Note: Descriptions are shown in the official language in which they were submitted.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
COMPOUNDS AND METHODS FOR INCREASING NEUROGENESIS
RELATED APPLICATIONS
This application is a continuation-in-part of Application No. 10/850,055 filed
May 19, 2004, which is a continuation-in-part of Application No. 10/718,071
filed November
20, 2003, which claim the benefit of provisional Application No. 60/427,912
filed November
20, 2002. All patents and patent applications cited in this disclosure are
hereby incorporated
by reference.
FIELD OF THE INVENTION
The invention is directed to in vitro and in vivo methods of modulating
neurogenesis.
Novel agents for modulating neurogenesis and novel Exendin analogs also
provided.
BACKGROUND OF THE INVENTION
Neural stem cells (NSC) are a source for new neurons in the mammalian CNS. NSC
are located within the ependymal and/or subventricular zone (SVZ) lining the
lateral ventricle
(Doetsch et al., 1999; Johansson et al., 1999b) and in the dentate gyrus of
the hippocampal
formation (Gage et al., 1998). Studies have revealed the potential for several
additional
locations of NSC within the adult CNS (Palmer et al., 1999). Asymmetric
division of NSC
maintains their starting number, while generating a population of rapidly
dividing precursor,
or progenitor cells (Johansson et al., 1999b). The progenitor cells respond to
a range of cues
that dictate the extent of their proliferation and their fate, both in terms
of differentiation and
positioning.
The NSC of the ventricular system in the adult are likely counterparts of the
embryonic ventricular zone stem cells lining the neural tube. The progeny of
these
embryonic cells migrate away to form the CNS as differentiated neurons and
glia (Jacobson,
1991). NSC persist in the adult lateral ventricle wall (LVW), generating
neuronal progenitors
that migrate down the rostral migratory stream to the olfactory bulb. There,
they differentiate
into granule cells and periglomerular neurons (Lois and Alvarez-Buylla, 1993).
Substantial
neuronal death occurs in the olfactory bulb, creating a need for continuous
replacement of
lost neurons which is satisfied by the migrating progenitors derived from the
LVW (Biebl et
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
2
al., 2000). In addition, there are indications that lost neurons from other
brain regions can be
replaced by progenitors from the LVW that differentiate into the phenotype of
the lost
neurons with appropriate neuronal projections and synapses with the correct
target cell type
(Snyder et al., 1997; Magavi et al., 2000).
In vitro cultivation techniques have been established to identify the external
signals
involved in the regulation of NSC proliferation and differentiation (Johansson
et al., 1999b;
Johansson et al., 1999a). The mitogens EGF and basic FGF allow cell culture
expansion of
neural progenitors isolated from the ventricle wall and the hippocampus
(McKay, 1997;
Johansson et al., 1999a). These dividing progenitors remain in an
undifferentiated state, and
grow into large clones of cells known as neuro spheres. Upon the withdrawal of
the mitogens
and the addition of serum, the progenitors differentiate into neurons,
astrocytes and
oligodendrocytes, which are the three cell lineages of the brain (Doetsch et
al., 1999;
Johansson et al., 1999b). Specific growth factors can be added to alter the
proportions of
each cell type formed. For example, CNTF acts to direct the neural progenitors
to an
astrocytic fate (Johe et al., 1996; Rajan and McKay, 1998). The thyroid
hormone,
triiodothyronine (T3), promotes oligodendrocyte differentiation (Johe et al.,
1996), while
PDGF enhances neuronal differentiation by progenitor cells (Johe et al., 1996;
Williams et
al., 1997). Recently, it has been shown that indeed adult regenerated neurons
are integrated
into the existing brain circuitry, and contribute to ameliorating neurological
deficits
(Nakatomi et al., 2002). Interestingly, observations have also shown that
neurogenesis is
occurring not only at the level of the olfactory bulb and hippocampus. In this
respect it has
been suggested by Zhao et al. that this process can also occur in the adult
mouse substantia
nigra, opening up a new field of investigation for the treatment of
Parkinson's disease (Zhao
et al., 2003)
The ability to expand neural progenitors and manipulate their cell fate has
enormous
implications for transplant therapies for neurological diseases where specific
cell types are
lost.
Parkinson's disease (PD), for example, is characterized by degeneration of
dopaminergic neurons in the substantia nigra. Previous transplantation
treatments for PD
patients have used fetal tissue taken from the ventral midbrain at a time when
substantia nigra
dopaminergic neurons are undergoing terminal differentiation (Herman and
Abrous, 1994).
These cells have been grafted onto the striatum where they form synaptic
contacts with host
striatal neurons, their normal synaptic target. This restores dopamine
turnover and release to
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
3
normal levels with significant functional benefits to the patient (Herman and
Abrous, 1994)
(for review see Bjorklund and Lindvall, 2000). However, the grafting of fetal
tissue is
limited by ethical considerations and a lack of donor tissue. The expansion
and manipulation
of adult NSC can potentially provide a range of well characterized cells for
transplant-based
strategies for neurodegenerative disease such as PD. To this aim, the
identification of factors
and pathways that govern the proliferation and differentiation of neural cell
types is
fundamentally important.
Studies have shown that intraventricular infusion of both EGF and basic FGF
induces
proliferation in the adult ventricle wall cell population. In the case of EGF,
extensive
migration of progenitors into the neighboring striatal parenchyma has been
observed (Craig et
al., 1996; Kuhn et al., 1997). EGF increases differentiation into glial
lineage and reduced the
generation of neurons (Kuhn et al., 1997). Additionally, intraventricular
infusion of BDNF in
adult rats increases the number of newly generated neurons in the olfactory
bulb and rostral
migratory stream, and in parenchymal structures, including the striatum,
septum, thalamus
and hypothalamus (Pencea et al., 2001). Thus, several studies have shown that
the
proliferation of progenitors within the SVZ of the LVW can be stimulated and
that their
lineage can be guided to neuronal or glial fates. Yet, the number of factors
known to affect
neurogenesis in vivo is small and their effects are adverse or limited.
BRIEF SUMMARY OF THE INVENTION
One embodiment of the invention is directed to a method for modulating
neurogenesis
in neural tissue of a patient that exhibits at least one symptom of a central
nervous system
disorder. The disorder may be, for example, neurodegenerative disorders,
ischemic disorders,
neurological traumas, and learning and memory disorders. More specifically,
the disorder
may be Parkinson's disease and Parkinsonian disorders, Huntington's disease,
Alzheimer's
disease, multiple sclerosis, amyotrophic lateral sclerosis, Shy-Drager
syndrome, progressive
supranuclear palsy, Lewy body disease, spinal ischemia, ischemic stroke,
cerebral infarction,
spinal cord injury, and cancer-related brain and spinal cord injury, multi-
infarct dementia,
geriatric dementia, cognition impairment, depression and traumatic injury. The
method
involves administrating at least one agent, such as, Thyrocalcitonin,
Calcitonin, Exendin, and
functional analogs, variants and combinations of these agents to the patient.
The Exendin
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
4
may be Exendin-3 or Exendin-4 and functional analogs and variants thereof. The
agent (or
agents) modulate neurogenesis in the patient, and modulate neurogenesis in the
neural tissue
of the patient. Modulating neurogenesis may be performed by an activation of a
GPCR
receptor in the neural tissue of the patient.
The agent may be a calcitonin analog such as katacalcin, calcitonin-gene-
related-
peptide, calcitonin-receptor-stimulating-peptides 1, calcitonin-receptor-
stimulating-peptides
2, calcitonin-receptor-stimulating-peptides 3, PHM-27, Intermedin, [Asp(17),
Lys(21)] side-
chain bridged salmon calcitonin, [Asp(17) Orn(21)] side-chain bridged salmon
calcitonin,
AC512 (Glaxo Wellcome and Amylin Pharmaceuticals), benzophenone-containing CT
analogs, [Arg11,18,Lys14] salmon calcitonin analog, eel calcitonin analog,
calcitonin 8-32,
or analogs or combinations thereof. The agent may be a calcitonin family
peptide member
such as CGRP 8-37, amylin amide, and analogs thereof. The agent may be an
Exendin
functional analog such as GLP-1 peptide, GLP-1 analog, CJC-1131, liraglutide,
pramlintide,
AVE-0010, or alpha-me-GLP-1. The Exendin functional analog, which includes at
least
Exendin-3 or Exendin-4 functional analogs, may be, for example, a peptide with
an amino
acid sequence of SEQ ID No:21, SEQ ID No:27, SEQ ID No:69, SEQ ID No:70, SEQ
ID
No:71, SEQ 113 No:72, SEQ ID No:73, SEQ ID No:74, SEQ ID No:75, SEQ ID No:76,
SEQ
ID No:77, SEQ ID No:78, SEQ ID No:79, SEQ ID No:80 or SEQ ID No:81. The agent
may
also be a GLP-1 receptor ligand peptide or a PACAP receptor ligand peptide.
The agent may be administered to achieve a tissue concentration in the patient
of
between 0.0001 nM to 50 nM. The amount of administered may be about 0.5
microgram to
about 100 micrograms per day, about 0.1 microgram to about 20 micrograms per
day, about
0.2 microgram to about 40 micrograms per day, about 5 micrograms to about 200
micrograms per day, about 10 micrograms to about 20 micrograms per day, about
20
micrograms to about 200 micrograms per day, about 50 micrograms to about 100
mg per day,
about 0.1 mg to about 200 mg per day, about 50 mg to about 200 mg per day, or
about 0.1 to
about 1 gram per day.
Another embodiment of the invention is directed to a method for modulating
neurogenesis in vitro. The method involves culturing a population of neural
cells comprising
neural stem cells; adding to the cultured cells at least one neurogenesis
modulating agent; and
repeating the adding step until a desired level of neurogenesis in achieved.
Neurogenesis
may be the increase in the amount of neural stem cells or adult neural stem
cells.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Another embodiment of the invention is directed to a method for alleviating a
symptom of a disease or disorder of the central nervous system in a patient.
The method
involves providing a population of neural stem cells or neural progenitor
cells; contacting the
neural stem cells or neural progenitor cells with at least one neurogenesis
modulating agent;
5 and delivering the cells to a patient to alleviate the symptom. The
method may include the
optional step of administering the at least one neurogenesis modulating agent
to the patient
before or after the delivery step.
Another embodiment of the invention is directed to a method for transplanting
a
population of cells enriched for neural stem cells from a donor to a
recipient. The method
involves contacting a population containing neural stem cells or neural
progenitor cells
derived from a donor with a neurogenesis modulating agent; and implanting the
selected cells
into a recipient. The contacting step may involve culturing a population of
neural cells
comprising neural stem cells from said donor; adding to the cultured cells at
least one
neurogenesis modulating agent; and repeating the adding step until a desired
level of
neurogenesis in achieved. In this method, the donor and recipient may be the
same patient
(e.g., person, mammal, organism).
Another embodiment of the invention is directed to a method for increasing
adult
neural stem cells in a patient with a disorder of the central nervous system
by administering
to the patient an amount of an Exendin or Exendin analog sufficient to
increase adult neural
stem .cells in the patient and reduce at least one symptom of the disorder.
The Exendin or
Exendin analog (including derivatives) may be a peptide with an amino acid
sequence of
SEQ ID No:21, SEQ ID No:27, SEQ ID No:69, SEQ ID No:70, SEQ ID No:71, SEQ lD
No:72, SEQ ID No:73, SEQ ID No:74, SEQ ID No:75, SEQ ID No:76, SEQ ID No:77,
SEQ
ID No:78, SEQ ID No:79, SEQ ID No:80, or SEQ ID No:81. The disorder may be any
central nervous system disorder listed in this specification including, at
least, Parkinson's
disease and Parkinsonian disorders, Huntington's disease, Alzheimer's disease,
multiple
sclerosis, amyotrophic lateral sclerosis, Shy-Drager syndrome, progressive
supranuclear
palsy, Lewy body disease, spinal ischemia, ischemic stroke, cerebral
infarction, spinal cord
injury, and cancer-related brain and spinal cord injury, multi-infarct
dementia, geriatric
dementia, cognition impairment, depression or traumatic injury. The Exendin or
Exendin
analog is administered at an amount of about 0.001 microgram to about 20
micrograms per
kilogram of body weight per day, about 0.01 microgram to about 2 micrograms
per kilogram
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
6
of body weight per day, or about 0.02 microgram to about 0.4 microgram per
kilogram of
body weight per day.
In all cases, the cell, neural tissue, or patient may be any mammal such as
rat, mice,
cat, dog, horse, pig, goat, cow and in particular human (adult, juvenile or
fetal).
BRIEF DESCRIPTION OF THE FIGURES
FIGURE 1: CREB phosphorylation following PACAP and cholera toxin treatment
occurs in a reproducible manner in both mouse and human adult neural stem
cells as shown
by Western blotting. The upper panel shows up-regulation of CREB
phosphorylation in
mouse and human adult neural stem cells after PACAP treatment. The lower panel
shows
up-regulation of CREB phosphorylation in both mouse and human adult neural
stem cells
after cholera toxin treatment.
FIGURE 2: plots the number of BrdU positive cells after an animal is
administered
Exendin-4, calcitonin, or vehicle (sham injected with saline).
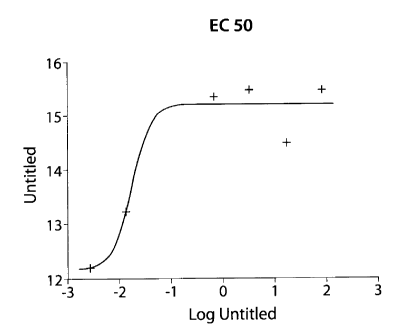
FIGURE 3: is a dose response curve showing that the EC50 value for calcitonin
is
0.03 nM.
FIGURE 4: is a dose response curve showing that the EC50 value for Exendin is
0.017 nM.
FIGURE 5: shows increase proliferation of adult human neural stem/progenitor
cells
in response to Exendin-4.
. FIGURE 6: shows that Exendi-4 independently increase dentate gyrys
proliferation.
FIGURE 7: shows that Exendin-4 significantly increase the number of
doublecortin
positive cells in rat hippocampus.
DETAILED DESCRIPTION OF THE INVENTION
Traditional treatments of neural diseases and injuries have focused on the
prevention
of neuronal death (i.e., apoptosis or necrosis). In contrast, this invention
is directed to novel
therapeutic treatments for neurological diseases and injuries based on
inducing neurogenesis,
in particular, neural stem cell, or progenitor cell proliferation. In
accordance with the
invention, key neurogenesis modulating agents have been identified to induce
proliferation
and/or differentiation in neural cells. Such "neurogenesis modulating agents,"
also
abbreviated as "agent" in this disclosure, are useful for effecting
neurogenesis for the
treatment of neurological diseases and injuries. As shown herein, increased
levels of cAMP
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
7
and/or Ca2+ elicit the proliferation of adult neural stem cells. In some
cases, this induction
follows the activation of G-protein coupled receptors (GPCRs). The data
disclosed herein
indicate that increasing intracellular cAMP and/or Ca2+ levels through various
compounds
(e.g., GPCR ligands) can be used to increase proliferation of adult neural
stem cells.
Furthermore, the data indicates that the progeny of the cells induced to
proliferate by all the
compounds analyzed, also retain their full neurogenic potential. Expression
data for the
GPCRs that bind to these ligands corroborate the importance of these two
second messengers
in promoting neurogenesis.
Proliferation data clearly shows that tissue culture cells and mice respond
positively to
the administration of neurogenesis modulating agents. The effects neurogenesis
modulating
agent administration includes neurogenesis in vivo and in vitro. See, e.g.,
the data presented
in the Examples section.
"Neurogenesis" is defined herein as proliferation, differentiation, migration,
or
survival of a neural cell in vivo or in vitro. In a preferred embodiment, the
neural cell is an
adult, fetal, or embryonic neural stem cell or progenitor cell. Neurogenesis
also refers to a
net increase in cell number or a net increase in cell survival. As used
herein, "NSC" would
include, at least, all brain stem cells, all brain progenitor cells, and all
brain precursor cells.
In this disclosure, the terms disease or disorder shall have the same meaning.
In this disclosure, the term analog shall also mean variants, fragments, and
mimetics.
All the methods of the invention'inay be used on mammals and mammalian cells.
In
a preferred embodiment, all the methods of the invention may be used on humans
or human
cells.
Neural tissue includes, at least, all the tissues of the brain and central
nervous system.
A neurogenesis modulating agent is defines as an agent or reagent that can
promote
neurogenesis. A number of novel neurogenesis modulating agent are disclosed in
this
invention including Exendin and calcitonin.
Exendin-4 is a naturally occurring endocrine hormone that was originally
isolated
from the salivary of the lizard Heloderma suspectum (Eng J et al, J Biol Chem
1992;
267:7402-5).
Exendin-4 exhibits several glucoregulatory effects including; glucose
dependent enhancement of insulin secretion; glucose dependent suppression of
high glucagon
secretion; slowing of gastric emptying, reduction in food intake; lowering of
blood pressure
(revived in Nielsen LL et al, Regulatory Peptides 2004 117;77-88). In mammals
Exendin-4
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
8
is resistant to degradation by dipeptidyl peptidase-IV (DPP-VI), whereas GLP-1
is degraded
with a halftime less than 2 min (Kieffer TJ et al, Endocrinology 1995;
136:3585-96).
Exendin-4 is currently under clinical investigation (phase II and III) for
treatment of Diabetes
type II by Amylin pharmaceutical in collaboration with Lilly under the name
exenatide:AC2993, AC002993, AC2993A, Exendin-4, or LY2148568 CAS# 141758-74-9
(Drugs RD 2004;5(1):35-40).
Studies have shown that intravenous injections of Exendin-4 pass the mouse
blood-
brain barrier (BBB) and reach the brain intact (Kastin AJ, Akerstrom V, Int J
Obes Relat
Metab Disord. 2003 Mar;27(3):313-8). Interestingly, the homozygous mice GLP-1R
knockout the animals shows contextual fear learning deficit. Additionally,
Rats over
expressing Glplr shows improved learning and memory. Glp 1r-deficient mice
also have
enhanced seizure severity and neuronal injury after kainate administration,
which was
reduced after Glplr hippocampal gene transfer. The finding suggests a role for
GLP1R and
=
its ligands in learning and neuro-protection.
Calcitonin is secreted from the thyroid C cells and inhibits both basal and
stimulated
resorption of bone and reduces osteoclast numbers. Calcitonin is a 32-amino-
acid-long
peptide belonging to the class II secretin like superfamily of GPCRs.
For the purposes of this application, calcitonin and thyrocalcitonin include
other
molecules that are their analogs, derivatives, and hybrid molecules including
calcitonin.
These include, at least, molecules described in US patents 6,713,452,
6,673,769, 6,617,423,
6,268,339, 6,265,534, 6,083,480, 6,028,168, 5,831,000, 4,658,014, 4,652,627,
4,644,054,
4,597,900, 4,497,731, 4,495,097, 4,451,395. These molecules include calcitonin
drug or
thyrocalcitonin drug which mean a drug possessing all or some of the
biological activity of
calcitonin or thyrocalcitonin respectively. These molecules also include
calcitonin fragments
or thyrocalcitonin fragments.
As used herein, the term "calcitonin" includes, at least, chicken calcitonin,
eel
calcitonin, human calcitonin, porcine calcitonin, rat calcitonin or salmon
calcitonin provided
by natural, synthetic, or genetically engineered sources.
As used herein, the term "calcitonin analog" or "thyrocalcitonin analog" means
calcitonin or thyrocalcitonin wherein one or more of the amino acids have been
replaced
while retaining some or all of the activity of the calcitonin or
thyrocalcitonin. The analog is
described by noting the replacement amino acids with the position of the
replacement as a
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
9
superscript followed by a description of the calcitonin. For example, "Pro2
calcitonin, human"
means that the glycine typically found at the 2 position of a human calcitonin
molecule has
been replaced with proline.
Calcitonin or thyrocalcitonin analogs may be obtained by various means, as
will be
understood by those skilled in the art. For example, certain amino acids may
be substituted
for other amino acids in the calcitonin structure without appreciable loss of
interactive
binding capacity with structures such as, for example, antigen-binding regions
of antibodies
or binding sites on substrate molecules. As the interactive capacity and
nature of calcitonin
defines its biological functional activity, certain amino acid sequence
substitutions can be
made in the amino acid sequence and nevertheless remain a polypeptide with
like properties.
As outlined above, amino acid substitutions are generally therefore based on
the
relative similarity of the amino acid side-chain substituents, for example,
their
hydrophobicity, hydrophilicity, charge, size, and the like. Exemplary
substitutions (i.e.,
amino acids that may be interchanged without significantly altering the
biological activity of
the polypeptide) that take the foregoing characteristics into consideration
are well known to
those of skill in the art and include, for example: arginine and lysine;
glutamate and aspartate;
serine and threonine; glutamine and asparagine; and valine, leucine and
isoleucine.
As used herein, the term "calcitonin fragment" means a segment of the amino
acid
sequence found in the calcitonin that retains some or all of the activity of
the calcitonin.
Similarly, the term "thyrocalcitonin fragment" means a segment of the amino
acid sequence
found in the thyrocalcitonin that retains some or all of the activity of the
thyrocalcitonin.
The capability of a cell to divide without limit and produce daughter cells
that
terminally differentiate into neurons and glia are stem cell characteristics.
Thus, the term
"stem cell" (e.g., neural stem cell), as used herein, refers to an
undifferentiated cell that can
be induced to proliferate using the methods of the present invention. The stem
cell is capable
of self-maintenance, meaning that with each cell division, one daughter cell
will also be a
stem cell. The non-stem cell progeny of a stem cell are termed progenitor
cells. The
progenitor cells generated from a single multipotent stem cell are capable of
differentiating
into neurons, astrocytes (type I and type II) and oligodendrocytes. Hence, the
stem cell is
multipotent because its progeny have multiple differentiation pathways.
The term "progenitor cell" (e.g., neural progenitor cell), as used herein,
refers to an
undifferentiated cell derived from a stem cell, and is not itself a stem cell.
Some progenitor
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
cells can produce progeny that are capable of differentiating into more than
one cell type. For
example, an 0-2A cell is a glial progenitor cell that gives rise to
oligodendrocytes and type II
astrocytes, and thus could be termed a bipotential progenitor cell. A
distinguishing feature of
a progenitor cell is that, unlike a stem cell, it has limited proliferative
ability and thus does
5 not exhibit self-maintenance. It is committed to a particular path of
differentiation and will,
under appropriate conditions, eventually differentiate into glia or neurons.
The term
"precursor cells", as used herein, refers to the progeny of stem cells, and
thus includes both
progenitor cells and daughter stem cells.
10 Neurogenesis modulating agents
One embodiment of the invention is directed to novel neurogenesis modulating
agents
that modulate intracellular levels of cAMP and/or Calf-. As used herein,
neurogenesis
modulating agent also include any substance that is chemically and
biologically capable of
increasing cAMP (e.g., by increasing synthesis or decreasing breakdown) and/or
Ca2+ (e.g.,
by increasing influx or decreasing efflux). These neurogenesis modulating
agents include
peptides, proteins, fusion proteins, chemical compounds, small molecules, and
the like.
Preferred for use with the invention are neurogenesis modulating agents
comprising cAMP
analogs, PDE inhibitors (e.g., cAMP-specific PDEs), adenylate cyclase
activators, and
activators of ADP-ribosylation of stimulatory G proteins.
