Note: Descriptions are shown in the official language in which they were submitted.
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
1
COATED ARTICLE
FIELD OF THE INVENTION
This invention relates to articles, particularly brass articles, having a
multi-layered decorative and protective coating having the appearance or
color of bronze, particularly antique bronze, thereon.
BACKGROUND OF THE INVENTION
It is currently the practice with various brass articles such as faucets,
faucet escutcheons, door knobs, door handles, door escutcheons and the
like to first buff and polish the surface of the article to a high gloss and
to
then apply a protective organic coating, such as one comprised of acrylics,
urethanes, epoxies and the like, onto this polished surface. This system has
the drawback that the buffing and polishing operation, particularly if the
article is of a complex shape, is labor intensive. Also, the known organic
coatings are not always as durable as desired, and are susceptible to attack
by acids. It would, therefore, be quite advantageous If brass articles, or
indeed other articles, either plastic, ceramic, or metallic, could be provided
with coating which provided the article with a decorative appearance as well
as providing wear resistance, abrasion resistance and corrosion resistance.
It is known in the art that a multi-layered coating can be applied to an
article
which provides a decorative appearance as well as providing wear
resistance, abrasion resistance and corrosion resistance. This multi-layer
coating includes a decorative and protective color layer of a refractory metal
nitride such as a zirconium nitride or a titanium nitride. This color layer,
when it is zirconium nitride, provides a brass color, and when it is titanium
nitride provides a gold color.
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
2
U.S. patent Nos. 5,922,478; 6,033,790 and 5,654,108, inter alia,
describe a coating which provides an article with a decorative color, such as
polished brass, provides wear resistance, abrasion resistance and corrosion
resistance. It would be very advantageous if a coating could be provided
which provided substantially the same properties as the coatings containing
zirconium nitride or titanium nitride but instead of being brass colored or
gold colored was bronze, particularly antique bronze, colored. The present
invention provides such a coating.
SUMMARY OF THE INVENTION
The present invention is directed to an article coated with a multi-
layer coating having a bronze color. The coating comprises a color and
protective stack layer comprised of layers of carbon-rich refractory metal or
refractory metal alloy carbonitride alternating with layers of nitrogen-rich
refractory metal or refractory metal alloy carbonitride. In another
embodiment, the alternating layers of the color stack layer may comprise
layers of carbon-rich refractory metal carbides or carbon-rich refractory
metal alloy carbides alternating with layers of nitrogen-rich refractory metal
nitrides or nitrogen-rich refractory metal alloy nitrides.
The present invention also is directed to an article such as a plastic,
ceramic, cermet or metallic article having a decorative and protective multi-
layer coating deposited on at least a portion of its surface. More
particularly,
it is directed to an article or substrate, particularly a metallic article
such as
stainless steel, aluminum, brass or zinc, having deposited on its surface
multiple superposed layers of certain specific types of materials. The coating
is decorative and also provides corrosion resistance, wear resistance and
abrasion resistance. The coating provides the appearance or color of
bronze, particularly antique bronze, i.e. has a two-tone color: dark gray and
dark yellow. Thus, an article surface having the coating thereon simulates a
bronze, particularly an antique bronze surface.
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
3
In the preferred embodiment, the article first has deposited on its
surface one or more basecoat layers. On top of the basecoat layers is then
deposited, by vapor deposition such as physical vapor deposition, one or
more vapor deposited layers. A first basecoat layer deposited directly on
the surface of the substrate is comprised of nickel or a polymeric material.
When the layer is nickel, it is an electroplated layer. The nickel may be
monolithic or it may consist of two different nickel layers such as, for
example, a semi-bright nickel layer deposited directly on the surface of the
substrate and a bright nickel layer superimposed over the semi-bright nickel
layer. Disposed over the nickel layers or polymeric layer is a strike layer
comprised of a refractory metal or metal alloy such as zirconium, titanium,
hafnium, tantalum or zirconium-titanium alloy, preferably zirconium, titanium
or zirconium-titanium alloy. In one embodiment disposed intermediate the
basecoat layer and the strike layer is a strengthening layer comprised of
chromium. Over the strike layer is a protective and decorative color layer
comprised of a stack layer comprised of layers of carbon-rich refractory
metal carbonitride or carbide alternating with layers of nitrogen-rich
refractory metal carbonitride or nitride, such as zirconium carbonitride,
titanium carbonitride, tantalum carbonitride and hafnium carbonitride, and
the carbonitrides of refractory metal alloys, such as a titanium-zirconium
alloy.
In another embodiment, these alternating layers may be a carbide
with no nitrogen content and a nitride with no carbon content.
These alternating layers of the stack layer may contain a small
percentage of oxygen in order to increase the dark appearance of the
coating. The small amount of oxygen ranges from about 2 to about 15
atomic percent. For zirconium, in the carbon-rich zirconium carbonitride
layer, the carbon content generally is between about 25 to about 50 atomic
percent, nitrogen content between about 5 to about 35 atomic percent, and
this layer has a dark gray color. In the nitrogen-rich zirconium carbonitride
layer, the nitrogen content is between about 25 to about 50 atomic percent,
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
4
carbon content between about 5 to about 35 atomic percent; and this layer
has a dark yellow color with a slight reddish tint. Neither of these two
layers
is thick enough by itself to make the coating have its own color. However,
when two or more of these layers are present, the overall color of the stack
layers mimics a dark gray and dark yellow two-tone antique bronze
appearance. On the top of this color layer, a very thin layer of refractory
metal oxide or refractory metal alloy oxide is deposited in order to improve
the corrosion and chemical resistance of the coating.
