Note: Descriptions are shown in the official language in which they were submitted.
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
DENTAL-BLEACHING COMPOSITIONS AND METHODS WITH REDUCED
PEROXIDE CONCENTRATION
FIELD
[0001] This application relates generally to dental-whitening methods and
compositions and, more particularly, to dental-bleaching methods and
compositions based
upon peroxide compounds.
BACKGROUND
[0002] Tooth whitening methods currently offered in dental practices often
involve
the use of take-home whitening kits containing a peroxide gel or paste. In one
type of kit, the
peroxide product is placed in a pre-fabricated tray and worn by the patient
for 1-2 weeks in
daily application times ranging from 30 minutes to overnight. In an attempt to
obtain faster
whitening, products containing high concentrations up to 22% carbamide
peroxide are now
available. Nevertheless, such products with high peroxide concentrations can
often produce
greater tooth and gingival irritation. Thus, there remains a continuing need
for new peroxide-
based tooth-whitening products.
SUMMARY
[0003] Accordingly, the present inventors have succeeded in developing methods
and
compositions for enhancing tooth-whitening with peroxide compounds that
accelerate the
tooth-whitening process, increase the whitening activity of the peroxide
compounds and
decrease oral irntation. The methods and compositions are based upon a tooth-
whitening
composition comprising a peroxide compound and one or more abrasive compounds,
said
composition having a pH of 9.0 or greater. Any one or more of an increased
rate of tooth-
whitening, increased effectiveness of tooth-whitening and decreased oral
irritation are
produced in comparison to that produced by a tooth-whitening composition
containing the
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
same or greater concentration of the peroxide compound at a pH of 7.0 or less
in absence of
the one or more abrasive compounds. The methods can involve increasing the pH
of the
peroxide-containing tooth-whitening composition from a pH of 7.0 or less to a
pH to 9.0 or
greater by combining, in a dual component system, a first component comprising
the
peroxide compound in an aqueous vehicle at a pH of about 7 or less and adding
a second
component comprising an alkaline compound produce a mixture having a pH of
about 9 or
greater. It is also possible to initially preparing the composition containing
both the peroxide
compound and the alkaline compound such that the composition has a pH of about
9 or
greater. The presence of the one or more abrasive compounds further increases
the
effectiveness of the composition. As a result, the whitening process is
accelerated whitening
at a lower concentration of the peroxide compound and with less oral irntation
than that
produced by a peroxide-containing composition having a pH of about 7 or less
in absence of
the one or more abrasive compounds.
[0004] Thus, in various embodiments, the present invention can involve a dual
component dentifrice system comprising first and second components. The first
component
can comprise a peroxide compound and a peroxide-compatible abrasive compound.
The
second component can comprise an alkaline compound. Combining the first and
second
components in a mixture produces a dentifrice composition having a pH of about
9 or greater
is produced. By combining the first and second components it is meant that the
first and
second components are mixed in their entireties or that predetermined portions
of the first and
second components are mixed, such as, for example, the first and second
components can be
combined in a 1:1 ratio by either weight or volume. The pH of the first
component can be in a
range of from about 4 to about 7 and the pH of the second component can be in
a pH range of
from at least about 9 to about 13. The peroxide compound in the dentifrice
composition can
2
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
be at a hydrogen-peroxide-equivalent concentration of not greater than about
5°lo by weight
or at a hydrogen-peroxide-equivalent concentration of not greater than about
3.5% by weight.
[0005] In various embodiments, the present invention can also include
dentifrice
compositions comprising a peroxide compound and a peroxide-compatible abrasive
compound. The pH of the composition can be 9.0 or greater and the
concentration of the
peroxide compound can be at a hydrogen-peroxide-equivalent concentration of
not greater
than about 5% by weight or at a hydrogen-peroxide-equivalent concentration of
not greater
than about 3.5% by weight. The composition can produces a tooth-whitening
effect equal to
or greater than that of a comparative tooth-whitening composition having a
hydrogen
peroxide concentration of about 7% or greater by weight.
[0006] In various embodiments, the present invention can also include methods
for
whitening a tooth in a mammal. The methods can comprise contacting the tooth
for an
effective tooth-whitening period with a tooth-whitening composition. The tooth-
whitening
composition can comprises a peroxide compound and a peroxide-compatible
abrasive
compound. The pH of the tooth-whitening composition can be at least about 9
and the
peroxide compound can be at a hydrogen-peroxide-equivalent concentration of
not greater
than about 5% by weight or at a hydrogen-peroxide-equivalent concentration of
not greater
than about 3.5% by weight. In various aspects, the composition can be prepared
from a dual
component system comprising a first component comprising a peroxide compound
and a
peroxide-compatible abrasive compound and a second component comprising an
alkaline
compound. The contacting is for a period of time which is effective in
whitening the tooth,
i.e. an effective tooth-whitening period, which is less than that required for
a comparative
composition comprising hydrogen peroxide at a concentration of about 7% by
weight or
greater and a pH of about 7.0 or less. Such effective tooth-whitening period
of the tooth-
whitening compositions of the present invention can be not more than about 15
minutes or
3
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
not more than about 20 minutes and that of comparative composition can be
about 30 minutes
or greater.
[0007] The present invention, in various embodiments, can also include methods
for
decreasing whitening time, increasing whitening effectiveness or decreasing
oral irritation in
a tooth-whitening process. The methods can comprise contacting a tooth for an
effective
tooth-whitening period with a tooth-whitening composition comprising a
peroxide compound
and a peroxide-compatible abrasive compound. The pH of the tooth-whitening
composition
can be at least about 9 and the peroxide compound can be present at a hydrogen-
peroxide-
equivalent concentration of not greater than about 5°!o by weight or at
a hydrogen-peroxide-
equivalent concentration of not greater than about 3.5% by weight. The tooth-
whitening
composition produces a decrease in whitening time, an increase in whitening
effectiveness or
a decrease in oral irritation or any combination thereof in comparison to a
reference
composition having a peroxide compound concentration of about 7% by weight or
greater
and a pH of about 7.0 or less. In various aspects of this embodiment, the
contacting can be for
an effective tooth-whitening period which is less than that required for a
comparative
composition comprising hydrogen peroxide at a concentration of about 7% by
weight or
greater and a pH of about 7.0 or less. For example, the effective tooth-
whitening period of the
tooth-whitening composition can be not more than about 15 minutes or not more
than about
20 minutes and that of comparative composition can be about 30 minutes or
greater. In
various aspects of this embodiment, the tooth-whitening composition can be
prepared from a
dual component system comprising first and second components in which the
first component
can comprise a peroxide compound and a peroxide-compatible abrasive compound
and the
second component can comprise an alkaline compound.
