Note: Descriptions are shown in the official language in which they were submitted.
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
END-CAPPED POLYMER CHAINS
AND PRODUCTS THEREOF
STATEMENT OF RELATED APPLICATION
[001] This application is related and claims priority to U.S. Utility Patent
Application
Number 10/776,681 filed February 11, 2004, and U.S. Provisional Patent
Application
Number 60/643,326, filed January 11, 2005, which are both incorporated herein
by
reference in their entireties.
FIELD OF THE INVENTION
[002] This invention relates to processes for end-capping a canonically
polymerized
polymer. More particularly this invention relates to processes for end-capping
a
cationically polymerized polymer with an anionic group, after which the
resulting
anionically terminated polymer can be used in subsequent anionic reactions,
including
anionic coupling and polymerization reactions. The present invention also
relates to
copolymers including a cationically polymerized polymer coupled to an
anionically
polymerized polymer.
BACKGROUND OF THE INVENTION
[003] It is well known that living polymerization (i.e., polymerization
proceeding in
the practical absence of chain transfer and termination) is a very useful
method for
designing polymer structures, permitting for example, versatile synthetic
routes for the
preparation of a wide variety of well-defined polymer structures, such as end-
functionalized polymers, star-shaped polymers and/or block copolymers and
control of
the molecular weight and molecular weight distribution of the polymer, as well
as
enabling functional groups to be positioned at desired points in the polymer
chain. Since
Szwarc et al. demonstrated the living nature of polystyryllithium formed from
the
reaction of sodium naphthalene and styrene in the 1950s, a wide variety of
living
polymerization schemes have been developed, including cationic, anionic,
radical, ring-
opening, and group transfer polymerization.
[004] Copolymers are an important class of polymers and have numerous
commercial
applications. For instance, their unique properties, whether in pure form, in
blends, in
melts, in solutions, and so forth, lead to their use in a wide range of
products, for
-1-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
example, compatiblilizers, adhesives and dispersants. Combining various
polymerization techniques (e.g., cationic and anionic polymerization
techniques in the
case of the present invention) may produce new copolymers, each with its own
unique
properties, which could not otherwise be prepared using a single
polymerization method.
[005] For example, polyisoolefins are attractive materials because the polymer
chain is
fully saturated and, consequently, the thermal and oxidative stability of this
polymer are
excellent. Polyisoolefins are prepared by cationic polymerization. Recently,
Muller et
al. reported that poly(alkyl methacrylate)-b-polyisobutylene and poly(alkyl
methacrylate)-b-polyisobutylene-b-poly(alkyl methacrylate) copolymers can be
prepared
by the combination of cationic and anionic polymerization techniques. See
Feldthusen,
J.; Ivan, B.; Miiller, A. H. E. Macromolecules, 1997, 30, 6989-6993;
Feldthusen, J.;
Ivan, B.; Miiller, A. H. E. Macromolecules 1998, 31, 578-585. In this process,
an end-
functionalized polyisobutylene (PIB), specifically 1,1-diphenyl-1-methoxy end-
functionalized polyisobutylene, , or 2,2-diphenylvinyl end-
functionalized polyisobutylene, , is prepared by the reaction of
living polyisobutylene with 1,1-diphenylethylene. The chain end of the
resulting
polymer is subsequently metallated with alkali metal compounds such as
sodium/potassium alloy or cesium in tetrahydrofuran at room temperature. The
thus
produced macroanion is capable of polymerizing monomer. This method, however,
is
inconvenient because of the complicated process for the metallation of the
polymer
chain using alkali metal compounds.
[006] A more recent attempt to combine cationic and anionic polymerization
techniques involves the preparation of end-functionalized polymers (e.g., end-
functionalized polyisobutylene) by reacting a carbocationically terminated
polymer with
a heterocyclic compound (e.g., thiophene) to provide an end-capped polymer
(e.g.,
-2-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
thiophene end-functionalized polyisobutylene). The end-capped polymer is then
reacted
with an organolithium compound to yield an anionically terminated polymer,
which is
subsequently reacted with an anionically polymerizable monomer such as tert-
butyl
methacrylate to produce a copolymer. See, Application Serial No. 60/480,121
filed June
20, 2003 and entitled "End-Capped Polymer Chains and Products Thereof ', and
Martinez-Castro, N,; Lanzendo 1 fer, M. G.; Muller, A. H. E.; Cho, J. C.;
Acar, M. H.;
and Faust, R. Macromolecules 2003, 36, 6985-6994. An advantage of this process
is
that simple and complete metallation is achieved. This process, however, is
also subject
to improvement. For example, in the case where thiophene end-functionalized
polyisobutylene is formed, to prevent coupling between thiophene
functionalized
polyisobutylene and living polyisobutylene, an excess of thiophene is used
while
functionalizing the polyisobutylene canon with the thiophene. Moreover, the
blocking
efficiency was found to be only about 80 % even when a low molecular weight
product
is targeted.
SUMMARY OF THE INVENTION
[007] According to one aspect of the present invention, a method is provided
in which
a double diphenylethylene compound is reacted with a polymer that contains a
carbocationically terminated chain, which chain contains a plurality of
constitutional
units corresponding to cationically polymerizable monomer species, thereby
providing a
1,1-diphenylene end-functionalized chain. Subsequently, an alkylating agent is
reacted
with the 1,1-diphenylene end-functionalized chain, resulting in the formation
of an
alkylated 1,1-diphenylene end-functionalized chain.
[008] The alkylating agent can be, but is not limited to an alkylaluminum
compound or
an alkylzinc compound, e.g., dimethyl-zinc. The double diphenylethylene
compound
can be, but is not limited to 1,4-bis(1-phenylethenyl)benzene.
[009] In certain embodiments, the above method further comprises optionally
combining a 1,1-diphenylorganolithium compound with the alkylated 1,1-
diphenylene
end-functionalized polymer to remove impurites. In other embodiments, the
method
further comprises reacting an organolithium compound with the alkylated 1,1-
diphenylene end-functionalized polymer, resulting in the formation of an
anionically
terminated polymer, which can be used, for example, in subsequent anionic
polymerization and coupling reactions.
-3-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0010] In some embodiments, the organolithium compound is of the formula RLi
in
which R is a hydrocarbon group containing from 1 to 20 carbon atoms per
molecule.
The hydrocarbon groups can be alkyl groups, aryl groups, or alky-aryl groups.
The
organolithium compound can be, but is not limited to, methyllithium,
ethyllithium,
isopropyllithium, n-butyllithium, sec-butyllithium, tert-butyllithium, tert-
octyllithium,
phenyllithium, 1-naphthyllithium,p-tolyllithium, cyclohexyllithium, and/or 4-
cyclohexylbutyllithium.
[0011] In some embodiments, the 1,1-diphenylorganolithium compound is of the
formula RC(Qj)2Li in which R is a hydrocarbon group containing 1 to 20 carbon
atoms
per molecule and Q~ is an unsubstituted or substituted aryl group. The 1,1-
diphenylorganolithium compound can be, but is not limited to, 1,1-
diphenylhexyllithium
or 1,1-diphenyl-4-methylpentyllithium.
[0012] In some embodiments, the method further includes contacting under
reaction
conditions the anionically terminated polymer with anionically polymerizable
monomer
species. In some embodiments, the cationically polymerizable monomer species
are
isoolefm monomer species and the anionically polymerizable monomer species are
methacrylate monomer species. In other embodiments, the cationically
polymerizable
monomer species are isoolefin monomer species and the anionically
polumerizable
monomer species are vinylpyridine monomer species.
[0013] According to another aspect of the present invention, a novel polymer
is
provided that comprises a polymer chain, which chain father comprises the
following:
(a) a plurality of constitutional units that correspond to cationically
polymerizable
monomer species and (b) an end-cap comprising a diphenylethylene group.
