Note: Descriptions are shown in the official language in which they were submitted.
CA 02553402 2013-11-14
25771-1225
Compositions for Inhibiting Sema7A and VLA-1 Interaction
and Methods of Using the Same
BACKGROUND OF THE INVENTION
1. TECHNICAL FIELD
This invention relates to compositions and methods of treating a cytokine
mediated
disease by inhibiting Sema7A and VLA-1 interaction.
2. BACKGROUND INFORMATION
Semaphorin family consists of a large number of phylogenetically conserved
soluble
and transmembrane proteins, many of which play diverse roles in axon guidance,
organogenesis, angiogenesis, vascularization, oncogenesis and immune responses
(1-3).
Sema7A, of which expression is observed in both nervous and immune systems, is
the
only GPI-anchored semaphorin, and it was originally identified in a search for
vertebrate homologues of virally-encoded semaphorins. Although Sema7A was
shown
to bind plexin-C1 (5), it has been suggested recently that Sema7A promotes
axon
outgrowth through pl-integrin in a plexin-C1-independent manner (6). However,
it
remains unclear how and to what extent Sema7A is involved in immune responses.
BRIEF SUMMARY OF THE INVENTION
It is therefore an object of the invention to provide a method of treating an
inflammatory
disease, by administering to a patient a composition which inhibits Sema7A ¨
VLA-1
interaction.
It is yet still another object of the invention to provide a method to
identify a compound
that controls interaction of Sema7A with VLA-1 activity in a cell, comprising:
(1)
contacting a cell with a putative regulatory compound, wherein the cell
includes a
Sema7A protein and a VLA-1 protein; and (2) assessing-the ability of the
putative
regulatory compound to inhibit the interaction of Sema7A with VLA-1.
1
CA 02553402 2013-11-14
25771-1225
Other aspects of the invention include:
- a method to identify a compound that inhibits interaction of Sema7A with
VLA-1, comprising: (1) contacting a cell with a putative regulatory compound,
wherein the
cell includes a Sema7A protein and a VLA-1 protein; and (2) assessing the
ability of the
putative regulatory compound to inhibit the interaction of Sema7A with VLA-1;
- a method to identify a compound that inhibits interaction of Sema7A with
VLA-1, comprising: (1) contacting a cell with a putative regulatory compound
and VLA-1,
wherein the cell includes a Sema7A protein; and (2) assessing the ability of
the putative
regulatory compound to inhibit the interaction of Sema7A with VLA-1; and
- a method to identify a compound that inhibits interaction of Sema7A with
VLA-1, comprising: (1) contacting a cell with a putative regulatory compound
and Sema7A,
wherein the cell includes a VLA-1 protein; and (2) assessing the ability of
the putative
regulatory compound to inhibit the interaction of Sema7A with VLA-1.
It is yet still another object of the invention to provide a composition that
controls interaction
of Sema7A with VLA-1 activity in a cell wherein the composition is
therapeutically useful in
treating an inflammatory disease.
BRIEF DESCRIPTION OF THE FIGURES
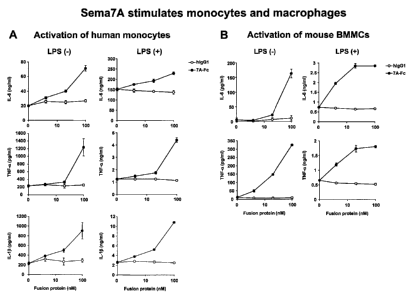
Figure 1: mouse Sema7A fused with the Fc portion of human IgG1
(Sema7A-Fc) induced production of inflammatory cytokines in not only mouse
bone marrow-
derived macrophages but also human monocytes.
Figure 2: the binding of Sema7A to human monocytic cells was inhibited not
only by anti-r31 antibody (Ab) and/or anti-al Ab but also by soluble a 1131-
integrin. Also
shown is the production of IL-6 induced by Sema7A which was blocked by the
Abs.
2
CA 02553402 2013-11-14
25771-1225
DETAILED DESCRIPTION OF EMBODIMENTS
In a first generic embodiment, there is provided a composition which inhibits
Sema7A and
VLA-1 interaction, for use in treating cytokine mediated disease.
The present inventors have identified that Sema7A, which is expressed on the
cell surface of
activated T-cells, is involved in activation of human monocytic cells. As
shown in Figure 1,
mouse Sema7A fused with the Fc portion of human IgG1 (Sema7A-Fc) induced
production of
inflammatory cytokines in not only mouse bone marrow-derived macrophages but
also human
monocytes. Sema7A-Fc also enhanced LPS-induced production of cytokines in
these cells.
