Note: Descriptions are shown in the official language in which they were submitted.
CA 02560417 2010-01-05
23940-1773
1
1,2-DIARYLIMIDAZOLE-4-CARBOXAMIDE DERIVATIVES
Field of invention
The present invention relates to certain 1,2-diarylimidazole-4-carboxamide
compounds of
formula I, to processes for preparing such compounds, to their use in the
treatment of
obesity, psychiatric and neurological disorders, to methods for their
therapeutic use and to
pharmaceutical compositions containing them.
Background of the invention
It is known that certain CBI modulators (known as antagonists or inverse
agonists) are
useful in the treatment of obesity, psychiatric and neurological disorders
(WOO 1/70700
and EP 656351).
W004/60367 and W02004/099130 disclose that certain diaryl imidazoles and
triazoles are
useful as COX-1 inhibitors useful in the treatment of inflammation. Compounds
exemplified in these applications are disclaimed from the claims of the
present invention.
DD 140966 discloses that certain imidazolecarboxylic acid anilides are useful
as plant
growth regulators. Compounds exemplified in this application are disclaimed
from the
claims of the present invention.
WO 03/007887 and W003/075660 disclose certain 4,5-diarytimidazole-2-
carboxamides as
CBI modulators.
W003/27076 and WO 03/63781 disclose certain 1,2-diarylimidazole-4-carboxamides
which are CBI modulators. Compounds exemplified in these applications are
disclaimed
from the claims of the present invention.
W003/40107 discloses certain 1,2-diarylimidazole-4-carboxamides as being
useful in the
treatment of obesity and obesity-related disorders.
However, there is a need for CBI modulators with improved physicochemical
properties
and/or DMPK properties and/or pharmacodynamic properties.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
2
Description of the invention
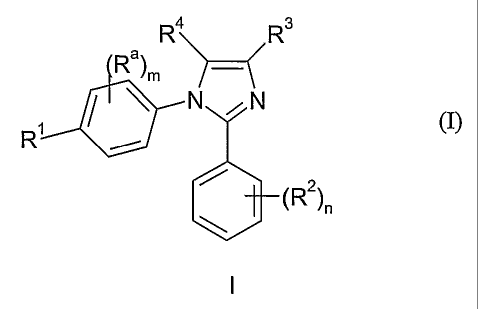
The invention relates to a compound of formula (I)
R4\ / R3
Ra) ~(
m / `
N ,N
Rt
(R2
and pharmaceutically acceptable salts thereof, in which
R1 represents a) a C1_6alkoxy group optionally substituted by one or more
fluoro b) a group
of formula phenyl(CH2)PO- in which p is 1, 2 or 3 and the phenyl ring is
optionally
substituted by 1, 2 or 3 groups represented by Z, c) a group R5S(O)20 or
R5S(O)2NH in
which R5 represents a C1_loalkyl group optionally substituted by one or more
fluoro, or R5
represents phenyl or a heteroaryl group each of which is optionally
substituted by 1, 2 or 3
io groups represented by Z or d) a group of formula (R6)3 Si in which R6
represents a Cl_
6alkyl group which may be the same or different;
Ra represents halo, a CI.3alkyl group or a C1_3alkoxy group;
m is 0, 1, 2 or 3;
R2 represents a C1_3alkyl group, a C1_3alkoxy group, hydroxy, nitro, cyano or
halo
nis0, 1,2or3;
R3 represents
a) a group X-Y-NR7R8
in which X is CO or SO2,
Y is absent or represents NH optionally substituted by a C1_3alleyl group;
and R7 and R8 independently represent :
a C1_6alkyl group optionally substituted by 1, 2, or 3 groups represented by
W;
a C3_15cycloalkyl group optionally substituted by 1, 2, or 3 groups
represented by W;
an optionally substituted (C3_15cycloalkyl)C1_3alkylene group optionally
substituted by 1, 2,
or 3 groups represented by W;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
3
a group -(CH2)r(phenyl ), in which r is 0,1, 2, 3 or 4, s is 1 when r is 0
otherwise s is 1 or 2
and the phenyl groups are optionally independently substituted by one, two or
three groups
represented by Z;
a saturated 5 to 8 membered heterocyclic group containing one nitrogen and
optionally
one of the following : oxygen, sulphur or an additional nitrogen wherein the
heterocyclic
group is optionally substituted by one or more CI-3alkyl groups, hydroxy or
benzyl ;
a group - (CH2)t Het in which t is 0,1, 2, 3 or 4, and the alkylene chain is
optionally
substituted by one or more C1_3alkyl groups and Het represents a heteroaryl
group
optionally substituted by one, two or three groups selected from a Cl_5alkyl
group, a Cl-
io sallcoxy group or halo wherein the alkyl and alkoxy group are optionally
independently
substituted by one of more fluoro;
or R7 represents H and R8 is as defined above;
or R7 and R$ together with the nitrogen atom to which they are attached
represent a
saturated or partially unsaturated 5 to 8 membered heterocyclic group
containing one
nitrogen and optionally one of the following : oxygen, sulphur or an
additional nitrogen;
wherein the heterocyclic group is optionally substituted by one or more CI-
3alkyl groups,
hydroxy, fluoro or benzyl;
or b) oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl,
thiadiazolyl, pyrrolyl,
pyrazolyl, imidazolyl, triazolyl, tetrazolyl, thienyl, furyl or oxazolinyl,
each optionally substituted by 1, 2 or 3 groups Z;
R4 represents H, a Cl_6alkyl group, a Cl_6alkoxy group or a
C1.6alkoxyC1_6alkylene group
which contains a maximum of 6 carbon atoms, each of which groups is optionally
substituted by one or more fluoro or cyano;
Z represents a C1.3aJkyl group, a C1.3alkoxy group, hydroxy, halo,
trifluoromethyl,
trifluoromethylthio, difluoromethoxy, trifluoromethoxy,
trifluoromethylsulphonyl, nitro,
amino, mono or di C1_3alkylamino, C1_3alkylsulphonyl, C1_3alkoxycarbonyl,
carboxy,
cyano, carbamoyl, mono or di C1_3allcyl carbamoyl and acetyl; and
W represents hydroxy, fluoro, a CI-3alkyl group, a C1_3allcoxy group, amino,
mono or di C1_
3alkylamino, or a heterocyclic amine selected from morpholinyl, pyrrolidinyl,
piperdinyl or
piperazinyl in which the heterocyclic amine is optionally substituted by a
C1_3allcyl group
or hydroxyl;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
4
with the proviso that when n is 1 then R2 is not methoxy in either the 2-
position or the 4-
position of the phenyl ring and the further proviso that R1 is not
methylsulfonylamino,
methoxy or CF3O-.
In a particular group of compounds of formula (I)
R4 R3
m r_~
Ra)
N ,N
R1
(R2)n
and pharmaceutically acceptable salts thereof, in which
R1 represents a) a C1_6alkoxy group optionally substituted by one or more
fluoro b) a group
of formula phenyl(CH2)pO- in which p is 1, 2 or 3 and the phenyl ring is
optionally
substituted by 1, 2 or 3 groups represented by Z, c) a group R5S(O)20 or
R5S(O)2NH in
which R5 represents a C1_6aIkyl group optionally substituted by one or more
fluoro, or R5
represents phenyl or a heteroaryl group each of which is optionally
substituted by 1, 2 or 3
groups represented by Z or d) a group of formula (R6)3 Si in which R6
represents a C1_
6alkyl group which may be the same or different;
Ra represents halo, a C1_3alkyl group or a C1_3alkoxy group;
m is 0, 1, 2 or 3;
R2 represents a Cl_3alkyl group, a C1.3alkoxy group, hydroxy, nitro, cyano or
halo
nis0, 1,2or3;
R3 represents
a) a group X-Y-NR7R8
in which X is CO or SO2,
Y is absent or represents NH optionally substituted by a C1_3alkyl group;
and R7 and R8 independently represent :
a Cl_6alkyl group optionally substituted by 1, 2, or 3 groups represented by
W;
a C3_15cycloalkyl group optionally substituted by 1, 2, or 3 groups
represented by W;
an optionally substituted (C3_lscycloalkyl)C1_3alkylene group optionally
substituted by 1, 2,
or 3 groups represented by W;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
a group -(CH2)r(phenyl ), in which r is 0,1, 2, 3 or 4, s is 1 when r is 0
otherwise s is 1 or 2
and the phenyl groups are optionally independently substituted by one, two or
three groups
represented by Z;
a saturated 5 to 8 membered heterocyclic group containing one nitrogen and
optionally
5 one of the following : oxygen, sulphur or an additional nitrogen wherein the
heterocyclic
group is optionally substituted by one or more CI-3alkyl groups, hydroxy or
benzyl ;
a group - (CH2)t Het in which t is 0,1, 2, 3 or 4, and the alkylene chain is
optionally
substituted by one or more CI-3alkyl groups and Het represents a heteroaryl
group
optionally substituted by one, two or three groups selected from a C1_5alkyl
group, a
C1_salkoxy group or halo;
or R7 represents H and R8 is as defined above;
or R7 and R$ together with the nitrogen atom to which they are attached
represent a
saturated or partially unsaturated 5 to 8 membered heterocyclic group
containing one
nitrogen and optionally one of the following : oxygen, sulphur or an
additional nitrogen;
is wherein the heterocyclic group is optionally substituted by one or more CI-
3alkyl groups,
hydroxy, fluoro or benzyl;
or b) oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl,
thiadiazolyl, pyrrolyl,
pyrazolyl, imidazolyl, triazolyl, tetrazolyl, thienyl, fiuryl or oxazolinyl,
each optionally substituted by 1, 2 or 3 groups Z;
R4 represents H, a C1.6alkyl group, a C1.6alkoxy group or a
C1_6alkoxyC1.6alkylene group
which contains a maximum of 6 carbon atoms, each of which groups is optionally
substituted by one or more fluoro or cyano;
Z represents a CI-3alkyl group, a C1.3alkoxy group, hydroxy, halo,
trifluoromethyl,
trifluoromethylthio, difluoromethoxy, trifluoromethoxy,
trifluoromethylsulphonyl, nitro,
amino, mono or di C1.3allcylamino, C1_3alkylsulphonyl, C1_3alkoxycarbonyl,
carboxy,
cyano, carbamoyl, mono or di CI-3alkyl carbamoyl and acetyl; and
W represents hydroxy, fluoro, a CI-3alkyl group, a C1.3alkoxy group, amino,
mono or di C1_
3alkylamino, or a heterocyclic amine selected from morpholinyl, pyrrolidinyl,
piperdinyl or
piperazinyl in which the heterocyclic amine is optionally substituted by a
C1_3allcyl group
or hydroxyl;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
6
with the proviso that when n is 1 then R2 is not methoxy in either the 2-
position or the 4-
position of the phenyl ring and the further proviso that R1 is not
methylsulfonylamino,
methoxy or CF3O-.
