Note: Descriptions are shown in the official language in which they were submitted.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
COMBINATION OF BENZOTHIAZOL-2-ONE BETA2 ADRENOCEPTOR AGONISTS AND
CORTICOSTEROIDS FOR THE TREATMENT OF RESPIRATORY DISEASES
This invention relates to organic compounds and their use as pharmaceuticals,
in particular for
the treatment of inflammatory or obstructive airways diseases.
In one aspect, the present invention provides a medicament comprising,
separately or together
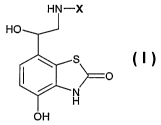
(A) a compound of formula I
H
in free or salt or solvate form, wherein
X is -R1-Ar-R2 or -R~-Y;
Ar denotes a phenylene group optionally substituted by halo, hydroxy, Ci-Cio-
alkyl, CmCio-
alkoxy, Ci-Cio-alkoxy-Ci-Cio-alkyl, phenyl, Ci-Cso-alkyl substituted by
phenyl, Ci-Cio-alkoxy
substituted by phenyl, Ci-Cio-alkyl-substituted phenyl or by Ci-Cio-alkoxy-
substituted phenyl;
R1 and RZ are attached to adjacent carbon atoms in Ar, and
either R1 is Ci-Cso-alkylene and R2 is hydrogen, Ci-Cio-alkyl, Cs-Cio-alkoxy
or halogen
or R1 and R2 together with the carbon atoms in Ar to which they are attached
denote a 5-, 6-
or 7-membered cycloaliphatic ring;
Ra is a bond or Ci-Cio-alkylene optionally substituted by hydroxy, Cs-C1o-
alkoxy, Cs-Cio-aryl
or C~-Ci4-aralkyl; and
Y is Ci-C1o-alkyl, Ci-Cio-alkoxy, Cz-Cio-alkenyl or Ca-C1o-alkynyl optionally
substituted by
halo, cyano, hydroxy, Cs-Cio-alkyl, Ci-CZO-alkoxy or halo-Ci-C1o-alkyl;
Ca-Cio-cycloalkyl optionally fused to one or more benzene rings and optionally
substituted by Ci-Cio-alkyl, Ci-Clo-alkoxy, Cs-Cso-cycloalkyl, C~-Ci4-aralkyl,
C7-Cia.-
aralkyloxy or C~-Cio-aryl, where Cs-Cio-cycloalkyl, C~-Ci4-aralkyl, C~-Ci4-
aralkyloxy
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
2
or Cs-Cio-aryl are optionally substituted by halo, hydroxy, Cl-Cio-alkyl, Ci-
Cso-alkoxy
or halo-Ci-Cio-alkyl;
Cs-Cio-aryl optionally substituted by halo, hydroxy, Ci-Cio-alkyl, Ci-Cso-
alkoxy, C1-
Cso-haloalkyl, phenoxy, CZ-Cio-alkylthio, Cs-Cio-aryl, 4- to 10- membered
heterocyclic
ring having at least one ring nitrogen, oxygen or sulphur atom, or by NRbR~
where Rb
and R~ are each independently Ci-Cio-alkyl optionally substituted by hydroxy,
Cs-Cio-
alkoxy or phenyl or R~ may additionally be hydrogen;
phenoxy optionally substituted by Ci-Cio-alkyl, Cl-Cio-alkoxy or by phenyl
optionally
substituted by Ci-Cio-alkyl or Ci-CZO-alkoxy;
a 4- to 10-membered heterocyclic ring having at least one ring nitrogen,
oxygen or
sulphur atom, said heterocyclic ring being optionally substituted by halo, Ci-
Cio-alkyl,
Ci-Cio-alkoxy, halo-Ci-Cio-alkyl, Cs-Cio-aryl, C~-C24-aralkyl, C~-Ci4-
aralkyloxy, C1-
Cio-alkoxycarbonyl or a 4- to 10-membered heterocyclyl-Ci-Cio-alkyl;
-NRdR° where Rd is hydrogen or Ci-Cio-alkyl and Re is Ci-Cio-alkyl
optionally
substituted by hydroxy, or Re is Cs-Cio-aryl optionally substituted by halo,
or Re is a 4-
to 10-membered heterocyclic ring having at least one ring nitrogen, oxygen or
sulphur
atom which ring is optionally substituted by phenyl or halo-substituted phenyl
or Re is
Cs-Cso-arylsulfonyl optionally substituted by Ci-Cio-alkylamino or di(C1-Cio-
alkyl)amino;
-SRfwhere Rf is Cs-CZO-aryl or C~-Cia-aralkyl optionally substituted by halo,
Ci-Cio-
alkyl, C1-Cio-alleoxy or Ci-Cio-haloalkyl; or
-CONHRg where Rg is CZ-Clo-alkyl, Cs-Cio-cycloalkyl or Cs-Cio-aryl; and
(B) a corticosteroid, for simultaneous, sequential or separate administration
in the treatment of
an inflammatory or obstructive airways disease, the molar ratio of (A) to (B)
being from 100:1
to 1:300.
In another aspect, the present invention provides a pharmaceutical composition
comprising a
mixture of effective amounts of (A) as hereinbefore defined and (B) as
hereinbefore defined,
optionally together with at least one pharmaceutically acceptable carrier.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
In a further aspect, the present invention provides a method of treating an
inflammatory or
obstructive airways disease which comprises administering to a subject in need
of such
treatment effective amounts of (A) as hereinbefore defined and (B) as
hereinbefore defined.
The invention further provides the use of (A) as hereinbefore defined and (B)
as hereinbefore
defined in the preparation of a medicament for combination therapy by
simultaneous,
sequential or separate administration of (A) and (B) in the treatment of an
inflammatory or
obstructive airways disease.
Terms used in the specification have the following meanings:
"Optionally substituted" as used herein means the group referred to can be
substituted at one
or more positions by any one or any combination of the radicals listed
thereafter.
"Halo" or "halogen" as used herein denotes a element belonging to group 17
(formerly group
VII) of the Periodic Table of Elements, which may be, for example, fluorine,
chlorine, bromine
or iodine. Preferably halo or halogen is fluorine or chlorine.
"Ci-Cio-alkyl" as used herein denotes straight chain or branched alkyl that
contains one to ten
carbon atoms. Preferably, Ci-Cio-alkyl is Ci-Ca-alkyl.
"Cs-Cio-alkylene" as used herein denotes a straight chain or branched alkylene
that contains
one to ten carbon atoms. Preferably Ci-Cio-alkylene is Ci-C4 alkylene,
especially ethylene or
methylethylene.
"Cz-Cio-alkenyl" as used herein denotes straight chain or branched hydrocarbon
chains that
contain two to ten carbon atoms and one or more carbon-carbon double bonds.
Preferably
"Cz-Cio-alkenyl" is "Cz-Ca-alkenyl".
"Cz-Cio-alkynyl" as used herein denotes straight chain or branched hydrocarbon
chains that
contain two to ten carbon atoms and one or more carbon-carbon triple bonds.
Preferably "Cz-
Cio-alkynyl" is "Cz-C4-alkynyl".
"Cs-Cio-cycloalkyl" as used herein denotes cycloalkyl having 3 to 10 ring
carbon atoms, for
example a monocyclic group such as a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cycloheptyl, cyclooctyl, cyclononyl or cyclodecyl, any of which can be
substituted by one or
CA 02552938 2006-07-17
WO 2005/074924 . PCT/EP2005/001241
more, usually one or two, Cs-Ca-alkyl groups, or a bicyclic group such as
bicycloheptyl or
bicyclooctyl. Preferably C3-Clo-cycloalkyl is Cs-C6-cycloalkyl, for example
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl.
"Ci-Cio-haloalkyl" as used herein denotes Cs-Cio-alkyl as hereinbefore defined
substituted by
one or more halogen atoms, preferably one, two or three halogen atoms.
"Ci-Cio-alkylamino" and "di(Ci-Clo-alkyl)amino" as used herein denote amino
substituted
respectively by one or two Ci-Cio-alkyl groups as hereinbefore defined, which
may be the same
or different. Preferably Ci-Cio-alkylamino and di(Ci-Cio-alkyl)amino are
respectively C1-C4-
alkylamino and di(Ci-C4-alkyl)amino.
"Ci-Cio-alkylthio" as used herein denotes straight chain or branched alkylthio
having 1 to 10
carbon atoms. Preferably, Ci-Cio-alkylthio is Ci-C4-alkylthio.
"Ci-Cio-alkoxy" as used herein denotes straight chain or branched alkoxy that
contains 1 to
carbon atoms. Preferably, Ci-Cio-alkoxy is Ci-C4-alkoxy.
"C1-Cio-alkoxy-Ci-Cso-alkyl" as used herein denotes Ci-Cio-alkyl as
hereinbefore defined
substituted by Ci-Cio-alkoxy. Preferably, Ci-Cio-alkoxy-Ci-Cio-alkyl is Ci-C4-
alkoxy-Cl-C4-
alkyl.
"Ci-Cio-alkoxycarbonyl" as used herein denotes CmCio-alkoxy as hereinbefore
defined linked
through an oxygen atom thereof to a carbonyl group.
"Cs-Cio-aryl" as used herein denotes a monovalent carbocyclic aromatic group
that contains 6
to 10 carbon atoms and which may be, for example, a monocyclic group such as
phenyl or a
bicyclic group such as naphthyl. Preferably C6-C1o-aryl is Cs-Cs-aryl,
especially phenyl.
"C~-Clo-arylsulfonyl" as used herein denotes Cs-Cio-aryl as hereinbefore
defined linked
through a carbon atom thereof to a sulfonyl group. Preferably Cs-Cso-
arylsulfonyl is Cs-Cs-
arylsulfonyl.
"C~-Cia.-aralkyl" as used herein denotes alkyl, for example CZ-C4-alkyl as
hereinbefore defined,
substituted by aryl, for example C6-Cio-aryl as hereinbefore defined.
Preferably, C~-Ci4-aralkyl
is C~-C1o-aralkyl such as phenyl-Cl-C4-alkyl, particularly benzyl or 2-
phenylethyl.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
"CrCi4-aralkyloxy" as used herein denotes alkoxy, for example Cl-C~-alkoxy as
hereinbefore
defined, substituted by aryl, for example Cs-Cio-aryl. Preferably, C~-Ci4-
aralkyloxy is C~-Cio-
aralkyloxy such as phenyl-Ci-C4-alkoxy, particularly benzyloxy or 2-
phenylethoxy.
Ar as used herein may be, for example, phenylene which is unsubstituted or
substituted by one
or more substituents selected from halogen, hydroxy, Ci-C1o-alkyl, Ci-Cio-
alkoxy, Ci-C1o-
alkoxy-Ci-Cio-alkyl, phenyl, or Cs-Cio-alkyl substituted by phenyl, Ci-Cio-
alkoxy substituted
by phenyl, Ci-Cio-alkyl-substituted phenyl and Ci-Cio-alkoxy-substituted
phenyl. Preferably Ar
is phenylene which is unsubstituted or substituted by one or two substituents
selected from
halogen, Ci-C4-alkyl, Cs-C4-alkoxy, or Cs-C4-alkoxy substituted by phenyl.
Preferably one
substituent in Ar is para to R1 and optional second and third substituents in
Ar are meta to R1.
"4- to 10-membered heterocyclic ring having at least one ring nitrogen, oxygen
or sulphur
atom" as used herein may be, for example, pyrrole, pyrrolidine, pyrazole,
imidazole, triazole,
tetrazole, thiadiazole, oxazole, isoxazole, thiophene, thiazole, isothiazole,
oxadiazole, pyridine,
pyrazine, pyridazine, pyrimidine, piperidine, piperazine, triazine, oxazine,
morpholino,
quinoline, isoquinoline, naphthyridine, indane or indene. Preferred
heterocyclic rings include
thiazole, pyrrolidine, piperidine, azacycloheptane and isoxazole.
"4 to 10-membered heterocyclyl-Cs-C1o-alkyl" denotes alkyl, for example Ci-Cio-
alkyl as
hereinbefore defined, substituted by a 4- to 10-membered heterocyclic ring as
hereinbefore
defined. Preferably, 4- to 10-membered heterocyclyl-Ci-Cio-alkyl is Ci-C4-
alkyl substituted by
a 4- to $-membered heterocyclic ring having at least one ring nitrogen, oxygen
or sulphur
atom.
"Ci-Ca-alkylsulfonyl" denotes sulfonyl substituted by Cs-C4-alkyl as
hereinbefore defined.
"Hydroxy-Ci-C4-alkyl" denotes Ci-C4-alkyl as hereinbefore defined substituted
by one or
more, preferably one, two or three hydroxy groups.
R1 and RZ together with the carbon atoms to which they are attached as a
cycloaliphatic ring
rnay be, for example, a cyclopentane ring, optionally substituted by one or
two Cl-C4-alkyl
groups, a cyclohexane ring, optionally substituted by one or two Ci-Ca-alkyl
groups, or a
cycloheptane ring, preferably a cyclopentane ring.
CA 02552938 2006-07-17
WO 2005/074924 6 PCT/EP2005/001241
Preferred compounds of formula I in free or salt or solvate form include those
wherein
X is -Ri-Ar-RZ or -Ra-Y;
Ar denotes a phenylene group optionally substituted by halo, Ci-Cio-alkyl, Cs-
Cio-alkoxy or by
Ci-CZO-alkoxy substituted by phenyl;
R~ and Rz are attached to adjacent carbon atoms in Ar, and
either Rl is C~-Cio-alkylene and R2 is hydrogen,
or Rl and RZ together with the carbon atoms in Ar to which they are attached
denote a S-, 6-
or 7-membered cycloaliphatic ring;
Ra is a bond or Ci-Cio-alkylene optionally substituted by hydroxy, C6-Cio-aryl
or C~-Clø-
aralkyl; and
Y is Ci-Cio-alkyl, Ci-Cio-alkoxy or CZ-Cio-alkynyl; Cs-Cio-cycloalkyl
optionally fused to one or
more benzene rings and optionally substituted by Ci-CZO-alkyl, C3-CZO-
cycloalkyl, C~-C14-
aralkyl, C~-Cm-aralkyloxy optionally substituted by halo, or by Cs-Cio-aryl
optionally
substituted by C1-C1o-alkyl or C1-C10-alkOxy; Cs-Cio-aryl optionally
substituted by halo,
hydroxy, Ci-Cio-alkyl, phenoxy, Cs-Cio-alkylthio, Cs-Cio-aryl, a 4- to 10-
membered
heterocyclic ring having at least one ring nitrogen atom, or by NRbR°
where Rb and R~ are each
independently Ci-Cio-alkyl optionally substituted by hydroxy or phenyl or Rb
may additionally
be hydrogen; phenoxy optionally substituted by Ci-Cio-alkoxy; a 4- to 10-
membered
heterocyclic ring having at least one ring nitrogen or oxygen atom, said
heterocyclic ring being
optionally substituted by Ci-Clo-alkyl, C6-Clo-aryl, C~-CZa-aralkyl, Ci-Clo-
alkoxycarbonyl or
by a 4- to 10-rnembered heterocyclyl-Cs-Cio-alkyl; -NRdRe where Rd is hydrogen
or C2-Cio-
alkyl and Re is Ci-Cio-alkyl, or Re is a 4- to 10-membered heterocyclic ring
having at least one
ring nitrogen or oxygen atom which ring is optionally substituted by halo-
substituted phenyl or
Re is Cs-Cio-arylsulfonyl optionally substituted by di(C1-Cio-alkyl)amino; -
SRS where RE is C6-
Cio-aryl or C~-Ci4-aralkyl optionally substituted by halo or Cs-Cio-haloalkyl;
or -CONHRg
where Rg is C3-Cio-cycloalkyl or Cs-Cio-aryl.
Especially preferred compounds of formula I in free or salt or solvate form
include those
wherein
X is -Rl-Ar-Rz or -Ra-Y;
Ar denotes a phenylene group optionally substituted by halo, Ci-Cø-alkyl, Ci-
C4-alkoxy or by
Ci-C4-alkoxy substituted by phenyl;
Rl and Rz are attached to adjacent carbon atoms in Ar, and
either Rl is Ci-C4-alkylene and Ra is hydrogen,
or Rl and RZ together with the carbon atoms in Ar to which they are attached
denote a S-, 6-
or 7-membered cycloaliphatic ring, especially a S-membered cycloaliphatic
ring;
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
7
Ra is a bond or Ci-Ca-alkylene optionally substituted by hydroxy, Cs-Cs-aryl
or C~-Cio-aralkyl;
and
Y is Ci-C4-alkyl, Ci-C4-alkoxy or Ca-C4-alkynyl; Cs-C6-cycloalkyl optionally
fused to one or
more benzene rings and optionally substituted by Cl-C6-alkyl, Cs-Cs-
cycloalkyl, C~-Cio-aralkyl,
CrCio-aralkyloxy optionally substituted by halo, or by C6-Cs-aryl optionally
substituted by Cl-
C4-alkyl or Ci-C4-alkoxy; Cg-Cs-aryl optionally substituted by halo, hydroxy,
Ci-Ca.-alkyl,
phenoxy, Ci-Ca-alkylthio, Cs-Cs-aryl, a 4- to 8-membered heterocydic ring
having at least one
ring nitrogen atom, or by NRbR~ where Rb and R~ are each independently Ci-C4-
alkyl
optionally substituted by hydroxy or phenyl or Rb may additionally be
hydrogen; phenoxy
optionally substituted by Cs-C4-alkoxy; a 4- to 8-membered heterocyclic ring
having at least
one ring nitrogen or oxygen atom, said heterocyclic ring being optionally
substituted by Cl-C4-
alkyl, Cs-Cs-aryl, C~-Cio-aralkyl, Ci-Ca-alkoxycarbonyl or by a 4- to 8-
membered heterocyclyl-
Ci-C4-alkyl; -NRdR° where Rd is hydrogen or Ci-C4-alkyl and Re is Ci-C4-
alkyl, or Re is a 4- to
8-membered heterocyclic ring having at least one ring nitrogen or sulphur atom
which ring is
optionally substituted by halo-substituted phenyl or Re is C6-Cs-arylsulfonyl
optionally
substituted by di(Cl-C4-alkyl)amino; -SRfwhere Rf is C6-Cs-aryl or C~-Cio-
aralkyl optionally
substituted by halo or Ci-Ca-haloalkyl; or -CONHRg where Rg is C3-C6-
cycloalkyl or C6-Cs-
aryl.