[0008] In various aspects of the embodiments of the present invention
involving a
dual component system, the first and second components can combine not more
than about
4
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
15 minutes, not more than about 10 minutes, not more than about 5 minutes or
not more than
about 2 minutes prior contacting a tooth with the composition to produce tooth
whitening.
[0009] In various of the embodiments of the present invention, the peroxide
compound can be hydrogen peroxide, an organic peroxide compound, a hydrogen
peroxide
generating compound or combinations thereof. Such organic peroxide compound
can be, for
example, urea hydrogen peroxide, glyceryl peroxide, benzoyl peroxide,
monoperoxyphthalate
or combinations thereof. The hydrogen peroxide generating compound can be, for
example,
sodium persulfate, sodium dipersulfate, sodium percarbonate, sodium
perphosphate, sodium
perborate, sodium persilicate, potassium persulfate, potassium dipersulfate,
potassium
percarbonate, potassium perphosphate, potassium perborate, potassium
persilicate, calcium
persulfate, calcium dipersulfate, calcium percarbonate, calcium perphosphate,
calcium
perborate, calcium persilicate, sodium peroxide, potassium peroxide and
calcium peroxide or
combinations thereof.
[0010] In various of the embodiments of the present invention the peroxide-
compatible abrasive comprises one or more calcium phosphate salts including,
for example
calcium pyrophosophate. The peroxide-compatible abrasive can be present in the
tooth-
whitening composition or in the dentifrice composition at a concentration of
at least about
10% by weight or at a concentration of at least about 20% by weight.
[0011] In various embodiments of the present invention, an alkaline compound
can be
present. Such alkaline compound can be, for example, sodium hydroxide,
potassium
hydroxide, ammonium hydroxide, calcium hydroxide, magnesium hydroxide, sodium
carbonate, potassium carbonate, ammonium carbonate, calcium carbonate,
magnesium
carbonate, sodium bicarbonate, potassium bicarbonate, ammonium bicarbonate,
calcium
bicarbonate, magnesium bicarbonate, urea, monoethanolamine, diethanolamine,
triethanolamine, mono(iso)propanolamine, di(iso)propanolamine,
tri(iso)propanolamine, 2-
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
amino-2-methylpropanol, 2-amino-2-methyl-1,3-propanediol, 2-amino-2-ethyl-1,3-
propanediol, tris(hydroxymethyl)aminomethane, N,N,N'N'-tetrakis(2-
hydroxypropyl)ethylenediamine, di(2-ethylhexyl)amine, triamylamine,
dodecylamine,
morpholine or combinations thereof.
[0012] In various embodiments of the present invention involving a dual
component
system, the second component can also comprise an abrasive compound. The
abrasive
compound in the second component can be one or more peroxide-incompatible
abrasive
compounds, such as, for example a silica compound or an alumina compound or
one or more
peroxide-compatible abrasive compounds, such as, for example one or more
calcium
phosphate salts including calcium pyrophosphate as well as a combination of
one or more
peroxide-incompatible and/or peroxide-compatible abrasive compounds. The total
concentration of abrasive compounds in the tooth-whitening compositions or the
dentifrice
compositions can be at least 10°7o by weight or at least 20°70
by weight.
[0013] In various embodiments of the present invention involving a dual
component
system, the second component can also comprise a color indicator such as, for
example,
group consisting of FD&C Red No. 3, FD&C Yellow No. 5, FD&C Yellow No. 6, FD&C
Green No. 3, FD&C Blue No. 1 and combinations thereof. In addition, either or
both of the
first or second components can further comprise a tooth-desensitizing compound
such as, for
example, a potassium salt of a weak acid or eugenol.
BRIEF DESCRIPTION OF THE DRAWINGS
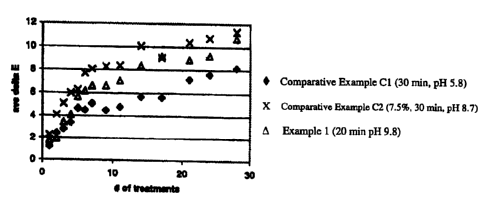
[0014] Figure 1 illustrates the in vitro treatment of extracted human teeth
for 28
treatment periods using the composition of Example 1 for 20 minute treatment
periods;
comparative example C1 for 30 minute treatment periods; and comparative
example C2 for
30 minute treatment periods.
6
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0015] Figure 2 illustrates the in vitro treatment of extracted human teeth
for 14 or
greater treatments using the compositions of Example 1 for 20 minute treatment
periods; the
composition of Example 5 for 15 minute treatment periods; comparative example
C1 for 30
minute treatment periods; and comparative example C2 for 30 minute treatment
periods.
DETAILED DESCRIPTION
[0016] The present invention, in various embodiments, can involve methods and
compositions for enhancing tooth-whitening with compositions containing one or
more
peroxide compounds and one or more abrasive compounds, wherein the
compositions can
have a pH of at least about 9Ø Such compositions can accelerate the tooth-
whitening
process, increase the whitening activity of the peroxide compounds and
decrease oral
irritation.
[0017] Reference herein to "a tooth" is intended to include the singular
(tooth) and
the plural (teeth). The tooth-whitening effect of the compositions is
detectable by visual
observation or by measurement using any of various instruments following a
given
application period or following successive application periods of the same
duration.
