Note: Descriptions are shown in the official language in which they were submitted.
CA 02555896 1999-05-06
r
TITLE
FAT PRODUCTS FROM HIGH STEARIC SOYBEAN OIL AND
A METHOD FOR THE PRODUCTION THEREOF
FIELD OF THE INVENTION
This invention concerns the fractionation of soybean oils and, in particular,
high stearic soybean oils to make a fat product useful in confectionary
applications. Also of concern is the fractionation of high stearic, high oleic
soybean oils to make a fat product useful in confectionary applications and
liquid,
high stability oils.
BACKGROUND OF THE INVENTION
Fats and oils play a major role in human nutrition and are recognized as
essential nutrients in both human and animal diets. Nutritional concerns have
led
to the replacement of animal-fat shortenings with vegetable oils as the major
source of lipids in human diets. The most commonly used vegetable oil
worldwide is soybean oil. Over 19 million metric tons of soybean oil were
consumed in 1995 alone. The use of soybean oil in the United States is
extremely
popular. In fact, over 80% of the vegetable oils consumed in the United States
are
soybean oils which are used in margarines, shortenings, salad and cooking
oils,
and commercial frying oils. About half of the soybean oil consumed is in the
form of margarines or shortenings and frying oils.
The specific performance and health attributes of edible oils in general are
determined largely by their fatty acid composition. Soybean oil is composed
primarily of palmitic (C 16:0), stearic (C 18:0), oleic (C18:1), linoleic (C
18:2) and
linolenic (C 18:3) acids and, in that regard, is similar to the other most
commonly
used vegetable oils including palm, sunflower, canola, cottonseed, peanut,
coconut, olive and palm kernel.
By comparison, soybean oil contains relatively high levels of both linoleic
and linolenic acid relative to some other vegetable oils. These fatty acids
are more
prone to oxidation than saturated and monounsaturated fatty acids. Without
modification, soybean oil is relatively unstable to oxidation reactions and
its use is
limited to applications that do not require a high degree of stability. Under
extended use, oxidized soybean oil develops off flavors and undergoes physical
changes such as increased viscosity and foaming.
Several methods are available to increase the stability of soybean oil. One
commonly used method is catalytic hydrogenation, a process that reduces the
number of double bonds and raises the melting point of the fat with the aid of
a
catalyst such as nickel. Specifically, catalytic hydrogenation reduces the
level of
polyunsaturated fatty acids, primarily linoleic (C 18:2) and linolenic (C
18:3) acids,
and increases oleic (C18:1) and stearic (C18:0) acids. This results in a
stable oil
1
CA 02555896 1999-05-06
}
suitable for food frying and specialized high stability oil applications clue
to the
reduction of the unsaturated fatty acid content. Also, the physical properties
of the
oil are changed because the fatty acid modifications increase the melting
point
resulting in a semi-liquid or solid fat at room temperature. A large
percentage of
the soybean oil consumed annually is partially hydrogenated soybean oil.
In general, soybean oil is produced using a series of steps involving the
extraction and purification of an edible oil product from the oil bearing
seed.
Soybean oils and soybean byproducts are produced using the generalized steps
shown in the diagram below.
Process Impurities Removed/
Byproducts Obtained
Soybean Seed
Oil Extraction I'm Meal
Degiuniming No. Lecithin
Alkali or Physical Refining ----- Gums, Free Fatty Acids, Pigments
Water Washing Soap
Bleaching =- Color, Soap, Metal
(Hydrogenation)
(Winterization) Stearine
Deodorization 01 FFA, Tocopherols, Sterols, Volatiles
Oil Products
2
CA 02555896 1999-05-06
y
Soybean seeds are cleaned. tempered, dehulled, and flaked which increases
the efficiency of oil extraction. Oil extraction is usually accomplished by
solvent
(hexane) extraction but can also be achieved by a combination of physical
pressure and/or solvent extraction. The resulting oil is called crude oil. The
crude
oil may be degununed by hydrating phospholipids and other polar and neutral
lipid complexes that facilitate their separation from the nonhydrating,
triglyceride
fraction (soybean oil). The resulting lecithin gums may be further processed
to
make commercially important lecithin products used in a variety of food and
industrial products as emulsification and release (antisticking) agents.
Degummed
oil may be further refined for the removal of impurities; primarily free fatty
acids,
pigments, and residual gums. Refining is accomplished by the addition of a
caustic agent that reacts with free fatty acid to form soap and hydrates
phosphatides and proteins in the crude oil. Water is used to wash out traces
of
soap formed during refining. The soapstock byproduct may be used directly in
animal feeds or acidulated to recover the free fatty acids. Color is removed
through adsorption with a bleaching earth that removes most of the chlorophyll
and carotenoid compounds. The refined oil can be hydrogenated resulting in
fats
with various melting properties and textures. Winterization (fractionation)
may be
used to remove stearine from the hydrogenated oil through crystallization
under
carefully controlled cooling conditions. Deodorization which is principally
steam
distillation under vacuum, is the last step and is designed to remove
compounds
which impart odor or flavor to the oil. Other valuable byproducts such as
tocopherols and sterols may be removed during the deodorization process.
Deodorized distillate containing these byproducts may be sold for production
of
natural vitamin E and other high-value pharmaceutical products. Refined,
bleached, (hydrogenated, fractionated) and deodorized oils and fats may be
packaged and sold directly or further processed into more specialized
products. A
more detailed reference to soybean seed processing, soybean oil production and
byproduct utilization can be found in Erickson, 1995, Practical Handbook of
Soybean Processing and Utilization, The American Oil Chemists' Society and
United Soybean Board.
Soybean oil is liquid at room temperature because it is relatively low in
saturated fatty acids when compared with oils such as coconut, palm, palm
kernel
and cocoa butter. Many processed fats, including spreads, confectionary fats,
hard
butters, margarines, baking shortenings, etc., require varying degrees of
solidity at
room temperature and can only be produced from soybean oil through alteration
of its physical properties. This is most commonly achieved through catalytic
hydrogenation.
3
CA 02555896 1999-05-06
Hydrogenation is a chemical reaction in which hydrogen is added to the
unsaturated fatty acid double bonds with the aid of a catalyst such as nickel.
High oleic soybean oil contains unsaturated oleic, linoleic, and linolenic
fatty
acids and each of these can be hydrogenated. Hydrogenation has two primary
effects. First, the oxidative stability of the oil is increased as a result of
the
reduction of the unsaturated fatty acid content. Second, the physical
properties
of the oil are changed because the fatty acid modifications increase the
melting
point resulting in a semi-liquid or solid fat at room temperature.
There are many variables which affect the hydrogenation reaction which
in turn alter the composition of the final product. Operating conditions
including pressure, temperature, catalyst type and concentration, agitation
and
reactor design are among the more important parameters which can be
controlled. Selective hydrogenation conditions can be used to hydrogenate the
more unsaturated fatty acids in preference to the less unsaturated ones. Very
light or brush hydrogenation is often employed to increase stability of liquid
oils. Further hydrogenation converts a liquid oil to a physically solid fat.
The
degree of hydrogenation depends on the desired performance and melting
characteristics designed for the particular end product. Liquid shortenings,
used
in the manufacture of baking products, solid fats and shortenings used for
commercial frying and roasting operations, and base stocks for margarine
manufacture are among the myriad of possible oil and fat products achieved
through hydrogenation. A more detailed description of hydrogenation and
hydrogenated products can be found in Patterson, H. B. W., 1994,
Hydrogenation of Fats and Oils: Theory and Practice. The American Oil
Chemists' Society.
Hydrogenated oils have also become controversial due to the presence of
trans fatty acid isomers that result from the hydrogenation process. Ingestion
of
large amounts of trans isomers has been linked with detrimental health effects
including increased ratios of low density to high density lipoproteins in the
blood
plasma and increased risk of coronary heart disease. It would be advantageous
to
produce foods that currently use hydrogenated oils in a form that would be
free of
trans fatty acids.
The term "substantially free of trans fatty acids" as used herein means a
non-health threatening level of trans fatty acids. For example, such a level
can
range from below 1% (i.e., an amount which cannot be reliably detected by
current methods for assessing trans fatty acid levels) to an upper limit which
does
not pose a health risk. In the near future, the Federal government is expected
to
place an upper limit on the levels of trans fatty acid isomers that can be
present in
foods and have the designation "trans fatty acid free".
4
CA 02555896 1999-05-06
It is believed that all of the oils, margarines and spread products of the
invention are expected to conform to whatever limits are imposed by
governmental authorities.
