Note: Descriptions are shown in the official language in which they were submitted.
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
IMPROVED FRACTURING FLUID AND METHOD OF USE
FIELD OF THE INVENTION
[0001] Improved aqueous fracturing fluids have now been discovered that are
particularly useful as well stimulation fluids to fracture tight (i.e., low
permeability)
subterranean formations. Gas wells treated with these fracturing fluids have
rapid
cleanup and enhanced well production. The fluids contain small but sufficient
amounts
of certain amine oxides to aid in the removal of the fracturing fluid from the
formation.
By facilitating the removal of fluid from the invaded zones, the amount of
damage to the
fracture faces in the formation is thereby minimized:
BACKGROUND OF THE INVENTION
[0002] Various amine oxides have been used as surfactants to create foams and
remove
"intrusion fluids from wellbores," according to USP 3,303,896 and they have
been used
as foam stabilizers, according to USP 3,317,430, Certain amine oxides have
also been
used in combination with quaternary ammonium compoiunds as foaming and silt
suspending agents. See, for example, USP 4,108,782 and USP 4,113,631. The use
of
amine oxide surfactants for chemical flooding enhanced oil recovery was
described in a
topical report by David K. Olsen in NIPER-417 (August 1989) for work performed
for
the US IDepartment of Energy under cooperative agreement DE-FC22-83FE60149 by
the
National Institute for Petroleum and Energy Research. However, to Applicants'
knowledge, the amine oxides have not been used to improve the properties.of
fracturing
fluids and to promote rapid cleanup, or to enhance well production from a well
stimulated
by hydraulic fracturing.
[0003] Hydraulic fracturing of subterranean formations has long been
established as an
effective means to stimulate the production of hydrocarbon fluids from a
wellbore. In
hydraulic fracturing, a well stimulation fluid (generally referred to as a
fracturing fluid or
a "frac fluid") is injected into and through a wellbore and against the
surface of a
subterranean formation penetrated by the wellbore at a pressure at least
sufficient to
create a fracture in the formation. - Usually a "pad fluid" is injected first
to create the
CONFIRMATION COPY
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
fracture and then a fracturing fluid, often bearing granular propping agents,
is injected at
a pressure and rate sufficient to extend. the fracture from the wellbore
deeper into the
formation. If a proppant is employed, the goal is generally to create a
proppant filled
= zone (aka, the proppant pack) from the tip of the fracture back to the
wellbore. In any
event, the hydraulically induced fracture is more permeable than the formation
and it acts
as a pathway or conduit for the hydrocarbon fluids in the formation to flow to
the
wellbore and then to the surface where they are collected. The methods of
fracturing are
well known and they may be varied to meet the user's needs, but most follow
this general
procedure (which is greatly overly simplified).
[00041 The fluids used as fracturing fluids have also been varied, but many if
not most
are aqueous based fluids that have been "viscosified" or thickened by the
addition of a
natural or synthetic polymer (cross-linked or uncross-linked). The carrier
fluid is usually
water, or a brine (e.g., dilute aqueous solutions of sodium chloride and/or
potassium
chloride). The viscosifying polymer is typically a solvatable (or hydratable)
polysaccharide, such as a galactomannan -gtim, a glycomannan gum, or a
cellulose
derivative. Examples of such polymers include guar,hydroxypropyl guar,
carboxymethyl
guar, carboxymethylhydroxyethyl guar, hydroxyethyl cellulose, carboxymethyl-
hydroxyethyl cellulose, hydroxypropyl cellulose, xanthan, polyacrylamides and
other
synthetic polymers. Of these, guar, hydroxypropyl guar and
carboxymethlyhydroxyethyl
guar are typically preferred because of commercial availability and cost
performance.
[0005] In many instances, if not most, the viscosifying polymer is crosslinked
with a
suitable crosslinking agent. The crosslinked polymer has an even higher
viscosity and is
even more effective at carrying proppant into the fractured formation. The
borate ion has
been used extensively as a crosslinking agent, typically in high pH fluids,
for guar, guar
derivatives and other galactomannans. See, for example, USP 3,059,909 and
numerous
other patents that describe this classic aqueous gel as a fracture fluid.