(The amino acid identity between mouse and human Sema7A is 90%.) In addition,
the
binding of Sema7A to human monocytic cells was inhibited not only by anti-f31
Ab and/or
anti-al Ab but also by soluble al 31-integrin (Fig. 2). Furthermore, the
production of IL-6
induced by Sema7A was significantly blocked by the Abs (Fig. 2). Collectively,
our finding
not only indicates a role of Sema7A in the immune system but also identifies
the involvement
of a1131-integrin (VLA-1) as a receptor for Sema7A in activation of monocytes.
Therefore,
2a
CA 02553402 2006-07-25
2 5 7 7 1-12 2 5
the interaction between Sema7A and a1l3 I -integrin (VLA-1) is a potential
therapeutic target for various inflammatory diseases.
Figure 1 shows that Sema7A is a potent stimulator for monocytes. (A) and (B)
Human
monocytes or mouse bone marrow-derived macrophages were cultured on Sema7A-Fc-
coated or human IgG1 -coated plates with or without LPS (100 ng/ml) for 24 hr.
Production of the indicated cytokines in the culture supernatants was measured
by
ELISA. Results were the means of triplicate determinations.
Method: Human monocytes were isolated from peripheral blood of volunteers by
using
RosseteSep human monocyte enrichment cocktail (StemCell Technologies). Mouse
bone marrow-derived macrophages were obtained from 5-day culture of bone
marrow
cells in the presence of G-CSF (50 ng/m1; Peprotec). Cells were plated onto
flat-
bottomed 96-well plates coated with the indicated concentrations of Sema7A-Fc
or
human IgG1 (1 x 105 cells/well in 200121 of RPMI1640 medium supplemented with
10% FCS) and incubated for 24 hours. The concentrations of cytokines in the
culture
supernatants were measured by ELISA kits (R&D Systems).
Figure 2 shows that al 13 1 integrin/VLA-1 is a functional receptor for
Sema7A.
(A) Adhesion of THP-1 cells, a human monocytic cell line, to Sema7A-coated
plates
was blocked with both anti-131 and anti-al integrin Abs. THP-1 cells were pre-
incubated with the indicated antibodies (25 g/ml) and subjected to adhesion
assays.
The number of cells attached to Sema7A-coated wells was quantified as
previously
described (6, 7).
(B) Adhesion of THP-1 cells to immobilized Sema7A was inhibited in the
presence of
soluble al f31 integrin proteins. Sema7A-coated wells were pre-treated with
the
indicated soluble integrin proteins before applying THP-1 cells.
(C) Sema7A-induced cytokine production by human monocytes was inhibited by
anti-
f31 and anti-al integrin Abs. Human monocytes were pretreated with 10 pg/m1 of
the
indicated Abs and then seeded on to Sema7A-coated plates. Production of IL-6
in the
culture supernatants were measured by ELISA.
Method: Tissue culture plates (96-well, suspension culture treated, SUMILON)
were
coated overnight with 100 of 10 nM fusion protein solutions, followed by
- 3 -
CA 02553402 2006-07-25
25771-1225
blocking with 10 mg/ml BSA in PBS buffer (200 41/well). Cells were added at 5
x 104
cells/well in 200 41 of Tyrode buffer (135 mM NaC1, 5.4 mM KC1, 1.0 mM MgC12,
5
mM NaOH-Hepes (pH7.4), 10 mM Glucose, 10 mg/ml BSA) and allowed to adhere for
1 hour at room temperature. Wells were then washed 3 times in prewarmed PBS
buffer.
The number of adherent cells was determined by CyQUANT cell proliferation
assay kit
(Molecular probe). For antibody blocking assays, THP-1 cells were incubated on
ice
with 25 jig/m1 of the indicated anti-integrin antibodies in Tyrode buffer for
30 mM.
Soluble forms of integrin proteins were used for pretreateatment of Sema7A-Fc-
coated
wells at 1 1..tM in Tyrode buffer and plates were incubated for 30 min at room
temperature.
