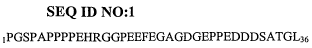Note: Descriptions are shown in the official language in which they were submitted.
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
HSV-2 TYPE-SPECIFIC IMMUNOASSAYS USING GLYCOPROTEIN G2 PEPTIDES
BACKGROUND OF THE INVENTION
[0001] Genital herpes, caused by infection with herpes simplex virus
type 2 (HSV-2),
is the most common sexually transmitted disease in humans. The current
prevalence of HSV-
2 infections is greater than 20% among adults in the United States (Ashley and
Wald, Clin.
Microbial. Rev. 12:1-8, 1999). This disease is a major concern in public
health due to its
morbidity, frequency of recurrence, and life-threatening severity in the case
of newborns
infected with the virus following intrapartum transmission.
[0002] The serological diagnosis for HSV-2 infection has been hampered,
however,
by the fact that there exists extensive cross-reactivity of HSV-2 antibodies
to herpes simplex
virus type 1 (HSV-1). The two subtypes of HSV have important differences in
epidemiology
and natural history: HSV-1 usually causes orolabial disease, whereas HSV-2
almost always
leads to genital disease. For a general review of HSV epidemiology and
diagnosis, see
Brugha et al., Int. J. Epidemiol. 26:698-709, 1997; Ashley and Wald, supra.
[0003] Various approaches have been developed in an effort to
identify HSV-2
specific antibodies. The most reliable method for a type-specific detection of
HSV-2
antibodies to date is an immunoblot assay, preferably a Western blot assay.
The significant
drawback of this method is that the procedure is labor-intensive and requires
the investigator
to have a certain level of skill in order to achieve unequivocal results. In
the last decade or
so, several HSV glycoproteins have been identified as the viral proteins that
contain type-
specific epitopes. Immunoassays have been developed based on these
glycoproteins for type-
specific determination of HSV-2 infection. See, e.g., Lee et al., J. Clin.
Microbial. 22:641-
644 (1985); Eis-Hubinger et al., J. Clin. Microbial. 37:1242-1246 (1999);
Groen et al., J.
Clin. Microbial. 36:845-847 (1998); Ashley et al., J. Clin. Microbial. 36:294-
295 (1998);
Hashido et al., J. Med. Viral. 53:319-323 (1997); and U.S. Patent No.
4,764,459.
[0004] As these methods show a varying degree of sensitivity and
specificity, there is
one common problem associated with these glycoprotein-based immunoassays for
HSV-2
antibody type-specific detection. The full length glycoproteins are obtained
through isolation
of either naturally-occurring viral proteins or recombinantly expressed
proteins. These
procedures can be costly and susceptible to impurities and thus cross-
reactivity.
CA 02558246 2010-08-17
[00051 Studies have indicated that peptides corresponding to partial
sequences of
certain viral proteins of HSV-2 may be useful in HSV-2 type-specific
detection, as these
peptides may represent some HSV-2 type-specific epitopes. See, e.g., Levi et
al., Clin.
Diagn. Lab. linnzunoL 3:265-269 (1996); Ackermann et al., J. Med. Viral.
55:281-287 (1998);
Marsden et al., J. Med. Virol. 56:79-84 (1998); Lijeqvist et al., J. Gen.
ViroL 79:1215-1224
(1998); Grabowska et al., J. Gen. ViroL 80:1789-1798 (1999); U.S. Patent Nos.
5,919,616
and 5,965,357. The present invention provides novel peptide sequences of HSV-2
glycoprotein G2 that can be used in HSV-2 type-specific diagnosis.
BRIEF SUMMARY OF THE INVENTION
[00071 In one aspect, the present invention relates to a composition
containing a
peptide that binds specifically to HSV-2 antibodies and reacts minimally to
HSV-1 specific
antibodies or antibodies to any other herpes family viruses. This peptide
consists of 24 to 36
contiguous amino acids of SEQ ID NO:l. In one preferred embodiment, the
peptide has a
sequence consisting of amino acids 5 to 32 of SEQ ID NO:l. In a more preferred
embodiment, the peptide has a sequence consisting of amino acids 9 to 32 of
SEQ ID NO: 1.
In a most preferred embodiment, the peptide has an amino acid sequence of SEQ
ID NO: 1.
[00081 In some embodiments, the peptide is dimerized. Such dimerization may
be
achieved via a disulfide bond. In other embodiments, the peptide is linked to
a carrier.
Examples of suitable carriers are carboxylated microspheres, preferably
carboxylated latex or
magnetic microspheres.
[0009) In some embodiments, the peptide is linked to a carrier via a
linker at the N-
terminus or the C-terminus of the peptide, whereas in other embodiments the
peptide is
linked to a carrier via a linker at an internal amino acid residue of the
peptide. In some cases,
the linker is or includes a heterologous peptide. In others, the linker is or
includes a
heterologous protein. In still other cases, both a heterologous protein and a
heterologous
peptide are used. In some other embodiments, the entire linker is a
heterologous peptide. In
one preferred embodiment, the heterologous protein is bovine serum albumin
(BSA), whereas
in another preferred embodiment, the heterologous protein is Keyhole Limpet
Hemocyanin
(KLH). In some embodiments, the heterologous peptide includes one cysteine
residue, one
2
CA 02558246 2010-03-23
lysine residue, and at least two glycine residues. In some other embodiments,
the linker
includes a branched amino acid polymer, whose structure is preferably that
shown below:
peptide¨ GGGG...
,K
peptide¨ GGGGr
K¨
peptide¨ GGGGN
7,K
peptide ¨ GGGG
[0010] In one preferred embodiment, the peptide has an amino acid
sequence of SEQ
ID NO:1, the carrier is a carboxylated magnetic microsphere, the linker
includes 4-
(maleimidomethyl)-1-cyclohexanecarboxylic acid (SMCC) and a heterologous
peptide with
an amino acid sequence of GGCK (SEQ ID NO:2), and the heterologous peptide is
attached to
the peptide at the C-terminus of the peptide. In other preferred embodiments,
the linker
further includes the heterologous protein BSA directly attached to the
carrier, and SMCC is
attached to BSA and, via the heterologous peptide, to the peptide.
[0011] In another preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 5 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
sequence of GGGGCK (SEQ ID NO:3), and the heterologous peptide is attached to
the
peptide at the C-terminus of the peptide. In other preferred embodiments, the
linker further
includes the heterologous protein BSA directly attached to the carrier, and
SMCC is attached
to BSA and, via the heterologous peptide, to the peptide.
[0012] In yet another preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
sequence of GGGGCK (SEQ ID NO:3), and the heterologous peptide is attached to
the
peptide at the C-terminus of the peptide. In other preferred embodiments, the
linker further
includes the heterologous protein BSA directly attached to the carrier, and
SMCC is attached
to BSA and, via the heterologous peptide, to the peptide.
[0013] In still another preferred embodiment, the peptide has an amino
acid sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
3
CA 02558246 2010-03-23
sequence of KCGGGG (SEQ ID NO:4), and the heterologous peptide is attached to
the
peptide at the N-terminus of the peptide. In other preferred embodiments, the
linker further
includes the heterologous protein BSA directly attached to the carrier, and
SMCC is attached
to BSA and, via the heterologous peptide, to the peptide.
10014] In one further preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated microsphere,
and the linker includes a branched amino acid polymer that has the following
structure and
further includes a short peptide of CK, which via the C residue is directly
attached to the last
K residue of the structure:
peptide ¨ GGGG
V K
peptide ¨ GGGG
K¨ C-K
peptide ¨ GGGG x
z K
peptide ¨ GGGG
[0015] In a second aspect, the present invention relates to a method for
type-specific
diagnosis of HSV-2 infection. The method for specific detection of HSV-2
antibodies in a
biological sample includes two steps. The first step is contacting the
biological sample with a
composition that includes a peptide consisting of 24 to 36 contiguous amino
acids of SEQ ID
NO:1, linked to a carrier. The second step is detecting whether antigen-
antibody binding has
occurred between the peptide and an antibody component of the biological
sample. In this
step, the detection of antigen-antibody binding indicates the presence of HSV-
2 antibodies in
the biological sample. In some preferred embodiments, the second step is
performed by flow
cytometry. It is also preferred that the biological sample be whole blood,
serum, plasma,
cerebrospinal fluid, tissue from a swab device, or vesicle fluid.
[0016] In one preferred embodiment, the peptide has a sequence consisting
of amino
acids 5 to 32 of SEQ ID NO: 1. In a more preferred embodiment, the peptide has
a sequence
consisting of amino acids 9 to 32 of SEQ ID NO: 1. In a most preferred
embodiment, the
peptide has an amino acid sequence of SEQ ID NO: 1.
[0017] In some embodiments, the peptide is dimerized. Such dimerization
may be
achieved via a disulfide bond. In some other embodiments, the peptide is
linked to a
carboxylated microsphere, preferably a carboxylated latex or magnetic
microsphere.
4
CA 02558246 2010-03-23
100181 In some embodiments, the peptide is linked to the carrier via a
linker at the N-
terminus or the C-terminus of the peptide, whereas in other embodiments the
peptide is linked
to a carrier via a linker at an internal amino acid residue of the peptide. In
some
embodiments, the linker includes a heterologous peptide. In some other
embodiments, the
linker includes a heterologous protein. In some other embodiments, the linker
includes a
heterologous protein in addition to a heterologous peptide. In some other
embodiments, the
linker is a heterologous peptide. In one preferred embodiment, the
heterologous protein is
BSA, whereas in another preferred embodiment, the heterologous protein is KLH.
In some
embodiments, the heterologous peptide includes one cysteine residue, one
lysine residue, and
at least two glycine residues. In some other embodiments, the linker includes
a branched
amino acid polymer, whose structure is preferably that shown below:
peptide ¨ GGGG
,
Z'
peptide ¨ GGGG K\
peptide¨ GGGGN /
7 K
peptide ¨ GGGG
[0019] In one preferred embodiment, the peptide has an amino acid
sequence of SEQ
ID NO:1, the carrier is a carboxylated magnetic microsphere, the linker
includes SMCC and a
heterologous peptide with an amino acid sequence of GGCK (SEQ ID NO:2), the
heterologous peptide is attached to the peptide at the C-terminus of the
peptide, and the
detection of antigen-antibody binding is achieved by flow cytometry. In other
preferred
embodiments, the linker further includes the heterologous protein BSA directly
attached to the
carrier, and SMCC is attached to BSA and, via the heterologous peptide, to the
peptide.