More especially preferred compounds of formula I in free or salt or solvate
form include:
4-hydroxy-7-( 1-hydroxy-2-{2-[4-(4-phenyl-butoxy)-phenyl]-ethylamino}-ethyl)-
3H-benzo-
thiazol-2-one; 7-[(R)-2-(1,1-dimethyl-2-phenyl-ethylamino)-1-hydroxy-ethyl]-4-
hydroxy-3H-
benzothiazol-2-one; 4-hydroxy-7-{(R)-1-hydroxy-2-[2-(5,6,7,8-tetrahydro-
naphthalen-2-yl)-
ethylaminol-ethyl}-3H-benzothiazol-2-one formate; 7-[(R)-2-((1S,2S)-2-
benzyloxy-cyclopentyl-
amino)-1-hydroxy-ethyl]-4-hydroxy-3H-benzo-thiazol-2-one; and 7-[(R)-2-
((1S,2R)-2-
benzyloxy-cyclopentylamino)-1-hydroxy-ethyl]-4-hydroxy-3H-benzothiazol-2-one.
In formula I the carbon atom alpha to the phenolic ring carries a hydroxy
group and so is
asymmetric, so the compound exists in individual optically active isomeric
forms or as
mixtures thereof, e.g. as racemic or diastereomeric mixtures. Compounds of
formula I embrace
both individual optically active R and S isomers as well as mixtures, e.g.
racemic or
diastereomeric mixtures, thereof.
Pharmaceutically acceptable acid addition salts of the compound of formula I
include those of
inorganic acids, for example, hydrohalic acids such as hydrofluoric acid,
hydrochloric acid,
CA 02552938 2006-07-17
WO 2005/074924 g PCT/EP2005/001241
hydrobromic acid or hydroiodic acid, nitric acid, sulfuric acid, phosphoric
acid; and organic
acids, for example aliphatic monocarboxylic acids such as formic acid, acetic
acid, trifluoro-
acetic acid, propionic acid and butyric acid, aliphatic hydroxy acids such as
lactic acid, citric
acid, tartaric acid or malic acid, dicarboxylic acids such as malefic acid or
succinic acid,
aromatic carboxylic acids such as benzoic acid, p-chlorobenzoic acid,
diphenylacetic acid or
triphenylacetic acid, aromatic hydroxy acids such as o-hydroxybenzoic acid, p-
hydroxy-
benzoic acid, 1-hydroxynaphthalene-2,-carboxylic acid or 3-hydroxynaphthalene-
2-carboxylic
acid, and sulfonic acids such as methanesulfonic acid or benzenesulfonic acid.
These salts may
be prepared from compounds of formula I by lenown salt-forming procedures.
Pharmaceutically acceptable solvates are generally hydrates.
Compounds of formula I in free or salt or solvate form may also be prepared
by:
(i) either (A) reacting a compound of formula II
R'-N-X
HO
S\
/ ,,.
O II
where X is as hereinbefore defined and R~ denotes a protecting group to
replace R~ by
hydrogen,
or (B) reacting a compound of formula III
F
R9 III
where X and R'' are as hereinbefore defined and R8 and R9 each independently
denote a
protecting group, to convert groups R~, R8 and R9 to hydrogen; and
(ii) recovering the compound of formula I in free or salt or solvate form.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
Where reference is made herein to protected functional groups or to protecting
groups, the
protecting groups may be chosen in accordance with the nature of the
functional group, for
example as described in Protective Groups in Organic Synthesis, T.W. Greene
and P.G.M.
Wuts, John Wiley ~ Sons Inc, Third Edition, 1999, which reference also
describes procedures
suitable for replacement of the protecting groups by hydrogen.
The protecting group R~ may be, for example, a group chosen from known amine-
protecting
groups. Preferred protecting groups R~ include araliphatic groups such as
benzyl and
trialkylsilyl e.g. trimethylsilyl. Protecting groups R$ and R9 may be chosen
from known
phenolic hydroxy - and alcoholic hydroxy-protecting groups respectively.
Preferred groups R8
and R9 include C1-C4-alkyl groups, particularly branched groups such as
isopropyl and tert-
butyl.
Process variant (A) may be effected, for example, using known procedures for
conversion of
amine-protecting groups to hydrogen or analogous procedures. For example,
where R~ is a
benzyl group it may be converted to hydrogen by hydrogenolysis of the compound
of formula
II, e.g. with a carboxylic acid such as formic acid, preferably in the
presence of a palladium
catalyst. This de-protection reaction may be carried out using procedures as
described
hereinafter in the Examples or analogous procedures.
Process variant (B) may be effected using known procedures for conversion of
hydroxy-
protecting groups to hydrogen or analogous procedures. For example, where, Rg
and R9 are
alkyl groups, R8 and R9 rnay be converted to hydrogen by hydrogenolysis of the
compounds of
formula IV, e.g. with a carboxylic acid such as formic acid preferably in the
presence of a
palladium catalyst, for example as hereinbefore described for conversion of R~
to hydrogen, or
by treatment with an acid alone such as formic acid, hydrochloric acid or
trifluoroacetic acid,
in either case the resulting 2-hydroxybenzothiazole compound being in
tautomeric equilibrium
with the benzothiazol-2-one form.
Compounds of formula II may be prepared by reduction of a compound of formula
IV
IV
CA 02552938 2006-07-17
WO 2005/074924 1o PCT/EP2005/001241
where X and R~ are as hereinbefore defined. The reduction may be effected
using known
methods for reduction of ketones to alcohols, or analogous methods, including
asymmetric
reductions. For example, the compounds of formula IV may be reacted with NaBH4
in an inert
solvent such as an aliphatic alcohol. Suitable reaction temperatures are from -
80° C to 100° C,
conveniently from -S° C to 5° C. The reduction may be effected
using known procedures or
analogously as described hereinafter in the Examples.
Compounds of formula III may be prepared by reacting a compound of formula V
O-R9 V
where R$ and R9 are as hereinbefore defined, with a compound of formula VI
R'
~N-X VI
H
where X and R~ are as hereinbefore defined. The reaction of compounds of
formulae V and VI
may be effected using known procedures for epoxide-amine reactions or
analogous procedures.
The reaction is optionally effected in an inert organic solvent, conveniently
an alcohol such a n-
butanol. Suitable reaction temperatures are, for example, from 0° C to
solvent reflux
temperature. The reaction may be effected conveniently using a procedure as
described
hereinafter in the Examples, or analogously.
Compounds of formula IV may be prepared by reacting a compound of formula VII
R' N-X
O
C
S
~>--O-R9 VII
'N
R8,0
where X, R~ , R$ and R9 are as hereinbefore described, with concentrated
hydrochloric or
hydrobromic acid. The reaction is preferably carried out in an inert organic
solvent such as an
aliphatic alcohol.
CA 02552938 2006-07-17
WO 2005/074924 11 PCT/EP2005/001241
Compounds of formula V and VI rnay be prepared by known methods or analogously
such as
hereinafter described in the Examples.
Compounds of formula VII may be prepared by reaction of a compound of formula
VIII
Q
S
/ ~ IC-O-R9 VII
~N
H
Rs, O
where Q is fluorine or chlorine and R8 and R9 are as hereinbefore defined,
with a strong base,
such as an alkyllithium, NaNHa or potassium tert-butoxide or a mixture of two
or more
thereof, and a compound of formula IX
R'
N-X
O
IX
N
H3C~ ~OCH3
where N and R~ are as hereinbefore defined. The reaction is preferably
effected in an inert
organic solvent, for example an ether such as tetrahydrofuran (THF). Suitable
reaction
temperatures may be, for example, from -80° C to 80° C. The
reaction may be effected using a
procedure as described hereinafter in the Examples or analogous procedures.
Compounds of formulae VIII and I~ may be prepared using known procedures or
analogously,
such as described hereinafter in the Examples.
Compounds of formula I in free form may be converted into salt form, and vice
versa, in a
conventional manner. The compounds in free or salt form can be obtained in the
form of
hydrates or solvates containing a solvent used for crystallisation. Compounds
of formula I can
be recovered from reaction mixtures and purified in a conventional manner.
Isomers, such as
enantiomers, may be obtained in a conventional manner, e.g. by fractional
crystallisation or
asymmetric synthesis from correspondingly asymmetrically substituted, e.g.
optically active,
starting materials.
The corticosteroid (B) may, for example, be a compound of formula X
CA 02552938 2006-07-17
WO 2005/074924 12 PCT/EP2005/001241
,.,a
~c
X
Xb
or a 1,2-dihydro derivative thereof, where
Ra is Ci-Ca-alkyl optionally substituted by halogen (preferably chlorine or
fluorine), hydroxy,
Cz-C4-alkoxy, acyloxy or by Ci-C4-acylthio, or Ra is C1-C4-alkoxy or Ci-C4-
alkylthio
optionally substituted by halogen, or R$ is 5-or 6-membered heterocyclylthio,
or Ra is C1-C4-
alkylthio optionally substituted by halogen (preferably chlorine or fluorine),
either Rb is acyloxy and R~ is hydrogen or Ci-C4-alkyl,
or Rb and R~ together denote a group of formula XI
,,~~~0/~Re
XI
.,~»O Ra
where Rd is CmC4-alkyl or Cs-C6-cycloalkyl and Re is hydrogen or Ci-C4-alkyl,
Xa and Xb are each independently hydrogen, chlorine or fluorine.
,When Ra is acyloxy-substituted CmC4-alkyl, the acyloxy group may be, for
example, C1-Cao-
alkylcarbonyloxy, e.g. acetyloxy, n-propionyloxy, isopropionyloxy or
hexadecanoyloxy, or C3-
Cs-cycloalkylcarbonyloxy, e.g. cyclohexylcarbonyloxy. When Ra is acylthio-
substituted C1-C4-
alkyl, the acylthio group may be, for example, Ci-Ca-alkylcarbonylthio, e.g.
acetylthio or n-
propionylthio. When Ra is 5-or-6-membered heterocyclylthio, the heterocyclyl
group may be an
O-heterocyclyl group, for example a furanonyl group.
When Rb is acyloxy, it may be, for example, Ci-Ca-alkylcarbonyloxy, e.g.
acetyloxy, n-
propionyloxy, or n-butyroyloxy, Cs-Cs-cycloalkylcarbonyloxy e.g.
cyclopropylcarbonyloxy, or
5-or 6-membered heterocyclylcarbonyloxy e.g. furoyloxy, or when Rb is acyloxy
it may be a
group -O-CO-T where T is a monovalent cyclic organic group having from 3 to 15
atoms in
the ring system. Preferably T is a carbocyclic group or a heterocyclic group
having one or more
ring hetero atoms selected from nitrogen, oxygen and sulfur.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
I3
When R~ is C1-C4-alkyl it may be in the alpha or beta conformation, more
usually in the alpha
conformation.
When Rb and R° together denote a group of formula XI, Ra as Ca-Cs-
cycloalkyl may be, for
example, cyclohexyl.
Corticosteroids of formula X and their 1,2-dihydro derivatives include
beclamethasone
dipropionate, budesonide, fluticasone propionate, mometasone furoate,
ciclesonide,
triamcinolone acetonide, flunisolide, rofleponide palmitate, butixocort
propionate,
icometasone enbutate and described in WO 03/042229, WO 03/035668, WO
02/100879, WO
02/088167.
In particularly preferred embodiments of the invention, the corticosteroid (B)
is budesonide,
fluticasone propionate, mometasone furoate or either of the following
compounds:
G
O \ F~ '~N
S
CH3
nCH~ nCH~
O O
F F
Budesonide, fluticasone propionate and mometasone furoate and their
preparation are
described in United States patent specifications US 3929768, US 4335121 and US
4472393
respectively.
Corticosteroids of formula X where Rb is -O-CO-T are preferably compounds of
formula XII
H3C
O
O
HO CH3 , O
O
CH3 "~~~~~ CH3
/ XII
w/
O
F
where T is a monovalent cyclic organic group having from 3 to 15 atoms in the
ring system.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
14
Preferably T is a carbocyclic group or a heterocyclic group having one or more
ring hetero
atoms selected from nitrogen, oxygen and sulfur.
In one embodiment, T is a cycloaliphatic group having 3 to ~ carbon atoms, for
example C3-
Cs-cycloalkyl such as cyclopropyl, methylcyclopropyl, cyclobutyl,
methylcyclobutyl,
cyclopentyl, cyclohexyl, methylcyclohexyl, dimethylcyclohexyl or cycloheptyl,
preferably Cs-
Cs-cycloalkyl.
In another embodiment, T is an at least partially saturated heterocyclic group
having 5 to 10
ring atoms, of which one or more are ring hetero atoms selected from nitrogen,
oxygen and
sulfur, preferably having 5 to 7 ring atoms, of which one or two are hetero
atoms selected
from nitrogen and oxygen, especially a 5-membered heterocyclic group having
one ring hetero
atom, such as a tetrahydrofuryl or oxotetrahydrofuryl group.
In a further embodiment, T is a carbocyclic or heterocyclic aromatic group
having S to 15
atoms in the ring system. For example, T may be such an aromatic group in
which the ring
system is unsubstituted or is substituted by one or more substituents selected
from halogen,
cyano, Ci-C4-alkyl, halo-Ci-C4-alkyl, Ci-C4-alkoxy, Ci-C4-alkylthio, hydroxyl,
Ci-C4-acyl, C1-
C4-acyloxy, amino, CrCøalkylarnino, di-(C1-C4-alkyl)amino, Ci-Ca-acylamino, Cs-
C4-acyl(C1-
C4-alkyl)-amino, Cs-C4-alkylsulfonyl(CrC4-alkyl)amino, Cs-C4-alkoxycarbonyl,
or 5-
membered heterocyclyl, usually N-heterocyclyl having one or two nitrogen
atoms. One
preferred class of such aromatic groups is phenyl or naphthyl optionally
substituted by one or
rr~ore, preferably one, two or three, substituents selected from cyano, Ci-Ca.-
alkyl, halo-C1-C4-
alkyl, Ci-C4 alkoxy, halogen, hydroxyl, Ci-C4-acyloxy, amino, Ci-C4-
alkylamino, di-Ci-C4-
alkylamino, Ci-C4-acyl-amino, Ci-C4-acyl(Ci-C4 alkyl)amino, C2-C4
alkylsulfonyl(C1-C4
alkyl)amino or Ci-C4-alkoxy-carbonyl, especially preferred such aromatic
groups including
phenyl, cyanophenyl, tolyl, dimethylphenyl, ethylphenyl,
(trifluoromethyl)phenyl, dimethoxy-
phenyl, diethoxyphenyl, hydroxyphenyl, (methylarnino)phenyl,
(methanesulfonylmethylamino)-phenyl and (methoxy-carbonyl)phenyl.
Another preferred class of such aromatic groups is a heterocyclic aromatic
group having a 6-
membered heterocyclic ring with one, two or three ring heteroatoms, preferably
nitrogen, the
heterocyclic ring being unsubstituted or substituted by one or more,
preferably one, two or
three, substituents selected from halogen, cyano, hydroxyl, Ci-C4-acyloxy,
amino, Ci-C4-alkyl-
amino, di-(C1-C4-alkyl)amino, Cs-C4-alkyl, hydroxy-Ci-C4-alkyl, halo-Cs-C4-
alkyl, C1-Ca-
alkoxy, or Ci-C4-alkylthio, and the heterocyclic ring being optionally fused
to a benzene ring.
CA 02552938 2006-07-17
WO 2005/074924 15 PCT/EP2005/001241
Preferred such heterocyclic aromatic groups include those in which the
heterocyclic group has
one or two nitrogen atoms in the ring, especially a pyridine, pyrimidine,
pyrazine or pyridazine
ring. Especially preferred heterocyclic aromatic groups are pyridyl,
pyrimidinyl and pyrazinyl
groups, optionally substituted by one or two substituents selected from
halogen (particularly
chlorine) or Ci-C4-alkyl (especially methyl or n-butyl).
