Note: Descriptions are shown in the official language in which they were submitted.
CA 02559275 2012-07-30
Autogenic Living Scaffolds and Living Tissue Matrices: Methods and Uses
Thereof
Technical Field
The present invention relates to "living scaffolds" particularly "autogenic
living
scaffolds" (ALS), that comprise suitable living cells and the extracellular
matrices these
living cells produce. The invention also relates to the use of such autogenic
living
scaffolds as templates and supporting structures for growth of the same cells
and tissue or
the growth of different cells and different tissue
Background Art
In the United States, millions of people are affected by tissue loss every
year.
Current treatments include tissue transfer from a healthy site in the same or
another
individual, use of medical devices to support the function of the lost tissue,
or
pharmacologic supplementation of the metabolic products of the lost tissue.
Problems
with these current treatments include potential tissue complications and
imperfect
matches including the possible dependence on immunosuppressants, limited
durability of
the mechanical devices, and the inconvenience and complexity of pharmacologic
supplementation. Current approaches for developing living tissue substitutes
make use of
1
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
a "scaffold" that serves as a physical support and template for cell
attachment and tissue
development. These scaffolds are ideally designed to resemble, both in
structure and
composition, the extracellular matrix that the cells are exposed to in vivo,
in order to
simulate the in vivo conditions. An early and widely used natural scaffold is
made of the
extracellular matrix protein collagen, while more recently, mechanically
stronger
artificial scaffolds made of substances such as poly-glycolic acid (PGA) and
poly-lactic
acid (PLA) have been used.
Some cell-scaffold compositions have multiple layers of biocompatible
materials
including extracellular matrix materials such as collagen, fibril-forming
collagen, Matrix
Gla protein, osteocalcin, or other biocompatible materials including marine
coral,
coralline hydroxyapatite ceramic, and mixtures thereof, and some such
scaffolds have
been seeded with cells, and then placed within a bioreactor having a means for
mechanically stimulating the cells at distinct frequencies (see U.S. Patent
Application No.
0040005297 to P. R. Connelly et a., filed July 8, 2002, published January 8,
2004).
In addition, living tissue equivalents (LTEs), notably cell-seeded collagen
and
fibrin gels, have been used extensively as in vitro wound-healing models as
well as
systems for studying tissue remodeling. More recently, LTEs have begun to gain
considerable attention as replacements for lost or damaged connective tissue
(e.g.,
Apligrafrm from Organogenesis, Inc.). LTEs have several advantages over
synthetic
alternatives including being a natural cell substrate, allowing cellularity to
be achieved
directly, and being conducive to cell spreading and extracellular matrix (ECM)
formation. LTEs are made by mixing cells with a soluble biopolymer solution
(e.g.,
collagen, fibrin, and/or proteoglycans). The cells invade, rearrange and
partially degrade
the biopolymer scaffold over the next few days as well as synthesize new
proteins
throughout the culture period. However, LTEs generally lack the physical
properties
necessary to resist in vivo mechanical forces, and are not true "living
tissues".
Over the last two decades, LTEs that are completely cell-derived have been
developed. However, to date they have been very thin and taken a long time to
grow,
generally on the order of months, whereas collagen gels and fibrin gels can be
developed
in only a few days. There is a need for completely biological cell-derived
LTEs, and
living scaffolds for use in wound repair and tissue regeneration in vitro and
in vivo.
2
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Summary of the Invention
=
Embodiments of the presently claimed invention provide a strong, thick, cell-
produced living tissue equivalent (LTE), comprising cells and ECM produced by
these
cells (this ECM is called cell-produced matrix or cell-derived matrix (CDM)),
that can be
developed in only three weeks for use in creating strong and completely
biological soft
connective tissue substitutes and for examining wound-healing and tissue
development in
vitro. The biomechanics and corresponding biochemical composition of cell-
produced
and cell-remodeled matrices are also provided, as are chemically-defined media
permissive to the self-production of extracellular matrix (ECM) by cells.
Other embodiments disclose placing fibroblasts in conditions that are
conducive
to the rapid production of extracellular matrix without an exogenous scaffold,
which
results in a significantly stronger and thicker 3-D construct than can be
obtained with
cell-remodeled matrices, such as fibroblast-populated collagen and fibrin
gels.
Thus, embodiments of the presently claimed invention provide the use of
autogenic living scaffolds, cell produced matrices (also referred to as cell-
derived
= matrices (CDMs), and living tissue matrices (LTMs) made entirely of
living cells and the
. extracellular matrices they produce in vitro, to promote differentiation,
dedifferentiation
and/or transdifferentiation of cells and formation of tissue in vitro and in
vivo, while at
the same time promoting cell growth, proliferation, migration and/or
acquisition of in
vivo-like morphology, none of which has been reported to date.
Embodiments in accordance with the presently claimed invention provide an
= autogenic living scaffold (ALS) or living tissue matrix (LTM) that is a
cell-produced
scaffold which provides mechanical, nutritional and/or developmental support
for cells,
tissues, organs, or combinations thereof. The cell-produced autogenic living
scaffold as
herein disclosed is smart, such that it is capable of adjusting to its
environment, and it is
= living, whereby it is biologically active and all components except
seeded cells and tissue
are naturally formed by the scaffold system itself, making the scaffold
autogenic, or self-
produced.
3
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
The Autogenic Living Scaffolds as disclosed herein are made by and comprise
living cells and the extracellular matrix (ECM) that the living cells produce.
The living
cells may be genetically engineered or otherwise modified. The ALS serves as a
blueprint, supporting structure, backbone, or scaffold for the same or other
cell lines or.
types. The ALS may also provide proper or supporting mechanical and chemical
environments, signals, or stimuli to other cells, to the cells that produce
the ALS, to
surrounding tissue at an implantation site, to a wound, or for in vitro
generation and
regeneration of cells, tissue and organs. The ALS may also provide other cells
with
nutrients, growth factors, and other necessary or useful components, may take
in or serve
as buffers for certain substances in the environment, and have the potential
to adapt to
new environments.
The cells of an Autogenic Living Scaffold may also be used to produce tissue
and/or organs such as cardiac muscle, when seeded with cells or tissue of
interest. For
example, liver tissue may be produced from an ALS that is seeded with
hepatocytes; and
= kidney, pancreas, spinal cord, and other organs and tissues of the body
may also be
produced by seeding the ALS with the desired cell or tissue type. Autogenic
Living
Scaffolds seeded with the appropriate cell types may thus be used to grow
implantable
tissue and organs in vitro, for later implantation into an in vivo site.
Many different types of cells may also be seeded in different parts of the
Living
Scaffold, or they could be sandwiched on top of each other. For example, a
Fibroblast
Autogenic Living Scaffold may first be grown in serum-free conditions
favorable to the
growth of the fibroblasts into tissue or an Autogenic Living Scaffold. This
Living
Scaffold is then seeded with astrocytes, and the serum-free growth conditions
(including
the media, pH, osmolarity, temperature, oxygen tension, and anything else
required) are
adjusted to be favorable to the growth of the astrocytes. If needed, other
components are
added to keep the Living Scaffold alive and healthy. Also, additional layers,
such as
skeletal muscle myocytes that might form into skeletal muscle tissue that is
innervated by
the already seeded nerves, may continue to be added, as desired.
In another embodiment, the Autogenic Living Scaffold may also be grown into
specific shapes by molds, and may also be reshaped to some degree. For
example, a sheet
of the above example of a Fibroblast Autogenic Living Scaffold, seeded first
with
4
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
astrocytes and then nerve cells, may be rolled into a cylinder. This cylinder
may then be
implanted into a spinal cord in vivo. The Autogenic Living Scaffold also
provides
mechanical support to the seeded cells. For example, in a particular
embodiment, a
Fibroblast Autogenic Living Scaffold seeded with neurons may be mechanically
stressed
and compressed, without major damage to the neurons, even though such a degree
of
mechanical stress and compression kills most neurons when grown in the absence
of an
ALS. The Autogenic Living Scaffold of other embodiments may also be introduced
to
mechanical stress or tension which may change the properties of the Living
Scaffold and
any cells or tissue that are growing on it.
In one particular embodiment, the fibers of a Fibroblast Autogenic Living
Scaffold may also be made to grow in parallel, which helps seeded nerve cells
to also
grown in parallel along these fibers, especially when Schwann cells are
previously seeded
onto the scaffolds and first start growing in parallel along these fibers.
This may be even
more useful when implanted in the spinal cord, since the implanted nerve cells
may then
be aligned in the same direction as the native nerve cells in the spinal cord.
In still
another embodiment, a sheet of Autogenic Living Scaffold with the seeded
neurons may
also be rolled into a cylinder prior to implantation to produce a structure
with layers of
neurons aligned in the same general direction as the native neurons in the
spinal cord.
Similar things may be done for implantation into other tissues and organs.
In other particular embodiments, cell to cell, tissue to tissue, and tissue to
cell
interactions may also be studied in vitro and in vivo with Autogenic Living
Scaffolds,
including by sandwiching different cells. In yet another embodiment, Autogenic
Living
Scaffolds may be used as in vitro biological models for studying the growth
and
development of cells, tissues, organs, systems, diseases, and different
responses in
organisms. For example, the wound response (in which fibroblasts play an
active role) on
different types of cells and tissues may be studied in vitro by using this
technology.
Fibroblasts (especially foreskin fibroblasts) secrete numerous growth factors
including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF),
and
neurotrophin-3 (NT-3), as well as fibroblast growth factor (FGF), and platelet-
derived
growth factor (PDGF), all of which promote neuron regeneration and survival.
The
embodiments of AISs described herein more closely mimic the extracellular
CA 02559275 2006-09-08
WO 2006/048783 PCT/IB2005/004091
environment that nerve cells are normally exposed to in vivo than any other
currently
available scaffolds, and even allow primary nerve cells to form active 3-D
neural
networks in vitro that can serve as in vitro 3-D models for potential
therapeutic agents for
=
neuronal regeneration, as may also be used to functionally replace injured
spinal cord
neurons in vivo.
In the case of particular embodiments of fALS of the present application, the
high
density of fibroblasts along with the insoluble ECM proteins collagen and
fibronectin of
the ALS work in conjunction to promote axon elongation and functional recovery
when
implanted into chronically injured spinal cord. In addition, the development
of a
functional neural network in vitro allows the nerve graft to have more utility
when
implanted, and is important for studying the effect of different
pharmacological agents
and Methods on the in vitro 3-D neural network model disclosed herein.
In other embodiments the effects of different nutritional supplements and
growth
factors on the development of the functional neural networks in the ALS may
also be
studied. Thus, in embodiments of the present invention, once the neurons are
seeded onto
the ALS, the growth media is changed from one that supports ALS growth to one
that
promotes neuronal growth and differentiation, while at the same time retards
the growth
of fibroblasts. This prevents the fibroblasts from over-running the neurons
and effectively
preventing neuronal development. In other embodiments, the fibroblasts may be
genetically engineered to secrete more growth factors such as NGF, BDNF, FGF-
2, and
bFGF to enhance neuron survival and development even further.
In still other embodiments, the ALS nerve graft has the flexibility of taking
on
almost any non-rigid shape and may be rolled up into a ball or cylinder.
Several thin
nerve grafts may also be layered on top of each other to form different
parallel layers of
neural networks, which in turn may again be rolled up into a cylinder or
formed into
some other shape.
Another embodiment provides an autogenic living scaffold that has been seeded
with cells, tissue or combinations thereof, including any of stem cells,
progenitor cells,
precursor cells; cells or tissue of a connective, epithelial, muscle, nerve
and/or glandular
origin; and cells of vascular and/or non-vascular organ origin such as
neuroblastomas,
nayoblasts, astrocytes, cardiomyocytes, skeletal muscle myoblasts,
hepatocytes,
6
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
chondrocytes, osteoblasts, fibroblasts, keratinocytes, Schwann cells, nerve
cells, glial
cells, epithelial cells, endothelial cells, smooth muscle cells, skeletal
muscle cells,
cardiac muscle cells, stromal cells, mesangial cells, mesenchymal cells,
hematopoietic
cells, dendritic cells, immune system cells, neural tissue, hepatic tissue,
aortic tissue,
venous tissue, capillary tissue, cartilage, bone, muscle, glands, and hair
follicles.
Another embodiment provides a Living Tissue Matrix (LTM) that closely
resembles in vivo generative/regenerative connective tissue since the cells
produce the
entire 3D matrix by themselves. In fact, LTMs are similar in composition to
the type of
fibroblast-populated connective tissue that first fills the wound bed in
embryonic wound
healing (and other non-scar forming tissue wound healing) that regenerates
without
scarring as opposed to wounds in neonatal or adult mammals that heal with
scarring.
Furthermore, this method produces a 3-D construct (the LTM) that is
significantly thicker
and stronger than those obtained using biopolymer gels, such as collagen or
fibrin gels.
The entire Living Tissue Matrix (cells and ECM) can also be made completely
autologous, thus preventing host rejection and making it completely
immunocompatible.
In another embodiment, the ECM produced in the LTM system provides an
optimal environment for de-differentiated or transdifferentiated autologous
adult cells
within the LTMs to create a regenerative environment and a virtual blastema.
In other
words, if the millions of fibroblasts within the LTM (the cells that produced
the LTM in
the first place) are de-differentiated or transdifferentiated, the LTM can
effectively
become a structurally sound implantable blastema-like structure for multi-
tissue type
regeneration.
Another embodiment provides a chemically defined media formulation (called
"fALS Media" or "Matrix Media") for growing an ALS or LTM that contains a 3:1
ratio
of DMEM (high glucose ¨ 4.5g/L ¨ with L-glutarnine and sodium pyruvate) and
Ham's
F12 medium (or 2:1 ratio of IMEM to Ham's F12 medium), supplemented with 4.2 x
10-
1 M Epidermal Growth Factor (in human serum albumin); 2.8 x 1040M Basic
Fibroblast
Growth Factor; 8.6 x 10-5M insulin;1.0 x 10-7M dexamethasone; 3.2 x 10-4M L-
ascorbic
acid phosphate magnesium salt n-hydrate; 2 x 10-1 M L-3,3',5-
triiodothyronine;104M
ethanolamine or other lipid precursor; 3.9 x 10-8M selenious acid; 4 x 10-3M
GlutamaxTM;
3.3 x 10-6M glutathione (reduced); and 1% penicillin/streptomycin/amphotericin
B. In
7
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
addition, other embodiments and variations of the above-listed medium may
contain
additional components, such as any one or more of: Platelet Derived Growth
factor
(PDGF); 100:68 ratio of glycine: L-proline; L-cysteine; and Trolox.
Concentrations may
vary as required, as long as the total osmolarity in the medium is kept at
acceptable levels
for growth of the ALS.
Other embodiments provide methods for growing tissue and/or organs using an
ALS or LTM, and methods for using an ALS or LTM to model a human biological
cellular system or tissue system of a combination thereof. In addition,
methods are
provided for using an ALS or LTM to assess the effect of one or more agents on
a
biological system being modeled, wherein one or more agents includes
pharmaceutical
agents, enzymes, hormones, small molecules, peptides, polypeptides, natural
products,
natural products extracts, inorganic salts, other cells, growth factors,
clotting factors,
toxins, poisons, nucleic acids, mechanical stress inducers, electrical current
generators,
electromagnetic field and pulse generators, and sonic wave inducers.
