NaHC03 and with water. )t was then
dried over anhydrous sodium sulfate and evaporated under reduced
pressure. The residue which remained was recrystallized from
dichloromethane.
Yield: 24.2 g (62% of theory). m.p.: 127 -128°C
'H-NMR: (300 MHz) 5.0 (s, 2H, CH2); 7.5-8.1 (4 d, 8H, ar)
MS: 311.1 (M+H)
Step 2: 4-Chlorobiphenylethane bromide
tert-Butylamineborane (27.5 g, 0.31 mol) was added, at 0°C, to a
stirred
suspension of AICI3 (20.0 g, 0.15 mol) in dichloromethane (500 ml). After
the mixture had been stirred at 0°C for 15 min, a solution of the
bromoketone from step 1 (16.0 g, 50 mmol) in dichloromethane (150 mi)
was added and the mixture was stirred at 0°C for a further 4 h. Cold
dilute
HCI (1 N, 30 ml) was added dropwise, after which the mixture was extracted
several times with ethyl acetate. The combined organic phases were
washed first with dilute HCI and then with a saturated solution of sodium
CA 02539731 2006-03-21
37
chloride, after which they were evaporated. An oily compound, which was
purified by flash chromatography on silica gel, was obtained.
Yield: 15 g (quantitative), m.p.: 142°C
'H-NMR: (300 MHz) 3.2; 3.78 (2t, 4H, CH2); 7.4-7.7 (4 d, 8H, ar)
MS: 296.2 (M+H)
Step 3: Sodium salt of the 4-chlorobiphenylethanesulfonic acid
The compound from step 2 (14.8 g, 50 mmol) was dissolved in a mixture of
ethanol and water (1:1, 200 ml). Sodium sulfite (9.5 g, 75 mmol) and
tetrabutylammonium iodide (1.8 g, 5 mmol) were added and the mixture
was heated under reflux for 16 h. After that, the liquid reaction mixture was
decanted off from a small quantity of a solid and the volume of this mixture
was reduced by partially evaporating the mixture under reduced pressure.
Then the mixture was cooled, the product crystallized out and was
subsequently filtered off and recrystallized from MeOH/H20. It was then
dried under reduced pressure.
Yield: 13.9 g (94% of theory).
'H-NMR: (300 MHz) 2.6; 2.95 (2 m, 4 H, CH2); 7.3-7.7 (4 d, 8H, ar)
Step 4: 4-Chlorobiphenylethanesulfonyl chloride
Phosphorus pentachloride (3.2 g, 15 mmol) was added to a suspension of
the compound from step 3 (4.8 g, 15 mmol) in phosphorus oxychloride
(50 ml). The mixture was heated at 60°C for 6 h and was subsequently
poured onto ice after methylene chloride had been added. This mixture was
neutralized with a saturated solution of sodium hydrogen carbonate and the
organic phase was separated off, dried and evaporated under reduced
pressure.
Yield: 5 g (quantitative) 'H-NMR: (300 MHz) 2.9 (m, 4H, CH2); 7.3-7.7
(4 d, 8H, ar)
Using chiral high pressure liquid chromatography (HPLC) to separate the
enantiomers of preparation example 3:
Column employed: Chiralpak ADR, 250 x 4.6 mm, 30°C, running
time: 30 min, injection volume: 5 NI, flow rate: 1 ml/min, solvent
MeOH/EtOH 1:1 isocratic, detection at 277 nm.
CA 02539731 2006-03-21
38
isomer 1 (example 14): running time (RT) 6.33 min, 50.48% and isomer 2
(example 15): RT 14.65 min, 49.52%.
Two-dimensional NMR spectroscopy was used to determine the structure
of isomer I. The methodology only makes it possible to determine the
relative stereochemistry, i.e. the positions of the chiral centers in relation
to
each other. This means that, when only one single enantiomer is present,
as is to be expected after an enantiomer separation, this enantiomer can
have either the absolute stereochemistry which is specified or else the
absolute stereochemistry which is the mirror image to it. The final structural
proof can only be obtained by carrying out X-ray structural analyses. This
was done both by means of cocrystallizing with MMP-13 and by means of a
single-crystal structural analysis. Both methods showed unambiguously
that the stereochemistry which is specified is in accordance with the actual
stereochemistry,
Chiral HPLC can likewise be used to carry out chiral separations of the
other compounds which represent such enantiomeric mixtures, i.e. which
have been prepared from the same decahydroisoquinoline derivatives.