In a particular group of compounds of formula (I)
4 / R3
R\
Ra)
m T~
N N
R1
(R2
and pharmaceutically acceptable salts thereof,
R1 represents a) a C3.6alkoxy group substituted by one or more fluoro or b) a
group of
formula phenyl(CH2)PO- in which p is 1, 2 or 3 and the phenyl ring is
optionally
substituted by 1, 2 or 3 groups represented by Z, or c) a group R5S(O)20 in
which R5
represents a C1.6alkyl group optionally substituted by one or more fluoro, or
R5 represents
phenyl or a heteroaryl group each of which is optionally substituted by 1, 2
or 3 groups
represented by Z;
Ra represents halo, a C1_3alkyl group or a C1.3alkoxy group;
mis0, 1,2or3;
R2 represents halo
n is 0, 1, 2 or 3;
R3 represents
a) a group X-Y-NR7R8
in which X is CO;
Y is absent or represents NH optionally substituted by a C1.3alkyl group;
and R7 and R8 independently represent :
a C1.6alkyl group optionally substituted by 1, 2, or 3 groups represented by
W;
a C3_15cycloalkyl group optionally substituted by 1, 2, or 3 groups
represented by W;
an optionally substituted (C3_15cycloalkyl)C1.3alkylene group optionally
substituted by 1, 2,
or 3 groups represented by W;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
7
a group -(CH2)r(phenyl )S in which r is 0,1, 2, 3 or 4, s is 1 when r is 0
otherwise s is 1 or 2
and the phenyl groups are optionally independently substituted by one, two or
three groups
represented by Z;
a saturated 5 to 8 membered heterocyclic group containing one nitrogen and
optionally
one of the following : oxygen, sulphur or an additional nitrogen wherein the
heterocyclic
group is optionally substituted by one or more C1_3alkyl groups, hydroxy or
benzyl ;
a group - (CH2)t Het in which t is 0,1, 2, 3 or 4, and the alkylene chain is
optionally
substituted by one or more C1_3alkyl groups and Het represents a heteroaryl
group
optionally substituted by one, two or three groups selected from a C1_salkyl
group, a
Ci_salkoxy group or halo;
or R7 represents H and R8 is as defined above;
or R7 and R8 together with the nitrogen atom to which they are attached
represent a
saturated or partially unsaturated 5 to 8 membered heterocyclic group
containing one
nitrogen and optionally one of the following : oxygen, sulphur or an
additional nitrogen;
wherein the heterocyclic group is optionally substituted by one or more
C1_3alkyl groups,
hydroxy, fluoro or benzyl;
R4 represents H, a C1_6alkyl group, a C1_6alkoxy group or a
C1_6alkoxyC1_6alkylene group
which contains a maximum of 6 carbon atoms, each of which groups is optionally
substituted by one or more fluoro or cyano;
Z represents a C1_3alkyl group, a C1_3alkoxy group, hydroxy, halo,
trifluoromethyl,
trifluoromethylthio, difluoromethoxy, trifluoromethoxy,
trifluoromethylsulphonyl, nitro,
amino, mono or di C1_3alkylamino, C1.3alkylsulphonyl, C1_3alkoxycarbonyl,
carboxy,
cyano, carbamoyl, mono or di C1_3alkyl carbamoyl and acetyl; and
W represents hydroxy, fluoro, a C1_3alkyl group, a C1_3alkoxy group, amino,
mono or di C1_
3alkylamino, or a heterocyclic amine selected from morpholinyl, pyrrolidinyl,
piperdinyl or
piperazinyl in which the heterocyclic amine is optionally substituted by a
C1.3alkyl group
or hydroxyl.
In a particular group of compounds of formula I, R1 represents a group
R5S(O)20 in which
R5 represents a C1_6alkyl group, particularly a C2_6alkyl group, each
optionally substituted
by one or more fluoro and in which R2, R3, R4, R, in and n are as previously
defined.
Ina particular group of compounds of formula I, R3 represents a group
CONHNR7R8 in
which NR7R8 represents piperidino and R1, R2, R4, Ra, in and n are as
previously defined..
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
8
It will be understood that where a substituent Z is present in more than one
group or where
more than one substituent Z is present in the same group that these
substituents are
independently selected and may be the same or different. The same is true for
W. Similarly
when m is 2 or 3 then the groups Ra are independently selected so that they
may be the
same or different and similarly when n is 2 or 3 then the groups R2 are
independently
selected so that they may be the same or different. Similarly when R5 and R7
and/or R8
contain a heteroaryl group the heteroaryl groups and their optional
substituents are
independently selected so that they may be the same or different.
The term C3_1scycloalkyl includes monocyclic, bicyclic, tricyclic and Spiro
systems for
example, cyclopentyl, cyclohexyl and adamantyl.
The term heteroaryl means an aromatic 5-, 6-, or 7-membered monocyclic ring or
a 9- or
10-membered bicyclic ring, with up to five ring heteroatoms selected from
oxygen,
nitrogen and sulfur. Suitable aromatic heteroaryl groups include, for example
furyl,
pyrrolyl, thienyl, oxazolyl, isoxazolyl, imidazolyl, pyrazolyl, thiazolyl,
isothiazolyl,
oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyridazinyl,
pyrimidinyl, pyrazinyl,
1,3,5-triazinyl, benzofuranyl, indolyl, benzothienyl, benzoxazolyl,
benzimidazolyl,
benzothiazolyl, indazolyl, benzofurazanyl, quinolyl, isoquinolyl,
quinazolinyl,
quinoxalinyl, cinnolinyl or naphthyridinyl. Preferably furyl, pyrrolyl,
thienyl, oxazolyl,
isoxazolyl, imidazolyl, pyrazolyl, oxazolyl thiazolyl, isothiazolyl,
oxadiazolyl,
thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl,
pyrazinyl or 1,3,5-
triazenyl and more preferably pyrrolyl, thienyl, imidazolyl, oxazolyl or
pyridyl.
Suitable saturated or partially unsaturated 5 to 8 membered heterocyclic
groups containing
one or more heteroatoms selected from nitrogen, oxygen or sulphur include, for
example
tetrahydrofuranyl, tetrahydropyranyl, 2,3-dihydro-1,3-thiazolyl, 1,3-
thiazolidinyl,
pyrrolinyl, pyrrolidinyl, morpholinyl, tetrahydro- 1,4-thiazinyl, 1-
oxotetrahydrothienyl, 1,1-
dioxotetrahydro- 1,4-thiazinyl, piperidinyl, homopiperidinyl, piperazinyl,
homopiperazinyl,
dihydropyridinyl, tetrahydropyridinyl, dihydropyrimidinyl or
tetrahydropyrimidinyl,
preferably tetrahydrofuranyl, tetrahydropyranyl, pyrrolidinyl, morpholinyl,
piperidinyl or
piperazinyl, more preferably tetrahydrofuran-3-yl, tetrahydropyran-4-yl,
pyrrolidin-3-yl,
morpholino, piperidino, piperidin-4-yl or piperazin-l-yl.
Suitable groups in which Rl represents a group R5S(O)20 in which R5 represents
a CI_
6alkyl group optionally substituted by one or more fluoro include
methanesulfonyloxy,
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
9
ethanesulfonyloxy, n-propylsulfonyloxy, n-butylsulfonyloxy, 3-methylbutane-1-
sulfonyloxy, 3,3-dimethylbutane-l-sulfonyloxy, fluoromethylsulfonyloxy,
difluoromethylsulfonyloxy, trifluoromethylsulfonyloxy, mono, di or tri
(fluoroethyl)sulfonloxy, 3,3,3-trifluoropropyl-l-sulfonyloxy, or 4,4,4-
trifluorobutyl-l-
sulfonyloxy,
Suitable groups in which R1 represents a C1_6alkoxy group optionally
substituted by one or
more fluoro include butoxy, pentyloxy, hexyloxy, fluoromethoxy,
difluoromethoxy,
trifluoroethoxy, 4,4,4 -trifluorobutoxy, 5,5,5,-trifluoropentyloxy and 6,6,6-
trifluorohexyloxy.
Suitable groups in which R1 represents a group R5S(O)20 in which R5 represents
phenyl
or a heteroaryl group each of which is optionally substituted by 1, 2 or 3
groups
represented by Z include phenylsulfonyloxy, thienylsulfonyloxy or
pyridylsulfonyloxy
optionally substituted by 1, 2 or 3 groups represented by Z.
A particular group of compounds of formula I is represented by formula IA
R4 R3
N N
R~
R2b
Rea
AA
in which R1 is
a) a C3_6alkoxy group substituted by one or more fluoro, b) a group of formula
phenyl(CH2)pO- in which p is 1, 2 or 3 and the phenyl ring is optionally
substituted by 1, 2
or 3 groups represented by Z, c) a group R5S(O)20 in which R5 represents a
C1_ioalkyl
group optionally substituted by one or more fluoro or R5 represents thienyl or
pyridyl each
of which is optionally substituted by one or more halo;
Rea represents H or chloro;
R2b represents H or chloro;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino or R3
represents
a group CONR7R8 in which R7 is H and R8 is pyridyl optionally substituted by
halo or
trifluoromethyl ; and
R4 represents a C1_3alkyl group.
5 Further values of Rea, R2b, R4, R5, R7, and R8 in compounds of formula I and
of formula IA
now follow. It will be understood that such values may be used where
appropriate with any
of the definitions, claims or embodiments defined hereinbefore or hereinafter.
Particularly in compounds of formula IA described immediately above R1
represents a
group R5S(O)20 in which R5 represents a C2_7alkyl group optionally substituted
by one or
10 more fluoro. Particularly in compounds of formula IA described immediately
above R1
represents a group R5S(O)20 in which R5 represents 2-thienyl optionally
substituted by
chloro or R5 represents 3-pyridyl. Particularly in compounds of formula IA
described
immediately above Rea represents chloro and R2b represents chloro.
Particularly in
compounds of formula IA described immediately above R3 represents a group
CONHNR7R8 in which NR7R8 represents piperidino. Particularly in compounds of
formula
IA described immediately above R3 represents a group CONR7R8 in which R7 is H
and R8
is pyridyl optionally substituted by trifluoromethyl. Particularly in
compounds of formula
IA described immediately above R4 is methyl.
A further particular group of compounds of formula I is represented by formula
IA
R4 R3
N ,N
R~
R2b
2a
A
in which R1 is
a) a C3_6alkoxy group substituted by one or more fluoro, b) a group of formula
phenyl(CH2)pO- in which p is 1, 2 or 3 and the phenyl ring is optionally
substituted by 1, 2
or 3 groups represented by Z, c) a group R5S(O)20 in which R5 represents a
C1.6alkyl
group optionally substituted by one or more fluoro;
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
11
Rea represents H or chloro;
R2b represents H or chloro;
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino; and
R4 represents a C1_3alkyl group.
Further values of R1 in compounds of formula I and of formula IA now follow.
It will be
understood that such values may be used where appropriate with any of the
definitions,
claims or embodiments defined hereinbefore or hereinafter.
In one group of compounds of formula I or formula IA, R1 is a C3_6alkoxy group
substituted by one or more fluoro. In a second group of compounds of formula I
or
formula IA, R1 is a C4_6alkoxy group optionally substituted by one or more
fluoro. In a
third group of compounds of formula I or formula IA, R1 is a C4_6alkoxy group
substituted
by one or more fluoro. In a fourth group of compounds of formula I or formula
IA, R1 is a
group of formula phenyl(CH2)pO in which p is 1, 2 or 3. In a fifth group of
compounds of
formula I or formula IA, R1 is a group RSS(O)20 in which Rs represents a
C1_6alkyl group
1s optionally substituted by one or more fluoro.
Particularly R1 is 4,4,4 -trifluorobutoxy, n-butylsulfonyloxy, n-
propylsulfonyloxy, n-
ethylsulfonyloxy, benzyloxy, 4,4,4-trifluorobutyl-l-sulfonyloxy or 3,3,3-
trifluoropropyl-l-
sulfonyloxy. More particularly R1 is 4,4,4 -trifluorobutoxy, n-
butylsulfonyloxy, n-
propylsulfonyloxy, ethylsulfonyloxy or benzyloxy.
Another particular group of compounds of formula I is represented by formula
IA
R4 3
N N
R R2a
IA
in which R1 is
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
12
a) a C3_6alkoxy group substituted by one or more fluoro or b) a group R5S(O)20
in which
R5 represents a C1_6alkyl group optionally substituted by one or more fluoro,
or R5
represents phenyl or a heteroaryl group each of which is optionally
substituted by 1, 2 or 3
groups represented by Z;
W' represents chloro;
R2b represents chloro;
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino; and
R4 represents a C1_3alkyl group.
In a particular group of compounds of formula IA, R1 represents a group
R5S(O)20 in
which R5 represents a C1_6aIkyl group optionally substituted by one or more
fluoro;
Rea represents chloro;
R2b represents chloro;
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino; and
R4 represents a C1_3alkyl group.
In a particular group of compounds of formula IA, R1 represents a C4_6alkoxy
group
optionally substituted by one or more fluoro;
Rea represents chloro;
R2b represents chloro;
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino; and
R4 represents a C1_3alkyl group.
