Note: Descriptions are shown in the official language in which they were submitted.
CA 02557452 2006-08-28
IMPROVED CATHETER AND SYSTEM FOR CONTROLLING SAME
FIELD OF THE INVENTION
This invention relates to catheters and in particular a haptically controlled
active catheter instrumented with shape memory alloy actuators and a three
degrees of
freedom force sensor.
BACKGROUND OF THE INVENTION
Angioplasty is a minimally invasive procedure that involves the insertion
of a catheter into the blood vessel for removal of blockages in blood flow. In
the
conventional approach, the catheter is inserted into the body through the
femoral artery
and is guided through the lumen of the blood vessel till it reaches a
blockage. A stent
(superelastic Shape Memory Alloy) is then deployed to open the blood vessel at
the
blockage and let normal blood flow resume. The surgeon is provided with images
that
are obtained either by X-ray imaging or by Magnetic Resonance Imaging. These
images enable the healthcare provider to track the end point of the catheter
(position in
absolute coordinates) in real time and determine the future course of
insertion.
There are a number of problems associated with the conventional way of
performing angioplasty. Specifically, the catheter insertion completely
depends on the
expertise and dexterity of the surgeon. In the case of intravascular
neurosurgery, the
catheter is pushed through extremely delicate and complex cranial blood
vessels to
treat aneurisms in the brain. The repeated insertion of the catheter through
several
1
CA 02557452 2006-08-28
trials could tear the blood vessel at the junction and cause heavy bleeding.
This could
also result in prolonged operating times and fatigue to clinicians and
patients.
The surgeon has no method of estimating the amount of force that is
being applied by the tip of the catheter on the walls of the blood vessel.
Excessive
pressure could rupture the blood vessel with dire consequences. Plaque could
also be
dislodged which may block blood vessels in the brain or heart and cause a
stroke or a
myocardial infarction.
The healthcare provider could have prolonged exposure to radiation or be
subjected to a high-level of noise caused by the machinery generating magnetic
fields
for MRI. These pose danger or discomfort to the healthcare providers who
perform the
procedure over a prolonged period of time.
Another problem with the present procedure of Angioplasty is restenosis.
The deployment of a stent at the site of a blockage only provides a temporary
solution
for resuming blood flow. In 40% of the procedures already done, the plaque
begins to
build up after a couple of years, a process called restenosis. In addition,
the stent,
made of superelastic Shape Memory Alloy, has a life of only 10 years. For
these
reasons, there may arise a need to perform repeated angioplasties for a single
patient.
Accordingly it would be advantageous to provide a catheter wherein the
movement of the tip can be controlled. Further it would be advantageous to
provide a
three degree of freedom force sensor that can be attached to a catheter.
SUMMARY OF THE INVENTION
2
CA 02557452 2006-08-28
The present invention describes a system for controlling a thin flexible
thermoplastic catheter. The system includes a plurality of shape memory alloy
filaments attached to the distal end of the catheter, each filament having a
phase and a
temperature; a means for receiving a strain value for at least one of the
filaments; a
means for determining the phase change that will result in the strain value,
whereby the
phase change is dependent on the temperature and the temperature is dependent
on a
voltage; and a means for setting the voltage in each filament thereby
resulting in the
bending of the catheter.
In another aspect of the invention there is provided a three dimensional
force sensor adapted to be attached to the distal end of a catheter. The
sensor
includes a two-dimensional optical position sensing detector, a spring, an
optical fibre
embedded within the catheter and a means for determining the position of the
beam
spot on the detector. The two-dimensional optical position sensing detector is
spaced
from the distal end of the catheter. The spring attaches the detector to the
catheter
whereby a force acting on the detector moves the detector relative to the
distal end of
the catheter. A light beam is emitted from the end of an optical fibre that is
embedded
within the lumen of the catheter.