Still other embodiments provide methods for using an ALS or LTM to treat
tissue
or organ damage or tissue or organ degeneration in a subject suffering from
Crohn's
disease; cancer, including lung, colon, stomach, liver, kidney, pancreas,
bone, brain;
= muscular dystrophy; ocular degeneration, diabetes, cardiac ischemia;
heart valve damage
or heart valve congenital defect comprising producing an ALS or LTM, and any
one of a)
regenerating a new organ or tissue of the tissue in vitro using the scaffold
and cells or
tissue of the type or class of the damaged or degenerated tissue or organ and
then
implanting such scaffold with regenerated tissue or tissue of the organ in the
subject at
the site of tissue or organ damage or degeneration ; or b) implanting the
scaffold and
cells or tissue of the type or class of the damaged or degenerated tissue or
organ in the
subject at the site of tissue or organ damage or degeneration and then
regenerating a new
organ or tissue of the organ in vivo using the scaffold having cells or tissue
of the type or
class of the damaged or degenerated tissue or organ; or c) regenerating a new
organ or
tissue of the organ as in a) or b) using the scaffold alone.
Other embodiments provide methods for using an ALS to generate viable
vertebrate neuronal tissue in vitro comprising seeding an ALS with vertebrate
primary
neural cells or neuronal tissue and maintaining the seed scaffold in culture
under
8
CA 02559275 2012-07-30
conditions where viable vertebrate neuronal tissue is generated. Such viable
vertebrate
neuronal tissue is then used in other embodiments to treat paralysis in a
subject by
contacting at least one site of spinal cord in the subject with an effect
amount of the ALS
with viable neuronal tissue so as to treat the paralysis.
Similarly, other embodiments provide methods for treating a neurodegenerative
disease in a subject, wherein the neurodegenerative disease is any one of
Parkinson's
disease, Huntington's disease, Alzheimer's disease, schizophrenia, dementia,
multiple
sclerosis, cerebral palsy, muscular dystrophy, Tay Sach's disease, Mad Cow
disease, or
Creutzfield-Jacob's disease, by contacting at least one site in the subject
with an effective
amount of an ALS or LTM with viable neuronal tissue so as to treat the
neurodegenerative disease.
Brief Description of the Drawings
The foregoing features of the invention will be more readily understood by
reference to the following detailed description, taken with reference to the
accompanying
drawings, in which:
Fig. 1 shows methylene blue staining of neurons that have differentiated from
neural
progenitor cells grown on a fibroblast ALS.
Fig. 2 shows a fluorescent live/dead assay performed on neural progenitor
cells grown on
a fibroblast ALS, where the fibroblasts had been killed prior to seeding with
human
neural progenitor cells.
Figs. 3 shows a cross-section of a 17-day fibroblast ALS system about 50-60 pm
thick
containing neuronal cells/tissue that are expressing anti-Hu MAb 16A11, an
early marker
of vertebrate neurogenesis that is expressed shortly after neuronal terminal
mitosis
(Marusich, M.F. et al (1994)1. Neurobiol. 25: 143-155). The marker is observed
in
nerve cell bodies. The ALS was allowed to grow for 4 weeks prior to seeding
with human
neuroprogenitor cells.
Fig. 4 shows another cross-sections of a 17-day fibroblast ALS system about 50-
60 p.m
thick containing neuronal cells/tissue that are expressing anti-Hu MAb 16A11,
an early
marker of vertebrate neurogenesis that is expressed shortly after neuronal
terminal mitosis
(Marusich, M.F. eta! (1994)1 Neurobiol. 25: 143-155). The marker is observed
in
9
CA 02559275 2012-07-30
nerve cell bodies. The ALS was allowed to grow for 4 weeks prior to seeding
with human
neuroprogenitor cells.
Figs. 5 shows cross-sections of a 31-day fibroblast ALS system about 216-230
[tm thick
containing neuronal cells/tissue that are expressing anti-Hu MAb 16A11. The
ALS was
allowed to grow for 3 weeks prior to seeding with human neuroprogenitor cells.
As can
be seen, varying-sized regions (different cross-sections of nerve cell bodies)
expressing
the anti-Hu MAb 16A 1 1 marker are indicated, ranging in size to over 20 [tm.
Fig. 6 shows another cross-sections of a 31-day fibroblast ALS system about
216-230 jim
thick containing neuronal cells/tissue that are expressing anti-Hu MAb 16A11.
The ALS
was allowed to grow for 3 weeks prior to seeding with human neuroprogenitor
cells. As
can be seen, varying-sized regions (different cross-sections of nerve cell
bodies)
expressing the anti-Hu MAb 16A11 marker are indicated, ranging in size to over
20 Jim.
Fig. 7 shows cross-sections of a 24-day fibroblast ALS system about 153 jim
thick
containing neuronal cells/tissue that are expressing anti-Hu MAb 16A11. The
ALS was
allowed to grow for 2 weeks prior to seeding with human neuroprogenitor cells.
As can
be seen, varying-sized regions (different cross-sections of nerve cell bodies)
expressing
the anti-Hu MAb 16A11 marker are indicated, ranging in size to almost 20 m.
Fig. 8 shows a section view of a negative control system for the neurogenesis
marker
experiments of Figures 3 through 7, where no primary Hu MAb 16A11 antibody was
present in the system. Thus, only the nuclei of the cells are visible, and no
expressed
neurogenesis markers are visible.
Fig. 9 shows a different section view of a negative control system for the
neurogenesis
marker experiments of Figures 3 through 7, where no primary Hu MAb 16A11
antibody
was present in the system. Thus, only the nuclei of the cells are visible, and
no expressed
neurogenesis markers are visible.
Fig. 10 shows a cross-sectional view of a positive control system for the
neurogenesis
marker experiments of Figures 3 through 7, using human brain sections.
Positive
indication that the marker anti-Hu MAb 16A11 attaches to nerve cell bodies is
indicated
by the large and numerous dye spots visible throughout the images. Most of the
cells that
did not stain for anti-Hu MAb 16A11 are glial cells.
Fig. 11 shows a different cross-sectional view of a positive control system
for the
neurogenesis marker experiments of Figures 3 through 7, using human brain
sections.
CA 02559275 2012-07-30
Positive indication that the marker anti-Hu MAb 16A11 attaches to nerve cell
bodies is
indicated by the large and numerous dye spots visible throughout the images.
Most of the
cells that did not stain for anti-Hu MAb 16A11 are glial cells.
Figs. 12 shows trichrome staining of a cross-section of a 24-day fibroblast
ALS system
containing muscle cells/tissue about 70 gm thick. The collagen fibers of the
ALS, the
nuclei of cells and muscle cells (differentiated from human skeletal muscle
myoblasts
seeded onto the ALS 2 weeks earlier) within the ALS are observed. The ALS was
allowed to grow for 1 week prior to seeding with human skeletal muscle
myoblasts.
Fig. 13 shows trichrome staining of a different cross-section of a 24-day
fibroblast ALS
system containing muscle cells/tissue about 115 gm thick. The collagen fibers
of the
ALS, the nuclei of cells and muscle cells (differentiated from human skeletal
muscle
myoblasts seeded onto the ALS 2 weeks earlier) within the ALS are observed.
The ALS
was allowed to grow for 1 week prior to seeding with human skeletal muscle
myoblasts.
Fig. 14 shows early nerve differentiation on Living Tissue Matrix. Human
neural cell
bodies stained with Hul6A11, a marker of early neurogenesis.
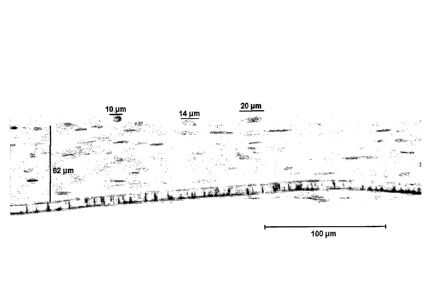
Fig. 15A shows Hematoxylin and Eosin (H&E) stained sections of fibroblast-
populated
collagen gel (CG), 84 gm thick, as measured by digital image analysis.
Fig. 15B shows fibroblast-populated fibrin gel (FG), 230 gm thick, as measured
by digital
image analysis.
Fig. 15C shows Living Tissue Matrix (LTM) fed with the same serum-supplemented
media that the CG and FG above were fed with, 110 gm thick, as measured by
digital
image analysis.
Fig. 15D shows Living Tissue Matrix (*LTM) fed with Matrix Media, 465 gm
thick, as
measured by digital image analysis.
11
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
= Fig. 15E shows Living Tissue Matrix (**LTM) fed with a modified Matrix
Media, 240
gm thick, as measured by digital image analysis. All micrographs for Figures A-
E were
taken at 200x. Scale bars = 100 gm.
Fig. 16A is a graphical representation of the results in Table 1 showing the
thickness
. ().Lm) of the fibroblast-populated collagen gel (CG) and fibrin gel (FG),
and cell-derived
matrix (CDM) fed with serum-supplemented medium; and cell-derived matrices
(*CDM
and **CDM) fed with Matrix Media, compared to human penile skin (HPS).
= Fig. 16B is a graphical representation showing the tensile strength (N/m)
of fibroblast-
populated collagen gel (CG) and fibrin gel (FG), and cell-derived matrix (CDM)
fed with
serum-supplemented medium; and cell-derived matrices (*CDM and **CDM) fed with
. Matrix Media, compared to human penile skin (HPS).
Fig. 16C is a graphical representation of the results in Table 1 showing the
total collagen
(mg) of fibroblast-populated collagen gel (CG) and fibrin gel (FG), and cell-
derived
:. matrix (CDM) fed with serum-supplemented medium; and cell-derived
matrices (*CDM
and **CDM) fed with Matrix Media, compared to human penile skin (HPS).
Fig. 16D is a graphical representation of the results in Table 1 showing the
total
proteoglycans and glycosaminoglycans (gg) of fibroblast-populated collagen gel
(CO)
. and fibrin gel (FG), and cell-derived matrix (CDM) fed with serum-
supplemented
medium; and cell-derived matrices (*CDM and **CDM) fed with Matrix Media,
. compared to human penile skin (HPS).
Fig. 16E is a graphical representation of the results in Table 1 showing the
total protein
(mg) of fibroblast-populated collagen gel (CG) and fibrin gel (FG), and cell-
derived
matrix (CDM) fed with serum-supplemented medium; and cell-derived matrices
(*CDM
and **CDM) fed with Matrix Media, compared to human penile skin (HPS).
Fig. 16F is a graphical representation of the results in Table 1 showing the
ultimate
tensile strength (kPa) of fibroblast-populated collagen gel (CG) and fibrin
gel (FG), and
cell-derived matrix (CDM) fed with serum-supplemented medium; and cell-derived
matrices (*CDM and **CDM) fed with Matrix Media, compared to human penile skin
(HPS).
Fig. 16G is a graphical representation showing the % collagen of fibroblast-
populated
collagen gel (CG) and fibrin gel (FG), and cell-derived matrix (CDM) fed with
serum-
12
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
, supplemented medium; and cell-derived matrices (*CDM and **CDM) fed with
Matrix
Media, compared to human penile skin (BPS).
Fig. 1611 is a graphical representation showing the % proteoglycans and
glycosaminoglycans of fibroblast-populated collagen gel (CG) and fibrin gel
(FG), and
cell-derived matrix (CDM) fed with serum-supplemented medium; and cell-derived
= matrices (*CDM and **CDM) fed with Matrix Media, compared to human penile
skin
(HPS).
Fig. 161 is a graphical representation of the results in Table 1 showing the
cell number
(millions) of fibroblast-populated collagen gel (CG) and fibrin gel (FG), and
cell-derived
matrix (CDM) fed with serum-supplemented medium; and cell-derived matrices
(*CDM
and **CDM) fed with Matrix Media, compared to human penile skin (HPS).
Fig. 16J is a graphical representation of the results in Table 1 showing the
failure strain
of fibroblast-populated collagen gel (CG) and fibrin gel (FG), and cell-
derived matrix
= (CDM) fed with serum-supplemented medium; and cell-derived matrices (*CDM
and
= **CDM) fed with Matrix Media, compared to human penile skin (HPS).
, Fig. 16K is a graphical representation of the results in Table 1 showing
the non-acid and
pepsin extractable collagen fraction (%) of fibroblast-populated collagen gel
(CG) and
fibrin gel (FG), and cell-derived matrix (CDM) fed with serum-supplemented
medium;
and cell-derived matrices (*CDM and **CDM) fed with Matrix Media, compared to
human penile skin (HPS).
Fig. 16L is a graphical representation of the results in Table 1 showing the
TITS/collagen
density (kPa/mg/cm3) of fibroblast-populated collagen gel (CG) and fibrin gel
(FG), and
cell-derived matrix (CDM) fed with serum-supplemented medium; and cell-derived
matrices (*CDM and **CDM) fed with Matrix Media, compared to human penile skin
(HPS).
Fig. 17A shows a TEM 12,000x; scale bar = 1 pm) of the distribution of
collagen fibrils
(arrows) in the collagen gel (CG), wherein most (from the collagen gel) are
about 56 t 5
nm in diameter with a small number (presumably secreted by fibroblasts I the
gel) about
46 5 nm in diameter. The collagen density was 81 4 fibrils/pm2.
13
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Fig. 178 shows a TEM 12,000x; scale bar = 1 gm) of the distribution of
collagen fibrils
(arrows) in the fibrin gel (FG) wherein the collagen fibrils have a diameter
of about 48
run in diameter. The collagen density was 28 t 4 fibrils/pm2, (p<0.01).
Fig. 17C shows a TEM (12,000x; scale bar = 1 gm) of the distribution of
collagen fibrils
(arrows) in the cell-derived matrix (CDM) wherein the collagen fibrils have a
diameter of
about 52 10 nm in diameter. The collagen density was 79 t 2 fibrils/pm2.
Fig. 17D shows a TEM 12,000x; scale bar = 1 gm) of the distribution of
collagen fibrils
(arrows) in the cell-derived matrix (*CDM) fed with Matrix Media wherein the
collagen
fibrils have a diameter of about 47 5 nm in diameter. The collagen density
was 80 2
fibrils/pm2.
Detailed Description of Specific Embodiments
=
Definitions. As used in this description and the accompanying claims, the
following terms shall have the meanings indicated, unless the context
otherwise requires:
"Autogenic Living Scaffold", or "ALS" as used herein means a 3-dimensional
structure comprising cells (or entities) and the ECM (or matrix) that has been
completely
produced and arranged by these cells (or entities) that supports the growth of
cells, tissue,
organs, or combinations thereof. "Autogenic" means it is a self-produced
scaffold,
providing mechanical and nutritional and developmental support for the cells,
tissues,
organs, or combinations thereof. "Living" means that the scaffold is smart ¨
it adjusts to
its environment. "Living" also means it is biologically active and all
components except
seeded cells and tissue are naturally formed, produced, and synthesized by the
scaffold
system itself¨ i.e., the scaffold is autogenic. "Scaffold" means that it
provides a
structural framework for cells that guide their direction of growth, enables
them to be
correctly spaced, prevents overcrowding and enables cells to communicate
between each
other, transmit subtle biological signals, receive signals from their
environment, and form
bonds and contacts that are required for proper functioning of all cells
within a unit such
as a tissue.
"Resembles" as used herein means there is physical, functional, compositional,
structural, phenotypic or other similarities between the materials or systems
being
14
CA 02559275 2012-07-30
compared, such that the objects are substantially equivalent. "Substantially
equivalent"
means that visible, microscopic, physical, functional, and other observations
and assays
do not easily or significantly distinguish the materials or systems. An easy
or significant
distinction would, for example, be a functional difference, a physical
difference, a
compositional difference, a structural difference immediately apparent, or
easily
detectable with standard assays and observational techniques such as staining,
microscopy, antibodies, etc.
"Extracellular Matrix" (ECM) or "Cell Derived Matrix" (CDM) or Cell-produced
Matrix as used interchangeably herein means a cell-derived secreted structural
substance
produced by and/or secreted from cells into the extracellular space. The
ECM/CDM
provides a growth template for any cell type to grow, differentiate, and
produce tissue.
