Note: Descriptions are shown in the official language in which they were submitted.
CA 02559699 2011-09-15
25771-1241(S)
1
PHARMACEUTICAL COMBINATIONS CONTAINING BENZOXAZINE FOR
TREATING RESPIRATORY DISEASES
The present invention relates to new medicament combinations which contain in
addition to one or more, preferably one, compound of general formula 1
OH 2
R R3
MAee
HN H õ R
O~O OH
wherein the groups R1, R2 and R3 may have the meanings given herein, at least
one
other active substance 2, processes for preparing them and their use as
pharmaceutical compositions.
Brief description of the drawings
Figure 1 shows a particularly preferred inhaler for using the pharmaceutical
combination according to the invention in inhalettes.
Detailed Description of the invention
The present invention relates to medicament combinations which contain in
addition
to one or more, preferably one, compound of general formula 1
OH 2
R R3
Me e
HN N n R'
O1 0 OH
wherein
n denotes 1 or 2, preferably 1;
CA 02559699 2011-09-15
25771-1241(S)
1a
R1 denotes hydrogen, halogen, Ci-C4-alkyl or -O-C1-C4-alkyl;
R2 denotes hydrogen, halogen, C1-C4-alkyl or -O-Cl-C4-alkyl;
R3 denotes CI-C4-alkyl, OH, halogen, -O-Ci-C4-alkyl, -O-Ci-C4-alkylene-COON,
-O-C1-C4-alkylene-CO-O-Cl-C4-alkyl,
at least one other active substance 2.
CA 02559699 2006-09-12
WO 2005/102349 2 PCT/EP2005/004073
Preferably the present invention relates to medicament combinations, which
contain, in
addition to one or more, preferably one, compound of formula 1 as a further
active
substance 2 one or more compounds selected from the categories of the
anticholinergics
(2a), PDE IV inhibitors (2b), steroids (Le), LTD4-antagonists (2d) and EGFR-
inhibitors
2e).
Preferred are medicament combinations which contain in addition to one or
more,
preferably one, compound of general formula 1, wherein
n denotes 1 or 2, preferably 1;
R' denotes hydrogen, fluorine, chlorine, methyl or methoxy;
R2 denotes hydrogen, fluorine, chlorine, methyl or methoxy;
R3 denotes C1-C4-alkyl, OH, fluorine, chlorine, bromine, -O-C1-C4-alkyl,
-0-C1-C4-alkylene-000H, -0-C1-C4-alkylene-CO-O-C1-C4-alkyl,
at least one other active substance 2.
Also preferred are medicament combinations which contain in addition to one or
more,
preferably one, compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen or C1-C4-alkyl;
R2 denotes hydrogen or C1-C4-alkyl;
R3 denotes C1-C4-alkyl, OH, -0-C1-C4-alkyl, -0-C1-C4-alkylene-COOH or
-0-C1-C4- alkylene-CO-O-C1-C4-alkyl,
at least one other active substance 2.
Also preferred are medicament combinations which contain in addition to one or
more,
preferably one, compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen, methyl or ethyl;
R2 denotes hydrogen, methyl or ethyl;
R3 denotes methyl, ethyl, OH, methoxy, ethoxy, -O-CH2-000H,
-O-CH2-COOmethyl or -0-CH2-COOethyl,
at least one other active substance 2.
CA 02559699 2006-09-12
WO 2005/102349 3 PCT/EP2005/004073
Also preferred are medicament combinations which contain in addition to one or
more,
preferably one, compound of general formula 1, wherein
n denotes 1;
R1 denotes hydrogen or methyl;
R2 denotes hydrogen or methyl;
R3 denotes methyl, OH, methoxy, -O-CH2-COOH or -O-CH2-COOethyl,
at least one other active substance 2.
Preferred medicament combinations according to the invention are those which
contain in
addition to one or more, preferably one, compound of general formula 1,
wherein
R3 denotes methoxy, ethoxy, -O-CH2-COOH, -O-CH2-COOmethyl or
-O-CH2-COOethyl,
and R', R2 and n may have the meanings given above, at least one other active
substance 2.
The present invention also relates to medicament combinations which contain in
addition
to one or more, preferably one, compound of general formula 1, wherein
n denotes 1;
R1 denotes halogen, C1-C4-alkyl or -O-Cl-C4-alkyl;
R2 denotes halogen, C1-C4-alkyl or -O-C1-C4-alkyl;
R3 denotes halogen, C1-C4-alkyl or -O-C1-C4-alkyl, at least one other active
substance 2.
The present invention also relates to medicament combinations which contain in
addition
to one or more, preferably one, compound of general formula 1, wherein
n denotes 1;
R1 denotes fluorine, chlorine, methyl or methoxy;
R2 denotes fluorine, chlorine, methyl or methoxy ;
R3 denotes fluorine, chlorine, methyl or methoxy
at least one other active substance 2.
In another preferred aspect the present invention relates to medicament
combinations
which contain in addition to one or more, preferably one, compound of general
formula 1,
wherein
n denotes 1;
R' denotes hydrogen;
CA 02559699 2006-09-12
WO 2005/102349 4 PCT/EP2005/004073
R2 denotes hydrogen, fluorine, chlorine or methyl;
R3 denotes methyl, ethyl, iso-propyl, tert.-butyl, OH, fluorine, chlorine,
bromine,
methoxy, ethoxy, -O-CH2-000H, -O-CH2-CH2-000H, -O-CH2-CH2-CH2-
COOH, O-CH2-COOmethyl, -O-CH2-COOethyl, -O-CH2-CH2-COOmethyl, -0-
CH2-CH2-COOethyl, -O-CI12-CI12-C12-COOmethyl, -0-CH2-CH2-CH2-COOethyl,
at least one other active substance 2.
Particularly preferred are medicament combinations which contain in addition
to one or
more, preferably one, compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen;
R2 denotes hydrogen, fluorine, chlorine or methyl;
R3 denotes OH, fluorine, chlorine, methyl, methoxy, ethoxy or -O-CH2-COOH, at
least one other active substance 2.
Also preferred are those medicament combinations which contain in addition to
one or
more, preferably one compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen;
R2 denotes halogen, Ci-C4-alkyl or -O-C1-C4-alkyl,
preferably fluorine, chlorine, methoxy or methyl;
R3 denotes halogen, Ci-C4-alkyl or -O-Ci-C4-alkyl,
preferably fluorine, chlorine, methoxy or methyl,
at least one other active substance 2.
In another preferred aspect the present invention relates to medicament
combinations
which contain in addition to one or more, preferably one, compound of general
formula 1,
wherein n = 1 , R' and R2 denote hydrogen and the group R3 may have the
meanings given
above, at least one other active substance 2.
In another preferred aspect the present invention relates to medicament
combinations
which contain in addition to one or more, preferably one, compound of general
formula 1,
wherein
n denotes 1;
R1 and R2 denote hydrogen;
CA 02559699 2006-09-12
WO 2005/102349 5 PCT/EP2005/004073
R3 denotes methyl, ethyl, iso-propyl, tert.-butyl, OH, fluorine, chlorine,
bromine,
methoxy, ethoxy, -O-CH2-000H, -O-CH2-CH2-000H, -O-CH2-CH2-CH2-
COOH, -O-CH2-COOmethyl, -O-CH2-COOethyl, -O-CH2-CH2-COOmethyl,
-O-CH2-CH2-COOethyl, -O-CH2-CH2-CH2-COOmethyl, -O-CH2-CH2-CH2-
COOethyl,
at least one other active substance 2.
Particularly preferred are medicament combinations which contain in addition
to one or
more, preferably one, compound of general formula 1, wherein
n denotes 1;
R' and R2 denotes hydrogen;
R3 denotes OH, fluorine, chlorine, methoxy, ethoxy,-O-CH2-COOH, preferably
OH, fluorine, chlorine, ethoxy or methoxy,
at least one other active substance 2.
Particularly preferred are medicament combinations which contain in addition
to one or
more, preferably one, compound of general formula 1, wherein
n denotes 1;
R' and R2 denotes hydrogen;
R3 denotes fluorine, chlorine, methoxy or ethoxy,
at least one other active substance 2.
The present invention also relates to medicament combinations which contain in
addition
to one or more, preferably one, compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen, halogen, Ci-C4-alkyl or -O-C1-C4-alkyl;
R2 denotes hydrogen, halogen, Ci-C4-alkyl or -O-C1-C4-alkyl;
R3 denotes hydrogen,
at least one other active substance 2.
Preferred are medicament combinations which contain in addition to one or
more,
preferably one, compound of general formula 1, wherein
n denotes 1;
R' denotes hydrogen, fluorine, chlorine, methyl or methoxy;
CA 02559699 2006-09-12
WO 2005/102349 6 PCT/EP2005/004073
R2 denotes hydrogen, fluorine, chlorine, methyl or methoxy;
R3 denotes hydrogen,
at least one other active substance 2.
The present invention also relates to medicament combinations which contain in
addition
to one or more, preferably one, compound of general formula 1, wherein
n denotes 1;
R1 denotes fluorine, chlorine, methyl or methoxy;
R2 denotes fluorine, chlorine, methyl or methoxy;
R3 denotes hydrogen,
at least one other active substance 2.
In the compounds of formula 1 the groups R' and R2, if they do not represent
hydrogen,
may each be in the ortho or meta position relative to the bond to the benzylic
"-CH2"-
group. If neither of the groups R1 and R2 denotes hydrogen, preferred
compounds of
formula 1 for the medicament combinations according to the invention are those
wherein
either the two groups R1 and R2 are in the ortho configuration or both groups
R1 and R2 are
in the meta configuration, while compounds in which both groups R1 and R2 are
in the
ortho configuration are of particular importance.
In the compounds of formula 1 wherein one of the groups R' and R2 does not
denote
hydrogen, it may be in the ortho or meta position relative to the bond to the
benzylic
"-CH2" group. In this case particularly preferred compounds of formula 1 for
the
medicament combinations according to the invention are those wherein the group
R' or
R2 which does not denote hydrogen is in the ortho configuration.
Also particularly preferred are medicament combinations which contain in
addition to one
or more, preferably one, compound of general formula 1 selected from the
compounds
} -
- 6-hydroxy-8- { 1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethyl amino] -
ethyl
4H-benzo[1,4]oxazin-3-one (L.1);
- 6-hydroxy-8- { 1-hydroxy-2-[2-( 4-phenoxyethyl -acetate)- 1,1 -dimethyl-
ethylamino]-
ethyl } -4H-benzo [1 ,4]oxazin-3 -one (1.);
- 6-hydroxy-8- 11-hydroxy-2-[2-(4-phenoxy-acetic acid)-1,1-dimethyl-ethyl
amino] -
ethyl }-4H-benzo[1,4]oxazin-3-one (L.3);
- 8-{2-[1,1-dimethyl-2-(2,4,6-trimethylphenyl)-ethyl amino] -1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1_4);
CA 02559699 2006-09-12
WO 2005/102349 7 PCT/EP2005/004073
}-
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-hydroxy-phenyl)-1,1-dimethyl-ethylamino] -
ethyl
4H-benzo[1,4]oxazin-3-one 1.5 ;
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-isopropyl-phenyl)-1,1dimethyl-ethylamino] -
ethyl}-
4H-benzo[1,4]oxazin-3-one (1.6);
- 8-{2-[2-(4-ethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-hydroxy-
4H-
benzo[1,4]oxazin-3-one (L.7);
- 8-{2-[2-(4-fluoro-3-methyl -phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-
ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L.8);
- 8- {2-[2-(4-fluoro-2-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl
} -6-
hydroxy-4H-benzo [ 1,4]oxazin-3 -one 1.9);
- 8-{2-[2-(2,4-difluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl) -6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L.10);
- 8-{2-[2-(3,5-difluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one 1.11 ;
- 8-{2-[2-(4-ethoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl }-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one (1.12);
- 8-{2-[2-(3,5-dimethyl-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1.13);
- 4-(4-{2-[2-hydroxy-2-(6-hydroxy-3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-8-yl)-
ethylamino]-2-methyl-propyl}-phenoxy)-butyric acid (1.14);
- 8-{2-[2-(3,4-difluoro-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one 1.15 ;
- 8-{2-[2-(2-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1.16);
- 8-{2-[2-(4-chloro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one (L.17);
- 8-{2-[2-(4-bromo-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl }-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one (L.18);
- 8-{2-[2-(4-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one (1.19);
- 8-{2-[2-(4-fluoro-3-methoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L20);
- 8-{2-[2-(4-fluoro-2,6-dimethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-
6-hydroxy-4H-benzo[1,4]oxazin-3-one (L21);
CA 02559699 2006-09-12
WO 2005/102349 8 PCT/EP2005/004073
- 8-{2-[2-(4-chloro-2-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1.22);
- 8-{2-[2-(4-chloro-3-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[I,4]oxazin-3-one (L23);
- 8-{2-[2-(4-chloro-2-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1.24);
- 8-{2-[2-(3-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L.25);
- 8-{2-[2-(2,6-difluoro-4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl) -6-hydroxy-4H-benzo[1,4]oxazin-3-one (1.26);
- 8- {2-[2-(2,5-difluoro-4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-6-hydroxy-4H-benzo[1,4]oxazin-3-one 1.27 ;
- 8-{2-[2-(4-fluoro-3,5-dimethyl-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-
ethyl}-
6-hydroxy-4H-benzo[1,4]oxazin-3-one 1.28 ;
- 8-{2-[2-(3,5-dichloro-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L29);
- 8-{2-[2-(4-chloro-3-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one (L30);
- 8-{2-[2-(3,4,5-trifluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[ 1,4]oxazin-3-one (1.31);
- 8- {2-[2-(3-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one 1.32 and
- 8- {2-[2-(3,4-dichloro-phenyl)-1,1-dimethyl-ethylamino] -1-hydroxy-ethyl } -
6-
hydroxy-4H-benzo[ 1,4] oxazin-3 -one (1.33)
at least one other active substance 2.
Particularly preferred are, especially, those medicament combinations which
contain in
addition to one or more, preferably one, compound of general formula 1
selected from the
compounds
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-
ethyl
}-
4H-benzo[1,4]oxazin-3-one (1.1);
- 6-hydroxy-8- {I -hydroxy-2 - [2-(4-phenoxyethyl -acetate)- 1, 1 -dimethyl -
ethyl amino] -
ethyl I -4H-benzo[ 1,4] oxazin-3 -one (1.2);
- 6-hydroxy-8- {I-hydroxy-2-[2-(4-phenoxy-acetic acid)- 1, 1 -dimethyl-
ethylamino]-
ethyl }-4H-benzo[1,4]oxazin-3-one (1_3);
CA 02559699 2012-01-20
25771-1241(S)
9
- 8-{2-[1,1-dimethyl-2-(2,4,6-trimethylphenyl)-ethylamino]-1-hydroxy-
ethyl}-6-hydroxy-4H-benzo[1,4]oxazin-3-one (1.4);
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-hydroxy-phenyl)-1,1-dimethyl-
ethylamino]-ethyl}-4H-benzo[1,4]oxazin-3-one (1.5);
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-isopropyl-phenyl)-1,1dimethyl-
ethylamino]-ethyl}-4H-benzo[1,4]oxazin-3-one (1_6);
- 8-{2-[2-(4-ethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1_7);
- 8-{2-[2-(4-ethoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo[1,4]oxazin-3-one (1.12);
- 4-(4-{2-[2-hydroxy-2-(6-hydroxy-3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazin-8-yl)-ethylamino]-2-methyl-propyl}-phenoxy)-butyric acid
(1.14) and
- 8-{2-[2-(3,4-difluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-6-hydroxy-4H-benzo[1,4]oxazin-3-one (1.15),
at least one other active substance 2.
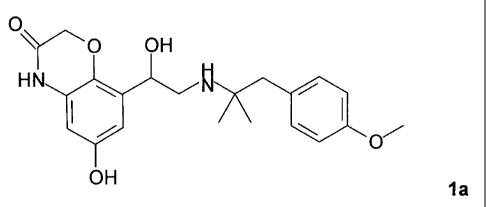
In a specific embodiment, there is provided a medicament combination which
contains in addition to a compound of formula 1a,
0
Y-~ O OH
HN
OH 1a
CA 02559699 2012-01-20
25771-1241(S)
9a
at least one other active substance 2 selected from the categories of
anticholinergics
(2a) and steroids (2c).
In an embodiment, 1a is in the form of the R-enantiomer. 1a may also be in the
form
of its hydrochloride acid addition salt.
In the medicament combinations according to the invention the compounds of
formula 1 may be present in the form of the individual optical isomers,
mixtures of the
individual enantiomers or racemates. Particularly preferred are those
medicament
combinations wherein one or more, preferably one compound of formula 1 is in
the
form of the enantiomerically pure compounds, preferably in the form of the
R-enantiomers. Methods of separating racemates into the various enantiomers
are
known in the art and may be used to prepare the enantiomerically pure R- or
S-enantiomers of the compounds of formula 1 analogously.
In another aspect the present invention relates to medicament combinations
which
contain the above-mentioned compounds of formula 1 in the form of the acid
addition
salts with pharmacologically acceptable acids as well as optionally in the
form of the
solvates and/or hydrates.
By acid addition salts with pharmacologically acceptable acids are meant for
example
salts selected from the group comprising the hydrochloride, hydrobromide,
hydroiodide, hydrosulphate, hydrophosphate, hyd rometha nesu I phonate,
hydronitrate,
hydromaleate, hydroacetate, hydrobenzoate, hydrocitrate, hydrofumarate,
hydrotartrate, hydrooxalate, hydrosuccinate, hydrobenzoate and hydro-p-
toluenesulphonate, preferably the
CA 02559699 2006-09-12
= WO 2005/102349 10 PCT/EP2005/004073
hydrochloride, hydrobromide, hydrosulphate, hydrophosphate, hydrofumarate and
hydromethanesulphonate. Of the above-mentioned acid addition salts, the salts
of
hydrochloric acid, methanesulphonic acid, benzoic acid and acetic acid are
particularly
preferred according to the invention.
Preferred medicament combinations contain in addition to one or more,
preferably one
compound of formula 1 as an additional active substance one or more,
preferably one
anticholinergic 2a, optionally in combination with pharmaceutically acceptable
excipients.
In the medicament combinations according to the invention the anticholinergic
2a is
preferably selected from among the tiotropium salts 2a.1 , oxitropium salts
2a.2 ,
flutropium salts 2a.3 , ipratropium salts (2a.4), glycopyrronium salts (2a.5),
trospium salts
2a.6 and the compounds of formulae 2a.7 to 2a.13.
In the above-mentioned salts 2a.1 to 2a.6 the cations tiotropium, oxitropium,
flutropium,
ipratropium, glycopyrronium and trospium are the pharmacologically active
constituents.
Explicit references to the above-mentioned cations are indicated by the
numerals 2a.1' to
2a.6'. Each reference to the above-mentioned salts 2a.1 to 2a.6 naturally
includes a
reference to the corresponding cations tiotropium 2a.1' , oxitropium 2a.2' ,
flutropium
2a.3' , ipratropium 2a.4' , glycopyrronium 2a.5' and trospium (2a.61).
By the salts 2a.1 to 2a.6 are meant according to the invention those compounds
which
contain in addition to the cations tiotropium 2a.1' , oxitropium (2a.21),
flutropium (2a.31),
ipratropium 2a.4' , glycopyrronium 2a.5' and trospium 2a.6' as counter-ion
(anion)
chloride, bromide, iodide, sulphate, phosphate, methanesulphonate, nitrate,
maleate,
acetate, citrate, fumarate, tartrate, oxalate, succinate, benzoate or p-
toluenesulphonate
contain, while the chloride, bromide, iodide, sulphate, methanesulphonate or p-
toluenesulphonate are preferred as counter-ions. Of all the salts the
chloride, bromide,
iodide and methanesulphonate are particularly preferred.
In the case of the trospium salts 2a.6) the chloride is particularly
preferred. Of the other
salts 2a.1 to 2a.5 the methanesulphonates and bromides are of particular
importance.
Of particular importance are medicament combinations which contain tiotropium
salts
2a.1 , oxitropium salts 2a.2 or ipratropium salts 2a.4 , while the respective
bromides
are particularly important according to the invention. Of particular
importance is the
tiotropium bromide 2a.1). The above-mentioned salts may optionally be present
in the
CA 02559699 2006-09-12
WO 2005/102349 11 PCT/EP2005/004073
medicament combinations according to the invention in the form of their
solvates or
hydrates, preferably in the form of their hydrates. In the case of tiotropium
bromide the
medicament combinations according to the invention preferably contain this in
the form of
the crystalline tiotropium bromide monohydrate, which is known from WO
02/30928. If
the tiotropium bromide is used in anhydrous form in the medicament
combinations
according to the invention, it is preferable to use the anhydrous crystalline
tiotropium
bromide which is known from WO 03/000265.
Examples of preferred medicament combinations according to the invention of
preferred
compounds of formula 1 with the above-mentioned anticholinergics 2a.1 to 2a.6
are
combinations containing the compounds 1.1 and 2a.1; 1.1 and 2a.2; 1.1 and
2a.3; 1.1 and
2a.4; 1.1 and 2a.5; 1_1 and 2a.6; 1.2 and 2a.1; 1.2 and 2a.2; 1_2 and 2a.3;
1.2 and 2a.4; 1_2
and 2a.5; 1.2 and 2a.6; 1.3 and 2a.1; 1.3 and 2a.2; 1.3 and 2a.3; 1.3 and
2a.4; 1.3 and 2a.5;
1.3 and 2a.6; 1.4 and 2a.1; 1.4 and 2a.2; 1.4 and 2a.3; 1.4 and 2a.4; 1.4 and
2a.5; 1.4 and
2a.6; 1.5 and 2a.1; 1.5 and 2a.2; 1.5 and 2a.3; 1.5 and 2a.4; 1_5 and 2a.5;
1.5 and 2a.6; 1.6
and 2a.1; 1.6 and 2a.2; 1_6 and 2a.3; 1.6 and 2a.4; 1.6 and 2a.5; 1.6 and
2a.6; 1.7 and 2a.1;
1.7 and 2a.2; 1.7 and 2a.3; 1_7 and 2a.4; 1.7 and 2a.5; 1.7 and 2a.6; 1.12 and
2a.1; 1.12
and 2a.2; 1.12 and 2a.3; 1.12 and 2a.4; 1.12 and 2a.5; 1.12 and 2a.6; 1.14 and
2a.1; 1.14
and 2a.2; 1.14 and 2a.3; 1.14 and 2a.4; 1.14 and 2a.5; 1.14 and 2a.6; 1.15 and
2a.1; 1.15
and 2a.2; 1.15 and 2a.3; 1.15 and 2a.4; 1.15 and 2a.5 or 1.15 and 2a.6, in
each case
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_l, 1.5, 1_6, or
1.12, while
those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2a one of the
compounds
2a.1, 2a.2 or 2a.4, while those combinations which contain the compound 2a.1
are
particularly important according to the invention.
The above-mentioned anticholinergics optionally have chiral carbon centres. In
this case
the medicament combinations according to the invention may contain the
anticholinergics
CA 02559699 2006-09-12
WO 2005/102349 12 PCT/EP2005/004073
in the form of their enantiomers, mixtures of enantiomers or racemates, while
enantiomerically pure anticholinergics are preferably used .
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the salts of
formula 2a.7
0-0P O
O
X HO /
S
S
2a.7
wherein
X - denotes an anion with a single negative charge, preferably an anion
selected
from among the fluoride, chloride, bromide, iodide, sulphate, phosphate,
methanesulphonate, nitrate, maleate, acetate, citrate, fumarate, tartrate,
oxalate, succinate, benzoate and p-toluenesulphonate,
optionally in the form of the racemates, enantiomers or hydrates thereof.
Preferred medicament combinations contain salts of formula 2a.7, wherein
X - denotes an anion with a single negative charge, preferably an anion
selected
from among the fluoride, chloride, bromide, methanesulphonate and p-
toluenesulphonate, preferably bromide,
optionally in the form of the racemates, enantiomers or hydrates thereof.
Preferred medicament combinations contain salts of formula 2a.7, wherein
X - denotes an anion with a single negative charge, preferably an anion
selected
from among the chloride, bromide and methanesulphonate, preferably
bromide,
optionally in the form of the racemates, enantiomers or hydrates thereof.
Particularly preferred medicament combinations contain the compound of formula
2a.7 in
the form of the bromide.
CA 02559699 2006-09-12
WO 2005/102349 13 PCT/EP2005/004073
Of particular importance are those medicament combinations which contain the
enantiomers of formula 2a.7-en
N
O O
O
X HO /
S
S
2a.7-en
wherein X - may have the above-mentioned meanings.
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.7 are combinations containing the
compounds 1.1
and 2a.7; 1.1 and 2a.7-en; 1.2 and 2a.7; 1.2 and 2a.7-en; 1.3 and 2a.7; 1.3
and 2a.7-en; 1.4
and 2a.7; 1.4 and 2a.7-en; 1.5 and 2a.7; 1.5 and 2a.7-en; 1.6 and 2a.7; 1.6
and 2a.7-en; 1.7
and 2a.7; 1.7 and 2a.7-en; 1.12 and 2a.7; 1.12 and 2a.7-en; 1.14 and 2a.7;
1.14 and 2a.7-
en; 1.15 and 2a.7; 1.15 and 2a.7-en, in each case optionally in the form of
the racemates,
enantiomers or diastereomers thereof and optionally in the form of the
pharmacologically
acceptable acid addition salts, solvates and/or hydrates thereof.
Of the above-mentioned combinations preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1.1, 1_5, 1_6, or
1.12,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain the compound 2a.7-en as
compound 2a.
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the salts of
formula 2a.8
CA 02559699 2006-09-12
WO 2005/102349 14 PCT/EP2005/004073
OH Me
LUNMe -
R X
Me/\Me
Me 2a.8
wherein R denotes either methyl 2a.8.1 or ethyl 2(a.8.2) and wherein X - may
have the
above-mentioned meanings. In an alternative embodiment the compound of formula
2a.8
is present in the form of the free base 2a.8-base
OH / Me
NMe
Me Me
Me 2a.8-base
The medicament combinations according to the invention may containthe
anticholinergic
of formula 2a.8 (or 2a.8-base in the form of the enantiomers, mixtures of
enantiomers or
racemates thereof . Preferably the anticholinergics of formula 2a.8 (or 2a.8-
base) are
present in the form of their R-enantiomers.
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.8 are combinations containing the
compounds 1.1
and 2a.8.1; 1.1 and 2a.8.2; 1.2 and 2a.8.1; 1.2 and 2a.8.2; 1.3 and 2a.8.1;
1.3 and 2a.8.2;
1.4 and 2a.8.1; 1.4 and 2a.8.2; 1.5 and 2a.8.1; 1.5 and 2a.8.2; 1.6 and
2a.8.1; 1.6 and
2a.8.2; 1.7 and 2a.8.1; 1.7 and 2a.8.2; 1.12 and 2a.8.1; 1.12 and 2a.8.2; 1.14
and 2a.8.1;
1.14 and 2a.8.2; 1.15 and 2a.8.1; 1.15 and 2a.8.2, in each case optionally in
the form of the
racemates, enantiomers or diastereomers thereof and optionally in the form of
the
pharmacologically acceptable acid addition salts, solvates and/or hydrates
thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1.1, 1_5, 1_6, or
1.12 ,
CA 02559699 2006-09-12
WO 2005/102349 15 PCT/EP2005/004073
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention.
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the
compounds of formula 2a.9
R2___+,R~
N X
H
A O O
R5 R4
R6 / R7 ~ s
R 2a.9
wherein
A denotes a double-bonded group selected from the groups
C-C C=C and
H2 H2 H H H O H
X - denotes one of the above-mentioned anions with a single negative charge,
preferably chloride, bromide or methanesulphonate,
Rl and R2 which may be identical or different denote a group selected from
methyl,
ethyl, n-propyl and iso-propyl, which may optionally be substituted by
hydroxy or fluorine, preferably unsubstituted methyl;
R3, R4, R5 and R6, which may be identical or different denote hydrogen,
methyl, ethyl,
methyloxy, ethyloxy, hydroxy, fluorine, chlorine, bromine, CN, CF3 or
NO2;
R7 denotes hydrogen, methyl, ethyl, methyloxy, ethyloxy, -CH2-F,
-CH2-CH2-F, -O-CH2-F, -O-CH2-CH2-F, -CH2-OH, -CH2-CH2-OH, CF3,
-CH2-OMe, -CH2-CH2-OMe, -CH2-OEt, -CH2-CH2-OEt, -0-COMe,
-0-COEt, -O-COCF3, -O-COCF3, fluorine, chlorine or bromine.
The compounds of formula 2a.9 are known in the art (WO 02/32899).
CA 02559699 2006-09-12
WO 2005/102349 16 PCT/EP2005/004073
Within the scope of the medicament combinations according to the invention
preferred
compounds of formula 2a.9 are those wherein
X - denotes bromide;
R' and R2 which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R3, R4, R5 and R6, which may be identical or different, denote hydrogen,
methyl,
methyloxy, chlorine or fluorine;
R7 denotes hydrogen, methyl or fluorine.
Of particular importance are medicament combinations which contain compounds
of
formula 2a.9, wherein
A denotes a double-bonded group selected from
\ / \\ /
H H and
H 0 H
Of particular importance are those medicament combinations which contain in
addition to
a compound of formula 1 one of the following compounds of formula 2a.9 :
- tropenol 2,2-diphenylpropionate methobromide 2a.9.1 ,
- scopine 2,2-diphenylpropionate methobromide (2a.9.2),
- scopine 2-fluoro-2,2-diphenylacetate methobromide 2a.9.3 ,
- tropenol 2-fluoro-2,2-diphenylacetate methobromide 2a.9.4 ,;
The compounds of formula 2a.9 may optionally in the form of the enantiomers,
mixtures
of enantiomers or racemates thereof, as well as optionally in the form of the
hydrates
and/or solvates thereof.
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.9 are combinations containing the
compounds 1.1
and 2a.9.1. 1.1 and 2a.9.2; 1.1 and 2a.9.3; 1.1 and 2a.9.4; 1.2 and 2a.9.1;
1.2 and 2a.9.2;
1.2 and 2a.9.3; 1.2 and 2a.9.4; 1.3 and 2a.9.1; 1.3 and 2a.9.2; 1_3 and
2a.9.3; 1.3 and
2a.9.4; 1.4 and 2a.9.1; 1.4 and 2a.9.2; 1.4 and 2a.9.3; 1.4 and 2a.9.4; 1_5
and 2a.9.1; 1_5
and 2a.9.2; 1.5 and 2a.9.3; 1.5 and 2a.9.4; 1_6 and 2a.9.1; 1.6 and 2a.9.2;
1.6 and 2a.9.3;
1.6 and 2a.9.4; 1.7 and 2a.9.1; 1.7 and 2a.9.2; 1.7 and 2a.9.3; 1.7 and
2a.9.4; 1.12 and
2a.9.1; 1.12 and 2a.9.2; 1.12 and 2a.9.3; 1.12 and 2a.9.4; 1.14 and 2a.9.1;
1.14 and 2a.9.2;
CA 02559699 2006-09-12
WO 2005/102349 17 PCT/EP2005/004073
1.14 and 2a.9.3; 1.14 and 2a.9.4; 1.15 and 2a.9.1; 1.15 and 2a.9.2; 1.15 and
2a.9.3; 1.15
and 2a.9.4, in each case optionally in the form of the racemates, enantiomers
or
diastereomers thereof and optionally in the form of the pharmacologically
acceptable acid
addition salts, solvates and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_1, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2a.9 one of the
compounds 2a.9.1 or 2a.9.2 , while those combinations which contain the
compound
2a.9.2 are particularly important according to the invention.