Further features of the invention will be described or will become
apparent in the course of the following detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described by way of example only, with
3
CA 02557452 2006-08-28
reference to the accompanying drawings, in which:
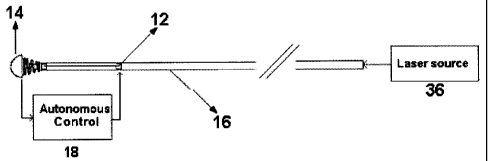
Fig. 1 is a schematic diagram of the autonomous control of the
instrumented catheter constructed in accordance with the present invention;
Fig. 2 is a schematic diagram of the instrumented catheter constructed in
accordance with the present invention and controlled by a haptic device;
Fig. 3A is a cross sectional view of the three-dimensional force sensor of
the present invention;
Fig. 3B is a perspective view of the three-dimensional force sensor of the
present invention;
Fig. 4 is a three dimensional rendering of the three-dimensional force
sensor of the present invention;
Fig. 5 is a cross sectional view of the active catheter showing multiple
shape memory alloy sections;
Fig. 6 is a cross sectional view of the active catheter showing patterned
shape memory alloy sections;
Fig. 7 is hysteresis characteristics of the shape memory alloy for varying
sinusoidal input;
Fig. 8 is a simulated result showing a closed loop shape memory alloy
response to a step reference input using the Linear Quadratic Regulator (LQR)
controller;
Fig. 9 is a simulated result showing a closed loop shape memory alloy
response to a sinusoidal reference input using the Linear Quadratic Regulator
(LQR)
4
CA 02557452 2006-08-28
controller;
Fig. 10 is an experimental result showing a shape memory alloy response
to a step reference input using the Linear Quadratic Regulator (LQR)
controller;
Fig. 11 is an experimental result showing a shape memory alloy response
to a sinusoidal reference input using the Linear Quadratic Regulator (LQR)
controller;
Fig. 12 is an experimental result showing a closed loop shape memory
alloy responses to two sinusoidal reference inputs with different DC offsets
using the
Linear Quadratic Regulator (LQR) controller;
Fig. 13 is an experimental result showing a closed loop shape memory
alloy responses to two sinusoidal reference inputs with different frequencies
using the
Linear Quadratic Regulator (LQR) controller;
Fig. 14 is a simulation result showing a closed loop shape memory alloy
responses to a step reference input using the H~ loop-shaping controller
Fig. 15 is a simulation result showing a closed loop shape memory alloy
response to a sinusoidal reference input using the H~ loop-shaping controller;
Fig. 16 is an experimental result showing a shape memory alloy response
to a step reference input using the H~ loop-shaping controller;
Fig. 17 is an experimental result showing a shape memory alloy response
to a sinusoidal reference input using the H~ loop-shaping controller;
Fig. 18 is a block diagram of a force control for autonomous guidance for
the catheter of the present invention;
Fig. 19 is a block diagram of a master-slave configuration for the catheter
5
CA 02557452 2006-08-28
of the present invention;
Fig. 20 is a picture of the haptic device for controlling the catheter of the
present invention; and
Fig. 21 is a cross-sectional diagram of the three-dimensional force sensor
of the present invention and showing a surgical tool attached thereto.
DETAILED DESCRIPTION OF THE INVENTION
Researchers in the past have tried to instrument the catheter with Shape
Memory Alloy actuators to actively control the tip of the catheter. This way
the catheter
can be smoothly guided into a branch in the blood vessel. The problem,
however, is
that the active catheters developed so far consist of actuators without any
position
feedback. Since most actuators used for the active catheter have a non-linear
behavior,
the control of the catheter by means of a joy-stick is extremely difficult and
inaccurate
and could cause damage to the blood vessels.
In one embodiment, this invention describes a completely autonomous
control of a catheter instrumented with Shape Memory Alloy (SMA) actuators 12
and a
novel three Degree Of Freedom (DOF) force sensor 14 on the tip of the catheter
16, as
shown in Figure 1. The force sensor 14 at the tip measures the magnitude and
direction of the force acting on the tip of the catheter 16 due to contact
with the blood
vessel when used in angioplasty. The force measured by the sensor 14 is fed to
a
control system to provide an input to the SMA actuators to minimize the force
acting at
the tip of the catheter. This way the force at the tip of the catheter is
minimized to
6
CA 02557452 2006-08-28
significantly reduce the likelihood of damage to epithelial cells of the blood
vessel.
