Note: Descriptions are shown in the official language in which they were submitted.
CA 02540501 2011-08-22
25771-1152
-1-
Quinoline derivatives and quinazoline derivatives, medicaments containing
said compounds, use thereof, and method for the production thereof
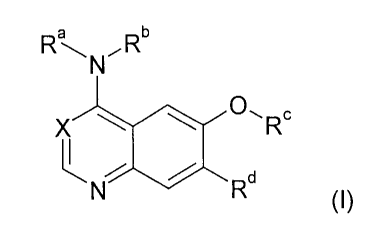
The present invention relates to bicyclic heterocycles of the general formula
Ra -, NRb
X 0,RC
d
N R
(I),
their tautomers, their stereoisomers, their mixtures and their salts, in
particular their
physiologically tolerable salts with inorganic or organic acids, which have
valuable
pharmacological properties, in particular an inhibitory action on the signal
transduction
mediated by tyrosine kinases, their use for the treatment of illnesses, in
particular of
oncoses and of benign prostate hyperplasia (BPH), of diseases of the lung and
of the
airways, and their preparation.
In the above general formula I
Ra is a hydrogen atom or a C1 -alkyl group,
Rb is a 1-phenylethyl group, in which the phenyl nucleus is in each case
substituted by
the groups R' to R3, where
R1 and R2, which can be identical or different, are in each case a hydrogen,
fluorine, chlorine, bromine or iodine atom,
a C1_4-alkyl, hydroxyl, C1.4-alkoxy, C2.3-alkenyl or C2_3-alkynyl group,
an aryl, aryloxy, arylmethyl or arylmethoxy group,
a heteroaryl, heteroaryloxy, heteroarylmethyl or heteroarylmethoxy group,
CA 02540501 2006-03-28
-2-
a methyl or methoxy group substituted by 1 to 3 fluorine atoms or
a cyano, nitro or amino group, and
R3 is a hydrogen, fluorine, chlorine or bromine atom or
a methyl or trifluoromethyl group,
Rc is a cyclobutyl, cyclopentyl or cyclohexyl group, which is in each case
substituted by
1o a group R4-N-R5, where
R4 is a hydrogen atom or a C1_3-alkyl group and
R5 is a hydrogen atom or a C1.3-alkyl group,
an aminocarbonyl-C1_3-alkyl, C1_3-alkylaminocarbonyl-C1.3-alkyl, di-(C1-3-
alkyl)-
aminocarbonyl-C1_3-alkyl, pyrrolidin-1-ylcarbonyl-C1_3-alkyl, piperidin-1-
ylcarbonyl-
C1_3-alkyl, homopiperidin-1-ylcarbonyl-C1_3-alkyl, morpholin-4-ylcarbonyl-
C1_3-alkyl, homomorpholin-4-ylcarbonyl-C1_3-alkyl, piperazin-1 -ylcarbonyl-
C1_3-alkyl, 4-C1_3-alkylpiperazin-1-ylcarbonyl-C1_3-alkyl, homopiperazin-1-yl-
carbonyl-C1_3-alkyl or a 4-C1_3-alkylhomopiperazin-1-ylcarbonyl-C1.3-alkyl
group,
a hydroxy-C2_4-alkyl, C1_3-alkyloxy-C2_4-alkyl, C1_4-alkyloxycarbonylamino-
C2_4-
alkyl, amino-C2_4-alkyl, C1_3-alkylamino-C2_4-alkyl, di-(C1_3-alkyl)amino-C2_4-
alkyl,
C1_3-alkylcarbonylamino-C2_4-alkyl, am inocarbonylamino-C2_4-alkyl, C1_3-alkyl-
aminocarbonylamino-C2_4-alkyl, di-(C1.3-alkyl)aminocarbonylamino-C2_4-alkyl,
pyrrolidin-1-ylcarbonylamino-C2_4-alkyl, piperidin-1-ylcarbonylamino-C2_4-
alkyl,
morpholin-4-ylcarbonylamino-C2_4-alkyl, C1_3-alkylsulphonyl-C2_4-alkyl or a
C1_3-alkylsulphonylamino-C2_4-alkyl group,
a (2-oxopyrrolidin-1-yl)-C2_4-alkyl, (2-oxopiperidin-1-yl)-C2_4-alkyl, (3-oxo-
morpholin-4-yl)-C2_4-alkyl, (2-oxoimidazolidin-1-yl)-C2_4-alkyl, (2-oxo-3-C1_3-
alkyl-
imidazolidin-1-yl)-C2_4-alkyl, (2-oxohexahydropyrimidin-1-yl)-C2_4-alkyl or a
(2-oxo-
3-C1_3-alkylhexahydropyrimidin-1-yl)-C2.4-alkyl group,
CA 02540501 2006-03-28
-3-
a C1_4-alkylsulphonyl, chloro-C1_4-alkylsulphonyl, bromo-C1_4-aikylsulphonyl,
amino-C1_4-aikylsulphonyl, C1_3-alkylamino-C1.4-aikylsulphonyl, di-(C1_3-
alkyl)-
amino-C1_4-alkylsulphonyl, (pyrrolidin-1-yl)-C1_4-alkylsulphonyl, (piperidin-1-
yl)-
C1_4-alkylsulphonyl, (homopiperidin-1-yl)-C1_4-alkylsulphonyl, (morpholin-4-
yl)-
C1_4-alkylsulphonyl, (homomorpholin-4-yl)-C1_4-alkylsulphonyl, (piperazin-1-
yl)-
C1_4-alkylsulphonyl, (4-C1_3-alkylpiperazin-1-yl)-C1.4-alkylsulphonyl, (homo-
piperazin-1-yl)-C1_4-alkylsulphonyl or a (4-C1_3-alkylhomopiperazin-1-yl)-
C1_4-alkylsulphonyl group,
a C1_4-alkyloxycarbonyl group,
a formyl, C1_4-alkylcarbonyl, C1_3-alkyloxy-C1_4-alkylcarbonyl,
tetrahydrofuranyl-
carbonyl, tetrahydropyranylcarbonyl, amino-C1_4-alkylcarbonyl, C1_3-alkylamino-
C1_4-alkylcarbonyl, di-(C1_3-alkyl)amino-C1_4-alkylcarbonyl, pyrrolidin-1-yl-
C1_4-alkylcarbonyl, piperidin-1-yI-C1_4-alkylcarbonyl, (homopiperidin-1-yl)-
C1_4-alkylcarbonyl, morpholin-4-yI-C1_4-alkylcarbonyl, (homomorpholin-4-yl)-
C1_4-alkylcarbonyl, (piperazin-1-yl)-C1.4-alkylcarbonyl, (4-C1_3-
alkylpiperazin-1-yl)-
C1_4-alkylcarbonyl, (homopiperazin-1-yl)-C1_4-alkylcarbonyl, (4-C1_3-alkyl-
homo-
piperazin-1-yl)-C1_4-alkylcarbonyl or a C1_3-alkylsulphonyl-C1_4-alkylcarbonyl
group,
a cyano, aminocarbonyl, C1_3-alkylaminocarbonyl, di-(C1_3-alkyl)aminocarbonyl,
(C1_3-alkyloxy-C2_4-alkyl)aminocarbonyl, N-(C1_3-alkyl)-N-(C1_3-alkyloxy-C2_4-
alkyl)-
aminocarbonyl, arylaminocarbonyl, pyrrolidin-1-ylcarbonyl, piperidin-1-
ylcarbonyl,
homopiperidin-1 -ylcarbonyl, morpholin-4-ylcarbonyl, homomorpholin-4-yl-
carbonyl, 2-oxa-5-azabicyclo[2.2.1 ]hept-5-ylcarbonyl, 3-oxa-8-
azabicyclo[3.2.1 ]-
oct-8-ylcarbonyl, 8-oxa-3-azabicyclo[3.2. 1 ]oct-3-ylcarbonyl, piperazin-1-yl-
carbonyl, 4-C1.3-alkylpiperazin-1-ylcarbonyl, homopiperazin-1-ylcarbonyl,
4-C1_3-alkylhomopiperazin-1-ylcarbonyl, aminosuiphonyl, C1_3-alkyl-
aminosulphonyl, di-(C1_3-alkyl)aminosulphonyl, pyrrolidin-1-yl-sulphonyl,
piperidin-1-ylsulphonyl, homopiperidin-1-ylsulphonyl, morpholin-4-ylsulphonyl,
homomorpholin-4-yl-sulphonyl, piperazin-1-ylsulphonyl, 4-C1_3-alkylpiperazin-1-
yisulphonyl, homo-piperazin-1-ylsulphonyl or a 4-C1_3-alkylhomopiperazin-1-yl-
suiphonyl group,
CA 02540501 2006-03-28
-4-
a cyclobutyl, cyclopentyl or cyclohexyl group, which is in each case
substituted by a
group R6, where
R6 is a 2-oxopyrrolidin-1-yl, 2-oxopiperidin-1-yl, 3-oxomorpholin-4-yl, 2-oxo-
imidazolidin-1-yl, 2-oxo-3-C1_3-alkylimidazolidin-1-yl, 2-
oxohexahydropyrimidin-1-
yl or a 2-oxo-3-C1_3-alkylhexahydropyrimidin-1-yl group,
an azetidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5
1o is defined as mentioned above,
a pyrrolidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5
is defined as mentioned above,
a piperidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5 is
defined as mentioned above,
a piperidin-4-yl group, which is substituted in the 1-position by the group
R5, where R5 is
defined as mentioned above,
or a tetra hydrofuran-3-yl, tetra hydropyran-3-yl or tetrahydropyran-4-yl