Agents that have been shown in the experiments detailed herein to increase
intracellular
levels of cAMP include:
Name Peptide sequence Identifier
Thyrocalcitonin salmon Cys-Ser-Asn-Leu-Ser-Thr-Cys-Val-Leu-Gly-Lys-Leu-Ser-
SEQ ID NO:1
Gln-Glu-Leu-His-Lys-Leu-Gln-Thr-Tyr-Pro-Arg-Thr-Asn-Thr-
Gly-Ser-Gly-Thr-Pro-NH2
Calcitonin (Human) Cys-Gly-Asn-Leu-Ser-Thr-Cys-Met-Leu-Gly-Thr-Tyr-Thr-
SEQ ID NO:2
Gln-Asp-Phe-Asn-Lys-Phe-His-Thr-Phe-Pro-Gln-Thr-Ala-lle-
Gly-Val-Gly-Ala-Pro
Exendin-3 His-Ser-Asp-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-
SEQ ID NO:3
Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-
Asn-Gly-Gly- Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
Exendin-4 His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-
SEQ ID NO:4
Glu-Glu-Glu-Ala-Va I-Arg-Leu-Phe-I le-Glu-Trp-Leu-Lys-Asn-
Gly-Gly- Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
Exemplary agents for increasing intracellular Ca2+ levels include, but are not
limited to
the agents summarized in the table below:
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
11
Name Peptide sequence Identifier
Amylin Receptor Val-Leu-Gly-Lys-Leu-Ser-Gln-Glu-Leu-His-
SEQ ID NO:5
Antagonist/Calcitonin(8-32)(Salmon). Lys-Leu-Gln-Thr-Tyr-Pro-Arg-Thr-Asn-Thr-
Gly-Ser-Gly-Thr-Pro
CGRP (8-37) (Human) (Selective Val-Thr-His-Arg-Leu-Ala-Gly-Leu-Leu-Ser-
SEQ ID NO:6
antagonist for CGRP receptor and Arg-Ser-Gly-Gly-Val-Val-Lys-Asn-Asn-Phe-
agonist for Calcitonin receptor). Val-Pro-Thr-Asn-Val-Gly-Ser-Lys-Ala-Phe-
NH2
amylin amide Lys-Cys-Asn-Thr-Ala-Thr-Cys-Ala-Thr-Gln- SEQ
ID NO:7
Arg-Leu-Ala-Asn-Phe-Leu-Val-H is-Ser-Ser-
Asn-Asn-Phe-Gly-Ala- Ile-Leu-Ser-Ser-Th r-
Asn-Val-Gly-Ser-Asn-Thr-Tyr
Calcitonin analogs also include, at least, the following: (1) Katacalcin; (2)
Calcitonin-
Gene-Related-Peptide (CGRP); (3) Calcitonin-Receptor-Stimulating-Peptides
(CRSP)1, 2 or
3; (4) Orphan peptide PHM-27 (hCT receptor agonist); (5) Intermedin; (6)
[Asp(17),
Lys(21)] and [Asp(17), Orn(21)] side-chain bridged salmon calcitonin (sCT) and
hCT
analogues; (7) AC512 (Glaxo Wellcome and Amylin Pharmaceuticals); (8)
Benzophenone-
containing CT analogs (Pharmacol Exp Ther. 1997 Nov;283(2):876-84); (9)
Analogs of
salmon calcitonin (sCT) [Arg11,18,Lys14]sCT; (10) Analogs of eel calcitonin
(eCT) (Eur J
Biochem. 1991 Nov 1;201(3):607-14). Each analog is described in more detail
below.
Katacalcin (KC) belongs to a small family of polypeptides encoded by the calc-
1 gene
and also include calcitonin (CT) and procalcitonin. Katacalcin includes the
amino acid
sequence Asp-Met-Ser-Ser-Asp-Leu-Glu-Arg-Asp-His-Arg-Pro-His-Val-Ser-Met-Pro-
Gln-
Asn-Ala-Asn (SEQ ID NO:8) and analogs thereof. See, e.g., J Bone Miner Res.
2002
Oct;17(10): 1872-82.
Human calcitonin gene related peptide includes the amino acid sequence: Ala-
Cys-Asp-
Thr-Ala-Thr-Cys-Val-Thr-His-Arg-Leu-Ala-Gly-Leu-Leu-Ser-Arg-Ser-Gly-Gly-Val-
Val-
Lys-Asn-Asn-Phe-Val-Pro-Thr-Asn-Val-Gly-Ser-Lys-Ala-Phe-NH2 (SEQ ID NO:9) and
analogs thereof.
Calcitonin receptor stimulating peptide 1 (CRSP-1) includes the amino acid
sequence
SCNTATCMTHRLVGLLSRSGSMVRSNLLPTKMGFKVFG (SEQ ID NO:10) and analogs
thereof. Calcitonin receptor stimulating peptide 2 (CRSP-2) includes the amino
acid
sequence SCNTASCVTHKMTGWLSRSGSVAKNNFMPTNVDSKIL (SEQ ID NO:11) and
analogs thereof. Calcitonin receptor stimulating peptide 3 (CRSP-3) includes
the amino acid
sequence SCNTAICVTHKMAGWLSRSGSVVKNNFMPINMGSKVL (SEQ ID NO:12) and
analogs thereof. See, e.g., Biochem Biophys Res Commun. 2003 Aug 29;308(3):445-
51.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
12
Histidine-methionine amide peptide hormone (PHM-27) includes the amino acid
sequence His-Ala-Asp-Gly-Val-Phe-Thr-Ser-Asp-Phe-Ser-Lys-Leu-Leu-Gly-Gln-Leu-
Ser-
Ala-Lys-Lys-Tyr-Leu-Glu-Ser-Leu-Met-NH2 (SEQ ID NO:13) and analogs thereof.
See,
e.g., Biochem Pharmacol. 2004 Apr 1;67(7):1279-84.
Intermedin includes the amino acid sequence Thr-Gln-Ala-Gln-Leu-Leu-Arg-Val-
Gly-
Cys-Val-Leu-Gly-Thr-Cys-Gln-Val-Gln-Asn-Leu-Ser-His-Arg-Leu-Trp-Gln-Leu-Met-
Gly-
Pro-Ala-Gly-Arg-Gln-Asp-Ser-Ala-Pro-Val-Asp-Pro-Ser-Ser-Pro-His-Ser-Tyr-NH2
(SEQ ID
NO:14) and analogs thereof. See, e.g., J Biol Chem. 2004 Feb 20;279(8):7264-
74.
Side-chain lactam-bridged analogs of human calcitonin (hCT) have been
described
(Kapurniotu, A.; et al. Eur. J. Biochem. 1999, 265, 606-618). Other side chain
analogs of
calcitonin, including a series of (Asp(17), Lys(21)) and (Asp(17), Orn(21))
side-chain
bridged salmon calcitonin (sCT) and hCT have been synthesized. [Asp17, Lys21]-
side-chain
bridged salmon calcitonin includes the sequence Cys-Ser-Asn-Leu-Ser-Thr-Cys-
Val-Leu-
Gly-Lys-Leu-Ser-Gln-Asp-Leu-Asp-Lys-Leu-Gln-Lys-Phe-Pro-Arg-Thr-Asn-Thr-Gly-
Ala-
Gly-Val-Pro (SEQ ID NO:15), wherein Asp17 and Lys21 are linked by a lactam-
bridge, and
analogs thereof. [Asp17, Orn21]-side-chain bridged salmon calcitonin includes
the sequence
Cys-Ser-Asn-Leu-Ser-Thr-Cys-Val-Leu-Gly-Lys-Leu-Ser-Gln-Asp-Leu-Asp-Lys-Leu-
Gln-
Orn-Phe-Pro-Arg-Thr-Asn-Thr-Gly-Ala-Gly-Val-Pro (SEQ ID NO:16), wherein Asp17
and
Orn21 are linked by a lactam-bridge, and analogs thereof. See, e.g., J Med
Chem. 2002 Feb
28;45(5):1108-21. For salmon calcitonin sequence and analogs, see, e.g.,
Hilton et al., 2000, ,
J. Endocrinol. 166:213-226. For side-chain bridged analogs, see, e.g., Taylor
et al., 2002, J.
Med. Chem. 45:1108-1121.
[Lysll-Bolton Hunter, Argl 8, Asn30, Tyr32]-salmon calcitonin (9-32) includes
the
sequence Leu-Gly-Lys-Leu-Ser-Gln-Asp-Leu-His-Arg-Leu-Gln-Thr-Phe-Pro-Arg-Thr-
Asn-
Thr-Gly-Ala-Asn-Val-Tyr (SEQ ID NO:17; also called AC512, Glaxo Wellcome and
Amylin
Pharmaceuticals), and analogs thereof.
Analogs of salmon calcitonin (sCT) have been synthesized (e.g., [Argl 1, 18,
Lys14]-
salmon calcitonin) to provide a free amino group for derivatization. [Argil,
18, Lys14]-
salmon calcitonin includes the sequence Cys-Ser-Asn-Leu-Ser-Thr-Cys-Val-Leu-
Gly-Arg-
Leu-Ser-Lys-Asp-Leu-His-Arg-Leu-Gln-Thr-Phe-Pro-Arg-Thr-Asn-Thr-Gly-Ala-Gly-
Val-
Pro (SEQ ID NO:18). The potency of [Argl 1, 18, Lys14]-sCT was found to be
equivalent to
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
13
that of sCT in activating adenylate cyclase in UMR 106-06 cells. The analog
was derivatized
with biotin, fluorescein, or 4-azidobenzoate without loss of activity. The
derivatized analog
was not degraded by lysine-specific endoprotease, whereas the underivatized
[Argl 1, 18,
Lys14]-sCT was cleaved at Lys-14. The derivatized analogs were purified by
HPLC and
subsequently shown to possess full biological activity. The photoactive analog
was used to
photolabel 88,000 and 71,000 molecular weight components of the calcitonin
receptor in rat
osteoclasts, but only an 88,000 molecular weight component was photolabeled in
the UMR
106-06 cells. See, e.g., Endocrinology. 1988 Sep;123(3):1483-8; J Endocrinol.
2000
Jul;166(1):213-26; Glaxo Wellcome; and Amylin Pharmaceuticals.
Benzophenone-containing calcitonin includes the calcitonin sequence Cys-Ser-
Asn-
Leu-Ser-Thr-Cys-Val-Leu-Gly-Lys-Leu-Ser-Gln-Asp-Leu-His-Lys-Leu-Gln-Thr-Phe-
Pro-
Arg-Thr-Asn-Thr-Gly-Ala-Gly-Val-Pro (SEQ ID NO:19), wherein all lysine
residues are
replaced with arginine, hydrophobic residues are replaced with a
lysine(epsilon-p-
benzoylbenzoyl) residues, Va18, Leul6 and Leul9 are replaced by lysine(epsilon-
p-
benzoylbenzoyl), and the N-terminus is acetylated by a p-Bz2 moiety.
Benzophenone-
containing calcitonin analogs are described in J Pharmacol Exp Ther. 1997
Nov;283(2):876-
84J Pharmacol Exp Ther. 1997 Nov;283(2):876-84.
Eel calcitonin analog includes the sequence Asu-Ser-Asn-Leu-Ser-Thr-Asu-Val-
Leu-
Gly-Lys-Leu-Ser-Gln-Glu-Leu-His-Lys-Leu-Gln-Thr-Tyr-Pro-Arg-Thr-Asp-Val-Gly-
Ala-
.20 Gly-Thr-Pro-NH2 (SEQ ID NO:20). See, e.g., Eur J Biochem. 1991 Nov
1;201(3):607-14.
Asu represents aminosuberic acid.
Exenatide (Exendin-4) is polypeptide with the amino acid sequence of
HGEGTFTSDLSKQM EEEAVRLFIEWLKNGGPSSGAPPPS (SEQ ID NO:21). Exenatide
(also called AC002993, AC2993A, AC 2993, LY2148568, or Synthetic Exendin-4, is
available from Amylin Pharmaceuticals (San Diego, CA, USA) and Eli Lilly and
Co.
(Indianapolis, IN, USA). Analogs of Exendin include, at least, the ones listed
herein.
The subject invention also provides an Exendin analog peptide agent of nine to
thirty
amino acids in length which is an analog of full length Exendin. In one
embodiment, the
peptide comprises an amino acid sequence selected from the group consisting of
HGEGTFTSD (SEQ ID No:27), HGEGTFTSDLSKQMEEEAVRL (SEQ ID No:69),
HSDGTFTSD (SEQ ID No:70), HSDGTFTSDXSK (SEQ ID No:71),
HSDGTFTSDXSKXLE (SEQ ID No:72), HSDGTFTSDXSKXLEXXXA (SEQ ID No:73),
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
14
HSDGTFTSDXSKXLEXXXAXK (SEQ ID No:74), HSDGTFTSDXSKXLEXXXAXKXFI
(SEQ ID No:75), HSDGTFTSDXSKXLEXXXAXKXFIXWL (SEQ ID No:76),
HSDGTFTSDLSK (SEQ ID No:77), HSDGTFTSDLSKXME (SEQ ID No:78),
HSDGTFTSDLSKXMEXXXAVK (SEQ ID No:79), HSDGTFTSDLSKXMEXXXAVKXFI
(SEQ ID No:80), and HSDGTFTSDLSKXMEXXXAVKXFIXWLLNG (SEQ ID No:81)
wherein X represents any amino acid.
GLP-1 (Glucagon-like peptide-1) has an amino acid sequence of His-Asp-Glu-Phe-
Glu-Arg-Hi s-Al a-Glu-Gly-Thr-Phe-Thr-S er-Asp-Val-S er-S
Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg-Gly (SEQ ID NO:22). Other GLP-
1
receptor ligand peptides include, HGEGTFTSDLSKMEE (SEQ ID NO:23),
HGEGTFTSDLSKMEEE (SEQ ID NO:24), HSEGTFTSDLSKMEE (SEQ ID NO:25),
HAEGTFTSDLSKMEE (SEQ ID NO:26), HGEGTFTSD (SEQ ID NO:27),
HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG (SEQ ID NO:28) and
HDEFERHAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG (SEQ ID NO:29). See, e.g.,
Diabetes 1998 47(2):159-69; Endocrinology. 2001 Feb;142(2):521-7; Curr Pharm
Des. 2001
Sep;7(14):1399-412.
GLP-1 analogs can exhibit one or more modifications of the N-terminal sequence
of
GLP-1, which includes the sequence HAEGTFTSDVS (SEQ ID NO:30). This
encompasses
[D-His1]-GLP-1, [Ac-His1]-GLP-1, desamino-GLP-1, [D-Ala2]-GLP-1, [Gly2]-GLP-1,
[Ser2]-GLP-1, [D-A1a2, D-Asp8]-GLP-1, [D-A1a2, D-Ser8]-GLP-1, and [D-A1a2, D-
Asp9]-
GLP-1. See, e.g., Siegel et al., 1999, Regul. Pept." 79:93-102; Drucker
et al.,
Gastroenterology. 2002 Feb;122(2):531-44. For these analogs, D represents a D-
amino acid,
Ac represents an acetylated amino acid, and the first residue is designated as
His 1. Other N-
terminal modifications of GLP-1(7-37) HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG
(SEQ ID NO:31) include [Thr81-GLP-1 (7-37), [G1y8]-GLP-1 (7-37), [Ser8]-GLP-1
(7-36),
and [Aib8]-GLP-1 (7-36). See, e.g., Deacon et al., 1998, Diabetologia 41:271-
278. For these
analogs, Aib represents 1-aminoisobutyric acid and the first residue is
designated as His7.
Other N-terminal modifications of GLP-1 include alpha-me-GLP-1 peptide with
the
structure:
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
0, 14
cfrtni*4LP-1
=
Additional N-terminal modifications of GLP-1 include:
N'tiN111
N
pia =atm
¨
14"JI/4 II0 112
\
II
g .r
d
fmna-01,-/ In0.01.P.1 dip1in4106431.P.4
See, e.g., Gallwitz et al Regul Pept. 2000 Jan 29;86(1-3):103-11.
5 CJC-
1131 includes the amino acid sequence HAEGTFTSDVSSYLEGQAAKEF
IAWLVKGRK (SEQ ID NO:32), which has a single amino acid substitution of L-A1a8
to D-
A1a8 and a Lys37 addition to the COOH-terminus with selective attachment of a
[2- [242-
maleimidopropionamido(ethoxy)ethoxy] acetamide to the epsilon amino group of
Lys37. For
this analog, the first residue is designated as His7. CJC-1131 has been
previously described
10 (Kim et al., 2003, Diabetes 52:751-759) and is available from ConjuChem
(Montreal,
Quebec, Canada).
Liraglutide (also called NN-2211 and [Arg34, Lys26]-(N- epsilon -(gamma-Glu(N-
alpha-hex adecanoy1))-GLP-1(7-37)) includes the
sequence
HAEGTFTSDVSSYLEGQAAKEFIAWKVRGRG (SEQ ID NO:33) and is available from
15 Novo Nordisk (Denmark) or Scios (Fremont, CA, USA). See, e.g., Elbrond
et al., 2002,
Diabetes Care. Aug;25(8):1398-404; Agerso et al., 2002, Diabetologia.
Feb;45(2):195-202.
Pramlintide (amylin analog) includes the sequence KCNTATCATQRLANFLVH
SSNNFGPILPPYNVGSNTY (SEQ ID NO:34) and is available from Amylin
Pharmaceuticals (San Diego, CA, USA) and Johnson and Johnson (New Brunswick,
NJ
USA.)). Pramlintide is also called 25,28,29-pro-h-amylin and Symilin. See,
e.g., Thompson
et al., 1998, Diabetes Care, 21:987-993; Maggs et al., 2003, Metabolism.
Dec;52(12):1638-
42; Whitehouse et al., 2002, Diabetes Care 25(4):724-30; Fineman et al., 2002,
Metabolism
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
16
51(5):636-41. Amylin is described in US 5,367,052 as including the sequence
KCNTATCATQRLANFLVHSSNN FGAILSSTNVGSNTY (SEQ ID NO:35).
AVE-0010 (also called ZP-10) is available from Aventis (France).
[Ser(2)]-Exendin (1-9) includes the sequence HSEGTFTSD (SEQ ID NO:36) and has
been described in Nature 1173-1179 (2003).
Still other neurogenesis modulating agents include PACAP receptors ligand
peptides
HSTGTFTSMDTSQLP (SEQ lD NO:37), HSTGTFTSMDT (SEQ ID NO:38),
HSTGTFTSMD (SEQ ID NO:39), QSTGTFTSMD (SEQ ID NO:40), QTTGTFTSMD (SEQ
ID NO:41) and HTTGTFTSMD (SEQ ID NO:42).
The neurogenesis modulating agents (also referred to as the agents) of this
disclosure
are as listed in this section. It is understood that these neurogenesis
modulating agent
(agents) may be used, individually or in any combinations, wherever
neurogenesis
modulating agent or agents is specified in this specification. In one aspect
of the invention
"neurogenesis modulating agent" means any agents listed in this section. In
another aspect of
the invention, the neurogenesis modulating agent increases or maintains the
amount of
doublecortin positive cells or the percentage of doublecortin positive cells
in a cell population
or neural tissue.
Production of Neurogenesis modulating agents
Neurogenesis modulating agents may be produced using known techniques of
chemical synthesis including the use of peptide synthesizers.
Neurogenesis modulating agents that are peptides and proteins may be
synthesized
chemically using commercially available peptide synthesizers. Chemical
synthesis of
peptides and proteins can be used for the incorporation of modified or
unnatural amino acids,
including D-amino acids and other small organic molecules. Replacement of one
or more L-
amino acids in a peptide or protein with the corresponding D-amino acid
isoforms can be
used to increase resistance to enzymatic hydrolysis, and to enhance one or
more properties of
biological activity, i.e., receptor binding, functional potency or duration of
action.
Introduction of covalent cross-links into a peptide or protein sequence can
conformationally and topographically constrain the peptide backbone for
increased potency,
selectivity, and stability. Other methods used successfully to introduce
conformational
constraints into amino acid sequences to improve their potency, receptor
selectivity, and
biological half-life include the use of (i) Ca-methylamino acids (see, e.g.,
Rose, et al., Adv.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
17
Protein Chem. 37: 1-109 (1985); Prasad and Balaram, CRC Crit. Rev. Biochem.,
16: 307-348
(1984)); (ii) Na-methylamino acids (see, e.g., Aubry, et al., hit. J. Pept.
Protein Res., 18:
195-202 (1981); Manavalan and Momany, Biopolymers, 19: 1943-1973 (1980)); and
(iii) a43-unsaturated amino acids (see, e.g., Bach and Gierasch, Biopolymers,
25: 5175-S192
(1986); Singh, et al., Biopolymers, 26: 819-829 (1987)). Additionally,
replacement of the C-
terminal acid with an amide can be used to enhance the solubility and
clearance of a peptide
or protein.
Alternatively, a neurogenesis modulating agent may be obtained by methods well
known in the art for recombinant peptide or protein expression and
purification. A DNA
molecule encoding the neurogenesis modulating agent can be generated. The DNA
sequence
is known or can be deduced from the amino acid sequence based on known codon
usage.
See, e.g., Old and Primrose, Principles of Gene Manipulation 3( ed., Blackwell
Scientific
Publications, 1985; Wada et al., Nucleic Acids Res. 20: 2111-2118(1992).
Preferably, the
DNA molecule includes additional sequences, e.g., recognition sites for
restriction enzymes
which facilitate its cloning into a suitable cloning vector, such as a
plasmid. Nucleic acids
may be DNA, RNA, or a combination thereof.
The biologically expressed neurogenesis modulating agent may be purified using
known purification techniques. An "isolated" or "purified" recombinant peptide
or protein, or
biologically active portion thereof, means that said peptide or protein is
substantially free of
cellular material or other contaminating proteins from the cell or tissue
source from which it
is derived. The language "substantially free of cellular material" includes
preparations in
which the peptide or protein is separated from cellular components of the
cells from which it
is isolated or recombinantly produced. In one embodiment, the language
"substantially free
of cellular material" includes preparations of peptide or protein having less
than about 30%
(by dry weight) of product other than the desired peptide or protein (also
referred to herein as
a "contaminating protein"), more preferably less than about 20% of
contaminating protein,
still more preferably less than about 10% of contaminating protein, and most
preferably less
than about 5% contaminating protein. When the peptide or protein, or
biologically active
portion thereof, is recombinantly produced, it is also preferably
substantially free of culture
medium, i.e., culture medium represents less than about 20%, more preferably
less than about
10%, and most preferably less than about 5% of the volume of the peptide or
protein
preparation.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
18
The invention also pertains to variants and derivatives of a neurogenesis
modulating
agent that function as either agonists (mimetics) or partial agonists.
Variants of a
neurogenesis modulating agent can be generated by mutagenesis, e.g., discrete
point
mutations. An agonist of a neurogenesis modulating agent can retain
substantially the same,
or a subset of, the biological activities of the naturally occurring form of
the neurogenesis
modulating agent. Thus, specific biological effects can be elicited by
treatment with a variant
with a limited function. In one embodiment, treatment of a subject with a
variant having a
subset of the biological activities of the naturally occurring form of the
neurogenesis
modulating agent has fewer side effects in a subject relative to treatment
with the non-variant
neurogenesis modulating agent.
Preferably, the analog, variant, or derivative neurogenesis modulating agent
is
functionally active. As utilized herein, the term "functionally active" refers
to species
displaying one or more known functional attributes of neurogenesis. "Variant"
refers to a
neurogenesis modulating agent differing from naturally occurring neurogenesis
modulating
agent, but retaining essential properties thereof.