One embodiment of the present invention relates to a method to identify a
compound
that controls interaction of Sema7A with VLA-1 activity in a cell, comprising:
(1)
contacting a cell with a putative regulatory compound, wherein the cell
includes a
Sema7A protein and a VLA-1 protein; and (2) assessing the ability of the
putative
regulatory compound to inhibit the interaction of Sema7A with VLA-1. The
assessment
step preferably involves studying the adhesion of THP-1 cells to Sema7A (for
example
by observing if adhesion of THP-1 cells to Sema7A is affected by presence of
the
putative regulatory compound), or equivalent methods known in the art.
The term "regulate" refers to controlling the activity of a molecule and/or
biological
function, such as enhancing or diminishing such activity or function.
The term "patient" includes both human and non-human mammals.
The teuns "treating" or "treatment" mean the treatment of a disease-state in a
patient,
and include:
(i) preventing the disease-state from occurring in a patient, in particular,
when such
patient is genetically or otherwise predisposed to the disease-state but has
not yet
been diagnosed as having it;
(ii) inhibiting or ameliorating the disease-state in a patient, i.e.,
arresting or slowing
its development; or
(iii) relieving the disease-state in a patient, i.e., causing regression or
cure of the
disease-state.
-4-
CA 02553402 2006-07-25
25771-1225
Yet another embodiment of the present invention
relates to an antibody or antibody binding site which binds
Sema7A, VLA-1 or fragments thereof. Embodiments of the
present invention further include polyclonal and monoclonal
antibodies. Preferred embodiments of the present invention
include a monoclonal antibody such as anti-VLA-1 monoclonal
antibody. The above antibody or antibody binding site which
binds Sema7A or VLA-1 inhibits binding of Sema7A with VLA-1.
Yet another embodiment of the present invention
relates to a biotherapeutic comprising Sema7A or VLA-1
protein or fragments thereof, wherein the biotherapeutic is
useful for treating an inflammatory disease. Certain
embodiments relate to use of a molecule capable of binding
to Sema7A or VLA-1 protein to stimulate cells and initiate a
signal transduction pathway, and to the use as described
herein wherein the molecule comprises an antibody
specifically binding to Sema7A, a Sema7A protein or soluble
fragment thereof; an antibody specifically binding to VLA-1,
a VLA-1 protein or soluble fragment thereof; or a compound
having a molecular mass of less than 1000 dalton.
The term "composition" as referred to herein
include a putative compound, or a substantially pure protein
selected from Sema7A and VLA-1 or fragments thereof, an
antibody or antibody binding site which binds Sema7A and
VLA-1 or fragments thereof, an expression vector encoding
Sema7A, VLA-1 or fragments thereof, a fusion protein
comprising Sema7A, VLA-1 or fragments thereof. In the
antibody binding site embodiments, the antibody binding site
may be: specifically immunoreactive with a mature protein
selected from the group consisting of the Sema7A and VLA-1;
raised against a purified or recombinantly produced human or
mouse Sema7A or VLA-1; in a monoclonal antibody, Fab, or
F(ab)2; immunoreactive with denatured antigen; or in a
-5-
CA 02553402 2006-07-25
' 25771-1225
labeled antibody. In certain embodiments; the antibody
binding site is detected in a biological sample by a method
of: contacting a binding agent having an affinity for Sema7A
or VLA-1 with the biological sample; incubating the binding
agent with the biological sample to form a binding agent:
Sema7A or VLA-1 protein complex; and detecting the complex.
In a preferred embodiment, the biological sample is human,
and the binding agent is an antibody.
Putative compounds as referred to herein include,
for example, compounds that are products of rational drug
design, natural products and compounds having partially
defined signal transduction regulatory properties. A
putative compound can be a protein-based compound, a
carbohydrate-based compound, a lipid-based compound, a
nucleic acid-based compound, a natural organic compound, a
synthetically derived organic compound, an anti-idiotypic
antibody and/or catalytic antibody, or fragments thereof. A
putative regulatory compound can be obtained, for example,
from libraries of
-5a-
CA 02553402 2006-07-25
25771-1225
natural or synthetic compounds, in particular from chemical or combinatorial
libraries
(i.e., libraries of compounds that differ in sequence or size but that have
the same
building blocks; see for example, U.S. Pat. Nos. 5,010,175 and 5,266,684 of
Rutter and
Santi) or by rational drug design.
In a rational drug design procedure, the three-dimensional structure of a
compound,
such as a signal transduction molecule can be analyzed by, for example,
nuclear
magnetic resonance (NMR) or x-ray crystallography. This three-dimensional
structure
can then be used to predict structures of potential compounds, such as
putative
regulatory compounds by, for example, computer modelling. The predicted
compound
structure can then be produced by, for example, chemical synthesis,
recombinant DNA
technology, or by isolating a mimetope from a natural source (e.g., plants,
animals,
bacteria and fungi). Potential regulatory compounds can also be identified
using SELEX
technology as described in, for example, PCT Publication Nos. WO 91/19813; WO
92/02536 and WO 93/03172.
