Note: Descriptions are shown in the official language in which they were submitted.
CA 02541871 2001-08-23
SELECTIVE ANDROGEN ~tECEPT0~2 MObrtLA~'~RS AND
METHOI1S OF USE TIiEFt>EOF
FIEUb OF 7NVI;NTION
looot] The present i~n'vention relates to a novel class of tissue-selective
androgen
receptor targeting agents (ABTA) which demonstrate androgenic and anabolic
activity .
of a nonsteroidal ligand for the androgen receptoz. The agents define a new
subclass of
compounds which are tissue-selective androgen receptor modulators (SARM) which
are
io useful for male hormone therapy such as oral testosterone replacarnent
therapy, male
contraception, rrtaintaining~ sexual desire in women, treating prostate
cancer, and
imaging prostate cancer. These agents are also administered to a subject for
the
treatment of sarcopenia, Lack of sexual libido, osteoporosis, erythropoiesis,
and fertility.
The agents may be used alone or in combination with a progestin or estrogen.
is
$ACICGROUND OF THE ZNYENT10N
]OOO21 The androgen receptor ("AR"') is a ligand-activated transcriptional
regulatory
protein that mediates induction of male sexual development and 'function
through its
activity with endogenous androgens. Androgens are generally known as the male
sex
ao hormones. However, androgens also play a pivotal tale in female physiology
and
reproduction. The androgenic hormones are stezoids which are produced in the
body by
the testis axed the cortex of the adrenal gland, or synthesized in the
laboratory.
Androgenic steroids play an important role in many physiologic processes,
including the
development and maintenance of male sexual characteristics such as muscle and
bona
as mass, prostate growth, spermatogenesis, and the male hair pattern
(Matsumoto,
. Endocrinol. Met_ Clip. N. Am. 23:857-75 (1994). The endogenous stezoidal
androgens
include testosterone and dihydrotestosterone ("1~HT''). Testosterone is the
principal
steroid secreted by the testes and is the primary circulating androgen found
in the plasma
of males. Testosterone is converted to DHT by the enzyme 5 alpha-reductase in
many
3o peripheral tissues. DHT is thus thought to serve as the intracellular
mediator for most
androgen actions (Zhou, et al., Molec. Endacrinoi. 9:208-18 (i995))_ Other
staroidal
androgens include esters of testosterone, such as the cypienata, propionate,
phenylpropionate, cyclopentytpropionate, isocarporate, enanthate, and
decanoate asters,
and other synthetic androgens such as 7-Methyl-hlortestosterone ("M.BNT"') and
its
CA 02541871 2001-08-23
acetate ester (Sundaram et al., "7 Alpha-Methyl-Norrestosterone(MBNT): The
Optima!
Androgen Bor Male Contraception," Ann. Med., 25;199-205 (1993) ("Sundaram")).
Because the AR is involved in male sexual development and function, the AR is
a likely
target for effecting male contraception or other forms of hormone replacement
therapy.
s The AR also regulates female sexual function (i.e., libido), bone formation,
and
erythropoiesis.
(0049] Worldwide. population growth and social awareness of family planning
have
stimulated a great deal of research in contraception. Contraception is a
difficult subject
~o under any circumstances. Xt is fraught with cultural and social stigma,
religious
implications, and, most certainly, significant health concerns. This situation
is only
exacerbated when the subject focuses on male contraception. Z3espite the
availability of
suitable contraceptive devices, historically, society has looked to women to
be
responsible for contraceptive decisions and their consequences. Although
health
15 concerns over sexually transmitted diseases have made men more aware of the
need to
develop safe and responsible sexual habits, ~ovomen still often bear the brunt
of
conttaceptire choice. Women have a number of choices, from temporary
mechanical
devices such as sponges and diaphragms to temporary chemical devises such as
spermicides. Women also have at their disposal more permanent options, such as
2o physical devices like IUDs and cervical caps as well as more permanent
chenuoal
treatments, such as birth control pills and subcutaneous implants. hlowever,
to date, the
only options available for men include the use of condoms or a vasectomy.
Condom use,
however is not favored by many men because of the reduced sexual sensitivity,
the
interruption in sexual spontaneity, and the significant possibility of
pregnancy caused by
zs breakage or misuse. 'V'asectorniee are also not favored. Zf more convenient
rnechods of
birth control ware available to men, particularly long term methods that
require no
preparative activity Immediately prior to a sexual act, such methods could
significantly
increase the likelihood that men would take more responsibility for
contraception.
30 (0004] Adminislratioxt of the male sex steroids (e.g., testosterone and its
derivatives)
has shown particular promise in this regard due to the combined gonadotropin-
suppressing and androgen-substituting properties ofthese cornpouztds
(Steinberger et al.,
2
CA 02541871 2001-08-23
"Effect of Chronic Administration of Testosterone Enanthate on Sperm
Production and
Plasma Testosterone, Follicle Stimulating Idormone, and Luteinizing Homnone
Levels:
A Preliminary Evaluation of a Possible Male Contraceptive", Fertility and
Sterility
28.1320- 28 (1977)). Chronic administration of high doses of testosterone
completely
s abolishes sperm production (azoospermia) or reduces it to a very low level
{oli.gospetrnia). The degree of spermatogenic suppression necessary to produce
infertility
is not precisely known. However, a recant report by the world Health
Organization
showed that weekly intratnuscular injections of testosterone enanthate- result
in
azoospermia or severe oligospermia (i.e., less than 3 million sperm par ml)
and infartiliry
o in 98% of men receiving therapy (World Health Organization Task Force on
Methods
Ar Regulation of Male Fertility, "Contraceptive Efficacy of Testosterone-
Induced
Azoospermia and Oligosperrnia in Normal Men," Fertility and Sterility 65:82)-
29
(1996)).
~ s 10005) A variety of testosterone esters have been developed that are more
slowly
absorbed after intramuscular injection and, thus, result in greater androgenic
effect.
Testosterone enanthate is the most widely used of these esters. While
testosterone
enanthate bas been valuable in terms of establishing the feasibility of
hormonal agents
fox male contraception, it has several drawbacks, including the need for
weekly
zo injections and the presence of supraphysiologic peak levels of testosterone
immediately
following intra~uscular injection (Wu, "Effects of Testosterone Enanthate in
Normal
Men; Experience From a Multicenter Contraceptive Efficacy Study," Fertility
and
Sterility 65:626-36 (1996)).
zs La006] Steroidal ligands vvhi~h_ bind the AR and act as androgens (e.g.
testosterone
enanthate) or as antiandrogens (e.g. cyproterone acetate) have been known for
many
years and are used clinicahy (Wu 1g88). Although nonsteroidal antiandrogens
are in
clinical use for hormone-dependent prostate cancer, rionsteroidai androgens
have not
been reported. For this reason, research on male contraceptives has focused
solely on
3o steroidal compounds.
3
CA 02541871 2001-08-23
SUM11~IAR~' OF THE 1<NVENT10N
[0oo7j 'this invention provides a novel class of tissue-selective androgen
receptor
targeting agents (ABTA). The agents define a new subclass of compounds which
axe
tissue-selective androgen receptor modulators (SARM), which are useful for
oral
testosterone replacement therapy, male contraception, maintaining sexual
desire in
women, osteoporosis, treating prostate cancer and imaging prostate cancer.
These
agents have art unexpected and tissue-selective in-vivo activity for an
androgenic arid
anabolic activity of a nonsteroidal ligand for the AR. These agents
selectively act as
to partial agonists in some tissues, While acting as full agonists in other
tissues, providing a
a novel and unexpected means for eliciting tissue-selective androgenic or
anabolic
effects. Those agents may be avtivc alone or in combination with progestins or
estrogens. The invention further provides a novel class of non-steroidal
agonist
compounds. The invention further provides compositions cbntaining the
selective
androgen modulator compounds or the non-steroidal agonist corr~pounds and
methods of
binding an A~, modulating spermatogenesis, bone formation and/or resorption,
treating
arid imaging prostate cancer, and providing hormonal therapy for androgen-
dependent
conditions.
[oohs) The present invention xelates to a selective aridrc~gen receptor
modulator
compound having tissue-selective in-vivo andragenic and anabolic activity of a
r<onsteroidal ligand for the androgen receptor, the selective androgen
receptor modulator
compound represented by the structure of formula I:
Z
0
y NCI X
R~ ~~'T
x
4
CA 02541871 2001-08-23
wherein 1 is a O, CHz, NH, Se, PR, or NR;
Z is N02, CN, COR, CObH or CONHR;
Y is I, CF3,13r, Cl, or SnRs;
s Q is alkyl, halogen, NRz, NHCOCH3, NHCOCF3, NHCOR, NHCONHR,
NHCOOR, OCONHR, CONHR> NHCSCH3, Nl-iCSCh3, NHCSR NHSOzCH3,
NHS02R, OR, CQR, OCQR, OSOzR, SOzR or SR
wherein R is a alkyl, aryl, hydroxy, C~-Ca alkyl, a C,-C4 haloalkyl,
phenyl, halo, alkenyl or hydroxyl; .
Io or Q together with the benzene ring to which it is attached is a fused ring
system represented by structure A, 8 or C:
NH 0 ~ NH D
I
1
A 13 C
!5 1~.~ )S Cf~3? CF3, CI~2CHg, Or CI3gCJ"~; and
T is OH, OR, -NHCOCH3, orN~COR wherein R is a C~-Cø alkyl, a C,-
C.~ haloalkyl, phenyl, halo, allzenyl or hydroxyl.
(OOpsj Xn one embodiment, Q is in the park. position. In another embodiment, X
is O.
za In another embodiment, Q is in the pare position and X is O. Tn yet another
embodiment, Q is pare alkyl, halogen, NRz, NHCC~CH~, NF3COC1~3, NHCOR,
NHCONHR, NHCOOR, OCOlV'HR, CONHR, NHCSCHs, NHCSCP3, NHCSR
NHSOaCH~, NHSOzR, OR, COR, OCO~, OSO,~JZ, SOzR or SR wherein R is a alkyl,
aryl, hydroxy, Cl-C4 alkyl, a Cl-Ca haloalkyl, ph~nyl> halo, alkenyl or
hydroxyl.
as
[00010] The present invention relates to a selective androgen receptor
modulator
compound having in-vivo androgenic and anabolic activity of a nonstaroidal
Jigand for
CA 02541871 2001-08-23
the androgen receptor, the selective androgen receptor modulator compound
represented
by the structure of formula TI:
H3C OT-1
NH ?~
I o ~ 1
Q
y Il
S
wherein ~ is a O, CH2, NH, Se, PR, or NR;
Z is a hydrogen bond acceptor, NOz, CN, COR, CONHR;
Y is a lipid soluble group, I, Cla3, Hr, Cl, SnR~;
R is an alkyl or aryl group or OH; and
o Q is acetamido-, tri~uroaaetamido-, alkylamiztes, ether, alkyl, N-
sulfonyl, O-sulfonyl, alJryfsulfonyi, carbonyl, or a ketone,
[ooo~ 1) The present invention also relates to a selective androgen receptor
modulator
compound having in-vivo androgenic and anabolic activity of a nonsteroidal
Jigand for
t s the androgen receptor the, selective androgen receptor modulator compound
represented
by the structure of formula ITI::
H3C OH
NH X.
o w ~ Q
y ITI
urhere X is a O, CHz, NH, $e, PR, or hlR;
z is N02, CN, COR, or CONIdR;
Y is I, CFs> 13r, Cl, or SnT~;
20 R is an alkyl, or aryl group or OH; and
Q is acetamido or trifluroacetamido.
[~oD12) Tha present invention also relates to a selective androgen receptor
modulator
compo>ynd ha'~ing tissue-selective in-vivo androgenic and anabolic activity of
a
6
CA 02541871 2001-08-23
nonsteroidal iigand for the androgen receptor, the selective androgen receptor
modulator
compound represented by the structure of formula 1V:
02N F
p ~ \
CF3 ~ NH O /
H3C .~~~'OH
1V
[O~ol3] The present invention also relates to a selective androgen receptor
modulator
compound hawing tissue-selective in~vivo androgertic and anabolic activity of
a
nonsteroidal ligand for the androgen receptor, the selective androgen receptor
modulator
to compound represented by the structure of formula V:
OzN ~ COCH3
\ I O I /
CF3 NH ,, ~ o
H3C~O~Y
V
(00014] The present invention also relates to a selective androgen receptor
modulator
compound having tissue-selective in-vivo androgenic and , anabolic activity of
a
5 nonsteroida~J iigand for the androgen receptor, the selective androgen
zeceptor modulator
corxipound represented by the structure of formula VI:
02N / O ~ COCF-IZCH~
CF3 \ NH~~O /
H.3 C OH
vl
CA 02541871 2001-08-23
(opo151 The present invention also relates to a selective androgen receptor
modulator
compound having tissue-selective in vivo androgenic and anabolic activity of a
nonsteroidal ligand for the androgen receptor, the selective androgen reeeptar
modulator
compound represented by the structure of formula VII:
OZN NHCOCI~
/ ~ 0.
