Note: Descriptions are shown in the official language in which they were submitted.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 1 -
TREATMENT OF HAEMORRHAGIC SHOCK USING COMPLEMENT Sa RECEPTOR
INHIBITORS
This application claims priority from Australian
provisional application No.2003902354 dated 15th I~ay 2003.
FIELD OF THE INVENTION
This invention relates to the treatment of
haemorrhagic shock with novel cyclic peptidic and
peptidomimetic compounds which have the ability to
modulate the activity of G protein-coupled receptors. The
compounds preferably act as antagonists of the C5a
receptor, and are active against C5a receptors on
polymorphonuclear leukocytes and macrophages.
BACKGROUND OF THE INVENTION
All references, including any patents or patent
applications, cited in this specification are hereby
incorporated by reference. No admission is made that any
reference constitutes prior art. The discussion of the
references states what their authors assert, and the
applicants reserve the right to challenge the accuracy and
pertinency of the cited documents. It will be clearly
understood that, although a number of prior art
publications are referred to herein, this reference does
not constitute an admission that any of these documents
forms part of the common general knowledge in the art, in
Australia or in any other country.
G protein-coupled receptors are prevalent
throughout the human body, comprising approximately 60% of
known cellular receptor types, and mediate signal
transduction across the cell membrane for a very wide
range of endogenous ligands. They participate in a
diverse array of physiological and pathophysiological
processes, including, but not limited to those associated
with cardiovascular, central and peripheral nervous
system, reproductive, metabolic, digestive, immunological,
inflammatory, and growth disorders, as well as other cell-
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
regulatory and proliferative disorders. Agents which
selectively modulate functions of G protein-coupled
receptors have important therapeutic applications. These
receptors are becoming increasingly recognised as
important drug targets, due to their crucial roles in
signal transduction (G protein-coupled Receptors, IBC
Biomedical Library Series, 1996).
~ne of the most intensively studied G protein-
coupled receptors is the receptor for CSa. C5a is one of
the most potent chemotactic agents known, and recruits
neutrophils and macrophages to sites of injury, alters
their morphology; induces degranulation; increases calcium
mobilisation, vascular permeability (oedema) and
neutrophil adhesiveness; contracts smooth muscle;
stimulates release of inflammatory mediators, including
histamine, TNF-oc, IL-1, IL-6, IL-8, prostaglandins, and
leukotrienes, and of lysosomal enzymes; promotes formation
of oxygen radicals; and enhances antibody production
(Gerard and Gerard, 1994).
Agents which limit the pro-inflammatory actions
of C5a have potential for inhibiting both acute and
chronic inflammation, and its accompanying pain and tissue
damage. Because such compounds act upstream from the
various inflammatory mediators referred to above, and
inhibit the formation of many of these compounds, they may
have a more powerful effect in alleviating or preventing
inflammatory symptoms .
In our previous applications No.PCT/AU98/00490,
we described the three-dimensional structure of some
analogues of the C-terminus of human CSa, and used this
information to design novel compounds which bind to the
human C5a receptor (CSaR), behaving as either agonists or
antagonists of CSa. It had previously been thought that a
putative antagonist might require both a C-terminal
arginine and a C-terminal carboxylate for receptor binding
and antagonist activity (Konteatis et a1, 1994). We
showed that in fact a terminal carboxylate group is not
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 3 -
generally required either for high affinity binding to
CSaR or for antagonist activity. Instead we found that a
hitherto unrecognised structural feature, a turn
conformation, was the lcey recognition feature for high
affinity binding to the human C5a receptor on neutrophils.
As described in our International application
PCT/AU0101427, we used these findings to design
constrained structural templates which enable hydrophobic
groups to be assembled into a hydrophobic array for
interaction with a C5a receptor. The entire disclosures
of these specifications are incorporated herein by this
reference .
Shock is a condition of major haemodynamic and
metabolic disturbance which may result from a number of
causes, and is characterised by failure of the circulatory
system to maintain adequate perfusion of vital organs with
blood. It may result from inadequate blood volume,
inadequate cardiac function or inadequate vasomotor tone.
Haemorrhagic shock caused by inadequate blood volume, also
known as hypovolaemic shock or volume deficiency shock,
results from major haemorrhage, which can have a very wide
range of underlying causes, such as trauma, uncontrollable
bleeding in relation to childbirth or as a result of a
nosebleed, blood-clotting disorders such as haemophilia,
surgical interventions, congenital defects such as
aneurysms, or gastrointestinal conditions such as
perforated ulcers.
In many cases, major haemorrhage is very
difficult to treat, and a variety of interventions has
been employed in addition to transfusion, restoration of
blood volume and other conventional supportive measures.
These include arterial embolization, emergency surgery,
and pharmacological agents such as sulprostone,
somatostatin, and vasopressin. The primary interventions
are directed to stopping the bleeding and to replacing the
lost blood volume, for example using blood transfusion,
infusion with isotonic or hypertonic saline, or blood
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 4 -
substitutes, and the secondary treatment is related to
alleviation or minimization of the sequelae of shock.
Treatment of haemorrhagic shock involves maintaining blood
pressure and tissue perfusion until bleeding is controlled.
Different resuscitation strategies have been used to
maintain the blood pressure in trauma patients until
bleeding is controlled. However, while maintaining blood
pressure may prevent shock, it may worsen bleeding.
Consequently a fine balance between these considerations
must be maintained. If shock is prolonged the
cardiovascular system may suffer damage, so that cardiac
output declines as the result of positive feedbacks and
th4 shock may become irreversible.
The treatments may be of limited effectiveness,
and may have serious side effects. For example, despite
successful surgery and intensive care support, the repair
of ruptured abdominal aortic aneurysm (RAAA) is associated
with a mortality rate of 50-75% (Adam et al, 1999). A
variety of agents, including immune regulating hormones
(Hollis-Eden Pharmaceuticals, Inc) and various blood
substitutes, such as diaspirin cross-linked haemoglobin
and other haemoglobin forms, are in various stages of
clinical trial, with mixed success.
The combined injury of haemorrhagic shock and
lower torso ischaemia-reperfusion injury initiates a
systemic inflammatory response syndrome, which is
characterised by increased microvascular permeability and
neutrophil sequestration, leading to multiple organ
dysfunction syndrome (MODS). MODS is the primary cause of
70% of such deaths, and a major contributory cause of the
remainder (Harris et al, 1991). Using a rat model of
ruptured abdominal aortic aneurysm, it has been shown that
haemorrhagic shock paired with supramesenteric aortic
clamping results in local intestinal and remote lung
injury, and that this can be attenuated by reducing
neutrophil adherence using a monoclonal antibody directed
against CD18 integrin (Boyd et al, 1999).
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 5 -
Reperfusion of the ischaemic lower torso
following prolonged ischaemia initiates a systemic
inflammatory response syndrome, characterised by pro-
inflammatory cytokines (Groeneveld et al, 1997) and
increased circulating polymorphonuclear leucocyte (PMN)
activation (Barry et al, 1997). Pulmonary sequestration
of activated neutrophils is followed by acute pulmonary
microvascular injury (Welbourne et al, 1991), acute
respiratory distress syndrome (Paterson et al, 1989), and
a high subsequent mortality. High circulating levels of
pro-inflammatory cytokines responsible for leukocyte
activation, such as tumour necrosis factor (TNF)-a,
interleukin-6 and interleukin-8, and of endotoxin (Baigrie
et al, 1993) have been demonstrated after repair of RAAA
(Roumen et al, 1993). We and others have previously shown
that lower limb ischaemia reperfusion injury is associated
with increased intestinal permeability, endotoxaemia, and
a systemic inflammatory response associated with acute
lung injury (Roumen et al, 1993; Harkin, D'Sa et al, 2001;
Harkin, Barros et al, 2001; Yassin et al, 1997).
Severe haemorrhage and trauma, in conjunction
with the syndrome of ischaemia-reperfusion injury,
activate the complement cascade, and the degree of
activation of the complement system correlates with the
severity of injury, and the likelihood of development of
multiple organ failure and ultimate death.
The complement system is a major contributor to
the inflammatory response in ruptured abdominal aortic
aneurysm (Lindsay et al, 1999), and has been reported to
mediate injury in experimental lower limb and intestinal
ischaemia-reperfusion injury (Rubin et al, 1990; Williams
et al, 1999).
Activated products of the classical complement
pathway, such as C5a and C3a, are potent inflammatory
mediators with myriad effects, including alteration of
blood vessel permeability and tone, leukocyte chemotaxis,
and activation of multiple inflammatory cell types. The
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 6 -
role of complement in some inflammatory tissue injury
conditions is supported by the attenuation of such injury
using anti-C5 antibody (Piccolo et al., 1999) and a C5a
receptor (CSaR) antagonist (Arumugam et al, 2003).
