Note: Descriptions are shown in the official language in which they were submitted.
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
I PREPARING SMALL CRYSTAL SSZ-32 AND ITS USE IN
2 A HYDROCARBON CONVERSION PROCESS
3
4 FIELD OF THE INVENTION
6, This invention is directed to a method of making a catalyst comprising a
small
7 crystal intermediate pore size zeolite, specifically SSZ-32. The catalyst is
8 suitable for use in isomerizing a feed which includes straight chain and
slightly
9 branched paraffins having 10 or more carbon atoms.
11 BACKGROUND OF THE INVENTION
12
13 The production of Group II and Group III base oils employing
hydroprocessing
14 has become increasing popular in recent years. Catalysts that demonstrate
improved isomerization selectivity and conversion are continually sought. As
16 discussed in U.S. Pat. No. 5,282,958, col. 1-2, the use of intermediate
pore
17 molecular sieves such as ZSM-22, ZSM-23,ZSM-35,SSZ-32, SAPO-1 1,
18 SAPO-31,SM-3,SM-6 in isomerization and shape-selective dewaxing is well-
19 known. Other typical zeolites useful in dewaxing include ZSM-48, ZSM-57,
SSZ-20,EU-I, EU-13, Ferrierite, SUZ-4, theta-1, NU-10, NU-23, NU-87,ISI-1,
21 ISI-4,KZ-1,and KZ-2.
22
23 U.S. Pat. No. 5,252,527 and 5,053,373 disclose a zeolite such as SSZ-32
24 which is prepared using an N-lower alkyl-N'-isopropyl-imidazolium cation as
a
template. 5,053,373 discloses a silica to alumina ratio of greater than 20 to
26 less than 40 and a constraint index, after calcination and in the hydrogen
form
27 of 13 or greater. The zeolite of 5,252,527 is not restricted to a
constraint
28 index of 13 or greater. 5,252,527 discloses loading zeolites with metals in
29 order to provide a hydrogenation- dehydrogenation function. Typical
replacing cations can include hydrogen ammonium, metal cations, e.g., rare
31 earth, Group IIA and Group VIII metals, as well as their mixtures. A method
32 for preparation of MTT-type zeolites such as SSZ-32 or ZSM-23 using small
33 neutral amines is disclosed in U.S. Pat. No. 5,707,601.
-1-
CA 02543283 2010-11-18
1 U. S. Pat. No. 5,397,454 discloses hydroconversion processes employing a
2 zeolite such as SSZ-32 which has a small crystallite size and a constraint
3 index of 13 or greater, after calcinations and in the hydrogen form. The
4 catalyst possesses a silica to alumina ratio of greater than 20 and less
than
40. U. S. Pat. No. 5,300,210 is also directed to hydrocarbon conversion
6 processes employing SSZ-32. The SSZ-32 of U. S. Pat. No. 5,300,210 is not
7 limited to a small crystal size.
8
9 SUMMARY OF THE INVENTION
11 The instant invention is directed to a small crystal SSZ-32 zeolite
(hereinafter
12 referred to as SSZ-32X) which is suitable for dewaxing a hydrocarbon feed
to
13 produce an isomerized product. It is also directed to a method of making
this
14 zeolite, and to dewaxing processes employing catalyst comprising SSZ-32X.
The feed to the process includes straight chain and slightly branched
paraffins
16 having 10 or more carbon atoms. The feed is contacted under isomerization
17 conditions in the presence of hydrogen with a catalyst comprising an SSZ-
18 32X. This catalyst possesses, in comparison with standard SSZ-32, less
19 defined crystallinity, altered Argon adsorption ratios, increased external
surface area and reduced cracking activity over other intermediate pore size
21 molecular sieves used for isomerization. Use of this catalyst in
isomerization
22 results in a higher lube product yield and lower gas production.