Other crosslinking
agents include, for example, titanium crosslinkers (USP 3,888,312), chromium,
iron,
aluminum, and zirconium (USP 3,301,723). Of these, the titanium and zirconium
crosslinking agents are typically preferred. Examples of commonly used
zirconium
-2-
CA 02540988 2007-08-29
crosslinking agents include zirconium triethanolamine complexes, zirconium
acetylacetonate, zirconium lactate, zirconium carbonate, and chelants of
organic
alphahydroxycorboxylic acid and zirconium. Examples of commonly used titanium
crosslinking agents include titanium triethanolamine complexes, titanium
acetylacetonate, titanium lactate, and chelants of organic
alphahydroxycorboxylic acid
and titanium.
Additional information on fracturing is found in the description by Janet
Gulbis
and Richard M. Hodge in Chapter 7 of the text "Reservoir Stimulation"
published by
John Wiley & Sons, Ltd, Third Edition, 2000 (Editors, Michael J. Economides
and
Kenneth G. Nolte). Some fracturing fluids have also been energized by the
addition of a
gas (e.g., nitrogen or carbon dioxide) to create a foam. See, for example, the
pioneering
work by Roland E. Blauer and Clarence J. Durborow in USP 3,937,283 ("Formation
Fracturing with Stable Foam"). The rheology of the traditional water-base
polymer
solutions and also complex fluids, such as foams, can be and typically is
modified and
augmented by several additives to control their performance. Fluid loss
additives are
typically added to reduce the loss of fracturing fluids into the formation.
The problems associated with the loss of fracturing fluid to the forrnation
are well
known. For example, in 1978 Holditch reported: "The fluid injected during the
fracturing
treatment will leak off into the formation and will reduce the relative
permeability to gas
in the invaded region. Near the fracture, the permeability to gas will be
reduced to zero."
In addition, Holditch said: "In some cases, the injected fracturing fluid may
reduce the
formation permeability in the invaded zone." Stephen A. Holditch, SPE 7561
(Presented
at the 53d Annual Fall Technical Conference and Exhibition of the Society of
Petroleum
Engineers of AIME, held in Houston, Texas, October 1-3, 1978). The damage to
the
formation could be severe, and the practical so what of that is reduced flow
of
hydrocarbons, low production and poor economics on the well. While the state
of the art
has advanced substantially since Holditch reported on the problems associated
with leak
off of fracturing fluid, the problems remain the same. See, for example,
Vernon G.
-3-
, CA 02540988 2007-08-29
Constien, George W. Hawkins, R.K. Prud'homme and Reinaldo Navarrete, Chapter 8
entitled "Performance of Fracturing Materials" and the other chapters on
fracturing and
well stimulation in "Reservoir Stimulation" published by John Wiley & Sons,
Ltd, Third
Edition, copyright Schlumberger 2000 (Editors, Michael J. Economides and
Kenneth G.
Nolte). These authors and others emphasize the importance of "cleanup" or
"fracture
cleanup" to optimize production of the hydrocarbon fluids from the well. The
term
"cleanup" or "fracture cleanup" refers to the process of removing the fracture
fluid
(without the proppant) from the fracture after the fracturing process has been
completed.
Techniques for promoting fracture cleanup often involved reducing the
viscosity of the
fracture fluid as much as practical so that it will more readily flow back
toward the
wellbore. So-called "breakers" have been used to reduce fluid viscosity in
many
instances. The breakers can be enzymes (oxidizers and oxidizer catalysts), and
they may
be encapsulated to delay their release. See, for example, USP 4,741,401
(Walles et al.),
assigned to Schlumberger Dowell. Another technique to aid in the cleanup,
albeit by a
contrarian approach, is found in USP 6,283,212 (Hinkel and England), which is
also
assigned to Schiumberger Dowell.
The need for improved fracturing fluids still exists, and the need is met at
least in
part by the following invention.
SUMMARY OF THE INVENTION
Improved aqueous fracturing fluids have now been discovered that are
particularly useful
as well stimulation fluids to fracture tight (i.e., low permeability)
subterranean
formations. Gas wells treated with these fracturing fluids have rapid cleanup
and
enhanced well production. The fluids contain small but sufficient amounts of
certain
amine oxides to aid in the removal of the fracturing fluid from the formation.
By
facilitating the removal of fluid from the invaded zones, the amount of damage
to the
fracture faces in the formation is thereby minimized. The amine oxides
correspond to the
formula
-4-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
R3
1-}-
~N
R 1/`1~
R2 (formula I)
wherein Rl'is an aliphatic group of from 6 to about 20 carbon atoms, and
wherein R2 and
R3 are each independently alkyl of from 1 to about 4 carbon atoms. The amine
oxides in
which Rl is an alkyl group are preferred, and those in which Rl is an alkyl
group of from
8 to 12 carbon atoms (in particular where Rl is a linear alkyl group), and R2
and R3 are
each methyl or ethyl groups are most preferred.
