Note: Descriptions are shown in the official language in which they were submitted.
CA 02540915 2010-09-10
365-10 CA/PCT
ALKOXYLATED FULLY HYDROGENATED JOJOBA WAX ESTERS
FIELD OF THE INVENTION
The present invention relates to novel alkoxylated fatty esters, their uses,
and methods
of manufacture thereof. More specifically, the present invention is related to
ethoxylated
monoesters, propoxylated monoesters and ethoxylated/propoxylated esters,
wherein the esters
are derived from natural products, such as plant and animal oils and waxes.
BACKGROUND
Natural products and their derivatives are increasingly favored in the
cosmetics
industry since consumers have become more environmentally sensitive. Further,
consumers
have recognized the value of many unique properties displayed by natural
products and their
derivatives.
One common method of producing natural products for the cosmetic industry is
to
extract an oil or wax from the seed of a plant. Oils and waxes are a group of
organic
substances that form an important and useful part of the cosmetic and other
industries.
Generally, waxes are solid and oils are liquid at ordinary room temperatures.
However, some
tropical products, which are liquids in their sites of origin, become solids
in cooler climates,
often retain the name originally given, e.g., palm oil and coconut oil. Waxes
and oils are
derived from both plant and animal sources.
Chemically most fats and oils are either simple or mixed glyceryl esters of
organic
acids belonging to the fatty-acid series (triglycerides). Triglycerides are
esters formed from
glycerol and three fatty acids that may be identical or different from each
other. In a simple
triglyceride such as tripalmitin or tristearin, all three fatty-acid groups
are identical. In a
mixed triglyceride, two or even three different fatty-acid groups are present.
Most oils and
waxes contain mixed triglycerides.
1
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
Waxes are often found as trace components of triglyceride oils or can be
extracted in
a more pure form from certain botanical and animal sources. Sunflower and corn
oils contain
natural waxes, while jojoba, carnauba and candelillia are examples of waxes
found naturally
in a more pure form. Beeswax and lanolin are examples of natural waxes of
insect and
animal origin. These example waxes range from the liquid, unsaturated jojoba
oil to the
almost completely saturated sunflower wax.
In order to control, or modify, various properties, such as solubility or
melting point,
of oils and waxes, certain modifications can be introduced into the
triglyceride and/or wax
ester structure. One such modification is the introduction of ethylene oxide
(ETO) and/or
propylene oxide (PO) units to the hydroxyl function of a hydrolyzed
triglyceride or wax ester.
It has been found that by controlling the number of units ETO or PO added,
various
properties such as solubility and melting point can be adjusted in the oil or
wax. Generally, it
has been found that compounds become more water soluble as the level of
ethoxylation
increases, but become more alcohol soluble, more oil soluble and more fluid as
the level of
propoxylation increases. Compounds that are ethoxylated, as well as
propoxylated, acquire
both water and alcohol solubility. Because ethoxylation also raises the
melting point of
materials, ethoxylates vary in form, depending on the level of ethoxylation.
For instance,
when a liquid starting material is ethoxylated with approximately 15 moles of
ethylene oxide,
it may become solid or semi solid at room temperature. Propoxylates, however,
are more
often liquids because they contain branched polyoxypropylene chains. Branching
tends to
keep materials fluid.
Another method of altering the melting points of oils and waxes is to add
hydrogen to
points of unsaturation within the oil or wax molecule. The addition of
hydrogen is typically
accomplished under several atmospheres of pressure at elevated temperatures
and in the
presence of metal catalysts, such as nickel or palladium. This hydrogenation
process can be
2
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
continued until all points of unsaturation within the oil or wax molecule are
saturated with
hydrogen, or the reaction can be stopped at some point short of achieving a
fully saturated oil
or wax. The melting point of the oil or wax generally increases as a linear
function of the
amount of hydrogen that has been added. A hydrogenation reaction is said to
yield "partially
hydrogenated" material when stopped short of achieving a "fully saturated" oil
or wax. The
melting point of these "partially hydrogenated" materials is less than the
melting point of the
"fully saturated" material and higher than the melting point of the starting
oil or wax.
Although partial hydrogenation is a means of adjusting the melting point of an
oil or
wax, this partial hydrogenation process results in the formation of unwanted
"trans" isomers.
These trans isomers have been shown to be harmful in human nutrition and have
an
inhibitory effect on the natural metabolic pathway whereby prostaglandins are
created in the
skin.
While alkoxylation is a process whereby the melting points and solubility of
various
oils and waxes can be modified, there is a need to expand the range of melting
points of
alkoxylated materials. Partial hydrogenation to achieve a higher melting point
is not
desirable because of the formation of unwanted trans isomers. Thus, there is a
need to have a
broad range of melting points of alkoxylated oils and waxes with elevated
melting points that
are produced from natural products, where any points of unsaturation are
originally in the cis
configuration.
SUMMARY OF INVENTION
It is an object of the present invention to provide a composition that is
useful for
cosmetic and other applications.
