Note: Descriptions are shown in the official language in which they were submitted.
CA 02552112 2012-10-11
PROTEIN DETECTION SYSTEM
Inventors: Dennis B. Jenkins, Edward B. Tucker and Timothy E. Kozikoski
FIELD OF THE INVENTION
[0001] The present invention relates generally to protein detection. In
particular
detection of protein in feline urine is discussed.
BACKGROUND OF THE INVENTION
[0002] The presence of protein in certain bodily fluids can be an indication
of illness.
For example, the presence of elevated protein levels in feline urine can
indicate kidney
disease, a significant cause of cat illness and death.
SUMMARY OF THE INVENTION
[0003] An aspect of the invention includes an animal litter additive
comprising: MB
Macroporous silica gel buffered at a pH below about 3 containing a mixture of
a protein-
sequestering agent and Phloxine, wherein said protein-sequestering agent is
present in an
amount effective at blocking the attachment of Phloxine to protein at a
predetermined
protein level,
[0004] Another aspect of the invention includes a protein detection method
comprising:
providing a sample; contacting said sample with Phloxine; and detecting the
presence of
protein in said sample, wherein a color change is observed if said sample
contains said
protein. Preferably, the Phloxine is buffered using a weak acid and optionally
a
corresponding salt.
[0004A] Preferably, the protein-sequestering agent is present in an amount
effective at
blocking the attachment of Phloxine to protein at a predetermined protein
level. More
preferably, the pretermined protein level is greater than 2,000 ppm.
[0004B] In another aspect, the sample is a urine sample, and more preferably
mammalian urine is selected from human, canine, feline, equine, bovine, lupine
or
rodent.
[0005] A further aspect of the invention includes an animal litter comprising:
an
absorbent material suitable for use as an animal litter, wherein at least a
portion of said
litter contains Phloxine, wherein said Phloxine-containing portion of litter
is below about
pH3.
1
CA 02552112 2012-10-11
BRIEF DESCRIPTION OF THE DRAWINGS
[0006] Further features and advantages will become apparent from the following
accompanying drawings.
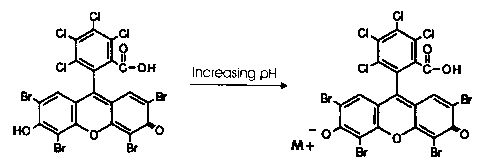
[0007] FIG. I shows the chemical structure of unbound Phloxine (colorless) and
bound
Phloxine (pink).
,
la
CA 02552112 2006-07-12
= =
100081 FIG. 2 shows a mechanism by which Phloxine attaches to protein.
[0009] FIG. 3 illustrates a mechanism by which a protein sequestering agent
inhibits
the attachment of Phloxine to protein.
DETAILED DESCRIPTION OF THE INVENTION
[0010] Protein levels in mammalian bodily fluids can be indicators of disease.
The
ability to detect these proteins in samples of various mammalian urine is
disclosed
herein. Bovine serum albumin is used as a representative protein to evaluate
the
various detection embodiments disclosed herein. The methods of the present
invention are effective for detecting protein in urine samples from mammals,
e.g.,
humans, felines, canines, and rodents. The animal litter disclosed herein
could be
used for common pets, cats, dogs, gerbils, guinea pigs, mice and hamsters,
rabbits,
ferrets and laboratory animals (e.g., mice, rats, and the like).
100111 Disclosed herein is the use of Phloxine B, i.e., Acid Red 92 (CAS NO.
18472-
87-2, C20H2Br4C14Na205. MOL WT. 829.64), hereinafter referred to as Phloxine,
as a
protein detector. Phloxine is a pH indicator that undergoes a visual color
change
based on it's pKa. Referring to FIG. 1, Phloxine goes from colorless (left) to
red
(right) between about pH 2-3.3, i.e., Phloxine is colorless below about pH 2,
red
above about pH 3.3 and varying shades of pink (pale to dark) between about pH
2-3.3.