The limit of detection for trans isomers of fatty acids in oils is around
0.1 % (The gas chromatography method for detecting trans fatty acids in oils
is
outlined in AOCS Ce I C-89), Reports of "low trans isomer oils" produced by
modifications of the hydrogenation method can achieve levels of 5-20% (w/w),
but usually at the cost of high saturated fatty acid levels (Allen, D. A.
(1998) Lipid
Technology, 10(2), 29-33). It is believed that the oils, fat products, and
blended
fat products, that are wholly or partially non-hydrogenated and non-chemically
modified, in the instant invention, should be substantially free of trans
fatty acids,
i.e., they should achieve trans fatty acid concentrations of below 20% (w/w),
preferably below 10%, more preferably below 5%, even more preferably below
3%, and again more preferably below I%, and most preferably below 0.5% of the
oil.
The term "non-hydrogenated" will be used to define oils that have not
been subjected to any physical or chemical hydrogenation process that causes
changes in, or is designed to alter, the naturally occurring fatty acid
composition
of the oil, including, but not limited to, all of the processes outlined in
the
background. The term hydrogenation will be used to define oils that have been
subjected to hydrogenation process(es) that alter the naturally occurring
fatty
acid composition of the oil, including, but not limited to, all of the
processes
outlined in the background.
The term "non-chemically modified" will be used to describe any oil that
has not undergone any chemical modification, including but not limited to
interesterification, that results in an alteration of the naturally occurring
complement and structure of the oil's fatty acids. The term "chemical
modification" will be used to describe any oil that has undergone any chemical
modification that results in the alteration of the naturally occurring
complement
and structure of the oil's fatty acids, including, but not limited to,
interesteri-
fication outlined in the background.
In addition, hydrogenated fats have their limitations. It is often very
difficult to produce fats with the appropriate plasticity across the wide
range of
temperatures required for a given application. Those with high melting points
impart an unpleasant mouth feel resembling wax. For example, the solids,
crystallization and melting requirements for confectionary fats such as cocoa
butter replacements and substitutes are notoriously difficult and expensive to
reproduce.
5
CA 02555896 1999-05-06
Interesterification refers to the exchange of the fatty acyl moiety between
an ester and an acid (acidolysis), an ester and an alcohol (alcoholysis) or an
ester
and ester (transesterification). Interesterification reactions are achieved
using
chemical or enzymatic processes. Random or directed transesterification
processes rearrange the fatty acids on the triglyceride molecule without
changing
the fatty acid composition. The modified triglyceride structure may result in
a
fat with altered physical properties. Directed interesterfication reactions
using
lipases are becoming of increasing interest for high value specialty products
like
cocoa butter substitutes. Products being commercially produced using
interesterification reactions include but are not limited to shortenings,
margarines, cocoa butter substitutes and structured lipids containing medium
chain fatty acids and polyunsaturated fatty acids. Interesterification is
further
discussed in Hui, Y.H., 1996, Bailey's Industrial Oil and Fat Products,
Volume 4, John Wiley & Sons.
Most confectionary fats have a high solid fat content at room temperature
but also must melt quickly in the mouth. Cocoa butter is a unique fat which
exhibits these types of physical properties. Products made with cocoa butter,
such
as chocolate, are solid at room temperature, have a desirable "snap" when
broken,
melt smoothly and rapidly in the mouth with no "waxy" or greasy impression,
and
provide a cooling sensation on the palate and good flavor release. Contraction
of
the fat upon cooling is also important for molded products. Cocoa butter is
excellent in this regard.
Cocoa butter is relatively expensive and subject to price fluctuation and
availability dependent on the volatility of the cocoa-bean market. It also
exhibits
an undesirable tendency towards "fat bloom" which appears on the surface of
the
product due to changes in the crystal structure of the fat. Products destined
for
tropical climates may need the addition of other fats or hard butters to
increase the
solidity of the product at higher ambient temperatures. As a result, a market
for
fat alternatives to cocoa butter, that exhibit many of the same physical
properties,
has developed.
Confectionary fats made from fats other than cocoa butter are designed to
have many of the positive attributes and properties of cocoa butter to make
them
suitable for these types of applications. They are, however, often expensive
to
produce and may only exhibit some of the desired physical properties.
Confectionary fats are produced from palm oil fractions, palm kernel oil and
its
fractions and from fractionated hydrogenated vegetable oils which contain a
high
trans fatty acid isomer content. Both dry and solvent fractionation have been
used
to produce products with different compositions. Often several processing
steps
6
CA 02555896 1999-05-06
including hydrogenation. fractionation and/or interesterification are employed
to
produce a product with the right melting characteristics.
The unique properties of cocoa butter result from the chemical
composition of the fat. Since it is a natural fat, its composition shows
normal
variation depending on what country (environment) the fat originates from. The
three major fatty acids of cocoa butter include palmitic (26%), stearic (34%),
and
oleic (34%). The physical characteristics of cocoa butter result from the
arrangement of these fatty acids on the triglyceride. There exists a high
degree of
symmetrical monounsaturated triglycerides which have the unsaturated fatty
acid
in the 2-position and saturated fatty acids in the 1- and 3- positions. These
triglycerides are most often 2-oleoyl-I-palmitoyl-3-stearoylglycerol (POS),
and
2-oleoyl- 1,3-distearolylglycerol (SOS), and 2-oleoyl-1,3-dipalmitoylglycerol
(POP), with POS being present in the largest amount. These three major
triglycerides have crystal forms with melting points just below body
temperature.
Other oils or their fractions can be used to produce confectionary fats such
as palm kernel, palm, illipe, shea, sal, coconut, and various vegetable fats.
These
oils have fatty acid compositions which differ from that of cocoa butter but
may
have similar physical properties.
Industry suppliers use a variety of terms to categorize confectionary fats of
which the more common terms included cocoa butter equivalents, cocoa butter
improvers, cocoa butter substitutes, cocoa butter replacers, hard butters,
coating
fats, compound coatings, center filling fats, and non-dairy fats. These fats
will
vary somewhat in melting behavior depending on the particular application for
which the fat is destined.
Cocoa butter extenders are generally based on illipe, shea, and/or palm oil.
The supply of these more "exotic" oils may be erratic. They are fractionated
and
mixed to achieve the proper melting characteristics. They may be used in any
proportion up to 100% with cocoa butter for complete replacement. Fats with
higher solids and melting points can be used to improve the properties of
cocoa
butter. The addition of up to 5% cocoa butter extender in chocolate products
(of
the total weight of the product) is permitted without label declaration in
some
countries.
Cocoa butter substitutes and replacers are usually described as lauric or
non lauric depending on the fat from which they are derived. Lauric cocoa
butter
substitutes are based mainly on palm kernel oil. The required physical
properties
are obtained by fractionation, blending, hydrogenation, interesterification or
a
combination of these. They have a high solid fat content at 20 C, do not
require
tempering, resist fat bloom, and have favorable thermal properties and
contract
upon cooling. However they are not completely compatible with cocoa butter in
7
CA 02555896 1999-05-06
that they may result in an undesirable softening of the mixed product,
Therefore,
lauric cocoa butter substitutes usually do not exceed 5-6% of the product.
They
also suffer from hydrolysis in products which contain a source of both water
and
lipases (e.g., cocoa powder, nuts, milk products, etc). Hydrolysis releases
free
lauric acid which gives the product an unpleasant soapy taste. Hydrolysis of
non-
lauric cocoa butter substitutes release longer chain fatty acids which do not
impart
this taste.
Non-lauric cocoa butter substitutes are generally produced by
hydrogenation of liquid oils and subsequent fractionation or blending. They
are
based on sunflower, canola, cottonseed, soybean, peanut, corn, safflower and
palm. Hydrogenation of these oils results in a high level of trans fatty
isomers
which, in addition to saturated fatty acids, results in fats with a higher
melting
point. Further fractionation results in fats with a narrower melting range.
They
can be used in greater proportion with cocoa butter (-25%) and are often used
for
coating because they have good gloss, long shelf life, and a high resistance
to
bloom. Their use is limited by poor eating quality, flavor release and mouth
feel.
U.S. Patent No. 5,557,037, issued to Fehr et al. on September 17, 1996,
describes soybeans having elevated contents of saturated fatty acids wherein
the
palmitie acid content is at least about 14% of the total fatty acid
composition and
the stearic acid content is at least about 20% or more of the total fatty acid
composition, Soybean varieties having sufficiently elevated palmitic and
stearic
acid contents are desirable, in that plastic fat (e.g., shortening and
margarine) can
be produced with the matrix stabilized in the B' form. There is no disclosure
that
high stearic soybean oils would be suitable for use in confectionary
applications.
