Note: Descriptions are shown in the official language in which they were submitted.
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
SYSTEMS AND METHODS OF PRODUCING A CRUDE PRODUCT
FIELD OF INVENTION
The present invention generally relates to systems and methods for treating
crude
feed, and to compositions that are produced, for example, using such systems
and
methods. More particularly, embodiments described herein relate to systems and
methods
for conversion of a crude feed that has a residue content of at least 0.2
grams of residue
per gram of crude feed to a crude product that is (a) a liquid mixture at 25
C and 0.101
MPa, and (b) has one or more properties that are improved in comparison to the
same
properties of the crude feed.
DESCRIPTION OF RELATED ART
Crudes that have one or more unsuitable properties that do not allow the
crudes to
be economically transported, or processed using conventional facilities, are
commonly
referred to as "disadvantaged crudes".
Disadvantaged crudes often contain relatively high levels of residue. Such
crudes
tend to be difficult and expensive to transport and/or process using
conventional facilities. =
High residue crudes may be treated at high temperatures to convert the crude
to coke.
Alternatively, high residue crudes are typically treated with water at high
temperatures to
produce less viscous crudes and/or crude mixtures. During processing, water
removal
from the less viscous crudes and/or crude mixtures may be difficult using
conventional
means.
Disadvantaged crudes may include hydrogen deficient hydrocarbons. When
processing of hydrogen deficient hydrocarbons, consistent quantities of
hydrogen
generally need to be added, particularly if unsaturated fragments resulting
from cracking
processes are produced. Hydrogenation during processing, which typically
involves the
use of an active hydrogenation catalyst, may be needed to inhibit unsaturated
fragments
from forming coke. Hydrogen is costly to produce and/or costly to transport to
treatment
facilities.
Coke may form and/or deposit on catalyst surfaces at a rapid rate during
processing
of disadvantaged crudes. It may be costly to regenerate the catalytic activity
of a catalyst
contaminated by coke. High temperatures used during regeneration may also
diminish the
activity of the catalyst and/or cause the catalyst to deteriorate.
Disadvantaged crudes may include acidic components that contribute to the
total
acid number ("TAN") of the crude feed. Disadvantaged crudes with a relatively
high
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
'IAN may contribute to corrosion ot metal components during transporting
and/or
processing of the disadvantaged crudes. Removal of acidic components from
disadvantaged crudes may involve chemically neutralizing acidic components
with various
bases. Alternately, corrosion-resistant metals may be used in transportation
equipment
and/or processing equipment. The use of corrosion-resistant metal often
involves
significant expense, and thus, the use of corrosion-resistant metal in
existing equipment
may not be desirable. Another method to inhibit corrosion may involve addition
of
corrosion inhibitors to disadvantaged crudes before transporting and/or
processing of the
disadvantaged crudes. The use of corrosion inhibitors may negatively affect
equipment
used to process the crudes and/or the quality of products produced from the
crudes.
Disadvantaged crudes may contain relatively high amounts of metal
contaminants,
for example, nickel, vanadium, and/or iron. During processing of such crudes,
metal
contaminants, and/or compounds of metal contaminants, may deposit on a surface
of the
catalyst or the void volume of the catalyst. Such deposits may cause a decline
in the
activity of the catalyst.
Disadvantaged crudes often include organically bound heteroatoms (for example,
sulfur, oxygen, and nitrogen). Organically bound heteroatoms may, in some
situations,
have an adverse effect on catalysts. Alkali metal salts and/or alkaline-earth
metal salts
have been used in processes for desulfurization of residue. These processes
tend to result
in poor desulfurization efficiency, production of oil insoluble sludge, poor
demetallization
efficiency, formation of substantially inseparable salt-oil mixtures,
utilization of large
quantities of hydrogen gas, and/or relatively high hydrogen pressures.
Some processes for improving the quality of crude include adding a diluent to
disadvantaged crudes to lower the weight percent of components contributing to
the
disadvantaged properties. Adding diluent, however, generally increases costs
of treating
disadvantaged crudes due to the costs of diluent and/or increased costs to
handle the
disadvantaged crudes. Addition of diluent to a disadvantaged crude may, in
some
situations, decrease stability of such crude.
U.S. Patent Nos. 3,136,714 to Gibson et al.; 3,558,747 to Gleim et al.;
3,847,797 to
Pasternak et al.; 3,948,759 to King et al.; 3,957,620 to Fukui et al.;
3,960,706 to
McCollum et al.; 3,960,708 to McCollum et al.; 4,119,528 to Baird, Jr. et al.;
4,127,470 to
Baird, Jr. et al.; 4,224,140 to Fujimori et al.; 4,437,980 to Heredy et al.;
4,591,426 to
Krasuk et al.; 4,665,261 to Mazurek; 5,064,523 to Kretschmar et al.; 5,166,118
to
Kretschmar et al.; 5,288,681 to Gatsis; 6,547,957 to Sudhakar et al.; and U.S.
Patent
2
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
Application Publication Nos. 20030000867 to Reynolds and 20030149317 to
Rendina,
describe various processes and systems used to treat crudes. The process,
systems, and
catalysts described in these patents, however, have limited applicability
because of many
of the technical problems set forth above.
In sum, disadvantaged crudes generally have undesirable properties (for
example,
relatively high residue, a tendency to corrode equipment, and/or a tendency to
consume
relatively large amounts of hydrogen during treatment). Other undesirable
properties
include relatively high amounts of undesirable components (for example,
relatively high
TAN, organically bound heteroatoms, and/or metal contaminants). Such
properties tend to
cause problems in conventional transportation and/or treatment facilities,
including
increased corrosion, decreased catalyst life, process plugging, and/or
increased usage of
hydrogen during treatment. Thus, there is a significant economic and technical
need for
improved systems, methods, and/or catalysts for conversion of disadvantaged
crudes into
crude products with properties that are more desirable.
SUMMARY OF THE INVENTION
Inventions described herein generally relate to systems and methods for
contacting
a crude feed with one or more catalysts to produce a total product comprising
a crude
product and, in some embodiments, non-condensable gas. Inventions described
herein
also generally relate to compositions that have novel combinations of
components therein.
Such compositions can be obtained by using the systems and methods described
herein.
The invention provides a method of preparing a crude product, comprising
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce the crude product, wherein one or more of the catalysts comprises a
catalyst
containing K3Fe10S14.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, at least one of the catalysts
comprising one or
more transition metal sulfides, and the crude feed having a residue content of
at least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307;
and
controlling contacting conditions such that the crude product has at most 0.05
grams of
coke per gram of crude product, the crude product has at least 0.001 grams of
naphtha per
gram of crude product, and the naphtha has an octane number of at least 70.
3
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
The invention also provides a method ot preparing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, at least one of the catalysts
comprising one or
more transition metal sulfides, and the crude feed having a residue content of
at least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307;
and
controlling contacting conditions such that the crude product comprises
kerosene, the
kerosene having at least 0.2 grams of aromatics per gram of kerosene, as
determined by
ASTM Method D5186, the kerosene having a freezing point at a temperature of at
most -
30 C, as determined by ASTM Method D2386, and the crude product having at
most 0.05
grams of coke per gram of crude product.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, at least one of the catalysts
comprising one or,
more transition metal sulfides, and the crude feed having a=residue content of
at least 0.2
grams of residue per gram of crude feed; and controlling contacting conditions
such that
the crude product has at most 0.05 grams of coke per gram of crude product
with a weight
ratio of atomic hydrogen to atomic carbon (H/C) in the crude product of at
most 1.75, as
determined by ASTM Method D6730.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, at least one of the catalysts
comprising one or
more transition metal sulfides, and the crude feed having a residue content of
at least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307,
and a
weight ratio of atomic hydrogen to atomic carbon (H/C) in the crude feed is at
least 1.5;
and controlling contacting conditions such that the crude product has an
atomic H/C ratio
of 80-120% of the atomic H/C ratio of the crude feed, the crude product having
a residue
content of at most 30% of the residue content of the crude feed, as determined
by ASTM
Method D5307, the crude product having at least 0.001 grams of naphtha per
gram of
crude product, and the naphtha having an octane number of at least 70.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts, to
4
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, at least one of the catalysts
comprising one or
more transition metal sulfides, and the crude feed has a residue content of at
least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307;
and
controlling contacting conditions such that the crude product has, per gram of
crude
product: at least 0.001 grams of naphtha, the naphtha having an octane number
of at least
70; at least 0.001 grams of kerosene, the kerosene comprising aromatics, the
kerosene
having at least 0.2 grams of aromatics per gram of kerosene, as determined by
ASTM
Method D5186, and the kerosene having a freezing point at a temperature of at
most -30
C, as determined by ASTM Method D2386; at least 0.001 grams of vacuum gas oil
(VGO), the VG0 having at least 0.3 grams of aromatics per gram of VGO, as
determined
by IP Method 368/90; and at most 0.05 grams of residue, as determined by ASTM
Method
D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts
comprising a transition metal sulfide catalyst to produce a total product that
includes the
crude product, wherein the crude product is a liquid mixture at 25 C and
0.101 MPa, the
transition metal sulfide catalyst having a total of at least 0.4 grams of one
or more
transition metal sulfides per gram of total transition metal sulfide catalyst,
the crude feed
having a residue content of at least 0.2 grams of residue per gram of crude
feed, as
determined by ASTM Method D5307; and controlling contacting conditions such
that the
crude product has at most 0.05 grams of coke per gram of crude product, and
the crude
product has a residue content of at most 30% of the residue content of the
crude feed, as
determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts
comprising a transition metal sulfide catalyst to produce a total product that
includes the
crude product, wherein the crude product is a liquid mixture at 25 C and
0.101 MPa, the
transition metal sulfide catalyst having a total of least 0.4 grams of one or
more transition
metal sulfides per gram of transition metal sulfide catalyst, the crude feed
having a
nitrogen content of at least 0.001 grams of nitrogen per gram of crude feed,
and the crude
feed having a residue content of at least 0.2 grams of residue per gram of
crude feed; and
controlling contacting conditions such that the crude product has a nitrogen
content of at
most 90% of the nitrogen content of the crude feed, and the crude product has
a residue
5
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
content of at most 30% of the residue content of the crude feed, wherein
nitrogen content
is as determined by ASTM Method D5762 and residue content is as determined by
ASTM
Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts
comprising a transition metal sulfide catalyst to produce a total product that
includes the
crude product, wherein the crude product is a liquid mixture at 25 C and
0.101 MPa, the
transition metal sulfide catalyst has a total of least 0.4 grams of one or
more transition
metal sulfides per gram of total transition metal sulfide catalyst, the crude
feed has a total
NiN/Fe content of at least 0.0001 grams of NiN/Fe per gram of crude feed, and
the crude
feed has a residue content of at least 0.2 grams of residue per gram of crude
feed; and
controlling contacting conditions such that the crude product has at most 0.05
grams of
coke per gram of crude product, the crude product has a total NiN/Fe content
of at most
90% of the NiN/Fe content of the crude feed, the crude product has a residue
content of at
most 30% of the residue content of the crude feed, and wherein NiN/Fe content
is as
determined by ASTM Method D5863, and residue content is as determined by ASTM
Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts
comprising a transition metal sulfide catalyst to produce a total product that
includes the
crude product, wherein the crude product is a liquid mixture at 25 C and
0.101 MPa, the
transition metal sulfide catalyst having a total of at least 0.4 grams of one
or more
transition metal sulfides per gram of total transition metal sulfide catalyst,
the crude feed
having a sulfur content of at least 0.001 grams of sulfur per gram of crude
feed, and the
crude feed having a residue content at least 0.2 grams of residue per gram of
cnide feed;
and controlling contacting conditions such that the crude product has a sulfur
content of at
most 70% of the sulfur content of the crude feed, and the crude product has a
residue
content of at most 30% of the residue content of the crude feed, wherein
sulfur content is
as determined by ASTM Method D4294 and residue content is as determined by
ASTM
Method D5307.
The invention also provides a method of producing a transition metal sulfide
catalyst composition, comprising: mixing a transition metal oxide and a metal
salt to form
a transition metal oxide/metal salt mixture; reacting the transition metal
oxide/metal salt
mixture with hydrogen to form an intermediate; and reacting the intermediate
with sulfur
6
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
in the presence of one or more hydrocarbons to produce the transition metal
sulfide
catalyst.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts
comprising a transition metal sulfide catalyst to produce a total product that
includes the
crude product, wherein the crude product is a liquid mixture at 25 C and
0.101 MPa, the
transition metal sulfide catalyst comprises a transition metal sulfide, the
crude feed having
a residue content of at least 0.2 grams of residue per gram of crude feed, as
determined by
ASTM Method D5307; controlling contact conditions such that the crude product
has a
residue content of at most 30% of the residue content of the crude feed; and
wherein the
transition metal sulfide catalyst is obtainable by: mixing a transition metal
oxide and a
metal salt to form a transition metal oxide/metal salt mixture; reacting the
transition metal
oxide/metal salt mixture with hydrogen to form an intermediate; and reacting
the
intermediate with sulfur in the presence of one or more hydrocarbons to
produce the
transition metal sulfide catalyst. =
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of one or more
catalysts to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, and the crude feed having at least 0.2
grams of
residue per gram of crude feed, as determined by ASTM Method D5307; producing
at
least a portion of the total product as a vapor; condensing at least a portion
of the vapor at
C and 0.101 MPa; and forming the crude product, wherein the crude product has,
per
gram of crude product: at least 0.001 grams of naphtha, the naphtha having an
octane
number of at least 70; at least 0.001 grams of VG0, the VGO having at least
0.3 grams of
25 aromatics per gram of VG0, as determined by IP Method 368/90; and at
most 0.05 grams
of residue, as determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
feed has a
residue content of at least 0.2 grams of residue per gram of crude feed, as
determined by
ASTM Method D5307, the crude product is a liquid mixture at 25 C and 0.101
MPa, and
the crude product has, per gram of crude product: at least 0.001 grams of
naphtha, the
naphtha having at least 0.001 grams of monocyclic ring aromatics per gram of
naphtha, as
7
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
determined by ASTM Method D6730; at least 0.001 grams of distillate; and at
most 0.05
grams of residue, as determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
feed has a
residue content of at least 0.2 grams of residue per gram of crude feed, as
determined by
ASTM Method D5307, the crude product is a liquid mixture at 25 C and 0.101
MPa, and
the crude product has, per gram of crude product: at least 0.001 grams of
diesel, and the
diesel has at least 0.3 grams of aromatics per gram of diesel, as determined
by IP Method
368/90; at least 0.001 grams of VGO, and the VGO has at least 0.3 grams of
aromatics per
gram of VG0, as determined by IP Method 368/90; and at most 0.05 grams of
residue, as
determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram a crude feed, as determined by ASTM Method D5307,
and the
crude feed has a monocyclic ring aromatics content of at most 0.1 grams of
monocyclic
ring aromatics per gram of crude feed; and controlling contacting conditions
such that
during the contacting at most 0.2 grams of hydrocarbons that are not
condensable at 25 C
and 0.101 MPa are formed per gram of crude feed, as determined by mass
balance, and
such that the crude product has a monocyclic ring aromatics content of at
least 5% greater
than a monocyclic ring aromatics content of the crude feed, wherein monocyclic
ring
aromatics content is as determined by ASTM Method D6730.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to a produce a total product that includes the crude product, wherein the
crude product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307,
and the
crude feed has an olefins content, expressed in grams of olefins per gram of
crude feed;
and controlling contacting conditions such that the crude product has an
olefins content of
at least 5% greater than the olefins content of the crude feed, wherein olefin
content is as
determined by ASTM Method D6730.
8
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed having a residue content
of at least
0.2 grams of residue per gram of crude feed, and the inorganic salt catalyst
exhibits an
emitted gas inflection of an emitted gas in a temperature range between 50 C
and 500 C,
as determined by Temporal Analysis of Products (TAP); and controlling
contacting
conditions such that the crude product has a residue content, expressed in
grams of residue
per gram of crude product, of at most 30% of the residue content of the crude
feed,
wherein residue content is as determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram of crude feed, the inorganic salt catalyst comprises
at least two
inorganic metal salts, and the inorganic salt catalyst exhibits an emitted gas
inflection of
an emitted gas in a temperature range, as determined by Temporal Analysis of
Products
(TAP), wherein the emitted gas inflection temperature range is between (a) a
DSC
temperature of at least one of the two inorganic metal salts and (b) a DSC
temperature of
the inorganic salt catalyst; and controlling contacting conditions such that
the crude
product has a residue content, expressed in grams of residue per gram of crude
product, of
at most 30% of the residue content of the crude feed, wherein residue content
is as
determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram of crude feed, as determined by ASTM Method D5307,
and the
inorganic salt catalyst exhibits an emitted gas inflection of an emitted gas
in a temperature
range between 50 C and 500 C, as determined by Temporal Analysis of Products
(TAP);
and producing the crude product such that a volume of the crude product
produced is at
least 5% greater than the volume of the crude feed, when the volumes are
measured at 25
C and 0.101 MPa.
9
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram of crude feed, and the inorganic salt catalyst
exhibits an emitted
gas inflection of an emitted gas in a temperature range between 50 C and 500
C, as
determined by Temporal Analysis of Products (TAP); and controlling contacting
conditions such that during the contacting at most 0.2 grams of hydrocarbons
that are not
condensable at 25 C and 0.101 MPa are formed per gram of crude feed, as
determined by
mass balance.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed having a residue content
of at least
0.2 grams of residue per gram of crude feed, and the inorganic salt catalyst
has a heat
transition in a temperature range between 200 C and 500 C, as determined by
differential scanning calorimetry (DSC), at a rate of 10 C per minute; and
controlling
Contacting conditions such that the crude product has a residue content,
expressed in
grams of residue per gram of crude product, of at most 30% of the residue
content of the
crude feed, wherein residue content is as determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed having a residue content
of at least
0.2 grams of residue per gram of crude feed, and the inorganic salt catalyst
has ionic
conductivity that is at least the ionic conductivity of at least one of the
inorganic salts of
the inorganic salt catalyst at a temperature in a range from 300 C and 500
C; and
controlling contacting conditions such that the crude product has a residue
content,
expressed in grams of residue per gram of crude product, of at most 30% of the
residue
content of the crude feed, wherein residue content is as determined by ASTM
Method
D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
3.0
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
grams of residue per gram of crude feed, the inorganic salt catalyst comprises
alkali metal
salts, wherein at least one of the alkali metal salts is an alkali metal
carbonate, and the
alkali metals have an atomic number of at least 11, and at least one atomic
ratio of an
alkali metal having an atomic number of at least 11 to an alkali metal having
an atomic
number greater than 11 is in a range from 0.1 to 10; and controlling
contacting conditions
such that the crude product has a residue content of at most 30% of the
residue content of
the crude feed, wherein residue content is as determined by ASTM Method D5307.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product, wherein the crude feed has a residue content of at
least 0.2
grams of residue per gram of crude feed, the inorganic salt catalyst comprises
alkali metal
salts, wherein at least one of the alkali metal salts is an alkali metal
hydroxide, and the
alkali metals have an atomic number of at least 11, and at least one atomic
ratio of an
alkali metal having an atomic number of at least 11 to an alkali metal having
an atomic
' number greater than 11 is in a range from 0.1 to 10; producing at least a
portion of the
total product as a vapor; condensing at least a portion of the vapor at 25 C
and 0.101
MPa; and forming the crude product, wherein the crude product has a residue
content of at
most 30% of the residue content of the crude feed.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product, wherein the crude feed has a residue content of at
least 0.2
grams of residue per gram of crude feed, the inorganic salt catalyst comprises
alkali metal
salts, wherein at least one of the alkali metal salts is an alkali metal
hydride, and the alkali
metals have an atomic number of at least 11, and at least one atomic ratio of
an alkali
metal having an atomic number of at least 11 to an alkali metal having an
atomic number
greater than 11 is in a range from 0.1 to 10; producing at least a portion of
the total
product as a vapor; condensing at least a portion of the vapor at 25 C and
0.101 MPa; and
forming the crude product, wherein the crude product has a residue content of
at most 30%
of the residue content of the crude feed.
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa, the crude feed has a residue content of
at least 0.2
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
grams of residue per gram of crude feed, the inorganic salt catalyst comprises
one or more
alkali metal salts, one or more alkaline-earth metal salts, or mixtures
thereof, wherein one
of the alkali metal salts is an alkali metal carbonate, wherein the alkali
metals have an
atomic number of at least 11; and controlling contacting conditions such that
the crude
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
The invention also provides a method of producing a crude product, comprising:
contacting a crude feed with a hydrogen source in the presence of an inorganic
salt catalyst
to produce a total product that includes the crude product, wherein the crude
product is a
The invention also provides a method of producing hydrogen gas, comprising:
contacting a crude feed with one or more hydrocarbons in the presence of an
inorganic salt
catalyst and water, the hydrocarbons have carbon numbers in a range from 1 to
6, the
12
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
The invention also provides a method of producing a crude product, comprising:
contacting a first crude feed with an inorganic salt catalyst in the presence
of steam to
generate a gas stream, the gas stream comprising hydrogen, wherein the first
crude feed
has a residue content of at least 0.2 grams of residue per gram of first crude
feed, as
determined using ASTM Method D5307, and the inorganic salt catalyst exhibits
an
emitted gas inflection of an emitted gas in a temperature range between 50 C
and 500 C,
as determined by Temporal Analysis of Products (TAP); contacting a second
crude feed
with a second catalyst in the presence of at least a portion of the generated
gas stream to
produce a total product that includes the crude product, wherein the crude
product is a
liquid mixture at 25 C and 0.101 MPa; and controlling contacting conditions
such that
one or more properties of the crude product change by at least 10% relative to
the
respective one or more properties of the second crude feed.
The invention also provides a method of generating a gas stream, comprising:
contacting a crude feed with an inorganic salt catalyst in the presence of
steam, wherein
the crude feed has a residue content of at least 0.2 grams of residue per gram
of crude
feed, as determined by ASTM Method 5307; and generating a gas stream, the gas
stream
comprising hydrogen, carbon monoxide, and carbon dioxide, and wherein a molar
ratio of
the carbon monoxide to the carbon dioxide is at least 0.3.
The invention also provides a method of producing a crude product comprising:.
conditioning an inorganic salt catalyst; contacting a crude feed with a
hydrogen source in
the presence of the conditioned inorganic salt catalyst to produce a total
product that
includes the crude product, wherein the crude product is a liquid mixture at
25 C and
0.101 MPa, the crude feed having a residue content of at least 0.2 grams of
residue per
gram of crude feed; and controlling contacting conditions such that the crude
product has a
residue content, expressed in grams of residue per gram of crude product, of
at most 30%
of the residue content of the crude feed, wherein residue content is as
determined by
ASTM Method D5307.
The invention also provides a crude composition, comprising hydrocarbons that
have a boiling range distribution between 30 C and 538 C (1,000 F) at 0.101
MPa, the
hydrocarbons comprising iso-paraffins and n-paraffins with a weight ratio of
the iso-
paraffins to n-paraffins of at most 1.4, as determined by ASTM Method D6730.
The invention also provides a crude composition having, per gram of
composition:
at least 0.001 grains of hydrocarbons with a boiling range distribution of at
most 204 C
(400 F) at 0.101 MPa, at least 0.001 grams of hydrocarbons with a boiling
range
13
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
distribution between 204 C and 300 C at 0.101 Mi'a, at teast u.uut grams oi
hydrocarbons with a boiling range distribution between 300 C and 400 C at
0.101 MPa,
and at least 0.001 grams of hydrocarbons with a boiling range distribution
between 400 C
and 538 C (1,000 F) at 0.101 MPa, and wherein the hydrocarbons that have a
boiling
range distribution of at most 204 C comprise iso-paraffins and n-paraffins
with a weight
ratio of the iso-paraffins to the n-paraffins of at most 1.4, as determined by
ASTM Method
D6730.