Any cell type, as used herein, includes stem cells, progenitor cells,
differentiated cells,
and any other type of cell or entity. The term also is intended to include the
matrix
material referred to as Extracellular Growth Matrix (ECGM) that is produced by
Radicari
entities, and Radicari pre-cells and cells, as described in US application
Serial Number
10/930,673 filed August 30, 2004 which claims priority from US provisional
application
Serial Number 60/499,142 filed August 29, 2003. The ECM allows cell attachment
and
cell migration, and promotes cell differentiation. The ECM also aids the
formation of
new tissue of a desired or existing cell type As used herein means, "Cell-
Produced
Matrix, also called Cell-Derived Matrix (CDM)" also means a 3-dimensional ECM
(or
matrix) structure that has been completely produced and arranged by cells (or
entities) in
vitro.
"Construct" as used herein means a physical structure with mechanical
properties
such as a matrix of scaffold. Construct encompasses both autogenic living
scaffolds and
living tissue matrices, ex-vivo cell-produced tissue and cell-derived matrix.
"Cell-derived" as used herein means that the source for the material, body, or
component is a cell or a collection of cells.
"Ex-vivo Cell-produced Tissue (ECT)" as used herein means, a functional tissue
comprising one or more types of cells (or entities) and the ECM (or matrix)
that has been
completely produced and arranged by some of these cells (or entities). For
example, an
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
- ex-vivo cell-produced neural graft consisting of an ALS that has been
seeded with
neuroprogenitor cells that differentiated in the ALS and formed neural tissue.
= "Living Tissue Matrix (LTM)" as used herein means, a 3-dimensional tissue
(or
= matrix) that is capable of being transformed into a more complex tissue
(or matrix) or a
completely different type of tissue (or matrix) that consists of cells (or
entities) and the
. ECM (or matrix) that has been completely produced and arranged by these
cells (or
= entities).
"Living Tissue Equivalent (L'TE)" as used herein means a construct containing
living cells that intends to mimic a certain type of native tissue. This
construct can
be produced by any means in vitro, including by the use of artificial
scaffolds.
"Supercontinent conditions" or "Superconfluency" means, within the context of
the present application, that cells are grown and maintained in high-density
growth
conditions such that cells are packed almost directly next to and top of each
other.
= "Hyperconfluent conditions" as used herein means conditions for in vitro
cell
= culture/growth such that cells and their associated ECM take up all the
space at the
bottom of the culture dish and have started to grow in the third dimension (on
top of each
other).
"Base media" as used herein means any cell culture medium that supports in
vitro
cultures of eukaryotic cells, including Dulbecco's Modification of Eagle's
Medium
(DMEM); Ham's F-12 (F12); Ham's F-10 (F10); Iscove's Modification of DMEM
(IDMEM); MEDIUM 199; Alpha MEM; Basal Medium Eagle with Earle's BSS;
Cyroprotective Medium (Freezing Medium); DMEM:F12 1:1; with L-Glutamine;
Glasgow's MEM with L-glutamine (GMEM); IMDM with HEPES, with or without L-
Glutamine; L-15 (Leibovitz), without L-Glutamine; McCoy's 5A Modified Medium;
Medium 199; MEM Eagle; MEM Eagle-Earle's BSS, with or without L-Glutamine;
MEM Eagle-Hanks BSS; NCTC-109; Richter's CM Medium; RPMI 1640 with HEPES;
RPMI 1640; or a combination of any of these.
"Glucocorticoid" as used herein, means dexamethasone, hydrocortisone,
corticosterone, cortisol, cortisone, prednisone, prednilisone,
methylprednisone,
16
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
budesonide, beclometasone or any other compound classified as, or commonly
referred
. to as, a glucocorticoid, or which interacts with the glucocorticoid
receptor.
"Growing a large number of fibroblasts" as used herein means growing a
plurality
of cultures of fibroblasts to obtain at least about 1,000 cells/mm3 to greater
than 200,000
cells/mm3, or growing a single culture to obtain at least about 1,000
cells/mm3 to greater
than 200,000 cells/mm3.
"Growth and development" as used herein, means ability to reproduce and be
. viable, and includes proliferation and differentiation and/or tissue
development.
"Fragility" as used herein, means a cell's tendency to be easily broken or
damaged, and refers to a cell's physical frailty and weakness, lack of
structural hardiness,
and inability to remain intact if subjected to physical stress.
"Conditions that promote three-dimensional tissue growth" as used herein,
means
in vitro or in vivo conditions that facilitate, aid, further or in any way
allow the
development of three-dimensional tissue growth. Conditions may include use of
specific
media, growth factors, minerals, incubation temperature, cell density,
aeration, agitation,
use of ALS "molds" to shape and contain growth of desired tissue, use of sub-
atmospheric pressure chambers such as SyntheconTM near-zero-gravity incubator
systems
(such as HARVs and STLVs) for growth of desired tissue, use of microcarrier
beads, use
of natural or biodegradable scaffolds, implanting a non-fibroblast-seeded
autogenic
living scaffold within an in vivo site such as in an organ or tissue such as
connective,
epithelial, muscle, and/or nerve tissue.
An "equivalent growth factor" as used herein means any growth factor that can
replace a specific growth factor in a fibroblast cell culture without
rendering the
fibroblast cells non-viable. For epidermal growth factor (EGF), such
equivalent growth
factors include, but are not limited to, basic fibroblast growth factor
(bFGF), fibroblast
= growth factor 2 (FGF-2), and transforming growth factor-a (TGF-a)
"Concentrations supportive of fibroblast growth and production of
extracellular
matrix" as used herein, means concentrations of growth media components,
whether
determined by molarity, weight/volume percent, weight/weight percent,
volume/volume
percent or any other standard means for measuring concentration, which allow
fibroblast
17
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
cells to grow, reproduce, proliferate, differentiate, or in any other way
remain viable and
produce extracellular matrix material.
"Regenerate tissue" as used herein means that autogenic living scaffolds with
or
without seeded cells and/or tissue, form, create, construct or otherwise
generate tissue
anew where previously there was none, or where previously the tissue was
partially or
completely non-functional.
"Supports the growth" as used herein means that growth is compatible with,
and/or promoted by, the system of interest. For example, the phrase "the
scaffold
supports the growth of cells" or "supports the growth of tissue" or "supports
the growth
of organs" means that the ALS is compatible with, and/or promotes, the growth
of cells,
tissue or organs within or on or throughout the scaffold.
"Genetically engineered" as used herein means that a cell or entity, by human
manipulation such as chemical, physical, stress-induced, or other means, has
undergone
mutation and selection; or that an exogenous nucleic acid has actually been
introduced to
= the cell or entity through any standard means, such as transfection; such
that the cell or
entity has acquired a new characteristic, phenotype, genotype, and/or gene
expression
= product, including but not limited to a gene marker, a gene product,
and/or a mRNA, to
endow the original cell or entity, at a genetic level, with a function,
characteristic, or
genetic element not present in non-genetically engineered, non-selected
counterpart cells
= or entities.
= "Smart" as used herein in regards to the autogenic living scaffold means
that the
living scaffold adapts to its environment, changes relative to its situation
and
surroundings, in both a reactive and active manner, interacting with and
reacting to
surrounding factors, cells, entities and structures through physical,
biochemical, cell-
signaling, enzymatic and/or genetic means. Being smart may also include
morphing into
a more complex, biochemically-, physically- and/or genetically- evolved
material,
system, scaffold or matrix; evolving functionally, physically, biochemically
and/or
genetically; and integrating functionally, phycically, biochemically and/or
genetically
with surround cells, entities and materials.
"Cell or cell type suitable for production of extracellular growth matrix" or
"produced by any suitable cell type" as used herein means any cell, cell type,
or cell
18
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
entity, including Radicari entities and Radicari pre-cells and Radicari cells
derived
. therefrom, that can be manipulated to produce extracellular matrix in
vitro.
"Manipulated to produce extracellular matrix in vitro" means that growth
conditions,
growth media, supplemental factors, and appropriate stimuli exist and may be
applied to
= these cells and entities to stimulate production of extracellular matrix
material, in contrast
= to non-suitable cell types and cell entities wherein conditions, media,
factors and stimuli
do not exist and cannot be applied to stimulate production of extracellular
matrix
material.
"Lipid precursor" as used herein means a small molecular precursor in lipid
- synthesis or biosynthesis that forms the structural backbone for
connection of the fatty
acid chains. Examples include ethanolamine or equivalents such as o-
phosphorylethanolamine, and also include any others that are interchangeable
with
ethanolamine in a serum-free medium for growing the ALS as herein disclosed.
=
The "Autogenic Living Scaffolds" (ALS) as disclosed herein are made and
composed of living cells and the extracellular matrix (ECM) that these living
cells
produce. The living cells that these Autogenic Living Scaffolds contain may be
= genetically engineered or otherwise modified. They serve as blueprints,
supporting
structures, backbones, or scaffolds for the same or other cell lines or types.
The ALS may
also provide proper or supporting mechanical and chemical environments,
signals, or
stimuli to other cells, to the cells that produce the ALS, to surrounding
tissue at an
implantation site, to a wound, or for in vitro generation and regeneration of
cells, tissue
and organs. They may also provide other cells with nutrients, growth factors,
and other
necessary or useful components. They may also take in or serve as buffers for
certain
= substances in the environment, and have also some potential at adapting
to new
environments.
The fibroblast produced matrix of the ALS, or "cell-derived matrix" (CDM), is
composed of a number of insoluble extracellular matrix (ECM) molecules
including
fibronectin, collagen-1 and collagen-3, possibly in ratios resembling those in
vivo. ALS
could thus provide a more "natural" environment, or an environment more
closely
resembling that of native tissue in vivo, for both fibroblasts and other cells
seeded onto
19
CA 02559275 2012-07-30
them in vitro, than artificial scaffolds and reconstituted extracellular
matrices such as
collagen gels provide. The ALS would thus potentially allow more accurate in
vitro
simulation of in vivo conditions to aid in better design of medical treatments
and in vitro
model systems.
In one embodiment, ALS is composed of slowly proliferating multilayered
fibroblasts surrounded by dense extracellular matrices produced by the
fibroblasts, a large
proportion of which consists of supermolecularly organized collagen. The
fibroblasts in
this self-produced mechanically stressed environment assume a synthetic
phenotype. The
synthetic phenotype of fibroblasts is the phenotype most commonly found in
connective
tissue in vivo, and is an activated cell phenotype characterized by low cell
proliferation,
high collagen accumulation, fibrillar fibronectin organization, and the
formation of actin
stress fibers and focal adhesions ( Kessler etal., 2001,1 Biol. Chem. 276:
36575-36585.
36575-36585.). This allows the ALS to be strong enough to provide structural
support for
other cell types seeded onto the matrix (and thus serve as a scaffold). The
slow
proliferation rate of the fibroblasts in the ALS keeps the fibroblasts from
overgrowing
other cell types. Changing the culture media from one that promotes fibroblast
growth to
one that promotes the growth and development of subsequently seeded cells
(while at the
same time possibly retarding the growth of the fibroblasts), also helps to
ensure that the
fibroblasts do not overgrow the seeded cells and/or tissue.
A chemically defined media allows more control of the ALS growth parameters
(and thus its mechanical and chemical properties), and allows the elimination
of non-
human components in the in vitro biological model and scaffold for cell
differentiation.
Several chemically defined media for fibroblast have been formulated over the
years
(Bettger etal., 1981, Proc. Natl. Acad. Sci. USA 78(9): 5588-5592; Shipley and
Ham,
1983, Exp. Cell Res. 146(2): 249-260; Parenteau, 1994, Skin Equivalents,
Keratinocyte
Methods, Leigh, I. and Watt, F. (eds), Cambridge University Press, Cambridge,
pp.45-54;
Vaccariello et al., 1999, Use of skin equivalent technology in a wound healing
model.
Methods in Molecular Medicine, Vol. 18: Tissue Engineering Methods and
Protocols.
Morgan, J.R. and Yarmush, M.L. Totowa, NJ, Humana Press Inc. 18: 391-405).
None
allows the efficient production of high-strength, high density, fast-growing
ECM, while
simultaneously keeping the growth of the fibroblast cells themselves
sufficiently slow so
that the fibroblasts do not over-run other cells seeded into the system. The
ALS Medium
CA 02559275 2012-07-30
allows the proper production of an autogenic "living" scaffold (ALS) system
for
producing cells and tissue of interest in vitro, for later implantation in
vivo, wherein all
components of the system are entirely defined, free from non-human components,
and the
20a
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
ALS thus-formed is of sufficient strength, adaptability, and thickness to
facilitate the
growth of cells and tissue of interest in an efficient and economical manner.
Autogenic Living Scaffolds made up of fibroblasts (these may be only a few
cells
thick, or over 1 mm in thickness) and other similar classes of cells (most of
the
anchorage-dependent class of cells) such as stromal cells, especially those
grown in
serum-free conditions and whose growth rate and other characteristics may be
controlled,
. may also serve another important role when implanted in vivo. For
example, Fibroblast
Autogenic Living Scaffolds (fALS) can grow and attach themselves to the site
of
implantation within a period of hours to days, and thus help to keep the
implanted cells
stationary with respect to the implantation site. This is very important, for
example, in
nerve grafts where a fraction of a degree change in the direction of a growing
axon
could/may prevent the nerves from making efficient connections with the
existing nerves
adjacent to the site of implantation. Fibroblast ALSs also have the capacity
to adjust to
their environments, making them "smart". For example, when a damaged portion
of a
heart is cut out and a fibroblast- or myofibroblast-ALS seeded with
cardiomyocytes.
and/or skeletal muscle myocytes is implanted into this site, the fibroblasts
or
myofibroblasts grow and attach themselves to the remaining portions of cardiac
muscle
around the site of the incision in a relatively short period of time and keep
the scaffold
almost completely stationary relative to the surrounding cardiac muscle. The
cardiomyocytes or skeletal muscle myocytes (or other seeded cells) then grow
and
develop into new cardiac muscle or into comparable cardiac muscle replacements
that
attach and join to the existing cardiac muscle. Using fibroblast- or
myofibroblast-ALSs
that are high in Collagen 111 relative to Collagen 1 (as is the case with the
secreted ECMs
from most foreskin fibroblasts), then scarring at the site of incision and
implantation may
be diminished.
The cells of an Autogenic Living Scaffold may also produce other useful and
beneficial components at ratios that more closely simulate or resemble the
natural
environments that cells are exposed to in vivo. This helps the seeded cells to
grow and
develop into tissue and organs that more closely resemble native tissue and
organs. When
implanted in vivo, the Autogenic Living Scaffold is expected to disappear over
time, at
. least partially, especially if the cells composing the Autogenic Living
Scaffold are from a
21
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
different host. However, the tissue or organ that develops from the cells
seeded onto the
Autogenic Living Scaffold is expected to remain. Apart from cardiac muscle and
the
heart, other organs or tissue may be produced using an ALS seeded with cells
or tissue of
interest. For example, liver tissue may be produced from an ALS that is seeded
with
hepatocytes; and kidney, pancreas, spinal cord, and other organs and tissues
of the body
may also be produced by seeding the ALS with the desired cell or tissue type.
