Note: Descriptions are shown in the official language in which they were submitted.
CA 02553074 2006-07-07
1
SOLID OXIDE FUEL CELL
[BACKGROUND OF THE INVENTION]
[0001 ] Field of invention
The present invention relates to a solid oxide fuel cell.
More particularly, the present invention relates to a solid oxide fuel cell
possessing excellent output performance and durability.
[0002] Backgiround art
Solid oxide fuel cells have a high operating temperature
(900 to 1000°C) and are expected as a highly efficient fuel cell.
Various
proposals have been made for realizing solid oxide fuel cells possessing
excellent output performance and durability.
[0003] For example, Japanese Patent Laid-Open Nos. 22821/2003
and 22822/2003 propose the addition of at least one oxide selected from
the group consisting of group 4A, 5A, 7A, and 4B elements into solid
oxide fuel cells, in order to improve oxygen ion conductivity stability and
high temperature strength stability of an electrolyte formed of zirconia
containing scandia in solid solution.
[0004] These publications have, however, no disclosure of a
2o combination of the oxide with an air electrode formed of a manganese
containing perovskite. Further, these publications describe that
manganese is added as an oxide of group 7A element Mn02. The
addition amount thereof, however, is not clear.
[0005] Further, Japanese Patent Laid-Open No. 187811/2003
proposes a mixed material composed of an electron conductive
perovskite oxide and a high-melting dielectric oxide which is provided
between an air electrode and an electrolyte in order to realize an efficient
reaction represented by formula (1) described later, in which oxygen gas
produced in the air electrode and the electrolyte is reacted with electrons
3o to produce oxygen ions. Lanthanum manganite containing Sr or Ca in
solid solution may be mentioned as a representative example of the
perovskite oxide used in this technique. Examples of compositions
thereof include (La, Sr)a_sMn03, (La, Ca)~_sMnOs, and (La, Sr)~_s(MnyFe~_
y)03. Further, cerium-containing oxides containing Sm203 or Gd203 in
solid solution are proposed as a typical example of the high-melting
dielectric oxide.
CA 02553074 2006-07-07
2
[0006] Japanese Patent Laid-Open No. 41674/1996 proposes, as
an air electrode in a solid oxide fuel cell, the use of a material, prepared
by mixing 40 to 60 parts by weight of zirconia containing yttria in solid
solution to a lanthanum manganite represented by (La~.x~Srx~)Mn03
wherein 0.1 < x1 < 0.4, in order to improve an electrode reaction
between an air electrode and an electrolyte, as well as to provide
excellent durability.
[0007] Japanese Patent Laid-Open No. 180886/1996 discloses a
thin layer formed of zirconia containing yttria in solid solution which is
1 o provided between an air electrode and an electrolyte, in order to reduce
contact resistance between the air electrode and the electrolyte and to
improve the output performance. The air electrode material used in this
technique is lanthanum manganite containing Sr in solid solution.
[0008] Japanese Patent Laid-Open No. 4424512000 discloses the
provision of a layer formed of a powder of a mixture of lanthanum
manganite containing Ca and/or Sr in solid solution with zirconia
containing yttria in solid solution between an air electrode and an
electrolyte. This is to reduce contact resistance between the air
electrode and the electrolyte and to improve the output performance.
2 0 [0009] Japanese Patent Laid-Open No. 173801 /2003 proposes
that, in a solid oxide fuel cell, a layer having a porosity of not more than
25% and formed of a cerium-containing oxide represented by Ce~_xLnx02_
s, wherein Ln represents a rare earth element; and 0.05 <_ x <_ 0.3, is
provided to prevent a reaction between an electrolyte and a fuel
2 5 electrode.
[0010] So far as the present inventors know, however, even in
these prior art techniques neither disclose nor suggest the suppression
of diffusion of manganese through the electrolyte.
[0011 ] On the other hand, Japanese Patent Laid-Open No.
30 134132/2002 proposes the provision of an oxide layer containing yttria,
zirconia, and ceria between an air electrode and an electrolyte in a solid
oxide fuel cell prepared by co-sintering an air electrode formed of a
manganese-containing peroviskite oxide and an electrolyte formed of
zirconia. This is to suppress the diffusion of manganese into a fuel
35 electrode. However, the oxide containing yttria, zirconia, and ceria is
poor in degree of sintering, and a sintering temperature of about
1500°C
g CA 02553074 2006-07-07
3
is necessary for the formation of a gas permeation-free electrolyte.
Therefore, it is considered that the control of the amount of manganese
diffused into the fuel electrode through the electrolyte is difficult.
[SUMMARY OF THE INVENTION]
[0012] The present inventors have now found that, in a solid oxide
fuel cell comprising an air electrode formed of a perovskite oxide
containing at least manganese, the content of manganese in the layer, in
contact with the fuel electrode, in its surface on the fuel electrode side
1 o greatly affects the performance of the fuel cell, and the regulation of
the
manganese content can provide an excellent fuel cell. The present
invention has been made base on such finding.
[0013] Accordingly, an object of the present invention is to provide
a solid oxide fuel cell possessing excellent output performance and
durability.
[0014] According to the present invention, there is provided a
solid oxide fuel cell comprising at least an electrolyte, an air electrode,
and a fuel electrode, characterized in that the air electrode comprises a
perovskite oxide containing at least manganese, and the content of
2o manganese in the surface of a layer which is in contact with the fuel
electrode is 0.3 to 4% by weight, where the surface is on the fuel
electrode side of the layer.
(BRIEF DESCRIPTION OF THE DRAWINGS]
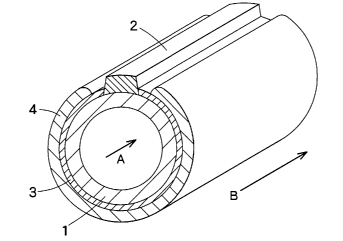
[0015] [FIG. 1] is a cross-sectional view of a cylindrical solid oxide
fuel cell.
[FIG. 2] is an enlarged cross-sectional view showing the
basic construction of a solid oxide fuel cell according to the present
invention. The solid oxide fuel cell according to the present invention
3 o has a basic construction comprising an air electrode support 1, an
electrolyte 3, and a fuel electrode 4. In this drawing, an air-side
electrode reaction layer 5 as one embodiment of the air electrode is
provided between the air electrode support 1 and the electrolyte 3, and a
porous layer 6 is provided between the electrolyte 3 and the fuel
electrode 4. Air (oxygen) is allowed to flow in a direction indicated by
an arrow into the air support 1 to bring air into contact with the air
' , CA 02553074 2006-07-07
y.
4
electrode. A fuel gas (for example, hydrogen, carbon monoxide, or
methane) is allowed to flow in a direction indicated by an arrow along the
fuel electrode 4 to bring the fuel gas into contact with the fuel electrode.
[FIG. 3] is an enlarged cross-sectional view of a solid oxide
fuel cell having the same construction as in Fig. 2, except that the
porous layer 6 is not provided, and a fuel-side electrode reaction layer
4a is provided between the electrolyte 3 and the fuel electrode 4.
[FIG. 4] is an enlarged view of a solid oxide fuel cell having
the same construction as in Fig. 3, except that the air-side electrode
1 o reaction layer 5 has a structure of a plurality of layers (5a, 5b).
[FIG. 5] is an enlarged view of a solid oxide fuel cell in
which, in the structure shown in Fig. 3, a porous layer 6 is additionally
provided between the fuel-side electrode reaction layer 4a and the
electrolyte 3.
[FIG. 6] is an enlarged view of a solid oxide fuel cell having
the same construction as in Fig. 5, except that the air-side electrode
reaction layer 5 has a structure of a plurality of layers (5a, 5b).
[FIG. 7] is a diagram having a cell construction for
measuring a reaction overvoltage for evaluating electrode characteristics.
[DETAILED DESCRIPTION OF THE INVENTION]
[0016] Basic construction of solid oxide fuel cell
The structure of the solid oxide fuel cell according to the
present invention is not particularly limited so far as the construction and
composition of the present invention which will be described later are
satisfied. For example, any of a flat plate type and a cylindrical type
may be adopted. The solid oxide fuel cell in the present invention can
also be applied to a microtube type (outer diameter: not more than 10
mm, more preferably not more than 5 mm). For example, a cylindrical
3o construction will be described below. Specifically, Fig. 1 is a cross-
sectional view of a cylindrical solid oxide full cell. This solid oxide fuel
cell is constructed so that a strip-shaped interconnector 2 and an
electrolyte 3 are provided on a cylindrical air electrode support 1 and,
further, a fuel electrode 4 is provided on the electrolyte 3 so as not to
come into contact with the interconnector 2. When air (oxygen) is
allowed to flow through the interior of the air electrode support while a
. CA 02553074 2006-07-07
fuel gas is allowed to flow through the outside, oxygen is converted to
oxygen ion at the interface of the air electrode and the electrolyte
according to the following reaction.
1/202 + 2e- -~ 02~ (1 )
5 This oxygen ion is passed through the electrolyte and
reaches the fuel electrode. In the fuel electrode in its part near the
electrolyte, the fuel gas is reacted with the oxygen ion to give water and
carbon dioxide. These reactions are expressed by the following
formulae.
1 o H2 + 02- -~ H20 + 2e- (2)
CO + 02- -~ C02 + 2e- (3)
Electricity can be taken out to the outside by connecting the
fuel electrode 4 to the interconnector 2.
[0017] Fig. 2 is an enlarged cross-sectional view showing the
basic construction of a solid oxide fuel cell according to the present
invention. The solid oxide fuel cell according to the present invention
has a basic structure comprising an air electrode support 1, an
electrolyte 3, and a fuel electrode 4. In Fig. 2, an air-side electrode
reaction layer 5 as one embodiment of the air electrode is provided
2 o between the air electrode support 1 and the electrolyte 3, and a porous
layer 6 is provided between the electrolyte 3 and the fuel electrode 4.
The air-side electrode reaction layer 5 and the porous layer 6 are not
indispensable to the present invention but is preferably provided.
[0018] Further, in another preferred embodiment of the present
invention, as shown in Fig. 3, the solid oxide fuel cell according to the
present invention may be provided with a fuel-side electrode reaction
layer 4a as one embodiment of the fuel electrode.
[0019] Further, in still another preferred embodiment of the
present invention, as shown in Fig. 4, the air-side electrode reaction
layer 5 in the solid oxide fuel cell may have a multilayer structure of a
plurality of layers (5a, 5b).
[0020] In a further embodiment of the present invention, there is
provided an embodiment comprising a combination of the above
constituent elements. For example, as shown in Fig. 5, there is
provided a solid oxide fuel cell comprising a porous layer 6 provided
between a fuel electrode 4 (an aspect including a fuel-side electrode
', CA 02553074 2006-07-07
a'
6
reaction layer 4a) and an electrolyte 3. In another embodiment of the
present invention, there is provided a solid oxide fuel cell having a
construction shown in Fig. 6 in which the air-side electrode reaction layer
has a multilayer structure of a plurality of layers.
[0021 ) The present invention is characterized in that the content
of manganese in a layer, in contact with the fuel electrode, in its surface
on the fuel electrode side is 0.3 to 4% by weight.
[0022] Accordingly, when the electrolyte is provided in contact with
the fuel electrode, and the content of manganese in the surface of the
1 o electrolyte on its fuel electrode side is 0.3 to 4% by weight. In a
preferred embodiment of the present invention, the content of
manganese in the surface of the electrode on its fuel electrode side is
preferably 0.6 to 3.5% by weight, more preferably 0.9 to 3% by weight.
Further, in this embodiment, the content of manganese in the surface of
the electrolyte on its air electrode side is preferably less than about 10%
by weight, more preferably less than 6% by Weight. In a further
preferred embodiment of the present invention, the content of
manganese in the surface of the electrolyte on its air electrode side is
larger than the content of the manganese component in the surface of
2 o the electrolyte on its fuel electrode side.
[0023] When a porous layer is provided between the fuel
electrode and the electrolyte, the content of manganese in the surface of
the porous layer on its fuel electrode side is 0.3 to 4% by weight. In a
preferred embodiment of the present invention, the content of
manganese in the surface of the porous layer on its fuel electrode side is
preferably 0.6 to 3.5% by weight, more preferably 0.9 to 3% by weight.
In a further preferred embodiment of the present invention, the content of
manganese in the surface of the electrolyte on its air electrode side is
larger than the content of manganese in the surface of the porous layer
3 o on its fuel electrode side.
[0024] In the present invention, the "content of manganese in the
surface of the layer" in the "content of manganese in the layer, in contact
with the fuel electrode, in its surface on the fuel electrode side" refers to
the content of manganese, in the layer in contact with the fuel electrode,
in its part of not more than 3 ~,m depth from the surface of the fuel
electrode. The content of manganese may be determined by any of
CA 02553074 2006-07-07
..
7
analysis from the fuel electrode side and analysis in which a section is
prepared and analysis is carried out from the sectional direction.
[0025] In the present invention as described above, in the layer in
contact with the fuel electrode, the content of manganese in the surface
on its fuel electrode side is regulated. It is considered that this
manganese is diffused from manganese-containing perovskite oxide
constituting the air electrode during sintering in the production of the
layer. The regulation of the diffusion amount can realize a solid oxide
fuel cell that possesses excellent output properties and, even when it
1 o undergoes a thermal cycle, can maintain the performance, that is,
possesses excellent durability. The reason why the regulation of the
manganese content can realize a good solid oxide fuel cell has not been
fully elucidated yet. Without wishing to be bound by theory, it is
believed that the content of manganese falls within the above-defined
range at the interface of the fuel electrode and the layer in contact with
the fuel electrode, satisfactory sintering can significantly improve the
adhesion between the two layers and, further, the electrolyte ensures
good ion conductivity to contribute to an improvement in the properties.
[0026] In the present invention, the content of manganese in the
2 0 layer, in contact with the fuel electrode, in its surface on the fuel
electrode side can be regulated by regulating the composition and
physical construction of the cell and production conditions. Elements
constituting the solid oxide fuel cell according to the present invention
including specific means for the regulation of the manganese content will
be described in detail.
[0027] Electrolyte
In the present invention, the electrolyte has high oxygen
ion (02-) conductivity at elevated temperatures, is a gas permeability-free
layer, and is preferably a layer formed of zirconia containing scandia
3o and/or yttria in solid solution. In the present specification, zirconia
containing scandia in solid solution will be referred to as "SSZ," zirconia
containing scandia and yttria in solid solution will be referred to as
"ScYSZ" or "SSZIYSZ," and zirconia containing yttria in solid solution will
be referred to as "YSZ."
[0028] In a preferred embodiment of the present invention, the
amount of solid solution of scandia in SSZ, the total amount of solid
, CA 02553074 2006-07-07
8
solution of scandia and yttria in ScYSZ, and the amount of solid solution
of yttria in YSZ are preferably about 3 to 12% by mole from the viewpoint
of realizing a high level of oxygen ion conductivity. More preferably, the
lower limit of the solid solution amount is about 8% by mole. In a
preferred embodiment of the present invention, in order to improve the
oxygen ion conductivity, at least one oxide selected from the group
consisting of Ce02, Sm203, Gd203, Yb203, Gd203, Er203, Nd203, Eu203,
Ey20s, Tm203, Pr203, La203, and Bi203 may be contained in solid
solution in a total amount of about 5% by mole or less. Further, for
1 o example, Bi203, A1203, and Si02 may be added to realize sintering at low
temperatures.
[0029] Further, in a preferred embodiment of the present invention,
the electrolyte in its film surface on the fuel electrode side has such a
crystal grain size distribution that 3% of the crystal grains has a diameter
of not less than 3 ~.m and 97% of the crystal grains has a diameter of not
more than 20 pm. When the crystal grain size distribution is in the
above-defined range, by virtue of good sinterability, an electrolyte can be
realized which is free from gas permeability and has good adhesion to
the fuel electrode.
2 0 [0030] The crystal grain size in the electrolyte surface on the fuel
electrode side refers to a grain size distribution determined by a
planimetric method. Specifically, at the outset, a photograph of the
electrolyte surface is taken with SEM. A known circle having an area
(S) is drawn on this photograph, and the number of grains N~ per unit
area is determined from the number of grains n~ present within the circle
and the number of grains n; present on the circumference by the
following equation:
N~ _ (n~ + 1/2n;)/(S/m2)
wherein m represents the magnification of the photograph.
3o Since 1/N~ represents the area occupied by one particle, when the
crystal grain diameter is an equivalent circle diameter, 2/~I(~N~) may be
adopted for the determination, while, in the case of square, 1hINc may
be adopted.
[0031 ] Further, in the present invention, 3% diameter of crystal
grains in the electrolyte refers to the grain diameter corresponding to the
third smallest grain, when the crystal grain diameter of 100 crystal grains
CA 02553074 2006-07-07
1~
9
is measured by a planimetric method and the grains are placed in
diameter ascending order, and 97% diameter refers to the grain diameter
corresponding to the 97th smallest grain.
[0032] In the present invention, that the electrolyte is free from
gas permeability can be specifically confirmed by providing a pressure
difference between the one side of the electrolyte and the opposite side
of the electrolyte and determining the amount of permeation of N2 gas
through the electrolyte. In a preferred embodiment of the present
invention, the gas permeation amount Q of the electrolyte is preferably Q
to < 2.8 x 10-9ms-'Pa-', more preferably Q <_ 2.8 x 10-'°ms-'Pa-'.
[0033] In the present invention, the thickness of the electrolyte
may be properly determined. Preferably, however, the thickness of the
electrolyte is about 10 ~m to 100 ~.m from the viewpoint of durability and
the like.
[0034] The electrolyte according to the present invention may be
prepared from a raw material powder of zirconia containing scandia
and/or yttria in solid solution. A raw material powder, which has been
regulated so that the BET value is 0.5 to 20 m2g~' and the particle size
distribution is such that the 3% diameter is not less than 0.1 ~.m, the 97%
2 o diameter is not more than 2 ~.m, and the average particle diameter is
about 0.3 to 1 ~,m, is more preferred from the viewpoint of preparing an
electrolyte which is free from gas permeability and can realize proper
crystal grain diameters. In the present invention, the BET value is
preferably a value as determined by measurement with a flow type
2 5 specific surface area measuring device Flow Sorb II 2300 manufactured
by Shimadzu Seisakusho Ltd. The particle size distribution is preferably
determined by measurement with a laser diffraction-type particle size
distribution measuring device SALD-2000 manufactured by Shimadzu
Seisakusho Ltd. Further, the average particle diameter is preferably a
3o median diameter (50% diameter) value determined by measurement with
a laser diffraction-type particle size distribution measuring device SALD-
2000 manufactured by Shimadzu Seisakusho Ltd.
[0035] The electrolyte may be prepared by any method without
particular limitation. Slurry coating, screen printing, and sheet bonding
35 are preferred from the viewpoints of excellent mass productivity and low
cost.
' . CA 02553074 2006-07-07
1~
[0036] The raw material for the electrolyte may be prepared by
any method without particular limitation so far as yttria and/or scandia
are homogeneously contained in solid solution. However,
coprecipitation is a common method and is preferred.
[0037] In another preferred embodiment of the present invention,
the electrolyte has a multilayer structure of at least two layers. The
multilayer structure may be such that a layer of zirconia containing yttria
in solid solution (YSZ) is provided on the air-side electrode layer reaction
side and a layer of zirconia containing scandia in solid solution (SSZ) is
1 o provided on the fuel electrode side. Alternatively, the multilayer
structure may be such that zirconia containing scandia in solid solution
(SSZ) is provided on the air-side electrode layer reaction side and a
layer of zirconia containing yttria in solid solution (YSZ) is provided on
the fuel electrode side.
[0038] Further, in still another preferred embodiment of the
present invention, the electrolyte have a multilayer structure of at least
three layers. In this case, a layer of SSZ, a layer of YSZ, and a layer of
SSZ may be stacked in that order.
[0039] In a further preferred embodiment of the present invention,
2o in the electrolyte, the proportion ratio of SSZ/YSZ may be varied. For
example, the proportion ratio may be such that, in the electrolyte on its
air electrode side, SSZ/YSZ = 3/1 while, in the electrolyte on its fuel
electrode side, SSZ/YSZ = 1/3. In another embodiment, the proportion
ratio may be varied from the air electrode side toward the fuel electrode
side so that SSZ/YSZ = 3/1, SSZ/YSZ = 1/3, and SSZ/YSZ = 3/1.
SSZ/YSZ = 3/1 represents the molar ratio between scandia and yttria
contained in solid solution in zirconia. For example, 88 mol Zr02-
9Sc203-3Y203 corresponds to this.
[0040] Air electrode
3 o In the present invention, the air electrode is preferably such
that, under an air atmosphere, the electron conductivity is on a high level,
the oxygen gas permeability is on a high level, and the oxygen ion can
be efficiently produced. In the present invention, the air electrode may
be constructed as an air electrode support which maintains the strength
of the cell and has a function as an air electrode.
[0041 ] In the present invention, the air electrode comprises a
'. CA 02553074 2006-07-07
11
perovskite oxide containing at least manganese. In a preferred
embodiment of the present invention, this air electrode is formed of a
lanthanum manganite represented by (La~_xAx)yMn03 wherein A
represents Ca or Sr; x satisfies 0.15 < x < 0.3; and y satisfies 0.97 < y <
1.
[0042] In a preferred embodiment of the present invention, the air
electrode or the air electrode support is formed of a mixed electrically
conductive ceramic material prepared by intimately mixing a perovskite
oxide containing manganese and nickel with an oxide having oxygen ion
1 o conductive properties. A preferred example thereof is a mixture of a
lanthanum manganite represented by (La~_xAx)y(Mn~_ZNiZ)Os wherein A
represents Ca or Sr; x satisfies 0.15 <_ x <_ 0.3; y satisfies 0.97 <_ y <_ 1;
and z satisfies 0.02 <_ z <_ 0.10, with SSZ. The proportion of the
perovskite oxide containing manganese and nickel is preferably 30 to
70% by weight. The air electrode has suitable pore diameter and
porosity from the viewpoints of oxygen and gas permeability. Preferably,
the pore diameter is not less than 0.5 ~,m, and the porosity is not less
than 5%. A composition which can highly suppress the diffusion of
manganese into the electrolyte is more preferred from the viewpoint of
2o improving the durability.
[0043] In a more preferred embodiment of the present invention,
the composition of the perovskite oxide containing manganese and
nickel is represented by (Ln~_XAx)y(Mn~_ZNiZ)03 wherein Ln represents one
or at least two elements selected from the group consisting of Sc, Y, La,
Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu; A
represents Ca or Sr; x satisfies 0.15 < x <_ 0.3; y satisfies 0.97 5 y < 1;
and z satisfies 0.02 <_ z < 0.10. When z is 0.02 s z 5 0.10,
advantageously, the solid solution is highly stable and the suppression of
the diffusion of manganese within the perovskite structure into other
3 o electrode on the highest level. When x satisfies 0.15 < x s 0.3, good
electron conductivity is ensured and, at the same time, oxygen ions can
be efficiently produced. When y satisfies 0.97 _< y s 1, advantageously,
the content of manganese within the perovskite structure may be
rendered proper and the excess lanthanum component absorbs moisture
and consequently is converted to lanthanum hydroxide to effectively
prevent a lowering in stability of the material per se.
CA 02553074 2006-07-07
12
[0044] The oxide having oxygen ion conductivity constituting the
air electrode is preferably at least zirconia-containing oxide, cerium-
containing oxide, and lanthanum gallate oxide. More preferred zirconia-
containing oxides include SSZ, ScYSZ, and YSZ.
[0045] The amount of solid solution of scandia in SSZ as the air
electrode is preferably in the range of 3 to 12% by mole. The total
amount of solid solution of scandia and yttria in ScYSZ is preferably in
the range of 3 to 12% by mole. The amount of solid solution of yttria in
YSZ is in the range of 3 to 12% by mole. When the amount of solid
1 o solution of scandia and yttria is excessive, regarding the crystal phase,
in addition to cubic crystals, rhombohedral crystals are produced,
resulting in lowered oxygen ion conductivity. Further, since scandia and
yttria are expensive materials, dissolution thereof to form a solid solution
to such an extent that the oxygen ion conductivity is low, is
disadvangeously impractical. Accordingly, care should be taken in the
use of scandia and yttria. Further, SSZ and ScYSZ may contain not
more than 5% by mole of at least one oxide selected from Ce02, Sm203,
Gd20s, Yb20s, and Er203 in solid solution from the viewpoint of ensuring
good oxygen ion conductivity.