The invention also provides a crude composition having, per gram of
composition:
at least 0.001 grams of naphtha, the naphtha having an octane number of at
least 70, and
the naphtha having at most 0.15 grams of olefins per gram of naphtha, as
determined by
ASTM Method D6730; at least 0.001 grams of kerosene, the kerosene having at
least 0.2
grams of aromatics per gram of kerosene, as determined by ASTM D5186, and the
kerosene having a freezing point at a temperature of at most -30 C, as
determined by
ASTM Method D2386; and at most 0.05 grams of residue, as determined by ASTM
Method D5307.
The invention also provides a crude composition having, per gram of
composition:
at most 0.15 grams of hydrocarbon gas that is non-condensable. at 25 C and
0.101 MPa, ,
the non-condensable hydrocarbon gas having at most 0.3 grams of hydrocarbons
with a
carbon number from 1 to 3 (Ci to C3), per gram of non-condensable hydrocarbon
gas; at
least 0.001 grams of naphtha, the naphtha having an octane number of at least
70; at least
0.001 grams of kerosene, the kerosene having a freezing point at a temperature
of at most -
C, as determined by ASTM Method D2386, and the kerosene having at least 0.2
grams of aromatics per gram of kerosene, as determined by ASTM Method D5186;
and at
most 0.05 grams of residue, as determined by ASTM Method D5307.
25 The invention also provides a crude composition, having, per gram of
composition:
at most 0.05 grams of residue, as determined by ASTM Method D5307; at least
0.001
grams of hydrocarbons with a boiling range distribution of at most 204 C (400
F) at
0.101 MPa; at least 0.001 grams of hydrocarbons with a boiling range
distribution between
204 C and 300 C at 0.101 MPa; at least 0.001 grams of hydrocarbons with a
boiling
30 range distribution between 300 C and 400 C at 0.101 MPa; at least
0.001 grams of
hydrocarbons with a boiling range distribution between 400 C and 538 C
(1,000 F) at
0.101 MPa; and wherein the hydrocarbons in a boiling range distribution
between 20 C
and 204 C comprise olefins having terminal double bonds and olefins having
internal
14
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
double bonds with a molar ratio of olefins having terminal double bonds to
oietins having
internal double bonds of at least 0.4, as determined by ASTM Method D6730.
The invention also provides a crude composition, having, per gram of
composition:
at most 0.05 grams of residue, as determined by ASTM Method D5307; and at
least 0.001
grams of a mixture of hydrocarbons that have a boiling range distribution
between 20 C
and 538 C (1,000 F), as determined by ASTM Method D5307, and the hydrocarbon
mixture has, per gram of hydrocarbon mixture: at least 0.001 grams of
paraffins, as
determined by ASTM Method D6730; at least 0.001 grams of olefins, as
determined by
ASTM Method D6730, and the olefins have at least 0.001 grams of terminal
olefins per
gram of olefins, as determined by ASTM Method D6730; at least 0.001 grams of
naphtha;
at least 0.001 grams of kerosene, the kerosene having at least 0.2 grams of
aromatics per
gram of kerosene, as determined by ASTM Method D5186; at least 0.001 grams of
diesel,
the diesel having at least 0.3 grams of aromatics per gram of diesel, as
determined by IP
Method 368/90; and at least 0.001 grams of vacuum gas oil (VG0), the VG0
having at
least 0.3 grams of aromatics per gram of VG0, as determined by IP Method
368/90.
The invention also provides a crude composition having, per gram of
composition:
at most 0.05 grams of residue, as determined by ASTM Method D5307; at least
0.001 =
grams of hydrocarbons with a boiling range distribution of at most 204 C (400
F) at
0.101 MPa; at least 0.001 grams of hydrocarbons with a boiling range
distribution between
204 C and 300 C at 0.101 MPa; at least 0.001 grams of hydrocarbons with a
boiling
range distribution between 300 C and 400 C at 0.101 MPa; and at least 0.001
grams of
hydrocarbons with a boiling range distribution between 400 C and 538 C
(1,000 F) at
0.101 MPa, as determined by ASTM Method D2887; and wherein the hydrocarbons
having a boiling range distribution of at most 204 C have, per gram of
hydrocarbons
having a boiling range distribution of at most 204 C: at least 0.001 grams of
olefins, as
determined by ASTM Method D6730; and at least 0.001 grams of paraffins, the
paraffins
comprising iso-paraffins and n-paraffins with a weight ratio of iso-paraffins
to n-paraffins
of at most 1.4, as determined by ASTM Method D6730.
The invention also provides a crude composition having, per gram of
composition:
at most 0.05 grams of residue, as determined by ASTM Method D5307; and at
least 0.001
grams of hydrocarbons with a boiling range distribution of at most 204 C (400
F) at
0.101 MPa; at least 0.001 grams of hydrocarbons with a boiling range
distribution between
204 C and 300 C at 0.101 MPa; at least 0.001 grams of hydrocarbons with a
boiling
range distribution between 300 C and 400 C at 0.101 MPa; and at least 0.001
grams of
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
hydrocarbons with a boiling range distribution between 400 C and 538 C
(1,000 F) at
0.101 MPa, as determined by ASTM Method D2887; and wherein the hydrocarbons
having a boiling range distribution between -10 C and 204 C comprise
compounds with
a carbon number of 4 (C4), the C4 compounds having at least 0.001 grams of
butadiene per
gram of C4 compounds.
The invention also provides a crude composition having, per gram of
composition:
at most 0.05 grams of residue; at least 0.001 grams of hydrocarbons with a
boiling range
distribution of at most 204 C (400 F) at 0.101 MPa, at least 0.001 grams of
hydrocarbons
with a boiling range distribution between 204 C and 300 C at 0.101 MPa, at
least 0.001
grams of hydrocarbons with a boiling range distribution between 300 C and 400
C at
0.101 MPa, and at least 0.001 grams of hydrocarbons with a boiling range
distribution
between 400 C and 538 C at 0.101 MPa; and greater than 0 grams, but less
than 0.01
grams of one or more catalyst, wherein the catalyst has at least one or more
alkali metals.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a crude feed
that: (a) has
,=
=4, not been treated in a refinery, distilled, and/or fractionally
distilled; (b) comprises
components having a carbon number above 4, and the crude feed has at least 0.5
grams of
= such components per gram of crude feed; (c) comprises hydrocarbons of
which a portion
has: a boiling range distribution below 100 C at 0.101 MPa, a boiling range
distribution
between 100 C and 200 C at 0.101 MPa, a boiling range distribution between
200 C
= and 300 C at 0.101 MPa, a boiling range distribution between 300 C and
400 C at 0.101
MPa, and a boiling range distribution between 400 C and 700 C at 0.101 MPa;
(d) has,
per gram of crude feed: at least 0.001 grams of hydrocarbons having a boiling
range
distribution below 100 C at 0.101 MPa, at least 0.001 grams of hydrocarbons
having a
boiling range distribution between 100 C and 200 C at 0.101 MPa, at least
0.001 grams
of hydrocarbons having a boiling range distribution between 200 C and 300 C
at 0.101
MPa, at least 0.001 grams of hydrocarbons having a boiling range distribution
between
300 C and 400 C at 0.101 MPa, and at least 0.001 grams of hydrocarbons
having a
boiling range distribution between 400 C and 700 C at 0.101 MPa; (e) has a
TAN; (f)
has from 0.2-0.99 grams, 0.3-0.8 grams, or 0.4-0.7 grams of residue per gram
of crude
feed; (g) comprises nickel, vanadium, iron, or mixtures thereof; (h) comprises
sulfur;
and/or (i) nitrogen containing hydrocarbons.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, the hydrogen
source that:
16
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
(a) is gaseous; (b) comprises molecular hydrogen; (c) comprises light
hydrocarbons; (d)
comprises methane, ethane, propane, or mixtures thereof; (e) comprises water;
and/or (f)
mixtures thereof.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
includes
conditioning the inorganic salt catalyst, wherein conditions the inorganic
catalyst
comprises: (a) heating the inorganic salt catalyst to a temperature of at
least 300 C; and/or
(b) heating the inorganic salt catalyst to a temperature of at least 300 C
and cooling the
inorganic salt catalyst to a temperature of at most 500 C.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
comprises
contacting a crude feed with one or more catalysts and controlling contacting
conditions:
(a) such that during the contacting at most 0.2 grams, at most 0.15 grams, at
most 0.1
grams, or at most 0.05 grams of hydrocarbons that are not condensable at 25 C
and 0.101
MPa are formed per gram of crude feed, as determined by mass balance; (b) such
that a
contacting temperature is in a range from 250-750 C or between 260-550 C;
(c)
' pressure is in a range from 0.1-20 MPa; (d) such that a ratio of a
gaseous hydrogen source ,
to the crude feed is in a range from 1-16100 or 5-320 normal cubic meters of
the hydrogen
source per cubic meter of the crude feed; (e) to inhibit coke formation; (f)
to inhibit
formation of coke in the total product or in the crude feed during the
contacting; (g) such
that the crude product also has at most 0.05 grams, at most 0.03 grams, at
most 0.01
grams, or at most 0.003 grams of coke per gram of crude product; (h) such that
at least a
portion of the inorganic salt catalyst is semi-liquid or liquid at such
contacting conditions;
(i) such that the crude product has a TAN of at most 90% of the TAN of the
crude feed; (j)
such that the crude product has a total NiN/Fe content of at most 90%, at most
50%, or at
most 10% of the NiN/Fe content of the crude feed; (k) such that the crude
product has a
sulfur content of at most 90%, at most 60%, or at most 30% of the sulfur
content of the
crude feed; (1) such that the crude product has a nitrogen content of at most
90%, at most
70%, at most 50%, or at most 10% of the nitrogen content of the crude feed;
(m) such that
the crude product has a residue content of at most 30%, at most 10%, or at
most 5% of the
residue content of the crude feed; (n) such that ammonia is co-produced with
the crude
product; (o) such that the crude product comprises methanol, and the method
further
comprises: recovering the methanol from the crude product; combining the
recovered
methanol with additional crude feed to form an additional crude feed/methanol
mixture;
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
ana neaung me aaamonai cruae reeannemanat mixture sueti tnat lAiN or me
aaartional
crude feed is reduced to below 1; (p) such that one or more properties of the
crude product
= change by at most 90% relative to the respective one or more properties
of the crude feed;
(q) such that an amount of catalyst in the contacting zone ranges from 1-60
grams of total
catalyst per 100 grams of crude feed; and/or (r) such that a hydrogen source
is added to the
crude feed prior to or during the contacting.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, contacting
conditions
that comprise: (a) mixing the inorganic salt catalyst with the crude feed at a
temperature
below 500 C, wherein the inorganic salt catalyst is substantially insoluble
in the crude
feed; (b) agitating the inorganic catalyst in the crude feed; and/or (c)
contacting the crude
feed with the inorganic salt catalyst in the presence of water and/or steam to
produce a
total product that includes the crude product that is a liquid mixture at STP.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
comprises
." 'contacting a crude feed with an inorganic salt catalyst and that
further comprises: (a)
providing steam to a contacting zone prior to or during contacting; (b)
forming an
emulsion of the crude feed with water prior to contacting the crude feed with
the inorganic
salt catalyst and the hydrogen source; (c) spraying the crude feed into the
contacting zone;
and/or (d) contacting steam with the inorganic salt catalyst to at least
partially remove
coke from the surface of the inorganic salt catalyst.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
comprises
contacting a crude feed with an inorganic salt catalyst to produce a total
product wherein
at least a portion of the total product is produced as a vapor, and the method
further
comprises condensing at least a portion of the vapor at 25 C and 0.101 MPa to
form the
crude product, the contacting conditions are controlled such that: (a) the
crude product
further comprises components with a selected boiling range distribution;
and/or (b) the
crude product comprises components having a selected API gravity.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
comprises
contacting a crude feed with an one or more catalysts and that the one or more
catalysts
are nonacidic.
18
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
in some emoocurnents, tne invenuon alSO prowues, iiico1ito1l1e111011 WILLI
uiie 01
more of the methods or compositions according to the invention, a K3Fe10S14
catalyst or a
transition metal sulfide catalyst that: (a) has a total of at least 0.4 grams,
at least 0.6 grams,
or at least 0.8 grams of at least one of transition metal sulfides per gram of
the K3Fe10S14
catalyst or the transition metal sulfide catalyst; (b) has an atomic ratio of
transition metal
to sulfur in the K3Fe10S14 catalyst or the transition metal sulfide catalyst
in a range from
0.2 to 20; (c) further comprises one or more alkali metals, one or more
compounds of one
or more alkali metals, or mixtures thereof; (d) further comprises one or more
alkaline-
earth metals, one or more compounds of one or more alkaline-earth metals, or
mixtures
thereof; (e) further comprises one or more alkali metals, one or more
compounds of one or
more alkali metals, or mixtures thereof wherein an atomic ratio of transition
metal to
sulfur in the K3Fe13S14 catalyst or the transition metal sulfide catalyst is
in a range from
0.5-2.5 and an atomic ratio of the alkali metals to the transition metal is in
a range from
above 0 to 1; (f) further comprises one or more alkaline-earth metals, one or
more
compounds of one or more alkaline-earth metals, or mixtures thereof an atomic
ratio of
transition metal to sulfur in the K3Fe10S14 catalyst or the transition metal
sulfide catalyst is
in a range from 0.5-2.5; and an atomic ratio of the alkaline-earth metal to
the transition
metal is in a range from above 0 to 1; (g) further comprises zinc; (h) further
comprises
KFe2S3; (i) further comprises KFeS2; and/or (j) is nonacidic.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, that the
K3Fe10S14
catalyst is formed in situ.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, one or more of
the
transition metal sulfides that or in which: (a) comprise one or more
transition metals from
Columns 6-10 of the Periodic Table, one or more compounds of one or more
transition
metals from Columns 6-10, or mixtures thereof; (b) comprise one or more iron
sulfides;
(c) comprises FeS; (d) comprises FeS2; (e) comprise a mixture of iron
sulfides, wherein
the iron sulfides are represented by the formula Fe(1b)S, where b is in a
range from above
0 to 0.17; (f) further comprises K3Fe10S14 after contact with the crude feed;
(g) at least one
of the transition metals of the one or more transition metal sulfides is iron;
and/or (h) are
deposited on a support, and the transition metal sulfide catalyst has at most
0.25 grams of
total support per 100 grams of catalyst.
19
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method of
forming a
transition metal sulfide catalyst composition the method comprising mixing a
transition
metal oxide and a metal salt to form a transition metal oxide/metal salt
mixture; reacting
the transition metal oxide/metal salt mixture with hydrogen to form an
intermediate; and
reacting the intermediate with sulfur in the presence of one or more
hydrocarbons to
produce the transition metal sulfide catalyst: (a) the metal salt comprises an
alkali metal
carbonate; (b) that further comprises dispersing the intermediate in the one
or more liquid
hydrocarbons while it is reacted with the sulfur; (c) in which one or more of
the
hydrocarbons have a boiling point of at least 100 C; (d) in which one or more
of the
hydrocarbons is VG0, xylene, or mixtures thereof; (e) in which mixing the
transition
metal oxide and the metal salt comprises: mixing the transition metal oxide
and the metal
salt in the presence of de-ionized water to from a wet paste; drying the wet
paste at a
temperature in a range from 150-250 C; and calcining the dried paste at a
temperature in
a range from 300-600 C; (f) in which reacting the intermediate with sulfur
comprises
heating the intermediate in the presence of at least one of the hydrocarbons
to a
temperature in the range from 240-350 C; and/or (g) that further comprises
contacting the
catalyst composition with a crude feed that comprises sulfur and a hydrogen
source.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that comprises: (a) one or more alkali metal carbonates, one or more alkaline-
earth metal
carbonates, or mixtures thereof; (b) one or more alkali metal hydroxides, one
or more
alkaline-earth metal hydroxides, or mixtures thereof (c) one or more alkali
metal
hydrides, one or more alkaline-earth metal hydrides, or mixtures thereof (d)
one or more
sulfides of one or more alkali metals, one or more sulfides of one or more
alkaline-earth
metals, or mixtures thereof (e) one or more amides of one or more alkali
metals, one or
more amides of one or more alkaline-earth metals, or mixtures thereof (f) one
or more
metals from Columns 6-10 of the Periodic Table, one or more compounds of one
or more
metals from Columns 6-10 of the Periodic Table, or mixtures thereof; (g) one
or more
inorganic metal salts, and wherein at least one of the inorganic metal salts
generates
hydride during use of the catalyst; (h) sodium, potassium, rubidium, cesium,
or mixtures
thereof (i) calcium and/or magnesium; (j) a mixture of a sodium salt and a
potassium salt
and the potassium salt comprises potassium carbonate, potassium hydroxide,
potassium
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
hydride, or mixtures thereoi, and the sodium salt comprises sodium carbonate,
sodium
hydroxide, sodium hydride, or mixtures thereof and/or (k) mixtures thereof
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that includes alkali metals in which: (a) the atomic ratio of an alkali metal
having an
atomic number of at least 11 to an alkali metal having an atomic number
greater than 11 is
in a range from 0.1 to 4; (b) at least two of the alkali metals are sodium and
potassium and
an atomic ratio of sodium to potassium is in a range from 0.1 to 4; (c) at
least three of the
alkali metals are sodium, potassium, and rubidium, and each of the atomic
ratios of
sodium to potassium, sodium to rubidium, and potassium to rubidium is in a
range from
0.1 to 5; (d) at least three of the alkali metals are sodium, potassium, and
cesium, and each
of the atomic ratios of sodium to potassium, sodium to cesium, and potassium
to cesium is
in a range from 0.1 to 5; (e) at least three of the alkali metals are
potassium, cesium,
rubidium, and each of the atomic ratios of potassium to cesium, potassium to
rubidium,
and cesium to rubidium is in a range from 0.1 to 5.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
comprising a support material, and: (a) the support material comprises
zirconium oxide,
calcium oxide, magnesium oxide, titanium oxide, hydrotalcite, alumina,
germania, iron
oxide, nickel oxide, zinc oxide, cadmium oxide, antimony oxide, or mixtures
thereof
and/or (b) incorporated in the support material are: one or more metals from
Columns 6-10
of the Periodic Table, one or more compounds of one or more metals from
Columns 6-10
of the Periodic Table; one or more alkali metal carbonates, one or more alkali
metal
hydroxides, one or more alkali metal hydrides, one or more alkaline-earth
metal
carbonates, one or more alkaline-earth metal hydroxides, one or more alkaline-
earth metal
hydrides, and/or mixtures thereof
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method
comprises
contacting a crude feed with an inorganic salt catalyst that: (a) the
catalytic activity of the
inorganic salt catalyst is substantially unchanged in the presence of sulfur;
and/or (b) the
inorganic salt catalyst is continuously added to the crude feed.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that exhibits: (a) an emitted gas inflection in a TAP temperature range, and
the emitted gas
21
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
comprises water vapor and/or carbon dioxide; (b) a neat transition in a
temperature range
between 200-500 C, 250-450 C, or 300-400 C, as determined by differential
scanning
calorimetry, at a heating rate of 10 C per minute; (c) a DSC temperature in a
range
between 200-500 C, or 250-450 C; (d) at a temperature of at least 100 C, an
x-ray
diffraction pattern that is broader than an x-ray diffraction pattern of the
inorganic salt
catalyst below 100 C; and/or (e) after conditioning, ionic conductivity, at
300 C, that is
less than ionic conductivity of the inorganic salt catalyst before
conditioning.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that exhibits an emitted inflection in a temperature range, as determined by
TAP, and the
contacting conditions are also controlled such that a contacting temperature
is: (a) above
T1, wherein T1 is 30 C, 20 C, or 10 C below the TAP temperature of the
inorganic salt
catalyst; (b) at or above a TAP temperature; and/or (c) at least the TAP
temperature of the
inorganic salt catalyst.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that or in which: (a) is liquid or semi-liquid at least at the TAP temperature
of the
inorganic salt catalyst, and the inorganic salt catalyst is substantially
insoluble in the crude
feed at least at the TAP temperature, wherein the TAP temperature is the
minimum
temperature at which the inorganic salt catalyst exhibits an emitted gas
inflection; (b) is a
mixture of a liquid phase and a solid phase at a temperature in a range from
50 C to 500
C; and/or (c) at least one of the two inorganic salts has a DSC temperature
above 500 C.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that when tested in the form of particles that can pass through a 1000 micron
filter, self-
deforms under gravity and/or under a pressure of at least 0.007 MPa when
heated to a
temperature of at least 300 C, such that the inorganic salt catalyst
transforms from a first
form to a second form, and the second form is incapable of returning to the
first form upon
cooling of the inorganic salt catalyst to 20 C.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, an inorganic
salt catalyst
that has, per gram of inorganic salt catalyst: (a) at most 0.01 grams of
lithium, or
compounds of lithium, calculated as the weight of lithium; (b) at most 0.001
grams of
22
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
halide, calculated as the weight of halogen; and/or (c) at most 0.001 grams ot
glassy oxide
compounds.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, the total
product that has
at least 0.8 grams of crude product per gram of total product.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a crude
product that: (a)
has at most 0.003 grams, at most 0.02 grams, at most 0.01 grams, at most 0.05
grams,
most 0.001 grams, from 0.000001-0.1 grams, 0.00001-0.05 grams, or 0.0001-0.03
grams
of residue per gram of crude product; (b) has from 0 grams to 0.05 grams,
0.00001-0.03
grams, or 0.0001-0.01 grams of coke per gram of crude product; (c) has an
olefins content
of at least 10% greater than the olefins content of the crude feed; (d) has
greater than 0
grams, but less than 0.01 grams of total inorganic salt catalyst per gram of
crude product,
as determined by mass balance; (e) has at least 0.1 grams, from 0.00001-0.99
grams, from
0.04-0.9 grams from 0.6-0.8 grams of VGO per gram of crude product; (f)
comprises
VG0 and the VG0 has at least 0.3 grams of aromatics per gram of VGO; (g) has
0.001
grams or from 0.1-0.5 grams of distillate; (h) an atomic H/C of at most 1.4;
(i) has an
atomic H/C of 90-110% of the H/C of the crude feed; (j) has a monocyclic ring
aromatic
content of at least 10% greater than the monocyclic ring aromatic content of
the crude
feed; (k) has monocyclic ring aromatics that comprise xylenes, ethylbenzene or
compounds of ethylbenzene; (1) has, per gram of crude product, at most 0.1
grams of
benzene, from 0.05-0.15 grams of toluene, from 0.3-0.9 grams of meta-xylene,
from 0.5-
0.15 grams of ortho-xylene, and from 0.2-0.6 grams of para-xylene; (m) has at
least
0.0001 grams or from 0.01-0.5 grams of diesel; (n) comprises diesel, and the
diesel has at
least 0.3 grams of aromatics per gram of diesel; (o) has at least 0.001 grams,
from above 0
to 0.7 grams, or from 0.001-0.5 grams of kerosene; (p) comprises kerosene, and
the
kerosene has at least 0.2 grams or at least 0.5 grams of aromatics per gram of
kerosene,
and/or a freezing point at a temperature of at most -30 C, at most -40 C, or
at most -50
C; (q) has at least 0.001 grams or at least 0.5 grams of naphtha; (r)
comprises naphtha,
and the naphtha has at most 0.01 grams, at most 0.05 grams, or at most 0.002
grams of
benzene per gram of naphtha, an octane number of at least 70, at least 80, or
at least 90,
and/or iso-paraffins and normal paraffins with a weight ratio of iso-paraffins
to normal
paraffins in the naphtha of at most 1.4; and/or (s) has a volume that is at
least 10% greater
than the volume of the crude feed.