It is another object of the present invention to provide an alkoxylated wax
ester,
where the wax ester is derived from natural products prior to being
alkoxylated.
3
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
wherein the alkoxylate is prepared using ethylene oxide.
It is still yet another object of the present invention to provide the
composition above
wherein the alkoxylate is prepared using propylene oxide.
It is a further object of the present invention to provide the composition
above
wherein the alkoxylate is prepared using a mixture of ethylene oxide and
propylene oxide.
It is yet a further object of the present invention to provide the composition
above
where the alkoxylation is roughly 150 mol equivalents.
It is still yet a further object of the present invention to provide an
alkoxylated wax
ester, where the wax ester is derived from natural products prior to being
alkoxylated and
where the wax ester is a partially saturated wax ester, the wax ester carbon-
carbon double
bonds primarily in the cis configuration prior to partial saturation.
The novel features that are considered characteristic of the invention are set
forth with
particularity in the appended claims. The invention itself, however, both as
to its structure
and its operation together with the additional object and advantages thereof
will best be
understood from the following description of the preferred embodiment of the
present
invention when read in conjunction with any accompanying drawings. Unless
specifically
noted, it is intended that the words and phrases in the specification and
claims be given the
ordinary and accustomed meaning to those of ordinary skill in the applicable
art or arts. If
any other meaning is intended, the specification will specifically state that
a special meaning
is being applied to a word or phrase.
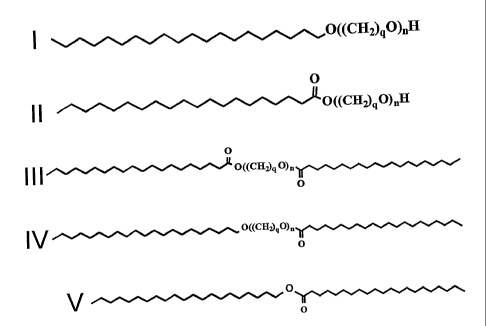
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 illustrates an example reaction process for a polyalkoxylation of a
hydroxy-free fatty
compound. The products illustrated show only majority products, it being
4
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
recognized that many additional minority components may be produced (but in
small concentrations).
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The present invention is a novel composition that is useful as an ingredient
in
pharmaceuticals, neutraceuticals, and cosmeceuticals. Preferably, the
compositions
according to the present invention are used in shampoos, hair conditioners,
skin conditioners,
deodorant sticks, lipsticks, toners, skin cleansers, and the like.
Additionally, the
compositions according to the present invention may be used as "water soluble"
adhesives
since their water solubility is greater than traditional, oil based,
adhesives, and have sufficient
stickiness and tackiness to hold items while in the solid state.
Preferably, the composition of the present invention comprises a family of
compositions that generally have melting points that range from 30-80 C,
preferably with
melting points between 55-58 C. At room temperature (-20 C), this family of
compositions
varies from pourable liquids, to soft creams, to pasty waxes, to a brittle
hard material.
However, the preferred embodiment is solid below 55 C. The composition of the
present
invention may be blended with different melting point components within the
family to form
other products with selected melting points and specific physical properties
or feel. These
compounds, whether used pure or when combined with other carrier and vehicle
components
(including other additives or binders) can form excellent carrier and vehicles
for delivery of
compositions for use in the cosmetic, personal care and/or pharmaceutical
field, including the
cosmeceutical field where cosmetic compositions also provide pharmaceutical or
other
therapeutic benefits. Typical materials with which the compound according to
the present
invention may be blended in accordance with the practice of the present
invention include,
but are not limited to, cosmetic oils and waxes, both natural and synthetic,
including
hydrogenated or partially hydrogenated oils, silicon oils, mineral oils, long
chain esters,
5
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
vitamins (especially vitamin E), long chain fatty acids, alcohols,
cosmeceuticals, pigments,
botanical extracts, esters and ethers, dimers, trimers, oligomers, and
polymers, and the like.
These blended compositions may of course be combined with the active
ingredients intended
to be delivered by the composition used in the present invention.
The compositions according to the present invention may be blended with other
waxes, oils, thickeners, abrasive materials, pigments, and the like to prepare
a "water soluble"
adhesive wax. This "water soluble" adhesive wax finds use in industries where
temporary
adhesives are needed, especially industries where the adhesives are needed to
be soluble
enough in water to wash residual adhesive off of attached surfaces.
Preferably, the present invention has a partially saturated component. Typical
alcohol-free fatty compounds are single ester waxes and oils such as jojoba
oil, rice bran wax,
sunflower wax, candellia wax, beeswax, corn oil wax, and the like. In their
natural state
(obtained directly from the source seed or nut), the liquid waxes and oils
typically contain
one or more double bonds (points of unsaturation). The partially saturated
component of the
instant invention is unique in that it has a single carbon-carbon double bond
that is a cis
isomer, resulting from the partially saturated component being derived from a
natural
product. This configuration is necessarily present or implicit in some of the
processes of
manufacture as disclosed herein. However alternate embodiments of the present
invention
utilize partially saturated compounds. Partial saturation accomplished via
partial
hydrogenation (an incomplete hydrogenation reaction) yields a quantity of the
trans isomer of
the carbon-carbon double bond. This is true even if the carbon-carbon double
bond is
originally in the cis isomer. However, when the partial saturation is
accomplished through
interesterification, as in the present invention, all carbon-carbon double
bonds originally in
the cis isomer remain in the cis isomer. Thus, since the starting unsaturated
compounds used
6
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
herein contain no trans carbon-carbon double bonds, but cis carbon-carbon
double bonds, any
partially saturated resultant prepared through interesterification will be
essentially trans free.