2 0 [0046] The cerium-containing oxide as the oxide having oxygen
ion conductivity in the air electrode is represented by general formula
(Ce02)~_2x~(J20s)x~ wherein J represents at least one element selected
from Sm, Gd, and Y; and X1 satisfies 0.05 <_ X1 <_ 0.15. This compound
has low sinterability, and, thus, a sintering temperature of 1500°C or
above is necessary for the formation of an electrolyte having no gas
permeability. Due to high temperature sintering, there is a tendency
toward an increase in diffusion of manganese from the manganese-
containing perovskite oxide into the electrolyte. The incorporation of
nickel suppresses the diffusion of manganese into the electrolyte.
3o [0047] The lanthanum gallate oxide as the oxide having oxygen
ion conductivity in the air electrode is preferably represented by general
formula La~_aDaGa~_bEb03 or La~_aDaGa~_b_~EbL~03 wherein D represents
one or at least two of Sr, Ca, and Ba; E represents one or at least two of
Mg, AI, and In; and L represents one or at least two of Co, Fe, Ni, and Cr.
Co-sintering with manganese-containing perovskite oxide causes mutual
dispersion which is likely to facilitate diffusion of manganese. The
CA 02553074 2006-07-07
13
incorporation of nickel can effectively suppress the diffusion of
manganese.
[0048] Fuel electrode
In the present invention, the fuel electrode may be any
conventional one used as the fuel electrode in a solid oxide fuel cell.
Specifically, the fuel electrode that, in a fuel gas atmosphere of the solid
oxide fuel cell, the electron conductivity and fuel gas permeability are on
a high level and a reaction between oxygen ions being moved through
the electrolyte and the fuel gas to give water and carbon dioxide
1 o proceeds with high efficiency, suffices for satisfactory results.
[0049] In a preferred embodiment of the present invention, the fuel
electrode is preferably formed of a sinter of nickel oxide and zirconia.
Nickel oxide is reduced under a fuel gas atmosphere to give nickel which
exhibits catalytic activity and electron conductivity.
25 [0050] In a preferred embodiment of the present invention, the fuel
electrode is preferably formed of nickel oxide and zirconia containing
yttrium in solid solution (NiO/YSZ), because this material has high
electron conductivity and low IR loss. The NiOIYSZ weight ratio is
preferably 50/50 to 90110 from the viewpoints of realizing high electron
2o conductivity and effectively preventing a lowering in durability caused by
aggregation of Ni particles.
[0051 ] In a preferred embodiment of the present invention,
zirconia containing NiO/SSZ and Ni0lcalucium in solid solution
(hereinafter referred to as "NiO/CSZ") may be mentioned as the material
25 for the fuel electrode. Since YSZ is more inexpensive than SSZ, YSZ is
preferred. CSZ is more inexpensive than YSZ, and, thus, NiO/CSZ is
most preferred from the viewpoint of cost. Also for NiO/CSZ, under a
fuel gas atmosphere of a solid oxide fuel cell, NiO/CSZ is converted to
Ni/CSZ.
30 [0052] The raw material for a fuel electrode may be prepared by
any method without particular limitation so far as fuel electrode materials
such as NiO/SSZ and NiO/YSZ are intimately mixed. Examples of such
methods include coprecipitation and spray drying.
[0053] In order to allow a reaction between oxygen ions and fuel
35 gas to efficiently proceed, a fuel-side electrode reaction layer is
preferably provided between the electrolyte and the fuel electrode. The
CA 02553074 2006-07-07
14
fuel-side electrode reaction layer will be described later in detail.
[0054] Air-side electrode reaction layer
In a preferred embodiment of the present invention,
preferably, the provision of an air-side electrode reaction layer between
the air electrode and the electrolyte is preferred from the viewpoint of
accelerating, at the interface of the air electrode and the electrolyte, a
reaction represented by
1 /202 + 2e- --~ 02-.
[0055] In the present invention, preferably, the air-side electrode
1 o reaction layer has high oxygen ion conductivity. The possession of
electron conductivity is more preferred because the above reaction can
be further accelerated. Further, preferred is a material that has a
coefficient of thermal expansion close to the electrolyte, has low
reactivity with the electrolyte and the air electrode, and has good
adhesion.
[0056] From the above viewpoints, in a preferred embodiment of
the present invention, the air-side electrode reaction layer is preferably a
layer formed of an intimate mixture of lanthanum manganite represented
by LaAMn03, wherein A represents Ca or Sr, with SSZ. In a preferred
2 o embodiment of the present invention, for example, from the viewpoints of
electron conductivity and material stability at 700°C or above, the
material has a composition represented by (La~_XAx)yMnOs wherein x and
y satisfy 0.15 <_ x <_ 0.3 and 0.97 <_ y <_ 1. In this composition range, high
electron conductivity can be ensured, the production of lanthanum
hydroxide can be prevented, and a high-output fuel cell can be realized.
[0057] In a preferred embodiment of the present invention, the
lanthanum manganite may contain, in addition to Sr or Ca, Ce, Sm, Gd,
Pr, Nd, Co, AI, Fe, Cr, Ni and the like in solid solution. In particular, a
composition of (La, A)(Mn, Ni)03 containing Ni in solid solution is
3 o preferred, because the production of an insulating layer called lanthanum
zirconate represented by La2Zr207 can be suppressed and, at the same
time, the diffusion of manganese can be suppressed.
[0058] SSZ as the air-side electrode reaction layer in the present
invention may further contain Ce02, Sm203, Gd203, Bi203 or the like in
solid solution in an amount of not more than about 5% by mole. Two or
more of them may be contained in solid solution. The dissolution of
~
. CA 02553074 2006-07-07
these materials in solid solution is preferred because an improvement in
oxygen ion conductivity can be expected. In a preferred embodiment of
the present invention, the amount of solid solution of scandia in SSZ in
the air-side electrode reaction layer in the present invention is preferably
5 about 3 to 12% by mole from the viewpoint of oxygen ion conductivity,
more preferably about 8 to 12 % by mole.
[0059] In a preferred embodiment of the present invention, the air-
side electrode reaction layer is a layer that is formed of an intimate
mixture of lanthanum manganite with SSZ and a cerium oxide
1o represented by general formula (Ce02)~_2x(B20s)x wherein B represents
Sm, Gd or Y; and X satisfies 0,05 <_ X <_ 0.15, and has interconnected
open pores. The presence of cerium oxide can be expected to
suppress the reaction between the air electrode and the electrolyte.
The mixing amount of cerium oxide is preferably about 3 to 30% by
15 weight based on the whole material from the viewpoints of inhibiting the
reaction between the air electrode and the electrolyte and ensuring
adhesion between both the air electrode and the electrolyte.
[0060] In another preferred embodiment of the present invention,
preferably, the air-side electrode reaction layer is formed of a mixed
2o electrically conductive ceramic that comprises a manganese- and nickel-
containing perovskite oxide, and zirconia-containing oxide, cerium oxide,
or a lanthanum- and gallium-containing perovskite oxide and has
interconnected open pores.
[0061 ] The manganese- and nickel-containing perovskite oxide
is preferably represented by (Ln~_xAX)y(Mn~_ZNiZ)03 wherein Ln represents
one or at least two elements selected from the group consisting of Sc, Y,
La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu; A
represents Ca or Sr; x satisfies 0.15 <_ x <_ 0.3; y satisfies 0.97 < y <_ 1;
z
satisfies 0.02 <_ z <_ 0.10.
[0062] The zirconia-containing oxide preferably refers to zirconia
containing scandia in solid solution or zirconia containing scandia and
yttria in solid solution.
[0063] The cerium oxide is preferably represented by formula
(Ce02)~_2x~(J2~s)x, wherein J represents Sm, Gd, or Y; and X1 satisfies
0.05<X1 <0.15.
[0064] In this embodiment, preferably, the content of the
CA 02553074 2006-07-07
16
manganese- and nickel-containing perovskite oxide in the air-side
electrode reaction layer is about 30 to 70% by weight.
[0065] In another preferred embodiment of the present invention,
the air-side electrode reaction layer has a multilayer structure of at least
two layers of a first layer on the air electrode side and a second layer on
the electrolyte side.
[0066] In this embodiment, the first layer is preferably a layer that
is formed of an intimate mixture of an electron conductive oxide with an
oxygen ion conductive oxide and has interconnected open pores.
[0067] The electron conductive oxide preferably has electron
conductivity and is stable in an air atmosphere of a solid oxide fuel cell,
and a specific example thereof is lanthanum manganite containing Sr or
Ca in solid solution. When a low level of diffusion of manganese into
the electrolyte and a high level of electron conductivity are taken into
consideration, more preferred is a lanthanum manganite represented by
(La~_xAx)yMn03 wherein A represents Ca or Sr; x satisfies 0.15 < x < 0.3;
and y satisfies 0.97 <_ y <_ 1. This lanthanum manganite may contain Ce,
Sm, Pr, Nd, Co, AI, Fe, Ni, Cr and the like in solid solution. In particular,
the dissolution of Ni in a solid solution form is preferred. The material
2o containing Ni in solid solution is preferably represented by (La~_XAx)y(Mn~-
ZNiZ)Os wherein A represents Ca or Sr; x satisfies 0.15 <_ x <_ 0.3; y
satisfies 0.97 < y _< 1; and z satisfies 0.02 <_ z <_ 0.10.
[0068] Satisfying the requirement that the oxide has oxygen ion
conductivity and is stable under an air atmosphere of the solid oxide fuel
2 5 cell, suffices for the oxide having oxygen ion conductivity in the first
layer.
Specific examples of such oxides include SSZ, ScYSZ, YSZ, cerium-
containing oxides, perovskite oxides containing at least lanthanum and
gallium (hereinafter referred to as "lanthanum gallate oxide").
[0069] The amount of solid solution of scandia in SSZ as the first
30 layer is preferably in the range of 3 to 12% by mole. The total amount
of solid solution of scandia and yttria in ScYSZ as the first layer is
preferably in the range of 3 to 12% by mole. The amount of solid
solution of yttria in YSZ preferable as the first layer is in the range of 3
to
12% by mole. When the amount of solid solution of scandia or yttria is
35 excessive, regarding the crystal phase, in addition to cubic crystals,
rhombohedral crystals are produced, resulting in lowered oxygen ion
CA 02553074 2006-07-07
conductivity. Further, since scandia and yttria are expensive materials,
dissolution thereof to form a solid solution to such an extent that the
oxygen ion conductivity is low, is disadvantageously impractical.
Accordingly, care should be taken in the use of scandia and yttria.
Further, SSZ and ScYSZ may contain not more than 5% by mole of at
least one oxide selected from CeOz, Sm20s, Gd203, Yb203, and Er203 in
solid solution from the viewpoint of ensuring good oxygen ion
conductivity.
[0070] The cerium-containing oxide as the first layer is
Zo represented by general formula (Ce02)~_Zx~(J2~3)x~ wherein J represents
Sm, Gd, or Y; and X1 satisfies 0.05 <_ X1 <_ 0.15.
[0071 ] The lanthanum gallate oxide as the first layer is preferably
represented by general formula La~_aDaGa~_bEb03 or La~_aDaGa~_b_~EbL~03
wherein D represents one or at least two of Sr, Ca, and Ba; E represents
one or at least two of Mg, AI and In; and L represents one or at least two
of Co, Fe, Ni, and Cr.
[0072] Electron conductive oxides and oxygen ion conductive
oxides, which are preferred as the first layer, have been mentioned
above. Oxides having both electron conductivity and oxygen ion
2o conductivity may also be used. Examples of such oxides include
lanthanum cobaltite oxides which are oxides containing at least
lanthanum and cobalt.
[0073] Preferably, the second layer has at least oxygen ion
conductivity, can suppress the diffusion of the manganese component
into the electrolyte, and has interconnected open pores.
[0074] The reason why the second layer preferably has at least
oxygen ion conductivity is for efficiently supplying oxygen ions
considered to be produced mainly in the first layer to the electrolyte.
Further, the reason why the second layer preferably has the effect of
3o suppressing the diffusion of the manganese component into the
electrolyte is that the development of electron conductivity in the
electrolyte can be suppressed and, further, a lowering in adhesion
between the electrolyte and the fuel electrode caused by the occurrence
of such a phenomenon that the size of particles on the fuel electrode-
side surface of the electrolyte is rendered excessively large by improved
sinterability, can be suppressed. The reason why the second layer
CA 02553074 2006-07-07
18
preferably has interconnected open pores is that, when there is no gas
permeability, the manganese component diffused from the first layer and
the air electrode is disadvantageously diffused with high efficiency. The
point of control of the diffusion amount of manganese resides in the
microstructure in the second layer, and the optimization of pore diameter,
porosity, and thickness is particularly important. The pore diameter is
preferably 0.1 to 10 Vim, the porosity is preferably 3 to 40%, and the
thickness is preferably 5 to 50 p.m.
[0075] In this embodiment, for the above reason, the second layer
1 o is preferably formed of a material that has high oxygen ion conductivity
but not have high sinterability, that is, a material that is less likely to
diffuse manganese into the electrolyte. Further, a material having the
effect of absorbing manganese diffused from the air electrode is
preferred. From this viewpoint, SSZ and cerium-containing oxides may
be mentioned as representative examples of such materials. The
utilization of ScYSZ is also preferred from the viewpoint of improving the
adhesion between the first layer and the electrolyte although the
sinterability is higher than that of SSZ. The reason why the second
layer preferably has the effect of absorbing manganese diffused from the
2 o air electrode is that the entry of manganese in the second layer leads to
the development of electron conductivity in the second layer that, as with
the first layer, can realize the production of oxygen ions. This
embodiment can be an advantageous embodiment of the present
invention in that, by virtue of this effect, higher output performance can
be realized.
[0076j fn this embodiment, SSZ and cerium-containing oxide as
the second layer may be the same as described above in connection
with the first layer. Further, ScYSZ may be the same as described
above in connection with the first layer. Preferably, however, the
3 o proportion of scandia to the total amount of scandia and yttria in ScYSZ
is not less than 20% by mole. When the amount of scandia is
excessively small, the effect of suppressing the dispersion of manganese
is reduced. Further, ScYSZ may contain not more than 5% by mole of
at least one oxide selected from Ce02, Sm203, Gd203, Yb203, and Er203
in solid solution.
[0077] Accordingly, embodiments of the present invention in which
CA 02553074 2006-07-07
19
the air-side electrode reaction layer has a two-layer structure include
an embodiment in which the first layer is formed of a
mixture of a manganese-containing perovskite oxide with zirconia
containing scandia andlor yttria in solid solution and has interconnected
open pores and the second layer is formed of zirconia containing scandia
in solid solution and has larger porosity than the electrolyte,
an embodiment in which the first layer is formed of a
mixture of a manganese-containing perovskite oxide with a cerium-
containing oxide and has interconnected open pores, and the second
layer is formed of zirconia containing scandia in solid solution and has a
larger porosity than the electrolyte,
an embodiment in which the first layer is formed of a
mixture of a manganese-containing perovskite oxide with a lanthanum-
and gallium-containing perovskite oxide and has interconnected open
pores, and the second layer is formed of zirconia containing scandia in
solid solution and has larger porosity than the electrolyte,
an embodiment in which the first layer is formed of a
lanthanum- and cobalt-containing perovskite oxide and has
interconnected open pores, and the second layer is formed of zirconia
2o containing scandia in solid solution and has larger porosity than the
electrolyte, and
an embodiment in which the first layer is formed of a
mixture of a manganese-containing perovskite oxide with zirconia
containing scandia andlor yttria in solid solution and has interconnected
open pores, and the second layer is formed of cerium oxide and has
larger porosity than the electrolye.
[0078] Further, in a preferred embodiment of the present invention,
in the embodiment in which the air-side electrode reaction layer has a
two-layer structure, the following relationship is preferably satisfied: d1 >
3o d2 > d3 wherein d1 represents the diameter of pores in the air electrode;
d2 represents the diameter of pores in the first layer; and d3 represents
the diameter of pores in the second layer, from the viewpoint of realizing
a fuel cell possessing excellent output performance.
[0079] According to another preferred embodiment of the present
invention, in the embodiment in which the air-side electrode reaction
layer has a two-layer structure, the following relationship is preferably
CA 02553074 2006-07-07
satisfied: a1 >_ a2 <_ a3 > a4 wherein a1 represents the porosity of the air
electrode; a2 represents the porosity of the first layer; a3 represents the
porosity of the second layer; and a4 represents the porosity of the
electrolyte.
5 [0080] The thickness of the first layer and the thickness of the
second layer may be properly determined. Preferably, the thickness of
the second layer is 5 to 50 Vim, and the thickness of the first layer is 5 to
50 pm.
(0081 ] Porous IaYer
1 o In a preferred embodiment of the present invention, a
porous layer is provided between the fuel electrode and the electrolyte.
In the present invention, this porous layer is formed of a zirconia-
containing fluorite oxide, has a thickness of 5 to 40 p,m, and has a larger
porosity than the electrolyte. As described above, the present invention
15 is characterized in that the content of manganese in the porous layer in
its surface on the fuel electrode side is 0.3 to 4% by weight.
[0082] Further, in a preferred embodiment of the present invention,
the content of the manganese component in the porous layer in its
surface on the fuel electrode side is preferably 0.6 to 3.5% by weight,
2 o more preferably 0.9 to 3% by weight.
[0083] In the present invention, this porous layer can suppress the
diffusion of manganese into the fuel electrode and, at the same time, has
the function of efficiently moving oxygen ions being moved through the
electrolyte into the fuel electrode. From this viewpoint, preferably, the
porous layer has high oxygen ion conductivity. The regulation of the
thickness of the porous layer is also important for preventing the
diffusion of manganese from the electrolyte into the fuel electrode and
for preventing a deterioration in output performance caused by
resistance of the material per se. In a preferred embodiment of the
3 0 present invention, the thickness of the porous layer is preferably 5 to 40
p.m. Further, regarding the porous layer, from the viewpoints of output
performance and durability, the porosity is preferably 3 to 30%, and the
pore diameter is preferably about 0.05 to 2 p,m. On the other hand, the
porous layer is preferably free from any hole which extends from the fuel
electrode side to the electrolyte, from the viewpoint of preventing H2 gas
from being transferred from the fuel electrode side and reaching the
CA 02553074 2006-07-07
21
electrolyte surface.
[0084] In a preferred embodiment of the present invention,
preferably, the following relationship is satisfied: a1 < a2 < a3 Wherein a1
represents the porosity of the electrolyte; a2 represents the porosity of
the porous layer formed of the fluorite oxide; and a3 represents the
porosity of the fuel electrode.
(0085] In a preferred embodiment of the present invention, the
zirconia-containing fluorite oxide constituting the porous layer is stable
under a fuel gas atmosphere of the solid oxide fuel cell and has high
oxygen ion conductivity. Preferred are SSZ, ScYSZ, and YSZ. These
SSZ, ScYSZ and YSZ may be the same as those for constituting the air-
side electrode reaction layer except for physical properties required of
the porous layer. This is true of the preferred embodiment.
[0086] Fuel-side electrode reaction layer
In a preferred embodiment of the present invention, the
provision of a fuel-side electrode reaction layer between the electrolyte
and the fuel electrode is preferred from the viewpoints of an efficient
reaction in the fuel electrode and improving output performance. In the
present invention, since the fuel-side electrode reaction layer is one
2o embodiment of the fuel electrode, in an embodiment in which a fuel-side
electrode reaction layer is provided, the term "layer in contact with the
fuel electrode" refers to a layer in contact with the fuel-side electrode
reaction layer.
[0087] In the present invention, preferably, the fuel-side electrode
reaction layer is formed of Ni0/SSZ or Ni/SSZ which is excellent in both
electron conductivity and oxygen ion conductivity. Here Ni0 is reduced
under a fuel atmosphere to Ni, and the fuel-side electrode reaction layer
is formed of Ni/SSZ.
[0088] In a preferred embodiment of the present invention, the
3o ratio of Ni0/SSZ is 10/90 to 50/50 in terms of weight ratio because good
electron conductivity and oxygen ion conductivity can be realized.
(0089] The amount of solid solution of scandia in SSZ constituting
the fuel-side electrode reaction layer is preferably about 3 to 12% by
mole, because the oxygen ion conductivity is high and the reaction in the
fuel electrode can be accelerated. SSZ may further contain one or at
least two of Ce02, Sm203, Gd203, and Bi203 in solid solution in an
CA 02553074 2006-07-07
22
amount of not more than 5% by mole. By virtue of the dissolution of
these oxides in a solid solution, not only an improvement in oxygen ion
conductivity under a fuel gas atmosphere but also an improvement in
electron conductivity can be expected.
[0090] In a preferred embodiment of the present invention, a layer
formed of a mixture prepared by intimately mixing NiO, SSZ and cerium
oxide together at a predetermined weight ratio (hereinafter referred to as
"NiOISSZ/cerium oxide) is preferred as the fuel-side electrode reaction
layer. This layer is advantageous in that, under a fuel gas atmosphere,
1 o the oxygen ion conductivity is high and the electron conductivity is high.
Ni0 is reduced under a fuel gas atmosphere to Ni, and, consequently,
this layer is formed of Ni/SSZ/cerium oxide. The cerium oxide is not
particularly limited so far as the oxide contains cerium. However, a
cerium oxide represented by general formula (CeO2)1-2x(8203)x, wherein
B represents Sm, Gd or Y; and X satisfies 0.05 <_ X <_ 0.15, is preferred
from the viewpoint of realizing high oxygen ion conductivity.
[0091 ] Interconnector
In an air atmosphere and a fuel gas atmosphere at a power
generation temperature in a solid oxide fuel cell, preferably, the
2o interconnector provided in the solid oxide fuel cell according to the
present invention has high electron conductivity, has no gas permeability,
and is stable against a redox atmosphere. Lanthanum chromite is
preferred from this viewpoint.
[0092] Lanthanum chromite is resistant to sintering, and, hence, it
is difficult to prepare a gas permeability-free interconnector at a firing
temperature (1500°C or below) of the solid oxide fuel cell. In order to
improve the sinterability, Ca, Sr, or Mg is prferably used in solid solution.
The dissolution of Ca in a solid solution form is preferred, because the
sinterability is the highest, and a film having no gas permeability can be
3 o prepared at substantially the same temperature as that used in sintering
of other electrode such as the electrolyte in the solid oxide fuel cell.
[0093] The electron conductivity enhances with increasing the
amount of solid solution of lanthanum chromite containing Ca in solid
solution used in the interconnector. In this case, however, since there
is a fear of causing a lowering in stability of the material, the amount of
solid solution is preferably about 10 to 40% by mole.
CA 02553074 2006-07-07
23
[0094] In a preferred embodiment of the present invention, a
precoat layer, which has a composition represented by (La~_XAx)yMn03,
wherein A represents Sr or Ca; x satisfies 0.15 <_ x < 0.3; and y satisfies
0.97 <_ y <_ 1, and is dense, may be provided between the air electrode
and the interconnector. Advantageously, this precoat layer can
effectively suppress fihe diffusion of a calcium chromite component as a
sintering aid component for lanthanum chromite containing Ca in solid
solution into the air electrode. The dense precoat layer is evaluated in
terms of the amount of permeated gas determined by providing a
1 o pressure difference between one side of the precoat layer and the
opposite side of the precoat layer and measuring the amount of gas
permeated through the precoat layer. The dense precoat layer
preferably refers to one having a gas permeability Q < 1.4 x 10-7ms-' Pa-'
or more.
[0095] When the solid oxide fuel cell is in a flat plate form, the
interconnector is called "separator," and the function of the separator is
the same as that of the interconnector. The separator may be formed of
a heat resistant metal such as ferrite stainless steel.