[0020] In another preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 5 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
sequence of GGGGCK (SEQ ID NO:3), the heterologous peptide is attached to the
peptide at
the C-terminus of the peptide, and the detection of antigen-antibody binding
is achieved by
flow cytometry. In other preferred embodiments, the linker further includes
the heterologous
protein BSA directly attached to the carrier, and SMCC is attached to BSA and,
via the
heterologous peptide, to the peptide.
CA 02558246 2010-03-23
[0021] In yet another preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
sequence of GGGGCK (SEQ ID NO:3), the heterologous peptide is attached to the
peptide at
the C-terminus of the peptide, and the detection of antigen-antibody binding
is achieved by
flow cytometry. In other preferred embodiments, the linker further includes
the heterologous
protein BSA directly attached to the carrier, and SMCC is attached to BSA and,
via the
heterologous peptide, to the peptide.
100221 In still another preferred embodiment, the peptide has an amino
acid sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated magnetic
microsphere, the linker includes SMCC and a heterologous peptide with an amino
acid
sequence of KCGGGG (SEQ ID NO:4), and the heterologous peptide is attached to
the
peptide at the N-terminus of the peptide. In other preferred embodiments, the
linker further
includes the heterologous protein BSA directly attached to the carrier, and
SMCC is attached
to BSA and, via the heterologous peptide, to the peptide.
[0023] In one further preferred embodiment, the peptide has an amino acid
sequence
consisting of amino acids 9 to 32 of SEQ ID NO:1, the carrier is a
carboxylated microsphere,
and the linker includes a branched amino acid polymer that has the following
structure and
further includes a short peptide of CK, which via the C residue is directly
attached to the last
K residue of the structure:
peptide¨ GGGG
V K
peptide ¨ GGGG
K¨ C-K
peptide GGGGN
K
peptide ¨ GGGG
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] Figure 1 shows SEQ ID NO:1,
1PGSPAPPPPEHRGGPEEFEGAGDGEPPEDDDSATGL36
[0025] Figure 2 shows the correlation between HSV-2 specific antibody
detection
using a commercial HSV-2 type-specific enzyme-linked immunoassay and using the
method
6
CA 02558246 2010-03-23
of the present invention where peptide 1-SMCC-BSA conjugate,
(PGSPAPPPPEHRGGPEEFEGAGDGEPPEDDDSATGLGGCK)-SMCC-BSA (SEQ ID NO: 5),
is used as an HSV-2 specific antigen.
[0026] Figure 3 shows the correlation between HSV-2 specific antibody
detection using a
commercial HSV-2 type-specific enzyme-linked immunoassay and using the method
of the
present invention where peptide 2-SMCC-BSA conjugate,
(APPPPEHRGGPEEFEGAGDGEPPEDDDSGGGGCK)-SMCC-BSA (SEQ ID NO:6), is used as
an HSV-2 specific antigen.
[0027] Figure 4 shows the correlation between HSV-2 specific antibody
detection using a
commercial HSV-2 type-specific enzyme-linked immunoassay and using the method
of the
present invention where BSA-SMCC-peptide 5 conjugate, BSA-SMCC-
(KCGGGGPEHRGGPEEFEGAGDGEPPEDDDS) (SEQ ID NO:7), is used as an HSV-2 specific
antigen.
[0028] Figure 5 shows the correlation between HSV-2 specific antibody
detection using a
commercial HSV-2 type-specific enzyme-linked immunoassay and using the method
of the
present invention where peptide 5, KCGGGGPEHRGGPEEFEGAGDGEPPEDDDS (SEQ ID
NO:8), is used as an HSV-2 specific antigen.
DETAILED DESCRIPTION OF THE INVENTION
I. Introduction
[0029] The present invention relates to peptides that consist of partial
sequences of HSV-
2 glycoprotein G2 and represent HSV-2 type-specific epitopes. Experiments have
shown that
HSV-2 peptides consisting of 24 to 36 contiguous amino acids of SEQ ID NO:1
bind HSV-2
antibodies with high specificity and sensitivity, while their cross-reactivity
to HSV-1 antibodies is
minimal. Thus these peptides are useful for type-specific serological
diagnosis of HSV-2
infection, particularly for differentiation of HSV-2 infection from HSV-1
infection.
[0030] Compositions comprising peptides of the present invention are also
provided for
type-specific serological diagnosis of HSV-2 infection. In preferred
embodiments, the peptides
of the present invention are linked to a carrier, such as a carboxylated latex
or magnetic
microsphere, via a linker that includes a heterologous peptide and/or protein,
such as bovine
serum albumin (BSA) and Keyhole Limpet Hemocyanin (KLH). Other linkers, such
as branched
amino acid polymers, straight or branched-chain carbon linkers, heterocyclic
carbon linkers (e.g.,
SMCC), or polyether linkers, may also be used in practicing
7
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
the present invention. An HSV-2 peptide of this invention may be conjugated to
a carrier at
the N- or C-terminus of the peptide, or via an internal amino acid residue.
[0031] Furthermore, methods for using these peptides for type-
specific detection of
HSV-2 antibodies are provided. In preferred embodiments, the detection of HSV-
2 specific
antibodies is performed by flow cytometry.
Definitions
[0032] The term "amino acid" refers to naturally occurring and
synthetic amino acids,
as well as amino acid analogs and amino acid mimetics that function in a
manner similar to
the naturally occurring amino acids. Naturally occurring amino acids are those
encoded by
the genetic code, as well as those amino acids that are later modified, e.g.,
hydroxyproline, -y-
carboxyglutamate, and 0-phosphoserine. The term "amino acid analogs" refers to
compounds that have the same basic chemical structure as a naturally occurring
amino acid,
i.e., an a carbon that is bound to a hydrogen, a carboxyl group, an amino
group, and an R
group, e.g., homoserine, norleucine, methionine sulfoxide, methionine methyl
sulfonium.
Such analogs have modified R groups (e.g., norleucine) or modified peptide
backbones, but
retain the same basic chemical structure as a naturally occurring amino acid.
"Amino acid
mimetics" refers to chemical compounds that have a structure that is different
from the
general chemical structure of an amino acid, but that function in a manner
similar to a
naturally occurring amino acid.
[0033] Amino acids may be referred to herein by either their commonly known
three
letter symbols or by the one-letter symbols recommended by the IUPAC-IUB
Biochemical
Nomenclature Commission.
[0034] The terms "polypeptide," "peptide" and "protein" are used
interchangeably
herein to refer to a polymer of amino acid residues. The terms apply to amino
acid polymers
in which one or more amino acid residue is an artificial chemical mimetic of a
corresponding
naturally occurring amino acid, as well as to naturally occurring amino acid
polymers and
non-naturally occurring amino acid polymers. As used herein, the terms
encompass amino
acid chains of any length, including full-length proteins, wherein the amino
acid residues are
linked by covalent peptide bonds. An HSV-2 peptide as used in the present
application refers
to a peptide that has a sequence corresponding to a segment of the HSV-2
glycoprotein G2.
[0035] A "carrier" as used herein refers to an inert solid support of
natural material,
such as glass and collagen, or synthetic material, such as acrylamide,
cellulose, nitrocellulose,
8
CA 02558246 2010-03-23
silicone rubber, polystyrene, polyethylene vinyl acetate, polypropylene,
polymethacrylate,
polyethylene, polysilicates, polyethylene oxide, polycarbonates, teflon,
fluorocarbons, nylon,
polyanhydrides, polyglycolic acid, polylactic acid, polyorthoesters,
polypropylfumarate,
glycosaminoglycans, and polyamino acids. Frequently, some functional groups,
e.g., carboxylic
acid (-COOH), free amine (-NH2), and sulfhydryl (-SH) groups, naturally
present on the surface
of a carrier can be used for peptide linkage. In case no such functional group
is naturally
available, a desired functional group, such as a carboxylic acid group, or a
moiety known to be a
partner of a binding interaction (such as avidin that is capable of binding
biotin) may be attached
to such solid support. A preferred carrier of the present invention is a
carboxylated latex or
magnetic microsphere.
1003 61 A "linker" as used herein refers to any means of connecting a
peptide of the
present invention to a carrier. The linkage may be located at either the N-
terminus or the C-
terminus of the peptide. In some embodiments, the linkage can be effectuated
through a side
group of an internal amino acid residue of the peptide, such as a second
carboxyl group of an
aspartate residue that is not located at the N- or C-terminus of the peptide.
A linker may
comprise a peptide (e.g., the short amino acid sequence of GGGGCK (SEQ ID
NO:3) or GGCK
(SEQ ID NO:2)) or a protein (e.g., BSA or KLH) that is heterologous to the
peptide of the present
invention. In addition to a heterologous peptide, a linker may further
comprise a heterologous
protein, such as BSA or KLH. Non-polypeptide linkers, such as straight or
branched-chain
carbon linkers, heterocyclic carbon linkers, or polyether linkers, may also be
suitable for the
purpose of connecting a peptide of the invention to a carrier, or as a part of
a multiple-component
linker. One preferred non-polypeptide linker is SMCC. Moreover, a linker may
comprise a
branched amino acid polymer that is capable of attaching more than one peptide
of the invention
to a carrier. Both heterobifunctional and monobifunctional linkers are
suitable in the practice of
the present invention. A linker may also comprise a covalent bond, such as a
peptide bond or a
disulfide bond, or a noncovalent bond, such as an ionic or hydrophobic bond,
in the case where a
peptide of the present invention is attached directly to a carrier. An example
of a non-covalent
bond is the interaction between antigen-antibody or biotin-avidin.