Table 1 shows the results:
15 ~6 17 CI
6 H 8 12 13
5 7- 9 1 / ~ 14
4 2 1 N~ 10 \
S
3 H18 O~ ~'O
HN O Molecular Weight =448.97
Exact Mass =448
OH Molecular Formula =C22H25CIN204S
Table 1: Chemical shift of isomer 1 at 300 K.
'H C
1 4.12 55.15
2 1.93 35.68
3 1.67/1.41 26.37
4 1.19 21.03
5 1.48/1.16 24.26
6 1.86/1.12 26.06
7 1.52 32.00
8 1.55/1.45 28.88
9 3.75/3.53 38.95
10 - 138.99
11 7.81 127.29
12 7.87 127.26
13 - 142.45
CA 02539731 2006-03-21
39
14 - 137.19
15 7.78 128.81
16 7.57 129.05
17 - 133.42
18 - 166.96
18-N H 10.86 -
18-NOH 8.88 ~ -
Preparation example 2: N-(4-Chlorobiphenylsulfonyl)decahydroiso-
quinoline-1-carboxylic acid
Decahydroisoquinolinecarboxylic acid was prepared and used as described
in US 5,430,023. The resulting imino acid (2.0 g, 9.1 mmol) was dissolved
or suspended in THF (20 ml) and the pH was adjusted to 10.5 with 1 molar
sodium hydroxide solution using an autotitrator. After that,
4-chlorobiphenylsulfonyl chloride (2.745 g, 9.6 mmol, 1.05 eq.), dissolved in
ml of THF, was added dropwise over a period of 2 hours while the pH
was maintained constant. After a further 2 hours, it was not possible to
10 observe any further consumption of sodium hydroxide solution. LC-MS,
which was carried out as a reaction control, confirmed this. The solution
was then adjusted to a pH of from 3 to 4 with dilute hydrochloric acid;
100 ml of ethyl acetate were then added and the solution was extracted by
being shaken. The aqueous phase was extracted a further 2 times with
small portions of ethyl acetate and the combined organic phases were
dried over sodium sulfate. After the solvent had been removed, there then
remained an oily residue which became solid under an oil pump vacuum.
Yield: 2.31 g (58% of theory). Analytical data: see Table 1.
Preparation example 3: N-(4-Chlorobiphenylsulfonyl)decahydroiso-
quinoline-1-(N-hydroxy)carboxamide
The carboxylic acid from Example 2 (2.3 g, 5.3 mmol) was dissolved in
50 ml of chloroform. Oxalyl chloride (1.345 g, 10.6 mmol, 0.924 ml) was
then added dropwise within the space of 10 min and the resulting reaction
mixture was heated at 45°C for one hour. After this time, a small
sample of
the reaction mixture (0.1 ml) was removed, for monitoring the reaction by
HPLC-MS, and treated with 0.05 ml of benzylamine. Subsequently, the
solvent was distilled off under reduced pressure and the resulting oily
residue was entrained with toluene for the purpose of removing any
possible oxalyl chloride residues or HCI and left under reduced pressure for
15 min. It was then once again taken up in chloroform (50 ml) after which
O-trimethylsilylhydroxylamine (2.23 g, 21.2 mmol, 2.593 ml) was added at
CA 02539731 2006-03-21
RT. After 2 hours, the solvent was removed under reduced pressure and
the residue was dissolved in a small quantity of a mixture of
acetonitrile/water/0.01 % trifluoroacetic acid for the purpose of direct
preparative RP-HPLC. Product fractions were combined, acetonitrile was
5 removed under reduced pressure and the remaining aqueous phase was
freeze-dried. Yield: 749 mg (32% of theory; 36 mg of another diastereomer
of the same molar mass is obtained in addition). Unreacted acid chloride
was reisolated in the form of the carboxylic acid. Analytical data: see
Table 1.
The following examples were prepared in analogy with the previously
mentioned protocols.
Table 2 shows the results.
