Note: Descriptions are shown in the official language in which they were submitted.
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
Improved Olefin Plant Recovery System
Employing a Combination of Catalytic Distillation
and Fixed Bed Catalytic Steps
Background of the Invention
The present invention relates to a method for'the production of
olefins and particularly to processing the cracking heater effluent to
more effectively recover the product and process the by-products.
In the production of ethylene and propylene through the
pyrolysis of a variety of feedstocks, several byproducts and
unsaturated diolefins and acetylenes are created. The net effluent
from the pyrolysis heaters, typically referred to as charge gas,
requires processing for the separation of the byproducts and removal
of the diolefins and acetylenes from the primary olefin products.
Removal of the C2 and heavier -diolefins and acetylenics from the
cracked gas is handled through a combination of separation via
distillation and reaction via hydrogenation. Specifically for acetylene,
separation alone would result in excessive loss of the ethylene
product since acetylene and ethylene have very similar relative
volatility. Currently, the distillatiQn and hydrogenation take place in
several distinct process steps that are designed to separate and
hydrogenate the C2, C3, and C4 compounds independently.
Separation of the different hydrocarbons before hydrogenation is
currently required for achieving better control over the hydrogenation,
prolonging catalyst life, and enhancing performance.
One disadvantage of this. widely practiced conventional
technology is the large energy consumption necessary to generate
the high pressures and cryogenic temperatures required to separate
first the hydrogen from the cracked gas and then subsequently the
molecules of higher carbon number. Additionally, the hydrogenation
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
2
steps for each of the hydrocarbon groups require an independent
reactor system consisting of several pieces of equipment driving up
the capital investment and complexity of the plant.
The invention outlined in the previous U.S. Patent 5,679,241
proposes the one-step conversion of all C2 to C5 and heavier
acetylenes and dienes without 'hydrogenation of the C2 or C3 olefins.
It is claimed that this is possible with one catalytic distillation unit
capable of treating the hot, relatively low pressure charge gas before
excessive compression and cryogenic cooling is performed. In
addition, if desired, this same one step process claims to be capable
of hydrogenating the C4 olefins to paraffins again without the loss of
C2 or C3 olefins. The patent relates to a system that is described as
being capable of removing 70% and more of the hydrogen in the
cracked gas prior to the required cryogenic separation by the
is hydrogenation of the C2 to C4 acetylenes and dienes and the C4 and
heavier olefins to paraffins. Removal of 70% or more of the
.hydrogen improves the economics :through a significant lowering of
the energy requirements for separation of the C2 and heavier
components. By reducing the hydrogen partial pressure, separation is
achieved at lower pressures and with reduced refrigeration.
However, it has been shown that such extensive hydrogenation in a
single step system cannot occur without substantial loss of ethylene
and propylene to paraffins by hydrogenation.
The process as described in USP 5,679,241 has significant
limitations. First, in the operation of an ethylene plant, the C2
acetylene specifically must be removed via hydrogenation since its
removal via distillation is extremely difficult requiring extensive
equipment and energy costs. Since acetylene is a polymerization
catalyst poison, it must be removed to low levels, often less than 1-2
ppm. The ability to hydrogenate; all of the C2 acetylene to that level
in a single catalytic distillation column while observing no ethylene
loss or preferably a gain was not possible at reasonable catalyst
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
3
volumes and commercially viable operating conditions. Second,
maintaining performance of the process during either a variation in
carbon monoxide flow, which impacts catalyst activity, and/or the
concentration of diene/acetylene in the feed was hard to manage and
would prove difficult to achieve at commercial scale. Third, methods
of handling eventual catalyst deactivation were limited. Since these
units must operate for long periods of time between shutdowns, the
only options were excessive catalyst or separate catalyst zones in the
reaction column that can be isolated and the catalyst replaced while
the other section remains in operation. When using larger catalyst
volumes, it is known that it is necessary to operate at lower
temperatures to avoid over-reaction while the catalyst is still active.
This negatively impacts the economics by requiring some refrigeration
to control operation at lower temperature and/or excessive recycles
of cool liquid within the column. Specifically pilot testing has shown
that:
a. When the single catalytic distillation column was
operated to remove greater than 95% of the C2
acetylene, the concurrent ethylene loss was above 1 %
by weight. This is undesirable economically.
b. When operating a single catalytic distillation column, if
the hydrogenation of C4 olefins is greater than 20%,
significant ethylene loss is to be expected with presently
available catalysts.
c. In order to achieve minimal ethylene and propylene
losses while operating a single catalytic distillation
column and maintaining extremely high conversions and
hydrogen removals, the design required excessive
catalyst as evidenced by low productivity and operation
at cooler temperatures requiring refrigeration.
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
4
d. A significant variation in catalyst activity will occur with
variations in the carbon monoxide in the feed. Such
variations if seen in a single step process, will result in
loss of acetylene removal efficiency and subsequent
products which do not meet specification. This impact
on performance d"ue to the loss of catalytic activity via
CO poisoning is equivalent to the impact on performance
due to catalyst aging.
e. A significant variation in feedstock to the ethylene
cracking heaters will result in substantial changes in
both the acetylenes and dienes as well as the hydrogen
flow. As the ratio of hydrogen to reactants changes, a
single step process has limited ability to follow such
changes. The results will be either a breakthrough of
acetylene leading to offspec ethylene product or a high
loss of valuable ethylene and propylene due to over-
reaction, unless th'Q system has substantial and
expensive overdesign which could be utilized to
surmount these process changes.