The present invention allows for the production of fatty compositions with
predetermined physical properties, such as melting point and solubility
profile. This is
important in the cosmetics and other industries where various formulations
require
components that melt either above or below normal skin or room temperatures or
which are
soluble in, or compatible with, a wide variety of cosmetic bases. Preferably,
the desired
melting characteristics of the present invention is accomplished by first
adjusting the physical
properties of the unsaturated fatty starting materials. This is accomplished
by controlling the
partial saturation of the unsaturated fatty compound through
transesterification. Then, when
the partially saturated fatty compound has the desired physical properties
(which are not
necessarily the same as the physical properties of the final product), the
physical properties of
the product of this invention may be tuned by varying the degree and
composition (ratios) of
the alkoxylate reactants. The result is an array of alkoxylated wax esters
whose physical
properties, such as melting points and solubility, are varied. Thus, the
present invention is a
composition that results from the polyalkoxylation of a fatty compound.
Preferably, this fatty
compound is derived from natural products, where any points of unsaturation
are in the cis
configuration, and alcohol free.
In one embodiment, the present invention is a composition that results from
the
addition of (CH2CH2O)X or (CH2CH2CH2O)y to a fully hydrogenated (saturated)
alcohol-free
fatty compound. This addition, in the absence of an alcohol ligand, is
accomplished using a
caustic catalyst (basic conditions), such as sodium methoxide (NaOCH3), or the
like to both
randomize and saponify the saturated wax esters prior to alkoxylation, as will
be described
below.
7
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
In one example embodiment of the present invention, double bonds in the waxes
first
are hydrogenated (saturated) to create the fully hydrogenated alcohol-free
fatty compound.
Then the hydrogenated waxes are saponified. The saponification step also
randomizes the
naturally occurring composition of the fatty acids and fatty alcohols in the
esters. Finally the
hydrogenated, saponified, and randomized waxes are alkoxylated, thereby
producing
compositions according to the present invention.
In another embodiment of the present invention, fully hydrogenated waxes and
unsaturated waxes are first mixed together. This mixture is then saponified,
said
saponification resulting in the randomization of the fatty acids and fatty
alcohols between,
and within, the hydrogenated and unsaturated waxes. The resultant compositions
are finally
alkoxylated, thereby producing further compositions according to the present
invention.
In yet another embodiment of the present invention, different amounts of
different
naturally derived fatty compounds are mixed together and catalytically
interesterified and
then followed by alkoxylation.
In still yet another embodiment of the present invention, partially or fully
hydrogenated wax esters are mixed with fatty compounds containing unsaturation
in the cis
isomer form. These mixtures are interesterified and alkoxylated, as described
above.
By mixing different amounts of different alcohol-free fatty compounds together
and
interesterifying the resultant mixture, physical properties, such as melting
point,
hydrophilic/lipophylic balance, slip, spread, and the like may be varied and
set. Alternately,
the physical and chemical properties of the interesterified materials can be
adjusted by
addition of ethylene oxide and/or propylene oxide, in different combinations
and ratios. This
would adjust the level and amount of randomized, but un-alkoxylated, cis
configuration,
interesterified starting material remaining in the final compositions, thereby
further adjusting
physical and chemical properties of the compositions according to the present
invention.
8
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
In one specific embodiment, 150 mole equivalents of the acyl oxide, preferably
ethylene oxide is reacted with each mole of fully hydrogenated alcohol-free
transesterified
fatty compound, hydrogenated jojoba oil, in the caustic conditions noted
above. Resulting
majority products, illustrated in Figure 1, are a combination of
polyalkoxylated fatty alcohols
(I), polyalkoxylated fatty acids (II), polyalkoxylated diethers (III),
polyalkoxylated ethers
(IV), and residual unreacted material (V). Other polyalkoxylated minority
products (not
shown) are possible, even likely, including fully hydrogenated and randomized
fatty
compounds. The preferred composition according to the present invention has
the total mole
equivalent amount of acyl oxide added to the hydrogenated alcohol free
composition,
molq,n, ,& 150. Y-molq,n may vary from 5 to 400 total mole equivalents and
still fall within the
scope of the present invention.
The product compositions of the present invention have been found to have
beneficial
physical properties, such as melting point, hardness, solubility profile,
wettability, adhesion,
and the like. The preferred composition is moderately soluble in oils and
produces a clear
solution when mixed with water, at concentrations up to 5%, may create a
cloudy mixture at
higher concentrations. The HLB number of the preferred composition is 9.6,
with a
penetrometer reading of 0.68 mm when tested at ambient (20 C) temperatures.