23
24 In accordance with another aspect, there is provided a zeolite having a
mole
ratio of silicon oxide to aluminum oxide greater than about 20:1 to less than
26 40:1, with crystallites having lath-like components in the range of 200-
400A,
27 and having the x-ray diffraction lines of Table 2(b) and wherein the
zeolite is
28 SSZ-32X.
29
In accordance with a further aspect, there is provided a method of preparing a
31 dewaxing catalyst suitable for use in a process for dewaxing a hydrocarbon
32 feed to produce an isomerized product, said catalyst possessing less
defined
33 crystallinity, reduced micropore volume, increased surface area and reduced
-2-
CA 02543283 2010-11-18
1 cracking activity over other intermediate pore size molecular sieves used
for
2 isomerization, the feed including straight chain and slightly branched chain
3 paraffins having 10 or more carbon atoms, the method of preparation
4 comprising the following steps:
(a) synthesizing a zeolite having a mole ratio of silicon oxide to
6 aluminum oxide greater than about 20:1 to less than 40:1, with
7 rystallites having lath-like components in the range of 200-400A,
8 and having the x-ray diffraction lines of Table 2(b), and wherein
9 the zeolite is SSZ-32X, by employing the following steps:
(i) combining the following reagents in the amounts
11 specified to form a mixture:
12 (1) 5 parts of an N-lower alkyl-N-methyl-N'-
13 isopropyl-imidazolium cation which has
14 been ion-exchanged to the hydroxide form;
(2) 20 parts of an alkali metal hydroxide;
16 (3) 100 parts of SiO2 to 3.5 parts of A1203; and
17 (4) 20 parts of an alkyl amine;
18 (ii) stirring the mixture of step (i) in an autoclave,
19 under autogenous pressure, in a range of from 500
to 1500 rpm for a period of from 0 to 5 hours;
21 (iii) maintaining the mixture from about 140 C to about
22 200 C for a period of from 40 to 120 hours to form
23 the crystals of the zeolite;
24 (iv) collecting the crystals of the zeolite by filtration or
centrifugation; and
26 (v) subjecting the crystals to calcinations and ion-
27 exchange;
28 (b) mixing the zeolite synthesized in stage (a) with a refractory
29 inorganic oxide carrier precursor and an aqueous solution to
form a mixture, the mixture having a molecular sieve content
31 from about 10 to about 90 wt%;
32 (c) extruding or forming the mixture from step (b) to form an
33 extrudate or formed particle;
-2a-
CA 02543283 2010-11-18
1 (d) drying the extrudate or formed particle of step (c);
2 (e) calcining the dried extrudate or formed particle of step (d);
3 (f) loading of the extrudate or formed particle of step (d) with a
4 hydrogenation component or other modifying metal or metals to
prepare a catalyst precursor, where other modifying metals are
6 selected from the groups consisting of magnesium, lanthanum,
7 and other rare earth metals, barium, sodium, praseodymium,
8 strontium, potassium, neodymium and calcium;
9 (g) drying the catalyst precursor of step (f); and
(h) calcining the dried catalyst precursor of step (f) to form a
11 finished bound dewaxing catalyst.
12
13 In accordance with another aspect, there is provided a zeolite prepared
from
14 an aqueous solution having a composition, as synthesized and in the
anhydrous state, in terms of mole ratios of oxides as follows: (0.05 to
16 2.0)Q20:(0.1 to 2.0)M20: AI2O3 (20 to less than 40) SiO2 wherein M is an
alkali
17 metal cation, and Q is the sum of Qa an N-lower alkyl-N'-
isopropylimidazolium
18 cation, and Qb, an amine, the zeolite having the X-ray diffraction lines of
Table
19 2(b), wherein the molar concentration of Qb is greater than the molar
concentration of Qa.
21
22 In accordance with a further aspect, there is provided a process for
dewaxing
23 a hydrocarbon feed thereby producing a maximized yield of isomerized
24 product and a minimized yield of light ends, the feed including straight
chain
and slightly branched paraffins having 10 or more carbon atoms, comprising
26 contacting the feed under isomerization conditions in the presence of
27 hydrogen with catalyst comprising an intermediate pore size molecular sieve
28 which is prepared according to the following steps:
29 (a) synthesizing a zeolite having a mole ratio of silicon oxide to
aluminum oxide greater than about 20:1 to less than 40:1, with
31 crystallites having lath-like components in the range of 200-
32 400A, and having the x-ray diffraction lines of Table 2(b), and
-2b-
CA 02543283 2010-11-18
1 wherein the zeolite is SSZ-32X, by employing the following
2 steps:
3 (i) combining the following reagents in the amounts
4 specified to form a mixture:
(1) 5 parts of an N-lower alkyl-N-methyl-N'-isopropyl-
6 imidazolium cation which has been ion-exchanged
7 to the hydroxide form;
8 (2) 20 parts of an alkali metal hydroxide;
9 (3) 100 parts of SiO2 to 3.5 parts of A1203; and
(4) 20 parts of an alkyl amine;
11 (ii) stirring the mixture of step (i) in an autoclave, under
12 autogenous pressure, in a range of from 500 to 1500 rpm
13 for a period of 0.5 to 5 hours;
14 (iii) maintaining the mixture from about 140 C to about 200 C
for a period of from 40 to 120 hours to form the crystals of
16 the zeolite;
17 (iv) collecting the crystals of the zeolite by filtration or by
18 centrifugation; and
19 (v) subjecting the crystals to calcination and ion-exchange;
(b) mixing the zeolite synthesized in stage (a) with a refractory
21 inorganic oxide carrier precursor and an aqueous solution to
22 form a mixture, the mixture having a molecular sieve content
23 from about 10 to about 90 wt%;
24 (c) extruding or forming the mixture from step (b) to form an
extrudate or formed particle;
26 (d) drying the extrudate or formed particle of step (c);
27 (e) calcining the dried extrudate or formed particle of step (d);
28 (f) loading of the extrudate or formed particle of step (d) with a
29 hydrogenation component to prepare a catalyst precursor;
(g) drying the catalyst precursor of step (f); and
31 (h) calcining the dried catalyst precursor of step (f) to form a
32 finished bound dewaxing catalyst.
-2c-
CA 02543283 2010-11-18
1 BRIEF DESCRIPTION OF THE FIGURES
2
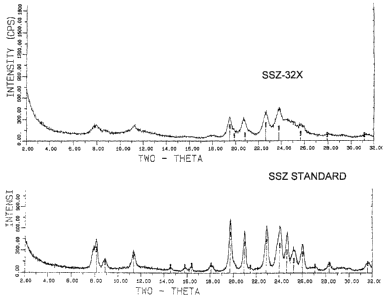
3 Figures 1(a), 1(b) and 1(c) illustrate comparisons between the x-ray
diffraction
4 patterns of standard SSZ-32 and SSZ-32X.