In a particular group of compounds of formula IA, R1 represents a C4_6alkoxy
group
substituted by one or more fluoro;
Rea represents chloro;
R2b represents chloro;
R3 represents a group CONHNR7R8 in which NR7R8 represents piperidino; and
R4 represents a C1_3alkyl group.
"Pharmaceutically acceptable salt", where such salts are possible, includes
both
pharmaceutically acceptable acid and base addition salts. A suitable
pharmaceutically
acceptable salt of a compound of Formula I is, for example, an acid-addition
salt of a
compound of Formula I which is sufficiently basic, for example an acid-
addition salt with
an inorganic or organic acid such as hydrochloric, hydrobromic, sulphuric,
trifluoroacetic,
citric or maleic acid; or, for example a salt of a compound of Formula I which
is
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
13
sufficiently acidic, for example an alkali or alkaline earth metal salt such
as a sodium,
calcium or magnesium salt, or an ammonium salt, or a salt with an organic base
such as
methylamine, dimethylamine, trimethylamine, piperidine, morpholine or
tris-(2-hydroxyethyl) amine.
Throughout the specification and the appended claims, a given chemical formula
or name
shall encompass all stereo and optical isomers and racemates thereof as well
as mixtures in
different proportions of the separate enantiomers, where such isomers and
enantiomers
exist, as well as pharmaceutically acceptable salts thereof and solvates
thereof such as for
instance hydrates. Isomers may be separated using conventional techniques,
e.g.
chromatography or fractional crystallisation. The enantiomers may be isolated
by
separation of racemate for example by fractional crystallisation, resolution
or HPLC. The
diastereomers may be isolated by separation of isomer mixtures for instance by
fractional
crystallisation, HPLC or flash chromatography. Alternatively the stereoisomers
may be
made by chiral synthesis from chiral starting materials under conditions which
will not
is cause racemisation or epimerisation, or by derivatisation, with a chiral
reagent. All
stereoisomers are included within the scope of the invention. All tautomers,
where
possible, are included within the scope of the invention. The present
invention also
encompasses compounds containing one or more isotopes for example 14C, 11C or
19F and their use as isotopically labelled compounds for pharmacological and
metabolic
studies.
The present invention also encompasses prodrugs of a compound of formula I
that is
compounds which are converted into a compound of formula I in vivo.
The following definitions shall apply throughout the specification and the
appended
claims.
Unless otherwise stated or indicated, the term "alkyl" denotes either a
straight or branched
alkyl group. Examples of said alkyl include methyl, ethyl, n-propyl,
isopropyl, n-butyl,
iso-butyl, sec-butyl and t-butyl . Preferred alkyl groups are methyl, ethyl,
propyl, isopropyl
and tertiary butyl.
Unless otherwise stated or indicated, the term "alkoxy" denotes a group O-
alkyl, wherein
alkyl is as defined above.
Unless otherwise stated or indicated, the term "halogen" shall mean fluorine,
chlorine,
bromine or iodine.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
14
Specific compounds of the invention are one or more of the following:
1-(4-benzyloxy-phenyl)-2-(2,4-dichlorophenyl)-5-methyl-1H-imidazole-4-
carboxylic acid
piperidin- 1 -ylamide;
ethanesulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-(piperidin-1-
ylcarbamoyl)-
imidazol-1-yl]phenyl ester;
propane-l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-(piperidin-1-
ylcarbamoyl)-
imidazol-1-yl]-phenyl ester;
butane- l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-(piperidin-1-
ylcarbamoyl)-
imidazol-1-yl] -phenyl ester;
2-(2,4-dichloro-phenyl)-5-methyl- l -[4-(4,4,4-trifluorobutoxy)phenyl]-1H-
imidazole-4-
carboxylic acid piperidin- l -ylamide;
3,3,3-trifluoropropane-l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-
(piperidin-l-
ylcarbamoyl)imidazol-l-yl]phenyl ester;
4,4,4-trifluorobutane-l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-
(piperidin-l-
ylcarbamoyl)imidazol-l-yl]phenyl ester;
4- {2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-l-ylamino)carbonyl]-1H-
imidazol-l -
yl}phenyl thiophene-2-sulfonate ;
4- {2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-l-ylamino)carbonyl]-1H-
imidazol-1-
yl}phenyl pyridine-3-sulfonate;
4-{2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-1-ylamino)carbonyl]-1H-
imidazol-1-
yl}phenyl pyridine-3-sulfonate ;
4- {2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-1-ylamino)carbonyl]-1H-
imidazol-1-
yl}phenyl 3-methylbutane- l -sulfonate;
4- { 2-(2,4-dichlorophenyl)-5 -methyl-4- [(piperidin- l -ylamino) carbonyl] -1
H-imidazol- l -
yl}phenyl 3,3-dimethylbutane-l-sulfonate;
4-[2-(2,4-dichlorophenyl)-5-methyl-4-(f [5-(trifluoromethyl)pyridin-2-yl]amino
} carbonyl)-
1 H-imidazol- l -yl]phenyl 3 ,3,3-trifluoropropane- l -sulfonate;
4-[2-(2,4-dichlorophenyl)-5-methyl-4-(1[5-(trifluoromethyl)pyridin-2-yl]
amino} carbonyl)-
1 H-imidazol- l -yl] phenyl 3 -methylbutane- l -sulfonate;
and pharmaceutically acceptable salts thereof.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
Methods of preparation
The compounds of the invention may be prepared as outlined below according to
any of
the following methods. However, the invention is not limited to these methods,
the
compounds may also be prepared as described for structurally related compounds
in the
5 prior art.
Compounds of formula I in which R1 represents a) a C3_6alkoxy group
substituted by one
or more fluoro or b) a group of formula phenyl(CH2)PO- in which p is 1, 2 or 3
and the
phenyl ring is optionally substituted by 1, 2 or 3 groups represented by Z, or
c) a group
R5S(O)20 may be prepared by reacting a compound of formula II
R4\f eR3
(
Ra)m `
N N
HO
(R2),
10 II
in which R2, R3, R4, R, in and n are as previously defined with a group R'A-X
in which
R1A represents a group such that R1AO represents R1 and X represents a leaving
group for
example halo, at a temperature in the range of -25 to 150 C, in the presence
of an inert
solvent, for example dichloromethane, and optionally in the presence of a base
for example
15 triethylamine or pyridine.
Compounds of formula I in which R, R1, R2, R4, in and n are as previously
defined and R3
represents a group X-Y-NR7R8 in which X is CO and Y, R7 and R8 are as
previously
defined may be prepared by reacting a compound of formula III
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
16
0
R4 OR10
Ra)
m
N ,N
R-
(R2)"
III
in which Ra, R1, R2, R4, in and n are as previously defined and R10 represents
H or a C1_
6alkyl group with a compound of formula IV or a salt thereof
R7 R8 YNH2 IV
in which Y, R7 and R8 are as previously defined, in an inert solvent, for
example toluene,
in the presence of a Lewis Acid, for example trimethylaluminium, at a
temperature in the
range of -25 C to 150 C when R10 is a C1_6alkyl group; or alternatively when
R10 is H by
reacting a compound of formula III with a chlorinating agent for example
oxalyl chloride,
and then reacting the acid chloride produced with an amine of formula IV in an
inert
solvent, for example dichloromethane, in the presence of a base, for example
triethylamine, at a temperature in the range of -25 C to 150 C.
Compounds of formula I in which Ra, R', R2, R4, in and n are as previously
defined and R3
represents a group X-Y-NR7R8 in which X is SO2 may be prepared by reacting a
compound of formula V
4 SO2A
Ra)
m
N ,N
R~
(R2),
V
in which Ra, R1, R2, R4, in and n are as previously defined and A represents a
leaving
group, for example halo eg chloro, with a compound of formula IV in which Y,
R7 and R$
are as previously defined or a salt thereof in an inert solvent, for example
THE or
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
17
dichloromethane, in the presence of a base, for example potassium carbonate,
triethylamine
or pyridine, at a temperature in the range of -25 C to 150 C.
Compounds of formula I in which R, R2, R3, R4, in and n are as previously
defined and R'
represents a group R5S(O)2NH may be prepared by reacting a compound of formula
VI
R4 )___~RRa)
m
N /IN
H2N
(R2),
VI
in which R', R2, R3, R4, m and n are as previously defined with a sulphonating
agent of
formula R5SO2L in which R5 is as previously defined and L represents a leaving
group,
for example chloro, in an inert solvent, for example dichloromethane, in the
presence of a
base, for example triethylamine, at a temperature in the range of -25 C to 150
C.
Certain intermediate compounds are believed to be novel and form part of the
present
invention, particularly compounds of formula III as defined above and
including each and
every definition of Rl given previously.
Compounds of formula II,III V and VI may be prepared by the general synthetic
route
shown at the end of the examples and adaptations thereof or by analogous
methods known
to those skilled in the art. It will be appreciated by those skilled in the
art that during the
reaction sequence certain functional groups will require protection followed
by
deprotection at an appropriate stage see "Protective Groups in Organic
Synthesis", 3rd
Edition (1999) by Greene and Wuts.
Pharmaceutical preparations
The compounds of the invention will normally be administered via the oral,
parenteral,
intravenous, intramuscular, subcutaneous or in other injectable ways, buccal,
rectal,
vaginal, transdermal and/or nasal route and/or via inhalation, in the form of
pharmaceutical
preparations comprising the active ingredient or a pharmaceutically acceptable
addition
salt, in a pharmaceutically acceptable dosage form. Depending upon the
disorder and
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
18
patient to be treated and the route of administration, the compositions may be
administered
at varying doses.
Suitable daily doses of the compounds of the invention in the therapeutic
treatment of
humans are about 0.001-10 mg/kg body weight, preferably 0.01-1 mg/kg body
weight.
Oral formulations are preferred particularly tablets or capsules which may be
formulated
by methods known to those skilled in the art to provide doses of the active
compound in
the range of 0.5mg to 500mg for example 1 mg, 3 mg, 5 mg, 10 mg, 25mg, 5 0mg,
100mg
and 250mg.
According to a further aspect of the invention there is also provided a
pharmaceutical
formulation including any of the compounds of the invention, or
pharmaceutically
acceptable derivatives thereof, in admixture with pharmaceutically acceptable
adjuvants,
diluents and/or carriers.
Pharmacological properties
The compounds of formula (I) are useful for the treatment of obesity or being
overweight,
(e.g., promotion of weight loss and maintenance of weight loss), prevention of
weight gain
(e.g., medication-induced or subsequent to cessation of smoking), for
modulation of
appetite and/or satiety, eating disorders (e.g. binge eating, anorexia,
bulimia and
compulsive), cravings (for drugs, tobacco, alcohol, any appetizing
macronutrients or non-
essential food items), for the treatment of psychiatric disorders such as
psychotic and/or
mood disorders, schizophrenia and schizo-affective disorder, bipolar
disorders, anxiety,
anxio-depressive disorders, depression, mania, obsessive-compulsive disorders,
impulse
control disorders (e.g., Gilles de la Tourette's syndrome), attention
disorders like
ADD/ADHD, stress, and neurological disorders such as dementia and cognitive
and/or
memory dysfunction (e.g., amnesia, Alzheimer's disease, Pick's dementia,
dementia of
ageing, vascular dementia, mild cognitive impairment, age-related cognitive
decline, and
mild dementia of ageing), neurological and/or neurodegenerative disorders
(e:.g. Multiple
Sclerosis, Raynaud's syndrome, Parkinson's disease, Huntington's chorea and
Alzheimer's
disease), demyelinisation-related disorders, neuroinflammatory disorders
(e.g., Guillain-
Barre syndrome).
The compounds are also potentially useful for the prevention or treatment of
dependence
and addictive disorders and behaviours (e.g., alcohol and/or drug abuse,
pathological
gambling, kleptomania), drug withdrawal disorders (e.g., alcohol withdrawal
with or
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
19
without perceptual disturbances; alcohol withdrawal delirium; amphetamine
withdrawal;
cocaine withdrawal; nicotine withdrawal; opioid withdrawal; sedative, hypnotic
or
anxiolytic withdrawal with or without perceptual disturbances; sedative,
hypnotic or
anxiolytic withdrawal delirium; and withdrawal symptoms due to other
substances),
alcohol and/or drug-induced mood, anxiety and/or sleep disorder with onset
during
withdrawal, and alcohol and/or drug relapse.
