Note: Descriptions are shown in the official language in which they were submitted.
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-1-
CONTROLLING BLANKING DURING MAGNETIC RESONANCE IMAGING
The invention relates to magnetic resonance imaging (MRI) techniques.
Magnetic resonance imaging (MRI) techniques make use of electromagnetic fields
to create images of a patient. MRI techniques permit the generation of high-
quality two-
or three-dimensional images of a patient's body, which can then be examined by
a
physician for diagnosis purposes. In particular, MRI techniques permit the
generation of
internal images of a patient's flesh, blood, bones, cartilage, blood vessels,
organs, and the
like. The generated images can then be examined by physicians in order to
diagnose
disease, disorders or injuries and facilitate patient care.
MRI devices typically subject a patient to a very strong static magnetic field
and a
pulsed gradient magnetic field, and then apply pulses or bursts of
electromagnetic
radiation (typically radio frequency (RF) radiation bursts) to an area of the
patient to be
imaged. The strong magnetic field generally orients the protons of the
patient's tissue in
particular directions. However, the RF radiation bursts cause some of the
patient's protons
to resonate, or spin, at a particular frequency depending on the local
magnetic field during
application of the radiation burst. The resonance frequency in MRI is referred
to as the
Larmour frequency which has a linear relationship with the local magnetic
field. When
the RF radiation burst is terminated, the resonating protons reorient
themselves in
accordance with the strong magnetic field of the MRI device, giving off energy
in the
process. The MRI device can detect the energy given off by the reorienting
protons in
order to create a high quality image of the patient's tissue.
A wide variety of implantable medical devices (IMDs) have also been developed
in
order to monitor patient conditions or possibly deliver therapy to the
patient. One
common example of an IMD is a pacemaker. A pacemaker typically includes one or
more
pacing and sensing leads for delivery of pacing pulses to a patient's heart.
Another
example of an IMD is a combination pacemaker-cardioverter-defibrillator. Other
examples include implantable brain stimulators, implantable gastric system
stimulators,
implantable nerve stimulators or muscle stimulators, implantable lower colon
stimulators,
implantable drug or beneficial agent dispensers or pumps, implantable cardiac
signal loops
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-2-
or other types of recorders or monitors, implantable gene therapy delivery
devices,
implantable incontinence prevention or monitoring devices, implantable insulin
pumps or
monitoring devices, and so on.
Conventionally, patients that use IMDs are generally discouraged or prohibited
from being subjected to MRI. For one thing, the strong static magnetic fields
associated
with MRI techniques may interact with the components of the IMD, possibly
causing
movement of the IMD within the patient because of magnetic attraction or
repulsion. The
interaction of the strong magnetic field with the IMD may cause trauma to the
patient.
However, reductions in the mass of IMDs, as well as use of non-magnetic
material or
other selected material in IMD construction, may reduce or eliminate the
interaction of
such magnetic fields with the IMD.
In general, the invention is directed to techniques for coordinating the
operation of
an IMD with MRI techniques. By coordinating the performance of MRI techniques
with
defined operation of the IMD, the use of MRI techniques on a patient that has
an IMD can
be facilitated. In particular, the timing of electromagnetic radiation bursts
emitted by an
MRI device can be communicated to the IMD. The IMD can respond to the timing
information by activating a "blanking period" during the time when the
electromagnetic
radiation bursts occur. A blanking period refers to a period during which one
or more
sensing components of the IMD, such as sensing amplifiers, are disabled within
the IMD
sensing circuitry. Blanking periods coordinated with MRI electromagnetic
radiation
bursts and gradients can avoid undesirable action by the IMD in response to
the
electromagnetic radiation bursts.
Even after solving problems associated with interaction between a strong
magnetic
Eeld of an MRI and an IMD in a patient, other problems may still limit the
ability to use
MRI in patients that have an IMD. In particular, the RF radiation bursts
associated with
MRI techniques may interfere with IMD operation, possibly causing
miscalculations by
the IMD, or worse yet, undesirable therapy to be delivered to the patient by
the IMD. By
causing the IMD to activate or otherwise enter blanking periods during the
application of
the electromagnetic radiation bursts, IMD operation may be more compatible
with MRI.