List et al., Journal of the American Oil Chemists' Society, Vol. 74, No. 3,
pages 468-472 (1997) discusses the effect of interesterification on the
structure
and physical properties of high stearic soybean oils. It was found that after
random interesterification, these oils exhibited solid fat index profiles and
dropping points suitable for soft tub margarine. There is no disclosure that
the
solid fat index profiles and dropping points of high stearic soybean oils
would be
suitable for use in confectionary applications.
European Patent Application Publication Number 245,076, published on
November 11, 1987, describes edible fats for confectionary applications made
by
rearrangement of unsaturated high oleic glyceride oils and fats under the
influence
of a lipase enzyme in the presence of saturated fatty acids or esters thereof
wherein the oils and fats consist substantially of 2-unsaturated triglycerides
at
least 80% of which are 2-oleoyl tricylcerides.
GB Patent Specification having number 827,172, published, February 3,
1960, describes cocoa butter substitutes in which at least a part of the cocoa
butter
8
CA 02555896 1999-05-06
is replaced with a fraction of palm oil having an iodine value not exceeding
45. a
dilatation at 20 C of not less than 1000 and a softening point between 30 and
45 C.
U.S. Patent No. 5,405,639, issued to Pierce et al. on April 1. 1995,
describes non-tempering confectionary fats.
"Confectionary Fats -- For Special Uses", Journal of the American Oil
Chemists' Society, Vol. 61, No. 3, pages 468-472 (March 1984) discusses what
is
new relative to fats and oils in the U.S. confectionary industry.
Kheiri, Formulation, Evaluation and Marketing of Cocoa Butter Replacer
Fats, Palm Oil Research Institute of Malaysia, No. 4, pages 1-53 (August
1982),
discusses the formation, evaluation and marketing of confectionary fats for
chocolate-based products.
PCT International Application having Publication Number WO 94/15478,
published on July 21, 1994, discloses an improved vegetable oil and
fractionation
process.
European Patent Application Publication Number 519,542, published on
December 23, 1992, describes a combined fractionation, refining and
interesterification process.
European Patent Application Publication Number 369,519, published on
May 23, 1990, describes an edible spread and processes for making such a
spread.
MPOPC, Specialty Fats Based on Palm Oil and Palm Kernel Oil,
pages 1-18, (February 24, 1998), describes, specialty fats designed to have
the
positive traits of cocoa butter or properties that make them more suitable for
specific applications.
New product development by confectioners challenges the fats and oils
producers to further their research and development efforts to produce
specialized
fats to fill the needs of the confectionary industry. Oil chemists and
researchers
continue to develop new technology to provide fats with characteristics more
closely resembling those of cocoa butter.
None of the references discussed above addresses the use of high stearic,
and/or high stearic plus high oleic, soybean oils to make fat products,whether
in a
blended or unblended form, suitable for confectionary applications.
SUMMARY OF THE INVENTION
The present invention concerns a fat product made from the fractionation
of a high stearic soybean oil having a C 18:0 content of at least 15% of the
fatty
acid moieties in the oil wherein said fat product is useful for confectionary
applications.
In another embodiment, this invention concerns a method for making a fat
product for confectionary applications which comprises fractionating a high
9
CA 02558386 2006-08-10
stearic soybean oil having oil having a C18:0 content of at least 15% of the
fatty
acid moieties in the oil under conditions suitable for obtaining a fat product
useful
for confectionary applications.
In still another embodiment, this invention concerns a method for making
two products wherein one product is for confectionary applications and the
second
product is a high oleic soybean oil having high oxidative stability, said
method
comprising fractionating a high stearic, high oleic soybean oil having a C
18:0
content of at least 15% of the fatty acid moieties in the oil and a C18:1
content of
greater than 55% of the fatty acid moieties in the oil under conditions
suitable for
obtaining a fat product useful for confectionary applications.
BIOLOGICAL DEPOSIT
The following soybean seed has been deposited with the American Type
Culture Collection (ATCC), 10801 University Boulevard, Manassas, VA
20110-2209, and bears the following designation, accession number and date of
deposit.
Soybean Accession Number Date of Deposit
Soybean L9216116-109 ATCC 203946 April 20, 1999
BRIEF DESCRIPTION OF THE FIGURES
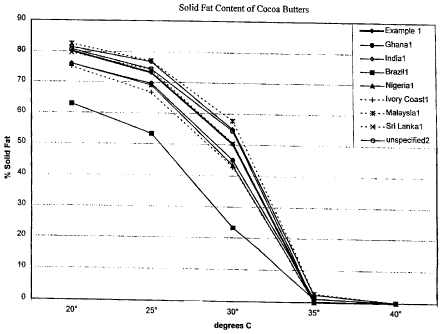
Figure 1 depicts the Solid Fat Content (SFC) profile of various cocoa
butters. There is natural variation in the SFC profile at a given temperature
for
different cocoa butter sources, however, the overall shape of the profile
remains
similar. The rapid decline in SFC profile between 25 and 35 C is a
distinctive
property of cocoa butter.
Figure 2 depicts the SFC profile of similar fractions obtained from
different soybean oils (e.g., high stearic acid; high stearic acid/high oleic
acid).
Fractionation of these oils yielded product(s) having an SFC profile
comparable to
that of cocoa butter. It was found that the SFC profile of the fractionated
oils
differed substantially from the SFC profile of the unfractionated oils.
DETAILED DESCRIPTION OF THE INVENTION
The present invention concerns a fat product made from the fractionation
of a high stearic soybean oil having a C 18:0 content of at least 15% of the
fatty
acid moieties, preferably at least 20% of the fatty acid moieties, and most
preferably at least 22% of the fatty acid moieties in the oil, wherein said
fat
product is useful for confectionary applications. Preferably, the fractionated
product should have a stearic acid content of greater than 35% of the fatty
acid
moieties, more preferably greater than 40% of the moieties, and most
preferably
greater than 50% of the moieties in the oil. It is also believed that the
starting oils
CA 02555896 1999-05-06
and the fractionated products used in the present invention can be in a non-
hydrogenated and non-chemically modified form, or can be used in a
hydrogenated and/or chemically modified form.
In another aspect this invention also concerns a method for making a fat
product for confectionary applications which comprises fractionating high
stearic
soybean oil having a C 18:0 content of at least 15% of the fatty acid
moieties,
preferably at least 20% of the fatty acid moieties, and most preferably at
least 22%
of the fatty acid moieties in the oil under conditions suitable for obtaining
a fat
product useful for confectionary applications.
In still another aspect, this invention concerns a confectionary which
comprises a fat product obtained from the fractionation of a high stearic
soybean
oil having a C18:0 content of at least 15% of the fatty acid moieties,
preferably at
least 20% of the fatty acid moieties, and most preferably at least 22% of the
fatty
acid moieties in the oil.
The term "fat product" as used herein refers to vegetable oils either in a
non-hydrogenated and non-chemically modified form, or in a hydrogenated and/or
chemically modified form, or fractions derived therefrom, either in a non-
hydrogenated and non-chemically modified form, or in a hydrogenated and/or
chemically modified form.
The term "mature seed" refers to a soybean that is no longer green that has,
or did have, a moisture content of less than 20%, and preferably less than
12%.
Furthermore, as used herein, "soybean" refers to the species Glycine max,
Glycine sofa, or any species that is sexually cross compatible with Glycine
max.
A "line" is a group of plants of similar parentage that display little or no
genetic
variation between individuals for a least one trait. Such lines may be created
by
one or more generations of self-pollination and selection, or vegetative
propagation from a single parent including by tissue or cell culture
techniques.
The term "non-hydrogenated" will be used to define oils that have not
been subjected to any physical or chemical hydrogenation process that causes
changes in, or is designed to alter, the naturally occurring fatty acid
composition
of the oil, including, but not limited to, all of the processes outlined in
the
background. The term hydrogenation will be used to define oils that have been
subjected to hydrogenation process(es) that alter the naturally occurring
fatty
acid composition of the oil, including, but not limited to, all of the
processes
outlined in the background.
The term "non-chemically modified" will be used to describe any oil that
has not undergone any chemical modification, including but not limited to
interesterification, that results in an alteration of the naturally occurring
complement and structure of the oil's fatty acids. The term "chemical
11
CA 02555896 1999-05-06
modification" will be used to describe any oil that has undergone any chemical
modification that results in the alteration of the naturally occurring
complement
and structure of the oil's fatty acids, including, but not limited to,
interesteri-
fication outlined in the background.
Fractionation processes of vegetable oils are well known in the literature.