CF3 ~ NH~O
H3C OH
VII
to [o~W sl The present invention z.lso relates to a method of binding a
selective androgen
receptor modulator compound to an androgen receptor, which includes contacting
the
androgen receptor with the selective androgen receptor modulator compound
under
conditions effective to bind tlxe selective androgen receptor modulator
compound to the
androgen receptor, xn one embodiment the compound is Compound I. In another
t5 embodiment the compound is Compound Ix. In another embodiment the compound
is
Compound II1. In another embodiment the compound is Compound 1V. In another
embodiment the eompou~nd is Compound V. In another embodiment the compound is
Compound yI. In another embodiment the compound is Compound VII. (n another
embodiment the eompo'und is Compound'V'Y31.
zo
(ooplTl Another aspect of the present invention relates to a method of
modulating
spermatogenesis in a subject, which includes contacting an androgen receptor
of the
subject with a setecti've androgen receptor modulator compound under
conditions
effective to increase or decrease Speztti producIion. In one embodiment the
compound is
35 Compound X. In another embodiment the compound is Compound II. In another
embodiment the compound is Compound III. In another embodiment the compound is
Compound fV'. Itt another embodiment the compound is Compound 'V'. !n another
8
CA 02541871 2001-08-23
embodiment the compound is Compound VI. In another embodiment the compound is
Compound VII. In another embodiment the oompound is Compound 'VIII.
[ooa1e] The present invention also relates to a method of bocmone therapy,
comprising
contacting an ar<drogen receptor of a subject with a selective androgen
receptor
modulator compound under conditions effective tn bind the selective androgen
receptor
modulator compound to the lndrogen receptor and effect a change in an androgen-
dependent condition, In one embodiment the compound is Compound 1. Yrt another
embodiment the compound is Compound II. In another embodiment the compound is
to Compound IrI. In another embodirr~ent the compound is Compound IV. In
another
eznbodirnent the compound is Compound V. In another embodiment the compound is
Compound VI. 1n another embodiment the compound is Compound VIZ. In another
embodiment the compound is Compound VIII.
Is l0~oZ9] The present invention also relates to $ method of treating a
subject having a
hormone related condition which comprises contacting an androgen receptor of
said
subject vc~ith a selective androgen xeceptor modulator compound under
conditions
effective to bind the selective androgen receptor modulator compound to the
androgen
receptor and effect a change in an apdrogen-dependent condition. In one
embodiment,
20 the selective androgen receptor modulator compound is selective for
androgen or
testosterone receptor. The present invention also relates to a method of oral
administration of the selective androgen receptor modulator compound.
(Ooo2p] The present invention also relates to a method of treating a subject
hawing a
25 chronic muscle yvastit~g disease state which comprises contacting an
androgen receptor
of said subject with a selective androgen receptor modulator compound as
described
herein under conditions effective to bind the selective androgen receptor
modulator
compound to the androgen receptor and effect a change iza an androgen-
dependent
condition. In one ernbodimetzt, the selective androgen receptor modulator
compound is
3o selective for androgen or testosterone receptor. The present invention also
relates to a
method of oral administration of the selective androgen receptor modulator
compound.
In one embodiment the compound is Compound I. In another embodiment the
9
CA 02541871 2001-08-23
compound is Compound II. In another embodiment the coritpound is Compound III.
In
another embodiment the cr~mpo~und is Compound IV. In another embodiment the
compound is Compound V. In another embodiment the compound is Compound VI. In
another embodiment the compound is Compound VII. In another embodiment the
s compound is Compound VIII.
(oo02t1 As defined herein, the disease state of "chronic muscl8 wasting" means
[00022) The present invention also relates to a method of treating a subject
having
fo prostate cancer vrhich comprises administering to a subject an effective
amount of a
selective androgen reoeptor modulator compound. Ixi one embodiment, the
selective
androgen receptor modulator compound is selective for androgen or testosterone
receptor. Iu one embodiment the compound is Compound I. In another embodiment
the
compound is Compound II. In another embodiment the compound is Compound III.
In
~ 5 another embodiment the compound Is Compound IV. hi another embodiment the
compound is Compound V. In another embodimenC the compound is Compeund VI. In
another embodiment the compound is Compound VII. In another embodiment the
compound is Compound VIII.
zo (00023] The present invention also relates to compositions and a
pharmaceutical
compositions which comprises a selective androgen receptor modulator alone or
in
combirxation with a progestin or estrogen and a suitable carrier, diluent or
salt. In one
embodiment the composition comprises Compound I. In another embodiment the
compound is Compound II, xti another embodiment the compound is Compound III.
In
as another embodiment the compound is Compound TV. In another embodiment the
compound is Compound ~. In another erxtbodiment the compound is Compound Vl.
In
another embodiment the compound is Compound VII. In another erxabodiment the
compound is Compound VIII.
30 1000241 'The present invention relates to a non-steroidal agonist compound,
the non.-
steroidal agonist compound represented by the structure of formula V1II:
O
~NH X'
R~ T I~
VIII
CA 02541871 2001-08-23
'uvhereiri X is a O, CHz> NH,. Se, PR, or NR;
R~ is CH3, CP3, CHZCHs, or CFZCF3;
T is OH, OR, -NHCOCH~, or N.~3COR wherein R is a C~-C4 alkyl, a C~-
C.~ haloalkyl, phenyl, halo, alkenyl ar hydroxyl;
s
A is a 5 oz 6 rnembered saturated, unsaturated or aromatic carbocyclic ox
heterocyclic ring represented by the structure;
Z p3._...Az ..AB
As'._
A4 . ,,,rAl-" 4r 2~ ..,,,A7
Y% :; Alo ,, ;
AS..... A6 / 'Ai' ; .
to $ is a 5 oz 6 mexnbered saturated, unsaturated or aromatic carbocyclic or
heterocyclic ring represented by the structure:
1\ _.... ~~ . B -_ .. se
9 .
aq~ ~'1- Or QI .
Q3~ i ~, B la
QzyC ~ < < ~ I,
wherein A1- Al l are each C, O, S orN;
B 1- B" are each C, O, 5 ox N;
Z is N02, CN, COOH CQR, or CONHR;
~'' is I, C~3, $r, Cl, or SnR3; and
zo Q1 and Q2 are independently of each other alkyl, halogen, NR,,
NHCOCH3, NHCOCF~, NHCOR, NHC4NHR, NHCOOR, OCONHR, CQNHR,
NHCSCF~I3, NHCSCF3, NHCSR NHSOzCH3, NI-ISOZR, OR, COR, OCOR,
OSOzR, S02R or SR ~vherarn R is a C,-Ca alkyl, a. C,-C~ haloalkyl, phenyl,
halo,
alkenyl or hydroxyl.
zs
CA 02541871 2001-08-23
[00025] The present inventiozl also relates to a composition and
pharmaceuracal
composition comprising the non-steroidal agonist compound alone or in
combination
with a progestin or estrogen and a suitable gamier, diluent or salt, In one
embodiment the
compound is Compound I. In another embodiment the compound is Compound IT. In
another embodiment the compound is Compound III. In another embodiment the
compound is Compound IV. Xn another embodiment the compound is Compound V. In
another embodiment the compound is Compound VT. rn another embodiment the
compound is Compound VII. In another embodiment the compound is Compound VTII,
(000261 The present invention also relates to a method of binding a non-
steroidal
agonist compound to ~.n androgen receptor comprising contacting the androgen
receptor
with the non-steraidal agonist compound under conditions effective to bind the
non-
stcroidal agoriist compound to the androgen receptor. In one embodiment the
compound
is Compound I. In another ernbodimerit the compound is Compound II. In another
embodiment the compound is Compouzad III. In another embodiment the compound
is
Compound IV. In another embodiment the compound is Compound V, In another
etnbodixnent the compound is Compound Vr. In another embodiment the compound
is
Compound VXI. In another embodiment the compound is Compound VIIx.
(0002] The present invention also relates to a method of modulating
spermatogenesis
in a subject comprising contacting an androgen receptor of the subject with a
non-
steroidal agonist compound under conditions effactir~e to increase or decrease
sperm
production. In one embodiment the compound is Compound I. In another
embodiment
the compound is Compound II. In another embodiment the compound is Compound
XT1..
as In anbther embodiment the compound i5 Compound IV. In another embodiment ~e
compound is Compound V. In mother embodiment the compound is Compound Vi, In
anothex embodiment the compound is Compound VII. xn another embodiment the
compound is Compound VIII.
[ooo2e] The present invention also relates to a method of hoxmone therapy
carnprising
contacting an androgen receptor of a subject with a non-steroidal agonist
under
conditions effective to bind the non-steroidal agonist compound to the
androgen receptor
IZ
CA 02541871 2001-08-23
and effect a change in an androgen-dependent condition. In one embodiment the
compound is Compound I. ~ another embodiment the compound is Compound 1I. In
another embodiment khe compound is Compound III. In another embodiment the
compound is Compound IV. In another embodiment the compound is Compound V. In
another embodiment the compound is Compound VI. In another embodiment the
compound is Compound VII. Iri another embodiment the compound is Compound
VIII.
(ooo2s] The present invention also relates to a method of treating a subjeot
having a
hormone related condition which comprises contacting an androgen receptor of
said
io subject with a non-steroidal agonist compound under conditions effective to
bind the
non-steroidal agonist compound to the androgen receptor and affect a change in
an
androgen-dependent condition. In one embodiment, the non-steroidal agonist
compound
is selective for androgen or testosterone receptor. The present invention alsp
relates to a
method of oral administration of the non-steroidal agonist compound. In one
is embodiment the compound is Compound I. In another embodiment the compound
is
Compound II. In another embodiment the compound is Compound III. In another
embodiment the compound is Compound IV. In another embodiment the compound is
Compound V. In anothez embodiment the compound is Compound VI_ In anothez
embodiment the compound is Compound VII. In another embodiment the compound is
as Compound VIII.
[00030] The present invention also relates to a method of treating a subject
having
prostate cancer which comprises aduzinisttating to a subject an effective
amount of a
non-steroida! agonist compound, In one embodiment, the non-steroidal agonist
zs compound is selective fer androgen or testosterone receptor. In one
embodiment the
compound is Compound 1'. In another embodiment the compound is Compound II. In
another embodiment the corrtpound is Compound .III. In another embodiment the
compound is Compound IV. In another ertibodiment the compound is Compound V.
In
another embodiment thn compound is Compound VI. Irt another embodiment the
3o compound is Compound VII. In another embodiment the compound is Compound
YII1.
[00031] Still another aspect of the present relates to a method of producing s
selective
!3
CA 02541871 2001-08-23
androgen receptor modulator or a non- steroidal AR agonist compound of the
present
inrrention. Tn one embodiment the compound is Compound I. In another
embodiment the
compound is Compound II. In another embodiment the compound is Compound Illi.
In
another embodiment the compound is Compound fV'. In another embodiment the
compound is Compound V. In another embodiment the compound is Compound 'VI_ In
another arribodiment the compound is Compound VII. In another embodiment the
compQUnd is Compound VIII.
i00o321 The present invention further relates to a method of determining the
presence
of a selective androgen modulator compound and/or a non-steroidal agonist
compound
of the present invention in a satrtple. 'Y'he method comprises the steps of
obtaining the
sample, and detecting the compound in the sample, thereby determining the
presence of
the compound in the sample. In one embodiment, the sample is a blood serum,
plasma,
urine, or saliva sample. In another ernbodirnent, the detection step comprises
measuring
the absorbance of the compound. In one embodiment the comppund is Compound I.
Tn
another embodiment the compound is Compound II. In another embodiment the
compound is Compound III. In another embodiment the compound is Compound N. Xn
another embodiment the compound is Compound 'V'. In another embodiment the
compound is Gompound VI. In another embodiment the compound is Compound VII.
In
2o another embodiment the compound is Compound VIII.
(00033) The novel selective androgen receptor modulator compounds and the non-
steroidal agonist compounds of the present invention, either alone or as a
composition,
are useful in males and females for tho treatment of a variety of hormone-
related
conditions, such as hypogonadism, sarcopenia, erythropoiesis, ereotile
function, tack of
libido, osteoporesis and fertility. blyzther, the selective androgen receptor
modulator
compounds arid the non- steroidal agorsist compounds are useful for oral
testosterone
replacement therapy, treating prostate cancer, imaging prostate cancer, and
maintaing
sexual desire in women. The agents may be used alone or in combination with a
~o pragestin or estrogen. In one embodiment the compound is Compound I. In
another
embodiment the compound is Compound II. In another embodiment the compound is
Compound III. In another emboditrtent the compound is Compo4nd N. 1n another
14
CA 02541871 2001-08-23
embodiment the compound is Compound V. In another embodiment the compound is
Compound VI, In another embodiment the compound is Compound VII. In another
embodiment the compound is Compound VIII.
s [00034] The selective androgen receptor modulator compounds and the non-
steroidal
agonist compounds of the present invention offer a significant advance over
steroidal
androgen treatment because the selective androgen receptor modulator compounds
a.nd
the non-steroidal agonist compounds of the present invention hava been shown
in-vivo
to have a tissue-selective androgenic and anabolic activity of a nonsteroidal
ligand for
Io the andxogen receptor. Moreover, the selecpve androgen receptorrnodulator
compounds
and the non-steroidal agonist compound$ of the present invention are not
accompanied
by serious side effects, labiliry to oxidative metabolism, inconvenient modes
of
administration, or high cQSts and still have the advantages of oral
bioavailability, lack of
cross-reactivity wig other steroid receptors, and long biological half lives.