[0018] The enhanced whitening effect of the compositions of the present
invention,
can be achieved at a reduced concentration of peroxide compounds compared to
concentrations normally used. Concentrations of peroxide compounds normally
used can be,
for example, 7% hydrogen peroxide by weight or 7.5% hydrogen peroxide by
weight or an
amount of a peroxide compound other than hydrogen peroxide in which the
peroxide
compound delivers a hydrogen peroxide ion or an organic peroxide ion in an
amount
equivalent to the hydrogen peroxide ion delivered by 7% by weight hydrogen
peroxide or
7.5% by weight hydrogen peroxide under the same conditions of temperature,
exposure to
7
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
light or other radiation, agitation and the like. Such equivalence is
sometimes referenced
herein as hydrogen-peroxide-equivalent concentration for a given peroxide
compound.
[0019] Thus, concentrations of peroxide compounds as used herein refer
concentrations that deliver the amount of bleaching activity equal to that
delivered by
hydrogen peroxide at the stated concentration. In various embodiments, the
compounds
deliver an amount of peroxide ion equal to that delivered by hydrogen peroxide
at the stated
concentration. For example, carbamide peroxide at a concentration of 10% by
weight in an
aqueous solution is generally considered to deliver the same amount of
hydrogen peroxide
ion as an aqueous solution of hydrogen peroxide at a concentration of about 3%
by weight.
Thus, for carbamide peroxide, a hydrogen-peroxide-equivalent concentration of
3.5% by
weight is intended to mean a carbamide peroxide concentration of about 12% by
weight, a
hydrogen-peroxide-equivalent concentration of 5% by weight is intended to mean
a
carbamide peroxide concentration of about 17% by weight and a hydrogen-
peroxide-
equivalent concentration of 7% by weight is intended to mean a carbamide
peroxide
concentration of about 22% by weight.
[0020] All amounts in the specification and claims are by weight unless
otherwise
indicated. Reference herein to amounts by weight can also be expressed as
(w/w) which
intended to mean the ratio of the number of grams of a particular component of
a composition
to the total number of grams of that composition.
[0021] The terms "comparative composition" and "reference composition" are
used
interchangeably herein. Concentrations of the comparative or reference
compositions as used
herein is intended to mean the concentrations that deliver an amount of
peroxide ion equal to
that delivered by hydrogen peroxide at the stated concentration. In various
embodiments, the
reference or comparative composition can be hydrogen peroxide. Other
comparative
compositions can, however, be used and in such instances, the concentrations
of the
8
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
comparative compositions are intended to mean the hydrogen-peroxide-equivalent
concentrations of the comparative compositions. The comparative compositions
can be at a
hydrogen-peroxide-equivalent concentration of about 3.5% by weight or greater,
about 5% by
weight or greater, about 7% by weight or greater, about 7.5% by weight or
greater, about
10% by weight or greater, or about 13% by weight or greater.
[0022] The hydrogen-peroxide-equivalent concentrations of the peroxide
compounds
of the present invention, show an enhanced whitening effect at hydrogen-
peroxide-equivalent
concentrations of not more than 3% by weight, not more than 3.5% by weight,
not more than
4% by weight, not more than 4.5% by weight, not more than 5% by weight, not
more than
5.5% by weight, not more than 6% by weight, and not more than 6.5% by weight.
[0023] In various embodiments of the present invention, the concentration of
the
peroxide compound can be viewed as a reduced concentration compared to the
reference
composition, i.e. the methods in certain embodiments, can involve a reduction
in the
concentration of the peroxide compound necessary to achieve the same whitening
effect in
duration of whitening period, effectiveness of whitening and degree of absence
of oral
irritation.
[0024] The peroxide compound can be any of a variety of peroxide-based
bleaching
agents, which deliver a hydrogen peroxide ion or an organic peroxide ion. Such
compound
include, for example, hydrogen peroxide, organic peroxide compounds, hydrogen
peroxide
generating compounds, organic peroxide generating compounds and combinations
thereof.
[0025] Organic peroxide compounds include, for example, urea hydrogen peroxide
(carbamide peroxide), glyceryl hydrogen peroxide as well as groups of
peroxides classified
according to the number and kind of organic functional groups attached to the
oxygen atoms,
such as, for example, alkyl hydrogen peroxide (R-O-O-H), dialkyl hydrogen
peroxide (R-O-
O-R') peroxy acids (RCO-O-O-H), peroxy esters (RCO-OOR'), and diacyl peroxides
(R-CO-
9
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
O-O-CO-R'). Among such peroxides used in dental whitening are the diacyl
peroxide,
benzoyl peroxide and the peroxy acid monoperoxyphthalate.
[0026] The hydrogen peroxide generating compound can be, for example, alkali
metal
and alkaline-earth metal persulfate, dipersulfate, percarbonate, perphosphate,
perborate, and
persilicate salts such as, for example, sodium persulfate, sodium
dipersulfate, sodium
percarbonate, sodium perphosphate, sodium perborate, sodium persilicate,
potassium
persulfate, potassium dipersulfate, potassium percarbonate, potassium
perphosphate,
potassium perborate, potassium persilicate, calcium persulfate, calcium
dipersulfate, calcium
percarbonate, calcium perphosphate, calcium perborate, calcium persilicate
salts as well as
sodium peroxide, potassium peroxide and calcium peroxide and combinations of
all of the
above hydrogen peroxide generating compounds.
[0027] The enhanced whitening effect is achieved at least in part due to the
high pH
of the tooth-whitening composition which is, in turn achieved by the presence
of at least one
alkaline compound in the composition. In dual component systems, the alkaline
compound
can be present in one component and the peroxide compound, in the other
component such
that upon combination prior to use, the tooth-whitening mixture has a pH of at
least about 9
or greater.