group,
Rd is a hydrogen atom or a fluorine, chlorine or bromine atom,
a hydroxyl group,
a C1_4-alkyloxy group,
a methoxy group substituted by 1 to 3 fluorine atoms,
an ethyloxy group substituted by 1 to 5 fluorine atoms,
6
a C2_4-alkyloxy group, which is substituted by the group R or R7, where
CA 02540501 2006-03-28
-5-
R6 is defined as mentioned above and
R7 is a hydroxyl, C1_3-alkyloxy, C3_6-cycloalkyloxy, amino, C1_3-alkylamino,
di-
(C1.3-alkyl)amino, bis(2-methoxyethyl)amino, pyrrolidin-1-yl, piperidin-1-yl,
homopiperidin-1-yl, morpholin-4-yl, homomorpholin-4-yl, 2-oxa-5-azabicyclo-
[2.2.1 ]hept-5-yl, 3-oxa-8-azabicyclo[3.2.1 ]oct-8-yl, 8-oxa-3-
azabicyclo[3.2.1 ]oct-3-
yl, piperazin-1-yl, 4-C1_3-alkylpiperazin-1-yl, homopiperazin-1-yl or C1_3-
alkyl-
homopiperazin-1-yl group, or
a formylamino, C1_4-alkylcarbonylamino, C1.3-alkyloxy-C1_3-alkylcarbonylamino,
C1_4-alkyloxycarbonylamino, aminocarbonylamino, C1.3-alkylaminocarbonylamino,
di-(C1_3-alkyl)aminocarbonylamino, pyrrolidin-1-ylcarbonylamino, piperidin-1-
yl-
carbonylamino, piperazin-1-ylcarbonylamino, 4-C1_3-alkylpiperazin-1-ylcarbonyl-
amino, morpholin-4-ylcarbonylamino or a C1_4-alkylsulphonylamino group,
a C3_7-cycloalkyloxy or C3_7-cycloalkyl-C1.4-alkyloxy group,
a tetra hydrofuran-3-yloxy, tetra hydropyran-3-yloxy or tetra hydropyran-4-
yloxy group,
a tetrahydrofuranyl-C1_4-alkyloxy or tetrahydropyranyl-C1_4-alkyloxy group,
a C1_4-alkoxy group, which is substituted by a pyrrolidinyl, piperidinyl or
homopiperidinyl
group substituted in the 1- position by the group R8, where
R8 is a hydrogen atom or a C1_3-alkyl group,
or a C1.4-alkoxy group, which is substituted by a morpholinyl group
substituted in the 4-
position by the group R8, where R8 is defined as mentioned above, and
X is a methine group substituted by a cyano group or is a nitrogen atom, and
where the aryl groups mentioned in the definition of the abovementioned
groupgroups
are in each case to be understood as meaning a phenyl group which is mono- or
disubstituted by R9, where the substituents can be identical or different and
CA 02540501 2006-03-28
-6-
R9 is a hydrogen atom, a fluorine, chlorine, bromine, or iodine atom or a
C1_3-alkyl, hydroxyl, C1_3-alkyloxy, difluoromethyl, trifluoromethyl,
difluoromethoxy,
trifluoromethoxy or cyano group,
the heteroaryl groups mentioned in the definition of the abovementioned
groupgroups
are to be understood as meaning a pyridyl, pyridazinyl, pyrimidinyl or
pyrazinyl group,
where the abovementioned heteroaryl groups are in each case mono- or
disubstituted
by the group R9, where the substituents can be identical or different and R9
is defined as
mentioned above, and
the abovementioned pyrrolidinyl, piperidinyl, piperazinyl and morpholinyl
groups can in
each case be substituted by one or two C1_3-alkyl groups, and
if not mentioned otherwise, the abovementioned alkyl groups can be straight-
chain or
branched.
Preferred compounds of the above general formula I are those in which
Ra is a hydrogen atom,
Rb is a 1-phenylethyl group,
R` is a cyclopentyl group, which is substituted in the 3-position by a group
R4-N-R5,
where
R4 is a hydrogen atom or a C1_3-alkyl group and
R5 is a hydrogen atom or a C1_3-alkyl group,
an aminocarbonyl-C1_3-alkyl, C1_3-alkylaminocarbonyl-C1_3-alkyl, di-(C1.3-
alkyl)-
aminocarbonyl-C1_3-alkyl, pyrrolidin-1-ylcarbonyl-C1_3-alkyl, piperidin-1-
ylcarbonyl-
C1_3-alkyl, piperazin-1-ylcarbonyl-C1_3-alkyl, 4-C1_3-alkylpiperazin-1-
ylcarbonyl-
C1.3-alkyl or morpholin-4-ylcarbonyl-C1_3-alkyl group,
CA 02540501 2006-03-28
-7-
a hydroxy-C2_4-alkyl, C1_3-alkyloxy-C2_4-alkyl, C1.4-alkyloxycarbonylamino-
C2_4-
alkyl, amino-C2_4-alkyl, C1_3-alkylamino-C2_4-alkyl, di-(C1_3-alkyl)amino-C2_4-
alkyl,
C1.3-alkylcarbonylamino-C2_4-alkyl, aminocarbonylamino-C2_4-alkyl, C1_3-alkyl-
aminocarbonylamino-C2_4-alkyl, di-(C1.3-alkyl)aminocarbonylamino-C2_4-alkyl,
morpholin-4-ylcarbonylamino-C2_4-alkyl, C1_3-alkylsulphonyl-C2.4-alkyl or
C1_3-alkylsulphonylamino-C2_4-alkyl group,
a (2-oxopyrrolidin-1-yl)-C2_4-alkyl, (2-oxopiperidin-1-yl)-C2_4-alkyl, (3-oxo-
morpholin-4-yl)-C2_4-alkyl, (2-oxoimidazolidin-1-yl)-C2_4-alkyl, (2-oxo-3-
methyl-
imidazolidin-1-yl)-C2_4-alkyl, (2-oxohexahydropyrimidin-1-yl)-C2_4-alkyl or (2-
oxo-
3-methylhexahydropyrimidin-1-yl)-C2_4-alkyl group,
a C1_3-alkylsulphonyl, chloro-C2_4-alkylsulphonyl, bromo-C2_4-alkylsulphonyl,
amino-C2_4-alkylsulphonyl, C1_3-alkylamino-C2_4-alkylsulphonyl, di-(C1_3-
alkyl)-
amino-C2_4-alkylsulphonyl, (pyrrolidin-1-yl)-C2_4-alkylsulphonyl, (piperidin-1-
yl)-
C2_4-alkylsulphonyl or (morpholin-4-yl)-C2_4-alkylsulphonyl group,
a C1_4-alkyloxycarbonyl group,
a formyl, C1_3-alkylcarbonyl, C1_3-alkyloxy-C1_3-alkylcarbonyl,
tetrahydrofuranyl-
carbonyl, tetrahydropyranylcarbonyl, amino-C1_3-alkylcarbonyl, C1_3-alkylamino-
C1_3-alkylcarbonyl, di-(C1_3-alkyl)amino-C1_3-alkylcarbonyl, pyrrolidin-1-yl-
C1_3-alkylcarbonyl, piperidin-1-yI-C1_3-alkylcarbonyl, piperazin-1-yI-C1_3-
alkyl-
carbonyl, 4-C1_3-alkylpiperazin-1-yI-C1_3-alkylcarbonyl, morpholin-4-yl-C1_3-
alkyl-
carbonyl or a C1_3-alkylsulphonyl-C1_3-alkylcarbonyl group,
a cyano, aminocarbonyl, C1_3-alkylaminocarbonyl, di-(C1_3-alkyl)aminocarbonyl,
(C1_3-alkyloxy-C2_4-alkyl)aminocarbonyl, N-(C1_3-alkyl)-N-(C1_3-alkyloxy-C2_4-
alkyl)-
aminocarbonyl, phenylaminocarbonyl, pyrrolidin-1 -ylcarbonyl, piperidin-1-yl-
carbonyl, morpholin-4-ylcarbonyl, C1_3-alkylmorpholin-4-ylcarbonyl, di-(C1_3-
alkyl)morpholin-4-ylcarbonyl, homomorpholin-4-ylcarbonyl, 2-oxa-5-azabicyclo-
[2.2.1]hept-5-ylcarbonyl, 3-oxa-8-azabicyclo[3.2.1 ]oct-8-ylcarbonyl, 8-oxa-3-
aza-
bicyclo[3.2. 1]oct-3-ylcarbonyl, piperazin-1-ylcarbonyl, 4-(C1_3-alkyl)-
piperazin-1-
ylcarbonyl, aminosulphonyl, C1_3-alkylaminosulphonyl, di-(C1_3-alkyl)amino-
CA 02540501 2006-03-28
-8-
sulphonyl, pyrrolidin-1-yl-sulphonyl, piperidin-1-ylsulphonyl or a morpholin-4-
ylsulphonyl group, or
a cyclopentyl group, which is substituted in the 3-position by a group R6,
where
R6 is a 2-oxopyrrolidin-1-yl, 2-oxopiperidin-1-yl, 3-oxomorpholin-4-yl, 2-oxo-
imidazolidin-1-yl, 2-oxo-3-methylimidazolidin-1-yl, 2-oxohexahydropyrimidin-1-
yl
or a 2-oxo-3-methylhexahydropyrimidin-1-yI group,
