Note: Descriptions are shown in the official language in which they were submitted.
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
COMPRESSION TREATMENT SYSTEM
BACKGROUND
1. Technical Field
The present disclosure generally relates to the field of vascular therapy for
application to a limb of a body, and more particularly, to a compression
treatment system
having a controller that regulates fluid flow.
2. Description of the Related Art
A major concern for immobile patients and persons alike are medical conditions
that
form clots in the blood, such as, deep vein thrombosis (DVT) and peripheral
edema. Such
patients and persons include those undergoing surgery, anesthesia, extended
periods of bed
rest, etc. These blood clotting conditions generally occur in the deep veins
of the lower
extremities and/or pelvis. These veins, such as the iliac, femoral, popiteal
and tibial return
deoxygenated blood to the heart. For example, when blood circulation in these
veins is
retarded due to illness, injury or inactivity, there is a tendency for blood
to accumulate or
pool. A static pool of blood is ideal for clot formations. A major risk
associated with this
condition is interference with cardiovascular circulation. Most seriously, a
fragment of the
blood clot can break loose and migrate. A pulmonary emboli can form blocking a
main
pulmonary artery, which may be life threatening.
The conditions and resulting risks associated with patient immobility may be
controlled or alleviated by applying intermittent pressure to a patient's
limb, such as, for
example, a leg including the thigh, calf and foot to assist in blood
circulation. Known
devices have been employed to assist in blood circulation, such as, one piece
pads and
compression boots. See, for example, U.S. Patent Nos. 6,290,662 and 6,494,852.
For example, sequential compression devices have been used, which consist of
an
air pump connected to a disposable wraparound pad by a series of air tubes.
The
wraparound pad is configured for placement about a portion of a patient's leg,
such as the
thigh, calf or foot. Multiple pads may be mounted to the leg to cover the
various portions of
1
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
the leg. Air is then forced into different parts of the wraparound pad(s) in
sequence,
creating pressure around the thigh, calf or foot, thereby improving venous
return.
These known devices may suffer from various drawbacks due to their bulk and
cumb.ersome nature of use. These drawbacks reduce comfort, compliance and may
disadvantageously prevent mobility of the patient as recovery progresses after
surgery.
Further, such known sequential compression devices typically include a
controller
assembly that regulates air flow and pressure in the wraparound pad(s). The
controller
assembly can be mounted to a bed and plugged into a wall outlet for power
during use: This
arrangement, however, can present challenges for example, when the patient
needs to
perforin certain tasks, e.g., bathroom, physical tlierapy, etc. In these
situations, the pads are
usually removed, thus disadvantageously discontinuing vascular therapy. Thus,
these
controller assemblies suffer from various drawbacks because they do not
accommodate
patient transport or mobility and are not typically adaptable for inflation of
thigh, calf and
foot pads.
Therefore, it would be desirable to overcome the disadvantages and drawbacks
of
the prior art with a compression treatment system having a controller that is
adaptable for
inflating thigh, calf and foot sleeves and accommodates patient transport and
mobility to
provide continuous vascular therapy. It would be desirable if the system
automatically
detects the types of sleeves connected tliereto. It would be highly desirable
if the system
included a pneumatic circuit that facilitates pressure monitoring with a
single pressure
transducer to achieve the advantages of the present disclosure. It is
conteinplated that the
compression treatment system is easily and efficiently manufactured.
SUMMARY
Accordingly, a compression treatment system is provided having a controller
that is
adaptable for inflating thigh, calf and foot sleeves and accommodates patient
transport and
mobility to provide continuous vascular therapy for overcoming the
disadvantages and
drawbacks of the prior art. Desirably, the system automatically detects the
types of sleeves
connected thereto. Most desirably, the system *includes a pneumatic circuit
that facilitates
pressure monitoring with a single pressure transducer to achieve the
advantages of the
present disclosure. The compression treatment system is easily and efficiently
fabricated.
2
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
The compression treatment system, in accordance with the principles of the
present
disclosure, can provide intermittent pneumatic compression for the prevention
of DVT. The
compression treatment system may also include venous refill detection, as will
be discussed,
and is compact, quiet, lightweight, and offers battery power. The compression
treatment
system also has the ability to provide sequential, gradient compression to
each limb
individually and the flexibility to provide compression to various sleeves,
which may, for
example, include three bladders. The sleeves may include thigh length tear-
away features
and knee length sleeves, as will be discussed. In addition, the compression
treatment
system can provide higher pressure, slow compression to a foot sleeve. The
compression
treatment system provides uninterrupted DVT prophylaxis as the system is used
throughout
a treatment facility, and can be worn and used continuously by the patient
during the entire
period of risk.
The compression treatment system may be portable to provide continuous therapy
for the patient at risk for DVT. This configuration advantageously facilitates
continuous
vascular therapy during patient activity and tasks such as, for example,
transport for testing,
bathroom, physical therapy, etc. Thus, the compression treatinent system
prevents
interruptions in therapy by providing a controller that will run on a battery
when it is not
plugged in, and will also be comfortable, compact, and light enough to move
with the
patient as needed.
The compression treatment system includes a controller, tubing sets, and
sleeves.
For example, the compression treatment system,delivers air through the tubing
sets to a pair
of disposable sleeves, one for each limb. The sleeves can have three bladders
each, which
correspond to the ankle, calf and thigh. The compression treatment system
independently
compresses one of the limbs, left or right. Inflation is alternated between
the two limbs
when both are connected. Alternatively, only one sleeve can be connected.
Alternatively, the compression treatment system is used as a slow compression
foot
device. In this configuration, the compression treatment system includes a
pair of single-
patient-use, single-bladder disposable foot garments alternative to the
sleeves. A single foot
garment may also be used. The compression treatment system also provides for
employment of a foot garment on a first limb and a sleeve on a second limb.
3
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
The compression treatment system includes tubing set connector ports that
interlock
with the mating geometry on the tubing sets. When the compression treatment
system is
initially powered, air is delivered through the ports until the system
recognizes which ports
are connected to a sleeve and what types of sleeves, i.e., leg sleeve or foot
garment, are
connected to those ports. Compression therapy is delivered to the ports with
the appropriate
sleeves connected.
For example, the compression treatment system provides clinical parameters for
vascular therapy such as an 11-second inflation cycle, followed by a vent
period of 20 to 60
seconds, depending on the venous refill measurement. The 11-second compression
time is
sequential: at 0 seconds a first bladder starts inflating. At 2.67 seconds a
second bladder
starts inflating, and at 5.67 seconds a third bladder starts inflating. After
11 seconds, all
three bladders vent. The pressures during the inflation period must remain
gradient with the
first bladder being greater than the second bladder, and the second bladder
being greater
than the third bladder. By way of example, the end of cycle pressures may be
45 mm Hg in
the first bladder, 40 mm Hg in the second bladder, and 30 mm Hg in the third
bladder.