6 Figures 2(a) and 2(b) illustrate a comparison of yield and VI
characteristics for
7 use of SSZ-32X in Isodewaxing, compared with that of standard SSZ-32.
-2d-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
I DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
2
3 Catalyst Preparation
4
Novel SSZ-32 zeolites can be suitably prepared from an aqueous solution
6 containing sources of an alkali metal oxide or hydroxide, an alkylamine
(such
7 as isobutylamine) an N-lower alkyl-N'- isopropyl-imidazolium cation
8 (preferably N,N'-diisopropyl-imidazolium cation or N-methyl -N'-isopropyl-
9 imidazolium cation) which is subsequently ion-exchanged to the hydroxide
form, an oxide of aluminum(preferably wherein the aluminum oxide source
11 provides aluminum oxide which is covalently dispersed on silica), and an
12 additional oxide of silicon. The reaction mixture should have a composition
in
13 terms of mole ratios falling within the following ranges:
14
Table I - Composition of mole ratios
16
Broad Preferred
Si02 /AI2O3 20-less than 40 30-35
OH- /Si02 0.10-1.0 0.20-0.40
Q/Si02 0.05-0.50 0.10-0.25
M+ /SiO2 0.05-0.30 0.15-0.30
H2 O/Si02 20-300 25-60
Q/Q+M+ 0.25-0.75 0.33-0.67
17
18 wherein Q is the sum of Qa and Qb.
19
Qa is an N-lower alkyl-N'-isopropyl-imidazolium cation (preferably an N,N'-
21 diisopropyl-imidazolium cation or N-methyl-N'- isopropyl-imidazolium
cation).
22 Qb is an amine. Isobutyl,neopentyl or monoethyl amine are suitable examples
23 of Qb, although other amines may be used. The molar concentration of
24 amine, Qb must be greater than the molar concentration of the imidazolium
compound, Qa. Generally the molar concentration of Qb is in the range from 2
26 to about nine times the molar concentration of Qa. U.S. Pat No. 5,785,947
-3-
CA 02543283 2010-11-18
1 describes how a zeolite synthesis method employing two organic sources,
2 one source being an amine containing from one to eight carbons provides
3 significant cost savings over a method in which the quaternary ammonium
4 ion source (such as imidazolium) is the only source of organic component.
The combination of the 2 organic nitrogen sources allows the possibility
6 of the primary template (used in smaller quantity) to nucleate the desired
7 zeolite structure and then the amine to contribute to fitting the pores in a
8 stabilizing manner, during crystal growth. Empty pores of high silica
9 zeolites are susceptible to re-dissolution under the synthesis, conditions.
The amine also can contribute to maintaining an elevated alkalinity for the
11 synthesis.
12
13 M is an alkali metal ion, preferably sodium or potassium. The organic
cation
14 compound which acts as a source of the quaternary ammonium ion employed
can provide hydroxide ion.
16
17 The cation component Q, of the crystallization mixture, is preferably
derived
18 from a compound of the formula
22 G~Ig
24 i-N N- c A.
26 o3s
27
28 wherein R is lower alkyl containing 1 to 5 carbon atoms and preferably -CH3
29 or isopropyl and an anion (AO) which is not detrimental to the formation of
the
zeolite. Representative of the anions include halogens, e.g., fluoride,
31 chloride, bromide and iodide, hydroxide, acetate, sulfate, carboxylate,
etc.
32 Hydroxide is the most preferred anion.
33
34 The reaction mixture is prepared using standard zeolitic preparation
techniques. Typical sources of aluminum oxide for the reaction mixture
36 include aluminates, alumina, and aluminum compounds, such as aluminum-
-4-
CA 02543283 2010-11-18
1 coated silica colloids (preferably NalcoTM 1056 colloid sol although other
2 brands may be used) AI2 (SO4.)3, and other zeolites.
3
4 In a preferred method of preparing zeolite SSZ-32X, we have found that
providing sources of aluminum oxide to a zeolite synthesis mixture wherein
6 the aluminum oxide is in a covalently dispersed form on silica allows
zeolites
7 with increased aluminum content to be crystallized. Increased alumina
content
8 promotes isomerization. In another approach zeolites of pentasil structure
9 and lower silica/alumina ratios (approximately 10) can be used as aluminum
oxide sources or feedstocks for the synthesis of zeolite SSZ-32X. These
11 zeolites are recrystallized to the new SSZ-32X zeolite in the presence of
the
12 organic sources Qa and Qb described above.
13
14 Mordenite and ferrierite zeolites constitute two such useful sources of
aluminum oxide or feedstocks. These latter zeolites have also been used in
16 the crystallization of ZSM-5 and ZSM-1 1 (U.S. Pat. No. 4,503,024).
