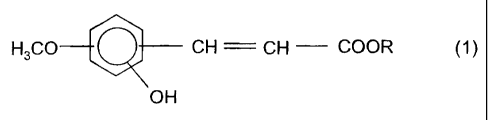Note: Descriptions are shown in the official language in which they were submitted.
CA 02545345 2006-04-28
Clariant Produkte (Deutschland) GmbH 2005DE420 Dr. KM
1
Description
Additives for low-sulfur mineral oil distillates, comprising aromatics which
bear a
hydroxyl group, a methoxy group and an acid function
The invention relates to additives for low-sulfur mineral oil distillates with
improved
cold flowability and paraffin dispersancy, comprising an additive based on
hydroxy-
methoxyphenylcarboxylic acid, to fuel oils additized therewith and to the use
of the
additive.
In view of the decreasing mineral oil reserves coupled with steadily rising
energy
demand, ever more problematic crude oils are being extracted and processed. In
addition, the demands on the fuel oils produced therefrom, such as diesel and
heating oil, are becoming ever more stringent, not least as a result of
legislative
requirements. Examples thereof are the reduction in the sulfur content and the
limitation of the final boiling point and also of the aromatics content of
middle
distillates, which force the refineries into constant adaptation of the
processing
technology. In middle distillates, this leads in many cases to an increased
proportion
of paraffins, especially in the chain length range of from C~$ to C24, which
in turn has
a negative influence on the cold flow properties of these fuel oils.
Crude oils and middle distillates, such as gas oil, diesel oil or heating oil,
obtained by
distillation of crude oils contain, depending on the origin of the crude oils,
different
amounts of n-paraffins which crystallize out as platelet-shaped crystals when
the
temperature is reduced and sometimes agglomerate with the inclusion of oil.
This
crystallization and agglomeration causes a deterioration in the flow
properties of
these oils or distillates, which may result in disruption in the course of
extraction,
transport, storage and/or use of the mineral oils and mineral oil distillates.
When
mineral oils are transported through pipelines, the crystallization phenomenon
can,
especially in winter, lead to deposits on the pipe walls, and in individual
cases, for
example in the event of stoppage of a pipeline, even to its complete blockage.
When
the mineral oils are stored and processed further, it may also be necessary in
winter
CA 02545345 2006-04-28
2
to store the mineral oils in heated tanks. In the case of mineral oil
distillates, the
consequence of crystallization may be blockages of the filters in diesel
engines and
boilers, which prevents reliable metering of the fuels and under some
circumstances
results in complete interruption of the fuel or heating medium feed.
In addition to the classical methods of eliminating the crystallized paraffins
(thermally, mechanically or using solvents), which merely involve the removal
of the
precipitates which have already formed, chemical additives (known as flow
improvers) have been developed in recent years. By interacting physically with
the
precipitating paraffin crystals, they bring about modification of their shape,
size and
adhesion properties. The additives function as additional crystal seeds and
some of
them crystallize out with the paraffins, resulting in a larger number of
smaller paraffin
crystals having altered crystal shape. The modified paraffin crystals have a
lower
tendency to agglomerate, so that the oils admixed with these additives can
still be
pumped and processed at temperatures which are often more than 20°C
lower than
in the case of nonadditized oils.
Typical flow improvers for crude oils and middle distillates are co- and
terpolymers of
ethylene with carboxylic esters of vinyl alcohol.
A further task of flow improvers is the dispersion of the paraffin crystals,
i.e. the
retardation or prevention of the sedimentation of the paraffin crystals and
therefore
the formation of a paraffin-rich layer at the bottom of storage vessels.
EP-A-0 061 895 discloses cold flow improvers for mineral oil distillates,
which
comprise esters, ethers or mixtures thereof. The esters/ethers contain two
linear
saturated C~o- to C3o-alkyl groups and a polyoxyalkylene group with from 200
to
5000 g/mol.
EP-0 973 848 and EP-0 973 850 disclose mixtures of esters alkoxylated alcohols
with more than 10 carbon atoms and fatty acids with 10 - 40 carbon atoms in
combination with ethylene copolymers as flow improvers.
EP-A-0 935 645 discloses alkylphenol-aldehyde resins as a lubricity-improving
CA 02545345 2006-04-28
30690-4
3
additive in low-sulfur middle distillates.
EP-A-0 857 776 and EP-A-1 088 045 disclose processes for improving the
flowability
of paraffin-containing mineral oils and mineral oil distillates by addition of
ethylene
copolymers and alkylphenol-aldehyde resins, and also optionally further
nitrogen-
containing paraffin dispersants.
WO-99/62973 discloses the use of copolymers of dialkylphenyl fumarate and a
comonomer selected from vinyl acetate, styrene, C3- to C3o-a-olefins, ethylene
and
carbon monoxide as a cold additive for oils.
The invention improves the flowability and especially the
paraffin dispersancy under cold conditions in mineral oils or mineral oil
distillates by
the addition of suitable cold additives.
It has now been found that, surprisingly, aromatic compounds which contain
hydroxyl, methoxy and carboxyl groups are highly suitable for paraffin
dispersancy in
mineral oils and mineral oil distillates.