BRIEF DESCRIPTION OF THE DRAWINGS
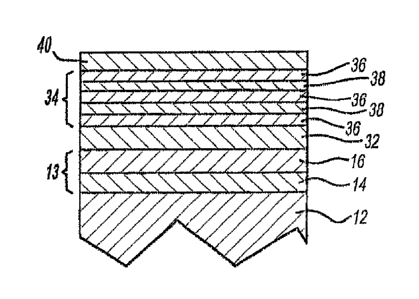
FIG. 1 is a cross sectional view, not to scale, of a portion of the
substrate having a multi-layer coating comprising a duplex nickel base coat,
a refractory metal layer, a stack layer comprised of alternating layers of
carbon-rich refractory carbonitride or carbide layers and nitrogen-rich
refractory carbonitride or nitride layers, and a thin refractory metal oxide
layer;
FIG. 2 is a view similar to FIG. 1 except that a strengthening
chromium layer is present intermediate the top basecoat layer and the
refractory metal strike layer.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT(S)
The article or substrate 12 can be comprised of any material onto
which a plated layer can be applied, such as plastic, e.g., ABS, polyolefin,
polyvinylchloride, and phenolformaldehyde, ceramic, cermet, metal or metal
alloy. In one embodiment it is comprised of a metal or metallic alloy such as
copper, steel, brass, zinc, aluminum, nickel alloys and the like.
In the instant invention, as illustrated in Figs. 1 and 2, a first layer or
series of layers is applied onto the surface of the article by plating such as
CA 02552357 2012-01-16
5 electroplating in the case of a nickel basecoat. In the case of a
polymeric basecoat,
the polymer is applied by conventional means. A second series of layers is
applied
onto the surface of the basecoat layer or layers by vapor deposition. The
polymer or
electroplated layers serve, inter alia, as a base coat which levels the
surface of the
article and as a corrosion barrier to improve corrosion resistance. In one
embodiment
of the instant invention a basecoat layer 13 may be deposited on the surface
of the
article. Where comprised of nickel, the basecoat layer 13 may be any of the
conventional nickels that are deposited by plating, e.g., bright nickel, semi-
bright
nickel, satin nickel, etc. The basecoat layer 13 may be deposited on at least
a portion
of the surface of the substrate 12 by conventional and well-known
electroplating
processes. These processes include using a conventional electroplating bath
such as,
for example, a Watts bath as the plating solution. Typically such baths
contain nickel
sulfate, nickel chloride, and boric acid dissolved in water. All chloride,
sulfamate and
fluoroborate plating solutions can also be used. These baths can optionally
include a
number of well known and conventionally used compounds such as leveling
agents,
brighteners, and the like. To produce specularly bright basecoat layer 13 at
least one
brightener from class I and at least one brightener from class ll is added to
the plating
solution. Class I brighteners are organic compounds which contain sulfur.
Class II
brighteners are organic compounds which do not contain sulfur. Class II
brighteners
can also cause leveling and, when added to the plating bath without the sulfur-
containing class I brighteners, result in semi-bright nickel deposits. These
class I
brighteners include alkyl naphthalene and benzene sulfonic acids, the benzene
and
naphthalene di- and trisulfonic acids, benzene and naphthalene sulfonamides,
and
sulfonamides such as saccharin, vinyl and allyl sulfonamides and sulfonic
acids. The
class II brighteners generally are unsaturated organic materials such as, for
example,
acetylenic or ethylenic alcohols, ethoxylated and propoxylated acetylenic
alcohols,
coumarins, and aldehydes. These class I and class II brighteners are well
known to
those skilled in the art and are readily
CA 02552357 2012-01-16
6
commercially available. They are described, inter alia, in U.S. Pat. No.
4,421,611
The basecoat layer 13 can be comprised of a monolithic layer such as semi-
bright nickel, satin nickel or bright nickel, or it can be a duplex layer
containing two
different nickel layers, for example, a layer comprised of semi-bright nickel
and a layer
comprised of bright nickel. The thickness of the basecoat layer 13 is
generally a
thickness effective to level the surface of the article and to provide
improved corrosion
resistance. This thickness is generally in the range of from about 2.5 pm,
preferably
about 4 pm, to about 90 pm.
As is well known in the art before the basecoat layer 13 is deposited on the
substrate the substrate is subjected to acid activation by being placed in a
conventional
and well known acid bath.
