Note: Descriptions are shown in the official language in which they were submitted.
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
FLUORESCENCE-BASED ADP DETECTION SYSTEM
FIELD OF THE INVENTION
[0001JThe present invention is in the field of nucleotide detection. In
particular, it
relates to the differential spectroscopic detection of nucleotides.
BACKGROUND OF THE INVENTION
(0002] In the last few years protein kinases have become a target for drug
development for pharmaceutical companies. It has been estimated that up to 20%
of all
the targets in the pharmaceutical industry are currently kinases. The reason
for this
interest has to do with the fact that kinases play a crucial role in
fundamental cellular
processes. Disturbances in kinase activity may cause or be an indication of
disease in
humans, and targeting kinase molecules or receptors with drugs may alter the
course
of the disease.
[0003] Kinases are enzymes (biochemical catalysts) that transfer a phosphate
group
from ATP (adenosine triphosphate) to a substrate. As a result of the phosphate
transfer, the ATP becomes dephosphorylated to form ADP (adenosine
diphosphate).
Once the substrate is phosphorylated in vivo, a biochemical pathway may be
activated.
A single kinase may have multiple substrates and, depending on which substrate
is
being phosphorylated, different pathways may be activated. Substrate
selectivity is thus
an important characteristic of kinases. The biochemical reaction that kinases
carry out
is the following:
[0004]ATP + Kinase + Substrate + ADP + Kinase + Substrate-P (I)
[0005]One of the requirements of the reaction is that the substrate have an
available
hydroxyl group to accept the phosphate group being transferred by the kinase
enzyme
from the molecule of ATP. Polypeptide or protein substrates thus generally
contain
tyrosine (tyrosine kinases) or serine/threonine (serine/threonine kinases) as
the
acceptor amino acid.
[0006] Detection of kinase activity in the clinical setting plays an important
role in
evaluating various states of human health. Creatine kinase (GK), for example,
is a
"leakage" enzyme present in high concentration in the cytoplasm of myocytes
and is
the most widely used enzyme for evaluation of neuromuscular disease. Current
methods for CK detection involve multiple steps including the use of various
other
enzymes.
[0007] Detection of kinase activity for the purposes of drug discovery and
drug profiling
presents an interesting challenge for the pharmaceutical industry. Coupled
detection
systems with multiple enzymes, which are currently used in industry, are not
practical
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
due to the need for counter screens to rule out the effects of drugs on
enzymes other
than the one of interest. For example, in looking for CK inhibitors,
hexokinase (HK), a
coupled enzyme, can be affected by drugs that interact with the ATP binding
site of CK
because HK contains its own ATP binding site.
[0008] One approach that investigators searching for inhibitors of kinases in
their drug
discovery programs have traditionally used is the detection of substrate
phosphorylation as a way to monitor kinase activity. The challenge is that the
substrate
for every kinase can be different and a single kinase may have multiple
phosphorylation
sites even within the same substrate. In every instance assay development has
to be
done in order to find the optimal conditions for the assay, which can be very
time
consuming. A currently used method to detect substrate phosphorylation is an
ELISA
based assay in which the detection of the phosphorylated substrate is done
using a
specific antibody. ELISA based systems show great sensitivity but many steps
are
required, a typical assay may run for hours, and many manipulations are
needed,
increasing the chance for error.
[0009]Another method currently used to monitor kinase activity is the
detection and
quantitation of the decrease of ATP as the assay progresses. This method is
limited by
the production of the product - in this case ADP - which, when accumulated,
may
inhibit the activity of the kinase. Application of such a methodology requires
extensive
assay development.
[0010] Detection systems traditionally used to monitor the progress of kinase
assays
utilize spectrometric techniques such as fluorescence spectrometry,
fluorescence
polarization (Panvera, Molecular Devices, Chromagen), time-resolved
fluorescence
(Cis-Bio), absorbance spectrometry (MDS Pharma Services, Upstate),
luminescence
spectrometry (Promega), and non-spectrometric techniques such as scintillation
(Perkin
Elmer, Amersham) and chromatography (Caliper).
BRIEF DESCRIPTION OF THE DRAWINGS
[0011 ] Figure 1 shows superimposed fluorescence spectra of ADP and ATP with
HEPES used as a control.
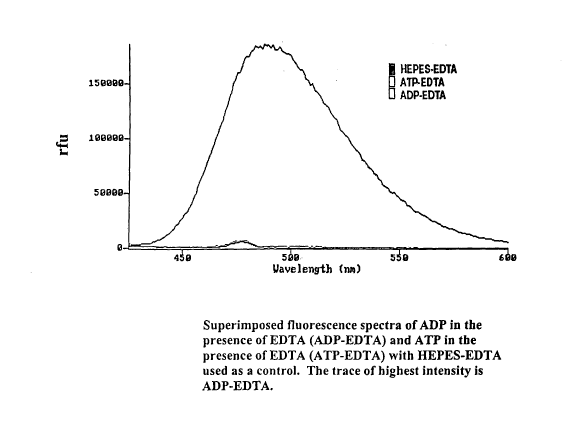
[0012] Figure 2 shows superimposed fluorescence spectra of ADP in the presence
of
EDTA (ADP-EDTA) and ATP in the presence of EDTA (ATP-EDTA) with HEPES-EDTA
used as a control.