This process involves the separation of solids from an oil under reduced
temperature. The most widely practiced form of fractionation is that of
crystallization wherein a mixture of triglycerides is separated into two or
more
different melting fractions based on solubility at a given temperature.
Fractionation may be described either as "wet" or "dry" depending on the
presence or absence of solvent. Dry fractionation is achieved by pressing the
liquid oil from the solid fat by means of hydraulic pressure. Solvent
fractionation
(wet) is used to describe a process for the crystallization of a fraction from
a
mixture of triglycerides dissolved in a solvent. Fractions may be selectively
crystallized at different temperatures after which the fractions are separated
and
the solvent removed.
Wet fractionations are especially well known in the literature wherein an
organic solvent such as a hydrocarbon, e.g., hexane or a ketone like acetone
is
used, are described at some length. In these common wet fractionations,
multiple
stages are often applied. An optional first fractionation at about 10 -25 C
may he
performed, in which a fat rich in stearin may be recovered. For oils that are
low in
stearin this step may be omitted. A fractionation at about -5 to +5 C is
routinely
performed to separate out a fat fraction with a relatively solid character. A
liquid
fraction remains, which may also be recovered. Solvent is removed to yield the
final fat fractions from these separations. In this way, up to three fractions
may be
obtained from the most conventional vegetable fats. A first fraction
containing
most of the fully saturated triglycerides (i.e., the stearin fraction),
another fraction
which is rich in triglycerides that are composed of mixed saturated and
unsaturated fatty acyl moieties, and a liquid fraction which is rich in
unsaturated
triglycerides. In the instant case no attempt was made to obtain a stearin
fraction.
Industry suppliers use a variety of terms to categorize confectionary fats.
The more common terns include cocoa butter equivalents, cocoa butter
improvers, cocoa butter substitutes, cocoa butter replacers, hard butters,
coating
fats, compound coatings, center filling fats, and non-dairy fats. These fats
will
vary somewhat in melting behavior depending on the particular application for
which the fat is destined.
Alternatives or substitutes for cocoa butter are desirable due to an
uncertain supplies and volatile prices of the fluctuating cocoa-bean market.
As
early as the 1930's, confectioners attempted to use fats other than cocoa
butter in
12
CA 02555896 1999-05-06
their formulations. With newer technology, oil chemists and researchers have
developed fats with characteristics more closely resembling those of cocoa
butter.
These fats are known today as hard butters, developed from domestic vegetable
oils, as well as palm kernel, coconut, and palm oils. In addition, exotic oils
from
other parts of the world, such as shea, sal and illipe, can also be utilized.
Industry suppliers use a variety of terms to categorize hard butters, with
the most commonly accepted terms being cocoa butter equivalents or extenders,
cocoa butter substitutes, partial replacers, total replacers, modifiers and
extenders.
Cocoa butter equivalents and extenders (CBE) are all vegetable,
nonhydrogenated specialty fats containing almost the same fatty acids and
symmetrical unsaturated triglycerides as cocoa butter. They often are fully
compatible with cocoa butter, and usually are tropical in origin. They are
prepared by blending, fractionation and/or interesterification of oils derived
from illipe, shea, palm, and sal fat. Careful blending of fractions can yield
hard
butters with chemical and physical properties closely matching those of cocoa
butter. Cocoa butter equivalents and extenders can be mixed with cocoa butter
in any proportion up to 100% without altering the final products' melting
characteristics. CBE can be used in chocolate-type and non-chocolate type
confections, in coating and as molded products, as cool melting center fats,
or as
an extra coating layer below a chocolate coating to protect the outer layer
from
center fat migration.
It is also possible to tailor CBEs to have a higher solid fat content than
cocoa butter and these products are generally referred to as cocoa butter
improvers (CBI). These products are often used to improve soft cocoa butter
products destined for tropical climates and even out variations in the
properties
of cocoa butter from different parts of the world. Cocoa butter improvers melt
a
little more slowly than cocoa butter. The addition of 5% CBE or CBI to
chocolate products is permitted without label declaration in may countries
including Japan, Canada, South Africa, Norway, Sweden, Finland, Great
Britain, Ireland and Denmark.
Cocoa butter substitutes (CBS) are available in two types, lauric and non-
lauric. Lauric CBS are not compatible with cocoa butter and must therefore be
used with low fat cocoa powder. Because these fats replace cocoa butter they
are also known as cocoa butter replacers (CBR). They are usually derived from
the fractionation, blending, hydrogenation and/or interesterification of palm
kernel or coconut oil. Lauric CBS are subject to two problems. Because they
form eutectics with cocoa butter, the presence of even small amount of cocoa
butter may result in undesirable softening and/or fat bloom of the final
product.
13
CA 02555896 1999-05-06
They are also subject to hydrolysis which results in the release of free
lauric acid
giving the product an unpleasant soapy taste.
Non lauric CBS are produced by hydrogenation of liquid oils, frequently
followed by fractionation and or blending. The raw materials may include
sunflower, canola, cottonseed, palm or soy oils. Hydrogenation is used to
increase the solids content of the oil followed by fractionation to achieve a
product with a steep melting curve. Their use may be limited by poor eating
quality, flavor release, and mouth feel. Careful fractionation of products can
result in better eating quality, however the product is more expensive.
All of the fats described above can be used in various specialty
applications including coating fats, center filling fats, binders, lubricating
fats,
and substitute dairy fats including coffee whiteners and ice cream ingredient
products. Coating fats have a rapid meltdown in the mouth and yet do not melt
on the fingers. These fats have a relatively high solid-fat content at room
temperature. This level should be near or above 50%, as lower levels can lead
to a greasy or tacky feel. Fats that melt away quickly and completely at mouth
temperature, typically near 34-37 C, are sought for many chocolate type
applications. Confectionary fillings are essentially mixtures of center
filling fats
and sugar, together with a selection of flavor contributing ingredients. The
fats
in cream fillings may contain various levels of solid fat at room temperature,
depending on the type of applications. The filling fat should be compatible
with
the coating fats since they can migrate to the coating or vice versa, and this
process can affect product integrity and appearance because of softening and
fat
bloom. Coating and center products that are similar in composition are less
likely to be affected by fat migration.
Most confectionary products have a high fat content and as a result the
meltdown in the mouth is extremely critical. The standard of excellence in
this
respect is cocoa butter. The unique properties of cocoa butter result from the
chemical composition of the fat. Cocoa butter is composed of predominantly
symmetrical triglycerides with oleic acid in the 2-position. Cocoa butter has
a
melting range of 32 -35 C (90-95 F) and softens around 30 -32 C (86 -90 F).
The completely liquid fat display a tendency to supercool, an important factor
in
chocolate enrobing and molding. Cocoa butter characteristics include a
brittle,
non-greasy texture at room temperature, quick meltdown at mouth temperature,
excellent keeping qualities such as resistance to oxidation and a high
coefficient of
contraction on crystallization.
Since it is a natural fat, its composition shows normal variation depending
on what country (environment) the fat originates from. The three major fatty
acids of cocoa butter include palmitic (26%), stearic (34%), and oleic (34%).
The
14
CA 02555896 1999-05-06
physical characteristics of cocoa butter result from the arrangement of these
fatty
acids on the triglyceride. There exists a high degree of symmetrical
monounsaturated triglycerides which have the unsaturated fatty acid in the
2-position and saturated fatty acids in the I - and 3- positions. These
triglycerides
are most often 2-oleoyl-l-palmitoyl-3-stearoylglycerol (POS), and 2-oleoyl-1,3-
distearolyl glycerol (SOS), and 2-oleoyl-l,3-dipalmitoylglycerol (POP), with
POS
being present in the largest amount. These three major triglycerides have
crystal
forms with melting points just below body temperature.
In order for a fat product made from the fractionation of a high stearic
soybean oil, which can be in a non-hydrogenated, non-chemically modified form,
such oil has a C 18:0 content of at least 15% of the fatty acid moieties in
the oil to
be useful for confectionary applications and it should have a solid fat
content
profile comparable to the solid fat content profile of a confectionary fat.
Examples of solid fat content profiles that are suitable for practicing the
invention
include, but are not limited to, the following: an SFC of between 60 and 90 at
10 C and less than 21 at 35 C, an SFC of between 60 and 90 at 10 C and less
than
15 at 35 C, an SFC of between 60 and 90 at 10 C and less than 10 at 35 C, an
SFC of between 60 and 90 at 10 C and less than 5 at 35 C, a SFC of between 75
and 90 at 10 C and less than 5 at 35 C, and an SFC of between 80 and 90 at 10
C
and less than 5 at 35 C.