!n one
embodiment the compound is Compound I. In another embodiment the compound is
Compound II. In another embodiment the compound is Compound III. In anothex
embodiment the compound is Compound IV. In another embodiment the compound is
Compound 'V, In another embodiment the compound is Compound VI. In another
embodiment the compound is Compound VII. In another embodiment the compound is
2o Compound VITI.
BRTE~' DESC~iIPT;<ON OF TT~E DRAWINGS
The presenk invention will be understood and appreciated more fully from the
25 following detailed description taken in conjunction with the appersded
drawings in
which:
Figure 1: Androgenic and Anabolio activity of (S)-G'I~t-007 in rats, Rats were
left
untreated (intact contxol), castrated (castrated control), treated with
3o testosterone propionate (TP), or treated with S-GTx-007, and the body
weight
gain as well as the weight of androgen-responsive tissues (prostate, semimal
vesicles and levator ani muscle) was determined.
CA 02541871 2001-08-23
F3guse Z: Androgenic arid Anabolic activity of S-GTx;007 in rats. Rats were
Ieft
untreated (intact control), castrated (castrated control), treated with 0.1,
0.3,
0.5, 0.75 and 1.0 mglday testosterone propionate (Tf), or treated with 0.1,
0.3,
s O.S, 0.75 and 1.0 mglday S-GTx-007, and the weight of androgen-responsive
tissues (prostate, semimal vesicles and levator ani muscle) was determined.
Figure 3: Andzogenic and Anabolic activity of S-GTx-014 in rats. Rats were
left
untreated (intact aontxol), castrated (castrated control}, treated with 0.1,
0.3,
tp 0.5, 0.7s and 1.0 mglday testosterone propionate (TP), or treated with 0,1,
0.3,
O.S, 0.75 and 1,0 mp~day S-GTx-014, and the weight of androgen-responsive
tissues (prostate, semimal vesicles and levator ani muscle) w$s determined.
Figure ~: Average plasma concentration-time profiles of S-GTx-007 in beagle
dogs
i s after 1V administration at 3 and 10 m~.,,lkg.
Figtrr~ 5: Average plasma concentration-time profiles of S-GTx-007 in beagle
dogs
after PO administration as solution at 10 mglkg.
2o FIg b; Average plasma concentration-time profiles of S-GTx-007 in beagle
dogs
after fV' administration as capsules at rng/kg.
Figure 7: hffects of GTx-014 and GTx-007 ort LH Levels.
2s .T~lgure 8: Fsffects of GTx-01~ and GTx-007 on FSH Levels.
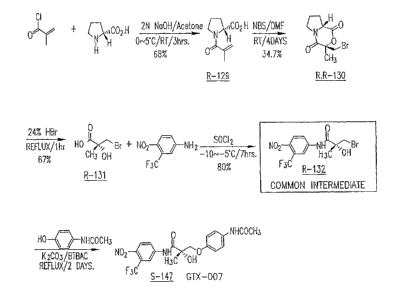
Figure ~: Synthesis scheme of GTX-007.
16
CA 02541871 2001-08-23
DETAILED DESCRIpTIOIV OF ~"FiE Y1~VEIV~TIOIrI
[00035] This invention provides a novel class of androgen reoeptor targeting
agents
(ABTA). The agents define a new subclass of compounds which are tissue-
selective
androgen receptor modulators (5A1~M) which are useful for oral testosterone
replacement therapy, male contraception, maintaining sexual desire in women,
treating
prostate cancer and imaging prostate cancer. These agents have an unexpected
tissue-
selective in-vivb activity for an androgenic and anabolic activity of a
nonsteraidal ligand .
for the androgen receptor. These agents may be active alone ar in, combination
with
to progestins or estrogens, The invention further provides a novel class of
non-steroids!
agotust compounds, The invention Filrther provides compositions containing the
selective androgen modulator compounds or the non-steroids! aganist compounds
and
methods of binding an ~.ndrdgen receptor, modulating sperznatagenesis,
treating and
imaging prostate cancer, and providing hozmonaI therapy for androgen-dependent
i5 conditions.
[00036) The compunds described herein, done a new class of selective androgen
receptor modulators (SARMS) that demonstrate potent anabolic effects (e.g.,
muscle
growth) with less androgenic activity (e.g,, prostatie growth). This new class
of drugs
2o has several advantages over non-selective androgens, °cluding
potential therapeutic
applications in males arid females for modulation of fertility,
erythropoiesis,
osteoporosis, sexual libido attd in men with or at high risk for prostate
cancez. Ln one
embodiment the compound is Compound I. In another embodiment the compound is
Compound II. In another embodiment the compound is Compound III. In another
25 embodiment the compound is CQrnpound IV. In another embodiment the compound
is
Compound V. Tn another embodiment the compound is Compound VI. Xn another
embodiment the compound is Compound VII. In another embodiment the compound is
Compound VII.
30 (00037] 'purther, in one embodiment the aompunds have tissue specific
pharmacologic
activity, As demonstaxted in )~igure 7 and 8, CTx-007 does not suppress LH
levels at
doses that era capable of eliciting maximal stimulation of levator ant muscle
growth and
17
CA 02541871 2001-08-23
does not suppress 1~SH levels at doses that are capable of eliciting maximal
stimulation
of levator ani muscle growth,
[00038] The present invention relates to a selective androgen receptor
modulator
s compound having tissue-selective in-vivo androgenic and anabolic activity of
a
nansteroida! iigand for the androgen receptor selective androgen receptor
tnodttlator
compound represented by the structyre of formula I:
2
Nli .~_ .~
R~ .~T
1
wherein ~C is a O, C~Ix, NH, Se, PR, or NR;
Z is N02, CN, COR, COON or CONHR;
~0 7' is I, CF3, )3r, Cl, or SnR3;
Q is alkyl, halogen, NRz, NHCOCH3, N~COCF3, NHCOR, NHCONHIZ,
NHGOOR, OCONHR, CONHR, NHCSCH3, NHCSCI~~, NHCS~. NHS02CH3,
NHS02R, OR, COR, OCOR, OS02R, SOzR or SR wherein R is an aryl, C~-C4
alkyl, a Ci-C4 haloalkyl, phenyl, halo, alkenyl or hydroxyl; or Q together
with
t 5 the benzene ring to which it is attached is a fused ring system
represented by
structure A, $ or C:
NH 0 / NH O NH
l ~ ,
A B C
18
CA 02541871 2001-08-23
Ri is CH3, CFa, CHzCH3, or CFzCF~; and
T is OH, OR, -NHCOC)~3, or NHCOR wherein R is a C,-Gq alkyl, a C,-
s Ca haloalkyl, phenyl, halo, alkenyl or hydroxyl.
[OOO3s) In one embodiment, Q is in the para position of the benzene ring to
whioh is is
attached. In another embodiment, Q is ii1 the para position arid ?C is 0. In
t~.tmthsr
embodiment, Q is in the para position and is .alkyl, halogen, NR2, NHCOCH3,
io NHCOCFa, NHCOR, NHCONHR, NHCOOR, OCONHR, CONHR, NHCSCHI,
NHCSCP'3, NHGSR NHSO2CH3, NHSO2I2, OR, COR, OCOR, OSOzR, SOzR or SR
wherein R is a, aryl, C,-C4 alkyl, a C~-C4 halaalkyl> phenyl, halo, alkenyl or
hydxoxyl.
(00040 The present invention relates to a Selective androgen receptor
modulator
1 s compound having in-vivo andragenic and anabolic activity of a nonsteroidal
Iigand for
the androgen receptor, the selective androgen receptor modulator oorrtpound
represented
by the structure of formula 11:
H3C OTC
NH ' X
0
Q
II
wherein ~ is a O, CHz, NH, Se, PR, or NR;
2 is a hydrogen bond acceptor, NO2, CN, COR, CONHR;
Y is a 1 ipid soluble group, I, C1~~, Br, Cl, SnR3;
R is an alkyl, or aryl group or OH; and
35 Q is acetamido-, Crifluroacetamido-, alkylamiries, ether, alkyl, N-
sulfonyl, O-sulfonyl, alkylsulfanyl, carbonyl, or a ketone.
19
CA 02541871 2001-08-23
jopo4la The present invention also relates to a selective androgen receptor
modulator
compound having inwivo androgenic arid anabolic activity of a rtonsteroidal
ligand for
the androgen receptor the, selective androgen receptor modulator compound
represented
by the structure of formula III:
hI3C OH
O \ ~ Q
111
s where X is a O, CHZ, NH, Se, PR, or NR;
2 is NOx, CN, COR, ~or CONHIt;
Y is T, CF3, Br, C(, or 5nR3;
R is an alkyl or aryl group or OH; 8.nd
Q is acetam[do or trifluroacetamido,
i0
[OD042j The present invention also relates to a selective androgen receptor
modulator
compound having tissue-selective in-vivo andro~enic and anabolic activity of a
nbnsteroidal ligand for the androgen receptor, the selective androgen receptor
modulator
compound represented by the structure of formula IV:
OyN y F
/ ~ O
CF3 \ NH~~ Q /
H3C 'OBI
T.Y
ao [0043] Tile present invention also relates to a selective androgen receptor
tttodulator
compound having tissue-selective in-viva androgenic and anabolic activity of a
nonsteroidal ligand fox the androgen receptor, the selective androgen receptor
modulator
compound represented by the stn.icture of formula 'V:
CA 02541871 2001-08-23
o~N r o ~ cocH3
C~3 ~ NH O
H3C .~.~~OH
V
(o00a4] The present invention also relates to a selective androgen receptor
modulator
compound having tissue-selective in-v;ivo androgenic and anabolic activity of
a
s nonsteroidal ligand for the androgen receptor, tkte selective androgen
receptor modulator
compound represented by the structure of formula V1;
OzN / O ~ COCHzCI-19
CF3 ~ Nki O
H3C 1~~~'OH
VI
[OOD45j The present invention also relates to a selective androgen receptor
modulator
compound having tissue-selective in-vivo androgenic and anabolic activity of a
1o nonsteroidal ligand for the androgen receptor, the selective androgen
receptor modulator
compound represented by the structure of formula VII:
ozN ~ o ~ NHCO~H
I
CF3 ~ NH O
HsC '.'y~'OT~.
V1I
[oop46j Then present invention relates to a non-steroidal agotlist compound
having the
1 s formula (Cottipound 'VIII):
21
CA 02541871 2001-08-23
Q
~NH X
lt~ T
VIII
s wherein ~ is a O, CHx. NH, Se, pR, or NR;
y 15 CH3, CF3, C~I2CH3, or CFzCF3:
T is OH, OR, -NHCOC.H~, or NHCOR wherein wherein R is a C~~Cq
alkyl, a Ci-C4 ha]oalkyl, phenyl, halo, a]kenyl or hydroxyl;
A is a 5 or 6 memberad saturated, unsaturated or aromatic earbocyc]ic or
to heterocyclic ring represented by the structure:
Z A3__... Aa p9 ,.,.. A$
' z
or
Ato ~ ~/.Ati.~~
B is a 5 or 6 iriembered saturated, unsaturated or aromatic carbocyclic or
heterocyciic ring represented by the structure:
IS
~~...__ as _.>38
B9-~- ,
or e,
Q2 ~ .._._pa, ~lQa~~t~~., '
?2
CA 02541871 2001-08-23
wherein At- Any sre each C, O, S or N;
Bt- B,~ axe each C, O, S or N;
Z is NOi, CN, COR, COON, or CONHR;
Y is I> CF3, Br, CI, or SnR3; and
and Q2 are independently of each other alkyl, halogen, NRz,
NHCOCH3> NbiCOCF3, NHCOR, NHCONHIt, NHCOOR, OCONH.R, CONHR,
NHCSCH3, NHCSC>~3, NHCSR NHSOiCH3, NHSO2R, OR, COR, OCOR,
to OSOZR, SOzR or SR wherein R is a C,-Ca alkyl, a C~-C4 haloalkyl, phenyl,
halo,
alkenyl or hydroxyl,
.[oa047] The substitutents Z and Y can 6e in any position of the fi~a or 6
membcred ring
carrying these substltutenks (hereinafter "A ring"). Similarly, the
snbstituent Q can he in
~5 any position of the five or 6 membered ring carrying this substitutent
(hereinafter "B
ring"), l;t is understood that when any of the ring members A,- A> > or B ~- B
i ~ are O or S,
then these ring members are unsubstituted. It is further understood that when
any of the
ring members A,- Air or )3t- Big are O or S, then the dotted line betyveen
said ring
members and other ring members represents a single bond.
ao
[ooo4s] In one embodiment, th.e A ring ir~oludes any type of saturated or
unsaturated
carbocyclic ring. In cne embodiment, the A ring is a 6 membered saturated
earbocyclic
ring, rwhich may be unsubstituted, monosubstituted or polysubstituted by any
of the
substitutents described horeinabove. rn one embodiment, the A ring is a S
membered
2s saturated carboeyclic ring, which may be unsubstituted, monosubstituced or
polysubstituted by any of the substitutents described hereinabove. In another
embodiment, the A ring is a 4 membered carbocyclic ring containing one or more
double
bonds, which ring may be urisubstituted, monosubstituted or polysubstituted by
any of
the substitutents described hereinabove, In another embodiment, the A ring is
a 5
3o mernbsrad carbocyclic ring containing one or more double bonds, which ring
may ba
unsubstituted, monosubsti.tuted or polysubstituted by any of the substitutents
described
hereinabove.