Table 2:
Example Struch~re Molecular ES+ 1H-NMR
weight
1 0 459.52 460.2 1.1-2.0 (4 m,12 H);
461.2 3.5-3.9 (m, 2 H); 4.15
\ ~ Q (d, 1 H); 7.88; 8.0;
8.07; 8.35 (4 d, 8 H);
0 0 10.9 (s, 1 H)
0
I
ai
2 a 433.95 433.11 1.2-2.1 (4 m, 12 H);
3.4-3.6 (m, 2 H); 4.2
/ ~ (d, 1 H); 7.60; 7.8; (2
w I d, 4 H); 7.9 (m, 4 H);
s
~o ~0 12.8 (s, 1 H)
HO" O
3 ci 448.97 449.15 1.1-2.0 (4 m, 12 H);
451.10 3.5-3.9 (m, 2 H); 4.15
i
I ~ (d, 1 H); 7.60; 7.7;
oos~~ \ 7.8; 7.9 (4 d, 8 H);
0
0 10.9 (s, 1 H)
OH
CA 02539731 2006-03-21
41
4 0 530.52 415.16
N \ \ N I / (ES-)
O ~S~~O
H O
O O F
OH
F
0 \ 545.54 ES- 1.1-2.0 (4 m,12 H);
N ~ ~ N I ~ 430.17 3.4-3.8 (2m, 2 H);
~~0 4.08 (d, 1 H); 7.2 (m,
0
"~ ° F 4 H); 7.45 (m, 2 H);
OH OH
F 8,1 (dd, 1 H); 8.45 (s,
1 H); 10.8 (s,1 H)
6 462.01 461.14 1.15-2.05 (4 m,12
H); 2.9-3.5 (mm,
about 6 H,
0 overlapping with
0
water); 3.85 (m, 1 H);
4.0 (d, 1 H); 7.48;
7.5;7.61;7.7 (4 d,8
H)
7 I 477.03 477.7 1.15-2.05 (m m, 12
H); 2.9-3.5 (mm,
about 6 H,
O~ ~~ \ overlapping with
'O water); 4.85 (m, 1 H);
I 7.48; 7.5; 7.65; 7.72
Oi
(4d,8H)
8 530.52 416.14 1.2-2.1 (4 m,12 H);
0 2.9.3 (mm, about 3
H, overlapping with
~N
Hp ~o o water); 7.3; 7.45;
F 7.95; 8.70 (4 m, 8 H);
F ~ 12.8 (s, 1 H)
F
9 545.54 431.15 1.1-2.0 (4 m, 12 H);
0 3.55 (m, 1 H); 3.8 (m,
1 H); 4.1 (d,1 H);
o '' 7.35; 7.45; 7.9; 8.70
F F (4 d, 8 H); 10.8 (s,
1H)
445.54 446.1
I o _ _ 447.1
N~II
to ~ ~ ~ ~
~o
CA 02539731 2006-03-21
42
11 460.55 461.2 1.1-1.95 (4m, 12 H);
_ _ 462.2 3.45 (m, 1 H); 3.7 (m,
1 H); 3.8 (s, 2 H);
\ O 4.05 (d, 1 H); 7.0 (m,
0
I 4 H); 7.1 (m, 2 H);
ai
7.7 (m, 2 H);10.8 (s,
1 H).
12 513.54 514.15
515.2
III \ ~ / °~ F
\ O I F
O F
~ 3 528.55 529.2 1.1-2.0 (4m, 12 H);
I o 530.2 3.45 (m, 1 H); 3.7 (m,
o F 1 H); 4.07 (d, 1 H);
o F 7.0 (d, 2 H); 7.17 (s,
F
4H);7.7(d,2H);
10.8 (s, 1 H).
14 hemistry 16 8.9726 9.15,4 1.1-2.0 (4 m, 12 H); 3.
1.10 .8 (2m, 2 H); 4.15 (d, 1
a
\ ~ H); 7.55-7.9 (3 m, 8 H)
H~ mss'' \ ~ 8.9 (s, 1 H); 10.9 (s, 1
NH~ p
I O H)
HO
15 hemistry 19 8.9726 9.15,4 1.1-2.0 (4 m, 12 H); 3.
H CHIF,AL
1.10 .8 (2m, 2 H); 4.15 (d, 1
a
\ / H); 7.55-7.9 (3 m, 8 H)
H /
.9 (s, 1 H); 10.9 (s, 1
"° H)
16 hemistry 35 00.6187 01.18 1.25 (d, 6 H); 1.2-1.