[0013] Figure 3 shows superimposed fluorescence spectra of ADP in the presence
of
EGTA (ADP-EGTA) and ATP in the presence of EGTA (ATP-EGTA) with HEPES-
EGTA used as a control.
2
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
[0014] Figure 4 shows the results of fluorescence data read at 500 nm showing
a 50
mM EDTA solution upon titration with ADP.
[0015] Figure 5 shows the results of fluorescence data showing ADP in the
presence of
EDTA upon titration with Ca2+ and Mg2+.
[0016] Figure 6 shows the results of fluorescence data showing ADP in the
presence of
EDTA upon titration with ATP.
[0017] Figure 7 shows the results of fluorescence data showing a series of ADP
and
ATP solutions of different concentrations upon addition of 50 mM of EDTA.
[0018] Figure 8 shows superimposed fluorescence spectra of selected
nucleotides in
the presence and absence of EDTA, with HEPES or HEPES-EDTA used as a control.
DETAILED DESCRIPTION OF THE INVENTION
[0019] The inventors have developed technology for the detection of
nucleotides in
assays. It may be used as a means of monitoring biochemical activity in
assays, such
as, for example, kinase activity in a kinase assay. It is a spectroscopic
detection
system which provides a mechanism to study any activity in which the
concentration of
a diphosphorylated nucleoside changes with time. It can be used, for example,
to
monitor the conversion of a di- or triphosphorylated nucleoside to a mono- or
diphosphorylated nucleoside respectively, or vice versa, independent of the
substrate
being used. A particular diphosphorylated nucleoside is ADP, and a particular
triphosphorylated nucleoside is ATP. In addition to replacing currently used
detection
methods, this technology may be used to screen kinase targets against
substrates for
which there currently are no available detection methods. In the clinic or
other situation,
this methodology could be used to detect and/or monitor the activity of
enzymes that
utilize ATP and generate ADP.
[0020]ATP and ADP are invariably present in any kinase reaction. Systems based
on
monitoring the consumption of ATP and/or production of ADP can thus permit the
monitoring of any kinase reaction, independent of the substrate. Since ADP is
not
present in the reaction before it starts, the monitoring of the production of
ADP can be
an effective method for monitoring the activity of a kinase.
[0021]The inventors have determined that there is a difference in the
spectroscopic
spectra of ATP and ADP which can be enhanced by the addition of a chelator.
More
specifically, under certain circumstances, there is a fairly consistent
difference in the
fluorescence emission at around 450 to 550 nm in the fluorescence spectra of
ADP and
ATP molecules, as demonstrated in Figure 1. The inventors have additionally
determined that, in the presence of a chelator such as
ethylenediaminetetraacetic acid
(EDTA) there is a substantial increase in ADP fluorescence in the 450 to 550
nm range
3
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
without a corresponding increase in ATP fluorescence, as shown in Figure 2.
Similarly,
as shown in Figure 3, there is a substantial increase in ADP fluorescence in
the
presence of the chelator ethylene glycol-bis (2-aminoethylether)-N,N,N',N,-
tetraacetic
acid (EGTA) without an increase in ATP fluorescence.
[0022] Figure 4 shows that the intensity of fluorescence at 500 nm is
proportional to the
amount of ADP added to a solution of EDTA.
[0023]As used herein, the term "chelator" refers to any molecule which
possesses at
least one functional group which can coordinate to a metal, either covalently
or non-
covalently. The chelator may be multidentate or coordinate in a unidentate
manner.
The chelator may be a macrocycle such as a porphyrin. The chelator may chelate
a
metal by donating or sharing pi electrons. Chelators further derivatized to
increase
spectroscopic detection are also included. The functional group of the
chelator may be
negatively or positively charged, or neutral. Examples of suitable functional
groups
include carboxylato, thiolato, hydrido, cyano, carbonato, thiocarcamato,
thiocarboxylato, thiophosphinato amino, phophoro, hydrazino, nitrilo,
hydrazido, oxime,
and thioether.
[0024] Examples of chelators include ethylenediaminetetraacetic acid (EDTA),
diethylenetriaminepentaacetic acid (DTPA), ethylene, and ethylene glycol-bis(2-
aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA).
