Note: Descriptions are shown in the official language in which they were submitted.
CA 02551561 2006-07-14
1
DRY-POWDER INHALER
FIELD OF THE INVENTION
This invention relates to a medical device for dry-powder drug inhalation.
Specifically, the present invention is a single-step inhaler where the act of
inhalation
releases the powder from a compressed solid form so that it can be inhaled
into the
lungs.
BACKGROUND OF THE INVENTION
Numerous drugs, medications and other substances are inhaled into the
lungs for rapid absorption in the blood stream. Inhaled drugs fall into two
main
categories: (1) liquids, including suspensions; and (2) powders. The present
invention relates to the latter category.
Dry-powder inhalers need to deliver a particle size that is predominantly
below 5 microns for maximum effectiveness. Such small particles are, however,
thermodynamically unstable due to their high surface area to volume ratio,
which
provides significant excess surface free energy and encourages particles to
agglomerate. In the inhaler, agglomeration of small particles and adherence of
particles to the walls of the inhaler are problems that result in the active
particles
leaving the inhaler as large agglomerates or being unable to leave the inhaler
and
remaining adhered to the interior of the inhaler. In an attempt to improve
that
situation, dry powders for use in dry powder inhalers often include particles
of an
excipient material mixed with the fine particles of active material. Fine
particles of
active material suitable for pulmonary administration have often been prepared
by
milling, for example, jet milling. However, once the particles reach a minimum
size
referred to as the critical size, they re-combine at the same rate as being
fractured,
or do not fracture effectively and therefore do not reduce further in size.
Thus,
manufacture of fine particles by milling can require much effort and there are
factors, which consequently place limits on the minimum size of particles of
active
material which can be achieved, in practice, by such milling processes.
Accordingly, one approach to the issue of maintaining a sub 5 micron particle
from dry-powder type inhalers is to use particles of an excipient material
mixed with
the fine particles of the active ingredient. For example, published
application
20040037785 describes a method of making particles for use in a pharmaceutical
composition for pulmonary administration, the method comprising a milling step
in
CA 02551561 2006-07-14
2
which particles of active material are milled in the presence of particles of
an
additive material which is suitable for the promotion of the dispersal of the
composite active particies upon actuation of an inhaler.
Another approach to this problem which is addressed in the prior art is the
production of the requisite powder size by means of scraping from a compressed-
powder directly before inhalation. US 5,617,845 describes an inhalation device
free
from propellent gas with a storage chamber for a powdered substance to be
inhaled.
This device employs a trigger-operated pump which can be manually primed
before
the inhalation process by means of a button and which can be actuated in
synchronism with the breathing, thereby generating a current of foreign air
which
disperses the metered substance. In this device, metering is carried out by
means
of a specially shaped metering notch as a metering chamber in the metering
punch,
which is rotated past a slightly compressed-powder charge. In this device,
although
compressed-powder is used, the step of scraping away the powder from the
compressed-powder is a preparatory step, where the inhalation of the powder
dose
is then performed in a second and separate step. In further prior art, US
5,887,586
describes a dry-powder aerosol generator, which is connected to a removable
nose
mask via a conduit system. The aerosol generator comprises a scraping
mechanism, by means of which powder can be scraped off a tablet of compressed-
powder, as well as means for aerosolizing the scraped-off powder in an air
flow.
Further prior art includes the Turbuhaler inhaler device (AstraZeneca PLC,
London,
UK) in which a dose of drug is scraped from a solid micronized drug matrix by
the
patient twisting the base of the device, prior to inhalation. However, in all
these
cases, the act of inhalation does not by itself cause the fine powder to be
scraped
away from the tablet of compressed-powder. On the contrary, the scraping of
powder from a reservoir of compressed-powder is a separate process, and one in
which complex generator elements are sometimes required.
This separation of the scraping process from the inhalation process in turn
leads to two additional problems with prior art devices: (1) the powder tends
to spill
prior to use if the inhaler is shaken; and (2) the dose may be lost if the
user blows
into the device rather than inhales.