(m, 11 H); 2.05 (m, 1
0
o s° ~ H); 3.44; 4.20; 4.90
Ho o I ~ m, 3-4 H); 7.6; 7.7 (dd
N o H); 7.87 (m, 4 H); 9.
(s, 1 H); 12.8 (s, 1 H)
CA 02539731 2006-03-21
43
17 hemistry 36 15.6334 16.19 1.25 (d, 6 H); 1.1-1.9
(m, 12 H); 3.55; 3.75
N~
.12;4.91 (4m,4H)
o ~ i I ~ o ..6-7.88 (2 dd, 8 H)
r
a~
18 hemistry 37 512.5524 13.13 1.1-2.0 (m, 12 H); 3.50
.75, 4.11; 4.83 (4 m,
I 0
H);7.2(d,2H);7.8(m
S 0 F
F H); 8.9 (s, 1 H); 10.
I F
(S, 1 H).
Chemistry 38 98.5253 50.08 1.1-2.0 (m, 12 H); 3.50
19 ~ o .75, 4.15 (3 m, 3 H)
N~
oS ~ .5(d,2H);7.9(m,
o ~ i I ~ F H); 8.9 (s, 1 H); 10.9 (s
O' F F
1 H).
0 hemistry 39 8.5174 9.24 1.1-2.0 (m, 12 H); 3.50
.75, 4.10 (3 m, 3 H)
N ~~
/ \ o .0-7.7 (4 m. 8 H); 8.
0
H IOH o / \ s,1 H); 10.9 (s, 1 H).
F
1 hemistry 44 8.5174 9.21 1.1-2.0 (m, 12 H); 3.50
0 ~ ~m 3.75, 4.10 (3 m, 3 H)
N\ ~ ~ I / F .0-7.7 (4 m. 8 H); 8.
o (s, 1 H); 10.9 (s, 1 H)
0
i
ai
hemistry 49 99.51 500.18 1.2-1.7 (m, 11 H); 2.0
/ ~ 0 ~ '1 F (m, 1 H); 3.5 (m, +/
N,~ ~ / Jc F H); 4.2 (d, 1 H); 7.1
00'' ~\F
p' '' .80 (4 m ("d"), 8 H)
0
12.7 (s, 1 H)
CA 02539731 2006-03-21
44
~3 hemistry 48 14.5247 15.21 1.1-2.0 (m, 12 H); 3.50
0 \ F 3.75, 4.10 (3 m, 3 H)
.1-7.8 (4 m ("d"), 8 H)
.9 (s, 1 H); 10.9 (s, 1
0
0 H)
I
24 hemistry 53 99.5129 00.24 1.1-2.0 (4 m, 12 H); 3.
.8 (2m,2H);4.15(d
~ 1 H); 7.4-8.0 (m, 9 H)
N~
O~SwO
p pH
Chemistry 52 14.5275 15.26 1.1-2.0 (4 m, 12 H); 3.
.8 (2m,2H);4.15(d
1 H); 7.4-8.0 (m, 9 H)
I
ors, ~ .9 (s, 1 H); 10.9 (s, 1
0
O NH )
OH
6 hemistry 55 55.5368 56.30 1.1-2.0 (m, 12 H); 3.50
O \ .75, 4.10 (3 m, 3 H)
7.2 (m, 4 H); 7.8-8.
~~ 00 ~ N (dd, 4 H); 8.9 (s, 1 H)
O 10.9 (s, 1 H)
CH
7 / O 13.4963 14.14 1.2-1.7 (m, 11 H); 2.0
(m, 1 H); 3.5 (m, +/-
H); 4.3 (d, 1 H); 7.5-7.
O
HO O (6 m, 7 H); 8.7 (s, 1 H)
8 Chemistry 57 28.511 29.21 1.1-2.0 (m, 12 H); 3.60
i ° .75, 4.10 (3 m, 3 H)
N~ ~ I ~ ~ 7.5-8.9 (7 m, 8 H); 10.
o' ''
0
HN O (S, 1 H)
I I
OH
CA 02539731 2006-03-21
29 hemistry 62 78.543879.22 1.0-1.95 (m)
and 2.6
.05 (m, together
14 H)
0 ~ ~ 0 / ~ .7(s,3H);6.7-7.8(
8 H); S.9
(s, 1 H)
0~ ~~0
10.9 (2 s,
0 1 H)
I
OH
Pharmacological examples
Determining the enzyme activity of the catalytic domain of human
5 collagenase-1 (MMP-1 ).