[0037] The term "heterologous" when used in the context of describing a
peptide or a
protein as a linker or a component of a linker indicates that the linker
peptide or linker protein
and the peptide of the invention, which is connected to the carrier via the
linker, are not found in
the same relationship with one another in nature. In other words, the linker
peptide and/or linker
protein and the peptide of the invention, as they are conjugated in any
particular
9
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
embodiment of the present invention, do not produce an amino acid sequence
that is found in
nature. As an example, the peptides of the present invention are derived from
HSV-2
glycoprotein G2, a heterologous peptide or protein is therefore not derived
from any sequence
of HSV-2 glycoprotein G2 that is contiguous to the sequence from which the
peptides of the
present invention are derived.
[0038] "Specific detection" as used herein refers to the fact that
detection of any
antibody bound to the peptides of the present invention is determinative of
the presence of
HSV-2 antibody, often in a heterogeneous population of other antibodies and
proteins. In
particular, the presence of HSV-1 antibodies will not result in a detectable
amount of
antibody bound to the peptides. Hence, the term "specific detection"
particularly
encompasses the use of HSV-2 peptides of the present invention to
differentiate HSV-2
infection from HSV-1 infection. Under designated immunoassay conditions, a
detectable
signal is designated as one that is at least twice the background signal.
Thus, specific
antibody-peptide binding should yield a signal at least two times, preferably
more than 10
times, and more preferably more than 100 times the background.
[0039] The term "dimerized" or "dimer" when used to describe a
peptide' of the
present invention refers to the complex formed by two molecules comprising the
same
peptide via a covalent bond, such as a disulfide bond, or a noncovalent bond,
such as via the
binding interaction between a known tag and tag-binder pair (e.g., biotin and
avidin). The
covalent bond or noncovalent bond typically occurs between the linker portions
of the two
peptide-containing molecules.
[0040] The term "an internal amino acid residue" as used herein
refers to any amino
acid residue that is not the first residue from either the N-terminus or the C-
terminus of an
HSV-2 peptide of the present invention.
[0041] The term "biological sample" refers to sections of tissues such as
biopsy and
autopsy samples, and frozen sections taken for histological purposes. Such
samples may
include whole blood, serum, plasma, cerebrospinal fluid, sputum, tissue,
cultured cells, e.g.,
primary cultures, explants, transformed cells, stool, urine, vesicle fluid,
mucus, and other
bodily secretion, or tissue that could be sampled with a swab device. A
biological sample is
typically obtained from a human who may have been infected with HSV-2.
[0042] The term "antibody" denotes a protein of the immunoglobulin
family or a
polypeptide including fragments of an immunoglobulin that is capable of
noncovalently,
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
reversibly, and in a specific manner binding a corresponding antigen. An
illustrative
antibody structural unit includes a tetramer. Each tetramer is composed of two
identical pairs
of polypeptide chains, each pair having one "light" (about 25 IcD) and one
"heavy" chain
(about 50-70 IcD), connected through one or more disulfide bonds. The
recognized
immunoglobulin genes include the K, X, a, 7, 5, Ã, and it constant region
genes, as well as the
myriad immunoglobulin variable region genes. Light chains are classified as
either K or X.
Heavy chains are classified as 7, pt, a, 8, or Ã, which in turn define the
immunoglobulin
classes, IgG, IgM, IgA, IgD, and IgE, respectively. The N-terminus of each
chain defines a
variable region of about 100 to 110 or more amino acids primarily responsible
for antigen
recognition. The terms variable light chain (VL) and variable heavy chain (VH)
refer to these
regions of light and heavy chains respectively.
[0043] The term "complementarity-determining domains" or "CDRs"
refers to the
hypervariable regions of VL and VH. The CDR is the target protein-binding site
of the
antibody chain that harbors specificity for that target protein. There are
three CDRs (CDR1-
3, numbered sequentially from the N-terminus) in each human VL or VH,
constituting about
15-20% of the variable domains. The CDRs are structurally complementary to the
epitope of
the target protein and are thus directly responsible for the binding
specificity. The remaining
stretches of the VL or VH, the so-called framework regions, exhibit less
variation in amino
acid sequence (Kuby, Immunology, 4th ed., Chapter 4, W.H. Freeman & Co., New
York, 2000).
[0044] The positions of the CDRs and framework regions are determined using
various well known definitions in the art, e.g., Kabat, Chothia, International
ImMunoGeneTics database (IMGT), and AbM (see, e.g., Johnson et al., Nucleic
Acids Res.,
29:205-206 (2001); Chothia and Lesk, J MoL Biol., 196:901-917 (1987); Chothia
et al.,
Nature, 342:877-883 (1989); Chothia et al., J. MoL Biol., 227:799-817 (1992);
Al-Lazikani et
al., J.MoLBioL, 273:927-748 (1997)). Definitions of antigen combining sites
are also
described in the following: Ruiz et al., Nucleic Acids Res., 28:219-221
(2000); and Lefranc,
M.P., Nucleic Acids Res., 29:207-209 (2001); MacCallum et al., J. MoL Biol.,
262:732-745
(1996); and Martin et al, Proc. NatL Acad. Sci. USA, 86:9268-9272 (1989);
Martin et al.,
Methods EnzymoL, 203:121-153 (1991); and Rees et al., In Sternberg M.J.E.
(ed.), Protein
Structure Prediction, Oxford University Press, Oxford, 141-172 (1996).
[0045] The terms "antibody light chain" and "antibody heavy chain"
denote the VL or
VH, respectively. The VL is encoded by the gene segments V (variable) and J
(junctional),
11
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
and the VH is encoded by V, D (diversity), and J. Each of VI, or VH includes
the CDRs as
well as the framework regions.
[0046] Antibodies exist as intact immunoglobulins or as a number of
well-
characterized fragments produced by digestion with various peptidases. Thus,
for example,
pepsin digests an antibody below the disulfide linkages in the hinge region to
produce F(
ob)'21
a dimer of Fab' which itself is a light chain joined to VH-CH1 by a disulfide
bond. The F(
ob)'2
may be reduced under mild conditions to break the disulfide linkage in the
hinge region,
thereby converting the F( dimer into an Fab' monomer. The Fab' monomer is
essentially
,'2
Fab with part of the hinge region (Paul, Fundamental Immunology 3d ed. 1993).
While
various antibody fragments are defined in terms of the digestion of an intact
antibody, one of
skill will appreciate that such fragments may be synthesized de novo either
chemically or by
using recombinant DNA methodology. Thus, the term antibody, as used herein,
also includes
antibody fragments either produced by the modification of whole antibodies, or
those
synthesized de novo using recombinant DNA methodologies (e.g., single chain
Fv) or those
identified using phage display libraries (see, e.g., McCafferty et al.,
Nature, 348:552-554
(1990))
[0047] For preparation of monoclonal or polyclonal antibodies, any
technique known
in the art can be used (see, e.g., Kohler & Milstein, Nature, 256:495-497
(1975); Kozbor et
al., Immunology Today, 4:72 (1983); Cole et al., Monoclonal Antibodies and
Cancer
Therapy, pp. 77-96. Alan R. Liss, Inc. 1985). Techniques for the production of
single chain
antibodies (U.S. Patent No. 4,946,778) can be adapted to produce antibodies to
polypeptides
of this invention. Also, transgenic mice, or other organisms such as other
mammals, may be
used to express humanized antibodies. Alternatively, phage display technology
can be used
to identify antibodies, and heteromeric Fab fragments, or scFv fragments that
specifically bind
to selected antigens (see, e.g., McCafferty et al., supra; Marks et al.,
Biotechnology, 10:779-
783, (1992)).
[0048] An "HSV-2 antibody" as used in this application refers to an
antibody that is
specifically reactive to HSV-2 antigens but not to antigens of any other
source, particularly
HSV-1.
12
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
III. Synthesis of Peptides
A. Synthesis of Peptides by Chemical Methods
[0049] The peptides of the present invention may be synthesized
chemically using
conventional peptide synthesis or other protocols well known in the art.
[0050] Peptides may be synthesized by solid-phase peptide synthesis methods
using
procedures similar to those described by Merrifield et al., J. Am. Chem. Soc.,
85:2149-2156
(1963); Barany and Merrifield, Solid-Phase Peptide Synthesis, in The Peptides:
Analysis,
Synthesis, Biology Gross and Meienhofer (eds.), Academic Press, N.Y., vol. 2,
pp. 3-284
(1980); and Stewart et al., Solid Phase Peptide Synthesis 2nd ed., Pierce
Chem. Co.,
Rockford, Ill. (1984). During synthesis, N-a-protected amino acids having
protected side
chains are added stepwise to a growing polypeptide chain linked by its C-
terminal and to a
solid support, i.e., polystyrene beads. The peptides are synthesized by
linking an amino
group of an N-a-deprotected amino acid to an a-carboxy group of an N-a-
protected amino
acid that has been activated by reacting it with a reagent such as
dicyclohexylcarbodiimide.
The attachment of a free amino group to the activated carboxyl leads to
peptide bond
formation. The most commonly used N-a-protecting groups include Boc, which is
acid
labile, and Fmoc, which is base labile.
[0051] Materials suitable for use as the solid support are well known
to those of skill
in the art and include, but are not limited to, the following: halomethyl
resins, such as
chloromethyl resin or bromomethyl resin; hydroxymethyl resins; phenol resins,
such as 4-(a-
[2,4-dimethoxyphenyl]-Fmoc-aminomethyl)phenoxy resin; tert-alkyloxycarbonyl-
hydrazidated resins, and the like. Such resins are commercially available and
their methods
of preparation are known by those of ordinary skill in the art.
[0052] Briefly, the C-terminal N-a-protected amino acid is first
attached to the solid
support. The N-a-protecting group is then removed. The deprotected a-amino
group is
coupled to the activated a-carboxylate group of the next N-a-protected amino
acid. The
process is repeated until the desired peptide is synthesized. The resulting
peptides are then
cleaved from the insoluble polymer support and the amino acid side chains
deprotected.
Longer peptides can be derived by condensation of protected peptide fragments.
Details of
appropriate chemistries, resins, protecting groups, protected amino acids and
reagents are
well known in the art and so are not discussed in detail herein (See, Atherton
et al., Solid
13
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
Phase Peptide Synthesis: A Practical Approach, IRL Press (1989), and
Bodanszky, Peptide
Chemistry, A Practical Textbook, 2nd Ed., Springer-Verlag (1993)).