Variants of the neurogenesis modulating agent that function as agonists
(mimetics)
can be identified by screening combinatorial libraries of mutants of the
neurogenesis
modulating agent for peptide or protein agonist or antagonist activity. In one
embodiment, a
variegated library of variants is generated by combinatorial mutagenesis at
the nucleic acid
level and is encoded by a variegated gene library. A variegated library of
variants can be
produced by, for example, enzymatically ligating a mixture of synthetic
oligonucleotides into
gene sequences such that a degenerate set of potential sequences is
expressible as individual
peptides, or alternatively, as a set of larger fusion proteins (e.g., for
phage display) containing
the set of sequences therein. There are a variety of methods that can be used
to produce
libraries of potential variants from a degenerate oligonucleotide sequence.
Chemical
synthesis of a degenerate gene sequence can be performed in an automatic DNA
synthesizer,
and the synthetic gene then ligated into an appropriate expression vector. Use
of a degenerate
set of genes allows for the provision, in one mixture, of all of the sequences
encoding the
desired set of potential sequences.
Derivatives and analogs of a neurogenesis modulating agent of the invention or
individual moieties can be produced by various methods known within the art.
For example,
the amino acid sequences may be modified by any number of methods known within
the art.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
19
See e.g., Sambrook, et al., 1990. Molecular Cloning: A Laboratory Manual, 2nd
ed., (Cold
Spring Harbor Laboratory Press; Cold Spring Harbor, NY). Modifications
include:
glycosylation, acetylation, phosphorylation, arnidation, derivatization by
known
protecting/blocking groups, linkage to an antibody molecule or other cellular
reagent, and the
like. Any of the numerous chemical modification methodologies known within the
art may
be utilized including, but not limited to, specific chemical cleavage by
cyanogen bromide,
trypsin, chyrnotrypsin, papain, V8 protease, NaBH4, acetylation, formylation,
oxidation,
reduction, metabolic synthesis in the presence of tunicamycin, etc.
Derivatives and analogs may be full length or other than full length, if said
derivative
or analog contains a modified nucleic acid or amino acid, as described infra.
Derivatives or
analogs of the neurogenesis modulating agent include, but are not limited to,
molecules
comprising regions that are substantially homologous in various embodiments,
of at least
30%, 40%, 50%, 60%, 70%, 80%, 90% or preferably 95% amino acid identity when:
(i)
compared to an amino acid sequence of identical size; (ii) compared to an
aligned sequence
in that the alignment is done by a computer homology program known within the
art (e.g.,
Wisconsin GCG software) or (iii) the encoding nucleic acid is capable of
hybridizing to a
sequence encoding the aforementioned peptides under stringent (preferred),
moderately
stringent, or non-stringent conditions. See, e.g., Ausubel, et al., Current
Protocols in
Molecular Biology, John Wiley and Sons, New York, NY, 1993.
Derivatives of a neurogenesis modulating agent of the invention may be
produced by
alteration of their sequences by substitutions, additions, or deletions that
result in functionally=
equivalent molecules. One or more amino acid residues within the neurogenesis
modulating
agent may be substituted by another amino acid of a similar polarity and net
charge, thus
resulting in a silent alteration. Conservative substitutes for an amino acid
within the
sequence may be selected from other members of the class to which the amino
acid belongs.
For example, nonpolar (hydrophobic) amino acids include alanine, leucine,
isoleucine, valine,
proline, phenylalanine, tryptophan, and methionine. Polar neutral amino acids
include
glycine, serine, threonine, cysteine, tyrosine, asparagine, and glutamine.
Positively charged
(basic) amino acids include arginine, lysine, and histidine. Negatively
charged (acidic)
amino acids include aspartic acid and glutamic acid.
Neurogenesis modulating agents also include functional mimetic. A functional
mimetic means a substance that may not contain an active portion of a protein
or peptide but,
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
and probably is not a peptide at all, but which has the property of binding to
a receptor for the
peptide or protein.
Compositions comprising neurogenesis modulating agent(s) and their
administration
Another embodiment of the invention is directed to pharmaceutical compositions
5 comprising a neurogenesis modulating agent of the invention. The
neurogenesis modulating
agents of the invention can be formulated into pharmaceutical compositions
that can be used
as therapeutic agents for the treatment of neurological diseases (disorders).
These
compositions are discussed in this section. It is understood that any
pharmaceutical
compositions and chemicals discussed in this section can be a component of a
pharmaceutical
10 composition comprising one or more neurogenesis modulating agents.
Neurogenesis modulating agents, derivatives, and co-administered agents can be
incorporated into pharmaceutical compositions suitable for administration.
Such
compositions typically comprise the agent and a pharmaceutically acceptable
carrier. As
used herein, "pharmaceutically acceptable carrier" is intended to include any
and all solvents,
15 dispersion media, coatings, antibacterial and antifungal agents,
isotonic and absorption
delaying agents, and the like, compatible with pharmaceutical administration.
The use of
such media and agents for pharmaceutically active substances is well known in
the art.
Except insofar as any conventional media or agent is incompatible with the
active compound,
use thereof in the compositions is contemplated. Supplementary active
compounds can also
20 be incorporated into the compositions. Modifications can be made to the
agents to affect
solubility or clearance of the peptide. Peptidic molecules may also be
synthesized with D-
amino acids to increase resistance to enzymatic degradation. In some cases,
the composition
can be co-administered with one or more solubilizing agents, preservatives,
and permeation
enhancing agents.
Preferably, the pharmaceutical composition is used to treat diseases by
stimulating
neurogenesis (i.e., cell growth, proliferation, migration, survival and/or
differentiation). For
treatment, a method of the invention comprises administering to the subject an
effective
amount of a pharmaceutical composition including an agent of the invention (1)
alone in a
dosage range of 0.001 ng/kg/day to 500 ng/kg/day, preferably in a dosage range
of 0.05 to
150 or up to 300 ng/kg/day, (2) in a combination permeability increasing
factor, or (3) in
combination with a locally or systemically co-administered agent.
The level of
administration may be at least 0.001 ng/kg/day, at least 0.01 ng/kg/day, 0.1
ng/kg/day, at least
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
21
1 ng/kg/day, at least 5 mg/kg/day, at least 10 mg/kg/day, or at least 50
mg/kg/day. In a
preferred embodiment, the administration raises the intracellular levels of
cAMP at least 20%
above normal. The administration may lead to tissue concentrations of the
agent of about
0.0001 nM to 50 nM.
A pharmaceutical composition of the invention is formulated to be compatible
with its
intended route of administration. Such compositions are known. The parenteral
preparation
can be enclosed in ampoules, disposable syringes or multiple dose vials made
of glass or
plastic.
Oral administration refers to the administration of the formulation via the
mouth
through ingestion, or via any other part of the gastrointestinal system
including the esophagus
or through suppository administration. Parenteral administration refers to the
delivery of a
composition, such as a composition comprising a neurogenesis modulating agent
by a route
other than through the gastrointestinal tract (e.g., oral delivery). In
particular, parenteral
administration may be via intravenous, subcutaneous, intramuscular or
intramedullary (i.e.,
intrathecal) injection. Topical administration refers to the application of a
pharmaceutical
agent to the external surface of the skin or the mucous membranes (including
the surface
membranes of the nose, lungs and mouth (in which case it may also be a form of
oral
administration, such that the agent crosses the external surface of the skin
or mucous
membrane and enters the underlying tissues. Topical administration of a
pharmaceutical
agent can result in a limited distribution of the agent to the skin and
surrounding tissues or,
when the agent is removed from the treatment area by the bloodstream, can
result in systemic
distribution of the agent.
In a preferred form of topical administration, the neurogenesis promoting
agent is
delivered by transdermal delivery. Transdermal delivery refers to the
diffusion of an agent
across the barrier of the skin. Absorption through intact skin can be enhanced
by placing the
active agent in an oily vehicle before application to the skin (a process
known as inunction)
and the use of microneedles. Passive topical administration may consist of
applying the
active agent directly to the treatment site in combination with emollients or
penetration
enhancers. Another method of enhancing delivery through the skin is to
increase the dosage
of the pharmaceutical agent. The dosage for topical administration may be
increased up to
ten, a hundred or a thousand folds more than the usual dosages stated
elsewhere in this
disclosure.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
22
In addition, the medicament and neurogenesis modulating agents of the
invention may
be delivered by nasal or pulmonary methods. The respiratory delivery of
aerosolized
medicaments is described in a number of references, beginning with Gamslen
(1925) Klin.
Wochenschr. 4:71 and including Laube et al. (1993) JAMA 269:2106-21-9; Elliott
et al.
(1987) Aust. Paediatr. J. 23:293-297; Wigley et al. (1971) Diabetes 20:552-
556. Corthorpe et
al. (1992) Pharm Res 9:764-768; Govinda (1959) Indian J. Physiol. Pharmacol.
3:161-167;
Hastings et al. (1992) J. Appl. Physiol. 73:1310-1316; Liu et al. (1993) JAMA
269:2106-
2109; Nagano et al. (1985) Jikeikai Med. J. 32:503-506; Sakr (1992) Int. J.
Phar. 86:1-7; and
Yoshida et al. (1987) Clin. Res. 35:160-166. Pulmonary delivery of dry powder
medicaments is described in U.S. Pat. No. 5,254,330. A metered dose inhaler is
described in
Lee and Sciara (1976) J. Pharm. Sci. 65:567-572. The intrabronchial
administration of
recombinant insulin is briefly described in Schlutiter et al. (Abstract)
(1984) Diabetes 33:75A
and Kohler et al. (1987) Atemw. Lungenkrkh. 13:230-232. Intranasal and
respiratory
delivery of a variety of polypeptides are described in U.S. Pat. No. 5,011,678
and Nagai et al.
(1984) J. Contr. Rel. 1:15-22.
Pharmaceutical compositions suitable for injectable use include sterile
aqueous
solutions (where water soluble) or dispersions and sterile powders for the
extemporaneous
preparation of sterile injectable solutions or dispersion. For intravenous
administration,
physiologically acceptable, suitable carriers include physiological saline,
bacteriostatic water,
Cremophor ELTM (BASF, Parsippany, N.J.) or phosphate buffered saline (PBS).
Physiologically acceptable carriers maybe any carrier known in the field as
suitable for
pharmaceutical (i.e., topical, oral, and parenteral) application. Suitable
pharmaceutical
carriers and formulations are described, for example, in Remington's
Pharmaceutical
Sciences (19th ed.) (Genarro, ed. (1995) Mack Publishing Co., Easton, Pa.).
Oral compositions generally include a physiologically acceptable, inert
diluent or an
edible carrier. They can be enclosed in gelatin capsules or compressed into
tablets. For the
purpose of oral therapeutic administration, the neurogenesis modulating agent
of the
invention can be incorporated with physiological excipients and used in the
form of tablets,
troches, or capsules.
A number of systems that alter the delivery of injectable drugs can be used to
change
the phannacodynamic and pharmacokinetic properties of therapeutic agents (see,
e.g., K.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
23
Reddy, 2000, Annals of Pharmacotherapy 34:915-923). Drug delivery can be
modified
through a change in formulation (e.g., continuous-release products, liposomes)
or an addition
to the drug molecule (e.g., pegylation). Potential advantages of these drug
delivery
mechanisms include an increased or prolonged duration of pharmacologic
activity, a decrease
in adverse effects, and increased patient compliance and quality of life.
Injectable
continuous-release systems deliver drugs in a controlled, predetermined
fashion and are
particularly appropriate when it is important to avoid large fluctuations in
plasma drug
concentrations. Encapsulating a drug within a liposome can produce a prolonged
half-life
and an increased distribution to tissues with increased capillary permeability
(e.g., tumors).
Pegylation provides a method for modification of therapeutic peptides or
proteins to
minimize possible limitations (e.g., stability, half-life, immunogenicity)
associated with these
neurogenesis modulating agents.
In accordance with the invention, one or more neurogenesis modulating agents
can be
formulated with lipids or lipid vehicles (e.g., micells, liposomes,
microspheres, protocells,
protobionts, liposomes, coacervates, and the like) to allow formation of
multimers. Similarly,
neurogenesis modulating agents can be multimerized using pegylation, cross-
linking,
disulfide bond formation, formation of covalent cross-links,
glycosylphosphatidylinositol
(GPI) anchor formation, or other established methods. The multimerized
neurogenesis
modulating agent can be formulated into a pharmaceutical composition, and used
to increase
or enhance their effects.
Systemic administration can also be by transmucosal or transdermal means. For
transmucosal or transdermal administration, penetrants appropriate to the
barrier to be
permeated are used in the formulation. Such penetrants are generally known in
the art, and
include, for example, for transmucosal administration, detergents, bile salts,
and fusidic acid
derivatives. Transmucosal administration can be accomplished through the use
of nasal
sprays or suppositories. For administration by inhalation, the neurogenesis
modulating
agents of the invention can be delivered in the form of an aerosol spray from
pressured
container or dispenser that contains a suitable propellant, e.g., a gas such
as carbon dioxide,
or a nebulizer. For transdermal administration, the neurogenesis modulating
agents of the
invention can be formulated into ointments, salves, gels, or creams as
generally known in the
art.
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
24
The neurogenesis modulating agents can also be prepared in the form of
suppositories
(e.g., with conventional suppository bases such as cocoa butter and other
glycerides) or
retention enemas for rectal delivery.
In one embodiment, the neurogenesis modulating agent of the invention are
prepared
with carriers that will protect the neurogenesis modulating agent against
rapid elimination
from the body, such as a controlled release formulation, including implants
and
microencapsulated delivery systems. These can be prepared according to methods
known to
those skilled in the art, for example, as described in U.S. Pat. No.
4,522,811.
Compositions that include one or more neurogenesis modulating agents of the
invention
can be administered in any conventional form, including in any form known in
the art in
which it may either pass through or by-pass the blood-brain barrier. Methods
for allowing
factors to pass through the blood-brain barrier include minimizing the size of
the factor,
providing hydrophobic factors which may pass through more easily, conjugating
the protein
neurogenesis modulating agent or other agent to a carrier molecule that has a
substantial
permeability coefficient across the blood brain barrier (see, e.g., U.S.
Patent 5,670,477).
In some instances, the neurogenesis modulating agent can be administered by a
surgical
procedure implanting a catheter coupled to a pump device. The pump device can
also be
implanted or be extracorporally positioned. Administration of the neurogenesis
modulating
agent can be in intermittent pulses or as a continuous infusion. Devices for
injection to
discrete areas of the brain are known in the art (see, e.g., U.S. Patent Nos.
6,042,579;
5,832,932; and 4,692,147).
Method for Reducing a Symptom of a Disorder by Administering Neurogenesis
modulating agent(s)
One embodiment of the invention is directed to a method for reducing a symptom
of a
disorder in a patient by administering a neurogenesis modulating agent of the
invention to the
patient. In that method, one or more neurogenesis modulating agent is directly
administered
to the animal, which will induce additional proliferation and/or
differentiation of a neural
tissue of said animal. Such in vivo treatment methods allows disorders caused
by cells lost,
due to injury or disease, to be endogenously replaced. This will obviate the
need for
transplanting foreign cells into a patient
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
A neurogenesis modulating agent of the invention can be administered
systemically to a
patient. In a preferred embodiment, the neurogenesis modulating agent is
administered
locally to any loci implicated in the CNS disorder pathology, i.e. any loci
deficient in neural
cells as a cause of the disease. For example, the neurogenesis modulating
agent can be
5
administered locally to the ventricle of the brain, substantia nigra,
striatum, locus ceruleous,
nucleus basalis Meynert, pedunculopontine nucleus, cerebral cortex, and spinal
cord.
Preferably, a central nervous system disorder includes neurodegenerative
disorders, ischemic
disorders, neurological traumas, and learning and memory disorders.
The method of the invention takes advantage of the fact that stem cells are
located in
10 the
tissues lining ventricles of mature brains offers. Neurogenesis may be induced
by
administering a neurogenesis modulating agent of the invention directly to
these sites and
thus avoiding unnecessary systemic administration and possible side effects.
It may be
desireable to implant a device that administers the composition to the
ventricle and thus, to
the neural stem cells. For example, a carmula attached to an osmotic pump may
be used to
15
deliver the composition. Alternatively, the composition may be injected
directly into the
ventricles. The cells can migrate into regions that have been damaged as a
result of injury or
disease. Furthermore, the close proximity of the ventricles to many brain
regions would
allow for the diffusion of a secreted neurological agent by the cells (e.g.,
stem cells or their
progeny).
20 ,
The invention provides a method for inducing neurogenesis in vivo or in vitro,
which
can be used to treat various diseases and disorders of the CNS as described in
detail herein.
The term "treating" in its various grammatical forms in relation to the
present invention refers
to preventing, curing, reversing, attenuating, alleviating, ameliorating
minimizing,
suppressing, or halting the deleterious effects of a neurological disorder,
disorder progression,
25
disorder causative agent (e.g., bacteria or viruses), injury, trauma, or other
abnormal
condition. Symptoms of neurological disorders include, but are not limited to,
tension,
abnormal movements, abnormal behavior, tics, hyperactivity, combativeness,
hostility,
negativism, memory defects, sensory defects, cognitive defects,
hallucinations, acute
delusions, poor self-care, and sometimes withdrawal and seclusion.
Abnormal movement symptoms include a wide variety of symptoms that can range
from unconscious movements that interfere very little with quality of life, to
quite severe and
disabling movements. Examples of symptoms which are seen associated with
neurological
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
26
disorders include involuntary tongue protrusions, snake-like tongue movements,
repetitive
toe and finger movements, tremors of extremities or whole body sections, tics,
muscular
rigidity, slowness of movement, facial spasms, acute contractions of various
muscles,
particularly of the neck and shoulder which may eventually lead to painful,
prolonged muscle
contraction, restlessness, distress and an inability to remain still. Abnormal
behavioral
symptoms, some of which are motor in nature, include irritability, poor
impulse control,
distractibility, aggressiveness, and stereotypical behaviors that are commonly
seen with
mental impairment such as rocking, jumping, running, spinning, flaying, etc.
Any of the methods of the invention may be used to alleviate a symptom of a
neurological disease or disorder such as Parkinson's disease (shaking palsy),
including
primary Parkinson's disease, secondary parkinsonism, and postencephalitic
parkinsonism;
drug-induced movement disorders, including parkinsonism, acute dystonia,
tardive
dyskinesia, and neuroleptic malignant syndrome; Huntington's disease
(Huntington's chorea;
chronic progressive chorea; hereditary chorea); delirium (acute confusional
state); dementia;
Alzheimer's disease; non-Alzheimer's dementias, including Lewy body dementia,
vascular
dementia, Binswanger's dementia (subcortical arteriosclerotic encephalopathy),
dementia
pugilistica, normal-pressure hydrocephalus, general paresis, frontotemporal
dementia, multi-
infarct. dementia, and AIDS dementia; age-associated memory impairment (AAMI);
amnesias, such as retrograde, anterograde, global, modality specific,
transient, stable, and
progressive amnesias, and posttraumatic amnesias, and Korsakoffs disease.
Other diseases and disorders include idiopathic orthostatic hypotension, Shy-
Drager
syndrome, progressive supranuclear palsy (Steele-Richardson-Olszewski
syndrome);
structural lesions of the cerebellum, such as those associated with infarcts,
hemorrhages, or
tumors; spinocerebellar degenerations such as those associated with
Friedreich's ataxia,
abetalipoproteinemia (e.g., Bassen-Kornzweig syndrome, vitamin E deficiency),
Refsum's
disease (phytanic acid storage disease), cerebellar ataxias, multiple systems
atrophy
(olivopontocerebellar atrophy), ataxia-telangiectasia, and mitochondrial
multisystem
disorders; acute disseminated encephalomyelitis (postinfectious
encephalomyelitis);
adrenoleukodystrophy and adrenomyeloneuropathy; Leber's hereditary optic
atrophy; HTLV-
associated myelopathy; and multiple sclerosis; motor neuron disorders such as
amyotrophic
lateral sclerosis, progressive bulbar palsy, progressive muscular atrophy,
primary lateral
sclerosis and progressive pseudobulbar palsy, and spinal muscular atrophies
such as type I
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
27
spinal muscular atrophy (Werdnig-Hoffmann disease), type II (intermediate)
spinal muscular
atrophy, type III spinal muscular atrophy (Wohlfart-Kugelberg-Welander
disease), and type
IV spinal muscular atrophy.
Further diseases and disorders include plexus disorders such as plexopathy and
acute
brachial neuritis (neuralgic amyotrophy); peripheral neuropathies such as
mononeuropathies,
multiple mononeuropathies, and polyneuropathies, including ulnar nerve palsy,
carpal tunnel
syndrome, peroneal nerve palsy, radial nerve palsy, Guillain-Barre syndrome
(Landry's
ascending paralysis; acute inflammatory demyelinating polyradiculoneuropathy),
chronic
relapsing polyneuropathy, hereditary motor and sensory neuropathy, e.g., types
I and II
(Charcot-Marie-Tooth disease, peroneal muscular atrophy), and type III
(hypertrophic
interstitial neuropathy, Dejerine-Sottas disease); disorders of neuromuscular
transmission,
such as myasthenia gravis; neuro-ophthalmologic disorders such as Homer's
syndrome,
internuclear ophthalmoplegia, gaze palsies, and Parinaud's syndrome; cranial
nerve palsies,
trigeminal neuralgia (Tic Douloureux); Bell's palsy; and glossopharyngeal
neuralgia;
radiation-induced injury of the nervous system; chemotherapy-induced
neuropathy (e.g.,
encephalopathy); taxol neuropathy; vincristine neuropathy; diabetic
neuropathy; autonomic
neuropathies; polyneuropathie;, and mononeuropathies; and ischemic syndromes
such as
transient ischemic attacks, subclavian steal syndrome, drop attacks, ischemic
stroke,
hemorrhagic stroke, and brain infarction.
For treatment of Huntington's disease, Alzheimer's disease, Parkinson's
disease, and
other neurological disorders affecting primarily the forebrain, one or more of
the disclosed
neurogenesis modulating agents, with or without growth factors or other
neurological agents
would be delivered to the ventricles of the forebrain to affect in vivo
modification or
manipulation of the cells. The disclosed neurogenesis modulating agents could
also be
delivered via a systemic route (oral, injection) but still execute their
effect at specific sites in
the brain (e.g. the ventricles). For example, Parkinson's disease is the
result of low levels of
dopamine in the brain, particularly the striatum. It would be advantageous to
induce a
patient's own quiescent stem cells to begin to divide in vivo, thus locally
raising the levels of
dopamine. The methods and compositions of the present invention provide an
alternative to
the use of drugs and the controversial use of large quantities of embryonic
tissue for
treatment of Parkinson's disease. Dopamine cells can be generated in the
striatum by the
administration of a composition comprising growth factors to the lateral
ventricle. A
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
28
particularly preferred composition comprises one or more of the neurogenesis
modulating
agents disclosed herein. While preferred embodiments of specific delivery have
been
disclosed, it is understood that the neurogenesis modulating agents disclosed
herein could
also be effective via systemic delivery using any of the methods of
administration discussed
in this disclosure.
For the treatment of MS and other demyelinating or hypomyelinating disorders,
and for
the treatment of Amyotrophic Lateral Sclerosis or other motor neuron diseases,
one or more
of the disclosed neurogenesis modulating agents, with or without growth
factors or other
neurological agents would be delivered to the central canal. In addition to
treating CNS
tissue immediately surrounding a ventricle, a viral vector, DNA, growth
factor, or other
neurological agent can be easily administered to the lumbar cistern for
circulation throughout
the CNS. Infusion of EGF or similar growth factors can be used with the
neurogenesis
modulating agents of the invention to enhance the proliferation, migration,
and differentiation
of neural stem cells and progenitor cells in vivo (see, e.g., U.S. Patent No.