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the
compounds of formula 2a.10
R~+,R1
N X
H
A R8 O O R7
R9 R11
R1 OH 12
R 2a.10
wherein
A, Y, R' and R2 may have the meanings given above and wherein
R', R8, R9, R10, R11 and R12 , which may be identical or different, denote
hydrogen, methyl,
ethyl, methyloxy, ethyloxy, hydroxy, fluorine, chlorine, bromine, CN, CF3 or
NO2, while
at least one of the groups R7, R8, R9, R10, R" and R12 may not be hydrogen.
The compounds of formula 2a.10 are known in the art (WO 02/32898).
Within the scope of the medicament combinations according to the invention
preferred
compounds of formula 2a.10 are those wherein
A denotes a double-bonded group selected from
CA 02559699 2006-09-12
WO 2005/102349 18 PCT/EP2005/004073
\ /
H H and
H O H
X - bromide;
R' and R2 which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R7, R8, R9, R10, R11 and R12, which may be identical or different, denote
hydrogen,
fluorine, chlorine or bromine, preferably fluorine, while at least one of the
groups R7, R8, R9, R10, R11 and R12 may not be hydrogen.
Of particular importance are those medicament combinations which contain in
addition to
a compound of formula 1 one of the following compounds of formula 2a.10 :
- tropenol 3,3',4,4'-tetrafluorobenzilate methobromide 2a.10.1 ,
- scopine 3,3',4,4'-tetrafluorobenzilate methobromide (2a.10.2),
- tropenol 4,4'-difluorobenzilate methobromide 2a.10.3 ,
- scopine 4,4'-difluorobenzilate methobromide (2a.10.4),
- tropenol 3,3'-difluorobenzilate methobromide (2a.10.5),
- scopine 3,3'-difluorobenzilate methobromide (2a.10.6).
The compounds of formula 2a.10 may optionally be presentin the form of the
enantiomers,
mixtures of enantiomers or racemates thereof, as well as optionally in the
form of the
hydrates and/or solvates thereof .
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.10 are combinations containing the
compounds
1.1 and 2a.10.1; 1_1 and 2a.10.2; 1.1 and 2a.10.3; 1.1 and 2a.10.4; 1.1 and
2a.10.5; 1.1 and
2a.10.6; 1.2 and 2a.10.1; 1.2 and 2a.10.2; 1.2 and 2a.10.3; 1.2 and 2a.10.4;
1.2 and
2a.10.5; 1.2 and 2a.10.6; 1.3 and 2a.10.1; 1.3 and 2a.10.2; 1.3 and 2a.10.3;
1.3 and
2a.10.4; 1.3 and 2a.10.5; 1.3 and 2a.10.6; 1.4 and 2a.10.1; 1.4 and 2a.10.2;
1.4 and
2a.10.3; 1.4 and 2a.10.4; 1.4 and 2a.10.5; 1.4 and 2a.10.6; 1.5 and 2a.10.1;
1.5 and
2a.10.2; 1.5 and 2a.10.3; 1.5 and 2a.10.4; 1.5 and 2a.10.5; 1.5 and 2a.10.6;
1.6 and
2a.10.1; 1.6 and 2a.10.2; 1.6 and 2a.10.3; 1.6 and 2a.10.4; 1.6 and 2a.10.5;
1.6 and
2a.10.6; 1.7 and 2a.10.1; 1.7 and 2a.10.2; 1.7 and 2a.10.3; 1.7 and 2a.10.4;
1.7 and
2a.10.5; 1.7 and 2a.10.6; 1.12 and 2a.10.1; 1.12 and 2a.10.2; 1.12 and
2a.10.3; 1.12 and
2a.10.4; 1.12 and 2a.10.5; 1.12 and 2a.10.6; 1.14 and 2a.10.1; 1.14 and
2a.10.2; 1.14 and
CA 02559699 2006-09-12
WO 2005/102349 19 PCT/EP2005/004073
2a.10.3; 1.14 and 2a.10.4; 1.14 and 2a.10.5; 1.14 and 2a.10.6; 1.15 and
2a.10.1; 1.15 and
2a.10.2; 1.15 and 2a.10.3; 1.15 and 2a.10.4; 1.15 and 2a.10.5; 1.15 and
2a.10.6, in each
case optionally in the form of the racemates, enantiomers or diastereomers
thereof and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_l, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations,
also preferred
according to the invention are those which contain as compound 2a.10 one of
the
compounds 2a.10.1, 2a.10.2, 2a.10.3 or 2a.10.4, while those combinations which
contain
the compounds 2a.10.1 or 2a.10.2 are particularly important according to the
invention.
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the
compounds of formula 2a.11
R2_'_~ + R1
X
N
H
A O O
R15
R13 3
Ttz::IJEEE:L:II:r R14'
2a.11
wherein
A and X - may have the meanings given above and wherein
R15 denotes hydrogen, hydroxy, methyl, ethyl, -CF3, CHF2 or fluorine;
R" and R2' which may be identical or different, denote CI-C5-alkyl, which may
optionally be substituted by C3-C6-cycloalkyl, hydroxy or halogen,
or
R1, and R2' together denote a -C3-C5-alkylene bridge ;
R13, R14, R13' and R14' which maybe identical or different, denote hydrogen, -
CI-C4-alkyl,
CA 02559699 2006-09-12
WO 2005/102349 20 PCT/EP2005/004073
-C 1-C4-alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2 or halogen.
The compounds of formula 2a.11 are known in the art (WO 03/064419).
Within the scope of the medicament combinations according to the invention
preferred
compounds of formula 2a.11 are those wherein
A denotes a double-bonded group selected from
\ / \\ /
H H and
H 0 H
X - denotes an anion selected from chloride, bromide and methanesulphonate,
preferably bromide;
R15 denotes hydroxy, methyl or fluorine, preferably methyl or hydroxy;
R1, and R2' which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R13, R14, R13' and R14' which may be identical or different, denote hydrogen, -
CF3, -CHF2
or fluorine, preferably hydrogen or fluorine.
Within the scope of the medicament combinations according to the invention
particularly
preferred compounds of formula 2a.11 are those wherein
A denotes a double-bonded group selected from
C ~ and
H H H 0 H
X - denotes bromide;
R15 denotes hydroxy or methyl, preferably methyl;
R1, and R2' which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R13, R14, R13' and R14' which may be identical or different, denote hydrogen
or fluorine.
Of particular importance are those medicament combinations which contain in
addition to
a compound of formula 1 one of the following compounds of formula 2a.11 :
- tropenol 9-hydroxy-fluorene-9-carboxylate methobromide 2a.11.1 ;
- tropenol 9-fluoro-fluorene-9-carboxylate methobromide 2a.11.2) ;
- scopine 9-hydroxy-fluorene-9-carboxylate methobromide 2a.11.3 ;
- scopine 9-fluoro-fluorene-9-carboxylate methobromide 2a.11.4 ;
CA 02559699 2006-09-12
WO 2005/102349 21 PCT/EP2005/004073
tropenol 9-methyl-fluorene-9-carboxylate methobromide 2a.11.5 ;
scopine 9-methyl-fluorene-9-carboxylate methobromide 2a.11 66 ;
The compounds of formula 2a.11 may optionally be presentin the form of the
enantiomers,
mixtures of enantiomers or racemates thereof, as well as optionally in the
form of the
hydrates and/or solvates thereof .
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.11 are combinations containing the
compounds
1.1 and 2a.11.1; 1.1 and 2a.11.2; 1.1 and 2a.11.3; 1_1 and 2a.11.4; 1_1 and
2a.11.5; 1_1 and
2a.11.6; 1.2 and 2a.11.1; 1_2 and 2a.11.2; 1.2 and 2a.11.3:1.2 and 2a.11.4;
1.2 and
2a.11.5; 1.2 and 2a.11.6; 1.3 and 2a.11.1; 1_3 and 2a.11.2; 1_3 and 2a.11.3;
1_3 and
2a.11.4; 1.3 and 2a.11.5; 1.3 and 2a.11.6; 1.4 and 2a.11.1; 1.4 and 2a.11.2;
1.4 and
2a.11.3; 1.4 and 2a.11.4; 1.4 and 2a.11.5; 1.4 and 2a.11.6; 1.5 and 2a.11.1;
1_5 and
2a.11.2; 1.5 and 2a.11.3; 1.5 and 2a.11.4; 1_5 and 2a.11.5; 1.5 and 2a.11.6;
1.6 and
2a.11.1; 1.6 and 2a.11.2:1.6 and 2a.11.3; 1_6 and 2a.11.4; 1_6 and 2a.11.5;
1.6 and
2a.11.6; 1.7 and 2a.11.1; 1.7 and 2a.11.2; 1.7 and 2a.11.3; 1_7 and 2a.11.4;
1_7 and
2a.11.5; 1.7 and 2a.11.6; 1.12 and 2a.11.1; 1.12 and 2a.11.2; 1.12 and
2a.11.3; 1.12 and
2a.11.4; 1.12 and 2a.11.5; 1.12 and 2a.11.6; 1.14 and 2a.11.1; 1.14 and
2a.11.2; 1.44 and
2a.11.3; 1.14 and 2a.11.4; 1.14 and 2a.11.5; 1.14 and 2a.11.6; 1.15 and
2a.11.1; 1.15 and
2a.11.2; 1.15 and 2a.11.3; 1.15 and 2a.11.4; 1.15 and 2a.11.5; 1.15 and
2a.11.6, in each
case optionally in the form of the racemates, enantiomers or diastereomers
thereof and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_1, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations,
also preferred
according to the invention are those which contain as compound 2a.11 one of
the
compounds 2a.11.2, 2a.11.4, 2a.11.5 or 2a.11.6 , while those combinations
which contain
the compounds 2a.11.5 or 2a.11.6 are particularly important according to the
invention.
CA 02559699 2006-09-12
WO 2005/102349 22 PCT/EP2005/004073
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the
compounds of formula 2a.12
R+,R
N X
4::~~ H
O O
R16
R17 D B R17'
R1s R " R1s'
Rx'
2a.12
wherein X - may have the meanings given above and wherein
D and B which may be identical or different, are preferably identical and
denote 0,
S, NH, CH2, CH=CH or
N(C 1-C4-alkyl);
R' 6 denotes hydrogen, hydroxy, -C 1-C4-alkyl, -C 1-C4-alkyloxy,
-C 1-C4-alkylene-halogen, -0-C 1-C4-alkylene-halogen,
-C 1-C4-alkylene-OH, -CF3, CHF2, -C 1-C4-alkylene-C 1-C4-alkyloxy,
-0-COC 1-C4-alkyl, -O-COC 1-C4-alkylene-halogen,
-C 1 -C4-alkylene-C3 -C6-cycloalkyl, -0-000F3 or halogen;
R1 and R2õ which may be identical or different, denote -C1-C5-alkyl, which may
optionally be substituted by -C3-C6-cycloalkyl, hydroxy or halogen,
or
R1õ and R2" together denote a -C3-C5-alkylene bridge ;
R'7, R18, R17' and R'8" which may be identical or different, denote hydrogen, -
C1-C4-alkyl,
-C 1-C4-alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2 or halogen;
Rx and Rx' which may be identical or different, denote hydrogen, -C1-C4-alkyl,
-C1-C4-alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2 or halogen,
or
RX and Rx' together denote a single bond or one of the double-bonded groups 0,
S, NH,
CH2, CH2-CH2, N(C 1-C4-alkyl), CH(C 1-C4-alkyl) and -C(C 1-C4-a1ky1)2.
The compounds of formula 2a.12 are known in the art (WO 03/064418).
CA 02559699 2006-09-12
WO 2005/102349 23 PCT/EP2005/004073
Within the scope of the medicament combinations according to the invention
preferred
compounds of formula 2a.12 are those wherein
X - denotes chloride, bromide or methanesulphonate, preferably bromide;
D and B which may be identical or different, are preferably identical and
denote 0,
S, NH or CH=CH;
R' 6 denotes hydrogen, hydroxy, -C 1-C4-alkyl, -C 1-C4-alkyloxy, -CF3, -CHF2,
fluorine, chlorine or bromine;
R'.. and R2" which may be identical or different, denote C 1-C4-alkyl, which
may
optionally be substituted by hydroxy, fluorine, chlorine or bromine,
or
R'" and R2" together denote a -C3-C4-alkylene bridge ;
R'7, R18, R'7 and R18" which may be identical or different, denote hydrogen,
C1-C4-alkyl,
C1-C4-alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2, fluorine, chlorine or
bromine;
Rx and Rx' which may be identical or different, denote hydrogen, C1-C4-alkyl,
C 1-C4-
alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2, fluorine, chlorine or bromine,
or
Rx and Rx' together denote a single bond or a double-bonded group selected
from 0, S,
NH- and CH2.
Within the scope of the medicament combinations according to the invention
particularly
preferred compounds of formula 2a.12 are those wherein
X - denotes chloride, bromide, or methanesulphonate, preferably bromide;
D and B which may be identical or different, preferably identical, denote S or
CH=CH;
R16 denotes hydrogen, hydroxy or methyl;
R'" and R2., which may be identical or different, denote methyl or ethyl;
R17, R18, R'7' and R18', which may be identical or different, denote hydrogen,
-CF3 or
fluorine, preferably hydrogen;
Rx and Rx' which may be identical or different, denote hydrogen, -CF3 or
fluorine,
preferably hydrogen, or
Rx and Rx' together denote a single bond or -0.
Within the scope of the medicament combinations according to the invention,
other
particularly preferred compounds of formula 2a.12 are those wherein
CA 02559699 2006-09-12
WO 2005/102349 24 PCT/EP2005/004073
X - denotes bromide;
D and B denotes -CH=CH-;
R16 denotes hydrogen, hydroxy or methyl;
R1,, and R2õ denotes methyl;
R17, R18, R17' and R18', which may be identical or different, denote hydrogen
or fluorine,
preferably hydrogen;
Rx and Rx' which may be identical or different, denote hydrogen or fluorine,
preferably
hydrogen, or
Rx and Rx' together denote a single bond or the group -0.
Of particular importance are those medicament combinations which contain in
addition to
a compound of formula 1 one of the following compounds of formula 2a.12
- cyclopropyltropine benzilate methobromide 2a.12.1 ;
- cyclopropyltropine 2,2-diphenylpropionate methobromide 2a.12.2 ;
- cyclopropyltropine 9-hydroxy-xanthene-9-carboxylate methobromide 2a.12.3 ;
- cyclopropyltropine 9-methyl-fluorene-9-carboxylate methobromide 2a.12.4 ;
- cyclopropyltropine 9-methyl-xanthene-9-carboxylate methobromide (2a.12.5);
- cyclopropyltropine 9-hydroxy-fluorene-9-carboxylate methobromide 2a.12.6 ;
- cyclopropyltropine methyl 4,4'-difluorobenzilate methobromide 2a.12.7 .
The compounds of formula 2a.12 may optionally be present in the form of the
enantiomers, mixtures of enantiomers or racemates thereof, as well as
optionally in the
form of the hydrates and/or solvates thereof .
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.12 are combinations containing the
compounds
1.1 and 2a.12.1; 1.1 and 2a.12.2; 1.1 and 2a.12.3; 1.1 and 2a.12.4; 1.1 and
2a.12.5; 1.1 and
2a.12.6; 1.1 and 2a.12.7; 1.2 and 2a.12.1; 1.2 and 2a.12.2; 1.2 and 2a.12.3;
1.2 and
2a.12.4; 1.2 and 2a.12.5; 1.2 and 2a.12.6; 1.2 and 2a.12.7; 1.3 and 2a.12.1;
1.3 and
2a.12.2-,1.3 and 2a.12.3. 1.3 and 2a.12.4; 1.3 and 2a.12.5; 1.3 and 2a.12.6;
1.3 and
2a.12.7; 1.4 and 2a.12.1; 1.4 and 2a.12.2; 1.4 and 2a.12.3; 1.4 and 2a.12.4;
1.4 and
2a.12.5; 1.4 and 2a.12.6; 1.4 and 2a.12.7; 1.5 and 2a.12.1; 1.5 and 2a.12.2;
1.5 and
2a.12.3; 1.5 and 2a.12.4; 1.5 and 2a.12.5; 1.5 and 2a.12.6; 1.5 and 2a.12.7;
1.6 and
2a.12.1; 1.6 and 2a.12.2; 1.6 and 2a.12.3; 1.6 and 2a.12.4; 1.6 and 2a.12.5;
1.6 and
2a.12.6; 1.6 and 2a.12.7; 1.7 and 2a.12.1; 1.7 and 2a.12.2; 1.7 and 2a.12.3;
1.7 and
CA 02559699 2006-09-12
WO 2005/102349 25 PCT/EP2005/004073
2a.12.4; 1.7 and 2a.12.5; 1.7 and 2a.12.6; 1.7 and 2a.12.7; 1.12 and 2a.12.1;
1.12 and
2a.12.2; 1.12 and 2a.12.3; 1.12 and 2a.12.4; 1.12 and 2a.12.5; 1.12 and
2a.12.6; 1.12 and
2a.12.7; 1.14 and 2a.12.1; 1.14 and 2a.12.2; 1.14 and 2a.12.3; 1.14 and
2a.12.4; 1.14 and
2a.12.5; 1.14 and 2a.12.6; 1.14 and 2a.12.7; 1.15 and 2a.12.1; 1.15 and
2a.12.2; 1.15 and
2a.12.3; 1.15 and 2a.12.4; 1.15 and 2a.12.5; 1.15 and 2a.12.6; 1.15 and
2a.12.7, in each
case optionally in the form of the racemates, enantiomers or diastereomers
thereof and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1.1, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2a.11 one of
the
compounds 2a.12.1, 2a.12.2 , 2a.12.5 or 2a.12.7 , while those combinations
which contain
the compounds 2a.12.1 or 2a.12.2 are particularly important according to the
invention.
In another preferred embodiment of the present invention the anticholinergics
2a contained
in the medicament combinations according to the invention are selected from
the
compounds of formula 2a.13
R2,,, +/R1,,, -
~N X
H
A' O O
iii:::ijEaEr:iir R 21'
2a.13
wherein X - may have the meanings given above and wherein
A' denotes a double-bonded group selected from
C H H and
H 0 H
CA 02559699 2006-09-12
= WO 2005/102349 26 PCT/EP2005/004073
R19 denotes hydroxy, methyl, hydroxymethyl, ethyl, -CF3, CHF2 or fluorine;
R1and R2which may be identical or different, denote C1-C5-alkyl, which may
optionally be substituted by C3-C6-cycloalkyl, hydroxy or halogen,
or
R' and R2.,, together denote a -C3-C5-alkylene bridge ;
R20, R21, R201 and R21' which may be identical or different, denote hydrogen, -
C1-C4-alkyl,
-C1-C4-alkyloxy, hydroxy, -CF3, -CHF2, CN, NO2 or halogen.
The compounds of formula 2a.13 are known in the art (WO 03/064417).
Within the scope of the medicament combinations according to the invention
preferred
compounds of formula 2a.13 are those wherein
A' denotes a double-bonded group selected from
\ / \ \ /
C H H and
H 0 H
X - denotes chloride, bromide or methanesulphnat, preferably bromide;
R19 denotes hydroxy or methyl;
R1 and R2,., which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R20, R21, R20' and R21' which may be identical or different, denote hydrogen, -
CF3, -CHF2
or fluorine, preferably hydrogen or fluorine.
Within the scope of the medicament combinations according to the invention
particularly
preferred compounds of formula 2a.13 are those wherein
A' denotes a double-bonded group selected from
\ /
H H and
H O H
X - denotes bromide;
R19 denotes hydroxy or methyl, preferably methyl;
R1and R2which may be identical or different, denote methyl or ethyl,
preferably
methyl;
R3, R4, R3' and R4' which maybe identical or different, denote hydrogen or
fluorine.
CA 02559699 2006-09-12
WO 2005/102349 27 PCT/EP2005/004073
Of particular importance are those medicament combinations which contain in
addition to
a compound of formula 1 one of the following compounds of formula 2a.13
- tropenol 9-hydroxy-xanthene-9-carboxylate methobromide (2a.13.1);
- scopine 9-hydroxy-xanthene-9-carboxylate methobromide 2a.13.2 ;
- tropenol 9-methyl-xanthene-9-carboxylate methobromide (2a.13.3);
- scopine 9-methyl-xanthene-9-carboxylate methobromide (2a.13.4);
- tropenol 9-ethyl-xanthene-9-carboxylate methobromide (2a.13.5);
- tropenol 9-difluoromethyl-xanthene-9-carboxylate methobromide 2a.13.6 ;
- scopine 9-hydroxymethyl-xanthene-9-carboxylate methobromide 2a.13.7).
The compounds of formula 2a.13 may optionally be present in the form of the
enantiomers, mixtures of enantiomers or racemates thereof, as well as
optionally in the
form of the hydrates and/or solvates thereof.
Examples of novel medicament combinations of preferred compounds of formula 1
with
the above-mentioned anticholinergics 2a.13 are combinations containing the
compounds
1.1 and 2a.13.1; 1.1 and 2a.13.2; 1.1 and 2a.13.3; 1.1 and 2a.13.4; 1.1 and
2a.13.5; 1.1 and
2a.13.6; 1.1 and 2a.13.7; 1.2 and 2a.13.1; 1.2 and 2a.13.2; 1.2 and 2a.13.3;
1.2 and
2a.13.4; 1.2 and 2a.13.5; 1.2 and 2a.13.6; 1.2 and 2a.13.7; 1.3 and 2a.13.1;
1.3 and
2a.13.2; 1.3 and 2a.13.3; 1.3 and 2a.13.4; 1.3 and 2a.13.5; 1.3 and 2a.13.6;
1.3 and
2a.13.7; 1.4 and 2a.13.1; 1.4 and 2a.13.2; 1.4 and 2a.13.3; 1.4 and 2a.13.4;
1.4 and
2a.13.5; 1.4 and 2a.13.6; 1.4 and 2a.13.7; 1.5 and 2a.13.1; 1.5 and 2a.13.2;
1.5 and
2a.13.3; 1.5 and 2a.13.4; 1.5 and 2a.13.5; 1.5 and 2a.13.6; 1.5 and 2a.13.7;
1.6 and
2a.13.1; 1.6 and 2a.13.2; 1.6 and 2a.13.3; 1.6 and 2a.13.4; 1.6 and 2a.13.5;
1.6 and
2a.13.6; 1.6 and 2a.13.7; 1.7 and 2a.13.1; 1.7 and 2a.13.2; 1.7 and 2a.13.3;
1.7 and
2a.13.4; 1.7 and 2a.13.5; 1.7 and 2a.13.6; 1.7 and 2a.13.7; 1.12 and 2a.13.1;
1.12 and
2a.13.2; 1.12 and 2a.13.3; 1.12 and 2a.13.4; 1.12 and 2a.13.5; 1.12 and
2a.13.6; 1.12 and
2a.13.7; 1.14 and 2a.13.1; 1.14 and 2a.13.2; 1.14 and 2a.13.3; 1.14 and
2a.13.4; 1.14 and
2a.13.5; 1.14 and 2a.13.6; 1.14 and 2a.13.7; 1.15 and 2a.13.1; 1.15 and
2a.13.2; 1.15 and
2a.13.3; 1.15 and 2a.13.4; 1.15 and 2a.13.5; 1.15 and 2a.13.6; 1.15 and
2a.13.7, in each
case optionally in the form of the racemates, enantiomers or diastereomers
thereof and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
CA 02559699 2006-09-12
WO 2005/102349 28 PCT/EP2005/004073
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1.1, 1_5, 1_6, or
1.12,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2a.11 one of
the
compounds 2a.13.2, 2a.13.3 , 2a.13.4 or 2a.13.5 , while those combinations
which contain
the compounds 2a.13.3 or 2a.13.4 are particularly important according to the
invention.
Within the scope of the present invention any reference to anticholinergics 1'
is to be taken
as a reference to the pharmacologically active cations of the various salts .
These cations
are tiotropium 2a.1' , oxitropium (2a.21), flutropium (2a.31), ipratropium
2a.4 ,
glycopyrronium 2a.5' , trospium (2a.6) and the cations shown below:
~N+ OH Me
O ~ \ NMe
H
HO R
S Me Me
S Me
2a.7'; 2a.8';
R +/R R +/R
H H
A 0 0 A R8 0 0 R
R5 R4 R9 R11
R6 R7 s R10 OH 12
R R
2a.9'; 2a.10'
CA 02559699 2006-09-12
WO 2005/102349 29 PCT/EP2005/004073
R2\+/R R2
N N
H H
A O O O O
R15 1s
R13 R13, R17 D B R17'
~
R14 R14' R18 Rx Rx' R 18
2a.11'; 2a.12';
R2,,. +,R
~N
H
A' O O
R19
R20 R20,
21 21'
or R O R 2a.13'.
Other preferred medicament combinations according to the invention contain as
an
additional active substance, in addition to one or more, preferably one
compound of
formula 1, one or more, preferably one, PDE IV-inhibitor 2b, optionally in
combination
with pharmaceutically acceptable excipients.
In such medicament combinations the PDE IV-inhibitor 2b is preferably selected
from
among enprofyllin, theophyllin, roflumilast, ariflo (Cilomilast), CP-325,366,
BY343, D-
4396 (Sch-351591), AWD-12-281 (GW-842470), N-(3,5-dichloro-l-oxo-pyridin-4-yl)-
4-
difluoromethoxy-3-cyclopropylmethoxybenzamide, NCS-613, pumafentine, (-)p-
[(4aR*,10bS*)-9-ethoxy-1,2,3,4,4a,1 Ob-hexahydro-8-methoxy-2-
methylbenzo[s][1,6]naphthyridin-6-yl]-N,N-diisopropylbenzamide, (R)-(+)-1-(4-
bromobenzyl)-4-[(3-cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidone, 3-
(cyclopentyloxy-
4-methoxyphenyl)-1-(4-N'-[N-2-cyano-S-methyl-isothioureido]benzyl)-2-
pyrrolidone,
cis[4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexane-l -carboxylic
acid], 2-
CA 02559699 2006-09-12
WO 2005/102349 30 PCT/EP2005/004073
carbomethoxy-4-cyano-4-(3-cyclopropylmethoxy-4-
difluoromethoxyphenyl)cyclohexan-l -
one, cis[4-cyano-4-(3-cyclopropylmethoxy-4-difluoromethoxyphenyl)cyclohexan-1-
ol],
(R)-(+)-ethyl[4-(3 -cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-ylidene]
acetate, (S)-(-)-
ethyl [4-(3 -cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-ylidene] acetate,
CDP840, Bay-
198004, D-4418, PD-168787, T-440, T-2585, arofyllin, atizoram, V-11294A, C1-
1018,
CDC-801, CDC-3052, D-22888, YM-58997, Z-15370, 9-cyclopentyl-5,6-dihydro-7-
ethyl-
3-(2-thienyl)-9H-pyrazolo[3,4-c]-1,2,4-triazolo[4,3-a]pyridine and 9-
cyclopentyl-5,6-
dihydro-7-ethyl-3-(tert-butyl)-9H-pyrazolo[3,4-c]-1,2,4-triazolo[4,3-
a]pyridine, optionally
in the form of the racemates, enantiomers or diastereomers thereof and
optionally in the
form of the pharmacologically acceptable acid addition salts, solvates and/or
hydrates
thereof.
In particularly preferred medicament combinations the PDE IV-inhibitor 2b is
selected
from the group comprising enprofyllin 2b.1), roflumilast 2b.2 , ariflo
(cilomilast) 2b.3),
AWD-12-281 (GW-842470) 2b.4 , N-(3,5-dichloro-l-oxo-pyridin-4-yl)-4-
difluoromethoxy-3-cyclopropylmethoxybenzamide 2b.5 , T-440 2b.6 , T-2585
2b.7),
arofyllin 2b.8 , cis[4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexane-l-
carboxylic acid] (1h.9), 2-carbomethoxy-4-eyano-4-(3-cyclopropylmethoxy-4-
difluoromethoxyphenyl)cyclohexan-I -one (1h.10), cis [4-cyano-4-(3 -
cyclopropylmethoxy-
4-difluoromethoxyphenyl)cyclohexan-l-ol] 2b.11 , PD-168787 2b.12 , atizoram
(1h.13),
V-1 1294A (2h.14), C1-1018 2b.15 , CDC-801 (2h.16), D-22888 (2b.17), YM-58997
2b.18 , Z- 15370 2b.19 , 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(2-thienyl)-9H-
pyrazolo[ 3,4-c] -1,2,4-triazolo[4,3-a]pyri dine 2b.20 and 9-cyclopentyl-5,6-
dihydro-7-
ethyl-3-(tert-butyl)-9H-pyrazolo[3,4-c]-1,2,4-triazolo[4,3-a]pyridine 2b.21 ,
optionally in
the form of the racemates, enantiomers or diastereomers thereof and optionally
in the form
of the pharmacologically acceptable acid addition salts, solvates and/or
hydrates thereof.
In particularly preferred medicament combinations the PDE IV-inhibitor 2b is
selected
from the group comprising roflumilast 2b.2), ariflo (cilomilast) 2b.3 ,
AWD-12-281 (GW-842470) 2b.4), arofyllin 2b.8), 2-carbomethoxy-4-cyano-4-(3-
cyclopropylmethoxy-4-difluoromethoxyphenyl)cyclohexan-l-one (2h.10), cis[4-
cyano-4-
(3-cyclopropylmethoxy-4-difluoromethoxyphenyl)cyclohexan-l-ol] (1h.11),
atizoram
2b.13 , Z-15370 (2b.19), 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(2-thienyl)-9H-
pyrazolo[3,4-c]-1,2,4-triazolo[4,3-a]pyridine (2b.20) and 9-cyclopentyl-5,6-
dihydro-7-
ethyl-3-(tert-butyl)-9H-pyrazolo[3,4-c]-1,2,4-triazolo[4,3-a]pyridine 2b.21 ,
while
CA 02559699 2006-09-12
WO 2005/102349 31 PCT/EP2005/004073
roflumilast (2k.2), Z-15370 2b.19 and AWD-12-281 2b.4 are of particular
significance,
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
By the acid addition salts with pharmacologically acceptable acids which the
compounds
2b may possibly be capable of forming are meant for example salts selected
from the
group comprising the hydrochloride, hydrobromide, hydroiodide, hydrosulphate,
hydrophosphate, hydromethanesulphonate, hydronitrate, hydromaleate,
hydroacetate,
hydrobenzoate, hydrocitrate, hydrofumarate, hydrotartrate, hydrooxalate,
hydrosuccinate,
hydrobenzoate and hydro-p-toluenesulphonate, preferably the hydrochloride,
hydrobromide, hydrosulphate, hydrophosphate, hydrofumarate and
hydromethanesulphonate.
