Note: Claims are shown in the official language in which they were submitted.
What we claim is:
1) The ionic liquid solvent used in step 1 is a salt for which the anion is a
bromide or a
chloride or a combination of bromide and chloride salts or a bromide, a
chloride or a
combination of bromide and chloride salts within a salt of a different anion.
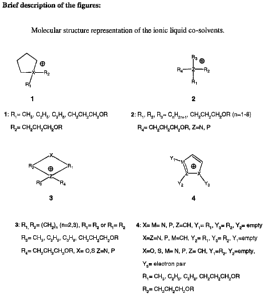
2) The ionic liquid solvent according to claim 1 is a salt for which the
cation is a
quarternized aliphatic or aromatic amine or phosphine, with one or more
heteroatom-containing substituent as shown in figure 1.
3) The ionic liquid solvent as described in claim 2 where one or more
substituent have a
common oxypropyl (-CH2-CH2-CH2-OR) motif, where the R group is a methyl, an
ethyl, an hydroxyethyl, a methoxyethyl, a repeating unit of the general
formula:
(CH2-CH2-O)n-H, (CH2-CH2-O)n-C p H2p1, (CH2-CH2-O)n-C p H2p
Where: n = 2, 3, 4; p=1, 2, 3, 4.
4) The ionic liquid solvent according to claim 3 where the oxypropyl
substituent
(-CH2-CH2-CH2-OR) originates from the corresponding chloride:
Cl-CH2-CH2-CH2-OR and used for the quaternization of the corresponding amine
or
phosphine according to claim 2. Where the R group is a methyl, an ethyl, an
hydroxyethyl, a methoxyethyl, a repeating unit of the general formula:
(CH2-CH2-O)n-H, (CH2-CH2-O)n-C p H2p+1, (CH2-CH2-O)n-C p H2p
Where: n = 2, 3, 4; p=1, 2, 3, 4.
5) The oxypropylchloride compound according to claim 4 which for R= CH2-CH2-OH
is obtained by reacting 1 equivalent of 1-chloropropan-1-ol with 1 to 4
equivalent of
ethylene carbonate, but preferably 1 to 1.5 equivalent, in the presence of 0.1
to 0.5
equivalent of base such as, but not limited to: sodium of potassium hydroxide,
pyridine or triethylamine.
6) The oxypropylchloride compound according to claim 5 which for
R=(CH2-CH2-O)n-H is obtained by repeating n times, successively the procedure
according to claim 5.
-6-
7) The oxypropylchloride compound according to claim 5 and 6 is isolated by
standard
work-up known to a person familiar with the art, followed by distillation.
Alkylation
by standard procedures known to a person familiar with the art provides the
remaining oxypropylchloride compound according to claim 4, where R=(CH2-CH2-
O)n-C p H2p+t, (CH2-CH2-O)n-C p H2p.
8) The cellulose solution, described in step 1 is an ionic liquid cellulose
solution of 1 to
25 % in weight concentration of cellulose and preferably 10 to 15 % in weight.
9) The process involved in dissolving the Cellulose as described in step 1,
may also
include:
a) Microwave irradiation of the solution, to facilitate dissolution.
b) Addition of a compatibilizer, such as but not limited to one or more fatty
acids
or one or more acid anhydrides, or a combination of one or more of the former
with one or more of the latter said compatibilizing agent.
10)The cellulose may be regenerated, microcrystalline, cotton fibres, paper or
wood
pulp, recycled paper or any other sources of cellulose, de-lignified prior to
use when
required.
11)The process involved in melting the polyolefin material (POM) as described
in step
2, may also include:
a) The use of an ionic liquid solvent as plasticizer to improve fluidity.
b) The use of an organic solvent as plasticizer to improve fluidity.
12) The process, according to claim 11 where the ionic liquid solvent can be:
the same
ionic liquid solvent used in step 1 and according to claims 1, 2 and 3; a
different
ionic liquid which may differ only by the cation, the anion or by both; a
combination
of one or more ionic liquids.
13) The process, according to claim 11 where the organic plasticizer is a
commercially
available solvent, varying in nature depending on POM used.
-7-
14) The polyolefin material (POM), as described in step 2 and according to
claim 11
wherein the POM is composed of low density polyethylene (LDPE), linear low
density polyethylene (LLDPE), high density polyethylene (HDPE), any other type
of
polyethylene resin, polypropylene (PP) or polystyrene (PS).
15) The polyolefin material (POM), as described in step 2 and according to
claim 14
wherein the POM is composed of a mixture of one or more of the resins
described in
claim 14.
16) The polyolefin material (POM), as described in step 2 and according to
claim 14
wherein the POM is composed of one or more resins according to claim 14 molten
separately.
17) The polyolefin material (POM), as described in step 2 and according to
claim 14, 15
and 16 wherein the source of one or more of the resins present in the POM may
be
virgin, pre-consumer, industrial or municipal post-consumer, reprocessed, in
the
form of pellets, powder, fibres or otherwise or from any other source and in
any
other form.
18) The polyolefin material (POM), according to claim 15 and 16 wherein the
POM may
be also composed of one or more compatibilizers.
19) The process involved in mixing the Cellulose and polyolefin material (POM)
as
described in step 3, may be achieved, but is not limited to the use of a co-
rotating
twin-screw extruder of a mixing tank.
20) The process, according to claim 18 and according to claim 11, 12,13 and 19
wherein
the mixing temperature is ~50°C about the melting temperature of the
said POM.
21) The process according to claim 19 and 20 where one or more compatibilizers
may be
added at one or more stages of the said extrusion.
-8-
22) The process, according to claim 19 and 20 where one or more of the molten
resins
according to claim 16 and 18 may be added at one or more stages of the said
extrusion.
23) The process as described in step 4, wherein the solvent for setting of the
CIS/PO
nano-dispersion is a Cellulose and PO non-solvent such as, but not limited to:
water,
methanol, ethanol, isopropanol, ethylene glycol or acetone.
24) The process as described in step 4 and according to claim 23, wherein
retrieving of
the said ionic liquid is achieved by evaporation with or without prior nano-
filtration
of the solution.
25) The process according to claim 23 and 24, wherein the said evaporation may
involve, but is not limited to rotatory evaporation, under vacuum or not,
spray-
drying, or a combination of these techniques.
26) The process as described in step 5 wherein moulding of CIS/PO material is
achieved
by standard methods, known to a person familiar with the art. The moulding
methods
include, but are not limited to: pressure moulding, low pressure moulding,
injection
moulding, blown moulding and die casting.
27) The process according to claim 26 wherein one or more plasticizers may be
used.
28) The process according to claim 27 wherein the plasticizer may be one or
more
hydrophilic ionic liquid as defined according to claims 1 to 7 inclusively.
29) The process according to claim 28 wherein the plasticizer is removed by
water
washings or by washings with a suitable organic solvent as described according
to
claim 23.
30) The process according to claim 29 wherein the plasticizer is retrieved
using methods
according to claims 23, 24 and 25.
-9-
31) The process according to claim 19 to 22, inclusively and 26 to 28
inclusively,
wherein at any of these stages, a dye or a combination of dyes may be added.
32) The process according to claim 19 to 22, inclusively, 26 to 28 inclusively
and claim
31, wherein at any of these stages, an antioxidant or a combination of
antioxidants
may be added.
-10-