Examples of
diphenylethylene groups include groups and
~"2 groups, where R is a branched or unbranched alkyl group,
typically containing from 1 to 20 carbons, more typically containing from 1 to
10
-4-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
\ ~\
/ /
c'
I
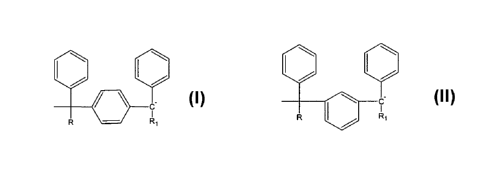
carbons. In other aspects, the end-cap comprises a R R~ group or a
group, where R is a branched or unbranched alkyl group
containing from 1 to 20 carbons and Rl is a branched, unbranched, or cyclic
alkyl group
or an aryl group, containing from 1 to 20 carbons
[0014] In some embodiments, Rl is n-pentyl or 2-methyl-butyl. In some
embodiments,
R is methyl or ethyl. In certain embodiments, the number average molecular
weight of
said polymer ranges from 5,000 to 500,000. In other embodiments, the chain
comprises
a plurality of constitutional units that correspond to two or more differing
cationically
polymerizable monomer species. In some embodiments, the polymer comprises two
or
more of said polymer chains. In certian embodiments, the constitutional units
correspond to isobutylene.
[0015] According to yet another aspect of the present invention, a novel
copolymer is
provided, which includes: (a) a first polymer block that comprises a plurality
of
constitutional units corresponding to cationically polymerizable monomer
species, (b) a
second polymer block that comprises a plurality of constitutional units
corresponding to
anionically polymerizable monomer species, and (c) a linking moiety which
links the
first and second polymer blocks together selected from a \ / R~ group
and a group (for example, a linking moiety selected from a
-5-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
(\
/ /
group and a group),
where R is a branched or unbranched alkyl group, typically containing from 1
to 20
carbons, more typically containing from 1 to 10 carbons, and where R~ is a
branched,
unbranched, or cyclic alkyl group or an aryl group, also typically containing
from 1 to
20 carbons, more typically containing from 1 to 10 carbons.
[0016] In some embodiments, the first polymer block includes a plurality of
constitutional units that correspond to two or more differing canonically
polymerizable
monomer species, e.g., a plurality of constitutional units that correspond to
isobutylene.
In other embodiments, the second polymer block includes a plurality of
constitutional
units that correspond to two or more differing anionically polyrnerizable
monomer
species, e.g., a plurality of constitutional units that correspond to a
methacrylate
monomer or a plurality of constitutional units that correspond to a
vinylpyridine
monomer. In certain embodiments, the polymer includes two or more second
polymer
blocks and two or more linking moieties. In come embodiments, the copolymer is
a
linear copolymer. In other embodiments, the copolymer is a radial-shaped
copolymer.
[0017] Other aspects of the present invention relate to novel copolymers that
comprise:
(a) a first polymer block that comprises a plurality of constitutional units
that correspond
to isobutylene; and (b) a second polymer block that comprises a plurality of
constitutional units that correspond to hydroxyethyl methacrylate. Examples
include
copolymers in which (a) the first block is a polyisobutylene block and (b) the
second
polymer block is a poly(hydroxyethyl methacrylate) block or is a random
polymer block
that contains constitutional units corresponding to hydroxyethyl methacrylate
and to
methyl methacrylate.
[0018] Still other aspects of the present invention relate to novel copolymers
that
comprise: (a) a first polymer block that comprises a plurality of
constitutional units that
correspond to isobutylene; and (b) a second polymer block that comprises a
plurality of
constitutional units that correspond to 2-vinylpyridine. In some embodiments,
the
copolymer also comprises (c) a linking moiety linking said first block polymer
region
with said second block polymer region, said linking moiety selected from a
-6-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
group and a group (for example, a linking
\ \
/ ~ /
moiety selected from a R R~ group and a
group), where R~ is a branched, unbranched, or cyclic
alkyl group or an aryl group, containing from 1 to 20 carbons.
[0019] In still other embodiments, the present invention relates to an article
of
manufacture which includes a copolymer including: (a) a first polymer block
that
comprises a plurality of constitutional units corresponding to cationically
polymerizable
monomer species, (b) a second polymer block that comprises a plurality of
constitutional
units corresponding to anionically polymerizable monomer species, and (c) a
linking
moiety which links the first and second polymer blocks together selected from
a
group and a group (for example, a linking
\ \
/ /
moiety selected from a R R~ group and a
group), where R is a branched or unbranched alkyl
group, typically containing from 1 to 20 carbons, more typically containing
from 1 to 10
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
carbons, and where R~ is a branched, unbranched, or cyclic alkyl group or an
aryl group,
also typically containing from 1 to 20 carbons, more typically containing from
1 to 10
carbons.
[0020] In still other aspects, the present invention is related to an article
of manufacture
which includes copolymers including: (a) a first polymer block that comprises
a plurality
of constitutional units that correspond to isobutylene; and (b) a second
polymer block
that comprises a plurality of constitutional units that correspond to
hydroxyethyl
methacrylate.
[0021] Still other aspects of the present invention relate to an article of
manufacture
which includes copolymers including: (a) a first polymer block that comprises
a plurality
of constitutional units that correspond to isobutylene; and (b) a second
polymer block
that comprises a plurality of constitutional units that correspond to 2-
vinylpyridine. In
some embodiments, the copolymer also comprises (c) a linking moiety linking
said first
block polymer region with said second block polymer region, said linking
moiety
selected from a \ / R~ group and a group (for
\ \
example, a linking moiety selected from a R R~ group and a
group), where Rl is a branched, unbranched, or cyclic
alkyl group or an aryl group, containing from 1 to 20 carbons.
[0022] These and other aspects, embodiments and advantages of the present
invention
will be more fully understood upon review of the Detailed Description to
follow.
DETAILED DESCRIPTION
[0023] As is well known, polymers are molecules that contain one or more
chains, each
containing multiple copies of one or more constitutional units. An example of
a
_g_
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
---f-HpC-CH
n
C
HC~ NCH
I I
common polymer is polystyrene Hc~CH ~H , where n is an integer, typically an
integer of 10 or more, more typically on the order of 10's, 100's, 1000's or
even more,
HpC=CH
C
HII ~IH
in which the constitutional units in the chain correspond to styrene monomers:
Hc~CH ~H
(i.e., they originate from, or have the appearance of originating from, the
polymerization
of styrene monomers--in this case the addition polymerization of styrene
monomers).
Copolymers are polymers that contain at least two dissimilar constitutional
units.
[0024] Methods for making copolymers, especially specific living
polymerization
methods (e.g., anionic and carbocationic living polymerizations) are generally
applicable
only to a limited number of monomers. The combination of different living
polymerization techniques, therefore, should lead to new and unique
combinations of
blocks in block copolymers.
[0025] As used herein a polymer "block" is defined as a grouping of 10 or more
constitutional units, commonly 20 or more, SO or more, 100 or more, 200 or
more, 500
or more, or even 1000 or more units, and can be branched or unbranched. A
"chain" is a
linear (unbranched) grouping of 10 or more constitutional units (i.e., a
linear block). In
the present invention, the constitutional units within the blocks and chains
are not
necessarily identical, but are related to one another by the fact that that
they are formed
in a common polymerization technique, e.g., a cationic polymerization
technique or
anionic polymerization technique.
[0026] In accordance with one aspect of the present invention, copolymers are
provided
which include (a) one or more blocks which contain a plurality of
constitutional units
that correspond to one or more cationically polymerizable monomer species and
(b) one
or more blocks which contain a plurality of constitutional units that
correspond to one or
more anionically polymerizable monomer species. Typically, these
constitutional units
occur within the copolymer molecule at a frequency of at least 10 times, and
more
typically at least 50, 100, 500, 1000 or more times.
[0027] An advantage of the present invention is that copolymers can be
prepared via the
combination of living cationic polymerization and living anionic
polymerization.
-9-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
Hence, copolymers containing one or more cationically polymerized blocks and
one or
more anionically polymerized blocks can be formed.
