Note: Descriptions are shown in the official language in which they were submitted.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
1
SALTS AND POLYMORPHS OF A PYRROLE-SUBSTITUTED INDOLINONE COMPOUND
This application claims the benefit of U.S. Provisional Application Serial No.
601508,104, filed October 2, 2003, the disclosure of which is incorporated
herein by reference in
its entirety.
Background of the Invention
This invention relates to salt forms and polymorphs of 5-[(Z)-(5-fluoro-2-oxo-
1,2-dihydro-
3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-
carboxamide that are useful in the treatment of abnormal cell growth, such as
cancer, in
mammals. This invention also relates to compositions including such salts, and
to methods of
using such compositions in the treatment of abnormal cell growth in mammals,
especially humans.
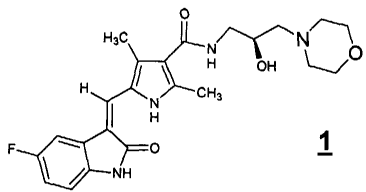
The compound 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-
[(2S)-2
hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide, shown
in structural
formula 1, is a potent, selective oral inhibitor of receptor tyrosine kinases
(RTKs) involved in
N O
1
signaling cascades that trigger tumor growth, progression and survival. In
vivo studies have
shown that this compound has anti-tumor activity in diverse preclinical solid
and hematopoietic
cancer xenograft models. This compound, its preparation and use are further
described in U.S.
patent application publication no. US 2003!0092917, published May 15, 2003,
the disclosure of
which is incorporated herein by reference in its entirety.
In its free base form, 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-
ylidene)methyl]-N
((2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide
is fairly
crystalline, chemically and enantiomerically stable, and relatively non-
hygroscopic. However, it
would be advantageous to have salt forms having improved properties, such as
improved
crystallinity and/or decreased hygroscopicity, while maintaining chemical and
enantiomeric
stability properties.
Summar5r of the Invention
In one embodiment, the invention provides a non-hygroscopic salt of 5-[(Z)-(5-
fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide. Hygroscopicity is determined by dynamic
moisture
sorption gravimetry (DMSG) using a controlled atmosphere microbalance at a
temperature of
25 °C. Samples ace analyzed over a relative humidity range of from 0 to
90°lo in 3% steps.
Each step is brought to equilibrium before moving to the next step, with
equilibrium assessed as
a weight change of less than 0.002 mg (0.02%) for five consecutive points at 1
point per 120
seconds. Using this measure of hygroscopicity, non-hygroscopic salts of the
invention exhibit a
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
2
water uptake of less than 5%, preferably less than 4%, more preferably less
than 3%, more
preferably less than 2%, more preferably less than 1 % by weight, at 80%
relative humidity.
In particular aspects of this embodiment, the salt is anhydrous, crystalline,
or both
anhydrous and crystalline.
In a particular aspect of this embodiment, the salt is a maleate salt,
preferably an
anhydrous maleate salt or a crystalline maleate salt, more preferably a
crystalline anhydrous
maleate salt.
In particular aspects of this embodiment, the salt is a crystalline anhydrous
maleate salt of
polymorphic Form 1 or polymorphic Form 2.
Polymorphic Form 1 is a crystalline, anhydrous polymorph having characteristic
powder X-
ray diffraction (PXRD) peaks at diffraction angles (2A) of 12.7 and
15.4°. More particularly,
polymorph Form 1 has a PXRD pattern including peaks as shown in Table 1.
Table 1: Polymorph Form 1 PXRD
2A () I/Im~ (%) 28 () I/Im~ (%)
10.85 15 20.18 14
12.68 20 20.42 16
14.48 13 22.37 68
15.35 41 24.74 52
17.06 17 25.73 25
18.14 100 26.84 44
19.04 58 27.41 53
One skilled in the art will appreciate that the peak positions (28) will show
some inter-apparatus
variability, typically as much as 0.1°. Further, one skilled in the art
will appreciate that relative
peak intensities will show inter-apparatus variability as well as variability
due to degree of
crystallinity, preferred orientation, prepared sample surface, and other
factors known to those
skilled in the art, and should be taken as qualitative measures only. Still
more particularly,
polymorph Form 1 has a PXRD pattern essentially the same as shown in Figure 1,
where
"essentially the same" encompasses typical peak position and intensity
variability as discussed
above.
Polymorphic Form 2 is a crystalline, anhydrous polymorph having characteristic
powder X
ray diffraction (PXRD) peaks at diffraction angles (26) of 13.1 and
15.9°. More particularly,
polymorph Form 2 has a PXRD pattern including peaks as shown in Table 2.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
3
Table 2: Polymorph Form 2 PXRD
28 () I/I",~ 28 () I/I
(%)
7.31 6 19.52 28
10.97 35 20.30 28
11.87 26 22.28 46
13.10 17 22.82 41
14.63 43 23.96 34
15.89 100 24.56 67
17.42 39 25.88 47
18.14 87 27.02 44
18.98 39
Still more particularly, polymorph Form 2 has a PXRD pattern essentially the
same as
shown in Figure 2, where "essentially the same" encompasses typical peak
position and
intensity variability as discussed above.
In another aspect of this embodiment, the salt is a crystalline anhydrous
maleate salt of
polymorphic Form 1 or polymorphic Form 2, wherein the polymorphic form is
substantially pure. A
"substantially pure" salt of polymorphic Form 1 includes less than 10%,
preferably less than 5%,
preferably less than 3%, preferably less than 1 % by weight of polymorph Form
2 or any other
poiymorphic form. Similarly, a "substantially pure" salt of polymorphic Form 2
includes less than
10%, preferably less than 5%, preferably less than 3%, preferably less than 1
% by weight of
polymorph Form 1 or any other polymorphic form.
In another aspect of this embodiment, the salt is a crystalline anhydrous
maleate salt that
is a mixture of polymorphic Form 1 and polymorphic Form 2. Preferably, the
mixture is a
substantially pure mixture, where a substantially pure mixture of polymorphic
forms 1 and 2
includes less than 10%, preferably less than 5%, preferably less than 3%,
preferably less than 1
by weight of any other polymorphic forms.
Mixtures of polymorph forms 1 and 2 will have diffraction peaks characteristic
of both
forms, particularly peaks at diffraction angles (28) of 12.7, 13.1, 15.4 and
15.9, more particularly
peaks at the positions shown in Tables 1 and 2, still more particularly a PXRD
pattern that is a
convolution of Figures 1 and 2.
In another embodiment, the invention provides a maleate salt of 5-[(Z)-(5-
fluoro-2-oxo-
1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-dimethyl-
1 H-pyrrole-3-carboxamide.
In particular aspects of this embodiment, the maleate salt is crystalline,
anhydrous, or
both crystalline and anhydrous.
In particular aspects of this embodiment, the maleate salt is a crystalline
anhydrous salt
of polymorph Form 1, preferably substantially pure polymorph Form 1, or
polymorph Form 2,
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
4
preferably substantially pure polymorph Form 2, or a mixture of polymorph
forms 1 and 2,
preferably a substantially pure mixture, where polymorph forms 1 and 2 are as
described above.