B. Producing Peptides by Recombinant Methods
[0053] As one skilled in the art will know, the peptides of the
present invention can
also be generated by recombinant means. Although it is often preferred to have
the peptides
synthesized chemically, according to the methods described above, there may be
some
advantages to obtain the peptides recombinantly in certain cases. For example,
when an
HSV-2 peptide of the present invention is to be conjugated with a heterologous
peptide
and/or a heterologous protein, the nucleic acid sequence encoding the HSV-2
peptide can be
introduced into a suitable expression vecto. r, and subsequently fused in-
frame with the coding
sequence(s) of the heterologous peptide and/or protein, so that upon
transfection or
transformation of an appropriate host cell line, the fusion polypeptide of the
HSV-2 peptide
and the heterologous peptide/protein can be produced and purified. A large
variety of
expression vectors and host cells well known to those skilled in the art can
be used for this
purpose.
C. Purification of Peptides
[0054] Purification of synthetic peptides is accomplished using
various methods of
chromatography, such as reverse phase HPLC, gel permeation, ion exchange, size
exclusion,
affinity, partition, or countercurrent distribution. The choices of
appropriate matrices and
buffers are well known in the art.
[0055] Purification of recombinantly produced peptides to substantial
purity can be
accomplished using standard techniques including selective precipitation with
such
substances as ammonium sulfate, column chromatography, immunopurification
methods and
others. See, e.g., Scopes, Protein Purification: Principles and Practice
(1982); U.S. Patent
No. 4,673,641; Ausubel et al., Current Protocols in Molecular Biology (1994);
and
Sambrook and Russell, Molecular Cloning: A Laboratory Manual 3d Ed. (2001). In
particular, when a recombinant polypeptide comprises an HSV-2 peptide fused to
a
heterologous protein with known molecular adhesion properties, it can be
purified with
relative ease and to a relatively high purity by passing through a column to
which a proper
binding partner is immobilized.
14
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
D. Confirmation of Peptide Sequence
[0056] The amino acid sequence of a peptide prepared for HSV-2 type-
specific
detection can be confirmed by a number of well established methods. For
example, the
conventional method of Edman degradation can be used to determine the amino
acid
sequence of a peptide. Several variations of sequencing methods based on Edman
degradation, including microsequencing, and methods based on mass spectrometry
are also
frequently used for this purpose.
E. Modification of Peptides
[0057] The peptides of the present invention can be modified to
achieve more
desirable properties. The design of chemically modified peptides and peptide
mimics which
are resistant to degradation by proteolytic enzymes or have improved
solubility or binding
ability is well known.
[0058] Modified amino acids or chemical derivatives of the peptides
used for HSV-2
type-specific detection may contain additional chemical moieties of modified
amino acids not
normally a part of the glycoprotein G2. Covalent modifications of the peptide
are within the
scope of the present invention. Such modifications may be introduced into a
peptide by
reacting targeted amino acid residues of the peptide with an organic
derivatizing agent that is
capable of reacting with selected side chains or terminal residues. The
following examples of
chemical derivatives are provided by way of illustration and not by way of
limitation.
[0059] The design of peptide mimics which are resistant to degradation by
proteolytic
enzymes is known to those skilled in the art. See e.g., Sawyer, Structure-
Based Drug Design,
P. Verapandia, Ed., N.Y. (1997); U.S. Patent Nos. 5,552,534 and 5,550,251.
Both peptide
backbone and side chain modifications may be used in designing secondary
structure
mimicry. Possible modifications include substitution of D-amino acids, Na-Me-
amino acids,
Ca-Me-amino acids, and dehydroamino acids. To this date, a variety of
secondary structure
mimetics have been designed and incorporated in peptides or peptidomimetics.
[0060] Other modifications include substitution of a natural amino
acid with an
unnatural hydroxylated amino acid, substitution of the carboxy groups in
acidic amino acids
with nitrile derivatives, substitution of the hydroxyl groups in basic amino
acids with alkyl
groups, or substitution of methionine with methionine sulfoxide. In addition,
an amino acid
of a peptide for type-specific detection of HSV-2 can be replaced by the same
amino acid but
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
of the opposite chirality, i.e., a naturally-occurring L-amino acid may be
replaced by its D-
configuration.
[0061] The peptides of the present invention can also be modified to
enhance their
ability to specifically bind to RSV-2 antibodies. For example, a linker, which
may comprise
a heterologous peptide, a heterologous protein, and/or chemicals of non-
polypeptide in
nature, may be introduced to either terminus of a peptide of the invention in
order to facilitate
the immobilization of the claimed peptide to a carrier, as well as to better
present the peptide
to HSV-2 antibodies. As another example, the peptides of the invention may be
dimerized to
achieve higher level of specific binding to HSV-2 antibodies. A variety of
means can be used
for dimerization, and the most commonly used method is through a disulfide
bond. Since
there is no naturally occurring cysteine residue in the peptides, a cysteine
residue can be
included as a part of the linker. The process of adding a linker to a peptide
of the present
invention will be discussed in detail in the next section.
IV. Immobilization of a Peptide to a Carrier
[0062] The peptides disclosed by the present invention for type-specific
detection of
HSV-2 antibodies are preferably immobilized to a solid support, or a carrier.
A carrier is
often a synthetic polymeric material, but may also be naturally-occurring.
Examples of
carrier material are acrylamide, cellulose, nitrocellulose, glass,
polystyrene, polyethylene
vinyl acetate, polypropylene, polymethacrylate, polyethylene, polysilicates,
polyethylene
oxide, polycarbonates, teflon, fluorocarbons, nylon, silicon rubber, collagen,
polyanhydrides,
polyglycolic acid, polylactic acid, polyorthoesters, polypropylfumarate,
glycosaminoglycans,
and polyamino acids. A carrier may be in one of the many useful forms
including thin films
or membranes, beads, bottles, dishes, fibers, woven fibers, shaped polymers,
particles, and
microparticles such as microspheres. Preferred forms of supports are plates
and beads. The
most preferred form of beads is magnetic beads or latex beads.
A. Attachment to a Linker
[0063] A peptide of the present invention can be attached to a
carrier via various
linkers. A linker can be attached to the N- or C-terminus of a peptide. In the
case of indirect
linkage between a peptide and a carrier, a linker may consist of a second
peptide that is
heterologous to the peptide of the invention, or may, in addition to a
heterologous peptide,
further comprise a protein heterologous to the peptide of the invention.
Branched amino acid
polymers can also be used as linkers, taking advantage of multiple functional
groups on
16
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
amino acid residues such as the two amine groups on a lysine residue. There
are other
suitable linkers well known to those of skill in the art, including but not
limited to, straight or
branched-chain carbon linkers, heterocyclic carbon linkers (e.g., SMCC), or
polyether
linkers. These linkers can be used in addition to or in place of a
heterologous peptide and/or
protein.
[0064] In the case of direct linkage, a linker can be a covalent bond
(e.g., a disulfide
bond) or a noncovalent bond (e.g., an ionic bond) between the peptide and the
carrier.
1. Indirect Linkage
[0065] When the peptides of the present invention are obtained
through synthetic
means, heterologous peptides serving as linkers may be included at the time of
peptide
...
synthesis. For example, a linker consisting of one cysteine residue, one
lysine residue, and at
least two glycine residues can be included as a part of the peptide sequence
to be synthesized,
either at the N- or C-terminus. Other linkers such as straight or branched-
chain carbon
linkers, heterocyclic carbon linkers, or polyether linkers may also be used to
join an HSV-2
peptide of the present invention alone or in combination with a heterologous
peptide and/or
protein. When a heterologous protein, such as BSA and KLH, is used in addition
to a
heterologous peptide in a linker, it may be joined to a peptide, to which a
heterologous
peptide is already added during synthesis, by chemical means following the
synthesis and
purification of the peptide. Such conjugation may be accomplished by joining
the peptide
and the heterologous protein through the a-carbon amino and carboxyl groups of
the terminal
amino acid residues, such as through a peptide bond; or by joining the amino
acid residues of
the peptide and the heterologous protein through their side groups, such as
through a disulfide
bond. Linkers such as heterologous peptides and/or proteins can also be joined
to the HSV-2
peptides of the present invention through a functional group of an internal
amino acid residue
(e.g., a second carboxyl group of an aspartate residue) of the HSV-2 peptides.
[0066] When peptides of the present invention are obtained through
recombinant
means, a nucleotide sequence encoding a heterologous peptide or protein can be
included
during the subcloning process and the resulting recombinant polypeptide will
thus already
have a proper linker attached.
[0067] One skilled in the art will recognize that a variety of other
linkers with
appropriate functional groups such as carbon linkers or polyether linkers may
also be useful
to practice the present invention. These linkers may be joined to a peptide's
constituent
17
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
amino acids through their side groups (for example, through a disulfide
linkage to cysteine).
The linkers may also be joined to the a-carbon amino or carboxyl groups of the
peptide's
terminal amino acids.
2. Direct Linkage
[0068] The peptides of the present invention can be immobilized to a
insoluble carrier
directly. The strategies of attaching a peptide, which usually contains a
variety of functional
groups such as carboxylic acid (¨COOH), free amine (¨NH2), or sulfhydryl (¨SH)
groups
that are available for reaction with a suitable functional group on the
carrier to result in a
linkage, are similar to some approaches of attaching a peptide or protein
linker to a carrier,
the detailed discussion of which is provided in the next section.
B. Attachment to a Carrier
1. Covalent Bonds
[0069] The peptides for HSV-2 type-specific detection, with or
without a linker, may
be attached to a carrier via a covalent bond. Frequently, a carrier has some
functional groups,
such as amine, carboxylic acid, and sulfhydryl groups, with which the
functional groups of a
peptide or a linker may easily react and establish a covalent bond that
conjugates the peptide
and the carrier. A covalent bond joining a peptide of the present invention
and a carrier can
exist between the carrier and a terminal amino acid residue of the peptide, or
between the
carrier and an internal amino acid residue of the peptide. In case there is no
functional group
naturally present on a carrier suitable for this purpose, the carrier may be
derivatized to
expose or to attach additional reactive functional groups prior to
conjugation. The
derivatization may involve attachment of any of a number of molecules such as
those
available from Pierce Chemical Company, Rockford, Illinois.