Autogenic
Living Scaffolds seeded with the appropriate cell types may thus be used to
grow
implantable tissue and organs in vitro, for later implantation into an in vivo
site. In
general, when embodiments of the invention comprise the cells and scaffold
they
produce, the material is referred to as a Living Scaffold or an "Autologous
Living
Scaffold (ALS)". When the scaffold is seeded with cells to produce a tissue or
organ, and
used as such, the material is referred to as "ex-vivo cell-produced tissue
(EC1) or organ
(ECO)".Embodiments of this invention demonstrate that certain cells such as
human skin
fibroblasts can be induced to produce an extensive 3-D structural filamentous
material,
. = referred to herein as cell-produced matrix or cell-derived matrix (CDM),
that forms a
grid-like or mesh-like matrix which serves to facilitate and aid in the
formation of new
= cell growth and tissue. The resulting Living Tissue Matrix (CDM + the
fibroblasts that
produced the CDM) is analogous to early connective tissue that forms prior to
the
migration and population of other cells to form tissues and organs composed of
those
particular cells. For example, during amphibian embryogenesis, the formation
of the
heart begins with the formation of this type of fibroblast-populated
connective tissue
followed by the transdifferentiation of these fibroblasts into cardiac cells
to ultimately
form the amphibian heart. In embryonic wound healing (and non-scar forming
tissue
wound healing), the wound bed is first filled with a type of fibroblast-
populated
connective tissue that is analogous to the Living Tissue Matrix followed by
migration and
repopulation of the wound site with cells of the damaged tissue. Preliminary
experiments
have indicated that LTMs provide the scaffolding and/or template for new cell
growth for
cells of any type (thus serving as "Living Scaffolds"), and serve to aid in
cell attachment,
migration, proliferation and differentiation, especially tissue generation and
regeneration,
at sites of tissue damage. Furthermore, the LTM can be transformed into an
"implantable
22
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
blastema" for multi-tissue regeneration in vitro by tansdifferentiation and
dedifferentation of the fibroblasts within the LTM.
The Living Tissue Matrix is produced by the culturing suitable cells such as
skin
fibroblasts at hyperconfluent density in a completely chemically defined
medium (no
serum added) such as Matrix Media which causes them to enter a synthetic phase
without
significant proliferation (prior to being placed under these special
conditions, the
fibroblasts are induced to proliferate rapidly and are passaged without ever
being allowed
to attain confluence). In this phase, the secreted extracellular matrix (ECM)
differs
markedly from that of typical fibroblast cultures and more closely resembles
the
extracellular matrix found in generating/regenerating environments in vivo.
There is a
high ratio of type HI collagen compared to type I along with high
concentrations of
hyaluronic acid and decorin.
Since the cells produce the entire 3D matrix by themselves, the LTMs appear to
more closely resemble in vivo generative/regenerative connective tissue than
any other
matrix, scaffold or structure currently in existence. In fact, LTMs are
similar in
= composition to the type of fibroblast-populated connective tissue that
first fills the wound
bed in embryonic wound healing (and other non-scar forming tissue wound
healing), that
regenerates without scarring as opposed to wounds in neonatal or adult mammals
that
heal with scarring. Furthermore, this method produces a 3-D construct (the
LTM) that is
significantly thicker and stronger than those obtained using biopolymer gels,
such as
collagen or fibrin gels. The entire Living Tissue Matrix (cells and ECM) can
also be
made completely autologous, thus preventing host rejection and making it
completely
immunocompatible.
The ECM produced in this system provides an optimal environment for de-
differentiated or transdifferentiated autologous adult cells within the LTMs
to create a
regenerative environment and a virtual blastema. In other words, if the
millions of
fibroblasts within the LTM (the cells that produced the LTM in the first
place) are de-
differentiated or transdifferentiated, the LTM effectively becomes a
structurally sound
implantable blastema for multi-tissue type regeneration.
Living Tissue Matrices (LTMs) have potential for being used as soft tissue
substitutes since they are produced solely from cells fed with a chemically-
defined
23
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
medium that does not contain animal components. The rapid growth and lack of
expensive and inherently variable serum makes the development of LTMs as soft
tissue
substitutes commercially viable. Due to the relative simplicity of the
chemically-defined
medium, these cell-produced Living Tissue Matrices can also serve as in vitro
biological
models for the effects of nutritional components and pharmaceutical products
on the
growth and development of soft tissues. They may also be useful for studying
numerous
in vivo conditions and processes such as wound healing, soft connective tissue
formation,
fibrosis, and the development and interaction of different cells in a
generating/regenerating tissue environment. The greater stability and
mechanical
integrity of LTMs over both collagen and fibrin gels allows them to retain
their structural
integrity longer in in vivo conditions than reconstituted ECM. The ability to
grow LTMs
thick and strong in a relatively short period of time in chemically-defined
conditions
enables the development of an attractive alternative to fibrin gels, collagen
gels and even
native tissues for tissue replacement.
Many different types of cells may also be seeded in different parts of the
Living
Scaffold, or they could be sandwiched on top of each other, to produce a
variety of ex-
= vivo cell-produced tissues (ECTs) or organs (ECOs). For example,
fibroblasts may first
be grown in serum-free conditions favorable to promote a fibroblast M.S. The
fibroblast
ALS is then seeded with astrocytes, and the serum-free growth conditions
(including the
media, pH, osmolarity, temperature, oxygen tension, and anything else
required) are
adjusted to be favorable to the growth of the astrocytes. If needed, other
components are
added to keep the ALS alive and healthy. This entire structure or system may
also be
grown on porous membranes, such as TransWellsTm or BD FalconTm/BioCoatTm Cell
Culture Inserts, which allows media to be added to both the basolateral and
apical sides
of the ECT complex. Thus, media components more favorable to the growth and
survival
of the original cells (for example, fibroblasts) of the ALS may be added to
the basolateral
side of the entire ECT system or complex, while media components more
favorable to the
growth of the cells and tissue that are seeded onto the ALS may be added to
the apical
side of the entire system. Another option is to use cell culture systems such
as OptiCellTm
that allows gas exchange to occur across the walls of the cell culture vessel
where the
cells can attach and grow, thus allowing an ALS or LTM grown up to a thickness
of
24
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
about 2mm to be developed in vitro. Once a favorable layer of astrocytes has
grown on
the layer of fibroblasts, they may both be referred to as together making up
the ECT, or
the fibroblasts alone may be referred to as the LTM. Nerve cells may then be
seeded on
top of the layer of astrocytes, and the serum-free growth conditions again
adjusted to be
favorable to the growth and development of the nerve cells. If needed, other
components
are again added to keep the ALS and astrocytes alive and healthy, and the
entire system
= may also be grown on a porous membrane to allow different media
components to be
added to the apical and basolateral sides of the entire system or ECT complex,
as
described above. Additional layers, such as skeletal muscle myocytes that
might form
into skeletal muscle tissue that is innervated by the already seeded nerves,
may continue
to be added in this or a similar fashion, as desired.
In another embodiment, the ALS or LTM may also be grown into specific shapes
by molds, and may also be reshaped to some degree. For example, a sheet of the
above
example of an ALS, seeded first with astrocytes and then nerve cells, may be
rolled into a
= cylinder. This cylinder may then be implanted into a spinal cord in vivo.
The ALS also
; provides mechanical support to the seeded cells. For example, in a
particular
. embodiment, an ALS seeded with neurons may be mechanically stressed and
compressed, without major damage to the neurons, even though such a degree of
mechanical stress and compression kills most neurons when grown in the absence
of an
. ALS or without formation of the ECT. The ALS of other embodiments may
also be
introduced to mechanical stress or tension which may change the properties of
the ALS
and any cells or tissue that are growing on it.
In one particular embodiment, the fibers of a Fibroblast Autogenic Living
Scaffold may also be made to grow in parallel, which helps seeded nerve cells
to also
grow in parallel along these fibers, especially when Schwann cells are
previously seeded
onto the scaffolds and first start growing in parallel along these fibers.
This may be even
more useful when implanted in the spinal cord, since the implanted nerve cells
may then
be aligned in the same direction as the native nerve cells in the spinal cord.
In still
another embodiment, a sheet of ALS with the seeded neurons may also be rolled
into a
cylinder prior to implantation to produce a structure with layers of neurons
aligned in the
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
same general direction as the native neurons in the spinal cord. Similar
things may be =
done for implantation into other tissues and organs.
In other particular embodiments, cell to cell, tissue to tissue, and tissue to
cell
interactions may also be studied in vitro and in vivo with Autogenic Living
Scaffolds and
LTMs, such as by sandwiching different cells. In yet another embodiment,
Autogenic
Living Scaffolds and LTMs may be used as in vitro biological models for
studying the
growth and development of cells, tissues, organs, systems, diseases, and
different =
responses in organisms. For example, the wound response (in which fibroblasts
play an
active role) on different types of cells and tissues may be studied in vitro
by using this
technology.
A current shortage of load-bearing tissue has created a demand for human-made
tissues that can withstand in vivo mechanical forces Most of the strength of
connective
tissues is due to the collagen content per unit mass of tissue. The high
tensile strength of
collagen is largely attributable to the presence of intermolecular covalent
cross-links
= between the collagen fibrils Strength increases with increasing collagen
fibril diameter
(which are typically in the order of up to 100 nm in diameter in native
tissue, and 40-60
nm in developing native connective tissue), as well as with increasing density
and cross-
= linking of collagen fibrils. For the collagen in fibroblast based ALSs
(fALS) and LTMs, a
density of over 100 collagen fibrils/m2 can be achieved with a high degree of
cross-
linking between the fibrils. The collagen fibril diameters in ALSs where the
fibroblasts
are derived from the dermis of neonatal human foreskin, have so far been in
the order of
= 40-50 nm, indicating similarity to young connective tissue in vivo that
has greater
regenerative potential than mature connective tissue. Mechanical strength of
collagen can
also be increased through magnetic alignment of the collagen fibrils and by
the addition
of lysyl oxidase that induces additional cross-links between the collagen
fibrils Strength
also increases with the addition of fibronectin, which in turn increases actin
organization
and regulates the composition of the ECM. For example, fibronectin induces a 5-
fold
increase in the ultimate tensile strength of fibroblast populated collagen
lattices In
particular embodiments, fibronectin is generally the second most abundant ECM
protein
after collagen in ALSs fed with the Matrix Media.
26
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Fibroblasts (especially foreskin fibroblasts) secrete numerous growth factors
including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF),
and
neurotrophin-3 (NT-3), as well as fibroblast growth factor (FGF), and platelet-
derived
growth factor (PDGF), all of which promote neuron regeneration and survival.
The
embodiments of ALSs and LTMs described herein more closely mimic the
extracellular
environment that nerve cells are normally exposed to in vivo than any other
currently
available scaffolds, and even allow primary nerve cells to form active 3-D
neural
networks in vitro that can serve as in vitro 3-D models for potential
therapeutic agents for
neuronal regeneration, as may also be used to functionally replace injured
spinal cord
neurons in vivo.
Previous experiments show that fibroblasts grafts obtained from an in vivo
site
have allowed axons to elongate -0.5imn/month at a density of -28 axons/1=2,
and have
provided functional recovery within 3 months of up to -10 on the BBB scale
(Basso,
Beattie and Bresnahan locomotor rating scale - scale range: 0-21, with 0
representing no
- function, and 21 representing complete locomotor functionality - in animals;
(Lu etal.,
(2002) Brain 125(Pt 1):14-21), when implanted into chronically injured spinal
cord in
rats and monkeys. When the fibroblasts are genetically modified to express 10x
more
BDNF and NGF, these numbers jump to -2.1mm/month at a density of -70
axons/pm2,
and functional recovery is further slightly improved. The fibroblasts stopped
proliferating
after they were implanted at high density, and the grafts also prevented the
formation of
fibrosis at the site of injury and implantation. Furthermore, the fibroblast
grafts promoted
rapid and extensive migration of Schwann cells into the grafts from the
peripheral
nervous system, which is considered to be a major and necessary factor of
functional
spinal cord regeneration.
Other research showed that collagen gels and collagen filament implants
allowed
axons to elongate up to 5 mm within a month, and provided functional recovery
within 3
months of up to -12 on the BBB scale when implanted into injured spinal cords
in rats.
Still others have shown that fibronectin mats have allowed axons to elongate
up to 4-5
mm and reach diameters of up to 3.5 gm within a month, and provided functional
recovery within 3 months of up to -10 on the BBB scale when implanted into
injured
spinal cords in rats. Implanted fibronectin has also been found to aid in the
proper
27
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
orientation of elongating neuronal axons at the site of implantation in the
damaged spinal
cord, as well as in attracting Schwann cells from the peripheral nervous
system.
In the case of particular embodiments of fALS of the present application, the
high
density of fibroblasts along with the insoluble ECM proteins collagen and
fibronectin of
the ALS work in conjunction to promote axon elongation and functional recovery
when
implanted into chronically injured spinal cord. It is within the realm of the
presently
= disclosed ALS systems that a particular ALS seeded with primary neuronal
cells that
produces differentiated nerve cells and/or tissue may allow axons to elongate
up to as
much as ¨10mm/month and may promote the beginnings of functional recovery
within 1
month, ideally increasing to a value approaching 20 on the BBB scale after 3
months. It
is also within the realm of embodiments of the presently disclosed ALS systems
that a
. particular ALS seeded with primary neuronal cells that produces
differentiated nerve
cells and/or tissue may allow between ¨0.5 min/month axon elongation and about
10 on
the BBB scale within 3 months, and up to about lOmm/month axon elongation and
preliminary functional recovery within 1 month, increasing to a value
approaching 20 on
the BBB scale after 3 months. Other particular embodiments of fALS systems
seeded
with primary neuronal cells are envisioned to allow intermediate axon
elongation,
anywhere from about 3-8 mm/month of axon elongation, with functional recovery
again
approaching about 20 on the BBB scale after 3 months.
It is known that implanted neural progenitor cells survive for long periods of
time
at the site of implantation in injured spinal cord and support axonal
elongation and
limited functional recovery, and that neural progenitor cells differentiate
into nerve and
glial lineages and survive for much longer than neuroblastomas (which usually
die before
maldng any active synaptic connections ¨ a requirement for survival and
nutritional
support from the glial cells). Neural progenitor cells are also deemed far
more safe and
predictive than stem cells, which are difficult to control and are thought to
be one of the
main promoters of cancer in the central nervous system. Unfortunately, neural
progenitor
cells differentiate only into astro- and oligodendro-glial lineages when
implanted into
injured spinal cord, or when cultured in vitro in the absence of ALS systems
according to
the present invention. When neural progenitor cells are seeded in vitro onto
an ALS in
accordance with embodiments of the present invention, however, the neural
progenitor
28
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
cells differentiate into nerve and glial lineages, as shown in Figures 1
through 7.
Furthermore, neuroblastomas seeded in vitro together with neural progenitor
cells
or astrocytes onto ALS according to embodiments herein, are envisioned to
allow the
long-term differentiation and survival of the neuroblastomas on the ALS and
also at the
site of in vivo implantation in injured spinal cord. Also, basic fibroblast
growth factor,
which is secreted by the fibroblasts of particular embodiments of the ALS
systems
described herein, induces the secretion of neural growth factor (NGF) by
astrocytes
grown on the ALS in serum-free culture conditions.
Research Design and Methods
Example: Using the ALS as a scaffold for creating a nerve graft:
There is currently no proper. treatment for spinal cord injury. Limited spinal
cord
regeneration has been achieved in a small number of patients through physical
rehabilitation and training, peripheral nerve grafts, and by transplanting
fetal spinal cord
tissue. Most patients are limited to being dependent on the use of a
wheelchair and even
on devices that sustain/replace lost autonomic function.
There are a number of approaches in current research into treatments for
spinal
cord injury. The current limitation of all these approaches is that they
restore only partial
and generally minimal functionality to the injured spinal cord. The first
clinical trials for
the treatment of spinal cord injury began on July 11, 2002, in Australia by
transplanting
olfactory ensheathing glia into patients' spinal cords. The limitation of this
approach is
that it takes several years to restore some of the functions lost due to the
spinal cord
injury, and the restoration of these functions is limited (from a 1 to 4.3 on
the Basso,
Beattie and Bresnahan (BBB) locomotor rating scale (scale range: 1-20, with 1
representing no function, and 20 representing complete locomotor
functionality) in
animals). Another clinical trial involving the vaccination of Copolymer-1 into
patients
with recent (<14 days) spinal cord injury is about to start in Israel and the
United States,
but this treatment is designed more to slow down and possibly reverse the
degeneration
of nerve cells in diseases such as Parkinson's disease rather than being
designed as a
means for regenerating injured spinal cord nerve cells.