In another embodiment, the invention provides a crystalline anhydrous maleate
salt of 5-
[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-
3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide. In particular aspects of
this embodiment, the
crystalline anhydrous maleate salt is a salt of polymorph Form 1, preferably
substantially pure
polymorph Form 1, or polymorph Form 2, preferably substantially pure polymorph
Form 2, or a
mixture of polymorph forms 1 and 2, preferably a substantially pure mixture,
where polymorph
forms 1 and 2 are as described above.
In another embodiment, the invention provides a crystalline anhydrous maleate
salt of
5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide having a powder X-ray
diffraction pattern
comprising peaks at diffraction angles (2A) essentially the same as shown in
Figure 1, where
"essentially the same" is as defined above.
In another embodiment, the invention provides a crystalline anhydrous maleate
salt of
5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide having a powder X-ray
diffraction pattern
comprising peaks at diffraction angles (28) essentially the same as shown in
Figure 2, where
"essentially the same" is as defined above.
In another embodiment, the invention provides a crystalline anhydrous maleate
salt of
5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrofe-3-carboxamide having a powder X-ray
diffraction pattern which
is a convolution of diffraction patterns comprising peaks at diffraction
angles (20) essentially the
same as shown in Figures 1 and 2, where "essentially the same" is as defined
above.
In another embodiment, the invention provides a pharmaceutical composition
comprising the salt of any of the preceding embodiments.
In another embodiment, the invention provides a capsule comprising any of the
pharmaceutical compositions of the invention. In particular aspects of this
embodiment, the
capsule comprises from 5 to 75 mg, preferably from 10 to 25 mg, free base
equivalent of the 5-
[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-
3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide salt.
In another embodiment, the invention provides a method of treating cancer in a
mammal, including a human, the method comprising administering to the mammal a
therapeutically effective amount of any of the pharmaceutical compositions of
the invention.
In another embodiment, the invention provides a method of treating cancer in a
mammal, the method comprising administering to the mammal, including a human,
any of the
capsules of the invention.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
In a particular aspect of any of the preceding method embodiments, the method
further
comprises administering one or more anti-tumor agents, anti-angiogenesis
agents, signal
transduction inhibitors, or antiproliferative agents.
The invention also relates to a method for the treatment of abnormal cell
growth in a
5 mammal, including a human, comprising administering to said mammal an amount
of a compound
of the formula 1, as defined above, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof, that is effective in treating abnormal cell growth. In one embodiment
of this method, the
abnormal cell growth is cancer, including, but not limited to, lung cancer,
bone cancer, pancreatic
cancer, skin cancer, cancer of the head or neck, cutaneous or intraocular
melanoma, uterine
cancer, ovarian cancer, rectal cancer, cancer of the anal region, stomach
cancer, colon cancer,
breast cancer, uterine cancer, carcinoma of the fallopian tubes, carcinoma of
the endometrium,
carcinoma of the cervix, carcinoma of the vagina, carcinoma of the vulva,
Hodgkin's Disease,
cancer of the esophagus, cancer of the small intestine, cancer of the
endocrine system, cancer of
the thyroid gland, cancer of the parathyroid gland, cancer of the adrenal
gland, sarcoma of soft
tissue, cancer of the urethra, cancer of the penis, prostate cancer, chronic
or acute leukemia,
lymphocytic lymphomas, cancer of the bladder, cancer of the kidney or ureter,
renal cell
carcinoma, carcinoma of the renal pelvis, neoplasms of the central nervous
system (CNS),
primary CNS lymphoma, spinal axis tumors, brain stem glioma, pituitary
adenoma, or a
combination of one or more of the foregoing cancers. In another embodiment of
said method,
said abnormal cell growth is a benign proliferative disease, including, but
not limited to, psoriasis,
benign prostatic hypertrophy or restinosis.
This invention also relates to a method for the treatment of abnormal cell
growth in a
mammal which comprises administering to said mammal an amount of a compound of
formula 1,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, that is
effective in treating
abnormal cell growth in combination with an anti-tumor agent selected from the
group consisting of
mitotic inhibitors, alkylating agents, anti-metabolites, intercalating
antibiotics, growth factor
inhibitors, cell cycle inhibitors, enzymes, topoisomerase inhibitors,
biological response modifiers,
antibodies, cytotoxics, anti-hormones, and anti-androgens.
This invention also relates to a pharmaceutical composition for the treatment
of abnormal
cell growth in a mammal, including a human, comprising an amount of a compound
of the formula
1, as defined above, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, that is
effective in treating abnormal cell growth, and a pharmaceutically acceptable
carrier. In one
embodiment of said composition, said abnormal cell growth is cancer,
including, but not limited to,
lung cancer, bone cancer, pancreatic cancer, skin cancer, cancer of the head
or neck, cutaneous
or intraocular melanoma, uterine cancer, ovarian cancer, rectal cancer, cancer
of the anal region,
stomach cancer, colon cancer, breast cancer, uterine cancer, carcinoma of the
fallopian tubes,
carcinoma of the endometrium, carcinoma of the cervix, carcinoma of the
vagina, carcinoma of the
vulva, Hodgkin's Disease, cancer of the esophagus, cancer of the small
intestine, cancer of the
endocrine system, cancer of the thyroid gland, cancer of the parathyroid
gland, cancer of the
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
6
adrenal gland, sarcoma of soft tissue, cancer of the urethra, cancer of the
penis, prostate cancer,
chronic or acute leukemia, lymphocytic lymphomas, cancer of the bladder,
cancer of the kidney or
ureter, renal cell carcinoma, carcinoma of the renal pelvis, neoplasms of the
central nervous
system (CNS), primary CNS lymphoma, spinal axis tumors, brain stem glioma,
pituitary adenoma,
or a combination of one or more of the foregoing cancers. In another
embodiment of said
pharmaceutical composition, said abnormal cell growth is a benign
proliferative disease, including,
but not limited to, psoriasis, benign prostatic hypertrophy or restinosis.
The invention also relates to a pharmaceutical composition for the treatment
of abnormal
cell growth in a mammal, including a human, which comprises an amount of a
compound of
formula 1, as defined above, or a pharmaceutically acceptable salt, solvate or
prodrug thereof, that
is effective in treating abnormal cell growth in combination with a
pharmaceutically acceptable
carrier and an anti-tumor agent selected from the group consisting of mitotic
inhibitors, alkylating
agents, anti-metabolites, intercalating antibiotics, growth factor inhibitors,
cell cycle inhibitors,
enzymes, topoisomerase inhibitors, biological response modifiers, anti-
hormones, and anti
androgens.