17
18 In another preferred approach, wherein the aluminum oxide is in a
covalently
19 dispersed form on silica is to use an alumina coated silica sol such as
that
manufactured by Nalco Chem. Co. under the product name 1056 colloid sol
21 (26% silica, 4% alumina). In addition to providing novel SSZ-32 X with
22 high aluminum content, use of the sol generates crystallites of less Thank
you
23 1000A (along the principal axis) with surprisingly high isomerization
capability.
24
Indeed, the catalytic performance of SSZ-32X (in the hydrogen form) for
26 cracking capability is manifested by Constraint Index values (as defined in
J.
27 Catalysis 67, page 218) of 13 or greater and preferably from 13 to 22.
28 Determination of Constraint index is also disclosed in U. S. Pat. No. 4,
29 481,177. In general, lowering the crystallite size of a zeolite leads to
decreased shape selectivity. This has been demonstrated for ZSM-5 reactions
31 involving aromatics as shown in J. Catalysis 99,327 (1986). In addition, a
32 zeolite ZSM-22, (U.S. Pat. No. 4,481,177) has been found to be closely
33 related to ZSM-23 (J. Chem. Soc. Chem. Comm. 1985 page 1117). In the
-5-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 above reference on ZSM-22 it was shown that ball-milling the crystallites
2 produced a catalyst with a constraint index of 2.6. This is a surprisingly
low
3 value for this material given other studies which indicate that it is a very
4 selective 10-ring pentasil (Proc. of 7th Intl. Zeolite Conf. Tokyo, 1986,
page
23). Presumably the ballmilling leads to a less selective but more active
6 catalyst, by virtue of producing smaller crystallites. So it is surprising
here,
7 that smaller crystallites maintain high selectivity in the case of SSZ-32X.
8 Typical sources of silicon oxide include silicates, silica hydrogel, silicic
acid,
9 colloidal silica, fumed silicas, tetraalkyl orthosilicates, and silica
hydroxides.
Salts, particularly alkali metal halides such as sodium chloride, can be added
11 to or formed in the reaction mixture. They are disclosed in the literature
as
12 aiding the crystallization of zeolites while preventing silica occlusion in
the
13 lattice.
14
The reaction mixture is maintained at an elevated temperature until the
16 crystals of the zeolite are formed. The temperatures during the
hydrothermal
17 crystallization step are typically maintained from about 140 C. to about
200
18 C., preferably from about 160 C. to about 180C. and most preferably from
19 about 170. degree. C. to about 180 C. The crystallization period is
typically
greater than 1 day and preferably from about 4 days to about 10 days.
21
22 The hydrothermal crystallization is conducted under pressure and usually in
23 an autoclave so that the reaction mixture is subject to autogenous
pressure.
24 The reaction mixture can be stirred while components are added as well as
during crystallization.
26
27 Once the zeolite crystals have formed, the solid product is separated from
the
28 reaction mixture by standard mechanical separation techniques such as
29 filtration or centrifugation. The crystals are water-washed and then dried,
e.g.,
at 90 C. to 150 C. for from 8 to 24 hours, to obtain the as-synthesized,
31 zeolite crystals. The drying step can be performed at atmospheric or
32 subatmospheric pressures.
33
-6-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 During the hydrothermal crystallization step, the crystals can be allowed to
2 nucleate spontaneously from the reaction mixture. The reaction mixture can
3 also be seeded with SSZ-32 crystals both to direct, and accelerate the
4 crystallization, as well as to minimize the formation of undesired
aluminosilicate contaminants.
6
7 SSZ-32X can be used as-synthesized or can be thermally treated (calcined).
8 Usually, it is desirable to remove the alkali metal cation by ion exchange
and
9 replace it with hydrogen, ammonium, or any desired metal ion. The zeolite
can be leached with chelating agents, e.g., EDTA or dilute acid solutions, to
11 increase the silica alumina mole ratio. SSZ-32X can also be steamed.
12 Steaming helps stabilize the crystalline lattice to attack from acids.
13
14 SSZ-32X can be used in intimate combination with hydrogenating
components for those applications in which a hydrogenation-dehydrogenation
16 function is desired. Typical replacing components can include hydrogen,
17 ammonium, metal cations, e.g. rare earth, Group IIA and Group VII metals,
as
18 well as their mixtures. Preferred hydrogenation components include
tungsten,
19 vanadium, molybdenum, rhenium, nickel, cobalt, chromium, manganese,
platinum, palladium (or other noble metals).
21
22 Metals added to affect the overall functioning of the catalyst (including
23 enhancement of isomerization and reduction of cracking activity) include
24 magnesium, lanthanum (and other rare earth metals), barium, sodium,
praseodymium, strontium, potassium and neodymium. Other metals that
26 might also be employed to modify catalyst activity include zinc, cadmium,
27 titanium, aluminum, tin, and iron.
28
29 Hydrogen, ammonium as well as metal components can be exchanged into
SSZ-32X. The zeolite can also be impregnated with the metals, or, the metals
31 can be physically intimately admixed with SSZ-32X using standard methods
32 known to the art. And, the metals can be occluded in the crystal lattice by
-7-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 having the desired metals present as ions in the reaction mixture from which
2 the SSZ-32 zeolite is prepared.
3
4 Typical ion exchange techniques involve contacting the SSZ-32X with a
solution containing a salt of the desired replacing cation or cations.