CA 02551561 2006-07-14
.
3
In view of these drawbacks and limitations of the prior art, what is needed is
a
simple and inexpensive inhaler without gas or other complex generators,
capable of
consistently delivering predominantly sub 5 micron particle sizes.
Therefore, it is an object of the invention to provide a simple, breath-
powered
inhaler where the act of inhalation causes the dry-powder to be scraped off a
compressed-powder volume.
It is a further object of the invention to provide a convenient and portable
housing for said inhaler.
It is a still further object of the invention to provide said specially
designed
device in a credit-card format.
It is a still further object of the invention to provide an ergonomic
mouthpiece
for miniature device, where said mouthpiece can be stored within a credit-card
format device.
It is a still further object of the invention to provide a dry-powder inhaler
which
synchronizes the drug release with the inhalation action of the patient, while
spreading the delivery over a defined duration of the breath and controlling
for
particle size.
It is further the object of the invention to provide a device that enables the
transporting of the drug separate from the device such that the patient can
load said
drug into the device.
It is further the object of the invention to provide a device that is
indifferent to
accidental air-blow into the device.
These and other objects of the present invention are achieved in the
preferred embodiments disclosed below by providing a breath-powered dry-powder
inhaler.
SUMMARY OF THE INVENTION
The inhaler device of the present invention provides an improved and
simplified mechanism for dry-powder drug inhalation, which ensures the
synchronization of fine-particle release during inhalation. The operating
principle of
said device is that the act of inhalation itself causes fine powder to be
scratched or
rubbed away from the surface of a compressed-powder volume, where the thus
released powder is inhaled directly. Advantageously, such an approach is
inherently free of the problems of prior art devices where a powder dose can
be
CA 02551561 2006-07-14
. . .
4
spilled or where exhaling into the device can disturb the powder. As the
powder for
inhalation is only produced during the inhalation, the synchronization of the
powder
inhalation with the breath is achieved inherently in this design. Depending on
the
drug type, said synchronization with the inhalation curve is extremely
important in
order to ensure that the drug is delivered to the required areas of the lungs.
Thus,
for several drugs, a too early or too late delivery results in extremely low
efficiency
of the administration, which in turn can affect the results of the treatment
and even
limit the use of certain devices from critical drugs. Additionally, in many
cases, a
pre-determined delay of the drug discharge to a certain point in the
inhalation curve
and the release of the drug over a defined period of that inhalation curve
(rather
than in a bolus) provides optimal results. By having the drug in a one-piece
form in
the device the management of the drug in the device become simpler and thus
enables a simpler and more compact mechanism.
The compressed-powder of the present invention shall refer to any form of
drug, vaccine or other therapeutic agent in which a powder is formed into a
solid
matrix. Said powder may be any kind of powder cake such as a freeze dried
cake,
or any kind of powder or micronized powder bonded or otherwise arranged into a
solid matrix.
The inhaler device of the present invention is a dry-powder inhaler device
comprising at least one air inlet, a flow chamber and an air outlet leading to
a
mouthpiece, said flow chamber further comprising at least one compressed-
powder
volume and at least one scraping surface; wherein the inhalation action of the
patient applied at said air outlet causes air to flow from said at least one
air inlet
through said flow chamber, said air flow generating relative motion between
said at
least one compressed-powder volume and said at least one scraping surface such
that fine particles of powder are scraped from the compressed-powder volume(s)
and inhaled by the patient.
In preferred embodiments of the present invention, said dry-powder inhaler
device comprises a multiplicity of air inlets.
In further preferred embodiments of the present invention, said dry-powder
inhaler device comprises a multiplicity of compressed-powder volumes.
CA 02551561 2006-07-14
. ~ ,
Preferably the scraping surface is a blade of an impeller, said blade
gradually
extending outwards as said impeller rotates, thereby ensuring a time lag
between
the start of said inhalation action and the first release of said fine
particles.