Another preferred class of such aromatic groups is a heterocyclic aromatic
group having a S-
membered heterocyclic ring with one, two or three ring hetero atoms selected
from nitrogen,
oxygen and sulfur, the heterocyclic ring being unsubstituted or substituted by
one or two
substituents selected from halogen, Ci-C4-alkyl, halo-Ci-C4-alkyl, Ci-Ca-
alkoxy, Ci-Ca.-alkyl-
thio, cyano or hydroxy-Ci-C4-alkyl and the heterocyclic ring being optionally
fused to a
benzene ring. Preferred such heterocyclic aromatic groups include those in
which the
heterocyclic ring has one nitrogen, oxygen or sulfur atom in the ring or one
oxygen and one or
two nitrogen atoms in the ring, or one sulfur and one or two nitrogen atoms in
the ring,
especially a pyrrole, furan, thiophene, oxazole, isoxazole, imidazole,
pyrazole, furazan, thiazole
or thiadiazole ring. Especially preferred heterocyclic aromatic groups are
pyrrolyl, furyl and
thienyl groups optionally substituted by one or two substituents selected from
halogen
(particularly chlorine or bromine), Ci-C4-alkyl (particularly methyl or
ethyl), halo-Ci-C4-alkyl
(particularly trifluoro-methyl), Ci-C4-alkoxy (particularly methoxy), C2-C4-
alkylthio
(particularly methylthio), cyano or hydroxy-Ci-C4-alkyl (particularly
hydroxymethyl);
isoxazolyl, imidazolyl, pyrazolyl, thiazolyl or thiadiazolyl groups optionally
substituted by one
or two Ci-C4-alkyl groups; and benzofuryl, benzothienyl and benzofurazanyl
groups.
In compounds of formula XII, the indicated methyl group in the 16 position of
the cortico-
steroid ring system may be in the alpha or beta conformation. 16-a-methyl
compounds are
preferred.
Especially preferred compounds of formula XII are those where the indicated 16-
methyl group
has the alpha conformation and T is S-methyl-2-thienyl, N-methyl-2-pyrrolyl,
cyclopropyl, 2-
furyl, 3-methyl-2-furyl, 3-methyl-2-thienyl, 5-methyl-3-isoxazolyl, 3,5-
dimethyl-2-thienyl, 2,5-
dimethyl-3-furyl, 4-methyl-2-furyl, 4-(dimethylamino)phenyl, 4-methylphenyl, 4-
ethylphenyl,
2,-pyridyl, 4-pyrimidyl or S-methyl-2-pyrazinyl or the indicated 16-methyl
group has the beta
conformation and R is cyclopropyl.
The compounds of formula XII and salts thereof where T contains a basic group
may be
prepared using the procedures described in international patent application WO
02/00679.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
16
The corticosteroid (B~ may, for example, also be a non-steroids!
glucocorticoid receptor
agonist, such as those described in DE 10261874, WO 00/00531, WO 02/10143, WO
03/82280, WO 03/82'787, WO 03/86294, WO 03/104195, WO 03/101932, WO 04/05229,
WO 04/18429, WO 04/19935 and WO 04/26248.
The medicament of tlae present invention may additionally contain one or more
AzA agonists,
Azs antagonists, antihistamines, caspase inhibitors, LTB4 antagonists, LTD4
antagonists, M3
antagonists and PDE inhibitors or anti-tussive drug substance.
Suitable AzA agonists include those described in EP 409595A2, BP 1052264, EP
1241176, WO
94/17090, WO 96/02543, WO 96/02553, WO 98/28319, WO 99/24449, WO 99/24450, WO
99/24451, WO 99138 877, WO 99/41267, WO 99/67263, WO 99/67264, WO 99/67265, WO
99/67266, WO 00/2,3457, WO 00/77018, WO 00/78774, WO 01/23399, WO 01/27130,
WO 01127131, WO O 1/60835, WO 01/94368, WO 02/00676, WO 02122630, WO 02/96462,
WO 03/086408, WO 04/039762, WO 04/039766, WO 041045618 and WO 041046083.
Suitable Azs antagonists include those described in WO 02/42298. Suitable
antihistamine drug
substances include cetirizine hydrochloride, acetaminophen, clemastine
fumarate,
promethazine, loratid3ne, desloratidine, diphenhydramine and fexofenadine
hydrochloride,
activastine, astemizole, azelastine, ebastine, epinastine, mizolastine and
tefenadine as well as
those disclosed in JP 2.004107299, WO 03/099807 and WO 0/026841. Suitable
caspase
inhibitors, including interleukin-I P converting enzyme (ICE) inhibitors,
include those that are
disclosed in Canadian patent specification 2109646, EP 519748, EP 547 699, EP
590 650, EP
628550, EP 644197, EP 644198, WO 93/05071, WO 93/14777, WO 93/16710, WO
94/00154, WO 94/03480, WO 94/21673, WO 95/05152, WO 95/35308, WO 97/22618, WO
97/22619, WO 981412,32, WO 99/06367, WO 99/65451, WO 01/119373, US 5411985, US
5416013, US 5430125, US 5434248, US 5565430, US 558535?, US 5656627, US
5677283, US
6054487, US 6531474, US 20030096737, GB 2,278,276 as well as those disclosed
in
international patent applications WO 98/10778, WO 98/11109, WO 98/11129 and WO
03/32918. Suitable LTB4 antagonists include those described in US 5451700.
Suitable LTD4
antagonists include m ontelukast and zafirlukast. Suitable M3 antagonists
include ipratropium
bromide, oxitropium bromide, tiotropium salts, CHF 4226 (Chiesi) and
glycopyrrolate, but
also those described in EP 424021, US 3714357, US 5171744, TWO 02/00652, WO
02/51841,
WO 02/53564, WO O 3/00840, WO 03/33495, WO 03/53966, WO 03/87094, WO
04/018422, WO 04/05285 and WO 04/96800, preferably compounds of the Examples
thereof.
The M3 antagonist is most preferably ipratropium bromide, oxitropium bromide
or a
tiotropium salt. The M3 antagonist may also be a dual beta-2 adrenoceptor
agonist l
CA 02552938 2006-07-17
WO 2005/074924 1,~ PCT/EP2005/001241
muscarinic antagonist such as those disclosed in US 2004/0167167, WO 04/74246
and WO
04/74812. Suitable PDE4 inhibitors include (Ariflo~ GSK), Roflumilast (Byk
Gulden),V-
11294A (Nappy, BAY19-8004 (Bayer), SCH-351591 (Schering-Plough), Arofylline
(Almirall
Prodesfarma), PD1896S9 / PD168787 (Parke-Davis), AWD-12-281 (Asta Medica), CDC-
801
(Celgene), SeICID(TM) CC-10004 (Celgene), VMSS4/UMS6S (Vernalis), T-440
(Tanabe),
KW-4490 (Kyowa Hakko Kogyo), and those disclosed in WO 92/19594, WO 93/19749,
WO
93/19750, WO 93/19751, WO 98/18796, WO 99/16766, WO 01/13953, WO 03/104204,
WO 031104205, WO 03/39544, WO 041000814, WO 04/000839, WO 04/005258, WO
04/018450, WO 041018451, WO 041018457, WO 04/018465, ~l0 04/018431, WO
04/018449, WO 04/018450, WO 04/018451, WO 04/018457, W O 04/018465, WO
04/019944, WO 041019945, WO 04/045607 and WO 04/037805.
Although the medicament of the invention contains a compound of formula I that
posseses
beta-2 adrenoceptor agonist activity the medicament may additionally contain
another beta-2
adrenoceptor agonist. Suitable additional such beta-2 adrenoceptor agonists
albuterol
(salbutamol), metaproterenol, terbutaline, salmeterol fenoterol, procaterol,
and especially,
formoterol, carmoterol and pharmaceutically acceptable salts thereof, and
compounds (in free
or salt or solvate form) of formula I of WO 0075114, which document is
incorporated herein
by reference, preferably compounds of the Examples thereof, especially a
compound of
formula
0
C H3
CH3
HO
N
H
OH
and pharmaceutically acceptable salts thereof, as well as compounds of EP
1440966, JP
05025045, WO 93/18007, WO 99/64035, US 2002/0055651, WO 01/42193, WO 01/83462,
WO 02/66422, WO 02/ 70490, WO 02/76933, WO 03/24439, ~O 03/42160, WO 03/42164,
WO 03/72539, WO 03/91204, WO 03/99764, WO 04/16578, WO 04/22547, WO 04/32,921,
WO 04/33412, WO 04/37768, WO 04/37773, WO 04/37807, WO 04/39762, WO 04/39766,
WO 04/45618 WO 04/46083 and WO 04/80964.
Administration of the medicament or pharmaceutical composition as hereinbefore
described,
i.e. with (A) and (B) in admixture or separate, is preferably by inhalation,
i.e. (A) and (B) are in
inhalable form. The inhalable form of the medicament may be, for example, an
atomizable
CA 02552938 2006-07-17
WO 2005/074924 1g PCT/EP2005/001241
composition such as an aerosol comprising the active ingredient, i.e. (A) and
(B) separately or
in admixture, in solution or dispersion in a propellant, or a nebulizable
composition
comprising a solution or dispersion of the active ingredient in an aqueous,
organic or
aqueous/organic medium. For example, the inhalable form of the medicament may
be an
aerosol comprising a mixture of (A) and (B) in solution or dispersion in a
propellant, or a
combination of an aerosol containing (A) in solution or dispersion in a
propellant with an
aerosol containing (B) in solution or dispersion in a propellant. In another
example, the
inhalable form is a nebulizable composition comprising a dispersion of (A) and
(B) in an
aqueous, organic or aqueous/organic medium, or a combination of a dispersion
of (A) in such
a medium with a dispersion of (B) in such a medium.
An aerosol composition suitable for use as the inhalable form of the
medicament may comprise
the active ingredient in solution or dispersion in a propellant, which may be
chosen from any
of the propellants known in the art. Suitable such propellants include
hydrocarbons such as n-
propane, n-butane or isobutane or mixtures of two or more such hydrocarbons,
and halogen-
substituted hydrocarbons, for example chlorine and/or fluorine-substituted
urethanes, ethanes,
propanes, butanes, cyclopropanes or cyclobutanes, such as
dichlorodifluoromethane (CFC 12),
trichlorofluoromethane (CFC11), 1,2-dichloro-1,1,2,2 -tetrafluoroethane
(CFC114) or,
particularly, 1,1,1,2-tetrafluoroethane (HFA134a) and 1,1,1,2,3,3,3-
heptafluoropropane
(HFA227), or mixtures of two or more such halogen-substituted hydrocarbons.
D~here the
active ingredient is present in suspension in the propellant, i.e. where it is
present in particulate
form dispersed in the propellant, the aerosol composition may also contain a
lubricant and a
surfactant, which may be chosen from those lubricants and surfactants known in
the art.
Other suitable aerosol compositions include surfactant-free or substantially
surfactant-free
aerosol compositions. The aerosol composition may contain up to about S% by
weight, for
example 0.0001 to 5%, 0.001 to 5%, 0.001 to 3%, 0.001 to 2%, 0.001 to 1%,
0.001 to
0.1%, or 0.001 to 0.01% by weight of the active ingredient, based on the
weight of the
propellant. Where present, the lubricant and surfactant may be in an amount up
to 5% and
0.5% respectively by weight of the aerosol composition. The aerosol
composition may also
contain a co-solvent such as ethanol in an amount up to 30% by weight of the
composition,
particularly for administration from a pressurised metered dose inhalation
device. The aerosol
composition may further contain a bulking agent, for example a sugar such as
lactose, sucrose,
dextrose, mannitol or sorbitol, in an amount, for example, of up to 20%,
usually 0.001 to 1%,
by weight of the composition.
CA 02552938 2006-07-17
WO 2005/074924 19 PCT/EP2005/001241
In another embodiment of the invention, the inhalable form is a dry powder,
i.e. (A) and (B)
are present in a dry powder comprising finely divided (A) and (B) optionally
together with at
least one particulate pharmaceutically acceptable carrier, which may be one or
more materials
known as pharmaceutically acceptable carriers, preferably chosen from
materials known as
carriers in dry powder inhalation compositions, for example saccharides,
including
monosaccharides, disaccharides, polysaccharides and sugar alcohols such as
arabinose, glucose,
fructose, ribose, mannose, sucrose, trehalose, lactose, maltose, starches,
dextran, rnannitol or
sorbitol. An especially preferred carrier is lactose. The compostion may also
contain a
compound that helps to protect against product performance deterioration due
to moisture e.g.
magnesium stearate.
The dry powder may be contained as unit doses in capsules of, for example,
gelatin or plastic,
or in blisters (e.g, of aluminium or plastic), for use in a dry powder
inhalation device, which
may be a single dose or multiple dose device, preferably in dosage units of
(A) and (B) together
with the carrier in amounts to bring the total weight of powder per capsule to
from S mg to 50
mg. Alternatively, the dry powder may be contained in a reservoir in a multi-
dose dry powder
inhalation device adapted to deliver, for example, 3-25mg of dry powder per
actuation.
In the finely divided particulate form of the medicament, and in the aerosol
composition where
the active ingredient is present in particulate form, the active ingredient
may have an average
particle diameter of up to about 10 p.m, for example 0.1 to 5 p.m, preferably
1 to S pm. The
particulate carrier, where present, generally has a maximum particle diameter
up to 300 p,m,
preferably up to 212, p,m, and conveniently has a mean particle diameter of 40
to 100 p,m, e.g.
50 to 75 pm. The particle size of the active ingredient, and that of a
particulate carrier where
present in dry powder compositions, can be reduced to the desired level by
conventional
methods, for example by grinding in an air-jet mill, ball mill or vibrator
mill, sieving, micro-
precipitation, spray-drying, lyophilisation or controlled crystallisation from
conventional
solvents or from supercritical media.
The inhalable medicament may be administered using an inhalation device
suitable for the
inhalable form, such devices being well known in the art. Accordingly, the
invention also
provides a pharmaceutical product comprising a medicament or pharmaceutical
composition
as hereinbefore described in inhalable form as hereinbefore described in
association with one
or more inhalation devices. In a further aspect, the invention provides an
inhalation device, or
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
a pack of two or more inhalation devices, containing a medicament or
pharmaceutical
composition as hereinbefore described in inhalable form as hereinbefore
described.
Where the inhalable form of the active ingredient is an aerosol composition,
the inha..lation
device may be an aerosol vial provided with a valve adapted to deliver a
metered dose, such as
10 to 100 p,l, e.g. 25 to 50 p.l, of the composition, i.e. a device known as a
metered dose
inhaler. Suitable such aerosol vials and procedures for containing within them
aerosol
compositions under pressure are well known to those skilled in the art of
inhalation therapy.
For example, an aerosol composition may be administered from a coated can, for
example as
described in EP 0642992 A. Where the inhalable form of the active ingredient
is a nebulizable
aqueous, organic or aqueous/organic dispersion, the inhalation device may be a
known
nebulizer, for example a conventional pneumatic nebulizer such as an airjet
nebulizer, or an
ultrasonic nebulizer, which may contain, for example, from 1 to 50 ml,
commonly 1 to 10 ml,
of the dispersion; or a hand-held nebulizer, sometimes referred to as a soft
mist or soft spray
inhaler, for example an electronically controlled device such as an AERx
(Aradigm, US) or
Aerodose (Aerogen), or a mechanical device such as a RESPIMAT (Boehringer
Ingelheim)
nebulizer which allows much smaller nebulized volumes, e.g. 10 to 100 pl, than
conventional
nebulizers. Where the inhalable form of the active ingredient is the finely
divided particulate
form, the inhalation device may be, for example, a dry powder inhalation
device,adapted to
deliver dry powder from a capsule or blister containing a dry powder
comprising a dosage unit
of (A) and (B) or a multidose dry powder inhalation (MDPI) device adapted to
deliver, for
example, 3-25 mg of dry powder comprising a dosage unit of (A) and (B) per
actuation.
Suitable such dry powder inhalation devices are well known. For example, a
suitable device for
delivery of dry powder in encapsulated form is that described in US 3991761,
while a suitable
MDPI device is that described in WO 97/20559.
The medicament of the invention is preferably a pharmaceutical composition
comprising a
mixture of (A) as hereinbefore defined and (B) as hereinbefore defined,
preferably together
with at least one pharmaceutically acceptable carrier as hereinbefore
described.
The molar ratio of the compound (A) to the steroid (B) may be, in general,
from 100.1 to
1:300, for example from 50:1 to 1:100 or from 2,0:1 to 1:50, preferably from
10:1 to 1:20,
more preferably from 5:1 to 1:10, from 3:1 to 1:7 or from 2:1 to 1:2. The
compound (A) and
the steroid (B) may be administered separately in the same ratio.