This invention also relates to a method for the treatment of a disorder
associated with
angiogenesis in a mammal, including a human, comprising administering to said
mammal an
amount of a compound of the formula 1, as defined above, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, that is effective in treating said disorder. Such
disorders include
cancerous tumors such as melanoma; ocular disorders such as age-related
macular
degeneration, presumed ocular histoplasmosis syndrome, and retinal
neovascularization from
proliferative diabetic retinopathy; rheumatoid arthritis; bone loss disorders
such as osteoporosis,
Paget's disease, humoral hypercalcemia of malignancy, hypercalcemia from
tumors metastatic to
bone, and osteoporosis induced by glucocorticoid treatment; coronary
restenosis; and certain
microbial infections including those associated with microbial pathogens
selected from
adenovirus, hantaviruses, Borrelia burgdorferi, Yersinia spp., Bordetella
pertussis, and group A
Streptococcus.
This invention also relates to a method of (and to a pharmaceutical
composition for)
treating abnormal cell growth in a mammal which comprise an amount of a
compound of
formula 1, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
and an amount of
one or more substances selected from anti-angiogenesis agents, signal
transduction inhibitors,
and antiproliferative agents, which amounts are together effective in treating
said abnormal cell
growth.
Anti-angiogenesis agents, such as MMP-2 (matrix-metalloprotienase 2)
inhibitors,
MMP-9 (matrix-metalloprotienase 9) inhibitors, and COX-II (cyclooxygenase II)
inhibitors, can
be used in conjunction with a compound of formula 1 in the methods and
pharmaceutical
compositions described herein. Examples of useful COX-II inhibitors include
CELEBREXT""
(alecoxib), valdecoxib, and rofecoxib. Examples of useful matrix
metalloproteinase inhibitors are
described in WO 96/33172 (published October 24, 1996), WO 96/27583 (published
March 7,
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
7
1996), European Patent Application No. 97304971.1 (filed July 8, 1997),
European Patent
Application No. 99308617.2 (filed October 29, 1999), WO 98/07697 (published
February 26,
1998), WO 98/03516 (published January 29, 1998), WO 98/34918 (published August
13, 1998),
WO 98/34915 (published August 13, 1998), WO 98/33768 (published August 6,
1998), WO
98/30566 (published July 16, 1998), European Patent Publication 606,046
(published July 13,
1994), European Patent Publication 931,788 (published July 28, 1999), WO
90/05719 (published
May 331, 1990), WO 99/52910 (published October 21, 1999), WO 99/52889
(published October
21, 1999), WO 99/29667 (published June 17, 1999), PCT International
Application No.
PCT/IB98/01113 (filed July 21, 1998), European Patent Application No.
99302232.1 (filed March
25, 1999), Great Britain patent application number 9912961.1 (filed June 3,
1999), United States
Provisional Application No. 60/148,464 (filed August 12, 1999), United States
Patent 5,863,949
(issued January 26, 1999), United States Patent 5,861,510 (issued January 19,
1999), and
European Patent Publication 780,386 (published June 25, 1997), all of which
are herein.
incorporated by reference in their entirety. Preferred MMP-2 and MMP-9
inhibitors are those that
have little or no activity inhibiting MMP-1. More preferred, are those that
selectively inhibit MMP-2
and/or MMP-9 relative to the other matrix-metalloproteinases (i.e. MMP-1, MMP-
3, MMP-4, MMP-
5, MMP-6, MMP-7, MMP-8, MMP-10, MMP-11, MMP-12, and MMP-13).
Some specific examples of MMP inhibitors useful in combination with the
compounds of
the present invention are AG-3340, RO 32-3555, RS 13-0830, and the compounds
recited in
/ the following list:
3-[[4-(4-fluoro-phenoxy)-benzenesulfonyl]-(1-hydroxycarbamoyl-cyclopentyl)-
amino]-
propionic acid;
3-exo-3-[4-(4-fluoro-phenoxy)-benzenesulfonylamino]-8-oxa-bicyclo[3.2.1
]octane-3-
carboxylic acid hydroxyamide;
(2R, 3R) 1-[4-(2-chloro-4-fluoro-benzyloxy)-benzenesulfonyl]-3-hydroxy-3-
methyl-
piperidine-2-carboxylic acid hydroxyamide;
4-[4-(4-fluoro-phenoxy)-benzenesulfonylamino]-tetrahydro-pyran-4-carboxylic
acid
hydroxyamide;
3-[[4-(4-fluoro-phenoxy)-benzenesulfonyl]-(1-hydroxycarbamoyl-cyclobutyl)-
amino]-
propionic acid;
4-[4-(4-chloro-phenoxy)-benzenesulfonylamino]-tetrahydro-pyran-4-carboxylic
acid
hydroxyamide;
3-[4-(4-chloro-phenoxy)-benzenesulfonylamino]-tetrahydro-pyran-3-carboxylic
acid
hydroxyamide;
(2R, 3R) 1-[4-(4-fluoro-2-methyl-benzyloxy)-benzenesulfonyl]-3-hydroxy-3-
methyl-
piperidine-2-carboxylic acid hydroxyamide;
3-[[4-(4-fluoro-phenoxy)-benzenesulfonyl]-(1-hydroxycarbamoyl-1-methyl-ethyl)-
amino]-
propionic acid;
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
8
3-[[4-(4-fluoro-phenoxy)-benzenesulfonyl]-(4-hydroxycarbamoyl-tetrahydro-pyran-
4-yl)-
amino]-propionic acid;
3-exo-3-[4-(4-chloro-phenoxy)-benzenesulfonylamino]-8-oxa-bicyclo[3.2.1
]octane-3-
carboxylic acid hydroxyamide;
3-endo-3-[4-(4-fluoro-phenoxy)-benzenesulfonylamino]-8-oxa-
bicyclo[3.2.1]octane-3-
carboxylic acid hydroxyamide; and
3-[4-(4-fluoro-phenoxy)-benzenesulfonylamino]-tetrahydro-furan-3-carboxylic
acid
hydroxyamide;
and pharmaceutically acceptable salts, solvates and prodrugs of said
compounds.
The compounds of formula 1, and the pharmaceutically acceptable salts,
solvates and
prodrugs thereof, can also be used in combination with signal transduction
inhibitors, such as
agents that can inhibit EGFR (epidermal growth factor receptor) responses,
such as EGFR
antibodies, EGF antibodies, and molecules that are EGFR inhibitors; VEGF
(vascular
endothelial growth factor) inhibitors; and erbB2 receptor inhibitors, such as
organic molecules or
antibodies that bind to the erbB2 receptor, for example, HERCEPTINT""
(Genentech, Inc. of
South San Francisco, California, USA).
EGFR inhibitors are described in, for example in WO 95/19970 (published July
27,
1995), WO 98/14451 (published April 9, 1998), WO 98/02434 (published January
22, 1998),
and United States Patent 5,747,498 (issued May 5, 1998). EGFR-inhibiting
agents include, but
are not limited to, the monoclonal antibodies C225 and anti-EGFR 22Mab
(ImClone Systems
Incorporated of New York, New York, USA), the compounds ZD-1839 (AstraZeneca),
BIBX-
1382 (Boehringer Ingelheim), MDX-447 (Medarex Inc. of Annandale, New Jersey,
USA), and
OLX-103 (Merck & Co. of Whitehouse Station, New Jersey, USA), VRCTC-310
(Ventech
Research) and EGF fusion toxin (Seragen Inc. of Hopkinton, Massachusettes).