The
measured melting point range of the preferred composition is 55-58 C, and the
Floratech slip
test angle, in a 5% solution, is 17 .
Example formulations using the product of the present invention are described
below.
Conditioning Shampoo
This composition lends viscosity and foam stabilization to this conditioning
shampoo.
Phase Trade Name INCI Name Supplier %wt/wt
A. Deionized Water Water Q.S.
Sodium Laureth Sulfate Sodium Laureth Sulfate Chemron 28.00
9
CA 02540915 2010-09-10
60%
Florasolvs PEG 150 PEG-150 Hydrogenated Floratech 4.00
Hydrogenated Jojoba Jojoba esters
B. Floraesters 20 Jojoba Esters Floratech 0.50
Sodium Lauryl Sulfate Sodium Lauryl Sulfate Chemron 6.00
30%
C. Lactic Acid Lactic Acid Inolex Q.S. to
pH 4
D. UCARET"i Polymer JR 30M Polyquaternium-10 Amerchol 0.30
Lexaine C Cocamidopropyl Betaine Inolex 6.00
Lexein X-250 Hydrolyzed Collagen Inolex 2.00
VerseneTM Na2 Disodium EDTA Dow 0.10
E. Preservative - - - - Q.S.
Fragrance Fragrance Q.S.
Color (optional) Q.S.
100.00
Mixing Procedure:
1. Heat water to 70 C and add Sodium Laureth Sulfate and Florasolves PEG-150
Hydrogenated Jojoba with agitation.
2. Pre-mix Phase B and add to Phase A with propeller agitation.
3. Adjust pH to 4 with Phase C and add UCARETM Polymer JR 30M slowly. Add
remaining ingredients of Phase D in order with propeller agitation.
4. At 40 C, add ingredients of Phase E with agitation. Cool to room
temperature.
Cream Conditioning Shampoo
A cream shampoo with excellent conditioning properties contributed by PEG 150
Hydrogenated Jojoba esters.
Phase Trade Name INCI Name Supplier %wt/wt
A. Deionized Water Water Q.S.
Florasolvs PEG-150 PEG-150 Jojoba Esters Floratech 5.00
Hydrogenated Jojoba
Sulfochem SLS Sodium Lauryl Sulfate Chemron 45.00
B. Lexemul 515 Glyceryl Stearate Inolex 3.00
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
Floramac 10 Ethyl Macadamiate Floratech 0.50
Floraesters IPJ Jojoba Esters (and) Isopropyl Floratech 0.50
Jojobate (and) Jojoba Alcohol
Floraesters 15 Jojoba Esters Floratech 0.50
C. Chembetaine CGF Cocamidopropyl Betaine Chemron 7.00
GE Silicone SF 1188 Dimethicone Copolyol GE Silicones 0.50
Abil Quat 3272 Quaternium-80 Goldschmidt 0.40
D. Citric Acid (to pH 6.5- Citric Acid Roche 0.10
6.8)
Fragrance Fragrance Shaw Mudge 0.50
Co.
Sodium Chloride Sodium Chloride Morton Salt 0.50
100.00
Mixing Procedure:
1. Heat water to 70 C. Dissolve the Florasolvs PEG-150 Hydrogenated Jojoba
with
propeller agitation. Add the Sulfochem SLS with propeller agitation.
2. Blend all ingredients of Phase B together with heat till melted. Add Phase
B to Phase A
with propeller agitation.
3. Lower temperature to 50 C. Add in Phase C ingredients in order with
propeller agitation.
4. Add in the Citric Acid powder with propeller agitation.
5. Add in Fragrance and Sodium Chloride with propeller agitation, and allow to
cool to
room temperature.
Spray Hair Detangler
This formula assists in detangling wet hair and leaving a conditioned, glossy
shine
contributed by use of PEG-150 Hydrogenated Jojoba esters.
Phase Trade Name INCI Name Supplier %wt/wt
A. Deionized Water Water - - - - - Q.S.
Propylene Glycol Propylene Glycol Dow 8.00
Chembetaine CGF Cocamidopropyl Betaine Chemron 3.00
Florasolvs PEG-150 PEG-150 Hydrogenated Jojoba Floratech 2.00
Hydrogenated Jojoba Esters
11
CA 02540915 2010-09-10
GE Silicone Fluid Dimethicone Copolyol GE Silicones 0.70
1188
Preservative - - - - - Q.S.
100.00
Mixing Procedure:
1. Add each of the ingredients in order, allowing time for each to completely
dissolve.
Hair Styling Wax
Florasolvs PEG 150 Hydrogenated Jojoba esters provide water-soluble jojoba
emolliency, and viscosity adjustment in this hair care product.