23
CA 02541871 2001-08-23
(oooG9] In another embodiment, the A ring includes any type of saturated,
unsaturated or
aromatic heterocyclic ring. )n another embodiment, the A ring is a 6 membered
saturated heterocyclic ring, which may be unsubstituted, monosubstituted or
polysubstituted by any of the substituents described hereinabove. Tn another
embodiment, the A ring is a 5 membered saturated heterocyclic ring, which may
be
unsubstituted, monosubstituted or polysubstituted by any of the substituextts
dascxibed
hereinabowe. In another embodiment, the A ring is a 6 membered heterocyclic
ring
cositaining one or more double bonds, ~cwhich ring may be unsubstituted,
monosubstituted
or polysubstituted by any of the substitutents described hereinabove. In
another
embodiment, the A ring is a 5 membered heterocyclic ring containing one or
more
double bands, which ring may be unsttbstituted, monosubstituted or
polysubstituted by
any of the substitutents described hareinabove. In another embodiment, the A
ring is a 6
mer~hered heteroaromatic ring which may be unsubstituted, monosubstituted or
1s polysubstituted by any of the substitutents described hereinabove. In
another
embodiment, the A ring (s a 5 membered heteroaromatic ring which may be
unsubstituted, monosubstituted or polysubstituted by any of the substitutents
described
hersina,bove.
(ooo5al Similarly, the B ring includes any type of saturated or unsaturated
carbocyclic
ring. In one embodiment, the H ring is a 6 mEmbered saturated carbocyclic
ring, which
may be unsubstituted, monosubstituted or polysubstituted by any of the
substitutents
described hereinabove. In one embodiment, the H ring is a 5 membered saturated
carbocyclic ring, which may be unsubstituted, monosubstituted or
polysubstituted by any
,5 of the substikutents described hereinabove. In another embodiment, the B
ring is a 6
membered carboeyclic ring containing one or zriore double bonds, Whiah ring
may be
unsubstituted, monosubstiiuted or polysubstituted by any of the substitutents
described
hereinabowe. In another embodiment, the B ring is a 5 mern6ered cazbocyclic
ring
containing one or more double bonds, which ring may be unsubstituted,
monosubstituted
or polysubstituted by any of the substitutents described hereinabove.
34
CA 02541871 2001-08-23
(ooo5lj rn another embodiment, the B ring includes any type of saturated,
unsaturated or
aromatic heterocyclic ring. Iri another embodiment, the B ring is a 6 membered
satuzated heterocyclic ring, Nrhich may be unsubstituted, monosubstituted ox
polysubstituted by any of the substituents described hereinabove. In another
s embodiment, the B ring is a 5 membered saturated t~eterocyclic ring, which
may be
unsubstituted, monosubsticuted or polysubstituted by any of the substituents
described
hereinabove. In another embodiment, the B Zing is a 6 membered boteroeyclic
ring
containing one or more double bonds, which ring may be unsubstituted,
~cnonos'ubstituted
or polysubstituted by any of the substitutents described hereinabove. In
another
to embodiment, the B ring is a 5 membered heterocyclic ring containing one or
more
double bonds, which ring may be unsubstituted, monosubstituted or
polysubstiiuted by
any of the substituter<ts described hereinabove. Tn another embodiment, the B
ring is a 6
membered heteroaromatic zing which naay be unsubstituted, monosubstituted or
polysubstituted by any of the substitutents described hereinabove. In another
15 embodiment, the ~ ring is a 5 membered heteroaromakic ring which may be
unsubstituted, monosubstituted or polysubstituted by any of the substitutents
described
hexeinabove.
Nonlimiting e~camplas of suitable A rings and/br $ rims era carbocyclic rings
such as
2o cyclopentane, eyclopentene, cyclo>;exane, and cyclohexene rings, and
heterocycIic rings
suci; as pyran, dihydropyrau, tetxahydropyran, pyrrole, dihydropyrrole,
tetrahydropyrrole, pyrazine, dihydxopyrazine, tetr~hydropyrazine, pyrimidine,
dihydropyrimidine, tetrahydropyrimidone, pyra2ol, dihydropyrazol,
tetrahydropyrazol,
piperidine, piperazine, pyridine, dihydropyridine, tetra.hydropyridine,
morpholine,
25 thiornorpholine, furan, dihydrotiuan, tetrai~ydrofuran, thiophene,
dihydrothiophene,
tetrahydrothiophene, thiazole, imidazole, isoxazole, and the like.
[ooos2j As used herein, receptors far extracellular signaling molecules are
collectively
refereed to as "cell signaling receptors". Ivlany cell signaling receptors arc
3o transmembrane proteins on a tell surface; When they bind an extracellular
signaling
molecule (i.e., a ligand), they become activated so as to generate a cascade
of
iatraa~Ilular signals that alter the behavior of the cell. In Lontrast, in
some cases, the
CA 02541871 2001-08-23
receptors are inside the cell and the signaling ligand has to enter the cell
to activate
thezh; these signaling moleoulas therefore must lie sufficiently small and
hydrophobic to
diffuse across the plasma membrane of the call. As used herein, these
receptors are
collectively roferred to as "ixttracellulax cell signaling receptors",
[00053) Steroid hormones are one example of small hydrophobic molecules that
diffuse directly across or are transported across the plasma membrane of
target cells and
bind to intracellular cell signaling receptors. These receptors are
structurally related and
constitute the intracellular receptor suparfamily (or steroid-hormone receptor
superfamily). Steroid hormone receptors include progesterone receptors,
estrogen
receptors, androgen receptors, glucocorticoid receptors, and
mineraloeorticoid, and
numerous orphan receptors. The present invention is particularly directed to
androgen
receptors and all of its isoforms.
is [00054] ln. addition to ligand binding to the receptors, the receptors can
be blocked to
prevent ligand binding. When a substance binds to a receptor, the three-
dimensional
structure of the substance fits into a space created by the three-dimensional
structure of
the receptor in a ball axtd socket configuration.
zo [000551 The better the ball fits into the socket, the more tightly it is
held. This
phenomenon is called affinity. If the affinity of a substance is su~ciently
high, it will
compete with the hormone and bind the binding site more frequently. The
binding of the
ligartd rnay also lead to tissue-selective recruitment of other important
proteins to
transduce the signal_ These proteins are known as coactivators and
carepressor,
2s participate in signal tra.rtsduction, and may be selectively induced or
inhibited by ligand
binding. Once bound, signals may be sent through the receptor into the cells,
causing
the cell to respond in some fashion. This is called activation. ~n activation,
the activated
receptor then directly regulates the trans4ription of specific genes_ But the
substance and
the receptor znay have cettain attribrltes, other than al:'flxzity, that
activate the cell.
so Chemical bonds between atoms of the substance arid the atoms of the
receptors may
form_ In some cases, this leads to a change in the configuration of the
receptor, which is
eno4gh to begin th.e activation process (called signal transductian), As a
result,
26
CA 02541871 2001-08-23
substances can be made which bind receptors and activate them (calfed receptor
agonists) or inactivate them (palled rec$ptor antagonists),
[ooo5s] The present invention is directed to selective androgen receptor
modulator
compounds which are agonist compounds, and are, therefore, useful in binding
to and
activating steroida) hormone receptors. The compounds are non-steroidal,
Preferably, the
agonist compound of the present invention is an agorust that binds the
androgen
receptor. Preferably, the compound his high afFlniky for the androgen
receptor, The
compound may bind either reversibly or irreversibly to the androgen receptor.
The
o compound of the present invention may contain a functional group (affinity
label) that
allows allcylation of the androgen receptox (i.e. covalent bond formation).
Thus, in this
case, the compound binds izreversibiy to the receptor and, accordingly, cannot
be
displaced by a steroid, suoh as the endogenous ligands dihydrotestosterone and
testosterone. Tt is preferable, however, for the compounds of the present
invention to
~ s reversibly bind the androgen receptor.
[000571 According to one aspect of the present invention, a method is provided
for
binding the selective androgen receptor zr~odulator compounds of the present
invention
to an androgen receptor by contacting the receptor with a selective androgen
receptor
zo modul~.tor compound under conditions effective to cause the selective
androgen receptor
modulator compound to bind the, androgen reoeptor. The binding of the
selective
androgen receptor modulator compounds to the androgen receptor enables the
compounds of the present invention to be useful in males and in females in a
number of
hormone therapies. The agonist compounds bind to and activate the androgen
receptor.
25 F3inding of the agonist compound is either reversible or irreversible,
preferably
reversible.
(o005s~ According to vne aspect of the present invention, a method is provided
for
modulating spermatogenesis by contacting an androgen receptor of a patient
with a
3o selective androgen receptor modulator compound under conditions effective
to bind the
selective androgen receptor modulator compound to the androgen receptor and
increase
or decrease sperm production.
27
CA 02541871 2001-08-23
[oUO59) According to another aspect of the present invention, a method is
provided for
hormonal therapy in a patient (i.e., suffering from an androgen- dependent
condition)
which includes contacting an androgen receptor of a patient with a selective
androgen
receptor modulator compound under conditions effective to bind the selective
androgen
receptor modulator compound to the androgen receptor and effect a change in an
androgen-dependent condition, Androgen-dependent conditions that may be
treated
according to the present invention include those conditions associated with
aging, such
ss hypogonadism, sarcopenia, erythropoiesis, osteoporosis, and any other
conditions
~0 later determined to be dependent upon low androgen (e.g., testosterone)
IaveJs. In one
embodiment, the selective androgen receptor modulator compound is administered
alone. In another embodiment, the selective androgen receptor modulator
compound is
administered in combination with pzogestin. Zn yet another embodiment, the
selective
androgen receptor modulator compound is administered in combination with
estrogen.
~5
[oooso) According to another aspect of the present invention, s method is
provided for
treating a subject having prostate cancer, The method comprises administrating
to a
subject an effective amount of a selective androgen receptor modulator
compound. In
one embodiment, the selective androgen receptor modulator compound is
selective foz
20 androgen ortestosterane receptor.
[00061) The present invention also relates to a method of treating a subject
hawing a
chronic muscle wasting disease state which comprises contacting a.n androgen
receptor
of said subject with a selective androgen receptor modulator compound under
conditions
2s effective to bind the selective androgen receptor modulator compound to the
androgen
receptor and effect a change in an androgen-dependent condition. In one
embodiment,
the selective androgen receptor modulator compound is selective fox androgen
or
testosterone receptor. The present invention also relates to a method of oral
administration of the selective androgen receptor modulator compound. In one
so embodiment the compound is Compound I. In another embodiment the compound
is
Compound II. In another embodiment the compound is Compound III. !n another
embodiment the compound is Compound IV. In another embodiment the compound is
?8
CA 02541871 2001-08-23
s
Compound V, in anothez embodiment the compound is Compound Vx. In another
ezr~bodiznent the compound is Compound 'VII. l:n another embodiment the
compound is
Compound 'VIII.
[OOO621 According to one aspect of the present invention, a method is provided
for
binding the non-steroidal aganist compounds of the present invention to an
androgen
receptor by contacting the receptor with a non-steroidal agonist compound
under
conditions effective to cause the non-stemidal agonist compound to bind the
androgen
to receptor. The binding of the non-steroidal agonist compounds to the
androgen receptor
enables the compounds of the present invention to be useful in males and in
females in a
number of hormone therapies. The agonist compounds bind to and activate the
androgen
receptor. Binding of the agonist compound is either reversible or
irreversible, preferably
reversible.
(OOOS3] According to one aspect of the present invention, a method is provided
for
modulating spermatogenesis by contacting an androgen receptor of a patient
with a non
sieroidal agonist compound under conditions effective to bind the selective
androgen
receptor modulator compound to the androgen receptor and increase or decrease
sperm
24 production.
(QOo64] According to another aspect of the present invention, a method is
provided for
hormonal therapy in a patient (i,e., one suffering from an androgen- dependent
condition) which Includes contaoting an androgen receptor of a patient with a
non-
zs steroidal agonist eompo~xnd under conditions effective to bind the non-
steroidal agonist
compound to the androgen receptor and effect $ change in an androgen-dependent
condition, Androgen-dependant conditions that may be treated according to the
present
invention include those conditions associated with aging, such as
hypogonadism,
sarcopenia, erythropoiesis. osteoporosis, lack of seal libido and any other
conditions
30 later deterrtmined to be dependent upon low androgen (e.g., testosterone)
levels. In one
embodiment, the non-sieroidal agonist compound is administered alone. In
another
embodiment, the non-steroidal agonist compound is administered in combination
with
29
CA 02541871 2001-08-23
progestin. In yet another embodiment, the non-steroidal agonist compound is
administered in combination With estrogen.