In particular, a naturally-occurring intracellular signal transduction
molecule can be
modified based on an analysis of its structure and function to form a suitable
regulatory
compound. For example, a compound capable of regulating the Sema7A or VLA-1
can
comprise a compound having similar structure to the amino acid residues in
their
respective binding domains. Such a compound can comprise a peptide, a
polypeptide or
a small organic molecule.
Putative regulatory compounds can also include molecules designed to interfere
with
Sema7A or VLA-1. For example, mutants of VLA-1 can be created that interfere
with
the coupling of the protein with Sema7A. Putative regulatory compounds can
include
agonists and antagonists of Sema7A or VLA-1. Such agonists and antagonists can
be
selected based on the structure of a naturally-occurring ligand to these
proteins.
The technology for producing monoclonal antibodies is well known. In general,
an
immortal cell line (typically myeloma cells) is fused to lymphocytes
(typically
splenocytes) from a mammal immunized with whole cells expressing a given
antigen,
-6-
CA 02553402 2006-07-25
P02-0245/CA
Osaka University
Boehringer Ingelheim International GmbH
e.g., Sema7A or VLA-1, and the culture supernatants of the resulting hybridoma
cells
are screened for antibodies against the antigen. See, generally, Kohler et
at., 1975,
Nature 265: 295-497, "Continuous Cultures of Fused Cells Secreting Antibody of
Predefined Specificity".
Immunization may be accomplished using standard procedures. The unit dose and
immunization regimen depend on the species of mammal immunized, its immune
status,
the body weight of the mammal, etc. Typically, the immunized mammals are bled
and
the serum from each blood sample is assayed for particular antibodies using
appropriate
screening assays. For example, anti-integrin antibodies may be identified by
immunoprecipitation of 125I-labeled cell lysates from integrin-expressing
cells.
Antibodies, including for example, anti-VLA-1 antibodies, may also be
identified by
flow cytometry, e.g., by measuring fluorescent staining of antibody-expressing
cells
incubated with an antibody believed to recognize VLA-1 molecules. The
lymphocytes
used in the production of hybridoma cells typically are isolated from
immunized
mammals whose sera have already tested positive for the presence of anti-VLA-1
antibodies using such screening assays.
Typically, the immortal cell line (e.g., a myeloma cell line) is derived from
the same
mammalian species as the lymphocytes. Preferred immortal cell lines are mouse
myeloma cell lines that are sensitive to culture medium containing
hypoxanthine,
arninopterin and thymidine ("HAT medium"). Typically, HAT-sensitive mouse
myeloma cells are fused to mouse splenocytes using 1500 molecular weight
polyethylene glycol ("PEG 1500"). Hybridoma cells resulting from the fusion
are then
selected using HAT medium, which kills unfused and unproductively fused
myeloma
cells (unfused splenocytes die after several days because they are not
transformed).
Hybridomas producing a desired antibody are detected by screening the
hybridoma
culture supernatants. For example, hybridomas prepared to produce anti-VLA-1
antibodies may be screened by testing the hybridoma culture supernatant for
secreted
antibodies having the ability to bind to a recombinant VLA-1 expressing cell
line.
To produce antibody homologs which are within the scope of the invention,
including
for example, anti-VLA-1 antibody homologs, that are intact immunoglobulins,
hybridoma cells that tested positive in such screening assays were cultured in
a nutrient
-7-
CA 02553402 2006-07-25
P02-0245/CA
Osaka University
Boehringer Ingelheim International GmbH
medium under conditions and for a time sufficient to allow the hybridoma cells
to
secrete the monoclonal antibodies into the culture medium. Tissue culture
techniques
and culture media suitable for hybridoma cells are well known. The conditioned
hybridoma culture supernatant may be collected and the anti-VLA-1 antibodies
optionally further purified by well-known methods.
Alternatively, the desired antibody may be produced by injecting the hybridoma
cells
into the peritoneal cavity of an unimmunized mouse. The hybridoma cells
proliferate in
the peritoneal cavity, secreting the antibody which accumulates as ascites
fluid. The
antibody may be harvested by withdrawing the ascites fluid from the peritoneal
cavity
with a syringe.