Phase Trade Name INCI Name Supplier %wt/wt
A. White Petrolatum Petrolatum Penreco 28.50
Yellow Beeswax SP 6P Beeswax Strahl & Pitsch 6.00
Bentone Gel VS-5PC Cyclomethicone (and) Rheox 5.00
Quaternium- 18 Hectorite
(and) SD Alcohol 40
Florasun 90 Helianthus Annuus Floratech 5.00
(Sunflower) Seed Oil
Floraesters 70 Jojoba Esters Floratech 3.00
Floraesters 60 Jojoba Esters Floratech 7.00
Talc Supra H Talc Luzenac 2.00
Carnauba Wax 91 Yellow Copernicia Cerifera Strahl & Pitsch 6.00
SP63 (Carnauba) Wax
B. Deionized Water Water - - - - - Q.S.
Florasolvs PEG-150 PEG-150 Hydrogenated Floratech 10.00
Hydrogenated Jojoba Jojoba Esters
Preservative - - - - - Q.S.
C. Fragrance (Bell J-6674-B) Fragrance Bell Q.S.
Covi-Ox T-70 Tocopherols Cognis 1.00
TOTAL: 100.00
Mixing Procedure:
1. Combine Phase A ingredients and heat to 90 C with propeller agitation. Mix
for 20
minutes.
12
CA 02540915 2010-09-10
2. Add Phase B ingredients and mix at 80 C with propeller agitation. When
Phase B is
completely mixed, add slowly to Phase A with fast propeller agitation.
3. Cool to 75 C and add Phase C components. Pour into containers will still
hot and fluid.
Allow time to cool to room temperature before use.
Skin Softening Lotion
This lotion spreads easily and absorbs quickly into the skin, leaving it
smooth, soft,
and supple as a result of the use of PEG 150 Hydrogenated Jojoba esters.
Phase Trade Name INCI Name Supplier %wt/wt
A. Floraesters 20 Jojoba Esters Floratech 2.50
Floramac Hawaiian Macadamia Floratech 2.00
Macadamia Nut Oil Integrifolia Seed Oil
Floraesters 30 Jojoba Esters Floratech 5.00
Lexemul 561 Glyceryl Stearate Inolex 3.00
(and) PEG-100
Stearate
Lanette 16 Cetyl Alcohol Cognis 1.00
Cosmowax P Stearyl Alcohol (and) Croda 0.50
Ceteareth-20
SF 96 - 200 Silicone Fluid Dimethicone GE Silicone 0.20
Preservative - - - - - Q.S.
B. Deionized Water Water Q.S.
Florasolvs PEG- 150 PEG-150 Floratech 4.00
Hydrogenated Jojoba Hydrogenated
Jojoba Esters
C. Propylene Glycol Propylene Glycol Dow 5.00
Kelgin HV Algin CP Kelco 0.20
Preservative - - - - - Q.S.
D. Fragrance Fragrance Q.S.
100.00
Mixing Procedure:
1. Heat Phase A to 75 C with agitation
2. Heat water of Phase B to 75 C with agitation. Add Florasolvs PEG-150
Hydrogenated
Jojoba.
13
CA 02540915 2010-09-10
3. Pre-mix Phase C with moderate agitation and add slowly to Phase B with
moderate high-
speed agitation. Mix for 15 minutes.
4. Add Phase A slowly to Phase BC with propeller agitation.
5. Force cool to 40 C with agitation. Add fragrance (if desired) and mix with
propeller
agitation to room temperature.
Deodorant Stick
Florasolvs PEG-150 Hydrogenated Jojoba esters clarify and improve the
application
properties of this clear deodorant stick.
Phase Trade Name INCI Name Supplier %wt/wt
A. Deionized Water Water Q.S.
Chloracel Sodium Aluminum Reheis 17.00
Chlorohydroxy Lactate
B. Propylene Glycol, Propylene Glycol Dow 33.00
USP
Irgasan DP300 Triclosan Ciba-Geigy 0.30
Sodium Stearate C-7 Sodium Stearate Crompton 8.00
C. Florasolvs PEG-150 PEG-150 Hydrogenated Floratech 5.00
Hydrogenated Jojoba Jojoba Esters
Carsamide CA Cocamide DEA Lonza 2.00
Polyglycol E-1450 PEG-32 Dow 4.20
D. Fragrance Fragrance Q.S.
Color (optional) Q.S.
TOTAL: 100.00
Mixing Procedure:
1. Heat water of Phase A to 75 C. Add Chloracel with agitation to dissolve.
Cool to 65 C.
2. Combine Phase B and heat to 75 C with agitation.
3. Add the ingredients of Phase C to Phase B in the given order and mix for 15
minutes.
Cool to 65 C.
4. Add Phase BC to A with agitation and mix until uniform.
5. Add color and fragrance.
6. Fill at 62-65 C. Cool in containers at room temperature.
14
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
Adhesive Composition
Florasolvs PEG-150 Hydrogenated Jojoba esters may be used essentially by
themselves, or in blended with other waxes, oils, thickeners, abrasive
materials, pigments,
and the like to prepare a "water soluble" adhesive wax. These adhesives are an
essentially
solvent-free water-soluble ester based adhesive. The adhesive composition
imparts soft
handle and hydrophilic/lipophylic properties as well as good resiliency and
crease resistance.