However, in contrast to this it has been reported that
lung injury induced by limb isehaemia is mediated by
leukotrienes, not by complement (Klausner et al, 1989).
Moreover, the role of complement in inflammatory tissue
injury after ruptured abdominal aortic aneurysm is still
largely unknown.
In studies using a rat model to examine
haemodynamic and metabolic recovery from prolonged and
profound haemorrhagic hypertension, it was found that
haemorrhage and resuscitation resulted in complement
consumption, and that prior depletion of circulating
complement levels protected the animals from shock, as
measured by mean arterial blood pressure and metabolic
acidosis. In contrast, administration of an exogenous
complement activator or inhibition of complement breakdown
exacerbated the injury. Although the authors concluded
that it was likely that C5a played a crucial role, they
considered that until agents which specifically neutralise
.CSa without affecting the activities of the parent
molecule C5 were available, this could not be confirmed.
In particular, C3a was also potentially implicated
(Younger et al, 2001). Others have found that the
intestinal and lung injury following haemorrhagic shock
and reperfusion can be minimised by reducing neutrophil
adherence with a monoclonal antibody directed against CD18
integrin (Boyd et al, 1999). The plethora of inflammatory
agents which have been identified in ischaemia-reperfusion
syndrome means that it is very difficult to identify the
most effective target for intervention. This difficulty
is reflected in the wide variety of candidate targets and
agents discussed at the 6th World Congress on Trauma,
Inflammation, Shock and Sepsis held in March 2004
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
7 -
(http://www.trauma-shock-sepsis-congress-munich-
2004.org/lis.html.
Therefore there is a great need in the art for
effective, non-toxic agents which preferably do not
require administration by injection, and which can be
produced at reasonable cost.
Glycoforms of the soluble complement receptor
type 1 (CR1) have been proposed for use in the treatment
of complement-mediated disorders and of shock. The
soluble CR1 fragments were functionally active, bound C3b
and/or C4b, and demonstrated factor I cofactor activity,
depending upon the regions they contained. Such
constructs inhibited the consequences of complement
activation, such as neutrophil oxidative burst,
complement-mediated haemolysis, and C3a and C5a production
(US patents No 5456909, No 5807844 and No 5858969).
However, to our knowledge none of these approved or
experimental agents for treatment of shock, and in
particular no small molecule agent, targets the C5a
receptor.
SUMMARY OF THE INVENTION
Due to the current uncertainty as to the nature
of the complement involvement in haemorrhagic shock, we
tested the possible inhibitory effects of a specific
complement inhibitor in an animal model of ruptured aortic
aneurysm, a condition which causes haemorrhagic shock. We
now show for the first time that a specific inhibitor of
the C5a receptor is able to ameliorate signs of damage in
an animal model of haemorrhagic shock. This is the first
reported case of a small molecule inhibitor of the
complement system being used to modulate pathology in a
model of haemorrhagic shock.
According to a first aspect, the invention
provides a method of treatment of haemorrhagic shock,
comprising the step of administering an effective amount
of an inhibitor of a C5a receptor to a subject in need of
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
_ g -
such treatment.
Preferably the inhibitor is a compound which
(a) is an antagonist of a C5a receptor,
(b) has substantially no agonist activity, and
(c) is a cyclic peptide or peptidomimetic compound of
Formula I
O
~N
N H
H
N
O NHS O
O
E
F
where A is H, alkyl, aryl, NHS, NH-alkyl,
N(alkyl)2, NH-aryl, NH-acyl, NH-benzoyl, NHS03, NHSO~-
alkyl, NHSO~-aryl, OH, O-alkyl, or 0-aryl;
B is an alkyl, aryl, phenyl, benzyl, naphthyl or
indole group, or the side chain of°a D- or L-amino acid
such as L-phenylalanine or L-phenylglycine, but is not the
side chain of glycine, D-phenylalanine, L-
homophenylalanine, L-tryptophan, L-homotryptophan, L-
tyrosine, or L-homotyrosine;
C is a small substituent, such as the side chain
of a D-, L- or homo-amino acid such as glycine, alanine,
leucine, valine, proline, hydroxyproline, or thioproline,
but is preferably not a bulky substituent such as
isoleucine, phenylalanine, or cyclohexylalanine;
D is the side chain of a neutral D-amino acid
such as D-Leucine, D-homoleucine, D-cyclohexylalanine, D-
homocyclohexylalanine, D-valine, D-norleucine, D-homo-
norleucine, D-phenylalanine, D-tetrahydroisoquinoline, D-
glutamine, D-glutamate, or D-tyrosine, but is preferably
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 9 -
not a small substituent such as the side chain of glycine
or D-alanine, a bulky planar side chain such as D-
tryptophan, or a bulky charged side chain such as D-
arginine or D-Lysine;
E is a bulky substituent, such as the side chain
of an amino acid selected from the group consisting of L-
phenylalanine, L-tryptophan and L-homotryptophan, or is L-
1-napthyl or L-3-benzothienyl alanine, but is not the side
chain of D-tryptophan, L-N-methyltryptophan,
L-homophenylalanine, L-2-naphthyl L-
tetrahydroisoquinoline, L-cyclohexylalanine, D-leucine, L-
fluorenylalanine, or L-histidine;
F is the side chain of L-arginine, L-
homoarginine, L-citrulline, or L-canavanine, or a
bioisostere thereof, ie. a side chain in which the
terminal guanidine or urea group is retained, but the
carbon backbone is replaced by a group which has different
structure but is such that the side chain as a whole
reacts with the target protein in the same way as the
parent group; and
X is - (CHI ) nNH- or (CHI ) n-S-, where n is an
integer of from 1 to 4, preferably 2 or 3; -(CH~)20-;
- ( CHI ) 30- ; - ( CH2 ) 3- ; - ( CHa ) 4- ; . -CH~COCHRNH-; or
-CHI-CHCOCHRNH-, where R is the side chain of any common or
uncommon amino acid.
In C, both the cis and trans forms of
hydroxyproline and thioproline may be used.
Preferably A is an acetamide group, an
aminomethyl group, or a substituted or unsubstituted
sulphonamide group.
Preferably where A is a substituted sulphonamide,
the substituent is an alkyl chain of 1 to 6, preferably 1
to 4 carbon atoms, or a phenyl or toluyl group.
In a particularly preferred embodiment, the
compound has antagonist activity against CSaR, and has no
C5a agonist activity.
The compound is preferably an antagonist of C5a
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 10 -
receptors on human and mammalian cells including, but not
limited to, human polymorphonuclear leukocytes and human
macrophages. The compound preferably binds potently and
selectively to C5a receptors, and more preferably has
potent antagonist activity at sub-micromolar
concentrations. Even more preferably the compound has a
receptor affinity IC50<25~1M, and an antagonist potency
IC50<1~,M.
Most preferably the compound is compound 1
(PMX53), compound 33 (AcF[OP-DPhe-WR]), compound 60
(AcF[OP-DCha-FR]) or compound 45 (AcF[OP-DCha-WCit])
described in International Patent Application
No. PCT/AU02/01427, or is HC-[OPdChaWR](PMX205),
AcF-[OPdPheWR](PMX273), AcF-[OPdChaWCitrulline]( PMX201)
or HC-[OPdPheWR]( PMX218).
The inhibitor may be used in conjunction with one
or more other agents for the treatment of haemorrhagic
shock, including but not limited to blood substitutes,
vasopressin, somatostatin, terlipresin and anti-nitric
oxide agents.
The compositions of the invention may be
formulated for oral, parenteral, inhalational, intranasal,
rectal or transdermal use, but parenteral, and especially
intravenous formulations are preferred. It is expected
that most if not all compounds of the invention will be
stable in the presence of metabolic enzymes, such as those
of the gut, blood, lung or intracellular enzymes. Such
stability can readily be tested by routine methods known
to those skilled in the art.
~ Suitable formulations for administration by any
desired route may be prepared by standard methods, for
example by reference to~well-known textbooks such as
Remington: The Science and Practice of Pharmacy, Vol. II,
2000 (20th edition), A.R. Gennaro (ed), Williams & Wilkins,
Pennsylvania.
It is contemplated that the invention is
applicable to the treatment of shock resulting from major
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 11 -
haemorrhage of any origin, including but not limited to
trauma, rupture of an aneurysm, uncontrollable epistaxis,
viral haemorrhagic fevers such as dengue, Lassa, Marburg
or Ebola virus, uterine haemorrhage during or after
delivery, haemorrhage during or after surgery, haemorrhage
resulting from gastrointestinal ulcers or oesophageal
varices, or of the lower gastrointestinal tract, eg.
diverticular haemorrhage, haemorrhage secondary to
invasion of cancer, haemorrhage resulting from bleeding
diatheses, eg. haemophilia, idiopathic thrombocytopaenic
purpura and the like, and haemorrhage associated with
thrombolytic therapy, eg. with agents such as warfarin,
aspirin, plasminogen activator, streptokinase or
urokinase.