[0025] Experiments showing that competitive displacement of EDTA from the ADP-
EDTA interaction destroys the increased fluorescence in the ranges of 450 to
500 nm
have also been conducted. As shown in Figure 5, there is a decrease in
fluorescence
at 500 nm when Mg2+ or Ca2+ is added to the reaction mixture, suggesting that
the
metal disrupts the interaction of ADP and EDTA. As well, as shown in Figure 6,
an
excess of ATP added to the reaction mixture attenuates the ADP-EDTA
interaction.
This result suggests that ATP may also directly interact with EDTA, but that
the
interaction does not cause an increase in fluorescence under these conditions.
[0026] Suitable spectrometric techniques include fluorescence spectrometry,
ultraviolet
and infrared absorption and transmission spectrometry, luminescence
spectrometry,
Raman spectrometry, and phosphorescence spectrometry. Most preferred is
fluorescence spectrometry. Suitable fluorescence spectrometry techniques
include the
excitation of a sample with, for example, a xenon lamp, laser-induced
fluorescence
using lifetime fluorescence in order to increase detection sensitivity,
fluorescent
polarization which may boost the desired signal and lower background noise to
improve
sensitivity, and time-resolved fluorescence.
4
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
(0027] Optionally, metals, such as lanthanides, may be used to change the
spectrometric characteristics, particularly the fluorescence spectrometric
characteristics, of nucleotide-chelator interactions. It is known that
tertiary complexes
may be formed between certain organic molecules, chelators, and lanthanides
(Diamandis EP, Christopoulos TK. Anal. Chem., 1990, 14:1149, Anal.
Christopoulos
TK, Diamandis EP. Chem. 1992, 64: 342, hereby incorporated by reference). Upon
formation of these tertiary complexes, an enhanced fluorescence signal was
observed.
Before now, such complexes had not been observed between nucleosides or
nucleotides and chelators and lanthanides where spectroscopic signals are
enhanced.
[0028] The following experimental examples are illustrative of the use of this
invention.
[0029] Experiments
[0030] Experimental Details
[0031 ] Spectrofluorometers
[0032]The peak generated by a fluorescence signal is typically broad, and the
ability to
precisely select of a wavelength of excitation and/or emission largely depends
on the
calibration of the instrument and the fluorescence detection system, or
reader, used.
For the experiments described herein, a Spex FluoroMax Spectrofluorometer
(serial
number 2093, Spex Industries, New Jersey) with a single well reader was used
with a
passband of 0.1 nm. In some cases, a Molecular Devices FLEXstation plate
reader
(serial number FX 01090, California) was used, which has a passband of 10 nm
and
therefore cannot detect the fluorescence signal as precisely as the SPEX
Fluoromax
scanner.
(0033] Reagents
[0034]The following reagents were used in the experiments described herein:
[0035]ADP (A-2754 Sigma Ultra, LOT#073k7007, Adenosine 5'-diphosphate sodium
salt);
[0036]ATP ( A-7699 Sigma Ultra, LOT#053k7042, Adenosine 5'-triphosphate
disodium
salt);
(0037] EGTA (E0396 Sigma Ultra, Ethylene glycol-bis(2-aminoethylether)-
N,N,N',N'-
tetraacetic acid);
(0038] EDTA Sigma-Aldrich (E2-628-2 Ethylenediaminetetraacetic acid);
[0039]HEPES (J848, AMRESCO, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic
acid);
[0040] CaCl2 (C-4901, Sigma, Calcium Chloride dehydrated); and
[0041] MgCl2 (M8266, Sigma, Magnesium Chloride Anhydrous).
5
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
[0042] Abbreviations
[0043] rfu = relative fluorescence unit
(0044] Experiment 1: Difference in Fluorescence Spectra of ATP and ADP in the
range
of 450 to 500 nm
[0045]A solution containing either 5 mM ADP or ATP in 10 mM HEPES pH 8.0
buffer
was analyzed for fluorescence emission within the ranges of 400 to 600 nm
using a
SPEX Fluoromax fluorescence scanner. The excitation wavelength used for this
experiment was 405 nm. This experiment shows that there is a weak fluorescence
emission difference between ADP and ATP measured at around 500 nm.
[0046] Experiment 2: Selective Interaction of EDTA and EGTA with ADP over ATP
[0047]A fluorescence scan was performed using a SPEX Fluoromax on a solution
containing 10 mM ADP or 10 mM ATP in 10 mM HEPES pH 8.0 buffer in the presence
or absence of 50 mM EDTA. As shown in Figure 2, the fluorescence spectra of
the
solution containing ADP is greatly enhanced in the presence of EDTA. By
contrast,
ATP does not show an increase in fluorescence in the presence of EDTA.
Similarly, as
shown in Figure 3, there is an increase in ADP fluorescence in the presence of
EGTA
which is not seen in the ATP fluorescence. The peak seen at 475 nm is part of
the
background spectrum.