Compression continues in this cyclical pattern until either the compression
treatment system
is turned off or the controller alarms.
By way of another non-limiting example, the foot compression parameters may
include a 5-second inflation cycle followed by the same vent period timing as
provided
above for the sleeve compression (20-60 seconds). The end of cycle pressure
for the foot
sleeve will have a set pressure target of 130 mm Hg by the end of the 5-second
inflation
period.
Venous refill detection may be employed with the compression treatinent
system.
Venous refill detection includes trapping a small amount of air in the second
bladder
described and monitoring the pressure increase as the veins in the limb of a
patient refill
witlz blood. As the compression treatment system reaches set pressure, and
every 30
minutes thereafter, the controller measures venous refill and adjusts the vent
time between
inflation cycles for any individual limb from 20 to 60 seconds. The longer of
the venous
refill measurements from both limbs will be used to adjust the vent time.
The compression treatment system benefits from several advantages including a
battery powered controller that is compact and lightweight for portability.
The compression
4
CA 02552353 2007-10-02
treatment system may also be used with one or two limbs and can provide slow
compression to a foot garment. The compression treatment system can also
detect the
type of sleeve connected and automatically apply the appropriate compression.
The compression treatment system also includes a pneumatic circuit designed
for use with the compression treatment system to allow for bladder inflation
and
pressure monitoring using only one transducer. Pressure monitoring from the
manifold-
side of the solenoid valves accounts for the pressure drop across the valves
with the
added advantage of only requiring one transducer to monitor any connected
bladder.
This configuration advantageously results in a lower manufacturing cost and
reduced
maintainance requirements, particularly with regard to transducer calibration.
In one aspect, in accordance with the principles of the present disclosure,
there
is provided a compression treatment system comprising: a first bladder
supported about
a limb; a second bladder supported about the limb, the first and second
bladders being
in fluid communication with a fluid source and the first and second bladders
being
inflated such that the first bladder is inflated for a first time period and
the second
bladder is inflated for a second time period, the second time period and
additional time
periods being initiated within the first time period; and a pneumatic control
circuit
located at a controller housed with a housing separately from the first and
second
bladders, the pneumatic control circuit including the controller, a single
pressure
sensor, a single check valve, the fluid source and a plurality of solenoid
valves, the
single pressure sensor located between the fluid source and the plurality of
solenoid
valves and communicating with the first bladder and the second bladder, and
the single
check valve operably connected to the fluid source and located between the
fluid source
and the plurality of solenoid valves, wherein the single check valve prevents
leakage
out of the first bladder, and the single pressure sensor measures bladder
pressure in
cooperation with the controller to calculate venous refill time at the first
bladder.
The compression treatment system may include a controller that communicates
with the pressurized fluid source and the pressure transducer. The controller
is
configured to monitor and regulate pressure in the bladders. The controller
may be
-5-
CA 02552353 2007-10-02
disposed with a housing that is portable. The housing may include a plurality
of ports
connectable to a plurality of bladders.
The pressure transducer can monitor pressure at each of the plurality of ports
to
determine if a bladder is connected thereto and sends a representative signal
to the
controller. The controller may include separate valves that regulate inflation
of the
bladders. The compression treatment system may define a pneumatic circuit. The
pressure transducer may be coupled to the pneumatic circuit and disposed
between the
pressurized fluid source and the valves in the pneumatic circuit.
The compression treatment system may include a third bladder supported about
a foot. The third bladder is in fluid communication with the fluid source and
the single
pressure sensor communicates with bladders. The pressurized fluid source can
alternately inflate the bladders disposed about the limb and the bladder
disposed about
the foot.
Thus, in another aspect, there is provided a compression treatment system
comprising: a first bladder supported about a limb; a second bladder supported
about
the limb, the first and second bladders being in fluid communication with a
fluid source
and the first and second bladders being inflated such that the first bladder
is inflated for
a first time period and the second bladder is inflated for a second time
period, the
second time period being initiated within the first time period; a third
bladder supported
about a foot, the third bladder being in fluid communication with the fluid
source; and a
pneumatic control circuit located at a controller housed separately from the
inflatable
bladders, the pneumatic control circuit including the controller, a single
pressure
sensor, a single check valve, the fluid source and a plurality of solenoid
valves, the
single pressure sensor, located between the fluid source and the plurality of
solenoid
valves, communicating with the first and second bladders and the single check
valve
operably connected to the fluid source and located between the fluid source
and the
plurality of solenoid valves, wherein the single check valve prevents leakage
out of a
bladder being measured, the single pressure sensor measures bladder pressure
in
cooperation with the controller to calculate venous refill time at the
measured bladder.
-6-
CA 02552353 2007-10-02
In another aspect, there is provided a compression treatment system
comprising:
a first plurality of bladders supported about a first limb; a second plurality
of bladders
supported about a second limb, the bladders being in fluid communication with
a fluid
source and the first and second plurality of bladders being inflated such
that: a first
bladder of the first plurality of bladders is inflated for a first time period
and a second
bladder of the first plurality of bladders is inflated for a second time
period, the second
time period being initiated within the first time period, and a first bladder
of the second
plurality of bladders is inflated for a third time period and a second bladder
of the
second plurality of bladders is inflated for a fourth time period, the fourth
time period
being initiated within the third time period; and a pneumatic control circuit
located at a
controller housed separately from the inflatable bladders, the pneumatic
control circuit
including the controller, a single pressure sensor, a single check valve, the
fluid source
and a plurality of solenoid valves, the single pressure sensor, located
between the fluid
source and the plurality of solenoid valves, communicating with the first and
second
plurality of bladders and the check valve operably connected to the fluid
source and
located between the fluid source and the plurality of solenoid valves, wherein
the single
check valve prevents leakage out of a bladder being measured, the single
pressure
sensor measures bladder pressure in cooperation with the controller to
calculate venous
refill time at the measured bladder.