As used herein "confectionary fat" includes cocoa butter extenders, cocoa
butter substitutes/equivalents, cocoa butter replacers, hard butters, coating
fats,
compounds coatings, center filling fats, non-dairy fats, and specialty fats.
In
another aspect, this invention concerns making blended fat products suitable
as
confectionary fats. These blended fat products comprise any of the fat
products of
the invention, blended with other fat products, to produce a blended fat
product
suitable for use as a confectionary fat. As those skilled in the art will
appreciate,
the choice of other fat products will depend upon the intended used. For
example,
cocoa butter, and untreated or hydrogenated/chemically modified forms of
sunflower, canola, cottonseed, palm, soybean, illipe, shea, sal, palm kernel,
or
coconut oils can be used in combinations with the oils of the instant
invention.
As was noted above, these fats will vary somewhat in melting behavior
depending on the particular application for which the fat is destined. The
solid fat
content profiles for these specialty fats are well known to those skilled in
the art.
Examples of some of these can be found in Tables 1 and 2 and in the indicated
references, Many other sources list physical properties of specialty fats; see
for
example Table I in Stauffer, C.E. (1998) Cereal Foods World 43(3): 124, and
(anonymous) (1984) JAOCS 61(3): 468-472.
CA 02555896 1999-05-06
TABLE I
Solid Fat Content Profiles of Cocoa Butter and Hard Butter
Solid fat content
20 25 300 350 40
cocoa butter- Ghana l 76 69.6 45 1.1
cocoa butter-India] 81.5 76.8 54.9 2.3
cocoa butter-Brazil1 62.9 53.3 23.3 1
cocoa butter-Nigeria] 76.1 69.1 43.3 0
cocoa butter-Ivory Coast 1 75.1 66.7 42.8 0
cocoa butter-Malaysia] 82.6 77.1 57.7 2.6
cocoa butter-Sri Lankal 79.7 74.2 50.4 0.1
cocoa butter-unspecified2 91.6 80.8 74.4 54.4 0 0
hard butter/coating fate 93 66 51 36 11 0
hard butter/coating fate 83 56 43 28 9 0
anti-bloom chocolate filling fat3 66 27 10 1
anti-bloom chocolate filling fat4 49 24 7 1
1Shukla, V.K.S. (1997) INFOIUlf 8(2), 152-162
2Bailey's Industrial Oil and Fat Products, Vol. 2, 4rth Ed., John Wiley &
Sons, New York (1982)
3Loders Croklaan Product Information Sheet, Prestine 34F
3Loders Croklaan Product Information Sheet, Prestine 12F
5
TABLE 2
Solid Fat Content Profiles and Melting Points of Specialty Fats
Solid fat content
Wiley
Melting
10 15 200 25 30 35 40 45 Pt
wafer filler fat 76(6) 60(5) 45(5) 32(4) 17(2) l (l) <I 35(l)
sandwich cookie 53(4) 39(3) 28(3) 2](2) 1](1) 5(l) <1 39(1)
filling fat
hard butter 88(5) 75(5) 61(5) 47(4) 32(4) 10(l) 0 39(I)
coating fat 88(5) 75(5) 61(5) 47(4) 32(4) 10(l) 0 39(l)
puff paste 47(4) 40(4) 33(3) 27(2) 21(2) 16(l) 11(1) 7(1) 34(2)
margarine
]Stauffer, C.E., Fats and Oils, Practical Guide for the Food Industry, Eagan
Press
A good quality cocoa butter substitute is hard at ambient temperature, has
sharp melting characteristics like cocoa butter and has a high degree of
10 compatibility with cocoa butter and/or cocoa butter-milk fat blends.
Thus, most confectionary fats have a high solid fat content at room
temperature but also must melt quickly in the mouth. Cocoa butter is a unique
fat
which exhibits these types of physical properties. As a result, products made
with
16
CA 02558386 2006-08-10
cocoa butter such as chocolate, are solid at room temperature, have a
desirable
"snap" when broken, melt smoothly and rapidly in the mouth with no "waxy" or
greasy impression, and provide a cooling sensation on the palate and good
flavor
release. Contraction of the fat upon cooling is also important for molded
products. Cocoa butter is excellent in this regard.
A common method for determining the solidity of fats at critical
temperatures is the AOCS standard method Cdl6b-93(97) for Solid Fat Content
(SFC). This measurement is determined by low-resolution nuclear magnetic
resonance (NMR). For confectionary fats such as cocoa butter and specialty
fats,
the direct, serial, stabilizing method is practiced. The SFC NMR direct method
measures and compares signals from the solid and liquid phases. SFC is defined
as the ratio between the NMR response obtained from the hydrogen nuclei in the
solid phase versus the response obtained from nuclei in both the solid and the
liquid phases of the sample. The serial, stabilizing method utilizes a single
set of
samples that are tempered by melting and storage at 100 C for 15 minutes,
holding at 60 C for 5 minutes, 0 C for 90 minutes, 26 C for 40 hours, and
returned to 0 C for 90 minutes. The samples are then held at each recording
temperature for 60 minutes and moved to the next higher temperature
immediately
after determination of SFC. For a given sample, the percentage of solids is
measured across temperatures generally ranging between 10 and 40 C. The
entire SFC curve is required in order to understand the properties of the fat
at
different temperatures. The functionality of the fat is based on both the
solids
content and on the slope of the SFC curve at critical temperatures, for
example,
between room and body temperature. In this way, the plasticity of the fat can
be
predicted for temperatures critical to performance.
The melting point of a fat is also an important measurement. Since fats are
made up of a mixture of triglycerides which have different melting points, a
sharp
determination is not always possible. There are several methods common to the
industry which measure melting point including capillary melting point (AOCS
Cc1-25-93), Wiley melting point (AOCS Cc2-38-91), slip point (AOCS
Cc3-25-93), and dropping point (AOCS Ccl 8-80-95).
Any high stearic soybean oils having a C 18:0 content of at least 15% of
the fatty acid moieties in the oil can be used to practice the instant
invention.
More preferably the C 18:0 content should be at least 20% and most preferably
at
least 22% of the fatty acid moieties in the oil. Examples of suitable high
stearic
soybean oils can be found in U.S. Patent No. 5,557,037 which issued on
September 17, 1996_.
Another soybean oil which can be used to practice the present invention is
a high stearic, high oleic soybean oil having high oxidative stability and a
C18:0
17
CA 02558386 2006-08-10
content of at least 15% of the fatty acid moieties in the oil and a C 18:1
content of
greater than 55% of the fatty acid moieties in the oil. When this high
stearic, high
oleic oil is fractionated under conditions suitable for obtaining a fat
product useful
for confectionary applications two products are obtained. One product is
useful
for confectionary applications such as that described above and the second
product
is a high oleic soybean oil having high oxidative stability such as that
described in
Applicants' Assignee's PCT Application having publication number
WO 97/40698 which was published on November 6, 1997 the disclosure of which
may be referred to herein. A soybean oil with "high oxidative stability"
is a soybean oil that is less susceptible to oxidative degradation when
compared to
normal soybean oil.
Thus, another aspect of this invention concerns a method for making two
products wherein one product is a fat product suitable for confectionary
applications and the second product is a high oleic soybean oil having high
oxidative stability, said method comprising fractionating a high stearic, high
oleic
soybean oil having a C 18:0 content of at least 15% of the fatty acid moieties
in the
oil and a C 18:1 content of greater than 55% of the fatty acid moieties in the
oil
under conditions suitable for obtaining a fat product useful for confectionary
applications. The fractionated high stearic, high oleic soybean oil has an OSI
(110) greater than 25 hours.
A number of methods are well known to those skilled in the art for
determining oxidative stability. A commonly used method to evaluate the
stability of commercial oils is the AOCS method Cdl2b-92(93) Oxidative
Stability Index (OSI) which is measured automatically using a machine
manufactured by Omnion, Inc. of Rockland, MA, USA.
The OSI machine works by bubbling air through oil heated to 1 10 C. As
the oil oxidizes, volatile organic acids, primarily formic acid, is formed
which can
be collected in distilled water in a cell. The machine constantly measures the
conductivity of the distilled water and the induction period is determined as
the
time it takes for this conductivity to begin a rapid rise.
EXAMPLES
The following Examples are intended to illustrate the present invention
and do not constitute a limitation thereon. All temperatures are given in
Celsius
unless indicated otherwise. Solid Fat Content profiles listed in these
Examples
report the percentage of the total fat moieties that were determined to be in
the
solid phase at the indicated temperature.