[0048] Experiment 3: ADP Titration in the Presence of EDTA
[0049]A solution 50 mM EDTA was titrated with ADP in 10 mM HEPES pH 8.0
buffer.
The fluorescence was read at 500 nm on a Molecular Devices FIexStation plate
reader.
As shown in Figure 4, the data points resulting from the titration of the EDTA
solution
with ADP follows a straight line over two log units. ADP below a concentration
of 100
uM was beyond the limit of detection of the technique used.
[0050] Experiment 4: Addition of Ca2+ and Mg2+ to Solutions Containing ADP and
EDTA
[0051] In this experiment, the effect of the addition of Ca2+ and Mg2+ to a
solution
containing 1 mM ADP and 30 mM EDTA in 10 mM HEPES pH 8.0 buffer was
examined. As shown in Figure 5, the fluorescence emission signal generated by
ADP
in the presence of EDTA was found to be reversed by the addition of 30 mM of
Mg2+ or
Ca2+, indicating that Mg2+ and Ca2+ can compete with the interaction of ADP
with
EDTA. Fluorescence was monitored at 500 nm.
[0052] Experiment 5: Titration of Solution Containing ADP and EDTA with ATP
[0053] Since ATP does not seem to fluoresce in the presence of EDTA, and ADP
does,
the effect of the presence of ATP on the EDTA-ADP induced fluorescence was
examined. To a solution containing 5 mM ADP and 10 mM EDTA in 200 u1 of 10 mM
6
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
HEPES pH 8.0 buffer, increasing concentrations of ATP were added. The
fluorescence
was read at 500 nm using an excitation wavelength of 410 nm . The samples were
read
on a FIexStation (Molecular devices) plate reader. As shown in Figure 6, 10 mM
ATP
completely disrupts the interaction between ADP and EDTA. This observation
suggests
that ATP can directly interact with EDTA; however, it does not cause an
increase in
fluorescence under these conditions.
[0054] Experiment 6: Solution of ADP Titrated with EDTA
[0055] In this experiment, a series of solutions of ADP and ATP in 10 mM HEPES
pH
8.0 buffer at various concentrations were treated with 50 mM EDTA. HEPES-EDTA
was used as a control. Figure 7 shows the data where the fluorescence reading
of the
control subtracted from each data point.
[0056]These experiments establish that the sensitivity of detection of the ADP-
chelator
is about 1 uM by fluorescence spectroscopy.
[0057] Experiment 7: Effect of EDTA on Fluorescence of Nucleotides
[0058]The fluorescence spectra of 5 mM solutions of ATP, ADP, guanidine
diphosphate (GDP), and guanidine triphosphate (GTP) were taken in the absence
and
presence of 50 mM of EDTA. The fluorescence emission signals were analyzed
between 450-600 nm. As shown in Figure 8, only ADP in the presence of EDTA
displays enhanced fluorescence, with a peak of fluorescence between 490-500
nm.
[0059] Experiment 8: Use of Lanthanides to Alter the Fluorescence Signal
Generated
by a Nucleotide in the Presence of a Chelator
[0060] In order to establish the use of a tertiary complex formed between a
lanthanide
metal, a chelator, and a nucleotide to alter the fluorescence signal generated
by the
nucleotide in the presence of the chelator, the following experiments will be
carried out.
[0061] (i) EDTA will be incubated in the presence and absence of terbium and
in
the presence or absence of increasing concentrations of nucleotides (ATP,
ADP).
Fluorescence emission will be monitored with the use of a SPEX Fluoromax
fluorescence scanner.
[0062] (ii) EDTA will be incubated with europium and in the presence or
absence
of increasing concentrations of nucleotides (ATP, ADP). Fluorescence emission
will be
monitored with the use of a SPEX Fluoromax fluorescence scanner.
[0063] (iii) A complex will be formed between an organic molecule such as
salicylic
acid, EDTA, and europium, yielding an increase in fluorescence. The effect of
the
addition of increasing concentration of nucleotides will be monitored by the
use of
SPEX Fluoromax fluorescence scanner.
7
CA 02553218 2006-07-11
WO 2005/069725 PCT/IB2005/000290
[0064] Given the results obtained in 8(i), 8(ii), or 8(iii), conditions for
monitoring
changes in the ADP concentrations with time will be optimized.
[0065] While preferred embodiments of the invention have been illustrated and
described, it will be appreciated that various changes and modifications can
be made
therein without departing from the spirit and scope of the invention as
defined by the
following claims.
[0066]Although various examples of combined elements of the invention have
been
described, it will also be understood that these are not intended to be
exhaustive and
features of one embodiment may be combined with those of another, and such
other
combinations are contemplated to be within the scope of the invention
disclosed herein.
[0067]All publications and other documents mentioned herein are hereby
incorporated
by reference into this document as though the entire contents thereof were
reproduced
herein.
8