FIG. 2 shows a mechanism by which Phloxine attaches to protein thereby causing
a
color change from colorless (unbound) to red (bound) without a pH change. The
pKa
of unbound Phloxine occurs at about pH 2.8-2.9, if Phloxine is bound to a
protein, the
pKa shifts to the lower pH value of about 2 -2.2. In a buffered system, this
pKa shift
changes the color of the indicator without a commensurate change in the pH.
Unbound Phloxine will appear colorless at pH levels between about 2.3-3,
whereas
The Clorox Company 2 430.204
CA 02552112 2006-07-12
[0012] Phloxine bound to protein will appear pink at pH levels between about
2.3 ¨3.
Thus, buffering a sample at a pH below about 3 and contacting that sample with
Phloxine enables the presence of protein to be detected in the sample via a
color
change from colorless to red. Optimally, the final combination of sample and
Phloxine will result in a pH between about 2 and 2.5. Thus, depending on the
conditions surrounding the Phloxine, the optimal buffering pH may vary, but
should
be below about 3.
[0013] Colorless to red as used herein means colorless to the entire spectrum
of pink
and red, including any and all shades of pink and red. Red shade color shifts
are also
possible if the starting color is something other than colorless. For example,
if the
starting color is blue, a red shade color shift to purple would be observed.
Test strips
containing Phloxine can be prepared by wetting a suitable paper substrate with
a
Phloxine solution and drying. Such test strips would be useful to those in the
medical
field.
Detection of protein in feline urine
[0014] Disclosed herein is the incorporation of a health indicating system
into an
animal litter. Animal litters have been used for decades as an effective way
of
managing the sanitation and odor control of waste. Since animal health
monitoring
often involves the testing of waste materials from the animal, it would be
desirable to
combine a health monitoring system with an animal litter. Problems associated
with
developing such a system are unique in that the animal waste material has to
be tested
"as is", i.e., without the benefit of any sample preparation or "clean-up".
For
example, protein-indicating strips exist, but there are a number of
limitations that
prevent this technology from being used in an animal litter. Specific
limitations with
The Clorox Company 3 430.204
CA 02552112 2006-07-12
existing protein-indicating test strips include the following. (1) Stability
of the
indicator. Many protein-indicating compounds are not stable in the presence of
elevated temperature, oxygen or moisture. (2) Stability of the color change.
Many
indicators do not hold the color change for long periods or when dry. Thus, a
color
change may occur without alerting the animal owner. (3) Color contrast. Many
indicating systems rely on a subtle shift in the shade of a color, creating a
system that
is difficult for the user to interpret.
[0015] Accompanying the fact that the waste material must be used "as is", is
the fact
that feline urine, itself, is unique in several aspects. Feline urine contains
high levels
of salt. Thus, in inadequately buffered systems, the high salt content and the
highly
buffering nature of feline urine tends to overwhelm these systems creating
numerous
false positives. The gradual changes in color that result from inadequately
buffered
systems are difficult for the user to detect. Furthermore, the urine of
healthy cats can
contain some protein. Thus, the detection of protein in feline urine can only
be an
indicator of cat health if (1) normal levels of protein (500 ppm) can be
distinguished
from disease levels (2000 ppm) and (2) the color change can be easily
interpreted.
For applications of Phloxine as an indicator of feline health, color changes
from
colorless to pink rather than red are preferred by users. Thus, much of the
discussion
will revolve around systems exhibiting color changes from colorless to pink.
However, it should be noted that, if desired, systems having color changes
from
colorless to red could readily be designed and substituted for those of
colorless to
pink.