The invention thus provides an additive for fuel oils, comprising at least one
copolymer of ethylene and an unsaturated ester, at least one oil-soluble polar
nitrogen compound which is a reaction product of amines of the formula NR6R'R$
in
which R6, R' and R8 may be the same or different, and at least one of these
groups
is C8-C36-alkyl, C6-C36-cycloalkyl, Cg-C36-alkenyl, especially C~2-C24-alkyl,
C~2-C24-
alkenyl or cyclohexyl, and the remaining groups are hydrogen, C~-C36-alkyl, C2-
C3s-
alkenyl, cyclohexyl or a group of the formulae -(A-O)X E or -(CH2)"-NYZ in
which A is
an ethyl or propyl group, x is from 1 to 50, E = H, C~-C3o-alkyl, C5-C~2-
cycloalkyl or
C6-C3o-aryl, and n = 2, 3 or 4, and Y and Z are each independently H, C~-C3o-
alkyl or
-(A-O)X with compounds which contain at least one acyl group, and at least one
aromatic compound of the formula 1,
CA 02545345 2006-04-28
4
H3C0 CH CH COOK (1 )
OH
in which R is H or C~- to C4-alkyl.
The invention further provides fuel oils comprising at least one copolymer of
ethylene
and an unsaturated ester, at least one oil-soluble polar nitrogen compound
which is a
reaction product of amines of the formula NR6R'R8 in which R6, R' and R$ may
be
the same or different, and at least one of these groups is C$-C36-alkyl, C6-
C36-cyclo-
alkyl, C$-C36-alkenyl, especially C~2-C24-alkyl, C~2-C24-alkenyl or
cyclohexyl, and the
remaining groups are hydrogen, C~-C36-alkyl, C2-C36-alkenyl, cyclohexyl or a
group of
the formulae -(A-O)X E or -(CH2)"-NYZ in which A is an ethyl or propyl group,
x is
from 1 to 50, E = H, C~-C3o-alkyl, C5-C~2-cycloalkyl or C6-C3o-aryl, and n =
2, 3 or 4,
and Y and Z are each independently H, C~-C3o-alkyl or -(A-O)X with compounds
which contain at least one acyl group, and at least one aromatic compound of
the
formula 1,
H3C0 CH CH COOK (1 )
OH
in which R is H or C~- to Ca-alkyl.
The invention further provides for the use of the inventive additives in
amounts of
from 5 to 10 000 ppm as a paraffin dispersent in fuel oils, preferably in
middle
distillates.
The invention further provides a process for improving the cold flow
properties of fuel
oils, comprising the addition of from 5 to 10 000 ppm of the inventive
additives to the
fuel oil.
The above-specified aromatic compounds of the formula 1 are referred to
CA 02545345 2006-04-28
hereinbelow as aromatic additives. Particular preference is given to 4-hydroxy-
3-
methoxyphenylpropionic acid or 4-hydroxy-3-methoxycinnamic acid.
The aromatic additives are added to middle distillates, irrespective of their
content of
5 copolymers of ethylene and an unsaturated ester, preferably in amounts of
from 10
to 1000 ppm, in particular from 20 to 500 ppm.
The additive for fuel oils is referred to hereinbelow as inventive additive.
In addition to the aromatic additive, the inventive additives also comprise
one or
more copolymers of ethylene and unsaturated esters and optionally further
olefinically unsaturated compounds. Suitable ethylene copolymers are in
particular
those which, in addition to ethylene, contain from 6 to 21 mol%, in particular
from 10
to 18 mol% of unsaturated esters. These copolymers preferably have melt
viscosities
at 140°C of from 20 to 10 000 mPas, in particular from 30 to 5000 mPas,
especially
from 50 to 2000 mPas.
The unsaturated esters are preferably vinyl esters, acrylic esters and/or
methacrylic
esters.
The further olefinically unsaturated compounds are preferably alkyl vinyl
ethers
and/or alkenes, which may be substituted by hydroxyl groups.
In addition to ethylene, one or more comonomers may be present in the
copolymer.
The vinyl esters are preferably those of the formula 2
CH2=CH-OCOR~ (2)
where R' is C~- to C3o-alkyl, preferably C4- to C~6-alkyl, especially C6- to
C~2-alkyl. In
a further embodiment, the alkyl groups mentioned may be substituted by one or
more hydroxyl groups.
In a further preferred embodiment, R' is a branched alkyl radical or a
neoalkyl radical
CA 02545345 2006-04-28
6
having from 7 to 11 carbon atoms, in particular having 8, 9 or 10 carbon
atoms.
Particularly preferred vinyl esters derive from secondary and especially
tertiary
carboxylic acids whose branch is in the alpha-position to the carbonyl group.
Suitable
vinyl esters include vinyl acetate, vinyl propionate, vinyl butyrate, vinyl
isobutyrate,
vinyl hexanoate, vinyl heptanoate, vinyl octanoate, vinyl pivalate, vinyl
2-ethylhexanoate, vinyl laurate, vinyl stearate and Versatic esters such as
vinyl
neononanoate, vinyl neodecanoate, vinyl neoundecanoate.
In a further preferred embodiment, these ethylene copolymers contain vinyl
acetate
and at least one further vinyl ester of the formula 4 where R' is C4- to C3o-
alkyl,
preferably C4- to C~6-alkyl, especially C6- to C~2-alkyl.