Said device preferably further comprises (a) a particle filter located between
said flow chamber and said outlet to ensure that large particles are not
inhaled and
(b) a mouthpiece attachable to said outlet. Said mouthpiece may either be an
integral part of said inhaler device or may be attached by the patient to said
outlet.
In the latter case, the inhaler device may further comprise a storage
compartment
for said mouthpiece. Regarding the scraping surfaces and the compressed-powder
volumes, either one is static and the other movable, or both or movable.
The inhaler device is preferably shaped like a credit-card, a conventional
hand-held inhaler, or have any other ergonomically suitable shape, including
that of
a cylinder, a prism, a disk, and an oval.
The invention will now be described in connection with certain preferred
embodiments with reference to the following illustrative figures so that it
may be
more fully understood.
With specific reference now to the figures in detail, it is stressed that the
particulars shown are by way of example and for purposes of illustrative
discussion
of the preferred embodiments of the present invention only and are presented
in the
cause of providing what is believed to be the most useful and readily
understood
description of the principles and conceptual aspects of the invention. In this
regard,
no attempt is made to show structural details of the invention in more detail
than is
necessary for a fundamental understanding of the invention, the description
taken
with the drawings making apparent to those skilled in the art how the several
forms
of the invention may be embodied in practice.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 presents isometric and planar views of a single use disposable
credit-card shape embodiment of the invention where the compressed-powder is
static;
Figure 2 presents isometric views of a multiple-use credit-card shape
embodiment of the invention where the compressed-powder is static;
Figure 3 presents isometric and cross-sectional views of an embodiment of
the invention where the compressed-powder is the moving element; and
CA 02551561 2006-07-14
6
Figure 4 presents isometric, planar and cross-sectional views of an
embodiment of the invention where the compressed-powder is embedded on the
film walls of the flow control chamber.
DETAILED DESCRIPTION OF THE DRAWINGS
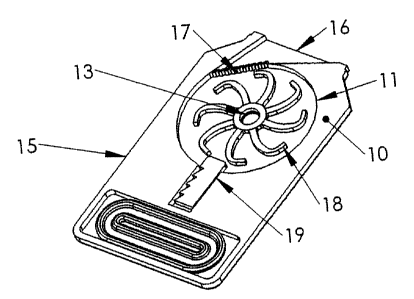
Referring now to Fig. 1, a preferred embodiment of the device of the present
invention is shown, in which a credit-card styie design is employed. Figure 1a
provides an overall isometric view of this preferred embodiment, shown ready
for
use, with a rubber mouthpiece 12 shown attached around the outlet 16. As an
inhaler 10 of this size and shape may not be convenient to place in the mouth,
said
rubber mouthpiece 12 is provided along with the inhaler device 10, and is
preferably
stored in a dedicated compartment thereof, as shown in Figure 1 b. To use the
mouthpiece 12, it is extracted from said compartment and stretched around the
drug
outlet 16. By so doing, the mouthpiece 12 deforms into the ergonomically
advantageous shape shown in Figure 1 a. The advantage of this approach is that
the device can easily be carried in a credit-card slot in a wallet, while
keeping the
mouthpiece from getting contaminated. It is also easier to clean and wash such
a
removable mouthpiece 12. Said mouthpiece 12 can be made from elastic polymers
such as Silicone Rubber or Santoprene . Alternatively, the outlet 16 can be
tapered into a narrow form (not shown) such that it can be inserted into a
mouth
more comfortably. In such a case, the need for a separate mouthpiece 12 can be
obviated.
Referring now to Figure 1c, an exploded isometric view of a preferred
embodiment of the inhaler device 10 of the present invention is provided. Said
device 10 comprises a body 15 sandwiched between two film walls 14, where at
least one of said walls 14 further comprises an air inlet 13. Said film walls
14 may
comprise multi-layer plastic film and/or metalized plastic films.