[0016] Disclosed herein is a stable health indicating system which allows for
the
detection of protein in feline urine using an easily interpretable colorless
to pink color
The Clorox Company 4 430.204
CA 02552112 2006-07-12
change. Although the disclosure and examples focus on feline urine, as
discussed
previously, the system is applicable to mammalian urine in general. A protein
sequestering agent, e.g., dodecyl(sulfophenoxy)benzenesulfonic acid
(hereinafter
DSB), is used to inhibit the color change at low levels of protein and allow
the color
change at higher levels. DSB competes strongly with the Phloxine for active
sites on
the protein and therefore, if present in a predetermined amount, the level of
protein
detection can be controlled. Referring to FIG. 3, DSB works by inhibiting the
attachment of the Phloxine molecule to the active sites on the protein
molecule
thereby inhibiting a color change from occurring. Since the sequestering agent
is
present at a fixed level, it is effective in blocking attachment at low levels
of protein,
but at higher levels of protein free sites become available for the Phloxine
to occupy,
thus allowing the color change to occur. Compounds believed to denature
proteins (as
opposed to binding to them as DSB) could be used in place of the sequestering
agent.
Some examples of include Tritann4 surfactant, Nacconollm, and 5-sulfosalicylic
acid.
[0017] Stock buffer solutions of citric acid which sometimes also contain
sodium
citrate (ranging from about 0.5-2.5M citric acid) can be used in preparing
phloxine/DSB litter compositions as discussed in the Examples herein. Both
Phloxine
and DSB are very stable, i.e., no refrigeration is required and shelf life is
not limited.
[0018] All or a portion of an absorbent litter material is coated or imbedded
with the
Phloxine/protein sequestering agent composition described. The
Phloxine/protein
sequestering agent composition can be added to the litter as a liquid spray, a
powder
coating or ingredient, a precoated particle, a speckle, or as part of an
agglomerate
litter. Suitable absorbent litter materials include minerals, fly ash,
absorbing
pelletized materials, perlite, silicas, other absorbent materials and mixtures
thereof.
The Clorox Company 5 430.204
= CA 02552112 2006-07-12
Preferred minerals include: bentonites, zeolites, fullers earth, attapulgite,
montmorillonite diatomaceous earth, opaline silica, Georgia White clay,
sepiolite,
calcite, dolomite, slate, pumice, tobermite, marls, attapulgite, kaolinite,
halloysite,
smectite, vermiculite, hectorite, Fuller's earth, fossilized plant materials,
expanded
perlites, gypsum and other similar minerals and mixtures thereof. The
absorbent
materials can be further processed, for instance by agglomeration. The lower
the
intrinsic pH of the substrate, the lower the amount of buffering agent needed.
Thus,
substrates having intrinsically low pHs relative to other substrates are
preferred.
When contacted with the animal urine, the Phloxine/protein sequestering agent
composition changes to a color in the presence of high levels of protein and
stays
colorless in the absence of protein or in the presence of low levels of
protein.
Additional base colorants can be added to create a color change from one color
to
another color of a redder shade (e.g., blue to purple, etc.). Filler materials
such as
limestone, sand, calcite, dolomite, recycled waste materials, zeolites, and
gypsum can
also be incorporated with the absorbent materials to reduce the cost of the
litter
without significantly decreasing the material's performance as a health
indicating
litter.
100191 Partial treatment of the litter can be accomplished by incorporating
"treated
speckles" into the base litter composition. Some problems associated with
identifying
effective speckle formulations include lack of color development, spontaneous
color
change, or bleeding of color. Effective speckle materials have the following
properties: (1) Porous and rapidly absorbent; (2) Able to hold a high salt
load; (3)
Moderate surface area (about 25-300 m2/g) and large pore size (greater than
400A);
(4) Moderate surface acidity (pH between about 1-7) (5) Light color.
Presumably, the
The Clorox Company 6
430.204
CA 02552112 2012-10-11
high buffer loading and the rapid absorptivity allows for less influence from
outside
the particle. One material which fit the above-listed criteria is large pore
size silica
gel or Type MB macroporous silica gel. For example, a suspension of Phloxine B
indicator, buffered at a pH of 2.1, with a trace of Rhodocal DSB-85, can be
sprayed at
a high loading rate (e.g., 20% or greater) onto Type MB Macroporous silica gel
to
form a "treated speckle". Incorporation of "treated speckles" into high pH
clays such
as bentonite can be accomplished, but care should be taken to avoid the
Phloxine
bleeding out of the speckle particles and turning red upon contact with the
bentonite.