In addition, the catheter 16 instrumented with SMA actuators 12 and the
3-DOF force sensor 14 can be used as a tool to perform minimally invasive
surgery and
therapy at remote locations. The instrumented catheter 12 can be interfaced to
a haptic
device 20 in a master-slave configuration, as shown in Figure 2. The haptic
device 20
should have a minimum of 3 DOF position output 24 and force input 26. The
surgeon
22, using the haptic device, provides the required position command to actuate
the
active catheter 16. The force felt at the tip of the catheter 16 is reflected
in 3DOFs to
the haptic device 20. In this way, the surgeon 22 has access to remote sites
in the
blood vessel using the instrumented catheter 16. The active catheter can also
be fitted
with microtools at the tip to perform on-site diagnosis, biopsies or plaque
removal. In
addition to application in angioplasty, the catheter can also be used for
fetal and
gastrointestinal surgeries.
The sections below describes the design for the 3-DOF force sensor, he
modeling of the Shape Memory Alloy Actuators, the LQR and H~ loop shaping
controller design, the autonomous control of the catheter, and the force
reflection to a
haptic device.
Sensor
Referring to figures 3 and 4, the sensor 14 designed in accordance with
the present invention is a miniaturized 3-DOF force sensor which is attached
to the tip
of the catheter 16. The sensor 14 is capable of measuring the magnitude and
direction
7
CA 02557452 2006-08-28
of the force acting on the tip. The sensor 14 consists of a spring 30
preferably a conical
spring (or any suitable elastic flexible material) - one end of which is
attached to an
optical fibre 32 which remains constrained to the catheter 16, the other end
is attached
to a 2-d Optical Position Sensing Detector (2d PSD) 34. The optical fibre 32
is coupled
to a source of LASER 36 or a high intensity LED. The 2d PSD, which could be a
quadrant detector or a lateral effect detector, consists of four independent
photodetectors, which measure the intensity of light falling on it. The
position sensor 34
provides four outputs from which the position of the beam spot on the surface
of the
PSD can be accurately calculated (Figure 3 and Figure 4). The position sensor
34 has
a hemispherical tip 35 attached thereto.
A force acting on the sensor 14 due to contact with the walls of the blood
vessel causes the spring 30 to bend. Since the optical fibre 32 and one end of
the
spring 30 are fixed to the catheter 16, the spring bends 30 relative to the
catheter 16
and as a result the position of the beam spot from the optical fibre 32 shifts
along the
surface of the 2d PSD 34. The deviation of the beam spot along the surface of
the 2d
PSD 34 can be used to calculate the amount of bending in the spring which is
proportional to the force acting on the tip of the catheter, by Hooke's Law.
Therefore,
the magnitude and direction of the force acting on the sensor can be measured
accurately. The total voltage from all four outputs is an indication of the
total intensity of
light falling laterally on the PSD.
The force sensor 14 is mounted on the tip of the catheter 16. The sensor
14 measures the force exerted by the artery walls on the tip of the catheter.
This force
8
CA 02557452 2006-08-28
reading can be used to autonomously guide the catheter to prevent damage to
the
epithelial cells of the artery or it can be reflected to a haptic device.
Actuators
Referring to figures 5 and 6, the actuators 12 guide the catheter 14
through the lumen of the blood vessel without causing damage to the inner
linings of
the blood vessel. The actuators 12 also facilitate easy movement of the
catheter 14 into
one of the blood vessels at a branch. The actuators 12 could be made of Shape
Memory Alloy (SMA) or Electro active polymers. SMA filaments (wire 40 and tube
44)
are used to build the actuators 12 on the catheter 14. The property of SMAs of
demonstrating a change in strain by a change in temperature is due to a solid-
state
phase change from the Martensite form to the Austenite form. Martensite is
relatively
softer and easily deformable and exists at a lower temperature. Heating by
means of
current or hot water results in a phase transition from the Martensite phase
to the
Austenite phase. In the Austenite phase, the SMA is more structured and is
harder and
stronger than in the Martensite phase.
The SMA actuators 12 on the instrumented catheter 16 could be either in
the form of a wire, tube or sheet. Since the machinability of SMA is low, the
wires or
filaments 40 should be microwelded to the stainless steel pads 42 which can be
glued
to the catheter, as illustrated in Figure 5. A minimum of three wires 40
placed at 1200 is
required to bend the catheter 16 in all directions. The bending can be created
by a
number of sections of SMA to create variable bending radius and angles. The
example
9
CA 02557452 2006-08-28
shown in Figure 1 shows one section of the SMA with one set of three wires 40
and the
example shown in Figure 5 shows two sections each having three wires 40. SMA
tubes and sheets can also be used to create the bending portion of the
catheter.
