Note: Descriptions are shown in the official language in which they were submitted.
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
NON-INVASIVE METHOD AND DEVICE FOR
DETECTING INSPIRATORY EFFORT
FIELD OF THE INVENTION
This invention relates generally to the diagnosis and treatment of breathing
disorders in sleeping and waking subjects. In one embodiment, the invention
relates to an
electrical device for monitoring and processing an electromyogram (EMG)
signal. In
another embodiment, the electrical device comprises non-invasive skin surface
electrodes
for the detection of EMG signals. In another embodiment, the electrical device
comprises a system for monitoring and recording of data by a patient such that
a
breathing disorder may be diagnosed by a clinician.
BACKGROUND
Over the past 30 years, clinicians and researchers have increasingly
recognized
the clinical importance of upper airway obstruction during sleep. Obstructive
sleep
apnea/hypopnea (OSA/H), a disorder affecting approximately 5% of the general
population, Young et al., "The Occurrence Of Sleep-Disordered Breathing Among
Middle-Aged Adults" NEfzgl JMed 328:1230-1235 (1993), is now understood to be
an
important cause of disturbed sleep and daytime sleepiness and a correlate of
hypertension, heart disease and stroke. Wolk et al., "Sleep-Disordered
Breathing And
Cardiovascular Disease" Circulation 108:9-12 (2003). Consequently, the number
of
clinical sleep laboratories has grown and technology has developed to
recognize and treat
upper airway obstruction during sleep.
During the past decade, however, it has become apparent that even mild levels
of
upper airway obstruction during sleep can have important clinical consequences
and
complicates sleep monitoring. This more subtle disorder, upper airway
resistance
syndrome (UARS), is characterized by only mild inspiratory airflow limitation
during
sleep, punctuated by arousals but not by significant involuntary abdominal
movements.
In UARS, by definition, few apneic or hypopneic events occur and they may be
entirely
absent. Patients with this disorder experience sleep onset insomnia, daytime
sleepiness or
fatigue and a variety of other functional complaints. Guilleminault et al., "A
Cause Of
Excessive Daytime Sleepiness. The Upper Airway Resistance Syndrome" Chest
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
104(3):781-7 (1993); Guilleminault et al. "Children And Nocturnal Snoring:
Evaluation
Of The Effects Of Sleep Related Respiratory Resistive Load And Daytime
Functioning"
Eur .I Pediatf° 139(3):165-71 (1982); and Gold et al., "The Symptoms
And Signs Of
Upper Airway Resistance Syndrome: A Link To The Functional Somatic Syndromes"
Chest 123(1):87-95 (2003).
Neither OSA/H or UARS are easily diagnosed without intrusive and
uncomfortable procedures. The physical nature of the necessary instrumentation
can
prevent the onset of sleep as well as the quality of sleep. Paradoxically, the
clinician has
no choice but to interfere with the very parameters involved in the diagnosis
of most
sleep disorders.
What is needed in the art, therefore, is a non-invasive, sensitive method to
diagnose breathing disorders that does not have a significant impact on a
patients' ability
for sleep. Moreover, there is a need for new technology for the diagnosis of
breathing
disorders that is sensitive, comfortable for a sleeping patient, and amenable
to
incorporation into medical devices for the diagnosis and treatment of sleep
disorders
outside clinical settings.
SUMMARY OF THE INVENTION
This invention relates generally to the diagnosis and treatment of breathing
disorders in sleeping and waking subjects. In one embodiment, the invention
relates to an
electrical device for monitoring and processing an electromyogram (EMG)
signal. In
another embodiment, the electrical device comprises non-invasive skin surface
electrodes
for the detection of EMG signals. In another embodiment, the electrical device
comprises a system for monitoring and recording of data by a patient such that
a
breathing disorder may be diagnosed by a clinician.
One embodiment of the present invention contemplates a method, comprising:
a) detecting an electrocardiogram signal within an electromyograrn signal,
said
electrocardiogram signal comprising a QRS complex, said QRS complex having an
amplitude; b) calculating an averaged amplitude of the QRS complex within said
electrocardiogram signal; c) comparing said averaged amplitude with a trigger
value and
generating a blanking pulse wherein said averaged amplitude exceeds said
trigger value,
2
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
said blanking pulse causing a blanker device to remove said electrocardiogram
signal
from said electromyogram signal. In one embodiment, said electromyogram signal
is
generated from skin surface electrodes connected to a subject. In one
embodiment, said
calculating of step (b) is performed by a microcontroller connected to said
electrodes.
One embodiment of the present invention contemplates a system, comprising:
a) a plurality of skin surface electrodes connected to a subj ect under
conditions such that
a electromyogram signal is generated, said electromyogram signal comprising a
contaminating electrocardiogram signal, said electrocardiogram signal
comprising a QRS
complex, said QRS complex having an amplitude; b) a microcontroller connected
to said
electrodes, said microcontroller capable of i) calculating an averaged
amplitude of the
QRS complex within said electrocardiogram signal, ii) comparing said averaged
amplitude, with a trigger value, and iii) generating a blanking pulse wherein
said averaged
amplitude exceeds said trigger value; and c) an EKG blanker configured to
receive said
blanking pulse, said EKG blanker capable of i) receiving said electromyogram
signal
comprising said electrocardiogram signal, and ii) removing said
electrocardiogram signal
from said electromyograrn signal.
One embodiment of the present invention contemplates a system, comprising:
a) a plurality of skin surface electrodes connected to a subject under
conditions such that
a contaminated electromyogram signal is generated, said contaminated
electromyogram
signal comprising a contaminating electrocardiogram signal, said
electrocardiogram
signal comprising a QRS complex, said QRS complex having an amplitude; b)
first and
second parallel filters configured for receiving said contaminated
electromyogram signal;
c) a microcontroller connected to said first filter so as to receive a
filtered
electrocardiogram signal, said microcontroller capable of i) calculating an
averaged
amplitude of the QRS complex within said filtered electrocardiogram signal,
ii) comparing said averaged amplitude with a trigger value, and iii)
generating a blanking
pulse wherein said averaged amplitude exceeds said trigger value; and d) an
EKG blanker
connected to said second filter so as to receive a filtered electromyogram
signal, said
EKG blanker further configured to receive said blanking pulse, said EKG
blanker capable
of i) receiving said filtered electromyogram signal comprising said
electrocardiogram
signal, and ii) removing said electrocardiogram signal from said
electromyogram signal.
3
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
One embodiment of the present invention contemplates a method for diagnosing a
breathing disorder, comprising: a) providing; i) a subject suspected of having
a breathing
disorder; ii) a plurality of skin surface electrodes capable of contacting
said subj ect,
wherein said electrodes are configured to generate a composite electromyogram
signal,
wherein said composite electromyogram signal comprises an electrocardiogram
artifact
signal; iii) a microcontroller connected to said electrodes and configured to
trigger a
blanking pulse upon calculation of a threshold average QRS peak from within
said
electrocardiogram artifact signal; and iv) an EKG blanker configured to
receive said
blanking pulse, wherein said blanker device is reconfigured to receive a
moving average
electromyogram signal; b) calculating said average QRS value from said
electrocardiogram artifact signal by said microcontroller, wherein said
threshold average
QRS value is detected; c) triggering said blanking pulse by said
microcontroller upon
detection of said threshold average QRS value; d) reconfiguring said EKG
blanker by
said blanking pulse to receive said moving average electromyogram signal; e)
displaying
said moving average electromyogram signal under conditions such that a
breathing
disorder is diagnosed. In one embodiment, the method further comprises the
step of
contacting said patient with said surface electrodes. In one embodiment, the
method
further comprises the step of filtering said electrocardiogram artifact signal
into a channel
to create an exaggerated electrocardiogram artifact signal. In one embodiment,
the
method further comprises the step of delaying said composite electromyogram
signal. In
one embodiment, said composite electrornyogram signal comprises a
diaphragmatic
electromyogram signal. In one embodiment, said reconfiguring of said EKG
blanker
replaces said electrocardiogram artifact signal with said moving average
electromyogram
signal. In one embodiment, at least one of said surface electrodes is
contacted with said
patient at the anterior axillary line. In another embodiment, at least one of
said surface
electrodes is contacted with said patient at the mid-axillary line.
One embodiment of the present invention contemplates an EMG monitoring
device for diagnosing a breathing order, comprising: a) an isolation amplifier
comprising
an input lead and an output lead, wherein said isolation amplifier input lead
is connected
to a plurality of skin surface electrodes; b) a first channel comprising a
band-pass filter
and an EKG gain amplifier, wherein said first channel is connected to said
isolation
4
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
amplifier output lead; c) a second channel comprising a high-pass band filter
and a
composite EMG gain amplifier wherein said second channel is connected to said
isolation
amplifier output lead; d) a first microcontroller comprising an EKG input lead
and aii
EKG output lead connected to said EKG gain amplifier, an EMG input lead and an
EMG
output lead connected to said EMG gain amplifier and a blanking pulse output
lead; e) a
second microcontroller comprising an input lead and an output lead wherein
said second
microcontroller input lead is connected to said EMG gain amplifier output
lead; f) an
EKG blanker comprising an analog switch, a composite EMG input lead connected
to
said second microcontroller output lead and a moving average EMG input lead,
wherein
said analog switch comprises an output lead and is connected to said first
microcontroller
blanking pulse output lead; g) a moving averager having an input lead and an
output lead,
wherein said moving averager output lead is connected to said EKG blanker
moving
average input lead and said moving averager input lead is connected to said
analog switch
output lead. In one embodiment, said second microcontroller further comprises
a digital
delay circuit. In one embodiment, the device further comprises a monitor
connected to
said output lead of said moving averager.
One embodiment of the present invention contemplates a system for diagnosing a
breathing disorder, comprising: a) a subject suspected of having a breathing
disorder
wherein said subject is contacted with a plurality of skin surface electrodes;
b) a
diagnostic device capable of activation by said subject and connected to said
electrodes,
wherein said diagnostic device comprises; i) an isolation amplifier capable of
receiving a
composite electromyogram signal from said electrodes; ii) a first channel
capable of
exaggerating an EKG artifact signal within said composite electromyogram
signal; iii) a
first microcontroller capable of triggering a blanking pulse upon detection of
a threshold
average QRS complex within said EKG artifact signal; iv) an EKG blanker
comprising
an analog switch, wherein said analog switch is reconfigured from receiving
said
composite EMG signal to receiving a moving averager output signal upon
detecting said
blanking pulse to create a clean electromyogram signal; v) a moving averager
capable of
calculating a moving average electromyogram signal from said clean
electromyogram
signal; vi) a positive pressure ventilation device capable of altering
positive pressure to
the respiratory system of said patient after receiving said moving average
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
electromyogram signal; and b) a data recorder capable of storing said moving
average
electromyogram signals and said altered positive pressures under conditions
such that a
breathing disorder may be diagnosed. In one embodiment, said surface
electrodes are
contacted with said patient by trained personnel. In one embodiment, said data
recorder
is further capable of storing said clean electromyogram signal, said
electrocardiogram
artifact signal and said composite electromyogram signal. In one embodiment,
the
system further comprises a computer reversibly connected to said data
recorder, wherein
said stored signals are downloaded for processing.
DEFINITIONS
The term "sleep disorder", as used herein, refers to any condition that
disrupts a
patient's ability to progress through the normal phases of sleep, as accepted
in the art. A
sleep disorder may prevent a patient from reaching Stage IV (i.e., for
example, rapid-eye-
movement (RElVl)) wherein a patient engages in dreaming (the most restful
stage of
sleep) when caused by either obstructive sleep apnea or centrally-mediated
sleep apnea..
A sleep disorder including, but not limited to, obstructive sleep apnea or
upper airway
resistance syndrome may modify the normally sinusoidal breathing pattern, such
that
paradoxical diaphragm and geniglossal muscle movement occur. Alternatively, a
sleep
disorder based upon a centrally-mediated sleep apnea may simply be expressed
as a
cessation of breathing. Other types of non-respiratory sleep disorders are
contemplated
by the present invention including, but not limited to, problems with staying
and falling
asleep, problems with staying awake, problems with adhering to a regular sleep
schedule
and sleep-disruptive behaviors.
The term "symptoms of a sleep disorder", as used herein; refers to clinical
manifestations consistent with a disruption of the normal phases of sleep.
These
symptoms include, but are not limited to, altered ventilation states, restless
leg
movements, bruxing, daytime fatigue, excessive daytime sleepiness,
irritability, high
blood pressure, low blood oxygen content, cardiac ischemia, stroke, awakening
in the
night, difficulty falling asleep, loud snoring, episodes of stopped breathing,
sleep attacks
during the day, depressed mood, anxiety, difficulty concentrating, apathy or
loss of
memory. The symptom expressed as an altered ventilation state comprises a
paradoxical
6
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
breathing pattern wherein the diaphragm contraction and geniglossal
contraction are not
properly synchronized.
The term "patient", as used herein, refers to any living mammal, human or non-
human.
The term "EKG blanker", as used herein, refers to any electronc device having
the capability to selectively remove any contaminating waveform that reduces
the
sensitivity and precision of an electromyogram (EMG). A contaminating waveform
may
comprise an electrocardiogram (EKG) artifact signal. An EKG blanker device, as
contemplated by the present invention, does not generate "flat spots" in a
cleaned EMG
that results in data loss in most currently used methods to remove EK.G
artifact.