This protein is obtained as an inactive proenzyme from Biocol, Potsdam
(catalog No. MMP1 ). Activation of the proenzyme:
2 parts by volume of proenzyme are incubated, at 37°C for 1 hour, with
1 part by volume of APMA solution. The APMA solution is prepared from a
10 10 mmol/l solution of p-aminophenylmercuric acetate in 0.1 mmol/I NaOH
by diluting with 3 parts by volume of tris/HCI buffer, pH 7.5 (see below).
The pH is adjusted to between 7.0 and 7.5 by adding 1 mmol/l HCI. After
the enzyme has been activated, it is diluted with the tris/HCI buffer to a
concentration of 2.5 ~g/ml.
15 In order to measure the enzyme activity, 10 ~I of enzyme solution are
incubated for 15 minutes with 10 ~I of a 3% (v/v) buffered solution of
dimethyl sulfoxide (reaction 1). In order to measure the enzyme inhibitor
activity, 10 ~.I of enzyme solution are incubated with 10 ~I of a 3% (v/v)
buffered solution of dimethyl sulfoxide which contains the enzyme inhibitor
20 (reaction 2).
Both in the case of reaction 1 and in the case of reaction 2, the enzyme
reaction is monitored by fluorescence spectroscopy (328 nm (extinction)/
393 nm (emission)) after adding 10 ~,I of a 3% (v/v) aqueous solution of
dimethyl sulfoxide which contains 0.3 mmol of the substrate/I.
25 The enzyme activity is presented as increase in extinction/minute.
The inhibitory effect is calculated as percentage inhibition using the
following formula: -
inhibition = 100 - [(increase in extinction/minute in reaction 2) / (increase
in extinction/minute in reaction 1 ) x 100].
30 The IC5o, i.e. the inhibitor concentration which is required for 50%
inhibition
of the enzyme activity, is determined graphically by plotting the percentage
inhibitions at different inhibitor concentrations.
CA 02539731 2006-03-21
46
The buffer solution contains 0.05% Brij (Sigma, Deisenhofen, Germany)
and also 0.1 mol of tris/HCl/l, 0.1 mol of NaCl/l, 0.01 mol of CaCl2/l
(pH=7.5).
The enzyme solution contains 2.5 ~g of the enzyme domain/ml.
The substrate solution contains 0.3 mmol of the fluorogenic substrate
(7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2',4'-dinitrophenyl)-L-
2,3-diaminopropionyl-Ala-Arg-NH2/I (Bachem, Heidelberg, Germany).
Preparation, and determination of the enzyme activity, of the catalytic
domain of human stromelysin (MMP-3) and neutrophilic collagenase
(MMP-8).
The two enzymes, i.e. stromelysin (MMP-3) and neutrophilic collagenase
(MMP-8), were prepared as described by Ye et al. (Biochemistry; 31 (1992)
pages 11231-11235). In order to measure the enzyme activity or the
inhibitory effect on the enzyme, 10 p.1 of enzyme solution were incubated,
for 15 minutes, with 10 ~I of a 3% (v/v) buffered solution of dimethyl
sulfoxide which contained the enzyme inhibitor, where appropriate. After
10 ~I of a 3% (v/v) aqueous solution of dimethyl sulfoxide which contained
1 mmol of substrate/l had been added, the enzyme reaction was monitored
by fluorescence spectroscopy (328 nm (ex)/393 nm(em)).
The enzyme activity is presented as increase in extinction/minute. The ICSo
values listed in Table 2 were determined as the inhibitor concentrations
which in each case resulted in the enzyme being inhibited by 50%.
The buffer solution contained 0.05% Brij (Sigma, Deisenhofen, Germany)
as well as 0.1 mol of tris/HCl/l, 0.1 mol of NaCl/l, 0.01 mol of CaCl2/l and
0.1 mol of piperazine-N,N'-bis[2-ethanesulfonic acidJ/l (pH= 7.5).
The MMP-3 enzyme solution contained 2.3 pg, and the MMP-8 enzyme
solution contained 0.6 pg, of one of the enzyme domains/ml prepared as
described by Ye et al. The substrate solution contained 1 mmol of the
fluorogenic substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-
(2',4'-dinitrophenyl)-L-2,3-diaminopropionyl-Ala-Arg-NH2/I (Bachem,
Heidelberg, Germany).