[0028] The alkaline compound can be, for example, an alkali metal, ammonium or
alkaline earth metal compound such as, for example, sodium hydroxide,
potassium
hydroxide, ammonium hydroxide, calcium hydroxide, magnesium hydroxide, sodium
carbonate, potassium carbonate, ammonium carbonate, calcium carbonate,
magnesium
carbonate, sodium bicarbonate, potassium bicarbonate, ammonium bicarbonate,
calcium
bicarbonate, magnesium bicarbonate or combinations thereof; an organic amine
such as urea,
alkanolamines such as monoethanolamine, diethanolamine, triethanolamine,
mono(iso)propanolamine, di(iso)propanolamine, tri(iso)propanolamine or 2-amino-
2-
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
methylpropanol; alkanediolamines such as 2-amino-2-methyl-1,3-propanediol or 2-
amino-2-
ethyl-1,3-propanediol; alkanepolyamines such as
tris(hydroxymethyl)aminomethane or
N,N,N'N'-tetrakis(2-hydroxypropyl)ethylenediamine; alkylamines such as di(2-
ethylhexyl)amine, triamylamine or dodecylamine; or amino ethers such as
morpholine.
[0029] The alkaline compound can be at a concentration of from about 0.1% by
weight to about 30% by weight, from about 0.2% by weight to about 10% by
weight, from
about 0.5% by weight to about 2% by weight or from about 0.5% by weight to
about 0.75%
by weight.
[0030] One or more abrasive compounds can be included in the tooth-whitening
compositions of the present invention. Such compounds impart cleaning and
polishing
'functions to the composition. In addition to such actions, the abrasive
compounds can
enhance the whitening effect of peroxide compounds.
[0031] The method of incorporating the abrasive compound into the tooth-
whitening
composition can depend upon the type of abrasive compound. For example,
certain abrasive
compounds can be considered incompatible with peroxide based bleaching
compounds
because they tend to produce a decomposition of the peroxide compound. This
can result not
only in a reduced efficacy, but also in premature gas evolution resulting in
swelling and/or
bursting of tubes containing products made up of a peroxide compound and a
peroxide-
incompatible abrasive.
[0032] Peroxide-compatible abrasives, i.e. abrasive compounds not considered
to
cause substantial decomposition of peroxide compounds, include calcium
phosphate abrasive
compounds (see for example, U.S. Patent No. 5,171,564, which is incorporated
in its entirety
by reference). Examples of such peroxide compatible abrasives include
dicalcium phosphate
dihydrate, anhydrous dicalcium phosphate, calcium pyrophosphate and mixtures
thereof.
11
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0033) Peroxide-incompatible abrasives include silica compounds and alumina
compounds. Such silica compounds include for example, hydrated silica, such as
Sorbosil
AC-35, marketed by INEOS Silicas Ltd (Warnngton, UK; formerly Crosfield
Chemicals), or
Zeodent 115 from Huber Company (Edison, N.J.). Alumina compounds can include,
for
example, alumina trihyhydrate, aluminum silicate, calcined alumina and
mixtures thereof.
[0034) Other abrasives can also be included in the compositions of the present
invention, such as, for example, hydroxyapatite, sodium metaphosphate,
potassium
metaphosphate, tricalcium phosphate, calcium carbonate, sodium bicarbonate,
bentonite, and
mixtures thereof.
[0035) Peroxide-incompatible abrasives can be combined with peroxide compounds
immediately prior to use without producing substantial decomposition of the
peroxide
compounds. Such mixtures can produce an enhanced whitening activity of the
peroxide
compound (see for example, U.S. Patent No. 5,766,574 which is incorporated in
its entirety
by reference).
[0036) Thus, in various embodiments, the tooth-whitening compositions of the
present invention can be in the form of a dual component system. The first
component can
comprise the peroxide compound and the second component can comprise the
alkaline
compound. In certain embodiments, the second component can also comprise a
peroxide-
incompatible abrasive compound. A peroxide-compatible abrasive compound can be
incorporated into either or both of the first or second components. In
particular, the first
component of a two component system of the present invention can comprise a
peroxide
compound and a calcium phosphate abrasive compound such as calcium
pyrophosphate and
the second component can comprise an alkaline compound. In various
embodiments, the
second component can also contain either or both of a peroxide-incompatible
abrasive
compound and a peroxide-compatible abrasive compound.
12
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0037] The first and second components can, independently, be in the form of a
liquid
or a semi-solid liquid such as a gel or paste.
[0038] The dual component system can be in the form of a kit which includes
the first
and second components along with instructions for combining the first and
second
components and/or instructions as to the method of use of the first and second
components
and the a mixture thereof. Such instructions as to the method of use can
include the amount to
be used, time period for applying the compositions, schedule for repeated
application and the
like.
[0039] Typically, equal amounts of the first component and the second
component are
combined in such a manner to effect mixing. Such mixing results in an increase
in the pH of
the mixture to about 9 or greater, about 9.5 or greater, or about 10 or
greater, or about 11 or
greater, or about 12 or greater up to a pH of about 13 or greater.
[0040] The time period for applying the compositions of the present invention
to
achieve tooth-whitening is sometimes referenced herein as "effective tooth-
whitening
period." This represents the time period during which the compositions contact
the tooth
during a single application. An "effective tooth-whitening period" can be
about 10 minutes or
less, about 15 minutes or less, about 20 minutes or less, about 25 minutes or
less or about 30
minutes or less or more than about 30 minutes.
[0041] The tooth whitening composition can be applied in a single application
or in
repeated application. Such repeated or successive applications can be
performed one or more
times during the day such as, for example, once a day, twice a day or three
times a day or less
frequently such as, for example once every two days, once every three days or
once a week.
The application period can continue, for example for about one week, about two
weeks, about
three weeks, or about four weeks or longer.
13
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0042) The compositions of the present invention, including the tooth-
whitening
compositions and the first and second component compositions of two-component
systems,
can, in various embodiments, contain a thickening agent which imparts a high
viscosity to the
composition. Typically the compositions have a viscosity of from less than
about 200 CPS up
to about 10,000 or greater or from about 1000 cps up to about 9000 cps or from
about 4000 to
about 6000 cps. In certain embodiments, the thickening agent can be a
hydrophilic block
copolymer of polyethylene oxide and polypropylene oxide such as a Pluronic~
compound,
for example Pluronic~ F127 which is a trade name of BASF obtained therefrom
(BASF
Corporation, Mount Olive, New Jersey). Pluronic~ F127 has a molecular weight
of about
4000. This substance can be present in the compositions at a concentration of
about 5% by
weight about 10% by weight, about 15% by weight, about 20% by weight, about
25% by
weight, about 30% by weight or greater. In particular, the Pluronic~ F127
concentration in
the first component containing the peroxide compound can be about 20% by
weight, in the
second component containing the alkaline compound, the concentration can be
about 17% by
weight and in the tooth-whitening mixture of the two, the concentration can be
about 18.5%
by weight.