The term "flat spots", as used herein, refers to regions on a "clean EMG" that
are
at or near baseline (i.e., no activity) following a non-selective removal of a
contaminating
waveform.
The term "clean EMG", as used herein, refers to an EMG signal from which
contaminating waveforms have been removed (i. e., for example, by replacement
with a
moving average signal). A clean EMG includes, but is not limited to, output
from an
EKG blanker to a moving averager as contemplated by the present invention.
The term "electrocardiography", "electrocardiogram" or "EKG", as used herein,
refers to a test that generates an electric signal (i.e., an EKG signal)
produced by the
sequential depolarization of the heart chambers. One of skill in the art will
recognize that
an electrocardiogram is inherently detected by surface skin electrodes
intended to detect a
diaphragmatic electromyogram (EMGdi); thus complicating an EMGdi analysis. An
averaged amplitude of the electrocardiogram's QRS complex (i.e., averaged QRS)
is
computed by a microcontroller and used to automatically trigger (i.e., for
example, by
generating a blanking pulse) a reconfiguration of input to an EKG blanker
device.
The term "QRS complex", as used herein, refers to a portion of an EKG
representing the actual successive atrial/ventricular contraction of the
heart.
The term "averaged QRS", as used herein, refers to an arithmetic average of
the
area-under-the-curve (i, e., integral) of the QRS portion of an EKG signal.
The
calculation of averaged QRS may be performed using peak detection (i. e, for
example, by
using a software algorithm). A peak detection algorithm may be based on a
simple first
7
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
difference approach by examining the variation between maximum QRS complex
amplitude and baseline EKG signal amplitude (i.e., for example, occuring
immediately
prior the QRS complex). The threshold used by the peak detection logic (i.e.,
resulting
the detection of a "threshold average QRS complex") is intially established
during a
patient initialization (i.e., for example, during electrode stabilization)
process. In one
embodiment, the threshold is a preset value (i. e, for example, a trigger
value) wherein the
present value is between approximately 50 - 90 % of the average QRS complex,
preferably between 60 - 80 % of the average QRS complex and more preferably
between
. In another embodiment, the threshold is not a fixed quantity and dynamic,
thereby
changing during the recording procedure. In one embodiment, the threshold is
determined from the overall amplitude of a pateint's typical QRS complex. This
is done
to allow for the variation in the QRS amplitude with respect to respiration,
body posture
etc. This is accomplished by computing the running average of QRS amplitudes
and
using the average amplitude to determine the threshold. Thus, there is a
feedback loop to
dynamically adjust the threshold as new QRS's are detected and identified. The
feedback
loop makes the system adaptive to the variations in patient EKG during the
analysis
period. In one embodiment, the present invention uses two different thresholds
to detect
QRS complexes. During the first pass over the data, a high threshold is used
to detect
only normal QRS complex amplitudes. Small QRS complex amplitudes, however, may
be missed but are recoverable by using a subsequent low threshold detection
pass. One
embodiment contemplates a QRS identification algorithm that identifies a lack
of a QRS
signal in a region of an EKG signal where a QRS signal is expected such that
the low
threshold detection pass is implemented.
The term "electromyography", "electromyogram" or "EMG", as used herein,
refers to a test that generates an electric signal (i.e., an EMG signal)
produced by the
depolarization of muscle tissue. One of skill in the art will recognize that
an
electromyogram will be detected by a set of skin surface electrodes resulting
from any
and all muscle depolarizations and thus may comprise an electrical signal or a
visual
representation of an electrical signal. As used herein, a surface EMG signal
is detected
by an empirical determination of the proper manner of placement and location
of skin
surface electrodes that minimizes the detection of inspiratory muscle
electromyograms
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
other than a diaphragmatic EMG (EMGdi). One empirically derived electrode
placement
contemplated by the present invention comprises skin surface electrodes placed
at the
seventh and eighth intercostal space along the axillary and mid-axillary chest
lines,
respectively.
The term "composite", as used herein, refers to a multiple waveform comprising
at least two individual waveforms. Individual waveforms include, but are not
limited to,
electromyogram signals and electrocardiogram signals.
The term "exaggerated" as used herein, refers to a composite waveform wherein
one waveform predominates. The present invention contemplates the exaggeration
of at
least one waveform in relation to a composite waveform by using a combination
of band
pass filters. The exaggeration process comprises a specific sequence of low-
pass band
filters and high-pass band filters (i. e., operating between approximately 14 -
4000 hertz
and -12 dB/octave). Exaggerated waveforms may be independently manipulated to
improve the gain and amplitude in preparation for triggering a blanking pulse.
The term "surface electrode", as used herein, refers to any electrically
conductive
component, that when properly placed on the outside epidermal layer (i.e,
skin) of a
patient, detects physiological electrical activity (i.e, for example, an EMG).
One of skill
in the art will recognize that the specific manner and location of electrode
placement is
determinant of the type and origin of the detected electrical activity.
The term "microcontroller", as used herein, refers to any electronic device
capable of receiving, processing and transmitting analog or digital signals
(i.e., for
example, a printed integrated circuit). For example, a microcontroller may be
configured
to use software programs to perform arithmetic calculations. Alternatively, a
microcontroller may be configured to use software programs to route electronic
signals to
specific destinations.
The term "input", as used herein, refers to any electrical signal that is
received by
an electrical component for reconfiguration and/or processing.
The term "output", as used herein, refers to any electrical signal that is
transmitted
by an electrical component after reconfiguration and/or processing.
The term, "channel", as used herein, refers to any electrical pathway used to
transmit an electrical signal within or between electronic devices. For
example, a
9
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
channel may include, but is not limited to, microchips comprising etched or
photoresist
electrically conductive pathways, shielded cables or metal alloy wires.
The term "connected", as used herein, refers to any electrical circuit
configured to
transmit a signal from one component to another component. It is not intended
to limit
the configuration to adjacent components. The present invention specifically
contemplates that non-adjacent components (i.e., those physically separated by
intervening components) may be connected.
The term "reconfiguring" or "reconfigured", as used herein, refers to any
change
in the routed pathway of an electrical signal within an electronic device. For
example,
reconfiguring may include, but is not limited to, an analog switch or a
digital component
(i. e., for example, a microchip).
The term "delaying" or "delayed", as used herein, refers to a transient
interruption
in a signal transmission through a microcontroller (i.e., for example, by use
of a digital
delay circuit). For example, a delay comprises approximately 50 milliseconds
(msec).
The term "transmission" or "transmitting", as used herein, refers to the
movement
of an electrical signal from one component to another component of an
electrical circuit
The term "moving averager", as used herein, refers to an electronic component
that is capable of computing (i.e., for example, by being configured with an
algorithm)
iterative averages over specific time intervals of a continuous waveform based
on the
frequency and amplitude (i.e., for example, an EMGdi waveform).
The term "displaying", as used herein, refers to any visual physical
representation
of an electrical signal (i.e., for example, an EKG or EMG). For example, such
physical
representations may include, but are not limited to, digital monitors, liquid
crystal
displays, light emitting diode displays, strip chart recorders or computer
haxdcopy
printouts.
The term "intercostals", as used herein, refers to any area between two ribs.
For
example, the seventh intercostal space comprises the area between the seventh
and eight
rib and the eighth intercostal space comprises the area between the eighth and
ninth ribs
(on either the left or right side of a patient's body).
The term "anterior axillary line", as used herein, refers to an imaginary
straight
vertical line continuing the line of the anterior axillary fold with the upper
limb in the
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
anatomical position.
The term "mid-axillary line", as used herein, refers to an imaginary straight
vertical line halfway between the anterior axillary line and the posterior
axillary line,
passing through the apex of the axilla.
The term "EMG monitor", as used herein refers to any electronic device that is
capable of calculating a maEMGdi without EKG artifact signals by detecting a
composite
EMG with surface electrodes.
The term "diagnostic device", as used herein, refers to an electronic device
that
may be operated by a patient and capable of monitoring, detecting and storing
physiological data that enables a skilled clinician to diagnose a breathing
disorder (i.e, for
example, sleep apnea or upper airway resistance syndrome). A diagnostic device
(i.e., for
example, an EMG monitor) is capable of providing input to automatically adjust
the
operation of a positive pressure ventilation device.
The term "positive pressure ventilation device", as used herein, refers to the
administration of a gas (i. e, for example, room air) to the lungs of a
patient exhibiting at
least one symptom of a breathing disorder (i.e., for example, a commercially
available
continuous positive airway pressure device; CPAP)).
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates an exemplary relationship between moving average
diaphragmatic EMG (~maEMGdi) measured with an esophageal electrode and
esophageal pressure (OPes) during a hypercapnic challenge. V = inspiratory
flow, Pga =
gastric pressure, Pdi = transdiaphragmatic pressure.
Figure 2 demonstrates one embodiment of the relationship between maEMGdi
and Pes.
Figure 3 shows an exemplary data tracing of an EMG signal that contains and
EKG artifact signal. Top trace: rectified composite EMG. Bottom trace: moving
average signal showing residual EKG artifact contamination.
Figure 4 shows an exemplary data tracing of an individual EKG artifact signal.
Figure S illustrates one example of surface electrode positioning for
measuring
OmaEMGdi as contemplated in one embodiment of the present invention. The
anterior
11
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
axillary line is defined by the lateral margin of the pectoralis (upper
arrowheads) while
the posterior axillary line is defined by the lateral border of the latissimus
dorsi (lower
arrowheads). In this embodiment, electrodes are shown placed in the lowest
interspace
intersecting the anterior axillary line and the next lower interspace in the
mid-axillary
line.
Figure 6 shows one embodiment of an EMG monitor.
Figure 7 illustrates one embodiment of an electronic schematic of an EMG
monitor.
Figure 8 demonstrates one example of a polygraph recording of a subject
breathing at increasing levels of nasal obsti action. Panel A: Level I - No
obstruction.
Panel B: Level II -1 +'/4 obstructed: Panel C: Level III -1 + %a obstructed.
Figure 9 illustrates exemplary correlations between ~maEMGdi and ~Pes for
eight subjects. Figure 9A presents data for Subjects 1 - 4 and Figure 9B
presents data for
Subjects 5 - 8. Y-Axis: ~maEMGdi (millivolts). X-Axis: ~Pes (cm H20)
Figure 10 demonstrates one possible relationship between ~maEMGdi and ~Pes
as' a function of body position as demonstrated in Subjects 3, 7 and 8. Y-
Axis:
~maEMGdi (millivolts). X-Axis: ~Pes (cm Hz0). Supine - 0 data point with a
solid
regression line; Right Side - o data point with a dashed regression line; Left
Side - x data
point with a dotted regression line.
Figures 11A and 11B demonstrate one possible relationship between maEMGdi
and Pes from four sleep disordered asleep subjects (A-D) undergoing positive
pressure
ventilation with a CPAP device. Y-Axis: dmaEMGdi (millivolts). X-Axis: OPes
(cm
H20). o data point with a solid regression line.
Figure 12 presents representative data showing a diagnosis of upper
respiratory
airway syndrome (UARS).
DETAILED DESCRIPTION OF THE INVENTION
This invention relates generally to the treatment of breathing disorders in
sleeping
and waking subjects. In one embodiment, the invention relates to an electrical
device for
monitoring and processing an electromyogram (EMG) signal. In another
embodiment,
the electrical device comprises non-invasive skin surface electrodes for the
detection of
12
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
EMG signals. In another embodiment, the electrical device comprises a system
for
monitoring and recording of data by a patient such that a sleep disorder may
be diagnosed
by a clinician.
This invention relates generally to the treatment of breathing disorders in
sleeping
and waking subjects. More particularly, the invention relates to the treatment
of
disorders emanating from upper airway obstruction and to methods and devices
for
detecting, evaluating, monitoring and ameliorating the adverse effects of such
obstructions. In one embodiment, the invention relates to an electrical device
(i.e., for
example, an EMG monitor) for monitoring and processing a composite
electromyogram
(EMG) signal. In another embodiment, the electrical device comprises non-
invasive skin
surface electrodes. One advantage of the device comprises an automatic
replacement of
an electrocardiogram (EKG) artifact signal (i.e., deemed as artifact in
regards to the
present invention) that one skilled in the art would consider rendering a
composite EMG
signal useless for quantitative analysis. Another advantage of the device is
that it is
useful for sleep studies or other applications where it is desirable to
measure human
diaphragm muscle activity. Another advantage of the device is that may be
operated by a
patient.
To establish that an upper airway obstruction is occurring during sleep
requires
the simultaneous measurement of inspiratory airflow and inspiratory effort.
The
reference standards for these measurements are the pneumotachygraph (a direct
determinant of airflow) and esophageal manometry (a direct determinant of
inspiratory
effort. Because these techniques are at least cumbersome, if not frankly
invasive, and
because they tend to interfere with a patient's sleep, they are not practical
as elements of
extra-clinical systems for monitoring and treating a breathing disorder.
Indeed,
pneumotachyography and esophageal manometry are not even used in routine
clinical
sleep testing and have little diagnostic application, except in research.
In place of the pneumotachygraph and esophageal manometry (collectively,
"polysomnography"), some clinical laboratories have adopted a less sensitive
approach
using thermocouples to measure inspiratory airflow and circumferential
movement
sensors to detect chest and abdominal movement to measure inspiratory effort.