23
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a method that
comprises
contacting a crude feed with a catalyst to form a total product that comprises
a crude
product, further comprising: (a) combining the crude product with a crude that
is the same
or different from the crude feed to form a blend suitable for transporting;
(b) combining
the crude product with a crude that is the same or different from the crude
feed to form a
blend suitable for treatment facilities; (c) fractionating the crude product;
(d) fractionating
the crude product into one or more distillate fractions, and producing
transportation fuel
from at least one of the distillate fractions; and/or (e) when the catalyst is
a transition
metal sulfide catalyst, treating the transition metal sulfide catalyst to
recover metals from
the transition metal sulfide catalyst.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a crude
product that has,
per gram of crude product: (a) at least 0.001 grams of VGO, and the VGO has at
least 0.3
grams of aromatics per gram of VGO; (b) at least 0.001 grams of diesel, and
the diesel has
at least 0.3 grams of aromatics per gram of diesel; (c) at least 0.001 grams
of naphtha, and r.
the naphtha: having at most 0.5 grams of benzene per gram of naphtha, an
octane number
of at least 70, and/or iso-paraffins and n-paraffins with a weight ratio of
the iso-paraffins
to the n-paraffins of at most 1.4; (d) a total of at least 0.001 grams of a
mixture of
components that have a boiling range distribution of at most 204 C (400 F),
and the
mixture having at most 0.15 grams of olefins per gram of mixture; (e) a weight
ratio of
atomic hydrogen to atomic carbon in the composition of at most 1.75, or at
most 1.8; (f) at
least 0.001 grams of kerosene, and the kerosene has: at least 0.5 grams of
aromatics per
gram of kerosene and/or has a freezing point at a temperature of at most -30
C; (g) from
0.09-0.13 grams of atomic hydrogen per gram of composition; (h) non-
condensable
hydrocarbon gases and naphtha, which, when combined, have at most 0.15 grams
of
olefins per gram of the combined non-condensable hydrocarbon gases and
naphtha; (i)
non-condensable hydrocarbon gases and naphtha, which, when combined, comprise
iso-
paraffins and n-paraffins with a weight ratio of the iso-paraffins to the n-
paraffins in the
combined naphtha and non-condensable hydrocarbon gases of at most 1.4; (j) the
hydrocarbons with a carbon number of up to 3 comprising: olefins and paraffins
with
carbon numbers of 2 (C2) and 3 (C3), and a weight ratio of the combined C2 and
C3 olefins
to the combined C2 and C3 paraffins is at most 0.3; olefins and paraffins with
a carbon
number of 2 (C2), wherein a weight ratio of the C2 olefins to the C2 paraffins
is at most
24
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
0.2; and/or olefins and paraffins with a carbon number of 3 (C3), wherein a
weight ratio of
the C3 olefins to the C3 paraffins is at most 0.3; (k) has butadiene content
of at least 0.005
grams; (1) has an API graving in a range from 15 to 30 at 15.5 C; (m) has at
most 0.00001
grams of total NiN/Fe per gram of composition; (n) a paraffins content of the
hydrocarbons having a boiling range distribution of at most 204 C in a range
from 0.7-
0.98 grams; (o) hydrocarbons with a boiling range distribution of at most 204
C that
have, per gram of olefins hydrocarbons having a boiling range distribution of
at most 204
C, from 0.001-0.5 grams of olefins (p) hydrocarbons with a boiling range
distribution of
at most 204 C that comprise olefins, and the olefins have at least 0.001
grams of terminal
olefins per gram of olefins; (q) hydrocarbons with a boiling range
distribution of at most
204 C that comprise olefins, and the olefins have a molar ratio of terminal
olefins to
internal olefins of at least 0.4; and/or (r) from 0.001-0.5 grams of olefins
per gram of
hydrocarbons in a boiling range distribution between 20 C and 204 C.
In some embodiments, the invention also provides, in combination with one or
more of the methods or compositions according to the invention, a crude
product that has
at least one of the catalysts comprising one or more alkali metals, in which:
(a) at least one
of the alkali metals is potassium, rubidium; or cesium, or mixtures thereof;
and/or (b) at
least one of the catalysts further comprises a transition metal, a transition
metal sulfide
and/or bartonite.
In further embodiments, features from specific embodiments of the invention
may
be combined with features from other embodiments of the invention. For
example,
features from one embodiment may be combined with features from any of the
other
embodiments.
In further embodiments, crude products are obtainable by any of the methods
and
systems described herein.
In further embodiments, additional features may be added to the specific
embodiments described herein.
BRIEF DESCRIPTION OF THE DRAWINGS
Advantages of the present invention will become apparent to those skilled in
the art
with the benefit of the following detailed description and upon reference to
the
accompanying drawings in which:
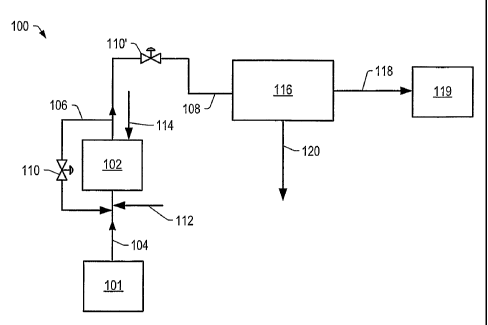
FIG. 1 is a schematic of an embodiment of a contacting system for contacting
the
crude feed with a hydrogen source in the presence of one or more catalysts to
produce the
total product.
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
FIG. 2 is a schematic of another embodiment of a contacting system tor
contacting
the crude feed with a hydrogen source in the presence of one or more catalysts
to produce
the total product.
FIG. 3 is a schematic of an embodiment of a separation zone in combination
with a
contacting system.
FIG. 4 is a schematic of an embodiment of a blending zone in combination with
a
contacting system.
FIG. 5 is a schematic of an embodiment of a separation zone, a contacting
system,
and a blending zone.
FIG. 6 is a schematic of an embodiment of multiple contacting systems.
FIG. 7 is a schematic of an embodiment of an ionic conductivity measurement
system.
FIG. 8 is a tabulation of properties of the crude feed and properties of crude
products obtained from embodiments of contacting the crude feed with the
transition metal
sulfide catalyst.
FIG. 9 is a tabulation of compositions of the crude feed and compositions of
non-
condensable hydrocarbons obtained from embodiments of contacting the crude
feed with
the transition metal sulfide catalyst.
FIG. 10 is a tabulation of properties and compositions of crude products
obtained
from embodiments of contacting the crude feed with the transition metal
sulfide catalyst.
FIG. 11 is a graphical representation of log 10 plots of ion currents of
emitted
gases of an inorganic salt catalyst versus temperature, as determined by TAP.
FIG. 12 is a graphic representation of log plots of the resistance of
inorganic salt
catalysts and an inorganic salt relative to the resistance of potassium
carbonate versus
temperature.
FIG. 13 is a graphic representation of log plots of the resistance of a
Na2CO3/K2CO3/Rb2CO3 catalyst relative to resistance of the potassium carbonate
versus
temperature.
FIG. 14 is a graphical representation of weight percent of coke, liquid
hydrocarbons, and gas versus various hydrogen sources produced from
embodiments of
contacting the crude feed with the inorganic salt catalyst.
FIG. 15 is a graphical representation of weight percentage versus carbon
number of
crude products produced from embodiments of contacting the crude feed with the
inorganic salt catalyst.
26
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
FIG. 16 is a tabulation of components produced from embodiments ot contacting
the crude feed with inorganic salt catalysts, a metal salt, or silicon
carbide.
While the invention is susceptible to various modifications and alternative
forms,
specific embodiments thereof are shown by way of example in the drawings and
will
herein be described in detail. The drawings may not be to scale. It should be
understood
that the drawings and detailed description thereto are not intended to limit
the invention to
the particular form disclosed, but on the contrary, the intention is to cover
all
modifications, equivalents, and alternatives falling within the spirit and
scope of the
present invention.
DETAILED DESCRIPTION OF THE INVENTIONS
Certain embodiments of the inventions are described herein in more detail.
Terms
used herein are defined as follows.
"Alkali metal(s)" refer to one or more metals from Column 1 of the Periodic
Table,
one or more compounds of one or more metals from Column 1 of the Periodic
Table, or
mixtures thereof.
"Alkaline-earth metal(s)" refer to one or more metals from Column 2 of the
Periodic Table, one or more compounds of one or more metals from Column 2 of
the
Periodic Table, or mixtures thereof.
"AMU" refers to atomic mass unit.
"ASTM" refers to American Standard Testing and Materials.
"C5 asphaltenes" refer to asphaltenes that are insoluble in pentane. C5
asphaltenes
content is as determined by ASTM Method D2007.
Atomic hydrogen percentage and atomic carbon percentage of crude feed, crude
product, naphtha, kerosene, diesel, and VG0 are as determined by ASTM Method
D5291.
"API gravity" refers to API gravity at 15.5 C. API gravity is as determined
by
ASTM Method D6822.
"Bitumen" refers to one type of crude produced and/or retorted from a
hydrocarbon formation.
Boiling range distributions for the crude feed and/or total product are as
determined by ASTM Methods D5307, unless otherwise mentioned. Content of
hydrocarbon components, for example, paraffins, iso-paraffins, olefins,
naphthenes and
aromatics in naphtha are as determined by ASTM Method D6730. Content of
aromatics in
diesel and VGO is as determined by IP Method 368/90. Content of aromatics in
kerosene
is as determined by ASTM Method D5186.
27
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
"Bronsted-Lowry acid" refers to a molecular entity with the ability to donate
a
proton to another molecular entity.
"Bronsted-Lowry base" refers to a molecular entity that is capable of
accepting
protons from another molecular entity. Examples of Bronsted-Lowry bases
include
hydroxide (OH), water (H20), carboxylate (RCO2¨), halide (Br, Cr, F, r),
bisulfate
(HSO4¨), and sulfate (S042).
"Carbon number" refers to the total number of carbon atoms in a molecule.
"Coke" refers to solids containing carbonaceous solids that are not vaporized
under
process conditions. The content of coke is as determined by mass balance. The
weight of
coke is the total weight of solid minus the total weight of input catalysts.
"Content" refers to the weight of a component in a substrate (for example, a
crude
feed, a total product, or a crude product) expressed as weight fraction or
weight percentage
based on the total weight of the substrate. "Wtppm" refers to parts per
million by weight.
"Diesel" refers to hydrocarbons with a boiling range distribution between 260
C
and 343 C (500-650 F) at 0.101 MPa. Diesel content is as determined by ASTM
Method D2887.
"Distillate" refers to hydrocarbons with a boiling range distribution between
204
C and 343 C (400-650 F) at 0.101 MPa. Distillate content is as determined by
ASTM
Method D2887. Distillate may include kerosene and diesel.
"DSC" refers to differential scanning calorimetry.
"Freeze point" and "freezing point" refer to the temperature at which
formation of
crystalline particles occurs in a liquid. A freezing point is as determined by
ASTM
D2386.
"GC/MS" refers to gas chromatography in combination with mass spectrometry.
"Hard base" refers to anions as described by Pearson in Journal of American
Chemical Society, 1963, 85, p. 3533.
"H/C" refers to a weight ratio of atomic hydrogen to atomic carbon. H/C is as
determined from the values measured for weight percentage of hydrogen and
weight
percentage of carbon by ASTM Method D5291.
"Heteroatoms" refer to oxygen, nitrogen, and/or sulfur contained in the
molecular
structure of a hydrocarbon. Heteroatoms content is as determined by ASTM
Methods
E385 for oxygen, D5762 for nitrogen, and D4294 for sulfur.
"Hydrogen source" refers to hydrogen, and/or a compound and/or compounds
when in the presence of a crude feed and the catalyst react to provide
hydrogen to one or
28
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
more compounds in the crude feed. A hydrogen source may include, but is not
limited to,
hydrocarbons (for example, CI to C6 hydrocarbons such as methane, ethane,
propane,
butane, pentane, naphtha), water, or mixtures thereof. A mass balance is
conducted to
assess the net amount of hydrogen provided to one or more compounds in the
crude feed.
"Inorganic salt" refers to a compound that is composed of a metal cation and
an
anion.
"IP" refers to the Institute of Petroleum, now the Energy Institute of London,
United Kingdom.
"Iso-paraffins" refer to branched-chain saturated hydrocarbons.
"Kerosene" refers to hydrocarbons with a boiling range distribution between
204
C and 260 C (400-500 F) at 0.101 MPa. Kerosene content is as determined by
ASTM
Method D2887.
"Lewis acid" refers to a compound or a material with the ability to accept one
or
more electrons from another compound.
"Lewis base" refers to a compound and/or material with the ability to donate
one
or more electrons to another compound.
"Light Hydrocarbons" refer to hydrocarbons having carbon numbers in a range
from 1 to 6.
"Liquid mixture" refers to a composition that includes one or more compounds
that
are liquid at standard temperature and pressure (25 C, 0.101 MPa, hereinafter
referred to
as "STP"), or a composition that includes a combination of one or more
compounds that
are liquid at STP with one or more compounds that are solid at STP.
"Micro-Carbon Residue" ("MCR") refers to a quantity of carbon residue
remaining
after evaporation and pyrolysis of a substance. MCR content is as determined
by ASTM
Method D4530.
"Naphtha" refers to hydrocarbon components with a boiling range distribution
between 38 C and 204 C (100-400 F) at 0.101 MPa. Naphtha content is as
determined
by ASTM Method D2887.
"NiN/Fe" refers to nickel, vanadium, iron, or combinations thereof
"NiN/Fe content" refers to NiN/Fe content in a substrate. NiN/Fe content is as
determined by ASTM Method D5863.
"Nm3/m3" refers to normal cubic meters of gas per cubic meter of crude feed.
"Nonacidic" refers to Lewis base and/or Bronsted-Lowry base properties.
29
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
"Non-condensable gas" refers to components and/or a mixture of components that
are gases at standard temperature and pressure (25 C, 0.101 MPa, hereinafter
referred to
as "STP").
"n-Paraffins" refer to normal (straight chain) saturated hydrocarbons.
"Octane number" refers to a calculated numerical representation of the
antiknock
properties of a motor fuel compared to a standard reference fuel. A calculated
octane
number of naphtha is as determined by ASTM Method D6730.
"Olefins" refer to compounds with non-aromatic carbon-carbon double bonds.
Types of olefins include, but are not limited to, cis, trans, terminal,
internal, branched, and
linear.
"Periodic Table" refers to the Periodic Table as specified by the
International
Union of Pure and Applied Chemistry (IUPAC), November 2003.
"Polyaromatic compounds" refer to compounds that include two or more aromatic
rings. Examples of polyaromatic compounds include, but are not limited to,
indene,
naphthalene, anthracene, phenanthrene, benzothiophene, and dibenzothiophene.
"Residue" refers to components that have a boiling range distribution above
538
C (1000 F) at 0.101 MPa, as determined by ASTM Method D5307.
"Semiliquid" refers to a phase of a substance that has properties of a liquid
phase
and a solid phase ofthe substance. Examples of semiliquid inorganic salt
catalysts include
a slurry and/or a phase that has a consistency of, for example, taffy, dough,
or toothpaste.
"SCFB" refers to standard cubic feet of gas per barrel of crude feed.
"Superbase" refers to a material that can deprotonate hydrocarbons such as
paraffins and olefins under reaction conditions.
"TAN" refers to a total acid number expressed as milligrams ("mg") of KOH per
gram ("g") of sample. TAN is as determined by ASTM Method D664.
"TAP" refers to temporal-analysis-of-products.
"TMS" refers to transition metal sulfide.
"VGO" refers to components with a boiling range distribution between 343 C
and
538 C (650-1000 F) at 0.101 MPa. VGO content is as determined by ASTM Method
D2887.
In the context of this application, it is to be understood that if the value
obtained
for a property of the composition tested is outside of the limits of the test
method, the test
method may be recalibrated to test for such property. It should be understood
that other
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
standardized testing methods that are considered equivalent to the referenced
testing
methods may be used.
Crudes may be produced and/or retorted from hydrocarbon containing formations
and then stabilized. Crudes are generally solid, semi-solid, and/or liquid.
Crudes may
include crude oil. Stabilization may include, but is not limited to, removal
of non-
condensable gases, water, salts, or combinations thereof, from the crude to
form a
stabilized crude. Such stabilization may often occur at, or proximate to, the
production
and/or retorting site.
Stabilized crudes typically have not been distilled and/or fractionally
distilled in a
treatment facility to produce multiple components with specific boiling range
distributions
(for example, naphtha, distillates, VG0, and/or lubricating oils).
Distillation includes, but
is not limited to, atmospheric distillation methods and/or vacuum distillation
methods.
Undistilled and/or unfractionated stabilized crudes may include components
that have a
carbon number above 4 in quantities of at least 0.5 grams of components per
gram of
crude. Examples of stabilized crudes include whole crudes, topped crudes,
desalted
crudes, desalted topped crudes, or combinations thereof. "Topped" refers to a
crude that
has been treated such that at least some of the components that have a boiling
point below
35 C at 0.101 MPa are removed. Typically, topped crudes have a content of at
most 0.1
grams, at most 0.05 grams, or at most 0.02 grams of such components per gram
of the
topped crude.
Some stabilized crudes have properties that allow the stabilized crudes to be
transported to conventional treatment facilities by transportation carriers
(for example,
pipelines, trucks, or ships). Other crudes have one or more unsuitable
properties that
render them disadvantaged. Disadvantaged crudes may be unacceptable to a
transportation carrier, and/or a treatment facility, thus imparting a low
economic value to
the disadvantaged crude. The economic value may be such that a reservoir that
includes
the disadvantaged crude that is deemed too costly to produce, transport,
and/or treat.
Properties of disadvantaged crudes may include, but are not limited to: a) TAN
of
at least 0.5; b) viscosity of at least 0.2 Pa=s; c) API gravity of at most 19;
d) a total NiN/Fe
content of at least 0.00005 grams or at least 0.0001 grams of Ni/V/Fe per gram
of crude; e)
a total heteroatoms content of at least 0.005 grams of heteroatoms per gram of
crude; f) a
residue content of at least 0.01 grams of residue per gram of crude; g) an
asphaltenes
content of at least 0.04 grams of asphaltenes per gram of crude; h) a MCR
content of at
least 0.02 grams of MCR per gram of crude; or i) combinations thereof In some
31
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
embodiments, disadvantaged crude may include, per gram of disadvantaged crude,
at least
0.2 grams of residue, at least 0.3 grams of residue, at least 0.5 grams of
residue, or at least
0.9 grams of residue. In certain embodiments, disadvantaged crude has 0.2-0.99
grams,
0.3-0.9 grams, or 0.4-0.7 grams of residue per gram of disadvantaged crude. In
certain
embodiments, disadvantaged crudes, per gram of disadvantaged crude, may have a
sulfur
content of at least 0.001 grams, at least 0.005 grams, at least 0.01 grams, or
at least 0.02
grams.
Disadvantaged crudes may include a mixture of hydrocarbons having a range of
boiling points. Disadvantaged crudes may include, per gram of disadvantaged
crude: at
least 0.001 grams, at least 0.005 grams, or at least 0.01 grams of
hydrocarbons with a
boiling range distribution between 200 C and 300 C at 0.101 MPa; at least
0.001 grams,
at least 0.005 grams, or at least 0.01 grams of hydrocarbons with a boiling
range
distribution between 300 C and 400 C at 0.101 MPa; and at least 0.001 grams,
at least
0.005 grams, or at least 0.01 grams of hydrocarbons with a boiling range
distribution
between 400 C and 700 C at 0.101 MPa, or combinations thereof.
= In some embodiments, disadvantaged crudes may also include, per gram of
disadvantaged crude, at least 0.001 grams, at least 0.005 grams, or at least
0.01 grams of
hydrocarbons with a boiling range distribution of at most 200 C at 0.101 MPa
in addition
to higher boiling components. Typically, the disadvantaged crude has, per gram
of
disadvantaged crude, a content of such hydrocarbons of at most 0.2 grams, or
at most 0.1
grams.
In certain embodiments, disadvantaged crudes may include, per gram of
disadvantaged crude, up to 0.9 grams, or up to 0.99 grams of hydrocarbons with
a boiling
range distribution of at least 300 C. In certain embodiments, disadvantaged
crudes may
also include, per gram of disadvantaged crude, at least 0.001 grams of
hydrocarbons with a
boiling range distribution of at least 650 C. In certain embodiments,
disadvantaged
crudes may include, per gram of disadvantaged crude, up to 0.9 grams, or up to
0.99 grams
of hydrocarbons with a boiling range distribution between 300 C and 1000 C.
Examples of disadvantaged crudes that can be treated using the processes
described herein include, but are not limited to, crudes from the following
countries and
regions of those countries: Canadian Alberta, Venezuelan Orinoco, U.S.
southern
Californian and north slope Alaska, Mexico Bay of Campeche, Argentinean San
Jorge
basin, Brazilian Santos and Campos basins, China Bohai Gulf, China Karamay,
Iraq
32
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
Zagros, Kazakhstan Caspian, Nigeria Offshore, United Kingdom North Sea,
Madagascar
northwest, Oman, and Netherlands Schoonebek.
Treatment of disadvantaged crudes may enhance the properties of the
disadvantaged crudes such that the crudes are acceptable for transportation
and/or
treatment. A crude and/or disadvantaged crude that is to be treated may be
referred to as
"crude feed". The crude feed may be topped as described herein. The crude
product
resulting from treatment of the crude feed, using methods described herein, is
suitable for
transporting and/or refining. Properties of the crude product are closer to
the
corresponding properties of West Texas Intermediate crude than the crude feed,
or closer
to the corresponding properties of Brent crude than the crude feed, and
thereby have
enhanced economic value relative to the economic value of the crude feed. Such
crude
product may be refined with less or no pre-treatment, thereby enhancing
refining
efficiencies. Pre-treatment may include desulfurization, demetallization,
and/or
atmospheric distillation to remove impurities from the crude product.
Methods of contacting a crude feed in accordance with inventions are described
herein. Additionally, embodiments to produce products with various
concentrations of
naphtha, kerosene, diesel, and/or VGO, which are not generally produced in
conventional
types of processes, are described.
The crude feed may be contacted with a hydrogen source in the presence of one
or
more of the catalysts in a contacting zone and/or in combinations of two or
more
contacting zones.
In some embodiments, the hydrogen source is generated in situ. In situ
generation
of the hydrogen source may include the reaction of at least a portion of the
crude feed with
the inorganic salt catalyst at temperatures in a range from 200-500 C or 300-
400 C to
form hydrogen and/or light hydrocarbons. In situ generation of hydrogen may
include the
reaction of at least a portion of the inorganic salt catalyst that includes,
for example, alkali
metal formate.
The total product generally includes gas, vapor, liquids, or mixtures thereof
produced during the contacting. The total product includes the crude product
that is a
liquid mixture at STP and, in some embodiments, hydrocarbons that are not
condensable
at STP. In some embodiments, the total product and/or the crude product may
include
solids (such as inorganic solids and/or coke). In certain embodiments, the
solids may be
entrained in the liquid and/or vapor produced during contacting.
33
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
A contacting zone typically includes a reactor, a portion of a reactor,
multiple
portions of a reactor, or multiple reactors. Examples of reactors that may be
used to
contact a crude feed with a hydrogen source in the presence of catalyst
include a stacked
bed reactor, a fixed bed reactor, a continuously stirred tank reactor (CSTR),
a spray
Contacting conditions typically include temperature, pressure, crude feed
flow,
total product flow, residence time, hydrogen source flow, or combinations
thereof.
Contacting conditions may be controlled to produce a crude product with
specified
properties.
Contacting temperatures may range from 200-800 C, 300-700 C, or 400-600 C.