Determining the enzyme activity of the catalytic domain of human
collagenase-3 (MMP-13).
This protein was obtained as an inactive proenzyme from INVITEK, Berlin
(catalog No. 30 100 803). Activation of the proenzyme:
2 parts by volume of proenzyme were incubated, at 37°C for 1.5 hours,
with
1 part by volume of APMA solution. The APMA solution was prepared from
CA 02539731 2006-03-21
47
a 10 mmol/I solution of p-aminophenylmercuric acetate in 0.1 mmol/l NaOH
by diluting the solution with 3 parts by volume of tris/HCl buffer, pH 7.5
(see
below). The pH was adjusted to between 7.0 and 7.5 by adding 1 mmol/l
HCI. After the enzyme had been activated, it was diluted with the tris/HCI
buffer to a concentration of 1.67 ~g/ml.
In order to measure the enzyme activity, 10 p,1 of enzyme solution were
incubated for 15 minutes with 10 p,1 of a 3% (v/v) buffered solution of
dimethyl sulfoxide (reaction 1 ). In order to measure the enzyme inhibitor
activity, 10 p1 of enzyme solution were incubated with 10 p1 of a 3% (v/v)
buffered solution of dimethyl sulfoxide which contained the enzyme inhibitor
(reaction 2).
Both in the case of reaction 1 and in the case of reaction 2, the enzyme
reaction was monitored by fluorescence spectroscopy (328 nm (extinction)/
393 nm (emission)) after 10 p.1 of a 3% (v/v) aqueous solution of dimethyl
sulfoxide which contained 0.075 mmol of the substrate/l had been added.
The enzyme activity was presented as increase in extinction/minute.
The effect of the inhibitor was calculated as a percentage inhibition in
accordance with the following formula:
inhibition = 100 - [(increase in extinction/minute in reaction 2) / (increase
in extinction/minute in reaction 1 ) x 100].
The ICSO, that is the concentration of inhibitor which is required for 50%
inhibition of the enzyme activity, was determined graphically by plotting the
percentage inhibitions at different inhibitor concentrations.
The buffer solution contained 0.05% Brij (Sigma, Deisenhofen, Germany)
and also 0.1 mol of tris/HCl/I, 0.1 mol of NaCI/I, 0.01 mol of CaCl2/l
(pH=7.5). The enzyme solution contained 1.67 ~g of the enzyme
domain/ml. The substrate solution contained 0.075 mmol of the fluorogenic
substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2',4'-
dinitrophenyl)-L-2,3-diaminopropionyl-Ala-Arg-NH2/I (Bachem, Heidelberg,
Germany).
Determining the enzyme activity of the catalytic domain of human
gelatinase A (MMP-2).
This protein was obtained as an inactive proenzyme from INVITEK, Berlin
(catalog No. 30 100 602). Activation of the proenzyme:
2 parts by volume of proenzyme were incubated, at 37°C for 0.5 hour,
with
1 part by volume of APMA solution. The APMA solution was prepared from
CA 02539731 2006-03-21
48
a 10 mmol/l solution of p-aminophenylmercuric acetate in 0.1 mmol/I NaOH
by diluting it with 3 parts by volume of tris/HCI buffer, pH 7.5 (see below).
The pH was adjusted to between 7.0 and 7.5 by adding 1 mmol/l HCI. After
the enzyme had been activated, it was diluted with the tris/HCI buffer to a
concentration of 0.83 ~g/ml.
In order to measure the enzyme activity, 10 p.1 of enzyme solution were
incubated for 15 minutes with 10 ~I of a 3% (v/v) buffered solution of
dimethyl sulfoxide (reaction 1 ). In order to measure the enzyme inhibitor
activity, 10 p.1 of enzyme solution were incubated with 10 ~,I of a 3% (v/v)
buffered solution of dimethyl sulfoxide which contained the enzyme inhibitor
(reaction 2).
Both in the case of reaction 1 and in the case of reaction 2, the enzyme
reaction was monitored by fluorescence spectroscopy (328 nm (extinction)/
393 nm (emission)) after 10 ~I of a 3% (v/v) aqueous solution of dimethyl
sulfoxide which contained 0.3 mmol of the substrate/l had been added.
The enzyme activity was presented as increase in extinction/minute.