18
CA 02555896 1999-05-06
EXAMPLE 1
Solid Fat Content determinations of cocoa butter and cocoa butter substitutes
Samples of 1) a commercial cocoa butter, 2) a hydrogenated, fractionated
lauric cocoa butter substitute, 3) a hydrogenated, interesterified lauric
cocoa
butter substitute, and 4) a confectioners coating, center fat were analyzed
for
SFC by AOCS method Cdl6b-93(97). The results are listed in Table 3. Sharp
drops in SFC between 30 and 35 C were indicative of a) the distinctive
behavior of cocoa butter and b) confectionary fats which maintain a
substantially
solid character at room temperature yet melt at a temperature close to body
temperature.
TABLE 3
Solid fat content of cocoa butter sample
Temperature: 10 15 20 25 30 35 40
SFC (%)
Cocoa butter 87.8 - 80.2 73.2 50.2 0 0
hydrogenated, fractionated lauric 89.5 85.6 80 70.9 36.4 0 0
cocoa butter substitute
hydrogenated, interesterified lauric 87.8 78.2 70.2 55 40 10.6 0
cocoa butter substitute
confectioners coating, center fat 88 81.4 72.9 66.4 50.5 20.4 1
Therefore, a fat product useful as a cocoa butter substitute, extender,
equivalent, improver, or replacer should have an SFC profile wherein the Solid
Fat Content is between 60 and 90 at 10 C, preferably between 75 and 90 at 10
C,
and most preferably between 80 and 90 at 10 C, and less than 21 at 35 C,
preferably less than 15 at 35 C, more preferably less than 10 at 35 C, and
most
preferably less than 5 at 35 C.
EXAMPLE 2
Preparation of oils from soybeans and analyses of fatty acid compositions
All of the oils used in these examples were prepared according to the
following laboratory scale method. Harvested soybeans were heated in the
microwave to 180 F, cooled to room temperature and cracked using a Roskamp
TRC 650-6 Crack and Roll. Soybean hulls were removed using a Kice Aspirator
and the remaining meats were heated to 180 F and flaked in a Roskamp TRC 912
Flake and Roll. Crude oil was extracted in a glass, water jacketed extraction
vessel heated to 60 for 45 minutes using a solvent to solids ratio of
approximately 4:1. The hexane/oil miscella was collected and the extraction
repeated. The miscella was desolventized using a rotary evaporator leaving
crude
oil.
19
CA 02555896 1999-05-06
A volume o1 an 85% phosphoric acid solution equal to 0.1 /t, (v/v) of the
crude oil was added and the solution heated to 65 -70 for 10 minutes while
stirring. Warm (60 ) NaOH (8% aqueous solution) was added dropwise to the oil
to neutralize the free fatty acids and the H3P04 with an additional 0.2% wt/wt
excess. The solution was stirred for five minutes and the solids separated by
centrifugation. The oil was water washed by adding hot water to 20% (v/v) as
the
sample was heated to 90 with rapid agitation. The oil and water were allowed
to
cool at room temperature for 10 minutes and then separated by centrifugation.
The oil was dehydrated using very rapid agitation under vacuum at 85 -95 for
30 minutes or until all moisture (bubbles, condensation) had been removed, The
vacuum was then broken with nitrogen. The oil was bleached by adding 2%
(,.vt/wt) Activated Bleaching Earth (AOCS ##Z1077) and the solution mixed
under
vacuum for 30 minutes at 85 -95 before cooling to 80 . The vacuum was broken
with nitrogen and I% (wt/wt) of diatomaceous earth was added and the mixture
filtered through a prepared bed of diatomaceous earth.
Citric acid was added to approximately 50 ppm, and the oil was
deodorized at 240 with steam (4 mL water per 100 g oil) in a glass deodorizer
for
approximately 1 hour. The oil was cooled to 80 with sparging, and it was
further
cooled to 40 under nitrogen. The refined, bleached, and deodorized oil was
stored frozen under a nitrogen atmosphere.
All of the fatty acid composition analyses described in these examples
were determined essentially by the methods described in AOCS Cc I c-89. Fatty
acid methyl esters were prepared as follows. Ten L oil or liquefied fat was
mixed with 1 mL hexane and 0.25 mL of a 3% sodium methoxide solution for
30 minutes. Acetic acid (0.1 mL of a 10% solution) was added, the sample was
mixed and the layers separated by centrifugation. The resulting fatty acid
methyl
esters extracted in the hexane layer were resolved by gas chromatography (GC).
Hewlett Packard 589000 (Wilmington, DE) equipped with a SP2340 column
(60 m, 0.25 mm ID, 0.20 micron film thickness) (Supelco, Bellefonte, PA).
Column temperature was 150 at injection and the temperature programmed from
150 to 200 at 2 C/min over 40 minutes. Injector and detector temperatures
were
215 and 230 , respectively. All compositional values reported are relative
values
calculated from the integrated areas measured by the GC detector.
EXAMPLE 3
Fractionation of a 22% stearic acid soybean oil
High stearic acid soybeans from the line designated L9216116-109 were
developed at DuPont from a pedigree (HST1.(HO2.HO4)).A2506 (which means
the progeny from a cross between H02 and H04, was crossed to FIST 1, and the
resulting progeny was crossed to a wild-type line, A2506 to produce
CA 02555896 1999-05-06
L9216116-109). The seeds produced have a higher stearic acid content and
lower linolenic acid content than conventional soybeans. The parents HST1,
H02, and H04 (see Table 6) are mutant lines selected by the mutagenesis
protocols outlined in U.S. Patent No. 5,710,365, except different soybean
lines
were used as starting materials for the mutagenesis, and selection was based
on
variation in fatty acid content instead of carbohydrate content. HSTI. is a
mutant
of line N85-2176 which is a high oleic, low linolenic line developed at North
Carolina State University by J. W. Burton (Kuhr et at., March 26, 1987 Release
Notice for N85-2124, N85-2131, and N85-2176. USDA Agric Res. Services).
HST1 differs from its parent N85-2176 by virtue of its abnormally high stearic
acid content (HST=high stearic), while retaining the lower linolenic acid
content of N85-2176. The high stearic mutation in HST1 suppresses (is
epistatic
to) the high oleic gene(s) that are present in the genetic background from
N85-2176. Therefore, HSTI is not high in oleic acid like N85-2176. The
stearic mutation in HST1 is allelic tofasa (see Table 6) in line A6 (W. Fehr
from Iowa State) and results in a similar phenotype when crossed into similar
backgrounds as the fasa allele. Crosses between N85-2176 and AS (W. Fehr
from Iowa State) confirm that N85-2176 contains an allele of the fan gene (see
Table 6) present in A5 that confers a similar low linolenic phenotype.
Therefore, HST1 contains both a high stearic mutation (allelic tofasa) and a
low
linolenic mutation (allelic to fan).
H02 is a DuPont proprietary mutant line selected from mutagenesis of the
line N85-2176. The mutagenesis protocol was essentially the same as the one
described in U.S. Patent No. 5,710,365, except that N85-2176 was used as the
starting material for tutagenesis, and selection was based upon variation in
fatty
acid content instead of carbohydrate content. H02 differs from N85-2176 (fan)
in
that it has a slightly higher oleic acid content (HO=high oleic), but retains
the low
linolenic acid content of N85-2176. Therefore, H02 contains an unnamed
mutation that confers higher oleic acid content than N85-2176 in addition to
the
fan gene.
H04 is a DuPont proprietary mutant line selected from mutagenesis of the
line A5. The mutagenesis protocol was essentially the same as the one
described
in U.S. Patent No. 5,710,365, except that A5 was used as the starting material
for
the mutagenesis, and selection was based upon variation in fatty acid content
instead of carbohydrate content. H04 differs from AS (fan) in that it has a
higher
oleic acid content (HO=high oleic), but retains the low linolenic acid content
of
AS. Therefore, H04 contains an unnamed mutation that confers higher oleic acid
content than A5 in addition to the fare gene.
21
CA 02555896 1999-05-06
It is believed that a derivative of A6 (fasa), or similar plant yielding seeds
with an oil composition comprising a high stearic and low linolenic acid
phenotype similar to that disclosed in the instant invention, would be useful
for
the methods described herein.
Mature seeds (5 1 g) from high stearic plants were dissolved in 510 ml
acetone and kept at 4 for 24 hours. The solid and liquid materials were
separated
using a cold jacketed Buchner funnel cooled to 4 un-ler vacuum. Acetone was
removed from both the solid and liquid fractions using rotary evaporation.
Solid
fat (5.8 g) and liquid oil (42 g) were recovered and analyzed for their
compositions.