In one embodiment as illustrated in Figs. 1 and 2, the basecoat layer 13 is
actually comprised of two different nickel layers 14 and 16. Layer 14 is
comprised of
semi-bright nickel while layer 16 is comprised of bright nickel. This duplex
nickel deposit
provides improved corrosion protection to the underlying substrate. The semi-
bright,
sulfur-free layer 14 is deposited by conventional electroplating processes
directly on the
surface of substrate 12. The substrate 12 containing the semi-bright nickel
layer 14 is
then placed in a bright nickel plating bath and the bright nickel layer 16 is
deposited on
the semi-bright nickel layer 14.
The thickness of the semi-bright nickel layer and the bright nickel layer is a
thickness at least effective to provide improved corrosion protection and/or
leveling of
the article surface. Generally, the thickness of the semi-bright nickel layer
is at least
about 1.25 pm (microns), preferably at least about 2.5 pm, and more preferably
at least
about 3.5 pm. The upper thickness limit is generally not critical and is
governed by
secondary considerations such as cost. Generally, however, a thickness of
about 40
pm, preferably about 25 pm, and more preferably about 20 pm should not be
exceeded.
The bright nickel layer 16 generally has a thickness of at
CA 02552357 2012-01-16
7
least about 1.2 pm, preferably at least about 3 pm, and more preferably at
least about 6
pm. The upper thickness range of the bright nickel layer is not critical and
is generally
controlled by considerations such as cost. Generally, however, a thickness of
about 60
pm, preferably about 50 pm, and more preferably about 40 pm should not be
exceeded.
The bright nickel layer 16 also functions as a leveling layer which tends to
cover or fill in
imperfections in the substrate.
In the instant invention, as illustrated in Figs. 1 and 2, basecoat layer 13
comprised of a polymer is applied onto the surface of the article 12 as a
basecoat layer.
A second series of layers is applied onto the surface of the basecoat layer 13
by vapor
deposition. The basecoat layer 13 serves, inter alia, as a base coat which
levels the
surface of the article and as a corrosion barrier to improve corrosion
resistance. In the
instant invention the basecoat layer 13 is deposited on the surface of the
article.
The basecoat layer 13 may be comprised of both thermoplastic and thermoset
polymeric or resinous material. These polymeric or resinous materials include
the well
known, conventional and commercially available polycarbonates, epoxy
urethanes,
polyacrylates, polymethacrylates, nylons, polyesters, polypropylenes,
polyepoxies,
alkyds and styrene containing polymers such as polystyrene, styrene-
acrylonitrile
(SAN), styrene-butadiene, acrylonitrile-butadiene-styrene (ABS), and blends
and
copolymers thereof.
The polycarbonates are described in U.S. Patent Nos. 4,579,910 and 4,513,037.
Nylons are polyamides which can be prepared by the reaction of diamines with
dicarboxylic acids. The diamines and dicarboxylic acids which are generally
utilized in
preparing nylons generally contain from two to about 12 carbon atoms. Nylons
can also
be prepared by additional polymerization. They are described in "Polyamide
Resins",
D.E. Floyc, Reinhold Publishing Corp., New York, 1958.
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
8
The polyepoxies are disclosed in "Epoxy Resins", by H. Lee and K.
Neville, McGraw-Hill, New York, 1957, and in U.S. Patent Nos. 2,633,458;
4,988,572; 4,680,076; 4,933,429 and 4,999,388
The polyesters are polycondensation products of an aromatic
dicarboxylic acid and dihydric alcohol. The aromic dicarboxylic acids
include terephthalic acid, 2,6-naphthalenedicarboxylic acid, and the like.
Dihydric alcohols include the lower alkane diols with from two to about 10
carbon atoms such as, for example, ethylene glycol, propylene glycol,
cyclohexanedimethanol, and the like. Some illustrative non-limiting
examples of polyesters include polyethylene terephthalate, polybutylene
terephthalate, polyethylene isophthalate, and poly (1,4-
cydohexanedimethylene terephthalate). They are disclosed in U.S. Patent
Nos. 2,645,319; 2,901,466 and 3,047,539
The polyacrylates and polymethacrylates are polymers or resins
resulting from the polymerization of one or more acrylates such as, for
example, methyl acrylate, ethyl acrylate, butyl acrylate, 2-ethylhexyl
acrylate, etc., as well as the methacrylates such as, for instance, methyl
methacrylates, ethyl methacrylate, butyl methacrylate, hexyl methacrylate,
etc. Copolymers of the above acrylate and methacrylate monomers are
also included within the term "polyacrylates or polymethacrylates" as it
appears therein. The polymerization of the monomeric acrylates and
methacrylates to provide the polyacrylate resins useful in the practice of the
invention may be accomplished by any of the well known polymerization
techniques.
The styrene-acrylonitrile and acrylonitrile-butadiene-styrene resins
and their preparation are disclosed, inter alia, in U.S. Patent Nos.
2,769,804;
2,989,517; 2,739,142; 3,991,136 and 4,387,179
CA 02552357 2012-01-16
9
The alkyd resins are disclosed in "alkyd Resin Technology", Patton,
Interscience
Publishers, NY, NY, 1962, and in U.S. Patent Nos. 3,102,866; 3,228,787 and
4,511,692.
The epoxy urethanes and their preparation are disclosed, inter alia, in U.S.
Patent
Nos. 3,963,663; 4,705,841; 4,035,274; 4,052,280; 4,066,523; 4,159,233;
4,163,809;
4,229,335 and 3,970,535.