Summary of the Invention
The present invention relates to an improved process for the
processing of the charge gas effluent from the pyrolysis of a variety
of feedstocks. The primary objective is still to remove a significant
fraction of the hydrogen in the effluent by hydrogenating the C2 to C5
diolefins and acetylenes in the feed while achieving essentially total
hydrogenation of the C2 acetylene without significant hydrogenation
of the ethylene and propylene. In the improved process, this is
achieved even with disturbances in the carbon monoxide
concentration, varying diene and 'acetylenic feed concentrations and
catalyst deactivation as well as other foreseeable processing upsets.
The invention relates to catalytic distillation with improved liquid
ABBLUM/260
CA 02553962 2009-11-12
77680-109
recycle in combination with fixed bed hydrogenation reactor systems.
Specifically,
the operating conditions of the catalytic distillation are maintained or
adjusted to
obtain the maximum hydrogenation of the acetylenes and dienes but without any
loss of ethylene and propylene and preferably with an ethylene gain by C2
5 acetylene hydrogenation. Maintaining a stable high conversion of all of the
C2
to C5 acetylenes and dienes with 100% conversion of the C2 acetylene (still
without hydrogenating the ethylene and propylene) under varying process
conditions is made possible by the fixed bed hydrogenation system in which the
remaining C2 acetylene is completely hydrogenated again without significant
ethylene or propylene hydrogenation.
According to one aspect of the present invention, there is provided a
method of processing a thermally cracked feed stream containing hydrogen,
ethylene, propylene, acetylene, methyl acetylene, propadiene and other C4, C5,
C6
and heavier unsaturated hydrocarbons to hydrogenate and convert essentially
all
of said acetylene in high proportion to ethylene and hydrogenate at least a
portion
of the methyl acetylene, propadiene and other C4, C5, C6 and heavier
unsaturated
hydrocarbons to olefins and to thereby consume a portion of said hydrogen
without hydrogenating ethylene and propylene comprising the steps of: a.
introducing said feed stream into a catalytic distillation column containing
at least
one hydrogenation catalyst bed and concurrently: (i) selectively hydrogenating
a
portion of said acetylene to form ethylene and hydrogenating portions of said
methyl acetylene, propadiene and C4, C;5, C6 and heavier unsaturated
hydrocarbons and controlling the hydrogenation conditions whereby said
ethylene
and propylene are not hydrogenated; and (ii) separating by fractional
distillation
said feed stream into lighter hydrocarbons and heavier hydrocarbons; b.
removing
substantially all of the remaining portion of said hydrogen and said lighter
hydrocarbons as a vapor phase overhead and substantially all of said heavier
hydrocarbons as bottoms from said catalytic distillation column; c.
introducing at
least a portion of said vapor phase overhead into a vapor phase fixed bed
reactor
system containing a hydrogenation catalyst and hydrogenating the remaining
portion of said acetylene to form further ethylene and hydrogenating further
portions of said methyl acetylene, propadiene and C4, C5, C6 and heavier
unsaturated hydrocarbons and controlling the hydrogenation conditions whereby
CA 02553962 2009-11-12
77680-109
5a
said ethylene and propylene are not hydrogenated; and d. removing mixed
product from said fixed bed reactor system.
Brief Description of the Drawings
Figure 1 is a flow diagram of the prior art involving catalytic
distillation alone with the bottoms recirculation for temperature control.
Figure 2 is a flow diagram illustrating the present invention.
Figure 3 is a graph illustrating the ethylene gain or loss versus the
diene output level for the present invention compared to the prior art.
Figure 4 is a flow diagram similar to Figure 2 but illustrating another
embodiment of the present invention.
Figure 5 is a flow diagram of an alternate embodiment of the present
invention.
Figure 6 is a flow diagram illustrating an alternate embodiment of the
process of Figure 5.
Figure 7 is a flow diagram similar to Figure 2 but illustrating an
alternate embodiment of the present invention.
Description of the Preferred Embodiments
For a better understanding of the present invention, the prior art as
represented by the process disclosed in U.S. Patent 5,679,241
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
6
will be briefly described. Figure 1 of the drawings of the present
invention is essentially a copy of a drawing from that prior patent
simplified to identify only those features relevant to the present
invention. The charge gas 150 is compressed and fed to the
catalytic distillation column .156. This column, as in the present
invention, simultaneously carries out a catalytic reaction and
distillation. The column has a stripping section 158 below the feed
and a rectifying/reaction section 160 above the feed containing the
catalyst beds 166, 168 and 170. Descending liquid is withdrawn as
sidestreams through the intercondensers 1 80 and reinjected back into
the column over the next lower catalyst bed. A portion of the heat
of reaction is removed by these 'intercondensers. A liquid recycle
stream 260 from the stripping section is recycled to the column
overhead. This recycle 260 may be a portion 262 of the bottoms
-5 and/or a portion 264 from within the stripping section.
The overhead from column 156 passes into condensers 186
and 188 and the partially condensed stream enters separation vessel
1 90. The product C2 to C5 vapor overhead 194, containing ethylene
and propylene, then passes out to subsequent separation while the
condensed hydrocarbons are utilized as reflux 196 for the column.
Since the objective of the invention is to completely remove the
acetylene impurities from ethylene with no loss of the ethylene
entering the column, this must be accomplished in this single step
operation (one catalytic distillation column). The overhead vapor
stream passes into additional fractionation (not shown) where the
individual carbon number fractions are isolated.