The (meth)acrylic esters are preferably those of the formula 3
CH2=CR2-COORS (3)
where R2 is hydrogen or methyl and R3 is C~- to C3o-alkyl, preferably C4- to
C~6-alkyl,
especially C6- to C~2-alkyl. Suitable acrylic esters include, for example,
methyl
(meth)acrylate, ethyl (meth)acrylate, propyl (meth)acrylate, n- and isobutyl
(meth)acrylate, hexyl, octyl, 2-ethylhexyl, decyl, dodecyl, tetradecyl,
hexadecyl,
octadecyl (meth)acrylate and mixtures of these comonomers. In a further
embodiment, the alkyl groups mentioned may be substituted by one or more
hydroxyl groups. An example of such an acrylic ester is hydroxyethyl
methacrylate.
The alkyl vinyl ethers are preferably compounds of the formula 4
CH2=CH-OR4 (4)
where R4 is C~- to C3o-alkyl, preferably C4- to C~6-alkyl, especially C6- to
C~2-alkyl.
Examples include methyl vinyl ether, ethyl vinyl ether, isobutyl vinyl ether.
In a further
embodiment, the alkyl groups mentioned may be substituted by one or more
hydroxyl groups.
The alkenes are preferably monounsaturated hydrocarbons having from 3 to
CA 02545345 2006-04-28
7
30 carbon atoms, in particular from 4 to 16 carbon atoms and especially from 5
to 12
carbon atoms. Suitable alkenes include propene, butene, isobutylene, pentene,
hexene, 4-methylpentene, octene, diisobutylene and norbornene and derivatives
thereof such as methylnorbornene and vinylnorbornene. In a further embodiment,
the alkyl groups mentioned may be substituted by one or more hydroxyl groups.
Apart from ethylene, particularly preferred terpolymers contain from 0.1 to 12
mol%,
in particular from 0.2 to 5 mol%, of vinyl neononanoate or of vinyl
neodecanoate, and
from 3.5 to 20 mol%, in particular from 8 to 15 mol%, of vinyl acetate, the
total
comonomer content being between 6 and 21 mol%, preferably between 12 and
18 mol%.
Further particularly preferred copolymers contain, in addition to ethylene and
from 8
to 18 mol% of vinyl esters, also from 0.5 to 15 mol% of alkenes as described
above.
Preference is given to using mixtures of two or more of the abovementioned
ethylene
copolymers. The polymers on which the mixtures are based more preferably
differ in
at least one feature.
For example, they may contain different comonomers, or different comonomer
contents, molecular weights and/or degrees of branching.
The mixing ratio between the aromatic additives, the ethylene copolymers and
the
oil-soluble polar nitrogen compounds may, depending on the application, vary
within
wide limits, the ethylene copolymers and the oil-soluble polar nitrogen
compounds
often constituting the major proportion. The inventive additives preferably
contain
from 2 to 70% by weight, preferably from 5 to 50% by weight of the aromatic
additive,
and also from 30 to 98% by weight, preferably from 50 to 95% by weight of
ethylene
copolymers, and also the oil-soluble polar nitrogen compounds. Examples of
concentrations are from 0.1 to 100 ppm, preferably from 1 to 50 ppm, in
particular
from 5 to 50 ppm of aromatic additive, from 20 to 500 ppm, preferably from 50
to 400
ppm, in particular from 75 to 350 ppm of ethylene copolymer and from 20 to 500
ppm, preferably from 50 to 400 ppm, in particular from 75 to 350 ppm of oil-
soluble
polar nitrogen compounds.
CA 02545345 2006-04-28
8
The suitable oil-soluble polar nitrogen compounds (constituent II) are
reaction
products of fatty amines with compounds which contain an acyl group. The
preferred
amines are compounds of the formula NR6R'R$ where R6, R' and R$ may be the
same or different, and at least one of these groups is C8-C36-alkyl, C6-C36-
cycloalkyl
or C$-C36-alkenyl, in particular C~2-C24-alkyl, C~2-C24-alkenyl or cyclohexyl,
and the
remaining groups are either hydrogen, C~-C36-alkyl, C2-C36-alkenyl,
cyclohexyl, or a
group of the formulae -(A-O)X-E or -(CH2)n-NYZ, where A is an ethyl or propyl
group, x is from 1 to 50, E = H, C~-C3o-alkyl, C5-C~2-cycloalkyl or C6-C3o-
aryl, and n =
2, 3 or 4, and Y and Z are each independently H, C~-C3o-alkyl or -(A-O)X. The
alkyl
and alkenyl radicals may each be linear or branched and contain up to two
double
bonds. They are preferably linear and substantially saturated, i.e. they have
iodine
numbers of less than 75 g of 12/g, preferably less than 60 g of 12/g and in
particular
between 1 and 10 g of 12/g. Particular preference is given to secondary fatty
amines
in which two of the R6, R' and R$ groups are each C$-C36-alkyl, C6-C36-
cycloalkyl,
C$-C36-alkenyl, in particular C~2-C24-alkyl, C~2-C24-alkenyl or cyclohexyl.
Suitable fatty
amines are, for example, octylamine, decylamine, dodecylamine,
tetradecylamine,
hexadecylamine, octadecylamine, eicosylamine, behenylamine, didecylamine,
didodecylamine, ditetradecylamine, dihexadecylamine, dioctadecylamine,
dieicosyl-
amine, dibehenylamine and mixtures thereof. The amines especially contain
chain
cuts based on natural raw materials, for example coconut fatty amine, tallow
fatty
amine, hydrogenated tallow fatty amine, dicoconut fatty amine, ditallow fatty
amine
and di(hydrogenated tallow fatty amine). Particularly preferred amine
derivatives are
amine salts, imides and/or amides, for example amide-ammonium salts of
secondary
fatty amines, in particular of dicoconut fatty amine, ditallow fatty amine and
distearylamine.