While the invention is not in any way restricted
to the treatment of any particular animal or species, it
is particularly contemplated that the method of the
invention will be useful in medical treatment of humans,
and will also be useful in veterinary treatment,
particularly of companion animals such as cats and dogs,
livestock such as cattle, horses and sheep, and zoo
animals, including non-human primates, large bovids,
felids, ungulates and canids.
The compound may be administered at any suitable
dose and by any suitable route. The route of
administration is preferably parenteral, for example i.v.,
so that effective blood concentrations of the drug are
reached as quickly as possible, because of the gravity of
the condition, and because shunting of the blood away from
the non-vital organs such as the stomach would reduce
absorption from enteral routes. In general i.v.
administration is preferred.
The effective dose will depend on the nature of
the condition to be treated, and the age, weight, and
underlying state of health of the individual treatment.
This will be at the discretion of the attending physician
or veterinarian. Suitable dosage levels may readily be
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 12 -
determined by trial and error experimentation, using
methods which are well known in the art.
BRIEF DESCRIPTION OF THE FIGURES
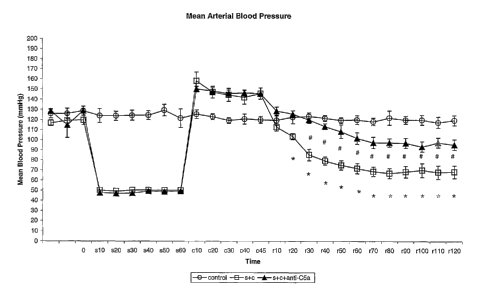
Figure 1 summarises the mean arterial pressure
results and fluid resuscitation requirements of the
animals in each group. A. Mean arterial blood pressure.
B. Fluid resuscitation requirements.
Figure 2 compares the lung permeability index
(LPI) in rats from each group.
Figure 3 shows the change in intestinal
permeability with time after removal of the clamp.
Figure 4 shows myeloperoxidase activity in
samples of lung and intestin. A. Lung. B. Intestine.
Figure 5 shows cytokine levels in samples of gut
tissue from animals of each group. A. TNF-oc. B. IL-6.
Figure 6 shows cytokine levels in lung tissue
from animals of each group. A. TNF-oc. B. IL-6.
DETAILED DESCRIPTION OF THE INVENTION
The invention will now be described by way of
reference only to the following general methods and
experimental examples.
In the claims which follow and in the description
of the invention, except where the context requires
otherwise due to express language or necessary
implication, the word "comprise" or variations such as
"comprises" or "comprising" is used in an inclusive sense,
i.e. to specify the presence of the stated features, but
not to preclude the presence or addition of further
features in various embodiments of the invention.
As used herein, the singular forms "a", "an'°, and
"the'° include plural reference unless the context clearly
dictates otherwise. Thus, for example, a reference to "an
enzyme'° includes a plurality of such enzymes, and a
reference to "an amino acid" is a reference to one or more
amino acids. Unless defined otherwise, all technical and
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 13 -
scientific
terms
used
herein
have
the same
meaning
as
commonl y understood by one of ordinary skill in the art
to
which his invention belongs. Although any materials and
t
methods similar or equivalent to those described herein
can be used to practice or test the present invention, the
preferr ed materials and methods are now described.
Abbreviations used herein are as follows:
AAA abdominal aortic aneurysm
Cit citrulline
dCha D-cyclohexylamine
DPhe D-phenylalanine
IL-6 interleukin-6
ip intraperitoneal
iv intravenous
LPS ~ lipopolysaccharide
MAP mean arterial pressure
MPO myeloperoxidase
PMN polymorphonuclear granulocyte
PMSF phenylmethylsulfonyl fluoride
sc subcutaneous
TNF-a tumour necrosis factor-a
Throughout the specification conventional single-
letter and three-letter codes are used to represent amino
acids.
For the purposes of this specification, the term
"alkyl" is to be taken to mean a straight, branched, or
cyclic, substituted or unsubstituted alkyl chain of 1 to
6, preferably 1 to 4 carbons. Most preferably the alkyl
group is a methyl group. The term "acyl" is to be taken
to mean a substituted or unsubstituted aryl of. 1 to 6,
preferably 1 to 4 carbon atoms. Most preferably the aryl
group is acetyl. The term "aryl" is to be understood to
mean a substituted or unsubstituted homocyclic or
heterocyclic aryl group, in which the ring preferably has
5 or 6 members.
A "common" amino acid is a L-amino acid selected
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 14 -
from the group consisting of glycine, leucine, isoleucine,
valine, alanine, phenylalanine, tyrosine, tryptophan,
aspartate, asparagine, glutamate, glutamine, cysteine,
methionine, arginine, lysine, proline, serine, threonine
and histidine.
An "uncommon" amino acid includes, but is not
restricted to, D-amino acids, homo-amino acids, N-alkyl
amino acids, dehydroamino acids, aromatic amino acids
other than phenylalanine, tyrosine and tryptophan, ortho-,
meta- or para-aminobenzoic acid, ornithine, citrulline,
canavanine, norleucine, y-glutamic acid, aminobutyric acid,
L-fluorenylalanine, L-3-benzothienylalanine, and
oc,oc-disubstituted amino acids.
Generally, the terms "treating", "treatment" and
the like are used herein to mean affecting a subject,
tissue or cell to obtain a desired pharmacological and/or
physiological effect. The effect may be prophylactic in
terms of completely or partially preventing a disease or
sign or symptom thereof, and/or may be therapeutic in
terms of a partial or complete cure of a disease.
"Treating" as used herein covers any treatment
of, or prevention of disease in a vertebrate, a mammal,
particularly a human, and includes: preventing the disease a
from occurring in a subject who may be predisposed to the
disease, but has not yet been diagnosed as having it;
inhibiting the disease, ie., arresting its development; or
relieving or ameliorating the effects of the disease, ie.,
cause regression of the effects of the disease.
The invention includes the use of various
pharmaceutical compositions useful for ameliorating
disease. The pharmaceutical compositions acc-ording to one
embodiment of the invention are prepared by bringing a
compound of formula I, analogue, derivatives or salts
thereof and one or more pharmaceutically-active agents or
combinations of compound of formula I and one or more
pharmaceutically-active agents into a form suitable for
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 15 -
administration to a subject using carriers, excipients and
additives or auxiliaries.
Frequently used carriers or auxiliaries include
magnesium carbonate, titanium dioxide, lactose, mannitol
and other sugars, talc, milk protein, gelatin, starch,
vitamins, cellulose and its derivatives, animal and
vegetable oils, polyethylene glycols and solvents, such as
sterile water, alcohols, glycerol and polyhydric alcohols.
Intravenous vehicles include fluid and nutrient
replenishers. Preservatives include antimicrobial, anti-
oxidants, chelating agents and inert gases. Other
pharmaceutically acceptable carriers include aqueous
solutions, non-toxic excipients, including salts,
preservatives, buffers and the like, as described, for
instance, in Remington's Pharmaceutical Sciences, 20th ed.
Williams & Wilkins (2000) and The British National
Formulary 43rd ed. (British Medical Association and Royal
Pharmaceutical Society of Great Britain, 2002;
http://bnf.rhn.net), the contents of which are hereby
incorporated by reference. The pH and exact concentration
of the various components of the pharmaceutical
composition are adjusted according to routine skills in
the art... See Goodman and Gilman's The Pharmacological
Basis for Therapeutics (7th ed., 195).
The pharmaceutical compositions are preferably
prepared and administered in dosage units. Solid dosage
units include tablets, capsules and suppositories. For
treatment of a subject, depending on activity of the
compound, manner of administration, nature and severity of
the disorder, age and body weight of the subject,
different daily doses can be used. Under certain
circumstances, however, higher or lower daily doses may be
appropriate. The dose can be administered either by
single administration in the form of an individual dosage
unit or in several smaller dosage units, or alternatively
by multiple administration of subdivided doses at specific
intervals.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 16 -
The pharmaceutical compositions according to the
invention may be administered locally or systemically in a
therapeutically effective dose. Amounts effective for
this use will, of course, depend on the severity of the
disease and the weight and general state of the subject.
Typically, dosages used in vitr~ may provide useful
guidance in the amounts useful for in situ administration
of the pharmaceutical composition, and animal models may
be used to determine effective dosages for treatment of
the cytotoxic side effects. Various considerations are
described, eg. in Langer, Science, 249: 1527, (1990).
Formulations for oral use may be in the form of hard
gelatin capsules, in which the active ingredient is mixed
with an inert solid diluent, for example, calcium
carbonate, calcium phosphate or kaolin. They may also be
in the form of soft gelatin capsules, in which the active
ingredient is mixed with water or an oil medium, such as
peanut oil, liquid paraffin or olive oil.