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-3-
In one embodiment, the invention provides a method of coordinating MRI
comprising blanking one or more components of an IMD during delivery of
electromagnetic radiation bursts to a patient.
In another embodiment, the invention provides an implantable medical device
comprising a receiver to receive a signal, and a control unit that in response
to the. signal,
blanks one or more components of an IMD during application of MRI
electromagnetic
radiation bursts.
In another embodiment, the invention provides an implantable medical device
(IMD) that disables one or more components during delivery of MRI
electromagnetic
radiation bursts to a patient.
In another embodiment, the invention provides a system comprising an MRI
device including a transmitter to transmit a signal relating to application of
an MRI
electromagnetic radiation burst, and an IMD including a receiver to receive
the signal, and
a control unit to blank one or more components of the IMD during application
of the MRI
electromagnetic radiation burst.
In another embodiment, the invention provides a system comprising a programmer
to define a timing for application of a magnetic resonance imaging (MRI)
electromagnetic
radiation burst, an MRI device to receive a first signal from the programmer
and apply the
electromagnetic radiation burst according to the timing, and an IMD to receive
a second
signal from the programmer and blank one or more components of the IMD during
application of the MRI electromagnetic radiation Burst.
In another embodiment, the invention provides an apparatus comprising means
for
receiving an indication of a timing of an application of an MRI
electromagnetic radiation
burst, and means for blanking one or more components of an IMD during
application of
the MRI electromagnetic radiation burst.
In another embodiment, the invention provides an MRI device that sends a
signal
to an IMD to cause the IMD to blank on or more components during application
of one or
more electromagnetic radiation bursts by the MRI device.
The different embodiments may be capable of providing a number of advantages.
For example, by coordinating the performance of MRI techniques with operation
of the
IMD, the use of MRI techniques on patients that have an IMD can be
facilitated. In
particular, blanking periods coordinated with MRI electromagnetic radiation
bursts can
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-4-
avoid undesirable action by the IMD in response to the electromagnetic
radiation bursts.
In general, by facilitating the use of M1RI techniques on patients that have
an IMD, patient
care can be improved.
The details of one or more embodiments of the invention are set forth in the
accompanying drawings and the description below. Other features, objects, and
advantages of the invention will be apparent from the description and
drawings, and from
'the claims.
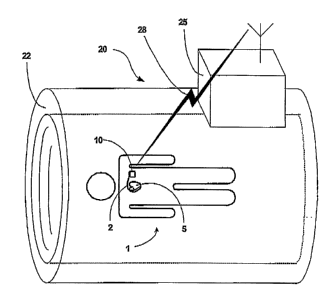
FIG. 1 is a conceptual diagram illustrating a magnetic resonance imaging (MRI)
device communicating with an implantable medical device (IMD).
FIG. 2 is a functional block diagram of an MhZI device communicating with an
IMD.
FIG. 3 is a flow diagram illustrating a technique for coordinating MIZI
techniques
with the operation of an IMD according to an embodiment of the invention.
FIG. 4 is another conceptual diagram illustrating an external programmer
coordinating an MRI device and an IMD in accordance with an embodiment of the
invention.
The invention is directed to techniques for coordinating the operation of an
implantable medical device (IMD) with magnetic resonance imaging (M1RI)
techniques.
Such coordination may improve, or possibly facilitate and allow the use of
MhRI
techniques on patients that have an IMD. In particular, timing of
electromagnetic
radiation bursts in M1RI techniques can be communicated to the IMD prior to
execution of
the electromagnetic radiation bursts. The IMD can respond to the timing
information by
activating "blanking periods" during the time when the electromagnetic
radiation bursts
occur. A blanking period refers to a period during which one or more sensing
components
of the IMD, such as sensing amplifiers are disabled from the IMD sensing
circuitry.
Synchronizing IMD blanking periods with times when MI2I electromagnetic
radiation
bursts occur can avoid undesirable action by the IMD in response to the
electromagnetic
radiation bursts. In particular, sensing and responsive stimulation to the
bursts may be
avoided. In some embodiments, a simple control signal can be sent to the IMD
to cause
activation of blanking periods, e.g., just prior to applying the
electromagnetic radiation
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-5-
bursts. Also, special protections of the IMDs sensitive circuits can be
initiated, or active
measures can be initiated to reduce RF and gradient susceptibility of the lead
system of the
IMD, which is another form of blanking.