In another aspect, there is provided a compression treatment system
comprising:
a first plurality of bladders being supported about a first limb and a second
plurality of
bladders being supported about a second limb; each bladder of the first
plurality of
bladders and the second plurality of bladders having a separate valve in
communication
therewith, the valves being in fluid communication with a fluid source and the
first and
second plurality of bladders being inflated such that: a first valve is open
such that a
first bladder of the first plurality of bladders is inflated for a first time
period and a
second valve is open such that a second bladder of the first plurality of
bladders is
inflated for a second time period, the second time period being initiated
within the first
time period, and a third valve is open such that a third bladder of the first
plurality is
nflated for a third time period, the third time period being initiated within
the second
time period, and a fourth valve is open such that a first bladder of the
second plurality
-7-
CA 02552353 2007-10-02
of bladders is inflated for a fourth time period and a fifth valve is open
such that a
second bladder of the second plurality of bladders is inflated for a fifth
time period, the
fifth time period being initiated within the fourth time period, and a sixth
valve is open
such that a sixth bladder of the second plurality is inflated for a sixth time
period, the
sixth time period being initiated within the fifth time period; a pneumatic
control circuit
located at a controller housed separately from the first and second plurality
of bladders,
the pneumatic control circuit including the controller, a single pressure
sensor, a single
check valve, the fluid source and a plurality of solenoid valves, the
controller
communicates with the pressurized fluid source and the single pressure sensor,
the
controller being configured to monitor and regulate pressure in the first and
second
plurality of bladders, the single pressure sensor, located between the fluid
source and
the plurality of solenoid valves, communicating with the first and second
plurality of
bladders and the check valve operably connected to the fluid source and
located
between the fluid source and the plurality of solenoid valves, wherein the
single check
valve prevents leakage out of a bladder being measured, the single pressure
sensor
measures bladder pressure in cooperation with the controller to calculate
venous refill
time at the measured bladder.
BRIEF DESCRIPTION OF THE DRAWINGS
The objects and features of the present disclosure, which are believed to be
novel, are set forth with particularity in the appended claims. The present
disclosure,
both as to its organization and manner of operation, together with further
objectives and
advantages, may be best understood by reference to the following description,
taken in
connection with the accompanying drawings, which are described below.
FIG. 1 is a front view of one particular embodiment of a compression treatment
system in accordance with the principles of the present disclosure;
FIG. 2 is a side view of the compression treatment system shown in FIG. 1;
FIG. 3 is a top view of the compression treatment system shown in FIG. 1;
- 7a -
CA 02552353 2007-10-02
FIG. 4 is a rear view of the compression treatment system shown in FIG. 1;
FIG. 5 is a schematic representation of a pneumatic circuit of the compression
treatment system shown in FIG. 1;
FIG. 6 is a plan view of a sleeve of the compression treatment system shown in
FIG. 1 being disposed about a limb;
FIG. 7 is an alternate embodiment of the sleeve shown in FIG. 6; and
FIG. 8 is another alternate embodiment of the sleeve shown in FIG. 6.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
The exemplary embodiments of the compression treatment system and methods
of operation disclosed are discussed in terms of vascular therapy including a
prophylaxis compression apparatus for application to a limb of a body and more
particularly in terms of a compression treatment system having a controller
that is
adaptable for inflating thigh, calf, ankle and foot sleeves and accommodates
patient
transport and mobility. It is contemplated
- 7b-
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
that the compression treatment system may be employed for preventing and
overcoming the
risks associated with patient immobility. It is - further contemplated that
the compression
treatment system alleviates the conditions arising from patient immobility to
prevent for
example, DVT, peripheral edema, etc. It is contemplated that the compression
treatment
system according to the present disclosure may be attributable to all types of
venous
compression systems, including, but not limited to a prophylaxis sequential
compression
apparatus. The term "prophylaxis sequential" shall not be construed as
limiting the general
venous compression treatment system described herein. It is envisioned that
the present
disclosure, however, finds application with a wide variety of immobile
conditions of
persons and patients alike, such as, for example, those undergoing surgery,
anesthesia,
extended periods of bed rest, obesity, advanced age, malignancy, prior
thromboembolism,
etc.
In the discussion that follows, the term "proximal" refers to a portion of a
structure
that is closer to a torso of a subject and the term "distal" refers to a
portion that is further
from the torso. As used herein the term "subject" refers to a patient
undergoing vascular
therapy using the compression treatment system. According to the present
disclosure, the
term "practitioner" refers to an individual administering the compression
treatment system
and may include support personnel.
The following discussion includes a description of the compression treatment
system, followed by a description of an exemplary method of operating the
compression
treatment system in accordance with the principles of the present disclosure.
Reference will
now be made in detail to the exemplary embodiments and disclosure, which are
illustrated
with the accompanying figures.
Turning now to the figures, wherein like components are designated by like
reference numerals throughout the several views. Referring initially to FIGS.
1-5, there is
illustrated a compression treatment system 10, constructed in accordance with
the principles
of the present disclosure. Compression treatment system 10 includes a housing
12.
Housing 12 encloses the components of a controller 14 (shown schematically in
FIG. 5)
disposed therein.
Housing 12 has a semi-circular configuration and has a handle cutout 16 along
its
apex 18 to facilitate transport and subject mobility. It is envisioned that
housing 12 may be
8
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
variously configured and dimensioned such as, for example, rectangular,
spherical, etc. It is
further envisioned that housing 12 may be assembled by any appropriate process
such as,
for example, snap fit, adhesive, solvent weld, thermal weld, ultrasonic weld,
screw, rivet,
etc. Alternatively, housing 12 may be monolithically fornied or integrally
assembled of
multiple housing sections and may be substantially transparent, opaque, etc.
Housing 12
may include ribs, ridges, etc. to facilitate manipulation of compression
treatment system 10.
The components of housing 12 can be fabricated from a material suitable for
medical applications, such as, for example, polymerics or metals, such as
stainless steel,
depending on the particular medical application and/or preference of a
clinician. Semi-rigid
and rigid polymerics are contemplated for fabrication, as well as resilient
materials, such as
molded medical grade polypropylene. However, one skilled in the art will
realize that other
materials and fabrication methods suitable for assembly and manufacture, in
accordance
with the present disclosure, also would be appropriate.
Housing 12 is portable to facilitate coritinuous vascular therapy to a subject
(not
shown). Housing 12 includes a bracket 20 that facilitates releasable mounting
of housing 12
with for example, a hospital bed, table, etc. Bracket 20 extends from a rear
portion 22 of
housing 12 and provides a hook configuration for suspending housing 12 from a
subject's
bed, etc. It is contemplated that bracket 20 may be suspended from various
structure for
releasable mounting of housing 12, or alternatively, that housing 12 does not
include a
bracket and may be placed on a floor or other supporting surface.
Alternatively, housing 12
includes a shoulder strap 24, as shown in FIG. 2, that allows housing 12 to be
worn on the
subject or practitioner during transport. Shoulder strap 24 may be employed
with or without
bracket 20 and may, for example, be secured to any portion of the housing 12
including
handle 16.