[0043] Polyethylene oxide of high molecular weight can also be used in the
compositions of the present invention as thickening agents. Such polyetheylene
oxide
thickeners have a number average molecular weight, a viscosity average
molecular weight, a
weight average molecular weight or a Z-average molecular weight of about
50,000 to about
5,000,000. One or more high molecular weight polyethylene oxide can be present
in a
concentration range of from about 0% to about 30% by weight, from about 10% by
weight to
about 25% by weight or from about 15% by weight to about 20% by weight.
[0044] One or more of glycerin, sorbitol and low molecular weight polyethylene
glycol can also be included in the compositions as carrier materials which can
also impart
14
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
effects on viscosity. The polyethylene glycol is a nonionic polymer of
ethylene oxide. The
polyethylene glycol when present in the compositions of the present invention
can have a
number average, weight average, or Z average molecular weight of from about
200 to about
1000, from about 400 to about 800 or about 600. The glycerin, sorbitol and
polyethylene
glycol can each independently be present in amounts of from about 0% to about
30% by
weight, from about 5% by weight to about 25% by weight, from about 10% by
weight to
about 20% by weight, or from about 15% by weight to about 20% by weight.
Typically,
glycerin and polyethylene glycol (600) are present in amounts of about 5% by
weight and
about 15% by weight, respectively in the first component composition, in
amounts of about
5% by weight and about 10% by weight, respectively in the second component
composition
and in amounts of about 5% by weight and about 12.5% by weight, respectively
in the tooth-
whitening composition.
[0045] The compositions of the present invention can also contain added water
in an
amount of from about 5% by weight to about 40% by weight, from about 10% by
weight to
about 30% by weight or from about 5% by weight to about 20% by weight.
[0046] Surfactants can also be included in the compositions of the present
invention
as solubilizing, dispersing and/or emulsifying agents. Such surfactants can
include nonionic
surfactants such as, for example, Tween 20 or anionic surfactants such as, for
example,
sodium lauryl sulfate or sodium dodecyl sulfate.
[0047) The compositions of the present invention can also contain flavoring
substances at a concentration of from about 0.05% by weight to about 5% by
weight. Such
flavoring substances include, for example, an essential oil, extract or
flavoring aldehyde,
ester or alcohol that imparts a flavor of spearmint, peppermint, wintergreen,
sassafras, clove,
sage, eucalyptus, marjoram, cinnamon, lemon, lime, grapefruit, orange, apple,
pear, peach,
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
strawberry, cherry, apricot, watermelon, banana, coffee, cocoa, menthol,
carvone, anethole or
combinations thereof.
[0048] One or more sweetening agents can also be included in the compositions
of
the present invention. Such sweetening agents can include sodium saccharin,
sodium
cyclamate, zylitol, aspartame and the like in a concentration of from about
0.01% by weight
to about 1.0% by weight.
[0049] The compositions of the present invention can also be formulated to
contain
one or more desensitizing agents to reduce tissue sensitivity and irritation
upon application of
the tooth-whitening compositions. Such sensitizers include, for example,
potassium salts of
weak acids, such as potassium citrate, potassium chloride, potassium tartrate,
potassium
bicarbonate, potassium oxalate, potassium nitrate as well as strontium salts
and eugenol (4-
allyl-2-methoxyphenol). One or more of the sensitizers can be present in the
compositions of
the present invention at a concentration of from about 0.05% by weight to
about 0.5% by
weight or from about 0.1% by weight to about 0.25% by weight for eugenol and
from about
1% by weight to about 10% by weight or from about 3% by weight to about 6% by
weight
for potassium salts of weak acids such as, for example potassium nitrate.
[0050] One or more redox color indicators that are oxidized by hydrogen
peroxide,
can also be included in the second component of the dual-component system. The
color
indicator can be a dye suitable for use in an tooth-bleaching composition such
as, for food
color additives certified under the Food Drug & Cosmetic Act for use in food
and ingested
drugs, including dyes such as FD&C Red No. 3 (sodium salt of
tetraiodofluorescein), FD&C
Yellow No. 5 (sodium salt of 4-p-sulfophenylazo-1-p-sulfophenyl-5-
hydroxypyrazole-3
carboxylic acid), FD&C Yellow No. 6 (sodium salt of p-sulfophenylazo-B-naphtol-
6-
monosulfonate), FD&C Green No. 3 (disodium salt of 4-{ [4-(N-ethyl-p-
sulffobenzylamino)-
phenyl]-(4-hydroxy-2-sulfoniumphenyl)-mewthylene }-[ 1-(N-ethyl-N-p-
sulfobenzyl)-D-3,5-
16
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
cyclohexadienimine], FD&C Blue No. 1 (disodium salt of
dibenzyldiethyldiaminotriphenylcarbinol trisulfonic acid of indigotin). The
dyes change
colors upon contacting the peroxide compound, thereby signaling the user when
the effective
whitening period is completed. The second component composition can contain
one or more
dyes at a concentration of from about 0.005% by weight to about 0.5% by weight
or from
about 0.025% by weight to about 0.15% by weight.
[0051] Agents that chelate metal ions can also be present in the compositions
of the
present invention. Such chelating agents include sodium acid pyrophosphate,
disodium
calcium ethylene diamine tetraacetic acid, phosphoric acid, citric acid,
sodium citrate,
potassium citrate, sodium pyrophosphate, potassium pyrophosphate, disodium
ethylenediamine tetraacetic acid and the like. The chelating agents can be
incorporated into
the compositions of the present invention in an amount of from about 0.1% by
weight to
about 8% by weight or from about 0.5% by weight to about 3.0% by weight.