VEGF inhibitors, for example SU-5416 and SU-6668 (Sugen Inc. of South San
Francisco, California, USA), can also be combined with a compound of formula
1. VEGF
inhibitors are described in, for example in WO 99/24440 (published May 20,
1999), PCT
International Application PCT/IB99/00797 (filed May 3, 1999), in WO 95/21613
(published
August 17, 1995), WO 99/61422 (published December 2, 1999), United States
Patent 5,834,504
(issued November 10, 1998), WO 98/50356 (published November 12, 1998), United
States Patent
5,883,113 (issued March 16, 1999), United States Patent 5,886,020 (issued
March 23, 1999),
United States Patent 5,792,783 (issued August 11, 1998), WO 99/10349
(published March 4,
1999), WO 97/32856 (published September 12, 1997), WO 97/22596 (published June
26, 1997),
WO 98/54093 (published December 3, 1998), WO 98/02438 (published January 22,
1998), WO
99/16755 (published April 8, 1999), and WO 98/02437 (published January 22,
1998), all of which
are herein incorporated by reference in their entirety. Other examples of some
specific VEGF
inhibitors are IM862 (Cytran Inc. of Kirkland, Washington, USA); anti-VEGF
monoclonal
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
9
antibody of Genentech, Inc. of South San Francisco, California; and angiozyme,
a synthetic
ribozyme from Ribozyme (Boulder, Colorado) and Chiron (Emeryville,
California).
ErbB2 receptor inhibitors, such as GW-282974 (Glaxo Wellcome plc), and the
monoclonal antibodies AR-209 (Aronex Pharmaceuticals Inc. of The Woodlands,
Texas, USA)
and 2B-1 (Chiron), may be administered in combination with a compound of
formula 1. Such
erbB2 inhibitors include those described in WO 98/02434 (published January 22,
1998), WO
99/35146 (published July 15, 1999), WO 99/35132 (published July 15, 1999), WO
98/02437
(published January 22, 1998), WO 97/13760 (published April 17, 1997), WO
95/19970
(published July 27, 1995), United States Patent 5,587,458 (issued December 24,
1996), and
United States Patent 5,877,305 (issued March 2, 1999), each of which is herein
incorporated by
reference in its entirety. ErbB2 receptor inhibitors useful in the present
invention are also
described in United States Provisional Application No. 60/117,341, filed
January 27, 1999, and
in United States Provisional Application No. 60/117,346, filed January 27,
1999, both of which
are herein incorporated by reference in their entirety.
Other antiproliferative agents that may be used with the compounds of the
present
invention include inhibitors of the enzyme farnesyl protein transferase and
inhibitors of the
receptor tyrosine kinase PDGFr, including the compounds disclosed and claimed
in the
following United States patent applications: 09/221946 (filed December 28,
1998); 09/454058
(filed December 2, 1999); 09/501163 (filed February 9, 2000); 09/539930 (filed
March 31,
2000); 09/202796 (filed May 22, 1997); 09/384339 (filed August 26, 1999); and
09/383755 (filed
August 26, 1999); and the compounds disclosed and claimed in the following
United States
provisional patent applications: 60/168207 (filed November 30, 1999);
60/170119 (filed
December 10, 1999); 60/177718 (filed January 21, 2000); 60/168217 (filed
November 30,
1999), and 60/200834 (filed May 1, 2000). Each of the foregoing patent
applications and
provisional patent applications is herein incorporated by reference in their
entirety.
A compound of formula 1 may also be used with other agents useful in treating
abnormal cell growth or cancer, including, but not limited to, agents capable
of enhancing
antitumor immune responses, such as CTLA4 (cytotoxic lymphocite antigen 4)
antibodies, and
other agents capable of blocking CTLA4; and anti-proliferative agents such as
other farnesyl
protein transferase inhibitors, for example the farnesyl protein transferase
inhibitors described in
the references cited in the "Background" section, supra. Specific CTLA4
antibodies that can be
used in the present invention include those described in United States
Provisional Application
60/113,647 (filed December 23, 1998) which is herein incorporated by reference
in its entirety.
The term "treating", as used herein, unless otherwise indicated, means
reversing,
alleviating, inhibiting the progress of, or preventing the disorder or
condition to which such term
applies, or one or more symptoms of such disorder or condition. The term
"treatment', as used
herein, unless otherwise indicated, refers to the act of treating as
"treating" is defined immediately
above.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
Brief Descrption of the Drawings
Figure 1 shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 1.
Figure 2 shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 2.
Figure 3A shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-
2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
10 dimethyl-1 H-pyrrole-3-carboxamide, including polymorph Form 3 in a
polymorph mixture.
Figure 3B shows a deconvoluted powder X-ray diffraction pattern of a maleate
salt of 5-
[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-
3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 3.
Figure 4 shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 4
Figure 5 shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 5.
Figure 6A shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-
2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, including polymorph Form 6 in a polymorph
mixture.
Figure 6B shows a deconvoluted powder X-ray diffraction pattern of a maleate
salt of 5-
[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-
3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 6.
Figure 7 shows a powder X-ray diffraction pattern of a maleate salt of 5-[(Z)-
(5-fluoro-2-
oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-((2S)-2-hydroxy-3-morpholin-4-
ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-carboxamide, polymorph Form 7.
Figure 8 shows the structural formulae of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-
3H-indol-3-
ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-
pyrrole-3-
carboxamide and the relative abundance of three metabolites in monkey plasma.
Figure 9 shows the structural formulae of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-
3H-indol-3-
ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-
pyrrole-3-
carboxamide and the relative abundance of several metabolites in human urine.
Figure 10A shows a dynamic moisture sorption gravimetry (DMSG) scan for a
first
hydrochloride salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-
ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
11
Figure 10B shows a dynamic moisture sorption gravimetry (DMSG) scan for a
second
hydrochloride salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-
ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 11 shows a dynamic moisture sorption gravimetry (DMSG) scan for an L-
malate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 12 shows a dynamic moisture sorption gravimetry (DMSG) scan for a
maleate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 13 shows a dynamic moisture sorption gravimetry (DMSG) scan for an L-
tartrate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 14 shows a dynamic moisture sorption gravimetry (DMSG) scan for a
tosylate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 15 shows a dynamic moisture sorption gravimetry (DMSG) scan for a
mandelate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Figure 16 shows a dynamic moisture sorption gravimetry (DMSG) scan for a
malonate
salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide.
Detailed Description Of The Invention
The compound 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-
[(2S)-2-
hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide can be
prepared
according to methods described in U.S. Patent No. 6,573,293 and U.S. patent
application
publication no. 2003/0092917, published May 15, 2003, the entire disclosures
of which are
incorporated herein by reference.
Salts of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-
2-hydroxy-3-
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide are readily
prepared by treating the
free base compound with a substantially equivalent amount of the chosen
mineral or organic acid
in an aqueous solvent medium or in a suitable organic solvent, such as
methanol or ethanol.
