Note: Descriptions are shown in the official language in which they were submitted.
r_
CA 02551658 2006-06-27
WO 2005/063591 PCT/EP2004/014726
91746pct
Method and device for filling the dosing chamber of an inhaler for the first
time
'The present invention relates to medicinal inhalers for medical purposes
which deliver a given
amount of a preferably pharmaceutical fluid over a fairly long period in the
form of a "soft"
aerosol mist for inhalation ("soft mistTM inhaler" or SMI for short). A device
of this kind may
be, for example, an inhaler of the Respimat~ type, which is described in more
detail in WO
97/12687. This type of inhaler is fitted with a cartridge containing a fairly
large amount of
the active substance formulation. The present invention relates to a further
development of
the Respimat~ system, in which the fitting of the cartridge into fiche device
is improved with a
view to speeding up the first use of the spray.
Prior art
The handy inhalers of the Respimat~ type or inhalers like the Respimat~ Soft~
MistTM Inhaler
(SMI), in which a small amount of an aqueous formulation is atomised in
amounts of a few
microlitres without the use of propellant gases to form an aerosol mist, are
one of the latest
innovative developments in the field of medical atomisation technology.
Because of its
cylindrical shape and handy size of less than 9 to 15 cm long and 2 to 4 cm
wide this device
can be taken anywhere by the patient, so that it is always available for
regular daily use in a
manner which is convenient to the patient, irrespective of the location.
The basic technical characteristics of these inhalers are disclosed for
example in WO
91/14468 or WO 97/12687, particularly in Figures 6a and 6b. In these inhalers,
the amount of
liquid pharmaceutical formulation to be nebulised by high pressure up to 500
bar is forced
through a micronozzle with preferably two nozzle outlets and thereby converted
into an
aerosol destined for the lungs. Reference is specifically made to the above-
mentioned
publications, within the scope of the present description.
The Respimat~ principle is based on two separate construction units: on the
one hand the
inhaler which contains all the mechanical components for producing the
aerosol, and on the
other hand a separate cartridge, which contains the pharmaceutical
formulation.
CA 02551658 2006-06-27
2
For use the cartridge is pushed onto a cannula formed in the inhaler. Liquid
is conveyed
through this cannula into a compressing and dosing chamber and from there
forced through a
micronozzle by the application of pressure.
The cartridge consists of a container filled with the fluid and a closure cap
therefor.
Essentially, a Respimat~ type nebuliser consists of an upper housing part at
the top, a lower
housing part which is rotatably mounted relative to the upper housing part and
defines the
bottom end, a pump housing, the nozzle, a locking clamping mechanism, a spring
housing, a
spring and the storage container.
The pump housing is in the upper housing part. At the top end of the pump
housing is located
the nozzle body with the nozzle or the nozzle arrangement. Below this is a
compression
chamber which may be part of a central tube in the form of a cylindrical bore.
Below the
compression chamber is the upper end of a cannula in the form of a hollow
piston, which
projects partially into the central tube and can move axially back and forth
therein in a stroke
action. The hollow piston is fixedly connected to a power takeoff flange
outside the central
tube. The power takeoff flange is located on the top end of a spring (helical
spring) and is
moved thereby. The helical spring is located in a spring housing, which is
rotatably mounted
on the upper housing part by means of a rotary bearing and can be tensioned
and released by
means of a locking clamping mechanism. All the components mentioned in this
paragraph
are located in the upper housing part. The hollow piston extends with its
lower, bottom end
into the inner space defined by the compression spring. This hollow space is
open at the
bottom. At the top it may be bounded by the power takeoff flange. The
pharmaceutical
cartridge is inserted from the bottom end into this cavity in the helical
spring and pushed onto
the cannula. The lower housing part is then pushed axially over the spring
housing. The
system comprising the hollow piston, central tube, compression chamber and
nozzle
constitutes a system for conveying a fluid. The connections between the
individual
components are sealed off to the outside. The fluid conveying system has only
two openings,
the lower opening in the hollow piston and the nozzle opening. One opening
serves to receive
a fluid, while the other, the nozzle opening, serves to deliver said fluid.
The nozzles used are special nozzles, as described for example in WO 94/07607
or WO
99/18530. Reference is made specifically to both these publications.
CA 02551658 2006-06-27
3
The nozzle in the nozzle body is preferably microstructured, i.e. produced by
micro-
engineering. Microstructured nozzle bodies are disclosed for example in WO-
94/07607;
reference is hereby made to the contents of this specification, especially
Figure l and the
associated description.