CA 02552938 2006-07-17
WO 2005/074924 21 PCT/EP2005/001241
A suitable daily dose of the compound (A), particularly a~ the maleate or
trifluoroacetate salt,
for inhalation may be from 10 ~,g to 2000 p,g, for example from 10 to 1500
p,g, frorn 10 to
1000 p,g, preferably from 20 to 800 p,g, e.g. from 20 to 600 p,g or from 20 to
500 E,~,g. A
suitable daily dose of steroid (B) for inhalation may be from 20 p,g to 5000
pg, for example
from 20 to 4000 fig, from 50 to 3000 fig, from 50 to 20r00 p.g, from 50 to
1000 ~t.g, from SO
to 500 ~.g, from 50 to 400 p.g, from 50 to 300 pg, frorr~ SO to 200 ~g or from
50 to 100 pg.
Where (B) is budesonide, a suitable daily dose may be from 25 to 4800 p.g, for
example from
25 to 4000 p,g, from 25 to 3200 p.g, from 25 to 2400 pg, from 25 to 1600 fig,
from 50 to 4800
pg, from 50 to 4000 pg, from 50 to 3200 p,g, from SO to 2400 p.g, from 50 to
1600 ~,g, from
100 to 4000 p,g, from 100 to 3200 p,g, from 100 to 2400 pg, from 100 to 1600
pg, from 100
to 800 p.g, from 100 to 400 p.g, from 200 to 4000 pg, from 200 to 1600 p,g,
from 200 to 800
p,g or from 200 to 400 ~,g, 100 to 1600 p,g being preferred. Where (B) is
mometasone furoate, a
suitable daily dose may be from SO ~g to 2000 p,g, for example from 100 to 200
fig, from 100
to 1600 pg, from 100 to 1000 p,g or from 100 to 800pg, preferably from 200 to
500 pg, for
instance from 200 to 400 pg. Where (B) is fluticasone propionate, a suitable
daily dose may be
for inhalation may be from 25 to 2000 p,g, for example from 25 to 1500 fig,
from 25 to 100 0
~.g, from 25 to 500 p.g, from 25 to 250 pg, from 50 to 1S 00 pg, from 50 to
1000 pg, from 54
to 500 ~,g, from SO to 250 pg, from 100 to 1500 p,g, from 100 to 1000 wg, from
100 to 500
pg, from 100 to 250 fig, from 200 to 1500 p.g, from 200 -to 1000 p,g or from
200 to 500 p,g,
100 to 1000 pg being preferred.
A suitable unit dose of compound (A), particularly as the maleate or
trifluoroacetate salt, may
be from 10 to 2000 p,g, for example from 10 to 1500 p,g, from 10 to 1000 pg,
preferably from
20 to 800 p,g, from 20 to 600 pg or from 20 to 500 p.g. Pi suitable unit dose
of budesonide
may be from 25 to 2400 ~,g, for example from 50 to 2400 pg, from SO to 2000
p,g, from SO to
1600 pg, from SO to 800 ~,g, from SO to 400 pg, from 50 to 200 pg, from 100 to
1600 p,g,
from 100 to 800 pg, from 100 to 400 p,g, from 100 to 200 p,g, from 200 to 1600
pg, from 200
to 800 p,g or from 200 to 400 p.g, 100 to 400 p,g being preferred. A suitable
unit dose of
mometasone furoate for inhalation may be from 25 to 2(>00 pg, for example from
50 p,g to
1500 p.g, from 50 to 1000 p,g, from 50 to 800 pg, from 5 0 to 400 p.g, from SO
to 200 p,g, from
50 to 100 p,g, from 100 to 800 ~.g, from 100 to 400 pg or from 100 to 200 p,g,
100 to 400 ~.i.g
being preferred. A suitable unit dose of fluticasone propionate for inhalation
may be from 25
to 1000 p,g, fox example from 25 to 500 p,g, from 25 to 250 pg, from 25 to 200
p,g, from SO to
1000 p,g, from 50 to 500 p,g, from 50 to 250 p,g, from SO to 200 pg, from 100
to 1000 pg,
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
22
from 100 to 500 p,g, frorn 100 to 250 p,g, from 100 to 200 p,g, from 150 to
500 ~.g or from
150 to 250 ~,g, 100 to 500 ~,g being preferred. These unit doses may be
administered once or
twice daily in accordance with the daily doses mentioned hereinbefore. The
precise unit and
daily dose used will of course depend on the condition to be treated, the
patient and the
efficiency of the inhalation device.
In one preferred embodiment of the invention, the medicament of the invention
is a
pharmaceutical composition which is a dry powder in a capsule containing a
unit dose of (A)
and (B), for example for inhalation from a single capsule inhaler, the capsule
suitably
containing a unit dose of (A) e.g. as hereinbefore described, and a unit dose
of (B), e.g. as
hereinbefore described, together with a pharmaceutically acceptable carrier as
hereinbefore
described in an amount to bring the total weight of dry powder per capsule to
between 5 mg
and 50 mg, for example 5 mg, 10 mg, 15 mg, 2,0 mg, 25 mg, 30 mg, 35 mg, 40 mg,
45 mg or
50 mg.
In another preferred embodiment of the invention, the medicament of the
invention is a
pharmaceutical composition which is a dry powder for administration from a
reservoir of a
mufti-dose dry powder inhaler adapted to deliver, for example, 3 mg to 25 mg
of powder
containing a unit dose of (A) and (B) per actuation, for example, where (A) is
in the form of
the maleate salt, a powder comprising, by weight, 20 to 2000 parts, for
example 60 to 1000
parts, 100 to 500 parts, or 100 to 300 parts of (A); 25 to 800 parts, e.g. 25
to 500 parts, 50 to
400 parts, or 100 to 400 parts of (B); and 2000 to 25000 parts, e.g. 4000 to
15000 parts or
4000 to 10000 parts of a pharmaceutically acceptable carrier as hereinbefore
described.
In a further preferred embodiment of the invention, the medicament of the
invention is a
pharmaceutical composition which is an aerosol comprising (A) and (B) e.g, in
a ratio as
hereinbefore described, in a propellant as hereinbefore described, optionally
together with a
surfactant and/or a bulking agent and/or a co-solvent such as ethanol as
hereinbefore
described, for administration from a metered dose inhaler adapted to deliver
an amount of
aerosol containing a unit dose of (A) and a unit dose of (B), or a known
fraction of a unit dose
of (A) and a known fraction of a unit dose of (B), per actuation. Thus if, for
example, the
inhaler delivers half of the unit doses of (A) and (B) per actuation, the unit
doses can be
administered by two actuations of the inhaler.
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
23
In accordance with the above, the invention also provides a pharmaceutical kit
comprising (A)
and (B) as hereinbefore defined in separate unit dosage forms, said forms
being suitable for
administration of (A) and (B) in effective amounts. Such a kit suitably
further comprises one or
more inhalation devices for administration of (A) and (B). For example, the
kit may comprise
one or more dry powder inhalation devices adapted to deliver dry powder from a
capsule,
together with capsules containing a dry powder comprising a dosage unit of (A)
and capsules
containing a dry powder comprising a dosage unit of (B). In another example,
the kit may
comprise a multidose dry powder inhalation device containing in the reservoir
thereof a dry
powder comprising (A) and a multidose dry powder inhalation device containing
in the
reservoir thereof a dry powder comprising (B). In a further example, the kit
may comprise a
metered dose inhaler containing an aerosol comprising comprising (A) in a
propellant and a
metered dose inhaler containing an aerosol comprising (B) in a propellant.
The medicaments of the invention are advantageous in the treatment of
inflammatory or
obstructive airways disease, exhibiting highly effective bronchodilatory and
anti-inflammatory
properties. For instance, it is possible using the combination therapy of the
invention to reduce
the dosages of a corticosteroid (B) required for a given therapeutic effect
compared with those
required using treatment with a corticosteroid alone, thereby minianising
possibly undesirable
side effects. In particular, these combinations, particularly where (.A) and
(B) are in the same
composition, facilitate achievement of a high anti-inflammatory effect, such
that the amount of
corticosteroid needed for a given anti-inflammatory effect may be reduced when
used in
admixture with a compound of formula I, thereby reducing the risk of
undesirable side effects
from the repeated exposure to the steroid involved in the treatment of
inflammatory or
obstructive airways diseases. Furthermore, using the combinations of the
invention,
particularly using compositions containing (A) and (B), medicaments which have
a rapid onset
of action and a long duration of action may be prepared. Moreover, using such
combination
therapy, medicaments which result in a significant improvement in lung
function may be
prepared. In another aspect, using the combination therapy of the invention,
medicaments
which provide effective control of obstructive or inflammatory airways
diseases, or a reduction
in exacerbations of such diseases, may be prepared. In a further aspect, using
compositions of
the invention containing (A) and (B), medicaments which reduce or eliminate
the need for
treatment with short-acting rescue medicaments such as salbutamo<I or
terbutaline, may be
prepared; thus compositions of the invention containing (A) and (P>)
facilitate the treatment of
an obstructive or inflammatory airways disease with a single medicament.
CA 02552938 2006-07-17
WO 2005/074924 24 PCT/EP2005/001241
Treatment of inflammatory or obstructive airways diseases in accordance with
the invention
may be symptomatic or prophylactic treatment. Inflammatory or obstructive
airways diseases
to which the present invention is applicable include asthma of whatever type
or genesis
including both intrinsic (non-allergic) asthma and extrinsic (allergic)
asthma, mild asthma,
moderate asthma, severe asthma, bronchitic asthma, exercise-induced asthma,
occupational
asthma and asthma induced following bacterial infection. Treatment of asthma
is also to be
understood as embracing treatment of subjects, e.g. of less than 4 or 5 years
of age, exhibiting
wheezing symptoms and diagnosed or diagnosable as "wheezy infants", an
established patient
category of major medical concern and now often identified as incipient or
early-phase
asthmatics. (For convenience this particular asthmatic condition is referred
to as "wheezy-
infant syndrome".)
Prophylactic efficacy in the treatment of asthma will be evidenced by reduced
frequency or
severity of symptomatic attack, e.g. of acute asthmatic or bronchoconstrictor
attack,
improvement in lung function or improved airways hyperreactivity. It may
further be
evidenced by reduced requirement for other, symptomatic therapy, i.e. therapy
for or intended
to restrict or abort symptomatic attack when it occurs, for example anti-
inflammatory (e.g.
corticosteroid) or bronchodilatory. Prophylactic benefit in asthma may in
particular be
apparent in subjects prone to "morning dipping". "Morning dipping" is a
recognised
asthmatic syndrome, common to a substantial percentage of asthmatics and
characterised by
asthma attack, e.g. between the hours of about 4 to 6 am, i.e. at a time
normally substantially
distant form any previously administered symptomatic asthma therapy.
Other inflammatory or obstructive airways diseases and conditions to which the
present
invention is applicable include acute/adult lung injury (ALI), adult/acute
respiratory distress
syndrome CARDS), cystic fibrosis, chronic obstructive pulmonary, airways or
lung disease
(COPD, COAD or COLD), including chronic bronchitis and emphysema,
bronchiectasis and
exacerbation of airways hyperreactivity consequent to other drug therapy, in
particular other
inhaled drug therapy. Further inflammatory or obstructive airways diseases to
which the
present invention is applicable include pneumoconiosis (an inflammatory,
commonly
occupational, disease of the lungs, frequently accompanied by airways
obstruction, whether
chronic or acute, and occasioned by repeated inhalation of dusts) of whatever
type or genesis,
including, for example, aluminosis, anthracosis, asbestosis, chalicosis,
ptilosis, siderosis,
silicosis, tobacosis and byssinosis.
The invention is illustrated by the following Examples.
CA 02552938 2006-07-17
WO 2005/074924 25 PCT/EP2005/001241
Preparation Example 1~ Compound E1
4-hydroxy-7-( 1-hydroxy-2-{2-[4-(4-phenyl-butoxy)-phenyl]-ethylar~ino}-ethyl)-
3H-
benzothiazol-2-one
Palladium black (0.2 g) is added portion-wise to a solution of 7-[2-~benzyl-{2-
[4-(4-phenyl-
butoxy)-phenyl]-ethyl}-amino)-1-hydroxy-ethyl]-4-hydroxy-3H-be~zothiazol-2-one
(0.29 g) in
formic acid (10 ml) at room temperature. After 1 hour the catalyst 3s removed
by filtration and
the filtrate partitioned between CHaCOaCHzCH3 and aqueous NaI~C03. Evaporation
of the
CHsCOaCHzCHs layers and recrystallisation from hexane l CH3COzCHaCH3 gives the
title
compound. MS (ES+) 479.
Preparation Example 2~ Compound E2
7-[(R)-2-(1,1-Dimethyl-2-phenyl-ethylamino)-1-hydroxy-ethyl]-4-hydroxy-3H-
benzothiazol-2-
one
A solution of (R)-1-(4-tert-Butoxy-2-isopropoxy-benzothiazol-7-yl)-2-
(l,ldimethyl-2-phenyl-
ethylamino)-ethanol in formic acid (10 ml) is stirred at room temperature. The
reaction is
shown to be complete by LCMS after 48 hours. Evaporation of the formic acid
and
purification by reverse phase chromatography (ISOLUTE FLASH C 18, 0-SO%
methylcyanide
in water (0.1% TFA)) gives the title compound. MS (ES+) rule 359 (MH+).
Preparation Example 3~ Compound E3
4-Hydroxy-7-{ (R)-1-hydroxy-2-[2-(5,6,7,8-tetrahydro-naphthalen-2. -yl)-
ethylamino]-ethyl}-3H-
benzothiazol-2-one formate
Palladium black (0.4 g) is added portion-wise to a solution of (R)-2-{benzyl-
[2-(5,6,7,8-
tetrahydro-naphthalen-2-yl)-ethyl]-amino}-1-(4-tert-butoxy-2-isoprQpoxy-
benzothiazol-7-yl)-
ethanol (0.40 g) in formic acid (5 ml) at room temperature. After 2~- hours
the catalyst is
removed by filtration. Evaporation of the formic acid and purification by
reverse phase
chromatography (ISOLUTE FLASH C18, 0-50% methylcyanide in water (0.1% formic
acid))
gives the title compound. MS (ES+) mle 385 (MH+).
Preparation Example 4~ Compound E4
7-[(R)-2-( ( 1 S,2 S )-2-B enzyloxy-cyclop entylamino )-1-hydroxy-ethyl]-4-
hydroxy-3 H-benzo-
thiazol-2-one
(R)-2-( ( 1 S,2S)-2-Benzyloxy-cyclopentylamino)-1-(4-tert-butoxy-2-isopropoxy-
benzothiazol-7-
yl)-ethanol (10 g, 20 mmol) is dissolved in isopropanol (100 ml). 1M HCl (SO
ml) is added and
the reaction mixture is heated at 80°C for 24 hours. The isopropanol is
removed in vacuo and
CA 02552938 2006-07-17
WO 2005/074924 26 PCT/EP2005/001241
the residue is partitioned between ethyl acetate (SO ml) and sat. NaHCOs. The
organic layer is
washed with brine (50 ml), dried over MgSOa, filtered and the solvent removed
i~ vacuo. The
title compound is purified by crystallisation from ethanol. MS (ES+) mle 401
(MH+).
Preparation Example 5: Compound ES
Example 154: 7-[(R)-2-((1S,2R)-2-Benzyloxy-cyclopentylamino)-1-hydroxy-ethyl-4-
hydroxy-
3H-benzothiazol-2-one
(R)-2-((1S,2R)-2-Benzyloxy-cyclopentylamino)-1-(4-tert-butoxy-2-isopropoxy-
benzothiazol-7-
yl)-ethanol (460 mg, 0.92 mmol) is dissolved in isopropanol (5 ml). 1M HCl
(2.SmL) is added
and the reaction mixture is heated at 80°C for 24 hours. The
isopropanol is removed i~z vacuo
and the residue is partitioned between ethyl acetate (50 ml) and sat. NaHC03.
Tie organic
layer is washed with brine (50 ml), dried over MgS04, filtered and the solvent
removed in
vacuo to give the title compound. MS (ES+) mle 401 (MH+).
Preparation of intermediate compounds
Certain compounds that are used to prepare compounds of the Examples that are
not readily
commercially available are prepared as follows:
Tert-butoxy-5-fluoro-phenylamine
A suspension of platinum oxide (17 g) in a solution of 1-tert-butoxy-4-fluoro-
2-riitro-benzene
(225 g, prepared by procedure T. F. Woiwode et al J. (7rg. Chew. 1998, 63,
9594.) in CHsOH
(1.5 1) is stirred under an atmosphere of hydrogen for 18 hours. Filtration
through CeliteTM
filter material and evaporation gives the title compound. 19F nmr (CDCIs, 376
M~~iz); -43.4.