29
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
There is somewhat of a consensus in the field of neuroregeneration that the
necessary elements for potential tissue engineered nerve constructs include:
1) a scaffold
for nerve growth and axonal migration; 2) support cells; 3) growth factors;
and 4)
extracellular matrix. To date, no system that includes all the elements has
been described
= or disclosed that successfully generates nerve cells in vitro, for later
in vivo implantation.
In addition, there are no in vitro 3-D models of neuronal growth or
regeneration. Since
= the ALS provides an environment that closely resembles the extracellular
environment
that neurons are normally exposed to in vivo, the proposed tissue engineered
nerve grafts
created using the ALS could be used for a variety of in vitro applications,
such as
studying the effects of potential therapeutic agents on neuronal regeneration.
In terms of
serving as a nerve graft for replacing damaged spinal cord, this system uses
only human
components and has the potential to replace/regenerate spinal cord that is
extensively
damaged over large areas and distances since it contains its own nerve
cells/neural tissue.
Preliminary studies have indicated that the majority of neurons grown within
an
= ALS in accordance with the present invention are still alive after a ¨200-
1.tm thick graft -
has been gently folded into itself and then gently unfolded, as determined by
a neuronal
viability assay (Molecular Probes LIVE/DEAD Viability/Cytotoxicity kit # L-
3224)
before and after handling of the nerve graft (data not shown). However, in
such an ALS, =
the neurons represent only a small fraction of the ¨2004tm thick graft. Thus,
in particular
embodiments, neurons may be seeded onto thinner supportive ALSs wherein the
ultimate
tensile strength (UTS) of the ALS is increased by small modification to the
ALS
Medium. Alternatively, in other embodiments the porosity of the ALS may also
be
increased, to allow neurons to migrate throughout thicker ALSs. The fAISs in
accordance with particular embodiments of the present invention are prepared
by seeding
human foreskin fibroblasts at high density onto wells or TransWellsTm, and
allowing the
fibroblasts to develop a 3-D matrix having a thickness of between 50 to
several hundred
micrometers over a period of several weeks. The fibroblasts are fed with a
defined
"chemical" media that does not contain any serum or animal components,
referred to
herein as Fibroblast Autogenic Living Scaffold Medium (fALS Medium), an
example of
which is Matrix Media, and described in Example 1. In a particular embodiment,
after
the 3-D matrix (ALS) is produced, the ALS is seeded with primary neuronal
cells. The
CA 02559275 2012-07-30
ALS with the seeded neuronal cells is then fed with another defined "chemical"
medium
(again, a medium with no serum or animal components) that supports the growth
of the
neurons and retards the growth of the fibroblasts. In one embodiment, the ALS
seeded
with primary neuronal cells is maintained in TransWellsTm, allowing the basal
side of the
ALS to continue being fed with the first media (fALS Media) while the apical
side of the
ALS is fed with the second media.
The development of a functional neural network in vitro allows the nerve graft
to
have more utility when implanted, and is important for studying the effect of
different
pharmacological agents and methods on the in vitro 3-D neural network model
disclosed
herein. To date, no functional neural networks grown in vitro have been able
to be
transferred and implanted into a host due to the frailty of these neural
networks ¨ nerves
are so frail that they cannot even support their own weight, and no scaffolds
(apart from
the fALS disclosed herein) have yet been developed that provide adequate
structural
support to these nerves. To optimize the production of functional neural
networks, the
nerve graft in one embodiment may be grown on a 64-microelectrode array, or
alternatively, voltage-sensitive dyes may be used to determine the
functionality (presence
of action potentials) and connectivity (presence of phasic bursting patterns)
of the neurons
(see Mistry etal., (2002) Proc Natl Acad Sci USA 99(3):1621-6 and Segev etal.,
(2003)
Phys Rev Lett 90(16):168101) in the ALS. In this way different types of
primary neurons
(such as neural progenitor cells and neuroblastomas) may be tested to
determine which
types differentiate and produce the most favorable functional neural networks
in the
particular embodiments of ALS as described herein.
In other embodiments the effects of different nutritional supplements and
growth
factors on the development of the functional neural networks in the ALS may
also be
studied in this way. For example, FGF-2 and BDNF have been shown to support
the
development of extensive and spontaneously active neural networks from primary
neurons within 3 weeks (see Mistry et al., 2002). Preliminary results with
ALSs seeded
with primary neuronal cells in accordance with the present invention show that
the
carbohydrate source galactose is favored over glucose by neurons, and that
galactose also
significantly retards the growth of the fibroblasts in the ALS. Thus, in
embodiments of
31
CA 02559275 2012-07-30
the present invention, once the neurons are seeded onto the ALS, the growth
media is
changed from one that supports ALS growth to one that promotes neuronal growth
and
differentiation, while at the same time retards the growth of fibroblasts.
This prevents the
fibroblasts from over-running the neurons and effectively preventing neuronal
development. In other embodiments, the fibroblasts may be genetically
engineered to
secrete more growth factors such as NGF, BDNF, FGF-2, and bFGF to enhance
neuron
survival and development even further.
Specific neuronal markers such as Anti-Hu MAb 16A11 (Marusich et al., (1994)
J. Neurobiol 25(2): 143-155) may be used to differentiate neurons from
fibroblasts (see
Figures 3 through 7, and 12 through 13). The degree of attachment and
differentiation of
neurons on ALS can be determined (no. of differentiated neurons/no, of primary
neurons
seeded), along with how far the axons elongate through the ALS (average length
of axons
in 4-10 fields of view (see Segev etal., 2003)) and the length of time that
the neurons are
viable on the ALS (from the time of seeding). Determining the length of time
that the
neurons are viable on the ALS allows the graft to be implanted days prior to
the
degeneration of the nerves, thereby ensuring that the nerves remain alive and
functional in
the host/patient to better the chances for neurogenesis. In addition,
histology and
transmission electron microscopy (tEM) may be used to observe the arrangement
and
connectivity of the neurons (Jin et al., (2002) Exp Neurol 177(1):265-75 and
Tuszynski et
al., (2002) J Comp Neurol 449(1):88-101) in the ALS.
It is expected that superior results may be achieved when a particular
embodiment
of an ALS nerve graft is implanted 2 weeks after spinal cord injury, since
this is when
BDNF-expressing and supporting microglia, as well as pen-wound sprouting
around the
site of injury, begin to reach significant levels. However, in particular
embodiments, the
ALS nerve graft contains active neurons, including primary neurons that have
begun to
differentiate and form active neural connections (see Figures 2-4, for
example), so
positive results are expected regardless of when the ALS nerve graft is
implanted.
In other embodiments, the ALS nerve graft has the flexibility of taking on
almost
any non-rigid shape and may be rolled up into a ball or cylinder. Several thin
nerve grafts
may also be layered on top of each other to form different parallel layers of
neural
32
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
networks, which in turn may again be rolled up into a cylinder or formed into
some other
shape. Alternative forms and structures of ALS nerve grafts are expected to
give better
results in terms of functional recovery for each type of spinal cord injury
(longitudinal,
transverse, shear, etc.). The requirement for immune-suppressants may also be
determined, although such agents are not expected to be required since nerve
cells are not
attacked by the immune system in humans and fibroblasts do not elicit much of
an
immune response. Alternatively, in other particular embodiments nude rats may
be used
as models for implanting human nerve grafts, to study functional recovery
promoted by
the ALS nerve grafts in accordance with embodiments of the present invention.
In still other embodiments, the ALS nerve grafts as herein described, may be
benchmarked against other current approaches, e.g., according to the extent of
functional
recovery in spinal cord injury-induced animals (using the BBB scale as a
standard), or
according to the extent of active functionality of implanted/regenerated nerve
cells (as
determined electrophysiologically), or according to the extent of
complications, the time
to functional recovery from the date when the procedure is started, and from a
cost/feasibility perspective.
EXAMPLES
Example la ¨ Serum-Free Chemically-Defined Medium for Growing Fibroblast ALS ¨
"fALS Medium "or "Matrix Media"
One embodiment of a chemically defined media formulation in accordance with
the present invention contains:
A 3:1 ratio of DMEM (high glucose (4.5g/L); with L-glutamine and sodium
pyruvate) and Ham's F12 medium supplemented with the following components:
= 4.2 x 10-16M Epidermal Growth Factor (in human serum albumin)
= 2.8 x 10-1 M Basic Fibroblast Growth Factor
= 8.6 x 10-6M insulin
= 1.0 x 10-7M dexamethasone
= 3.2 x 10-4M L-ascorbic acid phosphate magnesium salt n-hydrate
= 2 x 10-10M L-3,3',5-triiodothyronine
= 104M ethanolamine
= 3.9 x 10-8M selenious acid
= 4 x 10-3M Glutamaxn4
= 3.3 x 10-6M glutathione (reduced)
33
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
= = 1% penicillin/streptomycin/amphotericin B
In addition, other embodiments and variations of the above-listed medium may
contain additional components, such as any one or more of the following
components:
= Platelet derived growth factor (PDGF)
= 100:68 ratio of glycine : L-proline
= L-cysteine
= Trolox
Concentrations may vary as required, as long as the total osmolarity in the
medium is kept at acceptable levels for growth of the ALS. For example, L -
ascorbic acid
phosphate magnesium salt n-hydrate may range in concentration from 0.1 mM to 3
mM;
EGF may range in concentration from 0.002 nM to 2 nM; bFGF may range in
concentration from 0.03 nM to over 3 nM; insulin may range in concentration
from 10
pM to 1000 p.M; PDGF may range in concentration from 0.1 ng/mi to 10 ng/ml; L -
3,3',5-triiodothyronine may range in concentration from 0.1 nM to 10 nM;
ethanolamine
, may range in concentration from 1 pM to 10,000 p.M; selenious acid may range
in
concentration from 10 nM to 1000 nM; Glutamaxml may range in concentration
from 0
mIv1 to 10 mM; glycine:L-proline concentrations (still at a 100:68 ratio) may
be
supplemented with from 0 mM glycine:146 mM L-proline; to 2.675 mM
glycine:1.965
mM L -proline; dexamethasone concentrations may vary from 1 nM to 1000 nM; L-
cysteine concentrations may be supplemented with additional 0.1 mM to 1 mM.
Example lb ¨ Serum-Free Chemically-Defined Medium for Growing Neuronal
Cells/Tissue ¨ "Neural Medium"
One embodiment of a chemically defined media formulation in accordance with
the
present invention contains:
A 2:3 ratio of DMEM/F12 and Neurobasal Medium (see Gibco-Invitrogen
Corporation at www.invitrogen.com, or www.invitrogen.co.jp/focus/161006.pdf)
supplemented with the following components:
= 3 x 10-1 M Fibroblast Growth Factor 2
= 8.5 x 10-6M D(+)galactose
= 6.0 x 10-8M progesterone
= 6.0 x 10-7M retinyl acetate
34
CA 02559275 2012-07-30
= 9.0 x 10-8M corticosterone
= 1.0 x 10-4M putrescine
= 1.0 x 10-5M carnitine
= 1.3 x 10-5M linoleic Acid
= 4.3 x 10-6M linolenic Acid
= 4.0 x 10-6M biotin
= 4.0 x 10-6M Trolox
= 1% penicillin/streptomycin/amphotericin B
In addition, other embodiments and variations of the above-listed medium may
contain
additional components, and concentrations may vary as required.
Example 2. Production of Extracellular Matrix by Fibroblast Cells isolated
from the
dermis of newborn human foreskin
Large numbers of fibroblast cells were isolated from the dermis of newborn
human foreskin. Cells were proliferated in cell culture flasks fed with DMEM
Medium
with 10% Nu-Serum (or FBS or BCS) for several weeks. After a few passages, the
fibroblast cells were centrifuged at 1000 RPMs, the supernatant decanted, and
the cells
pooled. These pooled fibroblast cells were resuspended in ALS Medium as
described
above in Example 1, then seeded into TransWellsTm or BD FalconTm/BioCoatTm
Cell
Culture Inserts or 6- or 12-well plates or other small containers suitable for
in vitro cell
culture, at superconfluent conditions, whereby cell density was between about
200,000 to
greater than about 1,000,000 cells/cm2. The fibroblast cells were maintained
at
hyperconfluent conditions for about 3 weeks (or optionally about 1 week or
longer than
weeks), during which time they were observed to produce Extracellular Matrix,
thus
creating a Fibroblast ALS about 300 gm thick. See Figs. 3-9, and Figs. 12-13,
for
example, showing clearly visible extracellular matrices. Other embodiments may
include
Extracellular Matrices that have been produced by the fibroblasts resulting in
ALSs that
are 50 gm to 500 gm thick, or even up to or greater than 1 mm thick.
In one embodiment, the fibroblast ALS is grown on a circular porous
polyethylene
(Huang etal., (1993) Anna! Biomed Eng 21(3): 2890305 and Uysal et al., (2003)
Plast
Reconstr Surg 112(2):540-6) or ceramic support that exposes both sides of the
ALS to
media. This allows the ALS to grow faster and thicker due to the shorter
diffusion
distance for gases and
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
nutrients than when the ALS is exposed to media on only one side at the bottom
of a
culture well. Alternatively, the ALS is grown on 0.41.tm TransWellsTm
(Corning, 2003),
although this limits the subsequent seeding of other cell types to just one
side of the
CDM. The use of C)ptiCellsTM allows the ALS to grow up to about 2 mm thick in
vitro
due to the efficient gas exchange from both sides of the ALS. Another
alternative is to
use MINUCELISTm and MINUTISSUETm since they allow the constant perfusion of
media and oxygen to the cells and tissue of/on the ALS. Using 0.4pm
TransWellsTm or
center-well organ culture dishes (BD Biosciences # 353037) also allows more
nutrient
media to be used and permits the cells to be closer to an 02 source. The
availability of 02
may be further enhanced by increasing the partial pressure of 02 (p02) that
the cells and
= inside of the culture vessel are exposed to. The pores in the porous
polyethylene or
ceramic support provide adequate sites of ECM attachment for the ALS. Cells
are
prevented from growing on the wells and the outside circle of the porous
polyethylene or
ceramic supports by treating these areas with a non-binding substance such as
SigmaCote
(Sigma # SL-2), once the ALS is grown in a suspended or floating state.
Suspended ALSs
may be induced to have more dendritic fibroblast morphology, thereby allowing
more
flexibility in modifying the characteristics of the ALS, for example, through
the use of
= mechanical stimulation.
Example 3. In vitro Protocol for Generating Neuronal Cellsirissue
In one embodiment, a large number fibroblast cells (Cambrex CC-2509) were
centrifuged, at approximately 1000 rpm for about 8min, followed by removal of
the
supernatant. The resulting fibroblasts were then resuspended in ALS Medium and
seeded
into TransWellsTm or BD FalconTm/BioCoatTm Cell Culture Inserts at
superconfluency
(between about 1,000 cells/mm3 to greater than 200,000 cells/mm3), and fed
with ALS
Medium every 2-3 days for about 2-3 weeks or longer at hyperconfluent
conditions until
the desired amount and thickness of ALS had formed.
At this point, the ALS containing fibroblasts and extensive CDM was seeded
with
neural progenitor cells (Cambrex CC-2599), and the basolateral side of the
TransWellsTm
continued to be fed with fALS Medium while the apical side was fed with Neural
Medium every 2-3 days for another 2 ¨ 4 weeks or longer.
36
=
CA 02559275 2012-07-30
With time and appropriate growth factors, secreted by the fibroblasts in
response
to the presence of the seeded neural progenitor cells and so present in the
ALS (but they
may also be added as a supplement), the neural progenitor cells began to
differentiate into
both nerve cells, as evidenced by formation of nerve axons, and glial cells.