[0028] The copolymers of the present invention embrace a variety of
configurations,
including linear, cyclic, and branched configurations. Branched configurations
include
star-shaped configurations (e.g., radial configurations in which three or more
chains
emanate from a single region), comb configurations (e.g., graft copolymers
having a
main chain and a plurality of side chains), and dendritic configurations
(e.g., arborescent
or hyperbranched copolymers). The copolymers of the present invention embrace
(a)
one or more chains containing repeating constitutional units of a single type,
(b) one or
more chains containing randomly distributed constitutional units of two or
more types
(e.g., random or statistical copolymers), (c) one or more chains containing
two or more
types of constitutional units that repeat within an ongoing series (e.g.,
alternating
copolymers), and so forth.
[0029] Some examples of cationically polymerizable monomer species follow: (a)
olefins, including isomonoolefins with 4 to 18 carbon atoms per molecule and
multiolefins with 4 to 14 carbon atoms per molecule, for example, isobutylene,
2-
methylbutene, isoprene, 3-methyl-1-butene, 4-methyl-1-pentene, beta-pinene,
and the
like, (b) vinyl aromatics such as styrene, alpha-methyl styrene, para-
chlorostyrene, para-
methylstyrene, and the like, and (c) vinyl ethers such as methyl vinyl ether,
isobutyl
vinyl ether, butyl vinyl ether, N-vinyl carbazole, and the like.
[0030] In certain embodiments, the carbocationically terminated polymer is
formed at
low temperature (e.g., -80°C) in a reaction mixture that comprises: (a)
a solvent system
appropriate for cationic polymerization, many of which are well known in the
art (for
example, a mixture of polar and non-polar solvents, such as a mixture of
methyl chloride
and hexanes), (b) a monomer (e.g., isobutylene or another canonically
polymerizable
monomer such as those discussed above), (c) an initiator, for example, tent-
ester, tert-
ether, tert-hydroxyl or tert-halogen containing compounds, and more typically
cumyl
esters of hydrocarbon acids such as alkyl cumyl ethers, cumyl halides and
cumyl
hydroxyl compounds, as well as hindered versions of the same, for instance,
tert-butyl
dicumyl chloride and tert-butyl dicumyl chloride (5-tent-Butyl-1,3-bis(1-
chloro-1-
methylethyl)benzene) are used in the Examples below; and (d) a coinitiator,
typically a
Lewis acid such as boron trichloride or titanium tetrachloride.
Carbocationically
-10-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
terminated star polymers can be formed by selecting initiators having three or
more
initiation sites such as tricumyl chloride (1,3,5-tris(1-chloroy-1-
methylethyl)benzene).
[0031] In addition, an electron pair donor (e.g., dimethyl acetamide, dimethyl
sulfoxide
or dimethyl phthalate) or a proton-scavenger (e.g., 2,6-di-tert-butylpyridine,
4-methyl-
2,6-di-tert-butylpyridine, 1,8-bis(dimethylamino)-naphthalene or
diisopropylethyl
amine) can be added to the reaction mixture if desired.
[0032] Once a carbocationically terminated polymer is provided in an
appropriate
solvent system such as those discussed above (e.g., living cationic PIB
provided in a
CH3C1/n-hexane solvent system), a heterocyclic compound like those described
above
(e.g., thiophene) is added, and allowed to react with the carbocationically
terminated
polymer under appropriate reaction conditions (e.g., -78°C) to form an
end-capped
polymer (e.g., PIB-T).
[0033] Where a proton scavenger is used (for example, to scavenge protic
impurities and
thereby achieve a narrowing of the molecular weight distribution of the
carbocationically terminated polymer), the amount of proton scavenger is
preferably
held to a minimum, thereby avoiding reaction of more than one
carbocationically
terminated polymer with each heterocyclic compound. Preferably, the molar
ratio of
proton scavenger to carbocationically terminated polymer (which can be
approximated
by the initial initiator concentration) is 1:1 or less, for example, 0.75:1 or
less, 0.66:1 or
less, 0.5:1 or less, 0.25:1 or less, or even 0.1:1 or less.
[0034] Moreover, the molar ratio of Lewis acid to carbocationically terminated
polymer
(or initiator) is typically greater than 10, more typically greater than 20,
30, 40 or more
in order to improve reactivity with between the polymer and the heterocyclic
compound.
[0035] In some embodiments, these macroinitiators are used to synthesize star
polymers
(e.g., PIB stars) by reacting the macroinitiators (e.g., PIB-T-,Li+) with
coupling
molecules such as chlorosilanes (which have been used previously to couple
living
polybutadiene anionic chain ends to form star polymers; see Roovers, J. E. L.
and S.
Bywater (1972). "Macromolecules 1972, 5, 385).
[0036] Examples of anionically polymerizable monomer species include vinyl
aromatic
monomers such as styrene, styrene derivatives, alkyl substituted styrene and
divinyl
benzene, diphenylethylene, conjugated dimes such as isoprene and 1,3-
butadiene, N,N-
disubstituted acrylamides and methacrylamides such as N,N-dimethylacrylamide,
acrylates, alkyl acrylates and methacrylates such as isodecyl methacrylate,
glycidyl
-11-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
methacrylate and tert-butyl methacrylate, vinyl unsaturated amides,
acrylonitrile,
methacrylonitrile, vinyl pyridines, isopropenyl pyridines, other vinyl
monomers such as
n-alkyl isocyanates, heterocyclic monomers such as ethylene oxide, s-
caprolactone, L,L-
lactide, D,D-lactide, D,L-lactide, and mixtures thereof. Of particular benefit
are acrylate
or methacrylate monomers having the formula CHZ=CHCOZR or CHZ=C(CH3)COZR
where R is a substituted or unsubstituted, branched, unbranched or cyclic
alkyl groups
containing 1 to 20 carbons. Substituents for the alkyl groups include
hydroxyl, amino
and thiol functional groups, among others. In embodiments where monomers are
utilized that have functional groups, proper protection of the functional
group is
commonly needed during the course of anionic polymerization. Specifc examples
of
nonfunctional and protected functional methacrylate monomers include ethyl
methacrylate, methyl methacrylate, tert-butyl methacrylate, isodecyl
methacrylate,
dodecyl methacrylate, stearyl methacrylate, glycidyl methacrylate, 2-
[(trimethylsilyl)oxy]ethyl methacrylate, 2-[(tert-butyldimethylsilyl)oxy]ethyl
methacrylate, and 2-[(methoxymethyl)oxy]ethyl methacrylate. In some
embodiments,
the anionically polymerizable monomer species is 2-vinylpyridine.
[0037] The copolymers of the present invention tyically have a molecular
weight
ranging from 200 to 2,000,000, more typically from 500 to 500,000. The ratio
of
constitutional units corresponding to the cationically polymerized monomers
(e.g.,
isobutylene) relative to the constitutional units corresponding to the
anionically
polymerized monomers (e.g., methyl methacrylate) in the copolymer usually
ranges
from 1/99 to 99/1 w/w, e.g., from 30/70 to 95/S w/w. In some embodiments,
copolymers are provided which have a narrow molecular weight distribution such
that
the ratio of weight average molecular weight to number average molecular
weight
(Mw/Mn) (i.e., the polydispersity index) of the polymers ranges from about 1
to 10, or
even from about 1 to 2.
[0038] As a specific example, block copolymers of the formula X(PCA-C-PAN)"
are
formed in various embodiments of the invention, where X corresponds to the
initiator
species, C corresponds to the capping species, PCA is a polymer block
comprising a
plurality of constitutional units that correspond to one or more cationically
polymerizable monomer species, for example, a polyolefin block, PAN is a is
polymer
block comprising a plurality of constitutional units that correspond to one or
more
anionically polymerizable monomer species, for example, a poly(methyl
methacrylate)
-12-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
block, and n is a positive whole number. Linear block copolymers are formed
where
n=1 or n=2. Where n=2, the copolymers are sometimes referred to as triblock
copolymers. This terminology disregards the presence of the initiator
fragment, for
example, treating PCA -X- PCA as a single olefin block, with the triblock
therefore
denoted as PCA -PAN- PCA. Star shaped copolymers are formed where n=3 or more.
The value of n is typically dictated by the functionality of the initiator
molecule, with
monofunctional initiators corresponding to n=1, difunctional initiators
corresponding to
n=2, and so forth.