FIG 1 is a conceptual diagram of a patient 1 inside an MRI device 20. Patient
1
has an IMD 10. By way of example, IMD 10 is illustrated as a cardiac pacemaker
that
provides therapeutic electrical stimulation to heart 5. However, in accordance
with the
invention, IMD 10 may generally comprise any of a wide variety of medical
devices that
can be implanted in the body of a human or other life form. For example, IMD
10 may
alternatively take the form of an implantable cardioverter, an implantable
defibrillator, or
an implantable cardiac pacemaker-cardioverter-defibrillator. IMD 10 may
deliver pacing,
cardioversion or defibrillation pulses to a patient via electrodes disposed on
distal ends of
one or more leads 2. In other words, one or more leads 2 may position one or
more
electrodes with respect to various cardiac locations so that IMD 10 can
deliver pulses to
the appropriate locations.
In addition, the techniques described herein may useful to coordinate MRI
techniques with other IMDs, such as patient monitoring devices, or devices
that integrate
monitoring and stimulation features. Also, the invention may be used with a
neurological
device such as a deep-brain stimulation device or a spinal cord stimulation
device. In
other applications, the invention described herein may be used with devices
that provide
muscular~stimulation therapy, gastric system stimulation, nerve stimulation,
lower colon
stimulation, drug or beneficial agent dispensing, recording or monitoring,
gene therapy, or
the like. In short, the techniques described herein for coordinating MRI
techniques with
IMD operation may find useful applications in any of a wide variety IMDs.
MRI device 20 may assume a wide variety of shapes, sizes or configurations. In
the illustrated example of FIG. 1, MRI device 20 defines a relatively large
tubular cavity
22 into which patient 1 can be placed during performance of the MRI
techniques. In other
cases, however, MRI device 20 may define a much smaller cavity, e.g., for
insertion of a
patients arnl, leg, head, or the like. In any case, MRI device 20 includes a
set of MRI
components inside housing 25, such as circuitry, magnets, inductors and the
like, that
support operation of MRI device 20.
In particular, MRI device 20 makes use of electromagnetic fields to create
images
of patient 1. For example, MRI device 20 may subject a patient to a very
static strong
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-6-
magnetic fields and gradient fields via one or more permanent magnets or
electro magnets
located about cavity 22 or within housing 25. MRI device 20 then applies
radiation bursts,
e.g., pulses of electromagnetic radiation (typically radio frequency (RF)
radiation) to an
area of the patient 1 to be imaged. For example, housing 25 may house various
components that generate and apply RF radiation bursts at desired frequencies
associated
with the particular tissue of patient 1 to be imaged.
The strong magnetic field generally orients the protons of patient 1 in
particular
directions. However, the RF radiation bursts cause some of the patient's
protons to
resonate, or spin, at a particular frequency during the application of the RF
radiation
bursts. The resonance frequency applied by MRI device 20 is referred to as the
Larmour
frequency which has a linear relationship with the local magnetic field. When
an RF
radiation burst is terminated, the resonating protons reorient in accordance
with the strong
magnetic field of the MRI device, giving off energy in the process. MRI device
20 can
detect the energy given off by the local reorienting protons at the different
positions in
patient 1 to create a high quality image of the tissue or matter of patient 1.
In accordance with the invention, MRI device 20 and IMD 10 coordinate
operation
so as to avoid undesirable action by IMD 10 during MRI operation. In
particular, MRI
device 20 and IMD 10 coordinate to ensure that certain functions of IMD 10,
such as
sensing functions, are disabled or blanked, during the application of the RF
radiation
bursts. For example, one or more wireless signals 28 can be communicated
between IMD
10 and MRI device 20 to achieve such coordination. In this manner, it can be
ensured that
IMD 10 will not produce undesirable and incorrect sensing results because of
the presence
of the RF radiation field during the burst. Moreover, undesirable action by
IMD 10, such
as undesirable therapeutic pacing in response to sensing of the gradient and
the RF
radiation bursts can be avoided. Accordingly, such coordination between MRI
device 20
and IMD 10 may facilitate the use of MRI techniques with patient 1 that has
IMD 10.