BRIEF DESCRIPTION OF THE DRAWINGS
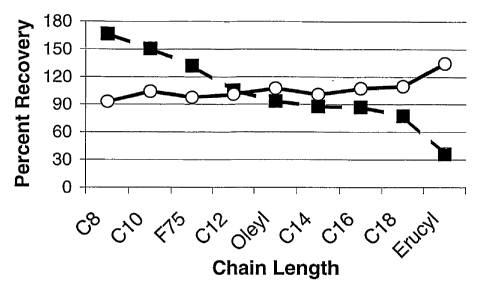
[001o] Figure 1 is entitled Gas Permeability vs. Chain Length. This Figure
plots the
Percent Recovery of permeability vs. the Chain Length of the aliphatic group,
Rl, of
several amine oxides.
DETAILED DESCRIPTION OF THE INVENTION
[0011] The amine oxides used in the present invention are known compounds and
many
are commercially available. They can be produced by various methods, one of
which is
by contacting a tertiary amine (corresponding to the formula R1R2R3N, wherein
Rl, R2
and R3 are as defined above) with a peroxide in a suitable aqueous reaction
medium. The
products thus produced are aqueous liqiuids having the amine oxides in up to
30 weight
percent concentration. The aqueous solutions of the amine oxides is an easy
and
preferred form of the product in this invention because they can be easily
pumped or
otherwise metered into the fracturing fluid or blended with the other
componenis of the
fracturing fluid.
[0012] Examples of the amine oxides of formula I above include, but are not
limited to,
those in which Rl is a straight chain alkyl group of 8 to 20 carbon atoms
(e.g., octyl,
nonyl, decyl, dodecyl, tetradecyl, octadecyl, and the like) or a straight
chain alkenyl
group of from 8 to 20 carbon atoms (e.g., oleyl, erucyl, and the like) and R2
and R3 are
each methyl, ethyl, n-butyl or 2-hydroxyethyl groups. The most preferred amine
oxides
are n-octyldimethylarnine oxide and n-decyldimethylamine oxide. While all of
the amine
-5-
CA 02540988 2007-08-29
oxides of formula I could reasonably be classified as surfactants, many are
known
foaming agents but the preferred amine oxides for use in the present invention
(e.g., n-
octyldimethylamine oxide and n-decyldimethylamine oxide) are not particularly
efficient
foaming agents. For instance, the preferred amine oxides have a foam half-life
of less
than one (1) minute when tested in 2 percent aqueous potassium chloride (2%
KCI)
solution, 3% hydrochloric acid, 0.2 % aqueous tetramethylammonium chloride
solution
or API brine. The foam half-life is determined by the tests are set forth in
USP 4,108,782, columns 5 and 6, under the headings "Initial Foam Volume Test"
and
"Foam Half-Life Test,". The preferred amine oxides thus do not promote the
formation
of emulsions (foams are a type of emulsion) in the presence of the formation
fluids and
they provide a desirable change (i.e., increase) in the contact angle.
The amine oxides are added to the fracturing fluids in small but sufficient
amounts to promote rapid clean-up. Normally, they are added as an aqueous
solution in
amounts of from about 0.01 to about I weight percent of amine oxide, weight-by-
weight
basis (w/w), and preferably from about 0.006 to about 0.024 weight percent.
The amine
oxides can be added "on-the-fly" to the fracturing fluid as it is being pumped
into the
wellbore or the amine oxides can be added to the so-called "frac tank" which
holds the
mix water for the fracturing fluid. The order of addition of the amine oxide
to the
fracturing fluid is not critical. The amine oxides seem to be compatible with
essentially
all ingredients of the fracture fluid, so far as Inventors are aware. They are
compatible
with acids (such as hydrochloric acid) and can, therefore, be used in so-
called "acid frac"
jobs where aqueous acid is used as the fracturing fluid (usually with acid
inhibitors
present). The amine oxides are also compatible with bases, and can be used in
fracturing
fluids having a basic pH which are common in fracturing fluids contain guar or
guar
derivatives (e.g., hydroxypropylguar ("HPG"), carboxymethyl guar,
carboxymethyl-
hydroxypropyl guar ("CMHPG")) as the viscosifiers; these fluids may be cross-
linked
with borates or zirconium or titanium cross-linking agents as well as other
species).