Fully human monoclonal antibody homologs against, for example Sema7A or VLA-1,
are another preferred binding agent which may block antigens in the method of
the
invention. In their intact form these may be prepared using in vitro-primed
human
splenocytes, as described by Boerner et al., 1991, J. Immunol. 147:86-95,
"Production
of Antigen-specific Human Monoclonal Antibodies from In Vitro-Primed Human
Splenocytes".
Alternatively, they may be prepared by repertoire cloning as described by
Persson et al.,
1991, Proc. Nat. Acad. Sci. USA 88: 2432-2436, "Generation of diverse high-
affinity
human monoclonal antibodies by repertoire cloning" and Huang and Stollar,
1991, J.
Immunol. Methods 141: 227-236, "Construction of representative immunoglobulin
variable region CDNA libraries from human peripheral blood lymphocytes without
in
vitro stimulation". U.S. Pat. No. 5,798,230 (Aug. 25, 1998, "Process for the
preparation
of human monoclonal antibodies and their use") describes preparation of human
monoclonal antibodies from human B cells. According to this process, human
antibody-
producing B cells are immortalized by infection with an Epstein-Barr virus, or
a
derivative thereof, that expresses Epstein-Barr virus nuclear antigen 2
(EBNA2).
EBNA2 function, which is required for immortalization, is subsequently shut
off, which
results in an increase in antibody production.
In yet another method for producing fully human antibodies, U.S. Pat. No.
5,789,650
(Aug. 4, 1998, "Transgenic non-human animals for producing heterologous
antibodies")
-8-
CA 02553402 2006-07-25
25771-1225
describes transgenic non-human animals capable of producing heterologous
antibodies
and transgenic non-human animals having inactivated endogenous immunoglobulin
genes. Endogenous immunoglobulin genes are suppressed by antisense
polynucleotides
and/or by antiserum directed against endogenous immunoglobulins. Heterologous
antibodies are encoded by immunoglobulin genes not normally found in the
genome of
that species of non-human animal. One or more transgenes containing sequences
of
unrearranged heterologous human immunoglobulin heavy chains are introduced
into a
non-human animal thereby forming a transgenic animal capable of functionally
rearranging transgenic immunoglobulin sequences and producing a repertoire of
antibodies of various isotypes encoded by human immunoglobulin genes. Such
heterologous human antibodies are produced in B-cells which are thereafter
immortalized, e.g., by fusing with an immortalizing cell line such as a
myeloma or by
manipulating such B-cells by other techniques to perpetuate a cell line
capable of
producing a monoclonal heterologous, fully human antibody homolog.
The conditions under which the cell of the present invention is contacted with
a putative
regulatory compound, such as by mixing, are conditions in which the cell can
exhibit
Sema7A or VLA-1 activity if essentially no other regulatory compounds are
present that
would interfere with such activity. Achieving such conditions is within the
skill in the
art, and includes an effective medium in which the cell can be cultured such
that the cell
can exhibit Sema7A or VLA-1 activity. For example, for a mammalian cell,
effective
media are typically aqueous media comprising RPMI 1640 medium containing 10%
fetal calf serum.
Cells of the present invention can be cultured in a variety of containers
including, but
not limited to, tissue culture flasks, test tubes, microtiter dishes, and
petri plates.
Culturing is carried out at a temperature, pH and carbon dioxide content
appropriate for
the cell. Such culturing conditions are also within the skill in the art. For
example, for
Ramos cells, culturing can be carried out at 37 C, in a 5% CO2
environment.
Acceptable protocols to contact a cell with a putative regulatory compound in
an
effective manner include the number of cells per container contacted, the
concentration
of putative regulatory compound(s) administered to a cell, the incubation time
of the
-9-
CA 02553402 2006-07-25
25771-1225
putative regulatory compound with the cell, the concentration of ligand and/or
intracellular initiator molecules administered to a cell, and the incubation
time of the
ligand and/or intracellular initiator molecule with the cell. Determination of
such
protocols can be accomplished by those skilled in the art based on variables
such as the
size of the container, the volume of liquid in the container, the type of cell
being tested
and the chemical composition of the putative regulatory compound (i.e., size,
charge
= etc.) being tested.