Blanking refers to a technique in which the functionality of one or more
components of an IMD 10 are temporarily disabled. A blanking period refers to
the period
of time during which such blanking occurs. Conventionally, blanking is used in
cardiac
pacemakers for a brief blancing period following application of a stimulus.
For example,
some conventional pacemakers enter a blanking period of approximately 20-50
msec.
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
following application of a electrical stimulus to the heart. If an electrical
event occurs
during this blanking period, the event will generally not be sensed.
In accordance with the invention, blanking periods can be coordinated with the
application of MRI electromagnetic radiation bursts and the application of
gradient fields
in order to ensure that electrical events associated with the radiation bursts
and gradients
are not sensed. If sensed, the radiation bursts or gradients might be
misinterpreted by IMD
10, possibly causing IMD 10 to respond in a manner that would be undesirable.
In
addition, sensing during the radiation bursts may cause saturation one or more
sensors,
which can take IMD 10 many milliseconds or even seconds to recover. By causing
IMD
10 to enter a blanking period during the time which the electromagnetic
radiation burst is
applied (or a larger blanking period that spans more time than the burst
period), electrical
events that occur during application of the radiation bursts can be ignored.
Also, the IMD
may change its internal impedance to the lead system, or perform actions to
reduce the
receiving performance of the lead, which is also a form of blanking. This can
ultimately
reduce currents at the electrodes of the lead system. In these ways, operation
of IMD 10
may be more compatible with MRI techniques, possibly allowing patients that
would have
been conventionally prohibited from obtaining an MRI to gain access to this
beneficial
medical imaging tool.
FIG. 2 is a block diagram illustrating a system 30 that includes an MRI device
20
and an IMD 10. In system 30, MRI device 20 communicates to IMD 10 via wireless
signals 28. In.particular, any of a wide variety of telemetry techniques may
be used to
facilitate transfer of information from MRI device 20 to IMD 10. The
transferred
information provides IMD 10 with an indication of the timing, e.g., the start
time and
duration, of one or more electromagnetic radiation bursts to be applied by MRI
device 20.
Accordingly, IMD 10 can use this information to define one,or more blanking
periods as
described herein. Alternatively, the MRI device 20 may simply communicate one
or more
control signals to cause IMD 10 to activate a blanking period, e.g., just
before application
of an electromagnetic radiation burst. The communication of timing information
may
provide absolute timing control, whereas the sending of control signals may
provide
relative timing control.
IMD 10 includes a receiver 32 and an antenna 34 to facilitate reception of
wireless
signals 28 from MRI device 20. IMD 10 also includes circuitry 36 for sensing
and/or
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
_g_
stimulating a patient for therapeutic purposes. For example,
sensing/stimulation circuitry
36 may include electrodes disposed on medical leads and implanted at locations
in a
patient where sensing and stimulation occurs. Sensing/stimulation circuitry 36
typically
includes one or more amplifiers to enhance the cardiac signals for effective
sensing or to
generate the electrical potentials needed for effective sensing and/or
stimulation.
IMD control unit 38 coordinates circuitry 36 so that sensing and stimulation
occurs
at proper times. In particular, IMD control unit 38 may define various sensing
and
stimulation algorithms that define the therapy to be provided. For example, if
IMD 10 is a
cardiac pacemaker, IMD control unit 38 may execute algorithms that interpret
sensed
information from circuitry 36 and determine whether an arrhythmia has occurred
in the
heart. If IMD control unit 38 identifies an arrhythmia, it may store this
information, and
possibly respond by causing circuitry 36 to provide stimulation therapy
specifically for the
identified arrhythmia. IMD control unit 38 may execute a number of algorithms
to
identify and respond to a wide variety of potential arrhythmias in the
patient's heart.
MRI, device 20 includes a transmitter 42 and an antenna 44 to facilitate
transmission of wireless signals 28 to IMD 10. MRI device 20 makes use of
electromagnetic fields to create images of a patient. In particular, MRI
techniques are
particularly useful in creating images of blood flow, images to facilitate
identification of
cancer, or other images that can not be easily generated via conventional
imaging
techniques such as X-ray techniques, or the like
MRI device 20 includes one or more magnetic field generators 45 and one or
more
electromagnetic radiation sources 46. In particular, magnetic field generator
45 generate a
relatively large magnetic field, e.g., in the range of 0.2 to 20 Tesla.