Particularly useful epoxy urethanes are those that are electrocoated onto the
article. Such electrodepositable epoxy urethanes are described in the afore-
mentioned
U.S. Patent Nos. 3,963,663; 4,066,523; 4,159,233; 4,035,274 and 4,070,258.
These polymeric materials may optionally contain the conventional and well
known fillers such as mica, talc and glass fibers.
The basecoat layer 13 may be applied onto the surface of the substrate by any
of
the well known and conventional methods such as dipping, spraying, brushing
and
electrodeposition.
The basecoat layer 13 functions, inter alia, to level the surface of the
substrate,
cover any scratches or imperfections in the surface of the article and provide
a smooth
and even surface for the deposition of the succeeding layers such as the vapor
deposited layers.
The basecoat layer 13 has a thickness at least effective to level out the
surface of
the article or substrate. Generally, this thickness is at least about 0.12 pm,
preferably at
least about 2.5 pm, and more preferably at least about 5 pm. The upper
thickness range
should not exceed about 250 pm.
In some instances, depending on the substrate material and the type of
polymeric
basecoat, the polymeric basecoat does not adhere sufficiently to the
substrate. In such
a situation a primer layer is deposited on the substrate to improve the
adhesion of the
polymeric basecoat to the substrate. The primer layer can be comprised, inter
alia, of
halogenated polyolefins. The halogenated polyolefins are conventional and well
known
polymers that are generally commercially available. The preferred
CA 02552357 2012-01-16
5
halogenated polyolefins are the chlorinated and brominated polyolenfins, with
the
chlorinated polyolenfins being more preferred. The halogenated, particularly
chlorinated,
polyolenfins along with methods for their preparation are disclosed, inter
alia, in U.S.
Patent Nos. 5,319,032; 5,840,783; 5,385,979; 5,198,485; 5,863,646; 5,489,650
and
4,273,894.
10 The
thickness of the primer layer is a thickness effective to improve the adhesion
of the polymeric basecoat layer to the substrate. Generally this thickness is
at least
about 0.25 pm. The upper thickness is not critical and generally is controlled
by
secondary considerations such as cost and appearance. Generally an upper
thickness
of about 125 pm should not be exceeded.
In one embodiment, as illustrated in Fig. 2, disposed between basecoat layer
13
and vapor deposited strike layer 32 are one or more metal alloy layer 22 which
function,
inter alia, as a strengthening layer. This metal alloy layer 22 may be
deposited by
electroplating or vapor deposition such as physical vapor deposition. This
metal alloy
layer 22 includes but is not limited to chromium. When metal alloy layer 22 is
comprised
of chromium it may be deposited on basecoat layer 13 by conventional and well
known
chromium electroplating techniques or conventional and well known physical
vapor
deposition techniques. The electroplating techniques along with various chrome
plating
baths are disclosed in Brassard, "Decorative Electroplating - A Process in
Transition",
Metal Finishing, pp. 105-108, June 1988; Zaki, "Chromium Plating", PF
Directory, pp.
146-160; and in U.S. patent Nos. 4,460,438; 4,234,396; and 4,093,522.
Chrome plating baths are well known and commercially available. A typical
chrome plating bath contains chromic acid or salts thereof, and catalyst ion
such as
sulfate or fluoride. The catalyst ions can be provided by sulfuric acid or its
salts and
fluosilicic acid. The baths may be operated at a
CA 02552357 2012-01-16
11
temperature of about 112 -116 F. Typically in chrome plating a current density
of about
150 amps per square foot, at about 5 to 9 volts is utilized.
The chrome layer generally has a thickness at least sufficient to function as
a
strengthening layer. Generally this thickness is at least about 0.05 pm,
preferably at
least about 0.12 pm, and more preferably at least about 0.2 pm. Generally, the
upper
range of thickness is not critical and is determined by secondary
considerations such as
cost. However, the thickness of the chrome layer should generally not exceed
about 1.5
pm, preferably about 1.2 pm, and more preferably about 1 pm.
Instead of metal alloy layer 22 being comprised of chromium it may be
comprised
of tin-nickel alloy, palladium-nickel alloy or nickel-tungsten-boron alloy.
Where metal alloy layer 22 is comprised of tin-nickel alloy, metal alloy layer
22
may be deposited on the surface of the substrate by conventional and well
known tin-
nickel electroplating processes. These processes and plating baths are
conventional
and well known and are disclosed, inter alia, in U.S. patent Nos. 4,033,835;
4,049,508;
3,887,444; 3,772,168 and 3,940,319 =
Where metal alloy layer 22 is comprised of tin-nickel alloy, metal alloy layer
22 is
preferably comprised of about 60-70 weight percent tin and about 30-40 weight
percent
nickel, more preferably about 65% tin and 35% nickel representing the atomic
composition SnNi. The plating bath contains sufficient amounts of nickel and
tin to
provide a tin-nickel alloy of the afore-described composition.