In one embodiment of the method of the present invention, a suitable number of
cells
are added to a 96-well tissue culture dish in culture medium. A preferred
number of
cells includes a number of cells that enables one to detect a change in VLA-1
or
Sema7A activity using a detection method of the present invention (described
in detail
below). A more preferred number of cells includes between about 1 and
lx106 cells per well of a 96-well tissue culture dish. Following addition of
the
cells to the tissue culture dish, the cells can be preincubated at 37 C, 5%
CO2
for between about 0 to about 24 hours.
A suitable amount of putative regulatory compound(s) suspended in culture
medium is
added to the cells that is sufficient to regulate the activity of a Sema7A or
VLA-1
protein in a cell such that the regulation is detectable using a detection
method of the
present invention. A preferred amount of putative regulatory compound(s)
comprises
between about 1 nM to about 10 mM of putative regulatory compound(s) per well
of a
96-well plate. The cells are allowed to incubate for a suitable length of time
to allow the
putative regulatory compound to enter a cell and interact with Sema7A or VLA-1
protein. A preferred incubation time is between about 1 minute to about 48
hours.
In another embodiment of the method of the present invention, cells suitable
for use in
the present invention are stimulated with a stimulatory molecule capable of
binding to
Sema7A or VLA-1 protein of the present invention to initiate a signal
transduction
pathway and create a cellular response. Preferably, cells are stimulated with
a
stimulatory molecule following contact of a putative regulatory compound with
a cell.
Suitable stimulatory molecules can include, for example, antibodies that bind
specifically to Sema7A or VLA-1 protein. A suitable amount of stimulatory
molecule to
add to a cell depends upon factors such as the type of ligand used (e.g.,
monomeric or
-10-
CA 02553402 2006-07-25
25771-1225
multimeric; permeability, etc.) and the abundance of Sema7A or VLA-1 protein.
Preferably, between about 1.0 nM and about 1 mM of ligand is added to a cell.
The method of the present invention include determining if a composition is
capable of
regulating Sema7A or VLA-1 protein activation. Such methods include assays
described in detail in the methods section. The method of the present
invention can
further include the step of performing a toxicity test to determine the
toxicity of the
composition.
Another aspect of the present invention includes a kit to identify
compositions capable
of regulating Sema7A or VLA-1 protein activity in a cell. Such a kit includes:
(1) a cell
comprising Sema7A or VLA-1 protein; and (2) a means for detecting regulation
of
either the Sema7A or VLA-1 protein. Such a means for detecting the regulation
of
Sema7A or VLA-1 protein include methods and reagents known to those of skill
in the
art, for example, VLA-1 protein activity can be detected using, for example,
activation
assays described herein-below. Means for detecting the regulation of Sema7A or
VLA-1
protein also include methods and reagents known to those of skill in the art.
Suitable
cells for use with a kit of the present invention include cells described in
detail herein. A
preferred cell for use with a kit includes a human cell.
METHODS OF THERAPEUTIC USE
As described above, the present inventors have found that Sema7A has a role in
the
immune system and have also identified the involvement of a1i31-integrin (VLA-
1) as a
receptor for Sema7A in activation of monocytes. Therefore, the interaction
between
Sema7A and al pl-integrin (VLA-1) will be a potential therapeutic target for
various
diseases mediated by cytokines such as inflammatory diseases.
The invention therefore provides a method of treating a cytokine mediated
disease, by
administering to a patient a composition which inhibits Sema7A and VLA-1
interaction.
A composition which would block the interaction of Sema7A with VLA-1 would
block
inflammatory cytokine production from cells. The inhibition of cytokine
production is
an attractive means for preventing and treating a variety of cytokine mediated
diseases
-11-
CA 02553402 2006-07-25
25771-1225
or conditions associated with excess cytokine production, e.g., diseases and
pathological
conditions involving inflammation, autoimmune responses or bone resorption.
Thus,
the compositions are useful for the treatment of diseases and conditions
including the
following:
osteoarthritis, atherosclerosis, contact dermatitis, bone resorption diseases
including
osteoporosis, reperfusion injury, asthma, multiple sclerosis, Guillain-Barre
syndrome,
Crohn's disease, ulcerative colitis, psoriasis, graft versus host disease,
systemic lupus
erythematosus and insulin-dependent diabetes mellitus, rheumatoid arthritis,
toxic shock
syndrome, Alzheimer's disease, diabetes, inflammatory bowel diseases, acute
and
chronic pain as well as symptoms of inflammation and cardiovascular disease,
stroke,
myocardial infarction, alone or following thrombolytic therapy, thermal
injury, adult
respiratory distress syndrome (ARDS), multiple organ injury secondary to
trauma, acute
glomerulonephritis, dermatoses with acute inflammatory components, acute
purulent
meningitis or other central nervous system disorders, syndromes associated
with
hemodialysis, leukopherisis, granulocyte transfusion associated syndromes, and
necrotizing entrerocolitis, complications including restenosis following
percutaneous
transluminal coronary angioplasty, traumatic arthritis, sepsis, chronic
obstructive
pulmonary disease and congestive heart failure. Said composition may also be
useful for
anticoagulant or flbrinolytic therapy (and the diseases or conditions related
to such
therapy).