Although
6 a wide variety of salts can be employed, chlorides and other halides,
nitrates,
7 and sulfates are particularly preferred. Representative ion exchange
8 techniques are disclosed in a wide variety of patents including U.S. Nos.
9 3,140,249; 3, 140,251; and 3, 140,253. Ion exchange can take place either
before or after SSZ-32X is calcined.
11
12 Following contact with the salt solution of the desired replacing cation,
SSZ-
13 32X is typically washed with water and dried at temperatures ranging from
65
14 C. to about 315 C. After washing, SSZ-32X can be calcined in air or inert
gas
at temperatures ranging from about 200 C. to 820 C. for periods of time
16 ranging from 1 to 48 hours, or more, to produce a catalytically active
product
17 especially useful in hydrocarbon conversion processes.
18
19 The SSZ-32X zeolite described above is converted to its acidic form and
then
is mixed with a refractory inorganic oxide carrier precursor and an aqueous
21 solution to form a mixture. The aqueous solution is preferably acidic. The
22 solution acts as a peptizing agent. The carrier (also known as a matrix or
23 binder) may be chosen for being resistant to the temperatures and other
24 conditions employed in organic conversion processes. Such matrix materials
include active and inactive materials and synthetic or naturally occurring
26 zeolites as well as inorganic materials such as clays, silica and metal
oxides.
27 The latter may occur naturally or may be in the form of gelatinous
precipitates,
28 sols, or gels, including mixtures of silica and metal oxides. Use of an
active
29 material in conjunction with the synthetic SSZ-32X i.e., combined with it,
tends
to improve the conversion and selectivity of the catalyst in certain organic
31 conversion processes.
32
-8-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 SSZ-32X is commonly composited with porous matrix materials and mixtures
2 of matrix materials such as silica, alumina, titania, magnesia, silica-
alumina,
3 silica- magnesia, silica-zirconia, silica-thoria, silica-beryllia, silica-
titania,
4 titania-zirconia as well as ternary compositions such as silica- alumina-
thoria,
silica-alumina-zirconia, silica-alumina-magnesia and silica-magnesia-zirconia.
6 The matrix can be in the form of a cogel. In the instant invention, the
7 preferred matrix materials are alumina and silica. It is possible to add
metals
8 for the enhancement of catalytic performance, during the actual synthesis of
9 SSZ-32X, as well as during later steps in catalyst preparation. Methods of
preparation include solid state ion exchange which is achieved by thermal
11 means, spray drying with a metal salt solution, and preparation of a slurry
in a
12 salt solution. The slurry may be filtered to retrieve the SSZ-32X, now
loaded
13 with metal.
14
Inactive materials can suitably serve as diluents to control the amount of,
16 conversion in a given process so that products can be obtained economically
17 without using other means for controlling the rate of reaction. Frequently,
18 zeolite materials have been incorporated into naturally occurring clays,
e.g.,
19 bentonite and kaolin. These materials e.g. clays, oxides, etc., function,
in
part, as binders for the catalyst. It is desirable to provide a catalyst
having
21 good crush strength, because in petroleum refining the catalyst is often
22 subjected to rough handling. This tends to break the catalyst down into
23 powders which cause problems in processing.
24
Naturally occurring clays which can be composited with the synthetic SSZ-
26 32X of this invention include the montmorillonite and kaolin families,
which
27 families include the sub-bentonites and the kaolins commonly known as
Dixie,
28 McNamee, Georgia and Florida clays or others in which the main mineral
29 constituent is halloysite, kaolinite, dickite, nacrite, or anauxite.
Fibrous clays
such as sepiolite and attapulgite can also be used as supports. Such clays
31 can be used in the raw state as originally mined or can be initially
subjected to
32 calcination, acid treatment or chemical modification.
33
-9-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
I The mixture of SSZ-32X and binder can be formed into a wide variety of
2 physical shapes. Generally speaking, the mixture can be in the form of a
3 powder, a granule, or a molded product, such as an extrudate having a
4 particle size sufficient to pass through a 2.5-mesh (Tyler) screen and be
retained on a 48-mesh (Tyler) screen. In cases where the catalyst is molded,
6 such as by extrusion with an organic binder, the mixture can be extruded
7 before drying, or dried or partially dried and then extruded. SSZ-32X can
also
8 be steamed. Steaming helps stabilize the crystalline lattice to attack from
9 acids. The dried extrudate is then thermally treated, using calcination
procedures.
11
12 Calcination temperature may range from 390 to 1100 F. Calcination may
13 occur for periods of time ranging from 0.5 to 5 hours, or more, to produce
a
14 catalytically active product especially useful in hydrocarbon conversion
processes.
16
17 The extrudate or particle may then be further loaded using a technique such
18 as impregnation, with a Group VIII metal to enhance the hydrogenation
19 function. It may be desirable to coimpregnate a modifying metal and Group
VIII metal at once, as disclosed in U.S. Pat. No. 4,094,821. The Group VIII
21 metal is preferably platinum, palladium or a mixture of the two. After
loading ,
22 the material can be calcined in air or inert gas at temperatures from 500
to
23 900 F.