5,851,832). In a
preferred embodiment EGF and FGF are administered together or sequentially
with the
neurogenesis modulating agents disclosed herein.
The blood-brain barrier can be bypassed by in vivo transfection of cells with
expression
vectors containing genes that code for neurogenesis modulating agents, so that
the cells
themselves produce the neurogenesis modulating agents. Any useful genetic
modification of
the cells is within the scope of the present invention. For example, in
addition to genetic
modification of the cells to express neurogenesis modulating agents, the cells
may be
modified to express other types of neurological agents such as
neurotransmitters. Preferably,
the genetic modification is performed either by infection of the cells lining
ventricular
regions with recombinant retroviruses or transfection using methods known in
the art
including CaPO4 transfection, DEAE-dextran transfection, polybrene
transfection, by
protoplast fusion, electroporation, lipofection, and the like see Maniatis et
al., supra. Any
method of genetic modification, now known or later developed can be used.
The methods of the invention can be used to treat any mammal, including
humans,
cows, horses, dogs, sheep, and cats. Preferably, the methods of the invention
are used to treat
humans. In one aspect, the invention provides a regenerative treatment for
neurological
disorders by stimulating cells (e.g., stem cells) to grow, proliferate,
migrate, survive, and/or
differentiate to replace neural cells that have been lost or destroyed. In
vivo stimulation of
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
29
such cells (e.g., stem cells) can be accomplished by locally administering
(via any route) a
neurogenesis modulating agent of the invention to the cells in an appropriate
formulation. By
increasing neurogenesis, damaged or missing cells can be replaced in order to
enhance blood
function. While the agents and methods of the invention is preferably used for
treating
humans, it is understood that these agents and methods are also suitable for
the treatment of
nonhuman mammals. For example, the agents of the invention may be used to
treat non-
human mammal conditions such as depression.
Methods for preparing the neurogenesis modulating agent dosage forms are
known, or
will be apparent, to those skilled in this art. The determination of an
effective amount of a
neurogenesis modulating agent to be administered in within the skill of one of
ordinary skill
in the art and will be routine to those persons skilled in the art. The amount
of neurogenesis
modulating agent to be administered will depend upon the exact size and
condition of the
patient, but will be at least 0.1 ng/kg/day, at least 1 ng/kg/day, at least 5
ng/kg/day, at least 20
ng/kg/day, at least 100 ng/kg/day, at least 0.5 Ilg/kg/day, at least 2
pg/kg/day, at least 5
pig/kg/day, at least 50 ptg/kg/day, at least 500 pig/kg/day, at least 1
mg/kg/day, at least 5
mg/kg/day, or at least 10 mg/kg/day in a volume of 0.001 to 10 ml. For any of
the minimum
doses listed in this specification, including minimum weight or minimum tissue
concentration, an optional maximum dose may be 200%, 500%, or 1000% of the
minimum
dose (weight or tissue concentration). In another method of dosage, the
modulator may be
administered so that a target tissue achieves a modulator concentration of
0.0001 nM to 50
nM, 0.001 nM to 50 nM, 0.01 nM to 50 nM, 0.1 nM to 50 nM, 0.1 nM to 100 nM, or
at least
1 nM, at least 50 nM, or at least 100 nM. Preferred dosages include
subcutaneous
administration of at least 10 mg twice a week or at least 25 mg twice a week;
subcutaneous
administration of at least 0.04 mg/kg/week, at least 0.08 mg/kg/week, at least
0.24
mg/kg/week, at least 36 mg/kg/week, or at least 48 mg/kg/week; subcutaneous
administration
of at least 22 mcg twice a week or 44 mcg twice a week; or intravenous
administration of at
least 3-10 mg/kg once a month. Particularly preferred dosage ranges are 0.04
mg/kg to 4
mg/kg and 0.05 mg/kg to 5 mg/kg. These dosages may be increased 10x, 100x, or
1000x in
transdermal or topical applications. For example, the dose at which the
compounds may be
administered to a human will depend upon the route of administration, the body
weight of the
patient, the severity of the conditions to be treated and the potency of the
compounds. For
example, the neurogenesis modulating agent disclosed in this patent
specifications may be
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
administered at daily doses of between: about 0.5 microgram to about 100
micrograms a day,
about 0.1 microgram to about 20 micrograms a day, about 0.2 micrograms to
about 40
micrograms per day, about 5 micrograms to about 200 micrograms per day, about
10
micrograms to about 20 micrograms per day, about 20 micrograms to about 200
micrograms
5 per day, about 50 micrograms to about 100 mg per day, about 0.1 mg to
about 200 mg per
day, about 50 mg to about 200 mg per day, and about 0.1 gram to about 1 gram a
day. The
required dosage may depend upon the severity of the condition of the patient
and upon such
criteria as the patient's height, weight, sex, age, and medical history.
Pharmaceutical compositions , suitable for use in the present invention
include
10 compositions wherein the active ingredients are contained in an
effective amount to achieve
its intended purpose. More specifically, a therapeutically effective amount
means an amount
effective to optimally stimulate or suppress cell (e.g., stem cell or
progenitor cell)
proliferation. It will be appreciated that the unit content of active
ingredient or ingredients
contained in an individual dose of each dosage form need not in itself
constitute an effective
15 amount since the necessary effective amount can be reached by
administration of a plurality
of dosage units (such as capsules or tablets or combinations thereof). In
addition, it is
understood that at some dosage levels, an effective amount may not show any
measurable
effect (the measurable effect could be lack of deterioration) until after a
week, a month, three
months, or six months of usage. Further, it is understood that an effective
amount may lessen
20 the rate of the natural deterioration that comes with age but may not
reverse the deterioration
that has already occurred. Determination of the effective amounts is well
within the
capability of those skilled in the art, especially in light of the detailed
disclosure provided
herein. The specific dose level for any particular user will depend upon a
variety of factors
including the activity of the specific neurogenesis modulating agent employed,
the age, the
25 physical activity level, general health, and the severity of the
disorder.
A therapeutically effective dose also refers to that amount necessary to
achieve the
desired effect without unwanted or intolerable side effects. Toxicity and
therapeutic efficacy
of a neurogenesis modulating agent of the invention can be determined by
standard
pharmaceutical procedures in cell cultures or experimental animals. Using
standard methods,
30 the dosage that shows effectiveness in about 50% of the test population,
the ED50, may be
determined. Effectiveness may be any sign of cell (e.g., stem cell)
proliferation or
suppression. Similarly, the dosage that produces an undesirable side effect to
50% of the
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
31
population, the SD50, can be determined. Undesirable side effects include
death, wounds,
rashes, abnormal redness, and the like. The dose ratio between side effect and
therapeutic
effects can be expressed as the therapeutic index and it can be expressed as a
ratio between
SD50/ED50. Neurogenesis modulating agents with high therapeutic indexes are
preferred, i.e.,
neurogenesis modulating agents that are effective at low dosage and which do
not have
undesirable side effects until very high doses. A preferred therapeutic index
is greater than
about 3, more preferably, the therapeutic index is greater than 10, most
preferably the
therapeutic index is greater than 25, such as, for example, greater than 50.
Furthermore,
neurogenesis modulating agents that do not have side effects at any dosage
levels are more
preferred. Finally, neurogenesis modulating agents that are effective at low
dosages and do
not have side effects at any dosage levels are most preferred. The exact
formulation, route of
administration and dosage can be chosen depending on the desired effect and
can be made by
those of skill in the art.
Dosage intervals can be determined by experimental testing. One or more
neurogenesis
modulating agents of the invention should be administered using a regimen
which maintains
cell (e.g., stem cell) proliferation at about 10% above normal, about 20%
above normal,
above 50% above normal such as 100% above normal, preferably about 200% above
normal,
more preferably about 300% above normal and most preferably about 500% above
normal.
In a preferred embodiment, the pharmaceutical composition of the invention may
comprise a
neurogenesis modulating agent of the invention at a concentration of between
about 0.001%
to about 10%, preferably between about 0.01% and about 3%, such as, for
example, about 1%
by weight.
Another suitable administration method is to provide a neurogenesis modulating
agent
of the invention through an implant or a cell line capable of expressing a
neurogenesis
modulating agent (e.g., peptide neurogenesis modulating agent) so that the
implant or cell
line can provide the neurogenesis modulating agent to a cell of the CNS.
In a preferred embodiment of the invention, the neurogenesis modulating agent
of the
invention induces neurogenesis in a patient. In a more preferred embodiment,
the
neurogenesis modulating agent induces neurogenesis in at least the lateral
ventricle wall
region or the hippocampus region of the brain. In a more preferred embodiment,
the
neurogenesis modulating agent induces neurogenesis in the lateral ventricle
wall but not in
the hippocampus.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
32
The methods of the invention may be used to detect endogenous agents in cells
(e.g.,
neural stem cells, neural progenitor cells) can be identified using RT-PCR or
in situ
hybridization techniques. In particular, genes that are up regulated or down
regulated in these
cells in the presence of one or more neurogenesis modulating agent of the
invention can be
identified. The regulation of such genes may indicate that they are involved
in the mediation
of signal transduction pathways in the regulation of neurogenesis function.
Furthermore, by
knowing the levels of expression of the these genes, and by analyzing the
genetic or amino-
acid sequence variations in these genes or gene products, it may be possible
to diagnose
disease or determine the role of cells (e.g., stem and progenitor cells) in
the disease. Such
analysis will provide important information for using cell-based 'treatments
for disease.
Another embodiment of the invention is directed to a method for increasing
adult
neural stem cells in a patient with a disorder of the central nervous system.
The method
comprises administering to said subject an amount of neurogenesis modulating
agent
sufficient to increase adult neural stem cells in said patient and reduce at
least one symptom
of said disorder. In one aspect, the neurogenesis modulating agent may be an
Exendin or a
functional analog or variant thereof. The disorder of the central nervous
system may be any
disorder disclosed in this specification and includes, at least, Parkinson's
disease, depression,
or minimal cognition impairment.
Another embodiment of the invention is directed to a method for increasing
adult
neural stem cells in a patient with a disorder of the central nervous system.
The method
comprises administering to the patient an amount of a neurogenesis modulating
agent
sufficient to increase adult neural stem cells in said patient. Preferably,
the method also
reduce at least one symptom of said disorder. In one aspect, the neurogenesis
modulating
agent may be an Exendin or Exendin analog. The disorder of the central nervous
system may
be any disorder disclosed in this specification and includes, at least,
Parkinson's disease,
depression, or minimal cognition impairment.
The administration method is discussed in detail in another section of this
disclosure.
In a preferred embodiment, the administration may be by injection (e.g.,
subcutaneous
injection). In another preferred embodiment, the amount administered is about
0.001
microgram to about 20 micrograms per kilogram of body weight per day, about
0.01
microgram to about 2 micrograms per kilogram of body weight per day, or about
0.02
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
33
microgram to about 0.4 microgram per kilogram of body weight per day. These
dosages are
also applicable for the other neurogenesis modulating agents of this
disclosure.
Method for Reducing a Symptom of a Disorder by Administering Cells Treated
with Neurogenesis modulating agent(s)
Harvesting Cells and Inducing Neurogenesis:
Another embodiment of the invention is directed to a method for inducing cells
(e.g.,
stem cells or progenitor cells) to undergo neurogenesis in vitro -- to
generate large numbers
of neural cells capable of differentiating into neurons, astrocytes, and
oligodendrocytes. The
induction of proliferation and differentiation of cells (e.g., stem cells or
progenitor cells) can
be done either by culturing the cells in suspension or on a substrate onto
which they can
adhere. The induced cells may be used for therapeutic treatment. For example,
therapy may
involve, at least, (1) proliferation and differentiation of neural cells in
vitro, then
transplantation, (2) proliferation of neural cells in vitro, transplantation,
then further
proliferation and differentiation in vivo, (3) proliferation in vitro,
transplantation and
differentiation in vivo, and (4) proliferation and differentiation in vivo.
Thus, the invention
provides a means for generating large numbers of cells for transplantation
into the neural
tissue of a host in order to treat neurodegenerative disease and neurological
trauma, for non-
surgical methods of treating neurodegenerative disease and neurological
trauma, and for
õ.
drug-screening applications.
Stem cell progeny can be used for transplantation into a heterologous,
autologous, or
xenogeneic host. Multipotent stem cells can be obtained from embryonic, post-
natal,
juvenile, or adult neural tissue, or other tissues. Human heterologous stem
cells may be
derived from fetal tissue following elective abortion, or from a post-natal,
juvenile, or adult
organ donor. Autologous tissue can be obtained by biopsy, or from patients
undergoing
surgery (e.g., neurosurgery) in which tissue is removed, for example, during
epilepsy surgery,
temporal lobectomies, and hippocampalectomies. Stem cells have been isolated
from a
variety of adult CNS ventricular regions and proliferated in vitro using the
methods detailed
herein. In various embodiments of the invention, the tissue can be obtained
from any animal,
including insects, fish, reptiles, birds, amphibians, mammals and the like.
The preferred
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
34
source of tissue (e.g., neural tissue) is from mammals, preferably rodents and
primates, and
most preferably, mice and humans.
In the case of a heterologous donor animal, the animal may be euthanized, and
the
neural tissue and specific area of interest removed using a sterile procedure.
Areas of
particular interest include any area from which neural stem cells can be
obtained that will
serve to restore function to a degenerated area of the host's nervous system,
particularly the
host's CNS. Suitable areas include the cerebral cortex, cerebellum, midbrain,
brainstem,
spinal cord and ventricular tissue, and areas of the PNS including the carotid
body and the
adrenal medulla. Preferred areas include regions in the basal ganglia,
preferably the striatum
which consists of the caudate and putamen, or various cell groups such as the
globus pallidus,
the subthalamic nucleus, the nucleus basalis which is found to be degenerated
in Alzheimer's
disease patients, or the substantia nigra pars compacta which is found to be
degenerated in
Parkinson's disease patients. Particularly preferred neural tissue is obtained
from ventricular
tissue that is found lining CNS ventricles and includes the subependyma. The
term
"ventricle" refers to any cavity or passageway within the CNS through which
cerebral spinal
fluid flows. Thus, the term not only encompasses the lateral, third, and
fourth ventricles, but
also encompasses the central canal, cerebral aqueduct, and other CNS cavities.
Cells can be obtained from donor tissue (e.g., neural tissue) by dissociation
of
individual cells from the connecting extracellular matrix of the tissue. The
donor tissue may
be tissue from any cell or organ that comprise neural tissue listed in this
application
including, at least, LV cells and hippcampus cells. Tissue from a particular
neural region is
removed from the brain using a sterile procedure, and the cells are
dissociated using any
method known in the art including treatment with enzymes such as trypsin,
collagenase and
the like, or by using physical methods of dissociation such as with a blunt
instrument.
Dissociation of fetal cells can be carried out in tissue culture medium, while
a preferable
medium for dissociation of juvenile and adult cells is low Ca.2+ artificial
cerebral spinal fluid
(aCSF). Regular aCSF contains 124 mM NaCl, 5 mM KC1, 1.3 mM MgC12, 2 mM CaCl2,
26
mM NaHCO3, and 10 mM D-glucose. Low Ca2+ aCSF contains the same ingredients
except
for MgC12 at a concentration of 3.2 mM and CaCl2 at a concentration of 0.1 mM.
Dissociated
cells are centrifuged at low speed, between 200 and 2000 rpm, usually between
400 and 800
rpm, and then resuspended in culture medium. The cells can be cultured in
suspension or on
a fixed substrate.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Methods for culturing neural cells are well known. See, US patents 5,980,885,
5,851,832, 5,753,506, 5,750376, 5,654,183, 5,589,376, 5,981,165, and
5,411,883, all
incorporated herein by reference. A preferred embodiment for proliferation of
stem cells is to
use a defined, serum-free culture medium (e.g., Complete Medium), as serum
tends to induce
5 differentiation and contains unknown components (i.e. is undefined). A
defined culture
medium is also preferred if the cells are to be used for transplantation
purposes. A
particularly preferable culture medium is a defined culture medium comprising
a mixture of
DMEM, F12, and a defined hormone and salt mixture. Conditions for culturing
should be
close to physiological conditions. The pH of the culture medium should be
close to
10 physiological pH, preferably between pH 6-8, more preferably between
about pH 7 to 7.8,
with pH 7.4 being most preferred. Physiological temperatures range between
about 30 C to
C. Cells are preferably cultured at temperatures between about 32 C to about
38 C, and
more preferably between about 35 C to about 37 C.
The culture medium is supplemented with at least one neurogenesis modulating
agent
15 of the invention. This ability of the neurogenesis modulating agent to
enhance the
proliferation of stem cells is invaluable when stem cells are to be harvested
for later
transplantation back into a patient, thereby making the initial surgery 1)
less traumatic
because less tissue would have to be removed 2) more efficient because a
greater yield of
stem cells per surgery would proliferate in vitro; and 3) safer because of
reduced chance for
20 mutation and neoplastic transformation with reduced culture time.
Optionally, the patient's
stem cells, once they have proliferated in vitro, could also be genetically
modified in vitro
using the techniques described below.
After proliferation Stem cell progeny can be cryopreserved until they are
needed by any
method known in the art. In a preferred embodiment of the invention, the cells
are derived
25 from the lateral ventricle wall region of the brain. In another
preferred embodiment of the
invention, the cells are derived from the CNS but not from the hippocampus.
Cellular Differentiation:
Included in the invention are methods of using the disclosed neurogenesis
modulating
agents to increase or maintain cell (e.g., stem cell or progenitor cell)
proliferation in vitro and
30 obtain large numbers of differentiated cells. Differentiation of the
cells can be induced by
any method known in the art. In a preferred method, differentiation is induced
by contacting
the cell with a neurogenesis modulating agent of the invention that activates
the cascade of
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
36
biological events that lead to growth and differentiation. As disclosed in
this invention, these
events include elevation of intracellular cAMF' and Ca2 .
Cellular differentiation may be monitored by using antibodies to antigens
specific for
neurons, astrocytes, or oligodendrocytes can be determined by
immunocytochemistry
techniques well known in the art. Many neuron specific markers are known. In
particular,
cellular markers for neurons include NSE, NF, beta-tub, MAP-2; and for glia,
GFAP (an
identifier of astrocytes), galactocerebroside (GalC) (a myelin glycolipid
identifier of
oligodendrocytes), and the like.
Differentiation may also be monitored by in situ hybridization histochemistry
that can
also be performed, using cDNA or RNA probes specific for peptide
neurotransmitter or
neurotransmitter synthesizing enzyme mRNAs. These techniques can be combined
with
immunocytochemical methods to enhance the identification of specific
phenotypes. If
necessary, additional analysis may be performed by Western and Northern blot
procedures.
A preferred method for the identification of neurons uses immunocytochemistry
to
detect immunoreactivity for NSE, NF, NeuN, and the neuron specific protein,
tau-1. Because
these markers are highly reliable, they will continue to be useful for the
primary identification
of neurons, however neurons can also be identified based on their specific
neurotransmitter
phenotype as previously described. Type I astrocytes, which are differentiated
glial cells that
have a flat, protoplasmic/fibroblast-like morphology, are preferably
identified by their
immunoreactivity for GFAP but not A2B5. Type II astrocytes, which are
differentiated glial
cells that display a stellate process-bearing morphology, are preferably
identified using
immunocytochemistry by their phenotype GFAP(+), A2B5(+) phenotype.
Administration of Cells treated with a Method of the Invention:
Following in vitro expansion and neurogenesis using a method of the invention
(see,
Example section for a detail description of these methods), the cells of the
invention can be
administered to any animal with abnormal neurological or neurodegenerative
symptoms
obtained in any manner, including those obtained as a result of mechanical,
chemical, or
electrolytic lesions, as a result of experimental aspiration of neural areas,
or as a result of
aging processes. Particularly preferable lesions in non-human animal models
are obtained
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
37
with 6-hydroxy-dopamine (6-0HDA), 1-methy1-4-phenyl- 1,2,3,6
tetrahydropyridine
(MPTP), ibotenic acid and the like.
The instant invention allows the use of cells (e.g., stem cells or progenitor
cells) that are
xenogeneic to the host. The methods of the invention are applied to these
cells (as shown in
the Examples) to expand the total number or total percent of neuronal stem
cells in culture
before use. Since the CNS is a somewhat immunoprivileged site, the immune
response is
significantly less to xenografts, than elsewhere in the body. In general,
however, in order for
xenografts to be successful it is preferred that some method of reducing or
eliminating the
immune response to the implanted tissue be employed. Thus recipients will
often be
immunosuppressed, either through the use of immunosuppressive drugs such as
cyclosporin,
or through local immunosuppression strategies employing locally applied
immunosuppressants. Local immunosuppression is disclosed by Gruber,
Transplantation
54:1-11 (1992). Rossini, U.S. Pat. No. 5,026,365, discloses encapsulation
methods suitable
for local immunosuppression.
Grafting of cells prepared from tissue that is allogeneic to that of the
recipient will most
often employ tissue typing in an effort to most closely match the
histocompatibility type of
the recipient. Donor cell age as well as age of the recipient have been
demonstrated to be
important factors in improving the probability of neuronal graft survival. In
some instances,
it may be possible to prepare cells from the recipient's own nervous system
(e.g., in the case
of tumor removal biopsies, etc.). In such instances the cells may be generated
from = -I-
dissociated tissue and proliferated in vitro using the methods described
above. Upon suitable
expansion of cell numbers, the cells may be harvested, genetically modified if
necessary, and
readied for direct injection into the recipient's CNS.
Transplantation can be done bilaterally, or, in the case of a patient
suffering from
Parkinson's disease, contralateral to the most affected side.
Surgery may be used to deliver cells throughout any affected neural area, in
particular,
to the basal ganglia, and preferably to the caudate and putamen, the nucleus
basalis or the
substantia nigra. Cells are administered to the particular region using any
method that \
maintains the integrity of surrounding areas of the brain, preferably by
injection cannula.
Injection methods exemplified by those used by Duncan et al. J. Neurocytology,
17:351-361
(1988), and scaled up and modified for use in humans are preferred.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
38
Although solid tissue fragments and cell suspensions of neural tissue are
immunogenic
as a whole, it could be possible that individual cell types within the graft
are themselves
immunogenic to a lesser degree. For example, Bartlett et al. (Prog. Brain Res.
82: 153-160
(1990)) have abrogated neural allograft rejection by pre-selecting a
subpopulation of
embryonic neuroepithelial cells for grafting by the use of immunobead
separation on the
basis of MHC expression. Thus, another approach is provided to reduce the
chances of allo-
and xenograft rejection by the recipient without the use of immunosuppression
techniques.
Cells when administered to the particular neural region preferably form a
neural graft,
wherein the neuronal cells form normal neuronal or synaptic connections with
neighboring
neurons, and maintain contact with transplanted or existing glial cells which
may form
myelin sheaths around the neurons' axons, and provide a trophic influence for
the neurons.
As these transplanted cells form connections, they re-establish the neuronal
networks which
have been damaged due to disease and aging.