Patterns 44 can be laser-machined on the SMA tubes or sheets and can be
directly
glued to the catheter, as shown in Figure 6.
In order to control the bending angle of the catheter, the amount of strain
generated in the SMA actuators 12 should be controlled. For this, the physical
behavior
of the SMA should be described in dynamic equations. The model of the SMA set
out
below is based on the laws of physics. The modeling equations are as follows:
Modelling of Phase Transformations
Since an SMA exists in two states, it can be modelled as a two-state
system. There is a similarity between this system and an electron, which can
exist only
in two states - the positive spin or the negative spin. The Fermi-Dirac
statistics are
used to describe the number of electrons in the two states depending on the
energy of
the electron. The state of an SMA in the Martensite and Austenite forms using
the
same statistics has been modeled.
Two modelling equations were used based on whether the alloy is being
heated or cooled due to hysteresis with two different transition temperatures.
Since the
SMA is in the Martensite form at lower temperatures, the phase transformation
equation during heating is described by analogy with the Fermi-Dirac
statistics in the
form:
CA 02557452 2006-08-28
r
Stn
S l + rxj~( r~-r + .Tti,~rr') (1)
where 4 is the fraction of the Austenite phase, 4, is the fraction of the
Martensite phase
prior to the present transformation from Martensite to Austenite, T is the
temperature,
Tfa is the transition temperature from Martensite to Austenite, 6a is an
indication of the
range of temperature around the transition temperature Tfa during which the
phase
change occurs, a is the stress and Ka is the stress curve-fitting parameter
which is
obtained from the loading plateau of the stress-strain characteristic with no
change in
temperature.
On cooling, the Austenite phase gets converted to the Martensite phase
and the modelling equation during cooling is described by analogy with the
Fermi-Dirac
statistics in the form:
c
~a
1 + c .r,P( Tr,,, -T' + li (7)
(2)
where Ea is the fraction of the Austenite phase prior to the present
transformation from
Austenite to Martensite, T is the temperature, Tf,n is the transition
temperature from
Austenite to Martensite, 6m is an indication of the range of temperature
around the
transition temperature Tfm during which the phase change occurs, 6 is the
stress and
Km is the stress curve-fitting parameter which is obtained from the unloading
part of the
11
CA 02557452 2006-08-28
stress-strain characteristic.
Since the SMA is modelled as a two-component system, at any given
time, the sum of the mole fractions of the Austenite and Martensite phase is
1, i.e.,
~' + (3)
The time derivatives of Eqns.(1) and (2) are as follows:
For heating:
~ - ~ [cxp( Tf - T + r~-'7)] [ t - r~~"T]
~,rra o'a (Tn (4)
For cooling:
~~
~, '~
- [r,rp( Tt,>, + 7)][ T
- - .~1 raa~']
"Ia (r" a', (5)
Modelling of Temperature Dynamics
An SMA actuator is heated by the process of Joules heating by applying a
voltage across the SMA. The loss of heat from the SMA is through natural
convection.
Mathematically the dynamics of the temperature are given by equation (6).
12
CA 02557452 2006-08-28
1 [i 2
- - h A (T-L)]
m.cp h" (6)
where m is the mass per unit length, cp is the specific heat capacity, V is
the voltage
applied across the SMA, R is the resistance per unit length, h is the
coefficient of
convection, A = 7rd is the circumferential area of cooling, d is the diameter
of the wire, T
is the temperature and Ta is the ambient temperature. The coefficient h is
assumed to
have the characteristics of a second-order polynomial to enhance the rate of
convection at higher temperatures as observed in open-loop results:
lz = 110 + 1-12l'2 (7)
Constitutive Equation
The constitutive equation relating changes in stress, strain, temperature
and mole fraction is given by the following equation:
,3~ = L)e + Otl' + Q~ (8)
where a is the stress in the SMA, D is the average of the Young's Moduli for
the
Martensite and Austenite phases, s is the strain, At is the thermal expansion
factor and
S2 is the phase transformation contribution factor. The model explains the
hysteresis as
well as the minor loops in the hysteresis, as illustrated in Figure 7.