The hydrogenation in catalytic distillation column 1 56 occurs in
the liquid phase. The column is operated such that liquid phase
composition is primarily C5 components. This minimizes the liquid
phase concentration of the 'ethylene and propylene and thus
minimizes their reaction. However, the concentration of these two
valuable olefins in the liquid will not be zero. The C2 acetylenes and
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
7
the C3 acetylenes and diolefins are more reactive than their olefin
counterpart. In the liquid phase, these are preferentially reacted to
ethylene and propylene respectively. However, as the reaction
moves to completion close to the desired 100% C2 acetylene
s conversion, there is no longer a significant concentration of more
reactive C2 acetylene present in the liquid phase. Under these
conditions, ethylene can react to form ethane (paraffin). This is the
point where ethylene loss occurs.
There are several options for reducing the reactivity in the
catalyst beds of this upper portion of the catalytic distillation column.
One option is to reduce the temperature. Reducing the temperature
in one section is difficult in a single column concept because the
distillation temperature is primarily controlled by the column pressure.
To do this, a second column operating at a lower pressure would be
required. A second option is to utilize a different catalyst in this
upper section. This catalyst would be designed to more selectively
hydrogenate acetylenes and diolefins. Both of these options would
however require higher volumes of catalyst within the column and
thus increase the column size and cost. A third option is to utilize an
intercooler as shown on the prior art.
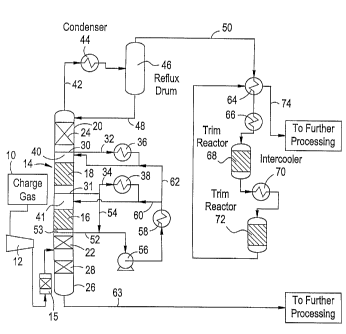
Referring now to Figure 2 that illustrates one embodiment of
the present invention, the charge gas 10 is compressed at 12 to
between 150 and 250 psig and then fed to the catalytic distillation
column 14. The charge gas may or may not be preheated to match
column temperatures. The charge gas would typically pass through
one or more guard beds 15 to remove such poisons as lead (Pb),
arsenic (As) and mercury (Hg). These are known catalyst poisons
and the guard beds would be employed in a known manner to protect
the catalytic distillation catalyst. Entering the catalytic distillation
column, the 8% to 20% by weight diene and acetylenic feed is
hydrogenated in catalyst beds 16 and 18 located in the rectification
section 20 of the column. The catalytic beds could be of the same or
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
8
different catalytic composition. The catalysts are known
hydrogenation catalysts consisting primarily of one or more Group
VIIIA metals (Ni, Pd, Pt) on a support. Additives such as Ag or Au
and/or alkali metals are typically used to control selectivity and
activity. Specific examples of selective hydrogenation catalysts
particularly suited for this service are disclosed in U.S. Patents
6,417,136; 5,587,348; 5,698,752 and 6,127,588. The catalytic
systems used within a catalytic distillation column can consist of
either a single catalyst, a catalyst with different metal loadings to
adjust activity located in different portions of the column, or mixtures
of catalysts of different metals located in different portions of the
column. The hydrogenation occurs in the liquid phase in catalytic
distillation fashion. Although only two reactive catalytic beds 16 and
18 are shown, this is only by way of example and could be any
number of beds depending on the requirements of any particular plant
or the desire to adjust catalyst activity through the use of more
complex catalyst systems. Fractionation internals 22 and 24, which
may be trays or packing, are provided in the rectification section 20.
Additional fractionation internals could be located between the
catalyst beds 16 and 18. The stripping section 26 contains
fractionation internals 28.
The overhead 42 from the column is cooled in the overhead
condenser 44 with cooling water or with refrigeration as needed and
the resulting vapor and liquid are separated in the reflux drum 46.
The resulting liquid from reflux drum 46 is fed through line 48 back
into the column as reflux. Similar to the prior art, the overhead vapor
50 contains most of the C5 and lighter compounds while the liquid
phase 48 is used to reflux the column. The vapor overhead 50
however does not pass into subsequent fractionation but into a fixed
bed reactor system consisting of one or more beds of catalyst with
provision for heating and/or cooling the vapor feed. Overhead 50 is
first exchanged against final fixed bed reactor system effluent 74 to
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
9
recover heat. It then passes to heater 66 where the temperature of
the vapor entering the first fixed bed reactor 68 is controlled. In
reactor 68, some portion of the Cz acetylene as well as some portion
of the C3 and heavier acetylenes and dienes that were not converted
in the catalytic distillation column are hydrogenated. The conditions
and the number of fixed bed reactors employed are such that the C2
acetylene is completely removed from effluent stream 74 with no
loss of ethylene and propylene over the entire system (catalytic
distillation plus fixed bed reactors). The addition of the fixed bed
reactor system to the catalytic distillation column dramatically
increases both the performance of the entire system and the ability of
that system to respond to process variations and catalyst
deactivation.
The operating criteria for the rectification section of the
-5 catalytic distillation column is that conditions be created wherein the
unsaturated hydrocarbons are hydrogenated to the extent possible
without any hydrogenation. of ethylene and propylene. This is
accomplished by:
1. Operating the column such that ethylene and propylene in the
liquid phase is minimized, and
2. Operating the catalytic distillation column such that there are
still unconverted C2 to C5 acetylenes and diolefins remaining in
column overhead 50.