Neural
progenitor cells implanted into mammalian spinal cord and grown in the absence
of an
ALS system do not differentiate into both nerve cells and glial cells; they
only
differentiate into glial cells. But as can be seen in Figure 2, neural
progenitor cells,
grown on a fibroblast ALS, have differentiated into both nerve axons and glial
cells.
Additionally, Figure 1 shows methylene blue staining of neurons that have
differentiated
from neural progenitor cells grown on a fibroblast ALS.
Figures 3 through 7 show sections of fibroblast ALS systems containing
neuronal
cells/tissue that are expressing anti-Hu MAb 16A11, an early marker of
vertebrate
neurogenesis that is expressed shortly after neuronal terminal mitosis. As can
be seen,
varying-sized regions (different cross-sections of nerve cell bodies)
expressing the anti-
Hu MAb 16A11 marker are indicated, ranging in size from 41.tm to over 20 [im.
Example 4. In vitro Protocol for Generating Muscle Cells/Tissue
In one embodiment, a large number fibroblast cells (Cambrex CC-2509) were
centrifuged, at approximately 1000 rpm for about 8min, followed by removal of
the
supernatant. The resulting fibroblasts were then resuspended in fALS Medium
and
seeded into TransWellsTm or BD FalconTm/BioCoatTm Cell Culture Inserts at
superconfluency (between about 1,000 cells/mm3 to greater than 200,000
cells/mm3), and
fed with fALS Medium every 2-3 days for about 2-3 weeks or longer at
hyperconfluent
conditions until the desired amount and thickness of ALS had formed.
At this point, the ALS containing fibroblasts and extensive CDM was seeded
with
human skeletal muscle myoblasts (Cambrex CC-2580), and the basolateral side of
the
TransWells continued to be fed with ALS Medium while the apical side was fed
with
Skeletal Muscle Medium (Cambrex CC-3160) every 2-3 days for another 2 ¨ 4
weeks or
longer. With time and appropriate growth factors, secreted by the fibroblasts
in response
to the presence of the seeded skeletal muscle myoblasts and so present in the
ALS (but
they may also be added as a supplement), the skeletal muscle myoblasts began
to
differentiate into skeletal muscle cells. Figures 12 and 13 show trichrome
staining of two
37
CA 02559275 2012-07-30
different sections of a fibroblast ALS system containing muscle cells/tissue,
one about 70
tm thick (Fig. 12) and the other section about 115 m thick (Fig. 13). The
fibroblast
extracellular matrix of collagen fibers, the nuclei of the ALS fibroblast
cells and the
young skeletal muscle cells within the ALS are observed.
Example 5. In vitro Protocol for Generating Liver Tissue
In one embodiment, a large number fibroblast cells (Cambrex CC-2509) were
centrifuged, at approximately 1000 rpm for about 8min, followed by removal of
the
supernatant. The resulting fibroblasts were then resuspended in fALS Medium
and
seeded into TransWellsTm or BD FalconTm/BioCoatTm Cell Culture Inserts at
superconfluency (between about 1,000 cells/mm3 to greater than 200,000
cells/mm3), and
fed with fALS Medium every 2-3 days for about 2-3 weeks or longer at
hyperconfluent
conditions until the desired amount and thickness of ALS had formed.
At this point, the ALS containing fibroblasts and extensive CDM was seeded
with
human hepatocytes (Cambrex CC-2591), and the basolateral side of the Trans
Wells
continued to be fed with fALS Medium while the apical side was fed with
Hepatocyte
Medium (Cambrex CC-3198) every 2-3 days for another 2 ¨ 4 weeks or longer.
With time
and appropriate growth factors, secreted by the fibroblasts in response to the
presence of
the seeded hepatocytes and so present in the ALS (but they can also be added
as a
supplement), the hepatocytes began to differentiate into albumin-secreting
liver cells and
tissue.
Example 6a. In vivo Protocol for Generating/Regenerating Neuronal Tissue Using
In
Vitro ALS Systems Seeded with Neural Progenitor Cells
Rat/Rodent Spinal Cord
In one embodiment of the present invention, the lower spinal cord of an adult
nude Fisher rat is partially severed under general anesthesia (a complete and
full 5mm
long spinal cord transection) such that the rat is paralyzed from below the
point of injury
38
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
(the site of injury is chosen such that the lower limbs are non-functional
after excision ¨
generally T9-T10). Neuronal cells/tissue generated in vitro within a
fibroblast ALS
system seeded with human neural progenitor cells or neuroblastomas or neuronal
stem
cells or other primary neural cell line, such as detailed in Example 3, are
next implanted
into the rat spinal cord at the site of injury.
The fibroblasts within the ALS begin attaching to the site of implantation
immediately, and continue attaching for days and weeks, as the neuronal cells
grow and
form active neural connections with existing nerves on each side of the
implantation site.
Any remaining primary neurons at the time of implantation appear to
differentiate into
functional nerve cells and tissue. The rat is maintained and observed for 8
weeks or
longer, and observed for indications of neurogenesis, such as the ability to
move its hind
legs, or even the ability to walk with erratic use of its hind legs, or the
ability to walk
using its hind legs for a short a distance. Experiments have so far resulted
in experimental
animals regaining sensation and movement corresponding to up to 14 on the BBB
scale,
as compared to an average of 2 on the BBB scale for control animals.
6b. In vivo Protocol for Generating/Regenerating Neuronal Tissue Using In
Vitro LTM
. Systems where the fibroblasts within the LTM are Transdifferentiated into
Neurons
Rat/Rodent Spinal Cord
In one embodiment of the present invention, the lower spinal cord of an adult
nude Fisher rat is partially severed under general anesthesia (a complete and
full 5mm
long spinal cord transection) such that the rat is paralyzed from below the
point of injury
(the site of injury is chosen such that the lower limbs are non-functional
after excision ¨
generally T9-T10). Neuronal cells/tissue generated in vitro within an LTM by
transdifferentiating the fibroblasts within the LTM into neurons (by placing
the LTM in
Neurogen media with cytochalasin B for 60h, followed by replacing the media
with fresh
Neurogen media for 2-3 days) are next implanted into the rat spinal cord at
the site of
injury.
The neuronal cells within the LTM grow and form active neural connections with
existing nerves on each side of the implantation site over several weeks.
Remaining
neurons on each side of the transection site appear to also start growing into
the LTM
39
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
over several weeks. The rat is maintained and observed for 8 weeks or longer,
and
observed for indications of neurogenesis, such as the ability to move its hind
legs, or even
the ability to walk with erratic use of its hind legs, or the ability to walk
using its hind
legs for a short a distance. Experiments have so far resulted in experimental
animals
regaining sensation and movement corresponding to up to 16 on the BBB scale,
as
compared to an average of 2 on the BBB scale for control animals.
6c. In vivo Protocol for Treating Spinal Cord Degeneration in an Animal
Fibroblast cells from human foreskin are cultured to produce an ALS or LTM
system having an extensive ECM, as detailed in Example 2. The ALS is then
seeded
with neural progenitor cells or neuroblastomas or neuronal stem cells or other
primary
neural cell line as described in Example 3, or the fibroblasts within the LTM
are
transdifferentiated into neurons as in example 6b, until nerve cells and/or
tissue are
evident. The ALS plus primary neural cells/tissue or neuronal LTM is next
implanted
into the animal's spinal cord near or at the site of degeneration, or at
several sites along
the spinal cord if degeneration is pervasive.
The treated animal is then maintained and observed for 8 weeks or longer, as
required, while monitoring for evidence of spinal cord regeneration, such as
new-found
movement, feeling and sensation. Several treatments with neuronal LTM or ALS
systems having primary neural cells and/or tissue may be performed, to
increase the
spinal cord regeneration seen, depending upon the severity of degeneration in
the animal
to be treated.
Such a procedure may be used on animals, including humans, suffering from a
wide-spectrum of neurodegenerative diseases such as Lou Gehrig's disease,
Huntington's
disease, spinal cord compression due to crushed vertebrae, spinal cord
severance, etc.
7. In vivo Protocol for Generating/Regenerating Cartilage Tissue Using In
Vitro ALS
Systems Seeded with Chondrocytes
In one embodiment, a large number fibroblast cells were centrifuged, at
approximately 1000 rpm for about 8min, followed by removal of the supernatant.
The
CA 02559275 2012-07-30
resulting fibroblasts were then resuspended in ALS Medium and seeded into
TransWellsTm or BD FalconTm/BioCoatTm Cell Culture Inserts at superconfluency
(between about 1,000 cells/mm3 to greater than 200,000 cells/mm3), and fed
with ALS
Medium every 2-3 days for about 2-3 weeks or longer at hyperconfluent
conditions until
the desired amount and thickness of ALS had formed. The ALS containing
fibroblasts
and extensive CDM was then seeded with chondrocytes (Cambrex CC-2550), and the
basolateral side of the TransWellsTm continued to be fed with ALS Medium while
the
apical side was fed with Chondrocyte Medium (Cambrex CC-3216) every 2-3 days
for
another 4 ¨ 6 weeks or longer. With time and appropriate growth factors,
secreted by the
fibroblasts in response to the present of the seeded chondrocytes and so
present in the
ALS (but supplemental factors can also be added as required or desired), the
chondrocytes began to differentiate into cartilage tissue.
The Autogenic Living Scaffold may also be first grown into specific shapes by
molds, and may also be reshaped to some degree. For example, a "balloon" or
hollow disc
of a Fibroblast Autogenic Living Scaffold may be prepared by growing the fALS
culture
around a TeflonTm disc or sphere with a thin coating of an easily degradable
protein or
other substance and then slitting one side to remove the disc or sphere and
thereby
creating the hollow space or "balloon" shape. After preparation of the ALS
"mold", it
may be filled with chondrocytes in a gel (such as an alginate gel) that may be
placed/injected into the open inner space of the ALS disc or balloon mold, and
then
closed back up to create dense, compact cartilage tissue over time. The ALS
allows
nutrients and gases to pass through it to the chondrocytes, while at the same
time
retaining most of the signaling molecules secreted by the chondrocytes and
secreting
certain growth factors and substances that promotes the formation of
functional cartilage
tissue. The high tensile strength of the Fibroblast ALS also creates
mechanical stresses
onto the chondrocytes and newly formed cartilage that promotes the development
of
functionally strong cartilage. The outside layer of ALS around the formed
cartilage disc
also promotes the attachment and integration of the cartilage disc once it is
implanted.
Such a procedure may be used to create, for example, cartilage discs for
replacement
within a vertebrate spinal column.
41
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Any desired shape or size may be made by creating the appropriate ALS mold,
and the resulting cartilage tissue formed within the ALS mold may be used to
treat an
animal, including a human, suffering from cartilage damage. The same is true
of bone
damage, where osteoblasts are used instead. In such treatments, the ALS mold
plus
interior seeded connective tissue cells, or plus interior cartilage tissue, is
placed/inserted
into the animal's joint, or placed/inserted near or at the site of injury
where cartilage
regeneration is desired, or at several sites.
The treated animal is then maintained and observed for several weeks and
months
or longer, as required, while monitoring for evidence of cartilage
regeneration by using
MRI imaging or other soft-tissue detection techniques, and by observation of
physical
indications such as joint function, levels of pain, strength, flexibility,
etc. Several
treatments with ALS molds and seeded chondrocytes may be performed, to
increase the
cartilage regeneration seen, depending upon the severity of the injury or
cartilage
degeneration in the animal to be treated.
Such a procedure may be used on animals, including humans, suffering from a
wide-spectrum of cartilage degeneration conditions such as cartilage
degradation/degeneration in the joints, including knees, ankles and fingers,
in the spinal
column, and in the nose, ear, throat, and elsewhere.
8. Living Tissue Matrix (LTM)
A strong and thick cell-produced Living Tissue Matrix (LTM) can be produced
within three weeks, making it a viable and attractive alternative to
reconstituted gels,
such as cell-populated fibrin or collagen gels. Data from comparative studies
show that a
completely cell-produced ECM is mechanically superior to reconstituted ECM,
and more
closely resembles native in vivo ECM than reconstituted ECM. This strength is
highly
correlated to the fraction of very stable collagen. In addition to closely
approximating
native generating/regenerating tissue, LTMs also support significantly faster
rates of cell
adhesion, migration, proliferation, differentiation, and acquisition of in
vivo-like
morphology than reconstituted ECM. The extracellular matrix (ECM) portion of
the
LTMs consists of several components such as collagen I, collagen HI,
fibronectin,
proteoglycans, sulfated glycosaminoglycans, hyaluronic acid, and decorin.
42
CA 02559275 2012-07-30
Extensive preliminary studies have indicated that these matrices are composed
of
numerous extracellular matrix proteins in amounts, ratios and arrangement that
more
closely resemble young, native, blastema-like tissue than any other matrices
or scaffolds
currently in existence. These matrices are embedded with multi-layered
fibroblasts that
created the LTM in the first place. Dedifferentiating these multi-layered
fibroblasts in the
matrix could essentially lead to a blastema-like structure that can be
implanted onto the
site of tissue injury for restoration of a functional multi-tissue type
structure.
Transdifferentiating some or all of these multi-layered fibroblasts in the
matrix could then
aid the blastema-like structure so formed to start developing and
differentiating along a
more controlled pathway.
Figure 14 shows an H&E stained cross-section of early nerve differentiation on
cell-produced Living Tissue Matrices. Human neural cell bodies having Hul 6A11
markers (a marker of early neurogenesis) as visible, with the stained markers
discernible
as dark stained regions in the neural cell bodies.
9. Genetically Engineering fibroblasts to make a stronger and thicker ALS or
LTM.
A human gene coding for fibroblast growth factor 2 (FGF-2) was inserted into
the
genome of human neonatal foreskin fibroblasts (obtained from the American Type
Culture Collection (ATCC, Manassas, VA)) under the regulation of a 13-actin
promoter.
These transformed or genetically engineered fibroblasts, produced a
significantly stronger
and thicker cell-derived matrix than control fibroblast cultured for a 3-week
period
according to the methods of example 2. The genetically engineered fibroblasts
were
found to secrete several-fold higher concentrations of FGF-2 into the
extracellular space
of the ALSs or LTMs than the wild-type fibroblasts. These higher FGF-2
concentrations
caused an increase in the rate of extracellular matrix protein synthesis by
the fibroblasts
within the ALSs or LTMs.
10. Methods for Preparing and Analyzing Living Tissue Matrices (LTMs) or
Autogenic
Living Scaffolds (ALSs) and their Cell-Derived Matrix (CDM) component versus
Fibroblast-Populated Collagen Gels and Fibrin Gels
43
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
A. Cells
=
= Human neonatal foreskin fibroblasts (HFFs, American Type Culture
Collection,
Manassas, VA) were cultured in T-300 tissue culture flasks (BD Biosciences,
Bedford,
' MA) with high glucose Dubelco's modified Eagle' medium (DMEM, Mediatech,
Herndon, VA) supplemented with 10 % bovine calf serum (BCS, Hyclone, Logan,
UT),
and 1 % penicillin/streptomycin/ampothericin B (Invitrogen, Carlsbad, CA) at
37 C in
humidified, 10 % CO2 conditions. Cells were harvested at 90 % confluency with
a 10 min
application of 0.25 % trypsin/0.05 % EDTA solution (Mediatech). Two million,
passage
cells were used for each sample in all experiments.
B. Standard Serum-Supplemented Medium
DMEM with 10 % fetal bovine serum (1413S, ATCC), 150 tig/m1 (519 tiM) L-
ascorbic acid phosphate magnesium salt n-hydrate (Wako Pure Chemicals, Japan),
and 1
% penicillin/streptomycin/amphotericin B (Invitrogen).