2. Non-covalent Bonds
[0070] Alternatively, a peptide can be linked to a carrier via the known
interaction of
a tag and a tag-binder. One of the partners of this binding interaction, e.g.,
a tag, can be
attached to the peptide as a linker whereas the other partner, e.g., a tag
binder, can be attached
to the carrier. A number of tags and tag binders can be used, based upon known
molecular
interactions well described in the literature. For example, where a tag has a
natural binder,
e.g., biotin, it can be used in conjunction with appropriate tag binders
(avidin, streptavidin,
neutravidin, etc.) Receptor-ligand interactions are also appropriate as tag
and tag-binder
18
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
pairs. For example, agonists and antagonists of cell membrane receptors (e.g.,
cell receptor-
ligand interactions such as transferrin, c-kit, viral receptor ligands,
cytokine receptors,
chemokine receptors, interleukin receptors, the cadherein family, the integrin
family, the
selectin family, and the like; see, e.g., Pigott & Power, The Adhesion
Molecule Facts Book I
(1993). Similarly, toxins and venoms, viral epitopes, hormones (e.g., opiates,
steroids, etc.),
intracellular receptors (e.g. which mediate the effects of various small
ligands, including
steroids, thyroid hormone, retinoids and vitamin D; peptides), drugs, lectins,
sugars, nucleic
acids (both linear and cyclic polymer configurations), oligosaccharides,
proteins,
phospholipids and antibodies can all interact with various cell receptors. In
addition, some
synthetic polymers, such as polyurethanes, polyesters, polycarbonates,
polyureas,
polyamides, polyethyleneimines, polyarylene sulfides, polysiloxanes,
polyimides, and
polyacetates can form an appropriate tag or tag binder as well.
[0071] A linker containing a tag can be attached to a peptide via a
number of ways as
described above. On the other hand, a tag binder can be fixed to a solid
substrate (i.e., a
carrier) using any of a variety of methods currently available. Solid
substrates are commonly
derivatized or functionalized by exposing all or a portion of the substrate to
a chemical
reagent which fixes a chemical group to the surface, and the chemical group is
in turn
reactive with a portion of the tag binder. For example, groups which are
suitable for
attachment to a longer chain portion would include amines, hydroxyl, thiol,
and carboxyl
groups. Aminoalkylsilanes and hydroxyalkylsilanes can be used to functionalize
a variety of
surfaces, such as glass surfaces. The construction of such solid phase
biopolymer arrays is
well described in the literature. See, e.g., Merrifield, J. Am. Chem. Soc.
85:2149-2154 (1963)
(describing solid phase synthesis of, e.g., peptides); Geysen et al., J.
Immun. Meth. 102:259-
274 (1987) (describing synthesis of solid phase components on pins); Frank &
Doring,
Tetrahedron 44:6031-6040 (1988) (describing synthesis of various peptide
sequences on
cellulose disks); Fodor et al., Science, 251:767-777 (1991); Sheldon et al.,
Clinical Chemistry
39(4):718-719 (1993); and Kozal et al., Nature Medicine 2(7):753759 (1996)
(all describing
arrays of biopolymers fixed to solid substrates). Non-chemical approaches for
fixing tag
binders to substrates include other common methods, such as heat, cross-
linking by UV
radiation, and the like.
19
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
V. Assays for Type-Specific HSV-2 Antibody Detection
A. Detection of HSV-2 Antibodies Using Peptides of the Present
Invention
[0072] In order for peptides of the present invention to be useful
for HSV-2 type-
specific detection, they must first be able to bind HSV-2 antibodies with
specificity. To test
such specific binding, a number of well known immunological binding assays can
be
performed. See, e.g., U.S. Patent Nos. 4,366,241; 4,376,110; 4,517,288; and
4,837,168. For
a general review of immunoassay methods, see also Asai, Methods in Cell
Biology, Volume
37: Antibodies in Cell Biology, Academic Press, Inc. NY (1993).
[0073] Typically, peptides of the present invention can be
immobilized and used as a
so-called "capture agent" for HSV-2 antibodies. Samples that are known to
contain HSV-2
antibodies but not HSV-1 antibodies may be used in binding assays to screen
for peptides that
can bind HSV-2 antibodies with specificity. The proper binding conditions are
well known
in the art and general instructions on performing such binding assays may be
found in many
scientific publications. See, e.g., Harlow and Lane, Antibodies: A Laboratory
Manual, Cold
Spring Harbor Laboratory (1988). Upon formation of the antibody-peptide
complex, a
labeling agent is used to indicate the presence of such complex. In the
present case, there are
several ways of using a labeling agent for this purpose. For instance, the
labeling agent may
be a second antibody that can recognize an antibody-peptide complex and bears
a label.
Alternatively, the second antibody may itself lack a label, but can in turn be
bound by a
labeled third antibody specific to antibodies of the species from which the
second antibody is
derived. The second antibody may also be modified with a detectable moiety,
such as biotin,
to which a third labeled molecule can bind with specificity, such as
streptavidin with a label.
In addition, other proteins capable of specifically binding immnunoglobulin
constant regions,
such as protein A or protein G, can also be used as labeling agents. These
proteins are
normal constituents of streptococcal bacteria cell walls, and exhibit a strong
non-
immunogenic reactivity toward immunoglobulin constant regions from a variety
of species.
See, generally, Kronval et al., J. Imniunol., 111:1401-1406 (1973); and
Akerstrom et al., J.
Immunol., 135:2589-2592 (1985).
[0074] Throughout the assays, incubation and/or washing steps may be
required after
each combination of reagents. Incubation steps can vary from about 5 seconds
to several
hours, preferably from about 5 minutes to about 24 hours. The incubation time
will vary,
depending upon the assay format, particular peptides, volume of solution,
concentrations, and
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
the like. The assays are frequently carried out at ambient temperature,
although they can be
conducted over a range of temperatures, such as from about 10 C to about 40 C.
[0075] Different means of labeling can be used for detection of
antibody-peptide
complex. A labeling moiety can be, e.g., a fluorescent molecule (such as
fluorescein,
rhodamine, Texas Red, and phycoerythrin) or an enzyme molecule (such as
horseradish
peroxidase, alkaline phosphatase, andi3-galactosidase) attached to a second or
a third
antibody, allowing detection based on fluorescence emission or a product of a
chemical
reaction catalyzed by the enzyme. Radioactive labels involving various
isotopes, such as 3H,
1251, 35s,
or - 1
2P, can also be attached to appropriate molecules, and detection of antibody-
peptide complex can thus be made by any suitable methods that registers
radioactivity, such
as autoradiography. See, e.g., Tijssen, "Practice and Theory of Enzyme
Immunoassays,"
Laboratory Techniques in Biochemistly and Molecular Biology, Burdon and van
Knippenberg Eds., Elsevier (1985), pp. 9-20. An introduction to labels,
labeling procedures,
and detection of labels can also be found in Polak and Van Noorden,
Introduction to
Immunocytochemistry, 2d Ed., Springer Verlag, NY (1997); and in Haugland,
Handbook of
Fluorescent Probes and Research chemicals, a combined handbook and catalogue
published
by Molecular Probes, Inc. (1996).
B. Flow Cytometry
[0076] Flow cytometry is one of the preferred methods for detecting
the presence of
HSV-2 type-specific antibodies, where the peptides of the present invention
are conjugated to
suitable particles and specific binding of HSV-2 antibodies is detected
through the binding of
a third molecule labeled with, e.g., fluorescence. Methods of and
instrumentation for flow
cytometry are known in the art, and can be used in the practice of the present
invention. Flow
cytometry in general resides in the passage of a suspension of the
microparticles as a stream
past a laser beam and the detection of fluorescent emission from each particle
by a photo
multiplier tube. Detailed descriptions of instrumentation and methods for flow
cytometry are
found in the literature. Examples are McHugh, "Flow Microsphere Immunoassay
for the
Quantitative and Simultaneous Detection of Multiple Soluble Analytes," Methods
in Cell
Biology 42, Part B (Academic Press, 1994); McHugh et al., "Microsphere-Based
Fluorescence Immunoassays Using Flow Cytometry Instrumentation," Clinical Flow
Cytometry, Bauer, K.D., et al., eds. (Baltimore, Maryland, USA: Williams and
Williams,
1993), pp. 535-544; Lindmo et al., "Immunometric Assay Using Mixtures of Two
Particle
21
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
Types of Different Affinity," J. Immunol. Meth. 126: 183-189 (1990); McHugh,
"Flow
Cytometry and the Application of Microsphere-Based Fluorescence Immunoassays,"
Immunochemica 5: 116 (1991); Horan et al., "Fluid Phase Particle Fluorescence
Analysis:
Rheumatoid Factor Specificity Evaluated by Laser Flow Cytophotometry,"
Immunoassays in
the Clinical Laboratory, 185-189 (Liss 1979); Wilson et al., "A New
Microsphere-Based
Immunoftuorescence Assay Using Flow Cytometry," J. Immunol. Meth. 107: 225-230
(1988); Fulwyler et al., "Flow Microsphere Immunoassay for the Quantitative
and
Simultaneous Detection of Multiple Soluble Analytes," Meth. Cell Biol. 33: 613-
629 (1990);
Coulter Electronics Inc., United Kingdom Patent No. 1,561,042 (published
February 13,
1980); and Steinkamp et al., Review of Scientific Instruments 44(9): 1301-1310
(1973).
[0077] The particles used in the practice of this invention are
preferably microscopic
in size and formed of a polymeric material. Polymers that will be useful as
microparticles are
those that are chemically inert relative to the components of the biological
sample and to the
assay reagents other than the binding member coatings that are affixed to the
microparticle
surface. Suitable microparticle materials will also have minimal
autofluorescence, will be
solid and insoluble in the sample and in any buffers, solvents, carriers,
diluents, or
suspending agents used in the assay, and will be capable of affixing to the
appropriate coating
material, preferably through covalent bonding. Examples of suitable polymers
are polyesters,
polyethers, polyolefins, polyalkylene oxides, polyamides, polyurethanes,
polysaccharides,
celluloses, and polyisoprenes. Crosslinking is useful in many polymers for
imparting
structural integrity and rigidity to the microparticle. The size range of the
microparticles can
vary and particular size ranges are not critical to the invention. In most
cases, the
microparticles will range in diameter from about 0.5 micrometers to about 100
micrometers,
and preferably from about 0.3 micrometers to about 40 micrometers.