1o a cyclohexyl group, which is substituted in the 3-position or in the 4-
position by a group
R4-N-R5, where R4 and R5 are defined as mentioned above,
a cyclohexyl group, which is substituted in the 3-position or in the 4-
position by a group
R6, where R6 is defined as mentioned above,
a pyrrolidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5
is defined as mentioned above,
a piperidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5 is
defined as mentioned above,
a piperidin-4-yl group, which is substituted in the 1-position by the group
R5, where R5 is
defined as mentioned above, or
a tetra hydrofuran-3-yl, tetra hydropyran-3-yl or tetra hyd ropyran-4-yl
group,
Rd is a hydrogen atom,
a C1_3-alkyloxy group,
a methoxy group, which is substituted by one to three fluorine atoms,
an ethyloxy group, which is substituted in the 2-position by a group R6 or R7,
where R6
is defined as mentioned above and
CA 02540501 2006-03-28
-9-
R7 is a hydroxyl, C1_3-alkyloxy, amino, C1_3-alkylamino, di-(C1_3-alkyl)amino,
bis(2-
methoxyethyl)amino, pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-yl, homo-
morpholin-4-yl, 2-oxa-5-azabicyclo[2.2.1 ]hept-5-yl, 3-oxa-8-azabicyclo[3.2.1
]oct-
8-yl, 8-oxa-3-azabicyclo[3.2.1]oct-3-yl, piperazin-1-yl or a 4-C1_3-
alkylpiperazin-1-
yl group, or
a formylamino, C1_4-alkylcarbonylamino, C1_3-alkyloxy-C1_3-alkylcarbonylamino,
C1_4-alkyloxycarbonylamino, aminocarbonylamino, C1.3-alkylaminocarbonylamino,
di-(C1_3-alkyl)aminocarbonylamino, pyrrolidin-1-ylcarbonylamino, piperidin-1-
ylcarbonylamino, piperazin-1 -ylcarbonylamino, 4-C1_3-alkylpiperazin-1-yl-
carbonylamino, morpholin-4-ylcarbonylamino or a C1_4-alkylsulphonylamino
group,
a propyloxy group, which is substituted in the 3-position by a group R6 or R7,
where R6
and R7 are defined as mentioned above, or
a butyloxy group, which is substituted in the 4-position by a group R6 or R7,
where R6
and R7 are defined as mentioned above, and
X is a nitrogen atom,
where, if not mentioned otherwise, the abovementioned alkyl groups can be
straight-
chain or branched,
their tautomers, their stereoisomers, their mixtures and their salts.
Particularly preferred compounds of the above general formula I are those in
which
Ra is a hydrogen atom,
Rb is a 1-phenylethyl group,
Rc is a cyclohexyl group, which is substituted in the 3-position or in the 4-
position by a
4 5
group R -N-R, where
CA 02540501 2006-03-28
-10-
R4 is a hydrogen atom, a methyl or ethyl group and
R5 is a hydrogen atom, a methyl, aminocarbonylmethyl, methylamino-
carbonylmethyl, dimethylaminocarbonylmethyl, pyrrolidin-1 -ylcarbonylmethyl,
piperidin-1-ylcarbonylmethyl, piperazin-1-ylcarbonylmethyl, 4-methylpiperazin-
1-
ylcarbonylmethyl, morpholin-4-ylcarbonylmethyl, 2-(morpholin-4-yl-
carbonyl)ethyl
or 3-(morpholin-4-yl-carbonyl)propyl group,
an ethyl, propyl, 2-hydroxyethyl, 3-hydroxypropyl, 2-methoxyethyl, 3-methoxy-
propyl, 2-(butyloxycarbonylamino)ethyl, 2-aminoethyl, 3-aminopropyl, 2-(acetyl-
amino)ethyl, 3-(acetylamino)propyl, 2-(ethylcarbonylamino)ethyl, 3-(ethyl-
carbonylamino)propyl, 2-(propylcarbonylamino)ethyl, 3-(propylcarbonylamino)-
propyl, 2-(ethylaminocarbonylamino)ethyl, 3-(ethylaminocarbonylamino)propyl,
2-(dimethylaminocarbonylamino)ethyl, 3-(dimethylaminocarbonylamino)propyl,
2-(morpholin-4-ylcarbonylamino)ethyl, 3-(morpholin-4-ylcarbonylamino)propyl,
2-(methylsulphonyl)ethyl, 3-(methylsulphonyl)propyl, 2-(methylsulphonylamino)-
ethyl or a 3-(methylsulphonylamino)propyl group,
a 2-(2-oxopyrrolidin-1-yl)ethyl, 2-(2-oxopiperidin-1-yl)ethyl, 2-(3-
oxomorpholin-4-
yl)ethyl, 2-(2-oxoimidazolidin-1-yl)ethyl, 2-(2-oxo-3-methylimidazolidin-1-
yl)ethyl,
2-(2-oxohexahydropyrimidin-1-yl)ethyl or a 2-(2-oxo-3-methyl
hexahydropyrimidin-
1-yl)ethyl group,
a 3-(2-oxopyrrolidin-1-yl)propyl, 3-(2-oxopiperidin-1-yl)propyl, 3-(3-
oxomorpholin-
4-yl)propyl, 3-(2-oxoimidazolidin-1-yl)propyl, 3-(2-oxo-3-methylimidazolidin-1-
yl)-
propyl, 3-(2-oxohexahydropyrimidin-1 -yl)propyl or a 3-(2-oxo-3-methyl-
hexahydropyrim id in-1-yl)propyl group,
a methylsulphonyl, ethylsulphonyl, 3-chloropropylsulphonyl, 2-(morpholin-4-yl)-
ethylsulphonyl or a 3-(morpholin-4-yl)-propylsulphonyl group,
a propyloxycarbonyl or butyloxycarbonyl group,
CA 02540501 2006-03-28
-11-
a formyl, acetyl, ethylcarbonyl, propylcarbonyl, methoxyacetyl, (2-methoxy-
ethyl)carbonyl, (3-methoxypropyl)carbonyl, tetra hydrofuran-2-ylcarbonyl,
tetra hydropyran-4-ylcarbonyl, aminoacetyl, methylaminoacetyl, dimethylamino-
acetyl, morpholin-4-ylacetyl, [2-(morpholin-4-yl)ethyl]carbonyl, [3-(morpholin-
4-
yl)propyl]carbonyl or a methylsuiphonylacetyl group,
a cyano, aminocarbonyl, methylaminocarbonyl, dimethylaminocarbonyl, ethyl-
aminocarbonyl, diethylaminocarbonyl, propylaminocarbonyl, (2-methoxyethyl)-
aminocarbonyl, N-methyl-N-(2-methoxyethyl)aminocarbonyl, (3-methoxypropyl)-
aminocarbonyl, N-methyl-N-(3-methoxypropyl)aminocarbonyl, phenylamino-
carbonyl, pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl, morpholin-4-
ylcarbonyl,
2-methylmorpholin-4-ylcarbonyl, 2,6-dimethylmorpholin-4-ylcarbonyl, homo-
morpholin-4-ylcarbonyl, 2-oxa-5-azabicyclo[2.2.1 ]hept-5-ylcarbonyl, 3-oxa-8-
azabicyclo[3.2. 1 ]oct-8-ylcarbonyl, 8-oxa-3-azabicyclo[3.2.1 ]oct-3-
ylcarbonyl,
4-methylpiperazin-1 -ylcarbonyl, aminosulphonyl, methylaminosulphonyl,
dimethylaminosulphonyl or a morpholin-4-ylsulphonyl group,
a cyclohexyl group, which is substituted in the 3-position or in the 4-
position by a group
R6, where
R6 is a 2-oxopyrrolidin-1-yl, 2-oxopiperidin-1-yl, 3-oxomorpholin-4-yl, 2-oxo-
imidazolidin-1-yi, 2-oxo-3-methylimidazolidin-1-yl, 2-oxohexahydropyrimidin-1-
yl
or a 2-oxo-3-methylhexahydropyrimidin-1-yl group,
a pyrrolidin-3-yl group, which is substituted in the 1-position by the group
R5, where R5
is defined as mentioned above,
a piperidin-3-yl group, which is substituted in the 1-position by the group
R5, where
R5 is defined as mentioned above,
a piperidin-4-yl group, which is substituted in the 1-position by the group
R5, where
R5 is defined as mentioned above,
a tetrahydrofuran-3-yl, tetrahydropyran-3-yl or tetrahydropyran-4-yl group,
CA 02540501 2006-03-28
-12-
Rd is a hydrogen atom,
a methoxy, difluoromethoxy or ethyloxy group,