Aqueous suspensions normally contain the active
materials in admixture with excipients suitable for the
manufacture of aqueous suspensions. Such excipients may
be suspending agents such as sodium carboxymethyl
cellulose, methyl cellulose, hydroxypropylmethylcellulose,
sodium alginate, polyvinylpyrrolidone, gum tragacanth and
gum acacia; dispersing or wetting agents, which may be
(a) a naturally occurring phosphatide such as
lecithin;
(b) a condensation product of an alkylene oxide
with a fatty acid, for example, polyoxyethylene stearate;
(c) a condensation product of ethylene oxide with
a long chain aliphatic alcohol, for example,
heptadecaethylenoxycetanol;
(d) a condensation product of ethylene oxide with
a partial ester derived from a fatty acid and hexitol such
as polyoxyethylene sorbitol monooleate, or
(e) a condensation product of ethylene oxide with
a partial ester derived from fatty acids and hexitol
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 17 -
anhydrides, for example polyoxyethylene sorbitan
monooleate.
The pharmaceutical compositions may be in the
form of a sterile injectable aqueous or oleaginous
suspension. This suspension may be formulated according
to known methods using suitable dispersing or wetting
agents and suspending agents such as those mentioned
above. The sterile injectable preparation may also a
sterile injectable solution or suspension in a non-toxic
parenterally-acceptable diluent or solvent, for example,
as a solution in 1,3-butanediol. Among the acceptable
vehicles and solvents which may be employed are water,
Ringer's solution, and isotonic sodium chloride solution.
In addition, sterile, fixed oils are conventionally
employed as a solvent or suspending medium. For this
purpose, any bland fixed oil may be employed, including
synthetic mono-or diglycerides. In addition, fatty acids
such as oleic acid may be used in the preparation of
injectables.
Compounds of formula I may also be administered
in the form of liposome delivery systems, such as small
unilamellar vesicles, large unilamellar vesicles, and
multilamellar vesicles. Liposomes can be formed from a
variety of phospholipids, such as cholesterol,
stearylamine, or phosphatidylcholines.
Dosage levels of the compound of formula I of the
present invention will usually be of the order of about
0.5mg to about 20mg per kilogram body weight, with a
preferred dosage range between about 0.5mg to about l0mg
per kilogram body weight per day (from about 0.5g to about
3g per patient per day). The amount of active ingredient
which may be combined with the carrier materials to
produce a single dosage will vary, depending upon the host
to be treated and the particular mode of administration.
For example, a. formulation intended for oral
administration to humans may contain about 5mg to 1g of an
active compound with an appropriate and convenient amount
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 18 -
of carrier material, which may vary from about 5 to 95
percent of the total composition. Dosage unit forms will
generally contain between from about 5mg to 500mg of
active ingredient.
It will be understood, however, that the specific
dose level for any particular patient will depend upon a
variety of factors including the activity of the specific
compound employed, the age, body weight, general health,
sex, diet, time of administration, route of
administration, rate of excretion, drug combination and
the severity of the particular disease undergoing therapy.
In addition, some of the compounds of the
invention may form solvates with water or common organic
solvents. Such solvates are encompassed within the scope
of the invention.
The compounds of the invention may additionally
be combined with other therapeutic compounds to provide an
operative combination. It is intended to include any
chemically compatible combination of pharmaceutically-
active agents, as long as the combination does not
eliminate the activity of the compound of formula I of
this invention.
General Methods
Peptide synthesis
Cyclic peptide compounds of formula I are
prepared according to methods described in detail in our
earlier applications No. PCT/AU98/00490 and
No. PCT/AU02/01427, the entire disclosures of which are
incorporated herein by this reference. While the
invention is specifically illustrated with reference to
the compound AcF-[OPdChaWR] (PMX53), whose corresponding
linear peptide is Ac-Phe-Orn-Pro-dCha-Trp-Arg, it will be
clearly understood that the invention is not limited to
this compound.
Compounds 1-6, 17, 20, 28, 30, 31, 36 and 44
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 19 -
disclosed in International patent application
No.PCT/AU98/00490 and compounds 10-12, 14, 15, 25, 33, 35,
40, 45, 43, 52, 55, 60~ fa6~ and 65-'70 disclosed for the
first time in International patent application
PCT/AU02/01427 have appreciable antagonist potency (IC50 <
1 ~M) against the C5a receptor on human neutrophils.
PMX53 and compounds 33, 45 and 60 of PCT/AU02/01427 are
most preferred.
We have found that all of the compounds of
formula I which have so far been tested have broadly
similar pharmacological activities, although the
physicochemical properties, potency, and bioavailability
of the individual compounds varies somewhat, depending on
the specific substituents.
The general tests described below may be used for
initial screening of candidate inhibitor of G protein-
coupled receptors, and especially of C5a receptors.
Drug preparation and formulation
The human C5a receptor antagonist AcF-[OPdChaWR]
(AcPhe[Orn-Pro-D-Cyclohexylalanine-Trp-Arg]) was
synthesized as described above, purified by reversed phase
HPLC, and fully characterized by mass spectrometry and
proton NMR spectroscopy. The C5a antagonist was prepared
in olive oil (10 mg/mL) for oral dosing and in a 30%
polyethylene glycol solution (0.6 mg/mL) for SC dosing.
It was prepared in a 50% propylene glycol solution (30
mg/kg) for IP injections.
Receptor-Binding Assay
Assays are performed with fresh human PMNs,
isolated as previously described (Sanderson et al, 1995),
using a buffer of 50 mM HEPES, 1 mM CaCl2, 5 mM MgCl2,
0.5% bovine serum albumin, 0.1% bacitracin and 100 uM
phenylmethylsulfonyl fluoride (PMSF). In assays performed
at 4°C, buffer, unlabelled human recombinant C5a (Sigma) or
peptide, Hunter/Bolton labelled 125I_C5a (~ 20 pM) (New
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 20 -
England Nuclear, MA) and PMNs (0.2 x 106) are added
sequentially to a Millipore Multiscreen assay plate (HV
0.45) having a final volume of 200 ~L/well. After
incubation for 60 min at 4°C, the samples are filtered and
the plate washed once with buffer. Filters are dried.,
punched and counted in an LKB gamma counter. Non-specific
binding is assessed by the inclusion of 1mM peptide or 100
nM CSa, which typically results in 10-15% total binding.
Data are analysed using non-linear regression and
statistics with Dunnett post-test.
Myeloperoxidase Release Assay for Antagonist Activity
Cells are isolated as previously described
(Sanderson et a1, 1995) and incubated with cytochalasin B
(5}lg/mL, 15 min, 37°C). Hank's Balanced Salt solution
containing 0.15% gelatin and peptide is added on to a
96 well plate (total volume 100 ~L/well), followed by
~.zL cells (4x106/mL). To assess the capacity of each
peptide to antagonise CSa, cells are incubated for 5 min
20 at 37°C with each peptide, followed by addition of C5a (100
nM) and further incubation for 5 min. Then 50 ~.1L of
sodium phosphate (0.1M, pH 6.8) is added to each well, the
plate was cooled to room temperature, and 25 uL of a fresh
mixture of equal volumes of dimethoxybenzidine (5.7 mg/mL)
25 and H202 (0.510) is added to each well. The reaction is
stopped at 10 min by addition of 2% sodium azide.
Absorbances are measured at 450 nm in a Bioscan 450 plate
reader, corrected for control values (no peptide), and
analysed by non-linear regression.
Statistical Analysis
Values are means ~ standard error mean (SEM), and
differences between group means were considered
significant at P<0.05. Data were analysed by a one-way
ANOVA, and individual group comparisons by Student's t
Test.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 21 -
EXAMPLE 1: Animal Model of Ruptured Aortic Aneurysm
Male Sprague-Dawley rats (350-500g) were used
throughout the experiment. All animals were anesthetized
with pentobarbital sodium (50 mg/l~g ip). For each rat, a
tail vein and the right carotid artery were cannulated
with 22-gauge angiocaths and sutured in place. The tail
vein was used to administer supplemental doses of
anesthetic, laSl-labeled albumin, CSaR antagonist, and
Ringer's lactate solution and for re-infusion of shed
blood. The carotid artery cannula provided continuous
monitoring of the mean arterial pressure (MAP) and was
used to haemorrhage animals.
Animals were randomised into two groups:
a) sham (n=6); and
b) shock + clamp (n=19).
Animals in the shock + clamp group were further
randomised into CSaR antagonist-treated (n=9) and control-
treated groups (n=10). In the treated group, the small
molecule CSaR antagonist, AcF-(OPdChaWR) (Promics Pty Ltd,
Queensland, Australia) was administered intravenously over
two minutes at the end of a haemorrhagic shock at a dose
of 1 mg/kg in endotoxin-free saline, whereas the control
group received saline infusion. In all cases the operator
was blinded to the treatment given.