24
Feeds
26
27 The instant invention may be used to dewax a variety of feedstocks ranging
28 from relatively light distillate fractions such as kerosene and jet fuel up
to high
29 boiling stocks such as whole crude petroleum, reduced crudes, vacuum tower
residua, cycle oils, synthetic crudes (e.g., shale oils, tars and oil, etc.),
gas
31 oils, vacuum gas oils, foots oils, Fischer-Tropsch derived waxes, and other
32 heavy oils. Straight chain n-paraffins either alone or with only slightly
33 branched chain paraffins having 16 or more carbon atoms are sometimes
-10-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
I referred to herein as waxes. The feedstock will often be a C10+ feedstock
2 generally boiling above about 350 F,since lighter oils will usually be free
of
3 significant quantities of waxy components. However, the process is
4 particularly useful with waxy distillate stocks such as middle distillate
stocks
including gas oils, kerosenes, and jet fuels, lubricating oil stocks, heating
oils
6 and other distillate fractions whose pour point and viscosity need to be
7 maintained within certain specification limits. Lubricating oil stocks will
8 generally boil above 230 C. (450 F.), more usually above 315 C. (600 F.).
9 Hydroprocessed stocks are a convenient source of stocks of this kind and
also of other distillate fractions since they normally contain significant
11 amounts of waxy n- paraffins. The feedstock of the present process will
12 normally be a C10 + feedstock containing paraffins, olefins, naphthenes,
13 aromatic and heterocyclic compounds and with a substantial proportion of
14 higher molecular weight n- paraffins and slightly branched paraffins which
contribute to the waxy nature of the feedstock. During the processing, the n-
16 paraffins and the slightly branched paraffins undergo some cracking or
17 hydrocracking to form liquid range materials which contribute to a low
18 viscosity product. The degree of cracking which occurs is, however, limited
19 so that the yield of products having boiling points below that of the
feedstock
is reduced, thereby preserving the economic value of the feedstock.
21
22 Typical feedstocks include hydrotreated or hydrocracked gas oils,
23 hydrotreated lube oil raffinates, brightstocks, lubricating oil stocks,
synthetic
24 oils, foots oils, Fischer-Tropsch synthesis oils, high pour point
polyolefins,
normal alphaolefin waxes, slack waxes, deoiled waxes and microcrystalline
26 waxes.
27
28 Conditions
29
The conditions under which the isomerization/dewaxing process of the
31 present invention is carried out generally include a temperature which
falls
32 within a range from about 392 F to about 800 F, and a pressure from about
33 15 to about 3000 psig. More preferably the pressure is from about 100 to
-11-
CA 02543283 2010-11-18
1 about 2500 psig. The liquid hourly space velocity during contacting is
2 generally from about 0.1 to about 20, more preferably from about 0.1 to
about
3 5. The contacting is preferably carried out in the presence of hydrogen. The
4 hydrogen to hydrocarbon ratio preferably falls within a range from about
2000
to about 10,000 standard cubic feet H2 per barrel hydrocarbon, more
6 preferably from about 2500 to about 5000 standard cubic feet H2 per barrel
7 hydrocarbon.
8
9 The product of the present invention may be further treated as by
hydrofinishing. The hydrofinishing can be conventionally carried out in the
11 presence of a metallic hydrogenation catalyst, for example, platinum on
12 alumina. The hydrofinishing can be carried out at a temperature of from
about
13 374 F to about 644 F and a pressure of from about 400 psig to about 3000
14 psig. Hydrofinishing in this manner is described in, for example, U. S.
Pat.
3,852, 207.
16
17 EXAMPLES
18
19 The synthesis of a broadline zeolite (in reference to the x-ray diffraction
pattern) SSZ-32X is really synonymous with crystallizing a very small crystal
21 example of the zeolite. The x-ray diffraction pattern broadens as the
22 crystallites are reduced in size. In general, for the system of MTT
structure
23 zeolites, of which standard SSZ-32 as well as SSZ-32X are examples, as the
24 Si02/AI2O3 ratio diminishes (greater wt% Al in the zeolite product) the
crystallite size also diminishes.
26
27 Figure 1(a) compares the SSZ-32X peak occurrence and relative intensity
28 with that of standard SSZ-32. Relative intensity is obtained when an
intensity
29 value is divided by a reference intensity and multiplied by 100%, or 100 x
I/lo.
31 Figures 1(b) and (c) superimpose the SSZ-32X plot on the standard SSZ-32
32 plot, clearly showing the match up of major peaks. Figure 1(c) shows a more
33 detailed portion of Figure 1(b). Table 2(a) shows the peak listing and
relative
-12-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 intensity of peaks of standard SSZ-32. Table 2(b) magnifies peak width so
2 that major peaks of SSZ-32X and standard SSZ-32 may be easily compared.