Acyl group refers here to a functional group of the following formula:
>C=0
Carbonyl compounds suitable for the reaction with amines are either low
molecular
weight or polymeric compounds having one or more carboxyl groups. Preference
is
CA 02545345 2006-04-28
9
given to those low molecular weight carbonyl compounds having 2, 3 or 4
carbonyl
groups. They may also contain heteroatoms such as oxygen, sulfur and nitrogen.
Suitable carboxylic acids are, for example, malefic acid, fumaric acid,
crotonic acid,
itaconic acid, succinic acid, C~-C4o-alkenylsuccinic acid, adipic acid,
glutaric acid,
sebacic acid and malonic acid, and also benzoic acid, phthalic acid,
trimellitic acid
and pyromellitic acid, nitrilotriacetic acid, ethylenediaminetetraacetic acid
and their
reactive derivatives, for example esters, anhydrides and acid halides. Useful
polymeric carbonyl compounds have been found to be in particular copolymers of
ethylenically unsaturated acids, for example acrylic acid, methacrylic acid,
malefic
acid, fumaric acid and itaconic acid; particular preference is given to
copolymers of
malefic anhydride. Suitable comonomers are those which confer oil solubility
on the
copolymer. Oil-soluble means here that the copolymer, after reaction with the
fatty
amine, dissolves without residue in the middle distillate to be additized in
practically
relevant dosages. Suitable comonomers are, for example, olefins, alkyl esters
of
acrylic acid and methacrylic acid, alkyl vinyl esters, alkyl vinyl ethers
having from 2 to
75, preferably from 4 to 40 and in particular from 8 to 20, carbon atoms in
the alkyl
radical. In the case of olefins, the alkyl radical attached to the double bond
is
equivalent here. The molecular weights of the polymeric carbonyl compounds are
preferably between 400 and 20 000, more preferably between 500 and 10 000, for
example between 1000 and 5000.
It has been found that oil-soluble polar nitrogen compounds which are obtained
by
reaction of aliphatic or aromatic amines, preferably long-chain aliphatic
amines, with
aliphatic or aromatic mono-, di-, tri- or tetracarboxylic acids or their
anhydrides are
particularly useful (cf. US 4 211 534). Equally suitable as oil-soluble polar
nitrogen
compounds are amides and ammonium salts of aminoalkylenepolycarboxylic acids
such as nitrilotriacetic acid or ethylenediaminetetraacetic acid with
secondary amines
(cf. EP 0 398 101 ). Other oil-soluble polar nitrogen compounds are copolymers
of
malefic anhydride and a,~-unsaturated compounds which may optionally be
reacted
with primary monoalkylamines and/or aliphatic alcohols (cf. EP-A-0 154 177,
EP 0 777 712), the reaction products of alkenyl-spiro-bislactones with amines
(cf.
EP-A-0 413 279 B1 ) and, according to EP-A-0 606 055 A2, reaction products of
terpolymers based on a,~-unsaturated dicarboxylic anhydrides, a,~3-unsaturated
CA 02545345 2006-04-28
compounds and polyoxyalkylene ethers of lower unsaturated alcohols:
The mixing ratio between the inventive additives and oil-soluble polar
nitrogen
compounds as constituent II may vary depending upon the application. Such
additive
5 mixtures preferably contain from 5 to 95% by weight, preferably from 10 to
90% by
weight of the inventive additive, and also from 5 to 95% by weight, preferably
from 10
to 90% by weight of oil-soluble polar nitrogen compounds.
In addition to the aromatic additives, the ethylene copolymers and the oil-
soluble
10 polar nitrogen compounds, further constituents may be present as
coadditives in the
fuel oils or in the inventive additive. For instance, olefin copolymers may be
used as
a coadditive in the inventive additives.
Suitable olefin copolymers as a coadditive for the inventive additive
(constituent III)
may derive directly from monoethylenically unsaturated monomers, or may be
prepared indirectly by hydrogenation of polymers which derive from
polyunsaturated
monomers such as isoprene or butadiene. Preferred copolymers contain, in
addition
to ethylene, structural units which derive from a-olefins having from 3 to 24
carbon
atoms and have molecular weights of up to 120 000 g/mol. Preferred a-olefins
are
propylene, butene, isobutene, n-hexene, isohexene, n-octene, isooctene, n-
decene,
isodecene. The comonomer content of olefins is preferably between 15 and
50 mol%, more preferably between 20 and 35 mol% and especially between 30 and
45 mol%. These copolymers may also contain small amounts, for example up to
10 mol%, of further comonomers, for example nonterminal olefins or
nonconjugated
olefins. Preference is given to ethylene-propylene copolymers. The olefin
copolymers
may be prepared by known methods, for example by means of Ziegler or
metallocene catalysts.
Further suitable olefin copolymers are block copolymers which contain blocks
composed of olefinically unsaturated aromatic monomers A and blocks composed
of
hydrogenated polyolefins B. Particularly suitable block copolymers have the
structure
(AB)~A and (AB)m, where n is from 1 to 10 and m is from 2 to 10.