Fracture fluids normally have a pH range of from about 4 to about 12, and the
amine
oxides can be used in such fluids. Fracture fluids with a basic pH tend to be
more
-6-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
thermally stable, and are thus generally preferred for use in fracturing low
permeability
formations. The fracturing fluids of the present invention can also contain
other additives
typically found in fracturing fluids. E.g., proppants, other fluid loss
additives, non-
emulsifiers, breaker systems, formation stabilizers, bactericides, and the
like.
[0014] The fracturing fluids of the present invention are used in accordance
with known
procedures to fracture the subterranean formations. See, for example, the
fracturing
procedures set forth in the text "Reservoir Stimulation" cited above.
EXAMPLES OF THE INVENTION
[0015] The following examples will further illustrate the invention:
Example 1-9:
[0016] Various amine oxides, and one commercial fluorocarbon surfactant
(identified as
F75N; not an example of the invention), were tested in certain fluids in core
flow tests
using the procedures set forth below. The amine oxides each corresponded to
Formula I:
R3
I -E. /N
R / ~ 1
R2
in which R2 and R3 are each methyl, and Rl is n-octyl, n-decyl, n-dodecyl, n-
tetradecyl,
n-hexadecyl, oleyl, or erucyl (the later two groups are alkenyl groups of 18
and 22 carbon
atoms, respectively). The data are plotted in Figure 1 where the open circles
correspond
to the flow recovery with a brine flow, and the solid squares to -the flow
recovery with
nitrogen. The detailed core flow procedures are given below. The data show
that the
amine oxides provide a percent recovery that differs with the chain length of
the aliphatic
Rl group on the amine oxide. Surprisingly, those amine.oxides in which the
aliphatic Rl
group had 8 or 10 carbon atoms performed better (i.e., had a higher percent
recovery)
than the commercial F75N surfactant, one of the best additives in the industry
for
promoting rapid cleanup. The data in Figure 1 also show that one can modify
the rate of
cleanup or permeability recovery by choosing an amine oxide with different
chain lengths
-7-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
for the aliphatic Rl group. This provides the user with a means to vary the
rate at which
cleanup is achieved and the well is produced. For example, if one wishes to
achieve a
rapid cleanup and production rate, the user would choose an amine oxide with a
lower
carbon number for Rl (e.g., n-octyl or n-decyl). If the user wanted a lower
cleanup rate
(to prevent, for example, channeling and possible incomplete return of the
fracture fluid),
then the user could select an amine oxide with a higher carbon number for Rl
(e.g., n-
hexadecyl or n-octadecyl or oleyl). It is expected that blends of such amine
oxides could
also be used to achieve any particularly desired cleanup result. The ability
to vary the
rate of flowback, and achieve a predictable and controllable means of cleaning
up
stimulation fluids to improve the post-treatment permeability to gas, is a
useful tool in the
arsenal of the engineer. The amine oxides are more environmentally "friendly"
than the
commercial fluorocarbon surfactant (F75N) and they are cost effective. It was
also noted
that the amine oxides in which Rl has a higher carbon number (e.g., 16 or 18
or higher)
were viscoelastic as well as surface active. This combination of surface
activity and
viscoelasticity makes these amine oxides effective in well treatments where
friction
reduction and good cleanup are particularly desirable. One industry leader
provides such
fracture fluids (i.e., aqueous viscoelastic fluids that do not contain guar or
any guar
derivative) under the identity of "slickwater" treatments.
Core Flow Procedures for Amine Oxide Evaluation
[0017] Brine Flow:
1. Pre-perm dry cores with N2 to match cores.
2. Saturate cores in DI water with 2% NaCI.
3. Determine initial permeability to 2% NaCl with flow in the.forward
direction for'
a total of 25 pore volumes.
4. Pump surfactant solution in the reverse direction for a total of five (5)
pore
volumes.
5. Determine regained permeability to 2% NaCI in the forward direction for a
total
of 25 pore volumes. ti -8-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
6. Determine ratio of the regained 2% NaC1 permeability to the initial 2% NaCl
permeability.
[0018] Kerosene Flow:
1. Pre-perm dry cores with N2 to match cores.
2. Saturate cores in DI water with 2% NaCI.
3. Determine, initial permeability to 2% NaCl with flow in the forward
direction for
a total of 25 pore volumes.