Compression treatment system 10 employs an electrical AC/DC switching power
supply for operation of its components. A power cord 26 is connected to
housing 12 for
conducting power to the components of controller 14. Power cord 26 accesses an
AC
power supply via a wall outlet, etc. Controller 14 may include a transformer
or other
electronics for connecting to the power supply. It is envisioned that power
cord 26 may be
wrapped around bracket 20 for storage and during transport and subject
mobility. It is
further envisioned that compression treatment system 10 may include a storage
capture
9
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
mechanism that retains power cord 26 with housing 12. The storage capture
mechanism
may include an elastic cord, pulley, etc.
Compression treatment system 10 also employs a battery 28 for powering the
components of controller 14 to facilitate transport and subject mobility.
Battery 28 is
disposed within a battery compartinent 30 of housing 12. It is contemplated
that battery 28
may include one or a plurality of cells. The battery cells may be lithium-ion
type, etc. It is
further contemplated that battery 28 is rechargeable and may be employed for
various
ranges of operation time, such as, for example, 6 hours, 8 hours, 10 hours,
etc. For
example, power cord 26 may be unplugged and captured by the storage capture
mechanism
of housing 12. Compression treatment system 10 then runs on battery 28 power
and the
subject is ambulatory.
It is envisioned that battery 28 may be mounted to an exterior surface of
housing 12
or separate therefrom. It is further envisioned that compression treatment
system 10 may
include alternate sources of power supply, such as, for example, solar, non-
electrical, etc.,
or alternatively may not include battery power.
Housing 12 has a control panel 32 disposed on a front surface 34 thereof.
Control
panel 32 includes controls and indicators for operation of compression
treatment system 10.
Control panel 32 has an LED display 36 that provides status indicia, messages,
etc. of the
various components of system 10, such as, for example, power, battery, sleeve
identification
and connection, inflation, venting, venous refill, errors, etc. Control panel
32 also includes
manually activated switches for powering system 10, etc. It is contemplated
that such
switches are membrane type actuated by finger pressure, etc.
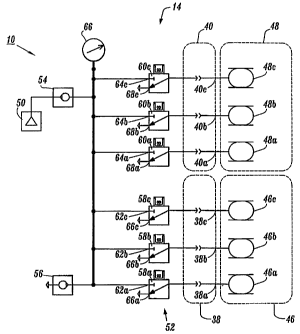
Rear portion 22 of housing 12 defines ports 38, 40 (FIG. 4). Ports 38, 40
include
output ports 38a, 38b, 38c, and output ports 40a, 40b, 40c, respectively.
Output ports 38a,
38b, 38c, and output ports 40a, 40b, 40c are in fluid communication with
inflatable
chambers 46a, 46b, 46c of a compression sleeve 46 and inflatable chambers 48a,
48b, 48c
of a compression sleeve 48, respectively, which are configured to fit around
the legs of a
subject, via a mating connector 42 and tubing set 44, as will be discussed.
Output ports
38a, 38b, 38c, 40a, 40b, 40c are configured for connection to tubing set 44.
Each of ports
38, 40 are connectable to a particular compression sleeve, for example, leg
sleeve, foot
sleeve, etc.
CA 02552353 2007-10-02
Ports 38, 40 are also connected with the components of controller 14 disposed
within housing 12 to facilitate inflation of selected compression sleeves, as
illustrated
in the pneumatic circuit shown in FIG. 5. Controller 14 includes a pressurized
fluid
source, such as, for example, a pump 50 that fluidly communicates with a valve
manifold 52 for connection with ports 38, 40, as will be discussed. Pump 50
includes a
motor that compresses air to valve manifold 52 via tubing or the like. The
speed of the
pump motor is electronically controlled to provide a corresponding compressor
speed
for respective output pressures as desired. It is contemplated that a power
supply board,
including the necessary electronics, circuitry, software, etc. known to one
skilled in the
art, is connected to the pump motor and other components of controller 14 to
regulate
power thereto. It is envisioned that pump 50 may be a diaphragm pump.
Controller 14 also includes a check valve 54 that prevents air leakage back
through pump 50 when monitoring bladder pressure during venous refill
detection, as
will be discussed. A pressure relief valve 56 is disposed with the pneumatic
circuit to
protect against over pressure in the compression sleeves. Pressure relief
valve 56 is
configured to bleed excess air pressure if necessary. It is contemplated that
various
types of valves may be employed such as, for example, spring loaded plunger
valves,
etc.
Valve manifold 52 includes solenoid valves 58a, 58b, 58c, 60a, 60b, 60c that
are coupled to output ports 38a, 38b, 38c, 40a, 40b, 40c, respectively.
Solenoid valves
58a, 58b, 58c, 60a, 60b, 60c each have an associated solenoid that is
electrically driven
via a control processor of controller 14. The solenoid is coupled to a valve
seat of each
particular solenoid valve 58a, 58b, 58c, 60a, 60b, 60c such that the seat is
operative to
open and close the respective solenoid valve upon actuation of the solenoid.
See, for
example, the solenoid valves described in U.S. Patent No. 5,876,359 to Bock et
al. It is
contemplated that the control processor of controller 14 includes the
necessary
electronics, circuitry, software, etc. known to one skilled in the art to
actuate solenoid
valves 58a, 58b, 58c, 60a, 60b, 60c in response to varying conditions of
compression
treatment system 10 and other indications and measurements sensed by the
components
of controller 14. It is envisioned that one or a plurality of solenoid valves
may be
employed, or alternatively, that other types of valves may be used.
-11-
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
Solenoid valves 58a, 58b, 58c, 60a, 60b, 60c and their associated valve
components
are mounted to ports 38, 40 on the interior of housing 12. Solenoid valves
58a, 58b, 58c,
60a, 60b, 60c are two position, three-way normally closed valves, which have
openings
62a, 62b, 62c, 64a, 64b, 64c, respectively. In the open position, air flows
through openings
62a, 62b, 62c, 64a, 64b, 64c to the associated output port 38a, 38b, 38c, 40a,
40b, 40c and
into inflatable chambers 46a, 46b, 46c of coinpression sleeve 46 and
inflatable chambers
48a, 48b, 48c of compression sleeve 48. In the closed position, openings 62a,
62b, 62c,
64a, 64b, 64c are blocked and air from compression sleeves 46, 48 flows back
through
output port 38a, 38b, 38c, 40a, 40b, 40c and through vent ports 66a, 66b, 66c,
68a, 68b, 68c
of the associated valve to deflate inflatable chambers 46a, 46b, 46c, 48a,
48b, 48c.