13
CA 02557452 2006-08-28
The dynamic characteristics of the SMA are completely defined by
equation (4) or (5) (heating or cooling), together with equations (6) and (8).
The ae is
defined as the integral of the error, i.e.,
L7e
(9)
where c is the strain of the SMA actuator and s,ef is the reference
trajectory. The
dynamic equations of the SMA along with equation (9) can be represented in the
state-
space form:
f~~' t) (10)
where
t
T
and u is the input voltage to the SMA wire. The nonlinear equations are
linearized
about a set of operating points (Eo, To, 40, uo) on the reference trajectory.
To obtain the operating points, so is chosen as the value of the reference
strain at that instant of time. To and 40 are obtained by integrating
equations (4) or (5)
14
CA 02557452 2006-08-28
and (8), depending on whether the SMA is being heated or cooled, for a given
value of
so. The value of uo is obtained from equation (6) for a given value of To,
assuming
steady-state conditions.
Equation (10) is linearized about the calculated operating points to obtain
linear models in the form:
.tl = A-7 + B ti (11)
= C,- (12)
where
A B
For the no-load case, which is the case considered here, a and 6 are equal to
zero. In
this case, the model given by (11) is not controllable since the number of
independently
controllable states is only 2. On removing the uncontrollable modes, the
following state
space model is obtained:
A'.z + B'u (13)
where
CA 02557452 2006-08-28
c
.!' =
Ãre
Controller Design
The force measured by the sensor at the tip of the catheter is provided as
input to the control strategy developed to provide an output to the SMA
actuators to
minimize the force at the tip of the catheter. The actuators could also be
activated by
the surgeon to guide the catheter into the branches of the blood vessel. The
control
scheme should provide fast and accurate control of the strain in the SMA. Two
control
strategies have been developed - a Gain Scheduled PI controller and a robust
H"' loop-
shaping controller using normalized coprime stabilization.
Gain Scheduled controller
For a PI controller, the feedback is of the form,
u = -.liP (c - f""f ~ - Iil0"~ + uo (14)
where Kp is the proportional gain and KI is the integral gain. Writing K=[KP
K,], the
gains are computed such that the resulting controller minimizes the quadratic
cost
function,
J(u) - ~~ (x TQx + JRu) dt
~
(15)
16
CA 02557452 2006-08-28
The choice of gains for minimizing J(u) are obtained by first solving for S in
the
algebraic Riccati equation:
A'TS+SA' -5B'R-1B'T8+t2=O
(16)
The matrix K is given by:
K = R-r B,7 S (17)
The simulation result for a step reference input is shown in Figure 8 and
that for a sinusoidal input is shown in Figure 9.
The experimental verification of the controller was done on a 700MHz
Windows based PC at a sampling rate of about 65Hz. Since the form of matrices
A'and B' are fairly simple, the solution for the Ricatti equation was obtained
in closed
form using MAPLE. This also greatly reduced the computation time by avoiding
matrix
decompositions like the Schur decomposition. The response of the SMA to a step
reference input is shown in Figure 10.
From the experimental results, it can be observed that the response time
for heating is approximately 1.0 second and for cooling is 2.1 seconds for a
0.012"
diameter SMA wire. The rate of heating and cooling is much higher for a
thinner wire
17
CA 02557452 2006-08-28
since the ratio of surface area to volume increases as the wire diameter is
reduced,
thereby increasing the rate of convectional cooling.
The experimental result for a sinusoidal reference input is shown in
Figure 11 which shows an excellent tracking of the reference trajectory by the
SMA in a
closed loop. The DC offset of the reference was also varied to check the
performance
of the controller in the entire operating range. The results are shown in
Figure 12. The
graphs show an excellent response over the entire operating region. The
frequency for
the reference input was also varied to check the performance of the controller
and the
SMA. The results are shown in Figure 13. The response of the controller is
satisfactory
for frequencies lower than 0.1 Hz.
Robust Controller
The LQR based PI controller shows excellent response to a step and a
sinusoidal reference input. The controller also has a good stability margin,
thereby
making the control law fairly insensitive to uncertainties in the parameters
of the model.