Further, these adhesives show good compatibility with various resins, and
therefore are
suitable for shape-memory finishing of cellulose fiber and the like.
Phase Trade Name INCI Name Supplier %wt/wt
Florasolvs PEG-150 PEG-150 Hydrogenated Floratech 97.00
Hydrogenated Jojoba Jojoba Esters
PEG-12 Carnauba PEG-12 Camauba Koster-Keunen 3.00
TOTAL: 100.00
Mixing Procedure:
1. Combine Florasolvs PEG-150 Hydrogenated Jojoba with PEG-12 Carnauba and
heat to
75 C with agitation.
2. Fill at 62-65 C. Cool in containers at room temperature.
In addition to the essential ingredients in the compositions of the present
invention,
further material may be present in the composition for functional or aesthetic
reason.
Antioxidants, including tocotrienols (compounds homologous to tocopherols that
differ by
the presence of three unsaturated bonds in the phytyl side chain), and
oryzanol (a mixture of
ferulic acid esters of sterols, e.g., beta-sitosteryl ferulate and methyl
ferulate, and triterpene
alcohols, e.g., 24-methylenecycloartenyl ferulate; see Bailey's Industrial Oil
and Fat
Products, 4th Ed., John Wiley, New York, 1979, volume 1, pages 407 to 409) may
be present.
Fragrances, colorants (e.g., dyes or pigments), topically applied medications,
UV absorbers,
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
whitening agents, emulsifying agents, binders, scrubbing particulates, and the
like may be
present.
Fatty elements used in combination with the present invention can be selected
from
mineral oils like paraffin or petroleum oils, silicon oils, vegetable oils
like coconut, almond,
apricot, corn, jojoba, olive, avocado, sesame, palm, eucalyptus, rosemary,
lavender, pine,
thyme, mint, cardamon, orange blossoms, soy beans, bran, rice, colza, and
castor oils, animal
oils and fats like tallow, lanolin, butter oil, fatty acid esters, fatty
alcohol esters, waxes whose
melting point is the same as the skin's (animal waxes like beeswax, botanical
waxes such as
camauba or candelilla waxes, mineral waxes like micro-crystalline waxes and
synthetic
waxes like polyethylene or silicone waxes). All acceptable oils used in
cosmetology can be
used, like the ones that have been mentioned in the CTFA's book, Cosmetic
Ingredient
Handbook, First edition, 1988, The Cosmetic, Toiletry and Fragrance
Association, Inc.,
Washington (hereinafter, "CTFA").
Cosmetically or dermatologically active substances may be added to the
composition
of the present invention, meaning active ingredients chosen from anti-acne
agents, anti-
microbial agents, anti-perspiration agents, astringents, deodorants, hair
removers, external
analgesics, agents for hair conditioning, skin conditioning, sun protection,
vitamins,
catechines, flavonoids, ceramides, fatty substances, polyunsaturated fatty
acids, essential fatty
acids, keratolytic agents, enzymes, anti-enzymes, moisteners, anti-
inflammatory substances,
detergents, perfumes, and mineral substances for synthetic coverings. These
substances may
represent from 1 to 20% by weight of the total weight of the composition.
Detergent or foaming agents, for example used in combination with the
preferred
embodiments, may include disodic cocoamphodiacetate salts; lauroylether
sulfosuccinate
disodic salts; the vegetable protein acylates; the cocoyl gutamate
triethanolamine salts; the
16
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
lauroyl sarcosinate sodium salts; the glucoside decyl-ethers; and the sodium
sulfate lauroyl
ethers.
Pasty active compounds like lanolin by-products (acetyl lanolin, lanolin, and
lanolin
alcohols; cholesterol by-products, like cholesterol esters (12 cholesteryl
hydroxy stearate);
pantaetythritol hydroxylated esters, linear mono-esters like butyl stearate,
arachidyl
propionate or stearyl heptanoate, and triglycerides with a fatty chain less
that C16 can also be
used. These substances may be water-soluble, lipid-soluble, or lipid-soluble
and water
soluble at the same time, or dispersible. They can be chosen from the
compounds that are
found in the CTFA dictionary at pages 51 to 101.
Surface active agents, cationic, anionic, non-ionic and/or Zwitterionic may be
used in
combination with the preferred embodiments of the present invention. These
surface agents
can be chosen, for example, from the hydrophilic surface agents, like glycols,
such as
hexylene glycol, butylene-1,2 glycol, ethyl-2-hexyl sulfosuccinate;
oxyethylene octylphenol
(and the salts derived from cocoyl and lauroyl collagen, sorbitan palmitate,
and the
polyoxyethylene byproducts of sorbitol palmitate esters, salts of fatty chain
quaternary
ammonium. Suitable anionic surfactants which may be used include water-soluble
alkali
metal or ammonium salts having alkyl radicals containing from about 8 to about
22 carbon
atoms, the term alkyl being used to include the alkyl portion of higher acyl
radicals.