The abdominal aorta was exposed by midline
laparotomy, and isolated at the superior mesenteric artery
and just proximal to the iliac bifurcation. A 5-cm
segment of jejunum, approximately 10 cm from the ligament
of Trietz, was isolated and cannulated at its proximal end
with an input cannula, and at its distal end with an
output cannula. The cannulas were exteriorized via two
incisions made in the right abdominal wall, and the
abdomen was sutured closed. The cannulated intestinal
segment was flushed with Ringer's lactate solution until
the output was devoid of solid particles. The intestinal
segment was perfused with Ringer's lactate solution at
37°C, at a rate of 0.3 ml/min with an infusion pump (model
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 22 -
AVI 480, 3M, St. Paul, MN) throughout the duration of the
experiment.
For the determination of intestinal and pulmonary
permeability, animals then received lzSl-albumin (~l~,Ci) via
the tail vein catheter, and were allowed to stabilize for
30 min to establish postoperative equilibrium. During the
stabilization and experimental periods, intestinal
perfusate was collected every 10 min. Throughout the
experimental period, samples of blood (0.3 ml) were
withdrawn at 1 h intervals. The blood samples were used
for the measurement of total albumin concentration, and
the specific activity of lzSI-albumin used for the
calculation of intestinal albumin loss, as described
below.
In appropriate groups, shock was induced by
withdrawal of blood into a plastic heparinized syringe
(500 U) to reduce and maintain MAP at 50 mmHg for 1 h.
The shed blood was maintained at room temperature on a
tube rocker during the shock period. After 60 min of
shock or the equivalent control period, clamps were
applied to the abdominal aorta just proximal to the
superior mesenteric artery and at the iliac bifurcation.
At this point, one-half of the shed blood was reinfused
into the tail vein. The clamps remained in place for 45
min. Just before clamp removal, the remainder of the shed
blood was reinfused. Additional Ringer's lactate solution
was also administered, as required, to resuscitate the
animals and maintain MAP at 100 mmHg. Reperfusion was
continued for 120 min, at which time the animals were
killed with an overdose of pentobarbital sodium.
The perfused intestinal segment was harvested,
weighed, and lyophilized to determine the intestinal dry
weight. Portion of the lung and liver and of the
intestine immediately distal to the perfused segment were
excised, washed in ice-cold saline, and rapidly frozen in
liquid nitrogen and stored at -70°C until analyzed for
myeloperoxidase (MPO) and cytokine levels, respectively.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 23 -
The MAP and fluid resuscitation requirements for
each group are summarised in Figure 1.
The mean arterial blood pressure (MAP) remained
stable throughout the entire experimental period in sham
animals, requiring minimal intravenous fluid. resuscitation
with Ringer's lactate solution, as summarised in Figure
1B. In the shock and clamp animals the MAP was reduced
during a haemorrhagic shock to <_ 50 mmHg for one hour as
defined by the protocol. On application of the
supramesenteric aortic clamp the MAP increased
significantly compared to pre-shock levels (158~9.0 versus
117~3.0 mmHg, p<0.001). In the shock and clamp animals
after removal of the aortic clamp, the MAP dropped
progressively during reperfusion to a nadir after 1~0
minutes of reperfusion (68~6.0 versus pre-shock 117~3.0,
p<0.001), despite vigorous fluid resuscitation by
intravenous infusion of Ringer's lactate solution
(69.3~8.5 ml).
Animals treated with C5a receptor antagonist
maintained significantly better MAP during reperfusion
compared to untreated shock and clamp animals (95~5.3
versus 68~6.0 mmHg, p<0.01), and required less intravenous
fluid resuscitation (60.0~7.0 ml versus 69.3~8.5 ml,
p<0.1, NS).
Throughout the experimental procedure the sham
animals maintained a stable blood pressure, with minimal
requirement for fluid resuscitation. Shock and clamp
animals required significant fluid resuscitation from the
start of the reperfusion period after aortio clamp release
in order to maintain MAP. After the initial response to
fluid resuscitation in the first hour of reperfusion,
shock refractory to fluid resuscitation developed in the
second hour of reperfusion, requiring large volumes of
intravenous fluid to maintain a blood pressure. Treatment
with the CSaR antagonist significantly prevented the
severe hypotension seen in the untreated group, and the
antagonist-treated animals required less fluid
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 24 -
resuscitation.
EXAMPLE 2: Determination of Pulmonary Permeability
The heart and lungs were excised in tot~, the
left lung was lavaged three times with 3.5 ml Ringer's
lactate solution, and the effluent bronchoalveolar lavage
(BAL) fluid was collected. Blood and BAL fluid were
weighed and. counted for 1~5I activity, and the lung
permeability index (LPI) was calculated using the
following formula:
LPI=BAL-1~5I (cpm/g) /blood-1251 (cpm/g)
The results are summarised in Figure 2.
The index of lung permeability (LPI) to lzSl-
labelled albumin was significantly increased in. the shock
and clamp group compared to the sham group (4.43~0.96
versus 1.30~0.17, p<0.01). This effect was blocked by
treatment with C5a receptor antagonist (1.74~0.50,
p<0.03).
EXAMPLE 3: Determination of Intestinal Permeability
Intestinal permeability was used as an index of
intestinal injury, and was measured as previously
described (Boyd et al, 1999).
To calculate intraluminal intestinal albumin
loss, all 10 min effluent collections from the intestinal
perfusion were weighed, and a 1 ml sample of each was
assayed for 1251-albumin activity with a gamma counter.
Each blood sample drawn during the experimental procedure
was centrifuged at 100,000 rpm, and 100 ~.l of plasma were
removed for determination of albumin content and 1~5I-
albumin activity. The level of 1251 in the blood samples
was regressed against time, and the slope of the curve was
used to determine the activity of this isotope in whole
blood. This was used to determine the specific activity
of lzSl per ~..1, gram of total albumin to calculate intestinal
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 25 -
albumin loss, expressed as milligrams per gram dry weight
of the perfused intestinal segment. The results are shown
in Figure 3.
The rate of intraluminal intestinal albumin loss,
the intestinal permeability index (IPI), remained stable
throughout the entire experimental period in sham animals.
In shock and clamp animals the IPI remained stable during
the stabilization, haemorrhage and clamp periods; however,
on reperfusion there was a statistically significant
increase in IPI. After 30 minutes of reperfusion, the IPI
was significantly increased in shock and clamp animals
compared to pre-shock levels (8.05x10-2~3.59x10-2 versus
0.72x10-z~0.51x10-2, p<0.0001), and compared to control
levels (8.05x10-~~3.59x10-2 versus 1.75x10-2~0.33x10-~,
p<0.0001), and remained at similar levels throughout the
120-min reperfusion period.
Treatment with the C5a receptor antagonist
significantly reduced the increase in IPI in early
reperfusion; after 30 minutes of reperfusion, the IPI was
significantly reduced in CSaR antagonist-treated animals
compared to untreated shock and clamp animals (2.82x10-
~~0.91x10-~ versus 8.05x10-~~3.59x10-2, p<0.01) . However, as
reperfusion progressed the .IPI increased even in the C5a
antagonist-treated group to mirror the~levels in untreated
shock and clamp animals.
EXAMPLE 4: Measurement of Neutrophil Sequestration
Lung and intestinal tissue samples were assayed
for myeloperoxidase (MPO) activity, an index of neutrophil
sequestration, as previously described (Boyd et al, 1999).
In brief, MPO activity was assessed at 37°C by monitoring
the change in absorbance at 655 nm over a 3-min period in
a Cobas FARA II centrifugal analyzer (Roche Diagnostic
Systems, Montclair, NJ). The reaction mixture contained
16 mmol/1 3,3',5,5'-tetramethylbenzidine dissolved in N,N-
dimethylformamide in 0.22 mol/1 phosphate buffered saline
which contained 0.11 mol/1 NaCl at pH 5.4. The reaction
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 26 -
was initiated by the addition of 3 mmol/1 hydrogen
peroxide. One unit of activity was defined as a one-unit
change in absorbance per minute at 37°C. The protein
content of pulmonary and intestinal samples was determined
by the bicinchoninic acid protein assay system (Pierce,
Rockford, IL). MPO activity was expressed as units per
milligram of protein. The results are shown in Figure 4.
As shown in Figure 4a, the lung tissue MPO
activity was significantly increased in the shock and
clamp groups compared to the sham group (2.41~0.34 versus
1.03~0.29 U/mg, p<0.009), and this increase was blocked. by
treatment with C5a receptor antagonist (1.11-!-0.09 U/mg,
p<0.006) .