[0078] To facilitate the particle recovery and washing steps of the assay,
the particles
preferably contain a magnetically responsive material, i.e., any material that
responds to a
magnetic field. Separation of the solid and liquid phases, either after
incubation or after a
washing step, is then achieved by imposing a magnetic field on the reaction
vessel in which
the suspension is incubated, causing the particles to adhere to the wall of
the vessel and
thereby permitting the liquid to be removed by decantation or aspiration.
Magnetically
responsive materials of interest in this invention include paramagnetic
materials,
ferromagnetic materials, ferrimagnetic materials, and metamagnetic materials.
Paramagnetic
22
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
materials are preferred. Examples are iron, nickel, and cobalt, as well as
metal oxides such as
Fe304, BaFe12019, CoO, NiO, Mn203, Cr203, and CoMnP.
[0079] The magnetically responsive material can be dispersed
throughout the
polymer, applied as a coating on the polymer surface or as one of two or more
coatings on the
surface, or incorporated or affixed in any other manner that secures the
material in to the
particle. The quantity of magnetically responsive material in the particle is
not critical and
can vary over a wide range. The quantity can affect the density of the microp
article,
however, and both the quantity and the particle size can affect the ease of
maintaining the
microparticle in suspension for purposes of achieving maximal contact between
the liquid
and solid phase and for facilitating flow cytometry. An excessive quantity of
magnetically
responsive material in the microparticles may produce autofluorescence at a
level high
enough to interfere with the assay results. It is therefore preferred that the
concentration of
magnetically responsive material be low enough to minimize any
autofluorescence emanating
from the material. With these considerations in mind, the magnetically
responsive material in
a particle in accordance with this invention preferably ranges from about
0.05% to about 75%
by weight of the particle as a whole. A more preferred weight percent range is
from about
1% to about 50%, a still more preferred weight percent range is from about 2%
to about 25%,
and an even more preferred weight percent range is from about 2% to about 8%.
[0080] Coating of the particle surface with the appropriate assay
reagent can be
achieved by electrostatic attraction, specific affinity interaction,
hydrophobic interaction, or
covalent bonding. Covalent bonding is preferred. The polymer can be
derivatized with
functional groups for covalent attachment of the assay reagent by conventional
means,
notably by the use of monomers that contain the functional groups, such
monomers serving
either as the sole monomer or as a co-monomer. Examples of suitable functional
groups are
amine groups (¨NH2), ammonium groups (¨NH3+ or ¨NR), hydroxyl groups (¨OH),
carboxylic acid groups (¨COOH), and isocyanate groups (¨NCO). Useful monomers
for
introducing carboxylic acid groups into polyolefins, for example, are acrylic
acid and
methacrylic acid.
[0081] Linkers can be used as a means of increasing the density of
antibody-
recognizable epitopes on the particle surface and decreasing steric hindrance.
This will
increase the range and sensitivity of the assay. Linkers can also be used as a
means of adding
specific types of reactive groups to the solid phase surface if needed to
secure the particular
23
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
coating materials of this invention. Examples of suitable useful functional
groups are
polylysine, polyaspartic acid, polyglutamic acid, and polyarginine.
[0082] In general, care should be taken to avoid the use of
particles that exhibit high
autofluorescence. Particles formed by conventional emulsion polymerization
techniques
from a wide variety of starting monomers are generally suitable since they
exhibit at most a
low level of autofluorescence. Conversely, particles that have been modified
to increase their
porosity and hence their surface area, i.e., those particles that are referred
to in the literature
as "macroporous" particles, are less desirable since they tend to exhibit high
autofluorescence. A further consideration is that autofluorescence increases
with increasing
size and increasing percentage of divinylbenzene monomer.
[0083] The labels used in the labeled binding members may be any
label that is
capable of emitting detectable signal. Preferred such labels are fluorophores.
A vast array of
fluorophores are reported in the literature and thus known to those skilled in
the art, and
many are readily available from commercial suppliers to the biotechnology
industry.
Literature sources for fluorophores include Cardullo etal., Proc. Natl. Acad.
Sci. USA 85:
8790-8794 (1988); Dexter, D.L., J. of Chemical Physics 21: 836- 850 (1953);
Hochstrasser et
al., Biophysical Chemistry 45: 133-141 (1992); Selvin, P., Methods in
Enzymology 246: 300-
334 (1995); Steinberg, I. Ann. Rev. Biochem., 40: 83- 114 (1971); Stryer, L.
Ann. Rev.
Biochem., 47: 819-846 (1978); Wang etal., Tetrahedron Letters 31: 6493-6496
(1990);
Wang etal., Anal. Chem. 67: 1197-1203 (1995).
[0084] The following is a list of examples of fluorophores:
4-acetamido-4'-isothiocyanatostilbene-2,2'disulfonic acid
acridine
acridine isothiocyanate
5-(2'-aminoethypaminonaphthalene-1-sulfonic acid (EDANS)
4-amino-N-[3-vinylsulfonyl)phenyl]naphthalimide-3,5 disulfonate
N-(4-anilino-1-naphthyl)maleimide
anthranilamide
BODIPY
Brilliant Yellow
coumarin
7-amino-4-methylcoumarin (AMC, Coumarin 120)
7-amino-4-trifluoromethylcoumarin (Coumarin 151)
cyanine dyes
cyanosine
4',6-diaminidino-2-phenylindole (DAPI)
5', 5"-dibromopyrogallol-sulfonaphthalein (Bromopyrogallol Red)
7-diethylamino-3-(4'-isothiocyanatopheny1)-4-methylcoumarin
24
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
diethylenetriamine pentaacetate
4,4'-diisothiocyanatodihydro-stilbene-2,2'-disulfonic acid
4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid
5-[dimethylamino]naphthalene-1-sulfonyl chloride (DNS, dansylchloride)
4-(4'-dimethylaminophenylazo)benzoic acid (DABCYL)
4-dimethylaminophenylazopheny1-4'-isothiocyanate (DABITC)
eosin
eosin isothiocyanate
erythro sin B
erythro sin isothiocyanate
ethidium
5-carboxyfluorescein (FA_M)
5-(4,6-dichlorotriazin-2-yDaminofluorescein (DTAF)
2' ,7'-dimethoxy-4'5 '-dichloro-6-carboxyfluorescein (JOE)
fluorescein
fluorescein isothiocyanate
fluorescamine
IR144
IR1446
Malachite Green isothiocyanate
4-methylumbelliferone
ortho cresolphthalein
nitrotyrosine
pararosaniline
Phenol Red
Phycobiliproteins (B-phycoerythrin, R-phycoerythrin, etc)
o-phthaldialdehyde
pyrene
pyrene butyrate
succinimidyl 1-pyrene butyrate
quantum dots
Reactive Red 4 (CibacronTM Brilliant Red 3B-A)
6-carboxy-X-rhodamine (ROX)
6-carboxyrhodamine (R6G)
lissamine rhodamine B sulfonyl chloride rhodamine (Rhod)
rhodamine B
rhodamine 123
rhodamine X isothiocyanate
sulforhodamine B
sulforhodamine 101
sulfonyl chloride derivative of sulforhodamine 101 (Texas Red)
N,N,N',N'-tetramethy1-6-carboxyrhodamine (TAMRA)
tetramethyl rhodamine
tetramethyl rhodamine isothiocyanate (TRITC)
riboflavin
rosolic acid
lanthanide chelate derivatives
[0085] The attachment of any of these fluorophores to the binding
molecules
described above to form assay reagents for use in the practice of this
invention is achieved by
CA 02558246 2010-08-17
conventional covalent bonding, using appropriate functional groups on the
fluorophores and
on the binding members. The recognition of such groups and the reactions to
form the
linkages will be readily apparent to those skilled in the art.
[00861 Similarly, methods of and instrumentation for applying and
removing a
magnetic field as part of an automated assay are known to those skilled in the
art and reported
in the literature. Examples of literature reports are Forrest et al., United
States Patent No.
4,141,687 (Technicon Instruments Corporation, February 27, 1979); Ithakissios,
United
States Patent No. 4,115,534 (Minnesota Mining and Manufacturing Company,
September 19,
1978); Vlieger, A.M., et al., Analytical Biochemistiy 205:1-7 (1992); Dudley,
Journal of
Clinical Immunoassay 14:77-82 (1991); and Smart, Journal of Clinical
Immunoassay 15:246-
251 (1992).
C. Non-Reactivity to HSV-1 Antibodies by Peptides of the Present
Invention
[0087] Another equally important aspect of the necessary
characteristics of peptides
to be used for HSV-2 type-specific detection is that they must not bind HSV-1
antibodies
with detectable specificity, particularly in the test formats used for HSV-2
antibody detection.
Once peptides are shown to react to HSV-2 antibodies specifically, they will
be further tested
for any possible cross-reactivity to HSV-1 antibodies and antibodies against
other viruses
such as the herpes family. To test their non-reactivity to HSV-1 antibodies,
the peptides are
immobilized and used as "capture agents" in immunoassays essentially identical
to those
described in last section, except that samples confirmed to contain HSV-1
antibodies (but not
HSV-2 antibodies) are used for the binding assays.
[0088] The following examples are provided for the purpose of
illustration and not
limitation.
26
CA 02558246 2010-03-23
EXAMPLES
I. Peptide Synthesis
[0089] Peptides listed in Table 1 were synthesized as monomers using the
N-a-
protecting group Boc or Fmoc.