Advantageously, this
construction enables the device 10 to benefit from the strength and excellent
barrier
properties that such film walls possess. The body 15 of said inhaler device 10
further comprises a flow chamber 11 containing an inhalable drug in a
compressed-
powder 19 form, an impelier 18, and an outlet filter 17. When air is inhaled
by the
patient, the resulting air flow from the air inlet 13, via the flow chamber 11
and out
through the outlet filter 17 to the outlet 16, causes the impeller 18 to
rotate. By
appropriate relative arrangement of the impeller 18 and the compressed-powder
CA 02551561 2006-07-14
7
and the employment of flexible blades on the impeller 18, said rotation causes
said
blades to stretch out such that the tip of one or more impeller 18 blades come
in
contact with the compressed-powder 19, causing said tip or tips to scrape
powder
off said compressed-powder 19. In this embodiment, said volume of compressed-
powder 19 is adhered to the inner circumferential wall of the flow chamber 11.
By
controlling the fiow parameters, and the impeller mechanical parameters a
control
on the delay and duration of the drug release can be achieved, resulting in
synchronization with the inhalation cycle. Additionally, by controlling the
mechanical
properties of the impeller 18 and in particular the mass and flexibility of
the blades
and the roughness of their tips and the properties of the compressed-powder
19, the
characteristics of the powder generated and inhaled can be controlled. The
outlet
filter 17 prevents large size powder particles from reaching the patient.
Advantageously, the overall result of this mechanism is the provision of a
breath-
powered, controllably-delayed drug delivery which can be sustained during the
breath of the patient.
In the above preferred embodiment, the impeller 18 can be made from
injection-molded thermoplastic materials such as polyurethane or
polycarbonate, or
alternatively from sheet metal spring materials. The outlet filter 17 can be
either be
an integrally-formed part of the body 15, or a separate component such as a
PorexTM piece (from Porex Corporation, Fairburn, GA, USA) or a non-woven mesh.
A centrifugal separation technology can be combined to allocate the large
particles
to specific area. Additionally, it will be obvious to one skilled in the art
that the outlet
filter 17 can be designed in many shapes and structures. For example, said
outlet
filter 17 can extend further than shown around the circumference of the flow
chamber 11, where the air passing through said filter 17 is channeled to the
outlet
16. Due to the use of film walls 14 with good barrier properties, provided
that a seal
(not shown) to the inlet 13 and outlet 16 is maintained in between uses, the
compressed-powder 19 will be maintained in an environment which protects it
against humidity and other pollutants. It will also be obvious to one skilled
in the art
that a mechanical restrictor can be implemented in the flow chamber 11 such
that it
will prevent the impeller 18 from turning backwards, thereby preventing
accidental
wasting of the drug.
CA 02551561 2006-07-14
8
Referring now to Figure 1 d, a planar view of a slight modification of this
embodiment is provided. Whereas in Figure 1 c the compressed-powder 19 is
adhered to the inner circumferential wall of the flow chamber 11, in this
preferred
embodiment said compressed-powder volume 19 is attached via teeth to the
plastic
forming the body 15 at that same location. The compressed dry powder 19 may
comprise any inhalable drug / carrier combination known in the field of drug
tablets
manufacturing, whether cold compressed into a solid form or otherwise. It is
obvious to those skilled in the art that the dispersion of drug powder in the
matrix
powder is controllable. For example, the powder (in the "teeth") that will
remain
unused in the present embodiment would preferably not contain any of the
active
drug ingredients. The compressed drug can be implemented on the chamber walls
as one piece by mechanical atachement or impregnated on the walls or on a
separate part that is introduced to the chamber such as a film strip.
Whereas in the above embodiment, the blades of the impeller 18 extend out
toward the compressed-powder during use, in an alternative embodiment, the
blades of the impeller 18 could remain fixed while the compressed-powder 19 is
spring-loaded to press forward into said blades or otherwise forced advanced
toward the impeller. For example a special mechanism can advance the dug
toward the impeller in response to the pressure in the chamber or the speed of
the
impeller or the rotations of the impeller.
The inhaler device 10 of the present invention may be provided in either
disposable or multiple-use embodiments. Referring now to Figure 2, a multiple-
use
variant of the approach described in connection with Figure 1 above is
presented.