As shown in Figure 4b, intestinal tissue MPO
activity was not significantly increased in the shock and
clamp animals compared to the sham animals (3.93~0.66
versus 3.34~0.53 U/mg, p=NS). Interestingly, the
intestinal MPO activity was significantly reduced. in C5a
receptor antagonist-treated animals compared both to
untreated shock and clamp animals (1.86~0.26 versus
3.93~0.66 U/mg, p<0.01), and compared to sham levels
(3.34~0.53 U/mg, p<0.017).
EXAMPLE 5: Measurement of Cytokines in Intestine and
Lung
One hundred mg of each tissue was homogenised in
1 mL of PBS (0.4 mol/L NaCl and 10 mmol/L
NaZHP04)containing protease inhibitors (0.1 mmol/L
phenylmethyl sulfonyl fluoride, 0.1 mmol/L benzethonium
chloride, 10 mmol/L ethylenediaminetetraacetic acid, and
20 KI aprotinin A) and 0.050 Tween 20. The samples were
then centrifuged for 10 minutes at 3000 g and the
supernatant immediately used for enzyme-linked
immunosorbent assay at a 1:2 dilution in assay dilution
buffer. The concentrations of TNF-oc and Interleukin-6 in
samples were measured using commercially-available
antibodies, according to the procedures supplied by the
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 27 -
manufacturer (R&D Systems, Minneapolis, MN). The protein
content of intestine and lung samples was determined by
the bicinchoninic acid protein assay system (Pierce,
Rockford, IL). Cytokine concentrations were expressed as
picograms per milligram of protein. The results are shown
in Figure 6.
As shown in Figure 5a, intestinal TNF-oc, levels
were significantly elevated in shock and clamp animals
compared to sham animals (73.02~10.12 versus 45.42~6.23
pg/mg protein, p<0.038), but not affected by treatment
with C5a receptor antagonist (72.00~13.95 pg/mg protein,
p=NS ) .
As shown in Figure 5b, intestinal IL-6 levels
were elevated in the shock and clamp group compared to the
sham group (280.91~35.95 versus 168.38~35.23 pg/mg
protein, p<0.04). Interestingly, the increase in
intestinal IL-6 levels was significantly less in the C5a
receptor antagonist-treated animals than in untreated
shock and clamp animals (196.30-!-23.68 pg/protein, p<0..05).
As shown in Figure 6a, lung TNF-oc levels were
significantly elevated in shock and clamp animals compared
to sham animals (89.70~13.83 versus 47.57~11.22 pg/mg
protein, p<0.03), but this increase was not prevented by
treatment with C5a receptor antagonist (78.71~15.78 pg/mg
protein, p=NS).
Lung IL-6 levels, shown in Figure 6b, were
elevated in the shock and clamp animals compared to the
sham animals (227.98~51.74 versus 144.81~26.31 U/mg
protein, p=NS). However, this increase did not reach
statistical significance. Interestingly, lung IL-6 levels
were significantly elevated in the shock and clamp group
treated with C5a receptor antagonist compared to the sham
groups (320.72~37.67 versus 144.81~26.31 U/mg protein,
p<0.002).
EXAMPLE 6: Further pre-clinical studies
The effects of therapeutic agents such as those
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
_ ~8 _
of the invention on haemorrhagic shock may be tested in a
variety of experimental models in addition to the one
described herein. The most common experimental species
used are pigs and rats, and, to a lesser extent, sheep and
mice. Because of their larger sire and the strong
similarities of their cardiovascular systems and
parameters to those of humans, pigs are the most commonly
used. Blood loss may be induced by a variety of methods,
and the specific method does not appear to have any
bearing on the outcome.
The C5a antagonist compounds of the invention may
be used in any of these models, subject to the caveat that
if the receptor affinity is lower in mice, sheep and pigs
than the affinity observed in rats, this may reduce the
potency or efficacy of the antagonist.
The test compound is administered following
induction of haemorrhagic shock. The route of
administration is preferably parenteral, for example i.v.,
so that effective blood concentrations of the drug are
reached as quickly as possible, because of the gravity of
the condition, and because shunting of the blood away from
the non-vital organs such as the stomach would reduce
absorption from enteral routes. In general i.v.
administration is used in these experiments.
The test compound is administered at various
doses and at various times after induction of haemorrhagic
shock, in order to ascertain the optimum regimen. Control
animals are treated with a sham injection, are left
untreated, or are treated with a comparative agent; this
may be another ant-inflammatory agent such as infliximab,
or may be another agent used for treatment of haemorrhagic
shock.
The effect of each treatment is monitored using
measurement of parameters such as
cardiac output (stroke volume x heart rate)
mean arterial pressure
fluid resuscitation requirements
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 29 -
neutrophil sequestration in tissues
levels of circulating or tissue cytokines such as
TNF~ and IL-1, IL-2, IL-6, and IL-8
reduction in intracellular ATP
blood haemoglobin levels
metabolic acidosis, and other changes such as
intestinal permeability
pulmonary permeability
Mean arterial pressure, fluid resuscitation
requirements, neutrophil sequestration in tissues,
intestinal permeability, pulmonary permeability, and
levels of TNFOC and IL-6 may be measured as described in
the preceding examples, or using other methods known in
the art. Physiological and biochemical parameters may be
measured by standard methods; for example, blood
haemoglobin is assessed by haematocrit, and metabolic
acidosis is assessed by measurement of pH of arterial
blood or by measurement of Pcoz - Levels of TNF-oc, IL-6 and
other cytokines may be measured using commercially-
available assays, such as immunoassays.
As far as possible the parameters chosen for
study are those which are accepted by regulatory
authorities such as the US Food and Drug Administration,
the European Agency for the Evaluation of Medicinal
Products, and the Australian Therapeutic Goods
Administration.
DISCUSSION
The development of specific monoclonal anti-
complement antibodies has renewed interest in complement
as a therapeutic target in the critically ill (Matis et
al, 1995). In the RA.AA model used herein, haemorrhagic
shock for 1 hour at a MAP of 50 mmHg followed by 45
minutes of supramesenteric aortic clamping and 120 minutes
of reperfusion resulted in significant intestinal and
pulmonary injury, and refractory shock despite vigorous
fluid resuscitation. In this study we have demonstrated
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 30 -
for the first time that the local and systemic injury
associated with RA.AA can be attenuated using a specific
small molecule C5a receptor, the cyclic peptide AcF-
(OPdChaWR), in a rat model.
Intestinal injury in this model was associated
with a significant increase in intestinal capillary
permeability to lzsl-albumin immediately after release of
the supra-mesenteric aortic clamp, an increase which
persisted throughout the 120 minute reperfusion period.
Treatment with the CSaR antagonist significantly prevented
the increase in intestinal permeability in early
reperfusion. However, in late reperfusion intestinal
permeability increased to levels similar to those in non-
treated shock and clamp animals. Increased intestinal
permeability has been reported after RAAA and elective
abdominal and thoracoabdominal aneurysm repair in humans,
and is associated with increased morbidity and mortality
(Van Damme et al, 2000; Lau et al, 2000; Harward et al,
1996).
Injury to the intestine is two-fold, in that the
initial global hypoxia during haemorrhagic shock is
compounded by the direct ischaemia-reperfusion injury
during and after.release of the supramesenteric aortic
clamp. Intestinal ischaemia-reperfusion injury is
associated with neutrophil sequestration and increased
microvascular permeability, and can be modulated by
neutrophil depletion or by antibodies directed against
neutrophil adhesion molecules (Hernandez et al, 1987). In
a recent study in the rat, the same C5a receptor
antagonist as that used in the present study was reported
to be effective in reducing intestinal ischaemia-
reperfusion injury and reduced the neutrophilic response
to ischaemia-reperfusion injury (Arumugam et al, 2002).
Complement activation occurs in the early stages
of inflammation, releasing the anaphylatoxins C3a, C4a,
C5a and the C5b-C9 membrane attack complex. These
activated complement components alter vascular tone and
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 31 -
permeability, and have been shown to be integral to
intestinal reperfusion injury (Williams et al, 1999),
while the membrane attack complex is directly lytic to
cells. The anaphylatoxins, particularly CSa,
chemotactically recruit and activate inflammatory cells
and lead to the release of the cytokines TNF-~t and IL-6.
By inhibiting the early interaction between the activated
complement component, CSa, and its target cells on the
intestinal vascular endothelium, and circulating immune
cells, we have reduced the severity of the initial gut
injury in this model.
Direct or indirect intestinal ischaemia-
reperfusion injury induces functional and morphological
changes in the gut associated with translocation of
bacterial fragments across a damaged intestinal capillary
barrier, with the resultant endotoxaemia producing an
exaggerated inflammatory response. This suggests that the
gut drives the inflammatory response to a variety of
critical illnesses (Bane et al, 1997). Complement has
been shown to be important in neutrophil activation in
response to endotoxin (van Deventer et al, 1991), and the
C5a antagonist used in this study blunts the oxidative
burst in PMNs following exposure to E. coli (Mollnes et .
al., 2002).