Upon careful evaporation of the solvent, the desired solid salt is readily
obtained. The desired acid
salt can also be precipitated from a solution of the free base in an organic
solvent by adding to the
solution an appropriate mineral or organic acid.
The maleate salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-
ylidene)methyl]-N-[(2S)-
2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide can
be produced with
good crystallinity using, for example, room temperature evaporation in ethanol
or acetonitrile/water
(1:1), heat evaporation in methanol/ethyl acetate, drowning in ethanoUhexane,
room temperature
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
12
slow evaporation in isopropanol, or room temperature slurry in acetonitrile or
isopropanol, to name
but a few acceptable methods and solvent systems. It was observed that samples
of fair or poor
crystallinity were produced using room temperature evaporation in water,
acetonitrile, isopropanol,
isopropanol/water (1:1) and methanol; room temperature slurry in acetonitrile
and isopropanol; and
drowning in ethanol/hexane.
The hydrochloride salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-
ylidene)methyl]-N-
[(2S)-2-hydroxy-3-morpholin~-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide
can be produced
with good crystallinity by distillation from acetonitrile/ethanol/water.
Seven polymorph forms of the maleate salt of 5-[(Z)-(5-fluoro-2-oxo-1,2-
dihydro-3H-indol-
3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-
pyrrole-3-
carboxamide have been identified and characterized. PXRD patterns for these
forms, denoted
Forms 1 through 7, are shown in Figures 1 through 7.
Polymorph Form 1 can be produced by cooling from 80 °C to 5 °C
in cyclopentanone or
nitrobenzene. The infrared spectrum (600 crri' to 4000 crri') of polymorph
Form 1 (peak table) is
shown in Table 3, and the PXRD pattern in Figure 1. Analysis by DSC and TGA
show that this
form melts with a peak melting point of 220 °C and onset melting point
of 215 °C; as the sample
melts, a simultaneous TGA weight loss occurs. Full decomposition starts at 315
°C.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
13
Table 3: Form 1, Infrared Peaks
~, (cm-')Transmittance~, (ctrl')Transmittance7~ (crti')Transmittance
(%) (%) (%)
3314.7 31 1479.4 6 1021.3 70
3158.4 42 1465.9 14 1002.0 79
3119.9 49 1454.3 10 979.8 57
3099.6 50 1445.6 8 956.7 83
3056.2 54 1412.9 54 935.5 76
3024.4 54 1387.8 43 922.9 69
2954.0 4 1377.2 37 917.1 70
2924.1 1 1359.8 25 902.7 83
2869.1 10 1323.2 6 887.3 74
2854.6 5 1302.9 36 878.6 73
2768.8 71 1294.2 47 866.0 23
2725.4 72 1280.7 27 808.2 35
2706.1 71 1261.4 18 800.5 42
2641.5 73 1235.4 30 780.2 53
2415.8 72 1213.2 43 773.5 53
1996.3 76 1196.8 11 759.0 70
1835.3 79 1169.8 38 731.0 67
1724.4 80 1154.4 20 725.2 72
1684.8 63 1135.1 31 698.2 76
1657.8 10 1112.9 53 668.3 23
1630.8 9 1099.4 39 650.0 62
1602.8 34 1077.2 51 626.9 61
1570.1 11 1065.7 52 605.6 57
1556.6 4 1058.0 38
1498.7 19 1037.7 37
Polymorph Form 2 can be produced by cooling from 80 °C to 5 °C
preferably in a polar
solvent, preferably cooled slowly (e.g., 0.6 °C/min), and preferably
using a long aging time (e.g., 48
hours). The infrared spectrum (600 cm' to 4000 crri') of polymorph Form 2
(peak table) is shown
in Table 4, and the PXRD pattern in Figure 2. Analysis by DSC and TGA show
that this form melts
with a peak melting point of 224 °C and onset melting point of 221
°C.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
14
Table 4: Form 2, Infrared Peaks
~ (cm-~)Transmittance~, (cm')Transmittance~ (cm-~)Transmittance
(%) (%) (%)
3313.7 34 1445.6 16 1021.3 63
3171.0 41 1428.3 40 979.8 54
3119.9 48 1412.9 52 955.7 79
3053.3 51 1386.8 43 935.5 72
3023.4 51 1377.2 40 925.8 71
2954.9 11 1359.8 32 921.0 70
2925.0 5 1322.2 14 913.3 65
2869.1 18 1302.9 39 902.7 80
2854.6 13 1294.2 46 887.3 72
2806.4 62 1278.8 33 877.6 71
2704.2 68 1260.5 24 865.1 28
2641.5 69 1234.4 35 813.0 43
2416.8 69 1213.2 45 807.2 43
1835.3 78 1196.8 20 780.2 55
1824.7 80 1168.9 40 773.5 55
1685.8 62 1157.3 31 749.3 70
1657.8 20 1135.1 34 731.0 64
1630.8 18 1112.0 51 724.3 68
1574.9 21 1098.5 40 698.2 74
1556.6 11 1095.6 41 668.3 31
1497.7 25 1077.2 48 651.0 62
1480.4 15 1065.7 51 629.6 63
1464.0 19 1058.0 40 606.6 62
1454.3 16 1037.7 39
Mixtures of polymorph forms 1 and 2 can be produced in ethanol and THF/water
solvents.
Polymorph Form 3 can be produced by cooling from 80 °C to 5 °C
in water. Attempts to
produce polymorph form 3 resulted in mixtures with forms 2 or 5. Figure 3A
shows a typical PXRD
pattern, and Figure 3B shows the pattern deconvoluted to show a calculated
pure polymorph Form
3 pattern. Polymorph Form 3 may be a hydrate; however, this was not confirmed.
Polymorph Form 4 can be produced by cooling from 80 °C to 5 °C
in 4-methyl morpholine
or triethylamine.
Polymorph Form 5 can be produced by cooling from 80 °C to 5 °C
preferably in a polar
solvents, preferably at a rapid cooling rate (e.g., 300 °C/min) and
short aging time (e.g., 1 hour).
Polymorph Form 6 can be produced by cooling from 80 °C to 5 °C
in a variety of solvents including
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
esters, ketones, alcohols, alkanes and amines, preferably using a rapid
cooling rate (e.g.,
300 °C/min) and a short aging time (e.g., 1 hour). Attempts to produce
polymorph Form 6 resulted
in mixtures with forms 2 or 5. Figure 6A shows a typical PXRD pattern, and
Figure 6B shows the
pattern deconvoluted to show a calculated pure polymorph Form 6 pattern.
5 Polymorph Form 7 can be produced by cooling from 80 °C to 5 °C
in propan-1,2-diol.
Polymorph form 7 may be a solvate; however, this was not confirmed.
Polymorph forms 3-7 are not stable, and convert over time to polymorph Form 2.