The effect of the inhibitor was calculated as percentage inhibition in
accordance with the following formula:
inhibition = 100 - [(increase in extinction/minute in reaction 2) / (increase
in extinction/minute in reaction 1 ) x 100].
The ICSO, that is the concentration of inhibitor which is required for 50%
inhibition of the enzyme activity, was determined graphically by plotting the
percentage inhibitions at different inhibitor concentrations.
The buffer solution contained 0.05% Brij (Sigma, Deisenhofen, Germany)
and also 0.1 mol of trisIHCl/l, 0.1 mol of NaCl/l, 0.01 mol of CaCl2/l
(pH=7.5). The enzyme solution contained 0.83 ~g of the enzyme
domain/ml. The substrate solution contained 0.3 mmol of the fluorogenic
substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2',4'
dinitrophenyl)-L-2,3-diaminopropionyl-Ala-Arg-NH2/I (Sachem, Heidelberg,
Germany).
Determining the enzyme activity of the catalytic domain of human
gelatinase B (MMP-9).
This protein was obtained as an inactive proenzyme from Roche,
Mannheim (catalog No. 1 758 896). Activation of the proenzyme:
2 parts by volume of proenzyme were incubated, at 37°C for 4 hours,
with
1 part by volume of APMA solution. The APMA solution was prepared from
a 10 mmol/l solution of p-aminophenylmercuric acetate in 0.1 mmol/I NaOH
by diluting it with 3 parts by volume of tris/HCl buffer, pH 7.5 (see below).
CA 02539731 2006-03-21
49
The pH was adjusted to between 7.0 and 7.5 by adding 1 mmol/l HCI. After
the enzyme had been activated, it was diluted with the tris/HCI buffer to a
concentration of 4.2 mU/ml.
In order to measure the activity of the enzyme, 10 ~I of enzyme solution
were incubated for 15 minutes with 10 ~I of a 3% (v/v) buffered solution of
dimethyl sulfoxide (reaction 1 ). In order to measure the enzyme inhibitor
activity, 10 ~I of enzyme solution were incubated with 10 ~I of a 3% (v/v)
buffered solution of dimethyl sulfoxide which contained the enzyme inhibitor
(reaction 2).
Both in the case of reaction 1 and in the case of reaction 2, the enzyme
reaction was monitored by fluorescence spectroscopy (328 nm
(extinction)/393 nm (emission)) after 10 p,1 of a 3% (v/v) aqueous solution of
dimethyl sulfoxide, which contained 0.15 mmol of the substrate/I, had been
added.
The enzyme activity was presented as increase in extinction/minute.
The inhibitory effect was calculated as percentage inhibition in accordance
with the following formula:
inhibition = 100 - [(increase in extinctionlminute in reaction 2)/(increase
in extinction/minute in reaction 1 ) X 100].
The ICSO, that is the concentration of inhibitor which is required for 50%
inhibition of the enzyme activity, was determined graphically by plotting the
percentage inhibitions at different inhibitor concentrations.
The buffer solution contained 0.05% Brij (Sigma, Deisenhofen, Germany)
and also 0.1 mol of tris/HCl/l, 0.1 mol of NaCI/I, 0.01 mol of CaCl2/I
(pH=7.5). The enzyme solution contained 4.2 mU of the enzyme
domain/ml. The substrate solution contained 0.15 mmol of the fluorogenic
substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2',4'-
dinitrophenyl)-L-2,3-diaminopropionyl-Ala-Arg-NH2/I (Sachem, Heidelberg,
Germany).
Table 3 below shows the results.
Table 3:
ExampleMMP-1 MMP-2 MMP-3 MMP-8 MMP-9 MMP-13
ICS ICS ICS ICS ICS IC
nnri nnn nm nnn nm nnri
3 400 30 40 3 100 3
5 70 2 28 2.5 1.2 1.8
7 600 4 65 13 3 7
CA 02539731 2006-03-21
9 1000 20 160 22 12 21
11 220 2 25 2.5 2 1.5
13 180 2.6 30 4 2 2
14 400 2 25 2.2 3.5 2
15 6000 2300 10000 2000 4000 2300
19 2100 3 59 10 5 3
20 41 2 26 3 2 2
21 21 1.5 23 2.3 0.45 1.3
23 290 2 50 9 2 2.1
26 450 3 41 16 3 3