The solid fat fraction was analyzed for SFC using AOCS Method
Cd 16b-93(97) at a variety of temperatures. Tables 4 and 5 show the
compositional and functional characteristics of the high stearic acid source
oil and
the solid and liquid fractions obtained from this oil. The solid fraction was
found
to be enriched in stearic acid (18:0) and arachidic acid (20:0) as compared to
the
source material. The SFC profile over the temperature range indicated a sharp
drop in the solid fat content between 30 and 35 , similar to the behavior of
authentic cocoa butter. This is in sharp contrast to the SFC profile of the
unfractionated soybean oil used as the starting material. The results obtained
in
this Example are compared to the SFC profile of cocoa butter found in Example
I
as shown in Figure 2.
TABLE 4
Fa acid compositions
16:0 18:0 18:1 18:2 18:3 20:0
22% stearic acid soybean oil 8.7 22.1 15.1 47.2 4.8 1.5
liquid fraction 9.1 17 16.6 49.7 5.1 1.4
solid fraction 4.8 55.1 7.3 27.2 2.5 2.5
TABLE 5
Solid fraction content as a function of temperature
Temperature 10 15 20 25 30 35 40
SFC of Unfractionated Oil 13.8 7.4 4.1 0.4 0 0 0
SFC of Solid Fraction 81.5 79.6 76.1 70 57.5 1.5 0
EXAMPLE 4
Soybeans with High Stearic and Hi h Oleic content
Crosses were performed between a soybean line which has elevated levels
of oleic acid in its seed fatty acids and a line which has elevated levels of
stearic
22
CA 02555896 1999-05-06
acid in its seed fatty acids. The high oleic line contains a transgene copy of
the
soybean fatty acid desaturase gene, gmFAD2-1 (Heppard, E.P. et al. (1996)
Plant
Phvsiol. 110:311-319), that results in co-suppression and therefore down
regulation of the gmFAD2-1 message level and is described in WO 97/40698.
Decreased expression of the FAD2-1 function leads to a decrease in activity of
delta-12 desaturase, and a decrease in the accumulation of poly-unsaturated
fatty
acids. The high oleic line is designated D2T and the typical fatty acid
profile of
its seed lipid is given in Table 6. The high stearate parent is a fatty acid
synthesis
mutant isolated from a mutagenized soybean seed population (U.S. Patent
No. 5,585,535), designated A6, and containing a fatty acid mutantfasa allele.
Its
typical seed lipid fatty acid profile is given in Table 6.
F 1 seeds obtained from the crosses were planted to obtain F 1 plants. The
Fl plants were then self pollinated to obtain F2 seeds that were segregating
for
both of the loci affecting the seed fatty acid profile. These F2 seeds were
planted
and the plants were self pollinated as in the previous generation. The
relative
content of the five main fatty acids in bulked seed samples from individual F2
plants was determined by gas liquid chromatography as described in
WO 94/11516. Remaining seed from F2 plants containing maximum stearic and
oleic acid content were selected, planted and allowed to self pollinate in
order to
obtain F3:4 seed. A sample of F4 seed from each F3 plant (F3:4 seed) was then
submitted for GC analysis so that individual F3:4 phenotypes could be
determined. F3:4 plant phenotypes tracing back to a common F2 plant ancestor
were then averaged to obtain mean phenotypes for F2-derived families (F2:4
family means). Single plants and family means that were highest in oleic and
stearic acid are shown in Table 6. The individual plant lines and the family
means
are presented in order of decreasing stearic acid in the seed fatty acids.
23
CA 02555896 1999-05-06
TABLE= 6
Seed fatty acid profiles from individual plants and F2:4 family means arising
from a
cross of the high oleic parent D2T and the high stearic parentJasa
Line ID or gene Individual Fatty Acid Content generation and type
modification I of total seed fatty acid) or reference
16:0 18:0 18:1 18:2 18:3
wild type 12 4 25 51 7 -
D2T 7 3 85 3 W094/11516
fasa 8 23 26 35 7 US 5,557,037
L9216 (1 16-109) 9 22 15 47 5 Example 3, this application
N85-2176 11 3 42 40 4 Kuhr et al., March 26, 1987.
USDA Agric. Res. Services
HSTI 9 28 15 43 5 Example 3, this application
H02 11 3 45 37 4 Example 3, this application
1404 11 3 45 37 4 Example 3, this application
fan 11 3 45 36 4 US 5,534,425
7SO-2334-1 7 26 61 1 3 F3:4 single plant
7SO-2293-2 6 26 61 2 3 F3:4 single plant
7SO-2293-1 6 25 62 2 3 F3:4 single plant
7SO-2303-5 6 24 66 0 2 F3:4 single plant
7S0-2339-1 6 24 64 I 3 F3:4 single plant
7SO-2295-1 6 24 64 I 3 F3:4 single plant
7SO-2331-3 7 24 63 I 3 F3:4 single plant
7SO-5097-3 6 20 67 2 3 F3:4 single plant
7SO-5097- I 6 20 66 2 3 F3:4 single plant
7SO-2356-1 6 18 72 0 3 F3:4 single plant
7SO-2306-2 6 18 71 2 3 F3:4 single plant
7SO-2305-3 6 18 70 2 2 F3:4 single plant
7SO-2310-3 6 18 70 2 3 F3:4 single plant
7SO-2293 6 24 63 2 3 F2:4 family mean of '9 plants
7SO-2334 6 23 65 I 3 F2:4 family mean of 8 plants
7SO-2339 6 23 65 I 3 F2:4 family mean of 7 plants
7SO-2379 6 19 70 1 2 F2:4 family mean of 3 plants
7SO-2305 6 18 71 2 3 F2:4 family mean of 10 plants
tThe Gene Modifications used in this Table refer to the following:
D2T refers to a delta-12 desaturase construct which is in a sense orientation,
the integration of
which results in a reduction of activity.
fasa refers to a gene for elevated seed stearic acid content.
L92161 16-109 refers to a line with high stearic acid content derived from
pedigree
(HSTI.(HO2.HO4)) .wild-type.
HSTI refers to a high stearic acid mutant line derived from N85-2176.
H02 refers to a high oleic acid mutant line derived from N85-2176.
H04 refers to a high oleic acid mutant line derived from AS.
24
CA 02555896 1999-05-06
The very low poly-unsaturated fatty acid phenotype arising from the D2T
parent is maintained in selected progeny from the cross as is the very high
level of
stearic acid coming from thefasa containing parent. The increased level of
stearic
acid in the selected progeny from the cross relative to the D2T parent causes
a
decrease in the oleic acid content.
The A6 mutant line of soybean which contains a defective fava allele and
was used in this cross is environmentally unstable. Environmentally unstable
describes a phenotype that is relatively variable, as a result of the
environmental
conditions in which a plant is grown. That instability leads to variation of
the
seed fatty acid composition in plants grown at different times and in
different
geographic locations. Oils produced from the D2T +fasa plants described in
this
example were found to vary in compositions as well. Examples 5 through 9
employ oils prepared from plants containing the D2T +fasa modifications as
starting materials for fractionations. Their compositions are given in Table
7.
TABLE 7
Compositions of oils used in Examples 5 through 9
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
Example 5 6.0 18.6 66.8 2.3 3.6 1.5
Example 6 5.4 17.6 67.6 3.4 3.7 1.4
Example 7 5.9 21.9 62.8 2.6 3.6 1.8
Example 8 5.2 16.2 70.8 1.7 3.9 1.2
Example 9 5.5 17.6 67.6 3.4 3.7 1.4
EXAMPLE 5
Fractionation of a high stearic acid /high oleic acid soybean oil
A sample of high stearic acid /high oleic acid soybean oil (50 g, containing
18.6% stearic acid and 66.8% oleic acid) as described in Table 7 above was
dissolved in 500 ml acetone and kept at 4 for 25 hours. The solid fat
fraction
(Solid Fraction, 9.3 g) was separated from the Liquid Fraction (37.4 g) using
a
cold jacketed Buchner funnel cooled to 4 under vacuum. Residual acetone was
removed from both the solid and liquid fractions as described in Example 3
prior
to weighing.
The fractions were analyzed for SFC and fatty acid composition as
described in Example 3. In addition. the dropping point of the solid fraction
was
determined using AOCS method Cc 18-80-95. The resulting data are presented in
Tables 8 and 9. The Solid Fraction recovered as described above was
CA 02555896 1999-05-06
characterized by a significant increase in the stearic acid content relative
to the
starting material, while the Liquid Fraction showed a modest enrichment of
oleic
acid from about 67% to about 77%. The SFC profile indicated the Solid Fraction
possessed a notable solid character from 10 -20 which then declined
particularly
rapidly in the temperature range 30 to 35 , indicating this fraction will
perform
well as a confectionary fat product.