Magnetic field
generator 45 may include a permanent magnet, an electromagnet, or the like,
and may also
include gradient field generators to impose gradient fields during the MRI. In
addition,
MRI device 20 includes one or more electromagnetic radiation sources 46, such
as radio
frequency (RF) radiation sources. As outlined above, MRI device 20 subjects a
patient to
a very strong magnetic field via magnetic field generator 45. Electromagnetic
radiation
source 46 of MRI device 20 then applies pulses or bursts of electromagnetic
radiation
(typically RF radiation) to an area of the patient to be imaged. The strong
magnetic field
of magnetic field generators 45 generally orients the protons of patient in
particular
directions, but the RF radiation bursts of electromagnetic radiation source 46
causes some
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-9-
of the patient's protons to resonate. When the RF radiation burst is
terminated, the
resonating protons reorient in accordance with the local strong magnetic field
of the
magnetic field generators 45, giving off energy in the process.
Imaging unit 48 of MRI device 20 can receive and detect the energy given off
by
the reorienting protons. Imaging unit 48 uses the detected energy given off by
the
reorienting protons to create one or more images of the tissue or matter of
the patient. In
this manner, MRI device 20 is used to create medical images.
MRI control unit 49 coordinates the application of RF radiation bursts by
electromagnetic radiation source 46, and the imaging by imaging unit 48. In
particular,
MRI control unit 49 may define the timing of the RF radiation bursts by
electromagnetic
radiation source 46, including the start time and duration of any given burst.
MRI control
unit 49 may perform one or more algorithms to coordinate and define the MRI
teclmiques
of MRI device 20. In addition, MRI control unit 49 may blank one or more
electrical
components of MRL device 20 during application of the RF radiation bursts,
e.g., to avoid
electrical interference or malfunction of the components.
In accordance with the invention, MRI device 20 communicates information (or a
control signal) to IMD 10 via transmitter 42 arid antenna 44. More
specifically,
information in MRI control unit 49 defining the timing of RF radiation bursts
to be applied
by electromagnetic radiation source 46 can be communicated to IMD 10 to via
transmitter
42 and antenna 44. This timing information may include a start time of a
burst, a duration
of a burst, information regarding sequence of bursts, or the like, that
defines when one or
more of the RF radiation bursts are to occur. Moreover, the information may
include
indication of gradient field application by MRI device 20. MRI control unit 49
may
generate this information specifically for sending to IMD 10, or may have
already
generated the information for purposes of blanking one or more components of
MRI
device 20 during application of the RF radiation bursts. In the latter case,
the same
information used by MRI control unit 49 to cause blanking of one or more
components of
MRI device 20 can be communicated to IMD 10 to facilitate blanking of one or
more
components of IMD 10. IMD 10 uses the timing information to blank sensing or
stimulation amplifiers within circuitry 36 during the application of RF
radiation bursts by
MRI device 20. Alternatively, MRI device may use the generated timing
information to
send one or more commands or control signals to IMD 10 to cause activation of
blanking.
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-10-
Importantly, blanking is activated during the electromagnetic radiation
bursts. If desired
an internal clock of MRI device 20 and IMD 10 may be synchronized to improve
timing
of the blanking periods. For example, clock synchronization may be
communicated
between the devices to achieve such synchronization. Alternatively, MRI
control unit 49
may send the information to an IMD programmer, which can coordinate blanking
in IMD
10.
FIG. 3 is a flow diagram illustrating a technique for coordinating MRI
techniques
with the operation of an IMD according to an embodiment of the invention. As
show in
FIG. 3, IMD 10 receives a signal indicating timing of a burst interval of MRI
(51). In
some cases, the signal may specify timing of applications of bursts and
gradients. For
example, IMD 10 may receive the signal from MRI device 20, or alternatively
from
another device such as an external programmer that coordinates MRI techniques
with IMD
operation. The timing of the burst interval may be defined, e.g., by a start
time and a
duration, although other variables may also be included in the timing such as
a timing
sequence that defines timing for a number of bursts. Upon receiving the signal
that
indicates the timing of the burst interval, IMD 10 subsequently initiates a
blanlcing period
just prior to the burst interval (52). Again, in alternative embodiments MRI
device 20 may
send a control signal that initiates the blanking. In any case, the blanking
period may be
defined to substantially correspond to the burst interval, or may be made
slightly larger
than the burst interval in order to ensure that the blanking period does not
begin late or
terminate early.