[0052] Fluoride compounds having anti-caries activity can also be incorporated
in the
compositions of the present invention. These substances release fluoride ions
in an aqueous
environment. Such fluoride compounds include salts such as, for example,
sodium fluoride,
potassium fluoride, cuprous fluoride, stannous fluoride, stannous
chlorofluoride, sodium
fluorosilicate, ammonium fluorosilicate, sodium monofluorophosphate, alumina
mono-
fluorophosphate and alumina difluorophosphate. The fluoride compounds, when
present, can
be at a concentration sufficient to release fluoride ion in an amount of from
about 15 to about
1500 ppm.
[0053] Anti-forming agents such as simethicone can also be present in the
compositions of the present invention at a concentration of from about 0% by
weight to
about 0.1% by weight.
17
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0054] Tooth whiteness can be visually observed or measured by any of various
instruments measured. One such instrument can be a colorimetry such as, for
example, a
Minolta portable Chroma-meter such as model CR-400 (Minolta Corp. Ramsey, New
Jersey).
This colorimeter can be programmed to measure Hunter lab values of "L", "a"
and "b" in
which "L" values represent lightness and "a" and "b" values represent the
chromaticity
coordinates. Lightness "L" values can represent dark to light color in which a
value of 0
represents black and a value of 100 represents white. Green to red can be
expressed by the
"a" value, the more positive value represented more red, and the more negative
value
represented more green color (-80 represents green and 100 represents red).
Blue to yellow
can be expressed by the "b" value, a more positive value represented more
yellow color in the
sample (-80 represents blue and 70 represents yellow). Typically "L" values
are used to
measure tooth-whiteness.
[0055] The invention can be further understood by reference to the examples
which
follow.
EXAMPLE 1
[0056] This Example illustrates a two component tooth-whitening system in
which
the concentration of the peroxide compound in the tooth-whitening composition
formed by
combining the two components is about 3.5% by weight.
[0057] The composition of the first, peroxide component, and the second
alkaline
component are as shown in Table 1.
18
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
Table 1. Tooth-Whitening Composition having 3.5% H202.
A B
Component Peroxide Component Alkaline Component
Ingredients Weight % Weight %
Deionized Water 14.3 31
Hydrogen peroxide 20 (35% solution) --
Potassium Nitrate -- 6
Glycerin 5 5
Polyethylene glycol 15 10
600
Pluronic F-127 20 17
Saccharin 0.2 --
Sodium hydroxide -- 0.5-0.75
FD+C Yellow #6 -- 0.025 - 0.15
Eugenol 0.25 --
Calcium pyrophosphate25 30
Sodium lauryl sulfate-- 0.4
Flavor 0.25 --
[0058] Components A and B were prepared in a Ross mixer (Charles Ross & Son
Company, Hauppauge, NY) as follows.
ComRonent A: Water, saccharin and 30% of the total volume of 35% hydrogen
peroxide
were stirred in a beaker until the saccharin dissolves. PEG 600 and glycerin
were added and
the mixture was placed in the Ross mixer along with Pluronic F127 and stirred
at high speed
under vacuum until a clear gel formed (approximately 45 minutes). Calcium
pyrophosphate
was added and the mixture stirred at medium speed for another 10 minutes under
vacuum.
The remaining 70% of the peroxide volume was added and stirred 10 minutes at
medium
speed. Next, the flavor was added and the mixture was stirred 5 minutes at low
speed. An
extrudible paste having a pH of 6.0 was obtained.
19
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
Component B: Water, sodium hydroxide potassium nitrate and FD+C Green #3 dye
were
mixed in a beaker until the potassium nitrate dissolved. PEG 600 and glycerin
were added to
the aqueous phase and the mixture was placed in the Ross mixing pot along with
Pluronic
F127. The mixture was stirred at high speed under vacuum until a clear gel
formed
(approximately 45 minutes). Calcium pyrophosphate, sodium lauryl sulfate,
sodium
bicarbonate and sodium hydroxide were added and the mixture stirred at medium
speed for
another 10 minutes under vacuum. An extrudable paste having a pH of 9.6 was
obtained.
EXAMPLE 2
[0059] This example illustrates the in vitro whitening effect of the tooth-
whitening
composition of Example 1 containing peroxide compound at a concentration of
3.5% by
weight and calcium pyrophosphate at a concentration of 27.5%.
[0060] Three compositions were tested. The composition of Example 1 was
prepared
by mixing component A with component B in approximately equal proportions.
Comparative
composition designated "C" was a simple pluronic gel prepared at approximately
the same
pH and peroxide concentration as the composition of Example 1, but having
water in place of
the calcium pyrophosphate. Comparative composition designated "C1" was a
commercially
available whitening composition comprised of a thickened Pluronic gel
containing about 7%
hydrogen peroxide at a pH of about 5.8 in addition to calcium pyrophosphate
and dicalcium
phosphate.
[0061] Extracted, naturally-stained human teeth were pumiced and initial color
determined using a Minolta Chromameter CR-241. Three teeth each were immersed
for
15 minutes at 37 °C in each of the three tooth-whitening compositions.
These 15 minute-
treatments were repeated 8 times for a total contact time of 2 hours. Tooth
shade was
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
measured midway and at the end of the total treatment period. The increase in
tooth
whiteness, represented as 0E values was calculated using the following
formula:
DE = [~L]2 + (0a)2 + (0b)2]1/2
wherein "L", "a" and "b" are Hunter lab values in which "L" values represent
lightness and
"a" and "b" values represent the chromaticity coordinates as described above.
Higher values
of 0E, indicate higher levels of tooth whiteness achieved. Results are shown
in Table 2
below.
Table 2. In vitro Immersion Study
Composition~H 0 E
4 treatments 8 treatments
Example 9.8 3.48 1.28 5.21 1.52
1
Comparative9.8 1.61 0.50 2.17 0.56
Example
C
Comparative5.8 2.60 0.47 4.23 0.97
Example
C 1
[0062] As shown in the table, the tooth-whitening effect of the composition of
Example 1 was greater than that of comparative composition C at both
measurement points.
The comparative composition C contained 3.5°lo by weight hydrogen
peroxide as did the
composition of Example 1, however, the composition of Example 1 also contained
calcium
pyrophosphate at a concentration 27.5%. This suggests that the calcium
pyrophosphate
contributed to the tooth-whitening effect of the composition of Example 1.