In embodiments in which the hydrogen source is supplied as a gas (for example,
hydrogen
gas, methane, or ethane), a ratio of the gas to the crude feed will generally
range from 1-
16,100 Nm3/m3, 2-8000 Nm3/m3, 3-4000 Nm3/m3, or 5-300 Nm3/m3. Contacting
typically
from 0.01-3 kilograms, 0.03-2.5 kilograms, or 0.1-1 kilogram of steam, per
kilogram of !..
crude feed. A flow rate of crude feed may be sufficient to maintain the volume
of crude
feed in the contacting zone of at least 10%, at least 50%, or at least 90% of
the total
FIG. 1 is a schematic of an embodiment of contacting system 100 used to
produce
embodiments, the crude feed may be heated to a temperature of at least 100 C
or at least
300 C prior to and/or during introduction of the crude feed to contacting
zone 102.
Typically, the crude feed may be heated to a temperature in a range from 100-
500 C or
200-400 C.
34
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In some embodiments, the catalyst is combined with the crude teed and
transferred
to contacting zone 102. The crude feed/catalyst mixture may be heated to a
temperature of
at least 100 C or at least 300 C prior to introduction into contacting zone
102. Typically,
the crude feed may be heated to a temperature in a range from 200-500 C or
300-400 C.
In some embodiments, the crude feed/catalyst mixture is a slurry. In certain
embodiinents,
TAN of the crude feed may be reduced prior to introduction of the crude feed
into the
contacting zone. For example, when the crude feed/catalyst mixture is heated
at a
temperature in a range from 100-400 C or 200-300 C, alkali salts of acidic
components
in the crude feed may be formed. The formation of these alkali salts may
remove some
acidic components from the crude feed to reduce the TAN of the crude feed.
In some embodiments, the crude feed is added continuously to contacting zone
102. Mixing in contacting zone 102 may be sufficient to inhibit separation of
the catalyst
from the crude feed/catalyst mixture. In certain embodiments, at least a
portion of the
catalyst may be removed from contacting zone 102, and in some embodiments,
such
catalyst is regenerated and re-used. In certain embodiments, fresh catalyst
may be added
to contacting zone 102 during the reaction process.
In some embodiments, the crude feed and/or a mixture of crude feed,with the
inorganic salt catalyst is introduced into the contacting zone as an emulsion.
The emulsion
may be prepared by combining an inorganic salt catalyst/water mixture with a
crude
feed/surfactant mixture. In some embodiments, a stabilizer is added to the
emulsion. The
emulsion may remain stable for at least 2 days, at least 4 days, or at least 7
days.
Typically, the emulsion may remain stable for 30 days, 10 days, 5 days, or 3
days.
Surfactants include, but are not limited to, organic polycarboxylic acids
(Tenax 2010;
MeadWestvaco Specialty Product Group; Charleston, South Carolina, U.S.A.), C21
dicarboxylic fatty acid (DIACID 1550; MeadWestvaco Specialty Product Group),
petroleum sulfonates (Hostapur SAS 30; Clarient Corporation, Charlotte, North
Carolina,
U.S.A.), Tergital NP-40 Surfactant (Union Carbide; Danbury, Connecticut,
U.S.A.), or
mixtures thereof. Stabilizers include, but are not limited to, diethyleneamine
(Aldrich
Chemical Co.; Milwaukee, Wisconsin, U.S.A.) and/or monoethanolamine (J. T.
Baker;
Phillipsburg, New Jersey, U.S.A.).
Recycle conduit 106 may couple conduit 108 and conduit 104. In some
embodiments, recycle conduit 106 may directly enter and/or exit contacting
zone 102.
Recycle conduit 106 may include flow control valve 110. Flow control valve 110
may
allow at least a portion of the material from conduit 108 to be recycled to
conduit 104
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
and/or contacting zone 102. In some embodiments, a condensing unit may be
positioned
in conduit 108 to allow at least a portion of the material to be condensed and
recycled to
contacting zone 102. In certain embodiments, recycle conduit 106 may be a gas
recycle
line. Flow control valves 110 and 110' may be used to control flow to and from
contacting zone 102 such that a constant volume of liquid in the contacting
zone is
maintained. In some embodiments, a substantially selected volume range of
liquid can be
maintained in the contacting zone 102. A volume of feed in contacting zone 102
may be
monitored using standard instrumentation. Gas inlet port 112 may be used to
allow
addition of the hydrogen source and/or additional gases to the crude feed as
the crude feed
enters contacting zone 102. In some embodiments, steam inlet port 114 may be
used to
allow addition of steam to contacting zone 102. In certain embodiments, an
aqueous
stream is introduced into contacting zone 102 through steam inlet port 114.
In some embodiments, at least a portion of the total product is produced as
vapor
from contacting zone 102. In certain embodiments, the total product is
produced as vapor
and/or a vapor containing small amounts of liquids and solids from the top of
contacting
zone 102. The vapor is transported to separation zone 116 via conduit 108. The
ratio of a
,hydrogen source to crude feed in contacting zone 102 and/or the pressure in
the contacting
zone may be changed to control the vapor and/or liquid phase produced from the
top of
contacting zone 102. In some embodiments, the vapor produced from the top of
contacting zone 102 includes at least 0.5 grams, at least 0.8 grams, at least
0.9 grams, or at
least 0.97 grams of crude product per gram of crude feed. In certain
embodiments, the
vapor produced from the top of contacting zone 102 includes from 0.8-0.99
grams, or 0.9-
0.98 grams of crude product per gram of crude feed.
Used catalyst and/or solids may remain in contacting zone 102 as by-products
of
the contacting process. The solids and/or used catalyst may include residual
crude feed
and/or coke.
In separation unit 116, the vapor is cooled and separated to form the crude
product
and gases using standard separation techniques. The crude product exits
separation unit
116 and enters crude product receiver 119 via conduit 118. The resulting crude
product
may be suitable for transportation and/or treatment. Crude product receiver
119 may
include one or more pipelines, one or more storage units, one or more
transportation
vessels, or combinations thereof. In some embodiments, the separated gas (for
example,
hydrogen, carbon monoxide, carbon dioxide, hydrogen sulfide, or methane) is
transported
to other processing units (for example, for use in a fuel cell or a sulfur
recovery plant)
36
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
and/or recycled to contacting zone 102 via conduit 120. In certain
embodiments, entrained
_solids and/or liquids in the crude product may be removed using standard
physical
separation methods (for example, filtration, centrifugation, or membrane
separation).
FIG. 2 depicts contacting system 122 for treating crude feed with one or more
catalysts to produce a total product that may be a liquid, or a liquid mixed
with gas or
solids. The crude feed may enter contacting zone 102 via conduit 104. In some
embodiments, the crude feed is received from the crude feed supply. Conduit
104 may
include gas inlet port 112. In some embodiments, gas inlet port 112 may
directly enter
contacting zone 102. In certain embodiments, steam inlet port 114 may be used
to allow
addition of the steam to contacting zone 102. The crude feed may be contacted
with the
catalyst in contacting zone 102 to produce a total product. In some
embodiments, conduit
106 allows at least a portion of the total product to be recycled to
contacting zone 102. A
mixture that includes the total product and/or solids and/or unreacted crude
feed exits
contacting zone 102 and enters separation zone 124 via conduit 108. In some
embodiments, a condensing unit may be positioned (for example, in conduit 106)
to allow
at least a portion of the mixture in the conduit to be condensed and recycled
to contacting
zone 102 for further processing. In certain embodiments, recycle conduit 106
may be a
gas recycle line. In some embodiments, conduit 108 may include a filter for
removing
particles from the total product. =
=
In separation zone 124, at least a portion of the crude product may be
separated
from the total product and/or catalyst. In embodiments in which the total
product includes
solids, the solids may be separated from the total product using standard
solid separation
techniques (for example, centrifugation, filtration, decantation, membrane
separation).
= = Solids include, for example, a combination of catalyst, used
catalyst, and/or coke. In some
embodiments, a portion of the gases is separated from the total product. In
some
embodiments, at least a portion of the total product and/or solids may be
recycled to
conduit 104 and/or, in some embodiments, to contacting zone 102 via conduit
126. The
recycled portion may, for example, be combined with the crude feed and enter
contacting
zone 102 for further processing. The crude product may exit separation zone
124 via
conduit 128. In certain embodiments, the crude product may be transported to
the crude
product receiver.
In some embodiments, the total product and/or crude product may include at
least a
portion of the catalyst. Gases entrained in the total product and/or crude
product may be
separated using standard gas/liquid separation techniques, for example,
sparging,
37
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
membrane separation, and pressure reduction. In some embodiments, the
separated gas is
transported to other processing units (for example, for use in a fuel cell, a
sulfur recovery
plant, other processing units, or combinations thereof) and/or recycled to the
contacting
zone.
In some embodiments, separation of at least a portion of a crude feed is
performed
before the crude feed enters the contacting zone. FIG. 3 is a schematic of an
embodiment
of a separation zone in combination with a contacting system. Contacting
system 130 may
be contacting system 100 and/or contacting system 122 (shown in FIGS. 1 and
2). The
crude feed enters separation zone 132 via conduit 104. In separation zone 132,
at least a
portion of the crude feed is separated using standard separation techniques to
produce a
separated crude feed and hydrocarbons. The separated crude feed, in some
embodiments,
includes a mixture of components with a boiling range distribution of at least
100 C, at
least 120 C or, in some embodiments, a boiling range distribution of at least
200 C.
Typically, the separated crude feed includes a mixture of components with a
boiling range
distribution between 100-1000 C, 120-900 C, or 200-800 C. The hydrocarbons
r. separated from the crude feed exit separation zone 132 via conduit 134
to be:transported to
other processing units, treatment facilities, storage facilities, or
combinations thereof.
At least a portion of the separated crude feed exits separation zone 132 and
enters
contacting system 130 via conduit 136 to be further processed to form the
crude product,
which exits contacting system 130 via conduit 138.
In some embodiments, the crude product produced from a crude feed by any
method described herein is blended with a crude that is the same as or
different from the
crude feed. For example, the crude product may be combined with a crude having
a
different viscosity thereby resulting in a blended product having a viscosity
that is between
the viscosity of the crude product and the viscosity of the crude. The
resulting blended
product may be suitable for transportation and/or treatment.
FIG. 4 is a schematic of an embodiment of a combination of blending zone 140
and contacting system 130. In certain embodiments, at least a portion of the
crude product
exits contacting system 130 via conduit 138 and enters blending zone 140. In
blending
zone 140, at least a portion of the crude product is combined with one or more
process
streams (for example, a hydrocarbon stream produced from separation of one or
more
crude feeds, or naphtha), a crude, a crude feed, or mixtures thereof, to
produce a blended
product. The process streams, crude feed, crude, or mixtures thereof, are
introduced
directly into blending zone 140 or upstream of the blending zone via conduit
142. A
38
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
mixing system may be located in or near blending zone 140. The blended product
may
meet specific product specifications. Specific product specifications include,
but are not
limited to, a range of or a limit of API gravity, TAN, viscosity, or
combinations thereof
The blended product exits blending zone 140 via conduit 144 to be transported
and/or
processed.
In some embodiments, methanol is generated during the contacting process using
the catalyst. For example, hydrogen and carbon monoxide may react to form
methanol.
The recovered methanol may contain dissolved salts, for example, potassium
hydroxide.
The recovered methanol may be combined with additional crude feed to form a
crude
feed/methanol mixture. Combining methanol with the crude feed tends to lower
the
viscosity of the crude feed. Heating the crude feed/methanol mixture to at
most 500 C
may reduce TAN of the crude feed to less than 1.
FIG. 5 is a schematic of an embodiment of a separation zone in combination
with a
contacting system in combination with a blending zone. The crude feed enters
separation
zone 132 through conduit 104. The crude feed is separated as previously
described to
form a separated crude feed. The separated crude feed enters contacting system
130
through conduit 136. The crude product exits contacting system 130 and enters
blending
zone 140 through conduit 138. In blending zone 140, other process stream
and/or crudes
introduced via conduit 142 are combined with the crude product to form a
blended
product. The blended product exits blending zone 140 via conduit 144.
FIG. 6 is a schematic of multiple contacting system 146. Contacting system 100
(shown in FIG. 1) may be positioned before contacting system 148. In an
alternate
embodiment, the positions of the contacting systems can be reversed.
Contacting system
100 includes an inorganic salt catalyst. Contacting system 148 may include one
or more
catalysts. The catalyst in contacting system 148 may be an additional
inorganic salt
catalyst, the transition metal sulfide catalyst, commercial catalysts, or
mixtures thereof
The crude feed enters contacting system 100 via conduit 104 and is contacted
with a
hydrogen source in the presence of the inorganic salt catalyst to produce the
total product.
The total product includes hydrogen and, in some embodiments, a crude product.
The
total product may exit contacting system 100 via conduit 108. The hydrogen
generated
from contact of the inorganic salt catalyst with the crude feed may be used as
a hydrogen
source for contacting system 148. At least a portion of the generated hydrogen
is
transferred to contacting system 148 from contacting system 100 via conduit
150.
39
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In an alternate embodiment, such generated hydrogen may be separated and/or
treated, and then transferred to contacting system 148 via conduit 150. In
certain
embodiments, contacting system 148 may be a part of contacting system 100 such
that the
generated hydrogen flows directly from contacting system 100 to contacting
system 148.
In some embodiments, a vapor stream produced from contacting system 100 is
directly
mixed with the crude feed entering contacting system 148.
A second crude feed enters contacting system 148 via conduit 152. In
contacting
system 148, contact of the crude feed with at least a portion of the generated
hydrogen and
the catalyst produces a product. The product is, in some embodiments, the
total product.
The product exits contacting system 148 via conduit 154.
In certain embodiments, a system that includes contacting systems, contacting
zones, separation zones, and/or blending zones, as shown in FIGS. 1-6, may be
located at
or proximate to a production site that produces disadvantaged crude feed.
After
processing through the catalytic system, the crude feed may be considered
suitable for
transportation and/or for use in a refinery process.
In some embodiments, the crude product and/or the blended product are
transported to a refinery and/or a treatment facility. The crude product
and/or the blended
product may be processed to produce commercial products such as transportation
fuel,
heating fuel, lubricants, or chemicals. Processing may include distilling
and/or
fractionally distilling the crude product and/or blended product to produce
one or more
distillate fractions. In some embodiments, the crude product, the blended
product, and/or
the one or more distillate fractions may be hydrotreated.
The total product includes, in some embodiments, at most 0.05 grams, at most
0.03
grams, or at most 0.01 grams of coke per gram of total product. In certain
embodiments,
the total product is substantially free of coke (that is, coke is not
detectable). In some
embodiments, the crude product may include at most 0.05 grams, at most 0.03
grams, at
most 0.01 grams, at most 0.005 grams, or at most 0.003 grams of coke per gram
of crude
product. In certain embodiments, the crude product has a coke content in a
range from
above 0 to 0.05, 0.00001-0.03 grams, 0.0001-0.01 grams, or 0.001-0.005 grams
per gram
of crude product, or is not detectable.
In certain embodiments, the crude product has an MCR content that is at most
90%, at most 80%, at most 50%, at most 30%, or at most 10% of the MCR content
of the
crude feed. In some embodiments, the crude product has a negligible MCR
content. In
some embodiments, the crude product has, per gram of crude product, at most
0.05 grams,
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
at most 0.03 grams, at most 0.01 grams, or at most 0.001 grams ot MCK.
lypically, the
crude product has from 0 grams to 0.04 grams, 0.000001-0.03 grams, or 0.00001-
0.01
grams of MCR per gram of crude product.
In some embodiments, the total product includes non-condensable gas. The non-
condensable gas typically includes, but is not limited to, carbon dioxide,
ammonia,
hydrogen sulfide, hydrogen, carbon monoxide, methane, other hydrocarbons that
are not
condensable at STP, or a mixture thereof.
In certain embodiments, hydrogen gas, carbon dioxide, carbon monoxide, or
combinations thereof can be formed in situ by contact of steam and light
hydrocarbons
with the inorganic salt catalyst. Typically, under thermodynamic conditions a
molar ratio
of carbon monoxide to carbon dioxide is 0.07. A molar ratio of the generated
carbon
monoxide to the generated carbon dioxide, in some embodiments, is at least
0.3, at least
0.5, or at least 0.7. In some embodiments, a molar ratio of the generated
carbon monoxide
to the generated carbon dioxide is in a range from 0.3-1.0, 0.4-0.9, or 0.5-
0.8. The ability
to generate carbon monoxide preferentially to carbon dioxide in situ may be
beneficial to
other processes located in a proximate area or upstream of the process. For
example, the
In some embodirnents, the total product as produced herein may include a
mixture
of compounds that have a boiling range distribution between -10 C and 538 C.
The
mixture may include hydrocarbons that have carbon numbers in a range from 1 to
4. The
mixture may include from 0.001-0.8 grams, 0.003-0.1 grams, or 0.005-0.01
grams, of C4
hydrocarbons per gram of such mixture. The C4 hydrocarbons may include from
0.001-
0.8 grams, 0.003-0.1 grams, or 0.005-0.01 grams of butadiene per gram of C4
hydrocarbons. In some embodiments, iso-paraffins are produced relative to n-
paraffins at
a weight ratio of at most 1.5, at most 1.4, at most 1.0, at most 0.8, at most
0.3, or at most
0.1. In certain embodiments, iso-paraffins are produce relative to n-paraffins
at a weight
ratio in a range from 0.00001-1.5, 0.0001-1.0, or 0.001-0.1. The paraffins may
include
iso-paraffins and/or n-paraffins.
In some embodiments, the total product and/or crude product may include
olefins
and/or paraffins in ratios or amounts that are not generally found in crudes
produced
and/or retorted from a formation. The olefins include a mixture of olefins
with a terminal
double bond ("alpha olefins") and olefins with internal double bonds. In
certain
embodiments, the olefin content of the crude product is greater than the
olefin content of
41
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
the crude feed by a factor of 2, 10, 50, 100, or at least 200. In some
embodiments, the
olefin content of the crude product is greater than the olefin content of the
crude feed by
factor of at most 1,000, at most 500, at most 300, or at most 250.
In certain embodiments, the hydrocarbons with a boiling range distribution
between 20-400 C have an olefins content in a range from 0.00001-0.1 grams,
0.0001-
0.05 grams, or 0.01-0.04 grams per gram of hydrocarbons having a boiling range
distribution in a range between 20-400 C.
In some embodiments, at least 0.001 grams, at least 0.005 grams, or at least
0.01
grams of alpha olefins per gram of crude product may be produced. In certain
embodiments, the crude product has from 0.0001-0.5 grams, 0.001-0.2 grams, or
0.01-0.1
grams of alpha olefins per gram of crude product. In certain embodiments, the
hydrocarbons with a boiling range distribution between 20-400 C have an alpha
olefins
content in a range from 0.0001-0.08 grams, 0.001-0.05 grams, or 0.01-0.04
grams per
gram of hydrocarbons with a boiling range distribution between 20-400 C.
In some embodiments, the hydrocarbons with a boiling range distribution
between
A weight ratio of alpha olefins to internal double bond olefins of the crudes
and
commercial products is typically at most 0.5. The ability to produce an
increased amount
of alpha olefins to olefins with internal double bonds may facilitate the
conversion of the
crude product to commercial products.
In some embodiments, contact of a crude feed with a hydrogen source in the
presence of an inorganic salt catalyst may produce hydrocarbons with a boiling
range
distribution between 20-204 C that include linear olefins. The linear olefins
have cis and
trans double bonds. A weight ratio of linear olefins with trans double bonds
to linear
olefins with cis double bonds is at most 0.4, at most 1.0, or at most 1.4. In
certain
embodiments, the weight ratio of linear olefins with trans double bonds to
linear olefins
with cis double bonds is in a range from 0.001-1.4, 0.01-1.0, or 0.1-0.4.
In certain embodiments, hydrocarbons having a boiling range distribution in a
range between 20-204 C have a n-paraffins content of at least 0.1 grams, at
least 0.15
grams, at least 0.20 grams, or at least 0.30 grams per gram of hydrocarbons
having a
boiling range distribution in a range between 20-400 C. The n-paraffins
content of such
42
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
hydrocarbons, per gram of hydrocarbons, may be in a range from 0.001-0.9
grams, 0.1-0.8
grams, or 0.2-0.5 grams. In some embodiments, such hydrocarbons have a weight
ratio of
the iso-paraffins to the n-paraffins of at most 1.5, at most 1.4, at most 1.0,
at most 0.8, or
at most 0.3. From the n-paraffins content in such hydrocarbons, the n-
paraffins content of
the crude product may be estimated to be in a range from 0.001-0.9 grams, 0.01-
0.8 grams,
or 0.1-0.5 grams per gram of crude product.
In some embodiments, the crude product has a total NiN/Fe content of at most
90%, at most 50%, at most 10%, at most 5%, or at most 3% of a NiN/Fe content
of the
crude feed. In certain embodiments, the crude product includes, per gram of
crude
product, at most 0.0001 grams, at most 1 x 10-5 grams, or at most 1 x 10-6
grams of
NiN/Fe. In certain embodiments, the crude product has, per gram of crude
product, a
total NiN/Fe content in a range from 1 x 10-7 grams to 5 x 10-5 grams, 3 x 1 e
grams to 2
x 10-5 grams, or 1 x 10-6 grams to 1 x 10-5 grams.
In some embodiments, the crude product has a TAN of at most 90%, at most 50%,
or at most 10% of the TAN of the crude feed. The crude product may, in certain
embodiments, have a TAN of at most 1, at most 0.5, at most 0.1, or at most
0.05. In some
embodiments, TAN of the crude product may be in a range from 0.001 to 0.5,0.01
to 0.2,
or 0.05 to 0.1.
In certain embodiments, the API gravity of the crude product is at least 10%
higher, at least 50% higher, or at least 90% higher than the API gravity of
the crude feed.
In certain embodiments, API gravity of the crude product is between 13-50, 15-
30, or 16-
20.
In some embodiments, the crude product has a total heteroatoms content of at
most
70%, at most 50%, or at most 30% of the total heteroatoms content of the crude
feed. In
certain embodiments, the crude product has a total heteroatoms content of at
least 10%, at
least 40%, or at least 60% of the total heteroatoms content of the crude feed.
The crude product may have a sulfur content of at most 90%, at most 70%, or at
most 60% of a sulfur content of the crude feed. The sulfur content of the
crude product,
per gram of crude product, may be at most 0.02 grams, at most 0.008 grams, at
most 0.005
grams, at most 0.004 grams, at most 0.003 grams, or at most 0.001 grams. In
certain
embodiments, the crude product has, per gram of crude product, a sulfur
content in a range
from 0.0001-0.02 grams or 0.005-0.01 grams.
In certain embodiments, the crude product may have a nitrogen content of at
most
90% or at most 80% of a nitrogen content of the crude feed. The nitrogen
content of the
43
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
crude product, per gram of crude product, may be at most 0.004 grams, at most
0.003
grams, or at most 0.001 grams. In some embodiments, the crude product has, per
gram of
crude product, a nitrogen content in a range from 0.0001-0.005 grams, or 0.001-
0.003
grams.
In some embodiments, the crude product has, per gram of crude product, from
0.05-0.2 grams, or 0.09-0.15 grams of hydrogen. The H/C of the crude product
may be at
most 1.8, at most 1.7, at most 1.6, at most 1.5, or at most 1.4. In some
embodiments, the
H/C of the crude product is 80-120%, or 90-110% of the H/C of the crude feed.
In other
embodiments, the H/C of the crude product is 100-120% of the H/C of the crude
feed. A
crude product H/C within 20% of the crude feed H/C indicates that uptake
and/or
consumption of hydrogen in the process is minimal.
The crude product includes components with a range of boiling points. In some
embodiments, the crude product includes: at least 0.001 grams, or from 0.001
to 0.5 grams
of hydrocarbons with a boiling range distribution of at most 200 C or at most
204 C at
0.101 MPa; at least 0.001 grams, or from 0.001 to 0.5 grams of hydrocarbons
with a
=boiling range distribution between 200 C and 300 C at 0.101 MPa; at least
0.001 grams,
= or from 0.001 to 0.5 grams of hydrocarbons with a boiling range
distribution between 300
C and 400 C at 0.101 MPa; and at least 0.001 grams, or from 0.001 to 0.5
grams of =
hydrocarbons with a boiling range distribution between 400 C and 538 C at
0.101 MPa.
In some embodiments, the crude product has, per gram of crude product, a
naphtha
content from 0.00001-0.2 grams, 0.0001-0.1 grams, or 0.001-0.05 grams. In
certain
embodiments, the crude product has from 0.001-0.2 grams or 0.01-0.05 grams of
naphtha.