Examples of novel preferred medicament combinations of preferred compounds of
formula
1 with the above-mentioned PDE IV inhibitors 2b are combinations containing
the
compounds 1.1 and 2b.1; 1.1 and 2b.2; 1_1 and 2b.3; 1_1 and 2b.4; 1.1 and
2b.5; 1.1 and
2b.6; 1.1 and 2b.7; 1_1 and 2b.8; 1_1 and 2b.9; 1.1 and 2b.10; 1_1 and 2b.11;
1_1 and
2b.12; 1.1 and 2b.13; 1.1 and 2b.14; 1.1 and 2b.15; 1.1 and 2b.16; 1.1 and
2b.17; 1.1 and
2b.18. 1.1 and 2b.19; 1.1 and 2b.20; 1.1 and 2b.21; 1.2 and 2b.1; 1.2 and
2b.2; 1.2 and
2b.3. 1.2 and 2b.4; 1.2 and 2b.5; 1.2 and 2b.6; 1.2 and 2b.7; 1.2 and 2b.8;
1.2 and 2b.9=
1.2 and 2b.10; 1.2 and 2b.11; 1.2 and 2b.12; 1.2 and 2b.13; 1_2 and 2b.14; 1.2
and 2b.15;
1.2 and 2b.16; 1.2 and 2b.17; 1.2 and 2b.18; 1_2 and 2b.19; 1.2 and 2b.20; 1_2
and 2b.21;
1.3 and 2b.1; 1.3 and 2b.2; 1.3 and 2b.3; 1.3 and 2b.4; 1.3 and 2b.5; 1_3 and
2b.6; 1_3 and
2b.7; 1.3 and 2b.8; 1.3 and 2b.9; 1.3 and 2b.10; 1_3 and 2b.11; 1.3 and 2b.12;
1_3 and
2b.13; 1_3 and 2b.14; 1_3 and 2b.15; 1.3 and 2b.16; 1_3 and 2b.17; 1.3 and
2b.18; 1.3 and
2b.19; 1.3 and 2b.20; 1.3 and 2b.21; 1.4 and 2b.1; 1_4 and 2b.2; 1_4 and 2b.3;
1_4 and
2b.4; 1.4 and 2b.5; 1.4 and 2b.6; 1.4 and 2b.7; 1.4 and 2b.8; 1_4 and 2b.9;
1.4 and 2b.10;
1.4 and 2b.11; 1.4 and 2b.12; 1.4 and 2b.13; 1.4 and 2b.14; 1_4 and 2b.15; 1_4
and 2b.16;
1.4 and 2b.17; 1.4 and 2b.18; 1.4 and 2b.19; 1.4 and 2b.20; 1.4 and 2b.21; 1_5
and 2b.1;
1.5 and 2b.2; 1.5 and 2b.3; 1.5 and 2b.4; 1.5 and 2b.5; 1.5 and 2b.6; 1.5 and
2b.7; 1.5 and
2b.8; 1.5 and 2b.9; 1.5 and 2b.10; 1.5 and 2b.11; 1.5 and 2b.12; 1.5 and
2b.13; 1.5 and
2b.14; 1.5 and 2b.15; 1.5 and 2b.16; 1.5 and 2b.17; 1.5 and 2b.18; 1.5 and
2b.19; 1.5 and
2b.20; 1.5 and 2b.21; 1.6 and 2b.1; 1.6 and 2b.2; 1.6 and 2b.3; 1.6 and 2b.4;
1.6 and 2b.5;
1.6 and 2b.6; 1.6 and 2b.7; 1.6 and 2b.8; 1.6 and 2b.9; 1.6 and 2b.10. 1.6 and
2b.11; 1.6
CA 02559699 2006-09-12
WO 2005/102349 32 PCT/EP2005/004073
and 2b.12; 1.6 and 2b.13; 1.6 and 2b.14; 1_6 and 2b.15; 1_6 and 2b.16; 1.6 and
2b.17; 1_6
and 2b.18; 1.6 and 2b.19; 1.6 and 2b.20; 1.6 and 2b.21; 1_7 and 2b.1; 1.7 and
2b.2; 1_7
and 2b.3; 1.7 and 2b.4; 1_7 and 2b.5; 1_7 and 2b.6; 1.7 and 2b.7; 1.7 and
2b.8; 1.7 and
2b.9; 1.7 and 2b.10. 1.7 and 2b.11; 1.7 and 2b.12; 1.7 and 2b.13; 1.7 and
2b.14; 1.7 and
2b.15; 1.7 and 2b.16; 1.7 and 2b.17-,1.7 and 2b.18; 1_7 and 2b.19; 1.7 and
2b.20; 1_7 and
2b.21; 1.12 and 2b.1; 1.12 and 2b.2; 1.12 and 2b.3; 1.12 and 2b.4; 1.12 and
2b.5; 1.12
and 2b.6; 1.12 and 2b.7; 1.12 and 2b.8; 1.12 and 2b.9; 1.12 and 2b.10; 1.12
and 2b.11;
1.12 and 2b.12; 1.12 and 2b.13; 1.12 and 2b.14; 1.12 and 2b.15; 1.12 and
2b.16; 1.12 and
2b.17; 1.12 and 2b.18; 1.12 and 2b.19; 1.12 and 2b.20; 1.12 and 2b.21; 1.14
and 2b.1;
1.14 and 2b.2; 1.14 and 2b.3; 1.14 and 2b.4; 1.14 and 2b.5; 1.14 and 2b.6;
1.14 and 2b.7;
1.14 and 2b.8; 1.14 and 2b.9; 1.14 and 2b.10; 1.14 and 2b.11; 1.14 and 2b.12;
1.14 and
2b.13; 1.14 and 2b.14; 1.14 and 2b.15; 1.14 and 2b.16; 1.14 and 2b.17; 1.14
and 2b.18;
1.14 and 2b.19; 1.14 and 2b.20; 1.14 and 2b.21; 1.15 and 2b.1; 1.15 and 2b.2;
1.15 and
2b.3; 1.15 and 2b.4; 1.15 and 2b.5; 1.15 and 2b.6; 1.15 and 2b.7; 1.15 and
2b.8; 1.15 and
2b.9; 1.15 and 2b.10; 1.15 and 2b.11; 1.15 and 2b.12; 1.15 and 2b.13; 1.15 and
2b.14;
1.15 and 2b.15; 1.15 and 2b.16; 1.15 and 2b.17; 1.15 and 2b.18; 1.15 and
2b.19; 1.15 and
2b.20 or 1.15 and 2b.21, in each case optionally in the form of the racemates,
enantiomers
or diastereomers thereof and optionally in the form of the pharmacologically
acceptable
acid addition salts, solvates and/or hydrates thereof, in each case optionally
in the form of
the racemates, enantiomers or diastereomers thereof and optionally in the form
of the
pharmacologically acceptable acid addition salts, solvates and/or hydrates
thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_11, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2b one of the
compounds
2b.2, 2b.3, 2b.4, 2b.8, 2b.10, 2b.11, 2b.13, 2b.19, 2b.20 or 2b.21, while
those
combinations which contain one of the compounds 2b.2, 2b.4 or 2b.19 are
particularly
important according to the invention.
Other preferred medicament combinations according to the invention contain as
an
additional active substance, in addition to one or more, preferably one
compound of
formula 1 , one or more, preferably one steroid 2c, optionally in combination
with
pharmaceutically acceptable excipients.
CA 02559699 2006-09-12
WO 2005/102349 33 PCT/EP2005/004073
In such medicament combinations the steroid 2c is preferably selected from
among
prednisolone 2c.1 , prednisone (1c.2), butixocortpropionate 2c.3 , RPR-106541
(1c.4),
flunisolide (2c.5), beclomethasone 2c.6 , triamcinolone 2c.7 , budesonide
(2c.,8),
fluticasone 2c.9 mometasone 2c.10 ,ciclesonide 2c.11 ,rofleponide (2c.12), ST-
126
2c.13 ,dexamethasone 2c.14 , (S)-fluoromethyl 6a,9a-difluoro-17a-[(2-
furanylcarbonyl)oxy]-11(3-hydroxy-16a-methyl-3-oxo-androsta-1,4-diene-17(3-
carbothionate (2c.15), (S)-(2-oxo-tetrahydro-furan-3S-yl)6a,9a-difluoro-11(3-
hydroxy-
16a-methyl-3-oxo-17a-propionyloxy-androsta-1,4-diene-17(3-carbothionate 2c.16
and
etiprednol-dichloroacetate (BNP-166, 2c.17 , optionally in the form of the
racemates,
enantiomers or diastereomers thereof and optionally in the form of the salts
and derivatives
thereof, the solvates and/or hydrates thereof.
In particularly preferred medicament combinations the steroid 2c is selected
from the
group comprising flunisolide 2c.5 , beclomethasone 2c.6 ,triamcinolone 2c.7 ,
budesonide (k.8), fluticasone 2c.9 , mometasone 2c.10 , ciclesonide 2c.11 11),
roflepon
(2c.12), ST-126 (2c.13), dexamethasone 2c.14), (S)-fluoromethyl 6a,9a-difluoro-
l7a-
[(2-furanylcarbonyl)oxy]-11(3-hydroxy-16a-methyl-3-oxo-androsta-1,4-diene-17(3-
carbothionate 2c.15 , (S)-(2-oxo-tetrahydro-furan-3S-yl)6a,9a-difluoro-11 [3-
hydroxy-
16a-methyl-3-oxo-l7a-propionyloxy-androsta-1,4-diene-17(3-carbothionate 2c.16
and
etiprednol-dichloroacetate (2c.17), optionally in the form of the racemates,
enantiomers or
diastereomers thereof and optionally in the form of the salts and derivatives
thereof, the
solvates and/or hydrates thereof.
In particularly preferred medicament combinations the steroid 2c is selected
from the
group comprising budesonide (2c.8), fluticasone 2c.9 , mometasone (2c.10),
ciclesonide
2c.11), (S)-fluoromethyl 6a,9a-difluoro-17a-[(2-furanylcarbonyl)oxy]-11 [3-
hydroxy-
16a-methyl-3-oxo-androsta-1,4-diene-17(3-carbothionate 2c.15 and etiprednol-
dichloroacetate 2c.17 , optionally in the form of the racemates, enantiomers
or
diastereomers thereof and optionally in the form of the salts and derivatives
thereof, the
solvates and/or hydrates thereof.
Any reference to steroids 2c includes a reference to any salts or derivatives,
hydrates or
solvates thereof which may exist. Examples of possible salts and derivatives
of the steroids
2c may be: alkali metal salts, such as for example sodium or potassium salts,
CA 02559699 2006-09-12
WO 2005/102349 34 PCT/EP2005/004073
sulphobenzoates, phosphates, isonicotinates, acetates, propionates, dihydrogen
phosphates,
palmitates, pivalates or furoates.
Examples of novel preferred medicament combinations of preferred compounds of
formula
1 with the above-mentioned steroids 2c are combinations containing the
compounds 1.1
and 2c.1; 1.1 and 2c.2; 1.1 and 2c.3; 1.1 and 2c.4; 1.1 and 2c.5; 1.1 and
2c.6; 1_1 and 2c.7;
1.1 and 2c.8; 1.1 and 2c.9; 1.1 and 2c.10; 1.1 and 2c.11; 1.1 and 2c.12; 1.1
and 2c.13; 1_1
and 2c.14. 1.1 and 2c.15. 1.1 and 2c.16. 1.1 and 2c.17. 1.2 and 2c.1. 1.2 and
2c.2. 1.2 and
2c.3; 1.2 and 2c.4. 1.2 and 2c.5-.1.2 and 2c.6; 1.2 and 2c.7; 1.2 and 2c.8;
1.2 and 2c.9; 1.2
and 2c.10. 1.2 and 2c.11; 1.2 and 2c.12; 1.2 and 2c.13; 1.2 and 2c.14; 1.2 and
2c.15; 1.2
and 2c.16; 1.2 and 2c.17; 1.3 and 2c.1; 1.3 and 2c.2; 1.3 and 2c.3; 1.3 and
2c.4; 1.3 and
2c.5; 1.3 and 2c.6-,1.3 and 2c.7; 1.3 and 2c.8; 1_3 and 2c.9; 1.3 and 2c.10;
1.3 and 2c.11;
1.3 and 2c.12; 1.3 and 2c.13, 1.3 and 2c.14, 1.3 and 2c.15, 1.3 and 2c.16; 1.3
and 2c.17;
1.4 and 2c.1; 1.4 and 2c.2; 1.4 and 2c.3; 1.4 and 2c.4; 1.4 and 2c.5; 1.4 and
2c.6; 1.4 and
2c.7; 1.4 and 2c.8; 1.4 and 2c.9; 1.4 and 2c.10. 1.4 and 2c.11; 1.4 and 2c.12;
1.4 and 2c.13;
1.4 and 2c.14. 1.4 and 2c.15; 1.4 and 2c.16; 1.4 and 2c.17. 1.5 and 2c.1; 1.5
and 2c.2; 1.5
and 2c.3; 1.5 and 2c.4; 1.5 and 2c.5; 1_5 and 2c.6; 1.5 and 2c.7; 1_5 and
2c.8; 1.5 and 2c.9;
1.5 and 2c.10. 1.5 and 2c.11; 1.5 and 2c.12; 1.5 and 2c.13; 1.5 and 2c.14; 1.5
and 2c.15;
1.5 and 2c.16; 1.5 and 2c.17; 1.6 and 2c.1; 1.6 and 2c.2; 1_6 and 2c.3; 1_6
and 2c.4; 1.6 and
2c.5; 1.6 and 2c.6-.1.6 and 2c.7; 1.6 and 2c.8; 1.6 and 2c.9; 1_6 and 2c.10;
1.6 and 2c.11;
1.6 and 2c.12; 1.6 and 2c.13; 1_6 and 2c.14; 1.6 and 2c.15; 1.6 and 2c.16; 1_6
and 2c.17;
1.7 and 2c.1; 1.7 and 2c.2; 1.7 and 2c.3; 1.7 and 2c.4; 1.7 and 2c.5; 1.7 and
2c.6; 1_7 and
2c.7; 1.7 and 2c.8; 1.7 and 2c.9; 1.7 and 2c.10. 1.7 and 2c.11; 1.7 and 2c.12;
1.7 and 2c.13;
1.7 and 2c.14; 1.7 and 2c.15; 1.7 and 2c.16; 1.7 and 2c.17; 1.12 and 2c.1;
1.12 and 2c.2;
1.12 and 2c.3; 1.12 and 2c.4; 1.12 and 2c.5; 1.12 and 2c.6; 1.12 and 2c.7;
1.12 and 2c.8;
1.12 and 2c.9; 1.12 and 2c.10; 1.12 and 2c.11; 1.12 and 2c.12; 1.12 and 2c.13;
1.12 and
2c.14; 1.12 and 2c.15; 1.12 and 2c.16; 1.12 and 2c.17; 1.14 and 2c.1; 1.14 and
2c.2; 1.14
and 2c.3; 1.14 and 2c.4; 1.14 and 2c.5; 1.14 and 2c.6; 1.14 and 2c.7; 1.14 and
2c.8; 1.14
and 2c.9; 1.14 and 2c.10; 1.14 and 2e.11; 1.14 and 2c.12; 1.14 and 2c.13; 1.14
and 2c.14;
1.14 and 2c.15; 1.14 and 2c.16; 1.14 and 2c.17; 1.15 and 2c.1; 1.15 and 2c.2;
1.15 and
2c.3; 1.15 and 2c.4; 1.15 and 2c.5; 1.15 and 2c.6; 1.15 and 2c.7; 1.15 and
2c.8; 1.15 and
2c.9; 1.15 and 2c.10; 1.15 and 2c.11; 1.15 and 2c.12; 1.15 and 2c.13; 1.15 and
2c.14; 1.15
and 2c.15; 1.15 and 2c.16 or 1.15 and 2c.17 , in each case optionally in the
form of the
racemates, enantiomers or diastereomers thereof and optionally in the form of
the
pharmacologically acceptable acid addition salts, solvates and/or hydrates
thereof.
CA 02559699 2006-09-12
WO 2005/102349 35 PCT/EP2005/004073
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_1, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2c one of the
compounds
2c.5, 2c.6, 2c.7, 2c.8, 2c.9, 2c.10, 2c.11, 2c.12, 2c.13, 2c.14, 2c.15, 2c.16
or 2c.17 , while
those combinations which contain one of the compounds 2c.8, 2c.9, 2c.10,
2c.11, 2c.15 or
2c.17 are particularly important according to the invention.
Other preferred medicament combinations according to the invention contain, as
an
additional active substance, in addition to one or more, preferably one
compound of
formula 1 , one or more, preferably one, LTD4 antagonist 2d, optionally in
combination
with pharmaceutically acceptable excipients.
In such medicament combinations the LTD4 antagonist 2d is preferably selected
from
among montelukast 2d.1 , 1-(((R)-(3-(2-(6,7-difluoro-2-
quinolinyl)ethenyl)phenyl)-3-(2-
(2- hydroxy-2-propyl)phenyl)thio)methylcyclopropane-acetic acid 2d.2 , 1-
(((1(R)-3(3-(2-
(2,3-dichlorothieno[3,2-b]pyridin-5-yI)-(E)-ethenyl)phenyl)-3-(2-(1-hydroxy- l
-
methylethyl)phenyl)propyl)thio)methyl)cyclopropanacetic acid 2d.3 , pranlukast
2d.4 ,
zafirlukast 2d.5 , [2-[[2-(4-tert-butyl-2-thiazolyl)-5-benzofuranyl]oxymethyl]-
phenyl]acetic acid (2A,.6), MCC-847 (ZD-3523) 2d.7 , MN-001 2d.8 , MEN-91507
(LM-
1507) 2d.9 , VUF-5078 (1g.10), VUF-K-8707 2d.11 and L-733321 (2A.12),
optionally
in the form of the racemates, enantiomers or diastereomers thereof, optionally
in the form
of the pharmacologically acceptable acid addition salts thereof as well as
optionally in the
form of the salts and derivatives thereof, the solvates and/or hydrates
thereof.
In preferred medicament combinations the LTD4 antagonist 2d is selected from
the group
comprising montelukast 2d.1 pranlukast 2d.4 , zafirlukast 2d.5 , MCC-847 (ZD-
3523)
2d.7 , MN-001 (2d.8), MEN-91507 (LM-1507) 24.9 , VUF-5078 (14.10), VUF-K-8707
2d.11 and L-733321 (2d.12), optionally in the form of the racemates,
enantiomers or
diastereomers thereof, optionally in the form of the pharmacologically
acceptable acid
addition salts thereof as well as optionally in the form of the salts and
derivatives thereof,
the solvates and/or hydrates thereof.
CA 02559699 2006-09-12
WO 2005/102349 36 PCT/EP2005/004073
In particularly preferred medicament combinations the LTD4 antagonist 2d is
selected
from the group comprising montelukast 2d.1 , pranlukast 2d.4 , zafirlukast
24.5 , MCC-
847 (ZD-3523) 2d.7 , MN-001 2d.8 and MEN-91507 (LM-1507) (2.d.9), while
montelukast 2d.1 , pranlukast 2d.4) and zafirlukast 2d.5 are particularly
preferred,
optionally in the form of the racemates, enantiomers or diastereomers thereof,
optionally in
the form of the pharmacologically acceptable acid addition salts thereof as
well as
optionally in the form of the salts and derivatives thereof, the solvates
and/or hydrates
thereof.
By the acid addition salts with pharmacologically acceptable acids which the
compounds
2d may possibly be capable of forming are meant for example salts selected
from the
group comprising the hydrochloride, hydrobromide, hydroiodide, hydrosulphate,
hydrophosphate, hydromethanesulphonate, hydronitrate, hydromaleate,
hydroacetate,
hydrobenzoate, hydrocitrate, hydrofumarate, hydrotartrate, hydrooxalate,
hydrosuccinate,
hydrobenzoate and hydro-p-toluenesulphonate, preferably the hydrochloride,
hydrobromide, hydrosulphate, hydrophosphate, hydrofumarate and
hydromethanesulphonate.
Examples of possible salts and derivatives which the compounds 2d may possibly
be
capable of forming include for example: alkali metal salts, such as for
example sodium or
potassium salts, alkaline earth metal salts, sulphobenzoates, phosphates,
isonicotinates,
acetates, propionates, dihydrogen phosphates, palmitates, pivalates or
furoates.
Examples of novel preferred medicament combinations of preferred compounds of
formula
1 with the above-mentioned LTD4-antagonists 2d are combinations containing the
compounds 1.1 and 2d.1; 1_1 and 2d.2; 1.1 and 2d.3; 1_1 and 2d.4; 1.1 and
2d.5; 1_1 and
2d.6; 1.1 and 2d.7; 1.1 and 2d.8; 1_1 and 2d.9; 1.1 and 2d.10; 1.1 and 2d.11;
1.1 and
2d.12; 1.2 and 2d.1; 1.2 and 2d.2; 1.2 and 2d.3; 1.2 and 2d.4; 1.2 and 2d.5;
1_2 and 2d.6;
1.2 and 2d.7, 1.2 and 2d.8; 1.2 and 2d.9; 1.2 and 2d.10. 1.2 and 2d.11. 1.2
and 2d.12; 1.3
and 2d.1; 1.3 and 2d.2; 1.3 and 2d.3; 1.3 and 2d.4; 1.3 and 2d.5; 1_3 and
2d.6; 1_3 and
2d.7-,1.3 and 2d.8; 1.3 and 2d.9; 1.33 and 2d.10; 1_3 and 2d.11; 1_3 and
2d.12; 1_4 and
2d.1; 1.4 and 2d.2; 1.4 and 2d.3; 1.4 and 2d.4; 1.4 and 2d.5; 1_4 and 2d.6;
1_4 and 2d.7;
1.4 and 2d.8; 1.4 and 2d.9; 1.4 and 2d.10. 1.4 and 2d.11; 1.4 and 2d.12; 1.5
and 2d.1= 1.5
and 2d.2; 1.5 and 2d.3; 1.5 and 2d.4; 1.5 and 2d.5; 1.5 and 2d.6; 1.5 and
2d.7; 1.5 and
2d.8; 1.5 and 2d.9; 1.5 and 2d.10. 1.5 and 2d.11; 1.5 and 2d.12; 1.6 and 2d.1;
1.6 and
2d.2; 1.6 and 2d.3; 1.6 and 2d.4; 1.6 and 2d.5; 1.6 and 2d.6; 1_6 and 2d.7;
1.6 and 2d.8;
CA 02559699 2006-09-12
WO 2005/102349 37 PCT/EP2005/004073
1.6 and 2d.9; 1.6 and 2d.10. 1.6 and 2d.11; 1.6 and 2d.12; 1.7 and 2d.1; 1.7
and 2d.2. 1.7
and 2d.3; 1.7 and 2d.4; 1.7 and 2d.5; 1.7 and 2d.6; 1_7 and 2d.7; 1.7 and
2d.8; 1.7 and
2d.9; 1.7 and 2d.10; 1.7 and 2d.11; 1.7 and 2d.12; 1.12 and 2d.1; 1.12 and
2d.2; 1.12 and
2d.3; 1.12 and 2d.4; 1.12 and 2d.5; 1.12 and 2d.6; 1.12 and 2d.7; 1.12 and
2d.8; 1.12 and
2d.9; 1.12 and 2d.10; 1.12 and 2d.11; 1.12 and 2d.12; 1.14 and 2d.1; 1.14 and
2d.2; 1.14
and 2d.3; 1.14 and 2d.4; 1.14 and 2d.5; 1.14 and 2d.6; 1.14 and 2d.7; 1.14 and
2d.8; 1.14
and 2d.9; 1.14 and 2d.10; 1.14 and 2d.11; 1.14 and 2d.12; 1.15 and 2d.1; 1.15
and 2d.2;
1.15 and 2d.3; 1.15 and 2d.4; 1.15 and 2d.5; 1.15 and 2d.6; 1.15 and 2d.7;
1.15 and 2d.8;
1.15 and 2d.9; 1.15 and 2d.10; 1.15 and 2d.11 or 1.15 and 2d.12, in each case
optionally in
the form of the racemates, enantiomers or diastereomers thereof and optionally
in the form
of the pharmacologically acceptable acid addition salts, solvates and/or
hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_l, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as compound 2d one of the
compounds
2d.1, 2d.4, 2d.5, 2d.7, 2d.8, 2d.9, 2d.10, 2d.11 or 2d.12, while those
combinations which
contain one of the compounds 2d.1, 2d.4, 2d.5, 2d.7, 2d.8 or 2d.9 are
particularly
important according to the invention, and those combinations which contain one
of the
compounds 2d.1, 2d.4 or 2d.5 are of exceptional importance.
Other preferred medicament combinations according to the invention contain as
an
additional active substance, in addition to one or more, preferably one
compound of
formula 1 , one or more, preferably one, EGFR-inhibitor 2e, optionally in
combination
with pharmaceutically acceptable excipients.
In such medicament combinations the EGFR-inhibitor 2e is selected for example
from the
group comprising 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-
oxo-2-
buten-l-yl]amino }-7-cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-
fluorophenyl)amino]-6- { [4-(N,N-diethylamino)-1-oxo-2-buten- l -yl] amino } -
7-
cyclopropylmethoxy-quinazoline, 4-[(3 -chloro-4-fluorophenyl)amino]-6- { [4-
(N,N-
dimethylamino)-1-oxo-2-buten-l-yl]amino}-7-cyclopropylmethoxy-quinazoline, 4-
[(R)-(1-
phenyl-ethyl)amino]-6- { [4-(morpholin-4-yl)-1-oxo-2-buten- l -yl] amino } -7-
cyclopentyloxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-6-
methyl-
CA 02559699 2006-09-12
WO 2005/102349 38 PCT/EP2005/004073
2-oxo-morpholin-4-yl)-1-oxo-2-buten-l-yl]amino }-7-cyclopropylmethoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino]-6- { [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-
1-oxo-2-
buten-l-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-quinazoline, 4-[(3-chloro-
4-fluoro-
phenyl) amino] -6- { [4-((R)-2-methoxymethyl-6-oxo-morpholin-4-yl)-1-oxo-2-
buten- l -
yl]amino }-7-cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-[2-
((S)-6-methyl-2-oxo-morpholin-4-yl)-ethoxy]-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-1-oxo-2-buten-
l -
yl}amino)-7-cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-
6-{[4-
(N,N-dimethylamino)-1-oxo-2-buten- l -yl] amino } -7-cyclopentyloxy-
quinazoline, 4-[(R)-
(1-phenyl-ethyl)amino]-6- { [4-(N,N-bis-(2-methoxy-ethyl)-amino)-1-oxo-2-buten-
l -
yl]amino}-7-cyclopropylmethoxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-
({4-[N-(2-
methoxy-ethyl)-N-ethyl-amino]-1-oxo-2-buten- l -yl } amino)-7-
cyclopropylmethoxy-
quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-
1-oxo-2-buten- l -yl } amino)-7-cyclopropylmethoxy-quinazoline, 4-[(R)-(1-
phenyl-
ethyl)amino]-6-({4-[N-(tetrahydropyran-4-yl)-N-methyl-amino]-1-oxo-2-buten-l-
yl} amino)-7-cyclopropylmethoxy-quinazoline, 4-[(3 -chloro-4-
fluorophenyl)amino]-6- {[4-
(N,N-dimethylamino)-1-oxo-2-buten- I -yl] amino } -7-((R)-tetrahydrofuran-3 -
yloxy)-
quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6- {[4-(N,N-dimethylamino)-1-
oxo-2-
buten-l-yl]amino}-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 4-[(3-chloro-4-
fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-1-oxo-2-buten-
l -
yl } amino)-7-cyclopentyloxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6-
{ [4-(N-
cyclopropyl-N-methyl-amino)-1-oxo-2-buten- l -yl] amino} -7-cyclopentyloxy-
quinazoline,
4-[(3-chloro-4-fluorophenyl)amino] -6- { [4-(N,N-dimethylamino)-1-oxo-2-buten-
l -
yl]amino}-7-[(R)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-chloro-4-
fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-yl]amino }-7-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6,7-
bis-(2-
methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-7-[3-
(morpholin-4-yl)-
propyloxy]-6-[(vinylcarbonyl)amino]-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-
6-(4-
hydroxy-phenyl)-7H-pyrrolo[2,3-d]pyrimidine, 3-cyano-4-[(3-chloro-4-
fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-yl]amino }-7-
ethoxy-
quinoline, 4- {[3 -chloro-4-(3-fluoro-benzyloxy)-phenyl] amino }-6-(5-{[(2-
methanesulphonyl-ethyl)amino]methyl } -furan-2-yl)quinazoline, 4-[(R)-(1-
phenyl-
ethyl)amino]-6- { [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten- l -yl]
amino) -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-
1-oxo-
2-buten-l-yl]amino) -7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-
chloro-4-
CA 02559699 2006-09-12
WO 2005/102349 39 PCT/EP2005/004073
fluorophenyl)amino]-6-({4-[N,N-bis-(2-methoxy-ethyl)-amino]-1-oxo-2-buten-l-
yl } amino)-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-ethynyl-
phenyl)amino]-6-
{[4-(5,5-dimethyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten-l-yl]amino }-
quinazoline, 4-[(3-
chloro-4-fluoro-phenyl) amino] -6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy] -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-
oxo-
morpholin-4-yl)-ethoxy]-7-[(R)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-
[(3-chloro-
4-fluoro-phenyl)amino] -7-[2-(2,2-di methyl-6-oxo-morpholin-4-yl)-ethoxy] -6-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino] -6- {2-[4-
(2-oxo-morpholin-4-yl)-piperidin-1-yl]-ethoxy} -7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-[l -(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-
yloxy)-
7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-
methanesulphonylamino-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-
4-
fluoro-phenyl)amino] -6-(tetrahydropyran-3-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl)amino] -6- {I - [ (morpholin-4-yl)carbonyl] -piperidin-
4-yloxyl -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-
[(methoxymethyl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl) amino] -6-(piperidin-3 -yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-4-fluoro-
phenyl)amino]-6-[ 1-(2-acetylamino-ethyl)-piperidin-4-yloxy]-7-methoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-ethoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino] -6-((S)-tetrahydrofuran-3 -yloxy)-7-hydroxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
(2-
methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-4-
[ (dimethylamino)sulphonyl amino] -cyclohexan- l -yloxy} -7-methoxy-
quinazoline, 4-[(3-
chl oro-4-fluoro-phenyl) amino] -6- {trans-4- [ (morphol in-4-yl) carbonyl
amino] -cyclohexan-
1-yloxy} -7-methoxy-quinazoline, 4-.[(3-chloro-4-fluoro-phenyl)amino]-6-
{trans-4-
[(morpholin-4-yl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl) amino] -6-(tetrahydropyran-4-yloxy) -7-(2-acetylamino-
ethoxy)-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
(2-
methanesulphonylamino-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6- { 1-
[(piperidin-1-yl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino] -6-(1-aminocarbonylmethyl-piperidin-4-yloxy)-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-
[(tetrahydropyran-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-
CA 02559699 2006-09-12
WO 2005/102349 40 PCT/EP2005/004073
4-fluoro-phenyl)amino]-6-(cis-4- {N-[(morpholin-4-yl)carbonyl] -N-methyl-amino
} -
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-(cis-
4- {N-[(morpholin-4-yl)sulphonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-
ethanesulphonylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-(1-
methanesulphonyl-piperidin-4-yloxy)-7-ethoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-(2-methoxy-ethoxy)-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-
piperidin-4-
yloxy]-7-(2-methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-
(cis-4-
acetylamino-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-ethynyl-
phenyl)amino]-6-
[ 1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-methoxy-quinazoline, 4-[(3-
ethynyl-
phenyl)amino]-6-(tetrahydropyran-4-yloxy]-7-methoxy-quinazoline, 4-[(3-chloro-
4-fluoro-
phenyl)amino] -6-(cis-4- {N-[(piperi din- l -yl)carbonyl] -N-methyl-amino } -
cyclohexan- l -
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-
[(4-
methyl-piperazin-1-yl)carbonyl]-N-methyl-amino } -cyclohexan- l -yloxy)-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl) amino] -6- {I - [2-(2-oxopyrrolidin- l -yl)ethyl]-piperidin-4-yloxy} -
7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(morpholin-4-
yl)carbonyl]-
piperidin-4-yloxy} -7-(2-methoxy-ethoxy)-quinazoline, 4- [(3-ethynyl-
phenyl)amino] -6-(1-
acetyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-
(1-
methyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-
(1-
methanesulphonyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7(2-methoxy-ethoxy)-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl)amino]-6-(1-isopropyloxycarbonyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methylamino-
cyclohexan-l -
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[N-
(2-
methoxy-acetyl)-N-methyl-amino]-cyclohexan-l-yloxy}-7-methoxy-quinazoline, 4-
[(3-
ethynyl-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-
ethynyl-
phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-piperidin-4-yloxy]-7-methoxy-
quinazoline, 4-[(3-
ethynyl-phenyl)amino]-6- { 1-[(morpholin-4-yl)carbonyl]-piperidin-4-yloxy} -7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(cis-2,6-dimethyl-
morpholin-4-
yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino] -6- { 1-[(2-methyl-morpholin-4-yl)carbonyl]-piperidin-4-yloxy} -
7-methoxy-
quinazoline, 4- [(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(S,S)-(2-oxa-5-aza-
CA 02559699 2006-09-12
WO 2005/102349 41 PCT/EP2005/004073
bicyclo[2.2.1 ]hept-5-yl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl) amino] -6- 11 - [ (N-methyl-N-2-methoxyethyl-
amino)carbonylI -
piperidin-4-yloxyl-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino] -
6-(1-
ethyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4- [ (3 -chloro-4-fluoro-
phenyl) amino] -6-
{ 1-[(2-methoxyethyl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline, 4-
[(3-chloro-4-
fluoro-phenyl)amino]-6- { 1-[(3-methoxypropyl-amino)-carbonyl]-piperidin-4-
yloxy} -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-
methanesulphonyl-
N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino] -6- [ ci s-4-(N-acetyl-N-methyl-amino)-cyclohexan-1-yloxy] -7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methylamino-
cyclohexan-l-
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-
(N-
methanesulphonyl-N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl) amino] -6-(trans-4-dimethyl amino-cyclohexan-l-yl oxy)-
7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4- {N-
[(morpholin-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-
4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-ethoxy] -7-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4- [(3 -chloro-4-fluoro-phenyl)
amino] -6-(1-
methanesulphonyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino] -6-(1-cyano-piperidin-4-yloxy)-7-methoxy-quinazoline, cetuximab,
trastuzumab, ABX-EGF and Mab ICR-62, optionally in the form of the racemates,
enantiomers or diastereomers thereof, optionally in the form of the
pharmacologically
acceptable acid addition salts thereof, the solvates and/or hydrates thereof.