In the catalytic distillation operation of the present invention,
the distillation function is designed and operated to distill essentially
all of the C5 and lighter components as overhead and essentially all of
the C6 and heavier components as. bottoms. Alternately, the split
could be at the C4 carbon number where essentially all of the C4 and
lighter components go overhead and the C5 and heavier components
leave as bottoms. In order = to selectively hydrogenate the C2
acetylenes, the C3 acetylenes and dienes, and the C4 and heavier
acetylenes, dienes and olefins while leaving the ethylene and
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
propylene un-hydrogenated, the rectification section 20 is operated
such that there is a substantial concentration gradient of C4 and C5
materials relative to C2 and C3 materials in 'the liquid phase where the
majority of the hydrogenation reaction occurs. This can be controlled
5 by variation of reboiler duty and reflux rate to achieve the desired
overhead and bottoms composition.
The choice of operation of the catalytic distillation column as
either a depentanizer or a debutanizer will be a function of both the
composition of the feed and the desired hydrogenation requirements
10 for the products. The preferred operating conditions for a
depentanizer will be a pressure of between 75 and 350 psig and a
catalyst bed temperature between 50 and 150 C. Similarly, the
preferred operating conditions for a debutanizer column will be a
pressure between 100 and 400 psig and a catalyst bed temperature
between 30 and 130 C.
In addition to controlling the overall fractionation, the
temperature and composition profiles over the reactive sections can
be controlled by adjusting the rate's of heat removal over the column
and by recirculation of liquid within and/or around the catalyst beds.
As shown in Figure 2, trays 30 and 31 collect the descending liquid
which is withdrawn as side streams 32 and 34. These streams may
or may not pass through the intercoolers 36 and 38 and then be
reinjected back into the column through the distribution headers 40.
This permits a portion of the heat of reaction to be removed in the
intercoolers. By arranging the intercoolers in this fashion, the cooling
medium can be water while the cooling in the overhead condensers
may need to be at least partly provided by mechanical refrigeration.
Hence, the use of the intercoolers can significantly reduce the portion
of the heat of reaction which needs to be- removed by mechanical
refrigeration.
The hydrogenation in the column 14 occurs in the liquid phase.
The extent of the reaction is dependent upon the relative reactivity of
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
11
the various components and the concentration of these components
in the liquid phase at any particular point in the column. The C2 and
C3 acetylenes and dienes are far more reactive than ethylene and
propylene so that they react first and rapidly. However, the relative
reactivities of ethylene, propylene and the C4 and heavier olefins,
dienes and acetylenes are very. close. In order to react a significant
quantity of the C4 and heavier olefins, dienes and acetylenes without
any significant loss of ethylene and propylene, the concentration of
the ethylene and propylene in the liquid phase must be minimized and
the concentration and temperature profiles from top to bottom must
be controlled. Since this stage of the hydrogenation occurs in a
fractionation column, this control can be accomplished by adjusting
the overhead reflux produced by the overhead condenser 44 and the
side stream reflux from the intercoolers 36 and 38. The liquid
-5 compositions of ethylene and propylene can be kept low in the
reactive zones through increases in the flow of reflux 48 and/or
increased interbed cooling at-36 'and 38.
In the catalytic distillation unit 14, the recycle and pumparound
circuits have been modified from the prior art as illustrated in Figure
1. That prior art shows simple intercoolers 180 and an overall
pumparound line 260. In the present invention, the system is
modified to provide the flexibility to have both uncooled and cooled
pumparounds in the catalyst zones 16 and 18 within the rectification
section 20. This improvement permits the desired temperature and
composition control with minimal disturbance to the overall
distillation. This is accomplished by drawing off pumparound liquid
immediately below the catalyst beds .as stream 52 and/or 54 from
withdrawal points 53 and 31 respectively and returning it through the
pump 56 and heat exchanger 58 to the top of the same bed as
streams 60 and/or 62. Alternatively, the liquid can be drawn from
the bottom most catalyst bed and returned to the highest bed via
stream 62. Cooling at 58 can be used, if necessary, to provide
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
12
combined composition adjustment and intercooling between reactive
beds. For example, while withdrawal intercooling stream 34 from
point 31, cooling that stream in exchanger 38 and returning the flow
to distribution system 41 will cool the liquid but not change the
composition. However, withdrawing the same liquid from 31,
passing it via line 54 through pump 56 to exchanger 58, cooling the
liquid and returning it to liquid distributor 40 above the catalyst bed
will change the composition profile within the column. This design
flexibility can be used to maximize the efficiency of the
hydrogenation. In this fashion, the option of cooling against a
warmer cooling medium available in the prior art is maintained with
the modified pumparound/intercooler reducing the expensive low level
cooling required in the overhead system. Further, the heat removed
by these pumparound streams can be utilized elsewhere in the
ethylene plant to reduce energy consumption. Another advantage of
the new pumparound scheme is that it allows for relatively large
liquid flow without affecting ,the overall column separation
performance due to heavies in the overhead as in the prior art. With
the large liquid flows, the pumparound can provide the necessary
liquid loading over the catalyst without the need for additional reflux.