[0096] Production~rocess of solid oxide fuel cell
2 o The solid oxide fuel cell according to the present invention
may be produced by an appropriate production process while taking into
consideration the shape of the solid oxide fuel cell and the like. A
cylindrical solid oxide fuel cell as shown in Fig. 1 can be produced as
follows.
[0097] An air electrode site which serves as a support is first
prepared as a high-strength and porous air electrode support by mixing a
perovskite oxide containing at least manganese and other components)
as raw materials preferably with a binder, extruding this mixture,
removing the binder from the extrudate at a temperature of about 300 to
500°C, and then firing the heat treated extrudate at about 1400 to
1500°C. A suspension firing method and a lateral sintering method may
be mentioned as the sintering method. The lateral sintering is preferred.
[0098) Subsequently, an air-side electrode reaction layer, an
electrolyte, an interconnector, and a fuel electrode are formed in a film
form onto the surface of the air electrode support. These electrodes
are preferably formed by a wet method from the viewpoint of cost. Wet
CA 02553074 2006-07-07
24
methods include a dipping method in which a slurry is prepared from a
raw material powder, a binder, and a solvent followed by dipping in this
slurry, to prepare the electrode, a screen printing method in which a film
is formed using paste having higher viscosity than slurry through a
screen, and a sheet bonding method in which a film sheeted on another
base material such as a PET film is applied onto the surface of the cell.
The production process may be properly selected depending upon the
shape of a part on which the film is to be formed. In the case of a
cylindrical cell shown in Fig. 1, the air-side electrode reaction layer and
1 o the electrolyte are preferably formed by the dipping method. On the
other hand, the interconnector and the fuel electrode are preferably
formed by a screen printing method or a sheet bonding method which is
a masking-free method.
[0099] Preferably, the cell formed in a film form by the above
method is heat treated at a temperature of about 300 to 500°C to remove
the binder, and the cell is then fired at a temperature below the firing
temperature of the air electrode support and in the range of about 1300
to 1500°C. Firing may be carried out by any of a successive firing
method in which individual layers are fired separately from each other,
2 o and a co-firing method in which a few layers are simultaneously fired.
The co-firing method is preferred from the viewpoint of cost. In the
present invention using a perovskite oxide containing at least
manganese as an air electrode support, there is a fear that the output
performance is significantly lowered by the diffusion of manganese. For
this reason, in some cases, the successive firing method is preferred.
[0100] Co-firing with the air electrode molded product is also
possible. In the case of firing of the air electrode support, firing is
carried out at a temperature above other electrode(s). Therefore, when
the diffusion of manganese is taken into consideration, successive firing
3 o is preferred.
[EXAMPLES]
[0101 ] The following Examples further illustrate the present
invention. However, it should be noted that the present invention is not
limited to these examples.
In the Examples, various properties, performance and the
like were determined by the following testing methods.
CA 02553074 2006-07-07
[0102] Measurement of diameter of crystal Grains on surface of
electrolyte film
The surface of the electrolyte film was observed under
SEM (S-4100, manufactured by Hitachi, Ltd.) to photograph the surface
5 of the electrolyte on its fuel electrode side at a magnification of 300
times.
The grain size distribution was determined by calculation using the
photograph by a planimetric method. Further, the average crystal grain
diameter was also determined. Specifically, a known circle having an
area (A) is drawn on the photograph, and the number of grains N~ per
1 o unit area is determined from the number of grains n~ present within the
circle and the number of grains n; present on the circumference by the
following equation:
N~ _ (n~ + 1/2n;)I(AIm2)
wherein m represents the magnification of the photograph.
15 Since 1 /N~ represents the area occupied by one particle, when the
crystal grain diameter is an equivalent circle diameter, 2/~l(~N~) may be
adopted for the determination, while, in the case of one side of square,
1l~No may be adopted.
In the grain size distribution in the film surface, 3%
2 o diameter of crystal grains in the electrolyte refers to the grain diameter
corresponding to the third smallest grain, when the crystal grain diameter
of 100 crystal grains is measured by a planimetric method and the grains
are placed in diameter ascending order, and 97% diameter refers to the
grain diameter corresponding to the 97th smallest grain. In the
25 measurement, upon sintering, even though particles were seen as if they
were bonded to one another, when a grain boundary was observed, they
were regarded as separate grains.
[0103] Gas leakage test
Before the power generation test, nitrogen gas was allowed
3o to flow into the air electrode support, and a pressure of 0.1 MPG was
applied from within the air electrode to determine the amount of gas
permeated through the electrolyte, and, based on the results, whether or
not the electrolyte is gas permeable was determined.
(0104] Power Generation test
A power generation test was carried out using the prepared
cell (fuel electrode effective area: 150 cm2). The power generation test
CA 02553074 2006-07-07
26
was carried out under the following operating conditions.
Fuel: (H2 + 11 % H20) : N2 = 1 : 2
Oxidizing agent: Air
Power generation temp.: 800°C
Current density: 0.3 Acm-2
[0105] Durabilit~r test
The cell was held under the same conditions as that in the
power generation test for 1000 hr. Thereafter, in such a state that the
current density was lowered to 0 A cm-2, the temperature was lowered to
1 o room temperature. The temperature was then again raised to 800°C,
and the cell was held under the same conditions for 500 hr. In such a
state that the current density was again reduced to 0 A cm-2, the
temperature was lowered to room temperature. Thereafter, the
temperature was raised to 800°C, and the cell was held under the same
conditions for 500 hr. Thus, a 2000-hr (total) durability test was carried
out including two heat cycles.
[0106] Compositional analysis of electro~te surface
The content of manganese in the electrolyte in its surface
on the fuel electrode side was determined for a cell prepared in the same
2 o manner as in the cell for the power generation test. The content of
manganese was measured with a Shimadzu electron probe micro
analyzer EPMA-8705 manufactured by Shimadzu Seisakusho Ltd. under
the following measurement conditions.
Acceleration voltage: 15 kW
Irradiation current quantity: 50 nA
Analyzing crystal: LiF
Analytical line: MnKa line (2.103 angstroms)
[0107] Porosity
The cell was cut, and the cut surface from the air electrode
3o toward the fuel electrode was polished for planishing. An SEM
photograph of the section from the electrolyte to the fuel electrode part
was taken, and a pore part and a particle part were traced using a
different color for each part on a transparent film. The film
distinguished by different colors was subjected to image processing to
calculate the proportion of the pore part to determine the porosity.
[0108] Pore diameter
CA 02553074 2006-07-07
27
The pore diameter was determined by the foilawing method.
The cell was cut, and the cut surface from the air electrode toward the
fuel electrode was polished for planishing. An SEM photograph of the
section from the air electrode to the electrode reaction layer part was
taken, and a pore part and a particle part were traced using a different
color for each part on a transparent film. The size of the pore part was
measured. For example, when the pore is an equivalent circle, the
diameter thereof is regarded as a pore diameter while, in an equivalent
square, the length of one side is regarded as a pore diameter. The pore
1 o diameter of 0.1 to 10 p,m refers to the 50th pore diameter in the range of
3rd to 97th pore diameter, when the diameter of 100 pores is measured
by the above method and the diameters are placed in ascending order.
That is, the pore diameter of 0.1 to 10 ~,m means that the diameter
corresponding to 50% diameter in pore diameters in the range of 3%
diameter to 97% diameter is 0.1 to 10 um.
[0109] Example A1: Fuel cell wherein electrolyte is layer formed of
SSZ
Example A1-1
(1 ) Preparation of air electrode support
2 o Lanthanum manganite containing Sr in solid solution and
having a composition represented by Lao,~SSro,25Mn03 was used as an
air electrode. After preparation by coprecipitation, heat treatment was
carried out to prepare a raw material powder for an air electrode. The
average particle diameter was 30 Vim. A cylindrical molded product was
, prepared by extrusion, and the molded product was then fired at
1500°C
to prepare an air electrode support.
(0110] (2) Preparation of air-side electrode reaction layer
Lao,75Sro.25Mno.95Nio.o54s/90 mol% Zr02-10 mol% Sc203 =
50/50 was used as an air-side electrode reaction layer. An aqueous
3 o solution of nitrate of La, an aqueous solution of nitrate of Sr, an
aqueous
solution of nitrate of Mn, an aqueous solution of nitrate of Ni, an aqueous
solution of nitrate of Zr, and an aqueous solution of nitrate of Sc were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
a regulated particle diameter. The average particle diameter was 2 ~.m.
CA 02553074 2006-07-07
28
This powder (40 parts by weight) was mixed With 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 100 mPas. The slurry was coated onto the air electrode
support (outer diameter 15 mm, wall thickness 1.5 mm, effective length
400 mm) to form a coating which was then sintered at 1400°C. The
sinter had a thickness of 20 ~,m.
[0111 ] (3) Preparation of slurry for electrolyte:
The electrolyte comprised 90 mol% Zr02-10 mol% Sc203.
An aqueous solution of nitrate of Zr and an aqueous solution of nitrate of
Sc were provided and were mixed with each other so as to give the
above composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
a regulated particle diameter. The average particle diameter was 0.5
~.m. This powder (40 parts by weight) was mixed with 100 parts by
weight of a solvent (ethanol), 2 parts by weight of a binder
(ethylcellulose), 1 part by weight of a dispersant (polyoxyethylene
2 o alkylphosphate), and 1 part by weight of an antifoaming agent (sorbitan
sesquioleate). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 140 mPas.
(0112] (4) Preparation of electrolyte
The slurry prepared above was coated onto the air-side
electrode reaction layer to form a coating which was then fired at
1400°C.
The thickness of the electrolyte thus formed was 30 ~.m. In this case,
the air electrode support in its part on which an interconnector film is to
be formed in a later step was masked so as not to be coated.
[0113] (5) Preparation of slurry for fuel-side electrode reaction
3 0 layer
The fuel-side electrode reaction layer comprised Ni0/90
mol% Zr02-10 mol% Sc2O3. An aqueous solution of nitrate of Ni, an
aqueous solution of nitrate of Zr, and an aqueous solution of nitrate of Sc
were provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was further carried out, and the particle diameter was
CA 02553074 2006-07-07
29
regulated to prepare a raw material. In this case, two types of
compositions for the fuel-side electrode reaction layer, that is, Ni0/90
mol% Zr02-10 mol% Sc203 = 20/80 and 50/50 were prepared. For both
the cases, the average particle diameter was 0.5 pm. This powder (100
parts by weight) was mixed with 500 parts by weight of an organic
solvent (ethanol), 10 parts by weight of a binder (ethylcellulose), 5 parts
by weight of a dispersant (polyoxyethylene alkylphosphate), 1 part by
weight of an antifoaming agent (sorbitan sesquioleate), and 5 parts by
weight of a plasticizes (DBP). The mixture was then thoroughly stirred
1 o to prepare a slurry. This slurry had a viscosity of 70 mPas.
[0114] (6) Preparation of slurry for fuel electrode
The fuel electrode comprised Ni0/90 mol% Zr02-10 mol%
Y203 = 70/30. An aqueous solution of nitrate of Ni, an aqueous solution
of nitrate of Zr, and an aqueous solution of nitrate of Y were provided
and were mixed with each other so as to give the above composition,
followed by coprecipitation with oxalic acid. Heat treatment was further
carried out, and the particle diameter was regulated to prepare a raw
material. The average particle diameter was 2 Vim. This powder (100
parts by weight) was mixed with 500 parts by weight of an organic
2o solvent (ethanol), 20 parts by weight of a binder (ethylcellulose), 5 parts
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate), and 5 parts by
weight of a plasticizes (DBP). The mixture was then thoroughly stirred
to prepare a slurry. This slurry had a viscosity of 250 mPas.
[0115] (7) Preparation of fuel electrode
The electrolyate prepared in the above step (4) was
masked so that the effective area was 150 cm2. The slurries for a fuel-
side electrode reaction layer were first coated on the electrolyte in the
order of Ni0/90 mol% ZrOz-10 mol% Sc20s (average particle diameter) _
20180 (0.5 ~.m) and 50/50 (0.5 ~.m). The film thickness (after firing) was
10 p.m. The slurry for a fuel electrode was coated thereon. The film
thickness (after firing) was 90 ~.m. The assembly was further fired at
1400°C.
[0116] (8) Preparation of interconnector:
The interconnector had a composition of lanthanum
chromite containing Ca in solid solution represented by Lao,BOCao.2oCrOs.
". CA 02553074 2006-07-07
A raw material powder was prepared by spray pyrolysis and was then
heat treated. The average particle diameter was 1 pm. This powder
(40 parts by weight) was mixed with 100 parts by weight of a solvent
(ethanol), 2 parts by weight of a binder ~ethylcellulose), 1 part by weight
5 of a dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of
an antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. An interconnector was formed by slurry coating using this slurry
and was then fired at 1400°C. The thickness of the interconnector after
1 o firing was 40 ~,m.
[0117] Exam~ale A1-2
A solid oxide fuel cell was prepared in the same manner
as in Example 1, except that the electrolyte firing temperature was
1360°C.
15 [0118] Example A1-3
A fuel cell was prepared in the same manner as in
Example 1, except that the electrolyte firing temperature was
1380°C.
[0119] Example A1-4
A fuel cell was prepared in the same manner as in
2 o Example 1, except that the electrolyte firing temperature was
1420°C.
[0120] ExampIeA1-5
A fuel cell was prepared in the same manner as in
Example 1, except that the electrolyte firing temperature was
1440°C.
[0121] Comparative Exam~leA1-1
25 A fuel cell was prepared in the same manner as in
Example 1, except that the electrolyte firing temperature was
1340°C.
[0122] Comparative Example A1-2
A fuel cell was prepared in the same manner as in
Example 1, except that the electrolyte firing temperature was
1460°C.
30 [0123] Example 2: Fuel cell in which electrolyte is layer of YSZ
Example A2-1
A fuel cell was prepared in the same manner as in
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-10 mol% Y2O3.
[0124] Example A2-2
A fuel cell was prepared in the same manner as in
CA 02553074 2006-07-07
31
Example A1-1,except that the electrolyte had
a composition of 90 mol%
Zr02-10 mol% Y203 and the firing temperature electrolyte
of the was
1350C.
[0125] Example
A2-3
A fuel cell was prepared in the manner as
same in
Example A1-1,except that the electrolyte had
a composition of 90 mol%
Zr02-10 moi% Y203 and the firing temperature electrolyte
of the was
1380C.
[0126] Example
A2-4
1 A fuel cell was prepared in the manner as
o same in
Example A1-1,except that the electrolyte had tion of 90
a composi mol%
Zr02-10 mol% Y203 and the firing temperature electrolyte
of the was
1410C.
[0127] Exam~~le
A2-5
A fuel cell was prepared in the manner as
same in
Example A1-1,except that the electrolyte had tion of 90
a composi mol%
Zr02-10 mol% Y2O3 and the firing temperature electrolyte
of the was
1420C.
[0128] Comparative
Example A2-1
2 A fuel cell was prepared in the manner as
o same in
Example A1-1,except that the electrolyte had tion of 90
a composi mol%
Zr02-10 mol% Y203 and the firing temperature electrolyte
of the was
1330C.
[0129] Comparative
Example A2-2
A fuel cell was prepared in the manner as
same in
Example A1-1,except that the electrolyte had tion of 90
a composi mol%
Zr02-10 mol% Y203 and the firing temperature electrolyte
of the was
1440C.
[0130] Examale te is layer
3: Fuel cell of
in which
electroy
SSZ/YSZ
Example A3-1
A fuel cell was prepared in the same manner as in
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-5 mol% Sc203-5 mol% Y203.
[0131] ExampIeA3-2
A fuel cell was prepared in the same manner as in
CA 02553074 2006-07-07
32
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-5 mol% Sc203-5 mol% Y203 and the firing temperature of the
electrolyte was1350°C.
[0132] Example A3-3
A fuel cell was prepared in the same manner as in
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-5 mol% Sc203-5 mol% Y203 and, the firing temperature of the
electrolyte was1380°C.
[0133] Example A3-4
1 o A fuel cell was prepared in the same manner as in
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-5 mol% Sc203-5 mol% Y203 and the firing temperature of the
electrolyte was1420°C.
[0134] Example A3-5
A fuel cell was prepared in the same manner as in
Example A1-1, except that the electrolyte had a composition of 90 mol%
Zr02-5 mol% Sc203-5 mol% Y203 and the firing temperature of the
electrolyte was1430°C.
[0135] The measurement of particle size distribution, a gas
leakage test, a power generation test, and a durability test were carried
out for the fuel cells thus obtained. The results were as shown in tables
bel ow.
[Table 1 ]
3% 97% Average Mn content,Gas perme-
diameter,diametercrystal wt% ability,
grain x 10''
m m diameter, ms~'Pa~'
m
Exam 1e 3 8 5 D.9 3.5
A1-1
Exam 1e 3 5 4 0.3 25.5
A1-2
Exam 1e 3 7 4.5 0.6 12.7
A1-3
Exam 1e 3 12 7.5 1.5 3.0
A1-4
Exam 1e 4 20 12 2.9 3.7
A1-5
Comparative1 4
2 0.1 320
Exam 1e
A1-1
Comparative5 26 15 4 5
3 5
Exam 1e . .
A1-2
2 5 [Table 2]
Initial After After After 2000
potential, 1000 1500 hr,
V hr, V hr, V V
Exam 1e A1-10.67 0.67 0.67 0.67
Exam 1e A1-20.65 0.65 0.65 0.65
Exam 1e A1-30.66 0.66 0.66 0.66
Exam 1e A1-40.67 0.67 0.67 0.67
CA 02553074 2006-07-07
33
[Table 3]
Exam 1e 0.66 0.66 0.66 0.66
A1-5
Comparative
0.45 0.44 43 0.42
0
Example .
A1-1
Comparative
0.64 0.64 0.63 0.62
Exam 1e
A1-2
3% 97% Average Mn Gas perme-
diameterdiametercrystal content,ability,
grain x 10''
m m diameter,wt% ms''Pa'
m
Exam 1e 3 13 7 1.3 1.5
A2-1
Exam 1e 3 5 4 0.5 3.1
A2-2
Exam 1e 3 8 5 0.9 2.2
A2-3
Exam 1e 4 16 9 2.5 0.8
A2-4
_
Exam 1e 5 20 12 4.0 0.9
A2-5
Comparative
Exam 1e 2 4 2.5 0.2 175
A2-1
Comparative
5 28 17 5 1
0 1
Exam 1e . .
A2-2
[Table 4]
Initial After 1000After 1500 After 2000
otential hr, hr, hr,
V V V V
Exam 1e 0.58 0.58 0.58 0.58
A2-1
Example 0.57 0.57 0.57 0.57
A2-2
Exam 1e 0.58 0.58 0.58 0.58
A2-3
Exam 1e 0.58 0.58 0.58 0.58
A2-4
Exam 1e 0.57 0.57 0.57 O.S7
A2-5
Comparative _
Exam 1e 0.42 0.41 0.40 0.39
A2-1
Comparative
Exam 1e 0.56 0.56 0.55 0.54
A2-2
[Table 5]
3% 97% Average Mn content,Gas perme-
diameter,diameter,crystal wt% ability,
grain x 10''
m m diameter, ms''Pa~'
m
Exam 1e 3 12 6 1.1 0.7
A3-1
Exam 1e 3 6 3.5 0.5 20
A3-2
Exam 1e 3 8 4.7 0.9 3.5
A3-3
Exam 1e 3 16 9 2.5 0.7
A3-4
Exam 1e 4 20 11 3.7 0.6
A3-5
Comparative
Exam 1e 2 4 2.3 0.2 280
A3-1
Comparative
Exam 1e 4 28 14 4.5 1. ~
A3-2
[Table 6]
Initial potential,After After After
V 1000 1500 2000
hr,
hr V hr, V V
Exam 1e 0.68 0.68 0.68 0.68
A3-1
Exam 1e 0.66 0.66 0.66 0.66
A3-2
CA 02553074 2006-07-07
34
Exam 1e 0.67 0.67 0.67 0.67
A3-3
Example 0.68 0.68 0.68 0.68
A3-4
Exam 1e 0.67 0.67 0.67 0.67
A3-5
Comparative
0.46 0.45 0.44 0.43
Exam 1e
A3-1
Comparative
0.66 0.66 0.65 0.64
Exam 1e
A3-2
[0136] Example A4: Fuel cell in which layer formed of SSZ on air
electrode side and layer formed of YSZ on fuel electrode side were
provided as electrolyte.
Example A4-1
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
1 o and the assembly was then fired at 1400°C. The thickness of the
electrolyte was 30 ~,m (layer formed of SSZ: 15 p,m, layer formed of YSZ:
p,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0137] Example A4-2
15 A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
and the assembly was then fired at 1350°C. The thickness of the
2 o electrolyte was 30 ~.m (layer formed of SSZ: 15 ~.m, layer formed of YSZ:
15 p.m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0138] Example A4-3
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Yz03 was formed on this layer by slurry coating,
and the assembly was then fired at 1380°C. The thickness of the
electrolyte was 30 ~.m (layer formed of SSZ: 15 ~,m, layer formed of YSZ:
3o 15 ~,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
CA 02553074 2006-07-07
[0139] Example A4-4
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
5 90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
and the assembly was then fired at 1415°C. The thickness of the
electrolyte was 30 p,m (layer formed of SSZ: 15 pm, layer formed of YSZ:
15 ~.m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
10 [0140] Examale A4-5
A layer formed of SSZ having a composition of 90 mol%
ZrOz-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
15 and the assembly was then sintered at 1425°C. The thickness of the
electrolyte was 30 p,m (layer formed of SSZ: 15 p.m, layer formed of YSZ:
15 ~,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0141 ] Comparative Example A4-1
2o A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
and the assembly was then fired at 1330°C. The thickness of the
25 electrolyte was 30 p,m (layer formed of SSZ: 15 p.m, layer formed of YSZ:
15 ~.m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0142] Comparative Example A4-2
A layer formed of SSZ having a composition of 90 mol%
3o Zr02-10 mol% Sc20s was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating,
and the assembly was then sintered at 1440°C. The thickness of the
electrolyte was 30 p.m (layer formed of SSZ: 15 p,m, layer formed of YSZ:
35 15 p,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
CA 02553074 2006-07-07
36
[0143) The measurement of particle size distribution, a gas
leakage test, a power generation test, and a durability test were carried
out for the fuel cells thus obtained. The results were as shown in tables
below.
[Table 7)
3~ 97% Average Mn content,Gas perme-
diameter,diametercrystal wt% ability,
grain x 10''
m m diameter ms''Pa''
m
Exam 1e 3 12 7 1.2 0.6
A4-1
Exam 1e 3 6 4 0.3 13
A4-2
Example 3 9 5 0.9 2,7
A4-3
Exam 1e 4 16 9 2.6 0.6
A4-4
Exam 1e 4 20 11 4.0 0.7
A4-5
Comparative
2 3 2.3 0 140
2
Exam 1e .
A4-1
Comparative
5 28 15 4.7 1.1
Exam 1e
A4-2
[Table 8)
Initial After 1000 After 1500After 2000
otential, hr, V hr, hr,
V V V
Exam 1e 0_.6_8 0.68 0.68 0.68
A4-1
Examle A4-20.67 0.67 0.67 0.67
Exam 1e 0.68 0.68 0.68 0.68
A4-3
Exam 1e 0.68 0.68 0.68 0.68
A4-4
Example 0.67 0.67 0.67 0.67
A4-5
Comparative
Exam 1e 0.46 0.45 0.44 0.43
A4-1
Comparative
Exam 1e 0.66 0.65 0.65 0.64
A4-2
[0144) Example A5: Fuel cell in which layer formed of YSZ on air
1 o electrode side and layer formed of SSZ on fuel electrode side were
provided as electrolyte.
Example A5-1
A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1400°C. The thickness of the
electrolyte was 30 ~m (layer formed of YSZ: 15 ~.m, layer formed of SSZ:
15 p.m). A fuel cell was prepared in the same manner as in Example
2 o A1-1 except for the above matter.