SEQ
Name ID SEQUENCE
NO:
1 9 PGSPAPPP PEHRGG = PEEFEGAGDG = EPPEDDDS = ATGL GG
CK
2 10 APPP PEHRGG PEEFEGAGDG EPPEDDDS
GGGG CK
3 11 PEHRGG PEEFEGAGDG EPPEDDDS
GGGG CK
4 12 PEEFEGAGDG EPPEDDDS
GGGG CK
8 KC GGGG PEHRGG PEEFEGAGDG EPPEDDDS
6 13 RGRAG RRRYAHPSVR
GGGG CK
7 14 WRGRAG RRRYAHPSVR Y
GGGG CK
8 15 RGRAG RRRYAHPSVR YVCLPPER D
GGGG CK
9 16 RRRYAHPSVR YVCLPPER D GGGG
CK --
17 KC GGGG WRGRAG RRRYAHPSVR
11 18 KC GGGG RGRAG RRRYAHPSVR
12 (PEHRGG PEEFEGAGDG EPPEDDDS
GGGG)4K3 CK _
13 (WRGRAG RRRYAHPSVR Y
GGGG)4K3 CK
II. Dimerization
[0090] An optional step of peptide dimerization is performed as follows:
a monomer
peptide was dissolved in either deionized water or 0.1 M sodium bicarbonate
and stirred at
4 C overnight. The resulting oxidized, dimeric peptide was purified by
preparative HPLC
using a C18 column.
III. Preparation of a Monomer Peptide-BSA conjugate
[0091] To a solution of Bovine Serum Albumin (BSA) in 0.1 mM borate
buffer
(pH8.0) (10 mg/ml), 154 j.ii of SMCC, N-hydroxysuccimide in dimethylsulfoxide
(DMSO)
(10mg/m1) was added. The mixture was incubated at room temperature for 2
hours, applied
to a Sephadex G-25 column (30 ml) pre-equilibrated with 10 rnM phosphate-
saline (PBS,
pH7.4), and eluted with PBS. The first peak was collected and the protein
concentration was
estimated by absorbance at 280 run.
[0092] The monomer peptide (1 mg) was mixed with 1 mg of the modified BSA
and
stored at 4 C overnight. The peptide-BSA conjugate was purified through size
exclusion
chromatography.
IV. Preparation of a Dimer Peptide-BSA Conjugate
[0093] A dimer peptide-BSA conjugate can be prepared in accordance with
the
following description.
27
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
1. SMCC-Peptide Dimer
[0094] 50 I of SMCC solution in DMSO (10 mg/ml) is added to 1 ml of
peptide
dimer solution (5 mg/ml) in 100 mM phosphate (pH 8.0). After incubation for 2
hours, the
modified peptide is purified by HPLC chromatography.
2. 2-Mercaptoacetyl-BSA
[0095] 50 p,1 of N-succinimidyl S-acetylthioacetate (SATA) (10 mg/ml)
in DMSO is
added to 1 ml of BSA (10mg/m1) in 100 mM phosphate (pH 8.0). After incubation
for 2
hours, 100 pi of 200 mM N-ethyl maleimide containing 10 mM EDTA is added. The
reaction mixture is incubated at room temperature for 1 hour and then applied
to a Sephadex
G-25 column. The modified BSA is eluted from the column with 10 mM PBS.
3. Peptide Dimer-BSA Conjugate
[0096] 4 mg of the modified BSA is mixed with 4 mg modified peptide
at 2-8 C and
the mixture is incubated overnight. The conjugated peptide is purified by size
exclusion
chromatography.
V. Preparation of Peptide or Peptide-BSA Coated Magnetic Beads
[0097] To 6 mg of magnetic beads washed with 25 mM 24N-
morpholinoiethanesulfonic acid (MES) (pH6.1) twice 588 ill of deionized water,
80 pl of 0.5
M MES (p116.1), 92 p.1 of 100 mg/m1N-hydroxysuccinimide (NHS) and 40 pl of 50
mg/ml 1-
ethy1-3-(3-dimethylaminopropyl)carbodiimide (EDC) in deionized water were
added and the
mixture was vortexed at room temperature for 30 minutes. The activated beads
were washed
twice with 25 mM MES (p116.1). Subsequently, 100 p.1 of 0.5 M MES (pH 6.1),
720 ftl of
deionized water, and 180[11 of Peptide (1 mg/ml) or Peptide-BSA conjugate (1
mg/ml) were
added and the mixture vortexed. The mixture was further incubated at room
temperature on a
rotator for 2 hours and centrifuged at 7000 rpm for 2 min. One ml of 1% BSA in
PBS
containing 20 mM Tris Base was added after discarding the supernatant, mixed
for 2 hour at
room temperature on a rotator and then centrifuged to remove the supernatant.
The beads
were washed with 1% BSA in 10 mM PBS twice and stored in 1 ml of 1% BSA.
Scheme 1
shows an illustrative process of conjugating an HSV-2 peptide via a
heterologous peptide
already attached to its C-terminus to SMCC, which is in turn attached to a
microsphere via
BSA:
28
CA 02558246 2010-08-17
0 0
0
BSA ¨NH 2 + BSA
0 d
st-1
COON
TH 2
NH2
s BSA -ism
Peptide 0
0-000H 0 COOH
sINH2
EDC/NHS 9
rospheres µ3/4111r-Cµ C¨NH ¨NH ¨C-0¨/N>--
Mic
0
SCHEME I
VI. Flow Cytometry Immunoassay (FCIA)
[00981 The peptides or peptide-BSA conjugates, such as those in Table
1, were
coupled to predefined magnetic beads (every peptide or peptide-BSA conjugate
was coupled
to magnetic beads whose fluorescence is characteristic of a particular region
of dye content)
and were mixed together at similar bead number for each region of magnetic
beads. The
mixed beads were diluted with 1% BSA in PBS with 0.1% Tween 2OTM to 1000
copies of each
region magnetic beads per ml.
[00991 To a microtiter tube 100 ill of sample diluent, 5 p1 of patient
sample and 100
11.1 of the bead mix were added and incubated at 37 C on a shaker for 20
minutes. After
magnetic separation, the beads were washed with wash buffer (10 mM PBS with
0.1% Tween
20) twice. 50 1.1.1 of' antihuman IgG (Fc specific) ¨B-phycoerythrin conjugate
was added.
Following a 10-minute incubation on a shaker at 37 C, the beads were washed
again with the
wash buffer twice and resuspended in 50 p1 of the wash buffer. The beads were
counted and
analyzed on Luminex 100 instrument. The amount of antibodies (IgG) bound to
the magnetic
beads was determined with antihuman IgG conjugated to phycoerythrin.
WI. Comparison with Commercially Available Assay Systems
[01001 Studies using 137 clinically defined patient samples have
demonstrated that
the assay system using the peptide-BSA conjugates of the present invention
performed better
than the commercially available gG-2 based HSV-2 type-specific IgG assay
systems and had
a 100% agreement with the confirmation test of Western Blot assays (Table 2).
29
CA 02558246 2006-08-31
WO 2005/098051
PCT/US2004/006780
Table 2. Peptide-Based HSV-2 Type-Specific Assay
Peptide-Based HSV-2 IgG Assay
Agreement Sensitivity Specificity
Meridian EIA 93.10% 86.20% 96.60%
MRL EIA 97.60% 93.10% 100%
Western Blot 100% 100% 100%
VIII. Non-reactivity to HSV-1 IgG
[0101]
HSV-2 type-specific immunoassay systems of the present invention are tested
for lack of cross-reactivity to antibodies against other viruses of the herpes
family,
particularly HSV-1. Table 3 demonstrates the lack of cross-reactivity to HSV-1
IgG as
confirmed by two commercially available HSV-2 type-specific assay systems and
Western
Blot assays.