In some embodiments, the naphtha has at most 0.15 grams, at most 0.1 grams, or
at most
0.05 grams of olefins per gram of naphtha. The crude product has, in certain
embodiments, from 0.00001-0.15 grams, 0.0001-0.1 grams, or 0.001-0.05 grams of
olefins
per gram of crude product. In some embodiments, the naphtha has, per gram of
naphtha, a
benzene content at most 0.01 grams, at most 0.005 grams, or at most 0.002
grams. In
certain embodiments, the naphtha has a benzene content that is non-detectable,
or in a
range from 1 x 10-7 grams to 1 x 10-2 grams, 1 x 10 grams to 1 x 10-5 grams, 5
x 10-6
grams to 1 x 104 grams. Compositions that contain benzene may be considered
hazardous
to handle, thus a crude product that has a relatively low benzene content may
not require
special handling.
In certain embodiments, naphtha may include aromatic compounds. Aromatic
compounds may include monocyclic ring compounds and/or polycyclic ring
compounds.
44
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
The monocyclic ring compounds may include, but are not limited to, benzene,
toluene,
ortho-xylene, meta-xylene, para-xylene, ethyl benzene, 1-ethy1-3-methyl
benzene; 1-ethyl-
2-methyl benzene; 1,2,3-trimethyl benzene; 1,3,5-trimethyl benzene; 1-methyl-3-
propyl
benzene; 1-methy1-2-propyl benzene; 2-ethyl-1,4-dimethyl benzene; 2-ethyl-2,4-
dimethyl
benzene; 1,2,3,4-tetra-methyl benzene; ethyl, pentylmethyl benzene; 1,3
diethy1-2,4,5,6-
tetramethyl benzene; tri-isopropyl-ortho-xylene; substituted congeners of
benzene,
toluene, ortho-xylene, meta-xylene, para-xylene, or mixtures thereof.
Monocyclic
aromatics are used in a variety of commercial products and/or sold as
individual
components. The crude product produced as described herein typically has an
enhanced
content of monocyclic aromatics.
In certain embodiments, the crude product has, per gram of crude product, a
toluene content from 0.001-0.2 grams, 0.05-0.15 grams, or 0.01-0.1 grams. The
crude
product has, per gram of crude product, a meta-xylene content from 0.001-0.1
grams,
0.005-0.09 grams, or 0.05-0.08 grams. The crude product has, per gram of crude
product,
an ortho-xylene content from 0.001-0.2 grams, 0.005-0.1 grams, or 0.01-0.05
grams. The
crude product has, per gram of crude product, a para-xylene content from 0.001-
009
grams, 0.005-0.08 grams, or 0.001-0.06 grams. .
An increase in the aromatics content of naphtha tends to increase the octane
number of the naphtha. Crudes may be valued based on an estimation of a
gasoline
potential of the crudes. Gasoline potential may include, but is not limited
to, a calculated
octane number for the naphtha portion of the crudes. Crudes typicallY have
calculated
octane numbers in a range of 35-60. The octane number of gasoline tends to
reduce the
requirement for additives that increase the octane number of the gasoline. In
certain
embodiments, the crude product includes naphtha that has an octane number of
at least 60,
at least 70, at least 80, or at least 90. Typically, the octane number of the
naphtha is in a
range from 60-99, 70-98, or 80-95.
In some embodiments, the crude product has a higher total aromatics content in
hydrocarbons having a boiling range distribution between 204 C and 500 C
(total
"naphtha and kerosene") relative to the total aromatics content in the total
naphtha and
kerosene of the crude feed by at least 5%, at least 10%, at least 50%, or at
least 99%.
Typically, the total aromatics content in the total naphtha and kerosene of
crude feed is
8%, 20%, 75%, or 100% greater than the total aromatics content in the total
naphtha and
kerosene of the crude feed.
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
In some embodiments, the kerosene and naphtha may have a total polyaromatic
compounds content in a range from 0.00001-0.5 grams, 0.0001-0.2 grams, or
0.001-0.1
grams per gram of total kerosene and naphtha.
The crude product has, per gram of crude product, a distillate content in a
range
from 0.0001-0.9 grams, from 0.001-0.5 grams, from 0.005-0.3 grams, or from
0.01-0.2
grams. In some embodiments, a weight ratio of kerosene to diesel in the
distillate, is in a
range from 1:4 to 4:1, 1:3 to 3:1, or 2:5 to 5:2.
In some embodiments, the crude product has, per gram of crude product, at
least
0.001 grams, from above 0 to 0.7 grams, 0.001-0.5 grams, or 0.01-0.1 grams of
kerosene.
In certain embodiments, the crude product has from 0.001-0.5 grams or 0.01-0.3
grams of
kerosene. In some embodiments, the kerosene has, per gram of kerosene, an
aromatics
content of at least 0.2 grams, at least 0.3 grams, or at least 0.4 grams. In
certain
embodiments, the kerosene has, per gram of kerosene, an aromatics content in a
range
from 0.1-0.5 grams, or from 0.2-0.4 grams.
In certain embodiments, a freezing point of the kerosene may be below -30 C,
below -40 C, or below -50 PC. An increase in the content of aromatics of the
kerosene P
portion of the crude product tends to increase the density and reduce the
freezing point of ,1/4
the kerosene portion of the crude product. kcrude product with a kerosene
portion having
a high density and low freezing point may be refined to produce aviation
turbine fuel with
the desirable properties of high density and low freezing point.
In certain embodiments, the crude product has, per gram of crude product, a
diesel
content in a range from 0.001-0.8 grams or from 0.01-0.4 grams. In certain
embodiments,
the diesel has, per gram of diesel, an aromatics content of at least 0.1
grams, at least 0.3
grams, or at least 0.5 grams. In some embodiments, the diesel has, per gram of
diesel, an
aromatics content in a range from 0.1-1 grams, 0.3-0.8 grams, or 0.2-0.5
grams.
In some embodiments, the crude product has, per gram of crude product, a VG0
content in a range from 0.0001-0.99 grams, from 0.001-0.8 grams, or from 0.1-
0.3 grams.
In certain embodiments, the VG0 content in the crude product is in a range
from 0.4-0.9
grams, or 0.6-0.8 grams per gram of crude product. In certain embodiments, the
VG0 has,
per gram of VG0, an aromatics content in a range from 0.1-0.99 grams, 0.3-0.8
grams, or
0.5-0.6 grams.
In some embodiments, the crude product has a residue content of at most 70%,
at
most 50%, at most 30%, at most 10%, or at most 1% of the crude feed. In
certain
embodiments, the crude product has, per gram of crude product, a residue
content of at
46
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
most 0.1 grams, at most 0.05 grams, at most 0.03 grams, at most 0.02 grams, at
most 0.01
grams, at most 0.005 grams, or at most 0.001 grams. In some embodiments, the
crude
product has, per gram of crude product, a residue content in a range from
0.000001-0.1
grams, 0.00001-0.05 grams, 0.001-0.03 grams, or 0.005-0.04 grams.
In some embodiments, the crude product may include at least a portion of the
catalyst. In some embodiments, a crude product includes from greater than 0
grams, but
less than 0.01 grams, 0.000001-0.001 grams, or 0.00001-0.0001 grams of
catalyst per
gram of crude product. The catalyst may assist in stabilizing the crude
product during
transportation and/or treatment in processing facilities. The catalyst may
inhibit corrosion,
inhibit friction, and/or increase water separation abilities of the crude
product. A crude
product that includes at least a portion of the catalyst may be further
processed to produce
lubricants and/or other commercial products.
The catalyst used for treatment of a crude feed in the presence of a hydrogen
source to produce the total product may be a single catalyst or a plurality of
catalysts. The
catalysts of the application may first be a catalyst precursor that is
converted to the catalyst
, in the contacting zone when hydrogen and/or a crude feed containing
sulfur is contacted =
=
. with the catalyst precursor.
The catalysts used in contacting the crude feed with a hydrogen source to
produce
the total product may assist in the reduction of the molecular weight of the
crude feed.
Not to be bound by theory, the catalyst in combination with the hydrogen
source may
reduce a molecular weight of components in the crude feed through the action
of basic
(Lewis basic or Bronsted-Lowry basic) and/or superbasic components in the
catalyst.
Examples of catalysts that may have Lewis base and/or Bronsted-Lowry base
properties
include catalysts described herein.
In some embodiments, the catalyst is a TMS catalyst. The TMS catalyst includes
a
compound that contains a transition metal sulfide. For the purposes of this
application,
weight of the transition metal sulfide in the TMS catalyst is determined by
adding the total
weight of the transition metal(s) to the total weight of sulfur in the
catalyst. An atomic
ratio of the transition metal to sulfur is typically in a range from 0.2-20,
0.5-10, or 1-5.
Examples of transition metal sulfides may be found in "Inorganic Sulfur
Chemistry";
Edited by G. Nickless; Elsevier Publishing Company; Amsterdam ¨ London ¨ New
York;
Copyright 1968; Chapter 19.
In certain embodiments, the TMS catalyst may include a total of at least 0.4
grams,
at least 0.5 grams, at least 0.8 grams, or at least 0.99 grams of one or more
transition metal
47
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
sulfides per gram of catalyst. In certain embodiments, the TMS catalyst has,
per gram of
catalyst, a total content of one or more transition metal sulfides in a range
from 0.4-0.999
grams, 0.5-0.9 grams, or 0.6-0.8 grams.
The TMS catalyst includes one or more transition metal sulfides. Examples of
transition metal sulfides include pentlandite (Fe4.5Ni4.5S8), smythite
(Fe6.75Ni2.25S11),
bravoite (Fe0.7Nio2C00.1S2), mackinawite (Fe0.75Ni0.25S0.9),
argentopentlandite
(AgFe6Ni2S8), isocubanite (CuFe2S3), isocalcopyrite (Cu8Fe9S16), sphalerite
(Zno:95Fe0.05S), mooihoekite (Cu9Fe9Si 6), chatkalite (Cu6FeSn2S8),
sternbergite (AgFe2S3),
chalcopyrite (CuFeS2), troilite (FeS), pyrite (FeS2), pyrrhotite (Fe (1,)S (x
= 0 to 0.17)),
heazlewoodite (Ni3S2) or vaesite (NiS2).
In some embodiments, the TMS catalyst includes one or more transition metal
sulfides in combination with alkali metal(s), alkaline-earth metal(s), zinc,
compounds of
zinc, or mixtures thereof. The TMS catalyst is, in some embodiments,
represented by the
general chemical formula Ac[MaSb]d, in which A represents alkali metal,
alkaline-earth
metal or zinc; M represents a transition metal from Columns 6-10 of the
Periodic Table;
and S is sulfur. An atomic ratio of a to b is in a range from 0.5 to 2.5, or
from 1 to 2. An
atomic ratio of c to a is in a range from 0.0001 to 1, 0.1 to 0.8, or 0.3 to
0.5. In some
embodiments, the transition metal is iron.
In some embodiments, the TMS catalyst may include generally known alkali
and/or alkaline-earth metals/transition metal sulfides (for example, bartonite
(K3FeloS14),
rasvumite (KFe2S3), djerfisherite (K6NaFe19Cu4NiS26C1), chlorobartonite
(1(6.1Fe24Cu0.2S26.1C10.7), and/or coyoteite (NaFe3S5'(420)2). In some
embodiments, the
TMS catalyst includes bartonite prepared in situ. Bartonite prepared in situ
may be
referred to as synthetic bartonite. Natural and/or synthetic bartonite may be
used as a
TMS catalyst in the methods described herein.
In some embodiments, the TMS catalyst may include at most 25 grams, at most 15
grams, or at most 1 gram of support material per 100 grams of the TMS
catalyst.
Typically, the TMS catalyst has from 0 to 25 grams, 0.00001 to 20 grams,
0.0001 grams to
10 grams of support material per 100 grams of the TMS catalyst. Examples of
support
materials that may be used with the TMS catalyst include refractory oxides,
porous carbon
materials, zeolites, or mixtures thereof. In some embodiments, the TMS
catalyst is
substantially free, or free, of support materials.
The TMS catalyst that includes alkali metal(s), alkaline-earth metal(s), zinc,
compounds of zinc, or mixtures thereof may contain one or more transition
metal sulfides,
48
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
bimetallic alkali metal-transition metal sulfides, higher valence transition
metal sulfides,
transition metal oxides, or mixtures thereof, as determined using x-ray
diffraction. A
portion of the alkali metal(s) component, alkaline-earth metal(s) component,
zinc
component and/or a portion of the transition metal sulfide component of the
TMS catalyst
may, in some embodiments, be present as an amorphous composition not
detectable by x-
ray diffraction techniques.
In some embodiments, crystalline particles of the TMS catalyst and/or mixtures
of
crystalline particles of the TMS catalyst have a particle size of at most 108
A, at most 103
A, at most 100 A, or at most 40 A. In normal practice, the particle size of
the crystalline
particles of the TMS catalyst will generally be at least 10 A.
The TMS catalyst that includes alkali metal(s), alkaline-earth metal(s), zinc,
compounds of zinc, or mixtures thereof may be prepared by mixing a sufficient
amount of
de-ionized water, a desired amount of a transition metal oxide, and desired
amount of
Columns 1-2 metal carbonate(s), Columns 1-2 metal oxalate(s), Columns 1-2
metal
acetate(s), zinc carbonate, zinc acetate, zinc oxalate, or mixtures thereof to
form a wet
i paste. The wet paste may. be dried at a temperature from 100-300 C or
150-250 C to
form a transition metal oxide/salt mixture. The transition metal oxide/salt
mixture may be
calcined at a temperature ranging from 300-1000 C, 500-800 C, or 600-700 C
to form a
transition metal oxide/metal salt mixture. The transition metal oxide/metal
salt mixture
may be reacted with hydrogen to form a reduced intermediate solid. The
addition of
hydrogen may be performed at a flow rate sufficient to provide an excess
amount of
hydrogen to the transition metal oxideh-netal salt mixture. Hydrogen may be
added over
10-50 hours or 20-40 hours to the transition metal oxide/metal salt mixture to
produce a
reduced intermediate solid that includes elemental transition metal. Hydrogen
addition
may be performed at a temperature of 35-500 C, 50-400 C, or 100-300 C, and
a total
pressure of 10-15 MPa, 11-14 MPa, or 12-13 MPa. It should be understood that
reduction
time, reaction temperature, selection of reducing gas, pressure of reducing
gas, and/or flow
rate of reducing gas used to prepare the intermediate solid is often changed
relative to the
absolute mass of the selected transition metal oxide. The reduced intermediate
solid may,
in some embodiments, be passed through a 40-mesh sieve with minimal force.
The reduced intermediate solid may be incrementally added to a hot (for
example,
100 C) diluent/elemental sulfur, and/or one or more compounds of sulfur,
mixture at a
rate to control the evolution of heat and production of gas. The diluent may
include any
suitable diluent that provides a means to dissipate the heat of sulfurization.
The diluent
49
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
may include solvents with a boiling range distributions of at least 100 C, at
least 150 C,
least 200 C, or at least 300 C. Typically the diluent has a boiling range
distribution
between 100-500 C, 150-400 C, or 200-300 C. In some embodiments, the
diluent is
VGO and/or xylenes. Sulfur compounds include, but are not limited to, hydrogen
sulfide
and/or thiols. An amount of sulfur and/or sulfur compounds may range from 1-
100
mole%, 2-80 mole%, 5-50 mole%, 10-30 mole%, based on the moles of Columns 1-2
metal or zinc in the Columns 1-2 metal salt or zinc salt. After addition of
the reduced
intermediate solid to the diluent/elemental sulfur mixture, the resulting
mixture may be
incrementally heated to a final temperature of 200-500 C, 250-450 C, or 300-
400 C and
maintained at the final temperature for at least 1 hour, at least 2 hours, or
at least 10 hours.
Typically, the final temperature is maintained for 15 hours, 10 hours, 5
hours, or 1.5 hours.
After heating to the elevated sulfurizing reaction temperature, the
diluent/catalyst mixture
may be cooled to a temperature in a range from 0-100 C, 30-90 C, or 50-80 C
to
facilitate recovery of the catalyst from the mixture. The sulfurized catalyst
may be
isolated in an oxygen-free atmosphere from the diluent using standard
techniques and
washed with at least a portion of a low boiling solvent (for example, pentane,
heptane, or
hexane) to produce the TMS catalyst. The TMS catalyst may be powdered using
standard
techniques. =
In some embodiments, the catalyst is an inorganic salt catalyst. The anion of
the
inorganic salt catalyst may include an inorganic compound, an organic
compound, or
mixtures thereof. The inorganic salt catalyst includes alkali metal
carbonates, alkali metal
hydroxides, alkali metal hydrides, alkali metal amides, alkali metal sulfides,
alkali metal
acetates, alkali metal oxalates, alkali metal formates, alkali metal
pyruvates, alkaline-earth
metal carbonates, alkaline-earth metal hydroxides, alkaline-earth metal
hydrides, alkaline-
earth metal amides, alkaline-earth metal sulfides, alkaline-earth metal
acetates, alkaline-
earth metal oxalates, alkaline-earth metal formates, alkaline-earth metal
pyruvates, or
mixtures thereof.
Inorganic salt catalysts include, but are not limited to, mixtures of:
Na0H/RbOH/Cs0H; KOH/RbOH/Cs0H; Na0H/KOH/RbOH; Na0H/KOH/Cs0H;
K2CO3/Rb2CO3/Cs2CO3; Na20/1(20/K2CO3; NaHCO3/KHCO3/Rb2CO3;
LiHCO3/KHCO3/Rb2CO3; KOH/RbOH/CsOH mixed with a mixture of
K2CO3/Rb2CO3/Cs2CO3; K2CO3/CaCO3; K2CO3/MgCO3; Cs2CO3/CaCO3; Cs2CO3/Ca0;
Na2CO3/Ca(OH)2; KH/CsCO3; KOCHO/Ca0; CsOCHO/CaCO3; CsOCHO/Ca(OCH0)2;
NaNH2/K2CO3/Rb20; K2CO3/CaCO3/Rb2CO3; K2CO3/CaCO3/Cs2CO3;
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
K2CO3/MgCO3/Rb2CO3; K2CO3/MgCO3/Cs2CO3; or Ca(OH)2 mixed with a mixture of
K2CO3/Rb2CO3/Cs2CO3.
In some embodiments, the inorganic salt catalyst contains at most 0.00001
grams,
=at most 0.001 grams, or at most 0.01 grams of lithium, calculated as the
weight of lithium,
per gram of inorganic salt catalyst. The inorganic salt catalyst has, in some
embodiments,
from 0 grams, but less than 0.01= grams, 0.0000001-0.001 grams, or 0.00001-
0.0001 grams
of lithium, calculated as the weight of lithium, per gram of inorganic salt
catalyst,
In certain embodiments, an inorganic salt catalyst includes one or more alkali
metal salts that include an alkali metal with an atomic number of at least 11.
An atomic
ratio of an alkali metal having an atomic number of at least 11 to an alkali
metal having an
atomic number greater than 11, in some embodiments, is in a range from 0.1 to
10, 0.2 to
6, or 0.3 to 4 when the inorganic salt catalyst has two or more alkali metals.
For example,
the inorganic salt catalyst may include salts of sodium, potassium, and
rubidium with the
ratio of sodium to potassium being in a range from 0.1-6; the ratio of sodium
to rubidium
being in a range from 0.1-6; and the ratio of potassium to rubidium being in a
range from
0.1-6. In another example; the inorganic salt catalyst includes a sodium salt
and a =
potassium salt with the atomic ratio of sodium to potassium being in a range
from 0.1 to 4.
= In some embodiments, an inorganic salt catalyst also includes metals from
Columns 8-10 of the Periodic Table, compounds of metals from Columns 8-10 of
the
Periodic Table, metals from Column 6 of the Periodic Table, compounds of
metals from
Column 6 of the Periodic Table, or mixtures thereof. Metals from Columns 8-10
include,
but are not limited to, iron, ruthenium, cobalt, or nickel. Metals from Column
6 include,
but are not limited to, chromium, molybdenum, or tungsten. In some
embodiments, the
inorganic salt catalyst includes 0.1-0.5 grams, or 0.2-0.4 grams of Raney
nickel per gram
of inorganic salt catalyst.
In certain embodiments, the inorganic salt catalyst also includes metal oxides
from
Columns 1-2 and/or Column 13 of the Periodic Table. Metals from Column 13
include,
but are not limited to, boron or aluminum. Non-limiting examples of metal
oxides include
lithium oxide (Li20), potassium oxide (K20), calcium oxide (CaO), or aluminum
oxide
(A1203).
The inorganic salt catalyst is, in certain embodiments, free of or
substantially free
of Lewis acids (for example, BC13, A1C13, and S03), Bronsted-Lowry acids (for
example,
H30+, H2SO4, HC1, and HNO3), glass-forming compositions (for example, borates
and
silicates), and halides. The inorganic salt may contain, per gram of inorganic
salt catalyst:
51
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
from 0 grams to 0.1 grams, 0.000001-0.01 grams, or 0.00001-0.005 grams of: a)
halides;
b) compositions that form glasses at temperatures of at least 350 C, or at
most 1000 C; c)
Lewis acids; d) Bronsted-Lowry acids; or e) mixtures thereof.
The inorganic salt catalyst may be prepared using standard techniques. For
example, a desired amount of each component of the catalyst may be combined
using
standard mixing techniques (for example, milling and/or pulverizing). In other
embodiments, inorganic compositions are dissolved in a solvent (for example,
water or a
suitable organic solvent) to form an inorganic composition/solvent mixture.
The solvent
may be removed using standard separation techniques to produce the inorganic
salt
catalyst.
In some embodiments, inorganic salts of the inorganic salt catalyst may be
incorporated into a support to form a supported inorganic salt catalyst.
Examples of
supports include, but are not limited to, zirconium oxide, calcium oxide,
magnesium
oxide, titanium oxide, hydrotalcite, alumina, germania, iron oxide, nickel
oxide, zinc
oxide, cadmium oxide, antimony oxide, and mixtures thereof. In some
embodiments, an
inorganic salt, a Columns 6-10 metal and/or a compound of a Columns 6-10 metal
may be
impregnated in the support. Alternatively, inorganic salts may be melted or
softened with
heat and forced in and/or onto a metal support or metal oxide support to form
a supported
inorganic salt catalyst.
A structure of the inorganic salt catalyst typically becomes nonhomogenous,
permeable, and/or mobile at a determined temperature or in a temperature range
when loss
of order occurs in the catalyst structure. The inorganic salt catalyst may
become
disordered without a substantial change in composition (for example, without
decomposition of the salt). Not to be bound by theory, it is believed that the
inorganic salt
catalyst becomes disordered (mobile) when distances between ions in the
lattice of the
inorganic salt catalyst increase. As the ionic distances increase, a crude
feed and/or a
hydrogen source may permeate through the inorganic salt catalyst instead of
across the
surface of the inorganic salt catalyst. Permeation of the crude feed and/or
hydrogen source
through the inorganic salt often results in an increase in the contacting area
between the
inorganic salt catalyst and the crude feed and/or the hydrogen source. An
increase in
contacting area and/or reactivity area of the inorganic salt catalyst may
often increase the
yield of crude product, limit production of residue and/or coke, and/or
facilitate a change
in properties in the crude product relative to the same properties of the
crude feed.