This permits operation of the catalytic distillation column at lower
reflux ratios than previously possible without the penalty in
distillation efficiency observed with the prior art. Reflux ratios in the
range of 0.5 to 1.8 by weight are satisfactory for producing the
necessary catalyst liquid wetting where values as high as 5 were
required with the prior art. In addition to the obvious reduction in
energy requirements, higher hydrogen partial pressures' due to the
lower reflux ratios will be available in the present invention resulting
in lower required catalyst volumes.
In a catalytic distillation colurnn, it is critical to keep the
catalyst wetted at all times to insure that the reaction occurs in the
liquid phase. The selectivity of a catalytic distillation system relies in
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
13
part on the reaction taking place in the liquid phase while certain
components that the operator wishes to remain unreacted such as
ethylene remain in the highest concentration in the vapor phase.
Maintaining a certain liquid traffic down the column is critical to
keeping the catalyst wetted. If the liquid traffic is greater than 800
lb liquid/hr/ft2 of column cross-section, the catalyst will be highly
wetted and reaction selectivity will be maintained.
A secondary control variable would be variation in the reflux
with associated variation in reboiler duty. In this way, both catalyst
to bed temperature and composition may be altered to achieve the
desired hydrogenation.
Additionally, a variable feed location allowing for a main feed
point below the stripping section 22 will provide some separation of
any heavy components present in the feed before reaching both the
catalyst bed 16 and the side stream 52 for the first pumparound. In
this way, circulating the heavy, potentially fouling components over
the catalyst bed is eliminated. In ,addition, feed points above the first
catalyst bed can be incorporated to allow for turndown operation and
thus avoid the problems of excess catalyst and resultant selectivity
loss under these lower flow conditions. The bottoms 63 from the
column 14 are sent for further processing as desired.
As shown in Figure 2, the present invention includes the
addition of a fixed bed trim reactor system providing further
hydrogenation of stream 50. This system is typically two reactors
with an intercooler but could be a series of reactors with intercoolers
between successive reactors. The fixed bed reactor system provides
four advantages:
1. The catalytic distillation column no longer needs to operate for
high levels of hydrogenation but can be operated for the maximum
productivity from the catalyst, = a net ethylene gain with high
acetylene, methyl acetylene, and propadiene conversion while
maintaining acetylene C2 specification.
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
14
2. Changes in the catalytic distillation overhead concentration of
acetylenics and dienes resulting from catalyst deactivation, carbon
monoxide content increase, or feedstock change can be
accommodated.
3. Hydrogen removal can be maintained during temporary upsets
and/or catalyst deactivation or poisoning thus stabilizing performance
of downstream refrigeration systems. If the quantity of hydrogen
from the system were to vary, the partial pressures of the
downstream distillation system would change and the required
quantity of refrigeration would vary. This would create process
upsets and be very undesirable.
4. Opportunity for catalyst regeneration by the use of spare fixed
bed reactors thus extending onstream operating life of the entire
system.
In addition to control of the temperature and composition
profile over the column, it is important to operate with less than
complete conversion of the acetylenes and dienes over the catalytic
distillation column. By doing so, ethylene and propylene gains can be
achieved. Further, this operation requires less catalyst than the full
hydrogenation of the prior art thus maximizing catalytic distillation
catalyst productivity. Operation with a fixed bed reactor system
following the column allows this to occur.
If the column were to be operated such that there is no more
than approximately 1 % ethylene liquid concentration in the reactive
beds, hydrogenation in excess of 95% of the C2 to C5 and heavier
dienes can be achieved. This results in 5000-7500 ppm dienes and
acetylenics in the vapor stream 50 from the reflux drum 46 and a
minimum ethylene loss of 1 %. To make 100% acetylene conversion,
ethylene losses would even be higher. This operation coincides to a
hydrogen removal of approximately 30-35% depending upon the feed
composition. However, when the overall conversions of the C2 to C5
and heavier dienes and acetylenics are reduced to between 80 and
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
95% resulting in 10,000 to 20,000 and typically 15,000 ppm C2 to
C5 diene and acetylene in the outlet stream 50, ethylene gains can be
achieved. Figure 3 is a plot of the dienes at the outlet in ppm versus
the ethylene gain or loss in weight percent for a catalytic distillation
5 unit (CDU) alone and for a CDU plus, a fixed bed hydrogenation
system. As can be seen from- Figure 3, allowing a certain quantity of
highly reactive acetylenes and dienes to remain unreacted in the
overhead from the catalytic distillation column, ethylene and
propylene losses can be eliminated while still obtaining 100%
10 conversion of acetylene overall.
With fixed bed reactors located after the catalytic distillation
column 14, C2 acetylene breakthrough with 10,000 to 55,000 and
typically 20,000 ppm combined C3 and heavier dienes and acetylenes
can be tolerated from the catalytic distillation column. A typical
15 system with two fixed bed hydrogenation reactors with intercooling
has been shown to hydrogenate 100% of the C2 acetylenes entering
the fixed bed reactor system,.=,.and approximately 75% of the
combined C3 and heavier dienes and acetylenes entering the fixed
bed reactor system. This results in 2500 to 14,000 and typically
only 5000 ppm breakthrough of dienes and acetylenics from the
combined system. This represents approximately 97% hydrogenation
of the total C2 and heavier acetylenes and diolefins in the feed. Such
operation allows for substantial overall ethylene gains of up to 0.5%
with 70% overall acetylene selectivity toward ethylene at 100%
acetylene conversion. This is a substantial improvement over the
prior art.