These,
somewhat less disruptive, technologies are adequate for the clinical
recognition of
13
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
OSA/H because all patients with this diagnosis manifest large reductions in
airflow and
most exhibit some degree of paradoxic thoraco-abdominal movement during
obstructive
apneas and hypopneas.
The thermocouples and movement sensors that are adequate for the diagnosis of
OSA/H patients, however, fail to distinguish LIARS patients from normals,
because
inspiratory airflow and effort are only slightly decreased in LIARS patients.
The
physiologic correlates of LIARS include, but axe not limited to, an
inspiratory airflow
plateau (demonstrable by pneumotachygraph) and an increased inspiratory effort
(demonstrable by esophageal manometry). Gold et al., "Upper Airway
Collapsibility
During Sleep In Upper Airway Resistance Syndrome" Claest 121:1531-1540 (2002);
and
Guilleminault et al., "A Cause Of Excessive Daytime Sleepiness. The Upper
Airway
Resistance Syndrome" Clzest 104(3):781-7 (1993).
One technological innovation has enabled effective LIARS diagnosis by
identifying mild levels of inspiratory airflow limitation during sleep that
includes the use
of a nasal cannula to make nasal/oral pressure measurements. The measurements
obtained from the cannula adequately demonstrate the plateau characteristic of
a mild
inspiratory airflow limitation. Hosselet et al., "Detection Of Flow Limitation
With A
Nasal Cannula/Pressure Transducer System" Am JRespir Crit Care Med 157(5 pt
1):1461-1467 (1998). A disadvantage of this less invasive approach, however,
is that the
sensitivity of inspiration effort measurements is not comparable to esophageal
manometry. Clearly, a reliable surrogate for esophageal manometry is needed to
improve
the quality of diagnosis for mild breathing disorders.
The present invention contemplates the diagnosis of LIARS by a method
comprising the detection of EMGdi in a patient. Subsequent to a LIARS
diagnosis, the
patient may be placed on a therapy comprising a positive pressure ventilation
device.
Although it is not necessary to understand the mechanism of an invention, it
is believed
that positive pressure ventilation therapy of patients diagnosed with LIARS
will provide
improvement for associated clinical conditions including, but not limited to,
irritable
bowel syndrome, migraine headaches, temporal mandibular joint dysfunction,
fibromyalgia, chronic fatigue syndrome and "Gulf War" syndrome. In
uncontrolled
testing, several subjects diagnosed with LIARS and treated with a positive
pressure
14
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
ventilation device have seen improvement in one or more associated clinical
conditions
within two weeks of therapy.
One hypothesis surrounding the present invention contemplates a correlation
between a peak inspiratory excursion of surface diaphragmatic EMG activity and
inspiratory effort measured as an inspiratory excursion of esophageal
pressure. Lopata et
al., "Quantification Of Diaphragmatic EMG Response To COZ Rebreathing In
Humans"
JAppl Physiol 43:262-270 (1977). Lopata et al. first demonstrated a close
correlation
between the peak excursion of moving average diaphragmatic EMG activity and
inspiratory effort as assessed by mouth occlusion pressures (R = 0.89) during
COa re-
breathing. Onal and associates, during research on progressive hypercapnea,
demonstrated an apparent correlation between the magnitude of the moving
average
EMG of the diaphragm (maEMGdi; measured with an esophageal electrode) and the
magnitude of esophageal pressure (Pes) as a function of carbon dioxide
concentration.
Onal et al., "Diaphragmatic EMG And Transdiaphragmatic Pressure Measurements
With
A Single Catheter" Am Rev Respir Dis 124:563-565 (1981). (See Figure 1). These
approaches failed to directly measure inspiratory Pes as done by contemporary
esophageal manometry and remain only as surrogate methods.
Percutaneous placement of diaphragmatic electrodes were used to calculate a
timed moving average EMGdi in anesthetized piglets. These data were compared
with
measurements of peak inspiratory flow and acceleration collected during
resistive
inductive plethysmography. A comparison of the two data sets validated using
an
analysis of breath waveforms, alone, to diagnosis sleep related disorders.
Sackner et al.,
"Method For Analyzing Breath Waveforms As To Their Neuromuscular Respiratory
Implications" United States Patent No. 6, 0153, 88; Filed: March 17, 1998.
Issued:
January 18, 2000. The use of alternative methodologies, such as body surface
sensors
(i.e., for example, impedance pneumography or Graseby capsules) were
identified as
unreliable. For example, Sackner et al, teaches that the Graseby capsules
measures
abdominal wall movement rather than an overall abdominal or rib cage
respiratory signal.
A significant improvement in the measurement of diaphragmatic EMG involved
the use of surface electrodes. Skin surface EMGdi was detected with
intercostal
electrodes (placed in the 6th and 7th interspaces anteriorly) in quadriplegic
patients
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
having nerve lesions above the first thoracic vertebra (i.e., the intercostal
muscles were
paralyzed). Gross et al., "The Effect Of Training On Strength And Endurance Of
The
Diaphragm In Quadriplegia" Am J. Med 68:27-35 (1980). These surface
diaphragmatic
EMGs display the same fatigue-related changes in the ratio of high to low
frequencies
demonstrated by EMG activity monitored with esophageal electrodes in both
normal and
quadriplegic patients. Gross et al., "Electromyogram Pattern Of Diaphragmatic
Fatigue"
JAppl Physiol 46:1-7 (1979). It will be recognized by those skilled in the art
that
artifacts within the EMGdi signal by EMG activity from other inspiratory
muscles of the
chest wall were not present because of the muscle paralysis in the
quadriplegic subjects.
The exact manner of placement and location of Gross et al. electrodes,
therefore, have a
large margin of error to detect reproducible signals.
Data from skin surface electrodes detecting EMGdi has been integrated with a
variety of other sensor inputs using a home-use sleep apnea diagnosis device.
Karakasoglu et al., "Multi-Channel Self Contained Apparatus And Method For
Diagnosis Of Sleep Disorders" United States Patent No. 6,171, ~5~; Filed:
October 8,
1998. Issued: January 9, 2001. Karakasoglu et al. integrate a variety of
sensory inputs
(including EMGdi) that calculates a respiratory disturbance index, generally
understood
in the art as representing to the number of apneas and hypopneas per hour.
Centrally-mediated sleep apnea in adults has been monitored for the presence
or
absence of diaphragmatic activity by surface EMGdi. Bradley et al., "The
Relation Of
Inspiratory Effort Sensation To Fatiguing Patterns Of The Diaphragm" Am Rev
Respir
Dis 134:119-1124 (1986). These EMGdi recordings were not used to quantify
inspiratory effort and are irrelevant in the diagnosis of centrally-mediated
sleep apnea.
Similarly, OSA/H in children have also been monitored using surface EMGdi as
an index
of diaphragmatic activity. Praud et al., "Diaphragmatic And Genioglossus
Electromyographic Activity At The Onset And At The End Of Obstructive Apnea In
Children With Obstructive Sleep Apnea" Pediatr Res 23:1-4 (1988); and Wulbrand
et al.,
"Submental And Diaphragmatic Muscle Activity During And At Resolution Of Mixed
And Obstructive Apneas And Cardiorespiratory Arousal In Preterm Infants"
Pediatr Res.
38:298-305 (1995). A disadvantage of these approaches was that inspiratory
effort was
not quantified.
16
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Surface diaphragmatic EMG has also been utilized for experimental research and
clinical applications. Recent studies have confirmed the correlation between
surface
EMGdi and invasively monitored diaphragmatic EMG activity. Experimentally,
surface
EMGdi has been used to investigate the effects of nasal pressure-support
ventilation on
diaphragmatic function, Nava et al., "Effect Of Nasal Pressure Support
Ventilation And
External PEEP On Diaphragmatic Activity In Patients With Severe Stable COPD"
Chest
103:143-150 (1993), and to investigate diaphragmatic dysfunction after
laparotomy.
Berdah et al., "Surface Diaphragmatic Electromyogram Changes After Laparotomy"
Clin
Phyisol Funct Iynaging 22:157-160 (2002). Clinically, surface EMGdi has been
used to
monitor diaphragmatic responses to operative neuromuscular blockade,
Hemmerling et
al, "Intramuscular Versus Surface Electromyography Of The Diaphragm For
Determining Neuromuscular Blockade" Anesth Analg 92:106-111 (2001), and to
monitor
the severity of expiratory airflow obstruction in asthmatic children who
cannot reliably
perform forced expiratory maneuvers. Maarsingh et al., "Respiratory Muscle
Activity
Measured With A Noninvasive EMG Technique: Technical Aspects And .
Reproducibility" .I Appl Physiol 88:1955-1961 (2000).
Recently, diagnosis of sleep apnea has been disclosed by evaluating phase
differences between the waveforms of abdominal and thoracic effort based upon
the
expansion and contraction of body circumference. Kumar et al., "Analysis Of
Sleep
Apnea" United States Patef~t Application 2003/0139691, Filed: January 22,
2003.
Published: July 24, 2003. In Kumar et al., the mechanical aspects of thoracic
and
abdominal effort is detected by piezo/PDF belts or inductance/impedance
measurements.
The signals are evaluated for separation of a calculated phase angle allowing
either a
diagnosis for sleep apnea or indicating a necessity for CPAP pressure
adjustments. This
approach did not detect or disclose any relationship between EMGdi and Pes.
Relative relationships between EMGdi and Pes were discussed in regards to a
method and device that generates a signal to adjust ventilatory support units.
In order to
obtain high quality EMG signals, other artifactual signals (i. e.,
electromotion, EKG,
generalized electrical interference and high frequency noise) are filtered.
Sinderly et al.,
"Method And Device Responsive To Myoelectrical Activity For Triggering
Ventilatory
Support", Ufaited States Patefat No. 6,588,423, Filed: June 22, 2001. Issued:
July 8,
17
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
2003. Sinderly et al. teaches that EMGdi is preferably measured by using an
esophageal
catheter which contains an number of electrodes. This catheter is intranasally
passed and
enters the diaphragm muscle in order to detect depolarization signals.
It has been shown that diaphragmatic fatigue does not occur during OSA/H
following data collection from esophageal EMGdi and gastric pressure
catheters. Cibella
et al., "Evaluation Of Diaphragmatic Fatigue In Obstructive Sleep Apnoeas
During Non-
REM Sleep" Thorax 52:731-735 (1997). This EMGdi data was converted into a
power
spectrum and compared to both diaphragmatic pressure time index and a maximum
transdiaphragmatic pressure relaxation rate. The matching profiles of these
three
parameters plotted across sequential breaths showed a lack of diaphragmatic
fatigue.
One of skill in the art will recognize that measurement of inspiratory effort
has
not been attempted by establishing a relationship between surface
diaphragmatic activity
and effort expended during an inspiration. Indeed, there is no suggestion in
the art that:
i) an a priori reason to believe that any such relationship exists and, ii) no
one has taught
that surface EMGdi could, or should, be used as an index of inspiratory
effort. In fact,
the American Academy Of Sleep Medicine teaches away from using EMGdi whether
measured by esophageal manometry, esophageal electrode or at the surface of
the body:
Diaphragm EMG is an indirect measurement of respiratory effort. It is a
difficult
signal to record reliably and continuously, and there is no direct way to
correlate
it with esophageal pressure or upper airway resistance. There are no data on
accuracy, reliability, or correlation with long term outcome in relation to
this
technique.
American Academy Of Sleep Medicine Task Force, "Sleep-Related Breathing
Disorders
In Adults: Recommendations For Syndrome Definition And Measurement Techniques
In
Clinical Research" Sleep 22:667-689 (1999).
One embodiment of the present invention contemplates that the magnitude of a
surface diaphragmatic moving average EMG change (OmaEMGdi) is positively
correlated in relation to the magnitude of an inspiratory esophageal pressure
change
(~Pes) in waking subjects with upper airway obstruction (i.e., for example,
upon resistive
loading of the nasal airway). One embodiment contemplates a method of
measuring a
correlation between ~maEMGdi and OPes comprising: surface electrodes, placed
intercostally (i.e., for example, within the seventh and eight interspaces),
under
conditions that detect diaphragmatic EMG from subjects with increased upper
airway
18
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
resistance that has a positive correlation with inspiratory effort measured by
esophageal
manometry. In one embodiment, the correlation is present at varying levels of
obesity.
In another embodiment, the correlation is present in recumbent individuals
irrespective of
whether the individual's body position is supine or recumbent on the left or
right sides.
One having skill in the art will recognize that this invention is especially
advantageous
because a surface EMGdi, as contemplated herein, is easy to record
continuously and less
cumbersome than state of the art polysomnographic piezoelectric belts.
A detected relationship between EMGdi activity and supraglottic pressure
measurements identified that OSA/H involves expiratory blockages as well as
inspiratory
blockages. Sauna et al., "Expiratory Supraglottic Obstruction During Muscular
Relaxation" Ghest 10:143-149 (1995). In Sauna et al., both supraglottic
pressure and
EMGdi were measured using nasal catheters. During negative ventilation, a
reduction in
EMGdi activity was positively correlated with increased supraglottic pressure,
thereby
resulting in expiratory and inspiratory blockages in normal subjects as well
as patients
having sleep apnea. The data suggested that upper airway muscles must be
activated to
preserve an open airway during both inspiration and expiration.