The nozzle body consists for example of two sheets of glass and/or silicon
securely fixed
together, at least one of which has one or more microstructured channels which
connect the
nozzle inlet end to the nozzle outlet end. At the nozzle outlet end there is
at least one round
or non-round opening 2 to 10 microns deep and 5 to 15 microns wide, the depth
preferably
being 4.5 to 6.5 microns and the length being 7 to 9 microns.
If there is a plurality of nozzle openings, preferably two, the directions of
spraying of the
nozzles in the nozzle body may run parallel to each other or may be inclined
relative to one
another in the direction of the nozzle opening. In the case of a nozzle body
having at least
two nozzle openings at the outlet end, the directions of spraying may be
inclined relative to
one another at an angle of 20 degrees to 180 degrees, preferably at an angle
of 60 to 150
degrees, most preferably 80 to 100°.
The nozzle openings are preferably arranged at a spacing of 10 to 200 microns,
more
preferably at a spacing of 10 to 100 microns, still more preferably 30 to 70
microns. A
spacing of 50 microns is most preferred.
'The directions of spraying therefore meet in the region of the nozzle
openings
For the nebulisation, the liquid pharmaceutical preparation hits the nozzle
body at an entry
pressure of up to 600 bar, preferably 200 to 300 bar and is atomised through
the nozzle
openings into an inhalable aerosol. The preferred particle sizes of the
aerosol are up to 20
microns, preferably 3 to 10 microns.
The hollow piston with valve body corresponds to a device disclosed in WO
97/12687. It
projects partially into the cylinder of the pump housing and is disposed to be
axially movable
in the cylinder. The valve body is preferably mounted on the end of the hollow
piston which
faces the nozzle body.
CA 02551658 2006-06-27
4
Reference is made particularly to Figures 1-4 - especially Figure 3 - and the
associated
passages of description. At the moment of release of the spring the hollow
piston with valve
body exerts, at its high pressure end, a pressure of 5 to 60 Mpa (about 50 to
600 bar),
preferably 10 to 60 Mpa (about 100 to 600 bar) on the fluid, the measured
amount of active
substance solution. Volumes of 10 to 50 microlitres are preferred, volumes of
10 to 20
microlitres are more preferable, whilst a volume of 10 to 15 microlitres per
actuation is
particularly preferred.
The locking clamping mechanism contains the spring, preferably a cylindrical
helical
compression spring, as a store for the mechanical energy. The spring acts on
the power take-
off flange as a spring member the movement of which is determined by the
position of a
locking member. The travel of the power take-off flange is precisely limited
by an upper stop
and a lower stop. The spring is preferably tensioned via a stepping-up gear,
e.g. a helical
sliding gear, by an external torque which is generated when the upper housing
part is turned
relative to the spring housing in the lower housing part. In this case, the
upper housing part
and the power take-off flange contain a single- or multi-speed spline gear.
The locking member with the engaging locking surfaces is arranged in an
annular
configuration around the power take-off flange. It consists for example of a
ring of plastics or
metal which is inherently radially elastically deformable. The ring is
arranged in a plane
perpendicular to the axis of the atomiser. After the locking of the spring,
the locking surfaces
of the locking member slide into the path of the power take-off flange and
prevent the spring
from being released. The locking member is actuated by means of a button. The
actuating
button is connected or coupled to the locking member. In order to actuate the
locking
clamping mechanism the actuating button is moved parallel to the annular
plane, preferably
into the atomiser, and the deformable ring is thereby deformed in the annular
plane. Details
of the construction of the locking clamping mechanism are described in WO
97/20590.
The lower housing part is pushed axially over the spring housing and covers
the bearing, the
drive for the spindle and the storage container for the fluid.
When the atomiser is operated, the upper part of the housing is rotated
relative to the lower
part, the lower part taking the spring housing with it. The spring meanwhile
is compressed
CA 02551658 2006-06-27
and biased by means of the helical sliding gear, and the clamping mechanism
engages
automatically. The angle of rotation is preferably a whole-number fraction of
360 degrees,
e.g. 180 degrees. At the same time as the spring is tensioned, the power take-
off component
in the upper housing part is moved along by a given amount, the hollow piston
is pulled back
inside the cylinder in the pump housing, as a result of which some of the
fluid from the
storage container is sucked into the high pressure chamber in front of the
nozzle.
The atomising process is initiated by gently pressing the actuating button.