A commercially available tin-nickel plating process is the NiColloyTm process
available from ATOTECH, and described in their Technical Information Sheet No:
NiColloy, Oct. 30, 1994
Where metal alloy layer 22 is comprised of tin-nickel alloy, the thickness of
the
metal alloy layer 22 is generally at least about 0.25 pm, preferably at least
about 0.5
pm, and more preferably at least about 1 pm. The upper thickness range is not
critical
and is generally
CA 02552357 2012-01-16
12
dependent on economic considerations. Generally, a thickness of about 50 pm,
preferably about 25 pm, and more preferably about 15 pm should not be
exceeded.
Where metal alloy layer 22 is comprised of nickel-tungsten-boron alloy, metal
alloy layer 22 may be deposited by plating such as electroplating or vapor
deposition
such as physical vapor deposition. If the metal alloy layer 22 is deposited by
electroplating, it is deposited by conventional and well known nickel-tungsten-
boron
electroplating processes. The plating bath is normally operated at a
temperature of
about 1150 to 125 F and a preferred pH range of about 8.2 to about 8.6. The
well known
soluble, preferably water soluble, salts of nickel, tungsten and boron are
utilized in the
plating bath or solution to provide concentrations of nickel, tungsten and
boron.
Where metal alloy layer 22 is comprised of nickel-tungsten-boron alloy, metal
alloy layer 22, generally contains at least 50, preferably at least about 55,
and more
preferably at least 57.5 weight percent nickel, at least about 30, preferably
at least
about 35, and more preferably at least 37.5 weight percent tungsten, and at
least about
0.05, preferably at least about 0.5, and more preferably at least about 0.75
weight
percent boron. Generally the amount of nickel does not exceed about 70,
preferably
about 65, and more preferably about 62.5 weight percent, the amount of
tungsten does
not exceed about 50, preferably about 45, and more preferably about 42.5
weight
percent, and the amount of boron does not exceed about 2.5, preferably about
2, and
more preferably about 1.25 weight percent. The plating bath contains
sufficient amounts
of the salts, preferably soluble salts, of nickel, tungsten and boron to
provide a nickel-
tungsten-boron alloy of the afore-described composition.
A nickel-tungsten-boron plating bath effective to provide a nickel-tungsten-
boron
alloy of which a composition is commercially available, such as the AmplateTM
system
from Amorphous Technologies International of Laguna Niguel, California. A
typical
nickel-tungsten-boron alloy contains about 59.5 weight percent nickel, about
39.5
weight percent tungsten, and about 1% boron. The nickel-tungsten-boron alloy
is an
amorphous/nano-
CA 02552357 2012-01-16
13
crystalline composite alloy. Such an alloy layer is deposited by the AMPLATE
plating
process marketed by Amorphous Technologies International.
Where metal alloy layer 22 is comprised of palladium-nickel alloy, the metal
alloy
layer 22 may be deposited by plating such as electroplating or vapor
deposition such as
physical vapor deposition. If the metal alloy layer 22 is deposited by
electroplating, it is
deposited by conventional and well known palladium-nickel electroplating
process.
Generally, they include the use of palladium salts or complexes such as nickel
amine
sulfate, organic brighteners, and the like. Some illustrative examples of
palladium/nickel
electroplating processes and baths are described in U.S. patent Nos.
4,849,303;
4,463,660; 4,416,748; 4,428,820 and 4,699,697.
The weight ratio of palladium to nickel in the palladium/nickel alloy is
dependent,
inter alia, on the concentration of palladium (in the form of its salt) in the
plating bath.
The higher the palladium salt concentration or ratio relative to the nickel
salt
concentration in the bath the higher the palladium ratio in the
palladium/nickel alloy.
Where metal alloy layer 22 is comprised of palladium-nickel alloy, the metal
alloy
layer 22 generally has a weight ratio of palladium to nickel of from about
50:50 to about
95:5, preferably from about 60:40 to about 90:10, and more preferably from
about 70:30
to about 85:15.
Over the metal alloy layer 22 is deposited, by vapor deposition such as
physical
vapor deposition or chemical vapor deposition, a protective and decorative
color layer
34. Color layer 34 is comprised of layers 36 of a carbon-rich refractory metal
carbonitride or refractory metal alloy carbonitride alternating with layers 38
of nitrogen-
rich refractory metal carbonitride or refractory metal alloy carbonitride,
such as, for
example, zirconium carbonitride, titanium carbonitride, hafnium carbonitride
and
tantalum carbonitride, and the carbonitrides of refractory metal alloys such
as a
titanium-zirconium alloy. These carbonitride layers may contain a small
percentage of
oxygen in order to increase the dark appearance of the coating. This small
amount of
oxygen ranges from about 2 to about 15
CA 02552357 2011-05-20
WO 2005/118282 PCT/US2005/018581
14
atomic percent. For zirconium, in the carbon-rich zirconium carbonitride
layer, the carbon content generally is between about 25 to about 50 atomic
percent, nitrogen content between about 5 to about 35 atomic percent,
giving this layer a dark gray color. In the nitrogen-rich zirconium
carbonitride
layer, the nitrogen content is between about 25 to about 50 atomic percent,
carbon content between about 5 to about 35 atomic percent, giving this
layer a dark yellow color with a slightly reddish tint.