(ouo65] . According to another aspect of the present invention, a method is
provided for
treatizlg a subject having prostate cancer, The method comprises
administrating to a
subject an effective amount of a non-steroidal agonist compound, In onB
embodiment,
the non-steroidal agonist compound is selective for androgen oc testosterone
receptor.
[ooo6s] The compounds afthe present invention have an assyrnetric center and
can be
Io the ]2 or S isomer, or a mixture of both. In one embodiment, the compounds
racemic
mixtures of the R and S enantiomers. Tn another embodiment, the compounds are
substantially pure R enantiomers. Xn another embodiment, the compounds are
substantially pure S enantiomers. "Substantially pure" is defined herein as
groater than
about 95% preponderance of one isomer, Where the above-described processes for
the
is preparation of the compounds of use in the invention give rise to mixtures
of
stereoisomers, these isomers may be separated by conventional techniques, such
as
pxeparatiwe chromatography. The compounds may be ptepared in racemic form, or
individual enantiomers may be prepared either by enantiospecific synthesis pr
by
resolution
[oooe7] As used hereixt, "pharmaceutical composition" means therapeutically
effective
amounts of the SARM or the non-steroidal agonlst cQmpourid of the present
invention,
together with suitable diluents, preservatives, solubilizers, emulsifiers,
adjuvant andlor
carriers. A. "therapeutically effective amount" as used herein refers to that
amount which
2s provides a therapeutic effect for a given condition and administration
regimen. Such
compositions are liquids or Lyophilized or otherwise dried formulations and
include
diluents of various buffer conteryt (e.g., Tris-HCI., acetate, phosphate), pH
and ionic
strength, additives such as albumin or gelatin to prevent absorption to
surfaces,
detergents (e.g., Tween 20, T1'Veen S0, Fluronic F68, bile acid salts),
solubilizing agents
ztf (e.g., glycerol, polyethylene glycerol), anti-oxidants (e.g., ascorbic
acid, sodium
metabisulfite), preservatives (e.g., Thimerosal, benzyl alcohol, parabens),
bulking
substances or tonicity modifiers (e.g., lactose, mannitol), covalent
attachment of
CA 02541871 2001-08-23
polymers such as polyethylene glycol to the protein, eomplexation ~uvikh metal
ions, or
incorporation of the material into or onto particulate preparations of
polymeric
compounds such as polylactic acid, polglycolic acid, hydrogels, etc, pr onto
liposomes,
microemulsions, micelles, unilamellar or multilamellar vesicles, erythrocyte
ghosts, or
spheropla9ts. Such compositions will influence the physical ~ state,
solubility, stability,
rate of iu vivo release, and rate of in vivo clearance_ Controlled or
sustained release
compositions include formulation in lipophilic depots (e.g., fatty acids,
waxes, oils).
Iooo~al Also comprehended by the invention are particulate compositions coated
with
to polymers (e.g., poloxamers or poloxatniries). Other embodiments of the
compositions of
the invention incorporate particulate forms, protective coatings, protease
inhibitors or
permeation enhancers for various routes of administration, including
parenteral,
pulmonary, nasal and oral. Xn one embodiment the pharrnaeeuti.cal composition
is
administered parenterally, patacanceraily, transmucosally, tcansdermally,
intramuscularly, intravenously, intradetTnally, subcutaneously,
intraperitonsaty,
intraventxicularly, intracrani$1~y and intratumorally.
10o06sJ Further, as used herein "pharmaceutically acceptable carriers" are
well lrnown
to those skilled in the art and include, but are not limited to, 0,01-0.1M and
preferably
zo O.OSM phosphate buffer or 0.$% saline. Additionally, such pharmaceutically
acceptable
carriers may be aqueous or non-aqueous solutions, suspensions, and emulsions.
Bxamples of non-aqueous solvents are propylene glycol, polyethylene glycol,
vegetable
oils such as olive ail, and injectable organic esters such as ethyl oleate.
Aqueous carriers
include water, alcoholiclaqueous solutions, emulsions or suspensions,
including saline
2s and buffered media.
(00070) Parenterat vehicles include sodium chloride solution, finger's
dextrose,
dextrose and sodium chloride, lactated Ringer's or fixed oils. Intravenous
vehicles
include fluid and nutrient replenishers, electrolyte replenishers such as
those based on
so Ringer's dextrose, and the like. Preservatives and other additlwes may also
be present,
such as, for example, antimicrobials, antioxidants, collating agents, inert
gases and the
like.
31
CA 02541871 2001-08-23
[00071] Controlled or sustained rele$se compositions include formulation in
lipophilic
depots (e.g, fatty acids, waxes, oils). Also comprehended by the invention are
particulate
compositions coated with polymers (c.g. poloxamers or poloxamines) axtd the
compound
coupled to antibodies directed against tissue-specific receptors, ligands or
antigens or
coupled to ligands of tissue-specific receptors.
[000721 Other ernboditrients of the compositions of the invention incorporate
particulate forms, protective coatings, protease inhibitors or permeation
enhancers for
to vaxious routes of administration, including parenteral, pulmonary, nasal
and oral.
Io0073j Compounds modified by the covalent attachment of water-soluble
polymers
such as polyethylene glycol, copolymers of polyethylene glycol and
polypropylene
glycol, carboxymethyl cellulose, dextran, polyvinyl alcohol,
polyvinylpyrrolidone or
is polyproline axe known to exhibit substantially longer half lives in blood
following
intravenous injection than do the corresponding unmodii!ied compounds
(Abuchowski et
al., 1981; Newmark et al., T~8Z; acrd Katre et al., 198'0. Such modifications
may also
increase the compound's solubility in aqueous solution, eliminate aggregation,
enhance
the physical and chemical stability of the compound, and greatly reduce the
2o immt~uogenicity and reactivity of the compound. As a result, the desired
iri vivo
biological activity play be achieved by the administration of such polymer-
compound
abducts lass frequently or in lower doses than with the unmodified compound,
[a0074j Tn yet another embodiment, the pharmaceutical composition can be
delivered
zs iri a controlled release system. 1~or example, the agent may be
administered using
intravenous infusion, an implantable osmotic ppmp, a transdezmal patch,
lipvsomes, or
other modes of administration. In one embodiment, a pump rnay be used (see
l;anger.
supra; Sefton, CRC Crit. Itef. Bior~aed. Eng. 1~:201 (1987); Buchwald et al"
Surgery
$8;507 (1980); Saudek et al., N. $tigl, r. Med. 321:574 (1989). In another
embodiment,
so polymeric materials can be used. In yet another embodiment, a controlled
release system
can 6e placed in proximity to the therapeutic target, i.e., the brain, thus
requiring only a
fiactiot< of the systemic dose (see, e.g., Goodsorl, in Medical Applications
of Controlled
32
CA 02541871 2001-08-23
Release, supra, vol. 2, pp. 115-138 (198x). Preferably, a controlled release
device is
intxoduced into a subject in proximity to the site of inappropriate immune
activation or a
tumor. Other controlled release systems are discussed in the review by banger
(Science
249.1527-1533 ( 1990).
[000751 The pharmaceutical preparation can comprise the selective androgen
receptor
modulator alone, or can further include a pharmaceutically acceptable carrier,
and can be
in solid or liquid form such as tablets, powders, capsules, pellets,
solutions, suspensions,
elixirs, emulsions, gels, creams, or suppositories, including rectal and
urethral
~ o suppositories. 1?hatmaceutically acceptable carriers include gums,
starches, sugars,
cellutosic materials, and mixtures thereof. The pharmaceutical preparation
containing
the selective androgen receptor modulator can be administered to a subject by,
for
example, subcutaneous implantation of a pellet; in a further embodiment, the
pellet
provides for controlled release of selective androgen receptor modulator ovex
a period of
~5 time. The preparation can also be adrtiinistered by inGcavenous,
[ntraarterial, or
iz~tramuscular injection of a liquid preparation, oral administration of a
liquid or solid
preparation, or by topical application. Administration can also be
accomplished by use
of a rectal suppository or a ureckual suppository.
zo [poo76] The pharmaceutical preparations of the invention can be prepared by
known
. dissolving, mixing, granulating, or tablet-forming processes. )xor oral
adr>iinistration, the
selective androgen receptor modulators or their physiologically tolerated
derivatives
such as salts, esters, N-oxides, and the like are mixed with additives
customary for this
puxpose, such as vehicles, stabilizers, or inerk diluents, and Converted by
customary
zs methods into a suitable form for administration, such as tablets, coated
tablets, hard or
salt gelatin capsules, aqueous, alcoholic or oily solutions. Examples of
suitable inert
vehicles are conventional tablet bases s~teb as lactose, sucrose, or
cornstarch in
combination with binders like acacia, cornstarch, gelatin, or with
disintegrating agents
such as cornstarch, potato starch, alginlc acid, or with x lubricant lik$
steaxic acid or
3o magnesium stearate.
[00077] Examples of suitable oily vehicles or solvents are vegetable or animal
oils
33
CA 02541871 2001-08-23
such as sunflower oil ox fish-liver oil. Preparations can be effeoted both as
dry and as
wet granules. For parentera.l administration (subcutaneous, intravenous,
intraarterial, or
intraxnusculax injection), the SARM agents or the non-steroidal agonist agents
or their
physiologicahy to]crated derivatives such. as s$]ts, esters, N-oxides, and the
like are
s converted into a solution, suspension, or emulsion, if desired with the
substances
customary and suitable for this purpose, for example, solubilizers or outer
auxiliaries.
Examples are; sterile liquids such as water and oils, with or without the
addition of a
surfactant and other pharmaceutically acceptable adjuvants. Illustrative oils
are those of
petroleum, animal, vegetable, or synthetic origin, for example, peanut oil,
soybean oil, or
~o mineral oil. In general, water, saline, aqueous dextrose and related sugar
solutions, and
glycols such as propylene glycols or polyethylene glycol are preferred liquid
carriers,
particu]arty fox injectable solutions.
[o007s1 The preparation of pharmaceutical compositions which contain an active
~s cot~ponent is well understood in the art. Typically, such compositions are
prepared as
aerosols of the polypeptide delivered to the nasopharynx or as injectables,
either as
liquid solutions or suspensions, however, solid forms suitable for solution
in, or
suspension in, li9uid prior to injection can a]so be prepared. The preparation
can also be
emulsified, The aotive therapeutic ingredient is often tuixed with excipients
that are
zo pharmaceutically acceptable and compatible with the active ingredient.
Suitable
excipiez~ts are, for example, water, saline, dextrose, g]yceroi, ethanol, or
the ]ike aad
combinations thereof
(ono79] :Ln addition, if desired, the composition can contain minor amounts of
auxiliary
z5 substances such as wetting or ert~ulsifying agents, pl-T buffering agents,
which enhance
the effectiveness of the active ingredient.
[000801 An active component can be formulated into the composition as
neutralized
pharmaceutically acceptable salt forms. Pharmaceutically acceptable salts
includt the
3o acid addition salts (formed with the free amino groups of the poiypeptide
or antibody
molecule), which axe formed with inorganic acids such as, for example,
hydrochloric or
phosphoric $cids, or such organic acids as acetic, oxalic, tartaric,
ntandelic, and the liko.
34
CA 02541871 2001-08-23
Salts formed from the free carboxyl groups can also be derived from inorganic
bases
such as, for example, sodium, potassium, ammonium, calcium, or ferxic
hydroxides, and
such organic bases as isoprapylatnine, tcimcthylamine, 2-ethylamino ethanol,
histidine,
procaine, and the like.
s
(Oooe1] For topical administration to body stufaees using, for example,
creams, gels,
drops, and the like, the SARM agents or the non-agonist steroidal compounds or
their
physiologically tolerated dexivatives such as salts, esters, ~1-oxides, and
the like are
prepared and applied as solutions, suspensions, or emulsions in a
physiologically
to acceptable diluent with or Without a pharmaceutical carrier.
(O~oe2] Tn another embodiment, the active compound caa be delivered in a
vesicle, in
particular a liposome (see Langer, Science 249:I527-1533 (1990); Treat et al.,
in
laiposomes in the Therapy of infectious Disease and Cancer, Lopez- Becestein
and Fidler
is (eds.), hiss, New 'York, pp. 353-365 (1989); hopez-Berestein, ibid., pp.
317-327; see
generally ibid).
lo0ve3] 1~or use in medicine, the salts of the SARM or the non-steroidal
agonist
compounds will be pharmaceutioally acceptable salts, Other salts may, however,
be
zo useful in the preparation of the compounds according to the invention or of
their
pharmaceutically a.eoeptable salts. Suitable pharmaceutically acceptable salts
of the
compounds of this invention include acid addition salts, which may, for
example, be
formed by mixing a solution of the compound according to the invention with a
sotution
of a pharmaceutically acceptable acid such as hydrochloric acid, sulphuric
said,
2s methanesulphonic a~eid, fumario acid, malefic acid, succinic acid, acetic
acid, berizeic
acid, oxalic acid, citric acid, tartaric acid, carbonic acid or phosphoric
acid.