1-Tert-butox~~-4-fluoro-2-isothiocyanato-benzene
Carbon disulphide (38.6 ml) is added to a solution of 2-tert-butoxy-5-fluoro-
phenylamine
(58.8 g) and triethylamine (89.5 ml) in toluene (66 ml) and the reaction
mixture stirred at
room temperature for 18 hours, then evaporated. Chloroform (200 ml) and trietl-
~ylamine (44.9
ml) are added to the residue, which is cooled before the addition of ethyl
chloro~ormate (30.8
ml). After 15 minutes at 0°C, the reaction mixture is washed
sequentially with aqueous 3N
HCI, saturated brine, saturated NaHCOs and saturated brine, then evaporated to
give the title
compound. 1H nmr (CDCIs, 400 MHz); 7.10-7.03 (m, 1H), 6.93-6.87 (m, 1H), x.86-
6.80 (m,
1H), 1.43 (s, 9H).
f 2-Tert-butoxy-5-fluoro-phenyl)-thiocarbamic acid O-isopropyl ester
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
27
A solution of 1-tert-butoxy-4-fluoro-2-isothiocyanato-benzene (50.0 g) and
triethylamine (31
ml) in isopropanol (170 ml) is refluxed for 48 hours. Evaporation of the
reaction mixture
followed by silica gel flash column chromatography, eluent 20:1 hexane:
CH3CO2CHaCH3
gives the title compound. 1H nmr (CDC13, 400 MHz); 8.60 (br s, 1H), 7.38 (br
s, 1H), 7.50-
6.87 (m, 1H), 6.67-6.58 (m, 1H), 5.64-5.50 (m, 1H), 1.43-1.32 (m, 6H), 1.32-
1.25 (s, 9H).
~benz~{2-[~4-phenyl-butoxYl-phen l~l-ether?-aminoLl~4-tert-butox, -~pro~ox~
benzothiazol-7-,~)-ethanone
A solution of tert. butyl lithium in pentane (12.3 ml, 1.7 M) is added to a
solution of (2-tert-
butoxy-5-fluoro-phenyl)-thiocarbamic acid O-isopropyl ester (3.20 g) in
tetrahydrofuran (10
ml) at -78° C, the solution warmed to -20° C over 1 hour then re-
cooled -78° C and a solution
of 2-(benzyl-{2-[4-(4-phenyl-butoxy)-phenyl]-ethyl}-amino)-N-methoxy-N-methyl-
acetamide in
tetrahydrofuran (10 ml) added at -78° C. The reaction mixture is warmed
to room
temperature and partitioned between aqueous NH4C1 and CHsCOzCHaCHs.
Evaporation of
the CHaCO2CHzCHa layers and silica gel column chromatography, eluent 4:1
hexane:
CH3COzCHzCH3, gives the title compound. MS (ES+) 665.
7-.f(benz~l-{2-[~4-phenyl-butoxYLphen~]-ether}-amino -acet~]-4-h dery-3H-
benzothiazol-
2-one
A solution of 2-(benzyl-{2-[4-(4-phenyl-butoxy)-phenyl]-ethyl}-amino)-1-(4-
tert-butoxy-2-
isopropoxy-benzothiazol-7-yl)-ethanone (2.49 g) in isopropanol (20 ml) and
concentrated
hydrobromic acid (20 ml) is heated at 50° C. After 3 hours the reaction
mixture is partitioned
between CHsCO2CH2CHs and water, and the CHsCOzCHaCHs layer washed with aqueous
NaHCO3 then brine. Evaporation of the CH3CO2CH2CH3 layers and silica gel
column
chromatography, eluent 4:1 hexane: CHsCOzCHzCHs, gives the title compd. MS
(ES+) 567.
7-f2-(benz r~{2-[4-(4-phen~~l-butoxY~phen~)-eth~~-amino -~ d~ -~~]-4-h drop
benzothiazol-2-one
NaBHa (2.67 g) is added portion-wise to a solution of 7-[(benzyl-{2-[4-(4-
phenyl-butoxy)-
phenyl]-ethyl}-amino)-acetyl]-4-hydroxy-3H-benzothiazol-2-one (0.40 g) in
CHaOH (15 ml) at
0° C. After 30 minutes the reaction mixture is partitioned between
CHsCOaCHzCHs and
water. Evaporation of the CHaCOaCHzCH3 layers and silica gel column
chromatography,
eluent 1:1 hexane: CH3COzCHzCH3, gives the title compound. MS (ES+) 569.
~4-Tert.butox~ -2-isopropoxy-benzothiazol-7-Xl)-2-chloro-ethanone
CA 02552938 2006-07-17
WO 2005/074924 28 PCT/EP2005/001241
Tert. butyllithium (22.7 ml, 1.7 M in pentane) is added dropwise to a solution
of (2-tert. -
butoxy-S-fluoro-phenyl)-thiocarbamic acid O-isopropyl ester (5.00 g) in THF
(20 ml) at -7~ °
C. This solution is then allowed to warm to -20~ C and a dried mixture of
lithium chloride
(2.12 g) and copper (I) cyanide (2.24 g) in THF (50 ml) is added. After 15
minutes chloroacetyl
chloride (4.36 g) is added and the reaction mixture allowed to warm to 0~ C.
This temperature
is maintained for 1 hour and then the reaction mixture is quenched by the
addition of
saturated aqueous NH4C1 (5 ml). The reaction mixture is partitioned between
ethyl acetate
(250 ml) and water (250 ml). The organic layer is washed with water (250 ml)
and brine (25 0
ml), dried over MgS04, filtered and the solvent removed in vacuo. The title
compound is
obtained by flash column chromatography (silica, iso-hexane / ethyl acetate
10:1). MS (ES+~
mle 341 (MH+).
(R)-1-(4-Tert-butox -2-isopropoxy-benzothiazol-7-' 1)-2-chloro ethanol
Borane-THF complex, (14.64 ml, 1M in THF) is added dropwise to a solution of
(1R, 2S)-(+ )-
1-amino-2-indanol (0.22 g) in THF (50 ml) and the solution is stirred at room
temperature for
15 minutes. A solution of 1-(4-tert.butoxy-2-isopropoxy-benzothiazol-7-yl)-2-
chloro-ethanorse
(5.00 g) in THF (50 ml) is then added dropwise over a period of 1 hour. The
reaction mixture
is stirred at room temperature for a further 15 minutes and then quenched by
the addition of
0.2M HaS04 (5 ml). The reaction mixture is partitioned between ethyl acetate
(200 ml) and
0.2M H2SO4 (200 ml). The organic layer is washed with water (200 ml) and brine
(2,00 ml),
dried over MgS04, filtered and the solvent removed in vacuo to give the title
compound. MS
(ES+) m/e 344 (MH+).
4-Tert.butox -2-isopropoxy-~R -oxiranyl-benzothiazole
A mixture of (R)-1-(4-tert.butoxy-2-isopropoxy-benzothiazol-7-yl)-2-chloro-
ethanol (4.70 g)
and potassium carbonate (7.48 g) in acetone (250 ml) is refluxed for 48 hours.
The reaction
mixture is allowed to cool, filtered and the solvent removed in vacuo to give
the title
compound. MS (ES+) m/e 308 (MH+).
IRl-2-lBenzyl-f2-(S 6 7 8-tetrahydro-naphthalen-2-vl -ether] aminol 1 (4 tert
butox,~2
isopropoxy-benzothiazol-7-,~Tl)-ethanol
A solution of 4-tert.butoxy-2-isopropoxy-7-(R)-oxiranyl-benzothiazole (3.50 g)
and benzyl-[2 -
(S,f,7,8-tetrahydro-naphthalen-2-yl)-ethyl]-amine (3.03 g) in 1-butanol (25
ml) is stirred at
110° C. The reaction is shown to be complete by TLC after 18 hours. The
title compound is
obtained after purification by flash column chromatography (silica, iso-hexane
/ ethyl acetate
10:1). 1H nmr (CDCl3, 400 MHz); 7.35-7.20 (m, SH), 7.00-6.95 (m, 3H), 6.90-
6.80 (m, 2H) ,
CA 02552938 2006-07-17
WO 2005/074924 29 PCT/EP2005/001241
5.45 (m, 1H), 4.70 (m, 1H), 3.95 (d, 1H), 3.55 (d, 1H), 2.85 (m, 6H), 2.70 (m,
4H), 1.75 (m,
4H), 1.45 (m, 6H), 1.35 (s, 9H).
f R)-~4-tert-Butox -~-isopropoxy-benzothiazol-7-~)-2 ~(1 ldimethyl-2-phen~
lamino~
ethanol
A solution of phentermine (0.728 g, 4.89 mmol) and N,O-
bis(trimethylsilyl)acetamide (0:496
g, 2.44 mmol) in dry DMF (1 ml) is stirred at room temperature for 30 minutes.
A solution of
4-tert.butoxy-2-isopropoxy-7-(R)-oxiranyl-benzothiazole (0.75 g, 2.44 mmol) in
dry DMF (1
ml) is added and the reaction mixture is stirred at 80° C. The reaction
is shown to be complete
by TLC after 18 hours. The title compound is obtained after purification by
flash column
chromatography (silica, iso-hexane / ethyl acetate 1:1). MS (ES+) hale 457
(MH+).
(R)-2-(jlS,2S -2-Benz~~yclopentylamino~4-tert-butox -y 2isopropoxy-
benzothiazol-7-
1 -ethanol
4-tert.butoxy-2-isopropoxy-7-(R)-oxiranyl-benzothiazole (13.2 g, 42.94 mmol)
and (1S,2S)-2-
Benzyloxy-cyclopentylamine (10.f8 g, 55.82 mmol) are placed in a flask with
Diglyme (40 ml)
and the reaction mixture is heated at 115°C. The reaction is shown to
be complete by TLC
after 17 hours. The reaction mixture is cooled and partitioned between heptane
(200 ml) and
water (200 ml). The organic layer is dried over MgSOa., filtered and the
solvent removed in
vac~o. The title compound is purified by crystallisation from isopropanol. MS
(ES+) mle 499
(MH+).
~1R.'2S)-2-Aminoc,~pentanol hydrochloride
The title compound is prepared from by the procedure of Schaus, Scott E.;
Larrow, Jay F.;
Jacobsen, Eric N. Practical Synthesis of Enantiopure Cyclic 1,2-Amino Alcohols
via Catalytic
Asymmetric Ring Opening of Meso Epoxides. Journal of Organic Chemistry (1997),
62,(12),
4197-4199.
llS,2R)-2-(2-H, d~,~ roxY-c,~pent,~}-isoindole-1,3-dione
(1R,2S)-2-Aminocyclopentanol hydrochloride (10 g, 72.73 mmol), phthalic
anhydride (10.76
g, 72.73 mmol) and diisopropylamine (11.26 g, 87.27 mmol) are placed in a
flask and heated
to 130°C. The reaction is shown to be complete by TLC after 2 hours.
The reaction mixture is
allowed to cool and is partitioned between ethyl acetate (200 ml) and 2M
hydrochloric acid
(200 ml). The organic layer is washed with water (1000 ml), sat. NaHC03 (100
ml) and brine
(100 ml), dried over MgS04, filtered and the solvent removed in vacuo. The
title compound is
obtained after purification by flash column chromatography (silica, iso-hexane
/ ethyl acetate
CA 02552938 2006-07-17
WO 2005/074924 3~ PCT/EP2005/001241
1:1). 1H nmr (CDCl3, 400 MHz); 7.85 (m, 2H), 7.70 (m, 2H), 4.50 (m, 1H), 4.30
(rn, 1H),
2.95 (d, 1H), 2.45 (m, 1H), 2.00 (m, 3H), 1.85 (m, 1H), 1.60 (m, 1H).
jlS.2R -~2-(2-Benz~~~~clopent~)-isoindole-1,3-dione
(1S,2R)-2-(2-Hydroxy-cyclopentyl)-isoindole-1,3-dione (7.50 g, 32.47 mmol) is
dissolved in
dry DMF (15 ml) under an atmosphere of argon. The solution is cooled to
0°C and sodium
hydride (0.78 g, 32.47 mmol) is added. The reaction mixture is stirred at room
temperature for
30 minutes and then cooled on ice. Benzyl bromide (6.11 g, 35.71 mmol) is
added dropwise
and the reaction mixture is stirred at room temperature for 18 hours. The
reaction is shown to
be complete by TLC. The reaction mixture is partitioned between ethyl acetate
(200 ml) and
water (200 ml). The organic layer is washed with brine (100 ml), dried over
MgSO4, filtered
and the solvent removed in vacuo. The title compound is obtained after
purification by flash
column chromatography (silica, iso-hexane / ethyl acetate 10:1). 2H nmr
(CDCIs, 400 MHz);
7.80 (m, 2H), 7.65 (m, 2H), 7.10 (m, SH), 4.65 (q, 1H), 4.50 (d, 1H), 4.35 (d,
1H), 4.00 (q,
1H), 2.70 (m, 1H), 2.00 (m, 4H), 1.50 (m, 1H).
jlS, R)-2-Benz,~~,~pentylamine
(1S,2R)-2-(2-Benzyloxy-cyclopentyl)-isoindole-1,3-dione S.SO g, 17.13 mmol) is
dissolved in
EtOH (17S ml). Acetic acid (3.08 g, 51.40 mmol) and hydrazine monohydrate
(2.57 g, 51.40
mmol) are added and the reaction mixture is refluxed for 2 hours, allowed to
cool, any solids
are filtered off and the solvent removed iv ~racuo. The residue is partitioned
between ethyl
acetate (100 ml) and 2M hydrochloric acid (100 ml). The aqueous layer is
basified to pHl2
with 2M NaOH and extracted with ethyl acetate (3 x SO ml). The organic layer
is washed with
brine (100 ml), dried over MgS04, filtered and the solvent removed in vacuo to
give the title
compound.1H nmr (CDCIs, 400 MHz); 7.80 (m, 2H), 7.65 (m, 2H), 7.10 (m, SH),
4.65 (q,
1H), 4.50 (d, 1H), 4.35 (d, 1H), 4.00 (q, 1H), 2.70 (m, 1H), 2.00 (m, 4H),
1.50 (m, 1H).
~R~((1S,2R -2-Benz~~~pentylamino~4-tert-butox -2-isopropoxy-benzothiazol-7-
1 -ethanol
This compound is prepared from (R)-1-(4-tert-butoxy-2-isopropoxybenzothiazol-7-
yl)-2-
chloro-ethanol and (1S,2R)-2-Benzyloxy-cyclopentylamine using procedures
analogous to those
used to prepare 4-tert.butoxy-2-isopropoxy-7-(R)-oxiranyl-benzothiazole MS
(ES+) mle 499
(MH+).
CA 02552938 2006-07-17
WO 2005/074924 31 PCT/EP2005/001241
Examples 1-60
Gelatin capsules suitable for use in a capsule inhaler such as that described
in US 3991761 or
EP 1270034 are prepared, each capsule containing a dry powder obtained by
mixing
Compound E1 and budesonide which have been ground to a mean particle diameter
of 1 to S
~m and lactose monohydrate having a particle diameter below 212 Vim, the
amounts being as
shown in the table below:
Example Compound E1 Budesonide Lactose
Parts _ Parts Parts
1 20 100 19880
2 40 100 19860
3 80 100 19820
4 100 100 19800
S 120 100 19780
6 140 100 19760
7 160 100 19740
8 180 100 19720
9 200 100 19700
220 100 19680
_
11 240 100 19660
12 300 100 19600
13 500 100 19400
14 1000 100 18900
2000 100 17900
' 16 20 100 24880
17 40 100 24860
18 80 100 24820
19 100 100 24800
120 100 24780
21 140 100 24760
22 160 100 24740
23 180 100 24720
24 200 100 24700
220 100 24680
26 240 100 24660
27 300 100 24600
28 500 100 24400
29 1000 100 23900
2000 100 22900
31 20 200 14780
32 40 200 14760
33 80 200 14720
34 100 200 14700
120 200 14680
36 140 200 14660
37 160 200 14640
38 ~ 180 ~ 200
14620
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
32
39 200 200 14600
40 220 200 14580
41 240 200 14560
42 300 200 14500
43 500 200 14300
44 1000 200 13800
45 2000 200 12800
46 20 200 24780
47 40 200 24760
48 80 200 24720
49 100 200 24700
50 120 200 24680
51 140 200 24660
52 160 200 24640
53 180 200 24620
54 200 200 24600
55 220 200 24580
56 240 200 24560
57 300 200 24500
58 500 200 24300
59 1000 200 23800
60 2000 200 22800
Examples 61-90
Examples 1-60 are repeated, but replacing budesonide with mometasone furoate,
and using
amounts as shown in the following table:
Example Compound E1 MF Lactose
Parts Parts Parts
61 20 100 24880
62 40 100 24860
63 80 100 24820
64 100 100 24800
65 12 100 2
0 4780
66 _ 100 _
.140 - 24760
67 160 100 24740
_. 68 _ ~ 8p- 100 24720
69 200 100 24700
70 220 100 24680
71 240 100 24660
72 300 100 24600
73 500 100 24400
74 1000 100 23900
75 2000 100 22900
76 20 200 14780
77 40 200 14760
- - -
78 80 200 14720
CA 02552938 2006-07-17
WO 2005/074924 33 PCT/EP2005/001241
79 ~ 100 ~ 200 14700
80 120 2 14680
00
81 140 _ 14660
200
82 160 200 14640
83 180 200 14620
84 200 200 14600
85 220 200 14580
86 240 200 14560
87 300 200 14500
88 500 ' 200 14300
89 1000 200 13800
90 2000 200 12800
Examples 91-135
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E1 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5 ~m and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table below:
Example Compound E1 FP Lactose
Parts Parts Parts
91 20 100 4880
92 40 100 4860
93 80 100 4820
94 100 100 4800
95 120 100 4780
96 140 100 4760
97 160 100 4740
98 180 100 4720
99 200 100 4700
100 220 100 4680
101 240 100 4660
102 300 100 4600
103 500 100 4400
104 1000 100 3900
105 2000 100 2900
106 20 200 9780
107 40 200 9760
108 80 200 9720
109 100 200 9700
110 ' 120 200 9680
111 140 200 9660
112 160 200 9640
113 180 200 9620
114 200 200 9600
115 220 200 9580
116 240 200 9560
117 300 200 9500
CA 02552938 2006-07-17
WO 2005/074924 34 PCT/EP2005/001241
118 500 200 9300
119 _1000 200 8800
120 2000 200 7800
121 2.0 250 14730
122 40 250 14710
123 80 250 14670
124 100 250 14650
_125 120 250 14630
126 140 250 14610
127 160 250 14590
128 180 250 14570
129 200 250 14550
130 220 250 14530
131 240 250 14510
132 300 250 14450
133 500 250 14250
134 1000 250 13750
135 2000 250 12750
Examples 13t~-180
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/2,0589 is prepared by mixing Compound E1 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5 ~m and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table directly above
but also
containing 0.5 % magnesium stearate by weight.