It is to be understood that in the practice of the instant invention each
of layers 36 and 38 is too thin, or not thick enough, to
provide or form
the color of the individual layer. However, layers 36 and 38 are used in
conjunction with each other and, when several layers are present, form a
color and provide protective stack layer 34. As a result, the overall color of
the stack layer 34 mimics or is a dark gray and dark yellow two-tone antique
bronze color.
The number of layers 36 and 38 in stack layer 34 is generally from
about 4 to about 50, preferably from about 8 to about 36. Each of layers 36
and 38 generally has a thickness of from about 30 A to about 200 A,
preferably from about 50 A to about 150 A.
The thickness of this color and protective stack layer 34 is a
thickness which is at least effective to provide the color of bronze,
particularly antique bronze, and to provide abrasion resistance, scratch
resistance, and wear resistance. Generally, this thickness is at least about
1,000 A, preferably at least about 1,500 A, and more preferably at least
about 2,500 A. The upper thickness range is generally not critical and is
dependent upon secondary considerations such as cost. Generally a
thickness of about 7500 A, preferably about 5000 A should not be
exceeded.
Layer 34 is deposited by conventional and well known techniques
including vapor deposition techniques such as cathodic arc evaporation
(CAE) or sputtering, and the like. Sputtering and CAE techniques and
equipment are disclosed, inter alia, in J. Vossen and W. Kern "Thin Film
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
Processes II", Academic Press, 1991; R. Boxman et at, "Handbook of
Vacuum Arc Science and Technology", Noyes Pub., 1995; and U.S. patent
Nos. 4,162,954 and 4,591,418
5 One method of depositing layer 34 is by physical vapor deposition
utilizing reactive sputtering or reactive cathodic arc evaporation. Reactive
cathodic arc evaporation and reactive sputtering are generally similar to
ordinary sputtering and cathodic arc evaporation except that a reactive gas
is introduced into the chamber which reacts with the dislodged target
10 material. Thus, in the case where zirconium carbonitride is the layer
34, the
cathode is comprised of zirconium and nitrogen and carbon-containing gas,
such as methane or acetylene, are the reactive gases introduced into the
chamber. When the carbon-rich zirconium carbonitride layer 36 is produced,
the carbon gas flow is momentarily increased meanwhile nitrogen gas
15 momentarily decreased. When the nitrogen-rich zirconium carbonitride
layer
38 is produced, nitrogen gas flow is momentarily increased meanwhile
carbon gas is momentarily decreased.
When a carbide layer 36 is formed, the carbon gas flow is increased
and the nitrogen gas flow is shut off. When a nitride layer 38 is formed, the
nitrogen gas flow is increased and the carbon gas flow is shut off.
In addition to the protective color stack layer 34 there may optionally
be present additional vapor deposited layers. These additional vapor
deposited layers may include a layer 32 comprised of refractory metal or
refractory metal alloy. The refractory metals include hafnium, tantalum,
zirconium and titanium. The refractory metal alloys include zirconium-
titanium alloy, zirconium-hafnium alloy and titanium-hafnium alloy. The
refractory metal layer or refractory metal alloy layer 32 generally functions,
inter alia, as a strike layer which improves the adhesion of the color layer
34
to the top electroplated layer. As illustrated in Fig. 1, the refractory metal
or
refractory metal alloy strike layer 32 is generally disposed intermediate the
color layer 34 and the top electroplated layer. As illustrated in Fig. 2, the
CA 02552357 2012-01-16
16
strike layer is disposed in the metal alloy layer 22. Layer 32 has a thickness
which is
generally at least effective for layer 32 to function as a strike layer.
Generally, this
thickness is at least about 60 A, preferably at least about 120 A, and more
preferably at
least about 250 A. The upper thickness range is not critical and is generally
dependent
upon considerations such as cost. Generally, however, layer 32 should not be
thicker
than about 1.2 pm, preferably about 0.5 pm, and more preferably about 0.25 pm.
The refractory metal or refractory metal alloy strike layer 32 is deposited by
conventional and well known vapor deposition techniques including physical
vapor
deposition techniques such as cathodic arc evaporation (CAE) or sputtering.
Briefly, in
the sputtering deposition process a refractory metal (such as titanium or
zirconium)
target, which is the cathode, and the substrate are placed in a vacuum
chamber. The air
in the chamber is evacuated to produce vacuum conditions in the chamber. An
inert
gas, such as Argon, is introduced into the chamber. The gas particles are
ionized and
are accelerated to the target to dislodge titanium or zirconium atoms. The
dislodged
target material is then typically deposited as a coating film on the
substrate.
In cathodic arc evaporation, an electric arc of typically several hundred
amperes
is struck on the surface of a metal cathode such as zirconium or titanium. The
arc
vaporizes the cathode material, which then condenses on the substrates forming
a
coating.
In a preferred embodiment of the present invention the refractory metal is
comprised of titanium or zirconium, preferably zirconium, and the refractory
metal alloy
is comprised of zirconium-titanium alloy.