The compounds are also potentially useful for the prevention or treatment of
neurologica_1
dysfunctions such as dystonias, dyskinesias, akathisia, tremor and spasticity,
treatment of
spinal cord injury, neuropathy, migraine, vigilance disorders, sleep disorders
(e.g.,
disturbed sleep architecture, sleep apnea, obstructive sleep apnea, sleep
apnea syndrome,
pain disorders, cranial trauma.
The compounds are also potentially useful for the treatment of immune,
cardiovascular
disorders (e.g. atherosclerosis, arteriosclerosis, angina pectoris, abnormal
heart rhythms,
and arrhythmias, congestive heart failure, coronary artery disease, heart
disease,
is hypertension, prevention and treatment of left ventricular hypertrophy,
myocardial
infarction, transient ischaemic attack, peripheral vascular disease, systemic
inflammation
of the vasculature, septic chock, stroke, cerebral apoplexy, cerebral
infarction, cerebral
ischaemia, cerebral thrombosis, cerebral embolism, cerebral hemorrhagic,
metabolic
disorders (e.g. conditions showing reduced metabolic activity or a decrease in
resting
energy expenditure as a percentage of total fat-free mass, diabetes mellitus,
dyslipidemia,
fatty liver, gout, hypercholesterolemia, hyperlipidemia, hypertriglyceridemia,
hyperuricacidemia, impaired glucose tolerance, impaired fasting glucose,
insulin
resistance, insulin resistance syndrome, metabolic syndrome, syndrome X,
obesity-
hypoventilation syndrome (Pickwickian syndrome), type I diabetes, type II
diabetes, low
HDL- and/or high LDL-cholesterol levels, low adiponectin levels), reproductive
and
endocrine disorders (e.g. treatment of hypogonadism in males, treatment of
infertility or as
contraceptive, menstrual abnormalities/emmeniopathy, polycystic ovarian
disease, sexual
and reproductive dysfunction in women and men (erectile dysfunction), GH-
deficient
subjects, hirsutism in females, normal variant short stature) and diseases
related to the
respiratory (e.g. asthma and chronic obstructive pulmonary disease) and
gastrointestinal
systems (e.g. dysfunction of gastrointestinal motility or intestinal
propulsion, diarrhea,
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
emesis, nausea, gallbladder disease, cholelithiasis, obesity-related gastro-
esophageal
reflux, ulcers).
The compounds are also potentially useful as agents in treatment of
dermatological
disorders, cancers (e.g. colon, rectum, prostate, breast, ovary, endometrium,
cervix,
5 gallbladder, bile duct), craniopharyngioma, Prader-Willi syndrome, Turner
syndrome,
Frohlich's syndrome, glaucoma, infectious diseases, urinary tract disorders
and
inflammatory disorders (e.g. arthritis deformans, inflammation, inflammatory
sequelae of
viral encephalitis, osteoarthritis) and orthopedic disorders. The compounds
are also
potentially useful as agents in treatment of (esophageal) achalasia.
10 In another aspect the present invention provides a compound of formula I as
previously
defined for use as a medicament.
In a further aspect the present invention provides the use of a compound of
formula I in
the preparation of a medicament for the treatment or prophylaxis of obesity or
being
overweight, (e.g., promotion of weight loss and maintenance of weight loss),
prevention of
15 weight gain (e.g., medication-induced or subsequent to cessation of
smoking), for
modulation of appetite and/or satiety, eating disorders (e.g. binge eating,
anorexia, bulimi a
and compulsive), cravings (for drugs, tobacco, alcohol, any appetizing
macronutrients or
non-essential food items), for the treatment of psychiatric disorders such as
psychotic
and/or mood disorders, schizophrenia and schizo-affective disorder, bipolar
disorders,
20 anxiety, anxio-depressive disorders, depression, mania, obsessive-
compulsive disorders,
impulse control disorders (e.g., Gilles de la Tourette's syndrome), attention
disorders like
ADD/ADHD, stress, and neurological disorders such as dementia and cognitive
and/or
memory dysfunction (e.g., amnesia, Alzheimer's disease, Pick's dementia,
dementia of
ageing, vascular dementia, mild cognitive impairment, age-related cognitive
decline, and
mild dementia of ageing), neurological and/or neurodegenerative disorders
(e.g. Multiple
Sclerosis, Raynaud's syndrome, Parkinson's disease, Huntington's chorea and
Alzheimer's
disease), demyelinisation-related disorders, neuroinflammatory disorders
(e.g., Guillain-
Barre syndrome).
In a further aspect the present invention provides the use of a compound of
formula I in the
preparation of a medicament for the treatment or prophylaxis of dependence and
addictive
disorders and behaviours (e.g., alcohol and/or drug abuse, pathological
gambling,
kleptomania), drug withdrawal disorders (e.g., alcohol withdrawal with or
without
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
21
perceptual disturbances; alcohol withdrawal delirium; amphetamine withdrawal;
cocaine
withdrawal; nicotine withdrawal; opioid withdrawal; sedative, hypnotic or
anxiolytic
withdrawal with or without perceptual disturbances; sedative, hypnotic or
anxiolytic
withdrawal delirium; and withdrawal symptoms due to other substances), alcohol
and/or
drug-induced mood, anxiety and/or sleep disorder with onset during withdrawal,
and
alcohol and/or drug relapse.
In a further aspect the present invention provides the use of a compound of
formula I in
the preparation of a medicament for the treatment or prophylaxis of
neurological
dysfunctions such as dystonias, dyskinesias, akathisia, tremor and spasticity,
treatment of
io spinal cord injury, neuropathy, migraine, vigilance disorders, sleep
disorders (e.g.,
disturbed sleep architecture, sleep apnea, obstructive sleep apnea, sleep
apnea syndrome),
pain disorders, cranial trauma.
In a further aspect the present invention provides the use of a compound of
formula I in
the preparation of a medicament for the treatment or prophylaxis of immune,
cardiovascular disorders (e.g. atherosclerosis, arteriosclerosis, angina
pectoris, abnormal
heart rhythms, and arrhythmias, congestive heart failure, coronary artery
disease, heart
disease, hypertension, prevention and treatment of left ventricular
hypertrophy, myocardial
infarction, transient ischaernic attack, peripheral vascular disease, systemic
inflammation
of the vasculature, septic chock, stroke, cerebral apoplexy, cerebral
infarction, cerebral
ischaemia, cerebral thrombosis, cerebral embolism, cerebral hemorrhagia,
metabolic
disorders (e.g. conditions showing reduced metabolic activity or a decrease in
resting
energy expenditure as a percentage of total fat-free mass, diabetes mellitus,
dyslipidemia,
fatty liver, gout, hypercholesterolemia, hyperlipidemia, hypertriglyceridemia,
hyperuricacidemia, impaired glucose tolerance, impaired fasting glucose,
insulin
resistance, insulin resistance syndrome, metabolic syndrome, syndrome X,
obesity-
hypoventilation syndrome (Pickwickian syndrome), type I diabetes, type II
diabetes, low
HDL- and/or high LDL-cholesterol levels, low adiponectin levels), reproductive
and
endocrine disorders (e.g. treatment of hypogonadism in males, treatment of
infertility or as
contraceptive, menstrual abnormalities/emmeniopathy, polycystic ovarian
disease, sexual
and reproductive dysfunction in women and men (erectile dysfunction), GH-
deficient
subjects, hirsutism in females, normal variant short stature) and diseases
related to the
respiratory (e.g. asthma and chronic obstructive pulmonary disease) and
gastrointestinal
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
22
systems (e.g. dysfunction of gastrointestinal motility or intestinal
propulsion, diarrhea,
emesis, nausea, gallbladder disease, cholelithiasis, obesity-related gastro-
esophageal
reflux, ulcers).
In a further aspect the present invention provides the use of a compound of
formula I in
the preparation of a medicament for the treatment or prophylaxis of
dermatological
disorders, cancers (e.g. colon, rectum, prostate, breast, ovary, endometrium,
cervix,
gallbladder, bile duct), craniopharyngioma, Prader-Willi syndrome, Turner
syndrome,
Frohlich's syndrome, glaucoma, infectious diseases, urinary tract disorders
and
inflammatory disoerders (e.g. arthritis deformans, inflammation, inflammatory
sequelae of
io viral encephalitis, osteoarthritis) and orthopedic disorders.
In a still further aspect the present invention provides a method comprising
administering a
pharmacologically effective amount of a compound of formula Ito a patient in
need
thereof for the prophylaxis or treatment of obesity or being overweight,
(e.g., promotion of
weight loss and maintenance of weight loss), prevention of weight gain (e.g.,
medication-
induced or subsequent to cessation of smoking), for modulation of appetite
and/or satiety,
eating disorders (e.g. binge eating, anorexia, bulimia and compulsive),
cravings (for drugs,
tobacco, alcohol, any appetizing macronutrients or non-essential food items),
for the
treatment of psychiatric disorders such as psychotic and/or mood disorders,
schizophrenia
and schizo-affective disorder, bipolar disorders, anxiety, anxio-depressive
disorders,
depression, mania, obsessive-compulsive disorders, impulse control disorders
(e.g., Gilles
de la Tourette's syndrome), attention disorders like ADD/ADHD, stress, and
neurological
disorders such as dementia and cognitive and/or memory dysfunction (e.g.,
amnesia,
Alzheimer's disease, Pick's dementia, dementia of ageing, vascular dementia,
mild
cognitive impairment, age-related cognitive decline, and mild dementia of
ageing),
neurological and/or neurodegenerative disorders (e.g. Multiple Sclerosis,
Raynaud's
syndrome, Parkinson's disease, Huntington's chorea and Alzheimer's disease),
demyelinisation-related disorders, neuroinflammatory disorders (e.g., Guillain-
Barre
syndrome).
In a still further aspect the present invention provides a method comprising
administering a
pharmacologically effective amount of a compound of formula Ito a patient in
need
thereof for the prophylaxis or treatment of dependence and addictive disorders
and
behaviours (e.g., alcohol and/or drug abuse, pathological gambling,
kleptomania), drug
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
23
withdrawal disorders (e.g., alcohol withdrawal with or without perceptual
disturbances;
alcohol withdrawal delirium; amphetamine withdrawal; cocaine withdrawal;
nicotine
withdrawal; opioid withdrawal; sedative, hypnotic or anxiolytic withdrawal
with or
without perceptual disturbances; sedative, hypnotic or anxiolytic withdrawal
delirium; and
withdrawal symptoms due to other substances), alcohol and/or drug-induced
mood, anxiety
and/or sleep disorder with onset during withdrawal, and alcohol and/or drug
relapse.
In a still further aspect the present invention provides a method comprising
administering a
pharmacologically effective amount of a compound of formula Ito a patient in
need
thereof for the prophylaxis or treatment of neurological dysfunctions such as
dystonias,
dyskinesias, akathisia, tremor and spasticity, treatment of spinal cord
injury, neuropathy,
migraine, vigilance disorders, sleep disorders (e.g., disturbed sleep
architecture, sleep
apnea, obstructive sleep apnea, sleep apnea syndrome), pain disorders, cranial
trauma.