[0145] Example A5-2
CA 02553074 2006-07-07
37
A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc20s was formed on this layer by slurry coating,
and the assembly was then fired at 1350°C. The thickness of the
electrolyte was 30 ~m (layer formed of YSZ: 15 p,m, layer formed of SSZ:
~.m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0146] Example A5-3
1 o A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1380°C. The thickness of the
15 electrolyte was 30 ~.m (layer formed of YSZ: 15 p,m, layer formed of SSZ:
15 p,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0147] Example A5-4
A layer formed of YSZ having a composition of 90 mol%
2o Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1420°C. The thickness of the
electrolyte was 30 pm (layer formed of YSZ: 15 p,m, layer formed of SSZ:
15 ~,m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0148) Exam~~le A5-5
A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
3 o electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1430°C. The thickness of the
electrolyte was 30 p,m (layer formed of YSZ: 15 p.m, layer formed of SSZ:
15 p.m). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0149] Comparative Example A5-1
CA 02553074 2006-07-07
38
A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1330°C. The thickness of the
electrolyte was 30 ~.m (layer formed of YSZ: 15 Vim, layer formed of SSZ:
Vim). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0150] Comparative Example A5-2
1 o A layer formed of YSZ having a composition of 90 mol%
Zr02-10 mol% Y203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of SSZ having a composition of
90 mol% Zr02-10 mol% Sc203 was formed on this layer by slurry coating,
and the assembly was then fired at 1450°C. The thickness of the
15 electrolyte was 30 ~m (layer formed of YSZ: 15 ~,m, layer formed of SSZ:
15 Vim). A fuel cell was prepared in the same manner as in Example
A1-1 except for the above matter.
[0151 J The measurement of particle size distribution, a gas
leakage test, a power generation test, and a durability test were carried
out for the fuel cells thus obtained. The results were as shown in tables
below.
[Table 9]
3% 97% Average Mn content,Gas perme-
diameter,diameter,crystal wt% ability,
grain x 10''
m m diameter, ms'~Pa'~
m
Exam 1e 3 10 6 1.0 1.7
A5-1
Exam 1e 3 6 4 0.3 13
A5-2
Exam 1e 3 8 5 0.7 5.5
A5-3
Exam 1e 3 15 9 2.1 1.3
A5-4
Exam 1e 4 20 11 4.0 1.5
A5-5
Comparative
1 3 2 0.2 260
Exam 1e
A5-1
Comparative
Exam 1e 4 27 15 4.6 1.6
A5-2
[Table 10]
Initial After 1500 After 2000
otential, After 1000 hr, hr,
V hr, V V V
Exam 1e 0.67 0.67 _ 0.67
A5-1 0.67
Exam 1e 0.66 0.66 0.66 0.66
A5-2
Exam 1e 0.67 0.67 0.67 0.67
A5-3
Exam 1e 0.67 0.67 0.67 0.67
A5-4
Exam 1e 0.66 0.66 0.66 0.66
A5-5
CA 02553074 2006-07-07
39
Comparative
0.45 0.44 0.43 0.42
Exam 1e
A5-1
Comparative
0.65 0.65 0.64 0.63
Example
A5-2
[0152] Example A6: Fuel cell in which electrolyte has three-layer
structure
Example A6-1
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
1 o mol% Sc203 was formed by slurry coating, and the assembly was then
fired at 1400°C. The thickness of the electrolyte was 30 ~m (layer
formed of SSZ on the air side: 10 Vim, layer formed of YSZ: 10 Vim,
layer formed of SSZ on the fuel electrode side: 10 ~.m). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
matter.
[0153] Example A6-2
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
mol% Sc203 was formed by slurry coating, and the assembly was then
fired at 1360°C. The thickness of the electrolyte was 30 ~,m (layer
formed of SSZ on the air side: 10 ~,m, layer formed of YSZ: 10 Vim,
layer formed of SSZ on the fuel electrode side: 10 ~.m). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
matter.
[0154] Example A6-3
A layer formed of SSZ having a composition of 90 mol%
3o Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 rnol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
CA 02553074 2006-07-07
mol% Sc203 was formed by slurry coating, and the assembly was then
fired at 1380°C. The thickness of the electrolyte was 30 ~m (layer
formed of SSZ on the air side: 10 Vim, layer formed of YSZ: 10 gm,
layer formed of SSZ on the fuel electrode side: 10 ~.m). A fuel cell was
5 prepared in the same manner as in Example A1-1 except for the above
matter.
[0155) Example A6-4
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
1 o electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
mol% Sc20s was formed by slurry coating, and the assembly Was then
fired at 1420°C. The thickness of the electrolyte was 30 ~,m (layer
15 formed of SSZ on the air side: 10 Vim, layer formed of YSZ: 10 ~,m,
layer formed of SSZ on the fuel electrode side: 10 ~,m). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
matter.
[0156] Example A6-5
2o A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc203 was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
25 mol% Sc203 was formed by slurry coating, and the assembly was then
fired at 1440°C. The thickness of the electrolyte was 30 ~m (layer
formed of SSZ on the air side: 10 ~.m, layer formed of YSZ: 10 ~,m,
layer formed of SSZ on the fuel electrode side: 10 Vim). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
30 matter.
[0157] Comparative Exam,~ole A6-1
A layer formed of SSZ having a composition of 90 mol%
Zr02-10 mol% Sc243 Was formed by slurry coating on the air-side
electrode reaction layer. A layer formed of YSZ having a composition of
35 90 mol% Zr02-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% Zr02-10
°
. CA 02553074 2006-07-07
41
mol% Scz03 was formed by slurry coating, and the assembly was then
sintered at 1330°C. The thickness of the electrolyte was 30 ~m (layer
formed of SSZ on the air side: 10 trm, layer formed of YSZ: 10 Vim,
layer formed of SSZ on the fuel electrode side: 10 Vim). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
matter.
[0158] Comparative Example A6-2
A layer formed of SSZ having a composition of 90 mol%
ZrOz-10 mol% Scz03 was formed by slurry coating on the air-side
1 o electrode reaction layer. A layer formed of YSZ having a composition of
90 mol% ZrOz-10 mol% Y203 was formed on this layer by slurry coating.
Further, a layer formed of SSZ having a composition of 90 mol% ZrOz-10
mol% Scz03 Was formed by slurry coating, and the assembly was then
sintered at 1450°C. The thickness of the electrolyte was 30 ~.m (layer
formed of SSZ on the air side: 10 ~,m, layer formed of YSZ: 10 ~.m,
layer formed of SSZ on the fuel electrode side: 10 ~,m). A fuel cell was
prepared in the same manner as in Example A1-1 except for the above
matter.
[0159] The measurement of particle size distribution, a gas
leakage test, a power generation test, and a durability test were carried
out for the fuel cells thus obtained. The results were as shown in tables
below.
[Table 11 ]
3% 97% Average Mn content,Gas perme-
diameter,diametercrystal wt% ability,
grain x 10''
m m diameter, ms''Pa''
m
Exam Ie 3 8 5 0.9 1.1
A6-1
Exam 1e 3 5 4 0.3 10.3
A6-2
Exam 1e 3 6 4 0.6 2.7
A6-3
Exam 1e 3 14 8 1.8 0.9
A6-4
Exam 1e 3 20 11 3.6 0.9
A6-5
Comparative
2 4 2.3 0.2 130
Example
A6-1
Comparative
Exam 1e 4 27 15 4.4 1.0
A6-2
[Table 12]
I After 1000 .After 1500Vfter 2000
l hr hr hr,
V V
o , ,
tential,
V
Exam 1e 0.69 0.69 0.69 0.69
A6-1
Exam 1e 0.67 0.67 0.67 0.67
A6-2
Exam 1e 0.68 0.68 0.68 0.68
A6-3
~
, CA 02553074 2006-07-07
42
Exam 1e 0.69 0.69 0.69 0.69
A6-4
Example 0.68 0.68 0.68 0.68
A6-5
Comparative
0.48 0.47 0.46 0.45
Exam 1e
A6-1
Comparative
I 0.67 ~ 0.67 0.66 ~ 0.65
Example
A6-2
[0160] Example A7: Thickness of electrolyte film
Example A7-1
A fuel cell was prepared in the same manner as in Example
A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc203-5 mol% Y203, was sintered at 1420°C, and had a
thickness of 8 ~,m.
[0161 ] Example A7-2
A fuel cell was prepared in the same manner as in Example
1 o A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc203-5 mol% Y203, was sintered at 1420°C, and had a
thickness of 10 ~,m.
[0162] Example A7-3
A fuel cell was prepared in the same manner as in Example
A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc20s-5 mol% Y203, was sintered at 1420°C, and had a
thickness of 15 ~,m.
[0163] Example A7-4
A fuel cell was prepared in the same manner as in Example
2 o A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc203-5 mol% Y203, was sintered at 1420°C, and had a
thickness of 30 ~,m.
[0164] Example A7-5
A fuel cell was prepared in the same manner as in Example
A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc20s-5 mol% Y203, was sintered at 1420°C, and had a
thickness of 50 ~,m.
[0165] Example A7-6
A fuel cell was prepared in the same manner as in Example
3o A1-1, except that the electrolyte film had a composition of 90 mol% Zr02
5 mol% Sc203-5 mol% Y20s, was sintered at 1420°C, and had a
thickness of 80 ~,m.
CA 02553074 2006-07-07
43
[0166] Example A7-7
A fuel cell was prepared in the same manner as in Example
A1-1, except that the electrolyte film had a composition of 90 mol% Zr02-
mol% Sc203-5 mol% Y203, was sintered at 1420°C, and had a
5 thickness of 100 ~,m.
[0167] Examale A7-8
A fuel cell was prepared in the same manner as in Example
A1-1, except that the electrolyte film had a composition of 90 mol% Zr02-
5 mol% Sc203-5 mol% Y20s, was sintered at 1420°C, and had a
1 o thickness of 120 Vim.
[0168] The measurement of particle size distribution, a gas
leakage test, a power generation test, and a durability test were carried
out for the fuel cells thus obtained. The results were as shown in tables
below.
[Table 13]
3% 97% Average Mn content,Gas perme-
diameter,diametercrystal wt% ability,
m m grain x 10''
diameter ms''Pa''
m
Exam 1e 5 8 7 4.0 28
A7-1
Exam 1e 5 10 8 3.8 20
A7-2
Exam 1e 5 13 9 3.4 7.5
A7-3
Exam 1e 3 16 9 2.5 0.7
A7-4
Exam 1e 3 11 6 1.5 0.6
A7-5
Example 3 8 5 1.0 0.5
A7-6
ExamleA7-73 5 4 0.6 0.4
Exam 1e 3 4 3.3 0.3 0.3
A7-8
[Table 14]
Initial After 1500After 2000
otential After 1000 hr, hr,
V hr, V V V
Exam 1e 0.61 0.61 0.61 0.61
A7-1
Exam 1e 0.64 0.64 0.64 0.64
A7-2
Exam 1e 0.66 0.66 0.66 0.66
A7-3
Exam (e 0.67 0.67 0.67 0.67
A7-4
Exam 1e 0.67 0.67 0.67 0.67
A7-5
Exam 1e 0.67 0.67 0.67 0.67
A7-6
Example 0.66 0.66 0.66 0.66
A7-7
Exam 1e 0.63 0.63 0.63 0.63
A7-8
[0169] Example B1
2 0 (1 ) Preparation of electrolyte
(1-1 ) Preparation of raw material powder for electrolyte
An SSZ material represented by 90 mol% Zr02-10 mol%
CA 02553074 2006-07-07
44
Sc203 was provided as an electrolyte material. Specifically, Zr02 was
dissolved in not less than 3 N concentrated nitric acid heated at
100°C,
and the solution was diluted with distilled water to give an aqueous
nitrate solution. Also for Sc203, an aqueous nitrate solution was
prepared in the same manner as described above. The aqueous nitrate
solutions thus obtained were mixed with each other so as to give the
above composition, followed by the addition of an aqueous oxalic acid
solution for coprecipitation. The liquid obtained by coprecipitation was
dried at about 200°C, was heat decomposed at 500°C, and was
further
1 o heat treated at 800°C to prepare a raw material powder. The average
particle diameter was 0.5 g.m.
[0170] (1-2) Preparation of pressed product
A binder PVA was added, to the powder, in an amount of
10% by weight based on the SSZ material. The mixture was kneaded
and was dried. The dried product was then monoaxially molded in a
disk-shaped mold and was subjected to pressing at 1000 kglcm2.
[0171 ] (1-3) Preparation of pressed sinter
The pressed product was sintered at 1430°C. Further,
after the sintering, the sinter was ground to a thickness of 1 mm.
[0172] (1-4) Measurement of porosity
The porosity of the pressed sinter was measured by an
Archimedes' method and was found to be 0.8%, confirming that the
electrolyte is not permeable to gas.
[0173] (2) Preparation of mixed electrically conductive ceramic
2 5 electrode
(2-1 ) Preparation of raw material
A mixed electrically conductive ceramic material prepared
by intimately mixing a manganese- and nickel-containing perovskite
oxide with an oxygen ion-conductive oxide was provided. The material
3o had a composition of (Lao.~SSro.2s)o.ss(Mno.ssNio.os)~s and SSZ
represented by 90 mol% Zr02-10 mol% Sc203 (hereinafter referred to as
"(Lao,~SSro.2s)o.se(Mno.ssNio.os)Os~90 mol% Zr02-10 mol% Sc203") at a
weight ratio of 50/50. (Lao.7sSro.zS)o.sa(Mno,95Nio.oS)03 was prepared as
follows. An aqueous solution of nitrate of La, an aqueous solution of
35 nitrate of Sr, an aqueous solution of nitrate of Mn, and an aqueous
solution of nitrate of Ni were prepared and were mixed together to
CA 02553074 2006-07-07
provide the above composition, and oxalic acid was added to the mixture
for precipitation. The precipitate was further heat treated. The
resultant raw material was ground and was then fired at 1300°C to
prepare a raw material powder. On the other hand, 90 mol% Zr02-10
5 mol% Sc203 was prepared as follows. Specifically, Zr02 was dissolved
in not less than 3 N concentrated nitric acid heated at 100°C, and the
solution was diluted with distilled Water to give an aqueous nitrate
solution. Also for Sc203, an aqueous nitrate solution was prepared in
the same manner as described above. The aqueous nitrate solutions
10 thus obtained were mixed with each other so as to give the above
composition, followed by the addition of an aqueous oxalic acid solution
for coprecipitation. The liquid obtained by coprecipitation was dried at
about 200°C, was heat decomposed at 500°C, and was further heat
treated at 1200°C to prepare a raw material powder. The raw material
15 powders thus obtained were mixed together, and the mixture was heat
treated at 1300°C to prepare a raw material powder. The particle
diameter was regulated to an average particle diameter of 2 ~.m.
(0174] (2-2) Preparation of paste
Ethylcellulose (10 parts by weight) as a binder and 90
2 o parts by weight of a-terpineol as a solvent were added to 100 parts by
weight of the raw material powder of (I_ap,75Sr0.25)0.98(Mn0.95N~0.05)03~9~
mol% Zr02-10 mol% Sc20s = 50/50, and the mixture was kneaded for 30
min to prepare a paste.
[0175] (2-3) Preparation of electrode
25 The paste was screen printed on one side of the electrolyte
in the pressed product so that the diameter was 6 mm, followed by
sintering at 1400°C. The thickness of the electrode after firing was 20
~~m. A platinum electrode was screen printed on this electrode and on
the pressed product in its side remote from the electrode so that the
3o diameter was 6 mm, followed by sintering at 1100°C to prepare a fuel
cell
specimen.
[0176] Example B2
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
3 5 electrode was prepared so as to have a composition of
(Lao.~SSro.2s)o.sa(Mno.ssNio.o~ )Os~90 mot% Zr02-10 mol% Sc203 = 50/50.
CA 02553074 2006-07-07
46
[0177] Example B3
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.75Sro.2s)o_sa(MnO.saNio.o2)43/90 mol% Zr02-10 mol% Sc203 = 50150.
[0178] Example B4
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
l o (Lao.75Sro.2s)o.sa(Mno.s2Ni0.0a)03190 mol% Zr02-10 mol% Sc203 = 50/50.
[0179] Example B5
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao,~5Sro.25)o.sa(Mno.soNio.,o)Os/90 mol% Zr02-10 mol% Sc203 = 50/50.
[0180] Example B6
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.~5Sro.zs)o.sa(Mno.s~Nio.,s)~sI90 mol% Zr02-10 mol% Sc203 = 50/50.
[0181 ] Comparative Example B1
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.7sSro.2s)o.saMn03/90 mol% Zr02-10 mol% Sc203 = 50/50.
[0182] Measurement of overvoltaae
The specimen prepared above was constructed
as shown in Fig. 7 for the measurement reaction of overvoltage.
Specifically, an electrode 11 formed of a mixed electrically conductive
3 o ceramic is provided on one side of an electrolyte 13 formed of an SSZ
material. A platinum electrode 12 is provided on the surface of the
electrode 11, and a counter electrode 14 of platinum is provided on the
electrode on its side remote from the electrode 11. A reference
electrode 15 of platinum is provided on the side face of the electrolyte 13.
Further, two lead wires16 are mounted on the platinum electrode 12, a
lead wire 17 is mounted on the counter electrode, and a lead wire 18 is
CA 02553074 2006-07-07
47
mounted on the reference electrode 15. The temperature of the cell
was raised to 800°C under the atmosphere, and the overvoltage was
then measured by a current chopping method. The current chopping
method is a method in which current flown into the cell is instantaneously
interrupted and, based on a voltage change at that time, reaction-derived
overvoltage and ohmic resistance-derived overvoltage are quantitatively
determined. In this test, the reaction overvoltage was measured under
conditions of 0.2 Acm-2. In general, it is the that, when the reaction
overvoltage is lower, the electrode characteristics are better.
[Table 15]
z value in Reaction
Lao,~SSro,2s o.9s(Mn~-zNiZ)03overvolta a mV
Exam 1e 0.05 25
B1
Exam 1e 0.01 70
B2
Exam 1e 0.02 45
B3
Exam 1e 0.08 24
B4
Exam 1e 0.10 38
B5
Exam 1e 0.13 60
B6
Comparative0 80
Exam 1e
B1
[0183] A comparison of Examples B1 to B6 and Comparative
Example B1 shows that the incorporation of Ni lowers the reaction
overvoltage. The reason for this is believed to reside in that the
incorporation of Ni suppresses the diffusion of manganese in the
electrolyte. Thus, it could be confirmed that the incorporation of Ni
suppresses the diffusion of manganese and can provide good electrode
characteristics. Regarding the Ni content, there is a tendency that an
Ni content of not less than 0.02 lowers the reaction overvoltage while an
2 o Ni content of more than 0.10 increases the reaction overvoltage. This
demonstrates that an Ni content in the range of 0.02 to 0.10 is more
preferred.
[0184] Tests were carried out below for
(Lao.~5Sro.25)y(Mno.ssNio.os)03190 mol% Zr02-10 mol% Sc203 = 50/50.
[0185] Example B7
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.~SSro.2s)o.ss(Mno.sSNio.oS)C3/90 mol% Zr02-10 mol% Sc203 = 50/50.
CA 02553074 2006-07-07
48
[0186] Example B8
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.75Sro.2s)o.s7(Mno.ssNio.os)Os~90 mol% Zr02-10 mol% Sc203 = 50/50.
[0187] Example B9
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.~SSro.25)o.ss(Mno.sSNio.os)Os~90
mol% Zr02-10
mol% Sc203
= 50150.
[0188] Example B10
A fuel cell specimen was prepared in the same
manner as
in Example B1, except that the mixed electrically conductive
ceramic
electrode was prepared so as to have a composition of
(Lao.~5Sro,25)(Mno,95Nio.os)Os~90
mol% Zr02-10
mol% Sc203
= 50/50.
[0189] Example B11
A fuel cell specimen was prepared in the same
manner as
in Example B1, except that the mixed electrically conductive
ceramic
electrode was prepared so as to have a composition of
~La0,75Srp,25~1.01~Mn0,95N~0.05~03~9~
mol% Zr02-10
mol% Sc203
= 50/50.
[0190] Overvoltage evaluation test
The reaction overvoltage was measured by the
same
overvoltage measurement method as described above. The results
were as summarized in the table below
2 5 [Table 16]
y value in Reaction
Lao.~SSro.2s Mno,95Nio.osovervolta e,
Os mV
Exam 1e B1 0.98 25
Exam 1e B7 0.96 70
Exam 1e B8 0.97 45
Exam 1e B9 0.99 17
Exam 1e B10 1.00 20
Exam 1e B11 1.01 45
[0191 ] The results show that, when y value is in the range of 0.97
to 1, the reaction overvoltage is low while, when y value is less than 0.97
and more than 1.00, a rapid increase in reaction overvoltage takes place,
3o confirming that y value is more preferably 0.97 s y < 1.00.
CA 02553074 2006-07-07
49
[0192] Next, tests were carried out with varied weight ratios.
[0193] Example B12
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
Lap,75Srp.25~0.98~Mn0.95N~0.05~~3/9~ mol% Zr02-10 mol% Sc203 = 20/80 in
terms of a weight ratio.
[0194] Example B13
A fuel cell specimen was prepared in the same manner as
1 o in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
Lap,75Sr0.25~0.98~Mn0.95N~p.05~~3/9~ mol% Zr02-10 mol% Sc203 = 30/70 in
terms of a weight ratio.
[0195] Exama~le B14
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
(Lao.~5Sro2s)o.ss(Mno.ssNio.os)Os/90 mol% Zr02-10 mol% Sc20s = 40/60 in
terms of a weight ratio.
2 o [0196] Example B 15
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
~La0.75Sr0.25~0.98~Mn0.95N~0.05~~3/9~ mol% Zr02-10 mol% Sc203 = 60/40 in
terms of a weight ratio.
[0197] Example B16
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
(Lao,75Sro.25)o.sa(Mno.sSNio.os)43/90 mol% Zr02-10 mol% Sc203 = 70/30 in
terms of a weight ratio.
[0198] Example B17
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared using as a material
(Lao.75Sro.2s)o.sa(Mno.ssNio.os)OsI90 mol% Zr02-10 mol% Sc203 = 80/20 in
CA 02553074 2006-07-07
terms of a weight ratio.
[0199] Comparative Example B2
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
5 electrode was prepared so as to have a composition of
(Lao,~5Sro.25)o.s8(Mno.sSNio.os)~s, and, after firing at 1300°C, the
average
particle diameter was regulated to 2 p,m.
[0200] Comparative Example B3
A fuel cell specimen was prepared in the same manner as
to in Example B1, except that an SSZ material having a composition of 90
mol% Zr02-10 mol% Sc203 was used as the material for the mixed
electrically conductive ceramic electrode, and, after firing at 1200°C,
the
average particle diameter was regulated to 2 ~.m.
[0201 ] Overvoltaae evaluation test
15 The reaction overvoltage was measured by the same
overvoltage measurement method as described above. The results
were as summarized in the table below
[Table 17]
Weight ratio, wt% of
(Lao,SSro_ZS)y(Mno.ssNio.os)~sReaction
in
(Lao,~SSro,zs?o.ss(Mno.ssNio.os)~s~90overvOltage,
mV
mol% Zr02~10 mol%Sc203
Exam 1e B1 50 25
Exam 1e B12 20 65
Exam 1e B13 30 39
Example B14 40 27
Exam 1e B15 60 27
Exam 1e B16 70 37
Exam 1e 817 80 56
Comparative
Exam 1e B2 100 205
Comparative
0 270
Exam 1e B3
20 [0202] When the weight ratio is in the range of 30 to 70% by
weight, there is a tendency that the overvoltage is reduced.
(0203] A test was carried out for the influence of rare earth
elements other than La.
[0204] Example B18
2S A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
CA 02553074 2006-07-07
51
electrode was prepared so as to have a composition of
(Y0.75Sr0.25)0.98(Mn0.95N~0.05)03~9~ mol% Zr02-10 mol% Sc20 = 50/50.
[0205] Example B19
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Smo,~SSro.2s)o.sa(Mno.95Nio.os)03~90 mol% Zr02-10 mol% Sc203 = 50150.