In such medicament combinations the EGFR-inhibitor 2e is preferably selected
from
among the 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-
buten-l-
yl]amino}-7-cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-
6-{[4-
(N,N-dethylamino)-1-oxo-2-buten-l-yl]amino) -7-cyclopropylmethoxy-quinazoline,
4-[(3-
chloro-4-fluorophenyl)amino] -6-{ [4-(N,N-dimethylamino)-1-oxo-2-buten- l -yl]
amino } -7-
cyclopropylmethoxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-{[4-(morpholin-
4-yl)-
1-oxo-2-buten-l-yl]amino }-7-cyclopentyloxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl) amino] -6- { [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-I-oxo-2-buten- l -
yl] amino) -7-
cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-
6-
methyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten- l -yl] amino) -7-[(S)-
(tetrahydrofuran-3-
yl)oxy]-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { [4-((R)-2-
methoxymethyl-6-
oxo-morpholin-4-yl)-I-oxo-2-buten-l-yl]amino}-7-cyclopropylmethoxy-
quinazoline, 4-
CA 02559699 2006-09-12
WO 2005/102349 42 PCT/EP2005/004073
[(3-chloro-4-fluoro-phenyl)amino] -6-[2-((S)-6-methyl-2-oxo-morpholin-4-yl)-
ethoxy] -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N-(2-methoxy-
ethyl)-N-
methyl-amino]-1-oxo-2-buten-1-yl } amino)-7-cyclopropylmethoxy-quinazoline, 4-
[(3-
chloro-4-fluorophenyl)amino] -6- { [4-(N,N-dimethylamino)-1-oxo-2-buten- l -
yl] amino) -7-
cyclopentyloxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-{[4-(N,N-bis-(2-
methoxy-
ethyl)-amino)-1-oxo-2-buten-l-yl]amino}-7-cyclopropylmethoxy-quinazoline, 4-
[(R)-(1-
phenyl-ethyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-ethyl-amino]-1-oxo-2-buten- l
-
yl } amino)-7-cyclopropylmethoxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-
({4-[N-(2-
methoxy-ethyl)-N-methyl-amino]-1-oxo-2-buten- l -yl } amino)-7-
cyclopropylmethoxy-
quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(tetrahydropyran-4-yl)-N-
methyl-
amino]-1-oxo-2-buten- l -yl } amino)-7-cyclopropylmethoxy-quinazoline, 4-[(3-
chloro-4-
fluorophenyl)amino] -6- { [4-(N,N-dimethylamino)-1-oxo-2-buten- l -yl] amino }
-7-((R)-
tetrahydrofuran-3-yloxy)-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6- {
[4-(N,N-
dimethylamino)-1-oxo-2-buten-l -yl]amino ) -7-((S)-tetrahydrofuran-3 -yloxy)-
quinazoline,
4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-
1-oxo-
2-buten-1-yl } amino)-7-cyclopentyloxy-quinazoline, 4-[(3-chloro-4-
fluorophenyl)amino]-
6- { [4-(N-cyclopropyl-N-methyl-amino)-1-oxo-2-buten- l -yl] amino } -7-
cyclopentyloxy-
quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-
oxo-2-
buten-l-yl]amino}-7-[(R)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-
chloro-4-
fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-yl]amino) -7-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6,7-
bis-(2-
methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-7-[3-
(morpholin-4-yl)-
propyloxy]-6-[(vinylcarbonyl)amino]-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-
6-(4-
hydroxy-phenyl)-7H-pyrrolo[2,3-d]pyrimidine, 3-cyano-4-[(3-chloro-4-
fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-yl]amino }-7-
ethoxy-
quinoline, 4-{[3-chloro-4-(3-fluoro-benzyloxy)-phenyl] amino) -6-(5-{[(2-
methanesulphonyl-ethyl)amino]methyl } -furan-2-yl)quinazoline, 4-[(R)-(1-
phenyl-
ethyl) amino] -6- {[4-((R)-6-methyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten-l-
yl]amino }-7-
methoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6- { [4-(morpholin-4-
yl)-1-oxo-
2-buten-l -yl]amino }-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-
chloro-4-
fluorophenyl)amino]-6-({4-[N,N-bis-(2-methoxy-ethyl)-amino]-1-oxo-2-buten- l -
yl } amino)-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-ethynyl-
phenyl)amino]-6-
{[4-(5,5-dimethyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten-l-yl]amino}-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl)amino] -6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy] -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-
oxo-
CA 02559699 2006-09-12
WO 2005/102349 43 PCT/EP2005/004073
morpholin-4-yl)-ethoxy]-7-[(R)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-
[(3-chloro-
4-fluoro-phenyl)amino]-7-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-ethoxy] -6-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4- [(3 -chloro-4- fluoro-phenyl)
amino] -6-{2-[4-
(2-oxo-morpholin-4-yl)-piperidin-1-yl]-ethoxy}-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-
yloxy)-
7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-
methanesulphonylamino-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-
4-
fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl)amino] -6- {I - [(morpholin-4-yl)carbonyl] -piperidin-4-
yloxyl -7 -
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-
[(methoxymethyl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-(piperidin-3-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-
4-fluoro-
phenyl)amino]-6-[ 1-(2-acetylamino-ethyl)-piperidin-4-yloxy]-7-methoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-ethoxy-
quinazoline, 4-
[(3 -chloro-4-fluoro-phenyl)amino] -6-((S)-tetrahydrofuran-3-yloxy)-7 -hydroxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
(2-
methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-4-
[(dimethylamino)sulphonylamino]-cyclohexan- l -yloxy} -7-methoxy-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl)amino] -6- {trans-4-[(morpholin-4-yl)carbonyl amino] -
cyclohexan-
1-yloxy} -7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-
4-
[(morpholin-4-yl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl) amino] -6-(tetrahydropyran-4-yloxy)-7-(2-acetylamino-
ethoxy)-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
(2-
methanesulphonylamino-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6- {I -
[(piperidin-1-yl)carbonyl]-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-(1-aminocarbonylmethyl-piperidin-4-yloxy)-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-
[(tetrahydropyran-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-
4-fluoro-phenyl)amino]-6-(cis-4- {N-[(morpholin-4-yl)carbonyl] -N-methyl-amino
} -
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-(cis-
4- {N-[(morpholin-4-yl)sulphonyl]-N-methyl-amino } -cyclohexan-1-yloxy)-7-
methoxy-
quinazoline, 4- [(3 -chloro-4-fluoro-phenyl)amino] -6-(trans-4-ethanesulphonyl
amino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-(1-
CA 02559699 2006-09-12
WO 2005/102349 44 PCT/EP2005/004073
methanesulphonyl-piperidin-4-yloxy)-7-ethoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-(2-methoxy-ethoxy)-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-
piperidin-4-
yloxy]-7-(2-methoxy-ethoxy)-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-
(cis-4-
acetylamino-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4- [ (3 -ethynyl-
phenyl) amino] -6-
[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-methoxy-quinazoline, 4-[(3-
ethynyl-
phenyl)amino]-6-(tetrahydropyran-4-yloxy]-7-methoxy-quinazoline, 4-[(3-chloro-
4-fluoro-
phenyl)amino]-6-(cis-4- {N-[(piperidin-1-yl)carbonyl]-N-methyl-amino } -
cyclohexan- l -
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-
[(4-
methyl-piperazin-1-yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6- { 1-[2-(2-oxopyrrolidin-1-yl)ethyl]-piperidin-4-yloxy} -7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(morpholin-4-
yl)carbonyl]-
piperidin-4-yloxy} -7-(2-methoxy-ethoxy)-quinazoline, 4-[(3-ethynyl-
phenyl)amino]-6-(1-
acetyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-
(1-
methyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-
(1-
methanesulphonyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino] -6-(1-methyl-piperidin-4-yloxy)-7(2-methoxy-ethoxy)-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl)amino]-6-(1-isopropyloxycarbonyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methylamino-
cyclohexan-1-
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[N-
(2-
methoxy-acetyl)-N-methyl-amino]-cyclohexan-l-yloxy}-7-methoxy-quinazoline, 4-
[(3-
ethynyl-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-
ethynyl-
phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-piperidin-4-yloxy]-7-methoxy-
quinazoline, 4-[(3-
ethynyl-phenyl)amino]-6- { 1-[(morpholin-4-yl)carbonyl]-piperidin-4-yloxy}-7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(cis-2,6-dimethyl-
morpholin-4-
yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6- { 1-[(2-methyl-morpholin-4-yl)carbonyl]-piperidin-4-yloxy} -7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(S,S)-(2-oxa-5-aza-
bicyclo[2.2.1 ]hept-5-yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl)amino]-6- {I -[(N-methyl-N-2-methoxyethyl-
amino)carbonyl]-
piperidin-4-yloxyl-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-(1-
ethyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4- [(3 -chloro-4-fluoro-
phenyl) amino] -6-
{ 1-[(2-methoxyethyl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline, 4-
[(3-chloro-4-
CA 02559699 2006-09-12
WO 2005/102349 45 PCT/EP2005/004073
fluoro-phenyl)amino]-6- {I -[(3-methoxypropyl-amino)-carbonyl]-piperidin-4-
yloxyI -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-
methanesulphonyl-
N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino] -6-[cis-4-(N-acetyl-N-methyl-amino)-cyclohexan-1-yloxy] -7-
methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methylamino-
cyclohexan-l -
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-
(N-
methanesulphonyl-N-methyl-amino)-cyclohexan-l-yloxy]-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl)amino]-6-(trans-4-dimethylamino-cyclohexan-1-yloxy)-7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4- {N-
[(morpholin-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-
4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-ethoxy] -7-
[(S)-
(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-(1-
methanesulphonyl-piperidin-4-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-methoxy-quinazoline, and
cetuximab,
optionally in the form of the racemates, enantiomers or diastereomers thereof,
optionally in
the form of the pharmacologically acceptable acid addition salts thereof, the
solvates
and/or hydrates thereof.
Particularly preferably, the EGFR-inhibitors 2a used within the scope of the
medicament
combinations according to the invention are selected from the group comprising
4-[(3-
chloro-4-fluorophenyl)amino]-6- { [4-(morpholin-4-yl)-1-oxo-2-buten- l -yl]
amino } -7-
cyclopropylmethoxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino] -6- { [4-
(morpholin-4-yl)-
1-oxo-2-buten-l-yl]amino}-7-cyclopentyloxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl) amino] -6- { [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-1-oxo-2-buten- l -
yl] amino } -7-
[(S)-(tetrahydrofuran-3-yl)oxy]-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-[2-
((S)-6-methyl-2-oxo-morpholin-4-yl)-ethoxy]-7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-1-oxo-2-buten-l
-
yl } amino)-7-cyclopropylmethoxy-quinazoline, 4-[(R)-(1-phenyl-ethyl)amino]-6-
({4-[N-
(tetrahydropyran-4-yl)-N-methyl-amino]-1-oxo-2-buten-1-yl } amino)-7-
cyclopropylmethoxy-quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N-(2-
methoxy-ethyl)-N-methyl-amino]-1-oxo-2-buten- l -yl } amino)-7-cyclopentyloxy-
quinazoline, 4-[(3-chloro-4-fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-
oxo-2-
buten-l-yl]amino}-7-[(R)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-
ethynyl-
phenyl)amino]-6,7-bis-(2-methoxy-ethoxy)-quinazoline, 4-[(R)-(1-phenyl-
ethyl)amino]-6-
(4-hydroxy-phenyl)-7H-pyrrolo[2,3-d]pyrimidine, 3-cyano-4-[(3-chloro-4-
CA 02559699 2006-09-12
WO 2005/102349 46 PCT/EP2005/004073
fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-oxo-2-buten- l -yl] amino } -
7-ethoxy-
quinoline, 4-[(R)-(1-phenyl-ethyl)amino]-6- { [4-((R)-6-methyl-2-oxo-morpholin-
4-yl)-1-
oxo-2-buten-l-yl]amino }-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluorophenyl)amino]-6-
{ [4-(morpholin-4-yl)-1-oxo-2-buten- l -yl] amino } -7-[(tetrahydrofuran-2-
yl)methoxy]-
quinazoline, 4-[(3-ethynyl-phenyl)amino]-6- { [4-(5,5-dimethyl-2-oxo-morpholin-
4-yl)-1-
oxo-2-buten-l-yl]amino }-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{2-
[4-(2-
oxo-morpholin-4-yl)-piperidin- l -yl]-ethoxy} -7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-yloxy)-7-methoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methanesulphonylamino-cyclohexan-
l -
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-
(tetrahydropyran-3-
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {I -
[(morpholin-4-
yl)carbonyl]-piperidin-4-yloxyl-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-(piperidin-3-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-[ 1-(2-acetylamino-ethyl)-piperidin-4-yloxy]-7-methoxy-
quinazoline, 4-
[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-ethoxy-
quinazoline, 4-
[(3 -chloro-4-fluoro-phenyl)amino] -6- {trans-4- [(morpholin-4-
yl)carbonylamino] -
cyclohexan-l-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6- { 1-
[(piperidin-1-yl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline, 4-[(3-
chloro-4-
fluoro-phenyl)amino] -6-(cis-4- {N-[(morpholin-4-yl)carbonyl]-N-methyl-amino }
-
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-
(trans-4-ethanesulphonylamino-cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-
chloro-
4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-(2-methoxy-
ethoxy)-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-
piperidin-4-
yloxy]-7-(2-methoxy-ethoxy)-quinazoline, 4- [(3 -ethynyl-phenyl) amino] -6-
(tetrahydropyran-4-yloxy]-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-
(cis-4- {N-[(piperidin- l -yl)carbonyl] -N-methyl- amino } -cyclohexan- l -
yloxy)-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl) amino] -6- {I - [2-(2-oxopyrrolidin- I -yl)ethyl]-piperidin-4-yloxy}-7-
methoxy-
quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline, 4-[(3-ethynyl-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-
yloxy)-7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-
4-
yloxy)-7(2-methoxy-ethoxy)-quinazoline, 4- [(3-ethynyl-phenyl)amino]-6- { 1-
[(morpholin-
4-yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-
CA 02559699 2006-09-12
WO 2005/102349 47 PCT/EP2005/004073
phenyl)amino]-6- {I -[(N-methyl-N-2-methoxyethyl-amino)carbonyl]-piperidin-4-
yloxyI -7-
methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-ethyl-piperidin-
4-yloxy)-
7-methoxy-quinazoline, 4- [(3 -chloro-4-fluoro-phenyl) amino] -6- [ cis-4-(N-
methanesulphonyl-N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline, 4-
[(3-
chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-acetyl-N-methyl-amino)-cyclohexan-1-
yloxy]-
7-methoxy-quinazoline, 4- [(3 -chloro-4-fluoro-phenyl) amino] -6-(trans-4-
methyl amino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-
6-
[trans-4-(N-methanesulphonyl-N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-dimethylamino-
cyclohexan-l-
yloxy)-7-methoxy-quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-
{N-
[(morpholin-4-yl)carbonyl]-N-methyl-amino } -cyclohexan-1-yloxy)-7-methoxy-
quinazoline, 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-
morpholin-4-
yl)-ethoxy]-7-[(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline, 4-[(3-chloro-4-
fluoro-
phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-methoxy-quinazoline,
4-[(3-
chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-methoxy-
quinazoline,
and 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(2-methoxyethyl)carbonyl]-
piperidin-4-
yloxy}-7-methoxy-quinazoline, optionally in the form of the racemates,
enantiomers or
diastereomers thereof, optionally in the form of the pharmacologically
acceptable acid
addition salts thereof, the solvates and/or hydrates thereof.
Particularly preferred medicament combinations according to the invention
contain as
EGFR-inhibitors 2e those compounds which are selected from the group
comprising
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
amino}-7-cyclopropylmethoxy-quinazoline 2e.1 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-6-methyl-2-oxo-morpholin-4-
yl)-1-
oxo-2-buten-l-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-quinazoline 2e.2 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-((S)-6-methyl-2-oxo-morpholin-4-yl)-
ethoxy]-7-methoxy-quinazoline 2e.3 ,
- 4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl}amino)-7-cyclopropylmethoxy-quinazoline 2e.4 ,
- 4-[(3-ethynyl-phenyl)amino]-6,7-bis-(2-methoxy-ethoxy)-quinazoline 2e.5 ,
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
amino}-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline 2e.6 ,
- 4-[(3-ethynyl-phenyl)amino]-6-{[4-(5,5-dimethyl-2-oxo-morpholin-4-yl)-1-oxo-
2-
buten-l-yl]amino }-quinazoline 2e.7),
CA 02559699 2006-09-12
WO 2005/102349 48 PCT/EP2005/004073
- 4-[(3-chloro-4-fluoro-phenyl)amino] -6-(trans-4-methanesulphonylamino-
cyclohexan-
1-yloxy)-7-methoxy-quinazoline 2e.8 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-methoxy-
quinazoline 2e.9 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {I -[(morpholin-4-yl)carbonyl]-
piperidin-4-yl-
oxyl-7-methoxy-quinazoline 2e.10 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[2-(2-oxopyrrolidin-1-yl)ethyl]-
piperidin-4-
yloxy}-7-methoxy-quinazoline 2e.11 ,
- 4-[(3-ethynyl-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
2e.12 ,
- 4-[(3-ethynyl-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
2e.13 ,
- 4-[(3-ethynyl-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline 2e.14 ,
- 4-[(3-ethynyl-phenyl)amino]-6- { 1-[(morpholin-4-yl)carbonyl]-piperidin-4-
yloxy}-7-
methoxy-quinazoline 2e.15 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {I -[(2-methoxyethyl)carbonyl]-
piperidin-4-
yloxyl-7-methoxy-quinazoline 2e.16 ,
- 4- [ (3 -chloro-4-fluoro-phenyl) amino] -6- [ cis-4-(N -methanesulphonyl-N-
methyl-
amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline 2e.17 ,
- 4- [(3 -chloro-4-fluoro-phenyl) amino] -6- [ cis-4-(N-acetyl-N-methyl -
amino)-cyclohexan-
1-yloxy]-7-methoxy-quinazoline 2e.18 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino] -6-(trans-4-methylamino-cyclohexan-l-
yloxy)-7-
methoxy-quinazoline (2e.19),
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(N-methanesulphonyl-N-methyl-
amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline (2e.20),
- 4-[(3-chloro-4-fluoro-phenyl)amino] -6-(trans-4-dimethylamino-cyclohexan-l-
yloxy)-
7-methoxy-quinazoline 2e.21 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4- {N-[(morpholin-4-
yl)carbonyl]-N-
methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline 2e.22 ,
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy]-7-[(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline (2e.23),
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-
methoxy-quinazoline 2e.24) and
CA 02559699 2006-09-12
WO 2005/102349 49 PCT/EP2005/004073
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-methoxy-
quinazoline 2e.25),
optionally in the form of the racemates, enantiomers or diastereomers thereof,
optionally in
the form of the pharmacologically acceptable acid addition salts thereof, the
solvates
and/or hydrates thereof.
By the acid addition salts with pharmacologically acceptable acids which the
compounds
2e may possibly be capable of forming are meant for example salts selected
from the group
comprising the hydrochloride, hydrobromide, hydroiodide, hydrosulphate,
hydrophosphate, hydromethanesulphonate, hydronitrate, hydromaleate,
hydroacetate,
hydrobenzoate, hydrocitrate, hydrofumarate, hydrotartrate, hydrooxalate,
hydrosuccinate,
hydrobenzoate and hydro-p-toluenesulphonate, preferably the hydrochloride,
hydrobromide, hydrosulphate, hydrophosphate, hydrofumarate and
hydromethanesulphonate.
Examples of novel preferred medicament combinations of preferred compounds of
formula
1 with the above-mentioned EGFR-inhibitors 2e are combinations containing the
compounds 1.1 and 2e.1; 1.1 and 2e.2; 1.1 and 2e.3; 1.1 and 2e.4; 1.1 and
2e.5; 1.1 and
2e.6; 1.1 and 2e.7; 1.1 and 2e.8; 1.1 and 2e.9; 1.1 and 2e.10; 1.1 and 2e.11;
1.1 and 2e.12;
1.1 and 2e.13; 1.1 and 2e.14; 1.1 and 2e.15; 1.1 and 2e.16; 1_1 and 2e.17; 1_1
and 2e.18;
1.1 and 2e.19; 1.1 and 2e.20; 1.1 and 2e.21; 1.1 and 2e.22; 1.1 and 2e.23; 1.1
and 2e.24;
1.1 and 2e.25; 1.2 and 2e.1; 1.2 and 2e.2; 1.2 and 2e.3; 1.2 and 2e.4; 1.2 and
2e.5; 1.2 and
2e.6; 1.2 and 2e.7; 1.2 and 2e.8; 1.2 and 2e.9; 1.2 and 2e.10; 1_2 and 2e.11;
1.2 and 2e.12;
1.2 and 2e.13; 1.2 and 2e.14; 1.2 and 2e.15; 1.2 and 2e.16; 1_2 and 2e.17; 1_2
and 2e.18;
1.2 and 2e1.9; 1.2 and 2e.20; 1.2 and 2e.21; 1.2 and 2e.22; 1_2 and 2e.23; 1_2
and 2e.24;
1.2 and 2e.25; 1.3 and 2e.1; 1.3 and 2e.2; 1.3 and 2e.3; 1_3 and 2e.4; 1.3 and
2e.5; 1.3 and
2e.6; 1.3 and 2e.7; 1.3 and 2e.8; 1.3 and 2e.9; 1.3 and 2e.10; 1_3 and 2e.11;
1.3 and 2e.12;
1.3 and 2e.13; 1.3 and 2e.14; 1.3 and 2e.15; 1.3 and 2e.16; 1.3 and 2e.17; 1.3
and 2e.18;
1.3 and 2e.19; 1.3 and 2e.20; 1.3 and 2e.21; 1.3 and 2e.22; 1.3 and 2e.23; 1.3
and 2e.24;
1.3 and 2e.25; 1.4 and 2e.1; 1.4 and 2e.2; 1.4 and 2e.3; 1.4 and 2e.4; 1.4 and
2e.5; 1.4 and
2e.6; 1.4 and 2e.7; 1.4 and 2e.8; 1.4 and 2e.9. 1.4 and 2e.10. 1.4 and 2e.11;
1.4 and 2e.12;
1.4 and 2e.13; 1.4 and 2e.14; 1.4 and 2e.15; 1.4 and 2e.16; 1.4 and 2e.17; 1_4
and 2e.18;
1.4 and 2e.19; 1.4 and 2e.20; 1.4 and 2e.21; 1.4 and 2e.22; 1.4 and 2e.23; 1.4
and 2e.24;
1.4 and 2e.25; 1.5 and 2e.1; 1.5 and 2e.2; 1.5 and 2e.3; 1.5 and 2e.4; 1_5 and
2e.5; 1.5 and
2e.6; 1.5 and 2e.7; 1.5 and 2e.8; 1.5 and 2e.9; 1.5 and 2e.10; 1_5 and 2e.11;
1_5 and 2e.12;
CA 02559699 2006-09-12
WO 2005/102349 50 PCT/EP2005/004073
1.5 and 2e.13; 1.5 and 2e.14; 1.5 and 2e.15; 1.5 and 2e.16; 1.5 and 2e.17; 1.5
and 2e.18;
1.5 and 2e.19. 1.5 and 2e.20, 1.5 and 2e.21; 1.5 and 2e.22; 1.5 and 2e.23; 1.5
and 2e.24;
1.5 and 2e.25; 1.6 and 2e.1. 1.6 and 2e.2, 1.6 and 2e.3, 1.6 and 2e.4; 1.6 and
2e.5; 1.6 and
2e.6; 1.6 and 2e.7; 1.6 and 2e.8; 1.6 and 2e.9; 1_6 and 2e.10; 1.6 and 2e.11;
1_6 and 2e.12;
1.6 and 2e.13; 1.6 and 2e.14; 1_6 and 2e.15; 1.6 and 2e.16; 1.6 and 2e.17; 1.6
and 2e.18;
1.6 and 2e.19; 1.6 and 2e.20; 1.6 and 2e.21; 1.6 and 2e.22; 1_6 and 2e.23; 1.6
and 2e.24;
1.6 and 2e.25; 1.7 and 2e.1; 1.7 and 2e.2; 1.7 and 2e.3; 1.7 and 2e.4; 1.7 and
2e.5; 1_7 and
2e.6; 1.7 and 2e.7; 1.7 and 2e.8; 1_7 and 2e.9; 1_7 and 2e.10; 1_7 and 2e.11;
1_7 and 2e.12;
1.7 and 2e.13; 1.7 and 2e.14. 1.7 and 2e.15; 1.7 and 2e.16; 1.7 and 2e.17; 1.7
and 2e.18;
1.7 and 2e.19; 1.7 and 2e.20; 1.7 and 2e.21; 1.7 and 2e.22; 1_7 and 2e.23; 1_7
and 2e.24;
1.7 and 2e.25; 1.12 and 2e.1; 1.12 and 2e.2; 1.12 and 2e.3; 1.12 and 2e.4;
1.12 and 2e.5;
1.12 and 2e.6; 1.12 and 2e.7; 1.12 and 2e.8; 1.12 and 2e.9; 1.12 and 2e.10;
1.12 and 2e.11;
1.12 and 2e.12; 1.12 and 2e.13; 1.12 and 2e.14-.1.12 and 2e.15; 1.12 and
2e.16; 1.12 and
2e.17; 1.12 and 2e.18; 1.12 and 2e.19; 1.12 and 2e.20; 1.12 and 2e.21; 1.12
and 2e.22; 1.12
and 2e.23; 1.12 and 2e.24; 1.12 and 2e.25; 1.14 and 2e.1; 1.14 and 2e.2; 1.14
and 2e.3;
1.14 and 2e.4; 1.14 and 2e.5; 1.14 and 2e.6; 1.14 and 2e.7; 1.14 and 2e.8;
1.14 and 2e.9;
1.14 and 2e.10; 1.14 and 2e.11; 1.14 and 2e.12; 1.14 and 2e.13; 1.14 and
2e.14; 1.14 and
2e.15; 1.14 and 2e.16; 1.14 and 2e.17; 1.14 and 2e.18; 1.14 and 2e.19; 1.14
and 2e.20; 1.14
and 2e.21; 1.14 and 2e.22; 1.14 and 2e.23; 1.14 and 2e.24; 1.14 and 2e.25;
1.15 and 2e.1;
1.15 and 2e.2; 1.15 and 2e.3; 1.15 and 2e.4; 1.15 and 2e.5; 1.15 and 2e.6;
1.15 and 2e.7;
1.15 and 2e.8; 1.15 and 2e.9; 1.15 and 2e.10; 1.15 and 2e.11; 1.15 and 2e.12;
1.15 and
2e.13; 1.15 and 2e.14; 1.15 and 2e.15; 1.15 and 2e.16, 1.15 and 2e.17; 1.15
and 2e.18; 1.15
and 2e.19; 1.15 and 2e.20; 1.15 and 2e.21; 1.15 and 2e.22; 1.15 and 2e.23;
1.15 and 2e.24
or 1.15 and 2e.25, in each case optionally in the form of the racemates,
enantiomers or
diastereomers thereof and optionally in the form of the pharmacologically
acceptable acid
addition salts, solvates and/or hydrates thereof.
Of the above-mentioned combinations, preferred ones according to the invention
are those
which contain as compound of formula 1 one of the compounds 1_1, 1_5, 1_6, or
1.12 ,
while those combinations which contain one of the compounds 1.1 or 1.12 are
particularly
important according to the invention. Of the above-mentioned combinations also
preferred
according to the invention are those which contain as the compound 2e one of
the
compounds 2e.1, 2e.2, 2e.3, 2e.4, 2e.10, 2e.11, 2e.14, 2e.16, 2e.17, 2e.18,
2e.19, 2e.20,
2e.21, 2e.22, 2e.23, 2e.24 or 2e.25 , while those combinations which contain
one of the
compounds 2e.2, 2e.3 or 2e.4 are particularly important according to the
invention.
CA 02559699 2006-09-12
WO 2005/102349 51 PCT/EP2005/004073
The novel medicament combinations comprising compounds of formula 1 with at
least one
other active substance 2 are not restricted to binary combinations of active
substances. The
combinations mentioned above, partly by way of example, which contain in
addition to a
compound of formula 1 one other active substance 2 , may also contain a third
or fourth,
preferably a third active substance, which is also selected from the above-
mentioned group
of anticholinergics (La), PDE-IV inhibitors 2b), steroids (Lc), LTD4-
antagonists (1d) and
EGFR-inhibitors (Le) .