an ethyloxy group, which is substituted in the 2-position by a group R6 or R7,
where R6
is defined as mentioned above and
R7 is a hydroxyl, methoxy, ethoxy, amino, dimethylamino, diethylamino, bis(2-
methoxyethyl)amino, pyrrolidin-l-yl, piperidin-1-yl, morpholin-4-yl, homo-
morpholin-4-yl, 2-oxa-5-azabicyclo[2.2.1]hept-5-yl, 3-oxa-8-
azabicyclo[3.2.1]oct-
8-yl, 8-oxa-3-azabicyclo[3.2.1]oct-3-yl, piperazin-1-yl, 4-methylpiperazin-1-
yl or
4-ethylpiperazin-l-yl group, or
an acetylamino, ethylcarbonylamino, propylcarbonylamino, butylcarbonylamino,
methoxyacetylamino, butyloxycarbonylamino, ethylaminocarbonylamino,
dimethylaminocarbonylamino, pyrrolidin-l-ylcarbonylamino, piperidin-l-yl-
carbonylamino, morpholin-4-ylcarbonylamino, methylsulphonylamino, ethyl-
sulphonylamino or butylsulphonylamino group,
a propyloxy group, which is substituted in the 3-position by a group R6 or R7,
where R6
and R7 are defined as mentioned above, or
a butyloxy group, which is substituted in the 4-position by a group R6 or R7,
where R6
and R7 are defined as mentioned above, and
X is a nitrogen atom,
where, if not mentioned otherwise, the abovementioned alkyl groups can be
straight-
chain or branched,
their tautomers, their stereoisomers, their mixtures and their salts.
Very particularly preferred compounds of the general formula I are those in
which
CA 02540501 2006-03-28
-13-
Ra is a hydrogen atom,
Rb is a 1-phenylethyl group,
Rc is a cyclohexyl group, which is substituted in the 4-position by an amino,
methylamino, dimethylamino, acetylamino, N-(acetyl)methylamino, methoxy-
acetylamino, N-(methoxyacetyl)methylamino, tetrahydropyran-4-ylcarbonylamino,
N-
(tetrahydropyran-4-ylcarbonyl)methylamino, tert-butyloxycarbonylamino, N-(tert-
butyloxycarbonyl)methylamino, N-(ethylaminocarbonyl)methylamino, dimethylamino-
1o carbonylamino, N-(dimethylaminocarbonyl)methylamino, N-(piperidin-1 -
ylcarbonyl)-
methylamino, morpholin-4-ylcarbonylamino, N-(morpholin-4-
ylcarbonyl)methylamino, N-
(4-methylpiperazin-1-ylcarbonyl)methylamino, methylsulphonylamino, N-(methyl-
sulphonyl)methylamino, ethylsulphonylamino, N-(ethylsulphonyl)methylamino,
dimethyl-
aminosulphonylamino, N-(dimethylaminosulphonyl)methylamino, morpholin-4-yl-
sulphonylamino or N-(morpholin-4-ylsulphonyl)methylamino group,
a pyrrolidin-3-yl group,
a pyrrolidin-3-yl group, which is substituted in the 1-position by a tert-
butyloxycarbonyl
or methylsuiphonyl group,
a piperidin-3-yl group,
a piperidin-3-yl group, which is substituted in the 1-position by a tert-
butyloxycarbonyl or
methylsulphonyl group,
a piperidin-4-yl group,
a piperidin-4-yl group, which is substituted in the 1-position by a methyl,
(aminocarbonyl)methyl, (dimethylaminocarbonyl)methyl, (morpholin-4-ylcarbonyl)-
methyl, 2-(tert-butyloxycarbonylamino)ethyl, 2-aminoethyl, 2-
(acetylamino)ethyl, 2-
(methylsulphonylamino)ethyl, cyano, acetyl, methoxyacetyl,
(dimethylamino)acetyl,
(morpholin-4-yl)acetyl, tetra hydropyran-4-ylcarbonyl, ethylaminocarbonyl,
isopropyl-
aminocarbonyl, dimethylaminocarbonyl, diethylaminocarbonyl, pyrrolidin-1-
ylcarbonyl,
CA 02540501 2006-03-28
-14-
piperidin-1-ylcarbonyl, morpholin-4-ylcarbonyl, 2-methylmorpholin-4-
ylcarbonyl, 2,6-di-
methylmorpholin-4-ylcarbonyl, homomorpholin-4-ylcarbonyl, 4-methylpiperazin-1 -
yI-
carbonyl, isopropyloxycarbonyl, tert-butyloxycarbonyl, methylsulphonyl,
dimethylamino-
sulphonyl or morpholin-4-ylsulphonyl group, or
a tetrahydrofuran-3-yl, tetra hydropyran-3-yl or tetrahydropyran-4-yl group,
Rd is a methoxy, ethyloxy or a 2-(methoxy)ethyloxy group,
1o and
X is a nitrogen atom,
their tautomers, their stereoisomers, their mixtures and their salts.
Especially preferred compounds of the general formula I are those in which
Ra is a hydrogen atom,
Rb is a 1-phenylethyl group,
Rc is a piperidin-4-yl group,
a piperidin-4-yl group, which is substituted in the 1-position by a methyl,
cyano, acetyl,
morpholin-4-ylcarbonyl, tert-butyloxycarbonyl or methylsulphonyl group,
Rd is a methoxy group
and
X is a nitrogen atom,
their tautomers, their stereoisomers, their mixtures and their salts.
CA 02540501 2006-03-28
-15-
The compounds of the general formula I can be prepared, for example, by the
following
processes:
a) reaction of a compound of the general formula
RaRb
N
O-H
X
d
N R
(II)
in which
1o Ra, Rb, Rd and X are defined as mentioned at the outset, with a compound of
the
general formula
Z' - Rc (III)
in which
Rc is defined as mentioned at the outset and Z' is a leaving group such as a
halogen
atom, e.g. a chlorine or bromine atom, a sulphonyloxy group such as a
methanesulphonyloxy or p-toluenesulphonyloxy group or a hydroxyl group.
Using a compound of the general formula III in which Z' is a hydroxyl group,
the
reaction is carried out in the presence of a dehydrating agent, preferably in
the
presence of a phosphine and of an azodicarboxylic acid derivative such as, for
example,
triphenylphosphine/diethyl azodicarboxylate, expediently in a solvent such as
methylene
chloride, acetonitrile, tetrahydrofuran, dioxane, toluene or ethylene glycol
diethyl ether
at temperatures between -50 and 150 C, but preferably at temperatures between -
20
and 80 C.
b) For the preparation of compounds of the general formula I, in which Rd is
one of the
optionally substituted alkyloxy groups mentioned at the outset:
CA 02540501 2006-03-28
-16-
reaction of a compound of the general formula
RaRb
N
O~Rc
X
N O-H (IV)
in which Ra, Rb, Rc and X are defined as mentioned at the outset, with a
compound of
the general formula
Z2 - Rd' (V)
1o in which Rd' is a C1_4-alkyl group, a methyl group substituted by 1 to 3
fluorine atoms, an
ethyl group substituted by 1 to 5 fluorine atoms, a C2_4-alkyl group
substituted by a
group R6 or R7, where R6 and R7 are defined as mentioned at the outset, a C1_4-
alkyl
group, which is substituted by a pyrrolidinyl, piperidinyl or homopiperidinyl
group
substituted in the 1-position by the group R8, or a C1.4-alkyl group, which is
substituted
by a morpholinyl group substituted in the 4-position by the group R8, where R8
is in each
case defined as mentioned at the outset, and
Z2 is a leaving group such as a halogen atom, an alkylsulphonyloxy,
arylsulphonyloxy or
a hydroxyl group.