One embodiment of the present invention contemplates a method to reduce
progressively increasing inspiratory effort during sleep apnea (i.e., for
example,
obstructive or central), upper airway resistive syndrome or other inspiratory
flow
limitation. In one embodiment, a progressive decrease in the magnitude and
variability
of inspiratory effort occurs by increasing pressure from a positive pressure
ventilation
device (i.e. for example, a nasal continuous positive airway pressure device;
CPAP) to
therapeutic levels. In one embodiment, therapeutic CPAP administration
decreases a
~maEMGdi value. (Figure 2).
Another embodiment of the present invention contemplates a method to remove
(i.e., for example, replace by blanking) electrical impulses from the heart
(i.e., for
example, EKG artifact signals) out of the surface EMGdi signal. It is known in
the art of
polysomnography that surface electrode EMG signals are contaminated by
electrocardiogram (EKG) artifact signals. Some somnographic methods and
devices
known in the art are capable of filtering out EKG artifact signals but lack
the necessary
sensitivity to provide accurate information required for diagnosis and
treatment of
19
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
breathing disorders (i.e., for example, sleep apnea and related conditions).
In one
embodiment, the present invention contemplates a method of diagnosis and
treatment of a
patient exhibiting at least one symptom of a subtle respiratory disturbance
(i.e., for
example, a breathing disorder). In one embodiment, the disturbance comprises
UARS.
One having skill in the art will recognize that the invention contemplates a
degree of
sensitivity, accuracy, reliability and automatic operability not currently
available in the
art. In fact, the present invention is capable of performing diagnosis and
changes in
treatment parameters to patients either on an outpatient basis or at home.
To facilitate the autonomy of the use of the present invention, one embodiment
contemplates a diagnostic device (i.e., for example, an EMG monitor)
comprising surface
electrodes integrated into an electronic circuit. In one embodiment, the
device comprises
a setup software function that is capable of automatically adjusting gain to
standardize
the amplitude of composite EMG and EKG artifact signals. In one embodiment,
the
composite EMG signal comprises a diaphragmatic EMG (EMGdi) signal. Although it
is
not necessary to understand the mechanism of an invention, it is believed that
a patient
might be expected to visit a local clinician's office for proper placement of
the electrodes
prior to a sleep session. Upon returning home, the patient would simply
connect the
electrodes to the input leads of the diagnostic device and power-up the
device. After an
appropriate stabilization period (i. e., for example, between 15 - 20
minutes), the
diagnostic device would automatically begin recording data. It is further
believed that
this stabilization period accommodates a physiological adaptation of the skin
cells to the
presence of the active electrodes (i.e., for example, stabilization of cell
membrane ion
channels). The present invention contemplates that during a patient's sleep an
associated
recording device (i. e., for example, a digital memory microchip) would store,
not only
sleep disorder related information (i. e., for example, diaphragmatic EMG),
but also basic
physiological parameters (i.e., for example, heart rate and respiration rate).
This
diagnostic device is operated by the patient and is contemplated to provide
data for the
diagnosis of breathing disorders. In another embodiment, a diagnostic device
operated by
the patient is contemplated as a system comprising a positive pressure
ventilation device
such that a diagnostic device provides real-time adjustments in the delivered
air pressure
by the positive pressure ventilation device.
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Another advantage of the present invention contemplates a method comprising:
providing a subject and an EMG monitor having an electronic circuit (i.e., for
example,
an EKG blanker) capable of replacing an EKG artifact signal within a patient's
composite
EMG signal. In one embodiment, a patient's EKG artifact signal is detected by
a
threshold amplitude of an average QRS complex. In another embodiment, an
electronic
circuit replaces the detected EKG artifact signal within a delayed composite
EMG signal
(i.e., for example, a delay of approximately 50 milliseconds) with moving
averager
output data.
The present invention contemplates an EMG monitor comprising a highly
sensitive and precise maEMGdi signal. In one embodiment, an EMG monitor
comprises
a channel having a composite electromyogram signal (i.e., for example, by
filtering
waveforms having a frequency of approximately between 50 - 3,000 Hz). In
another
embodiment, an EMG monitor comprises a channel having an exaggerated
electrocardiogram signal (i.e., for example, by filtering waveforms having a
frequency of
approximately between 1 - 50 Hz). One embodiment of the present invention
contemplates the individual optimization of a composite electromyogram and an
exaggerated electrocardiogram. In one embodiment, an exaggerated
electrocardiogram
signal identifies 100% of EKG artifact signals within a composite EMG signal.
Although
it is not necessary to understand the mechanism of an invention, it is
believed that
optimization of an individual EKG signal allows calculation of an average QRS
amplitude having a predetermined threshold (i.e, for example, when 75% of any
detected
QRS complex meets or exceeds a 1.5 volt peak-to-peak average). In one
embodiment,
detection of a threshold average QRS complex triggers a blanking pulse that
reconfigures
an analog switch within an EKG blanker to receive moving averager output as an
incoming signal. It is further believed that this moving averager output
"replaces" (i.e.,
blanks out) the EKG artifact signal within the incoming delayed composite EMG
signal.
The contamination of EMGdi signals with EKG artifact signals is a known
problem in the art. Another embodiment of the present invention replaces EKG
artifact
signal from composite EMGdi signals on a real-time basis. Prior efforts have
been
limited to iterative processes that matches (by linear regression) existing
EKG templates
(residing in a database) with the contaminating EKG artifact signal found
within the
21
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
recording of an expiratory EMGdi signal. This process requires approximately
twelve
hours of comparison effort to process and clean 30 minutes of EMGdi signal.
Levine et
al., "Description And Validation Of An ECG Removal Procedure For EMGdi Power
Spectrum Analysis" JAppl Physiol 60:1073-1081 (1986).
Certain embodiments of the present invention provide specific advantages over
a
prototype EKG blanker model (i.e., ECG Blanker Model SB-1; CWE, Inc).
Prototype
model SB-1 was commercially available until technological advances resulted in
the
obsolescence of specific integrated circuits. Unlike the present invention,
however, the
prototype Model SB-1 was limited to using an analog delay line to provide a
prediction
of when an EKG artifact signal would emerge within the delayed composite EMG
signal.
Unlike the present invention, the prototype Model SB-1 subtracted the EKG
artifact
signal from the EMG signal by: i) merely nulling-out the EMG signal during the
blanking
interval thereby creating nonsense "flat spots" or ii) substituting a portion
of the
undelayed EMG signal for the blanked signal. One having skill in the art will
recognize
that prototype Model SB-1 was subject to interference from the inevitable
switching
transients and discontinuities produced when cutting and pasting high-
frequency EMG
signals. Unlike the present invention, the prototype Model SB-1 utilized
highly
complicated circuitry in the microcontroller for gain adjustment and EMG
signal delays.
Certain embodiments of the present invention, however, comprise printed
integrated
circuit microcontrollers comprising simplified circuitry configured with
algorithms (i. e.,
software programs) that: i) automatically adjust EKG artifact signal gain and
composite
EMG signal gain independently; ii) digitally delay the composite EMG signal
and iii)
calculate an maEMGdi from a clean EMG signal. These advantages are facilitated
by the
integration of new generation very low-noise amplifier integrated circuits to
produce an
almost fully automatic EMG monitor.
Certain embodiments of the present invention utilize a digital delay circuit
that
delays a composite EMG signal thereby providing a surprising optimization of
the
blanking process. Specifically, some embodiments described herein utilize the
delayed
composite EMG signal to incorporate the moving average EMG calculations
directly into
the blanking process. For example, if an EKG artifact signal is detected by a
microprocessor (i.e., for example, by calculating a threshold average QRS
complex), the
22
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
EKG blanker may be reconfigured (i.e., for example, by an analog switch) to
receive
moving average EMG output signals at the same time the delayed composite EMG
signal
is received by the EKG blanker.
Initial attempts to optimize this blanking process were unsuccessful.
Specifically,
the EKG artifact signal, usually having a greater amplitude than the composite
EMG
signal, is sometimes reduced in size such that a ready discrimination between
the EMG
signal component and EKG artifact signal component by amplitude is not
possible (See
Figure 3). This situation causes erratic EKG-mediated triggering of blanking
pulses and
consequently poor EMG blanking performance. This problem is solved by one
embodiment of the present invention that employs frequency-specific band-pass
filters
which magnify (i. e., exaggerate) the EKG artifact signal component in
relation to the
composite EMG signal. This process allows an effective separation of the EKG
artifact
signal and composite EMG signal into individual channels that allows for
independent
processing (i.e., for example, gain adjustment). One embodiment of the present
invention
contemplates a method of band-pass filtering to create an EKG artifact signal
channel and
a composite EMG signal channel. In one embodiment, a composite EMG signal
channel
comprises two band-pass filters, a programmable gain amplifier configured to
interact
with a microcontroller configured with a gain-adjusting algorithm to perform
automatic
gain adjustment. In one embodiment, an individual EKG signal path comprises
one
band-pass filter, a programmable gain amplifier configured to interact with a
microcontroller configured with a gain-adjusting algorithm to perform
automatic gain
adjustment. In one embodiment, a microcontroller configured with a gain-
adjusting
algorithm interacts with an EKG artifact signal channel programmable gain
amplifier and
a composite EMG signal channel prograrmnable gain amplifier, wherein the
amplitude of
the EKG artifact signal and the amplitude of the composite EMG signal are
independently adjusted. Figure 4 shows a tracing from a representative
exaggerated EKG
artifact signal subsequent to filtering into an individual chamlel and optimal
gain
adjustment. One of skill in the art will recognize, however, that even in this
example of
an exaggerated EKG artifact signal there is some "leakage" of the high-
frequency
composite EMG signal (i.e., note the "ragged-edge" proEle) that, however, in
no way
affects the triggering of the blanking pulse by a microcontroller after
calculating a
23
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
threshold average QRS complex.
The present invention also solves a problem known in the art regarding the
validity of the surface diaphragmatic EMG due to contamination with EMG
activity from
other inspiratory muscles of the chest wall. The present invention
contemplates a method
of measuring EMGdi comprising placing a plurality of surface electrodes at the
seventh
and eighth interspaces on the anterior axillary line and mid-axillary line,
respectively.
Although it is not necessary to understand the mechanism of an invention, it
is believed
that the chest wall inspiratory muscles having the greatest potential to
interfere with
EMGdi are the parasternal internal intercostal muscles and the external
intercostal
muscles of the most rostral interspaces. De Troyer A., "The Respiratory
Muscles", In:
The Lung: Scientific Foundations, pp. 1203-1215, 2nd Ed., Eds. Crystal et al.,
Lippincott
- Raven, Philadelphia - New York ( 1997). It is also believed, therefore, that
placement
of the electrodes at the seventh and eighth interspaces is unlikely to detect
contaminating
EMG signals generated by the parasternal (internal or external) intercostal
chest wall
inspiratory muscles.
The present invention contemplates a method for detecting diaphragmatic
electromyograms using a plurality of skin surface electrodes. In one
embodiment, at least
one electrode is placed along the anterior axillary line of the chest. In
another
embodiment, at least one electrode is placed along the mid-axillary line of
the chest. One
advantage of the present invention contemplates an electrode placed in the
seventh
intercostal space. Another advantage of the present invention contemplates an
electrode
placed in the eighth intercostal space. An empirically derived method of
electrode
placement comprising a specific manner and location is necessary because the
contribution of intercostal inspiratory muscles to esophageal pressure may
vary between
NREM and REM sleep. Tusiewicz et al., "Mechanics Of The Rib Cage And Diaphragm
During Sleep" JAppl Physiol43:262-270 (1977).
In another embodiment, an electrode location overlies an area of opposition
between the diaphragm and the chest wall and minimizes the length of the
conduction
path between the diaphragm muscle and the electrodes. See Figure 5- showing
that the
diaphragm is sandwiched between the liver and the ribcage. The invention is
not limited,
however, by the site at which the electrodes are secured to the chest wall.
Other
24
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
embodiments are contemplated that comprise (as a non-limiting example) the
placement
of additional electrodes to acquire EMG signals from active non-diaphragm
inspiratory
muscles for use in decontaminating the diaphragmatic EMG signal by appropriate
signal
processing. It is also conceivable to use design-shaped surface electrodes
that
preferentially acquire diaphragmatic EMG signals.
As described above, the present invention contemplates a device for detecting
diaphragmatic EMG activity comprising an EMG monitor. It is not intended to
limit the
present invention by the following description of an EMG monitor device
because one
having skill in the art will recognize that many alternative designs are
possible to
facilitate similar signal processing. The EMG monitor described below is
intended only
as an example and comprises the following functional parts: i) an isolation
amplifier for
safely amplifying the signal received from skin electrodes; ii) a variable
gain amplifier
adjusted by a microcontroller configured with an algorithm; iii) a digital EKG
blanker to
replace the EKG artifact signal within the composite EMG signal and, iv) a
moving
averager for creating an envelope around the EMG activity. These functional
blocks, and
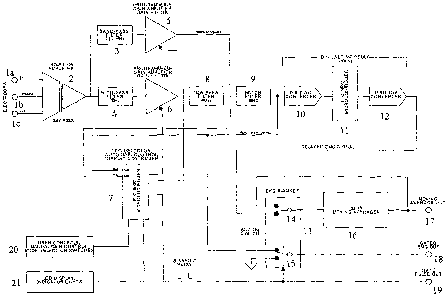
their relationships, are indicated in the accompanying diagram. See Figure 7.