Pharmaceutical compositions of the invention may, for example, be in a form
suitable for
oral administration as a tablet, capsule, pill, powder, sustained release
formulations, solution,
10 suspension, for parenteral injection as a sterile solution, suspension or
emulsion, for topical
administration as an ointment or cream or for rectal administration as a
suppository. The
pharmaceutical composition may be in unit dosage forms suitable for single
administration of
precise dosages. The pharmaceutical composition will include a conventional
pharmaceutical
carrier or excipient and a compound according to the invention as an active
ingredient. In addition,
15 it may include other medicinal or pharmaceutical agents, carriers,
adjuvants, etc.
Exemplary parenteral administration forms include solutions or suspensions of
active
compounds in sterile aqueous solutions, for example, aqueous propylene glycol
or dextrose
solutions. Such dosage forms can be suitably buffered, if desired.
Suitable pharmaceutical carriers include inert diluents or fillers, water and
various organic
solvents. The pharmaceutical compositions may, if desired, contain additional
ingredients such as
flavorings, binders, excipients and the like. Thus for oral administration,
tablets containing various
excipients, such as citric acid may be employed together with various
disintegrants such as starch,
alginic acid and certain complex silicates and with binding agents such as
sucrose, gelatin and
acacia. Additionally, lubricating agents such as magnesium stearate, sodium
lauryl sulfate and
talc are often useful for tableting purposes. Solid compositions of a similar
type may also be
employed in soft and hard filled gelatin capsules. Preferred materials,
therefor, include lactose or
milk sugar and high molecular weight polyethylene glycols. When aqueous
suspensions or elixirs
are desired for oral administration the active compound therein may be
combined with various
sweetening or flavoring agents, coloring matters or dyes and, if desired,
emulsifying agents or
suspending agents, together with diluents such as water, ethanol, propylene
glycol, glycerin, or
combinations thereof.
Preferred methods of formulating pharmaceutical compositions of the invention
are
described in U.S. provisional patent application no. 60/421,133, filed
September 10, 2002, the
disclosure of which is incorporated herein by reference in its entirety.
Methods of preparing various pharmaceutical compositions with a specific
amount of
active compound are known, or will be apparent, to those skilled in this art.
For examples, see
Reminqton's Pharmaceutical Sciences, Mack Publishing Company, Easter, Pa.,
15th Edition
(1975).
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
16
Examples
The examples and preparations provided below further illustrate and exemplify
particular
aspects of embodiments of the invention. It is to be understood that the scope
of the present
invention is not limited in any way by the scope of the following examples.
Methods and Materials
Differential Scanning Calorimetry (DSC): DSC measurements were performed with
a TA
Instruments model 2920 differential scanning calorimeter with a Thermal
Analyst 5000 controller.
Samples ranged in weight from 0.4 to 2 mg. The samples were placed in crimped
aluminum pans
and heated at a rate of 10 °C/min up to 320 °C. Dry nitrogen was
used as a purge gas.
Powder X-ray Diffraction (PXRD): PXRD data for Figures 1 and 2, and in the
following
examples, were collected using either a Scintag X2 or X1 Advanced Diffraction
System. The
system used a copper X-ray source maintained at 45 kV and 40 mA to provide
CuKa1 emission at
1.5406 A (0.15406 nm), and a solid state pettier cooled detector. Beam
aperture was controlled
using tube divergence and antiscatter slits of 2 and 4 mm, and detector
antiscatter and receiving
slits of 0.5 and 0.3 mm width. Data were normally collected from 2 ° to
40 ° two-theta (28) using a
step scan of 0.03 °/point and a 1 s/point count time. Scintag, round
top loading stainless steel
sample cups with a 12 mm or 9 mm aluminum tray insert or a quartz plate were
used to contain
samples. As necessary, samples were hand ground with a mortar and pestle
before analysis.
The intensity data in Tables 1 and 2 were roughly corrected for background by
subtracting the
approximate background counts per second from each point.
PXRD data for Figures 3-9 were collected using a high-throughput PXRD
screening
apparatus. Plates were mounted on a Bruker GADDS diffractometer equipped with
a Hi-Star area
detector. The data collection was carried out at room temperature using
monochromated CuKa
radiation in the region of 28 from 3 to 42°. The diffraction pattern of
each well was collected in two
theta ranges (3 5 2A < 21 ° for the first frame and 19 < 20 <_
42° for the second frame) with an
exposure time of 75 s for each frame. The carrier material used during PXRD
analysis was
transparent to X-rays and contributed only slightly to the background.
TGA measurements were performed using a TA Instruments model 2950 Hi-Res
analyzer
with a Thermal Analyst 5000 controller. Samples were placed onto a tared
platinum hanging pan
and heated to at least 165 °C at a rate of 10 °C/min. Dry
nitrogen was used as a purge gas.
HPLC conditions are given in the individual examples.
Example 1
Several salts of 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-
N-[(2S)-2-
hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide were
produced and
analyzed for hygroscopicity. Hygroscopicity was determined by dynamic moisture
sorption
gravimetry (DMSG) using a controlled atmosphere microbalance at a temperature
of 25 °C.
Samples were analyzed over a relative humidity range of from 0 to 90% in 3%
steps, in a
humidity profile of 36 -~ 0 ~ 90 ~ 0%, where the initial value of 36% reflects
the approximate
initial equilibrated relative humidity (not specifically measured). Each step
was brought to
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
17
equilibrium before moving to the next step, with equilibrium assessed as a
weight change of
less than 0.001 mg (0.01 %) or 0.002 mg (0.02%) for five consecutive points at
1 point per 120
seconds. The water uptake at 80% relative humidity (RH) was selected for
comparison
between the salts. The 80% RH values for each salt are shown in Table 5, and
the right column
of the table identifies the corresponding Figure showing the complete DMSG
scan. A range of
values given for the water uptake at 80% reflects hysteresis in the 0 ~ 90%
and 90 -~ 0%
scans.
Table 5
Salt Water Uptake at 80% Figure
RH (wt. %) No.
HCI, form 3 - 20 10A
1
HCI, form 1.2 10B
2
L-malate 12 -13 11
Maleate 0.8 12
L-tartrate 9 13
Tosylate 7 14
Mandalate 5 - 6 15
Malonate 12 16
For the hydrochloride salt, the initial measurement exhibited sorption with an
apparent
stepwise gain/loss of water; the sorption profile exhibited significant
hysteresis (see Fig. 10A).
This salt is designated "form 1" in Table 5. The final product (HCI "form 2")
was not the same
crystal form as the starting material, as evaluated by PXRD (not shown). DSC
and TGA
measurements on the HCI form 2 salt indicated a monohydrate with a melting
point of about
284 °C. TGA found 3.8% weight loss. The HCI form 2 salt was also
evaluated by DMSG, but
using a 0 ~ 81 ~ 0% relative humidity profile (Figure 10B). The form 2 salt
was relatively non-
hygroscopic and showed no significant hysteresis.
The L-malate salt was of poor crystallinity (PXRD not shown). The sample
melted at
about 182 °C; however, additional thermal events occurred at about 95
°C.
Initial data collected using a maleate salt are omitted. The initial maleate
salt was only of
fair crystallinity, exhibiting a unique PXRD pattern and having a melting
point of about 181 °C.