However, the controller is sensitive to unmodelled dynamics at high
frequencies and
thereby could lead to instability. For this reason, an H~ loop-shaping
controller using
normalized coprime stabilization is designed such that the gains are high when
the
model describes the SMA accurately and low at higher frequencies when the
model is
inaccurate. The loop-shaping controller, however, obtains a performance/robust
stability tradeoff.
The gain matrix K=[KP Ki]of the PI controller, as given by equation
18
CA 02557452 2006-08-28
(14), is computed by the loop-shaping procedure using normalized coprime
stabilization.
The nominal plant (SMA) is first shaped by using a precompensator W,
so that the singular values of the nominal plant G are shaped to a desired
open-loop
shape. The compensator also ensures that the shaped plant Gs = GW, is square
and
GS has no hidden modes.
For ensuring robust stabilization, sma, is calculated as,
-~.
1
~rnar = ll]f
1r-stabiEz.eaag K
(18)
[_~" ~i"] 11H ' < ~
(19)
where Ms and KS define the normalized coprime factors of G. An E= smaX is
selected
to form a stabilizing controller K~, which satisfies:
+GQK,s <~ f.-z = ~.
Itõ
X (20)
The final feedback controller K is obtained as the product of the
precompensator W,
19
CA 02557452 2006-08-28
and the H~ controller K, i.e.
.Fi = tV'j K,, (21)
The simulation of the robust controller and the SMA was done on
MATLABTM. The desired loop shape was chosen so that the gains are high at low
frequencies where the model describes the SMA accurately and the gains roll
off at -
20dB beyond the corner frequency, thereby ensuring a low gain at high
frequencies.
The value of the corner frequency was chosen to be 1000.424 rad.sec 1.
The simulation result for input consisting of step changes is shown in Figure
14 and for
a sinusoidal reference input is shown in Figure 15.
The experimental verification of the controller was done on a 700MHz
Windows based PC at a sampling rate of about 100Hz. The response of the SMA to
step changes is shown in Figure 16 and for sinusoidal reference input is shown
in
Figure 17.
Autonomous Control of the Active Catheter
In the previous section, modeling and robust control of SMA actuators
have been described. The simulation and experimental results show excellent
tracking
response for the SMA, thereby validating both the model and the control
scheme. In
autonomous control of an active catheter 16 fitted with SMA actuators 12 and a
3-DOF
CA 02557452 2006-08-28
force sensor 14, the force acting on the tip of the catheter 16 is regulated
so that there
is no damage to the epithelial cells of the artery. A force control algorithm
is
implemented to ensure that the tip interaction forces are minimized.
The miniature force sensor 14 at the tip of the catheter provides the
magnitude and direction of the force acting on the tip. This force is provided
as a
negative feedback signal to the robust control system, as shown in Figure 18.
The
sampling rate of the control algorithm is 100Hz, providing fast response to
minimize the
error between the force signal Fe and the desired force Fd. The autonomous
control of
an SMA actuator 12 based on the force felt at the tip prevents any damage to
the
epithelial cells of the arteries. In addition, the catheter autonomously
guides itself in the
proximity of the blockage to prevent accidental dislodging of the plaque due
to
application of excessive force. This ensures a higher success rate of
performing
angioplasty by reducing the risk of rupturing blood vessels or dislodging
plaque, which.
can cause a stroke or myocardial infarction if the plaque lodges itself in
minute arteries
of the heart or the brain.
Force reflection to a Haptic device
One of the major problems with the conventional way of performing
angioplasty is restenosis. In nearly 40% of procedures, the plaque begins to
rebuild at
the site after a couple of years, causing an obstruction to blood flow. In
addition, the
superelastic stent used for reopening the blood vessel has a lifetime of only
10 years.
21
CA 02557452 2006-08-28
Therefore, the patient would have to undergo multiple angioplasties during
his/her life
time. A solution to this problem is to perform microsurgery at the site of the
blockage.
The active catheter 16 fitted with SMA actuators 12 and a 3-DOF force
sensor 14 can be controlled by a master haptic device 20, as shown in Figure 2
and
Figure 20. The haptic device 20 provides position commands to the SMA
actuators 12.