[0039] In accordance with another aspect of the present invention, copolymers
are made
by a process that includes: (a) providing a 1,1-diphenylene end-functionalized
polymer
(which polymer contians one or more cationically polymerizable monomer
species); and
(b) reacting the 1,1-diphenylene end-functionalized polymer with an
organometallic
compound to yield an anionically terminated polymer (also referred to herein
as a
"macrocarbanion", or a "anionic macroinitiator" based on its ability to
initiate further
reactions such as coupling and polymerization reactions.
[0040] Thus, another advantage of the present invention is that end-capped
polymers
formed of cationically polymerizable monomers can be quantitatively reacted
with
organolithium compounds to form stable anionic macroinitiators, which are then
available for numerous anionic polymerization and coupling reactions.
(0041] For instance, in accordance with an embodiment of the present
inveniton, a
living macrocarbocation, e.g., living cationic polyisobutylene, is reacted
with a double
diphenylethylene, e.g., 1,3-bis(1-phenylethenyl)benzene (sometimes referred to
as meta-
double diphenylethylene) or 1,4-bis(1-phenylethenyl)benzene (sometimes
referred to as
para-double diphenylethylene), to produce a 1,1-diphenylethylene end-
functionalized
carbocationic polymer. The carbocation is then alkylated with a suitable
alkylating
agent, e.g., with an organometallic compound such as dimethylzinc, whereupon
the
resulting macromonomer is readily metallated with a suitable organometallic
compound
such as an alkyllithium compound, thereby providing a living anionic
macroinitiator in
near quantitative yield. A sterically hindered lithium compound, e.g., a 1,1-
diphenylalkyllithium species, is used in certain embodiments to remove
impurities that
may be present alongside the 1,1-diphenylethylene end-functionalized polymer,
thereby
preventing premature termination of the living macroanion.
-13-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0042] That is, once formed, the end-capped polymer is typically isolated and
purified.
After isolation and purification, the end-capped polymer is lithiated with an
organolithium compound, thereby yielding an anionically terminated polymer (or
macroinitiator). The organolithium compound is typically an alkyllithium
compound,
for example, methyllithium, ethyllithium, isopropyllithium, normal-, secondary-
and
tertiary-butyllithium, benzyllithium, allyllithium, and so forth.
[0043] Lithiation can be conducted, for example, at low temperatures (e.g., -
40°C) in a
reaction mixture that comprises: (a) a solvent system appropriate for
lithiation, many of
which are well known in the art (for example, a polar solvent such as THF or a
non-
polar solvent, such as hexane or toluene in the presence of an electron donor,
such as
N,N,N'N'-tetramethylethylenedieamine), (b) the end-capped polymer to be
lithiated, and
(c) the organolithium compound (e.g., an alkyllithium compound such as n-BuLi,
s-BuLi
or tert-BuLi).
[0044] The organolithium compound may be provided in a molar excess relative
to the
end-capped polymer. For example, the molar ratio of the organolithium compound
to
the end-capped polymer is beneficially 1.1:1, 1.5:1, 2:1, 4:1, or even
greater. Excess
organolithium compound can be removed, for example, by increasing the
temperature of
the same in the presence of a reactive solvent, for example, by increasing the
temperature to +30 °C or higher in the presence of THF.
[0045] In some embodiments, anionic macroinitiators formed in accordance with
the
present invention are used to synthesize star polymers (e.g., polyisobutylene
stars), for
example, by reacting the macroinitiators with coupling molecules such as
unhindered
chlorosilanes, e.g., SiCI"R4_n, or carbosilanes, such as [CInSiR3_"]4_mCR'm,
or more highly
branched structures, where n and m are integers between 1 and 4, R and R' can
independently be either hydrogen or an alkyl group. Chlorosilanes have been
used
previously to couple living anionic chain ends to form star polymers in
Roovers, J. E. L.
and S. Bywater, Macromolecules 1972, S, 385 and in Application Serial No.
60/480,121
filed June 20, 2003. Other linking agents include aromatic compounds like
benzene or
naphthalene carrying two or more chloromethyl or bromomethyl or
chlorodialkylsilyl
groups.
[0046] The formation of linear and star polymers is commonly carried out at a
temperature that is higher than that of prior steps (e.g., cationic
polymerization, end-
-14-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
capping and lithiation), for example, at room temperature (25°C), or
even greater (e.g.,
40°C).
[0047] In some embodiments, anionic macroinitiators formed in accordance with
the
present invention are used to efficiently initiate living polymerization of
ionically
polymerizable monomer species, e.g., acrylate or methacrylate monomers,
yielding
block copolymers with high blocking efficiency. The "blocking or crossover
efficiency"
is the percentage of macroanions that actually initiate polymerization (of
acrylate or
methacrylate monomers in this instance). The resulting block copolymers, e.g.,
diblock
polymers, triblock copolymers, radial-shaped block copolymers, etc., will
exhibit
properties that depend upon the canonically and anionically polymerizable
species found
within the block copolymer, as well as their absolute and relative amounts.
[0048] In other embodiments of the invention, block copolymers are reacted
(subsequent
to anionic polymerization and before anion quenching) with coupling molecules
such as
(di- or trichloromethyl)benzene or (di- or tribromomethyl)benzene, thereby
forming
larger-scale copolymers (e.g., PIB- PMMA stars) Application Serial No.
60/480,121
filed June 20, 2003.
[0049] The polymer products of the present invention may be used, for example,
as new
thermoplastic elastomers, dispersing agents, compatibilizers, emulsifiers,
nonionic
surfactants or biomaterials.
[0050] Further details are provided below.
Preparation of 1,1-diphenylethylene end-functionalized polymers.
[0051] In accordance with an embodiment of the present invention, 1,1-
diphenylethylene end-functionalized polymers are prepared from a living
carbocationic
polymer. Carbocationically terminated polymers are commonly formed at low
temperature from a reaction mixture that comprises: (a) an initiator, (b) a
Lewis acid
coinitiator, (c) a cationically polymerizable monomer, (d) an optional proton
scavenger
and (e) an optional diluent.
[0052] Suitable initiators include organic ethers, organic esters, and organic
halides.
Initiators may be monofunctional, difunctional, trifunctional and so forth,
thereby
producing, for example, diblock copolymers, triblock copolymers, and radial-
shaped
block copolymers, respectively. Specific examples include tert-alkyl chloride,
cumyl
ethers, alkyl cumyl ethers, cumyl halides, cumyl esters, alkyl cumyl esters,
cumyl
-15-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
hydroxyl compounds and hindered versions of the same, for instance, 2-chloro-
2,4,4-
trimethylpentane, cumyl chloride, dicumyl chloride, 5-tert-buty1,1,3-dicumyl
chloride
(i.e., 5-tert-butyl-1,3-bis(1-chloro-1-methylethyl)benzene), 5-tent-butyl-1,3-
bis(1-
methoxy-1-methylethyl)benzene, 5-tert-butyl-1,3-bis(1-acetoxy-1-
methylethyl)benzene,
1,3,5-tris(1-methoxy-1-methylethyl)benzene, and 1,3,5-tris(1-acetoxy-1-
methylethyl)benzene, and tricumyl chloride (i.e., 1,3,5-tris(1-chloro-1-
methylethyl)benzene).
[0053] Examples of suitable Lewis acid coinitiators include metal halides and
alkyl
metal halides such as boron trichloride, titanium tetrachloride and alkyl
aluminum
halides (e.g., chlorodiethyl aluminum, dichloroethyl aluminum, chlorodimethyl
aluminum, dichloromethyl aluminum). A commonly used coinitiator is titanium
tetrachloride. The coinitiator is usually used in concentrations equal to or
greater than
that of initiator, e.g., 1 to 100 times higher, e.g., 2 to 40 times higher
than that of
initiator.