Solenoid valves 58a, 58b, 58c, 60a, 60b, 60c are operated in sequence to
pressurize
inflatable chambers 46a, 46b, 46c, 48a, 48b, 48c and provide sequential
pressurization
thereof and venting of the chambers under the control processor of controller
14. It is
contemplated that solenoid valves 58a, 58b, 58c, 60a, 60b, 60c may be
selectively actuated
when cooling operation of the sleeves is desired, see for example, U.S. Patent
No. 5,876,359
to Bock et al.
Solenoid valves 58a, 58b, 58c, 60a, 60b, 60c are driven by pulse width
modulated
signals provided by the control processor of controller 14. The solenoid drive
signals are
initially at a higher power level for rapid and positive actuation of the
solenoid valves..After
initial actuation, the drive signals can be decreased, for example, by
approximately 70% to
maintain valve activation, thereby reducing power consumption. It is
envisioned that
solenoid valves 58a, 58b, 58c, 60a, 60b, 60c may be deactivated as desired. It
is further
envisioned that the control processor of controller 14 includes the ability to
verify the status
of solenoid valves 58a, 58b, 58c, 60a, 60b, 60c. As the condition of solenoid
valves 58a,
58b, 58c, 60a, 60b, 60c changes, the control processor verifies their status.
For example, if
a particular valve is detected to be shorted or open, compression treatment
system 10 will
go into a particular error mode, as will be discussed.
Controller 14 also includes a pressure transducer 66 disposed within housing
12.
Pressure transducer 66 is coupled to the pneumatic circuit and disposed
between pump 50
and solenoid valves 58a, 58b, 58c, 60a, 60b, 60c via tubing or the like.
Pressure transducer
66 is in fluid communication with inflatable chambers 46a, 46b, 46c, 48a, 48b,
48c for
12
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
monitoring pressure in each of inflatable chainbers 46a, 46b, 46c, 48a, 48b,
48c. The
control processor of controller 14 directs pressure transducer 66 to measure
any of inflatable
chambers 46a, 46b, 46c, 48a, 48b, 48c that are connected to their respective
solenoid valve
and thus in fluid communication therewith. Disposing pressure transducer 66
before the
solenoid valves, on the manifold side of the pneulnatic circuit,
advantageously facilitates
use of only a single pressure transducer for measuring the pressure in the
inflatable
chambers. This configuration facilitates inflation of one or a plurality of
inflatable
chambers. This configuration also advantageously reduces bulk of controller 14
to
contribute to the compact and lightweight design of compression treatment
system 10,
facilitates transport, patient mobility and reduces manufacturing costs.
For example, during a selected compression cycle, solenoid valves 58a, 58b,
58c,
60a, 60b, 60c are sequentially energized to the open position for
pressurizing, in sequence,
inflatable chambers 46a, 46b, 46c, 48a, 48b, 48c. In the open position,
solenoid valves 58a,
58b, 58c, 60a, 60b, 60c allow passage of air from pump 50 through the
respective output
ports 38a, 38b, 38c, 40a, 40b, 40c to the inflatable chambers. Pressure
transducer 66
monitors the pressure of each of inflatable chambers 46a, 46b, 46c, 48a, 48b,
48c of the
pneumatic circuit and provides an electrical signal input to the control
processor of
controller 14 for feedback control.
At the end of the selected compression cycle, solenoid valves 58a, 58b, 58c,
60a,
60b, 60c are simultaneously de-energized to the closed position for
disconnecting pump 50
from sleeves 46, 48. In the closed position, pump 5 0 air is blocked and
solenoid valves 58a,
58b, 58c, 60a, 60b, 60c vent sleeve pressure to the atmosphere via vent ports
66a, 66b, 66c,
68a, 68b, 68c on valve manifold 52. It is contemplated that compression
treatment system
10 can alternate inflation of the chambers between a first limb and a second
limb. It is
further conteinplated that compression treatment system 10 can individually
inflate each
bladder.
Referring to FIG. 6, compression treatment system 10, similar to that
described
above, is assembled and packaged for use. In operation, compression treatment
system 10
includes controller 14 disposed with housing 12, described above, and a sleeve
112. Sleeve
112 includes a thigh bladder 114, a calf bladder 116 and an ankle bladder 118.
Sleeve 112
includes a connector 120 that mates with mating connector 42, which is
comiected to port
13
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
38 via tubing 44. Connector 120 fluidly communicates with the chambers of
sleeve 112 via
tubing set 122. Thus, this configuration facilitates fluid communication
between bladders
114, 116, 118 and pump 50. It is contemplated herein that connector 120 may
further
include a valve mechanism to control fluid flow.
Sleeve 112 is provided and manipulated for disposal about leg L of the subject
(not
shown). Connector 120 is mated with mating connector 42 to establish fluid
communication between sleeve 112 and the pneumatic circuit. Sleeve 112 is
wrapped about
leg L and secured thereto via hook and loop pads 124, 126. It is contemplated
that
compression treatment system 10 may treat a second leg of a subject with a
compression
sleeve, similar to sleeve 112, via connection to port 40. The second leg is
treated in
compression cycles alternate to the compression cycles described below for
treatment of leg
L, as described below in the alternative.
The portable features of housing 12 and controller 14, described above,
provide a
compression treatinent system 10 that facilitates transport and subject
mobility. This
advantageous configuration provides uninterrupted DVT prophylaxis as the
system is used
throughout a treatment facility, and can be worn and used continuously by the
subject
during the entire period of risk. Compression treatment system 10
advantageously
facilitates continuous vascular therapy during subject activity and tasks such
as, for
example, transport for testing, bathroom, physical therapy, etc. Compression
treatment
system 10 prevents interruptions in therapy by providing controller 14 that
will run on
battery 28 when power cord 26 is not plugged in, and will also be comfortable,
compact,
and light enough to move with the subject as needed.
The manually activated switches of control panel 32 of controller 14 switch
compression treatment system 10 on for powering thereof. As compression
treatment
system 10 is initially switched on, a series of self-tests are conducted by
the control
processor of controller 14. The LED indicators of display 36 are illuminated
and audible
indicia are sounded to verify the operability of the visual and audible
indicators. Display 36
is illuminated to verify display operability. Controller 14 also verifies
operability of the
software of the control processor. If any of the verification fails, error
codes provide a
representative audible and/or visual indicia.
14
CA 02552353 2007-10-02
It is contemplated that if the control processor of controller 14 cannot
continue
normal software execution, an error code will be triggered. This causes
compression
treatment system 10 to reset and restart normal operation. Sleeve 112 would
vent
during a restart procedure. Audible and visual indicia may also engage to
represent the
condition.