(00084] The present invention further relates to a method of determining the
presence
of a selective androgen modulator compound andlor a non-steroidal agonist
compound
30 of the present invention in a sample. The method comprises the steps of
obtaining the
sample, and detecting the compound in the sample, thereby determining the
presence of
the cotnpouud in the sample.
CA 02541871 2001-08-23
joo085] In one embodiment, the sample is a blood serum sample. .In another
embodiment, the sample is a plasma sample. In another embodiment, the sample
is a
urine sample. In another embodiment, the sample is a saliva sample. In another
embodiment, the sample is any other tissue sample.
(00086] In one embodiment, the detention step comprises measuring the
absorbance of
the oompound at a predetermined wavelength. For example, the corttpounds of
the
present invention absorb in the ultraviolet region of the spectrum, with an
absorbency
to peak at 270 nm. Thus, in one embodiment of the present invention, the
compound is
detected by monitoring the W absorbance of the Sample at 270 nm. It should be
noted
that the present invention is net limited to U'V' absorption, and that any
other
spectrometric methods of identification are applicable. For example,
corupounds can be
detected by measuring their infra-red or visible absorbance.
IS
ioooBT] xn another embodiment, the present invention further provides a method
of
detem~ining the concentration of a selective androgen receptor modulator
compound
and/or a non-steroidal agonist compound of the present invention in a sample.
The
na.ethod comprises the steps of obtaining a sa,xnple; determining the level of
the
2o compound in the sar~lple, and caleulatitig the concentration of the
compound in the
sample by comparing the IeveI with a standard sample containing a known
concentration
of the compound. Calibration curves of known concentrations of the oompound in
the
sample, can be obtained, and the concentration of tho compound In the test
sample is
Calculated therefrom. By "level" it is meant the absorption level of the
Compound at the
25 measured vravelength~
jooos8] In another embodiment, the compound is detected in the sample by
contacting
th.e sample with a binding protein which specifically binds to the compound,
and
determining the amount of binding protein bound to the oQmpound. The
concentration
30 oP the compound can be determined by measuring the amount of binding
protein bound
to the compound, and comparing that amount to a standard sample containing a
known
concentration of the compound - binding protein complex.
36
CA 02541871 2001-08-23
(oo0gs] Protein levels can be determined according to standard techniques, as
described in Sambrook et al. Briefly, a sample obtained from a subject is
contacted with
a binding protein which specifically binds to a specific compound of the
present
s invention, and the amount of complex formed between the binding protein and
the
compound is determined. In one embodiment, the binding protein is an antibody
which
specifically binds to one or more compounds ofthe present invention. In
another
embodiment, the binding protein has a detectable label bound thereto, and the
complex
between the binding protein-label compound is determined by visuali2ing the
complex.
Io
[o0090] As defined herein, "contacting" means that the binding protein is
introduced
into the sample in a test tetbe, flask, tissue culture, chip, array, plate,
microplate,
capillary, or the like, and incubated at a temperature and time sufficient to
permit the
binding component to bind to a cell or a fraction thereof or plasma/serum or a
fractiotl
~s thereof containing the target. Methods for contacting the samples with the
binding
proteins, or other specif]c binding components are known to those skilled in
the art and
may be selected depending an the type of assay protocol ~to be run. Incubation
methods
are also standard and are known to those skilled in the art.
zo [oao91] "Visualizing" the complex may be carried out by any means known in
the
art, including, but not limited to, ELISA, radioimmunoassay, flow cytometry,
dot blots,
western immunoblotting combined with gel electrophoresis, immunohistochemistry
at
tight and electron pe levels, HPLC and mass spectrometry.
zs [ooos2] Either monoclonal or polyclonal antibodies (as welt as any
recombinant
antibodies) specific Per the selective androgen modulator compounds or the non-
steroidal agonist compounds of the present invention can be used iu the
various
immunbassays. The antibodies rrlay be delectably labeled, utilizing
conventional
labeling techniques wall-Imown to the art, As used herein, the term "label"
refers to a
3o molecule, vvhieh may be conjugated or otherwise attached (i.e., covalently
or non-
covalently) to a binding protein as defined herein. Labels are known to those
skilled in
the azt. Thus, the antibodies may be labeled with radioactive isotopes, non-
radioactive
37
CA 02541871 2001-08-23
isotopic labels, fluorescent labels, enzyme labels, chemiluminescent labels,
biolutrtinescent labels, free radical.labels, or bacteriophage labels, using
techniques
known in the art. Examples of radioisotopic labels are 3 ~I, 125 I,
t 31 I,
35 S, ,sup.I4 C, etc. Examples of non-radioactive isotopic labels are
55 Mn,
s ,sup.56 Fe, etc. Exaxaples of fluorescence labels are fluorescent labels
which are
directly labeled with the preferred fluorescence label, or fluorescent labels
which are
indirectly labeled with the preferred fluorescence label. !n the last case,
the preferred
fluoxescence label is conjugated to a secondary antibody, which is directed
against the
first antibody, such as an anti species Ig antibody. Typical fluorescent
labels include, but
1o are not limited to a fluorescein label, an isothiocyanate label, a
rhodamine label, a
phycoerythrin label, etc" for example fluorescein isothi.ocyattate (FITC,
International
Biological Supplies, Melbourne, FL), rhodamine, phycoerythrin (P.~., Coulter
Corp.,
Hialeah, FL)" phycocyanin, alophycocyanin, phycoerythrin-cyanin dye 5 (PECyS,
Coulter), label, a phycoeyanin label, an allophycocyanin label, an O-
phthaldehyde label,
1 s a tluorescarnine and Texas Red.
[o0os3a Examples of enzyme labels include alkaline phosphatase,
beta-galactosidase, glucose-6-phosphate dehydrogenase, tnaleate dehydrogenase,
and
peroxidase, Two principal types of enzyme immunoassay are the enzyme-linked
2o immunosorbent assay (ELISA), and the homogeneous enzyme immunoassay, also
knovcrn as enzyme-multiplied imrrtunoassay (EMT.T, Syva Corporation, Palo
Alto, CA).
In the ELISA system, separation may be achieved, for example, by the use of
antibodies
coupled to a solid phase. The EMIT system depends oil deactivation Qf the
etaayma iri
the tracer-antibody complex; the acti'~ity can thus be measured without the
need for a
25 separation step.
iooos4) Particularly suitable labels include those, which permit analysis by
flow
cytometry, e.g" fluoYoohromes. Qther suitable detectable labels include those
useful in
coloritnetric enzyme systems, e. g" horseradish peroxidase (HRF'} and
slkalir~e
3o phosphatase (AP). Other proximal enzyme systems are known to those of skilt
in the an,
including hexokinase in conjunction with glucose-6-phosphate dehydrogeriase,
38
CA 02541871 2001-08-23
(00095] Additionally, chemiluminesoent compounds rnay he used as labels.
Chemiluminescent labels, such as green fluorescent proteins, blue fluorescent
proteins,
and variants thereof are mown. Also bioluminescence or chemiluminescence can
be
detected using, respectively, NAD oxidoreductase with luciferase and
substrates NADH
s and FN)N or peroxidase'with luminol and substrate peroxide, Typioal
chemiluminescent
compounds include luminal, isoluminol, aromatic acridlnium esters,
irnidazoles,
acridinium salts, and oxalate esters. Similarly, biolumitlescent compounds
rr~ay be
utilized for labelling, the bioluminescent compounds including luciferin,
luciferase, and
aequotin. Once labeled, the antibody may be employed to identify and quantify
to ~ inununologie counterparts (antibody or antigenic polypeptide) utilizing
techniques
well-known to the art.
[ooos67 The following examples are presented in order to more fully illustrate
the
1 s preferred embodiments ef the invention. They should in no way be
oonsm.~ed, however,
as limiting the broad scope of the invention.
EXPERTMENTAIr DETAItLS SECT)(fJN
zo
EXAMPLE I
Nonsteroidat Llgsnds wzth Androgelnic and Anabolic Activity
z5 100p9i1 The SAZ2.M compounds provided herein wens designed, synthesized and
evaluated for in-vitro and in-vivo phatmacologlc activity. The in-vitto
androgen
receptor binding affinity and ability to maintain androgen dependent tissue
growth in
castrated animals was studied. Androgenic activity was monitored as the
ability of the
SARM compounds to maintain and~or stinnulatB the growth of the prostate and
seminal
3o vesicles, as measured by weight. Anabolic activity was monitored as the
ability of the
SAlZIt~ compounds to maintain and/or stimulate the gowth of the levator ani
muscle, as
measured 6y weight,
39
CA 02541871 2001-08-23
S~rnthetic Praaedures of Carnaounds I-VrY>(:
(ooosa] (2R)-1-Metttacryloylpyrrolfdin-2-F~rboxylic Acid (R-1z9), D-proline (R-
128, 14.93 g, 0. I3 mol) was dissolved in 71 mI. of 2 N NaOH and cooled in an
ice bath;
s the resulting alkaline solution was diluted with acetone ('71 mL). An
acetone solution (71
mL) of metacryloly chloride 1z7 (I3.56 g, 0.13 mol) and 2N NaOH solution (71
m~.)
were simultaneously added over 40 min to the aqueous solution of D-proiine in
an ice
bath. The pH ofthe mixture was kept at 10-11°C during the addition of
the metacryloly
chloride. After stirring (3 h, room temperature), the mixture was evaporated
in vaauo at
1o a temperature at 35-45 °C to remove acetone. The resulting solution
was washed with
ethyl ether arid was acidified to pH 2 with concentrated HCI. The acidic
mixture was
saturated with NaCI and was extracted with EtOAc (100 mL x 3). The combined
extracts
were dried over Naa504, filtered through Celite, and evaporated in vacuo to
give the
crude product as a colorless oil. Recrystallization of the oil from ethyl
ether and hexanes
~ s affoxded 16.2 (68%) of the desired compound as colorless crystals: mp 102-
103 °C (lit.
[214 mp 102.5-103,5 °C); the NMIt spectzum of this compound
demonstrated the
existence of two rotamers of the title compound. tH NIvIR (300 MHz, DMSO-ds} 8
5.28
(s) and 5:15 (s) for the first rotamer, 5.15 (s) and 5.03 (s) for the second
rotamer (totally
2H for both rotamers, vinyl Cl'lz), 4.48-4.44 for the first rotatner, 4.24-
4.20 (m) far the
24 second rotamer (totally Il-T for both rotamers, CH at the chiral eantex),
3.57-3.38 (tn, 2H,
CHz), 2.27-2.I2 (IH, CH)> I.97-1.72 (m, 6H, C~:f"~CH, Me); t3C NMR (7S MHz,
DMSO-ds) & for major rotarrier 173.3, 169.1, 140.9, 116.4, 58.3, 48,7, 28.9,
24.7, 19.5:
for minor rotarner 174.0, 170.0, I4I-6, 115.2, 60.3, 45.9, 31.0, 22.3, 19.7;
IR (KBr) 3437
(OH), 1737 (C=O), 1647 (CQ, COOH), 1584, 15p8, 1459, 1369, 1348, 1178 cm'';
as [oc]p 6 +80.8° (c - 1, MeOH); Anal. Calcd, for C9H~sNO3: C 59.00, H
?,15, N 7.65.
Found; C 59.13, H 7.19, N 7.61.
(Opp99j (3R,$aR)-3-Rromotnethyl-3-tnetltyl-tetrahydro-pyrrolo[2,y-
c](1,4]pxazine-1,4-dione (R, R-X30). A solution of NHS (23.Sg, O.I32 mot) in
I00 mlr
30 of DMF was added dropwise to a stirred solution ofcompound R-129 (l6.Ig, 88
mmol)
in 70 mL of hMF under argon at room temperature, and the resulting mixture Was
stirred 3 days. The solvent was removed in vacuo, and a yellow solid was
precipitated.
CA 02541871 2001-08-23
The solid was suspended in vJater, stirred overnight at room temperature,
filtered, and
dried to give i 8.6 (81%) (smalier weight when dried ~ 34%) of the title
compound as a
yellow solid: mp i52-154 °C (lit. [214] mp 107-109 °C for the S-
isomex);'$ NMR (300
MI3z, DMSO-db) s 4.69 (dd, I = 9.6 Hz, J = 5.7 Hz, 1H, Cli at the chiral
center), 4.02
(d, J = 11.4 Hz, 1 H, CHI°), 3.86 (d, J = 11.4 Hz, 1 H, CH1~lb), 3.53-
3.24 (m, 4H, CHZ),
2.30-2.20 (m, IH, CH), 2,04-1.72 (m, 3H, CHi and CH), 1.56 (s, 2pT, Me);'3CNMR
(75
MHz, DMSQ-ds) b 167.3, 163.1, 83.9, 57.2, 45.4, 37.8, 29.0, 22.9, 21.6; IR
(T~Br) 3474,
1745 (C=O), 1687 (C=O), 1448, 1377, 1360, 1308, 1227, 1159, 1062crri ~;
[a]pz6+124.5
° (C = 1.3, chloroform); Anal. Caltd. for CyH~2HrN03: C 41.24, H 4.6I,
N 5.34. Found:
C 41.46, H 4.64, N 5.32.