[0054] A proton scavenger, typically a Lewis base, typically provided to
ensure the
virtual absence of protic impurities, such as water, which can lead to
polymeric
contaminants in the final product. Examples of proton scavengers (also
referred to as
proton traps) include sterically hindered pyridines, for example, substituted
or
unsubstituted 2,6-di-tert-butylpyridines, such as 2,6-di-tert-butylpyridine
and 4-methyl-
2,6-di-tert-butylpyridine, as well as 2,6-dimethylpyridine, 1,8-
bis(dimethylamino)-
naphthalene and diisopropylethyl amine. The proton trap is usually used at the
concentration of 1 to 10 times higher than that of protic impurities in the
polymerization
system.
[0055] The varoius reactions of the present invention are tyically carried out
in the
presence of a diluent or a mixture of diluents. For the the cationic
polymerization and
end-capping reactions, typical diluents include (a) halogenated hydrocarbons
which
contain from 1 to 4 carbon atoms per molecule, such as methyl chloride and
methylene
dichloride, (b) aliphatic hydrocarbons and cycloaliphatic hydrocarbons which
contain
from S to 8 carbon atoms per molecule, such pentane, hexane, heptane,
cyclohexane and
methyl cyclohexane, or (c) mixtures thereof. For example, in some embodiments,
the
solvent system contains a mixture of a polar solvent, such as methyl chloride,
methylene
chloride and the like, and a nonpolar solvent, such as hexane, cyclohexane or
methylcyclohexane and the like.
-16-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0056] Regardless of the synthesis technique, once a desired living
carbocationically
terminated polymer is obtained, it is then available for 1,1-diphenylethylene
end-
funcitonalization using a double diphenylethylene species, for example, 1,3-
bis(1-
phenylethenyl)benzene, , or 1,4-bis(1-phenylethenyl)benzene,
. The 1,4-bis(1-phenylethenyl)benzene is tyically more
beneficial than the 1,3-bis(1-phenylethenyl)benzene for the functionalization
of both
living anionic and cationic polymers, because a coupled product is tyically
not generated
where the 1,4-bis(1-phenylethenyl)benzene is employed. In the present
invention,
double diphenylethylene is tyically employed at a concentration that is 1 to
10 times
higher than that of the initiator, more typically 1 to 6 times higher than
that of the
initiator. In this regard, it is known that l,l-diphenylethylene end-
functionalized
polyisobutylene can be prepared by the reaction of a living cationic polymer
such as
polyisobutylene with 1,3-bis(1-phenylethenyl)benzene or 1,4-bis(1-
phenylethenyl)benzene. See Bae, Y. C.; Faust, R. Macromolecules 1998, 31 (26),
9379-9383. Unfortunately, the quenching reaction of a living diphenyl
carbenium ion
(e.g., a polymer end-functionalized with 1,1-diphenylethylene carbocation)
with
methanol introduces a labile methoxy group at the chain end, which will lead
to side
reactions. Side reactions include the termination of subsequently added
organolithium
compounds as well as the macroinitiators that are formed from the subsequently
added
organolithium coupounds.
[0057] To prevent this, in various embodiments of the present invention, the
1,1-
diphenylethylene carbocation is subjected to an alkylation reaction. In
general, the
alkylation is carried out with an organometallic compound, such as an alkyl
aluminum
compound and an alkyl zinc compound which typically contains from 1 to 20
carbon
atoms, for example, selected from various branched or unbranched alkyl groups.
In the
present invention, the alkyl aluminum or alkyl zinc compound is typically used
at a
concentration ranging from 0.1 to 100 times the coinitiator concentration,
more typically
0.1 to 10 times the coinitiator concentration.
-17-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0058] Bae, Y. C.; Kim, I-J.; Faust, R. Polymer Bulletin 2000, 44(5-6), 453-
459, has
reported the methylation of with dimethylzinc to
form
[0059] Temperatures for the polymerization of the canonically polymerizable
monomer,
as well as the subsequent end-functionalization and alkylation of the
resulting living
polymer, will typically range from 0 °C to -150 °C, more
tyipcally from -10 °C to -90
°C. Reaction time for the cationic polymerization and the
functionalization and
alkylation of of the resulting living cationic polymer will typically range
from a few
minutes to 24 hours, more typically from 10 minutes to 10 hours.
[0060] The number average molecular weight of the resulting 1,1-
diphenylethylene end-
functionalized polymer will typically range from 1,000 to 1,000,000, more
typically
from 5,000 to 500,000.
[0061] A specific example of a procedure for the preparation of 1,1-
diphenylethylene
end-functionalized polymers follows. First, a living carbocationically
terminated
polymer, e.g., carbocationically terminated polyisobutylene, is obtained by
adding a
coinitiator into a polymerization zone (e.g., a flask), which contains
initiator, proton
trap, monomer and diluent as discussed above. After polymerizatoin of the
monomer is
complete, the resulting living cationic polymer, in this instance, living
carbocationically
H3
PIB-CHZ
terminated polyisobutylene (PIB), ~H3, is reacted with a double
diphenylethylene species, in this example 1,4-bis(1-phenylethenyl)benzene,
/ cH2 , for example, by dissolving the double diphenylethylene
-18-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
species in a diluent and charging it to the polymerization zone, whereupon a
carbenium
Hs C
PIB-CHZ ~ ~ CH
cation, e.g., ~"3 , is formed. An alkyl zinc or alkyl
aluminum compound, e.g., dimethylzinc (CH3)ZZn, is then supplied to alkylate
the
carbenium ion, for example, by dissolving it in a diluent and charging the
resulting
solution to the polymerization zone. Prechilled alcohol is then charged to the
polymerization zone to quench the reaction. The resulting l,l-diphenylethylene
end-
/
CH3
CH3
PIB-CHz ~ / CHI
functionalized polymer product, e.g., ~"_ , is then recovered.
Preparation of block copolymer using 1,1-diphenylethylene end-functionalized
macromer.
[0062] Once a 1,1-diphenylethylene end-functionalized macromer is provided, it
is
readily metallated with an organometallic compound, and the resulting anionic
macroinitiator is then available for a variety of reactions, including the
living anionic
polymerization reactions and anionic coupling reactions.
[0063] Organometallic compounds suitable for the metallation of the 1,1-
diphenylethylene end-functionalized macromer can be selected, for example,
from a
wide range of organolithium compounds of the formula RLi in which R is a
hydrocarbon
group, typically containing from 1 to 20 carbon atoms per molecule, for
example,
selected from unbranched alkyl groups, branched alkyl groups, cyclic alkyl
groups,
mono-ring aryl groups and multi-ring aryl groups. Specific examples of
suitable
organolithium compounds include methyllithium, ethyllithium, isopropyllithium,
n-
butyllithium, sec-butyllithium, tert-butyllithium, tert-octyllithium,
phenyllithium, 1-
naphthyllithium, p-tolyllithium, cyclohexyllithium, and 4-
cyclohexylbutyllithium.
Organolithium compounds are typically used at concentrations that are 1 to SO
times the
1,1-diphenylethylene end-functionalized macromer concentration, more typically
1 to 10
times the macromonomer concentration.
[0064] The metallation, as well as subsequent living anionic polymerization
and
coupling processes, are typically carned out in the presence of a diluent or
mixture of
-19-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
diluents. Suitable diluents include hydrocarbon solvents, for example,
paraffinic,
cycloparaffinic, and aromatic hydrocarbon solvents, and polar solvents, for
example,
ethers such as tetrahydrofuran, dimethylether, diethylether, dioxane, and 1,2-
dimethoxyethane.
[0065] Reaction times between the organolithium compound and the 1,1-
diphenylethylene end-functional polymer will typically range from a few
minutes to 24
hours, more typically from 1 hour to 12 hours. Temperatures for the reaction
between
the organolithium compound and the 1,1-diphenylethylene end-functional polymer
will
typically range from 30°C to -100°C, more typically from
30°C to -90°C.
[0066] In some embodiments, a small amount of a sterically hindered lithium
compound
is charged to the polymerization zone prior to introducing the alkyllithium
compound to
remove impurities that are frequently present, thereby preventing termination
during the
reaction of the alkyllithium compound with the 1,1-diphenyethylene end-
functionalized
polymer. Because the 1,1-diphenylalkyllithium cannot react with l,l-
diphenylethylene
end-functionalized polymer due to steric effects, its addition is effective
for purposes of
removing impurities that are present in the solution.