Upon completion of the self-test sequence compression for treatment system 10,
controller 14 begins a sleeve detection procedure to determine the type(s) of
sleeves
attached to ports 38, 40. Sleeve detection is performed during a first
inflation
(detection) cycle after controller 14 is initially powered on. During the
detection cycle,
air is delivered alternately through ports 38, 40 with pump 50 operating for
two
seconds, or until the pressure reaches a default threshold. One second later,
pressure
transducer 66 takes a pressure measurement to determine whether or not a
bladder is
connected to a particular output port, 38a, 38b, 38c, 40a, 40b or 40c under
sleeve
detection.
For example, the detection procedure is conducted for bladders 114, 116, 118
for each of sleeve ports 38, 40. If there is no backpressure at a particular
outlet port for
connection with a bladder, then the control processor of controller 14
determines that a
bladder is not being used with a particular outlet port. The control processor
adjusts the
compression therapy for the detected sleeve configuration accordingly. For the
3-
bladder sleeve, back pressure is detected at bladders 114, 116, 118 when
connected to
controller 14. It is contemplated that if no sleeves are detected by this
procedure at
either port 38 or 40, or if the detected configuration is not recognized, then
a low
pressure error is triggered with corresponding audible indicia. It is further
contemplated
that various timing periods may be employed for detection inflation and
pressure
measurement, according to the requirements of a particular application.
Alternatively, thigh bladder 114 is removable from calf bladder 116. For
example, calf bladder 116 is removably connected to thigh bladder 114 via a
perforated
attachment, see, for example, the sleeve described in U.S. Published Patent
Application
Serial No. 20050187503, filed on February 23, 2004 and entitled Compression
Apparatus. For the removable thigh bladder 114, the control processor of
controller 14
-15-
CA 02552353 2007-10-02
performs a similar sleeve detection procedure, as described above. The control
processor will detect a 3-bladder sleeve due to a flow-restricting valve (not
shown)
fitted with connector 120. See, for example, the flow-restricting valve
described in U.S.
Published Patent Application Serial No. 20050184264, filed on February 23,
2004 and
entitled Fluid Conduit Connector Apparatus. The flow restricting valve
simulates the
backpressure created by thigh bladder 114 when there is actually no bladder
connected.
Thus, the conversion from a 3-bladder thigh length sleeve to a 2-bladder knee
length
sleeve does not significantly impact the compression parameters, and
controller 14
continues vascular therapy as if thigh bladder 114 was still intact.
In an alternate embodiment, as shown in FIG. 7, sleeve 112 includes thigh
bladder 114 and a unitary second bladder 218. Second bladder 218 has a calf
portion
220 and an ankle portion 222. Pump 50 fluidly communicates with sleeve 112 via
valve
connector 224 and separate tubing 226, 228, for employment similar to that
described
above, including the optional removal of thigh bladder 114 via perforations or
the like.
In one particular compression cycle for compression treatment system 10, the
compression parameters include an 11-second inflation period for inflating
bladders
114, 116, 118 followed by 60 seconds of venting for deflating bladders 114,
116, 118.
The 11-second inflation period is sequential:
1) initially ankle bladder 118 is inflated for a first time period starting at
0
seconds;
2) thereafter and during the first time period, inflation of calf bladder 116
is
initiated for a second time period, the initiation of the second time period
coinciding
with approximately 2.67 seconds duration of the first time period;
3) thereafter and during the second time period, inflation of thigh bladder
114 is initiated for a third time period, the initiation of the third time
period at
approximately 3.0 seconds duration of the second time period and approximately
5.67
seconds of the first time period; and
-16-
CA 02552353 2007-10-02
4) after 11 seconds of the first time period, bladders 114, 116, 118 vent for
a minimum of 20 seconds and a maximum of 60 seconds. An example is illustrated
in
Table 1 below.
- 16a -
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
Table 1
Start of Se uence End of Sequence
Ankle Compression: 0 seconds 2 2/3 seconds
Ankle/Calf Compression: End of Ankle 5 2/3 seconds
Ankle/Calf/Thigh Compression: End of Ankle/Calf 11.0 seconds
Decompression/Vent: Minimum 20 seconds, maximum 60 seconds
It is contemplated that the vent period is measured from the end of one
inflation
cycle to the beginning of the next inflation cycle on leg L. It is further
contemplated that
both limbs of the subject may be treated and compression treatment system 10
alternates
vascular therapy from leg L to the second leg. It is envisioned that the time
period from the
end of the inflation cycle for leg L to the initiation of the inflation cycle
for the second leg
can range, for example, from 4.5-24.5 seconds.
During the initial inflation cycle for treating leg L, as described above,
pump 50
initiates a low default voltage so as to not over-inflate bladders 114, 116,
118 on the initial
cycle. Solenoid valves 58a, 58b, 58c are energized to the open position, as
described, such
that the valves open to deliver air to ankle bladders 118, then calf bladder
116, then thigh
bladder 114 of sleeve 112 using a desired cycle timing sequence. Pressure
transducer 66
monitors the pressure in each of bladders 114, 116, 118 throughout the 11-
second
compression cycle. At the conclusion of the inflation cycle, pump 50 stops and
solenoid
valves 58a, 58b, 58c de-energize to the closed position to allow bladders 114,
116, 118 to
deflate through vent ports 66a, 66b, 66c.
It is envisioned that if a second leg of the subject is treated for vascular
therapy,
solenoid valves 60a, 60b, 60c are energized to the open position, as
described, such that the
valves open to deliver air to corresponding bladders of a sleeve disposed
about the second
leg, similar to sleeve 112, using a desired cycle timing sequence. Pressure
transducer 66
monitors the pressure in each of the corresponding bladders throughout the 11-
second
compression cycle. At the conclusion of the inflation cycle, pump 50 stops and
solenoid
valves 60a, 60b, 60c de-energize to the closed position to allow the
corresponding bladders
to deflate through vent ports 68a, 68b, 68c. It is further envisioned that the
inflation cycle
for treatment of the second leg may be initiated approximately 24.5 seconds -
after
completion of the inflation cycle for treating leg L. This process may be
reiterated for
cycles pertaining to both legs. Other cycle times are contemplated.