Disorder of the inorganic salt catalyst (for example, nonhomogeneity,
permeability, and/or
52
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
mobility) may be determined using DSC methods, ionic conductivity measurement
methods, TAP methods, visual inspection, x-ray diffraction methods, or
combinations
thereof.
The use of TAP to determine characteristics of catalysts is described in U.S.
Patent
Nos. 4,626,412 to Ebner et al.; 5,039,489 to Gleaves et al.; and 5,264,183 to
Ebner et al.
A TAP system may be obtained from Mithra Technologies (Foley, Missouri,
U.S.A.). The
TAP analysis may be performed in a temperature range from 25-850 C, 50-500
C, or 60-
400 C, at a heating rate in a range from 10-50 C, or 20-40 C, and at a
vacuum in a range
from 1 x 10-13 to 1 x 10-8 torr. The temperature may remain constant and/or
increase as a
function of time. As the temperature of the inorganic salt catalyst increases,
gas emission
from the inorganic salt catalyst is measured. Examples of gases that evolve
from the
inorganic salt catalyst include carbon monoxide, carbon dioxide, hydrogen,
water, or
mixtures thereof. The temperature at which an inflection (sharp increase) in
gas evolution
from the inorganic salt catalyst is detected is considered to be the
temperature at which the
inorganic salt catalyst becomes disordered.
In some embodiments, an inflection of emitted gas from the inorganic salt
catalyst
, may be detected over a range of temperatures as determined using TAP.
The temperature
or the temperature range is referred to as the "TAP temperature". The initial
temperature
of the temperature range determined using TAP is referred to as the "minimum
TAP
temperature".
The emitted gas inflection exhibited by inorganic salt catalysts suitable for
contact
with a crude feed is in a TAP temperature range from 100-600 C, 200-500 C,
or 300-400
C. Typically, the TAP temperature is in a range from 300-500 C. In some
embodiments, different compositions of suitable inorganic salt catalysts also
exhibit gas
inflections, but at different TAP temperatures.
The magnitude of the ionization inflection associated with the emitted gas may
be
an indication of the order of the particles in a crystal structure. In a
highly ordered crystal
structure, the ion particles are generally tightly associated, and release of
ions, molecules,
gases, or combinations thereof, from the structure requires more energy (that
is more
heat). In a disordered crystal structure, ions are not associated to each
other as strongly as
ions in a highly ordered crystal structure. Due to the lower ion association,
less energy is
generally required to release ions, molecules, and/or gases from a disordered
crystal
structure, and thus, a quantity of ions and/or gas released from a disordered
crystal
53
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
structure is typically greater than a quantity of ions and/or gas released
from a highly
ordered crystal structure at a selected temperature.
In some embodiments, a heat of dissociation of the inorganic salt catalyst may
be
observed in a range from 50 C to 500 C at a heating rate or cooling rate of
10 C, as
determined using a differential scanning calorimeter. In a DSC method, a
sample may be
heated to a first temperature, cooled to room temperature, and then heated a
second time.
Transitions observed during the first heating generally are representative of
entrained
water and/or solvent and may not be representative of the heat of
dissociations. For
example, easily observed heat of drying of a moist or hydrated sample may
generally
occur below 250 C, typically between 100-150 C. The transitions observed
during the
cooling cycle and the second heating correspond to the heat of dissociation of
the sample.
"Heat transition" refers to the process that occurs when ordered molecules
and/or
atoms in a structure become disordered when the temperature increases during
the DSC
analysis. "Cool transition" refers to the process that occurs when molecules
and/or atoms
in a structure become more homogeneous when the temperature decreases during
the DSC
. . analysis: In some embodiments, the heat/cool transition of the
inorganic salt catalyst
occurs over a range of temperatures that are detected using DSC. The
temperature or
= temperature range at which the heat transition of the inorganic salt
catalyst occurs during a
second heating cycle is referred to as "DSC temperature". The lowest DSC
temperature of
the temperature range during a second heating cycle is referred to as the
"minimum DSC
temperature". The inorganic salt catalyst may exhibit a heat transition in a
range between
200-500 C, 250-450 C, or 300-400 C.
In an inorganic salt that contains inorganic salt particles that are a
relatively
homogeneous mixture, a shape of the peak associated with the heat absorbed
during a
second heating cycle may be relatively narrow. In an inorganic salt catalyst
that contains
inorganic salt particles in a relatively non-homogeneous mixture, the shape of
the peak
associated with heat absorbed during a second heating cycle may be relatively
broad. An
absence of peaks in a DSC spectrum indicates that the salt does not absorb or
release heat
in the scanned temperature range. Lack of a heat transition generally
indicates that the
structure of the sample does not change upon heating.
As homogeneity of the particles of an inorganic salt mixture increases, the
ability
of the mixture to remain a solid and/or a semiliquid during heating decreases.
Homogeneity of an inorganic mixture may be related to the ionic radius of the
cations in
the mixtures. For cations with smaller ionic radii, the ability of a cation to
share electron
54
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
density with a corresponding anion increases and the acidity of the
corresponding anion
increases. For a series of ions of similar charges, a smaller ionic radius
results in higher
interionic attractive forces between the cation and the anion if the anion is
a hard base.
The higher interionic attractive forces tend to result in higher heat
transition temperatures
for the salt and/or more homogeneous mixture of particles in the salt (sharper
peak and
increased area under the DSC curve). Mixtures that include cations with small
ionic radii
tend to be more acidic than cations of larger ionic radii, and thus acidity of
the inorganic
salt mixture increases with decreasing cationic radii. For example, contact of
a crude feed
with a hydrogen source in the presence of an inorganic mixture that includes
lithium
cations tends to produce increased quantities of gas and/or coke relative to
contact of the
crude feed with a hydrogen source in the presence of an inorganic salt
catalyst that
includes cations with a larger ionic radii than lithium. The ability to
inhibit generation of
gas and/or coke increases the total liquid product yield of the process.
In certain embodiments, the inorganic salt catalyst may include two or more
inorganic salts. A minimum DSC temperature for each of the inorganic salts may
be
.,determined. The minimurnDSC temperature of the inorganic salt catalyst may
be below
.the.minimum DSC temperature of at least one of the inorganic metal salts in
the inorganic
salt catalyst. For example, the inorganic salt catalyst may include potassium
carbonate
and cesium carbonate. Potassium carbonate and cesium carbonate exhibit DSC
temperatures greater than 500 C. A K2CO3/Rb2CO3/Cs2CO3 catalyst exhibits a
DSC
temperature in a range from 290-300 C.
In some embodiments, the TAP temperature may be between the DSC temperature
of at least one of the inorganic salts and the DSC temperature of the
inorganic salt catalyst.
For example, the TAP temperature of the inorganic salt catalyst may be in a
range from
350-500 C. The DSC temperature of the same inorganic salt catalyst may be in
a range
from 200-300 C, and the DSC temperature of the individual salts may be at
least 500 C
or at most 1000 C.
An inorganic salt catalyst that has a TAP and/or DSC temperature between 150-
500 C, 200-450 C, or 300-400 C, and does not undergo decomposition at these
temperatures, in many embodiments, can be used to catalyze conversion of high
molecular
weight and/or high viscosity compositions (for example, crude feed) to liquid
products.
In certain embodiments, the inorganic salt catalyst may exhibit increased
conductivity relative to individual inorganic salts during heating of the
inorganic salt
catalyst in a temperature range from 200-600 C, 300-500 C, or 350-450 C.
Increased
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
conductivity of the inorganic salt catalyst is generally attributed to the
particles in the
inorganic salt catalyst becoming mobile. The ionic conductivity of some
inorganic salt
catalysts changes at a lower temperature than the temperature at which ionic
conductivity
of a single component of the inorganic salt catalyst changes.
Ionic conductivity of inorganic salts may be determined by applying Ohm's law:
V
= IR, where V is voltage, I is current, and R is resistance. To measure ionic
conductivity,
the inorganic salt catalyst may be placed in a quartz vessel with two wires
(for example,
copper wires or platinum wires) separated from each other, but immersed in the
inorganic
salt catalyst.
FIG. 7 is a schematic of a system that may be used to measure ionic
conductivity.
Quartz vessel 156 containing sample 158 may be placed in a heating apparatus
and heated
incrementally to a desired temperature. Voltage from source 160 is applied to
wire 162
during heating. The resulting current through wires 162 and 164 is measured at
meter 166.
Meter 166 may be, but is not limited to, a multimeter or a Wheatstone bridge.
As sample
158 becomes less homogeneous (more mobile) without decomposition occurring,
the
resistivity of the sample should decrease and the observed current at meter
166 should
,
increase;
In some embodiments, at a desired temperature, the inorganic salt catalyst may
have a different ionic conductivit after heating, cooling, and then heating.
The difference
in ionic conductivities may indicate that the crystal structure of the
inorganic salt catalyst
has been altered from an original shape (first form) to a different shape
(second form)
during heating. The ionic conductivities, after heating, are expected to be
similar or the
same if the form of the inorganic salt catalyst does not change during
heating.
In certain embodiments, the inorganic salt catalyst has a particle size in a
range of
10-1000 microns, 20-500 microns, or 50-100 microns, as determined by passing
the
inorganic salt catalyst through a mesh or a sieve.
The inorganic salt catalyst may soften when heated to temperatures above 50 C
and below 500 C. As the inorganic salt catalyst softens, liquids and catalyst
particles may
co-exist in the matrix of the inorganic salt catalyst. The catalyst particles
may, in some
embodiments, self-deform under gravity, or under a pressure of at least 0.007
MPa, or at
most 0.101 MPa, when heated to a temperature of at least 300 C, or at most
800 C, such
that the inorganic salt catalyst transforms from a first form to a second
form. Upon
cooling of the inorganic salt catalyst to 20 C, the second form of the
inorganic salt
catalyst is incapable of returning to the first form of the inorganic salt
catalyst. The
56
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
temperature at which the inorganic salt transforms from the first form to a
second form is
referred to as the "deformation" temperature. The deformation temperature may
be a
temperature range or a single temperature. In certain embodiments, the
particles of the
inorganic salt catalyst self-deform under gravity or pressure upon heating to
a deformation
temperature below the deformation temperature of any of the individual
inorganic metal
salts. In some embodiments, an inorganic salt catalyst includes two or more
inorganic
salts that have different deformation temperatures. The deformation
temperature of the
inorganic salt catalyst differs, in some embodiments, from the deformation
temperatures of
the individual inorganic metal salts.
In certain embodiments, the inorganic salt catalyst is liquid and/or
semiliquid at, or
above, the TAP and/or DSC temperature. In some embodiments, the inorganic salt
catalyst is a liquid or a semiliquid at the minimum TAP and/or DSC
temperature. At or
above the minimum TAP and/or DSC temperature, liquid or semiliquid inorganic
salt
catalyst mixed with the crude feed may, in some embodiments, form ,a separate
phase from
the crUde feed. In some embodiments, the liquid or semiliquid inorganic salt
catalyst has
low solubility in the crude feed (for example, from 0 grams to 0.5 grams,
0.0000001-0.2
grams, or 0.0001-0.1 grams of inorganic salt catalyst per gram of crude feed)
or is
' insoluble in the crude feed (for example, from 0 grams to 0.05 grams,
0.000001-0.01
grams, or 0.00001-0.001 grams of inorganic salt catalyst per gram of crude
feed) at the
minimum TAP temperature.
In some embodiments, powder x-ray diffraction methods are used to determine
the
spacing of the atoms in the inorganic salt catalyst. A shape of the D001 peak
in the x-ray
spectrum may be monitored and the relative order of the inorganic salt
particles may be
estimated. Peaks in the x-ray diffraction represent different compounds of the
inorganic
salt catalyst. In powder x-ray diffraction, the D001 peak may be monitored and
the spacing
between atoms may be estimated. In an inorganic salt catalyst that contains
highly ordered
inorganic salt atoms, a shape of the D001 peak is relatively narrow. In an
inorganic salt
catalyst (for example, a K2CO3/Rb2CO3/Cs2CO3 catalyst) that contains randomly
ordered
inorganic salt atoms, the shape of the Dom peak may be relatively broad or the
D001 peak
may be absent. To determine if the disorder of inorganic salt atoms changes
during
heating, an x-ray diffraction spectrum of the inorganic salt catalyst may be
taken before
heating and compared with an x-ray diffraction spectrum taken after heating.
The Dow
peak (corresponding to the inorganic salt atoms) in the x-ray diffraction
spectrum taken at
temperatures above 50 C may be absent or broader than the D001 peaks in the x-
ray
57
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
diffraction spectrum taken at temperatures below 50 'C. Additionally, the x-
ray
diffraction pattern of the individual inorganic salt may exhibit relatively
narrow D001 peaks
at the same temperatures.
Contacting conditions may be controlled such that the total product
composition
(and thus, the crude product) may be varied for a given crude feed in addition
to limiting
and/or inhibiting formation of by-products. The total product composition
includes, but is
not limited to, paraffins, olefins, aromatics, or mixtures thereof. These
compounds make
up the compositions of the crude product and the non-condensable hydrocarbon
gases.
Controlling contacting conditions in combination with the catalyst described
herein
may produce a total product with lower than predicted coke content. Comparison
of the
MCR content of various crudes may allow crudes to be ranked based on their
tendency to
form coke. For example, a crude with a MCR content of 0.1 grams of MCR per
gram of
crude would be expected to form more coke than a crude with a MCR content of
0.061
grams of MCR per gram of crude. Disadvantaged crudes typically have MCR
contents of
at least 0.05 grams of MCR per gram of disadvantaged crude.
In some embodiments, the residue content and/or coke content deposited on the
=
catalyst during a reaction period may be at most 0.1 grams, at most 0.05
grams, or at most
0.03 grams of residue and/or coke per gram of catalyst. In certain
embodiments, the
weight of residue and/or coke deposited on the catalyst is in a range from
0.0001-0.1
grams, 0.001-0.05 grams, or 0.01-0.03 grams. In some embodiments, used
catalyst is
substantially free of residue and/or coke. In certain embodiments, contacting
conditions
are controlled such that at most 0.015 grams, at most 0.01 grams, at most
0.005 grams, or
at most 0.003 grams of coke is formed per gram of crude product. Contacting a
crude feed
with the catalyst under controlled contacting conditions produces a reduced
quantity of
coke and/or residue relative to a quantity of coke and/or residue produced by
heating the
crude feed in the presence of a refining catalyst, or in the absence of a
catalyst, using the
same contacting conditions.
The contacting conditions may be controlled, in some embodiments, such that,
per
gram of crude feed, at least 0.5 grams, at least 0.7 grams, at least 0.8
grams, or at least 0.9
grams of the crude feed is converted to the crude product. Typically, between
0.5-0.99
grams, 0.6-0.9 grams, or 0.7-0.8 grams of the crude product per gram of crude
feed is
produced during contacting. Conversion of the crude feed to a crude product
with a
minimal yield of residue and/or coke, if any, in the crude product allows the
crude product
to be converted to commercial products with a minimal amount of pre-treatment
at a
58
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
refinery. In certain embodiments, per gram of crude feed, at most 0.2 grams,
at most 0.1
grams, at most 0.05 grams, at most 0.03 grams, or at most 0.01 grams of the
crude feed is
converted to non-condensable hydrocarbons. In some embodiments, from 0 to 0.2
grams,
0.0001-0.1 grams, 0.001-0.05 grams, or 0.01-0.03 grams of non-condensable
hydrocarbons per gram of crude feed is produced.
Controlling a contacting zone temperature, rate of crude feed flow, rate of
total
product flow, rate and/or amount of catalyst feed, or combinations thereof,
may be
performed to maintain desired reaction temperatures. In some embodiments,
control of
the temperature in the contacting zone may be performed by changing a flow of
a gaseous
hydrogen source and/or inert gas through the contacting zone to dilute the
amount of
hydrogen and/or remove excess heat from the contacting zone.
In some embodiments, the temperature in the contacting zone may be controlled
such that a temperature in the contacting zone is at, above, or below desired
temperature
"T1". In certain embodiments, the contacting temperature is controlled such
that the
contacting zone temperature is below the minimum TAP temperature and/or the
minimum
DSC temperature. In certain embodiments, T1 may be 30 C below, 20 C below,
or 10 =
C below the minimum TAP temperature and/or the minimum DSC temperature. For,
example, in one embodiment, the contacting temperature may be controlled to be
370 C,
380 C, or 390 C during the reaction period when the minimum TAP temperature
and/or
minimum DSC temperature is 400 C.
In other embodiments, the contacting temperature is controlled such that the
temperature is at, or above, the catalyst TAP temperature and/or the catalyst
DSC
temperature. For example, the contacting temperature may be controlled to be
450 C,
500 C, or 550 C during the reaction period when the minimum TAP temperature
and/or
minimum DSC temperature is 450 C. Controlling the contacting temperature
based on
catalyst TAP temperatures and/or catalyst DSC temperatures may yield improved
crude
product properties. Such control may, for example, decrease coke formation,
decrease
non-condensable gas formation, or combinations thereof.
In certain embodiments, the inorganic salt catalyst may be conditioned prior
to
addition of the crude feed. In some embodiments, the conditioning may take
place in the
presence of the crude feed. Conditioning the inorganic salt catalyst may
include heating
the inorganic salt catalyst to a first temperature of at least 100 C, at
least 300 C, at least
400 C, or at least 500 C, and then cooling the inorganic salt catalyst to a
second
temperature of at most 250 C, at most 200 C, or at most 100 C. In certain
59
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
embodiments, the inorganic salt catalyst is heated to a temperature in a range
from 150-
700 C, 200-600 C, or 300-500 C, and then cooled to a second temperature in
a range
from 25-240 C, 30-200 C, or 50-90 C. The conditioning temperatures may be
determined by determining ionic conductivity measurements at different
temperatures. In
some embodiments, conditioning temperatures may be determined from DSC
temperatures obtained from heat/cool transitions obtained by heating and
cooling the
inorganic salt catalyst multiple times in a DSC. Conditioning of the inorganic
salt catalyst
may allow contact of a crude feed to be performed at lower reaction
temperatures than
temperatures used with conventional hydrotreating catalysts.
In some embodiments, a content of naphtha, distillate, VGO, or mixtures
thereof,
in the total product, may be varied by changing a rate of total product
removal from a
contacting zone. For example, decreasing a rate of total product removal tends
to increase
contacting time of the crude feed with the catalyst. Alternately, increasing
pressure
relative to an initial pressure may increase contacting time, may increase a
yield of a crude
product, may increase incorporation of hydrogen from the gases into a crude
product for a
given mass flow rate of crude feed or hydrogen source, or may alter
combinations of these
== effects. Increased contacting times of the crude feed with the catalyst
may produce an
increased amount of diesel, kerosene, or naphtha and a decreased amount of VG0
relative
to the amounts of diesel, kerosene, naphtha, and VG0 produced at shorter
contacting
times. Increasing the contacting time of the total product in the contacting
zone may also
change the average carbon number of the crude product. Increased contacting
time may
result in a higher weight percentage of lower carbon numbers (and thus, a
higher API
gravity).
In some embodiments, the contacting conditions may be changed over time. For
example, the contacting pressure and/or the contacting temperature may be
increased to
increase the amount of hydrogen that the crude feed uptakes to produce the
crude product.
The ability to change the amount of hydrogen uptake of the crude feed, while
improving
other properties of the crude feed, increases the types of crude products that
may be
produced from a single crude feed. The ability to produce multiple crude
products from a
single crude feed may allow different transportation and/or treatment
specifications to be
satisfied.
Uptake of hydrogen may be assessed by comparing H/C of the crude feed to H/C
of the crude product. An increase in the H/C of the crude product relative to
H/C of the
crude feed indicates incorporation of hydrogen into the crude product from the
hydrogen
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
source. Relatively low increase in H/C of the crude product (20%, as compared
to the
crude feed) indicates relatively low consumption of hydrogen gas during the
process.
Significant improvement of the crude product properties, relative to those of
the crude
feed, obtained with minimal consumption of hydrogen is desirable.
The ratio of hydrogen source to crude feed may also be altered to alter the
properties of the crude product. For example, increasing the ratio of the
hydrogen source
to crude feed may result in crude product that has an increased VG0 content
per gram of
crude product.
In certain embodiments, contact of the crude feed with the inorganic salt
catalyst in
the presence of light hydrocarbons and/or steam yields more liquid
hydrocarbons and less
coke in a crude product than contact of a crude feed with an inorganic salt
catalyst in the
presence of hydrogen and steam. In embodiments that include contact of the
crude feed
with methane in the presence of the inorganic salt catalyst, at least a
portion of the
components of the crude product may include atomic carbon and hydrogen (from
the
methane) which has been incorporated into the molecular, structures of the
components.
In certain embodiments, the volume of crude product produced from a crude feed
contacted with the hydrogen source in the presence of the inorganic salt
catalyst is at least
5% greater, at least 10% greater, or at least 15%, or at most 100% greater
than a volume of I
crude product produced from a thermal process at STP. The total volume of
crude product
produced by contact of the crude feed with the inorganic salt catalyst may be
at least 110
vol% of the volume of the crude feed at STP. The increase in volume is
believed to be
due to a decrease in density. Lower density may generally be at least
partially caused by
hydrogenation of the crude feed. =
In certain embodiments, a crude feed having, per gram of crude feed, at least
0.02
grams, at least 0.05 grams, or at least 0.1 grams of sulfur, and/or at least
0.001 grams of
NiN/Fe is contacted with a hydrogen source in the presence of an inorganic
salt catalyst
without diminishing the activity of the catalyst.
In some embodiments, the inorganic salt catalyst can be regenerated, at least
partially, by removal of one or more components that contaminate the catalyst.
Contaminants include, but are not limited to, metals, sulfides, nitrogen,
coke, or mixtures
thereof. Sulfide contaminants may be removed from the used inorganic salt
catalyst by
contacting steam and carbon dioxide with the used catalyst to produce hydrogen
sulfide.
Nitrogen contaminants may be removed by contacting the used inorganic salt
catalyst with
steam to produce ammonia. Coke contaminants may be removed from the used
inorganic
61
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
salt catalyst by contacting the used inorganic salt catalyst with steam and/or
methane to
produce hydrogen and carbon oxides. In some embodiments, one or more gases are
generated from a mixture of used inorganic salt catalyst and residual crude
feed.
In certain embodiments, a mixture of used inorganic salt (for example,
K2CO3/Rb2CO3/Cs2CO3; KOH/A1203; Cs2CO3/CaCO3; or Na0H/KOH/Li0H/Zr02),
unreacted crude feed and/or residue and/or coke may be heated to a temperature
in a range
from 700-1000 C or from 800-900 C until the production of gas and/or liquids
is
minimal in the presence of steam, hydrogen, carbon dioxide, and/or light
hydrocarbons to
produce a liquid phase and/or gas. The gas may include an increased quantity
of hydrogen
and/or carbon dioxide relative to reactive gas. For example, the gas may
include from 0.1-
99 moles or from 0.2-8 moles of hydrogen and/or carbon dioxide per mole of
reactive gas.
The gas may contain a relatively low amount of light hydrocarbons and/or
carbon
monoxide. For example, less than 0.05 grams of light hydrocarbons per gram of
gas and
less than 0.01 grams of carbon monoxide per gram of gas. The liquid phase may
contain
water, for example, greater than 0.5-0.99 grams, or greater than 0.9-0.9 grams
of water per
'gram of liquid.
=
In 42,me embodiments, the used catalyst and/or solids in the contacting zone
may
be treated to recover metals (for example, vanadium and/or nickel) from the
used catalyst =
and/or solids. The used catalyst and/or solids may be treated using generally
known metal
separation techniques, for example, heating, chemical treating, and/or
gasification.
EXAMPLES
Non-limiting examples of catalyst preparations, testing of catalysts, and
systems
with controlled contacting conditions are set forth below.