Particularly preferred combinations which contain two other active substances
in addition
to a compound of formula 1 are selected from the active substance
combinationslisted
below. These are medicament combinations which may contain, for example :
A) a compound of formula 1, an anticholinergic (1a), a PDEIV inhibitor (Lb);
B) a compound of formula 1, an anticholinergic (2a), a steroid (Lc);
C) a compound of formula 1, an anticholinergic (La), an LTD4 antagonist (Id D;
D) a compound of formula 1, an anticholinergic 2a , an EGFR inhibitor (Le);
E) a compound of formula 1, a PDEIV inhibitor 2b ), a steroi(2c);
F) a compound of formula a PDEIV inhibitor (2b), an LTD4 antagonist (L d);
G) a compound of formula 1, a PDEIV inhibitor (2b), an EGFR inhibitor (Le);
H) a compound of formula 1, a steroid (2,e), an LTD4 antagonist (L d);
I) a compound of formula 1, a steroid (2j e, an EGFR inhibitor (Le);
J) a compound of formula 1, an LTD4 antagonist (L d), an EGFR inhibitor (Le).
Particularly preferred examples of medicament combinations of the above-
mentioned
group A are selected from the group comprising the following combinations:
compounds 1.1 and 2a.1 and 2b.2; 1.1 and 2a.1 and 2b.4; 1.1 and 2a.1 and
2b.11; 1.1 and
2a.1 and 2b.19; 1.1 and 2a.9.1 and 2b.2; 1.1 and 2a.9.1 and 2b.4; 1.1 and
2a.9.1 and
2b.11; 1.1 and 2a.9.1 and 2b.19; 1.1 and 2a.9.2 and 2b.2; 1.1 and 2a.9.2 and
2b.4; 1.1 and
2a.9.2 and 2b.11; 1.1 and 2a.9.2 and 2b.19; 1.1 and 2a.10.1 and 2b.2; 1.1 and
2a.10.1 and
2b.4; 1.1 and 2a.10.1 and 2b.11; 1.1 and 2a.10.1 and 2b.19; 1.1 and 2a.10.2
and 2b.2; 1.1
and 2a.10.2 and 2b.4; 1.1 and 2a.10.2 and 2b.11; 1.1 and 2a.10.2 and 2b.19;
1.1 and
2a.11.1 and 2b.2; 1.1 and 2a.11.1 and 2b.4; 1.1 and 2a.11.1 and 2b.11; 1.1 and
2a.11.1 and
2b.19; 1.1 and 2a.11.6 and 2b.2; 1.1 and 2a.11.6 and 2b.4; 1.1 and 2a.11.6 and
2b.11; 1.1
and 2a.11.6 and 2b.19; 1.12 and 2a.1 and 2b.2; 1.12 and 2a.1 and 2b.4; 1.12
and 2a.1 and
2b.11; 1.12 and 2a.1 and 2b.19; 1.12 and 2a.9.1 and 2b.2; 1.12 and 2a.9.1 and
2b.4; 1.12
CA 02559699 2006-09-12
WO 2005/102349 52 PCT/EP2005/004073
and 2a.9.1 and 2b.11; 1.12 and 2a.9.1 and 2b.19; 1.12 and 2a.9.2 and 2b.2;
1.12 and 2a.9.2
and 2b.4; 1.12 and 2a.9.2 and 2b.11; 1.12 and 2a.9.2 and 2b.19; 1.12 and
2a.10.1 and
2b.2; 1.12 and 2a.10.1 and 2b.4; 1.12 and 2a.10.1 and 2b.11; 1.12 and 2a.10.1
and 2b.19;
1.12 and 2a.10.2 and 2b.2; 1.12 and 2a.10.2 and 2b.4; 1.12 and 2a.10.2 and
2b.11; 1.12
and 2a.10.2 and 2b.19; 1.12 and 2a.11.12 and 2b.2; 1.12 and 2a.11.12 and 2b.4;
1.12 and
2a.11.12 and 2b.11; 1.12 and 2a.11.12 and 2b.19; 1.12 and 2a.11.6 and 2b.2;
1.12 and
2a.11.6 and 2b.4; 1.12 and 2a.11.6 and 2b.11; 1.12 and 2a.11.6 and 2b.19, in
each case
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Particularly preferred examples of particularly preferred medicament
combinations of the
above-mentioned group B according to the invention are selected from the group
comprising the following combinations:
compounds 1.1 and 2a.1 and 2c.8; 1.1 and 2a.1 and 2c.9; 1_1 and 2a.1 and
2c.10; 1.1 and
2a.1 and 2c.11; 1.1 and 2a.1 and 2c.17; 1.1 and 2a.9.1 and 2c.8; 1_1 and
2a.9.1 and 2c9;
1.1 and 2a.9.1 and 2c.10; 1.1 and 2a.9.1 and 2c.11; 1_1 and 2a.9.1 and 2c.17;
1.1 and
2a.9.2 and 2c.8; 1.1 and 2a.9.2 and 2c.9; 1.1 and 2a.9.2 and 2c.10; 1.1 and
2a.9.2 and
2c.11; 1.1 and 2a.9.2 and 2c.17; 1.1 and 2a.10.1 and 2c.8; 1.1 and 2a.10.1 and
2c.9; 1_1
and 2a.10.1 and 2c.10; 1.1 and 2a.10.1 and 2c.11; 1_1 and 2a.10.1 and 2c.17;
1.1 and
2a.10.2 and 2c.8; 1.1 and 2a.10.2 and 2c.9; 1.1 and 2a.10.2 and 2c.10; 1_1 and
2a.10.2 and
2c.11; 1.1 and 2a.10.2 and 2c.17; 1.1 and 2a.11.1 and 2c.8; 1.1 and 2a.11.1
and 2c.9; 1.1
and 2a.11.1 and 2c.10; 1.1 and 2a.11.1 and 2c.11; 1.1 and 2a.11.1 and 2c.17;
1.1 and
2a.11.6 and 2c.8; 1.1 and 2a.11.6 and 2c.9; 1.1 and 2a.11.6 and 2c.10; 1_1 and
2a.11.6 and
2c.11; 1.1 and 2a.11.6 and 2c.17; 1.12 and 2a.1 and 2c.8; 1.12 and 2a.1 and
2c.9; 1.12 and
2a.1 and 2c.10; 1.12 and 2a.1 and 2c.11; 1.12 and 2a.1 and 2c.17; 1.12 and
2a.9.1 and
2c.8; 1.12 and 2a.9.1 and 2c.9; 1.12 and 2a.9.1 and 2c.10; 1.12 and 2a.9.1 and
2c.11; 1.12
and 2a.9.1 and 2c.17; 1.12 and 2a.9.2 and 2c.8; 1.12 and 2a.9.2 and 2c.9; 1.12
and 2a.9.2
and 2c.10; 1.12 and 2a.9.2 and 2c.11; 1.12 and 2a.9.2 and 2c.17; 1.12 and
2a.10.1 and
2c.8; 1.12 and 2a.10.1 and 2c.9; 1.12 and 2a.10.1 and 2c.10; 1.12 and 2a.10.1
and 2c.11-
1.12 and 2a.10.1 and 2c.17; 1.12 and 2a.10.2 and 2c.8; 1.12 and 2a.10.2 and
2c.9; 1.12 and
2a.10.2 and 2c.10; 1.12 and 2a.10.2 and 2c.11; 1.12 and 2a.10.2 and 2c.17;
1.12 and
2a.11.12 and 2c.8; 1.12 and 2a.11.12 and 2c.9; 1.12 and 2a.11.12 and 2c.10;
1.12 and
2a.11.12 and 2c.11; 1.12 and 2a.11.12 and 2c.17; 1.12 and 2a.11.6 and 2c.8;
1.12 and
2a.11.6 and 2c.9; 1.12 and 2a.11.6 and 2c.10; 1.12 and 2a.11.6 and 2c.11; 1.12
and
CA 02559699 2006-09-12
WO 2005/102349 53 PCT/EP2005/004073
2a.11.6 and 2c.17,, in each case optionally in the form of the racemates,
enantiomers or
diastereomers thereof and optionally in the form of the pharmacologically
acceptable acid
addition salts, solvates and/or hydrates thereof.:
Particularly preferred examples of medicament combinations of the above-
mentioned
group C are selected from the group comprising the following combinations:
compounds 1.1 and 2a.1 and 2d.1; 1.1 and 2a.1 and 2d.4; 1.1 and 2a.1 and 2d.5;
1.1 and
2a.1 and 2d.8; 1.1 and 2a.9.1 and 2d.1; 1.1 and 2a.9.1 and 2d.4; 1.1 and
2a.9.1 and 2d.5;
1.1 and 2a.9.1 and 2d.8; 1.1 and 2a.9.2 and 2d.1; 1.1 and 2a.9.2 and 2d.4; 1.1
and 2a.9.2
and 2d.5; 1.1 and 2a.9.2 and 2d.8; 1.1 and 2a.10.1 and 2d.1; 1.1 and 2a.10.1
and 2d.4; 1.1
and 2a.10.1 and 2d.5; 1.1 and 2a.10.1 and 2d.8; 1.1 and 2a.10.2 and 2d.1; 1.1
and 2a.10.2
and 2d.4; 1.1 and 2a.10.2 and 2d.5; 1.1 and 2a.10.2 and 2d.8; 1.1 and 2a.11.1
and 2d.1;
1.1 and 2a.11.1 and 2d.4; 1.1 and 2a.11.1 and 2d.5; 1.1 and 2a.11.1 and 2d.8;
1.1 and
2a.11.6 and 2d.1; 1.1 and 2a.11.6 and 2d.4; 1.1 and 2a.11.6 and 2d.5; 1.1 and
2a.11.6 and
2d.8; 1.12 and 2a.1 and 2d.1; 1.12 and 2a.1 and 2d.4; 1.12 and 2a.1 and 2d.5;
1.12 and
2a.1 and 2d.8; 1.12 and 2a.9.1 and 2d.1; 1.12 and 2a.9.1 and 2d.4; 1.12 and
2a.9.1 and
2d.5; 1.12 and 2a.9.1 and 2d.8; 1.12 and 2a.9.2 and 2d.1; 1.12 and 2a.9.2 and
2d.4; 1.12
and 2a.9.2 and 2d.5; 1.12 and 2a.9.2 and 2d.8; 1.12 and 2a.10.1 and 2d.1; 1.12
and 2a.10.1
and 2d.4; 1.12 and 2a.10.1 and 2d.5; 1.12 and 2a.10.1 and 2d.8; 1.12 and
2a.10.2 and
2d.1; 1.12 and 2a.10.2 and 2d.4; 1.12 and 2a.10.2 and 2d.5; 1.12 and 2a.10.2
and 2d.8;
1.12 and 2a.11.12 and 2d.1; 1.12 and 2a.11.12 and 2d.4; 1.12 and 2a.11.12 and
2d.5; 1.12
and 2a.11.12 and 2d.8; 1.12 and 2a.11.6 and 2d.1; 1.12 and 2a.11.6 and 2d.4;
1.12 and
2a.11.6 and 2d.5; 1.12 and 2a.11.6 and 2d.8, in each case optionally in the
form of the
racemates, enantiomers or diastereomers thereof and optionally in the form of
the
pharmacologically acceptable acid addition salts, solvates and/or hydrates
thereof.:
Particularly preferred examples of medicament combinations of the above-
mentioned
group D are selected from the group comprising the following combinations:
compounds 1.1 and 2a.1 and 2e.2; 1.1 and 2a.1 and 2e.3; 1.1 and 2a.1 and 2e.4;
1.1 and
2a.1 and 2e.10; 1.1 and 2a.9.1 and 2e.2; 1.1 and 2a.9.1 and 2e.3; 1.1 and
2a.9.1 and 2e.4;
1_1 and 2a.9.1 and 2e.10; 1.1 and 2a.9.2 and 2e.2; 1.1 and 2a.9.2 and 2e.3;
1.1 and 2a.9.2
and 2e.4; 1.1 and 2a.9.2 and 2e.10; 1.1 and 2a.10.1 and 2e.2; 1.1 and 2a.10.1
and 2e.3; 1.1
and 2a.10.1 and 2e.4; 1.1 and 2a.10.1 and 2e.10; 1.1 and 2a.10.2 and 2e.2; 1.1
and 2a.10.2
and 2e.3; 1.1 and 2a.10.2 and 2e.4; 1.1 and 2a.10.2 and 2e.10; 1.1 and 2a.11.1
and 2e.2;
1.1 and 2a.11.1 and 2e.3; 1.1 and 2a.11.1 and 2e.4; 1.1 and 2a.11.1 and 2e.10;
1.1 and
CA 02559699 2006-09-12
WO 2005/102349 54 PCT/EP2005/004073
2a.11.6 and 2e.2; 1.1 and 2a.11.6 and 2e.3. 1.1 and 2a.11.6 and 2e.4; 1.1 and
2a.11.6 and
2e.10; 1.12 and 2a.1 and 2e.2; 1.12 and 2a.1 and 2e.3; 1.12 and 2a.1 and 2e.4;
1.12 and
2a.1 and 2e.10; 1.12 and 2a.9.1 and 2e.2; 1.12 and 2a.9.1 and 2e.3; 1.12 and
2a.9.1 and
2e.4; 1.12 and 2a.9.1 and 2e.10; 1.12 and 2a.9.2 and 2e.2; 1.12 and 2a.9.2 and
2e.3; 1.12
and 2a.9.2 and 2e.4; 1.12 and 2a.9.2 and 2e.10; 1.12 and 2a.10.1 and 2e.2;
1.12 and
2a.10.1 and 2e.3; 1.12 and 2a.10.1 and 2e.4; 1.12 and 2a.10.1 and 2e.10; 1.12
and 2a.10.2
and 2e.2; 1.12 and 2a.10.2 and 2e.3; 1.12 and 2a.10.2 and 2e.4; 1.12 and
2a.10.2 and
2e.10; 1.12 and 2a.11.12 and 2e.2; 1.12 and 2a.11.12 and 2e.3; 1.12 and
2a.11.12 and 2e.4;
1.12 and 2a.11.12 and 2e.10; 1.12 and 2a.11.6 and 2e.2; 1.12 and 2a.11.6 and
2e.3; 1.12
and 2a.11.6 and 2e.4; 1.12 and 2a.11.6 and 2e.10, in each case optionally in
the form of the
racemates, enantiomers or diastereomers thereof and optionally in the form of
the
pharmacologically acceptable acid addition salts, solvates and/or hydrates
thereof.:
Particularly preferred examples of medicament combinations of the above-
mentioned
group E are selected from the group comprising the following combinations:
compounds 1.1 and 2c.8 and 2b.2; 1.1 and 2c.8 and 2b.4; 1.1 and 2c.8 and
2b.11; 1.1 and
2c.8 and 2b.19; 1.1 and 2c.9 and 2b.2; 1.1 and 2c.9 and 2b.4; 1.1 and 2c.9 and
2b.11; 1.1
and 2c.9 and 2b.19; 1.1 and 2c.10 and 2b.2; 1.1 and 2c.10 and 2b.4; 1.1 and
2c.10 and
2b.11; 1.1 and 2e.10 and 2b.19; 1.1 and 2c.11 and 2b.2; 1.1 and 2c.11 and
2b.4; 1.1 and
2c.11 and 2b.11; 1.1 and 2c.11 and 2b.19; 1.1 and 2c.17 and 2b.2; 1.1 and
2c.17 and 2b.4;
1.1 and 2c.17 and 2b.11; 1.1 and 2c.17 and 2b.19; 1.12 and 2c.8 and 2b.2; 1.12
and 2c.8
and 2b.4; 1.12 and 2c.8 and 2b.11; 1.12 and 2c.8 and 2b.19; 1.12 and 2c.9 and
2b.2; 1.12
and 2c.9 and 2b.4; 1.12 and 2c.9 and 2b.11; 1.12 and 2c.9 and 2b.19; 1.12 and
2c.10 and
2b.2; 1.12 and 2c.10 and 2b.4; 1.12 and 2c.10 and 2b.11; 1.12 and 2c.10 and
2b.19; 1.12
and 2c.11 and 2b.2; 1.12 and 2c.11 and 2b.4; 1.12 and 2c.11 and 2b.11; 1.12
and 2c.11 and
2b.19, 1.12 and 2c.17 and 2b.2; 1.12 and 2c.17 and 2b.4; 1.12 and 2c.17 and
2b.11; 1.12
and 2c.17 and 2b.19; in each case optionally in the form of the racemates,
enantiomers or
diastereomers thereof and optionally in the form of the pharmacologically
acceptable acid
addition salts, solvates and/or hydrates thereof.
Particularly preferred examples of medicament combinations of the above-
mentioned
group F are selected from the group comprising the following combinations:
compounds 1.1 and 2d.1 and 2b.2; 1.1 and 2d.1 and 2b.4; 1.1 and 2d.1 and
2b.11; 1.1 and
2d.1 and 2b.19; 1.1 and 2d.4 and 2b.2; 1.1 and 2d.4 and 2b.4; 1.1 and 2d.4 and
2b.11; 1.1
and 2d.4 and 2b.19; 1.1 and 2d.5 and 2b.2; 1.1 and 2d.5 and 2b.4; 1.1 and 2d.5
and 2b.11;
CA 02559699 2006-09-12
WO 2005/102349 55 PCT/EP2005/004073
1.1 and 2d.5 and 2b.19; 1.1 and 2d.8 and 2b.2; 1.1 and 2d.8 and 2b.4; 1.1 and
2d.8 and
2b.11; 1.1 and 2d.8 and 2b.19; 1.12 and 2d.1 and 2b.2; 1.12 and 2d.1 and 2b.4;
1.12 and
2d.1 and 2b.11; 1.12 and 2d.1 and 2b.19; 1.12 and 2d.4 and 2b.2; 1.12 and 2d.4
and 2b.4;
1.12 and 2d.4 and 2b.11; 1.12 and 2d.4 and 2b.19; 1.12 and 2d.5 and 2b.2; 1.12
and 2d.5
and 2b.4; 1.12 and 2d.5 and 2b.11; 1.12 and 2d.5 and 2b.19; 1.12 and 2d.8 and
2b.2; 1.12
and 2d.8 and 2b.4; 1.12 and 2d.8 and 2b.11; 1.12 and 2d.8 and 2b.19, in each
case
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Particularly preferred examples of medicament combinations of the above-
mentioned
group G are selected from the group comprising the following combinations:
compounds 1.1 and 2e.2 and 2b.2; 1.1 and 2e.2 and 2b.4; 1.1 and 2e.2 and
2b.11; 1.1 and
2e.2 and 2b.19; 1.1 and 2e.3 and 2b.2; 1.1 and 2e.3 and 2b.4; 1.1 and 2e.3 and
2b.11; 1.1
and 2e.3 and 2b.19; 1.1 and 2e.4 and 2b.2; 1.1 and 2e.4 and 2b.4; 1.1 and 2e.4
and 2b.11;
1.1 and 2e.4 and 2b.19; 1.1 and 2e.10 and 2b.2; 1.1 and 2e.10 and 2b.4; 1.1
and 2e.10 and
2b.11; 1.1 and 2e.10 and 2b.19; 1.12 and 2e.2 and 2b.2; 1.12 and 2e.2 and
2b.4; 1.12 and
2e.2 and 2b.11; 1.12 and 2e.2 and 2b.19; 1.12 and 2e.3 and 2b.2; 1.12 and 2e.3
and 2b.4;
1.12 and 2e.3 and 2b.11; 1.12 and 2e.3 and 2b.19; 1.12 and 2e.4 and 2b.2; 1.12
and 2e.4
and 2b.4; 1.12 and 2e.4 and 2b.11; 1.12 and 2e.4 and 2b.19; 1.12 and 2e.10 and
2b.2; 1.12
and 2e.10 and 2b.4. 1.12 and 2e.10 and 2b.11; 1.12 and 2e.10 and 2b.19, in
each case
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof.
Particularly preferred examples of medicament combinations of the above-
mentioned
group H are selected from the group comprising the following combinations:
compounds 1.1 and 2c.8 and 2d.1; 1.1 and 2c.8 and 2d.4; 1.1 and 2c.8 and 2d.5;
1.1 and
2c.8 and 2d.8; 1.1 and 2c.9 and 2d.1; 1.1 and 2c.9 and 2d.4; 1.1 and 2c.9 and
2d.5; 1.1 and
2c.9 and 2d.8. 1.1 and 2c.10 and 2d.1; 1.1 and 2c.10 and 2d.4. 1.1 and 2e.10
and 2d.5. 1.1
and 2c.10 and 2d.8; 1.1 and 2c.11 and 2d.1; 1.1 and 2c.11 and 2d.4; 1.1 and
2c.11 and
2d.5; 1.1 and 2c.11 and 2d.8; 1.1 and 2c.17 and 2d.1; 1.1 and 2c.17 and 2d.4;
1.1 and
2c.17 and 2d.5; 1.1 and 2c.17 and 2d.8; 1.12 and 2c.8 and 2d.1; 1.12 and 2c.8
and 2d.4;
1.12 and 2c.8 and 2d.5; 1.12 and 2c.8 and 2d.8; 1.12 and 2c.9 and 2d.1; 1.12
and 2c.9 and
2d.4; 1.12 and 2c.9 and 2d.5; 1.12 and 2c.9 and 2d.8; 1.12 and 2c.10 and 2d.1;
1.12 and
CA 02559699 2006-09-12
WO 2005/102349 56 PCT/EP2005/004073
2c.10 and 2d.4; 1.12 and 2c.10 and 2d.5; 1.12 and 2c.10 and 2d.8; 1.12 and
2c.11 and
2d.1; 1.12 and 2c.11 and 2d.4; 1.12 and 2c.11 and 2d.5; 1.12 and 2c.11 and
2d.8, 1.12 and
2c.17 and 2d.1; 1.12 and 2c.17 and 2d.4; 1.12 and 2c.17 and 2d.5; 1.12 and
2c.17 and
2d.8; in each case optionally in the form of the racemates, enantiomers or
diastereomers
thereof and optionally in the form of the pharmacologically acceptable acid
addition salts,
solvates and/or hydrates thereof.
Particularly preferred examples of medicament combinations of the above-
mentioned
group I are selected from the group comprising the following combinations:
compounds 1.1 and 2c.8 and 2e.2; 1.1 and 2c.8 and 2e.3; 1.1 and 2c.8 and 2e.4;
1.1 and
2c.8 and 2e.10; 1.1 and 2c.9 and 2e.2; 1.1 and 2c.9 and 2e.3; 1.1 and 2c.9 and
2e.4; 1.1 and
2c.9 and 2e.10; 1.1 and 2c.10 and 2e.2; 1.1 and 2c.10 and 2e.3; 1.1 and 2c.10
and 2e.4; 1.1
and 2c.10 and 2e.10; 1.1 and 2c.11 and 2e.2; 1.1 and 2c.11 and 2e.3; 1.1 and
2c.11 and
2e.4; 1.1 and 2c.11 and 2e.10; 1.1 and 2c.17 and 2e.2; 1.1 and 2c.17 and 2e.3;
1.1 and
2c.17 and 2e.4; 1.1 and 2c.17 and 2e.10; 1.12 and 2c.8 and 2e.2; 1.12 and 2c.8
and 2e.3;
1.12 and 2c.8 and 2e.4; 1.12 and 2c.8 and 2e.10; 1.12 and 2c.9 and 2e.2; 1.12
and 2c.9 and
2e.3; 1.12 and 2c.9 and 2e.4; 1.12 and 2c.9 and 2e.10; 1.12 and 2c.10 and
2e.2; 1.12 and
2c.10 and 2e.3; 1.12 and 2c.10 and 2e.4; 1.12 and 2c.10 and 2e.10; 1.12 and
2c.11 and
2e.2; 1.12 and 2c.11 and 2e.3; 1.12 and 2c.11 and 2e.4; 1.12 and 2c.11 and
2e.10, 1.12 and
2c.17 and 2e.2; 1.12 and 2c.17 and 2e.3; 1.12 and 2c.17 and 2e.4; 1.12 and
2c.17 and
2e.10; in each case optionally in the form of the racemates, enantiomers or
diastereomers
thereof and optionally in the form of the pharmacologically acceptable acid
addition salts,
solvates and/or hydrates thereof.
Particularly preferred examples of medicament combinations of the above-
mentioned
group J are selected from the group comprising the following combinations:
compounds 1.1 and 2d.1 and 2e.2; 1.1 and 2d.1 and 2e.3; 1.1 and 2d.1 and 2e.4;
1.1 and
2d.1 and 2e.10; 1.1 and 2d.4 and 2e.2; 1.1 and 2d.4 and 2e.3; 1.1 and 2d.4 and
2e.4; 1.1
and 2d.4 and 2e.10; 1.1 and 2d.5 and 2e.2; 1.1 and 2d.5 and 2e.3; 1.1 and 2d.5
and 2e.4;
1.1 and 2d.5 and 2e.10; 1.1 and 2d.8 and 2e.2; 1.1 and 2d.8 and 2e.3; 1.1 and
2d.8 and
2e.4; 1.1 and 2d.8 and 2e.10; 1.12 and 2d.1 and 2e.2; 1.12 and 2d.1 and 2e.3;
1.12 and
2d.1 and 2e.4; 1.12 and 2d.1 and 2e.10; 1.12 and 2d.4 and 2e.2; 1.12 and 2d.4
and 2e.3;
1.12 and 2d.4 and 2e.4; 1.12 and 2d.4 and 2e.10; 1.12 and 2d.5 and 2e.2; 1.12
and 2d.5
and 2e.3; 1.12 and 2d.5 and 2e.4; 1.12 and 2d.5 and 2e.10; 1.12 and 2d.8 and
2e.2; 1.12
and 2d.8 and 2e.3; 1.12 and 2d.8 and 2e.4; 1.12 and 2d.8 and 2e.10, in each
case optionally
CA 02559699 2006-09-12
WO 2005/102349 57 PCT/EP2005/004073
in the form of the racemates, enantiomers or diastereomers thereof and
optionally in the
form of the pharmacologically acceptable acid addition salts, solvates and/or
hydrates
thereof.
Of outstanding importance according to the invention are all those medicament
combinations disclosed within the scope of the present invention which contain
the
compounds of formula 1 in the form of the R-enantiomers thereof.
Unless otherwise stated, the alkyl groups are straight-chained or branched
alkyl groups
having 1 to 4 carbon atoms. The following are mentioned by way of example:
methyl,
ethyl, propyl or butyl. In some cases the abbreviations Me, Et, Prop or Bu are
used to
denote the groups methyl, ethyl, propyl or butyl. Unless otherwise stated, the
definitions
propyl and butyl include all the possible isomeric forms of the groups in
question. Thus,
for example, propyl includes n-propyl and iso-propyl, butyl includes iso-
butyl, sec.butyl
and tert.-butyl, etc.
Unless otherwise stated, the cycloalkyl groups are alicyclic groups with 3 to
6 carbon
atoms. They are the cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl groups
.
Cyclopropyl is particularly important within the scope of the present
invention.
Unless otherwise stated, the alkylene groups are branched and unbranched
double-bonded
alkyl bridges with 1 to 4 carbon atoms. Examples include: methylene, ethylene,
propylene
or butylene.
Unless otherwise stated, the alkylene-halogen groups are branched and
unbranched double-
bonded alkyl bridges with 1 to 4 carbon atoms which are mono-, di- or
trisubstituted,
preferably disubstituted, by a halogen. Accordingly, unless otherwise stated,
alkylene-OH-
groups are branched and unbranched double-bonded alkyl bridges with 1 to 4
carbon atoms
which are mono-, di- or trisubstituted, preferably monosubstituted, by a
hydroxy.
Unless otherwise stated, the term alkyloxy groups denotes branched and
unbranched alkyl
groups with 1 to 4 carbon atoms which are linked via an oxygen atom. Examples
include:
methyloxy, ethyloxy, propyloxy or butyloxy. In some cases the abbreviations
MeO, EtO,
PropO or BuO may be used to denote the methyloxy, ethyloxy, propyloxy or
butyloxy
groups. Unless otherwise stated, the definitions propyloxy and butyloxy
include all the
CA 02559699 2006-09-12
WO 2005/102349 58 PCT/EP2005/004073
possible isomeric forms of the groups in question. Thus, for example,
propyloxy includes
n-propyloxy and iso-propyloxy, butyloxy includes iso-butyloxy, sec.butyloxy
and tert.-
butyloxy, etc. In some cases the term alkoxy may be used instead of alkyloxy
within the
scope of the present invention. The groups methyloxy, ethyloxy, propyloxy or
butyloxy
may therefore also be referred to by the names methoxy, ethoxy, propoxy or
butoxy.
Unless otherwise stated, the term alkylene-alkyloxy refers to branched and
unbranched
double-bonded alkyl bridges with 1 to 4 carbon atoms which are mono-, di- or
trisubstituted, preferably monosubstituted, by an alkyloxy group.
Unless otherwise stated, the term -0-CO-alkyl groups refers to branched and
unbranched
alkyl groups with 1 to 4 carbon atoms which are linked by an ester group. The
alkyl groups
are attached directly to the carbonyl carbon of the ester group. The term
-0-CO-alkyl-halogen should be understood analogously. The group -0-CO-CF3
denotes
trifluoroacetate.
Halogen within the scope of the present invention denotes fluorine, chlorine,
bromine or
iodine. Unless stated otherwise, fluorine and bromine are the preferred
halogens. The
group CO denotes a carbonyl group.
Within the scope of the present invention by a pharmaceutical combination of
components
1 and 2 is meant the joint administration of both active substances in a
single preparation
or formulation or the separate administration of the two active substances in
separate
formulations. If the active substances 1 and 2 are administered in separate
formulations,
this separate administration may be done simultaneously or at different times,
i.e.
successively.
In one aspect the present invention relates to the above-mentioned medicament
combinations which contain in addition to therapeutically effective amounts of
1 and 2 a
pharmaceutically acceptable carrier. In one aspect the present invention
relates to the
above-mentioned pharmaceutical compositions which do not contain contain a
pharmaceutically acceptable carrier in addition to therapeutically effective
amounts of 1
and 2.
The present invention also relates to the use of therapeutically effective
amounts of the
CA 02559699 2006-09-12
WO 2005/102349 59 PCT/EP2005/004073
active substances 1 for preparing a pharmaceutical composition also containing
one or
more, preferably one active substance 2 for the treatment of inflammatory and
obstructive
respiratory complaints, for inhibiting premature labour in midwifery
(tocolysis), for
restoring sinus rhythm in the heart in atrioventricular block, for correcting
bradycardic
heart rhythm disorders (antiarrhythmic), for treating circulatory shock
(vasodilatation and
increasing the heart volume) as well as for the treatment of skin irritations
and
inflammation.
In a preferred aspect the present invention relates to the use of
therapeutically effective
amounts of the active substance 1 for preparing a pharmaceutical composition
also
containing one or more, preferably one, active substance 2 for the treatment
of respiratory
complaints selected from the group comprising obstructive pulmonary diseases
of various
origins, pulmonary emphysema of various origins, restrictive pulmonary
diseases,
interstitial pulmonary diseases, cystic fibrosis, bronchitis of various
origins,
bronchiectasis, ARDS (adult respiratory distress syndrome) and all forms of
pulmonary
oedema.