In a still further aspect the present invention provides a method comprising
administering a
pharmacologically effective amount of a compound of formula Ito a patient in
need
thereof for the prophylaxis or treatment of immune, cardiovascular disorders
(e.g.
atherosclerosis, arteriosclerosis, angina pectoris, abnormal heart rhythms,
and arrhythmias,
congestive heart failure, coronary artery disease, heart disease,
hypertension, prevention
and treatment of left ventricular hypertrophy, myocardial infarction,
transient ischaemic
attack, peripheral vascular disease, systemic inflammation of the vasculature,
septic chock,
stroke, cerebral apoplexy, cerebral infarction, cerebral ischaemia, cerebral
thrombosis,
cerebral embolism, cerebral hemorrhagia, metabolic disorders (e.g. conditions
showing
reduced metabolic activity or a decrease in resting energy expenditure as a
percentage of
total fat-free mass, diabetes mellitus, dyslipidemia, fatty liver, gout,
hypercholesterolemia,
hyperlipidemia, hypertriglyceridemia, hyperuricacidemia, impaired glucose
tolerance,
impaired fasting glucose, insulin resistance, insulin resistance syndrome,
metabolic
syndrome, syndrome X, obesity-hypoventilation syndrome (Pickwickian syndrome),
type I
diabetes, type II diabetes, low HDL- and/or high LDL-cholesterol levels, low
adiponectin
levels), reproductive and endocrine disorders (e.g. treatment of hypogonadism
in males,
treatment of infertility or as contraceptive, menstrual
abnormalities/emmeniopathy,
polycystic ovarian disease, sexual and reproductive dysfunction in women and
men
(erectile dysfunction), GH-deficient subjects, hirsutism in females, normal
variant short
stature) and diseases related to the respiratory (e.g. asthma and chronic
obstructive
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
24
pulmonary disease) and gastrointestinal systems (e.g. dysfunction of
gastrointestinal
motility or intestinal propulsion, diarrhea, emesis, nausea, gallbladder
disease,
cholelithiasis, obesity-related gastro-esophageal reflux, ulcers).
In a still further aspect the present invention provides a method comprising
administering a
pharmacologically effective amount of a compound of formula Ito a patient in
need
thereof for the prophylaxis or treatment of dermatological disorders, cancers
(e.g. colon,
rectum, prostate, breast, ovary, endometrium, cervix, gallbladder, bile duct),
craniopharyngioma, Prader-Willi syndrome, Turner syndrome, Frohlich's
syndrome,
glaucoma, infectious diseases, urinary tract disorders and inflammatory
disorders (e.g.
arthritis deformans, inflammation, inflammatory sequelae of viral
encephalitis,
osteoarthritis) and orthopedic disorders..
The compounds of the present invention are particulary suitable for the
treatment of
obesity or being overweight, (e.g., promotion of weight loss and maintenance
of weight
loss), prevention or reversal of weight gain (e.g., rebound, medication-
induced or
subsequent to cessation of smoking), for modulation of appetite and/or
satiety, eating
disorders (e.g. binge eating, anorexia, bulimia and compulsive), cravings (for
drugs,
tobacco, alcohol, any appetizing macronutrients or non-essential food items).
The compounds of formula (I) are useful for the treatment of obesity,
psychiatric disorders
such as psychotic disorders, schizophrenia, bipolar disorders, anxiety, anxio-
depressive
disorders, depression, cognitive disorders, memory disorders, obsessive-
compulsive
disorders, anorexia, bulimia, attention disorders like ADHD, epilepsy, and
related
conditions, and neurological disorders such as dementia, neurological
disorders(e.g.
Multiple Sclerosis), Raynaud's syndrome, Parkinson's disease, Huntington's
chorea and
Alzheimer's disease. The compounds are also potentially useful for the
treatment of
immune, cardiovascular, reproductive and endocrine disorders, septic shock and
diseases
related to the respiratory and gastrointestinal systems (e.g. diarrhea). The
compounds are
also potentially useful as agents in treatment of extended abuse, addiction
and/or relapse
indications, e.g. treating drug (nicotine, ethanol, cocaine, opiates, etc)
dependence and/or
treating drug (nicotine, ethanol, cocaine, opiates, etc) withdrawal symptoms.
The
compounds may also eliminate the increase in weight that normally accompanies
the
cessation of smoking.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
In another aspect the present invention provides a compound of formula I as
previously
defined for use as a medicament.
In a further aspect the present invention provides the use of a compound of
formula I in
the preparation of a medicament for the treatment or prophylaxis of obesity,
psychiatric
5 disorders such as psychotic disorders, schizophrenia, bipolar disorders,
anxiety, anxio-
depressive disorders, depression, cognitive disorders, memory disorders,
obsessive-
compulsive disorders, anorexia, bulimia, attention disorders like ADHD,
epilepsy, and
related conditions, neurological disorders such as dementia, neurological
disorders (e.g.
Multiple Sclerosis), Parkinson's Disease, Huntington's Chorea and Alzheimer's
Disease,
10 immune, cardiovascular, reproductive and endocrine disorders, septic shock,
diseases
related to the respiratory and gastrointestinal systems (e.g. diarrhea), and
extended abuse,
addiction and/or relapse indications, e.g. treating drug (nicotine, ethanol,
cocaine, opiates,
etc) dependence and/or treating drug (nicotine, ethanol, cocaine,
opiates,,etc) withdrawal
symptoms.
15 In a still further aspect the present invention provides a method of
treating obesity,
psychiatric disorders such as psychotic disorders such as schizophrenia and
bipolar
disorders, anxiety, anxio-depressive disorders, depression, cognitive
disorders, memory
disorders, obsessive-compulsive disorders, anorexia, bulimia, attention
disorders like
ADHD, epilepsy, and related conditions, neurological disorders such as
dementia,
20 neurological disorders (e.g. Multiple Sclerosis), Parkinson's Disease,
Huntington's Chorea
and Alzheimer's Disease, immune, cardiovascular, reproductive and endocrine
disorders,
septic shock, diseases related to the respiratory and gastrointestinal systems
(e.g. diarrhea),
and extended abuse, addiction and/or relapse indications, e.g. treating drug
(nicotine,
ethanol, cocaine, opiates, etc) dependence and/or treating drug (nicotine,
ethanol, cocaine,
25 opiates, etc) withdrawal symptoms comprising administering a
pharmacologically effective
amount of a compound of formula Ito a patient in need thereof.
The compounds of the present invention are particulary suitable for the
treatment of
obesity, e.g. by reduction of appetite and body weight, maintenance of weight
reduction
and prevention of rebound.
The compounds of the present invention may also be used to prevent or reverse
medication-induced weight gain, e.g. weight gain caused by antipsychotic
(neuroleptic)
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
26
treatment(s). The compounds of the present invention may also be used to
prevent or
reverse weight gain associated with smoking cessation.
The compounds of the present invention are suitable for use in treating the
above
indications in juvenile or adolescent patient populations.
Combination Therapy
The compounds of the invention may be combined with another therapeutic agent
that is
useful in the treatment of obesity such as other anti-obesity drugs, that
affect energy
expenditure, glycolysis, gluconeogenesis, glucogenolysis, lipolysis,
lipogenesis, fat
absorption, fat storage, fat excretion, hunger and/or satiety and/or craving
mechanisms,
appetite/motivation, food intake, or G-I motility.
The compounds of the invention may further be combined with another
therapeutic agent
that is useful in the treatment of disorders associated with obesity such as
hypertension,
hyperlipidaemias, dyslipidaemias, diabetes, sleep apnea, asthma, heart
disorders,
atherosclerosis, macro and micro vascular diseases, liver steatosis, cancer,
joint disorders,
and gallbladder disorders. For example, a compound of the present invention
may be used
in combination with a another therapeutic agent that lowers blood pressure or
that
decreases the ratio of LDL:HDL or an agent that causes a decrease in
circulating levels of
LDL-cholesterol. In patients with diabetes mellitus the compounds of the
invention may
also be combined with therapeutic agents used to treat complications related
to micro-
angiopathies.
The compounds of the invention may be used alongside other therapies for the
treatment of
obesity and its associated complications the metabolic syndrome and type 2
diabetes, these
include biguanide drugs, insulin (synthetic insulin analogues) and oral
antihyperglycemics
(these are divided into prandial glucose regulators and alpha-glucosidase
inhibitors).
In another aspect of the invention, the compound of formula I, or a
pharmaceutically
acceptable salt thereof may be administered in association with a PPAR
modulating agent.
PPAR modulating agents include but are not limited to a PPAR alpha and/or
gamma
agonist, or pharmaceutically acceptable salts, solvates, solvates of such
salts or prodrugs
thereof. Suitable PPAR alpha and/or gamma agonists, pharmaceutically
acceptable salts,
solvates, solvates of such salts or prodrugs thereof are well known in the
art.
In addition the combination of the invention may be used in conjunction with a
sulfonylurea. The present invention also includes a compound of the present
invention in
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
27
combination with a cholesterol-lowering agent. The cholesterol-lowering agents
referred to
in this application include but are not limited to inhibitors of HMG-CoA
reductase (3-
hydroxy-3-methylglutaryl coenzyme A reductase). Suitably the HMG-CoA reductase
inhibitor is a statin.
In the present application, the term "cholesterol-lowering agent" also
includes chemical
modifications of the HMG-CoA reductase inhibitors, such as esters, prodrugs
and
metabolites, whether active or inactive.
The present invention also includes a compound of the present invention in
combination
with an inhibitor of the ileal bile acid transport system (IBAT inhibitor).
The present
io invention also includes a compound of the present invention in combination
with a bile
acid binding resin.
The present invention also includes a compound of the present invention in
combination
with a bile acid sequestering agent, for example colestipol or cholestyramine
or
cholestagel.
is According to an additional further aspect of the present invention there is
provided a
combination treatment comprising the administration of an effective amount of
a
compound of the formula I, or a pharmaceutically acceptable salt thereof,
optionally
together with a pharmaceutically acceptable diluent or carrier, with the
simultaneous,
sequential or separate administration one or more of the following agents
selected from:
20 a CETP (cholesteryl ester transfer protein) inhibitor;
a cholesterol absorption antagonist;
a MTP (microsomal transfer protein) inhibitor ;
a nicotinic acid derivative, including slow release and combination products;
a phytosterol compound ;
25 probucol;
an anti-coagulant;
an omega-3 fatty acid ;
another anti-obesity compound for example sibutramine, phentermine, orlistat,
bupropion,
ephedrine, thyroxine;
30 an antihypertensive compound for example an angiotensin converting enzyme
(ACE)
inhibitor, an angiotensin II receptor antagonist, an adrenergic blocker, an
alpha adrenergic
blocker, a beta adrenergic blocker, a mixed alpha/beta adrenergic blocker, an
adrenergic
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
28
stimulant, calcium channel blocker, an AT-I blocker, a saluretic, a diuretic
or a
vasodilator;
a melanin concentrating hormone (MCH) modulator;
an NPY receptor modulator;
an orexin receptor modulator;
a phosphoinositide-dependent protein kinase (PDK) modulator; or
modulators of nuclear receptors for example LXR, FXR, RXR, GR, ERRa, 0, PPARa,
j3, y
and RORalpha;
a monoamine transmission-modulating agent, for example a selective serotonin
reuptake
inhibitor (SSRI), a noradrenaline reuptake inhibitor (NARI), a noradrenaline-
serotonin
reuptake inhibitor (SNRI), a monoamine oxidase inhibitor (MAOI), a tricyclic
antidepressive agent (TCA), a noradrenergic and specific serotonergic
antidepressant
(NaSSA);
an antipsychotic agent for example olanzapine and clozapine;
a serotonin receptor modulator;
a leptin/leptin receptor modulator;
a ghrelin/ghrelin receptor modulator;
a DPP-IV inhibitor;
or a pharmaceutically acceptable salt, solvate, solvate of such a salt or a
prodrug thereof,
optionally together with a pharmaceutically acceptable diluent or carrier to a
warm-
blooded animal, such as man in need of such therapeutic treatment.
According to an additional further aspect of the present invention there is
provided a
combination treatment comprising the administration of an effective amount of
a
compound of the formula I, or a pharmaceutically acceptable salt thereof,
optionally
together with a pharmaceutically acceptable diluent or carrier, with the
simultaneous,
sequential or separate administration of very low calorie diets (VLCD) or low-
calorie diets
(LCD).
Therefore in an additional feature of the invention, there is provided a
method for the
treatment of obesity and its associated complications in a warm-blooded
animal, such as
man, in need of such treatment which comprises administering to said animal an
effective
amount of a compound of formula I, or a pharmaceutically acceptable salt
thereof in
simultaneous, sequential or separate administration with an effective amount
of a
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
29
compound from one of the other classes of compounds described in this
combination
section, or a pharmaceutically acceptable salt, solvate, solvate of such a
salt or a prodrug
thereof.