Survival of the graft in the living host can be examined using various non-
invasive
scans such as computerized axial tomography (CAT scan or CT scan), nuclear
magnetic
resonance or magnetic resonance imaging (NMR or MN) or more preferably
positron
emission tomography (PET) scans. Post-mortem examination of graft survival can
be done by
removing the neural tissue, and examining the affected region macroscopically,
or more
preferably using microscopy. Cells can be stained with any stains visible
under light or
electron microscopic.,conditions, more particularly with stains that are
specific for neurons
and glia. Particularly useful are monoclonal antibodies that identify neuronal
cell surface
markers such as the M6 antibody, which identifies mouse neurons. Most
preferable are
antibodies that identify any neurotransmitters, particularly those directed to
GABA, TH,
ChAT, and substance P, and to enzymes involved in the synthesis of
neurotransmitters, in
particular, GAD. Transplanted cells can also be identified by prior
incorporation of tracer
dyes such as rhodamine- or fluorescein-labeled microspheres, fast blue,
bisbenzamide or
retrovirally introduced histochemical markers such as the lacZ gene, which
produces beta
galactosidase.
Functional integration of the graft into the host's neural tissue can be
assessed by
examining the effectiveness of grafts on restoring various functions,
including but not limited
to tests for endocrine, motor, cognitive and sensory functions. Motor tests
that can be used
include those that quantitate rotational movement away from the degenerated
side of the
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
39
brain, and those that quantitate slowness of movement, balance, coordination,
akinesia or lack
of movement, rigidity and tremors. Cognitive tests include various tests of
ability to perform
everyday tasks, as well as various memory tests, including maze performance.
Cells (e.g., stem cells or progenitor cells) can be produced and transplanted
using the
above procedures to treat demyelination diseases as described in detail
herein. Human
demyelinating diseases for which the cells of the present invention may
provide treatment
include disseminated perivenous encephalomyelitis, MS (Charcot and Marburg
types),
neuromyelitis optica, concentric sclerosis, acute, disseminated
encephalomyelitides, post
encephalomyelitis, postvaccinal encephalomyelitis, acute hemorrhagic
leukoencephalopathy,
progressive multifocal leukoencephalopathy, idiopathic polyneuritis,
diphtheric neuropathy,
Pelizaeus-Merzbacher disease, neuromyelitis optica, diffuse cerebral
sclerosis, central
pontine myelinosis, spongiform leukodystrophy, and leukodystrophy (Alexander
type).
Standard stereotactic neurosurgical methods may be used to inject cell
suspensions both
into the brain and spinal cord. Generally, the cells can be obtained from any
of the sources
discussed above. However, in the case of demyelinating diseases with a genetic
basis directly
affecting the ability of the myelin forming cell to myelinate axons,
allogeneic tissue would be
a preferred source of the cells as autologous tissue (i.e. the recipient's
cells) would generally
not be useful unless the cells have been modified in some way to insure the
lesion will not
continue (e.g. genetically modifying the cells to cure the demyelination
lesion).
Oligodendfocytes derived from dells proliferated and differentiated in vitro
may be
injected into demyelinated target areas in the recipient. Appropriate amounts
of type I
astrocytes may also be injected. Type I astrocytes are known to secrete PDGF
which
promotes both migration and cell division of oligodendrocytes (see, e.g.,
Nobel et al., Nature
333:560-652 (1988); Richardson et al., Cell, 53:309-319 (1988)).
A preferred treatment of demyelination disease uses undifferentiated cells
(e.g., stem
cells or progenitor cells). Neurospheres grown using a method of the invention
can be
dissociated to obtain individual cells that are then placed in injection
medium and injected
directly into the demyelinated target region. The cells differentiate in vivo.
Astrocytes can
promote remyelination in various paradigms. Therefore, in instances where
oligodendrocyte
proliferation is important, the ability of precursor cells to give rise to
type I astrocytes may be
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
useful. In other situations, PDGF may be applied topically during the
transplantation as well
as with repeated doses to the implant site thereafter.
Any suitable method for the implantation of cells near to the demyelinated
targets may
be used so that the cells can become associated with the demyelinated axons.
Glial cells are
5 motile and are known to migrate to, along, and across their neuronal
targets thereby allowing
the spacing of injections. Remyelination by the injection of cells is a useful
therapeutic in a
wide range of demyelinating conditions. It should also be borne in mind that
in some
circumstances remyelination by cells will not result in permanent
remyelination, and repeated
injections or surgeries will be required. Such therapeutic approaches offer
advantage over
10 leaving the condition untreated and may spare the recipient's life.
The term injection, throughout this application, encompasses all forms of
injection
known in the art and at least the more commonly described injection methods
such as
subcutaneous, intraperitoneal, intramuscular, intracerebroventricular,
intraparenchymal,
intrathecal, and intracranial injection. Where administration is by means
other than injection,
15 all known means are contemplated including administration by through the
buccal, nasal,
pulmonary or rectal mucosa. Commonly known delivery systems include
administration by
peptide fusion to enhance uptake or by via micelle or liposome delivery
systems.
The methods of the invention may be tested on animal models of neurological
diseases.
Many such models exist. For example, they are listed in Alan A Boulton, Glen B
Baker,
20 Roger F Butterworth "Animal Models of Neurological Disease" Humana Press
(1992) and
Alan A Boulton, Glen B Baker, Roger F Butterworth "Animal Models of
Neurological
Disease II" Blackwell Publishing (2000). Also, mouse models for the following
diseases
may be purchased by a commercial supplier such as the Jackson Laboratory:
Alzheimer's
disease, Amyotrophic Lateral Sclerosis (ALS), Angelman syndrome, astrocyte
defects, ataxia
25 (movement) defects, behavioral and learning defects, cerebellar defects,
channel and
transporter defects, defects in circadian rhythms, cortical defects, epilepsy,
fragile X mental
retardation syndrome, Huntington's disease, metabolic defects, myelination
defects, neural
tube defects, neurodegeneration, neurodevelopmental defects, neuromuscular
defects,
neuroscience mutagenesis facility strain, neurotransmitter receptor and
synaptic vesicle
30 defects, neurotrophic factor defects, Parkinson's disease, receptor
defects, response to
catecholamines, tremor, tremor defects, and vestibular and hearing defects.
(See, e.g.,
hypertext transfer protocol://jaxmice.jax.org/j
axmicedb/html/sbmodel_13.shtml; over 100
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
41
strains of mouse models of neurological diseases are listed in hypertext
transfer
protocol://jaxmice.jax.org/jaxmicedb/html/model_13.shtml). Rat models of
neurological
diseases are numerous and may be found, for example, in recent reviews (e.g.,
Cenci,
Whishaw and Schallert, Nat Rev Neurosci. 2002 Jul;3(7):574-9). One of skill in
the art,
reading this disclosure, would be able to use the results of this disclosure
to design animal
testing models to determine efficacy in vivo. See, also, Example 13. Other
animal models
include strains that contain the same mutations as the strains described above
but in a
different genetic background.
Another example, the neurogenesis modulating agents of this disclosure may be
tested
in the following animals models of CNS disease/disorders/trauma to demonstrate
recovery.
Models of epilepsy include at least electroshock-induced seizures (Billington
A et al.,
Neuroreport 2000 Nov 27;11(17):3817-22), pentylene tetrazol (Gamaniel K et
al.,
Prostaglandins Leukot Essent Fatty Acids 1989 Feb;35(2):63-8) or kainic acid
(Riban V et al,
Neuroscience 2002;112(1):101-11) induced seizures. Models of
psychosis/schizophrenia
include, at least, amphetamine-induced stereotypies/locomotion (Borison RL &
Diamond BI,
Biol Psychiatry 1978 Apr;13(2):217-25), MK-801 induced stereotypies (Tiedtke
et al., J
Neural Transm Gen Sect 1990;81(3):173-82), MAM (methyl azoxy methanol- induced
(Fiore
M et al., Neuropharmacology 1999 Jun;38(6):857-69; Talamini LM et al., Brain
Res 1999
Nov 13;847(1):105-20) or reeler model (Ballmaier M et al., Eur J Neurosci 2002
Apr;15(17):1197-205). Models of Parkinson's disease include, at least, MPTP
(Schmidt &
- _
Ferger, J Neural Transm 2001;108(11):1263-82), 6-0H dopamine (O'Dell &
Marshall,
Neuroreport 1996 Nov 4;7(15-17):2457-61) induced degeneration. Models of
Alzheimer's
disease include, at least, fimbria fornix lesion model (Krugel et al., Int J
Dev Neurosci 2001
Jun;19(3):263-77), basal forebrain lesion model (Moyse E et al., Brain Res
1993 Apr
2;607(1-2):154-60). Models of stroke include, at least, focal ischemia
(Schwartz DA et al.,
Brain Res Mol Brain Res 2002 May 30;101(1-2):12-22); global ischemia (2- or 4-
vessel
occlusion) (Roof RL et al., Stroke 2001 Nov;32(11):2648-57; Yagita Y et al.,
Stroke 2001
Aug;32(8):1890-6).
In addition, models of multiple sclerosis include, at least, myelin
oligodendrocyte
glycoprotein -induced experimental autoimmune encephalomyelitis (Slavin A et
al.,
Autoimmunity 1998;28(2):109-20). Models of amyotrophic lateral sclerosis
include, at least
pmn mouse model (Kennel P et at., J Neurol Sci 2000 Nov 1;180(1-2):55-61).
Models of
CA 02546843 2013-02-22
42
anxiety include, at least, elevated plus-maze test (Holmes A et al., Behav
Neurosci 2001
Oct;115(5):1129-44), marble burying test (Broekkamp et al., Eur J Pharmacol
1986 Jul
31;126(3):223-9), open field test (Pelleymounter et al., J Pharmacol Exp Ther
2002
Jul;302(1):145-52). Models of depression include, at least learned
helplessness test, forced
swim test (Shirayama Y et al., J Neurosci 2002 Apr 15;22(8):3251-61),
bulbectomy
(O'Connor et al., Prog Neuropsychopharmacol Biol Psychiatry 1988;12(1):41-51).
Model for
learning/memory include, at least, Morris water maze test (Schenk F & Morris
RG, Exp
Brain Res 1985;58(1):11-28). Models for Huntington's disease include, at
least, quinolinic
acid injection (Marco S et al., J Neurobiol 2002 Mar;50(4):323-32),
transgenics/knock-ins
(reviewed in Menalied LB and Chesselet MF, Trends Pharmacol Sci. 2002
Jan;23(1):32-9).
Models of aged animal include, at least, the use of old animals such as old
mice and old rats.
Other features of the invention will become apparent in the course of the
following
description of exemplary embodiments that are given for illustration of the
invention and are
not intended to be limiting thereof.
EXAMPLES
Unless noted otherwise, all experiments were performed using standard
molecular
biology techniques which are also described in co pending U.S. application
10/429,062 filed
May 2, 2003
EXAMPLE 1: Reagents
Chemicals for dissociation of tissue included trypsin, hyaluronidase, and
DNase (all
purchased from SIGMA). Medium (DMEM 4.5 mg/ml glucose, and DMEM/F12), B27
supplement, and trypsin/EDTA were purchased from GIBCO. All plastic ware was
purchased from CorningCostar. EGF for cell cultures was purchased from BD
Biosciences,
and the ATP-SL kit was purchased from BioThema.
For the test substances, the library was purchased from Phoenix
pharmaceuticals Inc.,
USA, variety Pack Peptide Library (#L-001). Compounds purchased from Sigma-
Aldrich
included forskolin (#F6886), rolipram (#R6520), n-6, 2-o-dibutyryladenosine
(#D0260),
cholera toxin (#C8052), MECA (#A024), HE-NECA (#H8034), nor-Binaltorphimine
(#N1771), and adrenocorticotropic hormone (#A0298).
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
43
EXAMPLE 2: Mouse neurosphere cultures
The anterior lateral wall of the lateral ventricle of 5-6 week old mice was
enzymatically dissociated in 0.8 mg/ml hyaluronidase and 0.5 mg/ml trypsin in
DMEM
containing 4.5 mg/ml glucose and 80 units/mIDNase at 37 C for 20 minutes. The
cells were
gently triturated and mixed with Neurosphere medium (DMEM/F12, B27 supplement,
12.5
mM HEPES pH7.4), 100 units/m1 penicillin and 100 g/m1 streptomycin. After
passing
through a 70 p.m strainer, the cells were pelleted at 200 x g for 4 minutes.
The supernatant
was subsequently removed and the cells were resuspended in Neurosphere medium
supplemented with 3 nM EGF. Cells were plated out in culture dishes and
incubated at 37 C.
Neurospheres were ready to be split at approximately 7 days after plating.
To split neurosphere cultures, neurospheres were collected by centrifugation
at 200 x
g for 4 minutes. The neurospheres were resuspended in 0.5 ml trypsin/EDTA in
HBSS (1x),
incubated at 37 C for 2 minutes, and triturated gently to aid dissociation.
Following another
3 minutes incubation at 37 C and trituration, the cells were pelleted at 220 x
g for 4 minutes.
Cells were resuspended in freshly prepared Neurosphere medium supplemented
with 3 nM
EGF and 1nM bFGF. Cells were plated out and incubated at 37 C.
,
EXAMPLE 3: ATP-assay
To determine proliferation, neurospheres were split and seeded in Neurosphere
medium as single cells in 96-well plates, at 10,000 cells/well. The following
experiment was
performed in sets of four parallel experiments (i.e., performed in
quadruplicate)-such that the
cells may be used for different assays. Substances to be tested were added and
cells were
incubated at 37 C for 4 days. Cells were lysed with 0.1 % Triton-X100 in Tris-
EDTA buffer.
Intracellular ATP was measured using an ATP-SL kit according to the
manufacturer's
instructions (BioThema, Sweden). Intracellular ATP was shown to correlate with
cell
number. For each experiment, wells were visually examined for signs of
neurogenesis and
counted to confirm the results of the assay. Results were repeatable and
statistically
significant.
EXAMPLE 4: cAMP detection method
For testing elevations in cAMP levels, the HitHunter EFC Cyclic AMP
Chemiluminescence Assay Kit was used (DiscoveRx,USA), as purchased from
Applied
Biosystems. Cells were dissociated as described earlier. Cells were then
seeded as non-
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
44
adherent neurosphere culture at 30,000 cells/well to permit reproducible
measurements of
cAMP levels. The cells were allowed to rest for 2 hours prior to addition of
the test
substances. Following the resting period, 1 mM IBMX (3 isobuty1-1-methil-
xanthine, Sigma)
was added to each well and incubated for 10 minutes in 37 C, according to
instructions of the
manufacturer. Test substances were incubated for 20 minutes at 37 C before the
cells were
lysed and cAMP was measured. Each substance was tested in 3 doses (100, 10, or
1 nM),
with each dose tested in quadruplicate. cAMP was measured according to kit
instructions,
and results were represented as pmol/well. Student's t-test was used to
calculate for
significance.
EXAMPLE 5: Ca2+ measurement using NFAT response element reporter system
Elevations in Ca2+ levels were determined using a vector construct that coded
for the
nuclear factor of activated T cells (NFAT) response element coupled to a
luciferase reporter.
NFAT was previously reported to be regulated in a Ca2 dependent manner (Rao et
al., 1997).
The luciferase signal was detected with the Staedy-Glo kit (Promega). After
dissociating the
cells (as described above), 4-6 x 106 cells were centrifuged at 250 x g for 4
minutes. The
supernatant was discarded and the cells were resuspended in 100 1.11
NucleofectorTM Solution
(Amaxa GmbH) and 10 lig NFAT-Luc vector DNA per 106 cells. The suspension was
transferred to a cuvette and electroporated. The transfected cells were seeded
at 50,000
cells/well as non-adherent neurosphere cultures. The cells were allowed to
rest over night
before being contacted with the test substances. Each substance was tested in
3-4 doses (100,
15, 1.5, or 0.15 nM), with each dose tested in quadruplicate. Luciferace was
measured
according to the manufacturer's instructions at 18-24 hours post-induction.
Results were
represented as fold induction compared to untreated control. Student's t-test
was used to
calculate significance compared to untreated control.
EXAMPLE 6: cDNA libraries and expression analysis
For the LVW cDNA library, RNA was isolated from the anterior lateral ventricle
of
adult mice (C57 black). An oligo dT-primed cDNA library was generated using
standard
procedures (Superscript One-Step RT-PCR with platinum Taq, Invitrogen), and
then
subjected to sequence analysis (9,000 sequences). For the Neurosphere cDNA
Library, RNA
was isolated from second generation neurospheres derived from the anterior
lateral ventricle
wall of adult mice (C57 black), and expanded using the growth factors EGF and
FGF2. An
oligo-dT-primed normalized cDNA library was generated using standard
procedures
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
(Superscript One-Step RT-PCR with platinum Taq, Invitrogen), and then
subjected to
sequence analysis (12,500 sequences).
Adult Human Neural Stem Cell (aHNSC) Cultures
A biopsy from the anterior lateral wall of the lateral ventricle was taken
from an adult
5 human patient and enzymatically dissociated in PDD (Papain 2.5 U/ml;
Dispase 1 U/ml;
DNase I 250 U/ml) in DMEM containing 4.5 mg/ml glucose and 37 C for 20 min.
The cells
were gently triturated and mixed with three volumes of DMEM/F12; 10% fetal
bovine serum
(FBS). The cells were pelleted at 250 x g for 5 mm. The supernatant was
subsequently
removed and the cells resuspended in DMEM F12 with 10% FBS, plated out on
fibronectin
10 coated culture dishes, and incubated at 37 C in 5% CO2. The following
day the expansion of
the culture was initiated by change of media to aHNSC culture media (DMEM/F12;
BIT
9500; EGF 20 ng/ml; FGF2 20 ng/ml). The aHNSC were split using trypsin and
EDTA
under standard conditions. FBS was subsequently added to inhibit the reaction
and the cells
collected by centrifugation at 250 x g for 5 min. The aHNSC were replated in
aHNSC
15 culture media.
RT-PCR
The cultures aHNSC were harvested and total RNA was extracted with an RNeasy
mini
kit (Qiagen) according to the manual. The primer pairs for the following genes
(see table
below) were designed and synthesized to identify their presence in aHNSC.
Gene GenBank Primers
name Acc. No.
ADORA2A NM_000675 5' - CAATGTGCTGGTGTGCTGG ( SEQ ID NO : 4 3 )
3' -TAGACACCCAGCATGAGCAG ( SEQ ID NO : 44 )
EDNRA NM 001957 5' - CAGGATCATTTACCAGAAC ( SEQ ID NO : 4 5 )
3' - GACGCTGCTTAAGATGTTC(SEQ ID NO : 4 6 )
CALCRL NM_005795 5' -AGAGCCTAAGTTGCCAAAGG ( SEQ ID NO : 4 7 )
3 " - GAATCAGCACAAATTCAATG ( SEQ ID NO : 4 8 )
MC1R NM_002386 5' -GAACCGGAACCTGCACTC ( SEQ ID NO : 4 9 )
3' - TGCCCAGCAGGATGGTGAG ( SEQ ID NO : 5 0 )
MC5R NM 005913 5' -GAGAACATCTTGGTCATAGG ( SEQ ID NO: 5 1 )
3 -AGCATTAAAGTGAGATGAAG ( SEQ ID NO : 52 )
VIPR1 NM 004624 5 ' -GCTACACCATTGGCTACGG ( SEQ ID NO : 5 3 )
3' -GACTGCTGTCACTCTTCCTG ( SEQ ID NO: 54)
VIPR2 NM 003382 5 ' -GATGTCTCTTGCAACAGGAAG ( SEQ ID NO : 5 5 )
3 ' -GCAAACACCATGTAGTGGAC ( SEQ ID NO : 5 6 )
SSTR1 NM 001049 5' -GGGAACTCTATGGTCATCTACGTGA ( SEQ ID NO : 5 7
)
3' -GAAATGTGTACAACACGAAGCCC ( SEQ ID NO : 5 8 )
SSTR2 NM 001050 5' -GGCAACACACTTGTCATTTATGTCA ( SEQ ID NO: 5 9 )
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
46
3'-AGGTAGCAAAGACAGATGATGGTGA(SEQ ID NO: 60)
ADCYAP I R1 NM_001118 5'-TACTTTGATGACACAGGCTGCT(SEQ ID NO: 61)
3'-AGTACAGCCACCACAAAGCCCT(SEQ ID NO:62)
One step RT-PCR (Life Technologies) was performed with the primers to detect
the
mRNA of the genes of interest. As a positive control, primers for the gene Flt-
1 were used.
The gene Flt-1 is known to be expressed in the aHNSC. As a negative control
primers for the
Flt-1 gene were used and Taq enzyme alone was added to ensure that the
material had no
genomic contamination. The PCR products were run on an 1.5% agarose gel
containing
ethidiurn bromide. The bands of the correct size were cut out and cleaned with
Qiagen's gel
extraction kit. To increase the amount of material for sequencing, the bands
were amplified
again with their corresponding primers and thereafter sequenced to confirm
their identity.
EXAMPLE 7: CREB phosphorylation assays
Briefly, NSC were split into a single cell suspension as described above. The
suspension was plated in 6-well plates coated with poly-D-lysine at a density
of 106
cells/well. Cells were incubated in media supplemented with 1% of fetal calf
serum (FBS)
and allowed to adhere overnight. The following morning, the media was
carefully replaced
with fresh DMEM/F12, and 100 nM PACAP or 100 nM cholera toxin was added to the
medium. CREB phosphorylation was determined at 15 minutes and 4 hours time
points after
PACAP treatment, and at 15 minutes, 4 hours, 6 hours, and 8 hours time points
after cholera
toxin treatment. Cell lysates were collected and Western blot analysis was
performed
following standard procedures (Patrone et al., 1999). The specific anti-
phospho-CREB
antibody (1:1000 dilution; Upstate Biotechnology) was utilized.
EXAMPLE 8: Flow cytometry analysis
Cells were split into as single cell suspensions, as described above. Cells
were plated
in 6-well-plates coated with poly-D-lysine at a density of 106 cells/well.
Following this, 1%
FBS was added to the media, and the cells were allowed to adhere over night.
The following
morning, the media was carefully replaced with fresh DMEM/F12, and the test
substance was
added to a predetermined final concentration. Cells were grown for 4 days in
the presence of
the substance. A complete media change was performed halfway through the
incubation
period. Cells were harvested by incubation with trypsin/EDTA for 5 minutes at
37 C and
gentle flushing with a 1000 I pipette. Cells were flushed and centrifuged
with 500 1 media
at 250 x g for 4 minutes.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
47
Following this, 2 x 105 cells were transferred into minicentrifuge tubes and
pelleted.
The pellet was carefully resuspended in 50 1 fixation buffer (Caltag) and
incubated for 15
minutes at room temperature. Next, 450 I PBS was added to the tube. The cells
were
centrifuged at 200 x g for 5 minutes, and the supernatant was removed. Cells
were
resuspended in 100 I permeabilization buffer (Caltag) and primary antibody
was added
(Doublecortin 1:200, Santa Cruz) for 20 minutes at room temperature. Cells
were washed as
above and resuspended in secondary antibody diluted in 100 1 PBS (FITC anti-
goat IgG,
1:500, Vector Laboratories). Cells were incubated in the dark for 20 minutes
at room
temperature. Thereafter, the cells were washed as above, resuspended in 100 I
PBS, and
transferred to tubes suitable for FACS analysis.
For FACS analysis, cells were analyzed on a FACSCalibur (Becton Dickinson).
Fluorescence signals from individual cells were excited by an argon ion laser
at 488 rim, and
the resulting fluorescence emissions from each cell was collected using
bandpass filters set at
530 30. Cell Quest Pro acquisition and analysis software was used to collect
the
fluorescence signal intensities, as well as forward and side scattering
properties of the cells.