The additional vapor deposited layers may also include refractory metal
compounds and refractory metal alloy compounds other than the above described
carbonitrides. These refractory metal compounds and refractory metal alloy
compounds
include the refractory metal oxides and refractory metal alloy oxides; the
refractory
metal nitrides and refractory
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
17
metal alloy nitrides; reaction products of (a) refractory metal or refractory
metal alloy, (b) oxygen, and (c) nitrogen; and the refractory metal
oxynitrides
and refractory metal alloy oxynitrides.
In one embodiment of the invention as illustrated in Figs. 1 and 2 a
layer 40 comprised of the reaction products of a refractory metal or metal
alloy, an oxygen containing gas such as oxygen, and nitrogen is deposited
onto layer 34. The metals that may be employed in the practice of this
invention are those which are capable of forming both a metal oxide and a
metal nitride under suitable conditions, for example, using a reactive gas
comprised of oxygen and nitrogen. The metals may be, for example,
tantalum, hafnium, zirconium, zirconium-titanium alloy, and titanium,
preferably titanium, zirconium-titanium alloy and zirconium, and more
preferably zirconium.
The reaction products of the metal or metal alloy, oxygen and
nitrogen are generally comprised of the metal or metal alloy oxide, metal or
metal alloy nitride and metal or metal alloy oxy-nitride.
Thus, for example, the reaction products of zirconium, oxygen and
nitrogen comprise zirconium oxide, zirconium nitride and zirconium oxy-
nitride. These metal oxides and metal nitrides including zirconium oxide and
zirconium nitride alloys and their preparation and deposition are
conventional and well known, and are disclosed, inter alia, in U.S. patent
No. 5,367,285
The layer 40 can be deposited by well known and conventional vapor
deposition techniques, including reactive sputtering and cathodic arc
evaporation.
In another embodiment instead of layer 40 being comprised of the
reaction products of a refractory metal or refractory metal alloy, oxygen and
nitrogen, it is comprised of refractory metal oxide or refractory metal alloy
oxide. The refractory metal oxides and refractory metal alloy oxides of
which layer 40 is comprised include, but are not limited to, hafnium oxide,
tantalum oxide, zirconium oxide, titanium oxide, and zirconium-titanium alloy
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
18
oxide, preferably titanium oxide, zirconium oxide, and zirconium-titanium
alloy oxide, and more preferably zirconium oxide. These oxides and their
preparation are conventional and well known.
Layer 40 is effective in providing improved chemical, such as acid or
base, resistance to the coating. Layer 40 containing (i) the reaction
products of refractory metal or refractory metal alloy, oxygen and nitrogen,
or (ii) refractory metal oxide or refractory metal alloy oxide generally has a
thickness at least effective to provide improved chemical resistance but is
not so thick as to obscure the color of color stack layer 34. Generally this
thickness is at least about 10 A, preferably at least about 25 A, and more
preferably at least about 40 A. Layer 40 should be thin enough so that it
does not obscure the color of underlying color layer 34. That is to say layer
40 should be thin enough so that it is non-opaque or substantially
transparent. Generally layer 40 should not be thicker than about 0.10 um,
preferably about 250 A, and more preferably about 100 A.
In order that the invention may be more readily understood, the
following example is provided. The example is illustrative and does not limit
the invention thereto.
EXAMPLE I
Brass faucets are placed in a conventional soak cleaner bath
containing the standard and well known soaps, detergents, defloculants and
the like which is maintained at a pH of 8.9-9.2 and a temperature of 180-
200 F. for about 10 minutes. The brass faucets are then placed in a
conventional ultrasonic alkaline cleaner bath. The ultrasonic cleaner bath
has a pH of 8.9-9.2, is maintained at a temperature of about 160-180 F.,
and contains the conventional and well known soaps, detergents,
defloculants and the like. After the ultrasonic cleaning the faucets are
rinsed
and placed in a conventional alkaline electro cleaner bath.
The electro cleaner bath is maintained at a temperature of about 140-
180 F., a pH of about 10.5-11.5, and contains standard and conventional
CA 02552357 2011-05-20
WO 2005/118282
PCT/US2005/018581
19
detergents. The faucets are then rinsed twice and placed in a conventional
acid activator bath. The acid activator bath has a pH of about 2.0-3.0, is at
an ambient temperature, and contains a sodium fluoride based acid salt.
The faucets are then rinsed twice and placed in a bright nickel plating bath
for about 12 minutes. The bright nickel bath is generally a conventional bath
which is maintained at a temperature of about 130-150 F., a pH of about
4.0, contains NiSO4, N1Cl2, boric add, and brighteners. A bright nickel layer
of an average thickness of about 10 jtm is deposited on the faucet surface.
The bright nickel plated faucets are rinsed three times and then
placed in a conventional, commercially available hexavalent chromium
plating bath using conventional chromium plating equipment for about seven
minutes. The hexavalent chromium bath is a conventional and well known
bath which contains about 32 ounces/gallon of chromic acid. The bath also
contains the conventional and well known chromium plating additives. The
bath is maintained at a temperature of about 112 -116 F., and utilizes a
mixed sulfate/fluoride catalyst. The chromic acid to sulfate ratio is about
200:1. A chromium layer of about 0.25 jm is deposited on the surface of
the bright nickel layer. The faucets are thoroughly rinsed in deionized water
and then dried.