Once the burst interval is done (yes branch of 53), IMD 10 terminates the
blanking
period (54). Thus, the sensing components that were disabled during the
blanking interval,
are reactivated following the blanking period. Accordingly, following
termination of the
blanking period, IMD 10 is fully capable of sensing and/or stimulating the
patient for
therapeutic purposes. This is very useful because if the RF radiation burst
caused negative
effects to the patient, or if an episode such as an arrhythmia in the heart
occurs when the
patient is in the MRI device 20, IMD 10 may be capable of sensing and
responding to the
episode. Accordingly, blanking IMD 10 only at selected times during MRI
techniques
may provide a number of advantages over a complete disabling of IMD 10, most
notably
that patient conditions can be monitored and therapy may be provided during
the MRI, if
necessary.
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-11-
Moreover, operation of IMD 10 itself may be used to improve the MRI process by
providing improved sensing and/or stimulation specifically for the MRI
process. In other
words, an IMD may'sense conditions or provide stimulation specifically for the
purpose of
enhancing MRI. For example, a cardiac pacemaker can be used to sense or
stimulate the
heart so as to more properly ensure that the heart is in a desired interval of
sinus rhythm
when MRI radiation bursts are applied for imaging. Such techniques of sensing
or
stimulating the heart to coordinate MRI radiation bursts at specific intervals
of sinus
rhythm may be used in conjunction with the techniques that define blanking
periods
during the radiation bursts. In contrast, if the IMD is disabled during the
MRI process,
such advantages associated with IMD operation in the MRI device could not be
achieved.
In any event, following termination of the blanking period, the process may
repeat.if
another MRI radiation burst is to be performed (yes branch of 55).
Alternatively, the
timing information in a received signal may define a number of MRI radiation
bursts, e.g.,
a sequence of bursts. In that case, a number of blanking periods may be
executed by IMD
10 in response to one received signal that communicates the sequence to IMD
10.
FIG. 4 is another conceptual diagram illustrating an alternative configuration
in
which an external programmer 60 coordinates MRI device 20 and IMD 10. In other
words, in system 70, programmer 60 defines the timing of MRI radiation bursts
and
communicates signals to IMD 10 and MRI device 20. First signal 71 may be a
wireless
signal, whereas the second signal may be transmitted over wire 72. In some
cases,
however, a wireless interface may be used between programmer 60 and MRI device
20.
The first and second signals sent from programmer 60 respectively to IMD 10
and MRI
device 20 may be substantially similar, may be specifically defined for
communication
with the different receiving device 10 or 20. In any case, MRI device 20
applies MRI
electromagnetic radiation bursts according to timing communicated from
programmer 60,
and IMD 10 enters blanking periods during such application of the radiation
bursts by
using the timing information communicated from programmer 60. Application of
gradient
fields by MRI device 20 and blanking by IMD 10 may also be coordinated.
In some cases, programmer 60 receive signals via wire 72 from MRI device 20
defining the timing of RF radiation bursts, and communicate signals 71 to IMD
10 so as to
forward this information for use by IMD 10 in blanking. Also, programmer 60
may also
CA 02540206 2006-03-24
WO 2005/035048 PCT/US2004/031578
-12-
use the received signals from MRI device 20 that define the timing in order to
ensure that
telemetry does not occur during the RF radiation bursts.
A number of embodiments of the invention have been described. However, one
slcilled in the art will appreciate that the invention can be practiced with
embodiments
other than those disclosed. For example, in other embodiments, IMD 10 may
measure or
detect the electromagnetic radiation bursts, and activate blanking upon such
detection.
The disclosed embodiments are presented for purposes of illustration and not
limitation,
and the invention is limited only by the claims that follow.