C. Chemically-Defined Medium
A 3:1 ratio of DMEM (high glucose (4.5 g/L); with L-glutamine and sodium
pyruvate (Mediatech) and Ham's F12 (Invitrogen) with the addition of 5 lig/int
insulin
(Sigma-Aldrich, St. Louis, MO), 5 ng/ml selenious acid (Sigma-Aldrich), 104M
ethanolamine (Sigma-Aldrich), 150 pg/ml L -ascorbic acid phosphate magnesium
salt n-
hydrate (Wako), 2.5 ng/ml epidermal growth factor (EGF (BD Biosciences)) in 5
pg/ml
human serum albumin, EM]) (Biosciences, San Diego, CA), 5 ng/ml basic
fibroblast
growth factor (bFGF (BD Biosciences)), 1.0 x 10-7M dexamethasone (Sigma-
Aldrich), 2
x 10-1 M L-3,3',5-triiodothyronine (Sigma-Aldrich), 4 x 103M GlutamaxTm
(Invitrogen), 1 p.g/m1 glutathione (reduced) (Sigma-Aldrich), and 1 %
penicillin/streptomycin/amphotericin B (Invitrogen). Growth factors were added
fresh at
each feeding, except for **CDM, where the growth factors were added at the
correct
concentration (2.5 ng/ml EGF and 5.0 ng/ml bFGF) into the entire stock medium
at the
start of the experiment.
D. Collagen Gel, Fibrin Gel and ALS/LTM Preparation
44
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Fibroblast-populated collagen gels (CGs) were prepared according to methods
known in the art by mixing 0.2 ml of collagen stock solution (5 mg/ml of 5 mM
HCl-
extracted rat tail tendon collagen in 5 inM acetic acid), 0.05 ml 5X DMEM
(Mediatech),
0.65 ml DMEM (Mediatech) with cells, 0.1 ml fetal bovine serum (FBS, ATCC),
150 jig
L -ascorbic acid phosphate magnesium salt n-hydrate (Wako) and 1 %
penicillin/streptomycin/amphotericin B (Invitrogen) at room temperature. One
milliliter
of the resulting solution was added into each 24 mm diameter well. The initial
collagen
concentration was 1.0 mg/ml, and the initial cell concentration was 2,000,000
cells/ml in
% FBS.
Fibroblast-populated fibrin gels (FGs) were prepared based on the methods
known in the art. Briefly, HFFs in standard serum-supplemented medium were
added to a
fibrinogen solution (Sigma-Aldrich F4753 type IV). One-ml samples were mixed
with 4
units of bovine thrombin (Sigma-Aldrich, T7513) at room temperature. One
milliliter of
the resulting solution was added into each 24 mm diameter well. The initial
fibrinogen
= concentration was 1.0 mg/ml, and the initial cell concentration was
2,000,000 cells/ml in
10% FBS.
ALSs/LTMs were prepared by mixing the 2 million, passage 5 HFFs with
= standard serum-supplemented medium or the chemically-defined medium
described
above (for *CG, *CDM and **CDM) at room temperature into a final volume of 1
ml per
sample. The initial cell concentration was 2,000,000 cells/ml in 10 % FBS. The
1 ml
samples of collagen gels, fibrin gels, and CDMs were pipetted onto 24mm
diameter,=
porous inserts (0.4 um TransWellsTm, Corning Life Sciences, Acton, MA)
suspended
above standard 6-well plates, and allowed to sit undisturbed at room
temperature. After a
1-hour period, 3 ml of standard serum-supplemented medium or chemically-
defined
medium (for *CD, *CDM and **CDM) was carefully added below, and 1 ml added
above, each sample and the samples were incubated at 37 C in humidified, 10 %
CO2
conditions. Samples were fed every other day (3 ml below and 2 nil above each
porous
insert) with the same medium for 3 weeks.
E. Mechanical Testing
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
After 3 weeks in culture, the samples were exposed to ddH20 for 1 hour to lyse
the cells and eliminate the contractile forces produced by the fibroblasts,
and then
= equilibrated in phosphate buffered saline (PBS, Mediatech) for
biomechanical testing.
= The human penile skin was obtained through Analytical Biological Serives
Inc.
= (Wilmington, DE) and shipped cold in RPM' medium with antibiotics and
tested within
12 h of removal from the subject. The thickness, failure tension, failure
strain, and
= ultimate tensile strength (UTS) of the samples were determined by a novel
tissue inflation =
device that measures the displacement and pressure at which a sample bursts
when
inflated with PBS at a constant rate of 1 ml/min. The sample is circularly
clamped at and
inflated through a 1-cm diameter opening, thus causing the sample to form a
spherical
= cap before failing. The increasing pressure applied to the sample was
measured by an on-
= board pressure transducer (model PX102-025GV, Omega Engineering,
Stamford, CT).
The displacement of the center of the cap was measured with a laser
displacement system
(LDS-080, Keyence, Woodcliff Lake, NJ). The LSD-080 also measured the
thickness of
= each sample after being slightly compressed by a small reflective disk
(1.3 g, 1.3 cm
diameter) for 1 minute. The maximum membrane tension, T, was calculated using
the
Law of Laplace for a spherical membrane:
T 2PR, (eq. 1)
where P is the pressure when the tissue bursts and R is the corresponding
radius at
the point of rupture, calculated assuming a spherical cap geometry by:
R= (w2 a2) / 2w, (eq. 2)
= where a is the radius of the clamp (5 mm) and w is the displacement at
the center
of the sample at failure measured by the laser.
The ultimate tensile strength (UTS) was calculated by:
= UTS = T 1 t,
(eq. 3)
where t is the initial thickness of the specimen before inflation. The actual
thickness at the time of bursting was less than this value. Thus the
calculated UTS
(engineering stress) is less than the true stress at failure, since the
thickness of the
specimens decreased as they were being inflated.
The ultimate tensile strength per collagen density (UTS / Collagen Density)
was
calculated by:
46
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
UTS / Collagen Density = UTS / (Total Collagen / 2rt(D/2)2)
= T / (Total Collagen / x(D/2)2),
(eq. 4)
where D is the diameter of the constructs (2.4 cm).
The failure strain was determined from the equibiaxial strain at the pole
estimated
from the displacement data using the approximate relationship:
Failure Strain = 2/3 (w / a)2 ¨ 2/15 (w / a)4 + 2/35 (w / a)6.
(eq. 5)
F. Biochemical Analysis
Following biomechanical testing, the samples were weighed (wet weight),
lyophilized overnight, and then weighed again (dry weight). Each lyophilized
sample was
solubilized in 1 ml of 0.5 M acetic acid and 1 mg/ml pepsin (Sigma-Aldrich)
and
incubated overnight at 20 C with rotation. This extraction step was repeated
twice to
achieve complete extraction of the acid and pepsin soluble fraction of
collagen. The
= samples were then centrifuged at 14,000 rpm for 1 hour at 15 C, and the
supernatant was
= combined with samples from the other two extractions and used for
determining non-acid
and pepsin extractable collagen content using the SircolTm Assay (Biocolor,
Belfast, N.
Ireland). The SircolTm Assay quantified the content of intact collagen
monomers in the
solution, and did not detect degraded collagen (these amounts were 5-10 % of
the actual
= collagen content (data not shown) determined by a hydroxyproline assay
(see methods
= below)). Total non-collagenous protein content of each extract was
determined with the
Tp-BlueTm Total Protein Assay (Biocolor) using Coomassie brilliant blue G.
Total
protein content was obtained by adding this value to the total amount of
collagen
obtained for each sample. The remaining pellets of each sample were digested
with
Proteinase K (Invitrogen), 50 jig in 500 1 solution of 10 mM EDTA and 0.1 M
sodium
phosphate (pH 6.5) (Fisher) overnight at 60 C. A 100 I aliquot of the digest
was used
for determining sulfated glycosaminoglycan and proteoglycan content (that did
not
include hyaluronic acid) with the BlycanTm Assay (Biocolor). A 10 1 aliquot
of the
digest was then used to determine DNA content, and thus cell number (assuming
8 pg of
DNA per cell), with Hoechst 33258 dye (Amersham Biosciences, Piscataway, NJ)
on a
DyNA Quant 200 fluorometer (Amersham Biosciences). 100-200 ttl aliquots of the
Proteinase K digests were used to determine the non-acid and pepsin
extractable collagen
47
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
content (insoluble collagen fraction) with a hydroxyproline assay. The
hydroxyproline
assay was based on the methods of Edwards and O'Brien (1980) and consisted of
hydrolyzing each sample in 6.0 M HC1 for 16 hours at 110 C, followed by drying
of the
samples under vacuum, reconstituting to 2.0 ml with assay buffer (consisting
of 5 g/I
citric acid (Sigma-Aldrich), 1.2 m1/1 glacial acetic acid (EMD Chemicals,
Gibbstown,
NJ), 12 g/1 sodium acetate (VWR, Bridgeport, NJ), and 3.4 g/1 sodium hydroxide
(VWR)), mixing with 1.0 ml of Chloramine-T reagent (made from 62 DIM
chloramine-T
solution (VWR) in 20.7 % ddH20, 26% n-propanol (VWR) and 53.3 % of assay
buffer)
for 20 minutes at room temperature, adding 1.0 ml of freshly prepared
dimethylaminobenzaldehyde reagent (made from 15 g of p-
dimethylaminobenzaldehyde
(Sigma-Aldrich) in 60m1 of n-propanol (VWR) and 26 ml of 60 % perchloric acid
(VWR)) and incubating each sample at 60 C for 15 minutes, cooling each sample
in tap
water for 5 minutes, and measuring the absorbance of each sample at 550 nm
within 45
minutes. Absorbance readings were correlated with collagen amount using a
standard
curve and a conversion factor of 10 jig collagen to 1 pg 4-hydroxyproline. The
standard
curve was created and the conversion factor determined with known amounts of
Trans-4-
hydroxy-L-proline (Sigma-Aldrich) and rat tail type I collagen.
G. Histology and Transmission Electron Microscopy
One sample from each group was prepared for histological evaluation by fixing
in
% zinc formalin (VWR) for 1 hour, followed by washing and storing in 4 C
ethanol
(VWR). The samples were embedded onto paraffin blocks, sectioned into 10 pm
thick
sections, and stained with hematoxylin and eosin (H&E). The stained sections
were
imaged and photographed at 200x with a Nikon Eclipse E600 microscope and Spot
digital camera (Diagnostic Instruments, Sterling Heights, MI). One sample from
each
group (except **CDM and penile dennis) was fixed for transmission electron
microscopy
(tEM) according to the methods of Gibson and Lang (1979). Briefly, the samples
were
fixed in 2 % glutaraldehyde for 1 hour, rinsed 3 times in sodium cacodylate
buffer, fixed
in osmium tetroxide for 10 minutes, and dehydrated in 10 minute ethanol (VWR)
steps
followed by two washes in propylene oxide (all from Electron Microscopy
Sciences,
Hatfield, PA). The samples were then embedded in epon-araldite resin (Electron
48
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Microscopy Sciences). Briefly, the samples were embedded in a 1:1 ratio of
propylene
oxide to Epon Araldite for 2 hours, followed by embedding in 100 % Epon
Araldite for 3
= hours, and then transferring each sample to embedding molds with fresh
100 % Epon
= . Araldite and cured in a 60 C oven overnight. 60-90 nm gold- and silver-
colored sections
== = were stained for 5 minutes with urinyl acetate saturated in 50 %
ethanol, followed by
staining for 20 minutes in lead citrate according to the methods of Venable
and
Coggesmall (1965). The stained sections from each of the samples were then
imaged with
a ZEISS electron microscope for observation of cell, collagen, and ECM
morphology.
The average collagen fibril diameter and density were calculated from the
average
= measured diameters and number of collagen fibrils per square micrometer
from 5
randomly chosen fields taken at high magnification (57,000X or 88,000X). The
collagen
diameters were measured at the thinnest point of cross-sectioned collagen
fibrils with a
. 5x magnified ruler. If a randomly chosen field fell on a grid, then
another field continued
to be randomly chosen until it fell solely on the sample.
=
H. Statistics
Error bars indicate one standard deviation in all figures. Statistical
differences
between groups were determined using ANOVA with Tukey HSD post hoc analysis
(SPSS, Inc., Chicago, IL). Differences were considered significant with p
<0.05. Linear
regression was performed and the R2-values were obtained for determining the
degree of
association between the independent and dependent variables (SPSS, Inc.).
Results and Discussion
Experiments were designed and carried out to determine whether the
extracellular
matrix (ECM) produced and assembled solely by hyperconfluent cells is
mechanically
superior to ECM generated by allowing cells to compact and remodel gels cast
from
purified solutions of collagen or fibrin. To reduce experimental variability,
all groups
were grown in parallel with two million human foreskin fibroblasts from the
same batch,
fed with the same standard serum-supplemented media at the same time, and
grown for
3-weeks next to each other on the same 6-well plates. The collagen gels (CGs)
and fibrin
49
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
gels (FGs) started out at a thickness of 2.2 mm and contracted to a thickness
83 5 pm
and 218 5 gm, respectively. The cell-derived matrices (CDMs), on the other
hand,
started out at slightly more than a single cell layer thick and grew to 125
6 gm, with the
synthesized ECM organizing into several alternating layers that were at
approximate
right-angles to each other, resembling native soft connective tissue (Fig.
17). After 3
weeks in culture, the CDMs were considerably stronger than the CGs and
significantly
stronger than the FGs (see Table 1).
Other measurements of CDM thickness vrizn determined using digital image
analysis, as indicated in Figure 15. Figures 15A-E show Hematoxylin and Eosin
(H&E) stained sections of fibroblast-populated collagen gel (CG), fibrin gel
(FG),
and three cell-derived matrices (CDM, *CDM, and **CDM), respectively. All
samples were grown for 3 weeks starting with the same initial number of
fibroblasts.
Fibroblasts were embedded in 1 mg/ml of collagen in the collagen gel, and in 1
mg/ml fibrin in the fibrin gel. The collagen and fibrin gels started at 1 mm
thickness
= and contracted to 84 gm thick and 230 gm thick, respectively, over the
first few days.
The cell-derived matrices started at 2-3 cell layers thick and grew to 110 gm
(CDM),
465 gm (*CDM), and 240 gm (**CDM) and in thickness, respectively, over the 3-
week culture period. The CDMs were at least 5 times stronger (120 N/m for LTM
versus 25 N/m for CG and FO) than the collagen and fibrin matrices (see Figure
1613)
and contained numerous extracellular matrix proteins in amounts, ratios and
arrangement that resembled young, native, blastema-like tissue. All
micrographs
were taken at 200x magnification. The thickness was measured by digital image
analysis. Scale bars = 100 pm. The values for the CGs and FGs are in line with
results obtained by other researchers and indicate that cell-produced ECM
(i.e. CDM)
can be made stronger than reconstituted ECM within a relatively short period
of time.
An embodiment of the presently defined invention also provides a relatively
simple chemically-defined medium for promoting high ECM synthesis by
multilayered
fibroblasts. Embodiments of the resulting minimal serum-free medium are
similar to a
serum-supplemented medium, except that in place of supplementation with serum,
the
chemically-defined medium is supplemented with basic fibroblast growth factor
(bFGF),
epidermal growth factor (EGF), dexamethasone and L-3,3',5-triiodothyronine,
and a few
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
other components that were determined to be necessary for cell growth that are
present in
serum (insulin, selenious acid, and a lipid precursor). The presence of these
few
components instead of serum resulted in a doubling (**CDMs: growth factors
added into
the stock medium at the start of the 3-week culture period) or tripling
(*CDMs: growth.
factors added fresh at each feeding) in thickness (see Table 1, and Figure
16A), and a
40% (*CDM) or 210% (**CDM) increase in the ultimate tensile strength (ITTS)
(Table 1,
= and Figure 16F) over standard serum-supplemented samples (CDM). The
matrix
produced using either version of the chemically-defined medium also resulted
in a
doubling of the failure strain (Table 1 and Figure 16J), a quadrupling of the
proliferation
rate (Table 1 and Figures 161), and a quintupling of the fraction of non-acid
and pepsin
extractable collagen (Table 1 and Figure 16K) ¨ a parameter that is highly
correlated
(adjusted R2 = 0.977) with the strength of the sample matrices. Interestingly,
when
collagen gels were fed with the chemically-defined medium (*CG), the
contractile forces
within the collagen gels became so great that they detached from the wells and
contracted
into themselves within 24 h of feeding them with the chemically-defined
medium. This
excessive contraction prevented a comparison of the effects of the chemically-
defined
medium and the serum-supplemented medium on the development of gels and is the
subject of further studies.