One embodiment of the EMG monitor comprises a self contained instrument for
monitoring and processing a composite EMG signal (i.e., for example, a
diaphragmatic
EMG signal). Preferably, the monitor comprises a medical grade isolation
amplifier with
direct electrode connections, a moving averager and a novel EKG artifact
signal
suppression function (i. e., for example, an EKG blanker connected to a
digital delay
circuit). Although it is not necessary to understand the mechanism of an
invention, it is
believed that the EMG monitor operates within the following parameters: i) an
isolation
voltage of either approximately 1500 volts continuous or approximately 2000
volts @
approximately 10 second pulse intervals; ii) a leakage current of
approximately 10
microamperes when receiving any input; iii) wideband noise (referred to input)
of
approximately < 7 microvolts peak-to-peak and approximately < 3 microvolts
root-mean-
square and iv) a common mode rejection of approximately > 100 dB @
approximately 60
hertz.
One embodiment of an EMG monitor contemplated by the present invention has
several advantages over prior attempts in the art to replace EKG artifact
signals within
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
composite EMG signals: i) a setup mode where the gain of the isolation
amplifier is
automatically adjusted to produce standardized signal levels; ii) a liquid
crystal display
(LCD) window showing current settings and operator messages; iii) an
integrated
measurement of heart rate and respiratory rate; and iv) a digital delay
circuit that delays
the composite EMG signal (i. e., for example, by approximately 50
milliseconds) which
allows a microcontroller to predict when a contaminating EKG artifact signal
will be
received by an EKG blanker thus allowing an effective replacement of the EKG
artifact
signal by a moving averager output signal. In one embodiment, the EMG monitor
comprises the dimensions of approximately 10 x 3.5 x 8 inches (i.e., width-
height-depth)
and a weight of approximately three pounds. See Figure 6. In one embodiment,
the
composite EMG signal delay is between approximately 30 - SO milliseconds,
preferably
between approximately 40 - 70 milliseconds and more preferably between
approximately
45 - 55 milliseconds. Output signals from an EMG monitor 100 include, but are
not
limited to, AMP OUT 105 (a raw, amplified composite EMG signal having a range
of
approximately + 2 volts @ approximately 10 milliamperes); GATED EMG OUT 110 (a
full-wave rectified clean EMG signal having a range between approximately 0 -
2 volts
@ approximately 10 milliamperes, with nulls (i.e., for example, "flat spots")
inserted
where the EKG artifact blanking occurs by reconfiguration of analog switch
15); GATE
PULSE 115 (an approximate 5 volt logic pulse that is TTL compatible coinciding
with
the blanking pulse interval that is synchronous with a detected EKG artifact
signal); and
M.A. OUT 120 (the moving average output signal having a range of approximately
0 - 2
volts @ approximately 10 milliamperes).
One embodiment of the present invention contemplates a method for performing
an EMG monitor setup routine comprising: a) plugging an input cable (i.e., for
example,
a three meter, fully shielded cable with snap electrode leads) into an INPUT
jack 125
(i.e., for example, a 7-pin Amphenol 703-91T-3475-001 operating at an input
impedance
of approximately > 1000 megaohms having a voltage range of approximately + 25
millivolts) on the front panel; b) placing at least three electrodes on the
skin of a patient;
c) snapping the electrode leads onto the input cable leads consistent with a
color code
(i. e., for example, (+) = white: (-) = black: (Common) = green) and d)
plugging a power
supply (i. e., for example, approximately + 5 volts @ approximately 1 ampere
or
26
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
approximately ~ 12 volts @ approximately 200 milliamperes) into the power jack
on the
rear panel (not shown). When the EMG monitor is first switched ON using the
rear panel
power switch (not shown), a sign-on message is shown with an LCD window 130.
After
a few seconds, the EMG monitor will begin operating.
Another embodiment of the present invention contemplates a method for
performing an EMG monitor operational routine comprising: a) connecting the
input
cable leads to the EMG monitor; b) stabilizing the electrode signals, wherein
said
stabilization time period is at least fifteen minutes; c) performing a method
comprising a
setup routine, wherein said routine optimizes the EKG artifact signal gain.
In one embodiment, EKG artifact signal gain is automatically optimized by
selecting the SETUP SWITCH 135 to AUTO on an EMG monitor front panel. In one
embodiment, the peak amplitudes of the EKG artifact signals are monitored
during
approximate 3.5 second epochs, wherein the gain is iteratively adjusted to
increase or
decrease the amplitude to provide an optimized EKG artifact signal. During the
automatic gain optimization process, an LCD window 130 shows the current EKG
artifact signal status including, but not limited to: [HI] - indicating that
the signal
amplitude is too large for processing; [LO] - indicating that the signal
amplitude is too
small for processing or [OK] - indicating that the signal amplitude is within
the target
range for processing. In another embodiment, the EKG artifact signal amplitude
is within
target range for processing when the PWR/AUX light 140 is flashing rapidly.
In another embodiment, the EKG artifact signal gain is manually optimized by
selecting SETUP SWITCH 135 to MANUAL on the EMG monitor front panel and
adjusting the gain setting by turning the ADJCTST knob 145. In one embodiment,
optimization of the EKG artifact signal is achieved when the signal at the AMP
OUT jack
105 is between approximately 1.00 - 2.00 volts peak-to-peak, preferably
between
approximately 1.25 -1.75 volts peak-to-peak and more preferably between
approximately 1.45 -1.55 volts peak-to-peak.
In one embodiment, the duration of a blanking pulse comprises approximately
between 100 -140 milliseconds, preferably between approximately 110 -130
milliseconds and more preferably between approximately 119 -121 milliseconds.
In one
embodiment, a blanking pulse duration may be either increased or decreased by
pressing
27
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
and turning the ADJUST knob 145, wherein the selected blanking pulse duration
automatically appears within an LCD window 130. Although it is not necessary
to
understand the mechanism of an invention, it is believed that if the blanking
pulse
duration is too short, some of the EKG artifact signal will "leak" into the
clean EMG
signal before transmission to the moving averager. It is further believed that
this
phenomenon will be indicated by bumps in the moving average output data.
In one embodiment, a composite EMG signal is monitored by selecting the
MONITOR switch 150 on the EMG monitor front panel, wherein a composite EMG
signal is automatically processed to minimize or replace an EKG artifact
signal. In one
embodiment, an LCD window 130 shows a computed heart rate (HR) and a
respiratory
rate (RR), wherein an EKG light 155 blinks in synchrony with the heart rate.
The proper functioning of one embodiment of a contemplated EMG monitor
device comprises the following areas of technical expertise:
Input and Amplification: A medical-grade isolation amplifier (i.e., for
example,
having isolated, differential instrumentation) provides a safe interface for
patient-
connected electrodes. The composite EMG signal output of the isolation
amplifier is
high-pass band filtered to remove any direct current components of the
recorded signal.
The composite EMG signal is then amplified by a programmable-gain amplifier
under
microcontroller control that results in a standardized signal under a variety
of recording
situations. The standardized composite EMG signal is then low-pass band
filtered and
transmitted through a notch filter that removes power line frequency
components.
Digital Tirne Delay: The standardized composite EMG signal generated
according to the above paragraph is next processed by a digital time delay
circuit that
optimally delays the signal for approximately 50 msec. The digital time delay
circuit
comprises an interconnected analog-to-digital converter, a microcontroller
with an
external memory buffer and a digital-to-analog converter. The standardized
composite
EMG signal is, therefore, delayed within the microcontroller as a digital
signal prior to
reconstruction into an analog signal. One of skill in the art will recognize
that the signal
may also be digitally full-wave rectified during the delay process.
EKG Blanker and Moving Avera_-eg-r:r: The rectified and delayed composite EMG
signal is then transmitted from the digital-to-analog converter to a moving
averager
28
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
circuit via an EKG blanker comprising a microcontroller-controlled analog
switch. This
analog switch is normally configured to transmit the rectified composite EMG
signal
from the digital-to-analog converter directly into the moving averager
circuit. In the
presence of blanking pulse, however, the analog switch is reconfigured to
provide input
to the moving averager circuit using the "last known" moving averager circuit
output (i. e,
the moving averager output is utilized as moving averager input during the
blanking
interval). This effectively clamps the moving average circuit output signal to
the signal
detected just prior to the blanking pulse (i.e., without any EKG artifact
signal). A
microcontroller monitors the real-time signal and automatically generates a
blanking
pulse upon detection of a threshold average QRS complex. One of skill in the
art will
recognize that a blanking pulse duration determines the length of time that
the analog
switch is reconfigured to accept moving averager output data. A predetermined
duration
of the blanking pulse is selected to sufficiently "envelop" the EKG artifact
signal within
the delayed EMG signal interval. A gated EMG signal is also provided as an
output to
verify that the proper interval is being blanked.
Operator Interface: The EMG monitor device includes a liquid crystal display
window to observe operational device conditions including, but not limited to,
amplifier
gain, amplitude of moving average, etc. The LCD window also may present
instructions
to the user for setup and operation. These instructions may include, for
example,
direction for operator controls of the built-in automatic setup functions or
manual
adjustment of certain parameters such as amplifier gain, blanking time, etc.
The LCD
window may also comprise indicators providing visual monitoring of proper
operation.
An EMG monitor device electronic schematic diagram is presented in Figure 7
and is not intended to limit the present invention but only to illustrate one
embodiment of
a breathing disorder diagnostic device. A composite EMG signal is detected by
skin
surface electrodes 1 A -1 C and increased in signal strength by isolation
amplifier 2. The
composite EMG signal is then processed by low-pass band filter 3 (i.e., having
a
frequency range of approximately 0.1 -18 Hz) that preferentially filters the
EKG artifact
signal and a high-pass band filter 4 (i. e., having a frequency range of
approximately 10
Hz) that preferentially filters the composite EMG signal. One of skill in the
art will
recognize that low-pass band filter 3 provides a significant exaggeration of
the EKG
29
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
artifact signal. Residual EKG artifact signal, however, remains within the
composite
EMG signal despite high-pass band filter 4. The gain of exaggerated EKG
artifact signal
and composite EMG signal may then be independently adjusted by programmable
gain
amplifier 5 and programmable gain amplifier 6 (i.e., having gain ranges of
approximately
1-100X), respectively. Each gain amplifier 5, 6 may receive input from printed
integrated circuit microcontroller 7 (i.e., for example, model 16F877), either
simultaneously or separately, to provide real-time monitoring and adjustment
of their
respective signal amplitudes. Microcontroller 7 maintains feed-back loops with
both the
composite EMG signal and the exaggerated EKG artifact signal via their
respective
programmable gain amplifiers 5, 6. Exaggerated EKG artifact signal input to
microcontroller 7 is received directly from the programmable gain amplifier 5,
while
composite EMG input to microcontroller 7 is indirectly received from the
programmable
gain amplifier 6 after further processing by low-pass band filter 8 (i.e.,
having a
frequency range of approximately 4000 Hz) and notch filter 9 (i. e., having a
frequency
range of approximately 60 Hz). The digital time delay circuit receives input
from notch
filter 9 wherein the composite EMG signal is first converted into a digital
signal by 12-bit
A/D converter 10. This digital composite EMG signal is thereafter delayed
approximately 50 milliseconds within printed integrated circuit
microcontroller 11 (i.e.,
for example, model 16F877) and reconverted to an analog signal by 12-bit D/A
converter
12. The EKG blanker 13 receives the delayed composite EMG signal by analog
switch
14. Analog switch 14 is reconfigured to receive output from moving averager 16
(i.e.,
providing an averaged signal data point over approximately 200 milliseconds of
signal
duration) upon receipt, and duration, of a blanking pulse generated by
microprocessor 7.
Depending upon the absence or presence of a blanking pulse, the EKG blanker
provides
input to moving averager 16 as either: i) a delayed composite EMG signal
(absence of
blanking pulse) or ii) output from moving avexager 16 (presence of blanking
pulse).
Synchronicity between the blanking pulse and the composite EMG signal is
verified by
comparing signals received at gated composite EMG output 17 (mediated by
analog
switch 1 S which is also reconfigured by the blanking pulse) with signals
received directly
from microcontroller 7 at gated blanking pulse output 18. User input controls
20 allow
manual gain control and/or alternative mode selection by a direct interface
with
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
microprocessor 7. Microprocessor 7 thereby returns status information for user
viewing
on LCD window 21.
The 16F877 microcontroller in the above example has port assignment
configurations as listed in Table I.
Table I. 16F877 Microcontroller Port Assi~mnents
' = out a0 EMG signal an
b0 =
' = out al MA signal an
bl =
' = lcd rs out a2 encoder A in
b2 =
' = lcd a out a3 an
b3 =
' = lcd d4 out a4 encoder B in
b4 =
' = lcd d5 out a5 encoder pb sw in
b5 =
' = lcd d6 out
b6
' = lcd d7 out
b7
' sw4 SETUP in d0 led2 EKG out
c0 =
=
c1 sw5 RESERVED in dl led3 PWR out
= =
' sw6 RES (TEST POINT)out d2 beeper out
c2 =
=
' ledl ERROR out d3 /rs, pot out
c3 =
=
' dta, pot out d4 COM2 in
c4 =
=
c5 clk, pot in d5 COM1 in
= =
c6 C1 analog sw out d6 led2 pcb green out
= =
' C2 analog sw , out d7 ledl pcb red out
c7 =
=
e0 swl SETUP/MONITOR in
=
' sw2 MAN/AUTO in
el
=
e2 sw3 BEEP ON/OFF in
=
Maintenance and execution of the proper relationships and interactions between
the above described components of the diagnostic device contemplates unique
and novel
software. Terminology utilized in some software embodiments contemplated
herein are
defined in Table II.