The clamping
mechanism then opens the way for the power take-off component. The biased
spring pushes
the piston into the cylinder in the pump housing. The fluid emerges from the
nozzle of the
atomiser in the form of a spray.
Further details of the construction are disclosed in PCT applications WO
97/12683 and WO
97/20590, to which reference is hereby made.
The storage container (cartridge) is preferably a container having a flange or
a closure cap via
which the hollow piston of the inhaler can be inserted into the interior. The
flange or the
closure cap contains a guide passage for the hollow piston with at least one
sealing point
which prevents air from getting into the container from outside along the
hollow piston or
fluid from escaping from the container by the same route. The flange or the
closure cap may
be designed to be releasably or non-releasably connected to the power takeoff
flange of the
inhaler. Preferably the container is constructed as a collapsible container
which is preferably
surrounded by a fixed, rigid second container which protects the collapsible
first container
from damage, inter alia. Suitable containers are described in EP 0775076 or WO
99/43571.
However, other suitable non-collapsible containers may also be used. The
storage container
constitutes a self contained system before it is fitted onto the hollow
piston, on which there
are no devices to which pressure is to be applied.
Before the first use the still sealed cartridge (the container) has to be
pushed onto the cannula
of the inhaler. In order to fill the region from the hollow piston to the
nozzle with fluid
completely for the first time the inhaler known from the prior art has to be
tensioned and
actuated several times.
CA 02551658 2006-06-27
Description of the invention
One aim of the present invention is to provide an inhaler of the Respimat~
type, which can be
operated more quickly than the device known from the prior art after the first
insertion of the
cartridge.
A further aim of the present invention is to shorten the steps prior to the
first operation of an
inhaler of the Respimat~ type.
A further aim of the present invention is to automate the steps for filling an
inhaler of the
Respimat~ type with fluid for the first time.
Detailed description of the invention
According to the invention the problem is to speed up and automate the process
of filling the
dead volume in the inhaler. The term dead volume refers to the volume which is
created by
the interior of the cannula above the fluid level, the inside of the valve,
the part of the cylinder
above it, including the pressure chamber, and the inner space of the nozzle,
minus the part of
the volume which is taken up by the region of the hollow piston. In other
words, that part of
the cannula volume which projects into the fluid after the completion of the
insertion process
of the cartridge, which is generally at least 90 vol% full, is not taken into
account. The
system of cannula, cylinder, pressure chamber, nozzle is hereinafter referred
to as the fluid
conveying system. Thus the dead volume corresponds to the inner volume of the
fluid
conveying system when the spring is relaxed, minus the proportion filled
solely by the
principle of the communicating tubes when the cartridge is pushed onto the
cannula. The
volume which is to be expelled through the atomiser is not included either.
This volume is
called the fill volume and is generated when the spring of the device is
tension and the piston
is moved out of the central tube without leaving it. The difference between
the two volumes
corresponds substantially to the amount of fluid that is to be nebulised
(delivery volume).
In detail, the preferred nebuliser may be described as follows. A pump housing
is located in a
cylindrical upper housing part. A holder for the atomiser nozzle is mounted on
its end. The
holder contains the nozzle body and optionally one or more filters. The nozzle
is located at
the upper end of a cylinder tube which is formed in the pump housing. The
hollow piston
fixed in a power takeoff flange of the locking clamping mechanism. At its end
the hollow
piston has a valve body. The hollow piston is sealed off to the outside by
means of a gasket.
CA 02551658 2006-06-27
7
Inside the upper housing part is a first stop on which the power takeoff
flange bears when the
spring is relaxed. On the power takeoff flange there is a second stop on which
the power
takeoff flange bears when the spring is relaxed. After the tensioning of the
spring a locking
member slides between the second stop and a support in the upper housing part.
An actuating
button is connected to the locking member. The upper housing part ends in a
mouthpiece and
is closed off by the push-on protective cap.
A cylindrical spring housing with compression spring is rotatably mounted on
the upper
housing part by means of snap-in lugs and rotary bearing. The cylindrical
lower housing part
is pushed over the spring housing. Inside the spring housing is the
exchangeable storage
container for the fluid which is to be atomised. The storage container is
sealed off by a
stopper through which the hollow plunger projects into the storage container
and is immersed
at its end in the fluid (supply of active substance solution).
To solve the problem according to the invention it is proposed to relax the
excess pressure
which has spontaneously formed in the storage vessel (container) or is present
therein with the
introduction of the storage vessel into the inhaler, with displacement of
fluid from the
container through the fluid conveying system comprising a hollow piston,
cylinder, pressure
chamber and nozzle, so that the dead volume of the fluid conveying system is
filled with fluid
and the nozzle is attached to the fluid supply with the exclusion of air.