This material was hygroscopic, having a moisture uptake of about 9% at 80%
relative humidity.
This salt is believed to be polymorph Form 5 or a mixture of Form 5 with one
ore more other
polymorphs, but this was not verified. A second maleate salt sample having
good crystallinity and
characterized as polymorph Form 2 was subsequently tested, and the results
from this polymorph
Form 2 sample are shown in Table 5.
The L-tartrate salt was of poor crystallinity (PXRD not shown). The sample
melted at
about 191 °C.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
18
The tosylate salt was of fair crystallinity (PXRD not shown). The sample
melted at about
190 °C. Uptake of about 2.5% water was observed at about 65% relative
humidity, with
subsequent loss at about 30% relative humidity. This weight change was
approximately equal to 1
mole of water.
The mandelate salt was of poor crystallinity (PXRD not shown). The sample
melted at
about 224 °C.
The malonate salt was of poor crystallinity (PXRD not shown). The sample
desolvated
(DSC) at about 133 °C and melted at about 261 °C. Solvent
content of about 15% was
determined by TGA.
Example 2
Chemical stability was assessed for two different samples of 5-[(Z)-(5-fluoro-
2-oxo-1,2-
dihydro-3H-indol-3-ylidene)methyl]-N-((2S)-2-hydroxy-3-morpholin-4-ylpropyl]-
2,4-dimethyl-1 H-
pyrrole-3-carboxamide maleate salt, polymorph Form 1. Samples were maintained
in a
temperature and humidity-controlled chamber at 25 °C and 60% relative
humidity (Tables 7 and
8) or at 40 °C and 75% relative humidity (Tables 9 and 10). At various
time intervals, material
was removed and tested for maleate counterion assay, impurities, water content
and
enantiomeric purity.
The maleate counterion assay was determined by ion exchange chromatography
(IEC).
Typical conditions were as follows:
Ionic Chromatograph: Dionex DX 600 with conductivity detector
Analytical Column: Anionic AS14, 250 x 4.0 mm, Dionex
Guard Column: Anionic AS14, 100 x 4.0 mm, Dionex
Column Temperature: 30 °C
Mobile Phase: 3.5 mM sodium carbonate plus 1 mM sodium hydrogen carbonate
Elution Mode: isocratic
Flow Rate: 1.2 mUmin
Injection Volume: 25 pL
A suitable amount of the maleate salt is dissolved in water (Milli Q grade)
and analyzed by IEC,
and compared to calibration samples. Under these conditions, the peak due to
malefic acid has
a retention time of about 11 minutes. The amount of malefic acid in the sample
is calculated as:
Assay = (CgMI) x 100
where Cg is the concentration of malefic acid found in the sample, and W is
the theoretical
sample concentration in the aqueous solution.
The amounts of impurities and degradation products were determined by HPLC,
using
a Perkin Elmer LC 200 system with a diode array detector. The analytical
column was a Waters
Xterra RP18, 5 Vim, 250 x 4.6 mm, and the guard column was a Waters Xterra
RP18, 5 Vim, 20
x 3.9 mm. Mobile phase A was a mixture of 90% 0.05 M ammonium acetate buffer
at pH 5.5
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
19
and 10% acetonitrile. Mobile phase B was a mixture of 10% 0.05 M ammonium
acetate buffer
at pH 5.5 and 90% acetonitrile. The solvent gradient is shown in Table 6.
Table 6
time (min) % A % B
0 80 20
15 80 20
40 50 50
41 20 80
46 20 80
47 80 20
60 80 20
Flow rate was 1 mUmin, injection volume 30 pL, room temperature, 435 nm
detection. Under
these conditions, the peak due to the maleate salt has a retention time of
about 24 minutes.
Impurities are characterized by their relative retention time (RRT). The
impurity at RRT = 0.26
is the E isomer. The other impurities were not characterized.
Enantiomeric purity was determined by HPLC, using a Chiralpak AD analytical
column,
10 pm, 250 x 4.6 mm (Daicel Che~ ' ~ ~ ~ "sihr, ~n oven temperature of 30
°C, a
mobile phase of 2-propanollh~:._t~ ~~:~~~~_; , - " an isocratic elution mode,
a flow
rate of 0.5 mUmin, injection volume of 50 ~L, detector wavelength of 265 nm,
dilution solvent 2
propanol/heptane (50/50) and a sample concentration of 0.3 mg/mL. Under these
conditions,
the S isomer has a retention time of about 15.5 minutes, and the R isomer has
a retention time
of about 22 minutes.
Water content was determined according to USP 25, <921 >, Method Ic.
The results are shown in Tables 7-10.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
Table 7: Sample A, at 25 °C and 60% Relative Humidity
Initial1 mo. 3 mo. 6 mo. 9 mo. 12
mo.
Appearance Ua Ua Ue Ua Ue
Assay (% free base equivalent98.7 97.5 98.4 98.4 98.4 98.2
)
Impurities (%)
RRT 0.26 0.09 0.28 0.05 0.07 0.15 0.06
RRT 0.51 0.40 0.41 0.40 0.42 0.36 0.33
RRT 0.71 0.05 0.06 ndb nd ndb nd
RRT 1.53 0.10 0.11 0.12 0.12 0.12 0.13
RRT 1.74 0.09 0.10 0.09 0.09 0.07 0.07
RRT 1.84 0.10 0.13 0.12 0.12 0.10 0.11
Total Impurities (%) 0.83 1.09 0.78 0.82 0.77 0.70
Enantiomeric Purity > 99.9> 99.9> 99.9> 99.9> 99.9> 99.9
(%)
Water Content (%) 0.55 0.53 0.68 0.46 0.42 0.74
a unchanged
b not detected
5 Table 8: Sample A, at 40 °C and 75% Relative Humidity
Initial1 mo. 3 mo. 6 mo.
Appearance Ua Ue Ue
Assay (% free base equivalent98.7 97.9 98.5 98.0
)
Impurities (%)
RRT 0.26 0.09 0.29 0.05 0.07
RRT 0.51 0.40 0.39 0.40 0.40
RRT 0.71 0.05 ndb ndb ndb
RRT 1.53 0.10 0.11 0.14 0.15
RRT 1.74 0.09 0.08 0.08 0.07
RRT 1.84 0.10 0.13 0.12 0.12
Total Impurities (%) 0.83 1.00 0.80 0.81
Enantiomeric Purity 100.0 100.0 100.0 100.0
(%)
Water Content (%) 0.55 0.57 0.69 0.42
a unchanged
b not detected
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
21
Table 9: Sample B, at 25 °C and 60% Relative Humidity
Initial1 mo. 3 mo. 6 mo.