[0206] Overvoltagie evaluation test
The reaction overvoltage was measured by the same
overvoltage measurement method as described above. The results
were as summarized in the table below
[Table 18]
Electrode material Reaction
overvolta e,
mV
Example ~La0.75Sr0.25~0.98~Mr1p,g5N10.05~~325
B1
/90 mol%Zr02.10 mol%Sc203
= 50/50
Example ~Yo.7ssr.25).sa(Mn_95Nip,5)4s
B18 30
/90 mol%Zr02.10 mol%Sc20
= 50/50
Example ~'Sm075Sr0.25~0.98(Mnp.sSNIp.05~0328
B19
/90 mol%Zr02~10 mol%Sc203
= 50/50
[0207] ft was confirmed that, when a perovskite oxide containing
at least manganese and nickel is represented by (Ln,_XAx)y(Mn~_ZNiZ)03,
Ln may be Sm (samarium) or Y (yttrium). It can easily be estimated
from this fact that the same effect can be attained also when Ln is one or
at least two elements selected from Sc (scandium), Y (yttrium), La
(lanthanum), Ce (cerium), Pr (praseodymium), Nd (neodymium), Prn
(promethium), Sm (samarium), Eu (europium), Gd (gadolinium), Tb
(terbium), Dy (dysprosium), Ho (holmium), Er (erbium), Tm (thulium), Yb
(ytterbium), and Lu (lutetium). That is, it could be confirmed that these
elements are prepared.
[0208] A test was carried out for the influence of materials having
2 5 oxygen ion conductive properties.
[0209] Example B20
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.~SSro.2s)o.ss(Mno.sSNio.os)Os~90 mol% Zr02-10 mol% Y20s = 50/50.
w CA 02553074 2006-07-07
52
[0210] Exams Ip a B21
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(La0.75Srp,25)0.98(Mn0.95N~0.05)O3~9O mol% Zr02-5 mol% Y203-5 mot%
Sc203 = 50/50.
[0211 j Example B22
The mixed electrically conductive ceramic electrode was
prepared using as a material (Lao.~sSro.2s)o.ss(Mno.ssNio.os)Os and a
1 o cerium-containing oxide represented by (Ce02)o,$(Sm203)o., (hereinafter
referred to as "(Lao.~sSro.2s)o.ss(Mno.ssNio.os)~s~(Ce02)o,8(Sm203)o.~") at a
weight ratio of 50150. (Ce02)o.a(Sm203)o.~ was prepared from a solution
of nitrate of Ce and a solution of nitrate of Sm by using coprecipitation
with oxalic acid and was heat treated at 1200°C, and the powder of
(Ce02)o.s(Sm203)o., was then mixed with the powder of
(Lao.7sSro.2s)o.sa(Mno.ssNio.os)~g, and the mixture was fired at
1300°C.
Further, this electrode was sintered at 1500°C. The electrode was
prepared in the same manner as in Example B1 except for the above
matter.
2 0 [0212] Example B23
The mixed electrically conductive ceramic electrode was
prepared using as a material (Lao.7sSro.2s)o.sa(Mno.ssNio.os)Os, 90 moi%
Zr02-10 mol% Sc203., and a cerium-containing oxide represented by
(Ce02)o.a(Sm243)o., (hereinafter referred to as
"(Lao.7sSro.2s)o.ss(Mno.ssNio.os)Os~90 mol% Zr02-10 mol%
Sc20s/(Ce02)o.8(Sm203)o.,") at a weight ratio of 50/25125.
(Lao,~sSro.2s)o.sa(Mno.ssNio.os)~3, 90 mol% Zr02-10 mol% Sc203, and
(Ce02)o.8(Sm20s)o.~ were prepared by coprecipitation. The powders
thereof Were mixed together, and the mixture was fired at 1300°C. A
3o fuel cell specimen was prepared in the same manner as in Example B1
except for the above matter.
[0213] Example B24
The mixed electrically conductive ceramic electrode was
prepared using a material (Lao.7sSro.2s)o.ss(Mno.ssNio.os)03 and a
lanthanum gallate represented by Lao,$Sro.2Gao.aMgo,203 (hereinafter
referred to as "(Lao,~sSro,2s)o.ss(Mno.ssNio.os)~3~Lao.aSro.2Gao,eMgo.203") at
CA 02553074 2006-07-07
53
a weight ratio of 50150. Lao,sSro,2Gao,aMgo.203 was prepared by mixing
La203, SrC03, Ga203, and Mg0 together so as to provide the above
composition, ball milling the mixture and then heat treating the mixture at
1200°C. The powder of Lao.aSro.2Gao.gMgo.203 was mixed with the
powder of (La0.75Srp,25)0.98(Mn0.95N~0.05)O3, and the mixture was fired at
1300°C. A fuel cell specimen was prepared in the same manner as in
Example B1 except for the above matter.
(0214] Comparative Example B4
A fuel cell specimen was prepared in the same manner as
to in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao,75Sro.2s)o.ssMn03/90 mol% Zr02-10 mol% Y203 = 50/50 (weight ratio).
[0215] Comparative Example B5
A fuel cell specimen was prepared in the same manner as
in Example B1, except that the mixed electrically conductive ceramic
electrode was prepared so as to have a composition of
(Lao.~SSro.2s)o.ssN1n03/90 mol% Zr02-5 mol% Sc203-5 mol% Y2O3 = 50/50
(weight ratio).
[0216] Comparative Exami~le B6
2 o A mixed electrically conductive ceramic electrode was
prepared so as to have a composition of
(Lao.~sSro.2s)o.saMn03/(Ce02)o.a(Sm20s)o.t = 50/50. (Ce02)o.s(Sm203)o.~
was prepared from a solution of nitrate of Ce and a solution of nitrate of
Sm by coprecipitation with oxalic acid and was heat treated at
1200°C.
The powder of (Ce02)o,a(Sm203)o.~ was then mixed with the powder of
(Lao.75Sro.2s)o.ssMnOs, and the mixture was fired at 1300°C. A fuel
cell
specimen was prepared in the same manner as in Example B22, except
for the above matter.
[0217] Comparative Example B7
3o A mixed electrically conductive ceramic electrode was
prepared so as to have a composition of (Lao.75Sro.2s)o.ssMn03190 mol%
Zr02-10 mol% Sc203/(Ce02)o,a(Sm203)o.~ - 50/25/25.
(Lao.~SSro.2s)o.saMn03, 90 mol% Zr02-10 mol% Sc20s, and
(Ce02)o.8(Sm203)o.~ were prepared by coprecipitation. The powder of
(Lao,~5Sro.25)o.ssMn03, the powder of 90 mol% Zr02-10 mol% Sc203, and
the powder of (Ce02)o_$(Sm20s)o.~ were mixed together, and the mixture
CA 02553074 2006-07-07
54
was fired at 1300°C. A fuel cell specimen was prepared in the same
manner as in Example B1, except for the above matter.
[0218) Comparative Example B8
A mixed electrically conductive ceramic electrode was
prepared so as to have a composition of
(Lao.~sSro.zs)o.ssMn03/Lao.aSro,2Gao.sMgo.20s - 50/50.
Lao,aSro.2Gao.8Mgo.203 was prepared by mixing La203, SrC03, Ga203, and
Mg0 together so as to give the above composition, ball milling the
mixture, and heat treating the ball milled mixture at 1200°C.
Thereafter,
to the power of (Lao.~sSro,25)o.98MnOs and the powder of
Lao.aSro,2Gao.sMgo.203 were mixed together, and the mixture was fired at
1300°C. A fuel cell specimen was prepared in the same manner as in
Example B1 except for the above matter.
[0219) Overvoltaae evaluation test
The reaction overvoltage was measured by the same
overvoltage measurement method as described above. The results
were as summarized in the table below
[Table 19)
Reaction
Electrode material overvoltage,
mV
Example B1 (Lao.~sSoo.zs)o.sa(Mno.ss 25
Nio.os)Os
/90 mol /ZrOz~10 mol /Scz03
= 50/50
Example B20 (Lap.75SrD 25)0.98(MnD.95N~0.05)~350
/90 mol%ZrOz.10 mol%Yz03 = 50/50
(Lao.~sSro.zs)o.sa(Mno.ssNio.os)~s
Example B21 /90 mol%ZrOz~5 mol%Y203.5 mol%Scz0335
=
50/50
Example B22 (Lap.75Sr0.2s)0.98(Mn0.95N~0.05)~325
/(CeOz)o.a(S~lz~s)o.,
(Lao.~sSro.zs)o.sa(Mno.ssNio.os)~s
Example B23 /90 mol%ZrOz.10 mol% 20
Scz03/ CeOz o.a SmzOs o.,
Example B24 (Lao.~sSro.zs)o.sa(Mno.ssNio.os)~s40
/Lao.aSro.zGao.aM o.zOs = 50/50
Comparative (Lao
~sSro
saMn03
zs)o
_ 105
Exam 1e B4 .
.
/90 mol%ZrOz.10 mol%Y203 = 50/50
Comparative (Lao.~sSro
saMn03/90 mol%
zs)o
Exam 1e B5 _
.
ZrOz~S mol% Scz03~5 mol%Y203
= 50/50
Comparative (Lao.~sSro zs)o
saMn03
Exam 1e B6 . ~5
/ CeOz o a Smz03 0., = 50/50
Comparative (Lao,~sSro
saMn03/90mo1%ZrOz~10mo1%
zs)o
Exam 1e B7 ,
.
Scz03/ CeOz o.a Smz03 0., =
50/25/25
Comparative (Lao.~sSro.zs)o
saMn03
Exam 1e B8 . 150
/Lao,aSro,zGao aM o.z~s = 50/50
'~ CA 02553074 2006-07-07
Pr
[0220] YSZ, ScYSZ, a cerium-containing oxide, a mixed material
composed of SSZ and cerium oxide, and lanthanum gallate oxide were
used as the material having oxygen ion conductive properties. It was
5 confirmed that, for all the cases where mixing with a perovskite oxide
containing at least manganese and nickel provides a low reaction
overvoltage, whereas, for the nickel-free material, the reaction
overvoltage is increased, and the incorporation of nickel in a
manganese-containing perovskite oxide can significantly improve
1 o electrode characteristics. It is considered that the electrode
characteristics were improved by suppression of the diffusion of
manganese in the electrolyte.
[0221 ] Pr,eJ~aration of solid oxide fuel cell
Example B25
15 (1 ) Preparation of air electrode support
Lanthanum manganite containing Sr in solid solution and
having a composition represented by Lao,~SSro.25MnOs was used as an
air electrode. After preparation by coprecipitation, heat treatment was
carried out to prepare a raw material powder for an air electrode. The
2 o average particle diameter was 30 ~.m. A cylindrical molded product was
prepared by extrusion, and the molded product was then fired at 1500°C
to prepare an air electrode support. The air electrode support had a
pore diameter of 14 pm, a porosity of 45%, and a wall thickness of 1.5
mm.
25 [0222] (2) Preparation of air-side electrode reaction layer
A layer formed of an intimate mixture of manganese- and
nickel-containing perovskite oxide with YSZ was prepared as an air-side
electrode reaction layer. In this case, a material having a composition
of (Lap,75Srp,25~(Mr10.95N~0.05~~3~g~ mol% Zr02-10 mol% Sc203 = 50/50
30 (weight ratio) was prepared and used. An aqueous solution of nitrate of
La, an aqueous solution of nitrate of Sr, an aqueous solution of nitrate of
Mn, an aqueous solution of nitrate of Ni, an aqueous solution of nitrate of
Zr, and an aqueous solution of nitrate of Y were provided and were
mixed with each other so as to give the above composition, followed by
35 coprecipitation With oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
~
. CA 02553074 2006-07-07
56
The average particle diameter was 5 Vim. This powder (40 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. The slurry was coated onto the surface of the air electrode
support (outer diameter 15 mm, wall thickness 1.5 mm, effective length
400 mm) to form a coating which was then sintered at 1400°C. The
layer thus formed had a pore diameter of 5 ~.m, a porosity of 28%, and a
thickness of 30 Vim.
[0223) (3) Preparation of slurry for electrolyte:
YSZ having a composition of 90 mol% Zr02-10 mol% Y20s
was prepared as a material for an electrolyte. An aqueous solution of
nitrate of Zr and an aqueous solution of nitrate of Y were provided and
were mixed with each other so as to give the above composition,
followed by coprecipitation with oxalic acid. Heat treatment was then
carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 0.5 ~,m. This powder (40
2 o parts by weight) was mixed with 100 parts by weight of a solvent
(ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part by weight
of a dispersant (polyoxyethylene alkyiphosphate), and 1 part by weight of
an antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 140
mPas.
[0224) (4) Preparation of electrolyte
The slurry prepared above was coated onto the surface of
the air-side electrode reaction layer prepared in the above step (2), and
the coating was sintered at 1400°C. The thickness of the electrolyte
3o thus formed was 30 Vim. In this case, the air electrode support in its
part on which an interconnector film is to be formed in a later step was
masked so as not to be coated.
[0225) (5) Preparation of slurry for fuel-side electrode reaction
layer
NiO/SSZ having a composition of
NiO/(Zr02)o.so(SczOs)o.,o was prepared as a material for a fuel-side
., CA 02553074 2006-07-07
57
electrode reaction layer. An aqueous solution of nitrate of Ni, an
aqueous solution of nitrate of Zr, and an aqueous solution of nitrate of Sc
were provided and were mixed with each other so as to give the above
composition, and oxalic acid was then added for precipitation. The
precipitate and the supernatant were dried, followed by heat treatment
and particle diameter regulation to prepare a raw material. In this case,
two types of compositions for the fuel-side electrode reaction layer, that
is, Ni0/(Zr02)o.so(Sc203)o.~0 - 20/80 and 50/50 (weight ratio) were
prepared. For both the cases, the average particle diameter was 0.5
1o pm. This powder (100 parts by weight) was mixed with 500 parts by
weight of an organic solvent (ethanol), 10 parts by weight of a binder
(ethylcellulose), 5 parts by weight of a dispersant (polyoxyethylene
alkylphosphate), 1 part by weight of an antifoaming agent (sorbitan
sesquioleate), and 5 parts by weight of a plasticizer (DBP). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 70 mPas.
[0226] (6) Preparation of fuel-side electrode reaction layer
The electrolyte layer formed in the above step (4) was
masked so that the effective area was 150 cm2. The slurry
2o Ni0/(Zr02)o.so(Sc203)o.~0 = 20/80 (average particle diameter 0.5 ~.m) and
the slurry NiOI(Zr02)o.so(Sc203)o.,0 = 50/50 (average particle diameter
0.5 ~.m) prepared in the above step (5) were coated on the electrolyte
layer in that order. The layer thickness (after sintering) was 10 ~,m.
[0227] (7) Preparation of slurry for fuel electrode:
Ni0/YSZ having a composition of Ni0/(Zr02)o.so(Y20s)o.~o
was prepared as a material for a fuel electrode. An aqueous solution of
nitrate of Ni, an aqueous solution of nitrate of Zr, and an aqueous
solution of nitrate of Y were provided and were mixed with each other so
as to give the above composition, and oxalic acid was then added for
3 o precipitation. The precipitate and the supernatant were dried, followed
by heat treatment and particle diameter regulation to prepare a raw
material. In this case, a material having a composition of
NiO/(Zr02)o.go(Y20s)o.~o - 70/30 (weight ratio) was prepared. The
average particle diameter was 2 ~.m. This powder (100 parts by weight)
was mixed with 500 parts by weight of an organic solvent (ethanol), 20
parts by weight of a binder (ethylcellulose), 5 parts by weight of a
~
, CA 02553074 2006-07-07
58
dispersant (polyoxyethylene alkylphosphate), 1 part by weight of an
antifoaming agent (sorbitan sesquioleate), and 5 parts by weight of a
plasticizer (DBP). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 250 mPas.
[0228] (8) Preparation of fuel electrode
The slurry prepared in the above step (7) was coated on
the fuel-side electrode reaction layer formed in the above step (6). The
film thickness (after sintering) was 90 ~~m. Further, the fuel-side
electrode reaction layer and the fuel electrode were co-sintered at
1400°C.
[0229] (9) Preparation of interconnector:
An interconnector having a composition of lanthanum
chromite containing Ca in solid solution represented by Lao.7oCao.3oCr03
was prepared. A raw material powder was prepared by spray pyrolysis
and was then heat treated. The average particle diameter was 1 ~,m.
This powder (40 parts by weight) was mixed with 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
2o was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 100 mPas. An interconnector was formed by slurry coating
using this slurry and was then sintered at 1400°C. The thickness of the
interconnector after sintering was 40 ~,m.
[0230] Comparative Example B9
An air-side electrode reaction layer was prepared so as to
have a composition of Lao.~sSro.2sMn03/90 mol%Zr02-10 mol% Y2O3 =
50/50 (weight ratio). An aqueous solution of nitrate of La, an aqueous
solution of nitrate of Sr, an aqueous solution of nitrate of Mn, an aqueous
solution of nitrate of Ni, an aqueous solution of nitrate of Zr, and an
3o aqueous solution of nitrate of Y were provided and were mixed with each
other so as to give the above composition, followed by coprecipitation
with oxalic acid. Heat treatment was then carried out to prepare a raw
material powder having a regulated particle diameter. The average
particle diameter was 5 ~.m. This powder (40 parts by weight) was
mixed with 100 parts by weight of a solvent (ethanol), 2 parts by weight
of a binder (ethylcellulose), 1 part by weight of a dispersant
CA 02553074 2006-07-07
r
59
(polyoxyethylene alkylphosphate), and 1 part by weight of an antifoaming
agent (sorbitan sesquioleate). The mixture was then thoroughly stirred
to prepare a slurry. This slurry had a viscosity of 100 mPas. The
slurry was coated onto the surface of the air electrode support to form a
coating which was then sintered at 1400°C. The sinter had a thickness
of 30 pm. A fuel cell was prepared in the same manner as in Example
B25 except for the above matter.
[0231 ] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
1o generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 20]
Gas
permeability,Mn content, Initial potential,
wt% V
x 1 O~,orpg.'
pa.,
Exam 1e B25 6.5 2.9 0.57
Comparative 6 5 0
5 5 48
Exam 1e B9 . . .
[Table 21]
After After Estimated
initial
potential,1000 After After potential,
hr 1500 2000
V , hr, hr, V after
V V 40,000
hr, V
Exam 1e B25 0.57 0.57 0.57 0.57 0.54
Comparative
0.48 0.48 0.48 0.475 0.38
Exam 1e B9
[0232] In Table 7, the estimated potential after 40000 hr is shown.
This is so because the service life required of stationary fuel cells is
40000 hr. It is generally the that a potential lowering rate of not more
than 10% at the time when a testing time of 40000 hr elapsed, poses no
2 o problem.
[0233] A test was carried out for the thickness of the air-side
electrode reaction layer.
[0234] Example B26
A fuel cell was prepared in the same manner as in Example
B25, except that the thickness of the air-side electrode reaction layer
was 3 p.m.
[0235] Example B27
A fuel cell was prepared in the same manner as in Example
CA 02553074 2006-07-07
B25, except that the thickness of the air-side electrode reaction layer
was 5 Vim.
[0236] Example B28
A fuel cell was prepared in the same manner as in Example
5 B25, except that the thickness of the air-side electrode reaction layer
was 20 ~,m.
[0237] Example B29
A fuel cell was prepared in the same manner as in Example
B25, except that the thickness of the air-side electrode reaction layer
1 o was 50 ~,m.
[0238] Example B30
A fuel cell was prepared in the same manner as in Example
B25, except that the thickness of the air-side electrode reaction layer
was 55 ~,m.
15 [0239] A gas leakage test, a power generation test, a durability
test, and a compositional analysis of the surface of the electrolyte were
carried out for the fuel cells prepared above in the same manner as
described above. The results were as shown in tables below.
[Table 22]
Thickness Gas perme- Mn
of
electrode abil ty, content,Initial
x 10~~ t
~ ti
l
V
reaction ms~ ~"~to~ en
layer, Pa~' a
,
po
m o
Exam 1e B25 30 6.5 2.9 0.57
Exam 1e B26 3 17.0 3.0 0.52
Exam 1e B27 5 12.5 3.6 0.55
Example B28 20 7.7 3.1 0.57
Exam 1e B29 50 4.4 2.8 0.56
Example B30 55 3.8 2.8--~ - 0.53
[Table 23]
After Estimated
initial
potentialAfter After After potential,
1000 1500 2000
, hr, V hr, V hr, V after
V 40,000
h r, V
Exam 1e 0.57 0.57 0.57 0.57 0.54
B1
Exam 1e 0.52 0.52 0.52 0.52 0.49
B26
Example 0.55 0.55 0.55 0.55 0.52
B27
Exam 1e 0.57 0.57 0.57 0.57 0.54
B28
Exam 1e 0.56 0.56 0.56 0.56 0.53
B29
Exam 1e 0.53 0.53 0.53 0.53 0.50
B30
[0240] The above results show that an air-side electrode reaction
~
. CA 02553074 2006-07-07
61
layer thickness in the range of 5 to 50 ~m is more preferred from the
viewpoints of output performance and durability performance.
[0241 ] Effect of adoption of two-layer structure in air-side electrode
reaction layer
Example B31 --
A second air-side electrode reaction layer formed of an
SSZ material having a composition of 90 mol% Zr02-10 mol% Sc203 was
formed. In this case, an aqueous solution of nitrate of Zr, and an
aqueous solution of nitrate of Sc were provided and were mixed with
1 o each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 2 p.m. This powder (40 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. The slurry was coated onto the surface of the air-side electrode
2 o reaction layer formed in the step (2) in Example B25 to form a coating
which was then sintered at 1400°C. The second layer had a pore
diameter of 1.5 pm and a porosity of 14% and a thickness of 10 ~.m. A
fuel cell was prepared in the same manner as in Example B25 except for
the above matter.
[0242] Example B32
A fuel cell was prepared in the same manner as in Example
B31, except that the thickness of the second air-side electrode reaction
layer was 3 p.m.
[0243] Example B33
3o A fuel cell was prepared in the same manner as in Example
B31, except that the thickness of the second air-side electrode reaction
layer was 5 pm.
[0244] Example B34
A fuel cell was prepared in the same manner as in Example
B31, except that the thickness of the second air-side electrode reaction
layer was 30 p,m.
~
. CA 02553074 2006-07-07
62
[0245] Example B35
A fuel cell was prepared in the same manner as in Example
B31, except that the thickness of the second air-side electrode reaction
layer was 50 Vim.
[0246] Example B36
A fuel cell was prepared in the same manner as in Example
B31, except that the thickness of the second air-side electrode reaction
layer was 55 Vim.
[0247] A gas leakage test, a power generation test, a durability
1 o test, and a compositional analysis of the surface of the electrolyte were
carried out for the fuel cells prepared above in the same manner as
described above. For the compositional analysis, in addition to the
electrolyte on its surface in contact with the fuel electrode, the content of
manganese in the electrolyte in its surface in contact with the second air
side electrode reaction layer was also determined in the same manner
as described above. The same measurement was carried out for
Comparative Example B9. The results were as shown in tables below.
[Table 24]
ThicknessGas perme-Air Fuel Initial
of secondability electrodeelectrodepotential,
x
layer , side side Mn
~m 10''ms'Pa''Mn,
, wt /o content,
wt /o
Exam 1e B25 0 6.5 8.0 2.9 0.57
Exam 1e B31 10 1.3 3.3 1.9 0.64
Exam 1e B32 3 0.8 6.2 2.8 0.58
Exam 1e B33 5 1.0 4.5 2.5 0.61
Exam 1e B34 30 2.8 2.2 0.9 0.65
Exam 1e B35 50 10.0 0.9 0.3 0.61
Exam 1e B36 55 17.5 0.6 0.2 0.57
Comparative
0 6.5 10.5 5.5 0.48
Example B9
[Table 25]
After Estimated
initial
potentialAfter After After potential,
1000 1500 2000
, hr, V hr, V hr, V after
V 40,000
h r, V
Example 0.57 0.57 0.57 0.57 0.54
B25
Example 0.64 0.64 0.64 0.64 0.61
B31
Example 0.58 0.58 0.58 0.58 0.55
B32
Example 0.61 0.61 0.61 0.61 0.58
B33
Example 0.65 0.65 0.65 0.65 0.62
B34
Example 0.61 _0_.61 0.61 0.61 0.58
B35
Example 0.57 0.57 0.57 0.57 0.54
B36 ~ ~
~
- CA 02553074 2006-07-07
63
[0248] The above results show that the provision of the second
air-side electrode reaction layer provides better results, and a thickness
in the range 5 to 50 ~m is more preferred.