CA 02545345 2006-04-28
11
The mixing ratio between the inventive additive and constituent III is
generally in
each case between 1:20 and 20:1, preferably in each case between 1:10 and 10:1
by weight.
In the inventive additive, comb polymers may also be used as a coadditive.
Suitable comb polymers as a coadditive for the inventive additive (constituent
IV)
may be described, for example, by the formula 5
A H G H
- C C C C - (5)
m ~ ~ n
D E M N
In this formula
A is R', COOR', OCOR', R"-COOR', OR';
D is H, CH3, A or R";
E is H, A;
G is H, R", R"-COOR', an aryl radical or a heterocyclic
radical;
M is H, COOR", OCOR", OR", COOH;
N is H, R", COOK", OCOR", an aryl radical;
R' is a hydrocarbon chain having from 8 to 50
carbon atoms;
R" is a hydrocarbon chain having from 1 to 10
carbon atoms;
m is from 0.4 to 1.0; and
n is from 0 to 0.6.
In a preferred embodiment, alkylphenol-aldehyde resins are used as further
constituents in the inventive additives (constituent V). Alkylphenol-aldehyde
resins
are known in principle and are described, for example, in Rompp Chemie
Lexikon,
9th edition, Thieme Verlag 1988-92, volume 4, p. 3351 ff. Suitable in
accordance
with the invention are in particular those alkylphenol-aldehyde resins which
derive
from alkylphenols having one or two alkyl radicals in the ortho- and/or para-
position
CA 02545345 2006-04-28
12
to the OH group. Particularly preferred starting materials are alkylphenols
which
bear, on the aromatic ring, at least two hydrogen atoms capable of
condensation with
aldehydes, and especially monoalkylated phenols whose alkyl radical is in the
para-
position. The alkyl radicals (for constituent V, this refers generally to
hydrocarbon
radicals as defined below) may be the same or different in the alkylphenol-
aldehyde
resins usable in the process according to the invention, they may be saturated
or
unsaturated and have 1 - 200, preferably 1 - 20, in particular 4 - 12 carbon
atoms;
they are preferably n-, iso- and tert-butyl, n- and isopentyl, n- and
isohexyl, n- and
isooctyl, n- and isononyl, n- and isodecyl, n- and isododecyl, tetradecyl,
hexadecyl,
octadecyl, tripropenyl, tetrapropenyl, poly(propenyl) and poly(isobutenyl)
radicals.
Suitable aldehydes for the alkylphenol-aldehyde resins are those having from 1
to 12
carbon atoms and preferably those having from 1 to 4 carbon atoms, for example
formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, 2-ethylhexanal,
benzaldehyde, glyoxalic acid and reactive equivalents thereof, such as
paraformaldehyde and trioxane. Particular preference is given to formaldehyde
in the
form of paraformaldehyde and especially formalin.
In the context of the present patent application, molecular weights are always
measured by means of gel permeation chromatography (GPC) against polystyrene
standards in THF.
The molecular weight of the alkylphenol-aldehyde resins is preferably 400 -
20 000 g/mol, especially 400 - 5000 g/mol. A prerequisite in this context is
that the
alkylphenol-aldehyde resins are oil-soluble at least in concentrations
relevant to the
application of from 0.001 to 1 % by weight.
In a preferred embodiment of the invention, the alkylphenol-formaldehyde
resins
contain oligo- or polymers having a repeat structural unit of the formula 3
CA 02545345 2006-04-28
13
(3)
where R5 is C~-C2oo-alkyl or -alkenyl and n is from 2 to 100. R5 is preferably
C4-C2o-
alkyl or -alkenyl and especially C6-C~6-alkyl or -alkenyl. n is preferably
from 2 to 50
and especially from 3 to 25, for example from 5 to 15.
For use in middle distillates such as diesel and heating oil, particular
preference is
given to alkylphenol-aldehyde resins having C2-C4o-alkyl radicals of the
alkylphenol,
preferably having C4-C2o-alkyl radicals, for example C6-C~2-alkyl radicals.
The alkyl
radicals may be linear or branched; they are preferably linear. Particularly
suitable
alkylphenol-aldehyde resins derive from linear alkyl radicals having 8 and 9
carbon
atoms. The average molecular weight, determined by means of GPC, is preferably
between 700 and 20 000 g/mol, in particular between 800 and 10 000 g/mol, for
example between 1000 and 2500 g/mol.
These alkylphenol-aldehyde resins are obtainable by known processes, for
example
by condensation of the appropriate alkylphenols with formaldehyde, i.e. with
from 0.5
to 1.5 mol, preferably from 0.8 to 1.2 mol of formaldehyde per mole of
alkylphenol.
The condensation may be effected without solvent, but is preferably effected
in the
presence of a water-immiscible or only partly water-miscible inert organic
solvent
such as mineral oils, alcohols, ethers and the like. Particular preference is
given to
solvents which can form azeotropes with water. Useful such solvents are in
particular
aromatics such as toluene, xylene, diethylbenzene and relatively high-boiling
commercial solvent mixtures such as ~Shellsol AB and Solvent Naphtha. The
condensation is effected preferably between 70 and 200°C, for example
between 90
and 160°C. It is catalyzed typically by from 0.05 to 5% by weight of
bases or acids.