Table 3. Non-Reactivity to HSV-1 Antibody Positive Samples
Meridian MRL Western Blot Present Method
Sample HSV-1 HSV-2 HSV-1 HSV-2 HSV-1 HSV-2
HSV-2
I.D. Value Result Value Result Value Result Value Result Result Result
Value Result
64 1.40 Pos 0.13 Neg 1.62 Pos 0.11 Neg Pos Neg 0.08 Neg
92 1.42 Pos 0.11 Neg 3.17 Pos 0.05 Neg Pos Neg 0.06 Neg
66 1.43 Pos 0.55 Neg 1.46 Pos 0.16 Neg Pos Neg 0.09 Neg
63 1.44 Pos 0.40 Neg 1.79 Pos 0.04 Neg Pos Neg 0.07 Neg
110 1.57 Pos 0.41 Neg 2.11 Pos 0.61 Neg Pos Neg 0.79 Neg
114 1.61 Pos 0.10 Neg 2.01 Pos 0.15 Neg Pos Neg 0.09 Neg
43 1.66 Pos 0.06 Neg 2.44 Pos 0.05 Neg Pos Neg 0.07 Neg
67 1.70 Pos 0.15 Neg 2.37 Pos 0.20 Neg Pos Neg 0.10 Neg
36 1.90 Pos 0.14 Neg 2.78 Pos 0.28 Neg Pos Neg 0.08 Neg
101 2.04 Pos 0.13 Neg 2.48 Pos 0.26 Neg Pos Neg 0.13 Neg
46 2.08 Pos 0.10 Neg 3.54 Pos 0.15 Neg Pos Neg 0.08 Neg
29 2.27 Pos 2.03 Pos 0.39 Neg 0.15 Neg
0.06 Neg
61 2.47 Pos 2.95 Pos 0.90 Equ 0.30 Neg
0.12 Neg
11 2.78 Pos 1.20 Neg 4.36 Pos 8.72 Pos
0.87 Neg
56 2.79 Pos 0.19 Neg 3.78 Pos 0.07 Neg Pos Neg 0.10 Neg
106 2.89 Pos 0.07 Neg 4.82 Pos 0.38 Neg Pos Neg 0.11 Neg
12 3.72 Pos 0.15 Neg 5.79 Pos 0.95 Equ Pos Neg 0.09 Neg
78 3.96 Pos 0.19 Neg 5.91 Pos 0.25 Neg Pos Neg 0.10 Neg
6 4.26 Pos 0.24 Neg 9.25 Pos 0.10 Neg Pos Neg 0.10 Neg
18 4.46 Pos 0.57 Neg 8.22 Pos 0.17 Neg Pos Neg 0.09 Neg
13 4.70 Pos 0.05 Neg 6.68 Pos 0.10 Neg Pos Neg 0.05 Neg
2 4.93 Pos 0.22 Neg 9.25 Pos 0.47 Neg Pos Neg 0.09 Neg
4.99 Pos 0.18 Neg 7.82 Pos 0.13 Neg Pos Neg 0.08 Neg
93 5.05 Pos 1.83 Pos 0.36 Neg 0.18 Neg
0.14 Neg
5.14 Pos 0.10 Neg 7.02 Pos 0.40 Neg Pos Neg 0.06 Neg
21 5.27 Pos 0.09 Neg 6.95 Pos 0.10 Neg Pos Neg 0.09 Neg
59 5.63 Pos 0.28 Neg 9.25 Pos 0.72 Neg Pos Neg 0.11 Neg
_ 88 5.79 Pos 0.13 Neg 6.93 Pos 0.08 Neg Pos Neg 0.08
Neg
44 6.08 Pos 0.19 Neg 8.55 Pos 0.33 Neg Pos Neg 0.12 Neg
62 6.16 Pos 0.12 Neg 9.25 Pos 0.24 Neg Pos Neg 0.09 Neg
83 6.25 Pos 0.10 Neg 7.96 Pos 0.15 Neg Pos Neg 0.06 Neg
45 6.54 Pos 0.11 Neg 9.25 Pos 0.44 Neg Pos Neg 0.42 Neg
51 6.55 Pos 0.11 Neg 9.25 Pos 0.08 Neg Pos Neg 0.06 Neg
CA 02558246 2010-03-23
Table 3. Non-Reactivity to HSV-1 Antibody Positive Samples
Meridian MRL Western Blot Present
Method
Sample HSV-1 HSV-2 HSV-1 HSV-2 HSV-1 HSV-2 HSV-2
I.D. Value Result Value Result Value Result Value Result Result Result
Value Result
7 6.56 Pos 0.16 Neg 9.25 Pos 0.15 Neg Pos Neg 0.10 Neg
25 6.56 _ Pos 0.14 Neg 9.25 Pos 0.12 Neg Pos
Neg 0.08 Neg
27 6.56 Pos 0.13 Neg 8.85 Pos 0.41 , Neg
Pos Neg 0.14 Neg
40 6.56 Pos 0.20 Neg 9.25 Pos 0.49 Neg Pos Neg 0.22 Neg
41 6.56 Pos 0.34 Neg 9.25 Pos 0.19 Neg Pos Neg 0.15 Neg
50 6.56 , Pos 0.15 Neg 9.25 Pos , 0.07 Neg Pos
Neg 0.08 Neg
58 6.56 Pos 0.73 Neg 8.45 Pos , 0.24 Neg Pos
Neg 0.10 Neg
74 6.56 Pos 0.14 Neg 9.25 Pos 0.09 Neg Pos Neg 0.09 Neg
75 6.56 Pos 0.15 Neg 8.78 Pos 0.17 Neg Pos Neg 0.07 Neg
77 6.56 , Pos 0.11 Neg 8.42 Pos 0.22 , Neg
Pos Neg 0.41 Neg
80 6.56 Pos 0.58 Neg 8.05 Pos 0.98 Equ Pos Neg 0.89 Neg
85 6.56 Pos 0.12 Neg 8.88 Pos 0.42 Neg Pos Neg 0.09 Neg
100 8.46 Pos 0.07 Neg 9.25 , Pos 0.08 Neg
Pos Neg 0.07 Neg
118 8.46 Pos 0.08 Neg 9.06 Pos 0.45 Neg Pos Neg 0.07 Neg
54 1.53 Pos 3.30 Pos 3.15 Pos 6.79 Pos
5.77 Pos
52 6.46 Pos 2.03 Pos 9.25 Pos 6.47 Pos
2.59 Pos
24 6.56 , Pos 2.09 Pos 6.62 Pos 8.72 Pos
5.53 Pos
37 6.56 Pos 2.78 Pos 9.25 Pos 6.73 Pos
2.17 Pos
42 6.56 Pos 5.85 Pos 9.25 Pos 8.72 Pos
6.41 Pos
57 6.56 Pos 4.28 Pos 9.14 Pos 5.54 Pos
4.43 Pos
SEQUENCE LISTING IN ELECTRONIC FORM
101021 This description contains a sequence listing in electronic form
in ASCII text
format. A copy of the sequence listing in electronic form is available from
the Canadian
Intellectual Property Office.
31
CA 02558246 2010-08-17
The sequences in the sequence listing in electronic form are reproduced in the
following Table.
SEQUENCE TABLE
<110> Bio-Rad Laboratories, Inc.
<120> RSV-2 Type-Specific Immunoassays Using Glycoprotein G2
Peptides
<130> 40330-2425
<140> CA 2,558,246
<141> 2003-03-04
<160> 18
<170> PatentIn Ver. 2.1
<210> 1
<211> 36
<212> PRT
<213> herpes simplex virus 2
<220>
<223> herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) amino acids 1-36
<400> 1
Pro Gly Ser Pro Ala Pro Pro Pro Pro Glu His Arg Gly Gly Pro Glu
1 5 10 15
Glu Phe Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser
20 25 30
Ala Thr Gly Leu
<210> 2
<211> 4
<212> pRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:heterologous
peptide linker
<400> 2
Gly Gly Cys Lys
1
32
CA 02558246 2010-08-17
<210> 3
<211> 6
<212> PRT
=
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:heterologous
peptide linker
<400> 3
Gly Gly Gly Gly Cys Lys
1 5
<210> 4
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:heterologous
peptide linker
<400> 4
Lys Cys Gly Gly Gly Gly
1 5
<210> 5
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:peptide
1-SMCC-BSA conjugate
<220>
<221> MOD RES
<222> (40)
<223> Xaa - Lys conjugated to
4-(maleimidomethyl)-1-cyclohexanecarboxylic acid
(SMCC)-bovine serum albumin (BSA)
<400> 5
Pro Gly Ser Pro Ala Pro Pro Pro Pro Glu His Arg Gly Gly Pro Glu
1 5 10 15
Glu Phe Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser
20 25 30
Ala Thr Gly Leu Gly Gly Cys Xaa
35 40
<210> 6
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:peptide
2-SMCC-BSA conjugate
<220>
<221> MOD RES
<222> (34)
33
CA 02558246 2010-08-17
<223> Xaa = Lys conjugated to
4-(maleimidomethyl)-1-cyclohexanecarboxylic acid
(SMCC)-bovine serum albumin (BSA)
<400> 6
Ala Pro Pro Pro Pro Glu His Arg Gly Gly Pro Glu Glu Phe Glu Gly
1 5 10 15
Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser Gly Gly Gly Gly
20 25 30
Cys Xaa
<210> 7
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial
Sequence:BSA-SMCC-peptide 5 conjugate
<220>
<221> MOD RES
<222> (1)¨
<223> Xaa = Lys conjugated to
4-(maleimidomethyl)-1-cyclohexanecarboxylic acid
(SMCC)-bovine serum albumin (BSA)
<400> 7
Xaa Cys Gly Gly Gly Gly Pro Glu His Arg Gly Gly Pro Glu Glu Phe
1 5 10 15
Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser
20 25 30
<210> 8
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 5
<400> 8
Lys Cys Gly Gly Gly Gly Pro Glu His Arg Gly Gly Pro Glu Glu Phe
1 5 10 15
Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser
20 25 30
<210> 9
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 1
34
CA 02558246 2010-08-17
<400> 9
Pro Gly Ser Pro Ala Pro Pro Pro Pro Glu His Arg Gly Gly Pro Glu
1 5 10 15
Glu Phe Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser
20 25 30
Ala Thr Gly Leu Gly Gly Cys Lys
35 40
<210> 10
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 2
<400> 10
Ala Pro Pro Pro Pro Glu His Arg Gly Gly Pro Glu Glu Phe Glu Gly
1 5 10 15
Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp Asp Ser Gly Gly Gly Gly
20 25 30
Cys Lys
<210> 11
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 3
<400> 11
Pro Glu His Arg Gly Gly Pro Glu Glu Phe Glu Gly Ala Gly Asp Gly
1 5 10 15
Glu Pro Pro Glu Asp Asp Asp Ser Gly Gly Gly Gly Cys Lys
20 25 30
<210> 12
<211> 24
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 4
<400> 12
Pro Glu Glu Phe Glu Gly Ala Gly Asp Gly Glu Pro Pro Glu Asp Asp
1 5 10 15
Asp Ser Gly Gly Gly Gly Cys Lys
35
CA 02558246 2010-08-17
<210> 13
<211> 21
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 6
<400> 13
Arg Gly Arg Ala Gly Arg Arg Arg Tyr Ala His Pro Ser Val Arg Gly
1 5 10 15
Gly Gly Gly Cys Lys
<210> 14
<211> 22
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 7
<400> 14
Trp Arg Gly Arg Ala Gly Arg Arg Arg Tyr Ala His Pro Ser Val Arg
1 5 10 15
Gly Gly Gly Gly Cys Lys
<210> 15
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 8
<400> 15
Arg Gly Arg Ala Gly Arg Arg Arg Tyr Ala His Pro Ser Val Arg Tyr
1 5 10 15
Val Cys Leu Pro Pro Glu Arg Asp Gly Gly Gly Gly Cys Lys
20 25 30
<210> 16
<211> 25
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 9
36
CA 02558246 2010-08-17
<400> 16
Arg Arg Arg Tyr Ala His Pro Ser Val Arg Tyr Val Cys Leu Pro Pro
1 5 10 15
Glu Arg Asp Gly Gly Gly Gly Cys Lys
20 25
<210> 17
<211> 23
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 10
<400> 17
Lys Cys Gly Gly Gly Gly Trp Arg Gly Arg Ala Gly Arg Arg Arg Tyr
1 5 10 15
Ala His Pro Ser Val Arg Tyr
<210> 18
<211> 21
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence:synthetic
herpes simplex virus type 2 (HSV-2) glycoprotein
G2 (gG-2) peptide 11
<400> 18
Lys Cys Gly Gly Gly Gly Arg Gly Arg Ala Gly Arg Arg Arg Tyr Ala
1 5 10 15
His Pro Ser Val Arg
37