Table II: Equates And Variables For Sorne Software Embodiments
sw-encoder porta.5 ' pb switch on encoder, 0 = on,
var l = off
select var portc.0 ' select pb sw, pressed = 0
sw
_ portc.2 ' test point r timing
test pt var fo
led err var portc.3 ' ERROR led
dta var portc.4 ' pot data
clk var portc.5 ' pot clock
cl var portc.6 ' analog sw Cl, gated EMG
c2 var portc.7 ' analog sw C2, M.A.
led_ekg var portd.0 ' EKG led
led~wr var portd.l ' PWR led
31
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
beeper var portd.2 ' piezo beeper
rs_pot var portd.3 ' pot /RS
com2 var portd.4
coml var portd.5
led_green portd.6
var
led red var portd.7
sw_setup porte.0 ' setup = 0, monitor = 1
var
man var porte.l ' manual = 0, auto = 1
sw
_ porte.2 ' beep on = 0, off = 1
sw beep var
EMG var word
ECG var word
MA var word
peak EMG word
var
peak ECG word
var
peak MA var word
trig level word ' ekg trigger threshold
var
RR_trig_level ' MA trigger level for resp rate
var word measurement
temp var word
tempb var word
temphi var word
templo var word
pot data word
var
HR var word ' heart rate, bpm
RR var word ' respiratory rate
target EMG word
var
hyst var word ' +/- range for EMG, used in gain
setting
z word
offset var
_ word[5] bankl' averaging array for peak follower
track
ma var
_ byte ' EMG amp gain value, 0 - 255
EMG gain
var
ECG gain byte ' ECG amp gain value, 0 - 255
var
pct gain byte ' amp gain, 0 - 100%
var
pct MA var byte ' MA, 0 - 100% of full scale
pct EMG var byte ' EMG, 0 - 100% full scale
time var byte ' blank time interval
blank
_ byte ' adc configuration data
config var
new var byte ' new encoder reading
old var byte ' old encoder reading
direct var byte ' encoder result: 0=CCW, 1=cw, 2=no
change
n var byte ' general purpose counter variable
x var byte ' general purpose counter variable-
y var byte ' general purpose counter variable
z var byte ' general purpose counter variable
nn var byte
timelb var byte ' timer low byte (mS counter)
timehb var byte ' timer high byte (mS counter)
btimehb var byte ~ ' timer b high byte
btimelb var byte ' timer b low byte
ctimehb var byte ' timer c high byte
ctimelb var byte ' timer c low byte
dtimehb var byte bank0 ' timer d high byte
dtimelb var byte bank0 ' timer d low byte
etimehb var byte bank0 ' timer a high byte
etimelb var byte bank0 ' timer a low byte
ftimehb var byte bank0 ' timer f high byte
ftimelb var byte bank0 ' timer f low byte
gtimehb var byte bank0 ' timer g high byte
32
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
gtimelbvar byte bank0 ' timer g low byte
wsave var byte $20 system
wsavelvar byte $a0 systemNecessary for devices with RAM in
' bankl
ssave var byte bank0 system
psave var byte bank0 system
fl bit ' flag = 1 if gain ok, = 0 if out of
EMG range
ok
var
_ bit ' flag = 1 if gain ok, = 0 if out of
_ range
ok
var
fl
ECG
_ bit ' flag for one-time operations
_
fl
one
time
var
_ bit ' blanking flag, 1 = blank in progress
_
blank
var
fl
_ var bit ' resp rate flag, 1 = first upward
RR cross of MA
fl
_ var bit ' arming flag for resp rate measurement
setl
fl
_ mon bit ' screen draw one time flag
draw var
fl
_ _ screen draw one time flag
fl setup
draw var
bit
'
_ _ screen draw one time flag
fl man
draw setup
var
bit
'
_ _ screen draw one time flag, blank
draw option time screen
fl var
bit
'
_ power on select of diagnostic mode
_
diagnostic
var
bit
'
fl
- con $Ofe ' lcd command prefix
I
w con 0 ' working register for instructions
f con 1 ' file register for instructions
asc con 48 ' offset for printing ascii characters
versioncon 120 ' software version x.xx
define INTHAND intserv ' defines interrupt service routine
goto init ' vector to jump around interrupt service
init:
device hs osc
DEFINEOSC 20 ' use 20MIiz crystal
DEFINEADC BITS 10 ' Set number of bits in
result
DEFINE_ CLOCIC 3 ' Set clock source (3=rc)
ADC
DEFINE_ _SAMPLEUS ' Set sampling time in
ADC 45 uS
DEFINELCD_DREG PORTB I
DEFINELCD _DBIT 4
DEFINELCD _RSREG PORTB
DEFINELCD _RSBIT 2
DEFINELCD _EREG PORTB
DEFINELCD _EBIT 3
DEFINELCD _BTTS 4
DEFINELCD _LINES 2
DEFINELCD _COMMANDUS '1500
1100
DEFINELCD DATAUS 70
'75
EEPROM_ ' default
0, gain,
[150, blank
150] time
The present invention contemplates novel software programs for the following
functions: Startup And Initialization (Table III); Main Program (Table IV);
Auto Setup
Mode (Table V); Auto Monitoring Mode (Table VI); Moving Average Peak Detection
(Table VII); Respiratory Rate Measurement (Table VIII); Manual Gain Set (Table
IX);
Blank Pulse Duration Set (Table X); Subroutines (Table XI); Rotary Encoder
(Table
XII); Welcome Screen (Table XIII); Main Monitoring Screen (Table IVX); Auto
Setup
Screen (Table XV); Manual Setup Screen (Table XVI); Blank Pulse Duration Setup
33
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Screen (Table XVII); Interrupt Service Routine (Table XVIII); and Counter
Updates &
Test Bit Toggles (Table T_X_X_).
Table III: Starts And Initialization
TRISA - %111111 ' configure ports
TRISB - %00000000
TRISC - %00000011 ' 0 = output, 1 = input
TRISD - %00110000
TRISE - %111
ADCONO = 0 ' set Aorta & port a for digital I/0
ADCON1 = %10000100 ' porta0, a1, a3 analog, rt. just.
ADRESH
'initializevariables, set
up timers and
interrupts
asm
bcf _led green
bcf _led_red
bcf _beeper
bcf _led~wr
bcf _led_ekg
bcf err -
led
clrf _ ; zero out time counters
_
timelb
clrf _
timehb
clrf _ ; first, just turn off all interrupts
INTCON
bcf T1CON, 1 ; select internal clock for tmrl
TMR1CS
bcf T1CON, 3 ; turn off internal tmrl oscillator
T1OSCEN
bsf INTCON, 7 ; global interrupts enable GIE
bsf INTCON, 6 ; peripheral interrupt enable
PEIE
bcf PIR1, 0 ; clear tmrl interrupt flag TMR1IF
bsf STATUS, RPO ; select bank l
bsf PIE1, 0 ; tmrl interrupt enable TMRlIE
bcf STATUS, RPO ; select bank 0
bsf TlCON, 0 ; enable tmrl TMR10N
movlw Oech ; preload tmrl for 1mS interrupt
movwf TMR1H
movlw 093h
movwf TMRiL
clrwdt ; clear wdt
movlw 11011011b ; prescale /l6, disable portb
pullups
option
bcf _fl_draw_setup ; clear flags
bcf draw_mon
fl
bcf _
_
draw man setup
fl
bcf _
_
_fl_draw option
bcf _fl_EMG_ok
bcf _fl_ECG_ok
bcf time
one
fl
bcf _ ; analog switch controls initial
_ state
_
_c1
bcf _c2
endasm
34
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
startup:
read 0, EMG-gain: read 1, blank_time
if sw_encoder = 0 then ' puts gain settings on line 4 of lcd
fl_diagnostic = 1
else
fl diagnostic = 0
endif
ECG_gain = EMG gain
peak_EMG = 0: peak MA = 0
trig-level = 590 '800 '750
target EMG = 945 ' approx 2.25V + 2.5V (signal goes from 2.5 - 5.OV)
hyst = 30 ' +/- hysteresis for target gain setting
z offset = 476 ' specific zero offset for this unit... check others!
gosub write-pot ' set amp gains to default values
pause 750
gosub welcome_screen
Table IV: Main Prod
' read switches and go to selected routines
main:
if sw_encoder = 0 then option_setup mode ' set blank time mode
if (sw setup = 1) then monitor ' monitor mode selected
if (sw setup = 0) and (sw man = 1) then auto_setup mode 'setup & auto selected
if (sw setup = 0) and (sw man = 0) then manual setup mode'setup & manual mode
goto main
Table V: Auto Setup Mode
' SETUP/MONTTOR switch = SETUP, AUTO/MAN switch = AUTO
' reads signal and automatically adjusts gain
Target is target_EMG +/- hyst (EMG channel)
' target ECG +/1 hyst (ECG channel)
Timer usage: btime (5 sec epoch timer)
' etime (led blink timer)
auto-setup mode:
' 128 EMG-1 Monitor v1.1
' 192 Peak EMG=999% <OIC>
' 148 MA=99% Gain=99%
' 212 STATUS: [AUTO SETUP]
if fl draw_setup = 0 then ' draw screen only once as needed
gosub draw_setup screen
fl_draw setup = 1
fl_draw mon = 0 ' reset other flags
fl_draw man'setup = 0 .