For example, the condensation catalyzed by amines, preferably tertiary amines,
for
example triethylamine, with subsequent neutralization by means of organic
sulfonic
CA 02545345 2006-04-28
14
acid leads to the suitable products. Preference is given in accordance with
the
invention to catalysis by organic sulfonic acids which, on completion of the
condensation with amines, are converted to the oil-soluble ammonium
sulfonates.
The mixing ratio of the alkylphenol-aldehyde resins as a coadditive to the
inventive
additive is generally between 20:1 and 1:20, preferably between 1:10 and 10:1.
In the inventive additive, polyoxyalkylene compounds may also be used as
coadditives.
Suitable polyoxyalkylene compounds as a coadditive for the inventive additive
(constituent VI) are, for example, esters, ethers and ether/esters which bear
at least
one alkyl radical having from 12 to 30 carbon atoms. When the alkyl groups
stem
from an acid, the remainder stems from a polyhydric alcohol; when the alkyl
radicals
come from a fatty alcohol, the remainder of the compound stems from a
polyacid.
Suitable polyols are polyethylene glycols, polypropylene glycols, polybutylene
glycols
and copolymers thereof having a molecular weight of from approx. 100 to
approx.
5000 g/mol, preferably from 200 to 2000 g/mol. Also suitable are alkoxylates
of
polyols, for example of glycerol, trimethylolpropane, pentaerythritol,
neopentyl glycol,
and the oligomers which are obtainable therefrom by condensation and have from
2
to 10 monomer units, for example polyglycerol. Preferred alkoxylates are those
having from 1 to 100 mol, in particular from 5 to 50 mol, of ethylene oxide,
propylene
oxide and/or butylene oxide per mole of polyol. Esters are particularly
preferred.
Fatty acids having from 12 to 26 carbon atoms are preferred for the reaction
with the
polyols to form the ester additives, and particular preference is given to
using C~$- to
C24-fatty acids, especially stearic and behenic acid. The esters may also be
prepared
by esterifying polyoxyalkylated alcohols. Preference is given to fully
esterified
polyoxyalkylated polyols having molecular weights of from 150 to 2000,
preferably
from 200 to 600. Particularly suitable are PEG-600 dibehenate and glycerol
ethylene
glycol tribehenate.
The mixing ratio between the inventive additive and the further constituent VI
is
CA 02545345 2006-04-28
generally between 1:10 and 10:1, preferably in each case between 1: 5 and 5:1.
The inventive additive may be used alone or in a mixture with one or more of
constituents) II, Ill, IV, V or VI.
5
The inventive additives may be used alone or else together with other
additives, for
example with other pour point depressants or dewaxing assistants, with
antioxidants,
flow improvers, cetane number improvers, dehazers, demulsifiers, detergents,
lubricity additives, dispersants, antifoams, dyes, corrosion inhibitors,
sludge
10 inhibitors, odorants and/or additives for lowering the cloud point. The
other additives
may be added directly to the fuel oil mixture or be introduced into the
mixture by
mixing different fuel oil components which have already been additized
individually
with one or more of the additives mentioned.
15 The inventive additives are suitable for improving the cold flow properties
of fuel oils
of animal, vegetable or mineral origin. In addition, they disperse the
paraffins which
precipitate out below the cloud point in middle distillates. In particular,
they are
superior to the prior art additives in problematic oils having a low aromatics
content
of less than 25% by weight, in particular less than 22% by weight, for example
less
than 20% by weight, of aromatics, and thus lower solubility for n-paraffins.
Middle
distillates refer in particular to those mineral oils which are obtained by
distillation of
crude oil and boil in the range from 120 to 450°C, for example
kerosene, jet fuel,
diesel and heating oil. Aromatic compounds refer to the totality of mono-, di-
and
polycyclic aromatic compounds, as can be determined by means of HPLC to DIN EN
12916 (2001 edition). The inventive additives are particularly advantageous in
those
middle distillates which contain less than 350 ppm of sulfur, more preferably
less
than 100 ppm of sulfur, in particular less than 50 ppm of sulfur and in
special cases
less than 10 ppm of sulfur. They are generally those middle distillates which
have
been subjected to refining under hydrogenating conditions and therefore
contain only
small fractions of polyaromatic and polar compounds. They are preferably those
middle distillates which have 90% distillation points below 360°C, in
particular 350°C
and in special cases below 340°C.
In view of decreasing world mineral oil reserves and the discussion about the
CA 02545345 2006-04-28
16
environmentally damaging consequences of the use of fossil and mineral fuels,
there
is increasing interest in alternative energy sources based on renewable raw
materials. These include in particular native oils and fats of vegetable or
animal
origin. These are generally triglycerides of fatty acids having from 10 to 24
carbon
atoms and a calorific value comparable to conventional fuels, but are at the
same
time classified as biodegradable and environmentally compatible.
Oils obtained from animal or vegetable material are mainly metabolism products
which include triglycerides of monocarboxylic acids, for example acids having
from
10 to 25 carbon atoms, and corresponding to the formula
H H H
H-~ ~-H
O C R O-C R O C R
O O O
where R is an aliphatic radical which has from 10 to 25 carbon atoms and may
be
saturated or unsaturated.
In general, such oils contain glycerides from a series of acids whose number
and
type vary with the source of the oil, and they may additionally contain
phospho-
glycerides. Such oils can be obtained by processes known from the prior art.