Therefore in an additional feature of the invention, there is provided a
method of treating
hyperlipidemic conditions in a warm-blooded animal, such as man, in need of
such
treatment which comprises administering to said animal an effective amount of
a
compound of formula I, or a pharmaceutically acceptable salt thereof in
simultaneous,
sequential or separate administration with an effective amount of a compound
from one of
the other classes of compounds described in this combination section or a
io pharmaceutically acceptable salt, solvate, solvate of such a salt or a
prodrug thereof.
According to a further aspect of the invention there is provided a
pharmaceutical
composition which comprises a compound of formula I, or a pharmaceutically
acceptable
salt thereof, and a compound from one of the other classes of compounds
described in this
combination section or a pharmaceutically acceptable salt, solvate, solvate of
such a salt or
a prodrug thereof, in association with a pharmaceutically acceptable diluent
or carrier.
According to a further aspect of the present invention there is provided a kit
comprising a
compound of formula I, or a pharmaceutically acceptable salt thereof, and a
compound
from one of the other classes of compounds described in this combination
section or a
pharmaceutically acceptable salt, solvate, solvate of such a salt or a prodrug
thereof.
According to a further aspect of the present invention there is provided a kit
comprising:
a) a compound of formula I, or a pharmaceutically acceptable salt thereof, in
a first unit
dosage form;
b) a compound from one of the other classes of compounds described in this
combination
section or a pharmaceutically acceptable salt, solvate, solvate of such a salt
or a prodrug
thereof; in a second unit dosage form; and
c) container means for containing said first and second dosage forms.
According to a further aspect of the present invention there is provided a kit
comprising:
a) a compound of formula I, or a pharmaceutically acceptable salt thereof,
together with a
pharmaceutically acceptable diluent or carrier, in a first unit dosage form;
b) a compound from one of the other classes of compounds described in this
combination
section or a pharmaceutically acceptable salt, solvate, solvate of such a salt
or a prodrug
thereof, in a second unit dosage form; and
CA 02560417 2010-01-05
23940-1773
c) container means for containing said first and second dosage forms.
According to another feature of the invention there is provided the use of a
compound of
the formula I, or a pharmaceutically acceptable salt thereof, and one of the
other
compounds described in this combination section, or a pharmaceutically
acceptable salt,
5 solvate, solvate of such a salt or a prodrug thereof, in the manufacture of
a medicament for
use in the the treatment of obesity and its associated complications in a warm-
blooded
animal, such as man.
According to another feature of the invention there is provided the use of a
compound of
the formula I, or a pharmaceutically acceptable salt thereof, and one of the
other
10 compounds described in this combination section, or a pharmaceutically
acceptable salt,
solvate, solvate of such a salt or a prodrug thereof, in the manufacture of a
medicament for
use in the treatment of hyperlipidaemic conditions in a warm-blooded animal,
such as man.
According to a further aspect of the present invention there is provided a
combination
treatment comprising the administration of an effective amount of a compound
of the
15 formula I, or a pharmaceutically acceptable salt thereof, optionally
together with a
pharmaceutically acceptable diluent or carrier, with the simultaneous,
sequential or
separate administration of an effective amount of one of the other compounds
described in
this combination section, or a pharmaceutically acceptable salt, solvate,
solvate of such a
salt or a prodrug thereof, optionally together with a pharmaceutically
acceptable diluent or
20 carrier to a warm-blooded animal, such as man in need of such therapeutic
treatment.
Furthermore, a compound of the invention may also be combined with therapeutic
agents
that are useful in the treatment of disorders or conditions associated with
obesity (such as
type II diabetes, metabolic syndrome, dyslipidemia, impaired glucose
tolerance,
hypertension, coronary heart disease, non-alcoholic steatohepatitis,
osteoarthritis and some
25 cancers) and psychiatric and neurological conditions.
It will be understood that there are medically accepted definitions of obesity
and being
overweight. A patient may be identified by, for example, measuring body mass
index
(BMI), which is calculated by dividing weight in kilograms by height in metres
squared,
and comparing the result with the definitions.
CA 02560417 2010-01-05
23940-1773
30a
The invention also relates to a commercial package comprising a
compound, salt or composition/formulation of the invention and associated
therewith instructions for the use thereof as defined above.
Pharmacological Activity
Compounds of the present invention are active against the receptor
product of the CB1 gene. The affinity of the compounds of the invention for
central cannabinoid receptors is
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
31
demonstrable in methods described in Devane et al, Molecular Pharmacology,
1988,
34,605 or those described in WOO 1/70700 or EP 656354. Alternatively the assay
maybe
performed as follows.
g of membranes prepared from cells stably transfected with the CB1 gene were
5 suspended in 200 l of 100mM NaCl, 5mM MgCl2, 1mM EDTA, 50mM HEPES (pH 7.4),
1mM DTT, 0.1% BSA and 100 M GDP. To this was added an EC80 concentration of
agonist (CP55940), the required concentration of test compound and 0.1 gCi
[35S]-GTPyS.
The reaction was allowed to proceed at 30 C for 45 min. Samples were then
transferred on
to GF/B filters using a cell harvester and washed with wash buffer (50mM Tris
(pH 7.4),
10 5mM MgC12, 50mM NaCl). Filters were then covered with scintilant and
counted for the
amount of [35S]-GTP7S retained by the filter.
Activity is measured in the absence of all ligands (minimum activity) or in
the presence of
an EC80 concentration of CP55940 (maximum activity). These activities are set
as 0%
and 100% activity respectively. At various concentrations of novel ligand,
activity is
is calculated as a percentage of the maximum activity and plotted. The data
are fitted using
the equation y=A+((B-A)/l+((C/x) LTD)) and the IC50 value determined as the
concentration required to give half maximal inhibition of GTP7S binding under
the
conditions used.
The compounds of the present invention are active at the CB 1 receptor (IC50
<1
micromolar). Most preferred compounds have IC50 <200 nanomolar. For example
the
IC50 of Example 1 is l8nM and Example 2 is 28nM
The compounds of the invention are believed to be selective CBI antagonists or
inverse
agonists. The potency, selectivity profile and side effect propensity may
limit the clinical
usefulness of hitherto known compounds with alleged CB 1 antagonistic/inverse
agonistic
properties. In this regard, preclinical evaluation of compounds of the present
invention in
models of gastrointestinal and/or cardiovascular function indicates that they
offer
significant advantages compared to representative reference CB1
antagonist/inverse
agonist agents.
The compounds of the present invention may provide additional benefits in
terms of
potency, selectivity profile, bioavailability, half-life in plasma, blood
brain permeability,
plasma protein binding (for example increasing the free fraction of drug) or
solubility
compared to representative reference CB 1 antagonists/inverse agonist agents.
CA 02560417 2010-01-05
23940-1773
32
The utility of the compounds of the present invention in the treatment of
obesity and
related conditions is demonstrated by a decrease in body weight in cafeteria
diet-induced
obese mice. Female C57B1/63 mice were given ad libitum access to calorie-dense
`cafeteria' diet (soft chocolate/cocoa-type pastry, chocolate, fatty cheese
and nougat) and
s standard lab chow for 8-1Oweeks. Compounds to be tested were then
administered
systemically (iv, ip, se or po) once daily for a minimum of 5 days, and the
body weights of
the mice monitored on a daily basis. Simultaneous assessment of adiposity was
carried by
means of DEXATM imaging at baseline and termination of the study. Blood
sampling was
also carried out to assay changes in obesity-related plasma markers.
Examples
Abbreviations
DMF dimethylformamide
DEA Diethylamine
EtOAc ethyl acetate
i s THE tetrahydrofuran
TLC thin layer chromatography
t triplet
s singlet
d doublet
q quartet
qvint quintet
m multiplet
br broad
bs broad singlet
dm doublet of multiplet
bt broad triplet
dd doublet of doublet
General Experimental Procedures
Mass spectra were recorded on either a MicromassTM ZQ single quadrupole or a
MicromassTM
LCZ single quadrupole mass spectrometer both equipped with a pneumatically
assisted
electrospray interface (LC-MS). 1H NMR measurements were performed on either a
CA 02560417 2010-01-05
23940-1773
33
Varian MercuryTM 300 or a Varian InovaTM 500, operating at 'H frequencies of
300 and 500
MHz respectively. Chemical shifts are given in ppm with CDC13 as internal
standard.
CDCI3 is used as the solvent for NMR unless otherwise stated. Purification was
performed
on a semipreparative HPLC with a mass triggered fraction collector, Shimadzu
QP 8000
s single quadrupole mass spectrometer equipped with 19 x 100 mm C8 column. The
mobile
phase used was, if nothing else is stated, acetonitrile and buffer (0.1 M
NH4Ac:acetonitrite
95:5).
For isolation of isomers, a KromasilTM CN E9344 (250 x 20 mm i.d.) column was
used.
Heptane:ethyl acetate: DEA 95:5:0.1 was used as mobile phase (1 ml/min).
Fraction
to collection was guided using a UV-detector (330 nm).
Examples of the Invention
Example 1
Step 1.
N-(44-Benzyloxyphenyl)-2,4-dichloro-benzamidine
is 4-Benzyloxyaniline hydrochloride (5.0 g, 21.2 mmol) was dropwise added to a
solution of
etbylmagnesium bromide (44.5 ml, 1M in THF, 44.5 mmol) in 25 nil dry THE under
nitrogen atmosphere. After stirring for 20 minutes a solution of 2,4-
dichlorobenzonitrile
(3.65 g, 21.2 mmol) in 25 ml THE was added. The reaction mixture was stirred
for 20
hours at room' temperature. Water (50 nil) was carefully added. Extraction
with EtOAc
20 (2x100 ml), drying (Na2SO4), filtration and evaporation to dryness afforded
7.7 g (98%) of
the title compound.
Step 2.
1-(4-Benzyloxyphenyl)-2-(2,4-dichlorophenyl)-5-methyl-lH-imidazole-4-
carboxylic acid
ethyl ester
25 To N-(4-bcnzyloxyphenyl)-2,4-dichlorobenzamidine, from Ex. 1, Step 1 (6.8 8
g, 18.5
mmol) dissolved in 50 ml THE was added potassium carbonate (2.56 g, 18.5
rnmol) and
the suspension was stirred for 10 minutes. Ethyl-3-bromo-2-oxobutanoate (4.65
g, 22.2
mmol) was dropwise added over 1 hour, and the mixture was stirred for 66 hours
at room
temperature. The solution was filtered and evaporated to dryness. The residue
was
30 dissolved in acetic acid and refluxed for 1 hour. The mixture was cooled to
room
temperature, 100 ml water added and the product extracted with EtOAc (2x200
ml). The
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
34
combined organic phases were washed with saturated sodium hydrogen carbonate,
dried
(Na2SO4), filtered and concentrated. Flash chromatography (silica,
hexane:EtOAc 70:30,
60:40) afforded 5.75 g (65%) of the title compound as a pale yellow solid.
'H NMR (CDC13): S 7.5-7.2 (8H, m), 7.1-6.9 (4H, m), 5.1 (2H, s), 4.5 (2H, q),
2.5 (3H, s),
1.5 (3H, t), MS m/z 504 (M+Na), 985 (2 M+Na)
Step 3.
1-(4-Benzyloxyphenyl)-2-(2,4-dichlorophanyl -5-methyl-1H-imidazole-4-
carboxylic acid
To a suspension of 1-(4-benzyloxyphenyl)-2-(2,4-dichlorophenyl)-5-methyl-lH-
imidazole-
4-carboxylic acid ethyl ester, from Ex. 1, Step 2 (3.62 g, 7.5 mmol) in 60 ml
methanol was
io added potassium hydroxide (4.05 g, 72 rnmol) in water (20 ml), and the
reaction mixture
was refluxed for 2 hours. The mixture was cooled to room temperature,
acidified to pH-2
with 1 M HCl and extracted with ethyl acetate (2x200 ml). The combined organic
phases
were dried (Na2S04), filtered and concentrated to give 3.38 g (99%) of the
title compound.