Since the majority
of the extrinsic staining was removed from the sample teeth with pumice, the
improved
whitening produced by calcium pyrophosphate is believed to result from removal
of intrinsic
tooth stain. Thus, the data above show that the calcium pyrophosphate provided
a beneficial
whitening benefit in addition to its effect on surface polishing and extrinsic
stain removal.
21
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0063] The whitening effect produced by the composition of Example 1 was
greater
than that produced by comparative composition, C1, which contained hydrogen
peroxide at a
concentration of about 7% and a pH of about 9.
EXAMPLE 3
[0064] This example illustrates the long-term in vitro effectiveness of the
tooth-
whitening composition of Example 1 containing peroxide compound at a
concentration of
3.5% by weight and calcium pyrophosphate at a concentration of 27.5%.
[0065] Three compositions were tested. The composition of Example 1 was
prepared
by mixing component A with component B in approximately equal proportions.
Comparative
composition designated "C1" was a commercially available whitening composition
comprised of a thickened Pluronic gel containing about 7% hydrogen peroxide at
a pH of
about 5.8 in addition to calcium pyrophosphate and dicalcium phosphate.
Comparative
composition "C2" was a commercially available whitening composition comprising
a
thickened Pluronic gel which contained about 7.5% hydrogen peroxide at a pH of
about 9 and
no added abrasive compounds.
[0066] Five human extracted teeth each were repeatedly treated with
comparative
composition "C1" for a periods of 30 minutes per treatment; or comparative
composition
"C2" for periods of 30 minutes per treatment. Another five human extracted
teeth were
treated with the composition of Example 1 for periods of 20 minutes per
treatment.
[0067] The study was carried out to 28 treatments, which is equivalent to 14
days of
twice daily treatments (a typical treatment regimen for at-home whitening).
Shade readings
were taken at various intervals with the Minolta Chromameter. Results are
shown in Figure 1.
As seen in the Figure 1, the tooth-whitening composition of Example 1 achieved
whitening
comparable to that of comparative composition C1 in half the number of
treatments
compared to that of the composition of Example 1. In addition, the composition
of Example
22
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
1 achieved whitening comparable to that of comparative composition C2 which
contained
more than twice the level of hydrogen peroxide at a concentration of 7.5%
compared to 3.5%
for the composition of Example 1.
EXAMPLE 4
[0068] This example illustrates the in vivo testing of the tooth-whitening
composition
of Example 1 containing peroxide compound at a concentration of 3.5% by weight
and
calcium pyrophosphate at a concentration of 27.5%.
[0069] The whitening efficacy of the composition of Example 1 was also
compared to
comparative composition C1 in a 2-week clinical study. One cell used
comparative
composition C1 30 minutes twice daily and the other cell used the composition
of Example 1
for 20 minutes once daily. Tooth shade was evaluated after 14 days using a
Vita shade guide.
The results are summarized in the Table 2.
23
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
Table 3: Clinical Testing of Composition of Example 1
Composition Hydrogen No. of SubjectsTotal Wear Shade Change
Peroxide Time
Concentration
Example 1 3.5% 13 4.7 hrs. 4.85 2.48
Comparative 7.5% 10 14.0 hrs. 4.56 2.65
Example C
1
[0070] As shown in the Table, there was no significant difference between
tooth
shade changes. Thus the composition of Example 1 provides the same whitening
effect as
comparative example C1, but at half the peroxide level and 1/3 the total wear
time. In
addition, subjects using comparative composition Cl reported significantly
(p=0.038) more
gum irntation than those using the composition of Example 1.
EXAMPLE 5
[0071] This Example illustrates a two component tooth-whitening system in
which
the concentration of the peroxide compound in the tooth-whitening composition
formed by
combining the two components is about 5°Io by weight.
[0072] The composition of the first, peroxide component, and the second
alkaline
component are as shown in Table 4.
24
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
Table 4. Tooth-Whitening Composition having 5% H202
A B
Component Peroxide Component Alkaline Component
Ingredients Weight % Weight %
Deionized Water 24 30.2
Hydrogen peroxide 10 --
Potassium Nitrate -- 10
Polyox (PEG 2M) 5 --
Glycerin S 25
Polyethylene glycol 10 3
600
Xanthan -- 0.7
Na carboxymethyl cellulose-- 0.5
Pluronic F-127 ~ 20 2
Sodium saccharin -- 0.2
Titanium dioxide -- 1
Sodium hydroxide (50%) -- 1
Ti02 --
1
FD+C Green #3 -- 0.025
Zeodent 115 (silica -- 17.5
abrasive)
Zeodent 165 (silica -- 3
thickener)
Sodium bicarbonate -- 5
Calcium pyrophosphate 25 --
Sodium lauryl sulfate -- 0.4
Flavor 0.5 0.5
Components A and B were prepared as described above in Example 1.
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
EXAMPLE 6
[0073] This example illustrates the in vitro whitening effect of the tooth-
whitening
composition of Example 5 containing peroxide compound at a concentration of 5%
by weight
and calcium pyrophosphate at a concentration of 12.5%.
[0074] In Vitro assessment of stain removal by the tooth-whitening composition
of
Example 5 was determined by an in vitro study procedure using human extracted
teeth as
described above in Example 2. The teeth were polished with a prophy paste to
remove any
surface stain. The root portions of five teeth were placed in a row and
immersed in an
impression compound. To mimic in vivo use, a tray for the sample teeth was
prepared by
placing a piece of tray material in a tray former and heated until the
material began to soften.
The softened tray material was then pulled down over the five teeth using a
vacuum and
allowed to harden to entrap the teeth in the hardened tray material.
[0075] Baseline Chromameter readings were taken of the teeth using a Minolta
Chromameter CR-241. Next, small dots of the tooth-whitening composition of
Example 5
composed of equal weight amounts of component A and B, so that the peroxide
content was
5% by weight, were placed in each tooth well in a dental tray. The tray was
weighed and
then placed over the teeth so that a thin film of the composition of Example 5
covered each
tooth. The tray covered teeth were placed in a 37°C incubator for 15
minute intervals and
thereafter removed, rinsed and measured with the Chromameter. This process was
repeated
for each time point. The DE values were determined for each time point as
described above.