Preferably the medicament combinations according to the invention are used as
specified
above for preparing a pharmaceutical composition for the treatment of
obstructive
pulmonary diseases selected from among bronchial asthma, paediatric asthma,
severe
asthma, acute asthma attacks, chronic bronchitis and COPD (chronic obstructive
pulmonary disease), while it is particularly preferable according to the
invention to use
them for preparing a pharmaceutical composition for the treatment of bronchial
asthma and
COPD.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of pulmonary
emphysema which
has its origins in COPD (chronic obstructive pulmonary disease) or al -
proteinase inhibitor
deficiency.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of restrictive
pulmonary diseases
selected from among allergic alveolitis, restrictive pulmonary diseases
triggered by work-
related noxious substances, such as asbestosis or silicosis, and restriction
caused by lung
tumours, such as for example lymphangiosis carcinomatosa, bronchoalveolar
carcinoma
CA 02559699 2006-09-12
WO 2005/102349 60 PCT/EP2005/004073
and lymphomas.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of interstitial
pulmonary diseases
selected from among pneumonia caused by infections, such as for example
infection by
viruses, bacteria, fungi, protozoa, helminths or other pathogens, pneumonitis
caused by
various factors, such as for example aspiration and left heart insufficiency,
radiation-
induced pneumonitis or fibrosis, collagenoses, such as for example lupus
erythematodes,
systemic sclerodermy or sarcoidosis, granulomatoses, such as for example
Boeck's disease,
idiopathic interstitial pneumonia or idiopathic pulmonary fibrosis (IPF).
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of cystic fibrosis or
mucoviscidosis.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of bronchitis, such
as for
example bronchitis caused by bacterial or viral infection, allergic bronchitis
and toxic
bronchitis.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of bronchiectasis.
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of ARDS (adult
respiratory
distress syndrome).
It is also preferable to use the medicament combinations according to the
invention for
preparing a pharmaceutical composition for the treatment of pulmonary oedema,
for
example toxic pulmonary oedema after aspiration or inhalation of toxic
substances and
foreign substances.
It is particularly preferable to use the compounds detailed above for
preparing a
pharmaceutical composition for the treatment of asthma or COPD. Also of
particular
importance is the above-mentioned use of medicament combinations according to
the
CA 02559699 2012-01-20
25771-1241(S)
61
invention for preparing a pharmaceutical composition for once-a-day treatment
of
inflammatory and obstructive respiratory complaints, particularly for the once-
a-day
treatment of asthma or COPD.
The present invention also relates to the use of therapeutically effective
amounts of
an active substance of formula 1 in combination with therapeutically effective
amounts of active substance 2 for preparing a pharmaceutical composition for
the
treatment of one of the above-mentioned diseases.
The present invention also relates to a process for treating one of the
above-mentioned diseases, which is characterised in that therapeutically
effective
amounts of active substance of formula 1 are administered in combination with
therapeutically effective amounts of active substance 2.
The invention also relates to a use of a compound of formula 1a,
0
O OH
HN
OH 1a
in combination with tiotropium bromide, in the treatment of COPD.
The invention also relates to a use of a compound of formula 1a,
0
O OH
HN IC
I X"'~I 1 0
OH 1a
CA 02559699 2012-01-20
25771-1241(S)
61a
in combination with tiotropium bromide, in the treatment of asthma.
The invention also relates to a use of a compound of formula 1a,
0
O OH
HN
OH 1a
in combination with ciclesonide, in the treatment of COPD.
The invention also relates to a use of a compound of formula 1a,
0
Y-"- O OH
HN
N/
OH 1a
in combination with ciclesonide, in the treatment of asthma.
The invention also relates to a use of: a compound of formula 1a;
0
Y-~- O OH
HN
OH 1a
in combination with titropium bromide; and ciclesonide, in the treatment of
COPD.
CA 02559699 2012-01-20
25771-1241(S)
61b
The invention also relates to a use of: a compound of formula 1a;
0
)_"~ O OH
HN
\ O",
OH 1a
in combination with titropium bromide; and ciclesonide, in the treatment of
asthma.
In the above-noted uses, 1a may be in the form of the R-enantiomer. 1a may
also be
in the form of its hydrochloride acid addition salt.
Within the scope of the medicament combinations according to the invention,
for
example, 0.1-1000 pg of a compound of formula 1 may be administered per single
dose. Preferably, 1-500 pg, particularly preferably 3-100 pg of the compound
of
formula 1 are administered per single dose, while a dosage range of from 5-75
pg,
preferably from 7-50 pg is preferred according to the invention. Particularly
preferably, the pharmaceutical compositions according to the invention are
administered in an amount such that 9-40 pg, particularly preferably 11-30 pg,
more
preferably 12-25 pg of the compound of formula 1 are administered per single
dose.
For example, and without restricting the present invention thereto, 5 pg, 7.5
pg,
10 pg, 12.5 pg, 15 pg, 17.5 pg, 20 pg, 22.5 pg, 25 pg, 27.5 pg, 30 pg, 32.5
pg, 35 pg,
37.5 pg, 40 pg, 42.5 pg, 45 pg, 47.5 pg, 50 pg, 52.5 pg, 55 pg, 57.5 pg, 60
pg,
62.5 pg, 65 pg, 67.5 pg, 70 pg, 72.5 pg or 75 pg of a compound of formula I
may be
administered per single dose.
The above-mentioned dosages relate to the compounds of formula 1 in the form
of
their free bases. If the compounds of formula 1 are administered in the form
of their
pharmaceutically acceptable acid addition salts, the skilled man can easily
calculate
the corresponding dosage ranges for the acid addition salts from the dosage
ranges
CA 02559699 2012-01-20
25771-1241(S)
61c
specified above, taking into account the molecular weight of the acids used.
Particularly preferably, the compounds of formula 1 are administered in the
above-mentioned dosage ranges in the form of the enantiomerically pure
compounds, particularly preferably in the form of the R-enantiomers thereof.
CA 02559699 2006-09-12
WO 2005/102349 62 PCT/EP2005/004073
If the compounds of formula 1 are administered in conjunction with an
anticholinergic 2a,
the amount of anticholinergic used will fluctuate considerably depending on
the choice of
active substance.
Without restricting the invention thereto, in the case of tiotropium 2a.1'
amounts of
anticholinergic 2a.1') may be administered such that each single dose contains
0.1 - 80 g,
preferably 0.5 - 60 g, particularly preferably about I - 50 g of 2a.1' . For
example and
without restricting the present invention thereto, 2.5 g, 5 g, Mpg,l8gg, 20 g,
36 g or
40 g 2a.1' maybe administered per single dose. The corresponding amount of
salt 2a.1 or
of any hydrate or solvate used in each case can easily be calculated by the
skilled man,
depending on the choice of anion. If for example tiotropium bromide is used as
the
preferred tiotropium salt 2a.1 according to the invention, the amounts of the
active
substance 2a.1' administered per single dose as specified by way of example
hereinbefore
correspond to the following amounts of 2a.1 administered per single dose: 3 g,
6 g,
12 g, 21.7 g, 24.1 g, 43.3 g and 48.1 g 2a.1. In the case of tiotropium 2a.1'
the dosages
specified above are preferably administered once or twice a day, while
administration once
a day is particularly preferred according to the invention.
Without restricting the invention thereto, in the case of the cation 2a.2'
amounts of
anticholinergic 2a.2') may be administered such that each single dose contains
1 - 500 g,
preferably 5 - 300 g, particularly preferably 15-200 gg 2a.2' . For example
and without
restricting the present invention thereto, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g,
45 g, 50 g,
55 g, 60 g, 65 g, 70 g, 75 g, 80 g, 85 g, 90 g, 95 g, 100 g, 105 g, 110 g, 115
g,
120 g, 125 g, 130 g, 135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g,
17511g, 180 g, 185 g, 190 g, 195 g or 200 g of 2a.2' maybe administered per
single
dose. The corresponding amount of salt 2a.2 used in each case or of any
hydrate or solvate
used can easily be calculated by the skilled man, depending on the choice of
anion. In the
case of oxitropium 2a.2' the dosages specified above are preferably
administered one to
four times a day, while administration two to three times a day is
particularly preferred
according to the invention.
Without restricting the invention thereto, in the case of the cation 2a.3'
amounts of
anticholinergic 2a.3') may be administered such that each single dose contains
1 - 500 g,
preferably 5 - 300 g, particularly preferably 15-200 g 2a.3' . For example
and without
CA 02559699 2006-09-12
WO 2005/102349 63 PCT/EP2005/004073
restricting the present invention thereto, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g,
45 g, 50 g,
55 g, 60 g, 65 g, 70 g, 75 g, 80 g, 85 g, 90 g, 95 g, 100 g, 105 g, 110 g, 115
g,
120 g, 125 g, 130 g, 135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g,
175 g, 180 g, 185 g, 190 g, 195 g or 200 gof 2a.3' may be administered per
single
dose. The corresponding amount of salt 2a.3 used in each case or of any
hydrate or solvate
used can easily be calculated by the skilled man, depending on the choice of
anion. In the
case of flutropium 2a.3' the dosages specified above are preferably
administered one to
four times a day, while administration two to three times a day is
particularly preferred
according to the invention.
Without restricting the invention thereto, in the case of the cation 2a.4'
amounts of
anticholinergic 2a.4') may be administered such that each single dose contains
1 - 500 g,
preferably 5 - 300 g, particularly preferably 20-200 gg 2a.4' . For example
and without
restricting the present invention thereto, 20 g, 25 g, 30 g, 35 g, 40 g, 45 g,
50 g, 55 g,
60 g, 65 g, 70 g, 75 g, 80 g, 85 g, 90 g, 95 g, 100 g, 105 g, 110 g, 115 g,
120 g,
125 g, 130 g, 135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g, 175 g,
180 g, 185 g, 190 g, 195 g or 200 g of 2a.4' may be administered per single
dose . The
corresponding amount of salt 2a.4 used in each case or of any hydrate or
solvate used can
easily be calculated by the skilled man, depending on the choice of anion. In
the case of
ipratropium 2a.4' the dosages specified above are preferably administered one
to four
times a day, while administration two to three times a day, more preferably
three times a
day, is particularly preferred according to the invention.
Without restricting the invention thereto, in the case of the cation 2a.5'
amounts of
anticholinergic 2a.5') may be administered such that each single dose contains
1 - 500 g,
preferably 5 - 300 g, particularly preferably 15-200 g . For example and
without
restricting the present invention thereto, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g,
45 g, 50 g,
55 g, 60 g, 65 g, 70 g, 75 g, 80 g, 85 g, 90 g, 95 g, 100 g, 105 g, 110 g, 115
g,
120 g, 125 g, 130 g, 135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g,
175 g, 180 g, 185 g, 190 g, 195 g or 200 g of 2a.5' may be administered per
single
dose. The corresponding amount of salt 2a.5 used in each case or of any
hydrate or solvate
used can easily be calculated by the skilled man, depending on the choice of
anion. In the
case of glycopyrronium 2a.5' the dosages specified above are preferably
administered one
to four times a day, while administration two to three times a day is
particularly preferred
according to the invention.
CA 02559699 2006-09-12
WO 2005/102349 64 PCT/EP2005/004073
Without restricting the invention thereto, in the case of the cation 2a.6'
amounts of
anticholinergic 2a.6') may be administered such that each single dose contains
1000 -
6500 g, preferably 2000 - 6000 g, particularly preferably 3000 - 5500 g,
particularly
preferably 4000 - 5000 g 2a.6' . For example and without restricting the
present invention
thereto, 3500 g, 3750 g, 4000 g, 4250 g, 4500 g, 4750 g, or 5000 g of 2a.6'
maybe
administered per single dose. The corresponding amount of salt 2a.6 used in
each case or
of any hydrate or solvate used can easily be calculated by the skilled man,
depending on
the choice of anion. In the case of trospiums 2a.6' the dosages specified
above are
preferably administered one to four times a day, while administration two to
three times a
day is particularly preferred according to the invention.
Without restricting the invention thereto, in the case of the cation 2a.7'
amounts of
anticholinergic 2a.7) may be administered such that each single dose contains
50 -
1000 g, preferably 100 - 800 g, particularly preferably 200 - 700 g,
particularly
preferably 300 - 600 g 2a.7' . For example and without restricting the present
invention
thereto, 300 g, 350 g, 400 g, 450 g, 500 g, 550 g, or 600 g of 2a.7' may be
administered per single dose. The corresponding amount of salt 2a.7 used in
each case or
of any hydrate or solvate used can easily be calculated by the skilled man,
depending on
the choice of anion. In the case of the cation 2a.7' the dosages specified
above are
preferably administered one to three times a day, while administration once or
twice a day,
more preferably once a day, is particularly preferred according to the
invention.
Without restricting the invention thereto, in the case of the cations 2a.9'
and 2a.10' ,
amounts of anticholinergic 2a.9' or 2a.10' may be administered such that each
single
dose contains 1 - 500 g, preferably 5 - 300 g, particularly preferably 15-200
g 2a.9' or
2a.10' . For example and without restricting the present invention thereto, 15
g, 20 g,
25 g, 30 g, 35 g, 40 g, 45 g, 50 g, 55 g, 60 g, 65 g, 70 g, 75 g, 80 g, 85 g,
90 g,
95 g, 100 g, 105 g, 110 g, 115 g, 120 g, 125 g, 130 g, 135 g, 140 g, 145 g,
150 g,
155 g, 160 g, 165 g, 170 g, 175 g, 180 g, 185 g, 190 g, 195 g or 200 g of
2a.9' or
2a.10' maybe administered per single dose. The corresponding amount of salt
2a.9' or
2a.10' or of any hydrate or solvate used in each case can easily be calculated
by the skilled
man, depending on the choice of anion. In the case of the cations 2a.9' or
2a.10' the
dosages specified above are preferably administered one to three times a day,
while
CA 02559699 2006-09-12
WO 2005/102349 65 PCT/EP2005/004073
administration once or twice a day, more preferably once a day, is
particularly preferred
according to the invention.
Without restricting the invention thereto, in the case of the cations 2a.11'
to 2a.13'
amounts of anticholinergic 2a.11', 2a.12' or 2a.13') may be administered such
that each
single dose contains 1 - 50011g, preferably 5 - 300 g, particularly
preferably 10-200 g
2a.11', 2a.12' or 2a.13'. For example and without restricting the present
invention thereto,
g, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g, 45 g, 50 g, 55 g, 60 g, 65 g, 70 g, 75
g,
80 g, 85 g, 90 g, 95 g, 100 g, 105 g, 110 g, 115 g, 120 g, 125 g, 130 g, 135
g,
10 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g, 175 g, 180 g, 185 g, 190
g, 195 g
or 200 g of 2a.11', 2a.12' or 2a.13' maybe administered per single dose. The
corresponding amount of salt 2a.11, 2a.12 or 2a.13 or of any hydrate or
solvate used in
each case can easily be calculated by the skilled man, depending on the choice
of anion.
In the case of the cations 2a.11, 2a.12 or 2a.13 the dosages specified above
are preferably
administered one to three times a day, while administration once or twice a
day, more
preferably once a day, is particularly preferred according to the invention.
If the compounds of formula 1 are administered in combination with a PDE IV-
inhibitor
2b , preferably about 1 - 10000 gg 2b are administered per single dose.
Preferably,
amounts of 2b are administered such that each single dose contains 10 - 5000
g,
preferably 50 - 2500 g, particularly preferably 100-1000 g of 2b . For
example and
without restricting the present invention thereto, 100 g, 115 g, 120 g, 125 g,
130 g,
135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g, 175 g, 180 g, 185 g,
190 g, 195 g, 200 g, 205 g, 210 g, 215 g, 220 g, 225 g, 230 g, 235 g, 240 g,
245 g, 250 g, 255 g, 260 g, 265 g, 270 g, 275 g, 280 g, 285 g, 290 g, 295 g,
300 g, 305 g, 310 g, 315 g, 320 g, 325 g, 330 g, 335 g, 340 g, 345 g, 350 g,
355 g, 360 g, 365 g, 370 g, 375 g, 380 g, 385 g, 390 g, 395 g, 400 g, 405 g,
410 g, 415 g, 420 g, 425 g, 430 g, 435 g, 440 g, 445 g, 450 g, 455 g, 460 g,
465 g, 470 g, 475 g, 480 g, 485 g, 490 g, 495 g, 500 g, 505 g, 510 g, 515 g,
520 g, 525 g, 530 g, 535 g, 540 g, 545 g, 550 g, 555 g, 560 g, 565 g, 570 g,
575 g, 580 g, 585 g, 590 g, 595 g, 600 g, 605 g, 610 g, 615 g, 620 g, 625 g,
630 g, 635 g, 640 g, 645 g, 650 g, 655 g, 660 g, 665 g, 670 g, 675 g, 680 g,
685 g, 690 g, 695 g, 700 g, 705 g, 710 g, 715 g, 720 g, 725 g, 730 g, 735 g,
740 g, 745 g, 750 g, 755 g, 760 g, 765 g, 770 g, 775 g, 780 g, 785 g, 790 g,
795 g, 800 g, 805 g, 810 g, 815 g, 820 g, 825 g, 830 g, 835 g, 840 g, 845 g,
CA 02559699 2006-09-12
WO 2005/102349 66 PCT/EP2005/004073
850 g, 855 g, 860 g, 865 g, 870 g, 875 g, 880 g, 885 g, 890 g, 895 g, 900 g,
905 g, 910 g, 915 g, 920 g, 925 g, 930 g, 935 g, 940 g, 945 g, 950 g, 955 g,
960 g, 965 g, 970 g, 975 g, 980 g, 985 g, 990 g, 995 g or 1000 g of 2b may be
administered per single dose. In the event that acid addition salts of 2b are
used, the
corresponding amount of salt used can easily be calculated by the skilled man
from the
values given hereinbefore, depending on the choice of acid.
If the compounds of formula 1 are administered in combination with a steroid
2c ,
preferably about 1 - 10000 g of 2c are administered per single dose.
Preferably, amouts
of 2c are administered such that each single dose contains 5 - 5000 g,
preferably 5 - 2500
g, particularly preferably 10-1000 g of 2c . For example and without
restricting the
present invention thereto, 10 g, 15 g, 20 g, 25 g, 30 g, 35 g, 40 g, 45 g, 50
g, 55 g,
60 g, 65 g, 70 g, 75 g, 80 g, 85 g, 90 g, 95 g, 100 g, 115 g, 120 g, 125 g,
130 g,
135 g, 140 g, 145 g, 150 g, 155 g, 160 g, 165 g, 170 g, 175 g, 180 g, 185 g,
190 g, 195 g, 200 g, 205 g, 210 g, 215 g, 220 g, 225 g, 230 g, 235 g, 240 g,
245 g, 250 g, 255 g, 260 g, 265 g, 270 g, 275 g, 280 g, 285 g, 290 g, 295 g,
300 g, 305 g, 310 g, 315 g, 320 g, 325 g, 330 g, 335 g, 340 g, 345 g, 350 g,
355 g, 360 g, 365 g, 370 g, 375 g, 380 g, 385 g, 390 g, 395 g, 400 g, 405 g,
410 g, 415 g, 420 g, 425 g, 430 g, 435 g, 440 g, 445 g, 450 g, 455 g, 460 g,
465 g, 470 g, 475 g, 480 g, 485 g, 490 g, 495 g, 500 g, 505 g, 510 g, 515 g,
520 g, 525 g, 530 g, 535 g, 540 g, 545 g, 550 g, 555 g, 560 g, 565 g, 570 g,
575 g, 580 g, 585 g, 590 g, 595 g, 600 g, 605 g, 610 g, 615 g, 620 g, 625 g,
630 g, 635 g, 640 g, 645 g, 650 g, 655 g, 660 g, 665 g, 670 g, 675 g, 680 g,
685 g, 690 g, 695 g, 700gg, 705 g, 710 g, 715 g, 720 g, 725 g, 730 g, 735 g,
740 g, 745 g, 750 g, 755 g, 760 g, 765 g, 770 g, 775 g, 780 g, 785 g, 790 g,
795 g, 800 g, 805 g, 810 g, 815 g, 820 g, 825 g, 830 g, 835 g, 840 g, 845 g,
850 g, 855 g, 860 g, 865 g, 870 g, 875 g, 880 g, 885 g, 890 g, 895 g, 900 g,
905 g, 910 g, 915 g, 920 g, 925 g, 930 g, 935 g, 940 g, 945 g, 950 g, 955 g,
960 g, 965 g, 970 g, 975 g, 980 g, 985 g, 990 g, 995 g or 1000 g of 2c may be
administered per single dose. In the event that salts or derivatives of 2c are
used, the
corresponding amount of salt/derivative used can easily be calculated by the
skilled man
from the values given hereinbefore, depending on the choice of
salt/derivative.
If the compounds of formula 1 are administered in combination with an LTD4-
antagonist
2d, preferably about 0,01 - 500 mg 2d are administered per single dose.
Preferably,
CA 02559699 2006-09-12
WO 2005/102349 67 PCT/EP2005/004073
amounts of 2d are administered such that each single dose contains 0.1 -
250mg, preferably
0.5 - 100 mg, particularly preferably 1-50 mg of 2d. For example and without
restricting
the present invention thereto, 1mg, 2.5mg, 5mg, 5.5mg, 7 mg, 7, 5mg, 10mg,
12.5mg,
15mg, 17.5mg, 20mg, 22.5mg, 25mg, 27.5mg, 30mg, 32.5mg, 35mg, 37.5mg, 40mg,
42.5mg, 45mg, 47.5mg or 50mg of 2d maybe administered per single dose. In the
event
that acid addition salts, salts or derivatives of 2d are used, the
corresponding amount of
salt/derivative used can easily be calculated by the skilled man from the
values given
hereinbefore, depending on the choice of salt/derivative.
If the compounds of formula 1 are administered in combination with an EGFR-
inhibitor
2e, preferably about 100 - 15000 Vg of 2e are administered per single dose.
Preferably,
amounts of 2e are administered such that each single dose contains 500 - 10000
g,
preferably 750 - 8000 Rg, particularly preferably 1000-7000 g of 2e . For
example and
without restricting the present invention thereto, 1000 g, 1150 g, 1200 g,
1250 g,
1300 g, 1350 g, 1400 g, 1450 g, 1500 g, 1550 g, 1600 g, 1650 g, 1700 g, 1750
g,
1800 g, 1850 g, 1900 g, 1950 g, 2000 g, 2050 g, 2100 g, 2150 g, 2200 g, 2250
g,
2300 g, 2350 g, 2400 g, 2450 g, 2500 g, 2550 g, 2600 g, 2650 g, 2700 g, 2750
g,
2800 g,2850 g,2900 g,2950 g,3000 g,3050 g, 3100 g, 3150 g, 3200 g, 3250 g,
3300 g,3350 g,3400 g,3450 g,3500 g,3550 g, 3600Rg,3650 g,3700Rg,3750Rg,
3800 g, 3850 g, 3900 g, 3950 g, 4000 g, 4050 g, 4100 g, 4150 g, 4200 g, 4250
g,
4300 g, 4350 g, 4400 g, 4450 g, 4500 g, 4550 g, 4600 g, 4650 g, 4700 g, 4750
g,
4800 g, 4850 g, 4900 g, 4950 g, 5000 g, 5050 g, 5100 g, 5150 g, 5200 g, 5250
g,
5300 g, 5350 g, 5400 g, 5450 g, 5500 g, 5550 g, 5600 g, 5650 g, 5700 g, 5750
g,
5800 g, 5850 g, 5900 g, 5950 g, 6000 g, 6050 g, 6100 g, 6150 g, 6200 g,
6250Rg,
6300 g, 6350 g, 6400 g, 6450 g, 6500 g, 6550 g, 6600 g, 6650 g, 6700 g, 6750
g,
6800 g, 6850 g, 6900 g, 6950 g, or 7000 g of 2e may be administered per single
dose.
In the event that acid addition salts of 2e are used, the corresponding amount
of the salt
used can easily be calculated by the skilled man from the values given
hereinbefore,
depending on the choice of acid.
The two active substance components 1 and 2 may be administered - together or
separately
- in each case by inhalation or by oral, parenteral or some other route, in
known manner, in
substantially conventional formulations such as for example plain or coated
tablets, pills,
granules, aerosols, syrups, emulsions, suspensions, powders and solutions,
using inert,
CA 02559699 2006-09-12
WO 2005/102349 68 PCT/EP2005/004073
non-toxic, pharmaceutically suitable carriers or solvents.
Suitable preparations for administering the compounds of formula 1 and 2
include tablets,
capsules, suppositories, solutions, powders, etc. The proportion of
pharmaceutically active
compound or compounds should be in the range from 0.05 to 90 % by weight,
preferably
0.1 to 50 % by weight of the total composition. Suitable tablets may be
obtained, for
example, by mixing the active substance(s) with known excipients, for example
inert
diluents such as calcium carbonate, calcium phosphate or lactose,
disintegrants such as
corn starch or alginic acid, binders such as starch or gelatine, lubricants
such as magnesium
stearate or talc and/or agents for delaying release, such as carboxymethyl
cellulose,
cellulose acetate phthalate, or polyvinyl acetate. The tablets may also
comprise several
layers.
Coated tablets may be prepared accordingly by coating cores produced
analogously to the
tablets with substances normally used for tablet coatings, for example
collidone or shellac,
gum arabic, talc, titanium dioxide or sugar. To achieve delayed release or
prevent
incompatibilities the core may also consist of a number of layers. Similarly
the tablet
coating may consist of a number or layers to achieve delayed release, possibly
using the
excipients mentioned above for the tablets.
Syrups or elixirs containing the active substances or combinations of active
substances
according to the invention may additionally contain a sweetener such as
saccharine,
cyclamate, glycerol or sugar and a flavour enhancer, e.g. a flavouring such as
vanilline or
orange extract. They may also contain suspension adjuvants or thickeners such
as sodium
carboxymethyl cellulose, wetting agents such as, for example, condensation
products of
fatty alcohols with ethylene oxide, or preservatives such as p-
hydroxybenzoates.
Solutions are prepared in the usual way, e.g. with the addition of isotonic
agents,
preservatives such as p-hydroxybenzoates, or stabilisers such as alkali metal
salts of
ethylenediamine tetraacetic acid, optionally using emulsifiers and/or
dispersants, whilst if
water is used as the diluent, for example, organic solvents may optionally be
used as
solvating agents or dissolving aids, and transferred into injection vials or
ampoules or
infusion bottles.
CA 02559699 2006-09-12
WO 2005/102349 69 PCT/EP2005/004073
Capsules containing one or more active substances or combinations of active
substances
may for example be prepared by mixing the active substances with inert
carriers such as
lactose or sorbitol and packing them into gelatine capsules.
Suitable suppositories may be made for example by mixing with carriers
provided for this
purpose, such as neutral fats or polyethyleneglycol or the derivatives
thereof.
Excipients which may be used include, for example, water, pharmaceutically
acceptable
organic solvents such as paraffins (e.g. petroleum fractions), vegetable oils
(e.g. groundnut
or sesame oil), mono- or polyfunctional alcohols (e.g. ethanol or glycerol),
carriers such as
e.g. natural mineral powders (e.g. kaolins, clays, talc, chalk), synthetic
mineral powders
(e.g. highly dispersed silicic acid and silicates), sugars (e.g. cane sugar,
lactose and
glucose), emulsifiers (e.g. lignin, spent sulphite liquors, methylcellulose,
starch and
polyvinylpyrrolidone) and lubricants (e.g. magnesium stearate, talc, stearic
acid and
sodium lauryl sulphate).
For oral administration the tablets may, of course, contain, apart from the
abovementioned
carriers, additives such as sodium citrate, calcium carbonate and dicalcium
phosphate
together with various additives such as starch, preferably potato starch,
gelatine and the
like. Moreover, lubricants such as magnesium stearate, sodium lauryl sulphate
and talc
may be used at the same time for the tabletting process. In the case of
aqueous suspensions
the active substances may be combined with various flavour enhancers or
colourings in
addition to the excipients mentioned above.
Preferably, even when the two components 1 and 2 are administered separately,
at least
component 1 is administered by inhalation. If component 1 is administered by
inhalation,
when the two active substances are taken separately, component 2 may also be
administered for example by oral or parenteral route using formulations
conventional in the
art such as plain or coated tablets, pills, granules, aerosols, syrups,
emulsions, suspensions,
powders and solutions, using inert, non-toxic, pharmaceutically suitable
carriers or
solvents.
Preferably, however, the medicament combinations according to the invention
are
administered by inhalation by means of a single preparation containing both
active
substances 1 and 2 or by means of separate preparations each containing only
one of the
active substances 1 and 2, suitable for administration by inhalation.
CA 02559699 2006-09-12
WO 2005/102349 70 PCT/EP2005/004073
Inhalable preparations include inhalable powders, propellant-containing
metered dose
aerosols or propellant-free inhalable solutions. Inhalable powders according
to the
invention containing the combination of active substances 1 and 2 may consist
of the active
substances on their own or of a mixture of the active substances with
physiologically
acceptable excipients. Within the scope of the present invention, the term
propellant-free
inhalable solutions also includes concentrates or sterile inhalable solutions
ready for use.
The preparations according to the invention may contain the combination of
active
substances 1 and 2 either together in one formulation or in two separate
formulations.
These formulations which may be used within the scope of the present invention
are
described in more detail in the next part of the specification.
A) Inhalable powder containing the combinations of active substances according
to
the invention:
The inhalable powders according to the invention may contain 1 and 2 either on
their own
or in admixture with suitable physiologically acceptable excipients. If the
active
substances 1 and 2 are present in admixture with physiologically acceptable
excipients, the
following physiologically acceptable excipients may be used to prepare these
inhalable
powders according to the invention: monosaccharides (e.g. glucose or
arabinose),
disaccharides (e.g. lactose, saccharose, maltose, trehalose), oligo- and
polysaccharides (e.g.
dextrans), polyalcohols (e.g. sorbitol, mannitol, xylitol), salts (e.g. sodium
chloride,
calcium carbonate) or mixtures of these excipients with one another.
Preferably, mono- or
disaccharides are used, while the use of lactose, trehalose or glucose is
preferred,
particularly, but not exclusively, in the form of their hydrates.
Within the scope of the inhalable powders according to the invention the
excipients have a
maximum average particle size of up to 250 m, preferably between 10 and 150 m,
most
preferably between 15 and 80 m. It may sometimes seem appropriate to add finer
excipient fractions with an average particle size of 1 to 9 m to the
excipients mentioned
above. These finer excipients are also selected from the group of possible
excipients listed
hereinbefore. Finally, in order to prepare the inhalable powders according to
the invention,
micronised active substance 1 and 2, preferably with an average particle size
of 0.5 to
10 m, more preferably from 1 to 6 m, is added to the excipient mixture.
Processes for
producing the inhalable powders according to the invention by grinding and
micronising
and finally mixing the ingredients together are known from the prior art. The
inhalable
CA 02559699 2006-09-12
WO 2005/102349 71 PCT/EP2005/004073
powders according to the invention may be prepared and administered either in
the form of
a single powder mixture which contains both 1 and 2 or in the form of separate
inhalable
powders which contain only 1 or 2.