3
4 Table 2(a) - Peak listing of standard SSZ-32
2 Theta d-spacing Relative Intensity (%)
(A) (1/10) x 100
7.9 11.2 19
8.2 10.8 24
8.9 10.0 11
11.4 7.8 20
14.7 6.05 2
15.9 5.59 5
11.4 5.41 4
18.2 4.88 12
19.6 4.52 69
20.1 4.43 11
20.9 4.25 70
21.4 4.15 9
22.8 3.90 100
23.9 3.73 53
24.0 3.70 58
24.7 3.61 50
25.2 3.53 36
26.0 3.43 42
28.2 3.16 11
29.4 3.03 7
31.6 2.83 13
6
-13-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 Table 2(b) - Peaks in as-made SSZ-32X
2
2 Theta d-spacing Relative
(A) Intensity (%)
(1/10 x 100)
8.03 11.0 33
8.83 10.0 6
11.30 7.83 20
15.71 5.64 3
16.34 5.42 3
18.09 4.90 7
19.54 4.54 33
19.67 4.51 20
20.81 4.27 31
21.21 4.18 14
22.74 3.91 63
23.91 3.72 100
24.54 3.62 24
25.09 3.55 34
25.87 3.44 31
26.91 3.31 5
28.10 3.17 4
29.34 3.04 5
31.46 2.84 8
31.94 2.80 3
34.02 2.63 1
35.22 2.55 17
36.29 2.47 16
3
-14-
CA 02543283 2010-11-18
1 EXAMPLE I SYNTHESIS OF SSZ-32X
2
3 A preparation of the desired material was synthesized as follows: A
Hastelloy
4 C liner for a 5 gallon autoclave unit was used for the mixing of reagents
and
then in the subsequent thermal treatment. At a rate of 1500 RPM and for a
6 period of 1/2 hour, the following components were mixed once they had been
7 added in the order of description. 300 grams of a 1 Molar solution of N,N'
8 Diisopropyl imidazolium hydroxide was mixed into 4500 grams of water. The
9 salt iodide was prepared as in US 4,483,835, Example 8, and then
subsequently was ion-exchanged to the hydroxide form using BioRadTM AG 1-
11 X8 exchange resin. 2400 grams of 1 N KOH were added. 1524 grams of
12 LudoxTM AS-30 (30wt% SO2) were added. 1080 grams of NaIcoTM 1056
13 colloid sol (26wt% S102 and 4 wt% A1203) were added. Last, 181 grams of
14 isobutylamine were stirred into the mixture. In general, the molar
concentration of the amine Qb must exceed the molar concentration of the
16 imidazolium compound, Qa.
17
18 Once the stirring was finished the autoclave head was closed up and the
19 reaction was taken up to 170 C with an 8 hour ramp up time. The system was
stirred at 150 RPM. The reaction was terminated so that a product was
21 collected after 106 hours of heating. The solids were collected by
filtration
22 (which goes very slowly; an indication of small crystals). It was
subsequently
23 washed several times and then dried. The material was analyzed by x-ray
24 diffraction and the pattern is shown in Table 3. A comparison is made with
the more standard SSZ-32 data presented in Table 2(a) and it can be seen
26 that the new product of Example 1 is essentially related to SSZ-32 but has
the
27 diffraction lines considerably broadened.
-15-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 Table 3
2
20 d-spacing Intensity Relative
(A) Intensity (%)
(1/lo x 100)
8.00 11.05 15 26
8.80 10.05 6 10
11.30 7.83 10 17
14.50 6.11 1 2
15.75 5.63 3 5
16.50 5.37 3 5
18.10 4.901 7 12
19.53 4.545 41 71
20.05 4.428 6 shoulder 10 shoulder
20.77 4.277 41 71
21.30 4.171 7 12
22.71 3.915 58 100
23.88 3.726 57 98
24.57 3.623 30 52
25.08 3.551 25 43
25.88 3.443 27 47
26.88 3.317 5 9
28.11 3.174 6 10
3
4 In a concern that the product might be a mix of small crystals and
considerable amorphous material, a TEM (Transmission Electron Microscopy)
6 analysis was carried out. The microscopy work demonstrated that the product
7 of Example I was quite uniformly small crystals of SSZ-32 (the product was
8 SSZ-32X) with very little evidence of amorphous material. The crystallites
-16-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 were characterized by a spread of small, broad lathe-like components in the
2 range of 200-400 Angstroms. The Si02/Al203 ratio of this product was 29.
3
4 EXAMPLE 2
6 The product of Example 1 was calcined to 1100 F in air with a ramp of 1 deg.
7 C/min(1.8F/min) and plateaus of 250 F for 3 hours, 1000 F for 3 hours and
8 then 1100 F for 3 hours. The calcined material retained its x-ray
crystallinity.
9 The calcined zeolite was subjected to 2 ion-exchanges at 200 F (using NH4
NO3) as has been previously described in US Pat. No. 5,252,527. The ion-
11 exchanged material was recalcined and then the microporosity measurements
12 were explored, using a test procedure also described in 5,252,527. The new
13 product, SSZ-32X, had some unexpected differences vs. conventional SSZ-
14 32.
16 The Ar adsorption ratio for SSZ-32X (Ar adsorption at 87K between the
17 relative pressures of 0.001 and 0.1)/(total Ar adsorption up to relative
18 pressure of 0.1) is larger than 0.5 and preferably in the range of 0.55 to
0.70.
19 In contrast for the conventional SSZ-32, the Ar adsorption ratio is less
than
0.5, typically between 0.35 and 0.45. The SSZ-32X of Examples I and 2
21 demonstrates an Argon absorption fraction of 0.62. The external surface
area
22 of the crystallites jumped from about 50 m2/g (SSZ-32) to 150 (SSZ-32X),
23 indicating the considerable external surface as a result of very small
crystals.