The specific hydrogenation reactivity of ethylene is just slightly
lower than the specific reactivity of propadiene. Thus close
observation of the C3 diene conversion provides a reliable indication
of the stability of the ethylene gain and can be used as a control
point for the system. For the catalytic distillation system alone,
when C3 diene conversion is between 40 and 60% and typically
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
16
45%, ethylene losses are observed. However, when operating at
conditions where the C3 diene conversion in the catalytic distillation
column is between 10 and 35% and typically 20%, ethylene gains
from 0.2% to 0.5 % are possible. With the present invention, the
propadiene conversion can be increased substantially while still
maintaining ethylene gain.
During the normal operation of an ethylene unit, variations in
the carbon monoxide content of charge gas 10 is experienced. In
addition, feedstock quality or operating severity may be changed that
will impact the acetylenic and diolefin content of charge gas. For a
fixed catalyst volume in the catalytic distillation column, increases in
carbon monoxide or inlet diene and acetylenic concentrations result in
lower conversion and thus higher releases of these undesired
products into stream 50. Compensation for such anticipated
disturbances would be difficult with the prior art alone as shown in
Figure 1. It would require increases in operating pressure or
temperature impacting the performance of the entire fractionation
system. In the improved process including a fixed bed reactor
system, the temperature of the vapor 50 entering the fixed bed
reactor system can be adjusted to either increase or decrease
reactivity of the reactor system and thus follow changes in catalytic
distillation reaction activity and maintain complete C2 acetylene
removal and high hydrogen removal efficiency.
Finally, a fixed bed hydrogenation reactor system is designed
to, include not only operating reactors but also spares. Catalyst
deactivation will occur in both the fixed bed system and the catalytic
distillation system. It is not possible to regenerate the catalytic
distillation catalyst without shutting down the process or installing a
parallel column. Both options are costly. However, a spare fixed bed
vapor phase reactor is a relatively inexpensive option. By utilizing a
fixed bed reactor system with a spare instead of the single column
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
17
concept of the prior art, onstream life of the process can be
substantially improved.
In the fixed bed hydrogenation system, the net overhead 50
from the catalytic distillation passes through the cross flow heat
exchanger 64 and inlet heater 66 into the first fixed bed reactor 68.
The effluent from the first reactor 68 goes through the intercooler 70
to the second fixed bed hydrogenation in reactor 72. A series of
fixed beds followed by intercoa'lers can be used in the same fashion
in order to achieve the necessary heat transfer when required. The
effluent from the last reactor 72 then goes back through the cross
flow heat exchanger 64 where heat is extracted and the feed 50 to
the fixed bed reactors is heated. The inlet temperature to the fixed
bed reactors can be quickly changed to either increase or decrease
the extent of hydrogenation in the fixed bed reactors. Such control is
necessary to successfully handle changes in carbon monoxide or
diene and acetylene feed concentration. Up to a maximum adiabatic
temperature rise of 80 F total for both beds, a stable fixed bed
operation with no ethylene loss is possible. A typical adiabatic rise of
351F is expected for normal operation. With an adiabatic
temperature rise of 70 to 80 and typically 80 F, handling of 35000
to 58000 and typically 43000 ppm acetylenes and dienes from the
catalytic distillation results in 9000 to 30000 and typically 10000
ppm C3 and heavier dienes and acetylenics in the final product stream
74 while maintaining 100% C2 acetylene conversion primarily to
ethylene.
In a similar fashion, the temperature control on the inlet to the
fixed bed reactors can provide for compensation for catalyst
deactivation providing the typical start-of-run and end-of-run
operating temperatures to the fixed bed system. In the prior art, this
could only be done by a temperature correction in the catalytic
distillation column. This requires a pressure change in the column
and thus the fractionation conditions will be altered. With both the
ABBLUM/260-
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
18
catalytic distillation column and fixed bed reactor system of the
present invention the catalytic distillation column can operate at
constant fractionation conditions and lower temperature corrections
for the fixed bed system will be required. This improves system
stability and allows for longer life of the catalyst.
Figure 4 presents an alternate embodiment of the present
invention. Instead of catalytic distillation column overhead stream 42
passing to exchanger 44 and then to reflux drum 44, overhead
stream 42 is passed directly to cross-flow exchanger 64 and into the
io fixed bed reactor system. Following the fixed bed reactor system,
the effluent is cooled at 65 and the reflux 48 for the column is
separated at 67 as a condensed liquid 69 and returned to the column.
Since the stream entering the fixed bed reactor system still
contains all of the reflux for the column, the operating temperature of
the fixed bed reactors will be somewhat higher to insure complete
vapor flow. This will change the design catalyst activity and space
velocity to insure stable operation, The advantage of this approach
will be a higher mass flow of hydrocarbon that will minimize
temperature rise across the fixed beds, a reduced hydrogen partial
pressure that will improve selectivity, and a higher space velocity that
will both improve selectivity and decrease catalyst costs.
Figure 5 illustrates an alternate embodiment of the present
invention incorporating a pre-reactor. This arrangement is
advantageous for bulk selective hydrogenation of feeds high in dienes
and acetylenes. Following compression at 12 and possible treatment
in a guard bed (not shown), the vapor phase feedstock is admixed
with recirculation liquid 76 from the pump 56 of column 14 and the
two phase mixture passed co-currently through a fixed bed reactor
78. Hydrogenation occurs and the presence of liquid serves to
control the temperature rise through vaporization. Hydrogenation
reactor 78 can be designed as an operating reactor plus a spare to
allow for extending the onstream operation of the system. Following
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
19
the pre-reactor, the liquid/vapor mixture can be either sent to the
column directly as a mixed feed or separated in a separation drum
and the liquid and vapor fed separately to the column. The latter is
preferred since any oligomers formed in the initial hydrogenation will
be in the liquid phase and can be fed to the column below the
catalyst beds thus reducing fouling.