c1 = 0: c2 = 0 ' be sure analog switches are set
endif
btimehb = 0: btimelb = 0 ' zero timerb
while ((btimehb * 256) + btimelb) < 3500 ' 3.5 sec monitoring interval
' check if mode still valid; if not, then exit
if (sw setup = 1) or (sw man = o) or (sw encoder = 0) then
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
gosub double_beep ' no, so exit this module
led~wr = 1 ' restore power led to default on
fl_EMG_ok = 0 ' reset flag
goto main
endif
adcin 0, EMG
adcin 3, ECG
adcin 1, MA
' if EMG < 512 then EMG = 511 + (512 - EMG) ' rectify signal
if EMG < z_offset then EMG = (z offset - 1) + (z offset - EMG) ' rectify
if ECG < z_offset then ECG = (z offset - 1) + (z offset - ECG) ' rectify
if EMG > peak EMG then peak EMG = EMG ' EMG peak detect
if ECG > peak ECG then peak ECG = ECG ' ECG peak detect
if MA > peak MA then peak MA = MA ' MA peak detect
if (fl EMG ok = 1) and (fl ECG ok = 1) then ' blink power LED if signals ok
if fl_one_time = 0 and ((etimehb * 256) + etimelb > 250)then
led-pwr = 0 ' turn off power led
fl onetime = 1
etimelb = 0: etimehb = 0 ' re-start timer
endif ' flag already set
if fl_one_time = 1 and ((etimehb * 256) + etimelb > 250) then
led~pwr = 1
fl onetime = 0
etimelb = 0: etimehb = 0 ' re-start timer
endif
else
led_pwr = 1 ' power led normally on
endif
wend
EMG gain = EMG gain min 252 ' adjust EMG channel gain
EMG gain = EMG gain max 3
if peak EMG < target_EMG
then
lcdout I, 208, "<LO>"
EMG gain = EMG gain + 2
else
lcdout I, 208, "<HI>"
EMG gain = EMG_gain - 2
endif
ECG-gain = ECG gain min 252 ' adjust EMG channel gain
ECG gain = ECG gain max 5
if peak_ECG < target_EMG
then
ECG gain = ECG_gain + 2
else
ECG gain = ECG gain - 2
endif
gosub write_pot ' update amplifier gains
' check if EMG is within window
amplitude
if (peak EMG > (target EMG and (peak EMG < (target EMG + hyst))
- hyst)) then
fl_EMG_ok = 1 ' s ignal is within target range
lcdout I, 208, "<OK>"
36
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
else
fl EMG ok = 0 ' signal out of range
endif
' check if ECG is within amplitude window
if (peak ECG > (target EMG - hyst)) and (peak ECG < (target EMG + hyst)) then
fl ECG ok = 1 ' signal is within target range
else
fl ECG_ok = 0 ' signal out of range
endif
gosub gain calc ' convert gain to percent
gosub MA_calc ' convert peak MA to percent
gosub EMG_calc ' convert peak EMG to percent
lcdout 1,165, DEC2 pct gain, "%'~
lcdout I, 201, DEC3 pct EMG, "%"
lcdout I, 151, DEC2 pct MA, "%"
if fl_diagnostic = l then
lcdout I, 212, "EMG=",DEC3 EMG_gain," ECG=",DEC3 ECG gain," "
~********
endif
peak_EMG = 0: peak_ECG = 0 ' reset peaks
peak_MA = (peak MA * 9) / 10 ' slow decay in peak
goto auto_setup mode
Table VI: Auto Monitorin_ Mode
SETUP/MONITOR switch = MONITOR
' timer usage:
timer (RR on hysteresis timer)
' timerb (MA peak detector droop timer)
' timerc (blank time)
timerd (heart rate)
' timere (RR time)
' timerf (EMG peak detector droop timer)
timerg (refractory timer to prevent double triggering)
<MONITORING> Screen
128 EMG-1 Monitor vl.l
192 HR=999 Resp=99
' 148 MA=100 Gain=99
' 212 STATUS: [MONITORING]
monitor:
if (sw setup <> 1) then ' exit if mode not selected
gosub double beep
goto main
endif
if sw encoder = 0 then option_setup mode ' set blank time mode
if fl draw_mon = 0 then ' draw screen only once as needed
gosub draw_main_screen
fl draw_mon = 1 ' set draw-once flag
fl draw setup = 0 ' reset other flags
37
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
fl_draw man setup = 0
fl_draw option = 0
c1 = 0: c2 = 0 ' be sure analog switches are set
gosub write_pot ' be sure amp gains are set
endif
adcin 1, MA
adcin 3, ECG ' read separate ECG channel
'if ECG < 512 then ECG = 511 + (512 - ECG) ' rectify signal
if ECG < ~ offset then ECG = (z offset - 1) + (z offset - ECG)' rectify signal
if ECG > peak ECG then peak ECG = ECG
' ECG peak detection
' moving average of peak detector for setting trig level
if ((ftimehb * 256) + ftimelb) > 1600 then ' 1800 peak detection time
' track ma [4] = track ma [3] : track ma [3] = track_ma [2]
track ma [2] = track -ma [1] : track ma [1] = track ma [0]
track ma[0] = peak ECG -
temp= (track ma [0] +track ma [1] +track ma [2] +track ma [3] +track ma [4] )
/ 5
temp= (track ma [0] +track ma [1] +track ma [2] ) / 3
tempb = temp - ~_offset
trig level = z_offset + ((7 * tempb) / 10)
ftimehb = 0: ftimelb = 0
'lcdout I, 212, "MA:",#temp," TL:",#trlg_leVel, " "
~**********
peak ECG = 0
endif
Table VII: Movin Average Peak Detection
if MA > peak MA then peak MA = MA
if ((btimehb'* 256) + btimelb) > 5000 then ' 5 sec MA peak detection time
peak MA = (9 * peak MA) / 10 ' let peak droop to 0.9 its value
btimehb = 0: btimelb = 0
eridif
RR trig level = (peak MA * 4) / 10 ' detect level
following diagnostic code, REM out for final release *****
if ECG > trig-level then
led_red = 1
else
led_red = 0
endif
' start blanking if ekg detect level above trigger AND refractory timer ok
' AND blanking not already in progress
if (fl blank = 0) and (ECG > trig level) and (((gtimehb * 256) +
gtimelb) > 350) then
fl blank = 1 ' set flag
ctimehb = 0: ctimelb = 0 ' reset blank time timer
gtimehb = 0: gtimelb = 0 ' reset refractory timer
38
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
led ekg = 1
ci = 1: c2 = 1 ' close analog switches
HR = 60000 / ((dtimehb * 256) + dtimelb) ' compute heart rate
gosub gain calc ' convert gain to percent
gosub MA_calc ' convert peak MA to percent
lcdout I, 195, DEC3 HR
lcdout I, 151, DEC2 pct MA, "%"
lcdout I, 165, DEC2 pct gain, "%"
dtimehb = 0: dtimelb = 0 ' reset heart rate timer
endif
' stop blanking
if (fl blank = 1) and ((ctimehb * 256) + ctimelb) >= blank_time then
fl blank = 0
led ekg = 0
c1 = 0: c2 = 0
if sw_beep = 0 then gosub short beep
endif
Table VIII: Respiratory Measurement
Following arms the ON trigger (fl setl) for resp rate measurement
' Flag is set on first upward crossing of MA, with no inspiration
' in progress (i.e., fl RR = 0), and one-time flag not already set
if (MA > RR_trig-level) and (fl setl = 0) and fl RR = 0 then
timehb = 0: timelb = 0 ' start hysteresis timer
fl_setl = 1 ' arm trigger
endif
' if arming flag is set and hysteresis time elapsed, then check if the MA
' is still above the trigger level, and no inspiration in progress
i.e., fl_RR = 0. If so, compute RR
if fl_setl = 1 and (((timehb * 256) + timelb) > 300) and fl RR = 0 then
adcin l, MA
if (MA > RR_trig_level) then ' still above trig?
RR = 60000 / ((etimehb * 256) + etimelb)
etimehb = 0: etimelb = 0 ' reset RR timer, peak MA
lcdout I, 209, DEC2 RR
fl_RR = 1 ' set flag for RR in progress
fl setl = 0 ' reset arming flag
led green = 1
else
fl_setl = 0
endif
endif
' check for next downward crossing of MA through trigger, and
' turn off inspiration
if (MA < RR_trig level) and (fl RR = 1) then
fl_RR = 0
led_green = 0
endif
39
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
toggle portb.l ' loop timing test point
goto monitor
Table IX: Manual Gain Set
' 128 Set GAIN...
' 192 Peak EMG=nnn%
' 148 Gain=nnn%
' 212 AUTO to exit
manual setup mode:
if fl draw_man_setup = 0 then ' draw screen only once as needed
gosub draw_man_setup screen
fl_draw man_setup = 1
fl_draw mon = 0 ' reset other flags
fl_draw setup = 0
fl_draw option = 0
c1 = 0: c2 = 0 ' be sure analog switches are set
gosub write_pot ' be sure amp gains are set
endif
set loop:
if (sw man = l) then ' MAN SETUP not selected, so exit
gosub double beep
goto main
endif
if sw encoder = 0 then option_setup mode ' set blank time mode selected
adcin 0, EMG
if EMG > peak EMG then peak EMG = EMG t
gosub gain talc
gosub EMG talc
if ((btimehb * 256) + btimelb) > 275 then ' write display each 300mS
lcdout I, 201, DEC3 pct EMG, "%"
lcdout I, 153, DEC3 pct_gain, "%"
btimehb = 0: btimelb = 0
peak EMG = peak EMG - ((10 * peak EMG) / 350) ' let peak droop
endif
if ctimelb > 50 then ' update encoder reading
gosub read_encoder
ctimehb = 0: ctimelb = 0
if direct = 2 then not_changed
if direct = 255 then
EMG gain = ((EMG_gain + 1) min 255)
ECG_gain = ((EMG gain + 1) min 255)
endif
if direct = 0 then
EMG gain = ((EMG gain - 1) max 1)
ECG_gain = ((EMG gain - 1) max 1)
endif
gosub write~pot
not changed:
endif
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
goto set loop
Table X: Blanking Pulse Duration Set
' 128 Set BLANKING TIME...
192 Time = xxx mS
' 148
' 212 Press SELECT to exit
option_setup mode:
if fl draw_option = 0 ' draw screen only once as needed
then
gosub draw_option_screen
draw option = 1
fl
_ ' reset other flags
fl_draw_man_setup = 0
fl_draw_mon = 0
draw-setup = 0
fl
_
endif
if sw_select = 0 then ' exit to main program by SEL
pb press
gosub double beep
goto main
endif
gosub read_encoder
if direct = 2 then no ' no encoder action
change2
_ = blank time + 1
if direct = 255 then blank
time
if direct = 0 then blank_timeblank_time - 1
=
time = blank_time min
l70
blank
_
time = blank_time max
90
blank
_
lcdout I, 199, DEC3 blank_time
write 1, blank time
no change2:
pause 75
goto option-setup mode
goto main
Table XI: Subroutines
' write gain to DS1267 digital pot
EMG amplification range: 0 = 316X, 255 = 31000X
' ECG amplification range: 0 = 320X, 255 = 6400X
' base gain = 316X
write~ot
pot data.byte0 = EMG gain: pot data.bytel = ECG gain
c~ bcf _clk - ' start with clock low
C~ bsf _rs_pot
shiftout dta, clk, 1, [0\1] ' first bit is stack select bit (0)
shiftout dta, clk, 1, [pot data\16] ' composite 16 bit data for pot
bcf rs~ot
return
41
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Table XII: Rotary Encoder
' Output A is porta.2, output B is porta.4
' Get direction of rotation by xor of left bit of old reading with
right
' bit of new reading. Returns direct = 255 (cw rotation),
' 0 (ccw rotation), 2 = no change
read_encoder:
new = Aorta & %00010100
if new = old then no_change ' branch around if no knob action
direct = (new & %00000100) xor (old & %00010000)
goto exit encoder
no_change:
direct = 2
exit_encoder:
old = new
return
clear_lcd:
lcdout I,1
pause 20
return
' compute percent gain from actual gain value
' enter with gain, returns pct gain
gain calc:
pct gain = (118 * EMG gain) / 30l ' = x .392
return
' compute percent MA from actual a/d units value
' enter with peak MA, returns pct MA
' MA units range is approx. 0 - 512
MA_calc:
pct MA = (peak MA * 10) / 26 ' was / 51 ' _ / 5.1
return
' compute percent peak EMG from actual a/d units value
' enter with peak_EMG, returns pct EMG
EMG units range is approx. 512 - 965
EMG_calc:
temp = peak_EMG max 512 ' limit bottom range
temp = temp - 512 ' correct for zero offset
pct_EMG = (peak EMG * 10) / 92 ' _ / 9.2
return
Table XIII: Welcome Screen
gosub clear_lcd
lcdout I, 128, " EMG-1 Monitor"
lcdout I, 192, " Version ", #(version / 100),".",#(version // 100)
lcdout I, 148, " (c) CWE,INC."
pause 2000
42
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
bsf _led_pwr
pause 250
bsf _led ekg
pause 250
bsf _led_err
pause 500
bcf _led_err
pause 250
C bcf _led_ekg
gosub short beep
pause 200
gosub short beep
pause 500
gosub short beep
pause 500
gosub clear-lcd
return
Table IVX: Main Monitoring Screen
' 128 EMG-1 Monitor v1.1
' 192 HR=999 Resp=99
' 148 MA=100 Gain=99
' 212 STATUS: [MONITORING]
gosub clear-lcd
lcdout I, 128, "EMG-1 Monitor v", #(version / 100), ".",
#(version // 100)
lcdout I, 192, "HR= Resp="
lcdout I, 148, "MA= Gain=" '
lcdout I, 212, "STATUS: [MONITOR]"
return
Table XV: Automatic Setup Screen
128 EMG-1 Monitor v1.1
192 Peak EMG=999%
' 148 MA=99% Gain=99%
' 212 STATUS : [AUTO SETUP]
gosub clear lcd
lcdout I, 128, "EMG-1 Monitor v", #(version / 100), ".", #(version // 100)
lcdout I, 192, "Peak EMG="
lcdout I, 148, "MA= Gain="
lcdout I, 212, "STATUS: [AUTO SETUP]"
return
Table XVI: Manual Setup Screen
' 128 Set GAIN...
' 192 Peak EMG=nnn%
' 148 Gain=nnn%
' 212 AUTO to exit
gosub clear lcd
43
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
lcdout I, 128, "Set GAIN..."
gosub gain calc
gosub EMG talc
lcdout I, 201, DEC3 pct_EMG, "%"
lcdout I, 153, DEC3 pct_gain, "%"
lcdout I, 192, "Peak EMG="
lcdout I, 148, "Gain="
lcdout I, 212, "AUTO to exit"
return
Table XVII: Blanking Pulse Duration Setup Screen
' 128 Set BLANKING TIME...
192 Time = xxx mS
' 148
' 212 Press SELECT to exit
gosub clear_lcd
lcdout I, 128, "Set BLANKING TIME..."
lcdout I, 192, "Time = mS"
lcdout I, 199, DEC3 blank_time ' put current value to start with
'lcdout I, 148, " "
lcdout I, 212, "Press SELECT to exit"
return
short beep:
Q bsf beeper
pause 10
bcf beeper
return
double_beep:
gosub short beep
pause 150
gosub short beep
return
Table XVIII: Interrupt Service Routine
movwf wsave ; Save the W register
; swapf STATUS, W
clrf STATUS ; Point to bank 0
movwf ssave ; Save STATUS with reversed nibbles
movf PCLATH, W ; Save PCLATH
movwf psave
Table IXX: Counter Update And Test Bit Toggle
intserv ; interrupt service routine
bcf PIRl, 0 ; clear tmrl int flag TMR1IF
incf _timelb,f ; increment time count to byte
btfsc status,2 ; zero set? N > 255?
incf timehb,f ; yes, increment hi byte of count
44
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
btfss test pt ; toggle the test
pin
goto setbit ; bit is clear
clrbit bcf _test~t ; clear bit 0
goto ldtmr ; load timer
setbit bsf _test_pt
set bit 0
ldtmr movlwOech ; preload for tmrl
movwf TMR1H ; tmrl hi byte
movlw 093h
movwf TMR1L ; tmrl to byte
incf _btimelb,f ; increment timer
b
btfsc status,2 ; zero set? N >
255?
incf _btimehb,f ; yes, increment byte
hi
'
incf ctimelb,f I increment timer
; c
btfsc _ ; zero set? N >
status,2 255?
incf ctimehb,f ; yes, increment byte
hi
incf _dtimelb,f ; increment timer
d
btfsc status,2 ; zero set? N >
255?
incf dtimehb,f ; yes, increment byte
hi
incf etimelb,f ; increment timer
a
btfsc _ ; zero set? N >
status,2 255?
incf etimehb,f ; yes, increment byte
hi
incf _ftimelb,f ; increment timer
f
btfsc status,2 ; zero set? N >
255?
incf ftimehb,f ; yes, increment byte
hi
incf _gtimelb,f ; increment timer
g
btfsc status,2 ; zero set? N >
255?
incf gtimehb,f ; yes, increment byte
hi
movf psave, w ; restore registers
movwf PCLATH ; restore PCLATH
swapf ssave, w ; put wsave into
w with reversed
nibbles
movwf STATUS ; restore STATUS
swapf wsave, f ; reverse nibbles wsave
of
swapf wsave, w ; reverse again,
leave in w retfie
endasm
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
EXPERIMENTAL
EXAMPLE 1
Diaplira~matic Movements And Inspiratory Effort Correlations In Awake Subjects
This example presents data showing the relationship between ~maEMGdi and
dPes in awake subjects. The study population of 8 subjects consisted of 7
health care
professionals having no sleep disordered breathing and one sleep disordered
breathing
patient. Each subjects' anthropornetric data is detailed in Table 1. The study
protocol
was approved by the institutional review boards of the DVA Medical Center -
Northport
and Stony Brook University and informed consent was obtained from each
subject.