If the leaving group is a halogen atom such as a chlorine, bromine or iodine
atom or an
alkylsulphonyloxy or arylsulphonyloxy group such as the methanesulphonyloxy or
p-
toluenesulphonyloxy group, the reaction is preferably carried out in the
presence of an
organic or inorganic base such as potassium carbonate, sodium hydride or N-
ethyl-
diisopropylamine. If the leaving group is a hydroxyl group, then the reaction
is carried
out in the presence of a dehydrating agent, preferably in the presence of a
phosphine
and of an azodicarboxylic acid derivative such as, for example, triphenyl-
phosphine/diethyl azodicarboxylate.
c) For the preparation of compounds of the general formula I, in which Rd is
one of the
CA 02540501 2006-03-28
-17-
alkyloxy groups mentioned at the outset, which is substituted by an optionally
substituted amino, alkylamino or dialkylamino group or by an optionally
substituted
heterocyclic group bonded via an imino nitrogen atom:
reaction of a compound of the general formula
Ra'-1/ Rb
X Rc
NO-(CH) z a-Z3 (VI)
in which Ra, Rb, Rc and X are defined as mentioned at the outset and Z3 is a
leaving
1o group such as a halogen atom, e.g. a chlorine or bromine atom or a
sulphonyloxy group
such as a methanesulphonyloxy or p-toluenesulphonyloxy group, with
ammonia, an appropriate, optionally substituted alkylamine, dialkylamine or an
imino
compound or their suitable salts or derivatives, such as, for example,
morpholine.
d) For the preparation of compounds of the general formula I, in which Rd is a
hydroxyl
group:
cleavage of a protective group from a compound of the general formula
RaRb
\ O
Rc
N Rd (VII)
in which Ra, Rb, Rc and X are defined as mentioned at the outset and Rd" is a
group
which can be converted into a hydroxyl group, for example an optionally
substituted
benzyloxy group, a trimethylsilyloxy, acetyloxy, benzoyloxy, methoxy, ethoxy,
tert-
butoxy or trityloxy group.
CA 02540501 2006-03-28
-18-
The cleavage of the protective group is carried out, for example,
hydrolytically in an
aqueous solvent, e.g. in water, isopropanol/water, acetic acid/water,
tetrahydrofuran/water or dioxane/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an alkali
base such as sodium hydroxide or potassium hydroxide or aprotically, e.g. in
the
presence of iodotrimethylsilane, at temperatures between 0 and 120 C,
preferably at
temperatures between 10 and 100 C.
The cleavage of a benzyl or methoxybenzyl group is carried out, for example,
1o hydrogenolytically, e.g. with hydrogen in the presence of a catalyst such
as pal-
ladium/carbon in a suitable solvent such as methanol, ethanol, ethyl acetate
or glacial
acetic acid optionally with addition of an acid such as hydrochloric acid at
temperatures
between 0 and 100 C, but preferably at room temperatures between 20 and 60 C,
and
at a hydrogen pressure of 1 to 7 bar, but preferably of 3 to 5 bar. The
cleavage of a
2,4-dimethoxybenzyl group, however, is preferably carried out in
trifluoroacetic acid in
the presence of anisole.
The cleavage of a tert-butyl or benzyl group is carried out, for example, by
treatment
with an acid such as trifluoroacetic acid, hydrochloric acid or hydrobromic
acid or by
treatment with iodotrimethylsilane optionally using a solvent such as
methylene chloride,
dioxane, methanol or diethyl ether.
e) For the preparation of compounds of the general formula I, in which R
contains an
-NH group:
cleavage of a protective group from a compound of the general formula
RaRb
X RC
N R
d (VIII)
in which Ra, Rb, Rd and X are defined as mentioned at the outset and R ' has
the
meanings mentioned at the outset for Rc with the proviso that Rc contains a
protected
CA 02540501 2006-03-28
-19-
nitrogen atom.
Customary protective groupgroups for an amino, alkylamino or imino group are,
for
example, the formyl, acetyl, trifluoroacetyl, ethoxycarbonyl, tert-
butoxycarbonyl, benzyl-
oxycarbonyl, benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group, where the
phthalyl
group is additionally possible for the amino group.
The cleavage of the protective group is carried out, for example,
hydrolytically in an
aqueous solvent, e.g. in water, isopropanol/water, acetic acid/water,
1o tetrahydrofuran/water or dioxane/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an alkali
base such as sodium hydroxide or potassium hydroxide or aprotically, e.g. in
the
presence of iodotrimethylsilane, at temperatures between 0 and 120 C,
preferably at
temperatures between 10 and 100 C.
The cleavage of a benzyl, methoxybenzyl or benzyloxycarbonyl group, however,
is
carried out, for example, hydrogenolytically, e.g. with hydrogen in the
presence of a
catalyst such as palladium/carbon in a suitable solvent such as methanol,
ethanol, ethyl
acetate or glacial acetic acid optionally with addition of an acid such as
hydrochloric
acid at temperatures between 0 and 100 C, but preferably at room temperatures
between 20 and 60 C, and at a hydrogen pressure of 1 to 7 bar, but preferably
of 3 to
5 bar. The cleavage of a 2,4-dimethoxybenzyl group, however, is preferably
carried out
in trifluoroacetic acid in the presence of anisole.
The cleavage of a tert-butyl- or tert-butyloxycarbonyl group is preferably
carried out by
treatment with an acid such as a trifluoroacetic acid or hydrochloric acid or
by treatment
with iodotrimethylsilane optionally using a solvent such as methylene
chloride, dioxane,
methanol or diethyl ether.
3o The cleavage of a trifluoroacetyl group is preferably carried out by
treatment with an
acid such as hydrochloric acid optionally in the presence of a solvent such as
acetic
acid at temperatures between 50 and 120 C or by treatment with sodium
hydroxide
solution optionally in the presence of a solvent such as tetrahydrofuran at
temperatures
between 0 and 50 C.
CA 02540501 2006-03-28
-20-
The cleavage of a phthalyl group is preferably carried out in the presence of
hydrazine
or of a primary amine such as methylamine, ethylamine or n-butylamine in a
solvent
such as methanol, ethanol, isopropanol, toluene/water or dioxane at
temperatures
between 20 and 50 C.
f) For the preparation of compounds of the general formula I, in which Rc
contains an
alkyl group substituted by an optionally substituted amino, alkylamino or
dialkylamino
group or by an optionally substituted heterocyclic group bonded via a nitrogen
atom:
reaction of a compound of the general formula
R a N,Rb
X Rcõ Z3
N /
Rd (IX)
in which Ra, Rb, Rd and X are defined as mentioned at the outset, Z3 is a
leaving group,
for example a halogen atom such as a chlorine or bromine atom, or a
sulphonyloxy
group such as a methanesulphonyloxy or p-toluenesulphonyloxy group, and R`"
has the
meanings mentioned at the outset for Rc, with the proviso that a hydrogen atom
bonded
to an aliphatic carbon atom is replaced by the group Z3,
with ammonia, an appropriate, optionally substituted alkylamine, dialkylamine
or an
imino compound or their suitable salts or derivatives, such as, for example,
morpholine.
If, according to the invention, a compound of the general formula I is
obtained which
contains an amino, alkylamino or imino group, then this can be converted into
a
corresponding acyl, cyano or sulphonyl compound of the general formula I by
means of
acylation, cyanation or sulphonylation, where suitable acylating agents are,
for example,
isocyanates, carbamoyl chlorides, carboxylic acid halides, carboxylic acid
anhydrides
and carboxylic acids with activating agents such as N,N'-carbonyldiimidazole,
N,N'-
3o dicyclohexylcarbodiimide or O-(benzotriazol-1-yl)-N,N,N'N'-
tetramethyluronium
CA 02540501 2006-03-28
-21 -
tetrafluoroborate, suitable sulphonylating agents are sulphonyl halides and
suitable
cyanating agents are cyanogen chloride or cyanogen bromide, and/or
a compound of the general formula I, which contains an amino, alkylamino or
imino
group, then this can be converted into a corresponding alkyl compound of the
general
formula I by means of alkylation or reductive alkylation and/or
a compound of the general formula I, which contains a chloro-C1_4-
alkylsulphonyl or
bromo-C1_4-alkylsulphonyl group, then this can be converted into a
corresponding
1o amino-C1_4-alkylsulphonyl compound by reaction with an amine
and/or
a compound of the general formula I, which contains a tert-
butyloxycarbonylamino, N-
alkyl-N-(tert-butyloxycarbonyl)amino or a N-tert-butyloxycarbonylimino group,
then this
can be converted into a corresponding amino, alkylamino or imino compound of
the
general formula I by means of treatment with an acid such as hydrochloric acid
or
trifluoroacetic acid.