For example, the litter can be layered to ensure that the Phloxine is bound up
in an
immobile layer, such as guar gum. Alternatively, a polymeric non-leaching
indicator
can be used in place of the Phloxine.
[0020i Non-litter applications of the combination of Phloxine/protein
sequestering
agent are also disclosed herein. For example, test strips containing Phloxine
and DSB
can be prepared and would be useful to those in the human or veterinarian
medicine
fields.
100211 The following examples illustrate the present invention. The examples
are for
illustrative purposes only and are not meant to limit the scope of the
invention in any
way.
EXAMPLES
Materials
[00221 Bovine serum albumin (BSA) was obtained from either EMD Pharmaceuticals
or Calbiochem was used as the protein detected in all examples listed below.
BSA/water and BSA/urine standards were prepared. The urine used in BSA/urine
standards was first treated by removing the native protein by precipitation
with
sulfosalicylic acid and filtration.
*Tra.de-mark 7
CA 02552112 2006-07-12
[0023] Phloxine was obtained from Aldrich Chemical. One percent phloxine
solutions were prepared by dissolving phloxine in a small amount of methanol
and
then diluting with water.
[0024] The DBS used was Rhodacal DSB-85.
[0025] Stock buffer solutions of citric acid and sodium citrate can be used in
preparing the phloxine/DSB litter compositions. For example, a buffer solution
was
prepared by adding citric acid to water and measuring the pH. Sodium citrate
was
then added to the citric acid solution, if necessary, until the pH reached the
desired
number.
Example 1 (Preparation of silica gel litter and speckles)
[0026] A Phloxine/Citric Acid/DSB solution comprising 0.1% Phloxine, 0.05%
Rhodacal DSB-85, and the remainder 2M citric acid buffered with sodium citrate
(adjusted to pH 2.15) was dripped on about 100g of Macropore B silica gel
until the
absorption capacity of the silica gel was reached. The Phloxine/DSB-treated
silica gel
was then air-dried.
Example 2 (Preparation of a clumping Georgia White Clay (GWC) litter)
[0027] Sample 2A- 4g of a 1% phloxine solution and 0.077g DSB were combined
with 150g of citric acid buffer (2M, pH 2). 35g were applied to 50g of GWC
using a
spray gun.
[0028] Sample 2B- 2.7g of the 1% phloxine solution were added to the solution
prepared in Sample A to increase the phloxine concentration to 5%. 35g of this
solution were applied to 50g GWC using a spray gun.
[0029] Sample 2C- 2g of the 1% phloxine solution were added to the solution
prepared in Sample B to increase the phloxine concentration to 7.5%. 35g of
this
solution were applied to 50g GWC using a spray gun.
[0030] Sample 2D- 1.125g of the 1% phloxine solution were added to the
solution
prepared in Sample C to increase the phloxine concentration to 10%. 35g of
this
solution were applied to 50g GWC using a spray gun.
The Clorox Company 8 430.204
= CA 02552112 2006-07-12
=
=
[0031] The GWC samples were allowed to dry for 2 days and then were tested
with
BSA/urine standards containing varying amounts of protein. The results are
compiled
in Table 1.
Table 1
700ppm BSA/urine 1200ppm BSA/urine 3200ppm BSA/urine
Sample 2A off-white off-white light pink
Sample 2B off-white light pink Pink
Sample 2C off-white light pink Pink
Sample 2D off-white light pink Pink
Example 3 (Preparation of silica gel litter with health indicating speckels)
100321 Type C silica gel with about 5% health indicating speckles was prepared
and
tested. Macropore B silica gel was prepared as described in Example 1 and then
added to a commercial Type C silica gel litter material.