TABLE 8
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
high stearic/high oleic acid soybean oil 6.0 18.6 66.8 2.3 3.6 1.5
Liquid Fraction 5.5 13.1 71.7 2.5 4.0 1.2
Solid Fraction 8.4 40.1 44.2 1.4 1.9 2.7
TABLE 9
SFC of Solid Fraction as a function of temperature
Temperature 100 150 200 25 30" 350 40
SFC of Unfractionated Oil 29.9 19.8 4.3 3.1 0.3 0.6 0.5
SFC of Solid Fraction 73.6 68.1 62.7 59.1 48.7 12.1 0
Dropping Point of Solid 31.6
Fraction
EXAMPLE 6
Single step fractionation of a high stearic acid /high oleic acid soybean oil
A sample of high stearic acid /high oleic acid soybean oil (50 g, containing
17.6% stearic acid and 67.6% oleic acid) as described in Table 7 above was
dissolved in 500 ml acetone and kept at 4 C for 25 hours. The solid fat
fraction
(Solid Fraction, 8.3 g) was separated by filtration under vacuum using a
jacketed
Buchner funnel cooled to 4 . A Liquid Fraction (38.1 g) was also recovered.
Residual acetone was removed from both the solid and liquid fractions as
described in Example 3 prior to weighing.
The fractions were analyzed as described in Example 5 and the resulting
data are presented in Tables 10 and 11. The Solid Fraction recovered as
described
above was characterized by a significant increase in the stearic acid content
relative to the starting material (17.6% to 39.6%), while the Liquid Fraction
showed a modest enrichment of oleic acid from about 68% to about 72% and a
concomitant decrease in the stearic acid content (to 12.5%). The SFC profile
indicated the Solid Fraction possessed a definite solid character from 10 -20
which then declined particularly rapidly in the temperature range 30 to 35 ,
indicating this fraction will perform well as a confectionary fat product.
26
CA 02555896 1999-05-06
TABLE 10
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
high stearic acid/high oleic acid 5.4 17.6 67.6 3.4 3.7 1.4
soybean oil
Liquid Fraction 5.1 12.5 72.4 3.7 4.1 1.2
Solid Fraction 7.4 39.6 44.6 2.0 1.9 2.8
TABLE 11
SFC of Solid Fraction as a function of temperature
Temperature 100 150 20 250 300 350 400
SFC of Solid Fraction 71.6 66.0 60.9 57.2 47.0 10.4 0
Dropping Point of Solid 32.2
Fraction
EXAMPLE 7
Fractionation of a high stearic acid /high oleic acid soybean oil
A sample of high stearic acid /high oleic acid soybean oil (50 g, containing
21.9% stearic acid and 62.8% oleic acid) as described in Table 7 above was
dissolved in 500 ml acetone and kept at 4 C for 23 hours. The solid fat
fraction
(Solid Fraction, 11.6 g) was separated from the Liquid Fraction (31.5 g) by
filtration under vacuum using a jacketed Buchner funnel cooled to 4 . Residual
acetone was removed from both the solid and liquid fractions as described in
Example 3 prior to weighing.
The fractions were analyzed as described in Example 5 and the resulting
data are presented in Tables 12 and 13. The Solid Fraction recovered as
described
above was characterized by a significant increase in the stearic acid content
relative to the starting material (21.9% to 40.1%), while the Liquid Fraction
showed an enrichment of oleic acid from about 63% to about 69% and a
concomitant decrease in the stearic acid content (to 15.5%). The SFC profile
indicated the Solid Fraction possessed a solid character from 10 -20 which
then
declined particularly rapidly in the temperature range 30 to 35 , indicating
this
fraction will perform well as a confectionary fat product.
TABLE 12
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
high stearic acid/high oleic acid soybean oil 5.9 21.9 62.8 2.6 3.6 1.8
Liquid Fraction 5.2 15.5 68.8 2.7 4.2 1.5
Solid Fraction 7.4 40.1 44.8 1.4 2.0 2.9
27
CA 02555896 1999-05-06
TABLE 13
SFC of Solid Fraction as a function of tcmpcraturc
Temperature 10 15 20 25 30 ' 35 40
SFC of Solid Fraction 71.7 65.5 59.8 56.2 45.9 9.8 0
Dropping Point of Solid 31.5
Fraction
EXAMPLE 8
Fractionation of a high stearic acid /high oleic acid soybean oil
A sample of high stearic acid /high oleic acid soybean oil (171 g,
S containing 16.2% stearic acid and 70.8% oleic acid) as described in Table 7
above
was dissolved in 1710 nil acetone and kept at 4 for 23 hours. The solid fat
fraction (Solid Fraction, 27 g) was separated from the Liquid Fraction by
filtration
under vacuum using a jacketed Buchner funnel cooled to 4 . Residual acetone
was removed from the solid fraction as described in Example 3 prior to
weighing.
The fraction was analyzed as described in Example 3 and the resulting data
are presented in Tables 14 and 15. The Solid Fraction recovered as described
above was characterized by a significant increase in the stearic acid content
relative to the starting material (16.2% to 35.3%). The SFC profile for the
solid
fraction recovered from this fractionation indicates that the material
possessed
solid character from 10--20 C and had minimal solidity at 35 and was
completely
melted at 40 , indicating this fraction will perform well as a confectionary
fat
product.
TABLE 14
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
high stearic/high oleic acid 5.2 16.2 70.8 1.7 3.9 1.2
soybean oil
Solid Fraction 6.9 35.3 50.8 1.1 2.1 2.2
TABLE 15
SFC of Solid Fraction as a function of temperature
Temperature 10 20 25 30 350 40
SFC of Solid Fraction 63.1 50.0 45.4 33.6 3.1 0
EXAMPLE 9
Fractionation of a high stearic acid /hiyh oleic acid soybean oil
A sample of high stearic acid /high oleic acid soybean oil (51 g, containing
17.6% stearic acid and 67.6% oleic acid) as described in Table 7 above was
dissolved in 510 ml acetone and kept at 4 for 24 hours. The solid fat
fraction
28
CA 02555896 1999-05-06
(Solid Fraction, 9.6 g) was separated from the Liquid Fraction by filtration
under
vacuum using a jacketed Buchner funnel cooled to V. Residual acetone was
removed from the solid fraction as described in Example 3 prior to weighing.
The fraction was analyzed as described in Example 3 and the resulting data
are presented in Tables 16 and 17. The solid fraction recovered as described
above was characterized by a significant increase in the stearic acid content
relative to the starting material (17.6% to 37.1%). The SFC profile for the
solid
fraction recovered from this fractionation indicates that the material
possessed
solid character from 10-20 C and had minimal solidity at 35 and was
completely
melted at 40 , indicating this fraction will perform well as a confectionary
fat
product.
TABLE 16
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0
high stearic/high oleic acid soybean oil 5.5 17.6 67.6 3.4 3.7 1.4
solid fraction 7.0 37.1 47.7 2.1 1.9 2.4
TABLE 17
SFC of Solid Fraction as a function of temperature
Temperature 10 20 25 30 350 40
SFC of solid fraction 66.4 54.1 49.8 38.5 4.6 0
EXAMPLE 10
Liquid Fractions From Fractionated Oils Exhibit High Oxidative Stability
Liquid fractions were prepared from high stearic and high stearic/high
oleic soybean oils as disclosed in Examples 3, 5, and 6 and were analyzed for
fatty
acid composition and oxidative stability by the method of OSI. The results are
shown in Table 18. The liquid fractions from high stearic/high oleic soybean
oils
were characterized by a significant decrease in linoleic acid (2.5%, 3.8% vs.
49.7%). The OSI values show an approximate five fold increase in oxidative
stability (33.2, 29.2, vs. 5.8) when compared with the liquid fraction from
high
stearic soybean oil.
29
CA 02555896 1999-05-06
TAB1,E- 18
Fatty acid composition and OSI values for liquid tractions
Fatty acid composition
16:0 18:0 18:1 18:2 18:3 20:0 OSI 0 10"C)
liquid fraction: high stearic 9.1 17.0 16.6 49.7 5.1 1.4 5.8
(22%) soybean oil
liquid frac'-on: high stearic 5.5 13.1 71.7 2.5 4.0 1.2 33.2
(19%)/ high oleic (67%)
soybean oil
liquid fraction: high stearic 5.1 12.5 72.4 3.8 4.1 1.2 29.2
(18%)/ high oleic (68%)
soybean oil