[OOO1oo) {2R)-3-Brpmo-2-hy'droxy-2-methylpropsnoic Acid (R-131). A mixture of
bromolactone R-13p (lB.Sg, 71 mmol) in 300 mL of 24% HEr was heated at reflex
for I
h. The resulting solution was diluted with brine {200 rnL), and was extracted
with ethyl
acetate (I00 mL x 4). The combined extracts were washed with saturated Nal-
1C03 (100
mL x 4). The aqueous solution was ecidif~ed with concentrated HCl to pH~ = 1,
which, in
tum, was extracted with ethyl acetate ( 100 ml; x 4). The combined organic
solution ~cvas
dried over Na~Spa,~ filtered khrough Celite, and evaporated in vacuo to
dryness.
Recry'stallizatiori from toluene afforded 10.2 g (86%) of the desired compound
as
zo colorless crystals: top 107-109 °C (lit, [214] mp 109-113 °C
for the S-isomer);'H NMR
(300 MHz, DMSO-d6) b 3.63 (d, J = 10.1 Hz, 1H, CHHg), 3.52 (d, J = 10.1 Hz,
IH,
CHHb), 1.35 (s, 3H, Me); rR (KBr) 3434 (OH), 3300-2500 (COOK), 1730 (C=O},
I449,
1421, 1380, 1292, 1193, 1085 cnri'; [a]p'6 +10,5° (c ~ 2.6, MeOH);
Arial. Calcd. fox
C4H~Bz03: C 26.25, H 3.86. Found: C 26.28, H 3,75_
zs
i0oo101j N-(4-Nitro-3-(trifluoromethyl]phenyl)-(2R)-3-6romo-Z-hydrOxy-2-
triett~yipropanasnide (R.-13Z}. Thionyl chloride (8.6 g, 72 ntmol) was added
dropwise
under argon to a solution of bromoacid R-13x (11.0 g, 60 mrnol) in 70 mL of
DMA at
5 to --10 °C. The resulting mixture was stirred for 2 h under the same
conditions. A
solution of 4-vitro-3-trifluvrornechyl-aniline (12.4 g, 60 mmol) in 80 mL of
DMA was
added dropwise to the above solution, and the resulting mixture was stirred
overnight at
room tenxpatature. The solvent was removed on Rotavapor using high vacuum oil
pump;
41
CA 02541871 2001-08-23
the residue was diluted with saturated NaHCO~ solution, and extracted with
ethyl ether
(I04 mL x 3). Combined extracts were dried over anllydxous NazSOa, filtered
through
Celite, and ptltified by flash chromatography on silica gel, using methylene
chloride as
etuent to afford 18.0 g (80%) of the desired compound: mp 98-100 °C
(Rr= 0.2 , silica
gel, CHzClz);'H NN1R (300 MH2, DMSO-d6) 8 I0.54 (s, IH, NH), 8.54 (d, J = 2.1
Hz,
1H, Arl~, 8.34 (dd, J = 9.0 Hz, J = 2.1 Hz, 1H, ArH), 8.18 (d, J ~ 9.0 Hz, 1H,
ArH),
6,37 (s, 1H, OH), 3.82, (d, J = 10.4 Hz, IH, CHHa), 3.58 (d, J ~ 10.4 Hz, 1H,
CHHr),
J .48 (s, 3H, Me); "C NMR (?5 MHz, DMSO-ds) S 173.6 (C=O), 143.0, 127.2,
123.2,
122.6 (q, J = 33.0 Hz), I22.0 (q, J = 271.5 Hz), 118.3 (q, J = 6.0 HZ), 74.4,
41.4, ? 4.9;1R
(KBr) 3344 (OH), 1680 (C=O), 1599, 1548 (C= C, Ar), 1427, 1363, 1161 cm''; MS
(~51~: m!z 370.8 (M)+; Anal. Calcd. for C~~H~oBrly~204: C 35.60, H 2.'72, N
7.55, pound:
C 35.68, H Z.72, N 7.49.
(ooo1D21 N-j4-nitro-3-fr9fluoromethyl)phe~yl~-(2S)-3-j4-(acetylamino)phenoxy]-
2-
~ s )aydroxy-2-msthylpropanamide (5..147). The title compound was prepared
from
compound R-13Z (0.37 g, L0 mmol)> 4-acetamidoplaenol (0.23 ~g, I.S mmol)
~.aC03
(0.28 g, 2.0 mmol), and 10% of ben2yltribt~tylammortium chloride as a phase
rxansfer
catalyst in 20 mL of methyl ethyl ketone vvas heated at rehux overnight under
argon.
The reaction was followed by TLC, the resulting mixture was filtered through
Celite,
2o and concentrated in vaceto to dryness. Purification by flash column
chromatography o~
silica gel (hexanes-ethyl acetate, 3:1) yielded 0.38 g ($6%) (Rf = 0.18
hexanes-ethyl .
acetate, 3:1) of the desired compound as a light yellow pov~rder: mp 70-74
°C; The solid
can be recrystalixed from ethyl acetate and hexane); ~H NMR (3DD MHz, DMSO-ds)
S
10.62 (s, 1H, NH), 9.75 (s, 1H, NH), 8.56 (d, J = 1.9 I3~z, 1H, ArH), 8.36
(dd, J = 9.1 Hz,
z5 J = 1.9 Hz, 1H, ArH), 8.18 (d, r = 9.1 Hz, 1H, ArH), 7.45-7.42 (m, 2pI,
ArH), 6.85-6.82
(m, 2H, ArH), 6.25 (s, 1H, OH), 4.17 (d, J ~ 9.5 Hz, 113, CHHa), 3.94 (d, J =
9.5 Hz, 1H,
CliHb), 1.98 (s, 3H, Me), 1.43 (s, 3H, M.e); tsC NMR (75 MHz, DMSO-ds) 8 I74.6
(C=Q), 167.7, 154.2, 143.3, 141.6, 132.5, 127.4, 123.0, 122.7 (g, J = 33.0
Hz), 122.1 (q,
T = 271.5 Hz), 120,1, 118.3 (q,1= 6.b Hx), 114.6, 74.9, 73.8, 23.8, 23.0; IR
(fix) 3364
3a (OH), 1668 (C=O), 1599, 1512 (C=C, Ar), 1457, 1415, I351, 1323, 1239, 1154
1046
cui ~; MS (BSr): rnlz 464.1 (MfNa)+; Anal. Calcd. for C~gH,eF3N3Os: C 51.71, H
4.11,
N 9.52. Fouled: C 52.33, H 4.40, N 9.01.
42
CA 02541871 2001-08-23
[puane insert]
[000103] The in-vitro activity of the SARM compounds, specifically compound
'VII,
~o demonsfxated high androgen receptor ,binding affinity (Ki = 7,5 nNi).
Animal studies
with the SARM compounds, specifically compound V, demonstrated that it is a
potent
androgenic and anabolic nonsteroidal agent. Four groups of rats were used for
these
studies: (1) intact controls, (2) castrated controls, (3) castrated animals
treated with
testosterone propionate (lOD p.g/day), and (4) castrated animals treated with
compound
1 s V (1 ODD ~c~day). Testosterone and compound VII were delivered at a
constant rate for
14 days via suhcutaneous osmotic pumps.
[000104] The results of these studies are shown in Figure 1. Castration
significantly
reduced the weight ofandroge~aic (e.g., prostate and seminal vesicles) aTad
anabolic (e.g.,
zo levator alai muscle) tissues, but had little effect on animal body weight
(B'VV). Treatment
of castrated animals With testosterone propionate of compound VxI maintained
the
weight of androgenic tissues to the same degree. Compound VII had similar
attdrogenic
activity as testosterone propionate (i.e., the prostate and seminal vesicle
weights were
the sarlle), but m~tGh greater efficacy &s an anabolic agent. Compound VII
showed
zs greater anabolic activity than testosterone propionate at the doses tested
(i.e., the levator
ani muscle maintained the same weight as intact control animals and was
greater than
that observed for testosterone). The experiments presented herein are the
first in-vivo
results which demonstrate tissue-selective androgenic and anabolio activity
(i.e.,
differing androgenic and anabolic potency) of a nonsteroidal ligand for the
androgen ,t
so receptor,
Q3
CA 02541871 2001-08-23
EXAMPLE Z
Nonsteroidal T.igands with A~droge~nie and Ansbolic Activity
(00o1o5j The in-vivo efficacy and acute toxicity of four novel rlonsteroidal
androgens
(compounds fV~, Y, V); and VIA in rats was examined. In-vitro essays
established that
these compounds bind the androgen receptor with very high affinity. The
structures and
names of the four compounds are presented below:
15
OzN ~ R
O ~ /
C);3 NH ~.~~, ,O
H3C OH
GTx-014 k~= F
OTx-015 R=COCkI~
GTx-a16 R=coC2~s
GTx-017 R~NIiCOCH3
EXPERrMENTAL METHODS
1000106] Materials. The S-isomers of compounds GTx-014 (compound ~, GTx-015
z5 (compound V), GTx-OIb (compound VI) and G'px-007 (compotuid VII rwherein R
is
N.~TCOCH3) and the R-isomer of GTx-014 were synthesized in accordance with the
scheme as set forth in Figure 9, Testosterone propionate (TP), polyethylene
glycol 3Qa
(PEG300, reagent grade) and neutral buffered formaiin (IO% w/w) were purchased
from
44
CA 02541871 2001-08-23
Sigma Chemical Company (St Louis, lvZO). Alzet osmotic pumps (model 2002} were
purchased fxom AJ2a Core. (Palo Alto, CA).
100010?] ~tnirr~c~ls, Immature male Spxague-pawley rats, weighing 90 to IOOg,
were
purchased from Harlan J~iosciences (7.ndianapolis, IN). The animals were
maintained on
a 12-hour light-dark cycle with food and water available ad libiturn.. The
animal pxotocol
was reviewed and approved by the rnstiiutional Laboratory Anirnal Care and
U'se
Camtnittee.
to [ooo~0sl Str~dyDesigri. Rats were randomly distributed into twenty-nine
(291 groups, with
S animals per group. Treatment goups are described in Table 1, One day prior
to the
start of drug treatment, animals ~ groups 2 through 29 were individually
removed from
the cage, weighed and anesthetized with an intraparitoneal dose o~
ketaminelxylazine
(g7/13 mg/kg; approximately I mL per leg). Where appropriately anesthetized
(i.e., no
response to toe pinch), the animals' ears were marked for identification.
purposes.
Animals were then placed on a sterile pad and their abdomen and scrotum washed
with
betadine and 70% alcohol. The testes were removed via a midline scrotal
incisioxt, with
sterile suture being used to legate supra-testicular tissue prior to surgical
removal of each
testis. The surgical wound site was olosed with sterile stainless steel wound
clips, and
24 the site cleaned with betadine. The animals were allowed to recover on a
stexiJe pad
(until able to stand) and then returned to theix cage.
(oo01 os] Twenty-four hours later, animals in groups Z through 29 were re-
anesthetized
with ketamineJxylazine, and an Alzet osmotic pumps) (model 2002) was placed
2s subcutaneouly in the scapular region. Xn this instance, the scapular region
was shaved
and cleaned (betadine and alcohol) and a small incision (I cm) made using a
sterile
scalpel. The osmotic pump was inserted and the wound closed with a sterile
stainless
steel wound clip. Animals were allowed to recover and were returned to their
cage.
Osmotic pumps contained the appropriate treatrdent (designated in Table I)
dissolved in
3o polyethylene glycol 300 (PEG300). Osmotic pumps were filled with the
appropriate
solution one day prior to implantation. Animals were mox~itoxed daily for
signs of acute
toxicity' to drug treatment (e.g., lethargy, rough coat).
CA 02541871 2001-08-23
(000110] After 14 days of drug treatment, rats were aneskhBtized with
ketaminelxylazine. Animals were then sacrificed by exsanguinations under
anesthesia.
A blood sample was collected byvenipuncture ofthe abdominal aorta, and
submitted for
complete blood cell analysis. A. portion of the blood was placed in a separate
tube,
s centrifuged at 12,OOOg for 1 minute, and the plasma layer removed and frozen
at -20°C.
The ventral prostates, seminal vesicles, levator ani muscle, liver, kidneys,
spleen, lungs,
and heart were removed, cleared of extraneous tissue, weighed, and placed in
vials
containing 10% neutral buffered formalin. preserved tissues were sent to GTx,
Tnc. for
histopathological analysis.
io
(ooottt] For data analysis, the weights of all organs were normalized to body
weight,
and analyzed for any statistical significant difference by single-factor
ANO'V'A, The
weights of prostate and seminal vesicle were used as indexes for evaluation of
androgeuit activity, and the levator ani muscle weight was used to evaluate
the anabolio
~s activity.
RES1ILTS
[ooo1~z] The androgenic and anabolic activities the S isomers cf compol~tds
GTx-0x4,
GTx-015, GTx-016 and G'15c-007, and the R isomer of GTx-014 'overe examined in
a
zo castrated rat model after 14 days of administration. Testosterone
propionate, at
increasing doses, was used as the positive control of anabolic and androgenic
effects.