Performing the fixed bed 'hydrogenations before the catalytic
distillation column 14 will allow for possibly higher catalyst utilization
without experiencing ethylene loss for that portion of the
hydrogenation due to the large amount of preferentially absorbed
dienes and acetylenes of higher reactivity available for hydrogenation.
At higher catalyst utilization, lower catalyst volumes would be
necessary making the process more economical. A catalytic
distillation unit is still required following a pre-reactor to reach
hydrogenation specifications. It is anticipated that a maximum of
50% and typically 20 % of the hydrogenation duty can be
accomplished in the pre-reactor.
Another advantage of a fixed bed hydrogenation reactor before
the catalytic distillation column 14 is that the reactor can be used as
a guard bed for catalyst poisons. The catalyst could either be nickel
or palladium. Nickel catalyst for example would be able to catalyze
the reaction of the sulfur compound thiophene with butadiene to
form a heavy mercaptan. This mercaptan would then be removed in
the stripping section 22 of column 14 and thus never contact the
palladium catalyst. A still further advantage is that the external pre-
reactor system could have a spare and thus allow for regeneration
without the requirement for shutting the entire plant down for
catalyst replacement.
Alternately, as shown in Figure 6, the liquid 76 from pump 56
can flow downwards through tF e'fixed bed 78 and the vapor stream
from the compressor 12 can flow upwards. Liquid from the bottom of
tho fixed bed reactor 78 then flows -to a lower portion of column 14
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
and vapor flows to a higher entry point. The advantage of this
counter-current process sequence is that oligomers resulting from
polymerization reactions of the unsaturated hydrocarbons are
removed from the catalyst bed as formed and do not pass over the
5 remaining portion of the catalyst bed. Also this liquid is sent to
column 26 at a lower entry point, minimizing any potential
contamination of the catalyst in column 14.
Oligomers which can foul the catalytic distillation catalyst are
easily separated and do not rise in the column to contaminate the
10 catalyst. Further, as in the co-current flow option, the pre-reactor
catalyst bed can have a spare, allowing for regeneration while the
rest of the system is operating. The ability to easily regenerate on-line
will increase system cycle lengths as the catalyst zone at the feed
inlet is expected to have the highest fouling rate.
15 To minimize fouling in the fixed bed pre-reactor, liquid flow
rates need to be sufficient to minimize local hot spots due to the high
heat of hydrogenation and to wn,sh any oligomers formed off the
catalyst. The operation of these, beds is preferably in the vapor
continuous zone. For cracked gas feeds that exhibit extreme fouling
20 tendencies, operating in the liquid continuous zone is also possible.
Figure 7 illustrates a further embodiment of the present
invention which incorporates fixed bed reactors within the liquid
pumparound or intercooler streams that are withdrawn from the
column 14. The fixed bed hydrogenation reactors 82 and 84 are
placed in the side stream from collecting tray 30 and the side stream
from collecting tray 31, respectively. These fixed beds 82 and 84
are in addition to the reactive hydrogenation sections 16 and 18 in
the hydrogenation sections 16 and 18 in the catalytic distillation
column 14. A mass transfer zone 85 in the form of structured
packing or trays is also added above the withdrawal point and below
the catalyst bed. This zone allows for hydrogen to be saturated into
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
21
the liquid phase and thus provide the hydrogen required for the
hydrogenation of the acetylenes and dienes in the withdrawn liquid.
The ability of the present invention to remove 30 to 40% of
the hydrogen from the charge gas prior to chilling and condensation
steps lowers the energy consumption and reduces capital cost. The
ability to hydrogenate 100% of the acetylene irrespective of the
carbon monoxide concentration without any C2 or C3 olefin losses
was not possible with the prior art. The combined fixed bed and
catalytic distillation steps provide superior handling of system upsets
while maintaining stable diene/acetylene hydrogenation and hydrogen
removal.
Following are some examples which illustrate the present
invention in its various embodiments as compared to the prior art.
The following Table 1 sets forth the feed composition used for all the
examples. Table 2 lists the results for each of the examples.
Table 1
Component wt%
Hydrogen 1.07
Lights 18.22
Total C2 24.21
C2 acetylene 0.22
Total C3 25.62
C3 diene and acetylenics 1.70
Total C4 13.96
C4 dienes 5.27
Total C5 4.83
C5 diene 2.10
C6 12.09
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
22
Table 2
Example Example Example Example Example
1 2 3 4 5
Column P (psig) 195 195 195 195 195
Average Catalyst T ( F) 2$0 232 228 231 228
C0/H2 ratio (mol%) 0.3 0.3 0.6 0.3 0.6
Catalyst Utilization 0.12 0.13 0.09 0.12 0.09
(Ibmole H2/ft3/hr)
Fixed Bed Inlet T ( F) - - - 129 140
Fixed Bed GHSV - - - 1800 2000
Conversions (%)
C2 acetylene 84 89 80 100 100
H2 conversions 28 30 20 34 31
C2 ethylene gain/loss 0 (0.6) loss 0.6 0.1 0.7
C3 propylene gain/loss 4.7 5.1 3.4 5.8 4.8
C4 olefin conversion 0.7 0.8 0.4 2.0 1.8
Ppm outlet (wt) Total 19070 12340 33860 3640 7740
C2 acetylene 370 240 460 0 0
C3 acetylene 2300 1600 3900 300 840
C3 diene 3400 2900 4500 2900 4000
C4 diene 11500 7000 21700 150 1500
C5 diene 1500 600 3300 290 1400
Example 1:
This example represents prior art outlined in the previous U.S.