Examples of suitable synthetic anionic surfactants are sodium or ammonium
alkyl sulfates,
especially those obtained by sulfating higher (C8-C1$) alcohols produced, for
example, from
tallow or coconut oil; alkyl (C9-C20) benzene sulfonates, particularly sodium
linear secondary
alkyl (C10-C15) benzene sulfonates; alkyl glyceryl ether sulfates, especially
those ethers of the
higher alcohols derived from tallow or coconut oil and synthetic alcohols
derived from
petroleum; coconut oil fatty monoglyceride sulfates and sulfonates; salts of
sulfuric acid
esters of higher (C8-C18) fatty alcohol-alkylene oxide, particularly ethylene
oxide reaction
17
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
products; the reaction products of fatty acids such as coconut fatty acids
esterified with
isoethionic acid and neutralized with sodium hydroxide; sodium and potassium
salts of fatty
acid amides of methyl taurine; alkane monosulfonates such as those derived
from reacting
alpha-olefins (C8-C20) with sodium bisulfite and those derived from reacting
paraffins with
SO2 and C12 and then hydrolyzing with a base to produce a random sulfonate;
and olefin
sulfonates which term is used to describe the material made by reacting
olefins, particularly
C10-C20 alpha-olefins, with SO3 and then neutralizing and hydrolyzing the
reaction product.
The preferred anionic surfactants are sodium or ammonium (C10-C18) alkyl
sulfates and (C10-
C18) alkyl polyethoxy (1-11 EO, ethylene oxide) sulfates and mixtures thereof
having
differing water solubilities.
Particularly preferred anionic surfactants comprise a mixture of a C10-C18
alkyl
sodium or ammonium sulfate or sulfonate or a C14-C18 alpha-olefin sodium or
ammonium
sulfonate (AOS) and a C8-C12 alkyl polyethoxy (2-4 EO) sodium or ammonium
sulfate.
Mixtures containing a major amount of the alkyl sulfates, olefin sulfonates or
alkyl alkoxy
sulfates with aryl sulfonates such as sodium cumene sulfonate, sodium xylene
sulfonate and
sodium benzene sulfonate are also optional.
The amount of anionic surfactant present in the composition will generally
range from
about 0 or 1 % or 4 to 12% by weight (total ingredients) by weight. The
amphoteric or
Zwitterionic surfactant, may optionally be present at a level of at least abut
0.1 or at least
about 0.25 percent by weight of the total composition, per 1 part by weight of
the content of
anionic surfactant present in the composition.
Examples of amphoteric surfactants that may be used in combination with the
composition of the invention are betaines and compounds that can be broadly
described as
derivatives of aliphatic secondary and tertiary amines in which the aliphatic
radical can be
straight chain or branched and wherein one of the aliphatic substituent
contains from abut 8
18
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
to 18 carbon atoms and one contains an ionic water solubilizing group, e.g.,
carboxy,
sulfonate, sulfate, phosphate, or phosphonate. Examples of compounds falling
within this
definition are sodium 3-dodecylaminopropionate, sodium 3-dodecylaminopropane
sulfonate,
N-alkyltaurines, such as prepared by reacting dodecylamine with sodium
isethionate, N-
higher alkyl aspartic acids and the products sold under the trade name
"Miranol".
Makeup or cosmetic compositions comprising the present invention may also
contain
as an optional ingredient, a film forming skin tightening agent, particularly
a plant derived
biological polysaccharide cosmetic ingredient that may be combined with a
casein
hydrolysate.
The polysaccharides that can be used in the practice of the invention include,
for
example, lecithin, pectin, karaya gum, locust bean gum, xanthan gum and
mixtures thereof.
The polysaccharides are preferably used in the present compositions in
combination with a
casein hydrolysate.
Suitable co-emulsifiers, which may be used in combination with compositions
according the present invention, are both known w/o (water in oil) and o/w
(oil in water)
emulsifiers. Typical examples of fats are glycerides while suitable waxes
include inter alia
beeswax, paraffin wax or microwaxes. Suitable thickeners are, for example,
crosslinked
polyacrylic acids and derivatives thereof, polysaccharides, more particularly
xanthan gum,
guar, agar, alginates and tyloses, carboxymethyl cellulose and hydroxyethyl
cellulose, also
fatty alcohols, monoglycrides and fatty acids, polyacrylates, polyvinyl
alcohol and polyvinyl
pyrolidone. In the context of the invention, biogenic agents are, for example,
plant extracts,
protein hydrolysates and vitamin complexes. Typical film formers are, for
example,
polyvinyl pyrolidone, vinyl pyrolidone/vinyl acetate copolymers, polymers of
the acrylic acid
series, quaternary cellulose derivatives and similar compounds. Suitable
preservatives are,
for example, formaldehyde solution, p-hydroxybenzoate or sorbic acid. Suitable
pearl esters
19
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
are, for example, glycol distearic acid esters, such as ethylene glycol
distearate, and also fatty
acids and fatty acid monoglycol esters. The dyes used may be selected from
many of the
substances that are permitted and suitable for cosmetic purposes, as listed
for example in the
publication "Kosmetische Farbemittel" of the Farbstoffkommission der Deutschen
pages 81-
106. These dyes are typically used in concentrations of 0.001 to 0.1 % by
weight, based on
the mixture as a whole.