Example 1. Preparation of a K-Fe Sulfide Catalyst. A K-Fe sulfide catalyst was
prepared by combining 1000 grams of iron oxide (Fe203) and 580 g of potassium
carbonate with 412 grams of de-ionized water to form a wet paste. The wet
paste was
dried at 200 C to form an iron oxide/potassium carbonate mixture. The iron
oxide/potassium carbonate mixture was calcined at 500 C to form an iron
oxide/potassium carbonate mixture. The iron oxide/potassium carbonate mixture
was
reacted with hydrogen to form a reduced intermediate solid that included iron
metal.
Hydrogen addition was performed over 48 hours at 450 C and 11.5-12.2 MPa
(1665-1765
psi). The intermediate solid was passed through a 40-mesh sieve with minimal
force.
62
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
The intermediate solid was added incrementally at a rate to control the
evolution of
heat and produced gas to a VGO/m-xylene/elemental sulfur mixture at 100 C.
After
addition of the intermediate solid, the resulting mixture was incrementally
heated to 300
C and maintained at 300 C for 1 hour. The solvent/catalyst mixture was cooled
to below
100 C and the sulfurized catalyst was separated from the mixture. The
sulfurized catalyst
was isolated by filtration in a dry-box under an argon atmosphere, and washed
with m-
xylene to produce 544.7 grams of the K-Fe sulfide catalyst. The K-Fe sulfide
catalyst was
powdered by passing the catalyst through a 40-mesh sieve.
The resulting K-Fe sulfide catalyst was analyzed using x-ray diffraction
techniques. From analysis of the x-ray diffraction spectrum, it was determined
that the
catalyst included troilite (FeS), K-Fe sulfide (KFeS2), pyrrhotite, and iron
oxides (for
example, magnetite, Fe304). A peak associated with iron disulfide (for
example, pyrite,
FeS2) was not observed in the x-ray diffraction spectrum.
Example 2. Contact of a Crude Feed With a Hydrogen Source in the Presence of a
K-Fe Sulfide Catalyst. A 600 mL continuously stirred tank reactor (composed of
316
stainless steel) was fitted with a bottom inlet feed port, a single vapor
effluent port, three
thermocouples located in the reactor interior, and a shaftdriven 1.25-inch
diameter six- ,
blade Rushton turbine.
The K-Fe sulfide catalyst (110.3 grams) prepared as described in Example 1 was
charged to the reactor. Hydrogen gas was metered at 8,000 Nm3/m3 (50,000 SCFB)
into
the reactor and mixed with bitumen (Lloydminster region of Canada). The
bitumen
entered the reactor through the bottom inlet feed port to form a
hydrogen/crude feed
mixture. During the reaction run period of 185 hours, hydrogen gas and crude
feed were
continuously fed into the reactor and product was continuously removed through
the
effluent vapor port of the reactor. Crude feed was fed at a rate of 67.0 g/hr
to maintain the
crude feed liquid level at 60% of the reactor volume. A 50 milli-curie 137Cs
gamma ray
source and a sodium iodide scintillation detector were used to measure the
liquid level in
the reactor.
The hydrogen gas/crude feed was contacted with the catalyst at an average
internal
reactor temperature of 430 C. Contacting of the hydrogen/crude feed with the
catalyst
produced a total product in the form of the reactor effluent vapor. The
reactor effluent
vapor exited the vessel through the single upper exit port. The reactor head
was
electrically heated to 430 C to prevent internal condensation of the reactor
effluent vapor
on the reactor head.
63
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
After exiting the reactor, the reactor etiluent vapor was cooled and separated
m a
high pressure gas/liquid separator and a low-pressure gas/liquid separator to
produce a
liquid stream and a gas stream. The gas stream was sent to a countercurrent
flow caustic
scrubber to remove acidic gases, and thereafter quantified using standard
chromatographic
techniques. The total product included, per gram of total product, 0.918 grams
of crude
product and 0.089 grams of non-condensable hydrocarbon gases. In the reactor,
0.027
grams of solids per gram of crude feed remained. Properties and compositions
of the
crude product and the non-condensable hydrocarbon gases produced by this
method are
summarized in Table 1 in FIG. 8, Table 2 in FIG. 9, and Table 3 in FIG. 10.
= This example demonstrates a method of contacting a crude feed with
hydrogen in
the presence of the transition metal sulfide catalyst to produce a total
product with
minimal concomitant generation of coke. The total product included a crude
product that
was a liquid mixture at STP and has at most 0.1 grams of non-condensable
hydrocarbon
gases per gram of total product.
By comparing the results of the MCR content for the crude feed (13.7 wt%) in
Table 1 to the solids formed during =the process (2.7 wt%), it is possible to
see.that the
combination of the controlled conditions and the catalyst produced a lower
quantity of .
coke than that indicated by the ASTM Method D4530.
The non-condensable hydrocarbons included C2, C3, and C4 hydrocarbons. From
the sum of the weight percentages of the C2 hydrocarbons listed in Table 2
(20.5 grams),
the ethylene content per gram of total C2 hydrocarbons may be calculated. The
C2
hydrocarbons of the hydrocarbon gases included 0.073 grams of ethylene per
gram of total
C2 hydrocarbons. From the sum of the weight percentages of the C3 hydrocarbons
listed
in Table 2 (23.9 grams), the propene content per gram of total C3 hydrocarbons
may be
calculated. The C3 hydrocarbons of the non-condensable hydrocarbon gases
included 0.21
grams of propene per gram of total C3 hydrocarbons. The C4 hydrocarbons of the
non-
condensable hydrocarbon gases had an iso-butane to n-butane weight ratio of
0.2.
This example demonstrates a method to produce a crude product that includes at
least 0.001 grams of hydrocarbons with a boiling range distribution of at most
204 C (400
F) at 0.101 MPa, at least 0.001 grams of hydrocarbons with a boiling range
distribution
between 204 C and 300 C at 0.101 MPa, at least 0.001 grams of hydrocarbons
with a
boiling range distribution between 300 C and 400 C at 0.101 MPa, and at
least 0.001
grams of hydrocarbons with a boiling range distribution between 400 C and 538
C
(1,000 F) at 0.101 MPa. The hydrocarbons that had a boiling range
distribution below
64
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
204 C included iso-paratiins and n-parattms, and the ratio ot such iso-
parattins to the n-
paraffins was at most 1.4.
The crude product included boiling point distributions that are associated
with
naphtha, kerosene, diesel, and VGO. The crude product had at least 0.001 grams
of
naphtha and the naphtha portion of the crude product had an octane number of
at least 70.
The naphtha portion of the crude product had a benzene content of at most 0.01
grams of
benzene per gram of naphtha. The naphtha portion of the crude product had at
most 0.15
grams of olefins per gram of naphtha. The naphtha portion of the crude product
had at
least 0.1 grams of monocyclic ring aromatics per gram of naphtha.
The crude product had at least 0.001 grams of kerosene. The kerosene portion
of
the crude product had a freezing point below -30 C. The kerosene portion of
the crude
product included aromatics, and the kerosene portion of the crude product had
an
aromatics content of at least 0.3 grams of aromatics per gram of kerosene. The
kerosene
portion of the crude product had at least 0.2 grams of monocyclic ring
aromatics per gram
of kerosene.
The crude product had at least 0.001 grams of diesel. The diesel fraction of
the ,
crude product included aromatics, and the diesel fraction of the crude product
had an
aromatics content of at least 0.4 grams of aromatics per gram of diesel.
The crude product had at least 0.001 grams of VGO. The VG0 portion of the
crude product included aromatics, and the VG0 had an aromatics content of at
least 0.5
grams of aromatics per gram of VG0.
Example 3. Preparation of a K-Fe Sulfide Catalyst in the Absence of
Hydrocarbon
Diluent. A K-Fe sulfide catalyst was prepared by combining 1000 g of iron
oxide and 173
g of potassium carbonate with 423 g of de-ionized water to form a wet paste.
The wet
paste was processed as described in Example 1 to form the intermediate solid.
The
intermediate solid was passed through a 40-mesh sieve with minimal force.
In contrast to Example 2, the intermediate solid was mixed with elemental
sulfur in
the absence of a hydrocarbon diluent. In a dry-box using an argon atmosphere,
the
intermediate solid was mixed with powdered elemental sulfur, placed in a
sealed carbon
steel cylinder, heated to 400 C, and maintained at 400 C for 1 hour. The
sulfurized
catalyst was recovered from the carbon steel reactor as a solid. The potassium-
iron sulfide
catalyst was crushed to a powder using a mortar and pestle such that the
resulting catalyst
powder passed through a 40-mesh sieve.
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
The resulting potassium iron sulfide catalyst was analyzed using x-ray
diffraction
techniques. From analysis of the x-ray diffraction spectrum, it was determined
that the
catalyst included pyrite (FeS2), iron sulfide (FeS), and pyrrhotite (FeiS).
Mixed
potassium-iron sulfide or iron oxide species were not detected using x-ray
diffraction
techniques.
Example 4. Contact of a Crude Feed With a Hydrogen Source in the Presence of a
K-Fe Sulfide Catalyst at an Increased Ratio of Gaseous Hydrogen to Crude Feed.
The apparatus, crude feed, and reaction procedure were the same as in Example
2, except
that the ratio of hydrogen gas to crude feed was 16,000 Nm3/m3 (100,000 SCFB).
The K-
Fe sulfide catalyst (75.0 grams), prepared as described in Example 3, was
charged to the
reactor.
Properties of the crude product produced from this method are summarized in
Table 1 in FIG. 8 and in Table 3 in FIG. 10. The weight percentage of VG0
produced in
Example 4 is greater than the weight percentage of VG0 produced in Example 2.
The
weight percentage of distillate produced in Example 4 is less than the weight
percentage of
distillate produced in Example 2. The API gravity of:the crude product
produced in =:
,µ
Example 4 is lower than the API gravity of the crude product produced in
Example 2. A
higher API gravity indicates hydrocarbons with a higher carbon number were
produced.
After contact with the crude feed, the TMS catalyst in the reactor was
analyzed.
From this analysis, the transition metal sulfide catalyst, after being in the
presence of the
crude feed and hydrogen, included K3Fe10S14.
Exam Ile 5. TAP Testin . of a K2CO3/Rb2CO3/Cs CO3 Catal st and the Individual
Inorganic Salts. In all TAP testing, a 300 mg sample was heated in a reactor
of a TAP
system from room temperature (27 C) to 500 C at a rate of 50 C per minute.
Emitted
water vapor and carbon dioxide gas were monitored using a mass spectrometer of
the TAP
system.
The K2CO3/Rb2CO3/Cs2CO3 catalyst supported on alumina showed a current
inflection of greater than 0.2 volts for emitted carbon dioxide and a current
inflection of
0.01 volts for emitted water from the inorganic salt catalyst at 360 C. The
minimum TAP
temperature was 360 C, as determined by plotting the log 10 of the ion
current versus
temperature. FIG. 11 is a graphical representation of log 10 plots of ion
current of emitted
gases from the K2CO3/Rb2CO3/Cs2CO3 catalyst ("log (I)") versus temperature
("T").
Curves 168 and 170 are log 10 values for the ion currents for emitted water
and CO2 from
66
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
the inorganic salt catalyst. Sharp inflections thr emitted water and CO2 from
the inorganic
salt catalyst occurs at 360 C.
In contrast to the K2CO3/Rb2CO3/Cs2CO3 catalyst, potassium carbonate and
cesium carbonate had non-detectable current inflections at 360 C for both
emitted water
and carbon dioxide.
The substantial increase in emitted gas for the K2CO3/Rb2CO3/Cs2CO3 catalyst
demonstrates that inorganic salt catalysts composed of two or more different
inorganic
salts may be more disordered than the individual pure carbonate salts.
Example 6. DSC Testing of an Inorganic Salt Catalyst and Individual Inorganic
Salts. In all DSC testing, a 10 mg sample was heated to 520 C at a rate of 10
C per min,
cooled from 520 C to 0.0 C at rate of 10 C per minute, and then heated from
0 C to
600 C at a rate of 10.0 C per min using a differential scanning calorimeter
(DSC) Model
DSC-7, manufactured by Perkin-Elmer (Norwalk, Connecticut, U.S.A.).
DSC analysis of a K2CO3/Rb2CO3/Cs2CO3 catalyst during second heating of the
sample shows that the salt mixture exhibited a broad heat transition between
219 C and
260 C. The midpoint of the temperature range was 250 C. The area under heat
=
transition curve was calculated to be -1.75 Joules per gram. The beginning of
crystal
disorder was determined to start at the minimum DSC temperature of 219 C.
In contrast to these results, no definite heat transitions were observed for
cesium
carbonate.
DSC analysis of a mixture of Li2CO3, Na2CO3, and K2CO3 during the second
heating cycle shows that the Li2CO3/Na2CO3/K2CO3 mixture exhibited a sharp
heat
transition between 390 C to 400 C. The midpoint of the temperature range was
385 C.
The area under heat transition curve was calculated to be -182 Joules per
gram. The
beginning of mobility is determined to start at the minimum DSC temperature of
390 C.
The sharp heat transition indicates a substantially homogeneous mixture of
salts.
Example 7. Ionic Conductivity Testing of an Inorganic Salt Catalysts or an
Individual Inorganic Salt Relative to K2CO3. A11 testing was conducted by
placing 3.81
cm (1.5 inches) of the inorganic salt catalysts or the individual inorganic
salts in a quartz
vessel with platinum or copper wires separated from each other, but immersed
in the
sample in a muffle furnace. The wires were connected to a 9.55 volt dry cell
and a
220,000 ohm current limiting resistor. The muffle furnace was heated to 600 C
and the
current was measured using a microammeter.
67
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
FIG. 12 is a graphical representation of log plots of the sample resistance
relative
to potassium carbonate resistance ("log (r K2CO3)") versus temperature ("T").
Curves
172, 174, 176, 178, and 180 are log plots of K2CO3resistance, CaO resistance,
K2CO3/Rb2CO3/Cs2CO3 catalyst resistance, Li2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst
resistance, and Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst resistance, respectively.
CaO (curve 174) exhibits relatively large stable resistance relative to K2CO3
(curve
172) at temperatures in a range between 380-500 C. A stable resistance
indicates an
ordered structure and/or ions that tend not to move apart from one another
during heating.
The K2CO3/Rb2CO3/Cs2CO3 catalyst, Li2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst, and
Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst (see curves 176, 178, and 180) show a
sharp
decrease in resistivity relative to K2CO3 at temperatures in a range from 350-
500 C. A
decrease in resistivity generally indicates that current flow was detected
during application
of voltage to the wires embedded in the inorganic salt catalyst. The data from
FIG. 12
demonstrate that the inorganic salt catalysts are generally more mobile than
the pure
inorganic salts at temperatures in a range from 350-600 'C.
FIG. 13 is a graphical representation of log plots of
Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst resistance relative to K2CO3 resistance
("log (r
K2CO3)") versus temperature ("T"). Curve 182 is a plot of a ratio of
Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst resistance relative to K2CO3 resistance
(curve
172) versus temperature during heating of the Na2CO3/K2CO3/Rb2CO3/Cs2CO3
catalyst.
After heating, the Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst was cooled to room
temperature and then heated in the conductivity apparatus. Curve 184 is a log
plot of
Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst resistance relative to K2CO3 resistance
versus
temperature during heating of the inorganic salt catalyst after being cooled
from 600 C to
25 C. The ionic conductivity of the reheated Na2CO3/K2CO3/Rb2CO3/Cs2CO3
catalyst
increased relative to the ionic conductivity of the original
Na2CO3/K2CO3/Rb2CO3/Cs2CO3
catalyst.
From the difference in ionic conductivities of the inorganic salt catalyst
during the
first heating and second heating, it may be inferred that the inorganic salt
catalyst forms a
different form (a second form) upon cooling that is not the same as the form
(a first form)
before any heating.
Example 8. Flow Property Testing of an Inorganic Salt Catalyst. A 1-2 cm thick
layer of powdered K2CO3/Rb2CO3/Cs2CO3 catalyst was placed in a quartz dish.
The dish
was placed in a furnace and heated to 500 C for 1 hour. To determine flow
properties of
68
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
the catalyst, the dish was manually tilted in the oven after heating. The
K2CO3/Rb2CO3/Cs2CO3 catalyst did not flow. When pressed with a spatula, the
catalyst
had a consistency of taffy.
In contrast, the individual carbonate salts were free flowing powders under
the
same conditions.
A Na2CO3/K2CO3/Rb2CO3/Cs2CO3 catalyst became liquid and readily flowed
(similar, for example, to water) in the dish under the same conditions.
Examples 9 - 10: Contact of a Crude Feed With a Hydrogen Source in the
Presence
of a 1C2CO3/1kÇLÇÇ!3 Catalyst 1 Steam. The following equipment and
general procedure was used in Examples 9-27 except where variations are
described.
Reactor: A 250 mL Hastelloy C Parr Autoclave (Parr Model #4576) rated at 35
MPa
working pressure (5000 psi) at 500 C, was fitted with a mechanical stirrer
and an 800
watt Gaumer band heater on a Eurotherm controller capable of maintaining the
autoclave
at + 5 C from ambient to 625 C, a gas inlet port, a steam inlet port, one
outlet port, and a
thermocouple to register internal temperature. Prior to heating, the top of
the autoclave
was insulated with glass cloth.
Addition Vessel: An addition vessel (a 250 mL, 316 stainless steel hoke
vessel) was
equipped with a controlled heating system, suitable gas control valving, a
pressure relief
device, thermocouples, a pressure gauge, and a high temperature control valve
(Swagelok
Valve # SS-4UW) capable of regulating flow of a hot, viscous, and/or
pressurized crude
feed at a flow rate from 0-500 g/min. An outlet side of the high temperature
control valve
was attached to the first inlet port of the reactor after crude feed was
charged to the
addition vessel. Prior to use, the addition vessel line was insulated.
Product Collection: Vapor from the reactor exited the outlet port of the
reactor and was
introduced into a series of cold traps of decreasing temperatures (dip tubes
connected to a
series of 150 mL, 316 stainless steel hoke vessels). Liquid from the vapor was
condensed
in the cold traps to form a gas stream and a liquid condensate stream. Flow
rate of the
vapor from the reactor and through the cold traps was regulated, as needed,
using a back
pressure regulator. A rate of flow and a total gas volume for the gas stream
exiting the
cold traps were measured using a wet test meter (Ritter Model # TG 05 Wet Test
Meter).
After exiting the wet test meter, the gas stream was collected in a gas bag (a
Tedlar gas
collection bag) for analysis. The gas was analyzed using GC/MS (Hewlett-
Packard Model
5890, now Agilent Model 5890; manufactured by Agilent Technologies, Zion
Illinois,
U.S.A.). The liquid condensate stream was removed from the cold traps and
weighed.
69
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
Crude product and water were separated from the liquid condensate stream. The
crude
product was weighed and analyzed.
Procedure: Cerro Negro (137.5 grams) was charged to the addition vessel. The
crude
feed had an API gravity of 6.7. The crude feed had, per gram of crude feed, a
sulfur
content of 0.042 grams, a nitrogen content of 0.011 grams, and a total NiN
content of
0.009 grams. The crude feed was heated to 150 C. The K2CO3/Rb2CO3/Cs2CO3
catalyst
(31.39 grams) was charged to the reactor.
The K2CO3/Rb2CO3/Cs2CO3 catalyst was prepared by combining of 16.44 grams
of K2CO3, 19.44 grams of Rb2CO3, and 24.49 grams of Cs2CO3. The
K2CO3/Rb2CO3/Cs2CO3 catalyst had a minimum TAP temperature of 360 C. The
K2CO3/Rb2CO3/Cs2CO3 catalyst had a DSC temperature of 250 C. The individual
salts
(K2CO3, Rb2CO3, and Cs2CO3) did not exhibit DSC temperatures in a range from
50-500
C. This TAP temperature is above the DSC temperature of the inorganic salt
catalyst and
below the DSC temperature of the individual metal carbonates.
The catalyst was heated rapidly to 450 C under an atmospheric pressure flow
of
methane of 250 cm3/min. After reaching the desired reaction temperature, steam
at a rate
of 0.4 mL/min, and methane at rate of 250 cm3/min, was metered to the reactor.
The
steam and methane were continuou ly metered during the addition of the crude
feed to the
reactor over 2.6 hours. The crude feed was pressurized into the reactor using
1.5 MPa
(229 psi) of CH4 over 16 minutes. Residual crude feed (0.56 grams) remained in
the
addition vessel after the addition of the crude feed was complete. A decrease
in
temperature to 370 C was observed during the addition of the crude feed.
The catalyst/crude feed mixture was heated to a reaction temperature of 450 C
and
maintained at that temperature for 2 hours. After two hours, the reactor was
cooled and
the resulting residue/catalyst mixture was weighed to determine a percentage
of coke
produced and/or not consumed in the reaction.
From a difference in initial catalyst weight and coke/catalyst mixture weight,
0.046
grams of coke remained in the reactor per gram of crude feed. The total
product included
0.87 grams of a crude product with an average API gravity of 13 and gas. The
gas
included unreacted CH4, hydrogen, C2 and C4-C6 hydrocarbons, and CO2 (0.08
grams of
CO2 per gram of gas).
The crude product had, per gram of crude product, 0.01 grams of sulfur and
0.000005 grams of a total Ni and V. The crude product was not further
analyzed.
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In Example 10, the reaction procedures, conditions, crude feed, and catalyst
were
the same as in Example 9. The crude product of Example 10 was analyzed to
determine
boiling range distributions for the crude product. The crude product had, per
gram of
crude product, 0.14 grams of naphtha, 0.19 grams of distillate, 0.45 grams of
VGO, and
residue content of 0.001 grams, and non-detectable amounts of coke.
Examples 9 and 10 demonstrate that contact of the crude feed with a hydrogen
source in the presence of at most 3 grams of catalyst per 100 grams of crude
feed produces
a total product that includes a crude product that is a liquid mixture at STP.
The crude
product had a residue content of at most 30% of the residue content of the
crude feed. The
crude product had a sulfur content and total NiN content of at most 90% of the
sulfur
content and NiN content of the crude feed.
The crude product included at least 0.001 grams of hydrocarbons with a boiling
range distribution of at most 200 C at 0.101 MPa, at least 0.001 grams of
hydrocarbons
with a boiling range distribution between 200-300 C at 0.101 MPa, at least
0.001 grams
of hydrocarbons with a boiling range distribution between 400-538 C (1000 F)
at 0.101
MPa.
Examples 11-12: Contact of a Crude Feed with a Hydrogen Source in the Presence
of the I( tMa1/3nl&emli. The reaction procedures,
conditions, and the K2CO3/Rb2CO3/Cs2CO3 catalyst in Examples 11 and 12 were
the same
as in Example 9, except that 130 grams of crude feed (Cerro Negro) and 60
grams of the
K2CO3/Rb2CO3/Cs2CO3 catalyst were used. In Example 11, methane was used as the
hydrogen source. In Example 12, hydrogen gas was used as the hydrogen source.
A
graphical representation of the amounts of non-condensable gas, crude product,
and coke
is depicted in FIG. 14. Bars 186 and 188 represent wt% coke produced, bars 190
and 192
represent wt% liquid hydrocarbons produced, and bars 194 and 196 represent wt%
gas
produced, based on the weight of the crude feed.
In Example 11, 93 wt% of crude product (bar 192), 3 wt% of gas (bar 196), and
4
wt% of coke (bar 188), based on the weight of the Cerro Negro, was produced.
In Example 12, 84 wt% of crude product (bar 190), 7 wt% of gas (bar 194), and
9
wt% of coke were produced (bar 186), based on the weight of the Cerro Negro.
Examples 11 and 12 provide a comparison of the use of methane as a hydrogen
source to the use of hydrogen gas as a hydrogen source. Methane is generally
less
expensive to produce and/or transport than hydrogen, thus a process that
utilizes methane
is desirable. As demonstrated, methane is at least as effective as hydrogen
gas as a
71
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
hydrogen source when contacting a crude feed in the presence of an inorganic
salt catalyst
to produce a total product.