[0067] Examples of sterically hindered organolithium compounds include
organolithium
compounds of the formula RC(fl~~)(Q~2)Li in which R is a hydrocarbon group,
typically
containing 1 to 20 carbon atoms per molecule, including unbranched alkyl
groups,
branched alkyl groups, cyclic alkyl groups, mono-ring aryl groups, and multi-
ring aryl
groups, and Qjl and Q~2 can be the same or different and are selected from
unsubstituted
or substituted, mono- or multi-ring, aryl groups. Commonly, the sterically
hindered
organolithium compound is a l,l-diphenylalkyllithium compound.
[0068] 1,1-Diphenylalkyllithium may be generated, for example, from the
reaction of an
alkyllithium compound and l,l-diphenylethylene at room temperature in the
presence of
diluent. 1,1-Diphenylethylene is typically used in concentrations equal to or
less than
that of the alkyllithium in this reaction. A example of one beneficial 1,1-
diphenylalkyllithium compound is 1 1,1-diphenylhexyllithium,
-20-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0069] The sterically hindered organolithium compound is typically added to a
solution
containing the 1,1-diphenylethylene end-functional polymer and a diluent or
mixture of
diluents, for example, at room temperature. Afterwards, the organolithium
compound is
added to the 1,1-diphenylethylene functional polymer, for instance, under
anionic
reaction conditions (e.g., at -78°C). After a stable living
macroinitiator is formed in this
fashion, any unreacted alkyllithium may be destroyed by heating, for example,
to 40°C
in the presence of a reactive species such as tetrahydrofuran (which can also
be used as a
diluent).
[0070] The resulting anionic macroinitiator is then available for subsequent
polymerization or coupling reactions, as desired. For example, in some
embodiments,
an anionically reactive species such as an anionically polyrnerizable monomer
is added
under polymerization conditions (e.g., at -78°C) to the macroinitiator.
After the desired
reaction is completed, purified alcohol is typically charged to the
polymerization zone to
quench the reaction.
[0071] Times for anionic polymerization will typically range from a few
minutes to 24
hours, more typically from 5 minutes to 12 hours. Temperatures for anionic
polymerization will typically range from 0°C to -100°C, more
typically from -10°C to -
90°C.
[0072] As a specific example, the reaction of a 1,1-diphenylethylene end-
functionalized
macromer, for example, 1,1-diphenylethylene end-functionalized polyisobutylene
(see
above), with an organolithium compound, for example, n-butyl lithium, results
in the
formation of a carbanion, e.g., . Subsequent exposure
of the carbanion to an anionically polymerizable monomer, e.g., a methacrylate
monomer such as methyl methacrylate (MMA), results in a copolymer having (a) a
cationically polymerized block, for example, a polyisobutylene block, and (b)
an
-21-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
anionically polymerized block, for example, a poly(methyl methacrylate) (PMMA)
block:
[0073] As another example, an exemplary carbanion, e.g.,
may be exposed to, e.g., 2-vinylpyridine, thus
resulting in a copolymer having (a) a cationically polymerized block, for
example, a
polyisobutylene block, and (b) an anionically polymerized block, for example,
a
~H2 PVPy
PIB-CE
\N
CH3
polyvinylpyridine (PVPy) block:
[0074] The invention is fizrther described with reference to the following non-
limiting
Examples.
EXAMPLES
[0075] Characterizations. 1H-NMR spectroscopy was carried out on a Bruker AC
250
MHz spectrometer at 25 °C in CDC13. Gel Permeation Chromatorgraphy
(GPC) was
carried out using a Waters HPLC system equipped with model 510 HPLC pump,
model
410 differential refractometer, model 486 UV/visible detector, model 712
sample
processor, and five ultra-Styragel columns connected in the series (500, 103,
104,105 and
100 ~). THF was used as an eluent at a flow rate of 1 mL/min.
[0076] Materials. 2,6-Di-tert-butylpyridine (Aldrich, 97%) was purified by
distillation
from CaH2. Isobutylene (Air Gas) was passed through in-line gas purifier
columns
packed with CaS04 and no. 13 molecular sieves and condensed at -15 °C
prior to
polymerization. Methyl chloride (CH3C1) was passed through in-line gas
purifier
-22-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
columns packed with Ba0/Drierite and condensed at -80 °C prior to
polymerization.
Methylene chloride (CHZC12) was purified by washing it with 10% aqueous NaOH
and
then with distilled water until neutral and dried over anhydrous MgS04
overnight. It
was refluxed for 24 h and distilled from CaH2, just before use. n-Hexane was
rendered
olefin free by refluxing it over concentrated sulfuric acid for 48 h. It was
washed with
10% aqueous NaOH and then with deionized water until neutral and stored over
MgS04
for 24 h. It was refluxed over CaH2 overnight and distilled. Titanium (IV)
chloride
(TiCl4, Aldrich, 99.9%) was used as received. 2-Chloro-2,4,4-trimethylpentane
was
prepared by hydrochlorination of 2,4,4-trimethyl-1-pentene (Fluka, 98 %) with
hydrogen
chloride gas in dry dichloromethane at 0 °C. Kaszas, G.; Gyor, M.;
Kennedy, J. P.;
Tiidos, F. J. Macromol.Sci., Chem 1983, A18,1367-1382. The product was dried
over
CaCl2 and distilled under reduced pressure before use. 5-tert-butyl-1,3-bis(1-
chloro-1-
methylethyl)benzene was synthesized following the procedure reported in Gyor,
M.
Wang., H. C.; Faust, R. J. J. Macromol.Sci., Pure Appl. Chem 1992, A29, 639.
Tetrahydrofuran (Merck p.a) was purified first by distillation under nitrogen
from CaH2
and then by refluxing over potassium. n-Butyllithium (n-BuLi, 2.5 M in hexane)
was
purchased from Aldrich and its concentration was titrated by a standard
method. See,
e.g., Reed, P. J.; Urwin, J. R. J. Organometal. Chem. 1972, 39, 1-10. Methyl
methacrylate (MMA) and 2-[(trimethylsilyl)oxy]ethyl methacrylate (TMSiOEMA),
in
which the hydroxyl group of 2-hydroxyethyl methacrylate (HEMA) is protected
with a
trimethylsilyl group, were dried over CaH2 for 24 h and then distilled over
triethylaluminum or trioctylaluminum under vacuum. The 1,4-Bis(1-
phenylethenyl)benzene is synthesized using known procedures, e.g., those
described in
U.S. Patent No. 4,182,818 to Tung, L. H. and Lo, G. Y.-S. 1,1-Diphenylethylene
purchased from Aldrich Chemical Company was purified by vacuum distillatin
under
potassium metal.
(0077] Synthesis of 1,1-diphenyhexyllithium. The preparation of 1,1-
diphenylhexyllithium is carried out under high vacuum conditions (< 10-6
mbar). 0.037
g of n-butyllithium (5.7 x 10~ mol) is added at -78 °C to a reactor
containing 0.01 mL of
1,1-diphenylethylene (5.7 x 10-5 mol) dissolved in tetrahydrofuran. After 5
minutes, the
cherry-reddish solution is brought to room temperature for 1 hour. During this
step,
unreacted n-butyllithium is decomposed by the reaction with tetrahydrofi~ran.
The
-23-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
solution is delivered into a graduated cylinder with a stopcock, which is
stored in a
refrigerator.
[0078] Synthesis of c~trrl,l-diphenylethylene end-functionalized
polyisobutylene.