4. Pump surfactant solution in the reverse direction for a total of five (5)
pore
volumes.
5. Determine regained permeability to kerosene in the forward direction for a
total of
25 pore volumes.
6. Determine ratio of the regained kerosene permeability to the initial 2%
NaCl
permeability.
(00197 Nitrogen Flow:
1. Pre-perm dry cores with N2 to match cores.
2. Saturate cores in DI water with 2% NaCI.
3. Determine initial permeability to 2% NaCl with flow in the forward
direction for
a total of 25 pore volumes.
4. Pump surfactant solution in the reverse direction for a total of five (5)
pore
volumes.
5. Determine regained permeability to nitrogen in the forward direction at 100
psi
for a total time equivalent to 25 pore volumes of brine at 1.0 mL/min (+/- 140
niinutes). .
6. Determine ratio of the regained nitrogen permeability to the initial 2%
NaCI
permeability. =
[0020] Pore Volume Calculations:
Assumption: .
Porosity is 15% .
-9-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
Volume Equation: CV ={3.1416 (D2) L} / 4
PV = CV (porosity) 100
Where PV is the Pore Volume in cc, CV is the Core Volume in cc, D is the Core
Diameter in cm and L is the Core Length in cm.
[0021] The above equation with the assumed 15% porosity, yields a pore volume
of 1.93
cc per one inch of core length. For simplicity, a pore volume will be rounded
up to 2.0 cc
per inch of core length.
Example 10:
[0022] A gas well is drilled into the Lobo 6 formation in western Texas to a
depth of
about 9,400 feet. The pay zone is in a low permeability sandstone. The bottom
hole
temperature is about 240 F and the reservoir pressure is about 4,450 pounds
per square
inch (psi). The well is cemented conventionally and is perforated using 4
shots per foot
of interval.. The well is broken down with dilute hydrochloric acid and balled
out. All
perforations appear to be accepting fluid. The well is then fracture
stimulated by
injecting sequentially, at a pump rate of 28 barrels per minute (BPM), a pad
fluid, a
proppant bearing fracture fluid, and a flush according to the pumping schedule
in Table 1
below:
a . .
-10-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
Table 1:
Stage Fluid Stage Volume Stage Proppant * Average Surface
(gallons) Concentration (pounds) Pressure (psi)
1 Fluid A 23,000 0.0 0.0 6,975
2 Fluid A 4,000 2.0 PPA 8,000 7,065
3 Fluid A 4,000 3.0 PPA 12,000 7,100
4 Fluid A 5,000 4.0 PPA 20,000 7,215
Fluid B 8,000 5.Q PPA 40,000 6,750
6 Fluid B 10,000 6.0 PPA 60,000 6,340
7 Fluid B 4,000 7.0 PPA 28,000 6,460
8 Fluid B 4,000 8.0 PPA 32,000 6,665
9 Fluid C 3,300 0.0 0.0 5,860
* The proppant is a commercial resin-coated sand proppant.
[0023] Fluid A is an aqueous plymer solution of a guar derivative (CMHPG at 40
pounds
of polymer per 1,000 gallons of fracture fluid), containing a
zirconate.crosslinker, a high
temperature gel stabilizer, a clay stabilizer and pH buffering agents. Fluid B
is an
aqueous plymer solution of a guar derivative (CMHPG at 35 pounds of polymer
per
1,000 gallons of fracture fluid), containing a zirconate crosslinker, a high
temperature gel
stabilizer, a clay stabilizer and a breaker for the gelled polymer. Fluids A
and B further
comprise the addition of n-decyl-N,N-dimethlyamine oxide so that each modified
fluid
contained the amine oxide at a concentration of 0.1 percent, weight-by-weight
basis. In
most cases, this corresponds to addign the surfactant at a ratio of between 1
and 2
gallons/thousand gallons; or 0.1-0.2 % (v/v).
[0024] In Stage 1, Fluid A is pumped as a pad fluid to fracture the formation.
[0025] In Stages 2-8, a proppant is added to the modified fracture fluids A
and B "on the
fly" as the fluids are being pumped and is ramped up from an initial
concentration of
2.0 PPA (pounds of proppant added) in Stage 2 to 8.0 PPA in Stage 8.
[0026] In Stage 9, Fluid C, a commercial fracture fluid, based on CMHPG at 35
pounds
of polymer per 1,000 gallons of fracture fluid, is used as a `flush" to
displace and push
-11-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
the proppant-bearing fracture fluid out of the tubing and into the formation.