Patent 5,679,241 (Figure 1) based on a one step catalytic distillation
column operating with a reflux ratio of 4.4. With a typical front end
acetylene hydrogenation catalyst containing palladium levels below
2000 ppm and operating at pressure of 195 psig and average
catalyst temperature of 230 F C2 acetylene conversion reached 84%
with 0% ethylene loss/gain. At the reactor outlet there were 370
ppm of C2 acetylene and a total of 19070 ppm dienes and
acetylenics. Total acetylene/diene conversion is 79.5%. This
example represents the case where the single column is operated for
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
23
no ethylene loss. As can be seen, there is substantial breakthrough
of C2 acetylene. This would produce off-spec ethylene product.
Example 2
This example also represents prior art and is based on the
single catalytic distillation column of Example 1 with higher catalyst
temperature and slightly lower reflux ratio of 4.1. The hydrogenation
severity of a single column can be increased to reach low C2
acetylene levels. This can be accomplished by raising temperature or
increasing catalyst activity. The higher temperature operation is
intended to reduce acetylene content and thus achieve specification
ethylene product. In this case all the diene and acetylenic
conversions are higher compared to Example I, however there is also
a 0.6% ethylene loss. At the reactor outlet there were 240 ppm of
C2 acetylene and a total of 12340 ppm dienes and acetylenics. Total
acetylene/diene conversion is 86.7%. As can be seen, increased C2
acetylene conversion is accompanied by increased ethylene loss that
is economically undesirable. Further, the ethylene product still does
not meet the specification limits of 1-2 ppm.
Example 3
This example represents prior art and is based on the single
catalytic distillation column of Example 1 with higher carbon
monoxide levels in the feed. Carbon monoxide acts as a catalyst
poison and therefore diene and acetylenic conversions were
substantially reduced. At carbon monoxide levels of 0.1 mol% in the
feed (0.6% carbon monoxide to hydrogen ratio) the product had 460
ppm of acetylene and 33860 ppm of total dienes and acetylenics.
The lower catalyst activity resulting from the CO is reflected on the
loss of catalyst productivity (0- 12 to 0.09 Ibmol/hr-ft3 catalyst
structure) and the lower overall acetylene/diene conversion (63.6%
versus 79.5% for the base case).
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
24
The response to this reduced activity would be to raise the
temperature of the catalyst within the catalytic distillation column.
This would require an increase in pressure beyond what is practical in
an operating unit. Thus options to compensate for CO increases are
limited for the prior art.
Example 4
This example represents the improved combined operation of
the catalytic distillation column and a fixed bed reactor described in
Figure 2. This combined operation is necessary in order to realize
ethylene gains with 100% C2 acetylene conversion and 50% to 95%
conversion of all other diene and acetylene compounds. Operation of
the catalytic distillation column at 195- psig and average catalyst
temperature of 230 F and 195 psig pressure resulted in 12,000 ppm
by weight dienes and acetylenes in the catalytic distillation overhead
which was then fed to the fixed bed hydrogenation reactor system.
Operation of the fixed bed hydrogenation reactors at gas
hourly space velocity (GHSV) of 1800 h-' and inlet bed temperatures
of 129 F was successful in converting 100% of the C2 acetylene
and provide enough additional hydrogenation resulting in 50% overall
C3 diene conversion (both catalytic and fixed bed hydrogenation), as
well as 96.1 % overall conversion of the dienes and acetylenes in the
feed to the combined system. This resulted in 0 ppm of C2 acetylene
and 3640 ppm C3 and heavier diene and acetylenes in the outlet.
Specification ethylene product can be produced with very high overall
conversion of the highly unsaturated species.
Example 5
This example represents the improved combined operation of
the catalytic distillation column an.d a fixed bed reactor at high carbon
monoxide levels in the feed. At carbon monoxide levels of 0.1 mol%
in the feed with constant operating conditions for the catalytic
ABBLUM/260
CA 02553962 2006-07-07
WO 2005/080530 PCT/US2004/001379
distillation column, only the fixed bed inlet temperature needed to be
adjusted in order to maintain product specifications. Specifically,
with an increase in carbon monoxide from 0.05 to 0.1 mol%, an
increase in the inlet fixed bed temperature from 129 to 140 F was
5 sufficient to maintain the conversion of the C2 acetylene. Further,
the C3 and heavier dienes and 'acetylenes were further hydrogenated
resulting in 7740 ppm wt. total, diene and acetylenes in the product.
The proposed improvement of the present invention will
perform 100% C2 acetylene hydrogenation with stable 90% +
10 hydrogenation of the C3 to Cs and heavier acetylenes, 90% +
hydrogenation of C4 and C5 dienes, and 50% + conversion of C3
diene in a feed stream without hydrogenating the C2 and C3 olefins.
The resulting hydrogen removal with the present invention will remain
steady at 30 to 40 and typically 30% depending on the feed
15 composition.
ABBLUM/260