[0249] A test was carried out for the construction of the electrolyte.
[0250] Example B37
ScYSZ having a composition of 90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 was prepared as a material for an electrolyte. An
aqueous solution of nitrate of Zr, an aqueous solution of nitrate of Y,
and an aqueous solution of nitrate of Sc were provided and were mixed
1 o with each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.5 Vim. A fuel cell was prepared in
the same manner as in Example B25 except for the above matter.
[0251 ] Example B38
SSZ having a composition of 90 mol% Zr02-10 mol% Sc20s
was prepared as a material for an electrolyte. An aqueous solution of
nitrate of Zr and an aqueous solution of nitrate of Sc were provided and
were mixed with each other so as to give the above composition,
2o followed by coprecipitation with oxalic acid. Heat treatment was then
carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 0.5 ~.m. A fuel cell was
prepared in the same manner as in Example B25 except for the above
matter.
[0252] Example B39
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. YSZ was coated by slurry
coating onto the surface of the air-side electrode reaction layer, and SSZ
was then coated by slurry coating onto the surface of YSZ, and the
assembly was sintered at 1400°C. The thickness of each of the layers
was 15 pm. A fuel cell was prepared in the same manner as in
Example B25 except for the above matter.
[0253] Example B40
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
~
CA 02553074 2006-07-07
64
provided as materials for an electrolyte. SSZ was coated by slurry
coating onto the surface of the air-side electrode reaction layer, YSZ was
then coated by slurry coating onto the surface of SSZ, and SSZ was
further coated by slurry coating onto the surface of YSZ. The layers
were co-sintered at 1400°C. The thickness of each of the layers was 10
p.m. A fuel cell was prepared in the same manner as in Example B25
except for the above matter.
[0254] A gas leakage test, a power generation test, a durability
test, and a compositional analysis of the surface of the electrolyte were
1 o carried out for the fuel cells prepared above in the same manner as
described above. The results were as shown in tables below.
[Table 26]
Gas permeability,Mn content,Initial
x 10' ms''Pa wt% potential,
V
Exam 1e B25 6.5 2.9 0.57
Exam 1e B37 5.7 2.7 0.60
Exam 1e B38 11.3 2.1 0.61
Exam 1e B39 6.5 2.1 0.61
~ Example B40 6.8 2.0 0.62
~
[Table 27]
After Estimated
initial After After After potential,
potential,1000 1500 2000 after 40,000
V hr, V hr, V hr, hr,
V V
Exam 1e 0.57 0.57 0.57 0.57 0.54
B25
Exam 1e 0.60 0.60 0.60 0.60 0.57
B37
Exam 1e 0.61 0.61 0.61 0.61 0.58
B38
Exam 1e __0.61 0.61 0.61 0.61 0.58
B39
Example 0.62 0.62 0.62 0.62 0.59
B40 ~
[0255] Example C1
(1 ) Preparation of air electrode support
Lanthanum manganite containing Sr in solid solution and
having a composition represented by Lao,75Sro.25MnOs was used as an
air electrode. After preparation by coprecipitation, heat treatment was
carried out to prepare a raw material powder for an air electrode. The
average particle diameter was 30 p.m. A cylindrical molded product was
prepared by extrusion, and the molded product was then fired at 1500°C
to prepare an air electrode support. The air electrode support had a
pore diameter of 14 pm, a porosity of 45%, and a wall thickness of 1.5
mm.
'~ CA 02553074 2006-07-07
[0256] (2) Formation of air-side electrode reaction layer (first layer)
A layer formed of an intimate mixture of (La~_XAx)yMn03
with YSZ was formed as a first layer using a material having a
composition of Lao,75Sro.25Mn03/90 mol% Zr02-10 mol% Y203 = 50/50
5 (weight ratio). An aqueous solution of nitrate of La, an aqueous
solution of nitrate of Sr, an aqueous solution of nitrate of Mn, an aqueous
solution of nitrate of Zr, and an aqueous solution of nitrate of Y were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
1 o treatment was then carried out to prepare a raw material powder having
a regulated particle diameter. The average particle diameter was 5 ~.m.
The powder for the first layer (40 parts by weight) was mixed with 100
parts by weight of a solvent (ethanol), 2 parts by weight of a binder
(ethylcellulose), 1 part by weight of a dispersant (polyoxyethylene
15 alkylphosphate), and 1 part by weight of an antifoaming agent (sorbitan
sesquioleate). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 100 mPas. The slurry was coated
onto the surface of the air electrode support (outer diameter 15 mm, wall
thickness 1.5 mm, effective length 400 mm) to form a coating which was
2 o then sintered at 1400°C. The first layer thus formed had a pore
diameter of 5 ~.m, a porosity of 28%, and a thickness of 20 ~.m.
[0257] (3) Formation of air-side electrode reaction layer (second layer)
SSZ having a composition of 90 mol% Zr02-10 mol%
Sc203 was prepared as a material for a second layer. An aqueous
25 solution of nitrate of Zr and an aqueous solution of nitrate of Sc were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
a regulated particle diameter. The average particle diameter was 2 Vim.
3o This powder (40 parts by weight) was mixed with 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
35 viscosity of 100 mPas. The slurry was coated onto the surface of the
first layer to form a coating which was then sintered at 1400°C. The
' CA 02553074 2006-07-07
r
66
second layer had a pore diameter of 1.5 Vim, a porosity of 14%, and a
thickness of 10 ~,m.
[0258] (4) Preparation of slurry for elecrolyte
YSZ was provided as a material for an electrolyte. The
composition of YSZ was 90 mol% Zr02-10 mol% Y203. An aqueous
solution of nitrate of Zr and an aqueous solution of nitrate of Y were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
1 o a regulated particle diameter. The average particle diameter was 0.5
~.m. This powder (40 parts by weight) was mixed with 100 parts by
weight of a solvent (ethanol), 2 parts by weight of a binder
(ethylcellulose), 1 part by weight of a dispersant (polyoxyethylene
alkylphosphate), and 1 part by weight of an antifoaming agent (sorbitan
sesquioleate). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 140 mPas.
[0259) (5) Preparation of electrolyte
The slurry prepared above was coated onto the second
layer to form a coating which was then sintered at 1400°C. The
2 o thickness of the electrolyte thus formed was 30 ~,m. In this case, the air
electrode support in its part on which an interconnector film is to be
formed in a later step was masked so as not to be coated.
[0260] (6) Preparation of slurry for fuel-side electrode reaction
layer
Ni0/SSZ having a composition of
Ni0/(Zr02)o_9o(Sc203)o.,o was prepared as a material for a fuel-side
electrode reaction layer. An aqueous solution of nitrate of Ni, an
aqueous solution of nitrate of Zr, and an aqueous solution of nitrate of Sc
were provided and were mixed with each other so as to give the above
3o composition, and oxalic acid was then added for precipitation. The
precipitate and the supernatant were dried, followed by heat treatment
and particle diameter regulation to prepare a raw material. In this case,
two types of compositions for the fuel-side electrode reaction layer, that
is, Ni0/(Zr02)o.so(Sc20s)o.~o - 20180 and 50/50 (weight ratio) were
prepared. For both the cases, the average particle diameter was 0.5
p.m. This powder (100 parts by weight) was mixed with 500 parts by
'~ CA 02553074 2006-07-07
67
weight of an organic solvent (ethanol), 10 parts by weight of a binder
(ethylcellulose), 5 parts by weight of a dispersant (polyoxyethylene
alkylphosphate), 1 part by weight of an antifoaming agent (sorbitan
sesquioleate), and 5 parts by weight of a plasticizes (DBP). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 70 mPas.
[0261 ] (7) Preparation of fuel-side electrode reaction layer
The electrolyte layer formed in the above step (5) was
masked so that the effective area was 150 cm2. The slurry
to NIO/(ZrO2)0.90(SC2O3)0.10 (average particle diameter) = 20/80 (0.5 ~.m)
and the slurry Ni0/(Zr02)o.so(Sc203)o.~0 = 50/50 (0.5 ~,m) were coated on
the electrolyte layer in that order. The layer thickness (after sintering)
was 10 Vim.
[0262] (8) Preparation of slurry for fuel electrode:
NiO/YSZ having a composition of NiO/(Zr02)o,9o(Y20s)o.~o
was prepared as a material for a fuel electrode. An aqueous solution of
nitrate of Ni, an aqueous solution of nitrate of Zr, and an aqueous
solution of nitrate of Y were provided and were mixed with each other so
as to give the above composition, and oxalic acid was then added for
2 o precipitation. The precipitate and the supernatant were dried, followed
by heat treatment and particle diameter regulation to prepare a raw
material. In this case, a material having a composition of
Ni0/(Zr02)o.so(Y20s)o.~o - 70/30 (weight ratio) was prepared. The
average particle diameter was 2 Vim. This powder (100 parts by weight)
was mixed with 500 parts by weight of an organic solvent (ethanol), 20
parts by weight of a binder (ethylcellulose), 5 parts by weight of a
dispersant (polyoxyethylene alkylphosphate), 1 part by weight of an
antifoaming agent (sorbitan sesquioleate), and 5 parts by weight of a
plasticizes (DBP). The mixture was then thoroughly stirred to prepare a
3o slurry. This slurry had a viscosity of 250 mPas
[0263] (9) Preparation of fuel electrode
The slurry for a fuel electrode was coated onto the fuel-side
electrode reaction layer. The film thickness (after sintering) was 90 p.m.
Further, the fuel-side electrode reaction layer and the fuel electrode were
co-sintered at 1400°C.
[0264] (10) Preparation of interconnector:
~
. CA 02553074 2006-07-07
68
An interconnector having a composition of lanthanum
chromite containing Ca in solid solution represented by Lao.~oCao.3oCr03
was prepared. A raw material powder was prepared by spray pyrolysis
and was then heat treated. The average particle diameter was 1 ~~m.
This powder (40 parts by weight) was mixed with 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkyl phosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
1o viscosity of 100 mPas. An interconnector was formed by slurry coating
using this slurry and was then sintered at 1400°C. The thickness of the
interconnector after sintering was 40 g,m.
[0265] Comparative Example C1
YSZ was prepared as a material for an air-side electrode
reaction layer. The composition of YSZ was 90 mol% Zr02-10 mol%
Y203 (weight ratio). An aqueous solution of nitrate of Zr, and an
aqueous solution of nitrate of Y were provided and were mixed with each
other so as to give the above composition, followed by coprecipitation
with oxalic acid. Heat treatment was then carried out to prepare a raw
2 o material powder having a regulated particle diameter. The average
particle diameter was 2 Vim. This powder (40 parts by weight) was
mixed with 100 parts by weight of a solvent (ethanol), 2 parts by weight
of a binder (ethylcellulose), 1 part by weight of a dispersant
(polyoxyethylene alkylphosphate), and 1 part by weight of an antifoaming
agent (sorbitan sesquioleate). The mixture was then thoroughly stirred
to prepare a slurry. This slurry had a viscosity of 100 mPas. The
slurry was coated onto the surface of the air electrode support to form a
coating which was then sintered at 1400°C. The sinter had a thickness
of 30 Vim. A fuel cell was prepared in the same manner as in Example
C1 except for the above matter.
[0266] Coma~arative Example C2
A layer formed of an intimate mixture composed of an
intimate mixture of (La~_xAx)yMn03 with YSZ and having a
composition of Lao.~5Sro.25MnOsI90 mol% Zr02-10 mol% Y2Os = 50/50
(weight ratio) was formed as an air-side electrode reaction layer. An
aqueous solution of nitrate of La, an aqueous solution of nitrate of Sr, an
~~
CA 02553074 2006-07-07
d
69
aqueous solution of nitrate of Mn, an aqueous solution of nitrate of Zr,
and an aqueous solution of nitrate of Y were provided and were mixed
with each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 5 Vim. This powder (40 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
1 o antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. The slurry was coated onto the surface of the air electrode
support, and the coating was then sintered at 1400°C. The thickness
was 30 Vim. A fuel cell was prepared in the same manner as in
Example C1 except for the above matter.
[0267] Comparative Examale C3
A layer formed of an intimate mixture composed of an
intimate mixture of (La~_XAx)yMnOs with a cerium-containing oxide
reprsented by general formula (Ce02)o.s(Y20s)o.~ (hereinafter referred to
2o as (Lay_xAx)yMn03/(Ce02)o.e(Y20s)o.~) was formed as an air-side
electrode reaction layer. The composition of the intimate mixture was
Lao,~SSro.25Mn03/(Ce02)o.a(YZOs)o.~ - 50/50 (weight ratio).
Lao,75Sro.25Mn03 was prepared by preparing an aqueous solution of
nitrate of La, an aqueous solution of nitrate of Sr, and an aqueous
solution of ntirate of Mn, mixing the aqueous solutions together to give
the above composition, then conducting coprecipitation with oxalic acid,
and further heat treating the precipitate at 1200°C. (Ce02)o,a(Y203)o.~
was prepared by preparing an aqueous solution of nitrate of Ce and an
aqueous solution of nitrate of Y, mixing the aqueous solutions together to
3o give the above composition, then conducting coprecipitation with oxalic
acid, and further heat treating the precipitate at 1200°C. The powder
of
Lao,~sSro,25MnOs and the powder of (CeOz)o.s(Y203)o.~ were mixed
together, and the mixture was then heat treated at 1400°C, and the
particle diameter was futher regulated to give a raw material powder.
The average particle diameter was 5 p.m. This powder (40 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
'~ CA 02553074 2006-07-07
Y
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkyl phosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. The slurry was coated onto the surface of the air electrode
support, and the coating was then sintered at 1400°C. The thickness
was 30 pm. A fuel cell was prepared in the same manner as in
Example C1 except for the above matter.
[0268] Comparative Example C4
1 o A fuel cell was prepared in the same manner as in
Comparative Example C3, except that the electrolyte was sintered at
1500°C.
[0269] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 28]
~erm,~ab~lity, Mn content, Initial
Gas
. wt% potential,
x 10 ms' Pa V
Exam 1e C1 1.8 2.8 0.61
Comparative 3 4 0
0 8 40
Exam 1e C1 . . .
Comparative 5 5 0
6 5 48
Exam 1e C2 . . .
Comparative 210 0 0
1 41
Exam 1e C3 . .
Comparative 17 4 0
5 8 54
Exam 1e C4 . . .
[Table 29]
After After Estimated
initial
potential000 After After potential,
hr 1500
, ~ hr, V 2000 after
, hr, 40,000
V
h r, V
Exam 1e 0.61 0.61 0.61 0.61 0.58
C1
Comparativep,40 0.40 0 0 0
40 395 30
Exam 1e . . .
C1
Comparative
0.48 0.48 0.48 0.475 0.38
Exam 1e
C2
Comparative
0.41 0.41 0.40 0.38 0
Exam 1e
C3
Comparative
0,51 0.51 0.51 0.505 0.41
Exam 1e
C4
[0270] A test was carried out for the pore diameter of the second
' ~ CA 02553074 2006-07-07
71
layer in the air-side electrode reaction layer.
[0271 ] Example C2
A fuel cell was prepared in the same manner as in Example
C1, except that the raw material for the second layer was regulated to an
average particle diameter of 0.5 ~m and was coated onto the surface of
the first layer by slurry coating, and the coating was sintered at
1350°C.
[0272] Example C3
A fuel cell was prepared in the same manner as in Example
C1, except that the raw material for the second layer was regulated to an
1 o average particle diameter of 0.5 ~m and was coated onto the surface of
the first layer by slurry coating, and the coating was sintered at
1380°C.
[0273] Example C4
A fuel cell was prepared in the same manner as in Example
C1, except that the raw material for the second layer was regulated to an
average particle diameter of 0.5 ~m and was coated onto the surface of
the first layer by slurry coating, and the coating was then sintered at
1400°C.
[0274] Example C5
A fuel cell was prepared in the same manner as in Example
2 o C1, except that the raw material for the second layer was regulated to an
average particle diameter of 2 ~m and was coated onto the surface of
the first layer by slurry coating, and the coating was then sintered at
1430°C.
[0275] Example C6
A fuel cell was prepared in the same manner as in Example
C1, except that the raw material for the second layer was regulated to an
average particle diameter of 5 pm and was coated onto the surface of
the first layer by slurry coating, and the coating was then sintered at
1430°C.
[0276] Example C7
A fuel cell was prepared in the same manner as in Example
C1, except that the raw material for the second layer was regulated to an
average particle diameter of 5 ~m and was coated onto the surface of
the first layer by slurry coating, and the coating was then sintered at
3 5 1450°C.
[0277] The determination of Mn content of the electrolyte in its
~
CA 02553074 2006-07-07
r
72
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 30]
Pore Gas perme-
diameter,o abi~~ty, Mn content,Initial
~m Porosity,x ~,,,to~opotential,
~0 10 V
ms~'Pa'
Exam 1e 2 14 1.8 2.8 0.61
C1
Exam 1e 0.2 6 1.5 2.3 0.61
C2
Exam 1e 0.1 3 1.1 4.0 0.60
C3
Exam 1e 0.08 2 0.6 4.6 0.55
C4
Exam 1e 5 25 3.2 3.5 0.60
C5
Exam 1e 10 40 8.5 3.2 0.60
C6
Exam 1e 12 43 14.5 4.4 0.55
C7
[Table 31 ]
After Estimated
initial After After After potential,
potential,1000 1500 2000 after
V hr, V hr, V hr, V 40,000
h r, V
Example 0.61 0.61 0.61 0.61 0.58
C1
Example 0.61 0.61 0.61 0.61 0.58
C2
Example 0.60 0.60 0.60 0.60 0.57
C3
Example 0.55 0.55 0.55 0.55 0.51
C4
Example 0.60 0.60 0.60 0.60 0.57
C5
Example 0.60 0.60 0.60 0.60 0.57
C6
Example 0.55 0.55 0.55 0.55 0.51
C7
[0278] The comparison of the gas permeability of the electrolyte
layer shows that, for Examples C5 to C7, the gas permeability is in the
to preferred gas permeability range Q < 2.8 x 10-9 ms-~Pa-~ but not in the
more preferred gas permeability range Q <_ 2.8 x 10-~° ms-~Pa-'. On the
other hand, for Examples C1 to C4, the gas permeability is in the more
preferred range Q <_ 2.8 x 10-x° ms-~Pa-~. When the gas permeability of
the electrolyte is taken into consideration, it is apparent that a
relationship represented by formula d1 > d2 > d3 wherein d1 represents
the pore diameter of the air electrode; d2 represents the pore diameter of
the first layer; and d3 represents the pore diameter of the second layer is
preferably satisfied.
Further, it is apparent that the porosity of the second layer
2 o is more preferably 3 to 40%.
[0279] A test was carried out for the thickness of the second layer
in the air-side electrode reaction layer.
~
. CA 02553074 2006-07-07
73
[0280] Example C8
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the second layer was 3 Vim.
[0281 ] Example C9
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the second layer was 5 ~.m.
[0282] Example C10
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the second layer was 30 ~.m.
[0283] Example C11
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the second layer was 50 ~,m.
[0284] Example C12
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the second layer was 55 ~.m.
[0285] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
2 0 [Table 32]
Thickness,Gas perme-~o Initial
~m ability, Mn content, potential,
x 10 wt% V
ms' Pa''
Exam 1e C1 10 1.8 2.8 0.61
Exam 1e C8 3 0.3 4.4 0.55
Exam 1e C9 5 0.8 3.9 0.59
Exam 1e C10 30 2.8 1.2 0.62
Exam 1e C11 50 10.0 0.3 0.60
Example C12 55 17.5 0.2 0.55
~
[Table 33]
After Estimated
initial
potentialAfter After After potential,
1000 1500 2000
, hr, V hr, V hr, V after
V 40,000
h r, V
Exam 1e 0.61 0.61 0.61 0.61 0.58
C1
Exam 1e 0.55 0.55 0.55 0.55 0.51
C8
Exam 1e 0.59 0.59 0.59 0.59 0.56
C9
Exam 1e 0.62 0.62 0.62 0.62 0.59
C10
Exam 1e 0.60 0.60 0.60 0.60 0.57
C11
Example 0.55 0.55 0.55 0.55 0.52
C12
[0286] The above results show that the thickness of the second
CA 02553074 2006-07-07
74
layer is more preferably in the range of 5 to 50 Vim.
Further, it is apparent that the content of the manganese
component in the fuel electrode-side surface of the electrolyte is more
preferably 0.3 to 4% by weight.
[0287] A test was carried out for the thickness of the first layer in
the air-side electrode reaction layer.
[0288] Example C13
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the first layer was 3 ~.m.
[0289] Example C14
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the first layer was 5 ~.m.
[0290] Example C15
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the first layer was 30 ~.m.
[0291 ] Example C16
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the first layer was 50 Vim.
[0292] Example C17
A fuel cell was prepared in the same manner as in Example
C1, except that the thickness of the first layer was 55 Vim.
[0293] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 34]
Gas ermeability,Mn content,Initial
Thickness,x 10~~ms~'Pa~'Wt~~o potential,
wm
Exam 1e 20 1.8 2.8 0.61
C1
Exam 1e 3 4.0 4.5 0.55
C13
Exam 1e 5 2.5 4.0 0.58
C14
Example 30 1.5 2.7 0.61
C15
Exam 1e 50 2.8 2.5 0.59
C16
Exam 1e 55 4.0 2.4 0.55
C17
CA 02553074 2006-07-07
r
[Table 35J
After After Estimated
After 1000 1500 After potential,
initial hr hr 2000
potential,, , hr, V after 40,000
V V V
h r, V
Example 0.61 0.61 0.61 0.61 0.58
C1
Exam 1e 0.55 0.55 0.55 0.55 0.51
C13
Exam 1e 0.58 0.58 0.58 0.58 0.55
C14
Exam 1e 0.61 0.61 0.61 0.61 0.58
C15
Exam 1e 0.59 0.59 0.59 0.59 0.56
C16
Exam 1e 0.55 0.55 0.55 0.55 0.52
C17
[0294] The above results show that the thickness of the first layer
is more preferably in the range of 5 to 50 Vim.
5 [0295] A test was carried out with the material for the first layer
and the material for the second layer in the air-side electrode reaction
layer being varied.
[0296] Example C18
ScYSZ was prepared as a material for the second layer.
1 o The composition of ScYSZ was 90 mol% Zr02-5 mol% Sc203-5 mol%
Y20s. An aqueous solution of nitrate of Zr, an aqueous solution of
nitrate of Sc, and an aqueous solution of nitrate of Y were provided and
were mixed with each other so as to give the above composition,
followed by coprecipitation with oxalic acid. Heat treatment was then
15 carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 2 ~.m. A fuel cell was
prepared in the same manner as in Example C1 except for the above
matter.
[0297] Example C19
2 o A layer formed of an intimate mixture composed of an
intimate mixture of (La~_xAx)yMn03 with SSZ was formed as a first layer.
The composition of the intimate mixture was Lao.75Sro.25Mn03/90 mol%
Zr02-10 mol% Sc203 = 50/50 (weight ratio). An aqueous solution of
nitrate of La, an aqueous solution of nitrate of Sr, an aqueous solution of
25 nitrate of Mn, an aqueous solution of nitrate of Zr, and an aqueous
solution of nitrate of Sc were provided and were mixed with each other
so as to give the above composition, followed by coprecipitation with
oxalic acid. Heat treatment was then carried out to prepare a raw
material powder having a regulated particle diameter. The average
3o particle diameter was 5 Vim. A fuel cell was prepared in the same
CA 02553074 2006-07-07
w
76
manner as in Example C1 exept for the above matter.