We hypothesized that C5a receptor blockade could
decrease the pro-inflammatory effects of endotoxin in
early reperfusion. However, as reperfusion continues
intestinal injury increases, most probably due to the
cellular effects of ischaemia-reperfusion injury and
parallel activation of cytokine and pro-inflammatory
mediator cascades. Our findings are in agreement with
previous reports that direct intestinal ischaemia-
reperfusion injury can be attenuated by blocking the
complement cascade at a variety of points (Hill et al,
1992; Arumugam et al, 2002).
The reduction in intestinal myeloperoxidase
concentration by the C5a antagonist compared to untreated
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 32 -
shock and clamp animals suggests that local complement-
induced neutrophil chemotaxis and activation, or target
cell opsonisation, may be crucial to neutrophil
sequestration and intestinal injury in this model.
Complement activation has been shown in vitr~ to stimulate
the rapid adhesion of neutrophils to endothelial target
cells (Marks et al, 199), and this effect is mediated via
the actions of CSa. In the present study, shock and clamp
animals have significantly increased intestinal levels of
the pro-inflammatory cytokine TNF-oc compared to sham
animals, and this is not altered by treatment with CSaR
antagonist. Although activated complement is known to
cause the release of TNF-oc from a variety of cell types,
including immune cells, by a receptor-mediated effect
(Barton et al, 1993), and intravenously-administered C5a
increases circulating TNF-oG levels in rats (Strachan et
al, 2000), a variety of other mediators released after
ischaemia-reperfusion injury such as arachidonic acid
metabolites, also stimulate cytokine release.
IL-6 is an important pleiotrophic cytokine, with
a variety of pro- and anti-inflammatory effects. High
serum levels of IL-6 have been associated with increased
morbidity and mortality after abdominal aortic aneurysm
repair (Groeneveld et al, 1997). In the present study we
have found that in this model, intestinal injury is
associated with increased intestinal tissue levels of IL-
6, and that treatment with CSaR antagonist prevents this
increase. This is important, as IL-6 has been shown to
upregulate the pro-inflammatory effects of TNF-a in
response to complement activation (Platel et al, 1996).
IL-6 has been shown to inhibit apoptosis of neutrophils,
prolonging their functional longevity and potential for
tissue injury (Biffl et al, 1996); therefore inhibition of
its release may reduce further neutrophil-mediated
intestinal injury. These data suggest that C5a and immune
cell receptor interaction may be integral to the release
of IL-6 in this model, or alternatively that this may also
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 33 -
reflect the decreased tissue sequestration of neutrophils
due to CSaR antagonism.
Ruptured abdominal aortic aneurysm is associated
with a non-cardiac acute interstitial pulmonary oedema and
associated hypoxemia, termed acute respiratory distress
syndrome CARDS), which carries a grave prognosis. In our
model of RAAA the shock and clamp group had significantly
increases in pulmonary permeability to 1~5I-albumin and in
lung myeloperoxidase levels. The suggestion that lung
injury in this syndrome is primarily due to neutrophil
adherence, sequestration and subsequent respiratory burst-
induced oxidative injury is supported by the observation
that lung injury can be attenuated with anti-CD18
monoclonal antibodies (Boyd et al, 1999).
In the model used in the present study,
antagonism of the C5a receptor on target cells reduces
neutrophil sequestration and subsequent microvascular
hyperpermeability, and the combination of the systemic
haemorrhagic shock injury compounded by lower torso
ischaemia-reperfusion produces a severe acute lung injury.
Abrogation of C5a-induced neutrophil chemotaxis and
activation in the pulmonary circulation (Solokin et al,
1985) may in part explain the attenuation of injury in
this study.
The C5a receptor antag~nist does not inhibit the
formation of the membrane attack complex (Arumugam et al,
2003). It is unlikely that C5a receptor blockade affects
the formation of the C5b-9 membrane attack complex, but,
regardless of this, it is unlikely that this large complex
could travel unmolested to the pulmonary circulation to
exert its lytic effects. In this acute model, modulation
of the early complement-dependent injury may be sufficient
to convert a lethal acute lung failure to a recoverable
acute lung dysfunction.
As with the intestine, TNF-oc levels in the lung
significantly increase after shock and clamp, and this is
not affected by treatment with CSaR antagonist. TNF-o~ is
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 34 -
produced after a variety of stresses, and has been shown
to induce direct lung injury (Welbourn et al, 1991). We
found that IL-6 levels in the lung are also increased
after shock and clamp compared to sham animals, although
this increase did not reach significant levels.
Interestingly, shock and clamp animals treated with. C5aR
antagonist have a highly significant increase in lung
tissue IL-6 compared to sham animals, and this suggests
that protection from complement-induced remote injury may
be independent of IL-6. Alternatively this may suggest
that IL-6 release has a beneficial anti-inflammatory
effect in the lung in this model, perhaps through
paracrine inhibition of inflammatory mediator release.
Haemorrhagic shock itself initiates a cascade of
pro-inflammatory mediator induction (Abraham, 1991), and
oxidative injury is associated with the degree of
complement activation in cardiac patients (Cavarocchi et
al, 1986). Aortic clamp release is associated with a
variety of vasoactive effects, including hypovolaemia due
to peripheral vasodilation and increased vascular
permeability, reperfusion of ischaemic tissues with
circulation of vasoactive mediators and metabolites, and
myocardial depressant factors (Barry et al, 1997). The
effect of the C5a receptor antagonist on reducing
microvascular permeability and immune cell activation via
complement receptor-specific pathways reduces the degree
and duration of hypotension in the present model. This is
probably a reflection of the reduced third space fluid
loss due to the prevention of complement activation and
complement-dependent increases in microvascular
permeability. Complement activation is also known to have
effects on vascular tone and histamine release (Ellis et
al, 1991), prevention of which may also help maintain
vascular resistance. Reduced organ injury, and perhaps
reduced myocardial depression, may also allow the animal
to better handle the fluid load required to maintain
target blood pressure.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 35 -
In conclusion, we have shown for the first time
that a small molecule C5a receptor antagonist can reduce
local intestinal and remote lung injury in a model of
ruptured abdominal aortic aneurysm. This treatment
appears to mediate its effects by reducing activated
complement-immune cell interaction, thus in turn reducing
the inflammatory stimulus to tissue neutrophil
sequestration. Antagonism of the human C5a receptor
therefore represents a realistic therapeutic target in
patients with haemorrhagic shock, and potentially
addresses a major clinical need.
It will be apparent to the person skilled in the
art that while the invention has been described in some
detail for the purposes of clarity and understanding,
various modifications and alterations to the embodiments
and methods described herein may be made without departing
from the scope of the inventive concept disclosed in this
specification.
References cited herein are listed on the
following pages, and are incorporated herein by this
reference.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 36 -
REFERENCES
Abraham E. Effects of stress on cytokine production.
Methods Achiev Exp Pathol 1991; 14:45-62.
Adam DJ, Mohan TV, Stuart WP, Bain M, Bradbury AW.
Community and hospital outcome from ruptured abdominal
aortic aneurysm within the catchment area of a regional
vascular surgical service. J Vasc Surg 1999; 30(5):922-
928.
Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie
DP, Taylor SM. A small molecule C5a receptor antagonist
protects kidneys from ischemia/reperfusion injury in rats.
Kidney Int 2003; 63(1):134-142.
Arumugam TV, Shiels IA, Woodruff TM, Reid RC, Fairlie DP,
Taylor SM. Protective effect of a new C5a receptor
antagonist against ischemia- reperfusion injury in the rat
small intestine. J Surg Res 2002; 103(2):260-267.
Baigrie RJ, Lamont PM, Whiting S, Morris PJ. Portal
endotoxin and cytokine responses during abdominal aortic
surgery [see comments]. Am J Surg 1993; 166(3):248-251.
Barry MC, Kelly C, Burke P, Sheehan S, Redmond HP,
Bouchier-Hayes D. Immunological and physiological
responses to aortic surgery: effect of reperfusion on
neutrophil and monocyte activation and pulmonary function.
Barton PA, Warren JS. Complement component C5 modulates
the systemic tumor necrosis factor response in murine
endotoxic shock. Infect Immun 1993; 61(4):1474-1481.
Baue AE. Multiple organ failure, multiple organ
dysfunction syndrome, and systemic inflammatory response
syndrome. Why no magic bullets? [see comments]. Arch Surg
1997; 132(7):703-707.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 37 -
Biffl WL, Moore EE, Moore FA, Barnett CC, Jr., Carl VS,
Peterson VN. Interleukin-6 delays neutrophil apoptosis.