As a consequence of the sometimes unsatisfactory physical properties of the
triglycerides, the industry has applied itself to converting the naturally
occurring
triglycerides to fatty acid esters of lower alcohols such as methanol or
ethanol. The
prior art also includes mixtures of middle distillates with oils of vegetable
or animal
origin (also referred to hereinbelow as "biofuel oils").
In a preferred embodiment, the biofuel oil, which is frequently also referred
to as
biodiesel or biofuel, comprises fatty acid alkyl esters composed of fatty
acids having
from 12 to 24 carbon atoms and alcohols having from 1 to 4 carbon atoms.
Typically,
a relatively large portion of the fatty acids contains one, two or three
double bonds.
CA 02545345 2006-04-28
17
The biofuel is more preferably, for example, rapeseed oil methyl ester and
especially
mixtures which comprise rapeseed oil fatty acid methyl ester, sunflower oil
fatty acid
methyl ester, palm oil fatty acid methyl ester, used oil fatty acid methyl
ester and/or
soya oil fatty acid methyl ester.
Examples of oils which are derived from animal or vegetable material and which
can
be used in the inventive fuel oils are rapeseed oil, coriander oil, soya oil,
cottonseed
oil, sunflower oil, castor oil, olive oil, peanut oil, maize oil, almond oil,
palm kernel oil,
coconut oil, mustardseed oil, bovine tallow, bone oil and fish oils. Further
examples
include oils which are derived from wheat, jute, sesame, shea tree nut,
arachis oil
and linseed oil, and can be derived therefrom by processes known from the
prior art.
It is also possible to use oils which have been obtained from used oils such
as deep
fat fryer oil. Preference is given to rapeseed oil, which is a mixture of
fatty acids
partially esterified with glycerol, since it is obtainable in large amounts
and is
obtainable in a simple manner by extractive pressing of rapeseeds. In
addition,
preference is given to the likewise widely available oils of sunflowers and
soya, and
also to their mixtures with rapeseed oil.
Useful lower alkyl esters of fatty acids are the following, for example as
commercial
mixtures: the ethyl, propyl, butyl and in particular methyl esters of fatty
acids having
from 12 to 22 carbon atoms, for example of lauric acid, myristic acid,
palmitic acid,
palmitolic acid, stearic acid, oleic acid, elaidic acid, petroselic acid,
ricinolic acid,
elaeostearic acid, linoleic acid, linolenic acid, eicosanoic acid, gadoleic
acid,
docosanoic acid or erucic acid, each of which preferably has an iodine number
of
from 50 to 150, in particular from 90 to 125. Mixtures having particularly
advantageous properties are those which comprise mainly, i.e. comprise at
least
50% by weight of, methyl esters of fatty acids having from 16 to 22 carbon
atoms,
and 1, 2 or 3 double bonds. The preferred lower alkyl esters of fatty acids
are the
methyl esters of oleic acid, linoleic acid, linolenic acid and erucic acid.
Commercial mixtures of the type mentioned are obtained, for example, by
hydrolyzing and esterifying animal and vegetable fats and oils, by
transesterifying
them with lower aliphatic alcohols. To prepare lower alkyl esters of fatty
acids, it is
advantageous to start from fats and oils having a high iodine number, for
example
CA 02545345 2006-04-28
18
sunflower oil, rapeseed oil, coriander oil, castor oil, soya oil, cottonseed
oil, peanut
oil or bovine tallow. Preference is given to lower alkyl esters of fatty acids
based on a
novel type of rapeseed oil, whose fatty acid component is derived to an extent
of
more than 80% by weight from unsaturated fatty acids having 18 carbon atoms.
When mixtures of middle distillate of mineral origin (A) and biofuels (B) are
used, the
A:B mixing ratio of the constituents may vary as desired. It is preferably
between
A:B = 99.9:0.1 and 0.1:99.9, in particular from 99:1 to 1:99, especially from
95:5 to
5:95, for example from 85:15 to 15:85 or from 80:20 to 20:80.
It is also possible to use mixtures of synthetic fuels, as are obtainable, for
example,
from the Fischer-Tropsch process, and a middle distillate of mineral origin A
and/or a
biofuel B as the fuel oil composition.
Examples
Table 1: Characterization of the test oils:
The test oils employed were current oils from European refineries. The CFPP
value
was determined to EN 116 and the cloud point to ISO 3015. The aromatic
hydrocarbon groups were determined to DIN EN 12916 (November 2001 edition)
CA 02545345 2006-04-28
19
Test Test Test Test Test
oil oil oil oil oil
1 2 3 4 5
Distillation
IBP [C] 166.3C 173.8C 240.7 173.8 166.6
90% - 20% cut [C] 147C 117C 64.4 116.6 102.5
FBP [C] 377.9C 345.7C 345.7 352.6 359.4
Cloud Point [C] -8.0 -6.7 -8.2 -6.9 -3.9
CFPP [C] -11.0 -8.0 -11 -9 -7
Sulfur [ppm] 308 210 1450 320 2.7
Density @15C [g/cm3] 0.826 0.831 0.841 0.827 0.845
Aromatics content [% 18.73 27.50 24.16 27.96 26.63
by wt.]
of which mono [% by wt.]14.31 22.22 15.76 22.58 23.89
di [% by wt.] 3.93 4.83 7.93 4.91 2.54
poly [% by wt.] 0.49 0.46 0.47 0.48 0.19
The following additives were used:
Characterization of the ethylene copolymers used as flow improvers
The ethylene copolymers used were commercial products having the properties
reported in Table 2. The products were used in the form of 65% and 50%
dilutions in
kerosene.