Step 4.
1-(4-Benzyloxyphenyl)-2-(2,4-dichlorophc:nyl)-5-methyl-1H-imidazole-4-
carboxylic acid
piperidin-1-vlamide
A solution of 1-(4-benzyloxyphenyl)-2-(2,4-dichlorophenyl)-5-methyl-1H-
imidazole-4-
carboxylic acid, from Ex 1, Step 3 (3.38 g, 7.5 mmol) in 60 ml CH2Cl2 was
added 3 drops
of DMF followed by oxalyl chloride (1.3 ml, 14.9 mmol). The mixture was
refluxed for 2
hours, cooled to room temperature and evaporated to dryness. The residue was
dissolved
in 50 ml CH2Cl2 and cooled to 0 C. T'riethylamine (2.1 ml, 14.9 mmol) was
added
followed by 1-aminopiperidine (0.9 ml, 8.2 mmol) and the mixture was stirred
at room
temperature for 2 hours. Water (300 ml) was added, the mixture extracted with
CH2Cl2
(3x100 ml), dried (Na2S04), filtered and concentrated. Flash chromatography
(silica,
hexane:EtOAc 1:2, EtOAc) afforded 2.94 g (74%) of the title compound as a
white solid.
1H NMR (CDC13): 6 7.5-7.2 (8H, m), 7.1-6.9 (4H, m), 5.1 (2H, s), 3.0-2.7 (4H,
m), 2.5
(3H, s), 1.9-1.7 (4H, m), 1.6-1.4 (2H, m). MS m/z 558 (M+Na). HPLC: 96.5%.
Example 2
Step 1.
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl- l H-imidazole-4-
carboxylic acid
piperidin-1-vlamide
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
1-(4-Benzyloxyphenyl)-2-(2,4-dichlorophenyl)-5-methyl-1H-imidazole-4-
carboxylic acid
piperidin-1-ylamide , from Ex. 1, Step 4 (2.78 g, 5.2 mmol) was dissolved in
80 ml
CH2C12 and cooled to 0 C. Boron tribromide solution (1 M in CH2C12, 10.4 ml,
10.4 mmol)
was added dropwise and the reaction mixture was stirred at room temperature
for 1 hour.
5 Water (200 ml) was added and the solution extracted with EtOAc (3x200 ml).
The
combined organic phases were dried (Na2SO4), filtered and concentrated. Flash
chromatography (silica, hexane:EtOAc 1:3, EtOAc) afforded 1.34 g (58%) of the
title
compound as a white solid.
'H NMR (CDC13): 8 8.6 (lH, bs), 7.4-7.1 (3H, m), 7.0-6.9 (4H, m), 3.0-2.8 (4H,
m), 2.5
10 (311, s), 1.8-1.6 (4H, m), 1.5-1.3 (2H, m).
Step 2
Ethanesulfonic acid 4-[2-(2 4-dichlorophenyl)-5-meths-(piperidin-1-
ylcarbamoyl)-
imidazol-1-yll-phenyl ester
A solution of 2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-1H-imidazole-
4-
15 carboxylic acid piperidin-1-ylamide, from Ex. 2, Step 1 (321 mg, 0.72 mmol)
in 10 ml
CH2C12 was cooled to 0 C. Triethylamine C101 p1, 0.72 mmol) was added followed
by
ethanesulfonyl chloride (69 Rl, 0.72 mmol) and the reaction mixture was
stirred at room
temperature overnight. Water was added, the mixture extracted with CH2C12
(3x20 ml),
dried (Na2S04), filtered and concentrated. Flash chromatography (silica,
hexane:EtOAc
20 1:3) afforded 230 mg (60%) of the title compound as a white solid.
'H NMR (CDC13): 8 7.9 (1H, broad s), 7.4-7.1 (7H, m), 3.3 (2H, q), 3.0-2.8
(4H, m), 2.5
(3H, s), 1.9-1.7 (4H, m), 1.5 (3H, t), 1.5-1.4 (2H, m). MS m/z 560 (M+Na).
HPLC: 97.0%.
Example 3
Propane- l-sulfonic acid 4-[2-(2 4-dichlorophenyl -5-methyl-4-(piperidin-l-
ylcarbamoyl)-
25 imidazol-l-yl]phenyl ester
A solution of 2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-lH-imidazole-
4-
carboxylic acid piperidin-l-ylamide, from Ex. 2, Step 1 (320 mg, 0.72 mmol) in
10 ml
CH2C12 was cooled to 0 C. Triethylamine (1 Q0 Rl, 0.72 mmol) was added
followed by 1-
propanesulfonyl chloride (81 RI, 0.72 mmol) and the reaction mixture was
stirred at room
30 temperature overnight. Water was added, the mixture extracted with CH2C12
(3x20 ml),
dried (Na2S04), filtered and concentrated. Flash chromatography (silica,
hexane:EtOAc
1:2) afforded 220 mg (56%) of the title compound as a white solid.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
36
'H NMR (CDC13): 6 7.9 (111, broad s), 7.4-7.1 (7H, m), 3.3 (2H, m), 3.0-2.8
(411, m), 2.5
(31-1, s), 2.1-1.9 (2H, m), 1.9-1.7 (4H, m), 1.5-1.4 (2H, m), 1.2 (3H, t).
MS m/z 574 (M+Na). HPLC: 97.0%.
Example 4
Butane- l-sulfonic acid 4-f2-(2,4-dichlorophenyl -5-methyl-4-(piperidin-1-
ylcarbamoyl)-
imidazol-1-yl]phen l ester
A solution of 2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-lH-
imi'dazole-4-
carboxylic acid piperidin-1-ylamide, Ex. 2, Step 1 (320 mg, 0.72 mmol) in 10
ml CH2C12
was cooled to 0 C. Triethylamine (100 l, 0.72 mmol) was added followed by 1-
butanesulfonyl chloride (93 l, 0.72 mmol) and the reaction mixture was
stirred at room
temperature overnight. Water was added and the mixture extracted with C112C12
(3x20 ml),
dried (Na2SO4), filtered and concentrated. Flash chromatography (silica,
hexane:EtOAc
1:2) afforded 230 mg (57%) of the title compound as a white solid.
'H NMR (CDC13): S 7.9 (1H, bs), 7.4-7.1 (71-1, m), 3.3 (2H, m), 3.0-2.8 (411,
m), 2.5 (311,
s), 2.1-1.9 (2H, m), 1.9-1.7 (4H, m), 1.6-1.4 (4H, m), 1.0 (3H, t)
MS m/z 588 (M+Na). HPLC: 96.0%.
Example 5
2-(2,4-Dichlorophenyl)-5-methyl- l - [4-(4,4,4-trifluorobutoxy)-phenyl] -1 H-
imidazole-4-
carboxylic acid piperidin-l-ylamide
1-Iodo-4,4,4-trifluorobutane (376 mg, 1.58 mmol) was added dropwise to a
suspension of
2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-1 H-imidazole-4-carboxylic
acid
piperidin-1-ylamide, from Ex 2, Step 1 (351 mg, 0.79 inmol) and K2C03 (218 mg,
1.58
mmol) in 50 ml acetone. The reaction mixture was refluxed overnight, cooled,
filtered and
concentrated. Flash chromatography (silica, hexane:EtOAc 1:2) afforded 200 mg
(46%) of
the title compound as a white solid.
'H NMR (CDC13): 6 8.0 (111, broad s), 7.4-7.2 (3H, m), 7.1-7.0 (2H, m), 6.9-
6.8 (21-1, m),
4.1-4.0 (211, m), 3.0-2.9 (41-1, m), 2.5-2.2 (511, m), 2.2-2.0 (2H, m), 1.9-
1.7 (4H, m), 1.6-1.4
(211, m). MS m/z 578 (M+Na). HPLC: 99.4%.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
37
Example 6
3,3,3-Trifluoropropane-l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-
4_(piperidin-l-
ylcarbainoyl)imidazol-l-yl]phen 1 este
2-(2,4-Dichlorophenyl)- 1-(4-hydroxyphenyl)-5-methyl- 1H-imidazole-4-
carboxylic
piperidin-1-ylamide, from Ex 2, Step 1, (0.89 g, 2.00 mmol) was dissolved in
dichloromethane (20 ml), cooled to 0 C and triethylamine (0.35 ml, 2.4 rnmol)
added
followed by 3,3,3-trifluoropropanesulfonyl chloride (prepared by an analogous
method to
that described in W000/010968 for the butyl homologue) (0.35 ml, 2.40 mmol).
The
reaction mixture was stirred at room temperature overnight. TLC showed
remaining
io starting material and so another portion of triethylamine and 3,3,3-
trifluoropropanesulfonyl
chloride was added and the reaction mixture stirred for additional 2 hrs.
Water was added
and the product was extracted with dichloromethane, dried (Na2SO4), filtered
and
concentrated. Flash chromatography (hexane : EtOAc 1 : 3 - EtOAc) followed by
recrystallisation (hexane : EtOAc) afforded 700 mg (59%) of the title compound
as a
is colorless solid.
1H NMR(CDC13): 5.7.40-7.10 (8H, m), 3.60-3.43 (2H, m), 3.02-2.70 (6H, in),
2.50 (3 H,
s), 1.92-1.70 (4 H, m), 1.57-1.40 (2 H, m). MS m/z 627 (M+Na). HPLC: 97.8: %
Example 7
4,4,4-Triuorobutane-l-sulfonic acid 4-[2-(2,4-dichlorophenyl)-5-methyl-4-
(piperidin-l-
2o ylcarbamoyl)imidazol-1-yll-phenyl ester
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-1 H-imidazole-4-carboxylic
piperidin-1-ylamide, from Ex 2, Step 1 (0.49 g, 1.20 mmol) was dissolved in
dichloromethane (20 ml), cooled to 0 C and triethylamine (0.67 ml, 4.8 rnmol)
added
followed by 4,4,4-trifluorobutane-l-sulfonyl chloride (prepared as described
in
25 W000/010968) (0.38 g, 1.80 mmol). The reaction mixture was stirred at room
temperature
for 3 hrs. TLC showed remaining starting material and another portion of
triethylamine and
4,4,4-trifluorobutane-l-sulfonyl chloride was added and the reaction mixture
stirred
overnight. Water was added, the product extracted with dichloromethane, dried
(Na2SO4),
filtered and concentrated. Flash chromatography (hexane : EtOAc 1 : 3 - EtOAc)
followed
30 by recrystallisation (hexane : EtOAc) afforded 0.45 g (61%) of the title
compound as a
colorless solid.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
38
'H NMR(CDC13): 6 7.35-7.19 (8H, m), 3.40 (2H, m), 3.05-2.90 (4H, m), 2.78-2.20
(7 H, s
and m), 1.92-1.70 (4 H, m), 1.57-1.40 (2 H, m). MS m/z 641 (M+Na). HPLC: 98.6%
Example 8
4- {2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin- l -ylamino)carbony-l]-1H-
imidazol- l -
ylphen thiophene-2-sulfonate
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-piperidin-1 -yl-1H-
imidazole-4-
carboxamide, prepared as described in Ex. 2, Step 1 (100 mg, 0.22 nimol) and
triethylamine (0.31 ml, 2.25 mmol) in dichloromethane (2.5 ml) were cooled to -
78 C. 2-
Thiophenesulfonyl chloride (287mg, 1.57mmol) dissolved in dichloromethane (2.5
ml)
io was carefully added to the reaction mixture. The resulting mixture was
stirred at -78 C for
1 h, and at room temperature overnight. Water was added to the reaction, the
phases were
separated and the organic phase washed with water and dried. The solvent was
removed
under reduced pressure and separation by preparatory HPLC gave the title
compound (110
mg, 83%) as a solid.
1H NMR (400 MHz) 6 7.89 (s, NH), 7.75-7.74 (m, 1H), 7.55-7.54 (m, 1H), 7.35-
7.34 (m,
1H), 7.30-7.25 (m, 2H), 7.13-7.11 (m, 1H), 7.07 (d, 4H), 2.90-2.86 (rn, 4H),
1.80-1.75 (m,
4H), 1.48-1.42 (m, 2H). MS ni/z 591 (M+H)+.