[0298] Example C20
A layer formed of an intimate mixture of (La~_xAX)Y(Mn~_
ZNiZ)03 with SSZ was formed as a first layer. The composition of the
intimate mixture was (Lao,~SSro,zS)(Mno.ssNio.os)~s/90 mol% Zr02-10 mol%
Sc203 = 50/50 (weight ratio). An aqueous solution of nitrate of La, an
aqueous solution of nitrate of Sr, an aqueous solution of nitrate of Mn, an
aqueous solution of nitrate of Ni, an aqueous solution of nitrate of Zr, and
an aqueous solution of nitrate of Sc were provided and were mixed with
1 o each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 5 Vim. A fuel cell was prepared in
the same manner as in Example C1 except for the above matter.
[0299] Exams Ip a C21
A layer formed of an intimate mixture of (La~_XAx)y(Mn~_
ZNiZ)03 with ScYSZ was formed as a first layer. The composition of the
intimate mixture was (Lao,~SSro.2s)(Mno.ssNio.oS)~s/90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 = 50/50 (weight ratio). An aqueous solution of
2 o nitrate of La, an aqueous solution of nitrate of Sr, an aqueous solution
of
nitrate of Mn, an aqueous solution of nitrate of Ni, an aqueous solution of
nitrate of Zr, an aqueous solution of nitrate of Y, and an aqueous solution
of nitrate of Sc were provided and were mixed with each other so as to
give the above composition, followed by coprecipitation with oxalic acid.
2 5 Heat treatment was then carried out to prepare a raw material powder
having a regulated particle diameter. The average particle diameter
was 5 p,m. A fuel cell was prepared in the same manner as in Example
C1 except for the above matter.
[0300] The determination of Mn content of the electrolyte in its
3 o surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 36]
Gas Mn content, Initial
permeability,
. wt% potential,
x 10 ms~'Pa V
Exam 1e C1 1.8 2.8 0.61
Exam 1e C18 1.0 3.0 0.61
' ~ CA 02553074 2006-07-07
77
Exam 1e C19 2.4 2.4 0.64
Exam 1e C20 1.8 1.5 0.69
LExam ple C21 1.0 1.7 0.68
~
[Table 37]
After Estimated
initial After After After potential,
potential,1000 1500 2000 after 40,000
V hr, V hr, hr, V h r, V
V
Exam 1e C1 0.61 0.61 0.61 0.61 0.58
Exam 1e C18 0.59 0.59 0.59 0.59 0.56
Exam 1e C19 0.64 0.64 0.64 0.64 0.61
Exam 1e C20 0.69 0.69 0 0.69 0.66
.69
Example C21 0.68 0.68 _ 0.68 0.65
~ ~ ~ 0.68
[0301 ] Construction of electrolyte
Example C22
ScYSZ having a composition of 90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 was prepared as a material for an electrolyte. An
aqueous solution of nitrate of Zr, an aqueous solution of nitrate of Y, and
an aqueous solution of nitrate of Sc were provided and were mixed with
1 o each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.5 pm. A fuel cell was prepared in
the same manner as in Example C1 except for the above matter.
i5 [0302] Example C23
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
was prepared as a material for an electrolyte. An aqueous solution of
nitrate of Zr and an aqueous solution of nitrate of Sc were provided and
were mixed with each other so as to give the above composition,
2 o followed by coprecipitation with oxalic acid. Heat treatment was then
carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 0.5 p.m. A fuel cell was
prepared in the same manner as in Example C1 except for the above
matter.
25 [0303] Examale C24
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. YSZ was coated by slurry
- CA 02553074 2006-07-07
r
r
78
coating onto the surface of the second layer, SSZ was then coated by
slurry coating onto the surface of YSZ, and the assembly was sintered at
1400°C. The thickness of each of the layers was 15 ~~m. A fuel cell
was prepared in the same manner as in Example C1 except for the
above matter.
[0304] Example C25
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. SSZ was coated by slurry
1 o coating onto the surface of the second layer, YSZ was then coated by
slurry coating onto the surface of SSZ, and SSZ was further coated by
slurry coating onto the surface of YSZ. The layers were co-sintered at
1400°C. The thickness of each of the layers was 10 pm. A fuel cell
was prepared in the same manner as in Example C1 except for the
above matter.
[0305] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
2 0 [Table 38]
Gas Initial
perm abil Mn content, wt% otential,
ty,
x 10~'~ms~]~a~'
Exam 1e 1.8 2.8 0.61
C1
Exam 1e 1.6 2.3 0.63
C22
Exam 1e 8.5 1.6 0.64
C23
Exam 1e 1.8 1.8 0.65
C24
~ Example 2.1 1.6 0.66
C25 ~
[Table 39]
After Estimated
initial
potentialAfter After After potential,
1000 1500 2000
, hr, V hr, V hr, V after
40,000
h r, V
Exam 1e 0.61 0.61 0.61 0.61 0.58
C1
Exam 1e 0.63 0.63 0.63 0.63 0.60
C22
Exam 1e 0.64 0.64 0.64 0.64 0.61
C23
Exam 1e 0.65 0.65 0.65 0.65 0.62
C24
Exam 1e 0.66 0.66 0.66 0.66 0.63
C25
[0306] Example D1
(1 ) Preparation of air electrode support
CA 02553074 2006-07-07
79
Lanthanum manganite containing Sr in solid solution and
having a composition represented by Lao.~SSro.25Mn03 was used as an
air electrode. After preparation by coprecipitation, heat treatment was
carried out to prepare a raw material powder for an air electrode. The
raw material powder had an average particle diameter of 30 Vim. The
raw material powder was extruded into a cylindrical form. The
cylindrical molded product was fired at 1500°C to prepare an air
electrode support. The air electrode support had a pore diameter of 14
Vim, a porosity of 45%, and a wall thickness of 1.5 mm.
[0307] (2) Preparation of air-side electrode reaction layer
A layer formed of an intimate mixture composed of an
intimate mixture of (La~_xAX)yMn03 with YSZ and having a
composition of Lao,~SSro,25Mn03/90 mol% Zr02-10 mol% Y203 = 50/50
(weight ratio) was formed as an air-side electrode reaction layer. An
aqueous solution of nitrate of La, an aqueous solution of nitrate of Sr, an
aqueous solution of nitrate of Mn, an aqueous solution of nitrate of Zr,
and an aqueous solution of nitrate of Y were provided and were mixed
with each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
2 o prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 5 Vim. This powder (40 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 100
mPas. The slurry was coated onto the surface of the air electrode
support, and the coating was then sintered at 1400°C. The thickness
was 30 ~,m.
[0308] (3) Preparation of slurry for elecrolyte:
YSZ was provided as a material for an electrolyte. The
composition of YSZ was 90 mol% Zr02-10 mol% Y203. An aqueous
solution of nitrate of Zr and an aqueous solution of nitrate of Y were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
CA 02553074 2006-07-07
a regulated particle diameter. The average particle diameter was 0.5
Vim. This powder (40 parts by weight) was mixed with 100 parts by
weight of a solvent (ethanol), 2 parts by weight of a binder
(ethylcellulose), 1 part by weight of a dispersant (polyoxyethylene
alkylphosphate), and 1 part by weight of an antifoaming agent (sorbitan
sesquioleate). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 140 mPas.
(0309] (4) Preparation of electrolyte
The slurry prepared above was coated onto the surface of
1 o the air-side electrode reaction layer to form a coating which was then
sintered at 1400°C. The thickness of the electrolyte thus formed was
30 ~,m. In this case, the air electrode support in its part on which an
interconnector film is to be formed in a later step was masked so as not
to be coated. The porosity was 1 %.
[0310] (5) Preparation of slurry for porous layer formed of zirconia-
containing fluorite oxide
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
2o aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.5 ~.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. This slurry had a viscosity of 200
mPas.
[0311 ] (6) Preparation of porous layer formed of zirconia-
containing fluorite oxide
The slurry prepared above was coated onto the surface of
the electrolyte layer, and the coating was sintered at 1400°C. The
thickness of the porous layer thus formed was 20 ~.m. In this case, the
air electrode support in its part on which an interconnector film is to be
' ' CA 02553074 2006-07-07
r
81
formed in a later step was masked so as not to be coated. The porous
layer had a porosity of 15% and a pore diameter of 0.3 ~,m.
[0312] (7) Preparation of slurry for fuel-side electrode reaction
layer
Ni0/SSZ having a composition of NiO/(Zr02)o.so(Sc20s)o.~o
was prepared as a material for a fuel-side electrode reaction layer. An
aqueous solution of nitrate of Ni, an aqueous solution of nitrate of Zr, and
an aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, and oxalic acid was then
1 o added for precipitation. The precipitate and the supernatant were dried,
followed by heat treatment and particle diameter regulation to prepare a
raw material. In this case, two types of compositions for the fuel-side
electrode reaction layer, that is, Ni01(Zr02)o.so(Sc203)o.~0 = 20/80 and
50/50 (weight ratio) were prepared. For both the cases, the average
particle diameter was 0.5 Vim. This powder (100 parts by weight) was
mixed with 500 parts by weight of an organic solvent (ethanol), 10 parts
by weight of a binder (ethylcellulose), 5 parts by weight of a dispersant
(polyoxyethylene alkylphosphate), 1 part by weight of an antifoaming
agent (sorbitan sesquioleate), and 5 parts by weight of a plasticizer
(DBP). The mixture was then thoroughly stirred to prepare a slurry.
This slurry had a viscosity of 70 mPas.
[0313] (8) Preparation of fuel-side electrode reaction layer
The porous layer formed in the above step (6) was
masked so that the effective area was 150 cm2. The slurry
NiO/(Zr02)o.so(Sc203)o.~0 (average particle diameter) = 20/80 (0.5 Vim)
and the slurry Ni0/(Zr02)o.so(Sc203)o.,0 = 50/50 (0.5 Vim) were coated on
the porous layer in that order. The layer thickness (after sintering) was
10 ~.m.
[0314] (9) Preparation of slurry for fuel electrode:
3o Ni0/YSZ having a composition of Ni0/(Zr02)o.so(YzOs)o.~o
was prepared as a material for a fuel electrode. An aqueous solution of
nitrate of Ni, an aqueous solution of nitrate of Zr, and an aqueous
solution of nitrate of Y were provided and were mixed with each other so
as to give the above composition, and oxalic acid was then added for
precipitation. The precipitate and the supernatant were dried, followed
by heat treatment and particle diameter regulation to prepare a raw
CA 02553074 2006-07-07
82
material. In this case, a material having a composition of
Ni0/(Zr02)o.so(Y2~s)o.~o - 70/30 (weight ratio) was prepared. The
average particle diameter was 2 t.~m. This powder (100 parts by weight)
was mixed with 500 parts by weight of an organic solvent (ethanol), 20
parts by weight of a binder (ethylcellulose), 5 parts by weight of a
dispersant (polyoxyethylene alkylphosphate), 1 part by weight of an
antifoaming agent (sorbitan sesquioleate), and 5 parts by weight of a
plasticizer (DBP). The mixture was then thoroughly stirred to prepare a
slurry. This slurry had a viscosity of 250 mPas.
[0315] (10) Preparation of fuel electrode
The slurry for a fuel electrode was coated onto the fuel-side
electrode reaction layer. The film thickness (after sintering) was 90 ~,m.
Further, the fuel-side electrode reaction layer and the fuel electrode were
co-sintered at 1400°C.
[0316] (11 ) Preparation of interconnector:
An interconnector having a composition of lanthanum
chromite containing Ca in solid solution represented by Lao,~oCao.3oCr03
was prepared. A raw material powder was prepared by spray pyrolysis
and was then heat treated. The average particle diameter was 1 Vim.
This powder (40 parts by weight) was mixed with 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 100 mPas. An interconnector was formed by slurry coating
using this slurry and was then sintered at 1400°C. The thickness of the
interconnector after sintering was 40 Vim.
The thickness referred to herein is a thickness determined
by cutting the fuel cell, observing a cut section between the air electrode
3o and the fuel electrode under SEM and calculating the thickness based on
the scale of the photograph.
[0317] Example D2
A fuel cell was prepared in the same manner as in Example
D1, except that the thickness of the porous layer was 5 g,m.
[0318] Example D3
A fuel cell was prepared in the same manner as in Example
CA 02553074 2006-07-07
r
83
D1, except that the thickness of the porous layer was 40 pm.
[Table 40]
Gas.~ermeab~lity,Mn content, Initial potential,
x 10 ms' Pa wt% V
Exam 1e 1.8 1.5 0.59
D1
Exam 1e 4.4 3.5 0.54
D2
Exam 1e 2.9 2.8 0.58
D3
Examle D4 2.8 0.9 0.58
Example 5.1 0.5 0.55
D5 ~
D1, except that the thickness of the porous layer was 10 pm.
[0319] Example D4
A fuel cell was prepared in the same manner as in Example
D1, except that the thickness of the porous layer was 30 pm.
[0320] Example D5
A fuel cell was prepared in the same manner as in Example
[Table 41 ]
After Estimated
initial After After After potential
1000 1500 2000 after
potential,hr, V hr, V hr, V ,
40
000 hr
V
V ,
,
Exam 1e 0.59 0.59 0.59 0.59 0.56
D1
Exam 1e 0.54 0.54 0.54 0.54 0.51
D2
Exam 1e 0.58 0.58 0.58 0.58 0.55
D3
Example 0.58 0.58 0.58 0.58 0.55
D4
Exam 1e 0.55 0.55 0.55 0.55 0.52
D5
[0321 ] A test was carried out with the porosity and pore diameter
of the porous layer being varied.
[0322] Example D6
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
2o coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.3 p.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
~
~ CA 02553074 2006-07-07
r
84
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 ~~m, a
porosity of 3%, and a pore diameter of 0.1 ~,m. A fuel cell was formed
in the same manner as in Example D1 except for the above matter.
[0323] Example D7
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc2O3. An aqueous solution of nitrate of Zr and an
1 o aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.3 ~.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
2 o surface of the electrolyte to form a coating which was then sintered at
1380°C. The porous layer thus formed had a thickness of 20 ~.m, a
porosity of 8%, and a pore diameter of 0.05 ~.m. A fuel cell was formed
in the same manner as in Example D1 except for the above matter.
[0324] Example D8
2 5 SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
3o coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 ~,m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
35 dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
CA 02553074 2006-07-07
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 t.~m, a
porosity of 15%, and a pore diameter of 0.8 N.m. A fuel cell was formed
5 in the same manner as in Example D1 except for the above matter.
[0325] Example D9
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
1o aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 Vim. This powder (20 parts by
15 weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
2o surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 ~,m, a
porosity of 20%, and a pore diameter of 2 ~,m. A fuel cell was formed in
the same manner as in Example D1 except for the above matter.
(0326] Example D10
25 SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
3o coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 pm. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
35 dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
CA 02553074 2006-07-07
86
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1350°C. The porous layer thus formed had a thickness of 20 Vim, a
porosity of 30%, and a pore diameter of 1.2 Vim. A fuel cell was formed
in the same manner as in Example D1 except for the above matter.
[0327] Example D11
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
1 o aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.2 Vim. This powder (30 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
2 0 surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 ~,m, a
porosity of 2%, and a pore diameter of 0.04 ~.m. A fuel cell was formed
in the same manner as in Example D1 except for the above matter.
[0328] Example D12
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
3o coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 2 ~.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
CA 02553074 2006-07-07
87
thoroughly
stirred
to
prepare
a
slurry.
The
slurry
was
coated
onto
the
surface h
of was
the then
electrolyte sintered
to at
form
a
coating
whic
1400C. thickness
The of
porous 20
layer urn,
thus a
formed
had
a
porosity A
of fuel
32%, cell
and was
a formed
pore
diameter
of
2.5
Vim.
in
the
same
manner
as
in
Example
D1
except
for
the
above
matter.
[Table
42]
Pore Gas Mn Initial
Porosity,diameter,permeability, content,potential,
%
wm x 10''ms~'Pa~' wt% V
Exam 1e 12 0.3 1.8 1.5 0.59
D1
Exam 1e 3 0.1 0.6 3.3 0.57
D6
Exam 1e 8 0.05 1.5 2.6 0.58
D7
Exam 1e 15 0.8 2.9 1.4 0.59
D8
Exam 1e 20 2 4.1 1.2 0.58
D9
Exam 1e 30 1.2 10.7 0.8 0.57
D10
Exam 1e 2 0.03 0.5 3.7 0.53
D11
Exam 1e 33 2.5 17.2 0.5 0.58
D12
[Table 43]
After After Estimated
initial After After
potential,1000 1500 2000 ptential,
V hr, hr, V hr, after 40,000
V V hr, V
Example 0.59 0.59 0.59 0.59 0.56
D1
Example 0.57 0.57 0.57 0.57 0.54
D6
Example 0.58 0.58 0.58 0.58 0.55
D7
Example 0.59 0.59 0.59 0.59 0.56
D8
Example 0.58 0.58 0.58 0.58 0.55
D9
Example 0.57 0.57 0.57 0.57 0.54
D10
Example 0.53 0.53 0.53 0.53 0.50
D11
Example 0.53 0.53 0.53 ~ 0.53 ~ 0.50
D12
[0329] A test was carried out for the material for the porous layer.
[0330] Example D13
ScYSZ was provided as a material for the porous layer.
The composition for ScYSZ was 90 mol% Zr02-5 mol% Sc203-5 mol%
Y203. An aqueous solution of nitrate of Zr, an aqueous solution of
nitrate of Sc, and an aqueous solution of nitrate of Y were mixed
together to give the above composition, followed by coprecipitation with
oxalic acid. A fuel cell was prepared in the same manner as in Example
D1 except for the above matter.
[0331] Example D14
2o YSZ was provided as a material for the porous layer. The
composition for YSZ was 90 mol% Zr02-10 mol% Y20s. An aqueous
°
CA 02553074 2006-07-07
88
solution of nitrate of Zr and an aqueous solution of nitrate of Y were
mixed together to give the above composition, followed by
coprecipitation with oxalic acid. A fuel cell was prepared in the same
manner as in Example D1 except for the above matter.
[0332] Comparative Example D5
A layer formed of a cerium-containing oxide represented
by (Ce02)o.s(Sm20s)o.~ was provided between an electrolyte and a fuel-
side electrode reaction layer. An aqueous solution of nitrate of Ce and
an aqueous solution of nitrate of Sm were provided and mixed together
1 o to give the above composition, followed by coprecipitation with oxalic
acid. Heat treatment was further carried out to prepare a raw material
powder having a regulated particle diameter. The raw material powder
had an average particle diameter of 0.5 p.m, a porosity of 18%, and a
pore diameter of 0.5 p.m. A fuel cell was prepared in the same manner
as in Example D1, except that this layer was provided.
[0333] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 44]
c~as Mn content,
~m il~t Initial potential,
V
~a.~ Wt
p 10' ~
Exam 1e D1 1.8 1.5 0.59
Exam 1e D13 1.3 1.7 0.58
Exam 1e D14 1.3 2.0 0.56
Comparative
4.0 0.2 0.55
Exam 1e D5
[Table 45]
After After After After Estimated
initial 1000 1500 2000 potential,
hr hr hr
potential,, , , after 40,000
V V V
V h r, V
Exam 1e D1 0.59 0.59 0.59 0.59 0.56
Exam 1e D13 0.58 0.58 0.58 0.58 0.55
Exam 1e D14 0.56 0.56 0.56 0.56 0.53
Comparative
p,55 0.55 0.545 0.54 0.35
Exam 1e D5
[0334] A test was carried out for a material for an air side
electrode reaction layer.
CA 02553074 2006-07-07
89
[0335]
Example
D15
A layer
formed
of
an
intimate
mixture
of
(La~_xAX)y(Mn~_
ZNiZ)03
with
SSZ
was
formed
as
an
air-side
electrode
reaction
layer.
The
composition
of
the
intimate
mixture
was
(Lao,~5Sro,25)(Mno.ssNio.os)~s~90
mol%
Zr02-10
mol%
Sc203
- 50/50
(weight
ratio).
An
aqueous
solution
of
nitrate
of
La,
an
aqueous
solution
of
nitrate
of
Sr,
an
aqueous
solution
of
nitrate
of
Mn,
an
aqueous
solution
of
nitrate
of
Ni,
an
aqueous
solution
of
nitrate
of
Zr,
and
an
aqueous
solution
of
nitrate
of
Sc
were
prepared
and
mixed
together
to
1 o
give
the
above
composition,
followed
by
coprecipitation
with
oxalic
acid.
Heat
treatment
was
further
carried
out
to
prepare
a raw
material
powder
having
a regulated
particle
diameter.
The
raw
material
powder
had
an
average
particle
diameter
of
5 pm.
A fuel
cell
was
prepared
in
the
same
manner
as
in
Example
D1,
except
for
the
above
matter.
[0336]
The
determination
of
Mn
content
of
the
electrolyte
in
its
surface
on
the
fuel
electrode
side,
a gas
leakage
test,
a power
generation
test,
and
a durability
test
were
carried
out
for
the
fuel
cells
thus
obtained.
The
results
were
as
shown
in
tables
below.
[Table
46]
Gas.~ermeab~lity,Mn content, Initial
0 wt% otential
' P V
a ,
ms p
x 1
Exam 1e D1 1.8 1.5 0.59
Example D15 1.8 1.2 ~ 0.66
[Table 47]
Estimated
After After After After potential,
initial 1000 1500 2000
potential,hr hr hr, V after 40,000
V V
V , , h r, V
Exam 1e 0.59 0.59 0.59 0.59 0.56
D1
Exam 1e 0.66 0.66 0.66 0.66 0.63
D15
[0337] A test was carried out with the construction of the
electrolyte being varied.
[0338] Example D16
ScYSZ having a composition of 90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 was prepared as a material for an electrolyte. An
aqueous solution of nitrate of Zr, an aqueous solution of nitrate of Y,
and an aqueous solution of nitrate of Sc were provided and were mixed
3o with each other so as to give the above composition, followed by
' CA 02553074 2006-07-07
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.5 Vim. A fuel cell was prepared in
the same manner as in Example D1 except for the above matter.
5 [0339] Example D17
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
was prepared as a material for an electrolyte. An aqueous solution of
nitrate of Zr and an aqueous solution of nitrate of Sc were provided and
were mixed with each other so as to give the above composition,
1 o followed by coprecipitation with oxalic acid. Heat treatment was then
carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 0.5 ~,m. A fuel cell was
prepared in the same manner as in Example D1 except for the above
matter.
15 (0340] Example D18
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. SSZ was coated by slurry
coating onto the surface of the air-side electrode reaction layer, and YSZ
2o was then coated by slurry coating onto the surface of SSZ, and the
assembly was sintered at 1400°C. The thickness of each of the layers
was 15 ~,m. A fuel cell was prepared in the same manner as in
Example D1 except for the above matter.
[0341 ] Example D19
25 SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. SSZ was coated by slurry
coating onto the surface of the air-side electrode reaction layer, YSZ was
then coated by slurry coating onto the surface of SSZ, and SSZ was
3o further coated by slurry coating onto the surface of YSZ. The layers
were co-sintered at 1400°C. The thickness of each of the layers was 10
Vim. A fuel cell was prepared in the same manner as in Example D1
except for the above matter.
[0342] The determination of Mn content of the electrolyte in its
35 surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
CA 02553074 2006-07-07
91
thus obtained. The results were as shown in tables below.
[Table 48)
Gas
perm~e~abil~ty,Mn content, p tential,
,~ wt% V
x 10 ms' Pa
Exam 1e D1 1.8 1.5 0.59
Exam 1e D16 1.6 1.1 0.61
Exam 1e D17 10.1 0.5 0.60
Exam 1e D18 2.7 1.1 0.63
Example D19 ~ 3.5 ~ 0.9 0.63
[Table 49)
Estimated
After After After After Potential,
initial 1000 1500 2000
potentialhr V hr after
V V hr V
, , , , 40,000
hr,
V
Exam 1e 0.59 0.59 0.59 0.59 0.56
D1
Exam 1e 0.61 0.61 0.61 0.61 0.58
D16
Exam 1e 0.60 0.60 0.60 0.60 0.57
D17
Exam 1e 0.6_3_ 0.63_ 0.63 0.63 0.60
D18
Example 0.63 ~ 0.63 0.63 0.63 0.60
D19 ~ ~
[0343) Example E1
A fuel cell was prepared in the same manner as in
Example D1, except that the following two-layer structure was adopted in
the air-side electrode reaction layer.