In the reaction as described above, reactive groups which may be present such
as
hydroxyl, amino, alkylamino or imino groups can be protected during the
reaction by
customary protective groups, which are cleaved again after the reaction.
For example, the trimethylsilyl, acetyl, trityl, benzyl or tetrahydropyranyl
group is
possible as a protective group for a hydroxyl group.
Possible protective groupgroups for an amino, alkylamino or imino group are,
for
example, the formyl, acetyl, trifluoroacetyl, ethoxycarbonyl, tert-
butoxycarbonyl, benzyl-
oxycarbonyl, benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group.
The optionally subsequent cleavage of a used protective group is carried out,
for
example, hydrolytically in an aqueous solvent, e.g. in water,
isopropanol/water, acetic
acid/water, tetrahydrofuran/water or dioxane/water, in the presence of an acid
such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an alkali
base such as sodium hydroxide or potassium hydroxide or aprotically, e.g. in
the
CA 02540501 2006-03-28
-22-
presence of iodotrimethylsilane, at temperatures between 0 and 120 C,
preferably at
temperatures between 10 and 100 C.
The cleavage of a benzyl, methoxybenzyl or benzyloxycarbonyl group, however,
is
carried out, for example, hydrogenolytically, e.g. with hydrogen in the
presence of a
catalyst such as palladium/carbon in a suitable solvent such as methanol,
ethanol, ethyl
acetate or glacial acetic acid optionally with addition of an acid such as
hydrochloric
acid at temperatures between 0 and 100 C, but preferably at room temperatures
between 20 and 60 C, and at a hydrogen pressure of 1 to 7 bar, but preferably
of 3 to 5
1o bar. The cleavage of a 2,4-dimethoxybenzyl group, however, is preferably
carried out in
trifluoroacetic acid in the presence of anisole.
The cleavage of a tert-butyl or tert-butyloxycarbonyl group is preferably
carried out by
treatment with an acid such as trifluoroacetic acid or hydrochloric acid or by
treatment
with iodotrimethylsilane optionally using a solvent such as methylene
chloride, dioxane,
methanol or diethyl ether.
The cleavage of a trifluoroacetyl group is preferably carried out by treatment
with an
acid such as hydrochloric acid optionally in the presence of a solvent such as
acetic
acid at temperatures between 50 and 120 C or by treatment with sodium
hydroxide
solution optionally in the presence of a solvent such as tetrahydrofuran or
methanol at
temperatures between 0 and 50 C.
Further, the compounds of the general formula I obtained, such as have already
been
mentioned at the outset, can be separated into their enantiomers and/or
diastereomers.
Thus it is possible, for example, to separate cis/trans mixtures into their
cis and trans
isomers, and compounds having at least one optically active carbon atom into
their
enantiomers.
3o Thus, for example, the cis/trans mixtures obtained can be separated by
chromatography
into their cis and trans isomers, the compounds of the general formula I
obtained, which
occur in racemates, can be separated into their optical antipodes by methods
known per
se (see Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry", Vol. 6,
Wiley
Interscience, 1971)) and compounds of the general formula I having at least 2
CA 02540501 2011-08-22
25771-1152
-23-
asymmetric carbon atoms can be separated on the basis of their physicochemical
differences by methods known per se, e.g. by chromatography and/or fractional
crystallization, into their diastereomers, which, if they occur in racemic
form, can
subsequently be separated into the enantiomers as mentioned above.
.5
The separation of enantiomers is preferably carried out by column separation
on chiral
phases or by recrystallizing from an optically active solvent or by reacting
with an
optically active substance forming salts or derivatives, such as, for example,
esters or
amides, with the racemic compound, in particular acids and their activated
derivatives or
alcohols, and separating the diastereomeric salt mixture or derivative
obtained in this
way, e.g. on the basis of different solubilities, where the free antipodes can
be liberated
from the pure diastereomeric salts or derivatives by the action of suitable
agents.
Particularly common, optically active acids are, for example, the D and L
forms of
tartaric acid or dibenzoyltartaric acid, di-O-p-tolyltartaric acid, malic
acid, mandelic acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. A possible
optically
active alcohol is, for example, (+)- or (-)-menthol and an optically active
acyl group in
amides is, for example, (+)- or (-)-menthyloxycarbonyl.
In addition, the compounds of the formula I obtained can be converted into
their salts, in
particular for pharmaceutical administration into their physiologically
tolerable salts with
inorganic or organic acids. Possible acids for this are, for example,
hydrochloric acid,
hydrobromic acid, sulphuric acid, methanesulphonic acid, phosphoric acid,
fumaric acid,
succinic acid, lactic acid, citric acid, tartaric acid or maleic acid.
Furthermore, the present invention relates to a process for the preparation of
a
medicament, wherein, by non-chemical means, a compound as described herein is
incorporated into one or more inert vehicles and/or diluents.
CA 02540501 2011-08-22
25771-1152
- 23a -
The compounds of the general formulae II to IX used as starting substances are
known
from the literature or can be obtained by processes known per se from the
literature or
the processes described above, optionally with additional introduction of
protective
groupgroups (e.g. compounds of the formula IV or VII and VIII).
As already mentioned at the outset, the compounds of the general formula I
according
to the invention and their physiologically tolerable salts have valuable
pharmacological
properties, in particular an inhibitory action on the signal transduction
mediated by the
epidermal growth factor receptor (EGF-R), where this can be caused, for
example, by
an inhibition of ligand binding, receptor dimerization or tyrosine kinase
itself. Moreover,
CA 02540501 2011-08-22
25771-1152
-24-
it is possible that the signal transmission to components lying further
downstream is
blocked.
The biological properties of the novel compounds were tested as follows:
The inhibition of human EGF receptor kinase was determined with the aid of the
cyto-
plasmatic tyrosine kinase domain (methionine 664 to alanine 1186 based on the
sequence published in Nature 309 (1984), 418). For this, the protein was
expressed in
Sf9 insect cells as a GST fusion protein using the Baculovirus expression
system.
The measurement of the enzyme activity was carried out in serial dilutions in
the
presence or absence of the test compounds. The polymer pEY (4:1) from SIGMA
was
used as a substrate. Biotinylated pEY (bio-pEY) was added as a tracer and
substrate.
Each 100 pl of reaction solution contained 10 pl of the inhibitor in 50% DMSO,
20 pl of
the substrate solution (200 mM HEPES pH 7.4, 50 mM magnesium acetate, 2.5
mg/ml
poly(EY), 5 pg/ml bio-pEY) and 20 pl of enzyme preparation. The enzyme
reaction was
started by addition of 50pl of a 100 pM ATP solution in 10 mM magnesium
chloride. The
dilution of the enzyme preparation was adjusted such that the phosphate
incorporation
into the bio-pEY was linear with respect to time and amount of enzyme. The
enzyme
preparation was diluted in 20 mM HEPES pH 7.4, 1 mM EDTA, 130 mM sodium
chloride, 0.05% Triton X-100, 1 mM DTT and 10% glycerol.
The enzyme assays were carried out at room temperature over a period of 30
minutes
and ended by addition of 50 pl of a stop solution (250 mM EDTA in 20 mM HEPES
pH
7.4). 100 pi were transferred to a streptavidin-coated microtitre plate and
incubated at
room temperature for 60 minutes. The plate was then washed with 200 pl of a
wash
solution (50 mM tris, 0.05% Tween 20). After addition of 100 pl of an HRPO-
labelled
anti-PY antibody (PY20H Anti-PTyr:HRP from Transduction Laboratories, 250
ng/ml)
the mixture was incubated for 60 minutes. The microtitre plate was then washed
three
times with 200 pi each of wash solution. The samples were then treated with
100 pl of a
TMB-peroxidase solution (A:B = 1:1, Kirkegaard Perry Laboratories). The
reaction was
stopped after 10 minutes. The extinction was measured at OD450nm using an
ELISA
reader. All data points were determined as triplicates.
*Trade-mark
CA 02540501 2006-03-28
-25-
The data were fitted by means of an iterative calculation using an analysis
program for
sigmoidal curves (Graph Pad Prism Version 3.0). All analyses had a correlation
coefficient of over 0.9. From the curves, the active compound concentration
was derived
which inhibits the activity of the EGF receptor kinase to 50%(l C50).
The compounds of the general formula I according to the invention inhibit the
signal
transduction by tyrosine kinases, for example of the human EGF receptor, and
are
therefore useful for the treatment of pathophysiological processes which are
caused by
hyperfunction of tyrosine kinases. These are, for example, benign or malignant
tumours,
1o in particular tumours of epithelial and neuroepithelial origin, formation
of metastases
and the abnormal proliferation of vascular endothelial cells
(neoangiogenesis).