The software was also used to set logical electronic gating parameters
designed to
differentiate between alive versus dead cells, as well as between positive and
negative cells.
A total of 10,000 cells per sample were analyzed.
EXAMPLE 9: cAMP levels correlate to neuronal stem cell proliferation
The aim of this investigation was to determine if cAMP and Ca2+ are important
regulators of proliferation in adult neuronal stem cells. The experiments
analyzed a large
number of test substances, most of which regulate cAMP and/or Ca2+ via GPCRs.
The
results of these experiments indicated that: 1) cAMP levels were correlated
with mouse
neural stem cells proliferation; 2) intracellular Ca2+ stimulation was
correlated with mouse
neural stem cell proliferation; 3) adult mouse stem cells retain their
potential to differentiate
towards any neuronal cell (phenotype); and 4) adult mouse and human neural
stem cells
showed similar, reproducible responses to cAMP stimulation.
To determine if cAMP pathways cause proliferation, adult neural stem cells
were
stimulated in vitro by incubation with a diverse set of cAMP cellular
activators (Table 1,
column 1). The results of these studies clearly demonstrate that induction of
cAMP in neural
stem cells leads to cell proliferation (Table 1, columns 2-6). Adult mouse
stem cells grown in
vitro were induced to proliferate following treatment with several compounds
belonging to a
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
48
chemical library of GPCR ligands (Example 1; Table 2, column 1). The cAMP
levels were
measured 15 minutes after the different treatments (Table 2, columns 5-6). ATP
levels, a
measure of cell number, were measured following 4 days of treatment (Table 2,
columns 3-
4). The results indicate a clear correlation between proliferation (ATP
levels) and cAMP
induction in all the substances analyzed. The GPCRs for the ligands listed in
Tables 1 and 2,
are shown in Table 3, columns 1-3. Expression data for the GPCRs was obtained
from
mouse neurospheres and lateral ventricle cDNA libraries (Table 3, columns 4-
5).
Table 1: Proliferation (ATP levels) and cAMP levels are closely correlated in
mouse
adult neural stem cells
Substance Conc. ATP Fold Induction cAMP Fold
Induction
(nMolar) (nM ATP/well) ATP (pmol/well ) cAMP
Vehicle 9.3 0.6 1.0 0.02 0.01
Forskolin 1000 10.4 2.4 1.1 0.07 0.01 3.1 **
Rolipram 100 10.4 0.4 1.1 * 0.09 0.03 3.8 *
N-6, 2-0-Dibutyryl- 100 13.9 1.1 1.5** 0.10 0.01 4=5***
adenosine
Cholera toxin 100 12.9 1.6 1.4 * 0.07 0.01 3.1 *** (10
nM)
Table 1 shows ATP levels, reflecting cell number, and cAMP levels, following
adult neural stem cell
treatment with cAMP chemical activators. Test substances were added to adult
mouse stem cell
cultures at the indicated doses, and after 15 minutes, cAMP levels were
measured. ATP levels were
measured after 4 days in culture. Fold induction was determined by comparison
to vehicle treated
cells. Data was represented as the mean SD value of quadruplicate tests in a
typical experiment.
The representative values were calculated based on two separate experiments.
*, P<0.05; **, P<0.005;
*** P<0.001 (Student's t test); n.s. = non significant.
Table 2: GPCR ligands that stimulate proliferation (ATP levels) and cAMP
activation
in mouse adult neural stem cells. Each agent is a neurogenesis modulating
agent.
Substance Conc. ATP Fold Induction cAMP Fold Induction
(nM) (nM ATP/well) ATP (pmol /well) cAMP
Vehicle 16.4 1.3 2.23 0.52
Adrenocortico- 10 18.6 1.0 1.1 * 6.36 2.58
2.8 * (100 nM)
tropic hormone
Vehicle 16.4 1.3 1.84 0.53
Endothelin-1 10 41.7 7.2 2.5 * 3.64 1.13
2.0 *
(human, porcine)
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
49
Substance Conc. ATP Fold Induction cAMP Fold Induction
(nM) (nM ATP/well) ATP (pmol /well) cAMP
Vehicle 4.5 0.6 1.84 0.53
MECA 100 7.4 0.7 1.6 ** 3.89 1.00 2.1 *
HE-NECA 1000 8.2 1.1 1.8** 3.32 0.28 1.8 *** (10 nM)
Vehicle 8.6 1.4 0.13 0.02
[Cys3,6, Tyr8, 100 11.2 0.4 1.3 ** 0.29 10 2.2 *
Pro9]-Substance P
Vehicle 8.6 1.4 0.13 0.02
[D-ArgO, Hyp3, 100 13.1 2.1 1.5 * 0.17 0.02 1.3 * (10 nM)
Ig15, D-1g17,
Oic8]-Bradylcinin
Vehicle 10.3 0.6 0.06 0.01
Adrenomedullin 100 11.6 0.8 1.1 * 0.150.3 2.5 **
(human)
Vehicle 8.8 0.9 0.03 0.01
[Des-Arg9, Leu8]- 10 9.80.4 1.1 * 0.09 0.02 2.6 * (1 nM)
Bradylcinin
[Des-Arg9]- 1 10.4 1.0 1.2 * 0.06 0.01 1.7 ***
Bradykinin
[D-Pen2-5]- 10 10.7 0.9 1.2 ** 0.060.01 1.7 *
Enkephalin
[D-pGlul, D- 100 11.1 0.4 1.3 *** 0.07 0.02 2.0 * (1 nM)
Phe2, D-Trp3,6]-
LH-RH
Vehicle 7.8 2.0 0.21 0.08
Adrenomedullin 1 11.4 0.7 1.5 ** 0.330.07 1.6 *
(26-52)
Adrenomedullin 100 . 12.3 1.1 1.6 ** 0.34 0.07 1.6 *
(22-52)
a -Neo-Endorphin 100 13.8 2.1 1.8 ** 0.36 0.09 1.7 * (1 nM)
Vehicle 10.3 2.2 0.17 0.04
13-MSH 100 13.6 1.6 1.3 * 0.23 0.02 1.3 ** (10 nM)
Vehicle 7.8 2.0 2.23 0.52
a-MSH 100 14.7 3.5 1.9 *** 5.820.86 2.6 ** (100 nM)
Vehicle 7.1 0.5 0.17 0.04
Thyrocalcitonin 1 9.20.7 1.3 * 0.63 0.23 3.8 * (1M)
(Salmon)
Vehicle 7.1 0.5 0.10 0.02
Calcitonin 100 9.9 1.6 1.4 * 0.350.15 3.3 *
(human)
CART (61-102) 100 8.3 0.4 1.2 ** 0.13 0.02 1.2 * (10nM)
Vehicle 8.8 0.9 0.09 0.03
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Substance Conc. ATP Fold Induction cAMP Fold Induction
(nM) (nM ATP/well) ATP (pmol /well) cAMP
Cholecystokinin 10 9.8 0.4 1.1 * 0.27 0.06 3.1 ** (100nM)
Octapeptide
[CCK(26-33)]
Vehicle 7.6 1.0 0.14 0.02
DTLET 10 9.2 0.9 1.2 * 0.20 0.02 1.4 * (100nM)
Vehicle 7.6 1.0 0.14 0.02
DDAVP 100 11.5 1.4 1.5* 0.27 0.02 1.9 *** (10nM)
Vehicle 8.5 1.5 0.84 0.11
Eledoisin 100 10.4 1.1 1.2 * 1.0 0.06 1.2 * (1 nM)
Vehicle 6.3 0.2 0.57 0.14
y-MSH 10 7.4 0.5 1.2 * 0.96 0.18 1.7 * (100 nM)
Vehicle 8.7 1.5 0.05 0.06
a-Neurokinin 100 11.0 1.4 1.3 * 0.11 0.03 2.3 * (10 nM)
Vehicle 9.4 1.4 0.03 0.01
PACAP-38 100 26.9 3.7 2.9 ** 0.13 0.03 4.2 **
Vehicle 10.3 2.2 0.17 0.04
Beta-ANP 100 13.6 2.1 1.3* 070 0.04 4.2***
Vehicle 6.3 0.2 0.57 0.14
Galanin (1-13)- 100 7.10 0.5 1.1 * 0.82 0.08 1.4
** (1 nM)
Spantide-Amide,
M40
Vehicle 12.5 1.8 0.07 0.06
[Sar9, Met (0)11]- 100 39.7 2.1 3.2 *** 0.16 0.05 2.2 *
Substance P
Vehicle 12.5 1.8 0.30 0.08
Sarafotoxin S6a 10 43.3 4.5 3.5 *** 0.41 0.06 1.4 *
Vehicle 15.2 3.2 0.07 0.06
Sarafotoxin S6b 100 43.0 7.8 2.8 ** 0.43 0.22 6.0 *
Sarafotoxin S6c 10 39.9 6.6 2.6 ** 0.21 0.03 3.0 **
Vehicle 13.5 1.9 0.06 0.01
[N1e8,18, Tyr34]- 1000 23.5 2.7 1.7 ** 0.16 0.05 2.6 * (10nM)
Parathyroid
Hormone (1-34)
Amide (Human)
ACTH (Human) 1000 15.7 1.3 1.2* 0.11 0.02 1.8 ** (100 nM)
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
51
Substance Conc. ATP Fold Induction cAMP Fold Induction
(nM) (nM ATP/well) ATP (pmol /well) cAMP
Glucagon-Like 1000 18.3 1.4 1.3 ** 0.08 0.01
1.4* (100 nM)
Peptide-1 (7-37)
(Human)
Vehicle 12.3 1.1 0.14 0.05
Exendin-3 100 14.2 1.0 1.2 * 0.21 0.03 1.5 * (10 nM)
Vehicle 12.3 1.1 0.30 0.08
Exendin-4 1000 16.0 2.0 1.3 * 0.49 0.04
1.6 *** (10 nM)
Vehicle 12.3 1.1 0.20 0.07
Urotensin II 100 14.3 1.1 1.2* 0.50 0.15 2.6 * (10 nM)
(Globy)
Vasoactive 1000 20.6 1.2 1.7*** 0.39 0.12
2.0 * (100 nM)
Intestinal Peptide
(Human, Porcine,
Rat)
Vehicle 13.4 1.8 0.97 0.46
Nor- 0.1 19.4 3.2 1.4 * 6.10 3.72 6.3 ** (0.01
nM)
Binaltorphimine
Vehicle 7.8 2.0 0.21 0.08
Agouti Related 10 11.2 1.7 1.4 * 0.5 0.20 2.4 *
Protein (87-132)-
Amide (Human)
Table 2 shows ATP levels, reflecting cell number, and cAMP levels. Test
substances were added to
adult mouse stem cell cultures at the indicated doses. After four days, values
for ATP and cAMP
were assayed. Fold induction was determined by comparison to vehicle treated
cells. Data was
represented as the mean SD value of quadruplicate tests in a typical
experiment. The representative
values were based on two separate experiments. *, P<0.05; **, P<0.005; ***,
P<0.001 (Student's t
test); n.s. = non significant. a Significant in lower concentration.
Table 3: Expression analysis of possible targets for the GPCR ligands listed
in Table 2
Official Name Locus Link Locus Link Mouse Mouse lateral Human
Symbol Symbol neurosphere ventricular wall
neurosphere
mouse Human Expression expression Expression
Adenosine Adora2a ADORA2A YES YES n.d.
A2a receptor
Adenosine Adora2b ADORA2B YES YES YES
A2b receptor
Adenosine A3 Adora3 ADORA3 n.d. n.d. n.d.
receptor
Adenylate Adcyap 1 r 1 ADCYAP 1R YES YES YES
cyclase 1
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
52
Official Name Locus Link Locus Link Mouse Mouse lateral
Human
Symbol Symbol neurosphere ventricular wall neurosphere
mouse Human Expression expression Expression
activating
polypeptide 1
receptor 1
Adrenomedulli Admr ADMR n.d. n.d. YES
n receptor
arginine Avpr2 AVPR2 n.d. n.d. n.d.
vasopressin
receptor 2
Bradykinin Bdkrb 1 BDKRB 1 n.d. n.d. n.d.
receptor, beta
1
Bradykinin Bdkrb2 BDKRB2 n.d. n.d. n.d.
receptor, beta
2
Calcitonin Calcr CALCR n.d. n.d. n.d.
receptor
Calcitonin Calcrl CALCRL n.d. n.d. YES
receptor-like
Cholecystokini Cckar CCKAR n.d. n.d. YES
n A receptor
Cholecystokini Cckbr CCKBR n.d. n.d. YES
n B receptor
Endothelin Ednra EDNRA YES YES YES
receptor type
A
Endothelin Ednrb EDNRB YES YES n.d.
receptor type B
Galanin Gain l GALR1 n.d. n.d. n.d.
receptor 1
Galanin Galr2 GALR2 n.d. n.d. n.d.
receptor 2
Galanin Galr3 GALR3 n.d. n.d. n.d.
receptor 3
Glucagon-like Glp 1 r GLP 1R n.d. n.d. n.d.
peptide 1
receptor
Gonadotropin Gnrhr GNRHR n.d. n.d. n.d.
releasing
hormone
receptor
Melanocortin 1 Mc lr MC 1R n.d. n.d. YES
receptor
Melanocortin 2 Mc2r MC2R n.d. n.d. n.d.
receptor
Melanocortin 3 Mc3r MC3R n.d. n.d. n.d.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
53
Official Name Locus Link Locus Link Mouse Mouse lateral Human
Symbol Symbol neurosphere ventricular wall
neurosphere
mouse Human Expression expression Expression
receptor
Melanocortin 4 Mc4r MC4R n.d. n.d. n.d.
receptor
Melanocortin 5 Mc5r MC5R n.d. n.d. YES
receptor
Natriuretic Nprl NPR1 n.d. n.d. n.d.
peptide
receptor 1
Natriuretic Npr2 NPR2 n.d. n.d. n.d.
peptide
receptor 2
Natriuretic Npr3 NPR3 n.d. n.d. n.d.
peptide
receptor 3
Opioid Oprdl OPRD 1 n.d. n.d. n.d.
receptor, delta
1
Opioid Oprkl OPRK1 n.d. n.d. n.d.
receptor, kappa
1
Tachykinin Tacrl TACR1 n.d. n.d. n.d.
receptor 1
Tachykinin Tacr2 TACR2 n.d. n.d. n.d.
receptor 2
Tachykinin Tacr3 TACR3 n.d. n.d. n.d.
receptor 3
, Vasoactive Viprl V1PR1 YES YES YES
intestinal
peptide
receptor 1
Vasoactive Vipr2 V1PR2 YES YES YES
intestinal
peptide
receptor 2
G protein- Gpr14 GPR14 n.d. n.d. n.d.
coupled
receptor 14
Parathyroid Pthrl PTHR1 n.d. n.d. n.d.
hormone
receptor 1
Table 3 shows that GPCRs were found to be expressed in adult mouse and/or
human stem cell
cultures. Gene expression in mouse cells or tissue was determined by cDNA
library analysis, and
human expression using RT-PCR.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
54
A number of compounds that were not previously identified as enhancers of
intracellular cAMP were tested for stimulation of neurogenesis. This test was
used to
determine: 1) if there were additional compounds that could stimulate
neurogenesis by any
mechanism; and 2) if there were additional compounds that could stimulate
neurogenesis by
increasing intracellular cAMP. Surprisingly, several of these compounds were
found to
stimulate neurogenesis even though they were not previously known increase
intracellular
cAMP levels. The compounds screened included: (Des-Arg9, Leu8)-Bradykinin,
(Des-
Arg9)-Bradykinin, Alpha-NeoEndorphin, CART (61-102), DTLET, Eledoisin,
Urotensin H,
[N1e8, 18, Tyr34]-Parathyroid Hormone (1-34) Amide, and [Cys3, 6, Tyr8, Pro9]-
Substance
P (see Table 2). Our review of the literature showed that these properties (of
elevating
intracellular cAMP, and inducing neurogenesis) were not previously known.
The experiments were repeated with visual examination of the wells for signs
of
neurogenesis and to confirm the results of the previous assay. The results
were repeatable.
The visual analysis confirmed our previous findings and did not reveal
anything that would
contradict the previous findings.
EXAMPLE 10: Ca2+ levels correlate to neuronal stem cell proliferation
To show that proliferation upon intracellular Ca2+increase in response to GPCR
ligands is upregulated in adult mouse stem cells grown in vitro, the cells
were treated with a
number of test substances (Table 4, column 1). Ca2+ was measured via
regulation of the
nuclear factor of activated T cells gene (NFAT; Example 5). The results show a
clear
correlation between ATP levels (Table 4, columns 3-4) and NFAT up-regulation
(Table 4,
columns 5-6). This indicates that Ca2+ levels are strongly correlated with
neural stem cells
proliferation. The GPCRs that trigger Ca2 for the ligands analyzed (Table 5,
columns 1-3)
were found to be present in the two cDNA libraries analyzed (Example 6; Table
5, columns
4-5). Tables 3 and 5 (columns 6) show GPCRs that were identified in human stem
cells
material using RT-PCR analysis. This corroborated our findings in adult mouse
stem cells,
and suggested that the activation of Ca2+is also important for triggering GPCR-
mediated
proliferation in human stem cells.
Table 4: GPCR ligands that regulate NFAT-Luciferace reporter (Ca2+) and ATP
(proliferation). Each one of these agents is a neurogenesis modulating agent.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Substance Conc ATP Fold NFAT Fold
(nMolar) (nM ATP/ Induction Luciferace Induction
well) ATP units NFAT
Vehicle 9.812.1 42.917.4 .
Amylin Receptor 100 15.0 2.2 1.5 * 57.3 5.4 1.3 ***
Antagonist/Calcitonin(8- (0.15 nM)
32)
Vehicle 9.811.6 42.917.4
ANP (human) 10 12.7 1.0 1.3 * 65.9 8.9 1.5 *
(1.5 nM)
Vehicle 8.810.9 21.114.1
CGRP (8-37) 100 10.4 0.5 1.2 ** 28.3 1.1 1.3 **
(at 15 nM)
Vehicle 4.510.6 3.410.8
Endothelin-1 (human, 10 14.4 2.4 3.2 * 8.3 2.5 2.4 *
Bovine, Canine, Mouse, (0.15nM)
Porcine, Rat)
Vehicle 6.310.2 2.411.4
y-MSH 10 7.4 0.5 1.2 * 4.8 1.5 2.0 *
(1.5 nM)
Vehicle 7.510.6 2.411.4
Growth Hormone 10 12.6 0.9 1.7 * 4.5 0.6 1.9 **
Releasing Factor (15 nM)
Vehicle 8.210.8 2.411.4
MGOP 27 100 10.2 1.2 1.2 * 4.0 0.4 1.7 *
(1.5 nM)
Vehicle 9.411.4 3.510.9
PACAP-38 10 22.0 0.9 2.3 *** 6.2 1.6 1.7 *
Vehicle 12.511.8 1.910.5
Sarafotoxin S6a 1 - = ' 38.9 3.2 3.1 *** 6.3 2.4 3.4
*
Vehicle 15.213.2 1.910.5
Sarafotoxin S6b 100 43.0 7.8 2.8** 13.4 7.0 7.2*
Sarafotoxin S6c 1 41.6 4.8 2.7 *** 8.3 2.0 4.4 *
Septide 100 25.1 3.1 1.7 * 3.7 0.9 2.0 *
,
Vehicle 14.011.8 1.910.5
Somatostatin-28 10 17.1 1.5 1.2 * 3.0 0.4 1.6 *
(100 nM)
Vehicle 9.310.06 8.210.7 _
Cholera toxin from 100 12.9 1.6 1.4* 11.6 1.0 1.4**
Vibrio Cholerae
Vehicle 9.812.1 5.610.5
Angiotensin II (human 11.7 0.6 1.2 * 12.0 3.7 2.1 *
synthetic)
Vehicle 8.810.9 5.610.5
[D-Pen2-5]-Enkephalin 10 10.7 0.9 1.2 * 10.3 2.1 1.8 *
(100 nM)
Vehicle 10.310.6 5.610.5
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
56
Adrenomedullin 100 11.6 0.8 1.1 *
12.4 0.9 2.2 **
Vehicle 28.1 5.3 8.2 0.7
Endothelin-1 (human, 10 35.3 3.7 1.3 *
13.3 1.3 1.6 **
Porcine,)
Table 4: Adult mouse neuronal stem cells were transiently transfected with
NFAT-Luciferace
construct and induced with test substances at the indicated doses. Cells were
analyzed 24 hours after
induction. NFAT-Luciferace activity and ATP was analyzed. Fold induction was
determined by
comparison to vehicle treated cells. The data was represented as the mean SD
value of
quadruplicate tests in a typical experiment. The representative values were
based on two separate
experiments. *. P<0.05; **. P<0.005; *** P<0.001 (Student's t test); n.s. =
non significant. a
Significant in lower concentration.
Table 5: Expression analysis of targets for the GPCR ligands listed in Table 4
Official Name Locus Link Locus Link Mouse Mouse Human
Symbol Symbol neurospher lateral
neurosphere
mouse human e ventricular
expression
expression wall
expression
Adenylate Adcyaplrl ADCYAP1R1 YES YES YES
cyclase activating
polypeptide 1
receptor 1
Angiotensin Agtrlb AGTR1 n.d. n.d. n.d.
receptor lb
Angiotensin II Agtr2 AGTR2 n.d. n.d. n.d.
receptor. type 2
Calcitonin Calcr CALCR n.d. n.d. n.d.
receptor
Calcitonin Calcrl CALCRL n.d. n.d. YES
receptor-like
Endothelin Ednra EDNRA YES YES YES
receptor type A
Endothelin Ednrb EDNRB YES YES n.d.
receptor type B
Growth hormone Ghrhr GHRHR n.d. n.d. n.d.
releasing
hormone receptor
Melanocortin 1 Mclr MC1R n.d. n.d. YES
receptor
Melanocortin 3 Mc3r MC3R n.d. n.d. n.d.
receptor
Melanocortin 4 Mc4r MC4R n.d. n.d. n.d.
receptor
Melanocortin 5 Mc5r MC5R n.d. n.d. YES
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
57
receptor
Natriuretic Nprl NPR1 n.d. n.d. n.d.
peptide receptor
1
Natriuretic Npr2 NPR2 n.d. n.d. n.d.
peptide receptor
2
Natriuretic Npr3 NPR3 n.d. n.d. n.d.
peptide receptor
3
Opioid receptor. Oprdl OPRD1 n.d. n.d. n.d.
delta 1
Somatostatin Sstrl SSTR1 YES YES YES
receptor 1
Somatostatin S str2 S STR2 YES YES YES
receptor 2
Somatostatin S str3 SSTR3 YES YES n.d.
receptor 3
Somatostatin S str4 S STR4 YES YES n.d.
receptor 4
Somatostatin S str5 S STR5 YES YES n.d.
receptor 5
Tachykinin Tacrl TACR1 n.d. n.d. n.d.
receptor 1
Vaso active Viprl VIPR1 YES YES YES
intestinal peptide
receptor 1
Vaso active Vipr2 V1PR2 YES YES YES
intestinal peptide
receptor 2
EXAMPLE 11: Human and mouse stem cell responses to cAMP stimulation
The experiments described above suggest that intracellular induction of cAMP
occurs
in proliferative mouse adult neural stem cells. To further investigate the
relevance of these
findings, the cAMP pathway was studied in human and mouse systems. Since CREB
phosphorylation is a well known downstream effector in the cAMP activation
pathway
(Lonze and Ginty. 2002), the phosphorylation state of this transcription
factor was
investigated in time course experiments. Two cAMP activators, PACAP and
cholera toxin,
were utilized (Example 7). PACAP and cholera toxin were added to the adult
human and
mouse neuronal stem cells. Western blot analysis showed similar up-regulation
in mouse as
in human neuronal stem cells (FIG. 1). The results clearly demonstrate that
the pattern of
CREB phosphorylation in both systems is responsive to PACAP and cholera toxin
in a
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
58
reproducible manner (FIG. 1). This suggests that mouse and human stem cells
respond in
similar ways following cAMP cell induction. GPCRs for which ligands were shown
to be
proliferative in mouse aNSCs were present also in human aNSCs (Table 3, column
6)
EXAMPLE 12: Adult neural stem cells retain their neuronal potential following
GPCRs proliferative stimuli.