The chromium plated faucets are placed in a cathodic arc
evaporation plating vessel. The vessel is generally a cylindrical enclosure
containing a vacuum chamber which is adapted to be evacuated by means
of pumps. A source of argon gas is connected to the chamber by an
adjustable valve for varying the rate of flow of argon into the chamber. In
addition, sources of nitrogen, methane and oxygen gases are connected to
the chamber by adjustable valves for varying the flow rates of nitrogen,
methane and oxygen into the chamber.
A cylindrical cathode is mounted in the center of the chamber and
connected to negative outputs of a variable D.C. power supply. The positive
side of the power supply is connected to the chamber wall. The cathode
material comprises zirconium.
CA 02552357 2011-05-20
WO 2005/118282
PCT/1152005/018581
The plated faucets are mounted on spindles, 16 of which are
mounted on a ring around the outside of the cathode. The entire ring
rotates around the cathode while each spindle also rotates around its own
axis, resulting in a so-called planetary motion which provides uniform
5 exposure to the
cathode for the multiple faucets mounted around each
spindle. The ring typically rotates at several rpm, while each spindle makes
several revolutions per ring revolution. The spindles are electrically
isolated
from the chamber and provided with rotatable contacts so that a bias
voltage may be applied to the substrates during coating.
10 The vacuum chamber
is evacuated to a pressure of about 104 to 10-7
torr and heated to about 150 C.
The electroplated faucets are then subjected to a high-bias arc
plasma cleaning in which a (negative) bias voltage of about -600 volts is
applied to the electroplated faucets while an arc of approximately 500
15 amperes is struck
and sustained on the cathode. The duration of the
cleaning is approximately five minutes.
Argon gas is introduced at a rate sufficient to maintain a pressure of
about 1 to 5 millitorr. A layer of zirconium having an average thickness of
about 0.1 Am is deposited on the chrome plated faucets during a three
20 minute period. The
cathodic arc deposition process comprises applying
D.C. power to the cathode to achieve a current flow of about 500 amps,
introducing argon gas into the vessel to maintain the pressure in the vessel
at about 1 to 5 millitorr and rotating the faucets in a planetary fashion
described above.
After the zirconium layer is deposited a zirconium carbonitride color
layer is deposited on the zirconium layer. Flows of nitrogen and methane
are introduced into the vacuum chamber while the arc discharge continues
at approximately 500 amperes. In order to increase the darkness of the
coating, a small flow of oxygen, amounted to 5 to 10 percent of the total gas
flow, may also be introduced into the chamber. To produce the dark gray
color carbon-rich zirconium carbonitride, the flow rate of methane is
CA 02552357 2011-05-20
WO 2005/118282
PCT/1JS2005/018581
21
momentarily increased meanwhile the flow rate of nitrogen is decreased,
and thus the resulting layer contains a carbon content between 25 to 50
atomic percent and nitrogen content between 5 to 35 atomic percent. To
produce the dark yellow nitrogen-rich carbonitride, the flow rate of nitrogen
is momentarily increased meanwhile the flow rate of methane is decreased,
and the resulting layer contains the nitrogen content between 25 to 50
atomic percent and carbon content between 5 to 35 atomic percent. Neither
these two layers is thick enough to make the coating bear its own color. As
a result, the overall color of the stack layers mimics a dark gray and dark
yellow two-tone antique bronze appearance. After this
zirconium
carbonitride layer is deposited, the nitrogen flow is terminated and a flow of
oxygen of approximately 100 to 500 standard liters per minute is introduced
for a time of about 10 to 60 seconds. A thin layer of zirconium oxide with a
thickness of about 20 to 100 A is formed. The arc is extinguished, the
vacuum chamber is vented and the coated articles removed.
EXAMPLE II
Other brass faucets were prepared according to the procedures of
Example I except that polymeric basecoats were used instead of nickel
basecoats. The initial cleaning procedures of Example I were followed.
After the ultrasonic cleaning the faucets are rinsed and dried.
A basecoat polymeric composition is applied onto the cleaned and
dried faucets by a standard and conventional high volume low pressure gun.
The polymer is comprised of 35 weight percent styrenated acrylic resin, 30
weight percent melamine formaldehyde resin, and 35 weight percent
bisphenol A epoxy resin. The polymer is dissolved in sufficient solvents to
provide a polymeric composition containing about 43 weight percent solids.
After the basecoat is applied onto the faucets the faucets are allowed to sit
for 20 minutes for ambient solvent flash off. The faucets are then baked at
375 F for two hours. The resulting cured polymeric basecoat has a
thickness of about 20 gm.
CA 02552357 2011-05-20
WO 2005/118282 PCT/US2005/018581
22
The polymeric coated faucets are rinsed three times and then placed
in a conventional, commercially available hexavalent chromium plating bath
using conventional chromium plating equipment according to the procedures
of Example I. The remaining procedures of Example I were followed to
produce coated articles having the same colored stack layer of Example I.
While certain embodiments of the invention have been described for
purposes of illustration, it is to be understood that there may be various
embodiments and modifications within the general scope of the invention.