A minimum of 3 DOFs in position and force are required for the haptic device
20. An
inverse dynamics control scheme under image guidance is used to control the
bending
of the active catheter. The force acting on the tip of the catheter 16 is
reflected to the
stylus of the haptic device 20. The surgeon can, therefore, feel these forces
caused by
interactions between the catheter 16 and the artery walls. In none of the
technologies
developed thus far has the surgeon had the capability of feeling the forces
exerted on
the tip of the catheter at a remote location. Micro tools 48 can be attached
to the tip of
the catheter 16 with 3 DOF position control and the ability to feel forces,
also in 3 DOF.
Using these tools, the surgeon can gently remove the plaque from the site
under image
guidance and force feedback, thereby leading to a permanent treatment of
plaque
buildup. This tool can also be used to perform various procedures like
biopsies and on-
site diagnosis, as shown in Figure 21.
In addition to the application in Angioplasty, the instrumented catheter 16
can also be used to perform minimally invasive fetal surgery. An abnormality
can arise
in some fetuses where the diaphragm separating the chest cavity from the
abdomen
does not completely develop. As a result the intestines begin to grow in the
chest
cavity, preventing the development of the lungs. This abnormality is called
Congenital
22
CA 02557452 2006-08-28
Diaphragmatic Hernia (CDH). In such cases, the surgeon can perform "fetal
endoscopic surgery" for CDH. In this procedure, a catheter along with surgical
instruments is inserted through tiny ports in the mother's womb. The surgeon
operates
on the fetus entirely within the womb. The trachea of the fetus is clipped to
block the
windpipe. This causes the trapped liquid in the lungs to aid in the growth and
expansion
of the lungs. This is a high risk operation and requires high dexterity and
maneuverability on the part of the surgeon to position the fetus and the
mother
accurately. In addition, it is extremely difficult to operate on a fetus
floating in the
amniotic fluid without any force or visual feedback. However, the procedure is
safer for
the mother and the fetus in comparison to open fetal surgery, where a large
incision
must be made in the uterus and in the amniotic sac, since it reduces the
chances of
pre-term labor. A premature birth would reduce the amount of time for the
fetus to
develop in the womb after prenatal surgery, and reduce its chances of
survival.
An instrumented catheter 16 as described in the invention would be
useful in guiding the catheter into the windpipe of the fetus under image and
haptic
guidance. This would reduce the time taken and the chances of injury to the
fetus in
inserting the instrumented catheter 16 into the fetus, thereby making the
procedure
more reliable and efficient and reducing the amount of stress to the surgeon.
The primary application of this invention is to insert a catheter
instrumented 16 with a 3-DOF force sensor 14 and Shape Memory Alloy actuators
12
smoothly into an artery and guide it through the lumen of the blood vessel
till the
catheter 16 reaches a blockage. The stent is then deployed to resume normal
blood
23
CA 02557452 2006-08-28
flow. The 3-Degrees of Freedom (DOF) force sensor 14 measures the magnitude
and
direction of the force being applied. It could be used for a number of other
applications.
Specifically it could be used at the tip of a catheter to measure the forces
exerted by
the walls of the blood vessel on the tip of the catheter. It could also be
used as a
minimally invasive tool to measure the force exerted by the tool during
surgery. As well
it could be used to study soft tissue properties by measuring accurately the
forces
exerted by the soft tissue during deformation of the tissue.
The Shape Memory alloy actuators 12 have a number of advantages.
Specifically, SMA actuators have large plastic deformations which can be
recovered;
can generate large load forces; have high power to weight ratio; have low
driving
voltages; have no moving parts; are noiseless; can be used in compact
workspace; and
are biocompatible and corrosion resistant.
The modeling and accurate control of the SMA actuators 12 enables its
use for a number of applications. Specifically it could be used as an active
catheter,
biomedical forceps, tissue stapler, stent, and minimally invasive tools.
As used herein, the terms "comprises" and "comprising" are to construed
as being inclusive and opened rather than exclusive. Specifically, when used
in this
specification including the claims, the terms "comprises" and "comprising" and
variations thereof mean that the specified features, steps or components are
included.
The terms are not to be interpreted to exclude the presence of other features,
steps or
components.
It will be appreciated that the above description related to the invention by
24
CA 02557452 2006-08-28
way of example only. Many variations on the invention will be obvious to those
skilled
in the art and such obvious variations are within the scope of the invention
as described
herein whether or not expressly described.