The viscosity was determined to ISO 3219/B with a rotational viscometer (Haake
RV20) with plate-cone measuring system at 140°C.
CA 02545345 2006-04-28
Table 2: Characterization of the ethylene copolymers used
Example Comonomer(s) V~ao
A1 13.6 mol% of vinyl acetate 130 mPas
A2 12.5 mol% of vinyl acetate and 1.2 150 mPas
mol% of
vinyl neodecanoate
A3 9.8 mol% of vinyl acetate and 0.6 170 mPas
mol% of vinyl
neodecanoate
5 Characterization of the further additives used (constituent II):
B1 ) Polar polymeric nitrogen compound consisting of a comb polymer prepared
from
1.3 mol% of 1-tetradecene, 1.3 mol% of hexadecene, 2.6 mol% of malefic
anhydride
and 0.2 mol% of allyl methyl polyglycol ether. The resulting polymer has a K
value of
10 15 and is reacted with 2.6 mol% each of distearylamine and dicoconut fatty
amine to
give the amide ammonium salt. The titratable base nitrogen content of the 50%
polymer solution is 0.69%.
Characterization of the aromatic additives
1 ) 4-Hydroxy-3-methoxyphenylpropionic acid
2) 4-Hydroxy-3-methoxycinnamic acid
3) 3-Hydroxy-4-methoxybenzaldehyde (comparison)
Effectiveness of the additives as cold flow improvers
To assess the effect of the inventive additives on the cold flow properties of
middle
distillates, the inventive additives were tested in middle distillates as
follows in the
short sediment test:
150 ml of the middle distillates admixed with the additive components
specified in the
CA 02545345 2006-04-28
21
table were cooled in 200 ml measuring cylinders in a cold cabinet at -
2°C/hour to
-13°C and stored at this temperature for 16 hours. Subsequently, volume
and
appearance, both of the sedimented paraffin phase and of the oil phase above
it,
were determined and assessed visually. A small amount of sediment and an
opaque
oil phase show good paraffin dispersancy.
In addition, the lower 20% by volume is isolated and the cloud point is
determined to
ISO 3015. Oniy a slight deviation of the cloud point of the lower phase (CP~c)
from
the blank value of the oil shows good paraffin dispersancy.
The aromatic additives reported are used in an amount of 50-150 ppm. A
dispersant
is used generally in the presence of a cold flow improver. In addition to the
inventive
additives, appropriate cold flow improvers were therefore used.
Results in test oil 1
The CFPP effectiveness and dispersing action of the inventive additives
(constituent I) were determined in a composition of (by parts by weight) 50
ppm of
the aromatic additive with 100 ppm of B1 and 400 ppm of A2.
Example Aromatic CFPP CPcc Visual assessment
additive [C] [C]
1 1 -26 -7.6 Homogeneously opaque, no sediment
2 2 -27 -7.9 Homogeneously opaque, no sediment
3(C) 3 (C) -22 -5.2 5 ml of sediment, homogeneously
opaque
Results in test oil 2
The CFPP effectiveness and dispersing action of the inventive additives
(constituent I) were determined in a composition of (by parts by weight) 100
ppm of
the aromatic additive with 150 ppm of B1 and 300 ppm of A2.
CA 02545345 2006-04-28
22
ExampleAromatic CFPP CPcc Visual assessment
additive [C] [C]
4 1 -27 -6.1 Homogeneously opaque, no sediment
2 -28 -6.5 Homogeneously opaque, no sediment
6 (C) 3 (C) -21 -4.7 8 ml of sediment
Results in test oil 3
The CFPP effectiveness and dispersing action of the inventive additives
5 (constituent I) were determined in a composition of (by parts by weight) 50
ppm of
the aromatic additive with 100 ppm of B1 and 200 ppm of A3.
ExampleAromatic CFPP CP~~ Visual assessment
additive [C] [C]
7 1 -23 -7.9 1 ml of sediment, homogeneously
opaque
8 2 -24 -8.1 Homogeneously opaque, no sediment
9 (C) 3 (C) -19 -6.8 12 ml of sediment, homogeneously
opaque
Results in test oil 4
The CFPP effectiveness and dispersing action of the inventive additives
(constituent I) were determined in a composition of (by parts by weight) 50
ppm of
the aromatic additive with 200 ppm of B1 and 300 ppm of A3.
ExampleAromatic CFPP CPcc Visual assessment
additive [C] [C]
10 1 -29 -3.2 Homogeneously opaque, no sediment
11 2 -28 -3.5 Homogeneously opaque, no sediment
12 (C) 3 (C) -23 -1.2 11 ml of sediment, homogeneously
opaque
CA 02545345 2006-04-28
23
Results in test oil 5
The CFPP effectiveness and dispersing action of the inventive additives
(constituent I) were determined in a composition of (by parts by weight) 100
ppm of
the aromatic additive with 200 ppm of B1 and 300 ppm of A1.
Example Aromatic CFPP CP~~ Visual assessment
additive [C] [C]
13 1 -25 -7.5 Homogeneously opaque, no sediment
14 2 -27 -7.1 Homogeneously opaque, no sediment
(C) 3 (C) -22 -4.9 15 ml of sediment, homogeneously
opaque