Appearance Ua Ue Ue
Assay (% free base equivalent97.8 98.2 98.2 97.7
)
Impurities (%)
RRT 0.26 0.21 0.06 0.18 0.05
RRT 0.51 0.68 0.62 0.61 0.56
RRT 0.71 0.07 0.09 0.09 0.05
RRT 1.53 0.11 0.09 0.08 0.08
RRT 1.74 0.07 0.06 0.05 0.06
RRT 1.84 0.10 0.08 0.09 0.09
Total Impurities (%) 1.24 1.00 1.10 0.90
Enantiomeric Purity > 99.9nm nmb nmb
(%)
Water Content (%) 0.45 0.50 0.54 0.47
a unchanged
b not measured
Table 10: Sample B, at 40 °C and 75% Relative Humidity
Initial1 mo. 3 mo. 6 mo.
Appearance Ue Ue Ua
Assay (% free base equivalent97.8 98.2 97.2 97.5
)
Impurities (%)
RRT 0.26 0.21 0.13 0.18 0.05
RRT 0.51 0.68 0.61 0.58 0.54
RRT 0.71 0.07 0.08 0.08 ndb
RRT 1.53 0.11 0.10 0.09 0.10
RRT 1.74 0.07 0.06 0.05 0.05
RRT 1.84 0.10 0.08 0.09 0.09
Total Impurities (%) 1.24 1.06 1.06 0.83
Enantiomeric Purity > 99.95nm' nm > 99.9
(%)
Water Content (%) 0.45 0.49 0.49 0.47
eunchanged
b not detected
not measured
Example 3
Crystal form stability was assessed for samples of 5-[(Z)-(5-fluoro-2-oxo-1,2-
dihydro-
3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
22
carboxamide maleate salt, polymorph Form 1, polymorph Form 2, and a mixture of
polymorph
Form 1 and Form 2. In each case, a sample of the substance was placed into a
controlled
temperature and humidity stability chamber at 40 °C and 75% relative
humidity. Samples were
taken several times during the duration of the test and analyzed for
qualitative changes in the
PXRD patterns. For Form 1, the duration of the test was 163 days, and no
crystal form
changes were observed in the PXRD pattern. For Form 2, the duration of the
test was 134
days, and no crystal form changes were observed in the PXRD pattern. For the
mixed Form
1/Form 2, the duration of the test was 6 weeks, and no crystal form changes
were observed in
the PXRD pattern.
Example 4
A six-week stability study was carried out on samples of 5-[(Z)-(5-fluoro-2-
oxo-1,2-
dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-
2,4-dimethyl-1 H-
pyrrole-3-carboxamide maleate salt, polymorph Form 1. The compound was exposed
.for 6
weeks, closed vial, at three stability test conditions: 5 °C, 25
°C/60% relative humidity and
40 °C/75% relative humidity, and analyzed for chemical and enantiomeric
stability as described
in Example 2, except that the HPLC instrument was an Agilent 1100 series. The
HPLC results
are shown in Table 11.
Table 11
Conditions Recovery (%) Total Impurity (%)
5 °C ~ 3 °C 98.00 1.67
°C ~ 2 °C, 60% RHa 97.52 1.85
40 °C ~ 2 °CC, 76% RHe 97.63 1.86
a relative humidity
20 The percent recovery was calculated based on the average response factor
obtained
from a reference standard throughout the HPLC run, and considering the E
isomer as the
compound and not as an impurity. Compounds in this class exhibit reversible E-
Z
isomerization, with the Z-isomer generally favored. The isomerization is often
an artifact of
sample preparation and analysis. Thermogravimetric analysis (TGA) showed
minimal water
25 content, and it was not considered in the percent recovery calculation. The
values shown in the
table are averages of several injections.
To determine the thermal stability and residual solvent content, the same
samples were
analyzed by DSC and TGA. The results are shown in Table 12.
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
23
Table 12
Sample Conditions TGA DSC
Wt. Loss Wt. Loss Peak Enthalpy
(%), (%), 1 (J/g)
25 C-125 25 C-275 (C)
C C
maleate 5 C 1.44 23.15 206.5 224.0
salt
maleate 25 C/60% 1.36 24.36 209.1 253.7
salt RHB
maleate 40 C/75% 1.66 20.61 208.3 243.3
salt RHe
relative humidity
The 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-
hydroxy-3
morpholin-4-ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide maleate salt was
stable under all
stability conditions investigated. The assay of the 6-week stability samples
was 97.1 - 98.5%
with a total impurity content of 1.67 - 1.85%. the assay and impurity profiles
did not show
significant change compared to the initial profiles under all conditions
studied. HPLC
quantitative analysis for malefic acid was consistent with the stoichiometric
ratio of free base to
acid of 1 : 1 (data not shown). Also, there was no chiral transformation
observed in any
samples. Enantiomeric stability was also confirmed in samples held at 80
°C for 2 weeks, with
no increase in the amount of R isomer observed.
DSC analysis of the 6-week samples showed a single sharp endothermic
melt/decomposition peak at 207-208 °C. TGA analysis showed a minimal
weight loss (less than
2%) between 25-125 °C. Significant weight loss (about 21-23%) occurred
at 207 °C,
corresponding to the endothermic peak at 218 °C observed by DSC.
PXRD analysis of the 6-week samples maintained at 40 °C/75% RH
showed no
crystalline changes compared to a control sample.
Example 6
In preclinical species (in vivo and in vitro) and in human hepatocytes and
microsomes,
5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-
hydroxy-3-morpholin-4-
ylpropyl]-2,4-dimethyl-1 H-pyrrole-3-carboxamide metabolism proceeds primarily
through an
oxidative pathway mediated by hepatic cytochrome P450. The primary site of
metabolism is the
morpholinyl group. Three metabolites have been identified in vivo following
single oral
administration (100 mg/kg) to cynomolgus monkeys (Fig. 8). These 3
metabolites, which were
detected at <16% relative abundance to parent in monkey plasma, were also the
major
products observed in human microsomes and hepatocytes. Relative abundance was
determined from monkey plasma following a single dose of 5-[(Z)-(5-fluoro-2-
oxo-1,2-dihydro-
3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-
dimethyl-1 H-pyrrole-3-
carboxamide (100 mg/kg) and based on (AUC0.,2 metabolite/AUCo-~2 parent) x
100, estimated
from the characteristic indole UV absorption at 440 nM. The N,O-dealkylation
metabolite has
been confirmed via nuclear magnetic resonance (NMR). This metabolite has
biochemical, but
not cellular, activity, suggesting that it lacks adequate permeability to
enter cells. The
CA 02540639 2006-03-29
WO 2005/033098 PCT/IB2004/003070
24
morpholinyl lactam metabolite is the product of oxygenation-dehydrogenation
and has also been
confirmed via NMR. The morpholinyl hemi-aminal metabolite is an unstable
metabolite
identified via an iminium-ion trapping experiment. Metabolites were also
determined in human
urine, following a single 12.5 mg dose; these metabolites are shown in Figure
9. Relative
abundance was determined by UV response at 428 nm.
While the invention has been illustrated by reference to specific and
preferred
embodiments, those skilled in the art will recognize that variations and
modifications may be
made through routine experimentation and practice of the invention. Thus, the
invention is
intended not to be limited by the foregoing description, but to be defined by
the appended
claims and their equivalents.