In order to understand if proliferating adult neural stem cells retained their
neuronal
potential following GPCR ligand treatment, analysis was performed to determine
the
expression of the early neuronal marker Doublecortin. Neural stem cells were
treated with
several GPCRs ligands for 4 days. Flow cytometric analysis was performed on
the cells with
an antibody against the early neuronal marker Doublecortin. As shown in Table
6, all GPCR
ligand-treated cells analyzed continued to express Doublecortin after four
days in culture (see
also Example 8). This indicated that the ligand-treated adult NSCs were still
able to
differentiate towards a neuronal phenotype.
Table 6: Adult neural stem cells retain their neuronal potential after
proliferation with
GPCR ligands.
Substance Concentration % Doublecortin- Fold
positive cells Induction
EGF/FGF 3 nM/1 nM 2.63 1.86 1
Forskolin 10 [tM 6.3 2.5
Cholera toxin from Vibrio Cholerae 100 nM 6.7 2.6
Endothelin I. human. porcine 10 nM 5.0 2.0
PACAP-38 100 nM 5.2 2.0
(D-Trp7.A1a8.D-Phel0)-a-Melanocyte 100 nM 5.3 2.1
stimulating hormone F:6-11/GHRP
a-Neurokinin 100 nM 4.6 1.8
Thyrocalcitonin salmon 100 nM 3.9 1.5
MECA 10 [tM 2.2 0.9
[Des-Arg9]-Bradykinin 100 nm 4.5 1.8
Eledoisin 100 nM 4.3 1.7
y-Melanocyte stimulating hormone 100 nM 4.1 1.6
[D-Pen2-5]-Enkephalin 100 nM 3.3 1.3
a-Neo-Endorphin (Porcine) 100 nM 4.0 1.6
CA 02546843 2013-02-22
59
Substance Concentration % Doublecortin- Fold
positive cells Induction
DTLET 100 riM 4.1 1.6
[D-ArgO. Hyp3. Ig15. D-1g17. Oic81- 100 nM 3.6 1.4
Bradykinin
[D-pGlul. D-Phe2. D-Trp3.6]-LH-RH 100 nM 3.4 1.3
Adrenomedullin (Human) 100 nM 4.2 1.6
Adrenomedullin (22-52) (Human) 100 nM 2.0 0.8
Agouti Related Protein (87-132)-Amide 100 nM 2.4 0.9
(Human)
Angiotensin II (Human) 100 nM 3.1 1.2
P-Melanocyte Stimulating Hormone 100nM 4.1 .1.6
CART (61-102)(Human. Rat) 100 nM 4.7 1.8
Cholecystokinin Octapeptide [CCK(26- 100 nM 3.2 1.3
33)](Non-sulfated)
DDAVP (enhances human learning and 100 nM 4.6 1.8
memory)
Sarafotoxin S6a (cardiotoxin isotoxin) 100 nM 3.2 1.3
Table 6: Cells proliferated by GPCR ligands maintained or increased their
potential to mature
towards a neuronal phenotype.
The sum of these results and previous studies on PACAP indication that
compounds (e.g.,
natural ligands, small chemical entities, affinity proteins, etc.) that
increase levels of cAMP
or Ca2+ can stimulate proliferation of adult neural stem cells in vitro and in
vivo. In some
cases, this stimulation may be mediated by GPCRs. In addition, cAMP elevation
alone (i.e.,
in a GPCR-independent-manner) can elicit an increase in the proliferation of
neural stem
cells. This increase was observed with various cAMP activators, including: 1)
cAMP-
derivatives, such as N-6.2-0-Dibutyryladenosine; 2) inhibitors of cAMP
phosphodiesterases,
such as 3-Isobuty1-1-Methylxanthine (IBMX) and rolipram; 3) adenylate cyclase
activators,
such as forskolin; and 4) compounds that elevate ADP-ribosylation of the alpha-
subunit of
the stimulatory G protein (Gs), such as cholera toxin. Cholera toxin and
related compounds
are believed to act by reducing GTPase activity and activating the alpha-
subunit. This leads
to an increase in the activity of adenylate cyclase resulting in increased
levels of cAMP.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Further, as shown herein, several ligands that act through GPCRs and increase
the
intracellular Ca2 content are also effective in promoting neurogenesis,
including cellular
proliferation.
These experiments show that cAMP or Ca2+ activation can be used in therapeutic
5 approaches to modulate proliferation, differentiation, survival, or
migration of adult neural
stem cells/progenitor cells in different physiological or pathological
conditions. The various
compounds (e.g., GPCRs ligands) described herein may display different
cellular specificities
and fate profiles, which make them suited for different physiological and
pathological
conditions. Importantly, adult neural stem cells retained their neuronal
potential following
10 GPCR ligand treatment. The sum of these findings implicate a broad range
of therapeutic
compounds for stimulating neurogenesis through the intracellular elevation of
cAMP and/or
Ca2+.
Example 13 Glp-1 Receptor and Calcitonin Receptor expression analysis by RT
PCR
15 Adult mouse brain tissue from lateral ventricular wall and cultured
adult mouse neural
stem cells (amNSC) were collected and total RNA was extracted with an RNeasy
mini kit
(Qiagen). The primer pairs for GLP-1 receptor (G1p1r) and Calcitonin receptor
(Calcr) were
synthesized:
Gene name Gene Bank Primers
Acc. No. .
Glplr NM 021332 5'GTACCACGGTGTCCCTCTCAGA (SEQ ID NO:65)
3'-GGCGGAGAAAGAAAGTGCGT (SEQ ID NO:66)
Calcr NM 007588 5' AACTGCAAAATGCGTACGTTCTTT (SEQ ID NO:63)
3'- GCATCCAGAAGTAGTTGCAAGACAT (SEQ ID NO:64)
One step RT-PCR (Platinum Taq lnvitrogen) was performed. As a negative
control,
primers were used and Taq enzyme alone was added to ensure that the material
had no
genomic contamination. RNA from total mouse brain was used as a positive
control since the
Glplr and Calcr genes are known to be expressed elsewhere in the brain. The
RNA was
DNase treated to eliminate possible DNA contamination. The RT-PCR reactions
were run as
follows: 1 cycle with incubation at 52 C for 30 minutes and at 94 C for 2
minutes; 35 cycles
with incubation at 94 C forl 5 seconds, at 56 C for 30 seconds, and at 72 C
for 30 seconds; 1
cycle with incubation at 72 C for 7 minutes. The PCR products were run on a
1.5% agarose
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
61
gel containing ethidium bromide. The PCR product was sequenced and, notably,
we found
that both Glp 1 r and Caler is expressed in mouse brain tissue from lateral
ventricular wall. In
addition, Glplr was expressed in cultured adult mouse neural stem cells.
Example 14 In vitro proliferation measured with ATP.
In order to examine proliferative activity of Exendin-4 and calcitonin we
incubated
neural stem cell cultures with either compound for 4 days. Unexpectedly, we
found that both
Exendin-4 and calcitonin significantly increased ATP (proliferation) of neural
stem cells as
compared to vehicle treated controls. For Exendin, the results show a ratio of
1.7-fold
induction compared to control/non treated cells (p= 0.049 student t-test; at
100 nM). At 10
M, calcitonin significantly increased the cell proliferation to 2.5-fold the
level of control cells
(p-value 0.027). The EC50 value for calcitonin is 0.03 nM as shown in the dose-
response
curve in FIG. 3.
Example 15 In Vivo Progenitor Cell Proliferation
Two neurogenesis modulating agents, Exendin-4 and calcitonin were separately
administered intraperitoneally to Male Wistar rats weighing about 270 g
(Harlan-
Winkelmann Germany n=10) at various concentrations (1 g/kg and 10 mg/kg
respectively in
0.1% RSA). The negative control (n=12), the vehicle group in FIG. 2, was
injected with
saline (in 0.1% RSA). Bromodeoxyuridine (BrdU; 50 mg/kg) was co-administrated
together
with the compounds. The intraperitoneally injections were given with a 12 hour
interval for 7
days. Animals were perfused on day 8. The rats were kept at 12 hours
light/dark regime.
Feeding: included standard pellets, and feeding and drinking was ad libitum.
Five animals
were included in standard cage (Macrolon typeM4).
In perfusion, animals were perfused transcardially with 50 ml of ice cold
phosphate
buffered saline (PBS) and then 100 ml of 4% paraformaldehyde in PBS. Brains
were fixed
after removal in 4% paraformaldehyde in PBS for 24 hours at 4 C, at least 3
days before
sectioning. Sections were prepared using a freezing microtome and stored in
cyroprotectant
at -20C before immunostaining for BrdU. Sections were immunostained for BrdU
with
mouse anti-BrdU paired with a biotinylated goat anti mouse IgG and visualized
using ABC
Elite kit (Vectorlabs. using manufactures directions). Standard light
microscope techniques
were used to count the total number of BrdU positive cells in each section and
in relevant
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
62
region of the brain. Analysis and quantification was performed for
proliferative brain
regions, subventricular zone, and the dentate gyros in hippocampus. Other
experimental
details not listed here are known to one of skill in the art and may be found
for example in
Pencea V et al. J. Neurosci Sept 1(2001). 21(17):6706-17.
Notably, we found that rats given intra-peritoneal infusion of Exendin-4 or
calcitonin
co-administrated with BrdU twice daily showed a significant increase
(nonparametric One-
way ANOVA) in the number of newborn cells (BrdU positive compared to sham
injected) in
highly neurogenic regions including the sub ventricular zone and the dentate
gyrus in the
hippocatnpus (FIG. 2A and 2B). These data indicate that Exendin-4 and
calcitonin, in
addition to previously described effects, also exhibit an unexpected neural
stem cell
proliferative effect pointing to neurogenesis.
Example 16 Progenitor Cell Proliferation
A neurogenesis modulating agent is administered intraperitoneally to adult
test
animals (n=12) at various concentrations from 0.01 to 100 mg/kg. Saline is
given as a
negative control. Starting two hours after neurogenesis modulating agent
administration,
animals are injected with four intraperitoneal injections of bromodeoxyuridine
(BrdU; 50
mg/kg each) at three hour intervals. Animals are perfused after 1, 2, or 3
days or after 1, 2, 3,
or 4 weeks after neurogenesis modulating agent administration. For animals
studied for more
than one day BrdU is administered by minipump.
In perfusion. animals are perfused transcardially with 50 ml of ice cold
phosphate
buffered saline (PBS) and then 100 ml of 4% paraformaldehyde in PBS. Brains
are fixed
after removal in 4% paraformaldehyde in PBS for 24 hours at 4 C for at least 3
days before
sectioning. Sections are prepared using a freezing microtome and stored in
cyroprotectant at
-20 C before immunostaining for BrdU.
Sections are immunostained for BrdU with mouse anti-BrdU paired with a
biotinylated goat anti-mouse IgG. Avidin-biotin-horseradish peroxidase (HRP)
complex is
applied to sections and immunoreactivity are visualized by reacting
diaminobenzidine with
the HRP. Standard techniques are used to estimate the total number of BrdU
positive cells in
each section and in each region of the brain.
Analysis and quantification is performed for proliferative brain regions,
migratory
streams, and areas of clinical relevance. Some, but not all. of these areas
are exemplified
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
63
below. This analysis is performed with DAB (diaminebenzidine) or fluorescence
visualization using one or several of the following antibodies: as neuronal
markers NeuN.
Tuj 1 , anti-tyrosine hydroxylase, anti-MAP-2, etc.; as glial markers anti-
GFAP, anti-S100,
etc.; as oligodendrocyte markers anti-GalC, anti-PLP, etc. For BrdU
visualization: anti-
BrdU. Quantification is performed in all areas of the brain using
stereological quantification.
In particular, the following regions are of particular interest: dorsal
hippocampus dentate
gyrus, dorsal hippocampus CAl/alveus, olfactory bulb (OB), subventricular zone
(SVZ), and
striatum. Quantification of double-staining with confocal microscope is
performed for every
structure (e.g., OB, DG, CAl/alveus, SVZ, wall-to-striatum) checking BrdU+ for
double-
staining with the lineage markers. Other experimental details not listed here
are known to
one of skill in the art and may be found, for example, in Pencea V et al. J.
Neurosci Sept 1
(2001). 21(17):6706-17. The experiment is performed with wild type animals as
well as an
animal model of a neurological disease. Such models are enumerated in the
detailed
discussion section. One preferred animal is the mouse.
Example 17 GLP-1R Receptor expression analysis by RT PCR
Cultured adult human neural stem cells (ahNSC) were collected and total RNA
was
extracted with an RNeasy mini kit (Qiagen). Total human hippocampus RNA was
purchased
from Ambion (catalog: 6870; lot: 013P011202039A) The primer pairs for GLP-1
receptor
(GLP1R) were synthesized:
Gene name Gene Bank Primers
Acc. No.
GLP1R NM 002062 5'-GATGTAGTTCCTGGTGCAGTGCA-3' (SEQ ID NO:65)
5'- CAGGTGAAGTGGTGGCAGTACCT-3' (SEQ ID NO:66)
5'-AATGGATCTTCAGGCTCTACGTGA-3'(SEQ ID NO:67)
5'-CTGTAAACAGCTTGATGAAGCGC-3' (SEQ ID NO:68)
One step RT-PCR (Platinum Taq Invitrogen) was performed. As a negative
control,
primers were used and Taq enzyme alone was added to ensure that the material
had no
genomic contamination. Total RNA from human pancreas (BD; Ref: 636577; Lot:
3110768)
was used as a positive control. The RNA was DNase treated to eliminate
possible DNA
contamination. The RT-PCR reactions were run as follows: 1 cycle with
incubation at 47 C
for 30 minutes and at 94 C for 2 minutes; 37 cycles with incubation at 94 C
for 15 seconds,
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
64
at 52 C for 20 seconds, and at 72 C for 60 seconds; 1 cycle with incubation at
72 C for 7
minutes. The PCR products were run on a 1.5% agarose gel containing ethidium
bromide.
We found that the human GLP1 receptor is expressed in human hippocampus brain
tissue and
is expressed in cultured adult human neural stem cells.
Example 18. Exendin-4 dose dependently increase proliferation in cultured
adult
mouse stem cells.
Different doses of Exendin-4 were added to amNCS cultures. The cells were
analyzed
for ATP. An ATP dose effect curve was calculated using XLfit program (ID
Business
solutions limited; Version: 2Ø6). An ATP EC50 was calculated to 0.017 nM
(Figure 4).
Example 19. Adult human neural stein/progenitor cell culture
A biopsy from the anterior lateral wall of the lateral ventricle was taken
from an adult
human patient and enzymatically dissociated in Collagenase 1mg/m1; Dispase
1.6mg/m1;
Trypsin 0.25mg/m1; DNase I 80 U/ml) in DMEM containing 4.5 mg/ml glucose and
37 C for
min. The cells were gently triturated and mixed with three volumes of DMEM/F12
with
10%FBS. The cells were pelleted at 250 x g for 5 mm. The supernatant was
subsequently
20 removed and the cells resuspended in DMEM/F12 with 10%FBS, plated out on
fibronectin
coated culture dishes and incubated at 37 C in 5% CO2. The following day the
expansion of
the culture was initiated by change of media to DMEM/F12; B27; EGF 2Ong/m1;
FGF2
2Ong/m1 (culture medium). The adult human neural stem/progenitor cells were
split using
trypsin and EDTA under standard conditions, and replated in culture medium.
Example 20. Intracellular human ATP assay
Intracellular ATP levels have previously been shown to correlate to cell
number
(Crouch, Kozlowski et al. 1993). Human neural stem/progenitor cells, cultured
as described
above, from various passages, were seeded in DMEM/F12 into a 96-well plate as
single cells
(1500 cells/well). After three days, cells were treated with substances
diluted in DMEM/F12
supplemented with B27 at the concentrations indicated. After 7 days
incubation, intracellular
ATP was measured using the ATP-HTS kit from BioThema, Sweden, according to the
manufacturer's instructions. Exendin-4 significantly (student t-test)
increased proliferation in
human neural stein/progenitor cells compared to non-treated cells (Fig 5).
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
Example 21. Exendin-4 increase the number of BrdU and doublecortin positive
cells in mouse
and rat brain
Animals
5
Male Wistar rats, ca.270g, Harlan-Winkelmann Germany; C57/black 6 mice, MTC,
KI, Sweden. Animal housing: 12 hours light /dark regime; feeding: standard
pellets; feeding
and drinking ad libitum.
Application of substances
10 The
application of substances was performed i.p., either with Exendin-4, 1
microgram/kg; 50mg/kg BrdU; 0,1% rat serum albumin, or with vehicle solution
50mg/kg
BrdU; 0,1% rat/mouse serum albumin. The mice had the additional groups with
doses of 0.1
and 0.01 microgram/kg of Exendin-4. The animals were injected twice daily;
early morning,
late afternoon, min 9h and max 12 hr apart. The rats were treated for 7 days
and the mice
15
were treated for 5 days. Perfusion was performed on day 8 for rats and day 5
for the mice.
Rats were put under anesthesia with chloralhydrate (4g/100m1; 6 ml/animal) and
transcardially perfused with NaC1 for ca 3-5 minutes (ca 60 ml); perfused with
paraformaldehyd (4%) solution (3-5 min, ca 60 ml), and decapitated. Mice were
anesthetized
with CO2 and decapitated Brains were cut coronally 25
and stored in phosphate-buffered
20 saline until immunostained.
Immunohistochemistry:DAB (diaminobenzidine) staining:
Incubated 12 hr, at 4 degrees celsius with mouse anti BrdU 1:100 in 0.1M
PBS/1%
DNS. or goat anti doublecortin (C-18, Santa Cruz). Secondary antibody for 1 h
at RT: donkey
25
anti rat or donkey anti goat biotinylated (Jackson)1:5000 in 0.1M PBS, and
stained with
Vectorlabs Elite Kit. For BrdU mouse:Primary antibody rat anti BrdU (Harlan-
Sera labs)
1:100.Secondary goat anti rat biotinylated (Vectorlabs) 1:200.
Analysis and BrdU quantification was performed in mouse hippocampus dentate
gyrys (DG). The result shows that Exendin-4, in a dosage dependent manner,
significantly
30
(0.01 microgram/kg and 0.1 microgram/kg) increased proliferation in DG
compared to non
treated animals (FIG 6) (non parametric One way ANOVA). Doublecortin
quantification
was performed in rat hippocampus dentate gyrys. The result shows that Exendin-
4
CA 02546843 2013-02-22
=
66
significantly increased the number of doublecortin positive cells compared to
the non-treated
animals (FIG 7) (student t-test).
Other features of the invention will become apparent in the course of the
following
description of exemplary embodiments that are given for illustration of the
invention and are
not intended to be limiting thereof. Throughout this specification, various
patents, published
applications, GenBank DNA and protein sequences, and scientific references are
cited to
describe the state and content of the art.
REFERENCES
Biebl M. Cooper CM. Winkler J. Kuhn HG (2000) Analysis of neurogenesis and
programmed cell death reveals a self- renewing capacity in the adult rat
brain.
Neurosci Lett 291:17-20.
Craig CG. Tropepe V. Morshead CM. Reynolds BA. Weiss S. van der Kooy D (1996)
In vivo
growth factor expansion of endogenous subependymal neural precursor cell
populations in the adult mouse brain. J Neurosci 16:2649-2658.
Doetsch F. Caille I. Lim DA. Garcia-Verdugo JM. Alvarez-Buylla A (1999)
Subventricular
zone astrocytes are neural stem cells in the adult mammalian brain. Cell
97:703-716.
Gage FH. Kempermann G. Palmer TD. Peterson DA. Ray J (1998) Multipotent
progenitor
cells in the adult dentate gyrus. J Neurobiol 36:249-266.
Herman JP. Abrous ND (1994) Dopaminergic neural grafts after fifteen years:
results and
perspectives. Prog Neurobiol 44:1-35.
Jacobson M (1991) Histosenesis and morphogenesis of cortical structures. In:
Developmental
Neurobiology. pp 401-451: Plenum Press. New York.
Johansson CB. Svensson M. Wallstedt L. Janson AM. Frisen J (1999a) Neural stem
cells in
the adult human brain. Exp Cell Res 253:733-736.
Johansson CB. Momma S. Clarke DL. Risling M. Lendahl U. Frisen J (1999b)
Identification
of a neural stem cell in the adult mammalian central nervous system. Cell
96:25-34.
Johe KK. Hazel TO. Muller T. Dugich-Djordjevic MM. McKay RD (1996) Single
factors
direct the differentiation of stem cells from the fetal and adult central
nervous system.
Genes Dev 10:3129-3140.
CA 02546843 2006-05-19
WO 2005/081619 PCT/1B2004/004451
67
Kuhn HG. Winkler J. Kempermann G. Thal U. Gage FH (1997) Epidermal growth
factor
and fibroblast growth factor-2 have different effects on neural progenitors in
the adult
rat brain. J Neurosci 17:5820-5829.
Lois C. Alvarez-Buylla A (1993) Proliferating subventricular zone cells in the
adult
mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad
Sci U S
A 90:2074-2077.
Lonze BE. Ginty DD (2002) Function and regulation of CREB family transcription
factors in
the nervous system. Neuron 35:605-623.
Magavi SS. Leavitt BR. Macklis JD (2000) Induction of neurogenesis in the
neocortex of
adult mice [see comments]. Nature 405:951-955.
McKay R (1997) Stem cells in the central nervous system. Science 276:66-71.
Nakatomi H. Kuriu T. Okabe S. Yamamoto S. Hatano 0. Kawahara N. Tamura A.
Kirino T.
Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic
brain injury by recruitment of endogenous neural progenitors. Cell 110:429-
441.
Neves SR. Ram PT. Iyengar R (2002) G protein pathways. Science 296:1636-1639.
Palmer TD. Markakis EA. Willhoite AR. Safar F. Gage FH (1999) Fibroblast
growth factor-2
activates a latent neurogenic program in neural stem cells from diverse
regions of the
adult CNS. J Neurosci 19:8487-8497.
Patrone C. Andersson S. Korhonen L. Lindholm D (1999) Estrogen receptor-
dependent
regulation of sensory neuron survival in developing dorsal root ganglion. Proc
Natl
Acad Sci U S A 96:10905-10910.
Pencea V. Bingaman KD. Wiegand SJ. Luskin MB (2001) Infusion of Brain-Derived
Neurotrophic Factor into the Lateral Ventricle of the Adult Rat Leads to New
Neurons in the Parenchyma of the Striatum. Septum. Thalamus. and Hypothalamus.
J
Neurosci 21:6706-6717.
Rajan P. McKay RD (1998) Multiple routes to astrocytic differentiation in the
CNS. J
Neurosci 18:3620-3629.
Rao A. Luo C. Hogan PG (1997) Transcription factors of the NFAT family:
regulation and
function. Armu Rev Immunol 15:707-747.