According to the invention the excess pressure is supposed to be sufficient to
more than
completely fill the dead volume with fluid on one side. On the other hand the
pressure is only
supposed to be high enough for preferably less than 100 microlitres to leave
the inhaler
through the nozzle as a result of the release of pressure. It is important
that at least one and a
half times more fluid is forced through the fluid conveying system than
corresponds to the
dead volume of the fluid conveying system. This compensates any tolerances
which may
occur as a result of the elasticity of the storage vessel.
In a first embodiment of the invention the excess pressure in the container is
generated
spontaneously by pushing the container onto the hollow piston of the non-
tensioned inhaler.
At least that part of the cannula of the inhaler which extends into the
container is of a different
construction from the embodiment known from the prior art. According to the
invention the
region of the cannula of the inhaler which extends into the container, should
be configured
CA 02551658 2006-06-27
such that this region displaces at least one and a half times, preferably
twice as much fluid as
the amount corresponding to the first dead volume. This measure ensures that
the pressure
which is produced by pushing the storage container onto the cannula inside the
container is
increased, with the result that the fluid is forced through the cannula
towards the nozzle under
higher pressure and hence more rapidly as a result of the excess pressure
inside the container.
The dead volume of the fluid conveying system of the known system is about 17
microlitres,
which is made up of about 10 microlitres of dead volume in the central tube
when the spring
is not under tension, including the dead space of the pressure chamber, 7
microlitres of dead
volume in the capillary (that is the proportion of the capillary volume which
is above the fluid
level when the totally full cartridge ~s fitted) and about 100 nanolitres of
dead volume of the
nozzle. This volume then has to be displaced from the region of the cannula
which penetrates
into the fluid when the cartridge is fitted onto the cannula. With an outer
diameter for the
cannula of 1.5 mm and a wall thickness of 1.1 mm, the part of the cannula that
is immersed in
the fluid has to be about 10.8, in order to displace a volume of 18
microlitres.
However, it has been found that these dimensions do not solve the problem, as
the flexible
container partially compensates the excess pressure produced.
The problem according to the invention is only completely solved if the
displacement volume
of that part of the hollow piston that penetrates into the interior of the
container is at least 23
microlitres, more preferably at least 34 microlitres.
In order to increase the displacement volume to the preferred levels mentioned
above, while
keeping the same internal and external diameters for the hollow piston, the
length of the
hollow piston projecting into the interior of the container must be increased
to at least 13.8
mm, preferably to at least 20.4 mm.
In another embodiment the external diameter of the cannula is increased, while
keeping the
internal diameter and the depth of penetration into the interior of the
container the same.
In this case an external diameter of at least 1.7 mm, preferably at least 2 mm
is useful.
This has the advantage that because of the broad effective punching surface of
the cannula the
initial pressure inside the container is built up more rapidly, so that the
pressure on the fluid to
escape through the cannula is initially increased more than by extending the
piston.
CA 02551658 2006-06-27
9
In another embodiment the piston may be extended and at the same time its
external diameter
is increased. Moreover, only that part of the capillary which dips into the
fluid can be shaped
accordingly, e.g. have a larger external diameter than the remainder of the
cannula.
In every case the length of the hollow piston of 44.2 mm outside the interior
of the container
should preferably be retained.
In another embodiment the container itself is acted upon by pressure when
filled with the
pharmaceutical formulation. This may be done for example by filling and
sealing the
container at low temperatures, e.g. from 4°C to 10°C (cold
filling). As it is heated to room
temperature the corresponding excess pressure is then generated by the
expansion of the fluid.
In yet another embodiment as the container is filled with the pharmaceutical
formulation an
excess pressure is generated by introducing the pharmaceutical formulation
under an excess
pressure atmosphere and leaving an air bubble of the corresponding order of
magnitude inside
the container. Then the container is sealed. In this process the air bubble is
compressed
during the filling. When the container is pierced with the cannula the air
bubble is freed from
tension and forces the fluid through the cannula. According to the above
remarks the volume
difference between the compressed and non-tensioned air bubble is preferably
at least 23
microlitres, more preferably at least 34 microlitres. Preferably an air bubble
of less than 100
microlitres is left in the container. In this embodiment too the cannula of
the inhaler has to
dip into the fluid.