Examples 181-208
Aerosol formulations are prepared by dispensing micronised active ingredients,
Compound E1
and mometasone furoate/ fluticasone propionate, and if required, lactose as
bulking agent into
a vial, sealing the vial with a metering valve, injecting the premixed
ethanol/propellant and
optional surfactant into the vial through the valve and subjecting the vial to
ultrasonic energy
to disperse the solid particles. The components and amounts used are shown in
the following
tables:
Ex. Cpd.E1 MF HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
181 2 10 36500 60750 2500 - 70
182 4 10 3410 6340 230 0.3 -
183 8 10 97000 - 2500 - 90
184 10 10 30500 67000 2500 0.5 100
CA 02552938 2006-07-17
WO 2005/074924 35 PCT/EP2005/001241
185 12 10 3150 6550 250 1 -
186 14 10 3700 6_050 250 0.8 -
187 16 10 3800 5900 230 0.4 -
188 18 10 4700 5050 250 1 -
189 20 20 3600 6150 225 1 -
190 22 20 3500 6200 230 1 -
191 24 20 98000 - 2500 1 -
192 30 20 3900 5900 250 1 -
193 2 20 30000 67000 2250 0.2 90
194 10 20 3500 6200 250 0.5 -
195 14 20 3200 6500 230 1 -
196 18 20 3100 6200 225 0.8 -
197 20 20 3150 6100 225 1 -
198 24 20 30000 60000 2000 0.8 -
Ex. Cpd.E1 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
199 4 10 34000 63000 2250 0.3 50
200 8 10 92000 - 2500 0.5 70
201 12 10 3000 5500 200 - -
202 16 10 2500 5000 200 0.3 -
203 20 10 2000 3000 150 0.2 -
204 30 10 2000 2000 150 0.2 -
205 8 20 20000 25000 1500 0.2 -
206 12 20 2500 2500 200 0.2 -
207 20 20 2000 2000 150 0.2 -
208 30 20 20000 20000 1500 0.2 -
Examples 209-244
The procedure of Examples 91-135 is repeated, but replacing fluticasone
propionate by
mometasone furoate, and using amounts as shown in the following table:
Example Compound E1 MF ~ Lactose
Parts Parts Parts
209 100 100 4800
210 200 100 4700
211 300 100 4600
212 400 100 4500
213 500 100 4400
214 600 100 4300
215 700 100 4200
216 800 100 4100
217 2000 100 2900
218 100 200 4700
219 200 200 4600
220 300 200 4500
CA 02552938 2006-07-17
WO 2005/074924 36 PCT/EP2005/001241
221 400 _ 200 4400
222 500 __ 200 4300
~
223 600 200 4200
224 700 200 4100
225 800 200 4000
226 1200 200 3600
227 100 400 4500
228 200 400 4400
229 300 400 4300
230 400 400 4200
231 500 400 4100
232 600 400 4000
233 700 400 3900
234 800 400 3800
235 100 100 9800
236 200 100 9700
237 300 100 900
238 400 100 9500
239 500 100 9400
240 100 200 9700
241 200 200 9600
242 300 200 9500
243 400 200 9400
244 500 200 9300
Examples 245-280
The procedure of Examples 91-135 is repeated, but replacing fluticasone
propionate by
mometasone furoate, using amounts as shown in the table directly above and
including O.S%
magnesium stearate by weight.
Examples 281-317
The procedures of Examples 181-208 is repeated, but using the amounts shown in
the
following table, the ethanol being omitted in some of the Examples:
Ex. Cpd.E1 MF HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
281 20 20 5000 - 200 O.S -
282 40 2 2500 2500 - - -
283 7S 2S 1500 3500 500 - 1
284 20 20 3600 6150 225 - O.S
285 2 20 30000 67000 - - -
I 286 14 ~ 20 ~ 3200 ~ 6500 ~ 1500 - ~ 4
I (
CA 02552938 2006-07-17
WO 2005/074924 3~ PCT/EP2005/001241
287_ 20 20 3150 6100 1500 4 -
288 10 20 4700 5050 500 - 0.2
289 60 20 10000 10000 - - -
290 60 20 10000 10000 200 - -
291 60 20 10000 10000 - 0.5 -
292 30 20 8000 12000 - 1 1
293 40 20 5000 15000 500 0.5 0.5
294 50 20 9000 11000 400 0.8 0.2
295 20 20 4600 5000 400 0.4 0.2
296 30 10 20000 25000 - - -
297 40 10 20000 30000 - - -
298 60 10 35000 65000 - - -
Ex. Cpd.E1 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts jParts Parts Parts Parts
299 20 10 5000 5000 - - 1
300 10 10 3650 6350 - - 1
301 30 10 3200 6800 100 0.5 0.5
302 30 20 7400 7600 100 - -
303 40 20 8300 6700 200 0.5 -
304 60 20 3100 6900 300 1 -
305 10 10 8000 12000 - - -
306 50 20 1600 3400 500 2 0.5
Ex. Cpd.E1 Bud HFA134a FA227 Ethanol OA Lactose
Parts H Parts Parts Parts Parts Parts
Parts
307 10 20 5500 4500 - - -
308 2 20 3500 6500 - - 1
309 1 20 2500 7500 - - 1
_310 20 20 3800 6100 100 0.5 -
311 15 20 3300 6600 100 0.5 0.5
312 30 20 3600 5900 500 4 -
313 40 20 4600 4900 500 3 -
314 30 10 3100 6800 100 0.2 0.5
315 40 10 1400 3100 500 0.2 -
316 60 10 8000 12000 - - 1
317 80 ~ 10 30000 70000 - - -
(
Example 318 - 326
The procedure of Examples 181-208 is repeated, but using sorbitan trioleate
(ST) as surfactant
in place of oleic acid, the amounts of the ingredients being as shown in the
following table:
Ex. Cpd.E1 MF HFA134a HFA227 Ethanol ST Lactose
Parts Parts Parts Parts Parts Parts Parts
318 60 40 10000 10000 300 4 -
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
38
319 60 20 ~ 8000 12000 200 8 -
320 50 20 12000 8000 400 10 -
321 40 20 5000 5000 600 2.5 1
322_ 30 20 3500 6500 - 4 2
323 20 20 6000 4000 - 3 3
324 10 20 4500 5500 100 2 1
325 20 10 4100 5900 SO 1 2
326 15 5 1550 3450 200 0.5 1
CA 02552938 2006-07-17
WO 2005/074924 39 PCT/EP2005/001241
Examples 327-386
Gelatin capsules suitable for use in a capsule inhaler such as that described
in IJS 3991761 or
EP 1270034 are prepared, each capsule containing a dry powder obtained by
mixing
Compound E2 and budesonide which have been ground to a mean particle diameter
of 1 to
Sam and lactose monohydrate having a particle diameter below 212 Vim, the
amounts being as
shown in the table below:
Example Compound E2 Budesonide Lactose
Parts Parts Parts
327 4 100 19880
328 8 100 19860
329 16 100 19820
330 20 100 19800
331 24 100 19780
332 28 100 19760
333 32 100 19740
334 36 100 19720
33S 40 100 19700
336 44 100 19680
337 48 100 19660
338 60 100 19600
339 100 100 19400
340 200 100 18900
341 400 100 17900
342 4 100 24880
343 8 100 24860
344 16 100 24820
34S 20 100 24800
346 24 100 24780
347 28 100 24760
348 32 100 24740
349 36 100 24720
3S0 40 100 24700
3S1 44 100 24680
3S2 48 100 24660
3S3 60 100 24600
3S4 100 100 24400
3SS 200 100 23900
3S6 400 100 22900
3S7 4 200 14780
3S8 8 200 14760
3S9 16 200 14720
360 20 200 14700
361 24 200 14680
362 28 200 14660
363 32 200 14640
364 36 200 14620
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
365 40 200 14600
366 44 200 14580
367 48 200 14560
368 60 200 14500
369 100 200 14300
370 200 200 13800
371 400 200 12800
372 4 200 24780
373 8. 200 24760
374 16 200 24720
375 20 200 24700
376 24 200 24680
377 28 200 24660
378 32 200 24640
379 36 200 24620
380 40 200 24600
381 44 200 24580
382 48 200 24560
383 60 200 24500
384 100 200 24300
385 200 200 23800
386 400 200 22800
Examples 3 87-41 G
Examples 327-386 are repeated, but replacing budesonide with mometasone
furoate, and using
amounts as shown in the following table:
Example Compound E2 MF Lactose
Parts Parts Parts
387 4 100 24880
388 8 100 24860
389 16 100 24820
390 20 100 24800
391 24 100 24780
392 28 100 24760
393 32 100 24740
394 36 100 24720
395 40 100 24700
396 44 100 24680
397 48 100 24660
398 60 100 24600
399 100 100 24400
400 200 100 23900
401 400 100 22900
402 4 200 14780
403 8 200 14760
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
41
404 16 ___ 200 14720
~
405 20 _ 14700
200
406 24 200 14680
407 28 200 14660
408 32 200 14640
409 36 200 14620
410 40 200 14600
411 44 200 14580
412 48 200 14560
413 60 200 14500
414 100 200 14300
415 200 200 13800
416 400 200 12800
Examples 417-461
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in ~0
97/20589 is prepared by mixing Compound E2 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-S~m and lactose monohydrate having a
particle
diameter below 212~m, the amounts being as shown in the table below
Example Compound E2 FP Lactose
Parts Parts Parts
417 4 __ ~~ 4880
100
418 8 100 4860
419 16 100 4820
420 20 100 4800
421 24 100 4780
422 28 100 4760
423 32 100 4740
424 36 100 4720
425 40 100 4700
426 44 100 4680
427 48 100 4660
428 60 100 4600
429 100 100 4400
430 200 100 3900
431 400 100 2900
432 4 200 9780
433 8 200 9760
434 16 200 9720
435 ~ 20 200 9700
436 24 200 9680
437 28 200 9660
438 32 200 9640
439 36 200 9620
440 40 200 9600
CA 02552938 2006-07-17
WO 2005/074924 42 PCT/EP2005/001241
441 - 44 200 9580
442 48 200 9560
443 60 200 ~ 9500
444 100 200 9300
445 200 200 8800
446 400 200 7800
__
447 4 250 14730
448 8 250 14710
449 16 250 14670
450 20 250 14650
451 24 250 14630
452 28 250 14610
453 32 250 14590
454 36 250 14570
455 40 250 14550
456 44 250 14530
457 48 250 14510
458 60 250 14450
459 100 250 14250
460 200 250 13750
461 ~ 400 250 12750
Examgles 462-506
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E2 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5~m and lactose monohydrate having a
particle
diameter below 212~m, the amounts being as shown in the table directly above
but also
containing 1.0% magnesium stearate by weight.
Examples 507-534
Aerosol formulations are prepared by dispensing micronised active ingredients,
Compound E2
and mometasone furoate/ fluticasone propionate, and if required, lactose as
bulking agent into
a vial, sealing the vial with a metering valve, injecting the premixed
ethanol/propellant and
optional surfactant into the vial through the valve and subjecting the vial to
ultrasonic energy
to disperse the solid particles. The components and amounts used are shown as
follows:
Ex. Cpd.E2 MF HFA134a FA227 Ethanol OA ~ Lactose
H
Parts Parts Parts _ Parts Parts Parts Parts
507 0.5 10 36500 60750 2500 - 70
CA 02552938 2006-07-17
WO 2005/074924 43 PCT/EP2005/001241
508 1 10 3410 6340 230 0.3 -
S09 2 10 97000 - 2500 - 90
510 2.S 10 30500 67000 2500 O.S 100
511 3 10 3150 6550 250 1 -
S12 3.S 10 3700 6050 250 0.8 -
S13 4 10 3800 5900 230 0.4 -
S14 4.S 10 4700 SOSO 250 1 -
S1S S 20 3600 6150 225 1 -
S16 S.S 20 3500 6200 230 1 -
S17 6 20 98000 - 2500 1 -
518 6.S 20 3900 5900 250 1 -
S19 0.S 20 30000 67000 2250 0.2 90
520 2.S 20 3500 6200 250 O.S -
521 3.S 20 3200 6500 230 1 -
S22 4.S 20 3100 6200 225 0.8 -
S23 S 20 3150 6100 225 1 -
524 S.S 20 30000 X0000 2000 0 8 -
~ ~
Ex. Cpd.E2 FP HFA134a FA227 Ethanol OA Lactose
Parts H Parts Parts Parts Parts Parts
Parts
S2S 1 10 34000 63000 2250 0.3 SO
526 2 10 _92000 - 2500 O.S 70
527 3 10 3000 SS00 200 -
S28 4 10 2500 5000 200 0.3 -
S29 S 10 2000 3000 150 0.2
S30 6 10 2000 2000 150 0.2 -
S31 2 ' 20 20000 25000 1500 0.2 -
S32 3 20 2500 2500 200 0.2 -
S33 S 20 2000 2000 150 0.2 -
534 6 ~ 20 20000 20000 1500 0 2 -
~
Examples 535-570
The procedure of Examples 417-461 is repeated, but replacing fluticasone
propionate by
mometasone furoate, and using amounts as shown in the following table.
Example Compound E2 MF Lactose
Parts Parts Parts
S3S 20 100 4800
536 40 100 4700
537 60 100 4600
538 80 100 4500
539 100 100 4400
540 120 100 4300
541 140 100 4200
542 160 100 4100
543 400 100 2900
CA 02552938 2006-07-17
WO 2005/074924 44 PCT/EP2005/001241
544 20 200 4700
S4S 40 200 4600
_
546 60 200 _
4500
547 80 200 4400
548 100 200 4300
549 120 200 4200
SSO 140 200 4100
551 160 200 4000
552 ~ 240 200 3600
553 20 400 4500
554 40 400 4400
SSS 60 400 4300
556 80 400 4200
557 100 400 4100
558 120 . 400 4000
559 140 400 3900
560 160 400 3800
561 20 100 9800
562 40 100 9700
563 60 100 9600
564 80 100 9500
S6S 100 100 9400
566 20 200 9700
567 40 200 9600
S6~ ~0 200 9500
569 80 200 9400
570 100 200 9300
Examples 571-606
The procedure of Examples 417-461 is repeated, but replacing fluticasone
propionate by
mometasone furoate, using amounts as shown in the table directly above and
including 1.0%
magnesium stearate by weight.