[ooo1t31 As shown in Figures 2 and 3, the weights of prostate, seminal
vesicle, and
levator ani muscle in casaated, vehicle-treated rats decreased significantly,
due tv the
as ablation of endogencus androgen production. Exogenous administration of
testosterone
prapionate, an androgenio , and anabolic steroid, increased the weights of
prostate,
seminal vesicle, and levatar ani muscle in castrated rats irt a dose-dependent
manner.
The R-isomer of GTx-014, attd S-isomers of GTx-015 and G'I~t-016 showed no
effect on
the weights of prostate, seminal vesicle, and levator ar~i muscle in castrated
animals
3U (data not shown). The S-isomers of GTx-007 (Figure 2: S-GTx-007) and GTx-
014
(Figure 3: S-GTx-014) resulted in dose-dependent increases in prostate,
seminal vesicle
and levator ani muscle weights. compared with testosterone propionate, S-
(',uTx-007
~#6
CA 02541871 2001-08-23
showed lower potency and intrinsic activity in increasing the weights of
prostate and
seminal vesicle, but a greater potency and intrinsic activity in increasing
the weight of
levatox ani muscle. Particularly, S-GTx-007, at a dose as low as 0.3
mp.,,lday, was able to
maintain the levator ani muscle weight of castrated animals in the same level
as that of
intact animals. Thus, S-GTx-ODD is a potent nonsteroidal anabolic agent with
less
androgenic activity but more anabolic activity than testosterone propionate.
This is a
significant improvement over previous clairixs, in that this compound
selectively
stimulates muscle growth and other anabolic effects white having less effect
on the
prostate and seminal vesicles_ This may be partieularly relevant in aging men
with
I o concerns related to the development or progression of prostate cancer.
(opo114] GTx-014 was less potent than GTx-007, but showed greater tissu~
selectivity
(compare effects on the prostate and seminal vesicles in Figures 2 and 3). GTx-
014
significantly increased levator ani muscle weights, but showed little to no
ability to
1s stimulate prostate and seminal vesicle growth (i.e_, the prostate and
seminal vesicle
tx~eights were less than 20% of that observed in intact animals or in animals
treated with
testosterone propionate),
(OOO115] results showed that none of the examined compounds produced
significant
Zo effect on body weight or the weights of outer organs (i.e., liver, kidneys,
spleen, Jungs
and heart). Nor did any compound produce any signs of acute toxicity, as
gauged by
diagnostic hernatolagy tests and visual examination of animals receiving
treatments.
Importantly, GTx-Ob7 did not suppress the production of luteinizang hormone
(L'f-I) or
follicle stimulating hormone (FSH) at a dose of 0.3 mglday (i.e" a dose that
exhibited
2s maxima( anabolic effects).
(ooolls] In summary, S-GTx-D07 exhibited exceptional anabolic activity in
animals by
maintaining the weight of levator aril muscle after removal of endogenous
androgen.
This discovery represents m~jox progress towards the development of
therapeutically
3o useful nonsteroidal androgens, and a major improvement (i.e., tissue
selectivity and
potency) fiver previous drugs in this class. S-GTx-O1~ and S-GTx-007 showed
selective
anabolic activity in comparison with testosterone propionate, an andragenic
and anabolic
47
CA 02541871 2001-08-23
steroid. The tissue-selective activity is actually one of the advantages of
nonsteroidal
androgens in terms of anabolic-related applications.
(000117) Despite similarities in structure and in-vitro functional activity,
the S-isomers
s of compounds GTx-014, GTx-015, GTx-016, and GTx-007 exhibited profound
differences in terms of their in-vivo activixy, GTX-007 the most efficacious
androgenic
and anabolic activity in animals, with the anabolic activity greater than that
of
testosterone propionate. GTx-014 showed a small degree of androgenic activity,
but an
anabolic activity comparable to testosterone propionate, In contrast, GTx-015
and GTx-
to OI6 failed to produce any androgenic or anabolic activity in-vivo,
[0001181 These studies show the discovery of two rr~emhers (GTx-014 and GTx-
007,
compounds, compounds IX attd Y respectively) of a new class of selective .
androgen
receptor modulators (SARMS) that demonstrate potent anabolic effects (e.g.,
nnuscle
15 growth) with less attdrogenic activity (e.g., prostatic growth). This new
class of drugs
has several advantages over non-selective androgens, including potential
therapeutic
applications in males and females for modulation of fertility, erythropoiesis,
osteoporosis, sexual libido and in men with or at high risk for prostate
cancer.
:0 [0001191 Fwther, Figures 7 axtd 8 demonstrate the effects of GTx-014 and
GTx-007 on
LH and FSH Levels in rats. Those restalts further demonstrate the novelty of
these
SARMs, due to their difFerential effects on these xeproductlve hormones, thus
demonstrating the tissue-specific phaxmacologic activity. In Fire 7, LH levels
in
castrated animals treated r.vith TP and GTx-014 were significantly lo~cwer
than those of
zs untreated animals (i.e., castrated controls) at doses greater than or equal
to 0.3 mglday.
However, higher doses (i.e., O.S mg/day or higher) of GTx-007 were required
before
significant decreases in LH levels were observed. Thus, GTx-007 does not
suppress ~,I-I
levels at doses that are capable of eliciting maximal stimulation of levator
ani muscle
growth, In 1 figure 8, FSH levels in castrated animals created with GTx-014
were
3o significantly lower than those of untreated animals (i,e" castrated
controls) at doses of
0.5 xnglday or higher. Similarly, lower FSH levels were observed in animals
treated with
TP. Ilowever, only this difference was only significant at a dose of 0.75
mg/day. 1~Sfl
48
CA 02541871 2001-08-23
levels in animals treated with GTx-007 wexe not significantly different from
those of
untreated animals at any dose level tested. Thus, G1k~007 does not suppress
FSH levels
at doses that are capable of eliciting maximal stimulation of levator ani
muscle growth.
Table 1. Animals Groups and Experimental Design
Grpup # CastratedhDrug Dpse # o~ animals
1 No None None ,5
2 Yes None Vehicle 5
only
3 Yes Testosterone0.1 mg/day5
4 Yes Testosterone.3 rng/day5
Yes Testosterone0.5 mg/day5
6 Yes Testosterone0.'15 mg/day5
? Yes Testosterone1.0 mg/day5
8 Yes It-OTx-OI4 1.0 mg/dayS
9 Yes S-GTx-014 0.1 mg/day5
'f'es S-GTx-014 0.3 mglday5
11 Yes S-GTx-014 0.5 mg/day5
2 Yes -GTx-014 D.7S mg/day5
13 Yes S-GTx-014 1.0 mglday5
14 Yes S-CrTx-015 0.1 mg/dayS
I S Yes S-GTac-015 D. mg/day 5
16 Yes S-GTx-015 0.5 m~day 5
I7 Yes S-GTx-015 0.75 mg/day5
18 Yes S-GTx-0i5 1.0 mglday5
19 Yes s-GTx-o16 a.! mg/day5
Yes S- Tx-016 0.3 rng/day5
zI es s-GTx-o1s o.s m~/d$y5
22 Yes S-Gxx-016 0.75 mg/day5
23 Yes S-GTx-O1 1.0 mglday5
24 Yes S-GTx 047 0.1 m~day 5
Yes S- Tx-007 4,3 m /day5
26 Yes S-GTx-007 0.5 mglday5
27 Yes S- Tx-007 0.75 mg/day5 i
2$ Yes S-GTx-0 1.0 mg/day5
7
29 Yes None Ve icle 5
only
~o
49
CA 02541871 2001-08-23
EXAMPLE 3
Pharxnacoltinetics of GTx-007 in Dogs
~o 10001201 The pharmacokinetics of S-~xTx-007, s. novel selective androgen
receptor
modulator (SARM), weze characterized in beagle dogs. A. four-treatment, foux-
period
crossover design was utilized in the study, which involved a total of six
beagle dogs,
three of each gender. Each animal received a 3 mg~kg IV dose, a.10 m~kg N
dose, a 10
m~kg PO dose in solution, arid a 10 mglkg p0 dose in capsule, ixr a randomly
assigned
i S order. There was an one-week washout period between treatments. Plasma
samples were
collected for up to 72 hr offer drug administration. plasma S-Gfx-007
concentrations
were analyzed by a. validated HP1.C method. The cIearanae (CL), volume of
distribution
(Vss), half life (T~ia), and other pharmacokinetic parameters were determined
by
noncompartmental methods. R$sults showed that S-GTx-007 was cleared from dog
zo plasma. with a tennibal T~~ of about ~ hr and a CL of 4.4 mL/min/kg a$er rY
administration. Figures 4, 5, and 6 show the plasma aflnoentration-time
prol:iles of S-
GTx-007 after administration of an intravenous sotutiori, oral solution, and
oral capsule,
respectively. The pharmacokinetics were dose. and gender-independent. The oral
bioavailability of S-G'Ik-007 varied with the dosage form, and averaged 3$%
and 19%
z5 for solution and capsule, respectively. Thus, S-GTx-007 demonstrated
modexate half
Iife, slow clearance and moderate bioavailabiiity in beagle dogs, identifying
it as the first
of a new class of orally bioavailable tissue.selactive androgen receptor
mvduiators.
ExaMpL~ a
GTx-U0T Analyses bY PLC
10o0121J A reversed phase high pressuxe liquid chromatograph (~iP?;C) assay
was
SO
CA 02541871 2001-08-23
developed to quantitate GTx-007 concentrations in dog plasma. Dog blood
simples were
obtained by venipuncture and centrifuged at I OOOg for 15 minutes. Samples
were stored
frozen at -20°C until analysis. Individual samples wexe rapidly thawed
and an aliquot
(0.5 ml) was spiked with intezrtal standard (20 ul of a 200 p.g/ml aqueous
solution of
CM-u-87). An aliquot of 1 ml of acetonitrile was added to the samples to
precipitate
plasma proteins. The samples were vortexed and then centrifuged at 10008 for
15
minutes. The supernatant was decanted into glass extraction tubes and 7.5 mi
of ethyl
acetate was added, The e~;traction mixture was left at room temperature for 20
minutes,
and vortexed several times during this interval. The samples were then
centrifuged at
to 10008 for 10 minutes, and the organic phase was removed and placed in
conical-
bottomed glass tubes. The organic phase was evaporated under nitrogen, The
samples
were reconstituted in 200 wl of rnobilG phase (35:65 acetonitrile:water) and
transferred to
an autosampler vial for HPLC injection (Waters 717 plus autosampler, Watexs
Carp.,
Ivlilford, MA). The isoeratic mobile phase of 35% (v/v) acetoriitrile in water
was
~ s puruped at a flow rate of 1 ml/min (Model 510, Waters Corp.). The
stationary phase was
a C18 reversed phase column (N'ovapak C18, 3.9 x 150 mm). Analytes were
monitored
with UV detection at 270 nm (Model 486 absorbance detector, Waters Corp.).
Retention
times for GTx-007 and CM-rI-87 were 11-1 and 16.9 minutes, respectively.
Chromatography data was collected and analyzed using Millennium software.
Plasma
24 concentrations of GTx-D07 in each sample were determined by comparison to
calibration curves. Calibration curves were constructed by adding known
amounts of
CrTx-007 to dog plasma. Final O~'x-007 concentrations in dog plasma samples
used in
the calibration ourves were 0.08, 0.2, 0.4, 2, 4, 10, and 20 ~g/rnl.
Calibration curves
were linear over this concentration range and exhibited correlation
eaefficients (r2) of
25 0.9935 or greater. Infra- and inter-day coeflaacients of variation for the
standards ranged
from 6.4% for 0,08 ~glrnl to 7.9% for 20 pg/ml.
1000'122) Melting points were dBtermined on a Thomas-Hoover capillary melting
point
apparatus and are uncorrected. Infrared spectra were recorded on a Perkin
Elmer System
30 2000 >~T-IR. Optical rotations were determined on an
Autopol°° TII Automatic
polarimeter (Rudolph Research Made1 ~-589-10, p'airfield, New Jersey). Proton
and
carbon-13 magnetic resonance spectra were obtained on a l3ruker AX 300
spectrometer
51
CA 02541871 2001-08-23
(300 and 75 MEIz for tTd and ~aC, respe~tivel~r). Chemical shift values were
reported as
parts per million (b) relative to tetramcthylsilat~e (TMS), Spectral data were
consistent
with assigned structures. Mass spectra were determined on a Broker-~1P Esquire
LC
System. ~lement2G1 atzalyses were performed by Atlantic Microlnb Inc,
(Norcross, GA),
s and found values were within 0.4 % of the theoretical values. )Zoutine thin-
layer
chromatography (TLC) vv~.s performed on silica gel on aluminum plates (silica
gel 60 F
254, 24 x 20 cm, AldriCh Chemical Company Inc., Milwaukee, WT). Flash
chromatography was performed on silica gel (Merck, grade 60, 230-400 mesh,
60).
Tetrahydrofuran (THp) was dried by distillation over sodium metal.
Acetonitrile
Io (MeCI~ and methylene chloride (CH2C12) were dried by distillation tom PzCs.
52