Further details of the filling operation can be found in the prior art
mentioned above.
As a result of the measures described, an excess pressure of preferably more
than 1 mbar,
particularly preferably more than 5 mbar, is built up inside the container.
The maximum
pressure built up should not exceed SO mbar.
In another embodiment it is not the inhaler but the matching cartridge, i.e.
the supply system
for the fluid consisting of a container and closure, which is physically
changed. A
displacement device is formed which when the cartridge is fitted onto the
cannula of the
inhaler is pushed into the inside of the container and thereby displaces some
of the fluid
CA 02551658 2006-06-27
through the fluid conveying system. Embodiments of this kind are hereinafter
illustrated in
more detail by means of Figures 1 to 5. The drawings are not to scale and are
in the nature of
sketches, in some cases.
A typical cartridge is described for example by Fig. 1. The closure (1)
comprises a device (2)
in the form of a connector. The connector can optionally displace some of the
contents of the
container (3) during the closing process. The immersion connector (2) for its
part comprises a
passage or guide (12) for the cannula (18) of the inhaler. The connector (2)
is initially sealed
at the bottom. The immersion connector (2) displaces fluid from the container
when the
closure cap is put on and thereby ensures that after sealing the container is
at least 90,
preferably 95 % full by volume. The closure cap also has an encircling bead
(4) on the inside
(crimp edge) which engages underneath a cylindrical ring (5) running round the
outside of the
neck of the container, at the lower edge of the closure cap (1) in the7closure
position. While
the closure cap (1) is pushed on the edge of the closure cap is expanded and
the bead (4) abuts
on the ring (5) to form a seal, so that the inside (7) of the cap only
communicates with the
outside through one or more vent openings (6). The vent openings) is (are)
arranged for
example in the outer part of the ring (5). In the closure position the gap
between the flat part
of the closure cap (1) and the upper edge of the neck of the container, which
is optionally
provided with an encircling rib (8) to improve the seal, is filled by a gasket
(9) and in this way
the interior of the container (3) is reliably sealed off from the interior (7)
of the cap, which
surrounds the sealing ring (9) and the neck of the container (3). The internal
diameter of the
sealing ring (9) is expediently chosen so as to fit tightly against the device
(2). The vent
openings) (6) may also be located elsewhere on the exterior of the cap, e.g.
laterally in the
cylindrical part of the cap. The immersion connector has a pierceable base
(10).
In a preferred embodiment the container (3) consists of a dimensionally stable
outer container
and a readily deformable inner bag (3b) which collapses when fluid is removed.
Containers
of this kind are described for example in European Patent 532 873, the
contents of which are
hereby incorporated by reference. The device (11) serves to attach the
deformable inner bag
(3b) to the inner wall of the outer rigid container (3a) facing the bag (3b).
Figure 2 shows a preferred embodiment of the closure cap according to the
invention, wherein
the inner chamber of the connector has a special guide (12) for a cannula for
removing fluid.
In the present instance, the vent openings (6) are provided on the upper part
of the container
(3). The vent openings may alternatively also be provided on the closure cap.
If desired the
guide (12) may be constructed as a press fit for the cannula (18) or an O-ring
seal (13) may be
mounted therein.
CA 02551658 2006-06-27
11
Figure 3a shows an embodiment of the invention wherein underneath the guide
(12) a cavity
is formed in the immersion connector (2), in which there is a displacement
member (14) in the
form of, for example, a stopper, cylinder, cork, etc., which is pushed at
least partly into the
container (3) when the cannula is passed through the guide (12) and thereby
helps to build up
the desired excess pressure inside the container. A displacement member of
this kind may be
located at any point in the guide (12). The shape of the displacement member
is preferably
cylindrical. The displacement member preferably consists of a plastic such as
polyethylene,
polypropylene, etc. On its side directed towards the top end of the closure
the displacement
member may have a recess in which the cannula can engage.
Preferably the displacement member i~ constructed as a punch which can only
partially
emerge from the guide (12). In this case at least part of the wall of the
displacement member
(14) and the wall of the guide (12) may interact to form a fluidtight seal. In
order that the
displacement member (14) cannot leave the guide (12), stop means in the form
of an
encircling edge, for example, may be formed at the upper end of the
displacement member
(14), which interact with stop means - e.g. again in the form of an encircling
edge - at the
lower end of the guide (12). This prevents fluid from flowing into the space
which was
previously filled by the displacement member (14), so that no pressure
compensation can take
place in this way. Alternatively, guide channels may be formed on the
displacement member
(14), which interact with complementary means on the guide, the guide channels
no longer
being formed at the top of the displacement member (14). Other stop means for
a system of
this kind are described inter alia under Figure 4 or in the prior art.