Snyder EY. Yoon C. Flax JD. Macklis JD (1997) Multipotent neural precursors
can
differentiate toward replacement of neurons undergoing targeted apoptotic
degeneration in adult mouse neocortex. Proc Natl Acad Sci U S A 94:11663-
11668.
CA 02546843 2006-05-19
WO 2005/081619
PCT/1B2004/004451
68
Williams BP. Park JK. Alberta JA. Muhlebach SG. Hwang GY. Roberts TM. Stiles
CD
(1997) A PDGF-regulated immediate early gene response initiates neuronal
differentiation in ventricular zone progenitor cells. Neuron 18:553-562.
Zhao M. Momma S. Delfani K. Carlen M. Cassidy RM. Johansson CB. Brismar H.
Shupliakov 0. Frisen J. Janson AM (2003) Evidence for neurogenesis in the
adult
mammalian substantia nigra. Proc Natl Acad Sci U S A 100:7925-7930.
1
CA 02546843 2006-05-19
68.1
SEQUENCE LISTING
<110> NeuroNovaAB
<120> Compounds and methods for increasing neurogenesis
<130> 840-MINT148
<140> Not Yet Known (based upon PCT/IB2004/004451)
<141> 2004-11-19
<160> 83
<170> PatentIn version 3.2
<210> 1
<211> 32
<212> PET
<213> artificial sequence
<220>
<223> Thyrocalcitonin (salmon)
<220>
<221> MOD_RES
<222> (32)..(32)
<223> AMIDATION
<400> 1
Cys Ser Asn Leu Ser Thr Cys Val Leu Gly Lys Leu Ser Gin Glu Leu
1 5 10 15
His Lys Leu Gin Thr Tyr Pro Arg Thr Asn Thr Gly Ser Gly Thr Pro
20 25 30
<210> 2
<211> 32
<212> PET
<213> artificial sequence
<220>
<223> Calcitonin (Human)
<400> 2
Cys Gly Asn Leu Ser Thr Cys Met Leu Gly Thr Tyr Thr Gin Asp Phe
1 5 10 15
Asn Lys Phe His Thr Phe Pro Gin Thr Ala Ile Gly Val Gly Ala Pro
20 25 30
<210> 3
<211> 39
<212> PRT
CA 02546843 2006-05-19
68.2
<213> artificial sequence
<220>
<223> Exendin-3
<220>
<221> MOD_RES
<222> (39)..(39)
<223> AMIDATION
<400> 3
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys Gin Met Glu Glu
1 5 10 15
Glu Ala Val Arg Leu Phe Ile Glu Trp Leu Lys Asn Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
<210> 4
<211> 39
<212> PRT
<213> artificial sequence
<220>
<223> Exendin-4
<220>
<221> MOD_RES
<222> (39)..(39)
<223> AMIDATION
<400> 4
His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Gin Met Glu Glu
1 5 10 15
Glu Ala Val Arg Leu Phe Ile Glu Trp Leu Lys Asn Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
<210> 5
<211> 25
<212> PRT
<213> artificial sequence
<220>
<223> Amylin Receptor Antagonist/Calcitonin(8-32)(Salmon)
CA 02546843 2006-05-19
68.3
<400> 5
Val Leu Gly Lys Leu Ser Gin Glu Leu His Lys Leu Gin Thr Tyr Pro
1 5 10 15
Arg Thr Asn Thr Gly Ser Gly Thr Pro
20 25
<210> 6
<211> 30
<212> PRT
<213> artificial sequence
<220>
<223> CGRP (8-37) (Human) (Selective antagonist for CGRP receptor and
agonist for Calcitonin receptor)
<220>
<221> MOD RES
<222> (30)..(30)
<223> AMIDATION
<400> 6
Val Thr His Arg Leu Ala Gly Leu Leu Ser Arg Ser Gly Gly Val Val
1 5 10 15
Lys Asn Asn Phe Val Pro Thr Asn Val Gly Ser Lys Ala Phe
20 25 30
<210> 7
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> amylin amide
<400> 7
Lys Cys Asn Thr Ala Thr Cys Ala Thr Gin Arg Leu Ala Asn Phe Leu
1 5 10 15
Val His Ser Ser Asn Asn Phe Gly Ala Ile Leu Ser Ser Thr Asn Val
20 25 30
Gly Ser Asn Thr Tyr
<210> 8
<211> 21
CA 02546843 2006-05-19
68.4
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Katacalcin (KC)
<400> 8
Asp Met Ser Ser Asp Leu Glu Arg Asp His Arg Pro His Val Ser Met
1 5 10 15
Pro Gin Asn Ala Asn
<210> 9
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Human calcitonin gene related peptide
(CGRP)
<220>
<221> MOD_RES
<222> (37)..(37)
<223> AMIDATION
<400> 9
Ala Cys Asp Thr Ala Thr Cys Val Thr His Arg Leu Ala Gly Leu Leu
1 5 10 15
Ser Arg Ser Gly Gly Val Val Lys Asn Asn Phe Val Pro Thr Asn Val
20 25 30
Gly Ser Lys Ala Phe
<210> 10
<211> 38
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Calcitonin receptor stimulating peptide
1 (CRSP-1)
<400> 10
Ser Cys Asn Thr Ala Thr Cys Met Thr His Arg Leu Val Gly Leu Leu
1 5 10 15
CA 02546843 2006-05-19
68.5
Ser Arg Ser Gly Ser Met Val Arg Ser Asn Leu Leu Pro Thr Lys Met
20 25 30
Gly Phe Lys Val Phe Gly
<210> 11
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Calcitonin receptor stimulating peptide
2 (CRSP-2)
<400> 11
Ser Cys Asn Thr Ala Ser Cys Val Thr His Lys Met Thr Gly Trp Leu
1 5 10 15
Ser Arg Ser Gly Ser Val Ala Lys Asn Asn Phe Met Pro Thr Asn Val
20 25 30
Asp Ser Lys Ile Leu
<210> 12
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Calcitonin receptor stimulating peptide
3 (CRSP-3)
<400> 12
Ser Cys Asn Thr Ala Ile Cys Val Thr His Lys Met Ala Gly Trp Leu
1 5 10 15
Ser Arg Ser Gly Ser Val Val Lys Asn Asn Phe Met Pro Ile Asn Met
20 25 30
Gly Ser Lys Val Leu
<210> 13
<211> 27
<212> PRT
<213> artificial sequence
<220>
CA 02546843 2006-05-19
68.6
<223> Amino acid sequence from Histidine-methionine amide peptide
hormone (PHM-27)
<220>
<221> MOD_RES
<222> (27)..(27)
<223> AMIDATION
<400> 13
His Ala Asp Gly Val Phe Thr Ser Asp Phe Ser Lys Leu Leu Gly Gin
1 5 10 15
Leu Ser Ala Lys Lys Tyr Leu Glu Ser Leu Met
20 25
<210> 14
<211> 47
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Intermedin
<220>
<221> MOD_RES
<222> (47)..(47)
<223> AMIDATION
<400> 14
Thr Gin Ala Gin Leu Leu Arg Val Gly Cys Val Leu Gly Thr Cys Gin
1 5 10 15
Val Gin Asn Leu Ser His Arg Leu Trp Gin Leu Met Gly Pro Ala Gly
20 25 30
Arg Gin Asp Ser Ala Pro Val Asp Pro Ser Ser Pro His Ser Tyr
35 40 45
<210> 15
<211> 32
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from [Asp17, Lys21]-side-chain bridged salmon
calcitonin
<220>
<221> SITE
<222> (17)..(21)
CA 02546843 2006-05-19
68.7
<223> lactam bridge
<400> 15
Cys Ser Asn Leu Ser Thr Cys Val Leu Gly Lys Leu Ser Gin Asp Leu
1 5 10 15
Asp Lys Leu Gin Lys Phe Pro Arg Thr Asn Thr Gly Ala Gly Val Pro
20 25 30
<210> 16
<211> 32
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from [Asp17, Orn21]-side-chain bridged salmon
calcitonin
<220>
<221> SITE
<222> (17)..(21)
<223> lactam bridge
<220>
<221> MOD_RES
<222> (21)..(21)
<223> Orn
<220>
<221> misc_feature
<222> (21)..(21)
<223> Xaa can be any naturally occurring amino acid
<400> 16
Cys Ser Asn Leu Her Thr Cys Val Leu Gly Lys Leu Her Gin Asp Leu
1 5 10 15
Asp Lys Leu Gin Xaa Phe Pro Arg Thr Asn Thr Gly Ala Gly Val Pro
20 25 30
<210> 17
<211> 24
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from [Lysll-Bolton Hunter, Arg18, Asn30,
Tyr32]-salmon calcitonin
<400> 17
Leu Gly Lys Leu Her Gin Asp Leu His Arg Leu Gin Thr Phe Pro Arg
1 5 10 15
CA 02546843 2006-05-19
68.8
Thr Asn Thr Gly Ala Asn Val Tyr
<210> 18
<211> 32
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from [Argil, 18, Lys14]-salmon calcitonin
<400> 18
Cys Ser Asn Leu Ser Thr Cys Val Leu Gly Arg Leu Ser Lys Asp Leu
1 5 10 15
His Arg Leu Gin Thr Phe Pro Arg Thr Asn Thr Gly Ala Gly Val Pro
20 25 30
<210> 19
<211> 32
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from benzophenone-containing calcitonin
<400> 19
Cys Ser Asn Leu Ser Thr Cys Val Leu Gly Lys Leu Ser Gin Asp Leu
1 5 10 15
His Lys Leu Gin Thr Phe Pro Arg Thr Asn Thr Gly Ala Gly Val Pro
20 25 30
<210> 20
<211> 32
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from eel calcitonin analog
<220>
<221> misc_feature
<222> (1)..(1)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (7)..(7)
<223> Xaa can be any naturally occurring amino acid
CA 02546843 2006-05-19
68.9
<220>
<221> MOD_RES
<222> (32)..(32)
<223> AMIDATION
<400> 20
Xaa Ser Asn Leu Ser Thr Xaa Val Leu Gly Lys Leu Ser Gin Glu Leu
1 5 10 15
His Lys Leu Gin Thr Tyr Pro Arg Thr Asp Val Gly Ala Gly Thr Pro
20 25 30
<210> 21
<211> 39
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exenatide (Exendin-4)
<400> 21
His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Gin Met Glu Glu
1 5 10 15
Glu Ala Val Arg Leu Phe Ile Glu Trp Leu Lys Asn Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
<210> 22
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 (Glucagon-like peptide-1)
<400> 22
His Asp Glu Phe Glu Arg His Ala Glu Gly Thr Phe Thr Ser Asp Val
1 5 10 15
Ser Ser Tyr Leu Glu Gly Gln Ala Ala Lys Glu Phe Ile Ala Trp Leu
20 25 30
Val Lys Gly Arg Gly
CA 02546843 2006-05-19
68.10
<210> 23
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 23
His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Met Glu Glu
1 5 10 15
<210> 24
<211> 16
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 24
His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Met Glu Glu Glu
1 5 10 15
<210> 25
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 25
His Ser Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Met Glu Glu
1 5 10 15
<210> 26
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 26
His Ala Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Met Glu Glu
1 5 10 15
<210> 27
<211> 9
<212> PRT
<213> artificial sequence
CA 02546843 2006-05-19
68.11
<220>
<223> Amino acid sequence of Exendin analog peptide agent/GLP-1
receptor ligand
<400> 27
His Gly Glu Gly Thr Phe Thr Ser Asp
1 5
<210> 28
<211> 31
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 28
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly Arg Gly
20 25 30
<210> 29
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of GLP-1 receptor ligand
<400> 29
His Asp Glu Phe Glu Arg His Ala Glu Gly Thr Phe Thr Ser Asp Val
1 5 10 15
Ser Ser Tyr Leu Glu Gly Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu
20 25 30
Val Lys Gly Arg Gly
<210> 30
<211> 11
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from GLP-1 analog with modified N-terminal
sequence
CA 02546843 2006-05-19
68.12
<400> 30
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser
1 5 10
<210> 31
<211> 31
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from GLP-1 analog with modified N-terminal
sequence
<400> 31
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gln Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly Arg Gly
20 25 30
<210> 32
<211> 31
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from GLP-1 analog CJC-1131
<400> 32
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gln Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly Arg Lys
20 25 30
<210> 33
<211> 31
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from GLP-1 analog Liraglutide (also called
NN-2211 and [Arg34, Lys26]-(N- epsilon
-(gamma-Glu(N-alpha-hexadecanoy1))-GLP-1(7-37))
<400> 33
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gln Ala Ala Lys Glu Phe Ile Ala Trp Lys Val Arg Gly Arg Gly
CA 02546843 2006-05-19
68.13
20 25 30
<210> 34
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from Pramlintide (amylin analog)
<400> 34
Lys Cys Asn Thr Ala Thr Cys Ala Thr Gin Arg Leu Ala Asn Phe Leu
1 5 10 15
Val His Ser Ser Asn Asn Phe Gly Pro Ile Leu Pro Pro Tyr Asn Val
20 25 30
Gly Ser Asn Thr Tyr
<210> 35
<211> 37
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from amylin
<400> 35
Lys Cys Asn Thr Ala Thr Cys Ala Thr Gin Arg Leu Ala Asn Phe Leu
1 5 10 15
Val His Ser Ser Asn Asn Phe Gly Ala Ile Leu Ser Ser Thr Asn Val
20 25 30
Gly Ser Asn Thr Tyr
<210> 36
<211> 9
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence from [Ser(2)]-Exendin (1-9)
<400> 36
His Ser Glu Gly Thr Phe Thr Ser Asp
1 5
CA 02546843 2006-05-19
68.14
<210> 37
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 37
His Ser Thr Gly Thr Phe Thr Ser Met Asp Thr Ser Gin Leu Pro
1 5 10 15
<210> 38
<211> 11
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 38
His Ser Thr Gly Thr Phe Thr Ser Met Asp Thr
1 5 10
<210> 39
<211> 10
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 39
His Ser Thr Gly Thr Phe Thr Ser Met Asp
1 5 10
<210> 40
<211> 10
<212> PRT
<213> artificial
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 40
Gin Ser Thr Gly Thr Phe Thr Ser Met Asp
1 5 10
<210> 41
<211> 10
<212> PRT
CA 02546843 2006-05-19
68.15
<213> artificial sequence
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 41
Gin Thr Thr Gly Thr Phe Thr Ser Met Asp
1 5 10
<210> 42
<211> 10
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of PACAP receptor ligand
<400> 42
His Thr Thr Gly Thr Phe Thr Ser Met Asp
1 5 10
<210> 43
<211> 19
<212> DNA
<213> artificial sequence
<220>
<223> primer for ADORA2A
<220>
<221> misc_feature
<222> (1)..(19)
<223> primer
<400> 43
caatgtgctg gtgtgctgg 19
<210> 44
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> primer for ADORA2A
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 44
tagacaccca gcatgagcag 20
CA 02546843 2006-05-19
68.16
<210> 45
<211> 19
<212> DNA
<213> artificial sequence
<220>
<223> Primer for EDNRA
<220>
<221> misc_feature
<222> (1)..(19)
<223> primer
<400> 45
caggatcatt taccagaac 19
<210> 46
<211> 19
<212> DNA
<213> artificial sequence
<220>
<223> Primer for EDNRA
<220>
<221> misc_feature
<222> (1)..(19)
<223> primer
<400> 46
gacgctgctt aagatgttc 19
<210> 47
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for CALCRL
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 47
agagcctaag ttgccaaagg 20
<210> 48
<211> 20
<212> DNA
<213> artificial sequence
CA 02546843 2006-05-19
68.17
<220>
<223> Primer for CALCRL
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 48
gaatcagcac aaattcaatg 20
<210> 49
<211> 18
<212> DNA
<213> artificial sequence
<220>
<223> Primer for MC1R
<220>
<221> misc_feature
<222> (1)..(18)
<223> primer
<400> 49
gaaccggaac ctgcactc 18
<210> 50
<211> 19
<212> DNA
<213> artificial sequence
<220>
<223> Primer for MC1R
<220>
<221> misc_feature
<222> (1)..(19)
<223> primer
<400> 50
tgcccagcag gatggtgag 19
<210> 51
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for MC5R
<220>
<221> misc_feature
,
CA 02546843 2006-05-19
68.18
<222> (1)..(20)
<223> primer
<400> 51
gagaacatct tggtcatagg 20
<210> 52
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for MC5R
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 52
agcattaaag tgagatgaag 20
<210> 53
<211> 18
<212> DNA
<213> artificial sequence
<220>
<223> Primer for VIPR1
<220>
<221> misc_feature
<222> (1)..(18)
<223> primer
<400> 53
ctacaccatt ggctacgg 18
<210> 54
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for VIPR1
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 54
gactgctgtc actcttcctg 20
,
CA 02546843 2006-05-19
68.19
<210> 55
<211> 21
<212> DNA
<213> artificial sequence
<220>
<223> Primer for VIPR2
<220>
<221> misc_feature
<222> (1)..(21)
<223> primer
<400> 55
gatgtctctt gcaacaggaa g 21
<210> 56
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for VIPR2
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 56
gcaaacacca tgtagtggac 20
<210> 57
<211> 25
<212> DNA
<213> artificial sequence
<220>
<223> Primer for SSTR1
<220>
<221> misc_feature
<222> (1)..(25)
<223> primer
<400> 57
gggaactcta tggtcatcta cgtga 25
<210> 58
<211> 23
<212> DNA
<213> artificial sequence
CA 02546843 2006-05-19
68.20
<220>
<223> Primer for SSTR1
<220>
<221> misc_feature
<222> (1)..(23)
<223> primer
<400> 58
gaaatgtgta caacacgaag ccc 23
<210> 59
<211> 25
<212> DNA
<213> artificial sequence
<220>
<223> Primer for SSTR2
<220>
<221> misc_feature
<222> (1)..(25)
<223> primer
<400> 59
ggcaacacac ttgtcattta tgtca 25
<210> 60
<211> 25
<212> DNA
<213> artificial sequence
<220>
<223> Primer for SSTR2
<220>
<221> misc_feature
<222> (1)..(25)
<223> primer
<400> 60
aggtagcaaa gacagatgat ggtga 25
<210> 61
<211> 22
<212> DNA
<213> artificial sequence
<220>
<223> Primer for ADCYAP1R1
<220>
<221> misc_feature
CA 02546843 2006-05-19
68.21
<222> (1)..(22)
<223> primer
<400> 61
tactttgatg acacaggctg ct 22
<210> 62
<211> 22
<212> DNA
<213> artificial sequence
<220>
<223> Primer for ADCYAP1R1
<220>
<221> misc_feature
<222> (1)..(22)
<223> primer
<400> 62
agtacagcca ccacaaagcc ct 22
<210> 63
<211> 24
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Calcr
<220>
<221> misc_feature
<222> (1)..(24)
<223> primer
<400> 63
aactgcaaaa tgcgtacgtt cttt 24
<210> 64
<211> 25
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Calcr
<220>
<221> misc_feature
<222> (1)..(25)
<223> primer
<400> 64
gcatccagaa gtagttgcaa gacat 25
CA 02546843 2006-05-19
68.22
<210> 65
<211> 22
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(22)
<223> primer
<400> 65
gtaccacggt gtccctctca ga 22
<210> 66
<211> 20
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(20)
<223> primer
<400> 66
ggcggagaaa gaaagtgcgt 20
<210> 67
<211> 24
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(24)
<223> primer
<400> 67
aatggatctt caggctctac gtga 24
<210> 68
<211> 23
<212> DNA
<213> artificial sequence
CA 02546843 2006-05-19
68.23
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(23)
<223> primer
<400> 68
ctgtaaacag cttgatgaag cgc 23
<210> 69
<211> 21
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<400> 69
His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Lys Gln Met Glu Glu
1 5 10 15
Glu Ala Val Arg Leu
<210> 70
<211> 9
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<400> 70
His Ser Asp Gly Thr Phe Thr Ser Asp
1 5
<210> 71
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (10)..(10)
<223> X = any amino acid
<220>
CA 02546843 2006-05-19
68.24
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<400> 71
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys
1 5 10
<210> 72
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(15)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<400> 72
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys Xaa Leu Glu
1 5 10 15
<210> 73
<211> 19
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(19)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<220>
CA 02546843 2006-05-19
68.25
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<400> 73
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys Xaa Leu Glu Xaa
1 5 10 15
Xaa Xaa Ala
<210> 74
<211> 21
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(21)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (20)..(20)
<223> Xaa can be any naturally occurring amino acid
<400> 74
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys Xaa Leu Glu Xaa
1 5 10 15
Xaa Xaa Ala Xaa Lys
CA 02546843 2006-05-19
68.26
<210> 75
<211> 24
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(24)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (20)..(20)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (22)..(22)
<223> Xaa can be any naturally occurring amino acid
<400> 75
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys Xaa Leu Glu Xaa
1 5 10 15
Xaa Xaa Ala Xaa Lys Xaa Phe Ile
<210> 76
<211> 27
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
CA 02546843 2006-05-19
68.27
<220>
<221> UNSURE
<222> (1)..(27)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (10)..(10)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (20)..(20)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (22)..(22)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (25)..(25)
<223> Xaa can be any naturally occurring amino acid
<400> 76
His Ser Asp Gly Thr Phe Thr Ser Asp Xaa Ser Lys Xaa Leu Glu Xaa
1 5 10 15
Xaa Xaa Ala Xaa Lys Xaa Phe Ile Xaa Trp Leu
20 25
<210> 77
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(12)
<223> X = any amino acid
CA 02546843 2006-05-19
68.28
<400> 77
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys
1 5 10
<210> 78
<211> 15
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(15)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<400> 78
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys Xaa Met Glu
1 5 10 15
<210> 79
<211> 21
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(21)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<400> 79
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys Xaa Met Glu Xaa
1 5 10 15
CA 02546843 2006-05-19
68.29
Xaa Xaa Ala Val Lys
<210> 80
<211> 24
<212> PRT
<213> artificial sequence
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(24)
<223> X = any amino acid
<220>
<221> misc_feature
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (22)..(22)
<223> Xaa can be any naturally occurring amino acid
<400> 80
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys Xaa Met Glu Xaa
1 5 10 15
Xaa Xaa Ala Val Lys Xaa Phe Ile
<210> 81
<211> 30
<212> PRT
<213> artificial
<220>
<223> Amino acid sequence of Exendin analog peptide agent
<220>
<221> UNSURE
<222> (1)..(30)
<223> X= any amino acid
<220>
<221> misc_feature
CA 02546843 2006-05-19
68.30
<222> (13)..(13)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (16)..(18)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (22)..(22)
<223> Xaa can be any naturally occurring amino acid
<220>
<221> misc_feature
<222> (25)..(25)
<223> Xaa can be any naturally occurring amino acid
<400> 81
His Ser Asp Gly Thr Phe Thr Ser Asp Leu Ser Lys Xaa Met Glu Xaa
1 5 10 15
Xaa Xaa Ala Val Lys Xaa Phe Ile Xaa Trp Leu Leu Asn Gly
20 25 30
<210> 82
<211> 23
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(23)
<223> primer
<400> 82
gatgtagttc ctggtgcagt gca 23
<210> 83
<211> 23
<212> DNA
<213> artificial sequence
<220>
<223> Primer for Glplr
<220>
<221> misc_feature
<222> (1)..(23)
<223> primer
CA 02546843 2006-05-19
68.31
<400> 83
caggtgaagt ggtggcagta cct 23