Examyles 607-643
The procedures of Examples 507-534 is repeated, but using the amounts shown in
the
following table, the ethanol being omitted in some of the Examples:
Ex. Cpd.E2 MF HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
607 S 20 5000 - 200 O.S -
~ 608 8 ~ 2 2500 2500 - -
~
CA 02552938 2006-07-17
WO 2005/074924 45 PCT/EP2005/001241
609 19 2S 1500 3500 _ 500 - 1
610 S 20 _ 6150 225 - O.S
3600
61_1 O.S 20 30000 67000 - - -
612 3.S 20 3200 6500 1500 - 4
613 S 20 3150 6100 1500 4 -
614 2.S 20 4700 SOSO 500 - 0.2
615 1S 20 10000 10000 - - -
616 1S 20 10000 10000 200 - -
617 1S 20 10000 10000 - O.S -
618 7.S 20 8000 12000 - 1 1
619 10 20 5000 15000 500 O.S O.S
620 12.5 20 9000 11000 400 0.8 0.2
621 S 20 4600 5000 400 0.4 0.2
622 7.S 10 20000 25000 - -
623 10 10 20000 30000 - - -
624 1S ~ 10 ~ 35000 65000 - ~ -
~ ~ ~
Ex. Cpd.E2 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts, Parts Parts Parts Parts Parts
625 5 10 5000 5000 - - 1
626 2.S 10 3650 6350 - - 1
627_ 7.S 10 3200 6800 100 O.S O.S
628 7.S 20 7400 7600 100 - -
629 10 20 8300 6700 200 O.S -
630 1S 20 3100 6900 300 1
631 2.S 10 8000 12000 - - -
632 12.5 20 1600 3400 500 2 O.S
Ex. Cpd.E2 Bud HFA134a HFA227 ~ EthanolOA Lactose
Parts Parts Parts Parts Parts Parts Parts
633 2.S 20 SS00 4500 - - -
634 O.S 20 3500 6500 - - 1
635 0.25 20 2500 7500 - - 1
636 S 20 3800 6100 - .100 O.S -
637 3.75 20 3300 6600 100 O.S O.S
638 7.S 20 3600 5900 500 4 -
639 10 20 4600 4900 500 3 -
640 7.S 10 3100 6800 100 0.2 O.S
641 10 10 1400 3100 500 0.2 -
642 1S 10 8000 12000 - - 1
643 20 10 30000 70000 - - -
Example 644 - 652
The procedure of Examples 507-534 is repeated, but using sorbitan trioleate
(ST) as surfactant
in place of oleic acid, the amounts of the ingredients being as shown in the
following table:
CA 02552938 2006-07-17
WO 2005/074924 46 PCT/EP2005/001241
Ex. Cpd.E2 MF ~ HFA134a FA227 Ethanol ST Lactose
Parts H Parts Parts Parts Parts Parts
Parts
644 15 40 10000 10000 300 4 -
645 1S 20 8000 12000 200 8 -
646 12.5 20 12000 8000 400 10 -
647 10 20 5000 5000 600 2.5 1
648 7.S 20 3500 6500 - 4 2
649 S 20 6000 4000 - 3 3
650 2.S 20 4500 SS00 100 2 1
651 S 10 4100 5900 SO 1 2
652 3.75 5 ~ 1550 ~ 3450 ~ 200 0 5 1
~ ~
Examples 653-712
Gelatin capsules suitable for use in a capsule inhaler such as that described
in US 3991761 or
EP 1270034 are prepared, each capsule containing a dry powder obtained by
mixing
Compound E3 and budesonide which have been ground to a mean particle diameter
of 1 to
S~m and lactose monohydrate having a particle diameter below 212 Vim, the
amounts being as
shown in the table below:
Example Compound E3 Budesonide Lactose
Parts Parts Parts
653 20 100 19880
654 40 100 19860
655 80 100 19820
656 100 100 19800
657 120 100 19780
. 658 140 100 19760
659 160 100 19740
660 180 100 19720
661 200 100 19700
662 220 100 19680
663 240 100 19660
664 300 100 19600
665 500 100 19400
666 1000 100 18900
667 2000 100 17900
668 20 100 24880
669 40 100 24860
670 80 100 24820
671 100 100 24800
672 120 100 24780
673 140 100 24760
674 ( 160 ~ 100 _
24740
CA 02552938 2006-07-17
WO 2005/074924 4~ PCT/EP2005/001241
675 180 100 24720
676 200 100 24700
677 220 100 24680
678 2,40 100 24660
679 300 100 24600
&80 500 100 24400
681 1000 100 23900
682 2000 100 22900
683 20 200 14780
684 40 200 14760
685 80 200 14720
686 100 200 14700
687 ~ 120 200 14680
688 140 200 14660
689 160 200 14640
690 180 200 14620
691 200 200 14600
692 220 200 14580
693 240 200 14560
694 300 200 14500
695 500 200 14300
696 1000 200 13800
697 2000 200 12800
698 20 200 24780
G99 40 200 24760
700 80 200 24720
701 100 200 24700
702 120 200 24680
703 140 200 24660
704 160 200 24640
705 180 200 24620
706 200 200 24600
707 220 200 24580
708 240 200 24560
709 300 200 24500
710 500 200 24300
711 1000 200 23800
712 2000 200 22800
Examples 713-742
Examples 1-60 are repeated, but replacing budesonide with mometasone furoate,
and using
amounts as shovcrn in the following table:
Example Compound E3 M F Lactose
Parts Parts ,Parts
713 20 100 24880
CA 02552938 2006-07-17
WO 2005/074924 4g PCT/EP2005/001241
714 40 100 ~ 24860
715 80 100 24820
716 100 100 24800
717 120 100 24780
718 140 100 24760
719 160 100 24740
720 180 100 24720
721 200 100 24700
722 220 100 24680
723 240 100 24660
724 300 100 24600
725 500 100 24400
726 1000 100 23900
727 2000 100 22900
728 20 200 14780
729 40 200 14760
730 80 200 14720
731 100 200 14700
732 120 200 14680
733 140 200 14660
734 160 200 14640
735 180 200 14620
736 200 200 14600
737 220 200 14580
738 240 200 14560
739 300 200 14500
740 500 200 14300
741 1000 200 13800
742 2000 200 12800
Examples 743-787
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E3 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5 ~m and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table below
Example Compound E3 FP Lactose
Parts Parts Parts
743 20 100 4880
744 40 100 4860
745 80 100 4820
746 100 100 4800
747 120 100 4780
748 140 100 4760
749 ~ 160 ~ 100 4740
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
49
750 180 100 4720
751 200 100 4700
752 220 100 4680
753 240 100 4660
754 300 100 4600
7SS 500 100 4400
756 1000 100 3900
757 2000 100 2900
758 20 200 9780
759 40 200 9760
760 80 200 9720
761 100 200 9700
762 120 200 9680
763 140 200 9660
764 160 200 9640
765 180 200 9620
766 200 200 9600
767 220 200 9580
768 240 200 9560
769 300 200 9500
770 500 200 9300
771 1000 200 8800
772 2000 200 7800
773 20 250 14730
774 40 250 14710
775 80 250 14670
776 100 250 14650
777 120 250 14630
778 140 250 14610
779 160 250 14590
780 180 250 14570
781 200 250 14550
782 220 250 14530
783 240 250 14510
784 300 250 14450
785 500 250 14250
786 1000 250 13750
787 2000 250 12750
Examples 788-832
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E3 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-S ~m and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table directly above
but also
containing O.S% magnesium stearate by weight.
CA 02552938 2006-07-17
WO 2005/074924 5~ PCT/EP2005/001241
Examples 833-860
Aerosol formulations are prepared by dispensing micronised active ingredients,
Compound E3
and mometasone furoate/ fluticasone propionate, and if required, lactose as
bulking agent into
a vial, sealing the vial yvith a metering valve, injecting the premixed
ethanol/propellant and
optional surfactant into the vial through the valve and subjecting the vial to
ultrasonic energy
to disperse the solid particles. The components and amounts used are shown in
the following
tables:
Ex. Cpd.E3 MF HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
833 2 10 36500 X0750 2500 - 70
834 4 10 3410 6340 230 0.3 -
83S 8 10 97000 - 2500 - 90
836 10 10 30500 67000 2500 O.S 100
837 12 10 3150 6550 250 1 -
838 14 10 3700 6050 250 0.8 -
839 16 10 3800 5900 230 0.4 -
840 18 10 4700 SOSO 250 1 -
841 20 20 3600 6150 225 1 -
842 22 20 3500 6200 230 1 -
843 24 20 98000 - 2500 1 -
844 30 20 3900 5900 250 1 -
84S 2 20 30000 67000 2250 0.2 90
846 10 20 3500 6200 250 O.S -
847 14 20 3200 6500 230 1 -
848 18 20 3100 6200 225 0.8 -
849 20 20 3150 6100 225 1 -
8S0 24 20 30000 60000 2000 0.8 -
Ex. Cpd.E3 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
851 4 10 34000 63000 2250 0.3 SO
852 8 10 92000 - 2500 O.S 70
853 12 10 3000 SS00 200 - -
8S4 16 10 2500 5000 200 0.3 -
8SS 20 10 2000 3000 150 0.2 -
8S6 30 10 2000 2000 150 0.2 -
8S7 8 20 20000 25000 1500 0.2
8S8 12 20 2500 2500 200 0.2 -
8S9 20 20 2000 2000 150 0.2 -
860 30 20 20000 20000 1500 0.2 -
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
51
Examples 861-896
The procedure of Examples 743-787 is repeated, but replacing fluticasone
propionate by
mometasone furoate, and using amounts as shown in the following table:
Example Compound E3 MF Lactose
Parts Parts Parts
861 100 100 4800
862 200 100 4700
863 300 100 4600
864 400 100 4500
865 500 100 4400
866 600 100 4300
867 700 100 4200
868 800 100 4100
869 2000 100 2900
870 100 200 4700
871 200 200 4600
872 300 200 4500
873 400 200 4400
874 500 200 4300
875 600 200 4200
876 700 200 4100
877 800 200 4000
878 1200 200 3600
879 100 400 4500
880 200 400 4400
881 300 400 4300
882 400 400 4200
883 500 400 4100
884 600 400 4000
885 700 400 3900
886 800 400 3800
887 100 100 9800
888 200 100 9700
889 300 100 9600
890 400 100 9500
891 500 100 9400
892 100 200 9700
893 200 200 9600
894 300 200 9500
895 400 200 9400
896 ~ 500 200 9300
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
52
Examples 897-932
The procedure of Examples 743-787 is repeated, but replacing fluticasone
propionate by
mometasone furoate, using amounts as shown in the table directly above and
including 0.5%
magnesium stearate by weight.
Examples 933-969
The procedures of Examples 136-163 is repeated, but using the amounts shown in
the
following table, the ethanol being omitted in some of the Examples:
Ex. Cpd.E3 MF HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
933 20 20 5000 - 200 0.5 -
934 40 2 2500 2500 - - -
935 75 25 1500 3500 500 - 1
936 20 20 3600 6150 225 - 0.5
937 2 20 30000 67000 - - -
938 14 20 3200 6500 1500 - 4
939 20 20 3150 6100 1500 4 -
940 10 20 4700 5050 500 - 0.2
941 60 20 10000 10000 - - -
942 60 20 10000 10000 200 - -
943 60 20 10000 10000 - 0.5 -
944 30 20 8000 12000 - 1 1
945 40 20 5000 15000 500 0.5 0.5
946 50 20 9000 11000 400 0.8 0.2
947 20 20 4600 5000 400 0.4 0.2
948 30 10 20000 25000 - - -
949 40 10 20000 30000 - - -
950 60 10 35000 65000 - - -
Ex. Cpd.E3 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
951 20 10 5000 5000 - - 1
952 10 10 3650 6350 - - 1
953 30 10 3200 6800 100 0.5 0.5
954 30 20 7400 7600 100 - -
955 40 20 8300 6700 200 0.5 -
956 60 20 3100 6900 300 1 -
957 10 10 8000 12000 - - -
958 SO 20 1600 3400 500 2 0.5
Ex. ~ Cpd.E3 ~ Bud ~ HFA134a ~ HFA227 ~ Ethanol ~ OA Lactose
CA 02552938 2006-07-17
WO 2005/074924 53 PCT/EP2005/001241
959 10 20 5500 4500 - - -
960 2 20 3500 6500 - - 1
961 1 20 2500 7500 - - 1
962 20 20 3800 6100 100 0.5 -
963 15 20 3300 6600 100 0.5 0.5
964 30 20 3600 5900 500 4 -
965 40 20 4600 4900 500 3 -
966 30 10 3100 6800 100 0.2 0.5
967 40 10 1400 3100 500 0.2 -
968 60 10 8000 12000 - - 1
969 80 10 30000 70000 - - -
Example 970 - 978
The procedure of Examples 833-860 is repeated, but using sorbitan trioleate
(ST) as surfactant
in place of oleic acid, the amounts of the ingredients being as shown in the
following table:
Ex. Cpd.E3 MF HFA134a HFA227 Ethanol ST Lactose
P arts Parts Parts Parts Parts Parts Parts
970 60 40 10000 10000 300 4 -
971 60 20 8000 12000 200 8 -
972 50 20 12000 8000 400 10 -
973 40 20 5000 5000 600 2.5 1
974 30 20 3500 6500 - 4 2
975 20 20 6000 4000 - 3 3
976 10 20 4500 5500 100 2 1
977 20 10 4100 5900 50 1 2
978 15 5 1550 3450 200 0.5 1
Examples 979-1038
Gelatin capsules suitable for use in a capsule inhaler such as that described
in US 3991761 or
EP 1270034 are prepared, each capsule containing a dry powder obtained by
mixing
Compound E4 and budesonide which have been ground to a mean particle diameter
of 1 to S
~.m and lactose monohydrate having a particle diameter below 212 ~tm, the
amounts being as
shown in the table below:
Example Compound E4 Budesonide Lactose
Parts Parts Parts
979 10 100 19880
980 20 100 19860
981 40 100 19820
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
54
982 SO _ 100 19800
983 60 100 19780
984 70 100 19760
985 80 100 19740
986 90 100 19720
987 10 0 100 19700
988 11 0 100 19680
989 120 100 19660
990 15 O 100 19600
991 25 0 100 19400
992 SO 0 100 18900
993 1000 100 17900
994 10 100 24880
995 20 100 24860
996 40 100 24820
997 50 100 24800
998 60 100 24780
999 7O 100 24760
1000 80 100 24740
1001 90 100 24720
1002 100 100 24700
1003 110 100 24680
1004 120 100 24660
1005 150 100 24600
1006 250 100 24400
1007 SOO 100 23900
1008 1000 100 22900
1009 10 200 14780
1010 20 200 14760
1011 40 200 14720
1012 50 200 14700
1013 60 200 14680
1014 70 200 14660
1015 80 200 14640
1016 90 200 14620
1017 100 200 14600
1018 110 200 14580
1019 120 200 14560
1020 150 200 14500
1021 250 200 14300
1022 500 200 13800
1023 100 0 200 12800
1024 10 200 24780
1025 20 200 24760
1026 40 200 24720
1027 SO 200 24700
1028 60 200 24680
1029 70 200 24660
1030 80 200 24640
1031 90 200 24620
1032 100 200 24600
CA 02552938 2006-07-17
WO 2005/074924 5$ PCT/EP2005/001241
1033 1 10 200 24580
1034 1 20 200 24560
1035 1 SO 200 24500
1036 250 200 24300
1037 500 200 23800
1038 1000 200 22800
Examples 1039-1068
Examples 979-1038 are repeated, but replacing budesonide with mometasone
furoate, and
using amounts as shown in the following table:
Example Compound E4 MF Lactose
Parts Parts Parts
1039 1 0 100 24880
1040 20 100 24860
1041 40 100 24820
1042 SO 100 24800
1043 60 100 24780
1044 70 100 24760
1045 8 0 100 24740
1046 90 100 24720
1047 100 100 24700
1048 110 100 24680
1049 120 100 24660
1050 1 SO 100 24600
1051 250 100 24400
1052 500 100 23900
1053 1000 100 22900
1054 1 0 200 14780
1055 20 200 14760
1056 40 200 14720
1057 S 0 200 14700
1058 60 200 14680
1059 '70 200 14660
1060 8 0 200 14640
1061 90 200 14620
1062 100 200 14600
1063 110 200 14580
1064 120 200 14560
1065 150 200 14500
1066 250 200 14300
1067 500 200 13800
1068 1000 200 12800
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
56
Examples 1069-1113
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E4 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5 ~tm and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table below:
Example Compound E4 FP Lactose
Parts Parts Parts
1069 10 100 4880
1070 20 100 4860
1071 40 100 4820
1072 SO 100 4800
1073 60 100 4780
1074 70 100 4760
1075 80 100 4740
1076 90 100 4720
1077 100 100 4700
1078 110 100 4680
1079 120 100 4660
1080 150 100 4600
1081 250 100 4400
1082 500 100 3900
1083 1000 100 2900
1084 10 200 9780
1085 20 200 9760
1086 40 200 9720
1087 50 200 9700
1088 60 200 9680
1089 70 200 9660
1090 80 200 9640
1091 90 200 9620
1092 100 200 9600
1093 110 200 9580
1094 120 200 9560
1095 1 SO 200 9500
1096 250 200 9300
1097 500 200 8 800
1098 1000 200 7800
1099 1 0 250 14730
1100 20 250 14710
1101 40 250 14670
1102 SO 250 14650
1103 60 250 14630
1104 70 250 14610
1105 8 0 250 14590
1106 90 250 14570
1107 100 250 14550
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
57
1108 110 250 14530
1109 120 250 14510
1110 150 250 14450
1111 250 250 14250
1112 500 250 13750
1113 1000 250 12750
Examples 1114-1158
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound E4 and fluticasone propionate which
have been
ground to a mean particle diameter of 1-S ~m and lactose monohydrate having a
particle
diameter below 212 ~tm, the amounts being as shown in the table directly above
but also
containing 1.0°1° magnesium stearate by weight.