The inhalable powders according to the invention may be administered using
inhalers
known from the prior art. Inhalable powders according to the invention which
contain a
physiologically acceptable excipient in addition to 1 and 2 may be
administered, for
example, by means of inhalers which deliver a single dose from a supply using
a
measuring chamber as described in US 4570630A, or by other means as described
in
DE 36 25 685 A. The inhalable powders according to the invention which contain
1 and 2
optionally in conjunction with a physiologically acceptable excipient may be
administered,
for example, using the inhaler known by the name Turbuhaler or using
inhalers as
disclosed for example in EP 237507 A. Preferably, the inhalable powders
according to the
invention which contain physiologically acceptable excipient in addition to 1
and 2 are
packed into capsules (to produce so-called inhalettes) which are used in
inhalers as
described, for example, in WO 94/28958.
A particularly preferred inhaler for using the pharmaceutical combination
according to the
invention in inhalettes is shown in Figure 1.
This inhaler (Handihaler ) for inhaling powdered pharmaceutical compositions
from
capsules is characterised by a housing 1 containing two windows 2, a deck 3 in
which there
are air inlet ports and which is provided with a screen 5 secured by a screen
housing 4, an
inhalation chamber 6 connected to the deck 3 on which there is a push button 9
provided
with two sharpened pins 7 and movable counter to a spring 8, and a mouthpiece
12 which
is connected to the housing 1, the deck 3 and a cover 11 via a spindle 10 to
enable it to be
flipped open or shut, and air through-holes 13 for adjusting the flow
resistance.
If the inhalable powders according to the invention are to be packaged in
capsules, in
accordance with the preferred method of administration described above, the
capsules
should preferably contain from 1 to 30 mg each. According to the invention
they contain
either together or separately the dosages per single dose specified for 1 and
2 hereinbefore.
B) Propellant gas-driven inhalation aerosols containing the combinations of
active
substances according to the invention:
CA 02559699 2006-09-12
WO 2005/102349 72 PCT/EP2005/004073
Inhalation aerosols containing propellant gas according to the invention may
contain
substances 1 and 2 dissolved in the propellant gas or in dispersed form. 1 and
2 may be
present in separate formulations or in a single preparation, in which 1 and 2
are either both
dissolved, both dispersed or only one component is dissolved and the other is
dispersed.
The propellant gases which may be used to prepare the inhalation aerosols
according to the
invention are known from the prior art. Suitable propellant gases are selected
from among
hydrocarbons such as n-propane, n-butane or isobutane and halohydrocarbons
such as
preferably chlorinated and fluorinated derivatives of methane, ethane,
propane, butane,
cyclopropane or cyclobutane. The propellant gases mentioned above may be used
on their
own or in mixtures thereof Particularly preferred propellant gases are
halogenated alkane
derivatives selected from TG 11, TG 12, TG 134a (1,1,1,2-tetrafluoroethane),
TG227
(1,1,1,2,3,3,3-heptafluoropropane) and mixtures thereof, the propellant gases
TG134a,
TG227 and mixtures thereof being preferred.
The propellant-driven inhalation aerosols according to the invention may also
contain other
ingredients such as co-solvents, stabilisers, surfactants, antioxidants,
lubricants and pH
adjusters. All these ingredients are known in the art.
The inhalation aerosols containing propellant gas according to the invention
may contain
up to 5 wt.-% of active substance 1 and/or 2. Aerosols according to the
invention contain,
for example, 0.002 to 5 wt.-%, 0.01 to 3 wt.-%, 0.015 to 2 wt.-%, 0.1 to 2 wt.-
%, 0.5 to
2 wt.-% or 0.5 to 1 wt.-% of active substance 1 and/or 2.
If the active substances 1 and/or 2 are present in dispersed form, the
particles of active
substance preferably have an average particle size of up to 10 m, preferably
from 0.1 to 6
pm, more preferably from 1 to 5 m.
The propellant-driven inhalation aerosols according to the invention mentioned
above may
be administered using inhalers known in the art (MDIs = metered dose
inhalers).
Accordingly, in another aspect, the present invention relates to
pharmaceutical
compositions in the form of propellant-driven aerosols as hereinbefore
described combined
with one or more inhalers suitable for administering these aerosols. In
addition, the present
invention relates to inhalers which are characterised in that they contain the
propellant gas-
containing aerosols described above according to the invention.
CA 02559699 2006-09-12
WO 2005/102349 73 PCT/EP2005/004073
The present invention also relates to cartridges which are fitted with a
suitable valve and
can be used in a suitable inhaler and which contain one of the above-mentioned
propellant
gas-containing inhalation aerosols according to the invention. Suitable
cartridges and
methods of filling these cartridges with the inhalable aerosols containing
propellant gas
according to the invention are known from the prior art.
C) Propellant-free inhalable solutions or suspensions containing the
combinations of
active substances 1 and 2 according to the invention:
Propellant-free inhalable solutions according to the invention contain for
example aqueous
or alcoholic, preferably ethanolic solvents, possibly ethanolic solvents in
admixture with
aqueous solvents. In the case of aqueous/ethanolic solvent mixtures the
relative proportion
of ethanol to water is not restricted, but the maximum limit is up to 70
percent by volume,
more particularly up to 60 percent by volume of ethanol. The remainder of the
volume is
made up of water. The solutions or suspensions containing 1 and 2, separately
or together,
are adjusted to a pH of 2 to 7, preferably 2 to 5, using suitable acids. The
pH may be
adjusted using acids selected from inorganic or organic acids. Examples of
particularly
suitable inorganic acids include hydrochloric acid, hydrobromic acid, nitric
acid, sulphuric
acid and/or phosphoric acid. Examples of particularly suitable organic acids
include
ascorbic acid, citric acid, malic acid, tartaric acid, maleic acid, succinic
acid, fumaric acid,
acetic acid, formic acid and/or propionic acid, etc. Preferred inorganic acids
are
hydrochloric acid and sulphuric acid. It is also possible to use the acids
which have
already formed an acid addition salt with one of the active substances. Of the
organic
acids, ascorbic acid, fumaric acid and citric acid are preferred. If desired,
mixtures of the
above acids may also be used, particularly in the case of acids which have
other properties
in addition to their acidifying qualities, e.g. as flavourings, antioxidants
or complexing
agents, such as citric acid or ascorbic acid, for example. According to the
invention, it is
particularly preferred to use hydrochloric acid to adjust the pH.
According to the invention, the addition of edetic acid (EDTA) or one of the
known salts
thereof, sodium edetate, as stabiliser or complexing agent is unnecessary in
the present
formulation. Other embodiments may contain this compound or these compounds.
In a
preferred embodiment the content based on sodium edetate is less than
I00mg/I00ml,
preferably less than 50mg/100 ml, more preferably less than 20mg/ 100 ml.
Generally,
CA 02559699 2006-09-12
WO 2005/102349 74 PCT/EP2005/004073
inhalable solutions in which the content of sodium edetate is from 0 to
10mg/I00ml are
preferred.
Co-solvents and/or other excipients may be added to the propellant-free
inhalable solutions
according to the invention. Preferred co-solvents are those which contain
hydroxyl groups
or other polar groups, e.g. alcohols - particularly isopropyl alcohol, glycols
- particularly
propyleneglycol, polyethyleneglycol, polypropyleneglycol, glycolether,
glycerol,
polyoxyethylene alcohols and polyoxyethylene fatty acid esters. The terms
excipients and
additives in this context denote any pharmacologically acceptable substance
which is not
an active substance but which can be formulated with the active substance or
substances in
the pharmacologically suitable solvent in order to improve the qualitative
properties of the
active substance formulation. Preferably, these substances have no
pharmacological effect
or, in connection with the desired therapy, no appreciable or at least no
undesirable
pharmacological effect. The excipients and additives include, for example,
surfactants
such as soya lecithin, oleic acid, sorbitan esters, such as polysorbates,
polyvinylpyrrolidone, other stabilisers, complexing agents, antioxidants
and/or
preservatives which guarantee or prolong the shelf life of the finished
pharmaceutical
formulation, flavourings, vitamins and/or other additives known in the art.
The additives
also include pharmacologically acceptable salts such as sodium chloride as
isotonic agents.
The preferred excipients include antioxidants such as ascorbic acid, for
example, provided
that it has not already been used to adjust the pH, vitamin A, vitamin E,
tocopherols and
similar vitamins and provitamins occurring in the human body.
Preservatives may be used to protect the formulation from contamination with
pathogens.
Suitable preservatives are those which are known in the art, particularly
cetyl pyridinium
chloride, benzalkonium chloride or benzoic acid or benzoates such as sodium
benzoate in
the concentration known from the prior art. The preservatives mentioned above
are
preferably present in concentrations of up to 50mg/I00ml, more preferably
between 5 and
20mg/ 100ml.
Preferred formulations contain, in addition to the solvent water and the
combination of
active substances 1 and 2, only benzalkonium chloride and sodium edetate. In
another
preferred embodiment, no sodium edetate is present.
CA 02559699 2006-09-12
WO 2005/102349 75 PCT/EP2005/004073
The propellant-free inhalable solutions according to the invention are
administered in
particular using inhalers of the kind which are capable of nebulising a small
amount of a
liquid formulation in the therapeutic dose within a few seconds to produce an
aerosol
suitable for therapeutic inhalation. Within the scope of the present
invention, preferred
inhalers are those in which a quantity of less than 100 L, preferably less
than 50 L, more
preferably between 10 and 30 L of active substance solution can be nebulised
in
preferably one spray action to form an aerosol with an average particle size
of less than
20 m, preferably less than 10 m, such that the inhalable part of the aerosol
corresponds to
the therapeutically effective quantity.
An apparatus of this kind for propellant-free delivery of a metered quantity
of a liquid
pharmaceutical composition for inhalation is described for example in
International Patent
Application WO 91/14468 and also in WO 97/12687 (cf. in particular Figures 6a
and 6b).
The nebulisers (devices) described therein are known by the name Respimat .
The above-mentioned examples of the active substances 2 are known in the art.
The
compounds of formula 1 by contrast are not known in the art.
The examples of synthesis described hereinafter serve to illustrate possible
methods of
synthesising the new compounds of formula 1. However, they are intended only
as
examples of procedures as an illustration of the invention without restricting
the invention
to the subject-matter described by way of example.
Example 1.1: 6-hydroxy-8- { 1-hydrox2-[2-(4-methoxy-phenyl)-1,1-dimethyl-
ethylamino]-ethyl } -4H-benzo[ 1,41 oxazin-3 -one
O~O H
H
HN N
Me Me
OMe
OH
a) 8-12-{ 1 1-dimethyl-2-(4-methoxy-phenyl)-ethylamino]-1-hydroxy-ethyl } -6-
benzyloxy-
4H-benzo[1,4]oxazin-3-one
7.5 g (6-benzyloxy-4H-benzo[1,4]oxazin-3-one)-glyoxalhydrate are added at 70 C
to a
solution of 3.6 g 1,1-dimethyl-2-(4-methoxyphenyl)-ethylamine in 100 mL
ethanol and the
CA 02559699 2006-09-12
WO 2005/102349 76 PCT/EP2005/004073
mixture is stirred for 15 minutes. Then 1 g sodium borohydride is added within
30 minutes
at 10 to 20 C. It is stirred for one hour, combined with 10 mL acetone and
stirred for a
further 30 minutes. The reaction mixture is diluted with 150 mL ethylacetate,
washed with
water, dried with sodium sulphate and evaporated down. The residue is
dissolved in 50 mL
methanol and 100 mL ethyl acetate and acidified with conc. hydrochloric acid.
After the
addition of 100 mL diethyl ether the product crystallises out. The crystals
are filtered off,
washed and recrystallised from 50 mL ethanol.
Yield: 7 g (68%; hydrochloride); m.p. = 232-234 C.
b) 82-[ 1 1-dimethyl-2-(4-methoxy-phen ll)-ethylaminol- l -hydroxy-ethyl } -6-
hydroxy-
4H-benzo[ 1,4loxazin-3-one
6.8 g of the benzyl compound obtained previously are hydrogenated in 125 mL
methanol
with the addition of 1 g palladium on charcoal (5%) at ambient temperature and
normal
pressure. The catalyst is filtered off and the filtrate freed from solvent.
After
recrystallisation of the residue from 50 mL acetone and some water a solid is
obtained
which is filtered off and washed.
Yield: 5.0 g (89 %; hydrochloride); m.p. = 155-160 C.
The (R)- and (S)-enantiomers of Example 1.1 may be obtained from the racemate,
for
example, by chiral HPLC (e.g. column: Chirobiotic T made by Messrs Astec, 250
x 22.1
mm, 5 m). Methanol with 0.05 % triethylamine and 0.05% acetic acid may be
used as the
mobile phase. The retention times of the R- or S-enantiomer are between 35 and
65 min
depending on the flow, while the R-enantiomer is eluted first. Of outstanding
importance
according to the invention is the R-enantiomer of Example 1.1.
Example 1.2: 6-hydroxy- 8-11 -hdroxy-2-[2-(4-phenoxyethvl-acetate)-1,1-
dimethyl-
ethlam mino]-ethyl }-4H-benzo[1,4loxazin-3-one
Oy 0 OH
H
HN N
Me Me / OEthyl
O"-r
OH O
a) 8- {2-[ 1 1-dimethyl -2_(4-phenoxyethyl-acetate)-ethylamino] -1-hydroxy-
ethyl}-6-
benzyloxy-4H-benzo[ 1,4loxazin-3-one
CA 02559699 2006-09-12
WO 2005/102349 77 PCT/EP2005/004073
Analogously to the method described in Example 1.1 a) the title compound is
obtained from
15 g (6-benzyloxy-4H-benzo[1,4]oxazin-3-one)-glyoxalhydrate and 11.8 g 1,1-
dimethyl-2-
(4-phenoxyethyl-acetate)-ethylamine hydrochloride.
Yield: 16.5 g (69%, hydrochloride); m.p. = 212-214 C.
b) 8- 12-[ 1,1 -dimeth l~ 2-(4-phenoxyethyl-acetate -ethylamino] -1-h d~y-
ethyl } -6-
hydroxy-4H-benzo[ 1,41 oxazin-3 -one
8 g of the benzylalcohol obtained previously are dissolved in 100 mL ethanol,
100 mL
methanol and 10 mL water and hydrogenated in the presence of 1 g palladium on
charcoal
(5%). After the absorption of the theoretically calculated amount of hydrogen
the catalyst
is filtered off and the filtrate is evaporated down. The product that
crystallises out on
distillation of the solvent is suction filtered and washed.
Yield: 5.5 g (81%; hydrochloride); m.p. = 137-140 C.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.3: 6-hydroxy-8- 11-h dY roxy-2-[2-(4 phenoxy-acetic acid)- 1, 1 -
dimethyl-
ethylaminol -ethyl } -4H-benzo[ 1,41oxazin-3-one
O~O H
H
HN N
Me Me I / n
O COOH
OH
11 g of 8-{2-[ 1,1-dimethyl-2-(4-phenoxyethyl-acetate)-ethylamino]-1-hydroxy-
ethyl }-6-
benzyloxy-4H-benzo[1,4]oxazin-3-one hydrochloride (Example 4a) are dissolved
in 125
mL methanol and hydrogenated in the presence of 1 g palladium on charcoal
(5%). After
the absorption of the theoretically calculated amount of hydrogen the catalyst
is filtered
off. 2.6 g sodium hydroxide dissolved in 20 mL water are added to the
filtrate. The mixture
is refluxed for 30 minutes, the methanol is distilled off and 10 mL water, 20
mL n-butanol
and 3.9 mL acetic acid. The solid precipitated is suction filtered and washed
with diethyl
ether.
Yield: 7 g (87%). The hydrochloride is obtained by recrystallisation from 0.5
molar
hydrochloric acid. M.p. = 152 C.
CA 02559699 2006-09-12
WO 2005/102349 78 PCT/EP2005/004073
The (R)- and (S)-enantiomers of this embodiment maybe obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.4: 8- {2-[ 1 1-dimethyl-2-(2 4 6-trimethylphenyl)-ethylaminol- l-
hydroxy-
ethyl)-6-h. dy roxy-4H-benzo[1,4]oxazin-3-one
O
~O OH Me
HN N
Me Me
Me Me
OH
a) 1-(6-benzyloxy-4H-benzo[ 1 4]oxazin-3-one)-2-[ 1, l -dimethyl-2-((2,4,6-
trimethylphenyl)-
eth liy mino]-ethanone
7.2 g (6-benzyloxy-4H-benzo[1,4]oxazin-3-one)-glyoxalhydrate and 3.6 g 1,1-
dimethyl-2-
(2,4,6-trimethylphenyl)-ethylamine are heated to 70 C for one hour in 100 mL
ethanol.
After cooling the precipitated crystals are filtered off and washed with
ethanol and diethyl
ether. Yield: 8.6 g (94%); m.p. = 175 C.
b) 8-{2-[1 1-dimethyl-2-(2 4 6-trimethylphenyl -ethylamino]-1-h dy rox -ey
thyl}-6-
benzyloxy-4H-benzo[ 1,41oxazin-3-one
8.6 g of the Schiff base obtained according to the prescribed method 1.4a) are
dissolved in
100 mL ethanol and 20 mL THF, combined with 0.7 g sodium borohydride within 30
min
at 10-20 C and stirred for one hour. After the addition of 10 mL acetone the
mixture is
stirred for 30 minutes and then diluted with ethyl acetate and water. The
product that
crystallises out during acidification with conc. hydrochloric acid is filtered
off and washed.
Yield: 7.4 g (80%, hydrochloride); m.p. = 235 C (decomposition).
c) 8-12- F 1 1 -dimethyl-2-(24,6-trimethylphenyl -ethylamino]-1-h dy roxy-
ethyl } -6-hydroxy-
4H-benzo[ 1,41oxazin-3 -one
7.4 g of the benzyl compound obtained in Step b) are hydrogenated in 125 mL
methanol
with the addition of 1 g palladium on charcoal (5%) at ambient temperature and
normal
pressure. Then the catalyst is filtered off and the filtrate evaporated down.
The product that
crystallises out on the addition of acetone is suction filtered and washed
with acetone and
diethyl ether. Yield: 5 g (78%, hydrochloride); m.p. 160 C (decomposition).
CA 02559699 2006-09-12
WO 2005/102349 79 PCT/EP2005/004073
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.5: 6-hydroxy-8- { 1-hydroxy-2-[2-(4-hydroxy-phenyl)-1,1-dimethyl-
ethylaminoLethyl } -4H-benzo[ 1,41oxazin-3 -one
O~O OH
H
HN / N
Me Me
OH
OH
a) 8-{2-[1 1-dimethyl-2-(4-hydrox -phenyl)-ethylamino]-1-h dy roxy-ethyl}-6-
benzyloxy-
4H-benzo[1,4]oxazin-3-one
The title compound is prepared from 10 g (6-benzyloxy-4H-benzo[ 1,4]oxazin-3-
one)-
glyoxalhydrate and 4.6 g 1,1-dimethyl-2-(4-hydroxy-phenyl)-ethylamine
analogously to
the procedure laid down for Example 1.1 a).
Yield: 9.0 g (64%, hydrochloride); m.p. = 255-258 C.
b) 8-{2-[1 1-dimethyl-2-(4-hydroxy_phenyl)-eth lay mino]-1-h dY roxy-ethyl}-6-
hydroxy.
4H-benzo[ 1,4]oxazin-3-one
5.7 g of the coupling product obtained previously are hydrogenated in the
presence of 0.6 g
palladium on charcoal (5%) in 100 mL methanol. After the absorption of the
theoretically
calculated amount of hydrogen the catalyst is filtered off and the filtrate
freed from
solvent. The residue is dissolved in ethanol with heating and then combined
with diethyl
ether. The product precipitated is suction filtered and recrystallised once
from water.
Yield: 3.6 g (72%, hydrochloride); m.p. = 159-162 C.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.6: 6-hydroxy-8- { 1-hydroxy-2-[2-(4-isopropyl-phenyl)-1,1 dimethyl-
ethylamino]-ethyl }-4H-benzo[ 1,41oxazin-3-one
CA 02559699 2006-09-12
WO 2005/102349 80 PCT/EP2005/004073
O
~O OH
H
HN N
OH
a)1_(4-i sopropyl-phenyl)-2-methyl -propan-2-ol
The reaction of a Grignard compound, prepared from 20 g (119 mmol)
4-isopropylbenzyl chloride, with 11.4 ml (155 mmol) acetone yields the target
compound
as a colourless oil. Yield: 13.0 g (57%); mass spectrometry: [M+H]+ = 193.
b) N-[2-(4-isopropyl-phenyl)-1,1-dimethyl-ethyl]-acetamide
A Ritter reaction is carried out with 10.2 g (53 mmol) 1-(4-isopropyl-phenyl)-
2-methyl-
propan-2-ol in the manner described for Example 1.7b). The reaction mixture is
poured
onto ice water and made alkaline with sodium hydroxide solution, during which
time a
solid is precipitated. This is suction filtered and dried.
Yield: 9.90 g (80%); mass spectrometry: [M+H]+ = 234.
c) 2-(4-isopropyl-phenyl)- 1,1-dimethyl-ethylamine
Reaction of 9.80 g (42 mmol) N-[2-(4-isopropyl-phenyl)-1,1-dimethyl-ethyl]-
acetamide
analogously to the procedure laid down for Example 1.7c).
Yield: 7.00 g (71 %, hydrochloride); m.p. 202-206 C.
d) 6-benzylox8-{1-hydrox2-[2-(4-isopropyl-phenyl)-1,1-dimethyl-ethylaminol-
ethyl)-
4H-benzo[ 1,41oxazin-3-one
2.18 g (6.1 mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[ 1,4]oxazin-
3 -one
and 1.1 g (5.8 mmol) 2-(4-isopropyl-phenyl)- 1, 1 -dimethyl-ethylamine are
stirred for one
hour at 50-80 C in 40 mL ethanol. After cooling to ambient temperature 0.24 g
(6.3 mmol)
sodium borohydride are added. The mixture is stirred for one hour, diluted
with 5 mL
acetone and stirred for a further 30 minutes. The reaction mixture is
acidified with
hydrochloric acid, combined with 100 mL water and 80 mL ethyl acetate and made
alkaline with ammonia. The organic phase is separated off, dried with sodium
sulphate and
freed from solvent. The residue is dissolved in 20 mL ethyl acetate and 10 mL
water,
acidified with conc. hydrochloric acid and diluted with diethyl ether. After
the addition of a
crystallisation aid the precipitated solid is suction filtered and washed.
White solid.
CA 02559699 2006-09-12
WO 2005/102349 81 PCT/EP2005/004073
Yield: 1.7 g (52 %, hydrochloride); m.p. 220-222 C.
e) 6-hydroxy-8- 1 1-hydroxy-2-[2-(4-isopropl-phenyl)-1.1 dimethyl-ethyl amino]
-ethyl}_
4H-benzo[ 1,41oxazin-3-one
1.6 g (3.0 mmol) 6-benzyloxy-8- 1 1-hydroxy-2-[2-(4-isopropyl-phenyl)-1,1-
dimethyl-
ethylamino]-ethyl }-4H-benzo[I,4]oxazin-3-one are dissolved in methanol and
hydrogenated with palladium on charcoal as catalyst at normal pressure and
ambient
temperature. The catalyst is suction filtered, the solvent is distilled off
and the residue is
recrystallised from isopropanol. White solid.
Yield: 1.1 g (85%, hydrochloride); m.p. 248-250 C; mass spectrometry: [M+H]+ =
399.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.7: 8- 2-[2-(4-ethyll-phenyl)-1,1-dimethyl-ethylaminol-l-hydroxy-
ethyl -6-
h dY roxy-4H-benzo[1,4]oxazin-3-one
O
O OH
HN N
OH
a) 1-(4-ethyll-phenyl)-2-methyl-propan-2-o1
14.8 g (90 mmol) 1-(4-ethyl-phenyl)-propan-2-one, dissolved in diethyl ether,
are added
dropwise to 39 mL of a 3 molar solution of methylmagnesium bromide in diethyl
ether,
while being cooled with the ice bath, such that the temperature does not
exceed 30 C.
After the addition has ended the reaction mixture is left to reflux for 1.5
hours and then
hydrolysed with 10% ammonium chloride solution. After separation of the
organic phase
the aqueous phase is extracted with diethyl ether. The combined ether phases
are washed
with water, dried with sodium sulphate and evaporated down. The oil thus
obtained is
further reacted directly. Yield: 15.5 g (90%).
b) N-[2-(4-ethyl-phenyl)-1,1-dimethyl-ethyl]-acetamide
CA 02559699 2006-09-12
WO 2005/102349 82 PCT/EP2005/004073
6.2 mL conc. sulphuric acid are added dropwise within 15 minutes to 15.5 g (87
mmol) 1-
(4-ethyl-phenyl)-2-methyl-propan-2-ol in 4.8 mL (91 mmol) acetonitrile and 15
mL glacial
acetic acid, while the temperature rises to 65 C. Then the mixture is stirred
for one hour,
diluted with ice water and made alkaline with conc. sodium hydroxide solution.
After
further stirring for 30 minutes the precipitated solid is suction filtered and
washed with
water. The crude product is dissolved in ethyl acetate, dried with sodium
sulphate and
evaporated down. The oil remaining is combined with petroleum ether, during
which time
a solid is precipitated, which is filtered off and dried. Yield: 16.3 g (85%);
m.p. 90-92 C.
c) 2-(4-ethyl-phenyl)- 1,1 -dimethyl-ethylamine
16.3 g (74 mmol) N-[2-(4-ethyl-phenyl)- 1, 1 -dimethyl-ethyl]-acetamide and
8.0 g
potassiumhydroxid are through 15 hours in 60 mL ethyleneglycol at reflux
temperature
heated. The reaction mixture is combined with ice water and extracted three
times with
diethyl ether. The combined organic phases are washed with water, dried with
sodium
sulphate and freed from solvent. To produce the hydrochlorides, the crude
product is
dissolved in acetonitrile and successively combined with ethereal hydrochloric
acid and
diethyl ether. The solid precipitated is suction filtered and dried. Yield:
11.0 g (69%,
hydrochloride); m.p. 165-167 C.
d) 6-benzylox8-12-[2-(4-ethyl-phenyl)-1,1-dimethvl-ethylamino]-1-hydroxy-
ethyl}-4H-
benzo[ 1,4]oxazin-3-one
The target compound is prepared analogously to the procedure laid down for
Example
1.6d) from 2.14 g (6.0 mmol) 6-benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-
benzo[ 1,4]oxazin-3 -one and 1.0 g (5.6 mmol) 2-(4-ethyl-phenyl)-1,1-dimethyl-
ethylamine.
White solid. Yield: 1.7 g (54%, hydrochloride); m.p. 210-214 C.
e) 8- {2-[2-(4-ethyl-phenyl)-1 1-dimethvl-ethylaminol-1-hydroxy-ethyl } -6-h
dy roxy-4H-
benzo[1,4]oxazin-3-one
The hydrogenolysis of 1.45 g (2.75 mmol) 6-benzyloxy-8-{2-[2-(4-ethyl-phenyl)-
1,1-
dimethyl-ethylamino] -1 -hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one according to
the
prescribed method for Example 1.6e) yields the target compound in the form of
a white
solid. Yield: 1.07 g (92%; hydrochloride); m.p. 266-269 C; mass spectrometry:
[M+H]+ _
385.
CA 02559699 2006-09-12
WO 2005/102349 83 PCT/EP2005/004073
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.8: 8-{2-[2-(4-fluoro-3-methyl-phenyl)-1,1-dimethyl-ethla~mino]-1-
hydroxy-
ethyl }-6-h day-4H-benzo[1,41oxazin-3-one
O
~O OH
HN N
F
OH
a) 1-fluoro-2-methyl=4-(2-methyl-propenyl)-benzene
100 mL of a 0.5 molar solution of 4-fluoro-3-methyl-phenylmagnesium bromide in
THE
are combined within 30 minutes with 4.7 mL (50 mmol) isopropylaldehyde, while
the
temperature rises to 45 C. The mixture is stirred for 30 minutes, refluxed for
1 hour and
then hydrolysed with 10% ammonium chloride solution. After separation of the
organic
phase the mixture is extracted with diethyl ether. The organic phases are
combined, dried
and evaporated down. The alcohol thus obtained is dissolved in 100 mL toluene,
combined
with 1 g p-toluenesulphonic acid monohydrate and refluxed for three hours
using the water
separator. The reaction mixture is poured onto water and made alkaline with
conc. sodium
hydroxide solution. After separation of the organic phase the latter is washed
with water,
dried with sodium sulphate and freed from solvent. Fractional distillation of
the residue
yields the product in the form of a colourless liquid (b.p. 80-85 C/10 mbar).
Yield: 4.1 g (50%).
b) N- [2-(4-fluoro-3-meth phenyl)-1,1-dimethyl-ethy]-formamide
4.9 mL conc. sulphuric acid are added dropwise at 5-15 C to 1.5 g (31 mmol)
sodium
cyanide in 5 mL glacial acetic acid. Then the mixture is combined with 3.9 g
(24 mmol) 1-
fluoro-2-methyl-4-(2-methyl-propenyl)-benzene, dissolved in 10 mL glacial
acetic acid,
and stirred for 1 hour at 50-60 C. The reaction mixture is diluted with ice
water, made
alkaline with conc. sodium hydroxide solution and extracted with
dichloromethane. The
organic phase is dried with sodium sulphate and freed from the solvent in
vacuo. The light
yellow oil thus obtained is further reacted directly. Yield: 4.3 g (87%).
CA 02559699 2006-09-12
WO 2005/102349 84 PCT/EP2005/004073
c) 2-(4-fluoro-3-methyll-phenyl)-1,1-dimethyl-eth lay mine
4.3 g (20.6 mmol) N- [2-(4-fluoro-3 -methyl-phenyl)- 1, 1 -dimethyl -ethyl] -
formamide, 20
mL cone. hydrochloric acid and 20 mL water are refluxed for 2 hours. The
reaction
mixture is diluted with water, made alkaline with conc. sodium hydroxide
solution and
extracted with dichloromethane. The organic phases are dried with sodium
sulphate and
evaporated down. The residue is dissolved in ethyl acetate, combined with
ethereal
hydrochloric acid and cooled. The crystals precipitated are suction filtered
and washed
with diethyl ether and dried. White solid.
Yield: 3.9 g (87%, hydrochloride); m.p. 196-198 C.
d) 6-benzyloxy-8- {2-[2-(4-fluoro-3-methyll-phenyl)-1 1-dimethyl-ethylaminol-l
-hydroxv-
ethyl } -4H-benzo[ 1,41 oxazin-3-one
1.10 g (3.1 mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[1,4]oxazin-
3-one
and 0.50 g (2.8 mmol) 2-(4-fluoro-3-methyl-phenyl)-1,1-dimethyl-ethylamine are
reacted
and worked up analogously to the procedure laid down for Example 1.6d). White
solid.
Yield: 0.75 g (47%, hydrochloride); m.p. 228-230 C.
e) 8-{2-[2-(4-fluoro-3-methyll-phenyl)-1 1-dimethyl-ethylaminol-1-hydroxv-
ethyl}-6-
hydroxy-4H-benzo[ 1,41oxazin-3-one
The hydrogenation of 0.70 g (1.4 mmol) 6-benzyloxy-8-{2-[2-(4-fluoro-3-methyl-
phenyl)-
1,1-dimethyl-ethylamino] -1-hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one yields
the target
compound as a white solid.