Example 4 (Preparation of clay litter with health indicating speckels1
[00331 A bentonite clay litter with about 3% health indicating speckles was
prepared
and tested. Macropore B silica gel was prepared as described in Example 1 and
then
added to a commercial bentonite litter material.
Example 5 (Detection of protein when contacted with sample litter)
[0034] The litter sample prepared in Example 3 was placed in a tray and tested
with 2
ml of base urine (low protein) and 2 ml of 10K (theoretical), 3-5K (as
analyzed)
BSA/urine. The samples were evaluated after 4 hours and after 24 hours. The
speckles contained in the section of sample tested with base urine remained
white
after 4 hours and remained unchanged after 24 hours. The speckles contained in
the
section of sample tested with a BSA/urine standard showed a pink hue after 4
hours
and remained unchanged after 24 hours.
[0035] Another sample as described in Example 3 was prepared with a larger
quantity
of urine, about 5 ml, and allowed to stand for one week. The speckles
contained in
the section of sample tested with base urine remained white. The speckles
contained
The Clorox Company 9
430.204
= ,4 CA 02552112 2006-07-12
in the section of sample tested with a BSA/urine standard contained regions of
light
pink and regions of bright pink. In all cases, the color was stable with
little to no
bleeding.
Example 6 (Detection of protein when contacted with sample litterl
[0036] The sample litter prepared in Example 4 was placed in a tray and tested
with 5
ml of base urine (low protein) and 5 ml of 10 K (theoretical), 3-5K (as
analyzed)
BSA/urine. The samples after 4 hours and after 24 hours. The speckles
contained in
the section of sample tested with base urine showed regions of white and pink
after 4
hours and remained unchanged after 24 hours. The speckles contained in the
section
of sample tested with a BSA/urine standard showed a few regions of white, some
regions of light pink and some regions of darker pink after 4 hours and
remained
unchanged after 24 hours. Due to the high alkalinity of bentonite clay, some
bleeding
of color was expected to occur. Thus, the speckles were only partially
successful in
avoiding the influence of surrounding material.
Example 7 (preparation of agglomerated health indicating clay liter)
[0037] 9.1 lbs of paste was prepared by mixing 7.8 lbs of guar gum and 1.3 lbs
of
citric acid, anhydrous and a small amount of water necessary to facilitate
mixing. The
paste was added to a mixture of: 69.9 lbs of Tennessee #10 Clay, 18.2 lbs of
Kentucky Stone Clay, 2.7 lbs of citric acid (anhydrous), and 0.10 lbs Phloxine
to form
a litter mixture. Other similar clay materials could be substituted for the
Tennessee
#10 Clay and the Kentucky Stone Clay. The litter mixture was agglomerated
through
extrusion (or other agglomeration means) to form an agglomerated health
indicating
litter material.
[0038] It is to be understood that the invention described herein is not
limited to
particularly exemplified systems or process parameters as such may, of course,
vary.
It is also to be understood that the terminology used herein is for the
purpose of
describing particular embodiments of the invention only, and is not intended
to limit
the scope of the invention in any manner.
The Clorox Company 10
430.204
CA 02552112 2012-10-11
[0039]
[0040] It must be noted that, as used in this specification and the appended
claims, the
singular forms "a," "an" and "the" include plural referents unless the content
clearly
dictates otherwise. Thus, for example, reference to a "buffering agent"
includes two
or more such agents.
[0041] All numbers expressing quantities of ingredients, constituents,
reaction
conditions, and so forth used in the specification and claims are to be
understood as
being modified in all instances by the term "about". Notwithstanding that the
numerical ranges and parameters setting forth the broad scope of the subject
matter
presented herein are approximations, the numerical values set forth in the
specific
examples are reported as precisely as possible. Any numerical value, however,
inherently contain certain errors necessarily resulting from the standard
deviation
found in their respective testing measurements.
[0042] A number of methods and materials similar or equivalent to those
described
herein can be used in the practice of the present invention. Many
modifications and
variations are possible in light of the above teaching.
11