In addition, the structural characteristics and biochemical composition
between
the five types of matrix were compared, as well as with native penile skin, to
determine
how these differences correlated with their biomechanical properties. These
studies were
done to provide further insight into the mechanisms of cell-mediated
strengthening of
ECM. Human penile skin was to compare native soft connective tissue to the 5
different
living tissue equivalents (LTEs) produced in this study, even though it is
several-fold
weaker than normal skin, since the fibroblasts used in making the LTEs were
derived
from neonatal human foreskin.
Although it is known that fibroblasts entrapped in purified collagen
synthesize far
less ECM proteins than in monolayer cultures, while fibroblasts entrapped in
purified
fibrin retain their ability to produce ECM protein, our data also surprisingly
demonstrate
that fibroblasts in self-produced collagen-rich matrices produce an even
greater amount
of ECM proteins such as collagen than in purified fibrin gels. Moreover,
although the
51
CA 02559275 2012-07-30
cell-derived matrices synthesized a similar amount of collagen as that of the
collagen
gels, the CDM-synthesized collagen did not appear to result in any inhibition
of ECM
synthesis in the cell-derived matrices. Specifically, the total protein
synthesized by the
cells in the cell-derived matrices was significantly more than the net
increase in total
protein in the collagen gels and fibrin gels (see Table 1 and Figure 16E).
These values are higher but in line with the results obtained by other
researchers
for gels and cell-produced matrices (Grinnell et a/.,1989, Exp. Cell Res.
181(2): 483-491;
Huang et aL, 1993, Annals of Biomedical Engineering 21(3): 289-305; Clark et
al., 1997,
Cell Physiol. 170(1): 69-80; Neidert et al., 2002, Development and
characterization of
improved tissue engineered valve-equivalents using chemical and mechanical
signaling.
Second Joint EMBS/BMES Conference, Houston, TX, IEEE). These differences could
be
due to the much greater cell density of our cultures that might have signaled
the
fibroblasts to synthesize more ECM as in the CDM cultures. These differences
might also
be due to the use of porous inserts that provided a shorter diffusion distance
for nutrients
for cells on the basal side of the samples (the use of porous inserts resulted
in a more than
50 % increase in thickness (but no effect on UTS) over samples grown on
regular 6-well
plates (data not shown)), or the 80% greater concentration of L-ascorbate, in
the more
stable form of L-ascorbic acid phosphate magnesium salt n-hydrate (1 week
stability
versus 24 h stability for L-ascorbate).
The cell-derived matrices also had a significantly greater fraction of non-
acid and
pepsin extractable collagen (insoluble collagen fraction) than the collagen
and fibrin gels
(see Table 1 and Figure 16K). The non-acid and pepsin extractable collagen
fraction was
the amount of collagen that was not solubilized by repeated 0.5 M acetic acid
and pepsin
(1 mg/ml) extraction steps over a 3-day period. The non-acid and pepsin
extractable
collagen density provides a measure of very stable collagen in the tissues
that is highly
cross-linked or bundled to resist extraction. The UTS / Collagen Density, a
metric that is
independent of the thickness of the samples and represents the strength of the
constructs
normalized per unit of collagen, was likewise significantly greater for the
cell-derived
matrices than the collagen gels (Table 1 and Figure 16L). The high value of
UTS /
Collagen Density for the fibrin gels (25.9 2.4 kPa/mg/cm3¨ see Table 1)
might be due
to ECM proteins other than collagen, such as fibrin, in the gel. However,
others have
found that the UTS of fibrin gels are correlated to the total collagen amount
in the
52
CA 02559275 2012-07-30
constructs (R2 = 0.77 at day 21), and our study found that the UTS correlated
to the
collagen density (R2 = 0.73 at day 21) not just in fibrin gels but also in
collagen gels and
52a
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
cell-derived matrices. Our results indicate that UTS of the CDMs of the
presently claimed
invention correlate even more closely to the non-acid and pepsin extractable
collagen
density (R2 = 0.993) than to collagen density. Thus, the strength of living
tissue
equivalents as described here, as well as native soft connective tissue, is
not just
correlated with collagen density, but more specifically to a very stable form
of collagen
represented by the non-acid and pepsin extractable collagen density.
The strength of soft connective tissues, as well as living tissue equivalents
such as
fibroblast-populated collagen gels, is generally attributed to the collagen
density and
average collagen fibril diameter. As summarized in Table 1, observation by
transmission
electron microscopy (tEM) revealed that the diameter of the collagen fibrils
in the CDMs,
*CDMs, collagen gels and fibrin gels were 46 5 nm, 48 5 nm, 52 10 tun and
47 5
nm, respectively, while the collagen density was significantly greater for the
CDMs (79 :I:.
2 fibrils/1=2), *CDMs (80 2 fibri1s4rm2) and collagen gels (81 4
fibrils/gm2) than for
the fibrin gels (28 : 4 fibrils/gm2, p <0.01). These diameters fall within
the range of
collagen fibril diameters (40-100 nm) found in native soft connective tissue.
In the present case, although most of the collagen in the collagen gel
consisted of
large, approximately 56 nm diameter fibrils, there was a small presence of
approximately
46 nm diameter fibrils; presumably newly synthesized collagen (see Fig. 17),
indicating
that some of the original collagen in the gel had been degraded since the
total amount of
collagen in the collagen gels remained unchanged over the 3-week period. And
although
the collagen gels disclosed herein had by far the greatest collagen density
and the largest
collagen fibril diameters, they were significantly weaker than the cell-
derived matrices
grown under identical conditions. As can be seen in Figures 17A-17D,
distribution of
collagen fibrils. CDMs, collagen gels, and *CDMs had a more than 2.5 times
greater
density of collagen fibrils at around 80 fibrils/ gm2 than fibrin gels at 28
fibrils/pm2.
Collagen fibrils are shown by arrows, and all micrographs were taken at
12,000x with the
scale bar = 1 gm. The collagen fibrils of the cell-derived matrices (Figures
17C and D)
appeared more parallel and to consist of more parallel alternating layers than
any of the
other groups (see upper portion of CDM where the micrograph consists of cross-
sections
of thousands of parallel collagen fibrils). The collagen fibrils of the
collagen gels (Figures
17A) appeared somewhat parallel but consisted of only a few alternating
layers, while the
53
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
collagen fibrils of the fibiin gels (Figure 17B) appeared only parallel in
small regions of
the ECM.
Without being limited to theory, this discrepancy could be due to a greater
presence of intra-molecular covalent cross-links between the collagen fibrils
or more
extensive bundling of the collagen fibrils into fibers in the cell-derived
matrices than in
the collagen gels and fibrin gels. The non-acid and pepsin extractable
collagen fraction
was closely correlated to the UTS / Collagen Density values for all these
groups (R2 =
= 0.93), except for the fibrin gels (R2 = 0.11). The *CDMs and **CDMs,
which had a
several-fold greater fraction of non-acid and pepsin extractable collagen than
any of the
other samples, also had a higher thermal and enzymatic stability than any of
the other
samples (data not shown). The very stable collagen represented by the non-acid
and
pepsin extractable collagen fraction appears to consist of highly cross-linked
or bundled
collagen that resists extraction and gives rise to a more resistant structure
that leads to
strengthening of the matrix.
Looking at Figure 16, a graphical depiction of the data in Table 1, it can be
seen
in Figures 16B and 16F that all types of cell-derived matrices were stronger
than collagen
gels (CG) and fibrin gels (FO). *CDMs and **CDMs were significantly thicker
(Figure
16A) and contained far more total protein (Figure 16E) than their serum-
supplemented
companions (CDMs, collagen gels and fibrin gels), but had only half the
percentage of
proteoglycans and glycosaminoglycans (Figures 16D and 1611). *CDMs and **CDMs
also contained a significantly higher fraction of non-acid and pepsin
extractable collagen
(Figure 16K), and were stronger per microgram of collagen (UTS/Collagen
Density) than
collagen gels or fibrin gels (Figure 16L). CDMs were more than twice as strong
as
collagen gels per microgram of collagen, indicating the possibility that the
collagen in the
CDMs was more organized for strength than in collagen gels. In addition to
collagen, a
substantial proportion of the strength of *CDMs and fibrin gels was also due
to other
ECM proteins. *CDMs and **CDMs were significantly more extensible than CDMs,
collagen gels and fibrin gels. Human penile skin (HPS) most closely resembled
**CDM
mechanically, and *CDM and **CDM biochemically.
The lowered growth factor concentration over time that occurs naturally in
vivo
during the wound healing response, where the high concentration of growth
factors in a
54
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
fresh clot decreases over time as it is invaded and reconstructed by
fibroblasts, was
. approximated by adding the growth factors (EGF and bFGF) directly into the
feeding
media at the start of the 3-week culture period and taking advantage of the
natural loss of
growth factor activity in media over time. This one-time addition of growth
factors at the
start of the 3-week period (**CDM) resulted in a matrix with about Y2 the
thickness of
parallel samples (*CDM) for which the growth factors were added fresh at every
feeding
(see Table 1 and Figure 16A). The thickness was highly correlated with total
protein
content (Race= 0.9), which in turn correlated with total cell number (Radj2=
0.874.
Interestingly, the **CDMs had significantly more total collagen than *CDMs,
although
the *CDMs had almost twice as much total protein as the **CDMs (Table 1 and
Figure
16C). Since collagen is the major extracellular matrix protein providing
strength, the
. **CDMs, with a more than two-fold greater fraction of collagen than the
*CDMs, were
also almost twice as strong as the *CDMs (Table 1 and Figure 16F).
Cell-derived matrices grown for longer periods (up to 6 weeks) further
continued
to increase in thickness and strength (data not shown). It is possible that
the **CDMs
developed mostly in thickness for the first part of the growth period followed
by
development in strength for the remainder of the growth period, since adding
lower
. concentrations of fresh growth factors at every feeding instead resulted
in matrices that
were significantly stronger than the *CDMs but significantly thinner than the
**CDMs
(data not shown). This effect appears to be due to the fact that EGF
stimulates the
synthesis of non-collagenous proteins but inhibits the transcription of type 1
collagen
genes, while high bFGF concentrations favor increased cell proliferation over
enhancing
the synthesis and strengthening of the ECM. Thus a decrease in total growth
factor
activity over time results in less synthesis of non-collagenous proteins, but
an increase in
the strength of the synthesized ECM by the resulting increase in collagen
synthesis. Many
growth factors, including bFGF, can also accumulate and retain their activity
for
relatively long periods of time within ECM. Thus some of the added growth
factors
might have accumulated in the ECM during the first part of the culture period
and then
used up continuously as the matrix developed. The failure strains for the
*CDMs and
**CDMs were also significantly greater than for their serum-supplemented
counterparts
(Table 1 and Figure 16J), closer mimicking native soft connective tissue.
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
These results demonstrate that living tissue equivalents are stronger and
thicker =
when they are cell-produced ¨ in other words, when the cells are allowed to
grow and
develop their own mechanical environment rather than being supplied with a
scaffold,
= such as gels or other biopolymer materials. This strength appears to be
due to a greater
fraction of very stable collagen and the significantly greater synthetic rate
of fibroblasts
in ALSs/LTMs than in gels. The LTMs and their cell-derived matrix component
(*CDM
and **CDM) produced and described herein have potential for use as soft
connective
tissue substitutes since they are produced solely from cells fed with a
chemically-defined
medium that does not contain animal components. The rapid growth and lack of
= expensive serum makes the development of LTMs as soft connective tissue
substitutes
commercially more viable, and opens the door to mainstream acceptance and
appeal.
Due to the relative simplicity of the chemically-defmed medium, these cell-
produced living tissue equivalents may also serve as in vitro biological
models for the
= effects of nutritional components and pharmaceutical products on the
growth and
== development of soft connective tissue, for examining the numerous in
vivo conditions and
= processes such as wound healing and connective tissue formation, and for
investigating
. the development and interaction of different cells and tissues in a soft
connective tissue
environment that is developed solely from cells in vitro. The higher thermal
and
= enzymatic stability and mechanical integrity of cell-derived matrices
over both collagen
and fibrin gels may also allow them to retain their structural integrity
longer in in vivo
conditions. The ability to grow LTMs thick and strong in a relatively short
period of time
in chemically-defined conditions provides a commercially viable option for
wound repair
and tissue regeneration as an attractive alternative to the use of fibrin
gels, collagen gels
and even native tissues.
56
CA 02559275 2006-09-08
WO 2006/048783
PCT/IB2005/004091
Table 1. Results from mechanical and biochemical analysis of human penile skin
and
cell-derived Matrices (CDMs, *CDMs, **CDMs), fibroblast-populated collagen
gels and
fibrin gels containing human foreskin fibroblasts. Numbers indicate mean +1-
SD.
Numbers in parentheses indicate approximate initial amount. Collagen fibril
diameters
and densities were measured in proximity to cell surfaces.
, I ,
_______ ,
1
Measure Collagen gel Fibrin gel CDM *CDM .
"CDM ' Penile Skin
Ultimate Tensile Strength 168.5 *43.1 133.2 * 10.6 223.2 *9
314.5 * 7.2 697.1 *36.1 713.0* 55.2 '
&Pa)
.
Thickness (pm) 83*5 218 * 5 125 * 6 395 6 225 7
651 *30
(2,220 pm) (2,220 pm) (-30 pm) (-30 pm) (-30 pm)
Failure Strain 0.13 *0.02 0.21 0.03 0.18 t 0.03
0.33 *0.03 0.31 * 0.06 0.88 0.28
Total Protein (mg) 1.08 0.03 1.58 0.04 1.25 0.06
4.40 0.08 2.65 0.07 6.74* 0.44
(1.0 mg) (1.0 mg)
Total Collagen (mg) 0.99 *0.02 0.51 *0.03 0.85 *0.03
1.40 0.04 1.78 0.06 3.98 *0.37
(1.0 mg)
Non-Acid and Pepsin 1.5 0.1 '1.3 0.1 2.3 0.2 12.8
0.5 13.1 *0.3 15.7 * 0.7 '
Extractable Collagen
Fraction (%) .
Collagen Density (mg/cm) 26.5 1.5 5.2 * 0.2 15.1 *0.9 7.8
* 0.2 17.4 *0.1 13.5 * 0.7
UTS / Collagen Density 6.4 1.9 25.9 2.4 14.5 * 1.1
40.3 0.4 ' 40.0 *1.9 52.9 * 3.1
(kPa/mg/cre)
. Collagen Fibril Diameter 52 *10 . 47* 5 46 * 5 48* 5 -
-
(nm) _
Collagen Fibril Density 81 *4 28 4 79 * 2 80 * 2 -
-
(11br11s/pm2)
Total Proteoglycans &
Glycosaminoglycans (pg)
38.4 *0.7 45.4 0.9 31.3 t 0.5 64.3 0.6 38.4* 0.4
83.0 *5.7
Wet Weight / Dry Weight 16.4 *0.6 20.1 0.4 13.0 *0.5
19.5 02 20.3 0.6 21.7 * 0.4
.
.
Cell Number (million) 2.8 * 0.1 4.0*0.1 2.8 0.1
6.1 *0.1 3.6 0.1 9.8 0.4
(2.0 million) (2.0 million) (2.0 million) (2.0 million) _
(2.0 million)
_
57