In a preferred embodiment the displacement member may have a bore (19) (Fig.
3b), in which
the cannula engages by the force of friction. This prevents the displacement
member from
dropping into the container after leaving the guide (12). Preferably, the bore
may have a press
fit or constriction (21 ) in which the cannula engages. The bore is shaped
such that the
cannula (18) is in contact with the fluid in the container. For this purpose
the bore may
constitute a linear passage.
In a variant of this embodiment the bore for receiving the cannula by
frictional engagement is
not a through-bore and is constructed so that the capillary can only be
partially pushed into
the bore. As a result a cavity (20) is formed underneath the capillary. For
this purpose a
constriction (21 ) may be formed in the bore, for example, preventing the
capillary from being
CA 02551658 2006-06-27
12
pressed forward any more. The displacement member then has further capillaries
(22) which
lead from the exterior of the displacement member to the cavity. If these
additional
capillaries of the displacement member are in the form of microcapillaries,
fluid is constantly
transported from outside into the cavity, thus ensuring that the cannula of
the inhaler is always
supplied with fluid even during the emptying of the container. An embodiment
along these
lines is shown, not to scale, in Figures 3c and 3d.
Preferably the displacement member in this variant is constructed as an
integral, capillaried,
open-pored, porous storage medium for fluid. In other words the displacement
member
simultaneously acts as a sponge conveying fluid into its interior. The
displacement member
may be a dimensionally stable body with a fluid-pervious wall, filled with
sintered or non- _
sintered powder, or a woven or knitted or non-woven structure or a wad of
fibres. It may
consist of plastics, ceramics, glass, metal or a natural material.
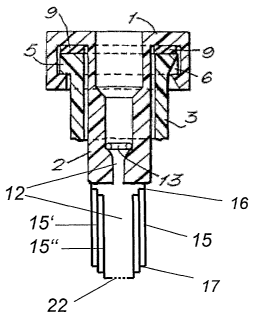
Figures 4a and 4b show another embodiment in which the immersion connector is
composed
of at least two sleeves (15, 15', 15") fitting telescopically one inside the
other. The inner
sleeves in each case have stop means (16) at their upper end, which cooperate
with
corresponding inwardly directed stop means at the lower end (17) of the outer
sleeve and thus
ensure that an inner sleeve cannot be pushed right through an outer sleeve.
The stop means
are preferably edges. Preferably, the individual cylinder-like sleeves fit
together to form a
seal. The bottom of the innermost sleeve is sealed to begin with, so that,
when the container
is pushed on, the cannula (18) pushes apart the sleeves which are still
nesting in one another
before it pierces the base (22) (Figure 4b). In this way the immersion
connector is extended
while the container is being pushed onto the cannula (18), and thus itself
acts as a
displacement member which builds up an excess pressure in the fluid-filled
interior of the
container.
The internal diameter of the innermost sleeve may be constructed as a press
fit for the sleeve,
or an O-shaped gasket is provided here, for example, to seal the cannula (18)
off to the
outside.
In the equivalent embodiment according to Figures Sa and Sb the lower region
of the
immersion connector (19) is pleated in the manner of an accordion (bellows)
(Figure Sa). The
guide (12) extends from the top part of the closure cap to the pleated end of
the immersion
CA 02551658 2006-06-27
13
connector. The bottom part of the immersion connector is constructed, as in
all the
embodiments, so that it can be pierced by the cannula. In this embodiment more
force is
needed to pierce the bottom part than to pull apart the bellows region. If the
cannula (18) is
passed through the guide (12), the pleated region is opened out before the
bottom part is
pierced, so that once again the enlarged immersion connector acts as a
displacement member
which creates excess pressure inside the container (Figure Sb). As the excess
pressure is only
slight there is little danger of the excess pressure being compensated by the
compression of
the extended bellows instead of by the displacement of the fluid through the
fluid conveying
system. This danger can additionally be countered by a suitable choice of
material.
In the alternative embodiments in Figures 4 and 5 the pressure
needed,_forpiercing is
controlled, for example, by means of the thickness of the bottom part.
Further details of the basic structure of the closure cap or the container can
be found in EP
0775076.
These latter embodiments of the invention described above may be constructed
analogously to
a closure system according to EP 1058657 in the form of a flange provided on a
container.