17
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
In this embodiment, the pressures, as measured by pressure transducer 66 a.nd
the
corresponding signal relayed to the control processor of controller 14, of
bladders 114, 116,
118 during the inflation cycle remain gradien-t with the pressure of ankle
bladder 118 being
greater than the pressure of calf bladder 116, and the pressure of calf
bladder 116 being
greater than the pressure of thigh bladder 1 L 4. The end of cycle pressures,
for example,
include 45 mm Hg in ankle bladder 118, 40 rnm Hg in calf bladder 116, and 30
mm Hg in
thigh bladder 114. An exanlple is illustrated in Table 2 below. It is
contemplated that
compression continues in this cyclical patterrn until either compression
treatment system 10
is turned off or controller 14 indicates and error code via audible or visual
indicia. Other
cycles pressures are contemplated.
Table 2
Thigh-Length Knee-Length Pressure (mmFig)
Sleeve Sleeve
Ankle bladder 118 Ankle Ankle 45 mmHg
Calf Calf Lower Calf 40 mmHg
bladder 116
Thigh bladder 114 Thi b Upper Calf 3 Q minH
For inflation cycles subsequent to the initial inflation cycle for leg L, as
described, a
pressure feedback adjustment can be made pursuant to the pressure measurement
taken by
pressure transducer 66. At the completion of the initial inflation cycle for
leg L, the end of
cycle pressure in ankle bladder 118 is measured by pressure transducer 66 and
compaxed by
the control processor of controller 14 with the set pressure of 45 mm Hg. If
the pressure of
ankle bladder 118 is higher or lower than the set pressure, then a
corresponding decrease or
increase in the speed of pump 50 is required to decrease or increase pressure
delivery. The
pump speed adjustment is based on the follo~ving calculation:
Adjustment = 145 - PI, where P = pressure at the ankle
If the pressure is less than the set pressure, then the pump speed for the
next cycle is
increased by the adjustment amount. If the pressure is greater than the set
pressure, then the
pump speed for the next cycle is decreased by the adjustment amount. It is
conteniplated
that the adjustment process continues even after the set pressure range is
reached_ It is
further contemplated compression treatment system 10 may adjust for separate
pump speeds
18
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
for each sleeve connected to controller 14. Other sequential compression
cycles are also
contemplated.
In an alternate embodiment, compression treatment system 10 performs venous
refill
time measurement. Venous refill time (VRT) measurement is an air
plethysmographic
technique that determines when the veins of a limb have completely refilled
with blood
following a compression cycle. See, for example, the venous refill time
measurernent
described in U.S. Patent No. 6,231,352 to Watson et al., the entire contents
of which is
hereby incorporated by reference herein. The VRT minimizes the amount of time
that the
blood remains stagnant inside the veins. The VRT will be substituted for the
default rest
time (60 seconds) as long as the VRT is between 20 and 60 seconds. If the VRT
is less than
seconds then the default of 20 seconds is used. If the VRT is greater than 60
seconds
then the maximum of 60 seconds is used. The VRT measurement is made when the
system
first reaches set pressure and once every 30 minutes thereafter. It is
contemplated that the
VRT technique and algorithm can be used for both sleeve and foot compression.
15 The VRT measurement uses an air plethysmographic technique where a low
pressure is applied to the calf bladders. As the veins fill with blood, the
pressures in the calf
bladders increase until a plateau is reached. The time that it takes for the
pressure to plateau
is the VRT. If two sleeves are connected to controller 14, then the VRT is
determined
separately for each limb being compressed and the greater of the two
measurements is used
20 as the new vent time of the compression cycle. The VRT measurement for each
sleeve is
made as each particular sleeve reaches set pressure independently. However,
the vent time
is not updated until VRT measurements have been calculated for both sleeves.
For example, compression treatment system 10 may employ the VRT measurement
after the system initiates vascular therapy. Subsequently, after 30 minutes
have elapsed, a
VRT measurement will be taken on the next full inflation cycle. After any of
the sleeves
described above inflates, the bladder(s) of the particular sleeve are vented
down to zero as
in the default inflation cycle.
It is contemplated that a selected bladder pressure is monitored and the vent
to the
bladder is closed when the pressure falls to 5-7 mm Hg. If the pressure in the
bladder is 5-7
nim Hg on a current cycle then a VRT measurement is taken. If the pressure in
the bladder
does not vent down to 5-7 mm Hg then the vent time will remain at its current
value and
19
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
another measurement will be made in 30 minutes. lEf an error occurs, a
corresponding alarm
provides audible and/or visual indicia.
The VRT measurement algorithm determiries when the pressures in the selected
bladders plateau after compression. The VRT will be determined separately for
both legs.
The longer of the two refill times will be used as the new vent time. If
compression is
applied to only one leg, the VRT for that leg is used as the new vent time.
The VRT
measurement algorithm initiates with a time counter started from the end of
the inflation
cycle, which occurs after the selected bladder reaches 5-7 mm Hg (enough
pressure to cause
the bladder to remain in contact with the surface of the leg) and the venting
is stopped. The
VRT measurement initiates with the time counter sta.rted from the end of the
inflation cycle.
The pressure in the selected bladder is the:n monitored. By way of example,
the
pressure is monitored with a 10-second, moving sarnple window. The window
moves in 1-
second intervals. When the difference between the first and last values in the
window is
less than approximately 0.3 mm Hg the curve has reached its plateau. The VRT
measurement is considered done, and the time interval is determined. The end
of the
window is considered to be the point at which the venous system in the limbs
has refilled.
Independent of the VRT measurement, the selected bladder is allowed to vent
for at
least 15 seconds before the next coinpression cycle an that saine limb is
started. As a safety
factor, 5 seconds are added to the measured refill time so the limb is not
compressed too
early. It is contemplated that the vent time may b e equivalent to the
measured refill time
plus 5 seconds. For example, as a result of patient inovement, the standard
deviation in the
sample window may be too high making the mea.surement erroneous. At this
point, the
calculation is discarded and the old value of the VRT is used. The VRT
measurement is
considered erroneous if at any time during the measurement, the pressure in
the selected
bladder is below 2 mmHg, the calculation is discarded, and the old value of
VRT is used.
This may occur if there is a leak in the system. I-t is contemplated that if
the pressure is
greater than 20 mmHg at any time during the VRT rneasurement the old value of
the VRT is
used. It is further contemplated that if the VRT calculation is done for both
legs, the longer
VRT of both legs is used. It is envisioned that if the VRT is calculated to be
greater than 60
seconds, a value of 60 seconds is used. If the VRT is calculated to be less
than 20 seconds,
a value of 20 seconds is used.