Examples 13-14: Producing a Crude Product with a Selected API Gravity. The
apparatus, reaction procedure and the inorganic salt catalyst were the same as
in Example
9, except that the reactor pressure was varied.
Example 13, the reactor pressure was 0.1 MPa (14.7 psi) during the contacting
period. A crude product with API gravity of 25 at 15.5 C was produced. The
total
product had hydrocarbons with a distribution of carbon numbers in a range from
5 to 32
(see curve 198 in FIG. 15).
In Example 14, the reactor pressure was 3.4 MPa (514.7 psi) during the
contacting
period. A crude product with API gravity of 51.6 at 15.5 C was produced. The
total
product had hydrocarbons with a distribution of carbon numbers in a range from
5 to 15
(see curve 200 in FIG. 15).
These examples demonstrate methods for contacting the crude feed with hydrogen
in the presence of an inorganic salt catalyst at various pressures to produce
a crude product
with a selected API gravity. By varying the pressure, a crude product with a
higher or
=
lower API gravity was produced.
Examples 15-16: Contact of a Crude Feed in the Presence of a
1(CO3/R13/CO3/Cs.2CO3Cata1yst or Silicon Carbide in the Absence of an External
Hydrogen Source. In Examples 15 and 16, the apparatus, crude feed, and
reaction
procedure were the same as in Example 9, except that the crude feed and
catalyst (or
silicon carbide) were directly charged into the reactor at the same time.
Carbon dioxide
(CO2) was used as a carrier gas. In Example 15, 138 grams of Cerro Negro was
combined
with 60.4 grams of the K2CO3/Rb2CO3/Cs2CO3 catalyst (same catalyst as in
Example 9).
In Example 16, 132 g of Cerro Negro was combined with 83.13 grams of silicon
carbide
(40 mesh, Stanford Materials; Aliso Viejo, CA). Such silicon carbide is
believed to have
low, if any, catalytic properties under the process conditions described
herein.
In each example, the mixture was heated to a reaction temperature of 500 C
over a
period of 2 hours. The CO2 was metered into the reactor at a rate of 100
cm3/min. Vapor
generated from the reactor was collected in the cold traps and a gas bag using
a back
pressure of 3.2 MPa (479.7 psi). Crude product from the cold traps was
consolidated and
analyzed.
72
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In Example 15, 36.82 grams (26.68 wt%, based on the weight ot the crude teed)
ot
a colorless hydrocarbon liquid with API gravity of at least 50 was produced
from contact
of the crude feed with the inorganic salt catalyst in the carbon dioxide
atmosphere.
In Example 16, 15.78 grams (11.95 wt%, based on the weight of the crude feed)
of
a yellow hydrocarbon liquid with an API gravity of 12 was produced from
contact of the
crude feed with silicon carbide in the carbon dioxide atmosphere.
Although the yield in Example 15 is low, the in-situ generation of a hydrogen
= source in the presence of the inorganic salt catalyst is greater than the
in-situ generation of
hydrogen under non-catalytic conditions. The yield of crude product in Example
16 is
one-half of the yield of crude product in Example 15. Example 15 also
demonstrates that
hydrogen is generated during contact of the crude feed in the presence of the
inorganic salt
and in the absence of a gaseous hydrogen source.
Examples 17-20: Contact of a Crude Feed with a Hydrogen Source in the Presence
of K CO3/Rb2CO3/Cs2CO3 Catalyst,Calcium Oxide and Silicon Carbide at
Atmospheric Conditions. The apparatus, reaction procedure, crude feed and the
inorganic salt catalyst were the same as in Example 9, except that the Cerrb
Negro was ,=
added directly to the reactor instead 'of addition through the addition vessel
and hydrogen =
gas was used as the hydrogen source. The reactor pressure was 0.101 MPa (14.7
psi) =
during the contacting period.. = The hydrogen gas flow rate was 250 cm3/min.
Reaction
temperatures, steam flow rates, and percentages of crude product, gas, and
coke produced
are tabulated in Table 4 in FIG. 16.
In Examples 17 and 18, the K2CO3/Rb2CO3/Cs2CO3 catalyst was used. In
Example 17, the contacting temperature was 375 C. In Example 18, the
contacting
temperature was in a temperature range from 500-600 C.
As shown in Table 4 (FIG. 16), for Examples 17 and 18, when the temperature
was
increased from 375 C to 500 C, production of gas increased from 0.02 grams
to 0.05
grams of gas per gram of total product. Coke production, however, decreased
from 0.17
grams to 0.09 grams of coke per gram of crude feed at the higher temperature.
The sulfur
content of the crude product also decreased from 0.01 grams to 0.008 grams of
sulfur per
gram of crude product at the higher temperature. Both crude products had H/C
of 1.8.
In Example 19, a crude feed was contacted with CaCO3 under conditions similar
to
the conditions described for Example 18. Percentages of crude product, gas,
and coke
production are tabulated in Table 4 in FIG. 16. Gas production increased in
Example 19
relative to the gas production in Example 18. Desulfurization of the crude
feed was not as
73
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
effective as in Example 18. The crude product produced in Example 19 had, per
gram of
crude product, 0.01 grams of sulfur as compared to the sulfur content of 0.008
grams per
gram of crude product for the crude product produced in Example 18.
Example 20 is a comparative example for Example 18. In Example 20, 83.13
grams of silicon carbide instead of the inorganic salt catalyst was charged to
the reactor.
Gas production and coke production significantly increased in Example 20
relative to the
gas production and coke production in Example 18. Under these non-catalytic
conditions,
0.22 grams of coke per gram of crude product, 0.25 grams of non-condensable
gas, and
0.5 grams of crude product were produced. The crude product produced in
Example 20
had 0.036 grams of sulfur per gram of crude product, compared to of 0.01 grams
of sulfur
per gram of crude product produced in Example 18.
These examples demonstrated that the catalysts used in Examples 17 and 18
provide improved results over non-catalytic conditions and conventional metal
salts. At
500 C, and a hydrogen flow rate of 250 cm3/min, the amounts of coke and non-
condensable gas were significantly lower than the amounts of coke and of non-
condensable gas produced under non-catalytic conditions.
In examples using inorganic salt catalysts (See Examples 17-18 in Table 4,
FIG.'
' 16), a decrease was observed in the weight percent of produced gas relative
to the
produced gas formed during the control experiment (for example, Example 20 in
Table 4,
FIG. 16). From the quantity of hydrocarbons in the produced gas, the thermal
cracking of
the crude feed is estimated to be at most 20 wt%, at most 15 wt%, at most 10
wt%, at most
5 wt%, or none, based on the total amount of crude feed contacted with a
hydrogen source.
Examples 21 and 22: Contact of a Crude Feed with a Gaseous Hydrogen Source In
the Presence of Water and a K2CO3/11b2CO3/Cs2CO3 Catalyst or Silicon Carbide.
Apparatus in Examples 21 and 22 were the same as in Example 9 except that
hydrogen gas
was used as the hydrogen source. In Example 21, 130.4 grams of Cerro Negro was
combined with 30.88 grains of the K2CO3/Rb2CO3/Cs2CO3 catalyst to form a crude
feed
mixture. In Example 22, 139.6 grams of Cerro Negro was combined with 80.14
grams of
silicon carbide to form the crude feed mixture.
The crude feed mixture was charged directly into the reactor. The hydrogen gas
was metered at 250 cm3/min into the reactor during the heating and holding
periods. The
crude feed mixture was heated to 300 C over 1.5 hours and maintained at 300
C for 1
hour. The reaction temperature was increased to 400 C over 1 hour and
maintained at
400 C for 1 hour. After the reaction temperature reached 400 C, water was
introduced
74
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
into the reactor at a rate ot 0.4 g/min in combination with the hydrogen gas.
Water and
hydrogen were metered into the reactor for the remaining heating and holding
periods.
After maintaining the reaction mixture at 400 C, the reaction temperature was
increased
to 500 C and maintained at 500 C for 2 hours. Generated vapor from the
reactor was
collected in the cold traps and a gas bag. Liquid product from the cold traps
was
consolidated and analyzed.
In Example 21, 86.17 grams (66.1 wt%, based on the weight of the crude feed)
of a
dark reddish brown hydrocarbon liquid (crude product) and water (97.5 g) were
produced
as a vapor from contact of the crude feed with the K2CO3/Rb2CO3/Cs2CO3
catalyst in the
hydrogen atmosphere.
In Example 22, water vapor and a small amount of gas was produced from the
reactor. The reactor was inspected, and a dark brown viscous hydrocarbon
liquid was
removed from the reactor. Less than 50 wt% of the dark brown viscous liquid
was
produced from contact of the crude feed with silicon carbide in the hydrogen
atmosphere.
A 25% increase in yield of crude product was observed in Example 21 relative
to a yield
of crude product produced in Example 22.
Example 21 demonstrates an improvement of the properties of the crude product
,
produced using methods described herein relative to a crude product produced
using hot
water. Specifically, the crude product in Example 21 was lower boiling than
the crude '
product from Example 22, as demonstrated by the crude product produced in
Example 22
not being able to be produced as a vapor. The crude product produced in
Example 21 had
enhanced flow properties relative to the crude product produced in Example 22,
as
determined by visual inspection.
Examples 23- 24: Contact of a Crude Feed with a Hydrogen Source in the
Presence
of a K2CO3/Rb2CO3/Cs2CO3 Catalyst to Produce a Crude Product with Increased
Volume Relative to a Crude Product Volume Produced under Non-Catalytic
Conditions. The apparatus, crude feed, inorganic catalyst, and reaction
procedure was the
same as described in Example 9, except the crude feed was directly charged to
the reactor
and hydrogen gas was used as the hydrogen source. The crude feed (Cerro Negro)
had an
API gravity 6.7 and a density of 1.02 g/mL at 15.5 C.
In Example 23, 102 grams of the crude feed (100 mL of crude feed) and 31 grams
of K2CO3/Rb2CO3/Cs2CO3 catalyst were charged to the reactor. A crude product
(87.6
grams) with an API gravity of 50 and a density of 0.7796 g/mL at 15.5 C (112
mL) was
produced.
CA 02548838 2006-06-08
WO 2005/066302
PCT/US2004/042120
In Example 24, 102 grams ot crude teed (100 mL ot crude teed.) ancl su grams
ot
silicon carbide were charged to the reactor. A crude product (70 grams) of
with an API
gravity of 12 and a density of 0.9861 g/mL at 15.5 C (70 mL) was produced.
Under these conditions, the volume of the crude product produced from Example
23 was approximately 10% greater than the volume of the crude feed. The volume
of the
crude product produced in Example 24 was significantly less (40% less) than
the volume
of crude product produced in Example 23. A significant increase in volume of
product
enhances a producer's ability to generate more volume of crude product per
volume of
input crude.
Example 25. Contact of a Crude Feed with a Hydrogen Source in the Presence of
a
K CO3/Rb CO3/Cs CO3 Catal st Sulfur, and Coke. The apparatus and reaction
procedure were the same as in Example 9, except that the steam was metered
into the
reactor at 300 cm3/min. The K2CO3/Rb2CO3/Cs2CO3 catalyst was prepared by
combining
27.2 grams of K2CO3, 322 grams of Rb2CO3 and 40.6 grams of Cs2CO3.
The crude feed (130.35 grams) and K2CO3/Rb2CO3/Cs2CO3 catalyst (31.6 grams)
was charged to the reactor. The Cerro Negro crude included, per gram of crude
feed, 0.04
grams total aromatics content in a boiling range distribution between 149-260
C (300,500
= F), 0.000640 grains of nickel and vanadium combined, 0.042 grams of
sulfur, and 0.56
grams of residue. API gravity of the crude feed was 6.7.
Contact of the crude feed with methane in the presence of the
K2CO3/Rb2CO3/Cs2CO3 catalyst produced, per gram of crude feed, 0.95 grams of
total
product, and 0.041 grams of coke.
The total product included, per gram of total product, 0.91 grams of crude
product
and 0.028 grams of hydrocarbon gas. The total gas collected included, per mole
of gas,
0.16 moles of hydrogen, 0.045 moles of carbon dioxide, and 0.025 moles of C2
and C4-C6
hydrocarbons, as determined by GC/MS. The balance of the gas was methane, air,
carbon
monoxide, and a trace (0.004 moles) of evaporated crude product.
The crude product was analyzed using a combination of gas chromatography and
mass spectrometry. The crude product included a mixture of hydrocarbons with a
boiling
range between 100-538 C. The total liquid product mixture included 0.006
grams ethyl
benzene (a monocyclic ring compound with a boiling point of 136.2 C at 0.101
MPa) per
gram of mixture. This product was not detected in the crude feed.
The used catalyst ("first used catalyst") was removed from the reactor,
weighed,
and then analyzed. The first used catalyst had an increase in weight from 31.6
grams to a
76
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
total weignt or /.36 grams an increase or 16 Wt/o, nasea on tne weignt or tne
original
K2CO3/Rb2CO3/Cs2CO3 catalyst). The first used catalyst included 0.15 grams of
additional coke, 0.0035 grams of sulfur, 0.0014 grams of NiN, and 0.845 grams
of
K2CO3/Rb2CO3/Cs2CO3 per gram of used catalyst.
Additional crude feed (152.71 grams) was contacted with the first used
catalyst
(36.63 grams) to produce 150 grams of recovered total product after losses.
The total
product included, per gram of total product, 0.92 grams of liquid crude
product, 0.058
grams of additional coke, and 0.017 grams of gas. The gas included, per mole
of gas, 0.18
moles of hydrogen, 0.07 grams of carbon dioxide, and 0.035 moles of C2-C6
hydrocarbons. The balance of the gas was methane, nitrogen, some air, and
traces of
evaporated oil product (<1% mole).
The crude product included a mixture of hydrocarbons with a boiling range
between 100-538 C. The portion of the mixture with a boiling range
distribution below
149 C included, per mole of total liquid hydrocarbons, 0.018 mole% of ethyl
benzene,
0.04 mole% of toluene, 0.03 mole% of meta-xylene, and 0.060 mole% of para-
xylene
(monocyclic ring compounds with a boiling points below 149 C at 0.101 MPa).
These
products were not detectable in the crude feed.
The used catalyst ("second used catalyst") was removed from the reactor,
weighed;
and then analyzed. The second used catalyst had an increase in weight from
36.63 grams
to a total weight of 45.44 grams (an increase of 43 wt%, based on the weight
of the
original K2CO3/Rb2CO3/Cs2CO3 catalyst). The second used catalyst included 0.32
grams
of coke, and 0.01 grams of sulfur, and 0.67 grams per gram' of second used
catalyst.
Additional crude feed (104 grams) was contacted with the second used catalyst
(44.84 grams) to produce, per gram of crude feed, 104 grams of total product
and 0.114
grams of coke was collected. A portion of the coke was attributed to coke
formation in the
addition vessel due to overheating the addition vessel since 104.1 grams of-
the 133 grams
of crude feed transferred was crude feed.
The total product included, per gram of total product, 0.86 grams of crude
product
and 0.025 grams of hydrocarbon gas. The total gas included, per mole of gas,
0.18 moles
of hydrogen, 0.052 moles of carbon dioxide, and 0.03 moles of C2-C6
hydrocarbons. The
balance of the gas was methane, air, carbon monoxide, hydrogen sulfide, and a
small trace
of evaporated oil.
The crude product included a mixture of hydrocarbons with a boiling range
between 100-538 C. The portion of the mixture with a boiling range
distribution below
77
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
149 'C included, per gram ot hydrocarbon mixture, 0.021 grams ethyl benzene,
0.027
grams of toluene, 0.042 grams of meta-xylene, and 0.020 grams of para-xylene,
determined as before by GC/MS.
The used catalyst ("third used catalyst") was removed from the reactor,
weighed,
and then analyzed. The third used catalyst had an increase in weight from
44.84 grams to
a total weight of 56.59 grams (an increase of 79 wt%, based on the weight of
the original
K2CO3/Rb2CO3/Cs2CO3 catalyst). Detailed elemental analysis of the third used
catalyst
was performed. The third used catalyst included, per gram of additional
matter, 0.90
grams of carbon, 0.028 grams of hydrogen, 0.0025 grams of oxygen, 0.046 grams
of
sulfur, 0.017 grams of nitrogen, 0.0018 grams of vanadium, 0.0007 grams of
nickel,
0.0015 grams of iron, and 0.00025 grams of chloride with the balance being
other
transition metals such as chromium, titanium and zirconium.
As demonstrated in this example, coke, sulfur, and/or metals deposited on
and/or in
the inorganic salt catalyst do not affect the overall yield of crude product
(at least 80% for
each run) produced by contact of a crude feed with a hydrogen source in the
presence of
the inorganic salt catalyst. The crude product had a monocyclic aromatics
content at least
100 times the monocyclic ring aromatics content of the crude feed in a boiling
range
distribution below 149 C.
For the three runs, the average crude product yield (based on the weight of
the
crude feed) was 89.7 wt%, with a standard deviation of 2.6%; the average coke
yield was
7.5 wt% (based on the weight of the crude feed), with a standard deviation of
2.7%, and
the average weight yield of gaseous cracked hydrocarbons was 2.3 wt% (based on
the
weight of the crude feed) with a standard deviation of 0.46%. The
comparatively large
standard deviation of both liquid and coke was due to the third trial, in
which the
temperature controller of the feed vessel failed, overheating the crude feed
in the addition
vessel. Even so, there is no apparent significant deleterious effect of even
the large
amounts of coke tested here on the activity of the catalyst system.
The ratio of C2 olefins to total C2 was 0.19. The ratio of C3 olefin to total
C3 was
0.4. The alpha olefins to internal olefins ratio of the C4 hydrocarbons was
0.61. The C4
cis/trans olefins ratio was 6.34. This ratio was substantially higher than the
predicted
thermodynamic C4 cis/trans olefins ratio of 0.68. The alpha olefins to
internal olefins ratio
of the C5 hydrocarbons was 0.92. This ratio was greater than the predicted
thermodynamic C5 alpha olefins to C5 internal olefins ratio of 0.194. The C5
cis/trans
78
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
olefins ratio was 1.25. This ratio was greater than the predicted
thermodynamic C5
cis/trans olefins ratio of 0.9.
Example 26: Contact of a Relatively High Sulfur Containing Crude Feed with a
Hydrogen the of the K2CO3/Thb CO3/Cs CO3 Catalyst. The
apparatus and reaction procedure were the same as described in Example 9,
except that the
crude feed, methane, and steam were continuously fed to the reactor. The level
of feed in
the reactor was monitored using a change in weight of the reactor. Methane gas
was
continuously metered at 500 cm3/min to the reactor. Steam was continuously
metered at 6
g/min to the reactor.
The inorganic salt catalyst was prepared by combining 27.2 grams of K2CO3,
32.2
grams of Rb2CO3 and 40.6 grams of Cs2CO3. The K2CO3/Rb2CO3/Cs2CO3 catalyst
(59.88
grams) was charged to the reactor.
A crude feed (bitumen, Lloydminster, Canada) having an API gravity of 9.4, a
sulfur content of 0.02 grams of sulfur, and a residue content of 0.40 grams,
per grain of
crude feed, was heated in the addition vessel to 150 C. The hot bitumen was
continuously metered from the addition vessel at 10.5 g/min to the reactor in
an attempt to
maintain the crude feed liquid level .of 50% of the reactor volume, however,
the rate was
insufficient to maintain that level.
The methane/steam/crude feed was contacted with the catalyst at an average
internal reactor temperature of 456 C. Contacting of the methane/steam/crude
feed with
the catalyst produced a total product (in this example in the form of the
reactor effluent
vapor).
A total of 1640 grams of crude feed was processed over 6 hours. From a
difference in initial catalyst weight and residue/catalyst mixture weight,
0.085 grams of
coke per gram of crude feed remained in the reactor. From contact of the crude
feed with
the methane in the presence of the K2CO3/Rb2CO3/Cs2CO3 catalyst, 0.93 grams of
total
product per grain of crude feed was produced. The total product included, per
gram of
total product, 0.03 grams of gas and 0.97 grams of crude product, excluding
the amount of
methane and water used in the reaction.
The gas included, per gram of gas, 0.014 grams of hydrogen, 0.018 grams of
carbon monoxide, 0.08 grams of carbon dioxide, 0.13 grams of hydrogen sulfide,
and 0.68
grams of non-condensable hydrocarbons. From the amount of hydrogen sulfide
generated,
it may be estimated that the sulfur content of the crude feed was reduced by
18 wt%. As
79
CA 02548838 2006-06-08
WO 2005/066302 PCT/US2004/042120
shown in this example, hydrogen, carbon monoxide, and carbon dioxide were
produced.
The molar ratio of carbon monoxide to carbon dioxide was 0.4.
The C2-05 hydrocarbons included, per gram of hydrocarbons, 0.30 grams of C2
compounds, 0.32 grams of C3 compounds, 0.26 grams of C4 compounds, and 0.10
grams
of C5 compounds. The weight ratio of iso-pentane to n-pentane in the non-
condensable
hydrocarbons was 0.3. The weight ratio of isobutane to n-butane in the non-
condensable
hydrocarbons was 0.189. The C4 compounds had, per gram of C4 compounds, a
butadiene
content of 0.003 grams. A weight ratio of alpha C4 olefins to internal C4
olefins was 0.75.
A weight ratio of alpha C5 olefins to internal C5 olefins was 1.08.
The data in Example 25 demonstrates that continuous processing of a
relatively.
high sulfur crude feed with the same catalyst in the presence of coke did not
diminish the
activity of the inorganic salt catalyst, and produced a crude product suitable
for
transportation.
Example 27: Contact of a Crude Feed with a Hydrogen Source in the Presence of
a
l_i2C0323 CO3n2CO3ELtAtALId Coke. The apparatus and reaction procedure was
performed using conditions as described in Example 26. The K2CO3/Rb2CO3/Cs2CO3
catalyst (56.5 grams) was charged to the reactor. A total of 2550 grams of
crude feed was
processed over 6 hours. From a difference in initial catalyst weight and
residue/catalyst
mixture weight, 0.114 grams of coke per gram of crude feed remained in the
reactor, based
on the weight of the crude feed. A total of 0.89 grams of total product per
gram of crude
feed was produced. The total product included, per gram of total product, 0.04
grams of
gas and 0.96 grams of crude product, excluding the amount of methane and water
used in
the reaction.
The gas included, per gram of gas, 0.021 grams of hydrogen, 0.018 grams of
carbon monoxide, 0.052 grams of carbon dioxide, 0.18 grams of hydrogen
sulfide, and
0.65 grams of non-condensable hydrocarbons. From the amount of hydrogen
sulfide
produced, it may be estimated that the sulfur content of the crude feed was
reduced by 14
wt%, based on the weight of the crude feed. As shown in this example,
hydrogen, carbon
monoxide, and carbon dioxide were produced. The molar ratio of carbon monoxide
to
carbon dioxide was 0.6.
The C2-C6 hydrocarbons included, per gram of C2-C6 hydrocarbons, 0.44 grams of
C2 compounds, 0.31 grams of C3 compounds, 0.19 grams of C4 compound and 0.068
grams of C5 compounds. The weight ratio of iso-pentane to n-pentane in the non-
condensable hydrocarbons was 0.25. The weight ratio of iso-butane to n-butane
in the
CA 02548838 2012-01-10
non-condensable hydrocarbons was 0.15. The C4 compounds had, per gram of C4
compounds, a butadiene content of 0.003 grams.
This example demonstrates that repeated processing of the a relatively high
sulfur
crude feed (2550 grams of crude feed) with the same catalyst (56.5 grams) in
the presence
of coke did not diminish the activity of the inorganic salt catalyst, and
produced a crude
product suitable for transportation.
The scope of the claims should not be limited by the preferred embodiments set
forth in the examples, but should be given the broadest interpretation
consistent with the
description as a whole.
81