Figure 2a shows an isometric view of an embodiment in which the compressed-
powder 19 is in a shape of a bar that can be incrementally advanced into the
flow
chamber 11 by a special mechanism (not shown) that engages with the ratchet
teeth. Similarly, Figure 2b provides an isometric view of an embodiment in
which
the compressed-powder 19 is in a shape of a disk that can be rotated between
uses, in order to expose another section of said disk to the impeller 18 each
time. In
this case, the compressed-powder 19 disk can be completely made of compressed-
powder or alternatively it can have a carousel structure comprising a rigid
framework
with compressed-powder volumes located at several points on its circumference.
Said rigid structure is preferably formed from plastics such as polypropylene.
Due
CA 02551561 2006-07-14
9
to the inexpensive nature of the design employed in Figures 1 and 2 above, the
device 10 presented can be a disposable one, whether intended for multiple-use
or
single use. Alternatively, the device 10 can be designed so that the
compressed-
powder 19 can be replaced by the user, thus making the device a permanent
multiple-use device. Advantageously, by enabling the patient to replace the
compressed-powder 19, the device 10 can be delivered separately from the drug
to
the user, pharmacist, or physician; thereby widening the flexibility of the
drug
distribution model. In a further multiple-use embodiment (not shown), the
compressed-powder volumes are stored individually in a strip, said strip being
advanced toward the scraping surface(s) and the powder exposed, as each next
dose is required.
Whereas Figures 1 and 2 present an embodiment wherein the compressed-
powder drug 19 is static and is ground into a powder by an element moving
against
it; referring now to Figure 3 a further preferred embodiment is illustrated in
which the
compressed-powder is the moving element and the powder is scraped away as said
compressed-powder moves against the static circumferential walls 32 of the
flow
chamber 11. This embodiment employs a plurality of air inlets 13 in the form
of
cantilevered sections of the film walls 14 that enclose the body 15, arranged
such
that said air inlets 13 impart a swirling air flow motion to the flow chamber
11 and
thereby make the disk 31 spin around said chamber 11. The exploded isometric
diagram of Figure 3d shows a preferred arrangement for such slots in the upper
film
wall 14, said arrangement being mirrored in the lower film wall as shown in
the
figures. Referring now to Figure 3a, an isometric view of a single-use
embodiment
of this approach is shown, in which the air entering from the inlet 13 flows
rotationally around the flow chamber 11 and then through the outlet filter 17
to the
outlet 16. Said rotational air flow causes a compressed-powder disk 31 to
rotate
along the circumferential wall 32 of the flow-chamber 11, thereby generating
fine
powder due to the friction between said wall 32 and said disk 31. Such disks
31 can
be replaced through the air inlet 13. In this figure the disk 31 is entirely
fabricated
from compressed-powder, and as described above, the active ingredients can be
controllably concentrated in the outer layer of said compressed-powder. Other
possibilities for the fabrication of said disk 31 include (a) the employment
of a hard
core 35 in the shape of a thin disk covered by upper and lower layers of
CA 02551561 2006-07-14
compressed-powder 34 (as shown in Figure 3b), and (b) a hard core 35 in the
shape of a disk whose circumference is covered by a layer of compressed-powder
34 (as per Figure 3c). The former embodiment produces fine powder as the disk
31
scratches against the flat walls of the flow chamber 11, said walls being made
suitable rough. Similarly, the latter embodiment produces fine powder as the
disk
31 scratches against the circumferential wall 32 of the flow chamber 11, said
wall
being made suitably rough. Referring now to Figure 3d, a further preferred
embodiment of the inhaler device of the present invention is shown, in which a
carousel component 36 serves to contain a multiplicity of the above described
disks
31, such that a new disk 31 can be exposed to the flow chamber 11 at each turn
of
the carousel 36. In this embodiment, exhausted disks can either be manipulated
back to the carousel 36 or can be disposed of via one of the air inlet holes
13.