[0344] (1 ) Formation of air-side electrode reaction layer (first layer)
A layer formed of an intimate mixture of (La~_XAX)yMn03
with YSZ was formed as a first layer using a material having a
composition of Lao,~5Sro.25MnOs/90 mol% Zr02-10 mol% Y2O3 = 50/50
(weight ratio). An aqueous solution of nitrate of La, an aqueous
25 solution of nitrate of Sr, an aqueous solution of nitrate of Mn, an aqueous
solution of nitrate of Zr, and an aqueous solution of nitrate of Y were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
2o a regulated particle diameter. The average particle diameter was 5 pm.
The powder (40 parts by weight) was mixed with 100 parts by weight of a
solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part by
weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part by
weight of an antifoaming agent (sorbitan sesquioleate). The mixture
~
. CA 02553074 2006-07-07
92
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 100 mPas. The slurry was coated onto the surface of the
air electrode support (outer diameter 15 mm, wall thickness 1.5 mm,
effective length 400 mm) to form a coating which was then sintered at
1400°C. The first layer thus formed had a pore diameter of 5 Vim, a
porosity of 28%, and a thickness of 20 ~.m.
[0345] (2) Formation of air-side electrode reaction layer (second layer)
SSZ having a composition of 90 mol% Zr02-10 mol%
Sc203 was prepared as a material for a second layer. An aqueous
1 o solution of nitrate of Zr and an aqueous solution of nitrate of Sc were
provided and were mixed with each other so as to give the above
composition, followed by coprecipitation with oxalic acid. Heat
treatment was then carried out to prepare a raw material powder having
a regulated particle diameter. The average particle diameter was 2 ~,m.
This powder (40 parts by weight) was mixed with 100 parts by weight of
a solvent (ethanol), 2 parts by weight of a binder (ethylcellulose), 1 part
by weight of a dispersant (polyoxyethylene alkylphosphate), and 1 part
by weight of an antifoaming agent (sorbitan sesquioleate). The mixture
was then thoroughly stirred to prepare a slurry. This slurry had a
viscosity of 100 mPas. The slurry was coated onto the surface of the
first layer to form a coating which was then sintered at 1400°C. The
second layer had a pore diameter of 1.5 Vim, a porosity of 14%, and a
thickness of 10 ~.m.
[0346] Example E2
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the porous layer was 5 Vim.
[0347] Example E3
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the porous layer was 10 ~,m.
[0348] Example E4
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the porous layer was 30 ~,m.
[0349] Example E5
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the porous layer was 40 ~,m.
[0350] The determination of Mn content of the electrolyte in its
. . CA 02553074 2006-07-07
93
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table
50]
Gas
perm~e~abil~ty,Mn content, Initial potential,
.~ wt% V
x 10 ms' Pa
Exam 1e 2.3 1.1 0.66
E1
Exam 1e 4.2 1.4 0.64
E2
Exam 1e 2.6 1.3 0.66
E3
Exam 1e 2.4 0.7 0.66
E4
Example 4 6 ~ 0.3 ~ 0.65
E5
[Table
51
]
After After After Estimated
initial After potential,
1000 1500 2000
potential,hr, hr, after 40,000
V V V hr, V h r, V
Exam 1e E1 0.66 0.66 0.66 0.66 0.63
Exam 1e E2 0.64 0.64 0.64 0.64 0.61
Exam 1e E3 0.66 0.66 0.66 0.66 0.63
Exam 1e E4 0.66 0.66 0.66 0.66 0.63
Examale E5 0.65 0.65 0.65 0.65 ~ 0.62
~
[0351 ] A test was carried out for the pore diameter of the second
layer in the air-side electrode reaction layer.
[0352] Example E6
A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 0.5 ~.m, the raw material was applied by
slurry coating onto the surface of the first layer, and the coating was then
sintered at 1350°C.
[0353] Example E7
A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 0.5 ~,m, the raw material was applied by
2 o slurry coating onto the surface of the first layer, and the coating was
then
sintered at 1380°C.
[0354] Example E8
A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 0.5 p,m, the raw material was applied by
CA 02553074 2006-07-07
94
slurry coating onto the surface of the first layer, and the coating was then
sintered at 1400°C.
[0355] Example E9
A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 2 ~,m, the raw material was applied by slurry
coating onto the surface of the first layer, and the coating was then
sintered at 1430°C.
[0356] Example E10
1 o A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 5 ~,m, the raw material was applied by slurry
coating onto the surface of the first layer, and the coating was then
sintered at 1430°C.
[0357] Example E11
A fuel cell was prepared in the same manner as in Example
E1, except that the average particle diameter of the raw material for the
second layer was brought to 5 ~,m, the raw material was applied by slurry
coating onto the surface of the first layer, and the coating was then
2 o sintered at 1450°C.
[Table 52]
Pore Porosity,Gas Mn Initial
diameter,% permeability,content,potential,
m x 10''ms wt% V
Pa''
Exam 1e 2 14 2.3 1.1 0.66
E1
Exam 1e 0.2 6 1.3 1.4 0.66
E6
Exam 1e 0.1 3 1.1 1.4 0.66
E7
Exam 1e 0.08 2 0.6 1.5 0.63
E8
Exam 1e 5 25 3.2 2.5 0.66
E9
Exam 1e 10 40 8.5 2.2 0.65
E10
Exam 1e 12 43 14.5 3.3 0.63
E11
[Table 53]
After After After After
initial Estimated potential,
1000 1500 2000
potentialhr, hr, hr, after 40,000
,V V V V hr, V
Example 0.66 0.66 0.66 0.66 0.63
E1
Example 0.66 0.66 0.66 0.66 0.63
E6
Example 0.66 0.66 0.66 0.66 0.63
E7
Example 0.63 0.63 0.63 0.63 0.60
E8
Example 0.66 0.66 0.66 0.66 0.63
E9
Example 0.65 0.65 0.65 0.65 0.62
E10
Example 0.63 0.63 0.63 0.63 0.60
E11
CA 02553074 2006-07-07
[0358] The comparison of the gas permeability of the electrolyte
shows that, for Examples 9 to 11, the gas permeability is in the
preferred gas permeability range Q <_ 2.8 x 10-9 ms-'Pa-' but not in the
5 more preferred gas permeability range Q <_ 2.8 x 10~'° ms-'Pa-'. On
the
other hand, for Examples E1 and 6 to 8, the gas permeability is in the
more preferred range Q < 2.8 x 10~'° ms-'Pa-'. When the gas
permeability of the electrolyte is taken into consideration, it is apparent
that a relationship represented by formula d1 > d2 > d3 wherein d1
10 represents the pore diameter of the air electrode; d2 represents the pore
diameter of the first layer; and d3 represents the pore diameter of the
second layer is preferably satisfied.
[0359] A test was carried out for the thickness of the second layer
in the air-side electrode reaction layer.
15 [0360] Example E12
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the second layer was 3 ~,m.
[0361 ] Example E13
A fuel cell was prepared in the same manner as in Example
2 o E1, except that the thickness of the second layer Was 5 Vim.
[0362] Example E14
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the second layer was 30 Vim.
[0363] Example E15
25 A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the second layer was 50 pm.
[0364] Example E16
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the second layer was 55 ~,m.
30 [0365] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 54]
Gas perme- Mn
'' Initial potential
Thickness, ability, content,,
~.m x 10 U
'
ms~ wt%
Pa''
~
~ CA 02553074 2006-07-07
96
Exam 1e E1 10 2.3 1.1 0.66
Example E12 3 0.3 1.5 0.62
Exam 1e E13 5 0.8 1.4 0.65
Exam 1e E14 30 2.8 0.7 0.66
Exam 1e E15 50 10.0 0.3 0.65
Exam 1e E16 55 17.5 0.2 0.62
[Table
55]
After After After After Estimated
initial1000 1500 2000 potential,
potential,hr, hr, hr, after 40,000
V V V V hr, V
Example E1 0.66 0.66 0.66 0.66 0.63
Exam 1e E12 0.62 0.62 0.62 0.62 0.59
Exam 1e E13 0.65 0.65 0.65 0.65 0.62
Exam 1e E14 0.66 0.66 0.66 0.66 0.63
Exam 1e E15 0.65 0.65 0.65 0.65 0.62
Exam 1e E16 0.62 0.62 0.62 0.62 0.59
[0366] A test was carried out for the thickness of the first layer in
the air-side electrode reaction layer.
[0367] Example E17
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the first layer was 3 pm.
[0368] Example E18
1 o A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the first layer was 5 Vim.
[0369] Example E19
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the first layer was 30 pm.
[0370] Example E20
A fuel cell was prepared in the same manner as in Example
E1, except that the thickness of the first layer was 50 Vim.
[0371 ] Example E21
A fuel cell was prepared in the same manner as in Example
2 o E1, except that the thickness of the first layer was 55 pm.
[0372] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
2 5 [Table 56]
Thickness, wm Gas Mn content, wt% Initial
~
~ CA 02553074 2006-07-07
97
permeability, potential,
x 10''
ms~'Pa~'
Example 20 2.3 1.1 0.66
E1
Exam 1e 3 4.0 3.3 0.62
E17
Exam 1e 5 2.5 2.6 0.65
E18
Exam 1e 30 1.5 0.8 0.66
E19
Exam 1e 50 2.8 0.4 0.65
E20
Exam 1e 55 4.0 0.3 0.62
E21
[Table
57]
After Estimated
initial After After After potential,
potential,1000 1500 2000 after 40,000
V hr, V hr, V hr, V h r, V
Exam 1e 0.66 0.66 0.66 0.66 0.63
E1
Exam 1e 0.62 0.62 0.62 0.62 0.59
E17
Exam 1e 0.65 0.65 0.65 0.65 0.62
E18
Exam 1e 0.66 0.66 0.66 0.66 0.63
E19
Exam 1e 0.65 0.65 0.65 0.65 0.62
E20
Exam 1e 0.62 0.62 0.62 0.62 0.59
E21
[0373] A test was carried out with the porosity and pore diameter
of the porous layer.
[0374] Example E22
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
1 o aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.3 ~.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
2 o surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 ~,m, a
porosity of 3%, and a pore diameter of 0.1 ~,m. A fuel cell was formed
in the same manner as in Example E1 except for the above matter.
[0375] Example E23
' ' CA 02553074 2006-07-07
98
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc2O3. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.3 p.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
1 o parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1380°C. The porous layer thus formed had a thickness of 20 ~.m, a
porosity of 8%, and a pore diameter of 0.05 ~,m. A fuel cell was formed
in the same manner as in Example E1 except for the above matter.
[0376] Example E24
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc20s. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation With oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 um. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
3o antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 ~.m, a
porosity of 15%, and a pore diameter of 0.8 ~,m. A fuel cell was formed
in the same manner as in Example E1 except for the above matter.
[0377) Exam~ole E25
' ' CA 02553074 2006-07-07
99
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc2O3. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 p.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
1 o parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1.5 1400°C. The porous layer thus formed had a thickness of 20 pm, a
porosity of 20%, and a pore diameter of 2 p.m. A fuel cell was formed in
the same manner as in Example E1 except for the above matter.
[0378] Example E26
SSZ was provided as a material for a porous layer formed
20 of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc2O3. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
25 prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 1 ~.m. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
3o antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1350°C. The porous layer thus formed had a thickness of 20 p.m, a
porosity of 30%, and a pore diameter of 1.2 p.m. A fuel cell was formed
35 in the same manner as in Example E1 except for the above matter.
[0379] Example E27
CA 02553074 2006-07-07
lob
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.2 p,m. This powder (30 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 2
1 o parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 pm, a
porosity of 2%, and a pore diameter of 0.04 p.m. A fuel cell was formed
in the same manner as in Example E1 except for the above matter.
[0380] Example E28
SSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of SSZ was 90
mol% Zr02-10 mol% Sc203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 2 Vim. This powder (20 parts by
weight) was mixed with 100 parts by weight of a solvent (ethanol), 5
parts by weight of a binder (ethylcellulose), 1 part by weight of a
dispersant (polyoxyethylene alkylphosphate), and 1 part by weight of an
3o antifoaming agent (sorbitan sesquioleate). The mixture was then
thoroughly stirred to prepare a slurry. The slurry was coated onto the
surface of the electrolyte to form a coating which was then sintered at
1400°C. The porous layer thus formed had a thickness of 20 p,m, a
porosity of 32%, and a pore diameter of 2.5 p.m. A fuel cell was formed
in the same manner as in Example E1 except for the above matter.
' ' CA 02553074 2006-07-07
101
[Table 58]
Pore Gas Mn Initial
Porosity,diameter,ermeabilit content,otential,
% m p 10'' ms~'P~a~'wt% V
Exam 1e E1 12 0.3 2.3 1.1 0.66
Exam 1e E22 3 0.1 0.6 2.2 0.65
Exam 1e E23 8 0.05 1.5 1.9 0.66
Exam 1e E24 15 0.8 2.9 0.9 0.66
Exam 1e E25 20 2 4.1 0.7 0.66
Exam 1e E26 30 1.2 10.7 0.4 0.65
Exam 1e E27 2 0.03 0.5 2.4 0.62
_
Example E28 33 2.5 17.2 0.3 _
0.62
[Table 59]
Estimated
After After After After Potential,
initial 1000 1500 2000 after
potential,hr, V hr, V hr, V 40,000
V hr,
V
Example E1 0.66 0.66 0.66 0.66 0.63
Example E22 0.65 0.65 0.65 0.65 0.62
Example E23 0.66 0.66 0.66 0.66 0.63
Example E24 0.66 0.66 0.66 0.66 0.63
Example E25 0.66 0.66 0.66 0.66 0.63
Example E26 0.65 0.65 0.65 0.65 0.62
Example E27 0.62 0.62 0.62 0.62 0.59
Example E28 ~ 0.62 __ 0.62 0.62 0.59
~ 0.62
[0381 ] A test was carried out for the material for the first layer and
the second layer in the air-side electrode reaction layer.
[0382] Example E29
ScYSZ was prepared as a material for the second layer.
The composition of ScYSZ was 90 mol% Zr02-5 mol% Sc203-5 mol%
1 o Y20s. An aqueous solution of nitrate of Zr, an aqueous solution of
nitrate of Sc, and an aqueous solution of nitrate of Y were provided and
were mixed with each other so as to give the above composition,
followed by coprecipitation with oxalic acid. Heat treatment was then
carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 2 p.m. A fuel cell was
prepared in the same manner as in Example E1 except for the above
matter.
[0383] Example E30
A layer formed of an intimate mixture composed of an
2o intimate mixture of (La~_xAx)yMr103 with SSZ was formed as a first layer.
The composition of the intimate mixture was Lao,~SSro.25MnOs/90 mol%
CA 02553074 2006-07-07
102
Zr02-10 mol% Sc203 = 50/50 (weight ratio). An aqueous solution of
nitrate of La, an aqueous solution of nitrate of Sr, an aqueous solution of
nitrate of Mn, an aqueous solution of nitrate of Zr, and an aqueous
solution of nitrate of Sc were provided and were mixed with each other
so as to give the above composition, followed by coprecipitation with
oxalic acid. Heat treatment was then carried out to prepare a raw
material powder having a regulated particle diameter. The average
particle diameter was 5 Vim. A fuel cell was prepared in the same
manner as in Example E1 except for the above matter.
[0384] Example E31
A layer formed of an intimate mixture of (La~_xAX)y(Mn~_
ZNiZ)03 with SSZ was formed as a first layer. The composition of the
intimate mixture was (Lao,~5Sro.25)(Mno.95Nio.os)~3/90 mol% Zr02-10 mol%
Sc203 = 50/50 (weight ratio). An aqueous solution of nitrate of La, an
aqueous solution of nitrate of Sr, an aqueous solution of nitrate of Mn, an
aqueous solution of nitrate of Ni, an aqueous solution of nitrate of Zr, and
an aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
2 o prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 5 Vim. A fuel cell was prepared in
the same manner as in Example E1 except for the above matter.
[0385] Example E32
A layer formed of an intimate mixture of (La~_xAX)y(Mn~_
ZNiZ)Os with ScYSZ was formed as a first layer. The composition of the
intimate mixture was (Lao.75Sro,2s)(Mno,9sNio.os)Os/90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 = 50/50 (weight ratio). An aqueous solution of
nitrate of La, an aqueous solution of nitrate of Sr, an aqueous solution of
nitrate of Mn, an aqueous solution of nitrate of Ni, an aqueous solution of
3o nitrate of Zr, an aqueous solution of nitrate of Y, and an aqueous solution
of nitrate of Sc were provided and were mixed with each other so as to
give the above composition, followed by coprecipitation with oxalic acid.
Heat treatment was then carried out to prepare a raw material powder
having a regulated particle diameter. The average particle diameter
was 5 ~,m. A fuel cell was prepared in the same manner as in Example
E1 except for the above matter.
' ' CA 02553074 2006-07-07
103
[0386] The determination of Mn content of the electrolyte in its
surface on the fuel electrode side, a gas leakage test, a power
generation test, and a durability test were carried out for the fuel cells
thus obtained. The results were as shown in tables below.
[Table 60]
Gas.~ermeab~lity,Mn content, Initial
x 10 ms' Pa wt% potential,
V
Exam 1e 2.3 1.1 0.66
E1
Exam 1e 1.4 1.3 0.65
E29
Exam 1e 2.6 1.0 0.69
E30
Exam 1e 2.4 0.8 0.72
E31
Exam 1e 1.8 0.9 0.71
E32
[Table 61
After Estimated
initialAfter After After potential,
potential,1000 1500 2000 after 40,000
V hr, V hr, V hr, V h r, V
Exam 1e 0.66 0.66 0.66 0.66 0.63
E1
Exam 1e 0.65 0.65 0.65 0.65 0.62
E29
Exam 1e 0.69 0.69 0.69 0.69 0.66
E30
Example 0.72 0.72 0.72 0.72 0.69
E31
Exam 1e 0.71 0.71 0.71 0.71 0.68
E32
[0387] A test was carried out for the material for the porous layer.
[0388] Example E33
ScYSZ was provided as a material for a porous layer
formed of zirconia-containing fluorite oxide. The composition of ScYSZ
was 90 mol% Zr02-5 mol% Sc203-5mol% Y203. An aqueous solution of
nitrate of Zr, an aqueous solution of nitrate of Sc, and an aqueous
solution of nitrate of Y were provided and were mixed with each other so
as to give the above composition, followed by coprecipitation with oxalic
acid. A fuel cell was formed in the same manner as in Example E1
except for the above matter.
[0389] Example E34
2 o YSZ was provided as a material for a porous layer formed
of zirconia-containing fluorite oxide. The composition of YSZ was 90
mol% Zr02-10 mol% Y203. An aqueous solution of nitrate of Zr and an
aqueous solution of nitrate of Y were provided and were mixed with each
other so as to give the above composition, followed by coprecipitation
with oxalic acid. A fuel cell was formed in the same manner as in
Example E1 except for the above matter.
' ' CA 02553074 2006-07-07
104
[0390] Comparative Example E8
A layer formed of a cerium-containing oxide represented by
(Ce02)o.s(Sm203)o.~ was provided between an electrolyte and a fuel-side
electrode reaction layer. An aqueous solution of nitrate of Ce and an
aqueous solution of nitrate of Sm were provided and mixed together to
give the above composition, followed by coprecipitation with oxalic acid.
Heat treatment was further carried out to prepare a raw material powder
having a regulated particle diameter. The raw material powder had an
average particle diameter of 0.5 Vim, a porosity of 18%, and a pore
1 o diameter of 0.5 pm. A fuel cell was prepared in the same manner as in
Example E1, except that this layer was provided instead of the porous
layer.
[Table 62]
Gas.~ermeab~lity,Mro content,Initial potential,
x 10 ms' Pa wt /o V
Exam 1e E1 2.3 1.1 0.66
Exam 1e E33 1.7 1.5 0.65
Exam 1e E34 1.7 1.7 0.63
Comparative 4.g 0.1 0.65
Exam 1e E8
[Table 63]
After After After After Estimated
initial
potential,1000 1500 2000 potential,
hr, hr, hr,
V V V V after 40,000
hr,
V
Exam 1e E1 0.66 0.66 0.66 0.66 0.63
Exam 1e E33 0.65 0.65 0.65 0.65 0.62
Exam 1e E34 0.63 0.63 0.63 0.63 0.60
Comparative
0.65 0.65 0.645 0.64 0.45
Exam 1e E8
[0391 ] A test was carried out with the construction of the
electrolyte being varied.
[0392] Example E35
2o ScYSZ having a composition of 90 mol% Zr02-5 mol%
Sc203-5 mol% Y203 was prepared as a material for an electrolyte. An
aqueous solution of nitrate of Zr, an aqueous solution of nitrate of Y, and
an aqueous solution of nitrate of Sc were provided and were mixed with
each other so as to give the above composition, followed by
coprecipitation with oxalic acid. Heat treatment was then carried out to
CA 02553074 2006-07-07
105
prepare a raw material powder having a regulated particle diameter.
The average particle diameter was 0.5 Vim. A fuel cell was prepared in
the same manner as in Example E1 except for the above matter.
[0393] Example E36
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
was prepared as a material for an electrolyte. An aqueous solution of
nitrate of Zr and an aqueous solution of nitrate of Sc were provided and
were mixed with each other so as to give the above composition,
followed by coprecipitation with oxalic acid. Heat treatment was then
1 o carried out to prepare a raw material powder having a regulated particle
diameter. The average particle diameter was 0.5 p.m. A fuel cell was
prepared in the same manner as in Example E1 except for the above
matter.
[0394) Example E37
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y20$ were
provided as materials for an electrolyte. YSZ was coated by slurry
coating onto the surface of the second layer, and SSZ was then coated
by slurry coating onto the surface of YSZ, and the assembly was sintered
2o at 1400°C. The thickness of each of the layers was 15 Vim. Afuel
cell
was prepared in the same manner as in Example E1 except for the
above matter.
[0395) Exami~le E38
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. SSZ was coated by slurry
coating onto the surface of the second layer, and YSZ was then coated
by slurry coating onto the surface of SSZ, and the assembly was sintered
at 1400°C. The thickness of each of the layers was 15 ~,m. A fuel cell
3o was prepared in the same manner as in Example E1 except for the
above matter.
[0396] Example E39
SSZ having a composition of 90 mol% Zr02-10 mol% Sc203
and YSZ having a composition of 90 mol% Zr02-10 mol% Y203 were
provided as materials for an electrolyte. SSZ was coated by slurry
coating onto the surface of the second layer, YSZ was then coated by
CA 02553074 2006-07-07
106
slurry coating onto the surface of SSZ, and SSZ was further coated by
slurry coating onto the surface of YSZ. The layers were co-sintered at
1400°C. The thickness of each of the layers was 10 Vim. A fuel cell
was prepared in the same manner as in Example E1 except for the
above matter.
[Table 64)
Gas Mn content,
p 10'~m il~t~a.~wt% Initial potential,
V
Exam 1e E1 2.3 1.1 0.66
Exam 1e E35 1.4 1.0 0.68
Exam 1e E36 10.1 0.4 0.68
Exam 1e E37 1.8 0.6 0.69
Exam 1e E38 2.5 0.6 0.69
Exam 1e E39 2.1 0.4 0.69
[Table 65j
After After After After Estimated
initial 1000 1500 2000 potential,
potential,hr, hr, hr, after 40,000
V V V V hr, V
Exam 1e E1 0.66 0.66 0.66 0.66 0.63
Exam 1e E35 0.68 0.68 0.68 0.68 0.65
Exam 1e E36 0.68 0.68 0.68 0.68 0.65
Exam 1e E37 0.69 0.69 0.69 0.69 0.66
Exam 1e E38 0.69 0.69 0.69 0.69 0.66
Exam 1e E39 0.69 0.69 0.69 0.69 0.66