Anti-cytokine activity can be demonstrated by using methods known in the art.
See for
example Branger et al., (2002) J Immunol. 168: 4070-4077, and the 46
references cited
therein.
A composition according to the invention will also be useful for treating
oncological
diseases. These diseases include but are not limited to solid tumors, such as
cancers of
the breast, respiratory tract, brain, reproductive organs, digestive tract,
urinary tract, eye,
liver, skin, head and neck, thyroid, parathyroid and their distant metastases.
Those
disorders also include lymphomas, sarcomas, and leukemias.
Examples of breast cancer include, but are not limited to invasive ductal
carcinoma,
invasive lobular carcinoma, ductal carcinoma in situ, and lobular carcinoma in
situ.
-12-
CA 02553402 2006-07-25
25771-1225
Examples of cancers of the respiratory tract include, but are not limited to
small-cell and
non-small-cell lung carcinoma, as well as bronchial adenoma and
pleuropulmonary
blastoma and mesothelioma.
Examples of brain cancers include, but are not limited to brain stem, optic
and
hypophthalmic glioma, cerebella and cerebral astrocytoma, medulloblastoma,
ependymoma, as well as pituitary, neuroectodermal and pineal tumor.
Examples of peripheral nervous system tumors include, but are not limited to
neuroblastoma, ganglioneuroblastoma, and peripheral nerve sheath tumors.
Examples of tumors of the endocrine and exocrine system include, but are not
limited to
thyroid carcinoma, adrenocortical carcinoma, pheochromocytoma, and carcinoid
tumors.
Tumors of the male reproductive organs include, but are not limited to
prostate and
testicular cancer.
Tumors of the female reproductive organs include, but are not limited to
endometrial,
cervical, ovarian, vaginal, and vulvar cancer, as well as sarcoma of the
uterus.
Tumors of the digestive tract include, but are not limited to anal, colon,
colorectal,
esophageal, gallblader, gastric, pancreatic, rectal, small-intestine, and
salivary gland
cancers.
Tumors of the urinary tract include, but are not limited to bladder, penile,
kidney, renal
pelvis, ureter, and urethral cancers.
Eye cancers include, but are not limited to intraocular melanoma and
retinoblastoma.
Examples of liver cancers include, but are not limited to hepatocellular
carcinoma (liver
cell carcinomas with or without fibrolamellar variant), hepatoblastoma,
cholangiocarcinoma (intrahepatic bile duct carcinoma), and mixed
hepatocellular
cholangiocarcinoma.
Skin cancers include, but are not limited to squamous cell carcinoma, Kaposi's
sarcoma,
malignant melanoma, Merkel cell skin cancer, and non-melanoma skin cancer.
-13-
CA 02553402 2006-07-25
P02-0245/CA
Osaka University
Boehringer Ingelheim International GmbH
Head-and-neck cancers include, but are not limited to laryngeal/
hypopharyngeal/nasopharyngeal/oropharyngeal cancer, and lip and oral cavity
cancer.
Lymphomas include, but are not limited to AIDS-related lymphoma, non-
Hodgkin's
lymphoma, Hodgkins lymphoma, cutaneous T-cell lymphoma, and lymphoma of the
central nervous system.
Sarcomas include, but are not limited to sarcoma of the soft tissue,
osteosarcoma,
Ewings sarcoma, malignant fibrous histiocytoma, lymphosarcoma, angiosarcoma,
and
rhabdomyosarcoma. Leukemias include, but are not limited to acute myeloid
leukemia,
acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic
myelogenous
leukemia, and hairy cell leukemia.
Plasma cell dyscrasias include, but are not limited to multiple myeloma, and
Waldenstrom's macroglobulinemia.
These disorders have been well characterized in man, but also exist with a
similar
etiology in other mammals, and can be treated by pharmaceutical compositions
of the
present invention.