Yield: 0.50 g (87%, hydrochloride); m.p. 278-280 C; mass spectroscopy: [M+H]+
= 389.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.9: 8-{2-[2-(4-fluoro-2-methyl-phenyl)-1,1-dimethyl-ethylaminol-l-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,41 ox azin-3 -one
~O OH
HN N
F
OH
CA 02559699 2006-09-12
WO 2005/102349 85 PCT/EP2005/004073
a) 1-(4-fluoro-2-methyl-phenyl)-2-methyl-propyl acetate
500 mL of a 0.5 molar solution of 4-fluoro-6-methylphenylmagnesium bromide and
23.2
mL (260 mmol) isopropylaldehyde are reacted analogously to Example 1.8a).
After
hydrolysis with 10% ammonium chloride solution the aqueous phase is separated
off and
extracted with diethyl ether. The combined organic phases are dried with
sodium sulphate
and evaporated down. The alcohol thus obtained is then dissolved in 50 mL
acetic
anhydride, combined with 1 mL conc. sulphuric acid and stirred for three hours
at reflux
temperature. Then the reaction mixture is poured onto water, stirred for a
further hour and
made alkaline. It is extracted with dichloromethane, the organic phases are
dried with
sodium sulphate and the solvents are distilled off. Fractional distillation of
the residue
yields the product in the form of a colourless liquid (b.p. 105-110 C/8 mbar).
Yield: 29.0 g (52%).
b) N-[2-(4-fluoro-2-methyl-phenyl)-1,1-dimethyl-ethyl]-formamide
29.0 g (130 mmol) 1-(4-fluoro-2-methyl-phenyl)-2-methyl-propyl acetate
are reacted and worked up analogously to the procedure laid down for Example
1.8b).
Yellow oil. Yield: 27.0 g (99%).
c) 2-(4-fluoro-2-methyl-phenyl)-1,1-dimethyl-ethylamine
In order to prepare the amine 27.0 g (130 mmol) N-[2-(4-fluoro-2-methyl-
phenyl)-1,1-
dimethyl-ethyl]-formamide are reacted as described in the procedure laid down
for
Example 1.8c). White solid. Yield: 15.5 g (55%, hydrochloride); m.p. 277-280
C.
d) 6-benzyloxy-8- {2-[2-[4-fluoro-2-methyll-phenyl)-1,1-dimethyl-ethylaminol-l
-hydroxy-
ethyl } -4H-benzo[ 1,41 oxazin-3 -one
Prepared analogously to the procedure laid down for Example 1.6d) from 0.95 g
(2.66
mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo [ 1,4] oxazin-3 -one
and 0.43 g
(2.37 mmol) 2-(4-fluoro-2-methyl-phenyl)-1,1-dimethyl-ethylamine.
Yield: 0.75 g (55%, hydrochloride); m.p. 233-236 C.
e) 8- {2-[2-(4-fluoro-2-methyll-phenyl)-1,1-dimethyl-ethyl amino] -1-hydroxy-
ethyl
h dy roxy-4H-benzo[1,4]oxazin-3-one
CA 02559699 2006-09-12
WO 2005/102349 86 PCT/EP2005/004073
The debenzylation of 0.70 g (1.36 mmol) 6-benzyloxy-8-{2-[2-[4-fluoro-2-methyl-
phenyl) - 1,1-dimethyl-ethylamino] -1-hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one
yields the
target compound in the form of a white solid.
Yield: 0.50 g (87%, hydrochloride); m.p. 278-280 C; mass spectroscopy: [M+H]+
= 389.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.10: 8 {2-[2-(2,4-difluoro-phenyl)-1 1-dimethyl-ethylaminol-1-hydroxy-
ethyl}-
6-h dy roxy-4H-benzo[1,41oxazin-3-one
O
~O OH F
HN N
11511 F
OH
a) I -(2 4-difluoro phenyl)-2-methyl_propan-2-ol
11.0 mL acetone, diluted with 50 mL diethyl ether, are added dropwise within
20 minutes
to a solution of 500 mL of 0.25 molar 2,4-difluorobenzylmagnesium bromide in
diethyl
ether. Then the mixture is stirred for 1.5 hours at reflux temperature and
then hydrolysed
with 10% ammonium chloride solution. The ether phase is separated off, washed
with
water, dried with sodium sulphate and evaporated down. The fractional
distillation of the
residues yields the alcohol as a colourless liquid (b.p. 70-73 C/ 2 mmbar).
Yield: 20.0 g (86%).
b) N-[2-(2 4-difluoro-phenyll-1,1-dimethyl-ethyll-formamide
Ritter reaction with 20 g (110 mmol) 1-(2,4-difluoro-phenyl)-2-methyl-propan-2-
ol
according to the process described for Example 1.8b). Yellow oil. Yield: 22.0
g (94%).
c) 2-(2 4-difluoro-phenyl)-1,1-dimethvl-ethylamine
Reaction of 22.0 g (100 mmol) N-[2-(2,4-difluoro-phenyl]-1,1-dimethyl-ethyl]-
formamide
analogously to the procedure laid down for Example 1.8c).
Yield: 16.0 g (72%, hydrochloride); m.p. 201-203 C.
CA 02559699 2006-09-12
WO 2005/102349 87 PCT/EP2005/004073
d) 6-benzyloxy-8-12-[2-(2 4-difluoro-phenyl)-1 1-dimethyl-ethylamino]-1-
hydroxy-ethyl}-
4H-benzo[ 1,41oxazin-3 -one
Reaction of 0.89 g (2.49 mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-
benzo[1,4]oxazin-3-one and 0.40 g (2.16 mmol) 2-(2,4-difluoro-phenyl)-1,1-
dimethyl-
ethylamine in the manner described for Example 1.6d).
Yield: 0.80 g (62%, hydrochloride); m.p. 245-247 C.
eL{2-[2-(2 4-difluoro-phenyl)-1 1-dimethyl-ethylaminol-l-hydroxyl}-6-hydroxy-
4H-benzo[ 1,41oxazin-3 -one
The hydrogenolysis of 0.70 g (1.35 mmol) 6-benzyloxy-8-{2-[2-(2,4-difluoro-
phenyl)-1,1-
dimethyl-ethylamino] -1 -hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one yields the
target
compound as a white solid.
Yield: 0.48 g (83%, hydrochloride); m.p. 279-280 C; mass spectroscopy: [M+H]+
= 393.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.11: 8-{2-[2-(3 5-difluoro-phenyl)-1 1-dimethyl-ethylaminol-l-h dy
roxy-ethyl}-
6-h, dy roxy-4H-benzo[1,41oxazin-3-one
O
~O OH
HN N F
OH F
a) 1-(3,5-difluoro-phenyl)-2-methyll-propan-2-ol
The target compound is obtained by reacting a Grignard compound, prepared from
25.0 g
(121 mmol) 3,5-difluorobenzylbromide, with 12.6 mL (171 mmol) acetone. Yellow
oil.
Yield: 13.5 g (60%).
b) 2-(3 ,5-difluoro-phenyl)-1,1-dimethyl-eth ly amine
The Ritter reaction of 5.5 g (29.5 mmol) 1-(3,5-difluoro-phenyl)-2-methyl-
propan-2-ol
and 1.8 g sodium cyanide yields 7.0 g formamide, which is treated with
hydrochloric acid
in order to cleave the formyl group. Light yellow oil. Yield: 4.6 g (75%).
CA 02559699 2006-09-12
WO 2005/102349 88 PCT/EP2005/004073
c) 6-benzyloxy-8-{2-[2-(3 5-difluorophenyl)-1 1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-
4H-benzo[ 1,41oxazin-3 -one
Prepared from 1.73 g (4.84 mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-
benzo[1,4]oxazin-3-one and 0.80 g (4.32 mmol) 2-(3,5-difluoro-phenyl)-1,1-
dimethyl-
ethylamine in the conventional manner.
Yield: 1.50 g (58 %, hydrochloride); m.p. 240-244 C.
d) 8-{2-[2-(3 5-difluoro-phenyl)-1 1-dimethyyl=ethylamino] -l-h ddroxy-ethyl}-
6-hydroxy-
4H-benzo[1,4]oxazin-3-one
Hydrogenolysis of 1.30 g (2.43 mmol) 6-benzyloxy-8-{2-[2-(3,5-difluoro-phenyl)-
1,1-
dimethyl-ethylamino]-1-hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one yields the
target
compound as a white solid.
Yield: 0.90 g (86%, hydrochloride); m.p. 150-158 C; mass spectroscopy: [M+H]+
= 393.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.12: 8-{2-[2-(4-ethoxy-phenyl)-1 1-dimethvl-ethylamino)-l-hday-ethyl}-
6-
hydroxy-4H-benzo[1,4]oxazin-3-one
O OH
H
HN I N
OH
a) benzyl [2-(4-ethoxy-phenyl)-1,1-dimethyl-ethyl]-carbamate
15.0 g (50 mmol) benzyl [2-(4-hydroxy-phenyl)-1,1-dimethyl-ethyl]-carbamate
are stirred with 7.5 mL (92 mmol) ethyl iodide and 21 g (150 mmol) potassium
carbonate
for 10 hours at 90-100 C. The reaction mixture is combined with ethyl acetate,
washed
twice with water and dried with sodium sulphate. After the solvents have been
distilled off
a yellow oil remains (15.0 g, 92%), which is further reacted directly.
CA 02559699 2006-09-12
WO 2005/102349 89 PCT/EP2005/004073
b) 2-(4-ethoxy_phenyl)-1,1-dimethyl-eth lay mine
A solution of 15.0 g (49 mmol) benzyl [2-(4-ethoxy-phenyl)-1,1-dimethyl-ethyl]-
carbamate in 100 mL glacial acetic acid is combined with 2 g palladium on
charcoal (10%)
and then hydrogenated at 5 bar and 40 to 50 C. The catalyst is filtered off
and the filtrate
freed from solvent. The residue is dissolved in a little water, made alkaline
with conc.
sodium hydroxide solution and extracted with ethyl acetate. The organic phase
is washed
with water, dried with sodium sulphate and evaporated down. The crude product
is
dissolved in acetonitrile and acidified with ethereal hydrochloric acid. The
solid
precipitated after the addition of diethyl ether is suction filtered and
dried.
Yield: 8.8 g (hydrochloride, 84%); m.p. 198-200 C.
c) 6-benzylox -88-{2-j2-(4-ethox -phenyl)-1 1-dimethyl-ethylamino]-1-hvdroxv-
ethyl}-
4H-benzo [ 1,4] oxazin-3 -one
2.14 g (6.0 mmol) 6-benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-
benzo[1,4]oxazin-3-one
and 1.0 g (5.2 mmol) 2-(4-ethoxy-phenyl)- 1, 1 -dimethyl-ethylamine are
stirred in 40 mL
ethanol for one hour at 50-80 C. After cooling to ambient temperature 0.23 g
(6.0 mmol)
sodium borohydride are added and the mixture is stirred for a further hour.
The reaction
mixture is combined with 5 ml acetone, stirred for 30 minutes, acidified with
glacial acetic
acid and evaporated down. The residue is combined with water and ethyl acetate
and made
alkaline. The organic phase is separated off, washed with water, dried with
sodium
sulphate and freed from solvent in vacuo. The residue is dissolved again in
ethyl acetate
and water, combined with cone, hydrochloric acid and diluted with diethyl
ether. The solid
precipitated is suction filtered and washed with diethyl ether. White solid.
Yield: 2.0 g (61 %, hydrochloride); m.p. 214-216 C.
d) 8- {2-[2-(4-ethoxy-phenyl)-1 1-dimethyl-ethylaminol-1-hydroxy-ethyl} -6-
hvdroxv-4H-
benzo[1,4]oxazin-3-one
1.5 g (2.8 mmol) 6-benzyloxy-8-{2-[2-(4-ethoxy-phenyl)-1,1-dimethyl-
ethylamino]-1-
hydroxy-ethyl}-4H-benzo[1,4]oxazin-3-one in 80 mL methanol are hydrogenated
with 250
mg palladium on charcoal (10 %) as catalyst at ambient temperature and normal
pressure.
The catalyst is suction filtered and the filtrate is evaporated down. The
residue is dissolved
in 5 mL ethanol by heating, inoculated and diluted with ethyl acetate. The
solid
precipitated is filtered off and washed. White solid.
Yield 1.0 g (83%, hydrochloride); m.p. 232-235 C; mass spectrometry: [M+H]+ =
401.
CA 02559699 2006-09-12
WO 2005/102349 90 PCT/EP2005/004073
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.13: 8-{2-[2-(3 5-dimethyl-phenyl)-1 1-dimethyl-ethylamino] -l-
hydroxy-
ethyl I -6-hydroxy-4H-benzor 1,41oxazin-3 -one
O
TO OH
HN N
OH
a) 1 -(3 5-dimethphenyl -2-methyl-propanol-2-ol
Obtained from the reaction of ethyl (3,5-dimethyl-phenyl)-acetate with
methylmagnesium
bromide.
b) 2-(3 5-dimethyl-phenyl)-1,1-dimethyl-eth lam mine
By reacting 6.00 g (34 mmol) 1-(3,5-dimethyl-phenyl)-2-methyl-propanol-2-ol
and 2.00 g
(41 mmol) sodium cyanide in a Ritter reaction, 2.40 g 2-(3,5-dimethyl-phenyl)-
1,1-
dimethyl-ethylformamide (35% yield) are obtained. To liberate the amine the
formamide
(2.40 g, 11.7 mmol) is treated with hydrochloric acid. The preparation and
working up are
carried out analogously to the procedure laid down for Example 1.8c). Oil.
Yield: 1.70 g (82%); mass spectroscopy: [M+H]+ = 178.
c) 6-benzyloxy-8-{2-[2-(3 5-dimethyl-phenyl)-1 1-dimethyl-ethylaminol-l-
hydroxy-
ethyl } -4H-benzo[ 1,41oxazin-3-one
Prepared analogously to the procedure laid down for Example 1.6d) from 1.47 g
(4.1
mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[ 1,4] oxazin-3 -one and
0.65 g
(3.7 mmol) 2-(3,5-dimethyl-phenyl)-1,1-dimethyl-ethylamine.
Yield: 1.1 g (51 %, hydrochloride); m.p. 220-222 C.
d) 8-{2-[2-(3 5-dimethyl-phenyl)-1 1-dimethyl-ethylaminol-l-h dxy-ethyl}-6-
hydroxy-
4H-benzo{ 1,41oxazin-3 -one
The target compound was obtained after hydrogenolysis of 0.90 g (1.71 mmol) 6-
benzyloxy-8- {2-[2-(3,5-dimethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl } -4H-
CA 02559699 2006-09-12
WO 2005/102349 91 PCT/EP2005/004073
benzo[1,4]oxazin-3-one and recrystallisation of the crude product from
isopropanol. White
solid.
Yield: 0.50 g (69%, hydrochloride); m.p. 235-238 C; mass spectroscopy: [M+H]+
= 385.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.14: 4-(4- {2-[2-h dy roxy-2-(6-hydroxy-3-oxo-3,4-dihydro-2H-
benzo[1 41oxazin-8-yl-ethylaminol-2-methyl-propyl}-phenoxy)-butyric acid
o-
0 OH
HN I N
O OH
OH O
a) ethyl 4-[4-(2-amino-2-methyl-propyl)-phenoxy] -butyrate
4.5 g (15.0 mmol) benzyl [2-(4-hydroxy-phenyl)-1,1-dimethyl-ethyl]-carbamate,
2.3 mL
(16.0 mmol) ethyl 4-bromo-butyrate, 2.3 g (16.6 mmol) potassium carbonate and
0.3 g (1.8
mmol) potassium iodide in 20 mL dimethylformamide are heated to 120 C for 13
h. The
reaction mixture is diluted with ethyl acetate and washed successively with
water, sodium
hydroxide solution and water. The organic phase is dried with sodium sulphate
and
evaporated down. The residue is purified by chromatography (eluant:
cyclohexane/ethyl
acetate = 9:1). 5.0 g of a yellow oil is isolated, which is dissolved in 50 mL
acetic acid and
hydrogenated with 1.0 g palladium on charcoal as catalyst at 40 C and 3 bar.
The catalyst
is filtered off and the filtrate is freed from solvent. The residue is
dissolved in diethyl ether
and combined with ethereal hydrochloric acid. The solid precipitated is
suction filtered and
dried. Yield: 2.9 g (66% over two steps, hydrochloride); m.p. = 103-105 C.
b) ethyl 4- 4-2-[2-(6-benzyloxy-3-oxo-3 4-dihydro-2H-benzo[1,4]oxazin-8-yl)-2-
hydroxy-ethylamino]-2-methyll-propyl } -phenoxy)-butyrate
1.20 g (3.36 mmol) benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[1,4]oxazin-
3-one
and 0.90 g (3.22 mmol) ethyl 4-[4-(2-amino-2-methyl-propyl)-phenoxy]-butyrate
are
reacted in the manner described for Example 1.6d). The crude product is
dissolved in 10
mL ethyl acetate and 10 mL water and combined with oxalic acid with stirring.
The
CA 02559699 2006-09-12
WO 2005/102349 92 PCT/EP2005/004073
solution is diluted with diethyl ether and the solid precipitated is suction
filtered and
washed with diethyl ether. Yield: 1.20 g (54%, oxalate); m.p. 223-227 C.
c)4-(4-{2-[2-(6-benzyloxy-3-oxo-3 4-dihydro-2H-benzo[1 4]oxazin-8-yl)-2-
hydroxy-
eth lyamino]-2-methyl-propyl}-phenoxy)-butyric acid
A solution of 1.00 g (1.73 mmol) ethyl 4-(4-{2-[2-(6-benzyloxy-3-oxo-3,4-
dihydro-2H-
benzo[ 1,4]oxazin-8-yl)-2-hydroxy-ethylamino]-2-methyl-propyl } -phenoxy)-
butyrate in 25
mL methanol is combined with 2.5 mL I N sodium hydroxide solution, refluxed
for 30
minutes and then neutralised with 1 N hydrochloric acid. The solution is
evaporated down
and the oil remaining is dissolved in 5 mL n-butanol by heating. After the
addition of a
crystallisation aid a solid is precipitated, which is suction filtered and
washed with acetone
and diethyl ether. Yield: 0.75 g (79%); m.p. 216-218 C.
d) 4-(4- {2-[2-hydroxy-2-(6-hydroxy-3-oxo-3 4-dihydro-2H-benzo[ 1,41oxazin-8-
yl)-
ethylamino]-2-methyll-propyl}-phenoxy)-butyric acid
0.70 g (1.28 mmol) 4-(4-{2-[2-(6-benzyloxy-3-oxo-3,4-dihydro-2H-
benzo[1,4]oxazin-8-
yl)-2-hydroxy-ethylamino]-2-methyl-propyl}-phenoxy)-butyric acid are dissolved
in 25
mL methanol and 2 mL acetic acid and hydrogenated in the presence of 150 mg
palladium
on charcoal (10%) at ambient temperature and normal pressure. The catalyst is
filtered off
and the filtrate is freed from solvent. The product is obtained by
crystallisation from a
methanol/acetone mixture.
Yield: 0.40 g (68%); m.p. 201-204 C; mass spectroscopy: [M+H]+ = 459.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.15: 8-{2-[2-(3 4-difluoro-phenyl)-1 1-dimethyl-ethylaminol-l-hydroxy-
ethyl}-
6-hydroxy-4H-benzo[ 1,41oxazin-3 -one
O
~O OH
HN I N ~~a F
F
OH
CA 02559699 2006-09-12
WO 2005/102349 93 PCT/EP2005/004073
a) 1-(3,4-difluoro-phenyl)-2-methyl-propan-2-ol
A Grignard reagent is prepared from 23.0 g (111 mmol) 3,4-
difluorobenzylbromide and is
then reacted with 11.6 mL (158 mmol) acetone. Light yellow oil.
Yield: 9.7 g (47%); Rf value: 0.55 (ethyl acetate/petroleum ether = 1:3).
b) N-[2-(3 4-difluoro-phenyl)-1,1-dimethyl-ethyl]-formamide
The target compound is obtained by a Ritter reaction with 4.0 g (21.5 mmol) 1-
(3,4-
difluoro-phenyl)-2-methyl-propan-2-ol. Light yellow oil.
Yield: 4.0 g (87%); mass spectrometry: [M+H]+ = 214.
c) 2-(3 4-difluoro-phenyl)-1,1-dimethyl-eth lam
4.00 g (18.5 mmol) N-[2-(3,4-difluoro-phenyl)-1,1-dimethyl-ethyl]-formamide
are
dissolved in ethanol, combined with conc. hydrochloric acid and heated
overnight at reflux
temperature. The reaction solution is poured onto ice water, made alkaline
with sodium
hydroxide and extracted with tert-butylmethylether. The organic phases are
washed with
water, dried with sodium sulphate and evaporated down. Yellow oil.
Yield: 3.2 g (92%); mass spectrometry: [M+H]+ = 186.
d) 8-{2-j2-(3 4-difluoro-phenyl)-1 1-dimethyl-ethylamino]-1-h dY roxy-ethyl)-6-
hydroxy-
4H-benzo[ 1,41oxazin-3 -one
357 mg (1 mmol) 6-benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[1,4]oxazin-
3-one
and 185 mg (1 mmol) 2-(3,4-difluoro-phenyl)-1,1-dimethyl-ethylamine are
stirred for 30
minutes in 5 mL tetrahydrofuran at ambient temperature. The mixture is cooled
to 0 C and
under an argon atmosphere 1.5 mL of a 2 molar solution of lithium borohydride
in
tetrahydrofuran is added dropwise thereto. The resulting mixture is stirred
for 30 min at
ambient temperature, combined with 10 mL dichloromethane and 3 mL water,
stirred for a
further hour and then filtered through Extrelut . The eluate containing the
ethanolamine is
freed from solvent. The residue is dissolved in methanol and hydrogenated with
palladium
on charcoal (10%) as catalyst at 2.5 bar and ambient temperature. Then the
catalyst is
separated off and the crude product is purified by chromatography. White
solid.
Yield: 31 mg (6%, trifluoroacetate); mass spectroscopy: [M+H]+ = 393.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
CA 02559699 2006-09-12
WO 2005/102349 94 PCT/EP2005/004073
Example 1.16: 8- 12-f 2-(2-chloro-4-fluoro-phenyl)-1 1-dimethyl-ethylaminol- l
-hydroxy-
eth ly}-6-hday-4H-benzo[1,4]oxazin-3-one
O
~O OH CI
HN N
F
OH
a) 1-(2-chloro-4-fluoro-phenyl)-2-methyl-propan-2-ol
Prepared from 20 g (97 mmol) methyl (2-chloro-4-fluoro-phenyl)-acetate and 98
mL of a 3
molar solution of methylmagnesium bromide analogously to the procedure laid
down for
Example 1.6a).
b) N_[2-2-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethyll-formamide
7.5 g (37 mmol) 1-(2-chloro-4-fluoro-phenyl)-2-methyl-propan-2-ol were reacted
and
worked up according to the procedure described for Example 1.8b). The oil thus
obtained
was chromatographed for further purification on a short silica gel column
(petroleum
ether/ethyl acetate = 9:1). Oil. Yield: 7.4 g (87%); mass spectrometry: [M+H]+
= 230/232.
c) 2-(2-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethylamine
Reaction of 7.4 g (32 mmol) N-[2-(2-chloro-4-fluoro-phenyl)-1,1-dimethyl-
ethyl]-
formamide as described in the procedure laid down for Example 1.15c). Brown
oil.
Yield: 5.14 g (79%); mass spectrometry: [M+H]+ = 202/204.
d) 8- {2-[2-(2-chloro-4-fluoro-phenyl)-1 1-dimethvl-ethylamino]-1-h ddroxy-
ethyl } -6-
hydroxy-4H-benzo[ 1,41oxazin-3 -one
357 mg (1 mmol) 6-benzyloxy-8-(2-ethoxy-2-hydroxy-acetyl)-4H-benzo[ 1,4]oxazin-
3 -one
and 202 mg (1 mmol) 2-(2-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethylamine are
reacted
with lithium borohydride analogously to the procedure laid down for Example
1.8d). To
debenzylate the ethanolamine thus obtained it is dissolved in 3 mL
dichloromethane and
cooled to -78 C. At this temperature 2 ml of a I molar solution of boron
tribromide in
dichloromethane is added dropwise and the mixture is slowly left to come up to
ambient
temperature. The reaction mixture is combined with 10 mL dichloromethane and 3
mL
water and filtered through Extrelut . The eluate is freed from solvent and the
residue is
purified by chromatography. White solid.
CA 02559699 2006-09-12
WO 2005/102349 95 PCT/EP2005/004073
Yield: 70 mg (13%, trifluoroacetate); mass spectroscopy: [M+H]+ = 409/11.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.17: 8-{2-[2-(4-chloro-phenyl)-1 1-dimethyl-ethylaminol-1-hydroxy-
ethyl}-6-
hydroxy-4H-benzo[ 1,41oxazin-3 -one
O
~O OH
HN N
/ CI
OH
A solution of 300 mg (0.91 mmol) 6-benzyloxy-8-(2,2-dihydroxy-acetyl)-4H-
benzo[ 1,4]oxazin-3 -one and 200 mg (1.09 mmol) 2-(4-chloro-phenyl)-1,1-
dimethyl-
ethylamine in 3 mL ethanol was combined with molecular sieve and stirred for
90 minutes
at 80 C. The mixture was allowed to cool to ambient temperature, 35 mg (0.91
mmol) of
sodium borohydride were added and the resulting mixture was stirred for 1
hour. Then the
reaction mixture was combined with sodium hydrogen carbonate solution and
extracted
with ethyl acetate. The combined organic phases were freed from solvent and
the residue
was chromatographed (eluant: hexane/ethyl acetate/methanol), thus yielding 305
mg
ethanolamine. This was dissolved in 3 mL dichloromethane and cooled to -78 C
under an
argon atmosphere. 3 mL of a 1 molar solution of boron tribromide in
dichloromethane
were added dropwise and the mixture was stirred for one hour at -78 C and 20
minutes at
ambient temperature. Then at -78 C 3 mL conc. ammonia solution were added
dropwise
and the mixture was stirred for 5 minutes. The reaction mixture was combined
with
ammonium chloride solution and extracted with ethyl acetate. The combined
organic
phases were evaporated down and the residue was chromatographed for further
purification (silica gel; eluant: dichloromethane/methanol + 1% ammonia).
Beige solid: 93
mg (26%); mass spectrometry: [M+H]+ = 391.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
CA 02559699 2006-09-12
WO 2005/102349 96 PCT/EP2005/004073
Example 1.18: 8-{2-[2-(4-bromo-phen )-1 1-dimethyl-ethylaminol-1-hydroxy-
ethyl}-6-
h dy roxy-4H-benzo[I,4]oxazin-3-one
0 ,) O OH
HN N
Br
OH
The preparation and debenzylation of the ethanolamine were carried out as
described for
Example 1.17 from 300 mg (0.91 mmol) 6-benzyloxy-8-(2,2-dihydroxy-acetyl)-4H-
benzo[ 1,4]oxazin-3 -one and 250 mg (1.09) mmol) 2-(4-bromo-phenyl)-1,1-
dimethyl-
ethylamine. Beige solid. Yield: 54 mg (14%); mass spectrometry: [M+H]+ = 435,
437.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
Example 1.19: 8-{2-[2-(4-fluoro-phenyl)-1 1-dimethyl-ethylaminol-1-hvdroxv-
ethyl}-6-
h dy roxy-4H-benzo[ 1,41oxazin-3-one
0 OH
HN N
F
OH
300 mg (0.91 mmol) 6-benzyloxy-8-(2,2-dihydroxy-acetyl)-4H-benzo[ 1,4]oxazin-3
-one
and 183 mg (1.09 mmol) 2-(4-fluoro-phenyl)-1,1-dimethyl-ethylamine were
dissolved in 3
ml ethanol. Molecular sieve was added and the mixture was heated to 80 C for
30 minutes.
After cooling to ambient temperature 35 mg (0.91 mmol) sodium borohydride were
added.
The mixture was stirred for 1 hour at ambient temperature ruhren, then sodium
hydrogen
carbonate solution was added to the reaction mixture and this was extracted
with ethyl
acetate. The organic phases were evaporated down and the residue was
chromatographed
(eluant: hexane/ethyl acetate/methanol). The ethanolamine thus obtained (223
mg) was
dissolved in methanol in order to cleave the benzyl protecting group and
hydrogenated
CA 02559699 2006-09-12
WO 2005/102349 97 PCT/EP2005/004073
with 150 mg palladium hydroxide as catalyst at ambient temperature and normal
pressure.
The catalyst was separated off by filtration through Celite , the filtrate was
freed from
solvent and the residue was chromatographed (silica gel; eluant:
dichloromethane/methanol). Beige solid.
Yield: 76 mg (22%); mass spectrometry: [M+H]+ = 375.
The (R)- and (S)-enantiomers of this embodiment may be obtained by separation
of the
racemate analogously to common methods known in the art.
The following compounds of formula 1 according to the invention may also be
obtained
analogously to the synthesis examples described hereinbefore:
Example 1.20: 8-{2-[2-(4-fluoro-3-methoxy-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-ethyl } -6-hydroxy-4H-benzo [ 1,4]oxazin-3-one;
Example 1.21: 8-{2-[2-(4-fluoro-2,6-dimethyl-phenyl)-1,1-dimethyl-ethylamino] -
1-
hydroxy-ethyl } -6-hydroxy-4H-benzo [ 1,4]oxazin-3-one;
Example 1.22: 8- {2-[2-(4-chloro-2-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-
ethyl }-6-hydroxy-4H-benzo[1,4]oxazin-3-one;
Example 1.23: 8- {2-[2-(4-chloro-3-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.24: 8-{2-[2-(4-chloro-2-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,4] oxazin-3 -one;
Example 1.25: 8-{2-[2-(3-chloro-4-fluoro-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.26: 8- {2-[2-(2,6-difluoro-4-methoxy-phenyl)-1,1-dimethyl-
ethylamino] -1-
hydroxy-ethyl } -6-hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.27: 8-{2-[2-(2.5-difluoro-4-methoxy-phenyl)-1,1-dimethyl-ethylamino]
-1-
hydroxy-ethyl}-6-hydroxy-4H-benzo[1,4] oxazin-3-one;
CA 02559699 2006-09-12
WO 2005/102349 98 PCT/EP2005/004073
Example 1.28: 8-{2-[2-(4-fluoro-3,5-dimethyl-phenyl)-1,1-dimethyl-ethylamino] -
1-
hydroxy-ethyl } -6-hydroxy-4H-benzo[ 1,4] oxazin-3-one;
Example 1.29: 8- {2-[2-(3,5-dichloro-phenyl)-1,1-dimethyl-ethyl amino] -1-
hydroxy-ethyl } -
6-hydroxy-4H-benzo[ 1,4] oxazin-3 -one;
Example 1.30: 8-{2-[2-(4-chloro-3-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.31: 8-{2-[2-(3,4.5-tri fluoro-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-
ethyl } -6-hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.32: 8-{2-[2-(3-methyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-
ethyl}-6-
hydroxy-4H-benzo[ 1,4]oxazin-3-one;
Example 1.33: 8-{2-[2-(3,4-dichloro-phenyl)-1,1-dimethyl-ethylamino] -1-
hydroxy-ethyl}-
6-hydroxy-4H-benzo[ 1,4]oxazin-3-one.