Examples 1159-1186
Aerosol formulations are prepared by dispensing micronised active ingredients,
Compound E4
and mometasone furoate/ fluticasone propionate, and if required, lactose as
bulking agent into
a vial, sealing the vial with a metering valve, injecting the premixed
ethanol/propellant and
optional surfactant into the vial through the valve and subjecting the vial to
ultrasonic energy
to disperse the solid particles. The components and amounts used are shown in
the following
tables:
Ex. Cpd.E4 MF HFA134a~ HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
~
1159 1 10 36500 60750 2500 - 70
1160 2 10 3410 6340 230 0.3 -
1161 4 10 97000 - 2500 - 90
1162 S 10 30500 67000 2500 O.S 100
1163 6 10 3150 6550 250 1 -
1164 7 10 3700 6050 250 0.8 -
1165 8 10 3800 5900 230 0.4 -
1166 9 10 4700 SOSO 250 1 -
1167 10 20 3600 6150 225 1 -
1168 11 20 3500 6200 230 1 -
1169 12 20 98000 - 2500 1 -
1170 13 20 3900 5900 250 1 -
1171 1 20 30000 67000 2250 0.2 90
1172 S 20 3500 6200 250 O.S -
1173 7 20 3200 6500 230 1 -
1174 9 20 3100 6200 225 0.8 -
CA 02552938 2006-07-17
WO 2005/074924 58 PCT/EP2005/001241
1175 10 20 3150 6100 225 1 -
1176 12 20 30000 60000 2000 0.8 -
Ex. Cpd.E4 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
_1_1772 10 34000 63000 2250 0.3 50
1178 4 10 92000 - 2500 0.5 70
_
1179_ 6 10 3000 5500 200 - -
1180 8 10 2500 5000 200 0.3 -
1181 10 10 2000 3000 150 0.2 -
1182 15 10 2000 2000 150 0.2 -
1183 4 20 20000 25000 1500 0.2 -
1184 6 20 2500 2500 200 0.2 -
1185 10 20 2000 2000 150 0.2 -
1186 15 20 20000 20000 1500 0.2 -
Examples 1187-1222
The procedure of Examples 1069-1113 is repeated, but replacing fluticasone
propionate by
mometasone furoate, and using amounts as shown in the following table.
Example Compound E4 MF Lactose
Parts Parts Parts
1187 50 100 4800
1188 100 100 4700
1189 150 100 4600
1190 200 100 4500
1191 250 100 4400
1192 300 100 4300
1193 350 100 4200
1194 400 100 4100
1195 1000 100 2900
1196 50 200 4700
1197 100 200 4600
1198 150 200 4500
1199 200 200 4400
1200 250 200 4300
1201 300 200 4200
1202 350 200 4100
1203 400 200 4000
1204 600 200 3600
1205 50 400 4500
1206 100 400 4400
1207 150 400 4300
1208 200 400 4200
1209 250 400 4100
1210 300 400 4000
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
59
1211 350 400 3900
1212 400 400 3800
1213 SO 100 9800
1214 100 100 9700
1215 150 100 9600
1216 200 100 9500
1217 250 100 9400
1218 50 200 9700
1219 100 200 9600
1220 150 200 9500
1221 200 200 9400
1222 250 200 9300
Examples 1223-1258
The procedure of Examples 10f9-1113 is repeated, but replacing fluticasone
propionate by
mometasone furoate, using amounts as shown in the table directly above and
including 1.0%
magnesium stearate by weight.
Examples 1259-1295
The procedures of Examples 1159-1186 is repeated, but using the amounts shown
in the
following table, the ethanol being omitted in some of the Examples:
Ex. Cpd.E4 MF HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
1259 10 20 5000 - 200 0.5 -
1260 20 2 2500 2,500 - - -
1261 37 25 1500 3500 500 - 1
1262 10 20 3600 6150 225 - 0.5
1263 1 20 30000 67000 - - -
1264 7 20 3200 6500 1500 - 4
1265 20 20 3150 6100 1500 4 -
1266 5 20 4700 5050 500 - 0.2
1267 30 20 10000 10000 - - -
1268 30 20 10000 10000 200 - -
1269 30 20 10000 10000 - 0.5 -
1270 15 20 8000 12000 - 1 1
1271 20 20 5000 15000 500 0.5 0.5
1272 25 20 9000 11000 400 0.8 0.2
1273 10 20 4600 5000 400 0.4 0.2
1274 15 10 20000 25000 - - -
1275 20 10 20000 3 0000 - - -
1276 30 10 35000 65000 - - -
CA 02552938 2006-07-17
WO 2005/074924 6~ PCT/EP2005/001241
Ex. Cpd.E4 FP HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
1277 10 10 5000 5000 - - 1
1278 S 10 3650 6350 - - 1
1279 15 10 3200 6800 100 0.5 0.5
128_0 15 20 7400 7600 100 - -
1281 20 20 8300 6700 200 0.5 -
1282 30 20 3100 6900 300 1 -
1283 S 10 8000 12000 - - -
1284 25 20 1600 3400 500 2 0.5
Ex. Cpd.E4 Bud HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts f Parts
1285 5 20 5500 4500 - - -
1286 1 20 3500 6500 - - 1
1287 0.5 20 2500 7500 - - 1
1288 10 20 3800 6100 100 0.5 -
1289 7.5 20 3300 6600 100 0.5 0.5
1290 15 20 3600 5900 500 4 -
1291 20 20 4600 4900 500 3 -
1292 15 10 3100 6800 100 0.2 0.5
1293 20 10 1400 3100 500 0.2 -
1294 30 10 8000 12000 - - 1
1295 40 ~ 10 ~ 30000 ~ 70000 - ~ - -
~ ~
Example 1296 -1304
The procedure of Examples 1159-1186 is repeated, but using sorbitan trioleate
(ST) as
surfactant in place of oleic acid, the amounts of the ingredients being as
shown in the following
table:
Ex. Cpd.E4 MF HFA134a IIFA227 Ethanol ST Lactose
- Parts Parts Parts Parts Parts Parts Parts
1296 30 40 10000 10000 300 4 -
1297 30 20 8000 12000 200 8 -
1298 25 20 12000 8000 400 10 -
1299 20 20 5000 5000 600 2.5 1
1300 15 20 3500 6500 - 4 2
1301 10 20 6000 4000 - 3 3
1302 S 20 4500 SS00 100 2 1
1303 10 10 4100 5900 50 1 2
1304 7.5 5 1550 3450 200 0.5 1
CA 02552938 2006-07-17
WO 2005/074924 61 PCT/EP2005/001241
Examples 1305-1364
Gelatin capsules suitable for use in a capsule inhaler such as that described
in US 3991761 or
EP 1270034 are prepared, each capsule containing a dry powder obtained by
mixing
Compound ES and budesonide which have been ground to a mean particle diameter
of 1 to
S~rn and lactose monohydrate having a particle diameter below 212 Vim, the
amounts being as
shown in the table below:
Example Compound ES Budesonide Lactose
Parts Parts Parts
1305 20 100 19880
1306 40 100 19860
1307 80 100 19820
1308 100 100 19800
1309 120 100 19780
1310 140 100 19760
1311 160 100 19740
1312 180 100 19720
1313 200 100 19700
1314 220 100 19680
1315 240 100 19660
1316 300 100 19600
1317 500 100 19400
1318 1000 100 18900
1319 2000 100 17900
1320 20 100 24880
1321 40 100 24860
1322 80 100 24820
1323 100 100 24800
1324 120 100 24780
1325 140 100 24760
1326 160 100 24740
1327 180 100 24720
1328 200 100 24700
1329 220 100 24680
1330 240 100 24660
1331 300 100 24600
1332 500 100 24400
1333 1000 100 23900
1334 2000 100 22900
1335 20 200 14780
1336 40 200 14760
1337 80 200 14720
1338 100 200 14700
1339 120 200 14680
1340 140 200 14660
1341 160 200 14640
1342 180 200 14620
CA 02552938 2006-07-17
WO 2005/074924 62 PCT/EP2005/001241
1343 200 200 14600
1344 220 200 14580
1345 240 200 14560
1346 300 200 14500
1347 500 200 14300
1348 1000 200 13800
1349 2000 200 12800
1350 20 200 24780
1351 40 200 24760
1352 80 200 24720
1353 100 200 24700
1354 120 200 24680
1355 140 200 24660
1356 160 200 24640
1357 180 200 24620
1358 200 200 24600
1359 220 200 24580
1360 240 200 24560
1361 300 200 24500
1362 500 200 24300
1363 1000 200 23800
1364 2000 200 22800
Examples 1365-1394
Examples 1305-1364 are repeated, but replacing budesonide with mometasone
furoate, and
using amounts as shown in the following table:
Example Compound ES MF Lactose
Parts Parts Parts
1365 20 100 24880
1366 40 100 24860
1367 80 100 24820
1368 100 100 24800
1369 120 100 24780
1370 140 100 24760
1371 160 100 24740
1372 180 100 24720
1373 200 100 24700
1374 220 100 24680
1375 240 100 24660
1376 300 100 24600
1377 500 100 24400
1378 1000 100 23900
1379 2000 100 22900
1380 20 200 14780
1381 40 200 14760
CA 02552938 2006-07-17
WO 2005/074924 63 PCT/EP2005/001241
1382 80 200 14720
1383 100 200 14700
1384 120 200 14680
1385 140 200 14660
1386 160 200 14640
1387 180 200 14620
1388 200 200 14600
1389 220 200 14580
1390 240 200 14560
1391 ~ 300 200 14500
_ 1392 ~ - 500 200 14300
1393 1000 '200 _
13800
1394 2000 200 12800
Examples 1395-1439
A dry powder suitable for delivery from the reservoir of the mufti-dose
inhaler described in WO
97/20589 is prepared by mixing Compound ES and fluticasone propionate which
have been
ground to a mean particle diameter of 1-5 ~m and lactose monohydrate having a
particle
diameter below 212 Vim, the amounts being as shown in the table below:
Example Compound ES FP Lactose
Parts Parts Parts
1395 20 100 4880
1396 40 100 4860
1397 80 100 4820
1398 100 100 4800
1399 120 100 4780
1400 140 100 4760
1401 160 100 4740
1402 180 100 4720
1403 200 100 4700
4104 220 100 4680
1405 240 100 4660
1406 300 100 4600
1407 500 100 4400
1408 1000 100 3900
1409 2000 100 2900
1410 20 200 9780
1411 40 200 9760
1412 80 200 9720
1413 100 200 9700
1414 120 200 9680
1415 140 200 9660
1416 160 200 9640
1417 180 200 9620
CA 02552938 2006-07-17
WO 2005/074924 64 PCT/EP2005/001241
1418 200 200 9600
1419 220 200 9580
1420 240 200 9560
1421 300 200 9500
1422 500 200 9300
1423 1000 200 8800
1424 2000 200 7800
1425 20 250 14730
1426 40 250 14710
1427 80 250 14670
1428 100 250 14650
1429 120 250 14630
1430 140 250 14610
1431 160 250 14590
1432 180 250 14570
1433 200 250 14550
1434 220 250 14530
1435 240 250 14510
1436 300 250 14450
1437 500 250 14250
1438 1000 250 13750
1439 2000 250 12750
Examples 1440-1484
A dry powder suitable for delivery from the reservoir of the multi-dose
inhaler described in WO
97/20589 is prepared by mixing Compound ES and flutacasone propionate which
have been
ground to a mean particle diameter of 1-5 ~m and lactose monohydrate having a
particle
diameter below 212 pm, the amounts being as shown in the table directly above
but also
containing O.S% magnesium stearate by weight.
Examples 1485-1512
Aerosol formulations are prepared by dispensing micronised active ingredients,
Compound ES
and mometasone furoate/ fluticasone propionate, and if required, lactose as
bulking agent into
a vial, sealing the vial with a metering valve, injecting the premixed
ethanol/propellant and
optional surfactant into the vial through the valve and subjecting the vial to
ultrasonic energy
to disperse the solid particles. The components and amounts used are shown in
the following
tables:
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
Ex. Cpd.ES MF HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
1485 2 10 36500 60750 2500 - 70
1486 4 10 3410 6340 230 0.3 -
1487 8 10 97000 - 2500 - 90
1488 10 10 30500 67000 2500 0.5 100
1489 12 10 3150 6550 250 1 -
1490 14 10 3700 6050 250 0.8 -
1491 16 10 3800 5900 230 0.4 -
1492 18 10 4700 5050 250 1 -
1493 20 20 3600 6150 225 1 -
1494 22 20 3500 6200 230 1 -
1495 24 20 98000 - 2500 1 -
1496 30 20 3900 5900 250 1 -
1497 2 20 30000 67000 2250 0.2 90
1498 10 20 3500 6200 250 0.5 -
1499 14 20 3200 6500 230 1 -
1500 18 20 3100 6200 225 0.8 -
1501 20 20 3150 6100 225 1 -
1502 24 ~ 20 30000 60000 2000 0.8 -
~
Ex. Cpd.ES FP HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
1503 4 10 34000 63000 2250 0.3 50
1504 8 10 92000 - 2500 0.5 70
1505 12 10 3000 5500 200 - -
1506 16 10 2500 5000 200 0.3 -
1507 20 10 2000 3000 150 0.2 -
1508 30 10 2000 2000 150 0.2 -
1509 8 20 20000 25000 1500 0.2 -
1510 12 20 2500 2500 200 0.2 -
1511 20 20 2000 2000 150 0.2 -
1512 30 20 20000 20000 1500 0.2 -
Examples 1513-1548
The procedure of Examples 1395-1439 is repeated, but replacing fluticasone
propionate by .
mometasone furoate, and using amounts as shown in the following table.
Example Compound ES MF Lactose
Parts Parts Parts
1513 100 100 4800
1514 200 100 4700
1515 300 100 4600
1516 400 100 4500
1517 500 100 4400
1518 600 100 4300
CA 02552938 2006-07-17
WO 2005/074924 66 PCT/EP2005/001241
1519 700 100 4200
1520 800 100 4100
1521 2000 100 2900
1522 100 200 4700
1523 200 200 4600
1524 300 200 4500
1525 400 200 4400
1526 500 200 4300
1527 600 200 4200
1528 700 200 4100
1529 800 200 4000
1530 1200 200 3600
1531 100 400 4500
1532 200 400 4400
1533 300 400 4300
1534 400 400 4200
1535 500 400 4100
1536 600 400 4000
1537 700 400 3900
1538 800 400 3800
1539 100 100 9800
1540 200 100 9700
1541 300 100 9600
1542 400 100 9500
1543 500 100 9400
1544 100 200 9700
1545 200 200 9600
1546 300 200 9500
1547 400 200 9400
1548 500 200 9300
Examples 1549-1584
The procedure of Examples 1395-1439 is repeated, but replacing fluticasone
propionate by
mometasone furoate, using amounts as shown in the table directly above and
including 0.5%
magnesium stearate by weight.
Examples 1585-1621
The procedures of Examples 1485-1512 is repeated, but using the amounts shown
in the
following table, the ethanol being omitted in some of the Examples:
CA 02552938 2006-07-17
WO 2005/074924 6~ PCT/EP2005/001241
Ex. Cpd.ES MF HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
1585 20 20 5000 - 200 0.5 -
1586 40 2 2500 2500 - - -
1587 75 25 1500 3500 500 - 1
1588 20 20 3600 6150 225 - 0.5
1589 2 20 30000 67000 - - -
1590 14 20 3200 6500 1500 - 4
1591 20 20 3150 6100 1500 4 -
1592 10 20 4700 5050 500 - 0.2
1593 60 20 10000 10000 - - -
1594 60 20 10000 10000 200 - -
1595 60 20 10000 10000 - 0.5 -
1596 30 20 8000 12000 - 1 1
1597 40 20 5000 15000 500 0.5 0.5
1598 SO 20 9000 11000 400 0.8 0.2
1599 20 20 4600 5000 400 0.4 0.2
1600 30 10 20000 25000 - - -
1601 40 10 20000 30000 - - -
1602 6~ 10 35000 65000 - - -
~
Ex. Cpd.ES FP HFA134a HFA227 Ethanol OA Lactose
P arts Parts Parts Parts Parts Parts Parts
1603 20 10 5000 5000 - - 1
1604 10 10 3650 6350 - - 1
1605 30 10 3200 6800 100 0.5 0.5
1606 30 20 7400 7600 100 - -
1607 40 20 8300 6700 200 O.S -
1648 60 20 3100 6900 300 1 -
1609 10 10 8000 12000 - - -
2610 50 20 1600 3400 500 2 0.5
Ex. Cpd.ES Bud HFA134a HFA227 Ethanol OA Lactose
Parts Parts Parts Parts Parts Parts Parts
1611 10 20 5500 4500 - - -
1612 2 20 3500 6500 - - 1
1613 1 20 2500 7500 - - 1
1614 20 20 3800 6100 100 0.5 -
1615 15 20 3300 6600 100 0.5 0.5
1616 30 20 3600 5900 500 4 -
1617 40 20 4600 4900 500 3 -
1618 30 10 3100 6800 100 0.2 0.5
1619 40 10 1400 3100 500 0.2 -
1620 60 10 8000 12000 - - 1
1621 80 10 30000 70000 - - -
CA 02552938 2006-07-17
WO 2005/074924 PCT/EP2005/001241
68
Example 1622 -1630
The procedure of Examples 1485-1512 is repeated, but using sorbitan trioleate
(ST) as
surfactant in place of oleic acid, the amounts of the ingredients being as
shown in the following
table:
Ex. Cpd.ES MF HFA134a HFA227 Ethanol ST Lactose
P arts Parts Parts Parts Parts Parts Parts
1622 60 40 10000 10000 300 4 -
1623 60 20 8000 12000 200 8 -
1624 50 20 12000 8000 400 10 -
1625 40 20 5000 5000 600 2.5 1
1626 30 20 3500 6500 - 4 2
1627 20 20 6000 4000 - 3 3
1628 10 20 4500 5500 100 2 1
1629 20 10 4100 5900 SO 1 2
1630 15 5 1550 3450 200 0.5 1