The compounds according to the invention are also useful for the prevention
and
treatment of diseases of the airways and of the lung which are accompanied by
increased or altered mucus production, which is caused by stimulation of
tyrosine
kinases, such as, for example, in inflammatory diseases of the airways such as
acute
bronchitis, chronic bronchitis, chronic obstructive bronchitis (COPD),
coughing,
pulmonary emphysema, allergic or non-allergic rhinitis or sinusitis, chronic
sinusitis or
rhinitis, asthma, allergic bronchitis, alveolitis, Farmer's disease,
hyperreactive airways,
bronchitis or pneumonitis caused by infections, such as, for example, bacteria
or
viruses, helminths, fungi or protozoa, paediatric asthma, bronchiectases,
acute respiratory distress syndrome (ARDS), pulmonary oedema, bronchitis or
pneumonitis or interstitial pneumonia or pulmonary fibrosis of various causes,
such as,
for example, as a result of aspiration, inhalation of toxic gases or vapours,
as a result of
heart failure, irradiation, chemotherapy, in lupus erythematosus, systemic
scleroderma,
asbestosis, silicosis, Besnier-Boeck disease or sarcoidosis, granulomatosis,
idiopathic pulmonary fibrosis (IPF),
cystic fibrosis or mucoviscidosis, or alphal-antitrypsin deficiency or
nasal polyps.
The compounds are also suitable for the treatment of diseases of the
gastrointestinal
tract and of the bile ducts and gall bladder, which are accompanied by a
disturbed
activity of the tyrosine kinases, such as are to be found, for example, in the
case of
chronic inflammatory changes, such as villous or adenomatous polyps of the
CA 02540501 2006-03-28
-26-
gastrointestinal tract, polyps in familial polyposis coli, intestinal polyps
in Gardner's
syndrome, polyps in Peutz-Jeghers syndrome, inflammatory pseudopolyps,
juvenile
polyps, polyps in colitis cystica profunda and in pneumatosis cystoides
intestinalis,
acute or chronic cholecystitis, Crohn's disease, ulcerative colitis, ulcers or
polyps of the
gastrointestinal tract, diseases of the gastrointestinal tract which are
accompanied by
increased secretion, such as Menetrier's disease, secretory adenoma or protein
loss
syndrome.
Moreover, the compounds of the general formula I and their physiologically
tolerable
1o salts can be used for the treatment of inflammatory diseases of the skin
and of the joints
which are caused by aberrant function of tyrosine kinases, such as, for
example
epidermal hyperproliferation (psoriasis) and arthritis, and for the treatment
of benign
prostate hyperplasia (BPH), diseases of the immune system and
hyperproliferation in
haematopoetic cells.
On account of their biological properties, the compounds according to the
invention can
be used alone or in combination with other pharmacologically active compounds,
for
example in tumour therapy in monotherapy or in combination with other
antitumour
therapeutics, for example in combination with topoisomerase inhibitors (e.g.
etoposide),
mitosis inhibitors (e.g. vinblastine), compounds interacting with nucleic
acids (e.g. cis-
platin, cyclophosphamide, adriamycin), hormone antagonists (e.g. tamoxifen),
inhibitors
of metabolic processes (e.g. 5-FU etc.), cytokines (e.g. interferons),
antibodies etc. For
the treatment of airway diseases, these compounds can be used alone or in
combination with other airway therapeutics, such as, for example, compounds
having
secretolytic activity (e.g. ambroxole, N-acetylcysteine), broncholytic
activity (e.g.
tiotropium or ipratropium or fenoterol, salmeterol, salbutamol) and/or anti-
inflammatory
activity (e.g. theophylline or glucocorticoids). For the treatment of diseases
in the region
of the gastrointestinal tract, these compounds can likewise be given alone or
in
combination with motility- or secretion-influencing substances. These
combinations can
3o be administered either simultaneously or sequentially.
The administration of these compounds either alone or in combination with
other active
compounds can be carried out intravenously, subcutaneously, intramuscularly,
CA 02540501 2006-03-28
-27-
intraperitoneally, intranasally, by inhalation or transdermally or orally,
aerosol
formulations being particularly suitable for inhalation.
In the case of pharmaceutical administration, the compounds according to the
invention
are as a rule used in warm-bloodied vertebrates, in particular in man, in
doses of 0.01-
100 mg/kg of body weight, preferably at 0.1-15 mg/kg. For administration,
these are
incorporated, e.g. with maize starch, lactose, sucrose, microcrysta I line
cellulose,
magnesium stearate, polyvinylpyrrolidone, citric acid, tartaric acid, water,
water/ethanol,
water/glycerol, water/sorbitol, water/polyethylene glycol, propylene glycol,
stearyl
1o alcohol, carboxymethylcelIulose or fat-containing substances such as hard
fat or their
suitable mixtures, into customary galenic preparations, such as tablets,
coated tablets,
capsules, powders, suspensions, solutions, sprays or suppositories.
The following examples are intended to illustrate the present invention in
greater detail
without restricting it:
Preparation of the final compounds:
Example 1
(R)-4-(1-Phenylethylamino)-6-[1-(tert-butyloxycarbonyl)piperidin-4-yloxy]-7-
methoxy-
quinazoline
N
N ~ 0--ON
o- /
N / O y o
~I
4.8 g of (R)-4-(1-phenylethylamino)-6-hydroxy-7-methoxyquinazoline (see WO
02/18351), 7.55 g of 1-(tert-butyloxycarbonyl)-4-(p-
toluenesulphonyloxy)piperidine, 5.7 g
of potassium carbonate and 50 ml dimethylformamide are stirred at 60 C for 48
hours.
The reaction mixture is diluted with 300 ml of ethyl acetate and extracted
three times by
shaking with water. The organic phase is washed with sodium chloride solution,
dried
CA 02540501 2006-03-28
-28-
and concentrated. The residue is purified by chromatography on a silica gel
column
using ethyl acetate/methanol/conc. aqueous ammonia.
Yield: 5.2 g (67 % of theory)
Rf: 0.53 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia = 95:5:1)
Mass spectrum (ESI+): m/z = 479 [M+H]+
Example 2
(R)-4-(1-Phenylethylamino)-6-(piperidin-4-yloxy)-7-methoxyquinazoline
dihydrochloride
EN
__ O
N
N 0
1
Prepared by treatment of (R)-4-(1-phenylethylamino)-6-[1-(tert-
butyloxycarbonyl)-
piperidin-4-yloxy]-7-methoxyquinazoline with 5M isopropanolic hydrochloric
acid in
methylene chloride at room temperature.
Rf: 0.52 (reversed phase silica gel RP8, acetonitrile/water/trifluoroacetic
acid = 50:50:1)
Mass spectrum (ESI+): m/z = 379 [M+H]+
Example 3
(R)-4-(1-Phenylethylamino)-6-(1-methylpiperidin-4-yloxy)-7-methoxyquinazoline
N
II
N __ O
N O N~
1
Prepared by treatment of (R)-4-(1-phenylethylamino)-6-(piperidin-4-yloxy)-7-
methoxy-
quinazoline dihydrochloride with formaldehyde, sodium triacetoxyborhydride and
N-ethyldiisopropylamine in tetrahydrofuran.
CA 02540501 2006-03-28
-29-
Rf: 0.48 (reversed phase silica gel RP8, acetonitrile/water/trifluoroacetic
acid = 50:50:1)
Mass spectrum (ESI+): m/z = 393 [M+H]+
Example 4
(R)-4-(1-Phenylethylamino)-6-(1-acetylpiperidin-4-yloxy)-7-methoxyquinazoline
N
~ ~ 0
INII
~N / 0 Nom/
1 0
Prepared by treatment of (R)-4-(1-phenylethylamino)-6-(pipe rid in-4-yloxy)-7-
methoxy-
1o quinazoline dihydrochloride with acetic anhydride and N-
ethyldiisopropylamine in
tetrahydrofuran.
Rf: 0.19 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 421 [M+H]+
The following compounds are obtained analogously to Example 4:
(1) (R)-4-(1-Phenylethylamino)-6-(1-methanesulphonylpiperidin-4-yloxy)-7-
methoxy-
quinazoline
N
O
N
N O
N-2S~
00
The reaction is carried out using methanesulphonic acid chloride.
Rf: 0.48 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 457 [M+H]+
CA 02540501 2006-03-28
-30-
(2) (R)-4-(1-Phenylethyl amino)-6-{1-[(morpholin-4-yl)carbonyl]-piperidin-4-
yloxy}-7-
methoxyquinazoline
N
N O
N Nr'O
i Y
O
The reaction is carried out using (morpholin-4-yl)carbonyl chloride.
Rf: 0.34 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 492 [M+H]+
(3) (R)-4-(1-Phenylethylamino)-6-(1-cyan opiperidin-4-yloxy)-7-
methoxyquinazoline
N
N ~ ~ O
N O N
The reaction is carried out using cyanogen bromide in methylene chloride.