For therapeutic use, the compositions may be administered in any conventional
dosage
form in any conventional manner. Routes of administration include, but are not
limited
to, intravenously, intramuscularly, subcutaneously, intrasynovially, by
infusion,
sublingually, transdermally, orally, topically or by inhalation. The preferred
modes of
administration are oral and intravenous.
The compositions may be administered alone or in combination with adjuvants
that
enhance stability of the inhibitors, facilitate administration of pharmaceutic
compositions containing them in certain embodiments, provide increased
dissolution or
dispersion, increase inhibitory activity, provide adjunct therapy, and the
like, including
other active ingredients. Advantageously, such combination therapies utilize
lower
dosages of the conventional therapeutics, thus avoiding possible toxicity and
adverse
side effects incurred when those agents are used as monotherapies. The above
described compositions may be physically combined with the conventional
therapeutics
or other adjuvants into a single pharmaceutical composition. Advantageously,
the
compositions may then be administered together in a single dosage form. In
some
-14-
CA 02553402 2006-07-25
t
25771-1225
embodiments, the pharmaceutical compositions comprising such combinations of
compositions contain at least about 5%, but more preferably at least about
20%, of a
composition (w/w) or a combination thereof. The optimum percentage (w/w) of a
composition of the invention may vary and is within the purview of those
skilled in the
art. Alternatively, the compositions may be administered separately (either
serially or in
parallel). Separate dosing allows for greater flexibility in the dosing
regime.
As mentioned above, dosage forms of the compositions described herein include
pharmaceutically acceptable carriers and adjuvants known to those of ordinary
skill in
the art. These carriers and adjuvants include, for example, ion exchangers,
alumina,
aluminum stearate, lecithin, serum proteins, buffer substances, water, salts
or
electrolytes and cellulose-based substances. Preferred dosage forms include,
tablet,
capsule, caplet, liquid, solution, suspension, emulsion, lozenges, syrup,
reconstitutable
powder, granule, suppository and transdermal patch. Methods for preparing such
dosage forms are known (see, for example, H.C. Ansel and N.G. Popovish,
Pharmaceutical Dosage Forms and Drug Delivery Systems, 5th ed., Lea and
Febiger
(1990)). Dosage levels and requirements are well-recognized in the art and may
be
selected by those of ordinary skill in the art from available methods and
techniques
suitable for a particular patient. In some embodiments, dosage levels range
from about
1-1000 mg/dose for a 70 kg patient. Although one dose per day may be
sufficient, up to
5 doses per day may be given. For oral doses, up to 2000 mg/day may be
required. As
the skilled artisan will appreciate, lower or higher doses may be required
depending on
particular factors. For instance, specific dosage and treatment regimens will
depend on
factors such as the patient's general health profile, the severity and course
of the
patient's disorder or disposition thereto, and the judgment of the treating
physician.
References
1) Kolodkin, A. L., Matthes, D. J., and Goodman, C. S. (1993). The semaphorin
genes
encode a family of transmembrane and secreted growth cone guidance molecules.
Cell
75, 1389-1399.
-15-
CA 02553402 2006-07-25
'
P02-0245/CA
Osaka University
Boehringer Ingelheim International GmbH
2) Pasterkamp, R. J., and Kolodkin, A. L. (2003). Semaphorin junction: making
tracks
toward neural connectivity. Curr Opin Neurobiol 13, 79-89.
3) Kilcutani, H., and Kumanogoh, A. (2003). Semaphorins in interactions
between T
cells and antigen-presenting cells. Nat Rev Immunol 3, 159-167.
4) Xu, X., Ng, S., Wu, Z. L., Nguyen, D., Homburger, S., Seidel-Dugan, C.,
Ebens, A.
and Luo, Y. (1998). Human semaphorin K1 is glycosylphosphatidylinositol-linked
and
defines a new subfamily of viral-related semaphorins. J Biol Chem 273, 22428-
22434.
5) Tamagnone, L., Artigiani, S., Chen, H., He, Z., Ming, G. I., Song, H.,
Chedotal, A.,
Winberg, M. L., Goodman, C. S., Poo, M. et al. (1999). Plexins are a large
family of
receptors for transmembrane, secreted, and GPI-anchored semaphorins in
vertebrates.
Cell 99, 71-80.
6) Pasterkamp, R. J., Peschon, J. J., Spriggs, M. K., and Kolodkin, A. L.
(2003).
Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424,
398-405.
7) Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002)
Identification of a factor that links apoptotic cells to phagocytes. Nature
417 182-7.
-16-