Example 9
4-{2-(2,4-dichlorophenyl)-5-meth yl-4-[(piperidin-1-ylamino)carbowI]-1H-
imidazol-1-
ylTphenyl pyridine-3-sulfonate
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-piperidin-1 -yl-1H-
imidazole-4-
carboxamide, prepared as described in Ex. 2, Step 1 (100 mg, 0.22 raLmol) and
triethylamine (0.31 ml, 2.25 mmol) in dichloromethane (5.0 ml) were cooled to -
78 C. 3-
Pyridinesulfonyl chloride (144 mg, 0.67 mmol) was added in small portions to
the reaction
mixture. The resulting mixture was stirred at -78 C for lh, and at room
temperature
overnight. Water was added to the reaction, the phases were separated and the
organic
phase washed with water and dried. The solvent was removed under reduced
pressure and
separation by preparatory HPLC gave the title compound (110 mg, 84%) as a
solid.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
39
'H NMR (400 MHz) S 8.96 (s, 1H), 8.92 (s, 1H), 8.09-8.06 (m, 1H), 7.89 (s,
1H), 7.51-
7.49 (m, 1H), 7.36 (d, 1H), 7.30-7.25 (m, 2H), 7.06 (s, 4H), 2.88-2.84 (m,
4H), 2.48 (s,
3H), 1.79-1.74 (m, 4H), 1.47-1.41 (m, 2H). MS m/z 586 (M+H)+.
Example 10
4-{2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-1-ylamino carbonyll-1H-
imidazol-l-
yl } phenyl 5-chlorothiophene-2-sulfonate
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-piperidin-1-yl-1 H-
imidazole-4--
carboxamide, prepared as described in Ex. 2, Step 1 (100 mg, 0.22 mmol) and
triethylamine (0.16 ml, 1.12 mmol) in dichloromethane (2.5 ml) were cooled to -
78 C. 5 -
Chlorothiophen-2-sulfonyl chloride (244 mg, 1.12 mmol) in dichloromethane (2.5
ml) was
carefully added to the reaction mixture. The resulting mixture was stirred at -
78 C for lh,
and at room temperature overnight. Water was added to the reaction, the phases
were
separated and the organic phase washed with water and dried. The solvent was
removed
under reduced pressure and separation by preparatory HPLC gave the title
compound (84
mg, 60%) as a solid.
1H NMR (400 MHz) S 7.90 (s, NH), 7.34-7.26 (m, 4H), 7.10 (d, 4H), 6.96 (d,
1H), 2.88-
2.85 (m, 4H), 2.49 (s, 3H), 1.79-1.74 (m, 4H), 1.47-1.42 (m, 2H). MS m/z 625
(M+H)+.
Example 11
4- {2-(2,4-dichlorophenyl)-5 -methyl=4- [(piperidin-1-ylamino)carbonyll -1 H-
imidazol-1-
yl}phenyl 3-methylbutane- l -sulfonate
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-piperidin-1-yl-1H-
imidazole-4--
carboxamide, prepared as described in Ex. 2, Step 1 (50 mg, 0.11 mmol) was
dissolved in
dichloromethane (3.0 ml), cooled to 0 C and triethylamine (20 l, 0.13 mmol)
was added
to the mixture. The resulting mixture was cooled to -78 C and 3-methylbutane-l-
sulfony-l
chloride (23 mg, 0.13 mmol) was carefully added. The reaction was stirred at -
78 C for
1.5h. Water was added to the reaction, the product extracted with
dichloromethane and
dried. The solvent was removed under reduced pressure and separation by
preparatory
HPLC gave the title compound (46 mg, 71%) as a solid.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
'H NMR (400 MHz) S 7.86 (s, NH), 7.30-7.20 (m, 5H), 7.12-7.09 (m, 2H), 3.26-
3.22 (m,
2H), 2.86-2.80 (m, 4H), 2.46 (s, 3H), 1.86-1.80 (m, 3H), 1.77-1.69 (m, 4H),
1.45-1.37 (m,
2H), 0.93 (d, 6H). MS m/z 579 (M+H)+.
Example 12
5 4-{2-(2,4-dichlorophenyl)-5-methyl-4-[(piperidin-1-ylamino)carbonyll-lH-
imidazol-l-
yl } phenyl 3 , 3 -dimethylbutane- l -sulfonate
2-(2,4-Dichlorophenyl)-1-(4-hydroxyphenyl)-5 -methyl-N-pip eridin- l -yl-1 H-
imidazole-4-
carboxamide, prepared as described in Ex. 2, Step 1 (50 mg, 0.11 mmol) was
dissolved in
dichloromethane (3.0 ml), cooled to 0 C and triethylamine (20 l, 0.13 mmol)
was added
io to the mixture. The resulting mixture was cooled to -78 C and 3,3-
dimethylbutane-l-
sulfonyl chloride (25 mg, 0.13 mmol) was carefully added. The reaction was
stirred at -
78 C for 2h. Water was added to the reaction, the product extracted with
dichloromethane
and dried. The solvent was removed under reduced pressure and separation by
preparatory
HPLC gave the title compound (46 mg, 69%) as a solid.
is 1H NMR (400 MHz) 8 7.85 (s, NH), 7.30-7.20 (m, 5H), 7.13-7.09 (m, 2H), 3.24-
3.18 (m,
2H), 2.86-2.81 (m, 4H), 2.46 (s, 3H), 1.86-1.80 (m, 2H), 1.76-1.69 (m, 5H),
1.44-1.37 (m,
2H), 0.92 (s, 9H).. MS m/z 593 (M+H)+.
Example 13
Step 1: 1 -[4-(Benz loxy)phenyl]-2-(2,4-dichlorophenyl -5-methyl-N-[5-
20 (trifluoromethyl)pyridin-2-yl]-1H-imidazole-4-carboxamide
2-Amino-5-(trifluoromethyl)pyridine (404 mg, 2.49 mmol) was dissolved in
dichloromethane (2.5 ml) under argon and trimethylaluminium (1.25 ml, 2.0 M in
toluene,
2.5 mmol) was carefully added during 5 min. The solution was stirred at
ambient
temperature for 1.5 h and, as a result, a 0.66 M solution of an amidation
reagent was
25 obtained. 3.75 ml (2.5 mmol) of this stock solution was added to ethyl 1-[4-
(benzyloxy)phenyl]-2-(2,4-dichlorophenyl)-5-methyl-1 H-imidazole-4-
carboxylate,
prepared as described in Ex. 1, Step 2, (400 mg, 0.83 mmol) and the reaction
solution was
stirred at 45 C overnight. The reaction solution was cooled to 0 C and
quenched with HCl
(aq, 2 M, 7.5 ml). The mixture was diluted with dichloromethane and
neutralized by
30 addition of KOH (aq, 2 M). The organic phase was separated and the aqueous
phase was
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
41
extracted further with dichloromethane. The collected organic phases were
washed with
H2O before drying with Na2SO4. The solvent was removed under reduced pressure
and
purification by preparatory HPLC gave the title compound (319 mg, 64%) as a
solid.
1H NMR (400 MHz) 8 9.89 (s, NH), 8.54 (s, 1H), 8.50 (d, 1H), 7.92-7.88 (m,
1H), 7.40-
7.33 (m, 5H), 7.27-7.20 (m, 3H), 7.03-6.91 (m, 4H), 5.02 (s, 2H), 2.50 (s,
3H).
MS m/z 597 (M+H)+.
Step 2: 2-(2,4-dichlorophenyl)-1-(4-hydroyphenyl)-5-meth l-N-15-
(trifluoromethyl)pyridin-2-yl] -1 H-imidazole-4-carb oxaniide
1-[4-(benzyloxy)phenyl]-2-(2,4-dichlorophenyl)-5-methyl-N-[5-
(trifluoromethyl)pyridin-
2-yl]-1H-imidazole-4-carboxamide (319 mg, 0.53 mmol) was dissolved in hydrogen
bromide (7.5 ml, 4.1 M in acetic acid, 30.75 mmol) and the reaction mixture
was stirred at
room temperature for 4h. The acetic acid was co-evaporated with ethanol, the
residue
neutralized with ammonia and dissolved in methanol. Purification by flash
chromatography gave the title compound (266 mg, 98%).
1H NMR (400 MHz) 8 10.36 (s, NH el. OH), 10.09 (s, NH el. OH), 8.89 (s, 1H),
8.69 (d,
1H), 8.48-8.43 (m, 1H), 7.87-7.80 (m, 2H), 7.67 (d, 1H), 7.41 (d, 2H), 7.06
(d, 2H), 2.65
(s, 3H). MS m/z 507 (M+H)+.
Step 3: 4-[2-(2,4-dichlorophenyl -5-meths({[5-(trifluoromethyl)pyridin-2-
yl]amino)carbonyl)-1H-imidazol-l-yllphenyl 3,3 3-trifluoropropane-1-sulfonate
2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-[5-
(trifluoromethyl)pyridin-2-yl]-
1H-imidazole-4-carboxamide (136 mg, 0.27 mmol) and triethylamine (40 l, 0.32
mmol)
in dichloromethane (4.0 ml) were cooled to -78 C. 3,3,3-trifluoropropane-l-
sulfonyl
chloride (63 mg, 0.32 mmol) was carefully added to the reaction mixture. The
resulting
mixture was stirred at -78 C for lh, and then allowed to reach room
temperature. Water
was added to the reaction, and the phases were separated. The organic phase
was washed
with NaHCO3, brine and dried with Na2SO4. The solvent was removed under
reduced
pressure and separation by preparatory HPLC gave the title compound (88 mg,
49%) as a
solid.
1H NMR (400 MHz) 8 9.87 (s, NH), 8.55 (s, 1H), 8.49 (d, 1H), 7.93-7.89 (m,
1H), 7.34-
7.18 (m, 7H), 3.53 (m, 2H), 2.85-2.73 (m, 2H), 2.54 (s, 3H). MS m/z 667
(M+H)+.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
42
Example 14
4-[2-(2,4-dichlorophenyl)-5-methyl-4-({[5-(trifluoromethyl)pyridin-2-yllamino}
carbonyl)-
1 H-imidazol- l-yl]phenyl 3-methylbutane- l-sulfonate
2-(2,4-dichlorophenyl)-1-(4-hydroxyphenyl)-5-methyl-N-[5-
(trifluoromethyl)pyridin-2-yl]-
1H-imidazole-4-carboxamide, prepared as described in Ex. 13, Step 3 (139 mg,
0.27
mmol) and triethylamine (46 l, 0.33 mmol) in dichloromethane (4.0 ml) were
cooled to -
78 C. 3-Methylbutane-l-sulfonyl chloride (56 mg, 0.33 mmol) was carefully
added to the
reaction mixture. The resulting mixture was stirred at -78 C for lh, and then
allowed to
reach room temperature. Water was added to the reaction, and the phases were
separated.
io The organic phase was washed with NaHCO3, brine and dried with Na2SO4. The
solvent
was removed under reduced pressure and separation by preparatory HPLC gave the
title
compound (81 mg, 46%) as a solid.
1H NMR (400 MHz) 6 9.87 (s, NH), 8.55 (s, 1H), 8.49 (d, 1H), 7.93-7.89 (m,
1H), 7.34-
7.14 (m, 7H), 3.29-3.24 (m, 2H), 2.53 (s, 3H), 1.88-1.81 (m, 2H), 1.79-1.69
(m, 1H), 0.95
(d, 6H). MS m/z 641 (M+H)+.
CA 02560417 2006-09-19
WO 2005/095354 PCT/GB2005/001153
43
General Synthetic Route
CN
CI
Br0
H NH
\ NHa I \
CI \ 0 / CI 0
01,
CI
0 0
0 OH rD
N N H2N
\ N N 'N
cr 0 ff CI cro~ CI
I
\ \I
CI CI
0 NJ 0 N--/
H H
NN
R-X
N ,N N .'IN
Cro CI HO CI
\ I \ I
CI CI
0 NO
N
R` ' \ N f N
0 CI
/I
CI