The amine
oxide of the invention is typically not needed in this displacement/flush
stage. After
flush, the job is over, and the well is shut in.
[0027] The job is pumped to completion without incident. A propped fracture
half-
length (Xf) of about 820 feet is obtained with an average conductivity (Kfw)
of about
1275 md.ft.
[0028] The well is then shut in for several hours and then flowed back. =
Cleanup is
substantially improved (20-25% or greater) over previous jobs performed in
offset wells
using comparable flowback parameters (pressure and choke size). Gas production
from
the well is also substantially enhanced over previous offset wells.
[0029] Similar results are obtained using the fracture fluids and procedure
set forth in
Example 10 above except the CMHPG polymer was crosslinked by a titanate
crosslinker.
[0030] ' Similar results are also obtained using the fracture fluids and
procedure set forth
in Example 10 above except guar is used as the viscosifier instead of CMHPG.
[0031] Similar results are also obtained using the fracture fluids and
procedure set forth
in Example 10 above except guar is used as the viscosifier instead of CMHPG
and a
titanate crosslinker is used instead of a zirconate crosslinker.
[0032] Similar results are also obtained using the fracture fluids and
procedure set forth
in Example 10 above except guar is used as the viscosifier instead of CMHPG
and a
borate crosslinker is used instead of a zirconate crosslinker. These fluids
have a basic pH.
Contact Angle:
[0033] As mentioned before, some preferred amine oxides, in presence of the
formation
fluids, provide a desirable increase of the contact angle. The contact angles
were,
measured according to a method consisting of packing finely divided solids
into a tube
and then measuring the rate at which a fluid penetrates into the pack. When an
aqueous
-12-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
fluid contacts the pack of finely divided solids, it will begin to move into
the pack as a
front.
[0034] Assuming that the pack consists of a bundle of capillaries, it is
possible to derive
an expression to describe the rate that the fluid moves into the pack.
According to Rosen,
in "Surfactants and Interfacial Phenomena", Second Edition, John Wiley and
Sons, 1989,
p. 247, the distance, 1, that a liquid with a viscosity, r/ , advances in
time, t, is given,by '
the following expression:
2 (kr)tylz cos 0
Z -
2q
where 'r is the mean capillary size of the voids through the powder and k is a
constant
relating to the tortuosity. Obviously, then, the quantity kr depends on the
packing of the
solids. The quantity kr is measured by passing a fluid with a known surface
tension
through the pack; water is a convenient choice. The contact angle of the fluid
of known
surface tension is also known or assumed to be 0, which is a good assumption
in the case
where the test fluid is water and the pack consists of sand, clay, and silica
flour. The
method assumes that neither flocculation, dissolution nor dispersion changes
the packing
of the particles. We also assume that the contact angle of the blank, 01, is
0. The method
further assumes that surfactant concentration never falls below the critical
micelle
concentration due to adsorption of the surfactant. Finally, since some amine
oxides may
increase the viscosity of the test solution, we must account for any viscosity
differences.
So, we will let q2 represent the viscosity of the test solution. If we further
use the
relative data for the length of the imbibition column, so l, = 1. We also know
that the
surface tension of water without surfactant, y, = 72 dynes/cm, which leads to
the
following formula for the contact angle (in radians):
z
62= Arccos 72lrer
y2
[0035] The following table relates the imbibition data to contact angle.
Sample 7 Dynes/cm l Relative 0 Degrees
-13-
CA 02540988 2006-03-31
WO 2005/040552 PCT/EP2003/010902
Attorney Docket No.: 56.0649
Inventors: Hinkel, et al
Blank 72 1.00 0
C8 DMAO 39.4 0.11 88.8
C10 DMAO 37.0 0.26 82.5
C 12 DMAO 43.1 .36 76.3
C 14 DMAO 42.6 .21 57.8
oleyl DMAO' 47.5 .2 <50
F75N (prior art) 21 0.41 54.8
X DMAO means an amine oxide according to formula I, where Rl is X and R2 and
R3 are methyl.
Where X= Cn than X is a linear alkyl chain of n carbons.
[0036] It is particularly remarkable that several of the tested surfactants of
the invention
form a contact angle significantly greater than the contact angle of the F75N
surfactant of
the prior art, in particular, form a contact angle greater than 60- Degrees,
and in some
cases greater than 80 Degrees, and actually approaching 90 Degrees.
-14-