Rf: 0.54 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia = 95:5:1)
Mass spectrum (ESI+): m/z = 404 [M+H]+
The following compounds can also be prepared analogously to the abovementioned
examples and other processes known from the literature:
CA 02540501 2006-03-28
-31 -
Example No. Structure
(1)
N
N O
IIII
N~
O
(2)
N
\ O
INI
N O N~
O~1
(3)
N
N \ \ O O
i /
N
(4)
N
INI O
N
(5)
N
N a O O
N
CA 02540501 2006-03-28
-32-
Example No. Structure
(6)
N
N O O
N~'A
N
O
(7)
N
\ O O
N ('-ON
N O
(8) I \
N
\
\ O
NI
N / O N'~"\N
(9) I \
N
O
N
~'ON O
O
(10)
N
' I I
N \ xo~~N
S\
1 0
CA 02540501 2006-03-28
-33-
Example No. Structure
(11)
i
N
a O
Nõ N \N/ o~
(12)
N
~ O
NõNi ON N
0
(13)
N
N O
N
~N^
0
O
X14)
N
N O\ ^ O
~N O ~lN
0
(15)
N
NN-,- O
i Ny
0
CA 02540501 2006-03-28
-34-
Example No. Structure
(16)
N
O
N / O N
0
(17)
N
O
O ON No
0
(18)
N
N -- O
i \N
N i y
0
(19)
N
O
N
~
O O
N~
101
(20)
N
N O N
i \N `N J
N i y
0
CA 02540501 2006-03-28
-35-
Example No. Structure
(21)
N
~ a O N
INI
N O S
0 O
(22)
N
II ~ O
\ N\ ~N~
N IS\\
O O
(23)
N
IN -- O\~ JN
~/
N 0
(24)
N
N ll'~
N O N
(25)
rN
~ a0O'aN
N ~N
CA 02540501 2006-03-28
-36-
Example No. Structure
(26)
o
N
~N O
(27) N
O
\ O
N O N
(28) N
\ O
N O N
(29) N
,
N N\ O O
0 NN
(30) N
O\
N \ ^ O
~N N1~1 N
CA 02540501 2006-03-28
-37-
Example No. Structure
(31)
N
N ~ ~ O O
II
O N'SO
(32)
N
N ~ ~ O O
II
~N / O N-S~-N'
OI
(33)
N
N O O
II
N i N/ O N
O
(34)
2N
IN
,
N xo
Example 5
Coated tablets containing 75 mg of active substance
Composition:
1 coated tablet core contains:
Active substance 75.0 mg
Calcium phosphate 93.0 mg
CA 02540501 2006-03-28
-38-
Maize starch 35.5 mg
Polyvinylpyrrolidone 10.0 mg
Hydroxypropylmethylcellulose 15.0 mg
Magnesium stearate 1.5 mg
230.0 mg
Preparation:
The active substance is mixed with calcium phosphate, maize starch,
polyvinylpyrrolidone, hydroxypropylmethylcellulose and half the stated amount
of
magnesium stearate. Pressings having a diameter of about 13 mm are prepared on
a
tabletting machine; these are grated through a sieve having a mesh width of
1.5 mm on
a suitable machine and mixed with the remaining amount of magnesium stearate.
These granules are pressed on a tabletting machine to give tablets having the
desired
shape.
Core weight: 230 mg
Die: 9 mm, convex
The coated tablet cores thus prepared are covered with a film which consists
essentially
of hydroxypropylmethylcellulose. The finished film-coated tablets are glazed
with
beeswax.
Coated tablet weight: 245 mg.
Example 6
Tablets containing 100 mg of active substance
Composition:
1 tablet contains:
Active substance 100.0 mg
Lactose 80.0 mg
Maize starch 34.0 mg
Polyvinylpyrrolidone 4.0 mg
CA 02540501 2006-03-28
-39-
Magnesium stearate 2.0 mg
220.0 mg
Preparation process:
Active compound, lactose and starch are mixed and uniformly moistened with an
aqueous solution of the polyvinylpyrrolidone. After sieving the moist mass
(2.0 mm
mesh width) and drying in a rack drying oven at 50 C it is sieved again (1.5
mm mesh
width) and the lubricant is admixed. The press-ready mixture is processed to
give
tablets.
Tablet weight: 220 mg
Diameter: 10 mm, biplanar having a facet on both sides
and breaking notch on one side.
Example 7
Tablets containing 150 mg of active substance
Composition:
1 tablet contains:
Active substance 150.0 mg
Lactose, powdered 89.0 mg
Maize starch 40.0 mg
Colloidal silicic acid 10.0 mg
Polyvinylpyrrolidone 10.0 mg
Magnesium stearate 1.0 mg
300.0 mg
Preparation:
The active substance mixed with lactose, maize starch and silicic acid is
moistened with
a 20% strength aqueous polyvinylpyrrolidone solution and pounded through a
sieve
having a mesh width of 1.5 mm.
The granules dried at 45 C are again grated through the same sieve and mixed
with the
stated amount of magnesium stearate. Tablets are pressed from the mixture.
CA 02540501 2006-03-28
-40-
Tablet weight: 300 mg
Die: 10 mm, flat
Example 8
Hard gelatine capsules containing 150 mg of active substance
Composition:
1 capsule contains:
Active compound 150.0 mg
Maize starch, dried about 180.0 mg
Lactose, powdered about 87.0 mg
Magnesium stearate 3.0 mg
about 420.0 mg
Preparation:
The active compound is blended with the excipients, passed through a sieve of
mesh
width 0.75 mm and mixed homogeneously in a suitable apparatus.
The final mixture is filled into hard gelatine capsules of size 1.
Capsule filling: about 320 mg
Capsule shell: hard gelatine capsule size 1.
Example 9
Suppositories containing 150 mg of active substance
Composition:
1 suppository contains:
Active compound 150.0 mg
Polyethylene glycol 1500 550.0 mg
Polyethylene glycol 6000 460.0 mg
Polyoxyethylene sorbitan monostearate 840.0 mg
2000.0 mg
CA 02540501 2006-03-28
-41 -
Preparation:
After melting the suppository mass, the active compound is dispersed
homogeneously
therein and the melt is poured into pre-cooled moulds.
Example 10
Suspension containing 50 mg of active substance
Composition:
100 ml of suspension contain:
Active compound 1.00 g
Carboxymethylcellulose Na salt 0.10 g
Methyl p-hydroxybenzoate 0.05 g
Propyl p-hydroxybenzoate 0.01 g
Sucrose 10.00 g
Glycerol 5.00 g
Sorbitol solution, 70% strength 20.00 g
Flavouring 0.30 g
Water, dist. to 100.00 ml
Preparation:
Distilled water is heated to 70 C. Methyl and propyl p-hydroxybenzoate and
also
glycerol and carboxymethylcellulose sodium salt are dissolved in this with
stirring. The
mixture is cooled to room temperature and the active compound is added with
stirring
and homogeneously dispersed. After adding and dissolving the sugar, the
sorbitol
solution and the flavouring, the suspension is evacuated with stirring for
deaeration.
5 ml of suspension contain 50 mg of active compound.
3o Example 11
Ampoules containing 10 mg of active substance
Composition:
CA 02540501 2006-03-28
-42-
Active compound 10.0 mg
0.01 N hydrochloric acid q. s.
Water, double-distilled to 2.0 ml
Preparation:
The active substance is dissolved in the required amount of 0.01 N HCl,
rendered
isotonic using sodium chloride, sterile-filtered and filled into 2 ml
ampoules.
Example 12
Ampoules containing 50 mg of active substance
Composition:
Active compound 50.0 mg
0.01 N hydrochloric acid q. s.
Water, double-distilled to 10.0 ml
Preparation:
The active substance is dissolved in the required amount of 0.01 N HCl,
rendered
isotonic using sodium chloride, sterile-filtered and filled into 10 ml
ampoules.
Example 13
Capsules for powder inhalation containing 5 mg of active substance
1 capsule contains:
Active substance 5.0 mg
Lactose for inhalation purposes 15.0 mg
20.0 mg
Preparation:
The active substance is mixed with lactose for inhalation purposes. The
mixture is filled
into capsules on a capsule machine (weight of the empty capsule about 50 mg).
CA 02540501 2006-03-28
-43-
Capsule weight: 70.0 mg
Capsule size: 3
Example 14
Inhalation solution for a hand nebulizer containing 2.5 mg of active substance
1 stroke contains:
Active substance 2.500 mg
Benzalkonium chloride 0.001 mg
1 N hydrochloric acid q.s.
Ethanol/water (50/50) to 15.000 mg
Preparation:
The active substance and benzalkonium chloride are dissolved in ethanol/water
(50/50).
The pH of the solution is adjusted using 1N hydrochloric acid. The adjusted
solution is
filtered and filled into containers (cartridges) suitable for the hand
nebulizer.
Filling mass of the container: 4.5 g