CA 02552353 2007-10-02
Alternatively, compression treatment system 10 may employ one, a plurality or
all of the following error codes to provide audible and/or visual indicia of
system error
or failure. These features advantageously enhance safety to the subject during
vascular
therapy. Several error conditions may cause compression treatment system 10 to
provide alarm and stop a particular compression cycle. It is contemplated that
compression treatment system 10 may flash error indicators, sound continuous
signals,
etc., causing a user to reset compression treatment system 10. Controller 14
may
provide an error alarm for one, a plurality or all of the following error
conditions: high
pressure error, including those pressures detected in excess of set pressure;
low
pressure error, including those pressures detected below set pressure and if
no sleeves
are detected; system pressure error, including pressure determined within an
inflation
cycle outside of desired parameters; valve error; software error; pump error;
vent and
deflation error; battery error; and temperature error, including temperatures
detected
outside of specified environmental conditions.
In an alternate embodiment, as shown in FIG. 8, compression treatment system
10, similar to that described above, includes a foot sleeve 312 configured to
provide
vascular therapy to the foot of the subject. Foot sleeve 312 includes a
bladder 314 that
is inflated with air to provide application of pressure to the foot and then
deflated. See,
for example, the sleeve described in U.S. Published Patent Application Serial
No.
20050187499, filed on February 23, 2004 and entitled Compression Apparatus.
Pump 50 fluidly communicates with foot sleeve 312. Sleeve 312 includes a
connector 316 that mates with mating connector 42, which is connected to port
40 via
tubing 44. Valve connector 316 fluidly communicates with bladder 314 of sleeve
312
via tubing 318. Thus, this configuration facilitates fluid communication
between
bladder 314 and pump 50. Foot sleeve 312 wraps about the side portions of the
foot via
a hook and loop type connector flap 320 that transverses the instep of the
foot and a
hook and loop type connector ankle strap 322.
-21-
CA 02552353 2007-10-02
Upon completion of the self-test sequence compression for treatment system 10,
similar to that described, controller 14 begins the sleeve detection procedure
to
determine the type(s) of sleeves attached to ports 38, 40. With regard to foot
sleeve
312, back pressure is detected by the control processor of controller 14
corresponding
to bladder 314, which is
-21a-
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
connected to outlet port 40b. It is contemplated that compression treatment
system 10 may
treat the foot of a second leg of a subject with foot sleeve 312 and also
treat leg L, as
described above, in alternate inflation cycles.
In one particular exemplary compression cycle for foot sleeve 312, the
compression
parameters include a 5-second inflation period followed by 60 seconds of
venting. An
example is illustrated in Table 3 below.
Table 3
Start of Se uence End of Se uence
Foot Compression: 0 Seconds 5.0 seconds
Decompression/Vent: Minimum 20 seconds, maximum 60 seconds
It is contemplated that the vent period is measured from the end of one
inflation
cycle to the beginning of the next inflation cycle on the foot of the subject.
It is further
contemplated that both limbs of the subject may be treated and compression
treatment
system 10 alternates vascular therapy from leg L to the second leg. It is
envisioned that the
time period from the end of the inflation cycle for leg L to the initiation of
the inflation
cycle for the second leg can range from 7.5-27.5 seconds.
During the initial inflation cycle for treating the foot of the subject, as
described
above, pump 50 initiates a low default voltage so as to not over-inflate
bladder 314 on the
initial cycle. Solenoid valve 60b is energized to the open position, as
described, such that
the valve opens to deliver air to bladder 314 using a desired cycle timing
sequence.
Pressure transducer 66 monitors the pressure in bladder 314 throughout the 5-
second
compression cycle. At the conclusion of the inflation cycle, pump 50 stops and
solenoid
valve 60b de-energizes to the closed position to allow bladder 314 to deflate
through vent
port 68b.
It is envisioned that if a second foot of the subject is treated for vascular
therapy,
solenoid valve 58b is energized to the open position, as described, such that
the valve opens
to deliver air to a corresponding bladder of a foot sleeve disposed about the
other leg,
similar to foot sleeve 312, using a desired cycle timing sequence. For
example, pressure
transducer 66 monitors the pressure in the corresponding bladder throughout
the 5-second
compression cycle. At the conclusion of the inflation cycle, pump 50 stops and
solenoid
22
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
valve 58b de-energizes to the closed position to allow the corresponding
bladder to deflate
through vent port 66b. It is further envisioned that the inflation cycle for
treatment of the
second foot may be initiated approximately 27.5 seconds after completion of
the inflation
cycle for treating the foot treated by foot sleeve 312. This process may be
reiterated for
cycles pertaining to both feet, or in the alternative, for foot s1eeve of a
first leg and. a leg
sleeve of a second leg. It is conteinplated that compressiorn treatment system
10 may
provide alternating compression to any combination of a sleeve and a foot
garment and that
if such a combination is employed, then, for example, a 6-second buffer of
additional vent
timing is added to all vent periods after the foot inflation cycle so that the
overall timing is
consistent with the default sleeve compression parameters_ Other cycle times
are
contenlplated.
In this embodiment, the target pressure, as measured by pressure transducer 66
and
the corresponding signal relayed to the control processor of controller 14, of
bladder 314 is,
for example, 130 mm Hg. It is contemplated that compressio-n continues in this
cyclical
pattern until either compression treatment system 10 is turned off or
controller 14 indicates
an error code via audible or visual indicia.
For inflation cycles subsequent to the initial inflatiorn cycle for foot
sleeve 312
described, a pressure feedback adjustment can be made pursuant to the pressure
measurement taken by pressure transducer 66. At the completion of the initial
inflation
cycle for foot sleeve 312, the end of cycle pressure in bladder 314 is
measured by pressure
transducer 66 and compared by the control processor of controller 14 with the
set pressure
of 130 mm Hg. If the pressure of bladder 314 is higher or lower than the set
pressure, then
a corresponding decrease or increase in the speed of pump 50 is required to
decrease or
increase pressure delivery. The pump speed adjustment is based on the
following
calculation:
Adjustment =1 130 - PI , where P = pressure at the foot
If the pressure is less than the set pressure, then the pumT speed for the
next cycle is
increased by the adjustment amount. If the pressure is greater tizan the set
pressure, then the
pump speed for the next cycle is decreased by the adjustment amount. It is
contemplated
that the adjustment process continues even after the set press-ure range is
reached. It is
further conteinplated that compression treatment system 10 may adjust for
separate pump
23
CA 02552353 2006-06-29
WO 2005/082314 PCT/US2005/005598
speeds for each sleeve connected to controller 14. Other sequential
compression cycles are
also contemplated.
It will be understood that various modifications may be made to the
embodiments
disclosed herein. Therefore, the above description should not be construed as
limiting, but
merely as exemplification of the various embodiments. Those skilled in the art
will
envision other modifications within the scope and spirit of the claims
appended hereto.
24