Table 1. Anthropometric Data
Subj ect # Age (years) Gender *BMI (kg/m )
1 36 M 31
2 37 M 26
3 48 M 29
4 38 M 28
27 F 28
6 57 M 39
7 38 F 22
8 38 M 27
Mean (SE) 40(3) -- 29(2)
*BMI = body mass index
Esophageal manometry was performed with a saline - filled catheter system and
placed in the middle-third of the esophagus. Baydur et al, "A Simple Method
For
Assessing the Validity Of The Esophageal Balloon Technique" Am Rev Respir Dis
126:788-791 (1982). An 8 French 42" infant feeding tube (Cat. # 85774,
Malinckrodt
Inc, St. Louis, MO) with lateral ports in the distal end (i.e., over the
terminal 1
centimeter) was connected to a calibrated, disposable, arterial line pressure
transducer
46
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
(Model # 041576504A, Argon Medical, Athens, TX). The infant feeding tube was
passed transnasally and swallowed until the distal end was in the stomach
(determined by
a positive pressure deflection with a strong sniff). The catheter was then
gradually
retracted until a strong sniff first resulted in a negative deflection (i. e.,
showing that the
distal 1 cm of the catheter was at the level of the diaphragm). From that
point, the
catheter was retracted an additional 5 centimeters and fastened to the nose
with surgical
tape. Observation of left atrial pressure artifact in the catheter trace was
used to validate
the position of the catheter tip in the middle third of the esophagus.
The surface maEMGdi was monitored using 2 disposable electrodes (type SP-00-
S, Medicotest A/S, Denmark) applied to the skin after very mild dermal
abrasion with
gauze. Positioning of the electrodes is illustrated in Figure 5. Specifically,
the electrodes
were positioned in the lowest right intercostal space intersecting the
anterior axillary line
(the 7th intercostal space) and the next inferior right intercostal space in
the rnid-axillary
line (the 8th intercostal space).The EMG signal was band-pass filtered (10-
1000 Hz),
amplified (Model 7P511 EEG amplifier, Grass Instrument Co, Quincy, MA), full-
wave
rectified and passed through a low-pass moving averager with a time constant
of 200
msec (Model 821, CWE Inc, Ardmore, PA) to obtain the maEMGdi. The EKG artifact
in
the rnaEMGdi was attenuated using a blanker device which senses the EKG signal
and
replaces the EKG artifact with an adjacent portion of the preceding moving
average EMG
signal (Model SB-1 EKG blanker, CWE Inc., Ardmore, PA).
While lying supine, each of the 8 subjects breathed through a nasal mask
connected with a pneumotachygraph (Hans Rudolph, Kansas City, MO). Each
subject
breathed 15 to 30 breaths at each of 4 levels of nasal obstruction: Level 1:
un-occluded
nose; Level 2: one nostril was completely occluded and the second nostril was
one-
quarter occluded; Level 3: one nostril was completely occluded and the second
nostril
was one-half occluded; Level 4: one nostril was completely occluded and the
second
nostril was three-quarters occluded. For each subject, nasal airflow,
esophageal pressure
(Pes) and maEMGdi were recorded. All signals were converted from analogue to
digital
with a sampling frequency of 720 Hz and stored for analysis using a data
analysis
program (DATAQ Instruments, Akron, OH). For both Pes and maEMGdi, a signal
excursion for each inspiration was determined by averaging,the region of the
peak signal
47
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
change and subtracting the baseline signal value, thereby calculating the
value of ~Pes
and ~ maEMGdi, respectively. For each subject, values of OPes and OmaEMGdi
were
plotted for all breaths pooled over all levels of nasal obstruction. The
relationship
between ~maEMGdi and OPes was characterized for each subject by fitting a
least
squares linear regression to the ~Pes values with intercept and slope on the
OmaEMGdi
values and computing the Pearson correlation coefficient. The relationship
between the
~maEMGdi and ~Pes values was then analyzed descriptively in terms of the
correlation
coefficients and the slopes and intercepts of the regression equations. EKG
artifact is
evident in the unprocessed EMGdi signal (raw EMGdi). See Figure 8. The data
clearly
show that with increasing nasal obstruction, inspiratory flow decreases and
inspiratory
effort measured as ~Pes increases. When the EKG is blanked, the OmaEMGdi
increases
in magnitude with increasing OPes.
To assess the effect of body position changes on the relationship of OPes to
~maEMGdi, 3 subjects (#3, #7 and #8) also performed the protocol while lying
recumbent on the left side or right side. See Table 2. Specifically, for
subjects 7 and 8
the y-intercepts in all positions were equally close to zero relative to the
spread of the
dmaEMGdi data. For subject 3, however, in the supine position the y-intercept
deviated
from zero to a much greater degree than observed when the subject is recumbent
on the
left or right side. In general, changes in body position did not interfere
substantially with
the relationship between OmaEMGdi and ~Pes. In some individuals, however,
there may
be an effect of body position depending upon the proportionality of the
relationship.
48
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Table 2. Characteristics of the linear relationship between ~maEMGdi and dPes
as a
function of body position.
Subject/PositionSlope Y-Intercept Corr. Coefficient
Subject 3 Left 1.8 -1.9 0.97
Subject 3 Right1.9 3.9 0.95
Subject 3 Supine2.0 25.0 0.95
Subject 7 Left 1.1 4.4 0.82
Subject 7 Right1.4 -3.5 0.95
Subject 7 Supine1.8 -4.6 0.93
Subject 8 Left 4.3 -8.5 0.88
Subj ect 8 Right3.1 -4.2 0.77
Subject 8 Supine3.9 -6.9 0.87
Figure 8 demonstrates a polygraph recording of the protocol for one subject.
Figure 9 plots 0 maEMGdi against OPes for each of the 8 subjects and
demonstrates that
there is a linear relationship between ~Pes and 0 maEMGdi for each subject.
There are
differences between subjects, however, in the slope of the relationship. For
all 8 subjects,
OPes and OmaEMGdi appear to be linearly related as shown in Table 3.
49
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
Table 3. Exemplary linear relationships between OmaEMGdi and 4Pes
Subject Number Of Y-Intercept Corr. Coefficient
Measurements
Subject 1 108 - 19.3 0.98
Subject 2 64 7.1 0.76
Subject 3 SS 25.0 0.95
Subject 4 72 11.2 0.68
Subject 5 81 4.9 0.84
Subject 6 92 - 15.1 0.92
Subject 7 73 - 4.6 0.93
Subject 8 41 ~ - 6.9 0.87
The slopes of the regression lines, however, vary. On average, the y-intercept
of
the regression lines is near zero suggesting a proportional relationship
(i.e., a positive
correlation) between ~Pes and OmaEMGdi.
In addition, for three of the subj ects, the regression line departs modestly
from
the origin (i. e., the point '0,0'). The data illustrate that ~Pes and b
maEMGdi are
positively correlated where increasing ~ maEMGdi correlates with increasing
~Pes. The
high correlation coefficients between the two parameters (average correlation
coefficient
= 0.85 ~ 0.10) mean that over a wide range of values for OPes, the change of ~
maEMGdi
for a given change in ~Pes is fairly constant. The above observation that the
slope of the
regression differs substantially between subjects prevents calculation of a
population
estimate of the value of ~Pes from dmaEMGdi measurements. Practically, this
means
that this method for measuring inspiratory effort based on ~maEMGdi
measurements is
subject-specific.
Given the observation that a change of ~ maEMGdi for a given change in OPes is
fairly constant, it is useful to investigate the extent to which a certain
percentage change
in ~maEMGdi corresponds to the same percentage change in ~Pes (i. e., whether
changes
in the two parameters are proportional). This correspondence would be true if
the y-
intercept of the regression line were zero. Figure 9 demonstrates that the y-
intercept is
generally not zero, but varies between 9.8 in Subject 3 and 12.6 in Subject
l.The y-
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
intercepts of five subjects (i.e., 2, 4, 5, 7 and 8) are relatively close to
zero. From these
observations, it can be concluded that while many subjects demonstrate
approximate
proportionality between the predicted percentage change in ~Pes and the
percentage
change in ~maEMGdi, one cannot be certain of the proportionality between
changes in
~Pes and ~maEMGdi for any individual subject.
Figure 10 plots dmaEMGdi against ~Pes for each of the 3 subjects who
performed the protocol supine and recumbent upon the right and left sides. As
discussed
above, the slopes of the regression in all three positions were similar and
the correlation
coefficients of the regression were high. See Table 2. The graphs of Subjects
7 and 8
demonstrate little change in the relationship with body position while the
graph of
Subject 3 demonstrates a deviation from the proportionality of the signal
excursions in
the supine position when compared to recumbent positions on the left or right
sides.
Thus, changes in body position did not interfere with the correlation between
~Pes and
OmaEMGdi. In some individuals, however, the precise relationship between the
two
parameters may vary with body position.
Although this example demonstrates a high correlation and approximate
proportionality between OmaEMGdi and ~Pes, it is suggested that ~maEMGdi
cannot be
used to predict dPes for any one subject. This observation is not surprising
because of
the nature of the relationship between diaphragmatic contraction and pleural
pressure
changes. If the diaphragmatic muscle fibers actually generated pleural
pressure, a strict
proportionality of the ~maEMGdi and OPes might be expected. The diaphragm,
however, is believed to decrease pleural pressure by increasing lung volume
and
increasing the lung's elastic recoil. It is suggested that because of this
indirect
relationship between diaphragmatic activity and pleural pressure changes the
value of one
does not predict the value of the other (the relationship between the two
parameters will
vary between subjects) and that the two do not change in a strictly
proportional fashion.
A second factor working against proportionality is the EKG artifact of EMGdi.
Although
we have attempted to blank the EKG signal in the moving average tracing, some
contamination with EKG persists and is visible in the moving average tracing
(Figure 5).
This EKG artifact constitutes a larger portion of the moving average trace at
low levels of
diaphragmatic activity than at high levels of diaphragmatic activity. Improved
blanking
51
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
methodology, as contemplated by the present invention, decreases the EI~G
artifact in the
diaphragmatic EMG signal and increases the degree of proportionality between
the
~maEMGdi and OPes. (see Example 2).
EXAMPLE 2
Diaphragmatic Movements And InspiratorX Effort Correlations In Asleep Su~iects
This example provides data on four sleeping subjects that have been diagnosed
with a sleeping disorder during non-rapid eye movement (NREM) stages of sheep.
Specifically, the data shows a positive correlation between esophageal
pressure and
maEMGdi.
The subjects were tested according to the procedure described in Example 1,
with
the exception that all subjects were administered positive pressure
ventilation with a
standard commercially available CPAP device.
Figure 11A shows data collected from Subject A, whereupon linear regression of
72 individual data points show a positive correlation between Pes and maEMGdi
(r =
0.87). Figure 11B shows data collected from Subject B, whereupon linear
regression of
95 individual data points show a positive correlation between Pes and maEMGdi
(r =
0.83). Figure 11C shows data collected from Subject C, whereupon linear
regression of
59 individual data points show a positive correlation between Pes and maEMGdi
(r =
0.89), Figure 11D shows data collected from Subject D, whereupon linear
regression of
68 individual data points show a positive correlation between Pes and maEMGdi
(r =
0.71).
These data show clearly that Pes and maEMGdi are significantly correlated in
subjects exhibiting a sleep disorder during administration of positive
pressure ventilation.
52
CA 02559857 2006-09-12
WO 2005/096924 PCT/US2005/009492
EXAMPLE 3
Diaphragmatic Movements And Inspiratory Effort Correlations In A UARS Subject
This example demonstrates the use an an EMG monitor in the diagnosis of a
subject having upper respiratory airway syndrome (LTARS).
Figure 12 shows one sixty (60) second data tracing from data collected with an
EMG-1 diagnostic device as contemplated by the present invention. Decreased
maEMGdi, decreased Pes and decreased inspiratory flow were positively
correlated.
Specfically, an inspection of the timeframe between 12:11:35 AM and 12:11:50
AM
clearly shows that a reduction in inspiratory flow (Flow tracing) positively
correlated
with a reduced maEMGdi (EMG averager tracing) and a reduced esophageal
pressure
(Pesoph tracing). These data allow the conclusion that the subject has an
upper airway
resistance.
53