Referring now to Figure 3a, a cross-sectional view is provided of the inhaler
device
of the present invention, in which a compressed powder disk as per Figure 3b
is
shown in contact with the above-described cantilevered sections of the film
walls of
the device which are serving to form the air inlets 13. In this embodiment,
the inner
side of said sections serves to scrape off powder 34 from the disk by
frictional action
against said disk.
While figures 1-3 presented powder generation of a static element working
against a moving element, one of them being the compressed-powder, it will be
obvious to those skilled in the art that the powder can also be generated by
the
interaction of two moving elements and such an embodiment is included in the
present invention providing that such interaction is driven by the inhalation.
Referring now to Figure 4a an isometric view of a further preferred
embodiment of the inhaler device of the present invention is shown. In this
embodiment the compressed-powder 42 is embedded in or otherwise deposited on
lowered sections 41 of the flat film wall 14 covering the flow chamber 11. As
per
Figure 3 above, said film wall 14 is preformed and cut in a way that these
lowered
sections 42 of the wall 14 have the shape of flexible fingers, whose embedded
powder 42 areas lightly touch the rotating disk. As per the previous
embodiment,
said sections also serve to swirl the incoming air so that the disk 31 rotates
around
the flow chamber 11. The difference from the previous embodiment is that in
this
case the disk does not contain a compressed-powder but only serves to scrape
off
CA 02551561 2006-07-14
11
the drug powder from said embedded powder areas 42. Referring now to Figure
4b,
a planar view of this preferred embodiment is presented in order to show the
line D-
D represented by the cross-sectional view shown in Figure 4c. Referring now to
Figure 4c, a disk 31 is shown on the left hand side, and the impregnated
compressed-powder area 42 that it will scratch against on contact is shown on
the
inside of the cantilever structure 41.
While the above embodiments describe credit-card shape designs, it will be
obvious to one skilled in the art that a number of device designs are
possible,
including a range of solutions for compressed-powder arrangements, and loading
and replacing solutions. For example, the device may be in the shape of a
prism, a
disk, an oval, or use the form-factor of existing, conventional hand-held
inhalers;
providing only that the internal volume is sufficient to allow the breath-
powered
scraping or rubbing action to liberate the fine powder as described above. It
should
also be apparent that the device of the present invention can further
incorporate a
number of standard drug-dosing device components or functions known in the
art.
These elements include a child-proof mechanism to protect against inadvertent
activation by a child; a counter display showing the number of inhalations,
shipping
seals, air-tight resealing plugs, etc. Further, it will be obvious to one
skilled in the art
that a number of drugs can be inhaled simultaneously using the device of the
present invention, whether by employing a multiplicity of compressed-powder
drug
volumes where different volumes contain different drugs, or by means of mixing
a
multiplicity of drugs within any given compressed-drug volume. Additionally,
where
a "magazine" of compressed drug volumes is used as per Figure 2a or Figure 3d,
each of said volumes may comprise a different drug or different drug
combination.
Advantageously, said arrangement enables the sequential administration of a
number of drugs.
It will also be obvious to those skilled in the art that, although primarily a
breath-powered device, it is possible to use a source of auxiliary power to
assist in
the scraping action and thereby increase its efficiency. Said auxiliary power
means
include the use of a lever with a spring that will add auxiliary force to the
impeller, a
compressed gas cylinder and an electric motor. Said auxiliary power source may
be
incorporated with in the device, or alternatively this power source can be
external.
For example by implementing a static magnet in the impeller of Figure 1 the
impeller
CA 02551561 2006-07-14
12
could gain power from an external electric or magnetic fieid. In another
configuration the impeller can incorporate a non-magnetic electric conductive
material that will be driven from an alternating magnetic field by means of an
Eddie
Current drive arrangement.
A dry-powder inhaler is described above. Various details of the invention may
be changed without departing from its scope. Furthermore, the foregoing
description of the preferred embodiment of the invention and the best mode of
practicing the invention are provided for the purpose of illustration only and
not for
the purpose of limitation--the invention being defined by the claims.