[0076] For purposes of comparison, the single component peroxide paste similar
to
component A alone but containing hydrogen peroxide at a concentration of 3.5%
and
designated composition "C1A" as described above was evaluated for tooth
whitening
efficacy following the procedure of the Example 3 above. The OE values of the
tray covered
teeth were measured at 30 minute intervals for composition C1A. For purposes
of further
26
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
comparison, a commercially available professional tooth whitening composition
was also
evaluated for tooth whitening efficacy with chromameter measurements being
performed also
at 30 minute intervals. The commercial whitening composition, designated
composition
"C2" as described above was a thickened Pluronic gel which contained 7.5%
peroxide at a of
about pH 9. No abrasives were present in the gel. The whitening study was
carried out to 14
treatments, which is equivalent to a typical home whitening regimen of twice
daily treatments
over a one week period. Results are shown in Table 5.
Table 5. Tooth Whitening Efficacy of Composition Containing 5% HZO2.
Composition Number reatments
of
T
4 8 12 14
Exam 1e 5 6.98 7.82 8.07 8.23
Comparative 2.71 4.26 5.55 5.84
Exam 1e ClA
Comparative 4.47 7.12 8.16 8.38
Example C2
[0077] The results recorded in Table 5 indicate that although the treatment
time (15
minutes) using the composition of Example 5 was half the treatment time (30
minutes) of
comparative composition C2, the composition of Example 5 achieved faster
maximum
whitening in half the number of treatments and half the application time in
spite of the fact
that the composition C2 contained 33% more peroxide whitening agent than that
of the
composition of Example 5 (7.5% v. 5.0%).
[0078] These results are further illustrated in Figure 2 which shows the
comparisons
above and in addition, the whitening effects of the composition of Example 1.
[0079] While not intending to be bound by any theory, the unexpected
enhancement
in whitening efficacy produced by the compositions of Examples 1 and 5 in
comparison to
the comparative composition C2 may be due to the presence in the composition
of calcium
pyrophosphate and silica abrasives which serve to aid in extrinsic stain
removal and, boost
the effective peroxide concentration delivered inside the tooth by the
increased density and
27
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
solid content of the compositions of Example 1 and 5 as compared to the
comparative
composition C2. Thus, larger amounts of peroxide are believed to have been
delivered by the
compositions of Examples 1 and 5 to the teeth per unit weight of paste. In
addition, the
peroxide ingredient in the compositions of Example 1 and 2 are believed to
have been
concentrated into the water soluble portion of the composition and it is
believed that this
concentrated portion may have diffused into the teeth to remove intrinsic
stain.
EXAMPLE 7
[0080] This example illustrates the in vivo assessment of whitening efficacy
of the
composition of Example 5.
[0081] The composition of Example 5 was prepared by combining equal weight
amounts of Components A and B. Whitening efficacy of the composition was also
compared
to that of comparative composition C2 in a two week human clinical study
wherein one cell
of 9 subjects used composition C2 thirty minutes twice daily according to the
manufacturer's
instruction. The other cell with 12 patients used the composition of Example 5
for fifteen
minutes once daily. Tooth shade was evaluated after 0, 5, 7 and 14 days using
a value-
ordered Vita shade guide. The results are summarized in Table 6.
Table 6. In vivo Whitening Efficacy of Composition Containing 5% H202.
Shade Guide
Chan a
Average
Average Shade
Guide
Average Shade Change
Guide
Shade (Day) (Day)
at
Com ositionBaseline
5 7 14 5 7 14
Exam 1e D3 7.67 6.15 4.88 3.58 4.92 6.19
ComparativeD3 8.06 6.11 4.22 2.28 3.92 5.66
Exam 1e
C2
28
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
[0082] The results recorded in Table 6 indicate that the overall whitening
efficacy of
the composition of Example 5 was directionally better than comparative
composition C2 and
statistically better at S days (p=0.025) evidencing faster whitening efficacy
since both the
application time and number of treatments using the composition of Example 5
was one
quarter that of comparative composition C2 (15 minutes once daily versus 30
minutes twice
daily).
[0083] In addition to enhanced efficacy, the patients in the tooth whitening
study
using the composition of Example 5 as described above reported less gingival
irritation and
tooth sensitivity than patients using comparative composition C2. The patients
involved in
the study rated their tooth sensitivity and gingival irritation on a scale of
zero (none) to 5, the
higher the number, the greater the tooth sensitivity and gingival irntation
experienced by the
patient involved in the study. Patient rated their perception of tooth
sensitivity and gingival
irritation using this scale before using the product (baseline) and after
using the product for 7
days and 14 days. The patient ratings of gum irritation and tooth sensitivity
minus the
baseline ratings are recorded in Table 7 below.
29
CA 02553672 2006-07-18
WO 2005/072692 PCT/US2005/002332
Table 7. Sensitivity and Irritation Study with Composition of Example 5.
0 Gum 0 Tooth
Irntation* Sensitivity**
(Day) (Day)
Com osition 7 14 7 14
Example 5 -0.08 0.00 -0.62 -0.46
Comparative +1.11 +1.13 0.00 +0.33
Exam 1e C2
* Change in gum irritation from baseline
** Change in tooth sensitivity from baseline
[0084] Ratings for gum irntation and tooth sensitivity were lower for the
composition
of Example 5 compared to that of comparative composition C2 at 7 and 14 days.
The levels
of tooth sensitivity reported for the composition of Example 5 at 7 and 14
days were negative
(lower than the reported baseline values), suggesting that the desensitizing
agent present in
the composition reduced tooth sensitivity over the treatment period.
[0085] Copending Application No. 10/065,244 filed September 27, 2002 is hereby
incorporated in its entirety by reference.
[0086] The description of the invention is merely exemplary in nature and,
thus,
variations that do not depart from the gist of the invention are intended to
be within the scope
of the invention. Such variations are not to be regarded as a departure from
the spirit and
scope of the invention.