The preparation of a difunctional macromonomer is carried out at -80 °C
under nitrogen
atmosphere. To a prechilled 500 mL 3-neck flask equipped with mechanical
stirrer are
added sequentially 187 mL of hexane, 111 mL of methyl chloride, 0.086 g of 5-
tert-
butyl-1,3-bis(1-chloro-1-methylethyl)benzene (3.0 x 10~ mol), 0.2 mL of 2,6-di-
tert-
butylpyridine (9.0 x 10-4 mol), and 21 mL of isobutylene (0.27 mol). Then, 1.2
mL of
titanium tetrachloride (1.1 x 10-2 mol) is added into the reactor to
polymerize the
isobutylene. After the completion of monomer polymerization, 0.34 g of 1,4-
bis(1-
phenylethenyl)benzene (1.2 x 10-3 mol) dissolved in methylene chloride is
added into the
reactor. After 2 hours, 5.15 g of dimethylzinc (5.4 x 10-2 mol) dissolved in
toluene is
added into the reactor. 2 hours later, 30 mL of prechilled methanol is added
into the
reactor to quench the reaction. The polymer solution is then poured into
ammonium
hydroxide/methanol (10/90, v/v). After the evaporation of solvents, the
polymer is
dissolved in hexane and inorganics are filtered. The polymer recovered by the
precipitation of the polymer solution into methanol. The polymer is then
dissolved
again in hexane and recovered again by the precipitation of the polymer
solution into
methanol, followed by drying in a vacuum.
[0079] According to 1H NMR and GPC measurements, functionalization and
methylation at polyisobutylene chain ends are essentially complete.
Essentially no
change in the number average molecular weight and polydispersity of the 1,1-
diphenylethylene functional polyisobutylene was observed, relative to those of
the
polyisobutylene precursor (Table 1), confirming that coupling reactions are
virtually
nonexistant.
Table 1.
Polymer M" MWlM"
Polyisobutylene 55000 1.03
a, ur l, l -Diphenylethylene 56800 1.04
end-functional polyisobutylene
-24-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
[0080] Synthesis of ro-l,l-diphenylethylene end-functionalized
polyisobutylene. The
preparation of monofunctional macromonomer is carried out at -80 °C
under nitrogen
atmosphere. To a prechilled 500 mL flask equipped with mechanical stirrer are
added
sequentially 198 mL of hexane, 118 mL of methyl chloride, 0.1 mL of 2-chloro-
2,4,4-
trimethylpentane (6.0 x 10-4 mol), 0.2 mL of 2,6-di-tert-butylpyridine (9.0 x
10-4 mol),
and 4.7 mL of isobutylene (0.06 mol). 1.2 mL of titanium tetrachloride (1.1 x
10-2 mol)
is then added into the reactor to polymerize the isobutylene. After the
completion of
monomer polymerization, 0.34 g of 1,4-bis(1-phenylethenyl)benzene (1.2 x 103
mol)
dissolved in methylene chloride is added into the reactor. After 2 hours, 5.15
g of
dimethylzinc (5.4 x 10-Z mol) is added into the reactor. After 2 more hours,
30 mL of
prechilled methanol is added into the reactor to quench the reaction. The
polymer
solution is then poured into ammonium hydroxide/methanol (10/90, v/v). After
the
evaporation of solvents, the polymer is dissolved in hexane and inorganics are
filtered.
The polymer solution is then precipitated into methanol to give solid polymer.
The solid
polymer is again dissolved in hexane and recovered again by the precipitation
of the
polymer solution into methanol, followed by drying under vacuum.
[0081] According to 1H NMR and GPC measurements, functionalization and
methylation at the polyisobutylene chain end are essentially complete. Number
average
molecular weight and polydispersity of 1,1-diphenylethylene functional
polyisobutylene
did not change substantiallly as compared with those of polyisobutylene (Table
2),
confirming a virtual absence of coupling reactions.
Table 2.
Polymer M~ MWlMn
Polyisobutylene 4500 1.09
erl,l-Diphenylethylene end- 4900 1.08
functional polyisobutylene
Example 1
[0082] All chemical purifications and acrylate polymerizations are carried out
under
high vacuum condition (< 10-6 mbar). 1.17 g (2.06 x 10-5 mol) of a,~l,l-
diphenylethylene end-functionalized polyisobutylene (M" = 56800, see above) in
250
mL of hexane is stirred over calcium hydride for 24 hours. The polymer
solution is then
-25-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
filtered to remove calcium hydride. The hexane solvent is evaporated, and 100
mL of
tetrahydrofuran are added to the remaining polymer. This polymer solution is
then
added to a reactor equipped with a stirrer. 1,1-diphenylhexyllithium in
tetrahydrofuran
(see above) is added into the reactor dropwise until the color of the polymer
solution
changes from colorless to yellowish. The amount of 1,1-diphenylhexyllithium
used for
this purpose is 0.0010 g (4.1 x 10-6 mol). The polymer solution is
subsequently cooled
down to -78 °C with vigorous stirring. After 10 minutes at this
temperature, 0.0090 g of
n-butyllithium (1.4 x 10-4 mol) in 27.5 mL of hexane is added into the
reactor. 12 hours
later, the polymer solution is heated up to 40 °C and kept at this
temperature for 1 hour.
The polymer solution is again cooled to -78 °C. After 10 minutes at
this temperature,
0.95 mL of methyl methacrylate (8.9 x 10-3 mol) is distilled into the reactor.
The
reactoin is quenched after S hours by adding purified degassed methanol to the
reactor.
The polymer solution is precipiated into methanol to give a white solid
polymer.
[0083] The blocking efficiency of the obtained block copolymer is measured
using GPC
and 1H NMR and is calculated to be at leaset 87 %. The product is immersed
into
hexane for 24 hours to isolate polyisobutylene homopolymer from the block
copolymer.
According to'H NMR and GPC measurements, the purified block copolymer has a
M"=109400, a MWlM" = 1.14, and the composition of isobutylene and methyl
methacrylate in the polymer is 57/43 w/w.
Example 2
[0084] 1.60 g (2.8 x 10-5 mol) of a,~crl,l-diphenylethylene end-functionalized
polyisobutylene (M" = 56800, see above) in 200 mL of hexane is stirred over
calcium
hydride for 24 hours. The polymer solution is then filtered to remove calcium
hydride.
Solvent is evaporated, and 100 mL of tetrahydrofuran is added to the remaining
polymer. The polymer solution is then added to a reactor equipped with a
stirrer, and
1,1-diphenylhexyllithium in tetrahydrofuran (see above) is added into reactor
dropwise
until the color of the polymer solution changes from colorless to yellowish.
The amount
of 1,1-diphenylhexyllithium used for this purpose is 0.0010 g (4.1 x 10-6
mol). The
polymer solution is subsequently cooled to -78 °C with vigorous
stirring. After 10
minutes at this temperature, 0.0122 g of n-butyllithium (1.9 x 10~ mol) in 40
mL of
hexane is added into the reactor. After an additional 12 hours, the polymer
solution is
heated to 40 °C and kept at this temperature for 1 hour. The polymer
solution is then
-26-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
cooled down to -78 °C. After 10 minutes at this temperature, 0.64 mL of
methyl
methacrylate (6.0 x 10-3 mol) is distilled into the reactor. 5 hours later,
purified
methanol is added to reactor to quench the reaction. The polymer solution is
poured into
methanol to yield a white solid polymer.
[0085] The blocking efficiency of the obtained block copolymer is measured
using GPC
and 1H NMR and is calculated to be at least 92 %. The block copolymer is
purified by
using hexane to remove polyisobutylene homopolymer. According to 1H NMR and
GPC measurements, the purified block copolymer had a M°=83400, a MW/Mp
1.30, and
the composition of isobutylene and methyl methacrylate in the polymer is 67/33
w/w.
Example 3
[0086] 1.96 g (3.45 x 10-5 mol) of a,a~-1,1-diphenylethylene end-
functionalized
polyisobutylene (Mn = 56800, see above) in 300 mL of hexane is stirred over
calcium
hydride for 24 hours. The polymer solution is then filtered to remove calcium
hydride.
The solvent is evaporated and 130 mL of tetrahydrofuran are added to the
remining
polymer. The resulting polymer solution is then added to a reactor equipped
with a
stirrer. l,l-diphenylhexyllithium in tetrahydrofuran (see above) is then added
into
reactor dropwise until the color of the polymer solution changes from
colorless to
yellowish. The amount of 1,1-diphenylhexyllithium used for this purpose is
0.0030 g
(1.2 x 10-5 mol). Afterwards, the polymer solution is cooled down to -78
°C with
vigorous stirring. After 10 minutes at this temperature, 0.0160 g of n-
butyllithium (2.5 x
10~ mol) in 40 mL of hexane is added into the reactor. 2 hours later, the
polymer
solution is heated to 40 °C and kept at this temperature for 1 hour.