Arch Surg 1996; 131(1):24-29.
Boyd AJ, Rubin BB, Walker PM, Romaschin A, Issekut~ TB,
Lindsay TF. A CD18 monoclonal antibody reduces multiple
organ injury in a model of ruptured abdominal aortic
aneurysm. Am J Physiol 1999; 277(1 Pt 2):H172-H182.
Cavarocchi NC, England MD, Schaff HV, Russo P, ~rs~ulalc
TA, Schnell WA, Jr. et al. ~xygen free radical generation
during cardiopulmonary bypass: correlation with complement
activation. Circulation 1986; 74(5 Pt 2):III130-III133.
van Deventer SJ, Hack CE, Wolbink CE, Voermans HJ, Strack
van Schijindel RJ, ten Cate JW et al. Endotoxin-induced
neutrophil activation--the role of complement revisited.
Prog Clin Biol Res 1991; 367:101-109.
Ellis SG, Bates ER, Schaible T, Weisman HF, Pitt B, Topol
EJ. Prospects for the use of antagonists to the platelet
glycoprotein IIb/IIIa receptor to prevent post-angioplasty
restenosis and thrombosis. J Am Coll Cardiol 1991; 17(6
Suppl B):89B-95B.
Fairlie, D.P., Abbenante, G. and March, D. Curr. Med.
Chem., 1995 2 672-705.
Fairlie, D. P., Wong, A. K.; West, M. W. Curr. Med. Chem.,
1998, 5, 29-62.
Gerard, C and Gerard, N.P. Ann. Rev. Immunol., 1994 12
775-808.
Groeneveld AB, Raijmakers PG, Rauwerda JA, Hack CE. The
inflammatory response to vascular surgery-associated
ischaemia and reperfusion in man: effect on postoperative
pulmonary function. Eur J Vasc Endovasc Surg 1997;
14 (5) :351-359.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 38 -
Harkin DW, Barros D'Sa AA, McCallion K, Hoper M, Halliday
MI, Campbell FC. Circulating neutrophil priming and
systemic inflammation in limb ischaemia-reperfusion
injury. Int Angiol 2001; 20(1):78-89.
Harkin DW, D'Sa AA, Yassin MM, Hoper M, Halliday MI. Gut
mucosal injury is attenuated by recombinant
bactericidal/permeability-increasing protein in hind limb
ischemia- reperfusion injury. Ann Vasc Surg 2001;
15(3):326-331.
Harris LM, Faggioli GL, Fiedler R, Curl GR, Ricotta JJ.
Ruptured abdominal aortic aneurysms: factors affecting
mortality rates. J Vasc Surg 1991; 14(6):812-818.
Harward TR, Welborn MB, III, Martin TD, Flynn TC, Huber
TS, Moldawer LL et al. Visceral ischemia and organ
dysfunction after thoracoabdominal aortic aneurysm repair.
A clinical and cost analysis. Ann Surg 1996; 223(6):729-
734.
Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM,
Granger DN. Role of neutrophils in ischemia-reperfusion-
induced microvascular injury. Am J Physiol 1987; 253(3 Pt
2):H699-H703.
Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore
FD, Jr. Soluble complement receptor type 1 ameliorates the
local and remote organ injury after intestinal ischemia-
reperfusion in the rat. J Immunol 1992; 149(5):1723-1728.
Klausner JM, Paterson IS, Kobzik L, Valeri CR, Shepro D,
Hechtman HB. Leukotrienes but not complement mediate limb
ischemia-induced lung injury. Ann Surg 1989; 209(4):462-
470.
Konteatis, Z.D., Siciliano, S.J., Van Riper, G.,
Molineaux, C.J., Pandya, S., Fischer, P., Rosen, H.,
Mumford, R.A., and Springer, M.S. J. Immunol., 1994 153
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 39 -
4200-4204.
Lau LL, Halliday MI, Lee B, Hannon RJ, Gardiner KR, Soong
CV. Intestinal manipulation during elective aortic
aneurysm surgery leads to portal endotoxaemia and mucosal
barrier dysfunction. Eur J Vasc Endovasc Surg 2000;
19(6):619-624.
Lindsay TF, Memari N, Ghanekar A, Walker P, Romaschin A.
Rupture of an abdominal aortic aneurysm causes priming of
phagocytic oxidative burst.
Marks RM, Todd RF, III, Ward PA. Rapid induction of
neutrophil-endothelial adhesion by endothelial complement
fixation. Nature 1989; 339(6222):314-317.
Matis LA, Rollins SA. Complement-specific antibodies:
designing novel anti-inflammatories. Nat Med 1995;
1(8):839-842.
Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D,
Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD.
Essential role of the C5a receptor in E coli-induced
oxidative burst and phagocytosis revealed by a novel
lepirudin-based human whole blood model of inflammation.
Blood. 2002; 100:1869-77.
Morgan EL, Ember JA, Sanderson SD, Scholz W, Buchner R, Ye
RD et al. Anti-C5a receptor antibodies. Characterization
of neutralizing antibodies specific for a peptide, CSaR-
(9-29), derived from the predicted amino-terminal sequence
of the human C5a receptor. 1993.
Paterson IS, Klausner JM, Pugatch R, Allen P, Mannick JA,
Shepro D et al. Noncardiogenic pulmonary edema after
abdominal aortic aneurysm surgery. Ann Surg 1989;
209(2):231-236.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 40 -
Piccolo, MT, Wang, Y, Sannomiya, P et al., Chemotactic
mediator requirements in lung injury following skin burns.
Exp. Mol. Pathol. 199; 66: 220-226
Platel D, Bernard A, Macl~ G, Guiguet M. Interleukin 6
upregulates TNF-alpha-dependent C3-stimulating activity
through enhancement of TNF-alpha specific binding on rat
liver epithelial cells. Cytokine 1996; 8(12):895-899.
Roumen RM, Hendril~.s T, van d, V, Nieuwenhuij~en GA,
Sauerwein RW, van der Meer JW et al. Cytokine patterns in
patients after major vascular surgery, hemorrhagic shock,
and severe blunt trauma. Relation with subsequent adult
respiratory distress syndrome and multiple organ failure.
Ann Surg 1993; 218(6):769-776.
Rubin BB, Smith A, Liauw S, Isenman D, Romaschin AD,
Walker PM. Complement activation and white cell
sequestration in postischemic skeletal muscle. Am J
Physiol 1990; 259(2 Pt 2):H525-H531.
Sanderson, S.D., Kirnarsky, L., Sherman, S.A., Vogen,
S.M., Prakesh, O., Ember, J.A., Finch, A.M. and Taylor,
S.M. J. Med. Chem., 1995 38 3669-3675.
Solomkin JS, Cotta LA, Satoh PS, Hurst JM, Nelson RD.
Complement activation and clearance in acute illness and
injury: evidence for C5a as a cell-directed mediator of
the adult respiratory distress syndrome in man. Surgery
1985; 97(6):668-678.
Strachan, AJ, Haaima, G, Fairlie, DP and SM Taylor.
Inhibition of the reverse passive Arthus reaction and
endotoxic shock in rats by a small molecule C5a receptor
antagonist. J. Immunol. 164: 6560-6565, 2000
Tyndall, J. D. A.; Fairlie, D. P. Curr. Med. Chem. 2001,
8, 893-907.
CA 02557615 2006-08-28
WO 2004/100975 PCT/AU2004/000642
- 41 -
Van Damme H, Creamers E, Limet R. Ischaemic colitis
following aortoiliac surgery. Acta Chir Belg 2000;
100(1):21-27.
Welbourn R, Goldman G, O'Riordain ~, Lindsay TF, Peterson
IS, Kob~ik L et al. Role for tumor necrosis factor as
mediator of lung injury following lower torso ischemia. J
Appl Physiol 1991; 70(6):2645-2649.
Welbourn CR, Goldman G, Peterson TS, Valeri CR, Shepro D,
Hechtman HB. Pathophysiology of ischaemia reperfusion
injury: central role of the neutrophil. Br J Surg 1991;
78 ( 6 ) : 652-655 .
Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore
FD, Jr. et al. Tntestinal reperfusion injury is mediated
by IgM and complement. J Appl Physiol 1999; 86(3):938-942.
Yassin MM, Barros D'Sa AA, Parks TG, McCaigue MD, Leggett
P, Halliday MI. Lower limb ischaemia-reperfusion injury
alters gastrointestinal structure and function. Br J Surg
1997; 84(10):1425-1429.
Younger JG, Sasaki N, Waite MD, Murray HN, Saleh EF,
Ravage ZA, Hirschl RB, Ward PA, Till GO. Detrimental
effects of complement activation in hemorrhagic shock. J
Appl Physiol 2001; 90:441-446.