The total percentage content of auxiliaries and additives may be 1 to 50% by
weight
and may be 5 to 40% by weight, based on the formulation. The formulations may
be
produced in known manner, i.e. for example by hot, cold, hot/cold or PIT
emulsification.
These are purely mechanical processes that do not involve a chemical reaction.
The cosmetic
and/or pharmaceutical formulations may have a water content of 25 to 95% by
weight and
preferably 50 to 75% by weight.
The following list of cosmetic category codes identifies fields of use for the
cosmetic
compositions and carriers of the present invention.
TABLE 1
FDA cosmetic category codes
01. Baby products.
A. Baby shampoos
B. Lotions, oils, powders and creams
C. Other baby products
02. Bath preparations
A. Bath oils, tablets, salts and beads
B. Bubble Baths
C. Bath capsules
D. Other bath preparations
03. Eye makeup preparations
A. Eyebrow pencil
B. Eyeliner
C. Eye shadow
D. Eye lotion
E. Eye makeup remover
F. Mascara
G. Other eye makeup preparations
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
04. Fragrance preparations
A. Cologne and toilet waters
B. Perfumes
C. Powders (dusting and talcum, excluding aftershave talc)
D. Sachets
E. Fragrance sticks
F. Other fragrance preparations
05. Hair preparations (non-coloring)
A. Hair conditioner
B. Hair spray (aerosol fixatives)
C. Hair straighteners
D. Permanent waves
E. Rinses (non-coloring)
F. Shampoos (non-coloring)
G. Tonics, dressings, and other hair grooming aids
H. Wave sets
1. Spiking gels, pomades and leave-in conditioners
J. Other hair preparations
06. Hair coloring preparations
A. Hair dyes and colors (all types requiring caution statements
and patch tests)
B. Hair tints
C. Hair rinses (coloring)
D. Hair shampoos (coloring)
E. Hair color sprays (aerosol)
F. Hair lighteners with color
G. Hair bleaches
H. Other hair coloring preparations
07. Makeup preparations (not eye)
A. Blushers (all types)
B. Face powders
C. Foundations
D. Leg and body paints
E. Lipstick
F. Makeup bases
G. Rouges
H. Makeup fixatives
I. Other makeup preparations
08. Manicuring preparations
A. Basecoats and undercoats
B. Cuticle softeners
C. Hair creams and lotions
D. Nail extenders
E. Nail polish and enamel
F. Nail polish and enamel removers
G. Other manicuring preparations
09. Oral hygiene products
A. Dentifrices (aerosol, liquid, pastes and powders)
B. Mouthwashes and breath fresheners (liquids and sprays)
21
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
C. Other oral hygiene products
10. Personal cleanliness
A. Bar soaps and detergents
B. Deodorants (underarm)
C. Douches
D. Feminine hygiene deodorants
E. Synthetic Detergent Preparations (Syn-Det Bars)
F. Other personal Cleanliness products
11. Shaving preparations
A. Aftershave lotion
B. Beard softeners
C. Men's talcum
D. Preshave lotions (all types)
E. Shaving cream (aerosol, brushless and lather)
F. Shaving soap (cakes, sticks, etc.)
G. Other shaving preparations products
12. Skin care preparations (creams, lotions, powder and sprays)
A. Cleansing (cold creams, cleansing lotions, liquids and pads)
B. Depilatories
C. Face and neck (excluding shaving preparations)
D. Body and hand (excluding shaving preparations)
E. Foot powders and sprays
F. Moisturizing
G. Night
H. Paste masks (mud packs)
1. Skin fresheners
J. Other skin products
13. Suntan preparations
A. Suntan gels, creams and liquids
B. Indoor tanning preparations
C. Self-Tanning Solutions and lotions
D. Other suntan preparations
The preferred embodiment of the invention is described above in the Drawings
and
Description of Preferred Embodiments. While these descriptions directly
describe the above
embodiments, it is understood that those skilled in the art may conceive
modifications and/or
variations to the specific embodiments shown and described herein. Any such
modifications
or variations that fall within the purview of this description are intended to
be included
therein as well. Unless specifically noted, it is the intention of the
inventor that the words
and phrases in the specification and claims be given the ordinary and
accustomed meanings
to those of ordinary skill in the applicable art(s). The foregoing description
of a preferred
22
CA 02540915 2006-03-30
WO 2005/061686 PCT/US2003/037112
embodiment and best mode of the invention known to the applicant at the time
of filing the
application has been presented and is intended for the purposes of
illustration and description.
It is not intended to be exhaustive or to limit the invention to the precise
form disclosed, and
many modifications and variations are possible in the light of the above
teachings. The
embodiment was chosen and described in order to best explain the principles of
the invention
and its practical application and to enable others skilled in the art to best
utilize the invention
in various embodiments and with various modifications as are suited to the
particular use
contemplated.
23