Then, the polymer
solution is again cooled to -78 °C. After 10 minutes at this
temperature, 2 mL of 2-
[(trimethylsilyl)oxy]ethyl methacrylate (9.2 x 10-3 mol) diluted with 2 mL of
tetrahydrofuran is added into the reactor. 3 hours later, purified methanol is
added to
reactor to quench the reaction. The polymer solution is precipitated into
methanol to
yield a white solid polymer.
[0087] The blocking efficiency is at least 90 %, as measured using GPC and 1H
NMR.
The obtained polymer is purified by using hexane to remove polyisobutylene
homopolymer. During the recovery step, the trimethylsilyloxy groups in the
block
copolymer are completely converted into hydroxyl groups. For'H NMR and GPC
measurements, the block copolymer is treated with benzoic anhydride to protect
the
-27-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
hydroxyl groups in the poly(2-hydroxylethyl methacrylate) blocks with a
benzoyl group.
According to 1H NMR and GPC measurements, the block copolymer treated with
benzoic anhydride had a M"=131900, a MW/M"=1.33, and the composition of
isobutylene
and 2-hydroxylethyl methacrylate in the polymer is 50/50 w/w.
Example 4
[0088] 0.93 g (1.9 x 10~ mol) of ~crl,l-diphenylethylene end-functionalized
polyisobutylene (M" = 4900, see above) in 200 mL of hexane is stirred over
calcium
hydride for 24 hours. Then, the polymer solution is filtered to remove calcium
hydride.
Solvent is evaporated, and 100 mL of tetrahydrofuran is added to the remaining
polymer. The polymer solution is added to a reactor equipped with a stirrer.
Unlike the
above examples, no 1,1-diphenylhexyllithium in tetrahydrofuran is then added
to the
reactor at this point. The polymer solution is cooled down to -78 °C
with vigorous
stirnng. After 10 minutes at this temperature, 0.0961 g of n-butyllithium (1.5
x 10-3
mol) is added into the reactor. 1 hour later, the polymer solution is heated
to 20 °C and
kept at this temperature for 1 hour. Then, the polymer solution is again
cooled to -78
°C. After 10 minutes at this temperature, 1.5 mL of methyl methacrylate
(1.4 x 10-2
mol) is charged into the reactor. 2 hours later, purified methanol is added to
reactor to
quench the reaction. The polymer solution is then poured into methanol to
yield a white
solid polymer.
[0089] The blocking efficiency is calculated to be 67 % based on GPC and'H NMR
results. The obtained polymer is purified using hexane to remove
polyisobutylene
homopolymer. According to 1H NMR and GPC measurements, the purified block
copolymer has a Mn 22300, a MW/Mp 1.26, and the composition of isobutylene and
methyl methacrylate in the polymer is 25/75 w/w.
Example 5
[0090] 0.24 g of ~crl,l-diphenylethylene end-functionalized polyisobutylene
(Mn =
4900, see above) in 200 mL of hexane is stirred over calcium hydride for 24
hours.
Then, the polymer solution is filtered to remove calcium hydride. Solvent is
evaporated
and 100 mL of tetrahydrofuran is then added to the remaining polymer. The
polymer
solution is added to a reactor equipped with a stirrer. No 1,1-
diphenylhexyllithium in
tetrahydrofuran is added to the reactor at this point. The polymer solution is
then cooled
-28-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
down to -78 °C with vigorous stirring. After 10 minutes, 0.0275 g of n-
butyllithium (4.3
x 10~ mol) is added into the reactor. 1 hour later, the polymer solution is
heated up to
20 °C and kept at this temperature for 1 hour. The polymer solution is
then cooled down
to -78 °C. After 10 minutes at this temperature, 0.6 mL of methyl
methacrylate (5.6 x
10-3 mol) is distilled into the reactor. 2 hours later, purified methanol is
added to reactor
to quench the reaction. The polymer solution is then poured into methanol to
yield a
white solid polymer.
[0091] The blocking efficiency is calculated to be 72 % based on GPC and 1H
NMR
results. The obtained polymer is purified using hexane to remove
polyisobutylene
homopolymer. According to 1H NMR and GPC measurements, the purified block
copolymer had a M"=31700, a MW/Mp 1.13, and the composition of isobutylene and
methyl methacrylate in the polymer is 18/82 w/w.
Example 6
[0092] 0.14 g of a~-1,1-diphenylethylene end-fiznctionalized polyisobutylene
(M" _
4900, see above) in 200 mL of hexane is stirred over calcium hydride for at
least 24
hours. The polymer solution is then filtered to remove calcium hydride.
Solvent is
evaporated and 100 mL of tetrahydrofuran is added to polymer. The polymer
solution is
added to a reactor equipped with a stirrer. No 1,1-diphenylhexyllithium in
tetrahydrofuran is added to the reactor at this point. The polymer solution is
then cooled
down to -78 °C with vigorous stirring. After 10 minutes at this
temperature, 0.016 g of
n-butyllithium (2.5 x 10~ mol) is added into the reactor. 1 hour later, the
polymer
solution is heated up to 20 °C and kept for 1 hour at this temperature.
The polymer
solution is again cooled down to -78 °C. After 10 minutes at this
temperature, 0.4 mL
of methyl methacrylate (3.7 x 10-3 mol) is charged into the reactor. 2 hours
later,
purified methanol is added to reactor to quench the reaction. The polymer
solution is
then poured into methanol to yield a white solid polymer.
[0093] The blocking efficiency is calculated to be 68 %, based on GPC and 1H
NMR
results. The obtained polymer is purified by using hexane to remove
polyisobutylene
homopolymer. According to 1H NMR and GPC measurements, the purified block
copolymer has a M"=36900, an MWlMn 1.20, and the composition of isobutylene
and
methyl methacrylate in the polymer is 15/85 w/w.
-29-
CA 02554528 2006-07-26
WO 2005/077987 PCT/US2005/004374
Example 7
(0094] All chemical purifications and anionic polymerizations are carned out
under high
vacuum condition (< 10-6 mbar).
[0095] 1.50 g (2.6 x 10-5 mol) of a,~-1,1-diphenylethylene end-functionalized
polyisobutylene (Mn = 56800, see above) in 200 mL of hexane is stirred over
calcium
hydride for 24 hours. The polymer solution is then filtered to remove calcium
hydride.
Solvent is evaporated, and 87 mL of tetrahydrofuran is added to the remaining
polymer.
The polymer solution is then added to a reactor equipped with a stirrer, and
1,1-
diphenylhexyllithium in tetrahydrofuran (see above) is added into reactor
dropwise until
the color of the polymer solution changes from colorless to yellowish. The
amount of
1,1-diphenylhexyllithium used for this purpose is 0.0010 g (4.1 x 10-6 mol).
The
polymer solution is subsequently cooled to -78 °C with vigorous
stirring. After 10
minutes at this temperature, 0.01 g of n-butyllithium (1.6 x 10-4 mol) in 33
mL of hexane
is added into the reactor. After an additional 12 hours, the polymer solution
is heated to
40 °C and kept at this temperature for 1 hour. The polymer solution is
then cooled down
to -78 °C. After 10 minutes at this temperature, 1.19 mL 2-
vinylpyridine (1.1 x 10-2
mol) is distilled into the reactor. 40 minutes later, purified methanol is
added to reactor
to quench the reaction. The polymer solution is poured into methanol to yield
a white
solid polymer.
[0096] The block copolymer is purified by using hexane to remove
polyisobutylene
homopolymer. According to GPC and 1H NMR measurements, the purified block
copolymer had an apparent Mp 67000, a MW/Mn 1.11, and the composition of
isobutylene and 2-vinylpyridine in the polymer is 68/32 w/w.
[0097] Although various embodiments are specifically illustrated and described
herein,
it will be appreciated that modifications and variations of the present
invention are
covered by the above teachings and are within the purview of the appended
claims
without departing from the spirit and intended scope of the invention.
-30-