24
At the same time, the micropore volume for SSZ-32X had dropped to about
26 0.035 cc/gm, as compared with about 0.06cc/gm for standard SSZ-32.
27
28 EXAMPLE 3
29
The ion-exchanged SSZ-32X was tested for cracking activity via the use of
31 the Constraint Index test. This test was an important parameter in showing
32 the unique selectivity of zeolite SSZ-32X. The test is described in U.S.
Pat.
33 No. 5252527, Example 9. Using the test conditions, standard SSZ-32
-17-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 typically provides a Constraint Index of 13-22 at 50% conversion and 800 F.
2 The product of Example 2 of the instant application, when run under the same
3 procedures yields a much lower conversion of about 12% while maintaining
4 shape-selective behavior.
6 Table 4 -- Characteristics
7 Comparison between standard SSZ-32 and SSZ-32X
8
Standard SSZ-32 SSZ-32X
Diisopropylimidazolium/silica 0.00 0.05
Product Silica/alumina ratio 35 28-30
Product Ar adsorption fraction* (0.35 - 0.45) (0.55 - 0.70)
Constraint index conversion Test 50% 12%
Crystal size, microns 0.17 0.01-0.04
9
* Ar adsorption at 87k between the relative pressures of 0.001 and 0.1) /
(total
11 Ar adsorption up to the relative pressure of 0.1)
12
13 EXAMPLE 4
14
The next surprise concerning this material came from the testing of
16 isomerization capability using n-hexadecane as feed and Pd metal on the
17 product from Example 2 of the instant invention. Pd ion-exchange was
18 carried out as was previously described in U.S. Pat. No. 5,282,958, Example
19 1. The catalyst was tested using the procedure described in U.S. Pat. No.
5,282,958, Example 1. Both the new catalyst and a standard SSZ-32 powder
21 at comparable Si02/AI203 gave about 96% conversion of n-C16 at 545 F. The
22 difference is that the new material of Example 2 of the instant invention
gave
23 an isomerization selectivity of 75.8% vs. a value of 64% for standard SSZ-
32,
24 as disclosed in U.S. Pat. No. 5,282,958, Example 1. So even though the
zeolite was shown to have considerably less cracking activity in Example 3,
26 the activity for hydroconversion equals that of the standard SSZ-32 and the
-18-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
I selectivity is considerably better. One of the main distinctions between the
2 catalysts was that the standard SSZ-32 produces about 13% material which is
3 C6 and lower, and the new zeolite reduces that number to 7%. Liquid yield is
4 increased and light end production is reduced.
6 EXAMPLE 5
7
8 The catalyst of Example 4 was made, in this instance with Pt rather than Pd.
9 Calcination of the Pt catalyst was at 550 F for 3 hours. 8 cc of catalyst
chips
(24-42 mesh size) were measured out and packed into a stainless steel
11 reactor after drying overnight in air at 500 F. The catalyst was then
reduced
12 at 500 F in flowing H2 at 2300 psig for 1 hour. After reduction of the
metal, the
13 catalyst was used to isomerize a waxy light neutral hydrocrackate feed, API
14 38.9, having a 33% wax content, and a pour point of 38 C. The whole liquid
product from the reactor was split in a stripper into two fractions. The
bottoms
16 product target was a -15 C pour point. A standard SSZ-32 catalyst was also
17 prepared and treated in an analogous manner. The data in Table 5 below
18 shows the improvement which is gained upon using the new SSZ-32X as an
19 isomerization catalyst. Once again a main advantage seems to be reduced
light gas production, which may be related to the lower overall intrinsic
acidity
21 of this new material.
22
23 Table 5
24
Zeolite Wt% Product C1-C4, C5- 250-
700+ VI At 4.1 wt% 250 F 550 F
CSt
Std SSZ-32 70.2 127 5.2 9.3 10
SSZ-32X 75.4 129 2.4 6 8.3
26
27
-19-
CA 02543283 2006-04-21
WO 2005/042144 PCT/US2004/036405
1 EXAMPLE 6
2
3 A bound catalyst was made using the SSZ-32X zeolite powder, using alumina
4 binder, extrusion, base drying and calcination, incipient wetness Pt
impregnation and final drying and calcining of the extrudate. The overall Pt
6 loading was 0.32 wt. %. After following loading and activation procedures
7 similar to the last example, a titration step was performed by adding 200
8 micromoles nitrogen (as tributyl amine) per gm catalyst. This catalyst was
9 then tested on a waxy 150N hydrocrackate feed containing 10% wax and a
pour point of +32 C. Process conditions used were a LHSV of 1.0 hr -1, 4000
11 scf/bbl gas to oil ratio and a total pressure of 2300 psig. In Figures 2(a)
and
12 2(b), the observed yield and VI obtained when a bottoms product (650 F +
13 cut) is contacted with a catalyst comprising SSZ-32X are compared to
results
14 obtained when the feed is contacted with a similar bound catalyst made from
standard SSZ-32 zeolite. An improved yield and VI over the range of -5 to -
16 25 C product pour point is shown.
-20-